Abstract
Polyamines are small polycationic alkylamines involved in many fundamental cellular processes, including proliferation, nucleic acid synthesis, apoptosis, and protection from oxidative damage. It has been proposed that in addition to these functions, elevated levels of polyamines promote longevity in various biological systems, including yeast, Drosophila, and murine models. A series of in vitro mechanistic studies by multiple investigators has led to the conclusion that addition of exogenous spermidine promotes longevity through autophagy induction; however, these experiments were confounded by the use of mammalian cell culture systems supplemented with fetal bovine serum. Using cell viability assays, LC3B immunoblots, and live-cell fluorescence microscopy, we report here that in the presence of ruminant serum, exogenously added polyamines are quickly oxidized by the copper-containing bovine serum amine oxidase. This polyamine oxidation resulted in the production of harmful byproducts including hydrogen peroxide, ammonia, and reactive aldehydes. Our data demonstrate that it is critically important to prevent confounding bovine serum amine oxidase–induced cytotoxicity in mechanistic studies of the roles of polyamines in autophagy.
Keywords: polyamine, spermidine, spermine, autophagy, amine oxidase, oxidative stress, beta-oxidation, hydrogen peroxide, bovine serum amine oxidase (BSAO), cytotoxicity
Introduction
Polyamines are small polycationic alkylamines involved in many fundamental cellular processes including cell growth and proliferation, nucleic acid synthesis, apoptosis, and protection from oxidative damage (1, 2). The naturally occurring eukaryotic polyamines, spermidine (SPD), spermine (SPM), and their diamine precursor putrescine are positively charged at physiological pH and thus interact with negatively charged molecules including DNA, RNA, and free radicals (3). Consequently, polyamines are involved in regulating chromatin structure and protecting nucleic acids from oxidative free radicals (4, 5). Polyamines naturally exist at millimolar concentrations inside of eukaryotic cells, although unbound concentrations are thought to be lower, and the metabolism of and requirement for polyamines is frequently dysregulated in cancer (6–8). Because polyamines have the ability to simultaneously affect multiple fundamental processes, the intracellular concentrations of naturally occurring polyamines are normally tightly regulated through coordinated biosynthesis, catabolism, and transport. The mammalian polyamine catabolic pathway includes three enzymes: spermidine/spermine N1-acetyltransferase (SSAT), N1-acetylpolyamine oxidase (PAOX), and spermine oxidase (SMOX), that are responsible for the back-conversion of SPD and SPM (Fig. 1) (9). SSAT is responsible for acetylating the primary N1 amines of SPD and SPM. These acetylated polyamines can be subsequently oxidized by PAOX to produce SPD or putrescine, depending on the starting substrate, with H2O2 and 3-acetoaminopropanal as byproducts (10–12). SMOX directly converts SPM to SPD while producing H2O2 and the aldehyde 3-aminopropanal (13). In addition to these enzymes, certain polyamines are substrates for a variety of other amine oxidases including copper-containing amine oxidases that catalyze the oxidative deamination of polyamines generating H2O2, ammonia, and aldehydes as toxic byproducts (14).
Figure 1.
The polyamine synthetic pathway begins with L-ornithine precursor and progresses to spermine following a series of enzymatic reactions. A, catabolism progresses using SMOX to back convert SPM to SPD, which can then be further broken down to putrescine using SSAT and PAOX. B, in serum-supplemented media, BSAO catalyzes the oxidative deamination of SPM and SPD, resulting in the rampant production of toxic byproducts including ammonia, hydrogen peroxide, and acrolein. BSAO activity can be inhibited by well-tolerated aminoguanidine (AG).
Cell culture is a facile and informative tool for completing in vitro mechanistic studies. Because of components necessary for cell growth, including proteins, growth factors, and hormones, bovid serum is a frequently used supplement in semidefined eukaryotic cell culture media. The composition of serum, however, is complex, and its components are not fully known or understood (15). The importance of considering off-target effects from serum components cannot be overemphasized. Extracellular supplementation of polyamines has been known to be cytotoxic when added in the presence of ruminant serum for over 60 years (16–19). The cytotoxicity is due to the production of multiple toxic metabolites including reactive oxygen species, ammonia, and aldehydes by an enzyme found in serum, bovine serum amine oxidase (BSAO). In the presence of ruminant serum, exogenously added polyamines are quickly oxidized by the copper-containing amine oxidase BSAO, resulting in the production of the metabolites (20). Both SPD and SPM are oxidized by BSAO (Fig. 1), and the formed aminoaldehydes undergo subsequent β-elimination to yield acrolein, a highly toxic aldehyde (21). Aminoguanidine (AG) is a one-carbon oxidase inhibitor capable of inhibiting BSAO activity (22). Supplementation of AG into cell culture medium is nontoxic to cells because AG is a well-tolerated inhibitor of BSAO that can be used to safely treat most cell types in millimolar concentrations (23, 24).
It has been proposed that in addition to promoting proliferation, elevated levels of polyamines, in particular SPD, promote longevity in various biological systems including yeast, Drosophila, and murine models (25). Ancillary mechanistic work concluded that the addition of exogenous SPD promotes longevity through the activation of autophagy. However, these experiments are potentially confounded by the fact that they were conducted in mammalian cell culture systems supplemented with bovine calf serum. Macroautophagy is a known protective response against oxidative stress, and as previously indicated, BSAO-mediated oxidation of SPD and SPM produces significant amounts of H2O2 (26–28). Consequently, considerable caution must be used when interpreting much of the published mechanistic data produced in in vitro cell culture systems because any observed autophagy included in such systems is likely the result of the toxic metabolites formed by BSAO following polyamine addition to the serum-containing medium and not a function of the polyamines themselves (25, 29–32). Therefore, we designed the current studies to determine the extent to which exogenously added polyamines contribute to the autophagic process in the presence and absence of serum amine oxidase activity.
Results
Cell viability following exogenous polyamine treatment in the presence of BSAO
It was previously demonstrated that the addition of exogenous polyamines is toxic in cell culture systems because of BSAO activity and that co-treatment with AG is sufficient to alleviate this toxicity (23, 24). Two cancer cell lines, A549 lung adenocarcinoma and HCT116 colon adenocarcinoma, were used to confirm that the addition of exogenous polyamines results in cell death that is preventable with AG treatment. The cells were treated for 24 h with concentrations of SPD ranging from 1 μm to 2 mm, with or without 1 mm AG. A549 cells show notable cell death following 75 μm of SPD with nearly complete cell death occurring by 500 μm of treatment (Fig. 2A). Noticeable HCT116 cell death occurs between 25 and 50 μm of SPD treatment (Fig. 2B). Treatment of both cell lines with 1 mm AG in addition to SPD was sufficient to prevent cytotoxicity up to the maximum concentration of 2 mm.
Figure 2.
Effects of exogenous polyamine addition on cellular viability in cancer cell lines. A and B, A549 lung cancer cells (A) and HCT116 colon cancer cells (B) were treated for 24 h with concentrations ranging from 1 μm to 2 mm of SPD either with or without supplementation of 1 mm AG. C and D, A549 (C) and HCT116 (D) were treated as above with concentrations ranging from 1 μm to 2 mm of SPM with or without AG supplementation. The results indicate the means of three biological replicates, each measured in triplicate. Error bars indicate S.D.
Similarly, both cell lines were treated for 24 h with concentrations of SPM ranging from 1 μm to 2 mm, with or without supplementation of 1 mm AG. A549 cells show less than 50% viability with 25 μm of SPM with complete loss of viability following 75 μm of SPM treatment (Fig. 2C). Co-treatment with 1 mm AG prevented the loss of cell viability up to the maximum 2 mm SPM dose. HCT116 cells show notable cell death with 25 μm of SPM treatment and complete loss of viability with 50 μm of SPM (Fig. 2D). Co-treatment with AG retained >75% cell viability up to 750 μm SPM treatment; however, at concentrations above 1 mm SPM, cell viability could not be maintained with AG co-treatment. These results provide support that A549 and HCT116 cells are susceptible to BSAO-induced cell death following polyamine supplementation and that these lines are an appropriate model system moving forward as has previously been reported for many cell types (16–19, 23, 24).
Effects of long-term exogenous polyamine treatment on cell viability
A549 and HCT116 cells were treated for 24, 48, 72, or 96 h with concentrations of SPD or SPM ranging from 1 to 100 μm. Both A549 (Fig. 3A) and HCT116 (Fig. 3B) show cell death following low-dose SPD that is alleviated with AG co-treatment. This is consistent over all time points. A549 cells treated with low-dose SPM show significant toxicity at 25 μm and all concentrations beyond (Fig. 3C). AG co-treatment alleviated all cellular toxicity for up to 4 days of treatment. HCT116 cells show significant toxicity between 25 and 50 μm of SPM treatment (Fig. 3D). AG supplementation alleviated all cellular toxicity up to 75 μm. The highest SPM concentration (100 μm) induced complete cellular toxicity with only a 50% rescue with concurrent AG treatment. This partial rescue at 100 μm was consistent across all time points and supports the lack of prevention observed at higher concentrations (Fig. 2D). HCT116 cells with a p53 deletion were treated with SPD and SPM to address a potential link between the macroautophagy and p53 pathways. HCT116 p53 null and parental HCT116 cells responded similarly to SPD and SPM treatment (Fig. S1), suggesting that the p53 pathway is not necessary for the cytotoxic response to exogenous polyamines added in the presence of BSAO. Putrescine, a diamine that is a poor substrate of BSAO, did not result in any toxicity among any tested cell line treated with concentrations up to 100 μm (Fig. S2). This emphasizes that only BSAO substrates produce the levels of oxidative stress necessary to induce autophagy.
Figure 3.
Effects of low-dose exogenous polyamine addition on long-term cellular viability in cancer cell lines. A and B, A549 lung adenocarcinoma cells (A) and HCT116 colon adenocarcinoma cells (B) were treated for 24, 48, 72, and 96 h with concentrations ranging from 1 to 100 μm of SPD either with or without supplementation of 1 mm AG. C and D, A549 (C) and HCT116 (D) cells were treated with concentrations ranging from 1 to 100 μm of SPM with or without AG supplementation. The results indicate the means of two biological replicates, each measured in triplicate. The error bars indicate S.E.
LC3B levels following exogenous addition of polyamines
Human microtubule-associated protein 1 light chain 3 (LC3B) is the main protein used as an autophagic marker (33). There are two forms of the LC3B protein. LC3B-I is explicitly cytosolic but can be processed to LC3B-II (34). LC3B-II is conjugated with phosphatidylethanolamine and can then bind tightly to autophagosomal membranes, associating increases in LC3B-II levels with autophagy induction (33, 34). A549 cells were treated with 25 μm SPD or SPM for 24 h with or without 1 mm AG. Rapamycin (100 nm) was used as a positive control for macroautophagy (35). Treatment with SPD or SPM in the presence of AG showed predominantly LC3B-I expression with very little LC3B-II expression (Fig. 4A and Fig. S3). However, when treated in the absence of AG, LC3B-II protein levels increased. LC3B-II protein levels were high in rapamycin-treated cells regardless of AG addition (Fig. 4A). The ratio of LC3B-II/LC3B-I is increased more than 1.5-fold by polyamine treatment when BSAO is active (Fig. 4B). Inhibition of BSAO by AG results in a reduction of the LC3B-II/LC3B-I ratio to mock levels (under 0.25-fold). These results indicate an increase in autophagy markers following exogenous polyamine addition only in the presence of BSAO.
Figure 4.
Effects of BSAO inhibition on LC3B expression following exogenous polyamine treatment. A549 cells were treated with 25 μm SPD or SPM for 24 h. A subset of cells were co-treated with 1 mm AG to inhibit BSAO activity. The lysates were run on a SDS-PAGE gel for Western blotting (Fig. S3). A, LC3B-II expression was quantified and normalized to actin. B, normalized LC3B-II and LC3B-I expression were used to produce the LC3BII:LC3BI ratio.
Effect of BSAO on autophagic response to exogenous polyamines
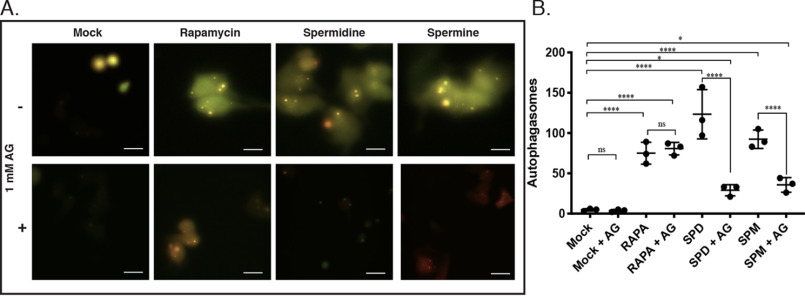
Puncta of fluorescent protein-tagged LC3 are used to visualize autophagosome formation in real time (36). The pEX-PK-hLC3 plasmid encodes both pH-sensitive pHluorin, a mutant GFP, and pH-stable mKate, a far-red fluorescent protein (37). Green fluorescence of pHluorin decreases as the pH decreases; therefore puncta that fluoresce both red and green represent autophagosomes and early autophagosome–lysosome fusion (37). Puncta that only emit far-red are autolysosomes that have acidified (37). A549 cells transiently expressing pEX-PK-hLC3 were treated with 25 μm SPD or SPM for 6 h. Treatment with either polyamine increased the number of yellow puncta, whereas co-treatment with AG decreased the number of yellow puncta to near baseline levels (Fig. 5A and Table S1). Rapamycin treatment increased the number of puncta regardless of AG co-treatment (Fig. 5A). Quantification of total co-fluorescing puncta shows a significant increase in total autophagosomes following SPD or SPM treatment in the presence of BSAO activity (Fig. 5B). Rapamycin treatment increased the total number of autophagosomes regardless of AG supplementation. Although rapamycin was used as a positive control, SPD treatment induced autophagosome formation significantly more than rapamycin. SPM induction is comparable with rapamycin induction. Inhibition of BSAO by AG yielded a 75% reduction in total autophagosomes formed after polyamine treatment, but the total number is still increased compared with baseline (Fig. 5B). In the presence of BSAO, both SPD and SPM are robust inducers of autophagosome formation. BSAO inhibition by AG prevents autophagosome formation following exogenous polyamine addition.
Figure 5.
Effects of AG supplementation on autophagosome formation following polyamine treatment. A549 cells transiently expressing the pEX-PL-hLC3 plasmid were treated with 25 μm SPD or SPM for 6 h. The cells were co-treated either with or without 1 mm AG. Treatment with 100 nm of rapamycin for 6 h was utilized as a positive control. A, following treatment, fluorescence was assessed by live-cell microscopy. The images are shown at 40× magnification. Scale bars, 10 μm. B, the total number of co-fluorescing puncta were quantified from 10× field photos of each treatment arm using Fiji SpotCounter. Lines represent the means of the three biological replicates. The error bars represent S.D. A table of all analysis of variance comparisons can be found in Table S1.
Discussion
Over the past decade, the role of polyamines, predominantly SPD, in aging and age-related disease has been widely studied (25, 29–32, 38–41). Although there is extensive valuable in vivo work documenting the potential for polyamine therapies in neurodegeneration and other age-related diseases, a common oversight in many studies is the addition of exogenous polyamines to mammalian cell culture systems containing ruminant serum. A component of bovine serum is BSAO, the enzyme responsible for the extracellular oxidation of higher-order polyamines. The toxic byproducts of this reaction result in cytotoxicity, as well as the activation of cellular stress responses. This inadvertent activation of stress responses can mimic or obscure biologically relevant responses.
The results of our cytotoxicity studies have recapitulated previous work showing the cytotoxicity of the polyamines in the presence of ruminant serum (16–19). Overall, both SPD and SPM were toxic to lung and colon adenocarcinoma cell lines when added in the presence of BSAO-containing serum. Low concentrations (micromolar range) of either polyamine is sufficient to induce cell death that is completely prevented with AG co-treatment. Treatment with SPM appears to be more toxic than SPD treatment (Fig. 2). SPM is toxic at lower concentrations, and its toxicity at high concentrations (millimolar) cannot be completely prevented by AG. Complete metabolism of SPM by BSAO (Fig. 1B) yields a dialdehyde that following spontaneous β-elimination yields two molecules of acrolein. By comparison, metabolism of SPD by BSAO yields an aldehyde that forms only one molecule of acrolein following b-elimination. This could, in part, explain the increased toxicity seen with SPM. Additionally, SMOX (Fig. 1A) is an intracellular enzyme that catabolizes SPM to SPD yielding H2O2 and the toxic aldehyde 3-aminopropanal. In response to SPM supplementation, cells up-regulate SMOX activity to maintain homeostasis (9). Consequently, the combined production of toxic products by the exogenous BSAO and the endogenous SMOX may exceed the cells' capacity for detoxification, thus leading to the greater toxicity observed with the higher concentrations of SPM that is not inhibited by AG.
Although SPD and SPM supplementation resulted in extreme toxicity to all tested cell lines, there is some variation in the sensitivity of each line to exogenous polyamine addition. HCT116 colon adenocarcinoma cells are more susceptible to SPD toxicity, showing more than a 50% reduction in viability with 75 μm of SPD treatment (Fig. 2). A549 lung adenocarcinoma cells show the same reduction with a slightly higher concentration of SPD. The toxicity of SPM is similar in A549 and HCT116 cells; however, AG is not able to prevent cell death in HCT116 cells exposed to more than 750 μm SPM (Fig. 2D). There are a few potential explanations for the difference in response to high concentrations of SPM, perhaps the most convincing being that different types of serum show varying BSAO activity levels. HCT116 cells are grown in fetal bovine serum, whereas A549 are grown in BCS, a supplemental serum with lower growth factor and enzymatic activity than fetal bovine serum (42).
Long-term (24–96 h) low-concentration polyamine supplementation was toxic to both A549 and HCT116 adenocarcinoma cells. As discussed above, SPM was toxic at lower concentrations than SPD, and HCT116 and A549 cells varied slightly in their susceptibility to polyamine supplementation toxicity. Notably, AG prevented polyamine-induced toxicity for up to 96 h, showing no additional toxicity from long-term AG culture (Fig. 3). The induction of autophagy has been linked with reciprocal interaction between the macroautophagy and p53 pathways (43, 44). HCT116 lung adenocarcinoma cells harboring a p53 null mutation behaved similarly to the HCT116 parental cells, suggesting that the toxicity of exogenous polyamine supplementation is p53-independent. Additionally, exogenous treatment with putrescine, a lower-order polyamine that is a poor substrate for BSAO, did not induce any cytotoxicity (Fig. S2). This supports the notion that cell death following exogenous SPD or SPM addition is attributable to BSAO activity.
Although treatment with low concentrations of either SPD or SPM induced LC3B-II expression and increased the LC3B-II:LC3B-I ratio, inhibition of BSAO by AG prevented the induction of LC3B-II protein levels (Fig. 4). This induction of LC3B is explained by the oxidative stress induced by oxidation of exogenous SPD and SPM. Treatment with either polyamine increased the total number of formed autophagosomes (Fig. 5). These results indicate that both SPD and SPM induce autophagosome formation in the presence of BSAO as well as or better than rapamycin, a known mTOR inhibitor and autophagy inducer. Treatment with AG significantly reduced the total number of autophagosomes following polyamine treatment. Although treatment with AG did not completely reduce autophagosome number to baseline levels, nearly all autophagosomes formed can be attributed to oxidative stress from BSAO oxidation of SPD or SPM. The remaining autophagosomes could be an indicator of low-level autophagy occurring independently of oxidative stress; however, it is likely that the AG concentration used did not fully inhibit BSAO activity. The results of this study once again confirm the cytotoxicity of exogenous polyamines in the presence of ruminant serum and indicate that autophagic responses to exogenous polyamines are a byproduct of BSAO oxidation.
Although the previously reported in vivo data suggesting that polyamines may have a role in longevity may be valid (25, 30), virtually all of the reported in vitro mechanistic studies are confounded by the addition of exogenous polyamines in the presence of BSAO. Our study confirms the long known, and consistently overlooked, toxic effects of exogenously added polyamines in the presence of serum-containing media and clarifies the relevance of BSAO as a confounder in autophagy studies in mammalian cell culture systems, underscoring the need for AG supplementation when studying exogenously added polyamines. It is critically important as in vitro mechanistic studies on the role, if any, of polyamines in autophagy progress that caution be taken to avoid the confounding epiphenomena of BSAO-induced cytotoxicity.
Experimental procedures
Cell lines and culture conditions
The lung adenocarcinoma line A549 was maintained in RPMI 1640 containing 10% bovine calf serum (Gemini Bio-Products, Sacramento, CA, USA) and penicillin/streptomycin at 5% CO2 and 37 °C. The colon adenocarcinoma line HCT116 was maintained in RPMI 1640 containing 10% fetal bovine serum (Gemini Bio-Products) and penicillin/streptomycin at 5% CO2 and 37 °C. p53 null HCT116 cells were kindly provided by Dr. Bert Vogelstein (Johns Hopkins University).
Cell viability assays
A549 or HCT116 cells were seeded in triplicate wells per condition of a 96-well plate and allowed to attach overnight. The cells were then treated with 100 μl of fresh medium containing the appropriate concentration of putrescine, SPD, SPM, and/or 1 mm AG. Following the indicated incubation times, cell viability was determined using the CellTiter-Blue cell viability assay (Promega). Fluorescence was measured using a SpectraMax M5 (Molecular Devices).
Western blotting analyses
Following treatment with SPD, SPM, or rapamycin (Sigma–Aldrich), the cells were lysed in 4% SDS containing protease inhibitors and passed through a homogenizer column (Zymo Research, Irvine, CA, USA). Protein was quantified using the Bio-Rad DC assay with interpolation based on a BSA standard curve. The reduced samples (50 μg/lane) were separated on 4–12% Bis-Tris BOLT gels (Invitrogen), transferred onto Immun-Blot polyvinylidene difluoride (Bio-Rad), and blocked in Odyssey blocking buffer (LI-COR, Lincoln, NE, USA) at room temperature for 2 h. The membranes were incubated with primary antibodies targeting LC3B (Cell Signaling Technology, Danvers, MA, USA) and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C. Species-specific, fluorophore-conjugated secondary antibodies were used for visualization and quantitation of bands using an Odyssey IR detection system and software (LI-COR).
LC3B transfection and live-cell fluorescence microscopy
For pEX-PK-hLC3 plasmid (Addgene, Cambridge, MA, USA) transfections, the cells were cultured in 12-well plates and transfected at 80% confluency. Transient transfections were completed with Lipofectamine 3000 (Invitrogen) according to the manufacturer's directions, and the cells were utilized 24 h after transfection. For fluorescence microscopy, A549 cells were transfected with pEX-PK-hLC3 as above, followed by 4 h of treatment with appropriate concentrations of rapamycin, SPD, SPM, and/or 1 mm AG. The cells were moved to live-cell imaging solution (Thermo Fisher Scientific), and fluorescence was visualized with an Axiovert 40 CFL (Zeiss, Oberkochen, Germany). Autophagic vesicles were quantified utilizing the SpotCounter plugin available through ImageJ Fiji (45) (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Total autophagic vesicles were quantified with SpotCounter and analyzed with a one-way analysis of variance. The false discovery rate associated with multiple comparisons was corrected for using the Benjamini–Hochberg adjustment. P value indications are as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001.
Data availability
All data are contained within this article.
Supplementary Material
This article contains supporting information.
Author contributions—C. E. H., M. D., T. M. S., and R. A. C. conceptualization; C. E. H., M. D., J. R. F., and T. M. S. data curation; C. E. H., J. R. F., and T. M. S. formal analysis; C. E. H., T. M. S., and R. A. C. validation; C. E. H., M. D., J. R. F., T. T. D., T. M. S., and R. A. C. investigation; C. E. H., M. D., J. R. F., T. T. D., T. M. S., and R. A. C. methodology; C. E. H., T. M. S., and R. A. C. writing-original draft; C. E. H., T. M. S., and R. A. C. project administration; C. E. H., M. D., J. R. F., T. T. D., T. M. S., and R. A. C. writing-review and editing; T. M. S. and R. A. C. supervision; R. A. C. resources; R. A. C. funding acquisition.
Funding and additional information—This work was funded by NCI, National Institutes of Health Grants R01CA204345 and R01CA235863 (to R. A. C.) and University of Pennsylvania Orphan Disease Center Million Dollar Bike Ride Grant MDBR-20-135-SRS (to R. A. C. and T. M. S.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- SPD
- spermidine
- BSAO
- bovine serum amine oxidase
- SPM
- spermine
- SSAT
- spermidine/spermine N1-acetyltransferase
- PAOX
- N1-acetylpolyamine oxidase
- SMOX
- spermine oxidase
- AG
- aminoguanidine.
References
- 1. Pegg A. E., and McCann P. P. (1982) Polyamine metabolism and function. Am. J. Physiol. 243, C212–C221 10.1152/ajpcell.1982.243.5.C212 [DOI] [PubMed] [Google Scholar]
- 2. Tabor C. W., and Tabor H. (1985) Polyamines in microorganisms. Microbiol. Rev. 49, 81–99 10.1128/MMBR.49.1.81-99.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pegg A. E. (2009) Mammalian polyamine metabolism and function. IUBMB Life 61, 880–894 10.1002/iub.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ha H. C., Sirisoma N. S., Kuppusamy P., Zweier J. L., Woster P. M., and Casero R. A. Jr. (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. U.S.A. 95, 11140–11145 10.1073/pnas.95.19.11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aikens D., Bunce S., Onasch F., Parker R. 3rd, Hurwitz C., and Clemans S. (1983) The interactions between nucleic acids and polyamines: II. Protonation constants and 13C-NMR chemical shift assignments of spermidine, spermine, and homologs. Biophys. Chem. 17, 67–74 10.1016/0301-4622(83)87015-X [DOI] [PubMed] [Google Scholar]
- 6. Pegg A. E. (1988) Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 48, 759–774 [PubMed] [Google Scholar]
- 7. Casero R. A. Jr., and Marton L. J. (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 6, 373–390 10.1038/nrd2243 [DOI] [PubMed] [Google Scholar]
- 8. Murray-Stewart T., Woster P. M., and Casero R. A. Jr. (2016) Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 473, 2937–2953 10.1042/BCJ20160383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casero R. A. Jr., and Pegg A. E. (2009) Polyamine catabolism and disease. Biochem. J. 421, 323–338 10.1042/BJ20090598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holst C. M., Nevesten P., Johansson F., Carlemalm E., and Oredsson S. M. (2008) Subcellular distribution of spermidine/spermine N1-acetyltransferase. Cell Biol. Int. 32, 39–47 10.1016/j.cellbi.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 11. Hölttä E. (1977) Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry 16, 91–100 10.1021/bi00620a015 [DOI] [PubMed] [Google Scholar]
- 12. Vujcic S., Liang P., Diegelman P., Kramer D. L., and Porter C. W. (2003) Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem. J. 370, 19–28 10.1042/BJ20021779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y., Devereux W., Woster P. M., Stewart T. M., Hacker A., and Casero R. A. Jr. (2001) Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 61, 5370–5373 [PubMed] [Google Scholar]
- 14. Averill-Bates D. A., Agostinelli E., Przybytkowski E., Mateescu M. A., and Mondovi B. (1993) Cytotoxicity and kinetic analysis of purified bovine serum amine oxidase in the presence of spermine in Chinese hamster ovary cells. Arch. Biochem. Biophys. 300, 75–79 10.1006/abbi.1993.1011 [DOI] [PubMed] [Google Scholar]
- 15. Gstraunthaler G., Lindl T., and van der Valk J. (2013) A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 65, 791–793 10.1007/s10616-013-9633-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch J. G. (1953) Spermine oxidase: an amine oxidase with specificity for spermine and spermidine. J. Exp. Med. 97, 345–355 10.1084/jem.97.3.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tabor C. W., Tabor H., and Rosenthal S. M. (1954) Purification of amine oxidase from beef plasma. J. Biol. Chem. 208, 645–661 [PubMed] [Google Scholar]
- 18. Blaschko H., and Hawes R. (1959) Observations on spermine oxidase of mammalian plasma. J. Physiol. 145, 124–131 10.1113/jphysiol.1959.sp006132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tabor C. W., Tabor H., and Bachrach U. (1964) Identification of the aminoaldehydes produced by the oxidation of spermine and spermidine with purified plasma and amine oxidase. J. Biol. Chem. 239, 2194–2203 [PubMed] [Google Scholar]
- 20. Gahl W. A., and Pitot H. C. (1982) Polyamine degradation in foetal and adult bovine serum. Biochem. J. 202, 603–611 10.1042/bj2020603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kimes B. W., and Morris D. R. (1971) Preparation and stability of oxidized polyamines. Biochim. Biophys. Acta 228, 223–234 10.1016/0005-2787(71)90562-4 [DOI] [PubMed] [Google Scholar]
- 22. Zeller E. A. (1975) Diamine oxidases. In The Enzymes, 3rd Ed., pp. 313–335, Academic Press, London [Google Scholar]
- 23. Gahl W. A., and Pitot H. C. (1978) Reversal by aminoguanidine of the inhibition of proliferation of human fibroblasts by spermidine and spermine. Chem. Biol. Interact. 22, 91–98 10.1016/0009-2797(78)90152-7 [DOI] [PubMed] [Google Scholar]
- 24. Parchment R. E., Lewellyn A., Swartzendruber D., and Pierce G. B. (1990) Serum amine oxidase activity contributes to crisis in mouse embryo cell lines. Proc. Natl. Acad. Sci. U.S.A. 87, 4340–4344 10.1073/pnas.87.11.4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eisenberg T., Knauer H., Schauer A., Büttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., Fussi H., Deszcz L., Hartl R., Schraml E., Criollo A., et al. (2009) Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 10.1038/ncb1975 [DOI] [PubMed] [Google Scholar]
- 26. Lemasters J. J., Nieminen A. L., Qian T., Trost L. C., Elmore S. P., Nishimura Y., Crowe R. A., Cascio W. E., Bradham C. A., Brenner D. A., and Herman B. (1998) The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366, 177–196 10.1016/s0005-2728(98)00112-1 [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez-Enriquez S., He L., and Lemasters J. (2004) Role of mitochondrial permeability transition pores in mitochondrial autophagy. Int. J. Biochem. Cell Biol. 36, 2463–2472 10.1016/j.biocel.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 28. Brunk U. T., Dalen H., Roberg K., and Hellquist H. B. (1997) Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 23, 616–626 10.1016/s0891-5849(97)00007-5 [DOI] [PubMed] [Google Scholar]
- 29. Morselli E., Mariño G., Bennetzen M. V., Eisenberg T., Megalou E., Schroeder S., Cabrera S., Bénit P., Rustin P., Criollo A., Kepp O., Galluzzi L., Shen S., Malik S. A., Maiuri M. C., Horio Y., et al. (2011) Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 192, 615–629 10.1083/jcb.201008167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bennetzen M. V., Mariño G., Pultz D., Morselli E., Faergeman N. J., Kroemer G., and Andersen J. S. (2012) Phosphoproteomic analysis of cells treated with longevity-related autophagy inducers. Cell Cycle 11, 1827–1840 10.4161/cc.20233 [DOI] [PubMed] [Google Scholar]
- 31. Chae Y., and Kim M. (2014) Activation of p53 by spermine mediates induction of autophagy in HT1080 cells. Int. J. Biol. Macromol. 63, 56–63 10.1016/j.ijbiomac.2013.10.041 [DOI] [PubMed] [Google Scholar]
- 32. Zhang H., Alsaleh G., Feltham J., Sun Y., Napolitano G., Riffelmacher T., Charles P., Frau L., Hublitz P., Yu Z., Mohammed S., Ballabio A., Balabanov S., Mellor J., and Simon A. K. (2019) Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell 76, 110–125.e9 10.1016/j.molcel.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aoki H., Kondo Y., Aldape K., Yamamoto A., Iwado E., Yokoyama T., Hollingsworth E. F., Kobayashi R., Hess K., Shinojima N., Shingu T., Tamada Y., Zhang L., Conrad C., Bögler O., et al. (2008) Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy 4, 467–475 10.4161/auto.5668 [DOI] [PubMed] [Google Scholar]
- 34. Wu J., Dang Y., Su W., Liu C., Ma H., Shan Y., Pei Y., Wan B., Guo J., and Yu L. (2006) Molecular cloning and characterization of rat LC3A and LC3B: two novel markers of autophagosome. Biochem. Biophys. Res. Commun. 339, 437–442 10.1016/j.bbrc.2005.10.211 [DOI] [PubMed] [Google Scholar]
- 35. Noda T., and Ohsumi Y. (1998) Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273, 3963–3966 10.1074/jbc.273.7.3963 [DOI] [PubMed] [Google Scholar]
- 36. Bains M., and Heidenreich K. A. (2009) Live-cell imaging of autophagy induction and autophagosome-lysosome fusion in primary cultured neurons. Methods Enzymol. 453, 145–158 10.1016/S0076-6879(08)04007-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanida I., Ueno T., and Uchiyama Y. (2014) A super-ecliptic, pHluorin-mKate2, tandem fluorescent protein-tagged human LC3 for the monitoring of mammalian autophagy. PLoS One 9, e110600 10.1371/journal.pone.0110600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wirth M., Benson G., Schwarz C., Köbe T., Grittner U., Schmitz D., Sigrist S. J., Bohlken J., Stekovic S., Madeo F., and Flörel A. (2018) The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 109, 181–188 10.1016/j.cortex.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 39. Saiki S., Sasazawa Y., Fujimaki M., Kamagata K., Kaga N., Taka H., Li Y., Souma S., Hatano T., Imamichi Y., Furuya N., Mori A., Oji Y., Ueno S. I., Nojiri S., et al. (2019) A metabolic profile of polyamines in Parkinson disease: A promising biomarker. Ann. Neurol. 86, 251–263 10.1002/ana.25516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Limon A., Delbruck E., Yassine A., Pandya D., Myers R. M., Barchas J. D., Lee F., Schatzberg F. L., Watson S. J., Akil H., Bunney W. E., Vawter M. P., and Sequeira A. (2019) Electrophysiological evaluation of extracellular spermine and alkaline pH on synaptic human GABAA receptors. Transl Psychiatry 9, 218 10.1038/s41398-019-0551-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu T.-T., Li H., Dai Z., Lau G. K., Li B.-Y., Zhu W.-L., Liu X.-Q., Liu H.-F., Cai W.-W., Huang S.-Q., Wang Q., and Zhang S.-J. (2020) Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging (Albany, NY) 12, 6401–6414 10.18632/aging.103035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang C., Wu C., Fang C.-L., Chen W., and Chen C. (2017) Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines. PLoS One 12, e0178960 10.1371/journal.pone.0178960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tasdemir E., Maiuri M. C., Galluzzi L., Vitale I., Djavaheri-Mergny M., D'Amelio M., Criollo A., Morselli E., Zhu C., Harper F., Nannmark U., Samara C., Pinton P., Vicencio J. M., Carnuccio R., et al. (2008) Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676–687 10.1038/ncb1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. White E. (2016) Autophagy and p53. Cold Spring Harb. Perspect. Med. 6, a026120 10.1101/cshperspect.a026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scindellin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within this article.