Abstract
Modulation of the viscoelastic properties of hydrogels is critical in tissue engineering applications. In the present study, a hyaluronate–alginate hybrid (HAH) was synthesized by introducing alginate to the hyaluronate backbone with varying molecular weights (700–2500 kDa), and HAH hydrogels were prepared in the presence of calcium ions at the same cross-linking density. The storage shear moduli of the HAH hydrogels increased with the concomitant increase in the molecular weight of hyaluronate in the HAH polymer. The HAH hydrogels were also modified with arginine–glycine–aspartic acid (RGD) and histidine–alanine–valine (HAV) peptides to enhance cell–matrix and cell–cell interactions, respectively. The chondrogenic differentiation of ATDC5 cells encapsulated within the HAH hydrogels was enhanced with the increase in the storage shear moduli of the gels in vitro as well as in vivo. This approach of regulating the viscoelastic properties of hydrogels using polymers of varying molecular weights at the same cross-linking density may prove to be useful in various tissue engineering applications including cartilage regeneration.
Introduction
Tissue engineering is an attractive technique for the replacement and regeneration of tissues and/or organs and is dependent on multidisciplinary collaboration for its successful execution.1 Articular cartilage is a specialized connective tissue that plays an important role in decreasing friction in the bone joints.2 Articular cartilage is mainly composed of 80% water and compact extracellular matrix (ECM).3 The ECM is made up of collagen type 2, water, and proteoglycans that exist in the form of a gel between the collagen matrices. Articular cartilage injury causes pain in the knee joint and inflammation, ultimately resulting in osteoarthritis.4,5 Numerous methods, such as intra-articular injection, drugs, and surgery, are available for treating cartilage tissue damage.6 However, the most promising method is a tissue engineering approach to create a natural cartilage tissue-mimicking environment by transplantation of chondrocytes with hydrogels.
Hydrogels have been widely used in tissue engineering applications such as cartilage repair and regeneration.7,8 They serve as appropriate scaffolds because of their water-swollen network structure that can encapsulate cells and bioactive molecules.9,10 Many biocompatible materials can serve as candidates for hydrogel preparation. Hydrogels are typically fabricated with water-soluble polymers using chemical and/or physical cross-linking methods, and they can be injected into the body using a syringe or endoscope.11 In particular, ionically cross-linked hydrogels are prepared from alginate, a natural polymer, in the presence of divalent cations.12 The carboxyl groups of l-guluronic acid form an ionic bond with divalent cations (e.g., Ca2+, Ba2+), which forms an egg-box model by enveloping these cations.13
In the present study, we used hyaluronate as a base material for hydrogel preparation to regenerate cartilage tissues. Hyaluronate (HA), a naturally existing polymer, is the main component of ECM. HA has been extensively utilized in tissue engineering because it plays important structural and signaling roles in many tissues including cartilage.14−16 HA is a linear polysaccharide consisting of glucuronic acid and N-acetylglucosamine.17 In general, HA can form a hydrogel by the chemical cross-linking method, and chemical cross-linking reagents may induce side effects such as toxicity. HA can be chemically modified with alginate to generate a hyaluronate–alginate hybrid (HAH), and HAH hydrogels that are physically cross-linked with calcium ions (i.e., without excipient chemical cross-linking reagents) do not elicit a severe inflammatory response in vivo.18 In addition, the HAH hydrogels have shown great potential as an injectable in cartilage regeneration.19 To design a tissue engineering scaffold, several factors need to be considered such as biocompatibility, biodegradability, mechanical stiffness, and cell-specific interactions.20 The success of cell-compatible hydrogels in tissue engineering is usually coupled with appropriate mechanical properties. The mechanical stiffness of a scaffold is very important to regulate cell adhesion, migration, growth, and differentiation.21,22
We hypothesized that the viscoelastic properties of the HAH hydrogels can be manipulated by varying the molecular weight of HA, which could in turn regulate cellular responses as well as subsequent cartilage regeneration in vivo. Controlling cell–cell and cell–matrix interactions within hydrogels is also crucial for cartilage regeneration, as these interactions critically influence cellular behavior.23 To achieve improved cell–matrix interactions, the arginine–glycine–aspartic acid (RGD) peptide was coupled with the HAH hydrogels, as it has been extensively used as an adhesion ligand that binds to the integrin receptor and promotes cell adhesion and differentiation.24 In addition, cadherin is a common transmembrane protein that enhances cell–cell interactions;25 therefore, the histidine–alanine–valine (HAV) peptide derived from cadherin was also conjugated to the HAH hydrogels. The density of both the RGD and HAV peptides in the HAH hydrogels was maintained constant, and the effect of the storage shear moduli of the gels prepared with HA of different molecular weights on the differentiation of mouse chondrocytes (ATDC5) was investigated in vitro. We also evaluated the efficacy of the HAH hydrogels with respect to cartilage regeneration in a mouse model using primary rabbit chondrocytes.
Results
Synthesis and Characterization of HAH
HAH was synthesized using a bifunctional linker such as adipic acid dihydrazide (ADH), which was first conjugated to the carboxyl group of hyaluronate (HA–ADH). Next, sodium alginate (ALG) modified with RGD and HAV peptides was coupled to the HA–ADH conjugate (Figure S1). The synthesis of peptide-modified HAH was confirmed by 1H NMR spectroscopy (Figure 1). The acetyl group of HA showed a peak at 1.9 ppm. Protons of carbohydrate-repeating units in HA and ALG were observed at 3.2–4.0 ppm. The methylene group of ADH exhibited proton peaks at 1.4 and 2.1 ppm, indicating that ADH was successfully conjugated to HA. We confirmed that more than 90% of ADH remained reactive in the HA–ADH conjugate by 1H NMR spectroscopy; this may indicate that intramolecular reaction or HA–HA coupling is not significant during the addition of ADH to HA.26
Figure 1.
(a) Chemical structure of hyaluronate–alginate hybrid (HAH) synthesized using adipic acid dihydrazide (ADH) as a linker. (b) 1H NMR spectra of hyaluronate (HA), ADH-conjugated HA (HA–ADH), peptide-modified alginate (ALG-P), and HAH.
The proton of arginine in the RGD peptide peaked at 3.1 ppm. Alanine and valine in the HAV peptide peaked at 1.2 and 0.9 ppm, respectively. This finding indicated that RGD and HAV peptides were successfully conjugated to ALG (Figures 1b, S2, and S3). We also quantified the integrated peak area for valine and arginine to determine the degrees of substitution of HAV and RGD peptides per 100 repeating units of ALG; these were found to be 4.7 and 4.8, respectively.26
Next, acid–base titration was performed to determine the conjugation efficiency of the HAH synthesis. The acid–base titration curve of HAH is shown in Figure S4. Only 5 mol % of the carboxyl groups in the HA backbone were initially activated to synthesize HAH, and the reduction in the number of carboxyl groups in HAH compared with the number of groups in HA was used to calculate the conjugation efficiency. HAH showed approximately 90% conjugation efficiency irrespective of the molecular weight of HA in HAH (Table 1).
Table 1. Conjugation Efficiency of HAH Polymer Determined by Acid–Base Titration.
| samplea | MWHA (kDa) | MWALG (kDa) | conjugation efficiency (%) |
|---|---|---|---|
| HAH700 | 700 | 250 | 93.6 |
| HAH1000 | 1000 | 250 | 91.2 |
| HAH1500 | 1500 | 250 | 88.8 |
| HAH2500 | 2500 | 250 | 91.2 |
The number suffixed after HAH denotes the molecular weight of HA (MWHA) before conjugation. The HA/ALG ratio was kept constant for all samples (HA/ALG = 2/1, weight ratio).
Preparation and Characterization of the HAH Hydrogel
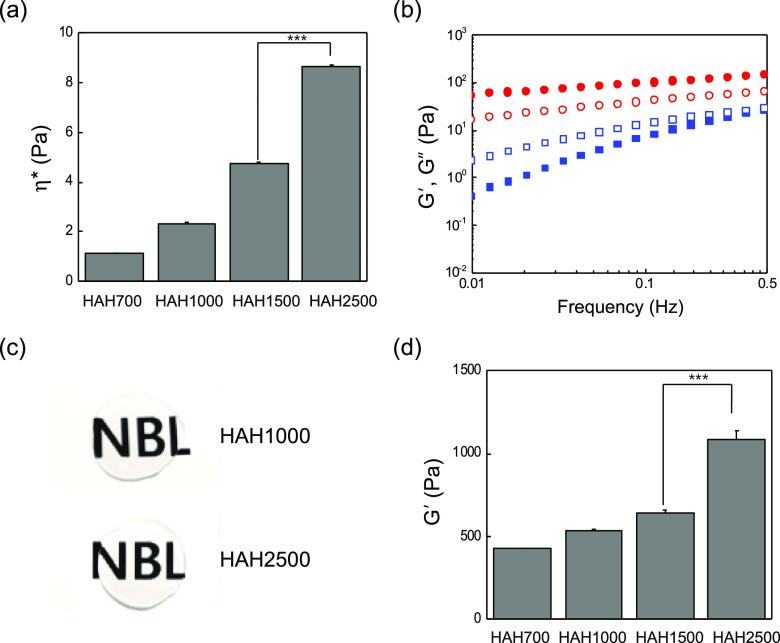
HAHs prepared using HA of various molecular weights (700, 1000, 1500, and 2500 kDa) were dissolved in Dulbecco’s phosphate-buffered saline (DPBS), and their complex viscosity (η*) was measured at 37 °C to investigate their viscoelastic properties. The viscosity of the HAH solution increased substantially with an increase in the molecular weight of HA (Figure 2a). Next, the value of the loss modulus (G″) of the HAH solution was measured at 37 °C using a frequency sweep mode, which was lower than that of the storage modulus (G′) at different frequencies (Figures 2b and S5). The HAH solution was able to form a transparent hydrogel in the presence of calcium ions ([CaSO4] = 60 mM) (Figure 2c), and the G′ value was higher than the G″ value at different frequencies (Figure 2b). The storage shear modulus of the HAH hydrogel was also dependent on the molecular weight of HA in HAH. The G′ value of the HAH hydrogel increased with an increase in the molecular weight of HA (Figure 2d). The storage modulus is a measure of elastic behavior of a material, often associated with the stiffness.
Figure 2.
Viscoelastic properties of hyaluronate–alginate hybrid (HAH) solution and the HAH hydrogel at 37 °C. (a) Changes in the complex viscosity (η*) of HAH solution depending on the molecular weight of HA. (b) Values of storage shear modulus (G′, filled symbol) and loss shear modulus (G″, open symbol) of the HAH2500 solution (square) and the HAH2500 hydrogel (circle) at different frequencies. (c) Images of HAH1000 and HAH2500 hydrogels. (d) Changes in the G′ value of the HAH hydrogel depending on the HA molecular weight ([HAH] = 2 wt %, [CaSO4] = 60 mM, ***P < 0.001).
Cell Viability
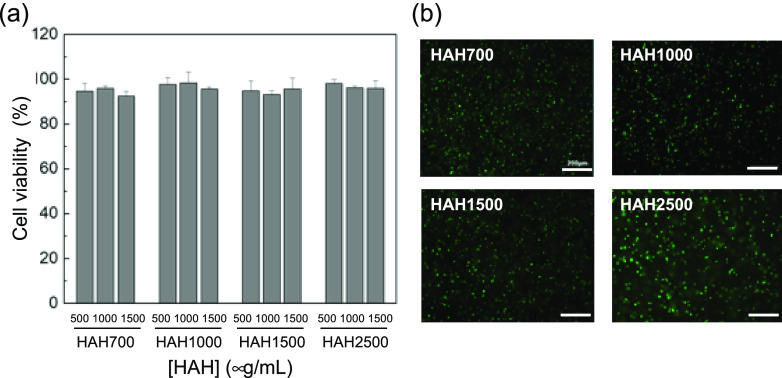
ATDC5 cells were treated with HAH solutions at various concentrations, and cell viability was tested by the Ez-Cytox assay ([ATDC5] = 5 × 103 cells/well, [HAH] = 500–1500 μg/mL). HAHs prepared with various molecular weights of HA showed good biocompatibility in vitro (Figure 3a). Next, cell viability within the HAH hydrogel was tested. ATDC5 cells were encapsulated in the HAH hydrogel and cultured in vitro ([cell] = 1 × 107 cells/mL, [polymer] = 2 wt %, [CaSO4] = 60 mM), and their viability was tested using the live/dead assay. The green and red signals indicate live and dead cells, respectively, in the gel. Cells were viable in the HAH hydrogels irrespective of the molecular weight of HA.
Figure 3.
(a) Relative viability (to cell-only control) of ATDC5 cells treated with the hyaluronate–alginate hybrid (HAH) solution ([ATDC5] = 5 × 103 cells/well) and (b) images of ATDC5 cells encapsulated within the HAH hydrogels ([ATDC5] = 1 × 107 cells/mL; scale bar, 200 μm) as seen in the live/dead assay. The green and red signals indicate live and dead cells, respectively, in the gel. Cell viability was evaluated after 24 h of culture.
Effect of Storage Shear Modulus on Chondrogenic Differentiation In Vitro
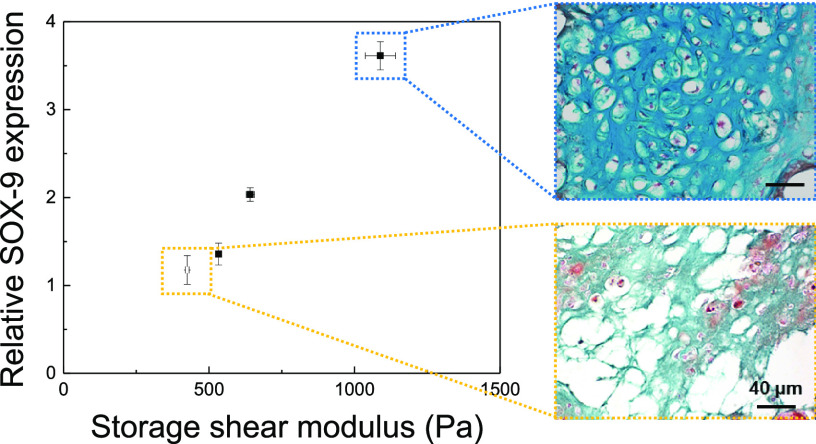
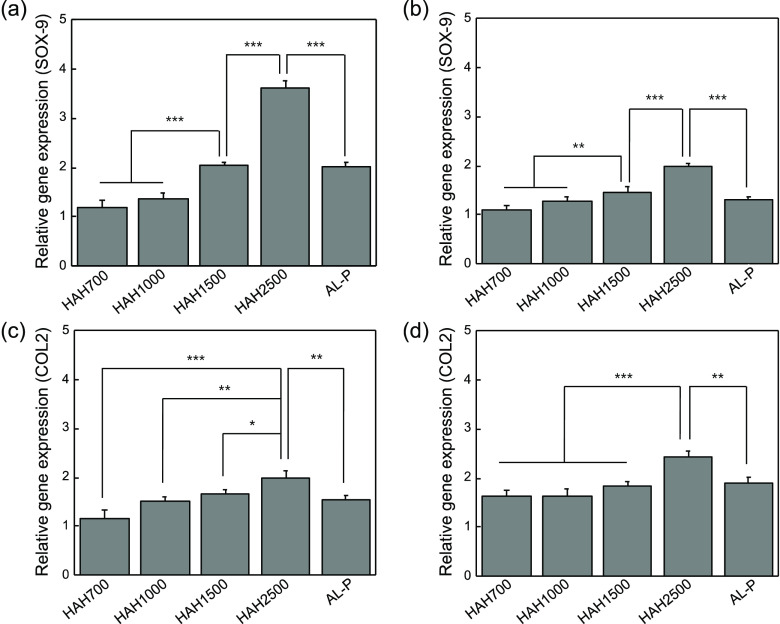
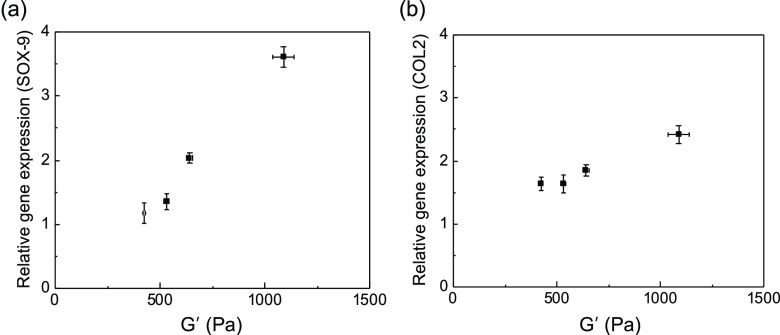
The chondrogenic differentiation of ATDC5 cells, encapsulated within the HAH hydrogels with varying degrees of storage shear moduli, was tested in vitro, as it is mainly influenced by the substrate stiffness.27 On days 7 and 21 of culture, the expression of typical chondrogenic marker genes such as SOX-9 and collagen type II (COL2) was evaluated by real-time polymerase chain reaction (RT-PCR) analysis (Figure 4). SOX-9 and COL2 expressions were significantly enhanced with an increase in the storage shear modulus of the HAH hydrogel. Therefore, we deduced that the stiffness of the HAH hydrogel is an important parameter for regulating the chondrogenic differentiation of ATDC5 cells, within the range of storage shear modulus tested in the present study (Figure 5). This finding may have critical implications for the design of hydrogel scaffolds for tissue engineering including cartilage regeneration.
Figure 4.
Quantitative analysis of SOX-9 and COL2 expressions in ATDC5 cells cultured within hyaluronate–alginate hybrid (HAH) hydrogels for (a, c) 7 days and (b, d) 21 days (*P < 0.05, **P < 0.01, and ***P < 0.001).
Figure 5.
Plots depicting the expressions of (a) SOX-9 (day 7) and (b) COL2 (day 21) with respect to the storage shear modulus of the hyaluronate–alginate hybrid (HAH) hydrogel.
In Vivo Cartilage Regeneration with the HAH Hydrogel
The efficacy of cartilage regeneration using primary chondrocytes encapsulated within the HAH hydrogel was evaluated in vivo. Primary chondrocytes, isolated from rabbits, were encapsulated within the HAH hydrogel, and the construct was subcutaneously injected into the dorsum region of mice. At 6 weeks after transplantation, regenerated tissues were retrieved. No significant change in the volume of the retrieved construct was observed compared to the initial injection volume.
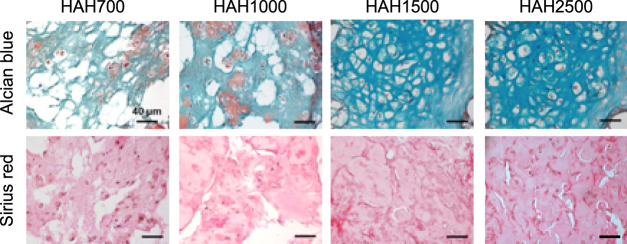
Tissue sections were stained with Alcian blue and Sirius red to detect glycosaminoglycan and collagen, respectively, which are essential for ECM formation in regenerated cartilage tissues. Lacuna, a typical structure present in native cartilage, was clearly observed when HAH1500 and HAH2500 hydrogels were used (Figure 6). The cells within the HAH2500 gels had more lacunae compared with those in other gels. Cartilage regeneration was found to be affected by the stiffness of the HAH hydrogels, and among the tested hydrogels, HAH2500 was the most efficient in promoting chondrogenic differentiation of primary chondrocytes in the animal model.
Figure 6.
Histological images of regenerated cartilage tissues stained with Alcian blue and Sirius red. The regenerated cartilage tissues were retrieved from mice after 6 weeks of transplantation with the primary chondrocyte-hyaluronate–alginate hybrid (HAH) hydrogel constructs (scale bar, 40 μm).
Quantification of Gene Expression Level and Sulfated Glycosaminoglycan (sGAG) Content In Vivo
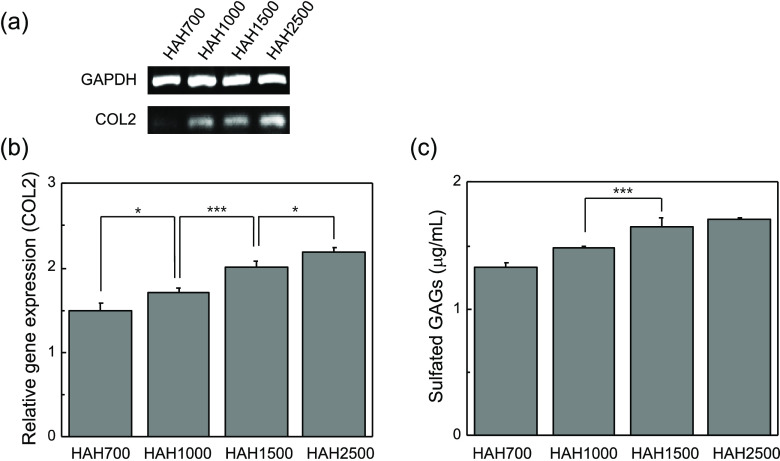
We investigated the expression of COL2 in regenerated tissues by RT-PCR and real-time PCR analyses, as COL2 is a major component of cartilage. We found that the expression of COL2 increased with the increase in the molecular weight of HA in HAH. The chondrocyte-HAH2500 hydrogel constructs showed the highest expression of COL2 among the tested constructs (Figure 7a,b). The amount of sGAGs, one of the major ECM components, was also determined in the regenerated tissues. It was found that the amount of sGAGs secreted by the transplanted chondrocytes increased with an increase in the molecular weight of HA. The amount of sGAGs was the highest in the group treated with the chondrocyte-HAH2500 gel compared to that in other groups (Figure 7c). These findings were consistent with the results of the histological analysis (Figure 6). Hence, we concluded that the HAH2500 gel may provide a favorable environment for transplanted chondrocytes and would also enhance cartilage regeneration. Importantly, the stiffness of the HAH hydrogels was found to be critical for the regulation of chondrogenic differentiation in vivo.
Figure 7.
(a) Expression of COL2 and (b) quantified expression of COL2 in regenerated cartilage tissues. (c) Amount of sGAGs in regenerated cartilage tissues (*P < 0.05 and ***P < 0.001).
Discussion
The objective of the present study was to test whether the viscoelastic properties of the HAH hydrogel would influence the regulation of chondrogenesis and cartilage regeneration in vivo. Biocompatibility, biodegradability, mechanical stiffness, and cell-specific interactions are the typical parameters to be considered when designing tissue engineering scaffolds including hydrogels.20 Among these parameters, scaffold stiffness is known to play a vital role in influencing cellular behavior.12,27−29 Generally, hydrogels possess mechanical and structural properties similar to those of the ECM, such as high water content, elasticity, and biocompatibility.13,29,30 However, hydrogels intrinsically have weak mechanical properties and are not suitable for load-bearing applications unless a supporting system is provided.31 A biocompatible HA suitable for cartilage regeneration was chemically modified using alginate to synthesize a HAH polymer, and its hydrogel was prepared with calcium ions (Figures 1 and 2).18 In addition, it was demonstrated that cell–matrix and cell–cell interactions of the HAH gel were enhanced by the introduction of the RGD and HAV peptides, respectively.23
The mechanical properties of hydrogels can be typically controlled by varying the cross-linking method (e.g., chemical or physical cross-linking), cross-linking density, and molecular weight of the polymer. In the present study, the storage shear moduli of the HAH hydrogels were regulated by varying the molecular weight of HA (i.e., 700, 1000, 1500, or 2500 kDa) (Table 1). The storage shear moduli of the HAH hydrogels increased with an increase in the molecular weight of HA at the same polymer composition and cross-linking density. The use of large amounts of cross-linkers generally increases the cross-linking density and the resultant mechanical stiffness of hydrogels. However, this method may not be suitable when cells are incorporated into the gels for tissue regeneration. Our approach of varying the molecular weight of the polymer used in hydrogel preparation can serve as a simple and useful method of regulating the mechanical stiffness of the gels at the same polymer concentration and same cross-linking density.
The regulation of cellular behavior by substrate stiffness, including hydrogel stiffness, is an important factor in tissue engineering approaches. The chondrogenic differentiation of ATDC5 cells encapsulated within the HAH hydrogels significantly increased in vitro with an increase in the storage shear moduli of the HAH hydrogels brought about by an increase in the molecular weight of HA in the HAH polymer (Figures 4 and 5). In addition, cartilage regeneration, as a result of transplantation with primary chondrocytes encapsulated within the HAH hydrogels, was substantially influenced by the viscoelastic properties of the HAH hydrogels. Histological analysis suggested that a stiff hydrogel promotes chondrogenic differentiation more efficiently than a soft hydrogel. As the stiffness of the hydrogel increased, a greater number of lacuna structures, which are indicative of cartilage formation, were observed (Figure 6). The enhanced levels of chondrogenic gene expression and of sGAG secretion with an increase in the storage shear moduli of the HAH hydrogels also support this finding (Figure 7).
However, in contrast to our findings, a study had previously reported that hydrogels with an elastic modulus of <2 to 3 kPa showed enhanced cellular aggregation and chondrogenesis, whereas those with a mechanical stiffness of >10 kPa resulted in poor chondrogenesis.27 Additionally, the shape and cytoskeletal structure of stem cells are known to be strongly dependent on the gel stiffness.32,33 The soft hydrogel promoted the chondrogenesis of mesenchymal stem cells.34,35 This difference may be attributed to the different chemical nature of the base biomaterials used and to the range of mechanical stiffness tested. In our study, we determined that stiff HAH hydrogels were more efficient at regenerating cartilage tissues in vivo in the following range of mechanical stiffness: G′ = 0.4–1.2 kPa.
HA degrades rapidly in vivo in the absence of chemical modification or cross-linking caused by endogenous hyaluronidase.36 Fast gel degradation may be unfavorable in the regeneration of many types of tissues, as gels should supply three-dimensional (3-D) structural environments and maintain their integrity as a scaffold during the new ECM development from delivered cells. Degradation of the HAH hydrogels by hyaluronidase was restricted by covalent conjugation of ALG to HA, leading to slow gel degradation (Figure S6). This might result in these HAH hydrogels being more appropriate for in vivo applications.
Conclusions
We demonstrated that the viscoelastic properties of the HAH hydrogel are a key factor in cartilage regeneration. The HAH hydrogels with different viscoelastic properties were fabricated by varying the molecular weight of HA in the HAH polymer at the same cross-linking density. The storage shear moduli of the HAH hydrogels increased with the increase in the molecular weight of HA. The HAH hydrogels encapsulating the ATDC5 cells effectively promoted chondrogenesis in vitro. In addition, primary chondrocyte-HAH hydrogel constructs were useful in regenerating cartilage tissues in mice. Consequently, chondrogenic differentiation and cartilage formation were enhanced when the mechanical stiffness of the HAH hydrogels increased. This finding may provide a useful method for designing scaffolding materials for many tissue engineering applications including cartilage regeneration.
Materials and Methods
Materials
Sodium hyaluronate was purchased from Lifecore (Mw = 700, 1500, 2500 kDa; Chaska, MN) and Humedix (Mw = 1000 kDa; Anyang, Korea). Sodium alginate (ALG) (Mw = 250 kDa, guluronate unit content = 66%) was purchased from FMC Biopolymer (Sandvika, Norway). RGD peptide (GGGGRGDSP) and HAV peptide (GGGGSHAVSS) were purchased from Anygen (Seoul, Korea). 1-Ethyl-3-(dimethylaminopropyl)carbodiimide (EDC) hydrochloride was purchased from Proteochem (Hurricane, UT). N-Hydroxysulfosuccinimide sodium salt (sulfo-NHS) was purchased from Covachem (Loves Park, IL). Adipic acid dihydrazide (ADH), 2-(N-morpholino)ethanesulfonic acid (MES) hydrate, calcium sulfate, deuterium oxide (D2O), Alcian blue, and direct red 80 were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco’s phosphate-buffered saline (DPBS), fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium nutrient mixture F-12 (DMEM/F-12), penicillin–streptomycin (P–S), and trypsin–ethylenediaminetetraacetic acid (EDTA) were procured from Gibco (Grand Island, NY). The Ez-Cytox kit was purchased from DoGenBio (Daejeon, Korea), and the Live/Dead Viability/Cytotoxicity kit was purchased from Invitrogen (Waltham, MA).
Modification of Alginate with Peptides
ALG was dissolved in 0.1 M MES buffer solution at room temperature (1 wt %, pH 6, 0.3 M NaCl). RGD and HAV peptides ([peptide] = 21 μmol/g alginate) were added to the alginate solution in the presence of EDC and sulfo-NHS ([COOH]/[EDC]/[sulfo-NHS] = 1:0.1:0.05, mole ratio). The reaction was carried out for 20 h at room temperature. After the completion of the reaction, the solution was dialyzed, treated with activated charcoal, sterilized through a 0.22 μm filter, and lyophilized.
Synthesis of HAH
HA with varying molecular weights (700, 1000, 1500, and 2500 kDa) was dissolved in 0.1 M MES buffer solution (1 wt %, pH 6.0, 0.3 M NaCl) and reacted with adipic acid dihydrazide (ADH) in the presence of EDC and sulfo-NHS ([COOH]/[EDC]/[sulfo-NHS] = 1:0.1:0.05, mole ratio). After 20 h, the reactant solution was precipitated with ethanol five times. The precipitated HA–ADH conjugate was filtered and lyophilized. Peptide-modified alginate (ALG-P) was dissolved in 0.1 M MES buffer (1 wt %, pH 6.0, 0.3 M NaCl) containing EDC and sulfo-NHS ([COOH]/[EDC]/[sulfo-NHS] = 1:0.1:0.05, mole ratio) and subsequently added to the HA–ADH solution (1 wt %, pH 6.0, 0.3 M NaCl) drop-by-drop to synthesize HAH. After 20 h, the solution was precipitated by ethanol, treated with activated charcoal, sterilized through a 0.22 μm filter, and lyophilized. HAH was synthesized at a ratio of 2:1 ([HA]/[ALG], weight ratio).
Characterization of HAH
To confirm the synthesis of HAH, the sample was dissolved in D2O at a concentration of 10 mg/mL, and 1H NMR analysis was performed (VNMRS 600 MHz; Varian, Palo Alto, CA). The conjugation efficiency of HAH was determined from the reduced number of carboxyl groups in the hybrid polymer compared with the carboxyl groups in HA by the acid–base titration method.26
Hydrogel Fabrication and Characterization
Hydrogels were fabricated by physical cross-linking with calcium ions. For the preparation of the HAH hydrogels, HAH was dissolved in DPBS ([polymer] = 2 wt %), and the solution was mixed with a slurry of calcium sulfate ([CaSO4] = 60 mM) using Luer lock syringes.19 The storage modulus (G′) of the HAH hydrogel was measured using a rotational rheometer (Bohlin Gemini 150; Malvern) equipped with a horizontal parallel plate (20 mm diameter, 800 μm gap from the bottom plate). The viscoelastic properties of the hydrogels were also measured using the frequency sweep mode (range 0.01–1 Hz). The complex viscosity was measured using a corn-and-plate fixture (4° cone angle, 40 mm diameter plate, 800 μm gap from the bottom plate). The temperature was maintained at 37 °C.
Cell Culture
ATDC5 mouse chondrogenic cells (ECACC) were cultured in DMEM/F-12 medium containing 5% FBS, 1% PS, 10 μg/mL human transferrin, and 3 × 10–8 M sodium selenite at 37 °C in an atmosphere containing 5% CO2. For the chondrogenic differentiation experiments, 10 μg/mL of bovine insulin was added to the culture medium, which was changed every other day.
Cell Viability
To evaluate the viability of cells treated with HAH solutions, ATDC5 cells were seeded in a 96-well tissue culture plate at a density of 5 × 103 cells/well and cultured using DMEM/F-12 medium containing 5% FBS, 1% PS, 3 × 10–8 M sodium selenite, and 10 μg/mL human transferrin at 37 °C in an atmosphere containing 5% CO2. After 24 h, the cells were treated with HAH solutions ([polymer] = 500–2000 μg/mL). Following this, 10 μL of Ez-Cytox was added to each well and incubated for 4 h and absorbance at 450 nm was measured using a spectrophotometer (SpectraMax M2; Molecular Devices, San Jose, CA).
Cell viability within the HAH hydrogels was also evaluated using the Live/Dead assay. In brief, ATDC5 cells were mixed with the HAH solution ([HAH] = 2 wt %) at a density of 1 × 107 cells/mL, followed by gelation with calcium ions to form hydrogel ([CaSO4] = 60 mM). The cells encapsulated within the gel disks (10 mm diameter, 1 mm thick) were incubated at 37 °C in an atmosphere containing 5% CO2. After 24 h of culture, the cell/hydrogel construct was treated with 20 μL of EthD-1 and 5 μL of calcein AM in 10 mL of DPBS for 30 min and observed by fluorescence microscopy (Nikon, Japan).
In Vitro Gene Expression
ATDC5 cells were encapsulated in the HAH hydrogels and cultured using chondrogenic differentiation medium for 3 weeks. Real-time PCR was performed to quantify the expression of the chondrogenic marker genes, namely, SOX-9 and collagen type II (COL2). Toward this end, the hydrogels encapsulating the cells were treated with EDTA (70 mM) and incubated for 30 min at 37 °C. RNA was isolated from the hydrogels using the RNAiso plus kit (Takara) and then reverse transcribed to cDNA using Maxime RT PreMix kit (iNtRON Biotechnology). The expression of the chondrogenic marker genes was quantified by an ABI PRISM 7500 real-time PCR system using real-time SYBR Green PCR technology (Applied Biosystems; South San Francisco, CA). The relative level of gene expression was determined by comparison with that of a housekeeping gene (β-actin). The primer sequences for real-time PCR were as follows: β-actin, 5′-CCCTGAACCCTAAGGCCAAC-3′, 5′-GCATACAGGGACAGCACAGC-3′; SOX-9, 5′-AAGTCGGAGAGCCGAGAGCG-3′, 5′-ACGAAACCGGGGCCACTTGC-3′; COL2, 5′-CACACTGGTAAGTGGGGCAAGACCG-3′, and 5-GGATTGTGTTGTTTCAGGGTTCGGG-3′.
Chondrocyte Isolation and Cartilage Regeneration In Vivo
All animal procedures were performed in accordance with the Hanyang University guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of Hanyang University. Primary chondrocytes were isolated from the knee joint of the hind leg of a New Zealand white rabbit (4-week-old, Samtako, Korea). They were digested with 4.5 mg/mL collagenase type II (Worthington) in DMEM/F-12 medium containing 10% FBS and 1% PS for 6 h at 37 °C in an atmosphere containing 5% CO2. Isolated cells were obtained by a cell strainer (40 μm; SPL Life Science) and cultured in DMEM/F-12 medium containing 10% FBS and 1% PS at 37 °C in an atmosphere containing 5% CO2; the medium was changed every other day.
The primary chondrocytes were dispersed in HAH solution ([polymer] = 2 wt %) at a density of 1 × 107 cells/mL, and the solution was mixed with 60 mM CaSO4 to form hydrogels. Then, the chondrocyte-hydrogel construct was injected (injection volume, 100 μL) into th e dorsum of BALB/C nude mice (6-week-old, Orient Bio, Korea). The tissue samples were retrieved 6 weeks postinjection (n = 4).
Histological Analysis
Retrieved tissues were fixed with 10% formalin for 24 h, washed with DPBS, and embedded in O.C.T. compound (Tissue Tek; Sakura Finetechnical) for histological analysis. The tissue blocks were cut by a cryotome (Leica) to obtain samples with 10 μm thickness and stained with Alcian blue and Sirius red. Histological images were observed by a light microscope (Carl Zeiss).
In Vivo Gene Expression
For evaluating the expression of chondrogenic marker genes, RNA was isolated from the retrieved cartilage tissue using an RNA isolation kit (Takara). Isolated RNA was reverse transcribed to cDNA by a reverse transcription master mix (iNtRON). RT-PCR and real-time PCR analyses were performed to confirm the expression of chondrogenic marker genes. For RT-PCR analysis, COL2 expression was evaluated by comparing it with the expression of a housekeeping gene (GAPDH) using conventional agarose gel electrophoresis. The primer sequences were as follows: GAPDH, 5′-TCACCATCTTCCAGGAGCGA-3′, and 5′-CACAATGCCGAAGTGGTCGT-3′; COL2, 5′-CAATCCTGGTGAACCTGGCG-3′, and 5′-TTTCCCCAGACTTTCCGGGC-3′.
Moreover, mRNA expression was quantified using a SYBR Green PCR kit in an ABI PRISM 7500 real-time PCR system (Applied Biosystems). Gene expression was determined by comparing it with the expression of a housekeeping gene (β-actin). The primer sequences for real-time PCR were as follows: β-actin, 5′-CCCTGAACCCTAAGGCCAAC-3′, and 5′-GCATACAGGGACAGCACAGC-3′; COL2, 5′-CACACTGGTAAGTGGGGCAAGACCG-3′, and 5-GGATTGTGTTGTTTCAGGGTTCGGG-3′.
Quantification of Sulfated Glycosaminoglycan Content
The content of sulfated glycosaminoglycans (sGAGs) in the regenerated cartilage tissue was quantified using a BlyscanTM sGAG assay kit (Biocolor). The retrieved tissues were homogenized in 500 μL of papain buffer solution (25 μg/mL papain, 2 mM cysteine, 50 mM sodium phosphate, 2 mM EDTA, pH 6.5) and incubated at 60 °C overnight. Then, 1 mL of 1,9-dimethylmethylene blue reagent (DMMB) was added to the sample and incubated for 30 min using a mechanical shaker. After centrifugation at 10 000g for 10 min, a dissociation reagent containing sodium salt of an anionic surfactant was added to the sample pellet, and absorbance was measured at 656 nm using a spectrophotometer (Molecular Devices, SpectraMax M2). Chondroitin 4-sulfate was used as the standard.
Statistical Analysis
All data are presented as the mean ± standard deviation (n = 4). Statistical analyses were performed using Student’s t test. *P < 0.05, **P < 0.01, and ***P < 0.001 were considered to be statistically significant.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A2C1012199).
Glossary
Abbreviations Used
- HAH
hyaluronate–alginate hybrid
- RGD
arginine–glycine–aspartic acid
- HAV
histidine–alanine–valine
- ECM
extracellular matrix
- HA
hyaluronate
- ADH
adipic acid dihydrazide
- MES
2-(N-morpholino)ethanesulfonic acid
- ALG
sodium alginate
- ALG-P
peptide-modified alginate
- sGAGs
sulfated glycosaminoglycans
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01763.
Synthetic procedure; 1H NMR spectra; acid–base titration curves; rheological measurements; and degradation behavior of hyaluronate–alginate hybrid hydrogels (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Guilak F.; Butler D. L.; Goldstein S. A. Functional tissue engineering - The role of biomechanics in articular cartilage repair. Clin. Orthop. Relat. Res. 2001, 391, S295–S305. 10.1097/00003086-200110001-00027. [DOI] [PubMed] [Google Scholar]
- Eleswarapu S. V.; Responte D. J.; Athanasiou K. A. Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. Plos One 2011, 6, e26178 10.1371/journal.pone.0026178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. R.; Erler J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Models Mech. 2011, 4, 165–178. 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker H.; Wild M.; Rath B.; Tingart M.; Driessen A.; Quack V.; Betsch M. Current overview of cartilage regeneration procedures. Der Orthopade 2017, 46, 907–913. 10.1007/s00132-017-3474-7. [DOI] [PubMed] [Google Scholar]
- Sophia Fox A. J.; Bedi A.; Rodeo S. A. The basic science of articular cartilage: Structure, composition, and function. Sports Health Multidiscip. Approach 2009, 1, 461–468. 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiku M. L.; Sabaawy H. E. Cartilage regeneration for treatment of osteoarthritis: a paradigm for nonsurgical intervention. Ther. Adv. Musculoskeletal Dis. 2015, 7, 76–87. 10.1177/1759720X15576866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherbiny I. M.; Yacoub M. H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38 10.5339/gcsp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkar P. M.; Kiick K. L.; Kloxin A. M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. 10.1039/C3CS60040H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest C.; Morrison D. W. G.; Ni B.; Rubin J.; Yadav V.; Mahdavi A.; Karp J. M.; Khademhosseini A. Microscale hydrogels for medicine and biology: Synthesis, characteristics and applications. J. Mech. Mater. Struct. 2007, 2, 1103–1119. 10.2140/jomms.2007.2.1103. [DOI] [Google Scholar]
- Ahmed E. M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy N.; Reddy R.; Jiang Q. R. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. 10.1016/j.tibtech.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Han F. X.; Zhu C. H.; Guo Q. P.; Yang H. L.; Li B. Cellular modulation by the elasticity of biomaterials. J. Mater. Chem. B 2016, 4, 9–26. 10.1039/C5TB02077H. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Mooney D. J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.; Fu C.; Li M.; Li X.; Wang M.; He L.; Zhang L.-M.; Peng Y. A pH-sensitive hyaluronic acid prodrug modified with lactoferrin for glioma dual-targeted treatment. Mater. Sci. Eng., C 2016, 67, 159–169. 10.1016/j.msec.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Tan H. P.; Chu C. R.; Payne K. A.; Marra K. G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H. S.; Lee E. A.; Yoon J. J.; Park T. G. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials 2005, 26, 1925–1933. 10.1016/j.biomaterials.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Sohn S. S.; Revuri V.; Nurunnabi M.; Kwak K. S.; Lee Y.-k. Biomimetic and photo crosslinked hyaluronic acid/pluronic F127 hydrogels with enhanced mechanical and elastic properties to be applied in tissue engineering. Macromol. Res. 2016, 24, 282–291. 10.1007/s13233-016-4029-1. [DOI] [Google Scholar]
- Park H.; Woo E. K.; Lee K. Y. Ionically cross-linkable hyaluronate-based hydrogels for injectable cell delivery. J. Control. Release 2014, 196, 146–153. 10.1016/j.jconrel.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Park H.; Lee K. Y. Cartilage regeneration using biodegradable oxidized alginate/hyaluronate hydrogels. J. Biomed. Mater. Res., Part A 2014, 102, 4519–4525. 10.1002/jbm.a.35126. [DOI] [PubMed] [Google Scholar]
- Zhu J. M.; Marchant R. E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou Y.-H.; Khoneisser J.; Huang P.-C.; Xu X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. 10.1016/j.bioactmat.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. Y.; Lai J. H.; Han L.-H.; Tong X. M.; Yang F. Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng., Part A 2014, 20, 2131–2139. 10.1089/ten.tea.2013.0531. [DOI] [PubMed] [Google Scholar]
- An H.; Lee J. W.; Lee H. J.; Seo Y.; Park H.; Lee K. Y. Hyaluronate-alginate hybrid hydrogels modified with biomimetic peptides for controlling the chondrocyte phenotype. Carbohydr. Polym. 2018, 197, 422–430. 10.1016/j.carbpol.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Bellis S. L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. 10.1016/j.biomaterials.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Lin S.; Sun Y.; Feng Q.; Li G.; Bian L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials 2016, 77, 44–52. 10.1016/j.biomaterials.2015.10.072. [DOI] [PubMed] [Google Scholar]
- Seo Y.; Lee H.; Lee J. W.; Lee K. Y. Hyaluronate-alginate hybrid hydrogels prepared with various linkers for chondrocyte encapsulation. Carbohydr. Polym. 2019, 218, 1–7. 10.1016/j.carbpol.2019.04.067. [DOI] [PubMed] [Google Scholar]
- Arora A.; Kothari A.; Katti D. S. Pericellular plasma clot negates the influence of scaffold stiffness on chondrogenic differentiation. Acta Biomater. 2016, 46, 68–78. 10.1016/j.actbio.2016.09.038. [DOI] [PubMed] [Google Scholar]
- Breuls R. G. M.; Jiya T. U.; Smit T. H. Scaffold stiffness influences cell behavior: opportunities for skeletal tissue engineering. Open Orthop. J. 2008, 2, 103–109. 10.2174/1874325000802010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.; Dong C.; Yang L.; Lv Y. 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 15790–15802. 10.1021/acsami.5b02662. [DOI] [PubMed] [Google Scholar]
- Yeom J.; Bhang S. H.; Kim B.-S.; Seo M. S.; Hwang E. J.; Cho I. H.; Park J. K.; Hahn S. K. Effect of cross-linking reagents for hyaluronic acid hydrogel dermal fillers on tissue augmentation and regeneration. Bioconjugate Chem. 2010, 21, 240–247. 10.1021/bc9002647. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Mooney D. J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Vining K. H.; Mooney D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D. E.; Janmey P.; Wang Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Kwon H. J. Chondrogenesis on sulfonate-coated hydrogels is regulated by their mechanical properties. J. Mech. Behav. Biomed. Mater. 2013, 17, 337–346. 10.1016/j.jmbbm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Park J. S.; Chu J. S.; Tsou A. D.; Diop R.; Tang Z.; Wang A.; Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 2011, 32, 3921–3930. 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X. Z.; Prestwich G. D.. Therapeutic Biomaterials from Chemically Modified Hyaluronan. In Chemistry and Biology of Hyaluronan; Garg H. G.; Hales C. A., Eds.; Elsevier: Boston, 2004; pp 485–504. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.