Abstract
Insulin signaling controls cell growth and metabolic homeostasis. Dysregulation of this pathway causes metabolic diseases such as diabetes. Insulin signaling pathways have been extensively studied. Upon insulin binding, the insulin receptor (IR) triggers downstream signaling cascades. The active IR is then internalized by clathrin-mediated endocytosis. Despite decades of studies, the mechanism and regulation of clathrin-mediated endocytosis of IR remain incompletely understood. Recent studies have revealed feedback regulation of IR endocytosis through Src homology phosphatase 2 (SHP2) and the mitogen-activated protein kinase (MAPK) pathway. Here we review the molecular mechanism of IR endocytosis and its impact on the pathophysiology of insulin resistance, and discuss the potential of SHP2 as a therapeutic target for type 2 diabetes.
Subject terms: Endocytosis, Mechanisms of disease, Homeostasis
Insulin: Keeping the receptors signaling
A potential cancer treatment also shows promise for treatment of type 2 diabetes. When insulin receptors (IRs) on cell surfaces bind to insulin, they send out signals that trigger glucose uptake, lowering blood sugar. The duration of IR signaling is crucial for metabolic health, but its regulation is poorly understood. Eunhee Choi, Columbia University, New York, and Hongtao Yu, Westlake University, Hangzhou, and a coworker have reviewed how IR signaling is controlled. They report that inhibiting the protein SHP2 may prolong IR signaling and improve how the body responds to insulin. Because SHP2 is also implicated in cancer, inhibitors have already been developed and could be tested for treatment of metabolic diseases. These results illuminate the fundamentals of a key metabolic pathway, and may help in treatment of type 2 diabetes.
Introduction
The pancreatic hormone insulin controls the metabolism of glucose and lipids in our body1,2. It promotes glucose uptake and its conversion into glycogen and lipids for energy storage in metabolic tissues, thereby enabling the maintenance of proper blood glucose levels. Normal circulating insulin levels are necessary for glucose homeostasis. Persistent hyperinsulinemia, an above normal level of insulin in the blood, is associated with insulin resistance. Insulin resistance is a hallmark of metabolic diseases, including type 2 diabetes and atherosclerosis1–6. Understanding the mechanisms of insulin resistance is therefore essential for the continued development of effective therapeutic strategies to treat these prevalent diseases.
The relationship between hyperinsulinemia and insulin resistance is complicated. The prevailing view is that the pancreas produces more insulin to compensate for the rise in blood glucose level caused by defective insulin signaling7–10. An alternative view is that hyperinsulinemia may initiate and expand insulin resistance11–14. These are not mutually exclusive concepts and probably act in parallel.
At the cellular level, insulin binds to the insulin receptor (IR) on the plasma membrane (PM) and triggers the activation of signaling cascades to regulate metabolism and cell growth. Following activation, insulin-bound IR can be internalized by clathrin-mediated endocytosis (CME)15–18. As a key CME adaptor, the assembly polypeptide 2 (AP2) complex links clathrin to both the cargo and lipids on the PM. The AP2 complex has four subunits: AP2A, AP2B1, AP2M1, and AP2S1. It has a large globular core consisting of the entirety of both AP2M1 and AP2S1 subunits, along with the N-terminal trunk domain of AP2A and AP2B1. The AP2 core recognizes sorting signals from the cargo, such as di-leucine and YXXΦ (X, any amino acids; Φ, hydrophobic residues) motifs. The C-terminal appendages of the AP2A and AP2B1 subunits extend from the core and bind to clathrin and other accessory proteins, thus promoting clathrin vesicle formation. The endocytosis of the IR–insulin complex is a key mechanism that regulates the intensity and duration of insulin signaling. In contrast, persistent hyperinsulinemia may accelerate IR endocytosis, thus decreasing the functional IR level at the PM. Biochemical and immunohistochemistry studies have shown that the level of IR at the PM might be reduced in diabetes patients19–21. These findings suggest that reduced IR levels at the PM might be a contributing factor to insulin resistance in human patients.
The spindle checkpoint ensures accurate chromosome segregation and prevents aneuploidy16,22–27. MAD2 and BUBR1 are critical spindle checkpoint proteins. In response to unattached kinetochores, they bind to CDC20 and BUB3 to form the mitotic checkpoint complex (MCC). MCC prevents chromosome segregation by directly inhibiting the anaphase-promoting complex/cyclosome28–34. When all kinetochores are attached to the bipolar spindle, p31comet binds to active MAD2 and inactivates the spindle checkpoint35–39.
Our previous studies have revealed an unexpected function of the spindle checkpoint in insulin signaling through regulating IR endocytosis. MAD2 binds to the C-terminal MAD2-interacting motifs (MIMs) of IR and recruits AP2B1 to IR through BUBR1-CDC2016,21,40 (Fig. 1a, b). p31comet prevents IR endocytosis by inhibiting the interaction between BUBR1-CDC20-AP2 and IR-bound MAD2. Liver-specific p31comet−/− mice have reduced IR levels on the PM of hepatocytes and develop whole-body insulin resistance40. Conversely, BUBR1 deficiency delays insulin-mediated IR endocytosis and improves insulin sensitivity in mice40,41. These findings suggest that the dysregulation of IR endocytosis is a potential mechanism underlying insulin resistance.
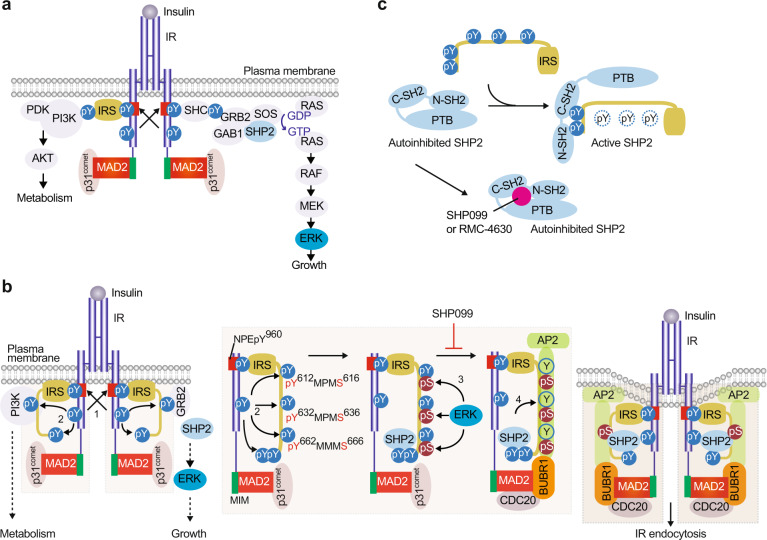
Fig. 1. IRS- and MAD2-dependent mechanisms collaborate to trigger IR endocytosis.
a The insulin receptor signaling pathway. b Model of the regulation of IR endocytosis by IRS and spindle checkpoint proteins. In the basal state, p31comet prevents IR endocytosis. Activated IR auto-phosphorylates multiple tyrosine residues on IR, including Y960 in the NPEY960 motif (1), recruits IRS proteins and initiates insulin signaling cascades. IR phosphorylates Y612/Y632/Y662 of the YXXΦ motifs on IRS1 (2). Activated ERK phosphorylates S616/S636/S666 on IRS1 (3). SHP2 binds to the C-terminal phosphotyrosine sites on IRS1 and dephosphorylates pY612/pY632/pY662 of the doubly phosphorylated IRS1 (pY/pS) to facilitate the IRS1–AP2 interaction (4). IR-bound MAD2 binds to BUBR1-CDC20, providing another binding surface for AP2. These two modules promote IR endocytosis. Inhibition of the feedback regulation prevents IR endocytosis, prolongs metabolic branch of insulin signaling, and improves insulin sensitivity. MIM, MAD2-interacting motif.
It has long been known that IR kinase activity is crucial for receptor endocytosis42,43, suggesting that IR endocytosis normally occurs after the receptor has been activated and has transduced signals downstream. However, how activated IR is selectively internalized remained largely unknown until our recent study. We have discovered a regulatory feedback mechanism of IR endocytosis through the SHP2–MAPK pathway21. Inhibition of this regulatory feedback delays IR endocytosis, prolongs metabolic signaling, and improves insulin sensitivity. Here we review this newly discovered regulatory mechanism of IR endocytosis, discuss its impact on pathophysiology, and highlight the key unanswered questions.
The SHP2–MAPK pathway in metabolic regulation
IR is a receptor tyrosine kinase (RTK) that is activated by insulin binding44. The binding of multiple insulin molecules to an IR destabilizes its autoinhibitory conformation, leading to its trans-autophosphorylation and activation45–47. The tyrosine-phosphorylated IR recruits and phosphorylates IR substrate (IRS) or SRC homology 2 domain-containing (SHC) proteins at several tyrosine residues48,49 (Fig. 1a). These tyrosine phosphorylation events recruit additional effectors and activate two major signaling cascades: (1) the phosphatidylinositol 3-kinase (PI3K)–protein kinase B/AKT (PI3K–PKB/AKT) pathway and (2) the MAPK pathway. The PI3K–PKB/AKT pathway is primarily responsible for controlling metabolism. The MAPK pathway mainly controls cell growth and proliferation. Accumulating evidence now suggests that the dysregulation of insulin-mediated MAPK pathway activation may contribute to insulin resistance50–53.
The phosphorylated IRS and SHC proteins bind to growth factor receptor-bound protein 2 (GRB2) and then recruit the guanine nucleotide exchange factor, son of sevenless (SOS), to activate the RAS–MAPK pathway54. SHP2, encoded by PTPN11, is a nonreceptor protein tyrosine phosphatase (PTP) and a scaffolding protein that controls SOS2-mediated MAPK pathway activation. SHP2 contains two tandem SH2 domains (N-terminal SH2 and C-terminal SH2), a PTP domain, and a C-terminal tail55 (Fig. 1c). In the basal state, the SH2 domains engage the catalytic pocket in the PTP domain and sterically block the active site. Upon insulin stimulation, the two SH2 domains in SHP2 interact with the phosphotyrosine sites in IRS proteins and GRB2-associated binder protein 1 (GAB1), thus breaking the autoinhibitory interface and rendering the active site available for substrates56,57. The phosphatase activity of SHP2 is required for the formation of the GAB1–GRB2–SOS1 complex, which in turn promotes RAS activation58 (Fig. 1a). Activated RAS binds to RAF and causes RAF translocation to the PM. RAF then activates the dual-specificity serine and threonine kinases, MEK1 and MEK2, which phosphorylate and activate extracellular signal-regulated kinase 1 and 2 (ERK1 and ERK2)59.
ERK1/2 are the best-characterized MAPK family members. ERK1/2 phosphorylate serine or threonine residues that are followed by a proline residue (S/T-P). Over 200 substrates of ERK1/2, including SREBP1, SREBP260,61, and peroxisome proliferator-activated receptor γ52, have been identified so far62–64. ERK1 and ERK2 share 75% amino acid identity and phosphorylate the same substrates with similar specificity in vitro. However, ERK1−/− mice are viable and fertile, but ERK2−/− mice are not viable65–68, suggesting that these kinases are not redundant and have tissue-specific roles. Although ERK1−/− mice have been shown to be more sensitive to insulin, diet-induced obesity mice and leptin-deficient (ob/ob) mice have elevated ERK activity51,69,70. Pharmacological inhibition of ERK improves insulin sensitivity in both diet-induced obesity and ob/ob mice52. Furthermore, the basal activity of ERK is elevated in human type 2 diabetes71–73, indicating that the MAPK pathway may be a potential therapeutic target for insulin resistance and metabolic disorders.
SHP2 is the first reported oncogenic tyrosine phosphatase. As an upstream regulator of the MAPK pathway, SHP2 promotes cell growth and proliferation. Conventional SHP2−/− mice are embryonic lethal74. Tissue-specific SHP2−/− mice survive and show that SHP2 controls metabolic homeostasis in multiple tissues. For example, striated and cardiac muscle-specific SHP2−/− mice display severe dilated cardiomyopathy, undergo premature death, and exhibit insulin resistance75. Neuronal SHP2 dysfunction causes early-onset obesity accompanied by high levels of leptin, insulin, glucose, and triglycerides76. On the other hand, liver-specific SHP2−/− mice exhibit enhanced insulin sensitivity77,78. Pharmacological inhibition of SHP2 markedly increased glucose and insulin sensitivity in a diet-induced obesity mouse model21. The introduction of adeno-associated viruses encoding SHP2 short-hairpin RNAs into the liver confirms a role of SHP2 in metabolic homeostasis in mice21. In addition, deficiency of GAB1, the binding partner of SHP2, in the liver exhibits improved insulin sensitivity, together with enhanced AKT and blunted MAPK pathway activation79. This finding suggests that the SHP2–MAPK pathway may offset certain aspects of insulin signaling in the liver, thus prolonging the metabolism signaling branch and improving whole-body insulin sensitivity.
The SHP2–MAPK pathway in IR endocytosis
How does the SHP2–MAPK pathway control metabolism? What are the main targets of SHP2 and MAPK in this pathway? IRS proteins are crucial adaptors that transduce signals from IR on the PM to intracellular downstream effectors and adaptors48. Insulin-activated IR phosphorylates its own NPEY960 motif in the juxtamembrane domain (Fig. 1a, b). The phosphotyrosine-binding domain of IRS proteins directly binds to the phosphorylated NPEY960 motif in IR80–84. The NPEY960 motif in IR had been implicated in AP2 binding and in IR endocytosis, but the mechanism remained unclear85,86. Activated IR phosphorylates several tyrosine residues in the IRS proteins, including multiple YXXΦ motifs in the middle region (Fig. 1b)87,88. These phosphotyrosine motifs interact with PI3K, facilitating the activation of the PI3K–PKB/AKT pathway. SHP2 binds directly to the C-terminal phosphotyrosine residues in IRS proteins and dephosphorylates the tyrosine residues in the YXXΦ motifs, thus negatively regulating PI3K activity89,90 (Fig. 1b, c). Multiple serine and threonine residues in IRS proteins can also be phosphorylated upon insulin stimulation91. The increased serine/threonine phosphorylation of IRS proteins is associated with insulin resistance in human and mouse models. ERK is one of the most well-known kinases of IRS proteins. Strikingly, the serine residues that follow the YXXΦ motifs are phosphorylated by ERK50,92. ERK-mediated phosphorylation of IRS proteins has been shown to reduce their tyrosine phosphorylation through negative feedback50,91,92.
We have recently shown that IRS1 and IRS2 bind directly to the clathrin adaptor AP2M1 through multiple YXXΦ motifs and promote insulin-activated IR endocytosis21 (Fig. 1b). Interestingly, these AP2M1-YXXΦ interactions are regulated by a phosphorylation switch mediated by ERK and SHP2. ERK-mediated serine/threonine phosphorylation promotes SHP2-mediated tyrosine dephosphorylation of the YXXΦ motifs, resulting in a switch from phosphotyrosine to phospho-serine/threonine. Only phospho-serine/threonine-containing IRS proteins can interact with AP2 and trigger IR endocytosis.
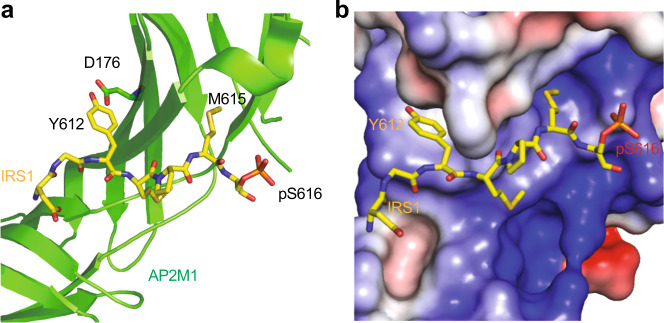
The crystal structure of AP2M1 bound to serine-phosphorylated Y612XXΦS616 motif in IRS1 (pS-IRS1) explains the structural basis of this phospho-switch (Fig. 2). Tyrosine (Y612) and methionine (M615) establish extensive hydrophobic interactions with AP2M1. The hydroxyl group at Y612 forms a hydrogen bond with D176 in AP2M1 (Fig. 2a). YXXΦ IRS1 mutants in which tyrosine was replaced with alanine or phenylalanine showed weakened interaction with AP2M1 in vitro and IR endocytosis was not restored in IRS1-depleted cells. Tyrosine phosphorylation of the YXXΦ motifs is expected to disrupt the IRS–AP2M1 interaction by introducing both static hindrance and unfavorable electrostatic interactions. In IRS1, pS616 is in the vicinity of a positively charged surface on the AP2M1, suggesting that this phospho-serine might participate in favorable electrostatic interactions with this basic region (Fig. 2b).
Fig. 2. Structure of AP2M1 bound to pS616-IRS1.
a Ribbon diagram of the crystal structure of AP2M1-pS616-IRS1 (PDB ID: 6BNT). b Surface drawing of AP2M1 colored by its electrostatic potential (blue, positive; red, negative; and white, neutral) with pS616-IRS1 shown as sticks.
Consistent with the pS-IRS1–AP2M1 structure, pS-IRS1 binds to AP2M1 with higher affinity, as compared to unphosphorylated IRS121. In addition, pS-IRS1 enhances the tyrosine dephosphorylation of IRS1 by SHP2. These data suggest that ERK-mediated serine phosphorylation of IRS proteins fosters AP2 interaction by directly enhancing the IRS–AP2M1 interaction and indirectly facilitating the SHP2-mediated tyrosine dephosphorylation of IRS, thus promoting clathrin-mediated IR endocytosis. Pharmacological SHP2 inhibition indeed blocks the insulin-stimulated IRS1-AP2 interaction in hepatocytes and delays IR endocytosis. Taken together, these findings establish a direct function of the MAPK pathway in IR endocytosis and, possibly, in metabolic regulation.
In summary, there are two regulatory modules of IR endocytosis: the IRS module and the mitotic checkpoint module (Fig. 1b). The IRS module is activated by the SHP2–MAPK pathway, which is, in turn, activated by insulin signaling. These two modules collaboratively promote the selective endocytosis of activated IR. Our studies suggest that targeting the feedback regulation of IR endocytosis might be beneficial for diabetes treatment. As SHP2 promotes IR endocytosis directly by removing IRS tyrosine phosphorylation and also indirectly by activating the MAPK pathway, inhibition of SHP2 is expected to disrupt the feedback loop, prolong insulin signaling at the PM, and improve insulin sensitivity.
The use of SHP2 inhibitors for the treatment of cancer and diabetes
Because of its role in growth factor receptor signaling, SHP2 has been implicated in the development of many diseases. Mutations of SHP2 are associated with multiple disorders, most notably Noonan syndrome and LEOPARD syndrome93. Somatic SHP2 mutations are also associated with cases of childhood leukemia, including juvenile myelomonocytic leukemia, myelodysplastic syndrome, and acute myeloid leukemia94,95. SHP2 overexpression is also causally related to cell proliferation dysfunction. Suppressing SHP2 expression in adult leukemia increases apoptosis and reduces the growth of leukemia cells96. Similarly, SHP2 levels are elevated in other types of cancer, including breast and ovarian cancers97,98. Due to the association of SHP2 with cancer cell proliferation, SHP2 has emerged as a potential target for cancer therapy.
SHP2 undergoes autoinhibition in the absence of an activator; when not stimulated by insulin, the N-SH2 domain binds the PTP domain and blocks its active site (Fig. 1c)99. The small-molecule SHP2 allosteric inhibitor SHP099 takes advantage of this natural regulatory mechanism by interacting with all three domains of SHP2 when it is in the autoinhibited configuration, locking it into the inactive form57. SHP099 binds to SHP2 with a high degree of specificity. Strikingly, SHP099 shows no inhibitory activity against SHP1, the closest homolog of SHP2. The effectiveness of SHP099 as an allosteric SHP2 inhibitor makes it a viable option for proof-of-principle studies on SHP2-induced inhibition as a cancer therapy.
Several clinical studies are currently exploring the use of SHP2 inhibitors to treat RTK-mutated cancers (Table 1). SHP099 has been used in conjunction with MEK inhibitors to decrease cancer cell proliferation in multiple types of cancer. This method successfully mitigated the development of the adaptive resistance to MEK inhibition that occurs when MEK inhibitors are used alone100. SHP099 is effective in decreasing tumor burden and promoting anti-tumor immunity in mice with grafted colon cancer cells101. These findings suggest that SHP099 is a promising candidate for cancer therapy, both as a monotherapy and in conjunction with other agents.
Table 1.
Ongoing clinical trials and preclinical studies of SHP2 inhibitors.
| Inhibitor | Cancer type(s) | Clinical status | Notesa |
|---|---|---|---|
|
JAB-3068 (Jacobio Pharmaceuticals) |
Non-small cell lung cancer (NSCLC) Head and neck cancer Esophageal cancer |
Phase 1/2a | NCT03565003 |
|
JAB-3312 (Jacobio Pharmaceuticals) |
Non-small cell lung cancer (NSCLC) Colorectal cancer Pancreatic ductal carcinoma Esophageal squamous cell carcinoma Head and neck squamous cell carcinoma Breast cancer Other solid tumors |
Phase 1 | NCT04045496 |
|
TN0155b (Novartis) |
Non-small cell lung cancer (NSCLC) Esophageal squamous cell cancer (SCC) Head and neck SCC Gastrointestinal stromal tumors |
Phase 1/1b | |
|
RMC-4630 (Revolution Medicines) |
Solid tumors (unspecified) | Phase 1b/2 | NCT03989115 |
|
RLY-1971 (Relay Therapeutics) |
Solid tumors (unspecified) | Phase 1 | NCT04252339 |
| SHP099 |
Esophageal cancer cells Hematopoietic cancer cells Colorectal cancer cells KRAS-mutant cancer cells Triple-negative breast cancer |
No clinical trials; research involves cell lines and mouse xenografts57,100,106. |
ahttps://clinicaltrials.gov/ct2/ (identification number).
bCombination with spartalizumab or ribociclib.
Studies on SHP2 and its associated signaling pathways have also revealed its involvement in the regulation of insulin signaling. Patients with LEOPARD syndrome-related SHP2 mutations exhibit resistance to diet-induced obesity, an improved overall metabolic profile, and insulin hypersensitivity102. This outcome suggests a possible role for SHP2 inhibition or modulation in treating insulin resistance. Our study has shown that SHP2 inhibitors can be potentially repurposed to treat type 2 diabetes21. These results follow the trend shown in previous research indicating a relationship between SHP2 and insulin signaling and suggest SHP2 inhibition as a promising therapeutic method for not only cancer treatment, but also treatment of insulin resistance and diabetes. Cancer and diabetes share common risk factors such as obesity, hyperinsulinemia, and aging. The number of patients suffering from both diseases has increased dramatically in recent years. SHP2 inhibitors may be particularly beneficial to patients who have both diabetes and cancer.
Targeting IR endocytosis for insulin resistance treatment
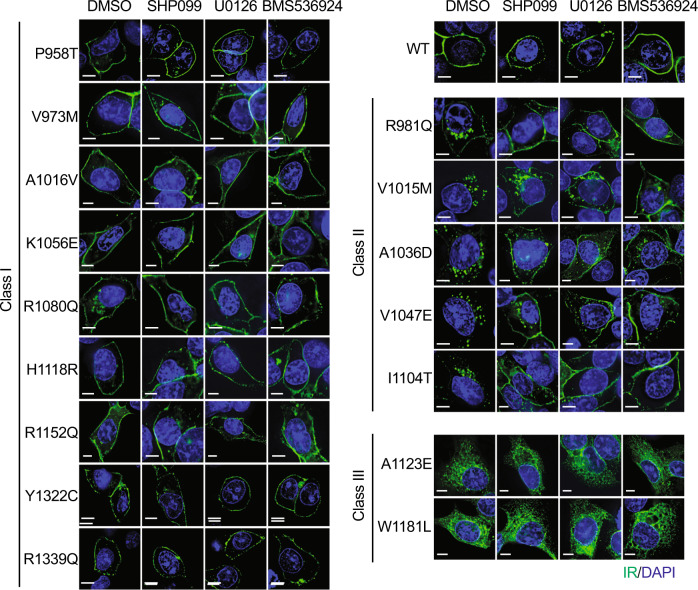
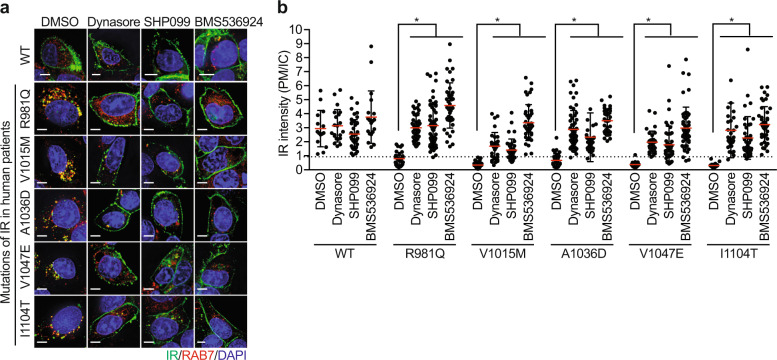
Mutations of IR are known to cause inherited severe insulin resistance syndromes103, but the mechanisms by which these mutations affect IR function have not been systematically explored. Using the missense mutations in the cytoplasmic region of IR found in patients with severe insulin resistance103, we defined three distinct classes of IR mutants based on their subcellular localization in the unstimulated state (Fig. 3 and Table 2). Class I mutants localize to the PM. Class II mutants show reduced signals at the PM and are enriched in RAB7-positive intracellular compartments (Fig. 4). Class III mutants remain in the ER and the Golgi apparatus, indicating that class III mutations affect IR processing and trafficking. The addition of dynasore, a chemical inhibitor of dynamin, elevated the IR level of the class II mutants at the PM (Fig. 4), suggesting that class II mutations cause premature CME of IR prior to insulin stimulation.
Fig. 3. Characterization of IR mutations found in human patients.
HepG2 cells expressing IR-GFP wild-type (WT) or mutants were starved for 14 h, treated with the indicated inhibitors for 4 h, and stained with anti-GFP (IR; green; 1181446001, Sigma) and DAPI (blue). SHP099 (SHP2 inhibitor, 10 μM, MedChemExpress), U0126 (MEK inhibitor, 40 μM, Cell Signaling), and BMS536924 (IR kinase inhibitor, 2 μM, Tocris). Scale bar, 5 μm.
Table 2.
Characterization of IR mutations found in human patients.
| Class | Mutation HGVSa nomenclature | Mutation mature, long isoform | Mutation mature, short isoform | Localizationb | Phenotype |
|---|---|---|---|---|---|
| I | P997T | P970T | P958T | PM | Rabson-Mendenhall syndrome |
| V1012M | V985M | V973M | PM | Type 2 diabetes | |
| A1055V | A1028V | A1016V | PM | Insulin resistance | |
| K1095E | K1068E | K1056E | PM | Type 2 diabetes | |
| R1119Q | R1092Q | R1080Q | PM | Leprechaunism | |
| H1157R | H1130R | H1118R | PM | Insulin resistance | |
| R1191Q | R1164Q | R1152Q | PM | Type 2 diabetes | |
| Y1361C | Y1334C | Y1322C | PM | Type 2 diabetes | |
| R1378Q | R1351Q | R1339Q | PM | Insulin resistance | |
| II | R1020Q | R993Q | R981Q | IC | Insulin resistance |
| V1054M | V1027M | V1015M | IC | Leprechaunism | |
| A1075D | A1048D | A1036D | IC | Insulin resistance | |
| V1086E | V1059E | V1047E | IC | Type 2 diabetes | |
| I1143T | I1116T | I1104T | IC | Rabson-Mendenhall syndrome | |
| III | A1162E | A1135E | A1123E | ER/Golgi | Insulin resistance |
| W1220L | W1193L | A1181L | ER/Golgi | Insulin resistance |
ER/Golgi endoplasmic reticulum/Golgi apparatus, IC intracellular compartment, PM plasma membrane.
aHuman Genome Variation Society, http://www.hgvs.org/rec.html.
bThe cellular localization of IR-GFP in the basal, unstimulated state.
Fig. 4. Characterization of class II IR mutations found in human patients.
a HepG2 cells stably expressing IR-GFP WT or Class II mutants were serum starved for 14 h, treated with the indicated inhibitors for 4 h, and stained with anti-GFP (IR; green) and anti-RAB7 (Red; D95F2, Cell Signaling) antibodies. Dynasore (dynamin inhibitor, 80 μM, Sigma), SHP099 (SHP2 inhibitor, 10 μM, MedChemExpress), and BMS536924 (IR kinase inhibitor, 2 μM, Tocris). Scale bar, 5 μm. b Quantification of the ratios of PM and IC IR-GFP signals of the cells shown in a (mean ± SD; *p < 0.0001).
Treating cells with SHP099 and U0126, a MEK inhibitor, significantly enhanced the PM levels of class II IR mutants, but not those of class I and III IR mutants. These results suggest that targeting IR endocytosis can potentially alleviate insulin resistance in patients with class II IR mutations and possibly in other type 2 diabetes patients. Future studies are required to determine the role of premature IR endocytosis in the pathogenesis of human insulin resistance. Our current study monitored IR endocytosis in HepG2 cells that expressed endogenous IR. Only patients with both alleles of IR mutated displayed insulin resistance phenotypes, whereas their parents, who each had a single mutated allele, did not exhibit these phenotypes. To mimic the situation of the patients, we depleted endogenous IR in HepG2 cells; however, due to high levels of cell death, we could not assess whether the premature endocytosis of IR mutants occurred in the absence of endogenous wild-type IR. It will be interesting to examine the IR PM levels before and after insulin stimulation in patient cell lines that harbor the particular class II mutations and to determine whether inhibitors of SHP2 or the MAPK pathway can recover the IR PM levels and insulin sensitivity.
Perspective
Our recent studies provide further mechanistic insight into IR endocytosis and raise many interesting questions as follows: (1) the core components of MCC, including MAD2, BUBR1, and CDC20, are assembled onto IR to control its endocytosis in interphase. Is the mitotic module regulated by the SHP2–MAPK pathway during insulin signaling? If it is, what is the main target of SHP2 and MAPK in this module? (2) The fact that an MCC-like complex is assembled onto IR suggests that IR might reciprocally control MCC assembly and spindle checkpoint signaling during cell division. Can insulin and the metabolic environment control genomic stability through IR? (3) How do the two modules–the IRS and mitotic checkpoint modules–cooperate to promote IR endocytosis? How many copies of the AP2 complex, BUBR1, or IRS1/2 are recruited to each IR dimer? Do two modules engage a single AP2 complex? This is theoretically possible because BUBR1 and IRS1/2 do not bind the same site on AP2: BUBR1 binds to AP2B1, and IRS1/2 bind to AP2M1. (4) What is the physiological function of the feedback regulation on human insulin resistance? Can hyperactivation of the MAPK pathway by altered metabolic stress reduce the IR levels at the PM? (5) Are the IR PM levels reduced in human patients harboring class II mutations? Can inhibitors of SHP2 or the MAPK pathway recover the IR PM levels in these patients and improve insulin sensitivity? Future studies aimed at answering these questions will provide further insight into the pathogenesis of insulin resistance.
Type 1 insulin-like growth factor receptor (IGF1R) belongs to the IR family. The intracellular domains of IR and IGF1R share over 80% of amino acid identity. Although IR and IGF1R also share several common adaptors and effectors for downstream signaling pathways, IGF1R only controls cell growth and proliferation, whereas IR controls both cell growth and metabolic homeostasis. The mechanisms by which these two highly homologous receptors achieve different signaling outcomes are largely unknown. In the context of endocytosis, IGF1R binds to IRS1–AP2 but does not have MIM16,40, suggesting that the internalization of IGF1R does not involve the mitotic checkpoint module and is solely controlled by the IRS module. Strikingly, Yoneyama et al.104 showed that IRS1, but not IRS2, negatively regulates IGF1R endocytosis. Most importantly, the timing of endocytosis after the activation of IGF1R is very different from that of IR. In contrast to activated IR, which is internalized within minutes, activated IGF1R can remain at the PM for over 1 h. Recent cryo-EM structural studies showed that the Γ-shaped asymmetric IGF1R dimer was bound to only one IGF1 molecule, while the T-shaped symmetric IR dimer was bound to multiple insulin molecules45,105. Future studies are required to explore whether these important structural differences upon ligand binding affect the endocytosis and downstream signaling of IR and IGF1R.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongtao Yu, Email: Hongtao.Yu@UTsouthwestern.edu.
Eunhee Choi, Email: EC3477@cumc.columbia.edu.
References
- 1.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018;19:31–44. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J. Clin. Endocrinol. Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 5.Lillioja S, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N. Engl. J. Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 6.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann. Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 8.Kasuga M. Insulin resistance and pancreatic beta cell failure. J. Clin. Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz SS, et al. The time is right for a new classification system for diabetes: rationale and implications of the beta-cell-centric classification schema. Diabetes Care. 2016;39:179–186. doi: 10.2337/dc15-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz SS, et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol. Metab. 2017;28:645–655. doi: 10.1016/j.tem.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Shanik MH, et al. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–268. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 12.Gregory JM, et al. Iatrogenic hyperinsulinemia, not hyperglycemia, drives insulin resistance in type 1 diabetes as revealed by comparison with GCK-MODY (MODY2) Diabetes. 2019;68:1565–1576. doi: 10.2337/db19-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knutson VP, Donnelly PV, Balba Y, Lopez-Reyes M. Insulin resistance is mediated by a proteolytic fragment of the insulin receptor. J. Biol. Chem. 1995;270:24972–24981. doi: 10.1074/jbc.270.42.24972. [DOI] [PubMed] [Google Scholar]
- 14.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017;23:804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2013;5:a017459. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi E, Yu H. Spindle checkpoint regulators in insulin signaling. Front Cell Dev. Biol. 2018;6:161. doi: 10.3389/fcell.2018.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traub LM, Bonifacino JS. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 2013;5:a016790. doi: 10.1101/cshperspect.a016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 19.Soll AH, Kahn CR, Neville DM., Jr. Insulin binding to liver plasm membranes in the obese hyperglycemic (ob/ob) mouse. Demonstration of a decreased number of functionally normal receptors. J. Biol. Chem. 1975;250:4702–4707. [PubMed] [Google Scholar]
- 20.Caro JF, et al. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J. Clin. Invest. 1986;78:249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi E, et al. Mitotic regulators and the SHP2-MAPK pathway promote IR endocytosis and feedback regulation of insulin signaling. Nat. Commun. 2019;10:1473. doi: 10.1038/s41467-019-09318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr. Biol. 2015;25:R1002–1018. doi: 10.1016/j.cub.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 23.Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia L, Kim S, Yu H. Tracking spindle checkpoint signals from kinetochores to APC/C. Trends Biochem Sci. 2013;38:302–311. doi: 10.1016/j.tibs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 25.London N, Biggins S. Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell Biol. 2014;15:736–747. doi: 10.1038/nrm3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfau SJ, Amon A. Chromosomal instability and aneuploidy in cancer: from yeast to man. EMBO Rep. 2012;13:515–527. doi: 10.1038/embor.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 29.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol. Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Luo X, Yu H. Protein metamorphosis: the two-state behavior of Mad2. Structure. 2008;16:1616–1625. doi: 10.1016/j.str.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izawa D, Pines J. The mitotic checkpoint complex binds a second CDC20 to inhibit active APC/C. Nature. 2015;517:631–634. doi: 10.1038/nature13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mapelli M, Musacchio A. MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr. Opin. Struct. Biol. 2007;17:716–725. doi: 10.1016/j.sbi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Choi E, et al. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009;28:2077–2089. doi: 10.1038/emboj.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagan RS, et al. p31(comet) acts to ensure timely spindle checkpoint silencing subsequent to kinetochore attachment. Mol. Biol. Cell. 2011;22:4236–4246. doi: 10.1091/mbc.E11-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia L, et al. Defining pathways of spindle checkpoint silencing: functional redundancy between Cdc20 ubiquitination and p31(comet) Mol. Biol. Cell. 2011;22:4227–4235. doi: 10.1091/mbc.E11-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia G, et al. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 2004;23:3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M, et al. p31comet blocks Mad2 activation through structural mimicry. Cell. 2007;131:744–755. doi: 10.1016/j.cell.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habu T, Kim SH, Weinstein J, Matsumoto T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 2002;21:6419–6428. doi: 10.1093/emboj/cdf659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi E, Zhang X, Xing C, Yu H. Mitotic checkpoint regulators control insulin signaling and metabolic homeostasis. Cell. 2016;166:567–581. doi: 10.1016/j.cell.2016.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker DJ, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 2008;10:825–836. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grako KA, Olefsky JM, McClain DA. Tyrosine kinase-defective insulin receptors undergo decreased endocytosis but do not affect internalization of normal endogenous insulin receptors. Endocrinology. 1992;130:3441–3452. doi: 10.1210/endo.130.6.1317784. [DOI] [PubMed] [Google Scholar]
- 43.Carpentier JL, et al. Two steps of insulin receptor internalization depend on different domains of the beta-subunit. J. Cell Biol. 1993;122:1243–1252. doi: 10.1083/jcb.122.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Meyts, P. The Insulin Receptor and Its Signal Transduction Network. In: Endotext (eds Feingold, K. R., Anawalt, B. & Boyce, A. et al.) (MDText.com, Inc: South Dartmouth (MA), 2000). [PubMed]
- 45.Uchikawa, E., Choi, E., Shang, G., Yu, H. & Bai, X. C. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife8, 10.7554/eLife.48630 (2019). [DOI] [PMC free article] [PubMed]
- 46.Scapin G, et al. Structure of the insulin receptor-insulin complex by single-particle cryo-EM analysis. Nature. 2018;556:122–125. doi: 10.1038/nature26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weis F, et al. The signalling conformation of the insulin receptor ectodomain. Nat. Commun. 2018;9:4420. doi: 10.1038/s41467-018-06826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boucher, J., Kleinridders, A. & Kahn, C. R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol6, a009191 10.1101/cshperspect.a009191 (2014). [DOI] [PMC free article] [PubMed]
- 49.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 50.De Fea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J. Biol. Chem. 1997;272:31400–31406. doi: 10.1074/jbc.272.50.31400. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y, et al. Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats. J. Endocrinol. 2009;203:337–347. doi: 10.1677/JOE-09-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banks AS, et al. An ERK/Cdk5 axis controls the diabetogenic actions of PPARgamma. Nature. 2015;517:391–395. doi: 10.1038/nature13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gehart H, Kumpf S, Ittner A, Ricci R. MAPK signalling in cellular metabolism: stress or wellness? EMBO Rep. 2010;11:834–840. doi: 10.1038/embor.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pronk GJ, McGlade J, Pelicci G, Pawson T, Bos JL. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J. Biol. Chem. 1993;268:5748–5753. [PubMed] [Google Scholar]
- 55.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 56.Furcht CM, Buonato JM, Lazzara MJ. EGFR-activated Src family kinases maintain GAB1-SHP2 complexes distal from EGFR. Sci. Signal. 2015;8:ra46. doi: 10.1126/scisignal.2005697. [DOI] [PubMed] [Google Scholar]
- 57.Chen YN, et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–152. doi: 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- 58.Nichols RJ, et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat. Cell Biol. 2018;20:1064–1073. doi: 10.1038/s41556-018-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cobb MH. MAP kinase pathways. Prog. Biophys. Mol. Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 60.Roth G, et al. MAP kinases Erk1/2 phosphorylate sterol regulatory element-binding protein (SREBP)-1a at serine 117 in vitro. J. Biol. Chem. 2000;275:33302–33307. doi: 10.1074/jbc.M005425200. [DOI] [PubMed] [Google Scholar]
- 61.Kotzka J, et al. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding Protein-2 at serine residues 432 and 455 in vivo. J. Biol. Chem. 2004;279:22404–22411. doi: 10.1074/jbc.M401198200. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Zheng L, Chng WJ, Ding JL. Comprehensive analysis of ERK1/2 substrates for potential combination immunotherapies. Trends Pharm. Sci. 2019;40:897–910. doi: 10.1016/j.tips.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Unal EB, Uhlitz F, Bluthgen N. A compendium of ERK targets. FEBS Lett. 2017;591:2607–2615. doi: 10.1002/1873-3468.12740. [DOI] [PubMed] [Google Scholar]
- 64.Wortzel I, Seger R. The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatano N, et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8:847–856. doi: 10.1046/j.1365-2443.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 66.Saba-El-Leil MK, et al. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao Y, et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc. Natl Acad. Sci. USA. 2003;100:12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pages G, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 69.Jager J, et al. Deficiency in the extracellular signal-regulated kinase 1 (ERK1) protects leptin-deficient mice from insulin resistance without affecting obesity. Diabetologia. 2011;54:180–189. doi: 10.1007/s00125-010-1944-0. [DOI] [PubMed] [Google Scholar]
- 70.Bost F, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- 71.Bouzakri K, et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- 72.Carlson CJ, Koterski S, Sciotti RJ, Poccard GB, Rondinone CM. Enhanced basal activation of mitogen-activated protein kinases in adipocytes from type 2 diabetes: potential role of p38 in the downregulation of GLUT4 expression. Diabetes. 2003;52:634–641. doi: 10.2337/diabetes.52.3.634. [DOI] [PubMed] [Google Scholar]
- 73.Ozaki KI, et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2016;310:E643–E651. doi: 10.1152/ajpendo.00445.2015. [DOI] [PubMed] [Google Scholar]
- 74.Saxton TM, et al. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Princen F, et al. Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Mol. Cell Biol. 2009;29:378–388. doi: 10.1128/MCB.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl Acad. Sci. USA. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuo K, et al. Altered glucose homeostasis in mice with liver-specific deletion of Src homology phosphatase 2. J. Biol. Chem. 2010;285:39750–39758. doi: 10.1074/jbc.M110.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagata N, et al. Hepatic Src homology phosphatase 2 regulates energy balance in mice. Endocrinology. 2012;153:3158–3169. doi: 10.1210/en.2012-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bard-Chapeau EA, et al. Deletion of Gab1 in the liver leads to enhanced glucose tolerance and improved hepatic insulin action. Nat. Med. 2005;11:567–571. doi: 10.1038/nm1227. [DOI] [PubMed] [Google Scholar]
- 80.White MF, et al. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell. 1988;54:641–649. doi: 10.1016/s0092-8674(88)80008-4. [DOI] [PubMed] [Google Scholar]
- 81.Eck MJ, Dhe-Paganon S, Trub T, Nolte RT, Shoelson SE. Structure of the IRS-1 PTB domain bound to the juxtamembrane region of the insulin receptor. Cell. 1996;85:695–705. doi: 10.1016/s0092-8674(00)81236-2. [DOI] [PubMed] [Google Scholar]
- 82.Gustafson TA, He W, Craparo A, Schaub CD, O’Neill TJ. Phosphotyrosine-dependent interaction of SHC and insulin receptor substrate 1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol. Cell Biol. 1995;15:2500–2508. doi: 10.1128/mcb.15.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He W, et al. Interaction of insulin receptor substrate-2 (IRS-2) with the insulin and insulin-like growth factor I receptors. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J. Biol. Chem. 1996;271:11641–11645. doi: 10.1074/jbc.271.20.11641. [DOI] [PubMed] [Google Scholar]
- 84.Wolf G, et al. PTB domains of IRS-1 and Shc have distinct but overlapping binding specificities. J. Biol. Chem. 1995;270:27407–27410. doi: 10.1074/jbc.270.46.27407. [DOI] [PubMed] [Google Scholar]
- 85.Backer JM, Kahn CR, Cahill DA, Ullrich A, White MF. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor beta-subunit. J. Biol. Chem. 1990;265:16450–16454. [PubMed] [Google Scholar]
- 86.Backer JM, Shoelson SE, Haring E, White MF. Insulin receptors internalize by a rapid, saturable pathway requiring receptor autophosphorylation and an intact juxtamembrane region. J. Cell Biol. 1991;115:1535–1545. doi: 10.1083/jcb.115.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun XJ, Crimmins DL, Myers MG, Jr., Miralpeix M, White MF. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol. Cell Biol. 1993;13:7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 89.Myers MG, Jr., et al. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 1998;273:26908–26914. doi: 10.1074/jbc.273.41.26908. [DOI] [PubMed] [Google Scholar]
- 90.Zhang SQ, et al. Receptor-specific regulation of phosphatidylinositol 3’-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hers I, Tavare JM. Mechanism of feedback regulation of insulin receptor substrate-1 phosphorylation in primary adipocytes. Biochem J. 2005;388:713–720. doi: 10.1042/BJ20041531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edouard T, et al. Functional effects of PTPN11 (SHP2) mutations causing LEOPARD syndrome on epidermal growth factor-induced phosphoinositide 3-kinase/AKT/glycogen synthase kinase 3beta signaling. Mol. Cell Biol. 2010;30:2498–2507. doi: 10.1128/MCB.00646-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tartaglia M, Gelb BD. Germ-line and somatic PTPN11 mutations in human disease. Eur. J. Med Genet. 2005;48:81–96. doi: 10.1016/j.ejmg.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Tartaglia M, et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 2003;34:148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 96.Xu R, et al. Overexpression of Shp2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia. Blood. 2005;106:3142–3149. doi: 10.1182/blood-2004-10-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aceto N, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat. Med. 2012;18:529–537. doi: 10.1038/nm.2645. [DOI] [PubMed] [Google Scholar]
- 98.Hu Z, Li J, Gao Q, Wei S, Yang B. SHP2 overexpression enhances the invasion and metastasis of ovarian cancer in vitro and in vivo. Onco Targets Ther. 2017;10:3881–3891. doi: 10.2147/OTT.S138833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grossmann KS, Rosario M, Birchmeier C, Birchmeier W. The tyrosine phosphatase Shp2 in development and cancer. Adv. Cancer Res. 2010;106:53–89. doi: 10.1016/S0065-230X(10)06002-1. [DOI] [PubMed] [Google Scholar]
- 100.Fedele C, et al. SHP2 inhibition prevents adaptive resistance to MEK inhibitors in multiple cancer models. Cancer Discov. 2018;8:1237–1249. doi: 10.1158/2159-8290.CD-18-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao M, et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm. Sin. B. 2019;9:304–315. doi: 10.1016/j.apsb.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tajan M, et al. LEOPARD syndrome-associated SHP2 mutation confers leanness and protection from diet-induced obesity. Proc. Natl Acad. Sci. USA. 2014;111:E4494–4503. doi: 10.1073/pnas.1406107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ardon O, Procter M, Tvrdik T, Longo N, Mao R. Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol. Genet. Metab. Rep. 2014;1:71–84. doi: 10.1016/j.ymgmr.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoneyama, Y. et al. IRS-1 acts as an endocytic regulator of IGF-I receptor to facilitate sustained IGF signaling. Elife7, e32893 10.7554/eLife.32893 (2018). [DOI] [PMC free article] [PubMed]
- 105.Li J, Choi E, Yu H, Bai XC. Structural basis of the activation of type 1 insulin-like growth factor receptor. Nat. Commun. 2019;10:4567. doi: 10.1038/s41467-019-12564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahmed TA, et al. SHP2 drives adaptive resistance to ERK signaling inhibition in molecularly defined subsets of ERK-dependent tumors. Cell Rep. 2019;26(65-78):e65. doi: 10.1016/j.celrep.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]