Abstract
Background.
Coronary physiology assessments have been shown by multiple trials to add clinical value in detecting significant coronary artery disease and predicting cardiovascular outcomes. Fractional flow reserve (FFR) obtained during invasive coronary angiography (ICA) has become the new reference standard for hemodynamic significance detection. Absolute myocardial blood flow (MBF) quantification by means of dynamic positron emission tomography (dPET) has high diagnostic and prognostic values. FFR is an invasive measure and as such cannot be applied broadly, while MBF quantification is commonly performed on standard vascular territories intermixing normal flow from normal regions with abnormal flow from abnormal regions and consequently limiting its diagnostic power.
Objective.
The aim of this study is to provide physicians with reliable software tools for the non-invasive assessment of lesion-specific physiological significance for the entire coronary tree by combining PET-derived absolute flow data and coronary computed tomography angiography (CTA)-derived anatomy and coronary centerlines.
Methods.
The Dynamic PET/CTA Myocardial Blood Flow Assessment with Fused Imagery (DEMYSTIFY) study is an observational prospective clinical study to develop algorithms and software tools to fuse coronary anatomy data obtained from CTA with dPET data to non-invasively measure absolute MBF, myocardial flow reserve and relative flow reserve across specific coronary lesions. Patients (N=108) will be collected from 4 institutions (Emory University Hospital, USA; Chonnam National University Hospital, South Korea; Samsung Medical Center, South Korea; Seoul National University Hospital, South Korea). These results will be compared to those obtained invasively in the catheterization laboratory and to a relatively novel non-invasive technique to estimate FFR based on CTA and computational fluid dynamics.
Conclusions.
Success of these developments should lead to the following benefits: 1) eliminate unnecessary invasive coronary angiography in patients with no significant lesions, 2) avoid stenting physiologically insignificant lesions, 3) guide percutaneous coronary interventions process to the location of significant lesions, 4) provide a flow-color-coded 3D roadmap of the entire coronary tree to guide bypass surgery, and 5) use less radiation and lower the cost from unnecessary procedures.
Keywords: dynamic PET, fractional flow reserve, coronary flow reserve, relative flow reserve, fusion imaging, microvascular dysfunction
Introduction
Coronary artery disease (CAD) is the leading cause of deaths attributed to cardiovascular disease1. More than 60% of patients with stable CAD referred for elective invasive coronary angiography (ICA) are found to have non-obstructive CAD2. Moreover, many patients with angiographically intermediate coronary lesions (50%−70% stenosis in one major epicardial vessel) often have non-hemodynamically significant stenosis. Many pivotal clinical trials, such as FAME3–5 and COURAGE6, have in fact shown the importance of assessing such lesions to guide revascularization decisions in patients with stable CAD. The FAME3–5 trials demonstrated that fractional flow reserve (FFR)-guided revascularization during ICA significantly improves outcomes and therefore FFR measures are now included in revascularization guidelines7. Yet, given its invasive nature, FFR cannot be applied to broad populations as a screening tool, nor during ICA are FFR measurements routinely performed for each potential lesion.
In the realm of non-invasive imaging, coronary computed tomography angiography (CTA) has gained support as a potential ICA substitute for anatomical assessment, while myocardial perfusion imaging (MPI) techniques continue to play a role as a powerful tool for the detection and quantification of perfusion defects. Additionally, in recent years positron emission tomography (PET) has been recognized as the superior nuclear perfusion modality compared to single photon emission computed tomography (SPECT)8,9. Its diagnostic and prognostic accuracy is significantly enhanced particularly in patients with multi-vessel disease and it has the unique capability of non-invasively quantifying absolute regional myocardial blood flow (MBF)10–12. Numerous studies have unequivocally established that reduced MBF and myocardial flow reserve (MFR) have strong prognostic and diagnostic value13–17 and PET-derived absolute flow quantification has proven its ability to extend the traditional investigations of advanced flow-limiting epicardial CAD to early stages of atherosclerosis and microvascular dysfunction12,18–20.
However, PET measurements of MBF and MFR are currently calculated either globally for the entire left ventricle or regionally to generic vascular or segmental territories. This approach is limited by the intermixing of normal flow from normal vessels with abnormal flows from diseased vessels thus reducing the measured degree of flow-impairment and limiting the diagnostic performance to detecting multi-vessels disease. Even when flow is measured in conventional vascular territories or myocardial segments, the variability of vessel paths between patients in relation to myocardial regions reduces the accuracy of the diagnosis21,22.
In previously published work by our team, we proved that the combined assessment of coronary anatomy from CTA and relative perfusion from MPI (either SPECT or PET) results in a diagnostically superior quantitative evaluation of CAD and myocardium-at-risk23 that exceeds stand-alone MPI or CTA assessment24 and has an excellent long-term predictor of adverse cardiac events25.
In the DEMYSTIFY study (Dynamic PET/CTA Myocardial Blood Flow Assessment with Fused Imagery), we propose to further enhance our multimodality approach by means of PET-derived non-invasive absolute MBF quantification. The 3D image fusion of dynamic PET (dPET) and CTA will allow to measure MBF, MFR and relative flow reserve (RFR)26–28 along patient-specific coronary trajectories and across specific lesions visible on the CTA images29. Differently from previous investigations, comprehensive invasive functional measurements, i.e. FFR, coronary flow reserve (CFR) and the index of microvascular resistance (IMR), will be obtained during catheterization and used as a reference standard to validate the accuracy of our methodology.
The rationale of this approach is to reduce or eliminate unnecessary catheterizations and stenting procedures while reducing patient risk, radiation exposure, and unnecessary costly invasive treatments. Dynamic PET/CTA image fusion will result in a game changing paradigm by: 1) eliminating unnecessary ICAs in patients with no significant lesions, 2) avoiding stenting non flow-limiting lesions, 3) guiding the percutaneous coronary intervention process to the location of significant lesions, and 4) providing a flow 3D roadmap of the entire coronary tree to guide bypass surgery when needed.
Study Objectives
The goal of this study is to provide a novel non-invasive methodology for image-guided therapy for patients with known or suspected CAD. Our diagnostic tool will assist physician’s decision in guiding referral for invasive procedures and particularly treatment selection to improve patients’ outcomes.
Study Hypothesis
Our primary hypothesis is that our novel multimodality 3D image fusion approach29 for vessel-specific MBF, MFR and RFR quantification will accurately and non-invasively predict lesion-specific ICA guidance for revascularization decisions with the long-term goal of providing a non-invasive tool to selectively identify who will benefit from catheterization and revascularization, thus eliminating unnecessary procedures.
Our secondary hypothesis is that our non-invasive lesion-specific and vessel-specific measurements of MBF, MFR, RFR are significantly more diagnostically accurate compared to the traditional approaches, such as relative database perfusion quantification approaches, traditional MBF assessment of standard vascular territories and segments, left ventricular MBF parametric quantification, and computational fluid dynamic (CFD)-based FFRCT30 quantification.
The study will also focus on the assessment of microvascular function and on its role31 in the identification of flow-limiting lesions, particularly in the comparison between FFRCT and PET-derived MBF quantification approaches.
Methods
I. Patient Enrollment, Imaging Protocols and Data Collection
The DEMYSTIFY study is an observational prospective clinical study to develop algorithms and software tools to fuse coronary anatomic data obtained from CTA with dPET data to non-invasively measure absolute MBF, MFR and RFR along vessels centerlines and across coronary lesions. These results will be compared to those obtained invasively in the catheterization laboratory and with the assessment of lesions hemodynamic significance evaluated by means of FFRCT.
Collaborating Centers and Institutions
Four institutions (Emory University Hospital, Atlanta, USA; Chonnam National University Hospital, Gwangju, South Korea; Samsung Medical Center, Seoul, South Korea; Seoul National University Hospital, Seoul, South Korea) will be participating in the study to create a database of comprehensive invasive and non-invasive imaging datasets and measurements. For each patient the following image data will be collected (Figure 1): dynamic PET, CTA and ICA images. Furthermore, during cardiac catheterization FFR, CFR and IMR for at least one major vessel will be measured. In collaboration with Heart Flow Inc, CFD-based computation of FFRCT will be performed.
Figure 1.
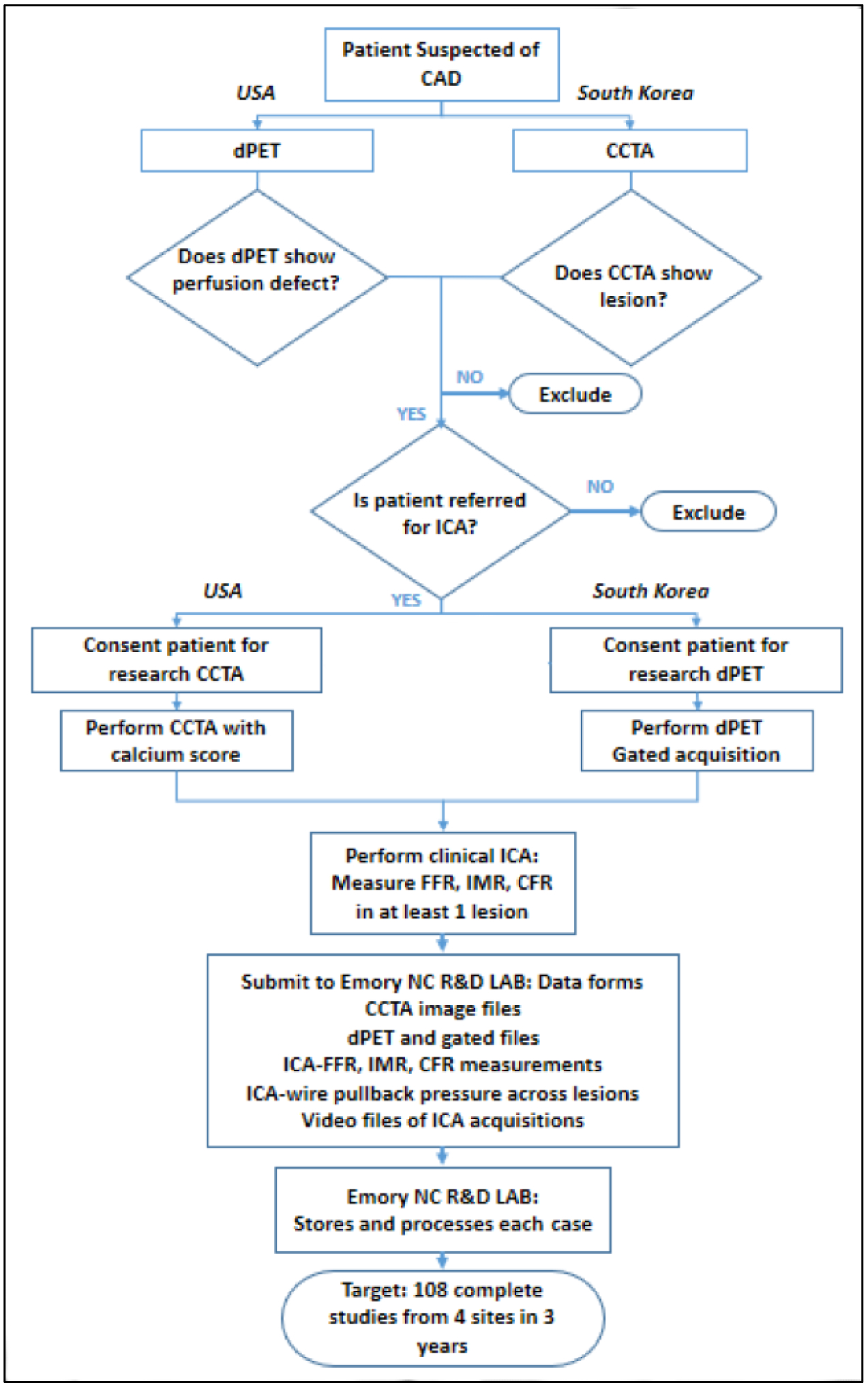
Study flow chart detailing patient enrollment for the DEMYSTIFY study in US and South Korea centers. CAD, coronary artery disease. dPET, dynamic positron emission tomography. CTA, coronary computed tomography angiography. ICA, invasive coronary angiography. FFR, fractional flow reserve. IMR, index of microcirculatory resistance. CFR, coronary flow reserve.
Patient Enrollment and Eligibility
Patients with ischemic heart disease symptoms referred to an initial imaging test for the assessment of CAD will be screened at each institution and considered for inclusion in the study. Different hospital policies and guidelines determine which initial test is commonly requested: at Emory University Hospital patients with known or suspected CAD who underwent a cardiac PET will be considered for enrollment; at South Korean centers patients who underwent a CTA will be screened and considered for enrollment. Patients with an abnormal initial diagnostic test (either PET or CTA) that are referred to clinically indicated cardiac catheterization will be invited to participate in the study.
Informed consent approved by the Institutional Review Board will be obtained and a second imaging test will be performed as a research procedure: a CTA at Emory University Hospital and a cardiac PET at the South Korean centers. During coronary angiography patients will undergo FFR evaluation as recommended by guidelines, while additional coronary physiology evaluation will be performed by measuring CFR and IMR for the same vessel as a research procedure.
The study will collect 108 datasets over three years of patient enrollment, i.e. 7 patients per year per center (84 subjects) plus 24 cases retrospectively collected at Seoul National University Hospital. At least one vessel per patient will be investigated during ICA. In case invasive measurements are not feasible (i.e. total obstruction) or deemed unsafe for the patient (i.e. tortuous vessels, severe calcifications, …) either the measurements will not be performed and the subject removed from the study, or a different compromised vessel will be investigated. All data will be collected, anonymized and analyzed by the investigators at the Emory Nuclear Cardiology R&D Laboratory.
Comprehensive inclusion and exclusion criteria are listed in Table 1.
Table 1:
Comprehensive inclusion and exclusion criteria for the DEMYSTIFY study.
| Eligibility Criteria |
|---|
| Inclusion criteria |
|
| Exclusion criteria |
|
CTA: Coronary computed tomography angiography; dPET: dynamic positron emission tomography
Medical Imaging Protocols
Since this is a multi-center study, different imaging devices and radiotracers will be used. Dynamic PET studies will be performed in South Korea with 13NH3, while with 82Rb at Emory University Hospital. Clinical guidelines will be followed by each center and agreements have been reached on performing identical protocols whenever possible, or equivalent in all other instances. In the following, we are illustrating the Emory University Hospital protocols for CTA, dPET and ICA.
A. Dynamic Cardiac PET (dPET).
Myocardial rest/stress perfusion imaging will be done using conventional clinical protocols. At Emory University Hospital, 82Rb PET will be performed. Image acquisition, reconstruction and processing will be done according to the ACC/AHA/ASNC guidelines for cardiac radionuclide imaging32. Patients will be asked to fast overnight and abstain from using methylxanthine- and caffeine-containing beverages for 24 hours prior to the test. Vasodilator medications, such as beta-blockers or calcium channel blockers, will be stopped for 24 hours. Before the resting perfusion phase, a single low-dose CT-based transmission scan is acquired for attenuation correction (AC) of all subsequent acquisitions. AC-CT images are automatically registered to the perfusion images, visually verified and manually corrected if necessary. Resting perfusion imaging started with the intravenous injection of a single bolus of 82Rb. Pharmacological stress imaging is obtained after rapid injection through a peripheral vein of 0.4 mg of regadenoson (5 mL solution, followed by a saline flush), followed by a second dose of 82Rb. Image reconstruction is achieved by means of ordered subset expectation maximization (OSEM) iterative method. The hemodynamic responses to rest/stress tests are collected in terms of mean heart rate, mean blood systolic pressures and diastolic at rest and stress. Dynamic, gated and ungated trans-axial reconstructions are saved in DICOM format for further analysis and processing at Core Laboratory.
B. Coronary Computed Tomography Angiography (CTA).
The CTA will be performed following established protocols according to the AHA clinical guidelines33. CTA examinations at Emory University Hospital will be performed on a third-generation dual-source CT scanner (SOMATOM Force, Siemens Healthineers, Forchheim, Germany). Briefly, after careful screening and informed consent, fasting patients will undergo a test for coronary calcium by CT; calcium scoring analysis will be done post image data acquisition using the manufacturer’s software. Nitroglycerine will be administered in all patients (sublingual administration prior to CTA initiation). CT acquisitions will be prospectively ECG-gated (30–80% of the cardiac cycle) with following technical parameters: adaptive detector collimation varying from 96–192 in step of 8× 0.6 mm; gantry rotation time 250ms; tube current-time product, 200–650 mAs (CARE Doses4D, Siemens), tube voltage 70–130 kV in 10-kV increments using an automated tube voltage selection algorithm (CARE kV; Siemens). The acquisition begins with a scout scan to identify the borders of the heart to minimize the field of view and exposure to the patient. A bolus of 60 mL nonionic contrast agent is then injected followed by 60 mL of saline at a rate of 4 mL/s to enhance signal from coronary arteries and blood chambers. In case of irregular heart rate, beta-blockers can be provided to keep optimal heart rate ~65–70 bpm. Trans-axial images are reconstructed by means of a filtered back-projection algorithm. For the present study, the diastolic phase (located between 60–75% of the cardiac cycle) will be selected for successive processing as it allows a relative motion free visualization of the main vessels and the myocardium.
Since CTA-derived FFR may compete with and/or complement our dPET-derived MBF measurements, we have entered into a collaboration agreement with Heart Flow Inc (Redwood City, CA) (Figure 2). After anonymization, CTA acquisitions will be uploaded to Heart Flow servers for processing and results made available for visualization and download.
Figure 2.
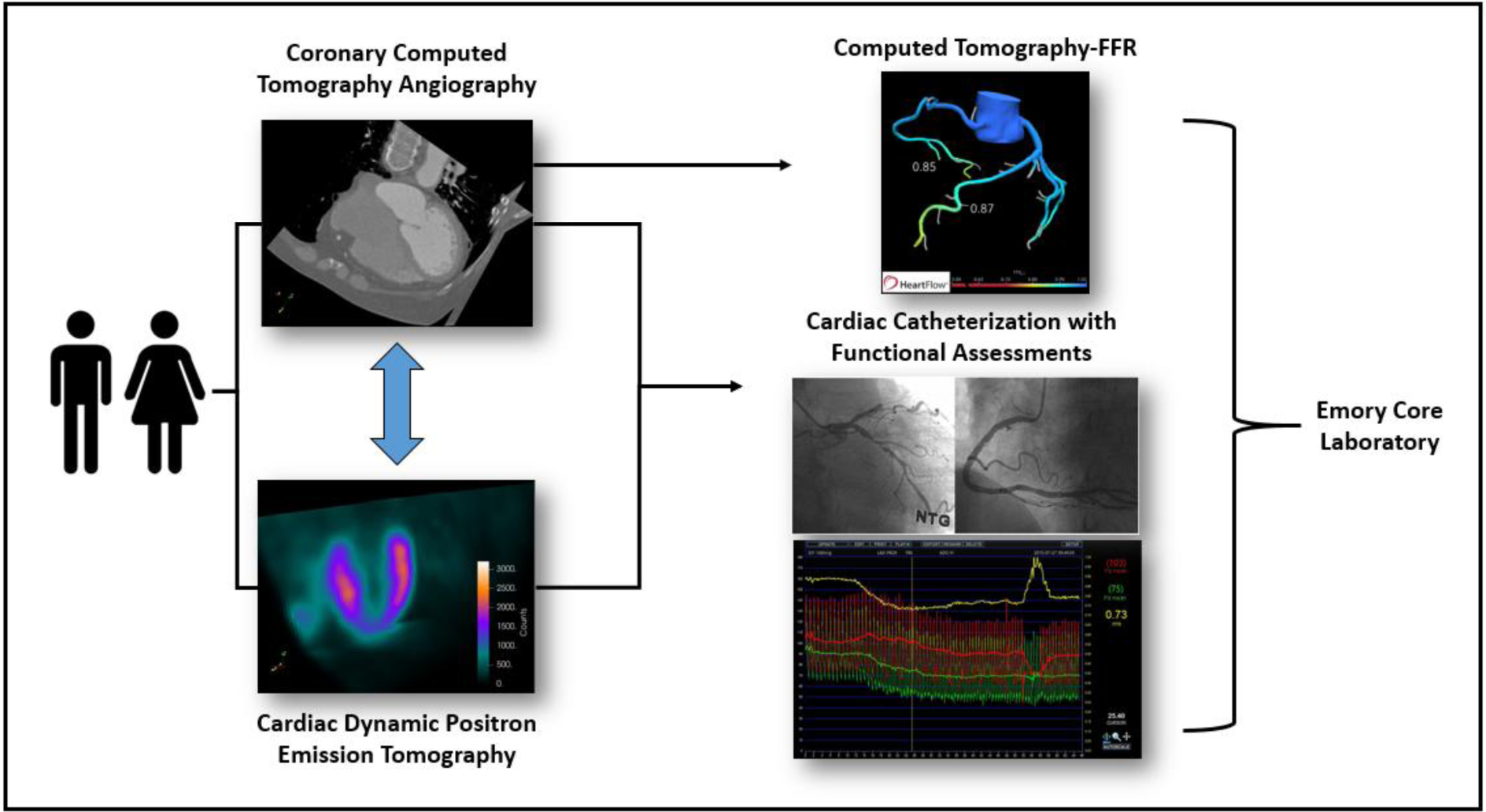
Imaging data and functional assessments collected per patient in the DEMYSTIFY study.
C. Invasive Coronary Angiography (ICA).
Coronary arteriography will be done using a standard coronary angiogram protocol. A diagnosis of obstructive CAD will be based on one or more of the major coronary vessels having at least one stenosis with ≥ 50% luminal narrowing or significant diffuse disease. Luminal-narrowing will be quantitatively assessed by an experienced interventional cardiologist. Invasive functional measurements will be performed to assess the functional significance of the culprit vessel by means of FFR, CFR and IMR. The culprit vessel will be identified as the one that most likely produced the perfusion abnormality on the PET images at Emory Hospital or the one that directed the referral to ICA on the basis of CTA images at the South Korean centers. In summary, a 5− to 7-F guide catheter without side holes is used to engage the coronary artery and a pressure-temperature sensor-tipped guidewire introduced (PressureWire X, Abbott medical, Lake Forest, IL). The pressure sensor is positioned at the distal segment of the target vessel, and intracoronary nitroglycerine (100–200 mg) administered before each measurement. To derive resting mean transit time (Tmn), a thermodilution curve is obtained by using 3 injections of 3 ml of room temperature saline. Hyperemia is induced via peripheral vein infusion of intravenous adenosine (140 mg/kg/min). Hyperemic proximal aortic pressure (Pa), distal arterial pressure (Pd), and hyperemic Tmn are measured during sustained hyperemia. FFR (Pd/Pa at hyperemia) is calculated as the lowest average of 3 consecutive beats during stable hyperemia. CFR is calculated as (resting Tmn / hyperemic Tmn). The uncorrected IMR is calculated by Pd × Tmn during hyperemia. All IMR values are also corrected by using Yong’s formula34 (IMRcorr = Pa × Tmn × ([1.35×Pd/Pa] − 0.32)) to adjust for the influence of collateral flow. An FFR pullback recording will also be performed distal to proximal as sensors are removed from the investigated vessel. The results from the ICA including all functional measurements will be used as standard reference to assess the performance of the non-invasive strategies (Figure 2).
II. Image Processing, Fusion Procedure and 3D Modeling
All images and data will be transferred to the Emory Core Laboratory for further processing. The following steps will be here briefly presented: CTA-derived anatomy retrieval, relative myocardial perfusion and absolute MBF quantification, dPET/CTA 3D image fusion and vessel-specific functional assessments.
A. Anatomy Retrieval.
A crucial step of our multi-modality image strategy resides in the extraction of heart anatomy from CTA images, particularly coronary centerlines and right and left myocardium. Our team has been continuously working on the development of semi-automated techniques for the time-efficient extraction of the myocardium and the coronaries centerlines. While we refer to more in-depth descriptions of our proposed methodologies35, the algorithms rely on a level set formulation and the extraction of shape priors from a set of training images that are then applied prospectively to new cases. An extensive validation on a database of clinical CTAs (n=70) is currently underway. The extraction of the coronaries is a substantially less laborious task and can be performed in few minutes. The final results of the anatomy retrieval consist of a 3D biventricular model of the myocardium and a patient-specific network of centerlines identifying the vessel trajectories (Figure 3A).
Figure 3: Image Processing, Fusion Procedure and 3D Modeling.
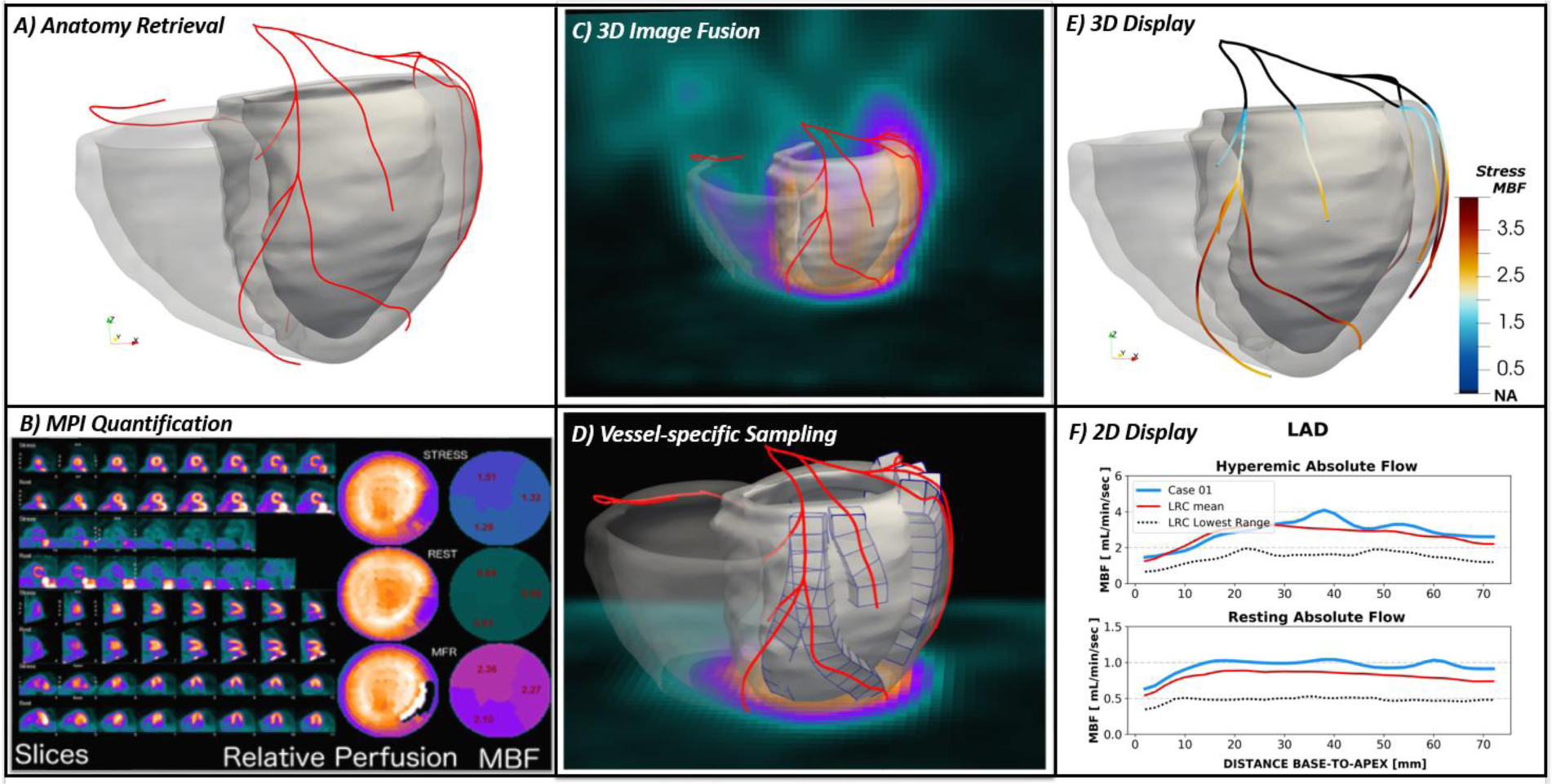
A) Coronary computed tomography angiography (CTA)-derived anatomy in terms of biventricular myocardium and coronary centerlines; B) Standard MPI quantification include polar maps of relative perfusion assessment, polar maps of rest/stress myocardial blood flow (MBF) [mL/min/g] and myocardial flow reserve (MFR) on standard vascular territories; C) Fusion display showing the CTA-derived anatomy superimposed to the MPI; D) identification of volumes of interest along the specific 3-dimensional trajectories of coronary arteries. Creation of column of contiguous volumetric elements within the CTA-derived myocardium along each vessel; E) 3-dimensional display of CTA-derived anatomy with the coronary centerlines color-coded with the computed vessel-specific hyperemic MBF; F) 2-dimensional plot of vessel-specific quantification of the left anterior descending artery (LAD) compared to normal ranges of MBF from base-to-apex.
B. Myocardial Perfusion and Absolute MBF Quantification.
The Emory Cardiac Toolbox36 (ECTb) will be used to automatically process all nuclear studies. The quantification of MBF will be performed by means of 1-tissue compartmental model with appropriate corrections for spillover and partial volume effects and tracer’s extraction fraction29. Relative perfusion as well as absolute MBF will be extracted according to the standard vascular territories and segments classification and displayed in conventional polar maps (Figure 3B).
C. Fusion Procedure and vessel-specific MBF assessments.
Our 2nd generation image fusion techniques23,37 will be used to register the CTA acquisition to the nuclear ones (Figure 3C). The procedure allows to spatially align the images and co-localize anatomical structures. Particularly, the myocardium subtended to each coronary 3D trajectory will be discretized in small volumetric elements to be used as new volumes of interest (VOIs) to sample the dPET data and calculate MBF. As the discretization follows the vessel from the base to the apex of the heart (Figure 3D), a vessel-specific profile of MBF can be derived to be displayed as on a 3D color-coded model (Figure 3E) or with a 2D plot (Figure 3F). Additional vessel-specific functional indexes will be computed, such as MFR and RFR29.
III. Statistical Analysis
Continuous variables will be presented as means, medians, standard deviations and ranges. Differences between groups will be assessed using the t-test for continuous variables and chi-square for categorical variables. For non-normally distributed variables, Mann-Whitney U test will be used to compare groups in unadjusted analyses.
Vessels as well as individual lesions will be divided into two subgroups based on invasive FFR value as indicated in clinical guidelines38: abnormal for FFR≤0.8 and normal for FFR>0.8. The clinically accepted threshold of IMR = 25 will be used to identify vascular beds exhibiting microvascular dysfunction or not.
The percentage of abnormal FFR lesions with abnormal RFR (based on normal limits) will be determined. The clinical thresholds for FFR and RFR per lesion location will be used to dichotomize both measures with a 5% level of significance; since a sample size of 44 vessels (with at least one lesion) yields a power of at least 95% to detect a Kendall’s Coefficient of Concordance of 0.8, the data collected during the DEMYSTIFY study (at least 108 vessels/lesions) will allow such analysis.
Sensitivity/specificity Analysis:
Power considerations are based on sensitivity and specificity, which are assumed to follow a binomial distribution. Using the two-sided 95% confidence interval, a minimum sample size of 44 patients will be required to confirm that the sensitivity will be higher than 80%, assuming the expected sensitivity will be approximately 90%. The expected value of specificity will also be about 90% so that the minimum sample size will also be 44 to confirm that the specificity will be higher than 80% with a 95% confidence level. In the study, we will have at least 108 complete evaluable vessels (with ICA FFRs, CFRs and IMRs) thus we will have enough sample size to carry out these tests of sensitivity and specificity, respectively to test our hypothesis on a per vessel basis.
Receiver Operating Characteristic (ROC) analysis:
The predictive discriminatory power of each of the 3 techniques (ICA, dPET/CTA, FFRCT) will be determined using ROC analysis and measured by the area under the curve (AUC). To investigate whether each technique has a significant predictive power, the AUC for each of the 3 techniques will be measured and tested as to whether it is significantly different from 0.5 (no discrimination ability). To test the difference in their predictive discriminatory powers among three techniques, comparison on AUCs of ROC curves using repeated measures analysis of variance (rANOVA) with Tukey’s post-hoc analysis. The analysis will be conducted for ICA, dPET/CTA, and FFRCT respectively. The anatomical and physiological findings during ICA will be the gold standard reference for comparison. Furthermore, to investigate whether there are significant predictive power differences when using 82Rb versus 13NH339, these ROC analyses will be performed in three stages: 1) with the data from both tracers merged, 2) only using the patients/vessels acquired with 82Rb, and 3) only using the patients/vessels acquired with 13NH3. Analogously the well-known impact40 of different stressors on peak hyperemia (at Emory Hospital regadenoson will be used; at South Korea centers adenosine will be administered) will be evaluated. Significance level will be set at 0.05. Similar analyses will be performed for the diagnostic performance of dPET/CTA, generic territories and the database approach.
Power and Sample Size Calculation:
The minimum expected AUC of ROC curve among all 3 three techniques is assumed to be more than 0.75. At the significance level of 0.05, a sample of 26 from the positive group and 26 from the negative group will achieve 91% power to detect a significant predictive power of each technique with an improvement in AUC of at least 0.25 higher than the null hypothesis of no predictive power (AUC= 0.50) using a two-sided z-test. For the pairwise comparisons, we expect that the technique with better predictive power will have an AUC of 0.94 or higher, and the one with worse predictive power will have an AUC of 0.8 or lower. A sample of 52 from the positive group and 52 from the negative group will achieve 80% power to detect a technique of a significantly better predictive power with a difference in AUC of at least 0.14 using a two-sided z-test at a significance level of 0.05. We will have at least 108 complete evaluable vessels out of the 104 needed.
Potential problems and alternative strategies.
As data will be collected from the US and South Korea, a brief comment related to the differences in population ethnicity is warranted. While patients at Emory University Hospitals can be considered very diverse, patients from South Korean centers will likely be mostly of Asian ethnicity. Even if we do anticipate that data and results from the 4 different sites will be consistent and generalizable, we will plan to include sensitivity analysis to explore the impact of ethnicity on our results.
If the accuracy of our PET/CTA fusion approach does not add value to the conventional relative perfusion approach or is not superior to the accuracy of the other techniques considered, two reasons can be suggested. First, the error in the PET-derived flow measurements can be greatly increased due to technical reasons such as patient motion, respiration and partial volume effects. We will study these issues and develop corrections methods to be applied to each PET dataset with expectations of reduced errors in the flow measures. Second, microvascular dysfunction may cause discrepancy between FFRCT by CTA and MBF/MFR/RFR by PET. We will explore its role subdividing the collected vessels/lesions data into two groups: A) IMR > 25 and B) IMR ≤ 2541. We hypothesize that our fusion framework will have a higher discriminatory power than FFRCT or Group A, whereas the two techniques will have similar power in detecting hemodynamically significant lesions for Group B.
Discussion
In response to a strong mandate for physiological blood flow assessments of coronary lesions to reduce improper referral of patients to catheterization42 and worse to improper revascularization3–5, we have evolved our fusion framework from databased relative quantification of SPECT studies43 to absolute flow measures from cardiac PET imges44–46. Our main goal is to develop a non-invasive method to localize physiologically significant coronary lesions suitable for revascularization anywhere in the coronary tree.
Although PET flow measurements have been developed by others, their single modality approaches are not lesion-specific, which is the main innovation of our fused dPET/CTA non-invasive approach. We anticipate that our lesion-specific approach will be significantly more diagnostically accurate compared to a) the traditional database relative perfusion quantification and b) absolute MBF assessment that relies on generic vascular territories that will likely mix normal and abnormal vascular areas reducing the accuracy of the flow measurements. Furthermore, as compared to other qualitative fusion approaches47, our fusion approach will provide comprehensive anatomical and physiological information obtained from fully quantitative analysis of stand-alone imaging modalities.
Our proposed non-invasive determination of MBF along the vascular tree is made possible and clinically feasible by: 1) robust epicardium and endocardium border definition, vessel centerline segmentation and complete 3D left ventricular rendering in clinically acceptable times, 2) PET fusion with CTA and 3D rendering allowing the registration of coronaries and myocardial tissue, 3) non-invasive extraction of lesion-specific and vessel-specific MBF/MFR/RFR and localization of physiologically significant lesions by comparison to normal flow limits and 4) automatic quality control, correction for motion and partial volume effects to more accurately measure MBF. These innovations have not been proposed or performed by anyone to date.
New Knowledge Gained
We anticipate the dPET/CTA MBF methodology will allow the non-invasive localization of physiologically significant coronary lesions suitable for revascularization anywhere in the coronary tree.
Supplementary Material
Source of Funding
The DEMYSTIFY study is an investigator-initiated clinical study that is funded by the National Institute of Health (NIH) Grant R01 HL143350-01 (PI: EV Garcia) from the National Heart, Lung, And Blood Institute of the NIH. The design, conduct of the study, all analyses, the drafting and editing of the paper, and its final contents are solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The DEMYSTIFY study has been registered on ClinicalTrials.gov with registration number NCT04221594
Abbreviations
- CFD
computational fluid dynamic
- CFR
coronary flow reserve
- DEMYSTIFY
Dynamic PET/CTA Myocardial Blood Flow Assessment with Fused Imagery
- dPET
dynamic cardiac PET
- FFR
fractional flow reserve
- ICA
invasive coronary angiography
- IMR
index of microvascular resistance
- MBF
myocardial blood flow
- MFR
myocardial flow reserve
- RFR
relative flow reserve
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Disclosure.
Dr. Ernest Garcia receives royalties from the sale of the Emory Cardiac Toolbox and have equity positions with Syntermed, Inc. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. The remaining authors did not report any conflicts of interest.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Peterson ED, Dai D, et al. Low Diagnostic Yield of Elective Coronary Angiography. New England Journal of Medicine. 2010;362(10):886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. Journal of the American College of Cardiology. 2010;55(25):2816–2821. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. The New England journal of medicine. 2012;367(11):991–1001. [DOI] [PubMed] [Google Scholar]
- 5.Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. Journal of the American College of Cardiology. 2010;56(3):177–184. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–1291. [DOI] [PubMed] [Google Scholar]
- 7.Lee AK, Qutub MA, Aljizeeri A, Chow BJ. Integrating anatomical and functional imaging for the assessment of coronary artery disease. Expert review of cardiovascular therapy. 2013;11(10):1301–1310. [DOI] [PubMed] [Google Scholar]
- 8.Sheth T, Yusuf S. Enhancing risk prediction with PET coronary flow reserve: can it be clinically useful? JACC Cardiovascular imaging. 2012;5(10):1035–1036. [DOI] [PubMed] [Google Scholar]
- 9.Machac J Cardiac positron emission tomography imaging. Seminars in nuclear medicine. 2005;35(1):17–36. [DOI] [PubMed] [Google Scholar]
- 10.Fiechter M, Ghadri JR, Gebhard C, et al. Diagnostic value of 13N-ammonia myocardial perfusion PET: added value of myocardial flow reserve. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53(8):1230–1234. [DOI] [PubMed] [Google Scholar]
- 11.Ziadi MC, Dekemp RA, Williams K, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19(4):670–680. [DOI] [PubMed] [Google Scholar]
- 12.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovascular imaging. 2010;3(6):623–640. [DOI] [PubMed] [Google Scholar]
- 13.Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50(7):1076–1087. [DOI] [PubMed] [Google Scholar]
- 14.Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. European heart journal cardiovascular Imaging. 2013;14(12):1203–1210. [DOI] [PubMed] [Google Scholar]
- 15.Bravo PE, Bengel FM. The role of cardiac PET in translating basic science into the clinical arena. Journal of cardiovascular translational research. 2011;4(4):425–436. [DOI] [PubMed] [Google Scholar]
- 16.Slart RH, Zeebregts CJ, Hillege HL, et al. Myocardial perfusion reserve after a PET-driven revascularization procedure: a strong prognostic factor. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52(6):873–879. [DOI] [PubMed] [Google Scholar]
- 17.Naya M, Murthy VL, Taqueti VR, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camici PG, Crea F. Coronary microvascular dysfunction. The New England journal of medicine. 2007;356(8):830–840. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. European heart journal. 2012;33(22):2771–2782b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schelbert HR. Anatomy and physiology of coronary blood flow. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2010;17(4):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereztol-Valdes O, Candell-Riera J, Santana-Boado C, et al. Correspondence between left ventricular 17 myocardial segments and coronary arteries. European heart journal. 2005;26(24):2637–2643. [DOI] [PubMed] [Google Scholar]
- 22.Liga R, Vontobel J, Rovai D, et al. Multicentre multi-device hybrid imaging study of coronary artery disease: results from the EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease (EVINCI) hybrid imaging population. European heart journal cardiovascular Imaging. 2016;17(9):951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccinelli M, Santana C, Sirineni GKR, et al. Diagnostic performance of the quantification of myocardium at risk from MPI SPECT/CTA 2G fusion for detecting obstructive coronary disease: A multicenter trial. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2018;25(4):1376–1386. [DOI] [PubMed] [Google Scholar]
- 24.Santana CA, Garcia EV, Faber TL, et al. Diagnostic performance of fusion of myocardial perfusion imaging (MPI) and computed tomography coronary angiography. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2009;16(2):201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pazhenkottil AP, Benz DC, Grani C, et al. Hybrid SPECT Perfusion Imaging and Coronary CT Angiography: Long-term Prognostic Value for Cardiovascular Outcomes. Radiology. 2018;288(3):694–702. [DOI] [PubMed] [Google Scholar]
- 26.Stuijfzand WJ, Uusitalo V, Kero T, et al. Relative flow reserve derived from quantitative perfusion imaging may not outperform stress myocardial blood flow for identification of hemodynamically significant coronary artery disease. Circulation Cardiovascular imaging. 2015;8(1). [DOI] [PubMed] [Google Scholar]
- 27.Lee JM, Kim CH, Koo BK, et al. Integrated Myocardial Perfusion Imaging Diagnostics Improve Detection of Functionally Significant Coronary Artery Stenosis by 13N-ammonia Positron Emission Tomography. Circulation Cardiovascular imaging. 2016;9(9). [DOI] [PubMed] [Google Scholar]
- 28.Johnson NP, Gould KL. Fractional Flow Reserve Returns to Its Origins: Quantitative Cardiac Positron Emission Tomography. Circulation Cardiovascular imaging. 2016;9(9). [DOI] [PubMed] [Google Scholar]
- 29.Piccinelli M, Cho SG, Garcia EV, et al. Vessel-specific quantification of absolute myocardial blood flow, myocardial flow reserve and relative flow reserve by means of fused dynamic (13)NH3 PET and CCTA: Ranges in a low-risk population and abnormality criteria. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. Journal of the American College of Cardiology. 2013;61(22):2233–2241. [DOI] [PubMed] [Google Scholar]
- 31.Garcia D, Harbaoui B, van de Hoef TP, et al. Relationship between FFR, CFR and coronary microvascular resistance - Practical implications for FFR-guided percutaneous coronary intervention. PloS one. 2019;14(1):e0208612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC Guidelines for the Clinical Use of Cardiac Radionuclide Imaging—Executive Summary. Circulation. 2003;108(11):1404–1418. [DOI] [PubMed] [Google Scholar]
- 33.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2012;60(24):e44–e164. [DOI] [PubMed] [Google Scholar]
- 34.Yong AS, Layland J, Fearon WF, et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovascular interventions. 2013;6(1):53–58. [DOI] [PubMed] [Google Scholar]
- 35.Dahiya N, Yezzi A, Piccinelli M, Garcia E. Integrated 3D anatomical model for automatic myocardial segmentation in cardiac CT imagery. Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization. 2019:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C. The increasing role of quantification in clinical nuclear cardiology: the Emory approach. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2007;14(4):420–432. [DOI] [PubMed] [Google Scholar]
- 37.Faber TL, Santana CA, Piccinelli M, et al. Automatic Alignment of Myocardial Perfusion Images With Contrast-Enhanced Cardiac Computed Tomography. IEEE transactions on nuclear science. 2011;58(5):2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2017;69(17):2212–2241. [DOI] [PubMed] [Google Scholar]
- 39.Murthy VL, Bateman TM, Beanlands DS, et al. Clinical quantification of myocardial blood flow using PET: Joint position paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Cardiol, 2018, 25(1):269–297. [DOI] [PubMed] [Google Scholar]
- 40.Johnson NP and Gould KL. Regadenoson vs dypirydamole hyperemia for cardiac PET imaging. JACC: Cardiovasc Imag, 2015, 8(4): 438–447. [DOI] [PubMed] [Google Scholar]
- 41.Melikian N, Vercauteren S, Fearon WF, et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2010;5(8):939–945. [PubMed] [Google Scholar]
- 42.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. The New England journal of medicine. 2010;362(10):886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia EV, Van Train K, Maddahi J, et al. Quantification of rotational thallium-201 myocardial tomography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1985;26(1):17–26. [PubMed] [Google Scholar]
- 44.Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. Journal of the American College of Cardiology. 2013;62(18):1639–1653. [DOI] [PubMed] [Google Scholar]
- 45.Tahari AK, Lee A, Rajaram M, et al. Absolute myocardial flow quantification with (82)Rb PET/CT: comparison of different software packages and methods. European journal of nuclear medicine and molecular imaging. 2014;41(1):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Tosh A, Votaw JR, Reichek N, Palestro CJ, Nichols KJ. The relationship between ischemia-induced left ventricular dysfunction, coronary flow reserve, and coronary steal on regadenoson stress-gated (82)Rb PET myocardial perfusion imaging. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013;20(6):1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaemperli O, Schepis T, Kalff V, et al. Validation of a new cardiac image fusion software for three-dimensional integration of myocardial perfusion SPECT and stand-alone 64-slice CT angiography. European journal of nuclear medicine and molecular imaging. 2007;34(7):1097–1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


