Weavers and Martin revisit Metchnikoff’s classic observations of inflammatory cell behavior in damaged tissues and update them with the latest cell biology studies.
Abstract
Tissue damage triggers a rapid and robust inflammatory response in order to clear and repair a wound. Remarkably, many of the cell biology features that underlie the ability of leukocytes to home in to sites of injury and to fight infection—most of which are topics of intensive current research—were originally observed in various weird and wonderful translucent organisms over a century ago by Elie Metchnikoff, the “father of innate immunity,” who is credited with discovering phagocytes in 1882. In this review, we use Metchnikoff’s seminal lectures as a starting point to discuss the tremendous variety of cell biology features that underpin the function of these multitasking immune cells. Some of these are shared by other cell types (including aspects of motility, membrane trafficking, cell division, and death), but others are more unique features of innate immune cells, enabling them to fulfill their specialized functions, such as encapsulation of invading pathogens, cell–cell fusion in response to foreign bodies, and their self-sacrifice as occurs during NETosis.
Introduction
To survive physical damage or other more subtle insults, our bodies have the remarkable capacity to repair and replace damaged tissues (Eming et al., 2014). Key to this, and superimposed upon the repair machinery, is a rapidly activated inflammatory response that has evolved to combat potential microbial invaders as well as our body’s own aberrant cells. A range of different traumas—from a scratch or surgical wound (with or without infection) through to UV damage from sunburn and even the initiation and progression stages of cancer—will all trigger a variation on a theme of this inflammatory response (Fig. 1 and Box 1).
Figure 1.
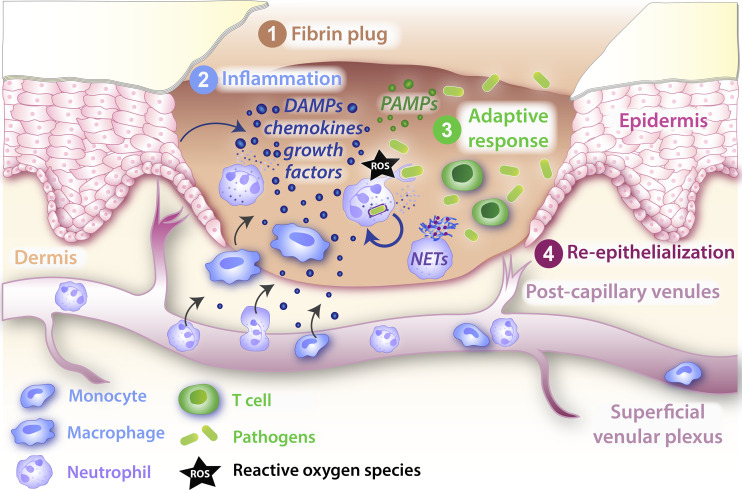
Schematic of a typical wound inflammatory response. Soon after a platelet plug has temporarily sealed a wound (1), the wound inflammatory response kicks in, with innate immune cells drawn from local resident populations and by recruitment from nearby vessels (2) in order to counter potential infections at this barrier breach. The recruited innate immune cells deploy a variety of antimicrobial weapons to kill or inactivate invading pathogens, including production of ROS and the release of NETs. There is an accompanying but less well-characterized adaptive immune cell response (3), and as these two defense mechanisms protect the exposed wound, tissue repair mechanisms, including resurfacing with a new epidermal layer, proceed in order to restore barrier integrity (4). DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern.
Box 1. Leukocyte subtypes and developmental origin
In this review, we focus mostly on two of Metchnikoff’s favorite cells, neutrophils and macrophages; however, for context, we provide a brief introduction to the larger family of inflammatory cell lineages, all of which derive from multipotential hematopoietic stem cells in the bone marrow, which in turn give rise to the common myeloid and common lymphoid progenitor lineages.
Mast cells and myeloblasts originate from common myeloid progenitors; myeloblasts in turn give rise to the three granulocyte lineages (basophils, eosinophils, and neutrophils) as well as monocytes, the latter of which are termed macrophages after they leave the vasculature. Collectively, these cells are the “innate immune cells.”
From common lymphoid progenitors come all of the adaptive immune cell lineages, including B and T cells.
At any site of tissue damage or infection, innate immune cells are recruited, first neutrophils and then monocyte/macrophages, from local tissue resident cells and by extravasation from vessels. Eosinophils are recruited also, but in significantly lower numbers. It is possible for T cells to be recruited in significant numbers, but adaptive immune cells generally appear to become significant players only when inflammation persists and becomes chronic (see Box 2).
In the case of a small local incisional wound involving damage to tissues that is deeper than the epidermis and transects subcutaneous blood vessels (Fig. 1), the defect will initially be plugged with a fibrin clot containing platelets and neutrophils from the spilled blood to make a transient protective scab (Martin, 1997). Various “alarm” signals (damage-associated molecular patterns; Niethammer, 2016) will then be released from damaged or necrotic cells; these signals, together with growth factor attractants from degranulating platelets, lead to the recruitment of leukocytes, both macrophages from the local environs and then later neutrophils and monocytes from nearby vessels. Immune cell recruitment subsequently becomes self-amplifying because these cells release chemokine attractants, which draw in more immune cells (Eming et al., 2007). If the wound becomes at all chronic or infected, then some degree of an adaptive immune response may also be triggered (see Box 2). After the tissue insult has ceased or been destroyed, then mechanisms for resolution of the inflammatory response are pivotal to prevent unnecessary host tissue damage caused by chronic inflammation.
Box 2. Cross-talk with the adaptive immune system
Initially, the study of innate and adaptive immune systems clashed amid rivalry over which was the pivotal system for protecting the body from infection. The award of the Nobel Prize in Medicine or Physiology jointly to Paul Ehrlich and Metchnikoff in 1908 was a clear indication that the general immunology community had realized that the two “immunities” were equally important.
Adaptive immune cells are much less often investigated than innate immune cells in a wound inflammatory context. However, it is clear that T reg cells are recruited to wounds, and their knockdown can significantly impair skin healing (Nosbaum et al., 2016). A sentinel subpopulation of γδ T cells, termed dendritic epidermal T cells (DETCs), reside within the epidermal layers of mammalian skin, and they rapidly switch morphology from sensory dendritic to an active, rounded phenotype in the vicinity of a wound. These activated DETCs play several roles in repair, and in mice, where this lineage is deleted or where it cannot become activated, wound repair is severely compromised (Havran and Jameson, 2010). DETCs directly influence wound edge keratinocyte survival and proliferation via Keratinocyte Growth Factor (KGF) and IGF-1 signals (Jameson et al., 2002; Ramirez et al., 2015); however, they also have significant impact on the wound inflammatory response, possibly via release of CCL3 and other inflammatory cell activators, since in their absence macrophage (but not neutrophil) recruitment to the wound is severely dampened (Ramirez et al., 2015). In human skin, γδ T resident cells appear to play a role similar to that of murine DETCs upon tissue damage; but in a chronic wound scenario, they and the later recruited T cell populations appear to lose their capacity for activation (Toulon et al., 2009), and this may indirectly impact their regulation of innate immune cell recruitment and behaviors at the wound site. In the example of granuloma formation in response to Tuberculosis (TB), significant numbers of T cells are recruited and differentiate into T helper type 1 effector cells that secrete IFN-γ and TNF-α to drive enhanced microbicidal activity by macrophages (Pagán and Ramakrishnan, 2018).
While the main functions of the inflammatory response are to destroy invaders (as well as aberrant cells) and to clear away cell and matrix debris, it has acquired other roles that mean it also orchestrates several responses by other cell lineages during the repair process, including wound angiogenesis and deposition of a collagen scar by wound fibroblasts (Gurevich et al., 2018; Eming et al., 2017). Not surprisingly, these inflammatory cells can also be subverted by some infective agents and by cancer cells to work for the “invader” in various ways, rather than being solely beneficial to the host (Pagán and Ramakrishnan, 2018; Swierczak and Pollard, 2020).
Elie Metchnikoff (1845–1916) is often described as the father of innate immunity and inflammation; he studied all aspects of the inflammatory response, often by live imaging studies, in a variety of organisms from Daphnia through to amphibia and some higher vertebrates. In this review, we revisit some of Metchnikoff’s original anecdotal observations described in his lectures of the late 1800s (translated in Metchnikoff, 1968) and update them with new cell and molecular insights derived from in vitro studies and in vivo observations (see Box 3); much of our new knowledge comes from translucent model organisms—no longer starfish and frogs as in Metchnikoff’s day (Fig. 2, A and B), but now the genetically tractable, early developmental stages of Drosophila and zebrafish (Fig. 2, C and D). We outline not only how the cells of the inflammatory response share much of their cell biology with other cell lineages, including aspects of motility, phagocytosis, trafficking, and signaling, but also how various leukocytic cell types possess some more unusual features that equip them for their specialized roles (e.g., neutrophil extracellular traps [NETs], encapsulation, and extravasation; Fig. 3). Given that too little or too much inflammation can be the root cause of many human pathologies, our aim here is to highlight those cell biology aspects of the inflammatory response that are potential targets for therapeutic modulation to make it better at killing tissue enemies and less harmful to host tissues.
Box 3. A revolution in imaging technologies has transformed our understanding of leukocyte cell biology
Recent technological advances have heralded a new era in state-of-the-art high-resolution 3D and 4D imaging, which has enabled many of these “modern updates” on Metchnikoff's observations. Confocal microscopy is now routine in most research laboratories, allowing living tissues to be imaged with high spatio-temporal resolution to discern molecular and cellular features in exquisite detail. Multiphoton microscopy uses even longer wavelength photons (which are lower in energy and penetrate more deeply), enabling deeper imaging of a sample while creating less tissue damage. More recently, light-sheet fluorescence microscopy has revolutionized in vivo biological imaging by using planar illumination strategies to further minimize phototoxicity while enabling rapid 3D imaging over longer time periods and at significant depth. These technological leaps, together with advances in cellular labeling approaches, mean that while in vivo imaging remains easiest in translucent laboratory organisms (such as Drosophila and zebrafish), “intravital microscopy” now enables dynamic cell behaviors to be visualized also in the normally opaque body tissues of more traditional mammalian models (such as the mouse). Moreover, electron microscopy methods can these days be integrated in a correlative way with live confocal (light) microscopy in the same sample to capture dynamic cellular processes (e.g., leukocyte diapedesis) at the ultrastructural level. There has also been an important move from qualitative to quantitative analysis of imaging data, and the increasing application of mathematical/computational modeling to study (and simulate) cell dynamics (Liepe et al., 2012) is beginning to transform our understanding of leukocyte behavior (see main text).
Figure 2.
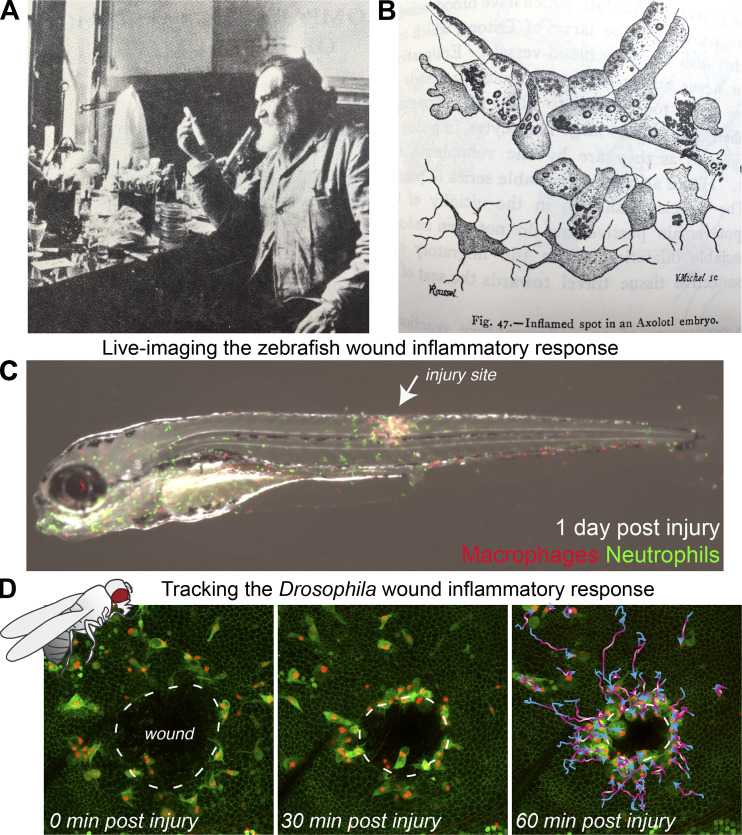
The “father of natural immunity,” Elie Metchnikoff made remarkable observations of the wound inflammatory response. (A and B) As early as 1892, Metchnikoff (A) used translucent organisms to observe the inflammatory response to damage or infection, as in B, where an axolotl fin was injured with a needle coated in carmine and migratory cells were seen to accumulate around the injured spot, “englobing” the colored granules and the debris of the dying cells. (C and D) In current research, translucent and genetically tractable models such as the zebrafish (C) and fruit fly Drosophila (D) are now adding further mechanistic insights into these inflammatory processes. (C) 3 d after fertilization, a translucent transgenic zebrafish larva with fluorescently tagged neutrophils (green) and macrophages (red) was subjected to a needle wound to the flank, enabling live imaging of the wound inflammatory response. (D) A series of time-lapse images from a wounded Drosophila pupal wing enables high-resolution spatio-temporal tracking of innate immune cell behavior. Images in A and B are adapted from Metchnikoff (1921) and Metchnikoff (1968), respectively. Images in C and D are courtesy of David Gurevich and Helen Weavers, respectively.
Figure 3.
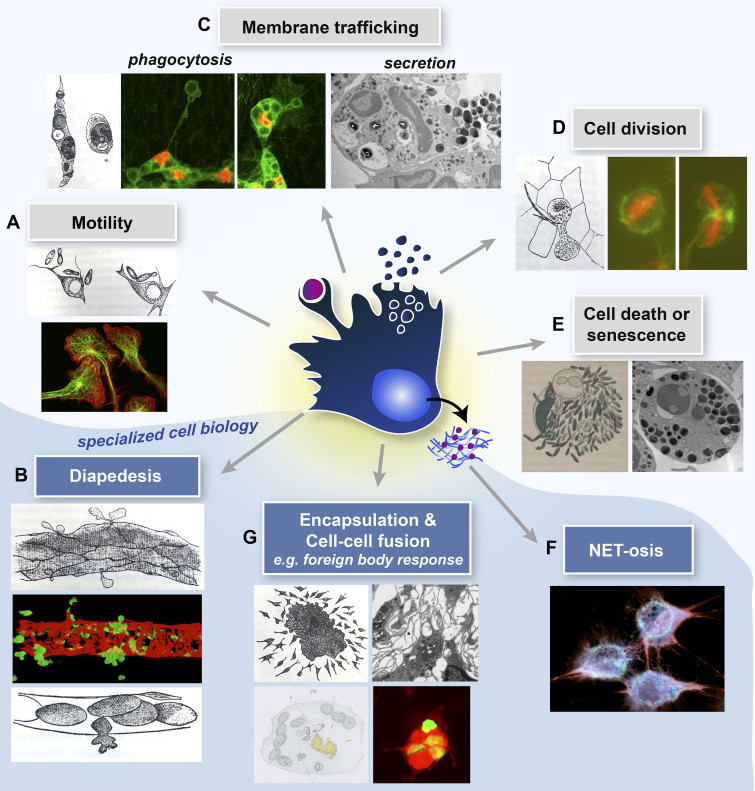
Innate immune cells exhibit both shared (with non-immune lineages) and specialized cell biology. A central schematized innate immune cell exhibiting both shared (upper panels) and specialized (lower, blue shaded zone) immune cell biology. Here, Metchnikoff’s drawings (Metchnikoff, 1893) are partnered against current light microscopic and TEM images of several aspects of cell biology that we discuss in this review article. (A) Modern images depict migrating macrophage cells, immunostained for tubulin (green) and actin (red; courtesy of Anne Ridley). (B) GFP neutrophils extravasating through the pericyte layer (red) of murine venules (courtesy of Sussan Nourshargh). (C) Phagocytosing Drosophila hemocytes with cytoplasm (green) and nuclei (red) labeled (courtesy of H. Weavers) and TEM image of a secreting neutrophil (courtesy of Natalia Hajdamowicz and Chris Hill). (D) Dividing Drosophila hemocytes with the actin (green) and tubulin (red) cytoskeleton labeled (courtesy of H. Weavers). (E) TEM of an apoptosing neutrophil (courtesy of Natalia Hajdamowicz and Chris Hill). (F) NETosing neutrophils extruding DNA/chromatin (red; courtesy of Borko Amulic). (G) TEM of macrophage aggregation (courtesy of Lali Ramakrishnan) and fusing zebrafish macrophages to give a foreign body giant cell with several GFP-tagged (green) nuclei in a common cytoplasm (red; courtesy of David Gurevich).
Leukocyte cell motility
Innate immune cells are motile from the outset. Their first migrations are to disperse themselves throughout the various embryonic tissues at developmental stages (Ginhoux and Guilliams, 2016; Wood and Martin, 2017). Subsequently, they can remain relatively stationary or patrol either locally or by traveling in the vasculature (Auffray et al., 2007). Innate immune cells tend only to migrate in a directed way if an epithelial barrier layer has been breached or an infection has arisen, and these inflammatory migratory episodes need to be very tightly regulated. Neutrophils and macrophages migrate within tissues using rather different strategies, amoeboid-like for neutrophils and mesenchymal with adhesion-dependent tethering and protrusion contraction for macrophages, making neutrophils somewhat faster (up to 6 µm/min), with macrophages lagging behind at speeds of seldom up to 2 µm/min in studies of translucent zebrafish larvae (Barros-Becker et al., 2017).
Metchnikoff and his colleagues realized that leukocytes were motile and described how they “put out protoplasmic processes to move from place to place” (Fig. 3 A). In fact, he and others undertook many experiments demonstrating how leukocytes had “chemotactic properties” in response to infection and tissue damage, while realizing that they did not yet have the tools to uncover what the attractant signals were. Now we know that bacterial components (including Lipopolysaccharide [LPS] and formylated peptides) as well as early tissue damage-associated molecular patterns (ATP, H2O2, and HMGB1) and later chemokines (interleukin [IL]-1β, IL-17, IL-8, and TNF-α) are just some of the attractants for inflammatory cells (Eming et al., 2007, 2014).
We also know much about how the responding cell’s cytoskeleton is regulated in order to move toward these cues; just as for other motile cells, Rho family small GTPases regulate their actin cytoskeleton (Jones et al., 1998). If Rac is inhibited in Drosophila macrophages, these cells fail to make proper lamellae and cannot efficiently migrate to a wound. Conversely, if Rho is inhibited, although macrophage-directed migration is unperturbed, the immune cells cannot contract to detach their trailing uropod and thus remain tethered to the spot (Stramer et al., 2005). Spatial activation of these small GTPase switches enables directed migration, for example, as highlighted in zebrafish larval experiments in which light activation of a genetically encoded Rac can artificially turn a neutrophil in vivo (Yoo et al., 2010).
Downstream effectors of Rho family signaling switches are critical in leukocyte migratory polarity; indeed, WASp, which coordinates actin polymerization via the Arp2/3 complex downstream of Cdc42, is pivotal for neutrophil-directed migration (Jones et al., 2013), and mutations in this gene lead to the clinical syndrome Wiskott Aldrich syndrome, characterized by chronic infections (Candotti, 2018). Most recently, studies in migrating Drosophila macrophages suggest that persistent migration toward a target is largely driven by flow of the actin network behind the leading edge of the cell (Yolland et al., 2019). However, actin is not the only cytoskeletal player involved in guiding leukocyte migration; disruptions in microtubule dynamics in innate immune cells of flies, zebrafish, and mammals all lead to a less directed, more “drunken walk” migration to targets (Redd et al., 2006; Xu et al., 2005; Stramer et al., 2010).
For no other migratory cell—barring perhaps germ cells or the axonal growth cones of neurons in a developing embryo—is the process of target navigation as complex as for a leukocyte en route to a site of inflammation. As will be discussed, before, during, and after extravasation they are exposed to multiple different chemoattractants with overlapping gradients in space and time. There is some evidence that there might be a hierarchy of signal integration and prioritization (Foxman et al., 1997, 1999; McDonald et al., 2010; Moreira et al., 2010), which may in turn be facilitated by receptor desensitization (see trafficking section below). There are likely to be other reinforcing strategies that enable a leukocyte to persist in its path toward a particular cue and to not be distracted; for example, ATP release by neutrophils themselves appears to amplify attractant signals from other sources (Chen et al., 2006), while controlled calcium influx appears to reinforce what is the leading edge in neutrophils migrating to a wound in a zebrafish larva (Beerman et al., 2015).
In most tissues, there is a resident population of some innate immune cell lineages, but in tissues served by vessels, local leukocytes become hugely augmented by cells drawn in from the circulation; these cells must first extravasate across the vessel wall (Fig. 3 B). Metchnikoff (1968) described how he and other researchers first observed in tadpoles and in the frog mesentery how leukocytes are drawn to the vessel periphery and then take active “passage” through the vessel wall at sites of inflammation. Using various experimental strategies, including some crude drug blocking experiments, they shrewdly inferred that this process, which they called “diapedesis,” also involved active “assistance” by the vessel wall cells.
Now, modern murine genetic approaches, combined with intravital microscopy and complemented by in vitro flow studies, have revealed many of the key steps that underpin diapedesis (Ley et al., 2007). A cascade of events beginning with tethering of leukocytes to the luminal aspect of the vessel wall is followed by firmer, integrin-mediated adhesion to endothelial cells and leukocytes crawling to find an exit point. Cells then migrate either through (transcellular) or between (paracellular) adjacent endothelial cells in the vessel wall, with breaching of junctions being the more common route and generally occurring at sites where three endothelial cells meet (Burns et al., 1997). Intravital microscopy has revealed that after traversing through the endothelial cell layer, leukocytes probe for regions in the basement membrane with lower ECM density corresponding to gaps in the pericyte sheath (Proebstl et al., 2012; Girbl et al., 2018). Adhesion and junction-related molecules expressed by both immune cells and endothelial cells are (not surprisingly) rate limiting, as revealed by mice deficient in the junctional adhesion molecules PECAM-1 (Thompson et al., 2001), JAM-A (Woodfin et al., 2007, 2009), and JAM-C (Woodfin et al., 2011), all of which exhibit severe deficiencies in diapedesis. As predicted by Metchnikoff and colleagues, the vessel wall cell layers themselves are active participants in diapedesis. Rho signaling is again pivotal for enabling endothelial cells to both pull apart from one another as immune cells squeeze through (Cerutti and Ridley, 2017) and for contraction of the transmigration pore at the end of diapedesis (Heemskerk et al., 2016). A recent study in Drosophila pupae identified a period when fly macrophages actively extravasate from circulating hemolymph through wing veins toward a laser wound, thus opening up this genetically tractable model as a tool for performing genome-wide screens to reveal more key players in this process (Thuma et al., 2018).
Metchnikoff was somewhat noncommittal about whether or not leukocytes were able to actively leave a site of inflammation after their purpose there was over, and still the mechanisms leading to inflammatory cell resolution—even whether it is an active process—are somewhat controversial. A large number of neutrophils drawn to a site of inflammation will undergo apoptosis and be phagocytosed by macrophages, but both neutrophils and macrophages also have the capacity to reverse migrate away from a site of inflammation rather than die (Mathias et al., 2006). For example, in a murine sterile hepatic injury, many neutrophils are seen leaving the wound site and reentering the vasculature and migrating via the lungs before entering the bone marrow (Wang et al., 2017). There also exists a complex array of disparate “resolvins,” from lipids through to protein families, that appear to actively encourage immune cell resolution (Serhan and Levy, 2018; Cash et al., 2014). And zebrafish studies are revealing additional mechanisms that either retain cells at the wound site or are part of the machinery that aids their resolution by sequestering or breaking down attractants (Pase et al., 2012; Isles et al., 2019).
Phagocytosis of pathogens, corpses, and debris
Unlike his contemporary pathologists, who perceived pathogen uptake by leukocytes to be a passive process, Metchnikoff’s early observations suggested that particle engulfment was an active process leading to pathogen destruction within vacuoles (Metchnikoff, 1968; Fig. 3 C). The term phagocytosis is now well established for describing the active cellular uptake of particulates within a plasma membrane “envelope” for removal of cell and matrix debris (e.g., apoptotic neutrophils) as well as pathogens in a receptor-mediated fashion (for recent reviews, see Gordon, 2016; Morioka et al., 2019). Macrophages are voracious phagocytes; Metchnikoff described seeing them “literally crammed full of foreign particles.” Phagocytosis occurs not only at sites of tissue damage and infection but also during developmental sculpting of many body tissues (Weavers et al., 2016a; Munro et al., 2019) and during normal tissue homeostasis (Arandjelovic and Ravichandran, 2015), whenever large numbers of unwanted or spent cells must be cleared (Morioka et al., 2019).
The molecular mechanisms underlying phagocytosis have been intensively studied over recent decades; it has emerged as a highly orchestrated process requiring a series of sequential overlapping steps, including but not limited to particle recognition by cell surface receptors (Ostrowski et al., 2016; Lim et al., 2017), engulfment, and phagosome maturation along with cytoskeletal remodeling (Gordon, 2016). The complexity of phagocytosis is becoming increasingly apparent and has been well reviewed elsewhere (Gordon, 2016; Morioka et al., 2019; Elliott and Ravichandran, 2016), so we only highlight a few interesting aspects here.
Leukocytes are equipped with a vast array of surface receptors, allowing them to recognize a huge variety of particles; the exact repertoire of these receptors varies across cell types as well as tissues and most likely reflects local adaptation. While macrophages are professional phagocytes and readily phagocytose various particles, neutrophils have a more varied skill set but are more limited in their phagocytic capacity (Yang et al., 2019). Not surprisingly, the exact mechanism of phagocytosis depends on the nature of the interaction between phagocytic cell and target. This can be directly mediated by detection of pathogen-associated molecular patterns on the pathogen surface (by toll-like receptors or C-type lectin receptors) or by recognition of phosphatidylserine on apoptotic cells via scavenger receptors (Gordon, 2016; Elliott and Ravichandran, 2016). Uptake can also occur indirectly via opsonins, which promote Fc region of IgG (FcγR)-mediated phagocytosis of IgG-opsonized particles or complement receptor–mediated phagocytosis (Gordon, 2016). Following particle recognition, short-lived dynamic podosome-like structures are formed within the nascent phagosome (mediated by local PtdIns(3,4,5)P3 production) to promote phagocytic receptor activation and facilitate membrane remodeling to envelope and engulf the target (Ostrowski et al., 2019). Studies in zebrafish have shown that phagocytic efficiency is both lineage and site specific, with macrophages efficiently clearing pathogens from within a fluid-filled body cavity (e.g., in blood), while neutrophils appear only able to clear surface-associated microbes (Colucci-Guyon et al., 2011).
Once activated, phagocytes use reactive oxygen and nitrogen metabolites to kill ingested microbes. Phagocytosis triggers the assembly of NADPH oxidase on the phagosome membrane for reactive oxygen species (ROS) production (via Rac and LC3-mediated stabilization of NOX2), which, together with delivered antimicrobials, creates a toxic environment for killing pathogens. ROS are also released extracellularly to kill pathogens that have not yet been phagocytosed (Dupré-Crochet et al., 2013). Strikingly, many pathogens are able to subvert phagosome maturation to aid their own growth and survival (Armstrong and Hart, 1971; Myrvik et al., 1984; Brubaker et al., 2015; Zaman and Colley, 1972); a classical example is Helicobacter pylori, which evades destruction by interfering with the targeting of NADPH oxidase to the phagosomal membrane (Allen et al., 2005). Mycobacterium tuberculosis also resists oxidative stress through the production of reductases that degrade phagocyte oxidants and the inhibition of the respiratory burst (Carranza and Chavez-Galan, 2019). Such sophisticated microbial evasion strategies were even observed by Metchnikoff, who stated that “in certain diseases the leucocytes take in a number of bacteria, such as tubercle bacilli or the bacilli of swine erysipelas…a few of which may be digested while the others resist the digestive action of the leucocytes, multiply in the cells and finally invade the whole organism” (Metchnikoff, 1968).
Despite these killing mechanisms, a significant proportion of pathogen proteins are not degraded and instead remain associated with a membrane fraction of macrophages, which enables presentation of antigen-derived peptides to cells of the adaptive immune system (Stuart and Ezekowitz, 2005). Remarkably, dendritic cells can even present bacterial antigens (e.g., those from Mycobacterium) derived from ingested infected neutrophils just as efficiently as those derived from direct pathogen uptake (Blomgran and Ernst, 2011). As eluded to earlier, phagocytosis not only functions to remove obsolete cells and pathogens, but it also has important regulatory functions within the engulfing leukocyte, including priming to become more wound responsive (Weavers et al., 2016a). Moreover, macrophages at wound sites are shifted into an anti-inflammatory tissue-remodeling state following phagocytosis of apoptotic neutrophils and undergo a dramatic metabolic shift that sustains actin polymerization and continued corpse uptake (Morioka et al., 2018).
Endocytic episodes
As leukocytes navigate to their target site, the adhesive contacts that they form with their substratum must be dynamically turned over to permit migration (Maritzen et al., 2015). Since the degradative turnover of adhesion receptors is rather slow (the half-life of surface-labeled integrins is 12–24 h), rapid turnover of adhesive contacts instead relies upon the more speedy endocytic recycling of adhesion molecules (Paul et al., 2015). It appears that calcium transients in migrating neutrophils may direct integrin recycling from the trailing to the leading edge and permit detachment of the leukocyte rear (Pierini et al., 2000; Lawson and Maxfield, 1995). As was discussed, a similar turnover of adhesion molecules is crucial during leukocyte extravasation through the vessel wall; here, endocytic turnover of adhesion molecules (such as selectins and integrins) helps leukocytes transition from weak transient adhesive contacts to firmer ones for wall arrest and final crawling across the vessel wall (Nourshargh and Alon, 2014).
Endocytic trafficking clearly also regulates leukocyte responsiveness to extracellular ligands (Lämmermann and Kastenmüller, 2019). Chemoattractant receptors (e.g., G protein coupled receptors, GPCRs) are often endocytosed following stimulation in order to promote desensitization to repeated stimulation with the same ligand and allow the neutrophil to navigate through complex environments of multiple overlapping chemoattractants (Foxman et al., 1999). The precise mechanisms underpinning receptor trafficking within the leukocyte (and whether this leads to degradation or recycling) is controlled by the Rab family of GTPases, β-arrestins, and the endosomal sorting complexes required for transport (ESCRT) machinery (Marchese, 2014). Intriguingly, chemokine-receptor complex internalization is also important for shaping extracellular chemotactic gradients within the host tissue by targeting the endocytosed ligand to the lysosome for degradation (Marchese, 2014). Consistent with this, many atypical chemokine receptors (e.g., D6) are now being discovered that lack signaling capacity but instead seem to act as important chemokine scavengers (Graham, 2009).
Secretion and exocytosis
Metchnikoff observed that after ingestion, “micro-organisms find within the leukocytes a very unfavorable medium” and “usually perish there” (Metchnikoff, 1905). Of course, we now know a clear hallmark of the “granulocyte” family of leukocytes is the presence of distinctive storage “granules,” which possess antimicrobial and other functions (Geering et al., 2013). Traditionally, granules are subdivided based on their resident cargoes: “azurophilic” (containing myeloperoxidase enzyme, defensins, and neutrophil elastase), “specific” (with lactoferrin and lysozyme), and gelatinase (with metalloproteases). Nevertheless, granule content is highly dynamic and is ultimately determined by the specific transcriptional program active at the time of formation so that, as neutrophils mature and change their transcriptional program, the granule content also changes (Cassatella et al., 2019).
Different granule types are released at different times during inflammation in a highly coordinated manner (Amulic et al., 2012). As neutrophils bind to selectins within the endothelium before extravasation, secretory vesicles fuse with the plasma membrane, exposing β2 integrins, which mediate firm adhesion and initiate the extravasation cascade. This signals the start of neutrophil activation and is soon followed by the release of gelatinase granules (containing metalloproteases) as the cell moves across the endothelium. Finally, once the neutrophil reaches the inflammatory site and becomes fully activated, it mobilizes the azurophilic and specific granules to fuse with either the plasma membrane or a phagosome to create an antimicrobial environment for killing invading pathogens (Cassatella et al., 2019). It is at this stage that the neutrophil unleashes its arsenal of antimicrobial weaponry and initiates the oxidative burst; secretory granules containing flavocytochrome b558 (a component of the NADPH oxidase machinery) move to internal or external membranes and promote assembly of the NADPH oxidase complex for ROS production (Nguyen et al., 2017). The importance of these antimicrobial responses is illustrated by severe immunodeficiency diseases such as chronic granulomatous disease, in which patients suffer life-threatening infections caused by inherited defects in the NADPH oxidase complex subunits (Curnutte et al., 1975).
It is not surprising, given the often indiscriminate nature of these highly reactive antimicrobials, that their release is exquisitely controlled to avoid significant bystander damage to host tissue (Soares et al., 2017). Indeed, it is now emerging that some chemoattractant molecules exert an important “priming” effect, only mildly stimulating the oxidative response on their own but dramatically enhancing the response to subsequent stimuli. For example, exposure of neutrophils to LPS alone induces only assembly of the NADPH oxidase machinery on the membrane, while subsequent fMLP (N-formylmethionyl-leucyl-phenylalanine) stimulation is required for robust activation of this machinery (El-Benna et al., 2008). Nevertheless, host tissues also up-regulate additional protective “resilience” pathways to further minimize damage from the “friendly fire” of a host inflammatory response (Weavers et al., 2019; Telorack et al., 2016).
As well as antimicrobial roles, neutrophil granule contents have important signaling functions. For example, neutrophils secrete cytokines (e.g., IL-8) to recruit other neutrophils, generate classical monocyte chemoattractants (e.g., CCL2, CCL3, CCL20, and CCL19), and release pro-inflammatory cytokines (e.g., IL-1β and TNF-α) to amplify leukocyte infiltration (Cassatella et al., 2019).
Despite lacking the granules characteristic of neutrophils, macrophages also secrete a considerable number of effector molecules in response to a challenge, many of which play critical roles during wound healing (Eming et al., 2017); these secretions change as repair progresses when the macrophage switches from pro- to anti-inflammatory, as will be discussed.
Leukocyte birth, life, and death
The topic of leukocyte origin, self-renewal, and longevity has been an area of considerable debate over the last decade, and as for most cell biological features, it is highly cell-type specific. Metchnikoff observed “undoubted mitotic division of the rabbit’s leucocytes…up to 2 per 1,000 leukocytes at any given time” as well as in the “migratory [leukocyte] cells of the larvae of Axolotl,” where “all the phases of the karyokinetic division may be studied” (Fig. 3 D).
Leukocytes originate from a common hematopoietic myeloid precursor (see Box 1), but each cell type has a very distinct life span (Hidalgo et al., 2019; Varol et al., 2015). Monocytes are generally short-lived and can remain in the circulation for up to 1–2 d, after which time, if they have not been recruited into a tissue as part of an inflammatory response, they will die and be removed (Yona et al., 2013). In contrast, macrophages can have a considerably longer life span, even the entirety of the host organism’s life. Indeed, it is now largely considered that there is very limited local self-renewal of adult tissue-resident macrophages (although this differs across different tissue populations); rather, the majority of macrophages in healthy tissues are established prenatally and self-maintain locally by a combination of longevity and limited proliferation (Ginhoux and Jung, 2014; Hashimoto et al., 2013). A small proportion of adult tissue macrophages are nevertheless derived from infiltrating monocytes that coexist with embryo-derived macrophages within certain tissues, such as the skin (Sieweke and Allen, 2013).
While differentiated tissue macrophage populations display a low steady-state proliferation rate, cell division strongly increases after macrophage depletion (Hashimoto et al., 2013) or under inflammatory challenge (Sieweke and Allen, 2013). Such homeostatic macrophage proliferation requires the growth factors CSF-1 and CSF-2 and is regulated by the transcription factors MafC and MafB (for review, see Sieweke and Allen, 2013). The accumulation of inflammatory monocytes in an inflamed tissue is mostly due to their influx from blood via diapedesis (Figs. 1 and 3 B) rather than by their proliferative ability (see earlier). However, it seems that subsets of inflammatory monocyte-derived macrophages can proliferate locally in specific inflammatory scenarios, such as during the resolution of zymosan-induced peritonitis (Davies et al., 2013). Although most inflammatory monocyte-derived macrophages die, some surviving cells can subsequently undergo in situ phenotype conversion and become M2-like tissue-resident macrophages (Hashimoto et al., 2013). Some of these cells appear to retain a “memory” of their past inflammatory experience and become “trained” monocytes or memory macrophages (Netea et al., 2011).
The lifetime of mammalian neutrophils remains highly contentious. They have historically been considered short-lived cells because of classical experiments from the 1950s/1960s suggesting a half-life in the circulation of ∼7–9 h (Dancey et al., 1976; Cartwright et al., 1964). More recent experiments, however, indicate significantly longer half-lives and that their lifetimes can be dramatically extended within inflamed conditions and by environmental conditions such as hypoxia, the release of inflammatory cytokines and growth factors (e.g., granulocyte-macrophage colony-stimulating factor, GM-CSF and TNF), and microbe-associated molecular patterns (e.g., LPS), as well as by viral infections (Hidalgo et al., 2019). Notwithstanding this, neutrophils must leave or be removed before they have significant detrimental bystander effects on host tissue (see motility and phagocytosis sections above). Indeed, Metchnikoff himself observed that “a great number of phagocytes perish and are englobed by other phagocytes, as can be seen in every case a few days after the onset of the inflammation” (Metchnikoff, 1968).
Fueling the inflammatory response: Immunometabolism
Metchnikoff of course could not observe molecular and metabolic changes in inflammatory cells, but he would have anticipated that leukocytes must adapt their energy production during an inflammatory episode. He observed, for example, that phagocytosis of apoptotic corpses “is evidently a much easier task for them and requires less activity on their part than does the struggle with parasites.” Indeed, it is now clear that leukocyte metabolism is precisely regulated (O’Neill et al., 2016) and, moreover, that metabolic adaptation is necessary for mounting an effective defense against bacterial and viral pathogens, since nearly all activated immune cells use glycolysis to “fuel” their functions in host defense (Gleeson and Sheedy, 2016). Bacterial-derived LPS, for example, induces the hypoxia-inducible factor HIF1α transcription factor to up-regulate enzymes involved in glycolysis (Tannahill et al., 2013). The switch to glycolysis not only provides a rapid means for boosting ATP production, but it also generates the biosynthetic intermediates that are necessary to support assembly of key cellular constituents (including serine, glycine, alanine, and acetyl coenzyme A [acetyl-CoA] for lipid synthesis) to ensure the cell can effectively perform functions such as phagocytosis and cytokine production (O’Neill et al., 2016).
Leukocytes also undergo a profound metabolic switch to aerobic glycolysis during phagocytosis of apoptotic corpses (Morioka et al., 2018); corpse uptake induces expression of the membrane transport protein SLC16A1 to support increased glucose uptake, and this aids in driving both actin polymerization and the synthesis of anti-inflammatory lactate (Morioka et al., 2018). The TCA cycle is also modified within inflammatory macrophages, leading to increased levels of citrate, which can support increased fatty acid biogenesis needed for membrane remodeling and prostaglandin and nitric oxide production, as well as synthesis of antimicrobials such as itaconic acid (Michelucci et al., 2013).
It is becoming increasingly clear that autophagy also plays a vital role in the differentiation and function of many leukocyte subtypes and may regulate a range of processes, including metabolism and selective degradation of substrates/organelles as well as cell survival (for a recent review, see Clarke and Simon, 2019). Pathogens such as Francisella tularensis (a highly virulent intracellular pathogen) can even induce autophagic pathways within host cells in order to scavenge host-derived amino acids (Steele et al., 2013). Strikingly, immune responses are shaped not only by endogenous host metabolites but also by metabolites derived from microbiota and infectious agents (Levy et al., 2016). Given that small molecules could easily target specific metabolic pathways and alter leukocyte phenotype, further research in this area could provide exciting opportunities for therapeutic leukocyte reprogramming in patients.
Specialized leukocyte functions
Superimposed on these classical cell biological features, the various leukocyte cell types are also equipped with highly specialized cell biology that enable them to perform specific functions during the inflammatory response (Fig. 3, lower blue zone). Remarkably, several of these were hinted at by Metchnikoff in his writing or can be discerned in his exquisite figures.
NETs
Alongside microbial killing by ROS release, neutrophils perform another extraordinary type of specialized antimicrobial activity involving release of NETs in a process termed “NETosis” (Brinkmann et al., 2004; Fig. 3 F). This is an active form of cell death that leads to the release of decondensed chromatin into the extracellular space, along with a high concentration of cytoplasmic and granular antimicrobial proteins (for a recent review, see Castanheira and Kubes, 2019). The exact mechanisms driving NET production and release have only recently been elucidated. It is clear that ROS are involved, as NADPH oxidase and Myeloperoxidase are required for NET formation (Metzler et al., 2011; Patel et al., 2010; Fuchs et al., 2007) as well as the Raf-MEK-ERK pathway and neutrophil elastase, which moves to the nucleus to promote histone degradation and chromatin decondensation (Papayannopoulos et al., 2010). Remarkably, in vivo studies in mouse skin suggest that neutrophils releasing NETs may not immediately die but can continue to perform functions such as chemotaxis and phagocytosis (Yipp and Kubes, 2013). It seems that other leukocytes, such as monocytes (Webster et al., 2010), macrophages (Mohanan et al., 2013), and eosinophils (Yousefi et al., 2008; Mukherjee et al., 2018), may also use extracellular traps to combat infections.
Clearly, NETosis has evolved as a mechanism for fighting infection, but there is emerging evidence that NET release might negatively impact wound repair and affect cancer progression. Indeed, levels of peptidyl arginine deiminase 4, which is essential for NET antimicrobial activity (Li et al., 2010), are markedly elevated within neutrophils from diabetic patients, and these cells appear primed for NET production. Moreover, blocking NETosis (via systemic DNase treatment or genetic peptidyl arginine deiminase 4 deficiency) accelerates wound repair in diabetic mouse models, suggesting that elevated NET release could be a major factor underpinning impaired wound healing in diabetic individuals (Wong et al., 2015). It is thought that NETs might amplify the pro-inflammatory state of macrophages in nonhealing wounds, as NET overproduction in diabetic wounds was associated with an activated NLRP3 inflammasome, as well as with induced IL-1β release in macrophages, relative to wounds from healthy patients and rats (Liu et al., 2019). There is now also good evidence that neutrophils recruited to various patient cancers can undergo NETosis, and this is associated with poor prognosis, suggesting that NETosis becomes a promising novel target to potentially dampen metastatic dissemination of cancer cells (Rayes et al., 2019).
Encapsulation and cell–cell fusion
One key specialization of monocyte-derived cell lineages is their capacity to drive and participate in the formation of a granuloma as a protective response to certain stimuli, including some infectious organisms or foreign bodies that cannot be efficiently “killed” and cleared by phagocytosis. A classic example is Metchnikoff’s experimental grafting of a tangerine tree thorn into a starfish embryo (Fig. 3 G). More recent studies have shown that following a TB mycobacterial infection of the lung, macrophages are recruited to the infection site and become reprogrammed to “seal off” the infected cells; they do this by flattening and assembling E-cadherin–rich interdigitations with one another to form an epitheliod “wall” (Pagán and Ramakrishnan, 2018). Granulomas can also trigger not just adhesion but fusion of macrophages to generate giant multinucleate cells, sometimes called foreign body giant cells (FBGCs), with obvious parallels to multinucleated, bone-dissolving osteoclasts, also from the myeloid cell lineage (Pereira et al., 2018). Metchnikoff’s student Tchistowitch “witnessed all the transition stages between mononuclear leukocytes…through to giant cells in the pulmonary alveoli of rabbits” (Metchnikoff, 1968). Having written that giant cells “represent a special form of phagocyte which are particularly energetic in the conflict with the microbes,” Metchnikoff would have been excited to learn from recent studies that FBGCs acquire an enhanced phagocytic capacity compared with unfused cells, in part through their increased membrane area but also through an altered receptor repertoire (Milde et al., 2015). Although it is still unclear which signals drive the macrophage fusion events, resulting in FBGC formation, more is understood about similar episodes in myoblast muscle fusions and osteoclast development, and it is likely that some of these mechanisms will turn out to be shared (Brukman et al., 2019). Although cell–cell fusion can clearly happen and has been captured in real time in the foreign body response of zebrafish larvae (Gurevich et al., 2019), it has now been shown that multinucleate macrophage-derived cells can also sometimes form instead through replicative stress stimuli forcing cell division without cytokinesis (Herrtwich et al., 2016; Gharun et al., 2017).
Presumably, the macrophage granuloma response evolved to advantage the host, but evolution has led to granulomas being subverted by the bacterium as a “safe house,” as is now clear from studies in zebrafish where disruption of the granuloma adhesions between macrophages allows increased neutrophilic influx and killing of the bacterium and, as a consequence, better host survival (Cronan et al., 2016).
Leukocyte reprogramming and phenotype switching
Other cell lineages at sites of tissue damage adopt subtle new programs of gene expression as, for example, epidermal wound edge cells when they transition from immotile to motile by undergoing a partial epithelial–mesenchymal transition (Nunan et al., 2015); however, immune cells are notoriously capable of dramatic switches in phenotype depending on the various microenvironments they find themselves in and the cues they are exposed to. Studies in Drosophila embryos indicate that innate immune cells must first be primed in order to even be able to sense a wound or infection-related attractant signals (Weavers et al., 2016a); and just as in earlier mammalian inflammation studies, one such priming signal can be engulfment of apoptotic corpses (Savill et al., 2002). Soon after initial recruitment to a wound, macrophages release pro-inflammatory molecules (e.g., IL-1, IL-6, TNF-α, nitric oxide, and ROS) to help amplify the inflammatory response and stave off infection, as well as matrix metalloproteinases MMP-2 and MMP-9 to remodel damaged matrix. However, as repair progresses, macrophages switch to a contrasting phenotype and release more anti-inflammatory molecules (e.g., IL-10, PDGF, IGF-1, and TGF-β) that have other functions and which may also help shut down various aspects of the repair process (Novak and Koh, 2013). A recent study in zebrafish has shown that the first influx of pro-inflammatory TNF-α+ve macrophages promote angiogenic sprouting at the wound site, in part via VEGF signaling; however, subsequently the wound macrophage phenotype switches to TNF-α−ve, and these cells now drive vascular regression and clearance of endothelial corpses (Gurevich et al., 2018).
To a degree, the popular M1/M2 (or “classic” versus “alternative”) activation state paradigm for macrophage polarization reflects the two extremes of phenotype that these cells adopt during a wound inflammatory response to influence other lineages at the wound site (Ploeger et al., 2013), although, in truth, it is likely that cells are dynamic and often mixed and transitioning in their phenotypes in tissues (Nguyen-Chi et al., 2015; Martinez and Gordon, 2014). Conditional knockdown of macrophages at various time points following murine wounding leads to a series of repair defects that support the idea of macrophages having a number of different functions at various phases during the repair process (Lucas et al., 2010). It is still not entirely clear whether these multiple roles can be achieved solely by individual cells switching phenotype or whether they necessitate influx of successive waves of new cells also; however, in vitro studies have shown that individual cells can switch phenotype, and in vivo live imaging studies in zebrafish also provide evidence for gene expression switching within individual cells during the repair process (Gurevich et al., 2018; Nguyen-Chi et al., 2015).
Neutrophils appear to switch phenotype during tissue repair also: a recent study of the inflammatory response to heart injury observed a first wave of “N1” pro-inflammatory neutrophils with high expression of cytokine genes (e.g., IL-1β, IL-6, and TNF-α) entering injured tissues, whereas “N2” anti-inflammatory neutrophils (expressing Arg1, IL-10, and Ym1) emerged as dominant at later stages (Ma et al., 2016). While the transcriptomic profiles for these dynamic switches in phenotype of macrophages and neutrophils are now being documented, it is still not entirely clear how this alters their full repertoire of behaviors and how plastic or permanent the switches might be. However, it is safe to assume that a better understanding of these switches and how to modulate them will lead us toward important therapeutic approaches.
Final remarks
Inflammation is essential to stave off infection and for coordinating various aspects of the repair and regeneration process; however, when it is misregulated or becomes chronic, it can cause severe tissue damage. Indeed, many pathologies, including arthritis, atherosclerosis, all of the fibrotic diseases, and even cancer, are now understood to be due to, or at least exacerbated by, an overexuberant and/or nonresolving inflammatory response. One route to discovering new therapies for targeting and managing these pathologies will be to more fully understand the cell biology of inflammatory cells and the inflammatory response, so as to reveal opportunities for dampening or enhancing specific components of their repertoire at key steps in the disease process.
Metchnikoff was the first to watch and describe the inflammatory response, and his legacy is our new understanding of how these cells work to maintain tissue homeostasis but also to drive pathology. Of course, Metchnikoff drew rather than photographed what he saw down the microscope, and this may explain his skill for unearthing the fine details, which are still a match and even a lead for many of our current photo-microscopy studies. Modern light microscopy offers much-increased resolution and opportunities for automated quantification, and such abundance of data can enable mathematical modeling (Box 3; Fig. 2 D; Weavers et al., 2016b), which, in turn, can reveal mechanistic details that he could only dream of. While Metchnikoff pioneered the use of translucent organisms for studying inflammation in situ and although it is now possible to image inflammation in situ in mammalian tissues, some of the new insights are again coming from translucent organisms, but this time ones that offer genetic tractability, such as Drosophila and zebrafish at early embryonic and later larval/pupal stages.
It is remarkable how often Metchnikoff anticipated our recent discoveries in the field. This was, of course, before the dawn of molecular biology; however, he spoke of “changes within the cells themselves” and how leukocytes have a “sensibility” and must be “excited positively,” which talks to what we now know to be altered gene expression as cells respond to inflammatory cues. What further insights are there to be gained from rereading his lectures? Because of vast leaps forward in light microscopy and complementary opportunities from transmission electron microscopy (TEM) studies (see Box 3), we are now able to image things that Metchnikoff could not; for example transgenic reporters of gene expression and of metabolic changes enable us to observe dynamic changes in innate immune cell activities as they respond to inflammatory cues, and we can now interrogate much about the signaling pathways that drive these changing activities and behaviors through genetic and chemical perturbations. But we still need to learn more about the interplay between various leukocytes and each other—particularly interactions between innate and adaptive immune cells (see Box 2)—as well as how they impact other tissue and cell lineages and how these interactions can be beneficially modulated to dampen or enhance inflammation and its consequences. Other novel avenues of potential clinical relevance include a fuller understanding of the ways in which tissues naturally protect themselves from the negative consequences of inflammation (Weavers et al., 2019; Soares et al., 2014; Schäfer et al., 2010).
Perhaps just as useful as his beautiful descriptions of inflammatory cell behavior and his uncanny grasp of what we now know to be crucial aspects of inflammatory cell biology is his pragmatism about what he did not know and his intuition for what the big questions were. There are cell biology lessons for all of us today, not only in Metchnikoff’s exquisite observational skills but also in his understated interpretation of his and his colleagues’ observations, constantly reminding his audience of the potential flaws in his arguments and what knowledge was not yet possible to prove definitively: for example, when he cautioned that “we have not sufficient evidence yet to justify…that the chemotaxis of leukocytes can only be excited by dead or injured bacteria…,” or when discussing leukocyte phagocytosis, he stated that “we are at present ignorant of the precise manner in which this digestive and destructive action is accomplished.” These words seem refreshingly modest by today’s standards.
Metchnikoff’s far-reaching legacy to the field of inflammation has led us toward a much clearer understanding of the cellular and molecular mechanisms underpinning how innate immune cells undertake their various tasks in tissues. The next steps will be to use this knowledge to develop therapeutic strategies that enhance the positive aspects of a robust inflammatory response while dampening down its negative consequences.
Acknowledgments
We thank members of H. Weavers’ and P. Martin’s laboratories for their intellectual support. P. Martin is grateful to Siamon Gordon for first introducing him to macrophage biology almost 30 years ago. We also thank Borko Amulic, Anne Ridley, Sussan Nourshargh, Natalia Hajdamowicz, Chris Hill, Lali Ramakrishnan, and David Gurevich for images and helpful discussions.
Work in our laboratories is supported by grants jointly from the Wellcome Trust and Royal Society (grant 208762/Z/17/Z to H. Weavers) and the Wellcome Trust (grant 217169/Z/19/Z to P. Martin).
The authors declare no competing financial interests.
Author contributions: H. Weavers and P. Martin equally wrote and edited the manuscript.
References
- Allen L.-A.H., Beecher B.R., Lynch J.T., Rohner O.V., and Wittine L.M.. 2005. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 174:3658–3667. 10.4049/jimmunol.174.6.3658 [DOI] [PubMed] [Google Scholar]
- Amulic B., Cazalet C., Hayes G.L., Metzler K.D., and Zychlinsky A.. 2012. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30:459–489. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- Arandjelovic S., and Ravichandran K.S.. 2015. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16:907–917. 10.1038/ni.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J.A., and Hart P.D.. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134:713–740. 10.1084/jem.134.3.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., and Geissmann F.. 2007. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science. 317:666–670. 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- Barros-Becker F., Lam P.Y., Fisher R., and Huttenlocher A.. 2017. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. J. Cell Sci. 130:3801–3808. 10.1242/jcs.206128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman R.W., Matty M.A., Au G.G., Looger L.L., Choudhury K.R., Keller P.J., and Tobin D.M.. 2015. Direct In Vivo Manipulation and Imaging of Calcium Transients in Neutrophils Identify a Critical Role for Leading-Edge Calcium Flux. Cell Rep. 13:2107–2117. 10.1016/j.celrep.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgran R., and Ernst J.D.. 2011. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 186:7110–7119. 10.4049/jimmunol.1100001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., and Zychlinsky A.. 2004. Neutrophil Extracellular Traps Kill Bacteria. Science. 303:1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Brubaker S.W., Bonham K.S., Zanoni I., and Kagan J.C.. 2015. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33:257–290. 10.1146/annurev-immunol-032414-112240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brukman N.G., Uygur B., Podbilewicz B., and Chernomordik L.V.. 2019. How cells fuse. J. Cell Biol. 218:1436–1451. 10.1083/jcb.201901017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A.R., Walker D.C., Brown E.S., Thurmon L.T., Bowden R.A., Keese C.R., Simon S.I., Entman M.L., and Smith C.W.. 1997. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J. Immunol. 159:2893–2903. [PubMed] [Google Scholar]
- Candotti F. 2018. Clinical Manifestations and Pathophysiological Mechanisms of the Wiskott-Aldrich Syndrome. J. Clin. Immunol. 38:13–27. 10.1007/s10875-017-0453-z [DOI] [PubMed] [Google Scholar]
- Carranza C., and Chavez-Galan L.. 2019. Several Routes to the Same Destination: Inhibition of Phagosome-Lysosome Fusion by Mycobacterium tuberculosis. Am. J. Med. Sci. 357:184–194. 10.1016/j.amjms.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Cartwright G.E., Athens J.W., and Wintrobe M.M.. 1964. Analytical Review: The Kinetics of Granulopoiesis in Normal Man. Blood. 24:780–803. 10.1182/blood.V24.6.780.780 [DOI] [PubMed] [Google Scholar]
- Cash J.L., Bass M.D., Campbell J., Barnes M., Kubes P., and Martin P.. 2014. Resolution mediator chemerin15 reprograms the wound microenvironment to promote repair and reduce scarring. Curr. Biol. 24:1406–1414. 10.1016/j.cub.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M.A., Östberg N.K., Tamassia N., and Soehnlein O.. 2019. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 40:648–664. 10.1016/j.it.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Castanheira F.V.S., and Kubes P.. 2019. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 133:2178–2185. 10.1182/blood-2018-11-844530 [DOI] [PubMed] [Google Scholar]
- Cerutti C., and Ridley A.J.. 2017. Endothelial cell-cell adhesion and signaling. Exp. Cell Res. 358:31–38. 10.1016/j.yexcr.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P.A., and Junger W.G.. 2006. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 314:1792–1795. 10.1126/science.1132559 [DOI] [PubMed] [Google Scholar]
- Clarke A.J., and Simon A.K.. 2019. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 19:170–183. 10.1038/s41577-018-0095-2 [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E., Tinevez J.-Y., Renshaw S.A., and Herbomel P.. 2011. Strategies of professional phagocytes in vivo: unlike macrophages, neutrophils engulf only surface-associated microbes. J. Cell Sci. 124:3053–3059. 10.1242/jcs.082792 [DOI] [PubMed] [Google Scholar]
- Cronan M.R., Beerman R.W., Rosenberg A.F., Saelens J.W., Johnson M.G., Oehlers S.H., Sisk D.M., Jurcic Smith K.L., Medvitz N.A., Miller S.E., et al. 2016. Macrophage Epithelial Reprogramming Underlies Mycobacterial Granuloma Formation and Promotes Infection. Immunity. 45:861–876. 10.1016/j.immuni.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J.T., Kipnes R.S., and Babior B.M.. 1975. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N. Engl. J. Med. 293:628–632. 10.1056/NEJM197509252931303 [DOI] [PubMed] [Google Scholar]
- Dancey J.T., Deubelbeiss K.A., Harker L.A., and Finch C.A.. 1976. Neutrophil kinetics in man. J. Clin. Invest. 58:705–715. 10.1172/JCI108517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L.C., Rosas M., Jenkins S.J., Liao C.T., Scurr M.J., Brombacher F., Fraser D.J., Allen J.E., Jones S.A., and Taylor P.R.. 2013. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat. Commun. 4:1886 10.1038/ncomms2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré-Crochet S., Erard M., and Nüβe O.. 2013. ROS production in phagocytes: why, when, and where? J. Leukoc. Biol. 94:657–670. 10.1189/jlb.1012544 [DOI] [PubMed] [Google Scholar]
- El-Benna J, Dang P.M., and Gougerot-Pocidalo M.A.. 2008. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 30:279–289. 10.1007/s00281-008-0118-3 [DOI] [PubMed] [Google Scholar]
- Elliott M.R.R., and Ravichandran K.S.S.. 2016. The Dynamics of Apoptotic Cell Clearance. Dev. Cell. 38:147–160. 10.1016/j.devcel.2016.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming S.A., Krieg T., and Davidson J.M.. 2007. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127:514–525. 10.1038/sj.jid.5700701 [DOI] [PubMed] [Google Scholar]
- Eming S.A., Martin P., and Tomic-Canic M.. 2014. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6 265sr6 10.1126/scitranslmed.3009337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming S.A., Wynn T.A., and Martin P.. 2017. Inflammation and metabolism in tissue repair and regeneration. Science. 356:1026–1030. 10.1126/science.aam7928 [DOI] [PubMed] [Google Scholar]
- Foxman E.F., Campbell J.J., and Butcher E.C.. 1997. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 139:1349–1360. 10.1083/jcb.139.5.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman E.F., Kunkel E.J., and Butcher E.C.. 1999. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J. Cell Biol. 147:577–588. 10.1083/jcb.147.3.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., and Zychlinsky A.. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231–241. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B., Stoeckle C., Conus S., and Simon H.-U.. 2013. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 34:398–409. 10.1016/j.it.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Gharun K., Senges J., Seidl M., Lösslein A., Kolter J., Lohrmann F., Fliegauf M., Elgizouli M., Alber M., Vavra M., et al. 2017. Mycobacteria exploit nitric oxide-induced transformation of macrophages into permissive giant cells. EMBO Rep. 18:2144–2159. 10.15252/embr.201744121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., and Guilliams M.. 2016. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 44:439–449. 10.1016/j.immuni.2016.02.024 [DOI] [PubMed] [Google Scholar]
- Ginhoux F., and Jung S.. 2014. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14:392–404. 10.1038/nri3671 [DOI] [PubMed] [Google Scholar]
- Girbl T., Lenn T., Perez L., Rolas L., Barkaway A., Thiriot A., Del Fresno C., Lynam E., Hub E., Thelen M., et al. 2018. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity. 49:1062–1076.e6: E6. 10.1016/j.immuni.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson L.E., and Sheedy F.J.. 2016. Metabolic reprogramming & inflammation: Fuelling the host response to pathogens. Semin. Immunol. 28:450–468. 10.1016/j.smim.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Gordon S. 2016. Phagocytosis: An Immunobiologic Process. Immunity. 44:463–475. 10.1016/j.immuni.2016.02.026 [DOI] [PubMed] [Google Scholar]
- Graham G.J. 2009. D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur. J. Immunol. 39:342–351. 10.1002/eji.200838858 [DOI] [PubMed] [Google Scholar]
- Gurevich D.B., Severn C.E., Twomey C., Greenhough A., Cash J., Toye A.M., Mellor H., and Martin P.. 2018. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 37 e97786 10.15252/embj.201797786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich D.B., French K.E., Collin J.D., Cross S.J., and Martin P.. 2019. Live imaging the foreign body response in zebrafish reveals how dampening inflammation reduces fibrosis. J. Cell Sci. 133 jcs236075 10.1242/jcs.236075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D., et al. 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 38:792–804. 10.1016/j.immuni.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W.L., and Jameson J.M.. 2010. Epidermal T cells and wound healing. J. Immunol. 184:5423–5428. 10.4049/jimmunol.0902733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk N., Schimmel L., Oort C., van Rijssel J., Yin T., Ma B., van Unen J., Pitter B., Huveneers S., Goedhart J., et al. 2016. F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signalling. Nat. Commun. 7:10493 10.1038/ncomms10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrtwich L., Nanda I., Evangelou K., Nikolova T., Horn V., Sagar D., Erny D., Stefanowski J., Rogell L., Klein C., et al. 2016. DNA Damage Signaling Instructs Polyploid Macrophage Fate in Granulomas. Cell. 167:1264–1280.e18: E18. 10.1016/j.cell.2016.09.054 [DOI] [PubMed] [Google Scholar]
- Hidalgo A., Chilvers E.R., Summers C., and Koenderman L.. 2019. The Neutrophil Life Cycle. Trends Immunol. 40:584–597. 10.1016/j.it.2019.04.013 [DOI] [PubMed] [Google Scholar]
- Isles H.M., Herman K.D., Robertson A.L., Loynes C.A., Prince L.R., Elks P.M., and Renshaw S.A.. 2019. The CXCL12/CXCR4 Signaling Axis Retains Neutrophils at Inflammatory Sites in Zebrafish. Front. Immunol. 10:1784 10.3389/fimmu.2019.01784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., and Havran W.L.. 2002. A role for skin γδ T cells in wound repair. Science. 296:747–749. 10.1126/science.1069639 [DOI] [PubMed] [Google Scholar]
- Jones G.E., Allen W.E., and Ridley A.J.. 1998. The Rho GTPases in macrophage motility and chemotaxis. Cell Adhes. Commun. 6:237–245. 10.3109/15419069809004479 [DOI] [PubMed] [Google Scholar]
- Jones R.A., Feng Y., Worth A.J., Thrasher A.J., Burns S.O., and Martin P.. 2013. Modelling of human Wiskott-Aldrich syndrome protein mutants in zebrafish larvae using in vivo live imaging. J. Cell Sci. 126:4077–4084. 10.1242/jcs.128728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T., and Kastenmüller W.. 2019. Concepts of GPCR-controlled navigation in the immune system. Immunol. Rev. 289:205–231. 10.1111/imr.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M.A., and Maxfield F.R.. 1995. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 377:75–79. 10.1038/377075a0 [DOI] [PubMed] [Google Scholar]
- Levy M., Thaiss C.A., and Elinav E.. 2016. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 30:1589–1597. 10.1101/gad.284091.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M.I., and Nourshargh S.. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678–689. 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- Li P., Li M., Lindberg M.R., Kennett M.J., Xiong N., and Wang Y.. 2010. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207:1853–1862. 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepe J., Taylor H., Barnes C.P., Huvet M., Bugeon L., Thorne T., Lamb J.R., Dallman M.J., and Stumpf M.P.H.. 2012. Calibrating spatio-temporal models of leukocyte dynamics against in vivo live-imaging data using approximate Bayesian computation. Integr. Biol. 4:335–345. 10.1039/c2ib00175f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.J., Grinstein S., and Roth Z.. 2017. Diversity and Versatility of Phagocytosis: Roles in Innate Immunity, Tissue Remodeling, and Homeostasis. Front. Cell. Infect. Microbiol. 7:191 10.3389/fcimb.2017.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Yang P., Gao M., Yu T., Shi Y., Zhang M., Yao M., Liu Y., and Zhang X.. 2019. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin. Sci. (Lond.). 133:565–582. 10.1042/CS20180600 [DOI] [PubMed] [Google Scholar]
- Lucas T., Waisman A., Ranjan R., Roes J., Krieg T., Müller W., Roers A., and Eming S.A.. 2010. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 184:3964–3977. 10.4049/jimmunol.0903356 [DOI] [PubMed] [Google Scholar]
- Ma Y., Yabluchanskiy A., Iyer R.P., Cannon P.L., Flynn E.R., Jung M., Henry J., Cates C.A., Deleon-Pennell K.Y., and Lindsey M.L.. 2016. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 110:51–61. 10.1093/cvr/cvw024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A. 2014. Endocytic trafficking of chemokine receptors. Curr. Opin. Cell Biol. 27:72–77. 10.1016/j.ceb.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritzen T., Schachtner H., and Legler D.F.. 2015. On the move: endocytic trafficking in cell migration. Cell. Mol. Life Sci. 72:2119–2134. 10.1007/s00018-015-1855-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. 1997. Wound Healing - Aiming for Perfect Skin Regeneration. Science. 276:75–81. 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- Martinez F.O., and Gordon S.. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6:13 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias J.R., Perrin B.J., Liu T.-X., Kanki J., Look A.T., and Huttenlocher A.. 2006. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80:1281–1288. 10.1189/jlb.0506346 [DOI] [PubMed] [Google Scholar]
- McDonald B., Pittman K., Menezes G.B., Hirota S.A., Slaba I., Waterhouse C.C., Beck P.L., Muruve D.A., and Kubes P.. 2010. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 330:362–366. 10.1126/science.1195491 [DOI] [PubMed] [Google Scholar]
- Metchnikoff E. 1893. Lectures on the Comparative Pathology of Inflammation Delivered at the Pasteur Institute in 1891. Starling, F.A. Starling, E.H. (Trans.) Kegan Paul, Trench, Trubner & Co, London. [Google Scholar]
- Metchnikoff E. 1905. Immunity in Infective Diseases. Harvard University Press, Cambridge, MA. [Google Scholar]
- Metchnikoff E. 1968. Lectures on the Comparative Pathology of Inflammation Delivered at the Pasteur Institute in 1891. Starling, F. A. Starling E.H. (trans.). Dover Publ. Inc., New York. [Google Scholar]
- Metchnikoff O. 1921. Life of Elie Metchnikoff 1845–1916. Houghton Mifflin , Boston & New York. [Google Scholar]
- Metzler K.D., Fuchs T.A., Nauseef W.M., Reumaux D., Roesler J., Schulze I., Wahn V., Papayannopoulos V., and Zychlinsky A.. 2011. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 117:953–959. 10.1182/blood-2010-06-290171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci A., Cordes T., Ghelfi J., Pailot A., Reiling N., Goldmann O., Binz T., Wegner A., Tallam A., Rausell A., et al. 2013. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA. 110:7820–7825. 10.1073/pnas.1218599110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde R., Ritter J., Tennent G.A., Loesch A., Martinez F.O., Gordon S., Pepys M.B., Verschoor A., and Helming L.. 2015. Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction. Cell Rep. 13:1937–1948. 10.1016/j.celrep.2015.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanan S., Horibata S., McElwee J.L., Dannenberg A.J., and Coonrod S.A.. 2013. Identification of macrophage extracellular trap-like structures in mammary gland adipose tissue: a preliminary study. Front. Immunol. 4:67 10.3389/fimmu.2013.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S., Stramer B., Evans I., Wood W., and Martin P.. 2010. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 20:464–470. 10.1016/j.cub.2010.01.047 [DOI] [PubMed] [Google Scholar]
- Morioka S., Perry J.S.A., Raymond M.H., Medina C.B., Zhu Y., Zhao L., Serbulea V., Onengut-Gumuscu S., Leitinger N., Kucenas S., et al. 2018. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 563:714–718. 10.1038/s41586-018-0735-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S., Maueröder C., and Ravichandran K.S.. 2019. Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity. 50:1149–1162. 10.1016/j.immuni.2019.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M., Lacy P., and Ueki S.. 2018. Eosinophil extracellular traps and inflammatory pathologies-untangling the web!. Front. Immunol. 9:2763 10.3389/fimmu.2018.02763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro D.A.D., Wineberg Y., Tarnick J., Vink C.S., Li Z., Pridans C., Dzierzak E., Kalisky T., Hohenstein P., and Davies J.A.. 2019. Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. eLife. 8 e43271 10.7554/eLife.43271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik Q.N., Leake E.S., and Wright M.J.. 1984. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am. Rev. Respir. Dis. 129:322–328. [PubMed] [Google Scholar]
- Netea M.G., Quintin J., and van der Meer J.W.M.. 2011. Trained immunity: a memory for innate host defense. Cell Host Microbe. 9:355–361. 10.1016/j.chom.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Nguyen G.T., Green E.R., and Mecsas J.. 2017. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 7:373 10.3389/fcimb.2017.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Chi M., Laplace-Builhe B., Travnickova J., Luz-Crawford P., Tejedor G., Phan Q.T., Duroux-Richard I., Levraud J.P., Kissa K., Lutfalla G., et al. 2015. Identification of polarized macrophage subsets in zebrafish. eLife. 4 e07288 10.7554/eLife.07288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P. 2016. The early wound signals. Curr. Opin. Genet. Dev. 40:17–22. 10.1016/j.gde.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosbaum A., Prevel N., Truong H.-A., Mehta P., Ettinger M., Scharschmidt T.C., Ali N.H., Pauli M.L., Abbas A.K., and Rosenblum M.D.. 2016. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 196:2010–2014. 10.4049/jimmunol.1502139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S., and Alon R.. 2014. Leukocyte migration into inflamed tissues. Immunity. 41:694–707. 10.1016/j.immuni.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Novak M.L., and Koh T.J.. 2013. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 183:1352–1363. 10.1016/j.ajpath.2013.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan R., Campbell J., Mori R., Pitulescu M.E., Jiang W.G., Harding K.G., Adams R.H., Nobes C.D., and Martin P.. 2015. Ephrin-Bs Drive Junctional Downregulation and Actin Stress Fiber Disassembly to Enable Wound Re-epithelialization. Cell Rep. 13:1380–1395. 10.1016/j.celrep.2015.09.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill L.A.J., Kishton R.J., and Rathmell J.. 2016. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16:553–565. 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski P.P., Grinstein S., and Freeman S.A.. 2016. Diffusion Barriers, Mechanical Forces, and the Biophysics of Phagocytosis. Dev. Cell. 38:135–146. 10.1016/j.devcel.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Ostrowski P.P., Freeman S.A., Fairn G., and Grinstein S.. 2019. Dynamic Podosome-Like Structures in Nascent Phagosomes Are Coordinated by Phosphoinositides. Dev. Cell. 50:397–410.e3: E3. 10.1016/j.devcel.2019.05.028 [DOI] [PubMed] [Google Scholar]
- Pagán A.J., and Ramakrishnan L.. 2018. The Formation and Function of Granulomas. Annu. Rev. Immunol. 36:639–665. 10.1146/annurev-immunol-032712-100022 [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V., Metzler K.D., Hakkim A., and Zychlinsky A.. 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191:677–691. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase L., Layton J.E., Wittmann C., Ellett F., Nowell C.J., Reyes-Aldasoro C.C., Varma S., Rogers K.L., Hall C.J., Keightley M.C., et al. 2012. Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Curr. Biol. 22:1818–1824. 10.1016/j.cub.2012.07.060 [DOI] [PubMed] [Google Scholar]
- Patel S., Kumar S., Jyoti A., Srinag B.S., Keshari R.S., Saluja R., Verma A., Mitra K., Barthwal M.K., Krishnamurthy H., et al. 2010. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide. 22:226–234. 10.1016/j.niox.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Paul N.R., Jacquemet G., and Caswell P.T.. 2015. Endocytic Trafficking of Integrins in Cell Migration. Curr. Biol. 25:R1092–R1105. 10.1016/j.cub.2015.09.049 [DOI] [PubMed] [Google Scholar]
- Pereira M., Petretto E., Gordon S., Bassett J.H.D., Williams G.R., and Behmoaras J.. 2018. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 131 jcs216267 10.1242/jcs.216267 [DOI] [PubMed] [Google Scholar]
- Pierini L.M., Lawson M.A., Eddy R.J., Hendey B., and Maxfield F.R.. 2000. Oriented endocytic recycling of α5β1 in motile neutrophils. Blood. 95:2471–2480. 10.1182/blood.V95.8.2471 [DOI] [PubMed] [Google Scholar]
- Ploeger D.T., Hosper N.A., Schipper M., Koerts J.A., de Rond S., and Bank R.A.. 2013. Cell plasticity in wound healing: paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun. Signal. 11:29 10.1186/1478-811X-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebstl D., Voisin M.-B., Woodfin A., Whiteford J., D’Acquisto F., Jones G.E., Rowe D., and Nourshargh S.. 2012. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med. 209:1219–1234. 10.1084/jem.20111622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K., Witherden D.A., and Havran W.L.. 2015. All hands on DE(T)C: Epithelial-resident γδ T cells respond to tissue injury. Cell. Immunol. 296:57–61. 10.1016/j.cellimm.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayes R.F., Mouhanna J.G., Nicolau I., Bourdeau F., Giannias B., Rousseau S., Quail D., Walsh L., Sangwan V., Bertos N., et al. 2019. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 4 e128008 10.1172/jci.insight.128008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd M.J., Kelly G., Dunn G., Way M., and Martin P.. 2006. Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil. Cytoskeleton. 63:415–422. 10.1002/cm.20133 [DOI] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Gregory C., and Haslett C.. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965–975. 10.1038/nri957 [DOI] [PubMed] [Google Scholar]
- Schäfer M., Dütsch S., auf dem Keller U., Navid F., Schwarz A., Johnson D.A., Johnson J.A., and Werner S.. 2010. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 24:1045–1058. 10.1101/gad.568810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., and Levy B.D.. 2018. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128:2657–2669. 10.1172/JCI97943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke M.H., and Allen J.E.. 2013. Beyond Stem Cells: Self-Renewal of Differentiated Macrophages. Science. 342 1242974 10.1126/science.1242974 [DOI] [PubMed] [Google Scholar]
- Soares M.P., Gozzelino R., and Weis S.. 2014. Tissue damage control in disease tolerance. Trends Immunol. 35:483–494. 10.1016/j.it.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Soares M.P., Teixeira L., and Moita L.F.. 2017. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 17:83–96. 10.1038/nri.2016.136 [DOI] [PubMed] [Google Scholar]
- Steele S., Brunton J., Ziehr B., Taft-Benz S., Moorman N., and Kawula T.. 2013. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog. 9 e1003562 10.1371/journal.ppat.1003562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B., Wood W., Galko M.J., Redd M.J., Jacinto A., Parkhurst S.M., and Martin P.. 2005. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168:567–573. 10.1083/jcb.200405120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B., Moreira S., Millard T., Evans I., Huang C.-Y., Sabet O., Milner M., Dunn G., Martin P., and Wood W.. 2010. Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J. Cell Biol. 189:681–689. 10.1083/jcb.200912134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart L.M., and Ezekowitz R.A.B.. 2005. Phagocytosis: elegant complexity. Immunity. 22:539–550. 10.1016/j.immuni.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Swierczak A., and Pollard J.W.. 2020. Myeloid Cells in Metastasis. Cold Spring Harb. Perspect. Med. 10 a038026 10.1101/cshperspect.a038026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., et al. 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 496:238–242. 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telorack M., Meyer M., Ingold I., Conrad M., Bloch W., and Werner S.. 2016. A Glutathione-Nrf2-Thioredoxin Cross-Talk Ensures Keratinocyte Survival and Efficient Wound Repair. PLoS Genet. 12 e1005800 10.1371/journal.pgen.1005800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.D., Noble K.E., Larbi K.Y., Dewar A., Duncan G.S., Mak T.W., and Nourshargh S.. 2001. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 97:1854–1860. 10.1182/blood.V97.6.1854 [DOI] [PubMed] [Google Scholar]
- Thuma L., Carter D., Weavers H., and Martin P.. 2018. Drosophila immune cells extravasate from vessels to wounds using Tre1 GPCR and Rho signaling. J. Cell Biol. 217:3045–3056. 10.1083/jcb.201801013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulon A., Breton L., Taylor K.R., Tenenhaus M., Bhavsar D., Lanigan C., Rudolph R., Jameson J., and Havran W.L.. 2009. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206:743–750. 10.1084/jem.20081787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C., Mildner A., and Jung S.. 2015. Macrophages: development and tissue specialization. Annu. Rev. Immunol. 33:643–675. 10.1146/annurev-immunol-032414-112220 [DOI] [PubMed] [Google Scholar]
- Wang J., Hossain M., Thanabalasuriar A., Gunzer M., Meininger C., and Kubes P.. 2017. Visualizing the function and fate of neutrophils in sterile injury and repair. Science. 358:111–116. 10.1126/science.aam9690 [DOI] [PubMed] [Google Scholar]
- Weavers H., Evans I.R., Martin P., and Wood W.. 2016a Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell. 165:1658–1671. 10.1016/j.cell.2016.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavers H., Liepe J., Sim A., Wood W., Martin P., and Stumpf M.P.H.. 2016b Systems Analysis of the Dynamic Inflammatory Response to Tissue Damage Reveals Spatiotemporal Properties of the Wound Attractant Gradient. Curr. Biol. 26:1975–1989. 10.1016/j.cub.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavers H., Wood W., and Martin P.. 2019. Injury Activates a Dynamic Cytoprotective Network to Confer Stress Resilience and Drive Repair. Curr. Biol. 29:3851–3862.e4. 10.1016/j.cub.2019.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster S.J., Daigneault M., Bewley M.A., Preston J.A., Marriott H.M., Walmsley S.R., Read R.C., Whyte M.K.B., and Dockrell D.H.. 2010. Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J. Immunol. 185:2968–2979. 10.4049/jimmunol.1000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A.B., Kahn C.R., and Wagner D.D.. 2015. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 21:815–819. 10.1038/nm.3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W., and Martin P.. 2017. Macrophage Functions in Tissue Patterning and Disease: New Insights from the Fly. Dev. Cell. 40:221–233. 10.1016/j.devcel.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A., Reichel C.A., Khandoga A., Corada M., Voisin M.-B., Scheiermann C., Haskard D.O., Dejana E., Krombach F., and Nourshargh S.. 2007. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood. 110:1848–1856. 10.1182/blood-2006-09-047431 [DOI] [PubMed] [Google Scholar]
- Woodfin A., Voisin M.-B., Imhof B.A., Dejana E., Engelhardt B., and Nourshargh S.. 2009. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 113:6246–6257. 10.1182/blood-2008-11-188375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A., Voisin M.-B., Beyrau M., Colom B., Caille D., Diapouli F.-M., Nash G.B., Chavakis T., Albelda S.M., Rainger G.E., et al. 2011. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 12:761–769. 10.1038/ni.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang F., Van Keymeulen A., Rentel M., and Bourne H.R.. 2005. Neutrophil microtubules suppress polarity and enhance directional migration. Proc. Natl. Acad. Sci. USA. 102:6884–6889. 10.1073/pnas.0502106102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Li Y., Xie Y., and Liu Y.. 2019. Different Faces for Different Places: Heterogeneity of Neutrophil Phenotype and Function. J. Immunol. Res. 2019 8016254 10.1155/2019/8016254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp B.G., and Kubes P.. 2013. NETosis: how vital is it? Blood. 122:2784–2794. 10.1182/blood-2013-04-457671 [DOI] [PubMed] [Google Scholar]
- Yolland L., Burki M., Marcotti S., Luchici A., Kenny F.N., Davis J.R., Serna-Morales E., Müller J., Sixt M., Davidson A., et al. 2019. Persistent and polarized global actin flow is essential for directionality during cell migration. Nat. Cell Biol. 21:1370–1381. 10.1038/s41556-019-0411-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S., Kim K.-W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., et al. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 38:79–91. 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.K., Deng Q., Cavnar P.J., Wu Y.I., Hahn K.M., and Huttenlocher A.. 2010. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell. 18:226–236. 10.1016/j.devcel.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E., Schmid I., Straumann A., Reichenbach J., Gleich G.J., et al. 2008. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14:949–953. 10.1038/nm.1855 [DOI] [PubMed] [Google Scholar]
- Zaman V., and Colley F.C.. 1972. Ultrastructural study of penetration of macrophages by Toxoplasma gondii. Trans. R. Soc. Trop. Med. Hyg. 66:781–782. 10.1016/0035-9203(72)90093-4 [DOI] [PubMed] [Google Scholar]