Abstract
Objective:
To evaluate the impact of physical, mental, and total health condition burden on functional outcome and life satisfaction up to 10 years after moderate-to-severe traumatic brain injury (TBI).
Setting:
Six TBI Model Systems centers.
Participants:
393 participants in the TBI Model Systems National Database.
Design:
Retrospective cohort study.
Main Measures:
Self-reported physical and mental health conditions at 10-years post-injury. Functional Independence Measure Motor and Cognitive subscales and the Satisfaction with Life Scale measured at 1, 2, 5, and 10 years.
Results:
In 10-year longitudinal individual growth curve models adjusted for covariates and inverse probability weighted to account for selection bias, greater physical and mental health co-morbidity burden were negatively associated with functional cognition and life satisfaction trajectories. Physical, but not mental, co-morbidity burden was negatively associated with functional motor trajectories. Higher total health burden was associated with poorer functional motor and cognitive trajectories and lower life satisfaction.
Conclusions:
This study offers evidence that co-morbidity burden negatively impacts longitudinal functional and life satisfaction outcomes after TBI. The findings suggest better identification and treatment of co-morbidities may benefit life satisfaction, functional outcome, reduced healthcare costs, and decreased re-injury. Specific guidelines are needed for the management of co-morbidities in TBI populations.
INTRODUCTION
Recognition of moderate-severe traumatic brain injury (TBI) as a chronic health condition1,2 has sparked interest in identifying factors that may contribute to change over time, particularly deterioration. Corrigan and colleagues3 found that more than half of individuals who receive inpatient rehabilitation for TBI will deteriorate or die during the time from rehabilitation discharge to 5 years post-injury. Mortality studies have found a variety of conditions associated with excess mortality,4 which has inspired investigation into how the presence of co-morbidities, or the concurrent medical conditions a patient has with TBI, may affect trajectories of recovery. Indeed, studies of older adults with TBI have consistently shown that the presence of co-morbid conditions is a substantial negative prognostic indicator.5,6
Several studies have found that co-morbid conditions, which include both pre-existing and new onset conditions, are quite common among individuals who seek medical care for TBI. A population-based study of older adults treated for a TBI in an Emergency Department (ED) found a majority suffered from at least two co-morbid conditions and almost one-third had three or more.7 Kumar and colleagues8 identified three clusters of conditions that most often co-occurred among adults over 50 who received rehabilitation for TBI characterized as: acute medical complications, chronic conditions, and substance use disorders.8 Another study of older adults who had received acute rehabilitation for TBI found those with fewer co-morbid conditions were more independent two years post-injury.6
Co-morbidity indices like the Elixhauser9 and the Charlson10 were designed to combine multiple co-morbidities into a single dimension predicting risk for healthcare utilization and/or mortality. These indices were developed for the general population of hospital inpatients and have been most widely used for predicting death. Their utility in TBI is unclear. At least one study of older adults with TBI admitted to a trauma service did not find the Elixhauser Index improved prediction of either mortality or discharge functional independence.5 An alternative method of quantifying chronic disease burden that has been widely used in clinical research involves calculating a simple sum score of health conditions,11–13 but this approach has not yet been applied to the study of TBI outcomes.
A recent TBI Model Systems (TBIMS) study14 examined the impact of individual co-morbid conditions independently on the trajectory of functional independence and life satisfaction. Among all prevalent co-morbidities (pre-existing and new onset conditions), those conditions present in at least 5% of the cohort that were associated with motor function were chronic heart conditions, asthma, cataracts, and depression.14 The trajectory of recovery for cognitive function was also altered by chronic heart conditions, asthma, and depression; but also included back pain, generalized anxiety disorder, and PTSD.14 Life satisfaction was associated with a host of physical and behavioral health conditions; specifically, rheumatoid arthritis, sleep disorders, fractures, back pain, alcoholism, generalized anxiety disorder, panic attacks, PTSD, depression, and bipolar disorder.14 In all cases, regardless the outcome being modeled, the presence of the co-morbidity was associated with worse outcome.
Converging evidence across multiple TBI studies suggests individual physical and mental health conditions negatively impact recovery. However, there remains little knowledge of the cumulative burden of co-morbidities among patients with TBI. Because health conditions do not occur in isolation, it is necessary to elucidate how the cumulative effects of diseases, including pre-existing and incident conditions, affect trajectories of recovery from TBI.
Evaluating long-term recovery of patients with TBI does require certain methodological considerations. Longitudinal TBI studies evaluating co-morbidities14–17 have not accounted for cohort selection, which could bias estimates because the unhealthiest participants are also generally the most likely to be deceased or lost to follow-up and excluded from longitudinal models. Widely used and well-validated epidemiological methods exist to address the selection and survival bias that is intrinsic in observational longitudinal research.18
The present study builds upon two prior TBIMS studies14,17 and aimed to examine the associations of physical, mental, and total health burden with longitudinal trajectories of motor, cognitive, and life satisfaction over the first 10 years following TBI in a sample of adults weighted to account for selection bias. We developed an interactive tool that dynamically plots longitudinal functional and life satisfaction trajectories after TBI based on individual demographic and injury variables, and physical, mental, and total health burden.
METHODS
Participants
Data for this project were gathered through a modular project of the TBIMS National Database, a multicenter longitudinal prospective cohort study. Criteria for inclusion in the TBIMS as follows: 16+ years old at time of injury; moderate or severe TBI [post-traumatic amnesia greater than 24 hours, intracranial neuroimaging abnormalities on computed tomography scan, loss of consciousness > 30 minutes, or Glasgow Coma Scale score in the ED < 13]. Participants presented to an acute care hospital within 72 hours of injury, and received inpatient rehabilitation at a designated TBIMS facility. Individuals were eligible for the current study if they came due for a 10 year follow-up interview between July 2013 and September 2017. For longitudinal modeling, participants were required to have non-missing outcome data for at least three follow-up time points. Two prior studies have used data from this TBIMS modular study.14,17 Each participating center obtained local Institutional Review Board approval.
Measures
Physical and Mental Health Conditions
We collected health information from participants or informants at 10-years post-injury. Specifically, we queried respondents about 27 physical health conditions from the National Health and Nutrition Examination Survey,19 and 17 mental health conditions from the National Co-morbidity Survey Replication.20 All questions were posed with the anchor, “Has a doctor ever told you that you had…”.
Covariates
Covariates in the primary analysis included sex, race/ethnicity, marital status, education, employment status, age at time of injury, time to first follow commands, discharge FIM™ Motor and Cognitive subscales, and rehabilitation length of stay (LOS). The covariates selected were designed to build upon the models presented in previous study by Malec and colleages.14 We grouped covariates to be consistent with prior studies using this dataset.14,17 Missing data for included covariates were low (<4%). We considered additional covariates to adjust for selection bias due to loss to follow-up.
Outcomes
The outcome measures of this study were the FIM21 Motor and Cognitive subscales and Satisfaction with Life Scale (SWLS).22 The FIM Motor measures functional motor capabilities, with scores ranging from 13-91. The FIM Cognitive measures functional ability in areas of comprehension, expression, social interaction, problem solving and memory, with scores ranging from 5-35. The SWLS is a 5-item scale designed to measure subjective report of one’s life satisfaction with scores ranging from 5-35.22 The SWLS required completion by only the person with TBI, and as a result, the number of cases with SWLS data is lower than other outcomes that could be completed by proxy. For all 3 measures, higher scores reflect better outcomes. The outcomes were gathered at 1, 2, 5, and 10 years post-injury.
Statistical Analysis
Sample characterization
Consistent with Malec et al.14, physical and mental health conditions with low prevalence (<10 subjects) were excluded, resulting in 28 health conditions (18 physical and 10 mental) for analyses (see Supplemental Table 1). We created physical, mental, and total health burden scores for each participant by summing the total number of conditions reported. We did not calculate burden scores for individuals with missing data on at least one health condition. As in prior studies,23,24 we defined “multimorbidity” as ever having two or more health conditions (excluding TBI) by 10 year post-TBI. We compared demographic and clinical characteristics by multimorbidity (0-1 vs. at least two) using chi-square tests for categorical characteristics, two sample t-tests for normally distributed continuous characteristics, and Wilcoxon rank-sum tests for markedly skewed continuous characteristics.
Individual Growth Curve (IGC) Longitudinal Models
We used IGC models to assess the relationship between each health burden score (physical, mental, and total) and the longitudinal trajectories of each of the three outcomes (FIM Motor, FIM Cognitive, SWLS) over time from 1-10 years post-injury. Previous work utilizing these data (Malec et al.)14 described quadratic relationships between FIM (Motor and Cognitive) and time, and a linear relationship between SWLS and time as the best functional form for the IGC trajectories. These relationships were critically assessed for this analysis and found to be appropriate. The health burden scores were modeled as continuous variables. The trajectories varied as a function of health burden score by including interaction effects between the intercept, linear, and quadratic growth parameters and health burden score. Within each model, a “joint” hypothesis test of the intercept and slopes tested if the “average” quadratic (or linear) trajectories varied as a function of the health burden score.
Application of Inverse Probability Weighting (IPW) for Selection Bias
Because selection into the analytic cohort required participants to survive to 10-years post-TBI and have at least three follow-up interviews, there exists potential for bias among individuals systematically selected out. To account for potential bias, we used IPW,18 a methodology that weights individuals based on the inverse of the predicted probability of inclusion into a cohort. The result of IPW is a pseudo-population that is balanced on relevant baseline demographic and clinical variables associated with inclusion.
Prediction of inclusion was based upon all individuals enrolled into the TBI-MS during the same window of injury dates and from the same six study sites. To identify relevant variables, we first descriptively compared individuals in the analytic cohort, deceased individuals, and 10-year survivors selected out of the analytic cohort for any reason. Covariates associated (p<0.1) with inclusion were considered in the IPW models. We performed two different multiple logistic regression models (with and without mortality group) predicting likelihood of inclusion. We used IPWs to calculate two weighted estimates of the IGC models. Because SWLS could only be completed by participants with TBI, we calculated separate IPWs for SWLS completion.
IGC Interactive Tool
To aid in the interpretation of this work, we developed an interactive tool based on our fitted IGC models. This tool allows stakeholders the opportunity to model individualized longitudinal trajectories real-time, based on patient demographics, injury characteristics, and physical, mental, and total health burden. For illustration purposes, we plotted hypothetical trajectories comparing the 99th, 90th, 75th, and 25th percentile of the total health burden score for three unique cases. We set demographic and clinical covariates to different clinically-relevant values for the three cases. All analyses were conducted using SAS v.9.425 assuming a 5% level of significance unless otherwise noted.
RESULTS
Sample characterization
The flow diagram for the derivation of our analytic cohort is shown in Figure 1. There were 404 individuals administered the health questionnaire, but 11 had missing data on at least one medical condition and were excluded from analyses. Therefore, the final analytic cohort includes 393 individuals. We characterized demographic and clinical variables by multimorbidity (Table 1). Multimorbidity rate in our cohort of 10-year survivors with TBI was 69.5%. Individuals with multimorbidity were on average older and more likely to be white and less likely to be Hispanic. There were no significant differences in injury characteristics by multimorbidity. Distributions of the physical, mental, and total health burden scores are provided in Figures 2a–c. The median physical health score was 2 (25th-75th percentile: 1-3). The median mental health score was 1 (25th-75th percentile: 0-2). The median total health score was 3 (25th-75th percentile: 1-5).
Figure 1:
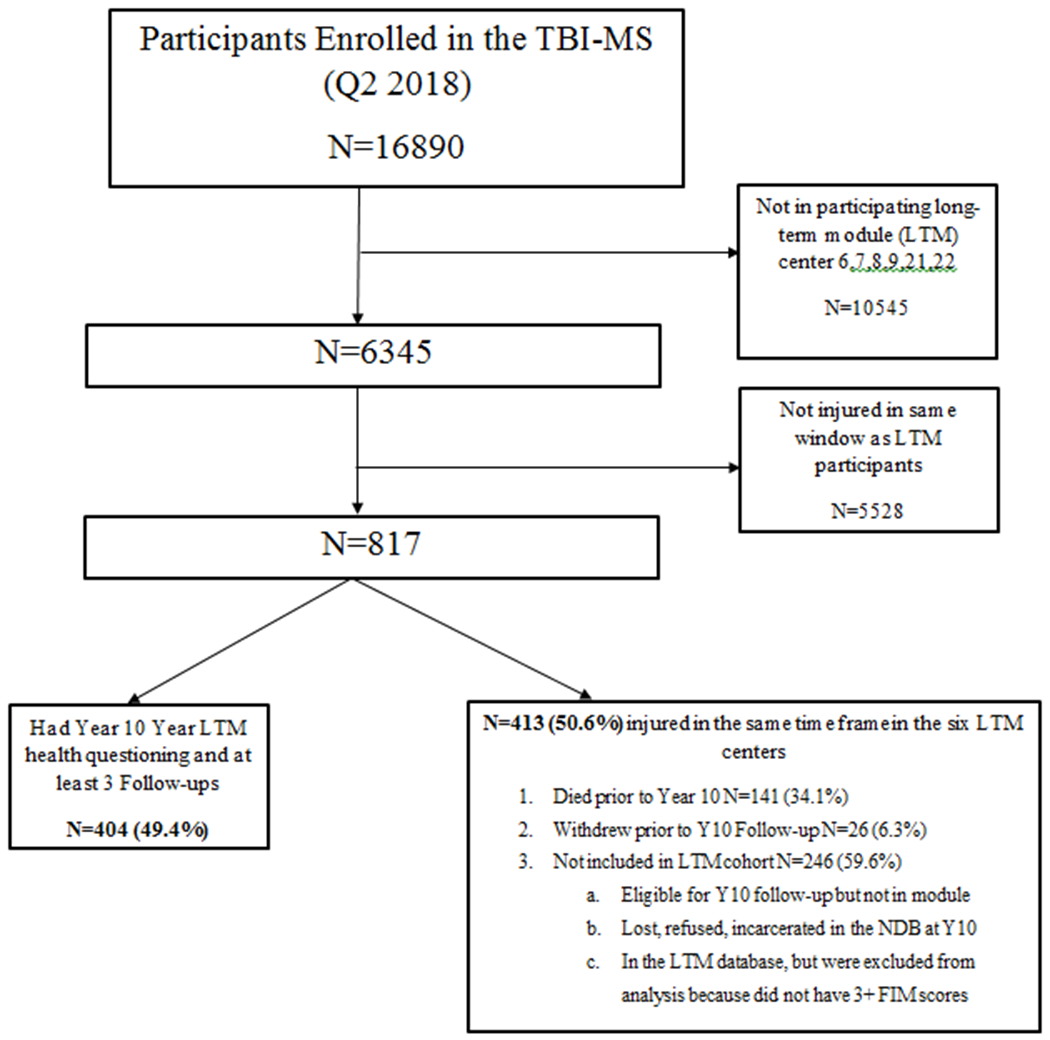
Flow Diagram of Participants in Analytic Sample
Table 1.
Demographic and Clinical Variables by Multimorbidity¥ Status
| Variable | No Multimorbidity (N=120) | Multimorbidity (N=273) | p-value |
|---|---|---|---|
| Age, Mean (SD) | 32.3 (13.7) | 40.0 (16.1) | <0.001* |
| Male Sex, Count (%) | 97 (80.8) | 202 (74.0) | 0.143 |
| Race, Count (%) | <0.001* | ||
| White | 70 (58.3) | 204 (74.7) | |
| Black | 23 (19.2) | 53 (19.4) | |
| Hispanic | 19 (15.8) | 11 (4.0) | |
| Other | 8 (6.7) | 5 (1.8) | |
| Marital Status, Count (%) | 0.159 | ||
| Married | 36 (30.0) | 102 (37.4) | |
| Not Married | 84 (70.0) | 171 (62.6) | |
| Education, Count (%) | 0.824 | ||
| Less than High School | 43 (35.8) | 90 (33.0) | |
| High School or GED | 34 (28.3) | 72 (26.4) | |
| Some College | 24 (20.0) | 65 (23.8) | |
| College or Higher | 19 (15.8) | 46 (16.9) | |
| Employment, Count (%) | 0.086 | ||
| Employed | 90 (75.0) | 181 (66.3) | |
| Not employed | 30 (25.0) | 92 (33.7) | |
| FIM™ Motor at Rehab Discharge, Mean (SD) | 70.4 (17.4) | 68.7 (18.3) | 0.379 |
| FIM™ Cognitive at Rehab Discharge, Mean (SD) | 23.1 (6.4) | 23.7 (6.6) | 0.354 |
| Length of stay in rehabilitation, Mean (SD) | 21 (14–33) | 21 (13–35) | 0.949 |
| Days to Follow Commands, Median (IQR) | 3 (1–14) | 2 (0.5–12) | 0.231 |
Multimorbidity is defined as having ≥ 2 health conditions, not including TBI; No Multimorbidity is 0 or 1 health condition, not including TBI; SD = standard deviation;
indicates statistically significant (α=0.05)
Figure 2A-C:
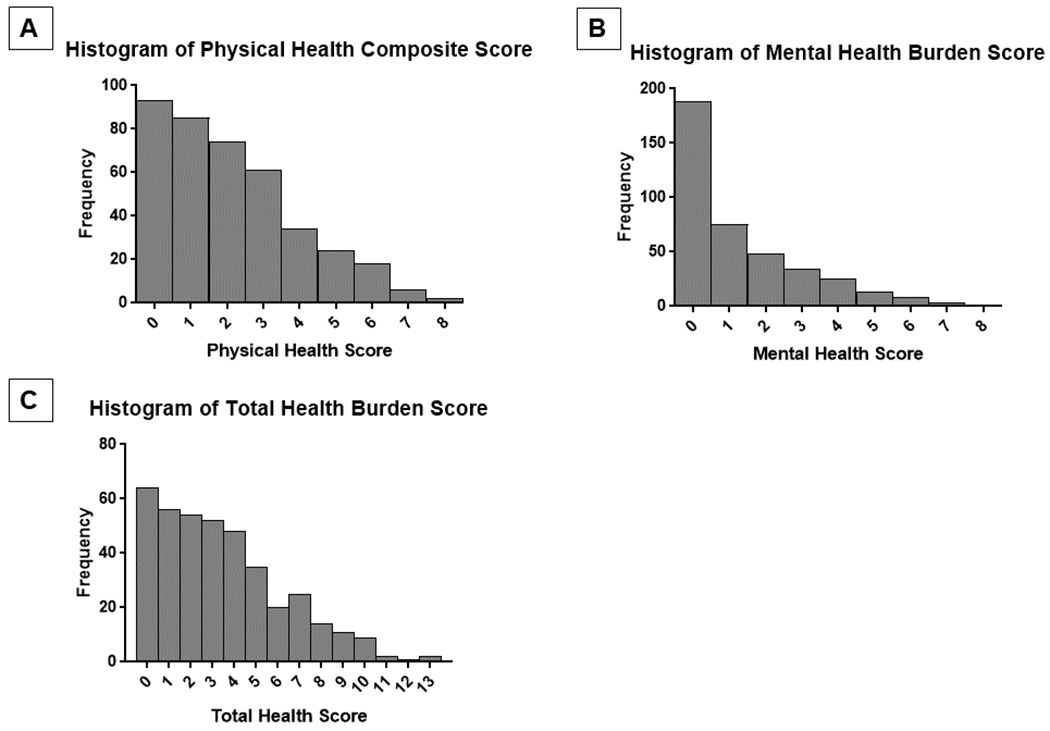
The histogram for physical, mental, and total health burden in plots 2a-c, respectively. The median (IQR) for the three health scores were as follows: A) Physical health score, Median=2 (IQR: 1,3); Mental health score, Median=1 (IQR: 0, 2); Total health score, Median=3 (IQR: 1, 5).
IGC Longitudinal Models
The results of the joint tests of interaction for the IGC models are presented in Table 2. Mean differences (75th vs. 25th percentile health score) in outcomes by follow-up period are presented in Supplemental Table 2.
Table 2:
Joint Test of Interactions between Time Parameters (Intercept and Slopes) and Health Burden Scores
| FIM Motor | FIM Cognitive | SWLS┼ | ||
|---|---|---|---|---|
| Health Burden Score | p-value | p-value | p-value | |
| Unweighted | Physical | 0.0197 | 0.0275 | 0.0266 |
| Mental | 0.1656 | < 0.0001 | < 0.0001 | |
| Total | 0.0469 | < 0.0001 | < 0.0001 | |
| Weighted (including mortality group) | Physical | 0.0173 | 0.0309 | 0.0225 |
| Mental | 0.2133 | < 0.0001 | < 0.0001 | |
| Total | 0.0552 | < 0.0001 | < 0.0001 | |
| Weighted (excluding mortality group) | Physical | 0.0197 | 0.0172 | 0.0243 |
| Mental | 0.1510 | < 0.0001 | < 0.0001 | |
| Total | 0.0485 | < 0.0001 | < 0.0001 | |
A separate IPW was calculated for the SWLS outcome, as that was a reduced cohort relative to FIM Motor and Cognitive
FIM Motor.
A quadratic trajectory was fit for FIM Motor models per previously reported model selection process.14 The joint test of the interaction (unweighted) between health burden score and the set of time parameters (intercept, linear slope, and quadratic slope) was statistically significant for the physical (p = 0.0197) and total (p = 0.0469) health burden scores, but was not significant for mental health burden (p = 0.1656). That is, the shape of the FIM Motor trajectory varied significantly as a function of the physical and total health burden, but not across mental health burden. In general, for those with low health burden scores, the FIM Motor trajectory had a concave downward shape, with FIM Motor scores improving between 1 and 5 years then declining somewhat by year 10, still remaining higher than at year 1. However, for those with higher health burden scores, FIM Motor scores decreased over time, with the greatest decline occurring 5-10 years post injury.
FIM Cognitive.
A quadratic trajectory was fit for all FIM Cognitive models. The joint test of the interaction (unweighted) between health burden score and the set of time parameters (intercept, linear slope, and quadratic slope) was statistically significant for the physical (p = 0.0275), mental (p < 0.0001), and total (p < 0.0001) health burden scores. That is, the shape of the FIM Cognitive trajectory varied as a function of the health burden scores. In general, for those with low health burden scores, the FIM Cognitive trajectory had a concave downward shape, with FIM Cognitive scores slightly improving between 1 and 5 years then declining to levels similar to year 1. However, for those with higher health burden scores, FIM Cognitive scores were lower at year 1 and declined over time. In particular, those with the highest mental and total health burden scores showed a more rapid decline during the first 5 years post-injury than 5-10 years post-injury.
SWLS.
SWLS data were available for only 326 of 393 subjects (83.0%) in this cohort. A linear trajectory was fit for all SWLS models. The joint test of the interaction (unweighted) between health burden score and the set of time parameters (intercept and linear slope) was statistically significant for the physical (p = 0.0266), mental (p < 0.0001), and total (p < 0.0001) health burden scores. That is, the shape of the SWLS trajectory varied significantly as a function of health burden score, primarily in terms of the intercept. In general, SWLS trajectory was constant over time for those with low health burden scores, but as health burden scores increased, SWLS scores were lower initially and declined slightly over time.
Application of IPW for Selection Bias
Of 817 individuals potentially eligible for inclusion, 404 (49.4%) were part of the cohort, 141 were deceased prior to year 10, and 272 survived but were selected out for any of the following reasons: withdrawal, lost to follow-up, or <3 follow-up visits. Demographic and clinical factors associated with these groups are provided in Supplemental Table 3. We constructed two IPW weights (with and without mortality group). The first IPW model (including mortality group) adjusted for age, race, marital status, rehabilitation payor, employment status, education, residence after discharge, and FIM Motor at rehabilitation discharge. The second IPW (not including mortality group) adjusted for race, marital status, rehabilitation payor, employment status, education, residence after discharge, FIM Cognitive and Motor at rehabilitation discharge, and DRS at rehabilitation discharge. After IPW adjustment, the estimates from the joint tests of interactions differed modestly (Table 2).
Interactive Tool and Case Examples
We developed an interactive tool using the fitted IGC models that can be used to plot longitudinal trajectories to understand the relationships between health burden and outcomes across various combinations of subject characteristics (see Supplemental Digital Content). We provide an illustration of the tool by plotting divergent longitudinal trajectories (weighted models excluding mortality group) among three hypothetical cases with four scenarios for health burden: one, five, seven, and 11 total health conditions (see Figures 3a–c).
Figure 3:
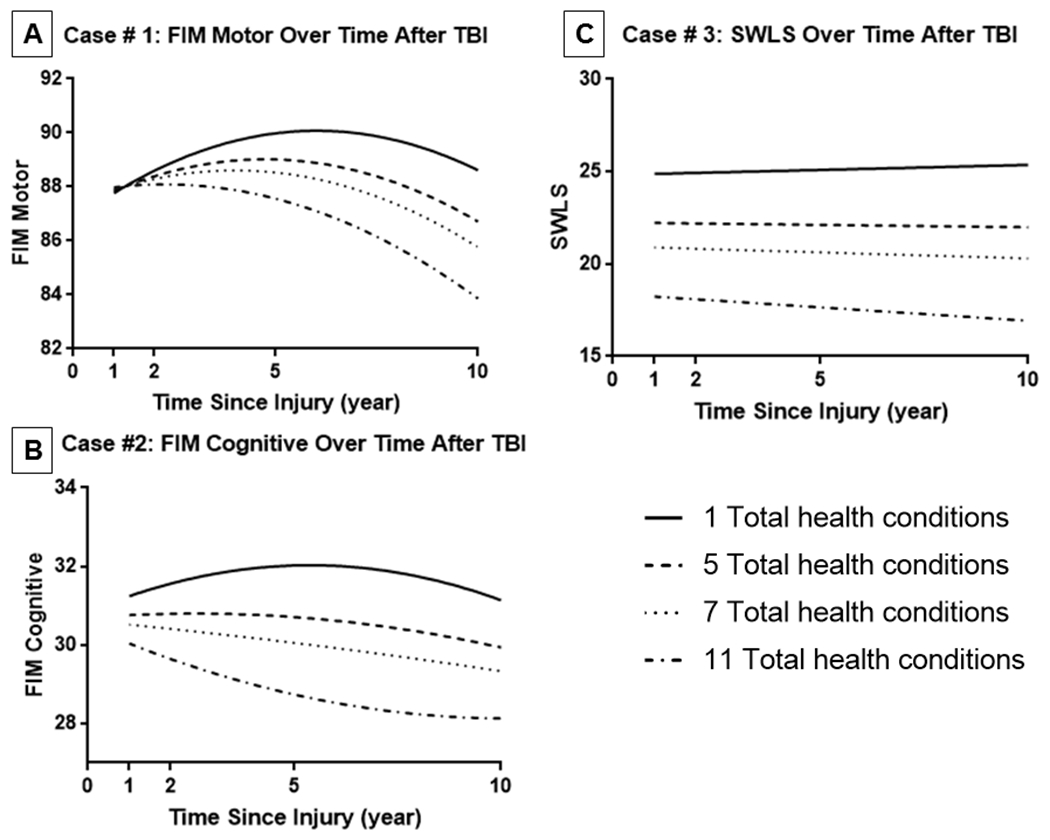
The following plots represent trajectories for three exemplar cases that exhibited the following demographic and clinical characteristics. A) Case 1: 37 year old, male, white race, not married, less than HS education, employed, 3 days to follow commands, 21 day rehab LOS, 72 FIM Motor rehab discharge, 25 FIM Cognitive at rehab discharge; B) Case 2: 55 year old, black female, married, less than HS education, not employed, 8 days to follow commands, 80 FIM motor, 30 FIM cognitive, 18 day rehab LOS; C) Case 3: 65 year old, Hispanic female, not married, less than HS education, employed, 12 days to follow commands, 78 FIM motor at rehab discharge, 28 FIM cognitive at rehab discharge, 20 day Rehab LOS. We plot 10-year trajectories in four counterfactual scenarios for a given case for three different outcomes (1, 5, 7, and 11 total health conditions).
Case #1 provides an illustration of FIM Motor trajectories for an average 37 year old white male. In plotting four hypothetical conditions of total health burden, we observed similar functional motor performance 1 year after injury, but under hypothetical conditions of very high total health burden (seven or 11 health conditions) there was considerable decline in FIM Motor from years 2-10. For case #2, a 55 year old black female, we determined FIM Cognitive roughly follows a concave down trajectory under a hypothetical scenario of one total health condition, but the opposite, a concave up shape under a hypothetical extreme scenario of 11 total health conditions. For case #3, a 65 year old Hispanic female, SWLS varied at 1 year post injury under different hypothetical total health burden scenarios, with greater health burden consistently corresponding to lower satisfaction. Life satisfaction remained mostly constant over time, with the exception of a slightly declining linear slope over time in a hypothetical extreme scenario of 11 total health conditions.
DISCUSSION
Using a sample of 10-year survivors that received acute inpatient rehabilitation for TBI, we found multimorbidity (2+ conditions not including TBI) occurred in nearly 70% of individuals. We found that greater physical health co-morbidity burden was negatively related to long-term functional motor, cognitive, and life satisfaction after TBI. Mental health co-morbidity was associated with functional cognition and life satisfaction trajectories. Health burden as measured in this study represents the accumulation of pre-injury and post-injury health conditions; therefore, current findings suggest that, regardless of its causal link to TBI, co-morbidity burden has long-term relevance for recovery from injury.
Several previous TBI studies have documented individual co-morbid conditions that are prevalent following TBI and that impact recovery from injury.17,26 Here, we characterized burden from multiple co-morbidities, which may better reflect real-world conditions. In practice, diseases rarely occur in isolation and are often co-occurring and may have common underlying causes. Physical and mental health conditions can be mutually exacerbating; that is, they can have interactive effects on health-related quality of life. For example, research in the general population of adults has shown that individuals with physical health conditions, including arthritis, diabetes, and asthma, were more likely to have depression than people without these conditions.27
The effect of health morbidities on functional outcome trajectories after TBI may be particularly significant for the prevention of secondary age-associated conditions. For example, chronic medical and psychiatric health conditions may be a modifiable point of intervention to prevent late-life dementia in TBI populations. Past studies that evaluated dementia risk among individuals with and without TBI, reported higher rates of diabetes, cerebrovascular, and cardiovascular disease among individuals with TBI, suggesting patients with TBI have greater rates of chronic diseases compared to individuals without TBI in the population.28,29 Researchers have long posited that aggressive management of hypertension, cholesterol, and diabetes may lower risk for dementia in late life.30 Others have theorized earlier-life depression may also have a causal link to incident dementia.31 Additionally, previous work from Dams-O’Connor and colleagues32 determined that cerebrovascular disease, depressive symptoms, and functional status predict risk for incident TBI among older adults, suggesting that accumulated health burden, and the associated functional decline, may also increase risk for re-injury in this already-vulnerable population.
There are limitations to this study that warrant consideration. We relied on self-report for measuring health, which requires awareness and memory of one’s conditions and may result in over- or under-reporting. While verification of self-report against medical records was not possible, the TBIMS cohort has shown good-to-excellent test-retest reliability for the health condition questions used in the present study.33 Other studies have found self-report and physician ratings have strong congruence and stability over time, with instances of misclassification in the direction of overestimating health (under-reporting).34 Only a select set of possible conditions were studied, and we did not have detailed information on the type, severity, or treatment. Our method of summing health conditions treated each condition equally and may not reflect the differential impact of individual health problems. We did not have access to administrative diagnosis codes or clinical data that might have allowed us to calculate validated co-morbidity indices.9,10,35 It is likely that disease burden increased over time but this was not considered in the present study because we did not have detailed information on timing of diagnoses. Mortality and attrition accounted for 50% of those otherwise eligible for this study. Our descriptive characterization of individuals not included in the analysis revealed that deceased individuals are systematically different (older age, more often white race, retired, living with other patients/residents/personal care attendants and living in nursing homes pre-injury) than survivors that were selected out for any reason. This descriptive analysis exemplifies issues of selection bias to generalizability of findings in longitudinal TBI research. We have incorporated the use of IPW models to help allay selection concerns in the present study.
The findings of the present study illustrate that, by negatively impacting rehabilitation outcome trajectories, cumulative burden of both physical and mental health conditions may impact domains of life that extend beyond the morbidity of the diseases themselves. This work lays the ground work for future research needed to improve co-morbidity care and outcomes for patients with TBI. Rates of co-morbid conditions need to be systematically evaluated among individuals with TBI and their demographically-similar counterparts in the general population. There also is need for a health burden score that differentially weights individual conditions based on their incremental effects on long-term outcomes from TBI– no such index has been validated in rehabilitation research. Another important consideration is identifying when individual conditions most often occur to better recognize opportunities to prevent accumulation of disease. Models of proactive, coordinated care aimed at decreasing health burden, minimizing polypharmacy, and improving rehabilitation outcome need to be developed and tested in TBI populations.
A majority of 10-year survivors of TBI have multimorbidity, and higher burden of disease is negatively related to long-term functional cognition and life satisfaction. These results highlight an urgent need to enhance education, prevention, surveillance, and treatment interventions that address TBI co-morbidities. The findings suggest that potential benefits of better identification and treatment of co-morbidities among individuals with TBI may include improved life satisfaction, functional outcome, reduced healthcare costs, and decreased re-injury.
Supplementary Material
Acknowledgements:
Disclosures
The contents of this manuscript were developed under grants from the National Institute of Disability, Independent Living, and Rehabilitation Research (NIDILRR) to Icahn School of Medicine at Mount Sinai (90DP0038 and 90DPTB0009), Craig (90DPTB0007), Ohio State (90DPTB0001), Indiana (90DP0036 and 90DRTB0002), and the Traumatic Brain Injury National Data and Statistical Center (90DP0084), and K01 HD074651-01A1 from the National Institute of Child Health and Development (NIH-NICHD). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this publication do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the Federal Government.
Footnotes
Conflict of Interests
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated. We also certify that all financial and material support for this research and work are clearly identified in the title page of the manuscript.
Contributor Information
Raj G. Kumar, Department of Rehabilitation & Human Performance, Icahn School of Medicine at Mount Sinai, New York, NY
Jessica M. Ketchum, Traumatic Brain Injury Model Systems National Data and Statistical Center, Englewood, CO; Research Department, Craig Hospital, Englewood, CO
John D. Corrigan, Department of Physical Medicine and Rehabilitation, Ohio State University, Columbus, OH
Flora M. Hammond, Department of Physical Medicine and Rehabilitation, Indiana University School of Medicine and Rehabilitation Hospital of Indiana, Indianapolis, IN
Mitch Sevigny, Traumatic Brain Injury Model Systems National Data and Statistical Center, Englewood, CO; Research Department, Craig Hospital, Englewood, CO.
Kristen Dams-O’Connor, Department of Rehabilitation Medicine, Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY.
References:
- 1.Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil. 2013;94(6):1199–1201. [DOI] [PubMed] [Google Scholar]
- 2.Masel BE, DeWitt DS. Traumatic Brain Injury: A Disease Process, Not an Event. J Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358 [DOI] [PubMed] [Google Scholar]
- 3.Corrigan JD, Cuthbert JP, Harrison-Felix C, et al. US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J Head Trauma Rehabil. 2014;29(6):E1–E9. [DOI] [PubMed] [Google Scholar]
- 4.Harrison-Felix C, Pretz C, Hammond FM, et al. Life expectancy after inpatient rehabilitation for traumatic brain injury in the United States. J Neurotrauma. 2015;32(23):1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson HJ, Dikmen S, Temkin N. Prevalence of comorbidity and its association with traumatic brain injury and outcomes in older adults. Res Gerontol Nurs. 2012;5(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lecours A, Sirois M-J, Ouellet M-C, Boivin K, Simard J-F. Long-term functional outcome of older adults after a traumatic brain injury. J Head Trauma Rehabil. 2012;27(6):379–390. [DOI] [PubMed] [Google Scholar]
- 7.Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: Characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005;20(3):215–228. [DOI] [PubMed] [Google Scholar]
- 8.Kumar RG, Juengst SB, Wang Z, et al. Epidemiology of Comorbid Conditions Among Adults 50 Years and Older With Traumatic Brain Injury. J Head Trauma Rehabil. 2018;33(1):15–24. doi: 10.1097/HTR.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 11.Friedman EM, Ryff CD. Living Well With Medical Comorbidities: A Biopsychosocial Perspective. J Gerontol Ser B. 2012;67(5):535–544. doi: 10.1093/geronb/gbr152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assari S, Lankarani MM. Chronic Medical Conditions and Negative Affect; Racial Variation in Reciprocal Associations Over Time. Front Psychiatry. 2016;7. doi: 10.3389/fpsyt.2016.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piazza JR, Charles ST, Almeida DM. Living With Chronic Health Conditions: Age Differences in Affective Well-Being. J Gerontol Ser B. 2007;62(6):P313–P321. doi: 10.1093/geronb/62.6.P313 [DOI] [PubMed] [Google Scholar]
- 14.Malec JF, Ketchum JM, Hammond FM, et al. Longitudinal Effects of Medical Comorbidities on Functional Outcome and Life Satisfaction After Traumatic Brain Injury: An Individual Growth Curve Analysis of NIDILRR Traumatic Brain Injury Model System Data. J Head Trauma Rehabil. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54(10):1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar RG, Olsen J, Juengst SB, et al. Comorbid Conditions Among Adults 50 Years and Older With Traumatic Brain Injury: Examining Associations With Demographics, Healthcare Utilization, Institutionalization, and 1-Year Outcomes. J Head Trauma Rehabil. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond FM, Corrigan JD, Ketchum JM, et al. Prevalence of Medical and Psychiatric Comorbidities Following Traumatic Brain Injury. J Head Trauma Rehabil. January 2019. doi: 10.1097/HTR.0000000000000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernán MA, Hernández-Díaz S, Robins JM. A Structural Approach to Selection Bias. Epidemiology. 2004;15(5):615–625. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; https://www.cdc.gov/nchs/nhanes/. Accessed November 8, 2018. [Google Scholar]
- 20.Kessler RC, Merikangas KR. The national comorbidity survey replication (NCS-R): Background and aims. Int J Methods Psychiatr Res. 2004;13(2):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127–132. [PubMed] [Google Scholar]
- 22.Pavot W, Diener E. The satisfaction with life scale and the emerging construct of life satisfaction. J Posit Psychol. 2008;3(2):137–152. [Google Scholar]
- 23.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. The Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 24.Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PloS One. 2014;9(7):e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SAS. Copyright (c) 2002-2012 by SAS Institute Inc, Cary, NC, USA. [Google Scholar]
- 26.Kumar RG, Juengst SB, Wang Z, et al. Epidemiology of comorbid conditions among adults 50 years and older with traumatic brain injury. J Head Trauma Rehabil. 2018;33(1):15–24. [DOI] [PubMed] [Google Scholar]
- 27.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet Lond Engl. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9 [DOI] [PubMed] [Google Scholar]
- 28.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312–319. doi: 10.1212/WNL.0000000000000616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol. 2014;71(12):1490–1497. doi: 10.1001/jamaneurol.2014.2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuller LH, Lopez OL. Cardiovascular disease and dementia risk: An ever growing problem in an aging population. Expert Rev Cardiovasc Ther. 2016;14(7):771–773. doi: 10.1080/14779072.2016.1185366 [DOI] [PubMed] [Google Scholar]
- 31.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dams-O’Connor K, Gibbons LE, Landau A, Larson EB, Crane PK. Health problems precede traumatic brain injury in older adults. J Am Geriatr Soc. 2016. http://onlinelibrary.wiley.com/doi/10.1111/jgs.14014/pdf. Accessed April 19, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogner JA, Whiteneck GG, MacDonald J, et al. Test-retest reliability of traumatic brain injury outcome measures: A traumatic brain injury model systems study. J Head Trauma Rehabil. 2017;32(5):E1–E16. [DOI] [PubMed] [Google Scholar]
- 34.Maddox GL, Douglass EB. Self-assessment of health: A longitudinal study of elderly subjects. J Health Soc Behav. 1973:87–93. [PubMed] [Google Scholar]
- 35.Horn SD, Smout RJ, DeJong G, et al. Association of Various Comorbidity Measures With Spinal Cord Injury Rehabilitation Outcomes. Arch Phys Med Rehabil. 2013;94(4):S75–S86. doi: 10.1016/j.apmr.2012.10.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


