Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age. Metabolic sequelae associated with PCOS range from insulin resistance to type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD). Insulin resistance plays a significant role in the pathophysiology of PCOS and it is a reliable marker for cardiometabolic risk. Although insulin sensitising agents such as metformin have been traditionally used for managing metabolic aspects of PCOS, their efficacy is low in terms of weight reduction and cardiovascular risk reduction compared with newer agents such as incretin mimetics and SGLT2 inhibitors. With current pharmaceutical advances, potential therapeutic options have increased, giving patients and clinicians more choices. Incretin mimetics are a promising therapy with a unique metabolic target that could be used widely in the management of PCOS. Likewise, bariatric procedures have become less invasive and result in effective weight loss and the reversal of metabolic morbidities in some patients. Therefore, surgical treatment targeting weight loss becomes increasingly common in the management of obese women with PCOS. Newer emerging therapies, including twincretins, triple GLP-1 agonists, glucagon receptor antagonists and imeglemin, are promising therapeutic options for treating T2DM. Given the similarity of metabolic and pathological features between PCOS and T2DM and the variety of therapeutic options, there is the potential to widen our strategy for treating metabolic disorders in PCOS in parallel with current therapeutic advances. The review was conducted in line with the recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018.
Keywords: bariatric surgery, dietary intervention, GLP-1, glucagon-like peptide-1, incretins hormone, lifestyle modifications, metabolic comorbidities, metabolic syndrome, obesity, physical activity, polycystic ovary syndrome (PCOS), SGLT2 inhibitors, sodium-glucose co-transporter2 inhibitors
Introduction
Metabolic consequences of PCOS
Polycystic ovary syndrome (PCOS) is a complex endocrine condition affecting women in the reproductive age, with a prevalence of 15–20%.1 PCOS is characterised by biochemical and clinical features of excess androgen levels (hirsutism and acne), menstrual irregularities and polycystic morphology of the ovaries.2 Women with PCOS have a higher prevalence of impaired glucose tolerance and insulin resistance, both drivers of type 2 diabetes mellitus (T2DM).3 Nearly 50% of women with PCOS progress to frank metabolic syndrome (MS).4 MS is defined as a group of conditions including dyslipidaemia, central adiposity, hypertension and impaired glucose tolerance, all predisposing to coronary heart disease and diabetes.5 The prevalence of MS in women with PCOS is significantly high, at around 40–50% compared with the general population.6 In addition, PCOS is associated with a spectrum of complications that include infertility, increased body weight, endometrial cancer and an increased risk of cardiovascular disease (CVD).7,8 A therapeutic approach targeting weight control is the cornerstone in the management of PCOS as effective weight loss is a positive predictor for long-term prevention of cardiometabolic disorders in PCOS.9 However, weight loss in women with PCOS remains challenging to achieve. Lifestyle modifications that include diet and physical activity are the first-line therapy. Still, it rarely reduces body weight by more than 10 kg,10 and the available evidence on lifestyle intervention is largely disappointing.11 Pharmacotherapies to prevent and manage metabolic comorbidities in PCOS exist; however, they are underutilised as their effectiveness remains relatively unexplored, and they are used primarily to treat other conditions such as T2DM. The surgical approach for weight loss has been an option for many decades, but it has not been used commonly as a therapeutic option in the management of PCOS. In this review, we have aimed to provide an overview of the treatment options that offer therapeutic and preventive advantages for the metabolic comorbidities in PCOS focussing on lifestyle modification, T2DM medications and bariatric surgery. The review was conducted in accordance with the recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.12
Treatment options for managing metabolic comorbidities
Lifestyle modification intervention
Over 50% of the women with PCOS are overweight or obese, with a higher propensity of central adiposity, due primarily to impaired glucose tolerance and insulin sensitivity in this population.13 Increased visceral fat deposition is associated with increased severity of PCOS and plays a pivotal role in high serum androgen production and reduced serum levels of sex-hormone-binding globulin (SHBG).14 Obese women with PCOS are more prone to an increased risk of metabolic abnormalities and severe cardiovascular conditions compared with women without PCOS.15 Even though the aetiology of PCOS not fully understood, PCOS has been associated strongly with obesity and insulin resistance.2 Given the links between obesity, insulin resistance and increased cardiometabolic risk factors, treating obesity is a priority, especially in obese women with PCOS.
In many cases, this can be achieved by modest weight loss and lifestyle modification interventions, including dietary management and physical activity, are strongly recommended, particularly for those who are categorised as prediabetes as this may delay the onset of T2DM.16,17 The recent international evidence-based guideline for the assessment and management of PCOS has emphasised the importance of physical activity and diet for managing PCOS-related symptoms and to prevent its metabolic complications.18 Weight reduction can benefit obese women with PCOS through reduced adiposity, androgen levels, insulin levels, improved ovulatory function, increased fertility and a reduction in the overall risk of CVD.2,16,19 Given the strong link between obesity, insulin resistance and metabolic problems, a low glycaemic index diet would seem to be an attractive option for weight reduction; however, no one single diet has proven to be better than another, although the trials to date have been small, of limited duration and heterogeneous in their design. A small study of women with PCOS assigned for a ketogenic, low-carbohydrate diet for 6 months reported significant improvement in their weight, hormonal profiles and fertility.20 Others have shown that a very modest reduction of carbohydrate intake from 55% to 41% of total energy reflected in favourable metabolic effects and a preferential decrease in fat mass among women with PCOS.21,22 A systematic review of relevant studies found that low carbohydrate diets improved the hormonal profile, reduced insulin levels and helped resume ovulation in women with PCOS.23 A survey of 14 women with PCOS administered a low ketogenic Mediterranean diet for 12 weeks, reported a significant reduction in serum insulin, blood glucose level and average weight loss of 9.4 kg.24 Previous studies showed that lifestyle modifications could be associated with significant improvement in symptoms of PCOS. A study assessing the effect of isocaloric diets on insulin sensitivity and insulin levels has demonstrated that moderate reduction in carbohydrate intake reduced fasting and challenged insulin levels amongst women with PCOS.25 A recent meta-analysis assessed the effect of different dietary compositions on metabolic and reproductive outcomes in women with PCOS that showed there was more significant weight loss with monounsaturated fat, improved menstrual problems with a low glycaemic index diet and a more substantial reduction in insulin resistance.26 Physical activity usually acts as an adjunct to dietary intervention for the management of PCOS. A systematic review evaluating exercise intervention in PCOS reported that moderate-intensity regular aerobic exercise over a short period of time significantly improved menstrual regularity and ovulation and contributed to reduced weight and insulin resistance in young and obese women with PCOS.27 A recent systematic review and meta-analysis that examined the effects of lifestyle interventions including exercise only or in combination with diet and behavioural therapy in women with PCOS, showed significant effects of exercise on the metabolic, anthropometric and cardiorespiratory outcomes.28
Insulin sensitising agents
The pathophysiology of PCOS includes defective insulin secretion and function.29 Hyperinsulinemia and insulin resistance are known to contribute to the high level of androgens in PCOS.30 Insulin helps to control ovarian function, and the ovaries are sensitive to high insulin levels.2 Theca cells produce high androgen as a response to excess insulin, which in turn contributes to the arrest of follicular maturation that predisposes to polycystic ovarian morphology, an indicator of PCOS.31 As well as its pivotal role in the pathology of PCOS, insulin resistance has a detrimental effect by predisposing PCOS patients to long-term health problems that including T2DM and CVD. Therefore, a therapeutic strategy to address insulin resistance, including pharmacotherapy and lifestyle intervention is crucial in the management of PCOS.
Thiazolidinediones
Pioglitazone is a thiazolidinedione that is peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonist. Pioglitazone principally acts by increasing peripheral glucose uptake, regulating adipogenesis and insulin action. Its effect in improving insulin resistance, hyperandrogenaemia and ovulatory dysfunction has been seen in women with PCOS.32 In a randomised control trial (RCT) investigating the effect of pioglitazone versus placebo in PCOS, pioglitazone resulted in significant reductions in fasting serum insulin and the free androgen index, whilst SHBG levels were increased.33 A meta-analysis comparing the effect of metformin and pioglitazone in treating PCOS reported a significant improvement in ovulation and menstrual cycle in the pioglitazone group. However, there was a marked increase in body mass index (BMI) score in the pioglitazone group compared with metformin.34 A randomised open-label study assessing the effect of pioglitazone, metformin and orlistat on mean insulin resistance (IR) and its biological variability in women with PCOS, reported a significant overall reduction in IR and IR variability.35 Despite the desirable effect of pioglitazone on the metabolic parameters in PCOS, there is considerable concern about the potential risk of myocardial damage, congestive heart failure and pulmonary oedema due to fluid retention.36 However, whilst the absolute risk is low in young women with PCOS, weight gain is a concern with thiazolidinediones in women with PCOS who are obese, and its use is an unlicensed indication.
Metformin
Metformin is a member of the biguanide family with proven safety and efficacy. Metformin has long been used in the management of T2DM and it is one of the insulin sensitising agents commonly used in the treatment of PCOS,37 though it is still an unlicensed indication in PCOS. The mechanism of action of metformin is through inhibition of hepatic glucose production, increased glucose uptake and increased insulin sensitivity in the peripheral tissues. The common side effects associated with metformin are nausea, vomiting, diarrhoea and abdominal bloating38; however, the prevalence of these symptoms is variable, and the severity of the side effects can be reduced by titrating the dose guided by the severity of the symptoms, or by using modified-release preparations. Women with PCOS are at an increased risk of having prediabetes or T2DM. Despite this clear association, obesity sometimes confounds the link between PCOS and T2DM. Thus, prevention of T2DM in this cohort is crucial, and there is reliable evidence for the use of metformin to reduce the risk of T2DM in high-risk women with PCOS. In a study comparing metformin and lifestyle intervention in women with PCOS, a significant reduction in BMI was observed in both groups; however, reduction in androgen levels was only seen in the metformin group.39 In an RCT of obese and morbidly obese women with PCOS assessing the effect of metformin on body weight, a significant decrease in BMI independent of lifestyle modification was reported.40 In a study of 3234 non-diabetic participants with elevated fasting plasma glucose randomised to either metformin or lifestyle intervention with a mean follow up nearly 3 years, lifestyle changes reduced the new incidence of T2DM by almost 60%. In contrast, metformin reduced it by just over 30 %41; however, this effect was lost entirely following the washout period. This was further confirmed in a similar study where the impact of metformin no longer existed after 12 months of withdrawal.42 Women with PCOS are also at an increased risk of CVD owing to the hyperinsulinemia, high androgen levels, obesity and dyslipidaemia.43 There is evidence that obesity and PCOS independently affect vascular endothelial function44; however, the association between high insulin levels and CVD is independent of obesity.45,46 Women with PCOS have worse lipid profiles compared with the healthy population and they typically have low high-density lipoprotein (HDL) and high triglyceride levels that are both strong predictors of CVD.47,48 Thus, the management of dyslipidaemia is crucial in PCOS. Metformin improves dyslipidaemia by either a direct effect on the hepatic metabolism of free fatty acids or indirectly by reducing hyperinsulinemia.49 Many studies have reported that metformin has a significant impact on dyslipidaemia;50,51 however, there was no beneficial effect of metformin on total cholesterol levels.52
In women with PCOS, metformin is usually prescribed at starting doses of 500–850 mg daily and can be titrated up to 2000 mg/day if tolerated.53 Higher metformin doses have been beneficial in reducing weight and improving lipid profiles, particularly in obese PCOS population.40 There is also evidence of the development of vitamin B12 deficiency with long-term metformin use.54–57 Therefore, due to metformin intolerance and its associated adverse events, it is important to consider other therapeutic options for the treatment of metformin intolerant women with PCOS.
Glucagon-like peptide-1 receptor analogue
Incretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are recognised stimulators for glucose-dependent insulin release, particularly following meal ingestion, known as the incretin effect.58 The impaired incretin effect is common in conditions of insulin resistance, particularly T2DM. A recent study has reported lower incretin hormone levels in patients with PCOS.59 Therefore, targeting this system has become a practical therapeutic approach in the treatment of T2DM,60 with both a glycaemic improvement and a reduction in weight in patients with T2DM.
Given that insulin resistance is the main driver for metabolic and endocrine dysfunction in PCOS, the therapeutic advantages of GLP-1 agonist therapy in this population is logical.61 Even though the primordial effect of GLP-1 is not to stimulate insulin secretion, they might indirectly improve the insulin sensitivity by their weight reduction effect. A recent study by Yaribeygi et al. suggested that these agents also enhance insulin sensitivity by acting on eight different molecular pathways including inflammatory, oxidative stress, lipid metabolism, GLUT-4 expression/translation, β-cells function, endoplasmic reticulum (ER) and insulin signalling pathways.62 Commercially available GLP-1 agonists include liraglutide, semaglutide, dulaglutide and exenatide. A once-daily injection of liraglutide (1.2 mg or 1.8 mg) has shown to improve levels of glycaemic control in patients with T2DM (HbA1c reduced by 1.5%) with concomitant weight loss.63 Similar results were illustrated with semaglutide treatment (HbA1c reduced by 1.4%), with significant weight reduction.64 Liraglutide showed a significant cardiovascular risk reduction in the LEADER study.65 A study that explored the effects of exenatide on cardiovascular risk markers in women with PCOS demonstrated substantial weight reduction and improvement in the endothelial markers.66 Of the several agents, the only one that has been used extensively in PCOS is liraglutide. A single observational study has explored the effect of liraglutide on weight loss in obese and overweight patients with PCOS, with promising results. In this trial (mean duration of treatment 27.8 weeks), 84 obese women with PCOS were treated with subcutaneous liraglutide daily injection (starting dose of 0.6 mg with daily increment to 1.2 mg and then 1.8 mg if tolerated) for 4 weeks and followed for over 27 weeks and showed a significant reduction in weight and BMI.67 A recent systematic review and meta-analysis that compared the efficacy of GLP-1 agonists and metformin in women with PCOS have shown a significant improvement in insulin sensitivity, reduced BMI and abdominal girth compared with metformin.68 However, similar studies reported positive results on weight reduction and a decrease in testosterone levels, but no significant effects on insulin levels and insulin sensitivity in women with PCOS treated with GLP-1 receptor analogues (RAs).69,70 In a trial that assessed the effects of treatment with liraglutide (1.8 mg od) on atherothrombotic risk in obese women with PCOS compared with control, reported a significant reduction in both weight and for atherothrombotic markers including endothelial function and clotting.71 Another trial that examined the effect of liraglutide (1.8 mg) on quality of life (QOL) and depression in obese PCOS patients showed a dramatic weight reduction which significantly improved QOL.72
Dipeptidyl peptidase-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors also known as gliptins (e.g. sitagliptin, vildagliptin, linagliptin, saxagliptin, alogliptin) are oral anti-hyperglycaemic agents for the treatment of T2DM; they are usually used as a second or third-line treatment after metformin.73 They work by inhibiting DPP-4 – a ubiquitous enzyme that is responsible for the degradation of internally produced GLP-1.74 Sitagliptin is the most studied class of this drug so far. A double-blind crossover study tested the effect of sitagliptin on blood glucose levels and visceral fat (VAT) in women with PCOS, and demonstrated a reduction in the maximal glucose response to the oral glucose tolerance and VAT.75 In a pilot randomised study that examined sitagliptin as a potential treatment option in metformin intolerant PCOS patients, sitagliptin improved β-cell function (HOMA-B) and insulin sensitivity.76 In an animal study, sitagliptin reduced fasting blood glucose, lowered androgen levels and improved ovarian fibrosis in rats with PCOS.77 The most reported adverse reactions to gliptin therapy are headache, breathing problems and pharyngitis.78 Hypersensitivity reactions, including angioedema, anaphylactic reaction and dermatological reaction, have been reported with saxagliptin therapy though they are rare.79
Sodium-glucose co-transporter-2 inhibitors
Sodium-glucose co-transporter-2 inhibitors (SGLT-2) (e.g. empagliflozin and dapagliflozin) are a class of oral medications used in the management of T2DM. Their mode of action is by inhibiting SGLT-2 in the proximal convoluted tubule (PCT) of the kidney that reduces glucose reabsorption and increases urinary glucose excretion.80 As glucose is eliminated, its plasma levels drop, leading to a significant improvement in glycaemic parameters.81 This mechanism of action is solely glucose-dependent, and unlike other agents, it is insulin-independent; therefore, the risk of hypoglycaemia is minimal.81 There is an emerging role of SGLT2 inhibitors for the treatment of obesity; their body weight effect is promising in addition to their protective advantages for cardiovascular and renal events.82 In addition to their glucose-lowering effect, they can also improve insulin sensitivity via several molecular pathways including reduction of glucotoxicity and lipotoxicity, enhance β-cells function, reduce the oxidative damage and inflammatory processes, improve caloric deposition and weight loss.83
Empagliflozin has demonstrated improvement in glycaemic control as monotherapy or in combination with other glucose-lowering agents. In a 24-week placebo-controlled study (EMPA-REG-MONO), empagliflozin at both doses of 10 mg and 25 mg showed significant reduction of HbA1c % (–1.43%, –1.44%, respectively) compared with placebo in T2DM patients.84 Empagliflozin, as an add-on to pioglitazone and compared with placebo in a 24-week study in patients with T2DM (EMPA-REG-PIO), demonstrated a significant reduction in fasting blood glucose and HbA1c compared with placebo.85 Treatment with SGLT2-inhibitors was also associated with significant weight reduction, with an average of 2–4 kg.86 Data from studies in patients with T2DM showed that 60–70% of the total weight reduction observed with dapagliflozin was attributed to the reduction in fat mass including both visceral, subcutaneous adipose tissues and body water.87,88 The EMPA-REG-OUTCOME trial was conducted in 7020 patients with T2DM with established CVDs and showed that the primary composite outcome (MACE) of cardiovascular death, myocardial infraction and stroke was significantly reduced in empagliflozin-treated patients.89 Similar results were seen in the CANVAS (for canagliflozin) trial, which enrolled 10,142 T2DM patients at high risk of CVD; the primary outcome (MACE) was reduced by 14%.90 However, even though data from DECLARE-TIMI trials (for dapagliflozin) did not show a significant effect on MACE outcome, it did demonstrate a lower rate of cardiovascular deaths and hospitalisation for heart failure.91 Treatment with SGLT2 inhibitors has also shown a reno-protective effect in high risk patients with T2DM. In the CANVAS trial, canagliflozin showed a 40% reduction in eGFR, reduced the need for dialysis and decreased deaths from renal causes, and the progression to albuminuria was reduced by 27%.90 Data from the canagliflozin trial in patients with impaired renal functions (CREDENCE) showed a significant reduction in renal composite outcomes by 34 %, including end-stage renal failure, doubling serum creatinine and deaths from renal causes.92
Recently, treatment with SGLT2 inhibitors have shown promising results in trials involving patients with PCOS. In a 12-week randomised open-label study of empagliflozin versus metformin in obese women with PCOS, treatment with empagliflozin demonstrated significant improvement of anthropometric parameters and body composition but no changes were observed in the metabolic parameters.93 This suggests that SGLT2 inhibitors could potentially be useful in the management of PCOS. Common adverse events reported for the SGLT2 inhibitors include genital infections, genitourinary tract infection, vulvovaginal candidiasis and vulvovaginitis.89
Myo-inositol
Inositol is a carbocyclic sugar that is present in abundance in the human and plant cells.94 In humans, it is synthesised principally in the liver and the kidney, and is naturally present in legumes, cereal, corn and meat.95 It can be found in nine different isomeric forms, with myo-inositol (MYO) and d-chiro-inositol being the most represented. MYO plays an essential role as the structural foundation for several second messengers in various intracellular signal transduction pathways, including insulin signal transduction. MYO-based second messenger activation increases the activity of glucose transport proteins and regulates glucose intake, while d-chiro-inositol-based second messenger activation stimulates glycogen synthesis. Abnormal inositol synthesis and metabolism has been reported in both type 1 and type 2 diabetes.96 Inositol has been used as a dietary supplement with increasing popularity in the treatment of various conditions, including T2DM, gestational diabetes (GD) and PCOS.97 A retrospective study reported a significantly low risk of GD in PCOS women treated with MYO compared with controls.98
MYO intake has shown beneficial effects in ovulation and the response to assisted fertilisation in otherwise infertile women with PCOS.99 A recent systematic review that assessed the impact of MYO in women with PCOS concluded that MYO supplements improved hormonal and reproductive problems of PCOS. In addition, it enhanced follicular development and oocyte maturation.100 Generally, MYO supplements are well tolerated at the currently recommended doses of 2–4 g/day with minimal safety issues; therefore, its clinical application in the management of PCOS is worth considering.
Statins
Dyslipidaemia, as reflected in elevated LDL-C, triglycerides and reduced HDL-C, is prevalent in women with PCOS and is a strong predictor of cardiovascular risk.101 Therefore, effective treatment of PCOS would encompass improvement in the lipid profile and subsequently reduced cardiovascular morbidity.
There is growing evidence that statins are beneficial in the treatment of PCOS.102 Statins (atorvastatin, fluvastatin, pravastatin, rosuvastatin and simvastatin) are an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase – a rate-controlling enzyme essential in the cholesterol biosynthesis pathway. Blocking this enzyme will stop the conversion of HMG-CoA to mevalonate and subsequently block cholesterol synthesis.103 In a randomised placebo-controlled study, atorvastatin significantly reduced insulin resistance, inflammatory markers and hyperandrogenaemia in women with PCOS compared with placebo.104 When both the atorvastatin and placebo group were followed up with metformin for another 12 weeks, atorvastatin pre-treatment to metformin in women with PCOS demonstrated significant improvements in HOMA-IR (homeostasis model assessment insulin resistance), free androgen index (FAI), total testosterone and sex hormone-binding globulin (SHBG) in atorvastatin pre-treated group compared with placebo pre-treated group, suggesting that atorvastatin augments the effect of metformin in PCOS patients.105 In this study, when the effect of atorvastatin on the markers of inflammation and adipose tissue dysfunction were examined, 12 weeks of treatment with atorvastatin significantly reduced acylation stimulating protein (ASP), interleukin-6 (IL-6) and monocyte-chemoattractant protein-1 (MCP-1). Subsequently, there was a substantial improvement in HOMA-IR and testosterone levels.106
In the same study, the effect of atorvastatin on pancreatic β-cell function (HOMA-β) was examined, which showed a significant reduction on HOMA-β. However, this result was maintained by metformin treatment for another 12 weeks, which indicated a potential improvement of insulin resistance and therefore, a reduction in β-cell requirement rather than an actual fall in β-cell function.107 Treatment with atorvastatin also significantly reduced serum malondialdehyde (MDA) – a marker of oxidative stress among obese women with PCOS.108 Furthermore, atorvastatin significantly reduced the levels of androstenedione and dehydroepiandrosterone sulphate (DHEAS) in this cohort of women with PCOS.109 A 12-week course of atorvastatin also significantly raised the concentration of serum vitamin D (25OHD) level among women with PCOS compared with placebo.105 However, until further robust data are available to clarify its efficacy, due to its potential teratogenicity it should not be used in young women of reproductive age.
Weight loss medications
Orlistat
Orlistat is both a gastric and pancreatic lipase inhibitor that reduces the absorption of prandial dietary fat by minimising the hydrolysis of triglyceride.110 Orlistat is recognised in obesity treatment with proven, though low, efficacy. A study evaluated and compared the effect of treatment with orlistat versus metformin on the biochemical and hormonal factors in women with PCOS, treatment with orlistat showed a significant reduction in weight and androgen level compared with metformin.111 A randomised open labelled parallel study compared the change in insulin resistance (IR) and its biological variability after treatment with orlistat, metformin and pioglitazone in obese patients with PCOS, orlistat significantly reduced both IR and its biological variability compared with metformin and pioglitazone.35 In a study, women with PCOS treated with orlistat were compared with metformin and lifestyle intervention, and there was an improvement in lipid profiles, weight, BMI and waist circumference.112 Also, orlistat reduced androgen levels, parameters of IR and total cholesterol.113,114 Orlistat also modestly reduces blood pressure and plays a role in the prevention of T2DM in this high-risk population, possibly by its effect on weight reduction.115 However, orlistat at the recommended dose of 120 mg up to 3 times a day taken with food has significant side effects, including fatty stool, diarrhoea, abdominal pain and flatulence.116 It may also cause fat-soluble vitamin deficiencies.117 While orlistat might have desirable effects in the management of obesity, its relevance in the control of the metabolic aspect of PCOS remains controversial.
Historical weight loss agents
Sibutramine
Sibutramine is an appetite suppressant used as an adjunct to lifestyle intervention in the treatment of obesity. It is a monoamine reuptake inhibitor that reduces the uptake of neurotransmitters such as serotonin, noradrenaline and dopamine.118 Therefore, it increases their availability in the synaptic clefts, which help reducing appetite, enhancing satiety and resulting in reduced food intake.119 An RCT reported significant weight reduction after 6 months of treatment with sibutramine at daily dose of 15 mg (7.8 ± 5.1 kg) compared with placebo (2.8 ± 6.2 kg) in women with PCOS.120 In addition, another RCT reported even greater weight reduction (−15.4 ± 1.1 versus –11.1 ± 1.9) with a lower daily dose of 10 mg.121 However, sibutramine has a significant cardiovascular risk through increased cardiovascular mortality, stroke and myocardial infarction.122 Therefore, the use of sibutramine for weight loss in women with PCOS with high cardiometabolic risk is questionable.
Rimonabant
Rimonabant is an anorectic, selective cannabinoid receptor 1 (CB1) blocker used for obesity treatment. A study assessed the impact of rimonabant on the markers of hepatic injury in obese women with PCOS without non-alcoholic fatty liver disease (NAFLD), rimonabant significantly reduced alanine aminotransferase (ALT) and weight.123 A trial comparing the effect of treatment with rimonabant and metformin on incretin hormones in obese women with PCOS showed a significant increase in GIP after 3 months of rimonabant treatment, no change was reported with metformin.124 Moreover, treatment with rimonabant augmented the weight loss effect and enhanced the metabolic benefit of metformin treatment in obese women with PCOS.125 However, rimonabant demonstrated superior impact in weight reduction, improved insulin resistance and a reduction in androgen levels compared with metformin in women with PCOS.126 Nevertheless, data from clinical trials showed that rimonabant caused severe psychiatric problems, including a depressive disorder, mood changes and suicidal ideation.127 Both sibutramine and rimonabant have been withdrawn from the market in view of their side effect profile.
Naltrexone/bupropion
Naltrexone is an opioid receptors antagonist with great affinity to the µ opiate receptor, which is implicated in eating behaviours. In experimental studies, naltrexone has shown an ability to block dopamine release and subsequently reduce food intake, food eating and binge eating behaviour. However, in clinical human studies, naltrexone as monotherapy has not produced consistent results. It has recently been approved by the United States (US) Food and Drug Administration (FDA) for the management of alcohol and drug addiction.128 Bupropion is an anti-depressant that is approved for the management of depression, seasonal affective disorder and to help with smoking cessation that acts by blocking dopamine reuptake. In clinical studies its main side effect was weight loss.129 Although these agents were not principally approved for management of obesity, clinical trials suggest that the combination of these agents induces significant weight loss. Therefore, the combination of naltrexone/bupropion (N/B), which is marketed as CONTRAVE pills, or COR for short, has recently been approved for obesity treatment both in the US and Europe.
In two double-blind placebo controlled clinical trials CONTRAVE Obesity Research (COR-I and COR-II) in overweight and obese patients, combination of N/B demonstrated significant weight loss (–8.1% and –8.2%, respectively) and showed an improvement in cardiometabolic parameters (–1.8% and –1.4%) compared with placebo.130,131 Furthermore, in the COR-BMOD (COR-Behavioural Modification) trial, patients treated with the combination of N/B in addition to an intensive behavioural modification programme or placebo showed significant weight loss with N/B+BMOD compared with placebo + BMOD (–11.5% versus –7.3%; p < 0.01, respectively).132 Furthermore, in the COR-diabetes study, where overweight and obese patients with T2DM were randomised to N/B or placebo, treatment with the N/B combination showed a significant weight loss effect regardless of concomitant diabetes medications (–5.9% versus –2.2%; p < 0.01 respectively) compared with placebo.133
The combination of N/B has not been used for PCOS treatment; therefore, there is no evidence of its efficacy in this cohort. However, a few studies have demonstrated positive effects of naltrexone monotherapy in the treatment of PCOS. In a RCT, 30 clomiphene-resistant obese women with PCOS treated with naltrexone(50 mg/day) for a duration of 6 months showed significant reductions in BMI, fasting serum insulin and luteinizing hormone to follicle-stimulating hormone (LH/FSH) ratio.134 It also showed significant effect in combination with pulsatile gonadotropin-releasing hormone (GnRH) by improving ovarian responsiveness to ovulation induction in obese women with PCOS compared with pulsatile GnRH alone.135 Thus, the combination of naltrexone and bupropion may have a significant clinical effect on weight management for the metabolic aspect of PCOS.
Bariatric surgery
Bariatric surgery has been around for decades; it ameliorates metabolic abnormalities, including insulin resistance and excess body weight. Although bariatric surgery may be undertaken using various procedures, the most commonly used is sleeve gastrectomy, Roux-en-Y gastric bypass (RYGB) and adjustable gastric banding.136 However, evidence has shown that RYGB is superior to sleeve gastrectomy in both obesity treatment and remission of T2DM.137,138 The current guidelines recommend bariatric surgery as a treatment option for obese patients with BMI of 40 kg/m2 or more, or BMI between 35 kg/m2 and 40 kg/m2 and other comorbidities such as T2DM or high blood pressure.139 Although PCOS is more prevalent in morbidly obese women at reproductive age, only a few studies have addressed the effect of bariatric surgery in this cohort. A prospective study followed 17 PCOS women with an average age of 30 years prospectively for 26 months after biliopancreatic bypass or a laparoscopic RYGB. Menstruation was normalised for most patients, and an average weight loss of 41 kg was reported. Moreover, there was a significant improvement in the parameters of insulin resistance (50% reduction of HOMA-IR), hirsutism and androgen profile.140 In addition, a retrospective study of 24 morbidly obese women with PCOS (average BMI 50 kg/m², mean age 34 ± 7 years and mean body weight of 306 ± 44 Ib) who had RYGB and followed for over two consecutive years was reported. All women resumed their menstrual cycle within 3.5 months, there was a weight loss of approximately 56.7% at 12 months and HbA1c reduced from 8.1% to 5.1% in 3 months.141 PCOS presents a great risk for MS since many women with PCOS are obese and bariatric surgery in selected women may provide a longer-term therapeutic option in selected subjects.142
Future treatment options
Dual GLP-1/GIP receptor agonist (Twincretins)
Twincretins is a GLP-1 and GIP RA named LY3298176 (a fatty acid-modified peptide). It is currently in phase II studies and has shown a promising effect in reducing A1c and weight in T2DM patients. In a randomised, placebo-controlled, double-blind trial investigating the efficacy and tolerability of LY3298176 compared with a selective GLP-1 agonist (dulaglutide) in T2DM patients, LY3298176 reduced fasting blood glucose and had a greater significant weight reduction than dulaglutide, with tolerability that was comparable with the GLP-1 agonist.143 A recent randomised, placebo-controlled double-blind phase IIa trial compared the efficacy and the safety profile of a novel dual-action product (NNC0090-2746) in inadequately controlled patients with T2DM. Patients were randomised to 1.8 mg of NNC0090-2746 by subcutaneous injection daily or placebo as one arm. Liraglutide 1.8 mg subcutaneous daily injection with 2 weeks titration was given as an open-label arm. The results showed that NNC0090-2746 significantly improved glycaemic control (HbA1c) and reduced body weight compared with placebo.144 From the current data, it would seem that twincretins are promising new therapies to enhance the management of T2DM and weight control with potential utility for PCOS treatment.
Dual GLP-1/glucagon agonist
GLP-1/glucagon co-agonists with enhanced metabolic efficacy have been developed recently as therapeutic options for diabetes and obesity treatment. In animal models and non-human primates, GLP-1/glucagon agonists have shown potency to induce glycaemic control, weight loss and reduce hepatic fat content.145 A novel GLP-1R/GCGR dual agonist used in diet-induced obese mice normalised glucose tolerance, improved adiposity and metabolic parameters,146 suggesting that this combination could be potentially beneficial in patients with diabetes and possibly women with PCOS.
Triple GLP-1/GIP/glucagon agonist
The potential success of dual GLP-1/GIP and GLP-1/glucagon agonists has inspired the invention of a single combination of an agonist of all three target receptors. In an animal model, the tri-agonist had a significant weight lowering effect that was higher than that of liraglutide.147 Moreover, it reduced plasma glucose and cholesterol levels.148 HM15211 is a glucagon agonist with the ability to target all three receptors that showed significantly higher weight loss effect compared with liraglutide, reducing hepatic fat mass and improving lipid profiles.149 Therefore, this could potentially be a therapeutic option in women with PCOS to improve metabolic risk if proven beneficial in clinical studies.
Glucagon receptor antagonist
Glucagon is a hormone produced by α-cells of the pancreas that potently regulates glucose homeostasis during fasting states by stimulating hepatic gluconeogenesis and glycogenolysis.150 High glucagon levels and increased glucagon to insulin ratio have been reported in patients with diabetes.151 Therefore, blocking glucagon receptors would be predicted to reduce hepatic glucose production and consequently improve glycaemic control. Glucagon has an opposing action to insulin; therefore, drugs targeting the inhibition of glucagon action are in development as potential therapies for T2DM, though what their utility may be in PCOS is unclear.
Imeglemin
Imeglemin is a novel class of glucose-lowering agents developed to treat T2DM, though its mechanism of action remains elusive. However, experimental studies suggest that it acts by blocking the oxidative phosphorylation that is a crucial step in hepatic gluconeogenesis.152 In addition, it increases insulin secretion and improves muscle glucose uptake.153 A recent study reported that imeglemin can improve insulin sensitivity through several molecular pathways including insulin signalling transduction via activating Akt phosphorylation.154
Imeglemin may also improve glucose homeostasis by improving β-cell function, suppressing gluconeogenesis, lowering insulin resistance, improving mitochondrial function and attenuating oxidative stress.155 This novel mechanism of action for imeglemin provides an additional benefit to patients with T2DM and potentially complementing other oral antidiabetes therapies. However, clinical trials are needed to examine its efficacy and tolerability in women with PCOS.
microRNA therapy
Evidence is emerging about the potential therapeutic effect of microRNAs (miRNAs) in the treatment of many diseases, including obesity-related disorders. MiRNAs are non-coding RNAs of approximately 22 nucleotides in length that post-transcriptionally regulate gene expression; miRNAs are differentially found in PCOS compared with normal controls.156,157 Their binding to target messenger RNA (mRNA) causes mRNA cleavage, translational repression and mRNA decay.158–161 miRNAs are released by many tissues, including adipose tissues, and act as both endocrine and paracrine messenger between various target organs. Furthermore, miRNAs are linked to adipocytes differentiation and, thus, might be potential biomarkers for obesity and its related diseases. Currently, there are a number of ongoing clinical trials assessing the potential therapeutic effects of targeting miRNAs in obesity and its associated metabolic disorders (e.g. T2DM, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis),162 which could also potentially benefit women with PCOS.
It should be emphasised that all these agents are contradicted during pregnancy and lactation due to their teratogenic effects. There is evidence that metformin is safe and does not increase congenital abnormalities as well as it being generally tolerable during pregnancy. Women with PCOS are relatively young and usually of reproductive age; therefore, it is imperative to mention that, apart from metformin, the use of all other pharmacological agents should be restricted to women with no desire for pregnancy and those using reliable methods of contraception. It should also be noted that these agents are not licensed for use in adolescents with PCOS. At present, the first-line therapy in women with PCOS should focus on lifestyle intervention including dietary modifications and physical activity with additional pharmacological agents as indicated.
Conclusion
There is a wide range of therapeutic options with potential advantages available for the management of the metabolic comorbidities in PCOS (Figures 1 and 2, Table 1). It is crucial to appreciate the efficacy and safety of metformin in providing metabolic benefits in PCOS, although it is still unlicensed for this indication. However, it is also imperative to acknowledge that no single agent can cover the entire spectrum of metabolic disorders in women diagnosed with PCOS. Metabolic benefits and improvement of parameters of metabolic comorbidities are superior with a combination of lifestyle intervention, metformin, GLP-1 RA, SGLT-2 inhibitors and bariatric surgery than monotherapy. SGLT-2 inhibitors and GLP-1 RA are promising therapeutic agents with significant potential advantages in improving metabolic abnormalities in women with PCOS and need further studies. Bariatric surgery appears to be a highly effective therapy that may have benefit in selected women with PCOS for the reversal of the metabolic abnormalities and the prevention of T2DM. There are several emerging therapies for the treatment of T2DM that may have direct utility in the management of the metabolic aspects of PCOS; however, clinical studies are needed to evaluate their clinical efficacy and safety in women with PCOS.
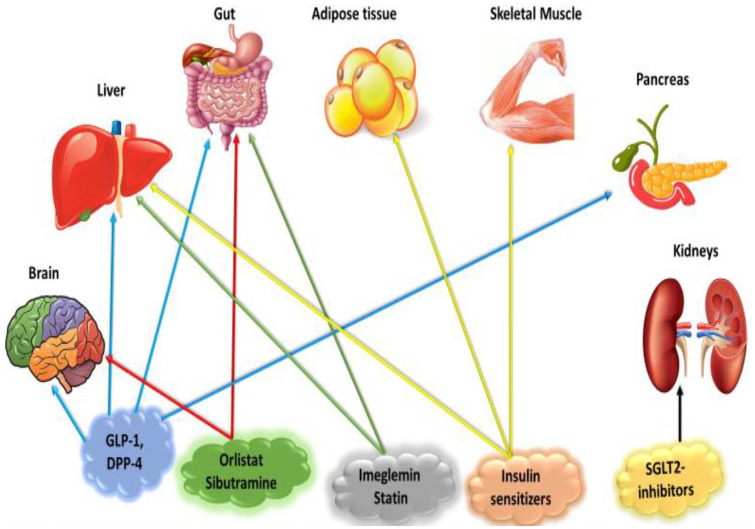
Figure 1.
Potential target organs for the therapeutic options in managing the metabolic aspects of PCOS.
DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; PCOS, polycystic ovary syndrome; SGLT2, sodium-glucose cotransporter 2.
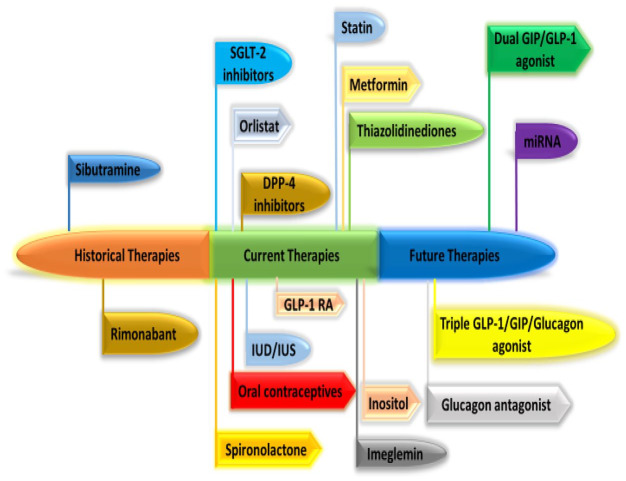
Figure 2.
Historical, present and potential future PCOS therapies.
DPP-4, dipeptidyl peptidase-4; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; PCOS, polycystic ovary syndrome; RA, receptor agonist; SGLT2, sodium-glucose cotransporter 2; IUD, intrauterine device; IUS, intrauterine system.
Table 1.
Currently available and novel therapies for management of the metabolic aspect of PCOS.
| Class | Drug example | Mechanism of action |
|---|---|---|
| Insulin sensitiser | ||
| Thiazolidinedione | Pioglitazone | Hepatic gluconeogenesis ⬇ |
| Insulin sensitivity ⬆ | ||
| Biguanide | Metformin | Hepatic gluconeogenesis ⬇ |
| Incretins | ||
| GLP-1, GIP & DPP-4 inhibitors, | Liraglutide, Sitagliptin | Appetite ⬇ |
| dual GLP-1/GIP-GLP-1/glucagon, | Gastric motility ⬇ | |
| triple GLP-1/GIP/glucagon agonist | Insulin release ⬆ | |
| Glucagon release | ||
| Weight ⬇ | ||
| SGLT2 inhibitors | Empagliflozin | Urinary glucose excretion ⬆ |
| Weight ⬇ | ||
| Statins | Atorvastatin | Cholesterol synthesis ⬇ |
| Oxidative phosphorylation | Imeglemin | Hepatic gluconeogenesis ⬇ |
| Inhibitor | ||
| Weight loss agents | Rimonabant | Appetite ⬇ |
| Orlistat | Cholesterol synthesis ⬇ | |
| Sibutramine | Appetite ⬇ | |
| Myo-inositol | Inositol | Insulin sensitivity ⬇ |
Drugs illustrated above are just an example and not the full list. The full list can be found elsewhere.
DPP-4, dipeptidyl peptidase-4; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; PCOS, polycystic ovary syndrome.
Footnotes
Author contribution(s): Mohammed Altigani Abdalla: Conceptualization; Writing-original draft; Writing-review & editing.
Harshal Deshmukh: Conceptualization; Methodology; Writing-review & editing.
Stephen Atkin: Conceptualization; Methodology; Supervision; Writing-review & editing.
Thozhukat Sathyapalan: Conceptualization; Methodology; Supervision; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Mohammed Altigani Abdalla  https://orcid.org/0000-0002-6016-3157
https://orcid.org/0000-0002-6016-3157
Contributor Information
Mohammed Altigani Abdalla, Department of Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School, University of Hull, Hull, UK.
Harshal Deshmukh, Department of Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School, University of Hull, Hull, UK.
Stephen Atkin, School of Postgraduate Studies and Research, RCSI Medical University of Bahrain, Kingdom of Bahrain.
Thozhukat Sathyapalan, Department of Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School, University of Hull, Hull, Kingston upon Hull, UK.
References
- 1. Barnard L, Ferriday D, Guenther N, et al. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod 2007; 22: 2279–2286. [DOI] [PubMed] [Google Scholar]
- 2. Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T 2013; 38: 336–355. [PMC free article] [PubMed] [Google Scholar]
- 3. Salley KES, Wickham EP, Cheang KI, et al. Glucose intolerance in polycystic ovary syndrome–a position statement of the androgen excess society. J Clin Endocrinol Metab 2007; 92: 4546–4556. [DOI] [PubMed] [Google Scholar]
- 4. Garruti G, Depalo R, Vita MG, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online 2009; 19: 552–563. [DOI] [PubMed] [Google Scholar]
- 5. Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol 2007; 50: 205–225. [DOI] [PubMed] [Google Scholar]
- 6. Apridonidze T, Essah PA, Iuorno MJ, et al. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90: 1929–1935. [DOI] [PubMed] [Google Scholar]
- 7. Goodarzi MO, Dumesic DA, Chazenbalk G, et al. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 2011; 7: 219–231. [DOI] [PubMed] [Google Scholar]
- 8. Mu N, Zhu Y, Wang Y, et al. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol 2012; 125: 751–757. [DOI] [PubMed] [Google Scholar]
- 9. Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005; 353: 2111–2120. [DOI] [PubMed] [Google Scholar]
- 10. Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011; 365: 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011; 2: CD007506. [DOI] [PubMed] [Google Scholar]
- 12. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018; 110: 364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab 2013; 98: 4655–4663. [DOI] [PubMed] [Google Scholar]
- 14. Kiddy DS, Sharp PS, White DM, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf) 1990; 32: 213–220. [DOI] [PubMed] [Google Scholar]
- 15. Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril 2003; 79: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 16. Kiddy DS, Hamilton-Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992; 36: 105–111. [DOI] [PubMed] [Google Scholar]
- 17. Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without type 2 diabetes. J Appl Physiol (1985) 2005; 99: 1220–1225. [DOI] [PubMed] [Google Scholar]
- 18. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018; 33: 1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moran LJ, Pasquali R, Teede HJ, et al. Treatment of obesity in polycystic ovary syndrome: a position statement of the androgen excess and polycystic ovary syndrome society. Fertil Steril 2009; 92: 1966–1982. [DOI] [PubMed] [Google Scholar]
- 20. Mavropoulos JC, Yancy WS, Hepburn J, et al. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond) 2005; 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gower BA, Chandler-Laney PC, Ovalle F, et al. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol (Oxf) 2013; 79: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goss AM, Chandler-Laney PC, Ovalle F, et al. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism 2014; 63: 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGrice M, Porter J. The effect of low carbohydrate diets on fertility hormones and outcomes in overweight and obese women: a systematic review. Nutrients 2017; 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med 2020; 18: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Douglas CC, Gower BA, Darnell BE, et al. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril 2006; 85: 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moran LJ, Ko H, Misso M, et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. J Acad Nutr Diet 2013; 113: 520–545. [DOI] [PubMed] [Google Scholar]
- 27. Harrison CL, Lombard CB, Moran LJ, et al. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 2011; 17: 171–183. [DOI] [PubMed] [Google Scholar]
- 28. Kite C, Lahart IM, Afzal I, et al. Exercise, or exercise and diet for the management of polycystic ovary syndrome: a systematic review and meta-analysis. Syst Rev 2019; 8: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Witchel SF, Oberfield SE, Peña AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc 2019; 3: 1545–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guzick D. Polycystic ovary syndrome: symptomatology, pathophysiology, and epidemiology. Am J Obstet Gynecol 1998; 179: S89–S93. [DOI] [PubMed] [Google Scholar]
- 31. Yildiz BO. Recent advances in the treatment of polycystic ovary syndrome. Expert Opin Investig Drugs 2004; 13: 1295–1305. [DOI] [PubMed] [Google Scholar]
- 32. Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998; 47: 507–514. [DOI] [PubMed] [Google Scholar]
- 33. Brettenthaler N, De Geyter C, Huber PR, et al. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2004; 89: 3835–3840. [DOI] [PubMed] [Google Scholar]
- 34. Xu Y, Wu Y, Huang Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS: a meta-analysis. Arch Gynecol Obstet 2017; 296: 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cho LW, Kilpatrick ES, Keevil BG, et al. Effect of metformin, orlistat and pioglitazone treatment on mean insulin resistance and its biological variability in polycystic ovary syndrome. Clin Endocrinol (Oxf) 2009; 70: 233–237. [DOI] [PubMed] [Google Scholar]
- 36. Jearath V, Vashisht R, Rustagi V, et al. Pioglitazone-induced congestive heart failure and pulmonary edema in a patient with preserved ejection fraction. J Pharmacol Pharmacother 2016; 7: 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jensterle M, Kravos NA, Ferjan S, et al. Long-term efficacy of metformin in overweight-obese PCOS: longitudinal follow-up of retrospective cohort. Endocr Connect 2020; 9: 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pasquali R. Metformin in women with PCOS, pros. Endocrine 2015; 48: 422–426. [DOI] [PubMed] [Google Scholar]
- 39. Curi DDG, Fonseca AM, Marcondes JAM, et al. Metformin versus lifestyle changes in treating women with polycystic ovary syndrome. Gynecol Endocrinol 2012; 28: 182–185. [DOI] [PubMed] [Google Scholar]
- 40. Harborne LR, Sattar N, Norman JE, et al. Metformin and weight loss in obese women with polycystic ovary syndrome: comparison of doses. J Clin Endocrinol Metab 2005; 90: 4593–4598. [DOI] [PubMed] [Google Scholar]
- 41. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palomba S, Falbo A, Russo T, et al. Insulin sensitivity after metformin suspension in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007; 92: 3128–3135. [DOI] [PubMed] [Google Scholar]
- 43. Akram T, Hasan S, Imran M, et al. Association of polycystic ovary syndrome with cardiovascular risk factors. Gynecol Endocrinol 2010; 26: 47–53. [DOI] [PubMed] [Google Scholar]
- 44. Kaya MG, Yildirim S, Calapkorur B, et al. Metformin improves endothelial function and carotid intima media thickness in patients with PCOS. Gynecol Endocrinol 2015; 31: 401–405. [DOI] [PubMed] [Google Scholar]
- 45. Mancini F, Cianciosi A, Reggiani GM, et al. Endothelial function and its relationship to leptin, homocysteine, and insulin resistance in lean and overweight eumenorrheic women and PCOS patients: a pilot study. Fertil Steril 2009; 91: 2537–2544. [DOI] [PubMed] [Google Scholar]
- 46. Mather KJ, Kwan F, Corenblum B. Hyperinsulinemia in polycystic ovary syndrome correlates with increased cardiovascular risk independent of obesity. Fertil Steril 2000; 73: 150–156. [DOI] [PubMed] [Google Scholar]
- 47. Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci 2013; 56: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wild RA. Dyslipidemia in PCOS. Steroids 2012; 77: 295–299. [DOI] [PubMed] [Google Scholar]
- 49. Sin HY, Kim JY, Jung KH. Total cholesterol, high density lipoprotein and triglyceride for cardiovascular disease in elderly patients treated with metformin. Arch Pharm Res 2011; 34: 99–107. [DOI] [PubMed] [Google Scholar]
- 50. Fleming R, Hopkinson ZE, Wallace AM, et al. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab 2002; 87: 569–574. [DOI] [PubMed] [Google Scholar]
- 51. Ng EH, Wat NM, Ho PC. Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene-resistant polycystic ovaries: a randomized, double-blinded placebo-controlled trial. Hum Reprod 2001; 16: 1625–1631. [DOI] [PubMed] [Google Scholar]
- 52. Sharpe A, Morley LC, Tang T, et al. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019; 12: CD013505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duleba AJ. Medical management of metabolic dysfunction in PCOS. Steroids 2012; 77: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Herbert L, Ribar A, Mitchell S, et al. Discovering metformin-induced vitamin B12 deficiency in patients with type 2 diabetes in primary care. J Am Assoc Nurse Pract. Epub ahead of print 18 October 2019. DOI: 10.1097/JXX.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 55. Longo SL, Ryan JM, Sheehan KB, et al. Evaluation of vitamin B12 monitoring in patients on metformin in urban ambulatory care settings. Pharm Pract (Granada) 2019; 17: 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alvarez M, Sierra OR, Saavedra G, et al. Vitamin B12 deficiency and diabetic neuropathy in patients taking metformin: a cross-sectional study. Endocr Connect 2019; 8: 1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alshammari AN, Iqbal R, Baksh IP. Vitamin B12 deficiency and the knowledge and practice of physicians regarding screening for vitamin B12 deficiency among type 2 diabetic patients on metformin in selected hospitals in Riyadh, Saudi Arabia. J Family Med Prim Care 2019; 8: 2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cefalu WT. The physiologic role of incretin hormones: clinical applications. J Am Osteopath Assoc 2010; 110(Suppl. 2): S8–S14. [PubMed] [Google Scholar]
- 59. Pontikis C, Yavropoulou MP, Toulis KA, et al. The incretin effect and secretion in obese and lean women with polycystic ovary syndrome: a pilot study. J Womens Health (Larchmt) 2011; 20: 971–976. [DOI] [PubMed] [Google Scholar]
- 60. Olansky L, Reasner C, Seck TL, et al. A treatment strategy implementing combination therapy with sitagliptin and metformin results in superior glycaemic control versus metformin monotherapy due to a low rate of addition of antihyperglycaemic agents. Diabetes Obes Metab 2011; 13: 841–849. [DOI] [PubMed] [Google Scholar]
- 61. Vrbikova J, Hill M, Bendlova B, et al. Incretin levels in polycystic ovary syndrome. Eur J Endocrinol 2008; 159: 121–127. [DOI] [PubMed] [Google Scholar]
- 62. Yaribeygi H, Sathyapalan T, Sahebkar A. Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sci 2019; 234: 116776. [DOI] [PubMed] [Google Scholar]
- 63. Zinman B, Gerich J, Buse JB, et al. ; LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009; 32: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 65. Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dawson AJ, Sathyapalan T, Vince R, et al. The effect of exenatide on cardiovascular risk markers in women with polycystic ovary syndrome. Front Endocrinol (Lausanne) 2019; 10: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rasmussen CB, Lindenberg S. The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Front Endocrinol (Lausanne) 2014; 5: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online 2019; 39: 332–342. [DOI] [PubMed] [Google Scholar]
- 69. Niafar M, Pourafkari L, Porhomayon J, et al. A systematic review of GLP-1 agonists on the metabolic syndrome in women with polycystic ovaries. Arch Gynecol Obstet 2016; 293: 509–515. [DOI] [PubMed] [Google Scholar]
- 70. Tzotzas T, Karras SN, Katsiki N. Glucagon-like peptide-1 (GLP-1) receptor agonists in the treatment of obese women with polycystic ovary syndrome. Curr Vasc Pharmacol 2017; 15: 218–229. [DOI] [PubMed] [Google Scholar]
- 71. Kahal H, Aburima A, Ungvari T, et al. The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocr Disord 2015; 15: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kahal H, Kilpatrick E, Rigby A, et al. The effects of treatment with liraglutide on quality of life and depression in young obese women with PCOS and controls. Gynecol Endocrinol 2019; 35: 142–145. [DOI] [PubMed] [Google Scholar]
- 73. Vella A. Mechanism of action of DPP-4 inhibitors–new insights. J Clin Endocrinol Metab 2012; 97: 2626–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab 2009; 23: 479–486. [DOI] [PubMed] [Google Scholar]
- 75. Devin JK, Nian H, Celedonio JE, et al. Sitagliptin decreases visceral fat and blood glucose in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 2020; 105: 136–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ferjan S, Janez A, Jensterle M. DPP4 inhibitor sitagliptin as a potential treatment option in metformin-intolerant obese women with polycystic ovary syndrome: a pilot randomized study. Endocr Pract 2018; 24: 69–77. [DOI] [PubMed] [Google Scholar]
- 77. Wang F, Zhang ZF, He YR, et al. Effects of dipeptidyl peptidase-4 inhibitors on transforming growth factor- β1 signal transduction pathways in the ovarian fibrosis of polycystic ovary syndrome rats. J Obstet Gynaecol Res 2019; 45: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Williams-Herman D, Round E, Swern AS, et al. Safety and tolerability of sitagliptin in patients with type 2 diabetes: a pooled analysis. BMC Endocr Disord 2008; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Frederich R, Alexander JH, Fiedorek FT, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med 2010; 122: 16–27. [DOI] [PubMed] [Google Scholar]
- 80. Chao EC. SGLT-2 inhibitors: a new mechanism for glycemic control. Clin Diabetes 2014; 32: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(Suppl. 1): S4–S11. [DOI] [PubMed] [Google Scholar]
- 82. Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs 2019; 79: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yaribeygi H, Sathyapalan T, Maleki M, et al. Molecular mechanisms by which SGLT2 inhibitors can induce insulin sensitivity in diabetic milieu: a mechanistic review. Life Sci 2020; 240: 117090. [DOI] [PubMed] [Google Scholar]
- 84. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208–219. [DOI] [PubMed] [Google Scholar]
- 85. Kovacs CS, Seshiah V, Swallow R, et al. ; EMPA-REG PIO™ trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014; 16: 147–158. [DOI] [PubMed] [Google Scholar]
- 86. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 87. Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 88. Fadini GP, Bonora BM, Zatti G, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol 2017; 16: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 90. Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 91. Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 92. Perkovic V, Jardine MJ, Neal B, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 93. Javed Z, Papageorgiou M, Deshmukh H, et al. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol (Oxf) 2019; 90: 805–813. [DOI] [PubMed] [Google Scholar]
- 94. Ostlund RE, Jr, McGill JB, Herskowitz I, et al. D-chiro-inositol metabolism in diabetes mellitus. Proc Natl Acad Sci U S A 1993; 90: 9988–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med 2011; 28: 972–975. [DOI] [PubMed] [Google Scholar]
- 96. Hong JH, Jang HW, Kang YE, et al. Urinary chiro- and myo-inositol levels as a biological marker for type 2 diabetes mellitus. Dis Markers 2012; 33: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Genazzani AD, Lanzoni C, Ricchieri F, et al. Myo-inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovary syndrome. Gynecol Endocrinol 2008; 24: 139–144. [DOI] [PubMed] [Google Scholar]
- 98. D’Anna R, Di Benedetto V, Rizzo P, et al. Myo-inositol may prevent gestational diabetes in PCOS women. Gynecol Endocrinol 2012; 28: 440–442. [DOI] [PubMed] [Google Scholar]
- 99. Garg D, Tal R. Inositol treatment and ART outcomes in women with PCOS. Int J Endocrinol 2016; 2016: 1979654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Unfer V, Nestler JE, Kamenov ZA, et al. Effects of inositol(s) in women with PCOS: a systematic review of randomized controlled trials. Int J Endocrinol 2016; 2016: 1849162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kodaman PH, Duleba AJ. HMG-CoA reductase inhibitors: do they have potential in the treatment of polycystic ovary syndrome? Drugs 2008; 68: 1771–1785. [DOI] [PubMed] [Google Scholar]
- 102. Sathyapalan T, Atkin SL. Evidence for statin therapy in polycystic ovary syndrome. Ther Adv Endocrinol Metab 2010; 1: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kodaman PH, Duleba AJ. Statins in the treatment of polycystic ovary syndrome. Semin Reprod Med 2008; 26: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sathyapalan T, Kilpatrick ES, Coady AM, et al. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab 2009; 94: 103–108. [DOI] [PubMed] [Google Scholar]
- 105. Sathyapalan T, Kilpatrick ES, Coady AM, et al. Atorvastatin pretreatment augments the effect of metformin in patients with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 2010; 72: 566–568. [DOI] [PubMed] [Google Scholar]
- 106. Sathyapalan T, Hobkirk JP, Javed Z, et al. The effect of atorvastatin (and subsequent metformin) on adipose tissue acylation-stimulatory-protein concentration and inflammatory biomarkers in overweight/obese women with polycystic ovary syndrome. Front Endocrinol (Lausanne) 2019; 10: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sathyapalan T, Coady AM, Kilpatrick ES, et al. The effect of atorvastatin on pancreatic beta cell requirement in women with polycystic ovary syndrome. Endocr Connect 2017; 6: 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sathyapalan T, Shepherd J, Coady AM, et al. Atorvastatin reduces malondialdehyde concentrations in patients with polycystic ovary syndrome. J Clin Endocrinol Metab 2012; 97: 3951–3955. [DOI] [PubMed] [Google Scholar]
- 109. Sathyapalan T, Smith KA, Coady AM, et al. Atorvastatin therapy decreases androstenedione and dehydroepiandrosterone sulphate concentrations in patients with polycystic ovary syndrome: randomized controlled study. Ann Clin Biochem 2012; 49: 80–85. [DOI] [PubMed] [Google Scholar]
- 110. Johnson S, Schwartz SM. Pharmacologic and pharmacodynamic equivalence of 2 formulations of orlistat. Clin Pharmacol Drug Dev 2018; 7: 773–780. [DOI] [PubMed] [Google Scholar]
- 111. Jayagopal V, Kilpatrick ES, Holding S, et al. Orlistat is as beneficial as metformin in the treatment of polycystic ovarian syndrome. J Clin Endocrinol Metab 2005; 90: 729–733. [DOI] [PubMed] [Google Scholar]
- 112. Kumar P, Arora S. Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J Hum Reprod Sci 2014; 7: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Panda SR, Jain M, Jain S, et al. Effect of orlistat versus metformin in various aspects of polycystic ovarian syndrome: a systematic review of randomized control trials. J Obstet Gynaecol India 2018; 68: 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Salehpour S, Hosseini S, Nazari L, et al. Effects of orlistat on serum androgen levels among Iranian obese women with polycystic ovarian syndrome. JBRA Assist Reprod 2018; 22: 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007; 334: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kujawska-Luczak M, Szulinska M, Skrypnik D, et al. The influence of orlistat, metformin and diet on serum levels of insulin-like growth factor-1 in obeses women with and without insulin resistance. J Physiol Pharmacol 2018; 69: 737–745. [DOI] [PubMed] [Google Scholar]
- 117. McDuffie JR, Calis KA, Booth SL, et al. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy 2002; 22: 814–822. [DOI] [PubMed] [Google Scholar]
- 118. Elfhag K, Rössner S, Carlsson AM, et al. Sibutramine treatment in obesity: predictors of weight loss including rorschach personality data. Obes Res 2003; 11: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 119. Rolls BJ, Shide DJ, Thorwart ML, et al. Sibutramine reduces food intake in non-dieting women with obesity. Obes Res 1998; 6: 1–11. [DOI] [PubMed] [Google Scholar]
- 120. Lindholm Å, Bixo M, Björn I, et al. Effect of sibutramine on weight reduction in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Fertil Steril 2008; 89: 1221–1228. [DOI] [PubMed] [Google Scholar]
- 121. Florakis D, Diamanti-Kandarakis E, Katsikis I, et al. Effect of hypocaloric diet plus sibutramine treatment on hormonal and metabolic features in overweight and obese women with polycystic ovary syndrome: a randomized, 24-week study. Int J Obes (Lond) 2008; 32: 692–699. [DOI] [PubMed] [Google Scholar]
- 122. James WPT, Caterson ID, Coutinho W, et al. ; SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010; 363: 905–917. [DOI] [PubMed] [Google Scholar]
- 123. Dawson AJ, Kilpatrick ES, Coady AM, et al. Endocannabinoid receptor blockade reduces alanine aminotransferase in polycystic ovary syndrome independent of weight loss. BMC Endocr Disord 2017; 17: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sathyapalan T, Cho L, Kilpatrick ES, et al. Effect of rimonabant and metformin on glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 in obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2010; 72: 423–425. [DOI] [PubMed] [Google Scholar]
- 125. Sathyapalan T, Cho LW, Kilpatrick ES, et al. Metformin maintains the weight loss and metabolic benefits following rimonabant treatment in obese women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 2009; 70: 124–128. [DOI] [PubMed] [Google Scholar]
- 126. Sathyapalan T, Cho LW, Kilpatrick ES, et al. A comparison between rimonabant and metformin in reducing biochemical hyperandrogenaemia and insulin resistance in patients with polycystic ovary syndrome (PCOS): a randomized open-label parallel study. Clin Endocrinol (Oxf) 2008; 69: 931–935. [DOI] [PubMed] [Google Scholar]
- 127. Moreira FA, Crippa JAS. The psychiatric side-effects of rimonabant. Braz J Psychiatry 2009; 31: 145–153. [DOI] [PubMed] [Google Scholar]
- 128. Tek C. Naltrexone HCI/bupropion HCI for chronic weight management in obese adults: patient selection and perspectives. Patient Prefer Adherence 2016; 10: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Patel K, Allen S, Haque MN, et al. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 2016; 6: 99–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Greenway FL, Fujioka K, Plodkowski RA, et al. ; COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010; 376: 595–605. [DOI] [PubMed] [Google Scholar]
- 131. Apovian CM, Aronne L, Rubino D, et al. ; COR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013; 21: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011; 19: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hollander P, Gupta AK, Plodkowski R, et al. ; COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36: 4022–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ahmed MI, Duleba AJ, El Shahat O, et al. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod 2008; 23: 2564–2569. [DOI] [PubMed] [Google Scholar]
- 135. Fulghesu AM, Ciampelli M, Belosi C, et al. Naltrexone effect on pulsatile GnRH therapy for ovulation induction in polycystic ovary syndrome: a pilot prospective study. J Endocrinol Invest 2001; 24: 483–490. [DOI] [PubMed] [Google Scholar]
- 136. Malik SM, Traub ML. Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World J Diabetes 2012; 3: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hofsø D, Fatima F, Borgeraas H, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: 912–924. [DOI] [PubMed] [Google Scholar]
- 138. Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14: 160–169. [DOI] [PubMed] [Google Scholar]
- 139. Stegenga H, Haines A, Jones K, et al. ; Guideline Development Group. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ 2014; 349: g6608. [DOI] [PubMed] [Google Scholar]
- 140. Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, et al. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 2005; 90: 6364–6369. [DOI] [PubMed] [Google Scholar]
- 141. Eid GM, Cottam DR, Velcu LM, et al. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis 2005; 1: 77–80. [DOI] [PubMed] [Google Scholar]
- 142. Sundararaman PG, Manomani R, Sridhar GR, et al. Risk of atherosclerosis in women with polycystic ovary syndrome: a study from South India. Metab Syndr Relat Disord 2003; 1: 271–275. [DOI] [PubMed] [Google Scholar]
- 143. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab 2018; 18: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Frias JP, Bastyr EJ, III, Vignati L, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab 2017; 26: 343–352.e2. [DOI] [PubMed] [Google Scholar]
- 145. Henderson SJ, Konkar A, Hornigold DC, et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab 2016; 18: 1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhou J, Cai X, Huang X, et al. A novel glucagon-like peptide-1/glucagon receptor dual agonist exhibits weight-lowering and diabetes-protective effects. Eur J Med Chem 2017; 138: 1158–1169. [DOI] [PubMed] [Google Scholar]
- 147. Finan B, Yang B, Ottaway N, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med 2015; 21: 27–36. [DOI] [PubMed] [Google Scholar]
- 148. Müller TD, Finan B, Clemmensen C, et al. The new biology and pharmacology of glucagon. Physiol Rev 2017; 97: 721–766. [DOI] [PubMed] [Google Scholar]
- 149. Choi I, Lee J, Kim J, et al. Potent body weight loss and efficacy in a NASH animal model by a novel long-acting GLP-1/Glucagon/GIP triple-agonist (HM15211). American diabetes association’s 77th scientific session, San Diego, CA, 2017, June 9. [Google Scholar]
- 150. Adeva-Andany MM, Funcasta-Calderón R, Fernández-Fernández C, et al. Metabolic effects of glucagon in humans. J Clin Transl Endocrinol 2019; 15: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hædersdal S, Lund A, Knop FK, et al. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clin Proc 2018; 93: 217–239. [DOI] [PubMed] [Google Scholar]
- 152. Vuylsteke V, Chastain LM, Maggu GA, et al. Imeglimin: a potential new multi-target drug for type 2 diabetes. Drugs R D 2015; 15: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab 2012; 14: 852–858. [DOI] [PubMed] [Google Scholar]
- 154. Yaribeygi H, Maleki M, Sathyapalan T, et al. Molecular mechanisms by which imeglimin improves glucose homeostasis. J Diabetes Res 2020; 2020: 8768954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Vial G, Chauvin MA, Bendridi N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes 2015; 64: 2254–2264. [DOI] [PubMed] [Google Scholar]
- 156. Sathyapalan T, David R, Gooderham NJ, et al. Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis. Sci Rep 2015; 5: 16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Butler AE, Ramachandran V, Hayat S, et al. Expression of microRNA in follicular fluid in women with and without PCOS. Sci Rep 2019; 9: 16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 2004; 23: 4051–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 160. Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009; 10: 126–139. [DOI] [PubMed] [Google Scholar]
- 161. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010; 11: 597–610. [DOI] [PubMed] [Google Scholar]
- 162. Ji C, Guo X. The clinical potential of circulating microRNAs in obesity. Nat Rev Endocrinol 2019; 15: 731–743. [DOI] [PubMed] [Google Scholar]