ABSTRACT
Background
Evidence suggests that the relations between intakes of individual fatty acids and risk of type 2 diabetes (T2D) vary. However, associations between intakes of different cooking oils as sources of fatty acids and incident T2D remain largely unknown.
Objectives
We aimed to evaluate relations between intakes of individual cooking oils and incident T2D in a nationwide Chinese cohort.
Methods
Overall 15,022 Chinese adults aged ≥20 y from the China Health and Nutrition Survey (CHNS) without self-reported T2D at entry in the 1997, 2000, 2004, 2006, or 2009 rounds were followed up until 2011. Consumption of various cooking oils/fats including lard, peanut oil, soybean oil, canola oil, sesame oil, and refined blended plant oil was assessed using 3-d 24-h records in each survey and the cumulative mean intake was calculated. Multivariable-adjusted Cox proportional hazards regression models were constructed to estimate the HRs of T2D.
Results
A total of 1014 cases were recorded after a median follow-up of 14 y. The intakes of animal and plant cooking oils/fats were both associated with higher T2D risk. Compared with nonconsumers, multivariable-adjusted HRs and 95% CIs for the highest tertiles were 1.31 (1.03, 1.67) for lard, 1.36 (1.10, 1.66) for peanut oil, 1.14 (0.91, 1.43) for soybean oil, 1.11 (0.87, 1.43) for canola oil, 1.02 (0.79, 1.32) for sesame oil, and 1.42 (1.12, 1.82) for refined blended plant oil. Substituting 1 tablespoon/d (8 g · 2000 kcal−1 · d−1) of soybean oil for the sum of lard, peanut oil, refined blended plant oil, and other plant oils was associated with a 3% (HR: 0.97; 95% CI: 0.95, 0.99) lower risk of T2D.
Conclusions
Intakes of lard, peanut oil, and refined blended plant oil but not soybean oil, canola oil, and sesame oil are associated with higher T2D risk. Reducing the consumption of cooking oils in general may be protective against T2D among the Chinese population.
This trial was registered at clinicaltrials.gov as NCT03259321.
Keywords: cooking oils, animal fats, plant oils, type 2 diabetes, China Health and Nutrition Survey
Introduction
Type 2 diabetes (T2D) has become a public health problem of broad concern, affecting 425 million (8.8% of adults) people worldwide (1). In addition, 352 million people are at risk of developing T2D. In China, the T2D prevalence steeply increased from 2.5% in 1994 (2) to 10.9% in 2013 (3) and it is expected to affect 120 million people in 2045 (1). This imposes a heavy burden on patients and health care systems. Evidence showing the importance of strategies of long-term lifestyle modifications, including dietary changes, for the prevention of T2D has recently raised extensive interest (4).
The quality or type of dietary fatty acids plays an important role in T2D development (5, 6). Growing evidence has suggested that higher intake of PUFAs and/or MUFAs could benefit by improving insulin sensitivity, whereas higher intake of SFAs and trans fatty acids might adversely affect glucose metabolism and insulin resistance (6, 7). However, the biological functions of the aforementioned specific fatty acids are unlikely to consistently translate to the health effects of cooking oils (8). Although animal fat is the main source of SFAs in modern diets, some animal fats, such as lard, contain higher contents of MUFAs than SFAs (9). Vegetable oils such as peanut oil, canola oil, and soybean oil typically consist of both PUFAs and MUFAs with different concentrations as well as a small proportion of SFAs (10). Besides, other bioactive ingredients in dietary oils such as lipophilic vitamins, minerals, and hydrophilic polyphenols may also contribute to their functions (11). Therefore, emerging interest has focused on evaluating the food-based health effects of individual cooking oils that represent complex matrixes of nutrients, food structure, and processing food.
Some intervention studies have shown that vegetable oils such as canola oil, safflower oil, and olive oil benefit glycemic control and insulin sensitivity (12–14). However, these clinical trials were conducted among patients with T2D or impaired glucose metabolism. Little is known about the consumption of these cooking oils in relation to the risk of T2D development among the general population. Data are also missing regarding other vegetable oils, including peanut oil, soybean oil, and refined blended plant oil. Lard is traditionally considered as an unhealthy source of animal fat and frequently used for a high-fat diet to induce an obese and diabetic phenotype in rodents (15). Nonetheless, limited evidence supports the negative impact of lard on developing T2D in humans.
In China, commonly used cooking oils include lard, peanut oil, canola oil, soybean oil, sesame oil, and various processed vegetable oils, which are made from refining mixtures of crude plant oils. However, it is unclear whether individual cooking oils are protective or detrimental for T2D development, especially considering the fact that cooking oils are commonly stir-fried which may potentially exert adverse health effects. Therefore, large population-based studies among Chinese people are warranted. Here we longitudinally examined the associations of individual cooking oil consumption in relation to T2D incidence in the China Health and Nutrition Survey (CHNS), which consists of a nationwide Chinese population.
Methods
Study population
Our study (NCT03259321) used data from the general population in the CHNS, a large-scale, household-based, open-cohort study initiated in 1989 and followed up every 2–3 y thereafter to evaluate the associations of the social and economic transformation with health and nutrition among the Chinese population. Detailed information on the CHNS has been described elsewhere (16). The survey used a multistage random-cluster sampling process to draw samples from 9 provinces, which represent 553 million people, to generally represent variations in economic development, geographic distribution, and health indicators throughout China. A total of 24 communities were randomly selected in each province as the primary sampling units and 20 households in each community were then selected. Household members were all interviewed by trained interviewers. A total of 9 rounds (1989, 1991, 1993, 1997, 2000, 2004, 2006, 2009, and 2011) of data collection have been conducted.
Information on T2D diagnosis has been collected from participants since 1997. Therefore, we utilized data from 6 rounds spanning 1997–2011 for the current analysis. Among 26,889 eligible participants, we excluded persons aged <20 y at entry, those without dietary data, participants who had extreme energy intake (<800 or >4200 kcal/d for men and <600 or >3500 kcal/d for women), and those who had diagnoses of T2D, myocardial infarction, or stroke at baseline. After exclusion, data from 15,022 participants including 7035 men and 7987 women were available for the final analysis (Supplemental Figure 1). The study was approved by the institutional review committees of the University of North Carolina and the National Institute of Nutrition and Food Safety from the Chinese Center for Disease Control and Prevention. All the participants provided written informed consent.
Dietary assessment and covariates
In each survey, dietary data were assessed by trained interviewers using weighing methods in combination with 3 consecutive 24-h dietary recalls at both the household level and the individual level (17, 18). In the 3-d 24-h dietary recalls, participants were asked to report in-home individual consumption and all food consumed away from home. In the home inventory, all foods, cooking oils, and condiments purchased from markets or picked from gardens, and food waste were weighed and recorded at the beginning and the end of the 3-d 24-h dietary recalls, which were randomly selected from Monday to Sunday and almost equally balanced across the 7 d of the week for each sampling unit.
We then allocated proportions of cooking oils consumed at the household level to each individual based on the proportion of specific cooking oils that he or she consumed. In Chinese cuisine, oils are added into all cooked dishes except rice and steamed flour products during cooking and preparation. Cooking oil consumption for each individual participant was then estimated from the individual proportional consumption of oil-containing foods using weighted household intake. Dietary intakes of nutrients and total energy were calculated using corresponding versions of the Chinese Food Composition Table for each round. Three-day average intakes of individual cooking oils/fats in each round were calculated, and cumulative mean intakes in all available rounds from baseline to the last round before T2D diagnosis or the end of follow-up were then further calculated to represent long-term diet and minimize within-person variation. In a validation study, the correlation coefficient between the total energy intake calculated by the dietary assessment method in the CHNS and that measured by the doubly labeled water method was 0.56 for men and 0.60 for women (both P < 0.01) (19). Oil consumed as part of restaurant food was not assessed but the rate of eating out was not high (20). In a CHNS study of 1013 adults from the 2011 survey year (20), the proportions of participants who reported eating out >2 times/wk in Western fast-food restaurants, Chinese full-service restaurants, Chinese fast-food restaurants, mobile food carts, cafes, canteens, and other restaurants were 0%, 8.5%, 6.9%, 4.3%, 0%, 8.1%, and 1.0%, respectively. Data on other demographic and lifestyle factors were also collected, including age, weight and height, marital status, income, education level, alcohol drinking, smoking, physical activity, history of hypertension, and site (urban or rural) (16).
Ascertainment of diabetes
Since 1997, participants have completed questionnaires on whether they had physician-diagnosed T2D and other detailed information, including the diagnosis date and hypoglycemic therapy, in each round thereafter. In the 2009 round, participants provided overnight-fasting blood samples and their plasma glucose and glycated hemoglobin (HbA1c) levels were measured with strict quality control (16). Individuals with a fasting plasma glucose ≥7.0 mmol/L or HbA1c ≥6.5% were then defined as having diabetes in addition to self-reported diabetes obtained from the questionnaire. Among 15,022 individuals in our current analysis, 7708 (51.3%) had data on plasma glucose collected in the 2009 round. The specificity of self-reported diabetes was assessed by a cross-sectional analysis among 10,215 participants. Of 9964 participants who reported being free of diabetes, 753 participants (7.6%) had diabetes according to fasting plasma glucose and HbA1c levels. Therefore, 92.4% of participants who did not report diagnosed diabetes were below the blood glucose threshold for diabetes, indicating a low rate of undiagnosed diabetes in this population.
Statistical analysis
Intakes of animal fats, plant oils, and other nutritional variables were divided by the daily calorie intake (g · 2000 kcal−1 · d−1) according to the nutrient density method (21). Based on the consumption of individual cooking oils/fats at the household level, lard, peanut oil, canola oil, soybean oil, sesame oil, and refined blended plant oil were selected as commonly consumed cooking oils/fats in the CHNS and separately analyzed. To consider the intakes of minor cooking oils in China and their associations with T2D risk, we categorized beef tallow, duck grease, and mutton tallow as other animal fats, whereas we classified cottonseed oil, sunflower seed oil, safflower oil, corn oil, tea seed oil, and olive oil as other plant oils. The correlations between consumption of individual cooking oils/fats were assessed by the Spearman correlation test. Consumers were divided into 3 groups according to tertiles of individual intakes, whereas nonconsumers were categorized as the reference category for the analyses. The consumption of other animal cooking fats was very low and thus was only divided into consumers and nonconsumers to maintain statistical power.
Person-years of follow-up for the individuals were calculated from the round at entry to the year of diagnosis of T2D, censoring at death, or the end of follow-up, whichever came first. We used time-dependent Cox proportional hazards regression models to estimate HRs and 95% CIs of T2D risk according to individual cooking oil/fat intake. In multivariable analyses, the model was adjusted for age, gender, marital status, BMI, income, education, physical activity, smoking, alcohol drinking status, site, history of hypertension, total energy intake, and consumption of red meat, white meat, vegetables, and fruit. When 1 type of oil/fat was analyzed, other types of oils/fats were simultaneously adjusted in this model. SEs and variance of the estimates were also adjusted by clustering at the community level to control for design effects. Tests for trend were computed by using the median values of each category as continuous variables in regression models. We also tested the potential nonlinearity using a likelihood ratio test which compared the model with only the linear term of specific oil consumption with the model with both the linear and the cubic spline terms.
We also estimated the change of T2D risk when hypothetically substituting 1 tablespoon/d (8 g · 2000 kcal−1 · d−1) of soybean oil, canola oil, or sesame oil for the sum of lard, peanut oil, refined blended plant oil, and other plant oils by simultaneously including these continuous variables in the same multivariable model, which also contained total energy intake and other nondietary covariates. The difference between regression coefficients and invariances and covariances was used to derive the HRs and 95% CIs of the substitution analyses.
Sensitivity analyses were also conducted to test the robustness of documented associations. We excluded persons who developed incident T2D during the initial 3 y of follow-up or further adjusted for health insurance to examine whether the results materially changed. In addition, an alternative healthy eating index (AHEI-2010) (22) was further adjusted to see whether the results were explained by the overall dietary pattern. We also analyzed the associations for women and men separately.
Statistical analyses were performed using the SAS statistical package (version 9.4; SAS Institute). Statistical tests were 2-sided and significance was defined as P < 0.05.
Results
Baseline characteristics
Table 1 summarizes baseline characteristics of participants by categories of plant cooking oil and animal cooking fat consumption. Compared with nonconsumers, those with higher animal cooking fat intake were thinner, less educated, and less likely to smoke, drink alcohol, and consume fruit, whereas they tended to exercise more, reside in rural areas, and consume more vegetables and red meat. Participants with higher plant cooking oil consumption were generally older, wealthier, and less physically active. They were more often females, urban residents, current smokers, and alcohol drinkers, and had a higher BMI. They also had a higher prevalence of hypertension and tended to consume more white meat and fruit but less red meat and vegetables. The mean intake in the highest tertile of consumption among consumers was 37.0 g · 2000 kcal−1 · d−1 for animal cooking fats and 50.3 g · 2000 kcal−1 · d−1 for plant cooking oils.
TABLE 1.
Baseline characteristics of participants in the China Health and Nutrition Survey according to categories of animal cooking fat and plant cooking oil consumption1
| Categories of animal cooking fat intake | Categories of plant cooking oil intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Consumers | Consumers | |||||||||
| Characteristics | Nonconsumers | T1 | T2 | T3 | P-trend2 | Nonconsumers | T1 | T2 | T3 | P-trend2 |
| n | 10,675 | 1449 | 1449 | 1449 | 855 | 4722 | 4723 | 4722 | ||
| Oil/fat intake, g · 2000 kcal−1 · d−1 | 0 | ≤9.6 | 9.7–22.1 | ≥22.2 | 0 | ≤23.4 | 23.5–34.9 | ≥35.0 | ||
| Male | 46.4 | 50.0 | 47.6 | 45.8 | 0.72 | 49.1 | 50.7 | 46.7 | 42.8 | <0.001 |
| Married | 80.2 | 85.1 | 80.9 | 79.7 | 0.60 | 67.6 | 78.0 | 83.0 | 83.5 | <0.001 |
| Age, y | 43.1 ± 0.1 | 42.8 ± 0.4 | 42.3 ± 0.4 | 43.9 ± 0.4 | 0.61 | 41.5 ± 0.6 | 41.2 ± 0.2 | 42.9 ± 0.2 | 45.4 ± 0.2 | <0.001 |
| BMI, kg/m2 | 22.5 ± 0.0 | 21.8 ± 0.1 | 21.4 ± 0.1 | 21.4 ± 0.1 | <0.001 | 21.3 ± 0.1 | 21.9 ± 0.0 | 22.2 ± 0.1 | 22.6 ± 0.1 | <0.001 |
| Individual income,3 yuan | 7329 ± 124 | 5651 ± 229 | 5269 ± 203 | 5570 ± 317 | <0.001 | 4680 ± 260 | 6523 ± 183 | 6752 ± 163 | 7465 ± 184 | <0.001 |
| High school or above | 14.4 | 11.0 | 7.5 | 6.6 | <0.001 | 6.4 | 12.0 | 13.0 | 14.1 | <0.001 |
| Moderate to vigorous activity level | 55.5 | 64.9 | 69.6 | 68.7 | <0.001 | 68.8 | 63.2 | 59.1 | 53.1 | <0.001 |
| Current smoker | 21.9 | 20.2 | 20.6 | 19.5 | 0.02 | 19.7 | 21.0 | 20.6 | 22.7 | 0.02 |
| Alcohol drinker | 26.6 | 22.0 | 22.6 | 22.0 | <0.001 | 22.5 | 24.6 | 25.7 | 26.2 | 0.01 |
| History of hypertension | 11.8 | 7.4 | 5.5 | 6.2 | <0.001 | 6.6 | 7.5 | 9.7 | 14.1 | <0.001 |
| Urban site | 39.6 | 37.6 | 27.7 | 23.5 | <0.001 | 22.9 | 33.4 | 37.0 | 42.0 | <0.001 |
| Dietary intake | ||||||||||
| Total energy, kcal/d | 2152 ± 5 | 2207 ± 12 | 2169 ± 12 | 2145 ± 13 | 0.47 | 2076 ± 20 | 2178 ± 7 | 2177 ± 7 | 2135 ± 7 | 0.24 |
| White meat, g · 2000 kcal−1 · d−1 | 57.3 ± 0.8 | 57.2 ± 1.4 | 40.3 ± 1.5 | 44.4 ± 1.7 | <0.001 | 41.1 ± 2.7 | 48.8 ± 1.2 | 57.7 ± 1.0 | 59.2 ± 1.0 | <0.001 |
| Red meat, g · 2000 kcal−1 · d−1 | 88.5 ± 0.9 | 96.9 ± 1.9 | 107.4 ± 2.2 | 108.7 ± 2.1 | <0.001 | 104.6 ± 4.9 | 94.4 ± 1.2 | 94.5 ± 1.2 | 88.2 ± 1.2 | <0.001 |
| Vegetables, g · 2000 kcal−1 · d−1 | 434 ± 2 | 459 ± 5 | 490 ± 5 | 490 ± 5 | <0.001 | 534 ± 11 | 450 ± 3 | 431 ± 3 | 446 ± 3 | <0.001 |
| Fruit, g · 2000 kcal−1 · d−1 | 49.9 ± 0.9 | 32.0 ± 1.5 | 23.1 ± 1.4 | 23.1 ± 1.7 | <0.001 | 25.4 ± 3.2 | 35.8 ± 1.1 | 47.5 ± 1.2 | 49.0 ± 1.4 | <0.001 |
n = 15,022. Values are means ± SEs or percentages unless otherwise indicated. T, tertile.
P-trend values for categorical variables were analyzed by Cochran–Armitage tests and the t test for slope was used for continuous variables in generalized linear models.
Individual income was inflated to 2009.
Consumption of individual cooking oils/fats
During 1997–2011, animal cooking fat consumption at household level gradually declined from 22.4% in 1997 to 11.6% in 2011, whereas plant cooking oil consumption rose from 77.6% in 1997 to 88.5% in 2011. Peanut oil, soybean oil, and canola oil were the top 3 most widely consumed plant cooking oils followed by refined blended plant oil, sesame oil, and other plant oils. For the animal cooking fats, lard consumption accounted for the predominant proportion (Supplemental Table 1). Animal cooking fat intake was weakly and negatively correlated with plant cooking oil intake (R = −0.35, P < 0.001). Intakes of individual cooking oils were all weakly correlated with each other (all |R|<0.4; Supplemental Table 2). As regards the preparation methods of cooking oils, stir-frying was the most common cooking method for lard, peanut oil, soybean oil, and canola oil consumption (Supplemental Table 3). Notably, refined blended plant oil was the most frequently deep-fried cooking oil (28.6%), whereas sesame oil was the only oil that had ever been consumed raw (46.2%).
Animal cooking fat consumption and risk of T2D
During a total of 167,698 person-years of follow-up, we documented 1014 incident cases of T2D. In age- and sex-adjusted models, animal cooking fat consumption was significantly associated with lower T2D risk (Table 2). However, the relation appeared positive after further adjusting for other covariates in multivariable-adjusted models; the vegetable intake was responsible for changing the direction of the association. Compared with nonconsumers, those in the highest tertile of animal cooking fat consumption had a 32% higher risk of T2D (HR: 1.32; 95% CI: 1.04, 1.68; P-trend = 0.004). This positive association was largely driven by lard consumption. Similarly, those consuming ≥22.2 g · 2000 kcal−1 · d−1 lard compared with nonconsumers had a 31% higher risk of T2D (HR: 1.31; 95% CI: 1.03, 1.67; P-trend = 0.007). Consumption of other animal cooking fats was not associated with T2D risk (HR: 0.93; 95% CI: 0.53, 1.61; P-trend = 0.78).
TABLE 2.
Associations between consumption of individual cooking oils/fats and type 2 diabetes risk among Chinese adults in the China Health and Nutrition Survey1
| Categories of cooking oil/fat intake | ||||||
|---|---|---|---|---|---|---|
| Consumers | ||||||
| Nonconsumers | T1 | T2 | T3 | P-trend | P nonlinearity | |
| Animal cooking fats | ||||||
| Fat intake, g · 2000 kcal−1 · d−1 | 0 | ≤9.6 | 9.7–22.1 | ≥22.2 | ||
| Cases/n | 709/10,675 | 119/1449 | 92/1449 | 94/1449 | ||
| Person-years | 115,364 | 17,508 | 17,387 | 17,447 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.09 (0.90, 1.33) | 0.85 (0.69, 1.06) | 0.79 (0.64, 0.98) | 0.02 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.33 (1.09, 1.64) | 1.26 (1.00, 1.59) | 1.32 (1.04, 1.68) | 0.004 | 0.06 |
| Lard | ||||||
| Fat intake, g · 2000 kcal−1 · d−1 | 0 | ≤9.7 | 9.8–22.1 | ≥22.2 | ||
| Cases/n | 715/10,730 | 117/1430 | 90/1431 | 92/1431 | ||
| Person-years | 115,990 | 17,300 | 17,198 | 17,218 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.09 (0.89, 1.32) | 0.84 (0.67, 1.04) | 0.78 (0.63, 0.97) | 0.02 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.33 (1.08, 1.63) | 1.24 (0.98, 1.56) | 1.31 (1.03, 1.67) | 0.007 | 0.07 |
| Other animal cooking fats3 | ||||||
| Fat intake, g · 2000 kcal−1 · d−1 | 0 | >0 | — | — | ||
| Cases/n | 1001/14,842 | 13/180 | — | — | ||
| Person-years | 165,451 | 2256 | — | — | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 0.88 (0.51, 1.52) | — | — | 0.65 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 0.93 (0.53, 1.61) | — | — | 0.78 | 0.65 |
| Plant cooking oils | ||||||
| Oil intake, g · 2000 kcal−1 · d−1 | 0 | ≤23.4 | 23.5–34.9 | ≥35.0 | ||
| Cases/n | 127/855 | 313/4722 | 238/4723 | 264/4722 | ||
| Person-years | 9607 | 54,127 | 53,020 | 50,951 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 2.44 (1.53, 3.88) | 3.07 (1.93, 4.87) | 3.64 (2.30, 5.76) | <0.001 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.82 (1.14, 2.91) | 2.13 (1.33, 3.41) | 2.37 (1.49, 3.79) | <0.001 | 0.12 |
| Peanut oil | ||||||
| Oil intake, g · 2000 kcal−1 · d−1 | 0 | ≤18.0 | 18.1–30.4 | ≥30.5 | ||
| Cases/n | 584/9730 | 159/1764 | 139/1764 | 132/1764 | ||
| Person-years | 108,050 | 20,487 | 19,744 | 19,425 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.35 (1.13, 1.61) | 1.23 (1.02, 1.48) | 1.07 (0.89, 1.29) | 0.08 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.18 (0.98, 1.43) | 1.42 (1.16, 1.73) | 1.36 (1.10, 1.66) | <0.001 | 0.16 |
| Soybean oil | ||||||
| Oil intake, g · 2000 kcal−1 · d−1 | 0 | ≤16.3 | 16.4–31.1 | ≥31.2 | ||
| Cases/n | 606/10,124 | 176/1632 | 111/1633 | 121/1633 | ||
| Person-years | 114,000 | 18,374 | 17,865 | 17,468 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.87 (1.58, 2.21) | 1.34 (1.09, 1.64) | 1.39 (1.15, 1.69) | <0.001 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.37 (1.14, 1.64) | 1.04 (0.83, 1.30) | 1.14 (0.91, 1.43) | 0.23 | 0.49 |
| Canola oil | ||||||
| Oil intake, g · 2000 kcal−1 · d−1 | 0 | ≤10.9 | 11.0–23.2 | ≥23.3 | ||
| Cases/n | 711/10,491 | 123/1510 | 95/1511 | 85/1510 | ||
| Person-years | 114,301 | 18,936 | 17,629 | 16,842 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.01 (0.83, 1.22) | 0.87 (0.70, 1.08) | 0.75 (0.60, 0.94) | 0.01 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.18 (0.96, 1.46) | 1.18 (0.94, 1.49) | 1.11 (0.87, 1.43) | 0.17 | 0.70 |
| Refined blended plant oil | ||||||
| Oil intake, g · 2000 kcal−1 · d−1 | 0 | ≤8.6 | 8.7–20.3 | ≥20.4 | ||
| Cases/n | 720/11,975 | 108/1015 | 96/1016 | 90/1016 | ||
| Person-years | 133,560 | 11,945 | 11,340 | 10,861 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.71 (1.39, 2.09) | 1.53 (1.24, 1.90) | 1.44 (1.15, 1.79) | <0.001 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.43 (1.16, 1.76) | 1.43 (1.15, 1.79) | 1.42 (1.12, 1.82) | <0.001 | 0.08 |
| Sesame oil | ||||||
| Oil intake, g · 2000 kcal−1 · d−1 | 0 | ≤0.4 | 0.5–1.7 | ≥1.8 | ||
| Cases/n | 781/12,906 | 94/705 | 68/706 | 71/705 | ||
| Person-years | 144,692 | 7881 | 7471 | 7663 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 2.38 (1.92, 2.95) | 1.66 (1.30, 2.13) | 1.52 (1.19, 1.94) | <0.001 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.54 (1.23, 1.92) | 1.04 (0.80, 1.34) | 1.02 (0.79, 1.32) | 0.43 | 0.44 |
| Other plant cooking oils4 | ||||||
| Oil intake, g · 2000 kcal−1 · d−1 | 0 | ≤5.0 | 5.1–11.2 | ≥11.3 | ||
| Cases/n | 766/12,642 | 86/793 | 77/794 | 85/793 | ||
| Person-years | 140,206 | 9812 | 9591 | 8097 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.50 (1.20, 1.87) | 1.38 (1.09, 1.75) | 1.89 (1.51, 2.36) | <0.001 | |
| Multivariable-adjusted HR (95% CI)2 | 1.00 | 1.43 (1.14, 1.80) | 1.16 (0.91, 1.47) | 1.60 (1.27, 2.01) | <0.001 | 0.046 |
HRs (95% CIs) were estimated using time-dependent Cox proportional hazards regression models. Consumers of other animal cooking fats were categorized into 1 group (T1). T, tertile.
The multivariable model was adjusted for age, sex, BMI (in kg/m2; <18.5, 18.5–23.9, 24–27.9, or ≥28), education (less than high school, high school, some college, or at least college), marital status (never married, married or living as married, widowed/divorced/separated, or unknown), income (quartile), site (urban or rural), physical activity (no regular activity, low to moderate activity, or vigorous activity), smoking (never, former, current, or unknown), alcohol drinking status (abstainer or drinker), history of hypertension (yes, no, or unknown), and intakes of total energy, white meat, red meat, fruit, vegetables, and remaining cooking oils/fats.
Other animal cooking fats include beef tallow, duck grease, and mutton tallow.
Other plant cooking oils include cottonseed oil, sunflower seed oil, safflower oil, corn oil, tea seed oil, and olive oil.
Plant cooking oil consumption and risk of T2D
Plant cooking oil consumption was associated with higher risk of T2D development in both age- and sex-adjusted and multivariable-adjusted models (Table 2). In comparison with nonconsumers, multivariable-adjusted HRs (95% CIs) across increasing tertiles of plant cooking oil consumption were 1.82 (1.14, 2.91), 2.13 (1.33, 3.41), and 2.37 (1.49, 3.79) (P-trend < 0.001). Regarding individual plant cooking oils, significant positive associations were observed for peanut oil and refined blended plant oil. Consumption of other plant cooking oils was nonlinearly associated with T2D risk (P-nonlinearity = 0.046). The HRs (95% CIs) for T2D risk comparing the highest category with the lowest (never/almost never) category were 1.36 (1.10, 1.66) for peanut oil, 1.42 (1.12, 1.82) for refined blended plant oil, and 1.60 (1.27, 2.01) for other plant cooking oils. In contrast, intakes of soybean oil, canola oil, and sesame oil did not show any associations with T2D risk in the multivariable-adjusted analysis. The HRs (95% CIs) comparing extreme categories of consumption were 1.14 (0.91, 1.43), 1.11 (0.87, 1.43), and 1.02 (0.79, 1.32) for soybean oil, canola oil, and sesame oil, respectively.
Substitution analysis
In hypothetical substitution analyses, each 8 g · 2000 kcal−1 · d−1 increment of soybean oil in replacement of the sum of lard, peanut oil, refined blended plant oil, and other plant cooking oils was associated with a 3% lower risk of T2D (HR: 0.97; 95% CI: 0.95, 0.99) (Figure 1). Substituting canola oil or sesame oil for the sum of lard, peanut oil, refined blended plant oil, and other plant cooking oils was not related to T2D risk.
FIGURE 1.
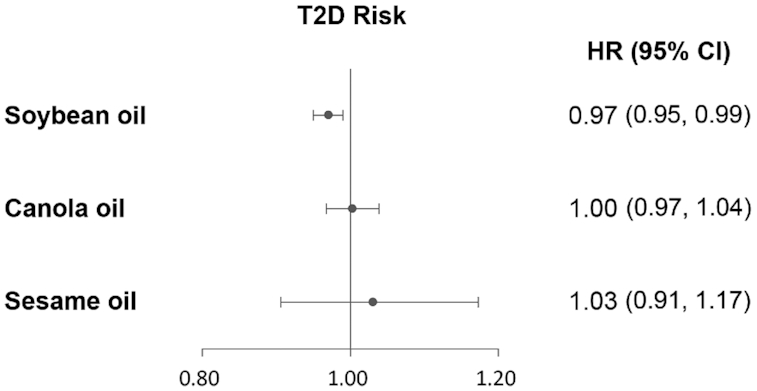
HRs (95% CIs) of T2D by hypothetical substitution of soybean oil, canola oil, or sesame oil for the sum of lard, peanut oil, refined blended plant oil, and other plant cooking oils (8 g · 2000 kcal−1 · d−1) among Chinese adults. HRs were adjusted for age, sex, BMI (in kg/m2; <18.5, 18.5–23.9, 24–27.9, or ≥28), education (less than high school, high school, some college, or at least college), marital status (never married, married or living as married, widowed/divorced/separated, or unknown), income (quartile), site (urban or rural), physical activity (no regular activity, low to moderate activity, or vigorous activity), smoking (never, former, current, or unknown), alcohol drinking status (abstainer or drinker), history of hypertension (yes, no, or unknown), and intakes of total energy, red meat, white meat, vegetables, and fruit. T2D, type 2 diabetes.
Sensitivity analyses
The documented associations between individual cooking oils and T2D risk remained largely unchanged after further adjusting for health insurance (Supplemental Table 4), AHEI-2010 (Supplemental Table 5), or excluding the T2D cases that occurred within the initial 3 y of follow-up (Supplemental Table 6). In addition, the documented associations did not appear significantly different for men and women (Supplemental Table 7).
Discussion
The present study reported the long-term associations of individual cooking oil/fat consumption with risk of T2D in China. We found positive associations of lard, peanut oil, and refined blended plant oil consumption with T2D risk. Substitution analyses showed lower T2D risk when lard, peanut oil, refined blended plant oil, and other plant cooking oils were together hypothetically replaced by soybean oil. Furthermore, canola oil and sesame oil intakes were not related to T2D risk.
Previous epidemiological data have highlighted the importance of limiting the intake of animal-sourced fatty acids in prevention and management of T2D (4). The adverse effect of animal-sourced fatty acids on T2D risk is generally thought to be due to the high content of SFAs, which has been associated with impaired glucose metabolism and insulin resistance (23, 24). In the Health Professionals Follow-Up Study, intake of SFAs was found to be correlated with higher T2D incidence (25). Consistently, we found animal cooking fat consumption was correlated with a modestly higher risk of T2D. Although lard also contains a high proportion of MUFAs, a positive relation with T2D risk was still observed. In contrast, other animal cooking fats (e.g., beef tallow and mutton fat) did not show any associations with T2D risk. Given that 75.9% of lard is stir-fried or deep-fried whereas almost 83.3% of other animal cooking fats are boiled, we suggest that the preparation method may be responsible for their various associations with T2D risk. In addition, the other components of fried as opposed to boiled dishes could also contribute to the differential associations. However, because the consumption of other animal cooking fats is very low, more investigations are needed to confirm our findings.
Unlike animal cooking fats, plant cooking oils were found to be inversely associated with incident T2D in older Iowa women (26), which may be ascribed to their high contents of MUFAs, PUFAs, and natural antioxidants like vitamin E and carotenoids. An updated review of existing evidence concluded that supplementation with 0.42–5.2 g PUFAs/d for ≥8 wk might be protective against T2D (27). Interventional studies using MUFAs also supported their beneficial effects on glycemic control and insulin resistance (24, 28–30). Olive oil, the major plant sources of MUFAs in Western countries, has been linked with lower T2D risk in several previous studies (13, 31). However, olive oil consumption was rarely reported during the study period in the CHNS. Unfortunately, similar to animal cooking fats, 80% of plant cooking oils were fried, including deep-fried, stir-fried, and griddle, whereas <1% were consumed raw in our population. Frying of plant cooking oils may produce changes harmful to health, including the increase of energy density, free fatty acid contents, the ratio of hypercholesterolemic to hypocholesterolemic fatty acids, and formation of trans fatty acids and triacylglycerol polymers and dimers (32, 33). Moreover, lipid peroxidation products from the frying process may in turn induce inflammation (34), which plays a crucial role in the pathogenesis of diabetes (35). A recent study linked fried-food consumption to incident T2D (36). Correspondingly, consumption of plant cooking oils which were commonly fried was associated with an obvious increase in T2D risk in the present study.
It was noteworthy that intakes of peanut oil and refined blended plant oil used for deep-frying were strongly associated with higher T2D risk in our study. In China, some plant oils used for cooking were refined oils processed from soybean, rapeseed, sunflower seeds, and rice bran, which could lead to a loss of phytochemicals. Refined blended plant oil was commonly used for stir-frying (28.6%) or deep-frying (28.6%) rather than salad dressing in China, which might contribute to the documented positive association with T2D incidence. Although peanut oil was shown to enhance insulin secretion in pancreatic β cells of diabetic mice (37), a human study in Myanmar reported that peanut oil consumption was positively associated with fasting plasma glucose concentrations compared with palm oil consisting of a high proportion of SFAs (38), which was consistent with our results. On the contrary, we found that sesame oil, which was rarely used for frying (7.7%), showed no significant association with T2D risk. In addition to abundant PUFAs and MUFAs, sesame oil also contains vitamin E and profuse sesame lignans, including episesamin, sesamin, sesamol, and sesamolin, which possess antioxidant properties (39, 40). An open-label study reported that 35 g sesame oil/d as a cooking medium for 2 mo was associated with 15% lower concentrations of plasma glucose among 60 T2D patients (41). A similar ameliorative effect of sesame oil on glucose metabolism was also observed in another larger trial consisting of 300 T2D patients (42). In our study, the nonsignificant association observed for sesame oil may result from the overall low intake amount in this population given that 95% of participants consumed <1.8 g · 2000 kcal−1 · d−1.
Here we also observed lower T2D risk when substituting soybean oil for other cooking oils. Soybean oil is rich in omega (ω)-6 PUFAs, mainly (51.7%) comprised of linoleic acid (LA; 18:2n–6) (43). Recently, we revealed a protective effect of LA intake on incident T2D among women (44), which was consistent with 2 other US cohort studies (26, 45). Importantly, a meta-analysis of 39,740 adults concluded that the biomarkers of ω-6 PUFAs were significantly related to lower risk of T2D (46). Experimental research in rodents showed that soybean oil could ameliorate hyperglycemia (47). In terms of putative mechanisms, LA could alleviate endotoxemia through the modulation of gut microbiota (44), whereas stigmasterol from soybean oil was able to increase glucose uptake via the induction and translocation of glucose transporter 4 (48).
A 3-mo clinical trial showed that canola oil supplementation (31 g/2000 kcal) improved glycemic control in 141 diabetic patients (12). Consistently, another 6-mo intervention study demonstrated that receiving canola oil for cooking (≤20 g/d) ameliorated insulin resistance in Asian Indians with nonalcoholic fatty liver disease (49). Canola oil typically contains a high content of ω-3 PUFAs, mainly α-linoleic acid (ALA; 18:3n–3) (8.4%) (43). Our previous investigation in the CHNS (44) and the Singapore Chinese Health Study (50) consistently showed a significant association of ALA intake with lower T2D risk. The plasma concentration of ALA was also inversely associated with T2D incidence (51). Metabolic research suggested that ALA rescued impaired glucose homeostasis in obese rats possibly via alleviating adipose inflammation and promoting insulin signaling (44, 52, 53). However, we did not find a protective association of canola oil consumption with T2D risk given that the beneficial effect of canola oil might be counterbalanced by the detrimental substances produced during frying.
The strengths of this study included the long duration of follow-up, large nationwide sample, and the use of repeatedly measured intakes of individual cooking oils. To further minimize reverse causation, we excluded incident T2D cases that occurred during the initial 3 y of follow-up in a sensitivity analysis and found similar results. Several limitations should be noted. First, the T2D incidence may be underestimated for participants (48.7% of all participants) with only self-reported T2D information from questionnaires. However, when evaluating the level of cooking oil consumption among undiagnosed T2D cases in 2009, we found no significant association between cooking oil consumption and fewer undiagnosed T2D cases. Participants with higher consumption of plant cooking oils had higher socioeconomic status and might get better access to health care, leading to fewer undiagnosed T2D cases. Nonetheless, when we further adjusted for health insurance, the outcomes persisted for the documented associations of plant cooking oil consumption with risk of T2D. Overall, the misclassification might occur virtually independently of cooking oil consumption and might not cause obvious bias in our study. Second, owing to the very low prevalence of nonfrying preparation methods we could not separately analyze the associations for fried oils as opposed to nonfried oils, which could have provided more implications. Third, measurement errors in individual cooking oil intakes were inevitable in spite of using the combination of 3 consecutive 24-h recalls and a weighing technique. However, we used cumulative mean intake of cooking oil consumption which reduced within-person variation and random measurement error as well as accommodated dietary changes over a long period of follow-up. Fourth, data on the cooking oils consumed away from home were not collected. Nonetheless, the proportion of participants who frequently dined out was low (20) and thus might not appreciably affect our findings. Fifth, the population in our study was relatively young (median age: 41 y; IQR: 31–53 y) and the Chinese dietary habit of frying cooking oils is distinctive, which may limit the generalizability of established findings to other populations. Finally, a causal association cannot be demonstrated given the observational nature of this study and residual confounding is still possible even after careful adjustment of multiple risk factors for T2D.
In conclusion, individual cooking oil/fat intakes were divergently associated with the risk of T2D. Our results indicated a potential adverse effect of lard, peanut oil, and refined blended plant oil on T2D management in China where frying methods are predominantly used for cooking with edible oils. Increasing consumption of soybean oil in replacement of lard, peanut oil, refined blended plant oil, and other plant cooking oils was associated with lower T2D risk. Further studies are needed to corroborate our findings that reducing the consumption amount of cooking oils may be protective against T2D among the Chinese population.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—JJ and YZ: designed the research, had primary responsibility for the final content, are the guarantors of this work, and, as such, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis; PZ and YZ: conducted the research and wrote the manuscript; LM, FW, and JW: performed the analysis and analyzed the data; and all authors: read and approved the final manuscript.
Notes
Supplemental Figure 1 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Supported by National Natural Science Foundation of China grant 81773419 (to JJ), China National Program for Support of Top-notch Young Professionals (to YZ), NIH grant R01 HD30880, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant P2C HD050924, National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK104371, NIH Fogarty grant D43 TW009077 for financial support for the China Health and Nutrition Survey (CHNS) data collection and analysis files since 1989, the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009, and Beijing Municipal Center for Disease Prevention and Control since 2011.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AHEI, alternative healthy eating index; ALA, α-linoleic acid; CHNS, China Health and Nutrition Survey; HbA1c, glycated hemoglobin; LA, linoleic acid; T2D, type 2 diabetes.
Contributor Information
Pan Zhuang, National Engineering Laboratory of Intelligent Food Technology and Equipment, Zhejiang Key Laboratory for Agro-Food Processing, Fuli Institute of Food Science, College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou, Zhejiang, China.
Lei Mao, Department of Nutrition, School of Public Health, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Fei Wu, Department of Nutrition, School of Public Health, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Jun Wang, National Engineering Laboratory of Intelligent Food Technology and Equipment, Zhejiang Key Laboratory for Agro-Food Processing, Fuli Institute of Food Science, College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou, Zhejiang, China.
Jingjing Jiao, Department of Nutrition and Food Hygiene, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu, China.
Yu Zhang, National Engineering Laboratory of Intelligent Food Technology and Equipment, Zhejiang Key Laboratory for Agro-Food Processing, Fuli Institute of Food Science, College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou, Zhejiang, China.
References
- 1. International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels (Belgium: ): International Diabetes Federation; 2017. [Google Scholar]
- 2. Pan XR, Yang WY, Li GW, Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care. 1997;20(11):1664–9. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D et al.. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44(7):805–17. [DOI] [PubMed] [Google Scholar]
- 6. Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Imamura F, Micha R, Wu JHY, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13(7):e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018;361:k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmad Nizar NN, Nazrim Marikkar JM, Hashim DM. Differentiation of lard, chicken fat, beef fat and mutton fat by GCMS and EA-IRMS techniques. J Oleo Sci. 2013;62(7):459–64. [DOI] [PubMed] [Google Scholar]
- 10. US Department of Health and Human Services (HHS) and USDA. 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington (DC): US HHS and USDA; 2015. [Google Scholar]
- 11. Chen B, McClements DJ, Decker EA. Minor components in food oils: a critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit Rev Food Sci Nutr. 2011;51(10):901–16. [DOI] [PubMed] [Google Scholar]
- 12. Jenkins DJA, Kendall CWC, Vuksan V, Faulkner D, Augustin LSA, Mitchell S, Ireland C, Srichaikul K, Mirrahimi A, Chiavaroli L et al.. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care. 2014;37(7):1806–14. [DOI] [PubMed] [Google Scholar]
- 13. Schwingshackl L, Lampousi AM, Portillo MP, Romaguera D, Hoffmann G, Boeing H. Olive oil in the prevention and management of type 2 diabetes mellitus: a systematic review and meta-analysis of cohort studies and intervention trials. Nutr Diabetes. 2017;7(4):e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norris LE, Collene AL, Asp ML, Hsu JC, Liu L-F, Richardson JR, Li D, Bell D, Osei K, Jackson RD et al.. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am J Clin Nutr. 2009;90(3):468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Putti R, Migliaccio V, Sica R, Lionetti L. Skeletal muscle mitochondrial bioenergetics and morphology in high fat diet induced obesity and insulin resistance: focus on dietary fat source. Front Physiol. 2016;6:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: the China Health and Nutrition Survey—monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol. 2010;39(6):1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Popkin BM, Lu B, Zhai F. Understanding the nutrition transition: measuring rapid dietary changes in transitional countries. Public Health Nutr. 2002;5(6A):947–53. [DOI] [PubMed] [Google Scholar]
- 18. Tee ES. Development and promotion of Malaysian Dietary Guidelines. Asia Pac J Clin Nutr. 2011;20(3):455–61. [PubMed] [Google Scholar]
- 19. Yao M, McCrory MA, Ma G, Tucker KL, Gao S, Fuss P, Roberts SB. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am J Clin Nutr. 2003;77(6):1409–16. [DOI] [PubMed] [Google Scholar]
- 20. Du WW, Su C, Wang H-J, Wang Z-H, Zhang J-G, Zhang J, Jiang H-R, Zhang Y-G, Zhang B. [Situation on ‘eating out’ and its related risk factors among 1013 Chinese adults in 3 provinces]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34(12):1159–63. [PubMed] [Google Scholar]
- 21. Willett WC. Food frequency methods; Reproducibility and validity of food-frequency questionnaires; Implications of total energy intake for epidemiologic analyses. In: Nutritional epidemiology. 3rd ed. Oxford (UK): Oxford University Press; 2012.pp. 273–97. [Google Scholar]
- 22. Wang Z, Adair LS, Cai J, Gordon-Larsen P, Siega-Riz AM, Zhang B, Popkin BM. Diet quality is linked to insulin resistance among adults in China. J Nutr. 2017;147(11):2102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morio B, Fardet A, Legrand P, Lecerf J-M. Involvement of dietary saturated fats, from all sources or of dairy origin only, in insulin resistance and type 2 diabetes. Nutr Rev. 2016;74(1):33–47. [DOI] [PubMed] [Google Scholar]
- 24. Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nälsén C, Berglund L, Louheranta A, Rasmussen BM et al.. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetologia. 2001;44(3):312–9. [DOI] [PubMed] [Google Scholar]
- 25. van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25(3):417–24. [DOI] [PubMed] [Google Scholar]
- 26. Meyer KA, Kushi LH, Jacobs DR Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care. 2001;24(9):1528–35. [DOI] [PubMed] [Google Scholar]
- 27. Coelho OGL, da Silva BP, Rocha DMUP, Lopes LL, Alfenas RCG. Polyunsaturated fatty acids and type 2 diabetes: impact on the glycemic control mechanism. Crit Rev Food Sci Nutr. 2017;57(17):3614–9. [DOI] [PubMed] [Google Scholar]
- 28. Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. Ann Nutr Metab. 2011;58(4):290–6. [DOI] [PubMed] [Google Scholar]
- 29. Qian F, Korat AA, Malik V, Hu FB. Metabolic effects of monounsaturated fatty acid–enriched diets compared with carbohydrate or polyunsaturated fatty acid–enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2016;39(8):1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pérez-Jiménez F, López-Miranda J, Pinillos MD, Gómez P, Paz-Rojas E, Montilla P, Marín C, Velasco MJ, Blanco-Molina A, Jiménez Perepérez JA et al.. A Mediterranean and a high-carbohydrate diet improve glucose metabolism in healthy young persons. Diabetologia. 2001;44(11):2038–43. [DOI] [PubMed] [Google Scholar]
- 31. Guasch-Ferré M, Hruby A, Salas-Salvadó J, Martínez-González MA, Sun Q, Willett WC, Hu FB. Olive oil consumption and risk of type 2 diabetes in US women. Am J Clin Nutr. 2015;102(2):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Narayanankutty A, Manalil JJ, Suseela IM, Ramavarma SK, Mathew SE, Illam SP, Babu TD, Kuzhivelil BT, Raghavamenon AC. Deep fried edible oils disturb hepatic redox equilibrium and heightens lipotoxicity and hepatosteatosis in male Wistar rats. Hum Exp Toxicol. 2017;36(9):919–30. [DOI] [PubMed] [Google Scholar]
- 33. Sayon-Orea C, Carlos S, Martínez-Gonzalez MA. Does cooking with vegetable oils increase the risk of chronic diseases?: a systematic review. Br J Nutr. 2015;113(Suppl 2):S36–48. [DOI] [PubMed] [Google Scholar]
- 34. Miller YI, Shyy JYJ. Context-dependent role of oxidized lipids and lipoproteins in inflammation. Trends Endocrinol Metab. 2017;28(2):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 36. Cahill LE, Pan A, Chiuve SE, Sun Q, Willett WC, Hu FB, Rimm EB. Fried-food consumption and risk of type 2 diabetes and coronary artery disease: a prospective study in 2 cohorts of US women and men. Am J Clin Nutr. 2014;100(2):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vassiliou EK, Gonzalez A, Garcia C, Tadros JH, Chakraborty G, Toney JH. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aung WP, Bjertness E, Htet AS, Stigum H, Chongsuvivatwong V, Soe PP, Kjøllesdal MKR. Fatty acid profiles of various vegetable oils and the association between the use of palm oil vs. peanut oil and risk factors for non-communicable diseases in Yangon Region, Myanmar. Nutrients. 2018;10(9):1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamashita K, Iizuka Y, Imai T, Namiki M. Sesame seed and its lignans produce marked enhancement of vitamin E activity in rats fed a low α-tocopherol diet. Lipids. 1995;30(11):1019–28. [DOI] [PubMed] [Google Scholar]
- 40. Sankar D, Rao MR, Sambandam G, Pugalendi KV. A pilot study of open label sesame oil in hypertensive diabetics. J Med Food. 2006;9(3):408–12. [DOI] [PubMed] [Google Scholar]
- 41. Sankar D, Ali A, Sambandam G, Rao R. Sesame oil exhibits synergistic effect with anti-diabetic medication in patients with type 2 diabetes mellitus. Clin Nutr. 2011;30(3):351–8. [DOI] [PubMed] [Google Scholar]
- 42. Devarajan S, Chatterjee B, Urata H, Zhang B, Ali A, Singh R, Ganapathy S. A blend of sesame and rice bran oils lowers hyperglycemia and improves the lipids. Am J Med. 2016;129(7):731–9. [DOI] [PubMed] [Google Scholar]
- 43. Yang Y, Wang G, Pan X. The 2002 Chinese Food Composition Table. Beijing: Medical Publishing House of Beijing University; 2002. [Google Scholar]
- 44. Zhuang P, Shou Q, Wang W, He L, Wang J, Chen J, Zhang Y, Jiao J. Essential fatty acids linoleic acid and α-linolenic acid sex-dependently regulate glucose homeostasis in obesity. Mol Nutr Food Res. 2018;62(17):e1800448. [DOI] [PubMed] [Google Scholar]
- 45. Salmerón J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73(6):1019–26. [DOI] [PubMed] [Google Scholar]
- 46. Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang W-S, de Oliveira Otto MC, Kröger J et al.. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sena CM, Proenca T, Nunes E, Santos MS, Seica RM. The effect of soybean oil on glycaemic control in Goto-Kakizaki rats, an animal model of type 2 diabetes. Med Chem. 2008;4(3):293–7. [DOI] [PubMed] [Google Scholar]
- 48. Wang J, Huang M, Yang J, Ma X, Zheng S, Deng S, Huang Y, Yang X, Zhao P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr Res. 2017;61(1):e1364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nigam P, Bhatt S, Misra A, Chadha DS, Vaidya M, Dasgupta J, Pasha QMA. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol Ther. 2014;16(4):255–61. [DOI] [PubMed] [Google Scholar]
- 50. Brostow DP, Odegaard AO, Koh W-P, Duval S, Gross MD, Yuan J-M, Pereira MA. Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2011;94(2):520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, Ye Z, Sluijs I, Guevara M, Huerta JM et al.. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct Case-Cohort Study. PLoS Med. 2016;13(7):e1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matravadia S, Herbst EAF, Jain SS, Mutch DM, Holloway GP. Both linoleic and α-linolenic acid prevent insulin resistance but have divergent impacts on skeletal muscle mitochondrial bioenergetics in obese Zucker rats. Am J Physiol Endocrinol Metab. 2014;307(1):E102–14. [DOI] [PubMed] [Google Scholar]
- 53. Matravadia S, Zabielski P, Chabowski A, Mutch DM, Holloway GP. LA and ALA prevent glucose intolerance in obese male rats without reducing reactive lipid content, but cause tissue-specific changes in fatty acid composition. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R619–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


