Abstract
Selective estrogen receptor modulators (SERMs) exhibit varying agonist/antagonist activities on estrogen receptors in tissues, although most SERMs, including ospemifene, have agonist effects on bone. In this article, outcomes in relation to bone homeostasis, bone mineral density (BMD), and osteopenia–osteoporosis are examined in postmenopausal women during treatment with ospemifene for vulvar and vaginal atrophy (VVA), a component of the genitourinary syndrome of menopause. In cases 1 and 2, the women had established osteopenia or osteoporosis before the start of ospemifene treatment. After 6 months’ and 7 months’ treatment, respectively, marked reductions were observed in bone resorption (decreased levels of carboxy-terminal cross-linking telopeptide of type-1 collagen). The woman in case 3 had normal bone marker data and BMD prior to starting treatment with ospemifene. After 1 year, bone biomarkers and densitometry indicated improved bone health. Ospemifene 60 mg daily for treatment of VVA in postmenopausal women appears to benefit bone health although, because current evidence is based mainly on bone biomarkers, long-term studies are required to confirm this potential.
Keywords: bone biomarkers, bone homeostasis, ospemifene, vulvar and vaginal atrophy
Introduction
Estrogen inhibits the formation of osteoclasts by regulating the receptor activator of nuclear factor-κB ligand/receptor activator of nuclear factor-κB interaction. In conditions of estrogen deficiency, osteoclast formation increases causing an increase in bone resorption, trabecular thinning and perforation, and a loss of connection between trabeculae.1 Declining estrogen levels during menopause predispose women to decreased bone mineral density (BMD) and osteoporosis, increasing the risk of fractures.2 Osteoporotic fractures cause substantial morbidity and mortality with significant associated economic burden3 and impaired quality of life.4 All menopausal women should receive appropriate guidance for prevention and management of osteoporosis.
Estrogen therapy plays a central role in the health management of menopausal women. Beyond relieving symptoms and improving quality of life, estrogen-based therapy decreases the risk of osteoporotic fractures and, in younger women (aged < 60 years at initiation of treatment), may protect against certain chronic diseases that affect women after menopause.5 However, not all women are candidates for estrogen therapy.
Selective estrogen receptor modulators (SERMs) are a class of agents exhibiting differential agonist/antagonist activity on estrogen receptors,6 most with varying agonist effects on bone.4 Raloxifene, a second-generation SERM, is approved for prevention and treatment of osteoporosis in postmenopausal women.7 Bazedoxifene, a third-generation SERM, is indicated for treatment of postmenopausal osteoporosis in women at increased risk of fracture.8 Significant reductions have been demonstrated with both agents in the incidence of vertebral, but not hip, fractures.7,8
Ospemifene is a third-generation SERM. In the USA, ospemifene is indicated for treatment of moderate-to-severe dyspareunia and moderate-to-severe vaginal dryness, symptoms of vulvar and vaginal atrophy (VVA) associated with genitourinary syndrome of menopause.9 In Europe, ospemifene is approved to treat moderate-to-severe symptomatic VVA in postmenopausal women, who are not considered candidates for local vaginal estrogen therapy.10
In preclinical models, ospemifene had a positive antiresorptive effect on bone, as indicated by analyses of bone mass, strength, and histomorphology.6 In ovariectomized rats (a model of menopause in women), ospemifene inhibited bone loss and improved mechanical strength with effects comparable to those of estradiol.11 In postmenopausal women, ospemifene was superior to placebo in reducing bone turnover, with effects on some biomarkers (osteocalcin, carboxy-terminal cross-linking telopeptide of type-1 collagen [CTX-1]) similar to those reported for raloxifene and bazedoxifene in placebo-controlled studies.4
Outcomes in relation to bone homeostasis, BMD, and osteopenia–osteoporosis are examined in three postmenopausal women during treatment with ospemifene for VVA. As patient-specific information was deidentified to ensure anonymity, patient consent was not required.
Case 1
Case 1 involves a 59-year-old woman (bodyweight 77 kg; body mass index [BMI] 30 kg/m2), who entered menopause at age 49 years. Relevant family history included a mother with vertebral crush.
At an annual gynecological review, vaginal sonography revealed an endometrial polyp (confirmed as benign by pathology), which was removed by surgical hysteroscopy. During surgery, we observed signs of vaginal dryness, absence of elasticity, and mucosal bleeding on contact with the speculum. At a postsurgical appointment 1 week later, the patient mentioned that sexual relations were difficult, and she was lacking sexual desire. She had been managing her vaginal symptoms with local estrogen (estriol 0.5 mg) vaginal cream twice weekly for 4 months, but with minimal success. Cytology was compatible with vaginal atrophy. Her vaginal pH was 6.1.
Mammography was normal (American College of Radiology [ACR] type B, Breast Imaging Reporting and Data System [BI-RADS] 2).
Patient-rated scores for symptom intensity during the previous 4 weeks were 4/10 for pain on sexual intercourse, 6/10 for vaginal dryness, 0/10 for itchiness, and 3/10 for sexual desire (on a 0–10 scale where 0 = none and 10 = most). She rated her quality of life at about 50% (0% = worst possible and 100% = best possible).
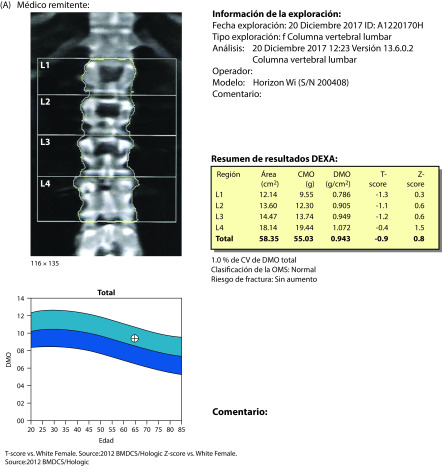
Taking into account the patient’s maternal history and her personal concerns about bone health, dual-energy x-ray absorptiometry was performed. T-scores were −2.5 for the lumbar spine (osteoporosis) and −1.7 for the hip (osteopenia). One year previously, the patient had been prescribed bazedoxifene 20 mg + calcium + vitamin D by her rheumatologist, although treatment was discontinued once T-scores improved (−1.88 in the lumbar spine and −0.94 in the hip). In view of her current bone densitometry results, blood tests were ordered to evaluate her bone status. A low serum vitamin D level (28 ng/mL) and elevated CTX-1 level (0.625 mg/L) suggested increased bone resorption.
To manage the patient’s VVA symptoms and preserve or improve her BMD in the context of osteoporosis/osteopenia and maternal history, ospemifene 60 mg + calcium + vitamin D was selected for treatment. Taking exercise (walking for at least an hour each day) was also recommended.
After 3 months’ treatment with ospemifene 60 mg + calcium + vitamin D, there was improvement in vaginal dryness and elasticity. Her vaginal pH was 4.7. The patient was able to have sexual intercourse and reported some return of sexual desire.
After 6 months’ treatment with ospemifene 60 mg + calcium + vitamin D, further improvement was observed. Her vaginal dryness was less severe, vulvovaginal tissues were more flexible, and dyspareunia was absent. She reported a level of sexual desire similar to that before menopause. Vaginal ultrasound indicated an endometrial thickness of 2 mm (normal for her menopausal status). Her serum vitamin D concentration had increased to 80 ng/mL (no longer deficient) and her CTX-1 level of 0.284 ng/mL indicated a reduction in bone resorption.
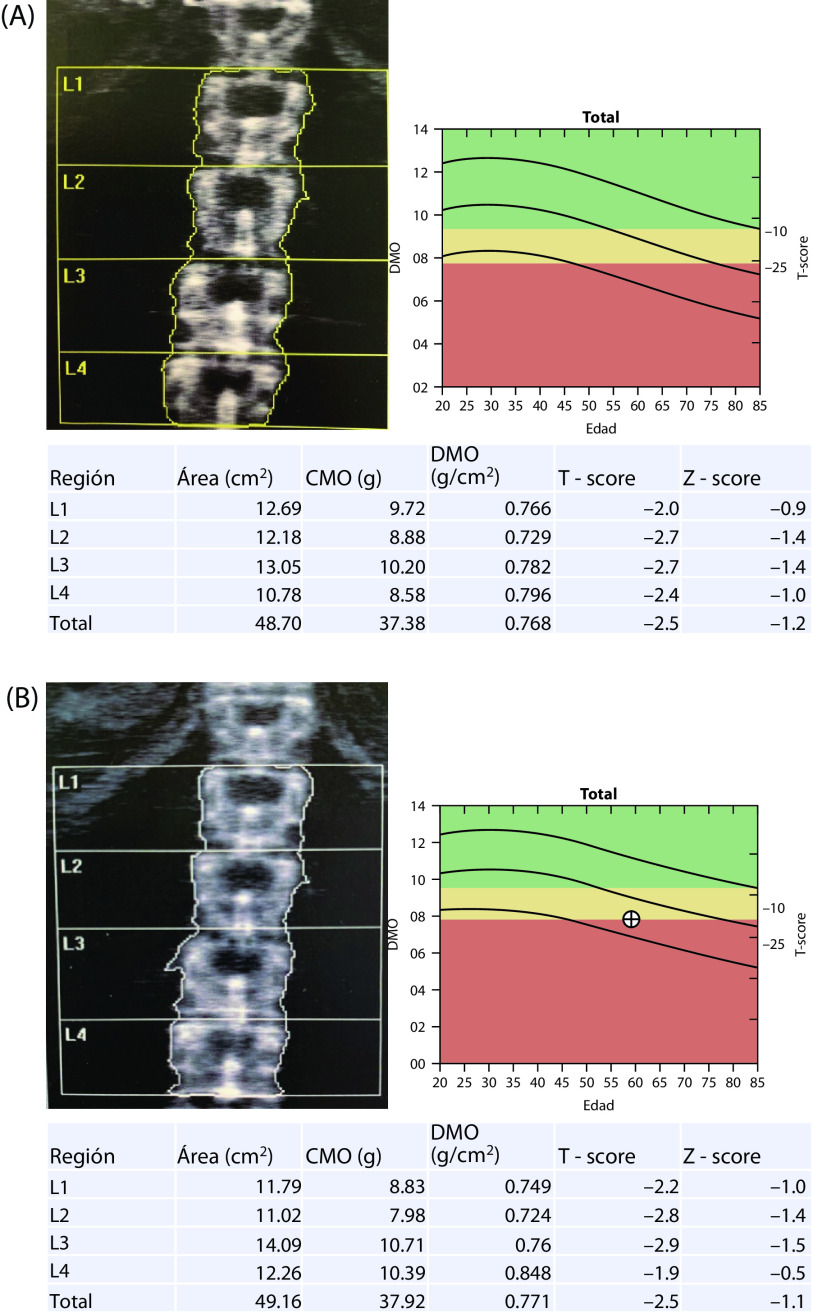
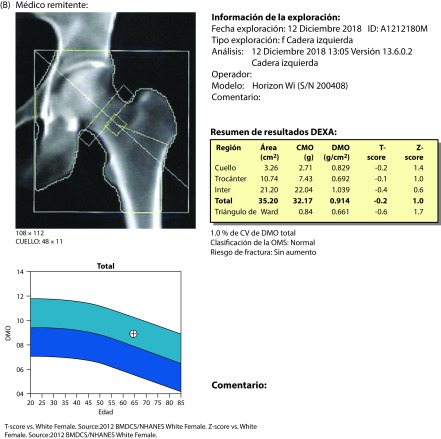
After 1 year of treatment with ospemifene 60 mg + calcium + vitamin D, a complete annual gynecological review showed no atrophy (by cytology) and an endometrial thickness of 2 mm. Mammography was normal (no change). T-scores were −2.5 for the lumbar spine (no change) and −1.4 for the hip (improvement) (Figure 1). Patient-rated scores for symptom intensity were 1/10 for dyspareunia, 0/10 for vaginal dryness, and 0/10 for itchiness. She rated her level of vaginal lubrication and ability to have sexual relations as acceptable, and scored her quality of life at about 88%.
Figure 1.
Densitometry results before (A) November 30, 2017 and after (B) November 20, 2018 treatment for 1 year with ospemifene 60 mg + calcium 600 mg + vitamin D3 1000 IU daily.
In this patient, treatment with ospemifene 60 mg + calcium + vitamin D for 1 year achieved several goals. VVA symptoms improved or disappeared, sexual desire was restored, the endometrium was preserved, and bone density was improved, in the absence of changes in breast density.
Case 2
Case 2 describes a 52-year-old woman (bodyweight 62 kg; height 1.62 m) with a gynecological history of two deliveries. Menarche was at 13 years, and menopause at 45 years. Medical history included hypothyroidism, which was treated with levothyroxine 50 μg/day, and a more recent diagnosis of Parkinson’s disease, which was treated with levodopa 50 mg/day, carbidopa 12.5 mg/day, and entacapone 200 mg/day.
The patient had previously been diagnosed with osteoporosis. At the time, T-scores were −2.51 for the lumbar spine (osteoporosis) and −1.53 for the femoral neck (osteopenia). She had no personal or family history of osteoporotic fractures; no other risk factors and no morphometric fractures were observed on radiological assessment of the spine (Figure 2). Calcium 500 mg + vitamin D3 400 IU daily were prescribed, but treatment had been discontinued 3 months prior to presentation.
Figure 2.
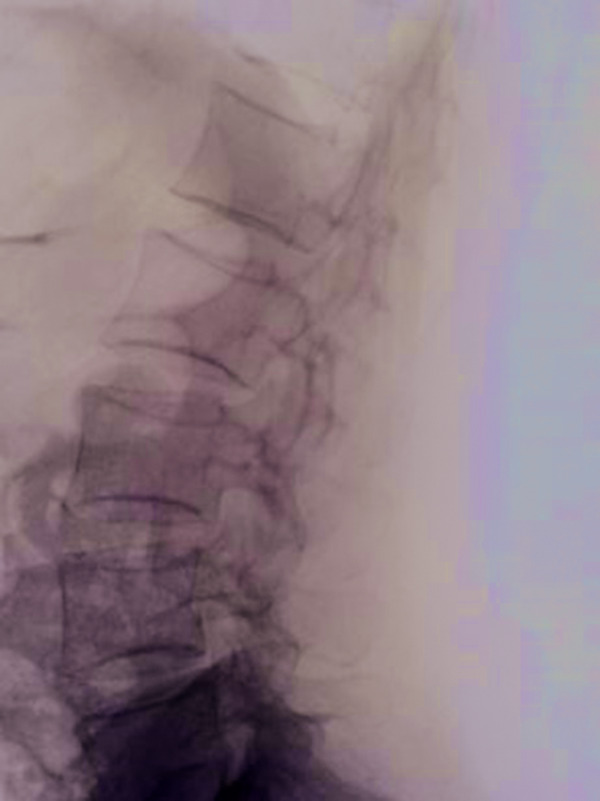
Lateral radiograph of the lumbar spine showing the absence of morphometric vertebral fractures.
At presentation in April 2017, a gynecological examination (Pap smear) showed atrophy (Figure 3). Her Vaginal Health Index score was 18/25 (lower scores indicate greater urogenital atrophy12), and she reported dyspareunia. During a quality-of-life assessment, she mentioned that her sexual dysfunction was causing relationship issues. Since onset of VVA symptoms at age 46 years, she had used local hormone therapy, but administration had become difficult due to her Parkinson’s disease. Taking into account the complete clinical picture including the need to reintroduce anti-osteoporotic measures, ospemifene 60 mg + calcium 600 mg + vitamin D3 1000 IU daily were selected for treatment.
Figure 3.
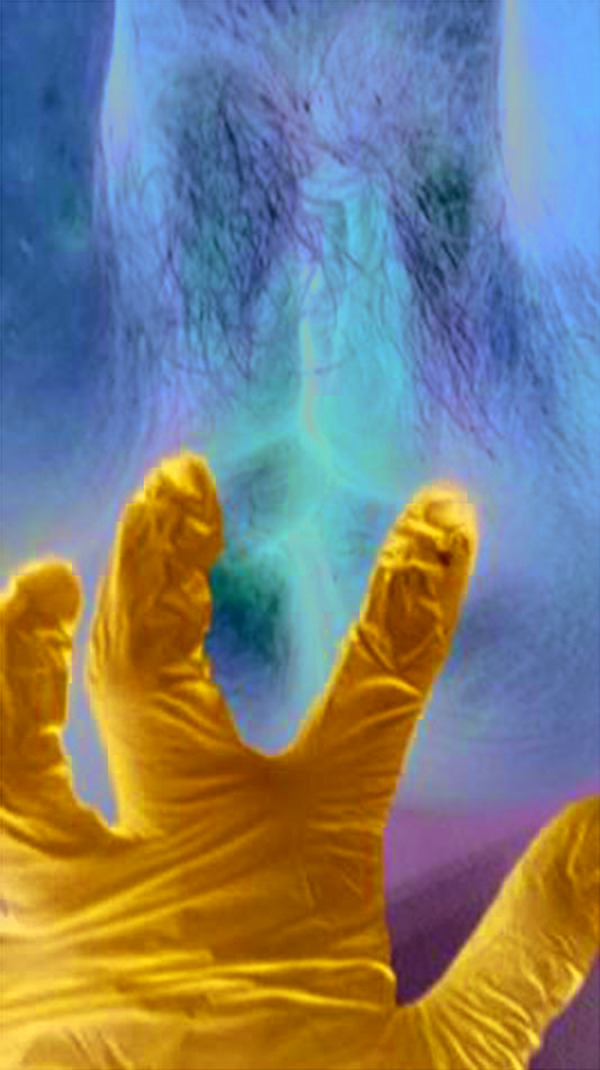
Vulvar inspection of the patient.
In November 2017, after 7 months’ treatment with ospemifene 60 mg + plus calcium + vitamin D3, the patient’s Vaginal Health Index score had improved to 21 and cytology revealed no atrophy. Re-evaluation of bone markers indicated improved bone health, although vitamin D levels remained low (<30 ng/mL) despite vitamin D administration. Improvements were observed in biomarkers of bone resorption (CTX-1, amino-terminal cross-linked telopeptide of type 1 collagen [NTX-1], pyridinoline, deoxipyridinoline) (Table 1).
Table 1.
Laboratory values before (baseline) and after 7 months’ treatment with ospemifene 60 mg + calcium 600 mg + vitamin D3 1000 IU daily.
| Variable | Baseline | 7 months | Change (%) |
|---|---|---|---|
| TSH (μIU/mL) | 1.317 | 1.149 | ↓ 12.7 |
| 25-hydroxy vitamin D (mg/L) | 25.19 | 25.42 | ↑ 0.9 |
| CTX-1 (mg/L) | 0.894 | 0.261 | ↓ 63.0 |
| NTX-1 (nM BCE/mM creatinine) | 92.8 | 33.4 | ↓ 64.0 |
| Pyridinoline, urinary (nmol/mmol creatinine) | 41.05 | 28.59 | ↓ 30.3 |
| Deoxypyridinoline, urinary (nmol/mmol creatinine) | 11.88 | 8.23 | ↓ 30.7 |
| Osteocalcin (mg/L) | 6.72 | 5.01 | ↓ 25.4 |
| P1NP (μg/L) | 79.43 | 49.34 | ↓ 37.9 |
CTX-1, carboxy-terminal cross-linking telopeptide of type-1 collagen; NTX-1, amino-terminal cross-linked telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal propeptide; TSH, thyroid stimulating hormone.
In choosing the most appropriate treatment for this patient, several factors were considered. At presentation, cytology indicated vaginal atrophy despite ongoing treatment with local estrogens, possibly reflecting poor (or worsening) adherence due to administration difficulties, although her Vaginal Health Index score suggested benefit from treatment. In patients with VVA, who are unsuited to receive topical estrogen treatment, ospemifene is a useful therapeutic alternative. After 7 months’ treatment with ospemifene, cytology showed vaginal trophism, which was reflected by the 3-point increase in her Vaginal Health Index score.
Case 3
Case 3 describes a 65-year-old woman (body weight 82 kg, BMI 32 kg/m2). Three pregnancies had resulted in two cesarean sections and one termination. Menopause occurred at 56 years. She had no exposure to menopausal hormone therapy.
The patient initially presented with VVA in 2012, at the age of 58 years. Local estrogen cream was used to manage symptoms, but without improvement. An estrogen vaginal ring inserted in 2015 brought no improvement after 3 months. Main symptoms of vaginal dryness and dyspareunia persisted and she continued to use local estrogen therapies.
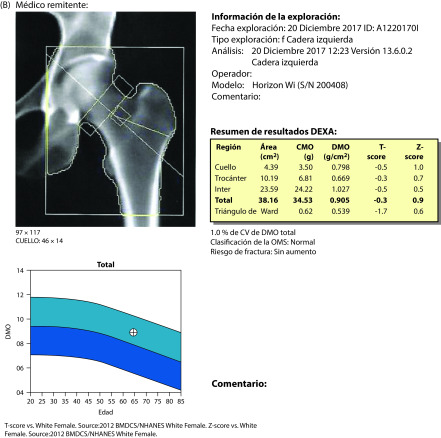
A gynecological assessment in December 2017 indicated atrophy of the uterus and ovaries. Breast ultrasound was normal (ACR type B2). Treatment began with oral ospemifene 60 mg/day. In view of her menopausal status and the documented antiresorptive effects of ospemifene, to monitor her progress, we performed bone densitometry and measured bone markers. T-scores were −0.9 for the lumbar spine and −0.3 for the hip (Figure 4). CTX-1 was 0.203 mg/L (normal <1.0 mg/L) and procollagen type 1 N-terminal propeptide (P1NP) was 37.09 mg/L (normal < 37.1 mg/L).
Figure 4.
Densitometry results in December 2017, before the start of ospemifene treatment: (A) lumbar spine; (B) hip.
After 3 months’ treatment with ospemifene, the patient reported improvement in vaginal dryness and dyspareunia, in the absence of treatment-related adverse effects.
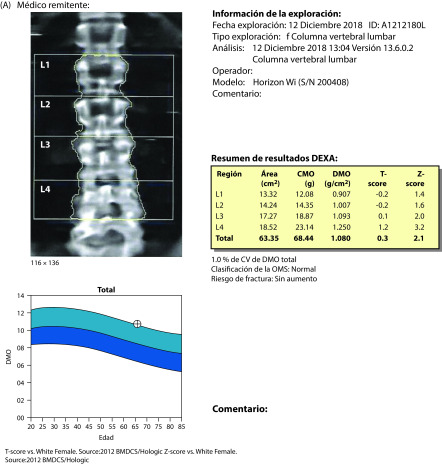
In December 2018, after 1 year’s treatment with ospemifene, she continued to be satisfied with treatment. A repeat gynecological ultrasound showed atrophy of the uterus and ovaries. Ultrasound breast mammography was normal (B2). T-scores were 0.3 for the lumbar spine and −0.2 for the hip (Figure 5). Bone mineral content was increased by 14.6% (from 0.943 to 1.080 g/cm2), CTX-1 was decreased by 15.7% (to 0.171 mg/L), and P1NP was increased by 12.9% (to 41.89 mg/L).
Figure 5.
Densitometry results in December 2018, 1 year after the start of ospemifene treatment: A) lumbar spine; B) hip.
Clinical overview
P1NP, procollagen type 1 C-terminal propeptide (P1CP), osteocalcin, and bone-specific alkaline phosphatase (bone ALP) are markers of bone formation. CTX-1, NTX-1, hydroxyproline, pyridinoline, and deoxypyridinoline are markers of bone resorption.13 As these processes are tightly coupled, increased indices of bone formation occur alongside increased bone resorption by osteoclasts.14 Increased markers of bone resorption may predict a subsequent fragility fracture.14
A comprehensive review of the effects of ospemifene on bone health, which examined cell-based studies, animal studies, and early clinical studies in healthy menopausal women, concluded that ospemifene may have bone protective effects based on an activity pattern on biochemical markers of bone turnover similar to those reported for raloxifene and bazedoxifene.1 In a recent placebo-controlled phase III trial involving 631 postmenopausal women with VVA of mean 8–9 years’ duration, ospemifene 60 mg/day for 12 weeks significantly improved VVA symptoms,15 and had positive effects relative to placebo on seven of nine biomarkers of bone resorption or bone formation, suggesting attenuation of bone loss.4 The benefits were similar irrespective of time since menopause (≥ 5 years or > 5 years) or baseline BMD (normal, osteopenia, osteoporosis).
The women in cases 1 and 2 had established osteopenia or osteoporosis before the start of ospemifene treatment. Reduced bone resorption during treatment, as shown by decreased CTX-1 levels, was particularly marked in these cases. Although post-treatment BMD data were not available for case 2, consistent favorable effects on biomarkers of bone resorption and bone formation were evident within 7 months from the start of ospemifene treatment. In case 3, BMD and bone marker data were within normal ranges prior to treatment. After 1 year’s treatment with ospemifene, densitometry and bone biomarker measurements indicated improved bone health; bone resorption (CTX-1) was slightly reduced and bone formation (P1NP) was slightly increased. Data reported for postmenopausal women enrolled in ospemifene clinical trials suggest that it has the potential to maintain bone health and possibly reduce fracture risk while treating VVA.4 However, as current evidence is based primarily on bone biomarkers, long-term studies are required to confirm this potential.
Other features of the case reports are noteworthy. One of the treatment goals in case 1, especially in view of an endometrial polyp, was to manage the patient’s VVA symptoms without impacting on the endometrium. In phase II/III clinical trials of ospemifene, no increase relative to placebo was observed in the incidence of endometrial hyperplasia or cancer among 1242 postmenopausal women exposed to ospemifene 60 mg for up to 52 weeks.16 Ospemifene is regarded as having weak partial agonist/antagonist activity on endometrial tissue.16,17 In case 2, the concomitant presence of Parkinson’s disease and osteoporosis/osteopenia is relevant as the symptoms of Parkinson’s disease (e.g., tremor at rest, bradykinesia, rigidity, postural instability) are recognized risk factors of reduced bone mass and associated conditions, such as osteoporosis or osteopenia, falls, and fractures.18 As observed in this patient, Parkinson’s disease symptoms can complicate the administration of local estrogen therapy. Oral ospemifene is a useful alternative for patients with VVA unsuited to receive local therapy.
Conclusion
As a systemic SERM, ospemifene 60 mg daily for treatment of VVA in postmenopausal women appears to have a positive effect on bone homeostasis irrespective of bone status, as observed in the three featured women who had osteoporotic/osteopenic or normal bone densitometry T-scores and some or no laboratory evidence of increased bone resorption. Improvement in bone biomarkers was evident within a few months of treatment start. Nevertheless, further studies are required to determine whether the effects of ospemifene on bone biomarkers translate into better bone health and reduced fracture risk in this at-risk population.
Acknowledgements
Medical writing assistance was provided by Kerry Dechant on behalf of Content Ed Net (Madrid, Spain). This article forms part of a Special Issue. All authors contributed to developing this Special Issue by sharing their experience with the benefit of patients in mind. The publication is expected to benefit gynecologists in their daily clinical practice by increasing knowledge and expertise.
Footnotes
Contributions: All authors contributed equally to the preparation of this case report. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Dr. Pingarron reports grants and personal fees from Pfizer, grants and personal fees from MSD, grants and personal fees from Shionogi, grants and personal fees from Theramex, grants and personal fees from Exeltis, grants and personal fees from FAES, grants and personal fees from IPRAD, and grants and personal fees from Effik, outside the submitted work. Dr. Gonzalez Rodriguez reports grants from Pfizer, grants from Amgen, grants from Gedeon Ritcher, grants from Exeltis, grants from Bayer, grants from MSD, grants from Procare Health, grants and personal fees from Shionogi, grants from Serelys, and personal fees from Mylan, outside the submitted work. Dr. Lilue reports personal fees from Shionogi, outside the submitted work. Dr. Palacios reports grants from Pfizer, grants from Amgen, grants from Gedeon Ritcher, grants from Exeltis, grants from Bayer, grants from MSD, grants from Procare Health, grants and personal fees from Shionogi, grants from Serelys, and personal fees from Mylan, outside the submitted work. The authors have also provided scientific support to Shionogi Spain by lecturing and/or taking part in Advisory Board meetings organized by Shionogi (Madrid, Spain). The authors’ time was compensated by Shionogi Spain according to local codes of practice. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/05/dic.2020-3-3-COI.pdf
Funding declaration: Medical writing assistance was funded by Shionogi (Madrid, Spain). This article forms part of a Special Issue funded by Shionogi (Madrid, Spain).
Correct attribution: Copyright © 2020 Pingarrón Santofimia MC, González Rodríguez SP, Lilue M, Palacios S. https://doi.org/10.7573/dic.2020-3-3. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 24 April 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23(6):638–644. doi: 10.1097/GME.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan SD, Lehman A, Nathan NK, Thomson CA, Howard BV. Age of menopause and fracture risk in postmenopausal women randomized to calcium + vitamin D, hormone therapy, or the combination: results from the Women’s Health Initiative Clinical Trials. Menopause. 2017;24(4):371–378. doi: 10.1097/GME.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komm BS, Chines AA. An update on selective estrogen receptor modulators for the prevention and treatment of osteoporosis. Maturitas. 2012;71(3):221–226. doi: 10.1016/j.maturitas.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 4.de Villiers TJ, Altomare C, Particco M, Gambacciani M. Effects of ospemifene on bone in postmenopausal women. Climacteric. 2019;22(5):442–447. doi: 10.1080/13697137.2019.1631789. [DOI] [PubMed] [Google Scholar]
- 5.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13(4):220–231. doi: 10.1038/nrendo.2016.164. [DOI] [PubMed] [Google Scholar]
- 6.Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78(12–13):1273–1280. doi: 10.1016/j.steroids.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Evista (raloxifene) Summary of product characteristics. 2017. [Accessed 11 November 2019]. Available at: https://www.medicines.org.uk/emc/product/3778/smpc.
- 8.Conbriza (bazedoxifene) Summary of product characteristics. [Accessed 11 November 2019]. Available at: https://www.ema.europa.eu/en/documents/product-information/conbriza-epar-product-information_en.pdf.
- 9.Osphena (ospemifene) Prescribing information. 2019. [Accessed 14 February 2020]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203505s015lbl.pdf.
- 10.Senshio (ospemifene) Summary of product characteristics. 2015. [Accessed 19 August 2019]. Available at: https://www.ema.europa.eu/en/documents/product-information/senshio-epar-product-information_en.pdf.
- 11.Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141(2):809–820. doi: 10.1210/endo.141.2.7342. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann G. Urogenital ageing: an old problem newly recognized. Maturitas. 1995;22( Suppl):S1–S5. doi: 10.1016/0378-5122(95)00956-6. [DOI] [PubMed] [Google Scholar]
- 13.Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017;5:18–26. doi: 10.1186/s40364-017-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017;63(2):464–474. doi: 10.1373/clinchem.2016.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderate-to-severe vaginal dryness: a phase 3, randomized, double-blind, placebo-controlled, multicenter trial. Menopause. 2019;26(6):611–621. doi: 10.1097/GME.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constantine GD, Goldstein SR, Archer DF. Endometrial safety of ospemifene: results of the phase 2/3 clinical development program. Menopause. 2015;22(1):36–43. doi: 10.1097/GME.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Pup L. Ospemifene: a safe treatment of vaginal atrophy. Eur Rev Med Pharmacol Sci. 2016;20(18):3934–3944. [PubMed] [Google Scholar]
- 18.Lee JY, Lim NG, Chung CK, Lee JY, Kim HJ, Park SB. Parkinson’s disease as risk factor in osteoporosis and osteoporotic vertebral fracture: prevalence study using National Inpatient Sample Database in Korea. J Korean Neurosurg Soc. 2019;62(1):71–82. doi: 10.3340/jkns.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]