Abstract
Paclitaxel-induced peripheral neuropathy (PIPN) and associated neuropathic pain are the most common and serious adverse effects experienced by cancer patients receiving paclitaxel treatment. These effects adversely impact daily activities and consequently the quality of life, sometimes forcing the suspension of treatment and negatively influencing survival. Patients are usually at high risk of developing PIPN if paclitaxel induces acute pain, which strongly suggests that an acute increase in the excitability of nociceptors underlies the chronic alterations of PIPN. KCNQ/Kv7 channels are widely expressed in the primary sensory neurons to modulate their excitability. In the present study, we show that targeting KCNQ/Kv7 channels at an early stage is an effective strategy to attenuate the development of PIPN. We found that paclitaxel did not decrease the expression level of KCNQ/Kv7 channels in the primary sensory neurons as detected by qRT-PCR and Western blotting. However, retigabine, which is a specific KCNQ/Kv7 channel opener, significantly attenuated the development of PIPN, as shown by both morphologic and behavioral evidence. We also observed that retigabine had no obvious effect on the chemosensitivity of breast cancer cells to paclitaxel. While retigabine has been approved by the FDA as an anticonvulsant, our study suggests that this drug can be repurposed to attenuate the development of PIPN.
Perspective
Paclitaxel-induced peripheral neuropathy and associated neuropathic pain are severe and resistant to intervention. The results of our study demonstrated that retigabine (a clinically available medicine) can be used to attenuate the development of paclitaxel-induced peripheral neuropathy.
Keywords: K+ channels, retigabine, hyperexcitability, paclitaxel, neuropathy, pain, prevention
1. Introduction
Paclitaxel is a commonly used chemotherapeutic agent for the treatment of breast and other cancers 12. Unfortunately, its use is often associated with significant neurotoxicity, especially resulting in sensory peripheral neuropathies that are often accompanied by neuropathic pain and are frequently severe and resistant to intervention. Paclitaxel-induced peripheral neuropathy (PIPN) results from damage to, or dysfunction of, peripheral nerves, including sensory, autonomic, and motor neurons. The incidence and severity of PIPN is duration- and dose-related, such that more than 60% of patients receiving paclitaxel-based chemotherapy experience peripheral neuropathy in first 3 months 58. However, neither analgesic medicine nor neuroprotective drugs yield promising effects on PIPN 19. Thus, the use of paclitaxel for cancer patients can be limited by unmanageable peripheral neuropathy, resulting in premature suspension of treatment and decreased survival.
PIPN is both persistent and refractory to therapy once it becomes chronic. Thus, preventing its development rather than palliation would be the best strategy. Recent studies indicate that paclitaxel induces inward currents and directly excites human dorsal root ganglion (DRG) neurons in vitro 39, 40. Paclitaxel also induces an immediate response of visceral and peripheral nociception in rodents 56. It is noteworthy that patients are usually at high risk of developing PIPN if paclitaxel induces acute pain 42, 53, which suggests that an acute increase in the excitability of nociceptors underlies the chronic alterations of PIPN. Thus, keeping neurons at rest or in a hyperpolarized state during paclitaxel administration could be a workable strategy to prevent/attenuate the development of PIPN and associated neuropathic pain.
Decreasing the hyperexcitability of peripheral neurons by calcium and magnesium infusions has been proposed as a strategy to prevent oxaliplatin-induced peripheral neuropathy 3, 23. However, this treatment has recently been evaluated as ineffective 27. An alternative therapeutic approach is to target K+ channels, which have an essential role in setting the resting membrane potential and thus controlling the excitability of neurons, including nociceptors. KCNQ channels (also called M channels) constitute the Kv7 subfamily (Kv7.1-Kv7.5) 32, 60. Many neurons in rat DRGs, including small neurons that respond to capsaicin (presumptive nociceptors), express Kv7.2, Kv7.3 and/or Kv7.5 49, 55. Agents that promote the opening of KCNQ/Kv7 channels have been shown to inhibit behavioral hypersensitivity in both inflammatory and other neuropathic pain models 5, 41, 49, 57, 65, and to provide some neuroprotection 6, 54. Notably, retigabine, a KCNQ/Kv7 channel opener 43, is already used clinically as an anticonvulsant 14. We thus tested whether early, brief application of retigabine will prevent the development of PIPN. Here we report that early activation of KCNQ channels with retigabine suppresses the degeneration of peripheral nerves and effectively reduces signs of chronic pain.
2. Materials and methods
All procedures conformed to the guidelines of the International Association for the Study of Pain, and were approved by the animal care and use committee of the University of Texas Medical Branch at Galveston. Male, adult, Sprague-Dawley rats (200–300g) were used in this study. Animals were housed 2 per wood chips bedding cage in a controlled environment (12 hour reversed light/dark cycle, 21 ± 1°C) with standard food and water. Cages were changed twice per week. The rats were allowed to adjust to their environment for a week before the experiment.
2.1. Administration of Paclitaxel and retigabine
The rats (200–300g) received intraperitoneal (i.p.) injections of clinically formulated paclitaxel (TEVA Pharmaceutical Inc, North Wales, PA) at a dosage of 2 mg/kg in 0.9% saline every other day for a total of four injections (Days 1, 3, 5, and 7, 8:00 to 10:00 am) 69. In order to match the clinical formulation of paclitaxel, the vehicle stock solution was made of equal parts of cremophor EL (Sigma) and Ethanol. Sham animals received an equivalent volume of the vehicle in 0.9% saline. The volume of paclitaxel and vehicle were injected according to their weight, i.e., a 250 g rat received 0.5 ml injection. For all of the experiments, rats were randomly divided into 3 groups: 1) Sham group – Rats received vehicle of paclitaxel and vehicle of retigabine (saline) in an identical schedule and volume as groups 2 and 3. 2) Paclitaxel plus retigabine – From day 1 to 10, paclitaxel-treated animals were administrated with retigabine (10 mg/kg, i.p.; Torondo Research Chemicals, Canada) twice daily. 3) Paclitaxel + vehicle group – From day 1 to 10, paclitaxel-treated animals received saline (vehicle of retigabine, i.p.) twice daily. On day 1, 3, 5, and 7, retigabine and its vehicle of morning session were injected 30 min prior to paclitaxel injection. The second injection of retigabine and its vehicle was performed at late afternoon.
2.2. Behavioral tests
All behavioral tests were performed in the active (dark) phase. Animals received tests for hindlimb reflex sensitivity prior to injection of paclitaxel (baseline) with or without retigabine treatment, and posttests on days 12, 28, and 35 after paclitaxel. All behavioral data were collected by staff blinded to any drug treatment during the animals’ active phase under red light. Prior to each test, the animal was habituated for 20 min per day in each of the testing chambers. Signs of mechanical sensitivity were tested with a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) delivered to the glabrous surface of the hindpaws. Thresholds were determined with the “up-down” method 11. Only one test series was applied to each hindpaw. Conditioned place preference (CPP) tests were performed as previously described 61, 66. Briefly, each rat was habituated to a CPP device (Med Associates Inc, VT) on the first day following a 3-day sequence of twice daily conditioning sessions (conditioning phase). In one session, the rat was restricted to the innately non-preferred white chamber for 60 min, starting 5 min after delivery of retigabine (10 mg/kg, i.p.; as an anesthetic for CPP test). In another daily session, the rat was placed for 60 min in the innately preferred black chamber 5 min after delivery (i.p.) of saline (vehicle of retigabine). In the testing phase, 1 day after the last conditioning session, the rat was placed (without any injection) in the open central gray chamber, and the time spent in each of the 3 chambers was recorded for 15 min. Conditioning to retigabine was indicated by greater time spent in the white compared to the black chamber during the testing phase.
2.3. Immunohistochemistry
After finishing all behavioral tests (day 28), a 3-mm glabrous skin section from the hindpaw was removed under deep anesthesia induced by injection of 75 mg/kg Beuthanasia (Merck Animal Health, Kenilworth, NJ) and fixed in Zamboni’s fixative overnight at 4 degrees. Tissues were transferred to 20% sucrose for at least 24 hours and frozen in optimal cutting temperature (OCT) compound. Tissues were then cut into 25 μm transverse sections. Sections were directly mounted onto gel-subbed glass slides, rinsed 3X in tris-buffered saline (TBS), and blocked with 5% serum for 1 hour. Sections were incubated with a combination of primary antibodies against PGP9.5 (Proteintech Group Inc, IL; Cat#17430) and collagen IV (Southernbiotech, AL; Cat# 1340) and then secondary antibodies. After rinsing in TBS, sections were mounted with coverslips with Vectashield (Vector Labs). Negative controls omitted primary antibodies. Labeled sections were examined with a Nikon Eclipse 600 (Nikon Instruments Inc, Melville, NY, USA) fluorescence microscope. The length of the epidermis within a field of view was measured using Image J software (NIH).
2.4. Quantification of intraepidermal nerve fibers
The intraepidermal nerve fibers were quantified as previously reported 7. Briefly, 4–5 slices from each animal and three fields of view from each slice were randomly selected. PGP9.5-stained nerve fibers that crossed the dermal/epidermal junction (stained with collagen IV) were counted, and the length of the epidermis within each field of view was measured. IENF density was calculated as the total number of fibers per unit length of epidermis (IENF/mm). Tissues were collected and marked by another technical staff member and treatment information was revealed only after IENF quantification.
2.5. Quantitative RT-PCR analysis
Total RNA was extracted from homogenized DRGs dissociated 12 days after initial paclitaxel treatment with on-column DNase digestion (E.Z.N.A. Total RNA Kit I) and cDNA was synthesized by MMLV reverse transcriptase (Invitrogen) using random primers. Rat KCNQ2 primers were 5’-CCGGCAGAACTCAGAAGAAG-3’ (forward) and 5’-TTTGAGGCCAGGGGTAAGAT-3’ (reverse) 55; rat KCNQ3 primers were 5’-ATACACATTTATCTGCTCTTCCTTTTA-3’ (forward) and 5’-TGCTCTCAGTTTATCCGAATCAA-3’ (reverse) 10, and rat GAPDH primers were 5’-CCCCCAATGTATCCGTTGTG-3’ (forward) and 5’-TAGCCCAGGATGCCCTTTAGT-3’ (reverse) 52; rat KCNQ5 primers were 5’-CCTGGCGTACACGAGAGT-3’ (forward) and 5’-TTTGACTGGGCGAACTGA-3’ (reverse). Abundance of mRNA was measured by real-time PCR (LightCycler 480; Roche) with SYBR Green Master Mix (Sigma). Preincubation at 95°C for 3 min was followed by 45 amplification cycles (95°C for 30 s, 57°C for 30 s, and 72°C for 30 s) and fluorescence collection at 60°C. Gene expression was normalized to GAPDH and averaged over three replicates from each of five animals in each group. Two independent experiments were performed.
2.6. Western Blotting
After finishing all behavioral tests, animals were deeply anesthetized with Beuthanasia and perfused with ice-cold PBS. The L4 and L5 of the spinal cords and dorsal root ganglions from each rat were removed and immediately placed in a 1.5 ml Eppendorf tubes on dry ice. Tissues were homogenized in 500 μl of lysis buffer (RIPA, Teknova) containing a protease inhibitor cocktail (Sigma). After homogenization, samples were sonicated 3 times (10 s pulses), and centrifuged at 14,000 rpm for 10 min at 4 °C. The protein concentration of lysates was determined by the BCA assay (Pierce BCA Protein Assay Kit). Samples were prepared for SDS-PAGE (Bio-Rad, 4–20% Tris-HCl) by 1:1 dilution with Laemmli sample buffer, and 30 μg of protein was loaded in each well. After electrophoresis, the gel was transferred to a PVDF membrane and blocked with 10% nonfat dry milk in PBS + 0.1% Tween 20 prior to incubation with antibody against GFAP (Millipore, USA; AB5541), KCNQ5 (Alomone Labs, Israel; APC-155), Iba-1 (Wako, 019–19741), and β-actin (Abcam, MA; ab8226) overnight at 4° C. The membrane was incubated with HRP-conjugated anti-rabbit or anti-mouse IgG (Jackson ImmnuoResearch, PA) for 1hour at room temperature and developed using the ECL kit (Pierce). Protein expression was quantified by optical density using Image J software (NIH). Color molecular weight standards were run on each gel and β-actin was detected as a loading control.
2.7. Electron microscopy and analysis of mitochondria
Analysis of axon mitochondria by electron microscopy was performed as previously described 21. Briefly, the tibial nerves (0.5 cm) excised from the lower leg of the rats were post-fixed for 2 hours in fixative containing 1% glutaraldehyde and 1% paraformaldehyde in 0.1 M PB (pH 7.4), followed by incubation in 10% sucrose at 4° C for 12 hours. Tissues were then incubated in 1% osmium tetroxide, dehydrated in ascending concentrations of alcohol and propylene oxide at room temperature, and embedded in Epon-Araldite. Ultrathin cross-sections of 70 nm were prepared with a microtome using a diamond knife, collected onto Formvar-coated grids, and counterstained. Four ultrathin cross-sections from each animal were randomly selected and sampled to take electron photomicrographs with a JEOL JEM-1400 transmission electron microscope (Jeol, USA) at three magnifications of X800, X3000 and X40000. Electron photomicrographs that were taken at X3000 magnification help us to identify A-fibers, C-fibers and Remak bundles, as photomicrographs taken at X40000 magnification were used to determine normal and abnormal morphology of mitochondria. Mitochondria in axons (A-fibers and C-fibers) were viewed and photographed randomly by same investigator blinded to treatment conditions. As previously reported 21, mitochondria in the axoplasm of each axon and in the cytoplasm of each myelinating and non-myelinating Schwann cell were counted. Normal mitochondria had at least one axis of at least 165 nm in length with intact double membranes. Mitochondria were counted as atypical if they had at least a two-fold increase in diameter and/or if 50% or more of the interior was electron-lucent. Nerve fibers containing one or more atypical mitochondria were counted.
2.8. Tumor cytotoxicity assay
SKBR3 cells (ACCT, VA) were seeded at a density of 2.5 × 104 cells/cm2 onto 24 well plates for experiments on the following day. The cell viability was assessed by measuring mitochondrial activity in reducing MTT (Thiazolyl blue tetrazolium bromide, Fisher scientific, 158990050) to formazan. After exposure to paclitaxel (0, 1, 5, 10, 20, 40, 60, 80, and 100 nM) with and without retigabine (10 μM) for 24, 48, or 72 hours, cells were washed with Dulbecco’s phosphate-buffered saline (DPBS), then 500 μl of medium and 50 μl of MTT assay solution (5 mg/ml) were added and incubated at 37°C in 5% CO2 for 4 hours. The incubation medium was carefully removed and re-suspend formazan in 500 μL DMSO. The amount of formazan dye was measured from the absorbance at 700 nm with a reference wavelength of 620 nm using a microplate reader.
2.9. Data Analysis
Analysis was performed with Sigmaplot 14 (Systat software, San Jose, CA) and Prism 7.0 (Graphpad, La Jolla, CA). Animal numbers were estimated on the basis of power analysis, past experience with each type of study, and published reports. Statistical data were presented as mean ± S.E.M. All comparisons among animal groups were tested for significance using one-way ANOVA with repeated measures followed by Bonferroni’s post-hoc tests, or repeated measures two-way ANOVA followed by Sidak’s multiple comparison tests. Abnormal mitochondria incidence among different group of animals was compared using Chi-square tests. For all statistical analyses, the α level is 0.05, P < 0.05 was considered to be statistically significant. Statistically significant differences were indicated in each figure (*, p < 0.05; **, p < 0.01; ***, p < 0.001). The n in all experiments is the number of rats tested in each condition except where otherwise indicated.
3. Results
3.1. The expression level of KCNQ channels in primary sensory neurons
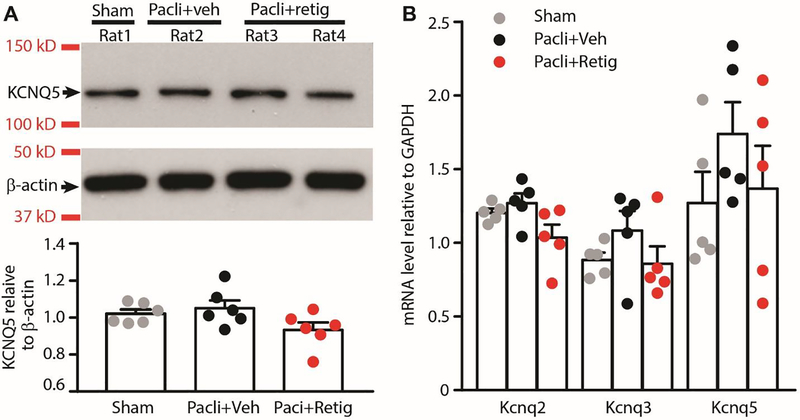
KCNQ channels are widely expressed in the primary sensory neurons 49, 55. However, their expression in primary sensory neurons was suppressed in some chronic pain models 55, 72. As an ideal target for PIPN treatment, one of the requirements is that there are enough channels remaining during PIPN development. Thus, we tested whether the expression of KCNQ channels are altered by treatment with paclitaxel. As KCNQ2, KCNQ3, and KCNQ5 are the major subtypes expressed in DRG neurons 49, 55, we found that the protein level of KCNQ5 in DRGs was unchanged after paclitaxel plus brief vehicle or retigabine treatment, as revealed by Western blot analysis. Although we were able to measure KCNQ5 protein levels in DRGs by Western blot, we did not find reliable antibodies against KCNQ2 and KCNQ3 to measure their protein levels. Instead, the qRT-PCR analysis was used to measure mRNA expression of KCNQ2 and KCNQ3 in DRGs. We found that paclitaxel treatment did not significantly alter KCNQ2, KCNQ3, and KCNQ5 mRNA levels in the L4 and L5 DRGs as compared to the levels from sham treated animals (Fig. 1). We then tested the expression of KCNQ channels after repeated retigabine application with paclitaxel. Retigabine did not affect significantly mRNA expression of either KCNQ2, KCNQ3, or KCNQ5 compared to control groups and paclitaxel-treated group respectively. Thus, these data indicate that the expression of KCNQ channels are not altered following paclitaxel treatment with or without brief retigabine co-administration.
Figure 1.
KCNQ channel expression in DRGs after repeated application of paclitaxel and retigabine. (A) Western blot analysis showing lack of effect of paclitaxel and retigabine on KCNQ5 protein expression in L4 and L5 DRGs. Upper panel: Bands of KCNQ5 and β-actin probed with specific antibodies. Lower panel: relative protein expression levels of KCNQ5 compared to β-actin. P = 0.089, F(2,15) = 2.855, one-way ANOVA with repeated measures followed by Bonferroni’s post-hoc tests. Dots in columns represent one animal each. Six animals were used for each group. (B) qRT-PCR analysis showing Kcnq2, Kcnq3 and Kcnq5 mRNA levels in L4 and L5 DRGs 28 days after initial paclitaxel/retigabine treatments. All data are normalized to GAPDH. Dots in columns represent one animal each. P = 0.0669, F(2,12) = 3.418 for KCNQ2; P = 0.2859, F(2,12) = 1.329 for KCNQ3; P = 0.3790, F(2,1) = 1.053 for KCNQ5. One-way ANOVA with repeated measures followed by Bonferroni’s post-hoc tests. Five animals were used for each group, and the experiments were performed twice independently. Pacli, paclitaxel; retig, retigabine.
3.2. Combining retigabine with paclitaxel mitigates the development of neuropathic pain.
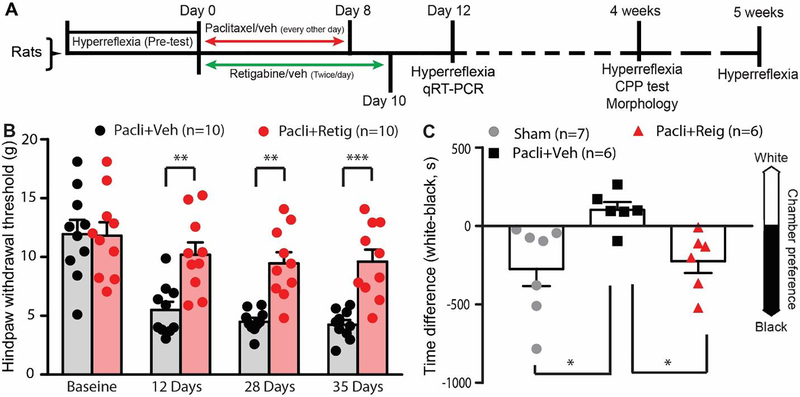
Recent evidence suggests that paclitaxel can acutely excite human primary sensory neurons 39. In addition, patients with paclitaxel-induced acute pain are usually at high risk of developing PIPN 42, 53. These results suggest that an acute increase in the excitability of nociceptors could underlie the chronic alterations of PIPN. We therefore co-delivered retigabine with paclitaxel to animals and measured behaviors reflective of nociceptor function. Behavioral pretests of intact animals were performed prior to paclitaxel treatment. Animals were then counterbalanced to different groups to ensure an equal starting point for the experiment. From day 1 to 10, animals were treated with paclitaxel plus brief retigabine (10 mg/kg in 1 ml saline, i.p.) or its vehicle twice daily. The animals received posttests 12, 28, and 35 days after initial treatment with paclitaxel (Fig. 2A). While paclitaxel induced mechanical hypersensitivity when brief vehicle (for retigabine) was co-administrated, mechanical hypersensitivity was significantly alleviated in animals when brief retigabine was co-administrated with paclitaxel at all time points tested (Fig. 2B). As compared to the paclitaxel plus brief vehicle treatment group, the hindpaw withdrawal threshold to von Frey stimulation was significantly increased in the brief retigabine plus paclitaxel group at three different time points (Fig. 2B). Cancer patients who developed PIPN usually report spontaneous pain, so we determined whether the development of spontaneous pain in PIPN animals was also attenuated by early brief application of retigabine using the conditioned place preference test (CPP) 28 days after initial paclitaxel treatment 61, 66. Similar to what we reported previously 61, no significant effects on CPP were found in sham animals when 3 daily conditioning retigabine (as analgesic) injections were paired with placement in a white chamber and identical conditioning saline injections were paired with the black chamber. However, the paclitaxel-treated rats that received early brief applications of the vehicle (paclitaxel + vehicle group), but not brief retigabine (paclitaxel + retigabine group), developed a significant preference for the innately less-preferred white chamber after pairing conditioned analgesic injections with placement in this chamber (Fig. 2C). These data suggest that brief application of retigabine during paclitaxel exposure attenuates the development of spontaneous pain and mechanical hypersensitivity.
Figure 2.
The effect of early repeated application of retigabine on the development of chronic behavioral hypersensitivity and spontaneous pain after paclitaxel. (A) Timeline for the experiments. (B) Mechanical hypersensitivity of hindpaws was measured 12, 28, and 35 days after initial paclitaxel treatment. Dots in columns represent one animal each. Repeated measures two-way ANOVA followed by Sidak’s multiple comparison test (treatment F(1, 9) = 21.58, p = 0.0012; time F(3, 27) = 34.16, p < 0.0001; interaction F(3, 27) = 4.546, P = 0.0105. Baseline, P > 0.999; 12 days, P = 0.0024; 28 days, P = 0.0014; 35 days, P = 0.0006. (C) Conditioned place preference tests were performed 4 weeks after treatment. Dots in each column represent individual rats tested in each condition. P = 0.039, F(2, 16) = 4.0, One-way ANOVA, Pacli, paclitaxel; retig, retigabine. *, p<0.05.
3.3. Combining retigabine with paclitaxel attenuates the morphological alterations of peripheral neuropathy
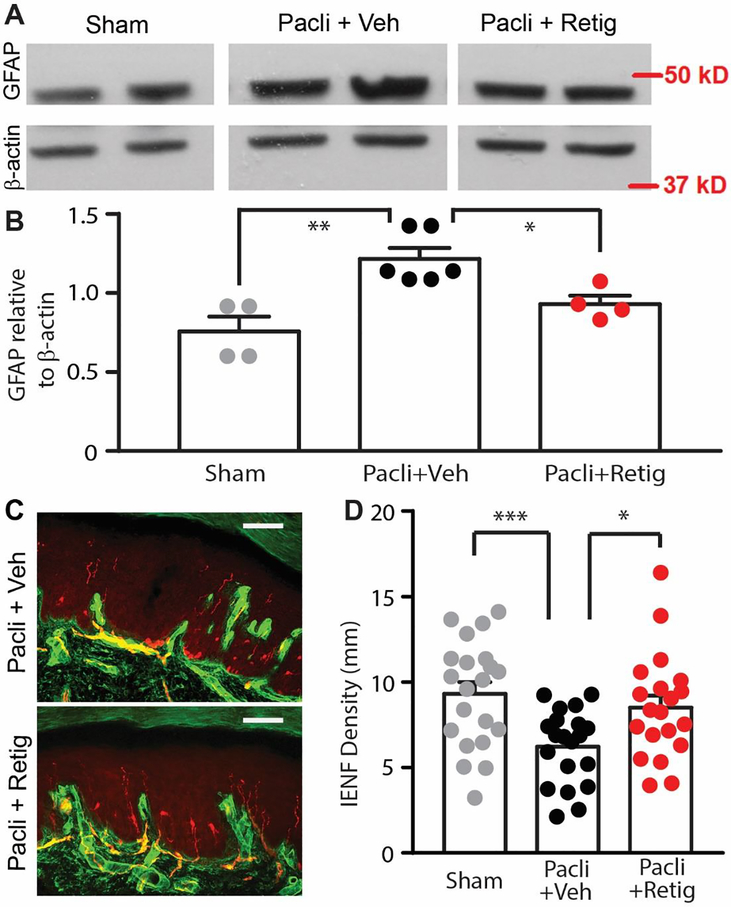
Because brief retigabine treatment significantly relieved PIPN associated pain, we were curious whether this was associated with reduced morphological changes characteristic of neuropathy. Thus, after behavioral testing, rats were euthanized and spinal segments L4-L5 along with glabrous skin from hindpaws were excised and processed to assess the effects of brief retigabine treatment on tissue properties. Because spinal cord astrocytes are activated after paclitaxel treatment 8, 69, 70, we used the marker GFAP to see if pretreatment with retigabine decreases the activation of astrocytes in the spinal cord 69. GFAP expression in the spinal cord from different groups was detected by Western blot. As compared to the sham group, we found that the protein level of GFAP was significantly increased in the spinal cords from the paclitaxel plus brief vehicle group, but not that from the paclitaxel plus brief retigabine group (Fig. 3A, B). Similar as reported 69, 70, however, we did not detect a significant difference in Iba-1 expression level among sham, vehicle- and retigabine-groups (data not shown). Because peripheral nerves in the epidermal region are particularly likely to degenerate as the result of excessive excitation 7, 62, reducing general excitation may preserve many of these peripheral terminals. Fluorescence staining of glabrous skin sections with PGP9.5, which marks intraepidermal nerve fibers, and collagen IV, which denotes the basal lamina between the dermal/epidermal junction, was performed 7. Ascending nerve fibers crossing the collagen-stained basal lamina into the epidermis were counted by measuring PGP9.5 density in skin sections. We found that IENF density in skin sections from the paclitaxel plus brief vehicle group were greatly reduced 4 weeks after paclitaxel treatment, whereas brief co-treatment with retigabine significantly attenuated paclitaxel-induced IENF degeneration as compared to the sham group (Fig. 3C and D). These data suggest that repeated application of retigabine during paclitaxel administration attenuates the degeneration of peripheral fibers.
Figure 3.
The effect of early, repeated application of retigabine on the paclitaxel-induced activation of astrocytes in the spinal cord and degeneration of peripheral nerves. (A) Representative images showing the expression of GFAP in L4/L5 spinal cords from sham, paclitaxel with vehicle, and paclitaxel with retigabine groups. Shown is a representative experiment from 3 independent experiments. (B) Quantification of GFAP expression normalized to β-actin in L4/L5 spinal cords. N shows the number of animals tested. P = 0.02, F(2,13) = 11.11, one-way ANOVA. (C) Representative images showing PGP9.5-stained intraepidermal nerve fibers (red) that crossed the collagen-stained dermal/epidermal junction (green) in the skin sections from paclitaxel with vehicle and paclitaxel with retigabine groups. Scale bars, 100 μm. (D) The effect of co-treatment with retigabine/vehicle on paclitaxel-induced PGP9.5-positive fiber loss in the epidermis. Dots in each column represents a section. 4–5 sections per animal were assessed. 4 sham, 5 brief vehicle-treated, and 5 brief retigabine-treated animals were used. P = 0.003, F(2,58) = 6.567, one-way ANOVA.
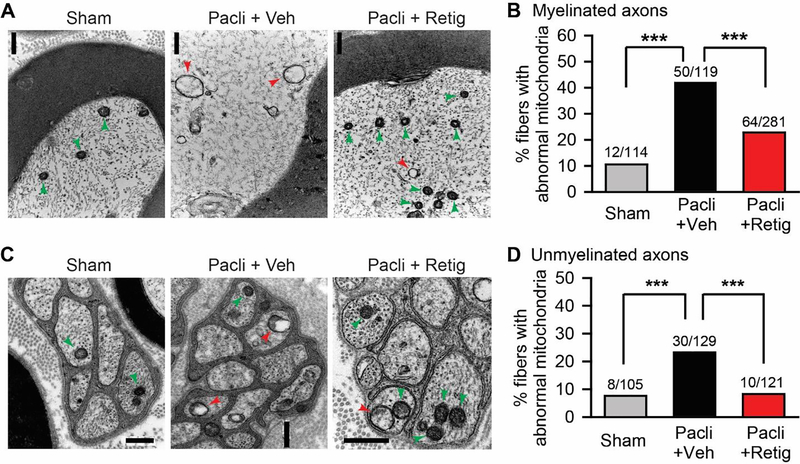
Since mitochondrial abnormalities in primary sensory neurons are linked to paclitaxel-induced peripheral neuropathy 21, 62, 63, we investigated whether retigabine pretreatment mitigates the paclitaxel-induced morphological alterations of mitochondria in primary sensory neurons. In the tibial nerve sections taken from the paclitaxel plus brief vehicle animals, swollen and vacuolated mitochondria were observed in both myelinated and unmyelinated fibers (Fig. 4A and C). However, the incidence of fibers with such abnormal mitochondria was greatly decreased in the nerves dissected from animals treated with paclitaxel plus brief retigabine (Fig. 4B and D). These data suggest that repeated application of retigabine can attenuate paclitaxel-induced mitochondrial abnormalities.
Figure 4.
The effect of early, repeated application of retigabine on the morphological alteration of mitochondria in tibial nerves after paclitaxel. Representative images of swollen mitochondria (red arrows) with vacuoles and small oval mitochondria (green arrows) with intact double membranes in myelinated axons (A) and unmyelinated axons (C) of tibial nerve sections from sham (left panel), paclitaxel plus vehicle (middle panel), and paclitaxel plus retigabine (right panel) groups. Scale bars, 0.5 μm. (B) and (D) Quantification of abnormal mitochondria in both myelinated axons and unmyelinated axons. P = 0.0003, X2 = 16.442 for unmyelinated fibers; P < 0.0001, X2 = 32.213 for myelinated fibers; Chi-square tests. N is the number of fibers tested. Four animals for each group were used. Data were collected 28 days after initial paclitaxel treatment. *, p<0.05; **, P<0.01, ***, P<0.001.
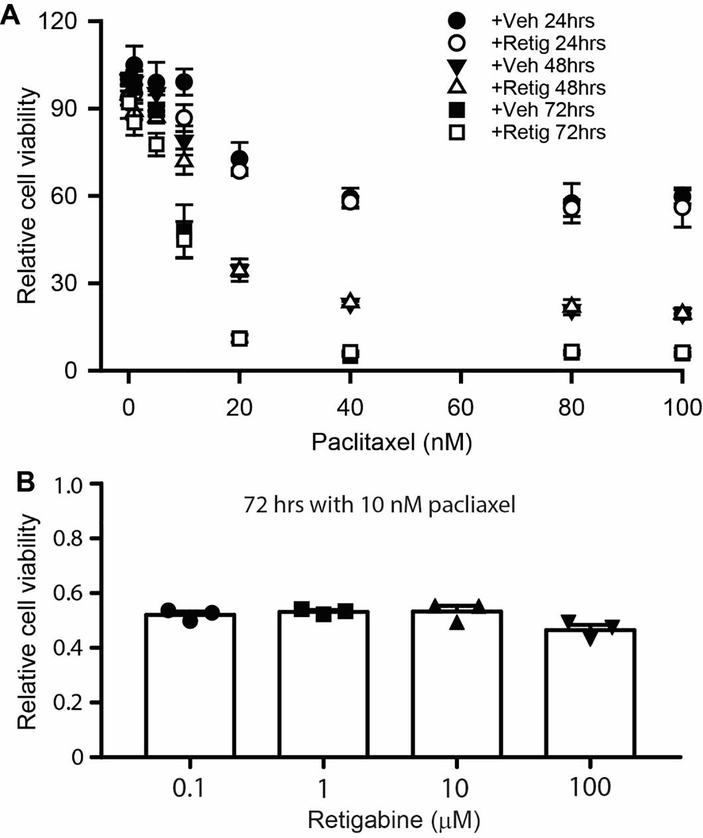
3.4. Retigabine does not alter chemosensitivity of breast cancer cells to paclitaxel
An ideal drug to treat PIPN should attenuates or prevent the pathological alterations with no interference to the chemosensitivity to cancer cells. We thus tested whether retigabine alters the anti-tumor effectiveness of paclitaxel. For these experiments, the viability of the human breast cancer cell line SKBR3 was assessed by mitochondrial enzyme activity using the MTT assay 45. Administration of 10 μM retigabine showed no obvious effects on cell viability at different times, indicating that retigabine is not toxic to these cells (Fig. 5A). In addition, co-administration of retigabine with 10 nM paclitaxel (the half-maximal inhibitory concentration, IC50) did not affect paclitaxel-induced cell death at 72 hours at any concentration tested (Fig. 5B). Thus, these data suggest that retigabine is unlikely to decrease the chemosensitivity of paclitaxel on breast cancer cells.
Figure 5.
The effect of retigabine on the chemosensitivity of SKBR3 breast cancer cells to paclitaxel. (A) MTT assay of SKBR3 cells treated with 10 μM retigabine plus different concentrations of paclitaxel (0 – 100 nM) at 24 hrs, 48 hrs, and 72 hrs. (B) MTT assay of SKBR3 cells treated with 10 nM paclitaxel plus different concentration of retigabine at 72 hrs. P = 0.09, F(3,6) = 3.487, one-way ANOVA. Dots in each column represent one independent experiment. Each experiment was performed three times.
4. Discussion
This study has shown that pharmacological activation of KCNQ channels with retigabine in primary sensory neurons could be a useful approach for mitigating the development of paclitaxel-induced peripheral neuropathy (PIPN). Due to the lack of an effective vascular permeability barrier in the DRG 1, 29, 33, sensory neurons in the DRGs have greater exposure to systemically applied paclitaxel compared to central nervous system (CNS) tissue or ventral roots 9, 63. Several mechanisms for PIPN have been proposed, including mitochondrial dysfunction 16, 21, 22, microtubule alterations 46, 59, 73, inflammation 40, 50, 51, ion channel modulation 25, 38, 39, 67, and oxidative stress 20. These mechanisms can directly or indirectly alter the excitability of primary sensory neurons. Patients are usually at high risk of developing PIPN if paclitaxel induces acute pain 42, 53, which suggests that an acute increase in the excitability of nociceptors could underlie the chronic alterations of PIPN. In this study, we show that decreasing neuronal excitability by activating KCNQ channels at an early stage can effectively attenuate both the pathological and behavioral alterations in a PIPN rodent model. It has been reported that activating KCNQ channels can attenuate cisplatin-induced axon conductance impairment and peripheral fiber loss 47. These studies suggest that neuronal hyperexcitation is a shared mechanism and that neuronal hyperpolarization may mitigate the development of peripheral neuropathy, regardless of the chemotherapy drug used.
KCNQ channels are widely expressed in primary sensory neurons 49, 55 and modulate their excitability 15, 49. Preventing PIPN by activating KCNQ channels requires that there are a sufficient number of channels available for activation during paclitaxel treatment. This was a significant concern as both bone cancer and peripheral nerve injury, but not spinal cord injury, downregulate KCNQ channel expression in primary sensory neurons 55, 61, 72. In fact, the expression of several ion channels is altered in the DRG neurons after paclitaxel treatment 25, 38, 39, 67. KCNQ2, KCNQ3, and KCNQ5 are the major KCNQ subtypes expressed in DRG neurons 49, 55. Our data indicate that paclitaxel exposure with or without brief retigabine treatment did not decrease mRNA expression of these subtypes or protein expression of KCNQ5 in the primary sensory neurons as compared to that from the sham group, which suggests that a sufficient number of channels remain available during treatment.
Multiple sites along pain pathways are altered in the PIPN rodent model. Most research into mechanisms underlying PIPN has focused on paclitaxel-induced plasticity in the spinal cord and primary sensory neurons, including glia cell activation in the spinal cord, inflammatory activity in the DRG, and degeneration of intraepidermal nerve fibers 8, 40, 50, 51, 62, 68, 69. Our data demonstrate that decreasing neuronal excitability can reduce both the activation of spinal cord astrocytes and the degeneration of IENF. The link between neuronal over-excitation and neuronal degeneration has been well studied. For example, topical application of capsaicin on human skin results in quick loss of epidermal nerve fibers 48. Many molecules in sensory fibers required for maintaining their structure and function are thought to be synthesized in, and transported from, DRG somata 13. Given the long journey from DRG somata to peripheral nerves 13, and the fact that maintaining membrane polarization alone accounts for more than 90% of neuronal energy consumption during their enhanced activity 17, 18, over-excitation of primary sensory neurons may thus result in energy exhaustion and degeneration of peripheral nerves because molecules required for metabolic loading fail to refresh in time 16. Thus, over-excitation of primary sensory neurons results in mitochondria dysfunction and affects the longest sensory nerves to the extremities in a “stocking and glove” distribution. Abnormal neuronal activity has an important role in spinal cord astrogliosis and microgliosis 64. Thus, spinal cord glia cells are activated even though the concentration of paclitaxel is extremely low in the CNS 9, 63. In addition, activated glial cells enhance neuronal excitability 44. DRG neurons terminate in the spinal cord, which may thus be affected by activated glial cells in the spinal cord to generate chronic discharge long after accumulated paclitaxel has dissipated 62, 63. Interrupting such a glial-neuronal loop is thus critical for preventing the development of PIPN, as shown by our morphological evidence and behavioral tests.
In addition to the interplay between neuronal excitability and inflammation, other mechanisms underlying neuropathy induced by paclitaxel are thought to include the alteration of neuronal function via microtubule effects 46, 59, 73, the perturbation of mitochondrial function 16, 59, 63, 71, alterations in channel expression 67, or the production of reactive oxygen species 4. It is unknown whether these are causative mechanisms for paclitaxel-induced peripheral neuropathy or common downstream alterations resulting from nonspecific damage to sensory neurons. Our data indicate that at least the alterations of mitochondrial morphology were attenuated when retigabine was briefly co-administrated with paclitaxel.
Decreasing neuronal excitability with calcium and magnesium infusion leads to a neuroprotective effect in the preclinical studies 3, 23, but the effect of this treatment on oxaliplatin chemosensitivity is uncertain 23, 24, 30, 36. Our data indicate that the KCNQ channel activator retigabine does not decrease the chemosensitivity of the breast cancer cell line SKBR3 to paclitaxel (Fig. 5). This suggests that retigabine might be a valuable approach to lessen PIPN in human patients.
In our study, only male rats were studied to remove the effect of estrus cycle stage on the treatments. While gender has no obvious effect on the development of paclitaxel-induced mechanical allodynia, it does affect the analgesic effect of low dose ketamine 31. The correlation between the risk of developing PIPN and paclitaxel-induced acute pain also occurs in female patients 53. There is no sex-related difference in the pharmacokinetics of retigabine 26, and retigabine dose-dependently inhibits bladder mechano- and nociceptive transduction of female SD rats 2. The neuroprotective effect observed in male rats in this study can thus be likely extended to female animals or female patients.
Several drugs failed to prevent/attenuate paclitaxel-induced neuropathy in clinical trials despite their promising preclinical observations 28, 35, 37. This may be due to the fact that reflexive signs other than spontaneous pain were often used to assess pain in these preclinical studies. However, reflexive signs of hypersensitivity are not necessarily correlated with pain levels in patients with neuropathy 34. Thus, in addition to reflexive tests, we performed conditioned place preference tests to assess spontaneous pain. Another possible reason underlying the different outcomes in preclinical studies and clinical trials could be that most preclinical studies were performed using healthy animals. Thus, the expression levels of treatment targets could be changed in cancer animals. In this regard, KCNQ2/KCNQ3 expression levels in primary sensory neurons from bone cancer animals are decreased 72. Thus, in the future we will extend this study to animals bearing breast cancer. Our findings are consistent with evidence that retigabine suppresses cisplatin-induced peripheral neuropathy 47, and encourage the exploration of retigabine to treat chemotherapy-induced peripheral neuropathy. It also provides an impetus for the identification of new classes of KCNQ activators for this purpose.
HIGHLIGHT.
Early repeated application of retigabine attenuates the development of paclitaxel-induced peripheral neuropathy.
Enough KCNQ channels are readily available for targeting during treatment.
Retigabine show no obvious interference to the chemosensitivity of cancer cells to paclitaxel.
Acknowledgments
We thank Dr. Patrick Dougherty from UT MD Anderson Cancer Center for providing technical support on IENF preparation and staining. We also thank Dr. Yong Zhou from UT Health at Houston for help with electron microscopy.
Footnotes
Disclosure: The project was supported by grants from the American Pain Society (Q. Y.), Department of Defense USAMRAA (Q.Y.), Craig H. Neilsen Foundation (Q.Y.), and NIH grants (CA208765 to Q.Y. and CA172129 to J.A.F.). The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 105:146–153, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Aizawa N, Wakamatsu D, Kida J, Otsuki T, Saito Y, Matsuya H, Homma Y, Igawa Y. Inhibitory effects of retigabine, a Kv7 channel activator, on mechanosensitive primary bladder afferent activities and nociceptive behaviors in rats. Neurourol Urodyn. 36:280–285, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong CM, Cota G. Calcium block of Na+ channels and its effect on closing rate. Proc Natl Acad Sci U S A. 96:4154–4157, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barriere DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin MA, Chapuis L, Salles J, Dubray C, Morio B. Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain. 153:553–561, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol. 460:109–116, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Boscia F, Annunziato L, Taglialatela M. Retigabine and flupirtine exert neuroprotective actions in organotypic hippocampal cultures. Neuropharmacology. 51:283–294, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 152:308–313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgos E, Gomez-Nicola D, Pascual D, Martin MI, Nieto-Sampedro M, Goicoechea C. Cannabinoid agonist WIN 55,212–2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur J Pharmacol. 682:62–72, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P, Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology. 21:389–393, 2000 [PubMed] [Google Scholar]
- 10.Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension. 59:877–884, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Crown J, O’Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist. 9 Suppl 2:24–32, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Devor M Unexplained peculiarities of the dorsal root ganglion. Pain. Suppl 6:S27–35, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Drug Enforcement Administration DoJ. Schedules of controlled substances: placement of ezogabine into Schedule V. Final rule. Fed Regist 76:77895–77899, 2011 [PubMed] [Google Scholar]
- 15.Du X, Hao H, Gigout S, Huang D, Yang Y, Li L, Wang C, Sundt D, Jaffe DB, Zhang H, Gamper N. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain. 155:2306–2322, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duggett NA, Griffiths LA, Flatters SJL. Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons. Pain. 158:1499–1508, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erecinska M, Nelson D, Yudkoff M, Silver IA. Energetics of the nerve terminal in relation to central nervous system function. Biochemical Society transactions. 22:959–965, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Erecinska M, Silver IA. Ions and energy in mammalian brain. Progress in neurobiology. 43:37–71, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Farquhar-Smith P Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care. 5:1–7, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Fidanboylu M, Griffiths LA, Flatters SJ. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PloS one. 6:e25212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 122:245–257, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flatters SJ, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett. 397:219–223, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamelin L, Boisdron-Celle M, Delva R, Guerin-Meyer V, Ifrah N, Morel A, Gamelin E. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. 10:4055–4061, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Gamelin L, Boisdron-Celle M, Morel A, Poirier AL, Berger V, Gamelin E, Tournigand C, de Gramont A. Oxaliplatin-related neurotoxicity: interest of calcium-magnesium infusion and no impact on its efficacy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 26:1188–1189; author reply 1189–1190, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hara T, Chiba T, Abe K, Makabe A, Ikeno S, Kawakami K, Utsunomiya I, Hama T, Taguchi K. Effect of paclitaxel on transient receptor potential vanilloid 1 in rat dorsal root ganglion. Pain. 154:882–889, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Hermann R, Ferron GM, Erb K, Knebel N, Ruus P, Paul J, Richards L, Cnota HP, Troy S. Effects of age and sex on the disposition of retigabine. Clin Pharmacol Ther. 73:61–70, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, American Society of Clinical O. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 32:1941–1967, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L, Wade JL, 3rd, Wong SF, Hortobagyi GN, Meyskens FL, Albain KS. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 31:2627–2633, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirakawa H, Okajima S, Nagaoka T, Kubo T, Takamatsu T, Oyamada M. Regional differences in blood-nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion. Neuroreport. 15:405–408, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Hochster HS, Grothey A, Childs BH. Use of calcium and magnesium salts to reduce oxaliplatin-related neurotoxicity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 25:4028–4029, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Hwang BY, Kim ES, Kim CH, Kwon JY, Kim HK. Gender differences in paclitaxel-induced neuropathic pain behavior and analgesic response in rats. Korean J Anesthesiol. 62:66–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 1:21–30, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Andrade JM, Herrera MB, Ghilardi JR, Vardanyan M, Melemedjian OK, Mantyh PW. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain. 4:10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalliomaki M, Kieseritzky JV, Schmidt R, Hagglof B, Karlsten R, Sjogren N, Albrecht P, Gee L, Rice F, Wiig M, Schmelz M, Gordh T. Structural and functional differences between neuropathy with and without pain? Experimental neurology. 231:199–206, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy BP, Rowland KM, Smith DA, Berg AR, Stella PJ, Loprinzi CL. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 19:1769–1777, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurniali PC, Luo LG, Weitberg AB. Role of calcium/ magnesium infusion in oxaliplatin-based chemotherapy for colorectal cancer patients. Oncology (Williston Park). 24:289–292, 2010 [PubMed] [Google Scholar]
- 37.Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber CH, Watanaboonyakhet P, Weiss M, Adams PT, Dockter TJ, Loprinzi CL, Alliance for Clinical Trials in O. North Central Cancer Treatment Group/Alliance trial N08CA-the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled study. Cancer. 120:1890–1897, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J Neurosci. 38:1124–1136, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Tatsui CE, Rhines LD, North RY, Harrison DS, Cassidy RM, Johansson CA, Kosturakis AK, Edwards DD, Zhang H, Dougherty PM. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain. 158:417–429, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. The journal of pain : official journal of the American Pain Society. 15:712–725, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linley JE, Rose K, Patil M, Robertson B, Akopian AN, Gamper N. Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J Neurosci. 28:11240–11249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Novotny PJ, Lachance DH. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 29:1472–1478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 58:253–262, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 10:23–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosmann T Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 65:55–63, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Nishio K, Arioka H, Ishida T, Fukumoto H, Kurokawa H, Sata M, Ohata M, Saijo N. Enhanced interaction between tubulin and microtubule-associated protein 2 via inhibition of MAP kinase and CDC2 kinase by paclitaxel. International journal of cancer. Journal international du cancer. 63:688–693, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Nodera H, Spieker A, Sung M, Rutkove S. Neuroprotective effects of Kv7 channel agonist, retigabine, for cisplatin-induced peripheral neuropathy. Neurosci Lett. 505:223–227, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 81:135–145, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 23:7227–7236, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Experimental neurology. 203:42–54, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Pevida M, Lastra A, Hidalgo A, Baamonde A, Menendez L. Spinal CCL2 and microglial activation are involved in paclitaxel-evoked cold hyperalgesia. Brain research bulletin. 95:21–27, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Piller N, Decosterd I, Suter MR. Reverse transcription quantitative real-time polymerase chain reaction reference genes in the spared nerve injury model of neuropathic pain: validation and literature search. BMC Res Notes. 6:266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Kelaghan J, Novotny PJ, Lachance DH, Loprinzi CL. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer. 118:5171–5178, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rekling JC. Neuroprotective effects of anticonvulsants in rat hippocampal slice cultures exposed to oxygen/glucose deprivation. Neurosci Lett. 335:167–170, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Rose K, Ooi L, Dalle C, Robertson B, Wood IC, Gamper N. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain. 152:742–754, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossato MF, Rigo FK, Oliveira SM, Guerra GP, Silva CR, Cunha TM, Gomez MV, Ferreira J, Trevisan G. Participation of Transient Receptor Potential Vanilloid 1 in Paclitaxel-Induced Acute Visceral and Peripheral Nociception in Rodents. Eur J Pharmacol. 2018 [DOI] [PubMed] [Google Scholar]
- 57.Roza C, Lopez-Garcia JA. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain. 138:537–545, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 155:2461–2470, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Shemesh OA, Spira ME. Paclitaxel induces axonal microtubules polar reconfiguration and impaired organelle transport: implications for the pathogenesis of paclitaxel-induced polyneuropathy. Acta neuropathologica. 119:235–248, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 282:1890–1893, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Wu Z, Li L, Xie F, Du J, Zuo Y, Frost JA, Carlton SM, Walters ET, Yang Q. Activation of KCNQ Channels Suppresses Spontaneous Activity in Dorsal Root Ganglion Neurons and Reduces Chronic Pain after Spinal Cord Injury. J Neurotrauma. 34:1260–1270, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 153:704–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao WH, Zheng H, Zheng FY, Nuydens R, Meert TF, Bennett GJ. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience. 199:461–469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 160:847–857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain. 6:49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Q, Wu Z, Hadden JK, Odem MA, Zuo Y, Crook RJ, Frost JA, Walters ET. Persistent pain after spinal cord injury is maintained by primary afferent activity. J Neurosci. 34:10765–10769, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Dougherty PM. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology. 120:1463–1475, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. The journal of pain : official journal of the American Pain Society. 17:775–786, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Yoon SY, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. The journal of pain : official journal of the American Pain Society. 13:293–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience. 176:447–454, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Experimental neurology. 232:154–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han JS, Xing GG. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 154:434–448, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Zhou J, Liu M, Aneja R, Chandra R, Joshi HC. Enhancement of paclitaxel-induced microtubule stabilization, mitotic arrest, and apoptosis by the microtubule-targeting agent EM012. Biochemical pharmacology. 68:2435–2441, 2004 [DOI] [PubMed] [Google Scholar]