Abstract
Feline hyperthyroidism is the most commonly diagnosed endocrine-related disease among geriatric housecats, but the causes remain unknown. Exposure to endocrine-disrupting compounds with thyroid targets, such as flame retardants (FRs), may contribute to disease development. Silicone passive sampling devices, or pet tags, quantitatively assessed the bioavailable FR exposures of 78 cats (≥7 y) in New York and Oregon using gas chromatography mass spectrometry. Pet tags were analyzed for 36 polybrominated diphenyl ethers, six organophosphate esters (OPEs), and two alternative brominated FRs. In non-hyperthyroid cats, serum free thyroxine (fT4), total T4 (TT4), total triiodothyronine, and thyroid-stimulating hormone concentrations were compared with FR concentrations. Tris(1,3-dichloro-2-isopropyl) phosphate (TDCIPP) concentrations were higher in hyperthyroid than non-hyperthyroid pet tags (adjusted odds ratio, p<0.07; Mantel-Cox, p<0.02). Higher TDCIPP concentrations were associated with air freshener use compared to no use (p<0.01), residences built since 2005 compared to pre-1989 (p<0.002), and cats preferring to spend time on upholstered furniture compared to no preference (p<0.05). Higher TDCIPP concentrations were associated with higher fT4 and TT4 concentrations (p<0.05). This study provides proof-of-concept data for the use of silicone pet tags with companion animals and further indicates that bioavailable TDCIPP exposures are associated with feline hyperthyroidism.
Keywords: Passive sampling, feline hyperthyroidism, endocrine disrupters, flame retardants, TDCIPP
Graphical Abstract
INTRODUCTION.
Feline hyperthyroidism is the most commonly diagnosed endocrine-related disease among senior and geriatric housecats (≥10 years).1 First clinically diagnosed in 1979, the prevalence of feline hyperthyroidism among US housecats 10 years or older has increased from one in 200 to one in 10 between 1980 and 2014.2, 3 Similar prevalence statistics are reported worldwide.4–7 In North America, an estimated two million cats are currently diagnosed with hyperthyroidism.3 The growing number of diagnoses is likely attributable to a true increase in prevalence, although increased awareness, improved diagnostic tools, and increased feline longevity may contribute.3
Domestic cats are the only nonhuman species frequently diagnosed with hyperthyroidism, known as toxic nodular goiter (TNG) in humans.3 Feline hyperthyroidism and TNG result from excessive circulating concentrations of the thyroid hormones thyroxine (T4) and triiodothyronine (T3).1, 3 These progressive diseases, which also share clinical symptoms, exhibit adenomatous hyperplasias with autonomous cell growth and hormone secretion.3, 8 Because of these similarities, hyperthyroid cats are recommended as animal models for TNG.
The underlying causes of feline hyperthyroidism remain unknown, but its development involves more than one risk factor.1 As with TNG,9 feline hyperthyroidism does not develop due to dietary iodine deficiency alone.1 However, iodine deficiency may function synergistically with other factors.1, 10 Reported risk factors for feline hyperthyroidism include increasing age,5–7, 11 canned cat food consumption,2, 4, 7, 11 and litter box use.4, 5
Researchers have hypothesized another risk factor for feline hyperthyroidism is household flame retardant (FR) exposures.12–16 The hypothesis emerged because the earliest diagnoses coincided with the introduction of polybrominated diphenyl ethers (PBDEs) as FRs during the mid-1970s.17 FRs are commonly used in textiles, polyurethane foam, plastics, and electronics to delay the ignition of a fire.17–19 To meet flammability standards, PBDEs emerged as a major FR series with the common commercial mixtures of pentaBDE, octaBDE, and decaBDE.17, 18 In 2004, the pentaBDE and octaBDE mixtures were voluntarily phased out of US manufacturing due to concerns of persistence, bioaccumulation, and potential to cause adverse health effects.18, 20 The decaBDE mixture phase-out began in 2010, but PBDEs detections continue in dust and biomonitoring samples.21–23 Additionally, products containing PBDEs are infrequently replaced, such that PBDE exposures will likely continue despite the phase-outs.17, 18, 24
In response to the PBDE phase-out, organophosphate ester (OPE) production has increased over the past 10 years.25 OPEs have been considered suitable alternatives to PBDEs for decades25–27 and include analytes such as tris(1,3-dichloroisopropyl) phosphate (TDCIPP), tris(1-chloro-2-propyl) phosphate (TCIPP), tris(2-chloroethyl)phosphate (TCEP), and triphenyl phosphate (TPHP). Evidence suggests that both PBDEs17, 28 and OPEs29–33 act as endocrine-disrupting chemicals (EDCs) with thyroid targets.
A fraction of household PBDEs and OPEs remain unbound and freely dissolved in the gas phase.25, 34, 35 These biologically relevant, or bioavailable, FRs are significant for inhalation and dermal contact exposure routes.35–38 By contrast, FRs bound to particulate matter (e.g. dust) are more significant for the ingestion pathway,34 but all three exposure routes can lead to potential adverse health effects. Both cats12–14 and young children28, 35 are hypothesized to experience FR exposures predominantly via dust ingestion and inhalation of gas phase FRs, such that researchers should consider using cats as sentinels for human FR exposures in the home.
To quantify the bioavailable FR exposures of housecats, this study used a novel silicone passive sampling devices (PSDs). PSDs sequester unbound volatile and semi-volatile organic compounds (VOCs, SVOCs) via diffusion because the PSD polymer mimics an organism’s phospholipid membrane.37–39 PSDs capture the bioavailable fraction of lipophilic organic chemicals and researchers can quantify exposures. Compared to active sampling devices, PSDs are lightweight, easy to use, and low-cost.39 Stationary PSDs underestimate individual exposures,40 leading to an increased interest in personalized PSDs.
In 2014, silicone wristbands were first modified to function as personalized PSDs.38–52 Worn against the skin, wristbands are capable of sequestering 1530 chemicals, including pesticides,45–49 polycyclic aromatic hydrocarbons,39, 40, 42, 44 personal care products,39, 41, 43–45 and FRs.38, 41, 50–52 In Hammel et al. 2016 (n=48), TDCIPP, TCIPP, and TPHP urinary metabolite concentrations were more strongly correlated with parent OPE concentrations in wristbands compared to hand wipes. In Hammel et al. 2018 (n=30), BDE-47, −99, −100, and −153 concentrations were strongly correlated between serum and wristbands. Together, these studies suggest that wristbands can act as strong predictors of FR body burden.51, 52
In this study, silicone pet tags are introduced as a new configuration of personalized PSDs and compare bioavailable FR exposures between hyperthyroid and non-hyperthyroid senior and geriatric cats. The objectives of this study were to (1) demonstrate the use of silicone PSDs on companion animals, (2) compare FR exposures of hyperthyroid and non-hyperthyroid cats, and (3) associate FR concentrations associated with feline hyperthyroidism to household variables and behaviors. This study further recommends the use of housecats as sentinels for human bioavailable FR exposures.
METHODS.
2.1. Materials
Solvents were Optima-grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). All analytical standards were purchased from Accustandard (New Haven, CT) as single analyte or composite solutions. For the full list of individual FR analytes and extraction surrogates, see Table S1 in Supporting Information (SI). Prior to use, glassware was rinsed in a base bath, washed with detergent in an automatic dishwasher, rinsed with 18 MΩ·cm water, and baked at >300 °C for 12 h. Air-tight polytetrafluoroethylene (PTFE) storage bags and closures were purchased from Welch Fluorocarbon, Inc. (Dover, NH, USA).
2.2. Silicone Tag Preparation
The silicone pet tags (3.0 cm wide by 2.5 cm long by 0.3 cm thick; ~2.7 g; https://24hourwristbands.com, Houston, TX, USA) were prepared as previously reported with minimal modifications.44 Briefly, the tags were conditioned in a vacuum oven at 270–300 °C for six hours at 0.1 Torr (Vacuum Oven, Blue-M, no. POM18VC, with Welch Duo-seal pump, no. 1405). Quality control (QC) samples were selected to evaluate for data quality objectives prior to storing the cat tags in sealed metal containers at 4 °C (see Section 2.5). Pet tags were transferred to PTFE bags before deployment.
2.3. Cat Population and Recruitment
Cat recruitment (n=78) occurred between December 2017 and October 2018. All protocols involving cats were approved by the Institutional Animal Care and Use Committees at Oregon State University (OSU ACUP 4963) and Columbia University (CU ACUP AC-AAAT5454). Hyperthyroid cats were recruited from the Animal Endocrine Clinic (nNY,hyperthyroid=22) and OSU’s Animal Teaching Hospital (nOR,hyperthyroid=17). Non-hyperthyroid cats were recruited from the New York Cat Hospital (nNY,non-hyperthyroid=16) and OSU’s Animal Teaching Hospital (nOR,non-hyperthyroid=23). For more details on cat recruitment, see SI.
2.4. Flame Retardant Extraction, Analysis, and Quality Control
The pet tags were cleaned, extracted, and analyzed as previously reported39 (see SI for details on extraction procedures, instrument analysis, and quality control). Additional details on instrument parameters, target analytes, and limits of detection and quantitation (LOD, LOQ) are given in SI and Table S2.
2.5. Statistical Analysis
Statistical analyses were performed using SAS statistical software (JMP Pro version 13.0.0; SAS Institute Inc., Cary, NC) and R free software (CRAN R Project version 3.5.2) for analytes detected in at least one pet tag. All FR concentrations were normalized to the average pet tag sampler mass (2.7 g), or the mass recorded during post-deployment cleaning if the tag was partially chewed. We substituted FR concentrations below the method LODs with a value equal to one-half the LOD. All concentrations were converted to moles per gram pet tag (pmol/g tag). During the PBDE congener comparison between cat tag and commercial mixture profiles, all concentrations were normalized using octanol-air partition coefficients to simulate the silicone-air partition coefficients.52
FR concentrations and thyroid hormone concentrations were approximately log-normally distributed (Kolmogorov’s test, p<0.05). Spearman’s correlation coefficients assessed bivariate comparisons for FR concentrations. Adjusted odds ratios for FR concentrations were calculated using logistic regression,53 where covariates were included if associated with the independent variable at p<0.15.
To confirm the significance of the adjusted odds ratios (OR), we performed two alternative analyses. A Kaplan-Meier procedure for censoring non-detected values compared hyperthyroid and non-hyperthyroid FR tag concentrations (Mantel-Cox χ2).54 Weighted quantile sum (WQS) regression for high-dimensional datasets assessed mixture effects in association with feline hyperthyroidism as the binary disease outcome (see SI).55–57 The WQS regression considered correlations between FR variables, enabled a generalized inference about the mixture effect, and identified individual FRs most strongly associated with feline hyperthyroidism.55–57 Chemical exposures occur as complex mixtures,41 yielding high-dimensional datasets in which some individual exposures may increase the risk of disease. During univariate analysis, identifying chemicals with the strongest association with the disease outcome can be complicated by strong correlations with other chemicals in the dataset.56 Because the FR dataset from this study demonstrated strong variable-variable correlations (Table S2, S3), WQS method application was appropriate as an alternative statistical approach. For the 21 FR components, the WQS method estimated a weighted linear index.56, 57 The 21 FR components were scored as ordinal variables into quantiles (n=4; quartiles) prior to being combined into the weighted index. The FR weights, which sum to 1, were empirically determined with bootstrap sampling (B=100). FRs with higher contributor weights had stronger associations with the outcome of feline hyperthyroidism.
A subset of FRs (adjusted OR, p<0.10) were selected from the logistic regressions for investigation with questionnaire variables using generalized linear models. For all multivariate linear models, we log10-transformed both FR and hormone concentrations to produce a more normal distribution. Again, covariates were included if associated with the independent variable at p<0.15.
RESULTS.
3.1. Participant Population
Of the 78 tags distributed, all tags were worn for seven days and all were returned (100% compliance). One cat owner did not complete a questionnaire (99% compliance). Select questions had up to 16 missing answers (79% compliance). All pet tags were included in the logistic regressions, but tags with missing questionnaire answers were excluded from the multivariate linear models. All pet tags detected at least one FR above LOQ. A summary of cat population demographics is given in Table 1.
Table 1.
Population demographics are reported between hyperthyroid and non-hyperthyroid study participants.
| Characteristic | Hyperthyroid n=39 | Non-Hyperthyroid n=39 | P-value |
|---|---|---|---|
| Location | 0.176 | ||
| New York | 22 (56%) | 16 (41%) | |
| Oregon | 17 (44%) | 23 (59%) | |
| Sex | 0.644 | ||
| Female | 20 (51%) | 22 (56%) | |
| Male | 18 (46%) | 16 (41%) | |
| Mean Age±SD (y) | 13.1±2.46 | 13.4±2.22 | 0.591 |
| Mean Weight±SD (kg) | 4.99±1.46 | 4.85±1.78 | 0.699 |
| Year adopted±SD | 2006±4.12 | 2007±4.22 | 0.511 |
| Time in residence±SD (y) | 7.50±4.61 | 6.00±4.56 | 0.169 |
| Breed | 0.999 | ||
| Domestic Short Hair | 31 (79%) | 26 (67%) | |
| Domestic Medium Hair | 4 (10%) | 5 (13%) | |
| Domestic Long Hair | 2 (5.1%) | 3 (10%) | |
| Manx | 1 (2.6%) | 0 (0.0%) | |
| Russian Blue | 0 (0.0%) | 1 (2.6%) | |
| Siamese/Siamese mix | 1 (2.6%) | 2 (5.1%) |
Due to missing questionnaire answers, not all percentages add up to 100%.
Potential confounding variables included location, age, bite marks on the pet tag, time spent outdoors, living in the same household as other recruited cats, and sampling season. Multiple pet tags (n=10, 13%) were returned with bite marks. Of the bitten tags, two tags (4%) were returned with sections missing, presumably chewed off by the recruited cat. Neither was found to be a confounder for any FRs (ANOVA, p>0.05; logistic regression, p>0.15). Unadjusted OR are shown in Table S3.
3.2. Flame Retardant Concentrations among Case-Control Cats
3.2.1. OPEs
All six OPEs were detected in at least one tag (Table 2). TPHP, TDCIPP, and TCIPP were detected in over 90% of pet tags in each group. TCEP and tri-n-butyl phosphate were detected over 50% of samples each, and tri-n-ethyl phosphate in fewer than 50% of samples. For the Spearman’s correlation, we included all six OPEs for a total of 15 coefficients, of which seven were significant (Table S4). This suggested the OPEs were unlikely to originate from a common source.
Table 2.
Detection frequencies, summary statistics, and adjusted odds ratios for flame retardants detected in at least one tag. Mantel-Cox χ2 values and WQS contributor weights are included as alternative analyses to confirm the adjusted odds ratio result.54–58 Larger WQS index weights indicate a larger contribution to the mixture effect and a stronger association with feline hyperthyroidism.
| Target Analyte | Detection Frequency (% samples) | Median (pmol/g tag) | Geometric Mean (pmol/g tag) | Adjusted Odds Ratio (95% CI) | P-value (odds ratio) | Mantel-Cox χ2 | P-value (χ2) | Weighted Quantile Sum Contribution | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyperthyroid | Non-Hyperthyroid | Hyperthyroid | Non-Hyperthyroid | Hyperthyroid | Non-Hyperthyroid | ||||||
| TNBPa,b,c | 71.8% | 74.4% | 160 | 160 | 66.6 | 68.5 | 0.711 (0.107, 4.61) | 0.716 | 0.549 | 0.459 | 0.137 |
| TNEPa,d,e | 41.0% | 35.9% | <LOD | <LOD | 20.8 | 23.4 | 0.921 (0.852, 9.24) | 0.943 | 0.078 | 0.780 | 0.070 |
| TCEPa,c,f | 51.3% | 56.4% | 212 | 222 | 56.4 | 76.0 | 0.206 (0.014, 2.58) | 0.222 | 1.17 | 0.280 | 0.016 |
| TCIPPa,e,g,h | 100% | 94.9% | 1810 | 2410 | 2070 | 1860 | 1.01 (0.926, 1.11) | 0.870 | 0.008 | 0.929 | 4.51*10−3 |
| TDCIPPa,d,f,h | 94.9% | 97.4% | 113 | 114 | 146 | 126 | 1.37 (0.986, 2.26) | 0.060* | 6.25 | 0.012** | 0.215 |
| TPHPa | 94.9% | 100% | 234 | 243 | 164 | 256 | 1.09 (0.427, 3.23) | 0.840 | 0.596 | 0.440 | 1.67*10−8 |
| Σ6OPEsa,e,g,h | 100% | 100% | 3060 | 3280 | 3270 | 3260 | 1.01 (0.932, 1.11) | 0.822 | 0.113 | 0.737 | -- |
| BDE-8 | 2.6% | 0.0% | <LOD | <LOD | <LOD | <LOD | -- | -- | -- | -- | 0.035 |
| BDE-12 | 2.6% | 0.0% | <LOD | <LOD | <LOD | <LOD | -- | -- | -- | -- | 0.035 |
| BDE-15 | 0.0% | 2.6% | <LOD | <LOD | <LOD | <LOD | -- | -- | -- | -- | 0.035 |
| BDE-17 | 0.0% | 5.1% | <LOD | <LOD | <LOD | <LOD | -- | -- | -- | -- | 0.035 |
| BDE-25 | 2.6% | 0.0% | <LOD | <LOD | <LOD | <LOD | -- | -- | -- | -- | 0.035 |
| BDE-28& BDE-33g | 5.1% | 10.3% | <LOD | <LOD | <LOD | <LOD | 0.949 (0.810, 1.02) | 0.186 | 2.60 | 0.107 | 0.035 |
| BDE-47g | 89.7% | 84.6% | 29.8 | 23.9 | 24.5 | 24.8 | 0.998 (0.990, 1.00) | 0.138 | 0.941 | 0.332 | 0.080 |
| BDE-49g | 10.3% | 10.3% | <LOD | <LOD | <LOD | <LOD | 0.975 (0.881, 1.02) | 0.338 | 2.70 | 0.101 | 0.035 |
| BDE-66 | 2.6% | 2.6% | <LOD | <LOD | <LOD | <LOD | 0.966 (0.773, 1.13) | 0.647 | 1.00 | 0.317 | 0.035 |
| BDE-99d,g | 74.4% | 69.2% | 19.8 | 23.4 | 12.4 | 12.1 | 0.997 (0.987, 1.00) | 0.356 | 1.37 | 0.243 | 0.090 |
| BDE-100g | 25.6% | 35.9% | <LOD | <LOD | 2.45 | 4.11 | 0.991 (0.977, 1.00) | 0.098 | 2.25 | 0.134 | 9.09*10−3 |
| BDE-138 | 0.0% | 2.6% | <LOD | <LOD | <LOD | <LOD | -- | -- | -- | -- | 0.035 |
| BDE-153g | 43.6% | 46.2% | <LOD | <LOD | 1.56 | 2.05 | 0.975 (0.911, 1.01) | 0.150 | 2.71 | 0.100 | 6.44*10−8 |
| BDE-154g | 23.1% | 28.2% | <LOD | <LOD | <LOD | 1.14 | 0.955 (0.832, 1.01) | 0.121 | 2.40 | 0.121 | 0.022 |
| Σ36BDEsg | 92.3% | 89.7% | 106 | 108 | 111 | 130 | 0.880 (0.585, 1.03) | 0.142 | 1.14 | 0.285 | -- |
| EH-TBBa,f,i | 7.7% | 17.9% | <LOD | <LOD | <LOD | 9.39 | 0.998 (0.993, 1.00) | 0.142 | 0.073 | 0.787 | 0.035 |
| Σ2BFRsa,f,i | 7.7% | 17.9% | <LOD | <LOD | <LOD | 10.8 | 0.998 (0.993, 1.00) | 0.142 | 0.073 | 0.787 | -- |
p<0.10;
p<0.05
Odds ratio calculated using nmol/g tag concentrations
Covariates included age,
breed,
sampling season,
years in current residence,
year adopted,
location,
time spent outdoors,
weight
TDCIPP concentrations were higher in hyperthyroid than non-hyperthyroid tags (Table 2; adjusted OR, p<0.07). The Mantel-Cox χ2 confirmed this result (Table 2; Figure S1; Mantel-Cox, p<0.02). The weighted quantile sum regression also indicated that TDCIPP was the largest contributor to the FR mixture effect and the FR most strongly associated with feline hyperthyroidism (contributor weight>0.20), although the entire FR mixture was not associated with the disease outcome (β=0.07; p>0.90). The remaining five OPE concentrations and Σ6OPEs demonstrated no difference between hyperthyroid and non-hyperthyroid tags (Table 2; adjusted OR, p>0.10; Mantel-Cox, p>0.10). This result suggested that besides TDCIPP, hyperthyroid and non-hyperthyroid cats experience similar OPE exposures.
3.2.2. PBDEs
Out of 36 PBDEs in the analytical method, 15 congeners were detected in at least one pet tag (Table 2). Low molecular weight (LMW) congeners (e.g. one through five bromines) were more frequently detected than high molecular weight (HMW) congeners (e.g. six through 10 bromines).
PBDE congeners were detected with similar frequency between hyperthyroid and non-hyperthyroid tags (Table 2). BDE-47 was the most frequently detected congener (>80%) in both hyperthyroid and non-hyperthyroid tags, followed by BDE-99 (>65%). BDE-100 and −153 were detected between 25% and 50% of samples in each group, while BDE-154, −49, −66, −28&−33, −8, −12, and −25 were detected in <25% of samples. The PBDE congener concentrations and Σ36PBDEs demonstrated no difference between hyperthyroid and non-hyperthyroid tags (Table 2; adjusted OR, p>0.10; Mantel-Cox, p>0.10). This result suggested that hyperthyroid and non-hyperthyroid cats experience similar PBDE exposures.
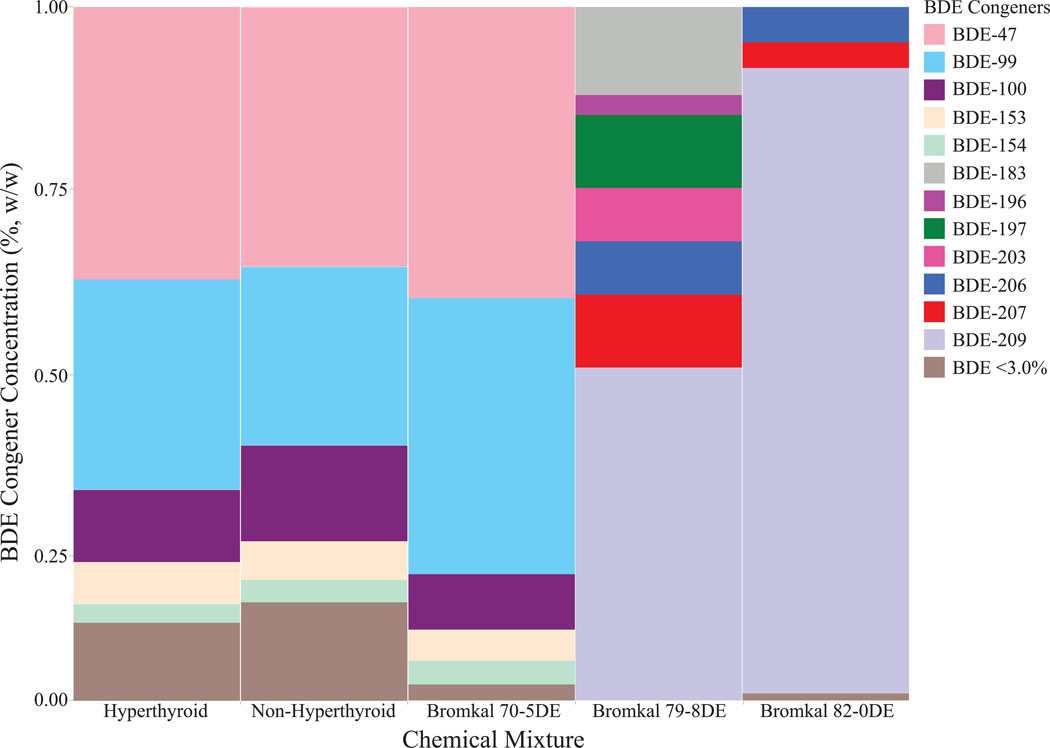
For the Spearman’s correlation, we included the six most frequently detected PBDE congeners for a total of 15 coefficients, all of which were statistically significant (Table S5). In contrast to OPEs, this result suggested pet tag PBDE congener profiles likely originated from a common source, like a commercial mixture. For example, the Bromkal series were common PBDE commercial formulations59 treated a wide variety of consumer products prior to the phase-outs.17, 18 The Bromkal congener compositions were compared to the mean hyperthyroid and non-hyperthyroid congener profiles from this study (Figure 2).59
Figure 2.
The mean PBDE congener profiles identified from hyperthyroid and non-hyperthyroid tags are compared to three commercial PBDE mixtures, known as the Bromkal formulation series.59 The pentaBDE mixture (Bromkal 70–5DE) matched the pet tag congener profiles more closely than the octaBDE (Bromkal 79–8DE) and decaBDE (Bromkal 82–0DE) mixtures.
3.2.3. Alternative BFRs
Two alternative BFRs were included in the analysis, of which only EH-TBB was detected. EH-TBB was detected less frequently in hyperthyroid than non-hyperthyroid tags. Neither EH-TBB nor Σ2BFR concentrations were different between the hyperthyroid and non-hyperthyroid cat tags (Table 2; adjusted odds ratio, p>0.10). This indicated the measured BFR exposures were not associated with feline hyperthyroidism. However, future studies may benefit by including additional BFRs.
3.3. Thyroid Hormone Concentrations
We investigated serum thyroid profile results from non-hyperthyroid cats (n=39) to assess correlations between FR concentrations and thyroid function. Only non-hyperthyroid cats were included in this analysis due to potential bias from hyperthyroid cat weight loss (see Discussion). Summary statistics for free T4 (fT4), total T4 (TT4), total T3 (TT3), and thyroid-stimulating hormone are reported in Table S1.
Multivariate associations between log10-transformed concentrations of OPEs and thyroid hormones (Table 3) were calculated for models with no covariates (Model A), models with cholesterol as the only covariate (Model B), and models with all covariates (Model C). Model C contained the largest number of statistically significant and predominantly positive associations. Compared to other OPEs in Model C, TDCIPP demonstrated the largest effect estimates (10β). For example, a 10% increase in TDCIPP pet tag concentrations corresponded with a 1.38% increase in fT4 hormone concentrations (95% CI: 1.15, 1.66; p<0.002). For fT4, TDCIPP and TPHP were positively associated (Table 3, p<0.002; p<0.03) and TCIPP negatively associated (Table 3, p<0.001). For TT4, TDCIPP and TCEP were positively associated (Table 3, p<0.01; p<0.002), with similar results for TT3 (Table 3, p<0.10; p<0.002).
Table 3.
Exponentiated beta (β) coefficients are shown for the multivariate linear models of log10-transformed OPE and thyroid hormone concentrations. The model was constructed using a stepwise variable selection procedure based on Akaike information criterion optimization.
| Model | TNBP | TNEP | TCEP | TCIPP | TDCIPP | TPHP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone | 10β (95% CI) | p | 10β (95% CI) | p | 10β (95% CI) | p | 10β (95% CI) | p | 10β (95% CI) | p | 10β (95% CI) | p |
| fT4 (ng/dL) | ||||||||||||
| Model A | 1.07 (0.982,1.17) | 0.116 | 0.954 (0.872,1.05) | 0.309 | 1.00 (0.896,1.12) | 0.976 | 1.01 (0.885,1.15) | 0.877 | 0.958 (0.683,1.34) | 0.801 | 0.926 (0.706,1.21) | 0.571 |
| Model B | 0.996 (0.933,1.06) | 0.909 | 0.969 (0.909,1.03) | 0.329 | 1.03 (0.952,1.11) | 0.458 | 0.897 (0.817,0.986) | 0.026 | 1.10 (0.869,1.39) | 0.418 | 1.21 (0.999,1.47) | 0.052 |
| Model C | 1.04 (0.945,1.14) | 0.422 | 1.00 (0.929,1.08) | 0.949 | 0.990 (0.927,1.06) | 0.763 | 0.806 (0.738,0.881) | <0.001 | 1.38 (1.15,1.66) | 0.001 | 1.27 (1.04,1.56) | 0.020 |
| TT4 (ug/dL) | ||||||||||||
| Model A | 1.12 (0.910,1.37) | 0.283 | 0.954 (0.776,1.17) | 0.645 | 1.11 (0.859,1.43) | 0.422 | 1.12 (0.831,1.52) | 0.437 | 1.26 (0.583,2.72) | 0.550 | 0.809 (0.436,1.50) | 0.491 |
| Model B | 1.03 (0.846,1.25) | 0.784 | 0.994 (0.823,1.20) | 0.953 | 1.13 (0.901,1.43) | 0.277 | 1.03 (0.780,1.37) | 0.817 | 1.36 (0.675,2.74) | 0.381 | 0.974 (0.549,1.73) | 0.928 |
| Model C | 1.04 (0.861,1.25) | 0.678 | 0.891 (0.764,1.04) | 0.132 | 1.25 (1.10,1.43) | 0.001 | 0.853 (0.715,1.02) | 0.076 | 1.70 (1.18,2.46) | 0.006 | 1.22 (0.816,1.82) | 0.323 |
| TT3 (ng/dL) | ||||||||||||
| Model A | 1.56 (0.869,2.79) | 0.133 | 0.725 (0.402,1.31) | 0.276 | 1.32 (0.643,2.73) | 0.437 | 1.98 (0.836,4.69) | 0.117 | 2.46 (0.274,22.2) | 0.411 | 0.429 (0.074,2.50) | 0.338 |
| Model B | 1.27 (0.717,2.23) | 0.407 | 0.804 (0.461,1.40) | 0.431 | 1.40 (0.715,2.76) | 0.315 | 1.60 (0.706,3.64) | 0.252 | 2.99 (0.384,23.2) | 0.287 | 0.682 (0.127,3.67) | 0.648 |
| Model C | 0.876 (0.499,1.54) | 0.636 | 0.784 (0.496,1.24) | 0.286 | 2.10 (1.41,3.12) | <0.001 | 0.961 (0.567,1.63) | 0.879 | 2.59 (0.859,7.80) | 0.089 | 1.72 (0.516,5.74) | 0.366 |
p<0.05
Model A: no covariates
Model B: cholesterol as covariate
Model C: all covariates – cholesterol, age, breed, sampling season, years in current residence, year adopted, location, time spent outdoors, and weight
3.4. Associations with Household and Feline Variables
TDCIPP log10-transformed concentrations from pet tags were associated with specific household and behavioral variables in a multivariate linear model, adjusted for confounders (Table 4). Reference groups are indicated in the effect estimate column (10β). For instance, the median TDCIPP pet tag concentrations in homes with weekly air freshener use was 61% higher compared to median concentrations in homes with no air freshener use (95% CI: 1.18, 2.20; p<0.002). For cleaning-related variables, TDCIPP concentrations were positively associated with monthly to weekly air freshener use (p<0.01), but not associated with vacuum frequency (p>0.05). Residence-specific variables associated with higher TDCIPP concentrations were residences built since 2005 compared to those built prior to 1989 (p<0.002) and residences containing upholstered furniture purchased between 2007 and 2012 compared to purchases prior to 2006 (p<0.01). Feline behavioral variables positively associated with TDCIPP were any consumption of commercial dry food (p<0.001) and cats who preferred to sleep on furniture compared to cats with no location preference (p<0.05).
Table 4.
Exponentiated beta (β) coefficients are shown for the multivariate linear model of log10-transformed TDCIPP concentrations with household variables. Covariates include sampling season, adoption year, and whether the cat spent any time outdoors. The model was constructed using a stepwise variable selection procedure based on Akaike information criterion optimization.
| Variable | 10β | 95% CI | p-value |
|---|---|---|---|
| Household Cleaning |
|||
| Air freshener use | |||
| Never | Reference | ||
| Annual | 0.653 | 0.330, 1.29 | 0.223 |
| Seasonal | 1.46 | 0.980, 2.19 | 0.066* |
| Monthly | 1.97 | 1.22, 3.18 | 0.007** |
| Weekly+ | 1.61 | 1.18, 2.20 | 0.004** |
| Vacuum frequency | |||
| 0 times/month | Reference | ||
| 1–4 times/month | 1.21 | 0.778, 1.88 | 0.402 |
| 5–8 times/month | 0.982 | 0.607, 1.59 | 0.942 |
| 9+ times/month | 1.06 | 0.646, 1.74 | 0.821 |
| Residence |
|||
| Number of people | 1.10 | 0.989, 1.22 | 0.085* |
| Residence built | |||
| Prior to 1989 | Reference | ||
| 1990–2004 | 1.25 | 0.888, 1.76 | 0.203 |
| 2005 to Present | 2.05 | 1.34, 3.13 | 0.001** |
| Last purchase of upholstered furniture | |||
| Prior to 2006 | Reference | ||
| 2007–2012 | 2.14 | 1.26, 3.62 | 0.006** |
| 2013-Present | 1.19 | 0.718, 1.97 | 0.499 |
| Feline Behaviors |
|||
| Any consumption of commercial dry food | 2.77 | 1.71, 4.49 | <0.001** |
| Preferred location in residence | |||
| No preference | Reference | ||
| Carpet/rug | 0.590 | 0.265, 1.31 | 0.198 |
| Cat bed/perch | 0.849 | 0.645, 1.12 | 0.247 |
| Furniture | 1.45 | 1.02, 2.05 | 0.040** |
| Human bed | 1.40 | 0.892, 2.21 | 0.146 |
p<0.10;
p<0.05
3.5. Intra-Household Comparison
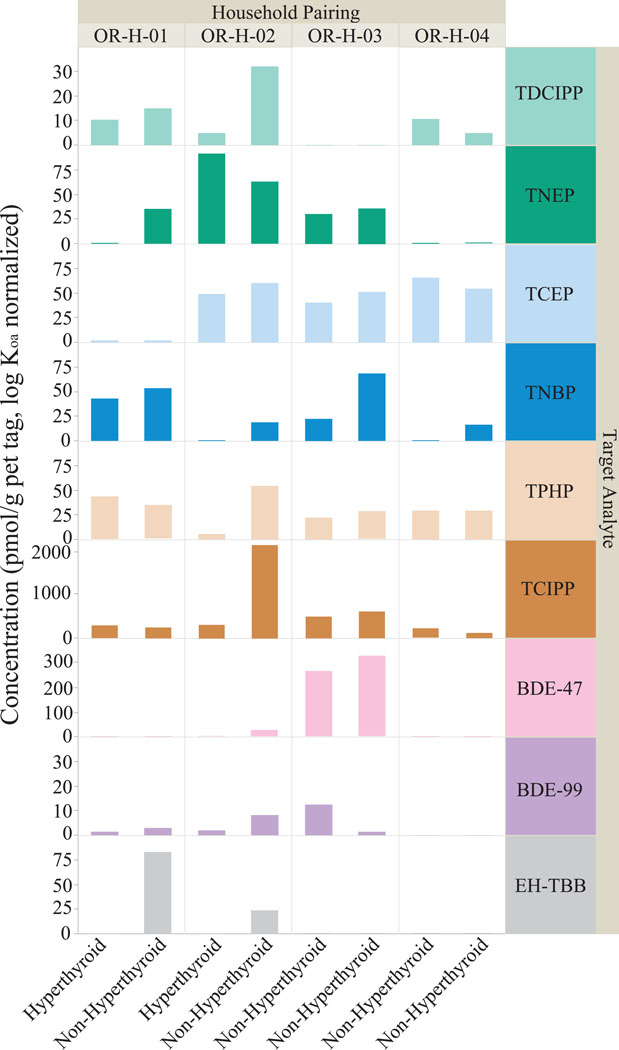
Of 78 recruited cats, four pairs of cats lived in the same household, enabling direct comparisons of feline exposures within a shared living space. Because household factors (e.g. air freshener use) were identical between cat pairs, FR exposure differences were attributable to specific feline behaviors. There were two pairs of hyperthyroid/non-hyperthyroid cats and two pairs of two non-hyperthyroid cats living in the same household. All FR concentrations were normalized using log Koa values to account for variable FR-silicone affinity.52 Each pet tag FR profile was unique, regardless of the shared household environment (Figure 3). Higher TDCIPP concentrations for one cat were observed between three of the four pairs (OR-H-01, OR-H-02, OR-H-04). In all three pairs, the cat with higher TDCIPP tag concentrations spent an additional one to six hours/day on upholstered furniture. Consistent with the results in Table 4, this suggested an association between elevated TDCIPP exposures and increased time spent on upholstered furniture (p<0.05).
Figure 3.
The FR profiles from four pairs of cats living in the same household were compared to identify individual variations. The FR profiles between non-hyperthyroid cats in a household (OR-H-03, OR-H-04) were visually more similar than profiles between a hyperthyroid and non-hyperthyroid cat (OR-H-01, OR-H-02). Each individual FR profile was unique, indicative of the sampler sensitivity to individual feline behaviors.
DISCUSSION.
4.1. Owner Feedback
The owner responses to this study were extremely positive. The most common written feedback were variations of “The tag didn’t bother her/him at all!.” Some recruited cats did not wear a collar on a daily basis, but owners frequently reported their cat became accustomed to wearing the collar and tag. This feedback indicated that pet tag use is low-stress and simple for both cat and owner when monitoring companion animal chemical exposures.
4.2. TDCIPP
4.2.1. This Study
To our knowledge, this is the first study to investigate bioavailable household OPE exposures between hyperthyroid and non-hyperthyroid cats. Of the OPEs, PBDEs, and BFRs detected, TDCIPP concentrations were higher in pet tags from hyperthyroid compared to non-hyperthyroid cats (Table 2; adjusted odds ratio, p<0.07; Mantel-Cox, p<0.02). In Table 3, TDCIPP concentrations were also positively associated with fT4 (p<0.002), TT4 (p<0.01), and TT3 (p<0.10) concentrations among non-hyperthyroid cats. TDCIPP also had the largest 10β coefficients, and therefore impacted fT4, TT4, and TT3 more strongly, compared to other OPEs in Table 3. This result was further suggestive of a link between TDCIPP exposure and thyroid function. Combined with historic use and altered thyroid hormone function in various organisms, this study provides new evidence that bioavailable household TDCIPP exposures may be linked to feline hyperthyroidism.
4.2.2. Background
Prior to the earliest diagnoses of feline hyperthyroidism, manufacturers introduced TDCIPP as a household FR.26, 29, 60 Known as Fyrol FR2,19, 26, 61 TDCIPP was initially applied to children’s sleepwear to meet US flammability standards in the mid-1970s. Concerns of mutagenicity26, 62 led to its discontinuation from sleepwear products in May 1977.29, 61 However, TDCIPP use continued in other consumer products, particularly upholstered furniture containing polyurethane foam.29, 63, 64 Annual US demand for TDCIPP expanded from an estimated 450 tons in 1997 to 22,700 tons in 2006.25, 33 TDCIPP use has continued to grow in the past decade with the subsequent PBDE phase-out.23, 63, 64
Additionally, a growing body of evidence has implicated TDCIPP as an EDC with thyroid targets.25, 29–31 TDCIPP exposures have been correlated with altered thyroid hormone levels in human men,65 as well as suspected neurotoxicity, developmental toxicity, and hepatotoxicity in various model organisms.25, 31 Although TDCIPP mechanisms of action remain unknown, there is interest in the downregulation of messenger RNA expression and ribosome protein genes along the hypothalamic-pituitary-thyroid axis.31–33 Ribosome biogenesis is suggested to drive cell growth, and the downregulation of ribosome protein genes may be important in TDCIPP-induced phenotypic alterations (e.g. decreased cell quantity).33 With historic TDCIPP use and exposure differences observed in this study, evidence of EDC mechanisms presents a converging line of evidence for the association of household bioavailable TDCIPP exposures with feline hyperthyroidism.
Widespread TDCIPP exposures among human populations, as documented by NHANES data,27 can potentially be monitored using cats as sentinels.66, 67 Such TDCIPP and other chemical exposure data may be particularly useful in homes with either a cat at risk of developing feline hyperthyroidism and/or a human at risk of developing TNG.
4.2.3. Household and Behavioral Variables
Associations between TDCIPP pet tag concentrations and household and behavioral variables suggested preventative health interventions that could be implemented to reduce TDCIPP exposures (Table 4). With cleaning-related variables, elevated TDCIPP concentrations with air freshener use may indicate OPE applications beyond flame retarding (e.g. plasticizers, anti-foaming agents).25, 27, 39 For instance, OPE use as anti-foaming agents27, 39 may have applications in gel air fresheners. However, to the authors’ knowledge, published studies have not reported emissions of SVOCs from gel air fresheners. Unexpectedly, vacuum frequency did not affect TDCIPP tag concentrations, in contrast to previously published results analyzing wristbands worn by preschool-aged children for FRs.38 Residence-specific variables (residences built since 2005 or which contain upholstered furniture purchased between 2007 and 2012) were potentially reflective of increasing OPE production volume in recent decades.25
More interestingly, select feline behaviors were associated with TDCIPP. Higher TDCIPP concentrations were associated with any consumption of commercial dry food (p<0.001), a result potentially related to chemical applications in pet food packaging1, 2, 5, 36 and warrants further study. TDCIPP concentrations were also positively associated with cats that preferred to sleep on furniture (p<0.05) compared to cats with no location preference, a result attributable to high flammability standards for human consumer products.63
FR concentrations beyond TDCIPP were not investigated using multivariate linear models because this study focused on FRs associated with feline hyperthyroidism. Exploring the remaining FRs may provide greater insight into how specific household and behavioral variables affect the pattern of bioavailable FR exposures among mature, senior, and geriatric cats.
4.3. PBDEs
4.3.1. Congeners
Similarities between the pet tags and the pentaBDE mixture (Figure 2) suggested that hyperthyroid and non-hyperthyroid cats experience similar PBDE exposures and that bioavailable PBDE exposures are not associated with feline hyperthyroidism. Both hyperthyroid and non-hyperthyroid pet tag profiles closely matched the pentaBDE mixture (Bromkal 70–5DE), compared to the octaBDE (Bromkal 79–8DE) and decaBDE (Bromkal 82–0DE) mixtures.59 These three profiles were dominated by BDE-47, −99, and −100, with minor contributions from BDE-153 and −154. By comparison, BDE-209 and other HMW congeners dominated the octa-and deca-mixtures, but were undetected in the pet tags. However, additional HMW congener exposures may occur via dust ingestion (e.g. feline grooming habits).14, 16 Although hyperthyroid and non-hyperthyroid pet tags demonstrated similar PBDE exposures in this study, silicone pet tags may still be applied in the future to identify risk factors associated with elevated PBDE concentrations in human home environments.
4.3.2. Previous Literature
Previous studies on feline PBDE exposures included samples of cat serum,12–16, 67–71 household dust,13, 14, 16, 69 and commercial cat food.12–14, 68 However, only three publications have positively associated hyperthyroidism diagnoses, serum PBDE concentrations, and dust PBDE concentrations.14–16
The strongest evidence for a link between PBDE exposures and feline hyperthyroidism came from Guo et al. 2016, a longitudinal case-control study (ntotal=22), where the median serum concentrations of 19 PBDE congeners were higher in hyperthyroid than non-hyperthyroid cats.15 Separately, Engdahl et al. 2017 demonstrated a significant association between dust and serum PBDE concentrations in homes with non-hyperthyroid cats (ntotal=17), but this was solely applicable for BDE-47, −99, and −153.16 To date, studies have only examined dust ingestion as the primary FR exposure route for indoor cats.14–16
4.4. Potential Biases of Serum Concentrations
Studies including serum PBDE and thyroid hormone concentrations from hyperthyroid cats may introduce unintentional bias. Serum concentrations represent body burden,20, 72 but over 90% of hyperthyroid cats experience moderate to extreme weight loss prior to treatment.73 Weight loss introduces potential overestimation bias into serum PBDE concentrations for hyperthyroid cats because PBDEs stored in fat can be released into serum.17 In contrast, the silicone pet tags effectively avoid bias resulting from weight loss while still indicative of body burden.42, 51, 52 Silicone pet tags may serve as a supplement to cat serum samples for future studies.
4.5. Intra-Household Variations
Each pet tag featured unique FR exposures for each cat in a shared household (Figure 3). These results suggested that silicone pet tags are highly sensitive to individual feline behaviors within a given household. In particular, elevated TDCIPP concentrations among cat pairs were associated with an additional one to six hours/day on upholstered furniture, consistent with results from Table 4. Despite the small sample size, this data also suggested that silicone pet tags and other personalized PSDs can effectively assess preventative health interventions.
The intra-household comparisons provide future opportunities to explore other feline chemical exposures, particularly additional EDCs with thyroid targets. As seen in Figure 3, among the hyperthyroid/non-hyperthyroid pairs, the non-hyperthyroid pet tags featured higher concentrations of TDCIPP, TNBP, and EH-TBB. Other potential EDCs, such as phthalates,41 were not captured by the FR analytical method and such data may provide insight into additional household chemical exposures.
4.6. Study Limitations
There were several limitations to this study. First, the study population was composed of non-random recruitments and may not be representative of the wider US cat population. The small sample size also limited the consideration of potential confounders. However, this is now the largest case-control study on feline hyperthyroidism related to household FR exposures. Second, silicone pet tags sample VOCs and SVOCs in the bioavailable phase, which can incorporate inhalation, dermal contact, and limited ingestion exposure pathways. For instance, silicone wristbands can detect caffeine after it has been ingested and sweat through the skin.39 However, the study objectives did not include isolating the FR concentrations attributed to specific exposure routes. Third, this study did not use performance reference compounds as in situ calibration standards to estimate environmental concentrations.74 Background air concentrations are difficult to calculate without the use of performance reference compounds, but this study investigated entire external exposures in the context to feline hyperthyroidism. Finally, feline behaviors in the home environment may change over time. Cats may reduce physical activity as they age and as the household dynamic changes (e.g. new furniture).
The results of this silicone pet tag study demonstrated that cats are exposed to bioavailable household FRs and elevated TDCIPP exposures are associated with the occurrence of feline hyperthyroidism. This study also demonstrated that TDCIPP pet tag concentrations positively associated with thyroid hormone concentrations among non-hyperthyroid cats. Evidence of EDC mechanisms and historic household use further strengthened these results linking TDCIPP exposures with feline hyperthyroidism. In future studies, cats can wear silicone pet tags to assess preventative health interventions and to act as sentinels for FR and EDC human exposures.
Supplementary Material
Figure 1.
The study compared flame retardant exposures using silicone pet tags between hyperthyroid (n=39) and non-hyperthyroid (n=39) mature, senior, and geriatric cats (e.g. ≥7 years).
ACKNOWLEDGMENT
I give special thanks to the cat owners and their cats for their assistance in this research. Special thanks is also given to the Institutional Animal Care and Use Committees, the OSU Superfund Research Program Core D for chemistry support, the veterinary clinicians, Richard Scott, Peter Hoffman, Clarisa Caballero-Ignacio, Holly Dixon, Michael Barton, Diana Rohlman, Amberlie Barnard, Jarvis Barnard, Elizabeth Gibson, Whitney Cowell, Anne Bozack, Courtney Barden, Candace Remcho, and Piper Poutasse.
Funding Sources
Funding is provided by the National Institute of Environmental Health Science Toxicology Training Grant (Grant T32ES007060–37) and the Food Safety and Environmental Stewardship Laboratory.
ABBREVIATIONS
- ACUP
Animal Care and Use Protocol
- EH-TBB
2-ethylhexyl-2,3,4,5-tetrabromobenzoate
- FR
flame retardant
- LOD
limit of detection
- LOQ
limit of quantitation
- OPE
organophosphate ester
- PBDE
polybrominated diphenyl ether
- PSD
passive sampling device
- PTFE
polytetrafluoroethylene
- TNG
toxic nodular goiter
- TCEP
tris(2-chloroethyl)phosphate
- TCIPP
tris(1-chloro-2-propyl) phosphate
- TDCIPP
tris(1,3-dichloroisopropyl) phosphate
- TPHP
triphenyl phosphate
- SVOC
semi-volatile organic compound
- VOC
volatile organic compound
Footnotes
Conflict of Interest
Kim A. Anderson, an author of this research, discloses a financial interest in MyExposome, Inc., which is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by OSU in accordance with its policy on research conflicts of interest. The authors have no other disclosures.
REFERENCES
- 1.Peterson M. What’s Causing This Epidemic of Thyroid Disease and Can We Prevent It? J Feline Med. Surg 2012, 14 (11), 804–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarlett JM; Moise NS; Rayl J. Feline Hyperthyroidism -a Descriptive and Case-Control Study. Prev. Vet. Med 1988, 6 (4), 295–309. [Google Scholar]
- 3.Peterson ME Feline Hyperthyroidism: An Animal Model for Toxic Nodular Goiter. J. Endocrinol 2014, 223 (2), T97–T114. [DOI] [PubMed] [Google Scholar]
- 4.Kass PH; Peterson ME; Levy J; James K; Becker DV; Cowgill L. Evaluation of Environmental, Nutritional, and Host Factors in Cats with Hyperthyroidism. J. Vet. Intern. Med 1999, 13 (4), 323–329. [DOI] [PubMed] [Google Scholar]
- 5.Wakeling J; Everard A; Brodbelt D; Elliott J; Syme H. Risk Factors for Feline Hyperthyroidism in the UK. J. Small Anim. Pract 2009, 50 (8), 406–414. [DOI] [PubMed] [Google Scholar]
- 6.Bree L; Gallagher BA; Shiel RE; Mooney CT Prevalence and Risk Factors for Hyperthyroidism in Irish Cats from the Greater Dublin Area. Irish Vet. J 2018, 71, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean JL; Lobetti RG; Mooney CT; Thompson PN; Schoeman JP Prevalence of and Risk Factors for Feline Hyperthyroidism in South Africa. J Feline Med. Surg 2017, 19 (10), 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson ME; Ward CR Etiopathologic Findings of Hyperthyroidism in Cats. Vet. Clin. N. Am 2007, 37 (4), 633. [DOI] [PubMed] [Google Scholar]
- 9.Derwahl M; Studer H. Nodular Goiter and Goiter Nodules: Where Iodine Deficiency Falls Short of Explaining the Facts. Exp. Clin. Endocr. Diab 2001, 109 (5), 250–60. [DOI] [PubMed] [Google Scholar]
- 10.Edinboro CH; Scott-Moncrieff JC; Glickman LT Feline Hyperthyroidism: Potential Relationship with Iodine Supplement Requirements of Commercial Cat Foods. J Feline Med. Surg 2010, 12 (9), 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin KM; Rossing MA; Ryland LM; DiGiacomo RF; Freitag WA Evaluation of Dietary and Environmental Risk Factors for Hyperthyroidism in Cats. J. Am. Vet. Med. Assoc 2000, 217 (6), 853–856. [DOI] [PubMed] [Google Scholar]
- 12.Dye JA; Venier M; Zhu L; Ward CR; Hites RA; Birnbaum LS Elevated Pbde Levels in Pet Cats: Sentinels for Humans? Environ. Sci. Technol 2007, 41 (18), 6350–6356. [DOI] [PubMed] [Google Scholar]
- 13.Mensching DA; Slater M; Scott JW; Ferguson DC; Beasley VR The Feline Thyroid Gland: A Model for Endocrine Disruption by Polybrominated Diphenyl Ethers (PBDEs)? Journal of Toxicological Health Part A 2012, 75 (4), 201–12. [DOI] [PubMed] [Google Scholar]
- 14.Norrgran J; Jones B; Bignert A; Athanassiadis I; Bergman A. Higher PBDE Serum Concentrations May Be Associated with Feline Hyperthyroidism in Swedish Cats. Environ. Sci. Technol 2015, 49 (8), 5107–14. [DOI] [PubMed] [Google Scholar]
- 15.Guo WH; Gardner S; Yen SM; Petreas M; Park JS Temporal Changes of PBDE Levels in California House Cats and a Link to Cat Hyperthyroidism. Environ. Sci. Technol 2016, 50 (3), 1510–1518. [DOI] [PubMed] [Google Scholar]
- 16.Engdahl JN; Bignert A; Jones B; Athanassiadis I; Bergman A; Weiss JM Cats’ Internal Exposure to Selected Brominated Flame Retardants and Organochlorines Correlated to House Dust and Cat Food. Environ. Sci. Technol 2017, 51 (5), 3012–3020. [DOI] [PubMed] [Google Scholar]
- 17.Birnbaum LS; Staskal DF Brominated Flame Retardants: Cause for Concern? Environ. Health Perspect 2004, 112 (1), 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EPA U. Polybrominated Diphenyl Ethers (PBDEs): Action Plan. In 2009. [Google Scholar]
- 19.Sanders HJ Flame Retardants. Chem. Eng. News 1978, 56 (17), 22–36. [Google Scholar]
- 20.Kim YR; Harden FA; Toms LML; Norman RE Health Consequences of Exposure to Brominated Flame Retardants: A Systematic Review. Chemosphere 2014, 106, 1–19. [DOI] [PubMed] [Google Scholar]
- 21.Allen JG; McClean MD; Stapleton HM; Webster TF Critical Factors in Assessing Exposure to PBDEs Via House Dust. Environ. Int 2008, 34 (8), 1085–1091. [DOI] [PubMed] [Google Scholar]
- 22.Dodson RE; Perovich LJ; Covaci A; Van den Eede N; Ionas AC; Dirtu AC; Brody JG; Rudel RA After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ. Sci. Technol 2012, 46 (24), 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammel SC; Hoffman K; Lorenzo AM; Chen A; Phillips AL; Butt CM; Sosa JA; Webster TF; Stapleton HM Associations between Flame Retardant Applications in Furniture Foam, House Dust Levels, and Residents’ Serum Levels. Environ. Int 2017, 107, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjodin A; Jones RS; Caudill SP; Wong LY; Turner WE; Calafat AM Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, and Persistent Pesticides in Serum from the National Health and Nutrition Examination Survey: 2003–2008. Environ. Sci. Technol 2014, 48 (1), 753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Veen I; de Boer J. Phosphorus Flame Retardants: Properties, Production, Environmental Occurrence, Toxicity and Analysis. Chemosphere 2012, 88 (10), 1119–1153. [DOI] [PubMed] [Google Scholar]
- 26.Gold MD; Blum A; Ames BN Another Flame Retardant, Tris-(1,3-Dichloro-2-Propyl)-Phosphate, and Its Expected Metabolites Are Mutagens. Science 1978, 200 (4343), 785–787. [DOI] [PubMed] [Google Scholar]
- 27.Ospina M; Jayatilaka NK; Wong LY; Restrepo P; Calafat AM Exposure to Organophosphate Flame Retardant Chemicals in the U.S. General Population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int 2018, 110, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darnerud P. Toxic Effects of Brominated Flame Retardants in Man and in Wildlife. Environ. Int 2003, 29 (6), 841–853. [DOI] [PubMed] [Google Scholar]
- 29.Dishaw LV; Macaulay LJ; Roberts SC; Stapleton HM Exposures, Mechanisms, and Impacts of Endocrine-Active Flame Retardants. Curr. Opin. Pharm 2014, 19, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojima H; Takeuchi S; Itoh T; Iida M; Kobayashi S; Yoshida T. In Vitro Endocrine Disruption Potential of Organophosphate Flame Retardants Via Human Nuclear Receptors. Toxicology 2013, 314 (1), 76–83. [DOI] [PubMed] [Google Scholar]
- 31.Farhat A; Crump D; Chiu S; Williams KL; Letcher RJ; Gauthier LT; Kennedy SW In Ovo Effects of Two Organophosphate Flame Retardants-TCPP and TDCPP-on Pipping Success, Development, mRNA Expression, and Thyroid Hormone Levels in Chicken Embryos. Toxicol. Sci 2013, 134 (1), 92–102. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q; Liang K; Liu J; Yang L; Guo Y; Liu C; Zhou B. Exposure of Zebrafish Embryos/Larvae to TDCPP Alters Concentrations of Thyroid Hormones and Transcriptions of Genes Involved in the Hypothalamic-Pituitary-Thyroid Axis. Aquat. Toxicol 2013, 126, 207–13. [DOI] [PubMed] [Google Scholar]
- 33.Li J; Giesy JP; Yu L; Li G; Liu C. Effects of Tris(1,3-Dichloro-2-Propyl) Phosphate (Tdcpp) in Tetrahymena Thermophila: Targeting the Ribosome. Sci. Rep 2015, 5, 10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liagkouridis L; Cousins AP; Cousins IT Physical-Chemical Properties and Evaluative Fate Modelling of ‘Emerging’ and ‘Novel’ Brominated and Organophosphorus Flame Retardants in the Indoor and Outdoor Environment. Sci. Total Environ 2015, 524, 416–426. [DOI] [PubMed] [Google Scholar]
- 35.Lorber M. Exposure of Americans to Polybrominated Diphenyl Ethers. J. Expo. Sci. Environ. Epidemiol 2008, 18 (1), 2–19. [DOI] [PubMed] [Google Scholar]
- 36.Weschler CJ; Nazaroff WW SVOC Exposure Indoors: Fresh Look at Dermal Pathways. Indoor Air 2012, 22 (5), 356–377. [DOI] [PubMed] [Google Scholar]
- 37.Anderson K; Hillwalker W. Bioavailability In Ecotoxicology, Jorgensen SE; Fath BD, Eds. Elsevier: Oxford, 2008; Vol. 1, 348–357. [Google Scholar]
- 38.Kile ML; Scott RP; O’Connell SG; Lipscomb S; MacDonald M; McClelland M; Anderson KA Using Silicone Wristbands to Evaluate Preschool Children’s Exposure to Flame Retardants. Environ. Res 2016, 147, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connell SG; Kincl LD; Anderson KA Silicone Wristbands as Personal Passive Samplers. Environ. Sci. Technol 2014, 48 (6), 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulik LB; Hobbie KA; Rohlman D; Smith BW; Scott RP; Kincl L; Haynes EN; Anderson KA Environmental and Individual PAH Exposures near Rural Natural Gas Extraction. Environ. Pollut 2018, 241, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon HM; Armstrong G; Barton M; Bergmann AJ; Bondy M; Halbleib ML; Hamilton W; Haynes E; Herbstman J; Hoffman P; Jepson P; Kile ML; Kincl L; Laurienti PJ; North P; Paulik LB; Petrosino J; Points GL; Poutasse CM; Rohlman D; Scott RP; Smith B; Tidwell LG; Walker C; Waters KM; Anderson KA Discovery of Common Chemical Exposures across Three Continents Using Silicone Wristbands. R. Soc. Open Sci 2019, 6 (2), 181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixon HM; Scott RP; Holmes D; Calero L; Kincl LD; Waters KM; Camann DE; Calafat AM; Herbstman JB; Anderson KA Silicone Wristbands Compared with Traditional Polycyclic Aromatic Hydrocarbon Exposure Assessment Methods. Anal. Bioanal. Chem 2018, 410 (13), 3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergmann AJ; Points GL; Scott RP; Wilson G; Anderson KA Development of Quantitative Screen for 1550 Chemicals with GC-MS. Anal. Bioanal. Chem 2018, 410 (13), 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson KA; Points III GL; Donald CE; Dixon HM; Scott RP; Wilson G; Tidwell LG; Hoffman PD; Herbstman JB; O’Connell SG Preparation and Performance Features of Wristband Samplers and Considerations for Chemical Exposure Assessment. J. Expo. Sci. Environ. Epidemiol 2017, 27 (6), 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergmann AJ; North PE; Vasquez L; Bello H; Ruiz M. d. C. G.; Anderson KA Multi-Class Chemical Exposure in Rural Peru Using Silicone Wristbands. J. Expo. Sci. Environ. Epidemiol 2017, 27 (6), 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donald CE; Scott RP; Blaustein KL; Halbleib ML; Sarr M; Jepson PC; Anderson KA Silicone Wristbands Detect Individuals’ Pesticide Exposures in West Africa. R. Soc. Open Sci 2016, 3 (8), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidi PA; Anderson KA; Chen HY; Anderson R; Salvador-Moreno N; Mora DC; Poutasse C; Laurienti PJ; Daniel SS; Arcury TA Personal Samplers of Bioavailable Pesticides Integrated with a Hair Follicle Assay of DNA Damage to Assess Environmental Exposures and Their Associated Risks in Children. Mutat. Res 2017, 822, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aerts R; Joly L; Szternfeld P; Tsilikas K; De Cremer K; Castelain P; Aerts JM; Van Orshoven J; Somers B; Hendrickx M; Andjelkovic M; Van Nieuwenhuyse A. Silicone Wristband Passive Samplers Yield Highly Individualized Pesticide Residue Exposure Profiles. Environ. Sci. Technol 2018, 52 (1), 298–307. [DOI] [PubMed] [Google Scholar]
- 49.Harley KG; Parra KL; Camacho J; Bradman A; Nolan JE; Lessard C; Anderson KA; Poutasse CM; Scott RP; Lazaro G. Determinants of Pesticide Concentrations in Silicone Wristbands Worn by Latina Adolescent Girls in a California Farmworker Community: The COSECHA Youth Participatory Action Study. Sci. Total Environ 2019, 652, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipscomb ST; McClelland MM; MacDonald M; Cardenas A; Anderson KA; Kile ML Cross-Sectional Study of Social Behaviors in Preschool Children and Exposure to Flame Retardants. Environ. Health 2017, 16, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammel SC; Hoffman K; Webster TF; Anderson KA; Stapleton HM Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol 2016, 50 (8), 4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammel SC; Phillips AL; Hoffman K; Stapleton HM Evaluating the Use of Silicone Wristbands to Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol 2018, 52 (20), 11875–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breslow NE; Day NE Statistical Methods in Cancer Research Vol. 1 The Analysis of Case-Control Studies. Distributed for IARC by WHO, Geneva, Switzerland: 1980; Vol. 1. [PubMed] [Google Scholar]
- 54.Helsel DR Nondetects and Data Analysis Statistics for Censored Environmental Data. Wiley-Interscience: 2005. [Google Scholar]
- 55.Carrico C; Gennings C; Wheeler DC; Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 2015, 20 (1), 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czarnota J; Gennings C; Wheeler DC Assessment of Weighted Quantile Sum Regression for Modeling Chemical Mixtures and Cancer Risk. Cancer Informatics 2015, 14 (Suppl 2), 159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czarnota J; Gennings C; Colt JS; De Roos AJ; Cerhan JR; Severson RK; Hartge P; Ward MH; Wheeler DC Analysis of Environmental Chemical Mixtures and Non-Hodgkin Lymphoma Risk in the NCI-SEER NHL Study. Environ. Health Perspect 2015, 123 (10), 965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helsel DR More Than Obvious: Better Methods for Interpreting Nondetect Data. In ACS Publications: 2005. [DOI] [PubMed] [Google Scholar]
- 59.La Guardia MJ; Hale RC; Harvey E. Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-, and Deca-PBDE Technical Flame-Retardant Mixtures. Environ. Sci. Technol 2006, 40 (20), 6247–6254. [DOI] [PubMed] [Google Scholar]
- 60.Council NR Toxicological Risks of Selected Flame Retardant Chemicals. National Academies Press: 2000. [PubMed] [Google Scholar]
- 61.Brozan N. Another Nightwear Chemical Causes a Safety Controversy. NY Times; 10/15/1977, 1977. [Google Scholar]
- 62.WHO Environmental Health Criteria 209, Flame Retardants: Tris (Chloropropyl) Phosphate and Tris (2‐Chloroethyl) Phosphate. In World Health Organisation Geneva: 1998. [Google Scholar]
- 63.Dodson RE; Rodgers KM; Carey G; Cedeno Laurent JG; Covaci A; Poma G; Malarvannan G; Spengler JD; Rudel RA; Allen JG Flame Retardant Chemicals in College Dormitories: Flammability Standards Influence Dust Concentrations. Environ. Sci. Technol 2017, 51 (9), 4860–4869. [DOI] [PubMed] [Google Scholar]
- 64.Stapleton HM; Sharma S; Getzinger G; Ferguson PL; Gabriel M; Webster TF; Blum A. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ. Sci. Technol 2012, 46 (24), 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGee SP; Cooper EM; Stapleton HM; Volz DC Early Zebrafish Embryogenesis Is Susceptible to Developmental TDCPP Exposure. Environ. Health Perspect 2012, 120 (11), 1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henriquez-Hernandez LA; Carreton E; Camacho M; Montoya-Alonso JA; Boada LD; Bernal Martin V; Falcon Cordon Y; Falcon Cordon S; Zumbado M; Luzardo OP Potential Role of Pet Cats as a Sentinel Species for Human Exposure to Flame Retardants. Frontiers in veterinary science 2017, 4, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walter KM; Lin YP; Kass PH; Puschner B. Association of Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) with Hyperthyroidism in Domestic Felines, Sentinels for Thyroid Hormone Disruption. BMC Vet. Res 2017, 13, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizukawa H; Nomiyama K; Nakatsu S; Iwata H; Yoo J; Kubota A; Yamamoto M; Ishizuka M; Ikenaka Y; Nakayama SM; Kunisue T; Tanabe S. Organohalogen Compounds in Pet Dog and Cat: Do Pets Biotransform Natural Brominated Products in Food to Harmful Hydroxlated Substances? Environ. Sci. Technol 2016, 50 (1), 444–52. [DOI] [PubMed] [Google Scholar]
- 69.Chow K; Hearn LK; Zuber M; Beatty JA; Mueller JF; Barrs VR Evaluation of Polybrominated Diphenyl Ethers (PBDEs) in Matched Cat Sera and House Dust Samples: Investigation of a Potential Link between PBDEs and Spontaneous Feline Hyperthyroidism. Environ. Res 2015, 136, 173–179. [DOI] [PubMed] [Google Scholar]
- 70.Norrgran J; Jones B; Lindquist NG; Bergman A. Decabromobiphenyl, Polybrominated Diphenyl Ethers, and Brominated Phenolic Compounds in Serum of Cats Diagnosed with the Endocrine Disease Feline Hyperthyroidism. Arch. Environ. Contam. Toxicol 2012, 63 (1), 161–8. [DOI] [PubMed] [Google Scholar]
- 71.Guo WH; Park JS; Wang YZ; Gardner S; Baek C; Petreas M; Hooper K. High Polybrominated Diphenyl Ether Levels in California House Cats: House Dust a Primary Source? Environ. Toxicol. Chem 2012, 31 (2), 301–306. [DOI] [PubMed] [Google Scholar]
- 72.Herbstman JB; Sjodin A; Kurzon M; Lederman SA; Jones RS; Rauh V; Needham LL; Tang D; Niedzwiecki M; Wang RY; Perera F. Prenatal Exposure to PBDEs and Neurodevelopment. Environ. Health Perspect 2010, 118 (5), 712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mooney CT; Peterson ME BSAVA Manual of Canine and Feline Endocrinology. British Small Animal Veterinary Association: 2004. [Google Scholar]
- 74.Booij K; Smedes F; Van Weerlee EM Spiking of Performance Reference Compounds in Low Density Polyethylene and Silicone Passive Water Samplers. Chemosphere 2002, 46 (8), 1157–1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.