Abstract
Gene therapy by expression constructs or down-regulation of certain genes has shown great potential for the treatment of various diseases. The wide clinical application of nucleic acid materials dependents on the development of biocompatible gene carriers. There are enormous various compounds widely investigated to be used as non-viral gene carriers including lipids, polymers, carbon materials, and inorganic structures. In this review, we will discuss the recent discoveries on non-viral gene delivery systems. We will also highlight the in vivo gene delivery mediated by non-viral vectors to treat cancer in different tissue and organs including brain, breast, lung, liver, stomach, and prostate. Finally, we will delineate the state-of-the-art and promising perspective of in vivo gene editing using non-viral nano-vectors.
Keywords: Gene delivery, Non-viral vectors, Cancer, in vivo, Gene editing, Clinical translation
Graphical abstract
1. Introduction
Since the elucidation of the molecular mechanisms of several diseases along with the discovery of nucleic acid structure, the replacement of defective genes with functional versions has been considered as a new therapeutic paradigm called “gene therapy” [1,2]. Gene therapy is carried out by expression constructs in order to increase the production of specific proteins inside the cells. On the other hand, down-regulation of specific genes has shown great potential for the treatment of various diseases [3]. Therefore, the modulation or silencing of such genes using antisense or siRNA has opened up new horizons for the introduction of a novel therapeutic strategy for incurable diseases [4]. Recently, the breakthrough of chimeric antigen receptor T cell immunotherapy and gene editing platforms have revolutionized the classic gene therapy approaches.
The broad clinical application of nucleic acid materials as a drug is significantly dependent on the progress of gene carriers with the capability to transfer nucleic acids into the target cells with low toxicity [5]. The evolution pathway of viruses enabled them to pack the genetic materials, protect them against degrading enzymes (e.g., nucleases) and transfer them into the target cells with high specificity. As of 2017, around 67% of all gene therapy clinical trials were carried out by viral vectors [6]. However, there are several significant concerns regarding the application of viruses as a cargo, including immunogenicity, insertional mutagenesis, as well as reports of deaths following the administration of viral vectors for gene delivery. Also, the limited capacity of viruses for gene delivery and expensive production methods of engineered viruses for large-scale production has hampered their application as a promising vector [7,8]. For example, Glybera (alipogene tiparvovec) which was approved in 2012 for the treatment of familial lipoprotein lipase deficiency (LPLD) withdrawn from the market due to the high price of 1 million US dollar per single injection which made it as the most expensive medicine in the world at that time [9]. Therefore, considerable attention has been directed to the application of a new class of carriers with the ability to mimic the virus properties for infection, promote the cellular entry of nucleic acids and their release inside the cells (e.g., cytosol or nucleus) [10,11]. These carriers are called as non-viral vectors and must be able to interact with nucleic acids to condense them outside the cells and protect the genetic materials from various degrading factors [12,13].
There are various enormous compounds widely investigated to be used as non-viral gene carriers, including peptides, lipids, and polymers. Among these different materials, polycationic polymers have been widely used due to their specific characteristics [14]. The molecular structure of these compounds is stable and enables them to act as a scaffold for further modifications in order to improve their properties for in vivo applications. For instance, cationic polymers contain several amine groups in their structure, making them as positively charged compounds. This charge is the critical factor for electrostatic interaction with the negatively charged nucleic acid materials and forming polyelectrolyte complexes (i.e., polyplex). These unique supramolecular assemblies could interact with the negatively-charged components on the plasma membrane and facilitate the cell entry via adsorptive endocytosis [15]. The translation of polyplexes from bench to bed is still in the beginning. However, there are some polycationic compounds in different phases of a clinical trial for the treatment of various diseases, including cystic fibrosis, AIDS, bladder and ovarian cancers, as well as melanoma and inherited TTR amyloidosis (Table 1, Table 2 ) [[16], [17], [18], [19]]. Various polycations have been used in these clinical trials, such as unmodified polyethylenimine (PEI), which is the most extensively investigated polycation for gene delivery. Also, the conjugated forms of PEI with cholesterol and mannose have been used for some clinical applications [20,21]. On the other hand, PEG conjugated, and transferrin-conjugated polylysine have been applied in human clinical trials [22]. The polycations used for human gene delivery showed that the application of such materials in human is highly dependent on the optimization of their intrinsic properties including cytotoxicity [23]. In other words, their clinical application might be hampered by the significant toxic effects result from their cationic nature, which is a prerequisite for the formation of nano-sized particles [24]. The dilemma between higher efficiency of gene transfer and cytotoxic effects of polycations has led researchers to seek for different conjugation strategies for improving the properties of these materials for human application [25]. In addition, learning from nature directs investigators to design precise and sequence-defined polymers. This novel class of polycationic compounds could be called artificial viruses since they are not a real virus particle. However, they contain the essential parts of a virus, which have shown crucial role in gene delivery. These particles must be able to pack nucleic acid materials and protect them in the extracellular environment as well as intracellular compartments. The artificial viruses also contain the targeting ligands in order to direct them into the specific cells or subcellular organelles [4,26,27]. On the other hand, the dissociation of nucleic acid from its cargo could be considered as the rate-limiting step in successful gene delivery. Although the association of nucleic acid and vehicle is essential for complex formation outside the cells, the release of nucleic acids in cytosol or nucleus is a determining factor for the biological effects of nucleic acid therapeutics. It seems that the bio-inspired polycationic carriers may open up new avenues for the clinical translation of non-viral gene delivery systems.
Table 1.
Examples of non-viral delivery systems used for cancer gene therapy in preclinical stages.
| Delivery system/device | Cancer type | Bioactive compound | Animal model | Safety | Major outcomes | Refs |
|---|---|---|---|---|---|---|
| Polymer hybrid NPs | Non-small cell lung cancer (NSCLC) | PLK1 siRNA | Subcutaneous A549 tumor model in male nude mice | Favorable gene delivery system | PHD/ PLL/siRNA NP showed excellent tumor growth inhibition rate | [73] |
| Gene-loaded microbubbles (MBs) | Lung cancer | miR-449a | Subcutaneous H1299-tumor model in Forty specific pathogen-free (SPF) BALB/C nude mice | Ultrasound MBs were showed the advantages of high safety, stability, and transfection efficiency |
Ultrasound-MB-mediated miR-449a protected the repressive effects of miR-449a on lung cancer progression | [74] |
| PEI-SP5-2 (PES) based polymer NPs | NSCLC | Human Wnt inhibitory factor-1 (hWIF-1) | Subcutaneous A549 tumor model in female BALB/c nude mice | Polymer NPs showed high biocompatibility in organ H&E and Hemolysis test | PES/hWIF-1 complexes inhibited the lung tumor growth | [75] |
| Liposome | Lung cancer | CYP1A1 siRNA | BALB/c nude xenografts | No noticeable toxicity | Inhibited tumor growth via down-regulation of CYP1A1 expression | [76] |
| Peptide-based cationic liposomes | Lung cancer | IGF-1R-siRNA | Lung cancer A549 cell xenografts | Induced pulmonary inflammation and liver injury at higher dosages. | Cationic peptide liposome was selectively delivered siRNA in the tumors of mice and efficiently inhibit tumor growth | [77] |
| Aptamer-nanocomplexes | NSCLC | AP/ES -Chloroquine /erlotinib/Survivin shRNA | Subcutaneous xenograft tumor model | Did not show apparent toxicity | Showed normalization of tumor vessels, which helps erlotinib/Survivin-shRNA delivery for reversal of erlotinib resistance in EGFR NSCLC. | [78] |
| Poly(ester amine) (PEA-NPs | Lung Cancer | Anti-MicroRNA-155 | Subcutaneous lung tumor model | PEA/anti-miR-155/HA-peptide complexes showed decent biocompatibility and stability | PEA/anti-miR-155/HA-peptide complexes showed excellent biocompatibility and lung tumor growth inhibition | [79] |
| Liposomes- PSH-DL | NSCLC | PFKFB3-shRNA& Docetaxel | Subcutaneous A459 lung tumor xenograft model | The highest apoptosis was observed for co-loaded liposomes rather than control group | PSH-DL showed promising tumor growth inhibition | [80] |
| Lipid-based NPs | Lung cancer | Plasmid DNA | Lung cancer-bearing BALB/c nude mice | Low cytotoxicity | Tf/HA-pDNA NLC was developed as an efficient and safe gene delivery system |
[81] |
| Glycerol propoxylate triacrylate spermine (GPT-SPE)- NPs |
Lung cancer | Importin 7 shRNA | K-rasLA1 lung cancer model | Low toxicity, high transfection efficiency and biocompatibility in vivo |
Down-regulation of importin 7 significantly inhibited lung tumor growth in vivo | [82] |
| Superparamagnetic iron oxide NPs (SPIONs) | Breast cancer | MIR376B microRNAs/AGO2 protein | Subcutaneous SKBR3 and MDA-MB-453 xenograft mouse models | NPs showed no detectable side-effects in histopathological examination of major organs | NPs selectively delivered microRNA into HER2-positive breast cancer cell lines in vivo and blocked autophagy | [83] |
| Polymer-inorganic hybrid –NPs | Breast cancer | Near-infrared (NIR-II)/plasmid | 4T1-Subcutaneous breast cancer | The H&E staining analysis of major organs and no noticeable body weight loss confirm the low in vivo cytotoxicity of tri-modal therapy | NPs showed a remarkable therapeutic effect of trimodal gene/PT/chemotherapy of malignant breast cancer treatment in vitro and in vivo | [84] |
| Graphene oxide nanoflakes with cationic lipids NPs | Breast cancer | DNA complexes | MDA-MB and MCF-7 cells | Showed high transfection efficiency with no appreciable cytotoxicity | Developed novel biocoronated gene delivery systems | [85] |
| Elastin like-recombinamer covalently conjugated to aptamer | Breast cancer | pDhMUC1 | Subcutaneous-MCF-7-breast cancer model | selective toxicity against cancer cells in in vitro and in vivo | Showed promising tumor growth inhibition in subcutaneous breast cancer model | [86] |
| Hydrogel | Breast cancer | RNA-triple-helix-& CXCR4siRNA | Subcutaneous breast cancer | Low toxicity | This gene delivery system delivered genes with high specificity and selectivity toward TNBCs | [87] |
| Linear polyethylenimine (LPEI)-Polyplexes | Breast cancer | CD49f-binding peptide CYESIKVAVS & plasmid DNA | 4T1 murine triple-negative breast cancer | No toxicity due to selective delivery | Polyplexes were well tolerated and resulted in measurable transgene expression in tumor areas. |
[88] |
| Magnetic- Fe3O4 NPs-b-MNP-PGEA | Breast cancer | PTT/p53gene | Subcutaneous mouse breast cancer model | No noticeable toxicity | Synergistic effects based on PTT-enhanced gene therapy was achieved | [89] |
| Hydrogel | Breast cancer | Survivin antisense oligonucleotide | Subcutaneous breast cancer | Reduced the possible adverse side effects | Sur-ASON/PHB-P/PF127 hydrogel significantly inhibited drug-resistant tumor growth | [90] |
| Mesoporous silica nanocapsules | Breast cancer | Doxorubicin/siRNA cocktail | Orthotopic breast cancer | MSNCs showed high biocompatibility | Doxorubicin/siRNA cocktail showed superior tumor growth inhibition in breast cancer. | [91] |
| PEGylated-PLGA/PIE NPs | Breast cancer | Ganciclovir (GCV) and CB1954 | Subcutaneous breast cancer | Minimum toxicity | GDEPT genes and prodrugs showed a significant reduction in tumor size (2.3-fold) compared with untreated control mice. | [92] |
| Immunoliposomes | Breast cancer | pcDNA3.1-CSF1-endostatin | Subcutaneous tumors | No differences were observed in mice behavior, no significant difference was detected in body weight and liver index |
Anti-CD105-mAb-conjugated immunoliposomes showed enhanced tumor targeting, imaging, and gene transfer applications with reduction of tumor growth | [93] |
| Branched polyethylenimine (BPEI) | Breast cancer | Plasmid DNA/ small interfering RNA (pololike kinase 1) | Subcutaneous tumors | Exhibited favorable biocompatibility, excellent targeting ability | SP-cross-linked BPEI/small interfering RNA (pololike kinase 1) polyplex showed favorable gene-silencing effects in vitro and satisfactory antitumor ability in vivo | [94] |
| Lipid-coated calcium phosphate (LCP) NPs | Breast cancer | Cell death control siRNA | MDA-MB-468 human breast xenografts | Showed no obvious cytotoxicity | Dual target LCP NPs significantly facilitated the tumor accumulation in vivo | [95] |
| Polymeric prodrugs- HPAA-MTX/MMP-9 | Breast cancer | MMP-9 shRNA plasmid/ methotrexate (MTX) | MCF7 subcutaneous tumors | No significant histological difference in vital organs | HPAA-MTX/MMP-9 co-delivery system exhibited significantly improved therapeutic efficacy to breast cancer | [96] |
| Rod-shaped active pure drug NPs | Breast cancer | microRNA lethal-7a (let-7a) | 4T1 tumors | No significant toxicity in H&E based in vital organs | Rod-shaped active NPs enable efficient and safe delivery of miRNA with synergistic treatment. |
[97] |
| Polypeptide NPs-PNLS | Breast cancer | siMDR1 | MCF-7/ADR tumors | Showed high biocompatibility & safety | PNLS combined with paclitaxel showed antitumor effects and high MDR1 gene silencing efficiency in the tumor-bearing nude mice | [98] |
| Polylysine-modified PEI polymer | Glioblastoma | HSV-TK and TRAIL | Intracranial C6 cell rat GBM model | double-transfected MSCs have increased the apoptosis in glioma of SD rats | Decline proliferation and angiogenesis, enhanced apoptosis | [99] |
| PEI-capped porous silicon NPs | Glioblastoma | MRP1-siRNA | Subcutaneous xenograft tumor model in nude mice | Biocompatible, No histopathological signs of acute damage | MRP1 knockdown, reduced GBM proliferation | [100] |
| Poly (l-lysine)-grafted polyethylenimine (PEI-PLL) NPs | Glioblastoma | HSV-TK DNA+ Angiopep-2 | Orthotopic U87MG-LUC GBM in nude mice model | Enhanced survival | Accumulation in striatum and cortex, inhibiting proliferation and inducing apoptosis, enhanced survival | [101] |
| Hyaluronic acid-decorated superparamagnetic iron oxide NPs | Glioblastoma | pDNA-TRAIL | Orthotopic glioma model in BALB/cAnN.Cg-Foxn1nu/CrlNarl mice | - | Activation of caspase-3 apoptotic signaling, prolonged survival, declined tumor size | [102] |
| Methoxy polyethylene glycol-polycaprolactone (MPEG-PCL) -DOTAP(DMC) nanomicelles | Glioblastoma | EZH2-siRNA | Subcutaneous Xerographic nude BALB/c mice and orthotopic glioma model in C57/BL6 mice | Normal histomorphology | High transfection efficacy, apoptosis, cell proliferation inhibition, enhanced anti-tumor efficacy, no changes in body weight | [103] |
| NickFect NPs- PEG2000 | Glioblastoma | pLuc2 | Intracranial U87MG and subcutaneous HT-1080 in nude mice | Elevated liver enzymes, no pathological changes in liver and lung | High transfection efficacy, better endosomal escape, higher bioactivity, accumulation in brain | [104] |
| Folate-conjugated cationic microbubbles | Glioblastoma | pFLuc | Intracranial C6 cell rat GBM model | Slight erythrocyte extravasation | Targeting potential, accumulation in brain, higher gene transfection and expression, accumulation in brain | [105] |
| Reducible poly(oligo-D- -arginine) | Glioblastoma | pEpo–NI2–SV–TK | Intracranial C6 cell rat GBM model | Less liver toxicity | Low cytotoxicity, higher anti-tumor potential, apoptosis | [106] |
| R7L10 peptide micelle | Glioblastoma | pEpo-NI2-SV- HSVtk | Subcutaneous C6 cell tumor model in Balb/c nude mice | Lower cytotoxicity than PEI | High apoptosis, higher antitumor effect, high transfection efficacy, reduced tumor size | [107] |
| R7L10 peptide micelles-curcumin | Glioblastoma | HSVtk | Subcutaneous C6 cell tumor model in Balb/c nude mice | - | High transfection efficacy, induced cell death, reduced tumor size | [108] |
| poly(β-amino ester)s (PBAEs) NPs | Glioblastoma | GFP DNA | Orthotopic murine model in nude athymic mice | - | Higher affinity to tumor cells, High transfection efficacy and expression | [109] |
| Self-assembling of DOTAP and MPEG-PLA (DMA) | Colorectal cancer (CRC) | IL-15 plasmid (pIL15) |
Subcutaneous and peritoneal models | Normal histological morphology, and no toxicity induced by the DMA-pIL15 on vital organ sections |
Inhibiting angiogenesis, promoting apoptosis, and reducing proliferation through activation of the host immune system |
[110] |
| Cationic fluorinated polymers (PFs) | Peritoneal metastasis of CRC | hTRAIL plasmid | Female BALB/c nude mice bearing peritoneal SW cells | The treatment did not cause any toxicity to normal tissues and organs |
Significant inhibiting of peritoneal metastasis of CRC |
[111] |
| Cationic poly (ω-pentadecalactone-co-N-methyldiethyleneamine-co-sebacate) (PPMS) polyplex (PPMS) |
CRC | G6PD shRNA | CRC cell line-based xenograft and patient-derived xenograft (PDX) models with high expression of G6PD * |
- | Increasing oxaliplatin-induced apoptosis in CRC by redox modulation | [112] |
| Self-assembling of DOTAP and MPEG-PLA (DMA) | CRC | IL-12 plasmid (pIL12) |
Subcutaneous and peritoneal models | No toxicity on vital organs induced by DMP-pIL12 | Suppressing tumor growth through preventing angiogenesis, enhancing apoptosis and inhibiting proliferation | [113] |
| Mixed micelleplexes containing PDMA-b-PCL and mPEG-PCL |
CRC | SN-38 (7-ethyl-10-hydroxycamptothecin), ultra-small superparamagnetic iron oxide NPs (USPIO), and VEGF siRNA | Xenograft LS174T tumor-bearing mouse | The mixed micelles more improved the in vivo biosafety than SN-38/USPIO-loaded siRNA-PEG micelleplexes | A theranostic micellar drug and gene delivery system, suppressing tumor growth, acting as a negative MRI contrast agent |
[114] |
| Self-assembled multi-arm polyrotaxanes | CRC | IL-12 plasmid (pIL12) |
C57BL/6 mice bearing subcutaneous MC38 tumor | No major systemic toxicity | Significant anti-tumor efficiency |
[115] |
| Poly(ethylene glycol)-ε-poly(caprolactone) block copolymer |
CRC | Co-loading of 5-fluorouracil (5-FU) and pEGFP | BALB/c nude mice bearing SW480 cells | The low toxicity of the pEGFP and the materials used in the formulation |
Significant inhibiting tumor growth |
[116] |
| PAMAM (G4 and G5) dendrimers modified by alkyl-carboxylate chain, PEG and cholesteryl chloroformate | Colon adenocarcinoma |
TRAIL plasmid | BALB/c mice bearing subcutaneous C26 tumor | No toxicity | Suppressing the tumor growth |
[117] |
| Fluorinated polymer (PF33) | Colon cancer | TRAIL gene | BALB/C nude mice bearing subcutaneous HCT 116 cells |
No systemic toxicity | Significant depletion of cancer stem cell-like cells (CSCL), remarkable inhibiting tumor growth |
[118] |
| Enteric-coated calcium pectinate microbeads |
CRC | p53 plasmid | Adult Wistar rats | - | Oral gene delivery as an effective novel alternative for CRC therapy |
[119] |
| CPIEDRPMC (RPM) peptide conjugated bioreducible polyethylenimine (SS-bPEIPEG-RPM) | Invasive colorectal cancer | pDNA | BALB/c-nu/nu mice bearing subcutaneous HT-29 cells |
Low toxicity | Specifically enhanced transfection efficiency in invasive colon cancer cells in in vivo |
[120] |
| Core/PEGylated shell (CPS) NPs comprised of a core of high molecular weight LPEI complexed with DNA and surrounded by a shell of polyethyleneglycol-modified (PEGylated) low molecular weight LPEI |
CRC | Plasmids | NOD-SCID-IL-2Rγ–deficient mice (NOG mice) bearing HCT116 through intrasplenic injection | Low toxicity | 16,000-fold increase tumor transfection, selectively transfected neoplastic cells rather than stromal cells within primary and metastatic tumors |
[121] |
| Electrotransfection | Colorectal adenocarcinoma | Plasmid DNA encoding miRNA-K-ras (pmiRNA-K-ras) |
SCID-C.B-17/IcrHsdPrkdcscid female mice bearing Subcutaneous LoVo cells | No side effects | Electrotransfection of LoVo cells with pmiRNA-K-ras indicated remarkable antitumor effectiveness, introducing the potential of miRNA molecules for local electrogene treatment of colorectal adenocarcinoma tumors |
[122] |
| Peptide-tagged cationic liposome–DNA NPs | Gastric cancer | pGFP | Athymic nude mice injected intraperitoneally with MKN-45P cells | Minimal accumulation in healthy control tissues | Enhanced tumor accumulation, preferential penetration of smaller tumor nodules, a highly clinically relevant target known to drive recurrence of the peritoneal cancer |
[123] |
| RGD peptides-conjugated pluronic triblock copolymers including poly(ethylene glycol)-block-poly(propylene glycol)- block-poly(ethylene glycol) (PEO-block-PPO-block-PEO, P123) | Gastric cancer | AP-2α expression plasmid | Female BALB/c mice bearing subcutaneous MGC803 cells (tumor xenograft mice) |
Low cytotoxicity | High anti-tumor efficacy by over-expression of AP-2α |
[124] |
| Oleylamine (OA)-modified disulfide-containing PEI | Liver cancer | Survivin-specific gene silencing | Nude mice carrying HepG2 xenografts | No toxicity to normal tissues | Enhanced tumor accumulation, Significant inhibiting tumor growth, |
[125] |
| Polymeric NPs composed of 2-((3- aminopropyl) amino) ethanol end-modified poly(1,5-pentanediol diacrylate-co-3-amino-1- propanol) (‘536’) |
Hepatocellular carcinoma (HCC) | pEGFP-N1 (eGFP) plasmid DNA | Subcutaneous HCC mouse model | Not cytotoxic to healthy hepatocytes | High and preferential DNA transfection in vivo |
[126] |
| Self-assembling peptide nanovesicle (SPV) | Liver cancer | Co-delivery of doxorubicin (DOX) and the acetylcholinesterase (AChE) gene | Liver cancer xenograft | - | Excellent drug/gene delivery, significant growth-suppressing effect |
[127] |
| Hydrodynamic gene delivery | HCC | Diphtheria toxin fragment A (DTA) gene-expressing plasmid and alpha-fetoprotein (AFP) promoter | YAP-induced HCC mice | No toxicity | Significant inhibition of HCC occurrence and the suppression of the tumor marker of AFP and des-gamma-carboxy prothrombin |
[128] |
| SP94-targeted triblock co-polymer NPs containing PLGA-PEG-PEI | HCC | Thymidine kinase-p53-nitroreductase triple therapeutic gene |
The xenograft tumor model bearing HepG2-FLuc cell |
Reduced toxicity | Strong expression of suicide genes selectively in tumors, inhibiting tumor growth after administration of GCV and CB1954 prodrugs |
[129] |
| Perfluoropentane/C9F17-PAsp(DET)/miR-122/PGA-g-mPEG ternary nanodroplets (PFP-TNDs/miR-122) or ultrasound-assisted polymeric nanodroplets | HCC | miR-122 | BALB/c nude mice (human HCC xenograft model) bearing SMMC-7721 cells | Excellent safety, all the mice remained alive without any side effects, and no significant weight loss |
Significantly enhanced miR-122 expression level 30-fold in human HCC xenografts, efficient inhibiting growth, migration and invasion of HCC cells and suppressing tumor proliferation |
[130] |
| ApoE-modified liposomes | HCC | Survivin promoter-driven HSVtk |
Human HCC xenograft mouse model | Liposome-HSVtk/GCV system is safe in vivo | Inhibiting the growth of xenograft tumors through an apoptosis-dependent pathway and extending the survival time of tumor-bearing mice |
[131] |
| Golgi membrane protein GP73 modified-liposome | HCC | Survivin promoter-driven HSVtk/ ganciclovir suicide gene | Human HCC xenograft mouse model |
Extended the survival of tumor-bearing mice without damaging the mice liver function | Significantly inhibiting the xenograft tumors growth via apoptosis-dependent pathway |
[132] |
| PEI-modified mesoporous silica NPs (PMSNs) |
HCC | Dual delivery of HNF4α and cisplatin | Male nonobese diabetic (NOD) severe combined immunodeficient (SCID) mice bearing subcutaneous Huh7 cells |
Mesoporous silica NPs (MSNs) have a good biocompatibility and low toxicity | Suppressing Cancer pluripotency and tumorigenicity in hepatoma-derived CD133-expressing stem cells |
[133] |
| Polyallylamine (PAA) mixed with partially oxidized alginate (OA) | HCC | miR-141 | Implanted HCC tumor model | NPX-glue delivers therapeutic miR-141 to solid tumors in a safe manner | Locoregional treatment of HCC is possible |
[134] |
| Magnetic mesoporous silica NPs (M-MSNs) | HCC | Herpes simplex virus thymidine kinase/ganciclovir (HSV-TK/GCV) | HepG2 xenograft-bearing nude mice | Decreased systemic toxicity | Theranostic nanoplatforms showed suicide gene therapy, magnetic hyperthermia therapy, and MRI simultaneously into a single system |
[135] |
| LPEI, polyethylene glycol (PEG) and synthetic peptide B6 (LPEI-PEG-B6) | HCC | Sodium iodide symporter (NIS) | HCC xenograft model bearing subcutaneous HuH7 | Markedly improved survival, improved safety of systemic NIS gene delivery |
Significant delay of tumor growth |
[136] |
| Polymer-based nanosystem (ROSE) | HCC | microRNA-34a | Mice bearing xenograft HCC tumors | ROSE/miR-34a could be used as a potential safe agent | Redox-responsiveness, oligopeptide-guided specificity, self-assembly, and enhanced transfection, Suppressing tumor growth |
[137] |
| RGD-PEG-DSPE/DOPA/CaP NPs | Prostate cancer | GRP78 siRNA and docetaxel (DTXL) | The PC-3 prostate cancer-bearing cells established in nude female BALB/c mice | LCP-RGD has a low hemolysis rate, good anticoagulation property, and immune safety | Good stability, Excellent biocompatibility, High drug and siRNA loading capacity, in vitro sustainable release profile |
[138] |
| Cationic nanobubbles (CNBs) conjugated with an A10-3.2 aptamer | Prostate cancer | FoxM1 siRNA | Xenografts tumors in nude-mouse model | Very low toxicity of siFoxM1-Apt-CNBs, without serious side effects | Significant inhibition of tumor growth with low toxicity, an obvious reduction in FoxM1 expression, and a higher apoptosis index | [139] |
| Sonoporation (sonodelivery) | Prostate cancer | IL-27 gene | They generated a model for the mouse IL-6Rα and aligned it to the human IL-6Rα crystal structure model | ART-1-directed liposomal IL-27 offered a higher safety profile and an improved therapeutic index, supporting the concept that peptides can be used to direct proteins or NPs for targeted delivery | Significant reduction in tumor growth, enhanced antitumor effects and higher accumulation of natural killer T (NKT) and CD8 effector cells in the tumors were observed. | [140] |
| Therapeutic-ultrasound (TUS) | Prostate cancer | Human tumor suppressor gene (hSef-b) | Xenograft model, Mouse models | Therapeutic ultrasound, considered safe for clinical applications | The results suggested that hSef-b acts in a cell autonomous as well as non-cell autonomous manner | [141] |
| TAT Modified and Lipid – PEI hybrid NPs | Prostate cancer | Docetaxel (DTX) and plasmid DNA (pDNA) | PC3 cancer cells and in a murine prostate cancer model | Safe TAT-DTX/pDNA LPNs improved safety of gene delivery | TAT-DTX/pDNA LPNs could be a promising co-delivery nano-system to achieve therapeutic efficacy for treatment of cancer | [142] |
| Dendrimeric RGD peptide and PEI grafted water soluble chitosan (RPgWSC) copolymer | Prostate cancer | pEGFP-N1 | Mouse xenograft model generated with PC3 prostate tumor cells by silencing BCL2 mRNA | RGD/PEI/WSC copolymer provides a safe and effective delivery of genetic material into cells | RGD/PEI/WSC copolymer for a good candidate as a simple and biocompatible gene carrier. | [143] |
| LPEI)-g-PEG as a career | Prostate cancer | VR1255C plasmid DNA encoding the gene for firefly luciferase | Metastatic prostate cancer-bearing mice | LPEI)-g-PEG as a career brings a safe delivery system in vitro and in vivo | lPEI-g-PEG with short PEG grafts (MW 500–700 Da) resulted in high colloidal stability, transfection activity in vitro and in vivo | [144] |
| Nanoghosts derived from mesenchymal stem cells | Metastatic orthotopic lung cancer and subcutaneous prostate cancer | Plasmid cDNA encoding for the C-terminal fragment of the human matrix metalloprotease-2, known as the hemopexin-like domain (PEX) | Prostate cancer xenograft model | The first evidence of the safe and effective transfection ability of MSC-NGs for cancer gene therapy | The NGs’ production scalability along with their uncompromising safety and efficient transfection ability as well as their versatile loading capacity, selective targeting of various pathologies, and shelf life stability can undoubtedly place them at the forefront of gene-delivery systems. | [145] |
| APT-PEG-PAMAM (APT-NPs) | Prostate cancer | miRNA-15a and miRNA-16-1 | Xenograft mouse model | To evaluate the safety of these NPs, body weight was monitored as a marker of overall toxicity. They resulted that the APT-NPs could be a safe gene delivery system for PCa treatment | A prototype for the safe and efficient delivery of miRNA expression vectors to PCa cells | [146] |
| Cationic hydroxyethylated cholesterol-based NPs | Prostate cancer | The plasmid pCMV-luc encoding the luciferase gene | Human prostate tumor PC-3 cells and xenograft models | Cationic hydroxyethylated cholesterol-based NPs transfer pCMV-luc in a safe manner | Potential non-viral DNA vector for the local treatment of tumor and in vitro | [147] |
* Patient-derived xenograft model is a tumor model in which the tumor cells from patients are implanted into the humanized or immunodeficient mouse model to obtain results that are more similar to the original patient.
Table 2.
Examples of non-viral carriers used in cancer gene therapy clinical trials.
| Non-viral carrier | Target | Bioactive compound | Clinical trial | Route of administration | National Clinical Trial (NCT) Identifier |
|---|---|---|---|---|---|
| PEG-PEI-cholesterol lipopolymer | Fallopian tube carcinoma, primary peritoneal carcinoma, recurrent ovarian carcinoma | Plasmid encoding IL-12 | Phase 2 | Intraperitoneal | NCT01118052 |
| Egen-001 (IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer | Recurrent or persistent ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer | IL-12 plasmid and - pegylated liposomal doxorubicin hydrochloride | Phase 1 | Pegylated liposomal doxorubicin hydrochloride intravenously (IV) and EGEN-001 intraperitoneally (IP) | NCT01489371 |
| Transferrin-cyclodextrin-oligocation | Solid tumors | siRNA against M2 subunit of ribonucleotide reductase (R2) | Phase 1 | Intravenous infusion | NCT00689065 |
| PEI | Bladder neoplasms | DNA plasmid that contains H19 gene regulatory sequences that drive the expression of an intracellular toxin [diphtheria toxin A (DTA) chain]only in cancer cells | Phase 2 | Intratumoral | NCT00711997 |
| PEI | Pancreatic ductal adenocarcinoma | Plasmid encoding somatostatin receptor subtype 2 named sst2 and deoxycitidine kinase :: uridylmonophosphate kinase named dck::umk | Phase 1 | Intratumoral | NCT01274455 |
| LPEI | Advanced/metastatic or recurrent solid tumors | MK-4621 with or without pembrolizumab |
Phase 1 | Intratumoral/Intralesional | NCT03739138 |
| DC-Chol liposomes | Advanced head and neck cancer | EGFR antisense | Phase 1 | Intratumoral | NCT00009841 |
| DOTMA/Cholesterol liposomes | Recurrent or refractory stage III or stage IV head and neck cancer | Interleukin-2 gene | Phase 2 | Intratumoral | NCT00006033 |
| Neutral liposome (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine or DOPC) | Advanced or recurrent solid tumors | EphA2 siRNA | Phase 1 | Intravenous infusion | NCT01591356 |
2. From bench to bedside: an overview
There are several intra- and extra-cellular barriers determining the pharmacokinetics and biodistribution of the non-viral gene carrier in the human body. These factors, along with the intrinsic characteristics of the carrier and nucleic acid material, play a crucial role in choosing the best and more efficient route for administration [4]. Various types of nucleic acids could be applied as therapeutic agents in gene therapy. The determining factor to choose the best nucleic acid material is the purpose of the treatment. In some diseases or pathologic conditions, the expression of specific genes may be reduced. Therefore, the essential need is to compensate the lower levels of gene expression by transferring a construct enabling the cells to up-regulate the specific gene. In these cases, a plasmid DNA (pDNA) could be considered as a tool to enhance gene expression. Plasmid DNA-based gene therapies could be categorized as the classic gene therapy in which the lack or loss of function in a cell is attributed to the low expression of a specific gene. The concept of using the pDNA as a therapeutic agent comes from the fact that this loss of function could be compensated by transferring the corrected or enhanced sequences expressing the functional protein in the target cell [[28], [29], [30], [31]]. Although the general idea of pDNA application for gene therapy seems to be simple, there are several problems hampering its clinical application. Naked pDNA delivery is not generally satisfactory due to the low uptake, degradation in the bloodstream, and poor pharmacokinetic properties. In addition, pDNA must be able to cross the nuclear membrane to access the transcriptional machinery of the cells [32]. A successful gene carrier must be able to pack the plasmid DNA outside the cells and protect it against degrading agents. On the other hand, the carrier system must allow the pDNA to be accessed by the transcriptional machinery of the cells for the production of mRNA. Therefore, an efficient plasmid DNA delivery system is needed to protect the plasmid outside the cells, particularly against degrading enzymes, enhance their cellular uptake, preferably to the target cells and improve their pharmacokinetic properties for in-vivo applications. Since pDNA delivery has shown some difficulties particularly in terms of in-vivo applications, an alternative strategy to improve the gene expression level is mRNA therapy [33]. mRNA therapy has shown great advantages compared with pDNA in recent years [34].The site of action for mRNA is cytoplasm, whereas the pDNA must be entered to cell nucleus for efficient gene expression [35]. Using mRNA does not need to overcome the nuclear envelope as one of the toughest barriers limiting gene delivery. Since the site of action for mRNA is the cytoplasm, the risk of insertional mutagenesis could be ignored. Although the immunogenic response against pDNA is limited to the CpG motif of plasmids by tool like receptors, the same responses against RNA sequences are remarkably lower. One more advantage of mRNA versus pDNA therapy is that the size of mRNA is smaller than pDNA. Therefore, it could be transferred to the host cells more easily. The last but not least advantage of mRNA application for gene therapy is the rapid responses following transfection. The transfection of pDNA takes several hours or days since the pDNA must enter the cell nucleus, be transcripted to mRNA, transferred to the cytosol and finally be accessed by the ribosome for the production of proteins. On the other hand, mRNA directly enters the cytoplasm and interacts with ribosome for protein production. These unique properties have made mRNA as a potential candidate not only for gene therapy but also for vaccine development particularly for the immunization against widespread viruses including SARS-CoV-2 [36]. However, the major concerns regarding the application of mRNA for gene therapy are its unstable nature and the existence of degrading enzymes such as RNases in the extra- and intra-cellular environments [37,38]. To overcome these problems, new developments, including SNIM (stabilized non-immunogenic mRNA), have been introduced in which the modified nucleotides could be incorporated into the mRNA structure to increase its stability and reduce its immunogenicity [4,[39], [40], [41]].
The aim of gene therapy is not just increasing the expression of certain gene as it was expected in previous decades. There are several pathological conditions related to the genes over-expression. In such conditions, the gene therapy goal would be silencing the target genes. The knock-down of such genes could be achieved by different nucleic acid materials, including antisense and siRNA. It must be considered that there are some differences between gene therapy and oligonucleotide therapy [42]. Oligonucleotide-based medications such as antisense do not need the transcriptional and translational machinery of the cells while the conventional gene therapy is based on the replacement of defected genes by the functional ones as well as the introduction of new gene into the cells including germlines or somatic cells [43]. Antisense technology is defined as a powerful tool to down-regulate a specific gene by transferring the antisense strand to the cells with the ability to interact with the sense strand. The base pairing between the sense and antisense strands results in the translational block [44,45]. On the other hand, RNAi technology employs several enzymes (e.g., dicer) and proteins (e.g., RISC complex) to interfere with the protein production. Antisense, miRNA, and siRNA are ribonucleic acid-based materials [46]. Therefore, the major concerns for RNA-based therapeutics do already exist for them. The successful delivery of such materials to the cells or tissues and organs need a delivery vehicle designed to circumvent the barriers for their efficient delivery [[47], [48], [49]].
A successful non-viral delivery system must have a favorable circulation time allowing the carrier to penetrate the target tissue with low toxic effects as well as biocompatibility and biodegradability of the carrier system [34,50]. Once taken up by the target cells, the delivery system must be able to be unpacked and release the therapeutic nucleic acid inside the cell. In other words, vector unpackaging inside the cells could be considered as an important factor for high transfection efficacy while the formation of stable complexes (i.e., packaging) outside the cells is a key factor for achieving successful gene delivery [5,30,51].
Another factor affecting the transfection efficiency is the size and zeta potential of the complexes. It seems that the particles with the size range of 50-100 nm and zeta potential of around ±10 mV have shown the best results to access the tumor microenvironment with the lowest uptake by the reticuloendothelial system (RES) [25,50,52,53]. The nucleic acid containing particles have shown short circulation half-life limiting their access to the target site while the larger complexes are not able to cross through the capillary fenestra to reach the tumor site. Prolonged blood circulation time is a prerequisite for gene delivery using non-viral gene carriers [54]. There are several various molecules conjugated on the surface of polymeric vehicles to make them as stealth carriers, including polyvinyl alcohol (PVA), poly (glycerol), poly-N-vinylpyrrolidone and poly (ethylene glycol) (PEG) [32,[55], [56], [57], [58]]. All these materials create a steric stabilization effect leading to the prolonged circulation half-life by prevention of immune-related proteins' opsonization. This type of carrier coating by forming a hydrophilic layer on the surface of the carriers reduces the risk of aggregation and increases colloidal stability. The reduction of the interaction between the stealth gene carriers and serum components reduces the recognition of the vehicles by mononuclear phagocyte system (MPS), including macrophages, which in turn leads to enhanced circulation time [58]. In order to direct the carriers into the precise site of action, smart gene carriers have been designed. These carriers could be targeted to the specific receptors by the conjugation of small molecules as well as macromolecules including monoclonal antibodies or aptamers [[59], [60], [61]]. Once the nano-carriers reach the cells, they may enter endosomal compartment, which degrades the nucleic acid therapeutics and leads to failed transfection. Hence, the promotion of proton sponge effect or the conjugation of membrane fusogenic compounds could be considered as brilliant strategies to overcome the endo/lysosomal barrier [62,63]. While the siRNA site of action is the cytosolic environment, plasmids must be able to cross the nuclear barrier. It has been shown that the molecules with the molecular mass of 40-70 kDa (10-25 nm) are able to passively diffuse via nuclear pores. However, the exact mechanism of nuclear entry is not completely understood [4,64]. It is not clear whether the polyplexes goes under vector unpackaging outside the nucleus or the transcriptional machinery of the cell dissociate the nucleic acids from the carrier inside the nucleus. Regardless of the mechanism, it has been demonstrated that cell cycle may have a crucial impact on the cell entry. The cells at the phases of S/G2 have shown the highest transfection efficiency. However, most cells are not in the dividing phase in vivo; therefore the alternative approaches, including the conjugation of nuclear localization signals (NLS), must be employed to increase nucleus entry [65,66]. The real value of these important findings is dependent on their translation to clinical application. The approval of patisiran (Onpattro®) as the first FDA approved siRNA based therapeutic for hereditary transthyretin-mediated (hATTR) amyloidosis opened up new horizons for the scientists to seek for the efficient delivery systems enabling the nucleic acids to be used as therapeutic agents. Patisiran has been formulated as lipid nanoparticles (NPs) and is used by intravenous infusions while the second approval for siRNA-based therapeutics belongs to givosiran (Givlaari®) [[67], [68], [69]]. Givosiran has been prepared as N-acetylgalactosamine (GalNAc) conjugated siRNA and is administrated subcutaneously. The first polymer-based gene therapy investigation in human was carried by Transferrin-polylysine (adenovirus-enhanced transferrinfection; AVET) carrier in order to transfer the plasmid encoding IL-2 gene for the treatment of melanoma [70]. In the first-ever human study of polyplexes, the ex-vivo gene transfer was performed to deliver the plasmid DNA into the patient cells. PEG conjugated polylysine was used to transfer the pDNA to treat cystic fibrosis as a nasal drug delivery system [16]. In another study to design a vaccine for HIV, mannose conjugated PEI was prepared as the carrier for the plasmid encoding various HIV antigens and used as a dermal formulation in a human clinical trials [71]. The intraperitoneal injection of PEG-PEI-Cholesterol to transfer IL-12 plasmid was also used for ovarian cancer treatment [72]. The intravenous injection of transferrin-cyclodextrin oligocation complexed with siRNA to silence ribonucleotide reductase M2(RRM2) was applied in various solid tumors [22]. Since various routes of administration have been used to transfer non-viral delivery systems for gene therapy, it seems that the route is highly dependent on the characteristics of the carrier and nucleic acids as well the prepared complex and the final formulation. It seems that there is no restrict limitation for a specific route of administration for non-viral gene delivery carriers at least in the theoretical aspect (Table 1, Table 2).
3. Lung cancer therapy
Despite advances in chemotherapy, surgery, and radiation therapy, lung cancer is one of the leading causes of cancer-related deaths globally [148,149]. Even though there is some initial response with present conventional chemotherapy, patients will develop resistance and exhibit poor survival with prolonged usage [150]. Several attempts were made to improve the survival of lung cancer patients using various combination therapies that have demonstrated that no further improvement observed, suggesting the need for specific, less toxic treatment approaches such as genetic alterations. Tumor suppressor genes and oncogenes are the two major genetic factors affecting the progression of the disease [151,152]. Hence, altering these explicit genes can advance the therapeutic benefit of present therapies. [153]. Numerous gene therapy strategies have been adopted, such as the deletions of oncogenes, immune stimulation, replacement of tumor-suppressor genes and transfer of genes that enhance conventional treatments [154]. Here, there are some examples of the recently reported non-viral gene carriers for lung cancer gene therapy [[155], [156], [157], [158]]. siRNA-encapsulated nanoformulations are being widely examined to find the suitable formulation, for lung cancer treatment [76,159]. For example, CYP1A1 is an important family member of cytochrome P450 enzymes involved in the metabolic pathways of cancer which is highly conserved in lung cancer. The investigators developed CYP1A1siRNA encapsulated cationic liposomes to inhibit the CYP1A1 gene in vivo. The cationic liposomes carrying CYP1A1siRNA efficiently silenced the CYP1A1 gene and inhibited tumor growth in BALB/c nude xenografts [77]. Recently, scientists demonstrated that peptide head groups containing lipids are more suitable than quaternary ammonium head groups containing lipids for gene delivery vectors for cancer therapy. Using this peptide-based IGF-1R-siRNA delivery system, the effective inhibition of tumor growth of the A549 cell xenografts was achieved [77].
Targeted delivery of the gene and drug to tumor cells is one of the important issues to reduce side effects on normal cells. Numerous approaches have been developed to improve the selectivity and safety of cancer treatments using small peptides, antibodies, and aptamers. For example, scientists developed Bcl-xL shRNA complexed PAMAM dendrimers containing aptamer as a targeting moiety for the treatment of lung cancer [160]. Yang et al. used a biodegradable polyester amine (PEA) and hyaluronic acid-coated gene delivery vehicle to deliver anti-miR-155 to lung tumors which showed promising results in both in vitro and in vivo (Fig. 1 ) [79]. Importantly, the Leaf Huang group developed VEGF-siRNA encapsulated polymetformin containing hyaluronic acid NPs which exhibited significant in vivo VEGF knockdown in lung cancer xenograft model (Fig. 2 ). The results exhibited that the non-viral delivery system for VEGF knockdown in a lung cancer xenograft model improved the efficiency of tumor suppression [161]. Recently, scientists developed a G11 peptide-functionalized supramolecular self-assembled pVEGF-shRNA loaded NPs for lung tumor-targeted therapy [162]. Zhao and his team also developed PLK1siRNA loaded poly(l-histidine) containing hybrid nanoplatforms to deliver PLK1 siRNA to NSCLC tumors [73]. Spermine is a tetra amine with outstanding biocompatibility. However, its usage in gene delivery is poor due to its low gene condensation capability. The researchers developed PEG-diacrylate modified spermine and folate functionalized NPs for gene therapy of lung cancer [163]. More recently, delivery and controlled regulation of genes via exosomes is recognized as a potential therapeutic method in the treatment of cancer. Researchers have developed an exosome-based microRNA-497 delivery platform for anti-cancer therapy in a microfluidic 3D lung cancer model [164]. In another study, scientists developed MDM2 siRNA loaded triazine-modified dendrimer NPs for gene delivery, which displayed remarkable tumor growth inhibition in the PC9 xenograft tumor model [165]. Scientists also used mesenchymal stem cells derived nanoghosts as a selective, safe non-viral gene delivery vehicle. pDNA complexed-nanoghosts inhibited the growth of metastatic orthotopic lung cancer, and significantly increased animal survival [145].
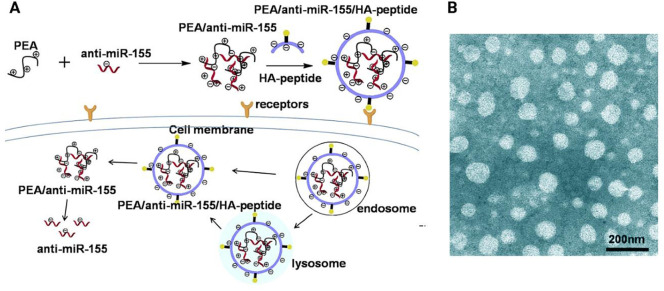
Fig. 1.
Formation and delivery progress of PEA/anti-miR-155/HA–peptide complexes into lung cancer cells. HA–peptide: CSNIDARAC peptide modified HA; CSNIDARAC peptide is a targeted peptide for lung tumor sites. (B) Transmission electron microscopy (TEM) image of surface morphologies of the carrier. Reprinted with permission from [79].
Fig. 2.
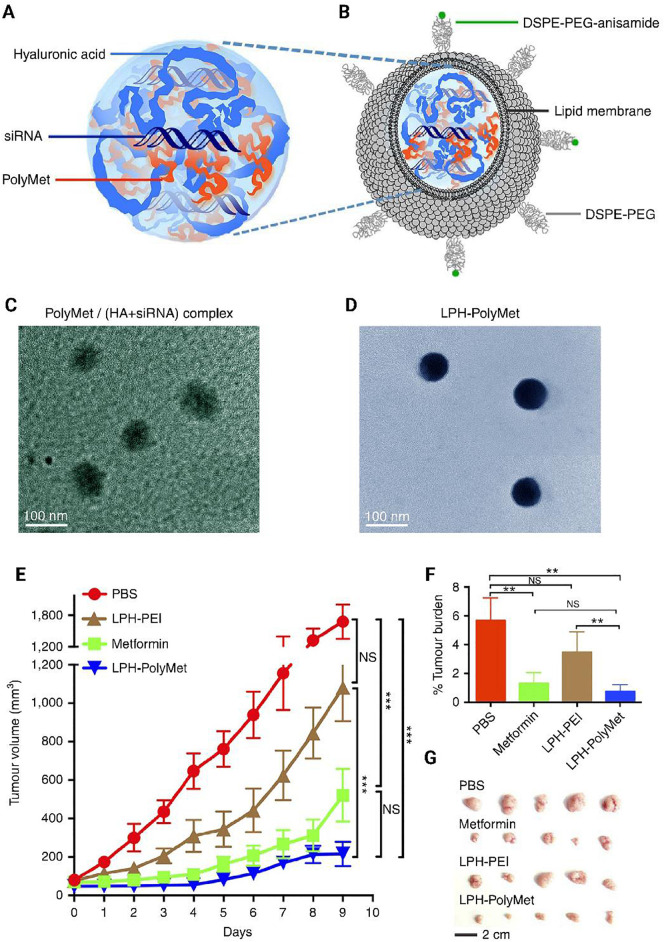
(A,C) Anionic HAsiRNA mixture was condensed by cationic PolyMet into a negatively charged PolyMet/(HAsiRNA) complex. (B,D) DOTAP/cholesterol cationic liposomes were added to the complex to form lipid coating, then DSPE-PEG and DSPE-PEG-anisamide were used to liposome by the post-insertion method to form LPH-PolyMet final NPs. (E) The daily calculated tumor volumes. (F) The daily calculated tumor weights. (G) Visual observations of the H460 tumor sizes in each treatment. DOTAP 1,2-dioleoyl-3-trimethylammonium-propane chloride salt. DSPE-PEG: 1,2-distearoryl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol-2000) ammonium salt. Reprinted with permission from [161].
There are several other non-viral vectors used for the delivery of various nucleic acid materials for lung cancer [[166], [167], [168], [169], [170], [171], [172], [173]]. Another most common genetic alteration happen in the lung cancer is associated with the tumor suppressor genes. For example, tumor suppressor gene TUSC2/FUS1 (TUSC2) is inactivated in lung cancer. However, no drug development approach is available for targeting the loss-of-function genetic deviations. Roth JA and his team developed a systemic gene therapy approach by using a TUSC2-expressing plasmid vector packaged in DOTAP:chol nanovesicles. They found that following the tumor treatment with DC-TUSC2, some major changes in the intrinsic pro-apoptotic pathway happened [174,175]. These nanovesicles were administered intravenously in the patients bearing lung cancer and the results showed an improvement in delivering TUSC2 genes to both human primary and metastatic tumors safely [176]. Among several existing polymeric transporters, PEI was mostly exploited to transfer genes for both in vitro and in vivo transfection. For example, scientists used PEI to develop a pH-sensitive in vivo selective gene delivery system to transfer p53DNA at the tumor site. A single administration of p53DNA nanocomplex along with laser radiation, significantly inhibited tumor growth and prolonged median survival [177]. Gold NPs also used to deliver p53DNA to lung cancer cells [178]. Several other studies also demonstrated that the p53-based gene delivery is able to improve the therapeutic outcome for lung cancer [[179], [180], [181], [182]]. In summary, based on these research updates, non-viral based gene therapy has shown promising potential for further developments towards lung cancer gene therapy.
The combination of physical approaches including ultrasound with non-viral vectors has shown great opportunity to enhance the transfection efficiency of these materials. For example, plasmid- binding cationic lipid microbubbles were combined with ultrasound mediated gene delivery to direct miR-133a to the tumor site. The results demonstrated that the transfection efficiency in cell cultivation and hind limb tumor xenografts significantly increased. The transfection enhancement could be associated with the potential of ultrasound in disturbing the cell membrane which facilitate the cell entry of nucleic acids [74].
4. Breast cancer therapy
There are several strategies to treat breast cancers based on the severity and the mechanisms involved in the pathogenesis including autophagy and apoptosis [183]. Although there are several non-viral vehicle for breast cancer gene delivery including cationic-liposomes, polymers, PLGA, inorganic material, exosomes, and engineered stem cells [184], we have focused on recent developments for designing novel carriers for breast cancer gene therapy.
Diverse categories of non-viral vehicles used for RNA (small interfering RNAs & microRNA) delivery. For instance, for the more sustained release of siRNA, Segovia et al. developed PBAE-siRNA biodegradable hydrogels in a framework built on PAMAM dendrimer cross-linked with dextran aldehyde. They observed significant levels of gene knockdown in the breast cancer tumor model [185]. In another study, the investigators established an inventive thermosensitive controlled release hydrogel loaded with a gene for breast cancer treatment (Fig. 3 ) [90]. Chol-VEGF-siRNA fused in high density lipoprotein (rHDL) for anti-angiogenic gene therapy of breast cancer [186]. Further, investigators used multi-functional mesoporous silica NPs (MSNP) for specific transfer of siRNA to the tumor site. In their study, they observed a safe delivery of Pgp-siRNA and Dox together with PEI-PEG-decorated MSNP at the tumor site while the tumor growth was reduced by inhibiting Pgp expression [187]. Pgp plays a crucial role in the induction of tumor resistance following the treatment with Dox and its down-regulation has attracted great attention for gene therapy. A similar study was carried out by another group using MSNs-TPGS NPs [188].
Fig. 3.
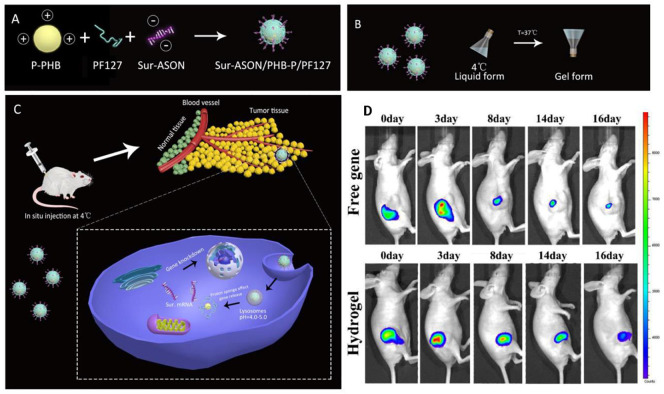
(A to C) Schematic presentation for the preparation of thermosensitive hydrogel and its in vivo therapeutic effect. (D) Retention of free gene and encapsulated gene in hydrogel at the local injection site after intradermal injection into mice and fluorescence emission. Sur-ASON: survivin antisense oligonucleotide; F127: Pluronic, poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) copolymer; PHB: Poly[(R)-3-hydroxybutyrate; PDMAEMA: 2-dimethylamino)ethyl methacrylate. Reprinted with permission from [90].
More recently, Zhang and his team developed a novel RNA-triple-helix hydrogel for the treatment of triple negative breast cancers (TNBCs). The researchers incorporated CXCR4siRNA and an RNA-triple-helix in the hydrogels NPs without synthetic polycationic reagents for the treatment of breast cancer [189]. Amorphous calcium carbonate fusion nanospheres fabricated with CaIP6 NPs were efficient in carrying genes to the tumor site. Scientists showed that AKT1 siRNA loaded CaCO3/CaIP6 nanocomplexes substantially inhibited tumor growth [190]. Similarly, a polypeptide containing LAH4-L1-siMDR1 loaded nanocomplexes displayed significant tumor growth inhibition when used along with PTX. In this study, high MDR1 gene silencing efficacy was observed in the tumor-bearing nude mice [98].
Enormous efforts are still underway for developing novel and effective gene delivery systems based on biocompatible nanomaterials to transfer the target genes to the tumor site [167,191,192]. For example, researchers have developed an elastin-like recombinant (ELR) and specific MUC1 aptamers for intracellular delivery of the MUC1 gene to breast tumors [193]. More recently, the same group developed a double protection tumor-specific nanomaterial device for gene therapy in breast cancer [86]. The functionalized peptides/ligands can also improve the delivery of nucleic acid-complexed NPs to tumors [95,194,195]. Recently, researchers established CD49f peptide-fabricated aerosol polyplexes for gene delivery to tumors of both breast and lung over-expressing the D49f gene [88]. In another study, scientists developed a polycation-decorated bowl-shaped magnetic assembly (b-MNP-PGEA) for magnetic resonance imaging (MRI)-guided synergistic gene therapy for the treatment of breast cancer [89]. Ruan et al. developed a cross-linked BPEI/plasmid DNA nanocomplexes, which resulted in great transfection efficiencies both in vitro and in vivo. Moreover, these polyplex have shown promising gene-silencing properties in vitro and significant antitumor activity [94]. Another group synthesized a novel PEGDGA-functionalized hPAMAM nanocomplex for effective gene delivery to breast cancer [196]. Cell-penetrating peptide (CPP)-containing and EGFR-siRNA loaded nanobubbles showed synergism with ultrasound irradiation mediated EGFR-siRNA delivery to TNBC [197]. Zhou et al also developed CD105-conjugated targeted cationic microbubbles for antiangiogenesis gene therapy for breast cancer [198]. Similarly, endostatin loaded and CD105 antibody conjugated immunoliposomes were prepared for antiangiogenic and imaging therapy [199]. Gu et al. also prepared CD44 antibody conjugated and anti-MDR1/P-gp short hairpin RNA complexed nanosystem for reversal of drug resistance. These nanocomplex enhanced the therapeutic efficacy of adriamycin in in vivo model [200]. Porous silicon NPs (pSi) were additionally reformed with PEI to yield pSi-PEI particles, which then complexed with siRNA for an effective treatment for breast cancer [201]. Recently, Devulapally et al. showed that PEGylated-PLGA/PIE NPs fused with the TK-NTR gene are able to decrease tumor growth when treated with other prodrugs in TNBC xenograft in vivo [92].
Recent discoveries may lead the researchers to redefine the role of p53 in breast cancer. Several studies have shown that p53 alterations increase the therapeutic efficacy of current chemotherapeutics. For example, Cationic β-cyclodextrin-PEI-Dox (PC-Dox) conjugates were prepared for carrying wt p53 plasmid in the form of PC-Dox/p53 nanocomplexes. This nanocomplex could inhibited the tumor growth synergistically and prolonged the survival of drug-resistant breast tumors mice [202]. In another similar study, the investigators proved that the co-delivery of p53 DNA and AVPI peptide enabled a complete arrest of tumor growth when used in combination with a reduced dose of Dox. In their study, they modified AVPI peptide not only to enable it to penetrate to tumor cells but also acts as a gene delivery vehicle by forming a nano complex with cationic R8 moiety [203]. There are several studies demonstrating that the p53 mediated gene therapy for breast cancer treatment is an efficient approach in cancer gene therapy [204,205]. Overall, the combination of chemotherapy along with gene therapy may enhance the therapeutic effects against breast cancer.
5. Brain tumor-targeted gene delivery
There are other categorization methods for brain tumors including primary and secondary tumors. Primary tumors originate from meninges, glands, nerve and other brain cells, while secondary tumors originate from other parts of the body and spread to the brain [206]. The most common brain cancers are glioma, neuroblastoma, meningioma, vestibular schwannoma and pituitary adenoma. The brain tumors can be primary diagnosed using MRI, CT scan, angiography, skull X-ray and biopsy. Despite enormous advances in the field of pharmaceutics and radiotherapy, the brain cancers cannot be completely cured.
Polymer-based carriers are accounted as one of the most effective carriers in drug delivery [[207], [208], [209]]. Active targeting with organic and inorganic NPs is the most effective strategy for drug delivery in cancer therapy [210,211]. Moreover, gene delivery is accounted as a hopeful strategy for brain cancer treatment. One of the most important obstacles in brain drug delivery is the blood-brain barrier (BBB). Therefore, there are many efforts to overcome this barrier including functionalization and modification of non-viral gene delivery vectors [212,213]. The modification leads to the transcytosis and endocytosis of vectors through cell-penetrating peptides (CPP) mediated transmembrane transport, adsorptive-mediated endocytosis and receptor-mediated endocytosis [214]. There are some receptors on the surface of brain capillary endothelial cells (BBB cells), including transferrin, insulin receptors and low-density lipoprotein receptor-related protein-1 (LRP1). Therefore, some molecules such as Angiopep-2, avidin, lactoferrin and transferrin are able to act as targeting ligands for these receptors and would be considered as promising molecules for transcytosis through the BBB. There are several reports indicating the role of cell-penetrating peptides and polyarginine (R8) to enhance the transcytosis of cargo through the BBB and cell uptake.
Several non-viral carriers have been investigated for gene delivery to the brain including monocytes owing to biocompatibility and passing through the BBB [101,215,216] as well as cationic polymers such as PEI [217], polyamidoamine (PAMAM) dendrimers, poly(amino acids cationic liposomes [218] and positive bubbles decorated with folate [105,214]. Despite significant advantages, each carrier system may suffer from drawbacks such as cytotoxicity and low transfection efficiency. [214]. PEGylated polyplexes have been developed to overcome the brain delivery of nucleic acids. These delivery systems not only decrease the cytotoxicity of polyplexes but also improve the gene transfection [219]. Abdallah et al. demonstrated that among the PEI with molecular weight of 25, 50 and 800 kDa, the PEI with 25 kDa has shown higher and prolonged gene transfection efficacy with less toxicity in mice brain [220]. However, modification of PEI with other molecules such as myristic acid enhances transfection and survival time in tumor animal models [217].
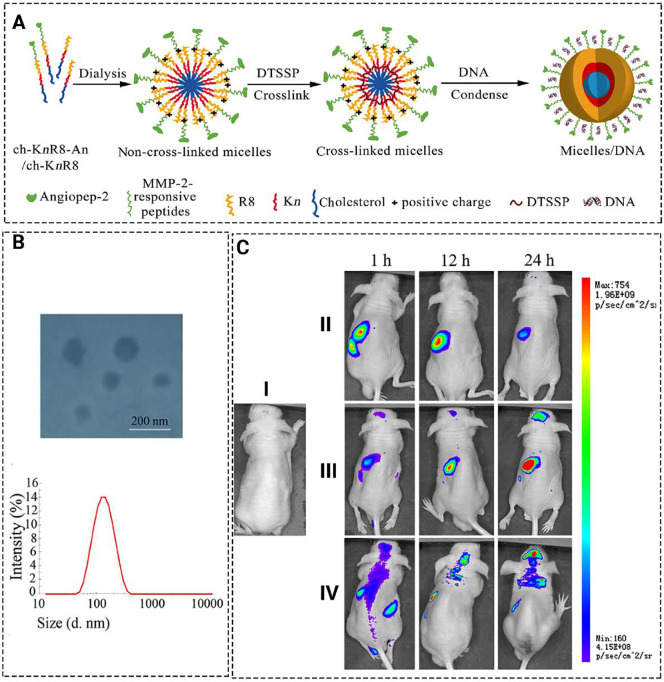
There are several various approaches to improve the transfection efficiency of non-viral carriers for brain delivery. For example, Jiao et al. [221] designed a multifunctional cargo for gene delivery (Fig. 4 ). They used angiopep-2 as a transcytosis factor and conjugated R8 to a targeting motif of MMP2 as an inducer of cell uptake and cancer microenvironment targeting agent. The polypeptide was supposed to be released from the MMP-2-responsive peptide since the MMP2 is upregulated in tumor microenvironment. They prepared a cholesterol coupled micelle containing lysine and arginine (ch-KnR8) with the particle size and zeta potential of 90-160 nm and +10-40 mV, respectively. The cargo showed high transfection efficacy and uptake in U251 cells and high accumulation in mice bearing glioma [221].
Fig. 4.
(A) Schematic illustration for the formation of micelle/DNA. (B) Size distribution and TEM image of the micelles. (C) Real-time in vivo fluorescence imaging of U251 tumor-bearing nude mice intravenously administrated with PBS(I), YOYO-1(II), ch-K5(s-s)R8/pEGFP-YOYO-1(III), and ch-K5(s-s)R8-An/pEGFP- YOYO-1 (IV). Reprinted with permission from [221].
Noteworthy, Shi et al. also used Angiopep-2 to enhance BBB penetration. They decorated a polymersome containing poly(ethylene glycol)-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate)-b-poly(ethylenimine) (ANG-CP) with Angiopeo-2 and loaded the cargo with anti- polo-like kinase 1 (PLK1) siRNA (N/P ratio of 0.4, siRNA loading 9.6 wt%, particle size of 115 ± 1.9 nm, zeta-potential + 0.4 mV). In vitro BBB transcytosis assay showed significantly higher transcytosis of targeted nanocarrier (ANG-CP-siRNA) as compared to naked siRNA and CP-siRNA. Interestingly, ANG-CP Scrambled siRNA induces 2.5 fold higher cell uptake compared to non-targeted CP siScramble on U-87 MG cells as a model cell line. Pharmacokinetic studies showed a significantly higher circulation time of targeted and non-targeted CP-siPLK1 compared to the naked siPLK1. However, targeted CP siPLK1 accumulated in tumor site and not in the brain parenchyma and the targeted nanocarrier significantly silenced the oncogene and decreased the tumor growth with no bodyweight loss compared to the CP siRNA and naked siRNA. This gene carrier system did not show toxic effects on the other tissues such as spline, liver, heart, kidney and lung [222].
Besides BBB transcytosis, multidrug resistance could be considered as one of the major obstacles in the efficacy of chemotherapeutic agents in glioblastoma multiforme (GBM). For example, multidrug resistance-associated protein 1 (MRP1) plays critical roles in chemo- and radio-resistance. Tong et al., prepared a PEI coated porous silicon NP with an average particle size of 169-173 nm and zeta potential of + 50 mV. They loaded NPs with the MRP1-siRNA with the release rate of 70% between 24 to 48 h and injected them into the mice bearing GBM (U87 cell). The release profile of NPs between 24 to 48 h was 70% [100]. PEI- Si NP- MRP1-siRNA showed significantly higher loading and cellular uptake in U87 cells as compared to the non-PEI NPs due to the higher positive charge. Furthermore, the NP exhibited S phase cell cycle arrest, MRP1 silencing and doxorubicin sensitivity in U87 cells treated with PEI- Si NP- MRP1-siRNA compared to non-siRNA cargo. Noteworthy, the knock-down of the multidrug transporter P-glycoprotein (Pgp) induces G2/M arrest in leukemia cells [223]. The investigation of MRP1 silencing in CD-1 nude mice bearing U87 cells showed that PEI-Si NP- MRP1-siRNA significantly decrease the level of MRP1 mRNA and protein compared to the non-siRNA cargo [100]. It seems that the release profile of siRNA between 24 to 48 h has critical role in gene delivery efficiency. On the other hand, NPs with the same size showed different gene delivery efficiency due to release profile between 24 to 48 h. For example, chitosan [224] and PLGA NPs [225] led to the only 10% release of siRNA between 24 to 48 h. There are some reports on the comparison of biocompatibility of PEI with other polymers. For example, Oh et al. revealed that the cytotoxicity of PEI vectors was significantly higher than R7L10. R7L10 is a short amphiphilic peptide micelle that is chemically synthesized [107]. They used a suicide gene, herpes simplex virus thymidine kinase (HSVtk), for the gene delivery to GBM. DNA with the negative charge interacts with the positive surface of R7L10 micelle, while hydrophobic drugs such as bevacizumab, an angiogenesis inhibitor, can be entrapped in the core. Erythropoietin (Epo) transcription enhances in hypoxia conditions (central core of GBM) while nestin intron 2 (NI2) leads to gene expression in glioblastoma and neural stem cells. It was demonstrated that the stability of pEpo–NI2–SV–HSVtk/R7L10 was considerably higher than pEpo–NI2–SV–HSVtk/PEI after heparin treatment. Another result obtained from the Oh et al. study was the high DNA protection from the nuclease and significantly less C6 cell toxic effects by pEpo–NI2–SV–HSVtk/R7L10 compared to the pEpo–NI2–SV–HSVtk/PEI. Moreover, the transfection efficacy of R7L10 was significantly less than PEI and lipofectamine, while PEI induced a significantly higher cytotoxic effect in the liver, kidney and lung. Besides, combination therapy of avastin with pEpo–NI2–SV–HSVtk/R7L10 had a synergistic effect on tumor growth inhibition. Hence, it seems that R7L10 is safer than PEI and conjugation with Epo enhances its gene and drug delivery efficacy in hypoxia condition [107].
Dendrimers have been considered as effective drug delivery carriers and polyamidoamine (PAMAM) is one the most well-known dendrimers in drug delivery. It seems that primary and tertiary amines in dendrimer play a critical role in DNA condensation and release [226]. However, there are controversial reports on the safety of dendrimers owing to their positive surface charge, especially for G2–G4 dendrimers [227,228]. It has been shown that PEGylated lactoferrin-dendrimer-DNA has shown significantly less toxicity and higher transfection efficacy than non-PEGylated ones. Interestingly, they showed that brain uptake and transfection efficacy of the lactoferrin conjugated complexes were significantly higher than the transferrin substituted ones [229]. Bai et al., prepared an arginine-PAMAM carrier to deliver human interferon beta (IFN-β) using human IFN-β plasmid to glioma tumors in mice. IFN-β plays anti-tumor efficacy through the induction of apoptosis in the tumor. Their findings showed that R-PAMAM- pORF-IFN-β plasmid DNA significantly decreases tumor size in xenograft brain tumor model induced by U87MG cells and cancer cells such as U87 and Neuro2a while did not decreases survival rate in HT22 cells. However, R-PAMAM- pORF-IFN-β induced significantly higher levels of IFN-β gene expression and apoptosis in the brain tumor models in mice compared to the R-PAMAM- pORF groups [230]. Furthermore, functionalization of PEGylated PAMAM/pEGFP with chlorotoxin (N/P= 3:1) significantly enhances the animal survival rate, biodistribution, gene expression and apoptosis following the intravenous injection in the brain tumor compared to non-chlorotoxin dendrimer in the tumor (C6)-bearing mice [231].
There is some reports showing that the PEGylation and modification of liposomes with OX26 (BBB transporting facilitator) and chlorotoxin (brain tumor targeting) containing the plasmid hTERTC27 (N/P=6:1, particle size 120 nm) leads to significantly decrease in tumor volume and enhanced survival rate as compared to liposome/C27, liposome/OX26/C27, liposome/ chlorotoxin/OX26/pEGFP. These findings confirmed the importance of dual targeting in a successful gene delivery [218]. Furthermore, Huang et al. developed a superparamagnetic iron oxide NPs decorated with hyaluronic acid and functionalized with TNF-related apoptosis-inducing ligand (TRAIL) and CD44. The complex significantly decreases the tumor size and enhanced survival rate in the orthotopic xenograft cancer BALB/cAnN.Cg-Foxn1nu/CrlNarl mice model [102].
As mentioned earlier, transferrin is a considerable receptor on the surface of brain blood endothelial cells and glioma while the presence of excess transferrin induces competition with endogenous transferrin molecules. Therefore, Kuang et al. developed a sequence that targets transferrin (His-Ala-Ile-Tyr-Pro-Arg-His) while interacts with the distinct binding site of transferrin receptor [232]. Since the transportation of T7 increases in the presence of excess transferrin, Kuang et al. attached the T7 via PEG to a peptide dendrimer (dendrigraft poly-l-lysines (DGLs)) and red fluorescent protein (RFP) plasmid was used as the reporter gene. They formed polyplexes with the particle size of 141.6 ± 52 nm and zeta potential of + 3.19 mV. The results showed the enhancement of U87 cellular uptake by the T7 complex as compared to the T7. [232].
There is a well-known method for the preparation of peptide carrier and template with a secure biological activity and stability. D-amino acids are more stable than L-amino acids while they show less biological activity. If the peptide sequence gets retro-inverse the biological activity will approach to the native sequence [233]. Wang et al. synthesized a retro-inverse peptide from the parent sequence of C-end rule (CendR) “RPPREGR” and conjugated it to modified PEI and PEG to prepare a non-viral vector. The sequence specifically recognizes neuropilin-1 receptor which is involved in angiogenesis and over expressed on glioma cells. Since the pORF-hTRAIL gene enhances survival time in U87 glioma-bearing BALB/c nude mice through the apoptosis of glioma cells, this plasmid was used to form the complexes with the peptide platform. The cell viability of the complex was significantly less than PEI on U87 cells. The peptide prepared in this study has shown higher stability, remarkable ligand-receptor affinity for glioma cells and biological activity than parent peptide. However, the transfection efficacy and anticancer effect of the complex containing RPPREGR was significantly higher than the parent RPPREGR vector owing to receptor targeting of the retro-inverse peptide [233]. Another example for the application of peptide motifs as gene delivery systems was reported by Zhan et al..They conjugated cyclic arginine-glycine-aspartic acid- (cyclic RGD) to a PEG-PEI polymer and the plasmid DNA (pORF-HTRAIL) was used for complexation. The complexes were formed with the average particle size of 73 nm. RGD as an important factor in neovascularization has shown high affinity for integrin αvβ3 and it could be used as a targeting ligand for glioblastoma cells (U87). However, the nanocarrier induces significantly prolonged survival time in glioblastoma bearing nude mice [234]. One major point is that the cyclic RGD has shown higher affinity and selectivity with its receptor compared to RGD through conformational restraint [235,236]. Lei et al. investigated whether the applying of disulfide bound to conjugate the RGD-PEG and PEI core may enhance the transfection efficacy in U87 brain tumor-bearing BALB/c nude mice. They used the plasmid pDsRED-N1 to form the complexes at N/P ratio of 12 with the particle size of 205.5 nm and zeta potential of +4.6 mV. The results indicated that the PEGylation decreased the particle size and zeta potential due to the reduced surface charge. Moreover, the transfection efficacy of RGD-PEG-SS-PEI/pDsRED-N1 was significantly higher than the non-sulfide vector due to the detachment of PEG from the complex following the cleavage of disulfide linker in GSH rich microenvironment at tumor cell [237]. Furthermore, other researchers designed a PEGylated peptide NP with CPP (stearylated transpartan 10 sequences) and nominated it as NickFect (NF). The structure was prepared using the attachment of Cys to Boc-l-Lys(Mtt)- OH. The negative charge of phosphorylated NF and increment of helicity lead to the enhancement of transfection efficacy. Moreover, the results of gene delivery in BALB/c mice bearing glioblastoma showed higher gene transfection efficacy than naked pDNA. [104]. Another example of gene delivery via peptide vehicles is the complexation of herpes simplex virus-thymidine kinase-ganciclovir (HSV-TK/GCV) plasmid and TRAIL plasmid into poly L-lysine-PEI. It has been shown that HSV-TK/GCV is a suicide gene which has synergistic effect while it is used with TRAIL [238]. They confirmed that the increase of polymer has a direct relationship with the decrease of cell viability and poly L-lysine enhances cell viability. Intratumoral injection of MSC (tumor tropism) transfected with polyplex-TRAIL- HSV-TK (N/P 1:3) enhances cell viability, rat survival and VEGF marker while decreases apoptosis as compared to the polyplex-TRAIL, polyplex-HSV-TK and PBS in glioma-bearing SD rats [99]. However, the complex containing SV-TK with erythropoietin and nestin intron 2 (NI2) showed that its complexation with reducible poly oligo D-arginine has significantly less cytotoxicity than PEI even at hypoxic condition. Furthermore, the polyplex induced significantly higher apoptosis and tumor size decrease in an intracranial glioblastoma rat model [106]. Overall, the targeting strategies might be considered as a prerequisite for non-viral vectors used for brain gene therapy.
6. Gastrointestinal cancer therapy
The focus of this section is on the synthetic non-viral delivery vectors evaluated in in vivo gastrointestinal cancers including colorectal and gastric cancers. These nano carriers have been employed as delivery vehicles for RNA silencing of oncogenes, DNA delivery of tumor suppressors, apoptosis inducers, suicide genes or immune-stimulatory molecules.
6.1. Colorectal cancer therapy
Colorectal cancer is the third most deadly diagnosed cancer in the world due to its metastasis [239,240]. Various types of non-viral carriers have been employed for colorectal cancer therapy [[241], [242], [243]]. However, combination therapy, including co-delivery of drug and gene by NPs have attracted more attention these years [244,245]. Wang et al. [116], investigated the potential of co-loaded NPs with anticancer drugs and genes as a promising strategy for colorectal cancer therapy. They used poly (ethylene glycol)-ε-poly(caprolactone) block copolymer for co-loading of 5-fluorouracil (5-FU) and pEGFP (DNA) as DFNC. Investigating in vivo gene transfection of NCs (nanocarrires) such as DNC (DNA nanocarrier) and DFNC showed more anticancer efficiency at 72 h rather than 24 h resulted from the NCs sustained release. The results of in vivo gene delivery indicated that around 60% of the cells were transfected by the gene. The in vivo study was done on BALB/c nude mice and qualitative and quantitative findings confirmed the efficiency of NCs for in vivo gene therapy of colon cancer. Antitumor efficacy of NCs was also exhibited significantly reduced the tumor growth in FNCs and DFNCs groups (around 320 mm3 at day 21) rather than free 5-FU (852 mm3).
Moreover, siRNA-based gene therapy is a promising alternative modality in colorectal cancer treatment. mPEG-PCL copolymer has been widely studied due to the biocompatibility and biodegradability as a carrier for different drugs [246,247]. Modifying this copolymer with amphiphilic DOTAP (DMP) has shown remarkable stability and safety for colon cancer gene therapy [248,249]. For example, the cationic self-assembled DOTAP and MPEG-PCL hybrid micelles safely and effectively deliver Bcl-xl siRNA and Mcl1 siRNA to C26 cells in BALB/c mice bearing colon cancer xenografts. DMP/siRNA also demonstrated significant therapeutic efficacy in inhibiting tumor growth induced by apoptosis activation. Bcl-xl and Mcl1 genes are anti-apoptotic genes from Bcl-2 family which play a crucial role in suppressing apoptosis.
DOTAP containing DMP micelles has shown great stability over 96 hours with remarkable transfection efficiency. Their hydrodynamic average size and zeta potential were 144.8 nm and +46.4 mV, respectively. The highest binding efficiency of siRNA was achieved at the highest DMP: siRNA (N/P) ratio of ≥ 30. Intratumoral injection of DMP/siRNA in C26 xenograft animal model showed significant reduced tumor weight, including DMP/siMcl1 complex (0.34 ± 0.06 g, p < 0.01), DMP/siBcl-xl complex (0.42 ± 0.08 g, p < 0.01) compared to control group (0.85 ± 0.09 g) and DMP group (0.76 ± 0.11 g), confirming the tumor growth inhibitory effects of DMP/siRNA complexes. Moreover, no significant changes were reported on the other organs such as heart, liver, spleen, lung, or kidney mainly due to partial masking of positive charges of DOTAP. During the self-assembly process of micelles, DOTAP is embedded inside the MP copolymer. This phenomenon causes the shielding of the positively charged head groups of DOTAP. Moreover, it is resulted in less serum protein binding and ultimately more transfection efficiency [248]. Also, DMP micelles were used for the delivery of the survivinT34A gene (S-T34A, a suicide gene) for colon cancer gene therapy [249]. Targeting the apoptosis pathways plays a critical role in cancer treatment. Survivin is an apoptosis inhibitor [126,250,251] through phosphorylation of its threonine 34 (Thr34) [247], resulting in the stimulation of tumor growth and resistance to cancer therapy. However, the lack of Thr34 phosphorylation leads to the breakdown of caspase-9-survivin protein complex and activation of caspase-dependent apoptosis [[247], [248], [249]]. In order to prepare a nonphosphorylated mimic of survivin, Thr34 was changed to Ala (T34A) via site-directed mutagenesis, [252,253]. Following the infection of cancer cells by survivin-T34A mutant, activation of the suicide effects and spontaneous apoptosis occurred. The mean particle size and the zeta potential of DMP were 46 ± 5.6 nm and +41.8 ± 0.5 mV, respectively. The highest DNA binding efficiency of DMP was observed at DMP: DNA ≥ 10 (weight:weight) ratio. The transfection efficiency was 37±2.5% compared to 32± 3% for the golden standard of PEI25kDa. Intraperitoneal injection of DMP/S-T34A (125 mg/5 mg) to female BALB/c mice with the abdominal cavity metastases of C-26 colon carcinoma resulted in the significant reduce of tumor weight and cancer-associated ascites. These findings indicated the therapeutic efficacy of DMP/S-T34A in suppressing the abdominal cavity metastases of C-26 colon carcinomas. It was suggested that apoptosis activation is the major anticancer mechanism of DMP/S-T34A in vivo.
Despite the advancement in targeted drugs, the metastatic CRC (mCRC) patients are still suffered from poor prognosis and more mortality. However, improved pharmacokinetic profiles of targeted drugs, such as siRNA can be considered as promising achievements in mCRC treatment. In this regard, Sousa et al. [254], reviewed the systemic siRNA delivery strategies in mCRC, focusing on PLGA NPs. They reviewed strategies to enhance the siRNA encapsulation efficiency into PLGA, including co-encapsulation by cationic polymers and the other less toxic materials. These co-encapsulants facilitate endosomal escape, which in turn improved delivery efficiency. For example, Sureban et al. [255], used PEI as a co-encapsulants to enhance encapsulation efficiency of siDCAMKL-1 in PLGA NPs for CRC therapy (Fig. 5 ). The particles has shown the average size of around 200 nm and practical loading efficiency of 7.45 μg/mg. NP-siDCAMKL-1 was injected intratumorally into HCT116 xenografts in male athymic nude mice (NCr-nu/nu). DCAMKL-1 (Doublecortin and CaM kinase-like-1) is a microtubule-associated protein kinase and has been proposed as the gastrointestinal stem cell marker with high expression levels in gastrointestinal cancers [256]. DCAMKL-1 silencing could be achieved by activating the tumor suppressors such as microRNAs (let-7a, miR-200a and miR-144) and the down-regulation of c-Myc, KRAS, ZEB1, ZEB2 and Notch-1. NP-siDCAMKL-1 administration into colorectal cancer tumor xenograft model inhibited the tumor growth by silencing the proto-oncogenes including Notch-1 and c-Myc through the activation of miR-144 and let-7a, respectively. NP-siDCAMKL-1 can also suppress cancer metastasis due to effect on epithelial–mesenchymal transition (EMT) by Snail, Slug, ZEB1, ZEB2 down-regulation via miR-200a activation [255]. EMT in human epithelial cells creates the phenotype of “stem cell-like” and CD44high/CD24low cell surface markers [257].
Fig. 5.
(A) Measured tumor volume size at different time. (B) Photograph of mice bearing the tumors. Reprinted with permission from [255].
Taken together, NP-siDCAMKL-1 as a novel promising anti-cancer therapeutics could inhibit tumorigenesis and metastasis of CRC by knocking down the specific oncogenes through the regulation of various miRNA-dependent mechanisms. Furthermore, the same group reported the liposomal carrier for siDCAMKL-1 in order to inhibit CRC xenograft growth previously. However, PLGA-siDCAMKL-1 has shown the same efficiency or more than the liposomal formulations in the silencing of oncogenes including c-Myc.
It has been extensively reported that dendrimers are promising nanomaterials for cancer gene therapy due to their unique properties. Dufes et al. [258] systemically administrated polypropyleneimine dendrimers (PPIG3) loaded with tumor necrosis factor A (TNFA) gene, under the control of telomerase gene promoters (hTR and hTERT) to LS174T colorectal adenocarcinoma. This treatment demonstrated the synergistic antitumor effects of TNFA-loaded PPIG3 compared with the alternative treatments. This delivery system led to the remarkable regression and long-term survival in 100% of tumor models.
PAMAM dendrimers were also used for the delivery of survivin antisense oligonucleotide (survivin-ASODN) in colorectal cancer subcutaneous xenograft models [259]. PAMAM-survivin- ASODN significantly down-regulated survivin expression and tumor growth.
Nanogels are another type of non-viral gene carriers used for the delivery of therapeutic oligonucleotide in vivo. Nanogel containing heparin and cell-penetrating peptide R8 were grafted to low-molecular-weight PEI for the delivery of human TNF-related apoptosis-inducing ligand plasmid (phTRAIL). HPR/phTRAIL complex exhibited safe and efficient hTRAIL plasmid delivery and significant tumor growth suppression in the in vivo model of the abdominal metastatic colon carcinoma [260].
Plasmids expressing vesicular stomatitis virus matrix protein (pVSVMP)-loaded heparin-polyethyleneimine (HPEI) nanogels were also showed significant anti-tumor efficacy. pVSVMP/HPEI complexes significantly arrested the C-26 colon carcinoma growth in both intraperitoneal and intravenous injection, which resulted in the inhibition of abdominal and pulmonary metastases, respectively. Tumor suppression is induced by apoptosis induction, resulting in the prolonged survival rate. pVSVMP/HPEI complexes showed high transfection efficiency, low cytotoxicity, and improved blood compatibility compared with PEI25kDa [261].
In another strategy, the combination of immunogenic chemotherapy was studied by oxaliplatin (OxP), the first-line chemotherapy of colorectal cancer, and plasmid DNA of PD-L1 trap- loaded lipid-protamine DNA NPs. This strategy led to a transient and local expression of PD-L1 trap in the tumor microenvironment and synergistically inhibited the tumor growth with OxP in an orthotopic colorectal cancer model. Interestingly, the combination of OxP and PD-L1 trap does not stimulate the spleen accumulation of Th17 cells, despite the combination of OxP and anti-PD-L1 mAb, representing its low immunogenicity. Finally, this system showed the efficient and safe cancer immunotherapy approach which overcomes the limitations of checkpoint inhibitor immunotherapy [262].
Liposomes have also been extensively used in gene therapy of gastrointestinal cancers due to remarkable properties, including biocompatibility [263]. An injectable plasmid DNA of telomerase-specific oncolytic adenovirus (TelomeScan) expressing GFP was loaded in liposome (Lipo-pTS) and investigated in HCT116 tumor-bearing mice to take the advantages of oncolytic virotherapy and overcome their limitations including elimination by the immune system. Lipo-pTS showed the strong tumor-specific antitumor effect independent of coxsackie and adenovirus receptor (CAR) and decreased adenovirus-neutralizing antibodies (AdNAbs) in immune-competent mice. [264]. In another study, cationic liposome-targeted the murine endostatin gene, antiangiogenic agent, (Lipo/mEndo) suppressed the colon cancer growth and prolonged survival times of intraperitoneally injected mice. Lipo/mEndo inhibited ascites formation and tumor foci numbers on mesentery of the mice resulting in the reduction of tumor burden in the abdominal cavity [265]. Cationic liposomes were also employed to encapsulate the plasmid encoding prostate apoptosis response protein 4 (par-4). The administration of this formulation resulted in the over-expression of par-4, activation of apoptosis and more susceptibility to 5-FU in hT29 tumor-bearing nude mice [266].
In addition to various materials used for the delivery of nucleic acids for colorectal carcinoma, electrotransfection is a promising route for facilitated delivery of genes into the target cells. In a study conducted by Vidic et al., the effect of miRNA to knock down the K-ras on K-ras expression level and the growth of colorectal carcinoma cell line was evaluated using electrotransfection. The results showed the potential of electroporation as a simple and reproducible method for local administration of miRNA-K-ras into the target cells with no side effects. Therefore, electro gene therapy could be considered as an effective strategy to direct the genes to the target cells and organs [122].
6.2. Gastric cancer therapy
Gastric cancer is the second most malignant cancer worldwide with the poor five-year survival of 30% [267]. A range of nanoparticulate systems have been investigated for efficient and safe delivery of genes to gastric cancer models. For example, calcium phosphate NPs (CPNPs) were used to deliver a novel fusion suicide gene, yCDglyTK, which is regulated by a cancer-specific CEA promoter and a CMV enhancer (CV) [[268], [269], [270]]. It was observed that CPNPs specifically delivered the suicide gene to the CEA positive gastric cancer cells and significantly inhibited the growth of gastric tumor xenograft models following the 5-FC administration. CPNPs-CV-yCDglyTK system can also be encouraging in the treatment of other cancers with CEA over-expression alone or in the combination with radiopharmaceuticals or other conventional therapies.
Furthermore, RNA-based nanoformulations have recently attracted considerable attention as a new paradigm of in vivo cancer therapy due to chemically and thermostatic stability and desirable and specific in vivo characteristics. For instance, Cui et al. constructed a multifunctional RNA NP to transfer BRCAA1 siRNA to gastric cancer MGC803 xenograft model. This targeted theranostic NPs composed of three-way junction (3WJ) of bacteriophage phi29 motor pRNA, folic acid as targeting ligand, Alexa647 as a fluorescent image marker and BRCAA1 siRNA. FA-pRNA-3WJ-BRCAA1 siRNA NPs significantly led to in vivo gastric cancer regression and in situ fluorescence imaging of tumor without toxicity in the non-targeted organs [271].
Drug resistance to anti-HER-2 medications in gastric cancer treatment resulted in the development of other treatment strategies. The combination of multiple therapies like gene-, immune-, and photothermal-therapy was reported through siRNA@CPG@Gold Nanoshell. Gold nanoshells were used for the delivery and photothermal release of HER-2-siRNA and immunoadjuvant of CpG oligodeoxynucleotide in MFC gastric cancer. Multidimensional treatment strategy based on gold nanoshell has shown more effectiveness rather than monotherapy in gastric cancer models [272].
Cationic polymers including linear or branched PEI have been extensively applied for gene therapy in vitro and in vivo. The branched form is preferable due to the high cationic charge and transfection efficiency [273]. A gastric cancer-targeting NP for siRNA delivery and MRI applications was synthesized by the conjugation of a single-chain variable fragment of CD44v6 (scFvCD44v6) to polyethylene glycol-grafted PEI modified with superparamagnetic iron oxide (PEG-g-PEI-SPION). The targeting of scFvCD44v6-PEG-g-PEI-SPION in vivo was confirmed by MRI, which was encouraging for simultaneous diagnosis and treatment of gastric cancer [274]. PEG-g-PEI-SPION with the promising results was also used by Chen et al. for the delivery of siRNA targeting CD44v6. This target was designed for the prevention and treatment of gastric cancer metastasis and in vivo MRI gene tracking [275].The results showed the capability of PEG-g-PEI-SPION as a highly efficient contrast agent in MRI applications in vivo.
7. Liver cancer therapy
Hepatocellular carcinoma (HCC) is another deadly cancer worldwide due to the late diagnosis and the impaired and insufficient treatments. Therefore, it is necessary to develop the carriers with enhanced targeted specificity, improved efficiency and safety [276,277]. Diez et al. [278], formulated one of the advanced non-viral carrier called targeted lipopolymer for encapsulation of interleukin (IL)-12. This system has demonstrated enhanced transfection efficiency and the higher survival in mice bearing BNL (undifferentiated murine hepatocarcinoma). The targeted lipopolymer consists of PLGA/DOTAP conjugated to asialofetuin (AF) ligand and loaded with IL-12 gene. The transfer of immunostimulatory gene is a potent strategy for cancer therapy. Interleukin (IL)-12 is one of the most powerful immunostimulatory cytokines with the considerable anticancer effects [279,280]. Asialofetuin (AF) is also an excellent ligand for the specific recognition of asialoglycoprotein receptor (ASGPR) over-expressing in the hepatocarcinoma cells. Targeted-NPs demonstrated five- to 12-fold improved transfection efficiency in comparison with non-targeted complexes or naked plasmid pCMV IL-12, respectively. This is associated with the maximal levels of IL-12 and interferon-γ in the mice sera on the day 14 after the injection. AF-NPs inhibited the tumor growth by stimulating the natural killer (NK) cells through the releasing of IFN-γ which is essential for antitumor activity of IL-12 [278].
Designing the targeted vectors for specific delivery to the liver with high transfection efficiency is the main obstacle for HCC gene therapy. To circumvent these limitations, Xue et al. [281], prepared dual targeting NPs to targeted delivery of RASSF1A gene to HCC via ASGPRs and external magnetic field (Fig. 6 ). In this regard, Gal-CMCS-Fe3O4-NPs were synthesized by modification of Fe3O4 NPs with biocompatible and biodegradable carboxymethyl chitosan (CMCS) and conjugation to galactose (Gal) ligands through free amino groups of CMCS. Ras Association Domain Family 1A (RASSF1A) is the prominent tumor suppressor gene which is involved in the Ras signaling pathway and have shown crucial role in apoptosis, microtubule stability, and cell-cycle regulations. Inactivation of RASSF1A through the hypermethylation of its promoter is associated with the risk of several cancers including HCC. It can also be a predictive factor for poor HCC prognosis. In vivo efforts to re-express RASSF1A has shown the arrest of HCC growth as well as the improved sensitivity of HCC cells to mitomycin [282].
Fig. 6.
(A) Schematic illustration of the entry of chitosan-Fe3O4-NPs inside the nucleus of cell. (B) Orthotopic transplantation of hepatocellular carcinoma in mice. The arrow marks the position of the small magnet. Reprinted with permission from [281].
Gal-CMCS-Fe3O4-NPs have shown the average size of 40.1± 5.3 nm and the zeta potential of +6.5 mV. This size limit is proper for uptaking by HCC cells [283]. Since receptor-mediated endocytosis of targeted carriers is affected by the NPs size [284], NPs with a diameter of < 50 nm are selectively targeted to the hepatocytes while NPs with a diameter of > 140 nm could be uptake by Kupffer cells. Gal-CMCS-Fe3O4-NPs were stable at pH=7 and demonstrated the strongest DNA binding at physiological pH. The optimal DNA binding was observed at the weight:weight ratio of 3:1. Transfection efficiency of pcDNA6.2mir-EGFP-loaded Gal-CMCS-Fe3O4-NPs in HCC tissue in the presence and the absence of an external magnetic field was about 40.8% and 29.7%, respectively, suggesting the efficiency of dual-targeting of NPs in the specific tumor accumulation. For in vivo studies, nude mice with orthotopically transplanted HCC were treated with intravenous injection of the Gal-CMCS-Fe3O4-NPs/pcDNA3.1(+) RASSF1A complex and intraperitoneal injection of MMC along with the applying an external magnetic field to the tumor site. These mice showed the smallest tumor size, the most percentage of apoptotic cells, and up-regulation of caspase-3 expression in tumor tissue compared with the other groups. Re-expressing of RASSF1A using dual targeting NPs proposes a new promising approach for HCC gene therapy through increasing the sensitivity of HCC cells to chemotherapy.
Using the gold NPs for miR-375 delivery has also been reported for the HCC gene therapy [284]. miR-375 regulates the gene expression and acts as a tumor suppressor macromolecule. It has been reported that miR-375 down-regulation is associated with different tumors, including gastrointestinal cancers [285]. However, re-expression of miR-375 suppresses hepatocarcinogenesis and HCC malignancy [247]. The surface of gold NPs was coated by a PEG layer for stabilizing the particles and covalent binding to miR-375 and labeled with Cy3 fluorescent dye for fluorescence imaging. Gold NP-miR-375 had an average size of 53 ± 8 nm and the zeta potential of -34 ± 1.8 mv. In vivo studies of gold NP-miR-375 in HepG2 xenograft tumor mouse model and primary HCC tumors demonstrated the safe and enhanced delivery of miR-375 to tumor tissue resulting in the significant increase of therapeutic efficacy.
Poly (beta-amino ester) (PBAE) NPs are another carrier which investigated by Zamboni et al. for specific and efficient DNA delivery to HCC. 2-((3-aminopropyl) amino) ethanol end-modified poly(1,5-pentanediol diacrylate-co-3-amino-1-propanol) (known as ‘536’) at carrier to plasmid ratio of 25 (w/w) demonstrated specific DNA delivery to a heterogenic HCC population and HCC xenograft model. pEGFP-N1 (eGFP) plasmid DNA was used as a reporter gene. The average hydrodynamic size and zeta potential of the carrier system at C/P ratio of 25 were 157 ± 3 nm and +18 ± 0.3 mV, respectively. The nano-vehicle was intratumorally injected to subcutaneous Huh-7 xenografts in athymic nude mice. It was suggested that biodegradable 536 NPs would also be appropriate for systemic or trans-arterial delivery due to its small size which preferentially localized in tumor through EPR effect [126].
In another effort for HCC gene therapy, a multifunctional NP targeted for HCC was designed to deliver TRAIL gene in mice [251]. These self-assembled lipid-bilayer structures (LCPP NPs) are composed of the calcium phosphate (CaP) and protamine core, which act as a pH stimuli-responsive and TRAIL nuclear localization agent, respectively. Moreover, The Ca ions released from CaP reverse the TRAIL resistance. HCC-targeting peptide (SP94) was also used for targeted delivery of NPs. Finally, TNF-related apoptosis induced by efficient TRAIL delivery and targeting of both the tumor and the adjacent tumor microenvironment resulted in the significant HCC tumor inhibition. Co-delivery of TRAIL and PTEN gene by zein NPs as an FDA-approved protein with a high proportion of hydrophobic and polar amino acids has also been reported [286]. The amphiphilic characteristics of these NPs have facilitated the interaction with DNA and cell membrane, thereby high bioavailability of loaded genes in HCC rat liver tissue has been observed. Besides TRAIL, other apoptotic inducers have been delivered for HCC gene therapy. DNA encoding tBid, a pro-apoptotic mitochondrial factor, was interacted with a nanopolymer based on folic acid grafted PEI600-CyD (Cyclodextrin) named H1. A modified human α-fetoprotein (AFP) promoter, EA4D, was fused with tBid to achieve pGL3-EA4D-tBid/H1. HCC tumor model studies showed the best activity and specificity in AFP-producing HCC treatment with the minimal toxicity in nude mice [287].
Various studies have shown the successful delivery of siRNA through NPs in HCC models in vivo, although they have not entered to clinical trials yet. siRNA-lipid NPs (siRNA-LNPs) were reported to target YAP (Yes-associated protein), an oncogenic transcription factor, in a genetically engineered mouse (GEM) HCC model. YAP suppression leads to the arrest of the rapid proliferation of tumor cells, and then acquire the characteristics of hepatocyte differentiation in advanced HCC [288]. Due to the high specificity and low toxicity of siYAP-LNPs, it is expected that this delivery system could be used for delivery of siRNA or shRNA to inhibit multiple targets in HCC.
In order to develop a novel approach for improved cancer immunotherapy, tumor-targeted lipid-dendrimer-calcium-phosphate (TT-LDCP) was designed for dual-targeting of siRNA against immunosuppressive factors (the immune checkpoint PD-L1) and pDNA encoding the immunostimulating cytokine IL-2. TT-LDCP NPs led to enhanced tumoral infiltration and stimulation of CD8+ T cells, improved cancer immunotherapy, and regression of HCC [289].
Another alternative strategy for hepatocellular carcinoma gene therapy is hydrodynamics-based gene delivery procedure. This method has been tested in several studies due to its simplicity, reproducibility and its potential to transfect around 30–40% of cells, mostly the hepatocytes in the liver. In a study conducted by Kamimura and his colleagues, a diphtheria toxin fragment A (DTA) gene-expressing plasmid was transferred using the hydrodynamics-based procedure. The results demonstrated a substantial inhibition of hepatocellular carcinoma occurrence in mice treated with hydrodynamic-based gene therapy 0 and 2 months after gene delivery [128]. Since ultrasound microbubble sonoporation have shown great potential for gene delivery, a ternary nanodroplet composed of perfluoropentane/C9F17-PAsp(DET)/miR-122/PGA-g-mPEG (PFP-7TNDs/miR-122) was prepared and evaluated for the transferring of microRNA-122 (miR-122) for hepatocellular carcinoma treatment. The results showed that the treatment of the cells with such system combined with ultrasound irradiation increased the miR-122 expression level by 30-fold in human HCC xenografts [130]. Hence, these methods have shown potential for further studies to develop safe and efficient gene therapy approaches.
8. Prostate cancer therapy
Prostate cancer is the fourth most common cancer and the second most extensive cancer in males leading to the mortality of around 300,000 individuals per year. Almost 200,000 new patients have been diagnosed per annum. The late diagnosis of prostate cancer is the primary cause of death [290,291]. Based on the stage and severity of the tumor, different treatments can be suggested to the patient including prostatectomy, radiotherapy, hormone therapy, chemotherapy, gene therapy, and a combination of them. The most recent procedure is gene therapy that mainly initiated via transferring a new gene to achieve destruction or fixation of cancerous cells [[292], [293], [294]]. Transferrin and lactoferrin are two iron-binding proteins that widely used as targeting ligands for prostate cancers [295,296]. Another promising approach for prostate targeting is using the integrins that can be attached to the extracellular matrix of prostate cancer microenvironment. Integrin receptors are supposed to be over-expressed on prostate cancer cells [297,298]. Prostate-specific membrane antigen (PSMA), integrins, and prostate stem cell antigen (PSCA) are the glycoprotein which could be targeted by various ligands [299,300].
Zhang et al. [138] studied an effective and biocompatible drug and gene delivery system using a RGD-PEG-DSPE/CaP. They achieved the LCP-RGD NPs by modifying the NPs, which contains a calcium phosphate (CaP) core, dioleoyl phosphatidic acid (DOPA) and RGD modified poly(ethylene glycol)-conjugated distearoyl phosphatidylethanolamine (RGD-PEG-DSPE). They used this system for co-delivery of GRP78 siRNA and docetaxel (DTXL) in other to cure the PC-3 CRPC. In another study, Dong et al. [142] tried to develop an effective co-delivery of docetaxel (DTX) and plasmid DNA (pDNA) for combination chemotherapy. They studied a cell-penetrating peptides (CPPs) modified lipid-PEI hybrid NPs (LPNs) and evaluated a modified RKKRRQRRR peptide (TAT), DTX, pDNA and LPNs (TAT-DTX/pDNA LPNs) in PC3 cancer cells (in vitro) and in a murine prostate cancer model (in vivo). Wang et al. [301] investigated in vitro and in vivo anti-tumor effects of nanobubbles carrying androgen receptor (AR) siRNA. In this study, they combined those nanobubbles with ultrasonic irradiation in order to test them on androgen-independent prostate cancer (AIPC). They concluded that those nanobubbles could be used as gene vectors for the treatment of AIPC. In a study by Wu et al. [139] they prepared an anti-tumor targeted FoxM1 siRNA-loaded cationic nanobubbles (CNBs) conjugated with an A10-3.2 aptamer (siFoxM1-Apt-CNBs). They concluded that their synthesized NPs could potentially act as a promising targeted gene delivery system for prostate cancer therapy. Rak et al. [302] suggested a group of cationic polyprenyl derivatives with different lengths of polyprenyl chains as DNA vehicles. They provided a group of lipofecting agents for in vitro and in vivo applications. Their results showed that these carriers could act as powerful gene carriers on DU145 human prostate cancer cells. In two different studies, Williford et al [144] and Wu et al. [146] worked on prostate tumor regression. Willfort et al. designed a PEGylated structure for DNA micellar NPs in order to achieve balanced colloidal stability and improved transfection activity [144]. On the other hand, Wu et al. evaluated the possibility of using aptamer-modified NPs (APT-NPs) to deliver miRNA to prostate cancer cells to demonstrate their tumor-targeting efficiency [146].
Some other studies tried to find a meaningful interaction between the prostate cancer and other organs using cytokines. For instance, Zolochevska [303] et al. analyzed the role of interleukin-27 (IL-27) in the interactions between prostate cancer and bone. They used the IL-27 gene delivery by applying sonoporation (sonodelivery) in vivo in order to treat and reduce the growth of prostate cancer at a bone metastatic site. Hattori et al. [304] achieved the elevated efficiency of transfection using the adhesion of extracellular matrix (ECM) to the complex of DNA/lipid (nanoplex). Once the ECM proteins coated on the nanoplex, they could improve DNA transfection activity in cells. They showed that Fn-coating nanoplexes could facilitate transfection of prostate tumor cells.
The use of alternative approaches including sonoporation for prostate cancer gene delivery has attracted great attention due to the high transfection efficiency and safety. For example, the delivery of IL-27 was carried out using sonodelivery with a biocompatible polymer complexed to pDNA to reduce prostate tumor growth in an immunocompetent TC2R C57/BL6 model [140]. Since there are several therapeutic ultra sound waves for clinical applications, those which operates at frequencies of 1-3 MHz and use relatively low intensities (0.1–2 W/cm2) could be considered for in vivo transfection. Using such therapeutic-ultrasound (TUS), a human tumor suppressor gene, hSef-b, was successfully transferred to prostate tumors in vivo [141]. These achievements have shown the potential of these routes for further studies towards the clinical translation.
9. Co-delivery systems
The most common treatment of cancers is chemotherapy while having various challenges and side effects including the lack of selectivity to the cancer cells and toxicity to the healthy cells [305]. Different approaches such as gene therapy and combination therapy have been suggested to circumvent these limitations [111,285,306]. Combination therapy may decrease the toxicity of each agent by reducing the individual drug-related dose. In this field, co-delivery of drug and gene-based NPs have attracted more attention [244,245]. The most common used nanosystems for co-delivery are polyplexes, which are made by the electrostatic interactions among the polymers, drugs, and nucleic acid materials. Wang et al. [116] investigated the potential of co-loaded NPs with anticancer therapeutics and genes as a promising strategy for the treatment of colorectal cancer. They used poly (ethylene glycol)-ε-poly(caprolactone) block copolymer for co-loading of pEGFP (DNA) and 5-fluorouracil (5-FU). The average hydrodynamic size of DNA and 5-FU co-loaded nanocarriers (DFNC) was increased to around 145 nm with a zeta potential of +15.4±3.2 mV in the case of co-loaded particles compared with +27.6±2.9 mV in the case of control nanocarriers. Electrostatic interaction of DNA with the outer layer of cationic NCs is the reason for increasing the size and neutralizing the surface charge. The gene entrapment efficiency was around 90%, indicating the high DNA-loading capacity resulting in the higher gene expression in vivo. Drug entrapment efficiency was also higher than 80%, suggesting the stability of NC. Moreover, the in vivo stability evaluation of NCs in the serum demonstrated no changes in the average size following the mixing with serum media. In vivo stability and the lack of protein corona may be induced by the PEG coating. Release studies exhibited that over 80 % of DNA was released at 48 h while the same release profile for the drug was achieved at 72 h, suggesting the faster release of DNA due to their orientation on the outer layer of NCs.
Interestingly, in a new promising strategy, a triblock copolymer micelle based on N-succinyl chitosan–poly-L-lysine–palmitic acid (NSC–PLL–PA) was employed by Zhang et al. [250] for co-delivery of doxorubicin and siRNA against P-glycoprotein. It has been observed that the emerged synergistic effect is even more efficient than co-treatment of chemotherapeutics and siRNA [116,193,244,285]. Dox–siRNA-micelle had an average size of 170 nm and a zeta potential of +3.2 mV. Furthermore, encapsulation and loading efficiency of DOX were 95.32 ± 2.06% and 16.09 ± 0.17%, respectively. siRNA binding efficiency was achieved at the best N/P ratio of 20:1. siRNA electrostatically interacted with the cationic backbone of PLL while the hydrophilic shell of NSC provides enhanced biocompatibility. On the other hand, DOX was encapsulated in the hydrophobic core of PLA. Following 24 h post-injection, tumor accumulation of Dox–siRNA-micelles was approximately complete due to the particle size and instability at low pH. In another study, researchers employed survivin shRNA and erlotinib (gene/drug) co-loaded delivery nanoformulation for the treatment of drug resistance EGFR-mutated non-small cell lung cancer [78]. More recently, this strategy was further improved by the other groups where the researchers used chitosan-based nanocomplex to deliver survivin shRNA, erlotinib, and heptamethine cyanine dye (Cy7, as a photothermal agent) in one stage for triple-combination therapy of NSCLC [307].
10. Future perspectives
In recent decades, considerable attention has been directed to the non-viral delivery of nucleic acid materials for gene therapy [308,309]. The breakthrough of immunotherapy, new advances in genomics and discoveries in re-programming the somatic cells to induced pluripotent stem cells (iPS) have created a new paradigm in medicine, which resulted in the re-introduction of gene therapy as a powerful tool for the treatment of several various diseases from cancer to viral infections. Recently, CRISPR (clustered regularly interspaced short palindromic repeat)/Cas (CRISPR-associated) genome editing systems have attracted considerable attention due to their potential to edit the genome based on RNA-guided nuclease [[310], [311], [312], [313]]. Among the several kinds of CRISPR/Cas systems, the type II systems attracted more attention for human applications. Only in these systems, Cas9 protein is an essential compartment for DNA interference. Generally, this system contains a nuclease protein (Cas9) and a guide RNA (gRNA) [213]. Since, the gRNA could be replaced by sgRNA (synthetic chimeric single guide RNA), the Cas9 protein could be directed to the target site using sgRNA which consequently leads to the induction of double-stranded DNA breaks (DSBs). Finally, the major pathways of repair mechanism in the cells are responsible for inducing the alterations. This simple, robust, user-friendly, specific, and efficient system has enabled researchers to create models for various diseases as well the novel therapeutic approaches [[314], [315], [316], [317]].
Generally, there are three different approaches for CRISPR/Cas 9 delivery [318]. The ultimate goal is to transfer the whole system into the cells. However, the ribonucleoprotein complex could be transferred to the cells via different routes. The first choice is to deliver sg RNA with Cas9 protein. This strategy is simple and straightforward which provides the Cas9 protein inside the target cells with no need for transcription or translation. The positive charge of Cas9 protein in physiological condition and the negative charge of sgRNA in the same environment may result in a major obstacle for efficient delivery of such ribonucleoprotein material. On the other hand, Cas9 protein is a large macromolecule with a molecular weight of ~160 kg/mol which might be obtained through the expression in bacterial hosts. The production of the protein in the bacterial hosts might be expensive and the endotoxin contamination could be considered as an additional obstacle for their large-scale production. Various non-viral carriers have been used to transfer such platforms into the target cells including gold NPs, graphene oxide, carboxylated branched poly (β-amino ester) NPs, β-cyclodextrin-conjugated low-molecular-weight PEI, microbubble-nanoliposomal particles, pH-responsive silica–metal-organic framework (SMOF) hybrid NPs consisting of both silica and zeolitic imidazole framework (ZIF) as well as cell-penetrating peptides and DNA nanoclews [[319], [320], [321], [322], [323], [324], [325], [326], [327], [328], [329], [330], [331]].
Since the difficulties for efficient delivery of Cas9 protein reduces the transfection efficiency of sgRNA and Cas9 protein, the alternative strategy is to use the Cas9 mRNA with sgRNA. For efficient delivery of Cas9 protein and sgRNA, the delivery platform must be able to transfer a large positively charged protein (Cas9) and a negatively charged nucleic acid (sgRNA) together. Designing such delivery systems is not simple. The second approach includes the delivery of two mRNA molecules with similar biophysical properties that facilitates the design of delivery systems. Besides, the introduction of Cas9 mRNA into cells does not need to be entered into the cell nucleus for subsequent transcription. Therefore, the main advantage for this approach is the quick onset of action. The transient expression of Cas9 mRNA in the cytosol along with the quick onset of Cas9 action make this approach an attractive way for the researchers to reduce the off-target effects associated with the long time presence of Cas9 protein inside the cells. However, the low stability of mRNA is the major hampering factor for this delivery method. Various non-viral delivery strategies have been employed to transfer Cas9 mRNA with the sgRNA together including zwitterionic aminolipid NPs [[332], [333], [334], [335]] and branched-tail lipid NPs.
Since there are several problems for efficient delivery of Cas9 protein, Cas 9 mRNA and sgRNA, the third method have been introduced which includes the design of a plasmid encoding Cas 9 and sgRNA inside the cells. The stability of plasmid-based CRISPR/Cas9 systems is really higher than protein or mRNA making these systems more attractive for in vivo applications. However, there are several major obstacles reducing its clinical applications. This system could be able to cross the nuclear membrane and access the transcriptional machinery of the cells. Since the transcription of the plasmids and the production of Cas9 protein and sgRNA need more time rather than the direct introduction of these macromolecules into the cells, the delay in the onset of therapeutic action is expected. In addition, the off-target effects associated with the long-term production of Cas9 protein is more probable rather than the previous methods. Also, the risk of the integration of plasmid into the genomic materials may reduce their potential for wide clinical applications. However, several non-viral delivery systems have been introduced for the efficient transfer of plasmid-based CRISPR/Cas9 systems including dendrimers, polymers, polypeptides and polysaccharides such as PLGA as well as lipid encapsulated gold NPs, PEI magnetic NPs and multifunctional nucleus-targeting core-shell artificial viruses [[336], [337], [338], [339], [340], [341]].
The physical approaches to transfer the CRISPR/Cas9 system for in vitro studies have shown great results. However, these strategies, including electroporation and microinjection, could not be used in human clinical trials [342]. The application of viruses (e.g., adeno-associated virus) is an efficient way to transfer these systems into the human target sites, but the drawbacks of the virus application as a gene carrier have raised several concerns [[343], [344], [345]]. Despite several obstacles hampering the efficient delivery of such systems into the cells, their tendency to the dividing cells versus post-mitotic non-dividing cells could be considered as an opportunity to transfer these platforms into the cancer cells. Altogether, the great potential of CRISP/Cas9 system for the treatment of several diseases and the development of various delivery approaches have opened up new horizons to translate the lab-scale achievements to the clinical applications.
11. Conclusions
In recent decades, various oligonucleotide-based therapeutics have been introduced for human clinical applications. This novel category of therapeutic materials includes antisense oligonucleotides and aptamers as well as siRNA-based medications. The clinical applications of these new drugs are the result of breakthrough discoveries in molecular biology. However, the translation of these achievements to the clinical applications is substantially dependent on the development of efficient and safe delivery systems. An optimized delivery system for nucleic acids should be able to form a stable structure outside the cells and release the payloads at the specific site of action. In addition, the toxicity of the delivery vehicle must be tolerable by the human cells. The biophysical properties and the pharmacokinetic characteristics of the vehicles are the other significant points which determine the potential of delivery system for human applications. In order to improve these properties, stealth technology using various materials such as PEG and targeting strategies have been introduced. Using these approaches, the biophysical characteristics of the carriers could be modified and their pharmacokinetic properties might be improved. Generally, polymer and dendrimer-based delivery systems have shown higher transfection efficiency [5,25,53]. However, their toxicity is the major concern for the further developments towards the clinical applications. For these carrier systems, the main modification strategy is focused on the reduction of cytotoxicity through the modulation of cationic charge or designs the biodegradable polycationic compounds. In addition, these materials suffer from the low targetability for the specific cells or tissues [346]. Therefore, the addition of targeting moieties on these materials could be considered as an effective way to improve their properties. These materials are appropriate delivery systems for the formation of complexes based on the electrostatic interaction between the nucleic acid and carrier. On the other hand, lipid-based carriers have demonstrated higher biocompatibility rather than the polymeric delivery systems [347,348]. These delivery systems have shown great potential for clinical applications due to their low toxicity. However, the transfection efficiency of such materials is generally lower than the polymeric compounds. Therefore, the major approaches to improve the properties of these vehicles are focused on the augmentation of their transfection efficiency. Similar to the polymeric delivery systems, lipid-based materials need the targeting moieties for efficient transfer of nucleic acid to the target cells or organs. Although the toxicity of lipid-based delivery systems is lower than the polycationic polymers or dendrimers, they may induce inflammatory responses following systemic administration. The translation of these materials for commercial application needs a scalable production process which leads to the commercial products with highest batch-to-batch uniformity. The most recent clinical trial on the application of mRNA as a potential vaccine for SARS-CoV-2 has been conducted by LNPs which shows the importance of this category of delivery system for human application [36]. To date, cationic lipids have shown great efficiency for the delivery of these materials compared with the other non-viral carriers. It seems that the rapid developments of gene editing platforms could not be translated to the clinical application, while the bottleneck of delivery systems is limiting their administration [349]. Therefore, the shoulder-to-shoulder development of these two fields is essential for the clinical translation of gene editing platforms.
Acknowledgments
The authors would like to thank Dr. Horacio Cabral (Department of Bioengineering, Graduate School of Engineering, The University of Tokyo, Japan) for his constructive comments.
Contributor Information
Ali Dehshahri, Email: dehshahria@sums.ac.ir.
Ali Zarrabi, Email: alizarrabi@sabanciuniv.edu.
References
- 1.Gorecki D.C. Prospects and problems of gene therapy: an update. Expert Opin. Emerg. Drugs. 2001;6(2):187–198. doi: 10.1517/14728214.6.2.187. [DOI] [PubMed] [Google Scholar]
- 2.Song S., Goudy K., Campbell-Thompson M., Wasserfall C., Scott-Jorgensen M., Wang J. Recombinant adeno-associated virus-mediated alpha-1 antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther. 2004;11(2):181–186. doi: 10.1038/sj.gt.3302156. [DOI] [PubMed] [Google Scholar]
- 3.Ashrafizadeh M., Fekri H.S., Ahmadi Z., Farkhondeh T., Samarghandian S. Therapeutic and biological activities of berberine: The involvement of Nrf2 signaling pathway. J. Cell. Biochem. 2020;121(2):1575–1585. doi: 10.1002/jcb.29392. [DOI] [PubMed] [Google Scholar]
- 4.Lächelt U., Wagner E. Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond) Chem. Rev. 2015;115(19):11043–11078. doi: 10.1021/cr5006793. [DOI] [PubMed] [Google Scholar]
- 5.Dehshahri A., Alhashemi S.H., Jamshidzadeh A., Sabahi Z., Samani S.M., Sadeghpour H. Comparison of the effectiveness of polyethylenimine, polyamidoamine and chitosan in transferring plasmid encoding interleukin-12 gene into hepatocytes. Macromol. Res. 2013;21(12):1322–1330. [Google Scholar]
- 6.Ginn S.L., Amaya A.K., Alexander I.E., Edelstein M., Abedi M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018;20(5) doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 7.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 8.Check E. Nature Publishing Group; 2002. A tragic setback. [DOI] [PubMed] [Google Scholar]
- 9.Senior M. Nature Publishing Group; 2017. After Glybera’s withdrawal, what’s next for gene therapy? pp. 491–492. [DOI] [PubMed] [Google Scholar]
- 10.Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011;6(12):815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 11.Tockary T.A., Foo W., Dirisala A., Chen Q., Uchida S., Osawa S. Single-Stranded DNA-Packaged Polyplex Micelle as Adeno-Associated-Virus-Inspired Compact Vector to Systemically Target Stroma-Rich Pancreatic Cancer. ACS Nano. 2019;13(11):12732–12742. doi: 10.1021/acsnano.9b04676. [DOI] [PubMed] [Google Scholar]
- 12.Lehrman S. Nature Publishing Group; 1999. Virus treatment questioned after gene therapy death. [DOI] [PubMed] [Google Scholar]
- 13.Wong S.Y., Pelet J.M., Putnam D. Polymer systems for gene delivery—past, present, and future. Prog. Polym. Sci. 2007;32(8–9):799–837. [Google Scholar]
- 14.De Smedt S.C., Demeester J., Hennink W.E. Cationic polymer based gene delivery systems. Pharm. Res. 2000;17(2):113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 15.Pack D.W., Hoffman A.S., Pun S., Stayton P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 16.Konstan M.W., Davis P.B., Wagener J.S., Hilliard K.A., Stern R.C., Milgram L.J. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum. Gene Ther. 2004;15(12):1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- 17.Walsh M., Tangney M., O’Neill M., Larkin J., Soden D., McKenna S.L. Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: implications for cancer gene therapy. Mol. Pharm. 2006;3(6):644–653. doi: 10.1021/mp0600034. [DOI] [PubMed] [Google Scholar]
- 18.Ohana P., Gofrit O., Ayesh S., Al-Sharef W., Mizrahi A., Birman T. Regulatory sequences of the H19 gene in DNA based therapy of bladder cancer. Gene Therapy & Molecular Biology. 2004;8(8):181–192. [Google Scholar]
- 19.Haussecker D. Current issues of RNAi therapeutics delivery and development. J. Control. Release. 2014;195:49–54. doi: 10.1016/j.jconrel.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 20.Fewell J.G., Matar M.M., Rice J.S., Brunhoeber E., Slobodkin G., Pence C. Treatment of disseminated ovarian cancer using nonviral interleukin-12 gene therapy delivered intraperitoneally. The Journal of Gene Medicine. 2009;11(8):718–728. doi: 10.1002/jgm.1356. [DOI] [PubMed] [Google Scholar]
- 21.Diebold S.S., Kursa M., Wagner E., Cotten M., Zenke M. Mannose polyethylenimine conjugates for targeted DNA delivery into dendritic cells. J. Biol. Chem. 1999;274(27):19087–19094. doi: 10.1074/jbc.274.27.19087. [DOI] [PubMed] [Google Scholar]
- 22.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadinejad R., Dadashzadeh A., Moghassemi S., Ashrafizadeh M., Dehshahri A., Pardakhty A. Shedding light on gene therapy: Carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs-A review. J. Adv. Res. 2019;18:81–93. doi: 10.1016/j.jare.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Tian B., Yin X., Zhang Y., Hu D., Hu Z. Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems. J. Biotechnol. 2007;130(2):107–113. doi: 10.1016/j.jbiotec.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Sabahi Z., Samani S.M., Dehshahri A. Conjugation of poly (amidoamine) dendrimers with various acrylates for improved delivery of plasmid encoding interleukin-12 gene. J. Biomater. Appl. 2015;29(7):941–953. doi: 10.1177/0885328214551010. [DOI] [PubMed] [Google Scholar]
- 26.Alemzadeh E., Dehshahri A., Izadpanah K., Ahmadi F. Plant virus nanoparticles: novel and robust nanocarriers for drug delivery and imaging. Colloids Surf. B: Biointerfaces. 2018;167:20–27. doi: 10.1016/j.colsurfb.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Alemzadeh E., Dehshahri A., Dehghanian A.R., Afsharifar A., Behjatnia A.A., Izadpanah K. Enhanced anti-tumor efficacy and reduced cardiotoxicity of doxorubicin delivered in a novel plant virus nanoparticle. Colloids Surf. B: Biointerfaces. 2019;174:80–86. doi: 10.1016/j.colsurfb.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Horn N.A., Meek J.A., Budahazi G., Marquet M. Cancer gene therapy using plasmid DNA: purification of DNA for human clinical trials. Hum. Gene Ther. 1995;6(5):565–573. doi: 10.1089/hum.1995.6.5-565. [DOI] [PubMed] [Google Scholar]
- 29.Hardee C.L., Arévalo-Soliz L.M., Hornstein B.D., Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes. 2017;8(2):65. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nouri F., Sadeghpour H., Heidari R., Dehshahri A. Preparation, characterization, and transfection efficiency of low molecular weight polyethylenimine-based nanoparticles for delivery of the plasmid encoding CD200 gene. Int. J. Nanomedicine. 2017;12:5557–5569. doi: 10.2147/IJN.S140734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oskouei R., Dehshahri A., Shier W.T., Ramezani M. Modified polyethylenimine: Self assemble nanoparticle forming polymer for pDNA delivery. Iranian Journal of Basic Medical Sciences. 2008;11(1):33–40. [Google Scholar]
- 32.Jiang C., Chen J., Li Z., Wang Z., Zhang W., Liu J. Recent advances in the development of polyethylenimine-based gene vectors for safe and efficient gene delivery. Expert Opinion on Drug Delivery. 2019;16(4):363–376. doi: 10.1080/17425247.2019.1604681. [DOI] [PubMed] [Google Scholar]
- 33.Tang X., Zhang S., Fu R., Zhang L., Huang K., Peng H. Therapeutic prospects of mRNA-based gene therapy for glioblastoma. Front. Oncol. 2019;9:1208. doi: 10.3389/fonc.2019.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merkel O.M., Kissel T. Quo vadis polyplex? J. Control. Release. 2014;190:415–423. doi: 10.1016/j.jconrel.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27(4):710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le T.T., Andreadakis Z., Kumar A., Roman R.G., Tollefsen S., Saville M. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 37.Wagner E. Biomaterials in RNAi therapeutics: quo vadis? Biomaterials Science. 2013;1(8):804–809. doi: 10.1039/c3bm60071h. [DOI] [PubMed] [Google Scholar]
- 38.Yoshinaga N., Cho E., Koji K., Mochida Y., Naito M., Osada K. Bundling mRNA Strands to Prepare Nano-Assemblies with Enhanced Stability Towards RNase for In Vivo Delivery. Angew. Chem. Int. Ed. 2019;58(33):11360–11363. doi: 10.1002/anie.201905203. [DOI] [PubMed] [Google Scholar]
- 39.Kormann M.S., Hasenpusch G., Aneja M.K., Nica G., Flemmer A.W., Herber-Jonat S. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29(2):154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 40.Bangel-Ruland N., Tomczak K., Fernández Fernández E., Leier G., Leciejewski B., Rudolph C. Cystic fibrosis transmembrane conductance regulator-mRNA delivery: a novel alternative for cystic fibrosis gene therapy. J. Gene Med. 2013;15(11–12):414–426. doi: 10.1002/jgm.2748. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Su H.-h., Yang Y., Hu Y., Zhang L., Blancafort P. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol. Ther. 2013;21(2):358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buss C.G., Bhatia S.N. Nanoparticle delivery of immunostimulatory oligonucleotides enhances response to checkpoint inhibitor therapeutics. Proc. Natl. Acad. Sci. 2020;117(24):13428–13436. doi: 10.1073/pnas.2001569117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin A.A. Treating disease at the RNA level with oligonucleotides. N. Engl. J. Med. 2019;380(1):57–70. doi: 10.1056/NEJMra1705346. [DOI] [PubMed] [Google Scholar]
- 44.Marwick C. First antisense drug will treat CMV retinitis. JAMA. 1998;280(10):871. [PubMed] [Google Scholar]
- 45.Smith R.J., Hiatt W.R. Two new drugs for homozygous familial hypercholesterolemia: managing benefits and risks in a rare disorder. JAMA Intern. Med. 2013;173(16):1491–1492. doi: 10.1001/jamainternmed.2013.6624. [DOI] [PubMed] [Google Scholar]
- 46.Zou Y., Zheng M., Yang W., Meng F., Miyata K., Kim H.J. Virus-mimicking chimaeric polymersomes boost targeted cancer siRNA therapy in vivo. Adv. Mater. 2017;29(42) doi: 10.1002/adma.201703285. [DOI] [PubMed] [Google Scholar]
- 47.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 48.Kota J., Chivukula R.R., O’Donnell K.A., Wentzel E.A., Montgomery C.L., Hwang H.-W. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalvati B., Dehshahri A. ShRNA-mediated knock-down of CD200 using the self-assembled nanoparticle-forming derivative of polyethylenimine. Nanomed. J. 2019;6(3):195–206. [Google Scholar]
- 50.Kullberg M., McCarthy R., Anchordoquy T.J. Systemic tumor-specific gene delivery. J. Control. Release. 2013;172(3):730–736. doi: 10.1016/j.jconrel.2013.08.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khalvati B., Sheikhsaran F., Sharifzadeh S., Kalantari T., Behzad Behbahani A., Jamshidzadeh A. Delivery of plasmid encoding interleukin-12 gene into hepatocytes by conjugated polyethylenimine-based nanoparticles. Artificial Cells Nanomed. Biotechnol. 2017;45(5):1036–1044. doi: 10.1080/21691401.2016.1202256. [DOI] [PubMed] [Google Scholar]
- 52.Amin Z.R., Rahimizadeh M., Eshghi H., Dehshahri A., Ramezani M. The effect of cationic charge density change on transfection efficiency of polyethylenimine. Iranian J. Basic Med. Sci. 2013;16(2):150–156. [PMC free article] [PubMed] [Google Scholar]
- 53.Dehshahri A., Oskuee R.K., Ramezani M. Plasmid DNA delivery into hepatocytes using a multifunctional nanocarrier based on sugar-conjugated polyethylenimine. Gene Ther. Mol. Biol. 2012;14:62–71. [Google Scholar]
- 54.Tong A.W., Jay C.M., Senzer N., Maples P.B., Nemunaitis J. Systemic therapeutic gene delivery for cancer: crafting Paris’ arrow. Curr. Gene Ther. 2009;9(1):45–60. doi: 10.2174/156652309787354630. [DOI] [PubMed] [Google Scholar]
- 55.Woodle M.C., Engbers C.M., Zalipsky S. New amphipatic polymer-lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjug. Chem. 1994;5(6):493–496. doi: 10.1021/bc00030a001. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi H., Kojima H., Yamamoto H., Kawashima Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J. Control. Release. 2001;75(1–2):83–91. doi: 10.1016/s0168-3659(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 57.Maruyama K., Okuizumi S., Ishida O., Yamauchi H., Kikuchi H., Iwatsuru M. Phosphatidyl polyglycerols prolong liposome circulation in vivo. Int. J. Pharm. 1994;111(1):103–107. [Google Scholar]
- 58.Romberg B., Hennink W.E., Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm. Res. 2008;25(1):55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dehshahri A., Sadeghpour H., Oskuee R.K., Fadaei M., Sabahi Z., Alhashemi S.H. Interleukin-12 plasmid DNA delivery using l-thyroxine-conjugated polyethylenimine nanocarriers. J. Nanopart. Res. 2014;16(5) [Google Scholar]
- 60.Sheikhsaran F., Sadeghpour H., Khalvati B., Entezar-Almahdi E., Dehshahri A. Tetraiodothyroacetic acid-conjugated polyethylenimine for integrin receptor mediated delivery of the plasmid encoding IL-12 gene. Colloids Surf. B: Biointerfaces. 2017;150:426–436. doi: 10.1016/j.colsurfb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Sadeghpour H., Khalvati B., Entezar-Almahdi E., Savadi N., Alhashemi S.H., Raoufi M. Double domain polyethylenimine-based nanoparticles for integrin receptor mediated delivery of plasmid DNA. Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-25277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall A., Lächelt U., Bartek J., Wagner E., Moghimi S.M. Polyplex evolution: understanding biology, optimizing performance. Mol. Ther. 2017;25(7):1476–1490. doi: 10.1016/j.ymthe.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sipe D.M., Jesurum A., Murphy R.F. Absence of Na+, K (+)-ATPase regulation of endosomal acidification in K562 erythroleukemia cells. Analysis via inhibition of transferrin recycling by low temperatures. J. Biol. Chem. 1991;266(6):3469–3474. [PubMed] [Google Scholar]
- 64.Pandey A.P., Sawant K.K. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater. Sci. Eng. C. 2016;68:904–918. doi: 10.1016/j.msec.2016.07.066. [DOI] [PubMed] [Google Scholar]
- 65.Liu G., Li D., Pasumarthy M.K., Kowalczyk T.H., Gedeon C.R., Hyatt S.L. Nanoparticles of compacted DNA transfect postmitotic cells. J. Biol. Chem. 2003;278(35):32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- 66.Khalil I.A., Kogure K., Akita H., Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006;58(1):32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 67.Hu B., Weng Y., Xia X.H., Xj Liang, Huang Y. Clinical advances of siRNA therapeutics. J. Gene Med. 2019;21(7) doi: 10.1002/jgm.3097. [DOI] [PubMed] [Google Scholar]
- 68.Dong Y., Siegwart D.J., Anderson D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rüger J., Ioannou S., Castanotto D., Stein C.A. Oligonucleotides to the (Gene) Rescue: FDA Approvals 2017–2019. Trends Pharmacol. Sci. 2019;41(1):27–41. doi: 10.1016/j.tips.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Stingl G., Bröcker E.-B., Mertelsmann R., Wolff K., Schreiber S., Kämpgen E. Phase I study to the immunotherapy of metastatic malignant melanoma by a cancer vaccine consisting of autologous cancer cells transfected with the human IL-2 gene. J. Mol. Med. 1997;75(4):297–299. doi: 10.1007/s001090050115. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez B., Asmuth D.M., Matining R.M., Spritzler J., Jacobson J., Mailliard R. Safety, Tolerability and Immunogenicity of Repeated Doses of DermaVir, a Candidate Therapeutic HIV Vaccine, in HIV Infected Patients Receiving Combination Antiretroviral Therapy. Results of the ACTG 5176 Trial. J. Acquir. Immune Defic. Syndr. 2013;64(4) doi: 10.1097/QAI.0b013e3182a99590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anwer K., Barnes M., Fewell J., Lewis D., Alvarez R. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther. 2010;17(3):360–369. doi: 10.1038/gt.2009.159. [DOI] [PubMed] [Google Scholar]
- 73.Shi M., Zhang J., Huang Z., Chen Y., Pan S., Hu H. Stimuli-responsive release and efficient siRNA delivery in non-small cell lung cancer by a poly(l-histidine)-based multifunctional nanoplatform. J. Mater. Chem. B. 2020;8(8):1616–1628. doi: 10.1039/c9tb02764e. [DOI] [PubMed] [Google Scholar]
- 74.Meng L., Yuan S., Zhu L., ShangGuan Z., Zhao R. Ultrasound-microbubbles-mediated microRNA-449a inhibits lung cancer cell growth via the regulation of Notch1. OncoTargets and Therapy. 2019;12:7437–7450. doi: 10.2147/OTT.S217021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y., Xue B., Shi K., Jia Y., Hao Y., Xiao Y. Synthesis and Application of a Novel Gene Delivery Vector for Non-Small-Cell Lung Cancer Therapy. J. Biomed. Nanotechnol. 2019;15(3):431–442. doi: 10.1166/jbn.2019.2695. [DOI] [PubMed] [Google Scholar]
- 76.Zhang M., Wang Q., Wan K.-W., Ahmed W., Phoenix D.A., Zhang Z. Liposome mediated-CYP1A1 gene silencing nanomedicine prepared using lipid film-coated proliposomes as a potential treatment strategy of lung cancer. Int. J. Pharm. 2019;566:185–193. doi: 10.1016/j.ijpharm.2019.04.078. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Y., Meng Y., Zhao Y., Zhu J., Xu H., Zhang E. Toxicological exploration of peptide-based cationic liposomes in siRNA delivery. Colloids Surf. B: Biointerfaces. 2019;179:66–76. doi: 10.1016/j.colsurfb.2019.03.052. [DOI] [PubMed] [Google Scholar]
- 78.Lv T., Li Z., Xu L., Zhang Y., Chen H., Gao Y. Chloroquine in combination with aptamer-modified nanocomplexes for tumor vessel normalization and efficient erlotinib/Survivin shRNA co-delivery to overcome drug resistance in EGFR-mutated non-small cell lung cancer. Acta Biomater. 2018;76:257–274. doi: 10.1016/j.actbio.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 79.Yang Y., Jia Y., Xiao Y., Hao Y., Zhang L., Chen X. Tumor-Targeting Anti-MicroRNA-155 Delivery Based on Biodegradable Poly(ester amine) and Hyaluronic Acid Shielding for Lung Cancer Therapy. ChemPhysChem. 2018;19(16):2058–2069. doi: 10.1002/cphc.201701375. [DOI] [PubMed] [Google Scholar]
- 80.Chowdhury N., Vhora I., Patel K., Doddapaneni R., Mondal A., Singh M. Liposomes co-Loaded with 6-Phosphofructo-2-Kinase/Fructose-2, 6-Biphosphatase 3 (PFKFB3) shRNA Plasmid and Docetaxel for the Treatment of non-small Cell Lung Cancer. Pharm. Res. 2017;34(11):2371–2384. doi: 10.1007/s11095-017-2244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang B., Zhang Y., Yu D. Lung cancer gene therapy: Transferrin and hyaluronic acid dual ligand-decorated novel lipid carriers for targeted gene delivery. Oncol. Rep. 2017;37(2):937–944. doi: 10.3892/or.2016.5298. [DOI] [PubMed] [Google Scholar]
- 82.Lee A.Y., Kim S., Lee S., Jiang H.-L., Kim S.-B., Hong S.-H. Knockdown of Importin 7 Inhibits Lung Tumorigenesis in K-rasLA1 Lung Cancer Mice. Anticancer Res. 2017;37(5):2181–2386. doi: 10.21873/anticanres.11576. [DOI] [PubMed] [Google Scholar]
- 83.Unal O., Akkoc Y., Kocak M., Nalbat E., Dogan-Ekici A.I., Yagci Acar H. Treatment of breast cancer with autophagy inhibitory microRNAs carried by AGO2-conjugated nanoparticles. J. Nanobiotechnol. 2020;18(1) doi: 10.1186/s12951-020-00615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao N., Fan W., Zhao X., Liu Y., Hu Y., Duan F. Polycation–Carbon Nanohybrids with Superior Rough Hollow Morphology for the NIR-II Responsive Multimodal Therapy. ACS Appl. Mater. Interfaces. 2020;12(10):11341–11352. doi: 10.1021/acsami.9b22373. [DOI] [PubMed] [Google Scholar]
- 85.Quagliarini E., Di Santo R., Palchetti S., Ferri G., Cardarelli F., Pozzi D. Effect of Protein Corona on The Transfection Efficiency of Lipid-Coated Graphene Oxide-Based Cell Transfection Reagents. Pharmaceutics. 2020;12(2) doi: 10.3390/pharmaceutics12020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piña M.J., Girotti A., Serrano S., Muñoz R., Rodríguez-Cabello J.C., Arias F.J. A double safety lock tumor-specific device for suicide gene therapy in breast cancer. Cancer Lett. 2020;470:43–53. doi: 10.1016/j.canlet.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 87.Ding L., Li J., Wu C., Yan F., Li X., Zhang S. A self-assembled RNA-triple helix hydrogel drug delivery system targeting triple-negative breast cancer. J. Mater. Chem. B. 2020;8(16):3527–3533. doi: 10.1039/c9tb01610d. [DOI] [PubMed] [Google Scholar]
- 88.Taschauer A., Polzer W., Alioglu F., Billerhart M., Decker S., Kittelmann T. Peptide-Targeted Polyplexes for Aerosol-Mediated Gene Delivery to CD49f-Overexpressing Tumor Lesions in Lung. Molecular Therapy - Nucleic Acids. 2019;18:774–786. doi: 10.1016/j.omtn.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang R., Dai X., Duan S., Zhao N., Xu F.-J. A flexible bowl-shaped magnetic assembly for multifunctional gene delivery systems. Nanoscale. 2019;11(35):16463–16475. doi: 10.1039/c9nr04763h. [DOI] [PubMed] [Google Scholar]
- 90.Zhao D., Song H., Zhou X., Chen Y., Liu Q., Gao X. Novel facile thermosensitive hydrogel as sustained and controllable gene release vehicle for breast cancer treatment. Eur. J. Pharm. Sci. 2019;134:145–152. doi: 10.1016/j.ejps.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 91.Wang S., Liu X., Chen S., Liu Z., Zhang X., Liang X.-J. Regulation of Ca2+ Signaling for Drug-Resistant Breast Cancer Therapy with Mesoporous Silica Nanocapsule Encapsulated Doxorubicin/siRNA Cocktail. ACS Nano. 2019;13(1):274–283. doi: 10.1021/acsnano.8b05639. [DOI] [PubMed] [Google Scholar]
- 92.Devulapally R., Lee T., Barghava-Shah A., Sekar T.V., Foygel K., Bachawal S.V. Ultrasound-guided delivery of thymidine kinase–nitroreductase dual therapeutic genes by PEGylated-PLGA/PIE nanoparticles for enhanced triple negative breast cancer therapy. Nanomedicine. 2018;13(9):1051–1066. doi: 10.2217/nnm-2017-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhuo H., Zheng B., Liu J., Huang Y., Wang H., Zheng D. Efficient targeted tumor imaging and secreted endostatin gene delivery by anti-CD105 immunoliposomes. J. Exp. Clin. Cancer Res. 2018;37(1):42. doi: 10.1186/s13046-018-0712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruan C., Liu L., Wang Q., Chen X., Chen Q., Lu Y. Reactive Oxygen Species-Biodegradable Gene Carrier for the Targeting Therapy of Breast Cancer. ACS Appl. Mater. Interfaces. 2018;10(12):10398–10408. doi: 10.1021/acsami.8b01712. [DOI] [PubMed] [Google Scholar]
- 95.Tang J., Howard C.B., Mahler S.M., Thurecht K.J., Huang L., Xu Z.P. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale. 2018;10(9):4258–4266. doi: 10.1039/c7nr08644j. [DOI] [PubMed] [Google Scholar]
- 96.Tang Q., Ma X., Zhang Y., Cai X., Xue W., Ma D. Self-sensibilized polymeric prodrug co-delivering MMP-9 shRNA plasmid for combined treatment of tumors. Acta Biomater. 2018;69:277–289. doi: 10.1016/j.actbio.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 97.Xin X., Pei X., Yang X., Lv Y., Zhang L., He W. Rod-shaped active drug particles enable efficient and safe gene delivery. Adv. Sci. 2017;4(11) doi: 10.1002/advs.201700324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J., Guo N., Gao C., Liu N., Zheng X., Tan Y. Effective Gene Silencing Mediated by Polypeptide Nanoparticles LAH4-L1-siMDR1 in Multi-Drug Resistant Human Breast Cancer. J. Biomed. Nanotechnol. 2019;15(3):531–543. doi: 10.1166/jbn.2019.2705. [DOI] [PubMed] [Google Scholar]
- 99.Malik Y.S., Sheikh M.A., Xing Z., Guo Z., Zhu X., Tian H. Polylysine-modified polyethylenimine polymer can generate genetically engineered mesenchymal stem cells for combinational suicidal gene therapy in glioblastoma. Acta Biomater. 2018;80:144–153. doi: 10.1016/j.actbio.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 100.Tong W.Y., Alnakhli M., Bhardwaj R., Apostolou S., Sinha S., Fraser C. Delivery of siRNA in vitro and in vivo using PEI-capped porous silicon nanoparticles to silence MRP1 and inhibit proliferation in glioblastoma. J. Nanobiotechnol. 2018;16(1) doi: 10.1186/s12951-018-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao S., Tian H., Xing Z., Zhang D., Guo Y., Guo Z. A non-viral suicide gene delivery system traversing the blood brain barrier for non-invasive glioma targeting treatment. J. Control. Release. 2016;243:357–369. doi: 10.1016/j.jconrel.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 102.Huang R.-Y., Lin Y.-H., Lin S.-Y., Li Y.-N., Chiang C.-S., Chang C.-W. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics. 2019;9(8):2411. doi: 10.7150/thno.29326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Hua Y., Xu G., Deng S., Yang D., Gao X. Targeting eZh2 for glioma therapy with a novel nanoparticle–sirNa complex. Int. J. Nanomedicine. 2019;14:2637. doi: 10.2147/IJN.S189871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freimann K., Arukuusk P., Kurrikoff K., Vasconcelos L.D.F., Veiman K.-L., Uusna J. Optimization of in vivo DNA delivery with NickFect peptide vectors. J. Control. Release. 2016;241:135–143. doi: 10.1016/j.jconrel.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 105.Fan C.-H., Chang E.-L., Ting C.-Y., Lin Y.-C., Liao E.-C., Huang C.-Y. Folate-conjugated gene-carrying microbubbles with focused ultrasound for concurrent blood-brain barrier opening and local gene delivery. Biomaterials. 2016;106:46–57. doi: 10.1016/j.biomaterials.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 106.Kim H.A., Lee H.-L., Choi E., Kim Y.-H., Lee M. Reducible poly (oligo-D-arginine) as an efficient carrier of the thymidine kinase gene in the intracranial glioblastoma animal model. J. Pharm. Sci. 2015;104(11):3743–3751. doi: 10.1002/jps.24576. [DOI] [PubMed] [Google Scholar]
- 107.Oh B., Han J., Choi E., Tan X., Lee M. Peptide micelle-mediated delivery of tissue-specific suicide gene and combined therapy with Avastin in a glioblastoma model. J. Pharm. Sci. 2015;104(4):1461–1469. doi: 10.1002/jps.24363. [DOI] [PubMed] [Google Scholar]
- 108.Park J.H., Han J., Lee M. Thymidine kinase gene delivery using curcumin loaded peptide micelles as a combination therapy for glioblastoma. Pharm. Res. 2015;32(2):528–537. doi: 10.1007/s11095-014-1482-4. [DOI] [PubMed] [Google Scholar]
- 109.Guerrero-Cázares H., Tzeng S.Y., Young N.P., Abutaleb A.O., Quiñones-Hinojosa A., Green J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano. 2014;8(5):5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu X., Li Y., Sun X., Muftuoglu Y., Wang B., Yu T. Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system. Theranostics. 2018;8(13):3490–3503. doi: 10.7150/thno.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li L., Deng R., Su Y., Yang C. Dual-targeting nanoparticles with excellent gene transfection efficiency for gene therapy of peritoneal metastasis of colorectal cancer. Oncotarget. 2017;8(52):89837. doi: 10.18632/oncotarget.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ju H., Lu Y., Wu Q., Liu J., Zeng Z., Mo H. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene. 2017;36(45):6282–6292. doi: 10.1038/onc.2017.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu X., Gao X., Zheng S., Wang B., Li Y., Zhao C. Modified nanoparticle mediated IL-12 immunogene therapy for colon cancer. Nanomedicine. 2017;13(6):1993–2004. doi: 10.1016/j.nano.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 114.Lee S.-Y., Yang C.-Y., Peng C.-L., Wei M.-F., Chen K.-C., Yao C.-J. A theranostic micelleplex co-delivering SN-38 and VEGF siRNA for colorectal cancer therapy. Biomaterials. 2016;86:92–105. doi: 10.1016/j.biomaterials.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 115.Ji Y., Liu X., Huang M., Jiang J., Liao Y.-P., Liu Q. Development of self-assembled multi-arm polyrotaxanes nanocarriers for systemic plasmid delivery in vivo. Biomaterials. 2019;192:416–428. doi: 10.1016/j.biomaterials.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Z., Wei Y., Fang G., Hong D., An L., Jiao T. Colorectal cancer combination therapy using drug and gene co-delivered, targeted poly (ethylene glycol)-ε-poly (caprolactone) nanocarriers. Drug Design Dev. Ther. 2018;12:3171. doi: 10.2147/DDDT.S175614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pishavar E., Attaranzadeh A., Alibolandi M., Ramezani M., Hashemi M. Modified PAMAM vehicles for effective TRAIL gene delivery to colon adenocarcinoma: in vitro and in vivo evaluation. Artificial Cells Nanomed. Biotechnol. 2018;46(sup3):S503–S513. doi: 10.1080/21691401.2018.1500372. [DOI] [PubMed] [Google Scholar]
- 118.Li L., Li X., Wu Y., Song L., Yang X., He T. Multifunctional nucleus-targeting nanoparticles with ultra-high gene transfection efficiency for in vivo gene therapy. Theranostics. 2017;7(6):1633. doi: 10.7150/thno.17588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhatt P., Khatri N., Kumar M., Baradia D., Misra A. Microbeads mediated oral plasmid DNA delivery using polymethacrylate vectors: an effectual groundwork for colorectal cancer. Drug Delivery. 2015;22(6):849–861. doi: 10.3109/10717544.2014.898348. [DOI] [PubMed] [Google Scholar]
- 120.Lee Y.M., Lee D., Kim J., Park H., Kim W.J. RPM peptide conjugated bioreducible polyethylenimine targeting invasive colon cancer. J. Control. Release. 2015;205:172–180. doi: 10.1016/j.jconrel.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 121.Yang J., Hendricks W., Liu G., McCaffery J.M., Kinzler K.W., Huso D.L. A nanoparticle formulation that selectively transfects metastatic tumors in mice. Proc. Natl. Acad. Sci. 2013;110(36):14717–14722. doi: 10.1073/pnas.1313330110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vidic S., Markelc B., Sersa G., Coer A., Kamensek U., Tevz G. MicroRNAs targeting mutant K-ras by electrotransfer inhibit human colorectal adenocarcinoma cell growth in vitro and in vivo. Cancer Gene Ther. 2010;17(6):409–419. doi: 10.1038/cgt.2009.87. [DOI] [PubMed] [Google Scholar]
- 123.Wonder E., Simón-Gracia L., Scodeller P., Majzoub R.N., Kotamraju V.R., Ewert K.K. Competition of charge-mediated and specific binding by peptide-tagged cationic liposome–DNA nanoparticles in vitro and in vivo. Biomaterials. 2018;166:52–63. doi: 10.1016/j.biomaterials.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang W., Liu Z., Sun P., Fang C., Fang H., Wang Y. RGD peptides-conjugated pluronic triblock copolymers encapsulated with AP-2α expression plasmid for targeting gastric cancer therapy in vitro and in vivo. Int. J. Mol. Sci. 2015;16(7):16263–16274. doi: 10.3390/ijms160716263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao Y., Lee R.J., Liu L., Dong S., Zhang J., Zhang Y. Multifunctional drug carrier based on PEI derivatives loaded with small interfering RNA for therapy of liver cancer. Int. J. Pharm. 2019;564:214–224. doi: 10.1016/j.ijpharm.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 126.Zamboni C.G., Kozielski K.L., Vaughan H.J., Nakata M.M., Kim J., Higgins L.J. Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma. J. Control. Release. 2017;263:18–28. doi: 10.1016/j.jconrel.2017.03.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liang X., Shi B., Wang K., Fan M., Jiao D., Ao J. Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials. 2016;82:194–207. doi: 10.1016/j.biomaterials.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 128.Kamimura K., Yokoo T., Abe H., Sakai N., Nagoya T., Kobayashi Y. Effect of Diphtheria Toxin-Based Gene Therapy for Hepatocellular Carcinoma. Cancers. 2020;12(2):472. doi: 10.3390/cancers12020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sukumar U.K., Rajendran J.C.B., Gambhir S.S., Massoud T.F., Paulmurugan R. SP94-Targeted Triblock Copolymer Nanoparticle Delivers Thymidine Kinase–p53–Nitroreductase Triple Therapeutic Gene and Restores Anticancer Function against Hepatocellular Carcinoma in Vivo. ACS Appl. Mater. Interfaces. 2020;12(10):11307–11319. doi: 10.1021/acsami.9b20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo H., Xu M., Cao Z., Li W., Chen L., Xie X. Ultrasound-Assisted miR-122-Loaded Polymeric Nanodroplets for Hepatocellular Carcinoma Gene Therapy. Mol. Pharm. 2019;17(2):541–553. doi: 10.1021/acs.molpharmaceut.9b00983. [DOI] [PubMed] [Google Scholar]
- 131.Mu X., Wang X., Wei Y., Wen C., Zhang Q., Xu C. ApoE-modified liposomes mediate the antitumour effect of survivin promoter-driven HSVtk in hepatocellular carcinoma. Cancer Gene Ther. 2019:1–14. doi: 10.1038/s41417-019-0145-3. [DOI] [PubMed] [Google Scholar]
- 132.Liu C., Wen C., Wang X., Wei Y., Xu C., Mu X. Golgi membrane protein GP73 modified-liposome mediates the antitumor effect of survivin promoter-driven HSVtk in hepatocellular carcinoma. Exp. Cell Res. 2019;383(1) doi: 10.1016/j.yexcr.2019.111496. [DOI] [PubMed] [Google Scholar]
- 133.Tsai P.-H., Wang M.-L., Chang J.-H., Yarmishyn A.A., Nhi Nguyen P.N., Chen W. Dual Delivery of HNF4α and Cisplatin by Mesoporous Silica Nanoparticles Inhibits Cancer Pluripotency and Tumorigenicity in Hepatoma-Derived CD133-Expressing Stem Cells. ACS Appl. Mater. Interfaces. 2019;11(22):19808–19818. doi: 10.1021/acsami.9b04474. [DOI] [PubMed] [Google Scholar]
- 134.Kim M.-K., Moon Y.-A., Song C.K., Baskaran R., Bae S., Yang S.-G. Tumor-suppressing miR-141 gene complex-loaded tissue-adhesive glue for the locoregional treatment of hepatocellular carcinoma. Theranostics. 2018;8(14):3891. doi: 10.7150/thno.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Z., Chang Z., Lu M., Shao D., Yue J., Yang D. Shape-controlled magnetic mesoporous silica nanoparticles for magnetically-mediated suicide gene therapy of hepatocellular carcinoma. Biomaterials. 2018;154:147–157. doi: 10.1016/j.biomaterials.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 136.Urnauer S., Klutz K., Grünwald G.K., Morys S., Schwenk N., Zach C. Systemic tumor-targeted sodium iodide symporter (NIS) gene therapy of hepatocellular carcinoma mediated by B6 peptide polyplexes. J. Gene Med. 2017;19(5) doi: 10.1002/jgm.2957. [DOI] [PubMed] [Google Scholar]
- 137.Hu Q., Wang K., Sun X., Li Y., Fu Q., Liang T. A redox-sensitive, oligopeptide-guided, self-assembling, and efficiency-enhanced (ROSE) system for functional delivery of microRNA therapeutics for treatment of hepatocellular carcinoma. Biomaterials. 2016;104:192–200. doi: 10.1016/j.biomaterials.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 138.Zhang X., He Z., Xiang L., Li L., Zhang H., Lin F. Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer. Drug Design Dev. Ther. 2019;13:1357–1372. doi: 10.2147/DDDT.S198400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu M., Zhao H., Guo L., Wang Y., Song J., Zhao X. Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of A10-3.2 aptamer targeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Delivery. 2018;25(1):226–240. doi: 10.1080/10717544.2017.1422300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Figueiredo M.L., Neto M.F., Salameh J.W., Decker R.E., Letteri R., Chan-Seng D. Ligand-mediated targeting of cytokine Interleukin-27 enhances its bioactivity in vivo. Mol. Ther. Methods Clin. Dev. 2020;17:739–751. doi: 10.1016/j.omtm.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mishel S., Shneyer B., Korsensky L., Goldshmidt-Tran O., Haber T., Machluf M. Delivery of the gene encoding the tumor suppressor Sef into prostate tumors by therapeutic-ultrasound inhibits both tumor angiogenesis and growth. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-12408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dong S., Zhou X., Yang J. TAT modified and lipid–PEI hybrid nanoparticles for co-delivery of docetaxel and pDNA. Biomed. Pharmacother. 2016;84:954–961. doi: 10.1016/j.biopha.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 143.Kim Y.-M., Park S.-C., Jang M.-K. Targeted gene delivery of polyethyleneimine-grafted chitosan with RGD dendrimer peptide in αvβ3 integrin-overexpressing tumor cells. Carbohydr. Polym. 2017;174:1059–1068. doi: 10.1016/j.carbpol.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 144.Williford J.-M., Archang M.M., Minn I., Ren Y., Wo M., Vandermark J. Critical length of PEG grafts on lPEI/DNA nanoparticles for efficient in vivo delivery. ACS Biomater. Sci. Eng. 2016;2(4):567–578. doi: 10.1021/acsbiomaterials.5b00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaneti L., Bronshtein T., Malkah Dayan N., Kovregina I., Letko Khait N., Lupu-Haber Y. Nanoghosts as a Novel Natural Nonviral Gene Delivery Platform Safely Targeting Multiple Cancers. Nano Lett. 2016;16(3):1574–1582. doi: 10.1021/acs.nanolett.5b04237. [DOI] [PubMed] [Google Scholar]
- 146.Wu X., Tai Z., Zhu Q., Fan W., Ding B., Zhang W. Study on the prostate cancer-targeting mechanism of aptamer-modified nanoparticles and their potential anticancer effect in vivo. Int. J. Nanomedicine. 2014;9:5431–5440. doi: 10.2147/IJN.S71101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hattori Y., W-x Ding, Maitani Y. Highly efficient cationic hydroxyethylated cholesterol-based nanoparticle-mediated gene transfer in vivo and in vitro in prostate carcinoma PC-3 cells. J. Control. Release. 2007;120(1–2):122–130. doi: 10.1016/j.jconrel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 148.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 149.Barta J.A., Powell C.A., Wisnivesky J.P. Global Epidemiology of Lung Cancer. Ann Global Health. 2019;85(1) doi: 10.5334/aogh.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lim Z.-F., Ma P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019;12(1) doi: 10.1186/s13045-019-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hanahan D., Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 152.Fouad Y.A., Aanei C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017;7(5):1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 153.Wang D., Gao G. State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications. Discov. Med. 2014;18(98):151–161. [PMC free article] [PubMed] [Google Scholar]
- 154.Goswami R., Subramanian G., Silayeva L., Newkirk I., Doctor D., Chawla K. Gene Therapy Leaves a Vicious Cycle. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ramamoorth M. Non Viral Vectors in Gene Therapy- An Overview. J. Clin. Diagn. Res. 2015;9(1):GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lundstrom K., Boulikas T. Viral and Non-viral Vectors in Gene Therapy: Technology Development and Clinical Trials. Technol Cancer Res. Treatment. 2016;2(5):471–485. doi: 10.1177/153303460300200513. [DOI] [PubMed] [Google Scholar]
- 157.Chen J., Guo Z., Tian H., Chen X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016;3 doi: 10.1038/mtm.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao Patil, Li Lin, Tian Dang. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019;20(21) doi: 10.3390/ijms20215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Itani R., Al Faraj A. siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ayatollahi S., Salmasi Z., Hashemi M., Askarian S., Oskuee R.K., Abnous K. Aptamer-targeted delivery of Bcl-xL shRNA using alkyl modified PAMAM dendrimers into lung cancer cells. Int. J. Biochem. Cell Biol. 2017;92:210–217. doi: 10.1016/j.biocel.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 161.Zhao Y., Wang W., Guo S., Wang Y., Miao L., Xiong Y. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu S., Bao X., Hai W., Shi S., Chen Y., Yu Q. Multi-functional self-assembled nanoparticles for pVEGF-shRNA loading and anti-tumor targeted therapy. Int. J. Pharm. 2020;575 doi: 10.1016/j.ijpharm.2019.118898. [DOI] [PubMed] [Google Scholar]
- 163.Zhang M., Kim Y.-K., Cui P., Zhang J., Qiao J., He Y. Folate-conjugated polyspermine for lung cancer–targeted gene therapy. Acta Pharm. Sin. B. 2016;6(4):336–343. doi: 10.1016/j.apsb.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jeong K., Yu Y.J., You J.Y., Rhee W.J., Kim J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip. 2020;20(3):548–557. doi: 10.1039/c9lc00958b. [DOI] [PubMed] [Google Scholar]
- 165.Huang Q., Li L., Li L., Chen H., Dang Y., Zhang J. MDM2 knockdown mediated by a triazine-modified dendrimer in the treatment of non-small cell lung cancer. Oncotarget. 2016;7(28) doi: 10.18632/oncotarget.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alhakamy N.A., Ishiguro S., Uppalapati D., Berkland C.J., Tamura M. AT2R Gene Delivered by Condensed Polylysine Complexes Attenuates Lewis Lung Carcinoma after Intravenous Injection or Intratracheal Spray. Mol. Cancer Ther. 2016;15(1):209. doi: 10.1158/1535-7163.MCT-15-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Piña M.J., Alex S.M., Arias F.J., Santos M., Rodriguez-Cabello J.C., Ramesan R.M. Elastin-like recombinamers with acquired functionalities for gene-delivery applications. J. Biomed. Mater. Res. A. 2015;103(10):3166–3178. doi: 10.1002/jbm.a.35455. [DOI] [PubMed] [Google Scholar]
- 168.Andey T., Marepally S., Patel A., Jackson T., Sarkar S., O’Connell M. Cationic lipid guided short-hairpin RNA interference of annexin A2 attenuates tumor growth and metastasis in a mouse lung cancer stem cell model. J. Control. Release. 2014;184:67–78. doi: 10.1016/j.jconrel.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mohammadi Z., Abolhassani M., Dorkoosh F.A., Hosseinkhani S., Gilani K., Amini T. Preparation and evaluation of chitosan–DNA–FAP-B nanoparticles as a novel non-viral vector for gene delivery to the lung epithelial cells. Int. J. Pharm. 2011;409(1–2):307–313. doi: 10.1016/j.ijpharm.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 170.Poddar A., Conesa J.J., Liang K., Dhakal S., Reineck P., Bryant G. Encapsulation, Visualization and Expression of Genes with Biomimetically Mineralized Zeolitic Imidazolate Framework-8 (ZIF-8) Small. 2019;15(36) doi: 10.1002/smll.201902268. [DOI] [PubMed] [Google Scholar]
- 171.Lee A.Y., Cho M.-H., Kim S. Recent advances in aerosol gene delivery systems using non-viral vectors for lung cancer therapy. Expert Opin. Drug Deliv. 2019;16(7):757–772. doi: 10.1080/17425247.2019.1641083. [DOI] [PubMed] [Google Scholar]
- 172.Kong L., Cai F.Y., Xm Yao, Jing M., Fu M., Jj Liu. RPV-modified epirubicin and dioscin co-delivery liposomes suppress non-small cell lung cancer growth by limiting nutrition supply. Cancer Sci. 2020;111(2):621–636. doi: 10.1111/cas.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Son S.-M., Yun J., Lee S.-H., Han H.S., Lim Y.H., Woo C.G. Therapeutic Effect of pHLIP-mediated CEACAM6 Gene Silencing in Lung Adenocarcinoma. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-48104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ji L., Roth J.A. Tumor Suppressor FUS1 Signaling Pathway. J. Thorac. Oncol. 2008;3(4):327–330. doi: 10.1097/JTO.0b013e31816bce65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Deng W.G., Kawashima H., Wu G., Jayachandran G., Xu K., Minna J.D. Synergistic Tumor Suppression by Coexpression of FUS1 and p53 Is Associated with Down-regulation of Murine Double Minute-2 and Activation of the Apoptotic Protease-Activating Factor 1-Dependent Apoptotic Pathway in Human Non-Small Cell Lung Cancer Cells. Cancer Res. 2007;67(2):709–717. doi: 10.1158/0008-5472.CAN-06-3463. [DOI] [PubMed] [Google Scholar]
- 176.Katoh M., Lu C., Stewart D.J., Lee J.J., Ji L., Ramesh R. Phase I Clinical Trial of Systemically Administered TUSC2(FUS1)-Nanoparticles Mediating Functional Gene Transfer in Humans. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tseng S.J., Liao Z.-X., Kao S.-H., Zeng Y.-F., Huang K.-Y., Li H.-J. Highly specific in vivo gene delivery for p53-mediated apoptosis and genetic photodynamic therapies of tumour. Nat. Commun. 2015;6(1) doi: 10.1038/ncomms7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abdel-Rashid R.S., Omar S.M., Teiama M.S., Khairy A., Magdy M., Anis B. Fabrication Of Gold Nanoparticles In Absence Of Surfactant As In Vitro Carrier Of Plasmid DNA. Int. J. Nanomed. 2019;14:8399–8408. doi: 10.2147/IJN.S226498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kamat C.D., Shmueli R.B., Connis N., Rudin C.M., Green J.J., Hann C.L. Poly(-amino ester) Nanoparticle Delivery of TP53 Has Activity against Small Cell Lung Cancer In Vitro and In Vivo. Mol. Cancer Ther. 2013;12(4):405–415. doi: 10.1158/1535-7163.MCT-12-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Moon C., Oh Y., Roth J.A. Current Status of Gene Therapy for Lung Cancer and Head and Neck Cancer. Clin. Cancer Res. 2003;9(14) [PubMed] [Google Scholar]
- 181.Li C., Hu J., Li W., Song G., Shen J. Combined bortezomib-based chemotherapy and p53 gene therapy using hollow mesoporous silica nanospheres for p53 mutant non-small cell lung cancer treatment. Biomater. Sci. 2017;5(1):77–88. doi: 10.1039/c6bm00449k. [DOI] [PubMed] [Google Scholar]
- 182.Rong J., Li P., Ge Y., Chen H., Wu J., Zhang R. Histone H2A-peptide-hybrided upconversion mesoporous silica nanoparticles for bortezomib/p53 delivery and apoptosis induction. Colloids Surf. B: Biointerfaces. 2020;186 doi: 10.1016/j.colsurfb.2019.110674. [DOI] [PubMed] [Google Scholar]
- 183.Rabiee S., Tavakol S., Barati M., Joghataei M.T. Autophagic, apoptotic, and necrotic cancer cell fates triggered by acidic pH microenvironment. J. Cell. Physiol. 2019;234(7):12061–12069. doi: 10.1002/jcp.27876. [DOI] [PubMed] [Google Scholar]
- 184.Tavakol S., Kiani V., Tavakol B., Derakhshan M.A., Joghataei M.T., Rezayat S.M. Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes. Elsevier; 2017. Toxicity concerns of nanocarriers; pp. 453–484. [Google Scholar]
- 185.Segovia N., Pont M., Oliva N., Ramos V., Borrós S., Artzi N. Hydrogel Doped with Nanoparticles for Local Sustained Release of siRNA in Breast Cancer. Adv. Healthcare Mater. 2015;4(2):271–280. doi: 10.1002/adhm.201400235. [DOI] [PubMed] [Google Scholar]
- 186.Ding Y., Wang Y., Zhou J., Gu X., Wang W., Liu C. Direct cytosolic siRNA delivery by reconstituted high density lipoprotein for target-specific therapy of tumor angiogenesis. Biomaterials. 2014;35(25):7214–7227. doi: 10.1016/j.biomaterials.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 187.Meng H., Mai W.X., Zhang H., Xue M., Xia T., Lin S. Codelivery of an Optimal Drug/siRNA Combination Using Mesoporous Silica Nanoparticles To Overcome Drug Resistance in Breast Cancer in Vitro and in Vivo. ACS Nano. 2013;7(2):994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao P., Li L., Zhou S., Qiu L., Qian Z., Liu X. TPGS functionalized mesoporous silica nanoparticles for anticancer drug delivery to overcome multidrug resistance. Mater. Sci. Eng. C. 2018;84:108–117. doi: 10.1016/j.msec.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 189.Ding L., Li J., Wu C., Yan F., Li X., Zhang S. A self-assembled RNA-triple helix hydrogel drug delivery system targeting triple-negative breast cancer. J. Mater. Chem. B. 2020;8:3527–3533. doi: 10.1039/c9tb01610d. [DOI] [PubMed] [Google Scholar]
- 190.Wang Dai Q., Zhou H., Wang L., Cheang T., Wei J. CaCO3/CaIP6 composite nanoparticles effectively deliver AKT1 small interfering RNA to inhibit human breast cancer growth. Int. J. Nanomedicine. 2015;10:4255–4266. doi: 10.2147/IJN.S73269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Arias F.J., Santos M., Ibanez-Fonseca A., Pina M.J., Serrano S. Elastin-Like Recombinamers As Smart Drug Delivery Systems. Curr. Drug Targets. 2018;19(4):360–379. doi: 10.2174/1389450117666160201114617. [DOI] [PubMed] [Google Scholar]
- 192.Changi K., Bosnjak B., Gonzalez-Obeso C., Kluger R., Rodríguez-Cabello J.C., Hoffmann O. Biocompatibility and immunogenicity of elastin-like recombinamer biomaterials in mouse models. J. Biomed. Mater. Res. A. 2018;106(4):924–934. doi: 10.1002/jbm.a.36290. [DOI] [PubMed] [Google Scholar]
- 193.Piña M.J., Girotti A., Santos M., Rodríguez-Cabello J.C., Arias F.J. Biocompatible ELR-Based Polyplexes Coated with MUC1 Specific Aptamers and Targeted for Breast Cancer Gene Therapy. Mol. Pharm. 2016;13(3):795–808. doi: 10.1021/acs.molpharmaceut.5b00712. [DOI] [PubMed] [Google Scholar]
- 194.Xiao Y., Shi K., Qu Y., Chu B., Qian Z. Engineering Nanoparticles for Targeted Delivery of Nucleic Acid Therapeutics in Tumor. Mol. Ther. Methods Clin. Develop. 2019;12:1–18. doi: 10.1016/j.omtm.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Taylor R.E., Zahid M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics. 2020;12(3) doi: 10.3390/pharmaceutics12030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hemmati M., Najafi F., Shirkoohi R., Moghimi H.R., Zarebkohan A., Kazemi B. Synthesis of a novel PEGDGA-coated hPAMAM complex as an efficient and biocompatible gene delivery vector: an in vitro and in vivo study. Drug Delivery. 2016;23(8):2956–2969. doi: 10.3109/10717544.2015.1132796. [DOI] [PubMed] [Google Scholar]
- 197.Jing H., Cheng W., Li S., Wu B., Leng X., Xu S. Novel cell-penetrating peptide-loaded nanobubbles synergized with ultrasound irradiation enhance EGFR siRNA delivery for triple negative Breast cancer therapy. Colloids Surf. B: Biointerfaces. 2016;146:387–395. doi: 10.1016/j.colsurfb.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 198.Zhou Y., Gu H., Xu Y., Li F., Kuang S., Wang Z. Targeted Antiangiogenesis Gene Therapy Using Targeted Cationic Microbubbles Conjugated with CD105 Antibody Compared with Untargeted Cationic and Neutral Microbubbles. Theranostics. 2015;5(4):399–417. doi: 10.7150/thno.10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhuo H., Zheng B., Liu J., Huang Y., Wang H., Zheng D. Efficient targeted tumor imaging and secreted endostatin gene delivery by anti-CD105 immunoliposomes. J. Exp. Clin. Cancer Res. 2018;37(1) doi: 10.1186/s13046-018-0712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gu J., Fang X., Hao J., Sha X. Reversal of P-glycoprotein-mediated multidrug resistance by CD44 antibody-targeted nanocomplexes for short hairpin RNA-encoding plasmid DNA delivery. Biomaterials. 2015;45:99–114. doi: 10.1016/j.biomaterials.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 201.Zhang M., Xu R., Xia X., Yang Y., Gu J., Qin G. Polycation-functionalized nanoporous silicon particles for gene silencing on breast cancer cells. Biomaterials. 2014;35(1):423–431. doi: 10.1016/j.biomaterials.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lu X., Wang Q.-Q., Xu F.-J., Tang G.-P., Yang W.-T. A cationic prodrug/therapeutic gene nanocomplex for the synergistic treatment of tumors. Biomaterials. 2011;32(21):4849–4856. doi: 10.1016/j.biomaterials.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 203.Wang H., Wang H., Liang J., Jiang Y., Guo Q., Peng H. Cell-Penetrating Apoptotic Peptide/p53 DNA Nanocomplex as Adjuvant Therapy for Drug-Resistant Breast Cancer. Mol. Pharm. 2014;11(10):3352–3360. doi: 10.1021/mp5001058. [DOI] [PubMed] [Google Scholar]
- 204.Rejeeth C., Vivek R. Comparison of two silica based nonviral gene therapy vectors for breast carcinoma: evaluation of the p53 delivery system in Balb/c mice. Artificial Cells Nanomed. Biotechnol. 2016;45(3):489–494. doi: 10.1080/21691401.2016.1175443. [DOI] [PubMed] [Google Scholar]
- 205.Rejeeth C., Salem A. Novel luminescent silica nanoparticles (LSN): p53 gene delivery system in breast cancer in vitro and in vivo. J. Pharm. Pharmacol. 2016;68(3):305–315. doi: 10.1111/j.2042-7158.2012.01547.x. [DOI] [PubMed] [Google Scholar]
- 206.Lights Verneda, Han S. Healthline. 2017. Brain Tumor. [Google Scholar]
- 207.Zare E.N., Jamaledin R., Naserzadeh P., Afjeh-Dana E., Ashtari B., Hosseinzadeh M., Vecchione R., Wu A., Tay F.R., Borzacchiello A., Makvandi P. Metal-Based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity, and Their Biomedical Applications. ACS Appl. Mater. Interfaces. 2020;1`2:3279–3300. doi: 10.1021/acsami.9b19435. [DOI] [PubMed] [Google Scholar]
- 208.Jamaledin R., Yiu C.K.Y., Zare E.N., Niu L., Vecchione R., Chen G., Gu Z., Tay F.R., Makvandi P. Advances in antimicrobial microneedle patches for combating infections. Adv. Mater. 2020 doi: 10.1002/adma.202002129. 2002129. [DOI] [PubMed] [Google Scholar]
- 209.Jamaledin R., Di Natale C., Onesto V., Taraghdari Z.B., Zare E.N., Makvandi P. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020;9(2):542. doi: 10.3390/jcm9020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jamaledin R., Yiu C.K.Y., Zare E.N., Niu L., Vecchione R., Chen G., Gu Z., Tay F.R., Makvandi P. Advances in antimicrobial microneedle patches for combating infections. Adv. Mater. 2020 doi: 10.1002/adma.202002129. [DOI] [PubMed] [Google Scholar]
- 211.Fekri H.S., Ranjbar M., Pardakhty A. A Systematic Study of Cu Nanospheres Embedded in Non-ionic Surfactant-Based Vesicle: Photocatalytic Efficiency and In Vivo Imaging Study. J. Clust. Sci. 2019;30(3):561–570. [Google Scholar]
- 212.Shakeri S., Ashrafizadeh M., Zarrabi A., Roghanian R., Afshar E.G., Pardakhty A. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines. 2020;8(1):13. doi: 10.3390/biomedicines8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mohammadinejad R., Sassan H., Pardakhty A., Hashemabadi M., Ashrafizadeh M., Dehshahri A. ZEB1 and ZEB2 gene editing mediated by CRISPR/Cas9 in A549 cell line. Bratisl. Lek. Listy. 2020;121(1):31–36. doi: 10.4149/BLL_2020_005. [DOI] [PubMed] [Google Scholar]
- 214.Liu Y., Huang R., Jiang C. Non-viral gene delivery and therapeutics targeting to brain. Curr. Nanosci. 2011;7(1):55–70. [Google Scholar]
- 215.Wu J., Yang S., Luo H., Zeng L., Ye L., Lu Y. Quantitative evaluation of monocyte transmigration into the brain following chemical opening of the blood–brain barrier in mice. Brain Res. 2006;1098(1):79–85. doi: 10.1016/j.brainres.2006.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mashal M., Attia N., Soto-Sánchez C., Martínez-Navarrete G., Fernández E., Puras G. Non-viral vectors based on cationic niosomes as efficient gene delivery vehicles to central nervous system cells into the brain. Int. J. Pharm. 2018;552(1–2):48–55. doi: 10.1016/j.ijpharm.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 217.Li J., Gu B., Meng Q., Yan Z., Gao H., Chen X. The use of myristic acid as a ligand of polyethylenimine/DNA nanoparticles for targeted gene therapy of glioblastoma. Nanotechnology. 2011;22(43) doi: 10.1088/0957-4484/22/43/435101. [DOI] [PubMed] [Google Scholar]
- 218.P-j Yue, He L., S-w Qiu, Li Y., Y-j Liao, Li X.-p. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Cancer. 2014;13(1):191. doi: 10.1186/1476-4598-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vinogradov S.V., Batrakova E.V., Kabanov A.V. Nanogels for oligonucleotide delivery to the brain. Bioconjug. Chem. 2004;15(1):50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Abdallah B., Hassan A., Benoist C., Goula D., Behr J.P., Demeneix B.A. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum. Gene Ther. 1996;7(16):1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 221.Jiao X., Yu Y., Meng J., He M., Zhang C.J., Geng W. Dual-targeting and microenvironment-responsive micelles as a gene delivery system to improve the sensitivity of glioma to radiotherapy. Acta Pharm. Sin. B. 2019;9(2):381–396. doi: 10.1016/j.apsb.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shi Y., Jiang Y., Cao J., Yang W., Zhang J., Meng F. Boosting RNAi therapy for orthotopic glioblastoma with nontoxic brain-targeting chimaeric polymersomes. J. Control. Release. 2018;292:163–171. doi: 10.1016/j.jconrel.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 223.Lehne G., De Angelis P., Den Boer M., Rugstad H. Growth inhibition, cytokinesis failure and apoptosis of multidrug-resistant leukemia cells after treatment with P-glycoprotein inhibitory agents. Leukemia. 1999;13(5):768–778. doi: 10.1038/sj.leu.2401392. [DOI] [PubMed] [Google Scholar]
- 224.Raja M.A.G., Katas H., Wen T.J. Stability, intracellular delivery, and release of siRNA from chitosan nanoparticles using different cross-linkers. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chen C., Mei H., Shi W., Deng J., Zhang B., Guo T. EGFP-EGF1-conjugated PLGA nanoparticles for targeted delivery of siRNA into injured brain microvascular endothelial cells for efficient RNA interference. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Santos J.L., Pandita D., Rodrigues J., Pêgo A.P., Granja P.L., Balian G. Receptor-mediated gene delivery using PAMAM dendrimers conjugated with peptides recognized by mesenchymal stem cells. Mol. Pharm. 2010;7(3):763–774. doi: 10.1021/mp9002877. [DOI] [PubMed] [Google Scholar]
- 227.Wang W., Xiong W., Zhu Y., Xu H., Yang X. Protective effect of PEGylation against poly (amidoamine) dendrimer-induced hemolysis of human red blood cells. J. Biomed. Mater. Res. 2010;93(1):59–64. doi: 10.1002/jbm.b.31558. [DOI] [PubMed] [Google Scholar]
- 228.Domański D., Klajnert B., Bryszewska M. Influence of PAMAM dendrimers on human red blood cells. Bioelectrochemistry. 2004;63(1–2):189–191. doi: 10.1016/j.bioelechem.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 229.Huang R., Ke W., Liu Y., Jiang C., Pei Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials. 2008;29(2):238–246. doi: 10.1016/j.biomaterials.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 230.Bai C.Z., Choi S., Nam K., An S., Park J.-S. Arginine modified PAMAM dendrimer for interferon beta gene delivery to malignant glioma. Int. J. Pharm. 2013;445(1–2):79–87. doi: 10.1016/j.ijpharm.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 231.Huang R., Ke W., Han L., Li J., Liu S., Jiang C. Targeted delivery of chlorotoxin-modified DNA-loaded nanoparticles to glioma via intravenous administration. Biomaterials. 2011;32(9):2399–2406. doi: 10.1016/j.biomaterials.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 232.Kuang Y., An S., Guo Y., Huang S., Shao K., Liu Y. T7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targeting. Int. J. Pharm. 2013;454(1):11–20. doi: 10.1016/j.ijpharm.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 233.Wang J., Lei Y., Xie C., Lu W., Wagner E., Xie Z. Retro-inverso CendR peptide-mediated polyethyleneimine for intracranial glioblastoma-targeting gene therapy. Bioconjug. Chem. 2014;25(2):414–423. doi: 10.1021/bc400552t. [DOI] [PubMed] [Google Scholar]
- 234.Zhan C., Meng Q., Li Q., Feng L., Zhu J., Lu W. Cyclic RGD–Polyethylene Glycol–Polyethylenimine for Intracranial Glioblastoma-Targeted Gene Delivery. Chemistry. 2012;7(1):91–96. doi: 10.1002/asia.201100570. [DOI] [PubMed] [Google Scholar]
- 235.Biris N., Abatzis M., Mitsios J.V., Sakarellos-Daitsiotis M., Sakarellos C., Tsoukatos D. Mapping the binding domains of the αIIb subunit: A study performed on the activated form of the platelet integrin αIIbβ3. Eur. J. Biochem. 2003;270(18):3760–3767. doi: 10.1046/j.1432-1033.2003.03762.x. [DOI] [PubMed] [Google Scholar]
- 236.Martin I., Dohmen C., Mas-Moruno C., Troiber C., Kos P., Schaffert D. Solid-phase-assisted synthesis of targeting peptide–PEG–oligo (ethane amino) amides for receptor-mediated gene delivery. Org. Biomol. Chem. 2012;10(16):3258–3268. doi: 10.1039/c2ob06907e. [DOI] [PubMed] [Google Scholar]
- 237.Lei Y., Wang J., Xie C., Wagner E., Lu W., Li Y. Glutathione-sensitive RGD-poly (ethylene glycol)-SS-polyethylenimine for intracranial glioblastoma targeted gene delivery. J. Gene Med. 2013;15(8–9):291–305. doi: 10.1002/jgm.2726. [DOI] [PubMed] [Google Scholar]
- 238.Beltinger C., Fulda S., Walczak H., Debatin K.-M. TRAIL enhances thymidine kinase/ganciclovir gene therapy of neuroblastoma cells. Cancer Gene Ther. 2002;9(4):372–381. doi: 10.1038/sj.cgt.7700448. [DOI] [PubMed] [Google Scholar]
- 239.Rawla P., Sunkara T., Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegla̜d Gastroenterologiczny. 2019;14(2):89. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G., Barzi A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 241.Marfavi Z.H., Farhadi M., Jameie S.B., Zahmatkeshan M., Pirhajati V., Jameie M. Glioblastoma U-87MG tumour cells suppressed by ZnO folic acid-conjugated nanoparticles: an in vitro study. Artificial Cells Nanomed. Biotechnol. 2019;47(1):2783–2790. doi: 10.1080/21691401.2019.1577889. [DOI] [PubMed] [Google Scholar]
- 242.Mirzaei-Parsa M.J., Najafabadi M.R.H., Haeri A., Zahmatkeshan M., Ebrahimi S.A., Pazoki-Toroudi H. Preparation, characterization, and evaluation of the anticancer activity of artemether-loaded nano-niosomes against breast cancer. Breast Cancer. 2020;27(2):243–251. doi: 10.1007/s12282-019-01014-w. [DOI] [PubMed] [Google Scholar]
- 243.Zahmatkeshan M., Gheybi F., Rezayat S.M., Jaafari M.R. Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model. Eur. J. Pharm. Sci. 2016;86:125–135. doi: 10.1016/j.ejps.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 244.Shao Z., Shao J., Tan B., Guan S., Liu Z., Zhao Z. Targeted lung cancer therapy: preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int. J. Nanomedicine. 2015;10:1223. doi: 10.2147/IJN.S77837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Piña M.J., Girotti A., Santos M., Rodríguez-Cabello J.C., Arias F.J. Biocompatible ELR-based polyplexes coated with MUC1 specific aptamers and targeted for breast cancer gene therapy. Mol. Pharm. 2016;13(3):795–808. doi: 10.1021/acs.molpharmaceut.5b00712. [DOI] [PubMed] [Google Scholar]
- 246.Wei X., Gong C., Gou M., Fu S., Guo Q., Shi S. Biodegradable poly (ɛ-caprolactone)–poly (ethylene glycol) copolymers as drug delivery system. Int. J. Pharm. 2009;381(1):1–18. doi: 10.1016/j.ijpharm.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 247.Gou M., Wei X., Men K., Wang B., Luo F., Zhao X. PCL/PEG copolymeric nanoparticles: potential nanoplatforms for anticancer agent delivery. Curr. Drug Targets. 2011;12(8):1131–1150. doi: 10.2174/138945011795906642. [DOI] [PubMed] [Google Scholar]
- 248.Lu Y., Zhong L., Jiang Z., Pan H., Zhang Y., Zhu G. Cationic micelle-based siRNA delivery for efficient colon cancer gene therapy. Nanoscale Res. Lett. 2019;14(1):193. doi: 10.1186/s11671-019-2985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Duan X., Wang P., Men K., Gao X., Huang M., Gou M. Treating colon cancer with a suicide gene delivered by self-assembled cationic MPEG–PCL micelles. Nanoscale. 2012;4(7):2400–2407. doi: 10.1039/c2nr30079f. [DOI] [PubMed] [Google Scholar]
- 250.C-g Zhang, W-j Zhu, Liu Y., Z-q Yuan, W-l Chen, Li J.-z. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci. Rep. 2016;6 doi: 10.1038/srep23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu C.H., Chern G.J., Hsu F.F., Huang K.W., Sung Y.C., Huang H.C. A multifunctional nanocarrier for efficient TRAIL-based gene therapy against hepatocellular carcinoma with desmoplasia in mice. Hepatology. 2018;67(3):899–913. doi: 10.1002/hep.29513. [DOI] [PubMed] [Google Scholar]
- 252.Aspe J.R., Wall N.R. Survivin-T34A: molecular mechanism and therapeutic potential. OncoTargets Ther. 2010;3:247. doi: 10.2147/OTT.S15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.O’Connor D.S., Grossman D., Plescia J., Li F., Zhang H., Villa A. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc. Natl. Acad. Sci. 2000;97(24):13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sousa A.R., Oliveira A.V., Oliveira M.J., Sarmento B. Nanotechnology-based siRNA delivery strategies for metastatic colorectal cancer therapy. Int. J. Pharm. 2019;568 doi: 10.1016/j.ijpharm.2019.118530. [DOI] [PubMed] [Google Scholar]
- 255.Sureban S.M., May R., Mondalek F.G., Qu D., Ponnurangam S., Pantazis P. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J. Nanobiotechnol. 2011;9(1):40. doi: 10.1186/1477-3155-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sureban S.M., May R., Ramalingam S., Subramaniam D., Natarajan G., Anant S. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009;137(2):649–659. doi: 10.1053/j.gastro.2009.05.004. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wahl G.M., Spike B.T. Cell state plasticity, stem cells, EMT, and the generation of intra-tumoral heterogeneity. NPJ Breast Cancer. 2017;3(1):1–13. doi: 10.1038/s41523-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dufes C., Keith W.N., Bilsland A., Proutski I., Uchegbu I.F., Schätzlein A.G. Synthetic anticancer gene medicine exploits intrinsic antitumor activity of cationic vector to cure established tumors. Cancer Res. 2005;65(18):8079–8084. doi: 10.1158/0008-5472.CAN-04-4402. [DOI] [PubMed] [Google Scholar]
- 259.Li Z., Huang Z.-H., Cui D.-X., Yao H., Yu J.-L., Li Q. Polyamidoamine dendrimer-mediated survivin antisense oligonucleotide inhibits the growth of subcutaneously transplanted colorectal cancer in nude mice. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(11):1935–1938. [PubMed] [Google Scholar]
- 260.Song L., Liang X., Yang S., Wang N., He T., Wang Y. Novel polyethyleneimine-R8-heparin nanogel for high-efficiency gene delivery in vitro and in vivo. Drug Deliv. 2018;25(1):122–131. doi: 10.1080/10717544.2017.1417512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gou M., Men K., Zhang J., Li Y., Song J., Luo S. Efficient inhibition of C-26 colon carcinoma by VSVMP gene delivered by biodegradable cationic nanogel derived from polyethyleneimine. ACS Nano. 2010;4(10):5573–5584. doi: 10.1021/nn1005599. [DOI] [PubMed] [Google Scholar]
- 262.Song W., Shen L., Wang Y., Liu Q., Goodwin T.J., Li J. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 2018;9(1):1–11. doi: 10.1038/s41467-018-04605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Das M., Huang L. Liposomal nanostructures for drug delivery in gastrointestinal cancers. J. Pharmacol. Exp. Ther. 2019;370(3):647–656. doi: 10.1124/jpet.118.254797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Aoyama K., Kuroda S., Morihiro T., Kanaya N., Kubota T., Kakiuchi Y. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-14717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lan K.-L., Ou-Yang F., Yen S.-H., Shih H.-L., Lan K.-H. Cationic liposome coupled endostatin gene for treatment of peritoneal colon cancer. Clin. Exp. Metastasis. 2010;27(5):307–318. doi: 10.1007/s10585-010-9328-x. [DOI] [PubMed] [Google Scholar]
- 266.Kline C.L., Shanmugavelandy S.S., Kester M., Irby R.B. Delivery of PAR-4 plasmid in vivo via nanoliposomes sensitizes colon tumor cells subcutaneously implanted into nude mice to 5-FU. Cancer Biol. Ther. 2009;8(19):1831–1837. doi: 10.4161/cbt.8.19.9592. [DOI] [PubMed] [Google Scholar]
- 267.Abdul Kuddus S. Nanoparticles to Deal with Gastric Cancer. J Gastrointestinal Cancer Stromal Tumors. 2017;2(112):2. [Google Scholar]
- 268.Czupryna J., Tsourkas A. Suicide gene delivery by calcium phosphate nanoparticles: a novel method of targeted therapy for gastric cancer. Cancer Biol. Ther. 2006;5(12):1691–1692. doi: 10.4161/cbt.5.12.3730. [DOI] [PubMed] [Google Scholar]
- 269.Liu T., Tang A., Zhang G., Chen Y., Zhang J., Peng S. Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biother. Radiopharm. 2005;20(2):141–149. doi: 10.1089/cbr.2005.20.141. [DOI] [PubMed] [Google Scholar]
- 270.Liu T., Zhang G., Chen Y.-H., Chen Y., Liu X., Peng J. Tissue specific expression of suicide genes delivered by nanoparticles inhibits gastric carcinoma growth. Cancer Biol. Ther. 2006;5(12):1683–1690. doi: 10.4161/cbt.5.12.3379. [DOI] [PubMed] [Google Scholar]
- 271.Cui D., Zhang C., Liu B., Shu Y., Du T., Shu D. Regression of gastric cancer by systemic injection of RNA nanoparticles carrying both ligand and siRNA. Sci. Rep. 2015;5 doi: 10.1038/srep10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhang J., Zhao T., Han F., Hu Y., Li Y. Photothermal and gene therapy combined with immunotherapy to gastric cancer by the gold nanoshell-based system. Journal of Nanobiotechnology. 2019;17(1) doi: 10.1186/s12951-019-0515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Patnaik S., Gupta K.C. Novel polyethylenimine-derived nanoparticles for in vivo gene delivery. Expert Opin. Drug Deliv. 2013;10(2):215–228. doi: 10.1517/17425247.2013.744964. [DOI] [PubMed] [Google Scholar]
- 274.Chen Y., Wang W., Lian G., Qian C., Wang L., Zeng L. Development of an MRI-visible nonviral vector for siRNA delivery targeting gastric cancer. Int. J. Nanomedicine. 2012;7:359. doi: 10.2147/IJN.S24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chen Y., Lian G., Liao C., Wang W., Zeng L., Qian C. Characterization of polyethylene glycol-grafted polyethylenimine and superparamagnetic iron oxide nanoparticles (PEG-g-PEI-SPION) as an MRI-visible vector for siRNA delivery in gastric cancer in vitro and in vivo. J. Gastroenterol. 2013;48(7):809–821. doi: 10.1007/s00535-012-0713-x. [DOI] [PubMed] [Google Scholar]
- 276.Baig B., Halim S.A., Farrukh A., Greish Y., Amin A. Current status of nanomaterial-based treatment for hepatocellular carcinoma. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.108852. [DOI] [PubMed] [Google Scholar]
- 277.Redd Bowman K.E., Lu P., Vander Mause E.R., Lim C.S. Advances in delivery vectors for gene therapy in liver cancer. Ther. Deliv. 2020;11(1):833–850. doi: 10.4155/tde-2019-0076. [DOI] [PubMed] [Google Scholar]
- 278.Díez S., Navarro G., de ILarduya C.T. In vivo targeted gene delivery by cationic nanoparticles for treatment of hepatocellular carcinoma. J. Gene Med. 2009;11(1):38–45. doi: 10.1002/jgm.1273. [DOI] [PubMed] [Google Scholar]
- 279.Lasek W., Zagożdżon R., Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014;63(5):419–435. doi: 10.1007/s00262-014-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hernandez-Alcoceba R., Poutou J., Ballesteros-Briones M.C., Smerdou C. Gene therapy approaches against cancer using in vivo and ex vivo gene transfer of interleukin-12. Immunotherapy. 2016;8(2):179–198. doi: 10.2217/imt.15.109. [DOI] [PubMed] [Google Scholar]
- 281.Xue W.-J., Feng Y., Wang F., Guo Y.-B., Li P., Wang L. Asialoglycoprotein receptor-magnetic dual targeting nanoparticles for delivery of RASSF1A to hepatocellular carcinoma. Sci. Rep. 2016;6 doi: 10.1038/srep22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Xue W.J., Li C., Zhou X.J., Guan H.G., Qin L., Li P. RASSF1A expression inhibits the growth of hepatocellular carcinoma from Qidong County. J. Gastroenterol. Hepatol. 2008;23(9):1448–1458. doi: 10.1111/j.1440-1746.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- 283.Popielarski S.R., Hu-Lieskovan S., French S.W., Triche T.J., Davis M.E. A nanoparticle-based model delivery system to guide the rational design of gene delivery to the liver. 2. In vitro and in vivo uptake results. Bioconjug. Chem. 2005;16(5):1071–1080. doi: 10.1021/bc0501146. [DOI] [PubMed] [Google Scholar]
- 284.Xue H.-Y., Liu Y., Liao J.-Z., Lin J.-S., Li B., Yuan W.-G. Gold nanoparticles delivered miR-375 for treatment of hepatocellular carcinoma. Oncotarget. 2016;7(52) doi: 10.18632/oncotarget.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sun B., Fang Y., Li Z., Chen Z., Xiang J. Advances in the application of nanotechnology in the diagnosis and treatment of gastrointestinal tumors. Mol. Clin. Oncol. 2015;3(2):274–280. doi: 10.3892/mco.2014.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.El Sharkawi F.Z., Ewais S.M., Fahmy R.H., Rashed L.A. PTEN and TRAIL genes loaded zein nanoparticles as potential therapy for hepatocellular carcinoma. J. Drug Target. 2017;25(6):513–522. doi: 10.1080/1061186X.2017.1289536. [DOI] [PubMed] [Google Scholar]
- 287.B-g Hu, L-p Liu, Chen G.G., Ye C.G., Leung K.K., Ho R.L. Therapeutic efficacy of improved α-fetoprotein promoter-mediated tBid delivered by folate-PEI600-cyclodextrin nanopolymer vector in hepatocellular carcinoma. Exp. Cell Res. 2014;324(2):183–191. doi: 10.1016/j.yexcr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 288.Fitamant J., Kottakis F., Benhamouche S., Tian H.S., Chuvin N., Parachoniak C.A. YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression. Cell Rep. 2015;10(10):1692–1707. doi: 10.1016/j.celrep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Huang K.-W., Hsu F.-F., Qiu J.T., Chern G.-J., Lee Y.-A., Chang C.-C. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020;6(3):eaax5032. doi: 10.1126/sciadv.aax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Freytag S.O., Stricker H., Movsas B., Kim J.H. Prostate cancer gene therapy clinical trials. Mol. Ther. 2007;15(6):1042–1052. doi: 10.1038/sj.mt.6300162. [DOI] [PubMed] [Google Scholar]
- 291.Tewari A.K., Whelan P., Graham J.D. John Wiley & Sons; 2013. Prostate cancer: diagnosis and clinical management. [Google Scholar]
- 292.Wirth T., Parker N., Ylä-Herttuala S. History of gene therapy. Gene. 2013;525(2):162–169. doi: 10.1016/j.gene.2013.03.137. [DOI] [PubMed] [Google Scholar]
- 293.Wang W., Li W., Ma N., Steinhoff G. Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 2013;14(1):46–60. [PubMed] [Google Scholar]
- 294.Bertrand N., Wu J., Xu X., Kamaly N., Farokhzad O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Altwaijry N., Somani S., Parkinson J.A., Tate R.J., Keating P., Warzecha M. Regression of prostate tumors after intravenous administration of lactoferrin-bearing polypropylenimine dendriplexes encoding TNF-α, TRAIL, and interleukin-12. Drug Delivery. 2018;25(1):679–689. doi: 10.1080/10717544.2018.1440666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Al Robaian M., Chiam K.Y., Blatchford D.R., Dufès C. Therapeutic efficacy of intravenously administered transferrin-conjugated dendriplexes on prostate carcinomas. Nanomedicine. 2014;9(4):421–434. doi: 10.2217/NNM.13.25. [DOI] [PubMed] [Google Scholar]
- 297.Fornaro M., Manes T., Languino L.R. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20(3–4):321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 298.Marelli U.K., Rechenmacher F., Sobahi T.R.A., Mas-Moruno C., Kessler H. Tumor targeting via integrin ligands. Front. Oncol. 2013;3:222. doi: 10.3389/fonc.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Panda P.K., Saraf S., Tiwari A., Verma A., Raikwar S., Jain A. Novel Strategies for Targeting Prostate Cancer. Curr. Drug Deliv. 2019;16(8):712–727. doi: 10.2174/1567201816666190821143805. [DOI] [PubMed] [Google Scholar]
- 300.Altwaijry N., Somani S., Dufès C. Targeted nonviral gene therapy in prostate cancer. Int. J. Nanomedicine. 2018;13:5753–5767. doi: 10.2147/IJN.S139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wang L., Zhang M., Tan K., Guo Y., Tong H., Fan X. Preparation of nanobubbles carrying androgen receptor siRNA and their inhibitory effects on androgen-independent prostate cancer when combined with ultrasonic irradiation. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Rak M., Ochałek A., Bielecka E., Latasiewicz J., Gawarecka K., Sroka J. Efficient and non-toxic gene delivery by anionic lipoplexes based on polyprenyl ammonium salts and their effects on cell physiology. J. Gene Med. 2016;18(11–12):331–342. doi: 10.1002/jgm.2930. [DOI] [PubMed] [Google Scholar]
- 303.Zolochevska O., Ellis J., Parelkar S., Chan-Seng D., Emrick T., Wei J. Interleukin-27 gene delivery for modifying malignant interactions between prostate tumor and bone. Hum. Gene Ther. 2013;24(12):970–981. doi: 10.1089/hum.2013.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hattori Y., Maitani Y. DNA/Lipid complex incorporated with fibronectin to cell adhesion enhances transfection efficiency in prostate cancer cells and xenografts. Biol. Pharm. Bull. 2007;30(3):603–607. doi: 10.1248/bpb.30.603. [DOI] [PubMed] [Google Scholar]
- 305.Tavakol S., Ashrafizadeh M., Deng S., Azarian M., Abdoli A., Motavaf M. Autophagy modulators: mechanistic aspects and drug delivery systems. Biomolecules. 2019;9(10):530. doi: 10.3390/biom9100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Du J., Li L. Which one performs better for targeted lung cancer combination therapy: pre-or post-bombesin-decorated nanostructured lipid carriers? Drug Delivery. 2016;23(5):1799–1809. doi: 10.3109/10717544.2015.1099058. [DOI] [PubMed] [Google Scholar]
- 307.Li Z., Zhu L., Liu W., Zheng Y., Li X., Ye J. Near-infrared/pH dual-responsive nanocomplexes for targeted imaging and chemo/gene/photothermal tri-therapies of non-small cell lung cancer. Acta Biomater. 2020;107:242–259. doi: 10.1016/j.actbio.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 308.Mohammadinejad R., Ashrafizadeh M., Pardakhty A., Uzieliene I., Denkovskij J., Bernotiene E. Nanotechnological Strategies for Osteoarthritis Diagnosis, Monitoring, Clinical Management, and Regenerative Medicine: Recent Advances and Future Opportunities. Curr. Rheumatol. Rep. 2020;22(4) doi: 10.1007/s11926-020-0884-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ashrafizadeh M., Ahmadi Z., Kotla N.G., Afshar E.G., Samarghandian S., Mandegary A. Nanoparticles targeting STATs in cancer therapy. Cells. 2019;8(10):1158. doi: 10.3390/cells8101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Fineran P.C., Charpentier E. Memory of viral infections by CRISPR-Cas adaptive immune systems: acquisition of new information. Virology. 2012;434(2):202–209. doi: 10.1016/j.virol.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 311.Wiedenheft B., Sternberg S.H., Doudna J.A. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482(7385):331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 312.Mohammadinejad R., Biagioni A., Arunkumar G., Shapiro R., Chang K.-C., Sedeeq M. EMT signaling: potential contribution of CRISPR/Cas gene editing. Cell. Mol. Life Sci. 2020:1–22. doi: 10.1007/s00018-020-03449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.McCarty N.S., Graham A.E., Studená L., Ledesma-Amaro R. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat. Commun. 2020;11(1):1–13. doi: 10.1038/s41467-020-15053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hwang W.Y., Fu Y., Reyon D., Maeder M.L., Tsai S.Q., Sander J.D. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013;31(3):227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bikard D., Euler C.W., Jiang W., Nussenzweig P.M., Goldberg G.W., Duportet X. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014;32(11):1146–1150. doi: 10.1038/nbt.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wu Y., Zhou H., Fan X., Zhang Y., Zhang M., Wang Y. Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Res. 2015;25(1):67–79. doi: 10.1038/cr.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Cho S.W., Kim S., Kim J.M., Kim J.-S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013;31(3):230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- 318.Rahimi H., Salehiabar M., Charmi J., Barsbay M., Ghaffarlou M., Razlighi M.R. Harnessing nanoparticles for the efficient delivery of the CRISPR/Cas9 system. Nano Today. 2020;34 [Google Scholar]
- 319.Mout R., Ray M., Yesilbag Tonga G., Lee Y.-W., Tay T., Sasaki K. Direct cytosolic delivery of CRISPR/Cas9-ribonucleoprotein for efficient gene editing. ACS Nano. 2017;11(3):2452–2458. doi: 10.1021/acsnano.6b07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Qiao J., Sun W., Lin S., Jin R., Ma L., Liu Y. Cytosolic delivery of CRISPR/Cas9 ribonucleoproteins for genome editing using chitosan-coated red fluorescent protein. Chem. Commun. 2019;55(32):4707–4710. doi: 10.1039/c9cc00010k. [DOI] [PubMed] [Google Scholar]
- 321.Ramakrishna S., Dad A.-B.K., Beloor J., Gopalappa R., Lee S.-K., Kim H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014;24(6):1020–1027. doi: 10.1101/gr.171264.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang Y., Shahi P.K., Xie R., Zhang H., Abdeen A.A., Yodsanit N. A pH-responsive silica–metal–organic framework hybrid nanoparticle for the delivery of hydrophilic drugs, nucleic acids, and CRISPR-Cas9 genome-editing machineries. J. Control. Release. 2020;324:194–203. doi: 10.1016/j.jconrel.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chen G., Abdeen A.A., Wang Y., Shahi P.K., Robertson S., Xie R. A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing. Nat. Nanotechnol. 2019;14(10):974–980. doi: 10.1038/s41565-019-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020;15(4):313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Shahbazi R., Sghia-Hughes G., Reid J.L., Kubek S., Haworth K.G., Humbert O. Targeted homology-directed repair in blood stem and progenitor cells with CRISPR nanoformulations. Nat. Mater. 2019;18:1124–1132. doi: 10.1038/s41563-019-0385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yue H., Zhou X., Cheng M., Xing D. Graphene oxide-mediated Cas9/sgRNA delivery for efficient genome editing. Nanoscale. 2018;10(3):1063–1071. doi: 10.1039/c7nr07999k. [DOI] [PubMed] [Google Scholar]
- 327.Alsaiari S.K., Patil S., Alyami M., Alamoudi K.O., Aleisa F.A., Merzaban J.S. Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J. Am. Chem. Soc. 2018;140(1):143–146. doi: 10.1021/jacs.7b11754. [DOI] [PubMed] [Google Scholar]
- 328.Sun W., Ji W., Hall J.M., Hu Q., Wang C., Beisel C.L. Self-assembled DNA nanoclews for the efficient delivery of CRISPR–Cas9 for genome editing. Angew. Chem. Int. Ed. 2015;54(41):12029–12033. doi: 10.1002/anie.201506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Rui Y., Wilson D.R., Choi J., Varanasi M., Sanders K., Karlsson J. Carboxylated branched poly (β-amino ester) nanoparticles enable robust cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci. Adv. 2019;5(12):eaay3255. doi: 10.1126/sciadv.aay3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wan T., Chen Y., Pan Q., Xu X., Kang Y., Gao X. Genome editing of mutant KRAS through supramolecular polymer-mediated delivery of Cas9 ribonucleoprotein for colorectal cancer therapy. J. Control. Release. 2020;322:236–247. doi: 10.1016/j.jconrel.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 331.Ryu J.-Y., Won E.-J., Lee H.A.R., Kim J.H., Hui E., Kim H.P. Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119736. [DOI] [PubMed] [Google Scholar]
- 332.Hajj K.A., Melamed J.R., Chaudhary N., Lamson N.G., Ball R.L., Yerneni S.S. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett. 2020 doi: 10.1021/acs.nanolett.0c00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Jiang C., Mei M., Li B., Zhu X., Zu W., Tian Y. A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Res. 2017;27(3):440–443. doi: 10.1038/cr.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Miller J.B., Zhang S., Kos P., Xiong H., Zhou K., Perelman S.S. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. 2017;56(4):1059–1063. doi: 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Finn J.D., Smith A.R., Patel M.C., Shaw L., Youniss M.R., van Heteren J. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22(9):2227–2235. doi: 10.1016/j.celrep.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 336.Kretzmann J.A., Ho D., Evans C.W., Plani-Lam J.H., Garcia-Bloj B., Mohamed A.E. Synthetically controlling dendrimer flexibility improves delivery of large plasmid DNA. Chem. Sci. 2017;8(4):2923–2930. doi: 10.1039/c7sc00097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wang P., Zhang L., Zheng W., Cong L., Guo Z., Xie Y. Thermo-triggered release of CRISPR-Cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew. Chem. Int. Ed. 2018;57(6):1491–1496. doi: 10.1002/anie.201708689. [DOI] [PubMed] [Google Scholar]
- 338.Rohiwal S., Dvorakova N., Klima J., Vaskovicova M., Senigl F., Slouf M. Polyethylenimine based magnetic nanoparticles mediated non-viral CRISPR/Cas9 system for genome editing. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-61465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jo A., Ringel-Scaia V.M., McDaniel D.K., Thomas C.A., Zhang R., Riffle J.S. Fabrication and characterization of PLGA nanoparticles encapsulating large CRISPR–Cas9 plasmid. J. Nanobiotechnology. 2020;18(1):1–14. doi: 10.1186/s12951-019-0564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Li L., Song L., Liu X., Yang X., Li X., He T. Artificial virus delivers CRISPR-Cas9 system for genome editing of cells in mice. ACS Nano. 2017;11(1):95–111. doi: 10.1021/acsnano.6b04261. [DOI] [PubMed] [Google Scholar]
- 341.Timin A.S., Muslimov A.R., Lepik K.V., Epifanovskaya O.S., Shakirova A.I., Mock U. Efficient gene editing via non-viral delivery of CRISPR–Cas9 system using polymeric and hybrid microcarriers. Nanomedicine. 2018;14(1):97–108. doi: 10.1016/j.nano.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 342.Cheng M.Q., Wahafu T.E.H.J., Jiang G.F., Liu W., Qiao Y.Q., Peng X.C. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016;6 doi: 10.1038/srep24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yu W., Mookherjee S., Chaitankar V., Hiriyanna S., Kim J.-W., Brooks M. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat. Commun. 2017;8(1):1–15. doi: 10.1038/ncomms14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Niu Y., Shen B., Cui Y., Chen Y., Wang J., Wang L. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156(4):836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 345.Xue W., Chen S., Yin H., Tammela T., Papagiannakopoulos T., Joshi N.S. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature. 2014;514(7522):380–384. doi: 10.1038/nature13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Dehshahri A., Sadeghpour H., Mohazzabieh E., Saatchi Avval S., Mohammadinejad R. Targeted double domain nanoplex based on galactosylated polyethylenimine enhanced the delivery of IL-12 plasmid. Biotechnol. Prog. 2020 doi: 10.1002/btpr.3002. e3002. [DOI] [PubMed] [Google Scholar]
- 347.Buck J., Grossen P., Cullis P.R., Jr Huwyler, Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13(4):3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- 348.Rui Y., Wilson D.R., Green J.J. Non-viral delivery to enable genome editing. Trends Biotechnol. 2019;37(3):281–293. doi: 10.1016/j.tibtech.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Chen F., Alphonse M., Liu Q. Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip. Rev. 2020;12(3) doi: 10.1002/wnan.1609. [DOI] [PubMed] [Google Scholar]