Abstract
Background:
Vasovagal syncope (VVS) significantly reduces quality of life yet lacks effective medical therapies. Pharmacological norepinephrine transporter (NET) inhibition increases synaptic norepinephrine reuptake, which may be able to prevent hypotension, bradycardia, and syncope.
Objective:
The objective of this systematic review was to evaluate the ability of three NET inhibitors – reboxetine, sibutramine, and atomoxetine – to prevent head-up-tilt-induced vasovagal outcomes in healthy participants and patients with VVS.
Methods:
Relevant studies were identified from MEDLINE, EMBASE, CENTRAL, and CINAHL without language restriction from database inception to August 2019. All randomized controlled trials comparing the benefit of NET inhibitor versus placebo in adult populations were selected for review and meta-analysis.
Results:
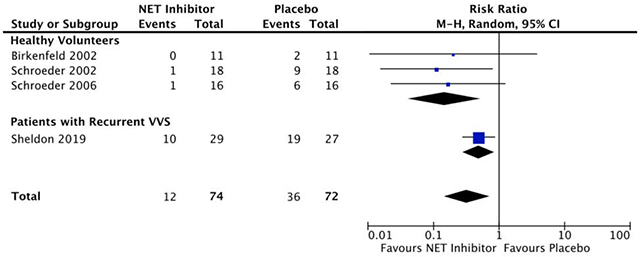
Four studies (101 participants) met inclusion criteria. The mean study size was 25 (range 11-56) participants. NET inhibition reduced the likelihood of vasovagal reactions marked by hypotension and bradycardia in healthy participants on head-up-tilt (Relative Risk (RR)=0.15 [0.04-0.52], p=0.003). This relative risk reduction also occurred in VVS patients on head-up-tilt with atomoxetine (RR=0.49 [0.28-0.86], p=0.01). This was achieved through heart rate compensation with NET inhibition towards the end of tilt testing (106±32bpm vs 60±22bpm, p<0.001), which in turn preserved cardiac output and mean arterial pressure (71±20mmHg vs 43±13mmHg, p<0.001) in the absence of significantly increased systemic vascular resistance.
Conclusion:
NET inhibition prevents severe vasovagal reactions and syncope induced by head-up-tilt testing in both healthy participants and patients with VVS. Pharmacological NET inhibition is a promising potential treatment for recurrent syncope.
Keywords: norepinephrine transporter inhibition, vasovagal syncope, meta-analysis, medication, atomoxetine, reboxetine, sibutramine, tilt table test
Graphical Abstract
INTRODUCTION
Vasovagal syncope is a common clinical condition that occurs at least once in over 35% of the general population and has a high recurrence rate. 1,2 It usually is caused by a fall in cardiac output and blood pressure (BP).1 Recurrent VVS can be a notable burden that is associated with physical and psychological morbidities impairing quality of life.3,4 Few pharmacological agents effectively prevent recurrent VVS, including beta-blockers,5,6 fludrocortisone,7 α1-adrenergic receptor agonists,8–12 and selective serotonin reuptake inhibitors.13,14 They are recommended for the prevention of syncope, despite only modest data supporting their effectiveness.1,15,16
The norepinephrine transporter (NET) protein provides a novel pharmacological target for syncope prevention. It is responsible for removing 25-90% of synaptic norepinephrine (NE),17 which modulates sympathetic activity for BP and heart rate (HR) regulation. The NET protein is a promising target for pharmacological inhibition in an attempt to maintain cardiac output and BP during orthostatic stress.17 Several studies assessed whether NET inhibitors prevented syncope and presyncope, using a variety of populations, methods, and outcomes.18–21 We performed a systematic review and meta-analysis to evaluate the efficacy of selective NET inhibitors for the prevention of severe vasovagal reactions and syncope induced by head-up-tilt (HUT) testing.
METHODS
The protocol for this systematic review was registered in PROSPERO (The International Prospective Register of Systematic Reviews) on August 4, 2019 (PROSPERO number 142650). The details include the search strategy, criteria for study selection, statistical methodology, and risk of bias assessments.
Literature search strategy
Multiple electronic databases were searched without language restriction from database inception to August 2019, including Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE), Cochrane Central Register of Controlled Trials (CENTRAL), and Cumulative Index to Nursing and Allied Health Literature (CINAHL). The Medical Subject Headings (MeSH) terms and keywords were related to vasovagal syncope, vasovagal reactions, and selective norepinephrine transporter inhibitors. Database-specific search terms and results are listed in Supplementary Table 1. Further screenings of Google Scholar, ClinicalTrials.gov, and references from selected articles were performed to identify relevant grey literature.
Eligibility criteria
All studies selected for review and meta-analysis were blinded, randomized crossover trials or randomized controlled parallel-group trials evaluating the benefit of pharmacological NET inhibition (with sibutramine, reboxetine, or atomoxetine) against matching placebo for the prevention of severe vasovagal reactions or syncope during HUT testing. Adult populations both with and without a history of recurrent VVS were included. Studies were required to report a dichotomous outcome of either frank syncope or a predefined syncope surrogate (i.e. severe presyncope with hypotension and bradycardia beyond predefined thresholds) for all participants.
Quality assessment
The risk of bias of each study was evaluated using the Cochrane Collaboration tool for assessing bias in randomized trials.22 The quality of evidence for each outcome was graded using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework.23
Statistical analysis
Meta-analysis was performed using Review Manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The risks of syncope within studies were expressed as relative risk (RR) ratios with 95% confidence intervals. Pooled RR were calculated using random-effects models. Heterogeneity was estimated with the I2 statistic.24 The 95% confidence intervals for I2 values were calculated using the test-based method proposed by Higgins et al.25
RESULTS
Study characteristics
There were 84 unique records identified for title and abstract screening. Ten full-text articles were assessed for eligibility and four studies met inclusion criteria, comprising a total of 101 participants (46% male; 31 ± 5 years; Figure 1). Characteristics of included studies are presented in Table 1. Of the four studies selected for meta-analysis, three assessed the ability of NET inhibition to prevent HUT-induced vasovagal reactions with severe hypotension and bradycardia in healthy volunteers,18–20 while one study directly investigated the efficacy of NET inhibition to prevent HUT-induced syncope in patients with VVS.21
Figure 1.
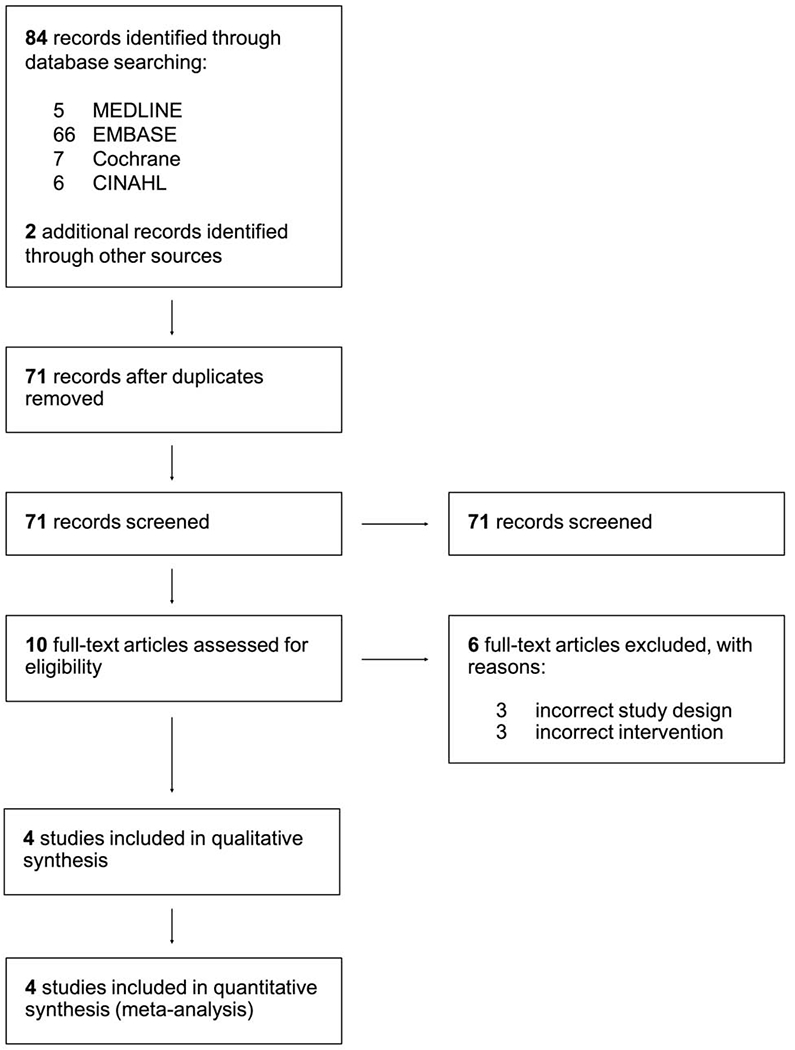
PRISMA flow diagram for systematic literature review and study selection.
Table 1.
Characteristics of included studies.
| Study | Study Design | Study Population | Interventions | Sample Size | Age in Years (Mean ± SD) | Head-up-tilt Protocol | Endpoint |
|---|---|---|---|---|---|---|---|
| Birkenfeld 2002 | Double-blind RCT Crossover | Healthy volunteers |
T: sibutramine 10 mg x2 + 20 mg C: matching placebo |
T: 11 C: 11 |
27 ± 2 | Graded HUT† | Vasovagal reaction with decrease in BP and HR‡ or intolerable symptoms§ |
| Schroeder 2002 | Double-blind RCT Crossover | Healthy volunteers |
T: reboxetine 8 mg x2 C: matching placebo |
T: 18 C: 18 |
30 ± 2 | Graded HUT† | Vasovagal reaction with decrease in BP and HR‡ |
| Schroeder 2006 | Double-blind RCT Crossover | Healthy volunteers |
T: reboxetine 8 mg x1 C: matching placebo |
T: 16 C: 16 |
26 ± 1 | 45° HUT for up to 30 min | Vasovagal reaction with decrease in BP¶ |
| Sheldon 2019 | Double-blind RCT | VVS patients |
T: atomoxetine 40 mg x2 C: matching placebo |
T: 29 C: 27 |
T: 35 ± 14 C: 38 ± 14 |
80° HUT for up to 60 min | Syncope |
Abbreviations: BP – blood pressure, C – control, HUT – head-up-tilt, HR – heart rate, RCT – randomized controlled trial, T – treatment, VVS – vasovagal syncope
Participants were raised to 15°, 30°, 45°, and 60° for 3 minutes each before reaching 75°, where they held for a maximum of 30 minutes
Decrease in blood pressure and heart rate of at least 30 mmHg and 20 bpm, respectively
Dizziness, nausea, or visual disturbances deemed sufficient to prematurely abort head-up-tilt testing by the investigators
Decrease in blood pressure of at least 20 mmHg
Quality of evidence assessments
All trials had low risks of bias across the six domains defined by the Cochrane Collaboration’s tool for assessing bias in randomized trials (Figure 2). There was high-quality evidence supporting each of the population-specific outcomes and moderate evidence for the pooled outcome. The pooled quality of evidence was downgraded due to overall inconsistencies in the way that HUT endpoints were defined across studies, the varied HUT protocols, and the differences in baseline population characteristics (Table 2).
Figure 2.
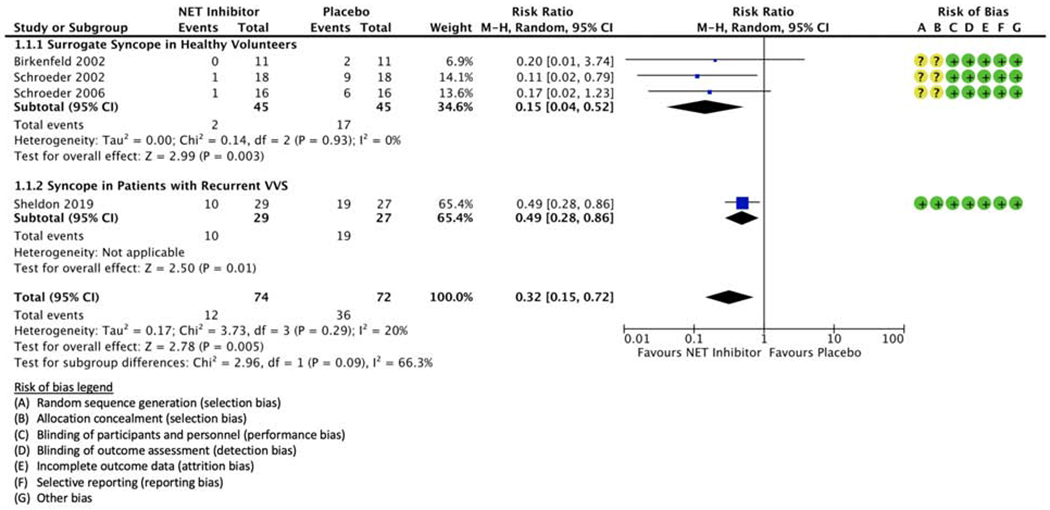
Random-effects model analysis of syncope risk in recurrent vasovagal syncope patients treated with a norepinephrine transporter (NET) inhibitor or matching placebo. Forest plots include risk of bias domains defined by the Cochrane Collaboration’s tool for assessing bias in randomized trials (green ‘+’, low risk of bias; red ‘−’, high risk of bias; yellow ‘?’, unclear risk of bias). Head-up-tilt-induced syncope is reported in both healthy volunteer and vasovagal syncope patient cohorts.
Table 2.
Summary of the GRADE (Grading of Recommendations Assessment, Development and Evaluation) quality of evidence assessment for each the predefined outcomes in both healthy volunteer and vasovagal syncope patient cohorts.
| Quality Assessment | Number of Patients | Relative Risk (95% CI) | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other | NET Inhibition | Control | ||
| Study Population: Pooled (Healthy Volunteers + Vasovagal Syncope Patients) | ||||||||||
| 4 | Double-Blind RCTs | Not serious | Serious | Not serious | Not serious | None | 74 | 72 | RR 0.32 [0.15, 0.72] | ⊕⊕⊕⊕ Moderate† |
| Study Population: Healthy Volunteers | ||||||||||
| 3 | Double-Blind RCTs | Not serious | Not serious | Not serious | Not serious | None | 45 | 45 | RR 0.15 [0.04, 0.52] | ⊕⊕⊕⊕ High |
| Study Population: Vasovagal Syncope Patients | ||||||||||
| 1 | Double-Blind RCTs | Not serious | Not serious | Not serious | Not serious | None | 29 | 27 | RR 0.49 [0.28, 0.86] | ⊕⊕⊕⊕ High |
Abbreviations: CI – confidence interval, RCT – randomized controlled trial, RR – relative risk
Moderate due to inconsistencies in syncope and syncope surrogate (i.e. severe vasovagal reaction with marked decreases in blood pressure and heart rate) outcome definitions, population characteristics, and head-up-tilt protocols
Overall relative risk of syncope with NET inhibition
The population RR with NET blockade was 0.32 [0.15, 0.72] (p = 0.005; Figure 2). The relative benefit of NET inhibition was statistically similar among studies regardless of participant population, as evidenced by the low-to-moderate between-study heterogeneity (I2 = 20% [0%, 66%], p = 0.3; Figure 2).
Relative risk of severe vasovagal reactions with NET inhibition in healthy volunteers
Three double-blind crossover randomized trials reported the prevention of severe HUT-induced vasovagal reactions in healthy volunteers (Table 1). The vasovagal reaction was classified based on the presence of prespecified threshold decreases in hemodynamics on HUT (below and Supplemental Table 2).
Birkenfeld et al. reported 11 participants (64% male, mean age of 27±2 years).18 who ingested sibutramine (10 mg taken 26 hours and 14 hours prior to HUT testing, followed by 20 mg taken 2 hours prior to HUT testing) against matching placebo.18 The specified endpoint was a vasovagal reaction with decreases in BP and HR of at least 30 mmHg and 20 bpm, respectively.18,19 In a graded HUT protocol the participants were raised to 15°, 30°, 45°, and 60° for three minutes each before reaching the final tilt angle at 75°, where they were held for up to 30 minutes or until reaching the prespecified endpoint.18 The RR of presyncope with hypotension and bradycardia was 0.20 [0.01, 3.74] with NET inhibition (Figure 2). Nine of 11 participants experienced side effects with sibutramine, compared to only 2 of 11 with placebo. Six subjects experienced sleeplessness and 3 complained of fatigue.
Schroeder et al. (2002) similar results in 18 healthy participants (44% male, mean age 30±2 years)20 who received reboxetine (8 mg taken 12 hours and 1 hour prior to HUT testing) versus matching placebo.18,20 The RR of presyncope with hypotension and bradycardia was 0.11 [0.02, 0.79] with NET inhibition (Figure 2). Nine of 18 participants complained of sleeplessness, and 6 complained of diaphoresis and piloerection with reboxetine, compared to only 2 of 18 with sleeplessness in the placebo group.
Schroeder et al. reported 16 participants (100% male, mean age of 26 ± 1 year) who underwent a 45° HUT for a maximum of 30 minutes or until reaching the prespecified vasovagal reaction endpoint.19 Individuals were given a single dose of reboxetine (8 mg taken 90 minutes prior to HUT testing) or matching placebo. This endpoint included vasovagal reactions with the formerly described threshold decreases in BP and HR, characterized by a BP decrease of at least 20 mmHg in the absence of bradycardia.19 The RR of vasovagal reactions was 0.17 [0.02, 1.23] with NET inhibition (Figure 2).
There was minimal heterogeneity between sample estimates in the healthy cohort subgroup (I2 = 0%). All three studies independently demonstrated a substantial risk reduction with selective NET inhibition (Figure 2). Due to the limited number of events in Birkenfeld et al. (RR = 0.20 [0.01, 3.74]) and Schroeder et al. (2006) (RR = 0.17 [0.02, 1.23]), there were high degrees of imprecision in the sample estimates, and these two studies only comprised 6.9% and 13.6% of the total effect estimate, respectively. Overall, there was a significantly reduced likelihood of positive HUT test outcomes with pharmacological NET inhibition in healthy volunteers (RR = 0.15 [0.04, 0.52], p = 0.003; Figure 2).
Relative risk of syncope with NET inhibition in patients with recurrent VVS
One double-blind, parallel-group randomized controlled trial investigated HUT-induced syncope occurrence in VVS patients given atomoxetine (40 mg taken 13 hours and 1 hour prior to HUT testing) or matching placebo.21 VVS patients (n = 56; 27% male) were randomized to receive the active drug (n = 29) or placebo (n = 27) treatments, with a mean age of 35 ± 14 years. Patients were subjected to an 80° HUT test for a maximum of 60 minutes or until frank syncope.21 The relative risk of syncope was substantially reduced with NET inhibition (RR = 0.49 [0.28, 0.86], p = 0.01; Figure 2). While the effect size was less in VVS patients compared to healthy participants, the sample estimate remained significant in this group.
Hemodynamic effects with NET inhibition
NET inhibition acted as a pharmacological pacemaker in the pooled study cohort, eliciting significantly elevated HR in the ten seconds preceding HUT abortion (106 ± 32 bpm vs 60 ± 22 bpm, p < 0.001; Figure 3A), which in turn preserved mean arterial pressure (71 ± 20 mmHg vs 43 ± 13 mmHg, p < 0.001; Figure 3B).18–21 One study in VVS patients reported that the preservation of cardiac output and BP during isolated presyncope with recovery versus presyncope with subsequent faint occurred in the absence of significant effects on stroke volume or systemic vascular resistance.21 It is possible that the NET inhibition had a splanchnic venoconstriction effect, in addition to the HR effect, and enhanced cardiac venous return and maintained stroke volume, which might otherwise have decreased with the increased HR.
Figure 3.
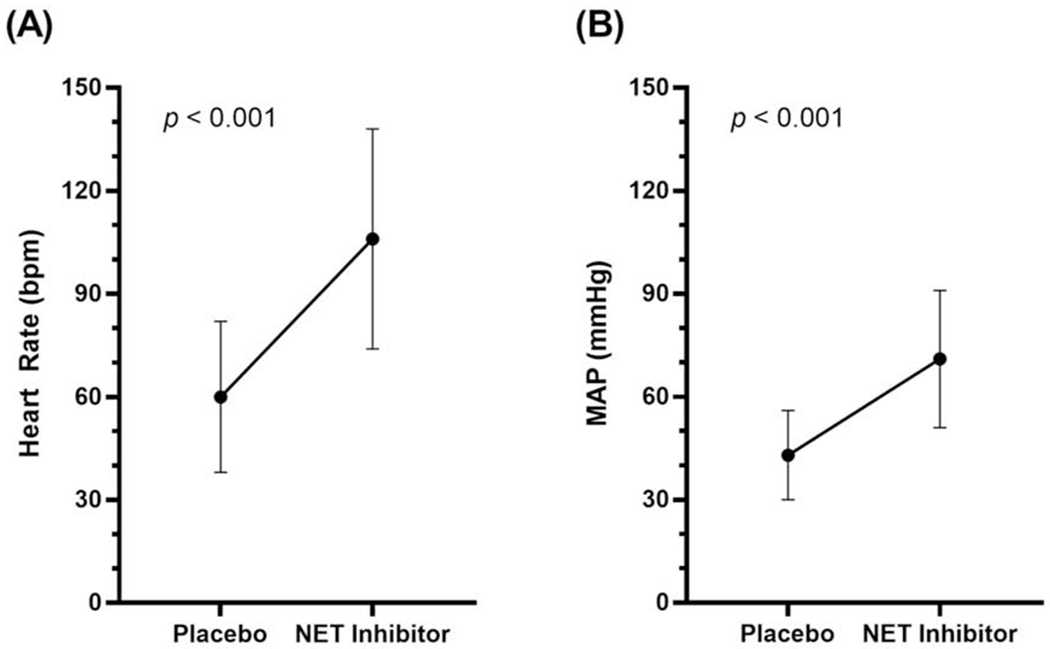
Mean ± SD (A) heart rates and (B) mean arterial pressures immediately prior to the end of head-up-tilt testing in a pooled cohort of healthy volunteers and vasovagal syncope patients.
DISCUSSION
There is moderate quality pooled evidence to suggest that pharmacological NET blockade reduces the risk of HUT-induced outcomes in both healthy volunteers and patients with a clinical history of VVS. Despite differences in the specific NET inhibitor administered, drug dosing, and HUT protocol, there was low statistical heterogeneity among studies. The consistency of the effect estimates across study conditions suggests that NET inhibitors as a class may provide protection against HUT-induced syncope.
The presynaptic NET protein is variably responsible for terminating the biological effects of NE, particularly in the heart where it removes up to 90% of synaptic NE.26 This suggests that inhibition of this transporter could markedly increase NE concentrations in the sinus node, leading to an increase in HR. All four studies reported significantly increased HR on HUT in participants receiving NET inhibitor.18–21
Drugs that inhibit the norepinephrine transporter may have other pharmacologic effects, and their selective potencies should be considered. For example, selective serotonin reuptake inhibitors (SSRIs) have been studied in vasovagal syncope. These drugs inhibit the serotonin transporter (SERT) but have little effect on NET. Serotonin-norepinephrine reuptake inhibitors (SNRIs) inhibit both SERT and NET but have more effects on SERT. For example, duloxetine is a SNRI with a SERT inhibitory constant [Ki] = 0.8 nM and a NET Ki = 7.5 nM. In contrast, the drugs included here are highly selective norepinephrine reuptake inhibitors, which inhibit NET at a lower concentration than is required to inhibit SERT. Reboxetine (NET Ki = 1 nM; SERT Ki = 129 nM) and atomoxetine (NET Ki = 5 nM; SERT Ki = 77 nM) are particularly potent NET inhibitors, while sibutramine (NET Ki = 283 nM; SERT Ki = 1811 nM) is a potent NET inhibitor with slightly more SERT inhibition than the others. For these reasons all three of sibutramine, reboxetine, and atomoxetine merited consideration for treatment of vasovagal syncope, although only atomoxetine is available in North America.
Syncope and presyncope outcomes with NET inhibition
Although there was only low heterogeneity among the sample estimates (I2 = 20%), trials were stratified by methodology for subgroup analysis to account for the large differences in study populations, HUT methodology, and predefined outcomes. Only one study included patients with history of VVS, while the other three contained healthy cohorts. Importantly, trials performed in healthy volunteers utilized surrogate syncope endpoints (i.e. severe vasovagal reactions), while frank syncope was reported in the single study of VVS patients. There was a high rate of vasovagal reactions noted in these healthy volunteers, which speaks to the frequency of vasovagal physiology, especially with the stress of intravenous access and instrumentation. While BP and HR depression reliably precede syncope, the vasovagal reaction may not progress to complete loss of consciousness, thus weakening its validity as a syncope surrogate. Consequently, studies that used hypotension and bradycardia to define their endpoint may have reported an exaggerated benefit of NET inhibition, particularly since HUT testing was aborted as soon as the prespecified hemodynamic criteria were met.
There is also an important distinction between vasovagal reactions defined using hemodynamic thresholds and symptomatic presyncope. Orthostatic symptoms indicative of the vasovagal reflex (for example, dizziness, nausea, and so on) may occur in the absence of marked hypotension and bradycardia, and we found that NET inhibition was not protective against presyncopal symptoms in patients with VVS.21
Adverse effects of NET inhibition
There was a significantly higher rate of adverse effects reported by participants while on sibutramine and reboxetine.18,20 These adverse effects must be weighed against potential benefits when deciding on potential pharmacological therapy for recurrent vasovagal syncope. Importantly, supratherapeutic doses were used in both physiological studies. In the sibutramine study, participants received 30 mg in the 14 hours before the study, while the labelled maximum daily dose was 15 mg.18 In the reboxetine study, participants received 16 mg in the 12 hours before the study, while the recommended maximum daily dose was 8 mg (4 mg BID).20
Clinical implications
The majority of patients with VVS show considerable improvement with physician reassurance, increased salt and fluid intake, and counterpressure maneuvers.27 However, patients with recurrent syncopal episodes refractory to lifestyle measures and conservative non-pharmacological therapies often experience marked reductions in quality of life across all dimensions of health, including significant psychological distress.3 The patients in this report resemble those studied in randomized clinical trials of drug therapy. They are predominantly young, female, and had moderately frequent vasovagal syncope in the preceding year. In this group, most patients improve remarkably with simple teaching and reassurance, and few agree to attempts at medical treatment. Partly this appears to be due to reassurance, partly to having to take medications daily for infrequently sporadic events, and partly due to the side effects of medications. This might include the sympatho-excitatory side effects of drugs such as atomoxetine. Indeed, in the POST trials many patients discontinued participation within a few months. Nonetheless, there is a lack of effective treatment options for recurrent VVS, especially for those with comorbid hypertension.
Currently, neither reboxetine nor sibutramine are available in North America, but atomoxetine is a generic drug used to treat attention deficit disorder. Much earlier we performed an open-label, dose-ranging uncontrolled clinical study of sibutramine in highly symptomatic VVS patients refractory to previous treatment.28 Sibutramine suppressed up to 92% of clinical syncopal episodes during the treatment period in a dose-dependent fashion relative to baseline, and the drug was generally well tolerated.28 Although there were limitations in the design of this small case series, the apparent clinical benefit of sibutramine is promising for the use of NET inhibitors as novel therapeutic agents for the management of recurrent VVS. It is necessary to conduct a formal randomized controlled trial with adequate follow-up to assess the advantages and side effects of a readily available drug of this class.
Importantly, patients with postural orthostatic tachycardia syndrome (POTS), a common form of chronic orthostatic intolerance, often present with recurrent presyncope. POTS is not primarily a syncopal disorder and can be differentiated from VVS by its manifestations of symptomatic orthostatic tachycardia (≥30 bpm within 10 minutes of standing). NET inhibitors should not be prescribed to patients with POTS as they potentiate upright tachycardia and worsen the symptom burden in these patients.29
The apparent effect mainly on HR also bears on the ongoing debate about the efficacy of permanent pacemaker therapy. The ISSUE-3 tilt test substudy suggested that very few patients with vasovagal syncope and profound bradycardia benefit from permanent pacing,30 while the SPAIN study suggests that most of the same patients might benefit.31 NET inhibition might offer support to stroke volume in addition to HR.
Limitations
The validity of the pooled risk reduction estimate is limited by the lack of independent high-quality, blinded controlled trials investigating the benefit of NET inhibition in VVS patients and by the small overall sample size. Turner et al. addressed the issue of sample size in meta analyses and defined an adequate meta-analysis as one with at least two component studies having at least 50% power to detect at least a 30% relative risk reduction.32 Therefore, despite having only four component studies, this analysis was adequately powered. Additionally, although this review demonstrated the ability of NET inhibition to considerably attenuate HUT-induced vasovagal reactions, these results should be interpreted with caution given previous concerns regarding the reproducibility of HUT tests and their ability to predict a patient’s response to pharmacological treatment.15,33,34 In order to evaluate the true clinical benefit of NET inhibitors, it is necessary to conduct a randomized controlled clinical trial comparing NET inhibitor against matching placebo for the prevention of clinical syncope recurrence within a prolonged follow-up period.
The studies did not report side effects such as palpitations and insomnia in the intervention arm, and therefore the degree of true blinding cannot be assessed. Given the apparent effect of placebo on the recurrence rate of vasovagal syncope, this may be an important limitation.27 The applicability of results from healthy subjects to clinical vasovagal syncope requires consideration. This limitation is increased because the studies on healthy personnel did not by design end with frank syncope. However, the studies targeted biological vasodepression, and were not clinical studies of efficacy. The reasons for the high outcome rates in the healthy subjects is unknown, and may reflect either site-specific effects such as dehydration or tilt test methodology,35 or that the subjects had a latent phenotype not yet clinically expressed. Lewis et al. reported that 35% of healthy young subjects had positive tilt tests without pharmacologic intervention, and this was markedly dependent on tilt test angle. This resembles the results of the earlier studies on NET inhibition.
CONCLUSIONS
NET inhibition is very effective at the prevention of HUT-induced syncope and severe vasovagal reactions with marked hypotension and bradycardia in both healthy participants and patients with VVS. Pharmacological NET inhibition holds promise as a novel treatment for syncope. A formal randomized controlled clinical trial is imperative to properly assess the clinical relevance of NET inhibition for the treatment of recurrent syncope.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank John and Leslie Bissett for their partial support of this project. Dr. Satish R Raj and Dr. Robert S Sheldon are investigators for the Cardiac Arrhythmia Network of Canada (CANet) as part of the Networks of Centres of Excellence (NCE).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflicts to disclose.
REFERENCES
- 1.Sheldon RS, Grubb BP II, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Hear Rhythm. 2015;12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, Van Dijk N. Lifetime cumulative incidence of syncope in the general population: A study of 549 Dutch subjects aged 35-60 years. J Cardiovasc Electrophysiol. 2006;17(11):1172–6. [DOI] [PubMed] [Google Scholar]
- 3.Ng J, Sheldon RS, Ritchie D, Raj V, Raj SR. Reduced quality of life and greater psychological distress in vasovagal syncope patients compared to healthy individuals. Pacing Clin Electrophysiol. 2019;42(2):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brignole M, Menozzi C, Bartoletti A, et al. A new management of syncope: Prospective systematic guideline-based evaluation of patients referred urgently to general hospitals. Eur Heart J. 2006;27(1):76–82. [DOI] [PubMed] [Google Scholar]
- 5.Sra JS, Murthy VS, Jazayeri MR, et al. Use of intravenous esmolol to predict efficacy of oral beta-adrenergic blocker therapy in patients with neurocardiogenic syncope. J Am Coll Cardiol. 1992;19(2):402–8. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon R, Connolly S, Rose S, et al. Prevention of Syncope Trial (POST): A Randomized, Placebo-Controlled Study of Metoprolol in the Prevention of Vasovagal Syncope. Circulation. 2006;113(9):1164–70. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon R, Raj SR, Rose MS, et al. Fludrocortisone for the Prevention of Vasovagal Syncope: A Randomized, Placebo-Controlled Trial. J Am Coll Cardiol. 2016;68(1):1–9. [DOI] [PubMed] [Google Scholar]
- 8.Ward CR, Gray JC, Gilroy JJ, Kenny RA. Midodrine: A role in the management of neurocardiogenic syncope. Heart. 1998;79(1):45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romme JJCM, Van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace. 2011;13(11):1639–47. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann H, Saadia D, Voustianiouk A. Midodrine in neurally mediated syncope: A double-blind, randomized, crossover study. Ann Neurol. 2002;52(3):342–5. [DOI] [PubMed] [Google Scholar]
- 11.Qingyou Z, Junbao D, Chaoshu T. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. J Pediatr. 2006;149(6): 777–80. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol. 2001;12(8):935–8. [DOI] [PubMed] [Google Scholar]
- 13.Grubb BP, Wolfe DA, Samoil D, Temesy-Armos P, Hahn H, Elliott L. Usefulness of Fluoxetine Hydrochloride for Prevention of Resistant Upright Tilt Induced Syncope. Pacing Clin Electrophysiol. 1993;16(3):458–64. [DOI] [PubMed] [Google Scholar]
- 14.Di Girolamo E, Iorio C Di, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: A randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1999;33(5):1227–30. [DOI] [PubMed] [Google Scholar]
- 15.Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope. J Am Coll Cardiol. 2017;70(5):e39–110. [DOI] [PubMed] [Google Scholar]
- 16.Brignole M, Moya A, De Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883–948. [DOI] [PubMed] [Google Scholar]
- 17.Tellioglu T, Robertson D. Genetic or acquired deficits in the norepinephrine transporter: Current understanding of clinical implications. Expert Rev Mol Med. 2001;3(29):1–10. [DOI] [PubMed] [Google Scholar]
- 18.Birkenfeld AL, Schroeder C, Boschmann M, et al. Paradoxical effect of sibutramine on autonomic cardiovascular regulation. Circulation. 2002;106(19):2459–65. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder C, Birkenfeld AL, Mayer AF, et al. Norepinephrine Transporter Inhibition Prevents Tilt-Induced Pre-Syncope. J Am Coll Cardiol. 2006;48:516–22. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder C, Tank J, Boschmann M, et al. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105(3):347–53. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon RS, Lei L, Guzman J, et al. A proof of principle study of atomoxetine for the prevention of vasovagal syncope: The prevention of syncope trial VI. Europace. 2019;21(11):1733–41. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein DS, Brush JE, Eisenhofer G, Stull R, Esler M. In Vivo Measurement of Neuronal Uptake of Norepinephrine in the Human Heart. Circulation. 1988;78(1):41–8. [DOI] [PubMed] [Google Scholar]
- 27.Pournazari P, Sahota I, Sheldon R. High Remission Rates in Vasovagal Syncope: Systematic Review and Meta-Analysis of Observational and Randomized Studies. JACC Clin Electrophysiol. 2017;3(4):384–92. [DOI] [PubMed] [Google Scholar]
- 28.Sheldon RS, Ritchie D, McRae M, Raj SR. Norepinephrine Transport Inhibition for Treatment of Vasovagal Syncope. J Cardiovasc Electrophysiol. 2013;24:799–803. [DOI] [PubMed] [Google Scholar]
- 29.Green EA, Raj V, Shibao CA, et al. Effects of norepinephrine reuptake inhibition on postural tachycardia syndrome. J Am Heart Assoc. 2013;2(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brignole M, Donateo P, Tomaino M, et al. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: An analysis from the third international study on syncope of uncertain etiology (ISSUE-3). Circ Arrhythmia Electrophysiol. 2014;7(1):10–6. [DOI] [PubMed] [Google Scholar]
- 31.Baron-Esquivias G, Morillo CA, Moya-Mitjans A, et al. Dual-Chamber Pacing With Closed Loop Stimulation in Recurrent Reflex Vasovagal Syncope: The SPAIN Study. J Am Coll Cardiol. 2017;70(14):1720–8. [DOI] [PubMed] [Google Scholar]
- 32.Turner RM, Bird SM, Higgins JPT. The Impact of Study Size on Meta-analyses: Examination of Underpowered Studies in Cochrane Reviews. PLoS One. 2013;8(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morillo CA, Leitch JW, Yee R, Klein GJ. A placebo-controlled trial of intravenous and oral disopyramide for prevention of neurally mediated syncope induced by head-up tilt. J Am Coll Cardiol. 1993;22(7):1843–8. [DOI] [PubMed] [Google Scholar]
- 34.Raviele A, Gasparini G, Di Pede F, Delise P, Bonso A, Piccolo E. Usefulness of head-up tilt test in evaluating patients with syncope of unknown origin and negative electrophysiologic study. Am J Cardiol. 1990;65(20):1322–7. [DOI] [PubMed] [Google Scholar]
- 35.Lewis DA, Zlotocha J, Henke L, Dhala A. Specificity of head-up tilt testing in adolescents: Effect of various degrees of tilt challenge in normal control subjects. J Am Coll Cardiol. 1997;30(4):1057–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


