Abstract
Objective
To measure the associations between newly initiated palliative care in the last six months of life, healthcare use, and location of death in adults dying from non-cancer illness, and to compare these associations with those in adults who die from cancer at a population level.
Design
Population based matched cohort study.
Setting
Ontario, Canada between 2010 and 2015.
Participants
113 540 adults dying from cancer and non-cancer illness who were given newly initiated physician delivered palliative care in the last six months of life administered across all healthcare settings. Linked health administrative data were used to directly match patients on cause of death, hospital frailty risk score, presence of metastatic cancer, residential location (according to 1 of 14 local health integration networks that organise all healthcare services in Ontario), and a propensity score to receive palliative care that was derived by using age and sex.
Main outcome measures
Rates of emergency department visits, admissions to hospital, and admissions to the intensive care unit, and odds of death at home versus in hospital after first palliative care visit, adjusted for patient characteristics (such as age, sex, and comorbidities).
Results
In patients dying from non-cancer illness related to chronic organ failure (such as heart failure, cirrhosis, and stroke), palliative care was associated with reduced rates of emergency department visits (crude rate 1.9 (standard deviation 6.2) v 2.9 (8.7) per person year; adjusted rate ratio 0.88, 95% confidence interval 0.85 to 0.91), admissions to hospital (crude rate 6.1 (standard deviation 10.2) v 8.7 (12.6) per person year; adjusted rate ratio 0.88, 95% confidence interval 0.86 to 0.91), and admissions to the intensive care unit (crude rate 1.4 (standard deviation 5.9) v 2.9 (8.7) per person year; adjusted rate ratio 0.59, 95% confidence interval 0.56 to 0.62) compared with those who did not receive palliative care. Additionally increased odds of dying at home or in a nursing home compared with dying in hospital were found in these patients (n=6936 (49.5%) v n=9526 (39.6%); adjusted odds ratio 1.67, 95% confidence interval 1.60 to 1.74). Overall, in patients dying from dementia, palliative care was associated with increased rates of emergency department visits (crude rate 1.2 (standard deviation 4.9) v 1.3 (5.5) per person year; adjusted rate ratio 1.06, 95% confidence interval 1.01 to 1.12) and admissions to hospital (crude rate 3.6 (standard deviation 8.2) v 2.8 (7.8) per person year; adjusted rate ratio 1.33, 95% confidence interval 1.27 to 1.39), and reduced odds of dying at home or in a nursing home (n=6667 (72.1%) v n=13 384 (83.5%); adjusted odds ratio 0.68, 95% confidence interval 0.64 to 0.73). However, these rates differed depending on whether patients dying with dementia lived in the community or in a nursing home. No association was found between healthcare use and palliative care for patients dying from dementia who lived in the community, and these patients had increased odds of dying at home.
Conclusions
These findings highlight the potential benefits of palliative care in some non-cancer illnesses. Increasing access to palliative care through sustained investment in physician training and current models of collaborative palliative care could improve end-of-life care, which might have important implications for health policy.
Introduction
Patients nearing the end of life often have high rates of costly healthcare, including emergency department visits and admissions to hospital, which could be avoidable.1 These potentially burdensome interventions are associated with poor quality of life.1 2 3 4 5 6 7 8 Consequently, the demand for palliative care is rapidly growing. The primary goal of palliative care is to improve quality of life and reduce symptom burden. One of the potentially beneficial consequences of palliative care might be to maximise high value care by reducing healthcare use and its associated costs.9 10
Current evidence for the many benefits of palliative care are skewed towards patients with cancer. A recent systematic review and meta-analyses of randomised controlled trials of palliative care interventions reported that healthcare use was decreased in 11 of 24 trials that measured this outcome. However, of all 43 trials included in the systematic review, nearly 70% were conducted in patients with cancer.9 This limitation might affect the applicability of findings to patients with non-cancer illness who have a trajectory of dying marked by frequent exacerbations and subsequent patterns of healthcare use.9 11 12 13 14 15 This unpredictable trajectory could make it difficult for patients and their healthcare providers to decide when to focus on a more comfort oriented approach to care. Evidence reported on the impact of palliative care on healthcare use in non-cancer illness primarily comes from a limited number of studies of patients with heart failure, dementia, or mixed illness. Conflicting findings have been reported as to whether palliative care reduces overall healthcare use.9 16 17 18 19 20 21 22 23 24 25 26 27 28
This study is novel because it examines the impact of palliative care on healthcare use in patients dying of non-cancer illness at a population level in a large healthcare system. Whereas a previous population level study examined home based palliative care,29 our study examines palliative care delivered across all care settings. This focus on non-cancer illness is distinct from studies that have previously measured patient reported outcomes such as quality of life or healthcare use in patients with cancer. For healthcare systems to achieve the greatest value for patients near the end of life (that is, improve patients’ experience and population health while reducing costs), it is important to define who might benefit from palliative care. The objective of this study was to measure the association between newly initiated physician delivered palliative care in the last six months of life and healthcare use in adults dying from non-cancer illness, and to compare this association with that for patients dying from cancer.
Methods
Study design, setting, and data sources
We conducted a population based cohort study in Ontario, Canada by using linked clinical and health administrative databases. Ontario is Canada’s most populous province with over 10 million adults. All residents of Ontario have access to hospital care and physician services free at the point of use, and those aged 65 years and older are given universal prescription drug insurance coverage. The administrative datasets used in this study were linked using encoded identifiers at the patient level at ICES (formerly the Institute of Clinical and Evaluative Sciences; eText1). These datasets are routinely used to conduct studies involving palliative care.1 30 31 32 33
Study cohort
Our cohort included all Ontario adults (aged ≥18 years) who died from cancer or selected non-cancer causes between 1 January 2010 and 31 December 2015. Cause of death was determined according to the ICD-10 (international classification of diseases 10th revision) code, which identified the disease that directly caused death as indicated by a physician on the death certificate. We defined non-cancer illness as death caused by heart failure, chronic obstructive pulmonary disease, end stage renal disease, cirrhosis, stroke, or dementia. These diseases represent the most common non-cancer conditions and some are also the most frequently studied in the palliative care literature.9 34 35 For the primary analysis, non-cancer illness was divided into chronic organ failure (heart failure, chronic obstructive pulmonary disease, end stage renal disease, cirrhosis, and stroke) or frailty (dementia), which are recognised as unique trajectories of functional decline at the end of life and could influence people’s healthcare needs and subsequent use of the healthcare system.12 13 14 15 For example, patients dying from cancer have a readily identifiable inflection point in their disease trajectory after the failure of adjuvant treatment, which could trigger palliative care referral earlier in the disease course. Conversely, it might be more difficult to determine when to start treatment aimed primarily at enhancing quality of life in patients with chronic organ failure or frailty who have exacerbations of their underlying disease with incomplete recovery in addition to a progressive decline towards death.
We excluded patients who received at least two palliative care visits in the year before the last six months of life. We considered that the approach to care of these patients probably involved engaging with palliative care. This new user design is used in pharmacoepidemiology studies and minimises bias by restricting analysis to patients who are starting treatment. Outcome risks are likely to vary over the time someone has been on treatment and so this design increases the likelihood that the study cohort would be similar at baseline.36
We also excluded people who received their first palliative care visit within seven days of death. Late referral to palliative care would not allow sufficient follow-up time to measure potential changes in healthcare use or to organise the substantial community based healthcare services needed to enable death at home.
Initiation of palliative care
The primary exposure was a patient’s first encounter with palliative care across all care settings within the last six months of life, which served as the study index date. We chose the last six months of life instead of the last year to minimise the effects of confounding by indication owing to time varying covariates. We identified the delivery of palliative care based on a set of unique physician claims fee codes (eText 3).1 4 13 30 31 33 37 38 39 40 These codes were created to specifically indicate the delivery of palliative care and are related to treatments not intended to be curative, such as symptom management or counselling.
In Ontario, over 70% of palliative care is delivered by general practitioners, which includes generalist and specialist palliative care physicians.37 Physicians were deemed to be palliative care specialists when their annual billing comprises more than 10% of palliative care fee codes, which is based on a previously validated method with a sensitivity of 76.0% and a specificity of 97.8%.37 Formal palliative care is predominantly provided by physicians and nurse practitioners in hospitals, outpatient clinics, and the home, and also includes home care services (such as nursing care and personal support workers). In general, patients require a referral from one of their physicians to access specialised palliative care services. Palliative care can also be provided by generalists (eg, family doctor or other non-palliative care specialists) without a referral.
Patient characteristics
We measured demographic and clinical variables, including age, sex, socioeconomic status, rural location of residence, comorbidities and chronic conditions,41 and hospital frailty risk score,42 using a five year lookback period. We also recorded year of death, use of acute health care services within one year before the study index date, and timing of the first palliative care consultation (or matched date in non-exposed patients) relative to death. We also determined the presence of functional decline in the year before the index date in a subset of adults who had completed home care assessments (eText 2). In patients who died from dementia, we determined if they were living in a nursing home using a five year lookback period for the dispensing of at least one drug in a nursing home during that time.43
Matching
To minimise confounding by indication, patients receiving newly initiated palliative care were directly matched to patients who did not receive palliative care (controls) in a 1:2 ratio by using baseline characteristics measured at six months before death. We directly matched on cause of death, frailty score category, presence of metastatic cancer, residential region (according to 1 of 14 local health integration networks), and the probability of receiving palliative care by using a propensity score derived from age and sex. When more than two matched controls were available, we chose patients with the closest year of death. Controls were assigned the index date of the matched patient to ensure equal follow-up time. We matched at six months before death rather than at study index date. Study index date was unique to each patient and it would be computationally too intensive to assign controls an index date and then iteratively find a match with the same index date for patients who received palliative care.
Outcomes
The primary outcomes were the rates of healthcare use, including unplanned emergency department visits, hospital admissions, and intensive care unit admissions after the study index date. Secondary outcomes included the location of death, which was in hospital, at home (including in a nursing home), or other (eText 4). Deaths that occurred in a dedicated palliative care unit or hospice were categorised as other because they cannot be distinguished from other subacute care beds such as those in a rehabilitation hospital. Currently, it is estimated that only 4300 palliative care unit and hospice beds exist in Ontario.44 Other secondary outcomes were the rates of potentially burdensome interventions,3 defined as positive pressure ventilation, cardiopulmonary resuscitation, and the initiation of dialysis (eText 5). We specifically chose these interventions because they are common, costly, associated with discomfort, are of limited benefit at the end of life, and are easily measured as quality indicators of end of life care by using administrative data.45 Incident use of dialysis was determined using a one year lookback from the index date to ensure that no previous exposure occurred.
Statistical analysis
The associations between palliative care and the rates of healthcare use and potentially burdensome interventions, and location of death were estimated using multivariable generalised linear models, accounting for matching. Outcomes for count data were modelled by using a stratified Poisson generalised linear model approach (unplanned emergency department visits, hospital admissions, intensive care unit admissions, and potentially burdensome interventions). Multilevel categorical outcomes were modelled by using a multinomial logistic generalised estimating equation approach (location of death: death at home v in hospital). All models were adjusted for age, sex, comorbidities, rural location of residence, neighbourhood income, hospital frailty risk score, and total number of hospital admissions within one year before the index date. The hospital frailty risk score (range 0-50) is a comprehensive and validated measure of a patient’s function and comorbidity that reflects global illness severity and identifies a group of patients who are at greater risk of adverse outcomes, including hospital admission and 30 day mortality.42 We categorised hospital frailty scores into four groups based on the distribution of scores within our cohort: 0, 0.1-8.9, at least 9, and not admitted to hospital.42 46 We did not account for clustering by physician or facility because most people receive end-of-life care from several physicians in multiple care settings. We performed two prespecified subgroup analyses that measured the primary outcome by cancer and by individual cause of death. We performed a post hoc analysis of healthcare use and location of death among patients who died from dementia, stratified by living in a nursing home. To provide a comparison of the outcomes between patients who died from cancer and those who died from non-cancer illness we measured effect modification by cause of death (cancer v organ failure v dementia) as an interaction term, with palliative care as the predictor variable.
To translate our findings into a more clinically meaningful measure, we calculated the associated number needed to treat for each healthcare use outcome for patients who received and those who did not receive palliative care. We used methods developed by Austin to calculate the crude rate difference of emergency department visits, hospital admissions, and intensive care unit admissions after bootstrapping randomly selected sets of paired patients 1000 times. We then used the inverse of the estimated crude rate difference and variance in each bootstrap sample to calculate the number needed to treat and corresponding 95% confidence intervals.47
We report balance diagnostics in our propensity score matched cohort by using weighted standardised differences to account for the 1:2 matching over statistical tests to assess balance between groups which are confounded with sample size.48 All analyses were performed by using SAS version 9.4 (SAS Institute, Cary, NC).
Patient and public involvement
Multiple patients with chronic serious illness were informally asked if they felt the results reported herein were reflective of their illness experience to check the validity of the findings.
Results
Baseline characteristics
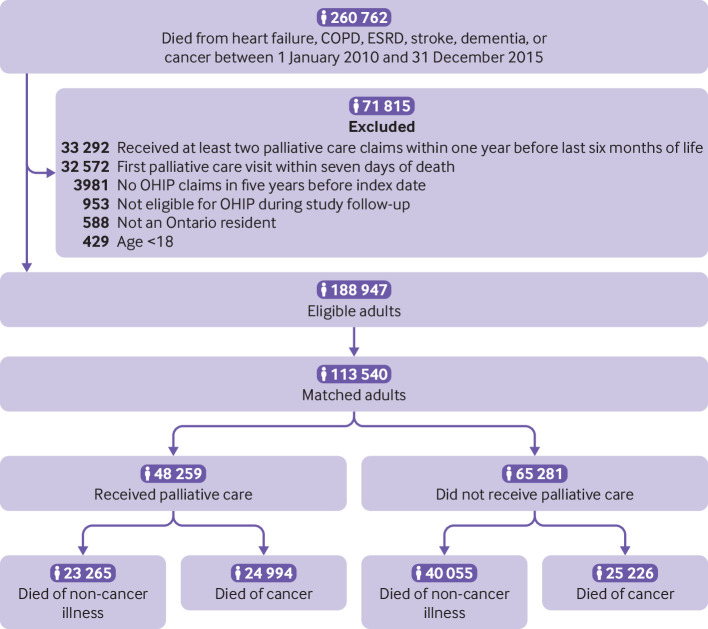
During the study period 260 762 adults died from cancer and non-cancer illness, of which 71 815 were excluded. The final cohort consisted of 113 540 adults; 63 320 (55.8%) died from non-cancer illness (fig 1). The median age of the study cohort was 83 years, 53.6% were women, and the median hospital frailty risk score was 4 (interquartile range 1-11; table 1 and eTable 1). Among patients with dementia, 72.1% (18 254) lived in a nursing home.
Fig 1.
Flow diagram for creation of study sample. All adults who died from heart failure, chronic obstructive pulmonary disease (COPD), end stage renal disease (ESRD), stroke, dementia, or cancer were assessed for inclusion in study. Patients who received their first consultation with palliative care at least seven days before death were included and matched in a 1:2 ratio to patients who did not receive palliative care. OHIP=Ontario health insurance plan
Table 1.
Baseline characteristics at six months before death of matched patients in the last six months of life who died from non-cancer illness in Ontario between 2010 and 2015 by receipt of palliative care. Data are number (%) of patients unless stated otherwise
| Received palliative care | Weighted standardised difference | ||
|---|---|---|---|
| Yes (n=23 265) | No (n=40 055) | ||
| Age (mean (standard deviation)) | 84.3 (9.0) | 84.1 (8.9) | 0.00 |
| Female sex | 13 700 (58.9) | 23 590 (58.9) | 0.00 |
| Cause of death | |||
| Chronic obstructive pulmonary disease | 4094 (17.6) | 7800 (19.5) | 0.00 |
| Dementia | 9255 (39.8) | 16 023 (40.0) | 0.00 |
| Cirrhosis | 333 (1.4) | 516 (1.3) | 0.00 |
| End stage renal disease | 2339 (10.1) | 3607 (9.0) | 0.00 |
| Congestive heart failure | 2768 (11.9) | 4261 (10.6) | 0.00 |
| Stroke | 4476 (19.2) | 7848 (19.6) | 0.00 |
| Rural residence | 2409 (10.4) | 5806 (14.5) | 0.09 |
| Hospital frailty score | |||
| Mean (standard deviation) | 8.9 (8.5) | 8.7 (8.3) | 0.00 |
| Median (IQR) | 7 (2-14) | 7 (2-13) | 0.00 |
| 0 | 2721 (11.7) | 4723 (11.8) | 0.00 |
| 0.1-8.9 | 8390 (36.1) | 14 513 (36.2) | 0.00 |
| ≥9 | 7503 (32.3) | 12 589 (31.4) | 0.00 |
| Not admitted to hospital | 4651 (20.0) | 8230 (20.5) | 0.00 |
| Chronic conditions | |||
| Arrhythmia | 5227 (22.5) | 7969 (19.9) | 0.05 |
| Primary cancer | 1494 (6.4) | 2117 (5.3) | 0.05 |
| Metastatic cancer | 66 (0.3) | 82 (0.2) | 0.00 |
| Chronic obstructive pulmonary disease | 4640 (19.9) | 7681 (19.2) | 0.04 |
| Congestive heart failure | 4691 (20.2) | 7146 (17.8) | 0.05 |
| Coronary artery disease | 3963 (17.0) | 6140 (15.3) | 0.04 |
| Dementia | 4881 (21.0) | 9570 (23.9) | 0.08 |
| Diabetes | 4926 (21.2) | 8413 (21.0) | 0.01 |
| Hypertension | 19 444 (83.6) | 32 720 (81.7) | 0.04 |
| Renal disease | 2653 (11.4) | 3859 (9.6) | 0.04 |
| Rheumatoid arthritis | 800 (3.4) | 1174 (2.9) | 0.03 |
| Stroke | 2578 (11.1) | 4114 (10.3) | 0.02 |
| Previous healthcare use (median (IQR))* | |||
| No of unique drug prescriptions | 15 (9-21) | 15 (9-22) | 0.01 |
| Emergency department visits | 1 (0-2) | 0 (0-2) | 0.13 |
| Hospital admissions | 0 (0-1) | 0 (0-1) | 0.12 |
IQR=interquartile range.
Previous healthcare use within one year of last six months of life.
At six months before death, the baseline characteristics of patients dying from non-cancer illness who received palliative care were similar to those who did not receive palliative care (controls). However, by the index date when patients received their first palliative care visit, some differences were evident compared with controls. A higher proportion of patients who received palliative care lived in urban areas, had multiple chronic conditions including metastatic cancer, and had frailty scores of at least 9 compared with controls. Patients who received palliative care also had a higher number of hospital admissions and emergency department visits within the year before the last six months of life compared with controls (table 2 and eTable 2).
Table 2.
Baseline characteristics at date of first palliative care visit (index date) of matched patients in the last six months of life who died of non-cancer illness in Ontario between 2010 and 2015 by receipt of palliative care. Data are number (%) of patients unless stated otherwise
| Received palliative care | Weighted standardised difference | ||
|---|---|---|---|
| Yes (n=23 265) | No (n=40 055) | ||
| Age (mean (standard deviation)) | 84.6 (9.0) | 84.5 (8.9) | 0.00 |
| Female sex | 13 700 (58.9) | 23 590 (58.9) | 0.00 |
| Cause of death | |||
| Chronic obstructive pulmonary disease | 4094 (17.6) | 7800 (19.5) | 0.00 |
| Dementia | 9255 (39.8) | 16 023 (40.0) | 0.00 |
| Cirrhosis | 333 (1.4) | 516 (1.3) | 0.00 |
| End stage renal disease | 2339 (10.1) | 3607 (9.0) | 0.00 |
| Congestive heart failure | 2768 (11.9) | 4261 (10.6) | 0.00 |
| Stroke | 4476 (19.2) | 7848 (19.6) | 0.00 |
| Rural residence | 2424 (10.4) | 5784 (14.4) | 0.09 |
| Hospital frailty score | |||
| Mean (standard deviation) | 12.3 (9.2) | 10.1 (8.7) | 0.30 |
| Median (IQR) | 11 (5-18) | 8 (3-15) | 0.30 |
| 0 | 1220 (5.2) | 3523 (8.8) | 0.14 |
| 0.1-8.9 | 7766 (33.4) | 13 945 (34.8) | 0.02 |
| ≥9 | 11 859 (51.0) | 15 205 (38.0) | 0.24 |
| Not admitted to hospital | 2420 (10.4) | 7382 (18.4) | 0.21 |
| Chronic conditions | |||
| Arrhythmia | 6914 (29.7) | 9135 (22.8) | 0.14 |
| Primary cancer | 1859 (8.0) | 2269 (5.7) | 0.09 |
| Metastatic cancer | 379 (1.6) | 158 (0.4) | 0.11 |
| Chronic obstructive pulmonary disease | 5768 (24.8) | 8593 (21.5) | 0.10 |
| Congestive heart failure | 6495 (27.9) | 8497 (21.2) | 0.14 |
| Coronary artery disease | 4803 (20.6) | 6670 (16.7) | 0.09 |
| Dementia | 7900 (34.0) | 11 201 (28.0) | 0.12 |
| Diabetes | 5908 (25.4) | 8999 (22.5) | 0.06 |
| Hypertension | 19 811 (85.2) | 32 975 (82.3) | 0.06 |
| Renal disease | 3705 (15.9) | 4566 (11.4) | 0.11 |
| Rheumatoid arthritis | 817 (3.5) | 1190 (3.0) | 0.03 |
| Stroke | 4778 (20.5) | 5120 (12.8) | 0.20 |
| Previous healthcare use (median (IQR))* | |||
| No of unique drug prescriptions | 17 (10-24) | 16 (10-23) | 0.06 |
| Emergency department visits | 2 (1-3) | 1 (0-2) | 0.36 |
| Hospital admissions | 1 (0-2) | 0 (0-1) | 0.44 |
| Functional decline† | 8978 (38.6) | 9551 (23.8) | 0.32 |
| Physician type | |||
| General practitioner | 19 778 (85.0) | — | — |
| Specialist | 3487 (15.0) | — | — |
| Palliative care specialist | 5543 (23.8) | — | — |
IQR=interquartile range.
Previous healthcare use within one year of last six months of life.
For people with a completed home care assessment within last two years of life.
Healthcare use
In patients dying from chronic organ failure, palliative care was associated with reduced rates of emergency department visits (crude rate 1.9 (standard deviation 6.2) v 2.9 (8.7) per person year; adjusted rate ratio 0.88, 95% confidence interval 0.85 to 0.91), hospital admissions (crude rate 6.1 (standard deviation 10.2) v 8.7 (12.6) per person year; adjusted rate ratio 0.88 (95% confidence interval 0.86 to 0.91), and intensive care unit admissions (crude rate 1.4 (standard deviation 5.9) v 2.9 (8.7) per person year; adjusted rate ratio 0.59, 95% confidence interval 0.56 to 0.62) compared with those who did not receive palliative care. In patients dying from dementia, palliative care was not associated with reduced rates of intensive care unit admission (crude rate 0.2 (standard deviation 2.1) v 0.2 (2.1) per person year; adjusted rate ratio 1.03, 95% confidence interval 0.96 to 1.11), but was associated with increased rates of emergency department visits (crude rate 1.2 (standard deviation 4.9) v 1.3 (5.5) per person year; adjusted rate ratio 1.06, 95% CI 1.01 to 1.12) and hospital admissions (crude rate 3.6 (standard deviation 8.2) v 2.8 (7.8) per person year; adjusted rate ratio 1.33, 95% confidence interval 1.27 to 1.39). However, differences in these outcomes were found to depend on whether the patient lived in a nursing home or in the community; no association was found for patients dying from dementia who lived in the community (eTables 7 and 8).
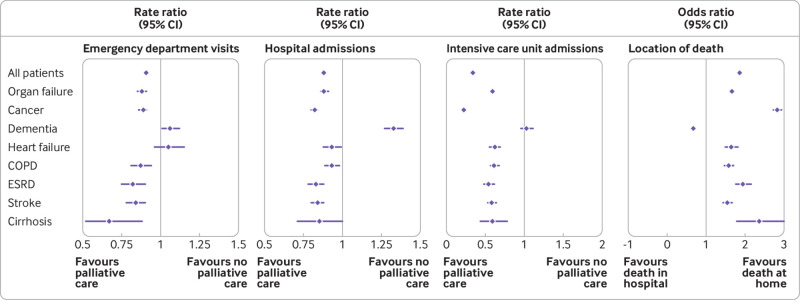
The magnitude of all associations was similar in patients dying from cancer and in those dying from chronic organ failure, except for rates of intensive care unit admission, which were lower in patients dying from cancer: emergency department visits (crude rate 2.5 (standard deviation 6.7) v 3.4 (8.4) per person year; adjusted rate ratio 0.89, 95% confidence interval 0.86 to 0.91), hospital admissions (crude rate 5.5 (standard deviation 8.8) v 7.5 (10.2) per person year; adjusted rate ratio 0.82, 95% confidence interval 0.80 to 0.83), and intensive care unit admissions (crude rate 0.4 (standard deviation 2.9) v 2.2 (6.8) per person year; adjusted rate ratio 0.22, 95% confidence interval 0.21 to 0.23; fig 2). Based on these results, in patients dying from chronic organ failure, palliative care was associated with one less emergency department visit for every 11 (95% confidence interval 6 to 32) patients who received it; one less hospital admission for every 4 (3 to 5) patients who received it; and one less intensive care unit admission for every 1 (1 to 2) patient who received it.
Fig 2.
Association between palliative care and healthcare use. Association between newly initiated palliative care and rates of emergency department visits not resulting in admission to hospital, hospital admissions, and intensive care unit admissions, or location of death among adults in the last six months of life dying from cancer and non-cancer illness in Ontario between 2010 and 2015. Locations of death were home (including nursing home), acute care (including hospital and intensive care unit), and other. Models were adjusted for age, sex, comorbidities, rural location of residence, neighbourhood income, frailty, and hospital admissions in the year before index date (index date defined as date of first palliative care visit). COPD=chronic obstructive pulmonary disease; ESRD=end stage renal disease
When we compared the effect of cancer related deaths and non-cancer (chronic organ failure or dementia) related deaths on healthcare use outcomes, we found variable results (eTable 9). Patients admitted to hospital had similar lengths of stay regardless of whether they received palliative care or not (7.8±14.1 v 6.3±11.4 days, respectively).
Location of death
Overall, 40 626 (35.8%) patients died in hospital or in the intensive care unit. Patients who died from chronic organ failure and received palliative care had increased odds of dying at home or in their nursing home than in hospital compared with those who did not receive palliative care (n=6936 (49.5%) v n=9526 (39.6%); adjusted odds ratio 1.67, 95% confidence interval 1.60 to 1.74). In patients dying from dementia, palliative care was associated with decreased odds of dying at home or in their nursing home (n=6667 (72.1%) v n=13 384 (83.5%); adjusted odds ratio 0.68, 95% confidence interval 0.64 to 0.73). However, an associated increased odds of dying at home was found for patients dying from dementia who lived in the community (adjusted odds ratio 1.35, 95% confidence interval 1.23 to 1.49; eTable 8). The magnitude of the association was higher among those dying from cancer (adjusted odds ratio 2.83, 95% confidence interval 2.73 to 2.94) than in those dying from non-cancer illness (fig 2).
Potentially burdensome interventions
Patients dying from chronic organ failure who received palliative care had a lower associated rate of potentially burdensome interventions compared with those who did not receive palliative care (composite adjusted rate ratio 0.66, 95% confidence interval 0.64 to 0.69). In patients dying from dementia, palliative care was associated with an increased rate of potentially burdensome interventions (composite adjusted rate ratio 1.18, 95% confidence interval 1.08 to 1.31). The magnitude of the association was smaller for patients dying from cancer (0.27, 0.26 to 0.28; eTables 5 and 6 and eFig 1).
Discussion
Principal findings
We conducted a matched population based study of 113 540 adults in Ontario, Canada who died from cancer and non-cancer illness. We found that in patients dying from chronic organ failure, physician delivered palliative care was associated with a 12%, 12%, and 41% reduction in the rate of emergency department visits, hospital admissions, and intensive care unit admissions, respectively. Palliative care was also associated with a 1.67 increased odds of death at home. We compared these associations between different trajectories of dying and found similar results in patients dying from cancer. Unexpectedly, we found increased rates of healthcare use associated with palliative care in those dying from dementia, which differed between those who lived in a nursing home compared with those who lived in the community; no association was found for patients dying from dementia who lived in the community.
Policy implications
Patients, caregivers, and healthcare systems struggle with the growing burden of medical complexity that is also associated with poor quality of life and high healthcare expenditure.1 2 3 4 5 6 7 8 49 End-of-life care that involves hospital admission and intensive care unit admission is costly and potentially burdensome. Our study supports the role palliative care has in providing high value end-of-life care to people dying from cancer and most non-cancer illness. We found that palliative care might reduce healthcare use and potentially burdensome interventions near the end of life.10 We also found an association between palliative care and an increased odds of dying at home, which is where most people would prefer to die and a recognised indicator of high quality end-of-life care.50 51 52 Our findings are consistent with previous literature on the association between home based palliative care and healthcare use outcomes, and with location of death in patients with cancer. Additionally our study adds to the knowledge about the associated effects in non-cancer illness across all care settings.4 29
Comparison with other studies
Most of the evidence that measures the effect of palliative care on healthcare use in non-cancer illness is conflicting and is limited to small studies of patients with heart failure, dementia, or mixed illness.9 16 17 18 19 20 21 22 23 24 25 26 27 28 53 Fourteen randomised control trials used palliative care interventions and measured their effect on rates of emergency department visits and hospital admissions. Three out of eight of these studies demonstrated a reduction in emergency department visits, and one out of 13 showed a reduction in hospital admissions. However, the interventions were heterogeneous in their design, the measures were all secondary analyses, and many of the trials were at high risk of bias and not powered to detect differences in these specific outcomes. Similar to our findings, a propensity matched cohort study of 6218 patients primarily with cancer (80%) but also non-cancer illness (20%) in the last six months of life in Ontario, Canada found that community based palliative care was associated with a 33% lower risk of emergency department visits and hospital admissions.4 Approximately 35% of our cohort died in hospital, which is similar to findings from a recent study in a large healthcare system.54 Our study extends these findings to patients with non-cancer illness at a population level in a universal healthcare system that includes palliative care delivered across all care settings.
Strengths and limitations
Our study is limited by the lack of information on patient and caregiver preferences for care, which we believe are crucial to providing high quality, patient goal directed palliative care. We assumed that patients received palliative care for issues related to their cause of death. In reality, many of these patients had multiple comorbidities, possibly including cancer, which probably contributed to their overall palliative needs. Previous work also shows that patients with metastatic cancer are more likely to receive palliative care than other disease groups.13 31 The observed heterogeneity in healthcare outcomes among the subgroups of patients dying from chronic organ failure could relate to differences in their underlying palliative needs (eg, symptoms) and non-palliative care needs (eg, difference in needs during an exacerbation of their underlying disease, such as ongoing dialysis). Patients who received palliative care were generally more unwell than those who did not, which could underestimate the magnitude of our results because these patients might be more likely to have increased healthcare use.
We used robust statistical methods to minimise the risk of confounding by indication, and consequently found only marginal differences between our unadjusted and adjusted results. To further minimise these effects, we made several decisions intended to minimise this risk: matching on several factors strongly associated with exposure to palliative care; selecting a cohort of patients who were in the last six months of life (to minimise the effects owing to time varying covariates and because baseline patient variables achieved a better balance at six months compared with 12 months); and a new user design to increase the likelihood that the groups of patients would be similar at baseline. However, patients with advanced illness often receive late referral to palliative care services that could limit several opportunities to relieve potentially avoidable suffering.
Current recommendations from several societies encourage the integration of palliative care early in the course of a patient’s disease, instead of at the end of life.11 55 56 In regions with limited healthcare access, some patients might not be able to receive care at home and avoid potential transfers to the emergency department or hospital, regardless of their preferences. Ontario lacks the rich infrastructure of hospice networks such as those found in many areas of the United States, which could limit the ability of patients with major care needs to die outside of the hospital setting.31 We also measured a physician delivered palliative approach to care across all care settings that includes generalist and specialist palliative care physicians. While this probably strengthens the generalisability of palliative care to real world care, it might underestimate the magnitude of the association for specialised palliative care delivered in the home.30 In other jurisdictions like the US, which use different funding mechanisms such as the Medicare Hospice Benefit, palliative care could be delivered by healthcare providers other than physicians, which might include nurse practitioners or social workers.25 Delivery of care by these providers and its association with important outcomes is not captured in our study using physician fee claims. However, the use of fee codes in administrative data as a means to capture delivery of palliative care is a strength of our study given that care classification has been less successful in health systems without universal coverage.57
Finally, by using the information on a patient’s death certificate we intentionally maximised specificity, but probably decreased the overall denominator in our study population. While this approach might result in inflated confidence intervals, we still found important differences in many outcomes. We were especially concerned that other approaches could introduce too much heterogeneity and other sources of bias.
Unanswered questions
Questions remain about the timing, location of initiation, and models of palliative care delivery to optimise end-of-life care for patients with non-cancer illness. These questions include the involvement of a patient’s primary care provider in the delivery of palliative care, which is often founded on a longitudinal and trusting relationship. Further study is also required to explain the differences found in healthcare use between patients dying from cancer and chronic organ failure and those dying from dementia. One explanation could be that many care decisions in patients with dementia are made by substitute decision makers and not the patients themselves. Dementia is often not recognised as a terminal illness in the same way as chronic organ failure and cancer, which makes it difficult to know when to focus on comfort over prolonging life. Additionally, recognising the cause of death could be more challenging in patients with dementia who die of its related complications (eg, pneumonia) when their dementia is less severe compared with patients with cancer. Therefore, the generalisability of our results could be limited to those with milder disease, such as those earlier in the course of their disease trajectory.
Alternatively, a palliative care physician might have been involved in situations involving complicated goals of care discussions if discordance in care plans existed between patients with dementia or their caregivers and their treating physicians. Previous work has shown a concerning rate of potentially burdensome interventions delivered in acute care settings near the end of life in this vulnerable population, especially for those who live in nursing homes.3 58 59 In our study, 72% of patients who died from dementia lived in a nursing home. We speculate that our findings could be related to differences in the care provided in nursing homes compared with that in the community. Several factors such as family pressure, physician workload, the capability of nursing home staff, and potential medicolegal concerns influence decisions to go to acute care, especially in the nursing home setting where many patients with dementia live.60
Conclusions
Palliative care was associated with reduced rates of healthcare use and an increased likelihood of a home death in people dying from chronic organ failure, but not dementia. These findings highlight the potential benefits of palliative care in some non-cancer illnesses. Increasing access to palliative care through sustained investment in physician training and current models of collaborative palliative care could improve end-of-life care, which might have major implications for health policy.
What is already known on this topic
Patients nearing the end of life often have high rates of potentially avoidable emergency department visits and hospital admissions, which are associated with poor quality of life
Palliative care improves the delivery of high value end-of-life care for patients with cancer, but the evidence for patients with non-cancer illness is lacking
What this study adds
Palliative care was associated with a 12%, 12%, and 41% reduction in the rate of emergency department visits, hospital admissions, and intensive care unit admissions, respectively, in patients dying from chronic organ failure (such as heart failure, cirrhosis, and stroke)
Overall palliative care was associated with increased rates of emergency department visits and hospital admissions in patients dying from dementia, but no association was found for those who lived in the community
Increasing access to palliative care through sustained investment in physician training and current models of collaborative palliative care could improve end-of-life care, which might have major implications for health policy
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: KLQ, TS, NMS, SI, PT, RG, PC, ASD, and CMB contributed to the study concept and design. AH was responsible for the acquisition of data. KLQ performed analyses of all data in this study. KLQ, TS, NMS, AH, SI, PT, RG, PC, DK, ASD, and CMB contributed to the interpretation of data. KLQ, TS, NMS, AH, SI, PT, RG, PC, DK, ASD, and CMB drafted the manuscript. KLQ, TS, NMS, AH, SI, PT, RG, PC, DK, ASD, and CMB contributed to the critical revision of the manuscript for important intellectual content. KLQ did the statistical analysis. KLQ and CMB obtained funding. KLQ is guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long term Care (MOHLTC). The analysis was supported by a research grant KLQ and CMB received from the Sinai Health System Research Foundation to perform this work. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. We thank IMS Brogan for use of their Drug Information Database. KLQ and NMS receive funding from the CIHR Vanier Scholarship Program, the Eliot Phillipson Clinician-Scientist Training Program, and the Clinician Investigator Program at the University of Toronto. DK receives research funding from the National Institutes of Health (K01HL133466), the Cystic Fibrosis Foundation, and the Milbank Foundation.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Institute for Clinical Evaluative Sciences (ICES) for the submitted work; the analysis was supported by a research grant KLQ and CMB received from the Sinai Health System Research Foundation to perform this work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Ethics approval was obtained from Sinai Health System’s research ethics board (ID 18-0015-E).
Data sharing: The dataset from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the programmes may rely on coding templates or macros that are unique to ICES.
The lead author (the manuscript's guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of this study will be disseminated to the academic community through presentation of the findings at relevant national and international meetings (eg, the annual meeting of the Canadian Society of Internal Medicine, and the Annual Scientific Meeting of the American Geriatrics Society); presenting the findings on The Rounds Table (where KLQ is a prior Director), a clinical research focused podcast downloaded by more than 7000 monthly listeners from 103 countries, and disseminating the results to networks of researchers associated with primary care, general internal medicine, geriatrics (including the Canadian Frailty Network), palliative care (including the Ontario Palliative Care Network), and health services research (including the Institute for Clinical and Evaluative Sciences). Strategies to disseminate the findings to healthcare organisations and policy makers include presenting the study findings to policy makers at the local (eg, University Health Network and Mount Sinai Hospital), provincial (eg, Ministry of Health and Long term Care of Ontario), and national (eg, Canadian Patient Safety Institute) levels. We will use these networks to encourage dialogue about the results, their implications for future research, and the potential for integration into existing clinical practice within our local institutions. Different versions of the final reports and a one page infographic summary will be created for the different stakeholder groups (policy makers, healthcare administrators, clinicians, and patients).
References
- 1. Tanuseputro P, Wodchis WP, Fowler R, et al. The health care cost of dying: a population-based retrospective cohort study of the last year of life in Ontario, Canada. PLoS One 2015;10:e0121759. 10.1371/journal.pone.0121759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yosick L, Crook RE, Gatto M, et al. Effects of a population health community-based palliative care program on cost and utilization. J Palliat Med 2019;22:1075-81. 10.1089/jpm.2018.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stall NM, Fischer HD, Fung K, et al. Sex-specific differences in end-of-life burdensome interventions and antibiotic therapy in nursing home residents with advanced dementia. JAMA Netw Open 2019;2:e199557. 10.1001/jamanetworkopen.2019.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014;348:g3496. 10.1136/bmj.g3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang JJ, Alam S, Cahill LE, et al. Global Burden of Disease Study trends for Canada from 1990 to 2016. CMAJ 2018;190:E1296-304. 10.1503/cmaj.180698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alla F, Briançon S, Guillemin F, et al. EPICAL Investigators Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail 2002;4:337-43. 10.1016/S1388-9842(02)00006-5 [DOI] [PubMed] [Google Scholar]
- 7. Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation--systematic review. Int J Chron Obstruct Pulmon Dis 2007;2:241-51. [PMC free article] [PubMed] [Google Scholar]
- 8. Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: Quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 2015;191:256-64. 10.1016/j.ijcard.2015.04.235. [DOI] [PubMed] [Google Scholar]
- 9. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016;316:2104-14. 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Courtright KR, Cassel JB, Halpern SD. A research agenda for high-value palliative care. Ann Intern Med 2018;168:71-2. 10.7326/M17-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kavalieratos D, Gelfman LP, Tycon LE, et al. Palliative care in heart failure: rationale, evidence, and future priorities. J Am Coll Cardiol 2017;70:1919-30. 10.1016/j.jacc.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA 2003;289:2387-92. 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 13. Seow H, O’Leary E, Perez R, Tanuseputro P. Access to palliative care by disease trajectory: a population-based cohort of Ontario decedents. BMJ Open 2018;8:e021147. 10.1136/bmjopen-2017-021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ 2005;330:1007-11. 10.1136/bmj.330.7498.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med 2010;362:1173-80. 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agar M, Luckett T, Luscombe G, et al. Effects of facilitated family case conferencing for advanced dementia: A cluster randomised clinical trial. PLoS One 2017;12:e0181020. 10.1371/journal.pone.0181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrison MB, Browne GB, Roberts J, Tugwell P, Gafni A, Graham ID. Quality of life of individuals with heart failure: a randomized trial of the effectiveness of two models of hospital-to-home transition. Med Care 2002;40:271-82. 10.1097/00005650-200204000-00003 [DOI] [PubMed] [Google Scholar]
- 18. Radwany SM, Hazelett SE, Allen KR, et al. Results of the promoting effective advance care planning for elders (PEACE) randomized pilot study. Popul Health Manag 2014;17:106-11. 10.1089/pop.2013.0017 [DOI] [PubMed] [Google Scholar]
- 19. Brumley R, Enguidanos S, Jamison P, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc 2007;55:993-1000. 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 20. Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med 2004;164:83-91. 10.1001/archinte.164.1.83. [DOI] [PubMed] [Google Scholar]
- 21. Zimmer JG, Groth-Juncker A, McCusker J. A randomized controlled study of a home health care team. Am J Public Health 1985;75:134-41. 10.2105/AJPH.75.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med 2006;9:111-26. 10.1089/jpm.2006.9.111. [DOI] [PubMed] [Google Scholar]
- 23. Wong FKY, Ng AYM, Lee PH, et al. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart 2016;102:1100-8. 10.1136/heartjnl-2015-308638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bekelman DB, Allen LA, McBryde CF, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med 2018;178:511-9. 10.1001/jamainternmed.2017.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: the PAL-HF Randomized, Controlled Clinical Trial. J Am Coll Cardiol 2017;70:331-41. 10.1016/j.jacc.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brännström M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail 2014;16:1142-51. 10.1002/ejhf.151. [DOI] [PubMed] [Google Scholar]
- 27. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med 2015;18:134-42. 10.1089/jpm.2014.0192. [DOI] [PubMed] [Google Scholar]
- 28. Ahronheim JC, Morrison RS, Morris J, Baskin S, Meier DE. Palliative care in advanced dementia: a randomized controlled trial and descriptive analysis. J Palliat Med 2000;3:265-73. 10.1089/jpm.2000.3.265 [DOI] [PubMed] [Google Scholar]
- 29. Maetens A, Beernaert K, De Schreye R, et al. Impact of palliative home care support on the quality and costs of care at the end of life: a population-level matched cohort study. BMJ Open 2019;9:e025180. 10.1136/bmjopen-2018-025180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanuseputro P, Beach S, Chalifoux M, et al. Associations between physician home visits for the dying and place of death: A population-based retrospective cohort study. PLoS One 2018;13:e0191322. 10.1371/journal.pone.0191322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanuseputro P, Budhwani S, Bai YQ, Wodchis WP. Palliative care delivery across health sectors: A population-level observational study. Palliat Med 2017;31:247-57. 10.1177/0269216316653524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown CR, Hsu AT, Kendall C, et al. How are physicians delivering palliative care? A population-based retrospective cohort study describing the mix of generalist and specialist palliative care models in the last year of life. Palliat Med 2018;32:1334-43. 10.1177/0269216318780223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gershon AS, Maclagan LC, Luo J, et al. End of life strategies among patients with advanced chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2018;198:1389-96. 10.1164/rccm.201803-0592OC. [DOI] [PubMed] [Google Scholar]
- 34. Singer AE, Goebel JR, Kim YS, et al. Populations and interventions for palliative and end-of-life care: a systematic review. J Palliat Med 2016;19:995-1008. 10.1089/jpm.2015.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaertner J, Siemens W, Meerpohl JJ, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357:j2925. 10.1136/bmj.j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915-20. 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 37. Barbera L, Hwee J, Klinger C, Jembere N, Seow H, Pereira J. Identification of the physician workforce providing palliative care in Ontario using administrative claims data. CMAJ Open 2015;3:E292-8. 10.9778/cmajo.20150005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seow H, Dhaliwal G, Fassbender K, Rangrej J, Brazil K, Fainsinger R. The effect of community-based specialist palliative care teams on place of care. J Palliat Med 2016;19:16-21. 10.1089/jpm.2015.0063. [DOI] [PubMed] [Google Scholar]
- 39. Qureshi D, Tanuseputro P, Perez R, Seow H. Place of care trajectories in the last two weeks of life: a population-based cohort study of Ontario Decedents. J Palliat Med 2018;21:1588-95. 10.1089/jpm.2018.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qureshi D, Tanuseputro P, Perez R, Pond GR, Seow HY. Early initiation of palliative care is associated with reduced late-life acute-hospital use: A population-based retrospective cohort study. Palliat Med 2019;33:150-9. 10.1177/0269216318815794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muggah E, Graves E, Bennett C, Manuel DG. The impact of multiple chronic diseases on ambulatory care use; a population based study in Ontario, Canada. BMC Health Serv Res 2012;12:452. 10.1186/1472-6963-12-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775-82. 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quinn KL, Campitelli MA, Diong C, et al. Association between physician intensity of antibiotic prescribing and the prescription of benzodiazepines, opioids and proton-pump inhibitors to nursing home residents: a population-based observational study. J Gen Intern Med 2019;34:2763-71. 10.1007/s11606-019-05333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Health Quality Ontario. Palliative care at the end of life. 2016.
- 45. De Schreye R, Houttekier D, Deliens L, Cohen J. Developing indicators of appropriate and inappropriate end-of-life care in people with Alzheimer’s disease, cancer or chronic obstructive pulmonary disease for population-level administrative databases: A RAND/UCLA appropriateness study. Palliat Med 2017;31:932-45. 10.1177/0269216317705099. [DOI] [PubMed] [Google Scholar]
- 46. McAlister F, van Walraven C. External validation of the hospital frailty risk score and comparison with the hospital-patient one-year mortality risk score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf 2019;28:284-8. 10.1136/bmjqs-2018-008661. [DOI] [PubMed] [Google Scholar]
- 47. Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol 2010;63:2-6. 10.1016/j.jclinepi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 48. Austin PC. Assessing balance in measured baseline covariates when using many-to-one matching on the propensity-score. Pharmacoepidemiol Drug Saf 2008;17:1218-25. 10.1002/pds.1674. [DOI] [PubMed] [Google Scholar]
- 49. Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med 2015;163:729-36. 10.7326/M15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care 2013;12:7. 10.1186/1472-684X-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Roo ML, Leemans K, Claessen SJJ, et al. EURO IMPACT Quality indicators for palliative care: update of a systematic review. J Pain Symptom Manage 2013;46:556-72. 10.1016/j.jpainsymman.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 52. Mizuno A, Miyashita M, Hayashi A, et al. Potential palliative care quality indicators in heart disease patients: A review of the literature. J Cardiol 2017;70:335-41. 10.1016/j.jjcc.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 53. Possin KL, Merrilees JJ, Dulaney S, et al. Effect of collaborative dementia care via telephone and internet on quality of life, caregiver well-being, and health care use: the care ecosystem randomized clinical trial. JAMA Intern Med 2019;30. 10.1001/jamainternmed.2019.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA 2018;320:264-71. 10.1001/jama.2018.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Braun LT, Grady KL, Kutner JS, et al. American Heart Association Advocacy Coordinating Committee Palliative care and cardiovascular disease and stroke: a policy statement from the American Heart Association/American Stroke Association. Circulation 2016;134:e198-225. 10.1161/CIR.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 56. Howlett JG, Chan M, Ezekowitz JA, et al. Canadian Cardiovascular Society Heart Failure Guidelines Panels The Canadian Cardiovascular Society heart failure companion: bridging guidelines to your practice. Can J Cardiol 2016;32:296-310. 10.1016/j.cjca.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 57. Hua M, Li G, Clancy C, Morrison RS, Wunsch H. Validation of the V66.7 code for palliative care consultation in a single academic medical center. J Palliat Med 2017;20:372-7. 10.1089/jpm.2016.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009;361:1529-38. 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med 2011;365:1212-21. 10.1056/NEJMsa1100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McDermott C, Coppin R, Little P, Leydon G. Hospital admissions from nursing homes: a qualitative study of GP decision making. Br J Gen Pract 2012;62:e538-45. 10.3399/bjgp12X653589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material