Abstract
Background
Patients who survive acute myocardial infarction (AMI) are at high risk for recurrence. We determined whether rehospitalizations after AMI further increased risk of recurrent AMI.
Methods and Results
The study included Medicare fee‐for‐service patients aged ≥65 years discharged alive after AMI from acute‐care hospitals in fiscal years 2009–2014. The outcome was recurrent AMI within 1 year of the index AMI. The Clinical Classifications Software (CCS) was used to classify rehospitalizations into disease categories. A Cox regression model was fit accounting for CCS‐specific hospitalizations as time‐varying variables and patient characteristics at discharge for the index AMI, adjusting for the competing risk of death. The rate of 1‐year recurrent AMI was 5.3% (95% CI, 5.27%–5.41%), and median (interquartile range) time from discharge to recurrent AMI was 115 (34–230) days. Eleven disease categories (diabetes mellitus, anemia, hypertension, coronary atherosclerosis, chest pain, heart failure, pneumonia, chronic obstructive pulmonary disease, gastrointestinal hemorrhage, renal failure, complication of implant or graft) were associated with increased risk of recurrent AMI. Septicemia was associated with lower recurrence risk. Hazard ratios ranged from 1.6 (95% CI, 1.55–1.70, heart failure) to 1.1 (95% CI, 1.04–1.25, pneumonia) to 0.6 (95% CI, 0.58–0.71, septicemia).
Conclusions
Patient risk of recurrent AMI changed based on the occurrence of hospitalizations after the index AMI. Improving post–acute care to prevent unplanned rehospitalizations, especially rehospitalizations for chronic diseases, and extending the focus of outcomes measures to condition‐specific rehospitalizations within 30 days and beyond is important for the secondary prevention of AMI.
Keywords: cardiovascular prevention, myocardial infarction, rehospitalization
Subject Categories: Quality and Outcomes
Clinical Perspective
What Is New?
This study addresses how subsequent hospitalizations after an acute myocardial infarction may influence the risk of a recurrent acute myocardial infarction.
We show that many types of subsequent hospitalizations can increase the risk of a recurrent acute myocardial infarction.
We are introducing the idea of dynamic risk prediction, in which subsequent events influence the likelihood of a future cardiovascular event.
What Are the Clinical Implications?
Risk‐stratification after an acute myocardial infarction is necessary to inform the choice of clinical strategies.
We are showing that risk prediction should be updated as new information becomes available. In this case, subsequent hospitalizations modify risk.
This study indicates the need to develop dynamic risk calculators that can be updated over time.
Nonstandard Abbreviations and Acronyms.
AMI acute myocardial infarction
CABG coronary artery bypass grafting
CAD coronary artery disease
CATH cardiac catheterization
CCS Clinical Classifications Software
COPD chronic obstructive pulmonary disease
DM diabetes mellitus
FY fiscal years
HR hazard ratio
HTN hypertension
ICD‐9‐CM International Classification of Diseases, Ninth Revision, Clinical Modification
IQR interquartile range
PCI percutaneous coronary intervention
UTI urinary tract infection
Despite the marked decline in the rate of recurrent AMI over recent decades,1, 2, 3, 4, 5 recurrence remains a significant threat to AMI survivors. Classifying patients according to their risk of recurrent AMI may be helpful in efforts to prevent the next AMI. Although patient baseline characteristics, postdischarge lifestyle, quality of care, and medication adherence are associated with risk of recurrent AMI,6, 7, 8, 9, 10 subsequent hospitalizations after the initial AMI may also increase the risk of recurrence and, therefore, may be important when considering a patient's risk. Identifying subsequent hospitalizations associated with recurrent AMI could better inform patients, families, and physicians about any further increases in the risk of recurrent AMI and help ensure intensive follow‐up and risk modification behaviors.
Efforts to identify risk markers and risk factors for long‐term outcomes after AMI have focused on patient characteristics available at the time of the initial hospitalization for AMI.8, 11, 12 Identification of postdischarge events as risk markers for adverse outcomes is limited, and many of the available data focus on mortality.13, 14, 15, 16 There is scant information on the association between subsequent hospitalizations and recurrent AMI. A comprehensive, contemporary, national evaluation of such rehospitalizations could provide important information for the prevention of recurrent AMI, particularly among Medicare beneficiaries who are a high‐risk population for AMI.
Accordingly, we used national Medicare inpatient claims data to assess the association between subsequent hospitalizations and recurrent AMI within 1 year after an initial AMI and identify clinically important hospitalizations that increased the risk of recurrence. This study, which was based on 100% national data and detailed follow‐up information about patients with AMI, is ideally positioned to generate information to update risk stratification for recurrent AMI in the year after hospital discharge.
Methods
Restricted by our Data Use Agreement with the Centers for Medicare & Medicaid Services (CMS), the Medicare data used for this study cannot be made publicly available to other researchers for purposes of reproducing the results or replicating the procedure. However, Medicare data are available from the Centers for Medicare & Medicaid Services upon request (https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/DUA_-_Forms.html).
Study Sample
We used the Centers for Medicare & Medicaid Services Medicare denominator files to identify all beneficiaries aged 65 years or older enrolled in the fee‐for‐service program for at least 12 months in fiscal years (FY) 2009–2014 (October 1, 2008 to September 31, 2014), a period in which all diagnosis codes were classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM). We linked these enrollment data to Medicare fee‐for‐service inpatient claims to identify beneficiaries who were discharged alive after hospitalization for AMI at an acute‐care hospital in the United States. This was designated the index AMI hospitalization. If a patient had >1 AMI hospitalization during the study period, we selected the first AMI during the study period as the index AMI. Data from FY 2008 were used to identify patients who were rehospitalized with AMI in FY 2009; FY 2015 data were used to ensure 1 year of follow‐up for patients hospitalized with AMI during FY 2014.
AMI was defined as an ICD‐9‐CM principal discharge diagnosis code of 410.xx. We excluded patients with ICD‐9‐CM codes 410.x2 because the codes represent subsequent episodes of care related to the index AMI. We also excluded patients who had a length of stay ≤1 day (because these patients were unlikely to have had an AMI), had conflicting dates of death and hospitalization, or were subsequently transferred to another acute‐care hospital for continuing care after the initial AMI.
Patient Baseline Characteristics
Patient baseline characteristics included age (continuous), sex, race (white, black, other), and clinical comorbidities identified using the method employed by the Centers for Medicare & Medicaid Services to profile hospital 30‐day mortality measures for AMI.17 We determined comorbidities from secondary diagnosis codes for the index AMI hospitalization as well as the principal and secondary diagnosis codes from all hospitalizations during the 12 months before the index AMI. Because the maximum number of diagnosis codes in Medicare data increased from 10 to 25 in 2011,18 we restricted the 2011–2015 data to the first 10 diagnosis codes to calculate comorbidities.
Outcome
The primary outcome was recurrent AMI within 1 year of discharge for the initial AMI. For patients with >1 recurrent AMI, the first recurrence was selected. Deaths during the 1‐year follow‐up period without a recurrent AMI hospitalization were treated as competing risks in the analysis. Secondary outcomes included 30‐day all‐cause mortality, 30‐day all‐cause readmission, and 1‐year all‐cause mortality using the index AMI discharge as the time zero. Mean length of stay and mean Medicare payment for the index AMI hospitalization were also assessed.
Subsequent Hospitalizations
We identified all subsequent hospitalizations within 1 year after discharge for the initial AMI. For patients with a recurrent AMI, subsequent hospitalizations were restricted to the period prior to the recurrent AMI. Because of the large volume of individual ICD‐9‐CM codes, we used the Clinical Classifications Software (CCS),19 a diagnosis and procedure categorization algorithm developed by the Agency for Healthcare Research and Quality, to characterize the subsequent hospitalizations. Using the CCS single‐level diagnosis‐specific algorithm, we collapsed >14 000 individual ICD‐9‐CM principal discharge diagnosis codes into 285 clinically homogeneous, meaningful, and mutually exclusive disease categories (Table S1). If a patient had >1 hospitalization for the same disease category, the first one was selected. We excluded CCS hospitalizations that occurred at a frequency <1% to avoid counting hospitalizations for less frequent diseases in the Medicare population.
Statistical Analysis
We divided patients into 2 samples, 2009–2011 and 2012–2014. We used the first sample to conduct the main analysis and the second to confirm the findings. We compared baseline characteristics between patients who had a recurrent AMI and those who did not have a recurrent AMI using the chi‐squared test for categorical variables and the t test for continuous variables. Using the 2009–2011 sample, for each patient, we estimated the baseline risk at the time of discharge of having a recurrent AMI within 1 year after discharge by fitting a Cox proportional hazards model with Markov Chain Monte Carlo simulations that modeled time to first recurrent AMI as a function of a patient's baseline characteristics described above. The model also included in‐hospital treatments (percutaneous coronary intervention, coronary artery bypass grafting, and cardiac catheterization), length of stay, and discharge to home (yes/no) because these variables may be associated with the outcome. We retained a variable in the model if the posterior probability of its nonzero coefficient was >0.95. We used the regression coefficients estimated from this model to calculate a baseline risk score for recurrent AMI for each patient. We standardized the score through the Z score method and stratified patients into 1 of 3 risk groups based on the risk score distribution: low (<10th percentile), average (10th–90th percentile), and high (>90th percentile). The baseline risk group represented a patient's risk of recurrent AMI at discharge. We used variables selected using the 2009–2011 data to calculate the score for patients in the 2012–2014 data as well.
We fit a single‐variable Cox regression model to describe the observed relationship between 1‐year recurrent AMI and a CCS‐specific condition‐related subsequent hospitalization, without accounting for patient baseline risk of recurrence. The time a hospitalization occurred was used as a time‐varying variable in the analysis. We repeated this analysis for each of the potential subsequent hospitalizations. To further assess the association between rehospitalizations and 1‐year recurrent AMI, we fit the Cox regression model with Markov Chain Monte Carlo simulations that modeled recurrent AMI as a function of all potential subsequent hospitalizations (event, yes/no, and time) as time‐varying variables, adjusted for the patient baseline risk score for recurrent AMI. We retained a rehospitalization in the model if the posterior probability of its nonzero coefficient was >0.95. To further assess the change in risk of recurrent AMI between patients with and without at least 1 subsequent rehospitalization, we fit the Cox model with a binary time‐varying indicator (1=had ≤1 subsequent CCS‐specific condition‐related rehospitalizations; 0=no rehospitalization), stratified by baseline risk group and further by age group. If a patient had >1 CCS‐specific condition‐related rehospitalization, the time that the first event occurred was used for the model.
Analyses were conducted using SAS version 9.4, 64‐bit Windows (SAS Institute Inc., Cary, NC). As of 2019, the data were 5 years old. Analyses were repeated using the 2012–2014 data. Deaths before recurrent AMI were addressed using the Fine and Gray20 method for competing risks. The Lee, Wei, and Amato method21 of robust sandwich variance matrix estimation was used to adjust for within‐hospital clustering of patients. All statistical testing was 2‐sided, and P<0.05 was considered statistically significant. The study followed the guidelines for cohort studies described in the Strengthening the Reporting of Observational Studies in Epidemiology Statement: Guidelines for Reporting Observational Studies.22 The Yale University Institutional Review Board reviewed the study protocol and granted a waiver of informed consent for the use of the deidentified database.
Results
Study Sample and Patient Baseline Characteristics
The study included 884 931 (447 690 in 2009–2011 and 437 241 in 2012–2014) unique patients who were discharged alive after AMI, were not transferred to another acute‐care hospital, and were hospitalized for >1 day during their index admission. Overall, patients had a mean age of 78.0 (SD, 8.6) years, and 47.7% were female. The most common comorbidities were chronic atherosclerosis (73.3%), hypertension (67.9%), diabetes mellitus (32.1%), and anemia (25.2%). During the index AMI hospitalization, 42.2% of patients had a percutaneous coronary intervention, 9.1% underwent coronary artery bypass grafting, and 58.2% had cardiac catheterization. The median length of stay was 4 (interquartile range [IQR], 2–7) days, and 57.5% of patients were discharged to home. Patient characteristics were no different between the 2009–2011 and 2012–2014 samples (Table 1).
Table 1.
Patient Baseline Characteristics by Study Sample
| Fiscal Year 2009–2011 | Fiscal Year 2012–2014 | |||||
|---|---|---|---|---|---|---|
| Aggregated (n=447 690) | Without Recurrent AMI (n=426 426) | With Recurrent AMI (n=21 264) | Aggregated (n=437 241) | Without Recurrent AMI (n=419 120) | With Recurrent AMI (n=18 121) | |
| Demographics, n (%) | ||||||
| Age, mean (SD) | 78.3 (8.6) | 78.2 (8.5) | 79.1 (9.0) | 77.8 (8.6) | 77.8 (8.6) | 78.3 (9.0) |
| Female | 218 034 (48.7) | 207 463 (48.7) | 10 571 (49.7) | 204 361 (46.7) | 195 846 (46.7) | 8515 (47.0) |
| White | 391 029 (87.3) | 373 133 (87.5) | 17 896 (84.2) | 378 338 (86.5) | 363 327 (86.7) | 15 011 (82.8) |
| Black | 35 049 (7.8) | 32 874 (7.7) | 2175 (10.2) | 35 158 (8.0) | 33 234 (7.9) | 1924 (10.6) |
| Other | 21 612 (4.8) | 20 419 (4.8) | 1193 (5.6) | 23 745 (5.4) | 22 559 (5.4) | 1186 (6.5) |
| Prior cardiovascular events, n (%) | ||||||
| Heart failure | 57 891 (12.9) | 53 063 (12.4) | 4828 (22.7) | 52 783 (12.1) | 48 970 (11.7) | 3813 (21.0) |
| AMI | 18 926 (4.2) | 16 607 (3.9) | 2319 (10.9) | 17 917 (4.1) | 15 946 (3.8) | 1971 (10.9) |
| Unstable angina | 11 499 (2.6) | 10 219 (2.4) | 1280 (6.0) | 10 356 (2.4) | 9375 (2.2) | 981 (5.4) |
| Chronic atherosclerosis | 326 924 (73.0) | 310 783 (72.9) | 16 141 (75.9) | 322 038 (73.7) | 308 135 (73.5) | 13 903 (76.7) |
| Cardiopulmonary respiratory failure or shock | 20 232 (4.5) | 18 717 (4.4) | 1515 (7.1) | 21 444 (4.9) | 20 102 (4.8) | 1342 (7.4) |
| Anterior MI (ICD‐9 410.00–410.19) | 40 964 (9.2) | 39 685 (9.3) | 1279 (6.0) | 36 106 (8.3) | 35 157 (8.4) | 949 (5.2) |
| Inferior/lateral/posterior MI (ICD‐9 410.20–410.69) | 60 244 (13.5) | 58 556 (13.7) | 1688 (7.9) | 56 186 (12.9) | 54 903 (13.1) | 1283 (7.1) |
| Comorbidities, n (%) | ||||||
| Hypertension | 299 244 (66.8) | 284 380 (66.7) | 14 864 (69.9) | 301 284 (68.9) | 288 320 (68.8) | 12 964 (71.5) |
| Stroke | 17 964 (1.8) | 16 798 (1.8) | 1166 (2.3) | 16 651 (1.7) | 15 600 (1.7) | 1051 (2.3) |
| Cerebrovascular disease | 57 891 (4.0) | 53 063 (3.9) | 4828 (5.5) | 52 783 (3.8) | 48 970 (3.7) | 3813 (5.8) |
| Renal failure | 52 288 (11.7) | 47 971 (11.2) | 4317 (20.3) | 54 285 (12.4) | 50 429 (12.0) | 3856 (21.3) |
| COPD | 88 711 (19.8) | 83 582 (19.6) | 5129 (24.1) | 85 379 (19.5) | 81 060 (19.3) | 4319 (23.8) |
| Pneumonia | 64 817 (14.5) | 61 108 (14.3) | 3709 (17.4) | 58 620 (13.4) | 55 605 (13.3) | 3015 (16.6) |
| Protein‐calorie malnutrition | 21 010 (4.7) | 20 090 (4.7) | 920 (4.3) | 21 924 (5.0) | 21 057 (5.0) | 867 (4.8) |
| Dementia | 48 997 (10.9) | 46 521 (10.9) | 2476 (11.6) | 24 691 (5.6) | 23 583 (5.6) | 1108 (6.1) |
| Functional disability | 10 927 (2.4) | 10 182 (2.4) | 745 (3.5) | 10 552 (2.4) | 9908 (2.4) | 644 (3.6) |
| Peripheral vascular disease | 27 609 (6.2) | 25 358 (5.9) | 2251 (10.6) | 24 431 (5.6) | 22 712 (5.4) | 1719 (9.5) |
| Metastatic cancer | 29 141 (6.5) | 27 664 (6.5) | 1477 (6.9) | 27 341 (6.3) | 26 094 (6.2) | 1247 (6.9) |
| Major trauma in past year | 26 039 (5.8) | 24 762 (5.8) | 1277 (6.0) | 22 871 (5.2) | 21 951 (5.2) | 920 (5.1) |
| Major psychiatric disorder | 9417 (2.1) | 8867 (2.1) | 550 (2.6) | 9679 (2.2) | 9210 (2.2) | 469 (2.6) |
| Chronic liver disease | 3009 (0.7) | 2835 (0.7) | 174 (0.8) | 3446 (0.8) | 3282 (0.8) | 164 (0.9) |
| Depression | 25 098 (5.6) | 23 821 (5.6) | 1277 (6.0) | 26 353 (6.0) | 25 187 (6.0) | 1166 (6.4) |
| Diabetes mellitus | 139 047 (31.1) | 130 064 (30.5) | 8983 (42.2) | 144 787 (33.1) | 136 443 (32.6) | 8344 (46.0) |
| Parkinson or Huntington disease | 6186 (1.4) | 5845 (1.4) | 341 (1.6) | 6045 (1.4) | 5771 (1.4) | 274 (1.5) |
| Anemia | 11 0791 (24.7) | 104 398 (24.5) | 6393 (30.1) | 112 477 (25.7) | 106 785 (25.5) | 5692 (31.4) |
| Asthma | 10 699 (2.4) | 10 178 (2.4) | 521 (2.5) | 11 153 (2.6) | 10 681 (2.5) | 472 (2.6) |
| In‐hospital procedures, n (%) | ||||||
| Percutaneous coronary intervention | 181 125 (40.5) | 174 586 (40.9) | 6539 (30.8) | 192 090 (43.9) | 185 794 (44.3) | 6296 (34.7) |
| Coronary artery bypass grafting | 41 365 (9.2) | 40 676 (9.5) | 689 (3.2) | 39 558 (9.0) | 38 948 (9.3) | 610 (3.4) |
| Cardiac catheterization | 260 515 (58.2) | 250 689 (58.8) | 9826 (46.2) | 263 534 (60.3) | 254 605 (60.7) | 8929 (49.3) |
| Discharge disposition, n (%) | ||||||
| Home | 253 528 (56.6) | 241 745 (56.7) | 11 783 (55.4) | 255 527 (58.4) | 245 028 (58.5) | 10 499 (57.9) |
| Home with care | 69 802 (15.6) | 65 712 (15.4) | 4090 (19.2) | 66 388 (15.2) | 63 034 (15.0) | 3354 (18.5) |
| Skilled nursing facility or intermediate care facility | 84 997 (19.0) | 80 767 (18.9) | 4230 (19.9) | 76 120 (17.4) | 72 897 (17.4) | 3223 (17.8) |
| Outcome | ||||||
| Length of stay, mean (SD) days | 6 (5.4) | 6 (5.5) | 5 (4.6) | 5 (5.1) | 5 (5.1) | 5 (4.2) |
| Medicare payment, median (IQR), $1000 | 10.8 (8.3–16.1) | 10.8 (8.4–16.2) | 10.3 (7.1–14.2) | 11.1 (8.5–16.7) | 11.1 (8.5–16.8) | 10.4 (7.1–14.8) |
| 30‐day mortality after discharge, n (%) | 23 061 (5.2) | 22 487 (5.3) | 574 (2.7) | 21 743 (5.0) | 21 240 (5.1) | 503 (2.8) |
| 1‐year mortality after discharge, n (%) | 86 692 (19.4) | 81 729 (19.2) | 4963 (23.3) | 76 542 (17.5) | 72 639 (17.3) | 3903 (21.5) |
| 30‐day all‐cause readmission after discharge, n (%) | 85 234 (19.0) | 76 967 (18.1) | 8270 (38.9) | 72 910 (16.7) | 65 913 (15.7) | 6997 (38.6) |
AMI indicates acute myocardial infarction; COPD, chronic obstructive pulmonary disease; ICD‐9, International Classification of Diseases, Ninth Revision; IQR, interquartile range; and MI, myocardial infarction.
Outcome
For the 2009–2011 and 2012–2014 samples, the rates of 1‐year recurrent AMI were 5.3% (95% CI, 5.27–5.41) and 4.6% (95% CI, 4.54–4.67), respectively (P<0.001). Among these patients who had a recurrent AMI, the median (IQR) days from discharge to a recurrent AMI was 115 (34–230) for the 2009–2011 sample and 106 (31–217) for the 2012–2014 sample. In the 2009–2011 and 2012–2014 samples, respectively, the median (IQR) survived days among patients who died within 1 year without a recurrent AMI were 56 (20–128) and 53 (19–121), and the median (IQR) survived days among patients who died with a recurrent AMI were 105 (49–188) and 97 (48–179).
All‐cause mortality rates after the index AMI and before a recurrent AMI were 17.5% (95% CI, 17.4–17.6) and 15.7% (95% CI, 15.6–15.8) for the 2009–2011 and 2012–2014 samples, respectively. Compared with patients without a recurrent AMI, patients with a recurrent AMI had a higher 30‐day postdischarge all‐cause mortality rate (5.3% versus 2.7%; P<0.001), higher 30‐day all‐cause readmission rate (38.9% versus 18.1%; P<0.001), higher 1‐year all‐cause mortality rate (23.3% versus 19.2%; P<0.001), lower median Medicare payment ($10 300 versus $10 500), and shorter mean (SD) length of stay (5 [4.6] days versus 6 [5.5] days). These observed outcomes were similar in the 2009–2011 and 2012–2014 samples (Table 1).
Association Between Patient Baseline Characteristics and Recurrent AMI
In the 2009–2011 sample, the 5 baseline characteristics most strongly associated with 1‐year recurrent AMI were AMI before the index admission (hazard ratio [HR], 1.8 [95% CI, 1.76–1.93]), unstable angina (HR, 1.5 [95% CI, 1.41–1.59]), diabetes mellitus (HR, 1.4 [95% CI, 1.39–1.47]), chronic atherosclerosis (HR, 1.3 [95% CI, 1.26–1.35]), and renal failure (HR, 1.2 [95% CI, 1.14–1.23]; Figure 1). Patients with a standardized risk <−1.2 times the SD, between −1.2 and 1.2, and >1.2 were stratified into low‐, average‐, and high‐risk groups, respectively (Figure 2). The mean (SD) estimated rates of 1‐year recurrent AMI were 1.7% (0.47) for the low‐risk group, 5.1% (2.03) for average risk, and 14.3% (5.31) for high risk. Within a risk group, the rate of recurrence increased with patient age (Figure 3, top panel). Results were similar for the 2012–2014 sample (Figure 3, bottom panel).
Figure 1.
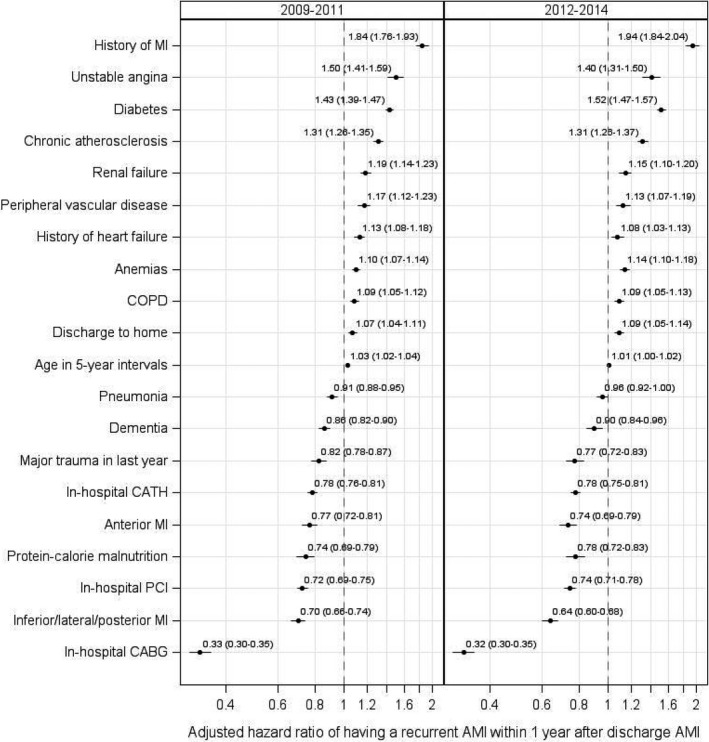
Patient baseline characteristics associated with recurrent AMI within 1 year after the initial AMI. AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; CATH, cardiac catheterization; COPD, chronic obstructive pulmonary disease; and PCI, percutaneous coronary intervention.
Figure 2.
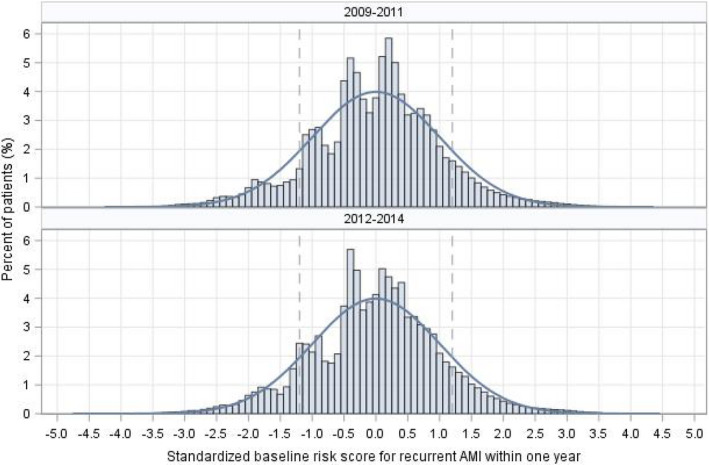
Distribution of baseline risk of recurrent acute myocardial infarction within 1 year after discharge for AMI.AMI indicates acute myocardial infarction.
Figure 3.
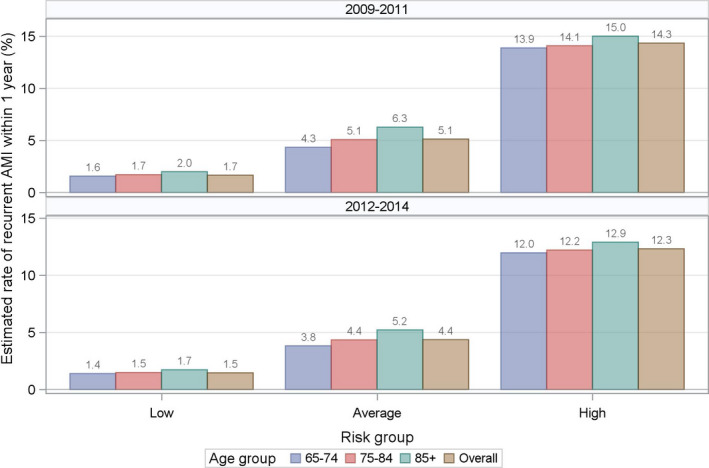
Baseline risk groups of recurrent acute myocardial infarction within 1 year after discharge for AMI.AMI indicates acute myocardial infarction.
Subsequent Hospitalizations
Among 285 CCS‐specific conditions, 19 occurred in at least 1% of patients and were included in the model for the 2009–2011 sample (Table 2). The median (IQR) tetrachoric correlations among parts of these conditions were low (0.11 [95% CI, 0.08–0.16] in 2009–2011 and 0.12 [95% CI, 0.09–0.18] in 2012–2014). The highest correlation occurred between chronic obstructive pulmonary disease and bronchiectasis (CCS‐127) and respiratory failure/insufficiency/arrest (CCS‐131), which was 0.40 in 2009–2011 and 0.41 in 2012–2014. Among 447 690 patients in the 2009–2011 sample, 36.0% (n=161 327) had at least 1 rehospitalization for 1 of these 19 CCS‐specific conditions before a recurrent AMI within 1 year. The 5 most common rehospitalization events were congestive heart failure (CCS‐108, 9.8%), coronary atherosclerosis and other heart disease (CCS‐101, 6.6%), septicemia (CCS‐2, 4.0%), cardiac dysrhythmias (CCS‐106, 3.7%), and pneumonia (CCS‐122, 3.4%). The 5 events that occurred soonest after discharge were complications of surgical procedures or medical care (CCS‐238; median, 53 [IQR, 12–165] days), coronary atherosclerosis and other heart disease (CCS‐101; median, 54 [IQR, 19–154] days), congestive heart failure (CCS‐108; median, 62 [IQR, 18–161] days), respiratory failure/insufficiency/arrest (CCS‐131; median, 88 [IQR, 28–198] days), and cardiac dysrhythmias (CCS‐106; median, 90 [IQR, 25–204] days; Figure 4, top panel). The findings were similar for the 2012–2014 sample (Figure 4, bottom panel). In‐hospital mortality for these CCS‐specific hospitalizations ranged from 0.3% (nonspecific chest pain, CCS‐102) to 21.9% (septicemia, CCS‐2).
Table 2.
Occurrence of 19 Targeted Types of CCS‐Specific Condition‐Related Subsequent Hospitalizations
| Rehospitalization, N (%) | FY 2009–2011 | FY 2012–2014 |
|---|---|---|
| N=447,690 | N=437,241 | |
| Septicemia (CCS‐2; except in labor) | 18 582 (4.2) | 19 333 (4.4) |
| Diabetes mellitus with complications (CCS‐50) | 5445 (1.2) | 4462 (1.0) |
| Fluid and electrolyte disorders (CCS‐55) | 7210 (1.6) | 5407 (1.2) |
| Deficiency and other anemia (CCS‐59) | 5159 (1.2) | 3971 (0.9) |
| Hypertension with complications and secondary hypertension (CCS‐99) | 6305 (1.4) | 6216 (1.4) |
| Coronary atherosclerosis and other heart disease (CCS‐101) | 31 034 (6.9) | 21 222 (4.9) |
| Nonspecific chest pain (CCS‐102) | 10 046 (2.2) | 6855 (1.6) |
| Cardiac dysrhythmias (CCS‐106) | 17 167 (3.8) | 14 521 (3.3) |
| Congestive heart failure (CCS‐108; non‐hypertensive) | 46 340 (10.4) | 37 766 (8.6) |
| Acute cerebrovascular disease (CCS‐109) | 8825 (2.0) | 7837 (1.8) |
| Pneumonia (CCS‐122; except that caused by tuberculosis or sexually transmitted disease) | 15 762 (3.5) | 12 688 (2.9) |
| Chronic obstructive pulmonary disease and bronchiectasis (CCS‐127) | 9949 (2.2) | 8012 (1.8) |
| Respiratory failure; insufficiency; arrest (CCS‐131; adult) | 8397 (1.9) | 7288 (1.7) |
| Gastrointestinal hemorrhage (CCS‐153) | 9592 (2.1) | 8825 (2.0) |
| Acute and unspecified renal failure (CCS‐157) | 11 172 (2.5) | 10 684 (2.4) |
| Urinary tract infections (CCS‐159) | 9389 (2.1) | 7232 (1.7) |
| Fracture of neck of femur (CCS‐226; hip) | 4804 (1.1) | 4002 (0.9) |
| Complication of device; implant or graft (CCS‐237) | 10 109 (2.3) | 8191 (1.9) |
| Complications of surgical procedures or medical care (CCS‐238) | 7680 (1.7) | 6306 (1.4) |
AMI indicates acute myocardial infarction; CCS, Clinical Classifications Software; and FY, fiscal years.
Figure 4.
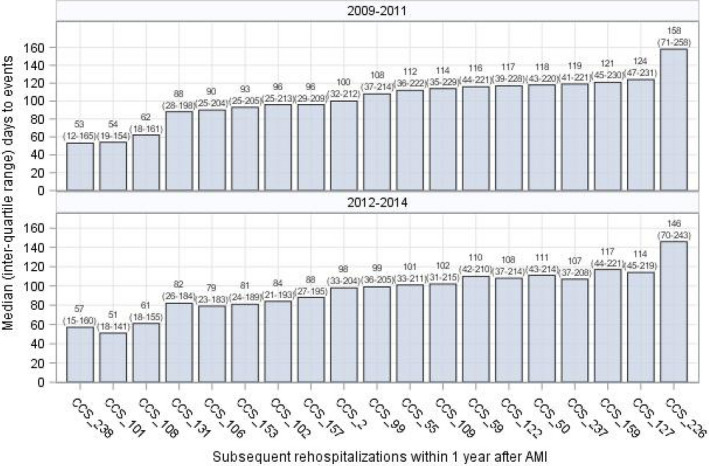
Median (interquartile range [IQR]) days to subsequent rehospitalizations within 1 year after discharge for index AMI.The median (IQR) days to recurrent AMI were 115 (34–230) in the 2009–2011 sample and 106 (31–217) in the 2012 to 2014 sample. AMI indicates acute myocardial infarction; CAD, coronary artery disease; CCS_101, Coronary atherosclerosis and other heart disease; CCS_102, Nonspecific chest pain; CCS_106, Cardiac dysrhythmias; CCS_108, Congestive heart failure; CCS_109, Acute cerebrovascular disease; CCS_122, Pneumonia; CCS_131, Respiratory failure; insufficiency; arrest; CCS_153, Gastrointestinal hemorrhage; CCS_157, Acute and unspecified renal failure; CCS_159, Urinary tract infections; CCS_2, Septicemia; CCS_226, Fracture of neck of femur; CCS_237, Complication of device; implant or graft; CCS_238, Complications of surgical procedures or medical care; CCS_50, Diabetes mellitus with complications; CCS_55, Fluid and electrolyte disorders; CCS_59, Deficiency and other anemia; CCS_99, Hypertension with complications and secondary hypertension; CCS, Clinical Classifications Software; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; and UTI, urinary tract infection
Association Between Subsequent Hospitalizations and Recurrent AMI
Most of the 19 subsequent hospitalizations were associated with an increased recurrent AMI risk in the descriptive analysis without accounting for patient baseline risk of recurrent AMI (Table 3). The Cox model based on the 2009–2011 data identified 12 CCS‐specific subsequent hospitalizations significantly associated to recurrent AMI risk (Figure 5). These hospitalizations were septicemia (CCS‐2), diabetes mellitus with complications (CCS‐50), deficiency and other anemia (CCS‐59), hypertension with complications and secondary hypertension (CCS‐99), coronary atherosclerosis and other heart disease (CCS‐101), nonspecific chest pain (CCS‐102), congestive heart failure (CCS‐108), pneumonia (CCS‐122), chronic obstructive pulmonary disease and bronchiectasis (CCS‐127), gastrointestinal hemorrhage (CCS‐153), acute and unspecified renal failure (CCS‐157), and complication of device (implant or graft; CCS‐237). All these rehospitalizations except septicemia (CCS‐2) were associated with increased risk of recurrent AMI; septicemia (CCS‐2) was associated with a lower risk of recurrent AMI (Figure 5). The HRs ranged from 1.6 (95% CI, 1.55–1.70, heart failure [CCS‐108]) to 1.1 (95% CI, 1.04–1.25, pneumonia [CCS‐122]); the HR for septicemia (CCS‐2) was 0.6 (95% CI, 0.58–0.71; Figure 5).
Table 3.
Observed Association Between a Targeted CCS‐Specific Condition‐Related Subsequent Hospitalization and 1‐Year Recurrent AMI Based on a Single‐Variable Cox Regression Model
| Rehospitalization | FY 2009–2011 | FY 2012–2014 |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Septicemia (CCS‐2; except in labor) | 0.80 (0.72–0.89) | 0.87 (0.78–0.97) |
| Diabetes mellitus with complications (CCS‐50) | 2.10 (1.85–2.37) | 2.50 (2.18–2.86) |
| Fluid and electrolyte disorders (CCS‐55) | 1.49 (1.31–1.68) | 1.74 (1.51–2.01) |
| Deficiency and other anemia (CCS‐59) | 1.87 (1.64–2.14) | 1.97 (1.68–2.31) |
| Hypertension with complications and secondary hypertension (CCS‐99) | 2.35 (2.11–2.62) | 2.39 (2.13–2.69) |
| Coronary atherosclerosis and other heart disease (CCS‐101) | 1.70 (1.61–1.80) | 1.94 (1.82–2.08) |
| Nonspecific chest pain (CCS‐102) | 1.79 (1.63–1.97) | 1.89 (1.68–2.13) |
| Cardiac dysrhythmias (CCS‐106) | 1.26 (1.16–1.37) | 1.25 (1.13–1.38) |
| Congestive heart failure (CCS‐108; non‐hypertensive) | 2.12 (2.03–2.21) | 2.00 (1.90–2.11) |
| Acute cerebrovascular disease (CCS‐109) | 1.03 (0.90–1.18) | 1.00 (0.86–1.17) |
| Pneumonia (CCS‐122; except that caused by tuberculosis or sexually transmitted disease) | 1.46 (1.33–1.59) | 1.57 (1.42–1.73) |
| Chronic obstructive pulmonary disease and bronchiectasis (CCS‐127) | 1.93 (1.75–2.13) | 1.75 (1.55–1.97) |
| Respiratory failure; insufficiency; arrest (CCS‐131; adult) | 1.54 (1.38–1.73) | 1.28 (1.11–1.47) |
| Gastrointestinal hemorrhage (CCS‐153) | 1.42 (1.28–1.58) | 1.48 (1.32–1.67) |
| Acute and unspecified renal failure (CCS‐157) | 1.60 (1.45–1.76) | 1.47 (1.31–1.63) |
| Urinary tract infections (CCS‐159) | 1.37 (1.22–1.54) | 1.35 (1.17–1.56) |
| Fracture of neck of femur (CCS‐226; hip) | 1.40 (1.18–1.66) | 1.26 (1.02–1.56) |
| Complication of device; implant or graft (CCS‐237) | 1.98 (1.80–2.17) | 2.13 (1.91–2.37) |
| Complications of surgical procedures or medical care (CCS‐238) | 0.90 (0.79–1.04) | 1.03 (0.88–1.20) |
AMI indicates acute myocardial infarction; CCS, Clinical Classifications Software; FY, fiscal years; and HR, hazard ratio.
Figure 5.
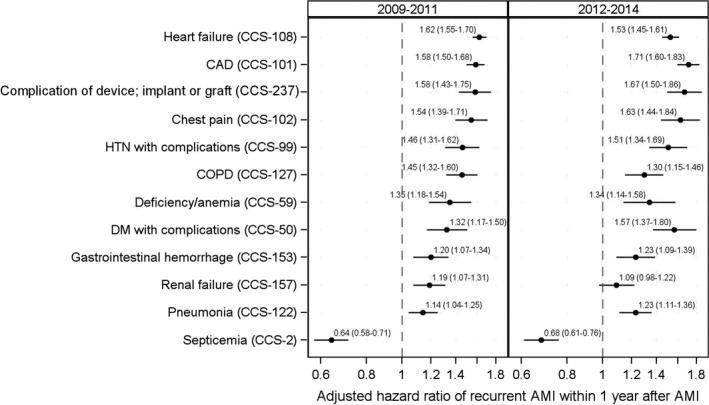
Association between subsequent rehospitalizations and recurrent AMI after discharge for index AMI, accounting for baseline risk of recurrence.AMI indicates acute myocardial infarction; CAD, coronary artery disease; CCS, Clinical Classifications Software; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; and HTN, hypertension.
Overall, 26.9% of patients in 2009–2011 and 22.5% of patients in 2012–2014 had at least 1 of the identified subsequent CCS‐specific hospitalizations significantly associated with increased risk of recurrent AMI. For the low‐, average‐, and high‐risk groups in the 2009–2011 sample, having at least 1 CCS‐specific hospitalization was associated with an increase in the risk of recurrent AMI by 210% (95% CI, 77%–149%), 73% (95% CI 66%–79%), and 43% (95% CI 34%–52%), respectively. The younger age group (65–74 years) in the average‐risk strata was most likely to have a recurrence with at least 1 CCS‐specific hospitalization (Figure 6, left panel). The findings were similar for the 2012–2014 cohort (Figure 6, right panel).
Figure 6.
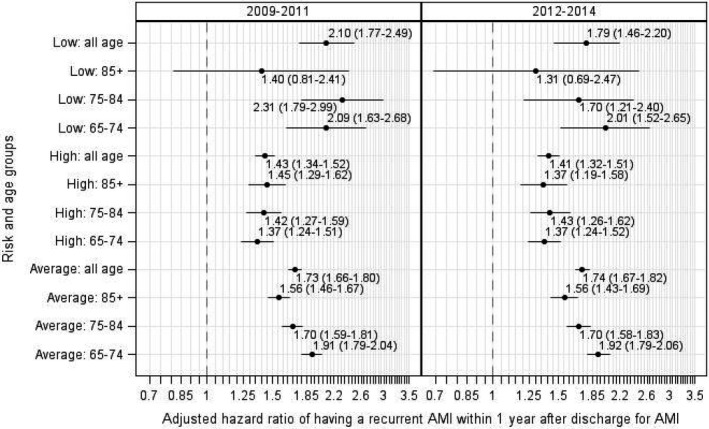
Association between at least 1 subsequent hospitalization and risk of recurrent AMI by patient baseline risk and age groups.AMI indicates acute myocardial infarction.
Discussion
In this study, we demonstrated that hospitalizations after AMI were associated with the risk of a subsequent AMI. Although patient baseline characteristics were also associated with the risk of a recurrent AMI, we showed that patient risk of recurrence was influenced by hospitalizations that occurred after discharge. Among the 12 rehospitalization categories identified in this study, 11 were associated with increased risk of recurrent AMI, with the increase in risk ranging from 14% (pneumonia) to 62% (heart failure). We found that patients who survived a hospitalization for septicemia had a lower risk of a recurrent AMI.
There are several potential explanations for the associations between subsequent hospitalizations and increased patient risk of recurrent AMI. It is possible that the hospitalization is a marker for the presence and severity of comorbidities. Sick patients tend to have more comorbidities23 and are more likely to be rehospitalized after AMI.24 We adjusted for baseline comorbidities, but information about the severity does not reside within the administrative codes in our Medicare database. Additionally, the hospitalizations may be a marker for postdischarge quality of care. Postdischarge care factors, such as continuity of care, type of care, and care providers, could impact AMI patient outcomes.25, 26, 27 Studies have identified associations between poor postdischarge care and subsequent hospitalizations,28, 29, 30 including recurrent AMI. Many subsequent hospitalizations identified by our study, including those for diabetes mellitus, anemia, hypertension, coronary atherosclerosis, chest pain, heart failure, pneumonia, chronic obstructive pulmonary disease and bronchiectasis, respiratory failure, gastrointestinal hemorrhage, renal failure, and complications of an implant or graft, have been individually identified as potential risk markers for recurrent AMI or major cardiovascular events in previous studies.6, 8, 31 Another possible explanation for our findings is that the hospitalization itself increased the risk. The reason for the hospitalization may have been associated with inflammation, a known contributor to AMI risk, or to other factors associated with AMI, such as stress or depression. It is also possible that the hospitalization led to the discontinuation of secondary preventive medications or the addition of medications to a patient's regimen, which may have resulted in nonadherence to the regimen.
The negative association of septicemia with recurrent AMI may represent a survivorship bias because patients who survived sepsis may have been healthier than those who died with sepsis and were therefore less likely to have a recurrent AMI. Our data showed that approximately 22% of AMI survivors rehospitalized for sepsis died during the sepsis hospitalization.
Our study, based on real‐world data, has several important characteristics. We focused on the first year after an initial AMI, a period that has the highest risk of recurrent AMI.4 Our findings provide real‐world empirical evidence of the importance of accounting for postdischarge rehospitalizations to help ensure better long‐term outcomes. We showed that patient risk stratification for recurrent AMI was a dynamic measure that could change immediately after discharge. The CCS categories allowed the grouping of similar medical conditions to provide hospitals and physicians a parsimonious, clinically meaningful, and practically useful composite measure of rehospitalizations. Such a composite measure could be used more easily than a traditional approach based on individual ICD diagnosis codes. The 12 subsequent hospitalizations identified in the study were based on the 285 CCS categories, which represent all principal diagnosis codes for rehospitalizations. These CCS categories are easy to collect and readily available at the time of discharge for the rehospitalization.
A model that combines rehospitalizations with patient baseline characteristics would allow hospitals and physicians to reevaluate patient risk for recurrent AMI throughout the first year and may help patients understand that their risk of recurrence depends not only on their baseline characteristics but also the sequence of rehospitalizations that occur after their initial AMI. The ability to identify individuals with the highest risk of recurrent AMI after a rehospitalization may aid in the provision of targeted, intensive, and higher‐quality longitudinal care after discharge. Additionally, insight regarding the long‐term risk of subsequent hospitalizations associated with recurrent AMI is important from a patient perspective as educating patients regarding their long‐term risk might provide an even stronger incentive to follow‐up and adhere to medications. Our study also provides evidence that hospitals and primary care physicians caring for patients with a history of AMI should be aware that subsequent hospitalizations can change patient risk of recurrent AMI.
Our study has several limitations. We considered only the first recurrent AMI and acknowledge that patients may experience multiple recurrent events, in which a recurrent event model can be fit. The subsequent hospitalizations identified in our study were based on the CCS categories, which represent multiple principal discharge diagnosis codes, while an individual rehospitalization only represents a single principal diagnosis code that could be more clinically important. We accounted for only inpatient rehospitalizations and did not consider outpatient care, observation stays, or emergency department visits. We treated subsequent rehospitalizations independently and acknowledge that some hospitalizations may have been related. Nevertheless, we found that the median tetrachoric correlation among these rehospitalizations was not high, indicating these rehospitalizations were not strongly related to each other. We did not address whether the association between a subsequent hospitalization and a recurrent AMI depended on the hospitalization‐free duration from an index AMI discharge to a rehospitalization, which could be clinically important. We restricted the 2011–2015 data to the first 10 diagnosis codes to align with the 2009–2010 data, which only contained 10 diagnosis codes. Accordingly, we may have missed some comorbidity information carried by the additional codes. Our study was limited by the availability of data resources, and therefore it did not incorporate information on medication adherence, nursing home stays, and home health services, which were associated with rehospitalizations and recurrent AMI in prior work.32, 33, 34 Moreover, we used comorbidity information from administrative data. These data lack detailed clinical information on patient functional status, left ventricular function, non–ST‐segment–elevation myocardial infarction, and ST‐segment elevation myocardial infarction, which could be important for assessing risk of recurrence and reducing measurement error.
In conclusion, patient risk of recurrent AMI changed on the basis of the occurrence of subsequent hospitalizations. Improving post–acute care to prevent unplanned rehospitalizations, especially those for chronic diseases, and extending the current focus on all‐cause 30‐day rehospitalizations to condition‐specific rehospitalizations beyond the 30‐day period are important for the secondary prevention of AMI. Moreover, there should be strong efforts to ensure that patients who experience these events have optimal secondary prevention strategies.
Disclosures
Dr Krumholz was a recipient of a research grant, through Yale, from Medtronic and the US Food and Drug Administration to develop methods for postmarket surveillance of medical devices; is a recipient of research agreements with Medtronic and Johnson & Johnson (Janssen), through Yale, to develop methods of clinical trial data sharing; was a recipient of a research agreement, through Yale, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; chairs a Cardiac Scientific Advisory Board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science and the Physician Advisory Board for Aetna; received payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation and from the Ben C. Martin Law Firm for work related to the Cook inferior vena cava filter litigation; and is the founder of Hugo, a personal health information platform. Drs Krumholz and Normand work under contract to the Centers for Medicare & Medicaid Services to develop and maintain performance measures that are publicly reported. The remaining authors have no disclosures to report.
Supporting information
Table S1
(J Am Heart Assoc. 2020;9:e014907 DOI: 10.1161/JAHA.119.014907.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Brown TM, Deng L, Becker DJ, Bittner V, Levitan EB, Rosenson RS, Safford MM, Muntner P. Trends in mortality and recurrent coronary heart disease events after an acute myocardial infarction among Medicare beneficiaries, 2001–2009. Am Heart J. 2015;170:249–255. [DOI] [PubMed] [Google Scholar]
- 2. Buch P, Rasmussen S, Gislason GH, Rasmussen JN, Kober L, Gadsboll N, Stender S, Madsen M, Torp‐Pedersen C, Abildstrom SZ. Temporal decline in the prognostic impact of a recurrent acute myocardial infarction 1985 to 2002. Heart. 2007;93:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaudhry SI, Khan RF, Chen J, Dharmarajan K, Dodson JA, Masoudi FA, Wang Y, Krumholz HM. National trends in recurrent AMI hospitalizations 1 year after acute myocardial infarction in Medicare beneficiaries: 1999–2010. J Am Heart Assoc. 2014;3:e001197 DOI: 10.1161/JAHA.114.001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smolina K, Wright FL, Rayner M, Goldacre MJ. Long‐term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5:532–540. [DOI] [PubMed] [Google Scholar]
- 5. Krumholz HM, Normand S‐LT, Wang Y. Twenty‐year trends in outcomes for older adults with acute myocardial infarction in the United States. JAMA Netw Open. 2019;2:e191938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilpin E, Ricou F, Dittrich H, Nicod P, Henning H, Ross J. Factors associated with recurrent myocardial infarction within one year after acute myocardial infarction. Am Heart J. 1991;121:457–465. [DOI] [PubMed] [Google Scholar]
- 7. McCormick D, Gurwitz JH, Lessard D, Yarzebski J, Gore JM, Goldberg RJ. Use of aspirin, β‐blockers, and lipid‐lowering medications before recurrent acute myocardial infarction. Arch Intern Med. 1999;159:561. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Li J, Zheng X, Jiang Z, Hu S, Wadhera RK, Bai X, Lu J, Wang Q, Li Y, et al. Risk factors associated with major cardiovascular events 1 year after acute myocardial infarction. JAMA Netw Open. 2018;1:e181079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shang P, Liu GG, Zheng X, Ho PM, Hu S, Li J, Jiang Z, Li X, Bai X, Gao Y, et al. Association between medication adherence and 1‐year major cardiovascular adverse events after acute myocardial infarction in China. J Am Heart Assoc. 2019;8:011793 DOI: 10.1161/JAHA.118.011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shore S, Jones PG, Maddox TM, Bradley SM, Stolker JM, Arnold SV, Parashar S, Peterson P, Bhatt DL, Spertus J, et al. Longitudinal persistence with secondary prevention therapies relative to patient risk after myocardial infarction. Heart. 2015;101:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long‐term mortality and health status outcomes. J Am Coll Cardiol. 2012;60:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roe MT, Chen AY, Thomas L, Wang TY, Alexander KP, Hammill BG, Gibler WB, Ohman EM, Peterson ED. Predicting long‐term mortality in older patients after non‐ST‐segment elevation myocardial infarction: the CRUSADE long‐term mortality model and risk score. Am Heart J. 2011;162:875–883.e1. [DOI] [PubMed] [Google Scholar]
- 13. Ketchum ES, Dickstein K, Kjekshus J, Pitt B, Wong MF, Linker DT, Levy WC. The Seattle Post Myocardial Infarction Model (SPIM): prediction of mortality after acute myocardial infarction with left ventricular dysfunction. Eur Heart J Acute Cardiovasc Care. 2014;3:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plakht Y, Shiyovich A, Gilutz H. Predictors of long‐term (10‐year) mortality postmyocardial infarction: age‐related differences. Soroka Acute Myocardial Infarction (SAMI) Project. J Cardiol. 2015;65:216–223. [DOI] [PubMed] [Google Scholar]
- 15. Smolderen KG, Buchanan DM, Gosch K, Whooley M, Chan PS, Vaccarino V, Parashar S, Shah AJ, Ho PM, Spertus JA. Depression treatment and 1‐year mortality after acute myocardial infarction: insights from the TRIUMPH Registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status). Circulation. 2017;135:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tu JV, Austin PC, Walld R, Roos L, Agras J, McDonald KM. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37:992–997. [DOI] [PubMed] [Google Scholar]
- 17. Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand S‐LT. An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–1692. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Medicare & Medicaid Services . 5010 implementation—Processing additional International Classification of Diseases, 9th Revision—Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes in Pricer, Grouper, and the Medicare Code Editor (MCE). Pub 100‐04. 2010. Available at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2010-Transmittals-Items/CMS1237956.html. Accessed January 16, 2020.
- 19. Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS), 2015. U.S. Agency for Healthcare Research and Quality. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/CCSUsersGuide.pdf. Accessed January 16, 2020.
- 20. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21. Lee EW, Wei LJ, Amato DA, Leurgans S. Cox‐type regression analysis for large numbers of small groups of correlated failure time observations In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Dordrecht: Kluwer Academic; 1992:237–247. [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radovanovic D, Maurer L, Bertel O, Witassek F, Urban P, Stauffer JC, Pedrazzini G, Erne P. Treatment and outcomes of patients with recurrent myocardial infarction: a prospective observational cohort study. J Cardiol. 2016;68:498–503. [DOI] [PubMed] [Google Scholar]
- 24. Ahmedani BK, Solberg LI, Copeland LA, Fang‐Hollingsworth Y, Stewart C, Hu J, Nerenz DR, Williams LK, Cassidy‐Bushrow AE, Waxmonsky J, et al. Psychiatric comorbidity and 30‐day readmissions after hospitalization for heart failure, AMI, and pneumonia. Psychiatric Serv. 2015;66:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Walraven C, Mamdani M, Fang J, Austin PC. Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Walraven C, Oake N, Jennings A, Forster AJ. The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract. 2010;16:947–956. [DOI] [PubMed] [Google Scholar]
- 27. Pereira Gray DJ, Sidaway‐Lee K, White E, Thorne A, Evans PH. Continuity of care with doctors—a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open. 2018;8:e021161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenblatt DY, Weber SM, OʼConnor ES, LoConte NK, Liou J‐I, Smith MA. Readmission after colectomy for cancer predicts one‐year mortality. Ann Surg. 2010;251:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lum HD, Studenski SA, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one‐year mortality in community‐dwelling older Medicare beneficiaries. J Gen Intern Med. 2012;27:1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stein LK, Kwon CS, Agarwal P, Jette N, Dhamoon MS. Readmission to another hospital following acute stroke admission is associated with worse outcomes: nationally representative data. Abstract m127. Ann Neurol. 2018;84:S149. [Google Scholar]
- 31. Qintar M, Spertus JA, Tang Y, Buchanan DM, Chan PS, Amin AP, Salisbury AC. Noncardiac chest pain after acute myocardial infarction: frequency and association with health status outcomes. Am Heart J. 2017;186:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Bartolomeo S, Marino M, Guastaroba P, Valent F, De Palma R. Self‐controlled case‐series study to verify the effect of adherence to beta‐blockers in secondary prevention of myocardial infarction. J Am Heart Assoc. 2015;4:e001575 DOI: 10.1161/JAHA.114.001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ortolani P, Di Bartolomeo S, Marino M, Vagnarelli F, Guastaroba P, Rapezzi C, De Palma R. Adherence to agents acting on the renin–angiotensin system in secondary prevention of non‐fatal myocardial infarction: a self‐controlled case‐series study. Eur Heart J Cardiovasc Pharmacother. 2015;1:254–259. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Pandolfi MM, Fine J, Metersky ML, Wang C, Ho S‐Y, Galusha D, Nuti SV, Murugiah K, Spenard A, et al. Community‐level association between home health and nursing home performance on quality and hospital 30‐day readmissions for Medicare patients. Home Health Care Manag Pract. 2016;28:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


