Abstract
Toxicity and poor adherence to treatment that favors the generation of resistance in the Leishmania parasites highlight the need to develop better alternatives. Here, we evaluated the in vitro effectiveness of hydrazone derived from chromanes 2-(2,3-dihydro-4H-1-benzothiopyran-4-ylidene) hydrazide (TC1) and 2-(2,3-dihydro-4H-1-benzopyran-4-ylidene) hydrazide (TC2) and the mixture of triterpene saponin hederagenin-3-O-(3,4-O-diacetyl-ß-D-xylopyranosyl-(1à3)-a-L- rhamnopyranosyl-(1à2)-a-L-arabinofuranoside, hederagenin-3-O-(3,4-O-diacetyl-a-L- arabinopyranosyl-(1à3)-a-L-rhamnopyranosyl-(1à2)-a-L-arabinofuranoside and, hederagenin-3-O-(4-O-acetyl-ß-D-xylopyranosyl-(1à3)-a-L-rhamnopyranosyl-(1à2)-a-L-arabinofuranoside from Sapindus saponaria (SS) on L. braziliensis and L. pifanoi. Mixtures of TC1 or TC2 with saponin were formulated for topical application and the therapeutic effectiveness was evaluated in the model for cutaneous leishmaniasis (CL) in golden hamster. The mode of action of these compounds was tested on various parasite processes and ultrastructural parasite modifications. TC1, TC2 and SS showed moderate cytotoxicity when tested independently but toxicity was improved when tested in combination. The compounds were more active against intracellular Leishmania amastigotes. In vivo studies showed that combinations of TC1 or TC2 with SS in 1:1 ratio (w/w) cured 100% of hamsters with no signs associated with toxicity. The compounds did cause changes in the mitochondrial activity of the parasite with a decrease in ATP levels and depolarization of membrane potential and overproduction of reactive oxygen species; nevertheless, these effects were not related to alterations in membrane permeability. The phagolysosome ultrastructure was also affected impacting the survival of Leishmania but the function of the lysosome nor the pH inside the phagolysosome did not change. Lastly, there was a protease inhibition which was directly related to the decrease in the ability of Leishmania to infect and multiply inside the macrophage. The results suggest that the combination of TC1 and TC2 with SS in a 1:1 ratio is capable of curing CL in hamsters. This effect may be due to the ability of these compounds to affect parasite survival and the ability to infect new cells.
Keywords: Chromane hydrazone, Leishmania braziliensis, L. pifanoi, Sapindus saponaria, ATP level, Mitochondria, Proteases, Morphology, Reinfection process
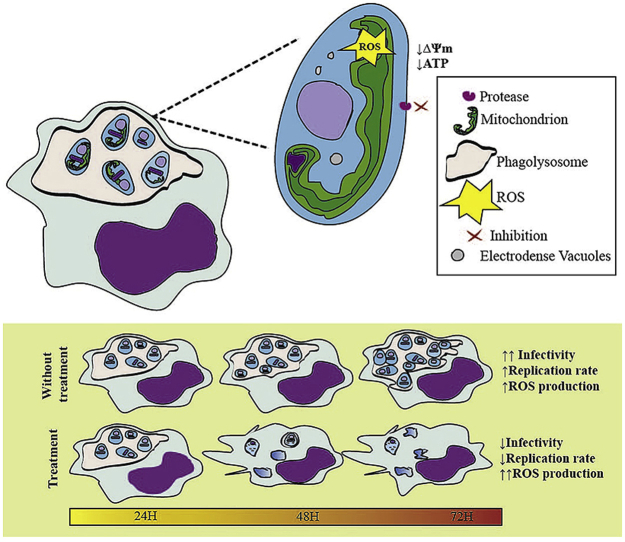
Graphical abstract
Highlights
-
•
TC1, TC2 and SS cytotoxicity was moderate and varied depending of the cell type.
-
•
TC1 or TC2 with SS in 1:1 ratio (w/w) are able to healing 100% of hamsters with no signs of toxicity.
-
•
TC1, TC2 and SS caused loss of phagolysosome bulkiness.
-
•
TC1, TC2 and SS affect the Leishmania ability to infect and reproduce inside the macrophages.
1. Introduction
Leishmaniasis is an infectious disease caused by parasites of the genus Leishmania. It is manifested by nodules, plaques, or ulcers in the skin; also by mucous membranes of the oral-nasopharyngeal region; or a typical clinical presentation consists of lesions in bone marrow, liver, spleen, and pancreas. These signs are clinically defined as cutaneous leishmaniasis (CL), mucosal leishmaniasis (ML), or visceral leishmaniasis (VL), respectively (WHO, 2010).
Leishmaniasis is endemic in 98 countries in the tropical and subtropical regions, with 350 million people at risk of getting the infection and 2 million people infected each year. No vaccine prevents infection, and therapeutic options for the treatment of the disease are scarce. The treatments require high doses of the medications, favoring toxicity with the consequent low adherence to the treatment and the appearance of resistance in the strains of circulating parasites (WHO, 2010). Because therapeutic options are insufficient, it is necessary to work decisively in the search for new and better therapeutic options that are not only more effective but also safer.
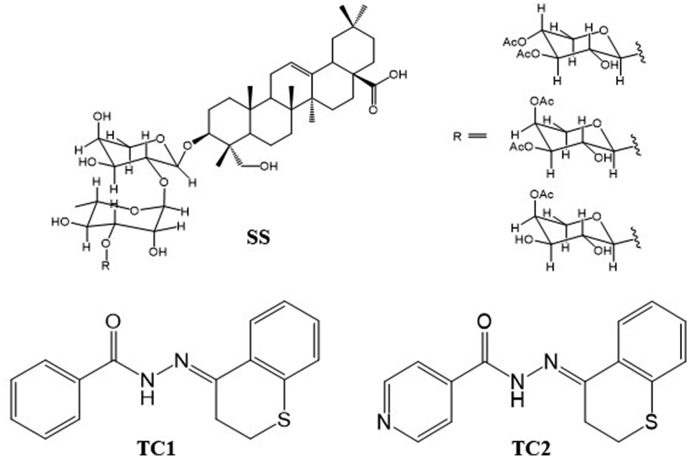
Hydrazones derived from thiochromanone (Vargas et al., 2018) and benzophenones (Al-Kahraman et al., 2012) have shown antileishmanial potential against L. panamensis and L. major, respectively. The 7-chloro-4-quinolinyl hydrazones have also shown activity against Leishmania species from the new and the old world (Coimbra et al., 2013). On the other hand, some saponins such as oleanolic acid and triterpene saponin PX6518 have shown promising results in vitro and in vivo for VL and CL (Melo et al., 2016; Inocencio da Luz et al., 2011), and the pure saponins of Sapindus saponaria resin hederagenin-3-O - (3,4-O-diacetyl -ß-D- xylopyranosyl - (1à3)-a-L- rhamnopyranosyl-(1à2)-a-L- arabinofuranoside, hederagenin-3-O-(3,4-O- diacetyl -a-L- arabinopyranosyl -(1à3)-a-L- rhamnopyranosyl -(1à2)-a-L- arabinofuranoside and hederagenin-3-O-(4-O-acetyl-ß-D- xylopyranosyl -(1à3)-a-L- rhamnopyranosyl -(1à2)-a-L- arabinofuranoside (SS) (Fig. 1) that demonstrated in vitro antileishmanial activity to L. panamensis, L. amazonensis and, but also in vivo activity to L. panamensis (Correa et al., 2014).
Fig. 1.
Structure of compounds. A. A mixture of saponins; B: Chroman hydrazone derivatives, TC1 and TC2.
For the last 15 years, the authors have focused on the quest for natural substances for the treatment of CL and their structural optimization by chemical transformations. The derivatization of 4-chromanone and 4-thiochromanone compounds with several hydrazides generated two chromane hydrazones 2-(2,3-dihydro-4H-1-benzothiopyran-4-ylidene) hydrazide (TC1) and 2-(2,3-dihydro-4H-1-benzopyran-4-ylidene) hydrazide (TC2), respectively (Fig. 1). Both compounds, formulated as 1% aqueous solution or 4% ointment, showed a high therapeutic response when administered topically to hamsters with CL caused by L. braziliensis (Upegui et al., 2019).
2. Materials and methods
Several formulations were prepared to optimize the therapeutic potential of the 4% TC1 and TC2 ointment, through the combination of each chroman hydrazide with the triterpene saponin mixture SS at ratios 1: 1 (w/w), 1: 3 or 3: 1, hydrazone:saponin, respectively. The new formulations of TC1 with SS, and TC2 with SS, were validated for their therapeutic response in the experimental CL model caused by L. braziliensis in the golden hamster. Finally, the effect of the compounds was evaluated individually, or in combination, on some biochemical processes of two species of Leishmania in various biological stages. Specifically, the effect on energy metabolism, response to cellular stress, interaction with the host cell, and activity in proteases were studied, as well as the reinfection process.
2.1. Synthesis of chromane hydrazones TC1 and TC2
Compounds were synthesized as reported (Vargas et al., 2018). Briefly, a solution of chromane or thiochromane-4-one, and the respective hydrazone were mixed in ethanol, and then, 5% acetic acid in ethanol was added dropwise. The reaction mixture was heated and then poured into an ice/water, filtered, dried and crystallized. Compounds were identified by 2D NMR and MS (Echeverri et al., 2015).
2.2. Isolation and purification of the triterpene saponin
The saponin SS was obtained according to the procedure reported by Correa et al. (2014) by the extraction of arillus with ethanol and purification by sephadex LH-20 followed by column chromatography using CH2Cl2–MeOH (9:1 v:v) on silica gel. A fraction containing an equimolar mixture of three saponins was obtained, differing on the substitutions of the glycosidic moiety of the molecule (Fig. 1).
2.3. Cells and culture
The U937 (human promonocyte, CRL-1593.2) and J774 (mouse macrophage, TIB-67) cell lines were grown under standard conditions in RPMI-1640 or DMEM, respectively, supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin and 0.1 mg/mL streptomycin). Hamster's peritoneal macrophages (haPDM) were obtained after intraperitoneal injection of 0.4% sodium thioglycollate in phosphate buffer saline (PBS) (Mesa et al., 2010) and cultured in RPMI medium with 10% FBS and 1% antibiotics. All cell types were incubated at 37 °C in the presence of 5% CO2.
2.4. Parasite strain and culture
Wild type L. braziliensis wild type (MHOM/CO/88/UA301-wt), L. braziliensis green fluorescent protein transfected (MHOM/CO/88/UA301-EGFP), L. braziliensis luciferase transfected (MHOM/CO/88/UA301-luc) and L. pifanoi (MHOM/VE/60/Ltrod) were used in the present work.
L. braziliensis was cultured as promastigotes in NNN (Novy-McNeal-Nicolle) biphasic medium at 26 °C, with a change of medium every 15 days. The L. pifanoi axenic amastigotes were grown in 199 medium supplemented with 1.25 g/L glucose, 2.5 g/L trypticase, 500 μL/L gentamicin, 0.375 g/L glutamine, 25 mg/L hemin and 10% of FBS (complete medium) and incubation at 32 °C in a wet chamber (Luque-Ortega and Rivas, 2010). The medium was changed every day to keeping parasites in exponential growth phase.
2.5. In vitro cytotoxic activity
Cytotoxicity of TC1, TC2, SS and TC1:SS and TC2:SS (1:1 ratio) was measured in U937, J774 and haPDM cells by inhibition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay as described elsewhere (Upegui et al., 2014). Cells exposed to doxorubicin and Amphotericin B (AMB) were used as positive (cytotoxicity) controls while unexposed cells were used as negative (viability) control. The negative control was performed using growth medium alone. Compounds were dissolved in DMSO (1 mg/mL, w/v) and then was prepared a stock solution of 200 μg/mL complete RPMI-1640 medium; serial four-fold dilutions were made from stock solution to give working concentrations of 200–0.78 μg/mL. Thus, the final concentration of DMSO in the tested dilutions was not higher than 0.2% which has been widely demonstrated not be toxic for cells (Hajighasemi and Tajic, 2017). Non-specific absorbance was corrected by subtracting absorbance (O.D) of the blank (RPMI-1640 plus 0.2% DMSO). Determinations were done by triplicate in at least two independent experiments.
2.6. In vitro antileishmanial activity
The activity of compounds was evaluated on promastigotes of L. braziliensis and L. pifanoi as well as in axenic amastigotes of L. pifanoi, using the MTT assay as described by others (Sereno and Lemesre, 1997). In this case, parasites at the log growth-phase were harvested and adjusted to 2 × 106 parasites/mL in complete RPMI-1640 (for promastigotes) or 199 media (for axenic amastigotes). Promastigotes were incubated at 26 °C, while axenic amastigotes were incubated at 32 °C for 72 and 96 h, respectively.
The leishmanicidal activity was also evaluated on intracellular amastigotes of L. braziliensis. Briefly, U-937 cells were adjusted to 3 × 105 cells/ml in complete RPMI-1640 medium and 0.1 μg/mL of phorbol 12 myristate 13 acetate. In each well of a 24 well plate containing a microscope cover glass, was dispensed 1 mL of cells and plates were incubated for 72 h at 37 °C and 5% CO2. Cells were then infected with promastigotes at 13:1 parasite/cell ratio. Cells exposed to parasites were incubated for 3 h at 34 °C, 5% CO2. Each well was washed three times with prewarmed PBS to remove non-internalized parasites, and new medium was added. After 24 h of infection, cells were exposed to four dilutions starting at 50 μg/mL of each compound or their combinations and incubated again for 72 h at the corresponding temperature. Cells were removed from the bottom well with trypsin/EDTA solution, washed by centrifuging with 1 mL/well of PBS and suspended in 500 μL/well of PBS. Plates were read in a flow cytometer (Cytomics FC 500MPL, Brea, CA), at 488 nm excitation and 525 nm emission and counting 10,000 events Pulido et al., 2012). Infected cells exposed to AMB were used as an internal assay control for leishmanicidal activity.
2.7. Formulations of TC1 and TC2 combined with SS
Ointments were prepared based on a mixture of lanolin and petrolatum 2: 1 (w/w) and 1% glycerin. For the preparation of the mixtures, the solids of the pure chromane hydrazones or the saponin mixture were combined and subsequently incorporated into the base ointment; those same solids were used for in vitro assays. In this way, three formulations were obtained, each containing 4% of the mixture of the active ingredients TC1 or TC2 and SS in a ratio 1: 1, 1: 3 and 3: 1 chromane hydrazone:saponin (w/w).
2.8. In vivo antileishmanial activity
The antileishmanial response of compounds was evaluated in juvenile male and female golden hamsters (Mesocricetus auratus) experimentally infected with L. braziliensis, using the method described by Robledo et al. (2012). In this model, those hamsters that self-limiting infections and do not develop an ulcer are excluded. Only hamsters that develop an ulcer with a diameter greater than 4 mm2 were randomly distributed in seven treatment groups (n = 5 animals per group). Seven groups were treated topically with 40 mg/day of each ointment preparation for 28 days while the remained group of hamsters was treated with meglumine antimoniate (MA), administered intralesional, at 200 μg/three times per week for 28 days (MA-28). After the end of treatment, animals were supervised for 90 additional days. From the beginning of the treatment, the hamsters were observed daily to record survival and changes in behavior. The effectiveness and toxicity of each compound was determined according to the evolution of the lesion size and body weight during the study. For this, the area (width and length) of the ulcer and the body weight were recorded before treatment (D0), the last day of treatment (TD28) and every 30 days during 3 months. The effectiveness of each treatment was assessed in terms of reduction or disappearance of the ulcer in each hamster. The outcome at the end of study was recorded as a cure (epithelial healing and emergence of fur); improvement (reduction of the lesion size by at least > 20%); relapse (reactivation of the lesion after initial improvement or cure); or failure (increase of the lesion size).
The Ethics Committee for Animal Experimentation at the Universidad de Antioquia approved all of the experiments conducted with the hamsters (Act No. 91 of September 25, 2014).
2.9. Effect of TC1, TC2, SS and their combinations in morphology and functions of Leishmania spp
2.9.1. Morphology of Leishmania pifanoi
The effect of the compounds was determined in both axenic and intracellular amastigotes of L. pifanoi but also in non-infected cells using TEM and fluorescence microscopy. The axenic amastigotes were grown under standard conditions, then adjusted to 2 × 106 amastigotes/mL and incubated with the compounds at a concentration equivalent to their effective concentration 70 (EC70) to ensure a response without causing a cytotoxic effect on the host cell.
On the other hand, 200,000 J774 cells/mL (DMEM medium with 10% FBS and 1% antibiotics) were seeded in each well of a 24-well plate containing microscope cover glass. The cells were incubated at 37 °C, 5% CO2 for 24 h and then macrophages were infected with axenic amastigotes in a 4:1 ratio (parasite per cell) and incubated for 4 h at 32 °C in a wet chamber. Non-internalized parasites were removed by PBS washing four times with 1 mL PBS; then 1 mL of RPMI-1640 medium with 10% SBF was added, and cells were incubated for additional 24 h.
For TEM analysis, axenic amastigotes and infected cells were washed and fixed with 3% glutaraldehyde (1 h, 4 °C) followed by increasing dehydration steps (Luque-Ortega and Rivas, 2010). The samples were embedded in Epon 812 resin and observed in TEM (JEOL-1230 USA Inc. MA, USA).
For fluorescence analysis, axenic amastigotes and infected cells were washed with Hanks-Buffer (155 mM NaCl, 1 mM MgCl2, 10 mM HEPES). The equivalent concentration of the treatment was put in the buffer solution to guarantee the maintenance of the system, and with this solution the washes and staining at 100 nM of Lysotracker network were carried out for 30 min, incubating at 32 °C, protected from light. The reading was done by observation under a fluorescence microscope (Nikon eclipse). Staining with nigericin (10 μM) during 10 min was used as positive control (Vercesi et al., 2000). Light and dark field photographs were registered.
2.9.2. Plasma membrane integrity
100 μL of the L. pifanoi axenic amastigotes (2 × 107 parasites/mL in Hanks-Glu medium plus 1 μM of the Sytox Green probe) were transferred to each well of a 96-well plate to determine the effect of the different compounds and mixtures of compounds on plasma membrane permeabilization. Next, 100 μL of each compound or combination thereof was added at 50 μg/mL. Plates were read every 60 s for 1 h at 485 nm excitation and 520 nm emission using a microplate reader (Polar Star Galaxy, BMG, Germany). To detect changes in fluorescence caused by the hybridization of the probe to the DNA, Triton X-100 (1%) was used as control to membrane permeabilization and to define the maximum fluorescence signal for each well.
The effect on membrane permeabilization was also evaluated in promastigotes of L. braziliensis-EGFP. In this case, the measurements were made at 1, 2 and 24 h. Here, 300 μL of the parasites were exposed to the compounds and their combinations and then centrifuged at 1000×g for 10 min and suspended in 300 μL of propidium iodide at 50 μg/mL. After 15 min of incubation at 25 °C and in the dark, fluorescence was recorded by reading at 488 nm excitation and 610 nm emission.
2.9.3. Intracellular ATP variation
Promastigotes of L. braziliensis-Luc in the log phase-growth were adjusted to 2 × 107 parasites/mL in Hansk-Glu solution. The ({D-luciferin-1 [- (4,5-dimethoxy-2-nitrophenyl) ethyl ester)}) (DMNPE) substrate - luciferin was added at 50 μM and then, 90 μL of the parasite solution was transferred to each well of a 96-well microplate; the luminescence was read, and then compounds or combinations at 50 μg/mL were added to each well. Plates were read every 60 s for 1 h in the microplate reader (Varioskan Flash, Thermo Fisher Scientific, USA). Parasites in untreated Hanks buffer supplemented with 10 mM D-glucose solutions were included as control. Triton X-100 (1%) was used as a permeability control and Naphthoquinone (1.5 μM) was used as control for mitochondrial involvement (Luque-Ortega et al., 2001).
2.9.4. Mitochondrial membrane potential (ΔΨm)
The effect in the mitochondrial membrane potential (ΔΨm) was evaluated in axenic amastigotes of L. pifanoi using the Rhodamine 123 (Rh 123) staining method (Carvalho et al., 2011) and in intracellular amastigotes of L. braziliensis using the Tetramethylrhodamine Methyl Ester (TMRM) probe.
Axenic amastigotes of L. pifanoi were adjusted to 2 × 107 parasites/mL in 199 medium and incubated in the presence of each compound at a concentration equivalent to the corresponding EC70 for 1 h and 4 h at 32 °C. Parasites incubated with 20 mM KCN for 40 min were used as control. Then, parasites were washed twice with PBS and centrifuged at 15,000×g for 4 min at 25 °C. The supernatant was discarded, and the pellet was resuspended in 1 mL of the Rh 123 solution at 0.3 μg/mL, incubated at 32 °C for 10 min and centrifuged again for washing with PBS and 1% bovine serum albumin (BSA). Pellet was suspended in 200 μL of PBS and transferred to a microplate for reading at 488 nm excitation and 520 nm emission.
The intracellular amastigotes of L. braziliensis were mechanically released from the U937 macrophages using a syringe plunger. The cell lysate was centrifuged at 100×g for 5 min; the supernatant was discarded and pellet was centrifuged again for 10 min at 1500×g. Free amastigotes were adjusted at 4 × 106 parasites/mL in RPMI-1640 medium free of phenol red, pH 5.5, and supplemented with 10% FBS and 1% antibiotics. In each well of a 96-well microplate were dispensed 100 μL of the parasite suspension and then, 100 μL of each compound at a concentration equivalent to EC90 were added. Plates were incubated for 1 h or 4 h. Parasites incubated with 20 mM NaCN for 30 min were used as control. Then, 100 μL of TMRM probe 100 μM were added and plates were incubated at 34 °C for 20 min. The plates were centrifuged at 1200×g for 8 min and resuspended in 100 μL of PBS, transferred to a dark 96-well plate and fluorescence was read at an excitation of wavelength 548 nm and 573 nm emission.
2.9.5. Reactive oxygen species production
The generation of reactive oxygen species (ROS) was assessed by flow cytometry in axenic amastigotes of L. pifanoi and mechanical released amastigotes of L. braziliensis, using the 2′7′-dihydrofluorescein (H2DCF-DA) probe (Carvalho et al., 2011). The dichlorofluorescein resulting from the oxidation of H2DCF-DA by ROS in L. pifanoi was quantified in the microplate reader (Varioskan Flash, Thermo Fisher Scientific, USA) at a wavelength of 495 nm excitation and 527 nm emission. In L. braziliensis, the amount of dichlorofluorescein was measured in a flow cytometer (LSR Fortesa, BD Biosciences, USA), according to the median fluorescence intensity (MFI). Antimycin (0.3 μg/mL) was used as control in L. pifanoi, while rotenone (50 μg/mL) was used as control in L. braziliensis experiments.
2.9.6. Lipid bodies formation
The formation of lipid bodies was determined in axenic amastigotes of L. pifanoi and mechanical released amastigotes of L. braziliensis and staining with Nile red, following the methodology described by Godinho et al. (2013). Plates were read in a microplate reader (Varioskan Flash, Thermo Fisher Scientific, USA) at an excitation wavelength of 485 nm and emission of 538 nm.
2.9.7. Proteolytic activity
Promastigotes of L. braziliensis adjusted at 2.5 × 106 parasites/mL of Schneider medium with 10% FBS, were incubated for 2 h at 26 °C, in the presence of each compound, at the equivalent concentration to EC90 determined in promastigotes. Proteins were quantified using the Pierce™ BCA Protein Assay Kit (Thermo Scientific) using BSA as protein standard. The reaction was read at 595 nm in a microplate reader (Varioskan Flash, Thermo Fisher Scientific, USA). Proteins were stored at −80 °C until use.
The zymographic study was carried out according to the methodology proposed by Cuervo et al. (2006), with some modifications. Briefly, 60 μg of Leishmania proteins were suspended in 60 μL of sample buffer containing 100 mM TRIS-HCl pH 8.0, 150 mM NaCl, 10% Glycerol, 0.6% Triton X-100, and bromophenol and incubated at room temperature for 1 h. Then, 20 μL of the buffer with 30 μg of protein was run in 10% SDS-PAGE copolymerized with 0.1% gelatin for 105 min at constant 120 V. After electrophoresis, gels were washed twice with 0.1 M sodium acetate pH 5.5 at 2.5% Triton X-100 for 30 min at 4 °C. After this, gels were washed four times with distilled water to remove detergent residue. Gels were placed in reaction buffer, 0.1 M sodium acetate, 1 mM DTT at pH 5.5, 37 °C for 24 h to allow protease activity. Finally, gels were stained with Coomassie blue for 20 min at room temperature. Protease activity was evidenced as uncolored zone in the gel due to the degradation of the gelatin.
This same procedure was performed in the presence of two cysteine protease inhibitor: leupeptin and trans-epoxysuccinyl-L-leucylamido-(4-guanidino)-butane (E−64). For this, after washing, gels were incubated in the reaction buffer containing 25 μM of leupeptin or ten μM of E−64 at 37 °C for 4 and 2 h, respectively. The gels were washed again as previously described and incubated in the reaction buffer at 37 °C for 24 h. Finally, gels were stained with Coomassie blue and the gels were visualized for the inhibition of the degradation of the gelatin caused by the corresponding family of proteases.
For cysteine proteases assay, mild log phase (day 3) promastigotes of L. braziliensis were washed two times in ice-cold PBS for10 min, 1,500×g in a Sorvall RT6000 centrifuge, and re-suspended in 100 mL of M199 medium supplemented with 10% FBS at 1 × 106 parasites/mL. Parasites were incubated with 50 μg/mL of compounds for 2 h at 25 °C. Controls were untreated or incubated with same volume of dimethyl sulphoxide (DMSO) used to re-suspend and adjust drug concentration (100 μL). For preparation of lysates, parasites were pelleted, washed in PBS, and re-suspended in fresh lysis buffer (20 mM Tris pH 8.0, 10 mM EDTA, pH 8.0, 4 mM NaCl) at 5 × 108 parasites per 300 μl of buffer. Lysates were left on ice for 30 min, sonicated in 3 cycles (30 s/cycle, 30 s cooling in between; 45 mv) and centrifuged at 21,000×g for 20 min to remove cell debris. Protein concentration was assessed by Pierce™ BCA Protein Assay kit.
The enzymatic activity of L. braziliensis lysates was assayed with the fluorogenic synthetic substrate Z-Phe-Arg-amidomethylcoumarin (AMC), as previously described (Somanna et al., 2002). Fluorescence of Z-Phe-Arg-AMC liberated from the substrates during proteolytic activity was determined by ELISA reader every 5 min up to 30 min at 460 nm emission and an excitation wavelength of 355 nm.
2.9.8. Reinfection capacity
Five x106 PMA activated U937 cells were infected with L. braziliensis-EGFP promastigotes as described above, 72 h post infection. The infected cells were exposed to each compound at the corresponding EC50 concentrations for 24 h. After that, cells were washed with PBS, and the amastigotes were mechanically released. Cell lysates were washed with PBS and centrifuged at 1500×g for 8 min. The amastigotes were adjusted to 2.25 × 106 amastigotes/mL in RPMI-1640 medium with 10% FBS. Four hundred μL of the parasite suspension was added in each well of a 24-well plate containing 100,000 U937 macrophages previously differentiated with PMA (3:1 parasite: cell ratio). After 24 h of incubation at 34 °C, 5% CO2 wells were washed with PBS to eliminate non-internalized parasites and cell debris, and the medium was replaced with fresh RPMI-1640 with 10% FBS. Plates were incubated for additional 96 h at 34 °C, 5% CO2. After this, cells were detached and the number of infected cells as well the number of parasites in the infected cells were determined according to the green fluorescent events and the MFI by flow cytometry, as previously described (Pulido et al., 2012).
2.10. Data analysis
Each experiment was performed in triplicate in at least two independent experiments. Data are expressed as the average value ± the SD. The cytotoxic and antileishmanial activity was determined according to the median lethal concentration (LC50) and the median effective concentration (EC50) obtained by Probit analysis, following the methodology described by Upegui et al. (2019). For the analysis of the data, comparisons were made between groups using two-way ANOVA, as well as the Bonferroni's test for comparisons between groups of parametric data or Kruskal Wallis test for non-parametric data. Differences with a p-value <0.05 were considered as statistically significant.
3. Results
3.1. Cytotoxicity and antileishmanial activity
The cytotoxicity showed by TC1, TC2, and SS compounds, both individually and in combination, on the U937 and J774 macrophage cell lines was classified as moderate, with LC50 values between 40 and 150 μg/mL (Table 1). For haPDM, TC1 and TC2 showed low cytotoxicity, with LC50 values > 200 μg/mL while SS showed high cytotoxic activity, with an LC50 of 33.9 ± 4.6 μg/mL; the cytotoxicity of the combinations in the haPDM was moderate, with LC50 values of 127.4 ± 10.2 and 181 ± 5.0 μg/mL for TC1:SS and TC2:SS, respectively). In turn, the compounds were active only in the amastigote stage, with activity between high (<25 μg/mL) and moderate (>25 and <50 μg/mL) (Table 1). The combination of the compounds showed reductions of their EC50 around 50% The highest activities were observed for intracellular amastigotes of L. braziliensis, but the SS showed low activity for axenic amastigotes of L. pifanoi.
Table 1.
Cytotoxicity and sensitivity of Leishmania to chromane derivatives TC1and TC2 and triterpene sapogenin.
| Compound | CL50 μg/mL |
CE50 μg/mL |
aIS | ||||
|---|---|---|---|---|---|---|---|
| L. braziliensis |
L. pifanoi |
||||||
| U937 | J774 | HaMDP | Promastigotes | Intracellular amastigotes | Axenic amastigotes | ||
| TC1 | 88.1 ± 3.6 | 83.7 ± 2.4 | >200 | 69.8 ± 6.7 | 12.1 ± 4.1 | 17.9 ± 3.1 | 7.3 |
| TC2 | 85.1 ± 3.7 | 72.9 ± 2.4 | >200 | 99.9 ± 4.8 | 25.5 ± 0.9 | 13.2 ± 4.2 | 3.3 |
| SS | 63.5 ± 1.2 | 43.9 ± 2.9 | 33.9 ± 4.6 | 135.5 ± 5.6 | 20.8 ± 6.4 | >50 | 3.1 |
| TC1:SS | 70.4 ± 1.8 | 81.2 ± 0.2 | 127.4 ± 10.2 | 108.7 ± 4.5 | 7.7 ± 2.4 | 13.4 ± 3.7 | 9.1 |
| TC2:SS | 99.7 ± 1.9 | 145.7 ± 9.9 | 181.0 ± 5.0 | 114.9 ± 8.5 | 13.6 ± 6.8 | 17.1 ± 5.3 | 7.3 |
| Doxorubicin | 0.13 ± 0.0 | 0.5 ± 0.01 | 0.06 ± 0.01 | NA | NA | NA | NA |
| Amphotericin B | 28.8 ± 1.2 | 52.7 ± 3.7 | 17.8 ± 3.2 | 3.4 ± 0.6 | 0.7 ± 0.1 | NA | 41.7 |
| Miltefosine | 104.6 ± 11.7 | 28.3 ± 3.3 | 3.9 ± 0.8 | NA | NA | 0.04 ± 0.0 | 261.5 |
Data represent the median lethal and effective concentration (LC50, EC50) in μg/mL for each compound ± SD.
IS: Index of selectivity calculated by CL50 in U937/CE50 in intracellular amastigotes.
3.2. In vivo therapeutic response of a combination of TC1 or TC2 and SS
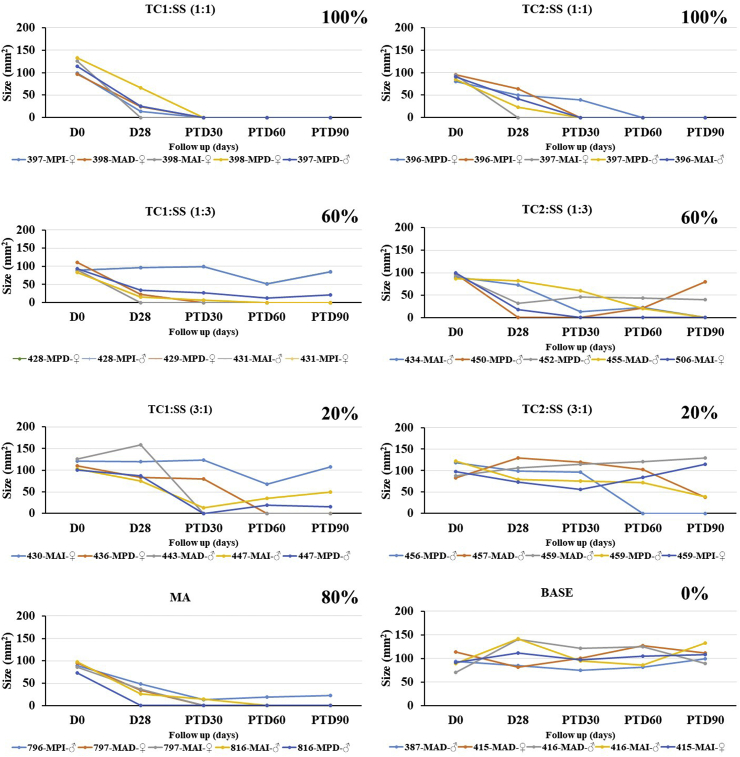
The combination of TC1 or TC2 with SS was evaluated in three different combinations 1:1, 1:3, and 3:1 (w/w ratio) (chromane hydrazone:saponin, respectively). Regarding TC1 combinations, the ratio 1:1 was the most effective treatment, with 100% cure when applied daily for 30 days. These results are similar to meglumine antimoniate (MA), used as antileishmanial control (Fig. 2). The other treatments were less effective, thus 1:3 combination caused a 60% cure in slow response, and the 3:1 combination showed the lowest efficacy, including several relapses. Concerning TC2 combinations, all treatments were less effective than TC1 combinations and cure process was slower. The other combinations with this compound, 1:3 and 3:1 were unsuccessful and included high relapsed cases. Treatment with intralesional MA every two days for 28 days (MA-28) produced cure in 3/5 hamsters (60%) and failure and improvement each in the remained two hamsters. In short, combinations of TC1:SS in 1:1 combination displayed a high and fast affectivity against L. The combinations in 3:1 ratio did produce the poorest therapeutic response, with only 40% and 20% cure for TC1: SS and TC2: SS, respectively.
Fig. 2.
Efficacy of an ointment of hydrazones TC1 and TC2 in combination with a mixture of triterpene saponin (SS) vs. meglumine antimoniate (MA) in a L. braziliensis-hamster model of cutaneous leishmaniasis. Data show the evolution of the lesion according to the size in mm2 after topical treatment with 40 mg of 4% ointments TC1:SS or TC2:SS (1:3), TC1:SS or TC2:SS (1:1) and TC1:SS or TC2:SS (3:1) every day for 28 days or MA (200 μg every three days, during 28 days) (MA-28). The effectiveness of each treatment was assessed, comparing the lesion sizes before and after treatment for everyone. The area (width and length) of the ulcer were recorded before treatment (D0), the last day of treatment (TD28) and every 15 days during 3 months. Nevertheless, only TD0, TD28 and post-treatment days (PTD) 90 are shown in the figure because these are the most relevant time points to show changes related to effectiveness of the treatment. The final outcome observed at the PTD90 was recorded as cure (epithelial healing and emergence of fur); improvement (reduction of the lesion size by at least > 20%); relapse (reactivation of the lesion after the initial improvement or cure); or failure (increase of the lesion size). All combinations were made w/w percentage; final percentage of compounds in the ointment was 4%. MA was administered by intralesional route. MAI, MAD, MPI and MPD refer to the brand that identifies each hamster and the number that accompanies these letters corresponds to the cage were hamsters are housed.
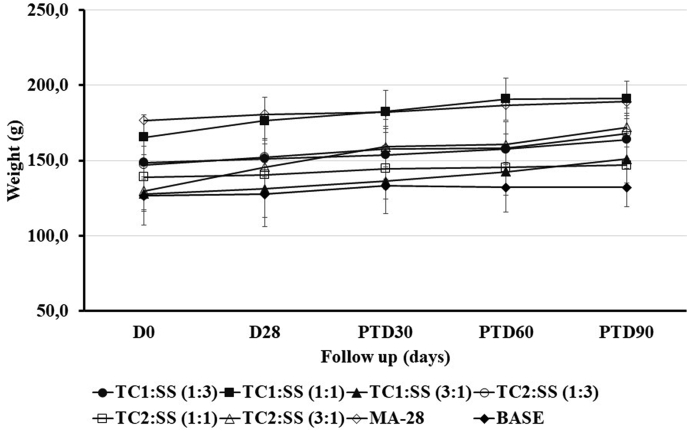
3.3. In vivo toxicity of treatments
Treatment with different combinations of compounds TC1 or TC2 with SS did not produce any detrimental effect on body weight in most hamsters, but rather a slight weight gain (4–30%) was observed in all groups of treatments. The evolution of the body weight in the treatment group is shown in Fig. 3. The parameters of renal and hepatic function ALT, creatinine, and BUN, did not show any alteration because of the treatment (data not shown).
Fig. 3.
Effect of the treatment on body weight. Golden hamsters (n = 5 each group) were treated for 28 days with 40 mg of three different combinations (1:3, 1:1 or 3:1) of hydrazonesTC1 or TC2 and SS, respectively, topically; MA-28 (200 μg every three days, 28 days, intralesional). The data represent the mean value ± SD of the weight in grams of the hamsters in each experimental group. p > 0.05. Bodyweight was measured before the treatment (D0) at the end of the treatment (D28) and every 30 days for three months, corresponding to post-treatment days (PTD) 30, 60, and 90.
3.4. Morphological changes in infected macrophages and axenic amastigotes of L. pifanoi
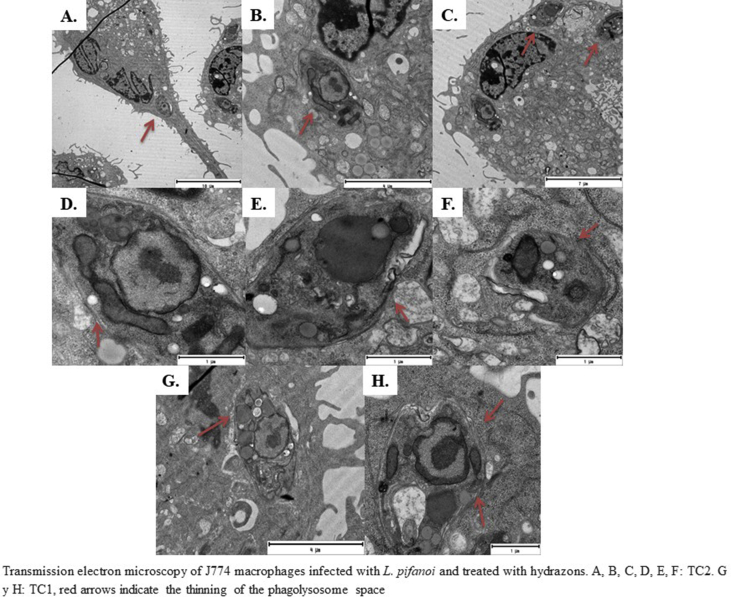
Infected J774 macrophages treated with TC1 and TC2 for 24 h showed an increase in cell size and elongation, with numerous degradation vacuoles. we also observed a decrease in phagolysosome size (Fig. 4A and B) and, apparently, loss of its content compared to the control without treatment (Fig. 4C). Abundant glycosomes were detected in the parasites as electro-dense structures. Infected macrophages treated with SS showed abundant empty vacuoles (Fig. 4D). In the combinations, empty vacuoles were abundant with little alteration of the phagolysosome morphology and degradation vacuoles; the parasitic load was low in TC1: SS (Fig. 4E). In the case of TC2: SS cells, more than one amastigotes were observed (Fig. 4F).
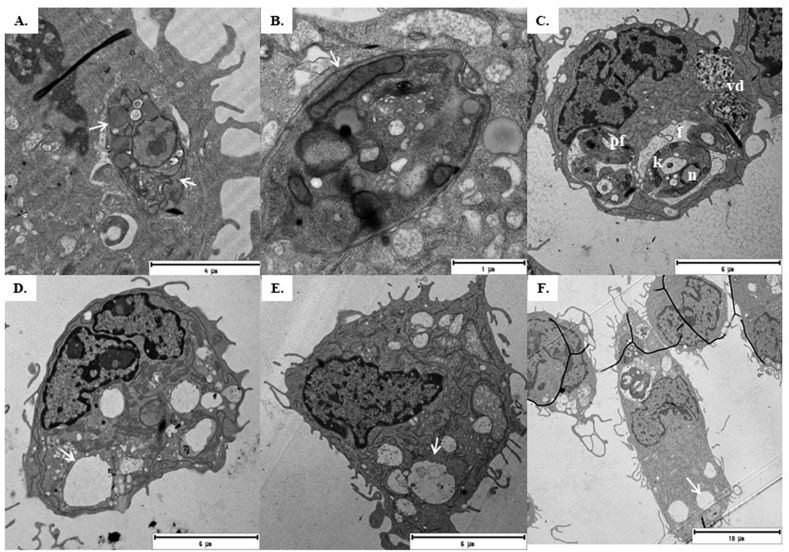
Fig. 4.
Transmission electron microscopy of J774 macrophages infected with L. pifanoi. A and B. TC1 and TC2 treatments caused loss of phagolysosome bulkiness, abundant electrodense vacuoles. C Control without treatment. D. SS treatments with empty vacuoles, E. TC1:SS (1:1), F. TC2:SS (1:1). The images are representative of the findings for each treatment group. f: Phagolysosome N: Macrophage nucleus n: L. pifanoi nucleus k: Kinetoplast pf: Flagellar pocket Vd: Parasitic degradation vacuums. The white arrows indicate the thinning of the phagolysosome (A) and the empty vacuoles (D, E and F).
No statistically significant association was found between the control of infected cells and the control of depolarization of nigericin acid compartments (Fig. 5A and B) with the treatments (5C-G) regarding the change in pH or the number of lysosomes, thus suggesting the absence of changes in the acidity of this compartment. However, compared to the controls of infected cells (Fig. 5A), a shift towards a single cell pole was observed mainly in cells treated with the combinations of TC1:SS and TC2:SS (Fig. 5F and G). An increase in the Arbitrary Fluorescence Units (AFU) were observed in cells treated with TC2:SS combination, which could suggest a better functionality of this compartment to eliminate the parasite.
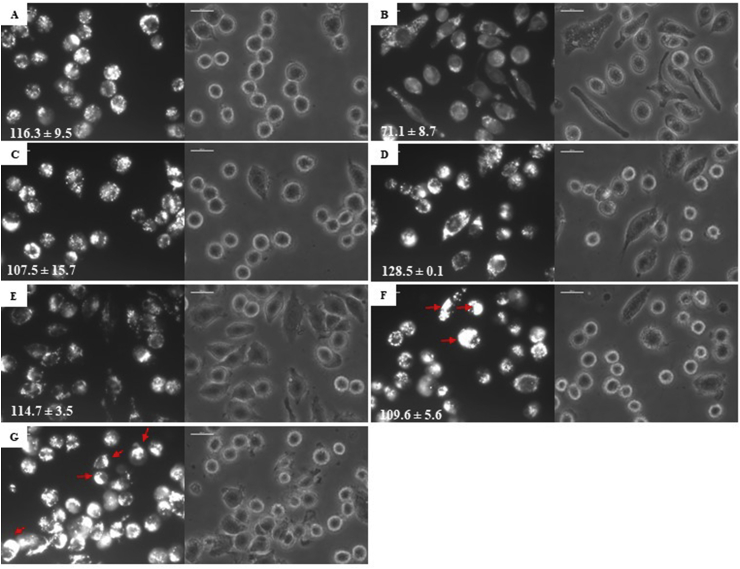
Fig. 5.
Characterization of acid compartments on parasitophorous vacuoles. The figure shows images of J774 macrophages with amastigotes of L. pifanoi labeled with LysoTracker. A. Control, B Positive control cells were incubated with Nigericin 10 μM. C: TC1, D: TC2, E: SS, F: TC1:SS, G: TC2:SS. Red arrows indicated the polar localization of the acid compartment in the cells. The numbers in the image correspond to the AUF (arbitrary units of fluorescence) by analysis in ImageJ. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The TEM analysis of axenic amastigotes showed three important facts: i) compounds, mainly SS, modified the morphology of amastigotes, even increasing their size (Fig. 6A and B), ii) there was an increase in the number of electro dense vacuoles in the treated parasites (Fig. 6B, D); and iii), the effect of SS in the mitochondria was confirmed structurally, evidenced as swelling of its membrane (Fig. 6C figs1).
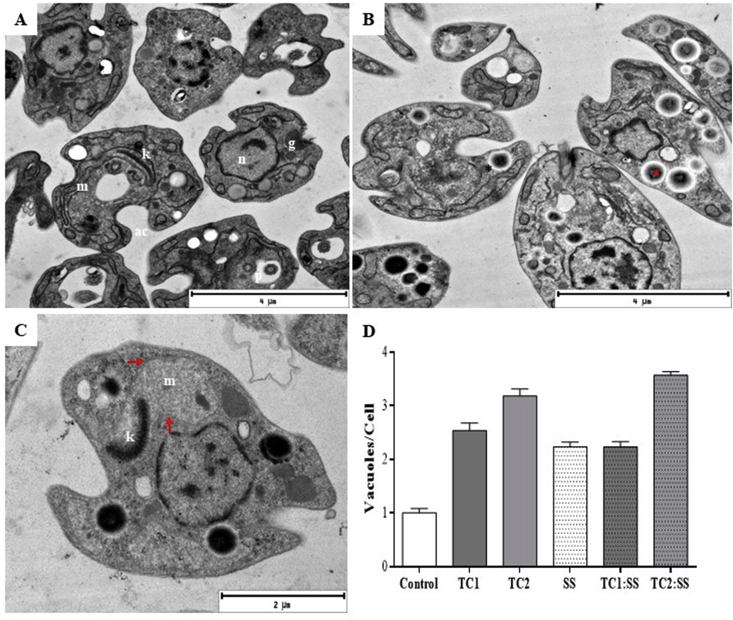
Fig. 6.
Transmission electron microscopy of axenic amastigotes of L. pifanoi after 24 h post-treatment. A. amastigotes without treatment, B: treated with TC1. C: Treated with SS. D: Bar graph of the ratio vacuoles per cell. The asterisk shows electro dense structures compatible with lipid bodies; the arrows show the swelling of the mitochondria.
3.5. Effect of chromane TC1 and TC2 and saponin SS in energy metabolism
The compounds combination produced a rapid decrease in ATP levels close to 30%. SS produced a strong depletion of ATP levels, even at levels comparable to naphthoquinone control (Fig. 7A). The integrity of the plasma membrane was evaluated, without finding significant effects on it in the different Leishmania species evaluated to determine the possible origin of this decrease (Fig. 7B). When the functionality of the mitochondria was studied after compounds addition, a decrease in intracellular accumulation of rhodamine 123 was observed due to changes in the mitochondrial electrochemical potential. The SS mixture caused a significant loss of mitochondrial membrane potential after 1 h of incubation, while TC1 and TC2 only caused this effect when used in combination with SS and after 4 h of incubation (Fig. 7C). This effect of loss of mitochondrial membrane potential was also observed in intracellular amastigotes of L. braziliensis when evaluated by staining with TMRM (Fig. 7D).
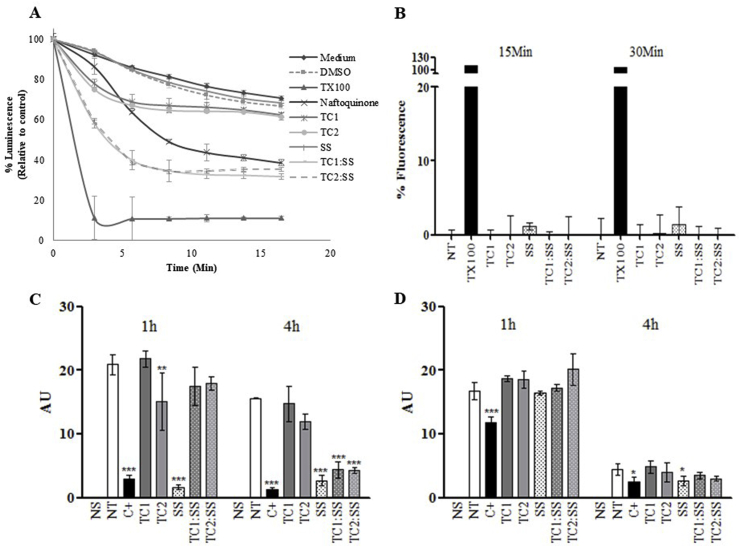
Fig. 7.
Energy metabolism in Leishmania after exposure to chromane hydrazones and a saponin mixture. Determination of the variation of ATP levels in promastigotes of L. braziliensis strain LUC. Under the conditions of the assay, luminescence levels are directly proportional to the intracellular concentration of ATP in the cytoplasm (A). Plasma membrane permeabilization test in amastigotes (L. pifanoi) and promastigotes (L. braziliensis): arbitrary units of fluorescence dependent the interaction between the SYTOX green probe and nucleic acids resulting from pore formation in the membrane, the compounds were applied at 50 μg/mL (B). Depolarization of the mitochondrial membrane in amastigotes of L. pifanoi (C) and amastigotes from L. braziliensis (D), the loss of the accumulation of the dye in the mitochondrial indicates changes in the membrane potential. AU: Arbitrary units; 1 h and 4 h: time of incubation of parasites in each treatment; NS: Non stained (negative control); NT: Non treated (negative control); C+: Positive control KCN (L pifanoi) NaCN (L. braziliensis).
3.6. Effect of chromane TC1 and TC2 and saponin SS in cell stress
Cellular stress was determined according to the production of ROS and the formation of lipid bodies. ROS production was measured as the appearance of fluorescence resulting from oxidation of the H2DCF-DA probe. All treatments induced oxidative stress, being more evident in axenic amastigotes of L. pifanoi, wherewith only 1 h of incubation, ROS levels increased up to five times compared to the control without treatment. On the other hand, L. braziliensis amastigotes showed a later and discreet response, generating at 1 h of incubation almost double the amount of ROS concerning basal production, which increased a little more at 4-hour post-exposure. For L. braziliensis, the evaluation was performed by flow cytometry, observing that at 1 h of incubation only 5% of the population increased the production of ROS, while the rotenone used as a control was 87%. At 4-hour post-treatment, the percentage of positive events was 95.5% for rotenone, 22.1% for TC1, 31.4% for TC2, 20.8% for SS, 23.1% for TC1:SS, and 25.2% for TC2:SS, respectively. In all treatments, there was an increase in UAF for lipid bodies detected by staining of Nile red, after 24 h of exposure (Table 2).
Table 2.
Induction of cellular stress by chromane derivatives (TC1and TC2) and triterpene saponin (SS).
| L. pifanoi |
L. braziliensis |
|||||
|---|---|---|---|---|---|---|
|
aROS |
Lipid bodiesb |
ROS |
Lipid bodies |
|||
| 1 h | 4 h | 24 h | 1 h | 4 h | 24 h | |
| +C | 1.8 ± 0.4c | 0.8 ± 0.1c | NA | 1.9 ± 0.2d | 5.5 ± 0.5d | NA |
| TC1 | 5.3 ± 0.9 | 2.0 ± 0.2 | 0.7 ± 0.02 | 1.2 ± 0.2 | 2.3 ± 0.1 | 1.3 ± 0.01 |
| TC2 | 6.9 ± 0.6 | 2.1 ± 0.1 | 0.9 ± 0.03 | 1.1 ± 0.4 | 2.5 ± 0.5 | 1.6 ± 0.05 |
| SS | 4.8 ± 0.5 | 1.7 ± 0.1 | 0.8 ± 0.04 | 2.7 ± 0.6 | 1.5 ± 0.3 | 0.4 ± 0.07 |
| TC1:SS | 6.0 ± 1.5 | 2.1 ± 0.1 | 1.2 ± 0.02 | 0.8 ± 0.2 | 1.5 ± 0.3 | 1.0 ± 0.01 |
| TC2:SS | 4.8 ± 0.1 | 2.2 ± 0.3 | 1.2 ± 0.04 | 0.6 ± 0.1 | 1.7 ± 0.4 | 1.0 ± 0.07 |
The table shows the production index (baseline control without treatment and treatment ratio).
ROS: Reactive oxygen species determined with the H2DCF-DA probe.
Lipid bodies determined by Red Nile stain.
Positive control Antimycin A (0.3 μg/mL).
Rotenone (50 μg/mL).
3.7. Effect of chromane TC1 and TC2 and saponin SS in proteases
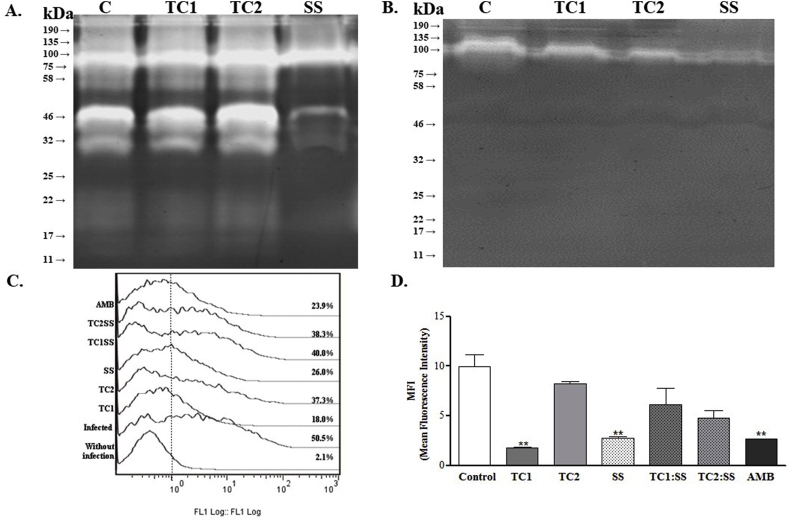
A complete inhibition of the proteases activity in promastigotes of L. braziliensis was observed after 2 h of exposure to SS. The inhibitory effect was evidenced by the disappearance of a band with a relative weight between 32 and 46 kDa (Fig. 8A). In contrast, hydrazones TC1 and TC2 only reduced the protease activity. Since a similar activity was observed for Leupeptin and E−64 protease inhibitors (Fig. 8B), the results suggest that the inhibitory effect caused by SS and hydrazones TC1 and TC2 occurs on cysteine proteases. This protease inhibition was related to a decrease in the parasite's ability to infect cells, as described below. Thus, the exposure of infected cells to compounds TC1 and SS for 24 h, and the subsequent release of the amastigotes to reinfection of cells, a significant decrease in the infective capacity of these pre-treated amastigotes, was observed. The percentage of infected cells in the absence of treatment was 47.5 ± 4.9 while in the treated cells was 15.6 ± 3.4 for TC1, 36.7 ± 0.8 for TC2, 23.1 ± 0.1 for SS, 36.6 ± 4.8 for TC1-SS and 34.1 ± 5.9 for TC2-SS (Fig. 8C).
Fig. 8.
Zymographic profiles on the protease activities. Enzymatic activities detectable in promastigote extracts after 24 h of reaction, Comassie stain. A. Protease activity of L. braziliensis treated. B. Protease activity of L. braziliensis treated and then exposed to 25 μM of Cysteine protease inhibitor Leupeptin. C. Histogram of cells infected with amastigotes released from cells exposed for 24 h to each of the treatments alone or in combination. D. Parasitic load measured as MFI average fluorescence index of cells infected with amastigotes previously exposed to treatments.
On the other hand, the percentage of cells infected with parasites pretreated with AMB was 23.4 ± 0.8, and the percentage of decrease in reinfection was 68% for TC1, 22.7% for TC2, 22.9% for TC1:SS, 28.2% for TC2:SS and 50.7% for parasites exposed to AMB. The most potent compound was TC1, while combinations or TC2 only achieved a response close to 30% of this value.
Besides, the decrease in the number of infected cells was directly proportional to the MFI. Cells infected with the pre-treated amastigotes pretreated with TC1 and SS showed 4 and 5 times less amount of parasites, respectively (Fig. 8D). The MFI values obtained for each treatment were: 9.9 ± 1.2 (untreated cells); 1.8 ± 0.1 (cells infected and treated with TC1); 8.3 ± 0.2 (cells infected and treated with TC2); 2.7 ± 0.2 (infected and treated cells with SS); 6.1 ± 1.7 (cells infected and treated with TC1-SS); 4.8 ± 0.7 (cells infected and treated with TC2-SS), and 2.6 ± 0.01 (cells infected and treated with amphotericin B, AMB).
In summary, the overall inhibitory potency of chromane or combination is TC1> SS > TC2: SS > TC1: SS > TC2. Even TC1 was more active than AMB, and SS was similar to this drug.
The evaluation of the inhibition of the enzymatic activity of cysteine proteases, in total lysates of promastigotes of L. braziliensis after 2 h of exposure to treatments, shows that there is a very subtle effect of all treatments on cysteine proteases (Table 3). The best effect was observed with TC1. Compound CA074 exerts a potent inhibitory action on the cysteine protease cathepsin B, thus indicating non-specific activity against this enzyme on the compounds assayed. These findings did not allow to correlate the inhibition observed in the zymography of L. braziliensis, with cathepsin B [ inhibition band of around 34 kDa], suggesting inhibition in other proteases or isoforms.
Table 3.
Inhibition of the enzymatic activity of Catepsin B from L. braziliensis.
| Compound | Time (minutes) |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | 20 | 25 | 30 | |
| TC1 | 36.7 ± 2.6 | 35.0 ± 2.4 | 34.9 ± 3.2 | 33.8 ± 2.5 | 33.4 ± 2.5 | 32.5 ± 2.6 | 32.0 ± 2.6 |
| TC2 | 19.1 ± 7.0 | 18.6 ± 9.4 | 17.5 ± 1.1 | 15.3 ± 9.9 | 15.0 ± 1.2 | 14.2 ± 1.2 | 12.9 ± 1.3 |
| SS | 19.4 ± 8.3 | 17.6 ± 9.8 | 16.0 ± 1.0 | 14.1 ± 1.1 | 12.7 ± 1.2 | 11.4 ± 1.3 | 9.7 ± 1.4 |
| DMSO | 17.7 ± 5.7 | 16.2 ± 6.6 | 14.4 ± 6.7 | 12.2 ± 7.8 | 11.0 ± 7.9 | 9.4 ± 1.8 | 8.2 ± 0.4 |
| CA074 | 84.6.2 ± 9.9 | 83.4 ± 7.9 | 83.2 ± 1.4 | 82.5 ± 1.3 | 82.0 ± 1.3 | 81.5 ± 1.5 | 81.1 ± 1.7 |
Data show the % inhibition of the enzymatic activity of cathepsin B after 2 h of exposure to 50 μg/mL of each compound.
4. Discussion
The WHO has classified 19 diseases as Neglected Tropical Diseases (NTD), which are associated with poverty in tropical and subtropical countries, and for which there are difficulties in accessing good and effective treatments. These diseases causing a global burden of 47.9 million people and 152,000 annual deaths (Malecela, 2019). In Colombia, the main NTDs caused by protozoa are leishmaniasis and Chagas disease.
A few therapeutic alternatives are available for the treatment of leishmaniasis. Although from the academic field, intense activity is developed in the search for new pharmacological alternatives, so far no alternatives have been generated to be developed clinically. In the last fifteen years, the authors of the present work have dedicated themselves to chemically optimized several molecules, starting from natural substances. After that, high curative activity of cutaneous leishmaniasis has been found both in vitro studies and the model of experimental LC in hamsters by application of several hydrazones and mixtures of triterpene saponins. Given the therapeutic potential, it is essential to know the biochemical effects that these substances exert in the parasite and eventually in the host cell, to understand the mode of action of these molecules and, therefore, its role as drug candidates.
The development of therapeutic alternatives for NTD, especially against cutaneous leishmaniasis, CL has been intensified in the last 15 years, and yet there is no new topical or oral treatment commercially available. A strategy to increase the effectiveness of medications is the use of combined therapies acting on different targets simultaneously (Sundar and Chakravarty, 2013). With this, in addition to increasing the effectiveness, reductions in the dose, the length of treatment, side effects, and especially the induction of resistance, also will be achieved, could with less health care costs.
Combined therapies in leishmaniasis have been studied both using endorsed medications or combinations of these with new compounds, finding few promising results (Momeni et al., 2002; Machado et al., 2018; Dastgheib et al., 2012; Firooz et al., 2006). The use of oral pentoxifylline plus glucantime IM for 20 days (Sadeghian and Nilforoushzadeh, 2006), and oral ketoconazole plus intralesional glucantime (El-Sayed and Anwar, 2010) are some examples of combined therapies that have shown better therapeutic efficacy are Even so, to date, no combination therapy is recommended in the pharmacological care guidelines.
This work was aimed to evaluate the therapeutic potential and mode of action of TC1 and TC2 chromane hydrazones, recently identified with antileishmanial potential for the topical treatment of CL (Upegui et al., 2019) and triterpene saponins previously reported also with antileishmanial activity (Correa et al., 2014). Different therapeutic targets were evaluated for the mode of action: energy metabolism, cell stress, protease activity, and the reinfection process.
The compounds proved to be selective on Leishmania spp. since all of them showed moderate cytotoxicity on macrophage lines and low cytotoxicity in primary cultures, the effectiveness was exclusive on the amastigote stage, with similar effects on the different Leishmania species evaluated. In the animal model, no toxic effect was evidenced after 90 days’ post-treatment, and the healing of hamster back injuries were 100% when using the 4% formulation in a 1: 1 ratio for combinations of TC1 or TC2 and SS. It was found that as the concentration of saponin decreases, the effectivity is reduced too, thereby showing that the SS has activity not only as a surfactant increasing the solubility of hydrazones but also has a direct effect on the viability of the parasite. The measurement of parasite load in healed lesions is not considered because this model follows the parameters established in clinical trials in humans where the outcome of the cure is purely clinical. This is because it has been shown that up to now, no medication, not even approved for the treatment of leishmaniasis gives sterility and parasites can persist without it being associated with relapses. In this model the follow-up is carried out for three months after the end of treatment, a time that has proved to be sufficient time for identify recurrences if they occur. Proof of this is that in the present study, one of the hamsters of the group treated with MA who was cured at the end of the treatment showed reactivation in the evaluation on the 90th day after treatment.
All compounds and their combinations affect processes associated with the functioning of mitochondria, an organelle that, in Leishmania, is primarily responsible for energy metabolism, since it provides 70% of ATP production. Also, it is involved in calcium homeostasis, redox balance, stress response and induction of apoptosis (Menna-Barreto and de Castro, 2014). Therefore, it has been defined as both essential and accessory targets in different trypanosomatids (Sundar and Singh, 2018). Several drugs like pentamidine affect the biochemistry of the mitochondria; this compound is used for the treatment of African trypanosomiasis and leishmaniasis resistant to pentavalent antimonials. A collapse in the mitochondrial membrane potential is induced, leading to an imbalance in the intracellular calcium content (Vercesi et al., 2000). In Leishmania, the intracellular calcium content is maintained due to the existing reserves between the endoplasmic reticulum, mitochondria, and acid-calcisomes (Docampo and Moreno, 2001). Pentamidine also inhibits mitochondrial complex II by inducing apoptosis (Mehta and Shaha, 2004). Likewise, miltefosine, a second-line medication for the treatment of leishmaniasis, affects the mitochondria by inhibiting the IV complex (cytochrome C oxidase), the last enzyme involved in electron transport (Luque-Ortega and Rivas, 2007). Its destabilization leads to the death of the parasite, although it also affects lipid metabolism since in trypanosomatids the synthesis of specialized fatty acids occurs in mitochondria (De Souza et al., 2009).
Of the evaluated compounds, only the SS produced functional effects in ΔΨm as well as structural effects, evidenced as swelling of the organelle. It was ruled out that this effect was the result of alterations at the level of the integrity of the plasma membrane, using the sytox green technique. The accumulation of ROS in promastigotes of L. amazonensis and mitochondrial dysfunction have been observed with some hydrazones obtained from other nucleous than chromans (Coimbra et al., 2018), but also in other chromans that directly affects the mitochondrial complex III (Monzote et al., 2011).
The present work also demonstrated the occurrence of oxidative stress induced by the assayed molecules as a consequence of the blockade of the electron transport chain, which redirects the electrons of the respiratory chain towards their incorporation into oxygen and production of reactive species. Said oxidative stress can act as a signaling stimulus, which in high concentrations supposes the induction of apoptosis (Menna-Barreto and de Castro, 2014). In the results obtained, it was observed that the axenic amastigotes of L. pifanoi and amastigotes released from L. braziliensis after 4 h of exposure to the compounds alone or in combination, maintain high levels of ROS, suggesting the parasite's inability to balance again the redox balance, which could lead to death. This mechanism has been documented in pentavalent antimonials and is complemented by the loss of mitochondrial membrane potential and increased cytosolic calcium (Sudhandiran and Shaha, 2003).
Also, it is important to highlight in this work the use of amastigotes in most the assays, since this stage in the vertebrate host is the target of the drug therapy. Usually, amastigotes are not used due to the difficulties for the axenic growth of the majority of the species and the complexity of working with infected macrophages. The amastigote of the Leishmania develops in the phagolysosome, a limited nutrient environment, in a metabolically quiescent state, and possess a higher resistance to different stress conditions, such as temperature and oxidative stress. In this compartment, there is a high degradation of proteins, free amino acids functioning as the main resource for survival (McConville and Naderer, 2011). On the other hand, the parasite is under the pressure of the oxidative defenses of the macrophage, which requires an adaptation to oxidative stress (Saunders et al., 2014). In this sense, we relate the results of oxidative stress in both axenic amastigotes and those released from the macrophage with the reduction of phagolysosome, which we believe would contribute to a greater concentration of intravacuolar ROS, with the subsequent reduction of parasite survival.
However, phagolysosome involvement was only structural; no alteration was found in the number of lysosomes that compose it or the pH, which means that there was no functional impairment. Besides, the results obtained with staining with Lysotracker network suggest a particular location of lysosomes towards one end of the infected macrophage treated with the combined therapy, an arrangement that may be promoting the elimination of the parasite. An increase in the lipid bodies also was observed in all treatments, suggesting the chemical pressure on the parasites at the intracellular level and in the axenic cultures. These types of structures have been described as a response to cellular stress in trypanosomatids after the use of different types of medicines (Rodrigues and de Souza, 2008).
A previous study demonstrated activity on cysteine protease for hydrazone-type compounds (Desai et al., 2004), suggesting that it reacts with the thiol group of cysteine and causes the inhibitory action. We found that both compounds TC1 and TC2 inhibited protease activity in promastigotes of L. braziliensis. Surprisingly, a strong protease inhibitory activity was also observed with the mixture of triterpene saponin SS, in L. braziliensis. Although the antileishmanial activity of saponins has been documented, protease inhibition in Leishmania has been little explored. Most studies use saponins as adjuvants and not as an active ingredient, due to their surfactant properties, facilitating absorption processes. However, some saponins have been reported as metalloproteinase inhibitors in the human keratinocyte model (Oh et al., 2015). Some experimental background on the mode of action of saponins suggests an interaction with CPY51 and inhibition of ergosterol synthesis (Melo et al., 2016). It is also recognized that saponins have destabilizing effects on cell membranes, even causing red cell lysis (Santos et al., 1997). Despite this, in the present work there were no alterations in the membrane permeability on the amastigote stage or promastigotes of different species after exposure to the triterpene saponin mixture isolated from S. saponaria.
Proteases in Leishmania are of crucial importance since they are related to vital processes of the parasite such as invasion, survival within the macrophage and modulation of the immune response (Silva-Almeida et al., 2014). In this work, it was found that amastigotes released from infected macrophages exposed for 24 h to the compounds, lose the ability to infect macrophages in vitro and decrease their survival inside the macrophage, after 96 h of infection. This effect is consistent with the survival functions within the macrophage and the modulation of the immune response, which has been documented in general for peptidases in Leishmania (Machado et al., 2019). We also demonstrated the decrease in infectivity and post-treatment survival, that there was a subtle effect of TC1 chromane derived hydrazones on the cysteine proteases of L. braziliensis. These enzymes that are expressed constitutively during the parasite's life cycle is crucial for survival within the macrophage (Somanna et al., 2002; Gerbaba and Gedamu, 2013; Siqueira-Neto et al., 2018) Cysteine proteases in protozoan parasites. PLoS Negl Trop Dis 12(8): e0006512.). This enzyme has been postulated as a therapeutic target and a vaccine candidate against Leishmania spp. More than three decades ago, findings in L. mexicana showed that parasites without this enzyme are unable to generate lesions in mice, which translates into a protective effect against infection (Alexander et al., 1998). Moreover, this effect has been applied to other trypanosomatids such as T. brucei, since the use of RNAi against this enzyme prevents mortality in infected animals, without splenomegaly, parasitological blood healing and increased survival (Abdulla et al., 2008).
In synthesis, both the chromane hydrazones and the saponin SS affect the mitochondrial functioning, the SS being a potent destabilizer of the membrane potential of this organelle, and caused a decrease in ATP levels. For both individual treatments and combinations no changes in the permeability of the cytoplasmic membrane were observed. Additionally, there is decompensation of the redox balance that can lead to the death of the parasite, an effect that is not immediate but gradual, consolidating a parasitostatic effect. At the ultrastructure level, chromane-derived hydrazones modify the appearance of phagolysosome, although both the pH and the number of lysosomes were biochemically not altered. Also, the compounds affect the infective capacity and the replication of amastigotes, by altering the functionality of proteases, enzymes that have been described as crucial for intracellular survival.
All these results suggest that the 4% ointment formulation containing TC1 or TC2 combined with SS in a 1:1 ratio, chromane hydrazone: saponin, has the potential to become an alternative for the topical treatment of CL. These results serve as the basis for future clinical trials that validate its safety and efficacy. A preliminary observational study in canines with LC is shown that the formulation of TC2: SS allows the healing of lesions in different anatomical locations and with different evolution times.
Funding
This work was supported by the Departamento de Ciencia y Tecnología - COLCIENCIAS [grant numbers 695-2014] and Redes de investigación cooperativa FEDER [grant RD16/0027/10]. Y.A.U was also supported by COLCIENCIAS [grant 727-2015]. L.G was supported by the Natural Sciences and Engineering Research Council of Canada (grant number 12034). Camila Dos Santos Meira was supported by Alberta Innovates Technology Futures Graduate Scholarship.
Declaration of competing interest
No conflict of interest is declared by the authors.
Acknowledgments
The work with Sapindus saponaria had “Permission of Access to Genetic Resources and its Derived Products” issued by ANLA-Colombia (Framework Contract No. 154 RGE0237).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.06.004.
Contributor Information
Fernando Echeverri, Email: fernando.echeverri@udea.edu.co.
Sara M. Robledo, Email: sara.robledo@udea.edu.co.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
References
- Abdulla M.H., O'Brien T., Mackey Z.B., Sajid M., Grab D.J., McKerrow J.H. RNA interference of Trypanosoma brucei cathepsin B and L affects disease progression in a mouse model. PLoS Neglected Trop. Dis. 2008;2:e298. doi: 10.1371/journal.pntd.0000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Coombs G.H., Mottram J.C. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- Al-Kahraman Y.M., Yasinzai M., Singh G.S. Evaluation of some classical hydrazones of ketones and 1,2-diketones as antileishmanial, antibacterial and antifungal agents. Arch Pharm. Res. (Seoul) 2012;35:1009–1013. doi: 10.1007/s12272-012-0608-7. [DOI] [PubMed] [Google Scholar]
- Carvalho L., Luque-Ortega J.R., López-Martín C., Castanys S., Rivas L., Gamarro F. The 8 aminoquinoline analogue sitamaquine causes oxidative stress in Leishmania donovanipromastigotes by targeting succinate dehydrogenase. Antimicrob. Agents Chemother. 2011;55:4204–4210. doi: 10.1128/AAC.00520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra E.S., Antinarelli L.M., da Silva A.D., Bispo M.L., Kaiser C.R., de Souza M.V. 7-Chloro-4-quinolinyl hydrazones: a promising and potent class of antileishmanial compounds. Chem. Biol. Drug Des. 2013;81:658–665. doi: 10.1111/cbdd.12112. [DOI] [PubMed] [Google Scholar]
- Coimbra E.S., Antinarelli L.M.R., de A Crispi M., Nogueira T.C.M., Pinheiro A.C., de Souza M.V.N. Synthesis, biological activity, and mechanism of action of 2-pyrazyl and pyridylhydrazone derivatives, new classes of antileishmanial agents. ChemMedChem. 2018;13:1387–1394. doi: 10.1002/cmdc.201800328. [DOI] [PubMed] [Google Scholar]
- Correa E., Quiñones W., Robledo S.M., Carrillo L., Archbold R., Torres F., Escobar G., Herrera N., Echeverri F. Leishmanicidal and trypanocidal activity of Sapindus saponaria. Bol. Latinoam. Caribe Plantas Med. Aromat. 2014;13:311–323. [Google Scholar]
- Cuervo P., Sabóia-Vahia L., Costa Silva-Filho F., Fernandes O., Cupolillo E., de Jesus J.B. A zymographic study of metalloprotease activities in extracts and extracellular secretions of Leishmania (Viannia) braziliensis strains. Parasitology. 2006;132:177–185. doi: 10.1017/S0031182005008942. [DOI] [PubMed] [Google Scholar]
- Desai P.V., Patny A., Sabnis Y., Tekwani B., Gut J., Rosenthal P., Srivastava A., Avery M. Identification of novel parasitic cysteine protease inhibitors using virtual screening. The ChemBridge database. J. Med. Chem. 2004;47:6609–6615. doi: 10.1021/jm0493717. [DOI] [PubMed] [Google Scholar]
- Dastgheib L., Naseri M., Mirashe Z. Both combined oral azithromycin plus allopurinol and intramuscular Glucantime yield low efficacy in the treatment of Old World cutaneous leishmaniasis: a randomized controlled clinical trial. Int. J. Dermatol. 2012;51:1508–1511. doi: 10.1111/j.1365-4632.2012.05610.x. [DOI] [PubMed] [Google Scholar]
- De Souza W., Attias M., Rodrigues J.C. Particularities of mitochondrial structure in parasitic protists (Apicomplexa and Kinetoplastida) Int. J. Biochem. Cell Biol. 2009;41:2069–2080. doi: 10.1016/j.biocel.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Docampo R., Moreno S.N. The acidocalcisome. Mol. Biochem. Parasitol. 2001;114:151–159. doi: 10.1016/s0166-6851(01)00246-8. [DOI] [PubMed] [Google Scholar]
- Echeverri F., Quinones W., Torres F., Archbold R., Escobar G., Robledo S.M., Velez I.D., Munoz D., Restrepo A., Daza A., Pulido S., Correa E. vol. 27. 2015. (Saponins and Chromans Derivatives Mixture Compositions against Leishmaniasis, Trypanosomiasis Americana, Malaria, Trypanosomiasis Africana and Fasciola Hepatica). USPTO 9168268B1. [Google Scholar]
- El-Sayed M., Anwar A.E. Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: a comparative study. J. Eur. Acad. Dermatol. Venereol. 2010;24:335–340. doi: 10.1111/j.1468-3083.2009.03417.x. [DOI] [PubMed] [Google Scholar]
- Firooz A., Khamesipour A., Ghoorchi M.H., Nassiri-Kashani M., Eskandari S.E., Khatami A., Hooshmand B., Gorouhi F., Rashighi-Firoozabadi M., Dowlati Y. Imiquimod in combination with meglumine antimoniate for cutaneous leishmaniasis: a randomized assessor-blind controlled trial. Arch. Dermatol. 2006;142:1575–1579. doi: 10.1001/archderm.142.12.1575. [DOI] [PubMed] [Google Scholar]
- Gerbaba T.K., Gedamu L. Cathepsin B gene disruption induced Leishmania donovani proteome remodeling implies cathepsin B role in secretome regulation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho J.L., Georgikopoulou K., Calogeropoulou T., de Souza W., Rodrigues J.C. A novel alkyl phosphocholine-dinitroaniline hybrid molecule exhibits biological activity in vitro against Leishmania amazonensis. Exp. Parasitol. 2013;135:153–165. doi: 10.1016/j.exppara.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Hajighasemi F., Tajic S. Assessment of cytotoxicity of dimethyl sulfoxide in human hematopoietic tumor cell lines. Iran. J. Blood Canc. 2017;9:48–53. [Google Scholar]
- Inocencio da Luz R.A., Vermeersch M., Deschacht M., Hendrickx S., Van Assche T., Cos P., Maes L. In vitro and in vivo prophylactic and curative activity of the triterpene saponin PX-6518 against cutaneousLeishmania species. J. Antimicrob. Chemother. 2011;66:350–353. doi: 10.1093/jac/dkq444. [DOI] [PubMed] [Google Scholar]
- Luque-Ortega J.R., Rivero-Lezcano O.M., Croft S.L., Rivas L. In vivo monitoring of intracellular ATP levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bioenergetic metabolism. Antimicrob. Agents Chemother. 2001;45:1121–1125. doi: 10.1128/AAC.45.4.1121-1125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Ortega J.R., Rivas L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007;51:1327–1332. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Ortega J.R., Rivas L. Characterization of the leishmanicidal activity of antimicrobial peptides. Methods Mol. Biol. 2010;618:393–420. doi: 10.1007/978-1-60761-594-1_25. [DOI] [PubMed] [Google Scholar]
- Machado P.A., Carneiro M.P.D., Sousa-Batista A.J., Lopes F.J.P., Lima A.P.C.A., Chaves S.P., Sodero A.C.R., de Matos Guedes H.L. Leishmanicidal therapy targeted to parasite proteases. Life Sci. 2019;219:163–181. doi: 10.1016/j.lfs.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Machado P.R.L., Ribeiro C.S., França-Costa J., Dourado M.E.F., Trinconi C.T., Yokoyama-Yasunaka J.K.U., Malta-Santos H., Borges V.M., Carvalho E.M., Uliana S.R.B. Tamoxifen and meglumine antimoniate combined therapy in cutaneous leishmaniasis patients: a randomised trial. Trop. Med. Int. Health. 2018;23:936–942. doi: 10.1111/tmi.13119. [DOI] [PubMed] [Google Scholar]
- Malecela M.N. Reflections on the decade of the neglected tropical diseases. Int. Health. 2019;11:338–340T. doi: 10.1093/inthealth/ihz048. [DOI] [PubMed] [Google Scholar]
- McConville M.J., Naderer T. Metabolic pathways required for the intracellular survival of Leishmania. Annu. Rev. Microbiol. 2011;65:543–561. doi: 10.1146/annurev-micro-090110-102913. [DOI] [PubMed] [Google Scholar]
- Melo T.S., Gattass C.R., Soares D.C., Cunha M.R., Ferreira C., Tavares M.T., Saraiva E., Parise-Filho R., Braden H., Delorenzi J.C. Oleanolic acid (OA) as an antileishmanial agent: biological evaluation and in silico mechanistic insights. Parasitol. Int. 2016;65:227–237. doi: 10.1016/j.parint.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Menna-Barreto R.F., de Castro S.L. The double-edged sword in pathogenic trypanosomatids: the pivotal role of mitochondria in oxidative stress and bioenergetics. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/614014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Shaha C. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J. Biol. Chem. 2004;279:11798–11813. doi: 10.1074/jbc.M309341200. [DOI] [PubMed] [Google Scholar]
- Mesa C.V., Muñoz D.L., Echeverry M., Velez I.D., Robledo S.M. Susceptibilidad in vitro a infección por Leishmania y sensibilidad a medicamentos difiere según tipo de macrófagos. Rev. Univ. Ind. Santander. Salud. 2010;42:200–211. [Google Scholar]
- Monzote L., Stamberg W., Patel A., Rosenau T., Maes L., Cos P., Gille L. Synthetic chromanol derivatives and their interaction with Complex III in mitochondria from bovine, yeast, and Leishmania. Chem. Res. Toxicol. 2011;24:1678–1685. doi: 10.1021/tx200233c. [DOI] [PubMed] [Google Scholar]
- Momeni A.Z., Reiszadae M.R., Aminjavaheri M. Treatment of cutaneous leishmaniasis with a combination of allopurinol and low‐dose meglumine antimoniate. Int. J. Dermatol. 2002;41:441–443. doi: 10.1046/j.1365-4362.2002.01527.x. [DOI] [PubMed] [Google Scholar]
- Oh S.J., Lee S., Kho Y.E., Kim K., Jin C.D., Lim C.J. Stereoselective suppressive effects of protopanaxadiol epimers on UV-B-induced reactive oxygen species and matrix metalloproteinase-2 in human dermal keratinocytes. Can. J. Physiol. Pharmacol. 2015;93:91–95. doi: 10.1139/cjpp-2014-0273. [DOI] [PubMed] [Google Scholar]
- Pulido S.A., Muñoz D.L., Restrepo A.M., Mesa C.V., Alzate J.F., Vélez I.D., Robledo S.M. Improvement of the green fluorescent protein reporter system in Leishmania spp. for the in vitro and in vivo screening of antileishmanial drugs. Acta Trop. 2012;122:36–45. doi: 10.1016/j.actatropica.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Robledo S.M., Carrillo L.M., Daza A., Restrepo A.M., Muñoz D.L., Tobón J., Murillo J.D., López A., Ríos C., Mesa C.V., Upegui Y.A., Valencia-Tobón D.L., Mondragón-Shem K., Rodríguez B., Vélez I.D. Cutaneous leishmaniasis in the dorsal skin of hamsters: a useful model for the screening of antileishmanial drugs. J. Vis. Exp. 2012;62:3533. doi: 10.3791/3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J.C.F., de Souza W. Ultrastructural alterations in organelles of parasitic protozoan induced by different classes of metabolic inhibitors. Curr. Pharmaceut. Des. 2008;14:925–938. doi: 10.2174/138161208784041033. [DOI] [PubMed] [Google Scholar]
- Santos W.R., Bernardo R.R., Peçanha L.M., Palatnik M., Parente J.P., Palatnik de Sousa C.B. Haemolytic activities of plant saponins and adjuvants. Effect of Periandra mediterranea saponin on the humoral response to the FML antigen of Leishmania donovani. Vaccine. 1997;15:1024–1029. doi: 10.1016/s0264-410x(96)00292-7. [DOI] [PubMed] [Google Scholar]
- Sadeghian G., Nilforoushzadeh M.A. Effect of combination therapy with systemic glucantime and pentoxifylline in the treatment of cutaneous leishmaniasis. Int. J. Dermatol. 2006;45:819–821. doi: 10.1111/j.1365-4632.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- Saunders E.C., Ng W.W., Kloehn J., Chambers J.M., Ng M., McConville M.J. Induction of a stringent metabolic response in intracellular stages of Leishmania mexicana leads to increased dependence on mitochondrial metabolism. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno D., Lemesre J.L. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob. Agents Chemother. 1997;41:972–976. doi: 10.1128/aac.41.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Almeida M., Souza-Silva F., Pereira B.A., Ribeiro-Guimarães M.L., Alves C.R. Overview of the organization of protease genes in the genome of Leishmania spp. Parasite Vectors. 2014;20(7):387. doi: 10.1186/1756-3305-7-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira-Neto J.L., Debnath A., McCall L.-I., Bernatchez J.A., Ndao M., Reed S.L., Rosenthal P.J. Cysteine proteases in protozoan parasites. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanna A., Mundodi V., Gedamu L. Functional analysis of cathepsin B-like cysteine proteases from Leishmania donovani complex. Evidence for the activation of latent transforming growth factor beta. J. Biol. Chem. 2002;277:25305–25312. doi: 10.1074/jbc.M203034200. [DOI] [PubMed] [Google Scholar]
- Sudhandiran G., Shaha C. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J. Biol. Chem. 2003;278:25120–25132. doi: 10.1074/jbc.M301975200. [DOI] [PubMed] [Google Scholar]
- Sundar S., Chakravarty J. Leishmaniasis: an update of current pharmacotherapy. Expet Opin. Pharmacother. 2013;14:53–63. doi: 10.1517/14656566.2013.755515. [DOI] [PubMed] [Google Scholar]
- Sundar S., Singh B. Emerging therapeutic targets for treatment of leishmaniasis. Expert Opin. Ther. Targets. 2018;22:467–486. doi: 10.1080/14728222.2018.1472241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upegui Y., Gil J.F., Quiñones W., Torres F., Escobar G., Robledo S.M., Echeverri F. Preparation of rotenone derivatives and in vitro analysis of their antimalarial, antileishmanial and selective cytotoxic activities. Molecules. 2014;19:18911–18922. doi: 10.3390/molecules191118911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upegui Y., Rios K., Quiñones W., Echeverri F., Archold R., Murillo J.D., Torres F., Escobar G., Vélez I.D., Robledo S.M. Chroman-4-one hydrazones derivatives: synthesis, characterization, and in vitro and in vivo antileishmanial effects. Med. Chem. Res. 2019;28:2184–2199. [Google Scholar]
- Vargas E., Echeverri F., Upegui Y.A., Robledo S.M., Quiñones W. Hydrazone derivatives enhance antileishmanial activity of thiochroman-4-ones. Molecules. 2018;23:1–12. doi: 10.3390/molecules23010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi A.E., Rodrigues C.O., Catisti R., Docampo R. Presence of a Na(+)/H(+) exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 2000;473:203–206. doi: 10.1016/s0014-5793(00)01531-3. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . 2010. Control of the Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. Geneva, 22-26 March WHO Technical Report Series 949. 2010. [Google Scholar]