Abstract
Acute liver failure (ALF) is an infrequent, unpredictable, potentially fatal complication of acute liver injury (ALI) consequent to varied etiologies. Etiologies of ALF as reported in the literature have regional differences, which affects the clinical presentation and natural course. In this part of the consensus article designed to reflect the clinical practices in India, disease burden, epidemiology, clinical presentation, monitoring, and prognostication have been discussed. In India, viral hepatitis is the most frequent cause of ALF, with drug-induced hepatitis due to antituberculosis drugs being the second most frequent cause. The clinical presentation of ALF is characterized by jaundice, coagulopathy, and encephalopathy. It is important to differentiate ALF from other causes of liver failure, including acute on chronic liver failure, subacute liver failure, as well as certain tropical infections which can mimic this presentation. The disease often has a fulminant clinical course with high short-term mortality. Death is usually attributable to cerebral complications, infections, and resultant multiorgan failure. Timely liver transplantation (LT) can change the outcome, and hence, it is vital to provide intensive care to patients until LT can be arranged. It is equally important to assess prognosis to select patients who are suitable for LT. Several prognostic scores have been proposed, and their comparisons show that indigenously developed dynamic scores have an edge over scores described from the Western world. Management of ALF will be described in part 2 of this document.
Keywords: acute liver failure, viral hepatitis, drug-induced liver injury, Wilson disease (WD), autoimmune hepatitis (AIH)
Abbreviations: ACLF, acute on chronic liver failure; AFLP, acute fatty liver of pregnancy; AKI, Acute kidney injury; ALF, Acute liver failure; ALFED, Acute Liver Failure Early Dynamic; ALT, alanine transaminase; ANA, antinuclear antibody; AP, Alkaline phosphatase; APTT, activated partial thromboplastin time; ASM, alternative system of medicine; ASMA, antismooth muscle antibody; AST, aspartate transaminase; ATN, Acute tubular necrosis; ATP, adenosine triphosphate; ATT, anti-TB therapy; AUROC, Area under the receiver operating characteristics curve; BCS, Budd-Chiari syndrome; BMI, body mass index; CBF, cerebral blood flow; CBFV, cerebral blood flow volume; CE, cerebral edema; CHBV, chronic HBV; CLD, chronic liver disease; CNS, central nervous system; CPI, clinical prognostic indicator; CSF, cerebrospinal fluid; DAMPs, Damage-associated molecular patterns; DILI, drug-induced liver injury; EBV, Epstein-Barr virus; ETCO2, End tidal CO2; GRADE, Grading of Recommendations Assessment Development and Evaluation; HAV, hepatitis A virus; HELLP, hemolysis; elevated liver enzymes, low platelets; HBV, Hepatitis B virus; HEV, hepatitis E virus; HLH, Hemophagocytic lymphohistiocytosis; HSV, herpes simplex virus; HV, hepatic vein; HVOTO, hepatic venous outflow tract obstruction; IAHG, International Autoimmune Hepatitis Group; ICU, intensive care unit; IFN, interferon; ICP, intracerebral pressure; ICH, intracerebral hypertension; IL, interleukin; IND-ALF, ALF of indeterminate etiology; INDILI, Indian Network for DILI; LC, liver cirrhosis; KCC, King's College Criteria; LT, liver transplantation; LDLT, living donor liver transplantation; MAP, mean arterial pressure; MHN, massive hepatic necrosis; MPT, mitochondrial permeability transition; MUAC, mid-upper arm circumference; NAPQI, n-acetyl-p-benzo-quinone-imine; NPV, negative predictive value; NWI, New Wilson's Index; ONSD, optic nerve sheath diameter; PAMPs, pathogen-associated molecular patterns; PCR, polymerase chain reaction; PELD, Pediatric End-Stage Liver Disease; PPV, positive predictive value; PT, prothrombin time; RAAS, renin–angiotensin–aldosterone system; sALI, severe acute liver injury; SIRS, systemic inflammatory response syndrome; SHF, subacute hepatic failure; SNS, sympathetic nervous system; TB, tuberculosis; TCD, transcranial Doppler; TGF, tumor growth factor; TJLB, transjugular liver biopsy; TLR, toll-like receptor; TNF, tumor necrosis factor; TSFT, triceps skin fold thickness; US, ultrasound; USALF, US Acute Liver Failure; VZV, varicella-zoster virus; WD, Wilson disease; YP, yellow phosphorus
Acute liver failure (ALF) is a common emergency in hepatology practice associated with high short-term mortality. The term is applied to a clinical presentation of encephalopathy within a few weeks after the onset of liver disease with associated coagulopathy.1,2 Epidemiology and presentation of ALF, as seen in India, is considerably different from that described in the Western literature.3 The most frequent cause of ALF in India is viral hepatitis, while it is paracetamol overdose in the West.4 The latter is rare, if ever seen in India, where drug hepatitis due to anti-tuberculosis (TB) drugs is the second most frequent cause.5 Consequently, the clinical presentation of ALF is also different in India. The interval from jaundice to encephalopathy longer than 7 days is rarely seen, and hence, classification of ALF into hyperacute and acute appears superfluous.6 In addition, it is important to be vigilant about the mimics of ALF, which are commonly seen in India. Several prognostic scores have been proposed, and their comparison shows that indigenously developed dynamic scores perform better than those scores described from the Western world.7 Therefore, Indian National Association for the Study of the Liver (INASL) felt it necessary to prepare a document to reflect the epidemiology, presentation, and prognosis of ALF from an Indian perspective.
For the development of consensus statements, the task force identified the main contentious issues on various aspects of ALF epidemiology, clinical presentation, pathology, and prognostication. Members of the task force reviewed the existing literature, especially from India, and developed consensus statements on each of these issues. The statements, along with supporting literature, were circulated to the group for peer review, and comments were responded to by authors. A 2-day roundtable discussion was held on 6th and 7th July 2019 to discuss, debate, and finalize the consensus statements. Only those statements that were unanimously approved by the members of the taskforce were accepted. The evidence and recommendations in these statements have been graded according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system with minor modifications (Table 1). The strength of recommendations (strong: 1, weak: 2) thus reflects the quality (grade) of underlying evidence (high, moderate, or low).8
Table 1.
Level of Evidence and Grade of Recommendations.
| Level of evidence | Confidence in the evidence | |
|---|---|---|
| 1. High | Data derived from meta-analyses or systematic reviews or from (multiple) randomized trials with high quality. | Further research is unlikely to change our confidence in the estimate of benefit and risk. |
| 2. Moderate | Data derived from a single RCT or multiple nonrandomized studies. | Further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate. |
| 3. Low | Small studies, retrospective observational studies, registries. | Any estimate of effect is uncertain. |
| Recommendations – Grade | Wording associated with the grade of recommendation | |
| 1. Strong | ‘‘must”, ‘‘should”, or ‘‘INASL recommends” | |
| 2. Weak | ‘‘can”, ‘‘may”, or ‘‘INASL suggests” | |
Adapted from GRADE system.
GRADE, Grading of Recommendations Assessment Development and Evaluation; INASL, Indian National Association for the Study of the Liver; RCT, randomized controlled trial.
Concept of liver failure
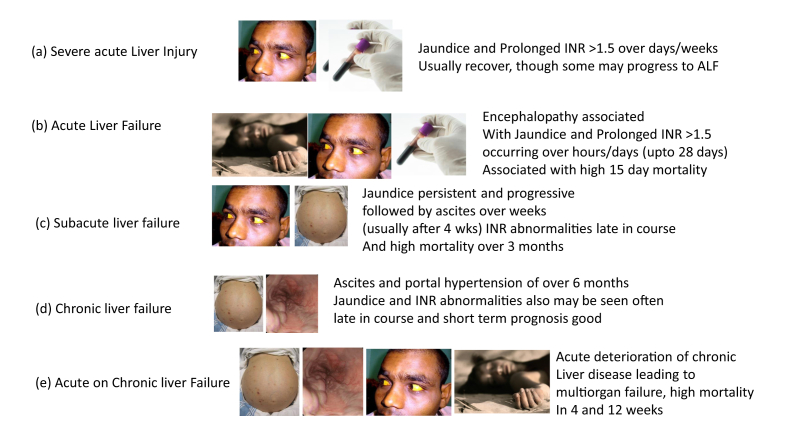
In health, the liver has myriads of functions, and liver failure usually implies loss of multiple functions of the liver, which may eventually be incompatible with life. The concept of liver failure should be clearly understood as it can present in several phenotypes (Figure 1). The commonest type of liver failure is a chronic liver failure as happens in cirrhosis of the liver. The natural progression is with sequential development of ascites, varices, variceal bleeding, followed by renal failure and encephalopathy over a period of months or years.9,10 If the deterioration in chronic liver disease (CLD) is precipitated rapidly (over a few days or weeks) by an acute event such as hepatitis B flare, variceal bleed, or superinfection, then it is termed as acute on chronic liver failure (ACLF) although there is no universally accepted consensus definition of this entity.11, 12, 13, 14, 15 However, the situation is different if the disease strikes for the first time in an apparently normal liver. Here the presentations of liver failure could be acute or subacute. If the appearance of liver dysfunction or jaundice is followed by encephalopathy with a few hours, days, or weeks, it is termed as ALF and diagnosis is mostly clinical based on the overall clinical presentation. On the other hand, if a patient presents with acute hepatitis with persistent or progressive jaundice for days to several weeks associated with coagulopathy (international normalized ratio (INR)>1.5), but has no evidence of encephalopathy, the term severe acute liver injury (sALI) is used.16 On the other hand, if persistent or progressive jaundice for over a month is followed by the appearance of ascites, we use the term subacute hepatic failure (SHF) for this presentation. All the three entities, ALF, SHF, and ACLF, are identified based on their phenotypic presentation with lack of evidence of any CLD in the former two and with direct or indirect, clinical/endoscopic/imaging or histological evidence of CLD in the latter. All these phenotypes must be clearly differentiated from one another because management approach, prognostic models, the timing of onset of various complications, and the underlying pathology of the liver differ in each of these three distinct phenotypes of liver failure (Table 2).
Figure 1.
Five phenotypes of liver failure. In (a), (b), and (c), there is no previously known liver disease. However, it is unclear if presence of a subclinical mild liver disease will change presentation, course, and outcome, e.g., in patients with nonalcoholic fatty liver, silent autoimmune hepatitis, Wilson disease, or inactive hepatitis B carrier state. Because classification is based on clinical presentation, phenotype concept helps to classify patients for planning management. ALF, acute liver failure; INR, international normalized ratio.
Table 2.
Clinical Differentiation Between Acute, Subacute, and Acute on Chronic Liver Failure.
| Criteria | ALF | SHF | ACLF |
|---|---|---|---|
| Previous liver status | Naïve – No h/o of previous liver disease | Naïve – No history of previous liver disease | Presence of underlying liver disease either in history or by evidence accrued at presentation |
| Clinical presentation: | |||
| 1. Encephalopathy | Present (definition) | Absent at presentation | Usually absent at presentation |
| 2. Jaundice | Usually present | Always present | Always present |
| 3. Overt features of cerebral edema | In 50–80% | Usually absent | Usually absent – occurs as a terminal event |
| 4. Ascites | Invariably absent | Always present | Always present |
| 5. Liver size | Small – not palpable – liver span reduced markedly | Usually not small – liver span normal or increased | Not small except when cirrhotic – may be palpable, span is not reduced in most |
| 6. Precipitating factors | Not identified – primary cause of liver damage causes liver failure | Not identified – primary cause with impaired regeneration causes liver failure | Usually present – sepsis, variceal bleed, super infection, superadded DILI, alcoholic binge, flare of underlying cause of chronic liver disease, idiopathic |
| Laboratory parameter | |||
| Transaminases | Markedly raised 15–30 times ULN | Moderately raised – 5-10 times ULN | Minimally or moderately raised depending on precipitating factors – 3-5 times ULN |
| INR | >1.5 s | Usually prolonged variably | Prolonged (>1.5 s as per APASL definition) |
| Bilirubin | Markedly raised | Markedly raised | Moderately raised |
| Albumin | Usually normal; may be decreased in pregnant women | Initially normal; reduces over time | Usually low than normal |
| Arterial ammonia | Markedly raised (100 μmol/L) | Not raised or moderately raised | Mildly raised – may be raised in flares or superadded liver injury (usually less than 100 μmol) |
| Natural course | |||
| Duration of disease course | Usually 2–7 days | Months – 4 weeks to 6 months | 4 weeks to 1 year |
| Imaging | Naïve small liver | Regenerating nodules – resulting in humps on the liver surface | Evidence of chronic liver disease with or without portosystemic collaterals |
| Endoscopy | No varices (but not usually done) | In 30%, small varices may present | More than half usually have varices |
| Histology | Features of acute hepatitis with submassive necrosis of the liver | Acute hepatitis with bridging necrosis | Features of chronic liver disease with or without superadded acute liver damage |
| Etiology | Mostly hepatitis viruses, ATT drug, | Hepatitis viruses, drugs | Alcohol, hepatitis virus, NAFLD, other cause of CLD, precipitating factors in pre-existing CLD |
Note: This table was prepared by the consensus group based on their expertise, experience, and literature. However, the present consensus is designed to rationalize various aspects of ALF in India and will focus on this single entity.
ALF, acute liver failure; ATT, anti-TB therapy; APASL, Asia Pacific Association for the Study of the Liver; CLD, chronic liver disease; INR, international normalized ratio; NAFLD, non-alcoholic fatty liver disease; SHF, subacute hepatic failure; ACLF, acute on chronic liver failure; ULN, upper limit of normal; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
Definition
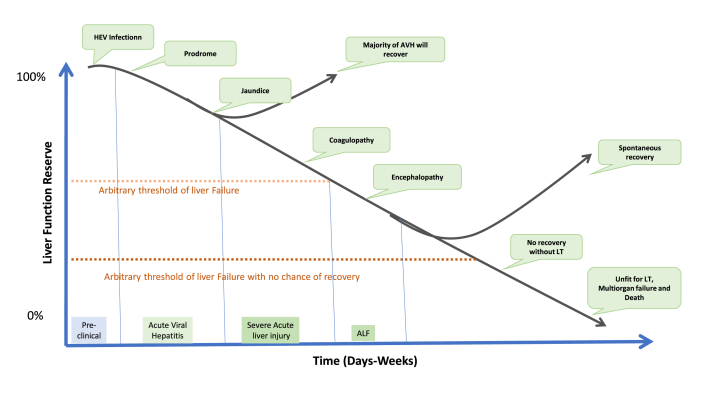
It is now apparent that ALF is a clinical syndrome characterized by a rapid deterioration in liver function resulting in encephalopathy and coagulopathy in a patient without any pre-existing liver disease. The syndrome has high mortality ranging from 60 to 80% depending on etiology, host factors, and place of care. ALF was first defined by Trey and Davidson in 1970, and today there are more than 40 definitions in published literature.1,17 The multiplicity of the definitions is primarily due to geographical variations in the etiology and clinical profile of ALF. Most definitions agree to define liver failure by the presence of encephalopathy (any grade) and coagulopathy (INR >1.5 or prothrombin time (PT) > 15 s) in the absence of pre-existing liver disease/cirrhosis (Table 3). They differ in the duration of the interval between the onset of symptoms or jaundice and encephalopathy.18, 19, 20, 21 This interval in various definitions has varied from 2 to 26 weeks. Chronic hepatitis B (vertically transmitted), AIH, and Wilson disease (WD) presenting with rapidly developing liver failure have been included as ALF in certain definitions despite the presence of CLD without overt cirrhosis.20,22 Superadded hepatitis A virus (HAV) or hepatitis E virus (HEV) infection or alcoholic bout in CLD have generally not been included as ALF in any definition.
Table 3.
Definitions of Acute Liver Failure Used in Clinical Practice
| Author | Encephalopathy | Coagulopathy | Jaundice encephalopathy interval |
|---|---|---|---|
| Trey & Davidson1 | Any grade | INR >1.5 | 8 weeks |
| AASLD (Lee)2 | Any grade | INR > 1.5 or PT > 15sec | 26 weeks |
| O'Grady19 | Any grade | INR > 1.5 | 12 weeks |
| IASL (Tandon)20 | Any grade | INR > 1.5 | 4 weeks |
| Japanese (Yamagishi)21 | Any grade | PTA <40% | 8 weeks |
Numbers in superscript indicate the reference number. INR, international normalized ratio; PT, prothrombin time; PTA, plasma thromboplastin antecedent.
INR, international normalized ratio; PT, prothrombin time; PTA, Plasma Thromboplastin Antecedent.
The Indian literature on ALF has been reviewed to arrive at a definition that will reflect the clinical profile of ALF as seen in India. Most series in India have documented viral hepatitis as the predominant etiology of ALF.23, 24, 25 Drugs including herbal medications constitute a significant proportion of cases in some geographical areas.24,25 Encephalopathy develops within four weeks of onset of jaundice in almost all cases of ALF associated with viral hepatitis in India.24,26 A minority of patients with severe liver injury develop encephalopathy between 5 and 8 weeks of onset of jaundice, and it has been seen in both viral hepatitis and drug-induced liver injury (DILI).26, 27, 28 In India, onset of liver failure after four weeks of onset of jaundice in the absence of CLD manifests with ascites rather than encephalopathy and is termed as SHF. SHF is a distinct histological entity and differs from ALF and ACLF in its clinical behavior (Table 2).
Hepatitis B virus (HBV) flare, AIH, and WD with underlying CLD may present with rapidly developing liver injury similar to the clinical syndrome of ALF (encephalopathy within four weeks of recognition of the disease, coagulopathy, high one-week mortality, infrequent ascites, or renal failure). These are included as ALF despite the possibility of underlying CLD as the presentation is phenotypically ALF and is clinically distinct from that of ACLF.
ALF has also been further subclassified depending on the interval between the appearance of encephalopathy and onset of jaundice into hyperacute (<7–10 days), acute (7–28 days), and subacute (5–12 weeks) presentation.21,22,27 This subclassification has been used as a clinical prognostic indicator with hyperacute having the best prognosis among the three presentations.26,27 However, the validity of this subclassification as a prognostic marker has been questioned and it is felt that jaundice to encephalopathy interval does not have any prognostic significance distinct from the cause of illness.2,24,29,30
Consensus Statement: Clinical Definition
-
1.
ALF is a clinical syndrome characterized by encephalopathy, jaundice, and prolonged PT (INR > 1.5) developing in a patient without pre-existing liver disease within four weeks of the onset of symptoms. A few patients presenting with sALI mostly due to DILI may develop encephalopathy later than 4 weeks up to 8 weeks (Grade of evidence: moderate; grade of recommendation: strong)
Disease burden
There is no central registry for the collection of data of patients with ALF in India. The only available data are published from individual centers, which have published results of patients with ALF evaluated at their respective centers. In addition, there are data from liver transplant (LT) centers in India regarding the number of transplants done for patients with ALF. However, there is no clarity regarding the total number of patients with ALF evaluated in LT centers as well as non-LT centers which have not published their results. Thus, the incidence of ALF in India is unclear. A summary of recent publications from tertiary care centers in India is shown in Table 4.31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 During epidemics of viral hepatitis, ~1% of clinical cases may develop ALF.
Table 4.
Different Etiologies of Acute Liver Failure Across Various Centers in India.
| Author, center, year | Cases (n) | HAV | HBV | HEV | Cryptogenic/non-A, non-E | Drugs | Other causes |
|---|---|---|---|---|---|---|---|
| Shalimar et al.,30 New Delhi, India, 1986–2015 | 1462 | 23 (2%) | 128 (8.8%) | 419 (28.7%) | 527 (36.0%) | ATT: 103 (7.0%) | Dual infection: 60 (4%), chronic markers: 138 (9%), no serology report: 64 (4%) |
| Alam et al.31 New Delhi, India, 2011–2016 | 109 | 43 (39.4%) | 0 | 2 (1.8%) | 16 (14.6%) | 12 (11%) ATT: 4, antibiotics: 3, CAM: 2, acetaminophen: 2, valproate: 1 |
Metabolic liver disease: 14 (13.2%), Parvovirus: 3 (2.7%), EBV: 1 (0.9%), VZV: 1 (0.9%), others: 17 (15.5%) |
| Das et al.,24 Dibrugarh, India, 2007–2015 | 255 | 76 (29.8%) | 8 (3.1%) | 34 (13.3%) | 112 (43.9%) | 0 | Amatoxin: 16 (6.2%), AIH: 2 (0.7%), combined viruses: 7 (2.7%) |
| Khuroo et al.,26 Kashmir, India, 1989–1996 | 180 | 4 (2.2%) | 25 (13.9%) | 79 (43.9%) | 56 (31.1%) | 1 | HDV: 2 (1.1%), HCV: 13 (7.2%) |
| Samanta et al,33 Kolkata, India, 2005–2007 | 45 | 9 (20%) | 4 (8.8%) | 6 (13.3%) | 10 (22.2%) | 1 (2.2%) | Wilson disease: (2.2%), malaria: 1 (2.2%), dual viral: 7 (15.5%) |
| Devarbhavi et al.,34 Bangalore, India, 1997–2017 | 128 | – | – | – | – | 128 (100%) ATT: 92 (72.4%), antiepileptic drugs: 11 (10%), dapsone: 7 (5.5%), hormones: 2, ferrous sulfate overdose: 2, acetaminophen: 2, antiretroviral: 2, CAM: 2, chemotherapy agents: 3, amoxicillin-clavulanic acid: 2, and others: 3 |
– |
| Poddar et al.,35 Lucknow, India, 2003–2010 | 52 | 12 (23%) | 6 (12%) | 12 (23%) | 8 (15%) | 8 (15%) All ATT |
Dual infections: 4 (8%), no serology report available: 2 (4%) |
| Mehrotra et al.,36 New Delhi, India, 2009–2015 | 36 | 4 (11.1%) | 1 (2.7%) | 7 (15.5%) | 14 (38.8%) | 4 (11.1%) All ATT |
Autoimmune: 1 (2.7%), Wilson disease: 5 (13.8%) |
| Pamecha et al.,37 New Delhi, India, 2011–2018 | 61 | 8 (13.1%) | 7 (11.4%) | 8 (13.1%) | 17 (27.8%) | 9 (14.7%) All ATT |
Others: 12 (19.6%) |
| Choudhary et al.,38 Haryana, India, 2017 | 18 | – | – | – | – | ATT: 14/18 (77.7%), others: 22.3% (orlistat: 1, flutamide: 1, and CAM: 2) | – |
| Dhiman et al.,39 Chandigarh, India, 1998 | 204 | Viral hepatitis: 186 (91.1%) | 15 (7.4%) All ATT |
Budd-Chiari syndrome: 1 (0.5%), Wilson disease: 1 (0.5%), malignant infiltration: 1 (0.5%) | |||
| Bernal et al., UK (1999–2008)40 | 422 | 2% | 5% | 1% | 17% | Paracetamol: 57%, other drugs: 11% |
7% |
| Lee 201218 | 1696 | 2% | 7% | 13% | Paracetamol: 46%, antimicrobial agents – ATT, antibiotics, antifungals, antiepileptics, NSAIDs, and antimetabolites: 12% | Autoimmune: 6.5%, ischemic: 5%, Wilson disease: 1%, Budd-Chiari syndrome: 1%, pregnancy: 1%, other causes: 5% |
|
| Ichai et al., France (1986–2006)41 | 363 | 5% | 28% | 18% | Paracetamol: 7%, other drugs: 21% |
21% | |
| Hadem et al.,42 Germany (2008–2009) | 109 | 4% | 10% | 4% | 24% | 32% (most importantly phenprocoumon: 23% of nonacetaminophen cases), valproate, NSAIDs, sertraline, clindamycin |
Autoimmune: 3%, Wilson disease: 3%, Budd-Chiari syndrome: 2%, malignancy: 3%, pregnancy: 3%, amanita, 2%, others: 4% |
| Gow et al.,43 Australia (1988–2001) [11] | 80 | 4% | 10% | 34% (non-A non-B) | Paracetamol: 36%, other drugs: 6% (nitrofurantoin sodium valproate, isoflurane, and ketorolac) |
Wilson disease: 7%, Budd-Chiari syndrome: 3% |
|
| Oketani et al,44 Japan (1998–2006) | 856 | 6% | 42% | 1% | 3% | 10% (ATT, acetaminophen), anticancer agents, allopurinol, and Acarbose | Autoimmune: 7%, unknown: 30% |
ATT, anti-TB therapy; CAM, complementary and alternative medicines; EBV, Epstein-Barr virus; VZV, varicella-zoster virus; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HEV, hepatitis E virus; AIH, autoimmune hepatitis; NSAIDs, nonsteroidal antiinflammatory drugs.
The various etiologies of ALF include hepatitis virus infections (the most common cause in India), drugs such as anti-TB therapy (ATT) drugs, metabolic causes, ischemia, and toxins.6,46 The details of individual etiologies of ALF are shown in Table 4. Paracetamol, the most common cause of ALF in the West, is virtually not seen as a cause of ALF in India. Pregnancy is a special situation; pregnant females are more susceptible to contract HEV infection, and there is also an increased risk of mortality.47 The details of various studies reporting acute viral hepatitis and ALF in pregnancy are shown in Table 5.33,48, 49, 50, 51, 52, 53
Table 5.
Studies Reporting AVH/ALF During Preganancy.
| Author, year, city | No of pregnant women/total population | No of AVH/ALF | HEV as the cause of AVH/ALF | Other causes of ALF in pregnant women | Overall outcome (deaths) | Outcome (maternal deaths) HEV | Relationship to different trimesters |
|---|---|---|---|---|---|---|---|
| Beniwal,47 2003, New Delhi, India | 97 consecutive P women with AVH/ALF | AVH: 69 ALF: 28 |
AVH: 25/69 (36%) ALF: 21/28 (75%) |
Non A-E: 5 HAV: 1 HBV: 1 |
Total: 24/97 AVH: 0 ALF: 24/97 (24.7%) Total: 24 18/24 (75%): HEV 5/24 (21%): non A-E 1/24 (4%): HAV |
HEV group: 18/46 (39.1%) expired 18/21 (85.7%) of HEV-ALF died |
– |
| Jaiswal,48 2001, Indore, India | 273 women 127 P 146 NP (controls) |
P: 83/127 (AVH) 44/127 (ALF) NP: 129/146 (AVH) 17/146 (ALF) |
P (AVH): 40/83 (48%) P (ALF): 33/44 (75%) 42/44 of ALF (2nd and 3rd trimester) |
HAV: 0 HBV: 4.5% HCV: 0 HDV: 2.3% Non-A, Non-E: 16% |
Total 24/273 P: AVH: 3/83 P: ALF: 21/43 (48.8%) 16/24 (HEV) 2/24: HBV 6/24 (Non A-E) |
P (AVH): 1/40 (2.5%) P (ALF): 15/33 (45.4%) |
Total 15 deaths in pregnancy: 1st: 1/2 (50%) 2nd: 4/12 (25%) 3rd: 10/19 (53%) |
| Rasheeda,49 2008, Chennai, India | 115 developed jaundice in 1,01,754 antenatal cases | AVH: 86/115 (75%) | – | 5/115 | 3.4% | – | |
| Patra,50 2007, New Delhi | 220 consecutive P females (2nd and 3rd trimester) | AVH: 129 ALF: 91 |
AVH: 59/129 (46%) ALF: 73/91 (80%) |
– | 60/220 AVH: 0 ALF: 60/91 |
HEV group: 54/132 (41%) | Mortality 2nd: 66% 3rd: 78% Non-E: 54% (NS) |
| Bhatia,51 2007, New Delhi, India | 1015 ALF P: 249 NP: 341 M: 425 |
HEV: 342/1015 (34.4%) P: 145/244 (HEV) 95/244 (Non-HEV) |
HAV: 2 (0.8%) HBV: 7 (2.9%) Dual acute: 4 (1.6%) Drugs: 6 (2.5%) No etiology: 68 (28%) |
575/1015 (56.7%) P: 134/249 (53.8%) NP: 195/341 (57.2%) M: 246/425 (57.9%) |
HEV: P: 74/145 (51%) NP: 46/100 (46%) M: 36/97 (37%) Non-E: P: 52/95 (55%) NP: 132/214 (62%) M: 184/293 (63%) NS |
1st: 3/5 (60%) 2nd: 92/171 (54%) 3rd: 39/70 (56%) NS |
|
| Khuroo,52 2003, Kashmir, India | P: 76 NP: 337 |
P (n = 76) AVH: 29/76 ALF: 47/76 NP: 337 AVH: 303/337 ALF: 34/337 |
P: 65/76 ALF: HEV: 45/47 (96%) NP: 140/337 HEV 14/34 (41.2%) |
P (ALF): 2/47 NP: Non-A, E 15/34 (44.1%) HBV: 5/34 (14.7%) |
P: 25/47 NP: 25/34 |
30/59 (HEV) 20/22 (non-HEV) |
The prevalence of HEV 1st trimester (76.9%), 2nd: 88.9%, 3rd: 83.8% and puerperium: 100% NS (P = 0.09) |
AVH, acute viral hepatitis; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HEV, hepatitis E virus; ALF, acute liver failure; P, pregnant; NP, not pregnant; M, men and boys; NS, not significant (difference).
Consensus Statement: Disease Burden of ALF
-
2.
ALF is uncommon; the exact burden in India is unknown. Hepatitis viruses are the most common cause of ALF in India. Among drugs, ATT drugs are the most common cause (Grade of evidence: moderate; grade of recommendation: strong)
Hepatitis A
Hepatitis A is not an uncommon cause of ALF in India. Limited data on the epidemiology of HAV-related ALF suggest that less than 1% of acute HAV infections result in ALF.54,55 Young children often have inapparent or subclinical hepatitis and have no symptoms or jaundice.56 In contrast, the infection is more severe in adults, with symptoms occurring in 70%.
There has been a change in the epidemiology of HAV infection in India, and similarly, there has been a proportionate change in the ALF burden due to HAV infection too. With economic improvement and better hygienic conditions prevailing in the communities, the age of acquiring HAV infection is shifting from early childhood to young adulthood.57 Such an epidemiological change has resulted in an increased incidence of symptomatic hepatitis A, with increased chances of development of hepatic failure. Data suggest that the population in India is no longer homogeneous for its HAV exposure profile. Occasional outbreaks of HAV and higher proportions of symptomatic cases are reported among older children and adults from different regions of the country.58,59 Besides, there is considerable geographical heterogeneity in the etiology of ALF from region to region in India; the proportion of ALF due to HAV infection varies from region to region. The geographical and age-related heterogeneities are highlighted in Table 6.35,38,60, 61, 62, 63, 64, 65 It is apparent that HAV infection in adults leading to ALF has progressively increased during the past three decades from 1.7% to very high in some reports.66
Table 7.
Prevalence of Acute Liver Failure due to Hepatitis A Virus in Adults.
| Authors | Centre/region | N [numbers] | Study period | ALF due to hepatitis A |
|---|---|---|---|---|
| Acharya et al29 | New Delhi [North India] | 423 | 1987–1995 | 1.7%¥ |
| Khuroo and Kamili26 | Srinagar [North India] | 180 | 1989–1996 | 2.2%¥ |
| Amarapurkar& Patel63 | Mumbai [Western India] | 28 | 2004–2005 | 10.7∗ |
| Das et al24 | Dibrugarh [Northeast] | 591 | 2005–13 | 33%¥ |
| Chidambaram et al25 | Pondicherry [South India] | 84 | 2015–16 | 27.4%¥ [62.1%]∗ |
| Mehrotra et al36 | New Delhi [North India] | 24 | 2009–2015 | 12.5%¥ |
Table 8.
Reports of DILI Causing ALF: Higher Incidence Among Women.
| Implicated drug | Country | Number | Women |
|---|---|---|---|
| Anti-TB111 | USA | 50 | 64% |
| Prescription medicines112 | Spain | 31 | 64% |
| Anti-TB30 | India | 103 | 70% |
| Prescription medicines113 | USA | 130 | 72% |
| Prescription medicines114 | Sweden | 42 | 62% |
| Anti-TB115 | India | 93 | 72% |
ALD, acute liver failure; DILI, drug-induced liver injury; TB, tuberculosis.
Table 6.
Prevalence of Acute Liver Failure due to Hepatitis A Virus in Children.
| Authors | Centre/region | n [numbers] | Study period | ALF due to hepatitis A |
|---|---|---|---|---|
| Arora et al58 | New Delhi [North India] | 44 | 1993–1994 | 37.5% |
| Srivastava et al59 | Lucknow [North India] | 41 | 1995–1996 | 2.4% |
| Poddar et al60 | Chandigarh [North India] | 67 | 1997–2000 | 65% |
| Samanta and Ganguly33 | Kolkata [Eastern India] | 45 | 2005–2007 | 35.5% |
| Kaur et al61 | New Delhi [North India] | 43 | 2008–2010 | 62.6% |
| Pandit et al62 | Vellore/Pune [Southern/Western India] | 76 | 2003–2005 | 36.3% |
| Mehrotra et al36 | New Delhi [North India] | 12 | 2009–2015 | 8.3% |
Consensus Statement: Etiology, Hepatitis A
-
3.
HAV is not an uncommon cause of ALF in India. The proportion of ALF due to HAV infection varies from region to region. There has been an increase in the incidence of ALF due to HAV infection over the last two decades (Grade of evidence: moderate)
Hepatitis B
In India, HBV infection is of intermediate endemicity, with nearly 2–4% of the population67 being affected by HBeAg-negative chronic HBV infection. Acute hepatitis B infection as an etiological agent of ALF has been reported in a small number of patients from several centers in India. In most series, the diagnosis of acute hepatitis B infection as an etiological agent has been based on serological testing of HbsAg and IgM anti-Hbc using 3rd-generation ELISA testing and has not included quantitative HBV DNA polymerase chain reaction (PCR) assays. Hepatitis B infection as a cause of ALF varied between 0.8% and 39% in different series.3,26,29,48,52,64,68, 69, 70, 71, 72, 73 One cannot distinguish cases of acute hepatitis B infection from flare of chronic hepatitis B infection or superinfection by another virus in the published series.
In 1984, Tandon et al67 reported that HBV infection was detected in 33% of the patients with ALF. By 2000, prevalence of HBsAg was seen in only 10.5% of ALF cases.71 Another interesting observation from this study was 23 of 59 cases (39%) of the presumed non-A-E patients with ALF had detectable HBV DNA in their serum even though they were anti–HBc-negative, raising the possibility of HBV core mutants playing an important role in causing ALF in the Indian subcontinent.
A study by Bhatia et al51 of 249 pregnant women presenting as ALF found that only 2 of 249 cases (0.8%) was due to acute HBV infection compared with 20 of 341 female patients (6.1%) who were nonpregnant. Barkotoki et al.73 reported from Delhi that acute HBV was found to be an etiologic agent in 2 of 160 cases (1.25%) of ALF in pregnant females. Das et al.24 reported that of the 255 cases of ALF seen in the Northeast, only 8 cases (3.13%) were due to HBV infection, but there were cases of combined HBV and HEV infection seen in 16.5% of cases. It is evident from the various reports that HBV infection is not the common etiologic agent for the ALF cases in India.
Flares of HBV can rarely lead to a picture of ALF which needs to be differentiated from acute hepatitis B. IgM anti-HBc titer, HBV DNA viral load, and anti-HBe estimation may help in such a situation. In a study by Dao et al.,74 based on 1602 patients with ALF, 27 cases (1.68%) were due to chronic HBV (CHBV) manifesting as ALF. Acute HBV–related ALF and chronic HBV infection–related ALF differed markedly in IgM anti-HBc titers, HBV viral loads, and prognosis, suggesting that two forms may be distinct entities and each may have unique pathogenesis. In another study by Lok et al.,75 exacerbation or reactivation of CHBV gave rise to icteric hepatitis and nonfatal hepatic failure in 22.2% and 3.7% of HBsAg-positive patients, respectively. The data suggested that patients who developed hepatitis during reinduction of cytotoxic therapy had greater risk of dying from hepatic failure. A study by Jindal et al76 from India based on severe hepatitis B reactivation reported that of 151 consecutive patients with HBV reactivation, 70 such cases (46.35%) presented as ACLF. The earlier studies from India which had reported the occurrence of acute HBV-ALF did not report HBV DNA viral load titers, and therefore, the possibility of including reactivation of chronic HBV cannot be ruled out.
Consensus Statement: Etiology, Hepatitis B
-
4.
HBV infection is an uncommon cause of ALF and is possibly decreasing over time (Grade of evidence: moderate)
Hepatitis E
Globally, genotype 1 and 2 HEV have been estimated to cause an estimated 20.1 million new infections annually in Asia and Africa.77 This is estimated to lead to around 3.4 million cases with acute hepatitis, 70,000 deaths related to ALF, and 3000 stillbirths.78 India is hyperendemic for HEV. HEV infection is the most common cause of acute sporadic and epidemic hepatitis. HEV accounts for 10–40% of acute hepatitis and 15–45% of ALF in India.79, 80, 81, 82 Almost all cases are related to genotype 1 in India.83 The mortality in HEV-related ALF has been reported as 51.9%.
In India and other endemic countries, despite common occurrence of clinical cases and outbreaks of HEV, the age-specific seroprevalence rates of anti-HEV are much lower (33–40%) than those for HAV and other feco-oral transmitted infections.84 HEV is a common cause for ALF across all parts of the country except the Northeast.24 Many epidemics have been reported from different parts of the country: Delhi (1955–: 29,300 cases), Aurangabad (1961–: 865 cases), Siliguri (1966: 4287 cases), Ahmadabad (1974: 2572 cases), Kanpur (1990: 79091 cases), Nellore (2008: 23915 cases). Hepatitis E shows seasonal variation, and most cases are documented between April and September every year.85 The mean age of presentation is the third and fourth decade, which is lower than other causes of ALF. The survival of patients with ALF of HEV etiology (55.1%) was significantly better than the survival of patients with ALF of other etiologies such as anti-TB treatment (30.0%), non-A non-E virus (38.1%), and HBV (35.9%).31
One of the most distinctive features of an epidemic and endemic hepatitis E in developing countries is increased incidence and severity of disease in pregnancy.33,52 Thus, the incidence of disease was 8 times higher and ALF occurred 13 times more in pregnant women than in age-matched men and nonpregnant women. ALF occurred in 44.4% among pregnant women in the third trimester. Clinical profile of ALF during pregnancy is characterized by a short pre-encephalopathy period, rapid development of severe encephalopathy, cerebral edema (CE), and cerebellar coning. HEV infection in pregnant women causes substantial fetal and perinatal mortality.51
The most common cause of ALF during pregnancy in India is HEV accounting for 60%.52 Pregnant women are more likely to develop ALF due to HEV. HEV is the causative agent of ALF among approximately 60% of pregnant women and girls, in contrast to 23% and 30% among age-matched men and boys and nonpregnant women and girls, respectively. Around 60% of women of childbearing age with ALF due to HEV are pregnant. In contrast, the fertility rate among women of childbearing age in the general population of India is 2.9%.86
Khuroo et al52 reported a mortality rate of 75% among pregnant women with HEV-related ALF, while a recent study51 showed a mortality of 51%. This mortality was similar to other causes of ALF among pregnant women (54.7%). The risk of development of ALF in symptomatic acute HEV during pregnancy is 8.8%, 19%, and 19% in 1st, 2nd, and 3rd trimester, respectively. However, once ALF develops, maternal outcomes are similar across all trimesters of pregnancy.52
Pregnant women with sarcopenia may be at higher risk of developing ALF due to HEV. Anthropometric parameters such as body mass index (BMI), mid-upper arm circumference (MUAC), and triceps skin fold thickness (TSFT) and serum levels of biochemical nutritional parameters such as albumin, globulin, prealbumin, and folate are significantly lower in the ALF group than in the acute viral hepatitis (AVH) group in pregnant patients with HEV infection.87 Reasons for the predisposition of pregnant women to develop acute HEV infection and ALF are unknown. Pregnancy is associated with changes in sex hormone levels, and the immune system is functioning at a lower key to protect the fetus. There is a shift from a Th1-dominant immune response to a Th-2 dominant one. This “Th2 bias” may help protect the fetus by suppressing macrophage activation.88 Impaired monocyte-macrophage function in pregnant women with ALF could contribute to an inadequate innate immune response and hence to the development and severity of ALF. Another study reported that high concentrations of cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-6, interferon [IFN]-γ, and tumor growth factor [TGF]-β1) may also be associated with an adverse pregnancy outcome.89
Women with ALF present a reduced expression of toll-like receptor (TLR) 3/TLR7/TLR9, a type of pattern recognition receptor (PRR) that plays a key role in the innate immune system, and have weaker phagocytic macrophages than women with acute viral hepatitis E.90 The concentrations of estrogen, progesterone, and β-human chorionic gonadotropin in HEV-positive pregnant women with acute liver failure (ALF) are higher than in HEV-negative pregnant ALF women or controls, although its association with HEV ALF is not clear.91
Consensus Statement: Etiology, Hepatitis E
-
5.
HEV is a common etiology of ALF in India. The most common cause of ALF in pregnancy is HEV in India. Pregnant women are more vulnerable to acute HEV and are at higher risk to develop ALF due to HEV (Grade of evidence: moderate; grade of recommendation: strong)
Indeterminate Etiology and Other Viruses
In several studies on ALF, in a significant proportion of cases, the infecting agent, although presumed to be viral, could not be detected with the currently available assays. In large series of ALF, in up to 47% cases, no etiological agent could be detected.3,25,73,92 In a recent study also, from Northeast India, any etiological agent could not be detected (called non A-E) in 44% of 255 patients with ALF.24 In this particular study, almost all of these patients had a history of herbal drug intake before development of encephalopathy. Most of these cases of ALF where the etiology could not be detected behaved clinically similar to a viral illness with a prodrome followed by jaundice and encephalopathy, suggesting some viral illness which was not routinely tested for. Herpes simplex virus is one such agent. In a large series of HCV hepatitis (n = 137), the patients had a stormy course with very poor prognosis with 74% progressing to death or (51% with acyclovir treatment and 88% without treatment). The diagnosis was made during postmortem in >50% as it was not suspected and tested for earlier.93 Most of the patients with herpes simplex virus (HSV)–related ALF have high HSV DNA loads, and the mortality may remain high even after transplantation.94 Other viruses that have been known to cause ALF and are not routinely tested for are the Epstein-Barr virus (EBV)95 and dengue virus. Recently, there has been a surge in the number of cases of dengue-related ALF seen during the seasonal epidemics in India both in children and adults, including cases of mixed infections.96,97 Another rare cause seen usually in immunocompromised individuals is a varicella-zoster virus (VZV). In these cases, the diagnosis is usually aided by the typical skin lesions.4
Among pediatric ALF population from India, a lower proportion (13–20%), as compared with adults, have indeterminate etiology.62,63 In all these cases of indeterminate etiology, the putative aetiologies could be some hitherto undetectable viruses, hepatotropic viruses undetectable by the present assays, some rare viral agents (that are routinely not tested), or cofactors such as herbal drugs or toxins. Thus, all those patients with ALF who clinically present similar to viral hepatitis and those in whom markers A to E are negative should be tested for other viruses such as HSV, EBV, dengue, and VZV.
Consensus Statement: Etiology, Other Virus and Indeterminate
-
6.
All etiological workup may be negative in about 15–47% of adults with ALF in India. In children, etiology remains indeterminate in 13–20%. Most of these patients may have an infection by other rare viruses, not tested for routinely, for example, dengue virus, HSV, EBV, VZV. Use of indigenous medicines as a cofactor cannot be ruled out (Grade of evidence: moderate)
Drug-Induced Liver Injury
Drugs have replaced viruses as the most common cause of ALF in high-income countries.4 O'grady et al19 have suggested that there are three distinct subgroups that incorporate distinct causes of ALF. Hyperacute liver failure (within 1 week) is generally caused by paracetamol or toxin intoxication; ALF (from 1 to 4 weeks) is generally caused by viruses and drugs; and subacute liver failure (from 4 to 12 weeks and beyond) is generally caused by drugs characterized by anti-TB drugs and complementary and alternative medicine. Subacute clinical course can mimic cirrhosis, clinically (ascites) and radiographically (irregular liver surface, nodularity).98 The nodularity on imaging is due to regenerating nodules.
Idiosyncratic DILI constitutes 5–13% of the cases of ALF worldwide.23,99 In India, one series reported that ~ 14% of all DILIs result in ALF.100 Classes of drugs causing idiosyncratic drug–induced ALF vary geographically. Paracetamol toxicity leading to ALF constitutes nearly half of all causes of ALF in the West,101 while in India, it constitutes ~1% of all causes of drug-induced ALF.102 Idiosyncratic drug–induced liver injury constitutes around 11% of all ALFs in the USA with antibacterials, antituberculosis agents, herbal and dietary supplements, antiepileptics, and nonsteroidal antiifammatory drugs, as top 5 causes.101 In India, anti-TB drugs, antiepileptic drugs (first generation), sulfonamides, and antiretroviral drugs are the most common drug classes causing drug-induced ALF.100 Anti-TB drug–induced ALF alone constitutes three-fourth of all DILI causes in a large single-center series and 63% in the Indian network on DILI. Anti-TB drug–induced ALF is also the commonest cause of drug-induced ALF leading to transplantation in India.100 This is not surprising as India accounts for one-fifth (21%) of all tuberculosis globally103 and three of the four first-line anti-TB drugs (isoniazid, rifampicin, and pyrazinamide) that are potentially hepatotoxic. Overall, antimicrobial agents are an important cause of drug-induced ALF worldwide.104
There is greater awareness of the contribution of alternative systems of medicine (ASMs) in India contributing to DILI, drug-induced ALF, and drug-induced ACLF.105, 106, 107 The Indian Network for DILI (INDILI) also found complementary and ASMs to be the second most common cause of DILI and drug-induced ALF.
Spontaneous survival in a large Indian series was only 34% and is driven partly by the higher mortality with anti-TB drugs which is almost two-thirds.100,108 Non-ATD and non-APAP drugs had a better survival (51%). INR and model for end-stage liver disease (MELD) predicted mortality. In patients with anti-TB drug–induced ALF, encephalopathy may not be evident initially and may set in days and weeks after the onset of jaundice. Other markers of severity such as ascites and coagulopathy occur before encephalopathy,100,109 highlighting the fact that the liver fails before encephalopathy sets in.110
ALF secondary to drugs occurs more commonly in women than in men.30,108,110,111,112,113 This phenomenon is particularly highlighted with regard to anti-TB drugs; although TB occurs more in men,103 the proportion of women who develop anti-TB drug–induced ALF is more in adverse outcomes (Table 9).30,111, 112, 113, 114, 115
Table 9.
Major Differences Between ALF and ACLF. TTC
| Characteristics | ALF | ACLF-APASL | ACLF-CLIF/AASLD |
|---|---|---|---|
| Acute insult | Hepatic | Hepatic | Hepatic or systemic |
| Time frame | 4–24 weeks | 4 weeks | 4–12 weeks |
| Chronic disease | No | CLD with or without cirrhosis | Cirrhosis |
| Hepatic decompensation | Yes | Yes | May or may not |
| Prior decompensation | No | No | Yes |
| Sepsis | Consequence | Consequence | Cause or consequence |
| Features of portal hypertension on endoscopy | No | May or may not | Yes |
| Evidence for chronicity on imaging | No | Yes | Yes |
| Spontaneous recovery | In nearly 40–60% cases | In nearly 40–50% cases | Very less |
| Hepatic reserve at baseline | Normal | Reduced | Grossly reduced |
| Hepatic reserve on long-term follow-up | Complete recovery | Partial recovery | No recovery |
Since there is no universally acceptable definition of ACLF, two commonly used definitions of ACLF have been considered.
ACLF, acute on chronic liver failure; ALF, acute liver failure; CLD, chronic liver disease; CLIF, chronic liver failure.
Drugs such as sulfonamides, aromatic antiepileptic drugs, and antiretroviral drugs are less common causes of DILI and DIALF. These drugs tend to cause immunoallergic liver injury with features of hypersensitivity such as cutaneous rashes, fever, lymphadenopathy, and eosinophilia.114 These extrahepatic features can be harbingers of severe disease and are important clues to early diagnosis and cessation of drug intake. Paracetamol hepatotoxicity which is very rare in India is a diagnosis of exclusion although paracetamol levels and adducts may aid diagnosis. Paracetamol hepatotoxicity can occur after single ingestion point or with staggered ingestion. Prognosis is worse in the latter.
Treatment of any drug-induced ALF includes prompt cessation of the offending drug and supportive care. Transfer to centers with facilities to manage liver failure and perform liver transplantation should be undertaken. Antidotes are few and are established for paracetamol toxicity (N-acetylcysteine) and leflunomide (cholestyramine). Early treatment with steroids may be effective in those with hypersensitivity phenomenon although controlled trails are lacking.116
Consensus Statement: Etiology, Drug-induced ALF
-
7.
ALF caused by drugs is a diagnosis of exclusion. Therefore, serological tests to rule out viral hepatitis A, B, C, and particularly E along with AIH should always be undertaken before a diagnosis of DIALF is considered. (Grade of evidence: moderate; grade of recommendation: strong)
-
8.
DILI, especially ATT-induced DILI, is the most frequent cause of DILI including DIALF in India. While TB is more common in males, ATT-induced DIALF and its related mortality is more common among females. (Grade of evidence: moderate; grade of recommendation: strong)
-
9.
Presence of hypersensitivity features is common in DILI resulting from some antiepileptic drugs such as carbamazepine and phenytoin and antibiotics such as dapsone and cotrimoxazole, and antivirals such as nevirapine. (Grade of evidence: moderate; grade of recommendation: strong)
-
10.
ALF caused by paracetamol drug–induced hepatotoxicity is rare in India. Similar to idiosyncratic drug–induced ALF, paracetamol hepatotoxicity is a diagnosis of exclusion, although paracetamol levels and adducts may aid diagnosis (Grade of evidence: moderate; grade of recommendation: strong)
ALF During Pregnancy
The incidence of ALF in the developed world is estimated to be less than 10 cases per million persons per year.117 The exact incidence of ALF in the developing world and in pregnancy is unknown; however, a large number of studies from India have shown that the occurrence of ALF in pregnancy is more common than that in general population.24,26,38,51,72 This is likely due to higher incidence of HEV infection in pregnancy and also certain pregnancy-specific conditions such as acute fatty liver of pregnancy (AFLP) and hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome causing ALF in the third trimester of pregnancy.118 It has been postulated that physiological stress coupled with poor nutrition of pregnant females in our country causes increased susceptibility to viral infections.
The most common cause of ALF in pregnancy is viral hepatitis, and the incidence of the same in pregnancy is higher than in general population. HEV infection contributes to more than half cases of icteric hepatitis in the pregnant population, while its prevalence in pregnant women with ALF is 60–90%.26,38,51,72 Other viral infections causing ALF in pregnancy include HBV, HAV, and rarely HSV and VZV. The other causes of ALF in pregnancy reflect similar trends as in the general population with some pregnancy-specific causes to be made a note of. These include AFLP; HELLP syndrome; and its complications such as acute hepatic rupture.
During an outbreak of HEV infection, it is observed that pregnant women have a higher likelihood to get infected (12–20%) and have a higher propensity to develop ALF (10–22%) than nonpregnant women and men (1–2%).119 In addition, this susceptibility increases in a linear fashion with increasing trimester of pregnancy.52,120 Several other studies from various parts of our country have confirmed this observation.39,72,121 Viral hepatitis in pregnancy carries a high mortality rate because of ensuing risk of liver failure with a poor maternal outcome.122,123 While Indian studies have clearly shown worse prognosis among pregnant patients with HEV-related ALF, a few reports from other countries found no significant difference in mortality among age-matched pregnant and nonpregnant population.124
A detailed history of recent travel, change of food habits, surgery or blood transfusion, alternative medication intake, and family history of liver-related disorders should be taken into consideration. Pregnancy-specific conditions such as AFLP and HELLP should be ruled out. Clinical features such as ascites and hypertension favor the diagnosis of pregnancy-associated liver diseases and can help differentiating it from viral hepatitis. In addition, it has been shown that ascites in such conditions is transient and may be related to portal hypertension.124,125,126 A history of previous pregnancy losses, third trimester of pregnancy, multiple gestations, male fetus, and primigravid status may suggest AFLP.127 Associated symptoms of itching, pedal edema, and hypertension may suggest HELLP syndrome.
Consensus Statement: Etiology, ALF in Pregnancy
-
11.
Pregnant women are more likely to have ALF than nonpregnant women and men because of increased frequency of HEV infection in pregnancy and occurrence of certain pregnancy-specific causes of liver failure (Grade of evidence: moderate; grade of recommendation: strong)
-
12.
HEV infection is a common cause of ALF in pregnancy in India and occurs more commonly in the third trimester of pregnancy. Pregnancy-specific causes of ALF at this time period include AFLP, HELLP syndrome, and acute hepatic (Grade of evidence: moderate; grade of recommendation: strong)
-
13.
The prognosis of ALF in pregnancy is similar to that in general population and is independent of the etiology of ALF or the trimester of pregnancy (Grade of evidence: moderate; grade of recommendation: strong)
Yellow Phosphorus
Yellow phosphorus (YP) is commonly used in India as a rat-killer poison. It is an inorganic waxy substance, highly combustible in the presence of oxygen, and is also commonly used in the manufacture of fireworks, pesticides, match heads, and military ammunition. YP poisoning is an uncommon cause of ALF.128,129 The toxic dose is 1 mg/kg body weight. Commonly used rat killers contain 3% zinc phosphide, which is available as a paste. Upon ingestion, zinc phosphide gets hydrolyzed by gastric acid and releases phosphine, a highly toxic gas that gets rapidly absorbed from the gastrointestinal system. Isotopic studies on rats have shown rapid absorption and deposition of 70% phosphorus in the liver within 3 h, leading to severe injury. The toxin may also get deposited in other organs such as the heart, kidney, and brain. Issues pertaining to management and prognosis of YP-ALF are not well known.
YP is commonly used as a suicidal agent in some parts of India. YP poisoning is predominently seen in the 2nd and 4th decade of life as also seen in some other parts of the world.130,131 Sublethal dose may not be associated with severe liver injury.132 The damaging effects on the liver after YP poisoning include hepatic steatosis due to impaired beta-oxidation resulting in the esterification of fatty acids. In addition, YP poisoning leads to hepatocyte cell death. The liver shows a mottled appearance with liver cell necrosis and collapsed liver plate and inflammatory cell infiltration of all zones.133,134
Patients can be asymptomatic in the first 48 h after oral ingestion. They may have nausea, vomiting, and abdominal discomfort. These nonspecific symptoms may mislead the clinician, and often patients get treated for gastroenteritis in the absence of proper clinical history. Mortality rates after YP poisoning are high with associated ALF and cardiac toxicity.135,136 Survival apart from clinical and biochemical factors is dependent on the amount ingested. Thus, patients who consumed a lower dose (6 g) survived than those who had consumed a higher dose of >10 g. In one series, severely deranged liver function and coagulopathy were common among nonsurvivors (36.3%). Hypoglycemia occurs in these patients probably related to increased activity of glucose-6-phosphatase. In a series from the Philippines, 4 of 15 patients with YP poisoning died (27%). Serum aminotransferases and PT were significantly high in the fatal group. Metabolic acidosis and hypoglycemia were significantly associated with high mortality.137 A high lactate level and low blood glucose levels are also associated with higher mortality. YP poisoning has been reported in children owing to accidental ingestion. In a series of 10 children with YP-related ALF, only one patient survived without major complications; 3 children died of renal and cardiac failure, 6 underwent living donor LT (LDLT), of whom 3 died within the first week. The rest survived and were well at a mean follow-up of 204 days.138
Consensus Statement: Etiology, Toxins
-
14.
Toxins that cause ALF include YP, mushrooms, and herbal food supplements, and they are rare causes of ALF (Grade of evidence: low; grade of recommendation: strong)
Vascular Causes
Budd-Chiari syndrome (BCS) as a cause of ALF is extremely rare. It accounts for 0.9%–1.5% of all cases.139,140 It results from hepatic venous outflow tract obstruction (HVOTO) due to either thrombus of hepatic veins (HVs) or inferior vena cava. Many such patients with BCS present subacutely over weeks due to partial occlusion of one or more HVs, but when all the three main HVs drain the liver clot simultaneously, there is diffuse and diverse intrahepatic ischemia with massive necrosis. The resultant ALF leaves inadequate time for collaterals to develop. It thus has a high mortality and a rapid clinical course, necessitating urgent management.
The largest series consisted of 19 cases of BCS manifesting as ALF from the US Acute Liver Failure (USALF) study group of 2344 enrolled with patients with ALF. Overall, these patients were young with a median age of 38 yrs (19–59), mostly Caucasian woman (84%). Polycythemia vera was the most commonly identified thrombophilic factor (37%), while some had a history of estrogen use. It may also arise secondarily when a liver cyst compresses all HV after infection or a rapid increase in the size due to bleed in the liver cyst. Some BCS have been described during pregnancy.141 However, in a large series of a consecutive patient with BCS, not a single case was documented to have ALF.142
All patients with ALF should undergo abdominal ultrasound (US) as part of the initial evaluation. The features suggestive of BCS include ascites, hepatomegaly, heterogeneous hepatic parenchyma, caudate lobe hypertrophy, thickened gallbladder, and splenomegaly. Doppler US shows inferior vena cava (IVC) compression, no flow and nonvisualization of HVs, and slow hepatofugal or bidirectional flow in portal and splenic vein with the absence of collaterals. These findings are also supported by computed tomography without diffuse liver atrophy or hepatic regenerative nodules as seen in chronic BCS.
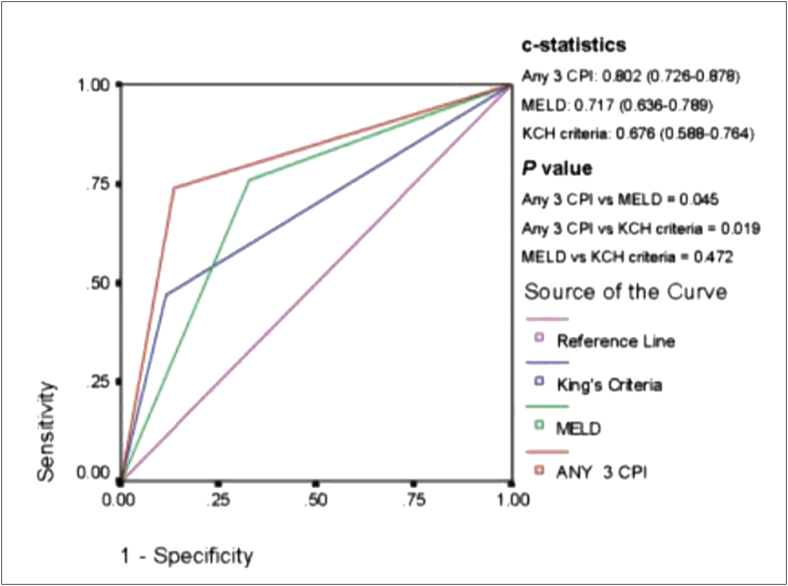
Serum biochemistry specially aspartate transaminase (AST)-to-alanine transaminase (ALT) ratio of >1 gives a clue to diagnosis of ALF due to BCS. This ratio is however reversed in other causes of ALF. Peak ALT is also associated with poor outcomes supporting the idea that greater ischemia portents worse patient outcomes.143 High peak creatinine also predicted poor outcomes. Both MELD and King's College Hospital (KCH) criteria liver failure were not predictive of survival in these patients. The recent report from India has defined a new All India Institute of Medical Sciences-hepatic venous outflow tract obstruction (AIMS-HVOTO) Score which has included therapy such as angioplasty and transjugular intrahepatic porto systemic shunt (TIPSS) to calculate prognostic score for liver transplant.142 Survival rates in ALF-BCS are poor (37–40%) in spite of LT. Anticoagulation should be initiated as soon as the diagnosis is made. Surgical shunts for decompressing the liver have been replaced by TIPSS. TIPSS has been an effective treatment in a series of 9 patients with ALF-BCS144 as well as from the series from India in which angioplasty with or without TIPSS had documented marked improvement in survival. Listing for LT should occur in parallel with anticoagulation and TIPSS. The prognostic model described from AIIMS may be used to select patients for LT. Some groups recommend TIPSS as first-line therapy in ALF with a survival of 50–80%.145
Consensus Statement: Etiology, Vascular Causes
-
15.
ALF due to vascular causes includes acute HVOTO, which is rare (Grade of evidence: moderate; grade of recommendation: strong)
Autoimmune Hepatitis
AIH seems to be an important cause of indeterminate etiology of ALF ranging from 8% to 30% of the total population of ALF.146, 147, 148 Most studies have looked at acute severe AIH and ALF-AIH as a combined single group, thereby including patients with and without encephalopathy, respectively. Studies have shown that 12.5–69% of such patients proceeded to have true ALF.149, 150, 151 AIH is suspected as an etiology of ALF in the presence of autoantibodies, high gamma globulin, and other autoimmune diseases.22 However, positivity of antinuclear antibody (ANA) or antismooth muscle antibody (ASMA) ranges from 29% to 88%152,153 and seronegativity seems to be not uncommon in ALF-AIH. A high positivity of autoantibodies was noted when low titers were included, whereas the positivity reduced if International Autoimmune Hepatitis Group (IAHG) criteria were applied. Similarly, normal levels of gamma globulins have been described in 25–39% of patients with acute severe AIH. Furthermore, the concentration of gamma globulin in the ALF-AIH group was significantly lower than that in the chronic AIH group with acute exacerbation (3.9 g/dl versus 4.7 g/dl). On the other hand, autoantibodies may be present in other etiological causes of ALF as well.154,155 Children with ALF-AIH usually have liver-kidney microsomal antibodies with poor response to immunosuppressive therapy and need an urgent transplant.156,157 Giant cell hepatitis in children has been shown to be associated with autoimmune hemolytic anemia.158 Drugs can be a superimposed insult on a background of autoimmune liver disease. In addition, DILI could have an immune basis. Of recent interest is the development of ALF with cancer immunotherapy – specifically the immune checkpoint inhibitors (e.g., nivolumab).159
Liver biopsy (transjugular) plays an important role in the diagnosis of AIH. Typical AIH histopathology includes portal inflammation with plasma cells, interface hepatitis, rosettes, and emperipolesis. Histopathology of ALF-AIH may not show these features and typically reveals centrilobular necrosis with portal inflammation developing later.160 As the ALF progresses, the necrosis can become confluent, making diagnosis difficult. Therefore, the timing of liver biopsy is important. The USALF study group has described 5 types of massive hepatic necrosis (MHN) as a characteristic finding in ALF and suggested MHN type 4 with centrizonal hemorrhagic necrosis and type 5 with confluent necrosis superimposed on chronic hepatitis as suggestive of AIH etiology.161 Presence of fibrosis implies chronicity, but this may be difficult to differentiate it from reticulin collapse and regeneration seen in massive necrosis. Special stains may help differentiate the two entities.162 Drug-induced ALF-AIH may have a predominance of eosinophils on liver biopsy.
Consensus Statement: Etiology, AIH
-
16.
Autoimmune etiology is an important cause of indeterminate ALF in up to one-third of patients (Grade of evidence: moderate; grade of recommendation: strong)
-
17.
In a patient with ALF, autoimmune etiology should be suspected in those with autoantibodies, high globulins, and other autoimmune diseases (Grade of evidence: moderate; grade of recommendation: strong)
-
18.
Autoantibodies may be absent, and gamma globulin may be normal in up to one-third of patients with ALF-AIH. Conversely, autoantibodies may be present in other etiologies of ALF, necessitating a liver biopsy (Grade of evidence: moderate; grade of recommendation: strong)
-
19.
Presence of high-grade hepatic encephalopathy, presence of high MELD scores, or lack of improvement within seven days of corticosteroids implies poor prognosis and necessitates a liver transplant. (Grade of evidence: moderate; grade of recommendation: strong)
Wilson Disease
WD can present as ALF-WD predominantly in childhood or adolescence in 8–20% of patients.163,164 The presentation starts as acute hepatitis but may rapidly deteriorate with deep jaundice, coagulopathy, ascites, and encephalopathy. Majority of patients presenting with clinical “ALF” have been found to have cirrhosis on explants and therefore are not true ALFs.165, 166, 167 Nevertheless, these patients with the first acute presentation and encephalopathy are considered as ALF despite evidence of chronicity.22 Presence of Coomb's negative hemolytic anemia with ALF is a useful clue to the diagnosis of WD.168 Alkaline phosphatase-to-total bilirubin ratio of <4 and the AST:ALT ratio of >2.2 have been described to have a sensitivity and specificity of 100% in ALF-WD in a study by Korman et al.167 The explanation of AST being more than ALT is that most patients with ALF-WD have a background of significant fibrosis. However, these ratios have not been validated in other studies.169
The diagnosis is important as the patient may be manageable with chelators d-penicillamine or trientine without the need for a transplant. Besides, even if a transplant is deemed to be necessary, it is important to rule out WD in the potential living donor who is often a close relative of the patient. In addition, screening of siblings and parents may diagnose presymptomatic disease. Serum ceruloplasmin is of little value in the setting of ALF-WD as may be falsely normal as it rises in the presence of inflammation being an acute-phase reactant. A very low value of <5, however, may be useful as the presence of significant liver disease would lower all proteins synthesized in the liver but not to this extent. A serum ceruloplasmin measured by the oxidase method was more specific than that measured by the nephelometry method.167 Serum copper is not reliable both in the setting of CLD; however, a value of >200 g/dl had a sensitivity of 75% and a specificity of 96% in the ALF-WD group.
Twenty-four hour urine copper may be underestimated if there is associated renal dysfunction and the urine output is low.170 On the other hand, it may be falsely raised in other causes of ALF as copper is released from the liver secondary to MHN of any cause.171 Slit lamp examination for Kayser–Fleischer (KF) rings is difficult in a patient with hepatic encephalopathy although hand held slit lamps are now available to be used by the bedside. KF rings may be present in only 50% of patients with ALF-WD.167 Liver biopsy to estimate liver copper as a diagnostic test is not reliable, and staining for copper may not be of value in the acute setting with significant cholestasis.172 Genetic mutation studies of the ATP7B gene are not a feasible option for diagnosis of ALF-WD as the results are not available at short notice.
Consensus Statement: Etiology, WD
-
20.
Acute presentation of WD with jaundice and encephalopathy should be considered as ALF and is usually seen in childhood or adolescence. Coomb's negative hemolytic anemia is almost always seen in the setting of ALF-WD (Grade of evidence: moderate; grade of recommendation: strong)
-
21.
New Wilson's Index (NWI) ≥11 has been used as a predictor of the need for LT. Rising bilirubin, hepatic encephalopathy, and acute hemolysis have been suggested as better predictors for LT than NWI, MELD, and PELD although they need validation (Grade of evidence: moderate; grade of recommendation: strong)
-
22.
Molecular adsorbent recirculating system (MARS) and total plasma exchange are newer modalities which rapidly remove copper and can be used as bridging therapy to LT (Grade of evidence: moderate; grade of recommendation: strong)
Other Rare Causes of ALF
Reye syndrome is characterized by acute noninflammatory encephalopathy and fatty degenerative liver failure. The pathogenesis of Reye syndrome appears to involve mitochondrial injury resulting in dysfunction that disrupts oxidative phosphorylation and fatty acid beta-oxidation in a virus-infected, sensitized host potentially with an underlying occult inborn error of fatty acid oxidation, urea cycle, or mitochondrial disorder. The mortality rate has decreased from 50% to less than 20% as a result of early diagnosis, recognition of mild cases, and aggressive therapy.173
Hemophagocytic lymphohistiocytosis (HLH) is a potentially fatal and likely underdiagnosed disease characterized by unregulated histiocyte proliferation, hypercytokinemia, and hemophagocytosis, causing life-threatening tissue damage and organ failure with high mortality. HLH is classified as either primary or familial HLH and secondary or acquired HLH. Liver injury is a common complication of HLH. In previous studies, ~85% of adult patients with secondary HLH had elevated ALT and AST levels, and ~50% of patients had hyperbilirubinemia. In patients with ALF induced by HLH, MHN can be found. It is considered that liver injury results from either infiltration of activated hemophagocytic histiocytes or overproduction of cytokines in patients with HLH. Liver injury may also be the result of underlying diseases. Bone marrow puncture biopsy and/or liver biopsy are helpful in the diagnosis of most cases of HLH. The prognosis of adult patients with ALF caused by HLH is very poor. Although the efficacy of the HLH 2004 protocol in such cases still remains to be demonstrated, early diagnosis and prompt combined treatment with steroids and cyclosporin A or etoposide must be emphasized.174
Extensive malignant infiltration of the liver, which can occur in metastatic breast cancer and lymphoma, can result in sALI or ALF. It is important to make this diagnosis early as these patients are not candidates for LT. In patients with a history of cancer or hepatomegaly, malignant infiltration should be ruled out with imaging and/or liver biopsy. Liver imaging has a pattern of diffuse infiltration as opposed to multiple deposits. The liver biochemistry classically shows an elevated alkaline phosphatase and gamma-glutamyl transferase but on occasions may present with marked increase in serum transaminases, caused by hepatocyte ischemia resulting upon the infiltration. In patients with lymphoma, a greater elevation of lactate dehydrogenase is observed compared with serum transaminases. Consideration of an underlying malignant process and potential infiltration should also be considered in acute presentations of BCS.175
Consensus Statement: Etiology, Rare Causes
-
23.
HLH, Reye syndrome, and extensive malignant infiltration are rare causes of ALF (Grade of evidence: low)
Pathogenesis of ALF
Liver Cell Death in ALF
ALF is characterized by sudden and massive, confluent loss of hepatocytes that stuns and overwhelms the otherwise extraordinarily brilliant ability of the liver to maintain homeostasis by regeneration to keep structural and functional integrity. “Cell drop out” – a classical histological feature of ALF – is not a passive, bland demise of cells. It is carried out by highly intricate mechanisms. Understanding these offers opportunities for evolving drug targets and potential predictive biomarkers in ALF.176,177
ALF caused by toxins, infections, and metabolic, ischemic, and genetic diseases leads to hepatocyte injury and subsequent death by broadly similar mechanisms. Consequential and accompanying immunological, metabolic, and hemodynamic alterations that seriously challenge organ function over a short time span and finally threaten survival are also similar. Necrosis and apoptosis are the classical death pathways.178 There are overlapping forms such as necroptosis which are relevant for ALF. A distinction of different cell death forms therefore not only is relevant semantically but also has important clinical implications when considering the therapeutic targeting of cell death processes.179
The relative contribution of apoptosis or necrosis to organ dysfunction in ALF remains controversial. Necrosis is typically the consequence of acute metabolic perturbation with adenosine triphosphate (ATP) depletion, whereas apoptosis represents an ATP-dependent cell death program. At low doses, a variety of injurious stimuli often induce apoptosis, but the same stimuli can result in necrosis at higher doses. Necrosis is viewed as a largely unregulated consequence of physicochemical stress, characterized by mitochondrial impairment, depletion of ATP, and subsequent failure of ATP-dependent ion pumps. This results in rapid swelling of cells and cell organelles (“oncosis”) accompanied by the formation of membrane “blebs” and ultimately cellular rupture. As a consequence, cellular constituents spill into the extracellular environment and elicit significant inflammatory responses, rendering necrosis an “immunogenic” form of cell death. Recent studies have highlighted that mitochondrial permeability transition (MPT) leads to the opening of a mitochondrial pore, triggering mitochondrial swelling and uncoupling of oxidative phosphorylation as a result of osmotic forces. Furthermore, lack of ATP may convert apoptotic death into secondary necrosis (also sometimes referred to as “necraptosis” or “aponecrosis,” to be distinguished from “necroptosis”. Diseases with cell death that used to be considered largely a consequence of unregulated necrosis, such as acetaminophen-induced liver injury and ischemia-reperfusion injury, can possibly be modulated by MPT inhibitor cyclosporin A or c-Jun N-terminal kinases (JNK) inhibitors, suggesting an important role of regulated necrosis in these settings.
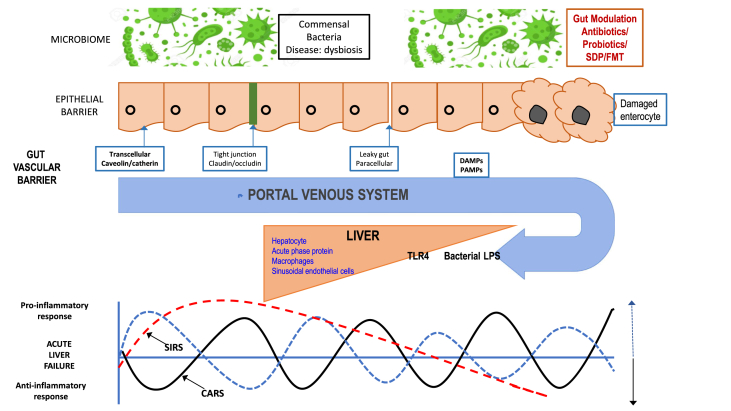
ALF is also associated with a massive immune response, with the recruitment of inflammatory cells from the peripheral circulation into the liver, the activation of stress and death receptors, and the clearance of apoptotic/necrotic debris, which lead to the perpetuation of hepatic inflammation and injury. Activated immune cells, as well as dying hepatocytes and stromal cells, are capable of secreting chemokines that lead to the further recruitment and retention of effector T and natural killer cells that amplify the inflammatory response often leading to a systemic inflammatory response syndrome (SIRS) that is the most common cause of death. Fas and TNF-α receptor activation are well-characterized processes leading to secretion of TNF-α that aggravates apoptosis and increased hepatic chemokines that recruit TNF-α–secreting neutrophils to the liver. Regardless of various etiologies, clinical evidence suggests that ALF is generally associated with significant and uncontrolled activation of systemic inflammation, which may consequently lead to multiple organ failure and poor prognosis.180, 181, 182
The prognosis of ALF is primarily dependent on the underlying etiology. During ALF, viral-mediated (i.e., direct cytopathic effects), cytokine- and/or immune-mediated (i.e., indirect cytopathic effects) hepatocyte necrosis, and apoptosis occur. A regenerative process is triggered, and replication of the remaining healthy hepatocytes ensues, in an attempt to restore hepatic architecture and function. This process is initiated or regulated, at least in part, by three major factors which include cytokines, growth factors, and metabolic signaling pathways. Quantum of liver injury, the tempo of its evolution as a destabilizer of homeostasis of cell and organelle, the cytokine – chemokine storm that it brings and the extent – as well as the timeframe of the activation of the regenerative machinery that happens are determinants of outcome after injury – the death of hepatocytes in ALF. Each of these phenomena are highly variable in quantity as well as nature and stems from an initiating event that perpetuates threat to cell viability and homeostasis. The primary modes of cell death have well-delineated pathways with multiple intracellular molecules that provide opportunities for intervention as a drug target and/or potential biomarker through its imprints in circulation.183,184
Consensus Statement: Pathogenesis of Liver Necrosis
-
24.
Different etiological agents cause ALF through similar mechanisms that result in massive hepatocyte death along with an immune and inflammatory response in both hepatic microenvironment and systemic circulation (Grade of evidence: low)
-
25.
Sudden, confluent but graded loss of hepatocyte occurs by a mixed mechanism of apoptosis and necrosis triggered by etiology and sustained by immune response (Grade of evidence: low)
-
26.
The cell death pathways are structured and interchangeable depending on the metabolic and immune milieu and are comprised of several molecules that may offer opportunities for the development of drug targets as well as predictive biomarkers (Grade of evidence: low)
-
27.
Available knowledge on the mechanism of necrosis is mostly based on experimental animals, and human data are needed (Grade of evidence: low; grade of recommendation: strong)
Cerebral Complications
Hepatic encephalopathy is the most dramatic clinical manifestation of ALF and includes headache, vomiting, asterixis, agitation, hyperreflexia, clonus, and varying degree of coma.185
Pathogenesis of encephalopathy is multifactorial. Several inflammatory markers and neurochemicals play an important role. Ammonia produced in the gut cannot be converted to urea by the liver as it normally does in health. A part of this ammonia is metabolized by combining with glutamate to form glutamine by the action of the enzyme glutamine synthetase which is present in the brain, kidney, and muscle.185 In patients with ALF, the arterial ammonia levels rapidly rise owing to loss of hepatocyte function. Ammonia has multiple actions on central nervous system (CNS) function that include direct effects of the ammonium ion (NH4+) on both excitatory and inhibitory neurotransmission, inhibition of glucose (pyruvate) oxidation and stimulation of glycolysis, altered mitochondrial function, and impairment of key cellular transport systems.186 High levels of ammonia are fixed by combining with glutamate to form glutamine in the brain. Glutamine itself is probably not responsible for the brain edema; however, it releases ammonia after crossing the mitochondrial membrane, thereby affecting the mitochondrial function.187 Increased brain lactate levels have been noted in animal models of ALF. Brain lactate levels have been found to have a direct correlation with both the development of hepatic encephalopathy as well as surges in intracerebral pressure (ICP) and mortality.188
The astrocyte is the most abundant glial cell in the brain and along with the microglia (macrophages) has been the main site of neurological dysfunction in ALF. Owing to liver necrosis and accumulation of ammonia, lactate, and cytokines in the astrocytes, there is downregulation of genes responsible for the synthesis of a number of structural proteins, neurotransmitters, transporter proteins, and receptor proteins.187 This results in the loss of viscoelastic properties of the astrocytes,189 accumulation of glutamate and glycine, and consequent brain excitation of the synapses, resulting in agitation and seizures.190 There is also upregulation of the glucose receptor causing an influx of glucose and water into the brain.191 Activation of microglia leads to the local release of inflammatory mediators such as TNF-α and IL-6. Both the latter processes cause brain edema and intracerebral hypertension (ICH).192
ALF is a state of immune paralysis, and sepsis is common (reported in 55–90% of patients with ALF) and is directly related to mortality.193 An SIRS results from the release of inflammatory cytokines from the necrotic liver in ALF as well as from sepsis.45 The cytokines (predominantly TNF-α, IL-1β, and IL-6) and endotoxins lead to alteration of the receptors present at endothelial cells and capillary tight junctions. This further increases the permeability of the blood–brain barrier. This systemic inflammatory state accentuates brain inflammation by stimulating the local microglia and adds to brain edema and ICH.193
Normally, cerebral blood flow (CBF) is tightly autoregulated to match metabolic demands of the brain. In ALF, both an increase and decrease in CBF can occur depending on the brain activity. Loss of cerebral autoregulation by the endothelial cells leads to an excess of fluid accumulating the brain. However, the cerebral vascular response to carbon dioxide remains intact, and thus, hyperventilation to induce hypocapnic vasoconstriction to decrease CBF can be utilized as a short-term strategy with those with raised ICP.194
Hyponatremia for unclear reasons has been associated with CE. One-fourth of patients with ALF from India have been reported to have hyponatremia.195 Use of sedatives, metabolic alkalosis, and sepsis are the other reversible factors which along with ammonia-lowering agents form the basis of treatment of ALF.
Clinical significance of CE is in being the most frequent cause of mortality in ALF. It has been documented to be present in 58% of patients in India.29 It is associated with mortality in 82% as compared with 44% in those without CE.29,196 Death due to CE is due to surges in ICP and coning. Recent literature with ICP monitoring has shown the presence of CE in most patients with HE at admission.197 CE manifests clinically as arterial hypertension, bradycardia, mydriasis, decerebrate posturing, and seizures. Seizures have been reported as a poor prognostic marker as they indicate advanced CE.198 Patients who are at higher risk of ICH are those with a hyperacute or acute presentation, shorter jaundice-to-encephalopathy interval, younger age, renal impairment, persistent elevation of arterial ammonia, and sepsis.199
ICP monitoring is the gold standard for monitoring ICH. However, there are no data to show that ICP monitoring has led to any survival benefit although randomized studies are lacking.200 The European Association for the Study of the Liver (EASL) recommends the use of ICP monitoring in patients at high risk of ICH,22 while the AASLD recommends its use only in patients likely to be subjected to transplant.18 ICP monitoring is associated with a significant risk of complications which include bleeding and infection.201 It has not found favor in any major hepatology center in India. Invasive monitoring may be carried out using subdural, epidural, or intraparenchymal catheters. Several noninvasive methods have been developed for ICP monitoring, and they include reverse jugular vein oxygen saturation, middle cerebral and transcranial artery Doppler, and optic nerve sheath diameter (ONSD) measurement.202, 203, 204
Grading of HE in ALF
Acute encephalopathy of ALF is labeled as type A encephalopathy as per the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) guidelines.205 The West Haven criteria are useful for discriminating grade 1 and 2 HE as they require subjective responses by the patient. More severe grades of encephalopathy are better graded with the Glasgow Coma Scale or the more recent full outline of unresponsiveness (FOUR) score for HE which can even be used in patients who are on a ventilator.206
Consensus Statement: Pathogenesis of CNS Complications
-
28.
Cerebral complications (HE and CE) are an integral part of ALF, and they determine the outcome of the disease (Grade of evidence: moderate; grade of recommendation: strong)
-
29.
The pathogenesis of ICH is multifactorial with an increase in ammonia and neuroinflammation being the central mechanism (Grade of evidence: moderate; grade of recommendation: strong)
-
30.
Most patients with high grades of encephalopathy have CE and ICH. The severity of ICH is directly related to the risk of mortality (Grade of evidence: moderate; grade of recommendation: strong)
-
31.
ICP monitoring is the gold standard to measure ICH as clinical signs of CE have low sensitivity (Grade of evidence: moderate; grade of recommendation: strong)
-
32.
West Haven criteria are the most accepted method to assess HE. The FOUR score can be used to assess patients on ventilators (Grade of evidence: moderate; grade of recommendation: strong)
Acute Kidney Injury in ALF
Acute kidney injury (AKI) in the setting of liver cirrhosis (LC) has recently been defined.207 It has been suggested that AKI in ALF should also be defined in the same manner,208 although no prospective studies applying this definition and severity grading have been reported so far. The occurrence of AKI in patients with ALF is a harbinger of poor outcomes as it results in prolongation of hospital stay (11 days vs. 6 days in the non-AKI cohort) as well as reduced spontaneous survival (36% vs. 84% in the non-AKI cohort).209
A high frequency of AKI in ALF, ranging from 40 to 80%, has been noted in reports from tertiary centers in the West.210 The frequency of AKI is higher in acetaminophen-related ALF (80%) than in non–acetaminophen-related ALF (51%).209 Surprisingly, the frequency of AKI in large series reported from northern India (Delhi, Srinagar, Chandigarh) has been low (3–10%), although a figure of 30% is reported in one study from Srinagar.23,26,29,51 Several risk factors for the development of AKI with patients with ALF have been identified. They include older age, SIRS, development of sepsis and hypotension with need for vasopressor support, severity of ALF, and acetaminophen etiology of ALF.
Limited data are available about the pathogenesis of AKI in ALF. It is thought that mechanisms responsible for AKI in ALF and AKI in sepsis may have much in common.
-
1.Acute tubular necrosis (ATN) is well described in patients with AKI in the setting of ALF. It may be either “toxic” or “ischemic.”
-
A.Direct nephrotoxicity: Incidence of renal failure is higher in cases of ALF owing to acetaminophen overdose and other hepatotoxins (e.g., Amanita phalloides poisoning and cotrimoxazole). Acetaminophen can produce a direct renal toxic effect, or it may induce ATN owing to glutathione depletion produced by toxic metabolites (n-acetyl-p-benzo-quinone-imine [NAPQI]) produced either within the kidney or by the liver. Acetaminophen-induced tubular apoptotic cell death has been described in vitro via an antiapoptotic protein Bcl-xL–dependent pathway. The oxidant stress and mitochondrial dysfunction characteristic of acetaminophen overdose may also contribute to the kidney injury.211 It has been suggested that toxicity may also occur due to cadmium nephrotoxicity. Cadmium from the environment that has accumulated in the liver is released in ALF and is redistributed to the kidneys, which are very sensitive to cadmium and exhibit tubular damage.212 ALF associated with heat stroke may be associated with significant rhabdomyolysis contributing to the development of toxic ATN and AKI.213
-
B.Ischemic injury to renal tubules and glomeruli can occur in ALF owing to reduction in renal blood flow or programmed necrosis pathways in ischemic conditions. Reduction in renal blood flow caused by factors such as hypotension, vasodilatation, and cardiac injury produces renal injury. The epithelial cells of the proximal tubules in the S3 segment in the outer medulla are particularly sensitive. Activation of the sympathetic nervous system (SNS) and the renin–angiotensin–aldosterone system (RAAS) contributes to renal vasoconstriction.214 Cell necrosis is now recognized as a regulated process in ischemic conditions, and a programmed necrosis pathways involving the poly(ADP-ribose) polymerase–calpain axis is thought to contribute to AKI.215
-
A.
-
2.
Systemic inflammation in ALF: Systemic inflammation is universal in ALF and may contribute to AKI. Elevated concentrations of proinflammatory cytokines (IL-1, TNF-a) may induce inflammation within the renal parenchyma. Damage-associated molecular patterns (DAMPs, e.g., cyclophilin A, HMBG1) released by necrosis within the kidney and the liver activate the renal innate immune system.216 DAMPs are ligands for cell surface TLRs, especially TLR-4, supporting a role for TLR-4 blockade in therapy. Occurrence of infections releases pathogen-associated molecular patterns (PAMPs) that augment AKI.
-
3.
Functional renal failure or hepatorenal syndrome, as seen in LC and ACLF, may also occur in ALF, especially the slowly evolving forms such as subacute liver failure, via similar mechanisms, i.e., renal vasoconstriction, activation of the RAAS and functional renal failure in the absence of any histological change.217
Consensus Statement: Pathogenesis of AKI
-
33.
AKI is common among patients with ALF in the West (40–80%) but is less common in India (3–30%) (Grade of evidence: moderate; grade of recommendation: weak)
-
34.
AKI is associated more commonly in patients with paracetamol overdose and is associated with poorer prognosis (Grade of evidence: moderate; grade of recommendation: strong)
-
35.
Other risk factors for AKI in ALF include older age, hypotension requiring vasopressor support, sepsis, and SIRS (Grade of evidence: moderate; grade of recommendation: strong)
Role of the Gut in ALF
There is a close intertwined connection between the liver, the gut, and the immune system. The human gastrointestinal tract is recognized as one of the largest immune organs in the body with the gut-associated lymphoid tissue which comprises almost 40% of the immune effector cells in the body, constituting up to 25% of the intestinal mucosal mass. The intestinal barrier system is composed of luminal factors, epithelial factors, and submucosal factors.218 Organisms, their endotoxins, and cytokines that cross the intestinal barrier are tackled by the liver. Uncontrolled release of these inflammatory cytokines secondary to bacterial endotoxin and DAMPs is responsible for multiorgan failure. Thus, rapid immune paralysis is well documented in patients with ALF.219 Infections have been reported in 55–90% of patients with ALF, and they constitute a common cause of death in such cases. Their impact on encephalopathy apart from organ failures has also been well documented in patients with ALF.220 A breach of the epithelial lining of the gut can be instrumental in sepsis-associated multiorgan failure or gut origin of sepsis, especially in the presence of host immune dysfunction (Figure 2). The gut can also be secondarily affected owing to multiorgan failure in patients with sepsis. This is caused by ischemia and reperfusion injury causing disruption of the splanchnic blood flow, apoptosis, necrosis of gut epithelial cells, and development of paralytic ileus with its effects on nutrient absorption.221 Animal studies have confirmed the role of a leaky gut in ALF.222
Figure 2.
Role of gut microbiome in modulating liver injury. Bacterial products (PAMPs) as well as endotoxins initiate a proinflammatory response leading to cytokine storm and SIRS. It cannot be handled by the liver's immune apparatus which is grappling with a huge amount of DAMPs released by liver cell necrosis. PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; LPS, lipopolysaccharaide; SIRS, systemic inflammatory response syndrome; TLR4, toll-like receptor 4; CARS, compensatory anti-inflammatory response syndrome.
Hepatic Regeneration in ALF
The liver can regenerate in response to hepatic damage. Unlike CLD, which is associated with disruption of hepatic architecture, in ALF, complete hepatic regeneration can occur in the surviving patients despite a significant loss of hepatocyte mass by the inciting event. The first experimental model of liver regeneration was the two-thirds partial hepatectomy model. However, regeneration failed after partial hepatectomy if there was a significantly greater percentage of resection.223 The same applies to ALF. Regeneration in ALF can be a slow process, and the likelihood of histological recovery appears to be minimal in the liver which develops total hepatocyte loss.224 Unlike partial hepatectomy, one major difference in liver regeneration in ALF, which must be considered, is the inflammatory component.225 The potential regenerative ability of the liver has been the basis of auxiliary liver transplantation (LT). The principle of auxiliary LT is to transplant a partial liver graft in an orthotopic position after performing a partial hepatectomy while leaving a part of the native liver.226 The graft helps the patient survive until the native liver regenerates. If the liver functions of the remnant native liver return to normal, immunosuppression is slowly decreased, leading to atrophy of the graft.227 While the concept of auxiliary LT is attractive, it is a more difficult procedure with higher complications and lower survival than LT. Auxiliary LT is therefore considered in patients with ALF with high potential of liver regeneration (young patients), ALF due to acute hepatitis A, or paracetamol poisoning.22
Liver biopsy in ALF
Liver biopsies are not required to diagnose ALF due to viral hepatitis; however, there have been instances where liver biopsies have resulted in establishing or reviewing an original diagnosis. Thus, Donaldson et al228 reviewed transjugular liver biopsies (TJLBs) in their cases of ALF and found that the presumed clinical diagnosis was confirmed in 34 of 54 patients (63%), the procedure served to clarify clinical uncertainty in 11, and the diagnosis was altered in 9 of 54 (16.7%). In an LT setting,229 liver biopsies confirmed the presumptive diagnosis in 76% of patients and a previously unsuspected diagnosis was raised in 11%. Hemochromatosis, WD, and acute alcoholic hepatitis were the most challenging diagnoses and had the most relevant implications in transplant decisions. In another seminal article, Singhal et al230 found that in 69 patients, clinical diagnosis was confirmed in 56 (81.2%). In the remaining 13, histological evaluation resulted in surprises such as giant cell hepatitis, Reye syndrome, mitochondrial disorder, malignant hemangioendothelioma, autoimmune hepatic failure, idiosyncratic drug reaction, and associated fibrosis.230 More recently, liver biopsies were found to confirm the clinical diagnosis in 62% of pediatric cases.231 The ALF Study Group of the United States published their series of 303 cases of ALF of indeterminate etiology (IND-ALF) as analyzed by an expert committee. Nearly half the cases could be assigned an etiology on review based on an algorithmic approach.232 Furthermore, in a country where viral hepatitis is the most common etiology of ALF, the etiology can be established without the use of a biopsy in a majority of instances.
Consensus Statement: Pathological Aspects
-
36.
Utility of liver biopsy in ALF in our country is currently limited and therefore not mandatory in routine workup (Grade of evidence: moderate; grade of recommendation: strong)
-
37.
Liver biopsy may, however, be necessary in isolated cases of ALF (Grade of evidence: moderate; grade of recommendation: strong)
-
38.
If liver biopsy is required in a case of ALF, transjugular biopsies appear to be a safe option (Grade of evidence: moderate; grade of recommendation: strong)
Prognosis
The histological hallmark of ALF is the severity of necrosis. Liver biopsies have been used to determine the extent of necrosis and thus predict the prognosis. In general, it is expected that the greater the extent of necrosis (>75%), the worse the outcome.233 The cutoffs for the extent of necrosis with the poor outcome have varied from 25% to 85%.234,235 The extent of necrosis can guide the requirement of LT. Other prognostic markers include significantly more Ki67-positive cells but fewer M30-positive cells in the liver of patients with ALF who recover spontaneously.236 However, sampling errors may occur in small biopsies, and hence, some authors have concluded that estimating necrosis in a liver biopsy may not be helpful with regard to its utility as a prognostic marker.237 From the aforementioned account, it appears that liver biopsies are not mandatory in the workup of ALF in India.
The general method of liver biopsy in ALF is the TJLB.238,239 No deaths due to TJLB were observed. The presence of neither ascites (6.6% complications) nor coagulopathy (platelets <50G/L and/or PT <50%; 4.8% complications) increased the risk for complications. Incidentally, minilaparoscopy with guided liver biopsy allows reliable and safe evaluation of liver disease in patients with severe coagulopathy.240
TJLB should also be considered in situations where LT is contemplated but there is a possibility that it may be contraindicated due to an undiagnosed associated disease.241 In many such cases, the diagnosis is not suspected. Thus, among 1910 patients from the ALF Registry, malignancy was the cause in 27 cases. Of these, only 44% had a mass on imaging. The most common malignancies included lymphoma or leukemia (33%), breast cancer (30%), and colon cancer (7%); 90% of the patients with lymphoma or leukemia had no history of cancer, compared with 25% of patients with breast cancer. Biopsies helped in the diagnosis in nearly half of these cases. In suspected AIH, biopsy would help in further management and may assist in the identification of a cause that may have precipitated liver failure.242 It will also be useful in drug-induced hepatitis243 and ALF due to obscure reasons where there is a possibility of etiology influencing management significantly and where ACLF is a strong differential diagnosis or cannot be excluded otherwise,244 and very rare instances in ALF due to metabolic disorders.245
Clinical features
The initial manifestation of ALF may range from constitutional symptoms such as malaise, fatigue, nausea, vomiting, and abdominal pain which evolve to hepatic encephalopathy, severe hypotension, and sepsis. The clinical course of ALF typically follows that of multiorgan failure. Indian studies have reported that approximately 80% of patients develop encephalopathy within 2 weeks of the onset of icterus, and all patients present within 4 weeks of onset of jaundice. The typical clinical course is shown in Figure 3. Encephalopathy in ALF includes a number of clinical manifestations ranging from drowsiness, slowed mentation, cognitive impairment, confusion, and euphoria to deep coma. Hepatic encephalopathy is usually classified based on the severity from grade 1 to grade 4. Prognosis is related to the grade of encephalopathy with higher levels of encephalopathy having worse prognosis.246 CE is seen in 65–75% of patients with ALF with grade 4 hepatic encephalopathy. In addition, patients have elevated prothrombin and partial thromboplastin times in ALF owing to decreased production of clotting factors II, V, VII, IX, and X by the injured liver. Intravascular coagulation and fibrinolysis leading to consumption of platelets may occur later in the course. More than 60% of patients with ALF have a platelet count of less than 150,000/ml. Vitamin K deficiency is seen in many patients with ALF. However, spontaneous bleeding in ALF rare and it is of capillary-type, usually from mucosal sites of the stomach, lungs, or genitourinary system. Similarly, spontaneous intracranial bleeding in the absence of ICP monitors has been reported in <1% of patients. The incidence of spontaneous bleeding in patients with ALF appears to have decreased in the last 30 years, probably as the result of improvements in intensive care unit (ICU) care.247
Figure 3.
Clinical course of acute liver failure as seen in Indian subcontinent after infection with a hepatitis virus. As liver dysfunction proceeds rapidly, patient may slide from stage of viral hepatitis to severe acute liver injury and then to (or often directly to) acute liver failure. Deterioration may be seen over a few hours, days or less often weeks. LT = liver transplantation.
Bacterial and fungal infections are common in ALF owing to alterations in immune defense mechanisms. Most common infections are bacterial pneumonia, urinary tract infection, intravenous catheter–induced sepsis, and spontaneous bacterial peritonitis.220,248,249 The presence of fungal infection is a poor prognostic sign in patients with ALF. Patients with ALF usually have multiple central and peripheral lines and indwelling catheters, which increase the risk of nosocomial infections.250
Hypoglycemia is an important complication of ALF. It contributes to altered mental status, and the true extent of hepatic encephalopathy cannot be ascertained in the presence of hypoglycemia. Two main mechanisms contribute to hypoglycemia in ALF: impaired gluconeogenesis in the injured liver in ALF and decreased uptake of insulin by the hepatocytes. This increases the insulin level in the peripheral blood, resulting in hypoglycemia. Hyperlactatemia can occur because of poor systemic microcirculation as well as owing to failure of the liver to clear lactate. Hyperlactatemia can aggravate hemodynamic instability and should be treated aggressively. Electrolyte abnormalities such as hyponatremia, hypokalemia, hypophosphatemia, and acid–base imbalances such as respiratory acidosis are commonly seen in ALF. Hyponatremia, when present, is usually due to hypervolemia. CNS-induced hyperventilation in ALF leads to respiratory alkalosis. This, in turn, causes the kidneys to absorb hydrogen ions in exchange for potassium, thus resulting in hypokalemia. These electrolyte abnormalities may rarely result in cardiac arrhythmias contributing to mortality.
ALF often results in circulatory dysfunction. The mechanism is multifactorial and is initially associated with hypovolemia due to a combination of poor oral intake and increased fluid loss. As ALF progresses, the release of circulatory cytokines and inflammatory mediators cause systemic vasodilation and worsens hypotension. The end result is the combination of low systemic vascular resistance, systemic hypotension, and increased cardiac output resembling septic shock. These hemodynamic derangements lead to decreased peripheral tissue oxygenation and eventually multiorgan failure.251
The combination of coagulopathy, increased serum transaminases, abnormal bilirubin, and altered levels of consciousness may be seen in patients with a variety of systemic disease processes. ALF may be confused with systemic illness such as severe sepsis, systemic lupus erythematosus, thrombotic purpura, and disseminated intravascular coagulation. It may be difficult to differentiate severe sepsis from ALF, and measurement of factor VIII levels may help as factor VIII levels are low in sepsis, while it is normal in patients with ALF. In tropical countries such as India, the differential diagnosis of ALF should include severe infections with Plasmodium malaria, dengue fever, leptospirosis, and sickettsial infections. Early recognition of these conditions is essential as specific therapies can cure most of these conditions.252
Consensus Statement: Clinical Presentation
-
39.
ALF manifest with or without constitutional symptoms and rapidly evolves over few hours, days, or weeks (usually within 4 weeks) into multiorgan failure characterized by encephalopathy, coagulopathy, hemodynamic alterations, metabolic abnormalities, sepsis, and renal failure (Grade of evidence: moderate; grade of recommendation: strong)
-
40.
ALF occurs in the absence of pre-existing liver disease except in cases of WD, AIH, hepatitis B, and (Grade of evidence: moderate; grade of recommendation: strong)
-
41.
The time of onset of encephalopathy may vary based on the etiology. ALF resulting from viral hepatitis occurs mostly between 1 and 4 weeks of onset of symptoms. Zinc phosphide (rodenticide), acetaminophen, and amanita poisoning usually lead to encephalopathy within the first week (Grade of evidence: moderate; grade of recommendation: strong)
-
42.
Presence of anicteric elevation of ALT, flu-like symptoms in an immunocompetent as well as immunocompromised patient, with evidence of mucocutaneous lesions, leucopenia, and thrombocytopenia should arouse the suspicion of HSV infection (Grade of evidence: low; grade of recommendation: strong)
-
43.
The combination of raised serum transaminases, abnormal bilirubin, and altered levels of consciousness with or without raised INR may be seen in patients with a variety of systemic disease processes. In tropical countries such as India, severe infections with dengue fever, leptospirosis, scrub typhus, and malaria need to be excluded (Grade of evidence: low; grade of recommendation: strong)
Differential diagnosis
ALF is a clinical syndrome with poor short-term outcome if not recognized and treated in time.253 Before committing all resources for the management of this condition, it is imperative that we mentally rule out common mimics of this syndrome. Major differential diagnosis includes ACLF and certain tropical mimics that often present with unconsciousness and jaundice. Ascites is usually minimal or absent in ALF and is difficult to detect clinically. If overt ascites is present, unusual etiology such as BCS or ACLF should be considered. Major differences from ACLF are outlined. Theoretically, ALF diagnosed in patients with chronic HBV, WD, and AIH can be considered ACLF; however, as most such patients phenotypically behave as ALF, they are classified as such. Diagnosis of these entities needs special attention. In addition, there are several tropical infections and systemic diseases where patients may present with jaundice and altered sensorium. These groups of patients characteristically may be febrile and have encephalopathy and jaundice with raised serum glutamic oxaloacetic transaminase (SGOT)/serum glutamic pyruvic transaminase (SGPT) (usually less than 10 times upper limit of normal (ULN)) and marginally abnormal/normal PT-INR, and a definitive treatment may improve outlook. Tropical infections such as falciparum malaria, dengue fever, enteric fever, leptospirosis (Weil disease), and scrub typhus should be evaluated depending on the epidemiological prevalence of these infections (Table 10). HLH is an uncommon but fatal presentation and often encountered in adults as secondary HLH. Persistent fever, splenomegaly, jaundice, and the pathologic finding of hemophagocytosis and often in association with EBV infection need to be diagnosed early.252
Table 10.
Differential Diagnosis From ALF Mimics in Tropics.
| Disease | Clinical and laboratory distinctiveness | Diagnostic test |
|---|---|---|
| Severe falciparum malaria | Paroxysmal fever with chills, enlarged spleen and/or liver, altered sensorium, seizure, coma, severe anemia, hemoglobinuria hypoglycemia, renal failure, DIC, ARDS, shock can be present. Predominant unconjugated bilirubin, mild increase in AST/ALT with a normal INR. |
Microscopy from peripheral blood smear or quantitative buffy coat test or the rapid diagnostic kit (ICT) alone or in combination can detect with a sensitivity and specificity of >95%. |
| Dengue Fever | High grade persistent fever, headache, retro-orbital pain, myalgia, arthralgia, rash (Dengue Fever). Or thrombocytopenia, skin, mucosal bleeds, rise in hematocrit i.e. (Dengue hemorrhagic fever -DHF) or with Hypotension. wide pulse pressure<20 mmHg, or complications like encephalitis, myocarditis, hepatitis, renal failure, ARDS, hemophagocytosis (Dengue shock syndrome) Mild rise in bilurubin, AST/ALT raised 5-20times, AST > ALT Evidence of muscle injury-raised CPK |
NS1 antigen detection IgM, IgG dengue serology |
| Leptospirosis | Fever with chills, headache, myalgia, abdominal pain, conjunctival suffusion, transient skin rash and the severe form, i.e., Weil's disease, with jaundice, proteinuria, hematuria, AKI, pulmonary hemorrhages, ARDS, myocarditis and hepatomegaly. | Raised creatinine phosphokinase levels, serologic test, microscopic agglutination test (MAT), IgM ELISA |
| Scrub typhus | Fever (prolonged, 1–3 weeks), headache and myalgia, breathing difficulty, delirium, cough, jaundice. Hepatomegaly common. Characteristic rash: eschar early in disease Jaundice –hepatocellular, AST/ALT raised <5 times. Normal PT-INR |
Indirect fluorescent antibody: “gold standard” Enzyme-linked immunosorbent assay (ELISA) for immunoglobulin G (IgG) and IgM antibodies: sensitivity and specificity > 90% Weil-Felix: poor sensitivity and specificity |
| Hemophagocytic lymphohistiocytosis (HLH) | Fever, hepatosplenomegaly, lymphadenopathy, neurological manifestations. | Cytopenias, elevated LDH, Serum ferritin, triglycerides and FDPs. Histologically evidenceof hemophagocytosis |
AST, aspartate transaminase; ALT, alanine transaminase; AKI, acute kidney injury; INR, international normalized ratio; DIC, disseminated intravascular coagulation; ARDSDIC, acute respiratory distress syndrome; ICT, immunochromatographic assay.
Consensus Statement: Differential Diagnosis
-
44.
ALF and ACLF differentiation is needed for prognostication and management decision. Clinical evaluation, laboratory investigation, and presence of gross ascites and varices on endoscopy will differentiate ACLF from ALF (Grade of evidence: moderate; grade of recommendation: strong)
Investigations on admission
Investigations for a newly admitted patient with suspected ALF will depend on clinical assessment to some extent. Investigations will be aimed at (a) confirming the diagnosis of ALF and for ruling out the differential diagnosis in the clinical setting; (b) once diagnosis of ALF has been confirmed, one looks for the cause of ALF and some investigations will be directed to that end; and finally, (c) some investigations will be needed to assess the severity of the liver failure, risk of complications, and for assessing prognosis in a given case. Table 11 gives some suggestions, which is by no means an exhaustive checklist, and most investigations will be guided by availability and clinical situation.
Table 11.
Summary of Investigations to be Done on Admission in Case Suspected to Have ALF.
| Confirming the diagnosis and to rule out differential diagnosis | For determining etiology | For determining complications and prognosis |
|---|---|---|
|
|
|
ALF, acute liver failure; PT, prothrombin time; HBV, hepatitis B virus; HAV, hepatitis A virus; HEV, hepatitis E virus; HSV, herpes simplex virus; VZV, varicella-zoster virus; EBV, Epstein-Barr virus; HIV, human immunovirus; INR, international normalized ratio; LFT, liver function tests; PCR, polymerase chain reaction; ECG, electrocardiogram; CT, computed tomography; AIH, autoimmune hepatitis; ANA, antinuclear antibody; ASMA, antismooth muscle antibody; HLA, human leukocyte antigen; ANCA, anti-neutrophilic cytoplasmic autoantibody; ABG, arterial blood gas test; IVC, inferior vena cava; CMV, Cytomegalovirus; CBC, complete blood counts.
Diagnosis is usually made on the basis of clinical picture (jaundice and encephalopathy) and confirmed by investigations showing a hepatitic pattern (ALT/AST raised >10 times ULN) and evidence of coagulopathy (INR raised above ULN). In rare cases, imaging or TJLB may be needed to rule out CLD. In a tropical country such as India, several other conditions may mimic ALF and may need to be ruled out by appropriate investigations based on the clinical picture.252
The commonest cause of ALF in India is viral hepatitis, and hence, all patients must undergo serological tests to determine etiology such as anti-HAV IgM, anti-HEV IgM, and HBsAg, as well as anti-HBc IgM (and HBV DNA if available); tests for hepatitis delta may also be conducted where available (the patient is detected positive for HBV). HSV is a rare cause of ALF, and anti-HSV IgM should also be done if this infection is suspected. In immunocompetent patients, tests for VZV IgM, Cytomegalovirus (CMV), EBV, HIV, parvovirus, or VZV PCR (in immunocompromised patients) are not warranted. Similarly, diagnosis of WD or AIH does not really change the approach to management but may have implications for posttransplant management and family screening and should only be done with these aims in mind. If BCS is suspected, liver imaging with color Doppler test would be required, while ischemic hepatitis related to cardiac failure will warrant echocardiography and cardiology evaluation. Paracetamol overdose is rarely if ever seen in India as a cause of ALF, and hence, routine toxicology screen in urine and paracetamol serum level is not routinely recommended.
Assessment of complications and prognosis is an ongoing process. Blood urea in ALF is usually low and does not reflect renal function, for which one must focus on urine output and creatinine levels. Arterial blood gas and a baseline arterial ammonia measurement is required in addition to assess prognosis. Sepsis is a common cause of death in patients with ALF, and hence, baseline complete blood counts (CBC), procalcitonin, cultures (respiratory, blood, urine), and chest X-ray should be done on admission. Prolongation of clotting tests with abnormal INR is a cardinal feature of ALF; however, bleeding is uncommon unless the platelet count is very low, combined with low fibrinogen, prolongation of activated partial thromboplastin time (APTT), factor V, and INR.254 There is evidence to suggest that there is balanced disturbance of both procoagulant and anticoagulant factors present in patients with ALF, and such patients may have procoagulant state in many cases.255 Thus, prophylactic administration of coagulation factors is not advised because it will alter the INR which is one of the prognostic factors that needs to be monitored. Clotting factor replenishment if considered should be guided by thromboelastogram. If hypovolemia is suspected, which is frequently present, adequacy of volume replacement can be measured by improvement in blood lactate levels and US evidence of IVC collapsibility.256,257 Blood sugar, serum amylase, lipase, and electrolytes also need to be done immediately on admission. In females of reproductive age group, one should ensure that diagnosis of pregnancy is not missed as it affects the management.
Consensus Statement: Investigations
-
45.
Patients who present with clinical picture suggestive of ALF should undergo liver function tests, INR, and liver imaging to confirm the diagnosis (Grade of evidence: moderate; grade of recommendation: strong)
-
46.
Tests to determine etiology will be dictated by the prevalence of various etiological factors in that area. Screening for viral hepatitis A, E, and B should be routinely (Grade of evidence: moderate; grade of recommendation: strong)
-
47.
If the atypical cause is suspected, other investigations such as IgM anti-HSV, tests for tropical infections, serum ceruloplasmin, autoimmune markers, and echocardiogram can also be considered. In immunosuppressed patients, additional tests for VZV, CMV, and EBV may also be done (Grade of evidence: moderate; grade of recommendation: strong)
-
48.
Investigations to determine the risk of complications and prognosis will include CBC, procalcitonin, cultures from blood, urine and sputum/broncho alveolar lavage (BAL), arterial ammonia, lactate, kidney function tests (KFT), blood sugars, serum electrolytes, amylase/lipase, and blood gas analysis. In selected cases, pregnancy tests and thromboelastogram may also be required (Grade of evidence: moderate; grade of recommendation: strong)
Monitoring in ALF
The monitoring in ALF can be broadly divided into the following components: (a) biochemical parameter monitoring, (b) hemodynamic monitoring, and (c) neurologic monitoring.
ALF is a life-threatening condition with a high short-term mortality; the following laboratory parameters need to be for monitored for early identification of complications and their effective management.
In addition to routine biochemical parameters such as CBC, liver function tests (LFT), and KFT (which can be done 12–24 hourly), special attention has to be paid to PT/INR. Because PT (INR) is the single most important factor predicting mortality and need for transplantation, it should be monitored at least 12 hourly. It is important to note that INR monitoring is not to determine coagulopathy but to assess the liver injury. For coagulation assessment, more holistic tests such as thromboelastography (TEG)/rotational thromboelastometry (ROTEM) can be used.258 AKI is a common occurrence during the course of ALF. Renal functions should be monitored everyday in the ICU. At the onset of AKI, effort should be made to define the type of injury (prerenal/hepatorenal syndrome (HRS) vs. ATN) as the management protocol would be different in each case.
Patients with ALF are at a very high risk of hypoglycemia. Blood sugar monitoring is recommended every 1–2 h if hypoglycemia is documented and every 2–4 h if no hypoglycemia is documented. Arterial blood gases should also be done at least twice a day. Specific attention has to be paid to pH (especially in case of acetaminophen poisoning), bicarbonate levels, and lactate. Serum lactate is a very important parameter, and high values may warrant renal support by itself (even in the absence of renal dysfunction). Serum electrolytes should be monitored, and sodium needs to be tightly controlled between 145 and 155 mEq/L (twice a day monitoring of sodium). Once the sodium levels reach above 150 mEq/L, it often becomes difficult to control the further rise. Hence, interventions to increase sodium should be stopped once it crosses 155 mEq/L. Hypomagnesemia and hypophosphatemia are commonly seen in ALF (hypercatabolic state) and should be tested for at least once in 24–48 h.
Arterial ammonia levels have been shown to have a direct correlation with outcomes in ALF. Many prognostic criteria now include ammonia as an important determinant.259 Recently pre-emptive renal replacement therapy has been recommended based on high ammonia as a sole criterion.260 Arterial ammonia should be monitored every 12–24 h in a patient with ALF. However, there is no consensus on whether fasting ammonia levels are necessary. The study on dynamic Acute Liver Failure Early Dynamic (ALFED) model that is followed in India described a cutoff of 123 μmoL/l based on fasting ammonia levels.259 However, it may be unethical to keep the patient with ALF fasting if we have to do frequent ammonia levels. That brings into question the validity of 123 μmol/l as the cutoff as ammonia levels are likely to increase in the fed state.
Continuous hemodynamic monitoring is required in all patients with ALF. Raised ICP may manifest as bradycardia, tachycardia, arrhythmias, or hypertension. A target mean arterial pressure (MAP) of 70–80 mmHg should be maintained. End tidal CO2 (ETCO2) monitoring is recommended in case of intubated patients (all grade 3–4 HE). In addition, hourly urine output should be monitored in all patients; it may be an early sign of hypovolemia or renal dysfunction. A continuous temperature monitoring should be done in all patients (core body temperature monitoring is recommended). Fever should be aggressively treated with cold blankets and/or paracetamol (prophylactic hypothermia has not been shown to decrease intracranial hypertension). Assessment of volume status in patients with ALF is mandatory as it has a direct correlation with the ICP. We recommend that central venous pressure (CVP) should not be used as a criterion owing to its obvious fallacies. IVC diameter assessment and stroke volume variation are better predictors of volume status and are recommended in preference to CVP.
The commonest cause of death in ALF is sepsis. Therefore, early recognition of infection is the cornerstone of management of ALF. Blood and urine cultures should be sent for all patients at admission. Infection should be considered at the time of any signs of deterioration in the overall clinical status of the patient. This should prompt repeat cultures (blood, urine, sputum/throat swab/ET) even if the initial cultures are negative. Surveillance cultures (even in the absence of suspected infection) are recommended although the frequency has not been adequately defined. We recommend surveillance cultures once every 48 h.
Neurologic Monitoring
Patients with ALF are at a very high risk of developing CE (especially in high-grade HE). The risk of CE is 25–35% in grade 3 HE and increases to ≥75% in grade 4 HE.261 Clinical examination (pupillary size and coma grade assessment) should be done. Sudden onset hemodynamic changes (rapid onset bradycardia, tachycardia, arrhythmias, hypertension, hypotension) and decerebrate posturing may signify raised ICP.
Invasive ICP monitoring is the most sensitive method to diagnose CE. However, it is associated with 1–10% risk of intracranial bleeding due to bolt insertion, limiting the overall benefit of this approach.201 Moreover, various studies have shown that although invasive ICP monitoring ensures more detection and treatment of episodes of ICP rise, but it has no effect on overall survival of these patients.262 There are two techniques of invasive ICP monitoring in use. Intraventricular pressure monitoring is the gold standard where a catheter is placed in the lateral ventricle and pressure measured by a transducer. It has the advantage of the most accurate monitoring as the catheter is in the cerebrospinal fluid (CSF). It can also be used to sample CSF in case of suspected infection. However, it is more invasive and has a higher risk of infection and bleeding than a subdural bolt. The other technique places a hollow bolt beneath the dura mater just next to the arachnoid membrane. It has an advantage of not causing injury to the brain parenchyma but has a higher risk of malfunction (brain herniation into the hollow of bolt). In addition, CSF cannot be drawn as it lies outside the brain. The expert panel of the INASL consortium does not recommend invasive ICP monitoring because of its complexity and complications. However, it can be done in patients with high-grade HE (grade 3 and 4), where the expertise is available.
Noninvasive methods of ICP monitoring include reverse jugular venous saturation, transcranial Doppler (TCD), near-infrared spectrophotometry, and ONSD. Of these, TCD and ONSD are easy to perform and can be done at all centers. However, their reliability and validity in patients with ALF have not been proven.263,264 TCD measures the blood flow velocity in major cerebral vessels (usually middle cerebral artery). It can be performed through the temporal window, orbital foramen, or foramen magnum. The CBF velocity has a direct correlation with CBF in the absence of vasoconstriction. The interpretation of cerebral blood flow volume (CBFV) needs a very careful assessment of cerebral vasospasm also as a rise in velocity may be seen in both cerebral hyperemia as well as in increased vasospasm. ICP calculation from TCD flow velocities has been shown to correlate well with invasive ICP measurements in a small study. Pulsatility index of cerebral artery measured by TCD is not as accurate as flow velocity in detecting ICP rise.265 ONSD is easy to do with an US on a patient's bedside. Normal value of ONSD is around 5 mm; any values above 5.5 mm are suspicious of ICP rise. In a published study, ONSD was not found to reliably detect ICP rise on invasive monitoring.265
Consensus Statement: Monitoring
-
49.
PT/INR should be monitored at least twice a day. (Grade of evidence: moderate; grade of recommendation: strong)
-
50.
Blood sugar monitoring should be done 1–4 hourly. Arterial ammonia should be monitored every 12–24 h in a patient with ALF (Grade of evidence: moderate; grade of recommendation: strong)
-
51.
Sodium levels should be monitored every 12 h and controlled between 145 and 150 meq/l.
-
52.
Urine output should be measured hourly. In case of AKI, effort should be made to define the type of injury (prerenal/HRS vs ATN) (Grade of evidence: moderate; grade of recommendation: strong)
-
53.
Surveillance cultures should be sent every 48 h or at any suspicion of infection (clinical deterioration of the patient) (Grade of evidence: moderate; grade of recommendation: strong)
-
54.
Continuous blood pressure monitoring is recommended with target MAP of 70–80 mmHg. Volume status can also be assessed though IVC diameter variability index (Grade of evidence: moderate; grade of recommendation: strong)
-
55.
Core body temperature monitoring should be done (Grade of evidence: moderate; grade of recommendation: strong)
-
56.
Routine use of invasive ICP monitoring is not recommended. Noninvasive ICP monitoring can be done in all patients with ALF (Grade of evidence: moderate; grade of recommendation: strong)
Prognostication in ALF
Prognostication in ALF is of crucial importance as the only treatment is known to improve survival is LT.266 Owing to the rapid and fulminant course of this syndrome, it is imperative to identify those cases early in the course of the disease (preferably within the first three days of presentation), which will not improve with medical management and may need emergency LT. At the same time, it is also important to identify cases, which are likely to improve with medical management and avoid unnecessary LT, depleting the already scarce donor pool and rendering the patient in need of lifelong immunosuppression. Hence, a good prognostic score should be able to identify patients who are
-
1.
Unlikely to survive without transplant: Need urgent LT (Prognostic models should have high sensitivity and specificity to predict death.)
-
2.
Likely to survive and gain functionality with medical management: (Do not need transplant)
-
3.
Too sick to transplant: (Transplant likely to be futile)
Assessment of prognosis and need for LT should begin on the first presentation at a medical facility and reviewed periodically. Multiple prognostic scores have been developed and validated since the recognition of this entity, namely, King's College Criteria (KCC),267 Clichy Criteria,268 MELD score,269 MELDNa score,270 clinical prognostic indicator (CPI) score,271 and ALFED score.259 There are many variables common to these scores such as coagulopathy, grades of encephalopathy, bilirubin, presence or absence of raised ICP, underlying etiology, age, and jaundice to encephalopathy interval. Newer prognostic markers such as serum lactate, phosphates, Gc globulin are under investigation; however, they need further validation. Most of these scores have high positive predictive value (PPV) for mortality; however, none of them are ideal scores that can segregate survivors from nonsurvivors with 100% sensitivity, specificity, PPV, and negative predictive value (NPV). Inability to identify sick patients who will benefit from emergency LT is the major limitation of these scores. In addition, dynamic assessment with these scores including clinical judgment should be the standard of care rather than one-time assessment at baseline.
King's College Criteria
KCC was developed by O'Grady et al3 for paracetamol-induced liver toxicity and non–paracetamol-induced liver failure. KCC showed PPV ranging from 70% to almost 100% and NPV ranging from 25% to 94%.271,272 In another pooled meta-analysis, sensitivity was determined to be between 68% and 69% and specificity from 82% to 92%,273 which is lower than that suggested in the original study. Later on, Bernal et al.274 found out that addition of postresuscitation lactate to KCC in 107 patients with paracetamol-induced ALF increased the sensitivity from 76% to 91% in the same cohort.
Clichy Criterion
In 1986, Clichy criterion268 used the degree of encephalopathy and factor V level less than 20% (age <30 years) or less than 30% (age >30 years) with a PPV of 82% and an NPV of 98%. Application of this criterion is limited in the Indian setting in view of frequent nonavailability of factor V level measurement; in addition, this criterion was developed in a specific population of patients with ALF due to hepatitis B infection which cannot be generalized to all patients with ALF.
MELD and MELD-Na
MELD score incorporates serum bilirubin levels, INR, and serum, which are easily available at every medical facility and also does not include any subjective variable such as encephalopathy. MELD was originally developed to assess the risk of mortality in patients undergoing elective TIPSS275 and later adopted by United Network for Organ Sharing (UNOS)/OPTN to prioritize organ for LT in 2002. In 2016, serum sodium, an independent predictor of mortality in cirrhotics, was incorporated into MELD score to improve prognostication and renamed MELD-Na score.269,270
Prognostic efficacies of KCC, Clichy's criteria, MELD, and Pediatric End-Stage Liver Disease (PELD) score were studied in 120 patients with ALF. Areas under the receiver operating characteristics curve (AUROCs) were significantly higher for MELD (0.95) and PELD (0.99) than for KCC (0.74) and Clichy's criteria (0.68).276
Early CPIs
In a retrospective study by Dhiman et al,271 six CPIs of adverse outcomes related to ALF were found on admission – age 50 years or older, jaundice-to-encephalopathy interval greater than 7 days, presence of CE, grade 3 or 4 encephalopathy, PT 35 s or greater, and creatinine 1.5 mg/dl or greater. By the construction of receiver operating characteristic (ROC) curves, the presence of any 3 of 6 CPIs and an MELD score of ≥33 was also found to best discriminate between survivors and nonsurvivors.
MELD score, when compared with the presence of any 3 of the 6 CPIs, had similar sensitivity (76.1% vs. 73.9%) but lower specificity (67.3% vs. 86.5%), PPV (80.5% vs. 90.7%), NPV (61.4% vs. 65.2%), and diagnostic accuracy (72.9% vs. 78.5%). When these CPIs were compared with KCC, it was determined that KCC had high specificity (88.5%) and PPV (87.8%) but lower sensitivity (46.7%) and diagnostic accuracy (61.8%) in direct comparison with previously reported data of 91% and 90% in the original study (O'grady 1989). This study concluded that the presence of any 3 of 6 CPIs was superior to MELD and KCH criteria in predicting the outcome (c-statistic [95% confidence interval]: CPI, 0.802 [0.726–0.878]; MELD, 0.717 [0.636–0.789]; and KCH criteria, 0.676 (0.588–0.764); P values: CPI vs. MELD, 0.045; CPI vs. KCH criteria, 0.019; and MELD vs. KCH criteria, 0.472) (Figure 4).
Figure 4.
MELD, Model For End-Stage Liver Disease.
ALFED score
This is one of the first dynamic models to assess and stratify patients with ALF dynamically over a period of 3 days rather than considering variables at baseline. The ALFED model study identified four prognostically significant variables: arterial ammonia, serum bilirubin, INR, and hepatic encephalopathy > grade II. (Table 12) This ALFED model had an AUROC of 0.91 in the derivation cohort and 0.92 in the validation cohort. The model showed a similar increase in mortality with increasing risk scores from 0 to 6. The performance of the ALFED model was found to be superior to the KCC and the MELD score, even when their 3-day serial values were considered. An ALFED score of ≥4 had a high PPV (85%) and NPV (87%) in the validation cohort. The pros of this model are the dynamic assessment of patient status, and as the study was conducted in a non–liver transplant center, the full natural history of this condition could be observed. Furthermore, in each patient, the model could stratify the risk of dying or surviving on day 3 (score 1 through 6) of hospitalization. Those with a score of 1–3 had a survival frequency of about 80% or more, and those with ≥4 had a mortality risk of >80%. These parameters at baseline were also independent predictors of mortality, but the dynamic assessment made these parameters as also a model for survival. In India, ALF etiology is hepatitis virus usually affecting individuals without any underlying chronic liver pathology. As discussed earlier, the liver regeneration simultaneously kicks off. Therefore, with liver regeneration, these predictive parameters change and hence are important in assessing for the maximal benefit from transplant as well as medical therapy.
Table 12.
ALFED Model (Score 0–6).259
| Variables over 3 days | Score assigned |
|---|---|
| Hepatic encephalopathy (persistent or progressed to grade >2) | 2 |
| INR (persistent or increased to ≥5) | 1 |
| Arterial ammonia (persistent or increased to ≥123 μmoL/l) | 2 |
| Serum bilirubin (persistent or increased to ≥15 mg/dl) | 1 |
ALFED, Acute Liver Failure Early Dynamic; INR, international normalized ratio.
In another analysis from the AIIMS, India,7 they compared dynamic changes among different prognostic scores in ALF related to viral hepatitis including MELD score, MELD-Na score, ALFED model, CLIF consortium ACLF score,16 and KCC. MELD, MELD-Na, ALFED, CLIF-C ACLF scores and KCH criteria were calculated at admission and day 3 of admission. The baseline values of prognostic scores (MELD, MELD-Na, ALFED, CLIF-C ACLF, and KCH) had modest (AUROC: 0.65–0.77) discriminatory capacity, whereas the AUROC increased on day 3 for all scores, except for KCH criteria. On day 3, ALFED score had the highest AUROC of 0.95, followed by CLIF-C ACLF (0.88), MELD (0.81), MELD-Na (0.77), and KCH (0.52) (Figure 5). This study further validated the ALFED score, thus concluding that the dynamic assessment of prognostic scores rather than single baseline value better predict outcomes in such patients. This model constitutes the important three aspects of an ideal prognostic model: evaluation of dynamicity of parameters dependent on hepatic regeneration, a model for survival, as well as death (Figure 6).
Figure 5.
Figure 6.
Figure explaines the possible process of selecting a patient with ALF for transplantation. Red lines indicate patients hospitalized with low ALFED scores, and blue line, those with mid-level ALFED score. Green lines indicate patients hospitalized with high ALFED score 6. All patients should be counseled about possible need for transplantation, and patients listed if ALFED is more than 3. In an LDLT setting, donor workup should be started. The patient should be reassessed every 12 h to determine his/her response to conventional medical treatment. Transplant decision can be tailor made to the patient's evolving condition. ALFED score at day 3 is the best predictor of prognosis (See text). ALF, acute liver failure; ALFED, Acute Liver Failure Early Dynamic Model; LDLT, living donor liver transplantation; Tx, transplantation.
Role of Ammonia in Prognosticating Patients with ALF
In a study by Bhatia et al277 where 80 consecutive patients with ALF were included and followed up until death or complete recovery, median ammonia levels were significantly higher in nonsurvivors than in survivors (174.7 μmol/L vs. 105.0 μmol/L; P < 0.001). An arterial ammonia level of >124 μmol/L was found to predict mortality with 78.6% sensitivity and 76.3% specificity and had 77.5% diagnostic accuracy. Higher ammonia levels also correlated with deeper encephalopathy (P = 0.055), CE (P = 0.020), need for ventilation (P = <0.001), and seizures (P = 0.006). Similarly, Bernal et al197 in their study also suggested that arterial ammonia level greater than >100 μmol/L was an independent risk factor for the development of both HE and ICH. Several studies have now shown that raised ammonia levels278 along with severe systemic inflammatory response lead to astrocyte swelling and brain edema responsible for raised ICP and possible subsequent brain herniation rendering all measures to save the patient futile and accounting for 20–30% of deaths.279 However, methods to detect ammonia (enzymatic vs. dipstick), timings, type of blood sample (arterial vs. venous vs. capillary), and cutoffs for serum ammonia levels still need validation and standardization in another center.
Consensus Statement: Prognostication
-
57.
ALF is a rapidly progressive illness with high short-term mortality. All patients with unequivocal diagnosis of ALF should be transferred to a specialist unit and assessment of prognosis and need for LT (Grade of evidence: moderate; grade of recommendation: strong)
-
58.
Dynamic models are a better predictor of mortality than the one-time assessment on admission (Grade of evidence: moderate; grade of recommendation: strong)
-
59.
Dynamic assessment of severity should be done by available scores namely ALFED or MELD. Transplantation should be considered in those patients fulfilling dynamic score(s) (Grade of evidence: moderate; grade of recommendation: strong)
-
60.
King's College Hospital Criteria are not a very sensitive or specific for ALF in India (Grade of evidence: moderate; grade of recommendation: strong)
-
61.
However, transplantation can be considered as a possible option in those patients who fulfill MELD score, KCH score, or CPI criteria on admission (Grade of evidence: moderate; grade of recommendation: strong)
-
62.
LT should not be offered to those patients who have evidence of compromised brainstem function, especially dilated pupils, decorticate/decerebrate spasms, or who have invasive fungal infection and rapidly escalating inotrope requirements (Grade of evidence: moderate; grade of recommendation: strong)
ALF: counseling of relatives
Patients with ALF have altered sensorium (varying from increased somnolence to frank coma); hence, they may not fully comprehend and be able to make decisions about their treatment. In patients who are not competent to make informed decision about their treatment, the “best interest principle” (i.e., providing treatment in the best interest of the patient) is to be taken by competent medical experts.280 The treatment should be undertaken in the best interests of the patient (who is incompetent to make a considered decision, in view of altered sensorium) by the treating team in consultation with the relative(s) of the patient.
The relatives of the patient with ALF need to be counseled about the seriousness of the illness (critically ill condition), its potentially poor prognosis (high short-term mortality), and the different treatment options. The patient should be managed in an ICU. Apart from the treatment provided for a critically ill patient, special emphasis is placed on reducing CE. LT may be needed in some patients. The proposed treatment options and its advantages as well as harmful effects need to be explained. The estimated cost of the treatment needs to be explained. Any alternative treatment options also need to be discussed.
ALF is a devastating illness of sudden onset, which often appears unexpectedly. The family members need help to cope with this sudden severe illness in the patient. It is useful to have trained professionals (social worker/nurse) to support the family in this regard.
Consensus Statement: Counseling of Relatives
-
63.
It is important to counsel relatives of the patient with ALF about the seriousness of the illness (critically ill patient), its poor prognosis (high short-term mortality), and the different treatment options. The patient needs to be managed in the (Grade of evidence: low; grade of recommendation: strong)
-
64.
Patients with ALF have altered sensorium; hence, they may not fully comprehend and be able to make informed decisions about their treatment. This clinical scenario necessitates that the consent for any treatment for the patient (e.g., emergency LT) can only be obtained from relatives of the patient, after discussion (Grade of evidence: low; grade of recommendation: strong)
-
65.
ALF is a devastating illness of sudden onset which often appears unexpectedly. The family members need help to cope with this sudden severe illness in the patient. It is useful to have trained professionals (social worker/nurse/doctor) to provide support to the family in this regard (Grade of evidence: low; grade of recommendation: strong)
In the first part of INASL consensus document, we have highlighted how epidemiology of ALF differs in India as compared with the West description. A major difference is that the majority of cases are due to viral etiology, while ALF related to anti-TB treatment is the next commonest cause. Paracetamol poisoning is extremely rare in India. We also need to keep in mind several tropical diseases where the clinical presentation may mimic ALF. Special predilection of hepatitis E for the pregnant women and relatively more severe illness among them due to this infection has also been highlighted. It has also been pointed out that KCC have not been found suitable for Indian patients, and physicians are advised to follow indigenously developed dynamic models such as ALFED score for selecting patients for transplantation. In the second part of this article, we will focus on the management of ALF in Indian conditions.
Authors' contribution
This is consensus document, produced by discussion and consensus of all the authors. All have contributed by reading and writing the manuscript.
Conflicts of interest
The authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2020.04.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Trey C., Davidson C.S. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–298. [PubMed] [Google Scholar]
- 2.Lee W.M., Squires R.H., Jr., Nyberg S.L. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya S.K., Panda S.K., Saxena A., Gupta S.D. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol. 2000 May;15:473–479. doi: 10.1046/j.1440-1746.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernal W., Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 5.Devarbhavi H., Patil M., Reddy V.V. Drug-induced acute liver failure in children and adults: results of a single-centre study of 128 patients. Liver Int. 2018;38:1322–1329. doi: 10.1111/liv.13662. [DOI] [PubMed] [Google Scholar]
- 6.Shalimar, Acharya S.K. Management of acute liver failure. J Clin Exp Hepatol. 2015;5:S104–S115. doi: 10.1016/j.jceh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalimar, Sonila U. Comparison of dynamic changes among various prognostic scores in viral hepatitis-related acute liver failure. Ann Hepatol. 2018;17:403–412. doi: 10.5604/01.3001.0011.7384. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt G.H., Oxman A.D., Vist G.E. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berzigotti A. Advances and challenges in cirrhosis and portal hypertension. BMC Med. 2017;15:200–2007. doi: 10.1186/s12916-017-0966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Amico G., Morabito A., D'Amico M. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12(suppl 1):34–43. doi: 10.1007/s12072-017-9808-z. [DOI] [PubMed] [Google Scholar]
- 11.Jalan R., Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–261. doi: 10.1159/000047017. [DOI] [PubMed] [Google Scholar]
- 12.Sarin S.K., Kumar A., Almeida J.A. Acute-onchronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand A.C., Dhiman R.K. Acute on chronic liver failure-what is in a 'definition'? J Clin Exp Hepatol. 2016 Sep;6:233–240. doi: 10.1016/j.jceh.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arroyo V., Moreau R., Kamath P.S. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primer. 2016;2:16041. doi: 10.1038/nrdp.2016.41. [DOI] [PubMed] [Google Scholar]
- 15.Jalan R., Yurdaydin C., Bajaj J.S. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4–10. doi: 10.1053/j.gastro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Koch D.G., Speiser J.L., Durkalski V. The natural history of severe acute liver injury. Am J Gastroenterol. 2017 Sep;112:1389–1396. doi: 10.1038/ajg.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wlodzimirow K.A., Eslami S., Abu-Hanna A., Nieuwoudt M., Chamuleau R.A.F.M. Systemic review: acute liver failure – one disease, more than 40 definitions. Aliment Pharmacol Ther. 2012;35:1245–1256. doi: 10.1111/j.1365-2036.2012.05097.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee W.M., Stravitz R.T., Larson A.M. Introduction to the revised American association for the study of liver diseases position paper on acute liver failure 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Grady J.G., Schalm S.W., Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 20.Tandon B.N., Bernauau J., O'Grady J. Recommendations of the international association for the study of the liver subcommittee on nomenclature of acute and subacute liver failure. J Gastroenterol Hepatol. 1999;14:403–404. doi: 10.1046/j.1440-1746.1999.01905.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamagishi Y., Saito H., Ebinuma H. A new prognostic formula for adult acute liver failure using computer tomography-derived hepatic volumetric analysis. J Gastroenterol. 2009;44:615–623. doi: 10.1007/s00535-009-0045-7. [DOI] [PubMed] [Google Scholar]
- 22.Wendon J. Clinical practical guidelines on the management of acute (fulminant) liver failure. European association for the study of the liver. J Hepatol. 2017;66:1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R., Shalimar, Bhatia V. Antituberculosis therapy–induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1665–1674. doi: 10.1002/hep.23534. [DOI] [PubMed] [Google Scholar]
- 24.Das A.K., Begum T., Kar P., Dutta A. Profile of acute liver failure from north-east India and its differences from other parts of the country. Euroasian J Hepato-Gastroenterol. 2016;6:111–115. doi: 10.5005/jp-journals-10018-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chidambaram V., Hamide A., Mohan P. Clinical and laboratory determinants of short-term transplant-free survival in acute liver failure in India—a prospective study. J Clin Exp Hepatol. 2017;7:S9. [Google Scholar]
- 26.Khuroo M.S., Kamili S. Aetiology and prognostic factors in acute liver failure in India. J Viral Hepat. 2003;10:224–231. doi: 10.1046/j.1365-2893.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 27.Dhiman R.K., Jain S., Maheshwari U. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-Stage Liver Disease (MELD) and King's College Hospital criteria. Liver Transplant. 2007;13:814–821. doi: 10.1002/lt.21050. [DOI] [PubMed] [Google Scholar]
- 28.Devarbhavi H., Patil M., Reddy V.V., Singh R., Joseph T., Ganga D. Drug-induced acute liver failure in children and adults: results of a single-centre study of 128 patients. Liver Int. 2017;38:1322–1329. doi: 10.1111/liv.13662. [DOI] [PubMed] [Google Scholar]
- 29.Acharya S.K., Dasarathy S., Tandon B.N. Should we redefine acute liver failure? Lancet. 1993;342:1421–1422. doi: 10.1016/0140-6736(93)92778-r. [DOI] [PubMed] [Google Scholar]
- 30.Shalimar, Kedia S., Gunjan D. Acute liver failure due to hepatitis E virus infection is associated with better survival than other etiologies in Indian patients. Dig Dis Sci. 2017;62:1058–1066. doi: 10.1007/s10620-017-4461-x. [DOI] [PubMed] [Google Scholar]
- 31.Alam S., Khanna R., Sood V., Lal B.B., Rawat D. Profile and outcome of first 109 cases of paediatric acute liver failure at a specialized paediatric liver unit in India. Liver Int. 2017;37:1508–1514. doi: 10.1111/liv.13370. [DOI] [PubMed] [Google Scholar]
- 32.Bechmann L.P., Manka P., Best J. Drug-induced liver injury as predominant cause of acute liver failure in a monocenter study. Dtsch Med Wochenschr. 2014 May;139:878–882. doi: 10.1055/s-0034-1369932. [Article in German] Epub 2014 Apr 23. [DOI] [PubMed] [Google Scholar]
- 33.Samanta T., Ganguly S. Aetiology, clinical profile and prognostic indicators for children with acute liver failure admitted in a teaching hospital in Kolkata. Trop Gastroenterol. 2007 Sep;28:135–139. [PubMed] [Google Scholar]
- 34.Devarbhavi H., Patil M., Reddy V.V., Singh R., Joseph T., Ganga D. Drug-induced acute liver failure in children and adults: results of a single-centre study of 128 patients. Liver Int. 2018;38:1322–1329. doi: 10.1111/liv.13662. [DOI] [PubMed] [Google Scholar]
- 35.Poddar B., Saigal S., Kumar A. Factors associated with outcome in acute liver failure in an intensive care unit. Indian J Gastroenterol. 2013 May;32:172–178. doi: 10.1007/s12664-012-0252-7. [DOI] [PubMed] [Google Scholar]
- 36.Mehrotra S., Mehta N., Rao P.S., Lalwani S., Mangla V., Nundy S. Live donor liver transplantation for acute liver failure: a single center experience. Indian J Gastroenterol. 2018 Jan;37:25–30. doi: 10.1007/s12664-017-0812-y. [DOI] [PubMed] [Google Scholar]
- 37.Pamecha V., Vagadiya A., Sinha P.K., Sandhyav R., Parthasarthy K., Sasturkar S. Live donor liver transplantation for acute liver failure - donor safety and recipient outcome. Liver Transplant. 2019 Mar 12;25(9):1408–1421. doi: 10.1002/lt.25445. [DOI] [PubMed] [Google Scholar]
- 38.Choudhary N.S., Saigal S., Saraf N. Good outcome of living donor liver transplantation in drug-induced acute liver failure: a single-center experience. Clin Transplant. 2017;31 doi: 10.1111/ctr.12907. [DOI] [PubMed] [Google Scholar]
- 39.Dhiman R.K., Seth A.K., Jain S., Chawla Y.K., Dilawari J.B. Prognostic evaluation of early indicators in fulminant hepatic failure by multivariate analysis. Dig Dis Sci. 1998 Jun;43:1311–1316. doi: 10.1023/a:1018876328561. [DOI] [PubMed] [Google Scholar]
- 40.Bernal W., Auzinger G., Dhawan A., Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/s0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 41.Ichai P., Samuel D. Etiology and prognosis of fulminat hepatitis in adults. Liver Transplant. 2008;14:s67–s79. doi: 10.1002/lt.21612. [DOI] [PubMed] [Google Scholar]
- 42.Hadem, J, Tacke F, Bruns T, et al. Etiologies and outcomes of acute liver failure in Germany. Clin Gastroenterol Hepatol, 10, 664–669.e2. [DOI] [PubMed]
- 43.Gow P.J., Jones R.M., Dobson J.L., Angus P.W. Etiology and outcome of fulminant hepatic failure managed at an Australian liver transplant unit. J Gastroenterol Hepatol. 2004;19:154–159. doi: 10.1111/j.1440-1746.2004.03273.x. [DOI] [PubMed] [Google Scholar]
- 44.Oketani M., Ido A., Tsubouchi H. Changing etiologies and outcomes of acute liver failure: a perspective from Japan. J Gastroenterol Hepatol. 2011;26:65–71. doi: 10.1111/j.1440-1746.2010.06574.x. [DOI] [PubMed] [Google Scholar]
- 45.Shalimar, Acharya S.K. Management of acute liver failure. J Clin Exp Hepatol. 2015;5:S104–S115. doi: 10.1016/j.jceh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shalimar, Acharya S.K. Hepatitis e and acute liver failure in pregnancy. J Clin Exp Hepatol. 2013 Sep;3:213–224. doi: 10.1016/j.jceh.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beniwal M., Kumar A., Kar P., Jilani N., Sharma J.B. Prevalence and severity of acute viral hepatitis and fulminant hepatitis during pregnancy: a prospective study from north India. Indian J Med Microbiol. 2003 Sep;21:184–185. [PubMed] [Google Scholar]
- 48.Jaiswal S.P., Jain A.K., Naik G., Soni N., Chitnis D.S. Viral hepatitis during pregnancy. Int J Gynaecol Obstet. 2001 Feb;72:103–108. doi: 10.1016/s0020-7292(00)00264-2. [DOI] [PubMed] [Google Scholar]
- 49.Rasheeda C.A., Navaneethan U., Jayanthi V. Liver disease in pregnancy and its influence on maternal and fetal mortality: a prospective study from Chennai, Southern India. Eur J Gastroenterol Hepatol. 2008 Apr;20:362–364. doi: 10.1097/MEG.0b013e3282f246d6. [DOI] [PubMed] [Google Scholar]
- 50.Patra S., Kumar A., Trivedi S.S., Puri M., Sarin S.K. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007 Jul 3;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005. [DOI] [PubMed] [Google Scholar]
- 51.Bhatia V., Singhal A., Panda S.K., Acharya S.K. A 20-year single-center experience with acute liver failure during pregnancy: is the prognosis really worse? Hepatology. 2008 Nov;48:1577–1585. doi: 10.1002/hep.22493. [DOI] [PubMed] [Google Scholar]
- 52.Khuroo M.S., Teli M.R., Skidmore S., Sofi M.A., Khuroo M.I. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252–255. doi: 10.1016/0002-9343(81)90758-0. [DOI] [PubMed] [Google Scholar]
- 53.Wasley A., Fiore A., Bell B.P. Hepatitis A in the era ofvaccination. Epidemiol Rev. 2006;28:101–111. doi: 10.1093/epirev/mxj012. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen K.H., Wiersma S.T. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653–6657. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 55.Romero R., Lavine J.E. Viral hepatitis in children. Semin Liver Dis. 1994;14:289–302. doi: 10.1055/s-2007-1007319. [DOI] [PubMed] [Google Scholar]
- 56.Murhekar M.V., Ashok M., Kanagasabai K. Epidemiology of hepatitis A and hepatitis E based on laboratory surveillance data-India, 2014-2017. Am J Trop Med Hyg. 2018 Oct;99:1058–1061. doi: 10.4269/ajtmh.18-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathur P., Arora N.K. Epidemiological transition of hepatitis A in India: issues forvaccination in developing countries. Indian J Med Res. 2008 Dec;128:699–704. [PubMed] [Google Scholar]
- 58.Arora N.K., Nanda S.K., Gulati S. Acute viral hepatitis types E, A, and B singly and in combination in acute liver failure in children in north India. J Med Virol. 1996 Mar;48:215–221. doi: 10.1002/(SICI)1096-9071(199603)48:3<215::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava K.L., Mittal A., Kumar A. Predictors of outcome in fulminant hepatic failure in children. Indian J Gastroenterol. 1998 Apr;17:43–45. [PubMed] [Google Scholar]
- 60.Poddar U., Thapa B.R., Prasad A., Sharma A.K., Singh K. Natural history and risk factors in fulminant hepatic failure. Arch Dis Child. 2002 Jul;87:54–56. doi: 10.1136/adc.87.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaur S., Kumar P., Kumar V., Sarin S.K., Kumar A. Etiology and prognostic factors of acute liver failure in children. Indian Pediatr. 2013 Jul;50:677–679. doi: 10.1007/s13312-013-0189-7. Epub 2012 Nov 5. [DOI] [PubMed] [Google Scholar]
- 62.Pandit A., Mathew L.G., Bavdekar A. Hepatotropic viruses as etiological agents of acute liver failure and related-outcomes among children in India: a retrospective hospital-based study. BMC Res Notes. 2015 Aug 27;8:381. doi: 10.1186/s13104-015-1353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amarapurkar D.N., Patel N.D. Differential diagnosis of acute liver failure in India. Ann Hepatol. 2006 Jul-Sep;5:150–156. [PubMed] [Google Scholar]
- 64.Chidambaram V., Hamide A., Mohan P. Clinical and laboratory determinants of short-term transplant-free survival in acute liver failure in India - a prospective study. J Clin Exp Hepatol. 2017;7:S9–S10. [Google Scholar]
- 65.Turk T., Al Saadi T., Sawaf B., Alkhatib M., Zakaria M.I., Daaboul B. Progressive liver failure post-acute hepatitis A, over a three-month period, resulting in hepatorenal syndrome and death. Gastroenterol Rep. 2017;5:161–164. doi: 10.1093/gastro/gow009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ray G. Current scenario of hepatitis B and its treatment in India. J Clin Translat Hepatol. 2017;5:277–296. doi: 10.14218/JCTH.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tandon B.N., Gandhi B.M., Joshi Y.K. Etiological spectrum of viral hepatitis and prevalence of markers of hepatitis A and B virus infection in north India. Bull World Health Organ. 1984;62:67–73. [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar A., Aggarwal R., Naik S.R., Saraswat V., Ghoshal U.C., Naik S. Hepatitis E virus is responsible for decompensation of chronic liver disease in an endemic region. Indian J Gastroenterol. 2004 Mar-Apr;23:59–62. [PubMed] [Google Scholar]
- 69.Monga R., Garg S., Tyagi P., Kumar N. Superimposed acute hepatitis E infection in patients with chronic liver disease. Indian J Gastroenterol. 2004 Mar-Apr;23:50–52. [PubMed] [Google Scholar]
- 70.Anand A.C., Gandhi B.M., Acharya S.K., Irshad M., Joshi Y.K., Tandon B.N. Hepatitis delta virus infection in India. J Gastroenterol Hepatol. 1988;3:425–429. [Google Scholar]
- 71.Acharya S.K., Panda S.K., Saxena A., Gupta S.D. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol. 2000 May;15:473–479. doi: 10.1046/j.1440-1746.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 72.Sahai S., Kiran R. Acute liver failure in pregnancy: causative and prognostic factors. Saudi J Gastroenterol. 2015;21:30–34. doi: 10.4103/1319-3767.151221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borkakoti J., Hazam R.K., Mohammad A., Kumar A., Kar P. Does high viral load of hepatitis E virus influence the severity and prognosis of acute liver failure during pregnancy? J Med Virol. 2013 Apr;85:620–626. doi: 10.1002/jmv.23508. [DOI] [PubMed] [Google Scholar]
- 74.Dao Y.D., Hynan S.L., Yuan J.H., Sanders C., Balko J., Attar N. Two distinct subtypes of hepatitis B virus-related acute liver failure are separable by quantitative serum immunoglobulin M anti-hepatitis B core antibody and hepatitis B virus DNA levels. Hepatology. 2012 Mar;55:676–684. doi: 10.1002/hep.24732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lok S.F.A., Liang H.S.R., Chiu K.W.E., Wong L.K., Chan T.K. Todd D Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991 Jan;100:182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 76.Jindal A., Kumar M., Sarin S.K. Predictors and outcomes of liver failure in severe hepatitis B reactivation. JCEH. 2013;3:106. doi: 10.1016/j.jceh.2013.03.106. [DOI] [Google Scholar]
- 77.Purdy M.A., Khudyakov Y.E. The molecular epidemiology of hepatitis E virus infection. Virus Res. 2011;161:31–39. doi: 10.1016/j.virusres.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 78.Amit Goel, Aggarwal Rakesh. Advances in hepatitis E – II: epidemiology, clinical manifestations, treatment and prevention. Expet Rev Gastroenterol Hepatol. 2016;10:1065–1074. doi: 10.1080/17474124.2016.1185365. [DOI] [PubMed] [Google Scholar]
- 79.Gupta E., Ballani N., Kumar M., Sarin S.K. Role of non-hepatotropic viruses in acute sporadic viral hepatitis and acute-on-chronic liver failure in adults. Indian J Gastroenterol. 2015;34:448–452. doi: 10.1007/s12664-015-0613-0. [DOI] [PubMed] [Google Scholar]
- 80.MoHFW, Who, ILBS . 2014. Technical Consultation: World Hepatitis Day. New Delhi : s.n. [Google Scholar]
- 81.Khuroo M.S. Discovery of hepatitis E: the epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Res. 2011;161:3–14. doi: 10.1016/j.virusres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Gupta N., Sarangi A.N., Dadhich S. Acute hepatitis E in India appears to be caused exclusively by genotype 1 hepatitis E virus. Indian J Gastroenterol. 2018 Jan;37:44–49. doi: 10.1007/s12664-018-0819-z. Epub 2018 Feb 5. PubMed PMID: 29399748. [DOI] [PubMed] [Google Scholar]
- 83.Arankalle V.A., Tsarev S.A., Chadha M.S. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 84.Satsangi S., Chawla Y.K. Viral hepatitis: Indian scenario. Med J Armed Forces India. 2016;72:204–210. doi: 10.1016/j.mjafi.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.India National Family Health Survey (NFHS-2) 1998-99. International Institute for population studies: Mumbai, India. http://www.nfhsindia.org/india2.html Available at:
- 86.Kumar A., Sharma S., Kar P. Impact of maternal nutrition in hepatitis E infection in pregnancy. Arch Gynecol Obstet. 2017;296:885–895. doi: 10.1007/s00404-017-4501-y. [DOI] [PubMed] [Google Scholar]
- 87.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 88.Pal R., Aggarwal R., Naik S.R., Das V., Das S., Naik S. Immunological alterations in pregnant women with acute hepatitis. E J Gastroenterol Hepatol. 2005;20:1094–1101. doi: 10.1111/j.1440-1746.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 89.Sehgal R., Patra S., David P. Impaired monocyte-macrophage functions and defective Toll-like receptor signaling in hepatitis E virus-infected pregnant women with acute liver failure. Hepatology. 2015;62:1683–1696. doi: 10.1002/hep.28143. [DOI] [PubMed] [Google Scholar]
- 90.Jilani N., Das B.C., Husain S.A. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–682. doi: 10.1111/j.1440-1746.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 91.Khuroo M.S. Acute liver failure in India. Hepatology. 1997;26:244–246. doi: 10.1002/hep.510260138. [DOI] [PubMed] [Google Scholar]
- 92.Norvell J.P., Blei A.T., Jovanovic B.D. Herpes Simplex Virus hepatitis: an analysis of the published literature and institutional cases. Liver Transplant. 2007;13:1428–1434. doi: 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- 93.Levitsky J., Duddenpudi A.T., Lakeman F.D. Detection and diagnosis of Herpes.Simplex Virus infection in adults with acute liver failure. Liver Transplant. 2008;14:1498–1504. doi: 10.1002/lt.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mellinger J.L., Rossaro L., Naugler W.E. Epstein-Barr virus ( EBV) related acute liver failure: a case series from the US acute liver failure study group. Dig Dis Sci. 2014;59:1630–1637. doi: 10.1007/s10620-014-3029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh L., Singh A., Agarwal M. Is Dengue emerging as an important cause of acute liver failure in endemic regions? World J Clin Cases. 2017;5:303–306. doi: 10.12998/wjcc.v5.i7.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taneja S., Borakokty A., Duseja A. Acute liver failure caused by hepatitis A virus with dengue confection. J Clin Exp Hepatol. 2016;6:164. doi: 10.1016/j.jceh.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brewer E.C., Hunter L. Acute liver failure due to disseminated Varicella zoster infection. Case Rep Hepatol. 2018 doi: 10.1155/2018/1269340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poff J.A., Coakley F.V., Qayyum A. Frequency and histopathologic basis of hepatic surface nodularity in patients with fulminant hepatic failure. Radiology. 2008;249:518–523. doi: 10.1148/radiol.2492072168. [DOI] [PubMed] [Google Scholar]
- 99.Ostapowicz G., Fontana R.J., Schiodt F.V. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 100.Devarbhavi H., Patil M., Reddy V.V. Drug-induced acute liver failure in children and adults: results of a single-centre study of 128 patients. Liver Int. 2018;38:1322–1329. doi: 10.1111/liv.13662. [DOI] [PubMed] [Google Scholar]
- 101.Tujios S.R., Lee W.M. Acute liver failure induced by idiosyncratic reaction to drugs: challenges in diagnosis and therapy. Liver Int. 2018;38:6–14. doi: 10.1111/liv.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choudhary N.S., Saigal S., Saraf N. Good outcome of living donor liver transplantation in drug-induced acute liver failure: a single-center experience. Clin Transplant. 2017;31:12907. doi: 10.1111/ctr.12907. [DOI] [PubMed] [Google Scholar]
- 103.Who . 2017. Global Tuberculosis Report 2017; pp. 1–249. [Google Scholar]
- 104.Devarbhavi H. Acute liver failure induced by anti-infectious drugs:causes and management. Curr Hepatol Rep. 2017;16:276–285. [Google Scholar]
- 105.Devarbhavi H. Ayurvedic and herbal medicine-induced liver injury: it is time to wake up and take notice. Indian J Gastroenterol. 2018 Jan;37:5–7. doi: 10.1007/s12664-018-0820-6. Epub 2018 Feb 8. [DOI] [PubMed] [Google Scholar]
- 106.Devarbhavi H., Choudhuri A., Kumar D.M. Drug-induced acute-on-chronic liver failure in asian patients. Am J Gastroenterol. 2019;114:929–937. doi: 10.14309/ajg.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 107.Philips C.A., Paramaguru R., Joy A.K. Clinical outcomes, histopathological patterns, and chemical analysis of Ayurveda and herbal medicine associated with severe liver injury-A single-center experience from southern India. Indian J Gastroenterol. 2018;37:9–17. doi: 10.1007/s12664-017-0815-8. [DOI] [PubMed] [Google Scholar]
- 108.Kumar R., Shalimar, Bhatia V. Antituberculosis therapy–induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1665–1674. doi: 10.1002/hep.23534. [DOI] [PubMed] [Google Scholar]
- 109.Devarbhavi H., Singh R., Patil M. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J Gastroenterol Hepatol. 2013;28:161–167. doi: 10.1111/j.1440-1746.2012.07279.x. [DOI] [PubMed] [Google Scholar]
- 110.Bernuau J., Benhamou J.P. Classifying acute liver failure. Lancet. 1993;342:252–253. doi: 10.1016/0140-6736(93)91809-z. [DOI] [PubMed] [Google Scholar]
- 111.Mindikoglu A.L., Magder L.S., Regev A. Outcome of liver transplantation for drug-induced acute liver failure in the United States: analysis of the United Network for Organ Sharing database. Liver Transplant. 2009;15:719–729. doi: 10.1002/lt.21692. [DOI] [PubMed] [Google Scholar]
- 112.Robles-Diaz M., Lucena M.I., Kaplowitz N. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147:109–118. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 113.Reuben A., Koch D.G., Lee W.M. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei G., Bergquist A., Broome U. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 115.Devarbhavi H. Gender enigma: increased occurrence of anti-tuberculosis drug-induced liver injury and acute liver failure in women despite tuberculosis being more common in men. Hepatology. 2017;66 6A (Abstract 17) [Google Scholar]
- 116.Devarbhavi H., Raj S. Drug-induced liver injury with skin reactions: drugs and host risk factors, clinical phenotypes and prognosis. Liver Int. 2019;5:802–811. doi: 10.1111/liv.14004. [DOI] [PubMed] [Google Scholar]
- 117.Bower W.A., Johns M., Margolis H.S., Williams I.T., Bell B.P. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007 Nov;102:2459–2463. doi: 10.1111/j.1572-0241.2007.01388.x. http://www.ncbi.nlm.nih.gov/pubmed/17608778 [Internet] [cited 2019 Mar 29] Available from: [DOI] [PubMed] [Google Scholar]
- 118.Acharya S.K. Hepatitis E and acute liver failure in pregnancy. J Clin Exp Hepatol [Internet] 2013;3:213–224. doi: 10.1016/j.jceh.2013.08.009. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Acharya S.K., Madan K., Dattagupta S., Panda S.K. Viral hepatitis in India. Natl Med J India. 2006 Jul-Aug;19:203–217. [PubMed] [Google Scholar]
- 120.Nayak N.C., Panda S.K., Datta R. Aetiology and outcome of acute viral hepatitis in pregnancy. J Gastroenterol Hepatol. 1989 Aug 1;4:345–352. doi: 10.1111/j.1440-1746.1989.tb00846.x. http://doi.wiley.com/10.1111/j.1440-1746.1989.tb00846.x [Internet] [cited 2019 Mar 27] Available from: [DOI] [PubMed] [Google Scholar]
- 121.Kumar A., Beniwal M., Kar P., Sharma J.B., Murthy N.S. Hepatitis E in pregnancy. Int J Gynaecol Obstet. 2004 Jun;85:240–244. doi: 10.1016/j.ijgo.2003.11.018. [Internet] [DOI] [PubMed] [Google Scholar]
- 122.Karnad D.R., Lapsia V., Krishnan A., Salvi V.S. Prognostic factors in obstetric patients admitted to an Indian intensive care unit. Crit Care Med [Internet] 2004 Jun 1;32:1294–1299. doi: 10.1097/01.ccm.0000128549.72276.00. http://www.ncbi.nlm.nih.gov/pubmed/15187509 [cited 2019 Mar 27] Available from. [DOI] [PubMed] [Google Scholar]
- 123.Rasheeda C.A., Navaneethan U., Jayanthi V. Liver disease in pregnancy and its influence on maternal and fetal mortality: a prospective study from Chennai, Southern India. Eur J Gastroenterol Hepatol. 2008 Apr 1;20:362–364. doi: 10.1097/MEG.0b013e3282f246d6. [DOI] [PubMed] [Google Scholar]
- 124.Jin H., Zhao Y., Zhang X., WangB Liu P. Case-fatality risk of pregnant women with acute viral hepatitis type E: a systematic review and meta-analysis. Epidemiol Infect. 2016 Jul 4;144:2098–2106. doi: 10.1017/S0950268816000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Devarbhavi H., Rao P., Patil M., Adarsh C.K. Characteristics of ascites in patients with pregnancy-specific liver diseases. Clin Gastroenterol Hepatol. 2012;10:559–562. doi: 10.1016/j.cgh.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 126.Devarbhavi H., Kremers W.K., Dierkhising R., Padmanabhan L. Pregnancy-associated acute liver disease and acute viral hepatitis: differentiation, course and outcome. J Hepatol. 2008;49:930–935. doi: 10.1016/j.jhep.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 127.Pandey C.K., Karna S.T., Pandey V.K., Tandon M. Acute liver failure in pregnancy : challenges and management. 2015;59(3):10–15. doi: 10.4103/0019-5049.153035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lakshmi C.P., Goel A., Basu D. Cholestatic presentation of yellow phosphorus poisoning. J Pharmacol Pharmacother. 2014;5:67–69. doi: 10.4103/0976-500X.124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mauskar A.M.K., Nagotkar L., Shanbag P. Acute hepatic failure due to yellow phosphorus ingestion. Indian J Pharmacol. 2011;43:355–356. doi: 10.4103/0253-7613.81500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simon F.A., Pickering L.K. Acute yellow phosphorus poisoning. "Smoking stool syndrome. J Am Med Assoc : J Am Med Assoc. 1976;235:1343–1344. [PubMed] [Google Scholar]
- 131.Caley J.P., Kellock I.A. Acute yellow phosphorus poisoning; with recovery. Lancet. 1955;268:539–541. doi: 10.1016/s0140-6736(55)91222-8. [DOI] [PubMed] [Google Scholar]
- 132.Kafeel S.I.C.V., Eswaran V.P. Role of N-acetylcysteine in outcome of patients with yellow phosphorus poisoning-an observational study. Nat J Emerg Med. 2012;1:35–40. [Google Scholar]
- 133.Brown C.A., Halpert B. Poisoning with yellow phosphorus. South Med J. 1957;50:740–742. doi: 10.1097/00007611-195706000-00006. [DOI] [PubMed] [Google Scholar]
- 134.Ghoshal A.K., Porta E.A., Hartroft W.S. Isotopic studies on the absorption and tissue distribution of white phosphorus in rats. Exp Mol Pathol. 1971;14:212–219. doi: 10.1016/0014-4800(71)90066-9. [DOI] [PubMed] [Google Scholar]
- 135.Santos O., Restrepo J.C., Velasquez L. Acute liver failure due to white phosphorus ingestion. Ann Hepatol. 2009;8:162–165. [PubMed] [Google Scholar]
- 136.Talley R.C.L.J., Trevino A.J. Acute elemental phosphorus poisoning in man: cardiovascular toxicity. Am Heart J. 1972;84:139–140. doi: 10.1016/0002-8703(72)90318-3. [DOI] [PubMed] [Google Scholar]
- 137.Fernandez O.U., Canizares L.L. Acute hepatotoxicity from ingestion of yellow phosphorus-containing fireworks. J Clin Gastroenterol. 1995;21:139–142. doi: 10.1097/00004836-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 138.Ates M., Dirican A., Ozgor D. Living donor liver transplantation for acute liver failure in pediatric patients caused by the ingestion of fireworks containing yellow phosphorus. Liver Transplant. 2011;17:1286–1291. doi: 10.1002/lt.22384. official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. [DOI] [PubMed] [Google Scholar]
- 139.Masudanayagam R., Shanmugam V., Gunson B. Etiology and outcomes of acute liver failure. HPB. 2009;11:429–434. doi: 10.1111/j.1477-2574.2009.00086.x. (Oxford) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parekh J., Mater V.M., Canas-Colo A., Friedman D., Lee W.M. Acute Liver Failure Study Group. Budd Chiary Syndrome causing acute livet failure: a multicenter case series. Liver Transplant. 2017;23:135–142. doi: 10.1002/lt.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Long J., Vaughan-Williams H., Moorhouse J., Sethi H., Kumar N. Acute Budd Chiary Syndrome due to a simle liver cyst. Annals R coll Engl. 2014;96:109E–111E. doi: 10.1308/003588414X13824511649698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shalimar Kumar A., Kedia S., Sharma H. Hepatic venous outflow tract obstruction: treatment outcomes and development of a new prognostic score. Aliment Pharmacol Ther. 2016;43:1154–1167. doi: 10.1111/apt.13604. [DOI] [PubMed] [Google Scholar]
- 143.Rautou P.E., Maucari R., Cazals-Hatem D. Levels and initial course of serum alanine aminotransferase can predict outcome of patients with Budd-Chiari syndrome. Clin Gastroenterol Hepatol. 2009;7:1230–1235. doi: 10.1016/j.cgh.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 144.Garcia-Pagan J.C., Heydtmann M., Raffa S. TIPS for Budd Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135:808–815. doi: 10.1053/j.gastro.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 145.Eapen C.E., Veliessaris D., Heydtmann M., Gunson B., Oliff S., Elias E. Favourable medium term outcome following hepatic vein reanalisation and/or transjugular intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut. 2006;55:878–884. doi: 10.1136/gut.2005.071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hiramatsu A., Takahashi S., Aikata H. Etiology and outcome of acute liver failure: retrospective analysis of 50 patients treated at a single center. J Gastroenterol Hepatol. 2008;23:1216–1222. doi: 10.1111/j.1440-1746.2008.05402.x. [DOI] [PubMed] [Google Scholar]
- 147.Fujiwara K., Yasui S., Yokosuka O. Autoimmune acute liver failure: an emerging etiology for intractable acute liver failure. HepatolInt. 2013;7:335–346. doi: 10.1007/s12072-012-9402-3. [DOI] [PubMed] [Google Scholar]
- 148.Mendizabal M., Marciano S., Videla M.G. Fulminant presentation of autoimmune hepatitis: clinical features and early predictors of corticosteroid treatment failure. Eur J Gastroenterol Hepatol. 2015;27:644–648. doi: 10.1097/MEG.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 149.Yeoman A.D., Westbrook R.H., Zen Y. Prognosis of acute severe autoimmune hepatitis (AS-AIH): the role of corticosteroids in modifying outcome. J Hepatol. 2014;61:876–882. doi: 10.1016/j.jhep.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 150.Moenne-Loccoz R., Severac F., Baumert T.F., Habersetzer F. Usefulness of corticosteroids as first-line therapy in patients with acute severe autoimmune hepatitis. J Hepatol. 2016;65:444–446. doi: 10.1016/j.jhep.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 151.Sonthalia N., Rathi P.M., Jain S.S. Natural history and treatment outcomes of severe autoimmune hepatitis. J Clin Gastroenterol. 2017;51:548–556. doi: 10.1097/MCG.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 152.Yasui S., Fujiwara K., Yonemitsu Y., Oda S., Nakano M., Yokosuka O. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J Gastroenterol. 2011;46:378–390. doi: 10.1007/s00535-010-0316-3. [DOI] [PubMed] [Google Scholar]
- 153.Fujiwara K., Fukuda Y., Yokosuka O. Precise histological evaluation of liver biopsy specimen is indispensable for diagnosis and treatment of acute-onset autoimmune hepatitis. J Gastroenterol. 2008;43:951–958. doi: 10.1007/s00535-008-2254-x. [DOI] [PubMed] [Google Scholar]
- 154.Bernal W., Ma Y., Smith H.M., Portmann B., Wendon J., Vergani D. The significance of autoantibodies and immunoglobulins in acute liver failure: a cohort study. J Hepatol. 2007;47:664–670. doi: 10.1016/j.jhep.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 155.Narkewicz M.R., Horslen S., Belle S.H. Prevalence and significance of autoantibodies in children with acute liver Failure.Pediatric acute liver failure study group. J Pediatr Gastroenterol Nutr. 2017;64:210–217. doi: 10.1097/MPG.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Q., Yang F., Miao Q., Krawitt E.L., Gershwin M.E., Ma X. The clinical phenotypes of autoimmune hepatitis: a comprehensive review. J Autoimmun. 2016;66:98–107. doi: 10.1016/j.jaut.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 157.Longhi M.S., Mieli-Vergani G., Vergani D. Autoimmune hepatitis. Curr Pediatr Rev. 2014;10:268–274. doi: 10.2174/1573396310666141114230147. [DOI] [PubMed] [Google Scholar]
- 158.Maggiore G., Sciveres M., Fabre M. Giant cell hepatitis with autoimmune hemolytic anemia in early childhood: long term outcome in 16 children. J Pediatr. 2011;159:127–132. doi: 10.1016/j.jpeds.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 159.Wang W., Lie P., Guo M., He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis of published data. Int J Canc. 2017;141:1018–1028. doi: 10.1002/ijc.30678. [DOI] [PubMed] [Google Scholar]
- 160.Abe K., Kanno Y., Okai K. Centrilobular necrosis in acute presentation of Japanese patients with type 1 autoimmune hepatitis. World J Hepatol. 2012;4:262–267. doi: 10.4254/wjh.v4.i9.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stravitz R.T., Lefkowitch J.H., Fontana R.J. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferrell L.D., Greenberg M.S. Special stains can distinguish hepatic necrosis with regenerative nodules from cirrhosis. Liver Int. 2007;27:681–686. doi: 10.1111/j.1478-3231.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 163.Walshe J.M., Yealland M. Wilson's disease: the problem of delayed diagnosis. J Neurol Neurosurg Psychiatry. 1992 Aug;55:692–696. doi: 10.1136/jnnp.55.8.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bayes F., Karim A.B., Helaly L. Spectrum of hepatic presentation of wilsons disease in children attending A tertiary care centre of dhaka city. Bangladesh J Child Health. 2014;38:86–93. [Google Scholar]
- 165.Schilsky M.L., Scheinberg I.H., Sternlieb I. Hepatic transplantation for Wilson's disease: indication and outcome. Hepatology. 1994;19:583–587. doi: 10.1002/hep.1840190307. [DOI] [PubMed] [Google Scholar]
- 166.Emre S., Atillasoy E.O., Ozdemir S. Orthotopic liver transplantation for Wilson's disease: a single center experience. Transplantation. 2001;72:1232–1236. doi: 10.1097/00007890-200110150-00008. [DOI] [PubMed] [Google Scholar]
- 167.Korman J.D., Volenberg I., Balko J., Webster J., Schiodt F.V., Squires R.H., Jr. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48:1167–1174. doi: 10.1002/hep.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walshe J.M. The acute haemolytic syndrome in Wilson's disease—a review of 22 patients. QJM. 2013;106:1003–1008. doi: 10.1093/qjmed/hct137. [DOI] [PubMed] [Google Scholar]
- 169.Eisenbach C., Sieg O., Stremmel W. Diagnostic criteria for acute liver failure due to Wilson disease. World J f Gastroenterol. 2007;13:1711–1714. doi: 10.3748/wjg.v13.i11.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ring-Larsen H., Pallazo U. Renal failure in fulminant hepatic failure and terminal cirrhosis: a comparison between incidence, types, and prognosis. Gut. 1981;22:585–591. doi: 10.1136/gut.22.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCullough A.J., Fleming C.R., Thistle J.L. Diagnosis of Wilson's disease presenting as fulminant hepatic failure. Gastroenterology. 1983;84:161–167. [PubMed] [Google Scholar]
- 172.Sato C., Koyama H., Satoh H. Concentrations of copper and zinc in liver and serum samples in biliary atresia patients at different stages of traditional surgeries. Tohoku J Exp Med. 2005;207:271–277. doi: 10.1620/tjem.207.271. [DOI] [PubMed] [Google Scholar]
- 173.Crocker J.F., Bagnell P.C. Reye's syndrome: a clinical review. Can Med Assoc J. 1981 Feb 15;124:375–382. 425. [PMC free article] [PubMed] [Google Scholar]
- 174.Lin Shide, Ying Li M.M., Jun Long B.S.M., Qichuan Liu M.M., Fangwan Yang M.M., Yihuai He M.M. Acute liver failure caused by hemophagocytic lymphohistiocytosis in adults A case report and review of the literature. Medicine. 2016;95:47. doi: 10.1097/MD.0000000000005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rich N.E., Sanders C., Hughes R.S. Malignant infiltration of the liver presenting as acute liver failure. Clin Gastroenterol Hepatol. 2015;13:1025–1028. doi: 10.1016/j.cgh.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bantel H., Schulze-Osthoff K. Mechanisms of cell death in acute liver failure. Front Physiol. 2012;79:6–13. doi: 10.3389/fphys.2012.00079. 2:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luedde T., Kaplowitz N., Schwabe R.F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schulze-Osthoff K., andBantel H. Necrosis versus apoptosis in acetaminophen-induced hepatotoxicity. Hepatology. 2011;53:1070. doi: 10.1002/hep.24027. [DOI] [PubMed] [Google Scholar]
- 179.Linkermann A., Green D.R. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu Z.X., Govindarajan S., Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 181.Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Karlmark K.R., Wasmuth H.E., Trautwein C., Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expet Rev Gastroenterol Hepatol. 2008;2:233–242. doi: 10.1586/17474124.2.2.233. Apr. [DOI] [PubMed] [Google Scholar]
- 183.Craig D.G., Lee P., Pryde E.A., Masterton G.S., Hayes P.C., Simpson K.J. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 2011;31:1127–1136. doi: 10.1111/j.1478-3231.2011.02528.x. [DOI] [PubMed] [Google Scholar]
- 184.Fischer U., Schulze-Osthoff K. New approaches and therapeutics targeting apoptosis in disease. PharmacolRev. 2005;57:187–215. doi: 10.1124/pr.57.2.6. [DOI] [PubMed] [Google Scholar]
- 185.Shawcross D.L., Wendon J.A. The neurological manifestation of acute liver failure. Neurochem Int. 2012;60:662–671. doi: 10.1016/j.neuint.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 186.Butterworth R.F. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab Brain Dis. 2002;17:221–227. doi: 10.1023/a:1021989230535. [DOI] [PubMed] [Google Scholar]
- 187.Butterworth R.D. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure. J Clin Exp Hepatol. 2015;5:S96–S103. doi: 10.1016/j.jceh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Butterworth R.F. Pathophysiology of brain dysfunction in hyperammonemic syndromes: the many faces of glutamine. Metab Genet Med. 2014;113:113–117. doi: 10.1016/j.ymgme.2014.06.003. Sep-Oct. [DOI] [PubMed] [Google Scholar]
- 189.Staub F., Baethmann, Peters J. Effects of lactic acidosis on glial cell volume and viability. J Cerebr Blood Flow Metabol. 1990;10:866–876. doi: 10.1038/jcbfm.1990.143. [DOI] [PubMed] [Google Scholar]
- 190.Belanger M., Desjardins P., Chatauret N., Butterworth R.F. Loss of expression of glial brillary acidic protein in acute hyperammonemia. Neurochem Int. 2002;41:155–160. doi: 10.1016/s0197-0186(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 191.Chaudhry F.A., Krisaj D., Larsson P., etal Coupled and uncoupled proton movement by amino acid transport system N. EMBO J. 2001;20:7041–7051. doi: 10.1093/emboj/20.24.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Belanger M., Desjardins P., Chatauret N. Selectively increased expression of the astrocytic/endothelial glucose transporter protein GLUT1 in acute liver failure. Glia. 2006;53:557–562. doi: 10.1002/glia.20310. [DOI] [PubMed] [Google Scholar]
- 193.Thrane V.R., Thrane A.S., Chanag J. Real-time analysis of microglial activation and motility in hepatic and hyperammonemic encephalopathy. Neuroscience. 2012;220:247–255. doi: 10.1016/j.neuroscience.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Strauss G.I., Hogh P., Moller K. Regional cerebral blood flow during mechanical ventilation in patients of acute liver failure. Hepatology. 1999;30:1368–1373. doi: 10.1002/hep.510300608. [DOI] [PubMed] [Google Scholar]
- 195.Murphy N., Auzinger G., Bernel W., Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39:464–470. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 196.Blei A.T. Brain edema in acute liver failure: can it be prevented? Can it be treated? J Hepatol. 2007;46:564–569. doi: 10.1016/j.jhep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bernal W., Hall C., Karvellas C.J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–1852. doi: 10.1002/hep.21838. [DOI] [PubMed] [Google Scholar]
- 198.Bhatia V., Batra Y., Acharya S.K. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure--a controlled clinical trial. JHepatol. 2004 Jul;41:89–96. doi: 10.1016/j.jhep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 199.Kumar R., Shalimar, Sharma H. Persistent hyperammonemia is associated with complications and poor outcomes in patients with acute liver failure. Clin Gastroenterol Hepatol. 2012;10:925–931. doi: 10.1016/j.cgh.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 200.Fortea J.I., Bañares R., Vaquero J. Intracranial pressure in acute liver failure: to bolt or not to bolt—that is the question. Crit Care Med. 2014;42:1304–1305. doi: 10.1097/CCM.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 201.Karvellis C.J., Fix O.K., Battenhouse H. Outcomes and complications of intracranial pressure monitoring in acute liver failure a retroscepctive cohort study. Crit Care Med. 2014;42:1157–1167. doi: 10.1097/CCM.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schell R.M., Cole D.J. Cerebral monitoring: jugular venous oximetry. Anesth Analg. 2000;90:559–566. doi: 10.1097/00000539-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 203.Abdo A., Pérez-Bernal J., Hinojosa R. Cerebral Haemodynamics patterns in patients with acute liver failureTransplant. SAVE Proc. 2015 Nov;47:2647–2649. doi: 10.1016/j.transproceed.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 204.Krishnamurty V., Beckmann K., Mueller M. Perioperative estimation of the intracranial pressure using the optic nerve sheath diameter during liver transplantation. Liver Transplant. 2013;19:246–249. doi: 10.1002/lt.23591. [DOI] [PubMed] [Google Scholar]
- 205.Bajaj J.S., Cordoba J., Mullen K.D. Review article: the design of clinical trials in hepatic encephalopathy — an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739–747. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wijdicks E.F., Rabinstein A.A., Bamlet W.R., Mandrekar J.N. FOUR score and Glasgow Coma Scale in predicting outcome of comatose patients: a pooled analysis. Neurology. 2011;77:84–85. doi: 10.1212/WNL.0b013e318220ac06. [DOI] [PubMed] [Google Scholar]
- 207.EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 208.Wong F., Nadim M.K., Kellum J.A. Working party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 209.Leithead J.A., Ferguson J.W., Bates C.M. The systemic inflammatory response syndrome is predictive of renal dysfunction in patients with non-paracetamol-induced acute liver failure. Gut. 2009;58:443–449. doi: 10.1136/gut.2008.154120. [DOI] [PubMed] [Google Scholar]
- 210.Betrosian A.P., Agarwal B., Douzinas E.E. Acute renal dysfunction in liver diseases. World J Gastroenterol. 2007;13:5552–5559. doi: 10.3748/wjg.v13.i42.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mazer M., Perrone J. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol. 2008;4:2–6. doi: 10.1007/BF03160941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hoet P., Haufroid V., Deumer G., Dumont X., Lison D., Hantson P. Acute kidney injury following acute liver failure: potential role of systemic cadmium mobilization? Intensive Care Med. 2012;38:467–473. doi: 10.1007/s00134-011-2449-0. [DOI] [PubMed] [Google Scholar]
- 213.Weigand K., Riediger C., Stremmel W., Flechtenmacher C., Encke J. Are heat stroke and physical exhaustion underestimated causes of acute hepatic failure? World J Gastroenterol. 2007;13:306–309. doi: 10.3748/wjg.v13.i2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mayeux P.R., Macmillan-Crow L.A. Pharmacological targets in the renal peritubular microenvironment: implications for therapy for sepsis-induced acute kidney injury. Pharmacol Ther. 2012;134:139–155. doi: 10.1016/j.pharmthera.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Linkermann A., De Zen F., Weinberg J., Kunzendorf U., Krautwald S. Programmed necrosis in acute kidney injury. NephrolDial Transplant. 2012;27:3412–3419. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 216.Rosin D.L., Okusa M.S. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Martin-Llahi M., Guevara M., Torre A. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488–496. doi: 10.1053/j.gastro.2010.07.043. e484. [DOI] [PubMed] [Google Scholar]
- 218.Jankowski J.A., Goodlad R.A., Wright N.A. Maintenance of normal intestinal mucosa: function, structure, and adaptation. Gut. 1994;35(1 suppl):S1–S4. doi: 10.1136/gut.35.1_suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rolando N., Wade J., Davalos M., Wendon J., Philpott-Howard J., Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 220.Vaquero J., Polson J., Chung C. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755–764. doi: 10.1016/s0016-5085(03)01051-5. [DOI] [PubMed] [Google Scholar]
- 221.Fukui H. Gut-liver axis in liver cirrhosis: how to manage leaky gut and endotoxemia. World J Hepatol. 2015 Mar 27;7:425–442. doi: 10.4254/wjh.v7.i3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gong S., Lan T., Zeng L. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol. 2018 Jul;69:51–59. doi: 10.1016/j.jhep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Panis Y., McMullan D.M., Emond J.C. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142–149. doi: 10.1016/s0039-6060(97)90283-x. [DOI] [PubMed] [Google Scholar]
- 224.Quaglia A., Portmann B.C., Knisely A.S. Auxiliary transplantation for acute liver failure:Histopathological study of native liver regeneration. Liver Transplant. 2008;14:1437–1448. doi: 10.1002/lt.21568. [DOI] [PubMed] [Google Scholar]
- 225.Goves C.D., Hughes R.D. Liver regeneration in relationship to acute liver failure. Gut. 1991;32(Suppl):S92–S96. doi: 10.1136/gut.32.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rajput I., Prasad K.R., Bellamy M.C., Davies M., Attia M.S., Lodge J.P. Subtotal hepatectomy and whole graft auxiliary transplantation for acetaminophen associated acute liver failure. HPB. 2014;16:220–228. doi: 10.1111/hpb.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Girlanada R., Vilca-Melendez H., Srinivasan P. Immunosuppression withdrawal after auxiliary liver transplantationfor acute liver failure. Transplant Proc. 2005;37:1720–1721. doi: 10.1016/j.transproceed.2005.03.141. [DOI] [PubMed] [Google Scholar]
- 228.Donaldson B.W., Gopinath R., Wanless I.R. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology. 1993;18:1370–1376. [PubMed] [Google Scholar]
- 229.Macedo G., Maia J.C., Gomes A., Teixeira A., Ribeiro T. The role of transjugular liver biopsy in a liver transplant center. J Clin Gastroenterol. 1999;29:155–157. doi: 10.1097/00004836-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 230.Singhal A., Vadlamudi S., Stokes K. Liver histology as predictor of outcome in patients with acute liver failure. Transpl Int. 2012;25:658–662. doi: 10.1111/j.1432-2277.2012.01470.x. Epub 2012 Apr 5. [DOI] [PubMed] [Google Scholar]
- 231.Chapin C.A., Mohammad S., Bass L.M., Taylor S.A., Kelly S., Alonso E.M. Liver biopsy can Be safely performed in pediatric acute liver failure to aid in diagnosis and management. J Pediatr Gastroenterol Nutr. 2018;67:441–445. doi: 10.1097/MPG.0000000000002096. [DOI] [PubMed] [Google Scholar]
- 232.Ganger D.R., Rule J., Rakela J. Acute liver failure study group. Acute liver failure of indeterminate etiology: a comprehensive systematic approach by an expert committee to establish causality. Am J Gastroenterol. 2018;113:1319. doi: 10.1038/s41395-018-0160-2. Epub 2018 Jun 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ndekwe P., Ghabril M.S., Zang Y., Mann S.A., Cummings O.W., Lin J. Substantial hepatic necrosis is prognostic in fulminant liver failure. World J Gastroenterol. 2017;23:4303–4310. doi: 10.3748/wjg.v23.i23.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Beckmann M.G., Bahr M.J., Hadem J. Clinical relevance of transjugular liver biopsy in comparison with percutaneous and laparoscopic liver biopsy. Gastroenterol Res Pract. 2009;2009:947014. doi: 10.1155/2009/947014. Epub 2009 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Miraglia R., Luca A., Gruttadauria S. Contribution of transjugular liver biopsy in patients with the clinical presentation of acute liver failure. Cardiovasc Intervent Radiol. 2006;29:1008–1010. doi: 10.1007/s00270-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 236.Dechêne A., Sowa J.P., Schlattjan M. Mini-laparoscopy guided liver biopsy increases diagnostic accuracy in acute liver failure. Digestion. 2014;90:240–247. doi: 10.1159/000366517. [DOI] [PubMed] [Google Scholar]
- 237.Hanau C., Munoz S.J., Rubin R. Histopathological heterogeneity in fulminant hepatic failure. Hepatology. 1995;21:345. [PubMed] [Google Scholar]
- 238.Wang M.C., Wandrer F., Schlué J. Transjugular diagnostics in acute liver failure including measurements of hepatocentral venous biomarker gradients. Hepatol Res. 2018;48:914–925. doi: 10.1111/hepr.13185. Epub 2018 Jun 5. [DOI] [PubMed] [Google Scholar]
- 239.Stift J., Semmler G., Walzel C. Transjugular aspiration liver biopsy performed by hepatologists trained in HVPG measurements is safe and provides important diagnostic information. Dig Liver Dis. 2019;51:1144–1151. doi: 10.1016/j.dld.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 240.Denzer U., Helmreich-Becker I., Galle P.R., Lohse A.W. Liver assessment and biopsy in patients with marked coagulopathy: value of mini-laparoscopy and control of bleeding. Am J Gastroenterol. 2003;98:893–900. doi: 10.1111/j.1572-0241.2003.07342.x. [DOI] [PubMed] [Google Scholar]
- 241.Rich N.E., Sanders C., Hughes R.S. Malignant infiltration of the liver presenting as acute liver failure. Clin Gastroenterol Hepatol. 2015;13:1025–1028. doi: 10.1016/j.cgh.2014.09.040. Epub 2014 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Buechter M., Manka P., Heinemann F.M. Potential triggering factors of acute liver failure as a first manifestation of autoimmune hepatitis-a single center experience of 52 adult patients. World J Gastroenterol. 2018;24:1410–1418. doi: 10.3748/wjg.v24.i13.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kleiner D.E. Histopathological challenges in suspected drug-induced liver injury. Liver Int. 2018;38:198–209. doi: 10.1111/liv.13584. Epub 2017 Sep 19. [DOI] [PubMed] [Google Scholar]
- 244.vanLeeuwen D.J., Alves V., Balabaud C. Liver Pathology Study Group TI. Acute-on-chronic liver failure 2018: a need for (urgent) liver biopsy? Expert Rev GastroenterolHepatol. 2018;12:565–573. doi: 10.1080/17474124.2018.1481388. Epub 2018 Jun 14. [DOI] [PubMed] [Google Scholar]
- 245.Alam S., Lal B.B. Metabolic liver diseases presenting as acute liver failure in children. Indian Pediatr. 2016;53:695–701. doi: 10.1007/s13312-016-0913-1. Epub 2016 Jun 1. [DOI] [PubMed] [Google Scholar]
- 246.Riegler J.L., Lake J.R. Fulminant hepatic failure. Med Clin. 1993;77:1057–1083. doi: 10.1016/s0025-7125(16)30210-3. [DOI] [PubMed] [Google Scholar]
- 247.Stravitz R.T., Kramer A.H., Davern T. Intensive care of patients with acute liver failure: recommendations of the acute liver failure study group. Crit Care Med. 2007;35:2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 248.Vaquero J., Chung C., Cahill M.E., Blei A.T. Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis. 2003 Aug;23:259–269. doi: 10.1055/s-2003-42644. [DOI] [PubMed] [Google Scholar]
- 249.Shalimar Kedia S., Sharma H., Vasudevan S., Sonika U., Upadhyaya A.D., Acharya S.K. Predictors of infection in viral-hepatitis related acute liver failure. Scand J Gastroenterol. 2017 Dec;52:1413–1419. doi: 10.1080/00365521.2017.1374449. [DOI] [PubMed] [Google Scholar]
- 250.Pyleris E., Giannikopoulos G., Dabos K. Pathophysiology and management of acute liver failure. Annal Gastroenterol North Am. 2010;23:257–265. [Google Scholar]
- 251.Stravitz R.T., Kramer D.J. Management of acute liver failure. Nat Rev Gastroenterol Hepatol. 2009;6:542–553. doi: 10.1038/nrgastro.2009.127. [DOI] [PubMed] [Google Scholar]
- 252.Anand A.C., Garg H.K. Approach to clinical syndrome of jaundice and encephalopathy in tropics. J Clin Exp Hepatol. 2015;5(suppl 1):S116–S130. doi: 10.1016/j.jceh.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bernal W., Lee W.M., Wendon J., Larsen F.S., Williams R. Acute liver failure: a curable disease by 2024? J Hepatol. 2015 Apr;62(1 suppl l):S112–S120. doi: 10.1016/j.jhep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 254.Habib M., Roberts L.N., Patel R.K., Wendon J., Bernal W., Arya R. Evidence of rebalanced coagulation in acute liver injury and acute liver failure as measured by thrombin generation. Liver Int. 2014;34:672–678. doi: 10.1111/liv.12369. [DOI] [PubMed] [Google Scholar]
- 255.Lisman T., Bakhtiari K., Adelmeijer J., Meijers J.C., Porte R.J., Stravitz R.T. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemostasis. 2012;10:1312–1319. doi: 10.1111/j.1538-7836.2012.04770.x. [DOI] [PubMed] [Google Scholar]
- 256.Bernal W. Lactate is important in determining prognosis in acute liver failure. J Hepatol. 2010;53:209–210. doi: 10.1016/j.jhep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 257.Ilyas A., Ishtiaq W., Assad S. Correlation of IVC diameter and collapsibility index with central venous pressure in the assessment of intravascular volume in critically ill patients. Cureus. 2017;9 doi: 10.7759/cureus.1025. e1025. Published 2017 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Stravitz R.T., Lisman T., Luketic V.A. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129–136. doi: 10.1016/j.jhep.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kumar R., Shalimar, Sharma H. Prospective derivation and validation of early dynamic model for predicting outcome in patients with acute liver failure. Gut. 2012;61:1068–1075. doi: 10.1136/gutjnl-2011-301762. [DOI] [PubMed] [Google Scholar]
- 260.Cardoso F.S., Gottfried M., Tujios S., Olson J.C., Karvellas C.J. US Acute Liver Failure Study Group. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology. 2018;67:711–720. doi: 10.1002/hep.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kok B., Karvellas C.J. Management of cerebral edema in acute liver failure. Semin Respir Crit Care Med. 2017 Dec;38:821–829. doi: 10.1055/s-0037-1608772. [DOI] [PubMed] [Google Scholar]
- 262.Vaquero J. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transplant. 2005;11:1581. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 263.Larsen F.S., Hansen B.A., Ejlersen E. Cerebral blood flow, oxygen metabolism and transcranialdopplersonography during high-volume plasmapheresis in fulminant hepatic failure. Eur J Gastroenterol Hepatol. 1996;8:261–265. doi: 10.1097/00042737-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 264.Nielsen H.B., Tofteng F., Wang L.P., Larsen F.S. Cerebral oxygenation determined by near-infrared spectrophotometry in patients with fulminant hepatic failure. J Hepatol. 2003;38:188–192. doi: 10.1016/s0168-8278(02)00377-x. [DOI] [PubMed] [Google Scholar]
- 265.Rajajee V., Williamson C.A., Fontana R.J., Courey A.J., Patil P.G. Noninvasive intracranial pressure assessment in acute liver failure. Neurocritical Care. 2018 Oct;29:280–290. doi: 10.1007/s12028-018-0540-x. [DOI] [PubMed] [Google Scholar]
- 266.Bernal W., Hyyrylainen A., Gera A. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013 Jul 1;59:74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 267.O'Grady J.G., Alexander G.J., Hayllar K.M., Williams R. Early indicators of prognosis in. Liver. Gastroenterology. 1989 Aug;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 268.Bernuau J., Goudeau A., Poynard T. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986 Jul;6:648–651. doi: 10.1002/hep.1840060417. [DOI] [PubMed] [Google Scholar]
- 269.Lee H.S., Choi G.H., Joo D.J. Prognostic value of model for end-stage liver disease scores in patients with fulminant hepatic failure. Transplant Proc Elsevier Inc. 2013;45:2992–2994. doi: 10.1016/j.transproceed.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 270.Kim W.R., Biggins S.W., Kremers W.K. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Dhiman R.K., Jain S., Maheshwari U. Early indicators of prognosis in fulminant hepatic failure: an assessment of the model for end-stage liver disease (MELD) and King's college hospital criteria. Liver Transplant. 2007;13:814–821. doi: 10.1002/lt.21050. [DOI] [PubMed] [Google Scholar]
- 272.Anand A.C., Nightingale P., Neuberger J.M. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King's criteria. J Hepatol. 1997;26:62–68. doi: 10.1016/s0168-8278(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 273.McPhail M.J.W., Wendon J.A., Bernal W. Meta-analysis of performance of Kings's College Hospital Criteria in prediction of outcome in non-paracetamol-induced acute liver failure. J Hepatol Eur Assoc Stud Liver. 2010;53:492–499. doi: 10.1016/j.jhep.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 274.Bernal W., Donaldson N., Wyncoll D., Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558–563. doi: 10.1016/S0140-6736(02)07743-7. [DOI] [PubMed] [Google Scholar]
- 275.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PCJ. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology;31:864–871. [DOI] [PubMed]
- 276.Yantorno S.E., Kremers W.K., Ruf A.E., Trentadue J.J., Podestá L.G., Villamil F.G. MELD is superior to King's college and Clichy's criteria to assess prognosis in fulminant hepatic failure. Liver Transplant [Internet] 2007 Jun;13:822–828. doi: 10.1002/lt.21104. [cited 2019 Mar 24] [DOI] [PubMed] [Google Scholar]
- 277.Bhatia V., Singh R., Acharya S.K. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut. 2006;55:96–104. doi: 10.1136/gut.2004.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tofteng F., Hauerberg J., Hansen B.A., Pedersen C.B., Jørgensen L., Larsen F.S. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cerebr Blood Flow Metabol. 2006;26:21–27. doi: 10.1038/sj.jcbfm.9600168. [DOI] [PubMed] [Google Scholar]
- 279.Stravitz R.T., Larsen F.S. Therapeutic hypothermia for acute liver failure. Crit Care Med [Internet] 2009 Jul;37(suppl.):S258–S264. doi: 10.1097/CCM.0b013e3181aa5fb8. [cited 2019 Mar 24] [DOI] [PubMed] [Google Scholar]
- 280.Writ Petition (Civil) 215/2005 Supreme Court of India 09 March. 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.