Abstract
Induced responses to above-ground and below-ground herbivores may interact via systemic signalling in plants. We investigated whether the impact of above-ground herbivory on root-knot nematode-induced responses depends on the nematode’s life cycle stages. Tomato plants were infected with the nematode (Meloidogyne incognita) for 5, 15 or 30 days before receiving Spodoptera exigua caterpillars above-ground. We collected root materials after 24 h of caterpillar feeding. We investigated phytohormones and α-tomatine levels, and the expression of defence and glycoalkaloid metabolism (GAME) marker genes in tomato roots. Nematode infection alone increased the endogenous root levels of jasmonic acid (JA), salicylic acid (SA), abscisic acid (ABA), α-tomatine and the expression of the GLYCOALKALOID METABOLISM 1 (GAME1) gene mostly at 30 days post-nematode inoculation. Caterpillar feeding alone upregulated Lipoxygenase D and downregulated Basic-β-1-glucanase and GAME1 expression in roots. On nematode-infected plants, caterpillar feeding decreased JA levels, but it increased the expression of Leucine aminopeptidase A. The induction patterns of ABA and SA suggest that caterpillars cause cross-talk between the JA-signalling pathway and the SA and ABA pathways. Our results show that caterpillar feeding attenuated the induction of the JA pathway triggered by nematodes, mostly in the nematodes’ reproduction stage. These results generate a better understanding of the molecular and chemical mechanisms underlying frequent nematode–plant–caterpillar interactions in natural and agricultural ecosystems.
Keywords: Above-ground–below-ground interaction, phytohormones, root-knot nematodes, Solanum lycopersicum, Spodoptera exigua, steroidal glycoalkaloids, systemic-induced responses
Root-knot nematodes are plant parasites that damage the roots. During their infection process in roots, the plants can be attacked on leaves by insects, e.g. caterpillars. Interestingly, the caterpillars might arrive on and feed on the plants when the root nematode is at different stages of its infection cycle. We investigated how the caterpillar might affect root defence responses triggered by root nematode at different stages of its life cycle. We demonstrated that caterpillar feeding on the nematode-infected plants decreases the jasmonic acid signalling when the nematode is at the reproduction stage. More studies are needed to progress this understudied field.
Introduction
Tomato is ranked the most consumed vegetable globally, with >170.8 million tons produced in 2017 alone (Omondi 2018; FAO 2019). This yield is ~30 % times more than a decade earlier (Oishimaya 2017). Like other crops, tomato plants experience high pest pressure by, e.g., nematodes, arthropods, bacterial and fungal pathogens. This pest pressure reduces the growth and limits tomato yield (Kumar et al. 2016; Garcia et al. 2018; van Dam et al. 2018). Root-knot nematodes (RKNs) are globally occurring, soil-borne pathogens that attack plants at their roots. The infective second-stage juveniles (J2s) hatch in the soil, where they locate and infect the roots of a susceptible host. Upon penetrating the roots, the J2s migrate intercellularly until they reach the vascular tissues. There they establish their permanent feeding sites (Niebel et al. 1994; Williamson and Gleason 2003; Gheysen and Mitchum 2011). Their infection impairs the translocation of water and minerals from the roots to the shoots, which can limit the plant’s productivity and fitness (Abad et al. 2008; Jones et al. 2013). At the same time, above-ground (AG) herbivores, such as leaf-chewing caterpillars, may be present on the plant. The leaf loss due to caterpillar feeding can adversely impact on primary plant processes, such as the rate of photosynthesis, which are directly related to the plant’s productivity (Meyer and Whitlow 1992; Mitchell et al. 2016). Together the damage caused by RKN and herbivorous insects can reduce crop production by ~20 % annually, making them agro-economically important crop pests (Karajeh 2008; van der Meijden 2015; Mitchell et al. 2016). Commonly, chemical pesticides are used to control crop pests, such as nematodes and insect herbivores. Although these pesticides might be effective, several of them are currently banned from use due to their detrimental effects on human health and the environment (Franco et al. 2015; Borel 2017). Efforts to identify natural plant resistance traits for AG and below-ground (BG) herbivores may help to develop sustainable pest management strategies.
Plants rely on constitutive and inducible defence responses to protect themselves against attackers. Constitutive responses are described as the physical barriers, such as thorns and trichomes, and chemical traits, such as alkaloids and glucosinolates, usually expressed independently of herbivore or pathogen attack (Wittstock and Gershenzon 2002). Induced defences are stimulated by herbivore feeding or pathogen attack, which results in the induction of specific plant phenotypic responses (Karban 2011; Boots and Best 2018). In addition, plants can tolerate herbivory via the re-allocation of resources to undamaged plant parts, followed by compensatory growth, or by increasing the rate of photosynthesis (Mauricio et al. 1997; Peterson et al. 1998; Retuerto et al. 2004; Boege et al. 2007; Núñez-Farfán et al. 2007; Fornoni 2011; Koch et al. 2016; Mitchell et al. 2016). These changes influence critical plant physiological processes and can adversely impact the performance of herbivores.
Plant hormonal signalling governs herbivore-induced defence responses. Among the many plant hormones described so far, jasmonic acid (JA), salicylic acid (SA), ethylene (ET) and abscisic acid (ABA) are the main signalling hormones that fine-tune plant defence responses upon attack (Pieterse et al. 2009, 2012). Interaction, or cross-talk, between phytohormonal pathways, results in specific defence responses, which tailor the defensive response to the particular attacker (Pieterse et al. 2009, 2012; Li et al. 2019). Induction of defence responses at the site of attack often results in systemic signalling to distal non-attacked plant parts, thereby protecting them against future attacks (Martínez-Medina et al. 2013; van Dam et al. 2018). Moreover, systemic-induced responses may influence the attraction, behaviour and performance of other organisms sharing the same host (Bruce 2014). As a consequence, induced responses play an essential role in indirect interactions between AG and BG herbivores feeding on the same plant (van Dam and Heil 2011).
Most studies investigating plant-mediated interactions between AG and BG herbivores focus on how AG herbivore-induced responses are affected by BG herbivory (Erb et al. 2009; Kumar et al. 2016; Arce et al. 2017; Hoysted et al. 2017; van Dam et al. 2018). Only a few studies analysed how AG-induced responses affect BG-feeding herbivores or pathogens. These studies report that AG herbivory induces systemic responses in the roots of crops (e.g., potato, tomato) and grass species (Kafle et al. 2017; Wang et al. 2017; Hoysted et al. 2018). Both primary and secondary metabolites play a role in plant-mediated interactions between AG and BG insect herbivores. For example, AG feeding by aphids changes potato root exudates by reducing amounts of glucose and fructose, which diminish cyst hatching (Hoysted et al. 2018). Defoliation by clipping increases nitrogen concentration in roots, which in return increases the total abundance of two species of root-feeding nematodes (Wang et al. 2017). Similarly, AG feeding by Manduca sexta on Nicotiana attenuata induces jasmonate-dependent facilitation of plant-parasitic nematode (PPN) abundance in the field, and RKN (Meloidogyne incognita) reproduction in a greenhouse (Machado et al. 2018). Collectively, these studies demonstrate that plant responses induced by AG herbivory can systemically affect BG defence responses.
The few studies available show that systemic-induced responses triggered by AG herbivory cause different effects on root feeders (Huang et al. 2013; Kafle et al. 2017; Wang et al. 2017; Hoysted et al. 2018; Machado et al. 2018). Partly the differences in the observed interaction outcomes are due to variation in the timing and sequence of arrival of both AG- and BG-feeding organisms (Erb et al. 2011; Kafle et al. 2017; Wang et al. 2017). In nature, root herbivores commonly colonize the plant before shoot herbivores arrive. This natural sequence of pest arrival follows from the fact that roots develop first (Bezemer and van Dam 2005). For PPNs, such as RKNs, these factors are particularly relevant. As obligate root feeders, RKNs undergo different distinct life cycle stages. In the invasion stage, J2s enter the root at the zone of elongation and move towards the vascular cylinder. Then they turn around and move several body lengths upwards before settling and initiating feeding (Robinson and Perry 2006). This movement occurs between the cells (intercellularly), which also reduces the elicitation of defence responses because only a few cells are damaged (Caillaud et al. 2008; Gheysen and Mitchum 2011). In the establishment stage, the juveniles become sedentary and inject various effectors to establish the so-called ‘giant cell’. This giant cell serves as their feeding site. The cells surrounding the giant cells undergo proliferation and enlargement, and, in due time, they become visible to the human eye as a gall or a ‘root-knot’ (Rodiuc et al. 2014; Escobar et al. 2015). We refer to this stage, in which the nematode establishes a feeding site, as the galling stage. At their feeding site, the nematodes acquire resources and develop through three molts to mature and reach the reproduction stage. The female nematode’s body swells up and becomes pear-shaped. When the eggs are ripe, the females release their eggs into the rhizosphere, and another cycle begins (Caillaud et al. 2008; Gheysen and Mitchum 2011). In each infection stage, the nematodes’ growth and development depend on the injection of different effectors into the host cells (Quentin et al. 2013; Favery et al. 2016; Gheysen and Mitchum 2019). These effectors trigger different hormonal signalling pathways, including JA, SA, ET and ABA (Caillaud et al. 2008; Kyndt et al. 2017; Gheysen and Mitchum 2019). Because hormones are generally involved in plant defence induction, systemic defence responses induced by AG herbivores might affect nematodes and the local responses they induce in the roots. Moreover, the effect that AG herbivores may have on BG defence signalling triggered by root herbivores may depend on the life cycle stage in which the nematodes are at the time point of AG attack.
Here, we used tomato (Solanum lycopersicum ‘Moneymaker’) and two generalist crop pests, the RKN M. incognita and larvae of Spodoptera exigua, as the study system to analyse the molecular mechanisms mediating interactions between AG herbivores and nematodes. Previous studies showed that interactions between RKN and shoot herbivores can be governed by JA-dependent responses, evidenced by changes in jasmonates levels in N. attenuata (Machado et al. 2018) and the production of trypsin protease inhibitors in tomato (Arce et al. 2017). These interactions may also involve cross-talk between hormonal pathways, such as JA–SA (van Dam et al. 2018) and JA–ABA (Erb et al. 2009; Kyndt et al. 2017). Therefore, we measured phytohormone concentrations (JA, SA, ABA) and the expression of several marker genes for hormonal signalling; Lipoxygenase D and Leucine aminopeptidase A (JA markers), Le4 (ABA marker) and Basic-β-1,3-glucanase (GluB) (ET marker) in roots [seeSupporting Information—Table S1]. Tomato is also known to produce steroidal glycoalkaloids, such as α-tomatine, as a defence to generalist herbivores (Friedman 2002; Cárdenas et al. 2015). Hence, we included measurements of α-tomatine and the expression of glycoalkaloid metabolism (GAME) genes Jasmonate-responsive Ethylene Response Factor 4 (JRE4) and GAME1. We specifically analysed how 24 h of AG feeding affected these defence-related traits in roots that were infected with M. incognita at 5, 15 and 30 days post-nematode inoculation (dpi). These time points coincide with the invasion (5 dpi), galling (15 dpi) and reproduction (30 dpi) stages of this nematode. With this approach, we aimed to assess whether the nature of the interaction between shoot- and root-induced responses depends on the developmental stage of the RKN.
Materials and Methods
Study plant, root and shoot organisms
In all our experiments, we used tomato (S. lycopersicum ‘Moneymaker’) as the model plant. The RKN M. incognita was used as root herbivore, and the larvae of the generalist herbivore S. exigua were used as shoot herbivores. We obtained M. incognita eggs from Rijk Zwaan (De Lier, The Netherlands) and maintained a glasshouse stock on tomato ‘Moneymaker’ for 8 weeks. Similar to a previous study (Martínez-Medina et al. 2017), we initiated the colony from a single egg mass, and 8 weeks later extracted eggs for use in the bioassay. We purchased S. exigua eggs from Entocare C.V. Biologische Gewasbescherming (Wageningen, The Netherlands) and maintained a laboratory colony on artificial diet, in a growth chamber set at 25 °C constant, 12-h photoperiod and 45 % relative humidity (RH).
Plant growth condition and herbivores infection
The tomato seeds were obtained from Intratuin B.V (Woerden, The Netherlands). Before germination, the seeds were surface-sterilized by immersion in 40 mL of 10 % sodium hypochlorite solution for 4 min. Afterward, the seeds were rinsed four times with water. Each round of rinsing was for 10 min. The sterilized seeds were placed on moistened glass beads and allowed to germinate at 27 °C in the dark for 3 days, followed by 4 days in a plant growth chamber (CLF PlantClimatic, CLF PlantClimatics GmbH, Wertingen, Germany). The growth conditions were 16-h:8-h day:night cycle, 55 % RH and 60 % (65 μmol s−1 m−2) light intensity. One-week-old seedlings were transplanted into sterilized 1:1 sand:soil mixture in 11 × 11 × 12 cm pots. They were grown in a glasshouse at 26 ± 3 °C:23 ± 3 °C day:night, with 16-h:8-h light:dark and RH was maintained at ~30 %. The plants were watered as required and supplemented weekly with 50 % strength Hoagland solution. The plants were grown for three more weeks before using them in bioassays. We randomly selected healthy plants of similar size and appearance for our experimental treatments. We divided the plants into two groups; one group was inoculated with M. incognita eggs (3000 eggs per mL), and the other group was mock-inoculated with water. In the M. incognita-inoculated plants, we set three time points to coincide with the main nematode life cycle stages. These were 5 dpi (invasion stage), 15 dpi (galling stage) and 30 dpi (reproduction stage). At each of these time points, plants were subjected to four different treatments, each with 10 biological replicates. The treatments were control (plants without herbivores or nematodes); BG infection (plants challenged with M. incognita); AG herbivory (plants challenged with S. exigua); and both BG infection and AG herbivory (plant challenged with M. incognita in the roots followed by S. exigua feeding on leaves). We infested the plants assigned to leaf feeding with one second-instar S. exigua caterpillar. The S. exigua caterpillars were confined to a 7-cm (diameter) round clip cage placed on one fully expanded leaf close to the tip (see Fig. 4D in Bandoly and Steppuhn (2016)). In plants without shoot herbivory, an empty clip cage was mounted on a leaf at a similar position to the one used in plants with shoot herbivory. The S. exigua larvae were allowed to feed for 24 h. Other studies showed that this time period suffices to affect defence metabolites and genes in roots. For example, 24 h of AG herbivory by M. sexta and Spodoptera littoralis on N. attenuata results in systemic induction of JA-related genes expression in roots (Fragoso et al. 2014). After this time, we harvested the roots by gently removing them from the pots. The soil was removed by soaking the whole root into a bucket filled with tap water. Then the roots were rinsed with running tap water and dried with filter paper. After quickly counting the number of galls (especially for roots collected at the galling and reproduction stages) [seeSupporting Information—Fig. S1], the roots were wrapped in clean labelled aluminium foil, and immediately shock-frozen in liquid nitrogen. The root samples were stored at –80 °C, pending molecular and metabolite analyses.
Quantitative reverse transcription-polymerase chain reaction analysis
Total RNA was extracted from ~100 mg fresh weight per root sample according to the method described by Oñate-Sánchez and Vicente-Carbajosa (2008). First-strand cDNA was synthesized from 1 µg DNase-free mRNA using Revert Aid H-minus RT (Thermo Fisher Scientific Baltic UAB, Vilnius, Lithuania) following the manufacturer’s instructions. Real-time qPCR reactions and relative quantification of specific mRNA levels were performed according to Martínez-Medina et al. (2017) by using a CFX 384 Real-Time PCR system (Bio-Rad Laboratories Inc., Singapore) and the gene-specific primers described in Supporting Information—Table S1. These genes were selected from previously published articles where their involvement in tomato biotic interactions is reported (Uppalapati et al. 2005; Martínez-Medina et al. 2013; Yan et al. 2013; Abdelkareem et al. 2017). The data were normalized using the housekeeping gene (SIEF X14449), which encodes for the tomato elongation factor-1α, a commonly used and stable reference gene for data normalization in studies on induced responses in tomato (Miranda et al. 2013; Martínez-Medina et al. 2017). Data were analysed by the 2−∆∆ct method (Livak and Schmittgen 2001).
Determination of phytohormone concentration
We extracted and quantified phytohormones following the protocol described by Machado et al. (2013). In brief, ~100 mg fresh weight per root sample was extracted with 1 mL ethyl acetate containing 40 ng of each of the following internal phytohormone standards: D6-JA and D6-SA, and D6-ABA. The extracts were vortexed for 10 min using a Thermomixer, then centrifuged at 15 000 × g, 4 °C for 20 min, the supernatants were transferred to a new tube and evaporated to dryness at room temperature using a SpeedVac (Labconco Co-operation, Kansas, MO, USA). Remaining pellets were resuspended in 200 µL methanol:water (70:30) using an ultrasonic bath for 5 min and centrifuged at 15 000 × g, 4 °C for 5 min. The supernatant was collected for phytohormone measurement using liquid chromatography (Bruker Advance UHPLC, Bremen, Germany) coupled to a mass spectrometer (Bruker Elite EvoQ Triple quadrupole, Bremen, Germany) (LC/MS EVOQ) (Schäfer et al. 2016). The separation was achieved on a Zorbax Eclipse XDB-C18 column (4.6 × 50 mm, 1.8 µm, 80 Å, Agilent technologies, Santa Clara, CA, USA). Mobile phase was composed of A (0.05 % (v/v) aqueous formic acid) and B (0.05 % (v/v) formic acid in 100 % acetonitrile). The following gradient was used: 0–0.5 min, 5 % B; 0.5–0.6 min, 5–50 % B; 0.6–2.5 min, 50–100 % B; 2.5–3.5 min, 100 % B; 3.5–3.55 min, 100–5 % B; 3.55–4.5 min, 5 % B at flow rate of 400 µL min−1. All solvents used were LC-MS grade. The column temperature was kept constant at 42 °C.
After separation, the compounds were nebulized by electron spray ionization in the negative mode using the following conditions: capillary voltage 4500 eV, cone gas 35 arbitrary units/350 °C, probe gas 60 arbitrary units/475 °C and nebulizing gas at 60 arbitrary units. The phytohormones were identified based on their retention time and the monitored mass to charge ratio (m/z) transition. The m/z ratio of the phytohormones of interest were; (m/z) 209.12 → 59.00 for JA; (m/z) 263.13 → 153.00 for ABA and (m/z) 137.02 → 93.00 for SA. Samples were analysed in a randomized sequence with acetonitrile samples in between as background controls. Data acquisition and processing were performed using the ‘MS data Review’ software (Bruker MS Workstation, version 8.2). Phytohormone levels were calculated based on the peak area of the corresponding internal standard and the amount of fresh mass of plant material (ng−1 mg−1 fresh weight).
Determination of the root α-tomatine concentrations
We extracted ~100 mg fresh weight of each root sample in a 2-mL Eppendorf tube with 1 mL solution containing 25 % of acetate buffer (2.3 mL acetic acid, 3.41 mg ammonium acetate dissolved in 1 L of Milli pure water, pH 4.8) and 75 % methanol. Tubes with extracts were inverted for 10 s and thoroughly mixed via shaking using a grinding ball mill (MM400, Retsch GmbH, Leipzig, Germany) set at 30 Hz for 5 min. To remove the solid particles in the extracts, we centrifuged them at 15 000 × g for 15 min, and the supernatant transferred into a new 2-mL Eppendorf tube, and the pellet was re-extracted as above. We mixed the first and second supernatant and transferred 200 µL of the combined extracts into a 2-mL HPLC vial and added 800 µL of the extraction buffer to obtain a 1:5 dilution for each sample. The extracts were stored at −20 °C, pending further analysis. Metabolites were characterized by injecting 1 µL of the extracts in a UPLC (Dionex 3000, Thermo Scientific). The chromatograph was equipped with a C18 column (Acclaim TM RSLC 120), 2.1 × 150 mm external dimension, 2.2 µm particle size and 120 Å pore size. The column was kept at 40 °C. The mobile phases (LC-MS grade solvents) were composed of solvent A: 0.05 % (v/v) aqueous formic acid and solvent B: 0.05 % (v/v) formic acid in acetonitrile. The multi-step gradient for solvent B was; 0–1 min 5 %, 1–4 min 28 %, 4–10 min 36 %, 10–12 min 95 %, 12–14 min 95 %, 14–16 min 5 %, 16–18 min 5 %. The flow was set to 400 µL min−1. We detected compounds using a maXis impact HD MS-qToF (Bruker Daltonics). Data were acquired in positive mode. Electron Spray Ionisation ion source conditions were; endplate offset = 500 V, capillary = 4500 V, nebulizer = 2.5 bar, dry gas = 11 L min−1, dry temperature = 220 °C. Transfer line conditions were: funnels 1 and 2 = RF 300 Vpp, isCID energy = 0 eV, hexapole = 60 Vpp, quadrupole ion energy = 5 eV, low mass = 50 m/z, collision cell energy = 10 eV, collision RF 500 Vpp, transfer time = 60 µs, pre-pulse storage = 5 µs. The mass spectrometer operated with a mass range of 50–1500 m/z and a spectral acquisition rate of 3 Hz. Sodium formate clusters (10 mM) were used for calibrating the m/z values. These sodium formate clusters were a mix consisting of 250 mL isopropanol, 1 mL formic acid, 5 mL 1 M NaOH and the final volume was adjusted to 500 mL. All analyses had a quality control sample, which was a pool of all the different experimental groups and time points. The quality control sample was analysed at the beginning and the end of the batch and after every 10 injections. The raw data files were processed using the program Compass DataAnalysis (Bruker Daltonics). The processing involved obtaining the extracted ion chromatogram (EIC) for a fragment of α-tomatine at the m/z value 578.4050 and m/z tolerance of ±0.1. We selected the option compound list to automatically calculate the peak areas of each EIC per sample per study time point. All the peak areas for α-tomatine were tabulated and used for multivariate statistical analysis.
Statistical analysis
We created two data sets combining (i) phytohormone and α-tomatine levels, and (ii) defence markers and glycoalkaloid metabolism genes. In each combined data set, we tested the effects of M. incognita (Mi; with vs. without), and S. exigua (Se; with vs. without), and their interactions on the defence variables (i.e. the plant defence traits; phytohormone, α-tomatine and marker genes). Each data set was analysed using the permutational multivariate analysis of variance (PERMANOVA). Permutational multivariate analysis of variance was chosen because our data lacked homogeneity of variance or normal distribution; PERMANOVA does not require this because it uses a distribution-free permutation approach to partition the variance among treatments (Anderson 2017). The PERMANOVA analysis was run for each data set using the Adonis function, with the Gower dissimilarities method among samples, and 999 permutations in R v 3.6.1 software (R Core Development Team 2019). Where the PERMANOVA output showed significant effects for either factor or their interaction [seeSupporting Information—Tables S2andS4], we performed separate factorial linear model ANOVAs on each dependent variable, with M. incognita and S. exigua and their interaction as fixed factors. Once the main effect significantly affected any of the dependent variables, the differences among the four experimental treatments were tested using Tukey’s Honest Significant Difference test for multiple comparisons.
Results
Root infection by M. incognita alone affects the expression of root-inducible defences at different life cycle stages
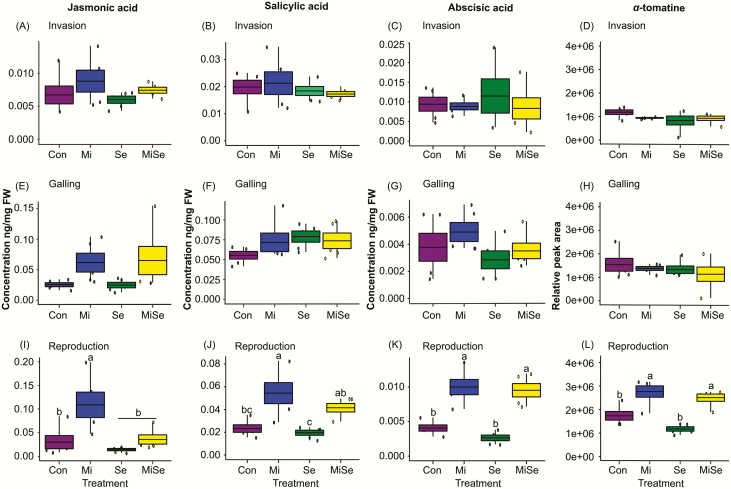
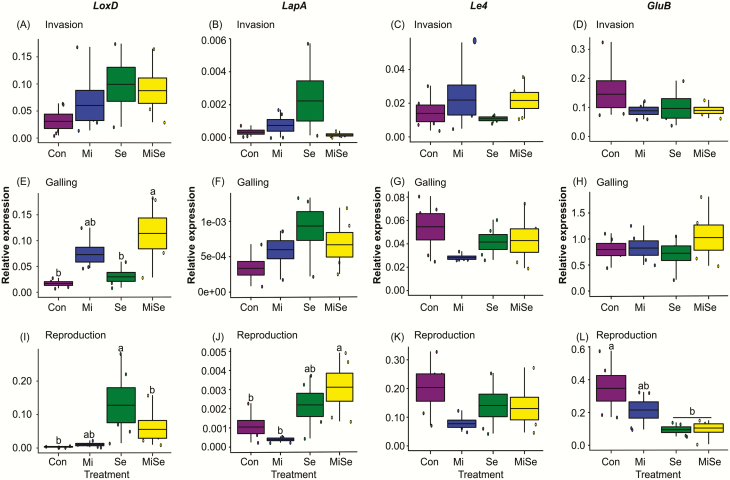
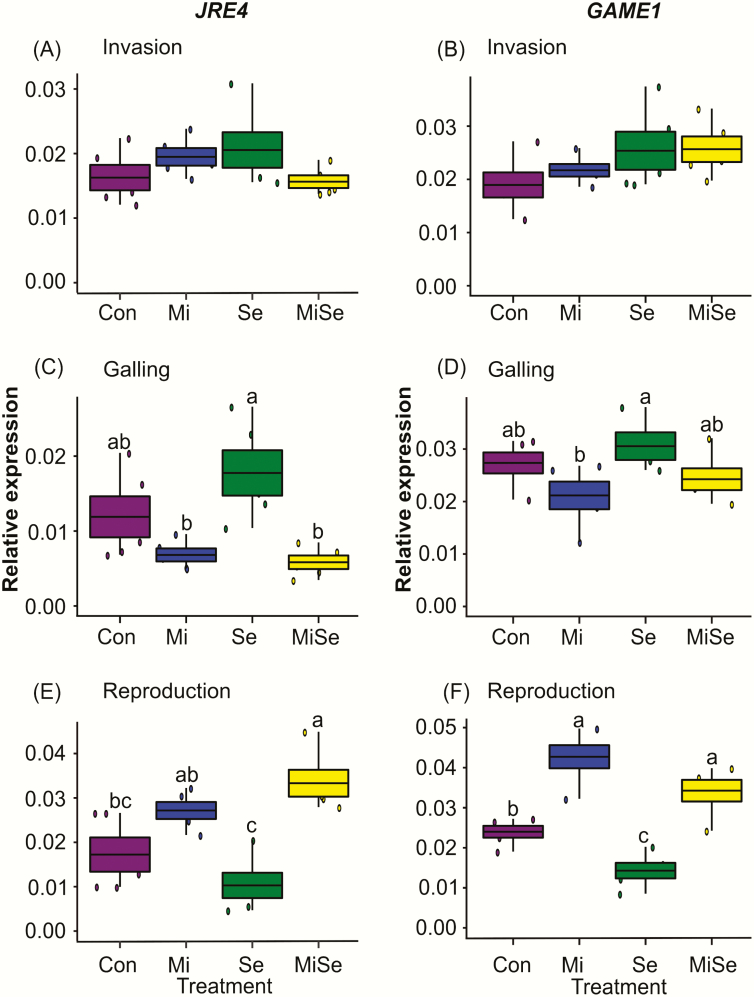
We first considered how the nematode affected root-inducible defences at the invasion, galling and reproduction stages. We found that M. incognita root infection enhanced the induction of JA, SA, ABA and α-tomatine progressively during the infection process. In particular, the JA response in M. incognita-infected plants became more pronounced with the progression of the nematode’s life cycle compared to controls (Fig. 1A, E and I, blue box plots). At both the invasion and galling stages, the levels of these signalling hormones were increased, but only at the reproduction stage, the increases became significant compared to control plants (Fig. 1I–L, blue box plots; seeSupporting Information—Table S3). In contrast, root infection by M. incognita did not trigger changes in the expression of the defence marker genes. We found that the expression of LoxD, LapA, Le4, GluB (Fig. 2, blue box plots; seeSupporting Information—Table S5) and JRE4 (Fig. 3, blue box plots; seeSupporting Information—Table S5) remained similar to those observed in control plants regardless of the nematodes’ root infection stage. However, we observed significant upregulation in the expression of the GAME1 transcripts at the nematodes’ reproduction stage compared to control plants (Fig. 3F, blue box plot; seeSupporting Information—Table S5). The increase in GAME1 transcripts correlated with an increase in α-tomatine concentrations in nematode-infected roots at the reproduction stage (Fig. 1L, blue box plot).
Figure 1.
Phytohormone concentrations and relative peak area of α-tomatine. Mean concentrations (ng mg−1 fresh weight) of phytohormones and the relative peak area of α-tomatine in tomato plants infected with Meloidogyne incognita (Mi), infested with Spodoptera exigua (Se) or both (MiSe). Con = plant without herbivory. Box plots are the mean (±SEM) of jasmonic acid (A, E, I); salicylic acid (B, F, J); abscisic acid (C, G, K); α-tomatine (D, H, L) per treatment (n = 5) measured at the nematodes’ invasion (A–D), galling (E–H) and reproduction (I–L) stages. Different lower-case letters above the box plots indicate significant differences in mean values between treatments, determined via multiple comparisons Tukey’s HSD test after ANOVA at P ≤ 0.05.
Figure 2.
Expression of defence marker genes. Relative expression of defence marker genes in tomato plants infected with Meloidogyne incognita (Mi), infested with Spodoptera exigua (Se) or both (MiSe). Con = plants without herbivory. Expression values are normalized over the expression of the housekeeping gene (SIEF X14449) encoding for tomato elongation factor-1α. Box plots are mean (±SEM) expression values of Lipoxygenase D (LoxD); Leucine aminopeptidase A (LapA); abscisic acid-responsive Le4 (Le4); Basic-β-1-glucanase (GluB) per treatment (n = 5) measured at the nematodes’ invasion (A–D), galling (E–H) and reproduction (I–L) stages, respectively. Different lower-case letters above the box plots indicate significant differences in mean expression among treatments, determined via multiple comparisons Tukey’s HSD test after ANOVA at P ≤ 0.05.
Figure 3.
Expression of steroidal glycoalkaloid metabolism genes. Relative expression of steroidal glycoalkaloid metabolism genes in tomato plants infected with Meloidogyne incognita (Mi), infested with Spodoptera exigua (Se) or both (MiSe). Con = plants without herbivory. Expression values are normalized over the expression of the housekeeping gene (SIEF X14449) encoding for tomato elongation factor-1α. Box plots are mean (±SEM) expression values of jasmonate-responsive ETHYLENE RESPONSE FACTOR 4 (JRE4; A, C, E); and glycoalkaloid metabolism 1 (GAME1; B, D, F) per treatment (n = 5) measured at the nematodes’ invasion (A and B), galling (C and D) and reproduction (E and F) stages, respectively. Different lower-case letters above the box plots indicate significant differences in mean expression among treatments, determined via multiple comparisons Tukey’s HSD test after ANOVA at P ≤ 0.05.
The impact of S. exigua feeding on root defence responses in tomato plants depends on plant age
Next, we analysed the impact of S. exigua leaf herbivory on root defences of tomato plants without nematode infection. Due to the experimental set-up, which was designed based on the life stages of the nematodes, the plants that received only caterpillars were 4.8 (coinciding with the invasion stage), 6.2 (coinciding with the galling stage) and 8 (coinciding with reproduction stage) weeks old. We found that S. exigua leaf herbivory did not affect the levels of JA, SA, ABA and α-tomatine in tomato roots compared to the control plants, regardless of plant age (Fig. 1, green box plots; seeSupporting Information—Table S3). In contrast, S. exigua herbivory triggered differential effects on the expression of the hormonal signalling and GAME marker genes (Figs 2 and 3, green box plots; seeSupporting Information—Table S5). In the 4.8 (invasion stage) and 6.2 (galling stage) weeks old plants, the expression of the marker genes was not significantly different from controls (Figs 2A–H and 3A–D, green box plots; seeSupporting Information—Table S5). Notably, when the plants were 8 weeks old, which coincided with the nematodes’ reproduction stage, the defence gene LoxD was upregulated compared to controls (Fig. 2I, green box plot; seeSupporting Information—Table S5). The LapA and Le4 expression levels were not significantly different compared to controls (Fig. 2J and K, green box plots; seeSupporting Information—Table S5), while GluB was significantly downregulated compared to controls (Fig. 2L, green box plot; seeSupporting Information—Table S5). The GAME gene JRE4 was not affected while the GAME1 was significantly downregulated compared to controls (Fig. 3E and F, green box plots; seeSupporting Information—Table S5).
Effects of S. exigua on M. incognita-induced responses depend on the nematodes’ infection stage
Because our primary interest was to analyse the effect of S. exigua AG feeding on root responses induced by M. incognita at different infection stages, we primarily focused on the comparison between M. incognita-infected plants (Mi treatment, blue box plots, Figs 1–3) with the double-infected plants (MiSe treatment, yellow box plots, Figs 1–3). We found that S. exigua herbivory on M. incognita-infected plants did not change JA levels at the invasion and galling stages compared to plants challenged with M. incognita alone (Fig. 1A and E, yellow box plots; seeSupporting Information—Table S3). Spodoptera exigua herbivory on the M. incognita-infected plants significantly decreased the JA levels at the nematodes’ reproduction stage compared to plants infected with M. incognita alone (Fig. 1I, yellow box plot; seeSupporting Information—Table S3). Spodoptera exigua feeding on M. incognita-infected plants did not affect SA, ABA and α-tomatine concentrations compared to plants challenged with M. incognita alone, regardless of the nematodes’ infection stage (Fig. 1B–D, F–H and J–L, yellow box plots; seeSupporting Information—Table S3). Overall, we observed that the local nematode-induced responses dominated the nature of SA, ABA and glycoalkaloid responses in roots (Fig. 1; seeSupporting Information—Table S3, main Mi effects). Similarly, S. exigua herbivory on M. incognita-infected plants triggered changes in the expression of marker genes depending on the nematodes’ root infection stages. At the invasion stage, the expression levels of both defence and GAME genes in double-infected plants were similar to those with M. incognita infection alone (Figs 2A–D, and 3A and B, yellow box plots; seeSupporting Information—Table S5). At the galling stage, the JA biosynthesis marker LoxD overall increased in plants infected with M. incognita (seeSupporting Information—Table S5, main Mi effect). Above-ground damage by S. exigua did not significantly alter this. A similar pattern was found for the expression levels of the other marker genes in plants with M. incognita and S. exigua; in the invasion and galling stage their expression levels were similar to plants with M. incognita infection alone (Figs 2E–H, and 3C and D, yellow vs. blue box plots; seeSupporting Information—Table S5). During the reproduction stage, S. exigua herbivory on M. incognita-infected plants significantly upregulated LapA (Fig. 2J, yellow box plot; seeSupporting Information—Table S5), whereas it had no significant effect on the other marker genes compared to plants infected with M. incognita alone (Figs 2I, K and L, and 3E and F, yellow box plots; seeSupporting Information—Table S5). By comparing the double-infected plants to control plants and those infected with S. exigua only, it became clear that the downregulation of GluB by S. exigua (Fig. 2L; seeSupporting Information—Table S5) is not affected by M. incognita infection. On the other hand, the significant main effects of M. incognita on the expression of JRE4 and GAME1 at the galling and reproduction stages were not changed by S. exigua feeding (Fig. 3C and D, and E and F, blue and yellow box plots; seeSupporting Information—Table S5). Therefore, our results collectively suggest that S. exigua can affect nematode-induced root responses, in particular via the JA-signalling pathway, depending on the nematodes’ infection stage.
Discussion
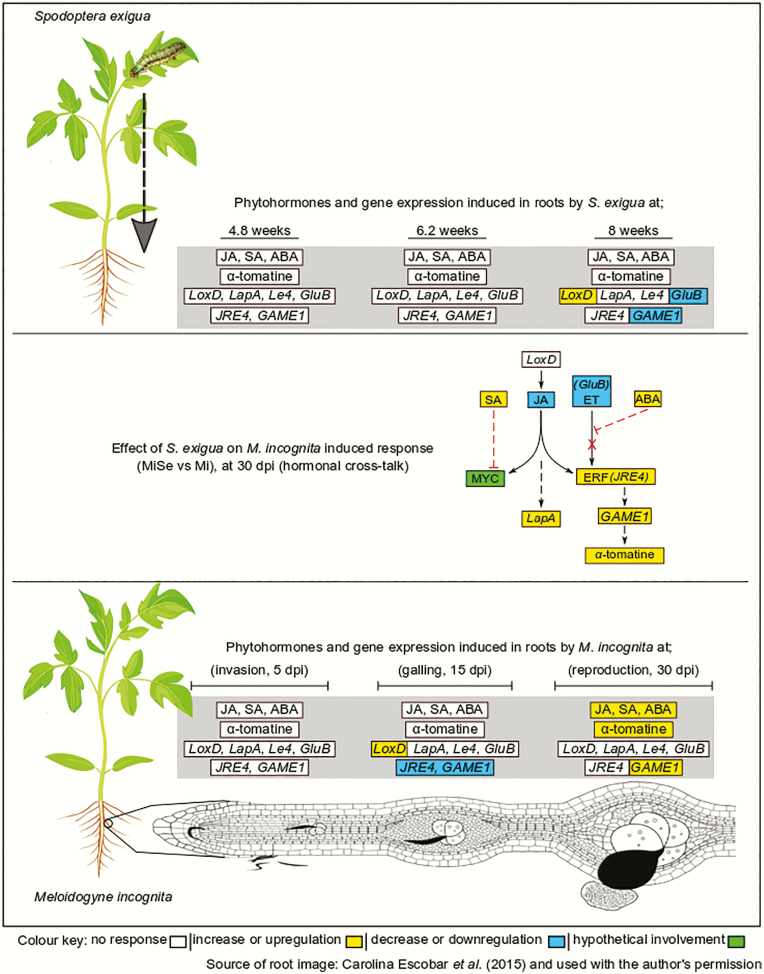
The goal of our study was to determine whether the impact of AG feeding on root defence responses induced by M. incognita depends on the nematodes’ life cycle. We tested this by exposing S. exigua caterpillars to tomato plants infected by M. incognita at different stages of the root infection cycle. We found that S. exigua affected M. incognita root-induced responses, mainly at the nematodes’ reproduction stage. In particular, the JA-signalling pathway was affected, as evidenced by lowered levels of JA in double-infected plants compared to plants infected with M. incognita alone. Jasmonic acid is known to regulate the GAME pathway in tomato via the JRE4 transcription factor (Thagun et al. 2016). In this study, the attenuation of the JA pathway did neither lower α-tomatine concentrations nor the expression of the GAME genes (JRE4 and GAME1) in double-infected plants compared to plants challenged with M. incognita alone (Fig. 4). This may be because the glycoalkaloid biosynthesis transcriptional coordinator JRE4 can act downstream of JA signalling (Abdelkareem et al. 2017). Caterpillar feeding also enhanced LapA expression in double-infected plants at the nematodes’ reproduction stage compared to plants challenged with M. incognita alone. LapA acts downstream of JA signalling as a modulator of late wound-induced responses (Fowler et al. 2009). LapA expression is induced by external application of ABA, methyl jasmonate (MeJA) and ET (Chao et al. 1999). Here, the levels of ABA in double-infected plants remained elevated, which could be related to the upregulation in LapA expression. Cross-talk between phytohormones is widely recognized as a mechanism to tailor herbivore-induced responses to specific combinations of attackers (Pieterse et al. 2009; Zamioudis and Pieterse 2012). Cross-talk between the JA-signalling pathway and both the SA and ABA pathways may also explain why glycoalkaloid levels remained increased in double-infected plants at the nematodes’ reproduction stage compared to plants infected with M. incognita alone, despite lowered JA levels, GluB expression and no effect on LoxD expression compared to M. incognita-infected roots (Fig. 4). This cross-talk of SA and ABA with the JA pathway might also occur downstream of JA biosynthesis, e.g. at the level of transcription factors like MYC or ERF and in our case, JRE4 (Fig. 4).
Figure 4.
Interactions between root defence responses upon root and leaf herbivory. Schematic illustration of induced defences in tomato roots including the phytohormones jasmonic acid (JA), salicylic acid (SA), abscisic acid (ABA), the glycoalkaloid α-tomatine and defence genes (Lipoxygenase D (LoxD), Leucine aminopeptidase A (LapA), Le4 abscisic acid-responsive, Basic-β-1-glucanase (GluB)) and glycoalkaloid metabolism (GAME) genes (jasmonate-responsive ETHYLENE RESPONSE FACTOR 4 transcription factor (JRE4) and GLYCOALKLOID METABOLISM 1 (GAME1)). The top panel represents phytohormones and gene expression induced in tomato roots by the caterpillar Spodoptera exigua on plants of different ages (4.8, 6.2 and 8 weeks). The bottom panel represents phytohormones and gene expression induced in tomato roots by the root-knot nematode (RKN) Meloidogyne incognita at different root infection cycle stages (invasion stage estimated at 5 days post-nematode inoculation (dpi), galling stage estimated at 15 dpi and reproduction stage estimated at 30 dpi). The middle panel shows the effect of S. exigua leaf feeding on root responses induced by M. incognita (MiSe) compared to those infected with M. incognita (Mi) alone at 30 dpi (hormonal cross-talk). White boxes: no response, yellow boxes: increase in trait levels or upregulation of gene expression, blue boxes: decrease in traits levels or downregulation of gene expression and green box: hypothetical involvement. In the proposed hormonal cross-talk schedule in the middle, dotted red lines show negative cross-talk, the black arrows show the steps in the JA pathway and the dashed black arrows represents several unknown steps. In our cross-talk model, we propose that the increase in SA affects the JA pathway negatively at the level of the MYC transcription factor. At the same time, the increase in ABA levels blocks the ethylene (ET) pathway, which regulates the ETHYLENE RESPONSIVE FACTOR (ERF) branch of the JA pathway. We hypothesize that the absence of ET promotes the activity of the JRE4 transcription factor, which enhances transcription of the GAME pathway. Based on the response of the defence marker gene LapA in MiSe plants at 30 dpi, we also hypothesize that this pathway leading to late JA responses is involved in the interaction.
To date, the elicitation of root defences by endoparasitic nematode infection at later time points in their life cycle is virtually undescribed; most papers focus on signalling events occurring at 1–7 days after infection (Kyndt et al. 2012a, b; Kammerhofer et al. 2015; Martínez-Medina et al. 2017). Here, we found that M. incognita infection at the invasion and galling stages did not elicit strong defence responses, either on the level of phytohormones, gene expression or glycoalkaloid production. The lack of significant defence induction during the invasion stage can be partly attributed to how the RKNs migrate inside the roots. Once the J2s of RKN are inside roots, they avoid damaging plant cells by moving intercellularly through soft tissues of the host plant root tissues (Gheysen and Mitchum 2011; Gheysen and Jones 2013). Also, RKNs secrete effector proteins that play an essential role during both the penetration (invasion) and the establishment and galling phases. These effectors suppress host defence responses and help the nematode to establish a permanent feeding site (Abad and Williamson 2010; Mitchum et al. 2013). For instance, the rice pathogenic nematodes M. graminicola and M. javanica excrete the effectors, Mg-MSP18 and Mj-MSP18, between 7 and 21 dpi to suppress the activation of their host’s immune responses, such as the hypersensitive response (Grossi-de-Sa et al. 2019). In our study, M. incognita did not induce significant root defences at the galling stage. We correlate this lack of defence induction to the fact that M. incognita utilizes effector proteins to repress plant responses in roots during the galling stage. For example, when feeding on A. thaliana, M. incognita secretes the effector Mi-CTR into the roots. This lowers pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) by suppressing the transcription of defense genes, such as WRKY33,29, PDF1.2 and pathogen related protein-1 (PR1) (Jaouannet et al. 2013). The effect of Mi-CTR occurs after root invasion and initiation of the giant cells 21 dpi most likely to ensure successful establishment (Jaouannet et al. 2013).
Interestingly, when M. incognita reached the reproduction stage, we observed an induction of defence responses. We found that M. incognita infection increased all phytohormone levels measured, as well as the concentration of α-tomatine and the expression of its biosynthesis gene GAME1. Possibly, the swelling of the female bodies with the ripening eggs intensifies the cell damage at the feeding sites, leading to the observed hormonal and defence responses. It is remarkable, however, that the expression patterns of defence-signalling marker genes are not affected in the same way. Possibly the expression of defence marker genes might be regulated by effector proteins that are only secreted by female RKN during the reproduction stage. For instance, the Misp12 effector is specific to M. incognita and secreted by mature females at least 28 dpi (Xie et al. 2016). Overexpression of Misp12 suppresses PR1 and phenylalanine ammonia-lyase-5 (PAL5) genes (SA pathway markers) in N. benthamiana. In Misp12-silenced plants, an upregulation of the proteinase inhibitor 2 (Pin2) (JA pathway marker) is reported. The authors suggest that Misp12 might be involved in the maintenance of giant cells during the reproduction stages (Xie et al. 2016).
The systemic effect of S. exigua feeding on root hormone levels and defence responses was much less pronounced than local nematode-induced responses. On the one hand, this may be because the caterpillars fed only for 24 h on the plant, while the nematodes were continuously feeding. In other studies, shoot feeding by herbivores, including S. exigua and Pieris rapae, was applied for 2–7 days before defence responses were observed in the roots (Danner et al. 2015; Kafle et al. 2017). Possibly, 24 h of AG feeding may have been too short to elicit strong systemic responses in tomato roots. Moreover, systemic responses are generally weaker than locally induced responses (van Dam et al. 2001; Babst et al. 2009; Ádám et al. 2018). For example, leaf feeding by diamondback moth caterpillars in Brassica oleracea elicited slight systemic JA responses in the roots compared to the local induction by Delia radicum (Karssemeijer et al. 2020). In another study, shoot feeding by P. rapae larvae on B. rapa plants elicits much lower root volatile emissions than local damage by D. radicum larvae (Danner et al. 2015).
Interestingly, we found that the age of the plant affects the systemic response as well. In our experimental set-up, we applied nematode eggs at one single time point. Consequently, the S. exigua caterpillars were placed on tomato plants that were at different ages and ontogenetic stages. The expression of some defence marker genes was significantly upregulated (Fig. 2I) or downregulated (Fig. 3F) by S. exigua feeding only in the last batch of plants, which were 8 weeks old and flowering. It has been reported that herbivore-induced plant responses can significantly change as a function of plant ontogenetic stage (Quintero and Bowers 2011, 2012). For instance, the concentration of iridoid glycosides in Plantago lanceolata roots after AG herbivory was twice as high in mature plants compared to young plants (Quintero and Bowers 2011).
In nature, plants are likely to interact with AG herbivores and RKN at the same time. Here we found that S. exigua herbivory differentially affects the root-induced responses by M. incognita in tomato roots. These effects occurred in dependence on the life cycle of the nematode, whereby the impact was the strongest in the reproductive stages. Herbivore identity and sequence of arrival on the target host plant are some of the critical factors shaping interactions between AG–BG herbivores (Erb et al. 2011; Sarmento et al. 2011; Kafle et al. 2017). We conducted our experiment by first infecting the plants with RKN. This is likely the natural sequence of arrival because the roots develop before the shoots after seed germination. Moreover, nematodes are ubiquitous in natural systems. Roots are therefore likely to be invaded with nematodes before herbivores arrive on AG organs (Hoysted et al. 2018; van Dam et al. 2018). Spodoptera exigua feeding on M. incognita-infected plants reduced JA but not SA concentrations. In a similar study, M. incognita were allowed to colonize tomato plants that had experienced 7 days of S. exigua feeding, followed by a lag phase of another 7 days (Kafle et al. 2017). The authors found that after 14 days of M. incognita infection, the root JA levels decreased in tomato plants that were previously damaged by S. exigua. Combining our results with this study, we conclude that it may not matter whether the nematode or the AG herbivore infects first; AG feeding seems always to reduce RKN-induced JA levels in the roots.
Jasmonates are essential regulators of systemic signalling between AG and BG tissues (Wasternack 2007; Wasternack and Hause 2013). It has been established that JAs regulate the steroidal glycoalkaloid metabolism pathway via the JRE4 transcription factor (De Geyter et al. 2012; Cárdenas et al. 2016; Thagun et al. 2016). Here the expression of JRE4 was not altered by S. exigua feeding alone, nor did the caterpillar alter the M. incognita-induced upregulation of this transcription factor. Notably, the expression of LapA (JA marker) was significantly upregulated in double-infected plants compared to plants infected with M. incognita only, while LoxD expression was similar when S. exigua co-occurred with M. incognita. Our results suggest that the interaction between M. incognita and S. exigua might rely on the induction of late wounding responses regulated by LapA downstream of JA synthesis, e.g., on transcription factor level (Fig. 4). Unfortunately, our experimental set-up did not allow us to precisely determine the role of LapA because the plants with RKN in different life cycle stages also differed in age. LapA might also be associated with plant development, especially in the flowering stage, as reported by Chao et al. (1999).
Finally, the induction of JA levels by M. incognita infection was accompanied by an increase in α-tomatine production. Increases in JA and α-tomatine concentrations upon nematode attack or exogenous application of elicitors, such as MeJA, have been reported in tomato and other plant species (Abdelkareem et al. 2017; Kafle et al. 2017). Glycoalkaloids are usually associated with increased generalist herbivore resistance (Ökmen et al. 2013; Abdelkareem et al. 2017). In our study, we did not measure the ecological consequences associated with these defence responses, e.g., for later arriving herbivores. Further studies to test the effects of α-tomatine on the performance of the RKNs may reveal their effectiveness as defences against this generalist herbivore.
Conclusions
Our study examined the impact of AG chewing herbivores on root-induced responses by RKN at different life cycle stages. We found that both the AG chewing herbivore and the RKN affect root defences. The effect of root infection by RKN alone, as well as the effect of AG herbivory on RKN-induced root defence responses, depends on the nematode’s life cycle stage. Studies testing the impact of long periods of AG herbivory on nematode-induced root responses are needed to reveal how the interactions with BG responses might change over longer interaction times. Such studies will help to optimize tomato breeding efforts towards cultivars with high resistance to AG and BG insect pests and pathogens.
Supporting Information
The following additional information is available in the online version of this article—
Figure S1. The number of root galls counted in tomato plants roots upon root infection by Meloidogyne incognita.
Table S1. List of primers sequences used for quantitative polymerase chain reaction (qPCR).
Table S2. Permutational multivariate analysis of variance (PERMANOVA) results based on Gower dissimilarities on phytohormone and α-tomatine data for herbivory effects of Meloidogyne incognita root-knot nematodes (RKNs) and the caterpillars of Spodoptera exigua.
Table S3. Two-way factorial analysis of variance (ANOVA) results on phytohormone and α-tomatine for the herbivory effects of Meloidogyne incognita root-knot nematode (RKN) and the caterpillars of Spodoptera exigua.
Table S4. Permutational multivariate analysis of variance (PERMANOVA) results based on Gower dissimilarities on gene expression data for herbivory effects of Meloidogyne incognita root-knot nematode (RKN) and the caterpillars of Spodoptera exigua.
Table S5. Two-way factorial analysis of variance (ANOVA) results on gene expression for the herbivory effect of Meloidogyne incognita root-knot nematode (RKN) and the caterpillars of Spodoptera exigua.
Data Availability
The data underlying this study are published as open access at the iDiv Data Repository (Mbaluto et al. 2020; http://idata.idiv.de/ddm/Data/ShowData/1816).
Acknowledgements
All authors thank Dr. Adriaan Verhage of Rijk-Zwaan, The Netherlands, for providing the root-knot nematode eggs, Dr. Katharina Grosser, Dr. Fredd Vergara and Anne Maedicke for their technical support in molecular and chemical analysis, Dr. Jitendra Gaikwad and the iDiv Data repository team for support in data preparation, quality check and uploading of the data to the iDiv repository. Lastly, all authors thank the two anonymous reviewers and the associate editor for their helpful and constructive comments on the earlier versions of the manuscript.
Sources of Funding
All authors gratefully acknowledge the support of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig funded by the German Research Foundation (FZT 118). C.M.M. acknowledges the Deutscher Akademischer Austauschdienst (DAAD) for a doctoral research grant (research grant no: 91607343). E.M.A. acknowledges the DAAD-German Egyptian Research Short-Term Scholarship Program (GERSS) scholarship awarded to conduct part of her Master of Science Research at iDiv. M.F. acknowledges the Go Global Self-Directed Research Award of the University of British Columbia for support to conduct research training at the iDiv facilities.
Contributions by the Authors
C.M.M., A.M.M. and N.M.v.D.: conception of the idea and experimental design; C.M.M.: execution, processing of samples, data analysis; E.M.A. and M.F.: processing of samples, data analysis; C.M.M.: writing of the initial manuscript; C.M.M.: deposition of data in iDiv data repository; A.M.M. and N.M.v.D.: critical revision of draft manuscripts and approval of the final manuscript for submission.
Conflict of Interest
The authors declare that this research article was conceptualized, designed and drafted in the absence of any commercial interest or financial obligations that could be construed as a potential conflict of interest.
Literature Cited
- Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Ségurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, Schiex T, Smant G, Weissenbach J, Wincker P. 2008. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnology 26:909–915. [DOI] [PubMed] [Google Scholar]
- Abad P, Williamson VM. 2010. Plant nematode interaction: a sophisticated dialogue. In: Kader J-C, Delseny M, eds. Advances in botanical research. Oxford, UK: Academic Press, 147–192. [Google Scholar]
- Abdelkareem A, Thagun C, Nakayasu M, Mizutani M, Hashimoto T, Shoji T. 2017. Jasmonate-induced biosynthesis of steroidal glycoalkaloids depends on COI1 proteins in tomato. Biochemical and Biophysical Research Communications 489:206–210. [DOI] [PubMed] [Google Scholar]
- Ádám AL, Nagy ZÁ, Kátay G, Mergenthaler E, Viczián O. 2018. Signals of systemic immunity in plants: progress and open questions. International Journal of Molecular Sciences 19:1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ. 2017. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online:1–15. [Google Scholar]
- Arce CCM, Machado RAR, Ribas NS, Cristaldo PF, Ataíde LMS, Pallini Â, Carmo FM, Freitas LG, Lima E. 2017. Nematode root herbivory in tomato increases leaf defenses and reduces leaf miner oviposition and performance. Journal of Chemical Ecology 43:120–128. [DOI] [PubMed] [Google Scholar]
- Babst BA, Sjödin A, Jansson S, Orians CM. 2009. Local and systemic transcriptome responses to herbivory and jasmonic acid in Populus. Tree Genetics and Genomes 5:459–474. [Google Scholar]
- Bandoly M, Steppuhn A. 2016. Bioassays to investigate the effects of insect oviposition on a plant’s resistance to herbivores. BIO-PROTOCOL 6:e1823. [Google Scholar]
- Bezemer TM, van Dam NM. 2005. Linking aboveground and belowground interactions via induced plant defenses. Trends in Ecology & Evolution 20:617–624. [DOI] [PubMed] [Google Scholar]
- Boege K, Dirzo R, Siemens D, Brown P. 2007. Ontogenetic switches from plant resistance to tolerance: minimizing costs with age? Ecology Letters 10:177–187. [DOI] [PubMed] [Google Scholar]
- Boots M, Best A. 2018. The evolution of constitutive and induced defences to infectious disease. Proceedings of the Royal Society B: Biological Sciences 285:20180658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel B. 2017. CRISPR, microbes and more are joining the war against crop killers. Nature 543:302–304. [DOI] [PubMed] [Google Scholar]
- Bruce TJA. 2014. Interplay between insects and plants: dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. Journal of Experimental Botany 66:455–465. [DOI] [PubMed] [Google Scholar]
- Caillaud MC, Dubreuil G, Quentin M, Perfus-Barbeoch L, Lecomte P, de Almeida Engler J, Abad P, Rosso MN, Favery B. 2008. Root-knot nematodes manipulate plant cell functions during a compatible interaction. Journal of Plant Physiology 165:104–113. [DOI] [PubMed] [Google Scholar]
- Cárdenas PD, Sonawane PD, Heinig U, Bocobza SE, Burdman S, Aharoni A. 2015. The bitter side of the nightshades: genomics drives discovery in Solanaceae steroidal alkaloid metabolism. Phytochemistry 113:24–32. [DOI] [PubMed] [Google Scholar]
- Cárdenas PD, Sonawane PD, Pollier J, Vanden Bossche R, Dewangan V, Weithorn E, Tal L, Meir S, Rogachev I, Malitsky S, Giri AP, Goossens A, Burdman S, Aharoni A. 2016. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nature Communications 7:10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao WS, Gu YQ, Pautot VV, Bray EA, Walling LL. 1999. Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiology 120:979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner H, Brown P, Cator EA, Harren FJ, van Dam NM, Cristescu SM. 2015. Aboveground and belowground herbivores synergistically induce volatile organic sulfur compound emissions from shoots but not from roots. Journal of Chemical Ecology 41:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter N, Gholami A, Goormachtig S, Goossens A. 2012. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends in Plant Science 17:349–359. [DOI] [PubMed] [Google Scholar]
- Erb M, Flors V, Karlen D, de Lange E, Planchamp C, D’Alessandro M, Turlings TC, Ton J. 2009. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. The Plant Journal 59:292–302. [DOI] [PubMed] [Google Scholar]
- Erb M, Robert CAM, Hibbard BE, Turlings TCJ. 2011. Sequence of arrival determines plant-mediated interactions between herbivores. Journal of Ecology 99:7–15. [Google Scholar]
- Escobar C, Barcala M, Cabrera J, Fenoll C. 2015. Overview of root-knot nematodes and giant cells. In: Escobar C, Fenoll C, eds. Advances in botanical research, plant nematode interactions: a view on compatible interrelationships. Oxford, UK: Academic Press, 1–32. [Google Scholar]
- FAO . 2019. Global production of vegetables in 2017, by type (in million metric tons). https://www.statista.com/statistics/264065/global-production-of-vegetables-by-type/ (4 April 2020).
- Favery B, Quentin M, Jaubert-Possamai S, Abad P. 2016. Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. Journal of Insect Physiology 84:60–69. [DOI] [PubMed] [Google Scholar]
- Fornoni J. 2011. Ecological and evolutionary implications of plant tolerance to herbivory. Functional Ecology 25:399–407. [Google Scholar]
- Fowler JH, Narváez-Vásquez J, Aromdee DN, Pautot V, Holzer FM, Walling LL. 2009. Leucine aminopeptidase regulates defense and wound signaling in tomato downstream of jasmonic acid. The Plant Cell 21:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso V, Rothe E, Baldwin IT, Kim SG. 2014. Root jasmonic acid synthesis and perception regulate folivore-induced shoot metabolites and increase Nicotiana attenuata resistance. The New Phytologist 202:1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco MFB, Pazin M, Pereira LC, Dorta DJ. 2015. Impact des pesticides sur la santé humaine. In: Andreazza AC, Scola G, eds. Toxicolgy studies; cells, drugs, environment. Rijeka, Croatia: InTech, 195–222. [Google Scholar]
- Friedman M. 2002. Tomato glycoalkaloids: role in the plant and in the diet. Journal of Agricultural and Food Chemistry 50:5751–5780. [DOI] [PubMed] [Google Scholar]
- Garcia PG, Neves Dos Santos F, Zanotta S, Eberlin MN, Carazzone C. 2018. Metabolomics of Solanum lycopersicum infected with Phytophthora infestans leads to early detection of late blight in asymptomatic plants. Molecules (Basel, Switzerland) 23:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen G, Jones JT. 2013. Molecular aspects of plant-nematode interactions. In: Perry RN, Moens M, eds. Plant nematology. Wallingford, UK: CABI Publishing, 274–296. [Google Scholar]
- Gheysen G, Mitchum MG. 2011. How nematodes manipulate plant development pathways for infection. Current Opinion in Plant Biology 14:415–421. [DOI] [PubMed] [Google Scholar]
- Gheysen G, Mitchum MG. 2019. Phytoparasitic nematode control of plant hormone pathways. Plant Physiology 179:1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi-de-Sa M, Petitot A-S, Xavier DA, Mezzalira I, Beneventi MA, Martins NF, Baimey HK, Albuquerque EVS, Grossi-de-Sa MF, Fernandez D. 2019. Rice susceptibility to root-knot nematodes is enhanced by the Meloidogyne incognita MSP18 effector gene. Planta 250:1215–1227. [DOI] [PubMed] [Google Scholar]
- Hoysted GA, Bell CA, Lilley CJ, Urwin PE. 2018. Aphid colonization affects potato root exudate composition and the hatching of a soil borne pathogen. Frontiers in Plant Science 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoysted GA, Lilley CJ, Field KJ, Dickinson M, Hartley SE, Urwin PE. 2017. A plant-feeding nematode indirectly increases the fitness of an aphid. Frontiers in Plant Science 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Siemann E, Yang X, Wheeler SG, Jianqing D. 2013. Facilitation and inhibition: changes in plant nitrogen and secondary metabolites mediate interactions between above-ground and below-ground herbivores. Proceedings of the Royal Society B: Biological Sciences 280:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaouannet M, Magliano M, Arguel MJ, Gourgues M, Evangelisti E, Abad P, Rosso MN. 2013. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Molecular Plant-Microbe Interactions 26:97–105. [DOI] [PubMed] [Google Scholar]
- Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM, Perry RN. 2013. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology 14:946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafle D, Hänel A, Lortzing T, Steppuhn A, Wurst S. 2017. Sequential above- and belowground herbivory modifies plant responses depending on herbivore identity. BMC Ecology 17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerhofer N, Radakovic Z, Regis JM, Dobrev P, Vankova R, Grundler FM, Siddique S, Hofmann J, Wieczorek K. 2015. Role of stress-related hormones in plant defence during early infection of the cyst nematode Heterodera schachtii in Arabidopsis. The New Phytologist 207:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karajeh M. 2008. Interaction of root-knot nematode (Meloidogyne Javanica) and tomato as affected by hydrogen peroxide. Journal of Plant Protection Research 48:181–187. [Google Scholar]
- Karban R. 2011. The ecology and evolution of induced resistance against herbivores. Functional Ecology 25:339–347. [Google Scholar]
- Karssemeijer PN, Reichelt M, Gershenzon J, van Loon J, Dicke M. 2020. Foliar herbivory by caterpillars and aphids differentially affects phytohormonal signalling in roots and plant defence to a root herbivore. Plant, Cell & Environment 43:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KG, Chapman K, Louis J, Heng-Moss T, Sarath G. 2016. Plant tolerance: a unique approach to control hemipteran pests. Frontiers in Plant Science 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Ortiz EV, Garrido E, Poveda K, Jander G. 2016. Potato tuber herbivory increases resistance to aboveground lepidopteran herbivores. Oecologia 182:177–187. [DOI] [PubMed] [Google Scholar]
- Kyndt T, Denil S, Haegeman A, Trooskens G, Bauters L, Van Criekinge W, De Meyer T, Gheysen G. 2012a. Transcriptional reprogramming by root knot and migratory nematode infection in rice. The New Phytologist 196:887–900. [DOI] [PubMed] [Google Scholar]
- Kyndt T, Nahar K, Haegeman A, De Vleesschauwer D, Höfte M, Gheysen G. 2012b. Comparing systemic defence-related gene expression changes upon migratory and sedentary nematode attack in rice. Plant Biology 14:73–82. [DOI] [PubMed] [Google Scholar]
- Kyndt T, Zemene HY, Haeck A, Singh R, De Vleesschauwer D, Denil S, De Meyer T, Höfte M, Demeestere K, Gheysen G. 2017. Below-ground attack by the root knot nematode Meloidogyne graminicola predisposes rice to blast disease. Molecular Plant-Microbe Interactions 30:255–266. [DOI] [PubMed] [Google Scholar]
- Li N, Han X, Feng D, Yuan D, Huang L-J. 2019. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering? International Journal of Molecular Sciences 20:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Machado RAR, Arce CCM, McClure MA, Baldwin IT, Erb M. 2018. Aboveground herbivory induced jasmonates disproportionately reduce plant reproductive potential by facilitating root nematode infestation. Plant, Cell & Environment 41:797–808. [DOI] [PubMed] [Google Scholar]
- Machado RA, Ferrieri AP, Robert CA, Glauser G, Kallenbach M, Baldwin IT, Erb M. 2013. Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. The New Phytologist 200:1234–1246. [DOI] [PubMed] [Google Scholar]
- Martínez-Medina A, Fernandez I, Lok GB, Pozo MJ, Pieterse CM, Van Wees SC. 2017. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. The New Phytologist 213:1363–1377. [DOI] [PubMed] [Google Scholar]
- Martínez-Medina A, Fernández I, Sánchez-Guzmán MJ, Jung SC, Pascual JA, Pozo MJ. 2013. Deciphering the hormonal signalling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Frontiers in Plant Science 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio R, Rausher MD, Burdick DS. 1997. Variation in the defense strategies of plants: are resistance and tolerance mutually exclusive? Ecology 78:1301–1311. [Google Scholar]
- Mbaluto CM, Martínez-Medina A, van Dam NM. 2020. Induced defense response in tomato roots infected by root-knot nematode infection at different life cycle stages and with leaf damage by caterpillar. iDiv Data Repository. doi: 10.25829/idiv.1816-16-2864. [DOI] [Google Scholar]
- Meyer GA, Whitlow TH. 1992. Effects of leaf and sap feeding insects on photosynthetic rates of goldenrod. Oecologia 92:480–489. [DOI] [PubMed] [Google Scholar]
- de Miranda V, Coelho R, Viana AA, de Oliveira Neto OB, Carneiro RMDG, Rocha TL, Grossi de Sa MF, Fragoso RR. 2013. Validation of reference genes aiming accurate normalization of qPCR data in soybean upon nematode parasitism and insect attack. BMC Research Notes 6:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Brennan RM, Graham J, Karley AJ. 2016. Plant defense against herbivorous pests: exploiting resistance and tolerance traits for sustainable crop protection. Frontiers in Plant Science 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL. 2013. Nematode effector proteins: an emerging paradigm of parasitism. The New Phytologist 199:879–894. [DOI] [PubMed] [Google Scholar]
- Niebel A, Gheysen G, Van Montagu M. 1994. Plant-cyst nematode and plant-root-knot nematode interactions. Parasitology Today 10:424–430. [DOI] [PubMed] [Google Scholar]
- Núñez-Farfán J, Fornoni J, Valverde PL. 2007. The evolution of resistance and tolerance to herbivores. Annual Review of Ecology, Evolution, and Systematics 38:541–566. [Google Scholar]
- Oishimaya SN. 2017. The world’s leading producers of tomatoes. https://www.worldatlas.com/articles/which-are-the-world-s-leading-tomato-producing-countries.html (21 October 2019).
- Ökmen B, Etalo DW, Joosten MH, Bouwmeester HJ, de Vos RC, Collemare J, de Wit PJ. 2013. Detoxification of α-tomatine by Cladosporium fulvum is required for full virulence on tomato. The New Phytologist 198:1203–1214. [DOI] [PubMed] [Google Scholar]
- Omondi S. 2018. The most popular vegetables in the world. https://www.worldatlas.com/articles/the-most-popular-vegetables-in-the-world.html (4 April 2020).
- Oñate-Sánchez L, Vicente-Carbajosa J. 2008. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Research Notes 1:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RKD, Higley LG, Haile FJ, Barrigossi JAF. 1998. Mexican bean beetle (Coleoptera: Coccinellidae) injury affects photosynthesis of Glycine max and Phaseolus vulgaris. Environmental Entomology 27:373–381. [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. 2009. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology 5:308–316. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC. 2012. Hormonal modulation of plant immunity. Annual Review of Cell and Developmental Biology 28:489–521. [DOI] [PubMed] [Google Scholar]
- Quentin M, Abad P, Favery B. 2013. Plant parasitic nematode effectors target host defense and nuclear functions to establish feeding cells. Frontiers in Plant Science 4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero C, Bowers MD. 2011. Plant induced defenses depend more on plant age than previous history of damage: implications for plant-herbivore interactions. Journal of Chemical Ecology 37:992–1001. [DOI] [PubMed] [Google Scholar]
- Quintero C, Bowers MD. 2012. Changes in plant chemical defenses and nutritional quality as a function of ontogeny in Plantago lanceolata (Plantaginaceae). Oecologia 168:471–481. [DOI] [PubMed] [Google Scholar]
- R Core Development Team . 2019. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Retuerto R, Fernandez-Lema B, Rodriguez-Roiloa S, Obeso JR. 2004. Increased photosynthetic performance in holly trees infested by scale insects. Functional Ecology 18:664–669. [Google Scholar]
- Robinson AF, Perry RN. 2006. Behaviour and sensory perception. In: Perry RN, Moens M, eds. Plant nematology. Wallingford, UK: CABI publishing, 210–233. [Google Scholar]
- Rodiuc N, Vieira P, Banora MY, de Almeida Engler J. 2014. On the track of transfer cell formation by specialized plant-parasitic nematodes. Frontiers in Plant Science 5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento RA, Lemos F, Bleeker PM, Schuurink RC, Pallini A, Oliveira MG, Lima ER, Kant M, Sabelis MW, Janssen A. 2011. A herbivore that manipulates plant defence. Ecology Letters 14:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M, Brütting C, Baldwin IT, Kallenbach M. 2016. High-throughput quantification of more than 100 primary- and secondary-metabolites, and phytohormones by a single solid-phase extraction based sample preparation with analysis by UHPLC-HESI-MS/MS. Plant Methods 12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thagun C, Imanishi S, Kudo T, Nakabayashi R, Ohyama K, Mori T, Kawamoto K, Nakamura Y, Katayama M, Nonaka S, Matsukura C, Yano K, Ezura H, Saito K, Hashimoto T, Shoji T. 2016. Jasmonate-responsive ERF transcription factors regulate steroidal glycoalkaloid biosynthesis in tomato. Plant & Cell Physiology 57:961–975. [DOI] [PubMed] [Google Scholar]
- Uppalapati SR, Ayoubi P, Weng H, Palmer DA, Mitchell RE, Jones W, Bender CL. 2005. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. The Plant Journal 42:201–217. [DOI] [PubMed] [Google Scholar]
- van Dam NM, Heil M. 2011. Multitrophic interactions below and above ground: en route to the next level. Journal of Ecology 99:77–88. [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT. 2001. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. Journal of Chemical Ecology 27:547–568. [DOI] [PubMed] [Google Scholar]
- van Dam NM, Wondafrash M, Mathur V, Tytgat TOG. 2018. Differences in hormonal signaling triggered by two root-feeding nematode species result in contrasting effects on aphid population growth. Frontiers in Ecology and Evolution 6:1–12. [Google Scholar]
- van der Meijden E. 2015. Herbivorous insects—a threat for crop production. In: Lugtenberg B, ed. Principles of plant-microbe interactions. Cham, Switzerland: Springer International Publishing, 103–114. [Google Scholar]
- Wang M, Biere A, Van Der Putten WH, Bezemer TM, Brinkman EP. 2017. Timing of simulated aboveground herbivory influences population dynamics of root-feeding nematodes. Plant Soil 415:215–228. [Google Scholar]
- Wasternack C. 2007. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany 100:681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111:1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson VM, Gleason CA. 2003. Plant-nematode interactions. Current Opinion in Plant Biology 6:327–333. [DOI] [PubMed] [Google Scholar]
- Wittstock U, Gershenzon J. 2002. Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology 5:300–307. [DOI] [PubMed] [Google Scholar]
- Xie J, Li S, Mo C, Wang G, Xiao X, Xiao Y. 2016. A novel Meloidogyne incognita effector Misp12 suppresses plant defense response at latter stages of nematode parasitism. Frontiers in Plant Science 7:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Zhai Q, Wei J, Li S, Wang B, Huang T, Du M, Sun J, Kang L, Li C-B, Li C. 2013. Role of tomato lipoxygenase D in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLoS Genetics 9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamioudis C, Pieterse CM. 2012. Modulation of host immunity by beneficial microbes. Molecular Plant-Microbe Interactions 25:139–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are published as open access at the iDiv Data Repository (Mbaluto et al. 2020; http://idata.idiv.de/ddm/Data/ShowData/1816).