Abstract
The formylpeptide receptor-like 1, now officially termed FPR2, in human and its mouse homolog mFPR2 mediate leukocyte migration in response to agonists associated with inflammation and immune responses. To clarify the in vivo role of the receptor, we generated mice deficient in mFPR2. mFPR2−/− mice showed markedly reduced severity in OVA/alum-induced allergic airway inflammation. This was associated with diminished recruitment of CD11c+ dendritic cells into the airway mucosa and secondary lymphoid organs, as well as reduced production of Type 2 cytokines and Igs. We also found that the bronchoalveolar lavage fluid from wild type mice with airway inflammation contained mFPR2 agonist activity. This study reveals a critical role for mFPR2 in the progression of allergic airway inflammation and immune responses.
The formylpeptide receptor (FPR) subfamily of the G protein-coupled chemoattractant receptors consists of at least three members in human and all were originally identified in phagocytic leukocytes (1, 2). The prototype FPR (FPR1) is a high-affinity receptor for the bacterial peptide formyl-methionyl-leucyl-phenylalanine (fMLF) and mediates fMLF-induced phagocyte chemotaxis and activation. In vivo, FPR is likely to play a role in antimicrobial host defense, because mice depleted of the FPR counterpart mFPR1 are more susceptible to infection by Listeria monocytogenes (3). The FPR variant formylpeptide receptor-like 1 (FPRL1 [FPR2]) and its mouse homolog mFPR2 are low affinity receptors for fMLF, but they recognize a plethora of agonist peptides associated with inflammation and immune responses (1, 2). Whereas the interaction of FPR2 (FPRL1) or mFPR2 with chemotactic peptides is believed to contribute to proinflammatory responses, a lipid mediator lipoxin A4 (LXA4) and the N-terminal peptides of annexin I have been shown to attenuate leukocyte recruitment at sites of inflammation (4, 5), and thus appear to elicit anti-inflammatory signals through FPR2 (FPRL1) or mFPR2 (6). The third human FPR subfamily member FPRL2 (FPR3) recognizes a peptide fragment derived from Heme-binding protein (7), and unlike FPR1 (mFPR1) and FPR2 (mFPR2) that are mainly expressed in neutrophils and monocytes with reduction in mature dendritic cells (DCs), functional FPR3 is more selectively expressed in human monocytes and DCs (8). The identity of mouse counterpart of FPRL2 (FPR3) is not entirely clear; however, recent studies have shown that mFPR2 is likely a mouse receptor that is a homolog of both human FPR2 and FPR3 (9, 10). Thus, mFPR2 might play an important and complex role in pathophysiologic conditions. To clarify the role of mFPR2, and possibly human FPR2 and FPR3, in disease states, we have generated mFPR2 knockout (mFPR2−/−) mice. In this study, we report a critical role for mFPR2 in the progression of allergic airway inflammation and type 2 immune responses.
Materials and Methods
Animals
Cre-loxp strategy (11) was used to deplete mouse (m) FPR2 gene (Fpr-rs2). (Supplemental Fig. 1). mFPR2−/− mice were backcrossed for five generations to wild type (WT) C57BL/6 mice before use in this study. Bone marrow transplantation was performed according to published methods (12). mFPR2 transgenic mice (mFPR2 Tg) were generated with human β-actin promoter in FVB background (G. Ying, unpublished observation).
Immunization and the measurement of Th2 cytokines and serum Ig
Immunization of mice with OVA/alum and airway challenge with OVA were performed as described (13). Control mice received OVA sensitization and airway challenge with PBS. The mice were euthanized on day 31. Cellularity in the bronchoalveolar lavage (BAL) liquid was analyzed morphologically, and cytokines were measured by ELISA (eBioscience, San Diego, CA). Mouse serum total Igs were determined by ELISA. The Igs are expressed as the mean ± SE of fold increase in serum Igs in immunized mice compared with naive mice.
Histology
Lung tissues fixed in formalin and embedded in paraffin were sectioned (5 mm). Tissue sections were stained with H&E or periodic acid-Schiff. Peribronchial cells and goblet cell hyperplasia were quantified using a five-point (scores, 0–4) grading system (13). At least three fields of coded lung sections were examined by pathologists without knowledge of sample identities. Mean scores were obtained from three to four mice after decoding the samples.
Immunohistochemistry and immunofluorescence
Eosinophils were identified by immunofluorescence using a rat anti-mouse major basic protein Ab (Dr. J. Lee, Mayo Clinic, Scottsdale, AZ), macrophages with rabbit anti-Iba1 (Wako Pure Chemical Industries, Osaka, Japan), neutrophils with Gr-1 Ab (eBioscience), and B lymphocytes with a rat anti-mouse CD45R/B220 followed by a biotinylated anti-Ig secondary Ab (BD Biosciences, San Diego, CA) and Streptavidin-HRP/DAB with hematoxylin counter staining (Surgipath, Richmond, IL). For immunofluorescence, frozen sections were stained with hamster anti-mouse CD11c and anti-mouse CD3ε Abs followed by biotinylated anti-Ig Abs (BD) with streptavidin-PE or streptavidin-FITC and DAPI (Invitrogen). Hamster IgG (eBioscience) was used as isotype control.
Chemotaxis
Chemotaxis of bone marrow cells (BMCs), neutrophils, and macrophages was measured as described (14). The results are expressed as the mean ± SE of the chemotaxis index, representing the fold increase in the number of migrated cells in response to chemoattractants over spontaneous cell migration.
RT-PCR
The expression of mFPR2 mRNA in mouse BMCs was examined by RT-PCR as described (15). β-Actin transcripts were used as a control.
Flow cytometry
The expression of BAL cell surface CD11c was analyzed by flow cytometry using hamster anti-mouse CD11c Ab and hamster IgG as the isotype control.
Statistical analysis
All experiments were performed at least three times. Representative and reproducible results are shown. The statistical significance of the difference between testing and control groups was analyzed by t test of the computer software Prism (WattMaster Controls, Parkville, MO). Values of p ≤ 0.05 was considered statistically significant.
Results and Discussion
Generation of mFPR2−/− mice
We used Cre-loxP strategy and 129/Svj embryonic stem cells to generate mFPR2−/− mice (Supplemental Fig. 1) (11). Southern blotting of tail DNA (Supplemental Fig. 1A) and RT-PCR analysis of BMC total RNA confirmed the disruption of mFPR2 gene and the absence of mFPR2 mRNA in BMCs (Supplemental Fig. 1B, 1C). An mFPR2 specific chemotactic peptide MMK-1 failed to induce migration of BMCs, neutrophils, and macrophages from mFPR2−/− mice (Fig. 1A). The expression of mFPR1 mRNA, another member of FPR family, was detected in mFPR2−/− neutrophils, and the cells migrated in response to fMLF, an agonist with relatively higher affinity for mFPR1 compared with mFPR2 (Supplemental Fig. 1C, 1D). Thus, mFPR2−/− neutrophils retained the expression of functional mFPR1. However, compared with WT neutrophils, cells from mFPR2−/− mice showed significantly reduced response to fMLF at low concentrations, which presumably interact mainly with mFPR1 (Supplemental Fig. 1D). These results suggest that the function of mFPR1 in neutrophils from mFPR2−/− mice may be affected, with mechanisms that are yet to be defined. In general, mFPR2−/− mice appeared to develop normally, and their lifespan in a pathogen-free facility was equivalent to WT (mFPR2+/+) littermates.
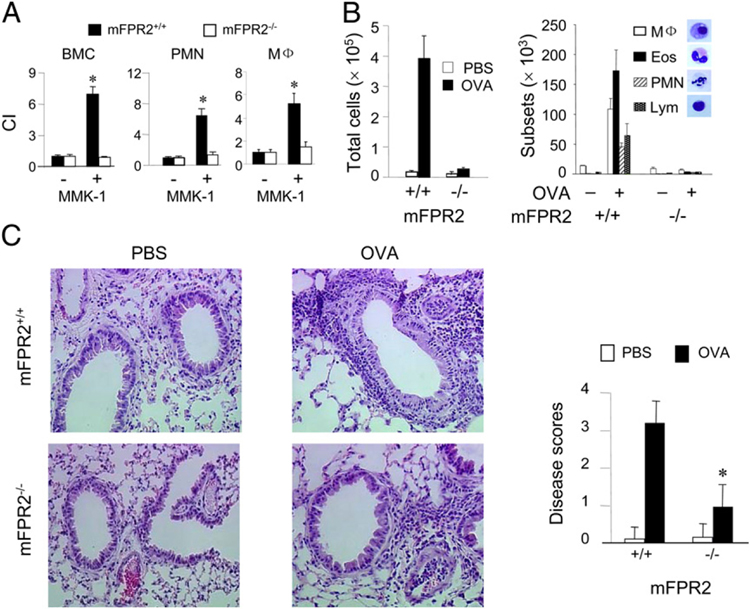
FIGURE 1.
Reduced severity of allergic airway inflammation in mFPR2−/− mice. A, Migration of BMCs, Casein-induced peritoneal neutrophils (PMNs) and thioglycolate-induced peritoneal macrophages (MF) from WT and mFPR2−/− mice in response to the mFPR2 ligand MMK-1 (1025 M). The results are expressed as chemotaxis index representing fold increase in cell response to MMK-1 versus medium control (−). Asterisk indicates significantly increased migration shown by BMCs, PMNs, or Mϕ; from WT mice (p < 0.01). B, Total number and differential counts of leukocytes contained in the BAL liquid from mice. Eos, eosinophil; Lym, lymphocyte. C, Histology showing infiltration of inflammatory cells in the perivascular and peribronchial regions of the lung tissues (H&E, original magnification ×200). The severity of lung inflammation was scored and the asterisk indicates significantly reduced severity in mFPR2−/− mice (p < 0.01). Mice used were 8-wk-old females.
Reduced severity of allergic airway inflammation in mFPR2−/− mice
Because mFPR2, similar to its human homolog FPR2, has been implicated in inflammatory and immune responses (1, 2), we examined the responses of mFPR2−/− mice in OVA/alum-induced allergic airway inflammation, which is characterized by leukocyte infiltration into the lung and type 2 immune responses. In OVA/alum-immunized and OVA aerosol-challenged mFPR2−/− mice, the severity of airway inflammation was markedly reduced, as evidenced by diminished exudation of leukocytes in the BAL liquid (Fig. 1B). There was a reduced infiltration of inflammatory cells in the lung tissue of OVA/alum-immunized and aerosol-challenged mFPR2−/− mice compared with WT littermates (Fig. 1C; Supplemental Fig. 2A). The airway epithelial layer of OVA/alum-immunized and OVA aerosol-challenged mFPR2−/− mice contained considerably fewer periodic acid-Schiff–positive goblet cells (Supplemental Fig. 2B), suggesting attenuated epithelial proliferation and secretory function.
Reduced type 2 cytokine and Ig production in mFPR2−/− mice
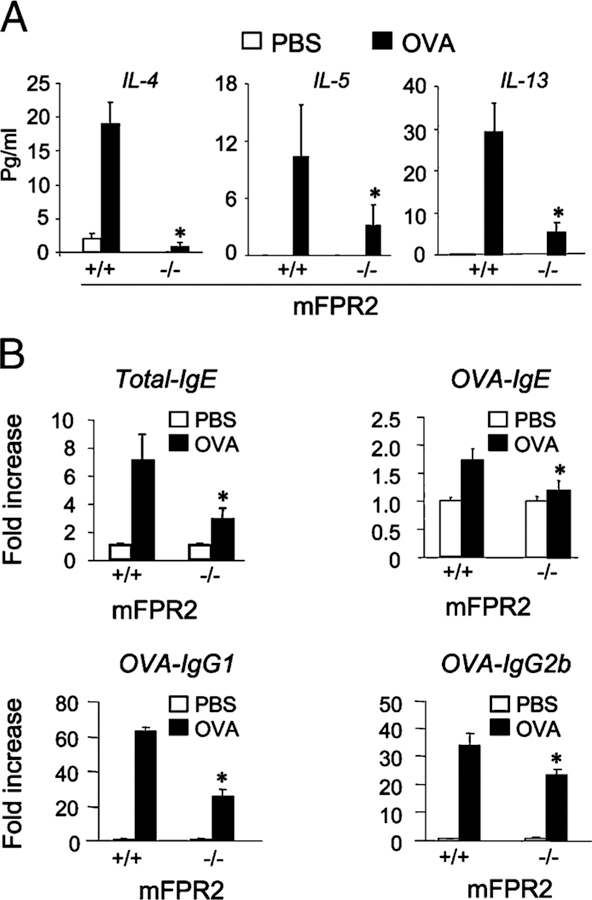
Examination of mouse immune responses revealed significantly lower levels of type 2 cytokines IL-4, IL-5, and IL-13 in the BAL liquid of OVA-immunized and aerosol-challenged mFPR2−/− mice compared with WT littermates (Fig. 2A), indicating diminished immune responses in mFPR2−/−mice. The deficiency in type 2 cytokine production in mFPR2−/− mouse airway was substantiated by reduced secretion of IL-4, IL-5, and IL-13 by splenocytes isolated from OVA/alum-immunized mFPR2−/− mice and then stimulated with OVA in vitro (Supplemental Fig. 3A). Moreover, OVA-immunized and aerosol-challenged mFPR2−/− mice produced lower levels of type 2 Igs in the sera, with reduction in total IgE as well as OVA-specific IgE, IgG1, and IgG2b (Fig. 2B). The production of secondary anti-OVA IgG1 and IgG2b in the sera by OVA-immunized mFPR2−/− mice was also reduced (Supplemental Fig. 3B). However, there were no differences in the production of type 1 cytokine interferon γ between mFPR2−/− and WT littermates after OVA treatment (Supplemental Fig. 3A).
FIGURE 2.
Reduced type 2 cytokine and Ig production in mFPR2−/− mice. A, The levels of type 2 cytokines IL-4, IL-5, and IL-13 measured in the BAL liquid of mice. Asterisk indicates significantly lower levels of cytokines in the BAL liquid of mFPR2−/− mice compared with WT littermates (p < 0.01). B, The levels of total IgE, OVA-specific IgE (OVA-IgE), IgG1 (OVA-IgG1), and IgG2b (OVA-IgG2b) detected in mouse sera. Asterisk indicates significantly reduced serum Ig levels in OVA-immunized and airway-challenged mFPR2−/− mice (p < 0.01). All mice used were 8-wk-old female littermates.
To verify the involvement of mFPR2 in airway inflammation, we transplanted bone marrow-nucleated cells from WT mice into mFPR2−/− mice. The chimeric mice showed a considerable restoration of airway inflammation and immune responses elicited by OVA/alum immunization and OVA challenge (Supplemental Fig. 4A–D), as evidenced by increased production of Th2 cytokines, exudation of eosinophils in the BAL liquid, and enhanced serum IgE. Furthermore, in FVB mice overexpressing human β-actin promoter-controlled mFPR2 transgene (G. Ying, unpublished observation), a significantly increased immature DC migration to the CCR7 ligand CCL21/SLC and a markedly increased response to OVA/alum immunization and airway challenge were observed, compared with WT FVB mice (Supplemental Fig. 5A–C). These results support the hypothesis that mFPR2 plays an important role in allergic airway inflammation and type 2 immune responses.
Reduced DC recruitment in OVA-immunized mFPR2 −/− mice
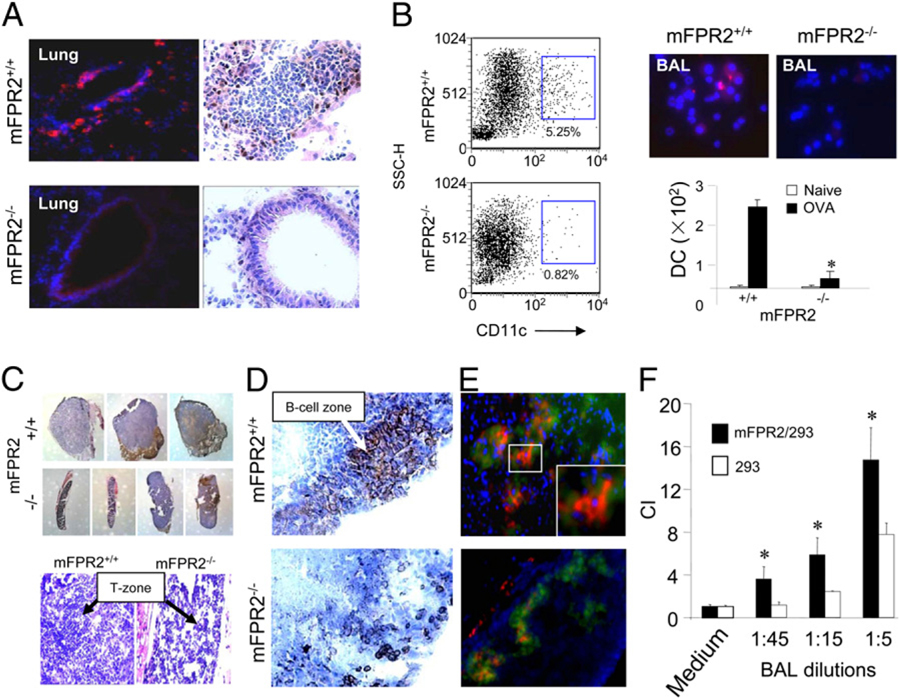
The impaired innate and type 2 immune responses in mFPR2−/− mice prompted us to investigate DC recruitment in vivo, which is crucial for proper immune responses. After OVA/alum immunization and airway challenge, the recruitment of CD11c+ DCs into the bronchial epithelial layer and DC exudation into the BAL liquid were markedly reduced in mFPR2−/− mice (Fig. 3A, 3B). The size of the mediastinal draining lymph nodes (MLNs; Fig. 3C) and their T/B lymphocyte zones (Fig. 3C, 3D) were considerably smaller in mFPR2−/− mice with reduced number of CD11c+ DCs in the T cell zone (Fig. 3E), indicating that the homing of DCs carrying Ag to the draining lymph nodes was diminished. These results demonstrate reduced trafficking of mFPR2−/− DCs into the airway and secondary lymphoid tissues in OVA-induced allergic airway inflammation.
FIGURE 3.
Reduction of DC recruitment in OVA-immunized mFPR2−/− mice. A, The recruitment of CD11c+ DCs to the airway mucosal region of OVA-sensitized and airway-challenged mice. CD11c immunofluorescence is shown in red; nuclei are shown by DAPI in blue (original magnification ×400). CD11c immunohistochemistry is shown in brown. B, Reduction of CD11c+ DCs in the BAL liquid of OVA sensitized and airway challenged mFPR2−/− mice. Left panels, FACS analysis of the percentage of CD11c+ DCs in the BAL liquid. Upper right panels, CD11c immunostaining in the BAL liquid shown in red; nuclei shown by DAPI in blue (original magnification ×400). Lower right panel, Numbers of CD11c+ DCs in the BAL liquid. Asterisk indicates significantly lower number of DCs in the BAL liquid of mFPR2−/− mice. C, The size and histology of the MLNs (original magnification ×35; H&E, original magnification ×200) from OVA-sensitized and airway-challenged mice. T zone, T cell zone. D, CD45R/B220+ B cells (brown) in MLNs (original magnification ×400). E, CD11c+ cells in MLNs (original magnification ×400) detected with red fluorescence. T cells were in green. Nuclei were stained with DAPI in blue. Insets, An amplified CD11c+ cell area among T cells in the MLN of WT mice (original magnification ×1000). F, Chemotaxis of mFPR2-transfected 293 cells and parental 293 cells in response to BAL liquid from immunized WT mice. *Significantly increased chemotaxis of mFPR2-transfected 293 cells (mFPR2/293) compared with the response of parental 293 cells (293).
To clarify the role of mFPR2 in sustaining DC function, we pulsed DCs from WT mice in vitro with OVA and transferred these DCs into mFPR2−/− mice via airway. This significantly restored the responses of mFPR2−/− mice to OVA challenge. In contrast, transfer of OVA-pulsed mFPR2−/− DCs did not increase the responses of WT mice (Supplemental Fig. 6). Thus, mFPR2 is involved in DC-mediated adaptive immune responses.
Increased mFPR2 agonist activity contained in the BAL of OVA/alum-immunized mice
A number of agonists for mFPR2 and its human counterpart FPR2 have been identified and most of these agonists are associated with inflammation and immune responses (2). We therefore asked whether mFPR2 agonist activity is produced in the airway in allergic inflammatory responses. We found that the BAL liquid from immunized WT mice contained high levels of mFPR2 agonist activity, because the BAL liquid exhibited a much more potent chemotactic activity for human embryonic epithelial cells (293) transfected to express mFPR2 (a gift of Dr. P. Murphy, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) than for the parental 293 cells (Fig. 3F). mFPR2/293 cells pretreated with a defined mFPR2 ligand MMK-1 showed reduced migration in response to the BAL liquid. In contrast, the migration of parental 293 cells was not attenuated by MMK-1 (Supplemental Fig. 7), indicating that the BAL liquid from immunized mice indeed contains mFPR2 agonists. Preliminary characterization of the nature of mFPR2 agonists in the BAL liquid suggests that the agonists are of polypeptide in nature of <3000 Da. Additional experiments are required to clarify the identity of the agonists.
Asthma is a type 2 inflammatory airway disease characterized by airway eosinophilia, increased mucus production by goblet cells, and structural remodeling of the airway wall. DCs are crucial for the initiation and progression of allergic airway inflammation (16). In such mouse asthmatic models, DCs take up Ag in the bronchial alveolar tissue (17) and enter the T cell zone of MLNs, where they become functionally mature to induce proliferation of naive T cells (18). In our study, mFPR2−/− mice exhibited markedly reduced severity of OVA-induced allergic airway inflammation accompanied by reduction in type 2 cytokine and Ab responses. These defects were associated with diminished recruitment of mDCs in the airway draining lymph nodes. Thus, reduction in DC homing might be responsible for attenuated airway inflammation seen in mFPR2−/− mice. In addition to reduced allergic airway inflammation, mFPR2−/− mice also exhibited diminished myeloid cell exudation into peritoneal cavity in response to thioglycollate and casein (data not shown), suggesting decreased inflammatory responses in another location than the airway in mFPR2−/− mice. Moreover, in an LPS/OVA-induced Th1 airway inflammation model (19), mFPR2−/− mice also showed diminished neutrophil exudation in the BAL liquid and infiltration in the lung (Supplemental Fig. 8A–C). Furthermore, in a model of LPS/OVA-induction of Th1 cytokines (20), mFPR2−/− mice showed reduced production of IFN-γ and IL-2 (Supplemental Fig. 8D). Thus, mFPR2 might play a role in both Th1 and Th2 responses.
FPR family members are differentially expressed in human and mouse myeloid cells. The human mFPR2 counterpart FPR2 is downregulated during the process of myeloid DC maturation (8, 21), whereas another FPR variant, FPR3, was persistently expressed (8). In mouse, mFPR2 has been shown to function as both FPR2 and FPR3 (9, 10) and is expressed in mouse myeloid DCs (K. Chen, unpublished observation). In fact, mFPR2 in mouse DCs was essential in mediating the signaling pathways elicited by a lipid agonist LXA4 that attenuates the function of the chemokine receptor CCR5, resulting in reduced lethal production of IL-12 in Toxoplasma gondii infection (15). In addition, injection of stable LXA4 analogs in T. gondii-infected mice reduced DC accumulation in the spleen (15). These observations suggest that manipulating mFPR2 function alters DC trafficking and subsequent host immune responses.
It is intriguing that activation of mFPR2 or human FPR2 has been shown to elicit both proinflammatory and anti-inflammatory signals depending on the nature of the ligands (2). Whereas our present study clearly demonstrated mFPR2 to be a proinflammatory immune response mediator, results obtained using anti-inflammatory mFPR2 ligands have shown protection of the host in some disease models. For example, LXA4 and annexin I peptides significantly reduced leukocyte infiltration of airway tissues and type 2 responses in the airway inflammation model (5). Clinically, the development of exercise-induced bronchoconstriction in asthmatic children was linked to reduced biosynthesis of endogenous LXA4 (22). However, it remains unclear how a single receptor such as mFPR2 is capable of mediating opposing signaling events elicited by different ligands. One possibility as suggested is that anti-inflammatory ligands such as LXA4 may bind to unique epitopes in mFPR2 (4). Alternatively, these ligands may exhibit the property of partial agonists that desensitize, and thus dampen, the proinflammatory function of mFPR2 or human FPR2 (1). In this context, although the chemical nature of the mFPR2 agonist activity contained the inflammatory BAL liquid remains to be elucidated, our present study has revealed a nonredundant role for mFPR2 in the development of innate and adaptive immune responses represented by allergic airway inflammation.
Supplementary Material
Acknowledgments
We thank Dr. J.J. Oppenheim, Dr. O.M.Z. Howard, and Dr. G. Trinchieri for critically reviewing the manuscript; C. Lamb for secretarial assistance; R. Matthai and K. Noer of Center for Cancer Research (National Cancer Institute at Frederick) for flow cytometric analysis; and Steve Stull for bone marrow transplantation.
This work was supported in part by federal funds from the National Cancer Institute and National Institutes of Health under Contract No. HHSN261200800001E and by the Intramural Research Program of the National Cancer Institute and National Institutes of Health. Y.L. was supported by Grant 2010CB529701 from the National Basic Research Program of China.
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BMC
bone marrow cell
- DC
dendritic cell
- Eos
eosinophil
- fMLF
formyl-methionyl-leucyl-phenylalanine
- FPR
formylpeptide receptor
- FPRL
formylpeptide receptor-like
- m
mouse
- Lym
lymphocyte
- MΦ
macrophages
- MLN
mediastinal draining lymph node
- PMN
peritoneal neutrophil
- WT
wild type
Footnotes
The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Animal studies were approved by the National Cancer Institute Frederick Animal Care and Use Committee. Animal care was provided in accordance with the procedures outlined in the Guide for Care and Use of Laboratory Animals (National Research Council, 1996, National Academy Press, Washington, DC).
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, and Murphy PM 2009. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev 61: 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2., Le Y, Murphy PM, and Wang JM 2002. Formyl-peptide receptors revisited. Trends Immunol 23: 541–548. [DOI] [PubMed] [Google Scholar]
- 3.Gao JL, Lee EJ, and Murphy PM 1999. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med 189: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang N, Fierro IM, Gronert K, and Serhan CN 2000. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med 191: 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, and Serhan CN 2002. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat. Med 8: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 6.Lim LH, and Pervaiz S 2007. Annexin 1: the new face of an old molecule. FASEB J 21: 968–975. [DOI] [PubMed] [Google Scholar]
- 7.Migeotte I, Riboldi E, Franssen JD, Grégoire F, Loison C, Wittamer V, Detheux M, Robberecht P, Costagliola S, Vassart G, et al. 2005. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J. Exp. Med 201: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang D, Chen Q, Gertz B, He R, Phulsuksombati M, Ye RD, and Oppenheim JJ 2002. Human dendritic cells express functional formyl peptide receptor-like-2 (FPRL2) throughout maturation. J. Leukoc. Biol 72: 598–607. [PubMed] [Google Scholar]
- 9.Devosse T, Guillabert A, D’Haene N, Berton A, De Nadai P, Noel S, Brait M, Franssen JD, Sozzani S, Salmon I, and Parmentier M 2009. Formyl peptide receptor-like 2 is expressed and functional in plasmacytoid dendritic cells, tissue-specific macrophage subpopulations, and eosinophils. J. Immunol 182: 4974–4984. [DOI] [PubMed] [Google Scholar]
- 10.Gao JL, Guillabert A, Hu J, Le Y, Urizar E, Seligman E, Fang KJ, Yuan X, Imbault V, Communi D, et al. 2007. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J. Immunol 178: 1450–1456. [DOI] [PubMed] [Google Scholar]
- 11.Holzenberger M, Lenzner C, Leneuve P, Zaoui R, Hamard G, Vaulont S, and Bouc YL 2000. Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucleic Acids Res 28: E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spangrude GJ 2001. Assessment of lymphocyte development in radiation bone marrow chimeras. Curr. Protoc. Immunol Chapter 4: Unit 4.6. [DOI] [PubMed]
- 13.Duan W, Chan JH, Wong CH, Leung BP, and Wong WS 2004. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J. Immunol 172: 7053–7059. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM, and Wang JM 2006. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J. Biol. Chem 281: 3651–3659. [DOI] [PubMed] [Google Scholar]
- 15.Aliberti J, and Sher A 2002. Role of G-protein-coupled signaling in the induction and regulation of dendritic cell function by Toxoplasma gondii. Microbes Infect 4: 991–997. [DOI] [PubMed] [Google Scholar]
- 16.Lambrecht BN 2005. Dendritic cells and the regulation of the allergic immune response. Allergy 60: 271–282. [DOI] [PubMed] [Google Scholar]
- 17.Stumbles PA, Thomas JA, Pimm CL, Lee PT, Venaille TJ, Proksch S, and Holt PG 1998. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med 188: 2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermaelen KY, Carro-Muino I, Lambrecht BN, and Pauwels RA 2001. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med 193: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, and Bottomly K 2002. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med 196: 1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, and Banchereau J 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol 167: 5067–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D, Chen Q, Le Y, Wang JM, and Oppenheim JJ 2001. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J. Immunol 166: 4092–4098. [DOI] [PubMed] [Google Scholar]
- 22.Tahan F, Saraymen R, and Gumus H 2008. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. J. Asthma 45: 161–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.