Abstract
Objective:
To determine how anti-retroviral therapy (ART/HAART) affects expression of apoptotic ligands and their death receptors in the blood and lymphoid tissues of HIV-infected patients and SIV-infected macaques.
Methods:
We analyzed mRNA expression of death molecules (TRAIL and FasL) and their receptors (DR5 and Fas) in blood and tonsils from HIV-infected patients (HIV+), HIV-infected patients receiving HAART (HAART) and HIV-uninfected (HIV−) donors in a cross-sectional study. We comparatively analyzed mRNA expression of TRAIL and DR5 in blood and lymph nodes collected longitudinally from SIV-infected macaques before and after ART.
Results:
Expression of TRAIL, FasL, DR5 and Fas were elevated in circulating CD4+ T cells from a group of HIV+ patients, compared to both HIV− donors and HAART patients. In a different study group, TRAIL, FasL, DR5 and Fas were increased in tonsils of HIV+ patients compared to HIV− donors, and HAART patients. However, tonsils from HAART patients showed reduced expression of TRAIL and FasL but not DR5 and Fas, compared to HIV+ patients. Similarly, data obtained in longitudinal study of SIV-infected macaques showed that ART reduced both TRAIL and DR5 in peripheral blood, but only TRAIL and not DR5, in lymph nodes from the same animals.
Conclusion:
These findings suggest that HAART/ART is ineffective in reducing expression of apoptotic death receptors in lymphoid tissue. However, analysis limited to blood leukocytes may not reveal such a defect. Our results highlight the persistence of an underlying immunologic condition that may prevent therapy-induced restoration of CD4+ T cells in lymphoid tissue.
Introduction
Current therapy for HIV-infected patients involves the combination of two or more antiretroviral drugs (highly active antiretroviral therapy, HAART), which reduces HIV-induced morbidity and mortality [1, 2]. HAART decreases HIV viral load [3], increases circulating CD4+ T cell counts, and reduces immune activation in blood and tonsils [4]. HAART also decreases apoptosis of CD4+ and CD8+ T cells in lymphoid tissue [5, 6], and was suggested to reduce the sensitivity of CD4+ T cells to Fas-mediated apoptosis [5]. HIV triggers FasL-mediated apoptosis of uninfected CD4+ T cells, whereas CD8+ T cell apoptosis may be driven by chronic immune activation [7]. Suppression of HIV replication reverses both of these effects, contributing to HAART-induced immune reconstitution.
TNF-related Apoptosis-Inducing Ligand (TRAIL), a TNF family member [8], induces apoptosis by binding to its death receptor (DR) 4 or 5 on target cells [9, 10]. CD4+ and CD8+ T cells from HIV-infected patients are more susceptible to TRAIL-induced apoptosis than uninfected CD4+ T cells, as shown in an in vitro human model [11], and in HIV-infected hu-PBL-NOD-SCID mice [12]. We previously reported that HIV induces TRAIL and DR5 expression on CD4+ T cells, leading to their apoptosis in vitro and ex vivo [13]. TRAIL expression is regulated by IFN-α produced by HIV-exposed plasmacytoid dendritic cells (pDC) [14]. Clinical studies reported that TRAIL levels and CD4+ T cells expressing DR5 are elevated in the blood of untreated HIV-infected patients [13].
Lymphoid tissues are the major sites of T cell depletion in HIV and SIV infection [15, 16]. We previously reported that tonsils of patients with progressive HIV disease express higher levels of IFN-α, TRAIL and DR5, compared to patients with non-progressing disease [17]. The T cell-rich areas of tonsils from progressing patients showed elevated IFN-α compared to non-progressing patients and uninfected controls [17]. These findings suggest that IFN-α induces expression of TRAIL in lymphoid tissue and potentially participates to apoptosis of DR5-expressing CD4+ T cells. However, the effects of HAART on TRAIL and DR5 expression in patients remain to be determined.
Our objective in this study was to determine whether HAART affects TRAIL/DR5 and FasL/Fas apoptotic pathways in blood and lymphoid tissues. Our findings indicate that TRAIL, FasL, DR5 and Fas mRNA expression were reduced in circulating CD4+ T cells from patients receiving HAART, compared to untreated patients. Surprisingly, in tonsils from HAART-treated patients, only expression of TRAIL and FasL, but not of their receptors, was significantly reduced compared to untreated patients. Similar results were obtained in a longitudinal study of ART-treated SIV-infected macaques. These findings raise a dichotomy between the effects of HAART on apoptotic ligands and their death receptors in lymphoid tissues.
Methods
Blood was collected from untreated HIV-infected (HIV+ patients, n=45), and from patients receiving HAART for more than 2 years (HAART patients, n=45) in a USAF natural history protocol at Wilford Hall Medical Center, Lackland AFB, TX, and from HIV-uninfected donors (HIV− donors) from the NIH Department of Transfusion Medicine under NCI and USAF IRB-approved protocols. We selected patients who successfully responded to HAART with the criteria of undetectable viral load and CD4 count >300.
CD4+ and CD8+ T cells were positively purified using CD4 or CD8 isolation kit (Miltenyi Biotech, Auburn, CA). Plasma TRAIL levels were measured by ELISA (Diaclone, Bensançon, France) according to the manufacturer’s instructions.
Tonsil biopsies were obtained from healthy seronegative individuals (HIV−; n=5), untreated HIV-infected patients (HIV+; n=6) or HAART patients (HAART; n=4) under a Karolinska University Hospital IRB-approved protocol. The clinical data of these patients were previously reported [18]. Untreated HIV+ patients had peripheral CD4 counts (cells per mm3) of 136–470 (mean, 341) and plasma viral loads (copies per ml) of 1,800–405,700 (mean, 155,860). HAART patients (at least 2 years of treatment) had peripheral CD4 counts of 340–1,220 (mean, 740) and undetectable plasma viral loads (<50). Immunohistochemical analysis of IFN-α from tonsil samples were performed as previously reported [17].
Colony-bred rhesus macaques that were 2–4 years of age, weighing 4–9 kg and seronegative for SIV, HTLV-1 and herpes virus B, were studied in compliance with NIH guidelines, and under an Animal Care and Use Committee approved-protocol (Advanced Biosciences Laboratories, Kensington, MD). Five macaques were infected intravenously with SIVmac251 as previously described [19], and antiretroviral therapy (ART) [20] was started 26 weeks later (see Fig. 2 legend). Leukocytes were harvested from blood and axillary lymph nodes (LN) collected prior to and 10 weeks after initiating ART.
Fig. 2.
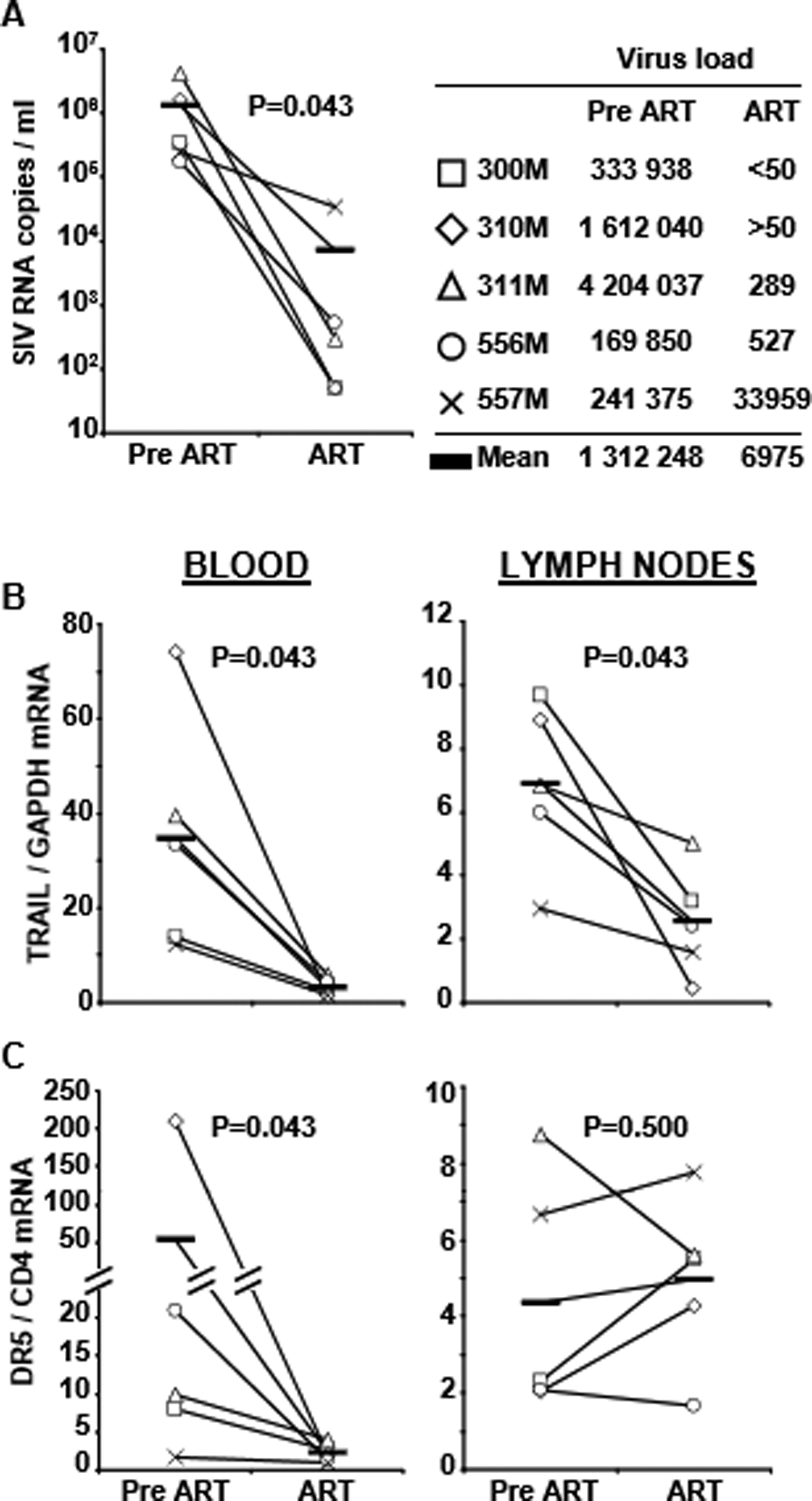
TRAIL and DR5 mRNA expression in leukocytes harvested from blood and axillary lymph nodes of 5 macaques before (Pre-ART) and 10 weeks after receiving therapy (ART). (A) Verification of ART-induced decrease in viral load, and clinical information of macaques in the study. (B) Comparison of TRAIL expression Pre-ART and ART in blood (left) and lymph nodes (right). (C) Comparison of DR5 expression Pre-ART and ART in blood (left) and lymph nodes (right). Horizontal bars indicate mean values of TRAIL and DR5
Total RNA was extracted from isolated human CD4+ and CD8+ cells, human whole tonsil tissue and macaque blood and lymph node leukocytes, and real time PCR was performed as previously reported [21]. Primers for human GAPDH, CD4, TRAIL, DR5, FasL and Fas were published in [17]. Primers for macaques GAPDH and CD4 we published in [21]. Primers for macques TRAIL were 5-TCCTGGGAATCATCAAGGAG-3 and 5-CCCTTTTCTTGGATGACCAG-3; primers for DR5 were 5-GTGCTTCGATGACTTTGCAG-3 and 5-CAGCTTTGGCCACCTTTATC-3.
Gene expression in biopsies was compared with the Mann–Whitney U test. Correlations were determined with Spearman’s rank correlation. A two-tailed P < 0.05 was considered to be significant.
Results
Plasma TRAIL protein concentrations were increased in HIV+ patients compared to HIV− donors and HAART patients (Fig. 1A left). We then compared mRNA levels of TRAIL in circulating CD4+ cells from HIV− (n=45), HIV+ (n=45) and HAART (n=45) (Fig. 1B left). TRAIL mRNA levels were increased in CD4+ T cells from HIV+ patients compared to those from HIV− donors. We observed decreased TRAIL mRNA in circulating CD4+ T cells in HAART patients, which was indistinguishable from HIV− donors’ cells. Importantly, levels of TRAIL protein and mRNA exhibit the same profile of expression in the three groups (Fig. 1A and 1B). Although the Fas/FasL pathway was reported to contribute to CD4+ T cell depletion [6], we did not observe differences in FasL mRNA levels among these three groups (Fig. 1C left).
Fig. 1.
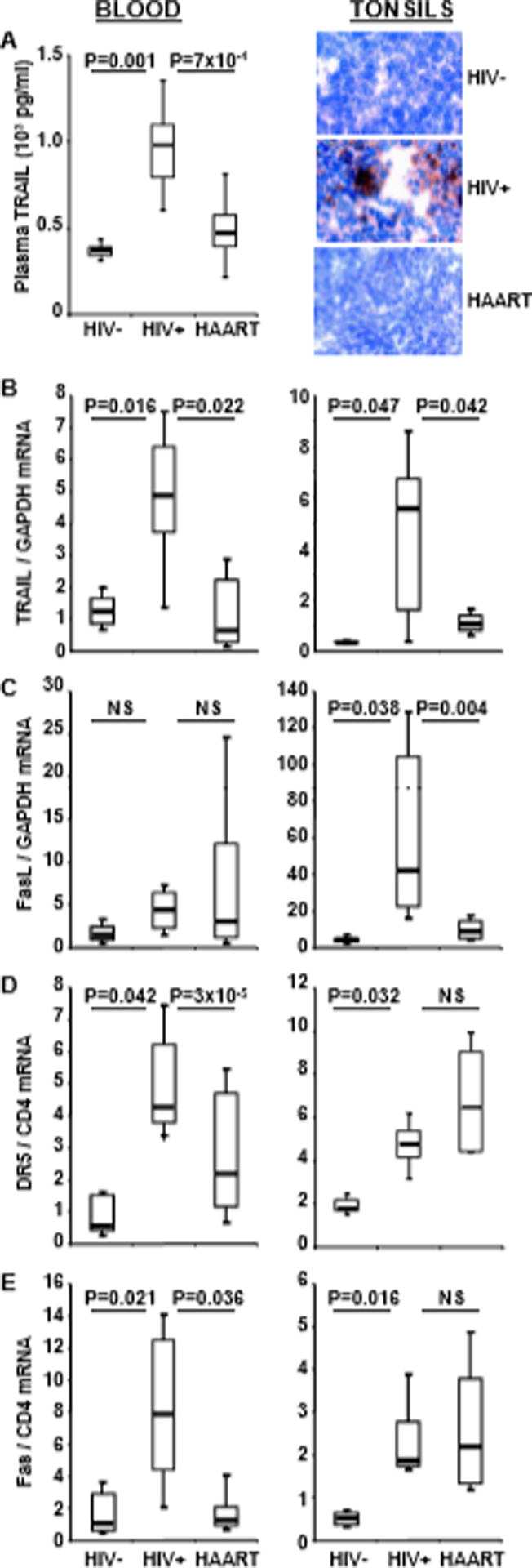
Plasma TRAIL, IFN-α and mRNA for apoptotic ligands and receptors in blood and tonsils. (A) left: Plasma TRAIL in HIV− donors, (n=45), HIV+ patients (n=45) and HAART patients (n=45). (A) right: IFN-α detected by immunohistochemistry in T cell-rich areas of tonsils from HIV− donors, HIV+ patients and HAART patients. mRNA expression for TRAIL (B), FasL (C), DR5 (D) and Fas (E) in isolated blood CD4+ cells (left) and in whole tonsil tissue (right) from HIV− donors, HIV+ patients and HAART patients. Data of B-E were compared by the Mann-Whitney test; NS = not significant (P>0.05). Horizontal bars: median values; upper and lower limits of boxes are 75th and 25th percentiles; vertical lines indicate 90th and 10th percentiles.
TRAIL and FasL induce apoptosis by binding to their respective receptors, DR5 and Fas. We found that mRNA levels for both DR5 (Fig. 1D left) and Fas (Fig. 1E left) were increased in CD4+ cells from HIV+ patients compared to HIV− donors and HAART patients. Importantly, no significant differences in DR5 or Fas were observed between HAART patients and HIV− donors. Also, no significant differences in TRAIL, FasL, DR5 and Fas expression were observed in CD8+ cells from the three groups (data not shown).
IFN-α is the main regulator of HIV-induced TRAIL expression [13, 14]. We recently reported that IFN-α, TRAIL and DR5 levels are elevated in tonsils of progressing compared to nonprogressing HIV+ patients [17] . Here we analyzed IFN-α expression by immunohistochemistry in tonsil biopsies from HIV+ patients who were or not receiving HAART, and from HIV− donors (Fig. 1A right). IFN-α was detected in the T cell-rich areas of tonsils from HIV+ patients, but not from HAART patients or HIV− donors.
We compared the tonsils of HIV−, HIV+ and HAART individuals for TRAIL and FasL mRNA expression. Higher levels of TRAIL (Fig. 1B right) and FasL (Fig. 1C right) mRNA were observed in tonsils of HIV+ patients compared to HIV− donors or HAART patients. In addition, we found that both TRAIL and FasL mRNA expression in tonsils correlated with patients plasma viral load (*R2=0.80, p=0.0001; R2=0.36, p=0.011, respectively).
Surprisingly, we found no difference in expression of the DR5 and Fas death receptors in tonsils from HIV+ and HAART patients, which both showed higher levels than those observed in HIV− donors (Fig. 1D and 1E right). Thus, in contrast to the reduced DR5 and Fas expression observed in circulating CD4+ cells in HAART patients, we found that neither DR5 nor Fas mRNA expression was reduced in the tonsils of HAART patients (compare Figs. 1D and 1E right with left). Because CD4 may be regulated by HAART, we also normalized Fas and DR5 mRNA using GAPDH housekeeping gene. We did not observed any dowregulation of Fas or DR5 mRNA in treated compared to untreated patients (Supplemental Fig 1) as we found using CD4 housekeeping gene. Furthermore, the high levels of death receptors in tonsils of HAART contrast with the reduced levels of death ligands in the same patients (compare Figs. 1B and 1C right with Figs. 1D and 1E right). This latter comparison supports the hypothesis that therapy was effective in reducing expression of both death ligands in the tonsils, but not of either death receptor in the same tissue samples.
Because these findings were unexpected, and the fact that our blood and tonsil studies were performed in different groups of patients, we longitudinally analyzed TRAIL and DR5 mRNA expression in leukocytes from blood and lymph nodes of five SIV-infected macaques before and 10 weeks after ART. The results indicate that ART reduced TRAIL in the blood of all five macaques (Fig. 2B left), and DR5 in the four that exhibited receptor expression prior to starting ART (Fig. 2C left). Similar to the human tonsil data, the macaque lymph node data showed that ART reduced TRAIL (Fig. 2B right), but not DR5 expression in the same animals (Fig. 2C right).
Discussion
CD4+ T cell depletion occurs mainly in lymphoid tissue during acute and chronic HIV infection [15], identifying lymphoid tissue and not blood as the primary site of T helper cell loss during HIV disease. Here we demonstrate that mRNA expression of the apoptotic ligands TRAIL and FasL in blood and tonsils of HAART-treated patients was reduced compared to untreated patients, complementing our previous findings of high plasma TRAIL in HIV+ patients with active viral replication [22] and increased tonsil IFN-α in progressing, but not non-progressing, HIV+ patients [17]. Similarly, expression of DR5 and Fas was reduced in the blood of HAART-treated patients. However, expression of these death receptors in patients’ tonsils was not affected by more than 2 years of HAART. We verified these human data in a SIV/macaque longitudinal study. Thus, TRAIL expression was reduced after 10 weeks of ART in blood and lymph nodes, whereas DR5 expression was reduced in blood, but not in the lymph nodes of these macaques. It is possible that DR5 is regulated with different kinetics than TRAIL, and may therefore require ART administration for more than the 10 weeks. However, the study conducted on human samples included patients who had received HAART for a minimum of 2 years, suggesting that the different effect of therapy on TRAIL and DR5 is not a mere consequence of limited length of therapy administration.
The failure of HAART to decrease apoptotic death receptor expression in lymphoid tissue could have important immunologic consequences. For example, other viruses can activate pDC to produce IFN-α, resulting in TRAIL and FasL expression [23]. Thus, if HAART reduces death ligands, but is ineffective in reducing death receptors in lymphoid tissue, coinfection or reactivation of latent non-HIV viruses could induce apoptotic ligands, which might result in the apoptosis of CD4+ T cell, that persistently express DR5.
Our comparison of apoptotic ligands and their receptors by CD4+ T cells in blood and tonsils show subtle but potentially important differences between HAART- and non-progressing patients. Non-progressing patients showed low tonsil expression of both death ligands and receptors [17], whereas death receptors were not reduced in tonsils of HAART patients (this study). This difference may partly account for the fact that HAART does not result in full immune restoration, despite controlling viral replication.
Supplementary Material
Acknowledgements
We thank the Fondation pour la Recherche Médicale (FRM) and Agence Nationale pour la Recherche sur le SIDA (ANRS) for its financial support. This research was supported by the Intramural Program of the Center for Cancer Research, NCI, NIH, and by the NIH Intramural AIDS Targeted Antiviral Program. JA is supported by the Swedish Foundation for Strategic Research, Swedish Cancer Foundation and Swedish Research Council. We also thank S. Anderson (Tri-Service AIDS Clinical Consortium) and M Dolan (Infectious Diseases Service), Wilford Hall Medical Center, Lackland AFB, Defense Institute for Medical Operations, Brooks City-Base, Tx for providing blood from HIV-1-infected patients.
JP Herbeuval and GM Shearer designed and performed research, wrote the paper and analyzed data. J Nilsson and A Boasso and AW Hardy performed human experiments, and analyzed data. A Boasso, M Vaccari, V Cecchinato and G Franchini designed, performed and analyzed macaque experiments. J Andersson and J Nilsson designed, performed and analyzed the human tonsil experiments.
References
- 1.Mouton Y, Alfandari S, Valette M, Cartier F, Dellamonica P, Humbert G, et al. Impact of protease inhibitors on AIDS-defining events and hospitalizations in 10 French AIDS reference centres. Federation National des Centres de Lutte contre le SIDA. Aids 1997,11:F101–105. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998,338:853–860. [DOI] [PubMed] [Google Scholar]
- 3.Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 1997,337:734–739. [DOI] [PubMed] [Google Scholar]
- 4.Dyrhol-Riise AM, Voltersvik P, Olofsson J, Asjo B. Activation of CD8 T cells normalizes and correlates with the level of infectious provirus in tonsils during highly active antiretroviral therapy in early HIV-1 infection. Aids 1999,13:2365–2376. [DOI] [PubMed] [Google Scholar]
- 5.Badley AD, Dockrell DH, Algeciras A, Ziesmer S, Landay A, Lederman MM, et al. In vivo analysis of Fas/FasL interactions in HIV-infected patients. J Clin Invest 1998,102:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyrhol-Riise AM, Stent G, Rosok BI, Voltersvik P, Olofsson J, Asjo B. The Fas/FasL system and T cell apoptosis in HIV-1-infected lymphoid tissue during highly active antiretroviral therapy. Clin Immunol 2001,101:169–179. [DOI] [PubMed] [Google Scholar]
- 7.Dyrhol-Riise AM, Ohlsson M, Skarstein K, Nygaard SJ, Olofsson J, Jonsson R, Asjo B. T cell proliferation and apoptosis in HIV-1-infected lymphoid tissue: impact of highly active antiretroviral therapy. Clin Immunol 2001,101:180–191. [DOI] [PubMed] [Google Scholar]
- 8.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999,179:859–870. [DOI] [PubMed] [Google Scholar]
- 9.Herbeuval JP, Lambert C, Sabido O, Cottier M, Fournel P, Dy M, Genin C. Macrophages From Cancer Patients: Analysis of TRAIL, TRAIL Receptors, and Colon Tumor Cell Apoptosis. J Natl Cancer Inst 2003,95:611–621. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997,277:818–821. [DOI] [PubMed] [Google Scholar]
- 11.Lichtner M, Maranon C, Vidalain PO, Azocar O, Hanau D, Lebon P, et al. HIV type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res Hum Retroviruses 2004,20:175–182. [DOI] [PubMed] [Google Scholar]
- 12.Miura Y, Misawa N, Maeda N, Inagaki Y, Tanaka Y, Ito M, et al. Critical Contribution of Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL) to Apoptosis of Human CD4(+) T Cells in HIV-1-infected hu-PBL-NOD-SCID Mice. J Exp Med 2001,193:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood 2005,106:3524–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M, Shearer GM. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 2005,102:13974–13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004,200:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, et al. SIV-specific CD8+T-cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, Anderson SA, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A 2006,103:7000–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol 2005,174:3143–3147. [DOI] [PubMed] [Google Scholar]
- 19.Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med 2000,6:1140–1146. [DOI] [PubMed] [Google Scholar]
- 20.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 2006,108:3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 2006,108:3808–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, Dolan MJ, et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood 2005,105:2458–2464. [DOI] [PubMed] [Google Scholar]
- 23.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol 2006,176:248–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


