Abstract
The management of diabetic retinopathy (DR) has evolved considerably over the past decade, with the availability of new technologies (diagnostic and therapeutic). As such, the existing Royal College of Ophthalmologists DR Guidelines (2013) are outdated, and to the best of our knowledge are not under revision at present. Furthermore, there are no other UK guidelines covering all available treatments, and there seems to be significant variation around the UK in the management of diabetic macular oedema (DMO). This manuscript provides a summary of reviews the pathogenesis of DR and DMO, including role of vascular endothelial growth factor (VEGF) and non-VEGF cytokines, clinical grading/classification of DMO vis a vis current terminology (of centre-involving [CI-DMO], or non-centre involving [nCI-DMO], systemic risks and their management). The excellent UK DR Screening (DRS) service has continued to evolve and remains world-leading. However, challenges remain, as there are significant variations in equipment used, and reproducible standards of DMO screening nationally. The interphase between DRS and the hospital eye service can only be strengthened with further improvements. The role of modern technology including optical coherence tomography (OCT) and wide-field imaging, and working practices including virtual clinics and their potential in increasing clinic capacity and improving patient experiences and outcomes are discussed. Similarly, potential roles of home monitoring in diabetic eyes in the future are explored. The role of pharmacological (intravitreal injections [IVT] of anti-VEGFs and steroids) and laser therapies are summarised. Generally, IVT anti-VEGF are offered as first line pharmacologic therapy. As requirements of diabetic patients in particular patient groups may vary, including pregnant women, children, and persons with learning difficulties, it is important that DR management is personalised in such particular patient groups. First choice therapy needs to be individualised in these cases and may be intravitreal steroids rather than the standard choice of anti-VEGF agents. Some of these, but not all, are discussed in this document.
Section 1: Scope
DR is a common cause of visual loss across the world, especially in the working-age group [1–9]. The best way of preventing visual loss in diabetes is early detection and treatment [4, 10, 11]. As such, the detection and treatment of this visual threatening problem is vital. The management of DR has evolved considerably over the past decade, with the availability of new technologies (diagnostic and therapeutic). As such, the existing Royal College of Ophthalmologists (RCOphth) DR Guidelines [12] (published in 2013) are outdated, and to the best of our knowledge are not under revision at present (personal communication, RCOphth). Furthermore, there are no other UK guidelines covering all available treatments, and there seems to be significant variation around the UK in the management of DMO. Developing an up to date consensus document/guidelines is especially necessary as contemporary management of DR generally, and DMO, in particular, involves multidisciplinary teams, and include non-medical Ophthalmic HCPs. Most HCPs lack the volume of experience that the medical retina specialist, including membership of this DR Working Group, has.
An expert DRS service is essential to ensure the right people are referred into the hospital service. It is known that there are variations in equipment used for screening, e.g. whether OCT is used or not. Referral rates from screening into hospital clinics vary across the country (e.g. 8% London, 2% Wales) [13]. With so many differences in screening and referrals to secondary care across the UK, it will be difficult to produce one protocol across the UK without consensus. The current screening programme seems to be clogging up hospital clinics, and any help to reduce this capacity demand will be appreciated by medical retina specialists. Furthermore, any protocol for DMO must include a discussion of the quality of screening. There needs to be reproducible standards of screening nationally, with clear intervention required at every level, as well as common access to OCT within screening programmes. It is also suggested that the adoption of ‘virtual clinics’, where possible, would help increase capacity.
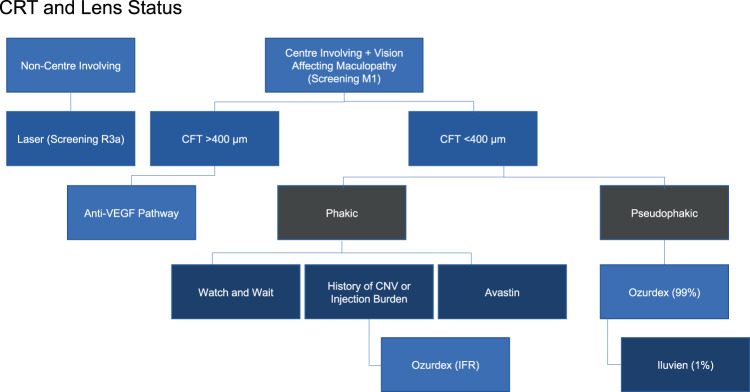
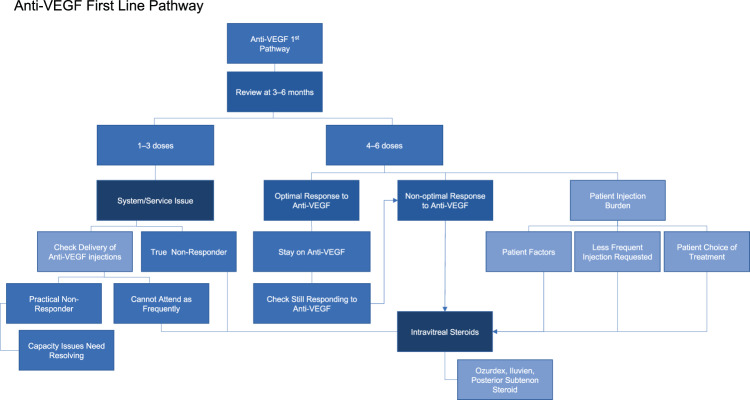
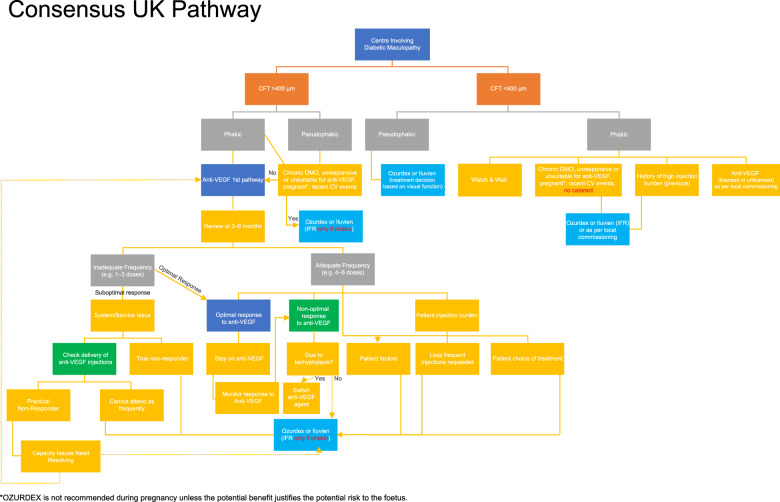
In the current pathway for DMO in the UK, there is no agreement in terms of assessing treatment response (Fig. 1). Definitions of response to therapies, and rationale for switching from one therapy to the other are not uniformly agreed upon. While it is agreed that visual acuity (VA) is a more standardised than OCT parameters (including CRT or central foveal thickness [CFT]), NICE assessed OCT parameters including CRT in developing guidelines for pharmacological treatments for DMO. NICE recommends pharmacological therapies for DMO in eyes with CRT >400 μm (Fig. 2). However, there is some ambiguity regarding the exact measurements of significance. The consensus amongst retinal specialists indicates that in DMO the relevant CRT measurement should be taken in the central 1 mm Early Treatment of Diabetic Retinopathy Study (ETDRS) circle from the fovea.
Fig. 1. Existing UK DMO pathway.
Pathway based on CRT and lens status. CFT central foveal thickness; CNV choroidal nevoascular membrane; DMO diabetic macular oedema; VEGF vascular endothelial growth factor.
Fig. 2. Existing UK DMO ‘anti-VEGF first-line’ pathway: based on NICE TAs for eyes with CFT > 400 μm.
NICE The National Institute for Health and Care Excellence; VEGF vascular endothelial growth factor.
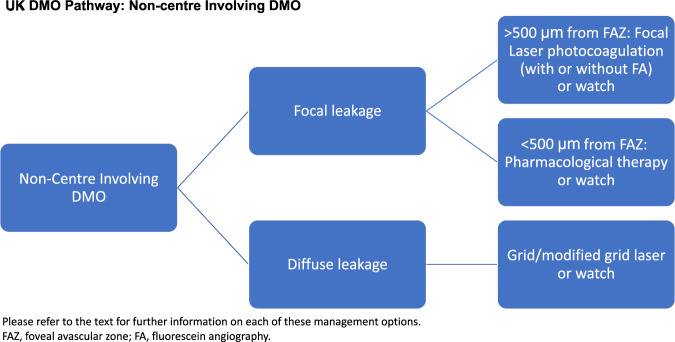
NICE does not recommend licensed pharmacological therapies for DMO in eyes with CRT <400 μm, as such treatments although clinically effective, are not considered cost-effective (NICE TA274; TA346; TA349) [14–16]. The SMC uses VA criteria rather than OCT parameters [17–19]. In particular, dexamethasone implant (Ozurdex, Allergan) is recommended only in eyes with DMO that are pseudophakic, and unresponsive to other therapies. There is variation in how patients with CRT <400 μm are treated across the UK. The population of DMO eyes in pregnancy, generally, cannot be treated with anti-VEGF therapies (on account of risk to the pregnancy and foetus). Similarly, laser photocoagulation may sometimes be inappropriate in such patients. Dexamethasone implant is a viable option in such patients. There is a population with DMO coexisting with cataracts who will eventually become pseudophakic. However, it is known that cataract surgery predisposes to, or worsens DMO. As such, the management of diabetic eyes with cataracts, particularly those with pre-existing DMO is important, as the CRT can change from <400 μm to >400 μm. Evidence from elsewhere supports treatment of such eyes with dexamethasone implant (Ozurdex) in the perioperative period, and best if given pre-cataract surgery [20, 21]. Currently, some clinicians resort to IFRs in order to treat patients with DMO and CRT <400 μm who are not pseudophakic. However, this can be cumbersome and challenging on account of rejection due to financial constraints or poor appreciation of the clinical need. Clinicians believe that agreed national guidelines would streamline processes for offering the best care to such patients.
Specialist opinion also agrees that symptom control is an important aspect of assessing response in DMO (e.g. consider control of diabetes, blood pressure, cholesterol). However, as there are now so few ophthalmology nurses, and other clinics are full, considering the patient ‘holistically’ is a challenge in the eye clinic.
This Working Group was formed in order to address the perceived variations or lack of uniformity in DMO management in the UK, after informal discussions amongst specialists at different advisory groups, and requests from several MR specialists. The groups include retinal specialists with expertise in managing diabetic eye disease. Other specialists including diabetologists, vitreoretinal surgeons, and public health aspects of DR were invited to join the group, in order to achieve a wider expertise and geographic representation.
Here, we seek to review the existing literature on pathophysiology, clinical features and investigation of DR, DR screening and treatment and provide guidance to clinicians who manage DR in the UK and elsewhere. The document used the existing RCOphth guidelines (2013) [12] and European Retina Guidance (2017) [20] as the backbone for its development and include the recommendation for virtual clinics to help reduce service pressures. It has made recommendations on currently available therapies, including laser photocoagulation, intravitreal steroids and anti-VEGFs, and provides definitions of optimal and suboptimal response to therapies.
For completeness, although our emphasis is on DMO, the management of proliferative diabetic retinopathy (PDR) and vitreous surgery in diabetic eye disease are briefly summarised in this document. However, in order not to make the document overwhelming, some aspects of care for DR patients, including Low Vision services, where recent changes are considered minor, are excluded from this document. That strategy does not reduce their importance.
Specific grading of evidence level is not provided for all the recommendations. Instead, the highest evidence level was adopted for each section. Where there is not enough evidence, a rationale is provided for the consensus statement. We have kept to the principles of the Appraisal of Guidelines for Research & Evaluation (AGREE) [22] and ensured that the guideline development is independent.
Sections included are:
The epidemiology of diabetic retinopathy-related vision loss in diabetes.
Public health and commissioning of diabetic eye services.
Pathophysiology of diabetic ocular disease.
The classification of diabetic retinopathy.
Systemic risk management of people with diabetes and effects on retinopathy.
Diabetic retinopathy in children and young adults.
Diabetic retinopathy and pregnancy.
Diabetic retinopathy screening.
Interface between screening and hospital eye service.
Virtual clinics and artificial intelligence in DMO.
The management of DMO.
Response to DMO therapies.
Treatment of PDR.
Vitrectomy in the management of diabetic retinopathy.
Management of cataract in diabetes mellitus and diabetic retinopathy.
Home monitoring as a useful extension of modern tele-ophthalmology.
Search strategy
Medline was used the Group Chair to retrieve relevant literature from the database up to 2019 and supplemented the by individual authors of each section using search terms relevant to the subject matter covered in each section. Previous editions of the RCOphth guidelines (2013), and the European Retina Guidelines (2017) were used as reference sources. The RCOphth guidelines formed the basis of our evidence and recommendation gradings.
Evidence is graded on three levels:
Level 1: evidence based on results of randomised controlled trials (RCTs), power calculations or other recognised means to determine the statistical validity of the conclusion.
Level 2: evidence based on results of case studies, case series or other non-randomised prospective or retrospective analysis of patient data.
Level 3: evidence based on expert opinion, consensus opinion or current recognised standard of care criteria where no formal case series analysis was available.
Recommendations for practice are based on treatment protocols and measures which were recognised to improve patient care and/or quality of life, and is graded on three levels:
Level A: where strength of evidence was universally agreed.
Level B: where the probability of benefit to the patient outweighed the risks.
Level C: where it was recognised that there was a difference of opinion as to the likely benefit to the patient and decision to treat would be based after discussion with the patient.
Section 2: The epidemiology of diabetic retinopathy-related vision loss in diabetes
Diabetes is one of the largest epidemics the world is facing, both in the developed and developing world. In 2016, the International Diabetes Federation (IDF) published data showing that diabetes affects 246 million people worldwide [23]. This estimate was revised upwards in 2010 to 285 million people [24], and again in 2019, where the IDF not only estimated that approximately 463 million adults (aged 20–79 years) were living with diabetes, but also projected that number would rise to 700 million by 2045 [25]. The proportion of people with type 2 diabetes is increasing in almost every country around the world: 79% of adults with diabetes live in low- or middle-income countries, and a further 232 million people—equating to half the people in the world with the disease again—are undiagnosed [25]. An accurate picture of the global burden of DM is hampered by the fact that very few developing nations have national data with ‘high quality’ prevalence surveys of diabetes mellitus; this is only available for 57% of the world’s 221 countries and territories, and only 19% of countries have oral glucose tolerance test-based prevalence data [26]. In the UK, the number of people diagnosed with diabetes has risen from 1.4 million in 1996 to 3.5 million [27–29] in 2015 with an estimated number in 2016 of 4.5 million [29–31] of which a further 1.1 million likely undiagnosed [32] and an estimated projection at the current rate of growth by 2025 of 5 million. The overwhelming majority—90%—of people with diabetes have type 2, 8% have type 1 with the remainder (up to 2%) are rarer manifestations of diabetes [33].
Prevalence and incidence of DR and DMO
With the increasing prevalence of DM and increasing lifespan of people diagnosed with DM, DR is set to be the leading global cause of vision loss in many countries [34]. Although prevalence is similar in men and women, they vary across ethnic groups, with the highest prevalence among Blacks and lowest among Asians, and as yet the cause remains uncertain for these apparent ethnic variations. Prevalence data across the globe varies [34–45] but a recent meta-analysis of 35 population-based performed between 1980 and 2009 across four continents calculated that in people with diabetes age between 20 and 79 years, the overall prevalence of any DR, PDR and DMO is 35%, 7.2% and 7.5%, respectively [46] The prevalence of DR, PDR and DMO were all considerably higher in individuals with type 1 diabetes as opposed to type 2 diabetes: (77%, 32% and 14% vs 32%, 3 and 6%)—and this was independent of the duration diabetes. However, the longer the duration of disease, the higher the prevalence of DR—from 20% in those with a diabetes duration of fewer than 10 years, to 76% in those with two decades or more disease duration.
There is a scarcity of data on the incidence and progression of DR and DMO. The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) reported the overall 10-year incidence of retinopathy was 74% amongst those with retinopathy at baseline of which 64% developed more severe retinopathy and 17% had progressed to PDR [47]. During the same time period, about 20% of type 1 DM and 14-25% of type 2 DM had developed DMO [1]. At the 25 year follow-up, 97% developed retinopathy with 42% developing PDR and 17% visually significant DMO [48, 49]. In the UK, the diabetic screening programme showed the 5-year cumulative incidence in type 2 DM of any DR was 36%, PDR 0.7% and DMO 0.6% approximately doubling at the 10-year time point to 66%, 1.5%, and 1.2% respectively [50].
The incidence and progression of DR and DMO can be seen to be related to a variety of risk factors, in particular control of the DM. The Diabetes Control and Complications Trials (DCCT) [51–53] showed in type 1 DM that intensive therapy reduced risk for development of DR, slowed the progression of DR and reduced the risk of development of severe non-proliferative DR (SNPDR) and PDR. Similarly, the United Kingdom Prospective Diabetes Study (UKPDS) [54–58] in type 2 DM showed the intensive controlled intensive control of blood glucose reduced the risk microvascular end-points including the risk for retinal photocoagulation. A systematic review [59] showed that since 1985, rates of prevalence and incidence have been progressively improving, with an increased awareness of retinopathy risk factors, earlier identification and initiation of care for patients with retinopathy as well as improved medical management of glucose, blood pressure [1, 48, 57, 60–66], and serum lipids [67–69], likely to have led to this reduced rate of incidence and progression.
Section 3: Public health and commissioning of diabetic eye services
Today, the global prevalence of diabetes is so high, it can accurately be described as a pandemic [70], and the prevalence of diabetes is set to double in the next 20 years. The need to tackle these epidemic proportions of diabetes now and in the future has spurred considerable efforts into better understanding this disease in order to better plan healthcare interventions in the future [33].
The investment in skills and resources for diabetes care has never been as important. In terms of UK healthcare spending, diabetes is costly. The latest NHS spending figures from 2019 show that £14 billion is spent on the management of diabetes and its complications—an amount that comprises 10% of the NHS budget for England and Wales, and equates to £25,000 spent per minute on diabetes [33]. The cost of diabetes should be considered not only in terms of detection, treatment and management of complications in healthcare terms as it also extends to absenteeism, early retirement and social benefits in macroeconomic terms [33].
The Diabetic Eye Screening service, along with other screening programmes in the UK are the responsibility of Public Health England (PHE) and the UK National Screening Committee (NSC). The NSC advises ministers in the NHS of the four UK countries about all aspects of population screening and supports the implementation of screening programmes [71]. The NSC looks at changes to screening such as quality standards, screening intervals, strategies to address non-attendance, review of IT systems, development of new screening programmes and improving access. The four countries of the United Kingdom were the first in the world to develop systematic national screening programmes for DR [72]. Prior to its introduction, services varied widely throughout the country. The NSC is independent of the National Institute for Clinical Excellence (NICE), as a screening service applies a test to ostensibly ‘healthy’ people as well as potentially ‘ill’ people. Management and governance of the service are under close scrutiny by PHE as the programmes contain large amounts of data and information. The challenges to the diabetic eye screening programme include cohort management (which is now automated in the GP2DRS screening programme), implementing failsafes and reporting software issues.
In the development of the screening service, the National Service Framework was focused on improving the quality of care for people with diabetes. It introduced targets in improvement, expansion and reform, and put in place registers and education. At the time the primary care trusts were responsible for delivering these standards [73].
By 2008 local retinal screening programmes covered the whole country, and since then have evolved to their current format. DR screening represents a small fraction of the money spent on diabetes care [33].
New commissioning structures moved screening services including retinal screening to the Clinical Commissioning Groups (CCG) in 2013. New grading criteria and a new common pathway were also rolled out [72]. The CCG, therefore, commissions both the screening services and treatment centres for diabetes.
In the UK, a comparison of the rates of registration for vision impairment has reduced in 1999–2000 compared to 2009–2010, so diabetes is no longer the leading cause of registration, having been overtaken by inherited retinal disease [74]. This is a clear indicator of the success of the retinal screening programmes in the UK.
In secondary care, the Ophthalmology department has the highest rate of attendance of all outpatient departments in the NHS, with 7.8 million attendances [75]. Developments in the management of the complications of diabetic eye diseases like DMO and PDR have improved patients’ outcomes. However, the price of new advanced imaging equipment, as well as the expense of new therapies have contributed to the increasing cost of managing diabetic eye disease in hospitals.
The coordinated care for patients becomes even more important for patients as they attend numerous appointments both in the community and in the hospitals to manage their complex condition [70].
There are new developments in the management of diabetes complications in all the subspecialties from medications to surgical options and HCP should be broadly aware of these when they are managing their patients [70].
The focus on diabetes eye care should be between prevention, early detection of complications and then managing complications. Rehabilitation and support services and long-term care of patients who are blind should also be taken into account when considering funding.
Section 4: Pathophysiology of diabetic ocular disease
Diabetes can affect any tissue in the eye, and typically, the ocular surface (i.e. the cornea and conjunctiva), lens, and retina are affected. Diabetic neuropathy is a common systemic sequelae of diabetes, and the eye is not immune from this: diabetics typically experience a progressive reduction of corneal sensitivity associated with increasing corneal nerve degeneration, and the neuropathy can also affect the oculomotor and optic nerves [76–82].
DR is the most common complication of diabetes [46]. DR is a microvascular disease, the presence of which is most closely related to the duration of diabetes [83, 84], but is heavily influenced by poor diabetes control (hyperglycaemia) [58, 85] and the associated cardiovascular comorbidities of hypertension [57], hyperlipidaemia [86], renal disease and smoking [3, 87, 88]. Genetic factors are thought to play a significant role in the development of DR [89, 90], although this is not as well-defined as it is in age-related macular degeneration.
How diabetes can affect the blood-retinal barrier
The normal retinal vascular system is designed to prevent leakage of fluid into retinal tissue, protecting the retina from excess fluid ingress (and some potentially harmful molecules circulating in the blood). This is achieved by the inner blood-retinal barrier (BRB), formed by the tight junctions between the single layer of tightly adherent endothelial cells (ECs), their basal lamina, and surrounding pericytes, astrocytes and microglia. Fluid flow from these retinal blood vessels is regulated by two main mechanisms: one involving the opening and closing of inter-endothelial tight junctions (the paracellular pathway), and the second involving the transport vesicles that travel through the ECs the (transcellular pathway). Diabetes and hyperglycaemia have significant metabolic effects on the cells of the retinal vasculature, as the glucose concentration in these cells reflects that in the blood and tissue fluid. Molecular alterations (summarised below) occur within the retinal vascular ECs and pericytes that result in increased vascular leakage (increased permeability), vascular occlusions, ischaemia, and subsequently angiogenesis if the ischaemia is substantial [3, 87, 88]. These changes manifest clinically as DR.
Recent studies have reported that changes occur in other retinal cells resulting in diabetic retinal neurodegeneration (DRN). However, unlike DR, this neurodegeneration is not clinically detectable [91], and consists of progressive inner retinal neuronal changes, which occur before clinically visible retinal microvascular abnormalities [46, 92–96]. Several investigators have reported that they have observed DRN on OCT images [97–103].
Retinal vascular abnormalities in diabetes (i.e. clinical DR) are well understood and have recognisable features and clinical stages [83, 84]. These mechanisms lead to a breakdown of the BRB which results in an increased permeability if the retinal vasculature, as summarised by Klaassen et al. [91]. This breakdown is what results in DMO. The increased IRF leads to progressive retinal dysfunction, and if left untreated, will result in permanent visual loss [91]. Until recently, there was a paucity of data on the contribution of the choroid to the clinical manifestations of diabetic eye disease, probably because of the inability to adequately visualise the choroid in vivo. The little information previously available suggested that diabetic choroidopathy occurs in the late stages of diabetic eye disease, and the largest contribution to this is likely to be choroidal EC alterations [104–107]. Recently, Vujosevic et al. [108] reported that peripapillary choroidal thickness was reduced in a manner that parallels the development and evolution of clinical DR. The presence of DMO did not seem to influence the changes in choroidal thickness. Yagzan et al. [109], on the contrary, suggest that choroidal thickness increases before the onset of clinical DR.
Kim et al. [110] reported that a subfoveal choroidal thickness increases as the severity of retinopathy worsens, that eyes with DMO have the thickest subfoveal choroid, and that choroidal thickness thins after PRP. However, the choroidal vascular index is significantly reduced as DR severity increases, and eyes with DMO have comparable choroidal vascular indices to those without it [111]. Similarly, Wang et al., [112] found increased DR severity reduced choroidal vascular density. These findings suggest that choroidal vascular index and thickness are different and that the vascular index is a more appropriate and accurate measurement in diabetic choroidopathy [111]. Rewbury et al. [113] suggested that SFCT increases with DR severity, but this association did not hold in the presence of DMO. Adhi et al. [114], reported that SFCT was significantly reduced in eyes with moderate and severe DR, and that the medium-sized vascular layer and choriocapillaris were significantly reduced in eyes with PDR and DMO.
Diabetic maculopathy can be ischaemic (due to perifoveal capillary closure), exudative or oedematous (again due to perifoveal capillary closure) in origin, and can occur at any stage of DR, but is more commonly seen in eyes with more advanced stages of DR including NPDR or PDR, and is influenced by higher baseline HbA1c and systolic blood pressure [48]. DMO is characterised by vascular leakage through both endothelial transcellular and paracellular routes, and this clinically manifests as tissue oedema and the deposition of exudates in the macula, which can be confirmed and quantified with FFA and/or OCT. DMO is responsible for significant visual impairment in diabetic patients [1–3, 46, 115].
Several morphological and biochemical changes are known to occur in DR. These changes, which are interlinked and modified by genetic factors, underpin the pathogenesis of DR.
Morphological changes in DMO
No retinal cell type is exempt from the damaging effects of hyperglycaemia in diabetes. Retinal capillary ECs become leaky (see above), retinal EC proliferation reduces while death rates through apoptosis increase (although this may take some time to be noticeable to the patient) [116, 117]. Similarly, there is increased pericyte loss (through apoptosis) and dysfunction [91, 118, 119]. The mechanism underlying pericyte apoptosis remains unclear, but has been attributed to the accumulation of stable advanced glycation end products, which are abundantly found in hyperglycaemia [3, 91]. There is thickening of the basement membrane (basal lamina) surrounding the retinal vascular ECs and pericytes, and the ECs may become thinner (as reviewed by Klassen) [91].
An early event in the pathogenesis of diabetic vasculopathy is leucocyte adherence to retinal vascular endothelium, resulting in EC death, vascular leakage, and capillary closure [120]. After a period of time, the ongoing cell loss results in acellular capillaries and microaneurysm formation. Occlusion of retinal capillaries and arterioles lead to retinal ischaemia and hypoxia which, depending on the severity, may progress to retinal neovascularisation [121–123]. Finally, retinal astrocytes are affected. Normally these cells help to improve barrier properties by inducing the production of tight junction proteins, but hyperglycaemia leads to significant loss of retinal astrocytes/glial cells, thereby contributing the DMO phenotype [91].
Molecular changes in DMO
The molecular changes in DMO are primarily a consequence of the overproduction of reactive oxygen species (ROS) in cellular mitochondria, leading to oxidative stress and tissue damage, which occur through several major mechanisms (reviewed in Amoaku et al, 2015) [124], some which are still not completely understood. It is thought that ROS, including peroxynitrite and methylglyoxal, lead to increased poly-ADP ribose polymerase (PARP) activation in the cytosols and nuclei of the retinal vascular ECs, setting up a cycle that results in reduction of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the cells, with subsequent changes that manifest as the clinical changes of DR (as reviewed by Klaasen [91]).
Hyperglycaemia leads to protein kinase C (PKC) isoform activation, overactivity of the hexamine pathway, an increased flux of glucose and other sugars through the polyol pathway, and an increased intracellular formation of advanced glycation products (AGEs) and increased receptor for AGEs (RAGE) expression [125]. Other pathways include the renin-angiotensin, and peroxisome proliferator-activated receptor gamma (PPAR-γ; also known as the glitazone receptor) pathways. This ROS increase leads to inflammation through the generation of IL-1, IL-6, IL-8, MCP-1, iNOS, IP-10, MMPs (especially MMP9), C5-9, and TNF-α [91]. In addition, endothelial adhesion molecules such as ICAM-1 (CD54) or E-Selectin (CD62E) [126–128] VCAM-1 (CD106) [129] and PECAM (CD31) are upregulated in ECs.
An early event in the pathogenesis of diabetic vasculopathy is leucocyte adherence to retinal vascular endothelium, resulting in EC death, vascular leakage, and capillary closure [120, 130–134]. The ROS directly affect the retinal neurovascular unit leading to an increased breakdown of the BRB. ROS significantly increase VEGF levels, which in turn increases retinal EC permeability through tight junction alterations. Similarly, the increased ROS levels lead to increased angiopoietin 2, reduced platelet-derived growth factor (PDGF), and reduced VE-Cadherin levels, which together result in pericyte loss [135]. Reduced EC proliferation and increased apoptosis also result from increased ROS generation.
Recent evidence confirms that DMO is not solely due to increased VEGF levels [136], and that VEGF-independent inflammatory pathways are important in the pathogenesis of DR [91, 120, 134, 137–141]. Roh et al. [142] showed that IL-6, IL-8, VEGF, and MCP-1 were significantly elevated in aqueous humour in eyes with clinically significant macular oedema (CSMO), and that elevation of these cytokines (IL-6, IL-8, and MCP-1) occurred with recurrences of CSMO after intravitreal injections of bevacizumab. Similarly, Funk et al. [143], and Sohn et al. [144], reported that IL-8, IP-10, MCP-1, and VEGF are significantly higher in the aqueous humour of DMO group than in controls, and that IL-6, IP-10, MCP-1, PDGF-AA, and VEGF were significantly decreased in eyes treated with intravitreal injections of triamcinolone (although only VEGF was reduced in the intravitreal bevacizumab-treated group). Funatsu et al. [145–147] reported a significant increase in vitreous ICAM-1 in eyes with DMO, as well as increased vitreous IL-6 and VEGF in DR, increased vitreous VEGF, and angiotensin II. Furthermore, VEGF’s effects on EC permeability are linked to angiopoietins [148, 149]. Angiopoietins (Ang) 1 and 2 are cytokines that regulate vascular function through their EC receptor, Tie2 [150, 151]. Ang2 [152] and Tie2 [153] are expressed by EC and levels are increased in response to inflammation and hypoxia, while Ang1 expression is often associated with mural cells and glia in induction and maintenance of the blood-retinal and blood-brain barriers. Ang2 leads to destabilisation [154] and is reportedly increased in high glucose and diabetic vascular dysfunction [155]. Ang2 levels are elevated in eyes with clinically significant DMO [156–158], as well as proliferative DR (PDR) [159, 160] and is thought to induce loss of vascular endothelial (VE)-cadherin through phosphorylation [135]. Stewart et al., [161] reported that high glucose levels resulted in the reduction of Ang1 secretion from human retinal vascular ECs in vitro, although Ang2 levels were consistently high. In vitro, dexamethasone was found to increase Ang1 and decrease Ang2 expression, indicating that the balance of Ang1/Ang2 may be important in determining functional changes in retinal vascular ECs under high glucose conditions [161]. As such, a rationale exists for targeting Ang2 in the treatment of DMO. The kallikrein-kinin system (KKS) has been shown to be dysregulated in DR [140, 162–164]. Kita et al., [140] have shown that VEGF and KKS contribute independently to DMO, and that increases in the KKS protein levels correlate better with severity of DMO than VEGF. Furthermore, injection of KKS proteins increases retinal oedema in animals with experimentally induced diabetes [162–164].
Proliferative DR
Proliferative retinopathy in diabetes manifests as retinal and optic disc neovascularisation. It occurs in the later stages of DR, secondary to microvascular occlusions and ischaemia. Retinal ischaemia in PDR was described several years ago by Wise in 1956 [165] and others, although the molecular mechanisms were unknown at the time; Ashton famously described ‘Factor X’ as the molecule that drove retinal neovascularisation [166]. Generally, diabetes leads to reduced cellular proliferation [161, 167, 168] and EC dysfunction, leading to defective angiogenesis [168, 169]. Several pro-angiogenic cytokines including insulin-like growth factor I (IGF-1), hepatocyte growth factor (HGF), basic fibroblast growth factor (b-FGF), PDGF, pro-inflammatory cytokines and angiopoietins, have been described as being involved in the pathogenesis of PDR, although VEGF is accepted as the most significant cytokine in driving PDR [170–173].
Anti-angiogenic factors including pigment epithelium-derived factor (PEDF), transforming growth factor-beta (TGF-β), thrombospondin (TSP) and somatostatin are synthesised locally within the retina [174–176]. Similarly, levels of several other pro-angiogenic cytokines are increased in the vitreous in eyes with PDR [177, 178], similar to the findings in DMO described above, so the rationale for anti-VEGF therapy in the treatment of PDR is therefore well established. A recent study by Klaassen et al. [177], showed that a network of cytokines was increased in the vitreous in PDR eyes, which included ‘neuregulin 1 (NRG1), nerve growth factor receptor (NGFR), placental growth factor (PlGF) and PDGF. Angiopoietin-2 (Ang2) concentration was strongly correlated to the degree of fibrosis, while PDGF was found to be extensively co-regulated with thrombospondin-1 and Ang2. Analysis of fibrovascular tissue derived from these PDR eyes showed mRNA levels of glial-derived and brain/derived neurotrophic factor (GDNF and BDNF) were elevated in the PDR membranes’ [177].
Section 5: The classification of diabetic retinopathy
DR is essentially, but not exclusively, a microvascular disease. The clinical features and classification of DR have been described in detail in other publications [179], so here we summarise existing DR classifications and discuss the role of newer imaging modalities in the assessment and classification of DR. The classification of DR is important in order to identify individuals’ risk of imminent visual impairment (e.g. clinically significant DMO, new vessel formation), as well as progression to sight-threatening DR, thereby assisting in the development of a management plan for an individual patient.
The features of nonproliferative DR (NPDR) are described in Table 1, with the earliest being the development of microaneurysms (MAs), which are localised saccular outpouchings of the retinal capillary wall. Table 2 describes the classification of the grades of NPDR, which were developed based on the clinical features present and FFA, prior to the development of OCT [179].
Table 1.
How non-proliferative retinopathy classification systems have evolved (adapted with permission from ref. [179] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6587728/).
| Classification System | Classification | |||||
|---|---|---|---|---|---|---|
| Airlie House 1968 (level) [300] | (0)None | (1) Mild to Moderate | (2) Moderate to severe | |||
| ETDRS, 1991 (level) [277] | (10) Diabetic retinopathy absent | (20) Microaneurysms only | (35) Mild non proliferative retinopathy | (43) Moderate non proliferative retinopathy | (47) Moderately severe non proliferative retinopathy | (53 A-D) Severe non proliferative retinopathy |
| ICDRSS, 2003 (level) [278] | No apparent retinopathy | Mild non proliferative retinopathy | Moderate non proliferative retinopathy | Severe non proliferative retinopathy | ||
| National Screening Committee UK, 2003 (level) [275] | (R0) None | (R1) Background diabetic retinopathy | (R2) Pre proliferative diabetic retinopathy | |||
| SDRGS, 2003 (level) [301] | (R0) None | (R1) Mild background diabetic retinopathy | (R2) Moderate background diabetic retinopathy | (R3) Severe background diabetic retinopathy | ||
| DR Deep Learning Algorithm, 2016 [297] | NA | Moderate non proliferative retinopathy | Severe non proliferative retinopathy | |||
ICDRSS International Clinical Diabetic Retinopathy Disease Severity Scale, SDRGS Scottish Diabetic Retinopathy Grading Scheme.
Table 2.
Non-proliferative retinopathy lesion types and potential relevance in NPDR diagnosis and characterisation (adapted with permission from ref. [179]).
| Lesion type | Description | Relevance in non-proliferative retinopathy diagnosis and characterisation |
|---|---|---|
| Microaneurysms and haemorrhages | Occur secondary to capillary wall outpouching as a result of pericyte loss | Number, size and distribution, and turnover of microaneurysms and haemorrhages are important for diagnosis and may help to determine progression rates to sight threatening diabetic retinopathy |
| Earliest clinical sign of diabetic retinopathy | ||
| Rupture of microaneurysms results in haemorrhages | ||
| Intraretinal microvascular abnormalities | Characterise remodelling of pre-existing vessels or growth of new vessels | Presence of intraretinal microvascular abnormalities is necessary for the diagnosis of moderate to severe non proliferative retinopathy |
| Intraretinal microvascular abnormalities are distinctive from the neovascularization observed in proliferative diabetic retinopathy in their larger size and broader arrangement | Unclear whether the distribution of intraretinal microvascular abnormalities is important in assessing severity | |
| Found adjacent to or surrounding areas of occluded capillaries | Intraretinal microvascular abnormalities originating via angiogenesis may be important for the development of proliferative diabetic retinopathy | |
| Visible as telangiectasia, dilated capillaries within the retina | ||
| Venous beading/loops/reduplications | Venous beading is produced by irregular constriction and dilation of venules in the retina | Evidence linking venous beading to proliferative diabetic retinopathy development is unequivocal |
| Venous loops and reduplications are rarer than venous beading and might result from accentuation of a bead, traction from Vitreoretinal adhesions or may be shunt vessels | Venous loops/reduplications do not appear to lead to sight threatening changes in the diseased retina | |
| Cotton Wool spots | Areas of nerve fibre ischaemia or infarction and axonal swelling induced by areas of retinal capillary closure | The early appearance of cotton wool spots helps in the early diagnosis of non-proliferative retinopathy but may lack predictive value for determining retinopathy progression |
| Signs of poor retinal perfusion and are easily visualised | ||
| Associated with systemic hypertension, diabetes and are common in diabetic retinopathy and hypertensive retinopathy | ||
| Hard exudates | Lipid and lipoprotein deposits, usually found in the outer layers of the retina | The presence of hard exudates plays a vital role in grading diabetic retinopathy into different stages, but their appearance was not found to be associated with diabetic retinopathy progression |
| Hard exudates have a ‘waxy’ appearance, with sharply defined borders, and result from leakage from abnormally permeable microaneurysms or capillaries in the retina |
OCT is now a very important imaging modality for the assessment of DMO, although imaging technology continues to advance at a rapid pace, with the advent of OCT angiography (OCT-A) and widefield FFA systems, which are now available in many hospital eye units. Widefield OCT-A imaging (where multiple OCT-A images are used to form a montage) has recently been developed, but is not in widespread use yet
PDR describes how the retina responds to extensive capillary closure—with angiogenesis. Angiogenesis occurs at the interface of perfused and non-perfused retina and occur either as ‘new vessels on the disc’ (NVD) or ‘new vessels elsewhere’ in the retina (NVE). NVD typically develop from venous circulation on either the disc or within 1-disc diameter (DD) of the disc. NVE typically form outside 1 DD from the disc. If macular ischaemia is widespread, it may add to the formation of NVD. NVE can sometimes be mistaken intraretinal microvascular anomalies (IRMA). IRMAs can be distinguished as occurring within regions of capillary occlusion, and typically occur within or flat on the retinal surface. They do not form fine loops. New vessels typically form in the border region between regions of capillary occlusion and healthy retinas [180].
Clinical assessment of diabetic maculopathy
DMO represents an accumulation of fluid within the macula area, due to breakdown of the blood retinal barrier (BRB), and may manifest as diffuse capillary leakage, or focal leakage from dilated capillaries or MAs. Intracellular (and extracellular) oedema may also occur due to retinal ischaemia. OCT is now in widespread use for the diagnosis, evaluation and monitoring of DMO. Spectral domain (SD-OCT) and swept-source (SS-OCT) have replaced the earlier time-domain OCT (TD-OCT) machines and are faster and provide much greater detailed information. OCT has the ability to provide information on CRT as well as distinct morphological features of the oedema. Additionally, it can show persistent morphological changes after DMO treatment. Most patients with DR attending the hospital eye service would now have an OCT examination at each visit as part of their routine clinical examination. The commercially available software from different OCT machines may give different readings of retinal thickness in the same patient, as different OCT manufacturers use different algorithms.
Several features of DMO can only be seen on OCT examination. As such, the descriptions of DMO have changed since the advent of OCT. Morphological signs of DMO may include subretinal fluid (SRF), which refers to ‘non-reflective spaces between the neurosensory retina and the retinal pigment epithelium, and intraretinal fluid (IRF) or cyst (IRC) which are minimally reflective round or oval spaces within the neurosensory retina’ [20]. Additional features are disorganisation of inner retinal layers (DRIL), other alterations to retinal integrity include changes to the inner and outer photoreceptor segments line and external limiting membrane (ELM), MA, exudates/hyperreflective foci, changes in choroidal thickness, and the status of the vitreomacular interface including epiretinal membranes. Disruption of the inner retinal layers, and/or photoreceptor disruption, and/or a thin subfoveal choroid at baseline may be poor prognostic factors for a treatment response [181, 182]. OCT is the most useful imaging modality for the evaluation and monitoring of individual treatment response to anti-VEGF treatment [183].
FFA remains the gold standard in assessing DMO prior to considering treatment and is still the only imaging modality that can detect vascular leakage. FFAs also show areas of capillary non-perfusion and enlargement of the FAZ and may be used in combination with OCT examination. These extra features could have a prognostic significance on any likely response to treatment. There is no consensus on whether FFA should be used in all cases of DMO prior to initiating treatment, but it would be a very important test prior to considering macular laser treatment in order to help determine exactly where to place the laser spots.
In the future, OCT-A would become important in assessing DMO, as it is better at demonstrating the different retinal/macular capillary layers, which are not individually visualised on FFA. OCT-A can determine areas of capillary non-perfusion and can demonstrate capillary dropout in the deep capillary plexus, something that is not shown with FFA. It very useful in assessing patients with DR and reduced vision without central oedema, as it may show capillary non-perfusion as the cause of reduced vision, potentially avoiding the need for FFA in that scenario. However, image artefacts can occur, and the presence of significant cystic changes can also make interpretation difficult. MA may not show up on OCT-A, even if they are perfused (and leak on fluorescein angiography), although improvements in software may help in this regard in the future. With the advent of OCT-A, it may, therefore, be reasonable to reserve FFA for assessing DMO cases where macular laser is being considered, or if inadequate OCT-A images are obtained. Most OCT-A equipment in current UK clinical practice does not permit imaging of the far retinal periphery, which is an additional advantage of FFA, but widefield OCT-A will mitigate this limitation when it becomes available for regular use around the UK.
Fundus autofluorescence
The role of fundus autofluorescence (FAF) in DR and DMO is still not fully clear, as FAF is a form of functional imaging which provides insights into the metabolic activity of the retinal pigment, rather than just a purely a method of visualising retinal anatomy [20].
The role of FAF may be defined by its ability to assessing the health of the underlying retinal pigment epithelium, and by inference, the health of the adjacent photoreceptor, meaning that it could be useful in judging the visual potential of patients with long-standing DMO. In healthy eyes, autofluorescence signals are almost absent at the optic disc and gradually increased centrifugally with a peak at fovea. Two patterns of FAF abnormalities have been described in centre-involving DMO: a ‘mosaic’ pattern consisting of granular or patchy hyper- and hypo-autofluorescence at the fovea, and ‘cystoid’ pattern where the cystoid spaces are outlined. Both the mosaic and cystoid patterns are associated with worse VA and thicker central subfield thickness on OCT [184]. Although autofluorescence can identify areas of the cysts with cystoid macular oedema (CMO), it is unlikely to replace the role of OCT. FAF is reported to increase with time in eyes treated with ‘barely visible laser photocoagulation’ [185]. However, such changes in subthreshold laser photocoagulation may not be very obvious [186, 187]. Its role in laser re-treatment decision making requires further elucidation, especially in subthreshold laser therapy.
Summary
OCT should be routinely used in the clinical assessment of patients with DR and maculopathy.
FFA should be considered on a case-by-case basis prior to macular laser treatment especially where the source of the leakage is not obvious, or the reduction in VA cannot be explained by the degree of clinically obvious maculopathy.
OCT-A is useful to determine areas of capillary non-perfusion and demonstrate capillary drop out especially in the deep capillary plexus avoiding the need for FFA.
Autofluorescence may have a role in laser retreatment of DMO, particularly with subthreshold laser or barely visible laser treatment where burns may not be clinically discernible yet easily apparent with autofluorescence [185–187].
Clinical assessment for PDR
Most NVE and NVD will be detected on careful clinical examination and are usually clearly visible on FFA. OCT examination can also be very helpful to determine if NV are present (at the posterior pole, where such imaging is possible) in doubtful cases by the presence of pre-retinal hyperreflective material [188]. OCT-A can also be very helpful in assessing areas of capillary non-perfusion and show flow within the new vessels. OCT-A may also be useful in determining the response of NV to treatment in terms of monitoring changes in flow. The advent of widefield OCT-A is likely to prove additionally helpful here. Widefield FFA is extremely useful in detecting areas of ischaemia, as well as detecting new vessels, but it can sometimes be difficult to distinguish very early new vessels from other sources of leakage and the use of structural OCT over the area in question in addition to the FFA can be helpful in these circumstances. Widefield FFA is also very helpful in guiding PRP treatment to the areas of capillary non-perfusion.
Summary
Most NVs are detectable on careful clinical examination but FFA, structural OCT and OCT-A can be very helpful in cases where there is uncertainty.
Widefield FFA is very helpful in planning PRP treatment by clearly showing the areas of capillary nonperfusion that may not be obvious on clinical examination or are likely to be missed on a standard multi-field FFA.
Section 6: Systemic risk management of people with diabetes and effects on retinopathy
To recap, diabetes is associated with increased incidence of microvascular (retinopathy, nephropathy and neuropathy) and macrovascular complications (heart disease, strokes and peripheral vascular disease), mortality and this imposes an increased economic burden to healthcare systems [189]. Effective management has led to collaborative diabetes care models between primary and specialist care with multidisciplinary teams being implemented globally. In many developed countries, over 90% of people with diabetes are managed in primary care [190]. Primary care is therefore well placed to screen for microvascular complications in people with diabetes. Overall, 12–19% of people with type 2 diabetes will have DR at diagnosis, with around 4% developing proliferative DR (PDR) after 20 years or more [54, 191, 192]. In most regions of the UK, DR screening is shared by the NHS DESP and primary care, with the overall uptake rate for screening being around 80% [193].
The risk factors for developing DR can include both modifiable (hyperglycaemia, hypertension, hyperlipidaemia and obesity) and non‐modifiable (duration of diabetes, puberty and pregnancy) factors [194]. Two landmark trials, The Diabetes Control and Complications Trial (DCCT in type 1 diabetes) [52] and UKPDS (in type 2 diabetes) [58] showed that tight glycaemic control (as measured by HbA1c level assessment) leads to a reduced risk of developing DR and its progression. Systematic review evidence suggests that intensive glycaemic control leads to a 20% reduction in risk of retinopathy (HR 0.80, 95% confidence interval [CI] 0.67–0.94) [195]. In people with type 2 diabetes, reductions in blood pressure is associated with 13% reduced risk of retinopathy (RR, 0.87; 95% CI 0.76–0.99) [196]. Furthermore, recent systematic evidence shows that renin-angiotensin system inhibitors reduce the risk of DR and the possibility of improving DR regression [197]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study showed that in people with type 2 diabetes who also received fenofibrate and simvastatin treatment was associated with less progression of DR (progression of three or more steps in the ETDRS scale) at 4 years compared to placebo (OR 0.60; 95% CI 0.42–0.87). However, these benefits were not sustained at 4 year follow-up [198]. Furthermore, the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study showed that in participants who received fenofibrate, there was a significant reduction in the need for laser therapy at 5 years for either DMO or PDR compared to placebo (HR 0.69; 95% CI 0.56–0.84)[199]. In addition, in people with DR, lipid-lowering agents have beneficial effects in progression of DR (OR = 0.77; 95% CI 0.62–0.96) and possibly reduce the risk of development of DMO (OR = 0.60; 95% CI 0.34–1.08) [200]. Primary care management of diabetes has resulted in overall improvements in retinopathy over the years. One key reason for this has been due to substantial improvements in risk factor (glycaemic, blood pressure and lipid control) management in primary care since the introduction of the Quality and Outcome Framework in the UK [201]. In view of variable uptake rates for DR screening, collaborative working, communication and coordination between general practitioners, specialist care and the ophthalmologists should be encouraged to reduce variations in retinopathy screening rates.
Recommendation
Systemic control of diabetes needs to be actively reviewed in patients with DR in order to modify progression. (Level 1, A) However, response to anti-VEGF therapies may not correlate directly with HbA1c levels.
Section 7: Diabetic retinopathy in children and young adults
DR, although a common complication in type 1 and type 2 diabetes, is rarely observed in children and young adults, and this has been attributed to the fact that several years are required from the onset of diabetes to the development of clinically significant retinal changes [202]. A retrospective analysis of 143 patients aged 12 years or younger, who attended diabetic eye screening for the first time in a Birmingham DR screening programme, identified only 12 patients (8.4%) with DR (mild DR without diabetic maculopathy); no patient was identified with sight-threatening DR at the initial assessment [202].
The current population eligible for DR screening in the UK includes all persons diagnosed with diabetes (type 1 or type 2) aged 12 years and over [203]. In the UK, the recommendation is to commence photographic screening in diabetic patients from the age of 12, and this is supported by a large study that reviewed data from 2125 children, aged 12 to 13 years at first screening, recruited from all four UK Nations (England, Scotland, Wales and Northern Ireland) [204]. The study identified that in children diagnosed with diabetes under the age of 2 years, 20.1% had signs of any retinopathy, compared with 6.3% of those diagnosed at the age of 10 years. However, only three children (0.14%) were identified as having referable DR at their first screening. Follow-up data was available for 1703 children, with 25 children (1.5%) graded as having referable retinopathy, with only three of whom (0.17%) graded as having PDR [204]. The median time from baseline screening to sight-threatening DR was 3.1 years [204].
Unfortunately, there is no literature detailing the treatment of DR in the paediatric population, given that all clinical trials for DR recruited subjects older than 18 years of age. However, it seems reasonable to consider focal or panretinal laser photocoagulation to treat DR for the same indications as in adult patients. However, the safety and efficacy of intravitreal anti-VEGF therapies in children and adolescents with DMO has not been established for ranibizumab (Lucentis, Novartis/Genentech) or Eylea (Bayer/Regeneron). Nevertheless, there is some limited data on the use of ranibizumab in adolescent patients between the ages of 12 to 17 for the treatment of choroidal neovascularisation, which showed that with Lucentis was well tolerated in this group [205]. Given the cataractogenic effects of intravitreal steroids, there is no relevant use of intravitreal steroids in the paediatric population affected by DR to date.
Evidence level: 2
Recommendation: Treatments for DR in the young and adolescent diabetics should be individually tailored, using evidence from treatments in adults, as there is limited RCT data to guide treatment decisions in this patient group.
Recommendation: Level B.
Section 8: Diabetic retinopathy and pregnancy
The prevalence of DM worldwide is increasing—as is the number of pregnant women with DM. There are two reasons for this: a general trend for increasing gestational age, combined with a younger age of onset of type 2 DM (T2DM). Indeed, DM is estimated to affect 17% of pregnancies worldwide [206]. The vast majority of these women have gestational DM and fortunately, gestational DM is not associated with an increased risk of developing DR during pregnancy. However, a smaller proportion of pregnant women have undiagnosed T2DM, and this subgroup may develop DR during or after pregnancy. The prevalence of DR in early pregnancy in T2DM is estimated at 14% [207] and a wide range has been reported in type 1 DM (T1DM): between 34 and 72% [208–216].
Few studies have investigated DR in pregnancy; some involved women with only T1DM or T2DM, and a low level of evidence underlies most findings. This reflects the relative rarity of women requiring treatment in pregnancy and the inherent difficulties of studies in pregnancy, both logistically and ethically. Nevertheless, there are some good practice points that these studies have established that can be used to guide a management pathway.
There are well-recognised risk factors for the progression of disease in the general diabetic population, but pregnancy is certainly an independent risk factor [211, 217]. Progression occurs at approximately double the rate compared to the non-pregnant population [218]. In the DCCT, 180 women became pregnant, and the odds ratio of progression were 2.48 in the conventionally treated and 1.63 in intensively treated groups [217].
The following factors have been implicated in the progression of disease in pregnancy:
Duration of DM
This is a strong association for durations of T1DM of less than 10 years, DR remained stable, but 10% of women with a 10–19-year disease duration experienced DR progression [213, 214]. The Diabetes in Early Pregnancy (DIEP) Study found that diabetes duration was particularly important in predicting the progression of PDR (R3); of those with DM of more than 15 years’ duration, 38% progressed to R3, compared with 18% with a disease duration of 15 years or less [209]. Duration is also a risk factor in T2DM—14% of women with an average of 6.7 years duration showed progression, compared with 3.3 years in those without progression [207]. However, long disease duration alone does not necessarily correlate with poor outcomes [214]—as is well recognised in the non-pregnant diabetic population.
Baseline level DR
In the DIEP study, progression of ≥2 stages occurred in 10.3% with R0 at baseline, 18.8% with mild nonproliferative diabetic retinopathy (NPDR) at baseline, and 54.8% with moderate NPDR or worse at baseline [209]. The risk of R3 developing where there was R0 or minimal R1 was rare (0.4%, and all of these were in patients with T1DM) although there have been case reports of rapid deterioration from minimal DR in early part of pregnancy to vitreous haemorrhage and DMO in the third trimester, even with stable glycaemic control [219].
Poor glycaemic control
As is well recognised in the non-pregnant diabetic population, poor glycaemic control has been shown to be a risk factor for progression in both pregnant women with T1DM [208, 209, 220–222] and T2DM [207] (although those with strict glycaemic control prior to conception had a lower risk of progression [217]). However, tightening of glycaemic control—especially if rapid—in those with pre-existing DR may in fact worsen the DR [51]. Rapid optimisation of glycaemic control is common because of improved non-ocular outcomes for mother and foetus, so there is a need to balance the risks here. Poor glycaemic control is associated with adverse outcomes for the foetus—there is an increased risk of congenital malformations, foetal mortality and morbidity—and for the mother, with an increased risk of renal failure and pre-eclampsia [223, 224]. The DCCT showed 53% of conventional treatment group who were changed to intensive control in early pregnancy had worsening of DR compared to 40% in initial intensive group [217].
Hypertension and pre-eclampsia
It is well documented that antihypertensive treatment reduces progression of retinopathy in the non-pregnant population [225]. Elevated blood pressure (BP) is also a recognised risk factor for DR progression in pregnancy [208, 215, 220, 222]—one in four (25%) of normotensive pregnant women with DM showed progression, compared with 61% with chronic hypertension, and 50% with pregnancy-induced hypertension [222]. A systolic BP of >115 mmHg compared in pregnant women (compared with <105 mmHg in non-pregnant women) has been quoted as a risk factor [208, 220]. However, not all studies have confirmed an association with BP, nor have proposed an optimal pressure level to aim for in pregnancy.
Another pregnancy-specific issue is pre-eclampsia (new hypertension from 20 weeks of gestation) which occurs more commonly in pregnant women with DM (than pregnant women without DM) [226]. In one study linking progression with sight-threatening deterioration, 50% of patients had pre-eclampsia compared to 8% without pre-eclampsia [221].
T1DM or T2DM
There is a low risk of progression in pregnant women with T2DM, although sight-threatening deterioration can occur from mild to R3+ DMO, although the cases described usually involved patients with other risk factors for progression such as poor pre-conception glycaemic control, low compliance and high BP in the first part of pregnancy [207, 214].
The mechanisms underlying the progression of DR in pregnancy are unknown, but some factors are likely to be involved.
Pregnancy is associated with major changes in systemic vasculature including increased cardiac output, plasma volume and decrease in peripheral resistance [227]. This could cause capillary endothelial damage [228, 229] and exacerbate the loss of autoregulation that occurs in DR due at least in part to pericyte loss. It is also possible that the local hypoxia associated with worsening retinopathy could cause a compensatory increase in blood flow which may then represent an epiphenomenon, rather than failure of autoregulation in pregnancy [230]. Doppler velocimetry studies have shown that retinal blood flow increases in pregnant women with DM who develop progression of retinopathy, and not in pregnant women with stable DR [228].
The hormone changes in pregnancy have also been implicated in the progression of DR. There is an increase in plasma human placental lactogen, oestrogen and progesterone. These hormones induce vascular changes that may contribute to progression of retinopathy, particularly human placental lactogen (hPL) that has growth hormone-like activity.
Pro-angiogenic growth factors such as insulin-like growth factor (IGF1) and VEGF expression levels increase during pregnancy. The stimulus for this may be the acute fall in retinal blood flow if metabolic control is improved rapidly [231]—one study showed progression independent of glycaemic control [211]. The possible role of insulin analogues such as Lispro in development of severe R3 in pregnancy has also been raised [232]. Lispro is a homologue of IGF-1, but this phenomenon has also been reported with other insulin-based drugs [219]. Two studies that investigated the progression of DR during and after pregnancy suggested that the probability of progression was associated with elevated IGF-1 levels in later pregnancy [233, 234].
Post-partum changes
Fortunately, retinopathy that progresses during pregnancy has a high tendency for regression post-partum, although how long regression might take to complete is not at all predictable [208, 217, 235–238].
The overall prevalence of DR in women with prior pregnancy was also shown to be similar to that of matched nulliparous women [217, 235, 239]. In the DCCT, DR levels after 6.5 years of follow-up were comparable both those who had been pregnant, and those who had not been pregnant [217]. It has been shown in other studies that pregnancy does not probability of progressing to R3 at 2 years post-partum, or having a requirement for laser photocoagulation therapy at 5 or 10 years post-partum [235]. One proposal to explain these observations was that if a women with DR was likely to experience DR progression, that woman was likely to do so during pregnancy (and therefore received appropriate therapy), or that these women derived a longer-term ‘metabolic memory’ benefit from improvements in glycaemic control during their pregnancy [235].
Similar outcomes were observed by the DCCT in women in the intensive control group, irrespective of pregnancy status [240]. A decade after the completion of the DCCT, women in the intensively treated group were observed to have a 53% greater reduction in further progression than women in the conventional treatment group, despite HbA1c levels being equal at the end of the trial’s follow-up period [241].
After long-term follow-up, it was found that women in the study who had pre-eclampsia or pregnancy-induced BP increases had a greater risk of developing DR that required laser after long-term follow-up than those without [242]—which suggests that raised BP during pregnancy might have a bigger impact on long-term DR levels than glycaemic control. It should be noted that these long-term follow-up studies included T1DM patients only.
Treatment
Studies have largely concentrated on the treatment of R3 (PDR) with limited data on DMO.
PDR should be treated with panretinal photocoagulation (PRP) [210, 224, 243] and ideally before the onset of pregnancy, in view of the risk of progression but also the considerable difficulties for patients that multiple appointments pose, and the risk therein of failure to attend all appointments and have their retinas adequately monitored [230, 244, 245]. There is level I evidence for DR for laser treatment for severe NPDR or PDR in pregnancy. Good evidence (level I/II) exists for high-risk PDR in pregnancy receiving PRP, although this is not specific to pregnancy. A high proportion of those who develop R3 may continue to progress post-partum so treatment may even need to be considered at the severe NPDR in some cases [244–246].
There may be post-partum regression of retinopathy so there may be a case for performing limited PRP in cases of less active disease. In some cases, however, the progression can be aggressive and response suboptimal [247]. As such, so treatment should be proportionate and sufficient to induce regression in each case.
Sight-threatening DMO can occur during pregnancy [215, 248] although data is limited; prevalence estimates for DMO at any time during pregnancy range from 5–27% in T1DM [215, 235, 249, 250] and 4% in T2DM [207, 249]. There are no studies published to date that use OCT to quantify. DMO does appear to spontaneously regress post-partum [207, 241]; a period of waiting/close observation may be reasonable.
In addition, given the relatively short duration of pregnancy and evidence of similar outcomes with delay in anti-VEGF in non-pregnant patients with delay in treatment [251, 252], delaying treatment of DMO during this time is likely to be justified in many cases, given the likelihood of resolution of the DMO post-partum. In particular, the use of intravitreal injections of anti-VEGF drugs in pregnancy is not recommended because of potential effects on the unborn child. When treatment is indicated in such cases, however, the use of intravitreal steroids, particularly dexamethasone implant is advised. Longer-acting steroids are not advised under such circumstances, as the pathology may be mitigated post-partum. Furthermore, that ensures that any systemic levels of steroids in breastfeeding mothers are low. For example, in cases of bilateral poor vision secondary to DMO—when this occurs in pregnancy, is thought to be due to an ischaemic capillariopathy and may be accompanied by R3 disease.
Anti-VEGF drugs
Neither anti-VEGF drug approved for use in DMO in the non-pregnant population in the UK have been studied in pregnancy and have been assigned Pregnancy Category C by The US Food and Drug Administration (FDA) (see Appendix A). The mechanism of action suggests that the anti-VEGF agents used for intravitreal injections may pose risk to developing embryo or foetus; it is therefore recommended that women wait at least 3 months after last treatment prior to conceiving [253–256]. There are some case reports also suggesting a possible effect on maternal BP also and an increased risk of pre-eclampsia [257, 258]. There are also some case reports of uneventful pregnancy following multiple injections [259].
Corticosteroids
Corticosteroids have been designated FDA Pregnancy Category B drugs although there is variation within the intravitreal agents; for example, triamcinolone acetonide in the form of Kenacort A-40 (triamcinolone acetonide) and Dexamethasone implant (in the form of Ozurdex) is designated category C, Triesence (triamcinolone acetonide) is designated category D [260].
After high-dose intravitreal triamcinolone (20–25mg) therapy, systemic serum levels of the drug were practically undetectable [261]. Dexamethasone has been used to accelerate foetal lung maturation in premature labour [262], but intravitreal triamcinolone [263] and dexamethasone [264, 265] have also been used to treat DMO with no reported systemic side effects in pregnancy. As dexamethasone (in the form of Ozurdex) is a NICE-approved drug in the UK for the treatment of DMO, it would be appropriate to consider using this in pregnancy, if treatment is deemed necessary. Although systemic corticosteroids have been found in breast milk, the systemic concentration of dexamethasone in the form of intravitreal Ozurdex is low. It is unknown whether intravitreal administration of steroids could result in sufficient systemic absorption to be detectable in breast milk, so caution is advised for use in breastfeeding. Ideally breastfeeding should be stopped before intravitreal anti-VEGF agents are used.
Fluorescein
Fluorescein is designated as an FDA category C drug (see Appendix A).
The product information states that there are insufficient studies to assess the safety in pregnancy [266].
One paper suggests that there is no evidence of an effect on the foetus [267], although fluorescein does cross the placenta [267] and is detectable in breast milk [268]. In practice, it would be advisable to avoid fluorescein angiography (FFA) in pregnancy and during breastfeeding, and in most cases there would be no justification for the use of FFA, as clinical examination and photography are usually sufficient to make a treatment decision.
Recommendations for the Management of DR in Pregnancy
There are a number DR screening and management in pregnancy guidelines across the world, but these are not supported with high levels of evidence. As RCTs are not possible generally in this group for ethical reasons, much of the data is an extrapolation from studies in the wider non-pregnant (and usually Caucasian) population. Guidelines are then largely based on expert opinion-based preferred practice guidelines only. There is a general recognition that an individual’s personal circumstances and comorbidities need to be considered. (Level 2, A)
Despite this lack of solid evidence, there is general consensus for:
Close collaboration between obstetrics, endocrinologists and ophthalmologists.
Counselling regarding the effect of pregnancy on the DR. Effect on the timing of pregnancy—generally advised women with T1DM particularly to plan pregnancies earlier as there is clear evidence of increased risk of disease progression with the duration of DM.
Glycaemic control should be optimised.
Increase screening during pregnancy—screening programmes in the UK already do so.
Screening in the postnatal period—up to 12 months would be advised if retinopathy has progressed into the third trimester. In DCCT, the increased risk of DR progression continued for 1 year after childbirth [217, 233, 246].
Those with gestational DM should be followed up medically to ensure it resolves and was not the result of underlying T2DM.
Severe NPDR or worse—scatter PRP should be considered, and the retinopathy stabilised if possible, prior to conception.
The best treatment option for progressive DMO in pregnancy is intravitreal injection of steroids.
DR should not be considered a contraindication to vaginal birth [246] (see Appendix B). In the rare cases of active R3 disease at full-term, it would be reasonable to inform a patient of the theoretical increased risk of vitreous haemorrhage with a vaginal birth. However, this should be a discussion between the patient, obstetrician and ophthalmologist, as the risk is likely to be low and all other factors involved in decision-making regarding labour should be taken into account.
Evidence grading: Level 2
Recommendation: A
Section 9: Diabetic retinopathy screening
The St Vincent Declaration, back in 1989 asserted that DR screening (DRS) is the cornerstone of DR management and treatment [269]. All patients with DM are screened for the presence of retinopathy, with the stated aim of the DRS programme being to reduce the incidence of blindness that results from DR—which is the commonest cause of blindness in the working-age population [1, 2]. Such blindness results from either proliferative DR (PDR) or DMO. The age of entry into the UK DRS programmes is currently 12 years, and the only exclusion criterion is an inability to consent and a VA of worse than PL. DRS standards are summarised in the National Framework document [73], and have subsequently been reviewed by Diabetes UK [270].
The UK now has a consensus grading system that underpins the DRS service in each of the four home nations, but differences do exist each scheme [271–274]. Despite this, DRS delivery is based on a few common principles, the most critical of which is quality assurance. The English and Welsh programmes require two photographs to be taken after mydriasis; one centred on the fovea, the other on the optic disc. The Scottish programme uses a single image and mydriasis is only used in some instances. In Northern Ireland, a similar strategy to the Scottish one is followed.
The principles underpinning the UK DRS are eloquently summarised in Scanlon (2017) [275] as follows:
Screening is a public health programme, not a diagnostic test.
Large numbers of apparently healthy individuals are invited for screening; some people may be harmed by the process, or falsely reassured.
There is an ethical and moral responsibility to ensure that the programmes are of high quality.
Quality assurance of screening programmes is therefore essential to ensure that the programme achieves the highest possible standards and minimises harm.
It is estimated that some grade of DR is present in more than a third of diabetics. Accurate clinical examination of the retina for disease detection in such a large population is logistically almost impossible. The situation is complicated further by the adoption of the original ‘gold’ standard imaging of diabetic fundus with the 7-field 30° stereographic retinal photography (as proposed by the Diabetic Retinopathy Study) [276] which was subsequently supported by the ETDRS [277]. The DRS imaging/grading system differentiates 13 complex levels of disease severity from 10 (no DR) to 85 (severe vitreous haemorrhage or retinal detachment involving the macula). This complexity makes it difficult to implement in a real-life situation, for the photographer, patient, and clinician alike. The International Clinical Diabetic Retinopathy Disease Severity Scale (ICDR) was developed as a simplified version, with the objective of making it useful in the clinical setting [278]. Here, DR is graded as ‘mild non-PDR’ (NPDR) if there were microaneurysms [MA] only; ‘moderate’ NPDR if there was more than one MA—i.e. dot and blot haemorrhages and cotton wool spots, and ‘severe’ NPDR if there was >20 haemorrhages in each of all four retinal quadrants or definite venous beading in ≥2 retinal quadrants or prominent intraretinal microvascular abnormality in one or more retinal quadrant; and finally PDR. DMO, if present, was graded in three levels based on the distance of retinal thickening and/or hard exudates from the fovea as seen in the photographs. However, for treatment, a clinical examination was essential to assess ‘retinal thickening’ as a marker for macular oedema. The term ‘CSMO’ was defined as any DMO which showed retinal thickening within 500 µm of the centre of the fovea, or yellow exudates within 500 µm of the centre of the fovea, with adjacent retinal thickening or one disc area of retinal thickening, any part of which is within 1 DD of the centre of the fovea [278].
For the classification of DR in screening the National Screening Committee (NSC) (updated in 2019) [279, 280] described the absence of diabetic retinopathy R0. The presence of MAs and small retinal haemorrhages or mild, non‐PDR as R1, moderate to severe non‐PDR R2, and PDR as R3.
The NSC classification of M1 represents referable maculopathy. This was based on what the ETDRS originally defined as CSMO. The NSC had to use this clinical definition to develop a set of features that can be reliably identified in a two-dimensional colour photograph. It was necessary to include a VA measurement as part of the assessment. The photographic criteria included:
-
(i)
exudate within 1 DD of the centre of the fovea
-
(ii)
a circinate or group of exudates within the macula
-
(iii)
any microaneurysm or haemorrhage within 1 DD of the centre of the fovea only if associated with a best VA of ≤ 6/12. The grades of R2, R3 and M1 are classified as sight‐threatening DR (STDR) or referable DR [278].
Of these only PDR (R3) is categorised as requiring urgent referral.
Recent publications have demonstrated this approach has resulted in a reduction in blindness in the UK [74]. This happened during a period where there has been a global increase in the prevalence of diabetes worldwide. Another interesting shift is that the blindness is now more likely to be related to DMO than PDR [281].
One way to improve DRS would be to utilise OCT in the screening process and improve early diagnosis of DMO. This practice is becoming increasingly recognised as the reference standard for assessment of DMO and can potentially provide a cost-effective solution for improving DMO detection. However, there is insufficient evidence of OCT alone as a tool to predict progression of visual loss that arises from DMO. It is therefore difficult to stage patients meaningfully in a mass screening programme and then refer them into a hospital eye service. There are other complex technologies like combined OCT and widefield imaging which can potentially give better sensitivity [282], but again, these instruments not yet widely adopted in clinics.
The use of automated image analysis and artificial intelligence (AI) to detect retinal pathologies has become a field of great interest in screening. This computerised approach, once fully validated, should offer great benefits in terms of quality assurance, cost and speed of assessment. However, there are significant hurdles that need to be cleared before the widespread acceptance of these techniques can begin—particularly on how to validate the techniques. Scotland was one of the early adopters of such automated image analysis techniques. In the US the first AI device received FDA approval for identifying DR in 2018—the IDx-DR. The device has 87% sensitivity and 90% specificity and 96% imageability. This seems to be the direction of travel; however, these products will need to be significantly improved before widespread acceptance/adoption.
Section 10: Interface between screening and hospital eye service
Having seamless arrangement between screening programmes and local ophthalmology departments is a crucial part of ensuring appropriate delivery of quality-assured clinical care to patients with diabetes [283–285]. It is possible that a screening programme boundary may overlap a number of hospital ophthalmology departments and similarly, a hospital ophthalmology department may receive referrals from more than one screening programme. It is important for each hospital eye service (HES) to understand the NHS diabetic eye screening programme (DESP)’s failsafe procedures as well as formulate their own patient pathway, standard operating procedures and failsafe standards for DR patients.
Ideally, hospital eye departments should identify a clinician as ‘Clinical Lead for DR’, who would have overall responsibility for the smooth running of hospital diabetic eye services—including laser and DMO treatment clinics, as well as the clinical governance of the service. The HES Clinical Lead would liaise with the local DESP clinical leads, referring patients in to HES and helping to prepare annual reports that are filed with DESP. It is essential that each hospital ophthalmology department has a dedicated person (a DESP co-ordinator) with administrative oversight of DR patient referrals and management. The DESP co-ordinator would liaise with each of the local DESP programme managers about key performance indicators and keep track of referrals and timely appointments in a hospital eye clinic. The Clinical Lead should review the arrangement of clinics including virtual clinics and work to ensure appropriate retinal imaging equipment—retinal photography, spectral domain (SD)-ocular coherence tomography (OCT) imaging (potentially with facilities for OCT angiography) and widefield retinal imaging—is available in the HES. Links with vitreoretinal consultant surgeons should be established for more severe cases needing surgical interventions.
The HES Clinical Lead and the co-ordinator should oversee and ensure that appropriate hardware and software are available to all clinical staff engaged in the management of DR patients. HES would need access to software used by each of the DESP in the clinics. It is increasingly becoming the norm that HES have an in-house electronic patient record (EPR) system—such as Medisoft (Medisoft Lt, Leeds, UK) or Openeyes (Apperta Foundation, Sunderland, UK). These EPR systems should link up with DESP software so that real-time data capture can take place and help prepare quarterly as well as annual reports for the programme for quality assurance. Regular communications—either automatic updates from EPR to DESP software or manual should take place between HES and DESP. Such information includes the acknowledgement of referrals arriving at the HES, patient attendances and their retinopathy grading, non-attendance, and especially patients that are discharged back to DESP. Minimum data capture standards should be agreed between the DESP and HES, but should include data on key performance indicators such as the number of referrals (stratified by retinopathy grading), waiting times for appointments, waiting time for laser treatment and the incidence of blind registrations caused by DR. The DESP website describes screening pathway and the themes (Population, Coverage, Uptake, Test, Diagnosis/intervention, Referral, Intervention/treatment, and Outcome) that make up the entire screening process [286].
Section 11: Virtual clinics and artificial intelligence in DMO
A virtual clinic can be defined as a clinic in which the face-to-face clinician consultation is removed [287]. The patient and clinician either interact in virtual real-time (synchronous model) or at different points in time (asynchronous model). One of the main drivers of the introduction of virtual clinics—particularly with respect to DR screening—is that they have been shown to increase service capacity. It has been recognised that, compared with a holistic ‘face-to-face’ consultations, twice the number of patients can be assessed by reviewing OCT image and VA data ‘virtually’ (in the absence of the patient) at a secondary, asynchronous event [282]. Virtual review of DMO patients’ maculopathy status fits well into this model, where OCT images and VA measurements are collected by nurses or technicians, and then later reviewed by ophthalmologists or trained healthcare professionals with the appropriate competencies, under the governance of a Consultant Ophthalmologist with medical retina sub-speciality expertise [288]. The capacity for assessing diabetic eyes can also be expanded by running data acquisition virtual clinics outside of normal clinic hours (when equipment stands unused) then the reviewing team assessing that data at a later stage.
Another driver of virtual clinic adoption by busy NHS practices is that there is a greater opportunity for Consultant Ophthalmologists to share their expertise in retinal image analysis in performing quality assurance of the decisions made by junior ophthalmologists or other trained healthcare professionals at a virtual review clinic (without patients) than in a busy face-to-face clinics, where patients wait in the clinic for their management plan during their consultation.
Patients with DMO also require the assessment of their peripheral DR status, which in traditional retina clinics typically occurs within the same patient episode as their maculopathy assessment. The use of virtual clinics in managing patients with DMO permits this by imaging both the macula and the peripheral retina in the same visit, something that is made easier if ultra-widefield (UWF) images are added to the macular OCT image. This is now a common approach for DR assessments within many hospital eye services [282, 289, 290]. However, in some cases, such as in patients with DMO receiving frequent intravitreal anti-VEGF injections (often as frequently as once per month in the first year of some anti-VEGF regimens), the acquisition of simultaneous macular OCT and peripheral UWF images is inefficient, as many peripheral UWF images will be acquired that are not needed, particularly given that intravitreal therapies also inherently protect against peripheral DR [291–293].
A more pragmatic approach for patients with DMO who require intravitreal injections of anti-VEGF drugs is to create a separate system of clinic reviews for peripheral DR that are driven by the severity of the disease and are independent of maculopathy status (where maculopathy is managed virtually). These peripheral retina clinic reviews can be an opportunity for a more holistic slit lamp assessment of the patient looking for other comorbidities (e.g. cataract progression) alongside retinopathy grading and in many patients (e.g. those with mild nonproliferative DR) might only be needed yearly. Equally, these peripheral assessments can themselves also be managed virtually with widefield colour retinal imaging [282, 290].
Virtual clinics are also effective at improving capacity for new referrals with DMO (e.g. M1 referrals from Diabetic retinopathy screening DRS). Some 75% of referrals with M1 status from DRS are false positive when OCT imaging has not occurred [294, 295]. OCT triage of referrals either within a DRS service or at the entry point to the HES reduces inappropriate referrals and is cost-effective [290, 295].
The safety and effectiveness of virtual clinic assessments in DMO patients instead of real-time clinical slit lamp examinations has been examined in a Cochrane review by Virgili et al. [296]. The review concluded that OCT images were more sensitive than slit lamp examinations in detecting DMO and, therefore, not highly accurate at diagnosing CSMO—the eligibility criterion for argon laser photocoagulation. However, the presence of CSMO does not necessarily mean a patient will require intravitreal anti-VEGF injections and the authors concluded that OCT-based diagnosis should be the new reference standard for DMO [296]. OCT diagnosis of DMO has also been proven to be superior to two-dimensional digital photographic retinal screening colour photographs as per UK DR screening protocols [295].
Further service capacity could be created if deep learning algorithms can be implemented to assess DMO using AI. Deep learning is a novel technique with potentially wide applications across medicine and is characterised by algorithms that can learn features of disease though exposure to large volumes of data, thereby extracting meaningful patterns from them; these algorithms are capable of picking up features and changes that are sometimes missed human assessments [297].
The assessment of DR, in general, has been a key area for the development of deep learning due to the huge unmet need for screening treatable diabetic retinopathies in very large populations, particularly in low-income countries [297]. The development of multiple commercially available ‘automated retinal image analysis systems’ (ARIAS) led to the eventual FDA approval in the USA of a DR detection algorithm combined with convolutional neural networks (CNN) facilitating deep learning known as the IDx-DR system [298]. IDx-DR has an 87.7% sensitivity and 96.8% specificity [298, 299].
Subsequent developments have included additional algorithms [275, 277, 278, 297, 300, 301] and their ongoing validation producing potentially even higher sensitivities and specificities (reviewed by Grzbowski et al. [295] in Table 1). While most available software uses colour fundus photographs to grade all retinopathy including maculopathy (with the ability to even predict quantitative disease metrics such as central subfield thickness from colour fundus photographs [302]) other recent automated image analysis systems examine OCT data [303]. The advances in automated analysis and the effects of deep learning on multimodal imaging would be powerful, particularly given the complexity of some DMO eyes that may be associated with OCT-A ischaemia and three-dimensional pathology such as vitreomacular traction.
The efficacy and accuracy of these deep learning systems depend on access to large volumes of data, as well as the ‘ground truth’ or benchmark used to confirm the correct diagnosis [304]. Unsettling for the clinician is the concept of the ‘black box’, where the computer system has reached a ‘conclusion’ or ‘diagnosis’ and the clinician cannot identify what retinal features led to that conclusion [304]. Future systems will be able to identify ‘heatmaps’—or regions of interest in the retina that led to the disease classification [304]. Finally, much more accessible systems possibly via smartphone image acquisition may revolutionise further DR screening [304].
Recommendation: Improved service capacity for DR assessments can be achieved by the usage of virtual clinics.
Section 12: The management of DMO
The treatment options for DMO have changed considerably over the last few years. We aim to give succinct guidance for UK ophthalmologists working in the NHS, where the availability of therapies is subject to restrictions based on NICE and SMC guidance. Attention to systemic factors, such as BP and glycaemic control, is always important in people with diabetes – especially in the context of macular oedema, where optimum control of BP can significantly reduce the oedema [48, 83, 305, 306]. Other conditions that are more likely to affect people with type II diabetes (such as sleep apnoea) need to be considered as well, as this may cause or exacerbate macular oedema [307–309].
Current treatment options include first-line intravitreal therapies with anti-VEGF drugs and then may include laser therapy or steroids (dexamethasone or fluocinolone implants) [20]. The rationale for anti-VEGF therapies is more obvious as VEGF-A is the most important cytokine responsible for retinal vascular leakage. The rationale for intravitreal steroid use comes from evidence that highlights the role of inflammation in the development of DMO, including leukostasis, upregulation of various inflammatory mediators such as ICAM-1 and IL-6 in addition to upregulation of VEGF-A (See ‘Pathophysiology’ section). Intravitreal steroid use is particularly relevant for eyes with chronic oedema that is insufficiently responsive to other therapies.
Clinically significant macular oedema
The term CSMO was developed at the time of the ETDRS study [310], which assessed laser photocoagulation as a treatment for DMO. CSMO was defined (based on the slit lamp examination), as:
Retinal thickening within 500 µm of the centre of the macula
Hard exudates within 500 µm of the centre of the macula if associated with thickening of the adjacent retina
Retinal thickening of >1-disc area in size, any part of which is located within 1-DD of the centre of the macula
It is important to note that this definition of CSMO is applicable only for laser treatment, and pre-dates the availability of OCT, and milder degrees of oedema are now detectable by OCT that may not have been seen before on slit lamp examination alone. Moreover, it is important to know whether the oedema involves the fovea/central subfield (i.e. centre-involving DMO [CI-DMO]), as this will determine what treatment options are appropriate.
CSMO without central involvement
In the ETDRS study, eyes with CSMO treated with laser had a 50% reduction in the risk of moderate visual loss compared to observation. However, very few eyes experienced an improvement in vision. The mechanism of action of macular laser treatment is not fully understood but may relate to cytokine release from the retinal pigment epithelium or Müller cells as reviewed by Bhagat et al. [3]. Since the original ETDRS study, a variety of different retinal laser delivery systems have been developed, and various different wavelengths can now be considered e.g. argon green (514 nm), yellow (577 nm), or diode (810 nm) in the treatment of DMO. Subthreshold grid laser therapy has also been developed (both at 577 and 810 nm wavelengths), with the aim of reducing the destructive effects of conventional macular laser photocoagulation. Some studies show similar efficacy of subthreshold laser to conventional laser [311, 312] and results from a UK-based randomised clinical trial [313] are awaited. At present, subthreshold laser is not in widespread use in the UK for DMO treatment.
UK current best-practice for treating CSMO without central involvement depends on the location of the leaking microvascular changes. If these are far from the fovea (>500 μm from the FAZ) with considerable associated fluid/exudate, then laser therapy intervention may be appropriate. However, observation until fluid involves the fovea may also be a reasonable option for non-centre involving CSMO. Many ophthalmologists from around the world advocate the latter approach. The particular issue in the UK is that NHS funding is not usually available to treat milder degrees of centre-involving oedema (defined as CST <400 μm) with intravitreal anti-VEGF drugs, or with steroids in phakic eyes. As such, considering laser treatment where it is safe to do so may sometimes be appropriate to try to prevent the fluid (or exudate) from deteriorating to involve the fovea.
Centre-involving CSMO (CI-DMO)
In UK NHS practice, the chief issues to consider when determining treatment decisions are the level of VA, central subfield thickness on OCT examination, and patient choice. It is noted that different OCT machines will show different thickness measurements for any given patient, with the Heidelberg Spectralis machine generally giving the greatest macular thickness measurements.
NICE guidance (applicable to England, Wales and Northern Ireland)
Ranibizumab. NICE TA274 [14] recommended it as an option for treating visual impairment due to DMO if the eye has a CRT of 400 μm or more at the start of treatment.
RESTORE study [314]. This study showed a mean best-corrected visual acuity (BCVA) gain overall of +6.1/+5.9 letters for the two ranibizumab-treated groups, compared with +0.8 letters in the laser monotherapy group. There was no benefit in the combination of laser and ranibizumab compared with ranibizumab monotherapy. The RESTORE extension study showed a mean BCVA gain of +8 letters at 3 years in the ranibizumab monotherapy group [252].
RISE and RIDE studies compared 0.3 mg and 0.5 mg ranibizumab vs. laser treatment. 10.9 and 12.5 mean change in BCVA at 2 years vs. laser (+2.6/+2.3 letters) [315].
Aflibercept. Recommended in NICE TA346 [16] for eyes with visual impairment due to DMO with more than 400 μm CRT at the start of treatment.
VIVID and VISTA studies: [316] These compared aflibercept with conventional laser treatment. In the aflibercept group, treatment was commenced with five injections at monthly intervals, then either 4 weekly or 8 weekly injections thereafter. Mean change in BCVA at 1 year was 11.6 letters for the 4 weekly aflibercept injections group, and 10.7 letters at 100 weeks. The 8 weekly injection regime BCVA changes were +10.7 letters after 52 weeks, and 10.3 letters after 100 weeks.
Fluocinolone acetonide (Iluvien). NICE TA301 [317] recommended it as an option for treating chronic DMO that is insufficiently responsive to available therapies in pseudophakic eyes. This recommendation was based on the FAME Study with Iluvien, where 34% of patients experienced a ≥15 letter the fluocinolone-treated group at 3 years vs. 13.4% in the sham treatment group [318, 319].
Dexamethasone implant (Ozurdex). NICE TA349 [15] recommended it as an option for treating chronic DMO that is insufficiently responsive to available therapies in pseudophakic eyes, based on the results from the MEAD study [320]. Ozurdex retreatment was administered every 6 months (although this may have led to relative under-treatment). In MEAD, 22.2% of patients achieved ≥15 letter gain in the Ozurdex treated group at 3 years compared with just 12% in the sham-treated group [320].
SMC guidance (applicable to Scotland only)
Ranibizumab and aflibercept are approved for the treatment of visual impairment due to DMO in adults where the BCVA is 75 letters or worse [17, 18].
For the dexamethasone and fluocinolone implants, these are approved for pseudophakic eyes where there has been insufficient response (or not suitable) for non-corticosteroid therapy [19, 321].
Treatments for CI-DMO
This section will present evidence-based guidance about which treatments to choose for UK-based NHS practice and which treatment regimens to consider. A separate chapter will deal with when to consider switching to intravitreal steroid treatment.
Visual acuity
The Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol V study [322] showed that ‘for eyes with CI-DMO and good VA (20/25 [6/7.5] or better), there was no significant difference in vision loss at 2 years, whether eyes were initially managed with aflibercept or with laser photocoagulation or observation (and given aflibercept only if the VA worsened)’. Therefore, in people with very good acuity (80 letters or more), it may be reasonable to observe initially, even if there is CI-CSMO, with later intervention if the VA deteriorates. This is less likely to be frequently relevant in the UK where anti-VEGF treatment cannot be given unless the CRT is 400 μm or more. However, it should be remembered that VA and central macular thickness (CMT) may not correlate, and that VA may be good in eyes with foveal thickness greater than 400 μm [323–326].
Eyes with CI-DMO and less than 400 μm CRT (or BCVA greater than 75 letters in Scotland)
Per the Protocol V results [322], if a patient’s VA is 80 letters or more, it may be reasonable to simply continue with observation. Conventional laser treatment can be considered if leaking microvascular changes are well away from the fovea, or alternatively, subthreshold grid laser treatment may be an option. In pseudophakic eyes where laser treatment has previously been given (or is not suitable) and there is persisting oedema, intravitreal steroid treatment use should be considered (based on NICE guidance). However, this option is not adopted universally in pseudophakic eyes with mild degrees of oedema because of the potential rise in intraocular pressure (IOP). In such circumstances, it is more common to observe until 400 μm central subfield thickness is reached at which point anti-VEGF treatment is considered (or if the VA decreases to75 letters or worse in Scotland).
Some units in the UK have approval for the use of bevacizumab for cases where there is CI-DMO but where the NICE or SMC criteria are not met (based on Protocol T results: see below).
Eyes with CI-DMO and more than 400 μm CRT (or BCVA ≤75 letters in Scotland)
Anti-VEGF therapy would be the first-line therapy, unless the patient does not wish to have regular intravitreal injections or is pregnant.
Which drug to choose when commencing anti-VEGF treatment?
DRCR.net Protocol T [327, 328]. This trial compared bevacizumab, aflibercept and 0.3 mg ranibizumab for the treatment of DMO. All eyes underwent a loading dose phase until week 24 (with observation if 85 letters and less than 250 μm) and then a flexible retreatment phase. For eyes with BCVA of 69 letters or better, there was no significant difference between the drugs at 1 and 2 years. For eyes with less than 69 letters, the 1-year results showed that aflibercept (+18.9 letters) was superior to ranibizumab (+14.2 letters) and bevacizumab (+11.8 letters). The 2-year results showed no statistical difference in mean BCVA between aflibercept and ranibizumab, but both were still superior to bevacizumab. It is noteworthy that ranibizumab 0.5mg which is the approved dose in the EU was not included in the DRCR Network protocol.
Using a crossover study design, the CADME study showed a statistically significant but small relative clinical benefit of ranibizumab 0.3 mg compared with bevacizumab for the treatment of DMO [329].
Given the area under the curve considerations from the Protocol T study, standard UK practice would be to commence aflibercept treatment for DMO, where VA is below 69 letters. With better VA, either ranibizumab or aflibercept would be NICE-approved options with bevacizumab as an off-label alternative. Systemic exposure is known to be greater for bevacizumab and aflibercept than for ranibizumab, however the clinical significance of this is still unclear. Overall, there is no evidence to suggest that there is significant difference in adverse event rates across any of the three medications based on the results of both in DMO and age-related macular degeneration (AMD) trials of these agents [330].
Choice of treatment regimens for anti-VEGF treatment
Licensed posology for aflibercept
The European posology for aflibercept in DMO is to administer one 2 mg injection every month for five consecutive doses, followed by one injection every 2 months [331]. After the first 12 months, the treatment interval may be further extended based on VA and anatomical measurements. The treating physician should determine the monitoring schedule.
Licensed posology for ranibizumab
‘Treatment in adults is initiated with one injection per month until maximum VA is achieved and/or there are no signs of disease activity [205]’.
Protocol T regimen
The Protocol T treatment regimen is comprised of an intensive initial treatment regimen with monthly dosing intervals (typically 5 to 6), followed by monitoring once no further improvement can be achieved, although treatment can be stopped earlier if there is a ‘complete response’ where VA improve to 85 letters and central subfield thickness (CST) drops below 250 μm on OCT [327]. ‘Improvement’, ‘worsening’ and ‘stability’ are defined as follows:
Improvement: ≥5-letter improvement in VA and/or ≥10% improvement in CST;
Worsening: ≥5-letter decrease in VA and/or ≥10% increase in CST;
Stability: no improvement or worsening in both VA and CST after two consecutive injections.
There is an extension of intervals once stability has been reached.
Treat-and-extend regimes
There is little evidence to date about the use of Treat-and-Extend (T&E) regimes for aflibercept for DMO from the outset, other than data from two studies, VIOLET and EVADE. The VIOLET study [332] examined the safety and effectiveness of intravitreal aflibercept for the treatment of DMO in patients who had already received a year’s worth of aflibercept therapy in the open-label, phase IV AQUA study of aflibercept in DMO (NCT02581995).
In VIOLET (NCT02818998), patients were randomised to either (a) fixed two-month treatment intervals, (b) pro re nata (PRN), or (c) treatment with increasing time intervals (Treat and Extend, T&E). No significant differences were observed in the visual results: In all, three treatment groups, patients were able to maintain the visual gains achieved with the first year of aflibercept therapy through to the end of the second year of treatment, although there were clear differences with respect to the number of doctor visits, despite comparable numbers of injections: the PRN treatment group required 14.4 clinic visits and 6.3 injections; the T&E treatment group, required 8 check-ups for 5.6 injections.
The Treat and Extend Versus Bi-monthly dosing with Aflibercept for the Treatment of Diabetic Macular Edema (EVADE Study) that compared the safety and efficacy of T&E and fixed interval (every 8 weeks) aflibercept dosing regimens (randomised in a 1:1 ratio) in subjects with DMO (n=50) receiving intravitreal aflibercept injections [333]. At one year, both treatment groups experienced significant VA gains; the study authors report that VA gains favoured the T&E arm, although this required more injections and visits than the fixed interval arm [333].
For ranibizumab, the RETAIN study compared a T&E 0.5 mg dosing regimen (with and without laser photocoagulation) with a 0.5 mg T&E regimen, and found that both T&E regimens was non-inferior to the pro re nata regimen [334].
UK ophthalmologists generally take an approach closer to the Protocol T regimen, with a proactive initial phase of dosing, followed by monitoring and extending thereafter [335].
Combination of anti-VEGF with macular laser treatment
The DRCR.net Protocol I study compared three regimens: (1) prompt and (2) deferred laser photocoagulation therapy in patients who also received ranibizumab, with (3) an initial 4 mg triamcinolone and prompt laser treatment regimen. They found that both ranibizumab regimens were significantly better in improving patient mean VA and mean CST at 1 year compared with the prompt laser/ triamcinolone regimen, but in the ranibizumab-treated patients, the timing of the laser therapy appeared to have no real impact on the visual or anatomical benefits achieved in these patients at one year. On the other hand, the deferred laser group was associated with a superior BCVA area under the curve, and a greater proportion of eyes experienced a >15 letter gain in the deferred laser group than in the prompt laser group [336]. Nevertheless, we conclude that routinely combining laser treatment with anti-VEGF therapy is not warranted for CI-DMO, although occasional adjunctive delayed laser treatment may sometimes be considered, especially where there are leaking microvascular changes well away from the fovea that persist, despite regular anti-VEGF treatment. There is also no evidence that peripheral scatter laser treatment reduces the need for intravitreal anti-VEGF injections for DMO [337].
Recommendation for anti-VEGF treatment for eyes meeting NICE or SMC criteria
VA <69 letters, consider anti-VEGF monotherapy with either aflibercept 2 mg or ranibizumab 0.5 mg as first choice, as there is no difference in long term outcomes with either drug
VA ≥69 letters, aflibercept 2 mg, or ranibizumab 0.5 mg monotherapy could all be considered.
Suggest considering a Protocol T-type treatment regimen.
Recommendation for eyes with CI-DMO not meeting NICE or SMC criteria
Observe,
or may sometimes consider laser if appropriate,
or consider trying to obtain funding approval for anti-VEGF treatment.
Occasionally intravitreal steroid treatment may be considered in pseudophakic patients where other treatments have not been effective.
The consideration for switching to intravitreal steroid treatment for insufficiently responsive eyes is discussed above (Fig. 3).
Fig. 3. Summary of recommended pathway for non-centre involving DMO: consensus recommendation.
DMO diabetic macular oedema.
Section 13: Response to DMO therapies
Several intravitreal pharmacological treatments are now available for the treatment of DMO, including three anti-VEGF agents, both licensed (ranibizumab, Novartis/Genentech; aflibercept, Regeneron/Bayer) and unlicensed (bevacizumab, Roche/Genentech), and steroids (dexamethasone implant [Ozurdex, Allergan] and fluocinolone implant [Iluvien, Alimera], and triamcinolone), and the efficacies of the individual treatments have been established in several clinical studies. Although these treatments are generally effective in DMO, they do not work in every eye—some eyes are more treatment-resistant to some drugs (and drug classes) than others.
The DRCR.net performed a landmark phase III clinical study that compared the use of ranibizumab (plus prompt or deferred laser) with triamcinolone (and prompt laser) for the treatment of DMO [336]. A significant number of ranibizumab-treated eyes failed to achieve LogMAR 0.0 vision. Furthermore, 50–70% ranibizumab-treated eyes failed to achieve a 2-line improvement [336], whereas 37% the ranibizumab-treated eyes had central subfield (CSF) thicknesses of >300 µm on TD-OCT at 12 months, and 40% ranibizumab eyes did not have complete resolution of macular oedema (MO) (<250 µm) at 24 months [327, 336]. Similarly in the VIVID and VISTA studies, Kaiser et al. [338], reported that eyes switched to aflibercept from other anti-VEGF therapies experienced similar vision and anatomic gains.
However, in the UK, the current DMO treatment pathway has no agreement in terms of assessing treatment response, or how such responses should be assessed, whether it be through VA assessment, retinal thickness assessments by OCT imaging, or a combination of both, and there is no UK-wide consensus on the rationale for switching from one therapy to the other. It is agreed that VA is a more standardised measure than OCT parameters (including CFT); the National Institute for Health and Care Excellence (NICE) assessed OCT parameters including CRT when developing guidelines for pharmacological treatments for DMO, and settled on recommending pharmacological therapies for DMO in eyes with a CRT >400 μm. However, there is some ambiguity regarding the exact measurements of significance. The consensus amongst retinal specialists indicates that in DMO, the relevant CRT measurement should be in the central 1 mm ETDRS circle from the fovea, with discretion based on local decision-making.
Evidence review
Analysis of the results from the randomised clinical trials of anti-VEGF drugs for the treatment of DMO indicate that the majority of VA gains were achieved within the initial 3–6 months of therapy, whereas the morphological response was slower [327, 336]. In Protocol T, after 2 years of treatment, 58% of eyes treated with bevacizumab for 2 years had residual fluid visible on OCT [336]. ‘Treatment failure’ was defined as persistent thickening of the macula and/or the loss of 10 ETDRS letters, despite 4-weekly injections [327]. ‘Non-response’ in this study was defined as bevacizumab-treated eyes that received at least three consecutive injections and showing <10% decrease in CST from baseline OCT, within the first 9 months of therapy.
Ashraf et al. [339] performed a retrospective study of 59 eyes from 45 patients with DMO that were treated with bevacizumab, and were, per the Protocol T definition, classed as non-responders, and were switched to aflibercept or ranibizumab. BCVA significantly improved in eyes switched to ranibizumab, and significant decreases in CST were observed in both the ranibizumab and aflibercept-treated eyes.
Another switching study by Bahrami et al. [340], in which DMO non-responder patients were switched to aflibercept therapy used the following criteria to define ‘non-responders’: CMT >300 µm despite at least four intravitreal injections of bevacizumab in the previous 6 months. In the retrospective study by Lim et al. [341], no definition of ‘non-response’ was provided, although patients were switched from ranibizumab or bevacizumab to aflibercept.
Rahimy et al. [342], in their retrospective study (of 50 eyes of 37 patients) on short-term functional and anatomic outcomes of persistent DMO converted patients to aflibercept from ranibizumab or bevacizumab, using the criteria of four consecutive injections at 4–6 weekly intervals prior to conversion (and performed aflibercept injections after conversion). They defined persistent DMO as: no reduction, incomplete resolution or increase in central subfield thickness after four consecutive injections. The investigators observed anatomic, but not functional improvements after the therapy switch.
Shah et al. [343], in a retrospective study reported on eyes with DMO that were switched from intravitreal bevacizumab or ranibizumab to intravitreal aflibercept, and defined persistent DMO as eyes with five persistent intraretinal cysts with or without exudates, (but no minimal CST criterion in their definition), after at least three injections of anti-VEGF at fixed intervals prior to switching from other anti-VEGFs to aflibercept.
The BOLT study categorised response of DMO eyes to bevacizumab injection as early responders with dry macula at 3 months, intermediate responders as those with dry macula at month 12, and late responders as eyes that were dry at 24 months [344, 345]. They further defined eyes with persistent MO at 24 months, and not dry at 4 or 12 months as ‘non-responders’ [345]. Clearly there is no consensus in the literature as to what constitutes a ‘non-responder’, and when to attempt to try an alternative anti-VEGF therapy.
In the DRCR Network’s Protocol U study [346], dexamethasone implant plus ranibizumab treatment was compared with ranibizumab and sham control treatment in patients who have continued DMO (i.e. anti-VEGF non-responders; those that received at least three anti-VEGF infections (aflibercept, bevacizumab, or ranibizumab) within the previous 20 weeks) and had CSF of approximately 300 µm (depending on the gender of the patient and the manufacturer of the OCT instrument), with the objective of seeing whether combined treatment with a corticosteroid and an anti-VEGF agent would improve vision and decrease MO. The investigators found that the addition of dexamethasone implant was more likely to reduce retinal thickness than continued ranibizumab alone (52% vs. 31% had normal CST, respectively, p = 0.02; mean CST change significant, p < 0.001). The authors concluded that the addition of dexamethasone did not statistically improve BCVA compared with the continuation of ranibizumab alone at 24 weeks despite these results (presented in Table 3).
Table 3.
DRCR Network’s Protocol U study 24-week study results.
| Change in vision | Dexamethasone implant + ranibizumab | Ranibizumab alone | Adjusted difference | p-value |
|---|---|---|---|---|
| ≥10 letter improvement | 22% | 14% | 6% | p = 0.3 |
| ≥15 letter improvement | 11% | 2% | 7% | p = 0.03 |
| ≥10 letter loss | 13% | 6% | 7% | p = 0.09 |
The currently available evidence suggests that each individual treatment modality in DMO does not result in a completely dry macula in approximately half of all cases sometimes, the response is better with one treatment compared to the other [124]. There is no unified definition of suboptimal or non-response to DMO pharmacotherapy as differing opinions on the definition of ‘non-response’ exist. Heier et al. [347], defined lack of improvement as <5 letters improvement in VA, and/or ≤10% decrease in CST after 6 injections at monthly intervals. In the study reported by Wood et al. [348], a majority of DMO eyes with persistent fluid on SD-OCT (despite regular intravitreal injections of ranibizumab 0.3 mg and/or bevacizumab 1.25 mg) showed a positive anatomic response to aflibercept 2 mg injections. The report by Klein et al. [349], defined recalcitrant DMO as eyes with DMO in which there was a decrease of <15% in CRT over 6 months despite at least four treatments including three anti-VEGF injections within 6 months, and >3 injections of aflibercept within 6 months after the switch.
The CHAMPLAIN Study recruited patients with treatment-refractory DMO. The definition of ‘refractory’ was not very obvious. These eyes had significant resorption of oedema, and improved BCVA (30.4% gaining ≥10 letters at 8 weeks; a mean of 6 letters at 8 weeks and 3 letters at 26 weeks respectively) [350]. However, IOP increased in 16% of eyes, although no patient required drainage surgery. In the PLACID study, the percentage of eyes gaining ≥10 letters was higher in the dexamethasone implant-treated group compared to laser monotherapy, although 15.2% of participants experienced an elevation IOP levels of >10 mmHg, and 4% experienced elevations of >35 mmHg [351]. In the FAME study, eyes with chronic DMO had a better response to steroid therapy where oedema was chronic than acute [352].
Bressler et al. [353] suggested that in Protocol T, the treatment response in eyes that were switched from one agent to another was good, irrespective of the CST and the number of previous anti-VEGF injections. The RISE and RIDE study, however, suggested that eyes that had received >4 previous injections were less responsive to anti-VEGF therapy on account of the chronicity of oedema [315].
Gonzalez et al. [354], have reported in a post-hoc analysis of Protocol I data, that it was possible to determine the response to anti-VEGF therapy (ranibizumab) after 3 injections at 4-weekly intervals of anti-VEGF in DMO. Suboptimal response was defined as <5 letters improvement compared to baseline. These authors suggest that eyes with early suboptimal visual responses are likely to have poor longer-term visual outcomes. There was a significant variation between subjects in each initial response group, so that later improvement may be slow, and was still possible, even in patients in the early suboptimal response group. The implication of this analysis that only 30% or fewer eyes that fail to show an early response to anti-VEGF therapy are likely to improve vision in the long term. A subset of 7% and 23% of the early suboptimal responders showed a 15-letter and 10-letter improvement respectively by week 52. Bressler et al. [353] reported that switching therapies for persistent DMO after 3 or more intravitreal injections of anti-VEGFs was not advisable, as continuing with the same anti-VEGF may result in subsequent (delayed) improvement in vision, and reduction of macular fluid. Similarly, Pieramici et al. [355], reported significant vision improvement at week 100 in only a small number of eyes with suboptimal early response (at week 12) in the VIVID and VISTA studies. They defined suboptimal outcomes as <10% reduction in CST and CST > 300 μm, and/or vision gain of <5 letters.
Dugel et al. [356], chose to define a ‘significant response to therapy’ as being a 20% reduction in CRT and following an analysis of anti-VEGF treated patients in Protocol I. They reported that around 65% were strong responders with 83% of those having a 20% reduction at 3 months continuing to do so at 3 years, whereas only 48% reached a 20% reduction at 3 years if they <20% reduction after the first 3 treatments.
A retrospective study by Busch et al. [357], reported that dexamethasone implant had a beneficial effect in early switch (after 3 injections of monthly anti-VEGF therapy) in eyes that were unresponsive to anti-VEGF therapy. A switch to dexamethasone later at 12 months still resulted in significant VA improvements—but less than those who were switched earlier, whereas eyes that continued to receive anti-VEGF therapy for the 24 months exhibited a delayed response.
Recommendations
It is recommended that when DMO treatment is commenced with intravitreal anti-VEGF injections, the same anti-VEGF is delivered at optimum intervals as recommended in the SmPC. (Level 1, A)
A preliminary assessment of response may be made at month 5–6 (i.e. 1 month after the initial loading injections). The eye should be considered as having a suboptimal or poor response if CST is reduced by less than 20% on OCT. (Level 2, A)
Caution has to be given when using VA as a measure of improvement, as reproducibility may be low, but consideration should be given when VA gains are seen on switching therapies, as small switch visual acuity gains are expected of around <5 letters, and where the CST is unchanged or continues to increase despite the initiation of three 4-weekly injections, an early decision to switch to a different therapy is advisable. Then
Consider switching to another anti-VEGF agent (Level 2, B).
Consider macular laser if appropriate.
Consider dexamethasone implant (Ozurdex) or fluocinolone implant (Iluvien), if eye pseudophakic. Eyes known for uncontrolled IOP rise with steroids (‘steroid response’) or eyes with uncontrolled glaucoma are excluded, unless agreed with a glaucoma specialist. (Level 1, A)
If the eye is NOT pseudophakic, consider phaco/IOL if cataract is present followed by Ozurdex or Iluvien or. (Level 1, B)
Ozurdex use is initially preferred by some because of shorter duration of action especially if unsure of IOP change. Ozurdex may be replaced with Iluvien later, if required. (Level 2, B)
Where the eye is NOT pseudophakic, and there is no significant cataract, and the DMO is chronic or inadequately responsive to anti-VEGFs, or the patient is pregnant or has other contraindications to anti-VEGF therapies including recent cardiovascular events, it is appropriate to consider dexamethasone implant (Ozurdex) injection. (Level 2, B)
Triamcinolone is not recommended for routine use as it is not licensed for intravitreal injection in the UK and has a shorter duration of action as well as a different safety profile from intravitreal dexamethasone or fluocinolone implant. (Level 1, A) (Fig. 4).
Fig. 4. Consensus Recommended Pathway for Management of Centre-involving DMO.
CFT central foveal thickness; DMO diabetic macular oedema; VEGF vascular endothelial growth factor.
Section 14: Treatment of PDR
The standard treatment for PDR has been PRP which was established over 40 years ago, thanks to the findings from the Diabetic Retinopathy Study (DRS) and the ETDRS. These were randomised clinical trials that compared the visual outcomes of patients treated with PRP with no treatment [358–362]. The DRS recruited patients whose eyes had PDR; they observed a 60% reduction in severe visual loss at two or more consecutive follow-up visits. Over a two year period, untreated eyes had a vision loss rate of 16.3%, whereas treated eyes only had a vision loss rate of 6.4% [358–361]. Patients with ‘non-PDR or PDR without high-risk characteristics’ were included in the ETDRS in order to determine at what stage PRP using an argon laser should be administered [361]. The results showed that overall, the 5-year risk of severe visual loss or vitrectomy was 2–6% in early PRP assigned eyes, compared with 4–10% in PRP deferral assigned eyes [362, 363]. Similarly, eyes with severe NPDR (ETDRS Level 53) benefited from PRP. This is particularly important in patients with severe NPDR where regular follow-ups may be problematic or inappropriate. PRP remains an effective treatment with effects lasting for several if properly applied [364, 365].
New laser technologies, and variations in treatment modalities from the original PRP techniques from the ETDRS have been reported, including diode, micropulse and pattern lasers [366]. A recent Cochrane review assessed the effects of these different (non-Argon) types of laser, and protocols (other than those established by the ETDRS), in PDR treatment [366]. The efficacy and adverse events of the different new laser systems/strategies compared with the standard treatment seem limited, although there is more data with the pattern laser systems [367–369]. The consensus is that the newer lasers are more comfortable to the patient, take less time to deliver the treatment, and have fewer adverse events—especially visual field changes [367–369]. In summary, the overall benefits and harms of different laser systems or strategies are similar compared with the standard treatment if applied judiciously.
Recently, the efficacy of anti-VEGF therapies including ranibizumab and aflibercept for the treatment of PDR have been evaluated in the DRCR.net Protocol S, and CLARITY studies [370, 371]. These confirmed that anti-VEGF therapies are efficacious in treating PDR. Protocol S confirmed that ranibizumab is an effective treatment alternative to PRP, and that there were no substantial safety concerns for at least 2 years. The 5-year Protocol S report showed that severe vision loss or serious PDR complications were uncommon, and similar between the ranibizumab and PRP groups [372]. However, ranibizumab-treated eyes had less frequent and less severe DMO [372]. This may be preferred initial treatment approach for some patients, for example, those who have both PDR and DMO [372]. Similarly, the CLARITY study was a non-inferiority trial that showed that PDR eyes treated with intravitreally administered aflibercept had improved VA (3.9 letters) at 1 year compared with PRP-treated eyes, and that aflibercept was not only non-inferior but superior to PRP [371]. Sameen et al. compared the effectiveness of PRP alone delivered with the PASCAL laser, with PASCAL PRP combined with IVT bevacizumab in a study of 76 eyes with PDR, on VA and CMT, finding that the combination therapy gave superior visual and anatomical outcomes compared with PRP alone in patients with combined PRP and DMO [373]. Where PDR exists in association with DMO, combination of IVT anti-VEGF with PRP is advisable [370–373].
However, further reports show that eyes with PDR treated exclusively with anti-VEGF therapy may experience marked disease progression with potentially devastating visual consequences if there are treatment interruptions – whether intentional or unintentional [17, 18, 374, 375]. Significant lapses in follow-up may be due to illness, financial hardship, failed appointments (capacity) or non-compliance, depending on the health system [374]. As such PRP remains the choice for treatment in PDR eyes.
In eyes with iris neovascularisation or neovascular glaucoma as part of proliferative diabetic eye disease, PRP provides longer-term control. Accordingly, where the cornea is clear, immediate PRP is recommended [376]. Where the cornea is opaque, or there is a significant elevation of the IOP, off-label treatment with intravitreal bevacizumab (Avastin) has been shown to lead to regression of iris neovascularisation within 3–5 days, and allows the application of PRP [377–382]. If IOP remains elevated, the expertise of a glaucoma specialist is required, who will likely consider drainage surgery with shunts or other glaucoma drainage devices, preferably at 1–2 weeks following the IVT bevacizumab [383, 384]. Repeat anti-VEGF injections may be required at completion of the glaucoma drainage surgery.
Recommendations
PRP remains the standard treatment for PDR. (Level 1)
Although the newer lasers are more comfortable, larger numbers of burns are required to control PDR as effectively as the originally described technology. Newer laser technologies require adequate treatment should be applied when new laser technologies (including, diode, micropulse, pattern lasers) are adopted. (Level 1)
Anti-VEGF therapies have been shown to be efficacious in treating PDR. (Level 1)
Eyes with severe NPDR may be treated with PRP. Similarly, the combination of PRP and intravitreal injections of anti-VEGF drugs has been shown to be effective in PDR. (Level 2) However, these anti-VEGF therapies are only useful if uninterrupted. (Level 2)
Section 15: Vitrectomy in the management of DR
The management of PDR and diabetic macular oedema has been transformed over the past few decades, as our enhanced understanding of the disease has progressed greatly, thanks, in part, to advances in retinal imaging, and the introduction intravitreally injected anti-VEGF agents [12]. Nevertheless there are still certain situations in which vitreoretinal surgeons have a role to play. This section provides a broad overview of vitrectomy in the management of diabetic eye disease.
The commonest indication for referring a patient with diabetic eye disease to a vitreoretinal surgeon is persistent or recurrent vitreous haemorrhage. The factors that determine the need and timing of surgery include the duration of haemorrhage, the amount of previous panretinal photocoagulation, the status of the fellow eye, and the patient’s ability to control their blood glucose levels [12, 385]. In general, patients have undergone adequate PRP, then it may be reasonable to observe with serial examinations using ultrasonography, if needs be. However, if the patient has not undergone adequate PRP, then early vitrectomy maybe beneficial.
The purpose of vitrectomy is to allow more rapid visual improvement than natural history, and to allow further PRP to be undertaken to reduce the risk of further haemorrhage. The improved visualisation will also allow a better evaluation of the macula. Further, if there is a pre-macular retro hyaloid haemorrhage, clearing this may reduce the likelihood of retinal toxicity.
Most of the time, modern micro-incisional vitrectomy surgery is performed while the patient is under local anaesthesia. The benefits of early visual rehabilitation with such a shorter procedure duration has meant that interventions are made earlier in the disease course than was made in the past, when larger gauge surgery was involved [386].
Vitrectomy is indicated to correct the macular anatomy due to complications of diabetic eye disease, for example in an eye with progressive tractional retinal detachment (TRD) involving the macula. Diabetic TRD can vary considerably in its progression, and it is important to exclude an ischaemic macula prior to embarking on a potentially challenging surgery, and a slowly progressing or a non-progressive macula sparing TRD may not require surgery. Patients may also have retinal breaks associated with the retinal traction, which require tractional and rhegmatogenous retinal detachment to be combined.
The surgery involves detachment of the posterior hyaloid from the retinal surface then careful relief of traction by means of desegmentation or actual removal of membranes from the retinal surface by means of delamination. The success of these procedures has improved significantly with micro-incision vitrectomy surgery and high-quality viewing systems.
Patients with DMO should always be evaluated for vitreo-macular interface (VMI) disease as this may negatively impact on their response to anti-VEGF drugs. If there is a taut posterior hyaloid face or an epiretinal membrane, then surgical intervention may allow a better response to intravitreal therapy [387–389].
Some studied have found that the use of anti-VEGF drugs pre- and intra-operatively results in less intra- and postoperative intraocular bleeding. However, they need to be used with caution because they are also associated with a potential risk of progression of fibrosed membranes and TRD. Similarly, the use of intraoperative steroids at the end of a diabetic vitrectomy procedure may allow a more favourable postoperative recovery. However, their use needs to be studied in more detail.
Section 16: Management of cataract in diabetes mellitus and diabetic retinopathy
Together, cataract and DR represent two of the top five leading causes of global blindness [390], and the global prevalence of both diseases continues to rise [391, 392]. Further, multiple population-based studies have identified a higher incidence and faster progression of cataract in patients with DM [393–398]. Increased glycated haemoglobin (HbA1c) levels have also been associated with a higher risk of cataract formation [393].
Cataract surgery in diabetic patients
In the UK, an analysis of 180,114 eyes from the Royal College of Ophthalmologists’ National Ophthalmology Database identified DR as the third most common (4.7%) ocular co-pathology for patients undergoing cataract surgery [399]. Outcomes after cataract surgery in diabetic patients are generally good [400, 401], but these patients’ visual outcomes can be less favourable than their non-diabetic counterparts, as has been shown by the UK Cataract National Dataset [402]. This observation is reinforced by the European Registry of Quality Outcomes for Cataract and Refractive Surgery analysis of 368,265 eyes, which reported that 28% of eyes with DR had worse VA after cataract surgery compared to 11.9% of those without ocular co-pathology [403].
There is a relative impairment of the blood-aqueous barrier in diabetic patients, with or without evidence of DR, which confers an increased risk of postoperative inflammation and MO after cataract surgery [404, 405]. Consequently, cataract surgery may accelerate the progression of pre-existing DR, induce rubeosis, or precipitate or initiate DMO [406–409]. Among those with DR, there is a positive correlation between DR severity and the degree of blood-aqueous barrier disruption [410] and poorer outcomes have been associated with operated eyes with active proliferative diabetic retinopathy (PDR) and/or pre-existing DMO [409, 411–413]. It is important to note that most of these prognostic studies looked at older techniques of cataract surgery (e.g. intra- or extracapsular cataract extraction).
Phacoemulsification of the crystalline lens is now the procedure of choice for routine cataract surgery [414, 415] and this approach is associated with less postoperative inflammation and more rapid visual rehabilitation when compared to intra- or extracapsular cataract surgery in diabetic eyes [416, 417]. Nonetheless, progression of pre-existing DR continues to be a significant issue after phacoemulsification surgery, with a reported progression rate of 21–32% for a follow-up period of 6–12 months [400, 401, 418–421].
Although DMO may be initiated and exacerbated by cataract surgery, assessing this can be difficult due to the fact that different clinical forms of MO can manifest after cataract surgery in diabetes, such as postoperative, inflammation-mediated cystoid MO (Irvine-Gass syndrome) which can occur alone or in combination with vasogenic DMO. Initiation and progression of pre-existing DMO have been reported in 29% of eyes with nonproliferative DR (NPDR) at a 6-month follow-up [145]. Krepler et al. reported that a similar proportion (31%) of eyes with NPDR developed CSMO after cataract surgery after 1-year of follow-up [422] Using OCT, Kim et al. reported a fairly similar incidence (22%) of postoperative MO (defined as an increase in centre point thickness on OCT >30% from preoperative baseline) after cataract surgery in diabetic patients [138]. A more recent evaluation of real-world data by the UK Diabetic Retinopathy Electronic Medical Record Users Group found that the rate of developing treatment-requiring DMO (defined as CMT of >400 μm on OCT) was 5.3% in the year after surgery [423].
Factors contributing to worsening visual outcomes
Pre-operative
Given that diabetes can affect every part of the eye [424], cataract surgery in diabetic patients poses a unique set of challenges to the operating surgeon. The most common ophthalmic complication of diabetes is DR. As highlighted above, there is a good body of evidence demonstrating a link between the presence of pre-existing DR and its subsequent progression after cataract surgery [401, 421]. DR can also be initiated by cataract surgery in diabetic patients who have no preoperative retinopathy [400, 425]. It is therefore prudent to counsel diabetic patients preoperatively regarding the risk of initiation or progression of DR.
DMO is the most common cause of vision loss among patients with diabetes [46]. There is a higher risk of developing DMO after cataract surgery compared with the normal population. This risk is further increased in the presence of pre-existing DR and rises proportionately with increasing DR severity [138, 423, 426]. A retrospective analysis of 81 984 eyes reported that diabetes, in the absence of DR, conferred a relative risk of 1.8 for postoperative CMO, and that this relative risk rose 6.23 in the presence of DR [426]. A further retrospective analysis of 4 850 eyes reported a sharp increase in treatment-requiring DMO after cataract surgery for all grades of DR, peaking in the 3 to 6 months’ postoperative period [423]. Pre-existing DMO also confers an increased risk of disease progression following cataract surgery [427] and is associated with worse visual outcomes 1 year after cataract surgery [412, 428].
Intra-operative
Corneal changes in diabetes include corneal hypaesthesia, increased epithelial fragility and impaired corneal wound healing [429]. Care should be taken to avoid corneal abrasion during or after surgery, as this may be slow to heal and result in recurrent corneal erosions.
Other anterior segment changes include poor pupil dilation from miosis secondary to diabetic neuropathy and accumulation of glycogen in the iris pigment epithelium [430]. Inadequate preoperative mydriasis may result in iris trauma and has been shown to double the risk of vitreous loss during cataract surgery [431]. Pupil enlargement can be facilitated via pharmacological (e.g. intracameral epinephrine) or mechanical strategies (e.g. iris hooks, pupil expansion devices or pupil-stretching techniques).
Increases in the duration and complexity of phacoemulsification surgery have been identified as important risk factors for DR progression and subsequent visual compromise [421]. Diabetic patients may also be more vulnerable to photic retinopathy, and intraoperative precautions such as reducing operating time with a senior surgeon should be considered to minimise this risk [432].
Posterior capsular opacification (PCO) is a common cause of decreased vision following cataract surgery. There is currently conflicting evidence regarding the link between diabetes and a higher incidence of PCO [433–436]. A large capsulorrhexis should be aimed for to prevent anterior capsular phimosis, which may hamper postoperative diagnosis and treatment of peripheral DR pathology [437–439].
Post-operative
Many of the postoperative risk factors overlap with those described above. A recent prospective Finnish population study by Ylinen et al. [440] further identified that younger patients and those with worse glycaemic control were at additional risk for postoperative CMO after routine cataract surgery.
Management of DMO in cataract surgery
Pre-operative
Focal laser photocoagulation represents an important treatment for nCI-DMO but its prophylactic role in prevention of DMO after cataract surgery remains questionable [441]. The EDTRS examined 270 eyes of 205 diabetic patients, of which about two-thirds received focal photocoagulation for DMO before cataract surgery. No significant difference was detected in the proportion of eyes with CSMO pre (29%) and post (31%) cataract surgery. In another prospective study looking at 154 eyes of diabetic patients undergoing small incision cataract surgery, 53% of these had worsening of CSMO despite preoperative macular laser therapy [442].
Recent real-world data have shown that good visual outcomes can be achieved with cataract surgery in diabetic eyes receiving intravitreal therapy (anti-VEGF and corticosteroid) for DMO [443]. However, an increased frequency of intravitreal therapy was reported in the 6 months before and after cataract surgery. In contrast to the well-recognised association of cataract progression following intravitreal corticosteroid administration particularly in the second year of treatment [320, 444–446], intravitreal anti-VEGF agents have been reported not to influence cataract progression in the short-term. Post-hoc analysis of cataract surgery outcomes from the Ranibizumab for Diabetic Macular Edema (RIDE and RISE) phase III trial data by Moshfeghi et al. revealed no difference in the frequency (11.9% vs. 14.0%) and timing of cataract surgery (average of 12 months from baseline) between ranibizumab- and sham-treated patients [447]. Similar findings have been reported by the phase III DMO trials of intravitreal aflibercept (VIVID and VISTA) which, over a 3-year period, found no increased incidence of cataract surgery among aflibercept-dosed patients compared to controls [347]. However, long-term outcomes of patients originally enroled in the BEVORDEX trial reported that over a 5-year period, cataract surgery will likely be required even in anti-VEGF treated eyes [448].
Intra-operative
Currently the standard of care, there has been a growing interest in the administration of intraoperative steroids and anti-VEGF agents as prophylactic treatment for DMO at the time of cataract surgery. The rationale for this is based on the observation of a positive correlation between aqueous inflammatory and angiogenic cytokine levels with postoperative macular thickness [449–451].
Triamcinolone acetonide is a potent corticosteroid that reduces the breakdown of the BRB and downregulates the production of prostaglandins and VEGF [452]. Subsequently, off-label intravitreal and subtenon administration of triamcinolone have been shown to reduce postoperative CMT and prevent the occurrence of CMO after cataract surgery in diabetic patients with pre-existing DR or DMO [453–458]. Its use may, however, be limited by the side effect of elevated IOP, with a reported incidence of between 12.5% and 23.5% following intravitreal administration [453, 456, 457].
A recent multi-centred randomised clinical trial (PREMED: PREvention of Macular Edema after Cataract Surgery), sponsored by the European Society of Cataract and Refractive Surgeons (ESCRS), compared the efficacy of different perioperative treatment strategies in reducing the risk of CMO after cataract surgery in diabetic patients [459]. This study, which involved 213 patients from 12 clinical sites across Europe, reported that diabetic patients receiving topical NSAIDs (bromfenac 0.09%) and a corticosteroid (dexamethasone 0.1%) combined with intraoperative subconjunctival injection of triamcinolone acetonide, had the lowest risk of postoperative macular thickening.
Compared to previous studies investigating the use of intravitreal triamcinolone acetonide, the ESCRS PREMED trial demonstrated that subconjunctival administration had a lower incidence of raised IOP (12.5–23.5% vs. 7.1% respectively) [459]. Additional benefits of subconjunctival route include its less invasive nature and better accessibility to surgical removal in case of a steroid response to normalise the IOP [460, 461]. Despite its convincing role in reducing postoperative cystoid MO, the ESCRS PREMED trial does not recommended the routine administration of subconjunctival triamcinolone acetonide in all diabetic patients given an increased incidence of elevated IOP (7.1%) in the context of a low overall incidence of postoperative CMO (4.5%) [459]. The decision to use this should be made on an individual basis after careful personalised risk and benefit assessment.
In addition to triamcinolone acetonide, there is also some evidence for the role of other intraocular steroid implants in diabetic patients undergoing cataract surgery. The efficacy of the intravitreal dexamethasone implant (Ozurdex, Allergan) in DMO has been demonstrated previously by the MEAD study [320]. Three studies have since investigated the intraoperative administration of Ozurdex in the context of phacoemulsification for patients with DMO. All of them found a significantly higher gain in BCVA and reduction in mean CMT in patients treated with Ozurdex at the time of cataract surgery [462–464]. The use of fluocinolone acetonide, another intravitreal steroid implant (Iluvien, Alimera Sciences), has also been reported to be ‘effective and well-tolerated in DMO patients undergoing cataract surgery’ by the Long-term Benefit of Sustained-Delivery Fluocinolone Acetonide Vitreous Inserts for Diabetic Macular Edema (FAME) phase III trial [465], although the longer duration of its action is also associated with a longer period of risk of raised IOP.
Intravitreal anti-VEGF agents also show promise in the perioperative management of DMO. Several authors have reported reduced rates of MO after cataract surgery over a follow-up period of 3 to 6 months with the use of intraoperative intravitreal anti-VEGF (bevacizumab or ranibizumab) in different diabetic populations with pre-existing NPDR and/or DMO [466–472]. Two other prospective, randomised studies have also concluded that off-label intravitreal bevacizumab significantly prevents further deterioration of pre-existing DMO, although there were no significant differences in mean CMT and BCVA between intervention and control groups [473, 474]. In contrast, the recent ESCRS PREMED trial reported no significant effect of intravitreal bevacizumab on postoperative CMT in diabetic patients mainly without pre-existing DR or DMO [459]. This disparity in effectiveness may reflect a difference in anti-VEGF drive between diabetic patients with and without ocular manifestation(s), with lower levels of anti-VEGF expected in the latter.
There are limited head-to-head trials comparing the different treatment modalities for DMO in the context of cataract surgery. The Diabetic Macular Edema at the time of Cataract Surgery Trial (DIMECAT) is a prospective, randomised clinical trial that has compared the effect of different adjunctive intravitreal treatment (bevacizumab versus triamcinolone acetonide) on DMO in the setting of cataract surgery [457, 475]. Both therapies resulted in improved BCVA at 6 months after cataract surgery, but only triamcinolone acetonide was associated with a sustained reduction of CMT. The incidence of elevated IOP in triamcinolone-treated patients increased, but this was associated with a reduced need for additional retreatment compared to bevacizumab-treated patients.
Post-operative
Pro-inflammatory prostaglandin (PG) expression has been reported to contribute to the development of postoperative MO after cataract surgery. Accumulation of PGs after cataract surgery leads to capillary leakage in the retinal tissue and subsequent macular oedema [476]. Nonsteroidal anti-inflammatory drugs (NSAIDs), which serve as PG antagonists, have been shown to reduce the incidence of CMO after cataract surgery [477] and preventative measures using them have been an area of considerable clinical success [478–482]. A meta-analysis involving seven randomised controlled studies concluded that a combination of topical NSAIDs and corticosteroids reduced the risk of postoperative CMO in diabetic patients, compared to those with topical corticosteroids alone [483]. This is further corroborated by two recent randomised, double-masked phase III trials involving 1220 patients with NPDR which reported a lower incidence of postoperative MO in eyes treated with topical NSAID compared to those without (4.1% vs. 15.9%) [484].
There is currently insufficient and inconsistent evidence connecting the use of PG analogues (a common first-line therapy for glaucoma) with an increased risk of CMO after cataract surgery [485–487]. There are also currently no published studies that have evaluated the association between PG analogues and pseudophakic CMO in a purely diabetic population. It is reasonable to continue with PG analogues with postoperative NSAID and steroid cover in diabetic patients.
Postoperative administration of intravitreal dexamethasone implant has been shown by the EPISODIC-2 study to confer benefit in patients with MO after cataract surgery [488]. This retrospective study, which included 100 eyes with pseudophakic CMO (Irvine-Gass syndrome), reported that significant gains in BCVA from baseline were maintained at 12 months after intravitreal dexamethasone implant with IOP rises being in general controlled with topical IOP-lowering therapy.
There remains a paucity of studies investigating the effect of combination treatment for DMO after cataract surgery. The ESCRS PREMED trial found no significant synergistic benefit of subconjunctival triamcinolone and intravitreal bevacizumab in the prevention of CMO after cataract surgery in diabetics [459].
Management of DR in cataract surgery
PRP is an effective treatment for PDR and the ETDRS has previously recommended that PRP should be performed before cataract surgery in patients with PDR [412]. Patients enroled in the ETDRS, however, underwent older techniques of cataract surgery (e.g. intra- and extracapsular cataract extraction) that were surgically more invasive and less relevant to the current standard of care. If there is no clear fundus view, then perioperative indirect panretinal laser photocoagulation is an option. Furthermore, PRP can be performed in the postoperative period once wounds have healed sufficiently.
The role of steroid therapy in the prevention of DR progression has largely been extrapolated from therapeutic trials in DMO. Previous DRCR.net trials have reported that intravitreal triamcinolone therapy resulted in slower progression from NPDR to PDR compared with macular laser treatment [489, 490]. Improvements in DR grading have also been observed with fluocinolone acetonide in the FAME trial [293]. The OZDRY trial has also reported improved DR grading with dexamethasone implants on DR progression in eyes treated for DMO.
Anti-VEGF agents may have a role in blunting DR progression following phacoemulsification. Cheema et al. found that patients treated with intravitreal bevacizumab during cataract surgery had significantly reduced DR progression at 6 months postoperatively compared to those without. In addition, a recent post-hoc analysis of the BEVORDEX trial showed a relatively low rate of new PDR events over 2 years in eyes that were treated with either intravitreal dexamethasone implant or bevacizumab [491]. Interestingly, there was a higher frequency of PDR events associated with the former, not reaching statistical significance, although the authors noted the original trial was not powered to study this specific relationship.
Compared to DMO, there remains a lack of evidence-based management of DR in the context of cataract surgery. Noted that the grading of DR and clinical endpoint of PDR in most of the above-mentioned studies was based on colour fundus photography (CFP). This is a surrogate endpoint for development of sight-threatening PDR; recent studies have shown a poor correlation between DR lesions on CFP and nonperfusion areas on fluorescein angiography (FA) [492, 493]. Future studies may benefit from employing multimodal imaging in DR grading of as well as adopting a more clinically relevant endpoint such as the development of new PDR events (e.g. vitreous haemorrhage). The introduction of newer imaging technologies such as swept-source widefield OCT angiography may allow detection of retinal neovascularisation and nonperfusion areas in DR in a less invasive manner than FA [493–496].
Discussion
There remain many unanswered questions concerning the optimal management of DMO and DR in the setting of cataract surgery. As is often the case when there is a plethora of treatment options, there is still no universally accepted single best treatment approach in such patients. Although the debate on the merits of each therapy continues, in the absence of high-level evidence, the presence of stable or treated DR and well-managed DMO need not be an indication for delaying cataract surgery when it is clinically indicated. Careful personalised risk assessment coupled with appropriately employed treatment and close monitoring after cataract surgery will afford diabetic patients the best possible visual and anatomic outcomes. These patients would not benefit from being placed in high volume cataract surgical pooled lists unless such considerations have been addressed in the clinic before surgical booking. Whereas cataract surgery can be considered a one-off intervention for many patients, in diabetics it needs to be considered in the context of a holistic management plan that also addresses their retinal and macular status.
RCTs remain the gold standard in evaluating the efficacy of different treatment modalities but may have limited clinical utility as they involve highly selected patient groups, take a long time to complete and are expensive. Structured registries that would allow individual centres to benchmark their performance against others and inform the evidence base in this area. Real-world studies that utilise large datasets from diverse patient populations hold promise in addressing this evidence gap and can complement data from RCTs.
Recommendations
Pre-operative
Where possible, manage DR and DMO before cataract surgery (Level 2)
Counselling that visual outcomes may not be as good as patients without DR (Level 2)
Peri-operative
Aim for a large capsulorrhexis (Level 2)
More likely to have to manage a small pupil (Level 2)
Senior surgeon to reduce surgery time (Level 2)
Not suitable for high volume cataract surgery lists (Level 2)
Consider topical NSAID in the peri-operative period for pre-existing DMO (Level 3)
Consider intravitreal anti-VEGF or steroid in the peri-operative period for pre-existing DMO (Level 2)
Consider anti-VEGF and/or PRP in the peri-operative period for eyes with or at high risk of PDR (Level 2)
Post-operative
Use of topical NSAID (Level 1)
Topical steroid drops may be required for longer than patients without DR (Level 2).
Section 17: Home monitoring as a useful extension of modern tele-ophthalmology
One of the pillars of modern medical care is patients self-measuring clinically relevant parameters in between consultations [497–499]. ‘Home measurement’ has repeatedly shown to improve the timely detection of disease worsening and enable prompt, targeted treatment [500–503] and can also lead to patients taking greater responsibility for the treatment of their disease and, presumably because of this commitment, showing a significantly higher adherence to treatment regimens [502]. Home assessment programmes may also reduce the number of emergency consultations and organisational inefficiencies in outpatient and inpatient care, although the evidence remains inconclusive [500].
Home measurements have been around for a long time and range from measuring body temperature during infection monitoring, to regular bodyweight measurements in heart failure or chronic kidney disease. More recently, studies of BP measurement for hypertension [497], peak-flow measurement for chronic obstructive pulmonary disease (COPD) [502], asthma [501] and glucose measurement for diabetes [503] have all shown the benefits of a home monitoring approach. Using simple therapy schemes, patients can make treatment adjustments on their own based on the measurements they take.
In the field of ophthalmology, the Amsler grid (from 1947) is one of the most widely used home monitoring instruments [504]. It identifies metamorphopsia, perceived distortions of visual stimuli that principally arise from a mechanically distorted retina. Such distortions are typical in advanced stages of AMD due to the presence of drusen, retinal pigment epithelial detachment, IRF, and SRF, or disorganisation of the inner and middle retinal microstructure in ERM; similarly, IRF, SRF, and disorganisation of the inner retina lead to metamorphopsia and reduced vision in DMO [505–507]. In these diseases, management is often guided by data obtained from sporadic outpatient visits. The dynamic fluctuations in chronic eye diseases contain valuable data that we cannot capture. Home monitoring with appropriate data (including combination of metamorphopsia, home OCT, and VA [see below]) by the patient themselves, generating additional measurements offers not only a novel quality of clinical data, but may also reduce patients’ need for physician visits, as well as enabling the collection of high-quality, structured data in an intramural environment for personalised and targeted management.
Recently, several digital home AMD measurement tests have come onto the market [508]. In recent years, preferential hyperacuity perimetry (PHP, Notal Vision Inc.) has established itself in patient self-testing for AMD [509]. A clinical study showed a lower reduction in VA compared to standard care using the PHP test [510]. The test runs on a standalone device connected to the company’s data centre via a wireless internet connection. myVisiontrack® and Alleye are the only two FDA-approved medical software applications that run on mobile devices. myVisiontrack® uses a shape discrimination task where respondents need to identify changes in the shape of circles; the app was able to accurately detect advanced stages of AMD [511]. The Alleye test has several similarities with myVisiontrack, but examines a much larger macular area [512, 513].
The implementation of home measurement programmes alone can have a positive effect on medical care. Ideally, home measurement systems are linked to the digitised processes of further care. As digitalisation progresses, completely new integrated care systems can emerge which are characterised by significantly higher efficiency than current best-practice [498]. Many experts believe that these new approaches can meet the challenges posed by demographic changes and the associated increase in the number of patients. It is estimated that the proportion of people over 60 will double by 2050 and that one in five will be 60 or older. This is crucial, as demand for ophthalmic services already exceeds supply [514].
Although the number of ophthalmologists being trained and entering clinical practice is increasing, the UK has one of the lowest ratios of ophthalmologists per capita in the developed world, and their number is growing only half as fast as the population over 60 years of age grows [515]. The challenge is to maintain timely and high quality care as resources become scarcer. The impact of this imbalance between supply and demand is illustrated by the recently published figures of 20 patients per month facing severe vision loss and waiting for access to ophthalmic services [516].
Over the past two decades, therefore, an important foundation has been laid for the efficient implementation of integrated digital ophthalmic care [517, 518]. Currently, the ‘store-and-forward’ model of teleophthalmology is now being used, where images are taken at a different time and place than are then assessed by a trained grader, and this is best illustrated by the UK National DESP [275]. DESP has improved access for patients, with 76% of patients eligible for screening receiving an annual retinal image. At the same time, at-risk populations of DR will be screened, in order to be identified for early treatment in hospital-based services. As a direct result of this programme, in 2009–2010, DR was no longer the leading cause of blindness in England and Wales—for the first time in five decades [74]. On the other hand, the introduction of the screening programme led to a large increase in referrals for further investigations, most of which did not reveal any abnormal clinical findings—something that placed an additional burden on the institutions due to the increased number of patients.
The analysis of these mechanisms led to the concept of the ‘virtual clinic’, which aims to provide additional capacity for unmet needs within the NHS [290]. Initial studies have shown that patients do not need personal interaction with a doctor every time they stay in hospital, and that a secure, efficient service can be offered virtually. Virtual clinics have been tested in several subspecialties, including medical retina, glaucoma and emergency ophthalmology, with a number of programmes developed over the last two decades. While it is clear that individual programmes have been successful, none has yet been scaled to a national level.
Despite several barriers to more widespread adoption of these practices being in place today, the future of teleophthalmology seems bright. The expansion of telemedical services is the logical response to current and future supply bottlenecks in ophthalmology. Further development will likely take place in stages and will include data-specific, technological and political steps.
From the point of view of data integration, the inclusion and provision of all care-relevant data is crucial. The inclusion of data from patient home measurements is a decisive and essential component, because the density of relevant data increases significantly and allows additional insights into disease progression, which are generally missing in classical data surveys. The inclusion of this extra data should enhance both efficiency and clinical outcomes. The increased involvement of patients and their ability to participate in the treatment of their own disease is therefore enhanced by the improvement of the downstream telemedical care paths.
An integrated telemedical software solution is necessary for a comprehensive introduction because several parallel software programmes lead either to data silos or interface problems. The technology must enable the collection of structured clinical information and the bi-directional flow of this information between patient, community and hospital. If these infrastructure requirements are met, automated classification systems such as AI algorithms can be used efficiently.
From a political point of view, a binding commitment is necessary. However, this seems to be the case since a recently published 10-year long-term plan of the NHS sets exactly these priorities. The plan aims at transforming services and overcoming the imbalance between supply and demand in health care. Digital technologies are described as a critical part to achieve this goal and to make care in the community as secure and possible as possible. An integrated telemedicine with patient home measurements maps these goals in an optimal way could form part of the future of monitoring disease activity in DR and help guide patients’ treatment.
Acknowledgements
This document, and its development was independent. None of the authors have received any funding for contributing to the development of this manuscript. The Group have retained final control of all the content and editorial decisions. KK acknowledges support from the National Institute for Health Research Applied Research Collaboration—East Midlands (NIHR ARC—EM) and the National Institute of Health Research (NIHR) Leicester Biomedical Research Centre. This study was supported by Allergan via an independent and unrestricted research grant. Allergan had the opportunity to review the final version of the manuscript to address any factual inaccuracies or request the redaction of information deemed to be proprietary or confidential and ensure that study support was disclosed.
Funding
Editorial assistance was provided to the authors by Dr Mark Hillen, BSc, PhD, through an unrestricted grant funding by Allergan International plc, Dublin, Ireland. Publication costs were also provided through an unrestricted education grant from Allergan International at the request of the lead author. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship and authors retained full control over the manuscript.
Appendix A
FDA Category B drugs
Animal reproduction studies have failed to demonstrate a risk to the foetus and there are no adequate and well-controlled studies in pregnant women.
FDA Category C drugs
Animal reproduction studies have shown an adverse effect on the foetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant the use of the drug in pregnant women despite potential risks.
FDA Category D drugs
There is positive evidence of human foetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.
Appendix B
Retinal assessment during pregnancy NICE 2015 [246]
1.3.24
Offer pregnant women with pre‑existing diabetes retinal assessment by digital imaging under tropicamide mydriasis following their first antenatal clinic appointment (unless they have had a retinal assessment in the last 3 months), and again at 28 weeks. If any diabetic retinopathy is present at booking, perform an additional retinal assessment at 16–20 weeks [2008, amended 2015].
1.3.25
Diabetic retinopathy should not be considered a contraindication to rapid optimisation of blood glucose control in women who present with a high HbA1C in early pregnancy [2008].
1.3.26
Ensure that women who have pre-proliferative diabetic retinopathy or any form of referable retinopathy diagnosed during pregnancy have ophthalmological follow‑up for at least 6 months after the birth of the baby [2008, amended 2015]
1.3.27
DR should not be considered a contraindication to vaginal birth [2008].
Author contributions
Abstract and Executive Summary—WA. Section 1. Scope—WA. Section 2. The Epidemiology of Diabetic Retinopathy-Related Vision Loss in Diabetes—RH. Section 3. Public Health and Commissioning of Diabetic Eye Services—RS. Section 4. Pathophysiology of Diabetic Ocular Disease—WA. Section 5. The Classification of Diabetic Retinopathy—DV, CB. Section 6. Systemic Risk Management of People with Diabetes and Effects on Retinopathy—KK. Section 7. Diabetic Retinopathy in Children and Young Adults—FQ. Section 8. Diabetic Retinopathy and Pregnancy—EP, SR. Section 9. Diabetic Retinopathy Screening—Sanjiv Banerjee, FG. Section 10. Interface between Screening and Hospital Eye Service—Sanjiv Banerjee, FG. Section 11. Virtual clinics and Artificial Intelligence in Diabetic Macular Oedema—LD. Section 12. The Management of Diabetic Macular Oedema—DV, CB. Section 13. Response to Diabetic Macular Oedema therapies—WA, RG. Section 14. Treatment of Proliferative Diabetic Retinopathy—WA. Section 15. Vitrectomy in the management of Diabetic Retinopathy—Somnath Banerjee. Section 16. Management of Cataract in Diabetes Mellitus and Diabetic Retinopathy—Peng Y Sim, DAS, HM. Section 17. Home monitoring as a useful extension of modern tele-ophthalmology—Livia Faes, Lucas M Bachmann, DAS.
Compliance with ethical standards
Conflict of interest
WMA has received honoraria for advisory board memberships from AbbVie, Alcon, Alimera, Allergan, Bayer, Bausch and Lomb, Novartis and Pfizer, speaker Fees from Alimera, Allergan, Bayer, Novartis and Pfizer, and Educational travel grants from Alimera, Allergan, Bayer, Novartis and Pfizer. He has undertaken clinical research sponsored by Allergan, Bayer, Gyroscope, and Novartis. His institution has received research funding from Allergan, Bayer, Boehringer Ingelheim, CenterVue, Novartis, and Optos plc. CB attended advisory boards of, and received lecture fees from Novartis, Bayer, Roche, Allergan, Alimera Sciences. Her employer Bristol Eye Hospital has received research funding from Boehringer Ingelheim, Roche, Bayer, Novartis, and Allergan. Sanjiv Banerjee has received speaker fees and travel grants from Novartis, and honoraria for advisory board memberships and travel grants from Allergan. Somnath Banerjee has received honoraria for Advisory Boards from Bayer and Alimera, and Educational Travel Grants from Allergan, Alimera, and DORC. LD has received honoraria for advisory board memberships from Alimera, Allergan, Bayer, Novartis and Thrombogenics, speaker fees from Alimera, Bausch and Lomb, Bayer and Novartis and educational travel grants from Allergan, Bayer and Novartis. Her institution has undertaken clinical research sponsored by Alimera, Allergan, Bayer, Novartis and Roche. RG has received travel grants from Allergan, Bayer and Novartis and research grants from Novartis and Bayer. He has received honoraria for advisory board memberships from Bayer, Novartis, Allergan, Alimera, and Roche. FG has received honorarium for consultancy-advisory boards from Alimera, Allergan, Bayer, Novartis, Oxford BioElectronics, Roche; educational travel grants from Allergan, Bayer, Novartis and departmental research grants from Allergan, Bayer, Boehringer Ingelheim, Chengdu Pharma, Novartis, PanOptica. RH has received educational travel awards from Allergan, Bayer, and Novartis. He has served on advisory boards of Allergan, Bayer, Novartis, and Roche. His institution has received research funding from Allergan, Novartis, Roche, Bayer, Thea, and Thrombogenics. KK has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Berlin-Chemie AG/Menarini Group, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi, Takeda, Servier and Pfizer, research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Pfizer. HM has received research grants, educational travel grants and honoraria for advisory board memberships from Allergan, Bayer, Novartis and Roche. EP has received honoraria for advisory board memberships from Alimera, Allergan, Educational sponsorships from Alimera, Allergan, and Bayer, and speaker fees from Allergan, Novartis. Her institution has received research funding from Bayer. FQ has received honoraria for advisory board memberships and speaker fees from Alimera Sciences, Allergan, Heidelberg Engineering, SIFI, Meagate and Novartis. He has received educational travel grants from Alimera Sciences, Allergan, Heidelberg Engineering, SIFI, Meagate and Novartis. SR has no declarations. RS has received educational travel grants and speaker fees from Allergan, Bayer, and Novartis. DS has received speaker fees and travel grants from Allergan, Bayer, Novartis, and Roche, and honoraria for advisory board memberships from Allergan and Big Picture Medical UK. DV has received honoraria for advisory board meetings and speaker fees from Allergan, Bayer and Novartis; travel sponsorships from Alimera, Allergan, Bayer and Novartis. Her institution has undertaken clinical research sponsored by Allergan, Alimera, Bayer, Roche, Boehringer Ingelheim, Chengdu Kanghong Biotechnology Co Ltd and Novartis.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic Macular Edema: Pathogenesis and Treatment. Survey of Ophthalmology. 2009;54:1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Abbate M, Cravedi P, Iliev I, Remuzzi G, Ruggenenti P. Prevention and Treatment of Diabetic Retinopathy: Evidence from Clinical Trials and Perspectives. Current Diabetes Reviews. 2011;7:190–200. doi: 10.2174/157339911795843168. [DOI] [PubMed] [Google Scholar]
- 5.Heng LZ, Comyn O, Peto T, Tadros C, Ng E, Sivaprasad S, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetic Medicine. 2013;30:640–50. doi: 10.1111/dme.12089. [DOI] [PubMed] [Google Scholar]
- 6.Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic Retinopathy. Primary Care: Clinics in Office Practice. 2015;42:451–64. doi: 10.1016/j.pop.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Colucciello M. Current intravitreal pharmacologic therapies for diabetic macular edema. Postgraduate Medicine. 2015;127:640–53. doi: 10.1080/00325481.2015.1052523. [DOI] [PubMed] [Google Scholar]
- 8.Gangwani RA, Lian JX, McGhee SM, Wong D, Li KK. Diabetic retinopathy screening: global and local perspective. Hong Kong Med J. 2016;22:486–95. doi: 10.12809/hkmj164844. [DOI] [PubMed] [Google Scholar]
- 9.Semeraro F, Morescalchi F, Cancarini A, Russo A, Rezzola S, Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019;45:517–27. doi: 10.1016/j.diabet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Doft BH, Kingsley LA, Orchard TJ, Kuller L, Drash A, Becker D. The Association Between Long-term Diabetic Control and Early Retinopathy. Ophthalmology. 1984;91:763–9. doi: 10.1016/s0161-6420(84)34235-x. [DOI] [PubMed] [Google Scholar]
- 11.Kollias AN, Ulbig MW. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107:75–83. doi: 10.3238/arztebl.2010.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ophthalmologists RCo. Diabetic Retinopathy Guidelines, December 2012. Available at: https://www.rcophth.ac.uk/wp-content/uploads/2017/03/Virtual-Glaucoma-Clinics.pdf.
- 13.Thomas RL, Luzio SD, North RV, Banerjee S, Zekite A, Bunce C, et al. Retrospective analysis of newly recorded certifications of visual impairment due to diabetic retinopathy in Wales during 2007-2015. BMJ Open. 2017;7:e015024. doi: 10.1136/bmjopen-2016-015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence. Ranibizumab for treating diabetic macular oedema. Technology appraisal guidance [TA274]. 2013. https://www.nice.org.uk/guidance/ta274.
- 15.National Institute for Health and Care Excellence. Dexamethasone intravitreal implant for treating diabetic macular oedema. Technology appraisal guidance [TA349]. 2015. https://www.nice.org.uk/guidance/ta349.
- 16.National Institute for Health and Care Excellence. Aflibercept for treating diabetic macular oedema. Technology appraisal guidance [TA346]. 2015. https://www.nice.org.uk/guidance/ta346.
- 17.Scottish Medicines Consortium. Aflibercept 40mg/mL solution for intravitreal injection (Eylea®) SMC No. (857/13). 2013. https://www.scottishmedicines.org.uk.
- 18.Scottish Medicines Consortium. Ranibizumab, 10mg/mL solution for injection (Lucentis®) SMC No. (711/11). 2012. https://www.scottishmedicines.org.uk/media/2214/ranibizumab_lucentis_resubmission_final_november_2012_for_website.pdf.
- 19.Scottish Medicines Consortium. Dexamethasone 700 micrograms intravitreal implant in applicator (Ozurdex®) SMC No. (1046/15). 2015. https://www.scottishmedicines.org.uk/media/1558/dexamethasone_ozurdex_final_april_2015_updated_060515_for_website.pdf.
- 20.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237:185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 21.Kodjikian L, Bellocq D, Bandello F, Loewenstein A, Chakravarthy U, Koh A, et al. First-line treatment algorithm and guidelines in center-involving diabetic macular edema. Eur J Ophthalmol. 2019;29:573–84. doi: 10.1177/1120672119857511. [DOI] [PubMed] [Google Scholar]
- 22.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Diabetes Federation. IDF Atlas, 3rd Edition. 2006. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/22-atlas-3rdedition.html.
- 24.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 25.International Diabetes Federation. IDF Atlas, 9th Edition. 2019. https://www.diabetesatlas.org/en/resources/.
- 26.Makaroff LE, Cavan D. Which biochemical assay is best for measuring diabetes prevalence? Lancet Diabetes Endocrinol. 2015;3:582–3. doi: 10.1016/S2213-8587(15)00203-X. [DOI] [PubMed] [Google Scholar]
- 27.Information Services Division Scotland. 2016. Quality & Outcomes Framework (QOF). https://www.isdscotland.org/Health-Topics/General-Practice/Quality-And-Outcomes-Framework/.
- 28.Welsh Government. General medical services contract: Quality and outcomes framework. http://gov.wales/statistics-and-research/general-medicalservices-contract/?tab=previous&skip=1&lang=en.
- 29.NHS Digital. Quality and Outcomes Framework (QOF). 2014. http://www.hscic.gov.uk/catalogue/PUB18887.
- 30.Diabetes UK. Diabetes Prevalence (November 2016). 2016. https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetesprevalence-2016.
- 31.Public Health England. Technical document for the diabetes prevalence model for England. 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/612307/Diabetesprevalencemodeltechnicaldocument.pdf.
- 32.Holman N, Forouhi NG, Goyder E, Wild SH. The Association of Public Health Observatories (APHO) Diabetes Prevalence Model: estimates of total diabetes prevalence for England, 2010-2030. Diabet Med. 2011;28:575–82. doi: 10.1111/j.1464-5491.2010.03216.x. [DOI] [PubMed] [Google Scholar]
- 33.Diabetes UK. Facts and Figures. 2020. https://www.diabetes.org.uk/Professionals/Position-statements-reports/Statistics.
- 34.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet (London, England) 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 35.Huang OS, Tay WT, Ong PG, Sabanayagam C, Cheng CY, Tan GS, et al. Prevalence and determinants of undiagnosed diabetic retinopathy and vision-threatening retinopathy in a multiethnic Asian cohort: the Singapore Epidemiology of Eye Diseases (SEED) study. Br J Ophthalmol. 2015;99:1614–21. doi: 10.1136/bjophthalmol-2014-306492. [DOI] [PubMed] [Google Scholar]
- 36.Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond) 2004;18:963–83. doi: 10.1038/sj.eye.6701476. [DOI] [PubMed] [Google Scholar]
- 37.Raman R, Ganesan S, Pal SS, Gella L, Kulothungan V, Sharma T. Incidence and Progression of Diabetic Retinopathy in Urban India: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS II), Report 1. Ophthalmic Epidemiol. 2017;24:294–302. doi: 10.1080/09286586.2017.1290257. [DOI] [PubMed] [Google Scholar]
- 38.Melo LGN, Morales PH, Drummond KRG, Santos DC, Pizarro MH, Barros BSV, et al. Current epidemiology of diabetic retinopathy in patients with type 1 diabetes: a national multicenter study in Brazil. BMC Public Health. 2018;18:989. doi: 10.1186/s12889-018-5859-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 40.Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115:1869–75. doi: 10.1016/j.ophtha.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy. The Beijing Eye Study 2006. Graefes Arch Clin Exp Ophthalmol. 2008;246:1519–26. doi: 10.1007/s00417-008-0884-6. [DOI] [PubMed] [Google Scholar]
- 42.Raymond NT, Varadhan L, Reynold DR, Bush K, Sankaranarayanan S, Bellary S, et al. Higher prevalence of retinopathy in diabetic patients of South Asian ethnicity compared with white Europeans in the community: a cross-sectional study. Diabetes Care. 2009;32:410–5. doi: 10.2337/dc08-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tapp RJ, Shaw JE, Harper CA, de Courten MP, Balkau B, McCarty DJ, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26:1731–7. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang FH, Liang YB, Zhang F, Wang JJ, Wei WB, Tao QS, et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology. 2009;116:461–7. doi: 10.1016/j.ophtha.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care. 2012;35:556 LP–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein R The epidemiology of diabetic retinopathy. In: Duh E editor. Diabetic retinopathy. Humana Press: Totowa, NJ; 2008. pp 67–108.
- 48.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859–68. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones CD, Greenwood RH, Misra A, Bachmann MO. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care. 2012;35:592–6. doi: 10.2337/dc11-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Early Worsening of Diabetic Retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874. [DOI] [PubMed]
- 52.Diabetes C, Complications Trial Research G, Nathan DM, Genuth S, Lachin J, Cleary P, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 53.The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus The Diabetes Control and Complications Trial. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 54.Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116:297–303. doi: 10.1001/archopht.116.3.297. [DOI] [PubMed] [Google Scholar]
- 55.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–53. [PubMed]
- 56.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England) 1998;352:854–65. [PubMed] [Google Scholar]
- 57.Group UPDS Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 58.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–63. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 59.Wong TY, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. 2009;32:2307–13. doi: 10.2337/dc09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM, Group UKPDS Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631–40. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 61.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(Suppl 2):B54–64. [PubMed] [Google Scholar]
- 62.Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet (London, England) 2008;372:1394–402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 63.Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet (London, England) 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- 64.Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet (London, England) 2008;372:1385–93. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 65.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beulens JW, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–36. doi: 10.1007/s00125-009-1457-x. [DOI] [PubMed] [Google Scholar]
- 67.Morgan CL, Owens DR, Aubonnet P, Carr ES, Jenkins-Jones S, Poole CD, et al. Primary prevention of diabetic retinopathy with fibrates: a retrospective, matched cohort study. BMJ Open. 2013;3:e004025. doi: 10.1136/bmjopen-2013-004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet (London, England) 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 69.Group AS, Group AES, Chew EY, Ambrosius WT, Davis MD, Danis RP, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gale R, Scanlon PH, Evans M, Ghanchi F, Yang Y, Silvestri G, et al. Action on diabetic macular oedema: achieving optimal patient management in treating visual impairment due to diabetic eye disease. Eye (Lond) 2017;31(S1):S1–S20. doi: 10.1038/eye.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Public Health England. Diabetic eye screening programme overview. 2017. https://www.gov.uk/guidance/diabetic-eye-screening-programme-overview.
- 72.Royal College of Opthalmologists. Focus: the NHS diabetic eye screening programme: new common pathway. 2012. https://www.rcophth.ac.uk/wpcontent/uploads/2014/08/Focus-Winter-2012.pdf.
- 73.Department of Health. National service framework for diabetes: delivery strategy. 2001. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/198836/National_Service_Framework_for_Diabetes.pdf.
- 74.Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4:e004015. doi: 10.1136/bmjopen-2013-004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.NHS Digital. Hospital Outpatient Activity 2018-2019. 2019. https://files.digital.nhs.uk/33/EF9007/hosp-epis-stat-outp-summ-rep-2018-19-rep.pdf.
- 76.Chang P-Y, Carrel H, Huang J-S, Wang I-J, Hou Y-C, Chen W-L, et al. Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. Am J Ophthalmol. 2006;142:488–90. doi: 10.1016/j.ajo.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 77.Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–8. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 78.Midena E, Brugin E, Ghirlando A, Sommavilla M, Avogaro A. Corneal Diabetic Neuropathy: A Confocal Microscopy Study. Journal of Refractive Surgery. 2006;22:S1047–S1052. doi: 10.3928/1081-597X-20061102-08. [DOI] [PubMed] [Google Scholar]
- 79.Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25:769–73. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 80.Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp. 2011;2194. [DOI] [PMC free article] [PubMed]
- 81.Tavakoli M, Petropoulos IN, Malik RA. Assessing corneal nerve structure and function in diabetic neuropathy. Clin Exp Optom. 2012;95:338–47. doi: 10.1111/j.1444-0938.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhivov A, Winter K, Hovakimyan M, Peschel S, Harder V, Schober HC, et al. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS One. 2013;8:e52157. doi: 10.1371/journal.pone.0052157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–32. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 84.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107:237–43. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- 85.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England) 1998;352:837–53. [PubMed] [Google Scholar]
- 86.Klein R, Marino EK, Kuller LH, Polak JF, Tracy RP, Gottdiener JS, et al. The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:84–90. doi: 10.1136/bjo.86.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhavsar AR. Diabetic retinopathy: the latest in current management. Retina. 2006;26(Supplement):S71–S79. doi: 10.1097/01.iae.0000236466.23640.c9. [DOI] [PubMed] [Google Scholar]
- 88.Bhavsar AR, Tornambe PE. 25 Years of Progress in the Treatment of Retinal Diseases: Where We Have Been, Where We Are Now, and Where We Will Be. Retina. 2006;26(Suppl):S1–S6. doi: 10.1097/01.iae.0000236450.70275.6d. [DOI] [PubMed] [Google Scholar]
- 89.Han J, Lando L, Skowronska-Krawczyk D, Chao DL. Genetics of Diabetic Retinopathy. Current Diabetes Reports. 2019;19:67. doi: 10.1007/s11892-019-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132:96–107. doi: 10.1001/jamaophthalmol.2013.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Simo R, Hernandez C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25:23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Simo R, Hernandez C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res. 2015;48:160–80. doi: 10.1016/j.preteyeres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment: Neuroretinal adaptation in diabetic retinopathy. Annals of the New York Academy of Sciences. 2014;1311:174–90. doi: 10.1111/nyas.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jindal V. Neurodegeneration as a primary change and role of neuroprotection in diabetic retinopathy. Mol Neurobiol. 2015;51:878–84. doi: 10.1007/s12035-014-8732-7. [DOI] [PubMed] [Google Scholar]
- 96.Barber AJ. Diabetic retinopathy: recent advances towards understanding neurodegeneration and vision loss. Sci China Life Sci. 2015;58:541–9. doi: 10.1007/s11427-015-4856-x. [DOI] [PubMed] [Google Scholar]
- 97.van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–5. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park HY, Kim IT, Park CK. Early diabetic changes in the nerve fibre layer at the macula detected by spectral domain optical coherence tomography. Br J Ophthalmol. 2011;95:1223–8. doi: 10.1136/bjo.2010.191841. [DOI] [PubMed] [Google Scholar]
- 99.Verma A, Raman R, Vaitheeswaran K, Pal SS, Laxmi G, Gupta M, et al. Does neuronal damage precede vascular damage in subjects with type 2 diabetes mellitus and having no clinical diabetic retinopathy? Ophthalmic Res. 2012;47:202–7. doi: 10.1159/000333220. [DOI] [PubMed] [Google Scholar]
- 100.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J Diabetes Res. 2013;2013:905058. doi: 10.1155/2013/905058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113:E2655–2664. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U, et al. Functional and Structural Findings of Neurodegeneration in Early Stages of Diabetic Retinopathy: Cross-sectional Analyses of Baseline Data of the EUROCONDOR Project. Diabetes. 2017;66:2503–10. doi: 10.2337/db16-1453. [DOI] [PubMed] [Google Scholar]
- 103.Chhablani J, Sharma A, Goud A, Peguda HK, Rao HL, Begum VU, et al. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Investigative Opthalmology & Visual Science. 2015;56:6333. doi: 10.1167/iovs.15-17334. [DOI] [PubMed] [Google Scholar]
- 104.Hidayat AA, Fine BS. Diabetic Choroidopathy. Ophthalmology. 1985;92:512–22. [PubMed] [Google Scholar]
- 105.Bischoff PM, Flower RW. Ten years experience with choroidal angiography using indocyanine green dye: a new routine examination or an epilogue? Documenta Ophthalmologica. 1985;60:235–91. doi: 10.1007/BF00157827. [DOI] [PubMed] [Google Scholar]
- 106.Fryczkowski AW, Hodes BL, Walker J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. International Ophthalmology. 1989;13:269–79. doi: 10.1007/BF02280087. [DOI] [PubMed] [Google Scholar]
- 107.Ishibashi T, Murata T, Kohno T, Ohnishi Y, Inomata H. Peripheral Choriovitreal Neovascularization in Proliferative Diabetic Retinopathy: Histopathologic and Ultrastructural Study. Ophthalmologica. 1999;213:154–8. doi: 10.1159/000027411. [DOI] [PubMed] [Google Scholar]
- 108.Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32:1781–90. doi: 10.1097/IAE.0b013e31825db73d. [DOI] [PubMed] [Google Scholar]
- 109.Yazgan S, Arpaci D, Celik HU, Dogan M, Isik I. Macular Choroidal Thickness May Be the Earliest Determiner to Detect the Onset of Diabetic Retinopathy in Patients with Prediabetes: A Prospective and Comparative Study. Curr Eye Res. 2017;42:1039–47. doi: 10.1080/02713683.2016.1264606. [DOI] [PubMed] [Google Scholar]
- 110.Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378–84. doi: 10.1167/iovs.12-11503. [DOI] [PubMed] [Google Scholar]
- 111.Gupta C, Tan R, Mishra C, Khandelwal N, Raman R, Kim R, et al. Choroidal structural analysis in eyes with diabetic retinopathy and diabetic macular edema—A novel OCT based imaging biomarker. PLoS One. 2018;13:e0207435. doi: 10.1371/journal.pone.0207435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang JC, Lains I, Providencia J, Armstrong GW, Santos AR, Gil P, et al. Diabetic Choroidopathy: Choroidal Vascular Density and Volume in Diabetic Retinopathy With Swept-Source Optical Coherence Tomography. Am J Ophthalmol. 2017;184:75–83. doi: 10.1016/j.ajo.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 113.Rewbury R, Want A, Varughese R, Chong V. Subfoveal choroidal thickness in patients with diabetic retinopathy and diabetic macular oedema. Eye (Lond) 2016;30:1568–72. doi: 10.1038/eye.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adhi M, Brewer E, Waheed NK, Duker JS. Analysis of Morphological Features and Vascular Layers of Choroid in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography. JAMA Ophthalmology. 2013;131:1267. doi: 10.1001/jamaophthalmol.2013.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 116.Lorenzi M, Cagliero E, Toledo S. Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes. 1985;34:621–7. doi: 10.2337/diab.34.7.621. [DOI] [PubMed] [Google Scholar]
- 117.Lorenzi M, Montisano DF, Toledo S, Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986;77:322–5. doi: 10.1172/JCI112295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Engerman R, Finkelstein D, Aguirre G, Diddie KR, Fox RR, Frank RN, et al. Ocular Complications. Diabetes. 1982;31(Suppl 1):82–88. doi: 10.2337/diab.31.1.s82. [DOI] [PubMed] [Google Scholar]
- 119.Engerman RL. Animal models of diabetic retinopathy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81(4 Pt 1):Op710–715. [PubMed] [Google Scholar]
- 120.Johnson MW. Etiology and treatment of macular edema. Am J Ophthalmol. 2009;147:11–21.e11. doi: 10.1016/j.ajo.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 121.Calles-Escandon J, Cipolla M. Diabetes and Endothelial Dysfunction: A Clinical Perspective. Endocrine Reviews. 2001;22:36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 122.Ciulla TA, Amador AG, Zinman B. Diabetic Retinopathy and Diabetic Macular Edema: Pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653–64. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 123.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye. 2009;23:1496–508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- 124.Amoaku WM, Saker S, Stewart EA. A review of therapies for diabetic macular oedema and rationale for combination therapy. Eye (Lond) 2015;29:1115–30. doi: 10.1038/eye.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brownlee M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 126.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, et al. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905–15. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Booth G, Stalker TJ, Lefer AM, Scalia R. Mechanisms of Amelioration of Glucose-Induced Endothelial Dysfunction Following Inhibition of Protein Kinase C In Vivo. Diabetes. 2002;51:1556–64. doi: 10.2337/diabetes.51.5.1556. [DOI] [PubMed] [Google Scholar]
- 128.Omi H, Okayama N, Shimizu M, Okouchi M, Ito S, Fukutomi T, et al. Participation of high glucose concentrations in neutrophil adhesion and surface expression of adhesion molecules on cultured human endothelial cells: effect of antidiabetic medicines. J Diabetes Complications. 2002;16:201–8. doi: 10.1016/s1056-8727(01)00163-5. [DOI] [PubMed] [Google Scholar]
- 129.Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376:517–9. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 130.Miyamoto K, Ogura Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin Ophthalmol. 1999;14:233–9. doi: 10.3109/08820539909069542. [DOI] [PubMed] [Google Scholar]
- 131.Chibber R, Ben-Mahmud B, Chibber S, Kohner E. Leukocytes in Diabetic Retinopathy. Current Diabetes Reviews. 2007;3:3–14. doi: 10.2174/157339907779802139. [DOI] [PubMed] [Google Scholar]
- 132.Chibber R, Ben-Mahmud BM, Coppini D, Christ E, Kohner EM. Activity of the glycosylating enzyme, core 2 GlcNAc (beta1,6) transferase, is higher in polymorphonuclear leukocytes from diabetic patients compared with age-matched control subjects: relevance to capillary occlusion in diabetic retinopathy. Diabetes. 2000;49:1724–30. doi: 10.2337/diabetes.49.10.1724. [DOI] [PubMed] [Google Scholar]
- 133.Chibber R, Ben-Mahmud BM, Mann GE, Zhang JJ, Kohner EM. Protein Kinase C 2-Dependent Phosphorylation of Core 2 GlcNAc-T Promotes Leukocyte-Endothelial Cell Adhesion: A Mechanism Underlying Capillary Occlusion in Diabetic Retinopathy. Diabetes. 2003;52:1519–27. doi: 10.2337/diabetes.52.6.1519. [DOI] [PubMed] [Google Scholar]
- 134.Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TC, Moore J, et al. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858–65. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 135.Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3784–91. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb j. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 138.Kim SJ, Equi R, Bressler NM. Analysis of Macular Edema after Cataract Surgery in Patients with Diabetes Using Optical Coherence Tomography. Ophthalmology. 2007;114:881–9. doi: 10.1016/j.ophtha.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 139.Kim YM, Kim YM, Lee YM, Kim HS, Kim JD, Choi Y, et al. TNF-related activation-induced cytokine (TRANCE) induces angiogenesis through the activation of Src and phospholipase C (PLC) in human endothelial cells. J Biol Chem. 2002;277:6799–805. doi: 10.1074/jbc.M109434200. [DOI] [PubMed] [Google Scholar]
- 140.Kita T, Clermont AC, Murugesan N, Zhou Q, Fujisawa K, Ishibashi T, et al. Plasma Kallikrein-Kinin System as a VEGF-Independent Mediator of Diabetic Macular Edema. Diabetes. 2015;64:3588–99. doi: 10.2337/db15-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Min JK, Cho YL, Choi JH, Kim Y, Kim JH, Yu YS, et al. Receptor activator of nuclear factor (NF)-kappaB ligand (RANKL) increases vascular permeability: impaired permeability and angiogenesis in eNOS-deficient mice. Blood. 2007;109:1495–502. doi: 10.1182/blood-2006-06-029298. [DOI] [PubMed] [Google Scholar]
- 142.Roh MI, Kim HS, Song JH, Lim JB, Kwon OW. Effect of intravitreal bevacizumab injection on aqueous humor cytokine levels in clinically significant macular edema. Ophthalmology. 2009;116:80–86. doi: 10.1016/j.ophtha.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 143.Funk M, Schmidinger G, Maar N, Bolz M, Benesch T, Zlabinger GJ, et al. Angiogenic and inflammatory markers in the intraocular fluid of eyes with diabetic macular edema and influence of therapy with bevacizumab. Retina. 2010;30:1412–9. doi: 10.1097/IAE.0b013e3181e095c0. [DOI] [PubMed] [Google Scholar]
- 144.Sohn HJ, Han DH, Kim IT, Oh IK, Kim KH, Lee DY, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152:686–94. doi: 10.1016/j.ajo.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 145.Funatsu H, Yamashita H, Ikeda T, Nakanishi Y, Kitano S, Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. American Journal of Ophthalmology. 2002;133:537–43. doi: 10.1016/s0002-9394(02)01323-5. [DOI] [PubMed] [Google Scholar]
- 146.Funatsu H, Yamashita H, Sakata K, Noma H, Mimura T, Suzuki M, et al. Vitreous Levels of Vascular Endothelial Growth Factor and Intercellular Adhesion Molecule 1 Are Related to Diabetic Macular Edema. Ophthalmology. 2005;112:806–16. doi: 10.1016/j.ophtha.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 147.Funatsu H, Yamashita H, Shimizu E, Kojima R, Hori S. Relationship between vascular endothelial growth factor and interleukin–6 in diabetic retinopathy. Retina. 2001;21:469–77. doi: 10.1097/00006982-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 148.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 149.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 150.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of Angiopoietin-1, a Ligand for the TIE2 Receptor, by Secretion-Trap Expression Cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 151.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 152.Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, et al. Angiopoietin-2 Is Critical for Cytokine-Induced Vascular Leakage. PLoS ONE. 2013;8:e70459. doi: 10.1371/journal.pone.0070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koehne P, Willam C, Strauss E, Schindler R, Eckardt KU, Buhrer C. Lack of hypoxic stimulation of VEGF secretion from neutrophils and platelets. Am J Physiol Heart Circ Physiol. 2000;279:H817–824. doi: 10.1152/ajpheart.2000.279.2.H817. [DOI] [PubMed] [Google Scholar]
- 154.Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Experimental Cell Research. 2013;319:1271–80. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 155.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, et al. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007;282:31038–45. doi: 10.1074/jbc.M704703200. [DOI] [PubMed] [Google Scholar]
- 156.Patel JI, Hykin PG, Gregor ZJ, Boulton M, Cree IA. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89:480–3. doi: 10.1136/bjo.2004.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peters S, Cree IA, Alexander R, Turowski P, Ockrim Z, Patel J, et al. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine. 2007;40:144–50. doi: 10.1016/j.cyto.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 158.Pfister F, Wang Y, Schreiter K, vom Hagen F, Altvater K, Hoffmann S, et al. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol. 2010;47:59–64. doi: 10.1007/s00592-009-0099-2. [DOI] [PubMed] [Google Scholar]
- 159.Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–81. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, et al. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–84. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 161.Stewart EA, Saker S, Amoaku WM. Dexamethasone reverses the effects of high glucose on human retinal endothelial cell permeability and proliferation in vitro. Exp Eye Res. 2016;151:75–81. doi: 10.1016/j.exer.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 162.Abdouh M, Talbot S, Couture R, Hasséssian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B1 and B2 receptors. British Journal of Pharmacology. 2008;154:136–43. doi: 10.1038/bjp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Clermont A, Chilcote TJ, Kita T, Liu J, Riva P, Sinha S, et al. Plasma Kallikrein Mediates Retinal Vascular Dysfunction and Induces Retinal Thickening in Diabetic Rats. Diabetes. 2011;60:1590–8. doi: 10.2337/db10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gao B-B, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nature Medicine. 2007;13:181–8. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 165.Wise GN. Retinal neovascularization. Trans Am Ophthalmol Soc. 1956;54:729–826. [PMC free article] [PubMed] [Google Scholar]
- 166.Ashton N. Pathological basis of retrolental fibroplasia. Br J Ophthalmol. 1954;38:385–96. doi: 10.1136/bjo.38.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Browning AC, Alibhai A, McIntosh RS, Rotchford AP, Bhan A, Amoaku WM. Effect of diabetes mellitus and hyperglycemia on the proliferation of human Tenon's capsule fibroblasts: Implications for wound healing after glaucoma drainage surgery. Wound Repair and Regeneration. 2005;13:295–302. doi: 10.1111/j.1067-1927.2005.00130312.x. [DOI] [PubMed] [Google Scholar]
- 168.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jain M, LoGerfo FW, Guthrie P, Pradhan L. Effect of hyperglycemia and neuropeptides on interleukin-8 expression and angiogenesis in dermal microvascular endothelial cells. J Vasc Surg. 2011;53:1654–1660.e1652. doi: 10.1016/j.jvs.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 170.Adamis AP, Miller JW, Bernal M-T, D'Amico DJ, Folkman J, Yeo T-K, et al. Increased Vascular Endothelial Growth Factor Levels in the Vitreous of Eyes With Proliferative Diabetic Retinopathy. American Journal of Ophthalmology. 1994;118:445–50. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 171.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AEB, Al-Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes/Metabolism Research and Reviews. 2003;19:442–55. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 172.Shima DT, Adamis AP, Ferrara N, Yeo KT, Yeo TK, Allende R, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182–93. [PMC free article] [PubMed] [Google Scholar]
- 173.Simo R, Lecube A, Segura RM, Garcia Arumi J, Hernandez C. Free insulin growth factor-I and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2002;134:376–82. doi: 10.1016/s0002-9394(02)01538-6. [DOI] [PubMed] [Google Scholar]
- 174.D'Amore PA. Vascular endothelial cell growth factor-a: not just for endothelial cells anymore. Am J Pathol. 2007;171:14–18. doi: 10.2353/ajpath.2007.070385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 177.Klaassen I, de Vries EW, Vogels IMC, van Kampen AHC, Bosscha MI, Steel DHW, et al. Identification of proteins associated with clinical and pathological features of proliferative diabetic retinopathy in vitreous and fibrovascular membranes. PLoS One. 2017;12:e0187304. doi: 10.1371/journal.pone.0187304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales' disease. Retina. 2008;28:817–24. doi: 10.1097/IAE.0b013e31816576d5. [DOI] [PubMed] [Google Scholar]
- 179.Sivaprasad S, Pearce E. The unmet need for better risk stratification of non-proliferative diabetic retinopathy. Diabet Med. 2019;36:424–33. doi: 10.1111/dme.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ghanchi F. Diabetic Retinopathy Guidelines Working G. The Royal College of Ophthalmologists' clinical guidelines for diabetic retinopathy: a summary. Eye (Lond) 2013;27:285–7. doi: 10.1038/eye.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250:61–70. doi: 10.1007/s00417-011-1774-x. [DOI] [PubMed] [Google Scholar]
- 182.Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–16. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 183.Rayess N, Rahimy E, Ying GS, Bagheri N, Ho AC, Regillo CD, et al. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2015;159:85–91. doi: 10.1016/j.ajo.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 184.Tan CS, Chew MC, Lim LW, Sadda SR. Advances in retinal imaging for diabetic retinopathy and diabetic macular edema. Indian J Ophthalmol. 2016;64:76–83. doi: 10.4103/0301-4738.178145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Muqit MM, Gray JC, Marcellino GR, Henson DB, Young LB, Patton N, et al. Barely visible 10-millisecond pascal laser photocoagulation for diabetic macular edema: observations of clinical effect and burn localization. Am J Ophthalmol. 2010;149:979–986. doi: 10.1016/j.ajo.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 186.Vujosevic S, Martini F, Longhin E, Convento E, Cavarzeran F, Midena E. Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema: Morphologic and Functional Safety. Retina. 2015;35:1594–603. doi: 10.1097/IAE.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 187.Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010;30:908–16. doi: 10.1097/IAE.0b013e3181c96986. [DOI] [PubMed] [Google Scholar]
- 188.Schwartz R, Khalid H, Sivaprasad S, Nicholson L, Anikina E, Sullivan P, et al. Objective evaluation of proliferative diabetic retinopathy using OCT. Ophthalmol Retina. 2020;4:164–74. doi: 10.1016/j.oret.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 189.Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabetic medicine : a journal of the British Diabetic Association. 2012;29:855–62. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 190.Seidu S, Davies MJ, Farooqi A, Khunti K. Integrated primary care: is this the solution to the diabetes epidemic? Diabetic medicine : a journal of the British Diabetic Association. 2017;34:748–50. doi: 10.1111/dme.13348. [DOI] [PubMed] [Google Scholar]
- 191.Olafsdottir E, Andersson DK, Dedorsson I, Stefansson E. The prevalence of retinopathy in subjects with and without type 2 diabetes mellitus. Acta Ophthalmol. 2014;92:133–7. doi: 10.1111/aos.12095. [DOI] [PubMed] [Google Scholar]
- 192.Looker HC, Nyangoma SO, Cromie D, Olson JA, Leese GP, Black M, et al. Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia. 2012;55:2335–42. doi: 10.1007/s00125-012-2596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lindenmeyer A, Sturt JA, Hipwell A, Stratton IM, Al-Athamneh N, Gadsby R, et al. Influence of primary care practices on patients' uptake of diabetic retinopathy screening: a qualitative case study. Br J Gen Pract. 2014;64:e484–492. doi: 10.3399/bjgp14X680965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–77. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 195.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898. doi: 10.1136/bmj.d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–15. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 197.Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL, et al. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. The lancet Diabetes & endocrinology. 2015;3:263–74. doi: 10.1016/S2213-8587(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 198.Action to Control Cardiovascular Risk in Diabetes Follow-On Eye Study G, the Action to Control Cardiovascular Risk in Diabetes Follow-On Study G Persistent Effects of Intensive Glycemic Control on Retinopathy in Type 2 Diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study. Diabetes Care. 2016;39:1089–1100. doi: 10.2337/dc16-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 200.Shi R, Zhao L, Wang F, Liu F, Chen Z, Li R, et al. Effects of lipid-lowering agents on diabetic retinopathy: a Meta-analysis and systematic review. Int J Ophthalmol. 2018;11:287–95. doi: 10.18240/ijo.2018.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khunti K, Gadsby R, Millett C, Majeed A, Davies M. Quality of diabetes care in the UK: comparison of published quality-of-care reports with results of the Quality and Outcomes Framework for Diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2007;24:1436–41. doi: 10.1111/j.1464-5491.2007.02276.x. [DOI] [PubMed] [Google Scholar]
- 202.Hamid A, Wharton HM, Mills A, Gibson JM, Clarke M, Dodson PM. Diagnosis of retinopathy in children younger than 12 years of age: implications for the diabetic eye screening guidelines in the UK. Eye (Lond) 2016;30:949–51. doi: 10.1038/eye.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Public Health England. Diabetic eye screening: programme overview. 2017. https://www.gov.uk/guidance/diabetic-eye-screening-programmeoverview#diabetic-retinopathy.
- 204.Scanlon PH, Stratton IM, Bachmann MO, Jones C, Leese GP. Four Nations Diabetic Retinopathy Screening Study G. Risk of diabetic retinopathy at first screen in children at 12 and 13 years of age. Diabet Med. 2016;33:1655–8. doi: 10.1111/dme.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lucentis® (ranibizumab solution for injection) [summary of product characteristics]. Dublin, Ireland: Novartis Europharm Ltd. 2018. https://www.medicines.org.uk/emc/medicine/19409.
- 206.International Diabetes Federation. IDF Atlas, 6th Edition. 2013. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/19-atlas-6thedition.html.
- 207.Rasmussen KL, Laugesen CS, Ringholm L, Vestgaard M, Damm P, Mathiesen ER. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia. 2010;53:1076–83. doi: 10.1007/s00125-010-1697-9. [DOI] [PubMed] [Google Scholar]
- 208.Axer-Siegel R, Hod M, Fink-Cohen S, Kramer M, Weinberger D, Schindel B, et al. Diabetic Retinopathy during Pregnancy. Ophthalmology. 1996;103:1815–9. doi: 10.1016/s0161-6420(96)30421-1. [DOI] [PubMed] [Google Scholar]
- 209.Chew EY, Mills JL, Metzger BE, Remaley NA, Jovanovic-Peterson L, Knopp RH, et al. Metabolic Control and Progression of Retinopathy: The Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18:631–7. doi: 10.2337/diacare.18.5.631. [DOI] [PubMed] [Google Scholar]
- 210.Dibble CM, Kochenour NK, Worley RJ, Tyler FH, Swartz M. Effect of pregnancy on diabetic retinopathy. Obstetrics and gynecology. 1982;59:699–704. [PubMed] [Google Scholar]
- 211.Klein BE, Moss SE, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13:34–40. doi: 10.2337/diacare.13.1.34. [DOI] [PubMed] [Google Scholar]
- 212.Phelps RL, Sakol P, Metzger BE, Jampol LM, Freinkel N. Changes in diabetic retinopathy during pregnancy. Correlations with regulation of hyperglycemia. Arch Ophthalmol. 1986;104:1806–10. doi: 10.1001/archopht.1986.01050240080044. [DOI] [PubMed] [Google Scholar]
- 213.Rahman W, Rahman FZ, Yassin S, Al-Suleiman SA, Rahman J. Progression of retinopathy during pregnancy in type 1 diabetes mellitus. Clin Exp Ophthalmol. 2007;35:231–6. doi: 10.1111/j.1442-9071.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 214.Temple RC, Aldridge VA, Sampson MJ, Greenwood RH, Heyburn PJ, Glenn A. Impact of pregnancy on the progression of diabetic retinopathy in Type 1 diabetes. Diabet Med. 2001;18:573–7. doi: 10.1046/j.1464-5491.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- 215.Vestgaard M, Ringholm L, Laugesen CS, Rasmussen KL, Damm P, Mathiesen ER. Pregnancy-induced sight-threatening diabetic retinopathy in women with Type 1 diabetes. Diabet Med. 2010;27:431–5. doi: 10.1111/j.1464-5491.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 216.Moloney JB, Drury MI. The effect of pregnancy on the natural course of diabetic retinopathy. Am J Ophthalmol. 1982;93:745–56. doi: 10.1016/0002-9394(82)90471-8. [DOI] [PubMed] [Google Scholar]
- 217.Effect of pregnancy on microvascular complications in the diabetes control and complications trial The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084–91. doi: 10.2337/diacare.23.8.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31:1060–79. doi: 10.2337/dc08-9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Joseph SE, McHugh D, Blott M, Amiel SA, Watkins PJ. Acceleration of diabetic retinopathy in pregnancy: a case report. Diabet Med. 2001;18:675–8. doi: 10.1046/j.1464-5491.2001.00525.x. [DOI] [PubMed] [Google Scholar]
- 220.Egan AM, McVicker L, Heerey A, Carmody L, Harney F, Dunne FP. Diabetic Retinopathy in Pregnancy: A Population-Based Study of Women with Pregestational Diabetes. Journal of Diabetes Research. 2015;2015:1–7. doi: 10.1155/2015/310239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lovestam-Adrian M, Agardh CD, Aberg A, Agardh E. Pre-eclampsia is a potent risk factor for deterioration of retinopathy during pregnancy in Type 1 diabetic patients. Diabet Med. 1997;14:1059–65. doi: 10.1002/(SICI)1096-9136(199712)14:12<1059::AID-DIA505>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 222.Rosenn B, Miodovnik M, Kranias G, Khoury J, Combs CA, Mimouni F, et al. Progression of diabetic retinopathy in pregnancy: association with hypertension in pregnancy. American journal of obstetrics and gynecology. 1992;166:1214–8. doi: 10.1016/s0002-9378(11)90608-5. [DOI] [PubMed] [Google Scholar]
- 223.Association AD 12. Management of Diabetes in Pregnancy. Diabetes Care. 2015;38(Supplement_1):S77–S79. doi: 10.2337/dc15-S015. [DOI] [PubMed] [Google Scholar]
- 224.Helen CC, Tajunisah I, Reddy SC. Adverse outcomes in Type I diabetic pregnant women with proliferative diabetic retinopathy. Int J Ophthalmol. 2011;4:443–6. doi: 10.3980/j.issn.2222-3959.2011.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kohner EM. Microvascular disease: what does the UKPDS tell us about diabetic retinopathy? Diabet Med. 2008;25(Suppl 2):20–24. doi: 10.1111/j.1464-5491.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- 226.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet (London, England) 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 227.Atkins AFJ, Watt JM, Milan P, Davies P, Crawford JS. A longitudinal study of cardiovascular dynamic changes throughout pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1981;12:215–24. doi: 10.1016/0028-2243(81)90012-5. [DOI] [PubMed] [Google Scholar]
- 228.Chen HC, Newsom RS, Patel V, Cassar J, Mather H, Kohner EM. Retinal blood flow changes during pregnancy in women with diabetes. Invest Ophthalmol Vis Sci. 1994;35:3199–208. [PubMed] [Google Scholar]
- 229.Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44:721–6. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- 230.Best RM, Chakravarthy U. Diabetic retinopathy in pregnancy. British Journal of Ophthalmology. 1997;81:249–51. doi: 10.1136/bjo.81.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schocket LS, Grunwald JE, Tsang AF, DuPont J. The effect of pregnancy on retinal hemodynamics in diabetic versus nondiabetic mothers. Am J Ophthalmol. 1999;128:477–84. doi: 10.1016/s0002-9394(99)00234-2. [DOI] [PubMed] [Google Scholar]
- 232.Kitzmiller JL, Main E, Ward B, Theiss T, Peterson DL. Insulin lispro and the development of proliferative diabetic retinopathy during pregnancy. Diabetes Care. 1999;22:874–6. doi: 10.2337/diacare.22.5.874. [DOI] [PubMed] [Google Scholar]
- 233.Lauszus FF, Klebe JG, Bek T, Flyvbjerg A. Increased serum IGF-I during pregnancy is associated with progression of diabetic retinopathy. Diabetes. 2003;52:852–6. doi: 10.2337/diabetes.52.3.852. [DOI] [PubMed] [Google Scholar]
- 234.Ringholm L, Vestgaard M, Laugesen CS, Juul A, Damm P, Mathiesen ER. Pregnancy-induced increase in circulating IGF-I is associated with progression of diabetic retinopathy in women with type 1 diabetes. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2011;21:25–30. doi: 10.1016/j.ghir.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 235.Arun CS, Taylor R. Influence of pregnancy on long-term progression of retinopathy in patients with type 1 diabetes. Diabetologia. 2008;51:1041–5. doi: 10.1007/s00125-008-0994-z. [DOI] [PubMed] [Google Scholar]
- 236.Serup L. Influence of pregnancy on diabetic retinopathy. Acta endocrinologica Supplementum. 1986;277:122–4. doi: 10.1530/acta.0.111s0122. [DOI] [PubMed] [Google Scholar]
- 237.Soubrane G, Canivet J, Coscas G. Influence of pregnancy on the evolution of background retinopathy. Preliminary results of a prospective fluorescein angiography study. Int Ophthalmol. 1985;8:249–55. doi: 10.1007/BF00137653. [DOI] [PubMed] [Google Scholar]
- 238.Sunness JS. The pregnant woman's eye. Surv Ophthalmol. 1988;32:219–38. doi: 10.1016/0039-6257(88)90172-5. [DOI] [PubMed] [Google Scholar]
- 239.Hemachandra A, Ellis D, Lloyd CE, Orchard TJ. The Influence of Pregnancy on IDDM Complications. Diabetes Care. 1995;18:950–4. doi: 10.2337/diacare.18.7.950. [DOI] [PubMed] [Google Scholar]
- 240.Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64:631–42. doi: 10.2337/db14-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Aiello LP. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:17–23. doi: 10.2337/dc13-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gordin D, Kaaja R, Forsblom C, Hiilesmaa V, Teramo K, Groop P-H. Pre-eclampsia and pregnancy-induced hypertension are associated with severe diabetic retinopathy in type 1 diabetes later in life. Acta Diabetologica. 2013;50:781–7. doi: 10.1007/s00592-012-0415-0. [DOI] [PubMed] [Google Scholar]
- 243.Hercules BL, Wozencroft M, Gayed II, Jeacock J. Peripheral retinal ablation in the treatment of proliferative diabetic retinopathy during pregnancy. Br J Ophthalmol. 1980;64:87–93. doi: 10.1136/bjo.64.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.American Academy of Ophthalmology Retina/Vitreous Panel. 2014. Diabetic Retinopathy. Preferred Practice Pattern Guidelines. https://www.aao.org/Assets/dba38b76-3095-4360-8cb6-00adab3aad68/635919125497230000/diabetic-retinopathy-ppp-pdf.
- 245.Chan WC, Lim LT, Quinn MJ, Knox FA, McCance D, Best RM. Management and outcome of sight-threatening diabetic retinopathy in pregnancy. Eye. 2004;18:826–32. doi: 10.1038/sj.eye.6701340. [DOI] [PubMed] [Google Scholar]
- 246.National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. NICE guideline [NG3]. 2015. https://www.nice.org.uk/guidance/ng3. [PubMed]
- 247.Conway M, Baldwin J, Kohner EM, Schulenburg WE, Cassar J. Postpartum Progression of Diabetic Retinopathy. Diabetes Care. 1991;14:1110–1. doi: 10.2337/diacare.14.11.1110. [DOI] [PubMed] [Google Scholar]
- 248.Sinclair SH, Nesler C, Foxman B, Nichols CW, Gabbe S. Macular Edema and Pregnancy in Insulin-Dependent Diabetes. American Journal of Ophthalmology. 1984;97:154–67. doi: 10.1016/s0002-9394(14)76085-4. [DOI] [PubMed] [Google Scholar]
- 249.Hampshire R, Wharton H, Leigh R, Wright A, Dodson P. Screening for diabetic retinopathy in pregnancy using photographic review clinics. Diabetic Medicine. 2013;30:475–7. doi: 10.1111/dme.12077. [DOI] [PubMed] [Google Scholar]
- 250.Ohrt V. The influence of pregnancy on diabetic retinopathy with special regard to the reversible changes shown in 100 pregnancies. Acta Ophthalmol (Copenh) 1984;62:603–16. doi: 10.1111/j.1755-3768.1984.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 251.Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study Report no. 4 The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27:265–72. doi: 10.1097/00004397-198702740-00006. [DOI] [PubMed] [Google Scholar]
- 252.Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121:1045–53. doi: 10.1016/j.ophtha.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 253.Australian Government, Department of Health, Therapeutic Goods Administration. Product Information for AusPAR: Lucentis, ranibizumab, Novartis Pharmaceuticals Australia, PM-2013-00985-1-5. 2014. https://www.tga.gov.au/sites/default/files/auspar-ranibizumab-141014-pi.pdf.
- 254.Genentech I Highlights of Prescribing Information: Avastin (bevacizumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0169lbl.pdf.
- 255.Australian Government, Department of Health, Therapeutic Goods Administration. Product Information for AusPAR: Eylea, aflibercept, Bayer Australia Ltd. PM-2013-04198-1-1. 2015. https://www.tga.gov.au/sites/default/files/auspar-aflibercept-150721-pi.pdf.
- 256.Petrou P, Georgalas I, Giavaras G, Anastasiou E, Ntana Z, Petrou C. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010;88:e136. doi: 10.1111/j.1755-3768.2009.01572.x. [DOI] [PubMed] [Google Scholar]
- 257.Rasier R, Artunay O, Yuzbasioglu E, Sengul A, Bahcecioglu H. The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye (Lond) 2009;23:1714–8. doi: 10.1038/eye.2008.360. [DOI] [PubMed] [Google Scholar]
- 258.Wu Z, Huang J, Sadda S. Inadvertent use of bevacizumab to treat choroidal neovascularisation during pregnancy: a case report. Annals of the Academy of Medicine, Singapore. 2010;39:143–5. [PubMed] [Google Scholar]
- 259.Tarantola RM, Folk JC, Boldt HC, Mahajan VB. Intravitreal bevacizumab during pregnancy. Retina. 2010;30:1405–11. doi: 10.1097/IAE.0b013e3181f57d58. [DOI] [PubMed] [Google Scholar]
- 260.Alcon Laboratories Inc. Highlights of Prescribing Information. Triesence™ (triamcinolone acetonide injectable suspension) 40 mg/mL. 2007. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/022223,022048lbl.pdf.
- 261.Degenring RF, Jonas JB. Serum levels of triamcinolone acetonide after intravitreal injection. American Journal of Ophthalmology. 2004;137:1142–3. doi: 10.1016/j.ajo.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 262.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement 1994; 12: 1-24. [PubMed]
- 263.Fazelat A, Lashkari K. Off-label use of intravitreal triamcinolone acetonide for diabetic macular edema in a pregnant patient. Clin Ophthalmol. 2011;5:439–41. doi: 10.2147/OPTH.S14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Concillado M, Lund-Andersen H, Mathiesen ER, Larsen M. Dexamethasone Intravitreal Implant for Diabetic Macular Edema During Pregnancy. American Journal of Ophthalmology. 2016;165:7–15. doi: 10.1016/j.ajo.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 265.Yoo R, Kim HC, Chung H. Dexamethasone intravitreal implant for diabetic macular edema in a pregnant patient. Int J Ophthalmol. 2016;9:1524–7. doi: 10.18240/ijo.2016.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Alcon Laboratories (Australia) Pty Ltd. Australian Product Information: Fluorescite. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf? OpenAgent&id=CP-2018-PI-02134-1&d=201911231016933.
- 267.Halperin LS, Olk RJ, Soubrane G, Coscas G. Safety of Fluorescein Angiography During Pregnancy. American Journal of Ophthalmology. 1990;109:563–6. doi: 10.1016/s0002-9394(14)70686-5. [DOI] [PubMed] [Google Scholar]
- 268.Mattern J, Mayer PR. Excretion of fluorescein into breast milk. Am J Ophthalmol. 1990;109:598–9. doi: 10.1016/s0002-9394(14)70694-4. [DOI] [PubMed] [Google Scholar]
- 269. Diabetes care and research in Europe: the Saint Vincent declaration. Diabet Med 1990; 7: 360. [PubMed]
- 270.Diabetes UK. The National service framework (NSF) for diabetes Five years on... are we half way there? 2008. https://www.diabetes.org.uk/resourcess3/2017-11/five_years_on_-_are_we_half_way_there2008.pdf.
- 271.English NHS Diabetic Eye Screening Programme. English national screening programme for diabetic retinopathy. 2020. http://www.retinalscreening.nhs.uk.
- 272.Scottish diabetic retinopathy screening collaborative. DRS Manual. 2014. http://www.ndrs.scot.nhs.uk/.
- 273.DRSSW. Diabetic retinopathy screening service for Wales. 2011. http://www.wales.nhs.uk/siteplus/docs/861/Item%206.1_Diabetes%Focus%20On1.pdf.
- 274.NIDRSP. Northern Ireland DR Screening Programme Annual report 2008–09. 2010. Available from http://publichealthwell.ie/node/18856.
- 275.Scanlon PH. The English National Screening Programme for diabetic retinopathy 2003-2016. Acta Diabetol. 2017;54:515–25. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Diabetic retinopathy study Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 1981;21(1 Pt 2):1–226. [PubMed] [Google Scholar]
- 277.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 278.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 279.Public Health England. Diabetic eye screening programme: standards. 2019. https://www.gov.uk/government/publications/diabetic-eye-screening-programme-standards.
- 280.Public Health England. PHE Screening Blog. 2019. https://phescreening.blog.gov.uk/2019/05/31/updated-national-diabetic-eye-screening-standardspublished/.
- 281.Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten Emerging Trends in the Epidemiology of Diabetic Retinopathy. Ophthalmic Epidemiol. 2016;23:209–22. doi: 10.1080/09286586.2016.1193618. [DOI] [PubMed] [Google Scholar]
- 282.Lee JX, Manjunath V, Talks SJ. Expanding the role of medical retina virtual clinics using multimodal ultra-widefield and optical coherence tomography imaging. Clin Ophthalmol. 2018;12:2337–45. doi: 10.2147/OPTH.S181108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Public Health England. Guidance. Diabetic eye screening: standards. 2019. https://www.gov.uk/government/publications/diabetic-eye-screeningprogramme-standards.
- 284.UK National Screening Committee. NHS Diabetic Eye Screening Programme Version 1.1. 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/395669/Failsafe_Desp_V1_1_Final_190913.pdf.
- 285.Public Health England. Programme Specific Operating Model for Quality Assurance of Diabetic Eye Screening Programmes. 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/653751/DES_PSOM.pdf.
- 286.Public Health England. Screening Pathway - DES. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/819366/Diabetic_eye_screening_pathway_and_themes_July_2019.pdf.
- 287.Royal College of Opthalmologists. Ophthalmic Services Guidance: Standards for Virtual Clinics in Glaucoma Care in the NHS Hospital Eye Service. https://www.rcophth.ac.uk/wp-content/uploads/2017/03/Virtual-Glaucoma-Clinics.pdf.
- 288.Royal College of Opthalmologists. Ophthalmic clinical competency framework: medical retina. 2020. https://healtheducationengland.sharepoint.com/:x:/g/Comms/Digital/Ec93F5CFScZEiosqZ4QN9HgBvnHC7rF3naDN_Ywj0viBrA?rtime=PIFjFGZ110g.
- 289.Manjunath V, Papastavrou V, Steel DH, Menon G, Taylor R, Peto T, et al. Wide-field imaging and OCT vs clinical evaluation of patients referred from diabetic retinopathy screening. Eye (Lond) 2015;29:416–23. doi: 10.1038/eye.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kortuem K, Fasler K, Charnley A, Khambati H, Fasolo S, Katz M, et al. Implementation of medical retina virtual clinics in a tertiary eye care referral centre. Br J Ophthalmol. 2018;102:1391–5. doi: 10.1136/bjophthalmol-2017-311494. [DOI] [PubMed] [Google Scholar]
- 291.Bressler SB, Liu D, Glassman AR, Blodi BA, Castellarin AA, Jampol LM, et al. Change in Diabetic Retinopathy Through 2 Years: Secondary Analysis of a Randomized Clinical Trial Comparing Aflibercept, Bevacizumab, and Ranibizumab. JAMA Ophthalmol. 2017;135:558–68. doi: 10.1001/jamaophthalmol.2017.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Iglicki M, Zur D, Busch C, Okada M, Loewenstein A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the 'DR-Pro-DEX Study'. Acta Diabetol. 2018;55:541–7. doi: 10.1007/s00592-018-1117-z. [DOI] [PubMed] [Google Scholar]
- 293.Wykoff CC, Chakravarthy U, Campochiaro PA, Bailey C, Green K, Cunha-Vaz J. Long-term Effects of Intravitreal 0.19 mg Fluocinolone Acetonide Implant on Progression and Regression of Diabetic Retinopathy. Ophthalmology. 2017;124:440–9. doi: 10.1016/j.ophtha.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 294.Royal College of Opthalmologists. The Way Forward. Options to help meet demand for the current and future care of patients with eye disease—agerelated macular degeneration and diabetic retinopathy. 2017. https://www.rcophth.ac.uk/wp-content/uploads/2015/10/RCOphth-The-Way-Forward-AMD-300117.pdf.
- 295.Leal J, Luengo-Fernandez R, Stratton IM, Dale A, Ivanova K, Scanlon PH. Cost-effectiveness of digital surveillance clinics with optical coherence tomography versus hospital eye service follow-up for patients with screen-positive maculopathy. Eye (Lond) 2019;33:640–7. doi: 10.1038/s41433-018-0297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Virgili G, Menchini F, Casazza G, Hogg R, Das RR, Wang X, et al. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD008081. doi: 10.1002/14651858.CD008081.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gulshan V, Rajan RP, Widner K, Wu D, Wubbels P, Rhodes T, et al. Performance of a deep-learning algorithm vs manual grading for detecting diabetic retinopathy in India. JAMA Ophthalmol. 2019. 10.1001/jamaophthalmol.2019.2004. [DOI] [PMC free article] [PubMed]
- 298.Grzybowski A, Brona P, Lim G, Ruamviboonsuk P, Tan GSW, Abramoff M, et al. Artificial intelligence for diabetic retinopathy screening: a review. Eye. 2020;34:451–60. doi: 10.1038/s41433-019-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Abramoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved Automated Detection of Diabetic Retinopathy on a Publicly Available Dataset Through Integration of Deep Learning. Invest Ophthalmol Vis Sci. 2016;57:5200–6. doi: 10.1167/iovs.16-19964. [DOI] [PubMed] [Google Scholar]
- 300.Davis MD, Norton EWD, Myers, FL. The Airlie classification of diabetic retinopathy. In: Goldberg MF, Fine SL, eds. Symposium on the Treatment of Diabetic Retinopathy. Public Health Service Publication No. 1890. Washington, DC: US Government Printing Office; 1969. p.7–22.
- 301.Scotland G, McKeigue P, Philip S, Leese GP, Olson JA, Looker HC, et al. Modelling the cost-effectiveness of adopting risk-stratified approaches to extended screening intervals in the national diabetic retinopathy screening programme in Scotland. Diabet Med. 2016;33:886–95. doi: 10.1111/dme.13129. [DOI] [PubMed] [Google Scholar]
- 302.Arcadu F, Benmansour F, Maunz A, Michon J, Haskova Z, McClintock D, et al. Deep Learning Predicts OCT Measures of Diabetic Macular Thickening From Color Fundus Photographs. Invest Ophthalmol Vis Sci. 2019;60:852–7. doi: 10.1167/iovs.18-25634. [DOI] [PubMed] [Google Scholar]
- 303.Schlegl T, Waldstein SM, Bogunovic H, Endstrasser F, Sadeghipour A, Philip AM, et al. Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. Ophthalmology. 2018;125:549–58. doi: 10.1016/j.ophtha.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 304.Raman R, Srinivasan S, Virmani S, Sivaprasad S, Rao C, Rajalakshmi R. Fundus photograph-based deep learning algorithms in detecting diabetic retinopathy. Eye (Lond) 2019;33:97–109. doi: 10.1038/s41433-018-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77:170–5. doi: 10.1034/j.1600-0420.1999.770211.x. [DOI] [PubMed] [Google Scholar]
- 306.Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Pract. 2013;100:298–305. doi: 10.1016/j.diabres.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 307.Chew M, Tan NYQ, Lamoureux E, Cheng CY, Wong TY, Sabanayagam C. The associations of objectively measured sleep duration and sleep disturbances with diabetic retinopathy. Diabetes Res Clin Pract. 2020;159:107967. doi: 10.1016/j.diabres.2019.107967. [DOI] [PubMed] [Google Scholar]
- 308.Mason RH, West SD, Kiire CA, Groves DC, Lipinski HJ, Jaycock A, et al. High prevalence of sleep disordered breathing in patients with diabetic macular edema. Retina. 2012;32:1791–8. doi: 10.1097/IAE.0b013e318259568b. [DOI] [PubMed] [Google Scholar]
- 309.Mason RH, Kiire CA, Groves DC, Lipinski HJ, Jaycock A, Winter BC, et al. Visual improvement following continuous positive airway pressure therapy in diabetic subjects with clinically significant macular oedema and obstructive sleep apnoea: proof of principle study. Respiration. 2012;84:275–82. doi: 10.1159/000334090. [DOI] [PubMed] [Google Scholar]
- 310.Treatment Techniques and Clinical Guidelines for Photocoagulation of Diabetic Macular Edema. Ophthalmology. 1987;94:761–74. [DOI] [PubMed]
- 311.Lavinsky D, Cardillo JA, Melo LA, Jr., Dare A, Farah ME, Belfort R., Jr Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52:4314–23. doi: 10.1167/iovs.10-6828. [DOI] [PubMed] [Google Scholar]
- 312.Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. British Journal of Ophthalmology. 2009;93:1341–4. doi: 10.1136/bjo.2008.146712. [DOI] [PubMed] [Google Scholar]
- 313.Lois N, Gardner E, Waugh N, Azuara-Blanco A, Mistry H, McAuley D, et al. Diabetic macular oedema and diode subthreshold micropulse laser (DIAMONDS): study protocol for a randomised controlled trial. Trials. 2019;20:122. doi: 10.1186/s13063-019-3199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 315.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 316.Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology. 2015;122:2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 317.National Institute for Health and Care Excellence. Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy. Technology appraisal guidance [TA301]. https://www.nice.org.uk/guidance/ta301.
- 318.Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term Benefit of Sustained-Delivery Fluocinolone Acetonide Vitreous Inserts for Diabetic Macular Edema. Ophthalmology. 2011;118:626–.e622. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 319.Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P, et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580–7. doi: 10.1016/j.ophtha.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 320.Boyer DS, Yoon YH, Belfort R, Bandello F, Maturi RK, Augustin AJ, et al. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology. 2014;121:1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 321.Scottish Medicines Consortium. Fluocinolone acetonide 190 micrograms intravitreal implant (Iluvien®) - SMC No. (864/13) (Resubmission). 2014. https://www.scottishmedicines.org.uk/medicines-advice/fluocinolone-acetonide-iluvien-resubmission-86413/.
- 322.Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, et al. Effect of Initial Management With Aflibercept vs Laser Photocoagulation vs Observation on Vision Loss Among Patients With Diabetic Macular Edema Involving the Center of the Macula and Good Visual Acuity: A Randomized Clinical Trial. JAMA. 2019;321:1880. doi: 10.1001/jama.2019.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Diabetic Retinopathy Clinical Research N, Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Edington M, Sachdev A, Morjaria R, Chong V. Structural-Functional Correlation in Patients with Diabetic Macular Edema. Retina. 2017;37:881–5. doi: 10.1097/IAE.0000000000001266. [DOI] [PubMed] [Google Scholar]
- 325.Shen Y, Liu K, Xu X. Correlation Between Visual Function and Photoreceptor Integrity in Diabetic Macular Edema: Spectral-Domain Optical Coherence Tomography. Curr Eye Res. 2016;41:391–9. doi: 10.3109/02713683.2015.1019003. [DOI] [PubMed] [Google Scholar]
- 326.Bressler NM, Odia I, Maguire M, Glassman AR, Jampol LM, MacCumber MW, et al. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the protocol T randomized clinical trial. JAMA Ophthalmol. 2019. 10.1001/jamaophthalmol.2019.1963. [DOI] [PMC free article] [PubMed]
- 327.Diabetic Retinopathy Clinical Research N, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123:1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wiley HE, Thompson DJ, Bailey C, Chew EY, Cukras CA, Jaffe GJ, et al. A Crossover Design for Comparative Efficacy: A 36-Week Randomized Trial of Bevacizumab and Ranibizumab for Diabetic Macular Edema. Ophthalmology. 2016;123:841–9. doi: 10.1016/j.ophtha.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS. Intravitreal Bevacizumab Versus Ranibizumab for Treatment of Neovascular Age-Related Macular Degeneration: Findings from a Cochrane Systematic Review. Ophthalmology. 2016;123:70–77.e71. doi: 10.1016/j.ophtha.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.European Medicines Agency. Eylea 40 mg/ml solution for injection in pre-filled syringe. Summary of Product Characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf.
- 332.Garweg J, Sivaprasad S, Štefanickova J, Hoyng C, Achcar C, Schmelter T, et al. A phase 3b, randomized study to compare three dosing regimens of intravitreal aflibercept for diabetic macular edema beyond the first year of treatment: primary outcomes of the VIOLET study. EURETINA. 2019.
- 333.Dhoot Dssnccacp, D; See, RF; Avery, RL Treat-and-Extend vs. Bimonthly Dosing With Aflibercept for the Treatment of DME: One-Year Outcomes (EVADE Study). AAO 2018; Chicago, 2018.
- 334.Prunte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnicka J, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100:787–95. doi: 10.1136/bjophthalmol-2015-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Pearce I, Bailey C, Fletcher E, Ghanchi F, Rennie C, Santiago C, et al. Translating evidence into practice: recommendations by a UK expert panel on the use of aflibercept in diabetic macular oedema. Eye. 2019;34:969–81. doi: 10.1038/s41433-019-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077.e1035. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Talks SJ, Bhatia D, Menon G, Cole A, Eleftheriadis H, Downey L, et al. Randomised trial of wide-field guided PRP for diabetic macular oedema treated with ranibizumab. Eye (Lond) 2019;33:930–7. doi: 10.1038/s41433-019-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kaiser PK Intravitreal aflibercept injection (IAI) in patients with prior therapy for diabetic macular edema (DME): 148-week outcomes from VISTA. Retina Society; October 8, 2015; Paris, France, 2015.
- 339.Ashraf M, Souka AA, ElKayal H. Short-Term Effects of Early Switching to Ranibizumab or Aflibercept in Diabetic Macular Edema Cases With Non-Response to Bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2017;48:230–6. doi: 10.3928/23258160-20170301-06. [DOI] [PubMed] [Google Scholar]
- 340.Bahrami B, Zhu M, Hong T, Chang A. Diabetic macular oedema: pathophysiology, management challenges and treatment resistance. Diabetologia. 2016;59:1594–608. doi: 10.1007/s00125-016-3974-8. [DOI] [PubMed] [Google Scholar]
- 341.Lim LS, Ng WY, Mathur R, Wong D, Wong EY, Yeo I, et al. Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol. 2015;9:1715–8. doi: 10.2147/OPTH.S81523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Rahimy E, Shahlaee A, Khan MA, Ying GS, Maguire JI, Ho AC, et al. Conversion to Aflibercept After Prior Anti-VEGF Therapy for Persistent Diabetic Macular Edema. Am J Ophthalmol. 2016;164:118–127 e112. doi: 10.1016/j.ajo.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 343.Shah CP, Heier JS. Aflibercept for Diabetic Macular Edema in Eyes Previously Treated With Ranibizumab and/or Bevacizumab May Further Improve Macular Thickness. Ophthalmic Surg Lasers Imaging Retina. 2016;47:836–9. doi: 10.3928/23258160-20160901-06. [DOI] [PubMed] [Google Scholar]
- 344.Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972–9. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 345.Sivaprasad S, Crosby-Nwaobi R, Heng LZ, Peto T, Michaelides M, Hykin P. Injection frequency and response to bevacizumab monotherapy for diabetic macular oedema (BOLT Report 5) Br J Ophthalmol. 2013;97:1177–80. doi: 10.1136/bjophthalmol-2013-303168. [DOI] [PubMed] [Google Scholar]
- 346.Maturi RK, Glassman AR, Liu D, Beck RW, Bhavsar AR, Bressler NM, et al. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:29–38. doi: 10.1001/jamaophthalmol.2017.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Heier JS, Korobelnik J-F, Brown DM, Schmidt-Erfurth U, Do VD, Midena E, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology. 2016;123:2376–85. doi: 10.1016/j.ophtha.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 348.Wood EH, Karth PA, Moshfeghi DM, Leng T. Short-term out- comes of aflibercept therapy for diabetic macular edema in patients with incomplete response to ranibizumab and/or bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2015;46:950–4. [DOI] [PubMed]
- 349.Klein KA, Cleary TS, Reichel E. Effect of intravitreal aflibercept on recalcitrant diabetic macular edema. Int J Retina Vitreous. 2017;3:16. doi: 10.1186/s40942-017-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li X-Y, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915–23. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 351.Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, et al. Dexamethasone Intravitreal Implant in Combination with Laser Photocoagulation for the Treatment of Diffuse Diabetic Macular Edema. Ophthalmology. 2013;120:1843–51. doi: 10.1016/j.ophtha.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 352.Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG, et al. Sustained Delivery Fluocinolone Acetonide Vitreous Implants. Ophthalmology. 2014;121:1892–.e1893. doi: 10.1016/j.ophtha.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 353.Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, et al. Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmology. 2018;136:257. doi: 10.1001/jamaophthalmol.2017.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Gonzalez VH, Campbell J, Holekamp NM, Kiss S, Loewenstein A, Augustin AJ, et al. Early and Long-Term Responses to Anti–Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema: Analysis of Protocol I Data. American Journal of Ophthalmology. 2016;172:72–79. doi: 10.1016/j.ajo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 355.Pieramici D, Singh RP, Gibson A, Saroj N, Vitti R, Berliner AJ, et al. Outcomes of Diabetic Macular Edema Eyes with Limited Early Response in the VISTA and VIVID Studies. Ophthalmol Retina. 2018;2:558–66. doi: 10.1016/j.oret.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 356.Dugel PU, Campbell JH, Kiss S, Loewenstein A, Shih V, Xu X, et al. Association between early anatomic response to anti–vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of Protocol I study data. Retina. 2019;39:88–97. doi: 10.1097/IAE.0000000000002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Busch C, Fraser-Bell S, Iglicki M, Lupidi M, Couturier A, Chaikitmongkol V, et al. Real-world outcomes of non-responding diabetic macular edema treated with continued anti-VEGF therapy versus early switch to dexamethasone implant: 2-year results. Acta Diabetol. 2019;56:1341–50. doi: 10.1007/s00592-019-01416-4. [DOI] [PubMed] [Google Scholar]
- 358.Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82–106. [DOI] [PubMed]
- 359.Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study The Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1979;97:654–5. doi: 10.1001/archopht.1979.01020010310003. [DOI] [PubMed] [Google Scholar]
- 360.Photocoagulation treatment of proliferative diabetic retinopathy Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 361.Indications for photocoagulation treatment of diabetic retinopathy: Diabetic Retinopathy Study Report no. 14 The Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27:239–53. doi: 10.1097/00004397-198702740-00004. [DOI] [PubMed] [Google Scholar]
- 362.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics ETDRS report number 7. Ophthalmology. 1991;98(Suppl 5):741–56. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 363.Early photocoagulation for diabetic retinopathy. ETDRS report number 9 Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(Suppl 5):766–85. [PubMed] [Google Scholar]
- 364.Chew EY, Ferris FL, 3rd, Csaky KG, Murphy RP, Agron E, Thompson DJ, et al. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: the early treatment diabetic retinopathy follow-up study. Ophthalmology. 2003;110:1683–9. doi: 10.1016/S0161-6420(03)00579-7. [DOI] [PubMed] [Google Scholar]
- 365.Bressler NM, Beck RW, Ferris FL., 3rd Panretinal photocoagulation for proliferative diabetic retinopathy. N Engl J Med. 2011;365:1520–6. doi: 10.1056/NEJMct0908432. [DOI] [PubMed] [Google Scholar]
- 366.Moutray T, Evans JR, Lois N, Armstrong DJ, Peto T, Azuara-Blanco A. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2018;3:Cd012314. doi: 10.1002/14651858.CD012314.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Muqit MM, Marcellino GR, Henson DB, Young LB, Turner GS, Stanga PE. Pascal panretinal laser ablation and regression analysis in proliferative diabetic retinopathy: Manchester Pascal Study Report 4. Eye (Lond) 2011;25:1447–56. doi: 10.1038/eye.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Chappelow AV, Tan K, Waheed NK, Kaiser PK. Panretinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Am J Ophthalmol. 2012;153:137–.e132. doi: 10.1016/j.ajo.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 369.Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91:251–8. doi: 10.1111/j.1755-3768.2011.02307.x. [DOI] [PubMed] [Google Scholar]
- 370.Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. Jama. 2015;314:2137–46. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet (London, England) 2017;389:2193–203. doi: 10.1016/S0140-6736(17)31193-5. [DOI] [PubMed] [Google Scholar]
- 372.Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:1138–48. doi: 10.1001/jamaophthalmol.2018.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sameen M, Khan MS, Mukhtar A, Yaqub MA, Ishaq M. Efficacy of intravitreal bevacizumab combined with pan retinal photocoagulation versus panretinal photocoagulation alone in treatment of proliferative diabetic retinopathy. Pak J Med Sci. 2017;33:142–5. doi: 10.12669/pjms.331.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wubben TJ, Johnson MW. Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Retinopathy: Consequences of Inadvertent Treatment Interruptions. Am J Ophthalmol. 2019;204:13–18. doi: 10.1016/j.ajo.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 375.Obeid A, Su D, Patel SN, Uhr JH, Borkar D, Gao X, et al. Outcomes of Eyes Lost to Follow-up with Proliferative Diabetic Retinopathy That Received Panretinal Photocoagulation versus Intravitreal Anti-Vascular Endothelial Growth Factor. Ophthalmology. 2019;126:407–13. doi: 10.1016/j.ophtha.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 376.West JA, Dowler JG, Hamilton AM, Boyd SR, Hykin PG. Panretinal photocoagulation during cataract extraction in eyes with active proliferative diabetic eye disease. Eye (Lond) 1999;13(Pt 2):170–3. doi: 10.1038/eye.1999.45. [DOI] [PubMed] [Google Scholar]
- 377.Wakabayashi T, Oshima Y, Sakaguchi H, Ikuno Y, Miki A, Gomi F, et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology. 2008;115:1571–80. doi: 10.1016/j.ophtha.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 378.Moraczewski AL, Lee RK, Palmberg PF, Rosenfeld PJ, Feuer WJ. Outcomes of treatment of neovascular glaucoma with intravitreal bevacizumab. Br J Ophthalmol. 2009;93:589–93. doi: 10.1136/bjo.2008.151472. [DOI] [PubMed] [Google Scholar]
- 379.Sun Y, Liang Y, Zhou P, Wu H, Hou X, Ren Z, et al. Anti-VEGF treatment is the key strategy for neovascular glaucoma management in the short term. BMC Ophthalmol. 2016;16:150. doi: 10.1186/s12886-016-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Tatsumi T, Yamamoto S, Uehara J, Sugawara T, Baba T, Inoue M, et al. Panretinal photocoagulation with simultaneous cryoretinopexy or intravitreal bevacizumab for neovascular glaucoma. Graefes Arch Clin Exp Ophthalmol. 2013;251:1355–60. doi: 10.1007/s00417-012-2236-9. [DOI] [PubMed] [Google Scholar]
- 381.Olmos LC, Sayed MS, Moraczewski AL, Gedde SJ, Rosenfeld PJ, Shi W, et al. Long-term outcomes of neovascular glaucoma treated with and without intravitreal bevacizumab. Eye (Lond) 2016;30:463–72. doi: 10.1038/eye.2015.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ciftci S, Sakalar YB, Unlu K, Keklikci U, Caca I, Dogan E. Intravitreal bevacizumab combined with panretinal photocoagulation in the treatment of open angle neovascular glaucoma. Eur J Ophthalmol. 2009;19:1028–33. doi: 10.1177/112067210901900620. [DOI] [PubMed] [Google Scholar]
- 383.Cheng Y, Liu XH, Shen X, Zhong YS. Ahmed valve implantation for neovascular glaucoma after 23-gauge vitrectomy in eyes with proliferative diabetic retinopathy. Int J Ophthalmol. 2013;6:316–20. doi: 10.3980/j.issn.2222-3959.2013.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hanna R, Tiosano B, Graffi S, Gaton D. Clinical Efficacy and Safety of the EX-PRESS Filtration Device in Patients with Advanced Neovascular Glaucoma and Proliferative Diabetic Retinopathy. Case Rep Ophthalmol. 2018;9:61–69. doi: 10.1159/000479363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Diabetic Retinopathy Preferred Practice Pattern(R) Ophthalmology. 2020;127:P66–P145. doi: 10.1016/j.ophtha.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 386.Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision Clinical application of results of a randomized trial–Diabetic Retinopathy Vitrectomy Study Report 4. The Diabetic Retinopathy Vitrectomy Study Research Group. Ophthalmology. 1988;95:1321–34. doi: 10.1016/s0161-6420(88)33014-9. [DOI] [PubMed] [Google Scholar]
- 387.Bressler SB, Melia M, Glassman AR, Almukhtar T, Jampol LM, Shami M, et al. Ranibizumab Plus Prompt or Deferred Laser for Diabetic Macular Edema in Eyes with Vitrectomy before Anti-Vascular Endothelial Growth Factor Therapy. Retina. 2015;35:2516–28. doi: 10.1097/IAE.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Diabetic Retinopathy Clinical Research Network Writing C. Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–1093 e1083. doi: 10.1016/j.ophtha.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Flaxel CJ, Edwards AR, Aiello LP, Arrigg PG, Beck RW, Bressler NM, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30:1488–95. doi: 10.1097/IAE.0b013e3181e7974f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Organisation WH. Blindness and vision impairment. 2019. Available at: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment.
- 391.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and vision (London, England) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Foster AE. Vision 2020: the cataract challenge. Community eye health. 2000;13:17–19. [PMC free article] [PubMed] [Google Scholar]
- 393.Klein BEK, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic Epidemiology. 1995;2:49–55. doi: 10.3109/09286589509071451. [DOI] [PubMed] [Google Scholar]
- 394.Klein BEK, Klein R, Moss SE. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985;92:1191–6. doi: 10.1016/s0161-6420(85)33877-0. [DOI] [PubMed] [Google Scholar]
- 395.Klein BEK, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. American Journal of Ophthalmology. 1995;119:295–300. doi: 10.1016/s0002-9394(14)71170-5. [DOI] [PubMed] [Google Scholar]
- 396.Nielsen NV, Vinding T. The prevalence of cataract in insulin-dependent and non-insulin-dependent-diabetes mellitus. Acta Ophthalmologica. 1984;62:595–602. doi: 10.1111/j.1755-3768.1984.tb03972.x. [DOI] [PubMed] [Google Scholar]
- 397.Rowe NG, Mitchell PG, Cumming RG, Wans JJ. Diabetes, fasting blood glucose and age-related cataract: the Blue Mountains Eye Study. Ophthalmic Epidemiology. 2000;7:103–14. [PubMed] [Google Scholar]
- 398.Mukesh BN, Le A, Dimitrov PN, Ahmed S, Taylor HR, McCarty CA. Development of Cataract and Associated Risk Factors: The Visual Impairment Project. JAMA Ophthalmology. 2006;124:79–85. doi: 10.1001/archopht.124.1.79. [DOI] [PubMed] [Google Scholar]
- 399.Day AC, Donachie PHJ, Sparrow JM, Johnston RL. Database oboasctTRCoONO. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29:552–60. doi: 10.1038/eye.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Sadiq SA, Sleep T, Amoaku WMK. The Visual Results and Changes in Retinopathy in Diabetic Patients following Cataract Surgery. European Journal of Ophthalmology. 1999;9:14–20. doi: 10.1177/112067219900900103. [DOI] [PubMed] [Google Scholar]
- 401.Zaczek A, Olivestedt G, Zetterström C. Visual outcome after phacoemulsification and IOL implantation in diabetic patients. British Journal of Ophthalmology. 1999;83:1036 LP–1041. doi: 10.1136/bjo.83.9.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Sparrow JM, Taylor H, Qureshi K, Smith R, Birnie K, Johnston RL, et al. The Cataract National Dataset electronic multi-centre audit of 55 567 operations: risk indicators for monocular visual acuity outcomes. Eye. 2012;26:821–6. doi: 10.1038/eye.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Lundström M, Barry P, Henry Y, Rosen P, Stenevi U. Visual outcome of cataract surgery; Study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. Journal of Cataract & Refractive Surgery. 2013;39:673–9. doi: 10.1016/j.jcrs.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 404.Oshika T, Kato S, Funatsu H. Quantitative assessment of aqueous flare intensity in diabetes. Graefe's Archive for Clinical and Experimental Ophthalmology. 1989;227:518–20. doi: 10.1007/BF02169443. [DOI] [PubMed] [Google Scholar]
- 405.Oshika T, Yoshimura K, Miyata N. Postsurgical inflammation after phacoemulsification and extracapsular extraction with soft or conventional intraocular lens implantation. Journal of Cataract & Refractive Surgery. 1992;18:356–61. doi: 10.1016/s0886-3350(13)80071-5. [DOI] [PubMed] [Google Scholar]
- 406.Sadiq SA, Chatterjee A, Vernon SA. Progression of diabetic retinopathy and rubeotic glaucoma following cataract surgery. Eye. 1995;9:728–32. doi: 10.1038/eye.1995.185. [DOI] [PubMed] [Google Scholar]
- 407.Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Survey of Ophthalmology. 2004;49:470–90. doi: 10.1016/j.survophthal.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 408.Pollack A, Dotan S, Oliver M. Progression of diabetic retinopathy after cataract extraction. British Journal of Ophthalmology. 1991;75:547 LP–551. doi: 10.1136/bjo.75.9.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Jaffe GJ, Burton TC, Kuhn E, Prescott A, Hartz A. Progression of Nonproliferative Diabetic Retinopathy and Visual Outcome After Extracapsular Cataract Extraction and Intraocular Lens Implantation. American Journal of Ophthalmology. 1992;114:448–56. doi: 10.1016/s0002-9394(14)71857-4. [DOI] [PubMed] [Google Scholar]
- 410.Liu Y, Luo L, He M, Liu X. Disorders of the blood-aqueous barrier after phacoemulsification in diabetic patients. Eye. 2004;18:900–4. doi: 10.1038/sj.eye.6701349. [DOI] [PubMed] [Google Scholar]
- 411.Hykin PG, Gregson RMC, Stevens JD, Hamilton PAM. Extracapsular Cataract Extraction in Proliferative Diabetic Retinopathy. Ophthalmology. 1993;100:394–9. doi: 10.1016/s0161-6420(93)31636-2. [DOI] [PubMed] [Google Scholar]
- 412.Chew EY, Benson WE, Remaley NA, Lindley AA, Burton TC, Csaky K, et al. Results After Lens Extraction in Patients With Diabetic Retinopathy: Early Treatment Diabetic Retinopathy Study Report Number 25. JAMA Ophthalmology. 1999;117:1600–6. doi: 10.1001/archopht.117.12.1600. [DOI] [PubMed] [Google Scholar]
- 413.Henricsson M, Heijl A, Janzon L. Diabetic retinopathy before and after cataract surgery. British Journal of Ophthalmology. 1996;80:789–93. doi: 10.1136/bjo.80.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Spalton D, Koch D. The constant evolution of cataract surgery. BMJ. 2000;321:1304. doi: 10.1136/bmj.321.7272.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Goldstein JL. How a jolt and a bolt in a dentist's chair revolutionized cataract surgery. Nature Medicine. 2004;10:1032–3. doi: 10.1038/nm1004-1032. [DOI] [PubMed] [Google Scholar]
- 416.Squirrell D, Bhola R, Bush J, Winder S, Talbot JF. A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. British Journal of Ophthalmology. 2002;86:565 LP–571. doi: 10.1136/bjo.86.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Romero-Aroca P, Fernández-Ballart J, Almena-Garcia M, Méndez-Marín I, Salvat-Serra M, Buil-Calvo JA. Nonproliferative diabetic retinopathy and macular edema progression after phacoemulsification: Prospective study. Journal of Cataract & Refractive Surgery. 2006;32:1438–44. doi: 10.1016/j.jcrs.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 418.Borrillo JL, Mittra RA, Dev S, Mieler WF, Pescinski S, Prasad A, et al. Retinopathy progression and visual outcomes after phacoemulsification in patients with diabetes mellitus. Transactions of the American Ophthalmological Society. 1999;97:435–49. [PMC free article] [PubMed] [Google Scholar]
- 419.Hong T, Mitchell P, de Loryn T, Rochtchina E, Cugati S, Wang JJ. Development and Progression of Diabetic Retinopathy 12 Months after Phacoemulsification Cataract Surgery. Ophthalmology. 2009;116:1510–4. doi: 10.1016/j.ophtha.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 420.Kato S, Fukada Y, Hori S, Tanaka Y, Oshika T. Influence of phacoemulsification and intraocular lens implantation on the course of diabetic retinopathy. Journal of Cataract & Refractive Surgery. 1999;25:788–93. doi: 10.1016/s0886-3350(99)00044-9. [DOI] [PubMed] [Google Scholar]
- 421.Mittra RA, Borrillo JL, Dev S, Mieler WF, Koenig SB. Retinopathy Progression and Visual Outcomes After Phacoemulsification in Patients With Diabetes Mellitus. JAMA Ophthalmology. 2000;118:912–7. [PubMed] [Google Scholar]
- 422.Krepler K, Biowski R, Schrey S, Jandrasits K, Wedrich A. Cataract surgery in patients with diabetic retinopathy: Visual outcome, progression of diabetic retinopathy, and incidence of diabetic macular oedema. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2002;240:735–8. doi: 10.1007/s00417-002-0530-7. [DOI] [PubMed] [Google Scholar]
- 423.Denniston AK, Chakravarthy U, Zhu H, Lee AY, Crabb DP, Tufail A, et al. The UK Diabetic Retinopathy Electronic Medical Record (UK DR EMR) Users Group, Report 2: real-world data for the impact of cataract surgery on diabetic macular oedema. British Journal of Ophthalmology. 2017;101:1673–8. doi: 10.1136/bjophthalmol-2016-309838. [DOI] [PubMed] [Google Scholar]
- 424.Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World Journal of Diabetes. 2015;6:92–108. doi: 10.4239/wjd.v6.i1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Jeng C-J, Hsieh Y-T, Yang C-M, Yang C-H, Lin C-L, Wang IJ. Development of diabetic retinopathy after cataract surgery. PloS one. 2018;13:e0202347–e0202347. doi: 10.1371/journal.pone.0202347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC. Risk Factors and Incidence of Macular Edema after Cataract Surgery: A Database Study of 81984 Eyes. Ophthalmology. 2016;123:316–23. doi: 10.1016/j.ophtha.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 427.Chen X-Y, Song W-J, Cai H-Y, Zhao L. Macular edema after cataract surgery in diabetic eyes evaluated by optical coherence tomography. International journal of ophthalmology. 2016;9:81–85. doi: 10.18240/ijo.2016.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Dowler JGF, Sehmi KS, Hykin PG, Hamilton AMP. The natural history of macular edema after cataract surgery in diabetes. Ophthalmology. 1999;106:663–8. doi: 10.1016/S0161-6420(99)90148-3. [DOI] [PubMed] [Google Scholar]
- 429.Sánchez-Thorin JC. The cornea in diabetes mellitus. International Ophthalmology Clinics. 1998;38:19–36. [PubMed] [Google Scholar]
- 430.Yanoff M, Fine BS, Berkow JW. Diabetic Lacy Vacuolation of Iris Pigment Epithelium: A Histopathologic Report. American Journal of Ophthalmology. 1970;69:201–10. doi: 10.1016/0002-9394(70)91280-8. [DOI] [PubMed] [Google Scholar]
- 431.Narendran N, Jaycock P, Johnston RL, Taylor H, Adams M, Tole DM, et al. The Cataract National Dataset electronic multicentre audit of 55 567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye. 2009;23:31–37. doi: 10.1038/sj.eye.6703049. [DOI] [PubMed] [Google Scholar]
- 432.Cetinkaya A, Yilmaz G, Akova YA. Photic retinopathy after cataract surgery in diabetic patients. Retina. 2006;26:1021–8. doi: 10.1097/01.iae.0000254895.78766.af. [DOI] [PubMed] [Google Scholar]
- 433.Ebihara Y, Kato S, Oshika T, Yoshizaki M, Sugita G. Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. Journal of Cataract & Refractive Surgery. 2006;32:1184–7. doi: 10.1016/j.jcrs.2006.01.100. [DOI] [PubMed] [Google Scholar]
- 434.Hayashi Y, Kato S, Fukushima H, Numaga J, Kaiya T, Tamaki Y, et al. Relationship between anterior capsule contraction and posterior capsule opacification after cataract surgery in patients with diabetes mellitus. Journal of Cataract & Refractive Surgery. 2004;30:1517–20. doi: 10.1016/j.jcrs.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 435.Praveen MR, Vasavada AR, Shah GD, Shah AR, Khamar BM, Dave KH. A prospective evaluation of posterior capsule opacification in eyes with diabetes mellitus: a case–control study. Eye. 2014;28:720–7. doi: 10.1038/eye.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Zaczek A, Zetterström C. Posterior capsule opacificationafter phacoemulsification in patients with diabetes mellitus. Journal of Cataract & Refractive Surgery. 1999;25:233–7. doi: 10.1016/s0886-3350(99)80132-1. [DOI] [PubMed] [Google Scholar]
- 437.Takamura Y, Tomomatsu T, Yokota S, Matsumura T, Takihara Y, Inatani M. Large capsulorhexis with implantation of a 7.0 mm optic intraocular lens during cataract surgery in patients with diabetes mellitus. Journal of Cataract & Refractive Surgery. 2014;40:1850–6. doi: 10.1016/j.jcrs.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 438.Kim EC, Hwang HS, Kim MS. Anterior Capsular Phimosis Occluding the Capsulorhexis Opening after Cataract Surgery in a Diabetic Patient with High Hemoglobin A1C. Seminars in Ophthalmology. 2013;28:68–71. doi: 10.3109/08820538.2012.754480. [DOI] [PubMed] [Google Scholar]
- 439.Kato S, Oshika T, Numaga J, Hayashi Y, Oshiro M, Yuguchi T, et al. Anterior capsular contraction after cataract surgery in eyes of diabetic patients. British Journal of Ophthalmology. 2001;85:21–23. doi: 10.1136/bjo.85.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Ylinen P, Laine I, Lindholm J-M, Tuuminen R. Poor glycemic control as a risk factor for pseudophakic cystoid macular edema in patients with diabetes. Journal of Cataract & Refractive Surgery. 2017;43:1376–82. doi: 10.1016/j.jcrs.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 441.Boscia F, Giancipoli E, D’Amico Ricci G, Pinna A. Management of macular oedema in diabetic patients undergoing cataract surgery. Current Opinion in Ophthalmology. 2017;28:23–28. doi: 10.1097/ICU.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 442.Raskauskas P, Walker J, Wing G, Fletcher D, Elsner A. Small Incision Cataract Surgery and Placement of Posterior Chamber Intraocular Lenses in Patients With Diabetic Retinopathy. Ophthalmic Surgery, Lasers Imaging Retina. 1999;30:6–9. [PubMed] [Google Scholar]
- 443.Bhandari S, Biechl AC, Nguyen V, Squirrell D, Mehta H, Barthelmes D, et al. Outcomes of cataract surgery in eyes with diabetic macular oedema: Data from the Fight Retinal Blindness! Registry. Clin Exp Ophthalmol. 2019. 10.1111/ceo.13707. [DOI] [PubMed]
- 444.Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, et al. A Randomized Clinical Trial of Intravitreal Bevacizumab versus Intravitreal Dexamethasone for Diabetic Macular Edema: The BEVORDEX Study. Ophthalmology. 2014;121:2473–81. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 445.Gillies M, Sutter F, Simpson J, Larsson J, Ali H, Zhu M. Intravitreal Triamcinolone for Refractory Diabetic Macular EdemaTwo-Year Results of a Double-Masked, Placebo-Controlled, Randomized Clinical Trial. Ophthalmology. 2006;113:1533–8. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 446.Fraser-Bell S, Lim LL, Campain A, Mehta H, Aroney C, Bryant J, et al. Bevacizumab or Dexamethasone Implants for DME: 2-year Results (The BEVORDEX Study) Ophthalmology. 2016;123:1399–401. doi: 10.1016/j.ophtha.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 447.Moshfeghi AA, Shapiro H, Lemmon LA, Gune S. Impact of Cataract Surgery during Treatment with Ranibizumab in Patients with Diabetic Macular Edema. Ophthalmology Retina. 2018;2:86–90. doi: 10.1016/j.oret.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 448.Cornish EE, Teo KYC, Gillies MC, Lim LL, McAllister I, Sanmugasundram S, et al. Five year outcomes of the Bevordex Study (a multicenter randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone). Investig Ophthalmol Vis Sci. 2018;59.
- 449.Dong N, Xu B, Wang B, Chu L, Tang X. Aqueous Cytokines as Predictors of Macular Edema in Patients with Diabetes following Uncomplicated Phacoemulsification Cataract Surgery. BioMed Research International. 2015;2015:1–8. doi: 10.1155/2015/126984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Hartnett ME, Tinkham N, Paynter L, Geisen P, Rosenberg P, Koch G, et al. Aqueous Vascular Endothelial Growth Factor as a Predictor of Macular Thickening Following Cataract Surgery in Patients With Diabetes Mellitus. American Journal of Ophthalmology. 2009;148:895–901.e891. doi: 10.1016/j.ajo.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Patel JI, Hykin PG, Cree IA. Diabetic cataract removal: postoperative progression of maculopathy—growth factor and clinical analysis. British Journal of Ophthalmology. 2006;90:697 LP–701. doi: 10.1136/bjo.2005.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Wilson CA, Berkowitz BA, Sato Y, Ando N, Handa JT, de Juan E., Jr Treatment With Intravitreal Steroid Reduces Blood-Retinal Barrier Breakdown due to Retinal Photocoagulation. JAMA Ophthalmology. 1992;110:1155–9. doi: 10.1001/archopht.1992.01080200135041. [DOI] [PubMed] [Google Scholar]
- 453.Ahmadabadi HF, Mohammadi M, Beheshtnejad H, Mirshahi A. Effect of intravitreal triamcinolone acetonide injection on central macular thickness in diabetic patients having phacoemulsification. Journal of Cataract & Refractive Surgery. 2010;36:917–22. doi: 10.1016/j.jcrs.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 454.Habib MS, Cannon PS, Steel DHW. The combination of intravitreal triamcinolone and phacoemulsification surgery in patients with diabeticfoveal oedema and cataract. BMC Ophthalmology. 2005;5:15. doi: 10.1186/1471-2415-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Kim S-Y, Yang J, Lee Y-C, Park Y-H. Effect of a single intraoperative sub-Tenon injection of triamcinolone acetonide on the progression of diabetic retinopathy and visual outcomes after cataract surgery. Journal of Cataract & Refractive Surgery. 2008;34:823–6. doi: 10.1016/j.jcrs.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 456.Lam DSC, Chan CKM, Mohamed S, Lai TYY, Lee VYW, Lai WW, et al. Phacoemulsification with intravitreal triamcinolone in patients with cataract and coexisting diabetic macular oedema: a 6-month prospective pilot study. Eye. 2005;19:885–90. doi: 10.1038/sj.eye.6701686. [DOI] [PubMed] [Google Scholar]
- 457.Lim LL, Morrison JL, Constantinou M, Rogers S, Sandhu SS, Wickremasinghe SS, et al. Diabetic Macular Edema at the time of Cataract Surgery trial: a prospective, randomized clinical trial of intravitreous bevacizumab versus triamcinolone in patients with diabetic macular oedema at the time of cataract surgery – preliminary 6 month results. Clinical & Experimental Ophthalmology. 2016;44:233–42. doi: 10.1111/ceo.12720. [DOI] [PubMed] [Google Scholar]
- 458.Takata C, Messias A, Folgosa MS, Lucena LR, Lucena DR, Scott IU, et al. Intravitreal injection versus subtenon infusion of triamcinolone acetonide during cataract surgery in patients with refractory diabetic macular edema. Retina. 2010;30:564–9. doi: 10.1097/IAE.0b013e3181c969b4. [DOI] [PubMed] [Google Scholar]
- 459.Wielders LHP, Schouten JSAG, Winkens B, van den Biggelaar FJHM, Veldhuizen CA, Murta JCN, et al. Randomized controlled European multicenter trial on the prevention of cystoid macular edema after cataract surgery in diabetics: ESCRS PREMED Study Report 2. Journal of Cataract & Refractive Surgery. 2018;44:836–47. doi: 10.1016/j.jcrs.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 460.Athanasiadis Y, Nithyanandrajah GAL, Kumar B, Sharma A. Reversal of steroid induced raised intraocular pressure following removal of subconjunctival triamcinolone for cataract surgery. Contact Lens and Anterior Eye. 2009;32:143–4. doi: 10.1016/j.clae.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 461.Kalina PH, Erie JC, Rosenbaum L. Biochemical Quantification of Triamcinolone in Subconjunctival Depots. JAMA Ophthalmology. 1995;113:867–9. doi: 10.1001/archopht.1995.01100070041022. [DOI] [PubMed] [Google Scholar]
- 462.Agarwal A, Gupta V, Ram J, Gupta A. Dexamethasone Intravitreal Implant During Phacoemulsification. Ophthalmology. 2013;120:211–211.e215. doi: 10.1016/j.ophtha.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 463.Panozzo GA, Gusson E, Panozzo G, Dalla Mura G. Dexamethasone Intravitreal Implant at the Time of Cataract Surgery in Eyes with Diabetic Macular Edema. European Journal of Ophthalmology. 2016;27:433–7. doi: 10.5301/ejo.5000920. [DOI] [PubMed] [Google Scholar]
- 464.Sze AM, Luk FO, Yip TP, Lee GK, Chan CK. Use of Intravitreal Dexamethasone Implant in Patients with Cataract and Macular Edema Undergoing Phacoemulsification. European Journal of Ophthalmology. 2014;25:168–72. doi: 10.5301/ejo.5000523. [DOI] [PubMed] [Google Scholar]
- 465.Yang Y, Bailey C, Holz FG, Eter N, Weber M, Baker C, et al. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye. 2015;29:1173–80. doi: 10.1038/eye.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Akinci A, Batman C, Ozkilic E, Altinsoy ALI. Phacoemulsification with intravitreal bevacizumab injection in diabetic patients with macular edema and cataract. Retina. 2009;29:1432–5. doi: 10.1097/IAE.0b013e3181b77422. [DOI] [PubMed] [Google Scholar]
- 467.Chae JB, Joe SG, Yang SJ, Lee JY, Sung KR, Kim JY, et al. Effect of combined cataract surgery and ranibizumab injection in postoperative macular edema in nonproliferative diabetic retinopathy. Retina. 2014;34:149–56. doi: 10.1097/IAE.0b013e3182979b9e. [DOI] [PubMed] [Google Scholar]
- 468.Chen C-H, Liu Y-C, Wu P-C. The Combination of Intravitreal Bevacizumab and Phacoemulsification Surgery in Patients with Cataract and Coexisting Diabetic Macular Edema. Journal of Ocular Pharmacology and Therapeutics. 2009;25:83–90. doi: 10.1089/jop.2008.0068. [DOI] [PubMed] [Google Scholar]
- 469.Fard MA, Abyane AY, Malihi M. Prophylactic Intravitreal Bevacizumab for Diabetic Macular Edema (thickening) after Cataract Surgery: Prospective Randomized Study. European Journal of Ophthalmology. 2010;21:276–81. doi: 10.5301/EJO.2010.1405. [DOI] [PubMed] [Google Scholar]
- 470.Lanzagorta-Aresti A, Palacios-Pozo E, Menezo Rozalen JL, Navea-Tejerina A. Prevention of vision loss after cataract surgery in diabetic macular edema with intravitreal bevacizumab: a pilot study. Retina. 2009;29:530–5. doi: 10.1097/IAE.0b013e31819c6302. [DOI] [PubMed] [Google Scholar]
- 471.Takamura Y, Kubo E, Akagi Y. Analysis of the Effect of Intravitreal Bevacizumab Injection on Diabetic Macular Edema after Cataract Surgery. Ophthalmology. 2009;116:1151–7. doi: 10.1016/j.ophtha.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 472.Udaondo P, Garcia-Pous M, Garcia-Delpech S, Salom D, Diaz-Llopis M. Prophylaxis of macular edema with intravitreal ranibizumab in patients with diabetic retinopathy after cataract surgery: a pilot study. Journal of ophthalmology. 2011;2011:159436. doi: 10.1155/2011/159436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Cheema RA, Al-Mubarak MM, Amin YM, Cheema MA. Role of combined cataract surgery and intravitreal bevacizumab injection in preventing progression of diabetic retinopathy: Prospective randomized study. Journal of Cataract & Refractive Surgery. 2009;35:18–25. doi: 10.1016/j.jcrs.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 474.Salehi A, Beni AN, Razmjoo H, Beni ZN. Phacoemulcification with Intravitreal Bevacizumab Injection in Patients with Cataract and Coexisting Diabetic Retinopathy: Prospective Randomized Study. Journal of Ocular Pharmacology and Therapeutics. 2011;28:212–8. doi: 10.1089/jop.2011.0069. [DOI] [PubMed] [Google Scholar]
- 475.Kandasamy R, Constantinou M, Rogers SL, Sandhu SS, Wickremasinghe S, Al-Qureshi S, et al. Prospective randomised clinical trial of intravitreal bevacizumab versus triamcinolone in eyes with diabetic macular oedema undergoing cataract surgery: 6-month results. Br J Ophthalmol. 2019;103:1753–8. [DOI] [PubMed]
- 476.Miyake K, Ibaraki N. Prostaglandins and Cystoid Macular Edema. Survey of Ophthalmology. 2002;47:S203–S218. doi: 10.1016/s0039-6257(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 477.Wolf EJ, Braunstein A, Shih C, Braunstein RE. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. Journal of Cataract & Refractive Surgery. 2007;33:1546–9. doi: 10.1016/j.jcrs.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 478.Elsawy MF, Badawi N, Khairy HA. Prophylactic postoperative ketorolac improves outcomes in diabetic patients assigned for cataract surgery. Clinical Ophthalmology (Auckland, NZ) 2013;7:1245–9. doi: 10.2147/OPTH.S39188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Endo N, Kato S, Haruyama K, Shoji M, Kitano S. Efficacy of bromfenac sodium ophthalmic solution in preventing cystoid macular oedema after cataract surgery in patients with diabetes. Acta Ophthalmologica. 2010;88:896–900. doi: 10.1111/j.1755-3768.2009.01582.x. [DOI] [PubMed] [Google Scholar]
- 480.Heier JS, Topping TM, Baumann W, Dirks MS, Chern S. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000;107:2034–8. doi: 10.1016/s0161-6420(00)00365-1. [DOI] [PubMed] [Google Scholar]
- 481.Miyake K, Masuda K, Shirato S, Oshika T, Eguchi K, Hoshi H, et al. Comparison of Diclofenac and Fluorometholone in Preventing Cystoid Macular Edema After Small Incision Cataract Surgery: A Multicentered Prospective Trial. Japanese Journal of Ophthalmology. 2000;44:58–67. doi: 10.1016/s0021-5155(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 482.Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery: The results of a meta-analysis. Ophthalmology. 1998;105:397–405. doi: 10.1016/S0161-6420(98)93018-4. [DOI] [PubMed] [Google Scholar]
- 483.Wielders LHP, Lambermont VA, Schouten JSAG, van den Biggelaar FJHM, Worthy G, Simons RWP, et al. Prevention of Cystoid Macular Edema After Cataract Surgery in Nondiabetic and Diabetic Patients: A Systematic Review and Meta-Analysis. American Journal of Ophthalmology. 2015;160:968–.e933. doi: 10.1016/j.ajo.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 484.Singh RP, Lehmann R, Martel J, Jong K, Pollack A, Tsorbatzoglou A, et al. Nepafenac 0.3% after Cataract Surgery in Patients with Diabetic Retinopathy: Results of 2 Randomized Phase 3 Studies. Ophthalmology. 2017;124:776–85. doi: 10.1016/j.ophtha.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 485.Fakhraie G, Mirghorbani M, Katz LJ, Mollazadeh A, Vahedian Z, Zarei R, et al. Cystoid macular edema with prostaglandin analogue use after uneventful cataract surgery in glaucoma patients. Journal of Cataract & Refractive Surgery. 2019;45:1436–45. doi: 10.1016/j.jcrs.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 486.Hernstadt DJ, Husain R. Effect of prostaglandin analogue use on the development of cystoid macular edema after phacoemulsification using STROBE statement methodology. Journal of Cataract & Refractive Surgery. 2017;43:564–9. doi: 10.1016/j.jcrs.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 487.Wendel C, Zakrzewski H, Carleton B, Etminan M, Mikelberg FS. Association of postoperative topical prostaglandin analog or beta-blocker use and incidence of pseudophakic cystoid macular edema. J Glaucoma. 2018;27:402–6. doi: 10.1097/IJG.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 488.Bellocq D, Pierre-Kahn V, Matonti F, Burillon C, Voirin N, Dot C, et al. Effectiveness and safety of dexamethasone implants for postsurgical macular oedema including Irvine–Gass syndrome: the EPISODIC-2 study. British Journal of Ophthalmology. 2017;101:333 LP–341. doi: 10.1136/bjophthalmol-2016-308544. [DOI] [PubMed] [Google Scholar]
- 489.Bressler SB, Qin H, Melia M, Bressler NM, Beck RW, Chan CK, et al. Exploratory Analysis of the Effect of Intravitreal Ranibizumab or Triamcinolone on Worsening of Diabetic Retinopathy in a Randomized Clinical Trial. JAMA Ophthalmology. 2013;131:1033–40. doi: 10.1001/jamaophthalmol.2013.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Diabetic Retinopathy Clinical Research Network A Randomized Trial Comparing Intravitreal Triamcinolone Acetonide and Focal/Grid Photocoagulation for Diabetic Macular Edema. Ophthalmology. 2008;115:1447–1459.e1410. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Mehta H, Lim LL, Nguyen V, Qatarneh D, Wickremasinghe SS, Hodgson LAB, et al. Development of New Proliferative Diabetic Retinopathy in the BEVORDEX Trial. Ophthalmology Retina. 2019;3:286–7. doi: 10.1016/j.oret.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 492.Bonnin S, Dupas B, Lavia C, Erginay A, Dhundass M, Couturier A, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. 2019;39:426–34. doi: 10.1097/IAE.0000000000002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Couturier A, Rey P-A, Erginay A, Lavia C, Bonnin S, Dupas B, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with Anti-Vascular Endothelial Growth Factor. Ophthalmology. 2019;126:1685–94. doi: 10.1016/j.ophtha.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 494.Hirano T, Kakihara S, Toriyama Y, Nittala MG, Murata T, Sadda S. Wide-field en face swept-source optical coherence tomography angiography using extended field imaging in diabetic retinopathy. British Journal of Ophthalmology. 2018;102:1199 LP–1203. doi: 10.1136/bjophthalmol-2017-311358. [DOI] [PubMed] [Google Scholar]
- 495.Salz DA, de Carlo TE, Adhi M, Moult E, Choi W, Baumal CR, et al. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared With Fluorescein Angiography and Normal Eyes. JAMA Ophthalmology. 2016;134:644–50. doi: 10.1001/jamaophthalmol.2016.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Sawada O, Ichiyama Y, Obata S, Ito Y, Kakinoki M, Sawada T, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology. 2018;256:1275–80. doi: 10.1007/s00417-018-3992-y. [DOI] [PubMed] [Google Scholar]
- 497.Noah B, Keller MS, Mosadeghi S, Stein L, Johl S, Delshad S, et al. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digit Med. 2018;1:20172. doi: 10.1038/s41746-017-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci Transl Med. 2015;7:283rv283. doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Whitehead L, Seaton P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J Med Internet Res. 2016;18:e97. doi: 10.2196/jmir.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369–78. doi: 10.1111/ijcp.12345. [DOI] [PubMed] [Google Scholar]
- 501.Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003:CD001117. [DOI] [PubMed]
- 502.Kaptein AA, Fischer MJ, Scharloo M. Self-management in patients with COPD: theoretical context, content, outcomes, and integration into clinical care. Int J Chron Obstruct Pulmon Dis. 2014;9:907–17. doi: 10.2147/COPD.S49622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Vas A, Devi ES, Vidyasagar S, Acharya R, Rau NR, George A, et al. Effectiveness of self-management programmes in diabetes management: a systematic review. Int J Nurs Pract. 2017;23. [DOI] [PubMed]
- 504.Amsler M. L'Examen qualitatif de la fonction maculaire. Ophthalmologica. 1947;114:248–61. [Google Scholar]
- 505.Kalinowska A, Nowomiejska K, Brzozowska A, Maciejewski R, Rejdak R. Metamorphopsia Score and Central Visual Field Outcomes in Diabetic Cystoid Macular Edema. Biomed Res Int. 2018;2018:4954532. doi: 10.1155/2018/4954532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Associations between metamorphopsia and foveal microstructure in patients with epiretinal membrane. Invest Ophthalmol Vis Sci. 2012;53:6770–5. doi: 10.1167/iovs.12-9683. [DOI] [PubMed] [Google Scholar]
- 507.Xu K, Gupta V, Bae S, Sharma S. Metamorphopsia and vision-related quality of life among patients with age-related macular degeneration. Can J Ophthalmol. 2018;53:168–72. doi: 10.1016/j.jcjo.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 508.Faes L, Bodmer NS, Bachmann LM, Thiel MA, Schmid MK. Diagnostic accuracy of the Amsler grid and the preferential hyperacuity perimetry in the screening of patients with age-related macular degeneration: systematic review and meta-analysis. Eye (Lond) 2014;28:788–96. doi: 10.1038/eye.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Loewenstein A, Malach R, Goldstein M, Leibovitch I, Barak A, Baruch E, et al. Replacing the Amsler grid: a new method for monitoring patients with age-related macular degeneration. Ophthalmology. 2003;110:966–70. doi: 10.1016/S0161-6420(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 510.Group AHSR. Chew EY, Clemons TE, Bressler SB, Elman MJ, Danis RP, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535–44. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Kaiser PK, Wang YZ, He YG, Weisberger A, Wolf S, Smith CH Feasibility of a novel remote daily monitoring system for age-related macular degeneration using mobile handheld devices: results of a pilot study. Retina. 2013;33:1863–70. [DOI] [PubMed]
- 512.Schmid MK, Faes L, Bachmann LM, Thiel MA. Accuracy of a Self-monitoring Test for Identification and Monitoring of Age-related Macular Degeneration: A Diagnostic Case-control Study. Open Ophthalmol J. 2018;12:19–28. doi: 10.2174/1874364101812010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Schmid MK, Thiel MA, Lienhard K, Schlingemann RO, Faes L, Bachmann LM. Reliability and diagnostic performance of a novel mobile app for hyperacuity self-monitoring in patients with age-related macular degeneration. Eye (Lond) 2019;33:1584–9. doi: 10.1038/s41433-019-0455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.WHO. Ageing and health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health 2018.
- 515.Stewart C Number of ophthalmologists per 10,000 population in Europe in 2017. https://www.statista.com/statistics/711061/number-of-ophthalmologists-in-european-union-eu/ 2019.
- 516.The Royal College of Ophthalmologists. Increasing demand on hospital eye services risks patients losing vision. Available from:https://www.rcophth.ac.uk/2016/03/increasing-demand-on-hospital-eye-services-risks-patients-losing-vision/ 2016.
- 517.Kern C, Fu DJ, Kortuem K, Huemer J, Barker D, Davis A, et al. Implementation of a cloud-based referral platform in ophthalmology: making telemedicine services a reality in eye care. Br J Ophthalmol. 2020;104:312–7. doi: 10.1136/bjophthalmol-2019-314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Sim DA, Mitry D, Alexander P, Mapani A, Goverdhan S, Aslam T, et al. The Evolution of Teleophthalmology Programs in the United Kingdom: Beyond Diabetic Retinopathy Screening. J Diabetes Sci Technol. 2016;10:308–17. doi: 10.1177/1932296816629983. [DOI] [PMC free article] [PubMed] [Google Scholar]