Abstract
Survival and growth of the bovine conceptus (embryo and associated extraembryonic membranes) are dependent on endometrial secretions or histotroph found in the uterine lumen. Previously, serial embryo transfer was used to classify heifers as high fertile (HF), subfertile (SF), or infertile (IF). Here, we investigated specific histotroph components [glucose, prostaglandins (PGs), and lipids] in the uterine lumen of day 17 pregnant and open fertility-classified heifers. Concentrations of glucose in the uterine lumen were increased by pregnancy but did not differ among fertility-classified heifers. Differences in expression of genes encoding glucose transporters and involved with glycolysis and gluconeogenesis were observed between conceptuses collected from HF and SF heifers. In the uterine lumen, PGE2 and PGF2α were increased by pregnancy, and HF heifers had higher concentrations of PGE2, PGF2α, and 6-keto-PFG1α than SF heifers. Differences were found in expression of genes regulating PG signaling, arachidonic acid metabolism, and peroxisome proliferator-activated receptor signaling among conceptuses and endometrium from fertility-classified heifers. Lipidomics was conducted exclusively in samples from HF heifers, and phosphatidylcholine was the main lipid class that increased in the uterine lumen by pregnancy. Expression of several lipid metabolism genes differed between HF and SF conceptuses, and a number of fatty acids were differentially abundant in the uterine lumen of pregnant HF and SF heifers. These results support the ideas that uterine luminal histotroph impacts conceptus survival and programs its development and is a facet of dysregulated conceptus–endometrial interactions that result in loss of the conceptus in SF cattle during the implantation period of pregnancy establishment.
Keywords: uterus, conceptus, embryo, lipids, prostaglandins, fertility
Summary Sentence: Conceptus–endometrium interactions differ in fertility-classified heifers and impact uterine luminal contents.
Introduction
The uterus clearly impacts conceptus (embryo/fetus and associated extraembryonic membranes) survival and development, thus affecting pregnancy success [1–5]. After hatching from the zona pellucida (days 9 and 10), the bovine blastocyst slowly grows into an ovoid or tubular form on days 12–14 and is then termed a conceptus [6]. The conceptus is about 2 mm in length on day 13, 6 mm by day 14, 60 mm (6 cm) by day 16, and 20 cm or more by day 19 [6, 7]. Peri-implantation growth of the conceptus is highly dependent on substances present in the uterine lumen. Uterine epithelia are present in the endometrium of all mammals [8], and their secretions constitute an important component of the histotroph, which is essential for preimplantation conceptus survival and development in sheep [9, 10]. Histotroph in the uterine lumen of cattle has been characterized [11–19] and is a complex mixture of amino acids, glucose, lipids, proteins, carbohydrates, vitamins, ions, cytokines, hormones, growth factors, among other substances. However, little is known about how different levels of specific constituents in the uterine lumen regulate pregnancy success in cattle.
Glucose is an essential nutrient for preimplantation conceptus development [20, 21] and increases in the uterine lumen during conceptus elongation in sheep [21]. The capacity of the uterus to generate free-glucose through gluconeogenesis is debatable [22–24], thus glucose transporters are thought to play an important role modulating intrauterine concentrations of glucose during pregnancy [20]. Mammalian cells express three families of glucose transporters, the facilitative glucose transporter family (solute carriers SLC2A/GLUT), the sodium/glucose cotransporter family (solute carriers SLC5A/SGLT) [25], and the recently discovered SWEET (SLC50) sugar transporter [26, 27]. The uterus expresses several members of these families [28], including SLC2A1, SLC5A1, and SLC5A11 whose expression increase in pregnant compared to cyclic endometrium in sheep [20].
Prostaglandins (PGs) are lipid hormones derived from arachidonic acid [29] and essential for conceptus development. In sheep, intrauterine infusion of meloxicam, a selective inhibitor of prostaglandin-endoperoxide synthase 2 (PTGS2), inhibited conceptus elongation [30]. Concentrations of PGs in the uterine lumen increase during early pregnancy in ruminants [20, 31], because both the conceptus and endometrium produce PGs, and endometrial production of PGs is further stimulated by interferon tau (IFNT) [32, 33], the signal for maternal recognition of pregnancy which is secreted predominantly by the trophectoderm of the elongating bovine conceptus after day 15 [34]. Classical signaling of PGs is through membrane-bound G protein-coupled receptors, and different types of PGs act through their specific receptor(s) and therefore regulate distinct biological processes [35, 36]. Nonetheless, select PGs can also activate nuclear receptors [37–39]. PGI2 signals through the peroxisome proliferator-activated receptor (PPAR) delta (PPARD) [40], and 15-deoxy-Δ12,14-PGJ2, a metabolite of PGD2, can activate PPAR gamma (PPARG) [41, 42]. PPARs are ligand-regulated transcription factors that upon heterodimerization with retinoid × receptor (RXR) and ligand binding regulate the expression of target genes and consequently cellular function [43].
Lipids are another important component present in the uterine lumen. In addition to being a precursor of eicosanoids [44], they play many critical roles in the body, serving as a source and storage for energy, providing substrates for membrane biogenesis, and acting as signal molecules [45]. The lipid content of the uterus fluctuates throughout the estrous cycle in several species [46], and the accumulation of lipid droplets in the endometrium luminal epithelium (LE) during the estrous cycle in sheep is attributed to progesterone from the ovarian corpus luteum [47–49]. This lipid accumulation has been hypothesized to serve as a reservoir to support increasing lipid demands of the endometrium and conceptus during early pregnancy [48, 50]. Progesterone increases the abundance of metabolites involved with membrane biogenesis in the uterine lumen during the onset of conceptus elongation in cattle [18] and the number of extracellular vesicles (EV) in the uterine luminal fluid during the period of conceptus elongation in sheep [51]. This increase in lipid availability during late diestrus is hypothesized to be important to support the growth of trophectoderm cells during conceptus elongation [52]. Fatty acids present in the uterine lumen likely participate in the crosstalk between the conceptus and endometrium during pregnancy, as saturated and unsaturated fatty acids can serve as ligands of PPAR receptors [43, 50, 53–55], which are expressed by both the bovine conceptus and endometrium [1, 56]. Although fatty acids are essential biological components, uterine lumen fatty acid composition remains poorly understood in ruminants.
To identify changes in the uterine lumen constituents associated with increased or reduced uterine capacity to support pregnancy, the current experiment utilized heifers that were previously fertility-classified as high fertile (HF; 100% pregnancy rate), subfertile (SF; 25–33% pregnancy rate), or infertile (IF; 0% pregnancy rate) using serial transfer of a single in vitro produced embryo (Grade 1) on day 7 followed by pregnancy determination on day 28 [1, 57]. Interestingly, conceptus development and survival on day 14 (7 days post-transfer) were not different among fertility-classified heifers, and only minimal differences in their endometrium transcriptome were observed on day 14 [57]. In a subsequent study, we found that on day 17 (10 days post-transfer), pregnancy rate was higher in HF (71%) and SF (90%) than in IF (20%) heifers. Day 17 conceptuses were longer in HF (mean 10.6 cm; range 1.2–32.2 cm) than in SF (mean 4.7 cm; range 1.5–13.5 cm) heifers, and the endometrial and conceptus transcriptomes were dysregulated in SF heifers [1]. The current study tested the hypothesis that specific histotroph constituents would be altered in the uterus of fertility-classified heifers with distinct uterine capacity to support pregnancy and be altered by pregnancy status. Because differences in endometrial transcriptome can translate into differences in the uterine lumen constituents [58], the focus of the current study was to investigate concentrations of glucose, PGs (PGE2, PGF2α, and 6-keto-PFG1α, a metabolite of PGI2 [59, 60]) and lipids in the uterine lumen from fertility-classified heifers.
Materials and methods
Animals
All animal procedures were conducted in accordance with the Guide for the Care and Use of Agriculture Animals in Research and Teaching and approved by the Institutional Animal Care and Use Committees of the USDA-ARS Fort Keogh Livestock and Range Research Laboratory and the University of Missouri. All fertility-classified heifers participating in the study were housed in the same pasture at the Beef Research and Teaching Farm of the University of Missouri and were subjected to the same management and diet.
Collection of the uterine luminal flush
Fertility-classified heifers (HF, n = 21; SF, n = 14; IF, n = 6) were synchronized to estrus (day 0) and received two in vivo-produced embryos on day 7. All heifers were slaughtered on day 17 (10 days post-transfer) at the University of Missouri slaughter facility, and reproductive tracts were collected within ~ 30 min of slaughter. Immediately after collection, the reproductive tracts were transported to the laboratory and the uterine lumen was gently flushed with 20 mL of sterile and filtered 1× PBS (pH 7.0). The conceptuses were removed, if present, the uterine luminal flush (ULF) clarified by centrifugation (3000×g at 4 °C for 15 min), and the supernatant was carefully removed with a pipette, mixed, divided into aliquots, frozen in liquid nitrogen, and stored at −80 °C until analyzed.
Glucose analysis
Concentrations of glucose in ULF and blood plasma were measured in samples collected from all the fertility-classified heifer enrolled in the study, which includes pregnant (HF, n = 15; SF, n = 9; IF, n = 1) and open (HF, n = 6; SF, n = 5; IF, n = 5) heifers. Glucose levels were determined using a glucose oxidase linked assay [61], that is based on the oxidation of glucose by glucose oxidase (Pointe Scientific, Canton, MI) forming gluconate and hydrogen peroxide, and H2O2 reacts with available phenol and 4-aminophenazone generating a quinoneimine dye that is measured at 500 nm. Briefly, each assay included a standard curve, and ULF (100 μL) and plasma (2 μL) samples were incubated at 37 °C with glucose oxidase (200 μL) for 45 and 15 min, respectively. Product formation was measured on a EnVision 2104 Multilabel plate reader (Perkin Elmer, Waltham, MA). Intra-assay coefficient of variation was 3.0 and 6.6% for ULF and plasma assays, respectively.
Statistical analyses of glucose levels in plasma and ULF were conducted in SAS (SAS Institute Inc., Cary, NC) by analysis of variance (ANOVA) using the General Linear Models (GLM) procedure. Post-test comparisons were conducted using the Least-Squares Means (LSMEANS) statement with the Fisher’s protected Least Significant Difference (LSD) option. Pearson’s correlation between glucose concentrations in plasma and in the ULF, as well as the correlation between conceptus size and glucose in the ULF, was determined using the CORR procedure. In all analyses conducted, conceptus length for heifers with two conceptuses was equal to the sum of both conceptuses present in the flush.
Prior to data analysis, glucose concentration was assessed for normality using the UNIVARIATE procedure, and both glucose in plasma and ULF were determined not normally distributed and therefore were log transformed for statistical analyses. Because glucose has been shown to increase in the ULF during conceptus elongation in sheep [21], we first tested whether pregnancy affected day 17 ULF glucose concentration in our combined dataset, which included samples from pregnant and open HF, SF, and IF heifers. The models used to test the effect of pregnancy on plasma and ULF glucose concentrations consisted of glucose levels as the depended variable and pregnancy status (pregnant vs. open) as the independent variable.
The effect of fertility group on glucose concentration was analyzed separated for pregnant and open heifers. Among pregnant heifers, the initial models used to test the effect of fertility classification on glucose levels were composed by ULF or plasma glucose concentrations as the dependent variable and fertility classification, conceptus number, conceptus length, the two-way interaction of fertility classification with conceptus number and conceptus length, and the three-way interaction of fertility classification, conceptus number, and conceptus length as independent variables. Nonsignificant (P > 0.05) variables were removed from the initial model by a manual backward stepwise elimination procedure. For ULF glucose, only the effect of fertility classification was retained in the final model. For plasma glucose, the variables retained in the final model were fertility classification, conceptus length, and the interaction of fertility group with conceptus length.
Among open heifers, the models used to test the effect of fertility classification on plasma and ULF glucose concentrations consisted of glucose levels as the depended variable and fertility classification (HF, SF, and IF) as the independent variable. Statistical significance was defined as P ≤ 0.05.
Eicosanoids analysis
The assay was conducted at the Eicosanoid Core Laboratory of Vanderbilt University on ULF samples from a subgroup of 25 heifers that were determined to be pregnant (HF, n = 5; SF, n = 5) or open (HF, n = 5; SF, n = 5; IF, n = 5) at day 17 slaughter. The selected 25 heifers used here are the same subgroup of animals which we have performed RNA sequencing (RNA-seq) of endometrial samples in a recent publication [1]. The selection of the pregnant heifers (HF, n = 5; SF, n = 5) used in these analyses was based on data of conceptus length and number, in an effort for selecting samples that represented well the overall data collected within each fertility group. For instance, in the complete data collected [1], 38.1% of HF heifers and 40% of SF heifers had two conceptuses, and the average conceptus length was 10.6 ± 7.6 cm (range: 1.2–32.2 cm) for HF heifers and 4.7 ± 4.2 cm (range: 1.5–13.5 cm) for SF heifers. In the selected subgroup of pregnant heifers, 2 HF and 3 SF heifers had two conceptuses, and the average conceptus size was 12.0 ± 8.1 cm (range: 1.3–25 cm) for HF and 6.4 ± 4.5 cm (range: 2.1–13.5 cm) for SF heifers.
To quantify PGs in the ULF, a total of 100 μL of each sample was placed in a microcentrifuge tube containing 25% methanol in water (500 μL) and internal standard (d4-PGE2 and d4-LTB4, 1 ng each). The sample was vortexed and then extracted on an Oasis MAX μElution plate (Waters Corp., Milford, MA) as follows. Sample wells were first washed with methanol (200 μL) followed by 25% methanol in water (200 μL). The sample was then loaded into the well and washed with 600 μL 25% methanol. Eicosanoids were eluted from the plate with 30 μL 2-propanol/acetonitrile (50/50, v/v) containing 5% formic acid into a 96-well elution plate containing 30 μL water in each well.
Samples were analyzed on a Waters Xevo TQ-S micro triple quadrupole mass spectrometer connected to a Waters Acquity I-Class UPLC (Waters Corp., Milford, MA). Separation of analytes was obtained using an Acquity pentafluorophenyl (PFP) column (2.1 × 100 mm) with mobile phase A being 0.01% formic acid in water and mobile phase B acetonitrile. Eicosanoids were separated using a gradient elution beginning with 30% B going to 95% B over 8 min at a flow rate of 0.250 mL/min.
Statistical analyses of PG concentrations in the ULF were conducted in SAS by ANOVA using the GLM procedure. Post-test comparisons were conducted using the LSMEANS statement with the Fisher’s protected LSD option. Among pregnant heifers, Pearson’s correlation between PG concentrations in the ULF and conceptus length was determined using the CORR procedure.
The models used to test the effect of pregnancy on PG concentrations in the ULF consisted of PGE2, PGF2α, or 6-keto-PFG1α as the depended variable and pregnancy status (pregnant vs. open) as the independent variable. Among pregnant heifers, a multivariate model that accounted for conceptus number and conceptus length was used to test the effect of fertility classification on ULF PG concentrations. The initial model was composed by PGE2, PGF2α, or 6-keto-PFG1α concentration as the dependent variable and fertility-classification, conceptus number, conceptus length, the two-way interaction of fertility classification with conceptus number and conceptus length, and the three-way interaction of fertility classification, conceptus number, and conceptus length as independent variables. Nonsignificant (P > 0.05) variables were removed from the initial model by a manual backward stepwise elimination procedure. For PGE2 and 6-keto-PFG1α, only the effect of fertility group was retained in the reduced model. For PGF2α, the effects of fertility group and conceptus number were retained in the reduced model.
Among open heifers, the models used to test the effect of fertility classification on ULF concentrations of PG consisted of PGE2, PGF2α, or 6-keto-PFG1α levels as the depended variable and fertility classification (HF, SF and IF) as the independent variable. Statistical significance was defined as P ≤ 0.05 and statistical tendencies 0.05 < P ≤ 0.10.
Fatty acids analysis
Total fatty acid analysis was conducted by high-resolution mass spectrometry at the Southeast Center for Integrated Metabolomics (SECIM) of the University of Florida on ULF samples collected from the same subgroup of 25 heifers described in the eicosanoid analysis. Unless otherwise stated, all reagents used were of Liquid chromatography–mass spectrometry (LC-MS) grade and obtained from Fisher Scientific (Fairlawn, NJ). A sample of 100 mg of ULF was weighed into a conical glass tube (5 mL volume) and 1 mL of acetonitrile containing 100 mg/L of butylated hydroxytoluene was added, followed by the addition of 0.5 mL of hydrochloric acid (37%). The sample was sonicated for 5 min and heated between 80 and 90 °C for 2 h in a heating block. The addition of hydrochloric acid and heating causes hydrolysis to release fatty acids from complex lipids. After cooling at room temperature, 2 mL of hexane was added, and the sample was centrifuged for 1 min at 3260×g. The top layer (1.5 mL) was removed, transferred to a clean glass culture tube, and dried under a gentle stream of nitrogen. The dried sample was reconstituted in 0.5 mL of 80/20 acetonitrile/5 mM ammonium acetate, and 10 μL of the injection standard mixture (DHA-D5, EPA-D5, α-LA-D14) was added to the reconstituted sample. The final sample was transferred to an LC vial for LC-MS analysis.
Fatty acid analysis was performed on a Thermo Q-Exactive Orbitrap Mass Spectrometry with Dionex Ultimate 3000 UHPLC and autosampler. Separation was achieved on a Waters HSS T3 column (150 × 2.1 mm, 1.8 μm) at a flow rate of 0.5 mL/min and column temperature of 30 °C with mobile phases A and B consisting of 1 mM ammonium acetate in water and 0.1% acetic acid in acetonitrile, respectively. Gradient elution started at 25% A and 75% B from 0 to 0.5 min with a linear increase to 90% B from 0.5 to 7 min, a further increase to 95% B from 7 to 8 min, and then held constant at 95% B from 8 to 21 min. The column was returned to initial conditions in 0.5 min and equilibrated for 4.5 min. The mass spectrometry was operated in negative heated electrospray ionization (HESI) mode with a resolution setting of 70 000 at m/z 200, collecting m/z 100–700. Data-dependent analysis was conducted on the top five most abundant peaks throughout the run with a resolution setting of 17 500, ion time of 75 ms, and collision-induced dissociation of 30, 50, and 70. The HESI settings were 3 kV, 100 °C probe temperature, 300 °C capillary temperature, 40 arb sheath gas, 5 arb auxiliary gas, and 1 arb sweep gas. Fatty acids were identified by accurate mass (<10 ppm accuracy), retention time, and tandem mass spectrometry.
Statistical analyses of ULF fatty acid data were conducted in SAS by ANOVA using the GLM procedure. Post-test comparisons were conducted using the LSMEANS statement with the Fisher’s protected LSD option. The models used to test the effect of pregnancy on ULF fatty acid abundance consisted of fatty acid peak area (Supplementary Dataset S5) as the depended variable and pregnancy status (pregnant vs. open) as the independent variable. Among pregnant heifers, the initial models used to test the effect of fertility classification on ULF fatty acid profile were composed by fatty acid data as the dependent variable and fertility classification, conceptus number, conceptus length, the two-way interaction of fertility classification with conceptus number and conceptus length, and the three-way interaction of fertility classification, conceptus number, and conceptus length as independent variables. Nonsignificant (P > 0.05) variables were removed from the initial model by a manual backward stepwise elimination procedure. The variables retained in the reduced models used to analyze ULF fatty acid data of pregnant heifers are described in Results section. Among open heifers, the models used to test the effect of fertility classification on ULF fatty acid profile consisted of fatty acid peak area as the depended variable and fertility classification (HF, SF and IF) as the independent variable. Statistical significance was defined as P ≤ 0.05.
Untargeted lipidomic analysis
To investigate changes in ULF lipidome that are normally induced by pregnancy, global lipidomics was conducted exclusively on ULF samples of HF heifers that were open (n = 5) or pregnant (n = 5). Lipidomic analysis was performed on the same subgroup of HF heifers which ULF samples were analyzed for PGs and fatty acids.
The assay was conducted at the SECIM of the University of Florida. Samples were extracted following a cellular extraction procedure without prenormalization to the sample protein content. Global lipidomics profiling was performed on a Thermo Q-Exactive Orbitrap mass spectrometer with Dionex UHPLC and autosampler. All samples were analyzed in positive and negative HESI with a mass resolution of 35 000 at m/z 200 as separate injections. Separation was achieved on an Acquity BEH C18 1.7 μm, 100 × 2.1 mm column with mobile phase A as 60:40 Acetonitrile:10 mM Ammonium formate with 0.1% formic acid in water and mobile phase B as 90:8:2 2-propanol:acetonitrile:10 mM ammonium formate with 0.1% formic acid in water. The flow rate was 500 μL/min with a column temperature of 50 °C with 5 μL was injected for negative ions and 3 μL for positive ions.
Data from positive and negative ion modes were separately analyzed using LipidMatch software. First, all MS2 raw files were converted to “ms2” and MS raw files to “MzXML” using MSConvert. A peak list was generated after running MzMine on all MzXML files. An input folder that included all “ms2” files and the peak list was used to run LipidMatch to identify features.
Statistical analysis of the lipidomics data was performed on MetaboAnalyst 4.0 [62–64]. A table matrix of m/z peak intensities with samples in columns and features in rows was created and imported to MetaboAnalyst 4.0, and data preprocessing consisted of autoscaling normalization [65]. Fold change (FC) analysis was conducted to identify features that increased or decreased in the ULF of HF heifers with pregnancy, and the standard FC threshold of 2 was used. Additionally, a t-test was used to investigate if features were differently (P < 0.05) abundant in the ULF of HF heifers that were pregnant or open.
Integration of uterine luminal content and endometrium and conceptus transcriptome
To further investigate the biology of subfertility, we integrated the present ULF data with transcriptome data from endometrium and conceptuses, which have been performed in the same group of animals and recently published [1]. The gene expression data generated in our previous publication [available in Gene Expression Omnibus database under the accession number GSE107891] were reanalyzed to address comparisons not performed in the original work. Differential gene expression analysis was conducted using edgeR-robust [66], and a false discovery rate (FDR) of <0.05 was used as the cutoff for determining the differently expressed genes (DEGs). After a list of DEG was generated for each comparison of interest, we use the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.genome.jp/kegg/) to verify if the genes associated with glucose, PGs, and lipid metabolism were differently express in our transcriptome data.
Relationship of endometrium transcriptome and uterine luminal flush composition
Two analyses were conducted to integrate data of ULF components and endometrium transcriptome. (1) The first one investigated the effect of pregnancy on endometrial expression of genes associated with synthesis and metabolism of glucose, PGs and lipids. This analysis was performed on endometrial transcriptome data generated from the same subgroup of pregnant (HF, n = 5; SF, n = 5) and open (HF, n = 5; SF, n = 5) heifers selected for ULF analyzes. The model used to test the effect of pregnancy on endometrial gene expression was composed by gene expression data as the dependent variable and pregnancy status (pregnant vs. open) as the independent variable. (2) The second analysis investigated endometrial expression of the genes of interest only in the subgroup of pregnant heifers (HF, n = 5; SF, n = 5) selected for ULF analyzes. One of the most important finding of our previous work was that the endometrial response to pregnancy was dysregulated in SF heifers [1]. Thus, this analysis aimed to explore if the diminished endometrial response to pregnancy observed in SF heifers affected the synthesis and metabolism of the ULF components of interested, as differences might provide insights on the biological mechanisms involved with pregnancy loss in SF heifers. The model used to test the effect of fertility group on endometrial gene expression of pregnant heifers was composed by gene expression data as the dependent variable and fertility group (HF vs. SF) as the independent variable.
Relationship of conceptus transcriptome and uterine luminal flush composition
A similar approach was used to investigate the relationship of ULF composition and the conceptus transcriptome. Because there is natural variation in conceptus length among conceptuses collected in the same day during the period of conceptus elongation in cattle [6, 56, 57, 67, 68] and the conceptus transcriptome changes as it develops [56], we first analyzed day 17 conceptus transcriptome data from HF (n = 15) and SF heifers (n = 7) that were either short (n = 11; mean length: 2.5 ± 0.4 cm) or long (n = 11; mean length: 14.5 ± 1.9 cm) to explore differences in the transcriptome of conceptuses that were likely due to stage of development. The model used to test the effect of conceptus length on conceptus transcriptome was composed by gene expression data as the dependent variable and category of conceptus size (short vs. long) as the independent variable. Then, the transcriptome of HF (n = 17) and SF (n = 10) conceptuses was compared for the same set of genes of interest to investigate the influence of ULF composition on conceptus transcriptome, in order to explore the mechanisms associated to the retarded growth of SF conceptuses and reduced pregnancy success in SF heifers. The model used to test the effect of fertility classification on conceptus transcriptome was composed by gene expression data as the dependent variable and fertility group (HF vs. SF) as the independent variable.
Results
Glucose concentrations in uterine luminal flush
Glucose concentrations in ULF were higher (P = 0.05) in pregnant compared to open heifers on day 17 (Figure 1A). Among pregnant heifers, there was no effect (P = 0.88) of fertility classification on ULF glucose concentrations (Figure 1B). Furthermore, ULF glucose did not differ (P = 0.66) among open fertility-classified heifers (Figure 1C).
Figure 1. Glucose concentrations in ULF were higher (P = 0.05) in pregnant compared to open heifers on day 17 (A). Among pregnant heifers, there was no effect (P = 0.88) of fertility classification on ULF glucose concentrations (B). ULF glucose did not differ (P = 0.66) among open fertility-classified heifers (C). Glucose in plasma was not different (P = 0.20) between pregnant and open heifers (D). Among pregnant heifers, plasma glucose was affected by the interaction between fertility group and conceptus length (P = 0.02; E). Among the open animals, IF heifers had higher (P = 0.04) plasma glucose concentrations compared to HF heifers (F).
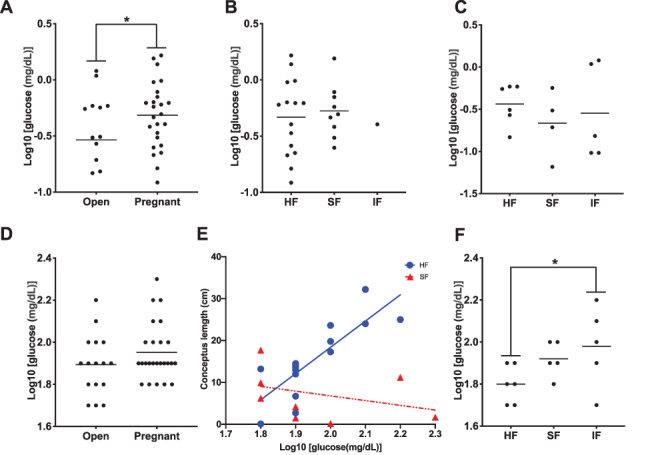
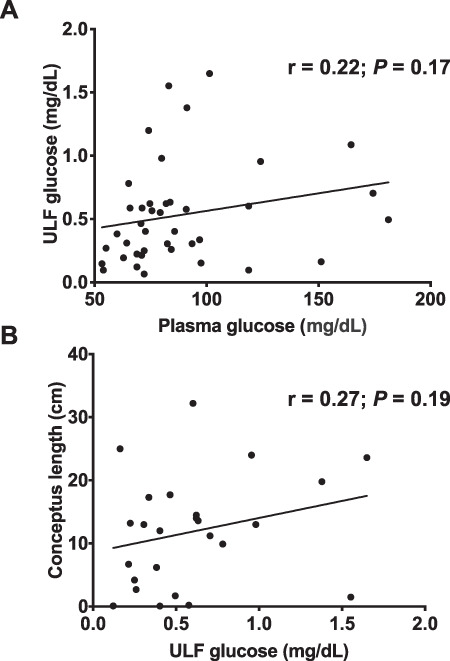
Circulating concentrations of glucose in plasma were not different (P = 0.20) between pregnant and open heifers (Figure 1D). Among pregnant heifers, plasma glucose was affected (P = 0.02) by the interaction of fertility group and conceptus length, as plasma glucose was positively correlated with conceptus length in HF heifers but not in SF heifers (Figure 1E). Among the open animals, IF heifers had higher (P = 0.04) plasma glucose concentrations than HF heifers (Figure 1F). Furthermore, ULF and plasma glucose concentrations were not correlated (Figure 2A; r = 0.22; P = 0.17), and there was no significant correlation (r = 0.27; P = 0.19) between conceptus length and concentrations of glucose in the ULF (Figure 2B).
Figure 2. Pearson correlation between glucose concentrations in plasma and in the ULF (A) and between conceptus size and glucose in the ULF (B).
Transcriptome analysis of endometrium and conceptuses for glucose transport and metabolism genes
Expression of genes encoding glucose transporters that increased in pregnant compared to open endometrium (SLC2A1, SLC5A1, SLC5A11, SLC35D2, and SLC5A4) was not different (FDR P ≥ 0.68) between pregnant HF and SF endometrium (Table 1). Interestingly, expression of other glucose transporters (SLC2A10, SLC2A3 and SLC37A4) decreased (FDR < 0.05) with pregnancy, but their expression was also not different (FDR P ≥ 0.65) in pregnant endometrium of HF and SF heifers (Table 1).
Table 1. Expression of genes in the endometrium involved with glucose transport.
| Gene name | Gene description | Pregnant vs. open (HF and SF) | Only pregnant HF vs. SF | |||||
|---|---|---|---|---|---|---|---|---|
| FPKMa Open | FPKMa Pregnant | FDR | FPKMa HF (P) | FPKMa SF (P) | FDR | |||
| SLC2A1 | Solute carrier family 2 (facilitated glucose transporter), member 1 | 68 ± 11 | 153 ± 19 | 0.00 | 174 ± 35 | 132 ± 13 | 0.68 | |
| Increased in pregnant | SLC5A1 | Solute carrier family 5 (sodium/glucose cotransporter), member 1 | 21 ± 3 | 35 ± 3 | 0.00 | 31 ± 5 | 38 ± 4 | 0.90 |
| Endometrium | SLC5A11 | Solute carrier family 5 (sodium/inositol cotransporter), member 11 | 0.02 ± 0.1 | 1 ± 0.1 | 0.02 | 1.2 ± 0.3 | 0.9 ± 0.1 | 0.94 |
| SLC35D2 | Solute carrier family 35 (UDP-GlcNAc/UDP-glucose transporter), member D2 | 12 ± 1 | 15 ± 1 | 0.04 | 15 ± 1 | 15 ± 1 | 1.00 | |
| Decreased in pregnant | SLC2A10 | Solute carrier family 2 (facilitated glucose transporter), member 10 | 1 ± 0.1 | 0.5 ± 0.1 | 0.00 | 0.6 ± 0.1 | 0.5 ± 0.0 | 1.00 |
| Endometrium | SLC2A3 | Solute carrier family 2 (facilitated glucose transporter), member 3 | 8 ± 0.5 | 5 ± 1 | 0.00 | 5 ± 1 | 6 ± 1 | 0.65 |
| SLC37A4 | Solute carrier family 37 (glucose-6-phosphate transporter), member 4 | 7 ± 0.2 | 6 ± 0.2 | 0.04 | 5 ± 0.3 | 6 ± 0.4 | 0.88 | |
aData are presented as FPKM ± standard error of the mean (SEM).
No differences were observed in conceptus transcripts encoding glucose transporters between short (mean length: 2.5 ± 0.4 cm) and long conceptuses (mean length: 14.5 ± 1.9 cm), and the five most abundant glucose transporters on day 17 conceptuses were SLC2A1, SLC2A5, SLC37A1, SLC2A8, and SLC2A13 (Supplementary Dataset S1). Interestingly, expression of SLC2A2 (GLUT2) and SLC2A4 (GLUT4) was higher in SF than in HF conceptuses (Supplementary Dataset S1).
For genes involved with glycolysis and gluconeogenesis according to the KEGG database, expression of 13 genes increased and 3 decreased in pregnant compared to open endometrium (Supplementary Dataset S2 and Table 2), and expression of one gene (FBP1, fructose-bisphosphatase 1) was increased in pregnant endometrium of HF compared to SF heifers (Supplementary Dataset S2 and Table 2). None of the selected genes involved with glycolysis and gluconeogenesis were differently expressed in short vs. long conceptuses (Supplementary Dataset S2), but expression of hexokinase 1 (HK1), FBP1, and galactose mutarotase (GALM) was increased in HF conceptuses and aldolase, fructose-bisphosphate B (ALDOB) and aldehyde dehydrogenase 3 family, member B1 (ALDH3B1) was increased in SF conceptuses (Supplementary Dataset S2 and Table 3).
Table 2. Differently expressed genes in the endometrium by pregnancy and between fertility-classified heifers for genes involved with glycolysis and gluconeogenesis.
| Comparisons | Gene name | Gene description | FPKMa | FPKMa | FDR |
|---|---|---|---|---|---|
| Pregnant vs. open | Open | Pregnant | |||
| LDHB | Lactate dehydrogenase B | 125 ± 5 | 101 ± 2 | 0.04 | |
| Increased in open | LDHA | Lactate dehydrogenase A | 21 ± 2 | 13 ± 1 | 0.00 |
| PFKM | Phosphofructokinase, muscle | 8 ± 0.4 | 5 ± 0.3 | 0.00 | |
| HK2 | Hexokinase 2 | 1 ± 0.3 | 2 ± 0.2 | 0.01 | |
| ALDOB | Aldolase, fructose-bisphosphate B | 1 ± 0.1 | 2 ± 0.4 | 0.00 | |
| ADPGK | ADP-dependent glucokinase | 9 ± 0.3 | 11 ± 0.5 | 0.00 | |
| PCK2 | Phosphoenolpyruvate carboxykinase 2 (mitochondrial) | 2 ± 0.1 | 5 ± 0.4 | 0.00 | |
| Increased in pregnant | PFKP | Phosphofructokinase, platelet | 11 ± 0.4 | 16 ± 0.9 | 0.00 |
| FBP1 | Fructose-bisphosphatase 1 | 4 ± 0.4 | 12 ± 3.7 | 0.02 | |
| HKDC1 | Hexokinase domain containing 1 | 12 ± 2 | 22 ± 3 | 0.00 | |
| ACSS2 | Acyl-CoA synthetase short-chain family member 2 | 12 ± 1 | 23 ± 2 | 0.00 | |
| AKR1A1 | Aldo-keto reductase family 1, member A1 (aldehyde reductase) | 30 ± 1 | 44 ± 5 | 0.00 | |
| ENO1 | Enolase 1 (alpha) | 95 ± 2 | 110 ± 5 | 0.01 | |
| PGK1 | Phosphoglycerate kinase 1 | 61 ± 2 | 85 ± 5 | 0.00 | |
| ACSS1 | Acyl-CoA synthetase short-chain family member 1 | 71 ± 7 | 106 ± 4 | 0.00 | |
| Only pregnant | SF | HF | |||
| HF vs. SF | FBP1 | Fructose-bisphosphatase 1 | 7 ± 4 | 16 ± 6 | 0.00 |
aData is presented as FPKM ± SEM.
Table 3. Expression of select genes involved with glycolysis and gluconeogenesis in conceptuses from fertility-classified heifers.
| Comparisons | Gene name | Gene description | FPKMa | FPKMa | FDR |
|---|---|---|---|---|---|
| Short conceptus | Long conceptus | ||||
| Short vs Long | – | – | – | – | – |
| HF vs. SF conceptuses | SF conceptus | HF conceptus | |||
| Increased in HF | HK1 | Hexokinase 1 | 9 ± 1 | 19 ± 3 | 0.01 |
| FBP1 | Fructose-bisphosphatase 1 | 0.4 ± 0.1 | 1.4 ± 0.4 | 0.02 | |
| GALM | Galactose mutarotase (aldose 1-epimerase) | 69 ± 14 | 207 ± 51 | 0.05 | |
| Increased in SF | ALDOB | Aldolase, fructose-bisphosphate B | 1.2 ± 0.4 | 0.4 ± 0.1 | 0.04 |
| ALDH3B1 | Aldehyde dehydrogenase 3 family member B1 | 2 ± 0.2 | 1 ± 0.1 | 0.04 |
aData are presented as FPKM ± SEM.
Prostaglandins in the uterine luminal flush
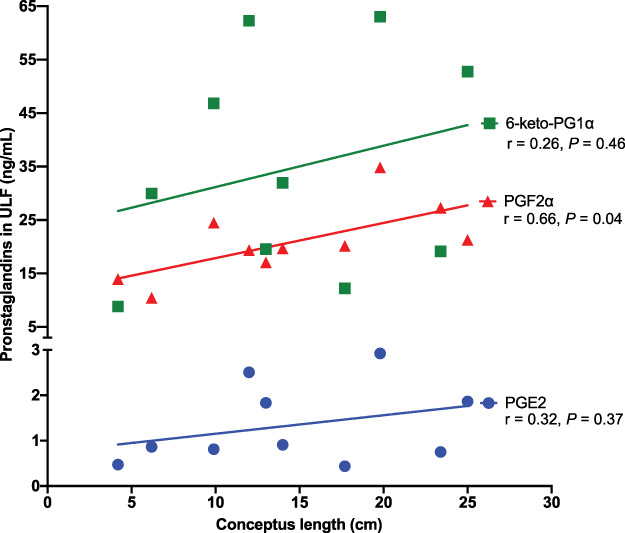
Pregnant heifers tended (P = 0.07) and had (P < 0.01) greater concentrations of PGE2 and PGF2α in the ULF than open heifers (Figure 3A and D, respectively). However, ULF concentrations of 6-keto-PFG1α was not affected (P = 0.18) by pregnancy status (Figure 3G). Although 6-keto-PFG1α concentrations did not differ between pregnant and open SF heifers (P = 0.61; Open = 29.7 ± 8.3 ng/mL, Pregnant = 23.5 ± 8.3 ng/mL), 6-keto-PFG1α was increased by 5.4 fold in the ULF of pregnant than open HF heifers (P < 0.01; Open = 8.5 ± 6.4 ng/mL, Pregnant = 45.8 ± 6.4 ng/mL). Among pregnant heifers, ULF concentrations of PGF2α (Figure 3E) was affected by fertility group (P = 0.01; HF = 25.31 ± 1.91; SF = 16.36 ± 1.91) and conceptus number (P = 0.02; one conceptus = 16.65 ± 1.91; two conceptuses = 25.02 ± 1.91). Additionally, concentrations of PGF2α in ULF was significantly correlated with conceptus length (r = 0.66, P = 0.04; Figure 4). Furthermore, ULF of HF pregnant heifers tended to have greater concentrations of PGE2 (P = 0.10; Figure 3B) and 6-keto-PFG1α (P = 0.08; Figure 3H) than ULF of pregnant SF heifers. Nonetheless, there was no significant correlation between PGE2 (r = 0.32, P = 0.37) and 6-keto-PFG1α (r = 0.26, P = 0.46) with conceptus length (Figure 4). Among open heifers, there was no effect (P ≥ 0.32) of fertility group on ULF PG concentrations (Figure 3C, F, and I).
Figure 3. Concentrations of PGE2 tended (P = 0.07) to be higher in ULF of pregnant compared to open heifers (A). Pregnant heifers had (P < 0.01) greater concentrations of PGF2α in the ULF compared to open heifers (D). ULF concentration of 6-keto-PFG1α was not affected (P = 0.18) by pregnancy status (G). Among pregnant heifers, concentration of PGF2α was higher (P = 0.01) in ULF of HF compared to SF heifers (E). Furthermore, ULF of HF pregnant heifers tended to have greater concentrations of PGE2 (P = 0.10; B) and 6-keto-PFG1α (P = 0.08; H) than ULF of pregnant SF heifers. Among open heifers, there was no effect (P ≥ 0.32) of fertility group on ULF PG concentrations (C, F, and I).
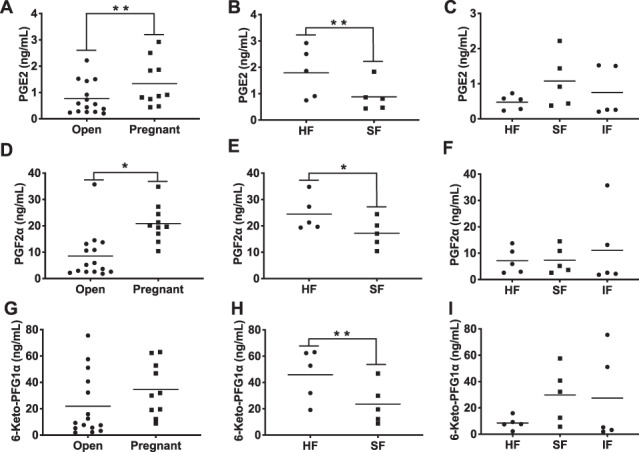
Figure 4. Pearson correlations between ULF PGs (PGE2, PGF2α, 6-keto-PFG1α) and conceptus length.
Transcriptome analysis of endometrium and conceptuses for prostaglandin signaling, arachidonic acid metabolism, and peroxisome proliferator-activated receptor signaling genes
Expression of selected genes involved with PG signaling by day 17 endometrium and conceptuses is summarized in Table 4. Expression of selected genes encoding PG synthases did not differ in the endometrium of pregnant HF vs. SF heifers or between conceptuses collected from HF or SF heifers. Expression of prostaglandin reductase (PTGR)1, PTGR3, prostaglandin F receptor (PTGFR), prostaglandin I2 receptor (PTGIR) and PPARG was very low [fragments per kilobase of transcript per million mapped reads (FPKM) < 1] in the endometrium. Among conceptuses, PGF receptor (PTGFR) expression was higher in HF than in SF conceptuses, and PTGFR was the overall highest PG receptor expressed by the conceptuses. Conceptus expression of prostaglandin E receptor (PTGER)1, PTGER2, PTGER3, PTGER4, PTGIR, and PPARA was very low (FPKM < 1). Additionally, the overall expression of PPAR receptors by the endometrium or conceptuses did not differ in all comparisons. Interestingly, the expression of the PG transporter SLCO2A1 was higher in HF than in SF conceptuses, but no differences were observed among short and long conceptuses or in the endometrium (Table 4).
Table 4. Expression of selected genes involved with PG synthesis or signaling in endometrium and conceptuses.
| Gene name | Gene description | HF vs. SF pregnant endometrium | HF vs. SF conceptus | ||||
|---|---|---|---|---|---|---|---|
| PG synthase | FPKMa HF (P) | FPKMa SF (P) | FDR | FPKMa HF | FPKMa SF | FDR | |
| PTGS2 | Prostaglandin-synthase 2 | 33 ± 4 | 40 ± 5 | 0.86 | 1071 ± 111 | 666 ± 95 | 0.13 |
| PTGES | Prostaglandin E synthase | 2 ± 0.5 | 2 ± 0.2 | 1.00 | 14 ± 2 | 18 ± 3 | 0.35 |
| PTGFS | Prostaglandin F synthase | 7 ± 6 | 2 ± 1 | 0.19 | 0.2 ± 0.1 | 0.5 ± 0.3 | 0.43 |
| PTGIS | Prostaglandin I2 synthase | 3 ± 0.4 | 4 ± 0.2 | 0.86 | 16 ± 1 | 12 ± 2 | 0.19 |
| Receptors | Open vs pregnant endometrium | HF vs SF pregnant endometrium | |||||
| Endometrium | FPKMa Open | FPKMa Preg. | FDR | FPKMa HF (P) | FPKMa SF (P) | FDR | |
| PTGER2 | Prostaglandin E receptor 2 | 5 ± 0.3 | 5 ± 0.4 | 0.29 | 6 ± 0.6 | 5 ± 0.4 | 0.90 |
| PTGER4 | Prostaglandin E receptor 4 | 3 ± 0.2 | 3 ± 0.2 | 0.06 | 2 ± 0.2 | 3 ± 0.3 | 0.59 |
| PPARA | Peroxisome proliferator-activated receptor alpha | 17 ± 1 | 15 ± 1 | 0.30 | 15 ± 1 | 15 ± 1 | 1.00 |
| PPARD | Peroxisome proliferator-activated receptor delta | 8 ± 0.2 | 8 ± 0.4 | 0.49 | 8 ± 0.5 | 8 ± 0.7 | 0.92 |
| Receptors | Long vs. short conceptus | HF vs. SF conceptus | |||||
| Conceptus | FPKMa Long | FPKMa Short | FDR | FPKMa HF | FPKMa SF | FDR | |
| PTGFR | Prostaglandin F receptor (Fp) | 4 ± 1 | 2 ± 1 | 0.18 | 4 ± 0.9 | 1 ± 0.2 | 0.04 |
| PPARD | Peroxisome proliferator-activated receptor delta | 5 ± 1 | 6 ± 1 | 0.73 | 6 ± 0.6 | 4 ± 0.4 | 0.07 |
| PPARG | Peroxisome proliferator-activated receptor gamma | 3 ± 0.3 | 2 ± 0.3 | 0.48 | 3 ± 0.2 | 2 ± 0.3 | 0.10 |
| Endometrium | Pregnant vs. open endometrium | HF vs SF pregnant endometrium | |||||
| PG transporter | FPKMa open | FPKMa pregnant | FDR | FPKMa HF (P) | FPKMa SF (P) | FDR | |
| SLCO2A1 | Solute carrier organic anion transporter family member 2A1 | 3 ± 0.3 | 3 ± 0.3 | 1.00 | 3 ± 0.5 | 3 ± 0.1 | 0.95 |
| Conceptus | Long vs. short conceptus | HF vs. SF conceptus | |||||
| PG transporter | FPKMa Long | FPKMa Short | FDR | FPKMa HF | FPKMa SF | FDR | |
| SLCO2A1 | Solute carrier organic anion transporter family member 2A1 | 7 ± 2 | 5 ± 1 | 0.63 | 7 ± 1 | 3 ± 1 | 0.02 |
aData are presented as FPKM ± SEM.
Expression of genes involved with arachidonic acid metabolism differed between pregnant and open endometrium. Expression of seven genes were increased in open endometrium [cytochrome P450 family 2 subfamily C member 18 (CYP2C18), arachidonate 15-lipoxygenase type B (ALOX15B), prostaglandin D2 synthase (PTGDS), prostaglandin I2 synthase (PTGIS), phospholipase A2 group IVB (PLA2G4B), ALOX15, and CYP2U1] and 10 genes increased in pregnant endometrium [CYP2J2, phospholipase A1 member A (PLA1A), ALOX12, carbonyl reductase 3 (CBR3), PLA2G3, hematopoietic prostaglandin D synthase (HPGDS), LOC506594 (prostaglandin F synthase 1-like), prostaglandin E synthase 3 (PTGES3), CBR1, and phospholipase A2 group IIA (PLA2G2A-2)] (Supplementary Dataset S3). However, none of the genes associated with arachidonic acid metabolism were differently expressed in endometrium of pregnant HF and SF heifers (Supplementary Dataset S3). In conceptuses, expression of only one gene (LOC506594; prostaglandin F synthase 1-like) was increased in short compared to long conceptuses (Supplementary Dataset S3), and expression of one gene (PLA2G15) increased and three (PLA2G1B, glutathione peroxidase 1 (GPX)1 and CBR1) decreased in HF compared to SF conceptuses (Supplementary Dataset S3).
Among genes involved with PPAR signaling, expression of 11 genes [phosphoenolpyruvate carboxykinase 2, mitochondrial (PCK2), perilipin 2 (PLIN2), acyl-CoA synthetase long chain family member 5 (ACSL5), fatty acid binding protein 3 (FABP3), stearoyl-CoA desaturase (SCD), oxidized low density lipoprotein receptor 1 (OLR1), ubiquitin C (UBC), SLC27A5, fatty acid desaturase 2 (FADS2), SLC27A6, and ACSL4] increased and 5 decreased [FABP7, phospholipid transfer protein (PLTP), stearoyl-CoA desaturase 5 (SCD5), RXRA, and carnitine palmitoyltransferase 1C (CPT1C)] in pregnant compared to open endometrium (Supplementary Dataset S4). However, expression of only one gene differed between pregnant HF vs. SF endometrium, as expression of FABP3 was increased by twofold in the endometrium of pregnant HF heifers (Supplementary Dataset S4).
In conceptuses, expression of one gene (FADS2) in the PPAR signaling pathway increased in long compared to short conceptuses (Supplementary Dataset S4) and expression of one gene increased (PDPK1; 3-phosphoinositide dependent protein kinase 1) and 6 decreased [acyl-CoA dehydrogenase long chain (ACADL), acyl-CoA dehydrogenase medium chain (ACADM), apolipoprotein A5 (APOA5), FABP3, lipoprotein lipase (LPL), and sorbin and SH3 domain containing 1 (SORBS1)] in HF compared to SF conceptuses (Supplementary Dataset S4).
Fatty acids in the uterine luminal flush
A total of 37 fatty acids were identified in the ULF of the fertility-classified heifers, and their concentrations in pregnant and open ULF are presented in Figure 5A. Two saturated fatty acids, palmitic acid (16:0) and stearic acid (18:0), were the predominant fatty acids detected in the ULF of both open and pregnant heifers (Figure 5A), accounting for about 90% of the total fatty acid content detected in the ULF (Figure 5B and C; Supplementary Dataset S5). The percentage of the subsequent most predominant fatty acids in the ULF relative to total fatty acid content for both open and pregnant heifers were 3.1% octacosaheptaenoic (28:7), 1.9% octacosaoctaenoic acid (28:8), 1.2% arachidate (20:0), 0.8% Behenate (22:0 and 18:1), 0.7% myristate (14:0), and 0.6% margaric (17:0) (Figure 5B and C). The remaining 28 fatty acids together accounted for only around 1.1% of the total fatty acids detected in ULF (Supplementary Dataset S5). A heatmap for fatty acid concentration in the ULF across samples is presented in Figure 5D. Surprisingly, there was no difference in fatty acid content between ULF from open and pregnant heifers. When HF and SF heifers were analyzed separately, the fatty acid content that was influenced by pregnancy was not consistent in HF and SF ULF (Table 5).
Figure 5. Concentrations of fatty acids identified in the ULF of the fertility-classified heifers (A). Proportion of the nine most abundant fatty acids in the ULF relative to the total fatty acid content detected for open (B) and pregnant (C) fertility-classified heifers. Heatmap (D) for fatty acid concentration (molar) in the uterine lumen flush (ULF) across samples.
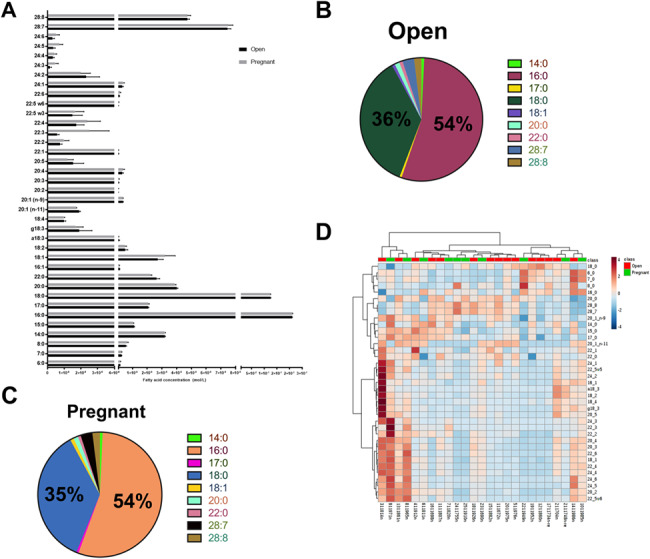
Table 5. Fatty acid differences in the ULF of fertility-classified heifers.
| Comparisons | Significantly different fatty acids in ULF | |
|---|---|---|
| Pregnant vs. open | None | – |
| HF pregnant vs. open | 226, 20:0, 20:1 n-11 | Higher in open than in pregnant |
| SF pregnant vs open | 24:3 | Higher in pregnant than in open |
| Only pregnant HF vs. SF | 15:0, 17:0, 24:2, 18:1, 22:5w6, 22:4, 22:2, 20:3, 20:2, 28:7, 28:8 | Higher in HF than in SF |
| 8:0, 6:0, 20:0 | Higher in SF than in HF | |
| Only open HF vs. SF vs. IF | 22:1 | Higher in HF than in SF |
| 22:0, 24:1 | Higher in HF than in SF and IF | |
In the comparison of only pregnant HF and SF heifers, concentrations of 11 fatty acids increased and 3 decreased in the ULF of HF compared to SF heifers (Table 5). Among the fatty acids that increased in the ULF of pregnant HF heifers, concentrations of 17:0, 28:7, and 28:0 were also affected by conceptus number (P < 0.01, P = 0.01, and P = 0.01; Figure 6A), by the interaction between conceptus number and fertility group (P < 0.01, P = 0.01, and P = 0.01), by the two-way interaction of conceptus length and fertility group (P < 0.01, P < 0.01, and P < 0.01), and by the three-way interaction of conceptus number, conceptus length, and fertility group (P < 0.01, P = 0.01, and P = 0.01; Figure 6B–D).
Figure 6. Description of the significant independent variables retained in the multivariable model investigating the effect of fertility classification on ULF fatty acid profile of pregnant heifers. For fatty acids that increased in the ULF of pregnant HF compared to SF heifers, conceptus number affected ULF concentrations of 17:0 (P < 0.01), 28:7 (P = 0.01), and 28:0 (P = 0.01) fatty acids, as heifers with two conceptuses had greater levels of these fatty acids than heifers with a single conceptus (A). Additionally, ULF concentration of 17:0, 28:7, and 28:0 fatty acids was affected by the interaction between conceptus number and fertility group (P < 0.01, P = 0.01, and P = 0.01), by the two-way interaction of conceptus length and fertility group (P < 0.01, P < 0.01, and P < 0.01), and by the three-way interactions of conceptus number, length, and fertility group (P < 0.01, P = 0.01, and P = 0.01; B–D). For fatty acids that increased in the ULF of pregnant SF than HF heifers, concentration of 20:0 was affected by the two-way interaction of conceptus number and fertility group (P = 0.01; E), and ULF concentration of 6:0 was affected by conceptus number (P < 0.01; one = 1.79 × 106 ± 3.84 × 103; two = 1.66 × 106 ± 8.15 × 103), by conceptus length (P < 0.01; F), by the two-way interaction of conceptus length and fertility group (P < 0.01), and by the three-way interaction of conceptus number, length, and fertility group (P < 0.01; G).
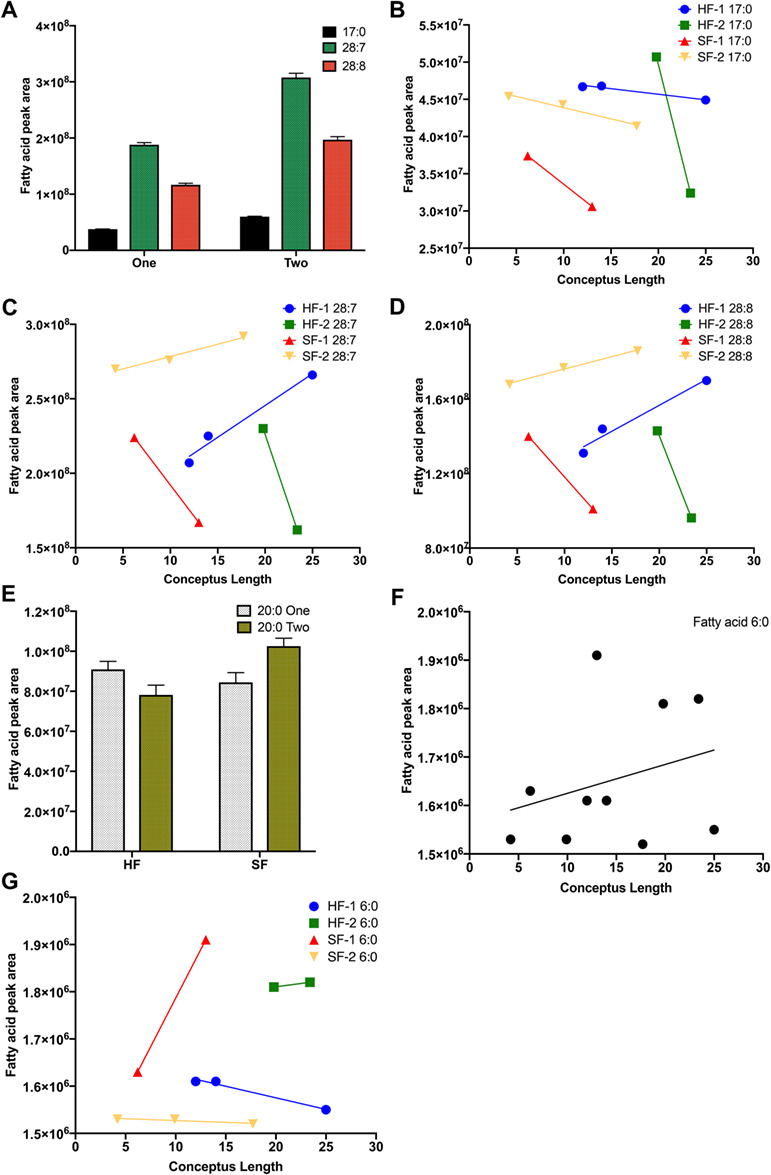
Furthermore, among the fatty acids that increased in the ULF of pregnant SF than HF heifers, concentration of 20:0 was affected by the two-way interaction of conceptus number and fertility group (P = 0.01; Figure 6E), and ULF concentration of 6:0 was affected by conceptus number (P < 0.01; one = 1.79 × 106 ± 3.84 × 103; two = 1.66 × 106 ± 8.15 × 103), conceptus length (P < 0.01; Figure 6F), and by the two-way interaction of conceptus length and fertility group (P < 0.01), and by the three-way interaction of conceptus number, length, and fertility group (P < 0.01; Figure 6G). For all the remaining analysis, only the effect of fertility group was retained in the reduced model.
Among the open HF, SF, and IF heifers, concentrations of three fatty acids were increased in the ULF of HF compared to SF heifers, and two fatty acids increased in the ULF of HF compared to IF heifers (Table 5).
Transcriptome analysis of endometrium and conceptuses for lipid metabolism genes
Expression of 66 genes associated with lipid metabolism differed between pregnant and open endometrium, with 41 genes increased and 25 decreased in the pregnant endometrium (Supplementary Dataset S6). Of note, expression of genes regulating fatty acid biosynthesis [fatty acid synthase (FASN), fatty acid desaturase 2 (FADS2), fatty acid elongase 1 (ELOVL1), and ELOVL6] and fatty acid transport (SLC27A5, SLC27A6, FABP3, and LOC100299715) was upregulated in the endometrium by pregnancy. However, expression of only three genes differed in the endometrium of pregnant HF and SF heifers, as FABP3, ST6 beta-galactoside alpha-2,6-sialyltransferase 2 (ST6GAL2) and polypeptide N-acetylgalactosaminyltransferase 16 (GALNT16) were increased in the endometrium of HF heifers (Supplementary Dataset S6).
In conceptuses, expression of six genes associated with lipid metabolism differed between short and long conceptuses (Supplementary Dataset S6). Expression of ELOVL7 and inositol polyphosphate-4-phosphatase type II B (INPP4B) was increased in short conceptuses, and expression of FADS1, FADS2, ELOVL5, and beta-1,3-galactosyltransferase 5 (B3GALT5) was increased in long conceptuses. Interestingly, expression of 24 genes differed between HF and SF conceptuses (Supplementary Dataset S6). Expression of 10 genes [ELOVL5, FADS1, glycerol-3-phosphate acyltransferase, mitochondrial (GPAM), choline kinase alpha (CHKA), chondroitin sulfate synthase 1 (CHSY1), cytochrome P450 family 11 subfamily A member 1 (CYP11A1), phosphatidylinositol 4-kinase beta (PI4KB), B3GALT5, xylosyltransferase 2 (XYLT2), and UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 5 (B3GNT5)] was increased in HF conceptuses, and 14 genes [ELOVL7, FABP3, SLC27A3, INPP4B, N-acylsphingosine amidohydrolase 1 (ASAH1), beta-1,4-galactosyltransferase 4 (B4GALT4), polypeptide N-acetylgalactosaminyltransferase 5 (GALNT5), acetyl-CoA acetyltransferase 1 (ACAT1), choline/ethanolamine phosphotransferase 1 (CEPT1), phosphatidylinositol glycan anchor biosynthesis class N (PIGN), N-deacetylase and N-sulfotransferase 3 (NDST3), inositol-trisphosphate 3-kinase A (ITPKA), ST6GAL2, and ceramide synthase 1 (CERS1)] increased in SF conceptuses.
Untargeted lipidomics
There was a total of 17,480 features detected from the positive mode and 2,020 features detected in the negative mode (Supplementary Dataset S7). Only features identified based on fragmentation spectra (ddMS2) were subjected to statistical analysis, which represents 22 features detected in the positive ion mode.
Fold change analysis found that abundance of nine lipids increased and one decreased (Supplementary Figure S1) in the uterine lumen by pregnancy, and six of the nine lipids which increased by pregnancy in the FC analysis were significantly different (P ≤ 0.05) in the t-test (Table 6). The differently abundant lipids in the FC analysis included phosphatidylcholines, triacylglycerides, and acylcarnitines.
Table 6. Lipids differentially abundant in the ULF of open and pregnant HF heifers.
| ID | Pregnant/open FC | log2 (FC) | P-value |
|---|---|---|---|
| Phosphatidylcholine (34:1) | 5.28 | 2.40 | 0.01 |
| Phosphatidylcholine (34:2) | 2.74 | 1.46 | 0.01 |
| Phosphatidylcholine (36:2) | 3.17 | 1.66 | 0.01 |
| Phosphatidylcholine (34:2) | 2.88 | 1.53 | 0.01 |
| Phosphatidylcholine (36:1) | 3.18 | 1.67 | 0.01 |
| Triacylglyceride (16:0_18:1_18:1) | 2.39 | 1.25 | 0.05 |
| Triacylglyceride (18:0_18:1_18:1) | 2.31 | 1.21 | 0.09 |
| Acylcarnitine (4:0) | 3.59 | 1.84 | 0.18 |
| Acylcarnitine (2:0) | 2.52 | 1.34 | 0.33 |
| Triacylglyceride (18:1_18:1_18:1) | 0.38 | −1.38 | 0.44 |
Discussion
The present study was conducted to investigate glucose, PGs, and lipids in the uterine lumen of fertility-classified heifers to gain insights in the mechanisms regulating uterine capacity for pregnancy. Conceptus elongation involves an increase in trophectoderm cell number that is driven by proliferation; therefore, sufficient energy and anabolic precursors are required to support cellular replication and specialization [69]. In mammalian embryos at the cleavage stage, ATP production is based on low levels of oxidation of pyruvate, lactate, and amino acids, and by the blastocyst stage, energy demands increase dramatically with the formation of the blastocoel and due to the increase in protein synthesis required for conceptus growth [70, 71]. The blastocyst preferably uses glucose as an energy source compared to embryos in earlier stages of development, and rather than direct glucose toward oxidative phosphorylation, aerobic glycolysis seems to be the preferred pathway, which is a metabolic adaptation of rapidly proliferative cells known as Warburg effect [72–75]. This same pattern of glucose metabolism was observed during conceptus elongation in sheep [76]. Additionally, the increase in available glucose during early pregnancy in cattle may be an important regulator of the onset of conceptus elongation and mediated by the glucose-AMPK-PPARγ interplay [77]. Thus, glucose availability in the uterine lumen and the expression of glucose transporters by the elongating conceptus are likely essential for pregnancy success in ruminants.
Gluconeogenesis appears not to occur in the uterus of mice and humans, because their uteri lack expression of rate-limiting gluconeogenic enzymes required for the synthesis of glucose from non-carbohydrate carbon substrates. For instance, phosphoenolpyruvate carboxykinase (PEPCK, also known as PCK), which converts oxaloacetate into phosphoenolpyruvate and carbon dioxide, was not detected in mouse uterus [24], and PCK and fructose-1,6-bisphosphatase (FBP1), which convert fructose-1,6-bisphosphate to fructose 6-phosphate, were also not detected in human myometrium and endometrium [23]. In the present study, the mRNA expression of rate-limiting gluconeogenic enzymes (G6PC, PCK2, FBP1) increased in pregnant compared to open endometrium. Similarly, expression of those same three enzymes were found to increase in the intercaruncular endometrium from days 28 to 42 of gestation in cattle [22], indicating that perhaps the bovine uterus is able to generate glucose through gluconeogenesis and that this gluconeogenic capacity of the uterus increases as pregnancy advances. Regardless of the gluconeogenic capacity of uterine cells, the uptake of glucose into the uterine lumen through glucose transporters expressed by the endometrium appears to be the main pathway regulating glucose in the uterine lumen of ruminants [20]. In fact, concentrations of glucose in the uterine lumen of ewes increased by sixfold from days 10 to 15 of pregnancy, which occurred concomitant with the increase in expression of glucose transporters (SLC2A1, SLC5A1, SLC5A11) by the endometrium [20, 21]. Those glucose transporters are nonclassical IFNT-stimulated genes [78] and were also increased by pregnancy in the endometrium in the present study, thus likely contributing to the higher glucose observed in the ULF of pregnant compared to open heifers. However, no differences in uterine lumen glucose were observed between pregnant HF and SF heifers, which were supported by the endometrium transcriptome data as only one gene (FBP1) involved with gluconeogenesis was increased in pregnant HF than in SF endometrium. Higher circulating progesterone levels have also been associated with increased uterine luminal concentrations of glucose [16], but plasma progesterone concentrations were not different between pregnant HF and SF heifers [1]. These findings indicated that the availability of glucose in the ULF is probably not a major factor influencing subfertility in this group of fertility-classified heifers. However, circulating plasma glucose was increased in IF than in HF heifers in the present study. Leane et al. [79] have recently reported that, in lactating dairy cows, increasing circulating glucose concentrations inhibited early conceptus development on day 14, despite not affecting ULF glucose concentrations. Thus, it is possible that higher circulating levels of glucose have systemic negative effects and may hinder fertility without affecting ULF glucose levels. Furthermore, no differences were observed between short and long conceptuses for genes encoding glucose transporters, but expression of SLC2A2 (GLUT2) and SLC2A4 (GLUT4) was higher in SF than in HF conceptuses, as five genes involved with glycolysis and gluconeogenesis (HK1, FBP1, GALM, ALDOB, ALDH3B1) were differently expressed between HF and SF conceptuses, which indicate dysregulated energy metabolism in SF conceptuses.
Prostaglandins are major regulators of female reproduction [80, 81] and modulate conceptus and endometrial gene expression during early pregnancy in ruminants [33]. In fact, PTGS2 was predicted as a key player regulating overall gene expression of day 17 bovine conceptuses [1]. In the present study, uterine luminal concentrations of PGE2, PGF2α, and 6-keto-PFG1α were higher in the ULF of pregnant HF than SF heifers. Although no differences in endometrium and conceptus mRNA expression of selected PG synthases (PTGS2, PTGIS, PTGES, PTGFS) were observed, the increase in uterine luminal concentrations of PGs in HF heifers is likely due to differences in conceptus-derived PGs, as HF heifers had longer conceptuses and therefore a larger number of cells producing PGs. Likewise, an earlier study observed no differences in IFNT mRNA between bovine conceptuses that were either long (>10 cm) or short (<5 cm), but IFNT protein was substantially increased in the uterine flush from long conceptuses [34].
Prostaglandins E2, PGF2α, and PGI2 signal through their designated receptors, termed EP, FP, and IP, respectively. There are four subtypes of PGE2 receptors (EP, also known as PTGER1 through 4) but only one PGF2α receptor (FP, also known as PTGFR) and one PGI2 receptor (IP, also known as PTGIR) [82, 83]. Among the PTGER receptors, PTGER2 and PTGER4 had the highest expression in the endometrium of open and pregnant cows. These receptors are members of the seven-transmembrane G protein-coupled receptors, which signal through cAMP [84], and the PGE2-cAMP pathway regulates important biological processes such as angiogenesis, vasodilatation, myometrial quiescence, and decidualization, which are essential for pregnancy establishment in several species [85]. In the present experiment, the expression of PGF synthase (PTGFS) by day 17 conceptuses was low, but the expression of the PGF2α receptor (PTGFR) was the highest among the PG receptors investigated in the conceptuses, suggesting that paracrine actions of endometrial PGF2α might be of particular importance regulating bovine conceptus elongation, and interestingly, HF conceptuses expressed higher PTGFR than SF conceptuses. Although the effect of PGF2α on embryonic and trophectoderm cell growth has not been investigated, PGF2α modulates endometrial cell proliferation, angiogenesis, and tissue remodeling in other systems [86, 87], which are important biological events occurring during conceptus elongation. Furthermore, higher expression of a PG transporter (SLCO2A1) was observed in HF than in SF conceptuses, possibly indicating higher PG signaling within HF conceptuses. The solute carrier organic anion transporter family member 2A1 (SLCO2A; also known as PGT) [88] is able to transport PGE1, PGE2, PGD2, PGF2α, and, to a lesser extent, TXB2 [89]. Although newly synthesized PGs may exit the cells by diffusion due to their lipophilic nature, SLCO2A1 is involved in the transport of PGs across membranes and plays an important role mediating the inactivation of the PG stimulus, which depends on the uptake of the PG molecule signaling through membrane bound receptors into the cytoplasm of the cell, where it is oxidized by 15-ketoprostaglandin dehydrogenase (15-PGDH) [90, 91]. Expression of another transmembrane PG transporter, the ATP-binding cassette, subfamily C member 4 (ABCC4; also known as MRP4) [92], which is expressed by the pig endometrium and conceptus [93], was not detected on day 17 bovine endometrium or conceptus in the present study.
Higher concentrations of 6-keto-PFG1α, a stable metabolite of PGI2, were detected in the uterine lumen of HF pregnant heifers, and PGI2 synthase was highly expressed by the endometrium in pregnant and open heifers as well as the conceptus. Although the expression of PGI2 synthase and PGI2 receptors by the endometrium and conceptus during the time of maternal recognition of pregnancy has been studied in sheep [94], the downstream effects of PGI2 signaling in the endometrium and conceptus is poorly understood. PGI2 is a strong vasodilator and a potent platelet inhibitor [95, 96]. PGI2 signaling via PPARD is essential for implantation and decidualization in mice [40]. PGI2 signaling through PPARD in sheep conceptuses does not seem to be important, as inhibition of PPARD in trophectoderm cells using morpholino antisense oligonucleotides did not affect conceptus elongation [97]. In contrast, the inhibition of PPARG using morpholino antisense oligonucleotides resulted in severely growth-retarded conceptuses [97], indicating that PPARG signaling is of great importance during conceptus elongation in ruminants. Furthermore, PPARG expression has been reported to increase during conceptus elongation in cattle, and its expression was correlated with the expression of several genes involved in lipid metabolism [56]. Of note, PPARA was the highest PPAR expressed by day 17 endometrium and regulates FA catabolism in several tissues [98], suggesting it may have critical roles modulating uterine luminal lipid content during early pregnancy. Among genes involved with PPAR signaling in the endometrium, the expression of fatty acid binding protein 3 (FABP3) was increased in pregnant compared to open heifers and in pregnant HF compared to SF heifers. FABP coordinates cellular lipid responses and reversely binds a variety of lipids, including eicosanoids and saturated and unsaturated long-chain fatty acids [99]. Of note, FABP3 modulates cell growth and proliferation [100] and is upregulated by pregnancy in the endometrium LE on days 15 and 18 of pregnancy in cattle [101].
Regarding the differentially expressed genes involved with arachidonic acid metabolism, expression of glutathione peroxidase 1 (GPX1), a major antioxidant enzyme [102], was increased in SF than in HF conceptuses and could indicate greater oxidative stress in SF conceptuses. Furthermore, expression of one phospholipase A2 (PLA2) was increased in HF conceptuses (PLA2G15) and another one (PLA2G1B) increased in SF conceptuses. The superfamily of PLA2 enzymes is characterized by their ability to hydrolyze fatty acids from the sn-2 position of glycerophospholipids producing a variety of free fatty acids and lysoglycerophospholipids [103]. PLA2G15 is localized in lysosomes and hydrolyses preferentially zwitterionic phospholipids (phosphatidylcholine—PC and phosphatidylethanolamine—PE) [103, 104], and PLA2G1B is described as a pancreatic lipase [103] expressed primarily by acinar cells, and in a much lesser extent by the lungs and kidney, and has higher affinity for anionic phospholipids (phosphatidic acid—PA, phosphatidylserine—PS, phosphatidylglycerol—PG) than PC [105, 106]. Those differences in PLA2 expression by HF and SF conceptuses perhaps contributed to the observed differences in ULF fatty acid composition between pregnant HF and SF heifers.
Simintiras et al. [18] have recently reported changes in the uterine fluid lipidome associated with progesterone and day of the estrus cycle during the period of onset of conceptus elongation (days 12–14) in heifers. Surprisingly, in the current study, there was no effect of pregnancy on the fatty acid profile of day 17 ULF. Palmitic acid (16:0) and stearic acid (18:0), which are the two most abundant saturated fatty acids in mammalian cells [49, 107], accounted for around 90% of the total fatty acid detected in the ULF. The low complexity of the ULF fatty acid profile observed in the present study may have been influenced by the amount of PBS used to perform the uterine flushes (20 mL), which perhaps diluted the fatty acid containing molecules in ULF, and thus biased the fatty acid profile towards the ones with highest intensity.
Two unusual ultralong-chain fatty acids (C ≥ 26) were the third (28:7) and fourth (28:8) most abundant fatty acids detected in the ULF of open and pregnant heifers, and their concentrations were increased in pregnant HF than SF ULF. Ultralong-chain polyunsaturated fatty acids (PUFAs) have also been found in the other mammalian tissues, such as brain, retina, skin, and testis [107, 108], and 28:5 and 30:5 are essential for sperm maturation and male fertility in the testis [109]. A number of long-chain (C11–20) and very long-chain (C21–25) fatty acids were increased in ULF of pregnant HF than SF heifers. However, the differently abundant fatty acids had overall low concentrations in the ULF, accounting together for only 1.2% of the total fatty acid detected. The source of long-chain saturated fatty acids in animals comes from the diet or from fatty acid de novo synthesis, which is mediated by FASN [110, 111]. Expression of FASN by the endometrium was increased by pregnancy, but it did not differ in the endometrium of pregnant HF and SF heifers or between HF and SF conceptuses. Fatty acid biosynthesis and catabolism involve dynamic processes in which the number of carbons and double bonds change under the influence of elongation, desaturation, and b-oxidation reactions [107]. Fatty acid extension depends on elongases (ELOVL), which are enzymes that catalyze carbon chain extension [112]. In the present study, ELOVL1 and ELOVL6 were upregulated in the endometrium by pregnancy. In addition, ELOVL5 was upregulated in long and in HF conceptuses, and ELOVL7 was upregulated in short and in SF conceptuses. Similarly, Barnwell et al. [67] also observed increased expression of ELOVL5 in long (24.7 ± 1.9 mm) compared to short (4.2 ± 0.1 mm) day 15 bovine conceptuses. In the present study, differences were also observed in the expression of fatty acid desaturases 1 and 2 (FADS1 and FADS2). Fatty acid desaturases control the degree of fatty acid unsaturation by catalyzing the insertion of double bonds into the fatty acid chain [107]. Fatty acid desaturases 1 and 2 (FADS1 and FADS2) are essential enzymes for PUFA biosynthesis [113, 114], and expression of FADS1 was increased in HF and long conceptuses and decreased in SF and short conceptuses. Furthermore, FADS2 expression was increased in long conceptuses and in pregnant endometrium. Moreover, a number of genes involved with lipid metabolism were differently expressed in HF and SF conceptuses, including genes involved with the transport of long-chain fatty acids (SLC27A3) and with the biosynthesis of glycerolipids (GPAM) [115], phosphatidylcholines (CHKA) [116], ceramides (CERS1, ASAH1) [117, 118], steroid hormones (CYP11A1) [119], and with cholesterol esterification (ACAT1) [120]. The observed differences in lipid metabolism among HF and SF conceptuses likely contributed to the observed differences in fatty acid content among pregnant fertility-classified heifers.
To identify changes in ULF lipidome that are normally induced by pregnancy, global lipidomics was conducted exclusively on ULF samples of HF heifers that were pregnant or not. This analysis found that phosphatidylcholines, a major component of the cell membrane in eukaryotes [121], were the main lipid class that increased in the uterine lumen with pregnancy, which is possibly related to the secretion of EV by the elongating conceptus [51, 52]. Phosphatidylcholines are the most abundant phospholipid in sheep endometrium on days 3, 12, and 15 of the estrus cycle and pregnancy [122] and the main lipid component of uterine epithelial cells during the peri-implantation period in mice [123]. It is likely that conceptus-derived EVs are responsible for the increased abundance of PC observed in day 17 pregnant ULF, as pregnancy appears to inhibit endometrial-derived EV through induction of interferon-stimulated gene 15 (ISG15) by IFNT [124].
In summary, the main findings of the current study are that concentrations of glucose, PGE2, PGF2α, and phosphatidylcholines were increased in the ULF by pregnancy and that ULF from pregnant HF heifers had overall higher concentrations of fatty acids, PGE2, PGF2α, and 6-keto-PFG1α than pregnant SF heifers. The observed differences in the metabolism of glucose, PG, and lipids, by the conceptus and endometrium from fertility-classified heifers, further reinforces our hypothesis of dysregulated conceptus–endometrium interactions in SF heifers [1], which consequently affects uterine luminal histotroph and negatively affects conceptus survival and development after day 17 and before day 28.
Conflict of Interest
The authors have declared that no conflict of interest exists
Supplementary Material
Acknowledgments
The authors greatly appreciate the help of Dr. William R. Lamberson with statistical analysis, Kenneth Ladyman and David Todd for their help caring for the animals, and Rick Disselhorst for coordinating the animal slaughter.
References
- 1. Moraes JGN, Behura SK, Geary TW, Hansen PJ, Neibergs HL, Spencer TE. Uterine influences on conceptus development in fertility-classified animals. Proc Natl Acad Sci USA 2018; 115:E1749–E1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, Rizos D, Lonergan P. Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 2009; 138:507–517. [DOI] [PubMed] [Google Scholar]
- 3. Koot YE, van Hooff SR, Boomsma CM, van Leenen D, Groot Koerkamp MJ, Goddijn M, Eijkemans MJ, Fauser BC, Holstege FC, Macklon NS. An endometrial gene expression signature accurately predicts recurrent implantation failure after IVF. Sci Rep 2016; 6:19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simon C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 2013; 100:818–824. [DOI] [PubMed] [Google Scholar]
- 5. Bauersachs S, Wolf E. Transcriptome analyses of bovine, porcine and equine endometrium during the pre-implantation phase. Anim Reprod Sci 2012; 134:84–94. [DOI] [PubMed] [Google Scholar]
- 6. Berg DK, van Leeuwen J, Beaumont S, Berg M, Pfeffer PL. Embryo loss in cattle between days 7 and 16 of pregnancy. Theriogenology 2010; 73:250–260. [DOI] [PubMed] [Google Scholar]
- 7. Betteridge KJ, Eaglesome MD, Randall GC, Mitchell D. Collection, description and transfer of embryos from cattle 10--16 days after oestrus. J Reprod Fertil 1980; 59:205–216. [DOI] [PubMed] [Google Scholar]
- 8. Spencer TE. Biological roles of uterine glands in pregnancy. Semin Reprod Med 2014; 32:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer TE, Gray CA. Sheep uterine gland knockout (UGKO) model. Methods Mol Med 2006; 121:85–94. [DOI] [PubMed] [Google Scholar]
- 10. Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124:289–300. [PubMed] [Google Scholar]
- 11. Forde N, McGettigan PA, Mehta JP, O'Hara L, Mamo S, Bazer FW, Spencer TE, Lonergan P. Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 2014; 147:575–587. [DOI] [PubMed] [Google Scholar]
- 12. Ulbrich SE, Schulke K, Groebner AE, Reichenbach HD, Angioni C, Geisslinger G, Meyer HH. Quantitative characterization of prostaglandins in the uterus of early pregnant cattle. Reproduction 2009; 138:371–382. [DOI] [PubMed] [Google Scholar]
- 13. Hugentobler SA, Sreenan JM, Humpherson PG, Leese HJ, Diskin MG, Morris DG. Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod Fertil Dev 2010; 22:684–694. [DOI] [PubMed] [Google Scholar]
- 14. Keller ML, Roberts AJ, Seidel GE Jr. Characterization of insulin-like growth factor-binding proteins in the uterus and conceptus during early conceptus elongation in cattle. Biol Reprod 1998; 59:632–642. [DOI] [PubMed] [Google Scholar]
- 15. Forde N, Simintiras CA, Sturmey R, Mamo S, Kelly AK, Spencer TE, Bazer FW, Lonergan P. Amino acids in the uterine luminal fluid reflects the temporal changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle. PLoS One 2014; 9:e100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simintiras CA, Sanchez JM, McDonald M, Martins T, Binelli M, Lonergan P. Biochemical characterization of progesterone-induced alterations in bovine uterine fluid amino acid and carbohydrate composition during the conceptus elongation windowdagger. Biol Reprod 2019; 100:672–685. [DOI] [PubMed] [Google Scholar]
- 17. Simintiras CA, Sanchez JM, McDonald M, Lonergan P. The influence of progesterone on bovine uterine fluid energy, nucleotide, vitamin, cofactor, peptide, and xenobiotic composition during the conceptus elongation-initiation window. Sci Rep 2019; 9:7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simintiras CA, Sanchez JM, McDonald M, Lonergan P. Progesterone alters the bovine uterine fluid lipidome during the period of elongation. Reproduction 2018; 157:399–411. [DOI] [PubMed] [Google Scholar]
- 19. Sponchiado M, Gonella-Diaza AM, Rocha CC, Lo Turco EG, Pugliesi G, Leroy JLMR, Binelli M. The pre-hatching bovine embryo transforms the uterine luminal metabolite composition in vivo. Sci Rep 2019; 9:8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen. Ii. Glucose transporters in the uterus and peri-implantation conceptuses. Biol Reprod 2009; 80:94–104. [DOI] [PubMed] [Google Scholar]
- 21. Gao H, Wu G, Spencer TE, Johnson GA, Li X, Bazer FW. Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 2009; 80:86–93. [DOI] [PubMed] [Google Scholar]
- 22. Moore SG, McCabe MS, Green JC, Newsom EM, Lucy MC. The transcriptome of the endometrium and placenta is associated with pregnancy development but not lactation status in dairy cows. Biol Reprod 2017; 97:18–31. [DOI] [PubMed] [Google Scholar]
- 23. Yanez AJ, Nualart F, Droppelmann C, Bertinat R, Brito M, Concha II, Slebe JC. Broad expression of fructose-1,6-bisphosphatase and phosphoenolpyruvate carboxykinase provide evidence for gluconeogenesis in human tissues other than liver and kidney. J Cell Physiol 2003; 197:189–197. [DOI] [PubMed] [Google Scholar]
- 24. Zimmer DB, Magnuson MA. Immunohistochemical localization of phosphoenolpyruvate carboxykinase in adult and developing mouse tissues. J Histochem Cytochem 1990; 38:171–178. [DOI] [PubMed] [Google Scholar]
- 25. Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics 2007; 8:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feng L, Frommer WB. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem Sci 2015; 40:480–486. [DOI] [PubMed] [Google Scholar]
- 27. Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med 2013; 34:183–196. [DOI] [PubMed] [Google Scholar]
- 28. Frolova AI, Moley KH. Glucose transporters in the uterus: an analysis of tissue distribution and proposed physiological roles. Reproduction 2011; 142:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011; 31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dorniak P, Bazer FW, Spencer TE. Prostaglandins regulate conceptus elongation and mediate effects of interferon tau on the ovine uterine endometrium. Biol Reprod 2011; 84:1119–1127. [DOI] [PubMed] [Google Scholar]
- 31. Spencer TE, Forde N, Dorniak P, Hansen TR, Romero JJ, Lonergan P. Conceptus-derived prostaglandins regulate gene expression in the endometrium prior to pregnancy recognition in ruminants. Reproduction 2013; 146:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charpigny G, Reinaud P, Tamby JP, Creminon C, Guillomot M. Cyclooxygenase-2 unlike cyclooxygenase-1 is highly expressed in ovine embryos during the implantation period. Biol Reprod 1997; 57:1032–1040. [DOI] [PubMed] [Google Scholar]
- 33. Brooks K, Burns G, Spencer TE. Conceptus elongation in ruminants: roles of progesterone, prostaglandin, interferon tau and cortisol. J Anim Sci Biotechnol 2014; 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson RS, Fray MD, Wathes DC, Lamming GE, Mann GE. In vivo expression of interferon tau mRNA by the embryonic trophoblast and uterine concentrations of interferon tau protein during early pregnancy in the cow. Mol Reprod Dev 2006; 73:470–474. [DOI] [PubMed] [Google Scholar]
- 35. Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 1994; 46:205–229. [PubMed] [Google Scholar]
- 36. Ide T, Egan K, Bell-Parikh LC, FitzGerald GA. Activation of nuclear receptors by prostaglandins. Thromb Res 2003; 110:311–315. [DOI] [PubMed] [Google Scholar]
- 37. Lim H, Dey SK. A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology 2002; 143:3207–3210. [DOI] [PubMed] [Google Scholar]
- 38. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 1997; 94:4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 1997; 94:4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev 1999; 13:1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995; 83:803–812. [DOI] [PubMed] [Google Scholar]
- 42. Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 1995; 83:813–819. [DOI] [PubMed] [Google Scholar]
- 43. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 2002; 53:409–435. [DOI] [PubMed] [Google Scholar]
- 44. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001; 294:1871–1875. [DOI] [PubMed] [Google Scholar]
- 45. Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 2007; 77:190–201. [DOI] [PubMed] [Google Scholar]
- 46. Beall JR. Uterine lipid metabolism—a review of the literature. Comp Biochem Physiol B 1972; 42:175–195. [DOI] [PubMed] [Google Scholar]
- 47. Brinsfield TH, Hawk HW. Control by progesterone of the concentration of lipid droplets in epithelial cells of the sheep endometrium. J Anim Sci 1973; 36:919–922. [DOI] [PubMed] [Google Scholar]
- 48. Boshier DP, Fairclough RJ, Holloway H. Assessment of sheep blastocyst effects on neutral lipids in the uterine caruncular epithelium. J Reprod Fertil 1987; 79:569–573. [DOI] [PubMed] [Google Scholar]
- 49. Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem 2001; 276:45358–45366. [DOI] [PubMed] [Google Scholar]
- 50. Ribeiro ES. Symposium review: lipids as regulators of conceptus development: implications for metabolic regulation of reproduction in dairy cattle. J Dairy Sci 2018; 101:3630–3641. [DOI] [PubMed] [Google Scholar]
- 51. Burns GW, Brooks KE, O'Neil EV, Hagen DE, Behura SK, Spencer TE. Progesterone effects on extracellular vesicles in the sheep uterus. Biol Reprod 2018; 98:612–622. [DOI] [PubMed] [Google Scholar]
- 52. Burns GW, Brooks KE, Spencer TE. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod 2016; 94:56. [DOI] [PubMed] [Google Scholar]
- 53. Gottlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci USA 1992; 89:4653–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 1999; 3:397–403. [DOI] [PubMed] [Google Scholar]
- 55. Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv Nutr 2012; 3:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ribeiro ES, Greco LF, Bisinotto RS, Lima FS, Thatcher WW, Santos JE. Biology of preimplantation conceptus at the onset of elongation in dairy cows. Biol Reprod 2016; 94:97. [DOI] [PubMed] [Google Scholar]
- 57. Geary TW, Burns GW, Moraes JG, Moss JI, Denicol AC, Dobbs KB, Ortega MS, Hansen PJ, Wehrman ME, Neibergs H, O'Neil E, Behura S et al. . Identification of beef heifers with superior uterine capacity for pregnancy. Biol Reprod 2016; 95:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brooks K, Burns GW, Moraes JG, Spencer TE. Analysis of the uterine epithelial and conceptus transcriptome and luminal fluid proteome during the peri-implantation period of pregnancy in sheep. Biol Reprod 2016; 95:88. [DOI] [PubMed] [Google Scholar]
- 59. Moncada S. Eighth Gaddum Memorial Lecture, University of London Institute of Education, December 1980. Biological importance of prostacyclin. Br J Pharmacol 1982; 76:3–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salmon JA, Smith DR, Flower RJ, Moncada S, Vane JR. Further studies on the enzymatic conversion of prostaglandin endoperoxide into prostacyclin by porcine aorta microsomes. Biochim Biophys Acta 1978; 523:250–262. [DOI] [PubMed] [Google Scholar]
- 61. Gochman N, Schmitz JM. Application of a new peroxide indicator reaction to the specific, automated determination of glucose with glucose oxidase. Clin Chem 1972; 18:943–950. [PubMed] [Google Scholar]
- 62. Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 2018; 46:W486–W494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xia J, Wishart DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc 2011; 6:743–760. [DOI] [PubMed] [Google Scholar]
- 64. Xia J, Wishart DS. Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr Protoc Bioinformatics 2011;34:10–14. [DOI] [PubMed] [Google Scholar]
- 65. Checa A, Bedia C, Jaumot J. Lipidomic data analysis: tutorial, practical guidelines and applications. Anal Chim Acta 2015; 885:1–16. [DOI] [PubMed] [Google Scholar]
- 66. Zhou X, Lindsay H, Robinson MD. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res 2014; 42:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barnwell CV, Farin PW, Ashwell CM, Farmer WT, Galphin SP Jr, Farin CE. Differences in mRNA populations of short and long bovine conceptuses on day 15 of gestation. Mol Reprod Dev 2016; 83:424–441. [DOI] [PubMed] [Google Scholar]
- 68. Ortega MS, Moraes JGN, Patterson DJ, Smith MF, Behura SK, Poock S, Spencer TE. Influences of sire conception rate on pregnancy establishment in dairy cattle. Biol Reprod 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Folmes CD, Terzic A. Metabolic determinants of embryonic development and stem cell fate. Reprod Fertil Dev 2014; 27:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod 2008; 14:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gardner DK, Wale PL. Analysis of metabolism to select viable human embryos for transfer. Fertil Steril 2013; 99:1062–1072. [DOI] [PubMed] [Google Scholar]
- 72. Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev 2012; 79:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Warburg O. On the origin of cancer cells. Science 1956; 123:309–314. [DOI] [PubMed] [Google Scholar]
- 75. Redel BK, Brown AN, Spate LD, Whitworth KM, Green JA, Prather RS. Glycolysis in preimplantation development is partially controlled by the Warburg effect. Mol Reprod Dev 2012; 79:262–271. [DOI] [PubMed] [Google Scholar]
- 76. Wales RG, Waugh EE. Catabolic utilization of glucose by the sheep conceptus between days 13 and 19 of pregnancy. Reprod Fertil Dev 1993; 5:111–122. [DOI] [PubMed] [Google Scholar]
- 77. Simintiras CA, Sanchez JM, McDonald M, Lonergan P. The biochemistry surrounding bovine conceptus elongation. Biology of Reproduction, 2019;101,328–337. [DOI] [PubMed] [Google Scholar]
- 78. Hansen TR, Sinedino LDP, Spencer TE. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction 2017; 154:F45–F59. [DOI] [PubMed] [Google Scholar]
- 79. Leane S, Herlihy MM, Curran F, Kenneally J, Forde N, Simintiras CA, Sturmey RG, Lucy MC, Lonergan P, Butler ST. The effect of exogenous glucose infusion on early embryonic development in lactating dairy cows. J Dairy Sci 2018; 101:11285–11296. [DOI] [PubMed] [Google Scholar]
- 80. Poyser NL. The control of prostaglandin production by the endometrium in relation to luteolysis and menstruation. Prostaglandins Leukot Essent Fatty Acids 1995; 53:147–195. [DOI] [PubMed] [Google Scholar]
- 81. Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997; 91:197–208. [DOI] [PubMed] [Google Scholar]
- 82. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 1999; 79:1193–1226. [DOI] [PubMed] [Google Scholar]
- 83. Woodward DF, Jones RL, Narumiya S. International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev 2011; 63:471–538. [DOI] [PubMed] [Google Scholar]
- 84. Regan JW, Bailey TJ, Pepperl DJ, Pierce KL, Bogardus AM, Donello JE, Fairbairn CE, Kedzie KM, Woodward DF, Gil DW. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol Pharmacol 1994; 46:213–220. [PubMed] [Google Scholar]
- 85. Arosh JA, Banu SK, Kimmins S, Chapdelaine P, Maclaren LA, Fortier MA. Effect of interferon-tau on prostaglandin biosynthesis, transport, and signaling at the time of maternal recognition of pregnancy in cattle: evidence of polycrine actions of prostaglandin E2. Endocrinology 2004; 145:5280–5293. [DOI] [PubMed] [Google Scholar]
- 86. Milne SA, Jabbour HN. Prostaglandin (PG) F(2alpha) receptor expression and signaling in human endometrium: role of PGF(2alpha) in epithelial cell proliferation. J Clin Endocrinol Metab 2003; 88:1825–1832. [DOI] [PubMed] [Google Scholar]
- 87. Kaczynski P, Kowalewski MP, Waclawik A. Prostaglandin F2alpha promotes angiogenesis and embryo-maternal interactions during implantation. Reproduction 2016; 151:539–552. [DOI] [PubMed] [Google Scholar]
- 88. Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science 1995; 268:866–869. [DOI] [PubMed] [Google Scholar]
- 89. Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA (hPGT). J Clin Invest 1996; 98:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat 2002; 68–69:633–647. [DOI] [PubMed] [Google Scholar]
- 91. Nakanishi T, Tamai I. Roles of organic anion transporting polypeptide 2A1 (OATP2A1/SLCO2A1) in regulating the pathophysiological actions of prostaglandins. AAPS J 2017; 20:13. [DOI] [PubMed] [Google Scholar]
- 92. Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci 2008; 29:200–207. [DOI] [PubMed] [Google Scholar]
- 93. Seo H, Choi Y, Shim J, Yoo I, Ka H. Prostaglandin transporters ABCC4 and SLCO2A1 in the uterine endometrium and conceptus during pregnancy in pigs. Biol Reprod 2014; 90:100. [DOI] [PubMed] [Google Scholar]
- 94. Cammas L, Reinaud P, Bordas N, Dubois O, Germain G, Charpigny G. Developmental regulation of prostacyclin synthase and prostacyclin receptors in the ovine uterus and conceptus during the peri-implantation period. Reproduction 2006; 131:917–927. [DOI] [PubMed] [Google Scholar]
- 95. Moncada S, Gryglewski RJ, Bunting S, Vane JR. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins 1976; 12:715–737. [DOI] [PubMed] [Google Scholar]
- 96. Dusting GJ, Moncada S, Vane JR. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins 1977; 13:3–15. [DOI] [PubMed] [Google Scholar]
- 97. Brooks KE, Burns GW, Spencer TE. Peroxisome proliferator activator receptor gamma (PPARG) regulates conceptus elongation in sheep. Biol Reprod 2015; 92:42. [DOI] [PubMed] [Google Scholar]
- 98. Yoon M. The role of PPARalpha in lipid metabolism and obesity: focusing on the effects of estrogen on PPARalpha actions. Pharmacol Res 2009; 60:151–159. [DOI] [PubMed] [Google Scholar]
- 99. Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 2008; 7:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Huynh H, Pollak M. Stabilization of mammary-derived growth inhibitor messenger RNA by antiestrogens. Clin Cancer Res 1997; 3:2151–2156. [PubMed] [Google Scholar]
- 101. Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Buttner M, Meyer HH, Spencer TE, Minten M, Sax G, Winter G, Wolf E. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod 2012; 86:46. [DOI] [PubMed] [Google Scholar]
- 102. Ufer C, Wang CC. The roles of glutathione peroxidases during embryo development. Front Mol Neurosci 2011; 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761; 2006:1246–1259. [DOI] [PubMed] [Google Scholar]
- 104. Fuly AL, de Miranda AL, Zingali RB, Guimaraes JA. Purification and characterization of a phospholipase A2 isoenzyme isolated from Lachesis muta snake venom. Biochem Pharmacol 2002; 63:1589–1597. [DOI] [PubMed] [Google Scholar]
- 105. Hui DY. Phospholipase A(2) enzymes in metabolic and cardiovascular diseases. Curr Opin Lipidol 2012; 23:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog Lipid Res 2011; 50:152–192. [DOI] [PubMed] [Google Scholar]
- 107. Sassa T, Kihara A. Metabolism of very long-chain fatty acids: genes and pathophysiology. Biomol Ther (Seoul) 2014; 22:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res 2010; 51:1624–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zadravec D, Tvrdik P, Guillou H, Haslam R, Kobayashi T, Napier JA, Capecchi MR, Jacobsson A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J Lipid Res 2011; 52:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci USA 2003; 100:6358–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Riezman H. The long and short of fatty acid synthesis. Cell 2007; 130:587–588. [DOI] [PubMed] [Google Scholar]
- 112. Jump DB. Mammalian fatty acid elongases. Methods Mol Biol 2009; 579:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stoffel W, Holz B, Jenke B, Binczek E, Gunter RH, Kiss C, Karakesisoglou I, Thevis M, Weber AA, Arnhold S, Addicks K. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J 2008; 27:2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Glaser C, Heinrich J, Koletzko B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 2010; 59:993–999. [DOI] [PubMed] [Google Scholar]
- 115. Yu H, Zhao Z, Yu X, Li J, Lu C, Yang R. Bovine lipid metabolism related gene GPAM: molecular characterization, function identification, and association analysis with fat deposition traits. Gene 2017; 609:9–18. [DOI] [PubMed] [Google Scholar]
- 116. Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res 2008; 49:1187–1194. [DOI] [PubMed] [Google Scholar]
- 117. Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life 2010; 62:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tirodkar TS, Lu P, Bai A, Scheffel MJ, Gencer S, Garrett-Mayer E, Bielawska A, Ogretmen B, Voelkel-Johnson C. Expression of ceramide synthase 6 transcriptionally activates acid ceramidase in a c-Jun N-terminal kinase (JNK)-dependent manner. J Biol Chem 2015; 290:13157–13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol 2015; 151:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Seo T, Oelkers PM, Giattina MR, Worgall TS, Sturley SL, Deckelbaum RJ. Differential modulation of ACAT1 and ACAT2 transcription and activity by long chain free fatty acids in cultured cells. Biochemistry 2001; 40:4756–4762. [DOI] [PubMed] [Google Scholar]
- 121. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008; 9:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Meier S, Trewhella MA, Fairclough RJ, Jenkin G. Changes in uterine endometrial phospholipids and fatty acids throughout the oestrous cycle and early pregnancy in the ewe. Prostaglandins Leukot Essent Fatty Acids 1997; 57:341–349. [DOI] [PubMed] [Google Scholar]
- 123. Burnum KE, Cornett DS, Puolitaival SM, Milne SB, Myers DS, Tranguch S, Brown HA, Dey SK, Caprioli RM. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J Lipid Res 2009; 50:2290–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Villarroya-Beltri C, Baixauli F, Mittelbrunn M, Fernandez-Delgado I, Torralba D, Moreno-Gonzalo O, Baldanta S, Enrich C, Guerra S, Sanchez-Madrid F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun 2016; 7:13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


