Abstract
Many insect families have evolved ears that are adapted to detect ultrasonic calls of bats. The acoustic sensory cues indicating the presence of a bat are then used to initiate bat avoidance behaviours. Background noise, in particular at ultrasonic frequencies, complicates these decisions, since a response to the background may result in costly false alarms. Here, we quantify bat avoidance responses of small rainforest crickets (Gryllidae, Trigoniinae), which live under conditions of high levels of ultrasonic background noise. Their bat avoidance behaviour exhibits markedly higher thresholds than most other studied eared insects. Their responses do not qualitatively differ at suprathreshold amplitudes up to sound pressure levels of 105 dB. Moreover, they also exhibit evasive responses to single, high-frequency events and do not require the repetitive sequence of ultrasonic calls typical for the search phase of bat echolocation calls. Analysis of bat and katydid sound amplitudes and peak frequencies in the crickets' rainforest habitat revealed that the cricket's behavioural threshold would successfully reject the katydid background noise. Using measurements of the crickets' echo target strength for bat predators, we calculated the detection distances for both predators and prey. Despite their high behavioural threshold, the cricket prey still has a significant detection advantage at frequencies between 20 and 40 kHz. The low-amplitude bat calls they ignore are no predation threat because even much louder calls would be detected before the bat would hear the cricket echo. This leaves ample time for evasive actions. Thus, a simple decision criterion based on a high-amplitude behavioural threshold can be adaptive under the high background noise levels in nocturnal rainforests, in avoiding false alarms and only missing detection for bat calls too far away to pose a risk.
This article is part of the theme issue ‘Signal detection theory in recognition systems: from evolving models to experimental tests’.
Keywords: bat avoidance, decision making, target strength, detection distance, ultrasound, receiver operating characteristic
1. Introduction
The interaction between aerial hawking bats and their insect prey is one of the best-studied predator–prey relationships. The pioneering studies of Roeder in the early sixties of the last century were the starting point [1,2], with a description of evasive actions of some moths in response to echolocation calls of bats, and the underlying sensory basis in the prey. Numerous following reports documented ultrasonic hearing and bat avoidance behaviours during flight in different taxonomic groups of insects [3–13]. In katydids and crickets, fossil evidence suggests that they could already produce sounds, and may have communicated acoustically about 165 Ma, long before the appearance of bats in the Miocene (65 Ma). The pattern that many katydids and crickets adopted a nocturnal lifestyle made them potential prey for insectivorous bats, when on the wing, which then may have driven the evolution of ultrasonic hearing and predator avoidance behaviour [14–16].
The few reports on katydids and crickets describe bat-evasive responses like those of other nocturnally flying insects [3,4,6,11,17]. Usually, the responses are graded with sound amplitude, with steering away (negative phonotaxis) from ultrasonic sound sources at relatively low amplitudes typical for distant bats, and erratic, unpredictable flight or cessation of flight at higher amplitudes (review in [13]; for two exceptions see [6,18,19]).
For the species-rich group of crickets (Gryllidae), extensive information is available for acoustic behaviour of different species in the context of intraspecific communication [20–22]. However, although predator avoidance behaviour of flying crickets and the underlying neurobiology are so well described, three reasons let us investigate the same system in a tropical cricket. First, most of our knowledge is based on only three species of field crickets, Gryllus bimaculatus, Teleogryllus oceanicus and Teleogryllus commodus ([3–5]; but see [18,19]), largely bypassing the tremendous global diversity of cricket taxa [23–26]. For example, within Mesoamerican habitats all neotropical cricket subfamilies are represented [23]. Second, species in these different subfamilies vary in several traits that are important for bat avoidance, such as their diel activity patterns, flight propensity and size [23,24,26]. Indeed, in a study of a small cricket species of the subfamily Nemobiinae, the threshold for eliciting an acoustic startle response (ASR) in flight was about 20 dB higher when compared with the field cricket species G. bimaculatus, T. oceanicus and T. commodus [18]. As a consequence of their small size, the amplitude of the reflected echo (the target strength) as an important cue for the hunting bat will be much lower compared with the larger field crickets, so that a negative relationship between size and ASR threshold was proposed [27]. In the current paper we investigate members of the Trigonidiinae, which are also extremely small species compared with field crickets. Finally, and most importantly, virtually all previous studies on predator avoidance have been performed under laboratory conditions with optimized acoustic signal-to-noise ratios. The acoustic predator avoidance behaviours have however evolved in natural habitats, such as tropical rainforests, where the density and diversity of bat species can be extremely high [28,29], and bat avoidance is complicated by the presence of high levels of background noise [30–32]. Calls of katydids, which mostly include the high sonic to ultrasonic frequencies also used by echolocating bats, are a particularly relevant source of background noise [33,34]. Responding to these calls with bat avoidance behaviour would represent a false alarm in terms of signal detection theory [35] and should be avoided.
We explored and test three possible hypotheses: (1) spectral separation of acoustic events in the background and bat calls; (2) temporal pattern recognition of the repetitive bat echolocation search phase calls; and (3) evolving an ASR with a high threshold to avoid responding to both distant predators and nearby ultrasound, excluding bat calls. We test these alternatives, which are not mutually exclusive, through a combination of behavioural tests and playback experiments, quantitative habitat sound analysis, cricket target strength measurements and acoustic detection range modelling. Based on the data on habitat acoustics and the threshold of the ASR we also perform a signal detection analysis by calculating a receiver operating characteristic curve (ROC) as a function of ASR threshold, to determine whether the insects maximize the ratio of hits to false alarms.
2. Material and Methods
(a). Study area and animals
The present study was conducted on Barro Colorado Island (BCI; 9°10′ N, 79°51′ W, Panama) during the dry season from January to April 2012 to 2014. Adult crickets of either sex were exclusively collected on the island. The Neotropical Panamanian cricket fauna is surprisingly rich and insufficiently known, with high regional but limited local diversity. The delimitation of genera and higher taxonomy are heavily disputed and in flux. This is especially true for members of the subfamily of Trigonidiinae (common name: sword-tailed crickets), for which more than 33 genera with 490 species have been described so far [36]. Characteristic for these crickets is their small size of 4–7 mm. Therefore, we have deposited voucher specimens at the Zoological Research Museum Alexander Koenig (Bonn, Germany) for subsequent taxonomic determination or reexamination and subsequent genetic barcoding analysis (sensu [37]). Individuals were usually collected at night at the lights of buildings on the island, kept in small plastic boxes and used within a maximum of 3 days for behavioural experiments. Fish food, apple slices and water were provided ad libitum.
(b). Behavioural experiments
Experiments were performed in the laboratory at a temperature of 25°C, typical for the nocturnal rainforest on BCI. Insects were tethered at the pronotum with a thin, 15 mm long copper wire during the day, and used for behavioural experiments starting with the following night. Insects were placed in a Faraday cage (1 × 0.7 × 0.7 m) lined with anechoic foam. The background noise level in the cage was below 28 dB sound pressure level (SPL) in a frequency range from 5 to 75 kHz.
Sound playback was done with Cool Edit Pro (v2.0 Syntrillium Software, Scottsdale AZ), which drove an Edirol A/D audio interface (Type Edirol Firewire Audio Capture FA-101; ROLAND Corporation, Hamamatsu, Japan) operated at a sampling rate of 192 kHz, attenuated (PA-5, Tucker Davis, Alachua, FL) and broadcast via an ultrasonic speaker (ScanSpeak with amplifier #70101; Avisoft Bioacoustics, Glienike, Germany). The resulting sound field reaching the tethered insect in the Faraday cage was calibrated by playing back each frequency continuously at maximum amplitude and recording it with a ¼ inch microphone (type 2540, Larson Davis, Depew, NY) at the position of the ears and a sound level meter (CEL 414, Casella, Bedford, UK) to determine the respective SPLs.
Insects were tethered dorsal side up 30 cm in front of a fan producing a wind speed of approximately 1.0–1.5 m s−1. After removing tarsal contact with a small paper ball, the animal immediately assumed a flight posture similar to that described by ter Hofstede & Ratcliffe [13] (see also electronic supplementary material, video). Playback experiments started only after the insect was in stable flight for at least 15 s. Individuals that did not fly steadily on a tether were not used but kept for further experimentation the following day. Flight activity was monitored through a nightshot camera (Sony Megapixel night shot handycam DCR-PC100E) placed above and behind the insect and a digital video recorder (Sony gv-1000e). A photodiode visible in the camera's field of view emitted a dim light at the onset of each acoustic stimulus allowing offline analysis of behavioural avoidance responses. Switching the LED light on and off never evoked a startle response. In three control experiments, the threshold of the ASR was determined twice, once with the LED and again without, and we found no difference.
Playback usually started with a presentation of 10 echolocation calls of the bat Saccopteryx bilineata repeated at 10 Hz, resembling an echolocation search phase sequence. We also tested the behavioural responses to a sequence of only five echolocation calls, or a single call. The original recording of the call had been digitized at a sampling rate of 192 kHz (courtesy of A. Surlykke 2013, University of Southern Denmark, Odense, Denmark). The same single echolocation call was repeated to create the five and 10 call sequence of Saccopteryx stimuli. When the insect showed an avoidance response, its tuning was tested with a series of 10 pure tone pulses (carrier frequencies from 15 to 75 kHz at increments of 5 kHz; pulse duration 5 ms) equally repeated at 10 Hz. The suprathreshold behavioural response of swordtail crickets was always a cessation of flight, and therefore this all-or-nothing response could be used directly to determine the behavioural threshold. Threshold was determined as the minimum SPL that just elicited a startle response. Experiments started at 90 dB SPL for each frequency, and when there was an ASR, the SPL was reduced by increments of 5 dB until no ASR was elicited. Then the SPL was increased by increments of 2 dB up to a suprathreshold response. The final threshold was then confirmed when a suprathreshold response occurred after two repetitions of the same SPL, and in the absence of a response 2 dB below. Because all 11 crickets tested ceased flying after several minutes of testing, thresholds at all 13 frequencies could not be determined within one single session. Thus, the experiments were continued and completed the following day with the same individuals.
In another series of experiments, calling songs of the following Panamanian rainforest katydids (recordings kindly provided by Hannah ter Hofstede, Dartmouth College, Hanover, Germany) were played back: Anapolisia colossea, Subria sylvestris and Copiphora brevirostris. These songs usually consist of short sound pulses with carrier frequencies in the high audio to ultrasonic range, and therefore overlap in frequency with echolocation calls of bats (figure 3a). Katydid songs represent the main source of background noise at frequencies higher than 10 kHz [31–33], and if rainforest crickets could not distinguish between these neutral events from echolocation calls the katydid background noise would elicit an ASR. A total of 25 cricket individuals were used to test the startle response to the three Saccopteryx calls and the katydid stimuli. Since not all individuals could be tested for all stimuli, and the response probability to the katydid calls was lower, each Saccopteryx stimulus was presented once to 10 individuals, and each katydid stimulus only to 20 individuals. All stimuli were broadcast at 95 dB SPL at the position of the tethered insect.
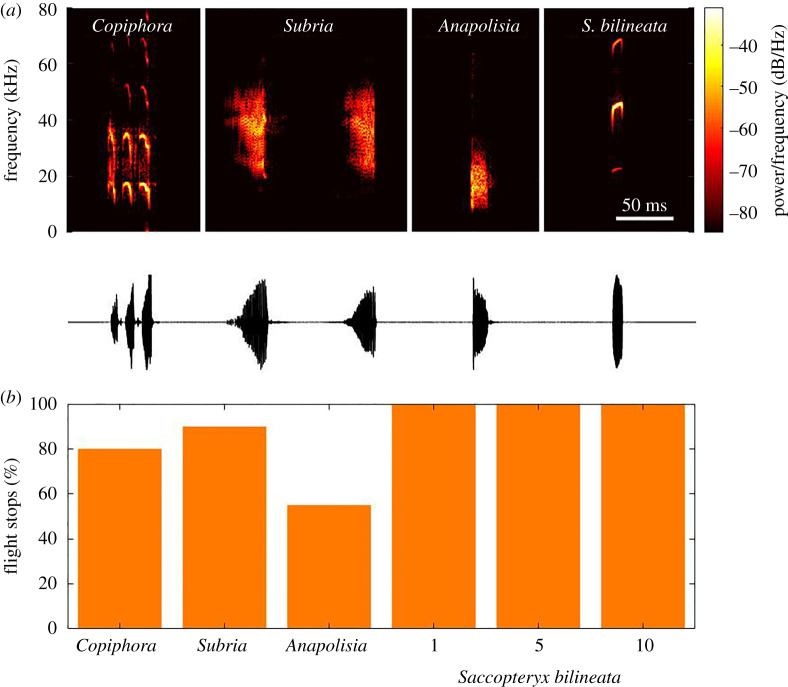
Figure 3.
(a) Example sonograms (window size 512, overlap 500, FFT size 1024, sampling frequency 192 kHz) and waveform of the calls of the three katydid species Anapolisia colossea, Subria sylvestris and Copiphora brevirostris and one single echolocation call of Saccopteryx bilineata used for playbacks. (b) Probability of flight stops of swordtail crickets in response to calls of the three katydid species and to single echolocation calls (1) or groups of five or 10 calls of Saccopteryx bilineata. All stimuli were broadcast at 95 dB SPL. N = 20 for the katydids and N = 11 for the bat. (Online version in colour.)
(c). Recordings of bat echolocation and katydid calls in the nocturnal rainforest
To determine the amplitudes of high-frequency and ultrasonic events resulting from katydid and bat echolocation calls, which potentially may elicit avoidance responses in flight, sound recordings were made at night, from 30 min after sunset to about midnight. Two different locations in the rainforest understorey were selected, where calling songs of crickets had been recorded in a previous study [38]. We used a condenser ultrasound microphone (CM16/CMPA, Avisoft Bioacoustics, Glienicke, Germany) with a moderately flat frequency response from 10 to 140 kHz, and UltraSoundGate 116H (Avisoft Bioacoustics) for sound recordings. This microphone was deployed at 2 m above the ground. Recordings were amplitude-calibrated by recording a series of pure tone 20 kHz sound pulses from 50 dB to 80 dB SPL at intervals of 5 dB (for calibration see above). Katydid calls and bat echolocation events were easily distinguishable in sonograms using Avisoft sound analysis software (SASLab Pro, Avisoft Bioacoustics). Peak amplitudes and peak frequencies of each recorded acoustic event were then measured manually and converted to peak-equivalent SPL (dB peSPL) by comparison with the peak amplitudes of the calibration recordings at 20 kHz. Example sonograms in figure 3 were created using the spectrogram function in MATLAB (R2018a, MathWorks, Natick, MA).
(d). Receiver operating characteristic analysis
As the ASR is an all-or-nothing response, we can create a simple bat versus katydid call classifier based on the recorded call amplitudes relative to the ASR threshold. The ROC of the proposed classifier was calculated using the amplitudes and peak frequencies of bat and katydid calls recorded in the habitat, and then comparing each call's respective amplitude with the behavioural ASR threshold measured for its peak frequency. A recorded call amplitude above the ASR threshold would count as a positive and below the threshold as a negative. Positives for bat calls are true positives, while positives for katydids are false positives. True and false positive rates were calculated as the percentage of acoustic events classified as positive, e.g. the false positive rate is the number of false positives divided by the total number of katydid calls. To calculate the ROC curve, we then systematically changed the ASR threshold curve by adding offsets from –50 dB to 10 dB in 5 dB steps and calculating the resulting true and false positive rates.
(e). Echo target strength of crickets
Target strength is a relative measure of how much lower the echo amplitude of an object is at a reference distance (10 cm) compared with the sound amplitude hitting the object. It is expressed in (typically negative) dB values, where 0 dB would indicate no difference between incident sound and echo amplitude. Individual crickets (hindwing length 6.8–7.3 mm) stored in 70% ethanol were completely dried before taking their echo measurements with their body in a natural resting position. Specimens were too brittle to extend the wings, so target strength reflects the body plus the folded wings. For most directions of sound incidence, a flying insect's body echo dominates its overall target strength [39], yet an extended wing ensonified perpendicularly would arguably have a higher peak target strength (amplitude glint), so the target strength measurements here might be considered partly conservative as they exclude glints.
Each specimen in turn was positioned on a 23 × 2 × 4 cm tower of sound absorber foam (Basotect W, BASF, Ludwigshafen, Germany) placed on a turntable (LT360, LinearX Systems, Battle Ground, WA). Echo measurements were taken from 32 cm distance with a sensor head comprising a ¼ inch ultrasound microphone with protective grid removed (type 26AB, GRAS Sound & Vibration, Holte, Denmark), pre-amplifier (type 2669 L), power supply (type 5935-L, both Brüel & Kjær, Nærum, Denmark) and a custom-made ring-shaped ferro electret foil loudspeaker (Emfit, Vaajakoski, Finland) driven by a PZD350 M/S high-voltage amplifier (TREK, Lockport, NY). The microphone was positioned in the central circular opening of the ring speaker (outer radius 10 mm, hole radius 4 mm) with speaker and microphone membrane in the same plane and both pointing at the centre of the specimen from an elevation angle of 10° above the horizontal. Three hundred and sixty echo measurements were taken, fully rotating the specimen in horizontal steps of 1° using the turntable. Specimens were ensonified with linear frequency modulated sweeps from 250 to 15 kHz of 10 ms duration, covering the range of frequencies used by bats. Echoes were sampled at 500 kHz with 16-bit resolution. Microphone, loudspeaker and turntable were connected to an NI-DAQ BNC-2110 card operated through LabVIEW v.16.0 (both National Instruments, Austin, TX) with custom-written scripts. Acoustic measurements were taken in a 2.9 × 2.7 × 2.3 m semi-anechoic audiometric room (IAC Acoustics, North Aurora, IL).
Complex spectral division (fast Fourier transforms) of cricket echoes with a calibration echo recorded perpendicularly from a 50 × 70 cm metal plate, and conversion to the conventional 10 cm reference distance by correcting for spherical spreading losses, were used to calculate spectral target strengths (custom-written scripts, MATLAB R2018a).
(f). Calculating predator–prey detection ranges of bats and crickets
Following methods in [40], we calculated frequency-dependent absorption (for average minimum night-time temperature 24°C and relative humidity 90%; e.g. 1.16 dB m−1 at 43 kHz, 0.16 dB m−1 at 15 kHz and 3.56 dB m−1 at 75 kHz) and spherical spreading losses for the range of ultrasonic frequencies tested here (15–75 kHz). Bat call source levels were set at 130 dB SPL at 10 cm [41]. Response range of crickets for these echolocating bats was then calculated (using frequency-dependent absorption and spreading losses) based on the crickets' flight cessation thresholds measured in dB SPL (see below). Equivalently, distances over which bats can detect crickets by echolocation were calculated using the same source level, absorption and spreading losses (factoring in absorption and spreading losses for both call emission and echo return), and using measured spectral target strengths to calculate at what distance the echo would have reached the bat's hearing threshold, set at 10 dB SPL at each respective frequency.
3. Results
(a). Ultrasound avoidance behaviour in tethered flight
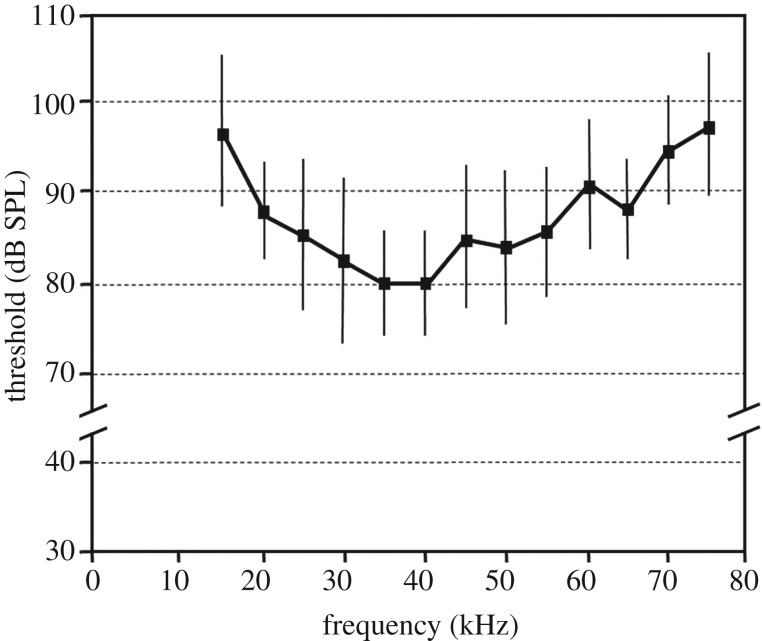
We tested 36 individuals in behavioural experiments (22 females and 14 males). Prior to acoustic stimulation, swordtail crickets readily assumed a flight posture as described for the field cricket T. oceanicus [4], with the forewings spread to either side, the hindwings flapping, and the abdomen and hindlegs extended. In response to all tested suprathreshold sound stimuli all individuals always fully ceased flight activity for a certain duration (electronic supplementary material, video). The behavioural tuning of flight cessation shows high thresholds (figure 1). At 35 and 40 kHz, thresholds were at 80 dB SPL, but values increased towards lower and higher frequencies up to 97 dB SPL.
Figure 1.
Audiogram of flight cessation of swordtail crickets in a flight paradigm in response to a series of 10 sound pulses (duration 5 ms, 100 ms interval) of different carrier frequencies (N = 11). Error bars show standard deviation.
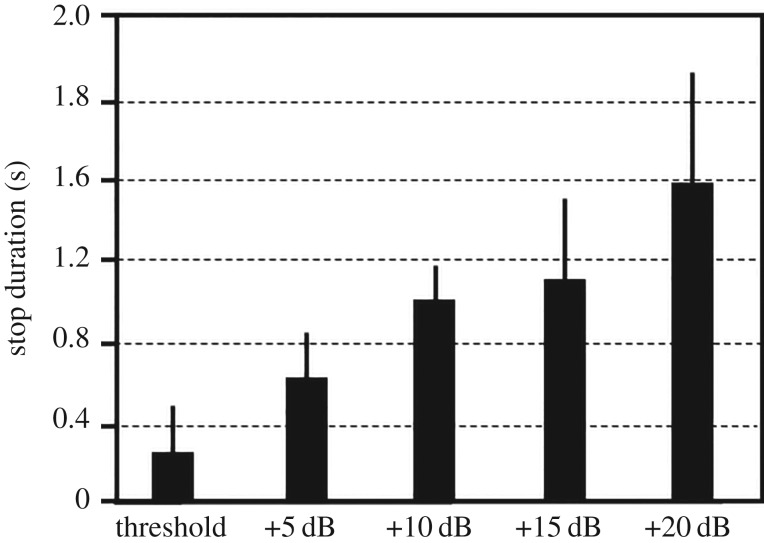
Notwithstanding that crickets showed consistent all-or-nothing flight cessation in response to ultrasound, rather than the repertoire of amplitude-graded avoidance behaviours of other insects, the duration of the crickets' suprathreshold flight cessation depended on sound amplitude: At the threshold amplitude, flight cessation was brief, lasting about 300 ms, and it increased almost linearly, reaching about 1.6 s at 20 dB above threshold (figure 2). Three of 11 tested individuals did not resume flight activity after responding to the highest playback amplitudes. The low frame rate of 25 s−1 of our videos prevents adequate ASR latency measurements. However, at a SPL of 20 dB above threshold the onset of the ASR often occurred in the second frame after the onset of the stimulus, thus with a latency below 100 ms.
Figure 2.
Duration of flight stops of crickets in response to a model of the echolocation call of Saccopteryx bilineata at different SPLs above threshold. N = 10 for threshold and +10 dB, and N = 8 for the +5 dB and +15 dB stimuli. Of 10 individuals tested with +20 dB stimuli, three stopped flying completely after stimulation, and are therefore not included in the flight stop calculation.
(b). Responses to katydid sounds
To test our first hypothesis, we quantified the response behaviour of these crickets to katydid calls, which represent the major source of background noise above 10 kHz in the nocturnal rainforest. Sonograms of the calls of three katydid species selected for the tests are shown in figure 3a. All three calls consist of brief sound calls with energy in a wide high sonic and ultrasonic frequency range, which overlaps with the ultrasonic frequency range of echolocating bats. When these katydid calls were presented to the crickets at 95 dB SPL during tethered flight, they frequently but not consistently ceased flight activity (80, 90, 55% with respectively, p ≪ 0.0001 each; figure 3b). These flight cessations could not be distinguished from those in response to bat calls (see electronic supplementary material, video).
To test our second hypothesis, that bat call sequences might be identified by their typical call repetition rates during the search phase, we compared responses to single echolocation calls with those to five or 10 Saccopteryx echolocation calls repeated at 10 Hz. At 95 dB SPL playback (approx. 10 dB above the hearing threshold at the call's peak frequency of approx. 45 kHz) all 11 individuals always responded with flight cessation, irrespective of the number of echolocation calls (figure 3b). These findings suggest that these rainforest crickets do not rely on spectral or temporal features to discern a bat predator from other ultrasonic events.
(c). Sound pressure levels of habitat sounds and response thresholds
Exploring our third hypothesis of discerning bats from katydids by an amplitude code, where any relevant ultrasonic frequency above its respective threshold would elicit a full response, requires respective analysis of the acoustic rainforest environment these crickets share with bats and other orthopterans. Whether or not ultrasonic events in the background noise of the nocturnal rainforest can potentially elicit bat avoidance behaviour was analysed using calibrated sound recordings performed in the rainforest understorey. The total duration of sound recordings was 8.5 h. Based on their typical temporal and frequency structure in the sonogram more than 98% of the recorded acoustic events could be clearly assigned to either katydid calls or echolocation calls of bats. We measured recorded SPLs of 828 katydid calls and 279 bat echolocation calls.
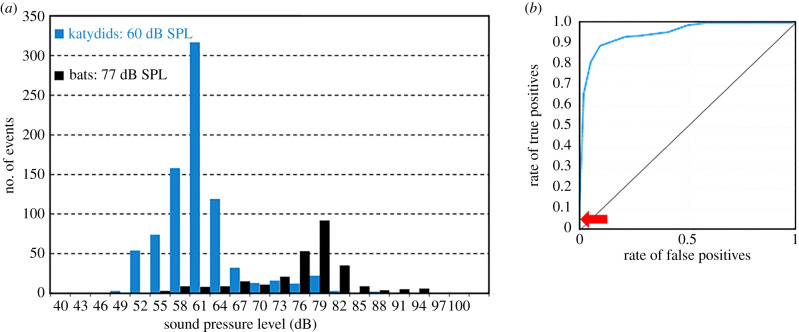
Recorded katydid calls had significantly lower peak frequencies than bat calls (katydids 19.9 ± 7.1 kHz, bats 47.7 ± 2.2 kHz, t1081 = −102, p ≪ 0.0001, two-sample heteroscedastic t-test), and recorded amplitudes of bat calls were significantly higher than those of katydid calls (figure 4; t396 = −30.8, p ≪ 0.0001, two-sample heteroscedastic t-test), with median values of 77 dB peSPL (73–79 dB peSPL interquartile range) and 60 dB peSPL (54–62 dB peSPL), respectively. However, the two distributions overlap widely, so crickets cannot use a single behavioural threshold criterion to separate the two.
Figure 4.
(a) Peak-equivalent sound pressure levels of katydid calls (blue, N = 828) and bat echolocation calls (black, N = 279) recorded at two locations in the rainforest. (b) ROC of a classifier of bat (true positive) versus katydid (false positive) calls recorded in the habitat. Classification is based on recorded sound pressure levels and peak frequencies compared with the cricket audiogram (figure 1) shifted by –50 to +10 dB in 5 dB steps. The actual audiogram has a true positive rate of 0.058 (arrow). (Online version in colour.)
(d). ROC performance for acoustic events recorded in the habitat
ROC curves plot the true positive rate (here percentage of bat calls triggering ASR) over the false positive rate (percentage of katydid calls triggering ASR) as a function of a threshold shift, and their shapes indicate classifier performance. The measured ROC curve rises steeply (figure 4b; electronic supplementary material) showing the classifier is effective. For example, at one certain threshold 84% of all bat calls would trigger an ASR compared with only 4.9% of katydid calls. This would, however, be the case had crickets been 15 dB more sensitive than observed. Remarkably, the crickets' actual threshold is at the exact value at and above which none of the recorded katydid calls would trigger an ASR. This comprehensive rejection of false positives comes at a cost though: only the loudest 5.8% of recorded bat calls trigger an ASR (94th percentile false negatives).
(e). Target strength and detection distances
We measured 360 spectral target strengths for each of five individuals of the unidentified swordtail cricket species, which had been used in behavioural experiments. We then calculated the average of the 360 spectral target strength measurements and the maximum at each frequency across the 360 spectral target strengths for each individual (figure 5a). The five individuals were very similar in their spectral target strength, with the maximum values at −30 dB for frequencies above 45 kHz and a gradual drop with frequency to −42 dB at 15 kHz. The mean spectral target strength was 6–14 dB lower than the maximum spectral target strength, ranging between −43 and −49 dB (figure 5a). Variation between individuals was higher in the mean than in the maximum spectral target strength (t56 = −5.8, p ≪ 0.0001).
Figure 5.
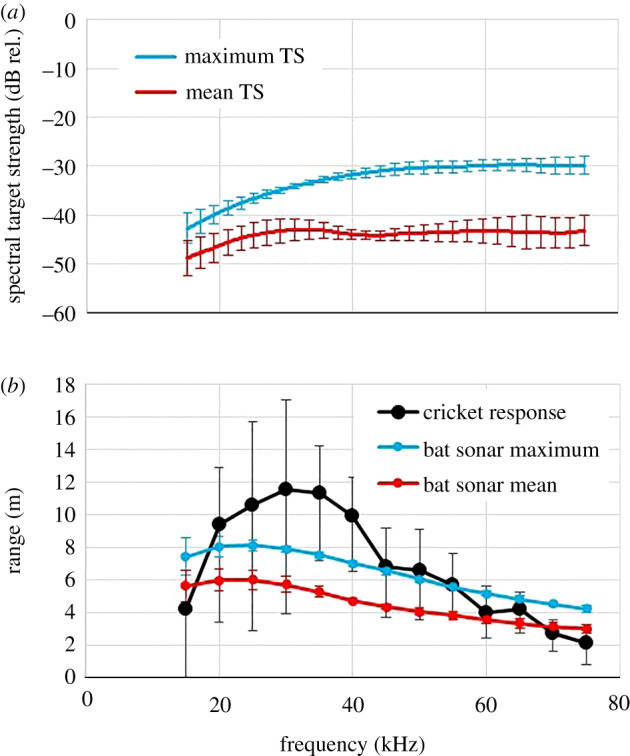
(a) Maximum (blue, upper curve) and mean (red, lower curve) spectral target strength (TS) of swordtail crickets (Trigonidiinae) (N = 5) over 360 echoes taken in one-degree steps from all directions in the frontal (horizontal) plane. (b) Calculated ranges over which bats first detect the maximum (blue) or mean (red) cricket echoes, and over which crickets would hear the echolocating bats (black). Error bars show standard deviation. (Online version in colour.)
Calculated maximum detection distances for crickets listening for bat calls and bats listening for cricket echoes as a function of frequency are compared in figure 5b. The maximum distance over which bats could detect the loudest cricket echoes was between 8 m at 20 kHz, gradually dropping to 4 m at 75 kHz. Based on the average cricket, calculated echo detection distances were reduced to 6 m at 20 kHz, gradually dropping to 3 m at 75 kHz. The crickets' maximum response distance to bat calls on average is between 10 and 12 m from 20 to 40 kHz. Above 40 kHz bat maximum detection distances were more similar to cricket response distances (difference of means 20–40 kHz = 2.8 ± 0.97 m versus 45–65 kHz = −0.16 ± 1.4 m; t4 = 4.49, p = 0.005, one-sided paired). The very high response threshold at 15 kHz means that a cricket would respond to an approaching bat calling at this very low frequency only after it detected the cricket's echo.
4. Discussion
The way field crickets detect and discriminate between predator cues and intraspecific signals, and the underlying sensory and neuronal processing, have entered classic textbooks in neuroethology, and made field crickets a model system for both behavioural contexts [20]. The decision heuristics reported for field crickets are based on categorical perception in the frequency domain, since when on the wing, crickets perform positive phonotaxis towards stimuli below 15 kHz, and fly away from sounds at high sonic and ultrasonic frequencies [42]. The large range of sound frequencies detectable by their ears is thus divided categorically between attractive and repulsive sounds, i.e. conspecific signals and predator cues, respectively. Such simple labelling of ‘good' and ‘bad’ frequencies in the decision heuristic finds its analogue in the activity of two large interneurons in the afferent auditory pathway of crickets, tuned either to the low frequencies of the conspecific calling song (AN1-neuron) or to ultrasound (AN2-neuron) [43].
Swordtail crickets must communicate and detect predators under rainforest conditions characterized by high levels of background noise [30–32]. A significant fraction of this background noise spectrum is from crickets calling below 10 kHz, which interferes with their own communication. The most important source of noise affecting predator detection are the calls of katydids, which mostly include high sonic to ultrasonic frequencies [34]. Responding to these calls with bat avoidance behaviour would represent a false alarm in terms of signal detection theory [35] and should be avoided. Notably, swordtail crickets respond differently to bat calls compared with most other eared insects, whose graded behavioural response changes from initial alterations in speed or flight direction (negative phonotaxis) to evasive action and flight stops as sound amplitude increases (review in [13]). The only response behaviour of swordtail crickets is complete flight cessation at any playback amplitude above their behavioural threshold (figure 1). Only two other orthopteran species show a similar response: the katydid Neoconocephalus ensiger [6] and the cricket Eunemobius carolinus (Gryllidae, Nemobiinae) [18].
Despite showing an all-or-nothing flight cessation, the swordtail crickets still exhibited some degree of graded response, because the duration of their flight interruptions increased with bat call amplitude. This creates a graded evasive response because brief flight interruptions would only result in an evasive drop in flight height, while the increasingly longer flight cessations as call amplitudes rise when the bat gets closer would lead to increasingly pronounced drops, eventually resulting in landing on the substrate.
Three candidate acoustic cues (spectral, temporal, and amplitude classification) were tested for how flying rainforest crickets might recognize and respond to predatory cues of bats while simultaneously avoiding false alarms to nocturnal background sounds. Regarding spectral separation, the same flight cessation shown in response to bat calls was observed in response to single katydid calls, albeit at a somewhat reduced response rate. Because katydid calls resemble bat calls in having similarly high sonic and ultrasonic frequencies and by their pulse-like temporal structure (figure 3), a comprehensive separation of bat calls and katydid background based on a cut-off frequency classification appears improbable, and the crickets' behavioural responses are in agreement with this. We cannot, however, rule out that some frequency information is included in the cricket́s behavioural decisions. The katydid community of Barro Colorado Island produces calls with most sound energy between 10 and 30 kHz [34,44], whereas bats on the island produce echolocation calls with peak frequencies between 20 and 80 kHz, with a median of 46.6 kHz [34]. Thus, the difference in the average spectra of katydid calls between 10 and 30 kHz (the acoustic background) and those of bat calls about 20 kHz higher has some discriminatory potential. Some evidence for this hypothesis comes from the probability of flight stops in response to the calls of the three selected katydid species (figure 3). The calls of S. sylvestris with most energy at about 40 kHz elicited flight stops with highest probability of 90%, in contrast to A. colossea with most energy between 10 to 20 kHz, and a flight stop probability of only 55%.
However, the idea of such a discrimination based on the spectra of background noise and predator cues conflicts with the likely neuronal substrate of the avoidance response in crickets during flight. An identified auditory interneuron (Int-1; synonymous with AN2) has been shown to elicit the steering away from ultrasound [5]. The neuron is broadly tuned to high sonic and ultrasonic frequencies; thus a separation of frequency ranges by a single neuron is not possible with this neuron's tuning. Future studies with sounds identical in temporal structure but differing in spectral composition are needed to confirm whether and how swordtail crickets can exploit spectral differences between background noise and bat calls.
Although swordtail crickets respond reliably with flight stops to a sequence of calls in a typical search phase of a bat, the repetition of calls was not necessary: the probability of responses did not drop even when only a single bat call was presented. Inevitably, classification based on the repetitive pattern of the bat call sequence faces a speed–accuracy trade-off as the accuracy of the decision depends on how much time is allocated to solving the task [45]. In the face of a deadly predator, any increase in response time is costly, and time invested in more accurate discrimination reduces time for escape. All insects tested in tethered flight under laboratory conditions indeed showed very short response latencies of 30–100 ms to bat echolocation sequences (see [46], Table 2]). Latencies below 100 ms do not even allow integration over two calls in a typical bat search call sequence (approx. 100 ms typical pause between calls). Not surprisingly, therefore, two katydid species [17,46] and a nemobiine cricket species [18] also respond to single, high-frequency sound pulses, with the consequence of a high probability of false alarms. Since most studies on bat avoidance have been conducted under laboratory conditions where background noise that might trigger false alarms is missing, response repertoires of bat prey should be studied under more natural acoustic settings.
The third and final hypothesis was clearly supported. Swordtail crickets have evolved a reduced sensitivity towards high-frequency or ultrasonic events, with the most sensitive threshold at 80 dB SPL. By comparison most other hearing insects have behavioural thresholds ranging from 40 to 70–80 dB SPL [46]. Only some flying beetles [9], the katydid N. ensiger [6], antlions (Myrmeleontidae) [47] and a nemobiine cricket species [18] have as high a response threshold to ultrasound as the swordtail crickets in our study.
ROC analysis revealed that the swordtail crickets' high response threshold is indeed highly adaptive in their noisy habitat (figure 4). On one hand, these crickets appear maximally risk averse against false alarms, as their very low sensitivity prevents ASRs to all katydid calls recorded in the habitat. Remarkably, any increase in sensitivity would result in such false alarms, underlining that the threshold criterion of the swordtail crickets is adaptive by avoiding false alarms in their noisy ultrasonic soundscapes. On the other hand, this comprehensive aversion of false alarms means that only the 5.8% loudest bat calls ever trigger an ASR, which seems counter-adaptive. Yet, distance-dependent losses as sound propagates mean that fainter calls (usually) are from more distant bats, which pose no immediate predation risk if the cricket remains outside the bats' biosonar range. Exclusively responding to calls that pose a real predation risk would be maximally adaptive, and indeed, detection range calculations as proxy of predation risk (figure 5b) show that the crickets' high response threshold still affords them a considerable detection range advantage over bat species calling at low ultrasonic frequencies (20–40 kHz; figure 5b). This detection range advantage might additionally serve against bats calling at reduced source levels (for example to actively shorten biosonar range in the dense rainforest understorey), which is known to reduce the detection range advantage of insects [39].
The most common bat calls in the natural soundscape (mainly S. bilineata) are slightly higher in frequency (approx. 43 kHz) and they elicit an ASR at or above 85 dB SPL. Two aspects of this are remarkable: first, these calls are exactly the loudest 5.8% of all recorded bat calls the swordtail crickets' maximally false alarm averse classifier would respond to as true positives, and secondly, 85 dB SPL signifies a bat no more than 7 m away, which is the exact maximum distance over which these bats would detect the swordtail crickets' echoes (figure 5b). In conclusion, the simple ultrasonic acoustic defence of the swordtail crickets with their all-or-nothing avoidance behaviour is remarkably elaborate and adaptive. Their high response threshold curve means they are maximally risk averse to false alarms and at the same time their small body size means that the small fraction of very loud bat calls they do respond to matches those calls that indicate they have been detected by the bat. Their classifier is doubly optimal with 0% false alarms and 100% response to calls indicating detection by their echolocating predators. Since the nemobiine cricket E. carolinus [18,19] shows similar ASR behaviour and thresholds to the swordtail crickets, both may have converged into the same acoustic niche under similar ecological conditions.
Sound frequencies in the audio range below 10 kHz do not elicit the ASR in crickets, but as shown for the flying nemobiine cricket E. carolinus using a two-tone suppression assay, the ASR can be suppressed by these frequencies [19]. Startle suppression was tuned to frequencies near 5 kHz, which is the most prominent frequency range in the nocturnal background noise in tropical rainforests [30–32]. However, the SPL of low-frequency stimuli required for suppression was greater than the SPL calculated for a dense population of singing crickets. Thus, we suggest that a suppression of the ASR by low-frequency sounds in E. carolinus functions to restrict startle responses to ultrasound events. For the swordtail crickets in our study it is therefore unlikely that the high threshold of the ultrasound-induced ASR is further increased by the low-frequency sounds of the many calling cricket species in the nocturnal rainforest.
In summary, swordtail crickets do not distinguish between cues of bats and acoustic background, since most high-frequency, short sound pulses elicit a flight stop (see electronic supplementary material, video). Rather, the insects employ a simple ‘amplitude classification rule' and treat any high-frequency event they hear as a potential predator. Factoring in the risk posed by bat calls of different amplitude shows that this strategy helps them achieve perfect false alarm rejections of background noise and perfect correct detection of dangerous bat signals. The common trade-off between correct detections and false alarms not only has fundamental implications for the evolution of communication [48] but also should be relevant for the evolution of decision criteria in response to predators.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to the Smithsonian Tropical Research Institute (STRI) and the Panamanian National Authority for the Environment (ANAM) for logistical support and research permits. We thank Inga Geipel for providing the Avisoft recording hardware, and Hannah ter Hofstede and Annemarie Surlykke for providing digital recordings of katydid and Saccopteryx calls, respectively. We also thank Marina Brunnhofer for help with the behavioural experiments, and Arne Schmidt and Bettina Erregger for their permanent logistic and other help in the project.
Ethics
The experiments reported in this paper comply with the current animal protection laws in Panama. According to these laws, studies on insects do not require approval by a review board institution or ethics committee (Institutional Animal Care and Use Committee Protocol). No specific permits were required for the described studies.
Data accessibility
Raw data are available in the electronic supplementary material.
Authors' contributions
M.H. measured the target strength of the crickets, calculated detection distances and performed the ROC analysis. H.R. performed behavioural experiments, as well as sound recordings and their analysis. Both authors contributed to the text.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by a grant from the Austrian Science Foundation FWF (grant no. P27145-B25 to H.R.), and by a Leverhulme Research Fellowship to M.H. (grant no. RF-2017-717).
References
- 1.Roeder KD. 1962. The behavior of free flying moths in the presence of artificial ultrasonic pulses. Anim. Behav. 10, 300–304. ( 10.1016/0003-3472(62)90053-2) [DOI] [Google Scholar]
- 2.Roeder KD. 1964. Aspects of the noctuid tympanic nerve response having significance in the avoidance of bats. J. Insect Physiol. 10, 529–546. ( 10.1016/0022-1910(64)90025-3) [DOI] [Google Scholar]
- 3.Popov AV, Shuvalov VF. 1977. Phonotactic of crickets. J. Comp. Physiol. A 119, 111–126. ( 10.1007/BF00655876) [DOI] [Google Scholar]
- 4.Moiseff A, Pollack GS, Hoy RR. 1978. Steering response of flying crickets to sound and ultrasound: mate attraction and predator avoidance. Proc. Natl Acad. Sci. USA 75, 4052–4056. ( 10.1073/pnas.75.8.4052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan TG, Hoy RR. 1984. Initiation of behavior by single neurons: the role of behavioral context. Science 226, 992–994. ( 10.1126/science.6505681) [DOI] [PubMed] [Google Scholar]
- 6.Libersat F, Hoy RR. 1991. Ultrasonic startle behavior in bushcrickets (Orthoptera; Tettigoniidae). J. Comp. Physiol. A 169, 507–514. ( 10.1007/BF00197663) [DOI] [PubMed] [Google Scholar]
- 7.Forrest TG, Farris HE, Hoy RR. 1995. Ultrasound acoustic startle response in scarab beetles. J. Exp. Biol. 198, 2593–2598. [DOI] [PubMed] [Google Scholar]
- 8.Hoy RR, Robert D. 1996. Tympanal hearing in insects. Annu. Rev. Entomol. 41, 433–450. ( 10.1146/annurev.en.41.010196.002245) [DOI] [PubMed] [Google Scholar]
- 9.Yager DD, Spangler HG. 1997. Behavioral response to ultrasound by the tiger beetle Cicindela marutha Dow combines aerodynamic changes and sound production. J. Exp. Biol. 200, 649–659. [DOI] [PubMed] [Google Scholar]
- 10.Fullard JH. 1998. The sensory coevolution of moths and bats. In Comparative hearing: insects (eds Hoy RR, Popper AN, Fay RR), pp. 279–326. New York, NY: Springer. [Google Scholar]
- 11.Yager DD. 1999. Structure, development, and evolution of insect auditory systems. Microsc. Res. Tech. 47, 380–400. () [DOI] [PubMed] [Google Scholar]
- 12.Greenfield MD. 2014. Acoustic communication in the nocturnal Lepidoptera. In Insect hearing and acoustic communication (ed. Hedwig B.), pp. 81–100. Berlin, Germany: Springer. [Google Scholar]
- 13.ter Hofstede HM, Ratcliffe JM. 2016. Evolutionary escalation: the bat–moth arms race. J. Exp. Biol. 219, 1589–1602. 10.1242/jeb.086686 [DOI] [PubMed] [Google Scholar]
- 14.Hoy RR. 1992. The evolution of hearing in insects as an adaptation to predation from bats. In The evolutionary biology of hearing (eds Webster DG, Popper AN, Fay RR), pp. 115–130. New York, NY: Springer. [Google Scholar]
- 15.Pollack GS. 2016. Hearing for defense. In Insect hearing. Springer handbook of auditory research, no. 55 (eds Pollack GS, Mason AC, Popper AN, Fay RR), pp. 81–98. Cham, Switzerland: Springer. [Google Scholar]
- 16.Conner WE, Corcoran AJ. 2012. Sound strategies: the 65-million-year-old battle between bats and insects. Annu. Rev. Entomol. 57, 21–39. ( 10.1146/annurev-ento-121510-133537) [DOI] [PubMed] [Google Scholar]
- 17.Schulze W, Schul J. 2001. Ultrasound avoidance behaviour in the bushcricket Tettigonia viridissima (Orthoptera: Tettigoniidae). J. Exp. Biol. 204, 733–740. [DOI] [PubMed] [Google Scholar]
- 18.Farris HE, Hoy RR. 2000. Ultrasound sensitivity in the cricket, Eunemobius carolinus (Gryllidae, Nemobiinae). J. Acoust. Soc. Am. 107, 1727–1736. ( 10.1121/1.428398) [DOI] [PubMed] [Google Scholar]
- 19.Farris HE, Hoy RR. 2000. Two-tone suppression in the cricket, Eunemobius carolinus (Gryllidae, Nemobiinae). J. Acoust. Soc. Am. 111, 1475–1485. ( 10.1121/1.1451069) [DOI] [PubMed] [Google Scholar]
- 20.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Greenfield MD. 2002. Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Balakrishnan R. 2016. Behavioral ecology of insect acoustic communication. In Insect hearing. Springer handbook of auditory research, no. 55 (eds Pollack GS, Mason AC, Popper AN, Fay RR), pp. 49–80. Cham, Switzerland: Springer. [Google Scholar]
- 23.Quintero DA, Aiello A. 1992. Insects of Panama and Mesoamerica. New York, NY: Oxford University Press. [Google Scholar]
- 24.Desutter-Grandcolas L. 2003. Phylogeny and the evolution of acoustic communication in extant Ensifera (Insecta, Orthoptera). Zool. Scripta 32, 525–561. ( 10.1046/j.1463-6409.2003.00142.x) [DOI] [Google Scholar]
- 25.Otte D. 2006. Eighty-four new cricket species (Orthoptera: Grylloidea) from La Selva, Costa Rica. Trans. Am. Entomol. Soc. 132, 299–418. ( 10.3157/0002-8320(2006)132[299:ENCSOG]2.0.CO;2) [DOI] [Google Scholar]
- 26.Robillard T, Desutter-Grandcolas L. 2004. Phylogeny and the modalities of acoustic diversification in extant Eneopterinae (Insecta, Orthoptera, Grylloidea, Eneopteridae). Cladistics 20, 271–293. ( 10.1111/j.1096-0031.2004.00025.x) [DOI] [PubMed] [Google Scholar]
- 27.Forrest TG, Farris HE, Hoy RR. 1995. Ultrasound acoustic startle response in scarab beetles. J. Exp. Biol. 198, 2593–2598. [DOI] [PubMed] [Google Scholar]
- 28.Altringham JD. 1996. Bats—biology and behaviour. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Kalko EKV, Handley CO, Handley D. 1996. Organization, diversity, and long-term dynamics of a Neotropical bat community. In Long-term studies of vertebrate communities (eds Cody ML, Smallwood JA), pp. 503–553. Burlington, MA: Academic Press. [Google Scholar]
- 30.Ellinger N, Hödl W. 2003. Habitat acoustics of a neotropical lowland rainforest. Bioacoustics 13, 297–321. ( 10.1080/09524622.2003.9753503) [DOI] [Google Scholar]
- 31.Lang A, Teppner I, Hartbauer M, Römer H.. 2005. Predation and noise in communication networks of neotropical katydids. In Animal communication networks (ed. McGregor P.), pp. 152–169. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Diwakar S, Balakrishnan R. 2007. The assemblage of acoustically communicating crickets of a tropical evergreen forest in southern India: call diversity and diel calling patterns. Bioacoustics 16, 113–135. ( 10.1080/09524622.2007.9753571) [DOI] [Google Scholar]
- 33.Morris GK, Mason AC, Wall P. 1994. High ultrasonic and tremulation signals in neotropical katydids (Orthoptera: Tettigoniidae). J. Zool. Lond. 233, 129–163. ( 10.1111/j.1469-7998.1994.tb05266.x) [DOI] [Google Scholar]
- 34.Symes LB, Martinson SJ, Hoeger L-O, Page RA, ter Hofstede HM. 2018. From understory to canopy: in situ behavior of Neotropical forest katydids in response to bat echolocation calls. Front. Ecol. Evol. 6, 227 ( 10.3389/fevo.2018.00227) [DOI] [Google Scholar]
- 35.Wiley RH. 2013. Signal detection, noise, and the evolution of communication. In Animal communication and noise (ed. Brumm H.), pp. 7–30. Berlin, Germany: Springer. [Google Scholar]
- 36.Eades DC, Otte D, Cigliano MM, Braun H. 2012. Orthoptera species file online. Version 2.0/4.1. See http://Orthoptera.SpeciesFile.org.
- 37.Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 270, 313–321. ( 10.1098/rspb.2002.2218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt AKD, Römer H, Riede K. 2012. Spectral niche segregation and community organization in a tropical cricket assemblage. Behav. Ecol. 24, 470–480. ( 10.1093/beheco/ars187) [DOI] [Google Scholar]
- 39.Neil TR, Shen Z, Robert D, Drinkwater BW, Holderied MW. 2020. Thoracic scales of moths as a stealth coating against bat biosonar. J. R. Soc. Interface 17, 20190692 ( 10.1098/rsif.2019.0692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goerlitz HR, ter Hofstede HM, Zeale MR, Jones G, Holderied MW. 2010. An aerial-hawking bat uses stealth echolocation to counter moth hearing. Curr. Biol. 20, 1568–1572. ( 10.1016/j.cub.2010.07.046) [DOI] [PubMed] [Google Scholar]
- 41.Surlykke A, Kalko EKV. 2008. Echolocating bats cry out loud to detect their prey. PLoS ONE 3, e2036 ( 10.1371/journal.pone.0002036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyttenbach RA, May ML, Hoy RR. 1996. Categorical perception of sound frequency by crickets. Science 273, 1542–1544. ( 10.1126/science.273.5281.1542) [DOI] [PubMed] [Google Scholar]
- 43.Pollack GS. 2015. Neurobiology of acoustically mediated predator detection. J. Comp. Physiol. A 201, 99–109. ( 10.1007/s00359-014-0948-5) [DOI] [PubMed] [Google Scholar]
- 44.ter Hofstede HM, Symes LB, Martinson SJ, Robillard T, Faure P, Madhusudhana S, Page RA. In press Calling songs of Neotropical katydids (Orthoptera, Tettigoniidae) from Panama. J. Orthop. Res.
- 45.Ings TC, Chittka L. 2008. Speed-accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr. Biol. 14, 1520–1524. ( 10.1016/j.cub.2008.07.074) [DOI] [PubMed] [Google Scholar]
- 46.Faure PA, Hoy RR. 2000. The sounds of silence: cessation of singing and song pausing are ultrasound-induced acoustic startle behaviors in the katydid Neoconocephalus ensiger (Orthoptera; Tettigoniidae). J. Comp. Physiol. A 186, 129–142. ( 10.1007/s003590050013) [DOI] [PubMed] [Google Scholar]
- 47.Holderied MW, Thomas LA, Korine C. 2018. Ultrasound avoidance by flying antlions (Myrmeleontidae). J. Exp. Biol. 221, jeb189308 ( 10.1242/jeb.189308) [DOI] [PubMed] [Google Scholar]
- 48.Green DM, Swets JA. 1966. Signal detection theory and psychophysics. New York, NY: Wiley; (Reprinted, with additions, by Krieger, New York, 1974) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available in the electronic supplementary material.