Abstract
This paper critically appraises the extrapolation of concentration‐response functions (CRFs) for fine and coarse particulate matter, PM2.5 and PM10, respectively, used in outdoor air pollution health impact assessment (HIA) studies to assess the extent of health impacts in communities exposed to volcanic emissions. Treating volcanic ash as PM, we (1) consider existing models for HIA for general outdoor PM, (2) identify documented health effects from exposure to ash in volcanic eruptions, (3) discuss potential issues of applying CRFs based on the composition and concentration of ash‐related PM, and (4) critically review available case studies of volcanic exposure scenarios utilizing HIA for outdoor air pollution. We identify a number of small‐scale studies focusing on populations exposed to volcanic ash; exposure is rarely quantified, and there is limited evidence concerning the health effects of PM from volcanic eruptions. That limited evidence is, however, consistent with the CRFs typically used for outdoor air pollution HIA. Two health assessments of exposure to volcanic emissions have been published using population‐ and occupational‐based CRFs, though each application entails distinct assumptions and limitations. We conclude that the best available strategy, at present, is to apply outdoor air pollution risk estimates to scenarios involving volcanic ash emissions for the purposes of HIA. However, due to the knowledge gaps on, for example, the health effects from exposure to volcanic ash and differences in ash composition, there is inherent uncertainty in this application. To conclude, we suggest actions to enable better prediction and assessment of health impacts of volcanic emissions.
Keywords: volcano, particulate matter, health impact assessment, health, exposure
Key Points
Ashfall from volcanic eruptions can disrupt life over extensive populated areas
The volcanic ash evidence base is too limited to undertake an ash‐specific HIA
Air pollution risk estimates may be used with caution for volcanic exposures
1. Introduction
Health impact assessment (HIA) is a linked set of approaches and tools for estimating, in advance of their occurrence, the implications for human health of proposed policies, programs, actions, or events. Generally, it includes making recommendations about how favorable health effects can be amplified, adverse consequences reduced, and equity improved (WHO, 1999). HIA also has been used to estimate the burden of mortality and disease attributable to an environmental factor at a point in time; the methodology is particularly well developed in relation to outdoor air pollution (Cohen et al., 2017). In volcanic settings, airborne, respirable emissions (ash particles, aerosols, and gases) could potentially affect human health, but there is limited epidemiological evidence from this context (Gudmundsson, 2011; Hansell & Oppenheimer, 2004; Horwell & Baxter, 2006), and detailed exposure measurements from such events are rarely available. Thus, civil protection and public health agencies may need to rely on extrapolations from other evidence when estimating health risks to communities.
As a significant proportion of outdoor air pollution sources are anthropogenic, ambient concentrations may be lessened by widespread behavior and policy change to reduce emissions, such as the encouragement of public transportation and active travel (e.g., walking, cycling) and the reduction of private car use (Nieuwenhuijsen & Khreis, 2016). These source prevention efforts are clearly not possible for volcanic emissions; therefore, it is important to understand potential health risks and to minimize population exposure through intervention, when necessary, which could be assisted by HIA methods. Yet, there are many substantive issues to consider before undertaking HIA of a volcanic eruption and, if deemed appropriate to do so, when interpreting results. Foremost is the absence of ash‐specific concentration‐response functions (CRFs), due to the lack of detailed exposure assessment and the sparsity of powerful context‐specific epidemiological studies. This dearth of data is compounded by the uniqueness of ash characteristics, which can vary by individual eruptions, and across volcanoes.
Additionally, ashfall from explosive eruptions can disrupt life over extensive populated areas in two main ways that may overlap, both of which provide challenges to the application of general air pollution HIA methods. First, single, large eruptions can result in blanketing of settlements in deep deposits of ash, which can create disastrous air pollution episodes capable of bringing normal living to a halt for days or weeks, due to the resuspension of ash by wind and vehicles. The daily ambient airborne concentrations of particulate matter of <2.5 μm (PM2.5) and PM10 during such an episode are much higher than those encountered normally from traffic and other common outdoor sources, and may greatly exceed regulatory air quality standards for particles, while being time limited, with natural rainfall and weathering processes, as well as official clean‐up measures, removing the deposits from inhabited areas (Moore et al., 2002; Searl et al., 2002). Special measures to protect human health and reduce exposure until the ash deposits have been removed include, as well as ash clearance, advice to stay indoors for vulnerable groups and mask wearing, with special attention being given to adults and children with preexisting respiratory conditions (see www.ivhhn.org). Recommendations to evacuate impacted areas, for at least the most susceptible groups, may be needed.
The second scenario is when smaller eruptions have intermittent, or continuous, emissions of lesser amounts of ash that may affect air quality for months (and even longer) while a volcano remains active, but without bringing transport and other essential functions to a standstill. Ground deposits may accumulate in the absence of, for example, daily cleaning of ash from streets and areas around houses. People will try to maintain normal activities as much as possible under these conditions, but questions may arise about the long‐term health risks from this more prolonged or chronic exposure to the moderately elevated average ambient concentrations of PM10 and PM2.5, based on the now well‐recognized morbidity and mortality effects from epidemiological studies from anthropogenic sources of particulate matter (PM) (World Health Organization (WHO), 2013b).
This paper will address the application of HIA in both scenarios, the latter of which hitherto has not been adequately addressed at any volcanic eruption, to our knowledge. HIAs could provide practical information in advance, to assist local health officials with preparing and mitigating adverse population health effects from volcanic eruptions. For short‐term exposure to high ambient concentrations from a given eruption, completing a relatively basic HIA could help estimate the required health resources under different eruption and exposure scenarios, as well as to inform thresholds of evacuation. In the long‐term (e.g., continuous eruptions and/or persistent ash resuspension), HIAs with more sophisticated modeling may be needed to determine potential health effects over a lifetime of exposure. This was done previously for volcanic silicosis risk, which is the development of lung fibrosis from the inhalation of crystalline silica dust (Hincks et al., 2006), and potentially for reduced life expectancy from PM2.5 exposures (e.g., Apte et al., 2018).
Given these acute and chronic exposure scenarios, we discuss the challenges of assessing associated health impacts using the following approach: (1) consider existing models for HIA for general outdoor PM; (2) identify documented health effects from exposure to ash in volcanic eruptions; (3) discuss potential issues of applying CRFs based on the composition and concentration of ash‐related PM; and (4) critically review available case studies of volcanic exposure scenarios utilizing HIA for outdoor air pollution before suggesting recommendations to generate stronger evidence, specific to volcanic localities, to improve the application and precision of HIA estimates.
2. HIA of Outdoor Air Pollution
The scientific process of an outdoor air pollution HIA is simplified by the fact that key relationships link ambient concentrations directly with human health (leading to CRFs; WHO, 2013a, 2013b) rather than being mediated through such complexities as the distribution of individual exposures or pharmacokinetic modeling of internalized dose, as is the case for some other exposures (e.g., butadiene, toluene, dioxins, and xylene; Sarigiannis et al., 2011). CRFs are based on Relative Risks (RRs) generated from epidemiological studies, which indicate the percentage increased risk per increment of ambient pollution. As an example, aggregated (meta‐analysis) results indicate a CRF of 1.062 for all‐cause mortality in populations subject to long‐term exposure to PM2.5 (i.e., a 6.2% increased risk for each 10 μg/m3 increment of annual average PM2.5 concentrations; Hoek et al., 2013). For a more complete list of CRFs, see WHO (2013a: 5–11).
HIA of outdoor air pollution is, in principle, a simple model with four component parts, as summarized diagrammatically in Figure 1, comprising relevant measures of the following:
Baseline and increase or decrease of the pollutant(s) of concern (e.g., PM2.5);
Population exposed to that pollutant (the “population at risk”);
Background rates of mortality/morbidity in the population at risk (i.e., the rates to which the percentage changes attributable to the pollutant [CRFs] are applied); and
CRF(s) to be used, which are typically expressed as a percentage change per unit of pollutant, linking various aspects of pollution with aspects of mortality and morbidity.
Figure 1.
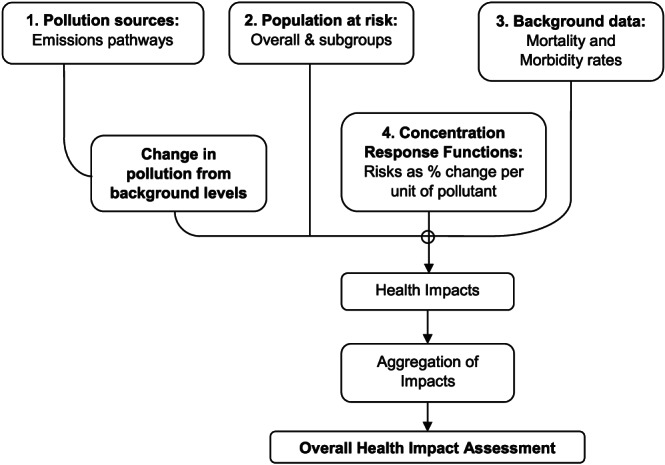
Schematic diagram of HIA of outdoor air pollution (adapted from Hurley & Vohra, 2010, their Figure 2).
While the above components may be readily available for outdoor air pollution HIAs, in other circumstances (e.g., a volcanic context), input data may be nonexistent or sparse and, therefore, may need to be estimated using available sources of information. We provide, in Appendix A, a more general background for HIA in the context of outdoor air pollution. The additional challenge presented by ash containing respirable crystalline silica and the associated risk of silicosis is described in Baxter et al. (2014).
3. Health Effects From Exposure to Volcanic Ash
Investigating the health impacts of a volcanic eruption is fraught with challenges, including outward and variable migration, and anxious populations and officials which, ultimately, can lead to biased information and results (Baxter et al., 2014). Health studies of ash exposure commenced, in earnest, with the Mount St. Helens (USA) eruption in 1980. Since then, available studies have identified mostly reversible, short‐term respiratory outcomes, with less clear‐cut results in longer‐term research, where few such studies have been undertaken, and where the majority of the studies were carried out prior to the development of the more recent research on CRFs for fine PM. A limited number of studies on mortality have been carried out, which have been predominantly in Japan, though these studies have had insufficient power and other methodological weaknesses to be able to provide compelling evidence of increased risks (Horwell & Baxter, 2006). The erratic nature and relatively short timeline (e.g., days to months) of most volcanic eruptions render it difficult to establish a long‐term cohort study, or at least one with chronic exposure, which contrasts to the continuous duration of exposure addressed in outdoor air pollution research (e.g., Pope et al., 2002). Instead, some studies have attempted to follow up individuals exposed at a point in time, as discussed below. A more comprehensive review can be found in Horwell & Baxter (2006).
Within the population at risk, individuals in specific subgroups may be particularly vulnerable to the health effects of volcanic ash inhalation, particularly those with respiratory conditions, such as asthma or bronchitis. The Mount St. Helens (USA) eruption in 1980 led to monthlong elevated concentrations of PM10 ranging from 30–100 μg/m3 for nonoccupational exposures and 50–570 μg/m3 for occupational exposures (Bernstein et al., 1986). A study of individuals with symptomatic asthma and acute bronchitis, and healthy controls, from communities heavily impacted by the eruption, supported the a priori hypothesis that preexisting respiratory diseases are important risk factors for adverse respiratory reactions to volcanic ash (Bernstein et al., 1986). Similarly, after the 2004 eruption of Mount Asama in Japan, a survey of 236 asthmatic adults found 43% experienced exacerbations, compared to 8% in an unexposed control area; those cases with more severe asthma did not experience such changes, as these individuals apparently tended to reduce their exposure by staying indoors with closed windows (Shimizu et al., 2007).
The Mount St. Helens, Soufrière Hills (1995 to ongoing; Montserrat), and Sakurajima (1955 to ongoing; Japan) eruptions are among the few volcanic contexts where numerous epidemiological and clinical studies have been conducted, including a limited number of long‐term follow‐up studies. After 4 years of follow‐up, loggers working in the vicinity of Mount St. Helens were deemed to have normal lung function (Buist et al., 1986); follow‐up was not continued beyond that period. After Soufrière Hills had been erupting intermittently for over 2 years, Forbes et al. (2003) conducted a survey of 440 schoolchildren and identified that the prevalence of wheeze in children aged 12 years and under was three to four times greater in those who had ever been heavily or moderately exposed to volcanic ash, compared to those exposed only to low levels. Once it became apparent that this specific volcano may erupt for years, with high cristobalite (crystalline silica) levels (Baxter et al., 1999; Horwell et al., 2003), a long‐term probabilistic health risk assessment was undertaken for early radiological signs of silicosis, which calculated up to a 4% lifetime risk for some outdoor occupational groups (particularly gardeners) and in children (Hincks et al., 2006). Cross‐sectional studies of towns near Sakurajima have identified no (Uda et al., 1999) or small (Yano et al., 1990) increases in respiratory diseases in chronically exposed areas. An ecological study reported higher levels of mortality over 35 years in a smaller town closer to the volcano relative to that in larger urban centers further away (Higuchi et al., 2012), though it is not possible to infer from the study if the differential mortality is attributable to exposures to the volcanic emissions, environmental, occupational, social, or some other exposure/characteristic differing among the study sites.
Eyjafjallajökull volcano, Iceland, erupted for 6 weeks in spring 2010. Two short‐term health studies were completed in the weeks (Carlsen et al., 2012) and months (Carlsen et al., 2012) following the event. Concentrations of PM10 exceeded the World Health Organization (WHO) 24‐hr average limit of 50 μg/m3 on 25 days during summer and autumn in that year. In the exposed population, half of all adults with asthma (n = 13) and all children with asthma (n = 5) experienced exacerbated symptoms shortly after the eruption (Carlsen et al., 2012). Six to nine months following the event, the exposed population was found to be more than twice as likely to experience symptoms as those unexposed, including tightness in the chest, cough, and phlegm (Carlsen et al., 2012). An additional follow‐up survey was completed in 2013 (Hlodversdottir et al., 2016), which found a continued increase in symptoms of wheezing and phlegm in the most exposed group; such increases over time were not observed in their children (Hlodversdottir et al., 2018).
A summary of selected studies of volcanic ash exposure and health, including and extending those listed in the preceding discussion, is presented in Table 1 (further detail on each study is provided in Appendix B). These studies illustrate (mainly) respiratory risks in the short and medium terms from exposure to volcanic ash; exposure concentrations and any risks of chronic disease development are still poorly understood. This overview indicates, similar to particulate air pollution, that volcanic ash is likely a respiratory hazard, but that there is insufficient evidence to quantify overall health risks, due to a dearth of research. The upshot is that these studies in no way provide sufficient evidence for development of a volcanic‐specific CRF for application in HIA; therefore, concentration‐response relationships from the most relevant analog exposure scenarios would need to be used instead. In the proceeding section, we discuss those of outdoor air pollution.
Table 1.
A Summary of the Health Effects From Exposure to Volcanic Ash
| Outcome | Author, year | Exposure | Population | Direction of findings a | |
|---|---|---|---|---|---|
| Asthma | Symptoms | Baxter et al. (1983) | Residence near Mount St. Helens | Asthma and bronchitis patients | ↑ |
| Sasayama et al. (2002) | Location of school to Mount Unzen Fugen | School children aged 6–11 years old | ↑↑ | ||
| Shimizu et al. (2007) | Residence in areas of Mount Asama ashfall with >100 g/m2, >10 g/m2 | Adults with asthma | ↑↑ | ||
| Uda et al. (1999) | Proximity of school to Mount Sakurajima | School children | ↔ | ||
| Bradshaw et al. (1997) | Exposed to ashfall from Mount Ruapehu | Adults with asthma | ↔ | ||
| Hospital admissions | Kraemar and McCarthy (1985) | Eruption period of Mount St. Helens | Children with asthma | ↑↑ | |
| Gordian et al. (1996) | Eruption period of Mount Spurr | General population of Anchorage, Alaska | ↔ | ||
| Non‐asthma respiratory disease | Symptoms | Baxter et al. (1983) | Residence near Mount St. Helens and received ashfall | Adult residents near Mount St. Helens | ↑ |
| Cowie et al. (2001) | Montserratians exposed to ashfall from Soufrière Hills | Emigrants (all ages) to the United Kingdom | ↑↑ | ||
| Forbes et al. (2003) | Residence in low/medium/high ashfall from Soufrière Hills | Children in Montserrat | ↑↑ | ||
| Carlsen et al. (2012) | Ashfall from Eyjafjallajökull | Residents (all ages) near Eyjafjallajökull | ↑↑ | ||
| Carlsen et al. (2012) | Exceedances of PM10 of 50 μg/m3 from Eyjafjallajökull during summer/fall 2010 | Residents (all ages) near Eyjafjallajökull | ↑↑ | ||
| Hlodversdottir et al. (2016) | PM10, low/medium/high exposure areas from Eyjafjallajökull ashfall | Adults residents (all ages) near Eyjafjallajökull | ↑↑ | ||
| Hlodversdottir et al. (2018) | PM10, low/medium/high exposure areas from Eyjafjallajökull ashfall | Residents (all ages) near Eyjafjallajökull | ↑↑ | ||
| Tobin and Whiteford (2004) | High ash/no evacuation, low ash/evacuation, no ash/no evacuation near Mount Tungurahua | Residents in three Ecuadorian communities | ↔ | ||
| Lung function decline | Cowie et al. (2002) | Residence in low/medium/high ashfall from Soufrière Hills | Workers on Montserrat | ↑↑ | |
| Johnson et al. (1982) | Exposed to ashfall from Mount St. Helens | School children | ↔ | ||
| Research committee on Volcanic emissions (1982) | Distance of towns to Mount Sakurajima | Adult residents near Mount Sakurajima | ↔ | ||
| Buist et al. (1983) | Respirable dust from Mount St. Helens averaged 0.17 mg/m3 | Children aged 8–13 years | ↔ | ||
| Buist et al. (1986) | Ashfall from Mount St. Helens | Loggers | ↔ | ||
| Rojas‐Ramos et al. (2001) | 0.5–1 mm ash from Popocatepetl | Nonsmoking farmers | ↔ | ||
| Hospital/clinic visits | Wakisaka et al. (1989) | <10 km, 10–15 km from Mount Sakurajima | General population near Mount Sakurajima | ↑ | |
| Malilay et al. (1996) | Communities near and within Cerro Negro volcano ashfall area | General population near Cerro Negro | ↑↑ | ||
| Naumova et al. (2007) | Eruption period (Guagua Pichincha) and afterward | Children ≤15 years of age in Quito, Ecuador | ↑↑ | ||
| Longo et al. (2010) | PM2.5, SO2 from Kilauea | General population in Ka'u District, Hawai'i | ↑↑ | ||
| Hickling et al. (1999) | Areas with >0.25 mm ashfall from Mount Ruapehu | General population |
↔ |
||
| Respiratory diseases—prevalence | Yano et al. (1986) | Distance of towns to Mount Sakurajima | Women, aged 30–59 years | ↑↑ | |
| Yano et al. (1990) | Distance of towns to Mount Sakurajima | Women, aged 30–59 years | ↑ | ||
| Mortality, bronchitis | Wakisaka et al. (1988) | 10, 20, 30 km from Mount Sakurajima | General population near Mount Sakurajima | ↑↑ | |
| Mortality, lung cancer | Higuchi et al. (2012) | Distance of towns to Mount Sakurajima | General population in Sakurajima town andTarumizu city | ↑↑ | |
| Other | Mortality, all‐cause | Oudin et al. (2013) | PM2.5 from Grimsvötn ashfall | General population in exposed areas of Sweden | ↔ |
| Preterm birth and birth weight | Balsa et al. (2016) | PM10 exposure from Puyehue volcano during pregnancy | Live births in Montevideo, Uruguay | ↑↑ | |
↑↑ = statistically significant increase; ↑ = suggestive increase; ↔ = no association.
4. Application of Outdoor Air Pollution HIA to Volcanic Ash: Challenges
To undertake an HIA for volcanic eruptions, there are a number of significant challenges that must be addressed relating to the issues identified in Figure 1 (i.e., CRFs, background rates of disease (e.g., cardiorespiratory hospital admissions), and pollutant concentrations).
4.1. CRFs
Here we assess whether it is possible to use urban air pollution‐derived CRFs as a proxy and examine the potential issues that need to be acknowledged before applying these methods. The main issues are that (i) the composition of PM from natural sources, such as volcanoes, may be very different to that of urban air pollution, and so the toxicity also may be distinct; and (ii) the concentrations experienced, at least in the short‐term, may be very high and outside the range of ambient PM levels for which the urban CRFs have been developed.
PM in outdoor air pollution is a mixture varying in composition by time and place. A review of 419 source apportionment datasets of urban ambient PM2.5, published during 1990–2014, generated the following global averages: 25% traffic, 15% industrial activities, 20% domestic fuel burning, 22% unspecified anthropogenic sources, and 18% from natural dust and salt (Karagulian et al., 2015). Similarly, the composition of dusts from volcanic eruptions may vary according to the chemical and physical properties of a given eruption and level of adsorption of volcanic, atmospheric, and anthropogenic elements (Damby et al., 2013; Durant et al., 2010; Horwell et al., 2013; Tomašek et al., 2016, 2018, 2019). While PM composition and toxicity are likely to vary by source, the evidence concerning how components affect toxicity, and to what extent, is not yet established. As such, WHO expert groups, and others (COMEAP, 2015; U.S. EPA, 2010; WHO, 2013a), maintain that it is not appropriate to quantify health impacts attributable to specific PM sources, although there may be differences in the resulting health effects, but the evidence to allow quantification of these is not yet available. In other words, for HIA of outdoor air pollution, it is recommended that health risks from PM2.5 be treated equivalently per unit exposure, regardless of source and composition. Nevertheless, research is ongoing to address this knowledge gap and examine PM speciation and, with the exception of elemental carbon, more evidence is still needed to attribute heightened or reduced risks to particular constituents of air pollution (Adams et al., 2015; Basagaña et al., 2015; WHO, 2013b). With respect to components of volcanic ash, the content of crystalline silica, particle size and surface area, and surface chemical composition and structure may all affect its bioreactivity, all of which depend on its eruptive origins (Hillman et al., 2012; Horwell et al., 2012; Horwell & Baxter, 2006) (see Figure 2); nevertheless, the general recommendation from various expert groups still applies.
Figure 2.
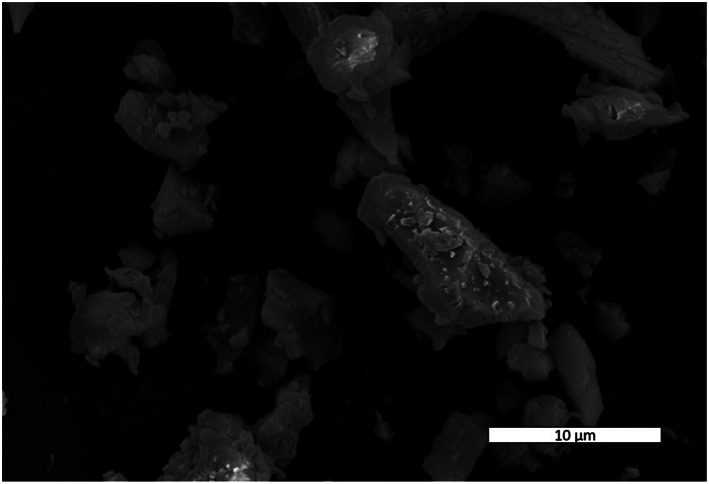
Ash from the eruption of Mount St. Helens, 18 May 1980. The scanning electron microscope image shows how ash particles are blocky and angular with diameters down to the submicrometer level.
Few studies have tried to tease apart health effects from volcanic ash and anthropogenic PM. An early example took place, serendipitously, in Montana, where 120 schoolchildren had their lung function measured before and after the Mount St. Helens eruption. No substantial difference was observed post‐eruption, though a decrease in lung function was apparent from subsequent exposure to relatively lower concentrations of urban air pollution (Johnson et al., 1982). More recently, an in vitro study using a multicellular lung culture found combined exposure to volcanic ash and diesel particles produced a greater, but minimal, pro‐inflammatory response than to that from ash alone (Tomašek et al., 2016), though a follow‐up study with gasoline exhaust found no such effect (Tomašek et al., 2018). Cardiorespiratory hospital admissions in Iceland were elevated on days of high PM10 concentrations (>50 μg/m3) caused by volcanic ash, but were only borderline significant after adjustment for such days (Carlsen et al., 2015).
A further complication related to differing PM composition is that volcanic ash tends to be coarser than urban air pollution (i.e., it contains a lower proportion of PM2.5). An analysis of 63 deposited ash samples found the mean ratio of sub‐2.5 to sub‐10 μm diameter particles to be 0.23 (Horwell, 2007). This contrasts with the higher ratios of PM2.5:PM10 typically identified for airborne urban air pollution (e.g., 0.65 for studies in Europe; WHO, 2013a), though proportions of ground‐deposited and airborne PM fractions may differ. Still, a potential overestimation may result of the health impacts from ash if based on CRFs for urban air PM10, given the smaller proportion of PM2.5 in volcanic ash; this is because PM2.5 entails more particles and surface area per unit mass and, importantly, a greater health risk per μg/m3 than PM10 (Lu et al., 2015).
Regarding ash concentrations, it is likely that, at least temporarily (and in some cases for months or years; Hincks et al., 2006), PM concentrations in the short‐term resulting from volcanic eruptions would be much higher (e.g., 24‐hr mean PM10 concentrations of 1,230 μg/m3 recorded after the 2010 Eyjafjallajökull eruption; Thorsteinsson et al., 2012) than those typical for outdoor air (e.g., <50 μg/m3; Basagaña et al., 2015) in North American and European cities (i.e., the locations where most studies of outdoor air pollution have been conducted and, thus, the source of CRFs for estimating health effects). This matters because, typically, CRFs for health effects of outdoor PM in North America and Europe are effectively linear at low concentrations whether for acute exposures (i.e., daily variations in ambient PM, as used in time series and panel studies) (WHO, 2013b) or chronic exposures (i.e., annual average PM, as used in cohort studies) (WHO, 2013a). Unless there are continual eruptions and/or minimal ash removal efforts resulting in ongoing airborne ash, exposures due to volcanic ash will be elevated for periods typically on the order of <6 months. In estimating resultant health impacts, this duration necessitates aggregating the effects of short‐term exposure (daily variations) and/or adapting the effects of long‐term exposure (annual average concentrations), to an intermediate time period. Aggregating estimated daily effects when ash concentrations are abnormally high for a sustained period, however, might exaggerate potential health effects of repeated short‐term exposures. This is due, in part, to mortality displacement, that is, deaths in those who would have otherwise died in the very near term (Costa et al., 2016), and consequent depletion of the population most at risk.
An alternative for the calculation of impacts using long‐term CRFs might be to apply the average concentration from the eruption episode as an annual average, then adjust downward the calculated estimates to match the actual time period (e.g., halving for 6 month exposures); comparing these two methods might provide a potential range of estimated effects. To further complicate ash exposure assessment, patterns of exposure arising from volcanic eruptions also differ from those on which the outdoor air pollution CRFs are based, with most of an individual's exposure arising from resuspension of volcanic ash deposits by wind and traffic and being highly dependent on the individual's activity within the affected area and measures that they might take to reduce exposure (e.g., wearing a facemask). Nevertheless, despite the discrepancy between the recorded ambient PM concentrations in volcanic and urban air pollution studies, the expert committees do not include an upper exposure limit for the application of CRFs (COMEAP, 2015; U.S. EPA, 2010; WHO, 2013a). Ultimately, the potential higher exposure settings and unique particle characteristics of volcanic ash will introduce additional uncertainty into the HIA process but, based on the best currently available information, these factors do not entirely preclude the use of outdoor air pollution CRFs to quantify health impacts related to volcanic ash exposure.
4.2. Background Rates of Health Outcomes
WHO guidance on conducting HIA for outdoor air pollution recommends the separate calculation of mortality from short‐ and long‐term exposure to PM2.5 (WHO, 2013a). In addition to excess deaths, the WHO (2013a) suggests to include the following short‐term morbidity outcomes: all‐cause mortality, hospital admissions for cardiovascular and respiratory disease, restricted activity days, work days lost, and incidence of asthma symptoms in asthmatic children. While these morbidities might be the most relevant health endpoints, detailed, routine surveillance and baseline health data are unlikely to be readily available or, at least, not at the local level. Also, there are issues in the transferability of corresponding CRFs for a given outcome, depending on healthcare systems and work patterns (e.g., for restricted activity days; Hunt et al., 2016). Examples of studies using available hospital surveillance data occurred after the 1980 Mount St. Helens (USA) (Baxter et al., 1981; Baxter et al., 1983) and 2000 Guagua Pichincha (Ecuador) (Naumova et al., 2007) eruptions. The latter documented a roughly doubling of respiratory admission rates for respiratory causes, evidencing the particular sensitivity of young children to ash exposure.
Many volcanoes are situated in parts of the world with poor healthcare facilities and where medical data are difficult to acquire; in this case, equivalent national data (the WHO publishes age‐standardized national mortality rates; WHO, 2015) may be the best available background data from which to calculate health impacts. If local baseline health data are not available, depending on resource availability, initiating a simple health surveillance system associated with volcanic ash exposure (e.g., hospital admissions and healthcare facility attendance) would be a useful first step for tracking both short and long‐term health trends, not least for improving the precision of future HIA work. The International Volcanic Health Hazard Network (www.ivhhn.org) has recently developed protocols for such studies, to facilitate rapid deployment of surveillance and the conduct of comparable studies (Mueller et al. 2020).
4.3. Pollutant Concentrations
For HIA, it is necessary to assess the incremental increase in pollutant concentrations from a given event, not just the absolute values at some point in time. At present, there are few monitoring stations surrounding active volcanoes. If stations exist, they may provide reliable baseline information, depending on the monitoring equipment used and its upkeep, as well as increases in concentrations during ashfall and subsequent resuspension. If ambient monitors are located in urban areas, background concentrations would reflect urban air pollution, though the incrementally higher readings following an eruption may reflect the contribution of volcanic PM (as seen in Gordian et al., 1996; Carlsen et al., 2015), with the increased concentrations used as inputs to the HIA. If monitors do not exist, it would be advisable to install equipment to measure PM concentrations to quantify the transient increased exposure concentrations, but often this does not happen. Once the ashfall event ends, exposure to ash may not fall correspondingly if it is still present in the environment (Baxter et al. 2014; Horwell & Baxter, 2006). In that case, the resuspension of ash may lead to significant, prolonged exposure to potentially harmful concentrations of PM, which could be up to threefold higher for smaller children than adults (Horwell et al., 2003). This continued exposure would intensify the contribution from individual behaviors and microenvironments, thus leading to the potential for exposure misclassification if using ambient PM concentrations in HIAs for volcanic scenarios. Nevertheless, in the absence of any air quality monitoring, best estimates for airborne concentrations could be based on expert judgment or modeling, based on experiences during other eruptions (e.g., Horwell et al. 2013; Moore et al. 2002; Searl et al. 2002), but use of urban PM concentrations may not be relevant.
To improve predictions from HIA and to take account of uncertainties in the findings, it would be of value to account for scenarios that may impact exposures on a population level. The overall extent and expediency of clean‐up activities, or lack thereof, as well as the large‐scale provision, and/or widespread ownership, of facemasks as well as other exposure reduction interventions such as staying indoors, could significantly increase or decrease concentrations to which the population is exposed; however, this will depend on the specific setting (e.g., homes in tropical, low income countries may be designed to optimize ventilation, thus providing less protection). These scenarios can be addressed by changing exposure assumptions in an HIA, particularly those that may be quantified, such as reduced ash exposure from wearing respiratory protection; reductions would depend on the amount and type of masks distributed to the population (Steinle et al., 2018), as well as the likelihood of uptake of such protection in communities (Covey et al., 2019).
5. Case Study Applications of Analog HIA to Volcanic Eruptions and Other Air Pollution Crises
To our knowledge, there is only one published HIA (Schmidt et al. 2011) and one health risk assessment (Hincks et al. 2006) for acute and chronic exposures, for volcanic emissions. It was not an aim of this paper to present a new case study, and indeed we do not have the relevant exposure, population, and morbidity data to do so. Instead, we discuss the key inputs of each of these assessments, in the light of the literature review and discussion so far, to help illustrate the possible approaches for an HIA of a given eruption scenario.
Schmidt et al. (2011) reported an HIA to estimate the excess mortality in Europe if the 2004 population, with its demographic structure and baseline air pollution levels, experienced a future Icelandic eruption similar to the Laki high impact sulfur gas‐dominated volcanic fissure eruption in 1783–1784. The course of the eruption was simulated using the Global Model of Aerosol Processes (GLOMAP) to estimate the magnitude, altitude, and timing of the release of volcanic SO2, which subsequently formed aerosolized sulfate (H2SO4). Sulfate PM2.5 is a component of general urban PM2.5, but is clearly different in composition, surface properties, and solubility from other components, such as directly emitted diesel PM. Nevertheless, in line with the recommendations of expert groups, Schmidt et al. (2011) based their HIA on published CRFs for PM2.5 derived from urban air pollution epidemiological studies, although in the accompanying informal assessment of uncertainties, they asserted that existing evidence suggested that sulfate aerosols are likely to cause greater health effects (on a mass basis) per μg/m3 than other aerosol components (Pope & Dockery, 2006). It is true that, if sulfate aerosol rather than PM2.5 is used as an indicator of the urban air pollution mixture, then higher coefficients (per μg/m3) will be found for sulfates than for PM2.5, simply because the same mixture effect is being represented by a pollutant (sulfate aerosol) which is part of PM2.5 and so has lower average concentrations. This does not imply that sulfate aerosols cause more health effects, on a mass basis (i.e., per μg/m3) than other aerosol components. Indeed, toxicological evidence suggests that pure sulfate aerosols are, in isolation, relatively inert, but they may potentiate the effects of, or be a marker for, other pollutants (WHO, 2013b: p. 19). From the viewpoint of the present paper, the important point, however, is that CRFs from urban air pollution epidemiology were used to quantify the health effects of a different kind of PM2.5, from a volcanic eruption.
Schmidt et al. (2011) estimated the effects of the volcanic eruption using CRFs for both short‐term exposures (daily average PM2.5) and long‐term exposures (annual average PM2.5). Short‐term CRFs for daily all‐cause mortality were based on the mean effect from several time series studies, equating to a 0.96% increase per 10 μg/m3 of PM2.5. Long‐term CRFs for excess cardiopulmonary mortality were adopted from the American Cancer Society cohort (Pope et al., 2002), based on a 6% increase, assuming a logarithmic exposure function to “flatten” the response curve at higher ambient concentration levels. Using WHO background rates of all‐cause and cardiopulmonary mortality across Europe, and population estimates from the History Database of the Global Environment (Goldewijk, 2001), it was estimated that a similar volcanic event to the Laki eruption would result in between 139,000 (95% CI 51,000 to 224,000) and 144,000 (95% CI 53,000 to 232,000) excess cardiopulmonary deaths from long‐term (1‐year) exposure to volcanic PM2.5 across Europe, depending on the meteorology. In practice, the actual timing of these deaths would not all occur within 1 year because some deaths would be delayed, for example, U.S. EPA (2011) and COMEAP (2010) both use the same lag time, spread unevenly over a 20‐year period. For aggregate shorter term (daily) exposure, between 27,500 (95% CI 22,600 to 32,200) and 30,100 (95% CI 24,800 to 35,300) all‐cause deaths were estimated over the 266 days of predicted elevated exposures following the eruption.
Hincks et al. (2006) reported a comprehensive assessment of lifetime risks related to long‐term exposure to crystalline silica‐rich respirable volcanic ash from the ongoing eruptions of the Soufrière Hills volcano in Montserrat, which commenced in 1995. Exposure to PM10 was modeled using a limited number of exposure measurements taken on the island during 1996–1999 and combined with synthetic time series data for volcanic activity, rainfall, ash deposition, and erosion using Monte Carlo simulation methods. Numerous assumptions were required to project exposures and calculate risks over an extended period of time: height above ground (relevant for children's exposure to resuspended ash; Horwell et al. 2003), meteorological and possible eruption conditions, and the fraction of cristobalite (crystalline silica) in PM10, an important characteristic of ash that is less relevant for urban PM, were but a few important inputs. Where model parameters could not be estimated empirically, expert elicitation methods were used, pooling best estimated (upper and lower) exposure values from a number of experts, which also weighted the level of knowledge of each expert. Similar methods of exposure estimation could be applied to HIA in other volcanic eruption scenarios if relevant exposure data are not available. Estimates of silicosis risk in workers and other population groups were calculated using CRFs based on comparable exposures from studies of occupational populations (diatomaceous earth workers; Hughes et al. 1998). Based on the Hincks et al. (2006) assessment, the median risk of silicosis for an average adult after 20 years' continuous exposure ranged from 0.5% to 1.6% across regions of Montserrat. The occupational group with the highest exposure to ash was gardeners, where risks ranged from 2% to 4%.
While there are many uncertainties in the results of these studies, including those already described in applying CRFs from urban air pollution (or other sources; e.g., occupational studies as in the latter example) in the context of volcanic ash, these studies are not unique. Other published HIAs, too, have applied urban air pollution CRFs to other exposure scenarios. For example, Goudarzi et al. (2017) used short‐term CRFs for PM10 based on a meta‐analysis of urban air pollution time series studies (Anderson et al., 2004) to estimate excess respiratory mortality and hospital admissions from dust storms in Iran. Kollanus et al. (2017) quantified short‐term deaths from PM2.5 originating from forest fires in Europe, basing CRFs on results of an epidemiological study of 112 U.S. cities that found a 0.98% nonaccidental mortality increase per 10 μg/m3 of PM2.5 (similar to that used by Schmidt et al., 2011; Zanobetti & Schwartz, 2009). As with the current discussion for volcanic ash, these two HIA studies applied urban air pollution‐based methods and CRFs to exposures involving mainly non‐anthropogenic PM sources. In summary, the case studies examined above demonstrate both the feasibility and usefulness of applying analog CRFs for the estimation of potential health impacts in the context of volcanic eruptions and other air pollution episodes. Application of such analogs certainly allows simulation of the scale of what clearly has the potential to be a major public health event.
6. Conclusions and Recommendations
The ability to perform HIA either in advance of, or following, an eruption, given an approximation of the potential concentrations and geographical extent of exposure, could be advantageous in providing quantitative information to help manage the situation, especially given the inherent difficulties in carrying out any level of health monitoring, data collection or basic scientific research during a volcanic crisis (due to immediate humanitarian needs requiring priority). However, the volcanic ash evidence base is currently too limited to undertake an HIA with ash‐specific CRFs. A number of short‐term studies, with varied methods, indicate ash to be a probable respiratory hazard, but the generally crude characterization of exposure, varied ash characteristics and toxicological properties, studies of small groups, inconsistent methodologies, and varying definitions of health effects restricts any quantitative comparisons with the CRFs used in outdoor air pollution HIAs. Although follow‐up of those exposed to a significant volcanic event at a point in time would be valuable, calls for study of impacts from long‐term exposure are likely to remain largely unheeded, due to the inconsistent nature of volcanic activity and the challenge of following dynamic populations over years and potentially decades.
As the best available strategy at this time, and adopting a precautionary approach, this review concludes that it is reasonable to apply outdoor air pollution risk estimates to scenarios involving volcanic ash‐PM emissions and, in the absence of robust evidence indicating otherwise, to do so with the same range of associated health endpoints. However, due to the knowledge gaps on the health effects from exposure to volcanic ash and to differences in ash composition and pathways of exposure, there is inherent uncertainty in this application. Meanwhile, local health authorities and researchers should continue to investigate which, if any, and to what extent, indicators of short‐term health (e.g., hospital admission rates) are increased after actual eruption episodes. Studies should obtain better exposure data to refine respiratory and other risks, as well as ascertain information on an expanded set of health outcomes, such as cardiovascular endpoints. We have summarized in Table 2 the key points to consider when completing an HIA of volcanic ash exposure based on air pollution CRFs.
Table 2.
Key Points to Consider Prior to Undertaking HIA of Volcanic Ash Exposure
| HIA component | Key considerations |
|---|---|
| 1. Pollution sources | Is the ash emission duration likely to be acute or chronic? |
| How can PM concentrations from ash be estimated? | |
| Is exposure likely to occur from (i) ashfall, (ii) resuspension of ash, (iii) clean‐up/occupationally, and/or (iv) another pathway? | |
| 2. Population at risk | What size of populated area will likely be exposed? |
| Will any exposure reduction activities be undertaken (e.g., evacuation, use of facemasks, remaining indoors)? | |
| 3. Background health data | Are baseline levels of respiratory (and other) diseases available for the population at risk? |
| 4. Concentration‐response functions | Are the concentrations of ash to which the population is exposed within the range of ambient PM concentrations on which CRFs are based? |
Additionally, we suggest the following actions to better prepare local authorities to predict and assess health impacts of a volcanic event using HIA methods:
Establish background rates of disease in areas surrounding volcanoes. Set up health surveillance systems to track associated health effects in populations exposed to ashfall or identify background rates of important health outcomes (e.g., respiratory diseases) in populations likely to be affected.
Improve exposure assessment. If possible, set up air quality monitors or sensors near volcanoes to record PM10 and PM2.5 or, at a minimum, examine options for monitors to be set up during ashfall events.
Validate HIA estimates. Document observed health impacts following an eruption episode through surveillance and epidemiological studies to compare with HIA outputs.
Finally, to supplement the findings of an HIA, actions could be taken to identify those likely to be at most risk from a volcanic eruption, for example, those more highly exposed to the ash (through measuring individual exposures, activity patterns, and infiltration of ash into housing, offices and schools) and monitoring of those more vulnerable to the effects of the ash (children, individuals with preexisting disease, migrant workers, etc.).
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Acknowledgments
This review represents part of a larger research project to develop evidence‐based advice to allow humanitarian agencies to distribute or recommend respiratory protection in volcanic crises (HIVE—A new evidence base for respiratory Health Interventions in Volcanic Eruption Crises, http://community.dur.ac.uk/hive.consortium). That larger project was led by one of the present authors, Claire Horwell, and was funded by Elrha's Research for Health in Humanitarian Crises (R2HC) program, which aims to improve health outcomes by strengthening the evidence base for public health interventions in humanitarian crises. R2HC is funded by the U.K. Department for International Development (DFID), Wellcome, and the U.K. National Institute for Health Research (NIHR). This project was funded by a grant from the Research for Health in Humanitarian Crises (R2HC) Programme (Grant 14048).
Appendix A. An Introduction to HIA and Outdoor Air Pollution
A.1. What Is HIA?
HIA is the name given to a linked set of approaches and tools for estimating, in advance of their occurrence, the implications for human health of proposed policies or programs. Here, “implications” includes not only both favorable and unfavorable consequences for human health, but also how those consequences are distributed across the population (i.e., the implications for health inequalities/health equity). Generally, it also includes making recommendations about how the favorable health effects can be amplified, adverse consequences reduced, and equity improved (WHO, 1999).
One established and widely used definition of HIA is “A combination of procedures, methods and tools by which a policy, program or project may be judged as to its potential effects on the health of a population, and the distribution of those effects within the population” (WHO, 1999). The term “HIA” is sometimes also used to include estimating the burden of mortality and disease attributable at a point in time to an environmental factor (i.e., to answer the question “How big is the problem?”) rather than simply for estimating the likely health consequences of doing something about it—see for example., COMEAP (2010) for a description of the difference and for its implications for quantification.
A.2. Is HIA Principally a Scientific Project or a Social Process?
HIA is necessarily both a scientific project and a social process; the extent to which one predominates depends in on the resources available to do it. These resources include the “state of the art” of science in the relevant areas linking environmental and social factors with health, the knowledge of the HIA team, the knowledge and willingness to engage of those potentially affected by the policy, program, project, or event (from now on, we will refer to “event”), the availability of relevant “local” data, the difficulties of modeling how the event may affect the environment and other factors, the time and money available to do the work, and the degree of accuracy and precision needed for that particular application.
Given that the purpose of an HIA is to inform planning for how to deal with an event should it occur, and what measures may be effective in supporting health and health equity, it may be that a “light touch” HIA will inform the planning process sufficiently. In these circumstances some well‐prepared workshops with key stakeholders, followed by wider consultation, may be what are needed. Major events, actually or potentially affecting a large number of people to a significant extent, may require more comprehensive modeling and analysis with associated more detailed scientific assessment within a framework where both the purpose of the HIA, and the level of detail needed, are determined largely by stakeholders (e.g., Briggs, 2008, for a model of how these can interact).
Either way requires not only interdisciplinary working of scientists from various backgrounds, but transdisciplinary collaboration of scientists with a wider stakeholder group and public. In our experience an appropriate balance can be achieved if (a) it is recognized from the outset that both aspects (and their interrelationship) are essential, and appropriate arrangements for collaboration put in place from the outset; and (b) if the HIA is carried out iteratively, with if needs be several iterations, from initial “framing the question” and screening to identify the broad set of issues that may need to be addressed, through a “light touch” HIA, or through various levels of more detailed assessment.
In summary then, HIA can be and is:
A scientific challenge, an expert‐driven assessment of health effects, often but not always quantitative, with a view to informing policy development. This is especially so where the evidence base is strong regarding the relevant determinants of health, and the extent to which they affect mortality and morbidity. In this context “success” is a good estimate, and one that is useful and used in the development of policy.
A social process, a way of involving stakeholders in the decision process, of “giving a voice to the voiceless”. From this viewpoint “success” is effective engagement with and influence on the decisions to be made, and in particular with a view to finding better solutions for health and health equity. This is relevant whether or not the underlying evidence is strong or quantified, though emphasis on HIA as a social process tends to be strongest when the scientific evidence base is limited or qualitative.
In practice, both aspects are essential and need to be integrated. For a more comprehensive discussion of all of these issues, see, for example, Hurley and Vohra (2010).
A.3. HIA of Air Pollution—Overview of the Component Parts
The scientific process of an air pollution HIA is simplified by the fact that key relationships link ambient concentrations directly with human health (to give CRFs) rather than being mediated through the distribution of individual exposures, or indeed through pharmacokinetic modeling of internalized exposure, dose and effect, as is the case for example for BTDX (butadiene, toluene, dioxins, and xylene; Sarigiannis et al., 2011).
Consequently, HIA of outdoor air pollution is in principle fundamentally a simple model with four component parts, comprising relevant measures of
Estimate of the increase or decrease of the pollutant(s) of concern (e.g., PM2.5, O3), that is to be evaluated;
Population exposed to that pollutant (the “population at risk”);
Background rates of mortality/morbidity in the population at risk (i.e., the rates to which the % changes attributable to the pollutant are applied); and
CRF(s) to be used, which are typically expressed as a % change per unit of pollutant, linking various aspects of pollution with aspects of mortality and morbidity.
This process has been summarized diagrammatically in Figure 1 in the main article.
Figure 1 Shows also a possible further step, in aggregating health impacts across various kinds of adverse effects. In order to do this, the various impacts need to be converted into a common “currency.” Typically, this is done using monetary valuation of mortality and morbidity, or following conversion to disability adjusted life years (DALYs).
A.4. Interrelationship of the Four Component Parts
While each component part has its own complexities—see later for CRFs—the complexity with which each of these components needs to be addressed depends in part on the other components also. Thus, for example, if a CRF has different values according to age, or gender, or socioeconomic status, or health status of the population, then in order to include this complexity in the analysis it is necessary that demographic data (specifically population size and background rates of morbidity or mortality) be available, or estimated, in corresponding detail. Similarly, the pollution metric underlying the CRF (e.g., for daily variations, 24‐hr average or 8‐hr daily max or 1‐hr daily max pollution) must be the same as, or translatable from, the metric used for pollution modeling. Analyses where CRFs are linear allow for simplifications which are not workable with nonlinear CRFs, including those with thresholds.
There are in practice many examples like this where linkage between the components is important—once again see, for example., Hurley and Vohra (2010). Not all of these are readily identifiable at the outset. They will however become obvious if an iterative approach is taken to the conduct of the HIA, and this we strongly recommend.
A.5. Some Examples of HIA of Outdoor Air Pollution
HIA of (changes in) air quality is the particular arena where environmental HIA (EHIA—those aspects of HIA that are mediated through changes in the environment) is most strongly established, both methodologically and in terms of practical applications for the development of policy. This is partly because (as outlined above) the underlying HIA model is relatively simple. Also, there has in the past 30 years or so been an enormous amount of research on air pollution and its health effects, and so the various component parts can be quantified more reliably than in many other areas of application; and quantification helps the integration of HIA into policy development, for example through cost‐benefit analysis of policy options. And finally, both the extensiveness of research and the translation into policy are driven by the very substantial public health effects of outdoor air pollution, estimated recently as causing over 4 million deaths annually (Cohen et al., 2017).
The fundamental framework for HIA of outdoor air pollution was developed initially by Bart Ostro (Ostro, 1994), in the context of burden of disease worldwide, but drawing on an evidence base for CRFs dominated by studies in North America and Europe. Greatest methodological development and most widespread use in the intervening 20+ years has been with a view to policy applications in North America and Europe. Much of the methodological development has related to updating of CRFs in the light of extensive new knowledge and understanding of air pollution and health, especially from epidemiological studies in North America and Europe, and consequently with greatest ease of application in those regions also. There was associated development of HIA methods also, notably with the application of life table methods when quantifying the effects on mortality of policies that reduce (or increase) long‐term exposure (Miller & Hurley, 2003).
The burden of disease (BoD) work, initially of WHO (World Health Organization, 1996), has been another main source of methodological development (Mathers et al., 2009). While once again drawing on epidemiological studies principally in North America and Europe, the BoD work has been intentionally focused on applications worldwide. This has led to very significant methodological developments in CRFs (Burnett et al., 2014) and in estimation of pollutant concentrations (Shaddick et al., 2018), and so is especially significant for applications to volcanic eruptions in Mexico, Japan and Indonesia. There also is an increasing base of air pollution HIAs in countries of Central and Latin America, and in Asia—see Hunt et al. (2016).
For a recent review of resources, especially computer packages, for HIA of outdoor air pollution, see, for example, Anenberg et al. (2016).
A.6. CRFs—Typical Pollutant‐Outcome Pairs
In 2012–3, WHO in Europe led two linked projects, HRAPIE (WHO, 2013a), and REVIHAAP (WHO, 2013b) and which together led to HRAPIE's recommendations for HIA of air pollution in Europe. These recommendations consisted of (i) a set of pollutant‐outcome pairs, and associated CRFs, for the three key pollutants PM2.5, ozone and NO2; and (ii) for sources of background rates of mortality and morbidity, for the various health outcomes identified. As is usual nowadays with HIAs of air pollution, HRAPIE's recommendations included CRFs relating both to short‐term exposures (i.e., the effects of daily variations in air quality, as identified via time series and panel studies) and longer‐term exposures (i.e., the effects of long‐term exposure to pollution, characterized as annual average concentrations close to residence, and identified via cohort studies).
Again as is typically the case, there was a wider range of pollutant‐outcome pairs for short‐term exposures than for longer‐term ones. The health outcomes where HRAPIE considered that the evidence of effects of daily variations in pollution was strong enough to merit quantification included mortality and hospital admissions for cardiovascular and respiratory disease. For longer‐term exposures HRAPIE recommended quantification of mortality in relation to PM2.5, ozone and NO2. Each CRF was classified as less, or more, uncertain, according to the strength of the underlying evidence base. The wider range and lesser uncertainty of pollutant‐outcome pairs for short‐term exposures identified by HRAPIE (WHO, 2013a) reflects the greater body evidence from time series and panel studies, which in turn reflects that they are easier to conduct, including being less resource‐intensive, than cohort studies of longer‐term exposures. It is however well established (e.g., Cohen et al., 2017), that much the greatest public health impacts arise from the effect on mortality of long‐term exposure to air pollution characterized as PM2.5.
In the Global Burden of Disease (GBD) project, Lim et al. (2012) used a smaller set of CRFs for HIA of outdoor air pollution worldwide. They focused in particular on the mortality effects of long‐term exposure to air pollution, characterized as annual average PM2.5. For this, they used cause‐specific rather than all‐cause mortality, specifically (list the causes)—it was necessary to use cause‐specific CRFs when transferring relationships from cohort studies in the USA for use in countries where there is a quite different mixture of causes of death. In Europe however the mixture of causes of death is sufficiently similar to that in the USA that all‐cause mortality can be and is used for quantification (WHO, 2013a).
GBD included just one pollutant‐outcome pair, that of PM2.5 and lower respiratory infections in children, to help quantify the effects of outdoor air pollution on morbidity. Hunt et al. (2016) considered what, if any, further pollutant‐outcome pairs could be used for quantification in OECD countries specifically, but in practice worldwide, and made some recommendations.
It is clear that, to some extent, the choice of CRFs depends in part on where the target population is located, and depends also on the judgment of the team doing the HIA analysis. There is no “one size fits all” formula such a quantification. Nevertheless, resources such as those listed above can and should be considered for quantification of the health effects of volcanic ash.
Appendix B. Summary of Studies Examining Exposure to Volcanic Ash and Health Effects
B.1.
Table B1.
A Summary of Studies Examining Exposure to Volcanic Ash and Health Effects
| Author, year | Volcano | Study design | Population | Outcome | Exposure | Result |
|---|---|---|---|---|---|---|
| Balsa et al. (2016) | Puyehue, Chile | Time series | 23 hospitals in Montevideo | Pregnancy and perinatal outcomes | PM10 exposure during pregnancy | Preterm birth per 10 μg/m3 in third trimester OR = 1.10 |
| Baxter et al. (1983) | Mount St. Helens, USA | Case–control | Asthma (n = 39) and Bronchitis (n = 44) patients | Asthma, bronchitis | Residence near Mount St. Helens | Patients more likely to have respiratory history, less likely to have cleaned ash |
| Baxter et al. (1983) | Mount St. Helens, USA | Case‐control | Chronic lung disease individuals (n = 97) | Symptom exacerbation | Residence near Mount St. Helens and received ashfall | 33% worsened in exposed versus 4% in unexposed |
| Bradshaw et al. (1997) | Mount Ruapehu, New Zealand | Case‐control | 1,392 asthmatics | Asthma symptoms and medication use via questionnaire | Exposed to ashfall from Mount Ruapehu | No significant associations in any of the outcomes. |
| Buist et al. (1983) | Mount St. Helens, USA | Longitudinal | Children's summer camp, n = 101 | Lung function (FEV1, FVC) | Respirable dust averaged 0.17 mg/m3 | No difference within or between days |
| Buist et al. (1986) | Mount St. Helens, USA | Longitudinal | Loggers at 4 camps, 2 exposed, 2 not | Lung function (FEV1, FVC), symptoms | Ashfall from Mount St. Helens | Short, reversible decline in lung function |
| Carlsen et al. (2012) | Eyjafjallajökull (Iceland) | Cross‐sectional | Residents, n = 1,148 exposed, n = 510 controls | Spirometry, questionnaires | Ashfall from Eyjafjallajökull | 50% reported eye and upper respiratory irritation. Higher lung function in exposed, but fewer smokers in exposed |
| Carlsen et al. (2012) | Eyjafjallajökull, Iceland | Longitudinal | Residents, exposed, n = 1,148, unexposed, n = 510 | Chronic, acute symptoms, GHQ‐12 | Exceedances of PM10 of 50 μg/m3 from Eyjafjallajökull during summer/fall 2010 | After 6–9 months, ↑ in exposed: OR > 2.0 chest tightness, cough, phlegm |
| Cowie et al. (2001) | Soufriere Hills, Montserrat | Cross‐sectional | Emigrants to United Kingdom, n = 465 | Respiratory symptoms | Compared to the U.K. population. | Slightly ↑ respiratory symptoms versus U.K. population |
| Cowie et al. (2002) | Soufriere Hills, Montserrat | Cross‐sectional | Workers (various) n = 421 | Lung function, respiratory symptoms, X‐rays | Residence in low/medium/high ashfall | Low prevalence of symptoms, ↓ lung function in gardeners and road workers |
| Forbes et al. (2003) | Soufriere Hills, Montserrat | Cross‐sectional | Schoolchildren | Respiratory symptoms | Residence in low/medium/high ashfall | 4 times as likely to report wheeze in most heavily exposed |
| Gordian et al. (1996) | Mount Spurr, Alaska | Time series | Population of Anchorage | Asthma, hospital visits, upper resp infections | Eruption period of Mount Spurr | Per 10 μg/m3: 3–6% ↑ asthma, 1–3% ↑ upper respiratory infection, but not after volcano |
| Hickling et al. (1999) | Mount Ruapehu, New Zealand | Time series | General population | Hospital visits | Areas with >0.25 mm ashfall | Similar rates in 3 months versus 7 years prior, borderline ↑ bronchitis |
| Higuchi et al. (2012) | Mount Sakurajima, Japan | Ecological | Populations in Sakurajima and Taramizu | Respiratory Mortality | Exposed versus less exposed | Elevated risk of lung cancer found in more exposed city (Sakurajima). |
| Hlodversdottir et al. (2016) | Eyjafjallajökull, Iceland | Longitudinal | Residents, exposed, n = 1,148, unexposed, n = 510 | Chronic, acute symptoms, GHQ‐12, stress | PM10, low/medium/high exposure areas—monitors, satellites | 3 years—↑ in 2013 versus 2010: OR > 2.0 phlegm, ↑ in exposed: OR > 2.0 wheezing, phlegm |
| Hlodversdottir et al. (2018) | Eyjafjallajökull, Iceland | Longitudinal | Children of exposed residents n = 433 (n = 200 unexposed) | Respiratory symptoms, headaches, anxiety, and others | PM10, low/medium/high exposure areas—monitor, satellites | Highly exposed children respiratory symptoms, OR = 1.52, worries OR = 2.77. No significant decrease in symptoms 2010–2013. |
| Johnson et al. (1982) | Mount St. Helens, USA | Longitudinal | School children | Lung function (FEV1, FVC) | Exposed to ashfall from Mount St. Helens | No difference before and after, though decrease from exposure to urban air pollution |
| Kraemar and McCarthy (1985) | Mount St. Helens, USA | Ecological | Spokane County, Washington | Asthma, childhood hospital admissions | Eruption period of Mount St. Helens | 2 times rates in year of volcano versus following year |
| Longo et al. (2010) | Kilauea, Hawai'i | Time series | General population | Clinic visits | PM2.5, SO2 (averaged 74.9 ± 51.9 ppbv/day) | 6 times ↑ airway issues after versus before, especially younger individuals |
| Malilay et al. (1996) | Cerro Negro, Nicaragua | Ecological | Proximal residents | Hospital visits | Communities near and within Cerro Negro volcano ashfall area | Acute respiratory visits in 2 communities: 3.6 times and 6 times increased versus before volcano |
| Naumova et al. (2007) | Guagua Pichincha, Ecuador | Time series | General population—children | Hospital visits—pediatric respiratory | Eruption period and afterward | 2.2 times and 1.7 times increases for lower and upper respiratory infections 3 weeks after eruption. 345 extra ER visits in 28 days following. |
| Oudin et al. (2013) | Grimsvötn, Iceland | Ecological | 21 regions in Sweden exposed to ashfall. | Mortality, all‐cause | Average PM2.5 increase of 10 μg/m3 | No significant differences. |
| Research committee on Volcanic emissions (1982) | Mount Sakurajima, Japan | Cross‐sectional | Residents of 2 towns | Pneumoconiosis, lung function | Distance of towns to Mount Sakurajima | 0 cases of pneumoconiosis, slight ↓ lung function in exposed women, but total suspended particulates (TSP) assumed too low to cause effect. |
| Rojas‐Ramos et al. (2001) | Popocatepetl, Mexico | Longitudinal | Farmers, n = 35 | Lung function (FEV1, FVC), symptoms | 0.5–1 mm ash | Short, reversible decline in lung function over 7 months |
| Sasayama et al. (2002) | Mount Unzen Fugen, Japan | Cross‐sectional | School children, aged 6–11 years | Asthma symptoms via parent questionnaire | Location of school to Mount Unzen Fugen | Some indication of deteriorated asthma. |
| Shimizu et al. (2007) | Mount Asama, Japan | Cross‐sectional | Adult asthma patients, n = 236 | Asthma, symptom exacerbation via questionnaire | Residence in areas of ashfall with >100 g/m2, >10 g/m2 | 42.9% exacerbations in 100 g/m2 ashfall area versus 8.3% in control area |
| Tobin and Whiteford (2004) | Mount Tungurahua, Ecuador | Cross‐sectional | Residents of 3 towns, n = 314 | Self‐reported symptoms | High ash/no evacuation, low ash/evacuation, no ash/no evacuation | No differences in reported respiratory or gastrointestinal illness, but differences in eye, skin, and throat |
| Uda et al. (1999) | Mount Sakurajima, Japan | Cross‐sectional | School children | Asthma (self‐administered questionnaire) | Proximity of school to Mount Sakurajima | No significant difference in asthma proportion between exposed and control groups |
| Wakisaka et al. (1988) | Mount Sakurajima, Japan | Ecological | 25 cities surrounding Mount Sakurajima | Respiratory mortality | 10, 20, 30 km from Mount Sakurajima | Deaths from bronchitis were elevated within 20 km, but not other respiratory causes. |
| Wakisaka et al. (1989) | Mount Sakurajima, Japan | Ecological | 4 districts located within 15 km of Mount Sakurajima | Clinical consultations for respiratory diseases | <10 km, 10–15 km from Mount Sakurajima | Higher rates of consultations in areas <10 km |
| Yano et al. (1986) | Mount Sakurajima, Japan | Cross‐sectional | Women in 3 towns | Respiratory diseases | Distance of volcano to towns | No difference in symptoms, overall respiratory disease prevalence low, but higher in town with highest TSP |
| Yano et al. (1990) | Mount Sakurajima, Japan | Cross‐sectional | Women in 2 towns | Respiratory diseases | Distance of volcano to towns | Respiratory disease slightly higher in town with higher TSP |
Mueller, W. , Cowie, H. , Horwell, C. J. , Hurley, F. , & Baxter, P. J. (2020). Health impact assessment of volcanic ash inhalation: A comparison with outdoor air pollution methods. GeoHealth, 4, e2020GH000256 10.1029/2020GH000256
References
- Adams, K. , Greenbaum, D. S. , Shaikh, R. , van Erp, A. M. , & Russell, A. G. (2015). Particulate matter components, sources, and health: Systematic approaches to testing effects. Journal of the Air & Waste Management Association, 65(5), 544–558. 10.1080/10962247.2014.1001884 [DOI] [PubMed] [Google Scholar]
- Anderson, H. R. , Atkinson, R. W. , Peacock, J. , Marston, L. , Konstantinou, K. , & World Health Organization (2004). Meta‐analysis of time‐series studies and panel studies of particulate matter (PM) and ozone (O3): Report of a WHO task group (No. EUR/04/5046026). Copenhagen: WHO Regional Office for Europe. [Google Scholar]
- Anenberg, S. C. , Belova, A. , Brandt, J. , Fann, N. , Greco, S. , Guttikunda, S. , Heroux, M. E. , Hurley, F. , Krzyzanowski, M. , Medina, S. , & Miller, B. (2016). Survey of ambient air pollution health risk assessment tools. Risk Analysis, 36(9), 1718–1736. 10.1111/risa.12540 [DOI] [PubMed] [Google Scholar]
- Apte, J. S. , Brauer, M. , Cohen, A. J. , Ezzati, M. , & Pope, C. A. III (2018). Ambient PM2.5 reduces global and regional life expectancy. Environmental Science & Technology Letters, 5(9), 546–551. 10.1021/acs.estlett.8b00360 [DOI] [Google Scholar]
- Balsa, A. I. , Caffera, M. , & Bloomfield, J. (2016). Exposures to particulate matter from the eruptions of the Puyehue volcano and birth outcomes in Montevideo, Uruguay. Environmental Health Perspectives, 124(11), 1816–1822. 10.1289/EHP235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basagaña, X. , Jacquemin, B. , Karanasiou, A. , Ostro, B. , Querol, X. , Agis, D. , Alessandrini, E. , Alguacil, J. , Artiñano, B. , Catrambone, M. , & Jesús, D. (2015). Short‐term effects of particulate matter constituents on daily hospitalizations and mortality in five south‐European cities: Results from the MED‐PARTICLES project. Environment International, 75, 151–158. 10.1016/j.envint.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Baxter, P. J. , Bonadonna, C. , Dupree, R. , Hards, V. L. , Kohn, S. C. , Murphy, M. D. , Nichols, A. , Nicholson, R. A. , Norton, G. , Searl, A. , Sparks, R. S. J. , & Vickers, B. P. (1999). Cristobalite in volcanic ash of the Soufriere Hills volcano, Montserrat, British West Indies. Science, 283(5405), 1142–1145. 10.1126/science.283.5405.1142 [DOI] [PubMed] [Google Scholar]
- Baxter, P. J. , Ing, R. , Falk, H. , French, J. , Stein, G. F. , Bernstein, R. S. , Merchant, J. A. , & Allard, J. (1981). Mount St Helens eruptions, May 18 to June 12, 1980: An overview of the acute health impact. JAMA, 246(22), 2585–2589. 10.1001/jama.1981.03320220035021 [DOI] [PubMed] [Google Scholar]
- Baxter, P. J. , Ing, R. , Falk, H. , & Plikaytis, B. (1983). Mount St. Helens eruptions: The acute respiratory effects of volcanic ash in a North American community. Archives of Environmental Health: An International Journal, 38(3), 138–143. 10.1080/00039896.1983.10543994 [DOI] [PubMed] [Google Scholar]
- Baxter, P. J. , Searl, A. S. , Cowie, H. A. , Jarvis, D. , & Horwell, C. J. (2014). Evaluating the respiratory health risks of volcanic ash at the eruption of the Soufriere Hills Volcano, Montserrat, 1995 to 2010. Geological Society, London, Memoirs, 39(1), 407–425. 10.1144/m39.22 [DOI] [Google Scholar]
- Bernstein, R. S. , Baxter, P. J. , Falk, H. , Ing, R. , Foster, L. , & Frost, F. (1986). Immediate public health concerns and actions in volcanic eruptions: Lessons from the Mount St. Helens eruptions, May 18–October 18, 1980. American Journal of Public Health, 76(Suppl), 25–37. 10.2105/AJPH.76.Suppl.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, L. , Fishwick, D. , Kemp, T. , Lewis, S. , & Rains, N. (1997). Under the volcano: Fire, ash and asthma? New Zealand Medical Journal, 110(1040), 90–91. 10.1111/j.1467-842x.2001.tb00549.x [DOI] [PubMed] [Google Scholar]
- Briggs, D. J. (2008). A framework for integrated environmental health impact assessment of systemic risks. Environmental Health, 7(1), 61. 10.1186/1476-069X-7-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist, A. S. , Johnson, L. R. , Vollmer, W. M. , Sexton, G. J. , & Kanarek, P. H. (1983). Acute effects of volcanic ash from Mount Saint Helens on lung function in children. American Review of Respiratory Disease, 127(6), 714–719. 10.1164/arrd.1983.127.6.714 [DOI] [PubMed] [Google Scholar]
- Buist, A. S. , Vollmer, W. M. , Johnson, L. R. , Bernstein, R. S. , & McCamant, L. E. (1986). A four‐year prospective study of the respiratory effects of volcanic ash from Mt. St. Helens 1–4. American Review of Respiratory Disease, 133(4), 526–534. 10.1164/arrd.1986.133.4.526 [DOI] [PubMed] [Google Scholar]
- Burnett, R. T. , Pope, C. A. III , Ezzati, M. , Olives, C. , Lim, S. S. , Mehta, S. , Shin, H. H. , Singh, G. , Hubbell, B. , Brauer, M. , & Anderson, H. R. (2014). An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environmental Health Perspectives, 122(4), 397 10.1289/ehp.1307049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen, H. K. , Gislason, T. , Benediktsdottir, B. , Kolbeinsson, T. B. , Hauksdottir, A. , Thorsteinsson, T. , & Briem, H. (2012). A survey of early health effects of the Eyjafjallajökull 2010a eruption in Iceland: A population‐based study. BMJ Open, 2(2), e000343 10.1136/bmjopen-2011-000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen, H. K. , Gislason, T. , Forsberg, B. , Meister, K. , Thorsteinsson, T. , Jóhannsson, T. , Finnbjornsdottir, R. , & Oudin, A. (2015). Emergency hospital visits in association with volcanic ash, dust storms and other sources of ambient particles: A time‐series study in Reykjavík, Iceland. International Journal of Environmental Research and Public Health, 12(4), 4047–4059. 10.3390/ijerph120404047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen, H. K. , Hauksdottir, A. , Valdimarsdottir, U. A. , Gíslason, T. , Einarsdottir, G. , Runolfsson, H. , Briem, H. , Finnbjornsdottir, R. G. , Gudmundsson, S. , Kolbeinsson, T. B. , & Thorsteinsson, T. (2012). Health effects following the Eyjafjallajökull volcanic eruption: A cohort study. BMJ Open, 2(6), e001851 10.1136/bmjopen-2012-001851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, A. J. , Brauer, M. , Burnett, R. , Anderson, H. R. , Frostad, J. , Estep, K. , Balakrishnan, K. , Brunekreef, B. , Dandona, L. , Dandona, R. , & Feigin, V. (2017). Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the global burden of diseases study 2015. The Lancet, 389(10082), 1907–1918. 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMEAP (2010). The mortality effects of long‐term exposure to particulate air pollution in the United Kingdom. London: Department of Health Committee on the Medical Effects of Air Pollutants. [Google Scholar]
- COMEAP (2015). Statement on the evidence for differential health effects of particulate matter according to source or components. London, UK: Committee on the Medical Effects of Air Pollutants. [Google Scholar]
- Costa, A. F. , Hoek, G. , Brunekreef, B. , & Ponce de Leon, A. C. (2016). Air pollution and deaths among elderly residents of Sao Paulo, Brazil: An analysis of mortality displacement. Environmental Health Perspectives, 125(3), 349–354. 10.1289/EHP98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey, J. , Horwell, C. J. , Rachmawati, L. , Ogawa, R. , Martin‐del Pozzo, A. L. , Armienta, M. A. , Nugroho, F. , & Dominelli, L. (2019). Factors motivating the use of respiratory protection against volcanic ashfall: A comparative analysis of communities in Japan, Indonesia and Mexico. International Journal of Disaster Risk Reduction, 35, 101,066 10.1016/j.ijdrr.2019.101066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie, H. A. , Graham, M. K. , Searl, A. , Miller, B. G. , Hutchison, P. A. , Swales, C. , Dempsey, S. , & Russell, M. (2002). A health survey of workers on the island of Montserrat. IOM Research Report TM/02/02. Edinburgh: Institute of Occupational Medicine. [Google Scholar]
- Cowie, H. A. , Searl, A. , Ritchie, P. J. , Graham, M. K. , Hutchison, P. A. , & Pilkington, A. (2001). A health survey of Montserratians relocated to the UK. Research report TM/01/07. Edinburgh: Institute of Occupational Medicine (IOM). [Google Scholar]
- Damby, D. E. , Horwell, C. J. , Baxter, P. J. , Delmelle, P. , Donaldson, K. , Dunster, C. , Fubini, B. , Murphy, F. A. , Nattrass, C. , Sweeney, S. , & Tetley, T. D. (2013). The respiratory health hazard of tephra from the 2010 centennial eruption of Merapi with implications for occupational mining of deposits. Journal of Volcanology and Geothermal Research, 261, 376–387. 10.1016/j.jvolgeores.2012.09.001 [DOI] [Google Scholar]
- Durant, A. J. , Bonadonna, C. , & Horwell, C. J. (2010). Atmospheric and environmental impacts of volcanic particulates. Elements, 6(4), 235–240. 10.2113/gselements.6.4.235 [DOI] [Google Scholar]
- Forbes, L. , Jarvis, D. , Potts, J. , & Baxter, P. J. (2003). Volcanic ash and respiratory symptoms in children on the island of Montserrat, British West Indies. Occupational and Environmental Medicine, 60(3), 207–211. 10.1136/oem.60.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldewijk, K. K. (2001). Estimating global land use change over the past 300 years: The HYDE database. Global Biogeochemical Cycles, 15(2), 417–433. [Google Scholar]
- Gordian, M. E. , Ozkaynak, H. , Xue, J. , Morris, S. S. , & Spengler, J. D. (1996). Particulate air pollution and respiratory disease in Anchorage, Alaska. Environmental Health Perspectives, 104(3), 290–297. 10.1289/ehp.96104290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi, G. , Daryanoosh, S. M. , Godini, H. , Hopke, P. K. , Sicard, P. , De Marco, A. , Rad, H. D. , Harbizadeh, A. , Jahedi, F. , Mohammadi, M. J. , & Savari, J. (2017). Health risk assessment of exposure to the middle‐eastern dust storms in the Iranian megacity of Kermanshah. Public Health, 148, 109–116. 10.1016/j.puhe.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Gudmundsson, G. (2011). Respiratory health effects of volcanic ash with special reference to Iceland. A review. The Clinical Respiratory Journal, 5(1), 2–9. 10.1111/j.1752-699X.2010.00231.x [DOI] [PubMed] [Google Scholar]
- Hansell, A. , & Oppenheimer, C. (2004). Health hazards from volcanic gases: A systematic literature review. Archives of Environmental Health: An International Journal, 59(12), 628–639. 10.1080/00039890409602947 [DOI] [PubMed] [Google Scholar]
- Hickling, J. , Clements, M. , Weinstein, P. , & Woodward, A. (1999). Acute health effects of the Mount Ruapehu (New Zealand) volcanic eruption of June 1996. International Journal of Environmental Health Research, 9(2), 97–107. 10.1080/09603129973236 [DOI] [Google Scholar]
- Higuchi, K. , Koriyama, C. , & Akiba, S. (2012). Increased mortality of respiratory diseases, including lung cancer, in the area with large amount of ashfall from Mount Sakurajima volcano. Journal of Environmental and Public Health, 2012, 257,831–257,831. 10.1155/2012/257831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman, S. E. , Horwell, C. J. , Densmore, A. L. , Damby, D. E. , Fubini, B. , Ishimine, Y. , & Tomatis, M. (2012). Sakurajima volcano: A physico‐chemical study of the health consequences of long‐term exposure to volcanic ash. Bulletin of Volcanology, 74(4), 913–930. 10.1007/s00445-012-0575-3 [DOI] [Google Scholar]
- Hincks, T. K. , Aspinall, W. P. , Baxter, P. J. , Searl, A. , Sparks, R. S. J. , & Woo, G. (2006). Long term exposure to respirable volcanic ash on Montserrat: A time series simulation. Bulletin of Volcanology, 68(3), 266–284. 10.1007/s00445-005-0006-9 [DOI] [Google Scholar]
- Hlodversdottir, H. , Petursdottir, G. , Carlsen, H. K. , Gislason, T. , & Hauksdottir, A. (2016). Long‐term health effects of the Eyjafjallajökull volcanic eruption: A prospective cohort study in 2010 and 2013. BMJ Open, 6(9), e011444 10.1136/bmjopen-2016-011444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlodversdottir, H. , Thorsteinsdottir, H. , Thordardottir, E. B. , Njardvik, U. , Petursdottir, G. , & Hauksdottir, A. (2018). Long‐term health of children following the Eyjafjallajökull volcanic eruption: A prospective cohort study. European Journal of Psychotraumatology, 9(sup2), 1442601 10.1080/20008198.2018.1442601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek, G. , Krishnan, R. M. , Beelen, R. , Peters, A. , Ostro, B. , Brunekreef, B. , & Kaufman, J. D. (2013). Long‐term air pollution exposure and cardio‐respiratory mortality: A review. Environmental Health, 12(1), 43 10.1186/1476-069x-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwell, C. J. (2007). Grain‐size analysis of volcanic ash for the rapid assessment of respiratory health hazard. Journal of Environmental Monitoring, 9(10), 1107–1115. 10.1039/b710583p [DOI] [PubMed] [Google Scholar]
- Horwell, C. J. , & Baxter, P. J. (2006). The respiratory health hazards of volcanic ash: A review for volcanic risk mitigation. Bulletin of Volcanology, 69(1), 1–24. 10.1007/s00445-006-0052-y [DOI] [Google Scholar]
- Horwell, C. J. , Baxter, P. J. , Hillman, S. E. , Calkins, J. A. , Damby, D. E. , Delmelle, P. , Donaldson, K. , Dunster, C. , Fubini, B. , Kelly, F. J. , & Le Blond, J. S. (2013). Physicochemical and toxicological profiling of ash from the 2010 and 2011 eruptions of Eyjafjallajökull and Grímsvötn volcanoes, Iceland using a rapid respiratory hazard assessment protocol. Environmental Research, 127, 63–73. 10.1016/j.envres.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Horwell, C. J. , Sparks, R. S. J. , Brewer, T. S. , Llewellin, E. W. , & Williamson, B. J. (2003). Characterization of respirable volcanic ash from the Soufrière Hills volcano, Montserrat, with implications for human health hazards. Bulletin of Volcanology, 65(5), 346–362. 10.1007/s00445-002-0266-6 [DOI] [Google Scholar]
- Horwell, C. J. , Williamson, B. J. , Donaldson, K. , Le Blond, J. S. , Damby, D. E. , & Bowen, L. (2012). The structure of volcanic cristobalite in relation to its toxicity; relevance for the variable crystalline silica hazard. Particle and Fibre Toxicology, 9(1), 44 10.1186/1743-8977-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, J. M. , Weill, H. , Checkoway, H. , Jones, R. N. , Henry, M. M. , Heyer, N. J. , Seixas, N. S. , & Demers, P. A. (1998). Radiographic evidence of silicosis risk in the diatomaceous earth industry. American Journal of Respiratory and Critical Care Medicine, 158(3), 807–814. 10.1164/ajrccm.158.3.9709103 [DOI] [PubMed] [Google Scholar]
- Hunt, A. , Ferguson, J. , Hurley, F. , & Searl, A. (2016). Social costs of morbidity impacts of air pollution, OECD Environment Working Papers (Vol. 99). Paris: OECD Publishing; 10.1787/5jm55j7cq0lv-en [DOI] [Google Scholar]
- Hurley, F. , & Vohra, S. (2010). Health impact assessment In Environmental medicine, (pp. 666–677). London: Hodder Arnold. [Google Scholar]
- Johnson, K. G. , Loftsgaarden, D. O. , & Gideon, R. A. (1982). The effects of Mount St. Helens volcanic ash on the pulmonary function of 120 elementary school children 1–3. American Review of Respiratory Disease, 126(6), 1066–1069. 10.1164/arrd.1982.126.6.1066 [DOI] [PubMed] [Google Scholar]
- Karagulian, F. , Belis, C. A. , Dora, C. F. C. , Prüss‐Ustün, A. M. , Bonjour, S. , Adair‐Rohani, H. , & Amann, M. (2015). Contributions to cities' ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmospheric Environment, 120, 475–483. 10.1016/j.atmosenv.2015.08.087 [DOI] [Google Scholar]
- Kollanus, V. , Prank, M. , Gens, A. , Soares, J. , Vira, J. , Kukkonen, J. , Sofiev, M. , Salonen, R. O. , & Lanki, T. (2017). Mortality due to vegetation fire‐originated PM2.5 exposure in Europe—Assessment for the years 2005 and 2008. Environmental Health Perspectives, 125(1), 30 10.1289/ehp194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer, M. J. , & McCarthy, M. M. (1985). Childhood asthma hospitalization rates in Spokane County, Washington: Impact of volcanic ash air pollution. Journal of Asthma, 22(1), 37–43. 10.3109/02770908509079882 [DOI] [PubMed] [Google Scholar]
- Lim, S. S. , Vos, T. , Flaxman, A. D. , Danaei, G. , Shibuya, K. , Adair‐Rohani, H. , AlMazroa, M. A. , Amann, M. , Anderson, H. R. , Andrews, K. G. , & Aryee, M. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. The Lancet, 380(9859), 2224–2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo, B. M. , Yang, W. , Green, J. B. , Crosby, F. L. , & Crosby, V. L. (2010). Acute health effects associated with exposure to volcanic air pollution (Vog) from increased activity at Kilauea Volcano in 2008. Journal of Toxicology and Environmental Health, Part A, 73(20), 1370–1381. 10.1080/15287394.2010.497440 [DOI] [PubMed] [Google Scholar]
- Lu, F. , Xu, D. , Cheng, Y. , Dong, S. , Guo, C. , Jiang, X. , & Zheng, X. (2015). Systematic review and meta‐analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environmental Research, 136, 196–204. 10.1016/j.envres.2014.06.029 [DOI] [PubMed] [Google Scholar]
- Malilay, J. , Mariana, G. R. , Ramirez Vanegas, A. , Non, E. , & Sinks, T. (1996). Public health surveillance after a volcanic eruption: Lessons from Cerro Negro, Nicaragua, 1992. Bulletin of PAHO, 30(3), 218–226. [PubMed] [Google Scholar]
- Mathers, C. , Stevens, G. , & Mascarenhas, M. (2009). Global health risks‐mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization. [Google Scholar]
- Miller, B. G. , & Hurley, F. (2003). Life table methods for quantitative impact assessments in chronic mortality. Journal of Epidemiology & Community Health, 57(3), 200–206. 10.1136/jech.57.3.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K. R. , Duffell, H. , Nicholl, A. , & Searl, A. (2002). Monitoring of airborne particulate matter during the eruption of Soufrière Hills volcano, Montserrat. In: T.H. Druitt and B.P. Kokelaar (Editors), The eruption of Soufrière Hills volcano, Montserrat, from 1995 to 1999. Geological Society, London, Memoir, 21(1), 557–566. 10.1144/GSL.MEM.2002.021.01.25 [DOI] [Google Scholar]
- Mueller, W. , Cowie, H. , Horwell, C. J. , Baxter, P. J. , McElvenny, D. , Booth, M. , Cherrie, J. W. , Cullinan, P. , Jarvis, D. , Ugarte, C. , & Inoue, H. (2020). Standardized epidemiological protocols for populations affected by volcanic eruptions. Bulletin of the World Health Organization, 98, 362–364. 10.2471/BLT.19.244509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova, E. N. , Yepes, H. , Griffiths, J. K. , Sempértegui, F. , Khurana, G. , Jagai, J. S. , Játiva, E. , & Estrella, B. (2007). Emergency room visits for respiratory conditions in children increased after Guagua Pichincha volcanic eruptions in April 2000 in Quito, Ecuador observational study: Time series analysis. Environmental Health, 6(1), 21 10.1186/1476-069x-6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen, M. J. , & Khreis, H. (2016). Car free cities: Pathway to healthy urban living. Environment International, 94, 251–262. 10.1016/j.envint.2016.05.032 [DOI] [PubMed] [Google Scholar]
- Ostro, B. D. (1994). Estimating the health effects of air pollutants: A method with an application to Jakarta (Vol. 1301). Washington, DC: World Bank publications. [Google Scholar]
- Oudin, A. , Carlsen, H. , Forsberg, B. , & Johansson, C. (2013). Volcanic ash and daily mortality in Sweden after the Icelandic volcano eruption of May 2011. International Journal of Environmental Research and Public Health, 10(12), 6909–6919. 10.3390/ijerph10126909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, C. A. III , Burnett, R. T. , Thun, M. J. , Calle, E. E. , Krewski, D. , Ito, K. , & Thurston, G. D. (2002). Lung cancer, cardiopulmonary mortality, and long‐term exposure to fine particulate air pollution. Journal of the American Medical Association, 287(9), 1132–1141. 10.1001/jama.287.9.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, C. A. III , & Dockery, D. W. (2006). Health effects of fine particulate air pollution: Lines that connect. Journal of the Air & Waste Management Association, 56(6), 709–742. 10.1080/10473289.2006.10464485 [DOI] [PubMed] [Google Scholar]
- Research Committee on Volcanic Emissions (1982). Research report on the human effect of volcanic exhaust (Sakurajima), 1980–1981 report. Tokyo: Tuberculosis and Chronic Disease Division, Public Health Bureau, Ministry of Health and Welfare. [Google Scholar]
- Rojas‐Ramos, M. , Catalan‐Vazquez, M. , Martin‐Del Pozzo, A. L. , Garcia‐Ojeda, E. , Villalba‐Caloca, J. , & Perez‐Neria, J. (2001). A seven months prospective study of the respiratory effects of exposure to ash from Popocatepetl volcano, Mexico. Environmental Geochemistry and Health, 23(4), 379–392. 10.1023/A:1012244311557 [DOI] [Google Scholar]
- Sarigiannis, D. A. , Karakitsios, S. P. , Gotti, A. , Liakos, I. L. , & Katsoyiannis, A. (2011). Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environment International, 37(4), 743–765. 10.1016/j.envint.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Sasayama, H. , Aoyagi, K. , Tagawa, Y. , Tagawa, M. , Mori, K. , Honda, S. , & Takemoto, T. I. (2002). The effects of volcanic disaster on the prevalence and severity of bronchial asthma. Acta Medica Nagasakiensia, 47(1–2), 31–36. [Google Scholar]
- Schmidt, A. , Ostro, B. , Carslaw, K. S. , Wilson, M. , Thordarson, T. , Mann, G. W. , & Simmons, A. J. (2011). Excess mortality in Europe following a future Laki‐style Icelandic eruption. Proceedings of the National Academy of Sciences, 108(38), 15,710–15,715. 10.1073/pnas.1108569108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searl, A. , Nicholl, A. , & Baxter, P. J. (2002). Assessment of the exposure of islanders to ash from the Soufriere Hills volcano, Montserrat, West Indies. Occupational and Environmental Medicine, 59(8), 523–531. 10.1136/oem.59.8.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddick, G. , Thomas, M. L. , Green, A. , Brauer, M. , Donkelaar, A. , Burnett, R. , Chang, H. H. , Cohen, A. , Dingenen, R. V. , Dora, C. , & Gumy, S. (2018). Data integration model for air quality: A hierarchical approach to the global estimation of exposures to ambient air pollution. Journal of the Royal Statistical Society: Series C (Applied Statistics), 67(1), 231–253. 10.1111/rssc.12227 [DOI] [Google Scholar]
- Shimizu, Y. , Dobashi, K. , Hisada, T. , Ono, A. , Todokoro, M. , Iijima, H. , Utsugi, M. , Kakegawa, S. , Iizuka, K. , Ishizuka, T. , & Morikawa, A. (2007). Acute impact of volcanic ash on asthma symptoms and treatment. International Journal of Immunopathology and Pharmacology, 20(2_suppl), 9–14. [DOI] [PubMed] [Google Scholar]
- Steinle, S. , Sleeuwenhoek, A. , Mueller, W. , Horwell, C. J. , Apsley, A. , Davis, A. , Cherrie, J. W. , & Galea, K. S. (2018). The effectiveness of respiratory protection worn by communities to protect from volcanic ash inhalation. Part II: Total inward leakage tests. International Journal of Hygiene and Environmental Health, 221(6), 977–984. 10.1016/j.ijheh.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Thorsteinsson, T. , Jóhannsson, T. , Stohl, A. , & Kristiansen, N. I. (2012). High levels of particulate matter in Iceland due to direct ash emissions by the Eyjafjallajökull eruption and resuspension of deposited ash. Journal of Geophysical Research, 117, B00C05 10.1029/2011JB008756 [DOI] [Google Scholar]
- Tobin, G. A. , & Whiteford, L. M. (2004). Chronic hazards: Health impacts associated with on‐going ash‐falls around Mt. Tungurahua in Ecuador. Papers of the Applied Geography Conferences, 27, 84–93. [Google Scholar]
- Tomašek, I. , Damby, D. E. , Horwell, C. J. , Ayris, P. M. , Delmelle, P. , Ottley, C. J. , Cubillas, P. , Casas, A. S. , Bisig, C. , Petri‐Fink, A. , Dingwell, D. B. , Clift, M. J. D. , Drasler, B. , & Rothen‐Rutishauser, B. (2019). Assessment of the potential for in‐plume sulphur dioxide gas‐ash interactions to influence the respiratory toxicity of volcanic ash. Environmental Research, 179(Pt a), 108798 10.1016/j.envres.2019.108798 [DOI] [PubMed] [Google Scholar]
- Tomašek, I. , Horwell, C. J. , Bisig, C. , Damby, D. E. , Comte, P. , Czerwinski, J. , Petri‐Fink, A. , Clift, M. J. , Drasler, B. , & Rothen‐Rutishauser, B. (2018). Respiratory hazard assessment of combined exposure to complete gasoline exhaust and respirable volcanic ash in a multicellular human lung model at the air‐liquid interface. Environmental Pollution, 238, 977–987. 10.1016/j.envpol.2018.01.115 [DOI] [PubMed] [Google Scholar]
- Tomašek, I. , Horwell, C. J. , Damby, D. E. , Barošová, H. , Geers, C. , Petri‐Fink, A. , Rothen‐Rutishauser, B. , & Clift, M. J. (2016). Combined exposure of diesel exhaust particles and respirable Soufrière Hills volcanic ash causes a (pro‐) inflammatory response in an in vitro multicellular epithelial tissue barrier model. Particle and Fibre Toxicology, 13(1), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda, H. , Akiba, S. , Hatano, H. , & Shinkura, R. (1999). Asthma‐like disease in the children living in the neighborhood of Mt. Sakurajima. Journal of Epidemiology, 9(1), 27–31. 10.2188/jea.9.27 [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency (2010). Quantitative health risk assessment for particulate matter. EPA‐452/R‐10‐005 Research Triangle Park: US Environmental Protection Agency. [Google Scholar]
- US Environmental Protection Agency (2011). The benefits and costs of the Clean Air Act from 1990 to 2020. Research Triangle Park: US Environmental Protection Agency. [Google Scholar]
- Wakisaka, I. , Yanagihashi, T. , Sato, M. , & Tomari, T. (1989). Health effects of volcanic air pollution. Nippon Eiseigaku Zasshi (Japanese Journal of Hygiene), 44(5), 977–986. 10.1265/jjh.44.977 [DOI] [PubMed] [Google Scholar]
- Wakisaka, I. , Yanagihashi, T. , Tomari, T. , & Ando, T. (1988). Effects of volcanic activity on the mortality figures for respiratory diseases. Nippon Eiseigaku Zasshi (Japanese Journal of Hygiene), 42(6), 1101–1110. 10.1265/jjh.42.1101 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1996). In Murray C. J. & Lopez A. D. (Eds.), The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: Summary. Geneva: WHO. [Google Scholar]
- World Health Organization (WHO) (1999). Health impact assessment: Main concepts and suggested approach. Gothenberg consensus paper. Geneva: WHO. [Google Scholar]
- World Health Organization (WHO) (2013a). Health risks of air pollution in Europe—HRAPIE project recommendations for concentration–response functions for cost–benefit analysis of particulate matter, ozone and nitrogen dioxide. World Health Organization Regional Office for Europe. Geneva, Switzerland: World Health Organization. [Google Scholar]
- World Health Organization (WHO) (2013b). Review of evidence on health aspects of air pollution–REVIHAAP project. Copenhagen, Denmark: World Health Organization. [Google Scholar]
- World Health Organization (2015). World Health Statistics. Geneva: World Health Organization. [Google Scholar]
- Yano, E. , Yokoyama, Y. , Higashi, H. , Nishii, S. , Maeda, K. , & Koizumi, A. (1990). Health effects of volcanic ash: A repeat study. Archives of Environmental Health: An International Journal, 45(6), 367–373. 10.1080/00039896.1990.10118757 [DOI] [PubMed] [Google Scholar]
- Yano, E. , Yokoyama, Y. , & Nishii, S. (1986). Chronic pulmonary effects of volcanic ash: An epidemiologic study. Archives of Environmental Health: An International Journal, 41(2), 94–99. 10.1080/00039896.1986.9937416 [DOI] [PubMed] [Google Scholar]
- Zanobetti, A. , & Schwartz, J. (2009). The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environmental Health Perspectives, 117(6), 898. [DOI] [PMC free article] [PubMed] [Google Scholar]


