Abstract
Molecular chaperones have a role to stabilize proteins or assist them in reaching their native fold. Heat shock proteins (HSPs) are a family of molecular chaperons that protect proteins from cellular stress during the assembly of protein complexes and also prevent the proteins from aggregation and disassembly. The immediate increase of HSPs is crucial for cellular adaptation to environmental changes and protection of other proteins from denaturation, thereby maintaining the cellular homeostasis and increasing the longevity of an organism. HSP70 and HSP90 are the most studied HSPs in this very large HSP family. Notably, HSP90 also stabilizes the disease-related proteins in neurodegenerative disorders. Therefore, small molecules that inhibit the HSP90 but also increase the HSP70 has been tested as potential drugs for neurodegenerative disorders.
Introduction
Heat shock proteins (HSPs) are the chaperones responsible for correct folding of proteins during normal conditions and for restoration and refolding of destructed polypeptides in the cells under stress exposure. HSP70 and HSP90 are the most studied HSPs in a very large HSP family [1]. Unlike enzymes with their finely tuned active sites that transduce the specific molecular signaling, chaperones are heavy-duty molecular machines that interact with a wide range of protein substrates.
Heat shock response of cells was first reported as a temperature-dependent change in the transcriptional activity in the fruit fly Drosophila melanogaster [2]. In 1974, the HSPs were brought to light when numerous new bands of proteins were noticed in different tissue samples of Drosophila followed by heat shock [3]. Almost a decade later, it was proved that HSPs prevent damage to the cells by binding to abnormal proteins resulting from heat shock and thus avoiding their accumulation [4]. The transcription of HSPs is mainly regulated by Heat Shock Factor 1 (HSF1). During unstressed conditions, HSF1 exists as an inactive monomer. Upon exposure to heat or other types of cellular stress, HSF1 is processed to be converted into a trimeric and transcriptionally active state. This allows HSF1 bind to promoters of downstream target genes, whereby initiating the transcription [5]. Apart from having role in stressed conditions, HSF1 also plays roles in gametogenesis [6], embryonic development [7], immune response [8], and neurogenesis of olfactory system [9].
Main cause of neurodegenerative diseases is protein misfolding. Various in-silico, in-vitro, and in-vivo studies have been undertaken to understand the misfolding mechanism and to develop therapeutics, and pharmacological induction of molecular chaperones has been found as a solution for prevention of the disease progression. In this review, we will summarize the roles of HSP70 and HSP90 in the onset and progression of neurodegenerative diseases and discuss the potentials for the interventions.
Chaperone machinery of HSP70
In both prokaryotic and eukaryotic organisms, HSP70 and HSP90 as well as their homologous proteins are highly expressed in many cell types. HSP70 comprises of two different domains; a 40 kDa N-terminal nucleotide-binding domain (NBD) that controls the interaction with the client protein, and a 25 kDa C-terminal substrate-binding domain (SBD) that identifies the hydrophobic regions in the client during initial stages of its folding [10,11]. These two domains are connected by a flexible linker. HSP70 possesses a below average ATPase activity when not bound to a client [12]. Thus a co-chaperon, J domain protein family channels client protein to HSP70 vitalizing its ATPase activity [13]. After the J protein leaves this complex, HSP70 is brought to its apo-form by a nucleotide-exchange factor liberating ADP from it. This conformation change makes the NBD free to engage ATP, leading the α-helical lid to “open” and releasing client [13,14]. This cycle continues until the client attains the native conformation or is shifted to other parts of chaperone machinery as shown in Figure 1.
Fig. 1.
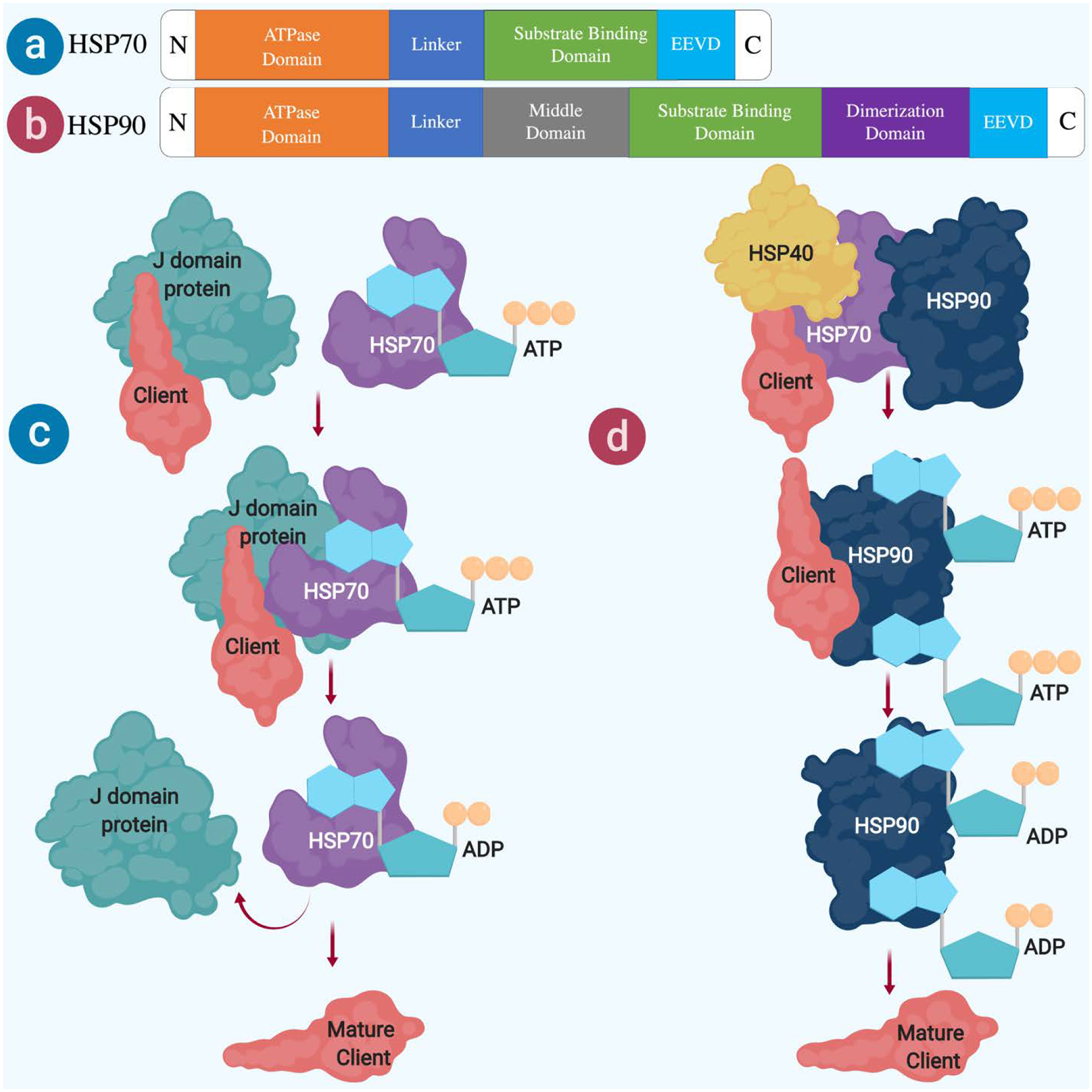
Schematic representation of HSP70 and HSP90. (a) HSP70 domains (b) HSP90 domains (c) HSP70 and (d) HSP90 co-chaperons mediated protein folding
HSP40 (also known as DNAJB1) is a main co-chaperon of J domain protein family that works with HSP70 by monitoring the activities such as; binding of the polypeptide to HSP70, eliminating polypeptide folding before maturation and the ATPase enzymatic function of HSP70 [15–17]. HSP40 is widely expressed in the brain and co-localized with HSP70 [18]. Especially dense co-immunolabeling of HSP40 and HSP70 was found in postsynaptic but not presynaptic compartment, suggesting the functional implication of postsynaptic chaperons in neuronal transmission. The recruitment of client proteins to HSP70 complex is commenced by interaction with another co-chaperon HSP40, followed by transfer of those client proteins to HSP90 complex via another co-chaperone STI1 (also called HOP or HSP-organizing protein in humans) [19–22].
Chaperone machinery of HSP90
In eukaryotes, HSP90 accounts for 1% of all proteins in a cell [4]. Higher eukaryotes contain four HSP90 paralogs: GRP94 in the endoplasmic reticulum, TRAP1 in mitochondria, and HSP90α and HSP90β in the cytosol. The active unit of all HSP90 paralogs is created by a homodimerization of three different regions that are linked via flexible linkers. The N-terminal domain is accountable for binding of a nucleotide, while the middle domain recognizes the client proteins and triggers hydrolysis of ATP. The C-terminal domain serves as the important site for the dimerization [19,23]. In the apo state, HSP90 adopts a V-shaped open conformation for ATP as shown in Figure 1. ATP binding activates a series of conformational changes including repositioning of the N-terminal lid region and a change in the N terminal-middle domain orientation. This allows the N-terminal region to support the dimerization and engages the middle domain in hydrolysis of ATP via a conserved arginine (R380 in yeast) [24]. HSP90 requires the ATP hydrolysis and structural rearrangement to reconfigure abnormally folded proteins to their normal states [25]. This process is governed by a group of co-chaperons such as stress inducible protein (STI1), cell division cycle 37 (Cdc37), protein phosphatase 5 (PP5), FK506-binding protein 51 (FKBP51), FK506-binding protein 52 (FKBP52) and cyclophilin 40 (Cyp40) [26].
Roles of HSP70 and HSP90 in neurogenerative diseases
Protein accumulation is the characteristic feature of various neurodegenerative disorders including Parkinson’s disease (PD), Huntington’s disease (HD), Amyotrophic lateral sclerosis (ALS), and Alzheimer’s disease (AD) [27] In this section, we will summarize the roles of HSPs in neurodegenerative diseases that were discovered by using animal models and in vitro cells.
PD is the second most prevalent neurodegenerative disorder, the major hallmark of which is the loss of dopaminergic neurons in the substantia nigra [28,29]. α-synuclein, a protein consisting of 140 amino-acids that localizes predominantly in presynaptic compartment [30], is linked genetically [31] and neuropathologically to PD [32–34]. It belongs to the synuclein family which also includes β and γ-synuclein. This family share a conserved KTKEGV repeat motifs at the N-terminus [35]. α-synuclein accumulates into intracellular filamentous inclusions with both phosphorylated and ubiquitinated forms [36–38]. HSP70 overexpression results in 50% reduction of α-synuclein species in the human neuroglioma cells [39]. Furthermore, 17-allylamino-17-demethoxygeldanamycin (17-AAG) that inhibits HSP90 and also increases HSP70 reduces oligomerization of α-synuclein and the neurotoxicity (Table 1)[40].
Table 1.
Drugs that target HSPs in the clinical trials.
HD is a neurodegenerative disease caused by a mutation in huntingtin (HTT) gene [41]. Long CAG repeats in the HTT gene that code for an extended polyglutamine (polyQ) stretch of the HTT protein [42] lead to the aggregation of the HTT protein [43]. Protein aggregates of the mutated HTT form inclusion bodies in the neurons of the spinal cord and several brain regions [44]. Although a study showed Hsp70.1/Hsp70.3 double knockout mice have increased size of the polyQ inclusion bodies in the cerebral cortex. The overexpression of the Hsp70 shows moderate effect on delaying the neurodegeneration in mouse models of HD [45–49]. Another study that used cell culture model of HD also demonstrated that overexpression of HSP70 and HSP40 inhibits polyQ accumulation [50].
ALS is a degenerative disorder of nervous system affecting the motor neurons of brainstem, cortex and spinal cord [51,52]. In most cases of the disease, an RNA binding protein called trans-active DNA binding protein-43 (TDP-43) which is normally found in the nuclear region, erroneously localize in the cytoplasm of neurons and glial cells forming aggregates [53]. The HSP70 is drastically reduced in the spinal cord tissues of patients with sporadic cases of the disease [54]. Knocking down HSP70 in human neuroblastoma cells considerably increases the toxic TDP-43 accumulated in the cytoplasm [55], suggesting roles of HSP70 in suppressing formation of the toxic forms.
AD is the most common type of dementia, mainly afflicting aging population [56–58]. A major hypothesis for the disease pathology posits the accumulation of misfolded proteins, Amyloid β and Tau in the forebrain [59,60]. In addition to clearly visible extracellular accumulation of Amyloid β [61,62], observation made in rodent models and human postmortem tissues demonstrated the intraneuronal accumulation [63,64]. Similarly, Tau accumulation is also found in both intracellular [65,66] and extracellular compartments [67]. Recent results reveal that Aβ and tau synergize to impair the functional integrity of neural circuits in vivo and suggest a possible cellular explanation contributing to disappointing results from anti-Aβ therapeutic trials [68]. Similar to other neurodegenerative diseases, AD-affected brains and animal models show an increase of HSPs and their co-chaperones, including HSP70 [69]. Inhibition of HSP90 by a classical inhibitor, Geldanamycin that had been placed to clinical trials of cancer, has been also tested in preclinical models of neurodegenerative disorders. Administration of Geldanamycin (GA) reduces phosphorylated Tau in vivo and in vitro, suggesting the protective role of HSP90 for hyperphosphorylated Tau against degradation in mouse brain (Table 1) [70].
As described above, misfolded proteins accumulate to form hard insoluble plaques and fibers that are a leading cause of neurodegeneration [71]. Clearance and refolding of these misfolded proteins are mediated by HSPs, especially by HSP70. On the other hand, the HSP90 rather plays a role in augmentation of neurodegeneration. For instance, pharmacological inhibition of HSP90 suppresses the progression of the neurodegeneration.
Pharmacological inhibition of HSP90 for treatment of neurodegenerative disorders
Based on the observations made in animal models that are described above, it is evident that HSP90 plays a major role in stabilizing the proteins and maintaining the pathology-associated changes, thereby leading to degeneration and dysfunction of neurons in neurodegenerative diseases. Given the proved effectiveness in animal models, HSP90 inhibitory drugs that have been utilized in cancer treatment were also considered for the application to neurodegenerative diseases. The GA and its derivative 17-AAG act as highly selective inhibitors of HSP90 via its specific binding to the ADP/ATP binding pocket so as to block later process that includes interaction with the co-chaperons [72,73]. Inhibition of HSP90 also promotes transcriptions of HSP encoding genes including Hsp70 and HSP40 through HSF-1 activation [74]. Unfortunately, 17-AAG failed the phase 1 clinical trials due to hepatotoxicity [75]. This means that many alternative strategies that target HSPs are still required; examples include 1) maintaining a balance between HSP70 and HSP90 activities; 2) targeting co-chaperons that are associated with HSP70 and HSP90 for modifying the activities; 3) testing drugs that target non-canonical pathways of HSF1.
Accumulations of Amyloid and Tau in AD have been suggested as the trigger and bullet in disease pathogenesis [76]. Based on this hypothesis, many attempts including tests of HSP70 inducer and HSP90 inhibitors, that target clearance of Tau and Amyloid β accumulations were made in animal studies [77–79]. Failure of the HSP90 inhibitors in clinical trials also suggests us to expand alternative approaches other than targeting the aggregation of misfolded proteins, such as the gene therapy of Amyloid Precursor Protein (APP) and Presenilin (PS) mutations [80].
Perspectives
Although the positive results of preclinical tests of HSP-targeted drugs encouraged the clinical application to the neurodegenerative diseases but there is no significant advancement happened in HSP90 inhibitor development process due to increased cytotoxity and lower effectiveness that were confirmed in phase 1 trials for cancer treatment. Therefore, current unmet need is to develop chaperon complex-targeted drugs with reduced toxicity, and this may be achieved by data science-assisted optimization for better pharmacological and pharmacokinetic characterization. This process would be accelerated by aggressive application of mathematical modeling and machine learning using accumulated big data [96–98]
Acknowledgements
This work was supported by National Institute of Health Grants R01AA025215, R01AA026272, and UH2AA026106 as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) (to K.H.-T.). The study was also funded by Scott-Gentle Foundation (to K.H.-T.).
References
- [1].Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE, Guidelines for the nomenclature of the human heat shock proteins, Cell Stress Chaperones. 14 (2009) 105–111. 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ritossa F, A new puffing pattern induced by temperature shock and DNP in Drosophila, Experientia. 18 (1962) 571–573. 10.1007/BF02172188. [DOI] [Google Scholar]
- [3].Tissières A, Mitchell HK, Tracy UM, Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs, J. Mol. Biol 84 (1974) 389–398. 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- [4].Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S, hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures, Mol. Cell. Biol 9 (1989) 3919–3930. 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cotto JJ, Kline M, Morimoto RI, Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation Evidence for a multistep pathway of regulation, J. Biol. Chem 271 (1996) 3355–3358. 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- [6].Jedlicka P, Mortin MA, Wu C, Multiple functions of Drosophila heat shock transcription factor in vivo, EMBO J. 16 (1997) 2452–2462. 10.1093/emboj/16.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Christians E, Davis AA, Thomas SD, Benjamin IJ, Embryonic development: maternal effect of Hsf1 on reproductive success, Nature. 407 (2000) 693 10.1038/35037669. [DOI] [PubMed] [Google Scholar]
- [8].Inouye S, Izu H, Takaki E, Suzuki H, Shirai M, Yokota Y, Ichikawa H, Fujimoto M, Nakai A, Impaired IgG production in mice deficient for heat shock transcription factor 1, J. Biol. Chem 279 (2004) 38701–38709. 10.1074/jbc.M405986200. [DOI] [PubMed] [Google Scholar]
- [9].Takaki E, Fujimoto M, Sugahara K, Nakahari T, Yonemura S, Tanaka Y, Hayashida N, Inouye S, Takemoto T, Yamashita H, Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice, J. Biol. Chem 281 (2006) 4931–4937. 10.1074/jbc.M506911200. [DOI] [PubMed] [Google Scholar]
- [10].Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B, Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries, EMBO J. 16 (1997) 1501–1507. 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bukau B, Weissman J, Horwich A, Molecular chaperones and protein quality control, Cell. 125 (2006) 443–451. 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- [12].Swain JF, Dinler G, Sivendran R, Montgomery DL, Stotz M, Gierasch LM, Hsp70 chaperone ligands control domain association via an allosteric mechanism mediated by the interdomain linker, Mol. Cell 26 (2007) 27–39. 10.1016/j.molcel.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Misselwitz B, Staeck O, Rapoport TA, J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences, Mol. Cell 2 (1998) 593–603. 10.1016/s1097-2765(00)80158-6. [DOI] [PubMed] [Google Scholar]
- [14].Schlecht R, Erbse AH, Bukau B, Mayer MP, Mechanics of Hsp70 chaperones enables differential interaction with client proteins, Nat. Struct. Mol. Biol 18 (2011) 345–351. 10.1038/nsmb.2006. [DOI] [PubMed] [Google Scholar]
- [15].Cyr DM, Lu X, Douglas MG, Regulation of Hsp70 function by a eukaryotic DnaJ homolog, J. Biol. Chem 267 (1992) 20927–20931. [PubMed] [Google Scholar]
- [16].Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU, Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones, Nature. 370 (1994) 111–117. 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- [17].Tsai J, Douglas MG, A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding, J. Biol. Chem 271 (1996) 9347–9354. 10.1074/jbc.271.16.9347. [DOI] [PubMed] [Google Scholar]
- [18].Suzuki T, Usuda N, Murata S, Nakazawa A, Ohtsuka K, Takagi H, Presence of molecular chaperones, heat shock cognate (Hsc) 70 and heat shock proteins (Hsp) 40, in the postsynaptic structures of rat brain, Brain Res. 816 (1999) 99–110. 10.1016/S0006-8993(98)01083-X. [DOI] [PubMed] [Google Scholar]
- [19].Taipale M, Jarosz DF, Lindquist S, HSP90 at the hub of protein homeostasis: emerging mechanistic insights, Nat. Rev. Mol. Cell Biol 11 (2010) 515–528. 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- [20].Lässle M, Blatch GL, Kundra V, Takatori T, Zetter BR, Stress-inducible, murine protein mSTI1. Characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases, J. Biol. Chem 272 (1997) 1876–1884. 10.1074/jbc.272.3.1876. [DOI] [PubMed] [Google Scholar]
- [21].Chen S, Smith DF, Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery, J. Biol. Chem 273 (1998) 35194–35200. 10.1074/jbc.273.52.35194. [DOI] [PubMed] [Google Scholar]
- [22].Johnson BD, Schumacher RJ, Ross ED, Toft DO, Hop modulates Hsp70/Hsp90 interactions in protein folding, J. Biol. Chem 273 (1998) 3679–3686. 10.1074/jbc.273.6.3679. [DOI] [PubMed] [Google Scholar]
- [23].Pearl LH, Prodromou C, Structure and mechanism of the Hsp90 molecular chaperone machinery, Annu. Rev. Biochem 75 (2006) 271–294. 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- [24].Cunningham CN, Southworth DR, Krukenberg KA, Agard DA, The conserved arginine 380 of Hsp90 is not a catalytic residue, but stabilizes the closed conformation required for ATP hydrolysis, Protein Sci. Publ. Protein Soc 21 (2012) 1162–1171. 10.1002/pro.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zuehlke A, Johnson JL, Hsp90 and co-chaperones twist the functions of diverse client proteins, Biopolymers. 93 (2010) 211–217. 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Allan RK, Ratajczak T, Versatile TPR domains accommodate different modes of target protein recognition and function, Cell Stress Chaperones. 16 (2011) 353–367. 10.1007/s12192-010-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kurtishi A, Rosen B, Patil KS, Alves GW, Møller SG, Cellular Proteostasis in Neurodegeneration, Mol. Neurobiol 56 (2019) 3676–3689. 10.1007/s12035-018-1334-z. [DOI] [PubMed] [Google Scholar]
- [28].Davie CA, A review of Parkinson’s disease, Br. Med. Bull 86 (2008) 109–127. 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- [29].Keifman E, Ruiz-DeDiego I, Pafundo DE, Paz RM, Solís O, Murer MG, Moratalla R, Optostimulation of striatonigral terminals in substantia nigra induces dyskinesia that increases after L-DOPA in a mouse model of Parkinson’s disease, Br. J. Pharmacol 176 (2019) 2146–2161. 10.1111/bph.14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maroteaux L, Campanelli JT, Scheller RH, Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal, J. Neurosci. Off. J. Soc. Neurosci 8 (1988) 2804–2815. 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Mutation in the α-synuclein gene identified in families with Parkinson’s disease, Science. 276 (1997) 2045–2047. 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- [32].Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M, α-Synuclein in Lewy bodies, Nature. 388 (1997) 839 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- [33].Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M, α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies, Proc. Natl. Acad. Sci 95 (1998) 6469–6473. 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baba M, Nakajo S, Tu P-H, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T, Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies., Am. J. Pathol 152 (1998) 879. [PMC free article] [PubMed] [Google Scholar]
- [35].Jakes R, Spillantini MG, Goedert M, Identification of two distinct synucleins from human brain, FEBS Lett. 345 (1994) 27–32. 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- [36].Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM, Fatal attractions: abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia, Cell Death Differ. 5 (1998) 832–837. 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- [37].Goedert M, Filamentous nerve cell inclusions in neurodegenerative diseases: tauopathies and alpha-synucleinopathies, Philos. Trans. R. Soc. Lond. B. Biol. Sci 354 (1999) 1101–1118. 10.1098/rstb.1999.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kawasawa YI, Mohammad S, Son AI, Morizono H, Basha A, Salzberg AC, Torii M, Hashimoto-Torii K, Genome-wide profiling of differentially spliced mRNAs in human fetal cortical tissue exposed to alcohol, Alcohol Fayettev. N 62 (2017) 1–9. 10.1016/j.alcohol.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ, Formation of toxic oligomeric alpha-synuclein species in living cells, PloS One. 3 (2008) e1867 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Putcha P, Danzer KM, Kranich LR, Scott A, Silinski M, Mabbett S, Hicks CD, Veal JM, Steed PM, Hyman BT, McLean PJ, Brain-permeable small-molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein-induced toxicity, J. Pharmacol. Exp. Ther 332 (2010) 849–857. 10.1124/jpet.109.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim EH, Thu DCV, Tippett LJ, Oorschot DE, Hogg VM, Roxburgh R, Synek BJ, Waldvogel HJ, Faull RLM, Cortical interneuron loss and symptom heterogeneity in Huntington disease: Interneuron Loss in HD Cortex, Ann. Neurol 75 (2014) 717–727. 10.1002/ana.24162. [DOI] [PubMed] [Google Scholar]
- [42].Fan H-C, Ho L-I, Chi C-S, Chen S-J, Peng G-S, Chan T-M, Lin S-Z, Harn H-J, Polyglutamine (PolyQ) Diseases: Genetics to Treatments, Cell Transplant. 23 (2014) 441–458. 10.3727/096368914X678454. [DOI] [PubMed] [Google Scholar]
- [43].MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, Taylor SA, James M, Groot N, MacFarlane H, Jenkins B, Anderson MA, Wexler NS, Gusella JF, Bates GP, Baxendale S, Hummerich H, Kirby S, North M, Youngman S, Mott R, Zehetner G, Sedlacek Z, Poustka A, Frischauf A-M, Lehrach H, Buckler AJ, Church D, Doucette-Stamm L, O’Donovan MC, Riba-Ramirez L, Shah M, Stanton VP, Strobel SA, Draths KM, Wales JL, Dervan P, Housman DE, Altherr M, Shiang R, Thompson L, Fielder T, Wasmuth JJ, Tagle D, Valdes J, Elmer L, Allard M, Castilla L, Swaroop M, Blanchard K, Collins FS, Snell R, Holloway T, Gillespie K, Datson N, Shaw D, Harper PS, A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes, Cell. 72 (1993) 971–983. 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- [44].Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP, Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation, Cell. 90 (1997) 537–548. 10.1016/S0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- [45].Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM, Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70, Nat. Genet 23 (1999) 425–428. 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- [46].Carmichael J, Chatellier J, Woolfson A, Milstein C, Fersht AR, Rubinsztein DC, Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington’s disease, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 9701–9705. 10.1073/pnas.170280697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jana NR, Tanaka M, h Wang G, Nukina N, Polyglutamine length-dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity, Hum. Mol. Genet 9 (2000) 2009–2018. 10.1093/hmg/9.13.2009. [DOI] [PubMed] [Google Scholar]
- [48].Krobitsch S, Lindquist S, Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 1589–1594. 10.1073/pnas.97.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ, Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer, Nat. Struct. Mol. Biol 11 (2004) 1215–1222. 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- [50].Sittler A, Lurz R, Lueder G, Priller J, Hayer-Hartl MK, Hartl FU, Lehrach H, Wanker EE, Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington’s disease, Hum. Mol. Genet 10 (2001) 1307–1315. 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- [51].Paez-Colasante X, Figueroa-Romero C, Sakowski SA, Goutman SA, Feldman EL, Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era, Nat. Rev. Neurol 11 (2015) 266–279. 10.1038/nrneurol.2015.57. [DOI] [PubMed] [Google Scholar]
- [52].Katz JS, Woolley SC, Amyotrophic Lateral Sclerosis, in: Sanders KM (Ed.), Physicians Field Guide Neuropsychol. Collab. Case Ex, Springer New York, New York, NY, 2019: pp. 255–265. 10.1007/978-1-4939-8722-1_15. [DOI] [Google Scholar]
- [53].Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling S-C, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW, Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43, Nat. Neurosci 14 (2011) 459–468. 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen H-J, Mitchell JC, Novoselov S, Miller J, Nishimura AL, Scotter EL, Vance CA, Cheetham ME, Shaw CE, The heat shock response plays an important role in TDP-43 clearance: evidence for dysfunction in amyotrophic lateral sclerosis, Brain J. Neurol 139 (2016) 1417–1432. 10.1093/brain/aww028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang Y-J, Gendron TF, Xu Y-F, Ko L-W, Yen S-H, Petrucelli L, Phosphorylation regulates proteasomal-mediated degradation and solubility of TAR DNA binding protein-43 C-terminal fragments, Mol. Neurodegener 5 (2010) 33 10.1186/1750-1326-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Panigrahi PP, Singla R, Bansal A, Junior MC, Jaitak V, Yennamalli RM, Singh TR, In silico screening and molecular interaction studies of tetrahydrocannabinol and its derivatives with acetylcholine binding protein, Curr. Chem. Biol 12 (2018) 181–190. [Google Scholar]
- [57].Kumar A, Bansal A, Integrated bioinformatics analysis of differentially expressed genes (degs) of alzheimer’s disease (ad) datasets from gene expression omnibus (geo), Alzheimers Dement. J. Alzheimers Assoc 13 (2017) P953 10.1016/j.jalz.2017.06.1270. [DOI] [Google Scholar]
- [58].Kumar A, Bansal A, Singh TR, ABCD: Alzheimer’s disease Biomarkers Comprehensive Database, 3 Biotech. 9 (2019) 351 10.1007/s13205-019-1888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Selkoe DJ, The molecular pathology of Alzheimer’s disease, Neuron. 6 (1991) 487–498. 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- [60].Bansal A, Salaria M, Singh TR, Tau Pathology: A Step Towards Understanding Neurodegenerative Disorders Network Complexity, in: Handb. Res. Crit. Exam. Neurodegener. Disord, IGI Global, 2019: pp. 217–234. 10.4018/978-1-5225-5282-6.ch010. [DOI] [Google Scholar]
- [61].Glenner GG, Wong CW, Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein, Biochem. Biophys. Res. Commun 120 (1984) 885–890. 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- [62].Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K, Amyloid plaque core protein in Alzheimer disease and Down syndrome, Proc. Natl. Acad. Sci 82 (1985) 4245–4249. 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR, Intraneuronal Aβ42 Accumulation in Human Brain, Am. J. Pathol 156 (2000) 15–20. 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Knobloch M, Konietzko U, Krebs DC, Nitsch RM, Intracellular Aβ and cognitive deficits precede β-amyloid deposition in transgenic arcAβ mice, Neurobiol. Aging 28 (2007) 1297–1306. 10.1016/j.neurobiolaging.2006.06.019. [DOI] [PubMed] [Google Scholar]
- [65].Weingarten MD, Lockwood AH, Hwo S-Y, Kirschner MW, A protein factor essential for microtubule assembly, Proc. Natl. Acad. Sci 72 (1975) 1858–1862. 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mandelkow E-M, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E, Tau domains, phosphorylation, and interactions with microtubules, Neurobiol. Aging 16 (1995) 355–362. 10.1016/0197-4580(95)00025-A. [DOI] [PubMed] [Google Scholar]
- [67].Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, Cirrito JR, Patel TK, Hochgräfe K, Mandelkow E-M, Holtzman DM, Neuronal activity regulates extracellular tau in vivo, J. Exp. Med 211 (2014) 387–393. 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Busche MA, Wegmann S, Dujardin S, Commins C, Schiantarelli J, Klickstein N, Kamath TV, Carlson GA, Nelken I, Hyman BT, Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo, Nat. Neurosci 22 (2019) 57–64. 10.1038/s41593-018-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Perez N, Sugar J, Charya S, Johnson G, Merril C, Bierer L, Perl D, Haroutunian V, Wallace W, Increased synthesis and accumulation of heat shock 70 proteins in Alzheimer’s disease, Brain Res. Mol. Brain Res 11 (1991) 249–254. 10.1016/0169-328X(91)90033-T. [DOI] [PubMed] [Google Scholar]
- [70].Dou F, Chang X, Ma D, Hsp90 maintains the stability and function of the tau phosphorylating kinase GSK3β, Int. J. Mol. Sci 8 (2007) 51–60. [Google Scholar]
- [71].Elbaum-Garfinkle S, Matter over mind: Liquid phase separation and neurodegeneration, J. Biol. Chem 294 (2019) 7160–7168. 10.1074/jbc.REV118.001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Piper PW, Millson SH, Mollapour M, Panaretou B, Siligardi G, Pearl LH, Prodromou C, Sensitivity to Hsp90-targeting drugs can arise with mutation to the Hsp90 chaperone, cochaperones and plasma membrane ATP binding cassette transporters of yeast, Eur. J. Biochem 270 (2003) 4689–4695. 10.1046/j.1432-1033.2003.03866.x. [DOI] [PubMed] [Google Scholar]
- [73].Gausdal G, Gjertsen BT, Fladmark KE, Demol H, Vandekerckhove J, Døskeland S-O, Caspase-dependent, geldanamycin-enhanced cleavage of co-chaperone p23 in leukemic apoptosis, Leukemia. 18 (2004) 1989–1996. 10.1038/sj.leu.2403508. [DOI] [PubMed] [Google Scholar]
- [74].Luo W, Sun W, Taldone T, Rodina A, Chiosis G, Heat shock protein 90 in neurodegenerative diseases, Mol. Neurodegener 5 (2010) 24 10.1186/1750-1326-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Grem JL, Morrison G, Guo X-D, Agnew E, Takimoto CH, Thomas R, Szabo E, Grochow L, Grollman F, Hamilton JM, Neckers L, Wilson RH, Phase I and Pharmacologic Study of 17-(Allylamino)-17-Demethoxygeldanamycin in Adult Patients With Solid Tumors, J. Clin. Oncol 23 (2005) 1885–1893. 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- [76].Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K, Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease: A Longitudinal Study, JAMA Neurol. 76 (2019) 915–924. 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Young ZT, Rauch JN, Assimon VA, Jinwal U, Ahn M, Li X, Dunyak BM, Ahmad A, Carlson G, Srinivasan SR, Zuiderweg ERP, Dickey CA, Gestwicki JE, Stabilizing the Hsp70-Tau Complex Promotes Turnover in Models of Tauopathy, Cell Chem. Biol 23 (2016) 992–1001. 10.1016/j.chembiol.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Abisambra J, Jinwal UK, Miyata Y, Rogers J, Blair L, Li X, Seguin SP, Wang L, Jin Y, Bacon J, Brady S, Cockman M, Guidi C, Zhang J, Koren J, Young ZT, Atkins CA, Zhang B, Lawson LY, Weeber EJ, Brodsky JL, Gestwicki JE, Dickey CA, Allosteric heat shock protein 70 inhibitors rapidly rescue synaptic plasticity deficits by reducing aberrant tau, Biol. Psychiatry 74 (2013) 367–374. 10.1016/j.biopsych.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Miyata Y, Li X, Lee H-F, Jinwal UK, Srinivasan SR, Seguin SP, Young ZT, Brodsky JL, Dickey CA, Sun D, Gestwicki JE, Synthesis and initial evaluation of YM-08, a blood-brain barrier permeable derivative of the heat shock protein 70 (Hsp70) inhibitor MKT-077, which reduces tau levels, ACS Chem. Neurosci 4 (2013) 930–939. 10.1021/cn300210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kametani F, Hasegawa M, Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease, Front. Neurosci 12 (2018). 10.3389/fnins.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhao Z, Faden AI, Loane DJ, Lipinski MM, Sabirzhanov B, Stoica BA, Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury, J. Cereb. Blood Flow Metab 33 (2013) 1897–1908. 10.1038/jcbfm.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Uchida S, Fujiki M, Nagai Y, Abe T, Kobayashi H, Geranylgeranylacetone, a noninvasive heat shock protein inducer, induces protein kinase C and leads to neuroprotection against cerebral infarction in rats, Neurosci. Lett 396 (2006) 220–224. 10.1016/j.neulet.2005.11.065. [DOI] [PubMed] [Google Scholar]
- [83].Yasuda H, Shichinohe H, Kuroda S, Ishikawa T, Iwasaki Y, Neuroprotective effect of a heat shock protein inducer, geranylgeranylacetone in permanent focal cerebral ischemia, Brain Res. 1032 (2005) 176–182. 10.1016/j.brainres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [84].Sittler A, Lurz R, Lueder G, Priller J, Lehrach H, Hayer-Hartl MK, Hartl FU, Wanker EE, Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington’s disease, Hum. Mol. Genet 10 (2001) 1307–1315. 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- [85].Gorska M, Popowska U, Sielicka-Dudzin A, Kuban-Jankowska A, Sawczuk W, Knap N, Cicero G, Wozniak F, Geldanamycin and its derivatives as Hsp90 inhibitors, Front. Biosci. Landmark Ed 17 (2012) 2269–2277. 10.2741/4050. [DOI] [PubMed] [Google Scholar]
- [86].McLean PJ, Klucken J, Shin Y, Hyman BT, Geldanamycin induces Hsp70 and prevents alpha-synuclein aggregation and toxicity in vitro, Biochem. Biophys. Res. Commun 321 (2004) 665–669. 10.1016/j.bbrc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- [87].Xiong R, Zhou W, Siegel D, Kitson RRA, Freed CR, Moody CJ, Ross D, A Novel Hsp90 Inhibitor Activates Compensatory Heat Shock Protein Responses and Autophagy and Alleviates Mutant A53T α-Synuclein Toxicity, Mol. Pharmacol 88 (2015) 1045–1054. 10.1124/mol.115.101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Alcazar A, Cid C, High cytotoxic sensitivity of the oligodendrocyte precursor cells to HSP90 inhibitors in cell cultures, Exp. Neurol 216 (2009) 511–514. 10.1016/j.expneurol.2008.12.022. [DOI] [PubMed] [Google Scholar]
- [89].Chen Y, Wang B, Liu D, Li JJ, Xue Y, Sakata K, Zhu L, Heldt SA, Xu H, Liao F-F, Hsp90 Chaperone Inhibitor 17-AAG Attenuates Aβ-Induced Synaptic Toxicity and Memory Impairment, J. Neurosci 34 (2014) 2464–2470. 10.1523/JNEUROSCI.0151-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G, 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration, Nat. Med 11 (2005) 1088–1095. 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- [91].Zuo Y, Wang J, Liao F, Yan X, Li J, Huang L, Liu F, Inhibition of Heat Shock Protein 90 by 17-AAG Reduces Inflammation via P2X7 Receptor/NLRP3 Inflammasome Pathway and Increases Neurogenesis After Subarachnoid Hemorrhage in Mice, Front. Mol. Neurosci 11 (2018). 10.3389/fnmol.2018.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ho SW, Tsui YTC, Wong TT, Cheung SK-K, Goggins WB, Yi LM, Cheng KK, Baum L, Effects of 17-allylamino-17-demethoxygeldanamycin (17-AAG) in transgenic mouse models of frontotemporal lobar degeneration and Alzheimer’s disease, Transl. Neurodegener 2 (2013) 24 10.1186/2047-9158-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Benitez MJ, Sanchez-Ponce D, Garrido JJ, Wandosell F, Hsp90 activity is necessary to acquire a proper neuronal polarization, Biochim. Biophys. Acta BBA - Mol. Cell Res 1843 (2014) 245–252. 10.1016/j.bbamcr.2013.11.013. [DOI] [PubMed] [Google Scholar]
- [94].Sha L, Wang X, Li J, Shi X, Wu L, Shen Y, Xu Q, Pharmacologic inhibition of Hsp90 to prevent GLT-1 degradation as an effective therapy for epilepsy, J. Exp. Med 214 (2017) 547–563. 10.1084/jem.20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chittoor-Vinod VG, Bazick H, Todd AG, Falk D, Morelli KH, Burgess RW, Foster TC, Notterpek L, HSP90 Inhibitor, NVP-AUY922, Improves Myelination in Vitro and Supports the Maintenance of Myelinated Axons in Neuropathic Mice, ACS Chem. Neurosci 10 (2019) 2890–2902. 10.1021/acschemneuro.9b00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hutchinson L, Steiert B, Soubret A, Wagg J, Phipps A, Peck R, Charoin J-E, Ribba B, Models and Machines: How Deep Learning Will Take Clinical Pharmacology to the Next Level, CPT Pharmacomet. Syst. Pharmacol 8 (2019) 131–134. 10.1002/psp4.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Vamathevan J, Clark D, Czodrowski P, Dunham I, Ferran E, Lee G, Li B, Madabhushi A, Shah P, Spitzer M, Zhao S, Applications of machine learning in drug discovery and development, Nat. Rev. Drug Discov 18 (2019) 463–477. 10.1038/s41573-019-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vakil V, Trappe W, Drug Combinations: Mathematical Modeling and Networking Methods, Pharmaceutics. 11 (2019). 10.3390/pharmaceutics11050208. [DOI] [PMC free article] [PubMed] [Google Scholar]


