Abstract
Background
COVID-19 infection is the most serious global public health crisis of the century. With no approved treatments against it, investigational treatments are being used despite limited safety data. Besides being at higher risk of complications of COVID-19 infection, patients with underlying cardiovascular disease are more likely to develop cardiac-related side effects of treatment. We present a case of sinus arrest with junctional escape related to lopinavir–ritonavir.
Case summary
A 67-year-old man, with underlying stable ischaemic heart disease, acquired COVID-19 infection. He had a prolonged duration of fever and cough. He subsequently developed acute respiratory distress and required intensive care unit (ICU) care. Given his severe infection, he was started on lopinavir–ritonavir. Hydroxychloroquine was not used as he had a prolonged QTc interval. During observation in the ICU, the patient developed recurrent episodes of sinus arrest with junctional escape. Initial concerns were of myocarditis, but he had no ST-segment changes on ECG, with mild elevations of highly sensitive troponin I and a normal transthoracic echocardiogram. A multidisciplinary team discussion involving the intensivist, infectious disease physicians, and cardiologist; the decision was made to stop treatment with lopinavir–ritonavir. Within 48 h, the bradyarrhythmia resolved. The patient did not require transvenous and permanent pacemaker insertion.
Conclusion
Current efficacy and safety evidence of lopinavir–ritonavir as a treatment in COVID-19 patients is limited. Although uncommonly reported, those with underlying cardiovascular disease are at increased risk of bradyarrhythmia-related adverse effects of lopinavir–ritonavir. When initiating investigational therapies, especially in patients with cardiovascular conditions, adequate counselling and close monitoring are required.
Keywords: COVID-19, Bradyarrhythmia, Sinus arrest, Lopinavir–Ritonavir, Antiretroviral, Case report
Learning points
Lopinavir–ritonavir is an antiviral drug used to treat HIV. Current efficacy and safety evidence for use in COVID-19 patients is limited. Those with underlying cardiovascular disease may be more prone to cardiac side effects of lopinavir–ritonavir, such as bradyarrhythmia, and require closer monitoring.
It is important to counsel the patient on risks and benefits and acknowledge the shortfalls when using investigational therapy for COVID-19.
Introduction
Much of the evidence for treatment of COVID-19 patients remains uncertain. In this rapidly evolving pandemic, time is of the essence. A myriad of treatments are being utilized despite inconclusive evidence. Treatment options include hydroxychloroquine, azithromycin, remdesivir, and lopinavir–ritonavir.1 Our patient, with COVID-19 pneumonia, was started on lopinavir–ritonavir and developed sinus arrest. We want to highlight the potentially fatal side effect of lopinavir–ritonavir-induced bradyarrhythmia with this case report.
Timeline
| Day of illness | Events |
|---|---|
| Day 1 | The onset of cough and fever. The patient visited his family physician. |
| Day 5 | Persistent fever and symptoms. COVID-19 swab testing done. |
| Day 6 | The test returns positive. He was admitted to hospital. |
| Day 9-10 | Haemoptysis started. Interval chest radiograph findings of pneumonia. The patient was started on lopinavir–ritonavir on day 10. |
| Day 11 | The patient was admitted to the ICU. |
| Day 12 | The patient required intubation and had sinus arrest. |
| Day 16 | Lopinavir–ritonavir was stopped. |
| Day 18 | Bradyarrhythmia resolved. |
Case presentation
A 67-year-old Chinese man presented with a 1-week history of fever and cough. He visited the family physician and was prescribed a week of co-amoxiclav 500 mg/125 mg t.i.d. He had no significant travel or contact history. He is an ex-smoker and has hypertension, hyperlipidaemia, and gout. He also has stable ischaemic heart disease (IHD), for which he presented with angina last year and underwent elective percutaneous coronary intervention at another hospital. His chronic medications included clopidogrel 75 mg o.m., rosuvastatin 20 mg o.n., bisoprolol 2.5 mg o.m., omeprazole 40 mg o.m., and cozaar (amlodipine 5 mg/losartan 50 mg) 1 tablet o.m. He has no family history of cardiac disease or arrhythmias.
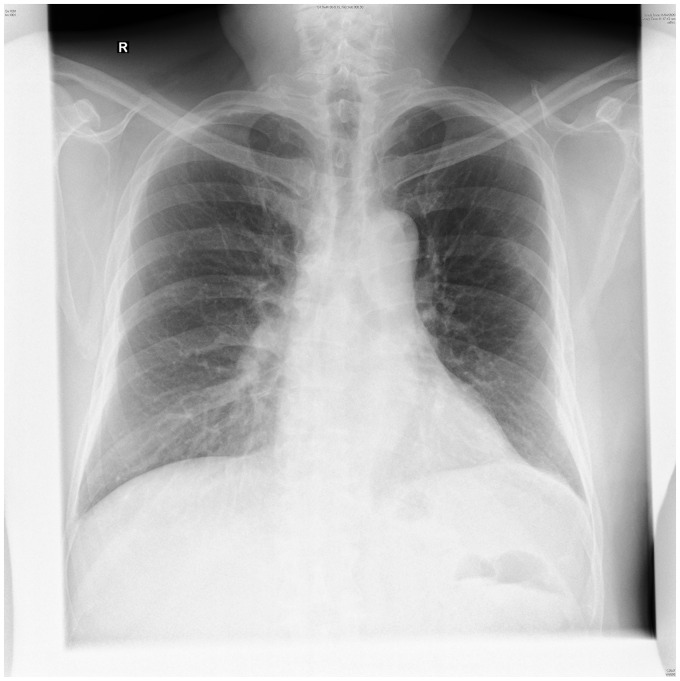
As his fever persisted, he revisited the clinic and was referred for COVID-19 testing. Test results were positive, and he returned to the hospital for further assessment and admission. Clinical examination was unremarkable. His vitals were normal except for a temperature of 38°C. Initial investigations revealed mild thrombocytopenia and lymphopenia. Chest radiograph did not show evidence of pneumonia (Figure 1). Baseline ECG was normal sinus rhythm with first-degree atrioventricular block (Figure 2).
Figure 1.
Normal chest radiograph on admission.
Figure 2.
Electrocardiogram on admission. Normal sinus rhythm with first-degree atrioventricular block.
On the third day, he had mild haemoptysis with saturations of 93–95% on room air. Repeat chest radiograph showed interval development of scattered bilateral consolidations (Figure 3). Inflammatory markers increased from a C-reactive protein of 16–54 mg/mL (normal <10 mg/mL) and ferritin of 410–1289 μg/L (normal <300 μg/L). Because of worsening clinical findings, hydroxychloroquine and azithromycin were considered but were deemed unsuitable as he had a prolonged QTc interval of 496 ms (Figure 2). The next day, he had temperatures up to 40°C and had intermittent blood-streaked sputum. He had no anaemia or coagulopathy. His oxygen saturations decreased to 91% and required 2 L of supplemental oxygen. He also developed bilateral lower zone crepitations. The infectious disease team was consulted, and he was started on lopinavir–ritonavir 400 mg/100 mg twice daily. He was brought to the intensive care unit (ICU) for closer monitoring and initiation of awake prone therapy. During the first day in the ICU, he had escalating oxygen requirements and respiratory distress which required intubation. He was concurrently started on intravenous piperacillin–tazobactam 4 g/0.5 g every 8 h for empirical coverage of bacterial superinfection.
Figure 3.
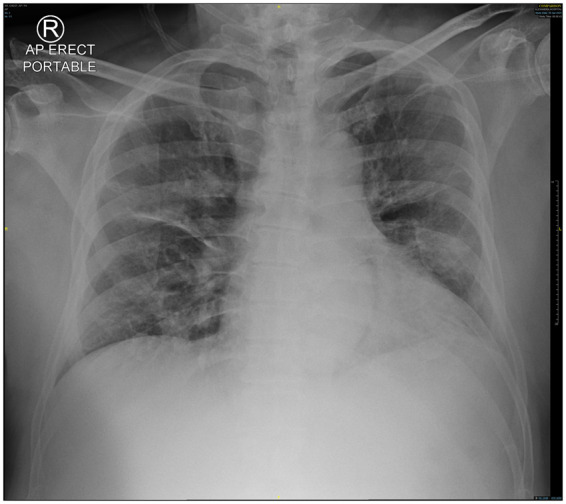
Development of bilateral consolidations.
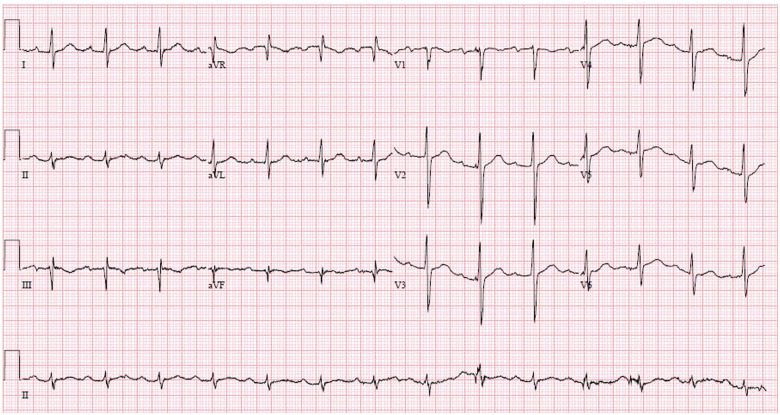
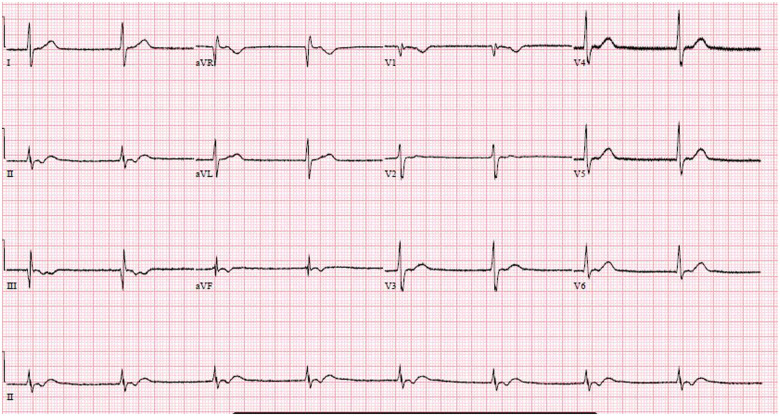
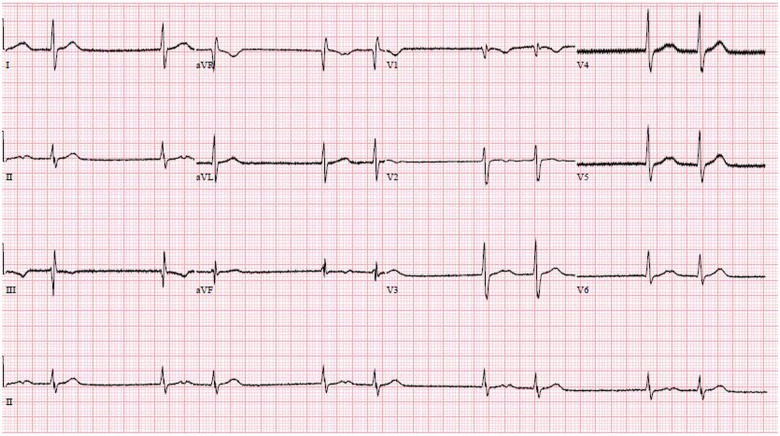
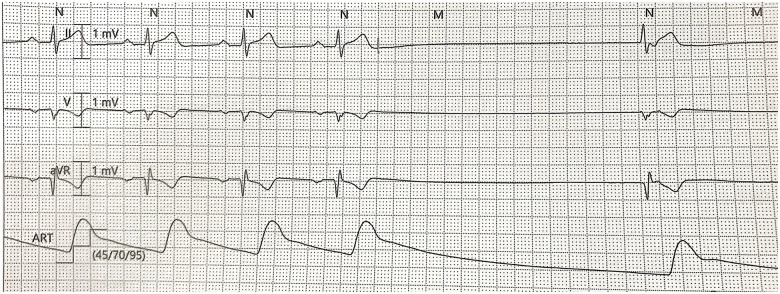
In the ICU, continuous ECG monitoring detected intermittent sinus pauses and junctional escape rhythm, but he remained normotensive (Figures 4 and 5). Importantly, his bisoprolol dose of 2.5 mg o.m. was stopped before the ICU admission. He also was not on ticagrelor, which may worsen bradyarrhythmias and interact with lopinavir–ritonavir. Electrolytes and thyroid function were normal. Despite optimization, he still had intermittent sinus arrests up to 3.2 s (Figure 6). The ICU team were worried about the possibility of COVID-19-related myocarditis. However, subsequent investigations suggested otherwise—there were no ST-segment evolving changes, and serial highly sensitive troponins were marginally elevated up to 33.6 ng/L (99th percentile upper reference limit 17.5 ng/L). Transthoracic echocardiogram showed preserved cardiac function and no regional wall motion abnormalities or significant pericardial effusion. After several days of supportive measures, he developed transient junctional escape rhythm of 35 b.p.m. with hypotension. He required emergency transcutaneous pacing with transition to intravenous dopamine infusion of 5 μg/kg/min. No obvious reversible causes of sick sinus syndrome were found. A multidisciplinary discussion, involving the intensivist, cardiologist, and infectious disease physicians, concluded that lopinavir–ritonavir was the likely cause of the bradyarrhythmia and was stopped. Although an uncommon side effect, lopinavir–ritonavir is associated with atrioventricular block in 0.1% of patients. It has a Food and Drug Administration warning for patients with IHD or conduction disease.2 Within 48 h, the bradycardic episodes resolved and he was extubated. After a day of observation, he was discharged from the ICU. Since then, he has recovered from COVID-19. He was last seen in the infectious disease outpatient clinic and reported no symptoms suggestive of bradycardia.
Figure 4.
Junctional rhythm.
Figure 5.
Escape–capture bigeminy.
Figure 6.
Sinus arrest with junctional escape rhythm.
Discussion
Lopinavir–ritonavir is a combination drug of a protease inhibitor with a CYP3A4 inhibitor used for the treatment of human immunodeficiency virus (HIV-1). It was effective in vitro against other coronaviruses, such as the 2003 SARS coronavirus.3 Like remdesivir, an antiviral drug developed for the treatment of the Ebola virus, both these drugs were purported to be effective against COVID-19, and diverted from their original purposes.
Within a short time, both positive and negative reports for lopinavir–ritonavir have been published.4–6 In the recent randomized controlled study in Wuhan China, no benefit was found in time to clinical improvement with 14-day treatment of lopinavir–ritonavir compared with standard of care.6 Virus RNA concentrations between the control and treatment subgroup were not significantly different. However, post-hoc subgroup analysis showed potential benefit if the treatment was started before 12 days. These early data are difficult to interpret due to the heterogeneous characteristics of the patients, disease severity, and confounding drugs. Other limitations include the non-blinded nature of the study and the use of glucocorticoids. Reported adverse events were predominantly gastrointestinal related. The authors reported an isolated case of prolonged QT interval but no bradyarrhythmias. Similar to the patients in that study, our patient was started on treatment before 12 days of symptom onset but he had to stop therapy due to adverse effects.
Before being used as a treatment for COVID-19, there have been case reports of lopinavir–ritonavir-induced cardiac arrhythmias in patients on treatment for HIV-1.7,8 As our patient had pre-existing IHD with first-degree atrioventricular block and prolonged interval QTc, he was at risk for cardiac-related side effects of lopinavir–ritonavir. Furthermore, as his baseline ECG was abnormal, he may have underlying genetic predisposition to arrhythmias, such as mutations of the SCN5A sodium channel. SCN5A polymorphisms modulate cardiac conduction velocity variability and increase susceptibility to a broad spectrum of arrhythmias.9 He was unfortunate to develop rare bradyarrhythmia side effects, but these resolved after stopping the offending medication. As the incidence and reports are few, it is difficult to postulate the mechanism of lopinavir–ritonavir-induced bradyarrhythmia. More reports and clinical trials are required to ascertain this.
Finally, we considered the potential of COVID-19 infection itself inducing arrhythmias. Although it is well reported that myocardial damage and myocarditis are caused by COVID-19, whether the virus itself causes arrhythmia is not known yet. Potential mechanisms of arrhythmias in COVID-19 may be due to acute cardiac injury from hypoxia, worsening of coronary perfusion, direct tissue damage, or a product of hyperacute systemic inflammatory response syndrome.10 In another study from China looking at the clinical characteristics of 138 patients with COVID-19, 17% of patients had arrhythmias. However, the arrhythmia types and treatment were not specified.11 Our patient did not have significant cardiac injury, ischaemia, or stress-induced cardiomyopathy, as evidenced by his preserved left ventricular ejection fraction and minimally elevated troponin. Myocarditis-related bradyarrhythmias are often related to atrioventricular block rather than sick sinus syndrome. In a recent case series publication, it was reported that two critically ill patients with COVID-19 developed new-onset sinus node dysfunction with haemodynamic instability, and the authors postulate that it was caused by the virus itself or myocardial inflammation. However, unlike our patient, both cases were not on investigational therapy and remained in stable sinus bradycardia.12
In unusual situations such as this pandemic, it is vital to discuss with patients the risk and benefits of drugs that lack conclusive evidence or guidelines. While racing against time to look for an effective treatment, many drugs will continue to surface as potential candidates with a promising hypothesis and in vitro or animal studies. However, the medical community needs to be hypervigilant for potential side effects which can be as serious as the COVID-19 infection. This case highlights the importance of considering lopinavir–ritonavir as a reversible cause of bradyarrhythmia in a COVID-19 patient, as well as the importance of a multidisciplinary team effort in the battle against COVID-19.
Lead author biography

Dr Laureen Wang is a practising clinician in cardiology and internal medicine at the National University Heart Centre, Singapore and Alexandra Hospital, Singapore. She graduated from the National University of Singapore, Yong Loo Lin School of Medicine in 2012. She was conferred membership of the Royal College of Physicians in 2014, and completed her residency in Internal Medicine in 2016. She subsequently did her cardiology training at the National University Hospital, Singapore. Dr Wang is also active on Preventive Medicine for chronic diseases and Women's health issues.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submissionand publication of this case report including image(s) and associatedtext has been obtained fromthe patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB.. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020;doi:10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 2.KALETRA (lopinavir/ritonavir) Label – FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021251s052_021906s046lbl.pdf (25 April 2020)
- 3. Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, Kao RTY, Poon LLM, Wong CLP, Guan Y, Peiris HSN, Yuen KYY.. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004;59:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young BE, Ong SWX, Kalimuddin S, Jenny GL, Seow YT, Jiashen L, Oon TN, Kalisvar M, Li WA, Tze MM, Sok KL, Danielle EA, Kian SC, Thean YT, Tong YN, Lin C, Zubaidah S, Lalitha K, Mark ICC, Monica C, Shawn V, Lin-Fa W, Boon HT, Raymond TPL, Vernon JML, Yee SL, David CL, for the Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020;323:1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Lin W, Cai W, Wen CY, Guan Y, Mo X, Wang J, Wang YP, Peng P, Chen SX, Hong W, Xiao G, Liu J, Zhang L, Hu F, Li F, Li F, Zhang F, Deng SX, Li L.. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI). medRxiv 2020;doi: 10.1101/2020.03.19.20038984. [Google Scholar]
- 6. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J.. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaubey SK, Sinha AK, Phillips E, Russell DB, Falhammar H.. Transient cardiac arrhythmias related to lopinavir/ritonavir in two patients with HIV infection. Sex Health 2009;6:254–257. [DOI] [PubMed] [Google Scholar]
- 8. Kikuchi Y, Genka I, Ishizaki A, Sunagawa K, Yasuoka A, Oka S.. Serious bradyarrhythmia that was possibly induced by lopinavir–ritonavir in 2 patients with acquired immunodeficiency syndrome. Clin Infect Dis 2002;35:488–590. [DOI] [PubMed] [Google Scholar]
- 9. Bezzina CR, Shimuzu W, Yang P, Koopman T, Tanck MWT, Miyamoto Y, Kamakura S, Roden DM, Wilde AAM.. Common sodium channel promoter haplotype in Asian subjects underlies variability in cardiac conduction. Circulation 2006;113:338–344. [DOI] [PubMed] [Google Scholar]
- 10. Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Crosbie MS, Chen Y, Han Y.. Cardiovascular manifestations and treatment considerations in covid-19. Heart 2020;doi: 10.1136/heartjnl-2020-317056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z.. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peigh G, Leya MV, Baman JR, Cantey EP, Knight BP, Flaherty JD.. Novel coronavirus 19 (COVID-19) associated sinus node dysfunction: a case series, Eur Heart J – Case Reports 2020;doi: 10.1093/ehjcr/ytaa132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.