Abstract
Central tolerance prevents autoimmunity, but also limits T cell responses to potentially immunodominant tumor epitopes with limited expression in healthy tissues. In peripheral antigen presenting cells (APCs), gamma-interferon-inducible lysosomal thiol reductase (GILT) is critical for MHC class II-restricted presentation of disulfide bond-containing proteins, including the self and melanoma antigen tyrosinase-related protein 1 (TRP1). The role of GILT in thymic antigen processing and generation of central tolerance has not been investigated. We found that GILT enhanced the negative selection of TRP1-specific thymocytes in mice. GILT expression was enriched in thymic APCs capable of mediating deletion, namely medullary thymic epithelial cells (mTECs) and dendritic cells, while TRP1 expression was restricted solely to mTECs. GILT facilitated MHC class II-restricted presentation of endogenous TRP1 by pooled thymic APCs. Using bone marrow chimeras, GILT expression in thymic epithelial cells (TECs), but not hematopoietic cells, was sufficient for complete deletion of TRP1-specific thymocytes. An increased frequency of TRP1-specific regulatory T (Treg) cells was present in chimeras with increased deletion of TRP1-specific thymocytes. Only chimeras that lacked GILT in both TECs and hematopoietic cells had a high conventional T:Treg cell ratio and were protected from melanoma challenge. Thus, GILT expression in thymic APCs, and mTECs in particular, preferentially facilitates MHC class II-restricted presentation, negative selection and increased Treg cells, resulting in a diminished anti-tumor response to a tissue-restricted, melanoma-associated self antigen.
Keywords: Antigen processing and presentation, Tolerance, Melanoma
Major histocompatibility complex (MHC) class II-restricted antigen presentation by epithelial and hematopoietic cells in the thymic stroma generates the CD4+ T cell repertoire and, thus, regulates autoimmune and anti-tumor responses to tissue-restricted antigens (reviewed in (1)). Ligation of T cell receptors (TCR) on CD4+CD8+ double positive (DP) thymocytes by self peptide/MHC complexes on cortical thymic epithelial cells (cTECs) leads to survival signals (positive selection) and downregulation of the unused coreceptor. CD4+ single positive (CD4SP) thymocytes migrate to the thymic medulla, where they interact with dendritic cells (DCs) and medullary thymic epithelial cells (mTECs) presenting self peptide:MHC class II complexes. Those thymocytes that bind self peptide:MHC complexes with high avidity die by apoptosis (negative selection) or alternatively escape negative selection and differentiate into Treg cells. However, the antigen processing pathways leading to MHC class II-restricted presentation in the thymus remain to be fully understood.
While expression of tissue-restricted antigens is limited to mTECs, direct MHC class II-restricted presentation by mTECs and indirect presentation by hematopoietic APCs, such as DCs, both contribute to negative selection. Expression of the transcriptional regulator, autoimmune regulator (Aire), is restricted to mature CD80hiMHC class IIhi mTECs and allows for the expression of a large portion of tissue-restricted antigens (2, 3). Aire also directs the selection of thymic regulatory T (Treg) cells (4). Previous studies have demonstrated transfer of intact cytosolic and transmembrane proteins, including MHC class II, from TECs to DC in vivo and in vitro (5, 6). Although not specifically demonstrated as a mechanism of antigen or MHC transfer between TECs and hematopoietic cells critical to thymic selection, possible mechanisms of transfer include exosomes (7), apoptotic bodies (8), tunneling nanotubules (6), trogocytosis (9, 10) and gap junctions (11). MHC class II-restricted presentation of mTEC-derived antigen has been demonstrated by mTECs and/or DCs ex vivo (5, 12). In multiple TCR transgenic models, mTECs are capable of mediating negative selection and selection of Treg cells through direct MHC class II-restricted presentation of neo-self antigens (13, 14). In other models, mTEC-derived antigen must be transferred to hematopoietic APCs, such as DCs, for indirect MHC class II-restricted presentation and negative selection (15, 16). In some cases, negative selection may be mediated by both hematopoietic and non-hematopoietic APCs, especially when the antigen is expressed in mTECs in high amounts (13, 17). Analysis of a fixed TCRβ model further supports a role for MHC class II-restricted presentation on both bone marrow-derived APCs and mTECs in mediating negative selection (18).
Gamma interferon-inducible lysosomal thiol reductase (GILT) is the only known reductase localized in the endosomal compartment and facilitates the enzymatic reduction of protein disulfide bonds (19, 20). In peripheral antigen presenting cells (APCs), GILT’s reductase activity facilitates the MHC class II-restricted presentation of a subset of epitopes from disulfide bond-containing proteins, including the melanocyte differentiation antigens tyrosinase and tyrosinase-related protein 1 (TRP1) (21–24). However, the importance of GILT expression in thymic APC populations and the impact on T cell development have not been fully explored. Here, we use the TRP1-specific TCR transgenic mouse model and GILT as tools to evaluate the antigen processing pathway leading to central T cell tolerance to an endogenously expressed melanocyte differentiation antigen, which is clinically relevant in melanoma and autoimmune vitiligo.
Materials and Methods
Mice
C57BL/6 wild-type and RAG1−/− mice were obtained from Jackson Laboratory (Bar Harbor, ME). GILT−/− mice were provided by Dr. Peter Cresswell (22). TRP1-deficient Tyrp1B-w RAG1−/− TRP1-specific TCR transgenic mice termed Ag-GILT+/+Tg were provided by Dr. Nicholas Restifo (25). TRP1-expressing RAG1−/− TRP1-specific TCR transgenic mice with and without GILT, termed Ag+GILT+/+Tg, and Ag+GILT−/−Tg mice, have been described (26). Thymuses, spleens, and inguinal, axillary and cervical lymph nodes were isolated as described (23). All animals were housed in microisolator cages. These studies were approved by the institutional review committee at the University of Arizona.
Flow cytometry and cell sorting
FcγRII/III was blocked with anti-CD16/CD32 mAb (clone 2.4G2, 1 μg per million cells). Cells were stained with FITC, phycoerythrin, phycoerythrin-Cy7, PerCP, Brilliant Violet 421™, or APC-conjugated mAbs against murine Vβ14 (14-2 or REA645), CD4 (RM4-5 or GK1.5), CD8 (53-6.7), CD45 (30-F11), CD11c (N418), I-Ab (AF6-120.1), EpCAM (G8.8), Ly51 (FG35.4), Foxp3 (FJK-16s), UEA-1, CCR7 (4B12), CD5 (53-7.3) and corresponding isotype controls (BD Biosciences; eBioscience; Biolegend; Miltenyi Biotec), as described (23). Dead cell exclusion was performed by staining with 7-AAD, fixable viability dye eFluor™ 780 (eBioscience), or propidium iodide (PI). Cells were analyzed on a BD LSRII or BD Canto II cytometer and sorted on BD FACSAria II and III cytometers. Data were analyzed with BD FACS Diva and Flow Jo (Tree Star) software. Absolute cell counts were determined by multiplying the cell count from the entire organ using a hemocytometer by the cell frequency determined by flow cytometry. This method for determination of the absolute cell count is less accurate than using counting beads and normalizing per unit of tissue mass.
Immunofluorescence microscopy
To investigate the cellular distribution of CCR7+ thymocytes, thymuses from the three transgenic mouse strains were isolated and fixed in 4% PFA at 4°C for overnight. The thymuses were then cryoprotected in 30% sucrose for 48 h before sectioning into 20 μm slices on a cryostat (Leica CM1950). Floating sections were collected, washed in PBS, and incubated in a blocking solution containing 5% normal goat serum (Cat# 16210064, ThermoFisher), 1% bovine serum albumin, and 0.2% Triton X-100 in PBS for 12 h. The sections were then switched to the blocking solution containing rabbit recombinant anti-CCR7 mAb antibody (clone Y59, Abcam, 1:500 dilution) for 24 h at 4°C with slow rotation. After three washes in PBS-Triton, secondary antibodies (goat anti-rabbit conjugated with Alexa Fluor 555, Invitrogen #A32732) were added for 12 h. The sections were extensively washed, mounted on a glass slide (SuperFrost, ThermoFisher), and covered with Vectashield mounting medium containing DAPI (H-1200, Vector Laboratories). The sections were imaged on a Zeiss LSM 710 confocal microscope. Images from each experiment were acquired with identical laser power, pinhole size, and photomultiplier tube detector gain settings. Images were exported in tiff format, and cropped/displayed using Adobe Creative Cloud.
Isolation of thymic stromal cells
Stromal cells were isolated as described (27). Minced thymuses were digested in media containing DNase I, collagenase D and dispase I. Cells were separated into light and heavy fractions by density gradient centrifugation with Percoll (GE Healthcare), representing predominantly thymic stromal cells and thymocytes, respectively. Thymic stromal cells were FACS-sorted to obtain purified populations of DCs (PI−, CD45+, CD11c+), cTECs (PI−, CD45−, EpCAM+, Ly51+, UEA-1−), and mTECs (PI−, CD45−, EpCAM+, Ly51−, UEA-1+).
Immunoblotting
GILT immunoblotting was performed on Percoll-enriched thymic stromal cells. Cells were lysed with 1% Triton X-100 in TBS for 30 min on ice. Lysates were resolved by SDS-PAGE (12% w/v acrylamide) under reducing conditions and electrophoretically transferred to Immobilon-P membrane (Millipore, Bedford, MA). The membrane was blocked in PBS with 0.2% Tween 20 and 5% dehydrated milk and incubated with primary Ab. GILT was immunoblotted with anti-mouse GILT polyclonal rabbit serum (1:1000, provided by Dr. P. Cresswell) (28). Rat anti-GRP94 mAb (1:5000; StressGen Biotechnologies, Victoria, Canada) served as a loading control. Membranes were then washed, incubated with HRP-conjugated goat anti-mouse or rat IgG (1:5000; Jackson ImmunoResearch Laboratories) and enhanced chemiluminescent substrate (SuperSignal West Pico; Pierce, Rockford, IL), and exposed to film.
Quantitative real-time PCR
RNA from sorted thymic stromal cell populations was purified using the Absolutely RNA Microprep kit (Stratagene). RNA from stromal fraction of the thymus, whole thymus, spleen, and skin was prepared using TRIzol (Invitrogen). RNA was reverse-transcribed into cDNA using oligo-dT (Invitrogen) and Superscript III reverse transcriptase (Qiagen). Ifi30 (GILT) transcript levels were assessed by real-time PCR using the exon 1–2 amplifying primers 5’-AGGCACAACCACCTGCAA-3’ and 5’-AGCGACAAGCTCCACACA-3’, and Tyrp1 was detected using the exon 6–7 amplifying primers 5’-CACTTTCACTGATGCGGTCTTTGAC-3’ and 5’-TGTCCAATAGGTGCGTTTTCCAAC-3’. Products were detected using SYBR green master mix (Applied Biosystems). Sample quantities were normalized to cyclophilin signal (SuperArray Biosciences). Real-time PCR reactions were run on a 7500 Fast Real-Time PCR System (Applied Biosystems), and data analyzed according to the standard curve method using Excel (Microsoft).
Antigen presentation assays
Pooled thymic stromal cells isolated by Percoll (GE Healthcare) density gradient centrifugation from wild-type and GILT-deficient mice were co-cultured with 1×105 primary, naive TRP1-specific T cells at the indicated APC:T cell ratios without the addition of exogenous antigen. Primary CD4+ TRP1-specific T cells were isolated from pooled lymph node and spleen cells from Ag-GILT+/+Tg mice using the EasySep mouse CD4-positive selection kit to achieve ≥ 95% purity (StemCell Technologies, Vancouver, Canada). T cells were isolated from TRP1-specific TCR Tg animals lacking TRP1 and RAG to prevent the influence of tolerance mechanisms and ensure TCR specificity, respectively. After 48 hours the IL-2 concentration in culture supernatants was determined by ELISA (mouse IL-2 ELISA, BD OptEIA; BD Biosciences). Thymic stromal APCs isolated from TRP1-deficient mice (TRP1Bw) were used as an antigen negative (Ag−) control. As a positive control TRP1109–130 peptide: NCGTCRPGWRGAACNQKILTVR (10 μg/ml) was added to some cultures.
Bone marrow chimeras
Bone marrow chimeras were generated by lethally irradiating 6-8 week old recipient mice with 1000 rad delivered as two doses of 500 rad spaced six hours apart. Bone marrow was harvested by flushing the marrow cavities of femurs, tibiae, and humeri. To deplete T cells, bone marrow was incubated with anti-CD4 (GK1.5) and anti-CD8 (53-6.7) for 15 minutes on ice followed by the addition of rabbit complement for one hour at 37°C. Irradiated recipients were injected with 1x107 T cell-depleted bone marrow cells via the tail vein. Mice were allowed to reconstitute for 8 weeks before thymuses and peripheral lymphoid organs were analyzed.
Tumor Challenge
Bone marrow chimeras were generated transferring Ag+GILT−/−Tg or Ag+GILT+/+Tg bone marrow into irradiated RAG−/− and GILT−/−RAG−/− mice. Eight weeks after bone marrow transfer, mice were injected subcutaneously with B16.F10 tumor cells (2.5x105) in 100 μl PBS into the right flank. Tumor growth was monitored two times weekly by measuring tumor length (L) and width (W). Tumor volume (mm3) was calculated using the formula π/6 × L × W2.
Results
GILT enhances the negative selection of TRP1-specific thymocytes
To study the role of GILT in central tolerance to an endogenously expressed self and tumor antigen, we used CD4+ TRP1-specific TCR transgenic mice on the RAG−/− background. Without endogenous TCRs and in the presence of TRP1 antigen and GILT (Ag+GILT+/+Tg), very few CD4+CD8lo and CD4SP cells develop (Fig. 1A and B). In the presence of TRP1 Ag and the absence of GILT (Ag+GILT−/−Tg), an increased frequency of CD4SP cells develop (Fig. 1A and B), consistent with our prior findings (26). In the absence of TRP1 antigen (Ag-GILT+/+Tg), and thus without negative selection, a higher frequency of CD4SP T cells develop than in the absence of GILT (Fig. 1A and B). No difference was found in the frequency of DP or CD4-CD8- (DN) thymocytes regardless of GILT or TRP1 expression (Fig. 1A), indicating clonal deletion is occurring in the CD4SP stage. In agreement with this observation, few total and Vβ14hi TRP1-specific CD4SP thymocytes develop in Ag+GILT+/+Tg mice (Fig. 1C and D). Overall, the absence of GILT (Ag+GILT−/−Tg) or TRP1 (Ag-GILT+/+Tg) resulted in an increased frequency of total and Vβ14hi TRP1-specific CD4SP thymocytes, in comparison to Ag+GILT+/+Tg mice (Fig. 1C and D), further supporting a role for GILT in thymic development. There was a small, but statistically significant, increase in Vβ14hi thymocytes in Ag+GILT−/−Tg mice compared to Ag-GILT+/+Tg mice, which might suggest a role for GILT in the development of Vβ14hi thymocytes beyond processing of TRP1.
Figure 1. GILT expression enhances negative selection of TRP1-specific thymocytes.
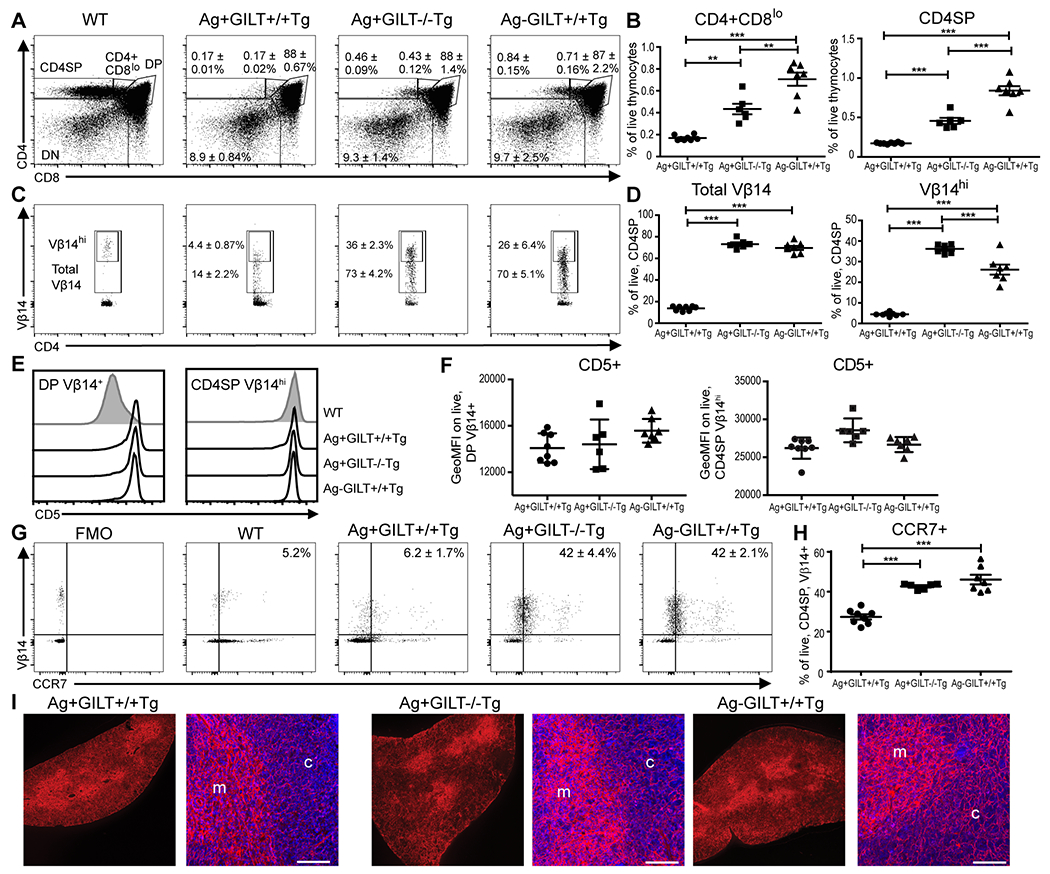
Thymic development of TRP1-specific (Vβ14+) T cells was analyzed in the presence or absence of GILT or Ag. All mice are on the C57BL/6 RAG1−/− background. (A) Representative profiles from each strain are shown with numbers indicating the mean ± SEM for the frequency of live, CD4SP, CD4+CD8lo, DP, and DN thymocytes. (B) Frequency of CD4+CD8lo and CD4SP thymocytes from (A). (C) Representative profiles from each strain indicating the mean ± SEM of the frequency of live, CD4SP total Vβ14+ and Vβ14hi thymocytes. (D) Frequency of total TRP1-specific (Vβ14+) and Vβ14hi thymocytes from (C). (E) Representative histograms of CD5 expression on live, DP Vβ14+ cells and CD4SP Vβ14hi cells in each transgenic strain. CD5 expression in wild-type (WT) mouse is gated on live, DP or CD4SP cells. (F) Geometric mean fluorescence intensity (GeoMFI) of CD5 on DP Vβ14+ cells and CD4SP Vβ14hi thymocytes from (E). (G) Representative profiles of CCR7 and Vβ14 expression in live, CD4SP cells for each strain with numbers indicating the mean frequency ± SEM. Fluorescence minus one (FMO) gating control is shown. (H) Mean frequency ± SEM of CCR7+ on CD4SP Vβ14+ thymocytes from (G). Data was compared by ANOVA with the Tukey correction for multiple comparisons. (I) Immunofluorescence confocal images of CCR7 staining in the thymus. In each transgenic strain, the image of the whole section (left) shows clear accentuation of CCR7 staining (red) in the medulla (inner region) of the thymus. The high power images (right) show CCR7 staining (red) at the boundary of the medulla (m) and cortex (c) along with DAPI staining of nuclei. Scale bar indicates 100 μm. **p < 0.01, ***p < 0.001.
The increase in TRP1-specific CD4SP cells in the absence of GILT could be due to enhanced positive selection or diminished negative selection. To address whether GILT alters positive selection, we evaluated CD5 expression in thymocytes. CD5 is a cell surface transmembrane protein that associates with the TCR complex. CD5 expression increases in the polyclonal thymocyte population from the DN to the DP to the CD4SP stage with high expression in the DP and CD4SP stages mediated by engagement of the TCR with positively selecting ligands. The level of CD5 expression on CD4SP thymocytes directly correlates with the avidity and signaling strength of the TCR-peptide:MHC interaction during positive selection (29). In the three strains that express the transgenic TCR known to undergo positive selection, the majority of DP Vβ14+ thymocytes express an equivalently high level of CD5, in comparison to DP thymocytes from a wild-type mouse in which only a fraction of DP thymocytes undergo positive selection and express a high level of CD5 (Fig. 1E and F). In wild-type mice, the CD5 expression level dramatically increases from the DP to the CD4SP stage, and there is an approximately 2-fold increase in the geometric MFI of CD5 expression levels from the DP to CD4SP stage in each of the transgenic strains. The level of CD5 expression on CD4SP Vβ14hi cells is similar among the three TCR transgenic strains, indicating that the absence of GILT or TRP1 Ag does not alter the positively selecting peptide:MHC ligands.
Upregulation of CCR7 expression on thymocytes is required for the migration of thymocytes from the thymic cortex to the medulla and maintenance of central tolerance to other tissue-restricted self antigens (30, 31). To begin to determine where negative selection is occurring, we evaluated CCR7 expression on TRP1-specific Vβ14+CD4SP T cells. In the presence of Ag and GILT (Ag+GILT+/+Tg), there is a low frequency of CCR7+Vβ14+CD4SP thymocytes (Fig. 1G and H). Loss of Ag or GILT results in a similar high frequency of CCR7+Vβ14+CD4SP thymocytes (Fig. 1G and H). These data suggest that GILT enhances the negative selection of TRP1-specific CD4SP thymocytes as they migrate into the thymic medulla. To distinguish whether TRP1-specific thymocytes are deleted prior to acquiring CCR7 expression or after acquisition of CCR7 expression and migration to the medulla, we evaluated CCR7 expression in the thymuses of the three transgenic strains using immunofluorescence microscopy (Fig. 1I). In each transgenic strain, CCR7 expression was enriched in the thymic medulla compared to the thymic cortex. Similar enrichment of CCR7 expression in the thymic medulla in Ag+GILT+/+Tg mice, in which Vβ14+ thymocytes undergo deletion, as well as Ag+GILT−/−Tg and Ag-GILT+/+Tg mice, supports that CCR7 expression is induced and CCR7+ thymocytes migrate to the medulla in each strain. These results, taken together, support that negative selection occurs after CCR7 upregulation and migration to the medulla.
GILT expression is enriched in thymic APCs capable of mediating negative selection
To further assess how GILT contributes to negative selection, we investigated GILT expression in thymic stromal cells. Immunoblot analysis revealed that GILT protein is expressed in a stromal cell-enriched fraction from wild-type thymuses, in which CD11c+ thymic DCs are the predominant cell type; stromal cells from GILT-deficient mice lacked GILT protein expression (Fig. 2A). Given the low frequency of stromal cell types, such as mTECs and cTECs, we determined GILT expression in FACS-sorted thymic APC populations by quantitative PCR. Transcripts in sorted mTECs, cTECs, and CD11c+ thymic DCs were compared with transcripts prepared from whole spleen and thymus tissues. GILT expression in the thymus was enriched among APC populations, most highly in mTECs and thymic DCs (Fig. 2B). The GILT mRNA expression levels identified in mTECs and thymic DCs were greater than that found in splenocytes (Fig. 2B), suggesting that there is sufficient GILT expression in mTECs and thymic DCs to function in antigen processing. As both mTECs and thymic DCs contribute to negative selection, the expression of GILT in mTECs and DCs is consistent with its observed contribution to negative selection in the TRP1-specific Tg system. cTECs, which primarily mediate positive selection in the thymic cortex, also express GILT transcripts (Fig. 2B).
Figure 2. GILT expression is enriched in mTECs and thymic DCs, and TRP1 expression is limited to mTECs.
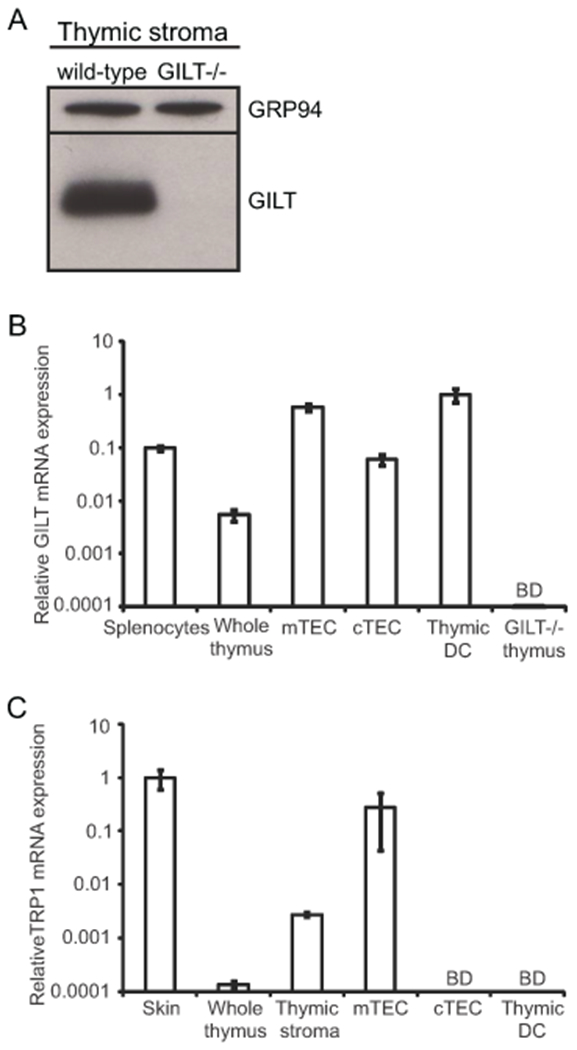
(A) Immunoblot analysis demonstrating GILT expression in total thymic stromal cells isolated by density gradient centrifugation. GRP94 was used as a loading control. (B) Thymic stromal cells were FACS-sorted to obtain purified populations of CD45+CD11c+ dendritic cells (DCs), CD45-EpCAM+Ly51-UEA-1+ medullary thymic epithelial cells (mTECs), and CD45-EpCAM+Ly51+UEA-1− cortical thymic epithelial cells (cTECs). GILT transcript levels assessed by quantitative PCR of splenocytes (positive control), whole thymus, mTECs, cTECs, thymic DCs, and GILT−/− thymus (negative control). Data were normalized to cyclophilin and shown relative to thymic DCs. (C) TRP1 transcript levels assessed by quantitative PCR of skin (positive control), whole thymus, total thymic stromal cells, mTECs, cTECs, and thymic DCs. Data were normalized to cyclophilin and shown relative to skin. Unless otherwise indicated, cells were obtained from wild-type mice. BD, below the limit of detection.
Thymic expression of TRP1 is restricted to mTECs
We next sought to determine TRP1 expression in the thymus by quantitative PCR. TRP1 signal was found in whole thymus extracts and the thymic stromal fraction, but not in thymocytes (Fig. 2C and data not shown). TRP1 transcript levels were relatively low in the whole thymus, as compared to the skin and brain, peripheral tissues expressing this antigen (Fig. 2C and data not shown). Similarly, several tissue-restricted antigens have been shown to have low levels of expression within the thymus and are nevertheless physiologically relevant to anti-tumor responses and autoimmune disease protection (32–37). Within sorted thymic stromal APC populations, TRP1 transcripts were restricted to mTECs and were below the limit of detection in cTECs and thymic DCs (Fig. 2C). mTECs expressed similar levels of TRP1 transcripts as skin tissue (Fig. 2C). mTEC-restricted expression is consistent with Aire-mediated transcriptional regulation of TRP1 (35). These findings demonstrate that mTECs are the source of endogenous TRP1 in the thymus and provide further support for TRP1-specific thymocytes undergoing negative selection when they migrate into the medulla (Fig. 1) and encounter TRP1.
GILT facilitates MHC class II-restricted presentation of endogenous TRP1 by pooled thymic APCs
Since GILT is critical for efficient MHC class II-restricted presentation of TRP1 by peripheral DCs and B cells (23), we evaluated the role of GILT in processing of TRP1 by thymic APCs. First, we established that we could detect presentation of endogenous TRP1 in the thymus. To this end, thymic APCs were enriched by density gradient centrifugation from thymuses of wild-type or TRP1-deficient (Ag-) mice. Ex vivo APCs were co-cultured with primary, naïve CD4+ TRP1-specific T cells without the addition of exogenous TRP1. IL-2 production was used as a measure of antigen presentation. We detected presentation of endogenous TRP1 by wild-type thymic APCs at both the 10:1 and 5:1 APC:T cell ratios compared to the negative controls (thymic APCs from Ag- mice co-cultured with T cells, wild-type APCs alone or T cells alone) (Fig. 3A). As expected, we observed lower levels of presentation of endogenous TRP1 in the thymus, as compared to presentation of exogenous TRP1 (23). To analyze the requirement of GILT on the processing of endogenous TRP1 in the thymus, thymic APCs from wild-type and GILT-deficient mice were co-cultured with CD4+ TRP1-specific T cells as above. Thymic APCs isolated from wild-type and GILT−/− mice had equivalent MHC class II (I-Ab) expression (Fig. 3B), suggesting that differences in presentation were due to intracellular antigen processing rather than cell-surface MHC class II expression. In contrast to presentation of endogenous TRP1 by wild-type thymic APCs, presentation of endogenous TRP1 was not detected by GILT−/− thymic APCs in comparison to the APC alone and T cell alone negative controls (Fig. 3C). Co-culture of TRP1-specific T cells with wild-type or GILT−/− thymic APCs in the presence of exogenous TRP1 peptide, which does not require intracellular processing, resulted in equivalent high IL-2 production (Fig. 3D). These data demonstrate that GILT facilitates the MHC class II-restricted processing of TRP1 in thymic APCs.
Figure 3. GILT facilitates MHC class II-restricted presentation of endogenous TRP1 by pooled thymic APCs.
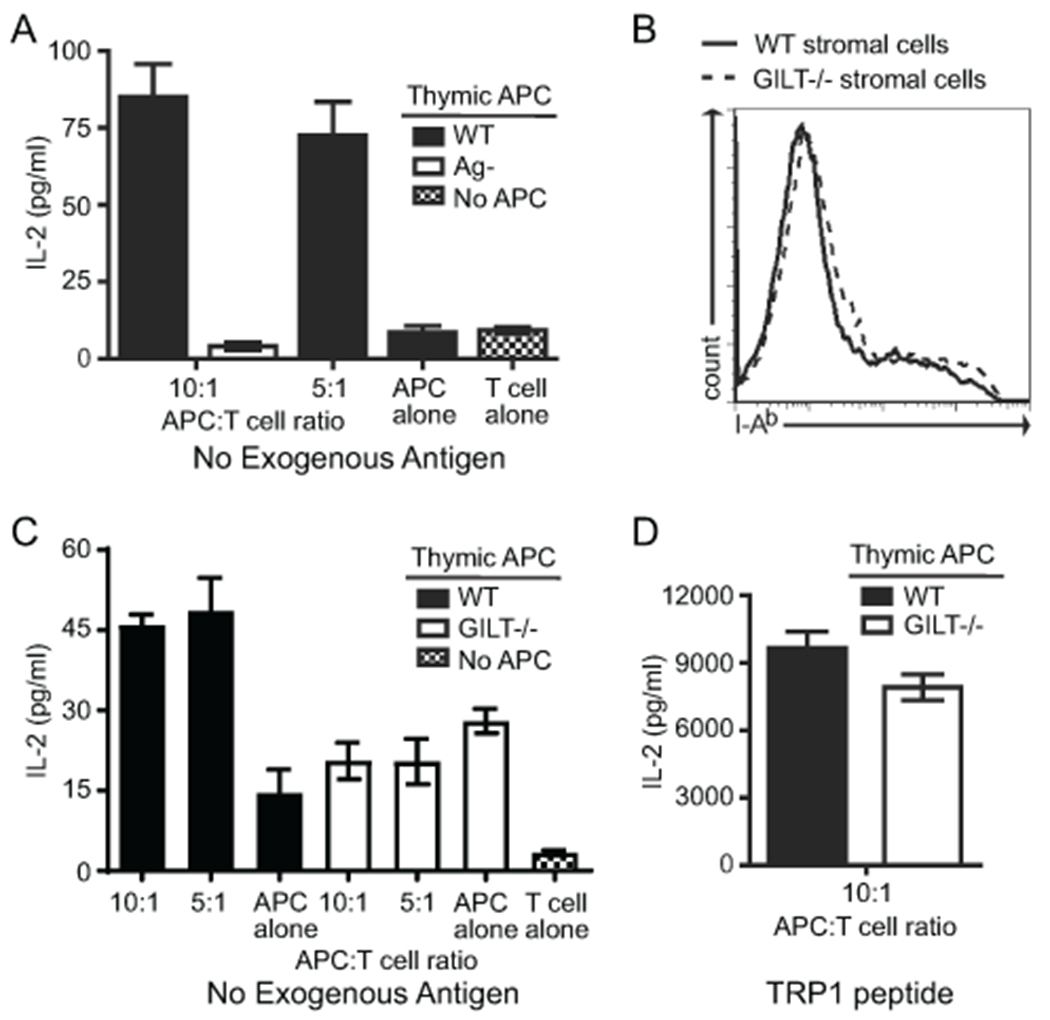
(A) Pooled thymic stromal cells isolated by density gradient centrifugation were co-cultured with primary, naive TRP1-specific T cells without additional antigen. Thymic APCs from TRP1-deficient (Ag−) mice served as a negative control. IL-2 production, as assessed by ELISA, was used as a measure of antigen presentation. Comparison of wild-type APCs (both 10:1 and 5:1) with each of the negative controls (thymic APCs from TRP1-deficient (Ag−) mice, wild-type APCs alone, and T cells alone) by one way ANOVA followed by Tukey’s multiple comparison test revealed p < 0.001 for each. (B) I-Ab expression on thymic stromal APCs from WT and GILT−/− mice. (C) Pooled thymic stromal cells from WT and GILT−/− mice were co-cultured with naive TRP1-specific T cells without additional antigen. Comparison of wild-type APCs 10:1 and 5:1 with wild-type APCs alone and T cells alone revealed p < 0.001 for each; no significant differences between GILT−/− APCs 10:1 and 5:1 with GILT−/− APCs alone and T cells alone. (D) Pooled thymic stromal cells from WT and GILT−/− mice were co-cultured with naive TRP1-specific T cells with TRP1 peptide (10 μg/ml) (positive control). No significant difference identified by an unpaired t test. Bars and error bars represent the mean ± SEM of triplicates in one experiment using the number of mice required to obtain sufficient cells. The data are representative of three independent experiments.
GILT in TECs is necessary for efficient deletion of TRP1-specific T cells
As technical limitations prevented us from assessing a role of GILT in antigen processing in mTECs using in vitro assays as in Figure 3, we, next, turned to bone marrow chimeras to assess the role of GILT in antigen processing in mTECs and thymic DCs and the subsequent outcome on thymic selection. Work in other mouse models has demonstrated that both mTECs and thymic DCs are capable of MHC class II-restricted presentation of mTEC-derived antigen and mediating thymic deletion of autoreactive T cells (13–18). In our model, both mTECs and thymic DCs express GILT (Fig 2B) and, therefore, have the capacity to efficiently process and present TRP1, although TRP1 expression is restricted to mTECs. The specific contribution of each cell type to the maintenance of central tolerance to this tissue-restricted antigen is unclear. To determine which thymic stromal APC populations require GILT expression for the negative selection of TRP1-specific T cells, we evaluated T cell development in bone marrow chimeras to isolate the contribution of GILT in radioresistant TECs and radiosensitive hematopoietic cells. CD4SP thymocytes and peripheral CD4+Vβ14+ T cells did not readily develop in chimeras in which GILT was expressed in both the TECs and bone marrow-derived APCs (Fig. 4A/D, upper left, B, C, E, F), consistent with the observation of negative selection of TRP1-specific T cells in Ag+GILT+Tg animals (Fig. 1A). There was a similar low frequency of CD4SP thymocytes and peripheral CD4+Vβ14+ T cells in chimeras in which GILT expression was restricted to TECs (Fig. 4A/D, lower left, B, C, E, F), indicating that GILT expression in TECs is sufficient for the deletion of TRP1-specific thymocytes. In contrast, a large frequency and absolute number of CD4SP thymocytes and peripheral CD4+Vβ14+ T cells developed in chimeras which lacked GILT expression in both TECs and bone marrow-derived APCs (Fig. 4A/D, lower right, B, C, E, F), consistent with our finding that TRP1-specific T cells escape negative selection in the absence of GILT (Fig. 1A). An intermediate frequency and absolute number of CD4SP thymocytes and peripheral CD4+Vβ14+ T cells developed in bone marrow chimeras in which GILT expression was limited to bone marrow-derived APCs (Fig. 4A/D, upper right, B, C, E, F), demonstrating that GILT expression in TECs is necessary for efficient deletion of TRP1-specific thymocytes and that GILT expression in hematopoietic cells can partially contribute to deletion. Together these data demonstrate that TEC-derived GILT preferentially supports efficient deletion of TRP1-specific autoreactive T cells.
Figure 4. GILT in TECs is necessary for efficient deletion of TRP1-specific T cells.
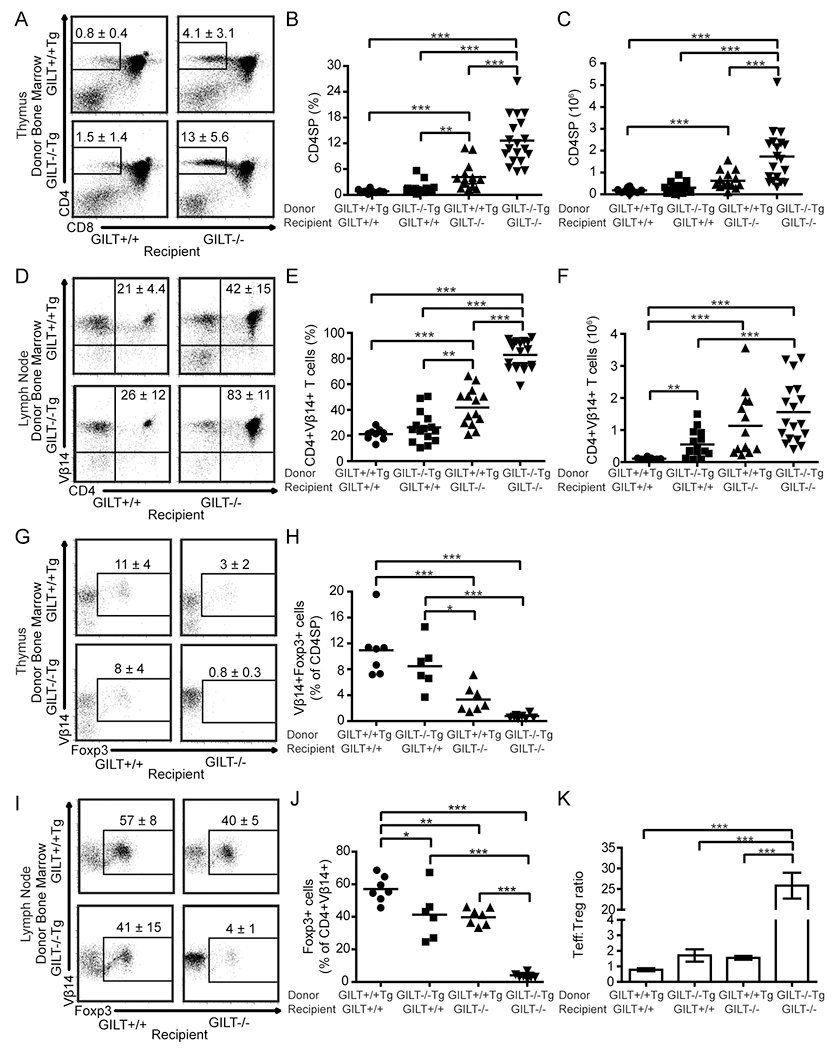
To limit T cell specificity to TRP1 and assess the development of TRP1-specific T cell tolerance, all donor and recipient animals in these studies were on the RAG−/− background and expressed TRP1 Ag. Lethally irradiated RAG−/− or GILT−/−RAG−/− mice were reconstituted with bone marrow from Ag+GILT+/+Tg or Ag+GILT−/−Tg mice. Thymocyte and lymph node cell subsets were analyzed eight weeks later. (A) Representative dot plots demonstrating the gating and mean frequency ± SEM of CD4SP cells in the thymus. (B) Frequency and (C) absolute number of CD4SP thymocytes (D) Representative dot plots demonstrating the mean frequency ± SEM of CD4+Vβ14+ lymph node cells. (E) Frequency and (F) absolute number of CD4+Vβ14+ lymph node cells. Data points (B, C, E, F) represent individual mice from three pooled experiments. (G) Representative dot plots demonstrating the mean frequency ± SEM of CD4SP thymocytes that are Vβ14+Foxp3+. (H) Frequency of Vβ14+Foxp3+ cells within CD4SP thymocytes. (I) Representative dot plots showing the mean frequency ± SEM of CD4+Vβ14+ lymph node cells that express Foxp3. (J) Frequency of TRP1-specific Treg cells in lymph nodes. (K) Tconv:Treg cell ratio in lymph nodes. Data points in (H, J, K) represent individual mice from two additional experiments. Groups were compared by ANOVA with the Bonferroni correction for multiple comparisons. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
Given that the activity of TRP1-specific T cells can be constrained by Treg cells (26, 38–41) and the role of Aire-expressing mTECs in promoting selection of Foxp3+ Treg cells (42), we evaluated Treg cells in additional sets of bone marrow chimeras transferring GILT−/−Tg or GILT+/+Tg bone marrow into GILT−/−RAG−/− or GILT+/+RAG−/− recipients as above. As in Fig. 4A–C, GILT expression in TECs was sufficient for deletion of TRP1-specific thymocytes, and the absence of GILT in TECs resulted in an increased absolute number of CD4SP cells (Supplemental Fig. 1A). Under conditions in which we observed the highest levels of negative selection (GILT+/+ and GILT−/− bone marrow into GILT+/+ recipients), we observed the highest frequency of Treg cells in the thymus and periphery (Fig. 4 G–J). Given the inverse relationship between CD4SP cells and the frequency of Treg cells, we did not observe a difference in the absolute number of Treg cells in the thymus (Supplemental Fig. 1B). While a statistically significant increase in the absolute number of Treg cells in the periphery was not observed (Supplemental Fig. 1C), there were substantial differences in the conventional T (Tconv):Treg cell ratio in lymph nodes (Fig. 4K). In GILT−/−Tg->GILT−/− chimeras, the Tconv:Treg cell ratio was approximately 25:1. In comparison, a Tconv:Treg cell ratio of 1–2:1 was observed in the other three chimeras. There is a similar pattern present in the thymus, where the ratio of antigen-specific CD4SP cells to Treg cells is 10-fold higher in the GILT−/−Tg->GILT−/− chimeras compared with the other three chimeras (Supplemental Fig. 1D). These data demonstrate that an increased frequency of Treg cells is observed in the same conditions that promote thymic deletion and that only GILT−/−Tg->GILT−/− chimeras have a substantial population of TRP1-specific peripheral Tconv cells.
Only TRP1-specific T cells that develop in the complete absence of GILT protect from melanoma challenge
Next, we tested the function of T cells that escape negative selection in TRP1-specific TCR Tg bone marrow chimeras due to differences in GILT expression, using prevention of melanoma tumor growth. TRP1-specific T cells are able to induce autoimmune vitiligo and have anti-melanoma activity (23, 26, 38, 41, 43); however, we were not able to use vitiligo as a measure of T cell function in the context of bone marrow chimeras, because irradiation itself leads to depigmentation (data not shown). CD4+ TRP1-specific TCR transgenic T cells have been shown to exhibit direct cytotoxicity of B16 melanoma cells, which is dependent on T cell-derived IFNγ production and induction of MHC class II expression on TRP1-expressing melanoma cells (41).
As in Fig. 4, we generated bone marrow chimeras by transferring GILT−/−Tg or GILT+/+Tg bone marrow into GILT−/−RAG−/− or GILT+/+RAG−/− recipients. After reconstitution, we challenged chimeras with TRP1-expressing B16 melanoma cells and assessed tumor growth (Fig. 5A, B). Tumor growth curves for individual mice in each group are shown in Supplemental Fig. 2. GILT−/−Tg → GILT−/− chimeras, which have a high Tconv:Treg ratio, were protected from melanoma challenge. Only one out of eight mice in this group developed a small 6 mm3 tumor, which did not progress in size. In contrast, chimeras which expressed GILT in either or both thymic compartments failed to control tumor growth, likely due to an impaired TRP1-specific T cell response. These data show that a robust anti-tumor response develops when GILT is completely absent during thymic development and that “intermediate” thymic selection through hematopoietic APC-restricted GILT expression is not sufficient to protect from tumor growth. Although GILT expression in peripheral APCs has the potential to contribute to immunity, as we have previously shown that GILT expression in the host enhances immunity following adoptive transfer of naïve TRP1-specific T cells (23), the dominant effect in the bone marrow chimeras appears to be due to T cell tolerance, as the control of tumor growth in the chimeras strongly correlates with the Tconv:Treg ratio present in the chimeras. While all seven out of seven GILT+/+Tg -> GILT−/− chimeras developed progressively enlarging tumors, in the GILT+/+Tg -> GILT+/+ and GILT−/−Tg -> GILT+/+ chimeras some mice did not develop tumors, some developed tumors very late, and some developed very small tumors that regressed. Radioresistant GILT+/+ Langerhans cells and dermal DCs (44, 45) in the GILT+/+Tg -> GILT+/+ and GILT−/−Tg -> GILT+/+ chimeras may have contributed to this partial immune control of tumor growth.
Figure 5. Only TRP1-specific T cells that develop in the complete absence of GILT protect from melanoma challenge.
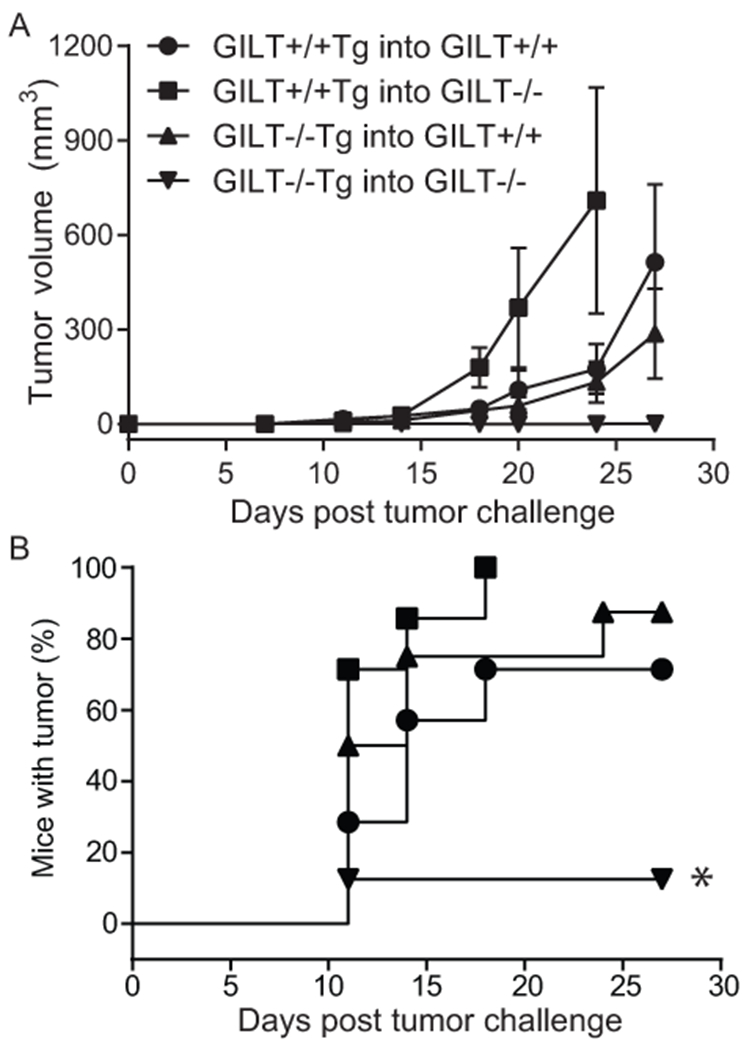
Lethally irradiated RAG−/− or GILT−/−RAG−/− mice were reconstituted with bone marrow from Ag+GILT+/+Tg or Ag+GILT−/−Tg mice. After eight weeks, mice were s.c. challenged with B16.F10 melanoma. (A) Tumor volume (mm3) and (B) tumor-free survival were monitored. Tumor-free survival curves were compared using the log-rank test. Data represent two pooled experiments with GILT+/+Tg into GILT+/+ n = 7 mice, GILT+/+Tg into GILT−/− n = 7 mice, GILT−/−Tg into GILT+/+ n = 8 mice, GILT−/−Tg into GILT−/− n = 8 mice; *, p < 0.05
Discussion
While GILT has a well-established function in facilitating MHC class II-restricted antigen processing in APCs in the periphery, in this study we addressed the role of GILT in antigen processing in the thymus. Some antigen processing pathway members are shared between TECs and hematopoietic APCs in the thymus and periphery, while others such as the thymoproteasome, cathepsin L and thymus-specific serine protease are unique to cTECs (1). Our data show that GILT facilitates the MHC class II-restricted presentation of endogenous TRP1 by pooled thymic stromal cells in vitro and enhances the negative selection of TRP1-specific thymocytes in vivo. In bone marrow chimeras, GILT expression in TECs is sufficient to achieve negative selection. The newly generated TRP1-specific T cells only protect from melanoma tumor growth, when GILT is absent in both TECs and hematopoietic cells, correlating with the highest Tconv:Treg cell ratio. Thus, we have demonstrated that GILT functions in the thymus to improve MHC class II-restricted presentation and modulate T cell selection and function.
Our data demonstrating that GILT expression limited to TECs is sufficient for thymic deletion, but GILT expression limited to bone marrow-derived cells results in partial thymic deletion, supports a more dominant role of TEC-derived GILT for antigen processing and subsequent T cell tolerance. Possible explanations for TEC-derived GILT, but not GILT in bone marrow-derived cells, being sufficient for thymic deletion are that 1) GILT-mediated reduction of TRP1 is more efficient in mTEC’s due to coexpression of the tissue restricted antigen and antigen processing machinery in the same thymic cell type, 2) mTECs may directly present TRP1 and mediate deletion, or 3) mTECs may load TRP1 peptide onto MHC class II for transfer to DCs. The advantage of TEC-derived GILT in the thymic presentation of the endogenous tissue-restricted antigen TRP1 may be due to limiting amounts of the self antigen available and the route of antigen processing. TRP1 expression in the thymus is restricted to mTECs. Expression of a particular tissue-restricted antigen is limited to 1-3% of total mTECs (46). At limiting antigen doses, GILT-mediated reduction of rare tissue-restricted antigens may occur more efficiently in mTECs, exposing GILT-dependent epitopes for MHC class II binding and direct presentation by mTECs or transfer to DCs. Another contributing factor for the advantage of TEC-derived GILT is that TRP1, as an integral membrane protein, is likely to intersect GILT-containing endocytic compartments and undergo lysosomal degradation in mTECs. Although the subcellular localization of TRP1 in mTECs is unknown, in melanocytes properly folded TRP1 transits through the Golgi to early endosomes en route to melanosomes directed by a dileucine-based endocytic sorting signal, and a small portion TRP1 reaches the cell surface and preferentially undergoes endocytic recycling (47). Consistent with this possibility, direct MHC class II-restricted presentation of a membrane-bound model antigen by mTECs was macroautophagy-independent (13). Alternatively, mTEC-derived GILT may be secreted or released in apoptotic bodies for uptake and use by DCs, but given that thymic DCs and mTECs express a similar level of GILT mRNA, it is unclear what advantage this strategy would offer.
We anticipate that the role of GILT-mediated reduction in facilitating central tolerance is broadly applicable to epitopes from protein disulfide bond-containing self antigens. Endogenous self antigens that are membrane-bound or secreted will intersect with GILT and the MHC class II loading compartment directly. Endogenous self antigens that are intracellular may use autophagy to intersect GILT and the MHC class II loading compartment. Exogenous self-antigens that are endocytosed access GILT and the MHC class II loading compartment. Thus, GILT is likely involved in shaping central tolerance to many self antigens.
A more detailed understanding of the antigen processing pathways used in the thymus not only increases the knowledge of requirements for the generation of the T cell repertoire, but also facilitates manipulation of thymic selection to alter T cell function. The newly developed T cell repertoire in bone marrow chimeras lacking GILT in both thymic stromal and hematopoietic cells exhibits 1) enhanced development of T cells recognizing a GILT-dependent MHC class II-restricted self and tumor antigen, 2) an increased Tconv:Treg cell ratio, and 3) enhanced protection from melanoma tumor growth. In other studies, Aire deficiency or mTEC depletion have been used in mouse models to rescue TRP1-specific T cells from thymic deletion leading to enhanced T cell-mediated anti-melanoma immunity (35, 36, 48). An improved understanding of antigen processing pathways in T cell tolerance may assist the development of novel therapeutic approaches in autoimmunity and cancer.
This study demonstrates a significant role for GILT in self antigen presentation in the thymus, especially in TECs, which improves MHC class II-restricted processing, enhances central T cell tolerance, and restricts the function of T cells recognizing a melanoma-associated self antigen.
Supplementary Material
Key Points.
GILT expression in thymic APCs enhances the negative selection of CD4+ T cells.
GILT facilitates MHC class II-restricted presentation by thymic APCs.
GILT expression in mTECs preferentially facilitates negative selection.
Acknowledgments
This work was supported by institutional start-up funds (to K.T.H.) and the National Institutes of Health (NIH) Cancer Biology Training Grant T32 CA09213 (to M.P.R. and L.R.M.).
References
- 1.Klein L, Kyewski B, Allen PM, and Hogquist KA. 2014. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 14: 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, and Mathis D. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science 298: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 3.Gray D, Abramson J, Benoist C, and Mathis D. 2007. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med 204: 2521–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, and Savage PA. 2016. Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity 44: 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koble C, and Kyewski B. 2009. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med 206: 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millet V, Naquet P, and Guinamard RR. 2008. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur J Immunol 38: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 7.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, and Bhardwaj N. 1998. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 188: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amigorena S, and Savina A. 2010. Intracellular mechanisms of antigen cross presentation in dendritic cells. Current opinion in immunology 22: 109–117. [DOI] [PubMed] [Google Scholar]
- 9.Harshyne LA, Watkins SC, Gambotto A, and Barratt-Boyes SM. 2001. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol 166: 3717–3723. [DOI] [PubMed] [Google Scholar]
- 10.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, and Cai Z. 1999. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 286: 952–954. [DOI] [PubMed] [Google Scholar]
- 11.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, and Neefjes J. 2005. Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434: 83–88. [DOI] [PubMed] [Google Scholar]
- 12.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, and Klein L. 2007. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8: 351–358. [DOI] [PubMed] [Google Scholar]
- 13.Aichinger M, Wu C, Nedjic J, and Klein L. 2013. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J Exp Med 210: 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, and Klein L. 2010. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol 11: 512–519. [DOI] [PubMed] [Google Scholar]
- 15.Gallegos AM, and Bevan MJ. 2004. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med 200: 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, and Anderson MS. 2012. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci U S A 109: 7847–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, and Heath WR. 2011. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 118: 2462–2472. [DOI] [PubMed] [Google Scholar]
- 18.Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, and Hsieh CS. 2014. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 41: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arunachalam B, Phan UT, Geuze HJ, and Cresswell P. 2000. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc Natl Acad Sci U S A 97: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phan UT, Arunachalam B, and Cresswell P. 2000. Gamma-interferon-inducible lysosomal thiol reductase (GILT). Maturation, activity, and mechanism of action. J Biol Chem 275: 25907–25914. [DOI] [PubMed] [Google Scholar]
- 21.Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, Phan UT, Maric M, Cresswell P, and Blum JS. 2002. Absence of g-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J Exp Med 195: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, Alfonso C, Karlsson L, Flavell RA, and Cresswell P. 2001. Defective antigen processing in GILT-free mice. Science 294: 1361–1365. [DOI] [PubMed] [Google Scholar]
- 23.Rausch MP, Irvine KR, Antony PA, Restifo NP, Cresswell P, and Hastings KT. 2010. GILT accelerates autoimmunity to the melanoma antigen tyrosinase-related protein 1. J Immunol 185: 2828–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hastings KT, Lackman RL, and Cresswell P. 2006. Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing. J Immunol 177: 8569–8577. [DOI] [PubMed] [Google Scholar]
- 25.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, and Restifo NP. 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rausch MP, and Hastings KT. 2012. GILT Modulates CD4(+) T-Cell Tolerance to the Melanocyte Differentiation Antigen Tyrosinase-Related Protein 1. J Invest Dermatol 132: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, and Anderson MS. 2008. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barjaktarevic I, Rahman A, Radoja S, Bogunovic B, Vollmer A, Vukmanovic S, and Maric M. 2006. Inhibitory Role of IFN-{gamma}-Inducible Lysosomal Thiol Reductase in T Cell Activation. J Immunol 177: 4369–4375. [DOI] [PubMed] [Google Scholar]
- 29.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, and Love PE. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med 188: 2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, Boyd RL, and Takahama Y. 2006. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity 24: 165–177. [DOI] [PubMed] [Google Scholar]
- 31.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, and Takahama Y. 2004. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med 200: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, and Anderson MS. 2006. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med 203: 2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeVoss JJ, LeClair NP, Hou Y, Grewal NK, Johannes KP, Lu W, Yang T, Meagher C, Fong L, Strauss EC, and Anderson MS. 2010. An autoimmune response to odorant binding protein 1a is associated with dry eye in the Aire-deficient mouse. J Immunol 184: 4236–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shum AK, DeVoss J, Tan CL, Hou Y, Johannes K, O’Gorman CS, Jones KD, Sochett EB, Fong L, and Anderson MS. 2009. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Science translational medicine 1: 9ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu ML, Nagavalli A, and Su MA. 2013. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res 73: 2104–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan IS, Mouchess ML, Zhu ML, Conley B, Fasano KJ, Hou Y, Fong L, Su MA, and Anderson MS. 2014. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med 211: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landegren N, Sharon D, Shum AK, Khan IS, Fasano KJ, Hallgren A, Kampf C, Freyhult E, Ardesjo-Lundgren B, Alimohammadi M, Rathsman S, Ludvigsson JF, Lundh D, Motrich R, Rivero V, Fong L, Giwercman A, Gustafsson J, Perheentupa J, Husebye ES, Anderson MS, Snyder M, and Kampe O. 2015. Transglutaminase 4 as a prostate autoantigen in male subfertility. Science translational medicine 7: 292ra101. [DOI] [PubMed] [Google Scholar]
- 38.Rausch MP, and Hastings KT. 2015. An exhaustion-like phenotype constrains the activity of CD4+ T cells specific for a self and melanoma antigen. PLoS One 10: e0123332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, Fulton A, Tamada K, Strome SE, and Antony PA. 2013. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol 190: 4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen SM, Twitty CG, Maston LD, Antony PA, Lim M, Hu HM, Petrausch U, Restifo NP, and Fox BA. 2012. Increased frequency of suppressive regulatory T cells and T cell-mediated antigen loss results in murine melanoma recurrence. J Immunol 189: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, and Allison JP. 2010. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, and Savage PA. 2013. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, and Antony PA. 2010. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 207: 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, Najfeld V, Phelps RG, Grosskreutz C, Scigliano E, Frenette PS, and Merad M. 2006. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med 203: 2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, and Engleman EG. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 3: 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derbinski J, Pinto S, Rosch S, Hexel K, and Kyewski B. 2008. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci U S A 105: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sitaram A, and Marks MS. 2012. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology 27: 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakhru P, Zhu ML, Wang HH, Hong LK, Khan I, Mouchess M, Gulati AS, Starmer J, Hou Y, Sailer D, Lee S, Zhao F, Kirkwood JM, Moschos S, Fong L, Anderson MS, and Su MA. 2017. Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity. JCI insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


