Abstract
High blood cholesterol is typically considered a feature of wealthy western countries1,2. However, dietary and behavioural determinants of blood cholesterol are changing rapidly throughout the world3 and countries are using lipid-lowering medications at varying rates. These changes can have distinct effects on the levels of high-density lipoprotein (HDL) cholesterol and non-HDL cholesterol, which have different effects on human health4,5. However, the trends of HDL and non-HDL cholesterol levels over time have not been previously reported in a global analysis. Here we pooled 1,127 population-based studies that measured blood lipids in 102.6 million individuals aged 18 years and older to estimate trends from 1980 to 2018 in mean total, non-HDL and HDL cholesterol levels for 200 countries. Globally, there was little change in total or non-HDL cholesterol from 1980 to 2018. This was a net effect of increases in low- and middle-income countries, especially in east and southeast Asia, and decreases in high-income western countries, especially those in northwestern Europe, and in central and eastern Europe. As a result, countries with the highest level of non-HDL cholesterol—which is a marker of cardiovascular risk—changed from those in western Europe such as Belgium, Finland, Greenland, Iceland, Norway, Sweden, Switzerland and Malta in 1980 to those in Asia and the Pacific, such as Tokelau, Malaysia, The Philippines and Thailand. In 2017, high non-HDL cholesterol was responsible for an estimated 3.9 million (95% credible interval 3.7 million–4.2 million) worldwide deaths, half of which occurred in east, southeast and south Asia. The global repositioning of lipid-related risk, with non-optimal cholesterol shifting from a distinct feature of high-income countries in northwestern Europe, north America and Australasia to one that affects countries in east and southeast Asia and Oceania should motivate the use of population-based policies and personal interventions to improve nutrition and enhance access to treatment throughout the world.
Subject terms: Cardiovascular diseases, Risk factors
From 1980 to 2018, the levels of total and non-high-density lipoprotein cholesterol increased in low- and middle-income countries, especially in east and southeast Asia, and decreased in high-income western countries, especially those in northwestern Europe, and in central and eastern Europe.
Main
Blood cholesterol is one of the most important risk factors for ischaemic heart disease (IHD) and ischaemic stroke4–6. Consistent and comparable information on cholesterol levels and trends in different countries can help to benchmark national performance in addressing non-optimal cholesterol, investigate the reasons behind differential trends and identify countries in which interventions are needed the most.
A previous global analysis7 reported trends in total cholesterol from 1980 to 2008, but did not analyse important lipid fractions—including HDL and non-HDL cholesterol—that are key to understanding the cardiovascular disease risk associated with non-optimal cholesterol. Dietary and behavioural determinants of cholesterol have changed throughout the world in the past decades, including a worldwide rise in adiposity8,9, divergent global trends in alcohol use10, a rise in the intake of animal-source foods in middle-income countries (especially in east Asia)3,11, and a replacement of saturated fats and trans fats with unsaturated fats in some high-income countries3,11,12. There is also considerable variation in how much different countries have adopted lipid-lowering medications13. These changes are likely to have influenced cholesterol levels substantially in the decade since the last estimates were made. Furthermore, HDL and non-HDL cholesterol, which have opposite associations with cardiovascular diseases4,5, respond differently to diet and treatment, and may therefore have different geographical patterns and trends over time14. Information on these major lipid fractions, which were not included in the previous global estimates, is essential for priority setting and intervention choice.
Here we pooled 1,127 population-based studies that measured blood lipids in 102.6 million individuals aged 18 years and older (Extended Data Figs. 1, 2 and Supplementary Table 1) and used a Bayesian hierarchical model to estimate trends from 1980 to 2018 in mean total, non-HDL and HDL cholesterol levels for 200 countries. We also estimated the number of deaths caused by IHD and ischaemic stroke that were attributable to high levels of non-HDL cholesterol using information on its hazards from epidemiological studies.
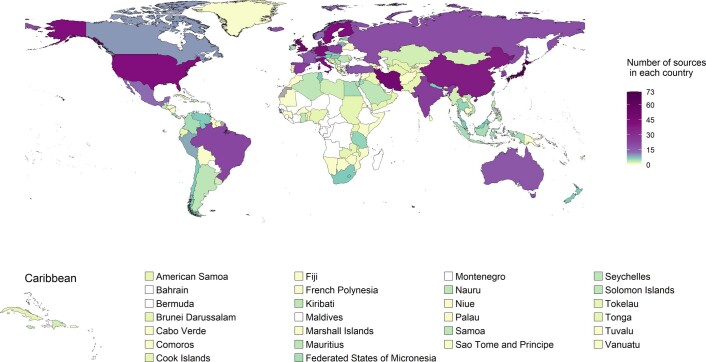
Extended Data Fig. 1. Number of data sources by country.
The colour indicates the number of data sources for each country used in the analysis. Countries and territories that were not included in the analysis are coloured in grey.
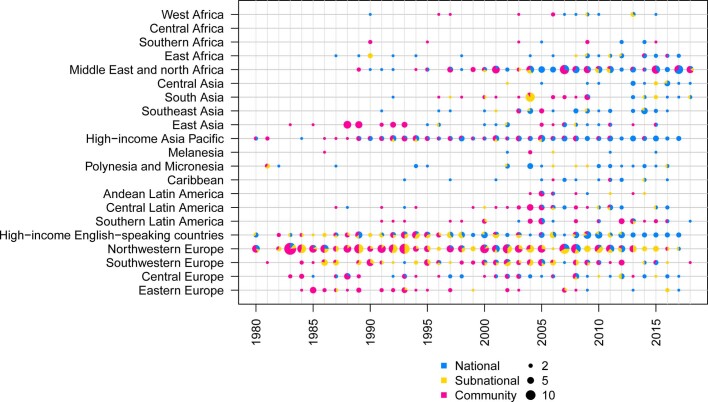
Extended Data Fig. 2. Number of data sources by region and year.
The size of each circle shows the number of data sources for each region and year, and the colours indicate the relative size of national, subnational and community data sources.
Trends in total cholesterol
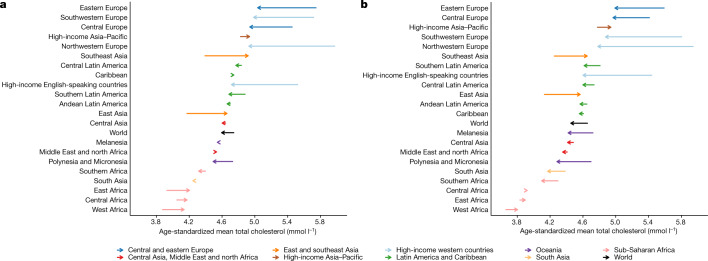
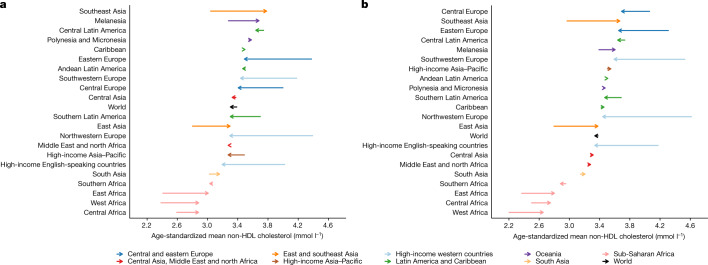
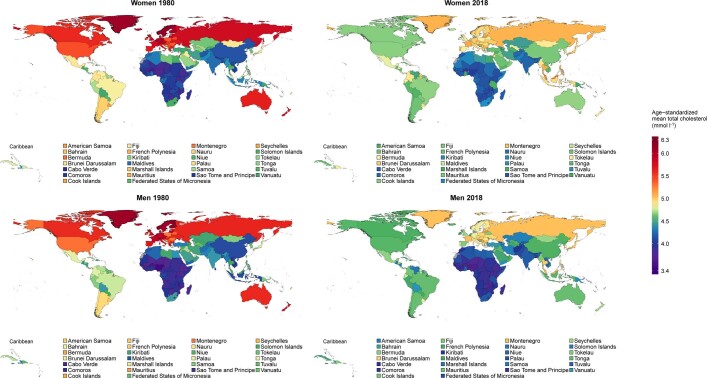
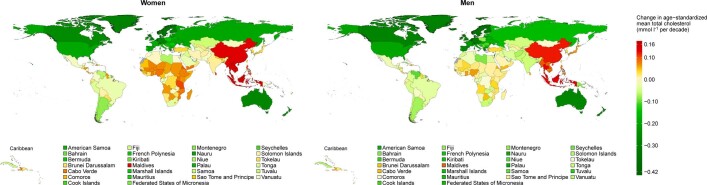
In 2018, global age-standardized mean total cholesterol was 4.6 mmol l−1 (95% credible interval, 4.5–4.7) for women and 4.5 mmol l−1 (4.3–4.6) for men. Global age-standardized mean total cholesterol changed little over these nearly four decades, decreasing by 0.03 mmol l−1 per decade (−0.02–0.08) in women and 0.05 mmol l−1 per decade (0.00–0.11) in men (posterior probability of the observed declines being true declines = 0.90 for women and 0.98 for men) (Fig. 1). Regionally, total cholesterol decreased the most in high-income western regions and in central and eastern Europe. The decrease was the largest (around 0.3 mmol l−1 per decade; posterior probability >0.9999) in northwestern Europe, where mean total cholesterol levels had been the highest in 1980. The decrease in total cholesterol in high-income western regions and central and eastern Europe was largely due to a decline in non-HDL cholesterol (Extended Data Fig. 4), which among women was offset partly by an increase in mean HDL cholesterol levels. Mean total cholesterol changed little in most of the other regions, with the notable exception of east and southeast Asia, where it increased by more than 0.1 mmol l−1 per decade in both women and men (posterior probability ≥0.95). The increase in east and southeast Asia was largely due to an increase in non-HDL cholesterol.
Fig. 1. Change in age-standardized mean total cholesterol between 1980 and 2018 by region for women and men.
a, Age-standardized mean total cholesterol in women. b, Age-standardized mean total cholesterol in men. The start of the arrow shows the level in 1980 and the head indicates the level in 2018. See Extended Data Fig. 3 for age-standardized mean HDL cholesterol. One mmol l−1 is equivalent to 38.61 mg dl−1.
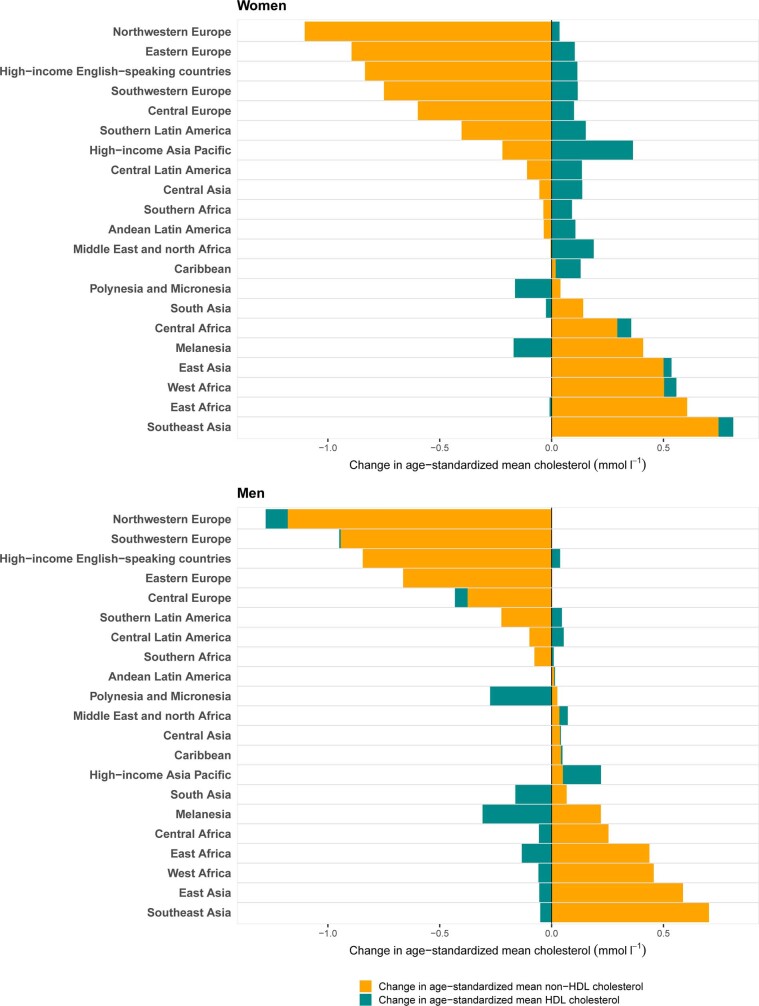
Extended Data Fig. 4. Change in age-standardized mean HDL and non-HDL cholesterol between 1980 and 2018 by region for women and men.
One mmol l−1 is equivalent to 38.61 mg dl−1.
Trends in non-HDL and HDL cholesterol
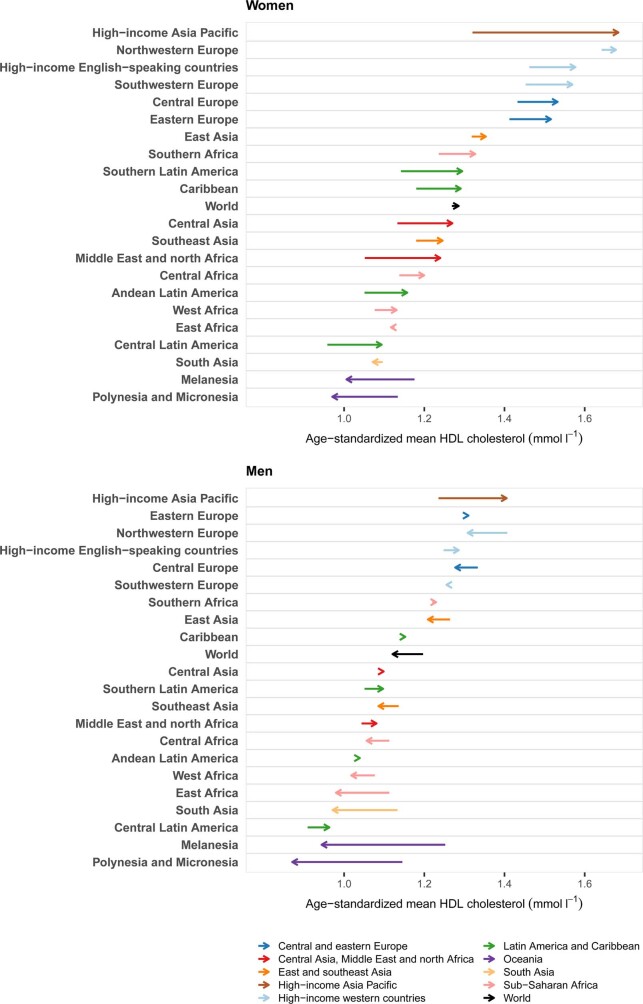
In 2018, global age-standardized mean non-HDL cholesterol was 3.3 mmol l−1 (3.2–3.4) for women and 3.3 mmol l−1 (3.3–3.4) for men; global age-standardized mean HDL cholesterol was 1.3 mmol l−1 (1.2–1.3) for women and 1.1 mmol l−1 (1.1–1.2) for men. Global age-standardized mean non-HDL cholesterol remained almost unchanged from 1980 to 2018, decreasing by only 0.02 mmol l−1 per decade (−0.02–0.06; posterior probability = 0.80) in women and 0.01 mmol l−1 per decade (−0.03–0.06; posterior probability = 0.72) in men. Global age-standardized mean HDL cholesterol remained unchanged for women and decreased slightly for men (by 0.02 mmol l−1 per decade, posterior probability = 0.91).
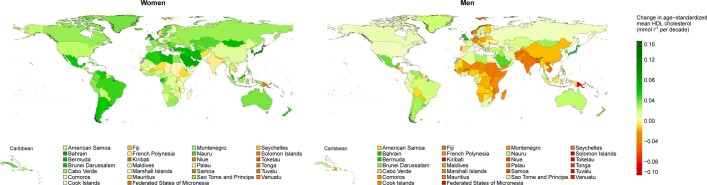
Regionally, non-HDL cholesterol decreased substantially in high-income western regions and central and eastern Europe. The largest decrease occurred in northwestern Europe (>0.3 mmol l−1 per decade; posterior probability >0.9999) (Fig. 2). By contrast, it increased in east and southeast Asia, parts of sub-Saharan Africa and Melanesia. The increase was the largest in southeast Asia, increasing by approximately 0.2 mmol l−1 per decade (posterior probability >0.9999). Mean HDL cholesterol increased in the high-income Asia–Pacific region, by as much as 0.1 mmol l−1 per decade in women (posterior probability >0.9999) but decreased in Melanesia, Polynesia and Micronesia (Extended Data Fig. 3).
Fig. 2. Change in age-standardized mean non-HDL cholesterol between 1980 and 2018 by region for women and men.
a, Age-standardized mean non-HDL cholesterol in women. b, Age-standardized mean non-HDL cholesterol in men. The start of the arrow shows the level in 1980 and the head indicates the level in 2018. See Extended Data Fig. 3 for age-standardized mean HDL cholesterol. One mmol l−1 is equivalent to 38.61 mg dl−1.
Extended Data Fig. 3. Change in age-standardized mean HDL cholesterol between 1980 and 2018 by region for women and men.
The start of the arrow shows the level in 1980 and the head shows the level in 2018. One mmol l−1 is equivalent to 38.61 mg dl−1.
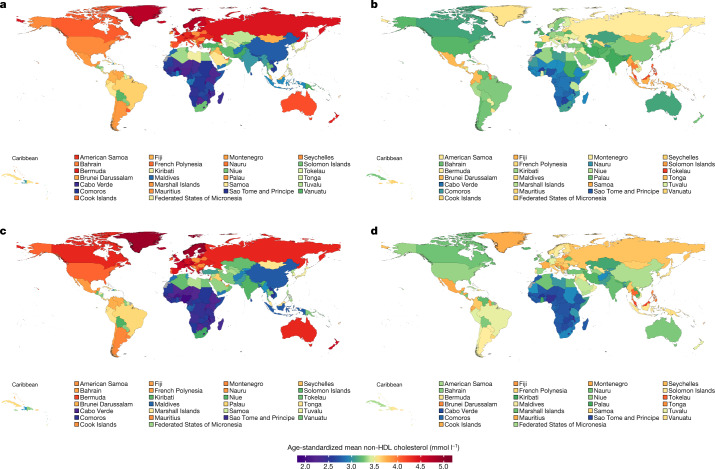
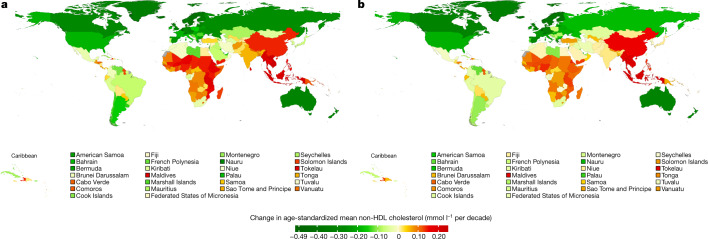
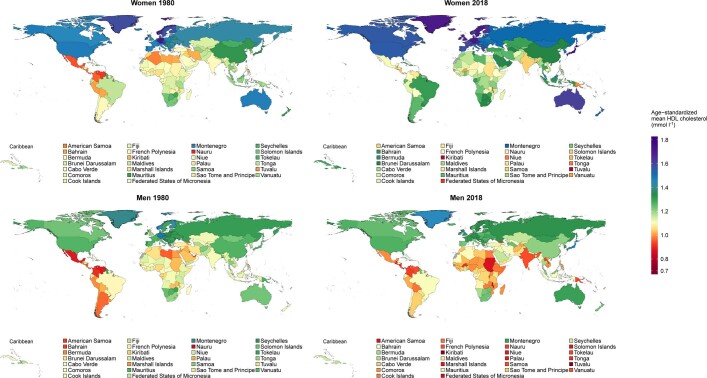
Belgium, Finland, Greenland, Iceland, Norway, Sweden, Switzerland and Malta had some of the highest non-HDL cholesterol levels in 1980 (>4.5 mmol l−1 in women and >4.7 mmol l−1 in men) but experienced some of the largest declines (Figs. 3, 4). At the extreme, mean non-HDL cholesterol declined by around 0.45 mmol l−1 per decade or more in Belgian and Icelandic women and men, changing their ranks from being in the top 10 countries in terms of non-HDL cholesterol in 1980 to being ranked in the lower half of the countries in 2018—below countries in southwestern Europe such as France and Italy. The largest increases were found in east Asian countries (for example, China) and southeast Asian countries (for example, Indonesia, Thailand, Malaysia, Cambodia and Lao PDR). In these countries, age-standardized mean non-HDL cholesterol increased by as much as 0.23 mmol l−1 per decade. As a result of these opposite trends, countries with the highest age-standardized mean non-HDL cholesterol levels in 2018 were all outside northwestern Europe: Tokelau, Malaysia, The Philippines and Thailand, all of which had mean non-HDL cholesterol around or above 4 mmol l−1. China, which had one of the lowest mean non-HDL cholesterol levels in 1980, reached or surpassed non-HDL cholesterol levels of many high-income western countries in 2018. Sub-Saharan African countries had the lowest mean non-HDL cholesterol in 2018, as low as 2.6 mmol l−1 in some countries, as they had in 1980. Not only did high-income countries benefit from decreasing non-HDL cholesterol levels, they had higher mean HDL cholesterol than low- and middle-income countries (Extended Data Fig. 6).
Fig. 3. Age-standardized mean non-HDL cholesterol by country in 1980 and 2018 for women and men.
a, Age-standardized mean non-HDL cholesterol in women in 1980. b, Age-standardized mean non-HDL cholesterol in women in 2018. c, Age-standardized mean non-HDL cholesterol in men in 1980. d, Age-standardized mean non-HDL cholesterol in men in 2018. See Extended Data Fig. 5 for age-standardized mean total cholesterol and Extended Data Fig. 6 for age-standardized mean HDL cholesterol. One mmol l−1 is equivalent to 38.61 mg dl−1.
Fig. 4. Change in age-standardized mean non-HDL cholesterol per decade by country for women and men.
a, Change per decade in age-standardized mean non-HDL cholesterol in women. b, Change per decade in age-standardized mean non-HDL cholesterol in men. See Extended Data Fig. 7 for change per decade in age-standardized mean total cholesterol and Extended Data Fig. 8 for change per decade in age-standardized mean HDL cholesterol. One mmol l−1 is equivalent to 38.61 mg dl−1.
Extended Data Fig. 6. Age-standardized mean HDL cholesterol by country in 1980 and 2018 for women and men.
One mmol l−1 is equivalent to 38.61 mg dl−1.
Deaths attributable to non-optimal cholesterol
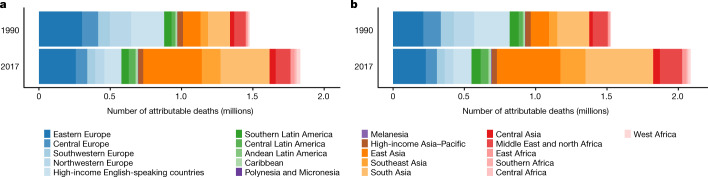
In 2017, high non-HDL cholesterol was responsible for an estimated 3.9 million (3.7–4.2 million) worldwide deaths from IHD and ischaemic stroke (Fig. 5), accounting for a third of deaths from these causes. From 1990 to 2017, the number of deaths caused by IHD and ischaemic stroke that were attributable to high non-HDL cholesterol increased by around 910,000 globally. This increase was a net result of a large decrease in western countries, from 950,000 (890,000–990,000) to 480,000 (430,000–530,000), and a large increase throughout Asia. In particular, the number of deaths attributable to high non-HDL cholesterol more than tripled in east Asia, from 250,000 (230,000–270,000) to 860,000 (770,000–940,000), and more than doubled in southeast Asia, from 110,000 (100,000–120,000) to 310,000 (290,000–330,000). As a result, by 2017 east, southeast and south Asia accounted for half of all deaths attributable to high non-HDL cholesterol, compared with a quarter in 1990.
Fig. 5. Deaths from IHD and ischaemic stroke attributable to high non-HDL cholesterol by region in 1990 and 2017 for women and men.
a, Deaths in women attributable to high non-HDL cholesterol. b, Deaths in men attributable to high non-HDL cholesterol.
Implications
Our results show that over the past nearly four decades, there has been a major global repositioning of lipid-related risk, with non-optimal cholesterol patterns shifting from being a distinct feature of high-income countries in northwestern Europe, north America and Australasia to one that affects middle-income countries in east and southeast Asia, as well as some countries in Oceania and central Latin America. This transition is especially noticeable for non-HDL cholesterol, which had not been quantified previously in a global analysis. This global repositioning has occurred as a consequence of opposing trends in high-income western countries and in Asia, which has led to some Asian countries having the highest worldwide non-HDL cholesterol levels in 2018.
The decrease in non-HDL cholesterol in western countries started in the 1980s, before statins were widely used15,16. This indicates that changes in diet, especially the replacement of saturated with unsaturated fats3,17–21 and reduction in trans fats12,17,22, are major contributors to this decline. Nonetheless, the increased use of statins from the late 1990s onwards15,16, may explain up to one half of the decrease in those countries in which statins are widely used19,23,24. In contrast to high-income western countries, the consumption of animal-source foods, refined carbohydrates and palm oil has increased substantially in east and southeast Asia3,25,26, where statin use remains low13,27. For example, the Pearson correlation coefficient between the change in non-HDL cholesterol and the change in a multi-dimensional score of animal-source foods and sugar3 was 0.69 for women and 0.67 for men using data from high-income western countries and countries in east and southeast Asia, the two regions that experienced the largest decrease and increase, respectively, in non-HDL cholesterol levels. Finally, changes in diet, especially a decrease in carbohydrate and an increase in fat intake28–31, may have contributed to the large increase in HDL cholesterol observed in the high-income Asia–Pacific region, where there was little increase in overweight and obesity relative to other regions8,9. By contrast, the large increase in diabetes32 and adiposity8 in Oceania may have contributed to the decrease in HDL cholesterol in this region. The Pearson correlation coefficient between the change in HDL cholesterol and the change in body-mass index8 was −0.87 for women and −0.69 for men using countries in the high-income Asia–Pacific region and Oceania, the two regions that had the largest increase and decrease, respectively, in HDL cholesterol; the Pearson correlation coefficient for the change in HDL cholesterol and change in diabetes prevalence32 was −0.84 for women and −0.69 for men. In the same regions, the Pearson correlation coefficient between the change in non-HDL cholesterol and the change in body-mass index8 was 0.77 for women and 0.62 for men; for the change in non-HDL cholesterol and the change in diabetes prevalence32, the Pearson correlation coefficient was 0.54 for women and 0.40 for men.
Although it has previously been documented that the prevalence of adiposity8,9, diabetes32 and high blood pressure33 is now higher in low- and middle-income countries than in high-income countries, higher cholesterol is commonly considered to be a feature of affluent western nations1,2. We show that, when focusing on non-HDL cholesterol, middle-income countries have emerged as the new global epicentre of non-optimal cholesterol as they did for other major cardiovascular disease risk factors, indicating that there is no such a thing as a western risk factor. At the same time, the populations of high-income countries would also benefit from further lowering non-HDL cholesterol. Therefore, population-based policies and personal interventions to improve nutrition and enhance treatment are now needed in all countries, especially as a part of the movement towards universal health coverage.
Methods
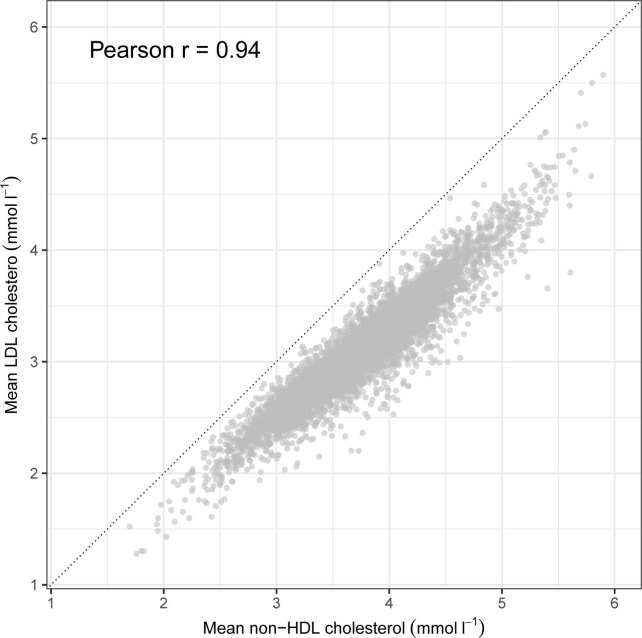
Our aim was to estimate trends in mean total, HDL and non-HDL cholesterol for 200 countries and territories (Supplementary Table 2). We used non-HDL cholesterol rather than low-density lipoprotein (LDL) cholesterol because most studies in our analysis had measured total cholesterol and HDL cholesterol, from which non-HDL cholesterol can be calculated through subtraction. By contrast, LDL cholesterol was directly measured in only around 14% of studies. When LDL cholesterol is not directly measured, its calculation requires data on triglycerides, which were available in approximately 64% of the studies. Furthermore, the most-commonly used estimation method—that is, the Friedewald equation—can be inaccurate, particularly at high levels of triglycerides34. Non-HDL and LDL cholesterol were highly correlated (Pearson correlation coefficient = 0.94) in studies with data on both variables (Extended Data Fig. 9), because LDL cholesterol constitutes most of non-HDL cholesterol. Furthermore, non-HDL cholesterol predicts IHD risk at least as well as LDL cholesterol5,35, and can be measured at a lower cost than LDL cholesterol, which is relevant for how widely it can be used in low- and middle-income countries. Although non-HDL cholesterol is now commonly used in clinical guidelines36–38, LDL cholesterol continues to be a key target for treatment36,37, possibly because the interpretation of non-HDL cholesterol is more complex than LDL cholesterol alone. Specifically, an increase in non-HDL cholesterol could be due to the increase in LDL cholesterol or very-low-density lipoprotein cholesterol39. Furthermore, there is some evidence that triglyceride levels are high in Asian populations, compared to levels seen in high-income western countries40. Therefore, data on non-HDL cholesterol can motivate dietary interventions to both reduce LDL cholesterol (for example, reducing saturated and trans fat intake) and triglyceride levels (for example, reducing refined carbohydrates and increasing omega-3 fatty acids) as well as treatments that lower LDL cholesterol (statins), alongside those that lower triglycerides (for example, fibrates).
Extended Data Fig. 9. The association between mean LDL and non-HDL cholesterol in studies that measured lipids in a laboratory that had data for both variables.
Each data point is one study–age–sex group (n = 6,864). One mmol l−1 is equivalent to 38.61 mg dl−1.
Data sources
We used a database of population-based data on cardiometabolic risk factors collated by the NCD Risk Factor Collaboration (NCD-RisC), a worldwide network of health researchers and practitioners that systematically monitors the worldwide trends and variations in non-communicable disease (NCD) risk factors. The database was collated through multiple routes for identifying and accessing data. We accessed publicly available population-based multi-country and national measurement surveys (for example, Demographic and Health Surveys and surveys identified through the Inter-University Consortium for Political and Social Research and European Health Interview & Health Examination Surveys Database). We requested, via the World Health Organization (WHO) and its regional and country offices, from ministries of health and other national health and statistical agencies to identify and access population-based surveys. Requests were also sent via the World Heart Federation to its national partners. We made a similar request to the co-authors of an earlier pooled analysis of cardiometabolic risk factors7,41–43, and invited the co-authors of the analysis to reanalyse data from their studies and join NCD-RisC. Finally, to identify major sources that were not accessed through the above routes, we searched and reviewed published studies as described in the Supplementary Information and invited all eligible studies to join NCD-RisC.
For each data source, we recorded the available information about the study population, start year and duration of measurement, sampling approach and measurement methods. The information about study population was used to establish that each data source was population-based, and to assess whether it covered the whole country, multiple subnational regions or one or a small number of communities, and whether it was rural, urban or combined.
We carefully checked all data sources in terms of how they met our inclusion and exclusion criteria listed below. We identified duplicate data sources by comparing studies from the same country and year. Additionally, all NCD-RisC members are asked periodically to review the list of sources from their country, to suggest additional sources not in the database, and to verify that the included data meet the inclusion criteria listed below and are not duplicates. The NCD-RisC database is continuously updated through the above routes and through regular contact with NCD-RisC members.
Anonymized individual record data from sources included in NCD-RisC were reanalysed according to a common protocol. Within each survey, we included participants aged 18 years and older who were not pregnant. We removed participants with implausible total cholesterol levels (defined as total cholesterol levels of <1.75 mmol l−1 or >20 mmol l−1, or total cholesterol values that were lower than HDL cholesterol values) (<0.05% of all participants with total cholesterol measurements) or HDL cholesterol levels (defined as HDL cholesterol levels of <0.4 mmol l−1 or >5 mmol l−1, or total cholesterol values that were lower than HDL cholesterol values) (<0.15% of all participants with HDL cholesterol measurements). When data on LDL cholesterol were also available, we removed individuals for whom the sum of LDL and HDL cholesterol level surpassed total cholesterol level by more than is plausible based on the limits to errors in their measurement (following the CDC Cholesterol Reference Method Laboratory Network (CRMLN) standards, these errors were set at 8.9% for total cholesterol, 13% for HDL cholesterol and 12% for LDL cholesterol) (<0.06% of all participants with total cholesterol and HDL cholesterol measurements)44–46.
We calculated mean total cholesterol, mean HDL cholesterol and mean non-HDL cholesterol, and associated standard errors and sample sizes, by sex and age group (18–19 years, 20–29 years, followed by 10-year age groups and 80+ years). All analyses incorporated appropriate sample weights and complex survey design in calculating age–sex-specific means when applicable. To ensure summaries were prepared according to the study protocol, computer code was provided to NCD-RisC members who requested assistance. All submitted data were checked independently by at least two researchers. Questions and clarifications were discussed with NCD-RisC members and resolved before the data were incorporated in the database.
Finally, we obtained data not accessed through the above routes by extracting data from published reports of all additional national health surveys identified through the above-described strategies, as well as eight sites of the WHO Multinational MONItoring of trends and determinants in CArdiovascular disease (MONICA) project that were not deposited in the MONICA Data Centre. Data were extracted from published reports only when reported by sex and in age groups no wider than 20 years. We also used data from a previous pooling study7 when such data did not overlap with those accessed through the above routes.
Data inclusion and exclusion
Data sources were included in NCD-RisC database if: (1) measured data on total, LDL, HDL cholesterol and/or triglycerides were available; (2) study participants were 10 years of age or older; (3) data were collected using a probabilistic sampling method with a defined sampling frame; (4) data were from population samples at the national, subnational (covering one or more subnational regions, more than three urban communities or more than five rural communities) or community (one or a small number of communities) level; (5) data were collected in or after 1950; and (6) data were from the countries and territories listed in Supplementary Table 2.
We excluded all data sources that included only hypercholesterolaemia or dyslipidaemia diagnosis history or medication status without measurement of cholesterol levels. We also excluded data sources on population subgroups for which the lipid profile may differ systematically from the general population, including: (1) studies that had included or excluded people on the basis of their health status or cardiovascular risk; (2) studies for which the participants were only from ethnic minorities; (3) studies that had recruited only specific educational, occupational or socioeconomic subgroups, with the exception noted below; and (4) studies that had recruited participants through health facilities, with the exception noted below.
We used school-based data in countries and for age–sex groups, for which secondary school enrolment was 70% or higher. We used data for which the sampling frame was health insurance schemes in countries in which at least 80% of the population was insured. Finally, we used data collected through general practice and primary-care systems in high-income and central European countries with universal insurance, because contact with the primary-care systems tends to be as good as or better than response rates for population-based surveys. We used data sources regardless of fasting status, because the differences between fasting and non-fasting measurements are negligible for total, non-HDL and HDL cholesterol39, and therefore non-fasting lipid profiles are now widely endorsed for the estimation of cardiovascular risk36,37.
Data used in the analysis
For this paper, we used data from the NCD-RisC database for years 1980 to 2018 and individuals aged 18 years and older. A list of the data sources that we used in this analysis and their characteristics is provided in Supplementary Table 1. The data comprised 1,127 population-based measurement surveys and studies that included measurements of blood lipids on 102.6 million participants aged 18 years and older. We had at least one data source for 161 of the 200 countries that we made estimates for, covering 92.4% of the world’s population in 2018 (Extended Data Fig. 1); and at least two data sources for 104 countries (87.5% of the world population). Of these 1,127 sources, 409 (36.3%) sampled from national populations, 250 (22.2%) covered one or more subnational regions, and the remaining 468 (41.5%) were from one or a small number of communities. Regionally, data availability ranged from around 2 data sources per country in sub-Saharan Africa to approximately 35 sources per country in the high-income Asia–Pacific region. In total, 454 data sources (40.3%) were from years before 2000 and the remaining 673 (59.7%) were collected from 2001 onwards.
Adjusting for the differences in mean cholesterol between portable device and laboratory measurements
In 112 (10%) of the 1,127 data sources used in our analysis (11.5% and 5.8% of age–sex-specific data points for total and HDL cholesterol, respectively) lipids were measured using a portable device. Some portable devices have narrower analytical ranges than laboratory methods, which results in truncations of blood cholesterol data that are outside their range (Supplementary Table 3). This may in turn affect the population mean. Although cholesterol concentrations that fall outside the analytical range are displayed as ‘high’ (above the measurement range) or ‘low’ (below the measurement range) by these devices, different surveys record and code cholesterol concentrations outside the analytical range in different ways, for example using ‘too low’, ‘too high’ and ‘error’ codes; assigning the minimum or maximum value to individuals whose cholesterol was below or above the analytical range, respectively; setting values outside the analytical range to missing; and so on. We used an approach that treated surveys with such data consistently.
Specifically, we first dropped all participants with cholesterol levels below and at the minimum, and at and above the maximum, values of the analytical range of each portable device before calculating the mean cholesterol (Supplementary Table 3). We then developed conversion regressions to adjust the mean cholesterol levels measured using a portable device (calculated over the restricted range, Supplementary Table 3) to the levels expected using laboratory measurements. The dependent variable in each regression was mean total, non-HDL or HDL cholesterol for the full range, and the main independent variable was mean total, non-HDL or HDL cholesterol over the above-mentioned restricted cholesterol range of the portable devices. The regression coefficients were estimated from data sources for which lipids were measured in a laboratory, and thus had the full range of measurement and could be used to calculate both dependent and independent variables. When estimating the regression coefficients, we constructed the dependent variable using the full data, and the independent variable by dropping the values outside the above-mentioned restricted cholesterol range of each device, mimicking those that would be expected if a portable device had been used. Separate models were developed according to the specific range of the different portable devices. All regressions included terms for age and sex, as well as interactions between predictors and age and sex, based on the Bayesian information criterion47. The regressions for mean non-HDL cholesterol also included mean total cholesterol and mean HDL cholesterol because non-HDL cholesterol is calculated from total cholesterol and HDL cholesterol. We excluded data points for which there were fewer than 25 individuals for the purpose of estimating the coefficients of these regressions. All sources of uncertainty in the conversion—including the sampling uncertainty of the original data, the uncertainty of the regression coefficients and residuals—were carried forward by using repeated draws from their respective distributions. The regression coefficients and number of data points used to estimate the coefficients are shown in Supplementary Table 4.
Statistical analysis
We used a statistical model to estimate mean total, non-HDL and HDL cholesterol by country, year, sex and age using all of the available data. The model is described in detail in a statistical paper and related substantive papers8,32,33,48; the computer code is available at http://www.ncdrisc.org/. In summary, we organized countries into 21 regions, mainly based on geography and national income; these regions were further aggregated into 9 ‘super-regions’ (Supplementary Table 2). The model had a hierarchical structure in which estimates for each country and year were informed by its own data, if available, and by data from other years in the same country and from other countries, especially countries in the same region or super-region with data for similar time periods. The extent to which estimates for each country-year are influenced by data from other years and other countries depends on whether the country has data, the sample size of data, whether or not they are national, and the within-country and within-region data variability. The model incorporated nonlinear time trends comprising linear terms and a second-order random walk. The age association of blood lipids was modelled using a cubic spline to allow nonlinear age patterns, which might vary across countries. The model accounted for the possibility that blood lipids in subnational and community samples might systematically differ from nationally representative ones; and/or have larger variation. These features were implemented by including data-driven fixed-effect and random-effect terms for subnational and community data. The fixed effects adjust for systematic differences between subnational or community studies and national studies. The random effects allow national data to have larger influence on the estimates than subnational or community data with similar sample sizes. The model also accounted for rural–urban differences in blood lipids, through the use of data-driven fixed effects for rural-only and urban-only studies. These rural and urban effects were weighted by the difference between study-level and country-level urbanization in the year in which the study was done. The proportion of the national population living in urban areas was also included as a predictor (covariate) in the model. The model for mean non-HDL and HDL cholesterol also used age-standardized mean total cholesterol as a covariate.
We fitted the statistical model with the Markov chain Monte Carlo (MCMC) algorithm, and obtained 5,000 post-burn-in samples from the posterior distribution of model parameters, which were in turn used to obtain the posterior distributions of mean total, non-HDL and HDL cholesterol. We calculated average change in mean total, HDL and non-HDL cholesterol across the 39 years of analysis (reported as change per decade). Age-standardized estimates were generated by taking weighted averages of age–sex-specific estimates, using the WHO standard population. Estimates for regions and the world were calculated as population-weighted averages of the constituent country estimates by age group and sex. The reported credible intervals represent the 2.5–97.5th percentiles of the posterior distributions. We also report the posterior probability that an estimated increase or decrease represents a truly increasing or decreasing trend as opposed to a chance observation. We performed all analyses by sex, because blood lipids levels and trends are different in men and women.
Validation of statistical model
We tested how well our statistical model predicts missing data, known as external predictive validity, in two different tests. In the first test, we held out all data from 10% of countries with data (that is, created the appearance of countries with no data where we actually had data). The countries for which the data were withheld were randomly selected from the following three groups: data rich (5 or more data sources, with at least one data source after the year 2000), data poor (1 data source) and average data availability (2–4 data sources). In the second test, we assessed other patterns of missing data by holding out 10% of our data sources, again from a mix of data-rich, data-poor and average-data countries, as defined above. For a given country, we either held out a random half of the data of a country or all of the 2000–2018 data of the country to determine, respectively, how well we filled in the gaps for countries with intermittent data and how well we estimated in countries without recent data. In both tests, we then fitted the model to the remaining 90% of the countries (test 1) or data sources (test 2) and made estimates of the held-out observations. We repeated each test five times, holding out a different subset of data in each repetition. In both tests, we calculated the differences between the held-out data and the estimates. We also calculated the 95% credible intervals of the estimates; in a model with good external predictive validity, 95% of held-out values would be included in the 95% credible intervals.
Our statistical model performed well in the external validation tests, that is, in estimating mean cholesterol when data were missing. The estimates of mean total, non-HDL and HDL cholesterol were unbiased, as evidenced with median errors that were very close to zero globally for every outcome and test, and less than ±0.30 mmol l−1 in every subset of withheld data except for women in the high-income Asia–Pacific region in test 1 for non-HDL cholesterol (median error 0.47 mmol l−1) and men in south Asia in test 2 for non-HDL cholesterol (median error −0.33 mmol l−1) (Supplementary Table 5). The 95% credible intervals of estimated means covered 83–92% and 75–83% of true data globally in the first and second tests, respectively. In subsets, coverage ranged from 47% to 100%, but was mostly greater than 75%, with coverage generally lower in test 2 than test 1. Median absolute errors ranged from 0.07 to 0.23 mmol l−1 globally for different outcomes and sexes, and were no more than 0.45 mmol l−1 in all subsets of withheld data, except for women in the high-income Asia–Pacific region for non-HDL cholesterol in test 1 (median absolute error 0.47 mmol l−1).
Calculation of the number of deaths attributable to high cholesterol
We estimated the number of deaths from IHD and ischaemic stroke attributable to high non-HDL cholesterol. For each country, year, sex and age group, we first calculated the population attributable fractions—that is, the proportion of deaths from IHD and ischaemic stroke that would have been prevented if non-HDL cholesterol levels were at an optimal level (defined as a mean of 1.8–2.2 mmol l−1) in the population6,49. For these calculations, we used age-specific relative risks from meta-analyses of prospective cohort studies4,5,50. The number of IHD and ischaemic stroke deaths attributable to high non-HDL cholesterol was calculated for each country–year–age–sex group by multiplying the cause-specific population attributable fractions by the cause-specific deaths from the Global Burden of Disease study in 1990 and 2017 (the earliest and latest years with cause-specific mortality data).
Strengths and limitations
The strengths of our study include its scope in making consistent and comparable estimates of trends in blood cholesterol and its cardiovascular disease mortality burden, over almost four decades for all of the countries in the world, including global estimates of non-HDL and HDL cholesterol. We used a large amount of population-based data, which came from countries in which 92% of the global adult population lives. We used only data from studies that had measured blood lipids to avoid bias in self-reported data. Data were analysed according to a consistent protocol, and the characteristics and quality of data from each country were rigorously verified through repeated checks by NCD-RisC members. We pooled data using a statistical model that took into account the epidemiological features of cholesterol, including nonlinear time trends and age associations. Our statistical model used all available data while giving more weight to national data than to subnational and community sources.
Similar to all global analyses, our study is affected by some limitations. Despite our extensive efforts to identify and access worldwide population-based data, some countries had no or few data sources, especially those in sub-Saharan Africa, the Caribbean, central Asia and Melanesia. Estimates for these countries relied mostly or entirely on the statistical model, which shares information across countries and regions through its hierarchy. Data scarcity is reflected in wider uncertainty intervals of our estimates for these countries and regions, highlighting the need for national NCD-oriented surveillance. The distribution of lipids measured in a population using a portable device, which was used in 10% of our studies, may be truncated and may therefore affect the population mean. To overcome this issue, we developed conversion regressions to adjust mean cholesterol levels measured using a portable device to the levels expected in laboratory measurements; the conversion regressions used for this purpose had good predictive accuracy. Although most studies had measured cholesterol in serum samples, around 7% had used plasma samples. As cholesterol measured in plasma and serum samples differ51 by only about 3%, adjusting for plasma-serum differences would have little effect on our results, as seen in a previous analysis14. Although methods to measure total and HDL cholesterol have evolved over time, since the 1950s there have been systematic efforts to standardize lipid measurements that have resulted in increased comparability between different methods. In our analysis, 90% of studies measured lipids in a laboratory; of these studies more than 60% for total cholesterol and more than 70% for HDL cholesterol participated in a lipid standardization programme or quality control scheme. We did not analyse emerging lipid markers such as apolipoprotein B and apolipoprotein A-I, because they are neither commonly measured in population-based health surveys, nor routinely used in clinical practice36.
Comparison with other studies
There are no global analyses on trends in lipid fractions for comparison with our results. Our findings for total cholesterol were largely consistent with the only other previous analysis7, but we estimated a larger decrease in mean total cholesterol in high-income western countries and central Europe, and a larger increase in southeast Asia, because we had an additional decade of data compared with the earlier global analysis. Therefore, although the highest mean total cholesterol levels reported previously7, for 2008, were still in high-income western countries, we estimated that in 2018 total cholesterol was equally high or higher in southeast Asia. Our findings on mean total cholesterol trends are also largely consistent with previous multi- and single-country reports14,15,17–21,52–73. Differences from previous studies—for example, in Italy61, Lithuania63, the Netherlands65, Russian Federation69 and in some countries that participated in the MONICA Project52—mostly arise because our study covered a longer period and used a larger number of data sources. Studies15,18,54,63,66,70,74–77 that have reported trends in lipid fractions for a period longer than 15 years have found changes in non-HDL cholesterol (or in LDL cholesterol for some studies) that were consistent with our results.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-020-2338-1.
Supplementary information
This file contains a Literature search for additional data sources and Supplementary Tables 1-5 and Supplementary Figure 1.
Acknowledgements
This study was funded by a Wellcome Trust (Biomedical Resource & Multi-User Equipment grant 01506/Z/13/Z) and the British Heart Foundation (Centre of Research Excellence grant RE/18/4/34215). C.T. was supported by a Wellcome Trust Research Training Fellowship (203616/Z/16/Z). The authors alone are responsible for the views expressed in this Article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Extended data figures and tables
Extended Data Fig. 5. Age-standardized mean total cholesterol by country in 1980 and 2018 for women and men.
One mmol l−1 is equivalent to 38.61 mg dl−1.
Extended Data Fig. 7. Change per decade in age-standardized mean total cholesterol by country for women and men.
One mmol l−1 is equivalent to 38.61 mg dl−1.
Extended Data Fig. 8. Change per decade in age-standardized mean HDL cholesterol by country for women and men.
One mmol l−1 is equivalent to 38.61 mg dl−1.
Author contributions
M.E. and G.D. designed the study and oversaw research. C.T., B.Z., H.B. and R.C.L. led the data collection. The other authors contributed to study design; and collected, reanalysed, checked and pooled data. C.T. analysed pooled data and prepared results. C.T., E.G. and M.E. wrote the first draft of the manuscript with input from the other authors.
Data availability
Estimates of mean total, non-HDL and HDL cholesterol by country, year and sex are available at http://www.ncdrisc.org/. Input data from publicly available sources can also be downloaded from http://www.ncdrisc.org/. For other data sources, contact information for data providers can be obtained from http://www.ncdrisc.org/.
Code availability
The computer code for the Bayesian hierarchical model used in this work is available at http://www.ncdrisc.org/.
Competing interests
M.E. reports a charitable grant from the AstraZeneca Young Health Programme, and personal fees from Prudential, Scor and Third Bridge, outside the submitted work. The other authors declare no competing interests.
Footnotes
Peer review information Nature thanks Frank Hu and Pekka Jousilahti for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of participants and their affiliations appears in the online version of the paper
Deceased: Konrad Jamrozik, Altan Onat, Robespierre Ribeiro, Jutta Stieber
Contributor Information
NCD Risk Factor Collaboration (NCD-RisC):
Cristina Taddei, Bin Zhou, Honor Bixby, Rodrigo M. Carrillo-Larco, Goodarz Danaei, Rod T. Jackson, Farshad Farzadfar, Marisa K. Sophiea, Mariachiara Di Cesare, Maria Laura Caminia Iurilli, Andrea Rodriguez Martinez, Golaleh Asghari, Klodian Dhana, Pablo Gulayin, Sujay Kakarmath, Marilina Santero, Trudy Voortman, Leanne M. Riley, Melanie J. Cowan, Stefan Savin, James E. Bennett, Gretchen A. Stevens, Christopher J. Paciorek, Wichai Aekplakorn, Renata Cifkova, Simona Giampaoli, Andre Pascal Kengne, Young-Ho Khang, Kari Kuulasmaa, Avula Laxmaiah, Paula Margozzini, Prashant Mathur, Børge G. Nordestgaard, Dong Zhao, Mette Aadahl, Leandra Abarca-Gómez, Hanan Abdul Rahim, Niveen M. Abu-Rmeileh, Benjamin Acosta-Cazares, Robert J. Adams, Imelda A. Agdeppa, Javad Aghazadeh-Attari, Carlos A. Aguilar-Salinas, Charles Agyemang, Tarunveer S. Ahluwalia, Noor Ani Ahmad, Ali Ahmadi, Naser Ahmadi, Soheir H. Ahmed, Wolfgang Ahrens, Kamel Ajlouni, Monira Alarouj, Fadia AlBuhairan, Shahla AlDhukair, Mohamed M. Ali, Abdullah Alkandari, Ala’a Alkerwi, Eman Aly, Deepak N. Amarapurkar, Philippe Amouyel, Lars Bo Andersen, Sigmund A. Anderssen, Ranjit Mohan Anjana, Alireza Ansari-Moghaddam, Hajer Aounallah-Skhiri, Joana Araújo, Inger Ariansen, Tahir Aris, Raphael E. Arku, Nimmathota Arlappa, Krishna K. Aryal, Thor Aspelund, Maria Cecília F. Assunção, Juha Auvinen, Mária Avdicová, Ana Azevedo, Fereidoun Azizi, Mehrdad Azmin, Nagalla Balakrishna, Mohamed Bamoshmoosh, Maciej Banach, Piotr Bandosz, José R. Banegas, Carlo M. Barbagallo, Alberto Barceló, Amina Barkat, Iqbal Bata, Anwar M. Batieha, Assembekov Batyrbek, Louise A. Baur, Robert Beaglehole, Antonisamy Belavendra, Habiba Ben Romdhane, Mikhail Benet, Marianne Benn, Salim Berkinbayev, Antonio Bernabe-Ortiz, Gailute Bernotiene, Heloisa Bettiol, Santosh K. Bhargava, Yufang Bi, Asako Bienek, Mukharram Bikbov, Bihungum Bista, Peter Bjerregaard, Espen Bjertness, Marius B. Bjertness, Cecilia Björkelund, Katia V. Bloch, Anneke Blokstra, Simona Bo, Bernhard O. Boehm, Jose G. Boggia, Carlos P. Boissonnet, Marialaura Bonaccio, Vanina Bongard, Rossana Borchini, Herman Borghs, Pascal Bovet, Imperia Brajkovich, Juergen Breckenkamp, Hermann Brenner, Lizzy M. Brewster, Graziella Bruno, Anna Bugge, Markus A. Busch, Antonio Cabrera de León, Joseph Cacciottolo, Günay Can, Ana Paula C. Cândido, Mario V. Capanzana, Eduardo Capuano, Vincenzo Capuano, Viviane C. Cardoso, Joana Carvalho, Felipe F. Casanueva, Laura Censi, Charalambos A. Chadjigeorgiou, Snehalatha Chamukuttan, Nish Chaturvedi, Chien-Jen Chen, Fangfang Chen, Shuohua Chen, Ching-Yu Cheng, Bahman Cheraghian, Angela Chetrit, Shu-Ti Chiou, María-Dolores Chirlaque, Belong Cho, Yumi Cho, Jerzy Chudek, Frank Claessens, Janine Clarke, Els Clays, Hans Concin, Susana C. Confortin, Cyrus Cooper, Simona Costanzo, Dominique Cottel, Chris Cowell, Ana B. Crujeiras, Semánová Csilla, Liufu Cui, Felipe V. Cureau, Graziella D’Arrigo, Eleonora d’Orsi, Jean Dallongeville, Albertino Damasceno, Rachel Dankner, Thomas M. Dantoft, Luc Dauchet, Kairat Davletov, Guy De Backer, Dirk De Bacquer, Giovanni de Gaetano, Stefaan De Henauw, Paula Duarte de Oliveira, David De Ridder, Delphine De Smedt, Mohan Deepa, Alexander D. Deev, Abbas Dehghan, Hélène Delisle, Elaine Dennison, Valérie Deschamps, Meghnath Dhimal, Augusto F. Di Castelnuovo, Zivka Dika, Shirin Djalalinia, Annette J. Dobson, Chiara Donfrancesco, Silvana P. Donoso, Angela Döring, Maria Dorobantu, Nico Dragano, Wojciech Drygas, Yong Du, Charmaine A. Duante, Rosemary B. Duda, Vilnis Dzerve, Elzbieta Dziankowska-Zaborszczyk, Ricky Eddie, Ebrahim Eftekhar, Robert Eggertsen, Sareh Eghtesad, Gabriele Eiben, Ulf Ekelund, Jalila El Ati, Denise Eldemire-Shearer, Marie Eliasen, Roberto Elosua, Rajiv T. Erasmus, Raimund Erbel, Cihangir Erem, Louise Eriksen, Johan G. Eriksson, Jorge Escobedo-de la Peña, Saeid Eslami, Ali Esmaeili, Alun Evans, David Faeh, Caroline H. Fall, Elnaz Faramarzi, Mojtaba Farjam, Mohammad Reza Fattahi, Francisco J. Felix-Redondo, Trevor S. Ferguson, Daniel Fernández-Bergés, Daniel Ferrante, Marika Ferrari, Catterina Ferreccio, Jean Ferrieres, Bernhard Föger, Leng Huat Foo, Ann-Sofie Forslund, Maria Forsner, Heba M. Fouad, Damian K. Francis, Maria do Carmo Franco, Oscar H. Franco, Guillermo Frontera, Yuki Fujita, Matsuda Fumihiko, Takuro Furusawa, Zbigniew Gaciong, Fabio Galvano, Jingli Gao, Manoli Garcia-de-la-Hera, Sarah P. Garnett, Jean-Michel Gaspoz, Magda Gasull, Andrea Gazzinelli, Johanna M. Geleijnse, Ali Ghanbari, Erfan Ghasemi, Oana-Florentina Gheorghe-Fronea, Anup Ghimire, Francesco Gianfagna, Tiffany K. Gill, Jonathan Giovannelli, Glen Gironella, Aleksander Giwercman, David Goltzman, Helen Gonçalves, David A. Gonzalez-Chica, Marcela Gonzalez-Gross, Juan P. González-Rivas, Clicerio González-Villalpando, María-Elena González-Villalpando, Angel R. Gonzalez, Frederic Gottrand, Sidsel Graff-Iversen, Dušan Grafnetter, Ronald D. Gregor, Tomasz Grodzicki, Anders Grøntved, Giuseppe Grosso, Gabriella Gruden, Dongfeng Gu, Pilar Guallar-Castillón, Ong Peng Guan, Elias F. Gudmundsson, Vilmundur Gudnason, Ramiro Guerrero, Idris Guessous, Johanna Gunnlaugsdottir, Rajeev Gupta, Laura Gutierrez, Felix Gutzwiller, Seongjun Ha, Farzad Hadaegh, Rosa Haghshenas, Hamid Hakimi, Ian R. Hambleton, Behrooz Hamzeh, Sari Hantunen, Rachakulla Hari Kumar, Seyed Mohammad Hashemi-Shahri, Jun Hata, Teresa Haugsgjerd, Alison J. Hayes, Jiang He, Yuna He, Marleen Elisabeth Hendriks, Ana Henriques, Sauli Herrala, Ramin Heshmat, Allan G. Hill, Sai Yin Ho, Suzanne C. Ho, Michael Hobbs, Albert Hofman, Reza Homayounfar, Wilma M. Hopman, Andrea R. V. R. Horimoto, Claudia M. Hormiga, Bernardo L. Horta, Leila Houti, Christina Howitt, Thein Thein Htay, Aung Soe Htet, Maung Maung Than Htike, José María Huerta, Ilpo Tapani Huhtaniemi, Martijn Huisman, Monica L. Hunsberger, Abdullatif S. Husseini, Inge Huybrechts, Nahla Hwalla, Licia Iacoviello, Anna G. Iannone, Mohsen M. Ibrahim, Norazizah Ibrahim Wong, Iris Iglesia, Nayu Ikeda, M. Arfan Ikram, Violeta Iotova, Vilma E. Irazola, Takafumi Ishida, Muhammad Islam, Aziz al-Safi Ismail, Masanori Iwasaki, Jeremy M. Jacobs, Hashem Y. Jaddou, Tazeen Jafar, Kenneth James, Konrad Jamrozik, Imre Janszky, Edward Janus, Marjo-Riitta Jarvelin, Grazyna Jasienska, Ana Jelakovic, Bojan Jelakovic, Garry Jennings, Gorm B. Jensen, Seung-lyeal Jeong, Anjani Kumar Jha, Chao Qiang Jiang, Ramon O. Jimenez, Karl-Heinz Jöckel, Michel Joffres, Jari J. Jokelainen, Jost B. Jonas, Torben Jørgensen, Pradeep Joshi, Farahnaz Joukar, Jacek Józwiak, Anne Juolevi, Anthony Kafatos, Eero O. Kajantie, Ofra Kalter-Leibovici, Nor Azmi Kamaruddin, Pia R. Kamstrup, Khem B. Karki, Joanne Katz, Jussi Kauhanen, Prabhdeep Kaur, Maryam Kavousi, Gyulli Kazakbaeva, Ulrich Keil, Sirkka Keinänen-Kiukaanniemi, Roya Kelishadi, Maryam Keramati, Alina Kerimkulova, Mathilde Kersting, Yousef Saleh Khader, Davood Khalili, Mohammad Khateeb, Motahareh Kheradmand, Alireza Khosravi, Ursula Kiechl-Kohlendorfer, Stefan Kiechl, Japhet Killewo, Hyeon Chang Kim, Jeongseon Kim, Yeon-Yong Kim, Jurate Klumbiene, Michael Knoflach, Stephanie Ko, Hans-Peter Kohler, Iliana V. Kohler, Elin Kolle, Patrick Kolsteren, Jürgen König, Raija Korpelainen, Paul Korrovits, Jelena Kos, Seppo Koskinen, Katsuyasu Kouda, Sudhir Kowlessur, Wolfgang Kratzer, Susi Kriemler, Peter Lund Kristensen, Steiner Krokstad, Daan Kromhout, Urho M. Kujala, Pawel Kurjata, Catherine Kyobutungi, Fatima Zahra Laamiri, Tiina Laatikainen, Carl Lachat, Youcef Laid, Tai Hing Lam, Christina-Paulina Lambrinou, Vera Lanska, Georg Lappas, Bagher Larijani, Tint Swe Latt, Lars E. Laugsand, Maria Lazo-Porras, Jeannette Lee, Jeonghee Lee, Nils Lehmann, Terho Lehtimäki, Naomi S. Levitt, Yanping Li, Christa L. Lilly, Wei-Yen Lim, M. Fernanda Lima-Costa, Hsien-Ho Lin, Xu Lin, Yi-Ting Lin, Lars Lind, Allan Linneberg, Lauren Lissner, Jing Liu, Helle-Mai Loit, Esther Lopez-Garcia, Tania Lopez, Paulo A. Lotufo, José Eugenio Lozano, Dalia Luksiene, Annamari Lundqvist, Robert Lundqvist, Nuno Lunet, Guansheng Ma, George L. L. Machado-Coelho, Aristides M. Machado-Rodrigues, Suka Machi, Ahmed A. Madar, Stefania Maggi, Dianna J. Magliano, Emmanuella Magriplis, Gowri Mahasampath, Bernard Maire, Marcia Makdisse, Fatemeh Malekzadeh, Reza Malekzadeh, Kodavanti Mallikharjuna Rao, Yannis Manios, Jim I. Mann, Fariborz Mansour-Ghanaei, Enzo Manzato, Pedro Marques-Vidal, Reynaldo Martorell, Luis P. Mascarenhas, Ellisiv B. Mathiesen, Tandi E. Matsha, Christina Mavrogianni, Shelly R. McFarlane, Stephen T. McGarvey, Stela McLachlan, Rachael M. McLean, Scott B. McLean, Breige A. McNulty, Sounnia Mediene-Benchekor, Parinaz Mehdipour, Kirsten Mehlig, Amir Houshang Mehrparvar, Aline Meirhaeghe, Christa Meisinger, Ana Maria B. Menezes, Geetha R. Menon, Shahin Merat, Alibek Mereke, Indrapal I. Meshram, Patricia Metcalf, Haakon E. Meyer, Jie Mi, Nathalie Michels, Jody C. Miller, Cláudia S. Minderico, G. K. Mini, Juan Francisco Miquel, J. Jaime Miranda, Mohammad Reza Mirjalili, Erkin Mirrakhimov, Pietro A. Modesti, Sahar Saeedi Moghaddam, Bahram Mohajer, Mostafa K. Mohamed, Kazem Mohammad, Zahra Mohammadi, Noushin Mohammadifard, Reza Mohammadpourhodki, Viswanathan Mohan, Salim Mohanna, Muhammad Fadhli Mohd Yusoff, Iraj Mohebbi, Farnam Mohebi, Marie Moitry, Line T. Møllehave, Niels C. Møller, Dénes Molnár, Amirabbas Momenan, Charles K. Mondo, Eric Monterrubio-Flores, Mahmood Moosazadeh, Alain Morejon, Luis A. Moreno, Karen Morgan, Suzanne N. Morin, George Moschonis, Malgorzata Mossakowska, Aya Mostafa, Jorge Mota, Mohammad Esmaeel Motlagh, Jorge Motta, Kelias P. Msyamboza, Maria L. Muiesan, Martina Müller-Nurasyid, Jaakko Mursu, Norlaila Mustafa, Iraj Nabipour, Shohreh Naderimagham, Gabriele Nagel, Balkish M. Naidu, Farid Najafi, Harunobu Nakamura, Jana Námešná, Ei Ei K. Nang, Vinay B. Nangia, Matthias Nauck, William A. Neal, Azim Nejatizadeh, Ilona Nenko, Flavio Nervi, Nguyen D. Nguyen, Quang Ngoc Nguyen, Ramfis E. Nieto-Martínez, Thomas Nihal, Teemu J. Niiranen, Guang Ning, Toshiharu Ninomiya, Marianna Noale, Oscar A. Noboa, Davide Noto, Mohannad Al Nsour, Irfan Nuhoğlu, Terence W. O’Neill, Dermot O’Reilly, Angélica M. Ochoa-Avilés, Kyungwon Oh, Ryutaro Ohtsuka, Örn Olafsson, Valérie Olié, Isabel O. Oliveira, Mohd Azahadi Omar, Altan Onat, Sok King Ong, Pedro Ordunez, Rui Ornelas, Pedro J. Ortiz, Clive Osmond, Sergej M. Ostojic, Afshin Ostovar, Johanna A. Otero, Ellis Owusu-Dabo, Fred Michel Paccaud, Elena Pahomova, Andrzej Pajak, Luigi Palmieri, Wen-Harn Pan, Songhomitra Panda-Jonas, Francesco Panza, Winsome R. Parnell, Nikhil D. Patel, Nasheeta Peer, Sergio Viana Peixoto, Markku Peltonen, Alexandre C. Pereira, Annette Peters, Astrid Petersmann, Janina Petkeviciene, Niloofar Peykari, Son Thai Pham, Rafael N. Pichardo, Iris Pigeot, Aida Pilav, Lorenza Pilotto, Aleksandra Piwonska, Andreia N. Pizarro, Pedro Plans-Rubió, Silvia Plata, Hermann Pohlabeln, Miquel Porta, Marileen L. P. Portegies, Anil Poudyal, Farhad Pourfarzi, Hossein Poustchi, Rajendra Pradeepa, Jacqueline F. Price, Rui Providencia, Jardena J. Puder, Soile E. Puhakka, Margus Punab, Mostafa Qorbani, Tran Quoc Bao, Ricardas Radisauskas, Salar Rahimikazerooni, Olli Raitakari, Sudha Ramachandra Rao, Ambady Ramachandran, Elisabete Ramos, Rafel Ramos, Lekhraj Rampal, Sanjay Rampal, Josep Redon, Paul Ferdinand M. Reganit, Luis Revilla, Abbas Rezaianzadeh, Robespierre Ribeiro, Adrian Richter, Fernando Rigo, Tobias F. Rinke de Wit, Fernando Rodríguez-Artalejo, María del Cristo Rodriguez-Perez, Laura A. Rodríguez-Villamizar, Ulla Roggenbuck, Rosalba Rojas-Martinez, Dora Romaguera, Elisabetta L. Romeo, Annika Rosengren, Joel G. R. Roy, Adolfo Rubinstein, Jean-Bernard Ruidavets, Blanca Sandra Ruiz-Betancourt, Paola Russo, Petra Rust, Marcin Rutkowski, Charumathi Sabanayagam, Harshpal S. Sachdev, Alireza Sadjadi, Ali Reza Safarpour, Saeid Safiri, Olfa Saidi, Nader Saki, Benoit Salanave, Diego Salmerón, Veikko Salomaa, Jukka T. Salonen, Massimo Salvetti, Jose Sánchez-Abanto, Susana Sans, Alba M. Santaliestra-Pasías, Diana A. Santos, Maria Paula Santos, Rute Santos, Jouko L. Saramies, Luis B. Sardinha, Nizal Sarrafzadegan, Kai-Uwe Saum, Savvas C. Savva, Norie Sawada, Mariana Sbaraini, Marcia Scazufca, Beatriz D. Schaan, Herman Schargrodsky, Christa Scheidt-Nave, Anja Schienkiewitz, Sabine Schipf, Carsten O. Schmidt, Ben Schöttker, Sara Schramm, Sylvain Sebert, Aye Aye Sein, Abhijit Sen, Sadaf G. Sepanlou, Jennifer Servais, Ramin Shakeri, Svetlana A. Shalnova, Teresa Shamah-Levy, Maryam Sharafkhah, Sanjib K. Sharma, Jonathan E. Shaw, Amaneh Shayanrad, Zumin Shi, Kenji Shibuya, Hana Shimizu-Furusawa, Dong Wook Shin, Youchan Shin, Majid Shirani, Rahman Shiri, Namuna Shrestha, Khairil Si-Ramlee, Alfonso Siani, Rosalynn Siantar, Abla M. Sibai, Diego Augusto Santos Silva, Mary Simon, Judith Simons, Leon A. Simons, Michael Sjöström, Tea Skaaby, Jolanta Slowikowska-Hilczer, Przemyslaw Slusarczyk, Liam Smeeth, Marieke B. Snijder, Stefan Söderberg, Agustinus Soemantri, Reecha Sofat, Vincenzo Solfrizzi, Mohammad Hossein Somi, Emily Sonestedt, Thorkild I. A. Sørensen, Charles Sossa Jérome, Aïcha Soumaré, Kaan Sozmen, Karen Sparrenberger, Jan A. Staessen, Maria G. Stathopoulou, Bill Stavreski, Jostein Steene-Johannessen, Peter Stehle, Aryeh D. Stein, Jochanan Stessman, Ranko Stevanović, Jutta Stieber, Doris Stöckl, Jakub Stokwiszewski, Karien Stronks, Maria Wany Strufaldi, Ramón Suárez-Medina, Chien-An Sun, Johan Sundström, Paibul Suriyawongpaisal, Rody G. Sy, René Charles Sylva, Moyses Szklo, E. Shyong Tai, Abdonas Tamosiunas, Eng Joo Tan, Mohammed Rasoul Tarawneh, Carolina B. Tarqui-Mamani, Anne Taylor, Julie Taylor, Grethe S. Tell, Tania Tello, K. R. Thankappan, Lutgarde Thijs, Betina H. Thuesen, Ulla Toft, Hanna K. Tolonen, Janne S. Tolstrup, Murat Topbas, Roman Topór-Madry, María José Tormo, Michael J. Tornaritis, Maties Torrent, Laura Torres-Collado, Pierre Traissac, Oanh T. H. Trinh, Julia Truthmann, Shoichiro Tsugane, Marshall K. Tulloch-Reid, Tomi-Pekka Tuomainen, Jaakko Tuomilehto, Anne Tybjaerg-Hansen, Christophe Tzourio, Peter Ueda, Eunice Ugel, Hanno Ulmer, Belgin Unal, Hannu M. T. Uusitalo, Gonzalo Valdivia, Damaskini Valvi, Rob M. van Dam, Yvonne T. van der Schouw, Koen Van Herck, Hoang Van Minh, Lenie van Rossem, Natasja M. Van Schoor, Irene G. M. van Valkengoed, Dirk Vanderschueren, Diego Vanuzzo, Anette Varbo, Patricia Varona-Pérez, Senthil K. Vasan, Lars Vatten, Tomas Vega, Toomas Veidebaum, Gustavo Velasquez-Melendez, Silvia J. Venero-Fernández, Giovanni Veronesi, W. M. Monique Verschuren, Cesar G. Victora, Dhanasari Vidiawati, Lucie Viet, Salvador Villalpando, Jesus Vioque, Jyrki K. Virtanen, Sophie Visvikis-Siest, Bharathi Viswanathan, Tiina Vlasoff, Peter Vollenweider, Ari Voutilainen, Alisha N. Wade, Aline Wagner, Janette Walton, Wan Mohamad Wan Bebakar, Wan Nazaimoon Wan Mohamud, Ming-Dong Wang, Ningli Wang, Qian Wang, Ya Xing Wang, Ying-Wei Wang, S. Goya Wannamethee, Niels Wedderkopp, Wenbin Wei, Peter H. Whincup, Kurt Widhalm, Indah S. Widyahening, Andrzej Wiecek, Alet H. Wijga, Rainford J. Wilks, Johann Willeit, Peter Willeit, Tom Wilsgaard, Bogdan Wojtyniak, Roy A. Wong-McClure, Andrew Wong, Tien Yin Wong, Jean Woo, Mark Woodward, Frederick C. Wu, Shouling Wu, Haiquan Xu, Liang Xu, Weili Yan, Xiaoguang Yang, Tabara Yasuharu, Xingwang Ye, Toh Peng Yeow, Panayiotis K. Yiallouros, Moein Yoosefi, Akihiro Yoshihara, San-Lin You, Novie O. Younger-Coleman, Ahmad Faudzi Yusoff, Ahmad A. Zainuddin, Seyed Rasoul Zakavi, Mohammad Reza Zali, Farhad Zamani, Sabina Zambon, Antonis Zampelas, Ko Ko Zaw, Tomasz Zdrojewski, Tajana Zeljkovic Vrkic, Zhen-Yu Zhang, Wenhua Zhao, Shiqi Zhen, Yingfeng Zheng, Bekbolat Zholdin, Baurzhan Zhussupov, Nada Zoghlami, Julio Zuñiga Cisneros, Edward W. Gregg, and Majid Ezzati
Extended data
is available for this paper at 10.1038/s41586-020-2338-1.
Supplementary information
is available for this paper at 10.1038/s41586-020-2338-1.
References
- 1.Danaei G, et al. The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 2013;127:1493–1502. doi: 10.1161/CIRCULATIONAHA.113.001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezzati M, et al. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2005;2:e133. doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentham J, et al. Multi-dimensional characterisation of global food supply from 1961 to 2013. Nat. Food. 2020;1:70–75. doi: 10.1038/s43016-019-0012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prospective Studies Collaboration Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 5.The Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. J. Am. Med. Assoc. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634–647. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farzadfar F, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 8.Risk NCD. Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risk NCD. Factor Collaboration (NCD-RisC). Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–264. doi: 10.1038/s41586-019-1171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manthey J, et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493–2502. doi: 10.1016/S0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- 11.Micha R, et al. Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open. 2015;5:e008705. doi: 10.1136/bmjopen-2015-008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micha R, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. Br. Med. J. 2014;348:g2272. doi: 10.1136/bmj.g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth GA, et al. High total serum cholesterol, medication coverage and therapeutic control: an analysis of national health examination survey data from eight countries. Bull. World Health Organ. 2011;89:92–101. doi: 10.2471/BLT.10.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risk NCD. Factor Collaboration (NCD-RisC). National trends in total cholesterol obscure heterogeneous changes in HDL and non-HDL cholesterol and total-to-HDL cholesterol ratio: a pooled analysis of 458 population-based studies in Asian and Western countries. Int. J. Epidemiol. 2020;49:173–192. doi: 10.1093/ije/dyz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME. Trends in lipids and lipoproteins in US adults, 1988–2010. J. Am. Med. Assoc. 2012;308:1545–1554. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 16.Walley T, Folino-Gallo P, Stephens P, Van Ganse E. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br. J. Clin. Pharmacol. 2005;60:543–551. doi: 10.1111/j.1365-2125.2005.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vartiainen E, Laatikainen T, Tapanainen H, Puska P. Changes in serum cholesterol and diet in North Karelia and all Finland. Glob. Heart. 2016;11:179–184. doi: 10.1016/j.gheart.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Miller JC, et al. Trends in serum total cholesterol and dietary fat intakes in New Zealand between 1989 and 2009. Aust. N. Z. J. Public Health. 2016;40:263–269. doi: 10.1111/1753-6405.12504. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson M, et al. Greater decreases in cholesterol levels among individuals with high cardiovascular risk than among the general population: the northern Sweden MONICA study 1994 to 2014. Eur. Heart J. 2016;37:1985–1992. doi: 10.1093/eurheartj/ehw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett DK, et al. Twenty-year trends in serum cholesterol, hypercholesterolemia, and cholesterol medication use: the Minnesota Heart Survey, 1980–1982 to 2000–2002. Circulation. 2005;112:3884–3891. doi: 10.1161/CIRCULATIONAHA.105.549857. [DOI] [PubMed] [Google Scholar]
- 21.Houterman S, Verschuren WM, Oomen CM, Boersma-Cobbaert CM, Kromhout D. Trends in total and high density lipoprotein cholesterol and their determinants in The Netherlands between 1993 and 1997. Int. J. Epidemiol. 2001;30:1063–1070. doi: 10.1093/ije/30.5.1063. [DOI] [PubMed] [Google Scholar]
- 22.Leth T, Jensen HG, Mikkelsen AA, Bysted A. The effect of the regulation on trans fatty acid content in Danish food. Atheroscler. Suppl. 2006;7:53–56. doi: 10.1016/j.atherosclerosissup.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Kypridemos C, et al. Quantifying the contribution of statins to the decline in population mean cholesterol by socioeconomic group in England 1991 - 2012: a modelling study. PLoS ONE. 2015;10:e0123112. doi: 10.1371/journal.pone.0123112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford ES, Capewell S. Trends in total and low-density lipoprotein cholesterol among U.S. adults: contributions of changes in dietary fat intake and use of cholesterol-lowering medications. PLoS ONE. 2013;8:e65228. doi: 10.1371/journal.pone.0065228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y, et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982–2012: a cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019;7:540–548. doi: 10.1016/S2213-8587(19)30152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolmarans P. Background paper on global trends in food production, intake and composition. Ann. Nutr. Metab. 2009;55:244–272. doi: 10.1159/000229005. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, et al. Prevalence of dyslipidemia and achievement of low-density lipoprotein cholesterol targets in Chinese adults: a nationally representative survey of 163,641 adults. Int. J. Cardiol. 2018;260:196–203. doi: 10.1016/j.ijcard.2017.12.069. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Joung H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr. Metab. Cardiovasc. Dis. 2012;22:456–462. doi: 10.1016/j.numecd.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Yoshiike N, Matsumura Y, Iwaya M, Sugiyama M, Yamaguchi M. National Nutrition Survey in Japan. J. Epidemiol. 1996;6:S189–S200. doi: 10.2188/jea.6.3sup_189. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura Y. Nutrition trends in Japan. Asia Pac. J. Clin. Nutr. 2001;10:S40–S47. [PubMed] [Google Scholar]
- 31.Kim S, Moon S, Popkin BM. The nutrition transition in South Korea. Am. J. Clin. Nutr. 2000;71:44–53. doi: 10.1093/ajcn/71.1.44. [DOI] [PubMed] [Google Scholar]
- 32.Risk NCD. Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risk NCD. Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017;389:37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin SS, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J. Am. Coll. Cardiol. 2013;62:732–739. doi: 10.1016/j.jacc.2013.01.079. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch. Intern. Med. 2001;161:1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mach F, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 38.Expert Dyslipidemia Panel of the International Atherosclerosis Society An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia—full report. J. Clin. Lipidol. 2014;8:29–60. doi: 10.1016/j.jacl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Nordestgaard BG, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016;37:1944–1958. doi: 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilen O, Kamal A, Virani SS. Lipoprotein abnormalities in South Asians and its association with cardiovascular disease: current state and future directions. World J. Cardiol. 2016;8:247–257. doi: 10.4330/wjc.v8.i3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danaei G, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 42.Danaei G, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 43.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cholesterol Reference Method Laboratory Network. Total Cholesterol Certification Protocol for Manufacturers. https://www.cdc.gov/labstandards/pdf/crmln/RevisedTCprotocolOct04.pdf (Cholesterol Reference Method Laboratory Network, 2004).
- 45.Cholesterol Reference Method Laboratory Network. HDL Cholesterol Certification Protocol for Manufacturers. https://www.cdc.gov/labstandards/pdf/crmln/HDL_Certification_Protocol-508.pdf (Cholesterol Reference Method Laboratory Network, 2018).
- 46.Cholesterol Reference Method Laboratory Network. LDL Cholesterol Certification Protocol for Manufacturers. https://www.cdc.gov/labstandards/pdf/crmln/LDL_Certification_Protocol-508.pdf (Cholesterol Reference Method Laboratory Network, 2018).
- 47.Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6:461–464. [Google Scholar]
- 48.Finucane MM, Paciorek CJ, Danaei G, Ezzati M. Bayesian estimation of population-level trends in measures of health status. Stat. Sci. 2014;29:18–25. [Google Scholar]
- 49.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 50.Singh GM, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS ONE. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Cholesterol Education Program. Recommendations on Lipoprotein Measurement. From the Working Group on Lipoprotein Measurement. NIH Publication No. 95-3044 (National Institutes of Health, National Heart, Lung, and Blood Institute, 1995).
- 52.Evans A, et al. Trends in coronary risk factors in the WHO MONICA project. Int. J. Epidemiol. 2001;30:S35–S40. doi: 10.1093/ije/30.suppl_1.s35. [DOI] [PubMed] [Google Scholar]
- 53.Bennett SA, Magnus P. Trends in cardiovascular risk factors in Australia. Results from the National Heart Foundation’s Risk Factor Prevalence Study, 1980–1989. Med. J. Aust. 1994;161:519–527. [PubMed] [Google Scholar]
- 54.Cífková R, et al. Longitudinal trends in major cardiovascular risk factors in the Czech population between 1985 and 2007/8. Czech MONICA and Czech post-MONICA. Atherosclerosis. 2010;211:676–681. doi: 10.1016/j.atherosclerosis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Sun JY, et al. The changing trend of serum total cholesterol in Beijing population aged 25 - 64 years during 1984 - 1999 (article in Chinese) Zhonghua Nei Ke Za Zhi. 2006;45:980–984. [PubMed] [Google Scholar]
- 56.Afzal S, Tybjærg-Hansen A, Jensen GB, Nordestgaard BG. Change in body mass index associated with lowest mortality in Denmark, 1976–2013. J. Am. Med. Assoc. 2016;315:1989–1996. doi: 10.1001/jama.2016.4666. [DOI] [PubMed] [Google Scholar]
- 57.Ferrières J, et al. Trends in plasma lipids, lipoproteins and dyslipidaemias in French adults, 1996–2007. Arch. Cardiovasc. Dis. 2009;102:293–301. doi: 10.1016/j.acvd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Truthmann J, et al. Changes in mean serum lipids among adults in Germany: results from National Health Surveys 1997–99 and 2008–11. BMC Public Health. 2016;16:240. doi: 10.1186/s12889-016-2826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sigfusson N, et al. Decline in ischaemic heart disease in Iceland and change in risk factor levels. Br. Med. J. 1991;302:1371–1375. doi: 10.1136/bmj.302.6789.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta R, et al. Twenty-year trends in cardiovascular risk factors in India and influence of educational status. Eur. J. Prev. Cardiol. 2012;19:1258–1271. doi: 10.1177/1741826711424567. [DOI] [PubMed] [Google Scholar]
- 61.Giampaoli S, et al. Cardiovascular health in Italy. Ten-year surveillance of cardiovascular diseases and risk factors: Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey 1998–2012. Eur. J. Prev. Cardiol. 2015;22:9–37. doi: 10.1177/2047487315589011. [DOI] [PubMed] [Google Scholar]
- 62.Iso H. Changes in coronary heart disease risk among Japanese. Circulation. 2008;118:2725–2729. doi: 10.1161/CIRCULATIONAHA.107.750117. [DOI] [PubMed] [Google Scholar]
- 63.Luksiene D, et al. Trends in prevalence of dyslipidaemias and the risk of mortality in Lithuanian urban population aged 45–64 in relation to the presence of the dyslipidaemias and the other cardiovascular risk factors. PLoS ONE. 2014;9:e100158. doi: 10.1371/journal.pone.0100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uusitalo U, et al. Fall in total cholesterol concentration over five years in association with changes in fatty acid composition of cooking oil in Mauritius: cross sectional survey. Br. Med. J. 1996;313:1044–1046. doi: 10.1136/bmj.313.7064.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koopman C, et al. Trends in risk factors for coronary heart disease in the Netherlands. BMC Public Health. 2016;16:835. doi: 10.1186/s12889-016-3526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metcalf P, et al. Trends in major cardiovascular risk factors in Auckland, New Zealand: 1982 to 2002–2003. N. Z. Med. J. 2006;119:U2308. [PubMed] [Google Scholar]
- 67.Sharashova E, Wilsgaard T, Brenn T. Resting heart rate on the decline: the Tromsø Study 1986–2007. Int. J. Epidemiol. 2015;44:1007–1017. doi: 10.1093/ije/dyv061. [DOI] [PubMed] [Google Scholar]
- 68.Pajak A, et al. Changes over time in blood lipids and their correlates in Polish rural and urban populations: the Poland–United States Collaborative Study in cardiopulmonary disease epidemiology. Ann. Epidemiol. 1997;7:115–124. doi: 10.1016/s1047-2797(96)00125-1. [DOI] [PubMed] [Google Scholar]
- 69.Vlasoff T, et al. Ten year trends in chronic disease risk factors in the Republic of Karelia, Russia. Eur. J. Public Health. 2008;18:666–673. doi: 10.1093/eurpub/ckn063. [DOI] [PubMed] [Google Scholar]
- 70.Bovet P, et al. Divergent fifteen-year trends in traditional and cardiometabolic risk factors of cardiovascular diseases in the Seychelles. Cardiovasc. Diabetol. 2009;8:34. doi: 10.1186/1475-2840-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serra-Majem L, et al. Trends in blood lipids and fat soluble vitamins in Catalonia, Spain (1992–2003) Public Health Nutr. 2007;10:1379–1388. doi: 10.1017/S1368980007000985. [DOI] [PubMed] [Google Scholar]
- 72.Berg CM, et al. Trends in blood lipid levels, blood pressure, alcohol and smoking habits from 1985 to 2002: results from INTERGENE and GOT-MONICA. Eur. J. Cardiovasc. Prev. Rehabil. 2005;12:115–125. doi: 10.1097/00149831-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Wietlisbach V, Paccaud F, Rickenbach M, Gutzwiller F. Trends in cardiovascular risk factors (1984–1993) in a Swiss region: results of three population surveys. Prev. Med. 1997;26:523–533. doi: 10.1006/pmed.1997.0167. [DOI] [PubMed] [Google Scholar]
- 74.Leiviskä J, et al. What have we learnt about high-density lipoprotein cholesterol measurements during 32 years? Experiences in Finland 1980–2012. Clin. Chim. Acta. 2013;415:118–123. doi: 10.1016/j.cca.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 75.Yokoyama S, et al. High-density lipoprotein levels have markedly increased over the past twenty years in Japan. J. Atheroscler. Thromb. 2014;21:151–160. doi: 10.5551/jat.20909. [DOI] [PubMed] [Google Scholar]
- 76.Hardoon SL, et al. Assessing the impact of medication use on trends in major coronary risk factors in older British men: a cohort study. Eur. J. Cardiovasc. Prev. Rehabil. 2010;17:502–508. doi: 10.1097/HJR.0b013e3283378865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carroll MD, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. J. Am. Med. Assoc. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains a Literature search for additional data sources and Supplementary Tables 1-5 and Supplementary Figure 1.
Data Availability Statement
Estimates of mean total, non-HDL and HDL cholesterol by country, year and sex are available at http://www.ncdrisc.org/. Input data from publicly available sources can also be downloaded from http://www.ncdrisc.org/. For other data sources, contact information for data providers can be obtained from http://www.ncdrisc.org/.
The computer code for the Bayesian hierarchical model used in this work is available at http://www.ncdrisc.org/.