The presence of Amyloid b (Ab) aggregates as ‘plaques’ in brains is a neuropathologic hallmark of Alzheimer’s disease (AD). Ab is generated by sequential cleavage of the amyloid precursor protein (APP) by b-site secretase enzyme (BACE-1) and g-secretase, with BACE-1-cleavage as the rate-limiting step. Both APP and BACE-1 are membrane-spanning proteins synthesized via the ERàGolgi pathway. Thereafter, both proteins are transported in vesicles, eventually leading to convergence of these two proteins, and cleavage of APP by BACE-1. Thus, physical proximity of APP and BACE-1 is a crucial and early step in Ab production; and it has been long recognized that deciphering the trafficking steps of APP and BACE-1 – leading up-to APP-cleavage – are critical for designing therapies to perturb Aβ production. In this short review, we discuss the known intracellular itineraries of APP and BACE-1, focusing on the trafficking/sorting pathways that lead to their co-localization and APP processing.
APP/BACE-1 convergence
Most studies on APP and BACE-1 trafficking have been done in nonneuronal cells. In these cells, APP is predominantly localized to the trans-Golgi network (TGN) and Golgi-derived vesicles, with low levels along the cell surface and endosomal compartments (Caporaso et al., 1994; Koo et al., 1996). BACE-1 is predominantly within the TGN and endosomal compartments, with relatively low levels along the cell surface (Huse et al., 2000; Yan et al., 2001). Thus the TGN, plasma membrane, and endosomes are the main sorting stations for APP and BACE-1, and likely sites where APP and BACE-1 converge. Colocalization studies show that APP and BACE-1 are present in the TGN and endosomal compartments (Greenfield et al, 1999, Prasad et al., 2015).
Few studies have directly looked at the physical convergence of APP and BACE-1. Using fluorescence resonance energy transfer (FRET), Kinoshita et al. found that APP and BACE-1 interact along plasma membrane and early endosomal compartments in H4 neuroglioma cells (Kinoshita et al., 2003). Recently, we developed an assay to visualize the physical convergence of APP and BACE-1 using fluorescence complementation (the optical convergence of APP and BACE-1, or OptiCAB assay, (Das et al., 2016)). Using this assay in cultured hippocampal neurons, we found that APP and BACE-1 interact in both ER/Golgi and endocytic compartments; particularly in recycling zones such as dendritic spines and presynaptic boutons. In other studies, we also found that after synthesis in the ER/Golgi, BACE-1 is selectively sorted into recycling endosomes (Das et al., 2013). Collectively, the data support a model where APP and BACE-1 are sorted into distinct vesicles after biogenesis. However, under conditions that promote amyloidogenesis, APP undergoes endocytosis and converges with BACE-1 in recycling endosomes, where β-cleavage occurs (Figure 1). Using the OptiCAB assay, Ferguson and colleagues recently reported an increase in APP/BACE-1 convergence in axons upon disruption of lysosomal transport (Gowrishankar et al., 2017). Also, blockage of BACE-1 recycling to the axonal plasma membrane by BIN1, or blockage of APP sorting in dendrites by CD2AP enhances APP/BACE-1 colocalization (Ubelmann et al., 2017). Interestingly, unlike dendrites, APP and BACE-1 were found to be colocalized in axons (Das et al., 2016); supporting the view that they are differentially regulated in these two domains. Collectively, the data indicate that APP/BACE-1 sorting events play key roles in their convergence, and further emphasize the importance of endosomes for APP/BACE-1 interaction and initiation of the amyloidogenic pathway.
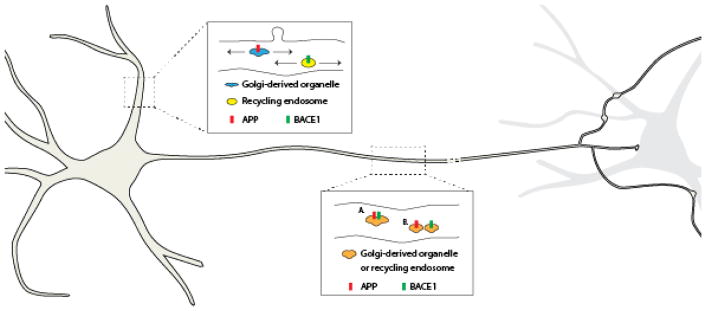
Figure 1. APP and BACE-1 transport in neurons.
In dendrites (upper inset), APP is mainly transported in Golgi-derived organelles, while BACE-1 moves in recycling endosomes. The initial segregation of APP and BACE-1 appears to occur in the soma after synthesis, where BACE-1 is sorted into recycling endosomes. The internalized fraction of BACE-1 in dendrites move in a persistently retrograde manner, while internalized dendritic APP is not very mobile (not shown). In axons (lower inset), moving APP and BACE-1 are co-localized, suggesting that they are either in a single vesicle (A.), or in closely spaced vesicles that cannot be resolved by conventional light microscopy (B.). The reason for this differential trafficking of APP and BACE-1 in axons and dendrites is unknown, but may be related to transcytosis. The precise identities of the axonal organelles in anterograde and retrograde transport are also unclear.
Intracellular itinerary of APP
APP is ubiquitously expressed in all tissues, with particularly high levels in the brain. It is a single-pass transmembrane protein with a large extracellular N-terminus and a short cytosolic C-terminus (Reinhard et al., 2005). Biogenesis and trafficking of APP has also been mostly studied in non-neuronal cells. After protein synthesis in ER-associated polysomes, APP is N-glycosylated in the ER and then transported to the Golgi for O- and N-glycosylation, phosphorylation and sulphonation at tyrosine sites (Lai et al., 1995). At steady state, most of the APP is found in the Golgi and trans-Golgi network (TGN), while a small portion is sorted to the plasma membrane (Kuentzel et al., 1993).
In neurons, tubulovesicular structures carrying APP are seen (Szodorai et al., 2009) in both dendrites in axons, but the transport behavior is very different in these two compartments. While the movement of dendritic APP vesicles is very interrupted – with short bursts of transport and frequent reversals – APP transport in axons is mostly persistent, with rare reversals (Das et al., 2016; Kaether et al., 2000; Tang et al., 2012). Axonal transport of APP is mediated by kinesin-1 (Ferreira et al., 1993; Kamal et al., 2001), and the retrograde transport is presumably dynein-dependent. Though the motors involved in dendritic transport are not established, kinesin-I may be involved, as the instantaneous velocity of moving APP vesicles is similar in axons and dendrites (compare APP velocity parameters in dendrites - Supp. Table 1 of (Das et al., 2013) to axons – Supp. Table 1 of (Das et al., 2016)). APP transport is regulated by the scaffold protein c-Jun N-terminal kinase-interacting protein 1 (JIP1), which links APP to kinesin slight chain-1 (Klc-1, (Matsuda et al., 2003)). JIP1 is also reported to regulate kinesin heavy chain – via phosphorylation of the S421 site in JIP1 – thereby controlling the directionality of APP transport (Fu and Holzbaur, 2013).
Through a pulse-chase analysis of newly synthesized APP, Toh et al recently found that APP can be directly transported from Golgi to early endosomes through secretory pathway (Toh et al., 2017). Only a small portion of APP is detected at plasma membrane because the cell surface APP is rapidly internalized (Koo and Squazzo, 1994). Along the plasma membrane, APP is cleaved by a-secretase via a “non-amyloidogenic” process (De Strooper and Annaert, 2000; Sisodia, 1992). Importantly, the a-secretase enzyme cuts APP within the Ab sequence, thereby precluding the BACE-1-mediated amyloidogenic pathway; thus α-cleavage is thought to be protective (Haass et al., 2012). APP internalization from the plasma membrane is mediated by clathrin-dependent endocytosis, which requires the canonical NPXY motif (Das et al., 2013; Lai et al., 1995). Though the precise fate of this internalized APP is still unclear, it is thought to return to the cell surface, degraded in lysosomes, or transported back to the TGN via a retromer-dependent pathway (Vieira et al., 2010). We found that in neurons, a substantial fraction of surface APP is routed into recycling endosomes, the latter enriched in BACE-1 (Das et al., 2013). However, a portion of the internalized APP is also routed into LAMP-1 positive late endosomes (Das et al., 2013). Since BACE-1 is not enriched in this compartment, we speculate that this might be a pathway by which excessive β-cleavage is avoided. Alternatively, this may also reflect post-endosomal sorting/retrieval of APP (see later).
Intracellular itinerary of BACE-1
BACE-1 is a transmembrane aspartyl protease with its catalytic domain at the extracellular site (Hong et al., 2000), and the enzyme is optimally active at acidic pH (Vassar et al., 1999). BACE-2, a homologue of BACE-1, has minimal effect on b-cleavage, though it may affect α-cleavage (Farzan et al., 2000). Importantly, BACE-1 knockout completely blocks b-cleavage and Ab generation (Cai et al., 2001), indicating that this is a critical rate limiting enzyme in the production of Aβ. BACE-1 is promiscuous, with many other substrates in addition to APP, such as voltage-gated sodium channel b2-subunit (Wong et al., 2005), neuregulin 1 (Willem et al., 2006), sialyltransferase ST6GAL1 (Kitazume et al., 2001), and P-selectin glycoprotein ligand-1 (Lichtenthaler et al., 2003). BACE-1 knockout mice show abnormal myelination (Hu et al., 2006; Willem et al., 2006) and reduced spine density in hippocampal pyramidal neurons (Savonenko et al., 2008), attesting to the roles of this enzyme outside the amyloidogenic pathway. Thus, enzymatic inhibition of BACE-1 – an approach pursued by most pharmaceutical companies – may not be the best strategy, and other avenues such as inhibition of APP/BACE-1 protein-protein interaction, or interference with APP/BACE-1 trafficking pathways before convergence, may be better alternatives.
Immature BACE-1 is synthesized in the ER with a short prodomain; and after transport to the Golgi, furin or furin-like proteases removes the prodomain (Creemers et al., 2001). Removal of the prodomain allows proper folding of the protease domain of BACE-1 and enhances its activity by two-fold (Shi et al., 2001). In neurons, a large proportion of BACE-1 is localized to recycling endosomes (Das et al., 2016). Cell surface BACE-1 is quickly internalized, and a dileucine motif at the cytoplasmic tail of BACE-1 is thought to facilitate this internalization (He et al., 2005). Thus, there are two routes by which BACE-1 is sorted to endosomal compartments – direct transport from TGN; or internalization from plasma membrane. After internalization, the dendritic BACE-1 undergoes retrograde transport, regulated by the Eps-15-homology-domain-containing (EHD) proteins, a family of endocytosis regulators (Buggia-Prevot et al., 2013). In addition, axonal BACE-1 is also retrogradely transported by dynein and adaptor Snapin, which is important for BACE-1 degradation (Ye and Cai, 2014; Ye et al., 2017).
Key regulators of APP/BACE-1 sorting/retrieval in amyloidogenic pathway
Internalization from the plasma membrane, and subsequent convergence of APP and BACE-1 is regulated by various adaptor/linker proteins (Figure 2). For example, APP interacts with the adaptor protein X11 through its YENPTY motif (Borg et al., 1996). This interaction retains APP in detergent-sensitive membranes; separating it from BACE-1, which is largely present in detergent-resistant membranes (Saito et al., 2008). Inhibition of this “APP sequestering” mechanism by knockout or phosphorylation of X11 causes a shift of APP into BACE-1-containing microdomains and Aβ overproduction (Saito et al., 2008; Sakurai et al., 2008; Sano et al., 2006). APP endocytosis is also regulated by the adaptor protein Dab2, via interaction with the APP NPXY endocytosis motif (Lee et al., 2008; Nordstedt et al., 1993). In contrast to APP, internalization of BACE-1 is thought to be regulated by ADP ribosylation factor 6 (Arf6) and AP-2 complex (Prabhu et al., 2012; Sannerud et al., 2011).
Figure 2. Sorting routes of endosomal APP and BACE-1.
APP and BACE-1 interact in acidic endosomes after internalization. Further sorting/retrieval pathways help degrade and/or recycle these proteins, limiting excessive APP β-cleavage in acidic endosomes. The retromer complex binds to the adaptor protein sorLA, which in turn binds to APP and mediates its retrieval to the trans-Golgi network. APP recycling back to plasma membrane is mediated by sorLA, SNX27, SNX15 and Par3/Numb. BECN1, CD2AP and PI3P/VPS34 modulate APP turnover and stability by sorting it into lysosomes. The retrieval pathway of BACE-1 is mediated by the retromer complex and sorting proteins GGA and PACS1. The recycling pathway of BACE-1 may be regulated by Rab11, and sorting of BACE-1 to lysosome for degradation is thought to be mediated by BIN1, Snapin, GGA3 and SNX4.
After APP and BACE-1 convergence in endosomes, they are further sorted/retrieved into various cellular compartments. Understanding these sorting/retrieval pathways could also offer new therapeutic avenues to decrease APP-cleavage products. Under physiological conditions, APP and BACE-1 can be further sorted via three trafficking pathways: 1) retrieval to TGN; 2) recycling back to the plasma membrane; and 3) trafficking into lysosomes for degradation. Abnormal residence time of APP and BACE-1 in endosomes can lead to increased cleavage of APP, and this might be the pathological mechanism of sporadic AD (Gowrishankar et al., 2017). Studies have begun to shed light into the sorting/retrieval pathway in AD. A key regulator of APP retrieval pathway is sorLA (sorting protein-related receptor with A-type repeats) (Andersen et al., 2005), a scaffold protein linking APP to the retromer complex (Fjorback et al., 2012). Overexpression of sorLA leads to the accumulation of APP in Golgi and reduction in Aβ production (Andersen et al., 2005). Genome-wide association studies (GWAS) also show an association of sorLA with sporadic, late-onset AD (Lambert et al., 2013). In mammals, the retromer complex is essential for retrieval transport, and it has been implicated in APP endosomal sorting in AD as well (Muhammad et al., 2008; Vieira et al., 2010).
Fjorback et al. found that the c-terminus FANSHY motif of sorLA interacts with the VPS26 subunit of the retromer complex, linking APP to the retromer (Fjorback et al., 2012). Disruption of this interaction redistributes APP to the endosomal network and increase amyloid processing. Knockdown of VPS35, the core element of the retromer complex, leads to accumulation of APP in endosomes along the processes of neurons and increases co-localization of APP and BACE-1 (Bhalla et al., 2012). In addition, the retrograde transport of the retromer complex is driven by motor protein dynein. Dysfunction of dynein blocks retrograde transport of retromer and APP, resulting in endosomal accumulation of APP and increased β-cleavage (Kimura et al., 2016). Endosomal APP can also recycle with the cell surface. The sorting nexins and the polarity protein Par3 play important roles in this process. Specifically, sorting nexin-27 (SNX27) positively regulates APP endosome-to-cell surface recycling by forming a ternary complex with APP and sorLA through interaction with c-terminus of sorLA (Huang et al., 2016). Similarly, SNX15 also increases cell surface level of APP by accelerating APP recycling (Feng et al., 2016). Par3 competes with the endocytic adaptor protein Numb to associate with APP, accelerating APP sorting to recycling endosomes, and recycling it back to plasma membrane (Sun et al., 2016). Acceleration of APP sorting to recycling endosomes and its recycling back to plasma membrane may protect APP from BACE-1-cleavage, reducing Aβ production. APP localized in early endosomes is further sorted into intraluminal vesicles (ILV) for degradation. Key molecules, CD2AP and phosphatidylinositol-3-phosphate (PI3P)/VPS34, were found to regulate this process (Morel et al., 2013; Ubelmann et al., 2017). Downregulation of these two pathways is thought to constrain APP at the limiting membrane of early endosomes, inhibiting APP sorting to ILV and increasing Aβ generation. The APP lysosome targeting is regulated by autophagy regulatory protein Beclin-1 (BECN1, see (Swaminathan et al., 2016)). BECN1 targets the internalized APP to lysosomes for degradation, while BECN1 phosphorylation at Ser295 inhibits BECN1/APP interaction and subsequent APP degradative sorting.
Regarding BACE-1 retrieval trafficking, the retromer component SNX6 is thought to interact with BACE-1, regulating its retrograde transport (Small et al., 2005). Disruption of other retromer components, VPS26 and VPS35, blocks BACE-1 retrograde transport (He et al., 2005; Wen et al., 2011). Also, the sorting proteins GGA and PACS1, which mediate protein trafficking between TGN and endosomes, interacts with cytosolic dileucine motif of BACE-1 for its retrieval trafficking (Bonifacino, 2004; He et al., 2005; Sun and Zhang, 2017; Wan et al., 1998). The interaction between BACE-1 and these sorting proteins requires the phosphorylation of BACE-1 at Serine 498 by casein kinase 1 and aPKC/Par3 complex (Sun and Zhang, 2017; von Arnim et al., 2004; Walter et al., 2001).
Regulators of the BACE-1 recycling pathway were recently identified by a Rab-GTPase screen, where the authors found that Rab11 could specifically regulate recycling of the endosomal BACE-1 back to the plasma membrane, and thus control Aβ generation (Udayar et al., 2013). Pathways of BACE-1 degradation are also becoming clear. BIN1, a genetic risk factor of late-onset AD found in GWAS studies, binds to BACE-1, regulating its lysosomal targeting (Lambert et al., 2013; Miyagawa et al., 2016). Depletion of BIN1 retains BACE-1 in early endosomes and reduces BACE-1 lysosomal degradation, leading to increased Aβ production. The SNARE-associated protein snapin is also thought to regulate BACE-1 trafficking from late endosomes to lysosomes (Ye and Cai, 2014). In addition, GGA3 and SNX4 were also found to regulate BACE-1 stability by sorting it to lysosome for degradation (Kim et al., 2017; Tesco et al., 2007). However, the cooperation between these pathways to regulate BACE-1 degradation is still unclear.
Summary
Pathways related to biogenesis and trafficking of APP and BACE-1 have been long recognized as important, and there has been significant progress. Yet many of the pathways are not firmly established, and their roles in α and β cleavage of APP are unclear. Cellular trafficking pathways are deeply intertwined, and the interrelationship between APP/BACE-1 biogenesis, trafficking, endocytosis and sorting is still uncertain. There are also some discrepancies in the literature that need to be resolved as we move forward. For instance, APP β-cleavage is thought to occur in early endosomes, and yet at steady-state, most of the BACE-1 in neurons appears to be in recycling endosomes.
One important issue is that a vast majority of the trafficking experiments have been done in nonneuronal cells that lack the unique morphology and polarization of neurons. For example, neurons have axons and dendrites that are several orders of magnitude longer than their minuscule cell bodies – the latter is presumably the anatomic correlate of a nonneuronal cell – and neurons also have pre- and post- synaptic specializations, where vesicle trafficking is known to be distinct from other compartments. While the utility of nonneuronal cells in unraveling the basic trafficking pathways of APP and BACE-1 is unquestionable, concepts emerging from these cells cannot simply be transferred to neurons. Furthermore, unbiased, discovery based studies that look at the entirety of APP/BACE-1 trafficking pathways are also needed. The translational importance of APP/BACE-1 convergence in AD, the many unanswered questions surrounding this key phenomenon, and the lack of any disease modifying agent to date is a perfect trifecta for the coming generations of scientific explorers.
Acknowledgments
Work in the Roy laboratory is supported by NIH grants R01NS075233, R01AG048218 and R21 AG052404.
References
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A, Vetanovetz CP, Morel E, Chamoun Z, Di Paolo G, Small SA. The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiology of disease. 2012;47:126–134. doi: 10.1016/j.nbd.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS. The GGA proteins: adaptors on the move. Nature reviews Molecular cell biology. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Borg JP, Ooi J, Levy E, Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Molecular and cellular biology. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggia-Prevot V, Fernandez CG, Udayar V, Vetrivel KS, Elie A, Roseman J, Sasse VA, Lefkow M, Meckler X, Bhattacharyya S, et al. A function for EHD family proteins in unidirectional retrograde dendritic transport of BACE1 and Alzheimer’s disease Abeta production. Cell Rep. 2013;5:1552–1563. doi: 10.1016/j.celrep.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nature neuroscience. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Caporaso GL, Takei K, Gandy SE, Matteoli M, Mundigl O, Greengard P, De Camilli P. Morphologic and biochemical analysis of the intracellular trafficking of the Alzheimer beta/A4 amyloid precursor protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:3122–3138. doi: 10.1523/JNEUROSCI.14-05-03122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers JW, Ines Dominguez D, Plets E, Serneels L, Taylor NA, Multhaup G, Craessaerts K, Annaert W, De Strooper B. Processing of beta-secretase by furin and other members of the proprotein convertase family. J Biol Chem. 2001;276:4211–4217. doi: 10.1074/jbc.M006947200. [DOI] [PubMed] [Google Scholar]
- Das U, Scott DA, Ganguly A, Koo EH, Tang Y, Roy S. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron. 2013;79:447–460. doi: 10.1016/j.neuron.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U, Wang L, Ganguly A, Saikia JM, Wagner SL, Koo EH, Roy S. Visualizing APP and BACE-1 approximation in neurons yields insight into the amyloidogenic pathway. Nature neuroscience. 2016;19:55–64. doi: 10.1038/nn.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. Journal of cell science. 2000;113(Pt 11):1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H. BACE2, a beta -secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9712–9717. doi: 10.1073/pnas.160115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Niu M, Ji C, Gao Y, Wen J, Bu G, Xu H, Zhang YW. SNX15 Regulates Cell Surface Recycling of APP and Abeta Generation. Molecular neurobiology. 2016;53:3690–3701. doi: 10.1007/s12035-015-9306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Caceres A, Kosik KS. Intraneuronal compartments of the amyloid precursor protein. J Neurosci. 1993;13:3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjorback AW, Seaman M, Gustafsen C, Mehmedbasic A, Gokool S, Wu C, Militz D, Schmidt V, Madsen P, Nyengaard JR, et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:1467–1480. doi: 10.1523/JNEUROSCI.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MM, Holzbaur EL. JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. The Journal of cell biology. 2013;202:495–508. doi: 10.1083/jcb.201302078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar S, Wu Y, Ferguson SM. Impaired JIP3-dependent axonal lysosome transport promotes amyloid plaque pathology. The Journal of Cell Biol. 2017 Aug 7; doi: 10.1083/jcb.201612148. pii: jcb.201612148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JP, et al. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer β-amyloid peptides. Proc Natl Acad Sci USA. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and Proteolytic Processing of APP. Cold Spring Harb Perspect Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Li F, Chang WP, Tang J. GGA proteins mediate the recycling pathway of memapsin 2 (BACE) The Journal of biological chemistry. 2005;280:11696–11703. doi: 10.1074/jbc.M411296200. [DOI] [PubMed] [Google Scholar]
- Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, Zhang XC, Tang J. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000;290:150–153. doi: 10.1126/science.290.5489.150. [DOI] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nature neuroscience. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Huang TY, Zhao Y, Li X, Wang X, Tseng IC, Thompson R, Tu S, Willnow TE, Zhang YW, Xu H. SNX27 and SORLA Interact to Reduce Amyloidogenic Subcellular Distribution and Processing of Amyloid Precursor Protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:7996–8011. doi: 10.1523/JNEUROSCI.0206-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer’s disease beta-secretase. The Journal of biological chemistry. 2000;275:33729–33737. doi: 10.1074/jbc.M004175200. [DOI] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Molecular biology of the cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- Kim NY, Cho MH, Won SH, Kang HJ, Yoon SY, Kim DH. Sorting nexin-4 regulates beta-amyloid production by modulating beta-site-activating cleavage enzyme-1. Alzheimer’s research & therapy. 2017;9:4. doi: 10.1186/s13195-016-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Samura E, Suzuki K, Okabayashi S, Shimozawa N, Yasutomi Y. Dynein Dysfunction Reproduces Age-Dependent Retromer Deficiency: Concomitant Disruption of Retrograde Trafficking Is Required for Alteration in beta-Amyloid Precursor Protein Metabolism. The American journal of pathology. 2016;186:1952–1966. doi: 10.1016/j.ajpath.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci. 2003;116:3339–3346. doi: 10.1242/jcs.00643. [DOI] [PubMed] [Google Scholar]
- Kitazume S, Tachida Y, Oka R, Shirotani K, Saido TC, Hashimoto Y. Alzheimer’s beta-secretase, beta-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. Journal of cell science. 1996;109(Pt 5):991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- Kuentzel SL, Ali SM, Altman RA, Greenberg BD, Raub TJ. The Alzheimer beta-amyloid protein precursor/protease nexin-II is cleaved by secretase in a trans-Golgi secretory compartment in human neuroglioma cells. The Biochemical journal. 1993;295(Pt 2):367–378. doi: 10.1042/bj2950367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Sisodia SS, Trowbridge IS. Characterization of sorting signals in the beta-amyloid precursor protein cytoplasmic domain. The Journal of biological chemistry. 1995;270:3565–3573. [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature genetics. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, van Kerkhof P, Marzolo MP, Bu G. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. The Journal of biological chemistry. 2008;283:11501–11508. doi: 10.1074/jbc.M800642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler SF, Dominguez DI, Westmeyer GG, Reiss K, Haass C, Saftig P, De Strooper B, Seed B. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. The Journal of biological chemistry. 2003;278:48713–48719. doi: 10.1074/jbc.M303861200. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuda Y, D’Adamio L. Amyloid beta protein precursor (AbetaPP), but not AbetaPP-like protein 2, is bridged to the kinesin light chain by the scaffold protein JNK-interacting protein 1. The Journal of biological chemistry. 2003;278:38601–38606. doi: 10.1074/jbc.M304379200. [DOI] [PubMed] [Google Scholar]
- Miyagawa T, Ebinuma I, Morohashi Y, Hori Y, Young Chang M, Hattori H, Maehara T, Yokoshima S, Fukuyama T, Tsuji S, et al. BIN1 regulates BACE1 intracellular trafficking and amyloid-beta production. Human molecular genetics. 2016;25:2948–2958. doi: 10.1093/hmg/ddw146. [DOI] [PubMed] [Google Scholar]
- Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C, Dall’Armi C, Simoes S, Point Du Jour KS, McCabe BD, et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nature communications. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, et al. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstedt C, Caporaso GL, Thyberg J, Gandy SE, Greengard P. Identification of the Alzheimer beta/A4 amyloid precursor protein in clathrin-coated vesicles purified from PC12 cells. The Journal of biological chemistry. 1993;268:608–612. [PubMed] [Google Scholar]
- Prabhu Y, Burgos PV, Schindler C, Farias GG, Magadan JG, Bonifacino JS. Adaptor protein 2-mediated endocytosis of the beta-secretase BACE1 is dispensable for amyloid precursor protein processing. Molecular biology of the cell. 2012;23:2339–2351. doi: 10.1091/mbc.E11-11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H, Rao R. The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. J Biol Chem. 2015;290:5311–5327. doi: 10.1074/jbc.M114.602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Hebert SS, De Strooper B. The amyloid-beta precursor protein: integrating structure with biological function. The EMBO journal. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Sano Y, Vassar R, Gandy S, Nakaya T, Yamamoto T, Suzuki T. X11 proteins regulate the translocation of amyloid beta-protein precursor (APP) into detergent-resistant membrane and suppress the amyloidogenic cleavage of APP by beta-site-cleaving enzyme in brain. The Journal of biological chemistry. 2008;283:35763–35771. doi: 10.1074/jbc.M801353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Kaneko K, Okuno M, Wada K, Kashiyama T, Shimizu H, Akagi T, Hashikawa T, Nukina N. Membrane microdomain switching: a regulatory mechanism of amyloid precursor protein processing. J Cell Biol. 2008;183:339–352. doi: 10.1083/jcb.200804075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannerud R, Declerck I, Peric A, Raemaekers T, Menendez G, Zhou L, Veerle B, Coen K, Munck S, De Strooper B, et al. ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E559–568. doi: 10.1073/pnas.1100745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Syuzo-Takabatake A, Nakaya T, Saito Y, Tomita S, Itohara S, Suzuki T. Enhanced amyloidogenic metabolism of the amyloid beta-protein precursor in the X11L-deficient mouse brain. The Journal of biological chemistry. 2006;281:37853–37860. doi: 10.1074/jbc.M609312200. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XP, Chen E, Yin KC, Na S, Garsky VM, Lai MT, Li YM, Platchek M, Register RB, Sardana MK, et al. The pro domain of beta-secretase does not confer strict zymogen-like properties but does assist proper folding of the protease domain. The Journal of biological chemistry. 2001;276:10366–10373. doi: 10.1074/jbc.m009200200. [DOI] [PubMed] [Google Scholar]
- Sisodia SS. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Kent K, Pierce A, Leung C, Kang MS, Okada H, Honig L, Vonsattel JP, Kim TW. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Annals of neurology. 2005;58:909–919. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- Sun M, Asghar SZ, Zhang H. The polarity protein Par3 regulates APP trafficking and processing through the endocytic adaptor protein Numb. Neurobiology of disease. 2016;93:1–11. doi: 10.1016/j.nbd.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zhang H. Par3 and aPKC regulate BACE1 endosome-to-TGN trafficking through PACS1. Neurobiology of aging. 2017;60:129–140. doi: 10.1016/j.neurobiolaging.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan G, Zhu W, Plowey ED. BECN1/Beclin 1 sorts cell-surface APP/amyloid beta precursor protein for lysosomal degradation. Autophagy. 2016;12:2404–2419. doi: 10.1080/15548627.2016.1234561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Muller U, Beyreuther K, et al. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J Neurosci. 2009;29:14534–14544. doi: 10.1523/JNEUROSCI.1546-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Scott DA, Das U, Edland SD, Radomski K, Koo EH, Roy S. Early and selective impairments in axonal transport kinetics of synaptic cargoes induced by soluble amyloid beta-protein oligomers. Traffic. 2012;13:681–693. doi: 10.1111/j.1600-0854.2012.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh WH, Tan JZ, Zulkefli KL, Houghton FJ, Gleeson PA. Amyloid precursor protein traffics from the Golgi directly to early endosomes in an Arl5b- and AP4-dependent pathway. Traffic. 2017;18:159–175. doi: 10.1111/tra.12465. [DOI] [PubMed] [Google Scholar]
- Ubelmann F, Burrinha T, Salavessa L, Gomes R, Ferreira C, Moreno N, Guimas Almeida C. Bin1 and CD2AP polarise the endocytic generation of beta-amyloid. EMBO reports. 2017;18:102–122. doi: 10.15252/embr.201642738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayar V, Buggia-Prevot V, Guerreiro RL, Siegel G, Rambabu N, Soohoo AL, Ponnusamy M, Siegenthaler B, Bali J, Aesg, et al. A paired RNAi and RabGAP overexpression screen identifies Rab11 as a regulator of beta-amyloid production. Cell reports. 2013;5:1536–1551. doi: 10.1016/j.celrep.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vieira SI, Rebelo S, Esselmann H, Wiltfang J, Lah J, Lane R, Small SA, Gandy S, da Cruz ESEF, da Cruz ESOA. Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Molecular neurodegeneration. 2010;5:40. doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim CA, Tangredi MM, Peltan ID, Lee BM, Irizarry MC, Kinoshita A, Hyman BT. Demonstration of BACE (beta-secretase) phosphorylation and its interaction with GGA1 in cells by fluorescence-lifetime imaging microscopy. Journal of cell science. 2004;117:5437–5445. doi: 10.1242/jcs.01422. [DOI] [PubMed] [Google Scholar]
- Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C. Phosphorylation regulates intracellular trafficking of beta-secretase. The Journal of biological chemistry. 2001;276:14634–14641. doi: 10.1074/jbc.M011116200. [DOI] [PubMed] [Google Scholar]
- Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- Wen L, Tang FL, Hong Y, Luo SW, Wang CL, He W, Shen C, Jung JU, Xiong F, Lee DH, et al. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. The Journal of cell biology. 2011;195:765–779. doi: 10.1083/jcb.201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. The Journal of biological chemistry. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Yan R, Han P, Miao H, Greengard P, Xu H. The transmembrane domain of the Alzheimer’s beta-secretase (BACE1) determines its late Golgi localization and access to beta -amyloid precursor protein (APP) substrate. J Biol Chem. 2001;276:36788–36796. doi: 10.1074/jbc.M104350200. [DOI] [PubMed] [Google Scholar]
- Ye X, Cai Q. Snapin-mediated BACE1 retrograde transport is essential for its degradation in lysosomes and regulation of APP processing in neurons. Cell reports. 2014;6:24–31. doi: 10.1016/j.celrep.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Feng T, Tammineni P, Chang Q, Jeong YY, Margolis DJ, Cai H, Kusnecov A, Cai Q. Regulation of Synaptic Amyloid-beta Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer’s Disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017;37:2639–2655. doi: 10.1523/JNEUROSCI.2851-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]