Highlights
-
•
Unhealthy alcohol use is common among persons living with HIV (PLWH).
-
•
In sub-Saharan Africa, there are limited treatment options for unhealthy alcohol use.
-
•
This RCT tests multiple interventions at reducing unhealthy alcohol use in SSA.
Keywords: Unhealthy alcohol use, Substance use, HIV, Zambia, Randomized controlled trial, Transdiagnostic therapy, Brief intervention
Abstract
Aims
Prevalence of unhealthy alcohol use and co-occurring mental health problems is high among persons living with HIV (PLWH) in sub-Saharan Africa (SSA). Yet, there is a dearth of evidence-based treatment options that can address both unhealthy alcohol use and comorbidities in SSA HIV care settings. Recent studies testing single-session alcohol brief interventions (BIs) among PLWH in SSA have suggested that more robust treatments are needed. This paper describes the protocol of a pilot randomized controlled superiority trial that will test the effectiveness of an evidence-based transdiagnostic multi-session psychotherapy, the Common Elements Treatment Approach (CETA), compared to a control condition consisting of a single session brief alcohol intervention (BI) based on CETA, at reducing unhealthy alcohol use, mental health problems, and other substance use among PLWH in urban Zambia.
Methods
The study is a single-blind, parallel, individually randomized trial conducted in HIV treatment centers in Lusaka. 160 PLWH who meet criteria for unhealthy alcohol use + mental health or substance use comorbidities and/or have a more severe alcohol use disorder are eligible. Participants are randomized 1:1 to receive the single-session BI or CETA. Outcomes are assessed at baseline and a six-month follow-up and include unhealthy alcohol use, depression, trauma symptoms, and other substance use.
Conclusions
The trial is a first step in establishing the effectiveness of CETA at reducing unhealthy alcohol use and comorbidities among PLWH in SSA. If effectiveness is demonstrated, a larger trial featuring long-term follow-ups and HIV treatment outcomes will be undertaken.
1. Introduction
Unhealthy alcohol use, including heavy and hazardous use, heavy episodic (binge) drinking, and alcohol use disorders (AUD), is a globally recognized public health problem (Saitz, 2005). In sub-Saharan Africa (SSA), alcohol is the most commonly consumed and distributed substance of abuse (Hahn, Dobkin, & Mayanja, 2012), and unhealthy use is attributable for 1% of all deaths among women and 4% of all deaths among men (Pithey and Parry, 2009, Rehm et al., 2009). Unhealthy alcohol use is common among persons living with HIV (PLWH) in SSA (Nouaman, Vinikoor, & Seydi, 2018), who are between two and four times more likely than the general population to have an AUD (Petry, 1999). In Zambia, preliminary studies found that 47% of male and 16% of female PLWH had unhealthy use during their first year on antiretroviral therapy (ART) (Vinikoor et al., 2015). Comorbidity between unhealthy use and mental health problems is very common: among Zambians who drank at unhealthy levels, over 50% met criteria for a severe AUD and/or mental health problems and over 30% reported other substance use (Kane et al., 2016, Kane et al., 2017).
Globally, there is recognition that unhealthy alcohol use is significant barrier to ending the HIV/AIDS epidemic (Bedoya et al., 2012, Hahn and Samet, 2010, Kalichman et al., 2008). Alcohol has been shown to impact HIV outcomes through both behavioral and biological mechanisms (Hahn & Samet, 2010), including: cognitive impairment (Vagenas et al., 2015), increased sexual risk behaviors (Gerbi, Habtemariam, Tameru, Nganwa, & Robnett, 2009), depression (Leserman, 2008), immunocompromise with increased susceptibility to infections (Hahn & Samet, 2010), and nutritional deficiencies (Fawzi et al., 2005, Watzl and Watson, 1992).
Despite the considerable burden of disease, treatment for unhealthy alcohol use is unavailable in many SSA HIV clinics. In high income country HIV clinic settings, brief alcohol interventions (BIs) have been tested for reducing unhealthy alcohol use (Aharonovich et al., 2006, Gilbert et al., 2008). BIs typically range from 1 to 4 sessions lasting anywhere between 10 and 60 min each. They are often administered in primary care settings by a nurse or physician and include components such as: feedback on use and the harm drinking may be causing, norm referencing, identifying coping strategies, and motivational enhancement for changing behavior (Jonas et al., 2012, Kaner et al., 2007, Kaner et al., 2011, Platt et al., 2016, SAMHSA, 2017). Literature from high income countries suggests that BIs in primary care settings can be both cost and clinically effective in reducing unhealthy alcohol use (Kaner et al., 2007, Platt et al., 2016, Wutzke et al., 2001), including among PLWH (Aharonovich et al., 2006, Chander et al., 2015, Gilbert et al., 2008). However, BIs were not designed to treat more severe AUD or comorbid mental health/substance use problems (NIAAA, 2005b, World Health Organization, 2012). Further, in SSA, evidence for the effectiveness of single-session BIs among PLWH is limited. Two studies conducted in Uganda and South Africa among HIV-affected populations found BIs were not superior than a control condition at achieving reduced alcohol consumption (Peltzer et al., 2013, Wandera et al., 2017). In Uganda, authors suggested that a single session may not have been a substantial enough dose and that additional sessions may be required to improve upon standard of care (Wandera et al., 2017); in South Africa, authors posited that the lack of treatment effect may have been due to behavioral reactivity in the control group, actual effectiveness of the active control intervention (a health education leaflet on alcohol use), or natural changes in alcohol use as a result of receiving standard TB treatment (Peltzer et al., 2013). Based on these results, it seems likely that a more comprehensive treatment approach, beyond the use of BIs alone, is needed to reduce the impact of unhealthy alcohol use on the HIV/AIDS response in SSA. One possible package of care could include a system in which patients receiving HIV treatment are screened for alcohol use, provided with a point-of-care brief intervention (if needed), and a referral to higher level treatment (if indicated). Such screening, brief intervention, and referral to treatment (SBIRT) public health programs in the U.S. have shown promise in treating alcohol and substance use problems and in preventing such problems from becoming more severe disorders (SAMHSA, 2017).
This paper describes the adaptation of an existing evidenced-based, transdiagnostic, 6–12 week psychotherapy treatment approach (CETA) for delivery in HIV care settings in SSA to address unhealthy alcohol use and comorbid mental health problems; the development of a novel single-session BI based on the alcohol treatment component of CETA (BI); and the protocol for testing CETA compared to the BI in a superiority randomized controlled trial in Zambia. The trial aims to evaluate the effectiveness of CETA compared to the BI in reducing unhealthy alcohol use, mental health, and other (non-alcohol) substance use problems among PLWH in Zambia. The study is a Stage 1 pilot/feasibility trial (NIH, 2017). Specific hypotheses include:
-
(1)
CETA and the BI can be feasibly delivered at HIV care settings in SSA.
-
(2)
Among PLWH with unhealthy alcohol use + comorbidities and/or a more severe AUD, CETA will be clinically superior in improving alcohol, mental health, and substance use outcomes compared to the single-session BI.
The purpose of the trial is to test the comparative effectiveness of CETA versus the BI with a goal of future studies and programs to potentially implement a stepped care system that includes an evidence-based BI (for unhealthy alcohol use alone) and more intensive treatment such as CETA (for unhealthy use + comorbidities and for more severe AUD) as part of an SBIRT program.
2. Methods
2.1. Design overview
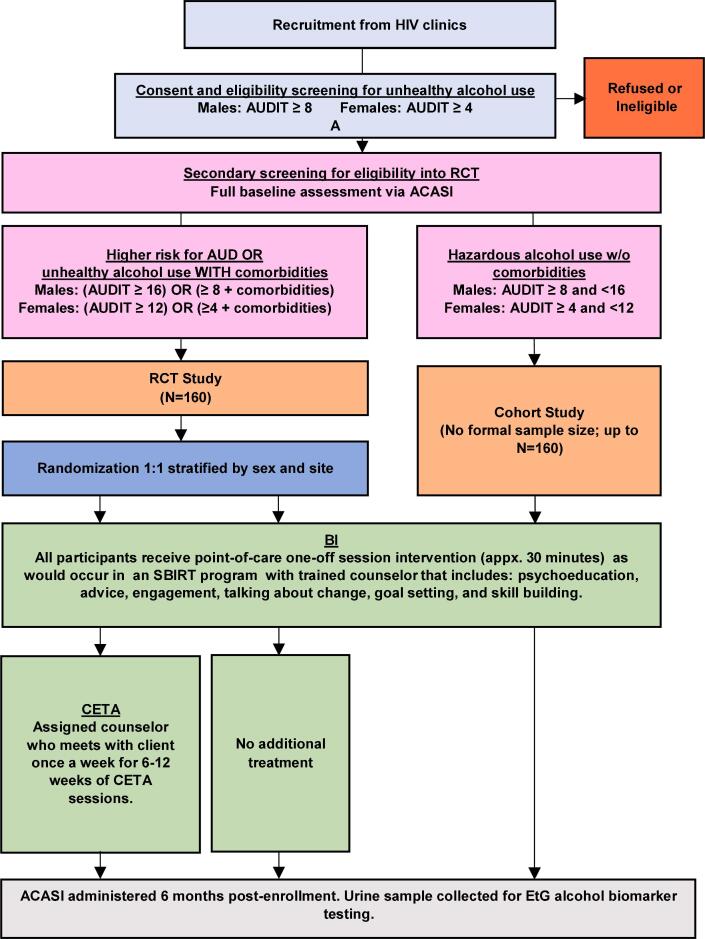
The Zambia CETA Alcohol Pilot (ZCAP) is a single-blind, parallel, individually randomized controlled trial comparing the effectiveness of a one-session brief alcohol intervention (BI) to a full course of multi-session CETA treatment (Fig. 1). Participants are adults living with HIV who have high risk alcohol use and possible mental health or other substance use comorbidities. Participants are recruited from two hospitals with large HIV clinics in Lusaka, Zambia during a regular HIV care visit, screened for eligibility, and randomized to receive the BI or CETA. Participants randomized to CETA also receive the BI after screening and before their first CETA session, as would be done in an SBIRT program. Assessment of the primary outcome (alcohol use) and secondary outcomes (mental health, other non-alcohol substance use) is completed at baseline and a six-month follow-up visit.
Fig. 1.
Flow diagram.
In addition to the high-risk group of participants in the RCT, we are also enrolling a lower risk group of individuals with HIV who have low-to-moderate risk unhealthy alcohol use and who do not have mental health or other substance use comorbidities (i.e., participants who do not meet symptom threshold criteria [see Measures] for depression, trauma, or substance use, and who have low-to-moderate risk alcohol use but do not meet alcohol use disorder [AUD] criteria). These lower risk participants are enrolled into an uncontrolled ‘cohort’ study and receive the BI; they are also assessed for outcomes at baseline and six-months post-baseline but are not randomized. The purpose of this cohort is to collect preliminary data to begin exploring the utility of the BI for lower risk clients under a hypothesis that, although the BI may not be sufficient for the higher risk participants (such as those with comorbidities and/or severe AUD), it may be sufficient and less costly for lower risk participants than a more intensive intervention such as CETA. It is beyond the scope of the present study to formally test the effectiveness of the BI among this lower risk group.
The study was approved by the Columbia University Medical Center Institutional Review Board (IRB), the Johns Hopkins Bloomberg School of Public Health IRB, the University of Zambia Biomedical Research Ethics Committee, and the National Health Research Authority in Zambia. The trial was registered on ClinicalTrials.gov (NCT03966885; Date of registration 05/29/2019) before participant enrollment. This protocol paper follows the SPIRIT guidelines for reporting of clinical trial protocols (Chan, Tetzlaff, & Altman, 2013).
2.2. Recruitment
Recruitment commenced in June 2019 at two large urban HIV clinics in Lusaka. Before recruitment, a series of meetings between study investigators and clinic personnel was conducted. Study aims and procedures were presented during these meetings and feedback was obtained. The goal was to integrate recruitment and other study procedures, including intervention provision, into HIV clinic systems with a minimal level of disruption to regular activities. Following these meetings, we conducted a training with clinic staff on eligibility criteria and participant recruitment.
For recruitment, regular health talks are given by lay healthcare workers in the HIV clinic waiting areas. The talks focus on the relationship between alcohol and HIV and also inform patients of the ongoing study. Patients are encouraged to talk to staff if they are interested and also told that their provider may give them some more information about the study. Clinic staff including doctors, nurses, and HIV peer educators (lay counselors from the catchment area) then further introduce the study in private to their patients with HIV who they believe might have unhealthy alcohol use. Potential participants who are interested in hearing more about the study are escorted to our on-site study room where they are met by a research assistant (RA). This strategy represents a likely entry point into alcohol interventions in a real-world (i.e., non-research) HIV care context.
2.3. Screening informed consent
A study RA meets with the potential participant and provides an overview of the study, including aims, purpose, procedures, burden, and risks and benefits. All participants are assured that their participation (or not) would not impact their regular HIV care services. The RA obtains written informed consent from participants for eligibility screening. Participants who are illiterate can provide a thumbprint instead of a signature and an impartial witness cosigns the consent form.
2.4. Eligibility screening and baseline assessment
Participants who provide informed consent are escorted by the RA to a separate room in the clinic to complete eligibility screening, which also serves as the baseline assessment.
Initial eligibility criteria for the overall study include:
-
•
Adults 18 years of age or older
-
•
HIV positive and receiving care at the HIV clinic
-
•
Unhealthy alcohol, measured by the Alcohol Use Disorders Identification Test (AUDIT). (Babor et al., 2001, Saunders et al., 1993)
Once age and HIV status are confirmed, the RA sets up a laptop computer that is pre-loaded with study assessment measures on an audio-computer assisted self-interviewing (ACASI) system. (Kane et al., 2016, Kane et al., 2016, Kane et al., 2018, Murray et al., 2018) ACASI permits participants to self-complete the questionnaire with the text presented on the laptop screen and also audially read to the participant through headphones. ACASI is programmed in English and the two most commonly spoken languages in Lusaka, Bemba and Nyanja. After the ACASI notebook has been setup, the participant completes the first two (out of five) assessment measures of the ACASI, including demographics (sex, age, education, employment, and marital status) and the AUDIT.
The ACASI automates a total AUDIT score and generates a screen for the RA informing them of the participant’s eligibility status. Participants are eligible if they meet criteria for hazardous drinking defined as a total AUDIT score ≥ 4 among women or ≥ 8 among men. Participants who do not meet this criterion exit the study. Participants who are eligible based on the AUDIT continue with three additional measures in the ACASI evaluating depression (CES-D), trauma symptoms (HTQ), and substance use (ASSIST; Table 1).
Table 1.
Outcome measures in the ZCAP trial.
| Outcome | Measure | Description | Clinically relevant cut-off score for eligibility |
|---|---|---|---|
| Unhealthy alcohol use (primary) | Alcohol Use Disorders Identification Test (AUDIT) (Babor et al., 2001, Saunders et al., 1993) | AUDIT is a 10-item measure of hazardous alcohol use. A total score is calculated across the items with a possible range of 0–40 and higher scores indicating more severe alcohol use problems. The AUDIT was previously validated for use in Zambia (Chishinga et al., 2011) | Initial eligibility for unhealthy use: ≥4 among women or ≥ 8 among men; eligibility for more severe problem/ higher risk of AUD: ≥12 among women or ≥ 16among men (Babor et al., 2001, NIAAA, 2005a) |
| Depression (secondary) | Center for Epidemiological Studies-Depression (CES-D) (Radloff, 1977) | CES-D is a 20-item measure of depression symptoms. Participants are asked how often they experienced each symptom over the past week (0 = never or less than one day; 1 = 1–2 days; 2 = 3–4 days; 3 = 5–7 days). A total score is calculated with a possible range of 0–60 and higher scores representative of more severe depression symptomatology. The CES-D was previously validated in Zambia. (Chishinga et al., 2011) | CES-D total score ≥ 16 (Vilagut, Forero, Barbaglia, & Alonso, 2016) |
| Trauma symptoms (secondary) | Harvard Trauma Questionnaire (HTQ) (Mollica et al., 1992) | HTQ is a 39-item scale assessing symptoms of post-traumatic stress. Participants are prompted to respond how often each symptom bothered them in the past week (1 = not at all; 2 = a little; 3 = quite a bit; 4 = extremely). An average item score is calculated with a possible range of 1–4 with higher scores indicative of greater trauma symptoms. A previous study in Zambia demonstrated strong internal reliability of the HTQ (α > 0.90) (Kane et al., 2017) | HTQ average item score ≥ 2.5 (Mollica et al., 1992) |
| Substance use (secondary) | Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) (Humeniuk, Ali, & Babor, 2008) | ASSIST is a 7-item measure that evaluates frequency of use, abuse, and dependence symptoms for a range of substance types, including tobacco, alcohol, marijuana, inhalants, cocaine, sedatives, hallucinogens, methamphetamines, and opioids. A specific substance involvement (SSI) score is calculated for each substance type that a participant reports ever having used in their lifetime. An SSI score can range from 0 to 39. The ASSIST was previously validated in Zambia (Kane, Murray, Bass, Johnson, & Bolton, 2016) | A non-alcohol/tobacco SSI score on the ASSIST of ≥ 27 (Humeniuk, Henry-Edwards, Ali, Poznyak, & Monteiro, 2010) |
The ACASI scores all three measures and generates a summary screen for the RA indicating whether the participant has met clinically relevant cut-off scores for each measure (Table 1). An algorithm built into ACASI then alerts the RA to the participant’s eligibility status with respect to the RCT or the cohort study. If a participant meets criteria for one or more of the CES-D, HTQ, or ASSIST measures and/or meets a threshold for higher risk of AUD according to the AUDIT, the participant is eligible for the RCT. If a participant does not meet symptom criteria for CES-D, HTQ, or ASSIST, and their AUDIT score suggests unhealthy use but not an AUD, then they are eligible for the cohort study. Thus, the RCT includes the highest risk group of participants and the cohort study includes a group with moderate risk.
For all participants, exclusion criteria for the study includes:
-
•
HIV negative status
-
•
Not receiving care at one of the study clinics
-
•
Currently psychotic or on an unstable psychiatric drug regimen
-
•
Actively suicidal and needing immediate hospitalization
-
•
Unable to provide informed consent
The study follows an approved safety protocol where participants who are identified as having safety concerns and/or psychotic symptoms are immediately seen by a CETA clinical supervisor for further assessment. Clinical supervisors then refer any clients in need of additional services to a psychiatrist at the University of Zambia Teaching Hospital (UTH).
2.5. Study informed consent
Following completion of ACASI, the RA obtains informed consent for the remaining study procedures; a separate consent form is used for those eligible for the cohort and those eligible for the RCT.
2.6. Randomization/blinding
Randomization of RCT participants is conducted following completion of the ACASI assessment and informed consent. Lists of randomization ID numbers were generated by a statistician not otherwise involved in the study before trial commencement. Four lists were generated: one each for males and females at the two study clinics. IDs within each list were randomly allocated on a 1:1 basis to BI or CETA using the ralloc procedures in Stata, version 15. (StataCorp, 2017) Randomization within each list was done in blocks of 20 to ensure approximately equal numbers in both treatment groups. RAs are blinded to the blocking sequence. The randomization assignments were recorded on separate pieces of paper and each was placed inside an opaque envelope that was sealed. The outside of the envelope contains the randomization ID. At each clinic there are two locked cabinets that house the randomization envelopes (one for male assignments and one for female) in sequential order. Once a randomization envelope is open, the RA informs the participant of the result and records the randomization ID in the participant’s study file, thus linking the study ID number and randomization ID number. Outcomes assessments are blinded in the study by the use of ACASI and data analysts will be also blinded. By nature of the interventions being tested, the study counselors, clinical team, study coordinator, and participants are not blinded.
2.7. Trial arms
2.7.1. Experimental arm: CETA
The Common Elements Treatment Approach (CETA) is a multi-problem, flexible, modular transdiagnostic treatment approach developed to address comorbid mental and behavioral health conditions with lay counselor delivery in low and middle income countries (LMIC) (www.cetaglobal.org) (Murray et al., 2014). This approach was chosen given its evidence-base in LMIC with lay counselors (Bolton et al., 2014, Weiss et al., 2015), its focus on comorbidity, which studies have shown is common among PLWH (Kane et al., 2018), and the potential for scale-up and systems-based implementation (Murray et al., 2011). CETA elements are grounded in cognitive behavioral therapy and the transdiagnostic, modular approach is based on methods developed in the United States (Chorpita and Weisz, 2009, Chorpita et al., 2005, Farchione et al., 2012, Weisz et al., 2012). CETA consists of nine key elements (Table 2), and originally focused on common mental health problems such as trauma/PTSD, depression, anxiety, behavioral issues (for youth), anger, and relationship and functioning problems. Randomized controlled trials in Iraq and Thailand demonstrated effectiveness for a range of mental health problems (Bolton et al., 2014, Weiss et al., 2015). CETA was later adapted to include elements aimed at reducing unhealthy alcohol use and preventing intimate partner violence (Kane et al., 2017).
Table 2.
CETA Elements.
| Element | Simplified name (Used in training) | Description |
|---|---|---|
| Psychoeducation and Engagement | Introduction and Encouraging Participation |
|
| Anxiety management strategies | Relaxation |
|
| Behavioral Activation | Getting Active (GA) |
|
| Cognitive Coping/Restructuring | Thinking in a Different Way –Part I and Part II(TDW1 and TDW2) |
|
| Imaginal Gradual Exposure | Talking about Trauma Memories (TDM) |
|
| In Vivo Exposure | Live Exposure |
|
| Suicide/Homicide/Danger Assessment and Planning | Safety |
|
| CBT for Substance Use and Relapse Prevention | Substance Use Element (SU) |
|
CETA was adapted in the ZCAP trial for use in HIV clinic settings by study staff and local counselors and supervisors. CETA training and ongoing supervision use the apprenticeship model for training (Murray et al., 2011). A 10-day live training of local HIV peer educators in Lusaka was conducted by study authors (LKM and SSvW) followed by small group practice (2 h weekly) in which the newly trained counselors practiced the CETA elements with the oversight of a CETA supervisor. Following the eight weeks of practice groups, counselors attend regular weekly supervision for 2–3 h per week for further practice, and review/support of CETA cases. Supervisors meet on a weekly basis with the study authors for clinical supervision for all active study clients (LKM and SSvW). Counselors and supervisors are trained to monitor session activities to ensure fidelity. Counselors document and report session activities to local supervisors, who document details in a clinical database, and this is reviewed with a CETA trainer. If counselors miss steps, they are expected to complete or re-do the component. A similar fidelity process is used for the BI intervention.
CETA is being implemented individually (one-on-one format) with flexibility of elements and number of sessions based on a client’s symptom presentation and severity. Treatment typically lasts from 6 to 12 sessions, usually one hour each, on average. In the ZCAP trial, all clients receive the CBT for Substance Use Reduction elements due to inclusion criteria. Prior or during the first session, the counselor and patient agree on when and where to conduct the subsequent weekly sessions. If sessions are missed, counselors follow up via phone (and home visit if necessary) to reschedule and assure safety.
Participants who are randomized to CETA also receive the single session BI (see description below) (Vinikoor et al., 2015)following screening and before their first CETA session, which typically occurs one week later. The rationale for providing the BI to CETA participants is that this is how we believe an SBIRT program would be administered in a real-world setting. That is, patients would be screened for alcohol use, provided with a BI on the spot (if indicated), and following the BI referred for higher-level treatment (i.e., CETA; if indicated). When possible, the same counselor who provides the BI to a participant also becomes their CETA counselor to facilitate continuity of treatment and build off the therapeutic relationship that was established in the BI session.
2.7.2. Control arm: BI
Treatment as usual in Zambia HIV settings for unhealthy alcohol use is currently an alcohol BI. However, in practice, the BI is inconsistently delivered with variable content and is not evidence-based. Preliminary unpublished data by our team found that health workers reported a lack of training and comfort in delivering any kind of alcohol reduction counseling. Given that the standard BI was not consistently delivered, the content varied considerably between and within clinic settings, was not evidence-based, and that providers did not feel comfortable providing it, we believed it was an inappropriately weak control condition to test in an RCT. Further, the variability within the control condition would have made a comparison with the experimental arm very difficult to interpret.
We therefore made the decision to enhance the existing BIs in HIV settings by developing a BI that was adapted from the evidence-based CETA element for Substance Use Reduction. Development of the BI was done by the study authors (CKD, LKM, SSvW) and with input from local partners working in HIV care settings. The BI consists of six components: 1) assessment/screening for alcohol and substance use; 2) understanding the impacts of use; 3) exploring possibilities for change; 4) goal setting; 5) identifying reasons for use; and 6) skill building (Table 3). It is designed to be one session of approximately 20–30 min, in response to local partners input on feasibility in HIV care settings. Counselors work to reach the goals of the session with clients by using a structured guide, the Improving Your Health (IYH) worksheet (included as supplemental material). The IYH worksheet was developed as a user-friendly tool to help counselors structure the BI session, observe the 20–30-minute time goal, and to assist clients with lower literacy rates. It was also hypothesized that the worksheet would reduce stigma for clients by allowing them to point or circle the response options instead of needing to discuss them out loud with the counselor.
Table 3.
BI elements.
| Element | Description |
|---|---|
| Assessment |
|
| Understanding impacts |
|
| Exploring Change |
|
| Goal Setting |
|
| Identifying the Reasons |
|
| Skill Building |
|
The BI training and supervision process is consistent with the apprenticeship model of training used for CETA (Murray et al., 2011). The same HIV peer educators trained as CETA counselors attended a 2-day live training on the BI held by one author (SSvW). Since counselors already knew the CETA element for substance use, this training focused on how to deliver it in a consolidated fashion, using the worksheet. Counselors participated in two 3-hour practice groups with supervisors at their sites. Once they started using the BI with clients, they received weekly supervision for the first five clients and continued to receive monthly supervision thereafter. The rationale for having the same counselors provide both the BI and CETA is that this resembles how we expect an SBIRT program would be run in a real-world setting. The training used a variety of methods including didactic, modeling, and small group role-plays to train participants in the BI.
2.8. Follow-up assessment
All participants (RCT and cohort) complete one follow-up assessment approximately six months post-baseline. For most participants, this should correspond to a regularly scheduled HIV care clinical visit, which are often approximately six months apart in Zambia. We permit this assessment to be completed as early as five months post-baseline and actively follow-up with participants to complete the assessment until seven months post-baseline. The assessment consists of the same ACASI questionnaire that was completed at screening/baseline. Following completion of the questionnaire, we ask participants for a urine sample to conduct a rapid ethyl glucuronide (EtG) biomarker test, which is an objective measure of recent alcohol consumption and can be used to confirm self-reported abstinence. The rapid EtG test was previously used in Zambia HIV care settings, where it had high specificity for alcohol use in the past three days. The test requires the participant to provide a small urine sample (appx. 100 ml). Following the sample collection, a research assistant inserts a dipstick into the sample and within 1–2 min the test provides a qualitative (positive/negative) result for alcohol consumption within the past 2–3 days (Vinikoor et al., 2018).
At follow-up, we will also conduct mixed methods interviews with approximately N = 30 participants. This will include a purposive sample of participants from a range of ages and both sexes who did and did not complete the BI and CETA. Focus group discussions with counselors and clinic staff who participated in the trial will also be conducted. Implementation factors to be explored from clients, counselors, and staff will include: acceptability, appropriateness, and feasibility, as well as the attitudes, thoughts, feelings, and barriers and facilitators related to implementation of SBIRT. We will systematically track throughout the course of the study: (1) the number of participants who successfully complete and decline BI; (2) the number of participants who successfully link to CETA, defined as attending the first CETA session; (3) the amount of time counselors dedicate to client tracking/retention; and (4) the number of clients who successfully complete CETA.
2.9. Data and safety monitoring
In addition to the ethical review boards, the trial is monitored by a three-person Data and Safety Monitoring Board (DSMB). All DSMB members reviewed and approved study procedures, as well as procedures for reporting and tracking all adverse events, overall study progress, and identifying any need for premature termination of the protocol. Progress reports are prepared for the DSMB every six months concerning enrollment, attrition, and adverse events and meetings are convened as needed. There are no plans for interim analyses.
2.10. Sample size and data analysis
The primary endpoint of the RCT is the difference in mean AUDIT score change from baseline to six-month follow-up between BI and CETA. We believe that an effect size of CETA ≥ 0.5 would be clinically significant and justify further investigation in a subsequent later Stage trial. Further assuming α = 0.05 and β = 80%, we require a total sample size of 128 (n = 64 per arm). To account for possible loss-to-follow-up/drop-out of 20% based on our previous studies with HIV-affected populations in Zambia, we inflated the sample size to 160 (n = 80 per arm). There is no sample size calculation for cohort study participants; however, the ceiling for the cohort is N = 160.
Primary analyses will be intent to treat (ITT). AUDIT score, along with other continuous outcomes (CES-D, HTQ, ASSIST scores) will be evaluated with linear mixed models. As a secondary analysis, we will also estimate generalized linear mixed effects models with binary AUDIT, CES-D, HTQ, and ASSIST outcomes using established cut-off values. Fixed effects in all models will include treatment arm (0 = -BI; 1 = CETA), time (0 = Baseline; 1 = 6-months post-baseline), and interaction terms between treatment arm and time. Clinic may also be an included variable to account for clustering. Random effects will include client ID and counselor ID. Robust standard errors will be estimated using a sandwich variance estimator (Huber, 1967, White, 1980). For linear models, we will estimate the mean difference in change in score between arms and 95% confidence intervals as well as the estimation of Cohen’s D effect size (Cohen, 1960). For generalized models, we will estimate relative risks. Covariates may be included if they differ meaningfully at baseline or predict significant change in the outcome. In addition to the ITT analysis, we will also conduct a per protocol analysis that includes all participants in the BI arm and only those participants in the CETA arm who completed CETA. We will also explore moderators (e.g., sex) of treatment effectiveness. Paired t-tests will be used to assess within group change among cohort participants. Sensitivity analysis will also be conducted after excluding participants who report abstinence at endline (AUDIT of 0 points) and who have a positive EtG result, indicative of underreporting.
2.11. Trial status
The trial is currently ongoing. All trial activities, including recruitment, treatment, follow-up, and data analysis are expected to be completed by the end of 2020.
3. Discussion
Research has shown that PLWH in SSA are at high risk for unhealthy alcohol use (Hahn et al., 2012) and that, in turn, unhealthy alcohol use can lead to poor HIV care outcomes. (Vagenas et al., 2015) Studies have also suggested that PLWH with unhealthy alcohol use often also have co-occurring mental health or other substance use problems (Hahn and Samet, 2010, Kane et al., 2018, Vagenas et al., 2015). Yet, evidence-based alcohol treatment services are rarely available in SSA HIV settings, particularly within the highly prevalent context of comorbid presentations (Kane et al., 2018). The ZCAP trial is an initial step to fill this gap by testing CETA, a multi-problem, modular, transdiagnostic evidence-based therapy that is designed for addressing unhealthy alcohol use and comorbidities. By developing and testing a brief alcohol intervention based on CETA (BI) as well, we aim to ultimately build an SBIRT system that can accurately and efficiently provide appropriate levels of care to PLWH in Zambia and throughout SSA.
The size, scope, and resource constraints contribute to notable limitations for this ongoing ZCAP trial. First, we do not have the resources to formally test the BI in reducing unhealthy alcohol use among PLWH who do not have comorbidities or a severe AUD; we aim to capture preliminary data on the BI amongst this population through the cohort we are enrolling as part of this project and formally test it in future studies. Second, the study has a risk of underreporting and social desirability bias and this may be stronger in the CETA arm because of the additional time spent with the study counselors, although it is also possible that a therapeutic alliance with counselors may have the reverse effect and increase the likelihood of accurate reporting. To reduce the effect of this bias should it occur, we are using ACASI to assess alcohol use and mental health. Also, we include the EtG test to detect participants who drink but report abstinence. It is possible that the participant’s knowledge of an upcoming EtG test could likewise result in behavioral reactivity and reduced alcohol consumption, however, we believe that an objective biomarker remains superior to self-report alone. Finally, our trial is occurring at two urban facilities in Zambia that are not representative of all HIV care environments in the country or in other SSA countries. The trial does not include PLWH in the community who do not access HIV clinics. Generalizability is also limited by the inability in this study to screen for alcohol among all patients in the HIV clinic. Future studies will be designed to address these generalizability limitations.
If the ZCAP study finds that the BI is feasible to deliver integrated within HIV care and that CETA is effective, we plan to conduct a hybrid implementation-effectiveness study (Curran, Bauer, Mittman, Pyne, & Stetler, 2012) to test an SBIRT system featuring the BI and CETA in several HIV care settings in Zambia and measure downstream HIV outcomes, including ART adherence, retention in care, and viral suppression.
Author Statement
The study was conceived and designed by JCK, MJV, AS, SS, LKM, and GC. LKM, SS, and CKD led intervention design, training, and oversight. JCK wrote the first draft of the manuscript. MEL led drafting of the Introduction. MJV, AS, SS, LKM, GC, MEL, TK, RP, JM, CKD, JC, and CC provided critical feedback on the first draft and contributed to writing of subsequent drafts. All authors contributed significantly to and edited all sections of the manuscript and have approved the final version.
Role of funding source
Funding for this study was provided by the National Institute on Alcohol Abuse and Alcoholism (R34AA027200: MPIs Kane and Vinikoor). The funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aharonovich E., Hatzenbuehler M.L., Johnston B. A low-cost, sustainable intervention for drinking reduction in the HIV primary care setting. AIDS Care. 2006;18(6):561–568. doi: 10.1080/09540120500264134. [DOI] [PubMed] [Google Scholar]
- Babor T.F., Higgins-Biddle J.C., Saunders J.B., Monteiro M. The alcohol use disorders identification test: Guidelines for use in primary care. Geneva. 2001 https://www.who.int/substance_abuse/publications/audit/en/ [Google Scholar]
- Bedoya C.A., Mimiaga M.J., Beauchamp G., Donnell D., Mayer K.H., Safren S.A. Predictors of HIV transmission risk behavior and seroconversion among Latino men who have sex with men in Project EXPLORE. AIDS and Behavior. 2012;16(3):608–617. doi: 10.1007/s10461-011-9911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, P., Lee, C., Haroz, E. E., et al. (2014). A Transdiagnostic community-based mental health treatment for comorbid disorders: Development and outcomes of a randomized controlled trial among burmese refugees in Thailand. PLoS Medicine 11(11). doi:10.1371/journal.pmed.1001757. [DOI] [PMC free article] [PubMed]
- Chan A.-W., Tetzlaff J.M., Altman D.G. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander G., Hutton H.E., Lau B., Xu X., McCaul M.E. Brief intervention decreases drinking frequency in HIV-infected, heavy drinking women. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;70(2):137–145. doi: 10.1097/QAI.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishinga N., Kinyanda E., Weiss H.A., Patel V., Ayles H., Seedat S. Validation of brief screening tools for depressive and alcohol use disorders among TB and HIV patients in primary care in Zambia. BMC Psychiatry. 2011;11:75. doi: 10.1186/1471-244X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita B.F., Daleiden E.L., Weisz J.R. Identifying and selecting the common elements of evidence based interventions: A distillation and matching model. Mental Health Services Research. 2005;7(1):5–20. doi: 10.1007/s11020-005-1962-6. [DOI] [PubMed] [Google Scholar]
- Chorpita B.F., Weisz J.R. Practicewise; Satellite Beach, FL: 2009. Match-Adtc: Modular approach to therapy for children with anxiety, depression, trauma, or conduct problems. [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- Curran G.M., Bauer M., Mittman B., Pyne J.M., Stetler C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care. 2012;50(3):217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farchione T.J., Fairholme C.P., Ellard K.K. Unified protocol for transdiagnostic treatment of emotional disorders: A randomized controlled trial. Behavior Therapy. 2012;43(3):666–678. doi: 10.1016/j.beth.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzi W., Msamanga G., Spiegelman D., Hunter D.J. Studies of vitamins and minerals and HIV transmission and disease progression. Journal of Nutrition. 2005;135(4):938–944. doi: 10.1093/jn/135.4.938. http://www.ncbi.nlm.nih.gov/pubmed/15795466. Accessed December 11, 2017. [DOI] [PubMed] [Google Scholar]
- Gerbi G.B., Habtemariam T., Tameru B., Nganwa D., Robnett V. The correlation between alcohol consumption and risky sexual behaviors among people living with HIV/AIDS. Journal of Substance Use. 2009;14(2):90–100. doi: 10.1080/14659890802624261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Ciccarone D., Gansky S.A. Interactive “video doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. Myer L, ed. PLoS ONE. 2008;3(4): doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J.A., Dobkin L.M., Mayanja B. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcoholism, Clinical and Experimental Research. 2012;36(5):854–862. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J.A., Samet J.H. Alcohol and HIV disease progression: Weighing the evidence. Current HIV/AIDS Reports. 2010;7(4):226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, P. J. (1967). The behavior of maximum likelihood estimates under nonstandard conditions. In: Vol. 1 of the proceedings of the fifth Berkeley symposium on mathematical statistics and probability. Berkeley, CA: University of California Press, pp. 221–233.
- Humeniuk R., Ali R., Babor T.F. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) Addiction. 2008;103(6):1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Humeniuk, R. E., Henry-Edwards, S., Ali, R. L., Poznyak, V., & Monteiro, M. (2010). The alcohol, smoking and substance involvement screening test (ASSIST): Manual for use in primary care.
- Jonas D.E., Garbutt J.C., Amick H.R. Behavioral counseling after screening for alcohol misuse in primary care: A systematic review and meta-analysis for the U.S. preventive services task force. Annals of Internal Medicine. 2012 doi: 10.7326/0003-4819-157-9-201211060-00544. [DOI] [PubMed] [Google Scholar]
- Kalichman S.C., Simbayi L.C., Vermaak R. Randomized trial of a community-based alcohol-related HIV risk-reduction intervention for men and women in Cape Town South Africa. Annals of Behavioral Medicine. 2008;36(3):270–279. doi: 10.1007/s12160-008-9067-2. [DOI] [PubMed] [Google Scholar]
- Kane J.C., Bolton P., Murray S.M., Bass J.K., Lakin D., Whetten K., Skavenski van Wyk S., Murray L.K. Psychometric evaluation of HIV risk behavior assessments using Audio Computer Assisted Self-Interviewing (ACASI) among orphans and vulnerable children in Zambia. AIDS Care. 2018;30(2):160–167. doi: 10.1080/09540121.2017.1384787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J.C., Murray L.K., Bass J.K., Johnson R.M., Bolton P. Validation of a substance and alcohol use assessment instrument among orphans and vulnerable children in Zambia using Audio Computer Assisted Self-Interviewing (ACASI) Drug and Alcohol Dependence. 2016;166:85–92. doi: 10.1016/j.drugalcdep.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J.C., Murray L.K., Sughrue S. Process and implementation of Audio Computer Assisted Self-Interviewing (ACASI) assessments in low resource settings: A case example from Zambia. Glob Mental Health. 2016;3 doi: 10.1017/gmh.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J.C., Skavenski Van Wyk S., Murray S.M. Testing the effectiveness of a transdiagnostic treatment approach in reducing violence and alcohol abuse among families in Zambia: Study protocol of the Violence and Alcohol Treatment (VATU) trial. Global Mental Health. 2017;4 doi: 10.1017/gmh.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J.C., Vinikoor M.J., Haroz E.E., Al-Yasiri M., Bogdanov S., Mayeya J.…Murray L.K. Mental health comorbidity in low-income and middle-income countries: A call for improved measurement and treatment. Lancet Psychiatry. 2018;5(11):864–888. doi: 10.1016/S2215-0366(18)30301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner E.F.S., Brown N., Jackson K. A systematic review of the impact of brief interventions on substance use and co-morbid physical and mental health conditions. Ment Heal Subst Use Dual Diagnosis. 2011 doi: 10.1080/17523281.2011.533449. [DOI] [Google Scholar]
- Kaner EFS, Dickinson HO, & Beyer FR, et al. (2007) Effectiveness of brief alcohol interventions in primary care populations. In: Kaner EFS, ed. Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd; 2007:CD004148. doi:10.1002/14651858.CD004148.pub3. [DOI] [PubMed]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine. 2008;70(5):539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Mollica, R. F., Caspi-Yavin, Y., Bollini, P., Truong, T., Tor, S., & Lavelle, J. (1992). The harvard trauma questionnaire. Validating a cross-cultural instrument for measuring torture, trauma, and posttraumatic stress disorder in Indochinese refugees. The Journal of Nervous and Mental Disease. 1992;180(2):111–116. http://www.ncbi.nlm.nih.gov/pubmed/1737972. Accessed December 11, 2011. [PubMed]
- Murray S.M., Bolton P.A., Kane J.C., Lakin D., Bass Judith K., Murray L.K. Measuring symptoms of psychopathology in Zambian orphans and vulnerable children: Scale validation and psychometric evaluation. Assessment. 2018;epub doi: 10.1177/1073191118780455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L.K., Dorsey S., Bolton P. Building capacity in mental health interventions in low resource countries: An apprenticeship model for training local providers. International Journal of Mental Health Systems. 2011;5(1):30. doi: 10.1186/1752-4458-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L.K., Dorsey S., Haroz E., Lee C., Alsiary M.M., Haydary A.…Bolton P. A common elements treatment approach for adult mental health problems in low- and middle-income countries. Cognitive Behavioral Practice. 2014;21:111–123. doi: 10.1016/j.cbpra.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. (2005). Helping patients who drink too much. https://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf.
- NIAAA. (2005). Brief interventions. https://pubs.niaaa.nih.gov/publications/aa66/aa66.htm. Accessed December 11, 2017.
- NIH. (2017). Behavioral & integrative treatment development program (R34). https://grants.nih.gov/grants/guide/pa-files/pa-16-073.html. [PubMed]
- Nouaman M.N., Vinikoor M., Seydi M. High prevalence of binge drinking among people living with HIV in four African countries. Journal of the International AIDS Society. 2018 doi: 10.1002/jia2.25202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K., Naidoo P., Louw J. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: Results from a cluster randomized controlled trial. BMC Public Health. 2013;13(1):699. doi: 10.1186/1471-2458-13-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N.M. Alcohol use in HIV patients: What we don’t know may hurt us. International Journal of STD and AIDS. 1999;10(9):561–570. doi: 10.1258/0956462991914654. [DOI] [PubMed] [Google Scholar]
- Pithey A., Parry C. Descriptive systematic review of sub-Saharan African studies on the association between alcohol use and HIV infection. Sahara Journal. 2009 doi: 10.1080/17290376.2009.9724944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L., Melendez-Torres G.J., O’Donnell A. How effective are brief interventions in reducing alcohol consumption: Do the setting, practitioner group and content matter? Findings from a systematic review and metaregression analysis. BMJ Open. 2016;6(8) doi: 10.1136/bmjopen-2016-011473. e011473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: A self report depression scale for research in the general. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rehm J., Samokhvalov A.V., Neuman M.G. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9(1):450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R. Unhealthy alcohol use. New England Journal of Medicine. 2005;352(6):596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2017). Screening, brief intervention, and referral to treatment (SBIRT). Rockville, MD. www.samhsa.gov/sbirt.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791-804. http://www.ncbi.nlm.nih.gov/pubmed/8329970. Accessed January 6, 2017. [DOI] [PubMed]
- StataCorp. (2017). Stata statistical software: Release 15. College Station, TX.
- Vagenas P., Azar M.M., Copenhaver M.M., Springer S.A., Molina P.E., Altice F.L. The impact of alcohol use and related disorders on the HIV continuum of care: A systematic review: Alcohol and the HIV continuum of care. Current HIV/AIDS Reports. 2015;12(4):421–436. doi: 10.1007/s11904-015-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilagut G., Forero C.G., Barbaglia G., Alonso J. Screening for depression in the general population with the center for epidemiologic studies depression (CES-D): A systematic review with meta-analysis. van der Feltz-Cornelis C, ed. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0155431. e0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor M., Masaninga M., Bolton Moore C., Munamunungu V., Siyunda A., Mulenga L.…Wandeler G. Clinical correlates of alcohol use disorders among HIV-infected adults in Zambia. Conference on Retroviruses and Opportunistic Infections. 2015 [Google Scholar]
- Vinikoor M.J., Zyambo Z., Muyoyeta M., Chander G., Saag M.S., Cropsey K. Point-of-care urine ethyl glucuronide testing to detect alcohol use among HIV-hepatitis B virus coinfected adults in Zambia. AIDS and Behavior. January 2018 doi: 10.1007/s10461-018-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandera B., Tumwesigye N.M., Nankabirwa J.I. Efficacy of a single, brief alcohol reduction intervention among men and women living with HIV/AIDS and using alcohol in Kampala, Uganda: A randomized trial. Journal of the International Association of Providers of AIDS Care. 2017;16(3):276–285. doi: 10.1177/2325957416649669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzl B., Watson R.R. Role of alcohol abuse in nutritional immunosuppression. Journal of Nutrition. 1992;122(3 Suppl):733–737. doi: 10.1093/jn/122.suppl_3.733. http://www.ncbi.nlm.nih.gov/pubmed/1542040. Accessed December 11, 2017. [DOI] [PubMed] [Google Scholar]
- Weiss W.M., Murray L.K., Zangana G.A.S. Community-based mental health treatments for survivors of torture and militant attacks in Southern Iraq: A randomized control trial. BMC Psychiatry. 2015;15(1):249. doi: 10.1186/s12888-015-0622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J.R., Chorpita B.F., Palinkas L.A. Testing standard and modular designs for psychotherapy treating depression, anxiety, and conduct problems in youth: A randomized effectiveness trial. Archives of General Psychiatry. 2012;69(3):274–282. doi: 10.1001/archgenpsychiatry.2011.147. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980 doi: 10.2307/1912934. [DOI] [Google Scholar]
- World Health Organization. (2012). Screening and brief intervention for alcohol problems in primary health care. World Health Organization. http://www.who.int/substance_abuse/activities/sbi/en/. Accessed December 11, 2017.
- Wutzke S.E., Shiell A., Gomel M.K., Conigrave K.M. Cost effectiveness of brief interventions for reducing alcohol consumption. Social Science Medicine. 2001;52(6):863–870. doi: 10.1016/s0277-9536(00)00189-1. http://www.ncbi.nlm.nih.gov/pubmed/11234861. Accessed December 11, 2017. [DOI] [PubMed] [Google Scholar]