Abstract
Aims
Several large studies have documented the outcome of transvenous lead extraction (TLE), focusing on laser and mechanical methods. To date there has been no large series addressing the results obtained with rotational lead extraction tools. This retrospective multicentre study was designed to investigate the outcomes of mechanical and rotational techniques.
Methods and results
Data were collected on a total of 2205 patients (age 66.0 ± 15.7 years) with 3849 leads targeted for extraction in six European lead extraction centres. The commonest indication was infection (46%). The targeted leads included 2879 pacemaker leads (74.8%), 949 implantable cardioverter-defibrillator leads (24.6%), and 21 leads for which details were unknown; 46.6% of leads were passive fixation leads. The median lead dwell time was 74 months [interquartile range (IQR) 41–112]. Clinical success was obtained in 97.0% of procedures, and complete extraction was achieved for 96.5% of leads. Major complications occurred in 22/2205 procedures (1%), with a peri-operative or procedure-related mortality rate of 4/2205 (0.18%). Minor complications occurred in 3.1% of procedures. A total of 1552 leads (in 992 patients) with a median dwell time of 106 months (IQR 66–145) were extracted using the Evolution rotational TLE tool. In this subgroup, complete success was obtained for 95.2% of leads with a procedural mortality rate of 0.4%.
Conclusion
Patient outcomes in the PROMET study compare favourably with other large TLE trials, underlining the capability of rotational TLE tools and techniques to match laser methods in efficacy and surpass them in safety.
Keywords: Rotational lead extraction, Evolution extraction sheath, Laser lead extraction, Complication, Technical success
What’s new?
Largest series of data published on clinical outcomes of a rotational lead extraction tool.
The Evolution rotational extraction sheath shows a good safety and efficacy performance, especially in long implanted leads.
The risk for superior vena cava injury when using the Evolution rotational extraction tool is very low.
Introduction
Transvenous lead extraction (TLE) plays an essential role in the long-term management of patients with cardiac implanted electronic devices. Fibrosis and calcification along implanted leads becomes increasingly likely with longer dwell times and simple traction is often not enough to achieve complete removal. Mechanical sheaths were the first widely used tool but were only moderately effective.1 Powered sheaths, utilizing either laser or radiofrequency energy to break down areas of fibrosis, were the next development. Of the two, laser powered sheaths gained the most traction and several large studies have shown them to achieve very high clinical success rates.2,3 Concerns have been raised about the risk of major complications when using laser sheaths.1,2,4
Rotational dissecting sheaths, characterized by a handle trigger-driven rotational dissecting tip at the end of a flexible sheath, have been available for this application for just over a decade.5 Rotational tools have been associated with greater efficacy than other non-laser methods in extracting all components of the targeted leads.6 Safety data from small case series have been promising,6 but data from larger series, such as that available for laser methods,3,7 have been lacking. The range of rotational tools has continued to expand, as has the range of accessories for use in association with them to enhance safety and efficacy.6,8–11
To date, there is a lack of large volume data on advanced mechanical lead extraction techniques and tools. The PROMET (Patient-Related Outcomes of Mechanical lead Extraction Techniques) study was designed to address this by collecting data from consecutive patients treated in high-volume extraction centres in which rotational tools were the preferred method for TLE in cases not amenable to simple traction.
Methods
Patient population
Patient data were collected from six European lead extraction centres that had maintained a comprehensive record of lead extraction procedures. Five of the six centres fulfilled the criteria of a high-volume extraction centre (>30 procedures/year). Depending on the availability of sufficient data sets in different study centres and based on the retrospective design of this study, the starting point of data collection differed between the centres from as early as January 2005. The local review board at all investigational sites approved the research protocol. The results obtained were analysed retrospectively with regard to efficacy and safety.
Definitions
Success and failure were defined according to the definitions of the 2017 Heart Rhythm Society and the 2018 European Heart Rhythm Association expert consensus.12 In brief, complete success for the extraction of an individual lead was defined as the removal of all components of that lead from the vascular space without the occurrence of a fatal or permanently disabling complication. A procedure was considered a clinical success if it attained the intended clinical outcome and did not involve the retention of any lead portion >4 cm in length.
Complications were adjudicated by a committee including representatives from each contributing centre. A complication was considered to have occurred if an undesired consequence of the extraction procedure suffering or disability, prolongation of the hospital admission, or a need for additional intervention or pharmacological therapy. Based on definitions in prior expert consensus papers,12–14 a complication was classed as serious if it led to the death of the patient or to persistent disability or if it required a substantial intervention such as cardiac surgery, pericardiocentesis, or vascular surgery. Fatalities were judged to be related to the extraction procedure if they occurred on the day of the procedure or as a consequence of a complication of the procedure.
Statistics
Categorical variables are presented as numbers and percentages. Continuous variables are presented as mean ± standard deviation or as median and interquartile range (IQR), as appropriate. Differences between groups were analysed by two-sample t-test. A P-value of <0.05 was considered significant.
Results
Patient and lead characteristics
Data were obtained for 2205 patients (69% male, age 66.0 ± 15.7 years; Table 1) with 3849 targeted leads for TLE. The mean dwell time of the targeted leads averaged 84.8 ± 61.9 months, with a median of 74 months (IQR 41–112). Seventy-five percent of the targeted leads were pacemaker leads, 25% implantable cardioverter-defibrillator leads.
Table 1.
Patient and lead characteristics
| Patient characteristics | |
|---|---|
| Patient number | 2205 |
| Age | 66.0 ± 15.7 years |
| Gender | |
| Male | 69% |
| Female | 30% |
| Unknown | 1% |
| Left ventricular ejection fraction | 38.3 ± 16.1% |
| NYHA class (n = 187) | |
| Class 1 | 18.7% |
| Class 2 | 25.1% |
| Class 3 | 40.6% |
| Class 4 | 15.5% |
| Diabetes mellitus (n = 419) | 22.7% |
| Chronic renal disease (n = 393) | 57.3% |
| Cardiomyopathy (n = 542) | |
| DCM | 40.8% |
| ICM | 59.2% |
| Previous cardiac surgery (n = 601) | 27.8% |
| Number of targeted leads | 3849 |
| 74.8% pacemaker leads | |
| 24.6% ICD leads | |
| 0.6% information not available | |
| Implant duration | |
| Mean implant duration | 84.7 ± 61.8 months |
| Median implant duration | 74 months (IQR 41–112) |
| Localisation of leads | |
| Right atrium | 1167 (30.3%) |
| Right ventricle | 2238 (58.1%) |
| Coronary sinus tributary | 395 (10.3%) |
| SVC | 8 (0.2%) |
| Fragment or unknown | 41 (1.1%) |
| Fixation mechanism | |
| Active fixation | 1560 (40.6%) |
| Passive fixation | 1795 (46.6%) |
| Information not available | 494 (12.8%) |
DCM, dilated cardiomyopathy; ICD, implantable cardioverter-defibrillator; ICM, ischaemic cardiomyopathy; IQR, interquartile range; NYHA, New York Heart Association; SVC, superior vena cava.
The most common indication for TLE was infection, present in 46%, but declined non-significantly over the course of the study, representing the primary indication in 47.0% in the first half of each centre’s experience compared to 44.0% in the second half.
Techniques and equipment
In all study centres a superior, subclavian approach was chosen as the primary option for lead extraction procedures. Most procedures (68.4%) were performed by cardiac surgeons, working in all cases in either a hybrid theatre (95.6%) or a standard operating theatre (4.4%). The remainder were performed by cardiologists/electrophysiologists, generally working in an electrophysiology laboratory (99.2%). All procedures not performed by a cardiac surgeon were performed with cardiac surgical stand-by including the availability of extracorporeal circulation and a perfusionist.
For leads that could not be extracted by simple traction, a multi-step lead extraction approach (individual to each study centre) was performed. A locking stylet (Liberator, Cook Medical, USA) was generally used as the first of these additional steps, making it the most commonly used item of specialized extraction equipment (Figure 1). For 940 leads with a median dwell time of 71 months (IQR 48–93), the locking stylet alone allowed enough additional traction to remove the lead. In other cases, this traction permitted the use of the lead as a rail to guide an extraction sheath.
Figure 1.
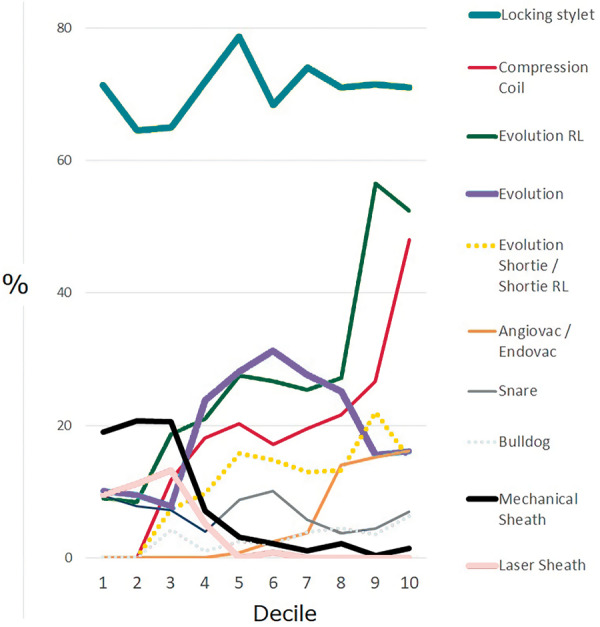
Temporal trends in the equipment used in the PROMET cohort. Laser equipment was almost abandoned by all centres within the first half of the experience. There was increasing uptake of rotational dissection sheaths instead of traction-only or laser methods, with a high and constant background usage of locking stylets.
Dissection sheaths included simple polypropylene models (Byrd Dilator Sheath, Cook Medical, USA), PTFE sheaths (Cook Medical, USA), laser sheaths (SLS II, Philips, USA), and rotational tools (Evolution and Evolution RL, Cook Medical, USA; Tightrail, Philips, USA). Laser sheaths were used for 139 leads (3.6%) with a median dwell time of 57 months (IQR 38–81). Their use was confined to the first half of the case series and they were used in only three centres, accounting for 21% of leads extracted in one centre, fewer than 1% in the other two.
Where a subclavian approach failed or was expected to be impossible, a femoral or an internal jugular approach was performed. In these cases, a variety of snares were used, predominantly the Needle’s Eye (Cook Medical, USA) which accounted for 70% of all snare usage.
Safety
Major complications occurred in 22 cases (1.0%; Table 3, Figure 2), and minor complications in 3.1%. Procedure-related mortality occurred in four patients (0.18%), three of whom had systemic infection as the indication for extraction.
Table 3.
Major complications
| Patient details | Device and lead number | Indication | Dwell time of oldest lead (months) | Equipment used | Complication and treatment | Outcomes |
|---|---|---|---|---|---|---|
| 80 years, male | Dual chamber pacemaker | Infection | 124 | Locking stylet, simple sheath | Cardiac avulsion | Full recovery after surgical repair |
| 83 years, female | Single chamber pacemaker | Infection | 151 | Locking stylet, simple sheath | Pericardial effusion, percutaneous drainage | Full recovery |
| 37 years, female | Dual chamber pacemaker with abandoned leads (4 leads total) | Infection, local | 228 | Locking stylets, femoral snare (needle eye) | Pericardial effusion post-procedure, percutaneous drainage | Full recovery |
| 77 years, male | Dual chamber pacemaker | Redundant lead | 178 | Locking stylet, Evolution (13F), femoral snare (needle eye) | Cardiac avulsion during use of femoral snare | Surgical repair, but died within 30 days |
| 65 years, female | CRT-D | Lead failure | 141 | Locking stylet, simple sheath, Evolution | Right ventricular injury | Full recovery after surgical repair |
| 46 years, male | Dual chamber pacemaker | Systemic infection | 47 | Locking stylet | Pericardial tamponade | Full recovery after surgical repair |
| 83 years, female | Dual chamber pacemaker with abandoned leads (total 4 leads) | Infection | >120 | Evolution, femoral snare | Pericardial effusion | Uncomplicated recovery after percutaneous drainage |
| 88 years, female | Dual chamber pacemaker (2 leads) | Perforated lead, lying in pleural space | 2 | Locking stylet | Oesophageal injury from transoesophageal echo probe | Recovered after long hospital stay |
| 80 years, female | Dual chamber pacemaker (2 leads) | Infection | 285 | Locking stylet, compression coil, Evolution RL (11F) | Peri-procedural stroke | Recovered |
| 43 years, male | Dual chamber ICD with abandoned leads (3 leads) | Infection | 66 | Evolution | Pericardial tamponade | Full recovery after surgical repair |
| 47 years, female | Single chamber ICD | Lead malfunction | 59 | Evolution RL | Right pleural collection of blood drained percutaneously at 1 day after extracting right sided device | Full recovery. No surgery, no transfusion required |
| 78 years, female | Dual chamber PPM | Local infection | Unknown | Locking stylet, compression coil, Evolution RL (11F) | Injury to right ventricle | Full recovery after surgical repair |
| 75 years, female | CRT-P (including Starfix lead) | Lead malfunction | 15 | Locking stylet, compression coil, Evolution RL (9F) | Injury to coronary sinus | Full recovery after surgical repair |
| 63 years, male | CRT-D | Lead malfunction | 83 | Locking stylet, compression coil, Bulldog, Evolution RL (13F) | Injury to inferior vena cava causing pericardial tamponade | Full recovery after surgical repair |
| 74 years, male | Dual chamber PPM, abandoned leads (total 4 leads) | Venous occlusion, redundant leads, need for upgrade | 356 | Locking stylets, Evolution 9F | Pericardial tamponade due to RV perforation | Full recovery after surgical repair |
| 78 years, female | Dual chamber pacemaker | Local infection | Unknown | Liberator, compression coil, Evolution 9F | Tamponade due to RV perforation | Full recovery after surgical repair |
| 79 years, male | CRT-D with redundant leads (total 7 leads) | Septicaemia | 79 | Liberator, compression coil, Evolution RL 13F | Septic shock | Peri-procedural death |
| 37 years, male | Dual chamber ICD | Lead malfunction | 83 | Simple stylet | Pericardial tamponade | Full recovery after surgical repair |
| 83 years, female | CRT-D | Local infection | 126 | Liberator, compression coil, Evolution RL 13F | Pericardial tamponade | Initial recovery after surgical repair, but died at 30 days |
| 65 years, male | CRT-D | Systemic infection | 105 | Liberator, compression coil, Evolution RL 13F | Left-sided haemothorax | Full recovery after surgical placement of a chest drain |
| 61 years, female | Single chamber pacemaker with redundant leads (3 leads in total) | Septicaemia | 208 | Liberator, compression coil, Evolution RL 13F | Injury to junction of SVC and RA leading to pericardial tamponade | Died at 2 days post-procedure despite surgical repair |
| 48 years, male | Single chamber ICD | Systemic infection | 4 | Liberator, compression coil, Evolution RL 13F | Damage to tricuspid valve requiring subsequent valve surgery | Full recovery after surgical repair |
CRT-D, cardiac resynchronization therapy with defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter-defibrillator; PPM, permanent pacemaker; RA, right atrium; RV, right ventricle.
Figure 2.
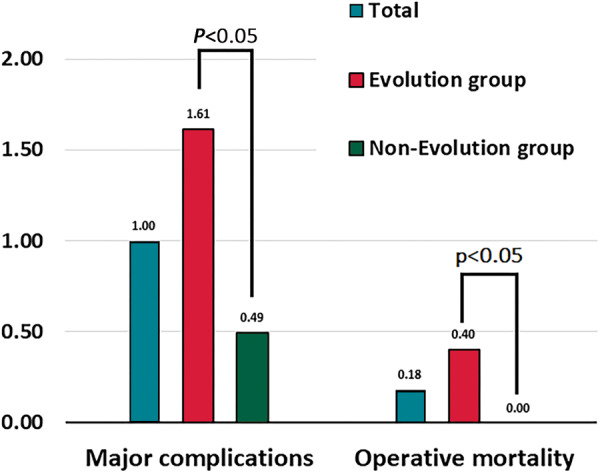
Safety endpoints of the PROMET study in the overall cohort and in the subset in whom rotational dissection sheaths were used.
Efficacy and efficiency
Among the entire study group (2205 patients/3849 leads), complete technical success was obtained in the extraction of 96.5% of leads, with a clinical success rate of 97.0% of procedures.
Rotational dissection tools
Subgroup analysis was performed for the use of the Evolution or the Evolution RL rotational TLE devices, which were used for 1552 leads in 992 patients. Other devices of the Evolution group including the Evolution Shortie, the Evolution Shortie RL, and the Evolution outer sheath were not included unless used in association with the full-length Evolution or Evolution RL. The Tightrail rotational extraction sheath was not included as the design of this tool is substantially different, and the device was used for only 12 leads.
Leads removed using the Evolution rotational tools had a longer dwell time than others (Table 2, Figure 3). Despite the implant duration of the leads extracted in the Evolution group, complete procedural success was only 2.1% lower than in the non-Evolution group (95.2% vs. 97.3%).
Table 2.
Subgroup analysis of cases with the use of the Evolution rotational sheath vs. cases without Evolution
| Evolution-group (992 patients/1552 leads) | Non-Evolution group (1213 patients/2297 leads) | P-value | |
|---|---|---|---|
| Median implant duration | 106 months (IQR 66–145) | 58 months (IQR 28–90) | <0.001 |
| Complete procedural success (per lead) | 95.2% | 97.3% | 0.003 |
| Major complications | 1.6% | 0.5% | <0.01 |
| Procedure-related mortality | 0.4% | 0% | <0.05 |
IQR, interquartile range.
Figure 3.
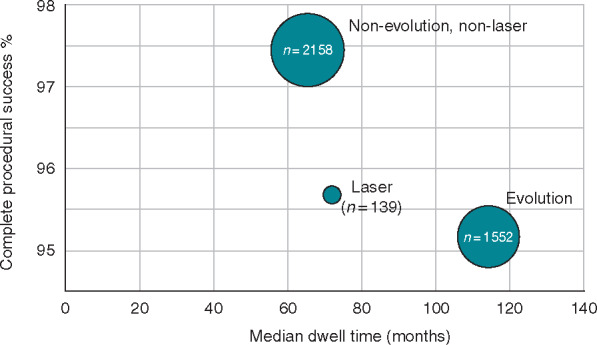
Complete procedural success in per lead extracted, according to the method used to extract the lead.
The major complications encountered in the Evolution group predominantly consisted of right ventricular injuries (Table 3). One case of injury to the right atrium and adjacent intrapericardial superior vena cava (SVC) occurred, and one late haemothorax requiring percutaneous drainage (Table 3) but no acute injuries to the extrapericardial part of the SVC were encountered.
Discussion
The PROMET study is the largest study to report on efficacy and safety outcomes in patients undergoing mechanical TLE and is comparable in size to the largest series of laser extraction (Table 4). The key findings are:
Table 4.
Comparison of the results of the PROMET study with other published large volume studies
| Patients/leads | Indications | Leads | Implant duration (months) | Success rates | Major complications | In-hospital mortality | |
|---|---|---|---|---|---|---|---|
| PROMET study | 2205/3849 | 46.0% infection | 74.8% pacemaker leads | Mean 84.7 ± 61.8 | 96.5% CPS | 1% | 1.7% (30-day mortality) |
| 54.0% non-infectious | 24.6% ICD leads | Median 74.0 | 97.0% | ||||
| 0.6% unknown | IQR (41.0–112.0) | CS | |||||
| LEXICON study | 1449/2405 | 56.9% | 70.0% pacemaker leads | Median 82.1 | 96.5% CPS | 1.4% | 1.86% |
| Infection | 29.2% ICD leads | IQR (0.4–356.8) | 97.7% | ||||
| 43.1% | 0.7% unknown | CS | |||||
| Non-infectious | |||||||
| ELECTRa study | 3510/4917 | 52.8% infection | 75.7% pacemaker leads | Mean 76.8 ± 64.8 | 95.7% CPS | 1.7% | 1.4% |
| 47.3% non-infectious | 24.3% ICD leads | Median 60.0 | 96.7% | ||||
| IQR (24.0–108.0) | CS |
CPS, complete procedural success; CS, clinical success; ICD, implantable cardioverter-defibrillator; IQR, interquartile range.
Mechanical tools can deliver a level of efficacy that is equivalent to that of laser powered sheaths.
The use of mechanical tools is associated with a low procedural risk for major and minor complications. The nature of major complications differs from that reported in any of the large series describing laser-based TLE methods, particularly the lower incidence of procedural injuries to the SVC.
Safety
The list of adverse events encountered in the PROMET cohort is remarkable for the low incidence of acute injuries to the SVC, which, in contrast, account for most of the fatal complications of laser TLE.
The low incidence of injury to the SVC demonstrated in the PROMET study constitutes a major advance in TLE. Even in the expert hands represented by the LEXICON and ELECTRa investigators, vascular tears/avulsion occurred in 6/1449 and 20/3510 cases, respectively. In real-world practice, this complication has clinical relevance, demonstrated by two published series of SVC events in the context of the use of compliant endovascular occlusion balloon: the initial series reported 35 surgically confirmed cases of laser-related SVC disruption in the USA during a 6-month period and the follow-up series reported 116 SVC events in a 25-month period.15,16 Even the lower of these estimates implies an average of more than 4 SVC events per month.
The reported safety results of the PROMET study are in line with the just recently published data of Diaz et al.,4 who compared the observed mortality between laser and rotational extraction sheaths based on the Manufacturer and User Facility Device Experience database. At different market share estimates and with adjustment for potential underreporting in cases with rotational sheaths the relative risk for mortality was significantly higher in laser sheath extractions than in procedures with the use of a rotational extraction sheath.
Efficacy
The clinical success rate and complete procedural success rates for leads targeted for removal in the PROMET series compare favourably with other large patient cohorts in TLE procedures such as the Lexicon study3 and the ELECTRa registry.17 The efficacy of the Evolution rotational TLE device was proven by the achievement of complete procedural success in more than 95% of leads with median implant durations of 106 months, substantially longer than in previous large TLE studies.3,17
Limitations
The major limitation of this study is its retrospective study design. The acquired data represents real-world data in a number of centres in different nations over a period of just over a decade. Due to the retrospective design and the individual data recording in the different participating centres, data recording may not have been complete in all cases. Despite this fact, the authors believe that this data is of relevant clinical importance for the lead extraction community and supports the hypothesis of a satisfactory safety profile and high efficacy of rotational extraction sheaths, as seen in previous smaller series.6,8,10,11,18
Conclusions
This large series of cases show that an extraction service based on rotational dissecting tools can deliver a procedural mortality rate of <0.2%, and that a service based on these products can deliver complete lead extraction in over 96% of leads targeted. In this series, rotational TLE tools showed a low incidence of major complications, especially of SVC injuries. The efficacy of rotational TLE tools was proven by the achievement of high success rates in leads with long implant durations.
Funding
This study was supported by a research grant of Cook Medical (USA).
Conflict of interest: C.T.S. has received consulting fees and travel expenses from Medtronic; consulting fees and research support from Biotronik; research support from Abbott; workshop fees, consulting fees, educational grants, and research support from Cook Medical; consulting fees from Spectranetics/Philipps; consulting fees from Angiodynamics. E.G. has received workshop fees and consulting fees from Cook Medical. O.A.R. has received workshop fees from Cook Medical. P.M. has received consulting fees from Boston Scientific, Abbott and Cook Medical. A.B. has received consulting fees from Abbott, Bayer Health Care, Biotronik, BMS/Pfizer, Boston Scientific, Daiichy Sankio, and Medtronic. Educational grants from Biosense Webster, Biotronik, and Actelion. Presenter fees from Abbott, Bayer Health Care, Biotronik, BMS/Pfizer, Boston Scientific, Daiichy Sankio, Medtronic, and Spectranetics/Philipps. J.S. has received consultant and/or speaker fees from Abbott, Amgen, Astra-Zeneca, Atricure, Bayer, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis, WebMD, and Zoll. He reports ownership of CorXL. J.S. has received grant support through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic. M.G. has received research funding from Medtronic and Attune medical and has acted as a consultant and paid speaker for Medtronic, Biosense Webster and Cook Medical. P.P.D. has received consultant and/or speaker fees from Abbott, Biotronik, Boston Scientific, Medtronic, EBR, MicroPort, and Cook Mediccal. All other authors declare no conflict of interest.
References
- 1. Diemberger I, Mazzotti A, Giulia MB, Cristian M, Matteo M, Letizia ZM. et al. From lead management to implanted patient management: systematic review and meta-analysis of the last 15 years of experience in lead extraction. Expert Rev Med Devices 2013;10:551–73. [DOI] [PubMed] [Google Scholar]
- 2. Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R. et al. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial1. J Am Coll Cardiol 1999;33:1671–6. [DOI] [PubMed] [Google Scholar]
- 3. Wazni O, Epstein LM, Carrillo RG, Love C, Adler SW, Riggio DW. et al. Lead extraction in the contemporary setting: the LExICon study. J Am Coll Cardiol 2010;55:579–86. [DOI] [PubMed] [Google Scholar]
- 4. Diaz CL, Guo X, Whitman IR, Marcus GM, Pellegrini CN, Beygui RE. et al. Reported mortality with rotating sheaths vs. laser sheaths for transvenous lead extraction. Europace 2019;341:1882. [DOI] [PubMed] [Google Scholar]
- 5. Hussein AA, Wilkoff BL, Martin DO, Karim S, Kanj M, Callahan T. et al. Initial experience with the Evolution mechanical dilator sheath for lead extraction: safety and efficacy. Heart Rhythm 2010;7:870–3. [DOI] [PubMed] [Google Scholar]
- 6. Domenichini G, Gonna H, Sharma R, Conti S, Fiorista L, Jones S. et al. Non-laser percutaneous extraction of pacemaker and defibrillation leads: a decade of progress. Europace 2017;19:1521–6. [DOI] [PubMed] [Google Scholar]
- 7. Yoshitake T, Goya M, Sasaki T, Shiohira S, Sekigawa M, Shirai Y. et al. Safety and efficacy of transvenous lead extraction with a high-frequency excimer laser—a single center experience. Circ J 2018;82:2992–7. [DOI] [PubMed] [Google Scholar]
- 8. Starck CT, Steffel J, Caliskan E, Holubec T, Schoenrath F, Maisano F. et al. Clinical performance of a new bidirectional rotational mechanical lead extraction sheath. Europace 2016;18:253–6. [DOI] [PubMed] [Google Scholar]
- 9. Starck CT, Stepuk A, Holubec T, Steffel J, Stark JW, Falk V.. Compression coil provides increased lead control in extraction procedures. Europace 2015;17:499–503. [DOI] [PubMed] [Google Scholar]
- 10. Sharma S, Ekeruo IA, Nand NP, Sundara Raman A, Zhang X, Reddy SK. et al. Safety and efficacy of transvenous lead extraction utilizing the evolution mechanical lead extraction system: a single-center experience. JACC Clin Electrophysiol 2018;4:212–20. [DOI] [PubMed] [Google Scholar]
- 11. Mazzone P, Migliore F, Bertaglia E, Facchin D, Daleffe E, Calzolari V. et al. Safety and efficacy of the new bidirectional rotational Evolution(R) mechanical lead extraction sheath: results from a multicentre Italian registry. Europace 2018;20:829–34. [DOI] [PubMed] [Google Scholar]
- 12. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R. et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–51. [DOI] [PubMed] [Google Scholar]
- 13. Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH. et al. Transvenous lead extraction: heart rhythm society expert consensus on facilities, training, indications, and patient management. Heart Rhythm 2009;6:1085–104. [DOI] [PubMed] [Google Scholar]
- 14. Bongiorni MG, Burri H, Deharo JC, Starck C, Kennergren C, Saghy L. et al. ; ESC Scientific Document Group. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace 2018;20:1217. [DOI] [PubMed] [Google Scholar]
- 15. Azarrafiy R, Tsang DC, Boyle TA, Wilkoff BL, Carrillo RG.. Compliant endovascular balloon reduces the lethality of superior vena cava tears during transvenous lead extractions. Heart Rhythm 2017;14:1400–4. [DOI] [PubMed] [Google Scholar]
- 16. Azarrafiy R, Tsang DC, Wilkoff BL, Carrillo RG.. Endovascular occlusion balloon for treatment of superior vena cava tears during transvenous lead extraction. Circ Arrhythm Electrophysiol 2019;12:e007266. [DOI] [PubMed] [Google Scholar]
- 17. Bongiorni MG, Kennergren C, Butter C, Deharo J-C, Kutarski A, Rinaldi CA. et al. ; ELECTRa Investigators. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J 2017;38:2995–3005. [DOI] [PubMed] [Google Scholar]
- 18. Delnoy PPHM, Witte OA, Adiyaman A, Ghani A, Smit JJJ, Ramdat Misier AR. et al. Lead extractions: the Zwolle experience with the Evolution mechanical sheath. Europace 2016;18:762–6. [DOI] [PubMed] [Google Scholar]


