Summary
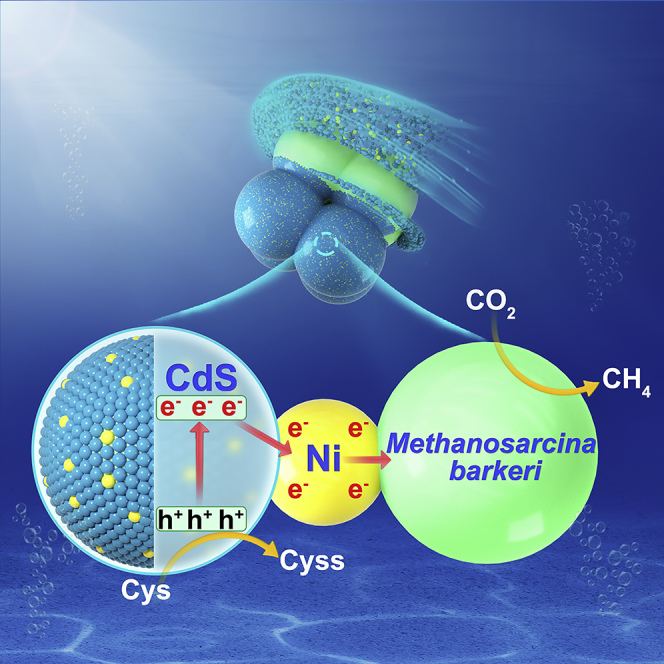
Semi-artificial photosynthesis (biohybrid) provides an intriguing opportunity for efficient CO2-to-CH4 conversion. However, creating a desirable semiconductor in biohybrids remains a great challenge. Here, by doping Ni into CdS nanoparticles, we have successfully developed the Methanosarcina barkeri-Ni:CdS biohybrids. The CH4 yield by the M. barkeri-Ni(0.75%):CdS biohybrids was approximately 250% higher than that by the M. barkeri-CdS biohybrids. The suitable Ni dopants serve as an effective electron sink, which accelerates the photoelectron transfer in biohybrids. In addition, Ni doping changes the metabolic status of M. barkeri and results in a higher expression of a series of proteins for electron transfer, energy conversion, and CO2 fixation. These increased proteins can promote the photoelectron capture by M. barkeri and injection into cells, which trigger a higher intracellular reduction potential to drive the reduction of CO2 to CH4. Our discovery will offer a promising strategy for the optimization of biohybrids in the solar-to-chemical conversion.
Subject Areas: Nanoparticles, Biochemistry, Energy Resources
Graphical Abstract
Highlights
-
•
M. barkeri-Ni:CdS biohybrids were successfully developed for CO2 reduction
-
•
A highest QE of 2.08% was achieved by the M. barkeri-Ni(0.75%):CdS biohybrids
-
•
Ni dopants effectively suppressed the electron-hole recombination in biohybrids
-
•
Ni doping changed the metabolic status of M. barkeri in biohybrids
Nanoparticles; Biochemistry; Energy Resources
Introduction
Solar-driven carbon dioxide (CO2) reduction into high-value biofuels such as methane (CH4) is a promising approach to alleviate both global energy challenge and greenhouse effect (Kim and Kwon, 2019). The traditional photocatalytic systems with various inorganic catalysts/enzymes are challenged by several critical drawbacks including poor CH4/H2 selectivity and lack of a self-replication ability (Nichols et al., 2015; Wagner et al., 2016). Semi-artificial photosynthesis (biohybrid), which synergistically combines the efficient light harvesting of semiconductors with the excellent biocatalytic capacity in methanogens, can provide a unique and intriguing opportunity for efficient CO2-to-CH4 conversion (Kornienko et al., 2018; Tremblay et al., 2020). The reducing equivalent [H] from photoelectrons in a semiconductor can be trapped by methanogens for metabolic activities via H2ase-mediated and cytochrome-mediated pathways (Ye et al., 2019). To achieve excellent photocatalytic performance for practical applications, the desirable semiconductor in biohybrids should be developed to enhance the photoelectron separation, transfer, and capture.
Metal chalcogenides such as cadmium sulfide (CdS) semiconductors are excellent candidates of light harvesters in biohybrids due to their distinct characteristics such as tunable band gaps, rich surface binding sites, excellent extinction coefficients and favorable conduction/valence band energies (Zhukovskyi et al., 2015). A variety of efforts have been made to effectively suppress the rapid and severe recombination of photo-induced electrons and holes in CdS semiconductors (Wei et al., 2018). For example, integration with other semiconductors has created heterostructures such as CdS/ZnSe (Grennell et al., 2017), MoS2/CdS (Yuan et al., 2018), and TiO2/CdS (Luo et al., 2012; Park et al., 2016), whereas increasing the spatial overlap between electron-donating and electron-accepting semiconductors remains a challenge. Although the addition of molecular linkers, e.g., 4-mercaptobenzoic acid, was proved to be effectively improve the charge transfer process among different semiconductors (Dibbell et al., 2009), the formed organic ligands may be unfavorable for shuffling photoelectrons for surface catalytic sites (Hines and Kamat, 2013). The separation of photogenerated charge carriers can be addressed by depositing noble metals such as Pd (Wu et al., 2012), Pt (Jiang et al., 2015), and Au (Serra et al., 2015) on the surface of CdS as cocatalysts to restrain the recombination of electron-hole pairs. However, the scarcity and high cost of these noble metals trigger the development of an alternative and noble-metal-free system.
Doping nickel (Ni) into CdS nanoparticles (Ni:CdS) has been proved to be a versatile approach to enhance the photocatalytic performance (Chai et al., 2016; Simon et al., 2014). The dopants are demonstrated to significantly improve the stability of CdS nanoparticles (Wang et al., 2018), because metal ions can interact with the CdS semiconductor to form a strong metal-sulfur binding in the lattice, which appears as an advantage over metal complex catalysts that effectively avoid the leaching of cations (Nag et al., 2012). In addition, Ni can serve as an effective electron outlet that promotes the migration of photoelectrons to the surface (Dong et al., 2015). Nevertheless, the excellent photocatalytic performance of Ni:CdS semiconductors, such as splitting alcohol into H2 and CO2 reduction with a high selectivity, was only demonstrated in the nonbiological systems (Wang et al., 2018). To our knowledge, little is known about the interaction between Ni:CdS semiconductors and microorganisms (methanogens). Particularly, the effect of the Ni-decorated semiconductor surface on the photoelectron transfer and capture by methanogens is unclear. Although the importance of Ni as an essential nutrient for methanogens was reported, such as the formation of Ni-Fe hydrogenases and cofactor F430 (Mulrooney and Hausinger, 2003), the metal toxicity on enzyme functions and structures may also inactivate methanogenic activity (Paulo et al., 2017). Therefore, it is of great interest to investigate the potential of Ni-decorated CdS to improve methanogenesis in biohybrids.
Methanosarcina barkeri was the methanogen that could participate in the direct interspecies electron transfer process (Rotaru et al., 2014), and its basic physiology and biochemistry was better understood than those of other methanogens (Kulkarni et al., 2018; Mand et al., 2018). Therefore, M. barkeri was selected to combine with Ni:CdS semiconductors to construct Ni-decorated M. barkeri-CdS (M. barkeri-Ni:CdS) biohybrids. The characterization of Ni:CdS semiconductors, such as the distribution, composition, and photoelectrochemical performance, was conducted. Importantly, the performance and mechanisms of M. barkeri-Ni:CdS biohybrids for methanogenesis were experimentally studied. This work was expected to offer a promising strategy to optimize biohybrids in the solar-to-chemical production.
Results and Discussion
Characterization of M. barkeri-Ni:CdS Biohybrids
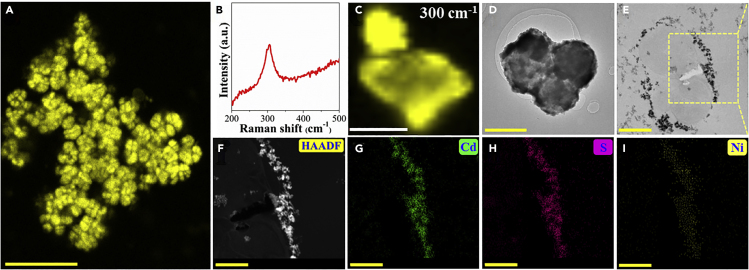
The Ni:CdS semiconductors were synthesized according to Wang et al. (2018), where the doped Ni atoms were efficiently embedded in CdS to capture photoelectrons at surface catalytic sites. When the growth of M. barkeri (DSM 800) reached an exponential phase (OD600∼0.2) in 50 mL anaerobic heterotrophic medium (Table S1), the successfully synthesized pure Ni:CdS semiconductors (Figures S1–S5) were added at a dosage of 0.6 mmol/L in a 100% N2 atmosphere according to the preliminary results (Figure S6). Then the mixture was placed in the shaker at 37°C for 3 days to construct M. barkeri-Ni:CdS biohybrids. Because the highest CH4 yield was achieved with an Ni weight ratio of 0.75% (Figure S7), the M. barkeri-Ni(0.75%):CdS biohybrids were chosen as the main representative of M. barkeri-Ni:CdS biohybrids to do the characterization. To verify the presence of semiconductors in the biohybrids, we examined the fluorescence change in vivo. Under UV irradiation, bright yellow fluorescence (a typical color of metal chalcogenides) was observed in the M. barkeri-Ni(0.75%):CdS biohybrids, whereas faint blue fluorescence was detected in the bare M. barkeri, which may be attributed to the cellular autofluorescence (Figure S8). Consistent results have been obtained by confocal laser scanning microscopic images, where bright yellow fluorescence well matched the shape of M. barkeri in the M. barkeri-Ni(0.75%):CdS biohybrids (Figure 1A), but it was missed in the bare M. barkeri (Figure S8). The identity of the yellow fluorescence was ascertained by micro-Raman spectroscopy (Figures 1B and 1C). Like that in Ni(0.75%):CdS semiconductors (Figure S1), a distinct peak at 300 cm−1 was detected in the M. barkeri-Ni(0.75%):CdS biohybrids, which was identified as the distinct signal of Cd-S bond (Ma et al., 2015). All these results imply the presence of fluorescent Cd-S semiconductors that were combined with M. barkeri in the biohybrids.
Figure 1.
Characterization of the M. barkeri-Ni(0.75%):CdS Biohybrids
Confocal laser scanning microscopic image (A); in situ Raman spectrum excited by a 532-nm laser (B); the Cd-S (at 300 cm−1) single-cell Raman mapping (C); TEM images of the non-sectioned (D) and thin-sectioned M. barkeri-Ni(0.75%):CdS biohybrids (E); high-angle annular dark-field image (F); and elements formed by Cd (G), S (H), and Ni (I) with energy-dispersive X-ray spectroscopic mapping. Scale bars: 20 μm in (A), 2 μm in (C and D), 1 μm in (E), and 0.5 μm in (F–I).
We further confirmed the distribution and composition of Cd-S semiconductors in the biohybrids. Transmission electron microscopic (TEM) images reveal that the clusters of black dots with an average size of 10–100 nm were closely connected to the cell surface of M. barkeri (Figures 1D and 1E). The high angle annular dark field (HAADF) scanning transmission electron microscopic and energy-dispersive X-ray spectroscopic mapping images show that these particles were mainly composed of Cd, S, and Ni elements (Figures 1F–1I), which is further corroborated by the full scan survey X-ray photoelectron spectra. Specifically, identical to those in Ni(0.75%):CdS semiconductors (Figure S3), the peaks of Cd 3d5/2 at 404.6 eV and Cd 3d3/2 at 411.4 eV in the M. barkeri-Ni(0.75%):CdS biohybrids are associated with Cd2+ species, whereas the peaks of S 2p3/2 at 161.5 eV and S 2p1/2 at 162.8 eV are assigned to S2− species (Yang et al., 2017). Importantly, four peaks for the chemical states of elemental Ni were demonstrated in the Ni 2p orbital spectrum (Figure S3), which was attributed to the Ni2+ state (Wang et al., 2018). In contrast, almost no nickel signal was detected on the X-ray photoelectron spectra of M. barkeri-CdS biohybrids (Figure S3), which demonstrates that nickel signal in the M. barkeri-Ni(0.75%):CdS biohybrids was from Ni(0.75%):CdS semiconductors. However, only CdS nanoparticles (PDF#75-0581) were identified with the X-ray diffraction (XRD) spectra and high-resolution TEM of Ni(0.75%):CdS semiconductors and M. barkeri-Ni(0.75%):CdS biohybrids. Three distinct diffraction peaks at 26.7°, 44.1°, and 52.2° were attributed to the (111), (220), and (311) crystal planes of CdS with d values of 0.35, 0.21, and 0.18 nm, respectively (Figures S4 and S5). There were no peaks for Ni nanocrystals or nickel compounds in XRD spectra, indicating that the low Ni doping amount did not significantly alter the cubic structure and crystallinity of CdS nanoparticles. This evidence suggests the successful decoration of Ni:CdS nanoparticles on the surface of M. barkeri.
Ni Doping Enhanced the Photocatalytic Performance of the Biohybrid
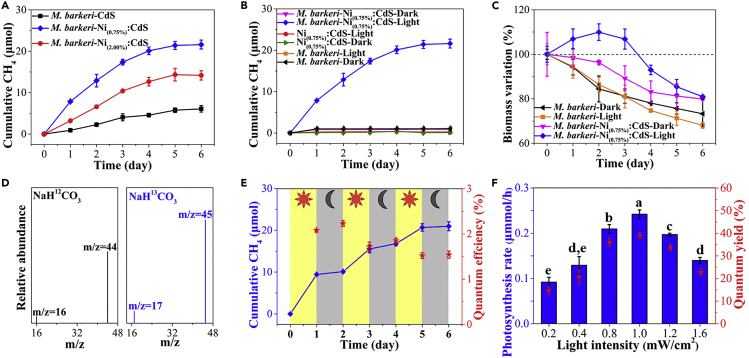
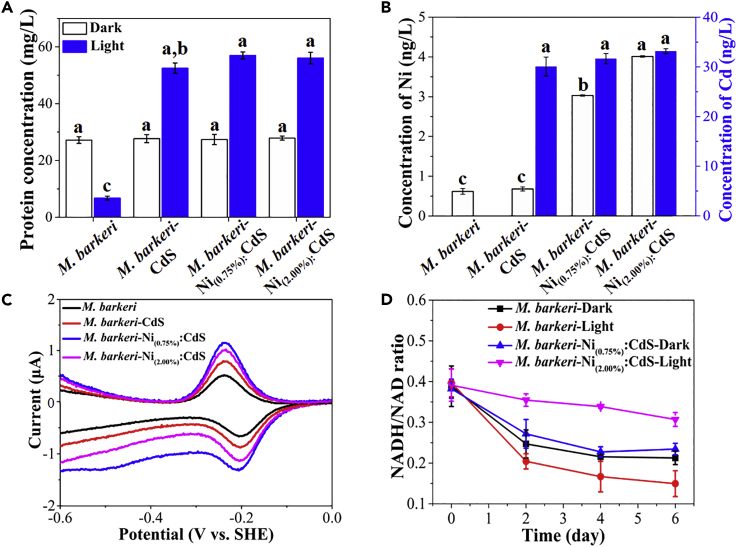
Photocatalytic CO2-to-CH4 conversion with the prepared biohybrids was performed at 35 ± 2°C under light irradiation. As shown in Figure 2A, only 6.13 ± 0.76 μmol CH4 was obtained with the M. barkeri-CdS biohybrid, likely due to the lack of active sites and high recombination rate of electron-hole pairs. In contrast, Ni doping significantly enhanced the CH4 yield to 21.50 ± 0.98 μmol with the M. barkeri-Ni(0.75%):CdS biohybrid, which is ∼250% higher than that with the M. barkeri-CdS biohybrids. The increase in CH4 production in M. barkeri-Ni(0.75%):CdS biohybrids was not caused by the addition of nickel alone, as almost no CH4 was produced after the addition of an identical amount of Ni2+ for M. barkeri with or without light irradiation (Figure S9). However, further increasing Ni concentration in biohybrids (M. barkeri-Ni(2.00%):CdS biohybrids) resulted in a lower CH4 yield, which suggests that the doping should be optimized to achieve the desired performance. In addition, the role of different components in the M. barkeri-Ni(0.75%):CdS biohybrids for CH4 production was evaluated including M. barkeri, Ni(0.75%):CdS, and light. Missing one of those factors led to negligible CH4 production under these conditions (Figure 2B) and a significant decrease in protein contents (indication of biomass growth) (Figure 2C), which demonstrates the key roles of those factors in the CO2-to-CH4 conversion. The isotopic labeling experiments show that only the peaks of 13CH4 (m/z = 17) and 13CO2 (m/z = 45) were detected with 13C-labeled NaHCO3 as a carbon source (Figure 2D), which suggests that the produced CH4 was derived from CO2 reduction. The XRD and X-ray photoelectron spectra demonstrate the structural stability of Ni(0.75%):CdS semiconductors in the biohybrids after 6 days of irradiation (Figures S3 and S4). However, the CH4 production by M. barkeri-Ni(0.75%):CdS biohybrids began to cease at day 4 (Figure 2A) with the decrease in biomass (Figure 2C). Due to sufficient CO2 in the headspace (Figure 2D), the possible reason may be the quick depletion of only sacrificial hole scavenger cysteine, which causes the oxidative photodamage of cells. To further prove this speculation, a second injection of cysteine was conducted after 4 days of irradiation. The CH4 production was enhanced with the addition of cysteine after the additional 5 days of irradiation (Figure S10). Therefore, the ceasing of CH4 production in M. barkeri-Ni(0.75%):CdS biohybrids can be attributed to the depletion of sacrificial hole scavenger cysteine.
Figure 2.
Photocatalytic Performance for CO2-to-CH4 Conversion with the Biohybrids
The CH4 yields by biohybrids with various Ni doping amounts (A); photocatalytic CH4 production by the M. barkeri-Ni(0.75%):CdS biohybrids and deletional controls (B); biomass variation of natural M. barkeri and M. barkeri-Ni(0.75%):CdS biohybrids (C); mass spectrometry of headspace gases with 12C-labeled NaHCO3 and 13C-labeled NaHCO3 as carbon sources in the media, respectively (D); photocatalytic CH4 yields and quantum efficiencies (% incident light) of the M. barkeri-Ni(0.75%):CdS biohybrids with a light-dark cycle of 1 day (E); photocatalytic CH4 production rates and quantum yields based on the initial Cys concentration under different light intensities (F). Data are represented as mean ± SEM (n = 3), and the different letters represented statistically significant difference (p < 0.05) in different groups.
The photocatalytic performance of the M. barkeri-Ni(0.75%):CdS biohybrids was further evaluated with a light-dark cycle of 1 day (Figure 2E). The continuous increase in CH4 concentration during several light-dark cycles suggests that the accumulated biosynthetic intermediates during the daytime can be used in the nighttime, which can effectively eliminate the catabolic energy loss that commonly occurs during the dark cycles in natural photosynthesis (Sakimoto et al., 2016; Larkum, 2010). A peak quantum efficiency of 2.08% ± 0.03% of total incident light was obtained with the M. barkeri-Ni(0.75%):CdS biohybrid, which was significantly higher than those of M. barkeri-CdS and M. barkeri-Ni(2.00%):CdS biohybrids (Figure S11). With the increase in light intensity, the CH4 production rate followed a volcano-type trend (Figure 2F). The maximum CH4 production rates with M. barkeri-Ni(0.75%):CdS biohybrids reached 0.21 and 0.24 μmol/h with a light intensity of 0.8 and 1.0 mW/cm2, respectively, which was higher than the reported CH4 production rate of 0.19 μmol/h (Table S2), with a quantum yield of 21.60% ± 0.97% and 39.04% ± 1.34% (based on the initial Cys concentration, Supplemental Information). More importantly, the dosage of Ni(0.75%):CdS semiconductor (0.6 mM) was lower than that in previous research (1.0 mM), which implies that better photocatalytic methanogenesis can be achieved by M. barkeri-Ni(0.75%):CdS biohybrids with a lower semiconductor dose and lower light intensity. Afterward, the CH4 production rate significantly decreased (p < 0.05), possibly related to the oxidative photodamage under high light intensities (Dumas et al., 2010) because photon energy under irradiation is the main driving force for the electron-hole separation in the M. barkeri-Ni(0.75%):CdS biohybrids. With the photon adsorption, the photoelectron (e−) transition to conduction band was performed in the Ni(0.75%):CdS semiconductor. Then, the remaining holes (h+) and consequently generated reactive oxygen species (ROS) such as hydrogen peroxide (2H2O + 2h+ → H2O2 + 2H+) and hydroxyl radical (H2O + h+ → ·OH + H+) (Nosaka and Nosaka, 2017) result in the oxidative stress for M. barkeri (Brioukhanov et al., 2000). At lower light intensity, sacrificial reagents Cys can effectively quench the photogenerated holes (2Cys + 2h+ → Cyss + 2H+) (Sakimoto et al., 2016) and ROS (He and Häder, 2002), which protect M. barkeri against oxidative stress. In contrast, a too high light intensity will quickly increase the concentrations of h+ and ROS, which become higher than the survival limit of M. barkeri (e.g., maximum 0.4 mM H2O2 stress, (Brioukhanov et al., 2006). A too high light intensity also causes the photocorrosion of the CdS semiconductor (CdS + 2h+ → Cd2+ + S, (Davis and Huang, 1991), which has a photoprotective role toward microorganisms. These results are consistent with the scanning electron microscopic images, where obvious cell shrinkage was observed in M. barkeri-Ni(0.75%):CdS biohybrids with light intensity of 1.6 mW/cm2 (Figure S12).
Ni Doping Improved the Photoelectron Separation and Transfer Efficiency
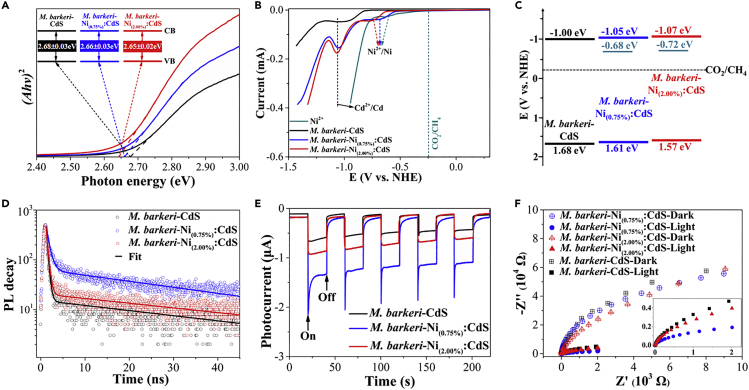
To understand the intrinsic reason for the excellent performance of the Ni-decorated M. barkeri-CdS biohybrid, we evaluated the band structures of different semiconductors that governed the photoelectron separation and transfer. UV-visible (UV-vis) spectra show that the band gaps of CdS and Ni(x):CdS semiconductors in biohybrids were approximately 2.65–2.68 eV (Figure 3A), which were close to those of pure CdS and Ni(x):CdS semiconductors (2.64–2.67 eV, Figure S13). The values were larger than that of the bulk CdS (approximately 2.42 eV) (Thambidurai et al., 2011). The reason can be the small size of the synthesized semiconductor (<5 nm, see Figure S5), which caused the size-induced quantum confinement effect (Takagahara and Takeda, 1992). Similar results were reported in previous studies, wherein the band gap of CdS semiconductors reached 2.51–2.72 eV (Sakimoto et al., 2016; Brown et al., 2016; Chavhan et al., 2008). However, the observed absorption trailing below 2.60 eV in the UV-vis spectra suggests more doping defects in the M. barkeri-Ni:CdS biohybrids than in the M. barkeri-CdS biohybrids (Figure S14), which can promote the efficient charge transfer from inside to the surface of the semiconductors (Huang et al., 2017). In addition to the reduction peak of Cd2+/Cd0 at approximately −1.00 V versus normal hydrogen electrode (NHE) (Simon et al., 2014), one more peak at approximately −0.70 V versus NHE was detected in the M. barkeri-Ni:CdS biohybrids by linear sweep voltammetry (Figure 3B), which was assigned to the reduction of Ni2+/Ni0 (Han et al., 2012). Thus, the surface Ni could be used as efficient catalytic sites to capture photoelectrons for the CO2 reduction. The cathodic photocurrents of M. barkeri-Ni(x):CdS biohybrids were higher than those of M. barkeri-CdS biohybrids, which indicates that the doping of Ni on CdS could further catalyze CO2 reduction. The estimated energy bands of the M. barkeri-CdS and M. barkeri-Ni:CdS biohybrids are shown in Figure 3C. The upshift of the valence band (VB) after Ni doping was consistent with the analysis from the VB-X-ray photoelectron spectra (Figure S15), which may be beneficial for photoelectron transfer.
Figure 3.
Photoelectrochemical Tests of the Biohybrids with Various Ni Doping Amounts
The band gaps of different biohybrids (A), linear sweep voltammetry curves of different biohybrids and free Ni2+ cations in water (B), energy band diagrams for different biohybrids (C), PL decay curves of different biohybrids (D), I-t curves under a light on/off cycle (20/20 s) (E), and Nyquist plots (F) with different biohybrids. Data are represented as mean ± SEM (n = 3).
To further comprehend the variation of the band structure on the charge behavior of biohybrids, we evaluated the recombination rate of electron-hole pairs in the biohybrids by the steady-state photoluminescence (PL) spectra. The PL peaks approximately at 550 nm were found in all samples (Figure S16A) and became weaker at approximately 650 nm in the presence of methyl viologen (MV2+), which sacrificed surface electrons (Figure S16B). The results indicate that the PL spectra mainly resulted from the radiative electron-hole recombination on the surface instead of in the bulk. Notably, the M. barkeri-Ni(0.75%):CdS biohybrids showed a stronger surface photoemission than the M. barkeri-CdS biohybrid, which demonstrates the enhanced efficiencies of separation and capture of photoelectrons on the surface after Ni doping. However, increasing Ni concentration (M. barkeri-Ni(2.00%):CdS biohybrid) decreased the PL peak intensity, which might be due to the nonradiative recombination of photoelectrons at bulk Ni sites. In addition, the time-resolved PL spectra show that the doped Ni (0.75 wt %) can serve as an effective electron sink on the surface to prolong the lifetime of photoinduced charge carriers by 213%, although an overly high Ni doping concentration can shorten the PL lifetime for surface radiative recombination (Figure 3D, Table S3). The results were corroborated by the amperometric I-t curves, where the M. barkeri-Ni(0.75%):CdS biohybrids had higher photocurrent intensity than the M. barkeri-CdS and M. barkeri-Ni(2.00%) biohybrids (Figure 3E). The Nyquist plot obtained from the M. barkeri-Ni(0.75%):CdS biohybrids show a smaller arc radius than the M. barkeri-CdS and M. barkeri-Ni(2.00%):CdS biohybrids with/without light irradiation, which confirms the enhanced electronic conductivity (Figure 3F). These results have illustrated that a suitable Ni doping amount can effectively accelerate the photoelectron transfer.
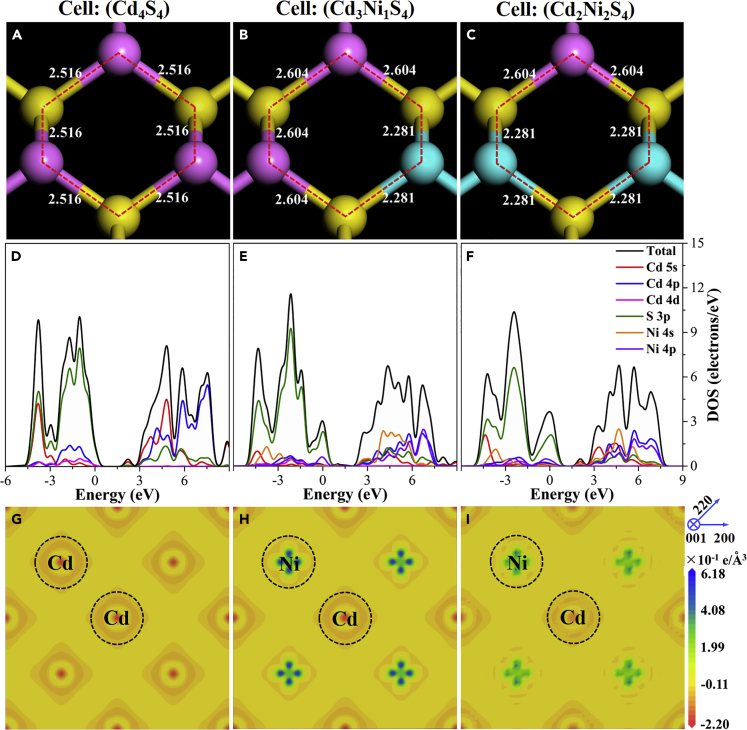
The density functional theory calculation was conducted to better understand the variation of the electronic band structure of semiconductors in the Ni-doped biohybrids. The doping models remained thermodynamically stable in theory with the relatively low change of total energy (1.10% and 2.21%) after structural relaxation (Figure S17). The lengths of Cd-S bonds in the Ni-doped semiconductors were slightly larger than those in bare CdS models (Figures 4A–4C), which might expand the crystal lattice (Huang et al., 2017). In addition, the Ni doping resulted in the increase of the valence band maximum (VBM) (Figure S18), which contributes to the narrower band gap of semiconductors in the M. barkeri-Ni:CdS biohybrids as shown in Figure 3A. Similar results were reported (Wu et al., 2011), where transition metal (Mn, Fe, and Ni) doping was proved to narrow the band gap of zinc blende CdS photocatalysts. We also calculated the density of states for Cd, S, and Ni orbitals. The conduction band minimum (CBM) and VBM in bare CdS are mainly composed of Cd 5s 4p and S 3p orbitals. However, after the substitution of Cd with Ni, more states of Ni 4s 4p were found in CBM states with the decreased contribution of Cd 5s 4p states (Figures 4D–4F). Thus, the photo-induced electrons preferred to migrate to Ni 4s 4p rather than Cd 5s 4p orbitals. In addition, higher electron transfer efficiencies were found in the electron density maps after Ni doping, which suggests the strong interaction between electron clouds of S and Ni atoms (Figures 4G–4I).
Figure 4.
The Band Structure Evolution of CdS Semiconductors after Ni Doping
Calculated bond distances (A–C), densities of state (D–F), and electron density difference maps (G–I) of the simulated Ni-doped CdS systems with Cd4S4, Cd3Ni1S4, and Cd2Ni2S4.
Ni Doping Promoted Electron Transfer from the Interface of Biohybrids to Cells
The isolation and analysis of membrane-bound proteins were conducted to evaluate the effect of Ni doping on electron transfer from the interface of biohybrids to microbial cells. It was found that no significant difference (p > 0.05) was observed between the concentrations of membrane-bound protein of different samples in the dark. In contrast, the concentrations of membrane-bound protein of M. barkeri were significantly increased after its interaction with semiconductors under light irradiation, with the highest value of 56.95 ± 0.25 mg/L with the M. barkeri-Ni(0.75%):CdS biohybrids, which could be attributed to both an increase of cell number (based on the flow cytometry results) and an increase of membrane-bound protein per cell (based on the normalization results, Figure S19). In addition, higher concentrations of Ni and Cd elements were detected with the Ni-decorated biohybrids (Figure 5B). These results imply that suitable Ni doping improved the biocompatibility of CdS semiconductors with M. barkeri. To evaluate the activity of redox-active mediators that catalyzed the electron transport on the interface, square wave voltammetry measurement was performed (Figure 5C). Two dominant redox centers with a mid-potential of −0.21 V (versus standard hydrogen electrode) were detected, which could be assigned to cytochromes b (Kühn and Gottschalk, 1983). In addition, a higher catalytic current was obtained with the M. barkeri-Ni(0.75%):CdS biohybrids compared with M. barkeri-CdS and M. barkeri-Ni(2.00%):CdS biohybrids, which demonstrates more redox-active mediators in the membrane-bound protein layer. The increased amount of redox-active mediators at the interface of biohybrids after Ni doping effectively promoted the photoelectron capture by M. barkeri and generation of excessive intracellular reducing power under light irradiation (Figure 5D).
Figure 5.
Characterization of the Membrane-Bound Protein and Activities of M. barkeri in Biohybrids with Various Ni Doping Amounts
Concentrations of membrane-bound protein (A), concentrations of Ni and Cd elements (B), square wave voltammetry curves of membrane-bound protein (C), and NADH/NAD ratio (D). Data are represented as mean ± SEM (n = 3), and the different letters represented statistically significant difference (p < 0.05) in different groups.
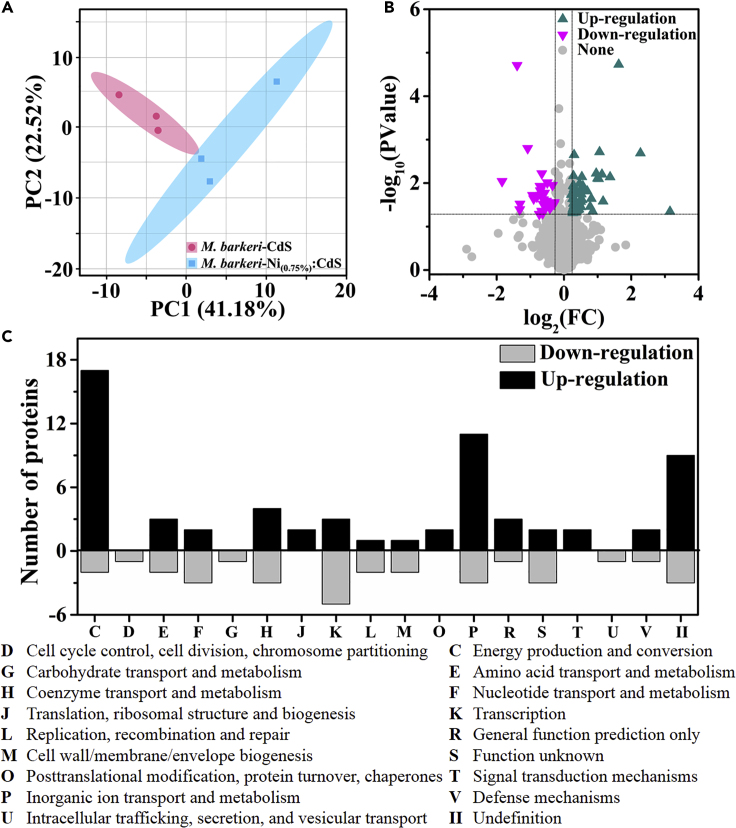
Proteins of M. barkeri in the M. barkeri-CdS and M. barkeri-Ni(0.75%):CdS biohybrids were further analyzed by the untargeted proteomic technology. In total, 1,457 proteins were detected in both biohybrids. The principal-component analysis shows that two distinct groups in the score plots were observed (Figure 6A), which indicates that the Ni doping significantly influenced the physiological state of M. barkeri in biohybrids under light irradiation. Compared with the M. barkeri-CdS biohybrids, the number of proteins with significant upregulation (fold change >1.20 or p < 0.05) and downregulation (fold change <0.80 or p < 0.05) in the M. barkeri-Ni(0.75%):CdS biohybrids reached 59 and 32, respectively (Figure 6B). The network analysis indicates that most of the 91 significantly changed proteins highly interacted with one another (Figure S20), which suggests that Ni doping led to systematic changes of the physiological status of M. barkeri such as electron transfer and energy conservation. A functional analysis of the significant proteins by the Clusters of Orthologous Groups (COG) database was further conducted (Figure 6C). In addition to energy production and conversion (C) and inorganic ion transport (P) proteins, other proteins with different functions were upregulated, including Cell-cycle-control/Cell-division/Chromosome-partitioning (D), Amino-acid-transport (E), Nucleotide-transport (F), Carbohydrate-transport (G), Coenzyme-transport (H), Translation/Ribosomal-structure/Biogenesis (J), Transcription (K), Replication/Recombination/Repair (L), Cell-wall/Membrane/Envelope-biogenesis (M), Posttranslational-modification/Protein-turnover/Chaperones (O), Signal-transduction-mechanisms (T), Intracellular-trafficking/Secretion/Vesicular-transport (U), and Defense-mechanisms (V).
Figure 6.
Assessment of Ni-Induced Physiological Perturbation of M. barkeri
Score plots (A) and volcano plots (B) of the proteome of M. barkeri-Ni(0.75%):CdS biohybrids compared with M. barkeri-CdS biohybrids; COG functional annotation for the 91 proteins with significant variations in M. barkeri-Ni(0.75%):CdS biohybrids compared with M. barkeri-CdS biohybrids (C).
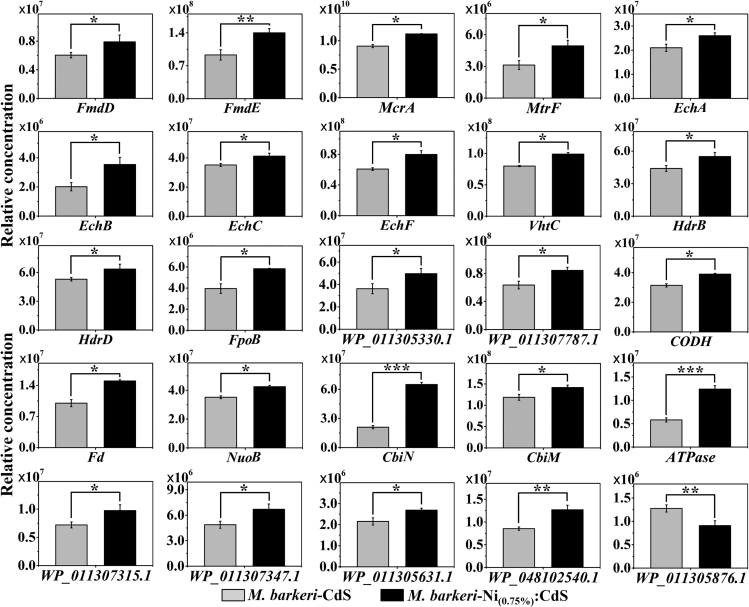
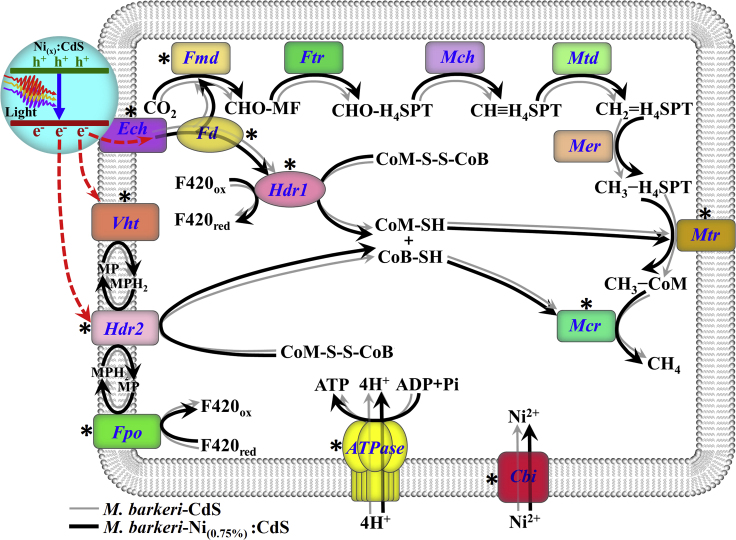
To better understand the mechanism, the proteins related to the charge transfer, carbon fixation, and energy conversion in M. barkeri were analyzed (Figures 7 and S21). Specifically, the carbon fixation during the CO2-to-CH4 conversion was catalyzed by different enzymes (Figure 8). Untargeted proteome data show that cytoplasmic formylmethanofuran dehydrogenase (Fmd) and methyl-S-CoM reductase (Mcr) were significantly upregulated in the M. barkeri-Ni(0.75%):CdS biohybrids compared with M. barkeri-CdS biohybrids (p < 0.05). In addition, membrane-associated methyl-H4SPT:CoM methyltransferase (Mtr), which can transmit methyl-tetrahydrosarcinapterin (CH3-H4SPT) into methyl-CoM (CH3-CoM), was also significantly upregulated in the M. barkeri-Ni(0.75%):CdS biohybrids. The membrane-associated proteins in M. barkeri also play an important role in the electron flow during methanogenesis, such as energy-converting [NiFe] hydrogenase (Ech, H2 + Fdox ⇌ Fdred2− + 2H+), methanophenazine-reducing [NiFe] hydrogenase (Vht, H2 + MP ⇌ MPH2), methanophenazine-dependent heterodisulphide reductase (Hdr, MPH2 + CoM-S-S-CoB ⇌ MP + HS-CoM + HS-CoB) (Thauer et al., 2008), and (Fpo, MP + F420H2 ⇌ MPH2 + F420) (Kulkarni et al., 2009). The significant upregulation (p < 0.05) of these enzymes in the M. barkeri-Ni(0.75%):CdS biohybrid is beneficial for the photoelectron harvest and transfer. Simultaneously, the significant upregulation of NADH-dependent oxidoreductase (WP_011305330.1, WP_011307787.1), carbon-monoxide dehydrogenase (CODH), and ferredoxin (Fd) indicate that the harvested photoelectrons can trigger the increase of reducing equivalents and accelerate in vivo reductive reactions in the cells (Zhang et al., 2020). In addition, Ni was demonstrated to act as cofactors for many enzymes (Paulo et al., 2015). The significant upregulation of nickel transport proteins (CbiN and CbiM, p < 0.05) in the M. barkeri-Ni(0.75%):CdS biohybrids and the increased membrane-associated ATP synthase (ATPase, p < 0.05) may stimulate the growth and metabolism of M. barkeri. Importantly, P-II family nitrogen regulator protein (WP_011307315.1) and ankyrin repeat domain-containing protein (WP_011307347.1), which functioned in signal transduction mechanisms, were significantly upregulated. ABC transporter ATP-binding proteins (WP_011305631.1, WP_048102540.1, WP_011305876.1), which are responsible for the defense mechanisms, were also significantly increased. These results indicate that M. barkeri adjusted the expression of related proteins to strengthen the stress resistance caused by Ni dopants.
Figure 7.
Relative Concentration of Proteins for Electron Transfer, Energy Conversion, and CO2 Fixation in M. barkeri-Ni(0.75%):CdS Biohybrids Compared with M. barkeri-CdS Biohybrids
Data are represented as mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Figure 8.
Pathways of Electron Transfer and CO2 Reduction in M. barkeri-Ni(0.75%):CdS Biohybrids and the Control
The degree of thickness of line (black and gray) represents the abilities of electron transfer and energy conversion. ∗p < 0.05.
Based on these results, a mechanism was proposed for the enhanced methanogenesis with the M. barkeri-Ni:CdS biohybrids (Figure 8). Ni:CdS semiconductors are decorated on the surface of M. barkeri and generate sufficient electrons under light irradiation. The photo-induced electrons migrate to Ni sites, which serve as an effective electron sink with Cys as a sacrificial reducing agent. In addition, Ni doping changes the metabolic status of M. barkeri and increases the concentration of proteins for electron transfer, energy conversion, and CO2 fixation. With these proteins, the photoelectrons on the interface of biohybrids can be more easily injected into cells to generate excessive intracellular reducing power, which enhances the CO2-to-CH4 conversion.
Limitations of the Study
A comprehensive study of the energy conversion pathways and intermediate metabolites of M. barkeri-Ni:CdS biohybrids should be further conducted. In addition, the reason for different responses of various subunits (operons) of the proteins in M. barkeri after Ni doping must be explored. This information is beneficial for better understanding and constructing the biohybrid system.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Shungui Zhou (sgzhou@soil.gd.cn).
Materials Availability
Any materials generated and used in this study are available for dissemination to others.
Data and Code Availability
This study did not generate a new code. All relevant data are available from the Lead Contact upon reasonable request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41925028, 41977281), the Project of Fujian Provincial Department of Science and Technology of China (2018J01748), the Fujian Agriculture and Forestry University Program for Distinguished Young Scholar (XJQ2017003), the Project of the Fuzhou Municipal Department of Science and Technology of China (No. 2019-G-32), and US National Science Foundation (1603190).
Author Contributions
J.Y. contributed to the experiments' designing and main manuscript writing; G.R. conducted the main experiments; L.K., Y.Z., and X.L. contributed to part of experiments; Z.H. and S.Z. helped to revise this manuscript. S.Z. contributed to the main funding acquisition. All authors reviewed and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101287.
Supplemental Information
References
- Brioukhanov A.L., Netrusov A.I., Eggen R.I. The catalase and superoxide dismutase genes are transcriptionally up-regulated upon oxidative stress in the strictly anaerobic archaeon Methanosarcina barkeri. Microbiol. 2006;152:1671–1677. doi: 10.1099/mic.0.28542-0. [DOI] [PubMed] [Google Scholar]
- Brioukhanov A., Netrusov A., Sordel M., Thauer R.K., Shima S. Protection of Methanosarcina barkeri against oxidative stress: identification and characterization of an iron superoxide dismutase. Arch. Microbiol. 2000;174:213–216. doi: 10.1007/s002030000180. [DOI] [PubMed] [Google Scholar]
- Brown K.A., Harris D.F., Wilker M.B., Rasmussen A., Khadka N., Hamby H., Keable S., Dukovic G., Peters J.W., Seefeldt L.C., King P.W. Light-driven dinitrogen reduction catalyzed by a CdS: nitrogenase MoFe protein biohybrid. Science. 2016;352:448–450. doi: 10.1126/science.aaf2091. [DOI] [PubMed] [Google Scholar]
- Chai Z.G., Zeng T.T., Li Q., Lu L.Q., Xiao W.J., Xu D.S. Efficient visible light-driven splitting of alcohols into hydrogen and corresponding carbonyl compounds over a Ni-modified CdS photocatalyst. J. Am. Chem. Soc. 2016;138:10128–10131. doi: 10.1021/jacs.6b06860. [DOI] [PubMed] [Google Scholar]
- Chavhan S.D., Senthilarasu S., Lee S.H. Annealing effect on the structural and optical properties of a Cd1-xZnxS thin film for photovoltaic applications. Appl. Surf. Sci. 2008;254:4539–4545. [Google Scholar]
- Davis A.P., Huang C.P. The photocatalytic oxidation of sulfur-containing organic compounds using cadmium sulfide and the effect on CdS photocorrosion. Water Res. 1991;25:1273–1278. [Google Scholar]
- Dibbell R.S., Youker D.G., Watson D.F. Excited-state electron transfer from CdS quantum dots to TiO2 nanoparticles via molecular linkers with phenylene bridges. J. Phys. Chem. C. 2009;113:18643–18651. [Google Scholar]
- Dong Y.T., Choi J.L., Jeong H.K., Son D.H. Hot electrons generated from doped quantum dots via upconversion of excitons to hot charge carriers for enhanced photocatalysis. J. Am. Chem. Soc. 2015;137:5549–5554. doi: 10.1021/jacs.5b02026. [DOI] [PubMed] [Google Scholar]
- Dumas E., Gao C., Suffern D., Bradforth S.E., Dimitrijevic N.M., Nadeau J.L. Interfacial charge transfer between CdTe quantum dots and gram negative vs gram positive bacteria. Environ. Sci. Technol. 2010;44:1464–1470. doi: 10.1021/es902898d. [DOI] [PubMed] [Google Scholar]
- Grennell A.N., Utterback J.K., Pearce O.M., Wilker M.B., Dukovic G. Relationships between exciton dissociation and slow recombination within ZnSe/CdS and CdSe/CdS dot-in-rod heterostructures. Nano Lett. 2017;17:3764–3774. doi: 10.1021/acs.nanolett.7b01101. [DOI] [PubMed] [Google Scholar]
- Han Z.J., Qiu F., Eisenberg R., Holland P.L., Krauss T.D. Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science. 2012;338:1321–1324. doi: 10.1126/science.1227775. [DOI] [PubMed] [Google Scholar]
- He Y.Y., Häder D.P. UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: protective effects of ascorbic acid and N-acetyl-L-cysteine. J. Photoch. Photobiol. B. 2002;66:115–124. doi: 10.1016/s1011-1344(02)00231-2. [DOI] [PubMed] [Google Scholar]
- Hines D.A., Kamat P.V. Quantum dot surface chemistry: ligand effects and electron transfer reactions. J. Phys. Chem. C. 2013;117:14418–14426. [Google Scholar]
- Huang H.M., Dai B.Y., Wang W., Lu C.H., Kou J.H., Ni Y.R., Wang L.Z., Xu Z.Z. Oriented built-in electric field introduced by surface gradient diffusion doping for enhanced photocatalytic H2 evolution in CdS nanorods. Nano Lett. 2017;17:3803–3808. doi: 10.1021/acs.nanolett.7b01147. [DOI] [PubMed] [Google Scholar]
- Jiang X.L., Fu X.L., Zhang L., Meng S.G., Chen S.F. Photocatalytic reforming of glycerol for H2 evolution on Pt/TiO2: fundamental understanding the effect of co-catalyst Pt and the Pt deposition route. J. Mater. Chem. A. 2015;3:2271–2282. [Google Scholar]
- Kim J., Kwon E.E. Photoconversion of carbon dioxide into fuels using semiconductors. J. CO2 Util. 2019;33:72–82. [Google Scholar]
- Kornienko N., Zhang J.Z., Sakimoto K.K., Yang P., Reisner E. Interfacing nature’s catalytic machinery with synthetic materials for semi-artificial photosynthesis. Nat. Nanotechnol. 2018;13:890–899. doi: 10.1038/s41565-018-0251-7. [DOI] [PubMed] [Google Scholar]
- Kühn W., Gottschalk G. Characterization of the cytochromes occurring in Methanosarcina species. Eur. J. Biochem. 1983;135:89–94. doi: 10.1111/j.1432-1033.1983.tb07621.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni G., Kridelbaugh D.M., Guss A.M., Metcalf W.W. Hydrogen is a preferred intermediate in the energy-conserving electron transport chain of Methanosarcina barkeri. Proc. Natl. Acad. Sci. U S A. 2009;106:15915–15920. doi: 10.1073/pnas.0905914106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni G., Mand T.D., Metcalf W.W. Energy conservation via hydrogen cycling in the methanogenic archaeon Methanosarcina barkeri. MBio. 2018;9:e01256-18. doi: 10.1128/mBio.01256-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum A.W.D. Limitations and prospects of natural photosynthesis for bioenergy production. Curr. Opin. Biotech. 2010;21:271–276. doi: 10.1016/j.copbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Luo J.S., Ma L., He T.C., Ng C.F., Wang S.J., Sun H.D., Fan H.J. TiO2/(CdS, CdSe, CdSeS) nanorod heterostructures and photoelectrochemical properties. J. Phys. Chem. C. 2012;116:11956–11963. [Google Scholar]
- Ma L.J., Liu M.C., Jing D.W., Guo L.J. Photocatalytic hydrogen production over CdS: effects of reaction atmosphere studied by in situ Raman spectroscopy. J. Mater. Chem. A. 2015;3:5701–5707. [Google Scholar]
- Mand T.D., Kulkarni G., Metcalf W.W. Genetic, biochemical, and molecular characterization of Methanosarcina barkeri mutants lacking three distinct classes of hydrogenase. J. Bacteriol. 2018;200:e00342-18. doi: 10.1128/JB.00342-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S.B., Hausinger R.P. Nickel uptake and utilization by microorganisms. FEMS Microb. Rev. 2003;27:239–261. doi: 10.1016/S0168-6445(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Nag A., Chung D.S., Dolzhnikov D.S., Dimitrijevic N.M., Chattopadhyay S., Shibata T., Talapin D.V. Effect of metal ions on photoluminescence, charge transport, magnetic and catalytic properties of all-inorganic colloidal nanocrystals and nanocrystal solids. J. Am. Chem. Soc. 2012;134:13604–13615. doi: 10.1021/ja301285x. [DOI] [PubMed] [Google Scholar]
- Nichols E.M., Gallagher J.J., Liu C., Su Y., Resasco J., Yu Y., Sun Y., Yang P., Chang C.J. Hybrid bioinorganic approach to solar-to-chemical conversion. Proc. Natl. Acad. Sci. U S A. 2015;112:11461–11466. doi: 10.1073/pnas.1508075112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka Y., Nosaka A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017;117:11302–11336. doi: 10.1021/acs.chemrev.7b00161. [DOI] [PubMed] [Google Scholar]
- Park H., Ou H.H., Kang U., Choi J., Hoffmann M.R. Photocatalytic conversion of carbon dioxide to methane on TiO2/CdS in aqueous isopropanol solution. Catal. Today. 2016;266:153–159. [Google Scholar]
- Paulo L.M., Ramiro-Garcia J., van Mourik S., Stams A.J., Sousa D.Z. Effect of nickel and cobalt on methanogenic enrichment cultures and role of biogenic sulfide in metal toxicity attenuation. Front. Microbiol. 2017;8:1341. doi: 10.3389/fmicb.2017.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo L.M., Stams A.J., Sousa D.Z. Methanogens, sulphate and heavy metals: a complex system. Rev. Environ. Sci. Bio. 2015;14:537–553. [Google Scholar]
- Rotaru A.E., Shrestha P.M., Liu F., Shrestha M., Shrestha D., Embree M., Zengler K., Wardman C., Nevin K.P., Lovley D.R. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energ. Environ. Sci. 2014;7:408–415. [Google Scholar]
- Sakimoto K.K., Wong A.B., Yang P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science. 2016;351:74–77. doi: 10.1126/science.aad3317. [DOI] [PubMed] [Google Scholar]
- Serra M., Albero J., García H. Photocatalytic activity of Au/TiO2 photocatalysts for H2 evolution: role of the Au nanoparticles as a function of the irradiation wavelength. ChemPhysChem. 2015;16:1842–1845. doi: 10.1002/cphc.201500141. [DOI] [PubMed] [Google Scholar]
- Simon T., Bouchonville N., Berr M.J., Vaneski A., Adrović A., Volbers D., Wyrwich R., Döblinger M., Susha A.S., Rogach A.L. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014;13:1013. doi: 10.1038/nmat4049. [DOI] [PubMed] [Google Scholar]
- Takagahara T., Takeda K. Theory of the quantum confinement effect on excitons in quantum dots of indirect-gap materials. Phys. Rev. B. 1992;46:15578. doi: 10.1103/physrevb.46.15578. [DOI] [PubMed] [Google Scholar]
- Thambidurai M., Muthukumarasamy N., Agilan S., Arul N.S., Murugan N., Balasundaraprabhu R. Structural and optical characterization of Ni-doped CdS quantum dots. J. Mater. Sci. 2011;46:3200–3206. [Google Scholar]
- Thauer R.K., Kaster A.K., Seedorf H., Buckel W., Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008;6:579–591. doi: 10.1038/nrmicro1931. [DOI] [PubMed] [Google Scholar]
- Tremblay P.L., Xu M., Chen Y., Zhang T. Nonmetallic abiotic-biological hybrid photocatalyst for visible water splitting and carbon dioxide reduction. Iscience. 2020;23:100784. doi: 10.1016/j.isci.2019.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T., Ermler U., Shima S. The methanogenic CO2 reducing-and-fixing enzyme is bifunctional and contains 46 [4Fe-4S] clusters. Science. 2016;354:114–117. doi: 10.1126/science.aaf9284. [DOI] [PubMed] [Google Scholar]
- Wang J., Xia T., Wang L., Zheng X.S., Qi Z.M., Gao C., Zhu J.F., Li Z.Q., Xu H.X., Xiong Y.J. Enabling visible-light-driven selective CO2 reduction by doping quantum dots: Trapping electrons and suppressing H2 evolution. Angew. Chem. Int. Ed. 2018;57:16447–16451. doi: 10.1002/anie.201810550. [DOI] [PubMed] [Google Scholar]
- Wei R.B., Huang Z.L., Gu G.H., Wang Z., Zeng L., Chen Y., Liu Z.Q. Dual-cocatalysts decorated rimous CdS spheres advancing highly-efficient visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2018;231:101–107. [Google Scholar]
- Wu J.C., Zheng J., Wu P., Xu R. Study of native defects and transition-metal (Mn, Fe, Co, and Ni) doping in a zinc-blende CdS photocatalyst by DFT and hybrid DFT calculations. J. Phys. Chem. C. 2011;115:5675–5682. [Google Scholar]
- Wu X., Song Q., Jia L., Li Q., Yang C., Lin L. Pd-Gardenia-TiO2 as a photocatalyst for H2 evolution from pure water. Int. J. Hydrogen Energ. 2012;37:109–114. [Google Scholar]
- Yang H., Jin Z., Fan K., Liu D., Lu G. The roles of Ni nanoparticles over CdS nanorods for improved photocatalytic stability and activity. Superlattice. Microst. 2017;111:687–695. [Google Scholar]
- Ye J., Yu J., Zhang Y.Y., Chen M., Liu X., Zhou S., He Z. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid. Appl. Catal. B Environ. 2019;257:117916. [Google Scholar]
- Yuan Y.J., Li Z.J., Wu S.T., Chen D.Q., Yang L.X., Cao D., Tu W.G., Zou Z.T., Zou Z.G. Role of two-dimensional nanointerfaces in enhancing the photocatalytic performance of 2D-2D MoS2/CdS photocatalysts for H2 production. Chem. Eng. J. 2018;350:335–343. [Google Scholar]
- Zhang R., He Y., Yi J., Zhang L., Shen C., Liu S., Liu L., Liu B., Qiao L. Proteomic and metabolic elucidation of solar-powered biomanufacturing by bio-abiotic hybrid system. Chem. 2020;6:234–249. [Google Scholar]
- Zhukovskyi M., Tongying P., Yashan H., Wang Y.S., Kuno M. Efficient photocatalytic hydrogen generation from Ni nanoparticle decorated CdS nanosheets. ACS Catal. 2015;5:6615–6623. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate a new code. All relevant data are available from the Lead Contact upon reasonable request.