Abstract
Idiopathic pulmonary arterial hypertension (IPAH) is a progressive and devastating disease characterized by vascular smooth muscle and endothelial cell proliferation leading to a narrowing of the vessels in the lung. The increased resistance in the lung and the higher pressures generated result in right heart failure. Nitric Oxide (NO) deficiency is considered a hallmark of IPAH and altered function of endothelial nitric oxide synthase (eNOS), decreases NO production. We recently demonstrated that glucose dysregulation results in augmented protein serine/threonine hydroxyl-linked N-Acetyl-glucosamine (O-GlcNAc) modification in IPAH. In diabetes, dysregulated glucose metabolism has been shown to regulate eNOS function through inhibition of Ser-1177 phosphorylation. However, the link between O-GlcNAc and eNOS function remains unknown. Here we show that increased protein O-GlcNAc occurs on eNOS in PAH and Ser-615 appears to be a novel site of O-GlcNAc modification resulting in reduced eNOS dimerization. Functional characterization of Ser-615 demonstrated the importance of this residue on the regulation of eNOS activity through control of Ser-1177 phosphorylation. Here we demonstrate a previously unidentified regulatory mechanism of eNOS whereby the O-GlcNAc modification of Ser-615 results in reduced eNOS activity and endothelial dysfunction under conditions of glucose dysregulation.
Keywords: Endothelial nitric oxide synthetase, O-GlcNAc modification, Pulmonary arterial hypertension, Nitric oxide
Highlights
-
•
Pulmonary arterial hypertension is characterized by Nitric Oxide deficient state.
-
•
Abnormal glucose metabolism occurs in pulmonary arterial hypertension.
-
•
Altered glucose metabolism leads to increased O-GlcNAc modifications of proteins.
-
•
O-GlcNAc modification of endothelial nitric oxide synthetase is increased in disease.
-
•
Site of modification suggests the mechanism of inactivation is unique.
1. Introduction
Pulmonary arterial hypertension (PAH) is a progressive, fatal disease that is clinically characterized by increased pulmonary arterial blood pressure and structural remodeling of the pulmonary vasculature [1]. This vascular remodeling leads to structural changes, occlusion of pulmonary arteries, and increased pulmonary vascular resistance. The increase in vascular resistance eventually leads to unusually high pressures that strain the right ventricle of the heart causing it to hypertrophy, which causes right heart failure. The pathology of PAH is strongly associated with the dysfunction of the pulmonary vascular endothelium and results in an imbalance of the production and release of vasodilators, such as nitric oxide (NO) and prostacyclin, and vasoconstrictors such as endothelin-1 and serotonin [2,3].
NO is a potent pulmonary arterial vasodilator that has many roles in the body [4]. In the vasculature, it causes smooth muscle relaxation, arterial vasodilation leading to increased blood flow, and is also involved in the inhibition of platelet activation and inhibition of vascular smooth muscle cell proliferation. We and others have shown that lower levels of exhaled NO are observed in PAH patients, and that lung NO production is inversely proportional to the degree of pulmonary hypertension [[5], [6], [7]]. The importance of NO in the PAH disease process has been highlighted in the eNOS knockout or overexpression animal models [[8], [9], [10]]. Transgenic mice that overexpress eNOS are protected from hypoxia-induced PAH; whereas, mild exposure of eNOS knockouts to hypoxia results in PAH. Additionally, in patients, increased survival with therapeutic interventions that target NO bioavailability has been observed. NO exerts its biological effects on multiple targets including binding to soluble guanylate cyclase (sGC), which results in the production of cGMP that leads to vasodilatation. Therapies have targeted either the inhibition of breakdown of cGMP (PDE5 inhibitors) or the activation of sGC [11,12]. However, NO affects multiple biological processes that may contribute to protection against hypoxia-induced vasoconstriction through the alteration of cellular respiration pathways, inhibition of smooth muscle proliferation, inhibition of platelet activation, and down-regulation of the vasoconstrictor, endothelial-1 [4]. Therefore, it is critical to understand the mechanism(s) that lead to reduced levels of NO production.
NO synthesis is regulated by three different isoforms of NO synthase enzymes, including endothelial NOSs (eNOS) [13]. eNOS is constitutively expressed in the endothelium and is important for vasodilation and therefore, changes in its activity directly lead to problems observed in PAH. Expression levels of eNOS are not altered in PAH suggesting that regulation at the post-translational level result in changes in its activity. Numerous post-translational modifications of eNOS regulate activity including phosphorylation, and the O-GlcNAc [14]. However, very little is known about the latter and its regulation of eNOS activity.
Distinct changes in glucose uptake and metabolism have been established in PAH [15]. We recently demonstrated that increased glucose flux into the hexosamine biosynthetic pathway resulted in increased O-GlcNAc modified proteins [16]. Over 1,500 proteins have been identified to receive the O-GlcNAc modification including those involved in vascular integrity, metabolism, cell cycle, signal transduction, and gene expression [17,18]. Multiple reports have demonstrated a reciprocal regulatory relationship between O-GlcNAc and phosphorylation, which have been shown to occupy the same or adjacent serine/threonine sites on protein.
2. Methods
2.1. Site directed mutagenesis
Human eNOS in pcDNA3 was used (a gift from William Sessa (Addgene plasmid # 22484)) for mammalian expression [19]. The eNOS S1177D clone was initially mutated to obtain eNOS (wt) and other clones were also created using the site-directed mutagenesis Quikchange XL kit (Agilent Technologies; Santa Clara, CA, USA) as directed by the manufacturer. The oligos used are shown in Table S1 and were ordered from IDT Technologies (Coralville, IO, USA). All clones were sequenced to determine the site(s) of modification.
2.2. Protein expression-purification (O-Linked N-Acetylglucosamine transferase (OGT)/eNOS)
BL21-DE3 cells were transformed using pCW-eNOS or mutant versions of eNOS using Ampicillin selection. Positive clones were then retransformed with pET-sOGT (a kind gift from Dr. Walker; Harvard University) and selected using both Ampicillin and Kanamycin resistance [20]. Proteins were expressed using a modified procedure for the production of eNOS and OGT [20,21]. Briefly, an overnight culture of bacteria was inoculated into fresh media (1:50 vol/vol). The cells were grown at 37°C until the OD600 reached 0.8–1.0. The media was then supplemented with 0.4 mM δ-aminolevulinic acid, and the induction was started with 0.5 mM IPTG. The culture was then transferred to an 18°C shaker and left to grow for 24hrs. Subsequently, the bacteria were harvested by centrifugation and frozen until purification was performed. Proteins were purified using an NTA-resin as described by Adak et al. [21]. Since both eNOS and OGT contained His tags, both proteins were co-purified using a nickel affinity chromatography.
2.3. Lung tissue, PAEC and PASMC isolation, and culture conditions
All explanted lungs were collected at the Cleveland Clinic, and Institutional Review Board approval was obtained. Human lung tissues used in this study were from donor lung explants not suitable for lung transplantation and idiopathic PAH patients. PAECs and PASMCs were cultured in endothelial cell growth media (EGM-2) and smooth muscle growth media (SmGM-2), respectively (Lonza; Walkerville MD).
2.4. Immunohistochemistry
For Immunohistochemistry, staining was performed as described by Barnes et al. [16].
2.5. Mammalian transfection
pcDNA-eNOS clones were transfected into HEK293T cells cultured in Opti-MEM with lipofectamine 2000 (Invitrogen, USA) using the manufacturers recommended protocol. Sixteen hours post-transfection, the media was changed and collected 24 h later for nitrite analysis. Nitrite was determined using a Sievers NOx Analyzer (Boulder, CO, USA) with the tri-iodide method [[22], [23], [24]] with standard curves generated using sodium nitrite.
2.6. Sample preparation and western blotting
Cells were cold lysed in buffer (50 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, and 1% Nonidet P-40) containing a protease inhibitor cocktail (Sigma, St. Louis, USA) and PUGNAC (50 μM; Sigma, St. Louis, MO) + Thiamet G (25nM, Sigma) and prepared for Western blot analysis as described above using 30 μg of protein for each sample. Samples were incubated at 95°C for 5 min with 4X Laemmli sample buffer and subjected to SDS-PAGE (4–15%) gradient gels (Bio-Rad, Hercules, CA, USA) followed by protein transfer to nitrocellulose for Western blot analysis. Membranes were probed with antisera for the following specific proteins: O-GlcNAc (1:4,000; clone CTD 110.6, Biolegend, San Diego, CA, USA) and eNOS (eNOS-total- 1:2000; Santa Cruz, CA, USA, phospho-Serine1177–1:2000; Millipore; Billerica, MA). Probed blots were developed using enhanced chemiluminescence (ECL, Amersham, Pittsburgh, PA, USA), while blots probed for β-Actin (1:10,000; Santa Cruz, CA, USA) were blocked, washed, and imaged using an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). For western blotting using anti-eNOS1177P (Abcam, Cambridge, MA) and were performed in triplicate from 3 different transfections. Film based westerns were quantified using the densitometry software from ImageJ [25]. For Immunoprecipitation, lysates were incubated with the anti-O-GlcNAC antibody (CTD110.6 and RL2) overnight at 4C and then bound to protein A/G sepharose beads (Genscript; Piscataway, NJ, USA), washed, and then separated using SDS-PAGE.
Concentration eNOS in lysates were calculated using a direct ELISA assay. Briefly, lysates were diluted in coating buffer (0.1 M NaHCO3, pH 9.6) and added to 96 well plates to bind for 24 hrs at 4°C. The plates were washed in washing buffer (50mM Tris/HCl, 150mM NaCl, 0.06% Tween 20) and probed with anti-eNOS (1:5000 in protein-free blocking buffer; (Thermo Scientific Pierce; Grand Island, NY, USA)) for 2hrs with shaking. Afterwards, the wells were washed 3 times with a wash buffer and probed with anti-rabbit-HRP (1:5000 in protein free blocking buffer) for another 2 h. After washing, the wells were analyzed using ultra-TMB reagent (Thermo Scientific Pierce; Grand Island, NY, USA) as suggested by the manufacturer). A standard curve was generated using purified eNOS protein to calculate the concentration of eNOS in the lysates. Each experiment was performed in triplicate. These concentrations were used to normalize the levels of nitrite in the culture media.
2.7. Size exclusion chromatography
Partially purified wild-type and mutant eNOS proteins were analyzed by fast protein liquid chromatography using an S200 gel filtration column (Bio-Rad; Hercules, CA, USA). The column was pre-equilibrated with Tris-buffered saline and calibrated with the following protein molecular mass standards: thyroglobulin, 675 kDa; γ-globulin, 158 kDa; bovine serum albumin, 67 kDa; ovalbumin, 44 kDa; and myoglobin, 17 kDa. Fractions of the eNOS wt and mutant proteins were collected and assessed by direct ELISA using an antibody specific for the reductase domain of eNOS.
2.8. Spectroscopy
eNOS proteins were incubated with 0.1mM Arginine and 25μM H4B (tetrahydrobiopterin), and left at 4°C for 30 min on ice as previously described [21]. Optical spectra were then recorded using a spectrophotometer. A difference in the ferrous heme-carbon monoxide (CO) spectrum was obtained by flushing the sample with CO gas, followed by dithionite reduction. As a reducing control, a non-reduced sample was used.
2.9. Mass spectroscopy
Partially purified proteins were reduced and alkylated. The proteins were then run on SDS PAGE gels and stained using GelCode blue (Pierce, Rockford, IL; USA). Protein bands were then excised for mass spectrometry as described [26,27] digested, and analyzed using an LC-MS with a Finnigan LTQ-Orbitrap Elite hybrid mass spectrometer system. The HPLC column was a Dionex 15 cm x 75 μm id Acclaim Pepmap C18, 2μm, 100 Å reversed-phase capillary chromatography column. A 5 μL volume of the extract was injected and the peptides eluted from the column by an acetonitrile/0.1% formic acid gradient at a flow rate of 0.3 μL/min and were introduced into the source of the mass spectrometer on-line. The data was analyzed using peaks studio (version 7.5) (Bioinformatics Solutions Inc. Waterloo, Canada) [28]. The mass tolerances were set to 5 ppm for precursor ion and 0.5 Da for fragment ion. For the modification conditions, we set the carbamidomethylation of cysteine as a fixed modification. N-Acetylhexosamine modification at a serine or threonine as well as oxidation of methionine residues were set as variables.
2.10. Statistical analysis
Statistical analysis was carried out using a paired t-test or 1 way ANOVA using Prism from GraphPad (Determined values are shown in the text).
3. Results
3.1. The O-GlcNAc modification of eNOS is elevated in the endothelium of IPAH patients
We previously demonstrated that increased flux through the hexosamine biosynthetic pathway leads to increased hyaluronan and global protein O-GlcNAc modification in PAH [29]. Here we show increased O-GlcNAc modification of protein staining in lungs from IPAH patients, in numerous lung regions including the endothelium (Fig. 1C). In diabetic models, increased O-GlcNAc and reduced NO production has been tied to reduced phosphorylation of eNOS at Ser-1177 [16,30]. Here we show that O-GlcNAc specifically modifies eNOS in IPAH patients. In Fig. 1A and B, the levels of the O-GlcNAc modification on eNOS were significantly elevated in IPAH patients compared to controls (1.17 ± 0.08 and 0.60 ± 0.12; p = 0.01). The functional consequence of the O-GlcNAc modification of eNOS is illustrated by the dose-dependent reduction of NO biosynthesis upon administration of a specific O-GlcNAcase inhibitor (Fig. 1D), which increases protein bound O-GlcNAc modification (1E). Interestingly, patient-derived IPAH PAECs had reduced amounts of phosphorylated Ser-1177 (Fig. 1F). Relative to the total eNOS protein expression, the phosphorylation of Ser-1177 was significantly decreased by over 50% (Fig. 1G; p = 0.079). These data suggest that the O-GlcNAc modification of eNOS may impact the phosphorylation of Ser-1177 in IPAH cells.
Fig. 1.
The O-GlcNAc modification of eNOS is elevated in IPAH patients. (A) Human PAEC lysates were immunoprecipitated with antiserum against eNOS and immunoblotted for the O-GlcNAc modification (red; upper panel). Total eNOS protein levels (green) and equal loading using antisera against β-actin (red; lower panel) was determined. (B) Densitometry analysis of eNOS O-GlcNAc levels from control and IPAH. (C) Immunohistochemical analysis of protein bound O-GlcNAc in the lungs from Control and IPAH patients. Lung sections were either stained with H&E (right) or with an anti-O-GlcNAc antibody (left). O-GlcNAc reactive proteins are increased in IPAH vasculature (brown) including the endothelium. (D) A dose-dependent increase in Thiamet-G (an OGA inhibitor) results in a decrease of nitrite production in HUVECs following 20 h of treatment (n = 6). ANOVA for multiple comparison and PAEC p < 0.0251 and HUVEC p < 0.0058. (E) A representative immunoblot, using antisera against global O-GlcNAc, demonstrates increased O-GlcNAc levels in HUVECs treated with and without the highest concentration (500nM) of Thiamet-G (TG) used in (D). In the lower panels the band of 120kDa O-GlcNAc modified protein was quantitated (ANOVA for multiple comparison is PAEC p < 0.0036 and HUVEC p < 0.054). (F,G) An immunoblot examining the phosphorylated state of eNOS Ser-1177 from control (n = 3) and IPAH patient (n = 3) PAEC lysates show a decrease in the levels of phosphorylated Ser-1177 on eNOS relative to total eNOS (p < 0.079). Ctrl = control and IPAH = idiopathic pulmonary arterial hypertension. Error bars represent the standard error of the mean (SEM) of independent triplicate experiments. The p-values are given based on a Student t-test determined from the independent triplicate experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
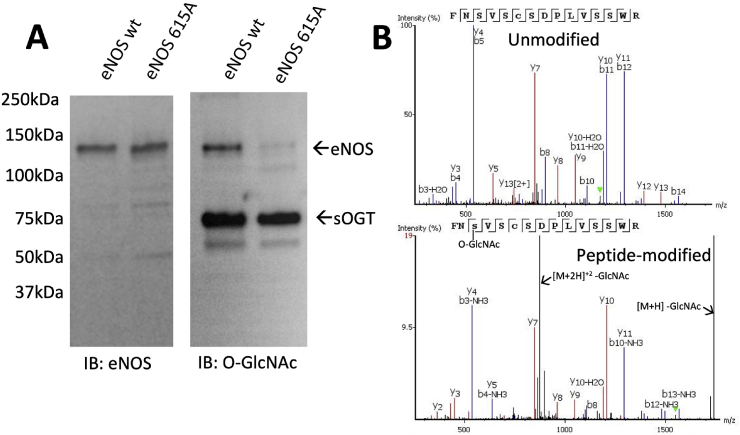
3.2. Co-expression of eNOS and OGT results in a previously unidentified O-GlcNAc modification site at Ser-615
Several reports have shown that the phosphorylation of Ser-1177 may be affected by O-GlcNAc [[30], [31], [32], [33]]. However, none of these reports performed a detailed O-GlcNAc site-map of eNOS. To determine possible O-GlcNAc modification sites, we co-expressed eNOS with soluble form of OGT (sOGT). We observed O-GlcNAc modification of both the sOGT and eNOS (Fig. 2A), which is consistent with previous reports [30,34]. Using mass spectrometry, we obtained approximately 99% coverage of the proteolyzed modified eNOS protein (Supplementary Figs. S1A–C), which covers all potential O-GlcNAc and phosphorylation sites (Thr-495, Ser-615, Ser-633, and Ser-1177). Only the peptides of eNOS containing Ser-615 were modified by O-GlcNAc (Fig. 2B). To validate our findings we mutated the Serine-615 to an Alanine (S615A) and found substantially lower O-GlcNAc modification compared to eNOS wt (Fig. 2A). Interestingly, a small amount of residual modified protein (<5%) was observed and may reflect promiscuous modification of sites adjacent to Ser-615 (Ser-617 or Ser-619) upon removal of the Ser-615 residue. It is also possible that another site of modification may exist at lower abundancy but escaped our MS detection. Nevertheless, it is clear that Ser-615, and not Ser-1177, is the prominent site of O-GlcNAc modification.
Fig. 2.
Bacterial co-expression of eNOS and OGT results in a previously unidentified O-GlcNAc modification site at Ser-615. (A) Bacterial lysates from co-expressed with eNOS wt/OGT or eNOS S615A/OGT were partially purified using NTA-resin, and subjected to SDS-PAGE followed by Western blot analysis for eNOS and O-GlcNAc. While eNOS protein levels were unaffected for eNOS wt and eNOS S615A (left panel), immunoreactivity for O-GlcNAc is only observed in the co-expression system with eNOS wt (right panel), which is negligible with eNOS S615A. Note that OGT auto O-GlcNAc modifies itself (right panel). (B) eNOS wt co-expressed with OGT was subjected to LC/MS/MS and analyzed for post translational modifications. A representative MS spectra shows an unmodified peptide (870.903 Da: Z = 2) and an O-GlcNAc modified peptide at Ser-615 (972.4427: Da: Z = 2), both covering residues 613–627. Due to the unstable nature of the GlcNAc moiety on the modified peptide, peaks of the neutral ion loss of GlcNAc are also observed.
3.3. Mutation of specific eNOS residues results in altered O-GlcNAc modification and eNOS activity
While bacterial expression studies suggested that Ser-615 was the primary site of O-GlcNAc modification on eNOS, mammalian cells may modify the eNOS protein differently. We, therefore, expressed eNOS mutants to determine the potential sites in mammalian cells. Expression of eNOS S615A or eNOS S1177A reduced the O-GlcNAc modification of eNOS compared to wild-type. This was also repeated with another anti-O-GlcNAc antibody (RL2) with similar results. The expression of eNOS S615D reduced the O-GlcNAc levels on eNOS by 86% (n = 2), which was greater than that observed with either S615A (49%) (n = 2) or S1177A (71%) (n = 2) (Fig. 3A).
Fig. 3.
Mutation of specific eNOS residues results in altered O-GlcNAc modification and eNOS activity. (A) A representative Immunoblot of eNOS mutants (S615A, S615D, and S1177A) expressed in Hek293T cells and Immunoprecipitated with an anti-O-GlcNAc antibody (CTD 110.6) followed by immunoblotting using antiserum against eNOS. As an input, cell lysate before immunoprecipitation was subjected to SDS-PAGE and immunoblot analysis. Comparison of the amount of O-GlcNAc on eNOS relative to eNOS wt is shown using the CTD110.6 and RL2 antibody. (B) Multiple eNOS mutants were expressed in Hek293T cells and media was collected to determine the activity of eNOS by detecting nitrite levels with Sievers NOx Analyzer using the tri-iodide method. Activity of the different eNOS mutants was recorded and compared to eNOS wt after quantification of eNOS protein from cell lysates by ELISA. Error bars represent the standard error of the mean (SEM). A comparison between the groups was determined using an ANOVA test, while Tukey’s post-hoc test was performed for individual comparisons. Asterisks represent statistical significance (*p < 0.005; **p < 0.001). (C) eNOS proteins from HEK293T expression were immunoprecipitated followed by immunoblotting to determine the phosphorylation state of eNOS Ser-1177. As an input, cell lysate before immunoprecipitation was collected and subjected to SDS-PAGE and immunoblot analysis. As a control, immunoreactivity of the eNOS-1177P antibody was tested against the S1177A eNOS mutant and no reactivity was observed. The quantitation relative to eNOS wt is shown derived from 3 independent transfections.
To further determine the loss- and gain-of-function of Ser-615 phosphorylation on eNOS activity, we measured the activity of multiple eNOS mutants (Fig. 3B). The expression of the S615A mutant resulted in a 60% reduction of eNOS activity (p < 0.005) (n = 7), which was comparable to the S1177A mutant (~70% reduction in eNOS activity; p < 0.005)(n = 7). Interestingly, the S615D mutant demonstrated greater than a four-fold increase in activity (p < 0.001) (n = 20) and was similar to the activity increases for both S1177D (p < 0.001) (n = 3) and the S615D:1177D (p < 0.001) (n = 3) mutants (~3- and 5-fold, respectively), which have been reported previously [19,35]. Upon double mutation, the S615A:S1177A resulted in an almost complete inactivation of eNOS (n = 4). Having either S615A:S1177D (n = 4) or S615D:S1177A (n = 4) rescued some activity but not to the same degree as having the serine residues at the reciprocal positions. Collectively, these data indicate that phosphorylation of Ser-615 impacts phosphorylation of other sites in the molecule.
We next examined whether the mutation of Ser-615 to aspartic acid itself was responsible for the activity change or it induced other changes in eNOS. HEK 293T cells have high levels of Akt activity, which targets both Ser-615 and Ser-1177 phosphorylation. We, therefore, investigated whether the O-GlcNAc modification of eNOS at Ser-615 altered Ser-1177 phosphorylation. As expected, eNOS wt was phosphorylated at Ser-1177 (Fig. 3C). However, the S615A mutant resulted in a 40% loss in Ser-1177 phosphorylation compared to eNOS wt but a 35% increase in phosphorylation of Ser-1177 was observed with the S615D compared to eNOS wt (Fig. 3C). Altogether, these data indicate that the phosphorylation state of Ser-615 impacts the phosphorylation of Ser-1177 in eNOS and that O-GlcNAc modification of that site would prevent its phosphorylation and so decrease Ser-1177 phosphorylation. It is also possible that GlcNAc modification of Ser-615 could interfere Ser-1177 phosphorylation.
3.4. Increased O-GlcNAc modification of eNOS Ser-615 leads to a reduction in eNOS dimerization, which is crucial for eNOS function
Besides phosphorylation, it has been widely recognized that the monomer/dimer formation of eNOS is a major regulatory mechanism that controls eNOS activity and function. Using size exclusion chromatography, we demonstrated that the O-GlcNAc modified eNOS wt (co-expressed with sOGT) contains less stable dimers as compared to the unmodified protein (eNOS wt) (Fig. 4A). Co-expression of eNOS S615A mutant with sOGT gave a similar dimer/monomer ratio to that observed in the eNOS wt (unmodified) (Fig. 4A). These data suggest that the O-GlcNAc modification at Ser-615 alters eNOS conformation and affects the stability of the dimer.
Fig. 4.
Increased O-GlcNAc modification of eNOS Ser-615 leads to a reduction in eNOS dimerization, which is crucial for eNOS function. (A) Partially purified eNOS wt, eNOS wt co-expressed with OGT, or eNOS S615A co-expressed with OGT from bacteria were subjected to size exclusion chromatography as described in the methods. eNOS wt (green) or eNOS S615A co-expressed with OGT (blue) predominately fractionate as a dimer with very little monomer. eNOS wt co-expressed with OGT (red), however, fractionates with a higher abundance of monomer compared to the eNOS wt alone or eNOS S615A mutant. * denotes the ~80 kDa peak that corresponds to the cleaved eNOS reductase domain upon fractionation of eNOS wt co-expressed with OGT. Inset in (A) shows a representative western blot of the column fractions of both eNOS wt and eNOS S615A + OGT. (B) The heme spectra of eNOS wt (0.64uM) and eNOS S615A (0.23uM) co-expressed with OGT was obtained as described in the methods. eNOS S615A co-expressed with OGT (blue) exhibits a single similar peak at 445nm, which is also shown in the eNOS wt co-expressed with OGT(red). However, a second peak at 420 nm, indicative of monomeric eNOS, is also shown for eNOS wt co-expressed with OGT (red). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To further confirm our findings, we subjected variants of eNOS co-expression with OGT to heme spectral analysis looking at the difference in the ferrous heme-carbon monoxide (CO) spectrum. The eNOS S615A mutant co-expressed with OGT formed a single peak at 445-nm, which was indicative of homodimer formation (Fig. 4B) [36]. However, eNOS wt co-expressed with OGT produced peaks at 420-nm and 445-nm, suggesting an altered heme environment (Fig. 4B), that possibly represents a mixture of both O-GlcNAc modified (monomer; 420-nm) and unmodified (dimer; 445-nm) protein. Altogether, these data suggest that the eNOS protein dimer formation and activity can be reduced by the presence of the O-GlcNAc modification at the Ser-615 position.
4. Discussion
The pathology of PAH is strongly associated with the dysfunction of the pulmonary vascular endothelium and an imbalance of the production and release of nitric oxide. The precise mechanism of this decreased NO production is unclear. In PAH it is clear that abnormal glucose metabolism occurs. In this study, we showed that O-GlcNAc levels were augmented in the IPAH endothelium and primary endothelial isolates compared to controls. Interestingly, the increased O-GlcNAc levels in the IPAH endothelial cells was inversely proportional to Ser-1177 phosphorylation. We further demonstrated that the sustained O-GlcNAc modification on eNOS results in a dose-dependent decrease in NO generation (Fig. 1D), which would be consistent with the decreased production of NO observed in PAH patients.
In type II diabetes, a disease with known endothelial dysfunction, increased O-GlcNAc modification of eNOS has been observed [30,32]. In these reports, increased O-GlcNAc modification of eNOS and its concomitant decreased activity was also linked to reduced phosphorylation at Ser-1177, consistent with our results (Fig. 1F and G). These reports have also suggested that Ser-1177 was the main site of O-GlcNAc modification. We used a systematic O-GlcNAc site-mapping strategy by mass spectrometry and demonstrated that Ser-615 is the primary site of O-GlcNAc (Fig. 2A and B). We also showed that co-expression of the wild-type eNOS with sOGT resulted in reduced dimer formation, which was not observed when eNOS S615A was co-expressed with OGT or when wild-type eNOS was expressed by itself (Fig. 4A). This finding was confirmed with heme spectra of the eNOS wt or eNOSS615A co-expressed with sOGT (Fig. 4B).
It is known that dimer formation is essential for eNOS function and is a mode of activity regulation. We could not purify enough eNOS from mammalian cells to determine the O-GlcNAc modification site. However, using O-GlcNAc immunoprecipitation, we demonstrated that both eNOS 1177A and 615A are less modified when expressed in HEK273T cells (Fig. 3A). The eNOS S615D mutation appeared to show a larger decrease (Fig. 3A). The protein determinant required for modification by OGT has not been fully characterized. It is apparent, however, that adjacent sites can be modified by O-GlcNAc. In OGT, we observe numerous sites of modification with many that are adjacent to each other, which suggests low specificity for the target site (data not shown). Therefore, it is likely that if Ser-615 is unavailable then adjacent sites (Ser-617 or Ser-619) may be utilized albeit with lower affinity. This would explain the residual modification on the eNOS S615A mutants. However, it is possible that some sites of modification may have been missed by our analysis and so the residual activity may be due to these unaffected residues.
The eNOS S615D mutant may deter the targeting of O-GlcNAc to that region due to the presence of the negative charge. Consistent with our results, previous reports demonstrate that eNOS mutant S1177A caused a reduction of O-GlcNAc levels on eNOS but did not completely abolish the modification [32]. This reports suggested that Ser-1177 as is the site of O-GlcNAc modification. However, Devika et al. showed the presence of intramolecular interactions between Ser-1177 and other major serine residues [37]; and altering these could have profound effects on the overall accessibility of these residues.
Ser-615 lies in the auto-inhibitory loop of eNOS [38]. From the structure, it is clear that the auto-inhibitory loop needs to move to allow for protein activity. We believe that phosphorylation of either Ser-615 altered the position and accessibility of Ser-1177 and vice-versa. Hence, mutating Ser-1177 to Alanine may alter accessibility of Ser-615 and thereby reduce its modification by O-GlcNAc. We explored the gain- and loss-of-function of eNOS through mutagenesis of several key serine residues that are known to receive phosphorylation. Our data clearly show a decrease in NO generation in the eNOS S1177A or S615A mutants suggesting its importance (Fig. 3B). With the eNOS S615A mutant, we also observed decreased phosphorylation of Ser-1177 (Fig. 3C), whereas, the eNOS S615D mutant increased eNOS activity (Fig. 3B). This finding was consistent with Tran and colleagues [39] where S615D or S1177D were more active than the wild-type protein, likely due to the reduced Ca2+ requirement for calmodulin binding [35]. Interestingly, loss of Ser-615 phosphorylation in the S615A:S1177D double mutant resulted in a lower activity than the S1177D alone and S615D:S1177D double mutant lead to maximal activity of eNOS. These combined results suggest that phosphorylation of Ser-615 impacts the Ser-1177 modification state. We postulate that the modification state of Ser-615 is a ‘checkpoint’ that may affect multiple regulatory mechanisms (i.e., phosphorylation of Ser-1177 and eNOS dimerization) of eNOS activity.
In this paper, our data puts forth a previously unidentified control mechanism of eNOS through the O-GlcNAc modification of Ser-615. The O-GlcNAc modification of eNOS at Ser-615, through increased glucose uptake and shunt into the hexosamine biosynthetic pathway, alters the phosphorylation state of Ser-1177. These findings have broader implications for diseases where glucose metabolism/uptake is altered such as pulmonary hypertension, diabetes, and cancer. This therefore represents a new target whereby inhibition of O-GlcNAc may augment eNOS activity.
Author contributions
These authors helped in designing research studies (KSA, JWB, RAD); conducting experiments (KSA, JWB LT, NEM, MMH, BW, LL, SCC); analyzing data (KSA, JWB LT, NEM, MMH, BW, LL, DSJ, RAD); providing reagents (MMH, SCC, DSJ); and writing/editing the manuscript (KSA, JWB LT, NEM, MMH, BW, LL, SCC, DSJ, RAD).
Declaration of competing interest
The authors have declared that no conflict of interest exists.
Acknowledgments
This work was supported by the National Institutes of Health (R00HL131866 to J.W.B. and R01HL130209 to R.A.D.).The Fusion Lumos instrument was purchased via an NIH shared instrument grant, 1S10OD023436-01.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101625.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Dweik R.A., Rounds S., Erzurum S.C., Archer S., Fagan K., Hassoun P.M. An official American Thoracic Society Statement: pulmonary hypertension phenotypes. Am. J. Respir. Crit. Care Med. 2014;189(3):345–355. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dweik R.A., Erzurum S.C. Update on pulmonary vascular diseases 2010. Am. J. Respir. Crit. Care Med. 2011;184(1):26–31. doi: 10.1164/rccm.201103-0394UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rondelet B., Kerbaul F., Van Beneden R., Motte S., Fesler P., Hubloue I. Signaling molecules in overcirculation-induced pulmonary hypertension in piglets: effects of sildenafil therapy. Circulation. 2004;110(15):2220–2225. doi: 10.1161/01.CIR.0000143836.40431.F5. [DOI] [PubMed] [Google Scholar]
- 4.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko F.T., Arroliga A.C., Dweik R.A., Comhair S.A., Laskowski D., Oppedisano R. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1998;158(3):917–923. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- 6.Cremona G., Higenbottam T., Borland C., Mist B. Mixed expired nitric oxide in primary pulmonary hypertension in relation to lung diffusion capacity. QJM. 1994;87(9):547–551. [PubMed] [Google Scholar]
- 7.Girgis R.E., Champion H.C., Diette G.B., Johns R.A., Permutt S., Sylvester J.T. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am. J. Respir. Crit. Care Med. 2005;172(3):352–357. doi: 10.1164/rccm.200412-1684OC. [DOI] [PubMed] [Google Scholar]
- 8.Steudel W., Ichinose F., Huang P.L., Hurford W.E., Jones R.C., Bevan J.A. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ. Res. 1997;81(1):34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Steudel W., Scherrer-Crosbie M., Bloch K.D., Weimann J., Huang P.L., Jones R.C. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J. Clin. Invest. 1998;101(11):2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagan K.A., Morrissey B., Fouty B.W., Sato K., Harral J.W., Morris K.G., Jr. Upregulation of nitric oxide synthase in mice with severe hypoxia-induced pulmonary hypertension. Respir. Res. 2001;2(5):306–313. doi: 10.1186/rr74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghofrani H.A., Humbert M., Langleben D., Schermuly R., Stasch J.P., Wilkins M.R., Klinger J.R. Riociguat: mode of action and clinical development in pulmonary hypertension. Chest. 2016;151(2):468–480. doi: 10.1016/j.chest.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Wardle A.J., Seager M.J., Wardle R., Tulloh R.M., Gibbs J.S. Guanylate cyclase stimulators for pulmonary hypertension. Cochrane Database Syst. Rev. 2016;8:CD011205. doi: 10.1002/14651858.CD011205.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowles R.G., Moncada S. Nitric oxide synthases in mammals. Biochem. J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiss E.H., Dirsch V.M. Regulation of eNOS enzyme activity by posttranslational modification. Curr. Pharmaceut. Des. 2014;20(22):3503–3513. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W., Koeck T., Lara A.R., Neumann D., DiFilippo F.P., Koo M. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104(4):1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes J.W., Tian L., Heresi G.A., Farver C.F., Asosingh K., Comhair S.A. O-linked beta-N-acetylglucosamine transferase directs cell proliferation in idiopathic pulmonary arterial hypertension. Circulation. 2015;131(14):1260–1268. doi: 10.1161/CIRCULATIONAHA.114.013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardiville S., Hart G.W. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metabol. 2014;20(2):208–213. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slawson C., Hart G.W. O-GlcNAc signalling: implications for cancer cell biology. Nat. Rev. Canc. 2011;11(9):678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulton D., Gratton J.P., McCabe T.J., Fontana J., Fujio Y., Walsh K. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarus M.B., Nam Y., Jiang J., Sliz P., Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469(7331):564–567. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adak S., Aulak K.S., Stuehr D.J. Chimeras of nitric-oxide synthase types I and III establish fundamental correlates between heme reduction, heme-NO complex formation, and catalytic activity. J. Biol. Chem. 2001;276(26):23246–23252. doi: 10.1074/jbc.M102509200. [DOI] [PubMed] [Google Scholar]
- 22.Samouilov A., Zweier J.L. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal. Biochem. 1998;258(2):322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 23.Dweik R.A., Laskowski D., Abu-Soud H.M., Kaneko F., Hutte R., Stuehr D.J. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J. Clin. Invest. 1998;101(3):660–666. [Google Scholar]
- 24.Dweik R.A., Comhair S.A., Gaston B., Thunnissen F.B., Farver C., Thomassen M.J. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl. Acad. Sci. U. S. A. 2001;98(5):2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aulak K.S., Koeck T., Crabb J.W., Stuehr D.J. Proteomic method for identification of tyrosine-nitrated proteins. Methods Mol. Biol. 2004;279:151–165. doi: 10.1385/1-59259-807-2:151. [DOI] [PubMed] [Google Scholar]
- 27.Aulak K.S., Miyagi M., Yan L., West K.A., Massillon D., Crabb J.W. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc. Natl. Acad. Sci. U. S. A. 2001;98(21):12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma B., Zhang K., Hendrie C., Liang C., Li M., Doherty-Kirby A. PEAKS: powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003;17(20):2337–2342. doi: 10.1002/rcm.1196. [DOI] [PubMed] [Google Scholar]
- 29.Aytekin M., Comhair S.A., de la Motte C., Bandyopadhyay S.K., Farver C.F., Hascall V.C. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295(5):L789–L799. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musicki B., Kramer M.F., Becker R.E., Burnett A.L. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2005;102(33):11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beleznai T., Bagi Z. Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation. Vasc. Pharmacol. 2012;56(3-4):115–121. doi: 10.1016/j.vph.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du X.L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Invest. 2001;108(9):1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gellai R., Hodrea J., Lenart L., Hosszu A., Koszegi S., Balogh D. The role of O-linked N-acetylglucosamine modification in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016;311(6):F1172–F1181. doi: 10.1152/ajprenal.00545.2015. [DOI] [PubMed] [Google Scholar]
- 34.Long Y., Yan J., Luo S., Liu Z., Xia Y. Measurement of O-GlcNAcylated endothelial nitric oxide synthase by using 2',5'-ADP-Sepharose pull-down assay. Anal. Biochem. 2017;537:8–12. doi: 10.1016/j.ab.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 35.McCabe T.J., Fulton D., Roman L.J., Sessa W.C. Enhanced electron flux and reduced calmodulin dissociation may explain "calcium-independent" eNOS activation by phosphorylation. J. Biol. Chem. 2000;275(9):6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 36.Panda K., Rosenfeld R.J., Ghosh S., Meade A.L., Getzoff E.D., Stuehr D.J. Distinct dimer interaction and regulation in nitric-oxide synthase types I, II, and III. J. Biol. Chem. 2002;277(34):31020–31030. doi: 10.1074/jbc.M203749200. [DOI] [PubMed] [Google Scholar]
- 37.Devika N.T., Amresh P., Hassan M.I., Ali B.M. Molecular modeling and simulation of the human eNOS reductase domain, an enzyme involved in the release of vascular nitric oxide. J. Mol. Model. 2014;20(10):2470. doi: 10.1007/s00894-014-2470-7. [DOI] [PubMed] [Google Scholar]
- 38.Garcin E.D., Bruns C.M., Lloyd S.J., Hosfield D.J., Tiso M., Gachhui R. Structural basis for isozyme-specific regulation of electron transfer in nitric-oxide synthase. J. Biol. Chem. 2004;279(36):37918–37927. doi: 10.1074/jbc.M406204200. [DOI] [PubMed] [Google Scholar]
- 39.Tran Q.K., Leonard J., Black D.J., Nadeau O.W., Boulatnikov I.G., Persechini A. Effects of combined phosphorylation at Ser-617 and Ser-1179 in endothelial nitric-oxide synthase on EC50(Ca2+) values for calmodulin binding and enzyme activation. J. Biol. Chem. 2009;284(18):11892–11899. doi: 10.1074/jbc.M806205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.