Highlights
-
•
Classic circuitry models of PTSD were evaluated alongside a new network-based model.
-
•
Functional connectivity literature is reviewed to evaluate both circuitry models.
-
•
Functional network literature in PTSD is reviewed to evaluate both circuitry models.
-
•
Global and network patterns of altered functional connectivity in PTSD are revealed.
Keywords: Posttraumatic stress disorder, Functional neuroimaging, Seed-based functional connectivity, Functional brain networks
Abstract
Classical neural circuitry models of posttraumatic stress disorder (PTSD) are largely derived from univariate activation studies and implicate the fronto-limbic circuit as a main neural correlate of PTSD symptoms. Though well-supported by human neuroimaging literature, these models are limited in their ability to explain the widely distributed neural and behavioral deficits in PTSD. Emerging interest in the application of large-scale network methods to functional neuroimaging provides a new opportunity to overcome such limitations and conceptualize the neural circuitry of PTSD in the context of network patterns. This review aims to evaluate both the classical neural circuitry model and a new, network-based model of PTSD neural circuitry using a breadth of functional brain organization research in subjects with PTSD. Taken together, this literature suggests global patterns of reduced functional connectivity (FC) in PTSD groups as well as altered FC targets that reside disproportionately in canonical functional networks, especially the default mode network. This provides evidence for an integrative model that includes elements of both the classical models and network-based models to characterize the neural circuitry of PTSD.
1. Introduction
More than two decades’ worth of traditional functional neuroimaging research has concentrated on understanding the influence of the brain’s structure and activity on emotion, behavior, and psychopathology, including posttraumatic stress disorder (PTSD). Early neuroimaging research implicated structural changes in the gray matter of brain regions including the hippocampus and prefrontal cortex as key components of the neurocircuitry of PTSD (Karl et al., 2006, Kitayama et al., 2005, Kühn and Gallinat, 2013, Li et al., 2014, Meng et al., 2014, Pitman et al., 2012). As functional magnetic resonance imaging (fMRI) techniques developed, analytic approaches examining univariate activation patterns of isolated brain regions began to contribute to the understanding of PTSD and have resulted in a widely accepted general neurocircuitry model of the disorder. The following review provides a detailed history of the development of “classical” neurocircuitry models in PTSD, beginning with univariate models and moving towards expansion of the classical model developed from bivariate circuitry investigations. Next, a discussion of the limitations of uni- and bivariate models for understanding neural deficits in PTSD follows, along with considerations for how reconceptualizing the brain through an integrative network framework may overcome such limitations. Section two contains a comprehensive review of existing bivariate neurocircuitry studies in PTSD populations, throughout which we evaluate support for both classical and network models from the evidence provided by bivariate investigations. Finally, we close with a review of studies from the nascent field of network neuroimaging in PTSD, again evaluating evidence for classical and network models from these studies and discussing future directions for neurocircuitry investigations in PTSD.
1.1. Classical models of PTSD neural circuitry (univariate models)
Several groups have proposed models of PTSD neural circuitry with varying degrees of complexity (Table 1). In 2006, two reviews forming the basis of the classical model of PTSD neural circuitry were published by an overlapping group of researchers, the first by Shin, Rauch, and Pitman (Shin et al., 2006) and the second by Rauch, Shin, and Phelps (Rauch et al., 2006). Both models highlight the role of the amygdala, hippocampus, and medial prefrontal cortex (mPFC; including the middle frontal gyrus, anterior cingulate cortex, and subcallosal cortex) in the canonical “fear circuit” and specify how alterations in the activity of these regions characterize neural deficits in PTSD. In the Shin model (Shin et al., 2006), it is argued that PTSD neural circuitry is characterized by hyperactivity of the amygdala and failure to activate the mPFC during trauma- and non-trauma related fearful cue presentations, and suggests a potential link between these two regions as an important factor in PTSD neural circuitry. The role of the hippocampus is less defined in this model, though the authors cite evidence of failure to activate the region during cognitive tasks and greater hippocampal activity at baseline in PTSD, potentially indicative of altered stimuli encoding and recollection. The researchers’ other model (Rauch et al., 2006) is consistent with this Shin model, emphasizing heightened reactivity of the amygdala during threatening cues, fear conditioning, and trauma reminders as well as failure to activate the mPFC and hippocampus during emotional Stroop and explicit memory tasks in individuals with PTSD. Here, however, the authors suggest that this altered circuitry contributes to fear learning deficits in PTSD, including enhanced acquisition of fear responses to conditioned stimuli and failure to extinguish learned fears. Though the authors present these models as specific to PTSD and not trauma exposure or symptom severity, per se, the authors discuss a need for future research to determine whether the abnormalities represented in this model are also related to symptoms and trauma exposure.
Table 1.
Characteristic features of classical neural circuitry models of PTSD. mPFC – medial prefrontal cortex; vmPFC – ventral medial prefrontal cortex; dmPFC – dorsal medial prefrontal cortex; dACC – dorsal anterior cingulate cortex; MFG – middle frontal gyrus; PCC – posterior cingulate cortex; R – right hemisphere; L – left hemisphere. Regions that are implicated in more than one model are denoted in italics.
| Model | Hyperactive Regions | Characteristic Deficits – Hyperactivity | Hypoactive Regions | Characteristic Deficits – Hypoactivity | Other Regions of Interest |
|---|---|---|---|---|---|
| Shin et al., 2006 | Amygdala | Not speculated | mPFC | Not speculated | Hippocampus |
| Rauch et al., 2006 | Amygdala | Enhanced acquisition of fear responses |
mPFC Hippocampus |
Failure to extinguish learned fears | |
| Pitman et al., 2012 |
Amygdala dACC Insula |
Enhanced acquisition of conditioned fear; heightened detection of bodily arousal | vmPFC | Failure to regulate amygdala, leading to attentional bias to threat, impaired extinction and emotional regulation, and enhanced fear responses | Hippocampus |
| Patel et al., 2012 |
L Amygdala Anterior Insula R Hippocampus L Putamen L Precuneus R MFG R Fusiform R Postcentral |
Enhanced fear conditioning that interferes with extinction learning; Intrusive trauma recollections |
R mPFC L Angular Gyrus R PCC L Supramarginal Gyrus L MFG Precentral Gyrus R Caudate |
Impaired top-down emotional regulation | |
| Admon et al., 2013 |
Amygdala dACC Insula |
Enhanced fear expression and interoception; hyperarousal; predisposing risk factor |
vmPFC Hippocampus |
Impaired fear inhibition; re-experiencing and avoidance symptoms; acquired risk factor | dmPFC Nucleus Accumbens |
| Liberzon and Abelson, 2016 | dACC | Enhanced cue-elicited fear responses |
vmPFC Hippocampus |
Deficits in context processing, triggering generalized emotional reactions; failures in safety learning | Thalamus |
Several years later, Pitman et al., 2012, Patel et al., 2012 added depth to the 2006 models of PTSD neural circuitry by publishing a review of findings from biological studies of PTSD and a meta-analysis of functional neuroimaging studies, respectively. The Pitman model (Pitman et al., 2012) supports the previous two models’ assertion that PTSD is characterized by exaggerated activity of the amygdala and reduced activity of the mPFC to trauma-related and threatening cues during fear conditioning and extinction. Additionally, however, the Pitman model notes differing activity profiles within regions of the mPFC in PTSD that were not delineated in the previous models. Specifically, they state that decreased activation of the vmPFC during both trauma- and non-trauma related stimuli presentation and increased activation of the dACC are evident during recall of extinction learning and cognitive tasks. Here, the authors suggest that a specific failure in activation of the vmPFC is associated with failure to recall extinction learning and reduced symptom improvement during cognitive behavioral therapy. Conversely, the authors posit that dACC activation during similar tasks is positively associated with PTSD symptom severity and fear expression. Additionally, the Pitman model implicates a role for the insula in PTSD neural circuitry, suggesting a positive association between insular cortex activation and PTSD symptom severity. The authors note, however, that this relationship may be characteristic of anxiety disorders generally and non-specific to PTSD. The final component of the Pitman neural circuitry model is the hippocampus which, similarly to the earlier models, has an unclear role in the neural profile of PTSD, with some evidence for enhanced activation during task and some evidence for reduced activation. Taken together, the Pitman model characterizes the neural circuity of PTSD as three regions of hyperactivation (amygdala, insular cortex, and dACC) and a hypoactive vmPFC, all of which work in a circuit underlying characteristic deficits in PTSD, including attentional bias towards threat, failures in extinction, and poor emotional regulation. Interestingly, the Pitman model also suggests that amygdala hyperactivity and mPFC hypoactivity is also correlated with PTSD symptom severity, adding a consideration for the role of symptoms in alterations within neural circuits.
The Patel model, which was based on the results of a meta-analysis of functional activation studies in trauma and PTSD, similarly implicates the left amygdala and bilateral anterior insula as reliably hyperactive clusters in PTSD in comparison to non-trauma exposed controls on a variety of tasks and the right mPFC as a reliably hypoactive cluster (Patel et al., 2012). Additional clusters of hyperactivity in PTSD groups compared to non-trauma exposed controls from this meta-analysis include the right hippocampus, left putamen, left precuneus, right middle frontal gyrus (MFG), right fusiform gyrus, and right postcentral gyrus. Additional clusters of hypoactivity include the left angular gyrus, right posterior parietal cortex (PCC), left supramarginal gyrus, left MFG, bilateral precentral gyrus, and right caudate. The Patel model also includes a consideration of the specific effect of PTSD symptoms by reporting a comparison of PTSD groups with trauma-exposed controls. Interestingly, the authors note that the dACC, precuneus, and middle temporal lobe were hyperactive in PTSD compared to trauma-exposed controls, suggesting that hyperactivity in some regions may be distinct to PTSD diagnosis and reflective of the other models of PTSD neural circuitry. Regions of hypoactivity in PTSD compared to trauma-exposed controls were also consistent with the other models and included the right mPFC, left parahippocampal gyrus, inferior and middle frontal gyri, dACC, and orbital frontal gyrus. While the Patel model supports the earlier models and the Pitman model, the finding of additional significant clusters outside of the fear circuit indicates neural factors external to the classical circuit that may influence neural deficits in PTSD.
Though the above models provide support for alterations in the fear circuit as characteristic deficits in PTSD neural circuitry, two remaining models add depth and context to the classical understanding of fear-related neural processing in PTSD. In 2013, Admon and colleagues (Admon et al., 2013) combined twin pair, environmental, and genetic studies to investigate both predisposing and acquired neural factors for vulnerability to PTSD and put forth a causal neural circuitry model of PTSD. Here, the authors review support for the classical models outlined above, noting that hyperactivity of the amygdala, dACC, and insula is related to enhanced fear expression and hyperarousal in PTSD. Additionally, this model holds that hypoactivity of the hippocampus and vmPFC is associated with deficits in fear inhibition and re-experiencing and avoidance symptoms. Interestingly, the authors suggest that regional hyperactivity, along with genetic profiles and life experiences are predisposing factors for the development of PTSD, whereas regional hypoactivity is an acquired factor that enhances risk vulnerability for PTSD. One of the strengths of the Admon model is the consideration of predisposing factors, including trauma exposure throughout the lifespan, separate from PTSD diagnosis and symptoms. This is a unique feature of the typical neural circuitry models of PTSD and provides some nuance to the distinct effects of trauma exposure from clinical symptoms.
Finally, a recent model put forth by Liberzon and Abelson conceptualizes PTSD neural circuitry through the lens of context processing (Liberzon and Abelson, 2016). The authors highlight the mPFC-hippocampus circuit as critical to context processing in PTSD, and that deficits in this circuit may explain characteristic symptoms of PTSD, such as generalized and situationally-inappropriate fear reactions (Kaczkurkin et al., 2017, Lopresto et al., 2016), failures in safety learning, and hyperarousal to fear cues (Duits et al., 2015, Norrholm et al., 2011, Pole, 2007, Wessa and Flor, 2007). Similar to the Pitman and Admon models, this Deficient Context Processing model delineates the mPFC into the dACC and vmPFC, which have opposing roles in deficient neural processing in PTSD (vmPFC is hyporeactive and dACC is hyperreactive). In contrast to all other classical models described above, the Deficient Context Processing model does not highlight hyperactivity of the amygdala as being characteristic of context processing deficits in PTSD. While the authors suggest that the amygdala may be involved in a deficient context processing circuit, explicit suggestion of its hyperactivity of this region driving PTSD-related symptomatology is missing from this model’s conceptualization. The Deficient Context Processing model’s characterization of PTSD neural circuitry exemplifies a general shift away from the domain-specific conceptualization of PTSD as a disorder of fear processing and towards a wider picture of higher-level neural and behavioral deficits.
1.2. Expansion of classical models of PTSD neural circuitry (bivariate functional connectivity)
While univariate activation studies previously dominated the field of neuroimaging research in PTSD, advancement in the development of neuroimaging techniques has allowed for investigations of coordinated activity and functional connectivity (FC) in neural circuits and how abnormal connectivity patterns are related to PTSD psychopathology. Thus, the traditional understanding of PTSD as a disorder characterized by altered univariate activation of isolated brain regions has been expanded to include alterations in bivariate FC between the regions of interest (ROIs) implicated in the classical model. Importantly, FC studies introduced resting-state brain organization into neural models of PTSD, as the methods utilized in bivariate FC analyses are not dependent on task-related voxelwise activation (Fox and Raichle, 2007). Understanding the brain’s organization during resting-state is essential because organization of functional networks during rest is hypothesized to support cognitive function (van den Heuvel and Hulshoff Pol, 2010), is highly correlated to functional organization during cognitive tasks (Bullmore and Sporns, 2012, Bullmore and Sporns, 2009), and is related to underlying structural connectivity networks (Bullmore and Sporns, 2009, Chen et al., 2008). Additionally, resting-state fMRI paradigms are relatively standardized and unconfounded by individual or group differences in task performance and thus may be more generalizable across other resting-state studies than task-based paradigms. The inclusion of resting-state neural circuitry into models of PTSD helps to identify intrinsic neural patterns that may contribute to the deficits outlined by the classical model. For example, bivariate resting-state studies implicate reduced FC between the amygdala and medial regions of the prefrontal cortex as a characteristic neural deficit in PTSD patients (Admon et al., 2013, Koch et al., 2016), in line with the univariate findings that formed the classical model and suggestive of alterations in baseline functional organization absent the influence of emotionally provocative stimuli. A detailed review of bivariate FC investigations in PTSD can be found in Section 2.
1.3. Limitations of classical and bivariate models of PTSD neural circuitry
The neural models of PTSD described above generally focus on isolated nodes of the brain or FC between two nodes within a circuit using the two most widely used neuroimaging analytic techniques: voxelwise functional activation and voxelwise seed-based FC analyses. The former approach assumes each brain region (e.g., amygdala) acts as an isolated unit, whereas the latter approach assumes there are only bivariate relationships between regions (e.g., amygdala-mPFC connectivity) that also operate in isolation from other bivariate relationships. As such, circuit- and seed-based approaches have considerable assumptions and limitations. Such limitations include the need to rely on a priori selection of brain regions for connectivity analyses rather than a more data-driven method (Cole et al., 2010, Tomasi and Volkow, 2010), inconsistency in node definition (Sohn et al., 2015), and ambiguity in neural activity outside of direct connectivity patterns between nodes or seeds of interest (van den Heuvel and Hulshoff Pol, 2010). Contemporary work is identifying some of these inconsistencies in FC research in PTSD, and findings suggest that the role of the amygdala and prefrontal regions, especially the vmPFC, may only be part of story (Koenigs and Grafman, 2009). For example, whereas PTSD neurocircuitry models based on univariate neuroimaging data would predict that vmPFC lesions should increase risk for PTSD, research in a large cohort of combat veterans demonstrated that vmPFC lesions actually decreased risk for PTSD (Koenigs et al., 2008), consistent with the view that focusing on isolated regions of interest is only one element of a wider picture. Similarly, other studies have found evidence for increased connectivity between the amygdala and prefrontal regions in trauma-exposed individuals (Thomason et al., 2015, Zhang et al., 2016, Zielinski et al., 2018), which contradicts and brings into question the classic neural circuitry model of PTSD, in which the vmPFC plays a role in top-down control over the amygdala. An additional limitation is that the studies forming the classical model of PTSD neural circuitry rely on data from adult participants with trauma exposure; thus, it is unclear what the role of development may be in the neural circuitry of pediatric PTSD.
1.4. Characterizing large-scale brain organization with networks
A more contemporary approach than the classical models to understanding the functionality of the human brain in health and disease conceptualizes the brain as an interconnected network of functional components that work as coordinated, rather than discrete, units. The recent inclusion of large-scale brain organization methods into the functional neuroimaging literature attempts to redefine the brain as a coordinated, dynamic graph with distinct functional components rather than a series of circuits functioning in isolation. Using network neuroscience methods, investigators can address the limitations of uni- and bivariate approaches, namely the narrowed focus on single brain regions or circuits in isolation, by examining large-scale patterns of connectivity in health and psychopathology. Conceptualizing the brain as a network allows for the testing of specific hypotheses about the brain on a hierarchical scale, from individual ROI connectivity up to whole-brain organization. Network neuroscience approaches that broaden the definition of brain function and connectivity have been employed in research on emotions (Pessoa, 2018), problem-solving (Ogawa et al., 2018), and intelligence (van den Heuvel et al., 2009) in subjects without psychopathology, as well as in disorders such as schizophrenia (Bordier et al., 2018, van den Heuvel and Fornito, 2014, Jafri et al., 2008, Ohta et al., 2018, Yu et al., 2012), major depression (Ye et al., 2015, Zhang et al., 2011), and autism (Rashid et al., 2018). These studies and others utilize tools, including Independent Component Analysis (ICA) and graph theory principles, to characterize the brain’s intercorrelations into meaningful units of connectivity. ICA isolates individual functional networks within the brain, and graph theory methods examine properties of networks, namely nodes and edges of a graph, and characterize these elements based on their intercorrelations. Analysis methods such as ICA and graph theory allow for testing hypotheses about the brain as a coordinated network of connectivity relationships, which can be altered in psychopathology. Further details of these methods are discussed in Sections 3.1 and 3.2, the Supplemental Material, and are depicted in Fig. 1.
Fig. 1.
Schematics of different functional organization methods discussed in review. The following review discusses four central methods for investigating functional organization with fMRI. A) Univariate voxelwise methods for ROI activation during task; B) Bivariate, seed-based functional connectivity for circuitry investigations during tasks and at rest; C) Independent Component Analysis for network structure and connectivity at rest and during task; and D) Graph Theory principles for determining node-, network-, and whole-brain level functional organization during task and at rest.
While several large-scale brain networks have been identified and studied in the context of mental health disorders, three canonical networks appear to play a particularly important role in cognitive function and dysfunction, and therefore are implicated in the “Triple Network Model” of psychopathology (Menon, 2011). These networks, referred to as the central executive network (CEN), salience network (SN), and default mode network (DMN), are commonly coupled both during tasks and at rest (Menon, 2013, Menon, 2011). The CEN is anchored in the dorsal lateral prefrontal cortex and posterior parietal cortex and plays a role in working memory, executive functioning, cognitive control, error encoding, goal-directed behavior, and attention (Bressler and Menon, 2010, Dosenbach et al., 2007, Seeley et al., 2007). The SN is anchored in the anterior insular cortex and dACC and is important for detecting and mapping internally and externally salient events, as well as dynamic switching between the CEN and DMN to meet extrinsic cognitive demands (Bressler and Menon, 2010, Menon and Uddin, 2010). Finally, the DMN is anchored in the PCC and mPFC and is important for self-referential mental activity, regulation of emotional state, and recollection of previous experience (Menon, 2011, Raichle, 2015). Disruptions in connectivity structure or activation profiles of these key networks may underlie the uni- and bivariate dysconnectivity observed in PTSD. Indeed, the classical univariate models of PTSD neural circuitry implicate altered activity of several of the nodes of the SN and DMN in PTSD symptom presentation. As stated above, classical models suggest that hyperactivity and hyperarousal symptoms are associated with increased activation of the amygdala and dACC, both of which are key nodes of the SN. Similarly, classical models suggest that re-experiencing symptoms and deficits in emotional regulation and fear extinction are associated with reduced activation of the hippocampus and vmPFC, both of which are nodes of the DMN. Though the CEN is thought to facilitate control of spatial attention (Scolari et al., 2015, Szczepanski et al., 2010) and emotional regulation (Sripada et al., 2014, Wessing et al., 2013), and cognitive impairments in PTSD patients including attentional bias to threat and deficits in emotional regulation are well-established (Koster et al., 2006, Sippel and Marshall, 2013), classical models of PTSD neural circuitry generally neglect to include CEN nodes. Only one model (Patel et al., 2012) highlights the observation of reduced functional activation of nodes within the CEN, including the inferior frontal gyrus and middle frontal gyrus, in PTSD neural circuitry. Because CEN nodes are often not considered within the classical neural circuitry model of PTSD, investigations of the role of the CEN within PTSD neural circuitry are limited. Thus, an integrated approach that includes classical and network-based models may be merited.
1.5. Purpose and predictions for review
The classical voxelwise activation model of PTSD neural circuitry has a wide body of supportive evidence from early functional neuroimaging studies of affective disorders. However, as research methods have advanced, reconceptualization of the brain as a coordinated network of intercorrelated units has challenged the interpretability of the classical model. Thus, the overarching goal of this review is to evaluate both the classical model, largely derived from univariate activation, and a new, network-based model of PTSD neural circuitry using a breadth of functional connectivity research in subjects with PTSD, including bivariate seed-based investigations and network-based approaches. It is relevant to mention that the classical model and network-based models operate at different resolutions; the classical model operates at a finer spatial resolution and attempts to explain specific activation or connectivity patterns within only a handful of neural structures. By contrast, network-based models by definition attempt to explain activation and connectivity patterns that are distributed across the brain. As such, the ultimate goal would be to combine and integrate knowledge and predictions from both models to a more comprehensive understanding of PTSD neural circuitry. Though no single neural circuitry model will explain every unique case of PTSD, the classical and network models of PTSD will be evaluated on predictions about what patterns might be observed in the human imaging literature on PTSD based on the central themes of each model. For example, if the central themes of the classical model are a reliable characterization of PTSD neural circuitry, patient groups will demonstrate altered FC mainly within the fronto-limbic circuit, represented by reduced FC from the mPFC to subcortical limbic regions and enhanced FC from the amygdala to the insula and dACC.
Conversely, if the central themes of a network model accurately characterize PTSD neural circuitry, patient groups will demonstrate patterns of altered organization on a global scale throughout the brain and within canonical networks that are implicated in psychopathology. Firstly, as the vmPFC and hippocampus are central nodes of the DMN (Menon, 2011, Raichle, 2015) and reduced connectivity of these nodes are thought to be associated with characteristic symptoms such as re-experiencing and difficulty in fear extinction and emotional regulation (Admon et al., 2013, Liberzon and Abelson, 2016, Pitman et al., 2012), a network-based model predicts that the DMN will demonstrate overall reduced connectivity in subjects with PTSD. Similarly, due to the CEN’s role in facilitating attention and emotional control, both of which are impaired in PTSD (Koster et al., 2006, Sippel and Marshall, 2013), and evidence suggesting reduced activity in important nodes of the CEN in PTSD (Patel et al., 2012), a network-level characterization of PTSD neural circuitry predicts reduced connectivity of the CEN. Finally, characteristic symptoms of PTSD including hyperarousal and enhanced acquisition of fear suggest hyperactivity of the key nodes of the SN, including the amygdala, anterior insula, and dACC (Admon et al., 2013, Bressler and Menon, 2010, Liberzon and Abelson, 2016, Menon and Uddin, 2010, Pitman et al., 2012); thus, a network-level characterization of PTSD neural circuitry predicts hyperconnectivity of the SN in PTSD patients.
To evaluate these models, we first present a comprehensive review of bivariate FC findings in subjects with trauma exposure and PTSD, covering over ten years of research on neural dysfunction in PTSD. We chose to utilize bivariate investigations of FC in PTSD because the seed-based FC method bridges the gap between univariate activation and network-based studies, therefore providing ample evidence to evaluate both potential models. In this section, we examine widespread FC patterns as revealed through qualitatively considering together results from bivariate FC studies. Second, we review the extant literature on altered large-scale neural network connectivity in PTSD from network-level computational methods in order to evaluate support for both models. Finally, we close with a discussion of implications for neural models of PTSD and considerations for future directions for functional brain organization research in affective psychopathology.
2. Comprehensive review of studies utilizing seed-based functional connectivity in PTSD
Seed-based functional connectivity (sbFC) is an analytical technique designed to investigate correlations in functional activity between a chosen brain region (i.e. “seed”) and the rest of the brain or a priori chosen brain regions (i.e. “targets”). This flexible analysis method can be used to determine functional connectivity (FC) patterns at rest and during tasks by producing correlation maps between the seed region and every other voxel in the brain (Fox et al., 2005) to evaluate synchronous brain activity. FC between a chosen seed and its targets represents the temporal correlation of activity between spatially distinct brain regions that may be altered in disease states (Cordes et al., 2000, Fox and Raichle, 2007). sbFC has been used in PTSD research for several years as a primary method for investigating altered bivariate connectivity patterns in the disorder; however, ambiguity in seed and target assignment along with the need to select a priori seeds of interest are substantial limitations of sbFC approaches. While using whole-brain analyses to examine seed FC to all voxels in the brain removes some experimenter bias in target selection, individual studies implicating a bivariate connection are unable to provide information on how the brain is behaving or organized outside of that connection, preventing meaningful interpretations of alterations outside of bivariate circuits. These methodological limitations result in a restricted understanding of the neural circuitry of PTSD, with researchers relying on classical models of univariate activation that implicate mainly fronto-limbic circuitry, to select seeds and targets. Despite the limitations of sbFC methods, this approach has been vital to expanding understanding of brain function from that of single regions active in isolation to coordinated circuits of two brain regions influencing one another to facilitate brain function.
The following sections first provide a review of results from 36 sbFC studies in trauma-exposed clinical groups (“Initial Review”). Though resting-state FC and task-related FC in PTSD are likely imperfectly correlated, evidence suggests that resting-state FC is predictive of fear extinction following a conditioned fear reminder (Feng et al., 2015), converges with task-based sbFC in identifying targets involved in social- and emotion-processing (Guell et al., 2018), and allows for the isolation of networks that highly correspond with task-evoked network patterns (Laird et al., 2013). As such, for the Initial Review, both resting-state and task-based fMRI studies are included in order to evaluate the classical neural circuitry models of PTSD, which are largely based in task-related activation. Next, in order to understand the overall patterns of connectivity revealed in these studies, we extracted the coordinates given in 23 of the 36 studies of resting-state sbFC in trauma-exposed samples with and without PTSD (“Resting-State Target Maps”). Here, only resting-state sbFC studies were included in order to standardize across fMRI paradigms, which was important because the sample size was restricted by the availability of coordinates from whole-brain corrected targets. Commonly chosen seeds for FC analyses in PTSD include the bilateral amygdalae; bilateral hippocampi; prefrontal regions including the anterior cingulate cortex, lateral prefrontal cortex, and medial prefrontal cortex; the insular cortex; and posterior cingulate cortex and precuneus. sbFC analyses are typically conducted between clinical groups, generally subjects with a PTSD diagnosis in comparison to trauma-exposed controls (TECs) or non-trauma exposed controls (NTCs) or between trauma-exposed participants without PTSD and NTCs.
For this section of the review, evidence for neural models of PTSD from sbFC studies will be evaluated according to predictions based on the central themes from the classical model and network model. According to the classical model of PTSD, the sbFC studies will overall demonstrate that individuals with PTSD exhibit altered FC mainly within the fronto-limbic circuit, represented by reduced FC from the mPFC to subcortical limbic regions (Admon et al., 2013, Patel et al., 2012, Pitman et al., 2012, Rauch et al., 2006, Shin et al., 2006) and enhanced FC from the amygdala to the insula (Admon et al., 2013, Patel et al., 2012, Pitman et al., 2012) and dACC (Admon et al., 2013, Liberzon and Abelson, 2016, Pitman et al., 2012). According to a network model perspective, it is anticipated that the sbFC studies will overall demonstrate patterns of altered connectivity to targets that reflect larger patterns of network structure at the whole-brain level, as well as altered FC to targets of the canonical networks implicated in the Triple Network model. Specifically, the classical model predicts reduced fronto-limbic FC, which may be evident in a network model by observing reduced FC between nodes of the DMN (e.g., vmPFC, hippocampus, and PCC) and the CEN (e.g., middle frontal gyrus and posterior parietal cortex), and limbic nodes, such as the amygdala. The classical model also suggests hyperactivity of SN nodes, including the anterior insular cortex and dACC; thus, according to a network model perspective, sbFC studies should demonstrate enhanced FC to these major nodes of the SN in the PTSD group, indicative of a hyperconnected SN.
2.1. Methods: Initial Review
2.1.1. Article selection and inclusion criteria
The article selection process is depicted in Fig. 2. Articles were initially selected through keyword searches on PubMed (www.pubmed.gov) and Google Scholar (scholar.google.com). Keyword searches included ‘ptsd AND fMRI’, ‘ptsd AND seed’, ‘ptsd AND functional connectivity’, and simply ‘ptsd.’ Articles were excluded if the authors did not use fMRI for FC or did not utilize a seed-based approach to determine FC. Both resting-state and task-based fMRI studies were included in the Initial Review. Articles were included if the PTSD group either met diagnostic threshold for PTSD or were sub-threshold but experienced a Criterion A traumatic event. Studies with both adult and pediatric patients were included. Due to the high comorbidity of disorders other than PTSD observed in trauma-exposed populations (i.e. depression and anxiety; Breslau et al., 2003, Kessler et al., 1995), articles were excluded if the only trauma-exposed group was specifically recruited to be absent of any psychopathology (i.e. subjects were excluded for meeting diagnostic criteria for a mental disorder) and there was no PTSD group. These articles were excluded because the trauma-exposed controls (TECs) likely do not accurately represent individuals who do develop PTSD following trauma.
Fig. 2.
Article inclusion flow-chart for initial review. Initial search terms on PubMed (www.pubmed.gov) and Google Scholar (scholar.google.com) included ‘ptsd AND fMRI’, ‘ptsd AND seed’, ‘ptsd AND functional connectivity’, and simply ‘ptsd.’ Articles were excluded if the authors did not use fMRI for FC or did not utilize a seed-based approach to determine FC. Patient groups of both adults and adolescents/children were included, but only if the patient group was not recruited specifically to lack psychopathology. Studies were included if the patient group was trauma-exposed yet had sub-threshold symptoms for PTSD, but only if an explicit control group was included for comparison. That control group could be either trauma-exposed without PTSD (TECs) or non-trauma exposed (NTCs). This resulted in a total of 36 articles included in the initial review.
Comparison groups from included articles were either “healthy” controls without trauma exposure or psychopathology (NTCs) or TECs who did not meet diagnostic criteria for any mental disorders. Articles were excluded if the study lacked a control group (i.e. only conducted comparisons within the trauma-exposed or PTSD group). Because the goal of this review is to evaluate models that classify group differences in neurocircuitry related to PTSD, articles with a trauma-exposed group with subthreshold PTSD symptoms were only included if the comparison group was NTCs. Articles with a PTSD group demonstrating above-threshold symptoms were included if the comparison group was either NTCs or TECs. Included articles for the initial review utilized both whole-brain and ROI-based approaches for determining targets of altered FC; however, results from each article were only included if targets of FC survived correction for multiple comparisons. Studies were only excluded from seed maps if the authors failed to provide xyz coordinates for their chosen seeds.
2.1.2. Direction of altered functional connectivity in PTSD groups
The overall directionality of altered FC between PTSD and control groups (i.e. either enhanced or reduced FC from the seed to the target) was recorded for each included study, regardless of task. For articles that reported increased or reduced negative FC or anticorrelation, “increased negative” FC was considered “reduced” and “reduced negative” FC was considered “enhanced.” The total number of targets from all studies and the total number of targets with reduced and enhanced FC, respectively, were determined. Directionality of altered connectivity was important for this review because classical models posit that some brain regions are hypoactive and some are hyperactive in PTSD; thus, evaluation of the merits of classical models is incomplete without examining the directionality of altered connectivity. Additionally, regardless of target or seed location, overall group differences in patterns of directionality of altered FC could suggest larger-scale disorganization in PTSD. Two-sample Z-tests with continuity correction were conducted using the prop.test function in R (https://www.rdocumentation.org/packages/stats/versions/3.6.1/topics/prop.test) to determine if the number of targets of reduced FC in PTSD groups relative to controls differed from the number of targets of enhanced FC. Comparisons were made on the total number of targets from all included studies in the initial review, regardless of volume correction method or whether the target coordinate was provided.
2.1.3. Spatial maps
Spatial maps of chosen seeds were constructed in Montreal Neurological Institute (MNI) space based on the xyz coordinates provided by the authors of each article. Coordinates that were given in Talariach space were converted to MNI space using Matlab’s tal2mni function. Each seed coordinate was placed with a 6-mm radius sphere onto the MNI template brain and visualized using AFNI (Cox, 1996) and MRIcroGL software (Version 23 June 2018 https://www.mccauslandcenter.sc.edu/mricrogl/).
2.2. Review findings: Initial Review
2.2.1. Included studies and sample characteristics
The initial comprehensive review summarizes results from 36 articles, published between 2008 and early 2019 (Table 2). The articles discussed below have a combined 1080 subjects in their respective PTSD groups and 1073 controls. The PTSD group is comprised of 168 children or adolescents (eight articles) and 912 adults (29 articles) with PTSD or trauma exposure and subthreshold PTSD symptoms, while the control group contains 176 children or adolescents and 897 adults and one article (Zielinski et al., 2018) evaluated subjects of both age groups. 110 patients and 111 controls were included from studies that allowed subthreshold PTSD symptoms in the PTSD group. Types of trauma exposure (e.g. interpersonal violence, motor vehicle accidents, natural disasters, etc.) varied within and between studies. Eight studies utilized task-based paradigms in fMRI, including processing of emotional faces (Keding and Herringa, 2016, Steuwe et al., 2015, Stevens et al., 2013) and other emotional or threatening stimuli (Marusak et al., 2015a, Rabellino et al., 2016, Wolf and Herringa, 2016) and fear conditioning (Linnman et al., 2011, Morey et al., 2015). The remaining 28 studies used resting-state fMRI. Only the studies that utilized whole-brain correction in their FC approaches (n = 23) were included in the target maps in Section 2.4.
Table 2.
Seed-based functional connectivity in PTSD. 36 articles discussing seed-based functional connectivity results in trauma-exposed subjects were included for drawing seed and target maps. Task, type and number (N) of subjects in the patient population, type and number (N) of subjects in the control population, seed origin(s), target name(s) and direction of connectivity (increased or decreased) in PTSD are included for each study. Only targets surviving statistical correction are included for each study. * Indicates ROI-based functional connectivity analysis; + Indicates whole-brain FC analysis. $Indicates studies not included on seed maps due to inability to locate seed coordinates. fMRI- functional magnetic resonance imaging; PTSD – posttraumatic stress disorder; L – left hemisphere; R – right hemisphere; B – bilateral; mPFC – medial prefrontal cortex; dmPFC – dorsal medial prefrontal cortex; vmPFC – ventral medial prefrontal cortex; dlPFC – dorsal lateral prefrontal cortex; lPFC – lateral prefrontal cortex; ACC – anterior cingulate cortex; rACC – rostral anterior cingulate cortex; dACC – dorsal anterior cingulate cortex; pgACC – perigenual anterior cingulate cortex; cACC – caudal anterior cingulate cortex; PCC – posterior cingulate cortex; IFG – inferior frontal gyrus; SFG – superior frontal gyrus; MFG – middle frontal gyrus; MTG – middle temporal gyrus; ITG – inferior temporal gyrus; SOG – superior occipital gyrus; OFG – orbital frontal gyrus; SFG – superior frontal gyrus; BLA – basolateral amygdala; CMA – central medial amygdala.
| Author(s), Date | Task | Population (N) | Control (N) | Seed Origin | Target Name | Direction of FC (contrast) | Notes |
|---|---|---|---|---|---|---|---|
| Amygdala seed | |||||||
| (Birn et al., 2014)+ | Resting State fMRI | Adult combat veterans with PTSD (17) | Adult combat veterans without PTSD (10) | R Amygdala | mPFC | ↓ | Predicted by CTQ |
| L SOG | ↓ | Predicted by CTQ | |||||
| R Cerebellum | ↑ | Predicted by CTQ | |||||
| R dmPFC | ↑ | Predicted by CAPS | |||||
| L Amygdala | R vlPFC/dlPFC | ↓ | Predicted by CTQ | ||||
| L rACC | ↓ | Predicted by CTQ | |||||
| L Insula | ↑ | Predicted by CAPS | |||||
| R dlPFC | ↑ | Predicted by CAPS | |||||
| R Insula | ↑ | Predicted by CAPS | |||||
| L dlPFC | ↑ | Predicted by CAPS | |||||
| R Cerebellum | ↓ | Predicted by CAPS | |||||
| (Zielinski et al., 2018)+ | Resting State fMRI | Adolescent girls (17) and adult women (14) with assault history | Healthy adolescent girls (19) and adult women (11) without assault history | L Amygdala | L Ventral ACC | ↓ | Adolescent assault survivors |
| ↑ | Adult assault survivors | ||||||
| (Jin et al., 2014)+$ | Resting State fMRI | Adult earthquake survivors with PTSD (57) | Adult earthquake survivors without PTSD (77) | B Amygdala | B MFG and medial frontal gyri | ↓ Positive | |
| (Sripada et al., 2012a)*$ | Resting State fMRI | Adult male combat veterans with PTSD (15) | Adult male combat veterans without PTSD (14) | R Amygdala | R Insula/STG | ↑ Positive | |
| R dACC | ↓ Anticorrelation | ||||||
| L Hippocampus | ↓ | ||||||
| L Inferior OFG | ↓ | ||||||
| rACC | ↓ Anticorrelation | ||||||
| L Amygdala | R Insula | ↑ Positive | |||||
| (Thomason et al., 2015)+$ | Resting State fMRI | Urban youth with trauma exposure (21) | Urban youth without trauma exposure (21) | CMA | sgACC | ↑ | |
| Precuneus | ↑ | ||||||
| dl Sensorimotor cortex | ↓ | ||||||
| Superficial Amygdala | SFG | ↑ | |||||
| Sensorimotor cortex | ↑ | ||||||
| ITG | ↑ | ||||||
| MTG | ↑ | ||||||
| L Insula | ↑ | ||||||
| dACC | ↓ | ||||||
| (Zhu et al., 2017)* | Resting State fMRI | Adults with PTSD (48) | Matched trauma-exposed healthy controls (34) | CMA | Thalamus | ↓ | |
| BLA | OFC | ↓ | |||||
| (van der Werff et al., 2013)+ | Resting State fMRI | Adults with reported childhood emotional maltreatment (44) | Healthy adults without childhood emotional maltreatment (44) | R Amygdala | B Occipital Cortex | ↓ Negative | |
| B Precuneus | ↓ Negative | ||||||
| Insular Cortex/OFC | ↓ Positive | ||||||
| L Hippocampus | ↓ Positive | ||||||
| L Putamen | ↓ Positive | ||||||
| (Rabinak et al., 2011) +$ | Resting State fMRI | Adult male combat veterans with PTSD (15) | Adult male combat veterans without PTSD (17) | R Amygdala | Insula | ↑ | |
| (Zhang et al., 2016)+ | Resting State fMRI | Adult earthquake survivors with PTSD (33) | Adult earthquake survivors without PTSD (33) | L Amygdala | R Hippocampus/parahippocampus | ↑ | |
| R vmPFC/ACC | ↑ | ||||||
| L STG | ↓ | ||||||
| R PCC/precuneus | ↓ | ||||||
| R Insula/MTG | ↓ Negative | ||||||
| R Amygdala | L Hippocampus/ parahippocampus | ↑ | |||||
| L MTG | ↑ | ||||||
| R Middle/ITG | ↓ | ||||||
| R Medial/IFG | ↓ | ||||||
| R IFG/Insula | ↓ | ||||||
| R Middle Orbital Frontal Gyrus | ↑ Negative | ||||||
| L Insula/MTG | ↓ Negative | ||||||
| (Aghajani et al., 2016)+$ | Resting State fMRI | Adolescents with PTSD and history of sexual abuse (19) | Age-, sex-, and IQ-matched healthy adolescents (23) | R BLA | R Frontal Pole | ↓ | |
| L mPFC | ↓ | ||||||
| L Occipital Cortex | ↑ | ||||||
| L Parietal Lobe | ↑ | ||||||
| L CMA | L OFC/subcallosal cortex | ↑ | |||||
| L Parahippocampal Gyrus | ↑ | ||||||
| L Temporal Pole | |||||||
| R CMA | B Parietal Cortex | ↓ | |||||
| B Occipital Cortex | ↓ | ||||||
| (Zhu et al., 2018)+ | Resting State fMRI | Adults with PTSD (24) | Trauma-Exposed Healthy Adults (26) | BLA | OFC | ↓ | |
| vmPFC | ↓ | ||||||
| CMA | OFC | ↓ | |||||
| (Brown et al., 2014)+$ | Resting State fMRI | Adult 9/11 Responders with PTSD (20) | Adult 9/11 Responders without PTSD (22) | L BLA | pgACC/dmPFC | ↑ | |
| R BLA | dACC | ↑ | |||||
| L IFG | ↓ | ||||||
| (Chen et al., 2018)+ | Resting State fMRI | Adult typhoon survivors with PTSD (27) | Adult typhoon survivors without PTSD (33) and non-exposed healthy adults (30) |
L Amygdala | L SMA | ↑ | PTSD vs. TEC |
| B vmPFC | ↓ | PTSD vs. HC | |||||
| R ITG | ↑ | PTSD vs. HC | |||||
| R Amygdala | L Precuneus/PCC | ↓ | PTSD vs. HC | ||||
| L Superior Parietal Lobule | ↓ | PTSD vs. HC | |||||
| (Morey et al., 2015)* | Preconditioning, fear conditioning, and generalization of facial stimuli | Adult combat veterans with PTSD (32) | Adult combat veterans without PTSD (35) | R Amygdala | Calcarine Sulcus | ↑ (generalization > preconditioning) | |
| vmPFC | ↓ (generalization > preconditioning) | ||||||
| (Stevens et al., 2013)+ | Emotional Faces Processing (neutral and fearful) | Adult, African American women with PTSD (20) | Adult, African American women with trauma exposure but no PTSD (20) | R Amygdala | sgACC | ↓ (fear > neutral) | |
| R Globus Pallidus | ↑ (fear > neutral) | ||||||
| L Amygdala | R dlPFC | ↑ (fear > neutral) | |||||
| (Keding and Herringa, 2016)* | Dynamic Emotional Faces Processing | Youth with PTSD (25) | Age- and sex-matched non-trauma-exposed youth (28) | R Amygdala | B dmPFC | ↓ (angry > neutral) | |
| ↑ (happy > neutral) | |||||||
| L vlPFC | ↓ (angry > neutral) | ||||||
| ↑ (happy > neutral) | |||||||
| (Marusak et al., 2015b)*$ | Emotional Conflict Interference | Adolescents with childhood trauma history (14) | Age-, sex-, and IQ-matched adolescents without trauma history (16) | Amygdala | pgACC | ↓ Negative (postincongruent incongruent -postcongruent incongruent) | |
| (Wolf and Herringa, 2016)+$ | Neutral Faces Processing Preceded by Trauma-Related Images | Youth with PTSD (24) | Age- and sex-matched non-trauma-exposed youth (24) | L Amygdala | B rACC/dmPFC | ↓ (threat – neutral) | |
| L dmPFC | ↓ (threat – neutral) | ||||||
| (Rabellino et al., 2016)*$ | Conscious and Subliminal Threat Processing with Trauma-Related Words | Adults with PTSD (26) | Healthy, matched adults without PTSD (20) | R CMA | SFG | ↑ (subliminal trauma > neutral words) | |
| R BLA | R Superior Colliculus | ↓ (subliminal trauma > neutral words) | |||||
| L CMA | L Pulvinar | ↑ (supraliminal trauma > neutral words) | |||||
| Posterior Cingulate Cortex (PCC) and Precuneus Seeds | |||||||
| (Jin et al., 2014) +$ | Resting State fMRI | Adult earthquake survivors with PTSD (57) | Adult earthquake survivors without PTSD (77) | L PCC | B Insula | ↑ Negative | |
| (Bluhm et al., 2008)+ | Resting State fMRI | Adult women with PTSD and childhood trauma (17) | Healthy matched control women (15) | PCC/Precuneus | R mPFC | ↓ | |
| L Angular Gyrus | ↓ | ||||||
| R Angular Gyrus/MTG | ↓ | ||||||
| R SFG | ↓ | ||||||
| B MFG | ↓ | ||||||
| R MTG | ↓ | ||||||
| B ITG | ↓ | ||||||
| Thalamus | ↓ | ||||||
| R Parahippocampus | ↓ | ||||||
| Cerebellum | ↓ | ||||||
| R Amygdala | ↓ | ||||||
| R Insula | ↓ | ||||||
| (Sripada et al., 2012b)*$ | Resting State fMRI | Adult combat veterans with PTSD (15) | Adult combat veterans without PTSD (15) and non-combat controls (15) | PCC | R Putamen | ↑ | |
| (Heyn et al., 2018)+ | Resting State fMRI | Youth aged 8–18 with PTSD (34) | Matched, typically developing youth (21) | B PCC | L Amygdala | ↓ | With Age |
| (Miller et al., 2017)+ | Resting State fMRI | Adult combat veterans with PTSD (69) | Adult combat veterans without PTSD (44) | L PCC | R Hippocampus | ↓ | |
| (DiGangi et al., 2016)+ | Resting State fMRI | Adult male combat veterans with (22) and without (18) PTSD | Healthy civilian male adults (13) | Precuneus | mPFC | ↓ | Both combat groups vs. controls |
| R Superior Parietal Lobule | ↓ | Both combat groups vs. controls | |||||
| (Qin et al., 2012)+ | Resting State fMRI | Adult vehicle accident victims with PTSD (17) | Age- and sex-matched vehicle victims without PTSD (15) | PCC | L mPFC | ↓ | Inversely correlated with CAPS Score |
| (Viard et al., 2019)+ | Resting State fMRI | Adolescents with PTSD only (14) | Typically developing adolescents without trauma history (24) | B PCC | L Middle Occipital Gyrus | ↓ | |
| Medial Prefrontal Cortex (mPFC) Seeds | |||||||
| (Jin et al., 2014)+$ | Resting State fMRI | Adult earthquake survivors with PTSD (57) | Adult earthquake survivors without PTSD (77) | L mPFC | L Postcentral Gyrus | ↓ Positive | |
| B PCC/Precuneus | ↓Positive | ||||||
| (Sripada et al., 2012b)*$ | Resting State fMRI | Adult combat veterans with PTSD (15) | Adult combat veterans without PTSD (15) and non-combat controls (15) | vmPFC | Precentral Sulcus/SMA | ↑ | |
| R Precentral Gyrus | ↑ | ||||||
| B Superior Temporal Sulcus | ↑ | ||||||
| rACC | ↓ | ||||||
| (DiGangi et al., 2016)+ | Resting State fMRI | Adult male combat veterans with (22) and without (18) PTSD | Healthy civilian male adults (13) | mPFC | Precuneus | ↓ | Both combat groups vs. controls |
| R Superior Parietal Lobule | ↓ | Both combat groups vs. controls | |||||
| (Heyn et al., 2018)+ | Resting State fMRI | Youth aged 8–18 with PTSD (34) | Matched, typically developing youth (21) | R vmPFC | L Amygdala | ↓ | With Age |
| (Miller et al., 2017)+ | Resting State fMRI | Adult combat veterans with PTSD (69) | Adult combat veterans without PTSD (44) | vmPFC | dACC | ↓Anticorrelation | |
| (Misaki et al., 2018)+ | Resting State fMRI | Adult male combat veterans with PTSD (39) | Age-matched non-trauma exposed healthy male controls (28) | L MFG | L IFG | ↓ | |
| L Frontal Operculum | ↓ | ||||||
| (Russman Block et al., 2017)+ | Resting State fMRI (with attention task component) | Adult male combat veterans with PTSD (36) | Age-matched male civilian controls (21) | MFG | R Amygdala | ↓ | With orienting effect |
| Hippocampus/Parahippocampus Seeds | |||||||
| (Birn et al., 2014)+ | Resting State fMRI | Adult combat veterans with PTSD (17) | Adult combat veterans with PTSD (10) | R Hippocampus | R dlPFC | ↓ | Predicted by CTQ |
| PCC/Precuneus | ↑ | Predicted by CTQ | |||||
| R lPFC | ↓ | Predicted by CTQ | |||||
| L rACC | ↓ | Predicted by CTQ | |||||
| L Hippocampus | PCC/Precuneus | ↑ | Predicted by CTQ | ||||
| ↓ | Predicted by CTQ | ||||||
| L Cerebellum | ↑ | Predicted by CTQ | |||||
| L mPFC | ↓ | Predicted by CTQ | |||||
| dmPFC | ↑ | Predicted by CAPS | |||||
| PCC | ↓ | Predicted by CAPS | |||||
| (Chen and Etkin, 2013)*$ | Resting State fMRI | Adults with PTSD (17) | Healthy matched controls (22) | Posterior Hippocampus | PCC | ↓ | |
| pgACC | ↓ | ||||||
| (Misaki et al., 2018)+ | Resting State fMRI | Adult male combat veterans with PTSD (39) | Age-matched non-trauma exposed healthy male controls (28) | L Parahippocampus | B Fusiform Gyrus | ↓ | |
| R Middle Occipital Gyrus | ↓ | ||||||
| L Middle Temporal Area | ↓ | ||||||
| R PCC | ↓ | ||||||
| (Zhu et al., 2018)+ | Resting State fMRI | Adults with PTSD (24) | Trauma-Exposed Healthy Adults (26) | Hippocampus | vmPFC | ↓ | |
| (Linnman et al., 2011)+ | Partial Reinforcement Classical Fear Conditioning | Adults with PTSD (19) | Matched, trauma-exposed controls (24) | L Parahippocampal Gyrus | R Inferior Parietal Lobule | ↓ (US > omitted US) | |
| L Medial Frontal Gyrus | ↓ (US > omitted US) | ||||||
| R Precentral Gyrus | ↓ (US > omitted US) | ||||||
| Anterior Cingulate Cortex (ACC) Seeds | |||||||
| (Zielinski et al., 2018)+ | Resting State fMRI | Adolescent girls (17) and adult women (14) with assault history | Healthy adolescent girls (19) and adult women (11) without assault history | dACC | L Precuneus | ↓ | Adolescent assault survivors |
| L Angular Gyrus | |||||||
| L Lingual Gyrus | |||||||
| rACC | R Precuneus | ↓ | Adolescent assault survivors | ||||
| R Ventral Anterior Superior Frontal Gyrus | ↑ | Adult assault survivors | |||||
| (van der Werff et al., 2013)+ | Resting State fMRI | Adults with reported childhood emotional maltreatment (44) | Healthy adults without childhood emotional maltreatment (44) | L dACC | R Angular Cortex | ↓ Negative | |
| R Precuneus | ↓ Negative | ||||||
| B Frontal Cortex | ↓ Positive | ||||||
| (Kennis et al., 2015)+ | Resting State fMRI | Male combat veterans with (31) and without (25) PTSD | Male civilian controls (25) | R cACC | R Precentral Gyrus | ↓ | Both combat groups vs. controls |
| L cACC | L Precentral Gyrus | ↓ | Both combat groups vs. controls | ||||
| R pgACC | R Superior Medial Gyrus | ↓ | Both combat groups vs. controls | ||||
| L pgACC | L Superior Medial Gyrus | ↓ | Both combat groups vs. controls | ||||
| L MTG | ↓ | Both combat groups vs. controls | |||||
| (Russman Block et al., 2017)+ | Resting State fMRI (with attention task component) | Adult male combat veterans with PTSD (36) | Age-matched male civilian controls (21) | dACC | vmPFC | ↓ | With orienting effect |
| PCC | ↓ | With orienting effect | |||||
| L SFG | ↓ | With orienting effect | |||||
| R SFG | ↓ | With orienting effect | |||||
| L STG | ↓ | With orienting effect | |||||
| R MTG/ITG | ↓ | With orienting effect | |||||
| (Keding and Herringa, 2016)* | Dynamic Emotional Faces Processing | Youth with PTSD (25) | Age- and sex-matched non-trauma-exposed youth (28) | dACC | B dmPFC | ↓ (angry > neutral) | |
| ↑ (happy > neutral) | |||||||
| Lateral Prefrontal Cortex (lPFC) Seeds | |||||||
| (Heyn et al., 2018)+ | Resting State fMRI | Youth aged 8–18 with PTSD (34) | Matched, typically developing youth (21) | L vlPFC | L Hippocampus | ↓ | With Age |
| L dlPFC | L Hippocampus | ↓ | With Age | ||||
| (Misaki et al., 2018)+ | Resting State fMRI | Adult male combat veterans with PTSD (39) | Age-matched non-trauma exposed healthy male controls (28) | L IFG | R SMA | ↓ | |
| L MFG | ↓ | ||||||
| L SFG | L Medial Frontal | ↓ | |||||
| L Middle Cingulate | ↓ | ||||||
| L Insula | ↓ | ||||||
| L Anterior Insula | ↓ | ||||||
| R IFG | ↓ | ||||||
| (Olson et al., 2018) + | Resting State fMRI | Adults with PTSD (21) | Trauma-exposed adults without PTSD (30) and healthy controls (36) | R dlPFC | Precuneus | ↑ Negative | |
| Insular Cortex Seeds | |||||||
| (Sripada et al., 2012b)*$ | Resting State fMRI | Adult combat veterans with PTSD (15) | Adult combat veterans without PTSD (15) and non-combat controls (15) | L Anterior Insula | L Peri-Insula/STG | ↑ | |
| R Hippocampus | ↑ | ||||||
| R Amygdala | ↑ | ||||||
| (Nicholson et al., 2016)* | Resting State fMRI | Adults with PTSD (44) | Age-matched healthy controls (40) | L Anterior Insula | R BLA | ↑ | |
| L Mid Insula | L BLA | ↑ | |||||
| L Posterior Insula | L BLA | ↑ | |||||
| R Anterior Insula | B BLA | ↑ | |||||
| R Mid Insula | L BLA | ↑ | |||||
| (Misaki et al., 2018)+ | Resting State fMRI | Adult male combat veterans with PTSD (39) | Age-matched non-trauma exposed healthy male controls (28) | L Insula | R Middle Cingulate | ↑ | |
Nineteen of the included studies examined FC from the amygdala in PTSD, eight used the posterior cingulate (PCC)/precuneus, seven utilized seeds within the medial prefrontal cortex (mPFC), five utilized the hippocampus/parahippocampus as a seed, five examined connectivity from the anterior cingulate cortex (ACC), three from the lateral prefrontal cortex (lPFC), and three from the insula (Fig. S1). Investigators also chose seeds in the thalamus (Yin et al., 2011), vestibular nuclei (Harricharan et al., 2017), superior colliculus and locus ceruleus (Steuwe et al., 2015), supplementary motor area (Misaki et al., 2018), and putamen (Linnman et al., 2011) for FC studies in PTSD (Table S1; Fig. S2). A detailed summary of FC targets from each seed is provided in the Supplemental Material and Table 2.
2.2.2. Spatial map of seed regions
A spatial map was constructed to visually display seed regions used in this line of research (Fig. 3). Eleven studies were excluded from the spatial map of seed regions because the authors failed to provide xyz coordinates for their chosen seed, leaving 25 studies for the seed map. The remaining studies provided coordinates for 16 amygdala seeds from ten studies, six PCC/precuneus seeds from six studies, five mPFC seeds from five studies, five hippocampus/parahippocampus seeds from four studies, nine ACC seeds from five studies, four lPFC seeds from three studies, six insular cortex seeds from two studies, and eight “other” seeds from five studies (Fig. 3, Table 2).
Fig. 3.

Seed map for initial review. 25 of the 36 studies provided xyz coordinates for their chosen seeds in seed-based functional connectivity analyses. Coordinates were used to draw 6-mm spheres in MNI space to visualize ROIs. The most commonly chosen seed was the amygdala (19 studies), followed by the posterior cingulate/precuneus (8), the medial prefrontal coretex (7), hippocampus/parahippocampus (6), anterior cingulate cortex (5), lateral prefrontal cortex (3), insula (3) and others including the thalamus and locus ceruleus.
2.3. Discussion and evaluation of models: Initial Review
The initial review of sbFC studies in trauma-exposed groups with and without PTSD reveals an unclear picture of altered FC patterns in PTSD groups. Predictably, based on the classical model, many studies chose to examine the amygdala as a seed. These studies suggest moderate support for the classical model in that one study noted reduced FC from the amygdala to the vmPFC during safety learning (Morey et al., 2015) and three studies observed enhanced FC from the amygdala to regions of the dACC and anterior insula (Birn et al., 2014, Thomason et al., 2015, Zhang et al., 2016); however, one of the same studies and one other observed evidence for reduced FC from the amygdala to the anterior insula and dACC (Thomason et al., 2015, van der Werff et al., 2013), which is not fully consistent with the classical model and hinders interpretability of these results. Two studies use the anterior insula as a seed and provide supportive evidence for the classical model by observing increased FC to the amygdala in PTSD groups (Nicholson et al., 2016, Sripada et al., 2012b); however, both of these studies utilize a priori targets with small-volume, rather than whole-brain correction, and thus are potentially biased in their observations. Of the seven studies that utilized mPFC seeds for FC analyses, two found evidence for reduced FC from the mPFC to the amygdala (Heyn et al., 2018, Russman Block et al., 2017), lending some support to the classical model. Though the classical model would imply a dominance of altered fronto-limbic circuitry in PTSD groups, the literature suggests that many other circuits are also associated with PTSD and trauma exposure, signifying a role of altered neural circuitry beyond the fronto-limbic circuit. In order to evaluate the evidence for a network model of PTSD neural circuitry, the following sections utilize the coordinates for the targets provided from resting-state, whole-brain corrected sbFC studies to visualize possible network structure from bivariate circuits.
2.4. Methods: Resting-state target maps
2.4.1. Article selection and inclusion criteria
Articles selected for the resting-state seed and target maps were a subset of the 36 articles from the Initial Review (see Fig. 4 for selection process). Articles were excluded from the resting-state target map section of the review if the fMRI paradigm involved a task, if the authors used small-volume correction on a priori targets, or if no target coordinates were provided. Due to the small number of task-based studies eligible for this section of the review and the evidence suggesting that resting-state organization is highly correlated with functional organization during tasks (Bullmore and Sporns, 2012, Bullmore and Sporns, 2009), only resting-state studies were included in this section of the analysis in order to standardize task paradigms in fMRI.
Fig. 4.
Article inclusion flow-chart for target maps. The 36 articles selected from the initial review were used for the basis of the target map analysis. Studies were excluded if the fMRI task used was any paradigm other than resting-state (8 articles). From the remaining 28 articles, five studies were excluded because they utilized a priori target regions with small-volume correction to define targets of altered FC in patient groups. The resultant target maps contained resting-state, whole-brain corrected targets from 23 studies.
2.4.2. Canonical network membership of whole-brain corrected targets
Target maps were constructed in MNI space based on the coordinates provided by the authors of each article. Coordinates that were given in Talariach space were converted to MNI space using Matlab’s tal2mni function. Each coordinate was placed with a 6-mm radius sphere onto the MNI template brain. Studies that did not provide coordinates for the targets were excluded from the maps produced in this review. In order to define anatomical locations of key networks in an unbiased manner, networks were defined using the cortical atlas by Yeo and colleagues (Yeo et al., 2011). The DMN, CEN, and SN were extracted from the Yeo et al. parcellation and projected onto an MNI template brain using AFNI (Cox, 1996) and MRIcroGL software (Version 23 June 2018 https://www.mccauslandcenter.sc.edu/mricrogl/). Each of the 6-mm radius targets from whole-brain corrected resting-state studies was displayed over the three networks and the target was classified as “DMN”, “CEN”, “SN” or “Other” based on anatomical overlap with the cortical parcellation. Finally, one-sample Pearson’s Chi-Squared tests conditioned on the total number of targets within the three canonical networks were conducted to determine differences in network membership of targets from each seed. Because each of the three canonical networks consists of a different number of voxels within the cortical atlas, expected proportions for all Chi-Squared tests were calculated based on the size of each network (in voxels). All tests were conducted with Yates’ Continuity Correction using the chisq.test function in R. Further details regarding the methods for this section are included in the Supplemental Material.
2.5. Review findings: Resting-state target maps
2.5.1. Included studies – Target maps
28 of the 36 studies included in the Initial Review conducted FC analyses from resting-state fMRI scans, while two used fear conditioning tasks and six used emotional- or threat-processing tasks in fMRI. A total of 13 studies were excluded from the original 36 due to task-based fMRI paradigms (n = 8) or a priori ROI approaches using small-volume correction (n = 5). This left a total of 23 studies included on the target maps for this review, all of which were whole-brain corrected, resting-state studies. Combined, the studies included on target maps represent 708 trauma-exposed patients with (n = 612) or without (n = 96) PTSD and 673 controls. 105 of the PTSD group and 108 of the controls were children or adolescents, while the remaining subjects were adults (n = 603 in PTSD group, n = 565 in control group).
2.5.2. Direction of altered functional connectivity in PTSD groups – Initial Review and target maps
A total of 199 targets were identified in the 36 studies included in the Initial Review. 119 of these targets demonstrated reduced FC from their seed in PTSD groups, while 74 demonstrated enhanced FC and six displayed both (Table 3). Following removal of targets for which evidence of both enhanced and reduced FC was found, this difference was significant (Χ2(1) = 20.1, p < .001), indicating that significantly more targets of altered FC in the PTSD group displayed reduced connectivity from their seeds relative to the number of enhanced FC targets (Fig. 5). This effect remains significant when removing targets from studies with subjects aged below 18 [167 targets total, 64 enhanced FC, 103 reduced FC (Χ2(1) = 17.29, p < .001)]. 97 of the 143 targets from articles utilizing resting-state whole-brain corrected analyses (i.e. the 23 studies included in Target Maps) demonstrated reduced FC compared to 46 that displayed enhanced FC (Χ2(1) = 55.5, p < .001; Fig. 6). This finding reflects the previous from the Initial Review in that PTSD groups displayed significantly more targets of reduced FC in comparison to targets of enhanced connectivity. Additional comparisons within each type of control group (i.e. PTSD vs. NTC, PTSD vs. TEC, and TEC vs. NTC) revealed that the PTSD group showed significantly more targets of reduced FC in the PTSD vs. NTC comparison with 62 targets; however, the was no difference between the groups in proportion of reduced FC targets in the PTSD vs. TEC studies or the TEC vs. NTC studies (Fig. S5, Table S2) suggesting that choice of control group may influence conclusions regarding directionality of altered FC.
Table 3.
Comparison of directionality of altered functional connectivity targets in patient groups. For targets of altered functional connectivity (FC) from the seed-based studies, patient groups demonstrated disproportionately reduced FC compared to enhanced. This effect was significant in both the Initial Review of all targets and the target map review, which included only resting-state, whole-brain corrected targets. For the initial review, six targets demonstrated both enhanced and reduced FC and therefore were excluded for comparisons. One such target was excluded from the target map review.
| Targets Enhanced FC Only | Targets Reduced FC Only | Χ2(1) | p | |
|---|---|---|---|---|
| All Included Targets in Initial Review (n = 199) | 74 | 119 | 20.06 | <.001 |
| Target Map Studies Only (n = 143) | 46 | 97 | 34.97 | <.001 |
Fig. 5.
Direction of altered seed-based functional connectivity in patient groups from initial review. 193 targets that displayed only enhanced or reduced functional connectivity (FC) in patient groups were compared using a two-sample Z-test to determine the difference in proportions of enhanced vs. reduced FC. Patients displayed disproportionately more targets of reduced FC from their seeds compared to enhanced. This effect remained significant when removing targets from studies that utilized subjects under the age of 18.
Fig. 6.

Direction of altered seed-based functional connectivity in patient groups from target maps. 143 resting-state, whole-brain corrected targets that displayed only enhanced or reduced functional connectivity (FC) in patient groups were compared using a two-sample Z-test to determine the difference in proportions of enhanced vs. reduced FC. Patients displayed disproportionately more targets of reduced FC from their seeds compared to enhanced. This effect remained significant when removing targets from studies that utilized subjects under the age of 18.
2.5.3. Network membership of targets of altered functional connectivity in PTSD
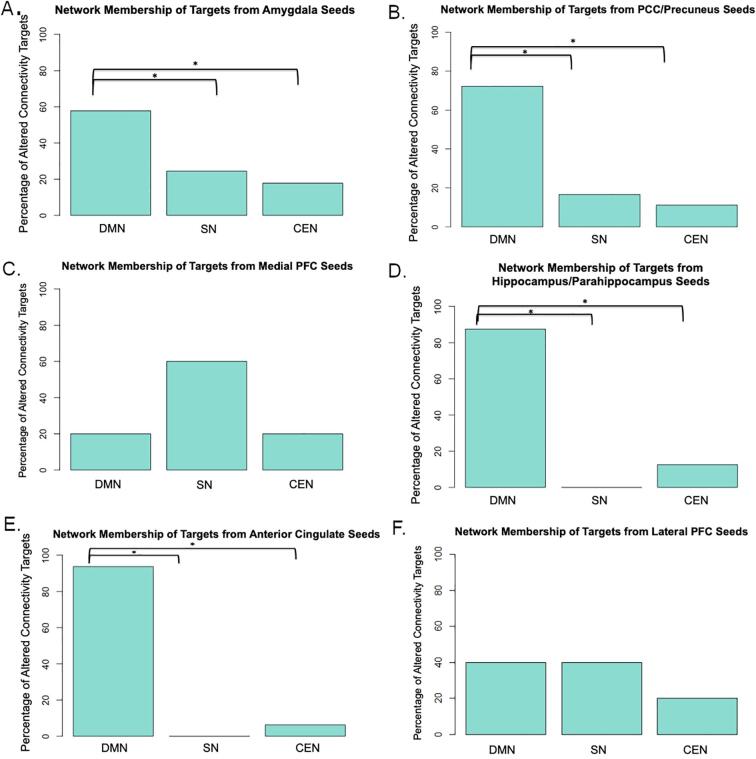
Fig. 7, Table 4, and Fig. S6 summarize the key findings for the network membership of targets with altered functional connectivity in PTSD. With the exception of lateral and medial prefrontal cortex seeds, targets of altered connectivity in PTSD from all seeds were located disproportionately within the DMN compared to the CEN and SN. This trend was the same for targets of both enhanced in reduced FC from the amygdala (Figs. S3 and S4). Specific details for each seed are provided in the Supplemental Material.
Fig. 7.
Network membership of targets of altered functional connectivity in PTSD patient groups. Each of the 6-mm radius targets from whole-brain corrected resting-state studies was displayed over the three networks of the Triple Network Model and the target was classified as “DMN”, “CEN”, “SN”or “Other”based on anatomical overlap with the cortical parcellation. Because the Yeo et al. cortical parcellation excludes subcortical regions from the atlas, most subcortical targets were excluded from network membership classification; however, the participation of the hippocampus within the default mode network is well-established (Greicius et al., 2009, Greicius et al., 2004, Raichle, 2015) and thus targets in thehippocampus were counted as part of the DMN in this analysis. One-sample Pearson’s Chi-Squared tests conditioned on the total number of targets within the three networks in total were conducted to determine differences in network membership of targets from each seed. Expected proportions for all Chi-Squared tests were calculated based on the size of each network (in voxels). A) 54 targets from the amygdala B) 23 targets from the PCC/Precuneus C) 7 targets from the mPFC D) 13 targets from the hippocampus/parahippocampus E) 20 targets from the ACC F) 10 targets from the lPFC. DMN = default mode network, CEN = central executive network, SN = salience network, PCC = posterior cingulate cortex, mPFC = medial prefrontal cortex, ACC = anterior cingulate cortex, lPFC = lateral prefrontal cortex.
Table 4.
Canonical network membership of targets with altered functional connectivity from common seeds. The number of altered connectivity targets in patient groups were counted and sorted by membership into canonical networks. Chi-squared tests were performed to assess significance of the proportion of targets in each network. DMN – default mode network; SN – salience network; CEN – central executive network.
| Seed Region | Total Resting-State Whole-Brain Targets in Canonical Networks | DMN Targets | SN Targets | CEN Targets |
|---|---|---|---|---|
| Amygdala | 45 | 26 | 11 | 8 |
| Χ2(1) | p | |||
| DMN vs. SN | 9.00 | 0.003 | ||
| DMN vs. CEN | 13.66 | <.001 | ||
| SN vs. CEN | 0.27 | 0.6054 | ||
| Posterior Cingulate Cortex (PCC)/Precuneus | 18 | 13 | 3 | 2 |
| Χ2(1) | p | |||
| DMN vs. SN | 9.11 | 0.003 | ||
| DMN vs. CEN | 11.43 | 0.001 | ||
| SN vs. CEN | 0 | 1 | ||
| Anterior Cingulate Cortex (ACC) | 16 | 15 | 0 | 1 |
| Χ2(1) | p | |||
| DMN vs. SN | 24.60 | <.001 | ||
| DMN vs. CEN | 21.13 | <.001 | ||
| SN vs. CEN | 0 | 1 | ||
| Lateral Prefrontal Cortex (lPFC) | 10 | 4 | 4 | 2 |
| Χ2(1) | p | |||
| DMN vs. SN | 0 | 1 | ||
| DMN vs. CEN | 0.24 | 0.626 | ||
| SN vs. CEN | 0.24 | 0.626 | ||
| Hippocampus/Parahippocampus | 8 | 7 | 0 | 1 |
| Χ2(1) | p | |||
| DMN vs. SN | 9.14 | 0.003 | ||
| DMN vs. CEN | 6.25 | 0.012 | ||
| SN vs. CEN | 0 | 1 | ||
| Medial Prefrontal Cortex (mPFC) | 5 | 1 | 3 | 1 |
| Χ2(1) | p | |||
| DMN vs. SN | 0.42 | 0.519 | ||
| DMN vs. CEN | 0 | 1 | ||
| SN vs. CEN | 0.42 | 0.519 | ||
2.5.4. Supplementary comparisons excluding studies with non-adult subjects
To address any concern about developmental effects of bivariate FC on network membership, all comparisons from the above sections were also conducted using only targets from studies with adult subjects (age 18+). The statistical significance of all comparisons except for the DMN vs. SN contrast from the amygdala seed was unchanged from the full review (Table S3). When targets from studies with youth were removed, there was no longer a significant difference in the proportion of amygdala targets in the DMN versus the SN, suggesting that participant age may partially contribute to the dominance of the DMN in the FC literature.
2.6. Discussion and evaluation of models: Resting-state target maps
sbFC studies of PTSD and trauma exposure reveal an inconsistent and often narrow picture of the neural profile of the disorder. Uni- and bivariate connectivity studies of PTSD suggest that PTSD may be associated with either hyper- or hypoactivation of threat-detection circuitry (Abdallah et al., 2019, Admon et al., 2013, Bryant et al., 2008, Koch et al., 2016), creating an unclear model of the neural correlates of the disorder. By examining many sbFC studies in concert, the current review addresses questions of directionality of neural alterations and suggests that PTSD is likely characterized by reduced FC to canonical networks. In fact, of the 143 resting-state whole-brain corrected targets reported in this review, reduced FC was observed to 97 targets, with seeds in all six of the common ROIs considered from the whole-brain corrected studies in this review.
Overall, globally reduced FC in individuals with PTSD may partially explain characteristic PTSD symptomology, including impaired fear extinction (Duits et al., 2015, Norrholm et al., 2011, Peri et al., 2000) and poor emotional regulation (Cloitre et al., 1997, Tull et al., 2007). Globally reduced FC in PTSD, as demonstrated by this analysis, may also contribute to resistance to cognitive behavioral therapy (CBT), which is the leading therapeutic treatment for PTSD (Cloitre, 2009). CBT has recently been shown to increase functional connectivity between the amygdala and various cortical regions involved in cognitive control in patients with social anxiety (Young et al., 2017) and PTSD (Shou et al., 2017), indicating that the efficacy of CBT in anxiety disorders may rest in strengthening functional connections throughout the brain. Patients with globally reduced FC may be less responsive to CBT compared to patients with stronger FC throughout the brain because the necessary functional connections for clinical improvement are reduced at baseline. Reduced FC across networks may also indicate increased functional modularity of the brain networks in which these seeds reside (limbic, DMN, and CEN, respectively) or globally reduced integration of neural processing in PTSD. Though some degree of modularity is necessary for economical brain network organization (Bullmore and Sporns, 2012), highly modular brain networks may result in failures in inter-network communication and inability to perform on cognitively-demanding tasks (Bertolero et al., 2015, Cohen and D’Esposito, 2016, Godwin et al., 2015, Yue et al., 2017). Maladaptive modular structure in PTSD could similarly contribute to cognitive and regulatory deficits that prevent efficacy of therapeutic interventions for PTSD patients. The limitations of sbFC methods preclude specific conclusions about global- and network-level modular structure; as such, investigating the hypothesis of altered modular structure in PTSD patients requires integrated approaches that consider both classical models and functional networks.
Another consistent observation from these studies is altered FC in trauma-exposed and PTSD patients from various seeds to regions comprising the DMN. In fact, targets of FC from four major seeds (amygdala, hippocampus/parahippocampus, PCC/precuneus, and ACC) preferentially fall into the DMN relative to the SN and CEN (Table 4). This result suggests a disproportionate role for the DMN in the neural circuitry of PTSD, which may contribute to re-experiencing and dissociative symptoms, fear generalization, and avoidance in PTSD patients (Akiki et al., 2017). Examining bivariate FC alone, which has been the norm for PTSD research for the last decade, obscures this important finding of DMN dominance in PTSD neural circuitry by neglecting the role of other nodes outside of the bivariate circuit. It is possible that the dominance of the DMN observed in this report is obvious only in resting-state, as the DMN is generally considered the “task-negative” network (Menon, 2011, Raichle, 2015). The limited number of task-based sbFC studies in PTSD that use whole-brain correction methods precluded examination of the role of the DMN in task-positive conditions; however, this is an important avenue for further research. Future studies should consider the role of the DMN and other canonical networks as whole, cohesive components rather than individual univariate parts. When compiled together into target maps, the sbFC studies contained in this review are showing clearly altered connectivity in large-scale networks in patient groups with PTSD and trauma exposure relative to healthy comparison subjects.
Examining sbFC studies together provides some support for the classical model of PTSD neural circuitry in that PTSD groups demonstrate generally reduced FC from prefrontal cortex seeds, and FC targets from seeds in the PFC did not preferentially fall into any specific canonical network. Additionally, two of the seven resting-state targets from the mPFC demonstrated reduced connectivity to the amygdala, further supporting the classical model; however, because a central theme of the classical model is reduced fronto-limbic connectivity in PTSD, one might expect for more than two of seven mPFC targets to show altered FC with the amygdala. This speaks to the univariate activation methods on which the classical models are based, indicating that fronto-limbic dominance may be most observable during tasks that are known activate the circuit, such as fear- or emotion-processing paradigms, or when a priori target selection biases results. Thus, an integrated methodology that considers the evidence for a fronto-limbic model alongside a whole-brain approach may be merited. Similarly, enhanced FC from the amygdala to the insula and dACC (or the SN under a network-based conceptualization) was observed in two studies that used resting-state, whole-brain corrected analyses, providing some support for the classical model; however, the majority of targets from the amygdala in this section of the review were nodes of the DMN rather than the SN. Again, the limited support for the classical model of PTSD neural circuitry from sbFC studies may be the consequence of the model’s basis in task-related activation or experimenter bias in target selection failing to reflect the brain’s organization in resting-state.
Taken together, the sbFC studies presented in this review provide strong evidence for a network model of PTSD neural circuitry. Firstly, the targets of altered FC in the PTSD group fall predominantly within canonical networks, specifically within the three networks implicated in the Triple Network Model of Psychopathology. Additionally, the finding of globally reduced FC in the PTSD group is supportive of a network model of PTSD neural circuitry because it indicates larger, coordinated patterns of altered brain organization outside of simple univariate activation. Secondly, the sbFC studies implicate a hypo-connected DMN in the PTSD group, supporting the prediction that a representative network model would demonstrate reduced FC to nodes of the DMN. While seven studies indicate reduced FC from various seeds to the CEN, the CEN does not emerge as a dominant network in PTSD neural circuitry and thus sbFC evidence does not support the prediction of reduced FC to the CEN in a network model. Similarly, while three studies observed enhanced FC from the amygdala to the SN, five studies actually observed reduced FC to SN nodes. This finding fails to support the prediction that a network model of PTSD neural circuitry would demonstrate enhanced FC to nodes of the SN in PTSD groups.
Overall, results from sbFC studies examined as a unit reveal network patterns of altered brain organization in subjects with PTSD, lending support to a network model of PTSD neural circuitry. Elements of the classical model of PTSD neural circuitry were also supported, suggesting that an integrated approach that bridges both classical and network-based models may be appropriate for future work. Recently, investigators have begun to utilize network-based analysis methods to explicitly examine network patterns of altered functional organization in PTSD patients. Though the extant research is limited thus far, the remainder of this review aims to summarize the network-level literature on PTSD neural circuitry in order to evaluate the evidence for both models.
3. Emerging network-based models of altered functional organization in PTSD
Computational methods in fMRI are vastly expanding the scope of answerable research questions in neuroscience. Elegant mathematical models and integration of graph theory and large-scale network concepts into fMRI analysis have allowed for the modeling of complex, coordinated brain systems from both resting-state and task timecourse data. Importantly, network-based approaches largely surpass the many limitations of sbFC and univariate activation models discussed above, namely the introduction of bias from choosing targets a priori and the resulting narrowed scope of investigation into simplistic circuits. The utilization of these methods for understanding the brain in health and disease has gleaned important insights into neural correlates underlying psychological disorders. To date, researchers have utilized two main tools for network-based analysis on neuroimaging data: Independent Component Analysis (ICA) and graph theory. From these studies, the functional structure of the human brain and its networks has been revealed. A 2011 study by Power and colleagues was one of the first to employ graph theoretical network principles to functional brain imaging to define a reliable set of nodes that fall consistently into large-scale networks within the healthy young adult brain (Power et al., 2011). The authors found that functional nodes of the brain do indeed fall into reliable subgraphs with high agreement between subjects and certain networks. The visual, default mode, central executive, and dorsal and ventral attention networks are particularly reliable in their partitioning between subjects and iterations (Power et al., 2011). This study was an important validation for graph network methods in neuroimaging, from which more precise conclusions can be drawn about normative and deviant structure.
Evidence suggests that functional organization follows a developmental trajectory such that children demonstrate less structurally (Baum et al., 2017) and functionally (Chen and Deem, 2015) segregated brain networks than young adults. Subsequently, older adults demonstrate more functional integration than do middle-aged adults (Onoda and Yamaguchi, 2013), indicating that brain network segregation may reach a peak during young adulthood and decline with normal ageing. While network organization follows a general developmental trajectory, the configuration of functional brain networks adjusts dynamically to meet the demands of cognitive tasks. Many networks that are involved in the execution of cognitive functions have a highly-connected node structure to coordinate with other functional networks to meet the demands of the task at hand (Power et al., 2011). Additionally, the number of functional networks that are involved in the execution of tasks increases with the cognitive demand of the tasks, likely facilitated by the highly-connected nodes found in networks that respond to high cognitive demands (Bertolero et al., 2015). Similarly, global functional connectivity adapts dynamically to tasks, decreasing during standard N-Back and sequence-tapping tasks (Cohen and D’Esposito, 2016) and during target detection (Godwin et al., 2015) to reduce network segregation and become more integrative to respond to the changing cognitive environment. Importantly, task performance may also be related to successful shifting of connectivity structure, as participants with more functionally segregated brains perform better on simple orienting tasks (Yue et al., 2017) while functional segregation was found to be negatively correlated with accuracy on complex tasks (Cohen and D’Esposito, 2016, Yue et al., 2017). These findings indicate that proper segregation and integration within and between functional networks is important for accurately meeting environmental demands. While the normative organization and development of large-scale brain networks at rest and during task is still under further investigation, the evidence garnered from individuals without psychopathology forms a crucial baseline from which deviations can be attributed to disordered functioning in PTSD.
The following sections describe the basic concepts behind both ICA and graph theory applications to fMRI, as well as discussion of the extant literature on network-based approaches in subjects with trauma exposure and PTSD. Subsequently, the evidence from this body of literature will be discussed in light of the central themes from each model of PTSD neural circuitry (i.e. classical univariate activation and network models). If the themes from the classical model are reliably detected in PTSD network literature, PTSD groups will display dominance of the amygdala and the mPFC in the altered neural profile. This may be observed through enhanced integration of the amygdala or reduced integration of the mPFC into large-scale networks of interest, or alterations in node characteristics such as clustering or participation coefficient of these two nodes. If the central themes of the network model reliably characterize PTSD neural circuitry, PTSD groups will demonstrate altered global connectivity patterns in the whole brain network, including changes in modularity or small-worldness. Additionally, a network model would predict reduced connectivity profiles of the DMN and CEN in PTSD groups, as well as enhanced connectivity of the SN.
3.1. Independent Component Analysis (ICA)
The development of Independent Component Analysis (ICA) methods for psychiatric neuroimaging has proven particularly advantageous, as this method allows for the isolation of neural networks in tasks with unknown neural models (please see the Supplemental Material for a description of ICA methodologies). Accordingly, ICA can be used in exploratory analyses to find large-scale neural correlates of health and disease without the need to rely on established paradigms. ICA can be used in the study of PTSD to isolate spatially-distributed networks during the same emotion- and threat-processing tasks used to develop the classical circuitry models as well as at rest. As such, ICA adds an additional tool for use in understanding the neural organization of PTSD whereby researchers can examine the function of spatially-distributed neural networks that may be driving the neural circuitry patterns observed in classical models.
Several studies have utilized ICA to understand alterations in functional networks in PTSD, starting with the canonical networks (DMN, CEN, and SN; Table 5). Within the DMN, PTSD is correlated with increased connectivity of the network at rest, the strength of which is also positively correlated with re-experiencing symptoms in PTSD patients (Patriat et al., 2016). During a threat-processing task, Rabellino and colleagues also observed increased connectivity with the DMN, in this case indicating higher integration of the amygdala with the DMN during subliminal threat processing (Rabellino et al., 2015). Alternatively, other groups have found evidence for decreased DMN functional connectivity during resting state in PTSD (Reuveni et al., 2016, Shang et al., 2014, Zhang et al., 2015), with one study observing an inverse association between DMN coupling and depersonalization and derealization symptoms (Tursich et al., 2015). In the Zhang et al. study, the authors observed decreased functional connectivity between the DMN and the SN, drawing an important link to the SN’s differential connectivity in PTSD. Because the SN is thought to facilitate the switch between the DMN and CEN to meet external cognitive demand (Menon, 2011, Menon and Uddin, 2010), the finding of decreased functional connectivity between the DMN and SN in PTSD indicates an impaired ability to switch out of the self-referential state and into a state of cognitive control or vice versa. During an emotional faces eye-gaze task, Thome and colleagues found more evidence for altered SN functional connectivity when they observed increased integration of the left amygdala and right insula into the SN of the PTSD group during direct eye gaze and a positive association between PTSD symptom severity and integration of the mid-cingulate into the SN (Thome et al., 2014). Finally, some evidence from ICA indicates decreased coupling and connectivity of certain regions of the CEN during threat-processing (Rabellino et al., 2015) and at rest (Shang et al., 2014) in PTSD, with one study identifying increased excitatory connections between the CEN and the posterior DMN in subjects with PTSD (Ke et al., 2018) adding to the picture of PTSD as a neural disorder associated with altered connectivity within and between major functional networks.
Table 5.
Studies of posttraumatic stress disorder using Independent Component Analysis in fMRI. Nine different studies utilized fMRI to investigate large-scale network alterations in PTSD patient groups. DMN – default mode network; SN – salience network; CEN – central executive network; FC – functional connectivity; SMA – supplementary motor area; ACC – anterior cingulate cortex; mPFC – medial prefrontal cortex; PCC – posterior parietal cortex; TEC – trauma-exposed control; HC – healthy control; dACC – dorsal anterior cingulate cortex.
| Author(s) and Year | Patient Group (N) | Control Group (N) | Task | Network(s) of Interest | Relationship with Population |
|---|---|---|---|---|---|
| (Patriat et al., 2016) | Youth with PTSD (29) | Non-Traumatized, aged-matched healthy youth (30) | Resting State | DMN | Increased FC within DMN DMN strength positively correlated with re-experiencing symptoms |
| (Reuveni et al., 2016) | Adults with PTSD (20) | Adults with Trauma Exposure and no PTSD (20) | Resting State | DMN | Decreased connectivity with right premotor cortex, lateral prefrontal cortex, and posterior medial frontal regions |
| (Shang et al., 2014) | Adult Earthquake Survivors with PTSD (18) | Adult Earthquake Survivors without PTSD (20) | Resting State | CEN | Decreased FC in medial frontal gyrus Increased FC in SMA |
| SN | Decreased FC in middle frontal gyri Increased FC in right insula |
||||
| DMN | Decreased within-network connectivity | ||||
| (Zhang et al., 2015) | Adult motor vehicle accident survivors with PTSD (20) | Matched healthy adults without PTSD (20) | Resting State | SN → posterior DMN | Increased Negative FC |
| SN | Decreased ACC Coupling | ||||
| DMN | Decreased mPFC and PCC coupling | ||||
| (Tursich et al., 2015) | Adults with PTSD and experience of childhood trauma (21) | None | Resting State | SN | Reduced within-network connectivity associated with increased hyperarousal symptoms |
| DMN | Reduced within-network connectivity associated with increased depersonalization/derealization symptoms | ||||
| CEN → DMN | Reduced between-network connectivity associated with increased depersonalization/derealization symptoms | ||||
| (Ke et al., 2018) | Adult typhoon survivors with PTSD (27) | Adult typhoon survivors without PTSD (33) and non-exposed healthy adults (30) | Resting State | CEN → Posterior DMN | Increased excitatory connection (PTSD vs. TEC and HC) |
| (Rabellino et al., 2015) | Adults with PTSD (26) | Healthy Adults without PTSD (20) | Threat-Processing | CEN | Hypercoupling of dACC and mPFC with the CEN |
| SN | Higher integration of dACC and mPFC with SN during subliminal threat processing | ||||
| DMN | Higher integration of the amygdala with the DMN during subliminal threat processing | ||||
| (Thome et al., 2014) | Adult females with PTSD and childhood trauma exposure (16) | Healthy adult females without PTSD (16) | Direct vs. Averted Eye Gaze with Emotional Faces | SN | Increased within-network FC during direct vs. averted eye gaze due to increased integration of left amygdala and right insula PTSD symptom severity positively correlated with mid-cingulate integration with SN |
| (Ross et al., 2018) | Adult females with PTSD and exposure to interpersonal violence (15) | Matched healthy adult females without PTSD (14) | Neutral Reinforcement Learning | Ventral Striatum/mPFC | Decreased encoding of prediction errors in PTSD |
| Anterior Insula | Decreased encoding of prediction errors in PTSD | ||||
3.2. Graph theory
Several principles of graph theory have been applied to investigations of brain network communication and dynamics. When using graph theory methods for fMRI analysis, investigators conceptualize the timecourse of functional activity within each brain region (or “node”) as one large, interconnected graph. The graph can then be analyzed in terms of local connectivity (i.e. connectivity profiles of individual nodes or circuits) and global connectivity (i.e. large-scale organization of the entire graph). The relevant definitions for local connectivity measures are located within Table 6. Please see the Supplemental Material for further descriptions of graph theory concepts and methods.
Table 6.
Definitions for relevant measures of local connectivity from graph theory analyses. Relevant references include: (Avena-Koenigsberger et al., 2018, Bassett et al., 2008, Bassett and Bullmore, 2006, Power et al., 2013, Puetz et al., 2017, Sporns, 2014, Sporns and Betzel, 2016, van den Heuvel et al., 2008).
| Local Connectivity Measure | Definition |
|---|---|
| Assortativity | Correlation between the degree of a node and the mean degree of its nearest neighbors; positive correlations indicate that the given node is likely to be connected to other nodes of the same degree |
| Betweenness Centrality | Measure of a node’s influence in a network based on the proportion of shortest paths that connect through that node; the number of times a node acts as a bridge between shortest paths of other nodes |
| Clustering Coefficient | Measure of the connectedness of a node to its nearest neighbors; nodes with high clustering coefficients are have neighbors that are mutually connected into communities |
| Efficiency | Metric of information transfer; inversely proportional to the average minimum path length |
| Hub | Highly clustered, central node that facilitates communication within and between functional brain networks |
| Node Degree | Measure of the number of edges that connect to each node. High-degree nodes have many edges connected to other nodes |
| Node Strength | Measure of the strength of the correlation between a node and other nodes based on its edge weight |
| Participation Coefficient | Measure of the distribution of a node’s connections across communities. Nodes with a high participation coefficient have connections across all communities in the network |
| Path Length | Determined by the number of edges between two mediating nodes; shorter path lengths facilitate efficient information transfer |
| Transitivity | Measure of the proportion of complete node triangles within a network; networks with high values of transitivity have many edges connected to many other nodes |
Studies that utilize graph theory approaches have a distinct advantage over ICA-based approaches because the use of graph theory can reveal information about the brain’s global connectivity in disease states, while ICA is more limited to distinct network patterns of connectivity. Using this data-driven approach, researchers have painted a global picture of the brain’s networks and connectome in PTSD, from which one can start to hypothesize whole-brain relationships with symptoms of the disorder (Table 7). As mentioned previously, the brain’s functional connectome is conceptualized as a small-world network and deviations from this configuration, including reduced global efficiency and reduced clustering, have been linked to trauma exposure in youth (Cisler et al., 2018, Suo et al., 2015, Xu et al., 2018) and PTSD in adults (Lei et al., 2015). Additionally, increased global modularity has been found to be associated with early life trauma in adolescents (Cisler, 2017, Cisler et al., 2018) and combat-related PTSD severity in adults (Akiki et al., 2018). At the individual network level, altered connectivity, decreased nodal and global efficiency, and increased clustering has been found in the DMN of adults with PTSD (Akiki et al., 2018, Zhang et al., 2017). Additionally, others have noted decreased connectivity and connectional density within a hippocampal-PFC network that could be predicted by re-experiencing symptom severity within an adult group of PTSD patients (Spielberg et al., 2015). Observations such as these provide evidence for investigating PTSD and similar pathologies as disorders of deviation from normative canonical network structure, which might be driving the observations of decreased binodal functional connectivity in traditional neurocircuitry models of the disorder. Taken together, findings from these studies provide promising new outlets for research and evidence for reconceptualization of PTSD neural circuitry through a large-scale network perspective.
Table 7.
Studies of posttraumatic stress disorder using measures from Graph Theory. Eleven different studies utilized concepts from graph theory in functional neuroimaging to investigate network-level alterations in PTSD patient groups. ELT - early life trauma; CTQ – Childhood Trauma Questionnaire; CAPS – Clinician-Administered PTSD Scale; DMN – default mode network; PFC – prefrontal cortex; FC – functional connectivity; IPV – interpersonal violence; ACC – anterior cingulate cortex; L – left hemisphere; MFG – middle frontal gyrus.
| Author(s) and Year | Task | Patient Group (N) | Control Group (N) | Metric | Relationship with Population |
|---|---|---|---|---|---|
| Global Connectivity | |||||
| (Cisler et al., 2018) | Facial Emotion Processing | Adolescent girls with experience of early life trauma (59) | Healthy adolescent girls without trauma history (29) | Modularity | Positive association with ELT |
| Assortativity | Positive association with ELT | ||||
| Global Efficiency | Negatively associated with ELT | ||||
| (Ohashi et al., 2017) | Resting State DTI | Young adults with three or more events of childhood maltreatment (1 4 0) | Young adults with no or low exposure to childhood maltreatment (1 2 2) | Global Efficiency, Strength, and Degree | Reduced in moderate-to-high childhood maltreatment exposure group |
| Small-worldness | Increased in moderate-to-high childhood maltreatment exposure group Increased path length in moderate-to-high exposure group |
||||
| (Puetz et al., 2017) | Resting State DTI | Children with documented maltreatment before age 3 (25) | Heathy children with no maltreatment exposure (24) | Whole-Brain General Node Strength | Decreased overall node strength in childhood maltreatment group |
| Global Assortativity and Transitivity | Decreased in childhood maltreatment group | ||||
| (Cisler, 2017) | Resting State fMRI | Adolescent girls with experience of early life trauma (26) | Healthy adolescent girls without trauma history (30) | Modularity | Global modularity is positively correlated with emotional abuse subscale scores on the CTQ |
| (Akiki et al., 2018) | Resting State fMRI | Adult combat veterans with PTSD (36) | Adult combat veterans without PTSD (35) | Modularity | Increased with increasing CAPS severity |
| Inversely related to DMN functional connectivity strength, predicted by CAPS severity | |||||
| (Xu et al., 2018) | Resting State fMRI | Youth earthquake survivors with PTSD (10) | Youth earthquake survivors without PTSD (16) | Clustering Coefficient | Reduced |
| Characteristic Path Length | Reduced | ||||
| Global Efficiency | Increased | ||||
| (Suo et al., 2015) | Resting State fMRI | Adolescent earthquake survivors with PTSD (24) | Adolescent earthquake survivors without PTSD (24) | Small-worldness | Increased clustering coefficient in PTSD group Normalized path length in PTSD Increased local efficiency in PTSD |
| (Lei et al., 2015) | Resting State fMRI | Adult earthquake survivors with PTSD (76) | Adult earthquake survivors without PTSD (76) | Small-worldness | Reduced path length in PTSD group Higher clustering, global, and local efficiency in PTSD group |
| Local Connectivity | |||||
| (Spielberg et al., 2015) | Resting State fMRI | Adult combat veterans with PTSD (2 0 8) | – | Connection Density in hippocampal-PFC network | Negative relationship between re-experiencing symptoms and connection density |
| Within-Network Connectivity | Negative relationship between hippocampal-PFC network connectivity and re-experiencing symptoms | ||||
| (Zhang et al., 2017) | Resting State fMRI | Adult earthquake survivors with PTSD (62) | Adult earthquake survivors without PTSD (62) | Within Network FC of DMN | Negative relationship with avoidance symptoms Decreased nodal efficiency in superior frontal gyrus Increased nodal efficiency in hippocampus/parahippocampus |
| (Roos et al., 2017) | T1 Structural | Adult women exposed to interpersonal violence (IPV; 18) | Matched healthy control women without interpersonal violence exposure (IPV; 18) | Betweenness Centrality of ACC-Precuneus network | Increased within IPV group |
| Regional Degree Connectivity | Increased in temporal lobe regions in IPV group | ||||
| Connection Density of FPN | Decreased in IPV group | ||||
| (Puetz et al., 2017) | Resting State DTI | Children with documented maltreatment before age 3 (25) | Heathy children with no maltreatment exposure (24) | Characteristic Path Length | Increased in frontal lobe of childhood maltreatment group |
| (Akiki et al., 2018) | Resting State fMRI | Adult combat veterans with PTSD (36) | Adult combat veterans without PTSD (35) | Functional Connectivity Strength of DMN | Reduced with increasing PTSD symptom severity |
| Global Efficiency of DMN | Reduced with increasing PTSD symptom severity | ||||
| Clustering Coefficient (DMN) | Increased with increasing PTSD symptom severity | ||||
| (Xu et al., 2018) | Resting State fMRI | Youth earthquake survivors with PTSD (10) | Youth earthquake survivors without PTSD (16) | Nodal Centrality | Increased – L MFG, Caudate, Hippocampus Decreased – L ACC, L Paracentral Lobule, Bilateral Thalamus |
3.3. Discussion and evaluation of models: Network-based literature
The extant body of literature utilizing network-based approaches to investigate functional brain organization in subjects with trauma exposure and PTSD provides limited support for the classical model of PTSD neural circuitry. While the themes of the classical model would predict the PTSD group to demonstrate altered connectivity profiles in the fronto-limbic circuit primarily, evidence from the network literature provides little support for the dominance of this circuit in PTSD groups. In fact, only two studies isolated the amygdala as having altered connectivity at a network level in PTSD groups. In both studies, investigators observed increased integration of the amygdala with canonical networks, one with the DMN during a threat-processing task (Rabellino et al., 2015) and the other with the SN during a direct eye gaze task (Thome et al., 2014). The latter finding of enhanced integration of the amygdala with the SN supports the classical model’s characterization of PTSD as a disorder associated with hyperactive amygdala and dACC; however, the former finding is not fully consistent with the classical model’s characterization that PTSD is associated with reduced mPFC activity, as enhanced integration of the amygdala with the DMN would imply the existence of a robust, coordinated circuit. The apparent lack of altered amygdala-mPFC connectivity in resting-state, network-based analyses suggests that the classical model may only be supported by task-related fMRI analyses in PTSD groups. Similarly, the second prediction from the classical model of alterations in nodal characteristics of the amygdala and mPFC in PTSD groups is not supported by the network-based literature. While some studies indirectly suggest altered node characteristics of the mPFC through its integration with the DMN, no individual node was observed to have an altered connectivity profile that drove connectivity changes in the larger network structure. It is important to keep in mind, however, that network-based literature has a different focus than uni- and bivariate literature and therefore the lack of fronto-limbic connectivity revealed in the network literature may be a question of resolution and does not inherently imply a negation of classical models.
The network-based literature in PTSD patient groups provides substantive support for a network model of PTSD neural circuitry according to the predictions evaluated in this report. Several studies observed alterations in global connectivity patterns in the brain networks of PTSD groups, including increased modularity (Akiki et al., 2018, Cisler, 2017, Cisler et al., 2018), decreased global efficiency (Cisler et al., 2018), alterations in clustering (Xu et al., 2018), and deviations from small-world structure (Lei et al., 2015, Suo et al., 2015). Taken together, these findings from both functional and structural connectivity studies support a characterization of PTSD neural circuitry as alterations in large-scale network connectivity structure. Additionally, the network-based literature supports the prediction that a network model of PTSD neural circuitry would be characterized by reduced connectivity of the DMN. Evidence from ICA studies report decreased within-network coupling of the DMN (Reuveni et al., 2016, Shang et al., 2014) that was inversely related to depersonalization and derealization symptoms (Tursich et al., 2015) and decreased FC between the DMN and SN (Zhang et al., 2015) during resting-state. Three other studies from the network literature observed alterations in the DMN in PTSD groups, two of which suggested inverse associations between FC coherence within the DMN and PTSD symptoms (Akiki et al., 2018, Zhang et al., 2017). Conversely, other studies provide evidence that fails to support the prediction of reduced connectivity in the DMN, with observations of enhanced clustering (Akiki et al., 2018) and increased FC strength (Patriat et al., 2016) of the network associated with PTSD symptoms. Similarly, though two studies do suggest decreased coupling of CEN nodes in PTSD groups (Rabellino et al., 2015, Shang et al., 2014), and two suggest enhanced integration of the SN during task (Rabellino et al., 2015, Thome et al., 2014), other studies in this literature have contrasting observations about the connectivity profiles of these networks in PTSD patient groups. Though the evidence is mixed for a network model of PTSD neural circuitry, in regard to the specific predictions discussed in this report, patterns of larger network connectivity emerge in support of network model.
4. Conclusions
To our knowledge, this review is the first of its kind to summarize over a decade’s worth of functional connectivity (FC) MRI research in PTSD. Studies utilizing bivariate seed-based FC approaches, a circuit-based method that bridges univariate and network-based investigations in fMRI, were used to evaluate the evidence for classical and network models of PTSD-related neural circuitry. Interestingly, two patterns emerge when examining bivariate seed-based functional connectivity in the disorder: overall reduced connectivity to targets in PTSD groups and disproportionate membership of altered FC targets within the default mode network. Results from network-level investigations reflect these bivariate findings by providing evidence for increased modularity and alterations in connectivity of the DMN in PTSD patients. This replication of findings provides an imperative for studying the directionality of bivariate FC alterations in PTSD, as the traditional models may be indicating dysfunctional connectivity between large-scale networks or altered graph structure rather than individual nodes contained within those networks. ICA and graph-theory based approaches can provide this information about the function of larger brain circuits and overall functional organization, which bivariate approaches are unable to provide.
Univariate activation models have dominated our understanding of PTSD neural circuitry for several decades. Though this “classical model” of a hyperactive amygdala and hypoactive mPFC is essential to understanding the foundations of PTSD neural circuitry, it may be overly simplistic when examining the breadth of the functional brain organization literature. Though some bivariate FC literature demonstrates reduced connectivity from the mPFC to limbic regions, and some bivariate findings suggest evidence for enhanced amygdala connectivity to the dACC and anterior insula, network-based studies fail to isolate these circuits as consistently altered in PTSD groups. Conversely, neural circuitry literature in trauma-exposed and PTSD patient groups provides better support for an integrated model of PTSD neural circuitry that both conceptualizes functional organization through a network perspective and also retains the finer spatial resolution of specific regional activation patterns specified by the classical model. Bivariate FC studies reveal a global pattern of reduced FC throughout the brain as well as dominance of a coordinated default mode network that shows altered connectivity from various seeds in the PTSD group. These patterns reflect global patterns of connectivity that a univariate model inherently cannot predict. The network-based literature also provides evidence for connectivity alterations within and between canonical networks, lending support to a neural network model of PTSD neural circuitry. Because the evidence from the seed-based FC and network-based organization literature lacks total cohesion, it is likely the case that PTSD neural circuitry is best represented through an integrative framework of regionally-specific alterations along with larger-scale changes in functional organization at the network level. Reconceptualizing neural deficits at this level in PTSD may be useful for shifting clinical focus away from the narrowed model of deficient fear processing and towards interventions that target widespread processing impairments in trauma-exposed patients.
5. Limitations and considerations for future work
The qualitative method employed in this report has many strengths in its ability to detect nuanced patterns of altered FC in PTSD; however, this report is limited by the dichotomization of groups into “PTSD” and “Controls.” This qualitative method was utilized for the sake of improving our power to detect group differences in FC patterns, yet may contribute to some ambiguity in conclusions. Similarly, one of the limitations of the classical and network-based models is that these models are often based on groups dichotomized into “PTSD” or “Control” and rarely include a discussion of symptom severity or other clinical factors of interest that may be related to alterations in neural circuitry. Future work should consider the role of PTSD symptom severity or symptom clusters in neural circuitry models of PTSD in order to gain some understanding of the distinct neural signatures of clinically-relevant symptoms. Finally, the integrative network model supported by the evidence examined in this report requires substantive testing before conclusions can be made about the utility of network models in PTSD. Future work might consider utilizing fMRI neurofeedback or connectome-wide approaches, similar to the methods described by Misaki and colleagues (Misaki et al., 2018) to examine the dynamic functional organization of large-scale networks during emotional regulation tasks. These approaches could help to determine if functional network organization can be targeted to modulate emotion processing deficits in trauma-exposed patients or if changes in PTSD symptom severity during clinical treatment are related to changes in underlying functional network structure.
Despite the many merits of the classical and network-based models of neural circuitry alterations in PTSD, some limitations and areas for improvement still exist. Firstly, most of the literature covered in this report discusses altered neural circuitry within the context of the adult brain, resulting in a dearth of understanding about the potential role for development in altered neural circuitry in PTSD. In fact, of the classical models described in Section 1.1, only the Admon model directly considers childhood trauma as a predisposing factor for altered neural circuitry, leaving the role of development within the models of PTSD neural circuitry unclear. Future work should rigorously investigate patterns of altered functional organization within pediatric PTSD in a similar manner to the adult-based models described in Section 1.1 in order to develop a neural circuitry model specific to pediatric PTSD. Additionally, conclusions drawn from combining bivariate FC investigations in PTSD are limited by methodological choices to transform FC correlation values. The type of transformation used may vary from study-to-study, preventing a comprehensive comparison of the strength of FC alterations between clinical groups. Future work would benefit from researchers providing raw correlation values to allow for cross-study comparison of FC strengths. Finally, the question of power in functional neuroimaging studies is an ever-present consideration. In order to reduce the bias implemented from a priori ROI FC studies, researchers should aim to conduct whole-brain analyses when power is sufficient to do so. If a study is underpowered for a whole-brain analysis, researchers should provide strong theoretical justification for the choice of ROIs and include appropriate corrections for multiple comparisons.
The development of methods for large-scale network models in neuroimaging has expanded the depth of investigations into psychological disorders. Using the brain’s small-world structure and canonical networks as a model of normative organization, researchers have identified new potential correlates for PTSD-related psychopathology in the form of deviations in both global and individual network modularity, dysfunctional connectivity between and within large-scale networks, and divergence from small-world structure and normative node characteristics. However, some limitations exist in this nascent field. In order to strengthen conclusions regarding neural dysfunction in PTSD, steps must be made to improve consistency in methodology and network definitions. Though the studies referenced in this review follow similar conceptual techniques within ICA and graph-theory methods, the execution of network modeling varies substantially from group to group in image processing, statistical modeling, atlas selection, module definition, treatment of correlation matrices, among other factors. In light of growing concerns towards transparency and reproducibility in neuroimaging, network-based methods must be standardized across the field in order to generate more confidence in our results. The first step towards standardizing these methods is to reconceptualize the brain’s functional profile from one of simple activation in isolated brain regions to one of a network of interconnected parts, dynamically communicating to properly meet internal and environmental demands. It is within this integrated framework that investigators should operate to strengthen inferences about the brain’s functioning in health and affective psychopathologies.
Acknowledgements
Research in this publication was supported by the National Institute of Mental Health and the National Institutes of Health under awards T32MH018931-31, T32GM007507, R01MH119132, and R21/R33 MH108753.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102319.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdallah C.G., Averill C.L., Ramage A.E., Averill L.A., Alkin E., Nemati S., Krystal J.H., Roache J.D., Resick P., Young-McCaughan S., Peterson A.L., Fox P., STRONG STAR Consortium Reduced salience and enhanced central executive connectivity following PTSD treatment. Chronic Stress Thousand Oaks Calif. 2019;3 doi: 10.1177/2470547019838971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R., Milad M.R., Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn. Sci. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Aghajani M., Veer I.M., van Hoof M.-J., Rombouts S.A.R.B., van der Wee N.J., Vermeiren R.R.J.M. Abnormal functional architecture of amygdala-centered networks in adolescent posttraumatic stress disorder. Hum. Brain Mapp. 2016;37:1120–1135. doi: 10.1002/hbm.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Abdallah C.G. A Network-based neurobiological model of PTSD: Evidence from structural and functional neuroimaging studies. Curr. Psychiatry Rep. 2017;19:81. doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Wrocklage K.M., Scott J.C., Averill L.A., Schweinsburg B., Alexander-Bloch A., Martini B., Southwick S.M., Krystal J.H., Abdallah C.G. Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. NeuroImage. 2018;176:489–498. doi: 10.1016/j.neuroimage.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena-Koenigsberger A., Misic B., Sporns O. Communication dynamics in complex brain networks. Nat. Rev. Neurosci. 2018;19:17. doi: 10.1038/nrn.2017.149. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E. Small-world brain networks. The Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E., Verchinski B.A., Mattay V.S., Weinberger D.R., Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum G.L., Ciric R., Roalf D.R., Betzel R.F., Moore T.M., Shinohara R.T., Kahn A.E., Vandekar S.N., Rupert P.E., Quarmley M., Cook P.A., Elliott M.A., Ruparel K., Gur R.E., Gur R.C., Bassett D.S., Satterthwaite T.D. Modular segregation of structural brain networks supports the development of executive function in youth. Curr. Biol. 2017;27:1561–1572.e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero M.A., Yeo B.T.T., D’Esposito M. The modular and integrative functional architecture of the human brain. Proc. Natl. Acad. Sci. 2015;112:E6798–E6807. doi: 10.1073/pnas.1510619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Patriat R., Phillips M.L., Germain A., Herringa R.J. Childhood maltreatment and combat post-traumatic stress differentially predict fear–related fronto-subcortical connectivity. Depress. Anxiety. 2014;31:880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 2008;8 [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Nicolini C., Forcellini G., Bifone A. Disrupted modular organization of primary sensory brain areas in schizophrenia. NeuroImage Clin. 2018;18:682–693. doi: 10.1016/j.nicl.2018.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Schultz L.R. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch. Gen. Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brown V.M., LaBar K.S., Haswell C.C., Gold A.L., Beall Shannon K., Van Voorhees Elizabeth, Marx Christine E., Calhoun Patrick S., Fairbank John A., Green Kimberly T., Tupler Larry A., Weiner Richard D., Beckham Jean C, Brancu Mira, Hoerle Jeffrey M., Pender Mary, Kudler Harold, Swinkels Cynthia M., Nieuwsma Jason A., Runnals Jennifer J., Youssef Nagy A., McDonald Scott D., Davison Rita, Yoash-Gantz Ruth, Taber Katherine H., Hurley Robin, McCarthy G., Morey R.A. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:361–369. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., Liddell B., Olivieri G., Peduto A., Gordon E., Williams L.M. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum. Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Chen A.C., Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38:1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Ke J., Qi R., Xu Q., Zhong Y., Liu T., Li J., Zhang L., Lu G. Increased inhibition of the amygdala by the mpfc may reflect a resilience factor in post-traumatic stress disorder: A resting-state fMRI granger causality analysis. Front. Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Deem M.W. Development of modularity in the neural activity of children’s brains. Phys. Biol. 2015;12 doi: 10.1088/1478-3975/12/1/016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.J., He Y., Rosa-Neto P., Germann J., Evans A.C. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb. Cortex. 2008;18:2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M. Childhood trauma and functional connectivity between amygdala and medial prefrontal cortex: A dynamic functional connectivity and large-scale network perspective. Front. Syst. Neurosci. 2017;11:29. doi: 10.3389/fnsys.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Privratsky A., Smitherman S., Herringa R.J., Kilts C.D. Large-scale brain organization during facial emotion processing as a function of early life trauma among adolescent girls. NeuroImage Clin. 2018;17:778–785. doi: 10.1016/j.nicl.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloitre M. Effective psychotherapies for posttraumatic stress disorder: a review and critique. CNS Spectr. 2009;14:32–43. [PubMed] [Google Scholar]
- Cloitre M., Scarvalone P., Difede J. Posttraumatic Stress Disorder, Self- and Interpersonal Dysfunction Among Sexually Retraumatized Women. J. Trauma. Stress. 1997;10:437–452. doi: 10.1023/A:1024893305226. [DOI] [PubMed] [Google Scholar]
- Cohen J.R., D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., Wendt G.J., Turski P.A., Moritz C.H., Quigley M.A., Meyerand M.E. Mapping functionally related regions of brain with functional connectivity MR imaging. Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. Int. J. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DiGangi J.A., Tadayyon A., Fitzgerald D.A., Rabinak C.A., Kennedy A., Klumpp H., Rauch S.A.M., Phan K.L. Reduced default mode network connectivity following combat trauma. Neurosci. Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P., Cath D.C., Lissek S., Hox J.J., Hamm A.O., Engelhard I.M., van den Hout M.A., Baas J.M.P. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety. 2015;32:239–253. doi: 10.1002/da.22353. [DOI] [PubMed] [Google Scholar]
- Feng P., Zheng Y., Feng T. Spontaneous brain activity following fear reminder of fear conditioning by using resting-state functional MRI. Sci. Rep. 2015;5:16701. doi: 10.1038/srep16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Essen D.C.V., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin D., Barry R.L., Marois R. Breakdown of the brain’s functional network modularity with awareness. Proc. Natl. Acad. Sci. 2015;112:3799–3804. doi: 10.1073/pnas.1414466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad. Sci. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cereb. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X., Gabrieli J.D.E., Schmahmann J.D. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. NeuroImage. 2018;172:437–449. doi: 10.1016/j.neuroimage.2018.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan S., Nicholson A.A., Densmore M., Théberge J., McKinnon M.C., Neufeld R.W.J., Lanius R.A. Sensory overload and imbalance: Resting-state vestibular connectivity in PTSD and its dissociative subtype. Neuropsychologia. 2017;106:169–178. doi: 10.1016/j.neuropsychologia.2017.09.010. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Fornito A. Brain Networks in Schizophrenia. Neuropsychol. Rev. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Stam C.J., Kahn R.S., Pol H.E.H. Efficiency of functional brain networks and intellectual performance. J. Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn, S.A., Keding, T.J., Ross, M.C., Cisler, J.M., Mumford, J.A., Herringa, R.J., 2018. Abnormal prefrontal development in pediatric posttraumatic stress disorder: a longitudinal structural and functional magnetic resonance imaging study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. https://doi.org/10.1016/j.bpsc.2018.07.013. [DOI] [PMC free article] [PubMed]
- Jafri M.J., Pearlson G.D., Stevens M., Calhoun V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Qi R., Yin Y., Hu X., Duan L., Xu Q., Zhang Z., Zhong Y., Feng B., Xiang H., Gong Q., Liu Y., Lu G., Li L. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol. Med. 2014;44:1927–1936. doi: 10.1017/S003329171300250X. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin A.N., Burton P.C., Chazin S.M., Manbeck A.B., Espensen-Sturges T., Cooper S.E., Sponheim S.R., Lissek S. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry. 2017;174:125–134. doi: 10.1176/appi.ajp.2016.15121549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A., Schaefer M., Malta L.S., Dörfel D., Rohleder N., Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Ke J., Zhang L., Qi R., Xu Q., Zhong Y., Liu T., Li J., Lu G., Chen F. Typhoon-related post-traumatic stress disorder and trauma might lead to functional integration abnormalities in intra- and inter-resting state networks: a resting-state fMRI independent component analysis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;48:99–110. doi: 10.1159/000491666. [DOI] [PubMed] [Google Scholar]
- Keding T.J., Herringa R.J. Paradoxical prefrontal-amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:2903–2912. doi: 10.1038/npp.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M., Rademaker A.R., van Rooij S.J.H., Kahn R.S., Geuze E. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum. Brain Mapp. 2015;36:99–109. doi: 10.1002/hbm.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kitayama N., Vaccarino V., Kutner M., Weiss P., Bremner J.D. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J. Affect. Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016;33:592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Koenigs M., Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Huey E.D., Raymont V., Cheon B., Solomon J., Wassermann E.M., Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat. Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster E.H.W., Crombez G., Verschuere B., Van Damme S., Wiersema J.R. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behav. Res. Ther. 2006;44:1757–1771. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol. Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Laird, A.R., Eickhoff, S.B., Rottschy, C., Bzdok, D., Ray, K.L., Fox, P.T., 2013. Networks of task co-activations. NeuroImage, Mapping the Connectome 80, 505–514. https://doi.org/10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed]
- Lei D., Li K., Li L., Chen F., Huang X., Lui S., Li J., Bi F., Gong Q. Disrupted Functional Brain Connectome in Patients with Posttraumatic Stress Disorder. Radiology. 2015;276:818–827. doi: 10.1148/radiol.15141700. [DOI] [PubMed] [Google Scholar]
- Li L., Wu M., Liao Y., Ouyang L., Du M., Lei D., Chen L., Yao L., Huang X., Gong Q. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci. Biobehav. Rev. 2014;43:163–172. doi: 10.1016/j.neubiorev.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Abelson J.L. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C., Zeffiro T.A., Pitman R.K., Milad M.R. An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol. Mood Anxiety Disord. 2011;1:8. doi: 10.1186/2045-5380-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresto D., Schipper P., Homberg J.R. Neural circuits and mechanisms involved in fear generalization: Implications for the pathophysiology and treatment of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2016;60:31–42. doi: 10.1016/j.neubiorev.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Etkin A., Thomason M.E. Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage Clin. 2015;8:516–525. doi: 10.1016/j.nicl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Martin K.R., Etkin A., Thomason M.E. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40:1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Qiu C., Zhu H., Lama S., Lui S., Gong Q., Zhang W. Anatomical deficits in adult posttraumatic stress disorder: a meta-analysis of voxel-based morphometry studies. Behav. Brain Res. 2014;270:307–315. doi: 10.1016/j.bbr.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 2013;17:627–640. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.R., Hayes S.M., Hayes J.P., Spielberg J.M., Lafleche G., Verfaellie M. Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2:363–371. doi: 10.1016/j.bpsc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M., Phillips R., Zotev V., Wong C.-K., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Connectome-wide investigation of altered resting-state functional connectivity in war veterans with and without posttraumatic stress disorder. NeuroImage Clin. 2018;17:285–296. doi: 10.1016/j.nicl.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Dunsmoor J.E., Haswell C.C., Brown V.M., Vora A., Weiner J., Stjepanovic D., Wagner H.R., VA Mid-Atlantic MIRECC Workgroup. LaBar K.S. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Sapru I., Densmore M., Frewen P.A., Neufeld R.W.J., Théberge J., McKinnon M.C., Lanius R.A. Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res. Neuroimaging. 2016;250:61–72. doi: 10.1016/j.pscychresns.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T., Olin I.W., Sands L.A., Karapanou I., Bradley B., Ressler K.J. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Aihara T., Shimokawa T., Yamashita O. Large-scale brain network associated with creative insight: combined voxel-based morphometry and resting-state functional connectivity analyses. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-24981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K., Anderson C.M., Bolger E.A., Khan A., McGreenery C.E., Teicher M.H. Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. NeuroImage. 2017;150:50–59. doi: 10.1016/j.neuroimage.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Nakataki M., Takeda T., Numata S., Tominaga T., Kameoka N., Kubo H., Kinoshita M., Matsuura K., Otomo M., Takeichi N., Harada M., Ohmori T. Structural equation modeling approach between salience network dysfunction, depressed mood, and subjective quality of life in schizophrenia: an ICA resting-state fMRI study. Neuropsychiatr. Dis. Treat. 2018;14:1585–1597. doi: 10.2147/NDT.S163132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, E.A., Kaiser, R.H., Pizzagalli, D.A., Rauch, S.L., Rosso, I.M., 2018. Regional prefrontal resting state functional connectivity in PTSD. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. https://doi.org/10.1016/j.bpsc.2018.09.012. [DOI] [PMC free article] [PubMed]
- Onoda K., Yamaguchi S. Small-worldness and modularity of the resting-state functional brain network decrease with aging. Neurosci. Lett. 2013;556:104–108. doi: 10.1016/j.neulet.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Patriat R., Birn R.M., Keding T.J., Herringa R.J. Default-mode network abnormalities in pediatric posttraumatic stress disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:319–327. doi: 10.1016/j.jaac.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri T., Ben-Shakhar G., Orr S.P., Shalev A.Y. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol. Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pessoa L. Understanding emotion with brain networks. Curr. Opin. Behav. Sci. 2018;19:19–25. doi: 10.1016/j.cobeha.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol. Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Lessov-Schlaggar C.N., Petersen S.E. Evidence for hubs in human functional brain networks. Neuron. 2013;79 doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz V.B., Parker D., Kohn N., Dahmen B., Verma R., Konrad K. Altered brain network integrity after childhood maltreatment: A structural connectomic DTI-study. Hum. Brain Mapp. 2017;38:855–868. doi: 10.1002/hbm.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Wang Z., Sun Y., Wan J., Su S., Zhou Y., Xu J. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Frewen P.A., Théberge J., McKinnon M.C., Lanius R.A. Aberrant functional connectivity of the amygdala complexes in PTSD during conscious and subconscious processing of trauma-related stimuli. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0163097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Tursich M., Frewen P.A., Daniels J.K., Densmore M., Théberge J., Lanius R.A. Intrinsic connectivity networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr. Scand. 2015;132:365–378. doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Welsh R.C., Kennedy A., Lyubkin M., Martis B., Phan K.L. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front. Psychiatry. 2011;2 doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Rashid, B., Blanken, L.M.E., Muetzel, R.L., Miller, R., Damaraju, E., Arbabshirani, M.R., Erhardt, E.B., Verhulst, F.C., Lugt, A. van der, Jaddoe, V.W.V., Tiemeier, H., White, T., Calhoun, V., 2018. Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum. Brain Mapp. https://doi.org/10.1002/hbm.24064. [DOI] [PMC free article] [PubMed]
- Rauch S.L., Shin L.M., Phelps E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol. Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reuveni I., Bonne O., Giesser R., Shragai T., Lazarovits G., Isserles M., Schreiber S., Bick A.S., Levin N. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum. Brain Mapp. 2016;37:589–599. doi: 10.1002/hbm.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Fouche J.-P., Stein D.J. Brain network connectivity in women exposed to intimate partner violence: a graph theory analysis study. Brain Imaging Behav. 2017;11:1629–1639. doi: 10.1007/s11682-016-9644-0. [DOI] [PubMed] [Google Scholar]
- Ross M.C., Lenow J.K., Kilts C.D., Cisler J.M. Altered neural encoding of prediction errors in assault-related posttraumatic stress disorder. J. Psychiatr. Res. 2018;103:83–90. doi: 10.1016/j.jpsychires.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russman Block S., King A.P., Sripada R.K., Weissman D.H., Welsh R., Liberzon I. Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cogn. Affect. Behav. Neurosci. 2017;17:422–436. doi: 10.3758/s13415-016-0488-2. [DOI] [PubMed] [Google Scholar]
- Scolari M., Seidl-Rathkopf K.N., Kastner S. Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr. Opin. Behav. Sci. 2015;1:32–39. doi: 10.1016/j.cobeha.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Lui S., Meng Y., Zhu H., Qiu C., Gong Q., Liao W., Zhang W. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: A resting-state fMRI study. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shou H., Yang Z., Satterthwaite T.D., Cook P.A., Bruce S.E., Shinohara R.T., Rosenberg B., Sheline Y.I. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage Clin. 2017;14:464–470. doi: 10.1016/j.nicl.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel L.M., Marshall A.D. Posttraumatic stress disorder and fear of emotions: the role of attentional control. J. Trauma. Stress. 2013;26:397–400. doi: 10.1002/jts.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.S., Yoo K., Lee Y.-B., Seo S.W., Na D.L., Jeong Y. Influence of ROI selection on resting state functional connectivity: an individualized approach for resting state fMRI analysis. Front. Neurosci. 2015;9 doi: 10.3389/fnins.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., McGlinchey R.E., Milberg W.P., Salat D.H. Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol. Psychiatry. 2015;78:210–216. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat. Neurosci. 2014;17:652–660. doi: 10.1038/nn.3690. [DOI] [PubMed] [Google Scholar]
- Sporns O., Betzel R.F. Modular brain networks. Annu. Rev. Psychol. 2016;67:613–640. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., Angstadt M., Kessler D., Phan K.L., Liberzon I., Evans G.W., Welsh R., Kim P., Swain J.E. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. NeuroImage. 2014;89:110–121. doi: 10.1016/j.neuroimage.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Garfinkel S.N., Wang X., Sripada C.S., Welsh R.C., Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C., Daniels J.K., Frewen P.A., Densmore M., Theberge J., Lanius R.A. Effect of direct eye contact in women with PTSD related to interpersonal trauma: Psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Res. Neuroimaging. 2015;232:162–167. doi: 10.1016/j.pscychresns.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Jovanovic T., Fani N., Ely T.D., Glover E.M., Bradley B., Ressler K.J. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res. 2013;47:1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo, X., Lei, D., Li, K., Chen, F., Li, F., Li, Lei, Huang, X., Lui, S., Li, Lingjiang, Kemp, G.J., Gong, Q., 2015. Disrupted brain network topology in pediatric posttraumatic stress disorder: A resting-state fMRI study: Suo et al. Hum. Brain Mapp. 36, 3677–3686. https://doi.org/10.1002/hbm.22871. [DOI] [PMC free article] [PubMed]
- Szczepanski S.M., Konen C.S., Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J. Neurosci. 2010;30:148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A., Tocco M.A., Vila A.M., McGarragle O., Rosenberg D.R. Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci. 2015;10:1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J., Frewen P., Daniels J.K., Densmore M., Lanius R.A. Altered connectivity within the salience network during direct eye gaze in PTSD. Borderline Personal. Disord. Emot. Dysregulat. 2014;1:17. doi: 10.1186/2051-6673-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Functional connectivity density mapping. Proc. Natl. Acad. Sci. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull M.T., Barrett H.M., McMillan E.S., Roemer L. A preliminary investigation of the relationship between emotion regulation difficulties and posttraumatic stress symptoms. Behav. Ther. 2007;38:303–313. doi: 10.1016/j.beth.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tursich M., Ros T., Frewen P.A., Kluetsch R.C., Calhoun V.D., Lanius R.A. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr. Scand. 2015;132:29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Stam C.J., Boersma M., Hulshoff Pol H.E. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. NeuroImage. 2008;43:528–539. doi: 10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- van der Werff S.J.A., Pannekoek J.N., Veer I.M., van Tol M.-J., Aleman A., Veltman D.J., Zitman F.G., Rombouts S.A.R.B., Elzinga B.M., van der Wee N.J.A. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol. Med. 2013;43:1825–1836. doi: 10.1017/S0033291712002942. [DOI] [PubMed] [Google Scholar]
- Viard A., Mutlu J., Chanraud S., Guenolé F., Egler P.-J., Gérardin P., Baleyte J.-M., Dayan J., Eustache F., Guillery-Girard B. Altered default mode network connectivity in adolescents with post-traumatic stress disorder. NeuroImage Clin. 2019;22 doi: 10.1016/j.nicl.2019.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M., Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am. J. Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Wessing I., Rehbein M.A., Postert C., Fürniss T., Junghöfer M. The neural basis of cognitive change: reappraisal of emotional faces modulates neural source activity in a frontoparietal attention network. NeuroImage. 2013;81:15–25. doi: 10.1016/j.neuroimage.2013.04.117. [DOI] [PubMed] [Google Scholar]
- Wolf R.C., Herringa R.J. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:822–831. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Chen F., Lei D., Zhan W., Sun X., Suo X., Peng Z., Wang T., Zhang J., Gong Q. Disrupted functional network topology in children and adolescents with post-traumatic stress disorder. Front. Neurosci. 2018;12 doi: 10.3389/fnins.2018.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Yang T., Qing P., Lei X., Qiu J., Liu G. Changes of functional brain networks in major depressive disorder: a graph theoretical analysis of resting-state fMRI. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0133775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Jin C., Hu X., Duan L., Li Z., Song M., Chen H., Feng B., Jiang T., Jin H., Wong C., Gong Q., Li L. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain Res. 2011;1411:98–107. doi: 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Young K.S., Burklund L.J., Torre J.B., Saxbe D., Lieberman M.D., Craske M.G. Treatment for social anxiety disorder alters functional connectivity in emotion regulation neural circuitry. Psychiatry Res. Neuroimaging. 2017;261:44–51. doi: 10.1016/j.pscychresns.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Plis S.M., Erhardt E.B., Allen E.A., Sui J., Kiehl K.A., Pearlson G., Calhoun V.D. Modular organization of functional network connectivity in healthy controls and patients with schizophrenia during the resting state. Front. Syst. Neurosci. 2012;5 doi: 10.3389/fnsys.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q., Martin R.C., Fischer-Baum S., Ramos-Nuñez A.I., Ye F., Deem M.W. Brain modularity mediates the relation between task complexity and performance. J. Cogn. Neurosci. 2017;29:1532–1546. doi: 10.1162/jocn_a_01142. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., He Y., Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry, Neural Connectivity, Ruminations, and Suicide. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang J., Wang L., Li R., Zhang W. Altered resting-state functional connectivity of the amygdala in Chinese earthquake survivors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;65:208–214. doi: 10.1016/j.pnpbp.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Zhang X.-D., Yin Y., Hu X.-L., Duan L., Qi R., Xu Q., Lu G.-M., Li L.-J. Altered default mode network configuration in posttraumatic stress disorder after earthquake. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu F., Chen Heng, Li M., Duan X., Xie B., Chen Huafu. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J. Affect. Disord. 2015;187:114–121. doi: 10.1016/j.jad.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Zhu X., Helpman L., Papini S., Schneier F., Markowitz J.C., Van Meter P.E., Lindquist M.A., Wager T.D., Neria Y. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress. Anxiety. 2017;34:641–650. doi: 10.1002/da.22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Suarez-Jimenez B., Lazarov A., Helpman L., Papini S., Lowell A., Durosky A., Lindquist M.A., Markowitz J.C., Schneier F., Wager T.D., Neria Y. Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with posttraumatic stress disorder. Depress. Anxiety. 2018;35:974–984. doi: 10.1002/da.22816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski M.J., Privratsky A.A., Smitherman S., Kilts C.D., Herringa R.J., Cisler J.M. Does development moderate the effect of early life assaultive violence on resting-state networks? An exploratory study. Psychiatry Res. Neuroimaging. 2018;281:69–77. doi: 10.1016/j.pscychresns.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.