Abstract
Background:
Human laboratory paradigms are a pillar in medications development for alcohol use disorders (AUD). Neuroimaging paradigms, in which individuals are exposed to cues that elicit neural correlates of alcohol craving (e.g. mesocorticolimbic activation), are increasingly utilized to test the effects of AUD medications. Elucidation of the translational effects of these neuroimaging paradigms on human laboratory paradigms, such as self-administration, are warranted. The current study is a secondary analysis examining whether alcohol cue-induced activation in the ventral striatum is predictive of subsequent alcohol self-administration in the laboratory.
Methods:
Non-treatment-seeking heavy drinkers of East Asian descent (n = 41) completed a randomized, placebo-controlled, double-blind, crossover experiment on the effects of naltrexone on neuroimaging and human laboratory paradigms. Participants completed 5 days of study medication (or placebo); on day 4, they completed a neuroimaging alcohol taste cue reactivity task. On the following day (day 5), participants completed a 60-minute alcohol self-administration paradigm.
Results:
Multilevel cox regressions indicated a significant effect of taste cue-elicited ventral striatum activation on latency to first drink, Wald χ2 = 2.88, p = 0.05, such that those with higher ventral striatum activation exhibited shorter latencies to consume their first drink. Similarly, ventral striatum activation was positively associated with total number of drinks consumed, F(1, 38) = 5.90, p = .02. These effects were significant after controlling for alcohol use severity, OPRM1 genotype, and medication. Other potential regions of interest (anterior cingulate, thalamus) were not predictive of self-administration outcomes.
Conclusions:
Neuroimaging alcohol taste cue paradigms may be predictive of laboratory paradigms such as self-administration. Elucidation of the relationships among different paradigms will inform how these paradigms may be used synergistically in experimental medicine and medications development.
Keywords: neuroimaging, human laboratory, alcohol self-administration, ventral striatum, cue-induced craving
INTRODUCTION
Development of effective treatments for alcohol use disorder (AUD) remain a high priority area which involves screening compounds in the laboratory before proceeding to clinical trials (Grodin and Ray, 2019; Ray et al., 2018a). Within this process, there is a need to develop and understand relationships among human laboratory paradigms to assess the potential efficacy of novel AUD treatments in early-stage clinical trials. To date, reviews of the human laboratory literature in AUD pharmacotherapy development indicate significant outcome variability based on experimental paradigm parameters, population of interest, and sample size, and suggest that these myriad variables contribute to the disconnect between laboratory effect sizes and treatment outcomes (Witkiewitz et al., 2019; Yardley and Ray, 2017).
Amidst the efforts to develop translational experimental paradigms, neuroimaging tasks are increasingly used to explore potential pharmacotherapy effects on neural correlates of alcohol-induced craving (Grodin and Ray, 2019). Alcohol consumption produces neuroadaptations in multiple circuits, including GABA-ergic regulation of traditional reward circuitry; alcohol craving is mediated by cortico-striatal-limbic activation, heightens relapse risk (Heinz et al., 2009), and can be triggered through internal and external stimuli associated with alcohol consumption (Seo and Sinha, 2014). For this reason, neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), have been used to explore these circuits as potential medication targets. Recent qualitative reviews and meta-analyses suggested that while such fMRI tasks vary in sensory experiences (e.g. taste vs visual cues) and scan parameters, mesocorticolimbic areas consistently exhibit task-based neural activity and may be viable tools in understanding mechanisms of AUD pharmacotherapy (Grodin and Ray, 2019; Schacht et al., 2013).
Based on this emerging literature, there is growing evidence that neural responses to alcohol cues and associated contexts are predictive of real-world consumption behavior and, potentially, clinical outcomes. For instance, among college students, alcohol cue-elicited blood oxygen level-dependent (BOLD) response in caudate, frontal cortex, and left insula predicted escalation to heavy drinking over a 1-year period (Dager et al., 2014). Further, insula and frontal gyrus activation in response to an emotion face recognition task similarly predicted alcohol-related problems five years later in young adults (Schuckit et al., 2016). Regarding treatment outcomes, increased ventral striatum activation in response to alcohol cues was associated with a faster time to relapse in a sample of abstinent AUD individuals (Reinhard et al., 2015). Comparisons of AUD treatment completers and non-completers in a community sample indicated that non-completers showed stronger associations between reported alcohol craving intensity and resting state functional connectivity between striatum and insula, relative to completers (Kohno et al., 2017). Of note, one study had contradicting results by reporting that relapsers, compared to successful alcohol abstainers and healthy controls, exhibited reduced alcohol cue-elicited activation in ventral striatum and midbrain (Beck et al., 2012).
Several studies have examined whether AUD pharmacotherapies alter neural responses to contexts that elicit alcohol craving, including alcohol cues, exposure to reward and emotional faces, and stress exposure. While significant variability exists in sample populations, examined tasks, modified areas of activation, and molecular targets of treatments, there is some consistent evidence that AUD pharmacotherapies may reduce reward-related activation in regions such as the ventral striatum, precuneus, and anterior cingulate (Grodin and Ray, 2019). Importantly, in one study of naltrexone, magnitude of reduction in alcohol cue-induced ventral striatum activation was associated with fewer instances of subsequent heavy drinking (Schacht et al., 2017). In support, Mann and colleagues (2014) have found that individuals with high ventral striatum cue reactivity demonstrate lower relapse rates when treated with naltrexone than those with low VS reactivity. Bach and colleagues (2019) have also identified that individuals with high alcohol cue-reactivity in the left putamen exhibit longer time to relapse when treated with naltrexone, compared to those with low reactivity. Together, these studies underscore reward circuitry (e.g. VS) as a key area in the translation of neural responses to clinical outcomes in AUD medication development (Nielsen et al., 2018).
Alcohol self-administration tasks in the laboratory are thought to capture alcohol use behavior in controlled settings that approximate consumption in real world settings. Studies have tested multiple variants of self-administration paradigms, including tasks that require participants to orally consume alcohol at the cost of monetary rewards per drink (McKee et al., 2009), and intravenous methods that can closely control breath alcohol concentration levels (e.g. computer-assisted self-infusion of ethanol (CASE); (Zimmermann et al., 2013). Studies have used self-administration methods to test genetic, physiological, and psychological risk factors for heavy drinking (Gowin et al., 2017; Green et al., 2019; Wardell et al., 2018). Self-administration tasks have also been used extensively in developing effective AUD pharmacotherapies (Hendershot et al., 2017; McKee et al., 2009). While both fMRI cue-reactivity tasks and alcohol self-administration tasks are widely used in alcohol research, the extent to which cue-reactivity predicts self-administration in the laboratory remains unknown.
In light of the emerging role of functional neuroimaging in predicting drinking behavior and AUD treatment outcomes, a remaining question is the nature of the relationship between neuroimaging task-induced neural activation and widely utilized laboratory paradigms considered proximal to real-world consumption, including self-administration tasks. To date, several studies have examined relationships of response across different laboratory paradigms (i.e. subjective response and self-administration) and have consistently identified that alcohol craving during intravenous alcohol administration mediates the relationship between alcohol-induced stimulatory effects and subsequent oral alcohol consumption (Bujarski et al., 2018; Green et al., 2019; Wardell et al., 2015). While relationships across human laboratory paradigms are recently delineated, no studies have yet investigated whether alcohol cue-induced BOLD response is predictive of responses within laboratory self-administration paradigms.
To address this gap in the literature and to further integrate neuroimaging and human laboratory paradigms for AUD, the current study examines whether alcohol taste cue-induced ventral striatum activation predicts subsequent oral alcohol self-administration in the laboratory. These secondary analyses are conducted in a within-subjects design whereby the same participants completed an fMRI cue-reactivity task followed by an alcohol-self administration task (one day later). As striatal activation is thought to underlie craving responses (Ray and Roche, 2018), we hypothesized that those with greater ventral striatum activation would consume their first drink faster than those with lower activation. Similarly, as previous research has demonstrated that mesolimbic activity predicts real-world heavy drinking, we hypothesized that ventral striatum activation would also be positively associated with the total number of drinks consumed during the self-administration paradigm.
MATERIALS AND METHODS
Participants
Participants for this secondary analysis of an experimental laboratory study on naltrexone (Lim et al., 2019; Ray et al., 2018b) were adult heavy drinkers of East Asian descent recruited from the Los Angeles metropolitan area through community fliers and online and print advertisements. Inclusion criteria were: 1) a score of 8 or higher on the Alcohol Use Disorders Identification Test (AUDIT; (Allen et al., 1997); 2) self-identification of East Asian ethnicity (i.e. Chinese, Japanese, Korean, or Taiwanese); and 3) between 21–55 years old. Exclusion criteria were: 1) history of Major Depressive Disorder with suicidal ideation; 2) lifetime psychotic disorder; 3) lifetime non-alcohol substance use disorder (with the exception of cannabis); 4) clinically significant levels of alcohol withdrawal (indicated by a score of 10 or higher on the Clinical Institute Withdrawal Assessment-Revised (CIWA-AR (Sullivan et al., 1989); 5) currently seeking AUD treatment; 6) history of epilepsy, seizures, or severe head trauma; 7) non-removable ferromagnetic objects in body; 8) claustrophobia; and 9) for women, pregnancy. The study was approved by the University of California Los Angeles Institutional Review Board.
Procedures
Recruitment
Interested individuals completed an in-person laboratory screening visit to learn about the study, provide written informed consent, and to assess for inclusion and exclusion criteria. Of note, this study collected information on genotypes encoding endogenous opioid receptors thought to mediate the stimulating effects of alcohol (rs1799971 SNP – Asn40Asp) (OPRM1), as well as those associated with metabolism of alcohol (ADH1B, ALDH2). Participants provided a saliva sample for DNA analyses and completed a medical screening that included a physical examination. Detailed information on recruitment procedures are available in the primary manuscripts from which the current study is based (Lim et al., 2019; Ray et al., 2018b). Detailed information on genotyping is available in Supplementary Materials. A study procedure flowchart can be seen in Figure 1.
Figure 1.
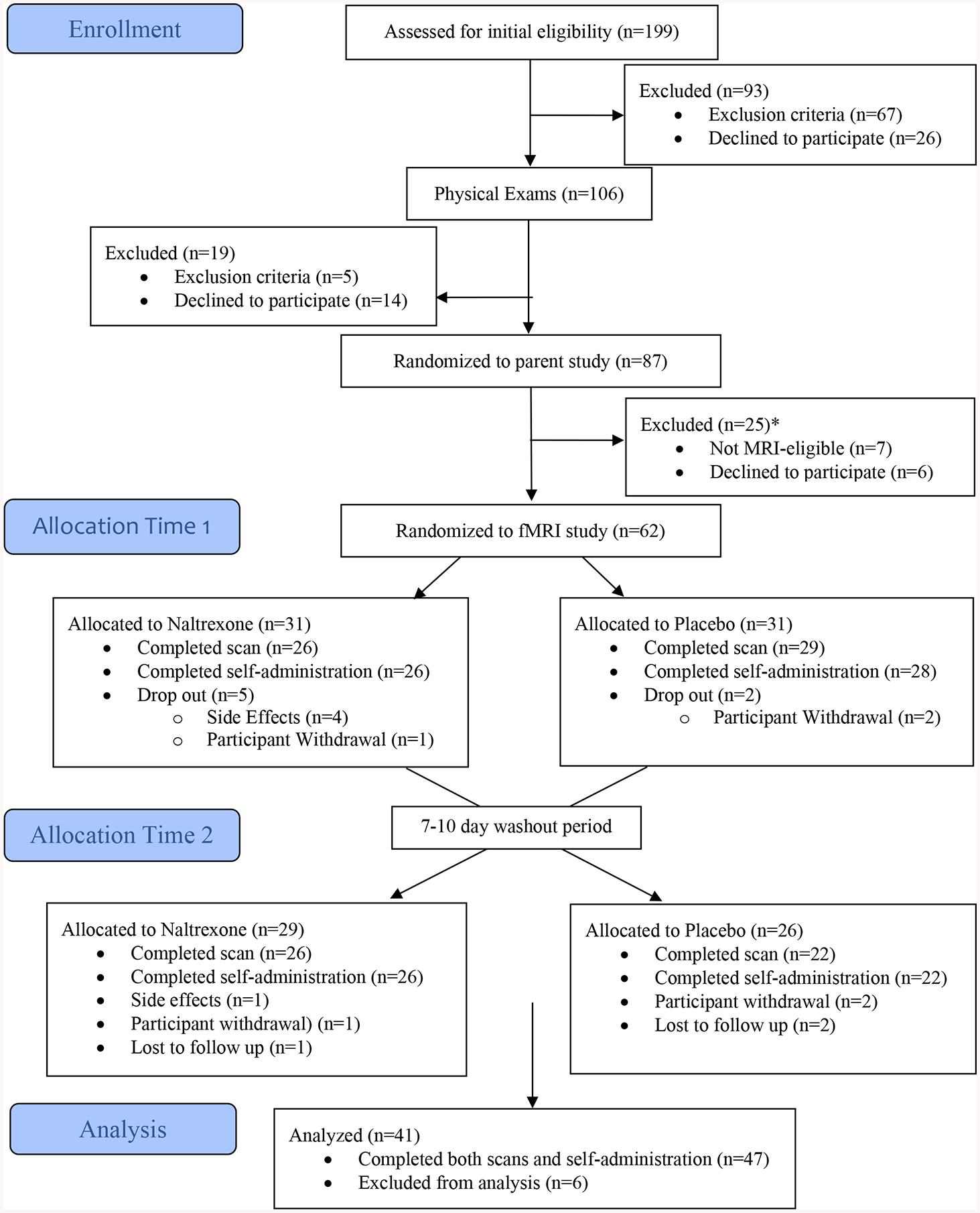
CONSORT Diagram *The scanner utilized for the study was upgraded towards the end of the study. Due to parameter compatibility concerns, scanning data was not collected from 12 MRI-eligible participants.
Medication Procedures
Study procedures followed a double-blind, randomized, placebo-controlled and counterbalanced design. Participants were assigned a medication sequence (placebo, naltrexone) based on a randomization pattern of ABBA. Within each medication condition, participants were titrated to the medication (or matched placebo) for 5 days (for naltrexone, 25 mg for days 1–2, 50 mg for days 3–5). Participants completed an fMRI scan on day 4 and an alcohol self-administration session on day 5 of the medication regimen. At the start of each experimental session, participants completed a urine toxicology screening; all participants tested negative for exclusionary substances during these screening periods. There was a minimum wash-out period between medication conditions of 7 days, with a range of 7–10 days. Regarding medication adherence, naltrexone and placebo capsules were packaged with 50mg of riboflavin. A visual inspection of riboflavin content under ultraviolet light indicated that all urine samples tested positive for riboflavin content.
fMRI Scanning Procedures
At the start of the scanning session (medication day 4), participants were required to have a BrAC of 0.00 g/dL, negative urine toxicology screen for all substances except cannabis, and negative pregnancy screen. Participants who smoked cigarettes (n = 12, 29% of the sample) were allowed to smoke 30 minutes prior to the scan to prevent acute nicotine withdrawal and craving.
Participants completed a modified version of the Alcohol Taste Cues Task in the scanner (Filbey et al., 2008b). Within each task trial, participants initially viewed a visual cue (the words “Alcohol” or “Water”) for 2 seconds, followed by a fixation cross (jittered with a mean of 3 seconds and range of 0.5 to 6 seconds). The word “Taste” then appeared, corresponding to oral delivery of the indicated liquid at the start of the trial (2mL alcohol or water; 5 second duration). Participants were also instructed to press a button on a button box to indicate the point at which the bolus of liquid was swallowed and this information was used to model motion associated with swallowing. There were two runs of this task, with 50 trials per run. Alcohol and water were delivered through Teflon tubing using a computer-controlled delivery system. Red or white wine, based on participant preference, was used as the alcohol stimulus; previous work from our group has demonstrated that this paradigm has been used to effectively elicit alcohol-related neural activation (Ray et al., 2014). Carbonated alcohol, such as beer, could not be systematically administered with the paradigm apparatus and was not offered as a drink option to participants. Visual stimuli and response collection were programmed using MATLAB (Mathworks, Natick, MA) and Psychtoolbox (www.psychtoolbox.org), and visual stimuli were presented using MRI-compatible goggles.
Self-Administration Procedures
Participants completed an oral alcohol self-administration paradigm on day 5 of medication titration. At the start of this session, participants were required to test negative for substance use (except cannabis) and to have a BrAC of 0.00 g/dl. Female participants were also required to test negative on a pregnancy test. Participants fasted for two hours prior to the session and were given a standardized meal before the alcohol administration. Participants initially completed an intravenous alcohol administration discussed in the primary manuscript (Ray et al., 2018b). After completing the alcohol infusion paradigm and reaching a target BrAC of 0.06 g/dl, the IV was removed and, after a standardized period of five minutes, participants subsequently began an oral self-administration session at the testing center. Notably, the alcohol dose of 0.06 g/dl prior to the self-administration period was higher than the typical 0.03 g/dl priming dose implemented in self-administration tasks (McKee et al., 2009; McKee et al., 2006) During the self-administration period, participants were provided 4 mini-drinks of their preferred alcoholic beverage and allowed to watch a movie over a 1-hour period. The 4 mini-drinks allowed participants to consume up to 0.04 g/dl alcohol in total, and were individualized by participant gender, weight, height, and alcohol content. Participants were also told that they would receive 1 dollar for each drink remaining at the end of the session. At the end of the session, participants were provided a meal and required to stay at the testing center until their BrAC dropped below 0.02 g/dl or to 0.00 g/dl if driving.
Data Analytic Plan
For the taste cues paradigm, information regarding image acquisition parameters and preprocessing steps are available in Supplementary Materials and are derived from the primary manuscript (Lim et al., 2019; Ray et al., 2018b). The main contrast of interest was the difference in activation corresponding to alcohol taste delivery and water delivery across the two task runs (Alcohol > Water), for each within-subject medication condition. Consistent with previous studies examining relationships among ventral striatum activity, subjective response to alcohol, and drinking behavior (Morales et al., 2018; Nikolova et al., 2016; Weafer et al., 2018), an anatomical bilateral ventral striatum region of interest was defined using the Harvard-Oxford atlas in standard MNI space and was transformed into participants’ respective native space using FSL’s FLIRT (see Figure 2). This ROI was selected because ventral striatum is most consistently elicited in alcohol cue and taste reactivity paradigms, as well as most frequently associated with behavioral measures and treatment response (Claus et al., 2011; Oberlin et al., 2016; Schacht et al., 2013). ROI selection was limited to one due to insufficient power to detect incremental model improvement with multiple ROIs. The mean contrast estimate values were extracted from this region for each subject and used in mixed models for group-level analysis (described below).
Figure 2.
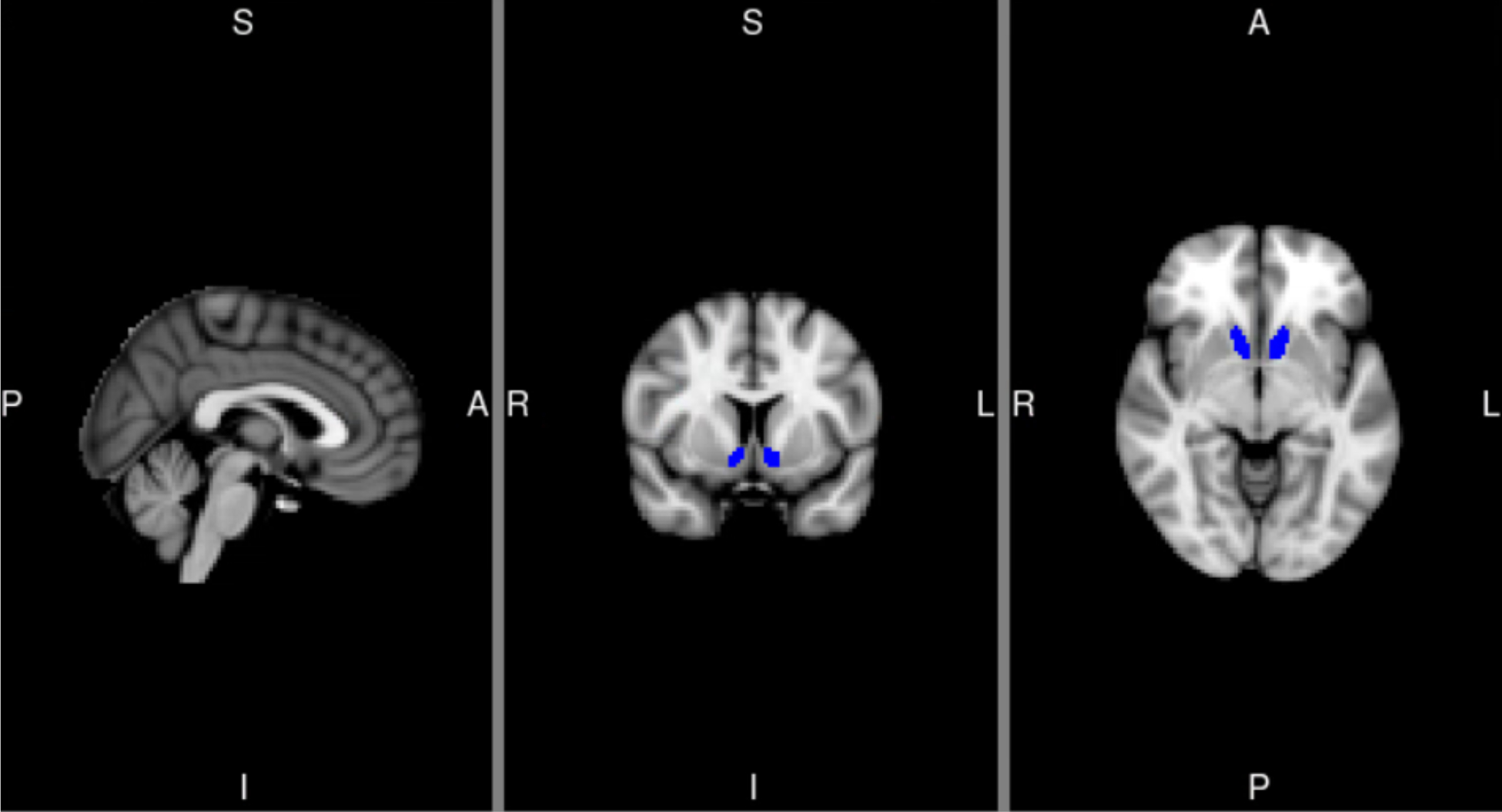
Anatomical region of interest mask for ventral striatum (left and right: 108 and 86 voxels, respectively). ROI extracted from the Harvard Oxford atlas thresholded at 25% based on the maximum probability labels. MNI coordinates for depicted slices are X=2 (left), Y=8 (middle), Z=−6 (right). L=Left, R=right, S=superior, I=inferior, A=anterior, P=posterior.
The self-administration paradigm yielded two outcome measures: (a) latency to first drink (in seconds, from the beginning of the session), and (b) total number of drinks consumed during the session (0–4 mini-drinks). To examine the relationship between alcohol taste-induced neural activation and self-administration, multilevel mixed poisson and cox (i.e. frailty) proportional hazard models were the primary analyses for total number of drinks and latency to first drink, respectively. Frailty models were fitted using a penalized partial likelihood approach available in SAS 9.4 (SAS Institute, Cary, NC). Primary analyses examined effects of variables of interest, including medication condition (naltrexone, placebo), alcohol consumption (30-day TLFB drinks per drinking day), and OPRM1. Due to concerns of overparameterization given the limited sample size, additional covariates of interest (medication randomization order, gender, alcohol abstinence days prior to scan, smoking status, consumption of preferred alcohol choice in scanner (yes/no)) were individually included in separate models to determine whether main effects of ventral striatum would be altered. Alpha corrections were not utilized in this exploratory study due to limited sample size and constrained power. Tests of proportional hazards are included in Supplementary Materials and Figures S1a–S1d. Survival plots for latency to first drink, controlling for covariates within the final model (drinks per drinking day, medication condition, and OPRM1), were generated to further explore ventral striatum activation in predicting latency to first drink. Of note, a dichotomous median-split ventral striatum variable was created for ease of visualization of these relationships, but ventral striatum activation was included as a continuous variable in all models.
As a final check to corroborate whether these results were specific to ventral striatum, final models for latency to first drink and total drinks were re-conducted for brain regions that demonstrated whole-brain activation clusters for the Alcohol > Water contrast. Specifically, anatomical anterior cingulate (ACC) and thalamus ROIs were defined using the Harvard-Oxford atlas and mean signal intensity was extracted for the Alcohol > Water contrast. The ventral striatum variable was individually replaced by ACC and thalamus activation in final models (with drinks per drinking day, medication, and OPRM1 as covariates).
RESULTS
Characteristics for the final sample of 41 participants who completed both fMRI and self-administration tasks are presented in Table 1. Study participants were, on average, younger adult heavy drinkers of Chinese or Korean descent, and a minority reported recent cigarette smoking and/or cannabis use.
Table 1.
Sample Characteristics (N=41)
| Variable | Statistic (M(SD)) |
|---|---|
| Age | 28.27 (6.94) |
| Sex (% Female) | 37% |
| Ethnicity (n(%)) | |
| Chinese | 17 (41.5%) |
| Japanese | 3 (7.3%) |
| Korean | 19 (46.3%) |
| Taiwanese | 2 (8%) |
| AUDIT Total | 14.46 (5.19) |
| 30-Day TLFB Drinking Days | 13.66 (6.56) |
| 30-Day TLFB Drinks Per Drinking Day | 4.79 (2.29) |
| Cigarette Smokers (n(%)) | 12 (29%) |
| 30-Day TLFB Cigarettes Per Day | 4.00 (4.89) |
| Cannabis Users (n(%)) | 4 (10%) |
| ADH1B (AA/AG/GG) | 5/7/19 |
| ALDH2 (AA/AG/GG) | 0/6/35 |
| OPRM1 (AA/AG/GG) | 18/17/6 |
| Placebo Self-Administration % who drank (n(%)) | 39 (53%) |
| Placebo Self-Administration Latency to First Drink (median) | 180 s |
| Naltrexone Self-Administration % who drank (n(%)) | 31 (41%) |
| Naltrexone Self-Administration Latency to First Drink (median) | 180 s |
| Placebo TLFB Pre-scan Days since Last Drink | 2.39 (2.20) |
| Placebo Alcohol > Water Ventral Striatum Activation | 1.44 (7.42) |
| Naltrexone TLFB Pre-scan Days since Last Drink | 2.85 (1.65) |
| Naltrexone Alcohol > Water Ventral Striatum Activation | 2.83 (9.08) |
| Washout Period TLFB Drinks Per Drinking Day | 4.86 (3.01) |
| Washout Period TLFB Cigarettes Per Day | 3.80 (4.23) |
Note. AUDIT = Alcohol Use Disorders Identification Test. TLFB = Timeline Follow-Back. Ventral Striatum contrast estimate units of measure are arbitrary units; higher values correspond to greater activation.
Fisher’s exact tests tested the association between medication condition and 24 possible side effects as indicated by the SAFTEE checklist (Levine and Schooler, 1986). These tests indicated a significant association between medication and nausea (p < .01), such that 20% of individuals on naltrexone and 0% of individuals on placebo reported experiencing nausea. Similarly, there was a significant association between medication and fatigue (p < .01), such that 25% of individuals on naltrexone and 0% of individuals on placebo reported experiencing fatigue. There were no other significant associations among the remaining 22 side effects and medication.
Ventral striatum activation and self-administration outcomes are also presented in Table 1 by medication condition. Of note, the two primary manuscripts from which this data is derived did not identify significant effects of naltrexone on ventral striatum activation or self-administration outcomes (total number of drinks and latency to first drink) (Lim et al., 2019; Ray et al., 2018b). Ventral striatum activation demonstrated moderate reliability (ICC = .47) and are consistent with other studies examining striatum in fMRI (Peters and Crone, 2017; Vetter et al., 2017). Ventral striatum activation was also not significantly associated with any of the covariate variables used in the following analyses (ps = .11–.86).
Latency to First Drink
The distribution of latencies to first drink was non-normal. Across medication conditions, 52% of individuals refrained from drinking throughout the paradigm, 29% consumed a drink within the first three minutes of the paradigm, and 19% of individuals consumed their first drink at some point during the remainder of the session. Cox regressions for latency to first drink indicated a significant effect of ventral striatum activation, Wald χ2 = 2.88, p = 0.05, such that those with lower ventral striatum activation exhibited longer latencies to first drink (see Figure 3). Significant covariates included medication condition, Wald χ2 = 5.99, p = 0.01, such that naltrexone was associated with longer latency to first drink. OPRM1 was also significant, Wald χ2 = 3.31, p = 0.03, such that Asn40Asn homozygotes exhibited shorter latency to first drink. Other covariates of interest (e.g. medication randomization order, gender, medication side effects) were not associated with latency to first drink (ps=.15–.98). There were also no interactions of medication X gender on self-administration outcomes. Finally, neither ACC nor thalamus effects were significantly associated with latency to first drink in separate models (ps > .46).
Figure 3.
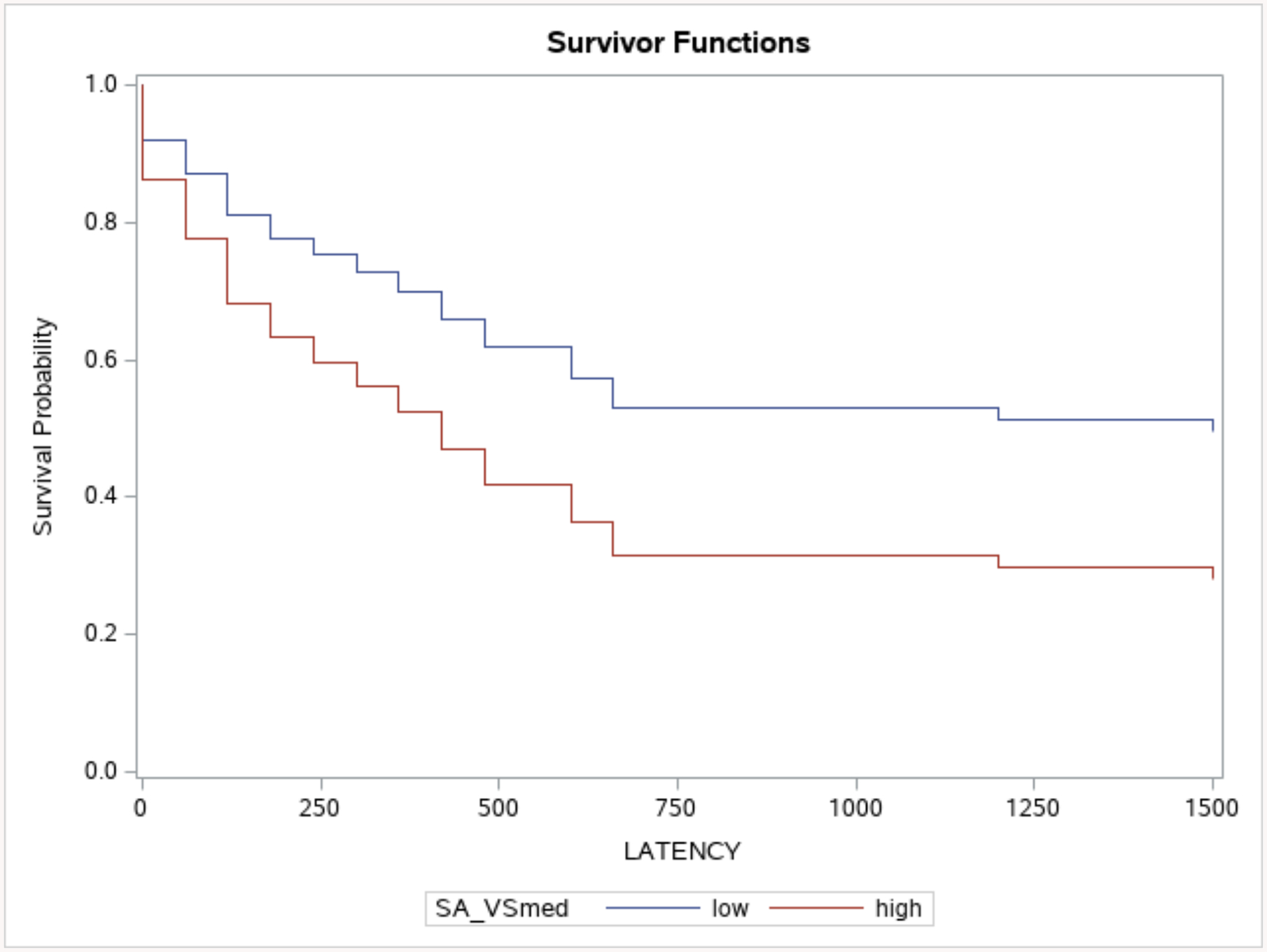
Multilevel cox regressions depicting the relationship between alcohol-elicited ventral striatum activation and subsequent latency to first drink (seconds), controlling for medication, OPRM1, and Timeline Follow-Back drinks per drinking day. Ventral striatum median-split activation (SA_VSmed; 0 = below median, 1 = above median) is for visualization purposes only.
Total Number of Drinks
Multilevel Poisson analyses for total number of consumed drinks indicated a significant effect of ventral striatum activation, F(1, 38) = 5.90, p = .02. Significant covariates included medication, F(1, 38) = 7.93, p = .01, with naltrexone yielding lower consumption (B(SE) = −.60(.21). OPRM1 genotype was also significant, F(1, 38) = 5.37, p = .03, such that Asn40Asn homozygotes consumed a greater number of drinks. Drinks per drinking day were not associated with consumption, F(1, 38) = 3.58, p = .07. Other covariates of interest (e.g. medication randomization order, gender, medication side effects) were also not associated with total number of drinks, ps=.13–.54. Finally, both ACC and thalamus effects were not significantly associated with total number of drinks in separate models (ps > .23).
DISCUSSION
This study examined the relationship between alcohol cue-induced ventral striatum activation and alcohol self-administration in the laboratory. Results from this heavy-drinking sample of East Asians indicated that higher ventral striatum activation was associated with a shorter latency to first self-administered drink. Similarly, ventral striatum activation was positively associated with the total number of drinks consumed during the self-administration paradigm in this sample. These results remained significant after controlling for severity of drinking patterns, OPRM1, and medication condition. The results were also not replicated in other brain regions that were activated during the Alcohol > Water contrast (i.e. ACC and thalamus), corroborating that these findings may be VS-specific.
Overall, this is the first study to examine whether neuroimaging outcomes of interest can predict responses within laboratory paradigms commonly used in the alcohol literature. This foundational work adds important validity to the hypothesized interplay between neural bases of alcohol craving and behavioral measures of alcohol seeking, namely alcohol self-administration in the human laboratory. These associations contribute to a growing literature on the translational value of neuroimaging paradigms in alcohol treatment, particularly in elucidating potential mechanisms through which self-administration paradigms in AUD research are related to real world alcohol consumption (Grodin and Ray, 2019; Hendershot et al., 2017). Such work is aligned with current efforts in behavioral treatments utilizing neuroimaging to study mechanisms of behavior change for substance use disorders; identifying those individuals with severe orbitofrontal cortex deficits, for instance, may be useful in guiding them away from treatments focused on increasing the salience of future negative consequences of substance use (Morgenstern et al., 2013). In a similar fashion, adjunctive fMRI has been used to train individuals with substance use disorders through resonance-based breathing to reduce visual processing of drug cues and increase activation in areas implicated in internally directed cognition (Bates et al., 2019). Elucidating the translational value of these various experimental paradigms is strongly indicated, as AUD medications can exhibit differential results based on the utilized paradigm (e.g. alcohol challenge or self-administration; (Chukwueke and Le Foll, 2019)) and such variability may in turn inform precision medicine efforts. Expanding the study of inter-experimental paradigms may also shed light on aspects of alcohol consumption unique to individual paradigms. For instance, a greater understanding of individuals’ experiences in the transition between the first and subsequent drinks may be an important point of clinical interventions when discussing naltrexone use.
While the primary aim of this study was not focused on genetic determinants of self-administration, it is notable that genotypes encoding the binding potential of mu-opioid receptors (OPRM1) were associated with self-administration outcomes. While it is theorized that individuals with at least one copy of the G-allele for OPRM1 (Asn40Asp) exhibit greater vulnerability to developing AUD, meta-analyses have been mixed, with findings that such an association may not be reliable (Kong et al., 2017; Sloan et al., 2018), are population specific (Chen et al., 2012), or that Asn40Asp confers a modest protective effect on general substance dependence in European-ancestry cohorts (Schwantes-An et al., 2016). In this study, Asn40Asp individuals exhibited lower total consumption relative to Asn40Asn at a statistical trend level, as well as slower latency to first drink. This finding is consistent with the primary analyses for this data (Ray et al., 2018b), which indicated that those with Asn40Asp also reported less severe drinking history and lower AUDIT scores compared to Asn40Asn homozygotes and may, in turn, help to explain these findings. In sum, we accounted for genetic factors in these analyses given their theoretical and practical salience (Hart and Kranzler, 2015), particularly in this population (Cservenka et al., 2017). And while the genetic findings are notable and largely consistent with the literature, the primary focus on the study is on the fMRI to human laboratory association. This is the area in which the present analyses make a substantive contribution to the literature by supporting a long hypothesized, yet rarely tested, association between brain and behavior.
Finally, this study identified significant effects of naltrexone in increasing latency to first drink and decreasing total alcohol consumption. Notably, while these contrast the primary study (N=77) results from which the data are derived (Ray et al., 2018b) the current study is a secondary analysis of a subsample of participants (N=41) who had completed both neuroimaging sessions. While inclusion of VS activation may have helped to improve model fit, the primary study had greater power in order to test pharmacogenetic effects. For these reasons, while it is possible that consideration of neuroimaging outcomes help elucidate AUD pharmacotherapy effects, replication using larger samples is warranted.
On balance, this study should be interpreted in light of its strengths and limitations. Strengths included assessment of multiple experimental procedures used in the medication development literature and consideration of multiple psychiatric and genetic predictors of self-administration in the statistical analyses. Another strength is the test of hypothesis at the within-subjects level of analysis. As argued by Curran and Bauer (2011), several psychological processes which are inherently within-person processes, such as the relationship between how one’s brain processes alcohol cues and how much s/he wants to drink in the future, are presumed to be explained in between-subjects models, when in fact, within-subject analyses provide a more representative test of the process at hand (Curran and Bauer, 2011). Thus, a within-subjects approach represents a more robust, and methodologically adequate, test of the association between brain and behavior. One of the most important limitations of the current study is a constrained sample and power – alpha corrections were not implemented in this exploratory study. Similarly, models that solely included ventral striatum indicated a nonsignificant association with latency to first drink (p = .08) and a significant positive association with total number of drinks (p = .04); while covariate selection was based on incorporating important factors in the study design (e.g. medication, OPRM1), replication using larger sample sizes and similar models is particularly needed to elucidate these relationships and the impact of covariates. A limitation of the taste cues fMRI paradigm used in this study is that it was modified to reduce trial duration in order to increase the number of trials for analysis; in contrast to the original task (Filbey et al., 2008a), a whole-brain analysis of the task did not elicit significant clusters of mesocorticolimbic, including ventral striatum, activation. Therefore, replication using other tasks that more strongly elicit ventral striatum activation are needed, both to induce significant enough variability to test medication effects and also to translate such effects into another subsequent experimental modality. Variations of the Monetary Incentive Delay task that administer beer may be particularly useful in disentangling whether anticipation, relative to receipt, of alcohol taste are differently discriminant in predicting self-administration (Groefsema et al., 2019). Relatedly, the taste cues paradigm was limited to the choice of red or white wine, which did not always correspond with participants’ drink of choice; while this correspondence was not a significant covariate in self-administration outcomes, administering drink of choice may increase external validity of the imaging task. Another potential weakness is that medication effects from the primary manuscripts were null; future studies are needed to corroborate that medication effects are consistent across paradigms, particularly in identifying significant such effects. An additional warranted question is whether such consistency of medication effects in laboratory studies would translate directly to clinical outcomes and treatment-seeking populations. Lastly, the “priming dose” that preceded the self-administration period was higher than the usual 0.03 g/dl reported in the literature. While the higher priming dose of alcohol in this study did not suppress alcohol self-administration, it may be interpreted differently in that participants were seeking to self-administer to reach high levels of BrAC, perhaps binge-like levels. If that was the case, results would remain highly relevant and consistent with recent efforts to phenotype binge-drinking in the human laboratory (Gowin et al., 2017).
Limitations notwithstanding, the present findings provide proof-of-concept that neuroimaging and laboratory paradigms may be closely linked. Further, neuroimaging may be a useful tool to explore in greater detail how different paradigms are related to real world consumption behavior. Future studies are warranted to replicate the current results and to identify, refine, and implement translational paradigms in AUD research.
Supplementary Material
Table 2.
Outcomes for latency to first drink and total number of drinks
| Outcome: Latency to First Drink | ||
|---|---|---|
| Variable | Wald Chi-Square | Adjusted p-Value |
| Ventral Striatum | 2.88 | .05 |
| Medication | 5.99 | .01 |
| OPRM1 | 3.31 | .03 |
| TLFB Drinks Per Drinking Day | 6.39 | .003 |
| Outcome: Total Number of Drinks | ||
| Variable | Estimate (SE) | p-Value |
| Ventral Striatum | .03(.01) | .02 |
| Medication | −.60(.21) | .01 |
| OPRM1 | .78(.34) | .03 |
| TLFB Drinks Per Drinking Day | .13(.07) | .07 |
Note.TLFB = Timeline Follow-Back. Latency to first drink outcomes generated from cox frailty models that produce adjusted degrees of freedom and p-values. Total number of drinks outcomes generated from multilevel poisson models.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (R01AA021744) and the UCLA Clinical and Translational Science Institute (CTSI) (grants UL1RR033176 and UL1TR000124). This work is also supported by the Tobacco Related Disease Research Program Grants T29DT0371 &T30DT0950; the National Institute on Alcohol Abuse and Alcoholism Grants F32AA027699, 3R01AA026190-02S1 & K24AA025704. LAR has received study medication from Pfizer and Medicinova and consulted for GSK. The authors report no other conflict of interest.
References
- Allen JP, Litten RZ, Fertig JB, Babor T (1997) A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 21:613–619. [PubMed] [Google Scholar]
- Bach P, Weil G, Pompili E, Hoffmann S, Hermann D, Vollstadt-Klein S, Mann K, Perez-Ramirez U, Moratal D, Canals S, Dursun SM, Greenshaw AJ, Kirsch P, Kiefer F, Sommer WH (2019) Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict Biol. [DOI] [PubMed] [Google Scholar]
- Bates ME, Lesnewich LM, Uhouse SG, Gohel S, Buckman JF (2019) Resonance-Paced Breathing Alters Neural Response to Visual Cues: Proof-of-Concept for a Neuroscience-Informed Adjunct to Addiction Treatments. Front Psychiatry 10:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A (2012) Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry 69:842–852. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Jentsch JD, Roche DJO, Ramchandani VA, Miotto K, Ray LA (2018) Differences in the subjective and motivational properties of alcohol across alcohol use severity: application of a novel translational human laboratory paradigm. Neuropsychopharmacology 43:1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Liu L, Xiao Y, Peng Y, Yang C, Wang Z (2012) Ethnic-specific meta-analyses of association between the OPRM1 A118G polymorphism and alcohol dependence among Asians and Caucasians. Drug Alcohol Depend 123:1–6. [DOI] [PubMed] [Google Scholar]
- Chukwueke CC, Le Foll B (2019) The Human Laboratory and Drug Development in Alcohol Use Disorder: Recent Updates. Methods Mol Biol 2011:195–219. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE (2011) Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Yardley MM, Ray LA (2017) Review: Pharmacogenetics of alcoholism treatment: Implications of ethnic diversity. Am J Addict 26:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ (2011) The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol 62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, Austad CS, Raskin SA, Tennen H, Wood RM, Fallahi CR, Pearlson GD (2014) Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 109:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE (2008a) Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 33:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE (2008b) Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res 32:1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. Am J Psychiatry 174:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Grodin E, Lim AC, Venegas A, Bujarski S, Krull J, Ray LA (2019) The Interplay Between Subjective Response to Alcohol, Craving, and Alcohol Self-Administration in the Human Laboratory. Alcohol Clin Exp Res 43:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Ray LA (2019) The Use of Functional Magnetic Resonance Imaging to Test Pharmacotherapies for Alcohol Use Disorder: A Systematic Review. Alcohol Clin Exp Res 43:2038–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groefsema MM, Engels R, Voon V, Schellekens AFA, Luijten M, Sescousse G (2019) Brain responses to anticipating and receiving beer: Comparing light, at-risk, and dependent alcohol users. Addict Biol:e12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Kranzler HR (2015) Alcohol Dependence Genetics: Lessons Learned From Genome-Wide Association Studies (GWAS) and Post-GWAS Analyses. Alcohol Clin Exp Res 39:1312–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J (2009) Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol 14:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Samokhvalov AV, Rehm J (2017) Effects of naltrexone on alcohol self-administration and craving: meta-analysis of human laboratory studies. Addict Biol 22:1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Dennis LE, McCready H, Hoffman WF (2017) Executive Control and Striatal Resting-State Network Interact with Risk Factors to Influence Treatment Outcomes in Alcohol-Use Disorder. Front Psychiatry 8:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Deng H, Gong S, Alston T, Kong Y, Wang J (2017) Lack of associations of the opioid receptor mu 1 (OPRM1) A118G polymorphism (rs1799971) with alcohol dependence: review and meta-analysis of retrospective controlled studies. BMC Med Genet 18:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler NR (1986) SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull 22:343–381. [PubMed] [Google Scholar]
- Lim AC, Ghahremani DG, Grodin EN, Green R, Bujarski S, Hartwell EE, Courtney KE, Hutchison K, Miotto K, Ray LA (2019) Neuroimaging findings from an experimental pharmacology trial of naltrexone in heavy drinkers of East Asian descent. Drug Alcohol Depend 200:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN (2014) Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res 38:2754–2762. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS (2006) Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 189:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Jones SA, Ehlers A, Lavine JB, Nagel BJ (2018) Ventral striatal response during decision making involving risk and reward is associated with future binge drinking in adolescents. Neuropsychopharmacology 43:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Naqvi NH, Debellis R, Breiter HC (2013) The contributions of cognitive neuroscience and neuroimaging to understanding mechanisms of behavior change in addiction. Psychol Addict Behav 27:336–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L, Riddle M, King JW, Aklin WM, Chen W, Clark D, Collier E, Czajkowski S, Esposito L, Ferrer R, Green P, Hunter C, Kehl K, King R, Onken L, Simmons JM, Stoeckel L, Stoney C, Tully L, Weber W (2018) The NIH Science of Behavior Change Program: Transforming the science through a focus on mechanisms of change. Behav Res Ther 101:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Knodt AR, Radtke SR, Hariri AR (2016) Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol Psychiatry 21:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Harezlak J, Kudela MA, Tran SM, Soeurt CM, Yoder KK, Kareken DA (2016) Corticostriatal and Dopaminergic Response to Beer Flavor with Both fMRI and [(11) C]raclopride Positron Emission Tomography. Alcohol Clin Exp Res 40:1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Crone EA (2017) Increased striatal activity in adolescence benefits learning. Nat Commun 8:1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Roche DJO, Magill M (2018a) Overcoming the “Valley of Death” in Medications Development for Alcohol Use Disorder. Alcohol Clin Exp Res 42:1612–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Hutchison KE, Mackillop J, Galvan A, Ghahremani DG (2014) Initial evidence that OPRM1 genotype moderates ventral and dorsal striatum functional connectivity during alcohol cues. Alcohol Clin Exp Res 38:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Green R, Roche DJO, Bujarski S, Hartwell EE, Lim AC, Rohrbaugh T, Ghahremani D, Hutchison K, Miotto K (2018b) Pharmacogenetic Effects of Naltrexone in Individuals of East Asian Descent: Human Laboratory Findings from a Randomized Trial. Alcohol Clin Exp Res 42:613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Roche DJ (2018) Neurobiology of Craving: Current Findings and New Directions. Current Addiction Reports 5:102–109. [Google Scholar]
- Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Heinz A, Kiefer F, Smolka MN, Wellek S, Mann K, Vollstadt-Klein S (2015) A comparison of region-of-interest measures for extracting whole brain data using survival analysis in alcoholism as an example. J Neurosci Methods 242:58–64. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H (2013) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF (2017) Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology 42:2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Paulus MP, Tapert SF, Simmons AN, Tolentino NJ, Shafir A (2016) The Ability of Functional Magnetic Resonance Imaging to Predict Heavy Drinking and Alcohol Problems 5 Years Later. Alcohol Clin Exp Res 40:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwantes-An TH, Zhang J, Chen LS, Hartz SM, Culverhouse RC, Chen X, Coon H, Frank J, Kamens HM, Konte B, Kovanen L, Latvala A, Legrand LN, Maher BS, Melroy WE, Nelson EC, Reid MW, Robinson JD, Shen PH, Yang BZ, Andrews JA, Aveyard P, Beltcheva O, Brown SA, Cannon DS, Cichon S, Corley RP, Dahmen N, Degenhardt L, Foroud T, Gaebel W, Giegling I, Glatt SJ, Grucza RA, Hardin J, Hartmann AM, Heath AC, Herms S, Hodgkinson CA, Hoffmann P, Hops H, Huizinga D, Ising M, Johnson EO, Johnstone E, Kaneva RP, Kendler KS, Kiefer F, Kranzler HR, Krauter KS, Levran O, Lucae S, Lynskey MT, Maier W, Mann K, Martin NG, Mattheisen M, Montgomery GW, Muller-Myhsok B, Murphy MF, Neale MC, Nikolov MA, Nishita D, Nothen MM, Nurnberger J, Partonen T, Pergadia ML, Reynolds M, Ridinger M, Rose RJ, Rouvinen-Lagerstrom N, Scherbaum N, Schmal C, Soyka M, Stallings MC, Steffens M, Treutlein J, Tsuang M, Wall TL, Wodarz N, Yuferov V, Zill P, Bergen AW, Chen J, Cinciripini PM, Edenberg HJ, Ehringer MA, Ferrell RE, Gelernter J, Goldman D, Hewitt JK, Hopfer CJ, Iacono WG, Kaprio J, Kreek MJ, Kremensky IM, Madden PA, McGue M, Munafo MR, Philibert RA, Rietschel M, Roy A, Rujescu D, Saarikoski ST, Swan GE, Todorov AA, Vanyukov MM, Weiss RB, Bierut LJ, Saccone NL (2016) Association of the OPRM1 Variant rs1799971 (A118G) with Non-Specific Liability to Substance Dependence in a Collaborative de novo Meta-Analysis of European-Ancestry Cohorts. Behav Genet 46:151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Sinha R (2014) The neurobiology of alcohol craving and relapse. Handb Clin Neurol 125:355–368. [DOI] [PubMed] [Google Scholar]
- Sloan ME, Klepp TD, Gowin JL, Swan JE, Sun H, Stangl BL, Ramchandani VA (2018) The OPRM1 A118G polymorphism: converging evidence against associations with alcohol sensitivity and consumption. Neuropsychopharmacology 43:1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- Vetter NC, Steding J, Jurk S, Ripke S, Mennigen E, Smolka MN (2017) Reliability in adolescent fMRI within two years - a comparison of three tasks. Sci Rep 7:2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell JD, Le Foll B, Hendershot CS (2018) Preliminary evaluation of a human laboratory model of impaired control over alcohol using intravenous alcohol self-administration. J Psychopharmacol 32:105–115. [DOI] [PubMed] [Google Scholar]
- Wardell JD, Ramchandani VA, Hendershot CS (2015) A multilevel structural equation model of within- and between-person associations among subjective responses to alcohol, craving, and laboratory alcohol self-administration. J Abnorm Psychol 124:1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Ross TJ, O’Connor S, Stein EA, de Wit H, Childs E (2018) Striatal activity correlates with stimulant-like effects of alcohol in healthy volunteers. Neuropsychopharmacology 43:2532–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Litten RZ, Leggio L (2019) Advances in the science and treatment of alcohol use disorder. Sci Adv 5:eaax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Ray LA (2017) Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addict Biol 22:581–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 13:315–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


