Abstract
Objectives:
To examine the association of lifetime history of traumatic brain injury (TBI) with later-life physical impairment (PI) and functional impairment (FI), and to evaluate the impact of neurobehavioral symptoms that frequently co-occur with TBI on these relations.
Participants:
1148 respondents to the 2014 Wave of the Health and Retirement Study (HRS), a nationally representative survey of older community-dwelling adults, randomly selected to participate in a TBI exposure survey. They reported no prior TBI (n=737) or prior TBI (n=411).
Design:
Cross-sectional survey study.
Main Measures:
PI (self-reported difficulty with ≥1 of 8 physical activities); FI (self-reported difficulty with ≥1 of 11 activities of daily living); Self-reported current neurobehavioral symptoms (pain, sleep problems, depression, subjective memory impairment); Ohio State University TBI Identification Method (OSU-TBI-ID)- short form.
Analyses:
Stepwise logistic regression models (1. unadjusted; 2. adjusted for demographics and medical comorbidities; 3. additionally adjusted for neurobehavioral symptoms) compared PI and FI between TBI groups.
Results:
TBI-exposed (mean 33.6 years post-injury) respondents were younger, less likely to be female, reported more comorbidities and neurobehavioral symptoms. While TBI was significantly associated with increased odds of PI and FI in unadjusted models and models adjusted for demographics/comorbidities (adjusted OR, 95% Confidence Interval [CI]: PI 1.62, 1.21–2.17; FI 1.60, 1.20–2.14), this association was no longer statistically significant after further adjustment for neurobehavioral symptoms.
Conclusion:
History of TBI is associated with substantial physical and functional impairment among community-dwelling older adults. Further research is warranted to determine whether aggressive management of neurobehavioral symptoms in this population may mitigate long-term physical and functional impairment in this population.
Keywords: Traumatic Brian Injury, Physical Impairment, Functional Impairment
INTRODUCTION
Up to 40% of adults in the US are estimated to have experienced a traumatic brain injury (TBI) [1, 2]. TBI may cause both immediate and long-term cognitive, physical, and functional impairment [3–5]. Common sequelae of TBI include physical impairment [6] and neurobehavioral symptoms including pain [7], sleep problems [8, 9], depression [10, 11], and subjective [12] and objective [4] cognitive problems, which may uniquely and additionally contribute to long-term functional disability after TBI [13, 14]. If these symptoms precede TBI, they may worsen post-TBI outcomes ([15–17]). Older adults are particularly vulnerable to the effects of TBI, which occur at higher rates in older adulthood than at any other time [18]. Understanding the impact of lifetime TBI exposure on physical and functional impairment in older adults is essential because of the implications for their ability to maintain independence and quality of life.
Older age is a risk factor for poor acute and sub-acute functional and neurobehavioral outcomes after TBI [18]. Additionally, TBI may interact with the aging process to result in increased risk for dementia [19–26], although most individuals who experience a TBI do not develop dementia [23, 25]. Previous population-based and representative studies have documented worse functional status [1] and higher rates of disability [27, 28] poor health, and health-related functional impairment [9] in TBI-exposed adults of all ages compared to those without history of TBI. Even among adults receiving acute inpatient rehabilitation after TBI, most (57%) were moderately or severely disabled five years post-injury [29]. However, the impact of lifetime history of TBI (including very remote TBI) on the daily functioning of community dwelling older adults without dementia is unknown. Previous research examining a small cohort of older military Veterans without dementia (mean age: 78 years) has shown that those with a history of TBI exposure (on average 32 years prior to study participation) had worse cognitive function [3], overall daily function [4], and motor function [6] compared to those without history of TBI. However, few studies have examined this question in a large, nationally representative cohort of community-dwelling older adults.
Our group’s previous work demonstrated that among community-dwelling older civilians without dementia, a history of TBI is associated with subjective but not objective cognitive impairment in the domains of episodic memory, language, working memory, or calculations [12]. It remains unclear, however, whether a history of TBI is associated with physical and functional impairment in this population and how demographic and medical factors and neurobehavioral symptoms may affect these relations.
This study aimed to illuminate the long-term physical and functional consequences of a history of TBI in a nationally representative sample of community-dwelling older adults without dementia and to understand the role co-occurring conditions play in these phenotypes and associated, possibly treatable, syndromes. Our primary goal was to determine whether history of TBI is an independent risk factor for impairment in physical function and basic and instrumental activities of daily living (ADLs and IADLs) among older adults after accounting for demographic features and medical comorbidities. Our secondary aim was to investigate the contribution of several, potentially modifiable, neurobehavioral symptoms (that frequently co-occur with TBI) to physical and functional impairment. We hypothesized that history of TBI would result in increased risk of physical and functional impairment, and that co-occurring neurobehavioral conditions would further increased risk of physical and functional impairment in this group.
METHODS
Design and Protocol Approval
Data for this study were deidentified, publicly available data from the Health and Retirement Study (HRS); Therefore, the study was deemed exempt by the University of California San Francisco Human Research Committee. All HRS respondents provided oral consent prior to data collection.
Data Source and Sampling
Data were drawn from the HRS, a longitudinal survey study of community-dwelling older adults (age 51 years and older) and their spouses which was launched in 1992 and has continued with repeat surveys of approximately N=20,000 older adults every two years. HRS employs national area probability sampling of U.S. households with supplemental oversampling of black individuals, Hispanic individuals, and Florida state residents in order to reflect United States population demographics and increase representation of minorities in the survey cohort.
In each survey year, all respondents first complete an extensive “core survey” and then may be offered participation in one of several randomly-selected optional shorter year-specific “module surveys” focused on a topic of interest. The core survey is repeated every two years to as many of the same respondents as possible. In addition, new respondents are added in each survey wave to reflect the growing US population of adults 51 and older. Only the 2014 wave of the HRS survey was used in the current study because the TBI module was conducted only in 2014. One to two years after completion of each survey, de-identified data are made publicly available. Detailed information about the sampling procedures, study design, instruments, and data access can be found online (http://hrsonline.isr.umich.edu).
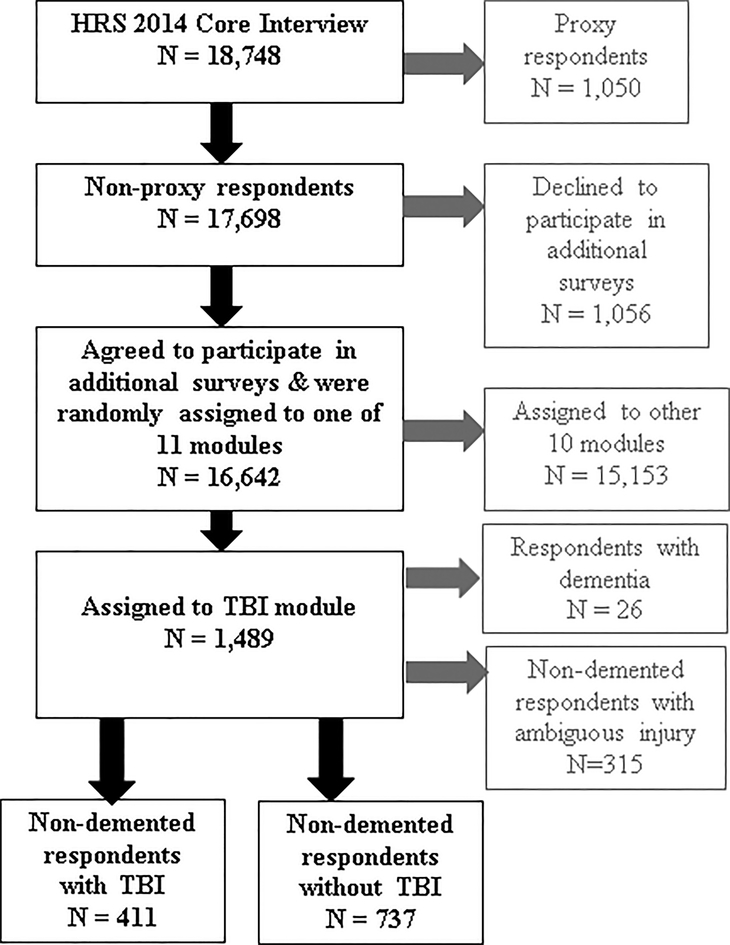
For the present study, we used data from the TBI module survey, administered to a random sub-sample of the 2014 survey non-proxy respondents (n=1,489 of the 16,642 non-proxy respondents; see Figure 1 and Gardner et al., 2017 [12], for additional details about the sample). Compared to the n=16,642 non-proxy respondents who completed the 2014 core interview (i.e., the main survey covering aspects of health, lifestyle, finances, and psychosocial factors administered to all HRS respondents), the random sub-set of n=1,489 non-proxy respondents who additionally completed the TBI module were slightly more educated (mean (SD) years education 13.6 (8.0) vs. 13.2 (6.7), p<0.01) but did not differ significantly on age, race, or ethnicity.
Figure 1. Sampling of respondents.
Figure Legend: TBI is defined as injury to the head or neck resulting in LOC and/or PTA. Treatment in a hospital for the head or neck injury was not required in order for a participant to be categorized as having had a TBI. The study sample was identified in the following manner: respondents whose data were analyzed for this study were non-proxy respondents to the 2014 wave of the Health and Retirement Study (HRS) randomly assigned to participate in a traumatic brain injury (TBI) module. Text in grey represents respondents excluded from this study.
TBI Exposure
Participants in the TBI module responded to questions from the short form of the Ohio State University TBI Identification Method (OSU-TBI-ID [30]). Because our goal was to examine the impact of lifetime TBI exposure, we utilized self-report of TBI history, as do most other studies in this area [1, 9, 11, 12, 27, 28, 31, 32]. Medical record confirmation of TBI exposure was not possible in this study due to the HRS data use agreement which precludes contacting respondents or attempting to de-identify their data. Additionally, self-report is necessary to establish total lifetime exposure, particularly among older adults in whom TBI exposure may be extremely remote and in whom medical record documentation may have never or may no longer exist (e.g., older adults who experience TBI are less likely to present for medical treatment compared to younger individuals with TBI [33]. The OSU-TBI-ID measure, which has demonstrated excellent reliability and predictive validity (interclass correlations all >.80; strong predictive validity for TBI recency/severity/frequency and cognitive and/or social functioning [30]), is recommended by the National Institute of Neurological Disorders and Stroke (NINDS) for retrospective report of lifetime exposure to TBI in clinical research [34] and has been selected by expert consensus as a TBI Common data element. The short form used in this study differs from the standard form as it omits questions assessing periods of “multiple, repeated impacts to the head”.
In the present study, TBI was defined as any injury to the head or neck resulting in loss of consciousness (LOC; i.e., “were you knocked out or did you lose consciousness?”) or peri-traumatic amnesia/feeling dazed (PTA; i.e., “were you dazed, or did you have a gap in your memory?”) or both. “No TBI” was defined as no prior head or neck injury of any kind. To reduce the likelihood of misclassification of TBI exposure, respondents who reported any prior head or neck injury that did not result in LOC or PTA were classified as having “ambiguous injury.” This definition of TBI is based on the validated wording of the OSU-TBI-ID, which employs the language “head or neck” when querying possible injuries in order to increase sensitivity.
Participant Selection
Of the 1,489 respondents to the TBI module, 26 were excluded based on self-reported diagnosis of Alzheimer’s disease or non-AD dementia. Because diagnosis of Parkinson’s disease (PD) was not queried in HRS, respondents with a PD diagnosis could not be excluded. We also excluded participants with ambiguous injury to the head or neck not meeting criteria for TBI (n=315, 21.5% of the 1,463 non-demented respondents to the TBI module). The final study cohort consisted of 411 respondents with TBI and 737 respondents with no TBI. Because we did not aim to estimate national population prevalence of TBI in this sample, HRS survey weights were not used.
Outcomes
Physical impairment:
Respondents who reported difficulty on ≥1 of 8 physical activities “because of a health problem” were classified as having physical impairment. The activities queried were walking 1 block, arising from a chair, climbing 1 flight of stairs, stooping, reaching, pulling/pushing heavy items, lifting 10 pounds, and picking up a dime.
Functional impairment:
Respondents who reported difficulty on ≥1 of 11 basic or instrumental ADLs “because of a health or memory problem” were classified as having functional impairment. This impairment criterion was selected based on previous research using HRS (i.e., [35, 36]). The basic ADLs were dressing, bathing, eating, toileting, and getting into and out of bed. The instrumental ADLs questioned were: using a map, hot meal preparation, grocery shopping, making phone calls, taking medications, and managing money.
Demographics, Medical Comorbidities, and Neurobehavioral Symptoms
We examined the following demographic and medical comorbidity variables, which may be associated with TBI exposure and/or physical and functional outcome: age, sex, ethnicity, race, years of education, military Veteran status, and self-report of medical comorbidities diagnosed by a physician (arthritis, cancer, diabetes, heart disease, hypertension, lung disease, and stroke). We also examined the following neurobehavioral symptoms which, although nonspecific are frequently associated with TBI and were endorsed by respondents as being current: pain, sleep problems, depression, and subjective memory impairment. Given our prior study that identified subjective but not objective memory impairment in the HRS TBI module participants with history of TBI [20], we did not include the HRS objective cognitive battery in the present analysis.
Sleep was assessed using a modified version of the four-item Jenkins Sleep Problems Questionnaire [37]. In accordance with previously published research using the HRS database [38], respondents were conservatively categorized as having sleep problems if they reported difficulty with sleep initiation, nighttime awakenings, or early-morning awakenings “most of the time,” or answered “rarely or never” to feeling rested upon awakening. Pain was assessed with a single question (“are you often troubled by pain?”). In accordance with published literature [39], “depression” was defined as a score of 3 or higher on the Center for Epidemiological Studies Depression Scale (CESD-8 [39]), an eight-item scale addressing depressive symptoms over the past week (score 0–8). Subjective memory impairment was assessed using a self-assessment of current memory function (“How would you rate your memory at the present time? Excellent, very good, good, fair, or poor?”). Participants who rated their current memory functioning as fair or poor were categorized as having subjective memory impairment. Missingness was under 1% for all variables.
Data Analysis
All analyses were conducted using SPSS Version 25 [40]. The assumptions of all statistical tests regarding distributions and noncollinearity of predictor variables were met. Demographics, medical comorbidities, and neurobehavioral symptoms were compared between respondents with and without TBI using t-tests for continuous variables and chi-square tests for categorical variables. The association between TBI exposure and functional and physical impairment was assessed using three levels of logistic regression models to evaluate the effect of adjustment for different types of covariates on the magnitude of the risk estimate. Level 1. Unadjusted; Level 2. Adjusted for demographic and medical comorbidities that significantly differed between groups at p<0.01 in univariate analyses; Level 3. Additionally adjusted for neurobehavioral symptoms (i.e., pain, sleep problems, depression, and subjective memory impairment) that differed between groups at p<0.10 individually, and entered into the model together in one step. In the Level 3 models investigating neurobehavioral contributions to the association of TBI with functional impairment, physical impairment was also included as a predictor given the complex relations between older adults’ physical function and basic and instrumental ADLs [41], and between these outcomes and TBI. Exploratory analyses examining a possible dose effect of TBI severity (no TBI, TBI without LOC, TBI with LOC) and frequency (no TBI, one TBI, more than one TBI) on odds of physical and functional impairment were also undertaken using logistic regression with dummy-coded severity and frequency predictor variables. Similar models using ordinally-coded predictors were built to assess the presence of a trend across levels in increasing TBI severity and/or frequency on greater risk of physical and functional impairment.
We calculated confidence intervals for the B coefficient using bootstrapping with 1000 replications to allow for more robust and accurate estimation [42]. Model fit was evaluated using measures of effect size, Hosmer-Lemeshow goodness of fit, and −2 log likelihood statistics. Statistical significance was set at p<0.05.
RESULTS
Characteristics of respondents with versus without TBI are shown in Table 1. Those with TBI were younger, more likely to be male, and reported significantly higher rates of multiple medical comorbidities (heart disease, lung disease, arthritis) and neurobehavioral symptoms (pain, sleep problems, depression) and physical impairment; Table 1). Among respondents with TBI, most reported experiencing a single injury, endorsed losing consciousness as a result of TBI, and reported having received hospital treatment for a TBI (Table 2). Average time since most recent TBI was 33.6 years (range: 0–88 years).
Table 1.
Characteristics of respondents with and without TBI
| Values are mean (SD) or % | No TBI (n=737) | TBI (n=411) | p |
|---|---|---|---|
| Demographics | |||
| Age, years | 68.22 (10.99) | 64.97 (10.05) | <0.001 |
| Military Veteran | 8.8 | 16.5 | <.001 |
| Female | 66.2 | 46.7 | <0.001 |
| Hispanic | 16.4 | 12.2 | 0.052 |
| Race | 0.736 | ||
| White | 71.4 | 72.3 | |
| Black | 19.9 | 18.2 | |
| Other/Unknown | 8.7 | 9.5 | |
| Education, years | 13.18 (6.33) | 14.03 (9.88) | 0.074 |
| Medical Comorbidities | |||
| Hypertension | 60.1 | 65.5 | 0.154 |
| Diabetes | 24.7 | 24.6 | 0.522 |
| Cancer | 15.9 | 14.1 | 0.426 |
| Lung Disease | 7.2 | 13.1 | 0.001 |
| Heart Disease | 18.7 | 27.3 | 0.001 |
| Stroke | 5.7 | 8.3 | 0.093 |
| Arthritis | 56.0 | 67.6 | <0.001 |
| Neurobehavioral Symptoms | |||
| Pain | 30.3 | 53.0 | <0.001 |
| Sleep problems | 40.4 | 49.4 | 0.003 |
| Depression | 14.1 | 22.4 | <0.001 |
| Subjective memory impairment | 27.3 | 31.9 | 0.094 |
Abbreviations: SD = standard deviation.
Table 2.
Traumatic brain injury features
| mean (SD) or % | |
|---|---|
| Frequency | |
| 1 | 64.2 |
| 2 | 24.1 |
| >2 | 11.7 |
| Treated in Hospital | |
| Yes | 62.3 |
| No | 37.7 |
| Severity | |
| Dazed/PTA only (no LOC) | 33.6 |
| LOC | 66.4 |
| Time since Injury | |
| Years since first TBI | 41.13 (18.83) |
| Years since last TBI | 33.55 (19.94) |
Abbreviations: SD = standard deviation.
Prevalence of physical and functional impairment on specific tasks among respondents with and without TBI is shown in Table 3. Impairment on all physical tasks queried except the fine motor task of “picking up a dime” was significantly more prevalent for respondents with history of TBI and they were more likely to report any physical impairment. Regarding ADLs, participants with history of TBI were more likely to report problems with dressing, getting into/out of bed, and any ADL impairment. Participants with history of TBI were also more likely to have instrumental ADL impairment in shopping for groceries, preparing a hot meal, taking medication, managing money, and any impairment. Respondents who reported a history of TBI were significantly more likely to meet criteria for physical and functional impairment (Table 4). In models adjusted for demographics/medical history that differed between groups at p<.10, respondents with TBI had an estimated 62% increased odds of physical impairment and 60% increased odds of functional impairment.
Table 3.
Physical and functional impairment profile of respondents with and without TBI
| Values are % | No TBI | TBI | p |
|---|---|---|---|
| Physical Impairment | N=731 | N=409 | |
| Walking 1 block | 12.6 | 17.3 | 0.031 |
| Arising from a chair | 33.9 | 47.7 | <0.001 |
| Climbing 1 flight of stairs | 16.1 | 22.4 | 0.009 |
| Stooping | 43.7 | 56.9 | <0.001 |
| Reaching | 14.7 | 23.4 | <0.001 |
| Pull/pushing | 23.9 | 30.2 | 0.020 |
| Lifting | 23.2 | 29.0 | 0.033 |
| Picking up a dime | 7.9 | 9.5 | 0.338 |
| Any physical impairment | 57.0 | 68.2 | <0.001 |
| Functional Impairment | N=737 | N=411 | |
| Basic ADLs | |||
| Dressing | 8.8 | 16.1 | 0.007 |
| Bathing | 4.7 | 8.0 | 0.123 |
| Eating | 2.3 | 3.6 | 0.417 |
| Toileting | 4.1 | 6.6 | 0.239 |
| Getting into/out of bed | 3.7 | 8.8 | 0.003 |
| Any ADL impairment | 12.8 | 22.6 | <0.001 |
| Instrumental ADLs | |||
| Using map | 13.3 | 13.4 | 0.943 |
| Preparing hot meal | 3.1 | 5.6 | 0.040 |
| Shopping for groceries | 4.6 | 8.3 | 0.012 |
| Making phone calls | 3.3 | 2.7 | 0.584 |
| Taking medications | 1.4 | 3.2 | 0.036 |
| Managing money | 4.7 | 8.3 | 0.016 |
| Any IADL impairment | 20.2 | 26.3 | 0.017 |
Table 4.
Effect of traumatic brain injury status on physical and functional impairment, after adjustment for neurobehavioral symptoms of interest
| No TBI (ref) | TBI | B (SE)** | 95% CI for B** | Odds Ratio (95% CI) | p | |
|---|---|---|---|---|---|---|
| Physical Impairment | N=731 | N=409 | ||||
| Level 1: Unadjusted | 57.0 | 68.2 | .48 (.13) | .23-.75 | 1.62 (1.25–2.08) | 0.002 |
| Level 2: Adjusted for demographics and medical comorbidities* | 57.0 | 68.3 | .48 (.15) | .18-.79 | 1.62 (1.21–2.17) | 0.001 |
| Level 3: Additionally adjusted for: | ||||||
| Pain | 57.0 | 68.2 | .18 (.16) | −.13–.51 | 1.20 (.87–1.65) | 0.274 |
| Sleep Problems | 57.1 | 68.3 | .44 (.16) | .16-.77 | 1.56 (1.15–2.11) | 0.004 |
| Depression | 57.0 | 68.3 | .43 (.16) | .11-.75 | 1.53 (1.14–2.07) | 0.005 |
| Subjective Memory Impairment | 57.1 | 68.2 | .45 (.15) | .17-.76 | 1.56 (1.16–2.11) | 0.003 |
| All of the above | 57.0 | 68.2 | .13 (.17) | −.19–.44 | 1.13 (.82–1.57) | 0.458 |
| Functional Impairment | N=737 | N=411 | ||||
| Level 1: Unadjusted | 26.9 | 36.3 | .44 (.13) | .17-.69 | 1.55 (1.20–2.01) | 0.001 |
| Level 2: Adjusted for demographics and medical comorbidities* | 26.9 | 36.3 | .47 (.15) | .16-.77 | 1.60 (1.20–2.17) | 0.002 |
| Level 3: Additionally adjusted for: | ||||||
| Pain | 26.9 | 36.3 | .28 (.16) | −.05–.60 | 1.33 (.98–1.80) | 0.068 |
| Sleep Problems | 26.9 | 36.3 | .43 (.15) | .14-.72 | 1.53 (1.14–2.06) | 0.004 |
| Depression | 26.9 | 36.3 | .36 (.16) | .04-.66 | 1.43 (1.06–1.93) | 0.020 |
| Subjective Memory Impairment | 26.9 | 36.1 | .43 (.15) | .14-.74 | 1.54 (1.14–2.08) | 0.005 |
| Physical Impairment | 27.0 | 36.4 | .37 (.17) | .06-.70 | 1.45 (1.07–1.97) | 0.017 |
| All of the above | 27.0 | 36.3 | .17 (.17) | −.16-.52 | 1.19 (.85–1.65) | 0.306 |
Adjusted for age, sex, Hispanic ethnicity, heart disease, lung disease, arthritis, and stroke.
Abbreviations: CI = confidence interval, SE=standard error.
Bootstrapping based on 1000 repetitions.
Examination of the further adjusted (Level 3) models revealed that the direct effect of TBI exposure on physical and functional impairment was weakened by additional adjustment for current pain, sleep problems, depression, and subjective memory impairment – and for models of functional outcome, additional adjustment for physical impairment – either individually or all together (see Table 4). Adjustment for pain individually or all symptoms together, however, had the largest effect on weakening the association between TBI exposure and both outcomes, rendering the association non-significant (Table 4).
The overall models containing all demographic, medical, and neurobehavioral predictors as well as TBI status fit the data well (physical impairment: −2 Log Likelihood=1122.72, p<.001, Hosmer-Lemeshow chi square 4.27, p=.832; functional impairment: −2 Log Likelihood=1083.90, p=.001, Hosmer-Lemeshow chi square 11.91, p=.155) and accounted for substantially more variance in the outcomes (based on Nagelkerke R2 statistics) compared to the original unadjusted and partially adjusted (for demographics and medical comorbidities) models (physical impairment: 40% of the variance accounted for in fully adjusted model, compared to 1.6% in the original unadjusted model and 24% in the partially adjusted model; functional impairment: 34% of the variance accounted for, compared to 1.3% in the original unadjusted model and 14% in the partially adjusted model).
In exploratory analyses examining the dose-response effect of increasing TBI severity (no TBI, TBI without LOC, TBI with LOC) and frequency (no TBI, single TBI, more than one TBI) on risk of physical and functional impairment, we found a statistically significant trend for increasing odds of physical and functional impairment with increasing TBI severity and frequency (p<.001 for trend across levels for all) in unadjusted models. After staged adjustment, these findings were robust after adjustment for medical and demographic characteristics, although the strength of the effects was diminished, but did not survive adjustment for neurobehavioral conditions (see Supplemental Table 1).
DISCUSSION
In this cross-sectional, nationally representative study, we investigated the association of self-reported lifetime history of TBI with physical and functional impairment among older adults (age 51+) without dementia residing in the community, as well as the role of neurobehavioral symptoms in these relations. We found that a reported history of TBI is associated with increased risk of physical (but not fine motor) and any functional impairment in this relatively high-functioning cohort of community-dwelling older adults. This association persists even after adjustment for demographic and health variables including age, sex, Hispanic ethnicity, and common medical symptoms. We also identified a statistically significant trend for increasing risk of physical and functional impairment with increasing TBI severity and increasing TBI frequency. This dose-response finding warrants further research in larger samples, particularly to elucidate the possible association of mild TBI with physical and functional impairment. In each analysis, the strength of the association between TBI and physical impairment is diminished, and ultimately rendered non-significant, after accounting for a variety of frequently co-occurring neurobehavioral symptoms. Adjustment for pain had the strongest effect on weakening the association between TBI and physical/functional impairment.
Our results are consistent with the few other large population-based or representative studies that examine the impact of TBI exposure on long-term functional impairment outcomes among adults of all ages in select U.S. states [1, 5, 27, 43, 44], showing high rates of long-term disability associated with remote history of TBI. Several of these studies [1, 27, 28] identified TBIs using the same self-report method as the current study (i.e. OSU-TBI-ID). Whiteneck and colleagues [1] compared rates of disability (defined as any activity limitations related to TBI) in respondents with and without TBI. However, this study of adults of all ages in a single U.S. state (Colorado) did not adjust for demographics or medical or neurobehavioral symptoms. A related study [28] using the same sample found increased risk of disability and other negative outcomes (which included poor life satisfaction and impaired memory) associated with lifetime history of TBI. Yi and colleagues [27] also studied the association of lifetime history of TBI with disability (categorized as visual, cognitive, mobility, self-care, or independent living disability) among adults of all ages in a single U.S. State (Ohio). They found increased disability in participants with a history of TBI as well as a dose-effect relation between TBI severity and odds of having any disability. This study adjusted for demographics but not neurobehavioral conditions. Other studies [5, 43, 44] examined only individuals who received medical treatment for TBI (vs. self-report of all TBIs), meaning many mild injuries were likely missed, and there was no comparison to individuals without TBI. In addition, although these studies stratified analyses by demographic variables and used comorbid medical symptoms as predictors of disability, they did not adjust for neurobehavioral symptoms.
One recent representative study of adults of all ages in Ohio [11] examined the association of a lifetime history of TBI with neurobehavioral conditions and showed that after adjustment for demographics TBI history is associated with increased alcohol misuse, smoking, and depression. However, this study did not examine functional outcomes. A recent related study of the same sample [9] showed that TBI with loss of consciousness is associated with increased likelihood of poor sleep, in addition to worse overall health and having activities limited by poor health.
Several recent population-based studies examine the association between lifetime history of TBI and functional and/or neurobehavioral outcomes in nationally representative samples of adults of all ages, in France [45], Canada [46, 47], and Australia [48]. Results of these studies document higher rates of functional disability and neurobehavioral conditions in individuals with a lifetime history of TBI compared to those without. However, no nationally representative studies of the association between lifetime TBI history and functional status have been conducted with U.S. samples.
Our work furthers the findings of these previous studies by using data from a U.S. nationally representative sample, looking specifically at an older adult population, including all reported TBIs (even those that did not require medical attention), and undertaking a more detailed examination of functional status and contributions of co-occurring neurobehavioral symptoms.
Additional previous studies using convenience rather than representative sampling have prospectively examined the impact of TBI exposure on functional outcomes for older adults in the shorter term (i.e., post-rehabilitation discharge and up to 9 months post-discharge follow-up), and shown that older adults experience substantial short-term functional impairment after TBI [49–54]. However, these previous studies are not representative and generally only report discharge disposition (e.g. home vs. institution) or scores on the Functional Independence Measure as outcomes, rather than a detailed, geriatric-appropriate, assessment of basic and instrumental ADLs and physical function as we report here.
Our findings suggest that the currently symptomatic self-reported neurobehavioral symptoms we studied substantially contribute to physical and functional impairment for older adults without dementia self-reporting history of TBI, and that physical impairment further contributes to functional impairment in this population. Pain, sleep problems, depression, and subjective cognitive impairment are negative outcomes which, while nonspecific, are clearly associated with TBI [7–12]. They may contribute to functional disability after TBI and, if preceding TBI, may increase individuals’ vulnerability to sustaining a TBI [13, 14]. However, although previous research has examined the neurobehavioral sequelae of TBI in older adults [55–57], these symptoms have not been considered in relation to functional impairment. Additionally, most extant research on TBI and functional impairment outcomes, including representative and population-based studies of long term post-TBI outcomes for adults of all ages and shorter-term studies focused on older adults, does not consider neurobehavioral symptoms as predictors or covariates. Our results suggest that the neurobehavioral symptoms studied here should be included in future studies. Further, our findings raise the possibility that treating the neurobehavioral symptoms we investigated, which are common sequelae of TBI and/or may precede TBI and contribute to risk of negative post-TBI outcomes, could improve physical and daily function among TBI-exposed older adults.
We identified an increased prevalence of pain in respondents who self-reported a history of TBI compared to those without history of TBI. There may be several explanations for this finding: the high comorbidity of extracranial trauma with TBI [58], TBI-related pain such as headaches [59], and preexistence of chronic pain resulting in increased susceptibility to falls and increasing risk for TBI [60]. We also found that pain was an especially strong predictor of both physical and functional impairment in our sample of TBI-exposed community-dwelling older adults. The association between TBI and chronic pain has been well-studied in a variety of populations [7]. Additionally, prior studies have shown that chronic pain is strongly associated with functional disability in older adults [61, 62]. There is a dearth of information, however, about the functional impact of pain in individuals with history of TBI, particularly older adults. Our results suggest that pain is extremely common among older adults with a history of TBI, is a highly relevant factor in determining community-dwelling, TBI-exposed older adults’ functioning, and warrants further dedicated study to identify potential points of intervention.
Limitations of this cross-sectional survey study include potential for self-report bias on all self-reported measures including TBI exposure and outcomes, as well as recall bias in categorization of TBI exposure; limited information about TBI severity (i.e., lack of sufficient data to categorize TBI into mild vs. moderate/severe); single-item measures of some neurobehavioral symptoms (pain and subjective memory impairment); possible misclassification of trauma to the neck producing interruption of blood supply to the brain resulting in LOC, PTA or alteration of consciousness which is not technically due to traumatic injury to the brain; the exclusion of participants with dementia and those who completed the survey with proxy respondents and may have the highest levels of functional disability; and inability to determine onset of neurobehavioral symptoms and physical/functional impairment relative to TBI exposure because of lack of longitudinal data. Unique strengths of this study include a large, nationally representative sample of older adults; use of a frequently used, recommended (by NINDS and as part of the TBI Common Data Elements) TBI identification method; detailed, geriatric-appropriate self-report assessment of functional status; and adjustment for multiple demographic, medical, and neurobehavioral variables.
In conclusion, this nationally representative cohort study highlights the potential long-term adverse impact of TBI – sustained, on average, three decades earlier – on physical and daily function, even among relatively high-functioning older adults without dementia residing in the community. This study also elucidates the effect of active complaints of pain, sleep problems, depression, and subjective memory impairment on physical and functional impairment in this population. Recognition of the importance of pain, sleep problems, and depression in determining TBI-exposed older adults’ functional status is important because these symptoms may be treatable, and these older adults may be candidates for targeted intervention/treatments that could promote optimal functioning. Pain appears to be a particularly strong contributing factor to physical and functional impairment in this sample, suggesting that treatment of chronic pain may improve both physical and daily functioning in this sample of older adults, and may reduce functional limitations both directly and through its effect on physical impairment. These results underscore the importance of TBI prevention and raise the hope that aggressive management of neurobehavioral symptoms in older adults with a history of TBI may mitigate long-term physical and functional impairment in this vulnerable population.
Supplementary Material
Acknowledgments
The Health and Retirement Study is sponsored by the National Institute on Aging (U01 AG009740) and is conducted at the Institute for Social Research, University of Michigan.
Dr. Kornblith was supported by Department of Veterans Affairs Office of Academic Affiliation TBI/Polytrauma Rehabilitation Research Fellowship.
Dr. Langa was supported by grants from the National Institute on Aging (P30 AG053760 and P30 AG024824).
Dr. Yaffe was supported by National Institute on Aging K24AG031155.
Dr. Gardner was supported by the National Institute of Neurological Disorders and Stroke Beeson K23 NS095755 and the American Federation for Aging Research.
Footnotes
Conflict of interest: No conflict of interest exists.
REFERENCES
- 1.Whiteneck GG, et al. , Prevalence of self-reported lifetime history of traumatic brain injury and associated disability: a statewide population-based survey. The Journal of head trauma rehabilitation, 2016. 31(1): p. E55–E62. [DOI] [PubMed] [Google Scholar]
- 2.P Veitch D, E Friedl K, and W Weiner M, Military risk factors for cognitive decline, dementia and Alzheimer’s disease. Current Alzheimer Research, 2013. 10(9): p. 907–930. [DOI] [PubMed] [Google Scholar]
- 3.Peltz CB, et al. , Neurobehavioral characteristics of older veterans with remote traumatic brain injury. The Journal of head trauma rehabilitation, 2017. 32(1): p. E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaup AR, et al. , Neuropsychological profile of lifetime traumatic brain injury in older veterans. Journal of the International Neuropsychological Society, 2017. 23(1): p. 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selassie AW, et al. , Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil, 2008. 23(2): p. 123–31. [DOI] [PubMed] [Google Scholar]
- 6.Gardner RC, et al. , Remote traumatic brain injury is associated with motor dysfunction in older military veterans. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 2017. 72(9): p. 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nampiaparampil D, Prevalence of chronic pain after traumatic brain injury: A systematic review. JAMA, 2008. 300(6): p. 711–19. [DOI] [PubMed] [Google Scholar]
- 8.Mathias J and Alvaro P, Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep medicine, 2012. 13(7): p. 898–905. [DOI] [PubMed] [Google Scholar]
- 9.Manchester K, et al. , Current health status and history of traumatic brain injury among Ohio adults. Injury prevention, 2019: p. injuryprev-2018–043056. [DOI] [PubMed] [Google Scholar]
- 10.Osborn A, Mathias J, and Fairweather-Schmidt A, Depression following adult, non-penetrating traumatic brain injury: a meta-analysis examining methodological variables and sample characteristics. Neuroscience & Biobehavioral Reviews, 2014. 47: p. 1–15. [DOI] [PubMed] [Google Scholar]
- 11.Bogner J, et al. , Lifetime History of Traumatic Brain Injury and Behavioral Health Problems in a Population-Based Sample. The Journal of head trauma rehabilitation, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Gardner RC, Langa KM, and Yaffe K, Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study. PLoS medicine, 2017. 14(3): p. e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman JM, et al. , Understanding pain after traumatic brain injury: impact on community participation. Am J Phys Med Rehabil, 2007. 86(12): p. 962–9. [DOI] [PubMed] [Google Scholar]
- 14.McMahon PJ, et al. , Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. Journal of neurotrauma, 2014. 31(1): p. 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preece MHW and Geffen GM, The contribution of pre-existing depression to the acute cognitive sequelae of mild traumatic brain injury. Brain injury, 2007. 21(9): p. 951–961. [DOI] [PubMed] [Google Scholar]
- 16.Mooney G, Speed J, and Sheppard S, Factors related to recovery after mild traumatic brain injury. Brain Injury, 2005. 19(12): p. 975–987. [DOI] [PubMed] [Google Scholar]
- 17.MacMillan PJ, et al. , Pre-injury status and adaptation following traumatic brain injury. Brain Injury, 2002. 16(1): p. 41–49. [DOI] [PubMed] [Google Scholar]
- 18.Thompson HJ, McCormick WC, and Kagan SH, Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. Journal of the American Geriatrics Society, 2006. 54(10): p. 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortimer J, et al. , Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. International journal of epidemiology, 1991. 20(Supplement_2): p. S28–S35. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, et al. , Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology, 2000. 55(8): p. 1158–1166. [DOI] [PubMed] [Google Scholar]
- 21.Guo Z, et al. , Head injury and the risk of AD in the MIRAGE study. Neurology, 2000. 54(6): p. 1316–1323. [DOI] [PubMed] [Google Scholar]
- 22.Wang H-K, et al. , Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry, 2012. 83(11): p. 1080–1085. [DOI] [PubMed] [Google Scholar]
- 23.Gardner RC, et al. , Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA neurology, 2014. 71(12): p. 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y-K, et al. , Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PloS one, 2013. 8(5): p. e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes DE, et al. , Traumatic brain injury and risk of dementia in older veterans. Neurology, 2014. 83(4): p. 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordström P, et al. , Traumatic brain injury and young onset dementia: a nationwide cohort study. Annals of neurology, 2014. 75(3): p. 374–381. [DOI] [PubMed] [Google Scholar]
- 27.Yi H, et al. , Lifetime history of traumatic brain injury and current disability among Ohio adults. The Journal of head trauma rehabilitation, 2018. 33(4): p. E24–E32. [DOI] [PubMed] [Google Scholar]
- 28.Whiteneck GG, et al. , Risk of negative outcomes after traumatic brain injury: a statewide population-based survey. The Journal of head trauma rehabilitation, 2016. 31(1): p. E43–E54. [DOI] [PubMed] [Google Scholar]
- 29.Corrigan JD, et al. , US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. The Journal of head trauma rehabilitation, 2014. 29(6): p. E1–E9. [DOI] [PubMed] [Google Scholar]
- 30.Corrigan JD and Bogner J, Initial reliability and validity of the Ohio State University TBI identification method. The Journal of head trauma rehabilitation, 2007. 22(6): p. 318–329. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson PL, et al. , Prevalence of traumatic brain injury among prisoners in South Carolina. The Journal of head trauma rehabilitation, 2012. 27(3): p. E11–E20. [DOI] [PubMed] [Google Scholar]
- 32.Cuthbert JP, et al. , The reliability of a computer-assisted telephone interview version of the Ohio State University Traumatic Brain Injury Identification Method. The Journal of head trauma rehabilitation, 2016. 31(1): p. E36–E42. [DOI] [PubMed] [Google Scholar]
- 33.Setnik L and Bazarian JJ, The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Inj, 2007. 21(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 34.Common Data Elements: Traumatic Brain Injury. 2017, National Institute of Neurological Disorders and Stroke: Bethesda, Maryland. [Google Scholar]
- 35.Brown RT, et al. , Functional impairment and decline in middle age: a cohort study. 2017. 167(11): p. 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RT, et al. , Association of Functional Impairment in Middle Age With Hospitalization, Nursing Home Admission, and Death. 2019. [DOI] [PMC free article] [PubMed]
- 37.Jenkins CD, et al. , A scale for the estimation of sleep problems in clinical research. Journal of clinical epidemiology, 1988. 41(4): p. 313–321. [DOI] [PubMed] [Google Scholar]
- 38.Min Y, Nadpara PA, and Slattum PW, The Association between Sleep Problems, Sleep Medication Use, and Falls in Community-Dwelling Older Adults: Results from the Health and Retirement Study 2010. Journal of Aging Research, 2016. 2016: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turvey CL, Wallace RB, and Herzog R, A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics, 1999. 11(2): p. 139–148. [DOI] [PubMed] [Google Scholar]
- 40.IMBCorp., IBM SPSS Statistics for Windows, Version 24.0. 2016, IBM Corp.: Armonk, NY. [Google Scholar]
- 41.Gill TM, Williams CS, and Tinetti ME, Assessing risk for the onset of functional dependence among older adults: the role of physical performance. Journal of the American Geriatrics Society, 1995. 43(6): p. 603–609. [DOI] [PubMed] [Google Scholar]
- 42.Salibian-Barrera M and Zamar RH, Bootrapping robust estimates of regression. The Annals of Statistics, 2002. 30(2): p. 556–582. [Google Scholar]
- 43.Zaloshnja E, et al. , Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil, 2008. 23(6): p. 394–400. [DOI] [PubMed] [Google Scholar]
- 44.Brown AW, et al. , A survey of very-long-term outcomes after traumatic brain injury among members of a population-based incident cohort. Journal of neurotrauma, 2011. 28(2): p. 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jourdan C, et al. , Disability and health consequences of traumatic brain injury: national prevalence. American journal of physical medicine & rehabilitation, 2018. 97(5): p. 323–331. [DOI] [PubMed] [Google Scholar]
- 46.Ilie G, et al. , Associations between self-reported lifetime history of traumatic brain injuries and current disability assessment in a population sample of Canadian adults. PloS one, 2018. 13(1): p. e0188908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ilie G, et al. , Associations between a history of traumatic brain injuries and current cigarette smoking, substance use, and elevated psychological distress in a population sample of Canadian adults. Journal of neurotrauma, 2015. 32(14): p. 1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osborn A, et al. , Anxiety and comorbid depression following traumatic brain injury in a community-based sample of young, middle-aged and older adults. Journal of affective disorders, 2017. 213: p. 214–221. [DOI] [PubMed] [Google Scholar]
- 49.Dijkers M, et al. , Inpatient rehabilitation for traumatic brain injury: the influence of age on treatments and outcomes. NeuroRehabilitation, 2013. 32(2): p. 233–252. [DOI] [PubMed] [Google Scholar]
- 50.Graham JE, et al. , Influence of sex and age on inpatient rehabilitation outcomes among older adults with traumatic brain injury. Archives of physical medicine and rehabilitation, 2010. 91(1): p. 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang P-FJ, et al. , Ethnic differences in discharge destination among older patients with traumatic brain injury. Archives of physical medicine and rehabilitation, 2008. 89(2): p. 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frankel JE, et al. , A follow-up study of older adults with traumatic brain injury: taking into account decreasing length of stay. Archives of physical medicine and rehabilitation, 2006. 87(1): p. 57–62. [DOI] [PubMed] [Google Scholar]
- 53.Susman M, et al. , Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. Journal of Trauma and Acute Care Surgery, 2002. 53(2): p. 219–224. [DOI] [PubMed] [Google Scholar]
- 54.Cifu DX, et al. , Functional outcomes of older adults with traumatic brain injury: a prospective, multicenter analysis. Archives of physical medicine and rehabilitation, 1996. 77(9): p. 883–888. [DOI] [PubMed] [Google Scholar]
- 55.Yi A and Dams-O’Connor K, Psychosocial functioning in older adults with traumatic brain injury. NeuroRehabilitation, 2013. 32(2): p. 267–273. [DOI] [PubMed] [Google Scholar]
- 56.Menzel JC, Depression in the elderly after traumatic brain injury: a systematic review. Brain Injury, 2008. 22(5): p. 375–380. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein FC, et al. , Cognitive and neurobehavioral functioning after mild versus moderate traumatic brain injury in older adults. J Int Neuropsychol Soc, 2001. 7(3): p. 373–83. [DOI] [PubMed] [Google Scholar]
- 58.Reid MC, Eccleston C, and Pillemer K, Management of chronic pain in older adults. bmj, 2015. 350: p. h532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seifert TD and Evans RW, Posttraumatic headache: a review. Current pain and headache reports, 2010. 14(4): p. 292–298. [DOI] [PubMed] [Google Scholar]
- 60.Stubbs B, et al. , Pain and the risk for falls in community-dwelling older adults: systematic review and meta-analysis. Archives of physical medicine and rehabilitation, 2014. 95(1): p. 175–187. e9. [DOI] [PubMed] [Google Scholar]
- 61.Shega JW, et al. , Relationship Between Persistent Pain and 5‐Year Mortality: A Population‐Based Prospective Cohort Study. Journal of the American Geriatrics Society, 2013. 61(12): p. 2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdulla A, et al. , Guidance on the management of pain in older people. Age and ageing, 2013. 42: p. i1–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.