Abstract
Voltage-gated Kv7 potassium channels, encoded by KCNQ genes, have major physiological impacts cardiac myocytes, neurons, epithelial cells, and smooth muscle cells. Cyclic adenosine monophosphate (cAMP), a well-known intracellular secondary messenger, can activate numerous downstream effector proteins, generating downstream signaling pathways that regulate many functions in cells. A role for cAMP in ion channel regulation has been established, and recent findings show that cAMP signaling plays a role in Kv7 channel regulation. Although cAMP signaling is recognized to regulate Kv7 channels, the precise molecular mechanism behind the cAMP-dependent regulation of Kv7 channels is complex. This review will summarize recent research findings that support the mechanisms of cAMP-dependent regulation of Kv7 channels.
Keywords: Kv7 (KCNQ), cAMP, PKA, EPAC, physiology
cAMP-Dependent Signaling
Regulators of cAMP: G Protein-Coupled Receptors
First discovered by Dr. Earl W. Sutherland in 1958 (Rall and Sutherland, 1958; Sutherland and Rall, 1958), cyclic adenosine monophosphate (cAMP) is well-recognized as an important intracellular secondary messenger molecule that can induce a cascade of events to influence cellular function (Patra and Brady, 2018). cAMP orchestrates numerous signal transduction pathways through the activation of several downstream effector proteins, resulting in a wide variety of cellular processes including gene transcription, cell growth and cell differentiation (Dumont et al., 1989; Sassone-Corsi, 1995; Mayr and Montminy, 2001; Sands and Palmer, 2008; Bacallao and Monje, 2015).
cAMP is created from ATP through the action of Adenylate cyclases (AC), of which there are nine membrane-bound isoforms (AC 1-9) and one soluble isoform (sASC). The characteristics of each isoform are summarized effectively in several reviews (Taussig and Gilman, 1995; Simonds, 1999; Hanoune and Defer, 2001; Sadana and Dessauer, 2009; Tresguerres et al., 2011; Halls and Cooper, 2017). Membrane-bound ACs are stimulated downstream of Gs-specific G protein-coupled receptor (GPCR) activation, while the sAC is insensitive to GPCR-dependent regulation (Braun and Dods, 1975; Braun et al., 1977; Forte et al., 1983). When a GPCR is activated by its extracellular ligand, it causes a conformational change in the receptor which activates the associated heteromeric G protein complex, consisting of an α, ß and γ subunit. Subsequently, the Gsα dissociates from the receptor and Gßγ subunits, activating AC and increasing cAMP levels (see Pierce et al., 2002 for a detailed review of this signaling pathway). ACs can also be regulated by other intracellular signals, including Ca2+ and PKC (see Halls and Cooper, 2017 for a detailed review).
Phosphodiesterase’s (PDEs) remain the only known route of cAMP degradation. The expression and localization of PDEs within a cell controls the magnitude and duration of cAMP-dependent events, as well as the compartmentalization and intracellular gradients of cAMP (Baillie, 2009). Thus, the expression and localization of PDEs in a cell contributes to the specificity of the cAMP-response following activation.
Effectors of cAMP: PKA and EPAC
cAMP mediates its effect by activation of effector proteins including protein kinase A (PKA), Epac (Exchange protein directly activated by cAMP), or cyclic nucleotide-gated channels (CNGCs) (Shabb, 2001; Bos, 2006; Biel and Michalakis, 2009). For many years it was believed that the effects of cAMP were mediated exclusively through the activation of intracellular PKA. PKA is a heterotetrameric holoenzyme consisting of two regulatory (R) subunits and two catalytic (C) subunits (Krebs and Beavo, 1979; Taylor et al., 2013; Turnham and Scott, 2016). There are two types of regulatory subunits, RI and RII, each consisting of two isoforms, RIα, RIβ, RIIα, RIIβ, each having different tissue expression, subcellular localization and cAMP binding affinity (Bechtel et al., 1977; Corbin et al., 1977, 1978; Zoller et al., 1979; Rannels and Corbin, 1980; Ringheim and Taylor, 1990; Taylor et al., 2012; Weigand et al., 2017). PKA that contains either RI or RII, form the two classes of PKA termed type I or type II, respectively. Binding of four cAMP molecules to each R subunit induces a conformational change and activates the kinase, resulting in the free catalytic subunits phosphorylating serine and threonine residues in specific substrate proteins (Welch et al., 2010).
Scaffolding proteins, such as A Kinase Anchoring Proteins (AKAPs; reviewed in Rubin, 1994; Scott et al., 2013; Dema et al., 2015) bind to the PKA-R subunits at the dimerization/docking domain at the N-terminus and targets PKA to specific subcellular locations to ensure specificity in signal transduction by placing PKA close to its appropriate substrate target, allowing compartmentalization of PKA (Scott et al., 1990; Carr et al., 1991; Bauman et al., 2006; Gold et al., 2006). AKAPs also bind other proteins such as protein kinase C and PDEs (Moleschi and Melacini, 2014). The AKAP family has more than 50 members and can be classified according to their binding specificity for the PKA-R subunits (Wong and Scott, 2004). The majority of the AKAPs bind to PKA-RII, however, several AKAPs bind to PKA-RI (Kinderman et al., 2006; Sarma et al., 2010). Only a few AKAPs have dual-specificity and can bind to both PKA-RI and PKA-RII, although with lower affinity for PKA-RI (Burns et al., 2003). This lower affinity for PKA-RI is due to the structural difference in the dimerization/docking domain in the N-terminal of RI (Huang et al., 1997; Gold et al., 2006). AKAPs are expressed in different tissues of the body and can facilitate tissue-specific signaling in many cells types including neurons (Glantz et al., 1992; Moita et al., 2002; Dell’Acqua et al., 2006), heart (Fink et al., 2001; Marx et al., 2002; Ruehr et al., 2004), and pancreas (Lester et al., 1997).
cAMP also activates Epac (Exchange protein directly activated by cAMP) (de Rooij et al., 1998; Kawasaki et al., 1998). Epac proteins, stimulated by cAMP binding, activate the Ras superfamily of small GTPases, Rap1 and Rap2. There are two known Epac proteins, Epac1 and Epac2, which are both present in almost all tissues but have different expression levels (Roscioni et al., 2008). Epac acts as guanine-nucleotide exchange factors and catalyzes the exchange of GDP for GTP on Rap1 and Rap2 with subsequent activation of these two GTPases (Cheng et al., 2008). This leads to activation of further downstream pathways, which will result in a variety of cellular functions, depending on specific tissue and cell type. In cardiac myocytes, for example, Epac plays a role in the cardiac Ca2+ regulation by increasing Ca2+ release from the sarcoplasmic reticulum (SR) via ryanodine receptor 2 (RyR2) and thereby effecting contraction (Fujita et al., 2017). Furthermore, a signaling pathway in which cAMP acts via Epac to modulate ion channel function has been identified (Renström et al., 1997; Kang et al., 2006; Stott et al., 2016). Additionally, Epac enhances the synaptic release of neurotransmitters following cAMP elevation in neurons (Shariati et al., 2016).
Cyclic nucleotide-gated channels (CNGCs) are also activated directly by cAMP signaling (Ludwig et al., 1998; Santoro et al., 1998). CNGCs are non-selective cation channels expressed in many tissues, including the heart, lung, kidney, pancreas, liver, spleen, testis and various neuronal systems (Kaupp and Seifert, 2002; Biel and Michalakis, 2007; Biel, 2009). CNGCs are activated by direct binding of cAMP or cGMP to the channel and activation of the channel results in the influx of extracellular cations causing depolarization of the cell membrane. One example of a CNGC is the hyperpolarization-activated and cyclic nucleotide–gated channels (HCN), which are expressed in the heart and the nervous system. These channels mainly conduct Na+ and K+ and are activated by a hyperpolarized membrane potential and stimulated by intracellular cyclic nucleotides. HCN channels are responsible for the “funny current” (If) in the heart and neurons, where they regulate pacemaker activity and neuronal firing (Difrancesco, 1986; DiFrancesco and Tortora, 1991; Moosmang et al., 1999; DiFrancesco, 2020).
KCNQ-Encoded Potassium Channels
There are 12 sub-families of voltage-gated potassium channels (Kv1-Kv12), within which the Kv7 potassium channel family consists of five isoforms encoded by the KCNQ1-5 genes (Kv7.1-Kv7.5) (Robbins, 2001; Gutman et al., 2005). Like all Kv channels, Kv7 channels are voltage-sensing channels and open and close upon changes in transmembrane potential to selectively let K+ ions pass through the channel. The Kv7 channels are comprised of four α-subunits, with each α-subunit consisting of six transmembrane (TM) spanning domains (S1-S6). S1-S4 form the voltage-sensing domain, while S5-S6 form the pore domain. Each α-subunit contains cytoplasmic amino (N) – and carboxyl (C) – termini, which are essential for channel tetramerization and channel regulation (Barros et al., 2012). In the C-terminus of the channel, a coiled-coil sequence motif is the major determinant of channel assembly (Jenke et al., 2003; Schwake et al., 2003, 2006). Assembly of all four α-subunits placed in a cylindrical arrangement creates a pore through which the K+ ions are conducted selectively (Heginbotham et al., 1992; Doyle et al., 1998). This assembly can be done with four identical α-subunits from the same Kv7 isoform to form homomeric channels, or by combining at least two types of α-subunits with other Kv7 isoforms to form heteromeric channels. Kv7.1 was thought to only form as a homotetrameric channel (Schwake et al., 2000); however, heterotetrameric assembly with Kv7.5 has been suggested (Oliveras et al., 2014). Kv7.3 homomers are weakly effective but form functional heterotetramers with Kv7.2 (Schroeder et al., 1998; Wang et al., 1998), Kv7.4 (Kubisch et al., 1999) and Kv7.5 (Schroeder et al., 2000a) subunits, and Kv7.4 heterotetramizes with Kv7.5 (Bal et al., 2008; Brueggemann et al., 2014b; Chadha et al., 2014; Jepps et al., 2015). Kv7 channels also interact with KCNE-encoded single transmembrane-spanning auxiliary protein subunits [KCNE1-5; also called MinK and MinK-related peptides (MiRPs)] that function as β or ancillary subunits (Abbott and Goldstein, 1998; McCrossan and Abbott, 2004; Abbott, 2016; Jepps, 2017). These KCNE proteins cannot form functional channels themselves but coassemble with Kv7 channels to modulate several functional properties of the channel, including membrane trafficking, voltage dependence, and kinetics of channel opening and closing (McCrossan and Abbott, 2004; Kanda and Abbott, 2012). Finally, Kv7 channels have a large cytoplasmic C-terminal composed of four segments (helix A-D) (Haitin and Attali, 2008; Xu et al., 2013; Barrese et al., 2018). In addition to coordinating channel tetramerization, several regulatory molecules interact with the C-terminal region to modulate Kv7 channel function, including calmodulin (CaM), phosphatidylinositol 4,5-biphosphate (PIP2), protein kinase C (PKC), protein kinase A (PKA), A-kinase-anchoring protein (AKAP), ankyrin G (Ank-G) and Nedd4-2 (summarized by Haitin and Attali (2008) and Barrese et al., 2018).
This review will now focus on the mechanisms of cAMP-dependent regulation of Kv7 channels and explore a possible role for this mechanism for therapeutic targeting in diseases associated with Kv7 channel dysfunction.
cAMP Regulation of Kv7 Channels
Kv7.1
Kv7.1 channels are expressed in several tissues throughout the body including the heart, uterus, and epithelial cells of the inner ear, pancreas, airways, gastrointestinal tract and kidneys. In the heart, Kv7.1 associates with the ancillary subunit KCNE1 to constitute the channel responsible for the late repolarizing current termed IKs (Barhanin et al., 1996; Sanguinetti et al., 1996). Kv7.1 also associates with KCNE1 in the inner ear, where they regulate auditory function and pancreatic acinar cells where activation of the Kv7.1-KCNE1 channel provides the driving force for Cl– secretion (Kim and Greger, 1999; Köttgen et al., 1999; Warth et al., 2002). In addition, Kv7.1 channels in the pancreatic ß-cells contribute to the regulation of insulin secretion, and loss of function mutations in the KCNQ1 gene leads to impaired ß-cell insulin secretion, which is associated with type 2 diabetes (Ullrich et al., 2005; Unoki et al., 2008; Yasuda et al., 2008; Yamagata et al., 2011; Liu et al., 2014; Torekov et al., 2014; Min Lee et al., 2017; Zhang et al., 2020). Furthermore, association of Kv7.1 with KCNE3 in epithelial tissues of the colon, small intestine and airways regulates the transport of water and salts (Schroeder et al., 2000b; Grahammer et al., 2001a, b; Vallon et al., 2005).
Kv7.1-KCNE1 channels have been studied extensively in cardiac myocytes where the channel is important for delayed cardiac repolarization and shapes action potential duration (Terrenoire et al., 2005). In addition, it is enhanced by sympathetic nerve activity through activation of β-adrenergic receptors (Bennett and Begenisch, 1987; Yazawa and Kameyama, 1990). Stimulation of the β-adrenergic receptor by noradrenaline released from the cardio-accelerator nerve causes an elevation of cAMP that increases IKs via PKA-dependent phosphorylation (Yazawa and Kameyama, 1990).
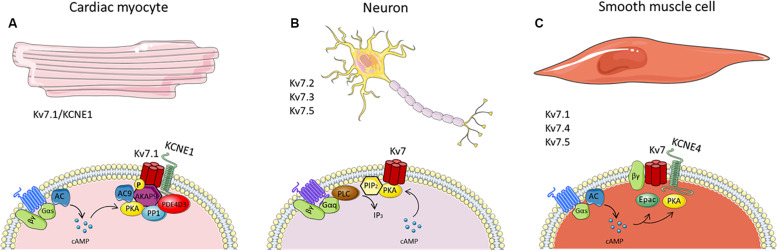
As mentioned previously, PKA actions are regulated by AKAPs, by positioning PKA and other cAMP-responsive enzymes in proximity to their substrates. In the heart, several AKAPs are expressed, including AKAP79/150 (human AKAP79/murine AKAP150), AKAP15/18δ and AKAP9. AKAP9, better known as yotiao, facilitates PKA-phosphorylation of serine 27 in the Kv7.1 N-terminus required for the proper functioning of the channel (Walsh et al., 1988; Walsh and Kass, 1991; Potet et al., 2001; Marx et al., 2002). Yotiao also associates with protein phosphatase 1 (PP1), phosphodiesterase (PDE) and AC to control IKs phosphorylation status (Terrenoire et al., 2009; Li et al., 2012). Yotiao binds directly to Kv7.1 C-terminus via a leucine zipper motif, an amino acid sequence in the C terminus of Kv7.1, and disruption of the complex by mutations in yotiao or KCNQ1 are associated with Long QT Syndrome (Kass et al., 2003; Fodstad et al., 2004; Chen et al., 2007). Besides acting as a scaffold protein, yotiao has a functional role that occurs after IKs phosphorylation. Studies revealed that cAMP-dependent activation of Kv7.1-KCNE1 depends not only on serine 27 phosphorylation but also requires the direct binding of yotiao to the Kv7.1 at the leucine zipper motif (Kurokawa et al., 2004; Chen et al., 2005; Chen and Kass, 2006). Furthermore, it was revealed that yotiao itself is a substrate for PKA phosphorylation and can be phosphorylated upon β adrenoceptor stimulation (Chen et al., 2005). Serine 43 was identified as target residue for PKA phosphorylation on yotiao, with mutations of this residue dampening cAMP effects on the IKs current, even though yotiao binding and phosphorylation of Kv7.1 channels were not affected. Taken together, these studies conclude that AKAP protein yotiao has a crucial and complex regulatory role on the cAMP-dependent activity of IKs channels (see Figure 1A).
FIGURE 1.
Schematics of known cAMP-dependent Kv7 channel regulation in panel cardiac (A), neurones (B) and smooth muscle cells (C). The figure summarizes many of the known interaction partners involved in the cAMP-mediated regulation of Kv7 channels in the different cells types, thereby highlighting the heterogeneity of cAMP-Kv7 channel signaling. It should be noted that, although AKAP79/150 is associated with Kv7.2 channels, it has not been included in the figure since there is no evidence of its involvement in PKA phosphorylation of the channel.
Although PKA activation of Kv7.1 following β adrenergic stimulation can increase IKs, chronic β adrenergic stimulation downregulates the IKs response (Mona et al., 2014). Chronic β adrenergic stimulation led to a significant decrease in KCNE1 but not KCNQ1 mRNA expression in guinea pig cardiomyocytes. Furthermore, chronic in vitro and in vivo isoprenaline stimulation reduced KCNE1 protein expression and KCNE1 membrane expression in guinea pig cardiomyocytes. This effect was mediated via Epac1 and not PKA activation, leading to increased translocation of nuclear factor of activated T cell (NFAT), which was responsible for KCNE1 downregulation (Mona et al., 2014).
In the inner ear, cAMP activates Kv7.1-KCNE1 channels, which results in the secretion of K+, necessary for normal hearing (Wang et al., 1996; Neyroud et al., 1997). However, the precise mechanisms underlying the cAMP-regulated increase in K+ conductance in the inner ear are unclear (Sunose et al., 1997). In the intestine, cAMP enhances Kv7.1-KCNE3 currents and stimulates Cl– secretion by hyperpolarizing the cell membrane and thereby amplifying the driving force for Cl– exit through cystic fibrosis transmembrane conductance regulator Cl– channels (Lohrmann et al., 1995; Diener et al., 1996; Suessbrich et al., 1996; Devor et al., 1997; Rufo et al., 1997; Schroeder et al., 2000b; Bajwa et al., 2007). An essential role for KCNE3 in cAMP-driven Cl– secretion has been suggested from the observation that KCNE3 knockdown reduced cAMP-mediated Cl– secretion across tracheal and intestinal epithelia without altering Kv7.1 expression (Preston et al., 2010) but the precise mechanism by which cAMP stimulates Kv7.1-KCNE3 channels in these cells is still unknown. Like for intestine epithelial cells, airway epithelial cells secrete Cl– stimulated by the cAMP-signaling pathway, where blockade of Kv7.1 channels suppress the cAMP-mediated Cl– secretion (Mall et al., 2000; Grahammer et al., 2001b; MacVinish et al., 2001; Cowley and Linsdell, 2002; Kim et al., 2007). However, the direct mechanism how cAMP mediates Kv7.1 channel activation responsible for Cl– secretion needs to be further investigated. In pancreatic β-cells, Kv7.1 channels contribute to insulin secretion. Besides, a link between cAMP and insulin secretion has established (Malaisse and Malaisse-Lagae, 1984; Seino and Shibasaki, 2005). However, it remains to be determined if Kv7.1 channels play a role in this cAMP-dependent mechanism.
Modifiers of cAMP-Mediated Regulation of Kv7.1 Channels
Although the direct regulation of cAMP signaling on Kv7.1 channel activity is well described, several factors can modulate this interaction indirectly. For instance, Nicolas et al. (2008) determined that the PKA-dependent regulation of IKs was microtubule-dependent (Nicolas et al., 2008). Cytoskeletal microtubules are essential for proper trafficking of cardiac ion channels to the plasma membrane (Steele and Fedida, 2014), and although a physical interaction between Kv7.1 and the microtubule forming protein, β-tubulin, was shown, disruption of the microtubules did not modify the Kv7.1-KCNE1 channel membrane density or baseline currents in transfected COS-7 cells or cardiomyocytes. However, microtubule disruption decreased the IKs response to PKA-mediated stimulation. This was not due to altered channel phosphorylation, yotiao phosphorylation or the interaction between both proteins, suggesting that microtubules play an important role in the coupling of PKA-dependent Kv7.1 phosphorylation and its channel activation (Nicolas et al., 2008).
The effect of cAMP on the trafficking of Kv7.1 channels has also been investigated. PKA inhibition in Madin-Darby canine kidney (MDCK) cells reduces Kv7.1 channel membrane expression and increases intracellular accumulation of the Kv7.1 protein in late endosomes/lysosomes (Andersen et al., 2015). This suggests a role for PKA-mediated trafficking of Kv7.1 channels, although this was not a result of channel phosphorylation. A previous study by the same group found that two cAMP phosphorylation residues on Kv7.1, serine 27 and serine 92, were not crucial for trafficking Kv7.1 (Lundby et al., 2013). Instead the E3 ubiquitin ligase Nedd4-2 is required in this PKA-dependent trafficking pathway (Andersen et al., 2015). Nedd4-2 is an ubiquitin-protein ligase that binds to ion channels containing a C-terminal proline-rich segment (PY motif) (Manning and Kumar, 2018). Among the Kv7 channels, Kv7.1 is the only isoform containing this sequence motif. Binding of Nedd4-2 to the C-terminal PY motif in Kv7.1 channels regulates the ubiquitylation of the channel and its internalization (Jespersen et al., 2007). Andersen et al. (2015) concluded that PKA regulated Nedd4-2-dependent trafficking of Kv7.1 but the precise mechanism how PKA influences Nedd4-2 needs to be determined (Andersen et al., 2015).
Kv7.2 and Kv7.3
Kv7.2 and Kv7.3 channels are widely expressed in neuronal tissues, where they form a heteromeric channel that generates the “M-current” (Brown and Adams, 1980; Wang et al., 1998; Wickenden et al., 2001; Roche et al., 2002; Shah et al., 2002). Kv7.5 channels are also a component of the M-channel in heteromeric formation with Kv7.3 (Lerche et al., 2000; Schroeder et al., 2000a; Tzingounis et al., 2010). The M-current in neurons maintains the negative resting membrane potential to limit neuronal firing and excitability (Marrion, 1997; Jentsch, 2000). M-currents are inhibited by Gq/11 coupled GPCRs such as muscarinic acetylcholine receptors M1, M3 and M5, or bradykinin B2 receptors. Stimulation of these receptors decreases M-current density resulting in depolarization, which subsequently increases neuronal excitability (Brown and Selyanko, 1985; Cruzblanca et al., 1998). It is well established that disruption of the M-current due to mutations in either KCNQ2 or KCNQ3 genes causes excessive neuronal excitability, leading to neuronal diseases, such as benign familial neonatal convulsions and epileptic encephalopathy (Biervert et al., 1998; Charlier et al., 1998; Singh et al., 1998; Jentsch, 2000; Castaldo et al., 2002; Borgatti et al., 2004; Weckhuysen et al., 2012, 2013).
Kv7.2/Kv7.3 currents in overexpressed Xenopus oocytes are enhanced by cAMP, which relies on PKA-mediated phosphorylation of serine 52 in the Kv7.2 N-terminus (Schroeder et al., 1998). Furthermore, Cooper et al. (2000) identified an interaction of Kv7.2 and PKA subunits in human brain samples by co-immunoprecipitation and affinity chromatography (Cooper et al., 2000). In addition, AKAP79/150 is associated with Kv7.2 channels (Hoshi et al., 2003, 2005; Zhang and Shapiro, 2012). Although the core AKAP79/150 complex contains PKA (Gold et al., 2006), it has not yet been determined whether AKAP70/150 facilitates PKA phosphorylation of Kv7.2 channel; however, AKAP79/150 is essential for the recruitment and phosphorylation of Kv7.2 channels by PKC (Hoshi et al., 2003; Higashida et al., 2005; Zhang and Shapiro, 2012).
Multiple other phosphorylation sites of Kv7.2/Kv7.3 channels have been identified using mass spectrometry. However, the responsible kinase for this phosphorylation remains elusive as not only PKA but also PKC and src tyrosine kinase can regulate Kv7.2/Kv7.3 channel phosphorylation (Gamper et al., 2003; Hoshi et al., 2003; Li et al., 2004; Surti et al., 2005). A recent study by Salzer et al. (2017) found 13 phosphorylation sites for human Kv7.2 using mass spectrometry, one already identified (serine 52) located at the N-terminus, whereas the remaining 12 were located in the C-terminus. Using in vitro phosphorylation assays the authors identified the protein kinases responsible for C-terminus Kv7.2 phosphorylation. Only two of the 12 residues (serine 438 and serine 455) were phosphorylated by PKA. Inhibition of PKA reduced Kv7.2 phosphorylation, which decreased channel sensitivity to PIP2 depletion, thereby attenuating Kv7 channel regulation via M1 muscarinic receptors. Thus, phosphorylation of the Kv7.2 channel is necessary to maintain a reduced affinity for PIP2 (Salzer et al., 2017; Figure 1B).
Kv7.5 channels are expressed in some regions of the brain, where they coassemble with Kv7.3 channels. Kv7.5/Kv7.3 channels show similar currents to the Kv7.2/Kv7.3 currents and are also inhibited by M1 muscarinic receptor activation (Schroeder et al., 2000a). Kv7.5 can be phosphorylated and stimulated by PKA on serine 53 on the N-terminus (Brueggemann et al., 2018); however, it is still unclear whether the heterotetrameric Kv7.3/Kv7.5 channel found in neurones are regulated in the same way by cAMP.
Kv7.4 and Kv7.5
Kv7.4 channels are expressed in the inner ear and auditory nerves (Kharkovets et al., 2000), the substantia nigra (Hansen et al., 2008), skeletal muscle cells (Iannotti et al., 2010), the mitochondria of cardiac myocytes (Testai et al., 2016) and in smooth muscle cells extensively (see Barrese et al., 2018 for a recent review of the smooth muscle literature). Kv7.5 channels are expressed in the brain, where they play a role in the regulation of neuronal excitability, and in skeletal and smooth muscle (Schroeder et al., 2000a; Roura-Ferrer et al., 2008; Iannotti et al., 2010; Haick and Byron, 2016).
In smooth muscle, there is evidence Kv7.4 and Kv7.5 channels exist as both homomers and heteromers (Brueggemann et al., 2011, 2014b; Chadha et al., 2014; Jepps et al., 2015). The first indication that smooth muscle Kv7 channels were activated by cAMP came from gastric smooth muscle cells. In these cells, β adrenoceptor stimulation with isoprenaline induced a current that resembled the neuronal M current, although at this time the molecular identity of the M current was still unknown (Sims et al., 1988).
In vascular smooth muscle, the use of pharmacological inhibitors and molecular interference has identified a role for Kv7 channels in receptor-mediated relaxations. The first identification was for β adrenoceptor-mediated vasodilatation in the renal vasculature (Chadha et al., 2012). Inhibition of Kv7 channels by XE991 or linopirdine and knockdown of Kv7.4 with KCNQ4-targeted small interfering RNA (siRNA), attenuated arterial relaxations to the β adrenoceptor agonist, isoprenaline (Chadha et al., 2012). More recent, experiments with morpholino-mediated suppression of Kv7.4 translation corroborated these findings (Stott et al., 2018). Furthermore, isoprenaline increased Kv7 currents in vascular smooth muscle cells isolated from rat arteries and in A7r5 cells (a rat aortic smooth muscle cell line) (Chadha et al., 2012; Stott et al., 2015; Mani et al., 2016; Brueggemann et al., 2018).
In hypertension, Kv7.4 protein expression is diminished causing reduced hyperpolarization and relaxation of the smooth muscle cells. Furthermore, in renal arteries from spontaneously hypertensive rats, where Kv7.4 expression is reduced, receptor-mediated relaxations were diminished (Jepps et al., 2011; Chadha et al., 2012). Interestingly, in the cerebral arteries from hypertensive rats, there is no compromise of Kv7.4 channel expression and CGRP receptor-mediated relaxations are unaffected (Chadha et al., 2014).
Although targeting Kv7.4 in isolated vessels with siRNA- and morpholino-induced knockdown inhibits isoprenaline- and CGRP-induced relaxations (Chadha et al., 2014; Stott et al., 2018), there is evidence that Kv7.5 is required to elicit cAMP-dependent relaxations. In A7r5 cells, stimulation of β adrenoceptors and the downstream pathway enhanced the currents produced by Kv7.5 channels and heterotetrameric Kv7.4/Kv7.5 channels expressed exogenously, but not when homomeric Kv7.4 channels were expressed (Mani et al., 2016), suggesting cAMP-mediated regulation of Kv7.4 and Kv7.5 depends on the stoichiometry of the channel.
The precise Kv7 channel stoichiometry eliciting cAMP-dependent relaxations in vascular smooth muscle is still unknown. Kv7.4 channels are important for proper Kv7 channel function in arteries, highlighted by their downregulation in arteries from hypertensive animals, whereas both Kv7.4 and Kv7.5 may play a role in the cAMP-dependent activation. To further complicate this mechanism, KCNE4 co-assembles with Kv7.4 and Kv7.5 in vascular smooth muscle cells to alter the biophysical properties and cellular localization of these channels. Targeted knockdown of Kcne4 in rat arteries depolarized the smooth muscle resting membrane potential and reduced vasorelaxations to Kv7 channel activators. Interestingly, in Kcne4 knockout mice, only the males displayed attenuated Kv7 channel function, but both male and female Kcne4 knockout mice had attenuated responses to isoprenaline. Given that Kcne4 expression has been shown in several arterial beds, it could be facilitating the Kv7 channel-dependent cAMP relaxations (Jepps et al., 2015; Abbott and Jepps, 2016). The expression of KCNE4 in A7r5 cells is yet to be determined but including KCNE4 subunits in future studies investigating the cAMP-dependent Kv7.4/Kv7.5 channel activation could give valuable insight into this mechanism.
Both PKA and Epac enhance Kv7 channel activity in vascular smooth muscle (Khanamiri et al., 2013; Mani et al., 2016; Stott et al., 2016). Isoprenaline-mediated relaxations were attenuated by PKA inhibition in renal arteries, whilst the isoprenaline relaxations in mesenteric arteries were not affected (Stott et al., 2016). In the mesenteric artery, isoprenaline-induced linopirdine-sensitive relaxations were elicited through activation of Epac, which is present in both rat renal and mesenteric arteries, but more predominant in mesenteric arteries. With electrophysiology experiments, Kv7 currents were increased with direct Epac stimulation in smooth muscle cells isolated from renal and mesenteric arteries (Stott et al., 2016). These data highlight that cAMP-dependent stimulation of Kv7 channels in vascular smooth muscle is not only dependent on the Kv7 channel architecture but also the channel’s association with downstream cAMP effector proteins, all of which are likely to be artery specific (see Figure 1C).
Kv7 channels are also expressed in airway smooth muscle cells (ASMCs) of rodents and humans. Pharmacological activators of Kv7 channels, including retigabine, flupirtine and S-1, relax airway smooth muscle cells from guinea pigs and human, and are important regulators of airway diameter (Brueggemann et al., 2012; Evseev et al., 2013). β2-adrenergic receptor agonists are commonly used for the treatment of asthma to relieve hyperconstriction of the airway (Cazzola et al., 2013), and are able to enhance Kv7 currents in ASMCs thereby inducing relaxation of rat airways (Brueggemann et al., 2014a). A later study by Brueggemann et al. (2018) revealed that β adrenoceptor stimulation activated the Kv7.5 channels in cultured human ASMCs, a response which was mediated by the PKA pathway (Brueggemann et al., 2018). This study identified serine 53 on the Kv7.5 N-terminus to be responsible for the increased Kv7.5 currents in response to cAMP elevation, which could be a common mechanism for β adrenoceptor/cAMP activation of Kv7.5 channels in vascular smooth muscle cells. Recent findings show that phosphorylation of serine 53 on the N-terminus of Kv7.5 increases its affinity for PIP2, corresponding to enhanced channel activation (Brueggemann et al., 2020).
Kv7.4 and Kv7.5 are also expressed in the smooth muscle of the gastrointestinal tract (Ohya et al., 2002; Jepps et al., 2009; Ipavec et al., 2011; Adduci et al., 2013), bladder (Streng et al., 2004; Rode et al., 2010; Svalø et al., 2012, 2013; Afeli et al., 2013; Anderson et al., 2013), penis (Jepps et al., 2016) and uterus (McCallum et al., 2009) where important functional roles have been established. Whether Kv7 channels in these tissues are subjected to the same cAMP-dependent regulation as in vascular and airway smooth muscle remains to be determined. Given that, even within the vasculature, there are differences in the cAMP-dependent mechanisms that lead to Kv7 channel stimulation, the Kv7 channels in these smooth muscle tissues cannot be assumed to behave in the same way as has been described in airway and vascular smooth muscle. Future work should investigate how Kv7 channels are subjected to cAMP-dependent regulation in these tissues, which could lead to novel therapeutic strategies for the treatment of various smooth muscle disorders, such as constipation, preeclampsia, erectile dysfunction and incontinence.
Modifiers of cAMP-Mediated Regulation of Kv7.4 Channels
Gβγ subunits have a regulatory role on the modulation of Kv7.4 channels in vascular smooth muscle cells, which is Gβ subunit-specific (Stott et al., 2015, 2018; Greenwood and Stott, 2020). Gβγ subunit binding to Kv7.4 channels enhances the current in both HEK and CHO cells expressing Kv7.4, and in rat renal arterial smooth muscle cells. In addition, Gβγ subunit interaction is critical for the β adrenergic dependent activation of Kv7 channels in rat renal arteries, but not in rat mesenteric arteries. Contrarily, inhibition of Gβγ subunits attenuated the CGRP dependent relaxations in mesenteric arteries, but showed no effect on CGRP relaxation in cerebral arteries, highlighting the role of Gβγ in Kv7-dependent vasorelaxations to be artery specific and dependent on the vasorelaxant (Stott et al., 2018).
The microtubule network has also been implicated in the Kv7 channel-dependent cAMP-mediated relaxations in rat mesenteric and renal arteries (Lindman et al., 2018). Disruption of the microtubule network with colchicine increased the membrane expression of Kv7.4 channels, which enhanced membrane hyperpolarization, decreases in intracellular Ca2+ and vasorelaxation to isoprenaline and forskolin in a Kv7 channel-dependent manner, specifically (Lindman et al., 2018). Further work in this area is required to determine whether the microtubule network regulates cAMP signaling in smooth muscle cells.
Conclusion
The second messenger cAMP regulates Kv7 channel activity in many cell types, including cardiac myocytes, smooth muscle and neurons. However, cAMP-mediated regulation of Kv7 channels is complex and many aspects remain to be elucidated. Generation of cAMP results from stimulation of AC, of which 9 isoforms exist. Depending on the isoforms expressed in a cell type, extracellular signals through GPCR activation can be integrated differently. Identifying how different AC isoforms contribute to cAMP-dependent activation of Kv7 channels in different tissue types could lead to targeting of a particular isoform providing therapeutic benefits with fewer adverse effects. Furthermore, the recent discovery of Epac activation of Kv7.4/Kv7.5 channels in vascular smooth muscle adds more complexity to this pathway. Determining whether this cAMP/Epac pathway plays a role in the activation of other Kv7 channels will be necessary to fully understand the physiological impact of cAMP signaling through Kv7 channels. Finally, the importance of the KCNE subunits in the cAMP-dependent regulation of Kv7 channels has often been overlooked but is likely to have a substantial role. By better defining the cAMP regulation of Kv7 channels in different tissues, it may become possible to exploit subtle tissue-specific differences in the activation pathways in order to generate novel therapeutics.
Author Contributions
All authors contributed to the drafting, revision and final approval of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. TJ received funding from the Lundbeck Foundation (R307-2018-3674). JH received funding from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement No. 801199.

References
- Abbott G. W. (2016). KCNE4 and KCNE5: K(+) channel regulation and cardiac arrhythmogenesis. Gene 593 249–260. 10.1016/j.gene.2016.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott G. W., Goldstein S. A. (1998). A superfamily of small potassium channel subunits: form and function of the MinK-related peptides (MiRPs). Q. Rev. Biophys. 31 357–398. 10.1017/s0033583599003467 [DOI] [PubMed] [Google Scholar]
- Abbott G. W., Jepps T. A. (2016). Kcne4 deletion sex-dependently alters vascular reactivity. J. Vasc. Res. 53 138–148. 10.1159/000449060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adduci A., Martire M., Taglialatela M., Arena V., Rizzo G., Coco C., et al. (2013). Expression and motor functional roles of voltage-dependent type 7 K+ channels in the human taenia coli. Eur. J. Pharmacol. 721 12–20. 10.1016/j.ejphar.2013.09.061 [DOI] [PubMed] [Google Scholar]
- Afeli S. A. Y., Malysz J., Petkov G. V. (2013). Molecular expression and pharmacological evidence for a functional role of Kv7 channel subtypes in guinea pig urinary bladder smooth muscle. PLoS One 8:e75875. 10.1371/journal.pone.0075875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen M. N., Hefting L. L., Steffensen A. B., Schmitt N., Olesen S.-P., Olsen J. V., et al. (2015). Protein kinase A stimulates Kv7.1 surface expression by regulating Nedd4-2-dependent endocytic trafficking. Am. J. Physiol. Cell Physiol. 309 C693–C706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson U. A., Carson C., Johnston L., Joshi S., Gurney A. M., McCloskey K. D. (2013). Functional expression of KCNQ (Kv7) channels in guinea pig bladder smooth muscle and their contribution to spontaneous activity. Br. J. Pharmacol. 169 1290–1304. 10.1111/bph.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacallao K., Monje P. V. (2015). Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination. PLoS One 10:e0116948. 10.1371/journal.pone.0116948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie G. S. (2009). Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 276 1790–1799. 10.1111/j.1742-4658.2009.06926.x [DOI] [PubMed] [Google Scholar]
- Bajwa P. J., Alioua A., Lee J. W., Straus D. S., Toro L., Lytle C. (2007). Fenofibrate inhibits intestinal Cl- secretion by blocking basolateral KCNQ1 K+ channels. Am. J. Physiol. Liver Physiol. 293 G1288–G1299. [DOI] [PubMed] [Google Scholar]
- Bal M., Zhang J., Zaika O., Hernandez C. C., Shapiro M. S. (2008). Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis. J. Biol. Chem. 283 30668–30676. 10.1074/jbc.m805216200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romey G. (1996). KvLQT1 and IsK (minK) proteins associate to form the IKS cardiac potassium current. Nature 384 78–80. 10.1038/384078a0 [DOI] [PubMed] [Google Scholar]
- Barrese V., Stott J. B., Greenwood I. A. (2018). KCNQ-encoded potassium channels as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 58 625–648. 10.1146/annurev-pharmtox-010617-052912 [DOI] [PubMed] [Google Scholar]
- Barros F., Dominguez P., de la Peña P. (2012). Cytoplasmic domains and voltage-dependent potassium channel gating. Front. Pharmacol. 3:49. 10.3389/fphar.2012.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman A. L., Soughayer J., Nguyen B. T., Willoughby D., Carnegie G. K., Wong W., et al. (2006). Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell 23 925–931. 10.1016/j.molcel.2006.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. (1977). Purification and characterization of catalytic subunit of skeletal muscle adenosine 3’:5’ monophosphate dependent protein kinase. J. Biol. Chem. 252 2691–2697. [PubMed] [Google Scholar]
- Bennett P. B., Begenisch T. B. (1987). Catecholamines modulate the delayed rectifying potassium current (IK) in guinea pig ventricular myocytes. Pflugers Arch. 410 217–219. 10.1007/bf00581919 [DOI] [PubMed] [Google Scholar]
- Biel M. (2009). Cyclic nucleotide-regulated cation channels. J. Biol. Chem. 284 9017–9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M., Michalakis S. (2007). Function and dysfunction of CNG channels: insights from channelopathies and mouse models. Mol. Neurobiol. 35 266–277. 10.1007/s12035-007-0025-y [DOI] [PubMed] [Google Scholar]
- Biel M., Michalakis S. (2009). Cyclic Nucleotide-Gated Channels. In CGMP: Generators, Effectors and Therapeutic Implications. Berlin: Springer, 111–136. [Google Scholar]
- Biervert C., Schroeder B. C., Kubisch C., Berkovic S. F., Propping P., Jentsch T. J., et al. (1998). A potassium channel mutation in neonatal human epilepsy. Science 279 403–406. 10.1126/science.279.5349.403 [DOI] [PubMed] [Google Scholar]
- Borgatti R., Zucca C., Cavallini A., Ferrario M., Panzeri C., Castaldo P., et al. (2004). A novel mutation in KCNQ2-associated with BFNC, drug resistant epilepsy, and mental retardation. Neurology 63 57–65. 10.1212/01.wnl.0000132979.08394.6d [DOI] [PubMed] [Google Scholar]
- Bos J. L. (2006). Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31 680–686. 10.1016/j.tibs.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Braun T., Dods R. F. (1975). Development of a Mn-2+-sensitive, ‘soluble’ adenylate cyclase in rat testis. Proc. Natl. Acad. Sci. U.S.A. 72 1097–1101. 10.1073/pnas.72.3.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Frank H., Dods R., Sepsenwol S. (1977). Mn2+-sensitive, soluble adenylate cyclase in rat testis. Differentiation from other testicular nucleotide cyclases. Biochim. Biophys. Acta 481 227–235. 10.1016/0005-2744(77)90155-3 [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. (1980). Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone [18]. Nature 283 673–676. 10.1038/283673a0 [DOI] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. (1985). Membrane currents underlying the cholinergic slow excitatory post-synaptic potential in the rat sympathetic ganglion. J. Physiol. 365 365–387. 10.1113/jphysiol.1985.sp015777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L. I., Cribbs L. L., Byron K. L. (2020). Structural determinants of Kv7.5 potassium channels that confer changes in phosphatidylinositol 4,5-bisphosphate (PIP2) affinity and signaling sensitivities in smooth muscle cells. Mol. Pharmacol. 97 145–158. 10.1124/mol.119.117192 [DOI] [PubMed] [Google Scholar]
- Brueggemann L. I., Cribbs L. L., Schwartz J., Wang M., Kouta A., Byron K. L. (2018). Mechanisms of PKA-dependent potentiation of Kv7.5 channel activity in human airway smooth muscle cells. Int. J. Mol. Sci. 19:2223. 10.3390/ijms19082223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L. I., Haick J. M., Neuburg S., Tate S., Randhawa D., Cribbs L. L., et al. (2014a). KCNQ (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 306 L476–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L. I., Kakad P. P., Love R. B., Solway J., Dowell M. L., Cribbs L. L., et al. (2012). Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am. J. Physiol. Lung Cell. Mol. Physiol. 302 L120–L132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L. I., Mackie A. R., Cribbs L. L., Freda J., Tripathi A., Majetschak M., et al. (2014b). Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J. Biol. Chem. 289 2099–2111. 10.1074/jbc.m113.527820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L. I., Mackie A. R., Martin J. L., Cribbs L. L., Byron K. L. (2011). Diclofenac distinguishes among homomeric and heteromeric potassium channels composed of KCNQ4 and KCNQ5 subunits. Mol. Pharmacol. 79 10–23. 10.1124/mol.110.067496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L. L., Canaves J. M., Pennypacker J. K., Blumenthal D. K., Taylor S. S. (2003). Isoform specific differences in binding of a dual-specificity A-kinase anchoring protein to type I and type II regulatory subunits of PKA. Biochemistry 42 5754–5763. 10.1021/bi0265729 [DOI] [PubMed] [Google Scholar]
- Carr D. W., Stofko-Hahn R. E., Fraser I. D. C., Bishop S. M., Acott T. S., Brennan R. G., et al. (1991). Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J. Biol. Chem. 266 14188–14192. [PubMed] [Google Scholar]
- Castaldo P., Giudice E. M., del Coppola G., Pascotto A., Annunziato L., Taglialatela M. (2002). Benign familial neonatal convulsions caused by altered gating of KCNQ2/KCNQ3 potassium channels. J. Neurosci. 22:RC199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M., Page C. P., Rogliani P., Matera M. G. (2013). β2-agonist therapy in lung disease. Am. J. Respir. Crit. Care Med. 187 690–696. 10.1164/rccm.201209-1739pp [DOI] [PubMed] [Google Scholar]
- Chadha P. S., Jepps T. A., Carr G., Stott J. B., Zhu H.-L., Cole W. C., et al. (2014). Contribution of Kv7.4/Kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler. Thromb. Vasc. Biol. 34 887–893. 10.1161/atvbaha.114.303405 [DOI] [PubMed] [Google Scholar]
- Chadha P. S., Zunke F., Zhu H.-L., Davis A. J., Jepps T. A., Olesen S. P., et al. (2012). Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired β-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension 59 877–884. 10.1161/hypertensionaha.111.187427 [DOI] [PubMed] [Google Scholar]
- Charlier C., Singh N. A., Ryan S. G., Lewis T. B., Reus B. E., Leach R. J., et al. (1998). A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 18 53–55. 10.1038/ng0198-53 [DOI] [PubMed] [Google Scholar]
- Chen L., Kass R. S. (2006). Dual roles of the A kinase-anchoring protein Yotiao in the modulation of a cardiac potassium channel: a passive adaptor versus an active regulator. Eur. J. Cell Biol. 85 623–626. 10.1016/j.ejcb.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Chen L., Kurokawa J., Kass R. S. (2005). Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J. Biol. Chem. 280 31347–31352. 10.1074/jbc.m505191200 [DOI] [PubMed] [Google Scholar]
- Chen L., Marquardt M. L., Tester D. J., Sampson K. J., Ackerman M. J., Kass R. S. (2007). Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 104 20990–20995. 10.1073/pnas.0710527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Ji Z., Tsalkova T., Mei F. (2008). Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim. Biophys. Sin. 40 651–662. 10.1111/j.1745-7270.2008.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E. C., Aldape K. D., Abosch A., Barbaro N. M., Berger M. S., Peacock W. S., et al. (2000). Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proc. Natl. Acad. Sci. U.S.A. 97 4914–4919. 10.1073/pnas.090092797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., Lincoln T. M., Keely S. L. (1977). Compartmentalization of adenosine 3’:5’ monophosphate and adenosine 3’:5’ monophosphate dependent protein kinase in heart tissue. J. Biol. Chem. 252 3854–3861. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., West L., Flockhart D. A., Lincoln T. M., McCarthy D. (1978). Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3’:5’-monophosphate-dependent protein kinase. J. Biol. Chem. 253 3997–4003. [PubMed] [Google Scholar]
- Cowley E. A., Linsdell P. (2002). Characterizatin of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J. Physiol. 538 747–757. 10.1113/jphysiol.2001.013300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzblanca H., Koh D. S., Hille B. (1998). Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc. Natl. Acad. Sci. U.S.A. 95 7151–7156. 10.1073/pnas.95.12.7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua M. L., Smith K. E., Gorski J. A., Horne E. A., Gibson E. S., Gomez L. L. (2006). Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur. J. Cell Biol. 85 627–633. 10.1016/j.ejcb.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Dema A., Perets E., Schulz M. S., Deák V. A., Klussmann E. (2015). Pharmacological targeting of AKAP-directed compartmentalized cAMP signalling. Cell. Signal. 27 2474–2487. 10.1016/j.cellsig.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Devor D. G., Singh A. K., Gerlach A. C., Frizzell R. A., Bridges R. J. (1997). Inhibition of intestinal Cl- secretion by clotrimazole: Direct effect on basolateral membrane K+ channels. Am. J. Physiol 273 C531–C540. [DOI] [PubMed] [Google Scholar]
- Diener M., Hug F., Strabel D., Scharrer E. (1996). Cyclic AMP-dependent regulation of K+ transport in the rat distal colon. Br. J. Pharmacol. 118 1477–1487. 10.1111/j.1476-5381.1996.tb15563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difrancesco D. (1986). Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature 324 470–473. 10.1038/324470a0 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. (2020). A brief history of pacemaking. Front. Physiol. 10:1599. 10.3389/fphys.2019.01599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. (1991). Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351 145–147. 10.1038/351145a0 [DOI] [PubMed] [Google Scholar]
- Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., et al. (1998). The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280 69–77. 10.1126/science.280.5360.69 [DOI] [PubMed] [Google Scholar]
- Dumont J. E., Jauniaux J. C., Roger P. P. (1989). The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem. Sci. 14 67–71. 10.1016/0968-0004(89)90046-7 [DOI] [PubMed] [Google Scholar]
- Evseev A. I., Semenov I., Archer C. R., Medina J. L., Dube P. H., Shapiro M. S., et al. (2013). Functional effects of KCNQ K(+) channels in airway smooth muscle. Front. Physiol. 4:277. 10.3389/fphys.2013.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M. A., Zakhary D. R., Mackey J. A., Desnoyer R. W., Apperson-Hansen C., Damron D. S., et al. (2001). AKAP-mediated targeting of protein kinase A regulates contractility in cardiac myocytes. Circ. Res. 88 291–297. 10.1161/01.res.88.3.291 [DOI] [PubMed] [Google Scholar]
- Fodstad H., Swan H., Laitinen P., Piippo K., Paavonen K., Viitasalo M., et al. (2004). Four potassium channel mutations account for 73% of the genetic spectrum underlying long−QT syndrome (LQTS) and provide evidence for a strong founder effect in Finland. Ann. Med. 36 53–63. 10.1080/17431380410032689 [DOI] [PubMed] [Google Scholar]
- Forte L. R., Bylund D. B., Zahler W. L. (1983). Forskolin does not activate sperm adenylate cyclase. Mol. Pharmacol. 24 42–47. [PubMed] [Google Scholar]
- Fujita T., Umemura M., Yokoyama U., Okumura S., Ishikawa Y. (2017). The role of Epac in the heart. Cell. Mol. Life Sci. 74 591–606. 10.1007/s00018-016-2336-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N., Stockand J. D., Shapiro M. S. (2003). Subunit-specific modulation of KCNQ potassium channels by Src tyrosine kinase. J. Neurosci. 23 84–95. 10.1523/jneurosci.23-01-00084.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz S. B., Amat J. A., Rubin C. S. (1992). cAMP signaling in neurons: patterns of neuronal expression and intracellular localization for a novel protein, AKAP 150, that anchors the regulatory subunit of cAMP-dependent protein kinase IIβ. Mol. Biol. Cell 3 1215–1228. 10.1091/mbc.3.11.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. G., Lygren B., Dokurno P., Hoshi N., McConnachie G., Taskén K., et al. (2006). Molecular basis of AKAP specificity for PKA regulatory subunits. Mol. Cell 24 383–395. 10.1016/j.molcel.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Grahammer F., Herling A. W., Lang H. J., Schmitt-Gräff A., Wittekindt O. H., Nitschke R., et al. (2001a). The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology 120 1363–1371. 10.1053/gast.2001.24053 [DOI] [PubMed] [Google Scholar]
- Grahammer F., Warth R., Barhanin J., Bleich M., Hug M. J. (2001b). The small conductance K+ channel, KCNQ1. Expression, function, and subunit composition in murine trachea. J. Biol. Chem. 276 42268–42275. 10.1074/jbc.m105014200 [DOI] [PubMed] [Google Scholar]
- Greenwood I. A., Stott J. B. (2020). The Gβ1 and Gβ3 subunits differentially regulate rat vascular Kv7 channels. Front. Physiol. 10:1573. 10.3389/fphys.2019.01573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Chandy K. G., Grissmer S., Lazdunski M., McKinnon D., Pardo L. A., et al. (2005). International union of pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 57 473–508. 10.1124/pr.57.4.10 [DOI] [PubMed] [Google Scholar]
- Haick J. M., Byron K. L. (2016). Novel treatment strategies for smooth muscle disorders: targeting Kv7 potassium channels. Pharmacol. Ther. 165 14–25. 10.1016/j.pharmthera.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Haitin Y., Attali B. (2008). The C-terminus of Kv7 channels: a multifunctional module. J. Physiol. 586 1803–1810. 10.1113/jphysiol.2007.149187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halls M. L., Cooper D. M. F. (2017). Adenylyl cyclase signalling complexes – pharmacological challenges and opportunities. Pharmacol. Ther. 172 171–180. 10.1016/j.pharmthera.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Hanoune J., Defer N. (2001). Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 41 145–174. [DOI] [PubMed] [Google Scholar]
- Hansen H. H., Waroux O., Seutin V., Jentsch T. J., Aznar S., Mikkelsen J. D. (2008). Kv7 channels: Interaction with dopaminergic and serotonergic neurotransmission in the CNS. J. Physiol. 586 1823–1832. 10.1113/jphysiol.2007.149450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., Abramson T., MacKinnon R. (1992). A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science 258 1152–1155. 10.1126/science.1279807 [DOI] [PubMed] [Google Scholar]
- Higashida H., Hoshi N., Zhang J.-S., Yokoyama S., Hashii M., Jin D., et al. (2005). Protein kinase C bound with A-kinase anchoring protein is involved in muscarinic receptor-activated modulation of M-type KCNQ potassium channels. Neurosci. Res. 51 231–234. 10.1016/j.neures.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Hoshi N., Langeberg L. K., Scott J. D. (2005). Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat. Cell Biol. 7 1066–1073. 10.1038/ncb1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N., Zhang J.-S., Omaki M., Takeuchi T., Yokoyama S., Wanaverbecq N., et al. (2003). AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat. Neurosci. 6 564–571. 10.1038/nn1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. J. S., Durick K., Weiner J. A., Chun J., Taylor S. S. (1997). Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J. Biol. Chem. 272 8057–8064. 10.1074/jbc.272.12.8057 [DOI] [PubMed] [Google Scholar]
- Iannotti F. A., Panza E., Barrese V., Viggiano D., Soldovieri M. V., Taglialatela M. (2010). Expression, localization, and pharmacological role of Kv7 potassium channels in skeletal muscle proliferation, differentiation, and survival after myotoxic insults. J. Pharmacol. Exp. Ther. 332 811–820. 10.1124/jpet.109.162800 [DOI] [PubMed] [Google Scholar]
- Ipavec V., Martire M., Barrese V., Taglialatela M., Currò D. (2011). KV7 channels regulate muscle tone and nonadrenergic noncholinergic relaxation of the rat gastric fundus. Pharmacol. Res. 64 397–409. 10.1016/j.phrs.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenke M., Sánchez A., Monje F., Stühmer W., Weseloh R. M., Pardo L. A. (2003). C-terminal domains implicated in the functional surface expression of potassium channels. EMBO J. 22 395–403. 10.1093/emboj/cdg035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J. (2000). Neuronal KCNQ potassium channels:physislogy and role in disease. Nat. Rev. Neurosci. 1 21–30. 10.1038/35036198 [DOI] [PubMed] [Google Scholar]
- Jepps T. A. (2017). Unravelling the complexities of vascular smooth muscle ion channels: fine tuning of activity by ancillary subunits. Pharmacol. Ther. 178 57–66. 10.1016/j.pharmthera.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Jepps T. A., Carr G., Lundegaard P. R., Olesen S.-P., Greenwood I. A. (2015). Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J. Physiol. 593 5325–5340. 10.1113/jp271286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps T. A., Chadha P. S., Davis A. J., Harhun M. I., Cockerill G. W., Olesen S. P., et al. (2011). Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 124 602–611. [DOI] [PubMed] [Google Scholar]
- Jepps T. A., Greenwood I. A., Moffatt J. D., Sanders K. M., Ohya S. (2009). Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am. J. Physiol. Gastrointest. Liver Physiol. 297 G107–G115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps T. A., Olesen S. P., Greenwood I. A., Dalsgaard T. (2016). Molecular and functional characterization of Kv7 channels in penile arteries and corpus cavernosum of healthy and metabolic syndrome rats. Br. J. Pharmacol. 173 1478–1490. 10.1111/bph.13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen T., Membrez M., Nicolas C., Pitard B., Staub O., Olesen S., et al. (2007). The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc. Res. 74 64–74. 10.1016/j.cardiores.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Kanda V. A., Abbott G. W. (2012). KCNE regulation of K(+) channel trafficking – a sisyphean task? Front. Physiol. 3:231. 10.3389/fphys.2012.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G., Chepurny O. G., Malester B., Rindler M. J., Rehmann H., Bos J. L., et al. (2006). cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J. Physiol. 573 595–609. 10.1113/jphysiol.2006.107391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Kurokawa J., Marx S. O., Marks A. R. (2003). Leucine/isoleucine zipper coordination of ion channel macromolecular signaling complexes in the heart. Roles in inherited arrhythmias. Trends Cardiovasc. Med. 13 52–56. 10.1016/s1050-1738(02)00211-6 [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Seifert R. (2002). Cyclic nucleotide-gated ion channels. Physiol. Rev. 82 769–824. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Springett G. M., Mochizuki N., Toki S., Nakaya M., Matsuda M., et al. (1998). A family of cAMP-binding proteins that directly activate Rap1. Science 282 2275–2279. 10.1126/science.282.5397.2275 [DOI] [PubMed] [Google Scholar]
- Khanamiri S., Soltysinska E., Jepps T. A., Bentzen B. H., Chadha P. S., Schmitt N., et al. (2013). Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertens 62 1090–1097. 10.1161/hypertensionaha.113.01244 [DOI] [PubMed] [Google Scholar]
- Kharkovets T., Hardelin J. P., Safieddine S., Schweizer M., El-Amraoui A., Petit C., et al. (2000). KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc. Natl. Acad. Sci. U.S.A. 97 4333–4338. 10.1073/pnas.97.8.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. K., Yoo H. Y., Kim S. J., Hwang Y.-S., Han J., Kim J. A., et al. (2007). Effects of sevoflurane on the cAMP-induced short-circuit current in mouse tracheal epithelium and recombinant Cl- (CFTR) and K+ (KCNQ1) channels†. Br. J. Anaesth. 99 245–251. 10.1093/bja/aem123 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Greger R. (1999). Voltage-dependent, slowly activating K+ current (I(Ks)) and its augmentation by carbachol in rat pancreatic acini. Pflugers Arch. Eur. J. Physiol. 438 604–611. 10.1007/s004240051083 [DOI] [PubMed] [Google Scholar]
- Kinderman F. S., Kim C., Daake S., von Ma Y., Pham B. Q., Spraggon G., et al. (2006). A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol. Cell 24 397–408. 10.1016/j.molcel.2006.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köttgen M., Hoefer A., Kim S. J., Beschorner U., Schreiber R., Hug M. J., et al. (1999). Carbachol activates a K+ channel of very small conductance in the basolateral membrane of rat pancreatic acinar cells. Pflugers Arch. 438 597–603. 10.1007/s004240051082 [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. (1979). Phosphorylation-dephosphorylation of enzymes. Annu. Rev. Biochem. 48 923–959. 10.1146/annurev.bi.48.070179.004423 [DOI] [PubMed] [Google Scholar]
- Kubisch C., Schroeder B. C., Friedrich T., Lütjohann B., El-Amraoui A., Marlin S., et al. (1999). KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96 437–446. 10.1016/s0092-8674(00)80556-5 [DOI] [PubMed] [Google Scholar]
- Kurokawa J., Motoike H. K., Rao J., Kass R. S. (2004). Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 101 16374–16378. 10.1073/pnas.0405583101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche C., Scherer C. R., Seebohm G., Derst C., Wei A. D., Busch A. E., et al. (2000). Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J. Biol. Chem. 275 22395–22400. 10.1074/jbc.m002378200 [DOI] [PubMed] [Google Scholar]
- Lester L. B., Langeberg L. K., Scott J. D. (1997). Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc. Natl. Acad. Sci. U.S.A. 94 14942–14947. 10.1073/pnas.94.26.14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen L., Kass R. S., Dessauer C. W. (2012). The A-kinase anchoring protein yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart. J. Biol. Chem. 287 29815–29824. 10.1074/jbc.m112.380568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Langlais P., Gamper N., Liu F., Shapiro M. S. (2004). Dual phosphorylations underlie modulation of unitary KCNQ K+ channels by Src tyrosine kinase. J. Biol. Chem. 279 45399–45407. 10.1074/jbc.m408410200 [DOI] [PubMed] [Google Scholar]
- Lindman J., Khammy M. M., Lundegaard P. R., Aalkjær C., Jepps T. A. (2018). Microtubule regulation of Kv7 channels orchestrates cAMP-mediated vasorelaxations in rat arterial smooth muscle. Hypertension 71 336–345. 10.1161/hypertensionaha.117.10152 [DOI] [PubMed] [Google Scholar]
- Liu L., Wang F., Lu H., Ren X., Zou J. (2014). Chromanol 293B, an inhibitor of KCNQ1 channels, enhances glucose-stimulated insulin secretion and increases glucagon-like peptide-1 level in mice. Islets 6:e962386. 10.4161/19382014.2014.962386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann E., Burhoff I., Nitschke R. B., Lang H. J., Mania D., Englert H. C., et al. (1995). A new class of inhibitors of cAMP-mediated Cl- secretion in rabbit colon, acting by the reduction of cAMP-activated K+ conductance. Pflügers Arch. Eur. J. Physiol. 429 517–530. 10.1007/bf00704157 [DOI] [PubMed] [Google Scholar]
- Ludwig A., Zong X., Jeglitsch M., Hofmann F., Biel M. (1998). A family of hyperpolarization-activated mammalian cation channels. Nature 393 587–591. 10.1038/31255 [DOI] [PubMed] [Google Scholar]
- Lundby A., Andersen M. N., Steffensen A. B., Horn H., Kelstrup C. D., Francavilla C., et al. (2013). In vivo phosphoproteomics analysis reveals the cardiac targets of beta-adrenergic receptor signaling. Sci. Signal. 6:rs11. 10.1126/scisignal.2003506 [DOI] [PubMed] [Google Scholar]
- MacVinish L. J., Guo Y., Dixon A. K., Murrell-Lagnado R. D., Cuthbert A. W. (2001). Xe991 reveals differences in K(+) channels regulating chloride secretion in murine airway and colonic epithelium. Mol. Pharmacol. 60 753–760. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. (1984). The role of cyclic AMP in insulin release. Experientia 40 1068–1075. 10.1007/bf01971453 [DOI] [PubMed] [Google Scholar]
- Mall M., Wissner A., Schreiber R., Kuehr J., Seydewitz H. H., Brandis M., et al. (2000). Role of K(v)LQT1 in cyclic adenosine monophosphate-mediated Cl- secretion in human airway epithelia. Am. J. Respir. Cell Mol. Biol. 23 283–289. 10.1165/ajrcmb.23.3.4060 [DOI] [PubMed] [Google Scholar]
- Mani B. K., Robakowski C., Brueggemann L. I., Cribbs L. L., Tripathi A., Majetschak M., et al. (2016). Kv7.5 potassium channel subunits are the primary targets for PKA-dependent enhancement of vascular smooth muscle Kv7 currents. Mol. Pharmacol. 89 323–334. 10.1124/mol.115.101758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. A., Kumar S. (2018). Physiological functions of Nedd4-2: lessons from knockout mouse models. Trends Biochem. Sci. 43 635–647. 10.1016/j.tibs.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Marrion N. V. (1997). Control of M-current. Annu. Rev. Physiol. 59 483–504. [DOI] [PubMed] [Google Scholar]
- Marx S. O., Kurokawa J., Reiken S., Motoike H., D’Armiento J., Marks A. R., et al. (2002). Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295 496–499. 10.1126/science.1066843 [DOI] [PubMed] [Google Scholar]
- Mayr B., Montminy M. (2001). Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2 599–609. 10.1038/35085068 [DOI] [PubMed] [Google Scholar]
- McCallum L. A., Greenwood I. A., Tribe R. M. (2009). Expression and function of Kv7 channels in murine myometrium throughout oestrous cycle. Pflugers Arch. Eur. J. Physiol. 457 1111–1120. 10.1007/s00424-008-0567-5 [DOI] [PubMed] [Google Scholar]
- McCrossan Z. A., Abbott G. W. (2004). The MinK-related peptides. Neuropharmacology 47 787–821. 10.1016/j.neuropharm.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Min Lee S., Baik J., Nguyen D., Nguyen V., Liu S., Hu Z., et al. (2017). Kcne2 deletion impairs insulin secretion and causes type 2 diabetes mellitus. FASEB J. 31 2674–2685. 10.1096/fj.201601347 [DOI] [PubMed] [Google Scholar]
- Moita M. A. P., Lamprecht R., Nader K., LeDoux J. E. (2002). A-kinase anchoring proteins in amygdala are involved in auditory fear memory. Nat. Neurosci. 5 837–838. 10.1038/nn901 [DOI] [PubMed] [Google Scholar]
- Moleschi K., Melacini G. (2014). Signaling at crossroads: the dialogue between PDEs and PKA is spoken in multiple languages. Biophys. J. 107 1259–1260. 10.1016/j.bpj.2014.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mona A., Xiao-Yan Q., Ling X., Balazs O., Artavazd T., Xiaobin L., et al. (2014). Exchange protein directly activated by cAMP mediates slow delayed-rectifier current remodeling by sustained β-adrenergic activation in guinea pig hearts. Circ. Res. 114 993–1003. 10.1161/circresaha.113.302982 [DOI] [PubMed] [Google Scholar]
- Moosmang S., Biel M., Hofmann F., Ludwig A. (1999). Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biol. Chem. 380 975–980. 10.1515/bc.1999.121 [DOI] [PubMed] [Google Scholar]
- Neyroud N., Tesson F., Denjoy I., Leibovici M., Donger C., Barhanin J., et al. (1997). A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat. Genet. 15 186–189. 10.1038/ng0297-186 [DOI] [PubMed] [Google Scholar]
- Nicolas C. S., Park K.-H., Harchi A., El Camonis J., Kass R. S., Escande D., et al. (2008). IKs response to protein kinase A-dependent KCNQ1 phosphorylation requires direct interaction with microtubules. Cardiovasc. Res. 79 427–435. 10.1093/cvr/cvn085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S., Asakura K., Muraki K., Watanabe M., Imaizumi Y. (2002). Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 282 G277–G287. [DOI] [PubMed] [Google Scholar]
- Oliveras A., Roura-Ferrer M., Solé L., La Cruz A., De Prieto A., Etxebarria A., et al. (2014). Functional assembly of Kv7.1/Kv7.5 channels with emerging properties on vascular muscle physiology. Arterioscler. Thromb. Vasc. Biol. 34 1522–1530. [DOI] [PubMed] [Google Scholar]
- Patra C., Brady M. F. (2018). Biochemistry, cAMP. Treasure Island, FL: StatPearls. [PubMed] [Google Scholar]
- Pierce K. L., Premont R. T., Lefkowitz R. J. (2002). Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3 639–650. [DOI] [PubMed] [Google Scholar]
- Potet F., Scott J. D., Mohammad-Panah R., Escande D., Baró I. (2001). AKAP proteins anchor cAMP-dependent protein kinase to KvLQT1/IsK channel complex. Am. J. Physiol. Circ. Physiol. 280 H2038–H2045. [DOI] [PubMed] [Google Scholar]
- Preston P., Wartosch L., Günzel D., Fromm M., Kongsuphol P., Ousingsawat J., et al. (2010). Disruption of the K+ channel beta-subunit KCNE3 reveals an important role in intestinal and tracheal Cl- transport. J. Biol. Chem. 285 7165–7175. 10.1074/jbc.m109.047829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall T. W., Sutherland E. W. (1958). Formation of a cyclic adenine ribonucleotide by tissue particles. J. Biol. Chem. 232 1065–1076. [PubMed] [Google Scholar]
- Rannels S. R., Corbin J. D. (1980). Studies of functional domains of the regulatory subunit from cAMP-dependent protein kinase isozyme I. J. Cyclic Nucleotide Res. 6 201–215. [PubMed] [Google Scholar]
- Renström E., Eliasson L., Rorsman P. (1997). Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J. Physiol. 502(Pt 1) 105–118. 10.1111/j.1469-7793.1997.105bl.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringheim G. E., Taylor S. S. (1990). Dissecting the domain structure of the regulatory subunit of cAMP-dependent protein kinase I and elucidating the role of MgATP. J. Biol. Chem. 265 4800–4808. [PubMed] [Google Scholar]
- Robbins J. (2001). KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol. Ther. 90 1–19. 10.1016/s0163-7258(01)00116-4 [DOI] [PubMed] [Google Scholar]
- Roche J. P., Westenbroek R., Sorom A. J., Hille B., Mackie K., Shapiro M. S. (2002). Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K + channels. Br. J. Pharmacol. 137 1173–1186. 10.1038/sj.bjp.0704989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode F., Svalø J., Sheykhzade M., Rønn L. C. B. (2010). Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur. J. Pharmacol. 638 121–127. 10.1016/j.ejphar.2010.03.050 [DOI] [PubMed] [Google Scholar]
- de Rooij J., Zwartkruis F. J. T., Verheijen M. H. G., Cool R. H., Nijman S. M. B., Wittinghofer A., et al. (1998). Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396 474–477. 10.1038/24884 [DOI] [PubMed] [Google Scholar]
- Roscioni S. S., Elzinga C. R. S., Schmidt M. (2008). Epac: effectors and biological functions. Naunyn. Schmiedebergs. Arch. Pharmacol. 377 345–357. 10.1007/s00210-007-0246-7 [DOI] [PubMed] [Google Scholar]
- Roura-Ferrer M., Solé L., Martínez-Mármol R., Villalonga N., Felipe A. (2008). Skeletal muscle Kv7 (KCNQ) channels in myoblast differentiation and proliferation. Biochem. Biophys. Res. Commun. 369 1094–1097. 10.1016/j.bbrc.2008.02.152 [DOI] [PubMed] [Google Scholar]
- Rubin C. S. (1994). A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim. Biophys. Acta 1224 467–479. [PubMed] [Google Scholar]
- Ruehr M. L., Russell M. A., Bond M. (2004). A-kinase anchoring protein targeting of protein kinase A in the heart. J. Mol. Cell. Cardiol. 37 653–665. 10.1016/j.yjmcc.2004.04.017 [DOI] [PubMed] [Google Scholar]
- Rufo P. A., Merlin D., Riegler M., Ferguson-Maltzman M. H., Dickinson B. L., Brugnara C., et al. (1997). The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances: demonstration of efficacy in the intact rabbit colon and in an in vivo mouse model of cholera. J. Clin. Invest. 100 3111–3120. 10.1172/jci119866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana R., Dessauer C. W. (2009). Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 17 5–22. 10.1159/000166277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer I., Erdem F. A., Chen W.-Q., Heo S., Koenig X., Schicker K. W., et al. (2017). Phosphorylation regulates the sensitivity of voltage-gated Kv7.2 channels towards phosphatidylinositol-4,5-bisphosphate. J. Physiol. 595 759–776. 10.1113/jp273274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands W. A., Palmer T. M. (2008). Regulating gene transcription in response to cyclic AMP elevation. Cell. Signal. 20 460–466. 10.1016/j.cellsig.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Curran M. E., Zou A., Shen J., Specter P. S., Atkinson D. L., et al. (1996). Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKS potassium channel. Nature 384 80–83. 10.1038/384080a0 [DOI] [PubMed] [Google Scholar]
- Santoro B., Liu D. T., Yao H., Bartsch D., Kandel E. R., Siegelbaum S. A., et al. (1998). Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93 717–729. 10.1016/s0092-8674(00)81434-8 [DOI] [PubMed] [Google Scholar]
- Sarma G. N., Kinderman F. S., Kim C., Daake S., von Chen L., Wang B. C., et al. (2010). Structure of D-AKAP2:PKA RI complex: insights into AKAP specificity and selectivity. Structure 18 155–166. 10.1016/j.str.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P. (1995). Transcription factors responsive to cAMP. Annu. Rev. Cell Dev. Biol. 11 355–377. 10.1146/annurev.cb.11.110195.002035 [DOI] [PubMed] [Google Scholar]
- Schroeder B. C., Hechenberger M., Weinreich F., Kubisch C., Jentsch T. J. (2000a). KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J. Biol. Chem. 275 24089–24095. 10.1074/jbc.m003245200 [DOI] [PubMed] [Google Scholar]
- Schroeder B. C., Kubisch C., Stein V., Jentsch T. J. (1998). Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature 396 687–690. 10.1038/25367 [DOI] [PubMed] [Google Scholar]
- Schroeder B. C., Waldegger S., Fehr S., Bleich M., Warth R., Greger R., et al. (2000b). A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403 196–199. 10.1038/35003200 [DOI] [PubMed] [Google Scholar]
- Schwake M., Athanasiadu D., Beimgraben C., Blanz J., Beck C., Jentsch T. J., et al. (2006). Structural determinants of M-type KCNQ (Kv7) K+ channel assembly. J. Neurosci. 26 3757–3766. 10.1523/jneurosci.5017-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M., Jentsch T. J., Friedrich T. (2003). A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep. 4 76–81. 10.1038/sj.embor.embor715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M., Pusch M., Kharkovets T., Jentsch T. J. (2000). Surface expression and single channel properties of KCNQ2/KCNQ3, M-type K+ channels involved in epilepsy. J. Biol. Chem. 275 13343–13348. 10.1074/jbc.275.18.13343 [DOI] [PubMed] [Google Scholar]
- Scott J. D., Dessauer C. W., Taskén K. (2013). Creating order from chaos: cellular regulation by kinase anchoring. Annu. Rev. Pharmacol. Toxicol. 53 187–210. 10.1146/annurev-pharmtox-011112-140204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D., Stofko R. E., McDonald J. R., Comer J. D., Vitalis E. A., Mangili J. A. (1990). Type II regulatory subunit dimerization determines the subcellular localization of the cAMP-dependent protein kinase. J. Biol. Chem. 265 21561–21566. [PubMed] [Google Scholar]
- Seino S., Shibasaki T. (2005). PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 85 1303–1342. 10.1152/physrev.00001.2005 [DOI] [PubMed] [Google Scholar]
- Shabb J. B. (2001). Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 101 2381–2411. [DOI] [PubMed] [Google Scholar]
- Shah M. M., Mistry M., Marsh S. J., Brown D. A., Delmas P. (2002). Molecular correlates of the M-current in cultured rat hippocampal neurons. J. Physiol. 544 29–37. 10.1113/jphysiol.2002.028571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariati B., Thompson E. L., Nicol G. D., Vasko M. R. (2016). Epac activation sensitizes rat sensory neurons through activation of Ras. Mol. Cell. Neurosci. 70 54–67. 10.1016/j.mcn.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F. (1999). G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 20 66–73. 10.1016/s0165-6147(99)01307-3 [DOI] [PubMed] [Google Scholar]
- Sims S. M., Singer J.J., Walsh J. V., Jr. (1988). Antagonistic adrenergic-muscarinic regulation of M current in smooth muscle cells. Science 239 190–193. [DOI] [PubMed] [Google Scholar]
- Singh N. A., Charlier C., Stauffer D., DuPont B. R., Leach R. J., Melis R., et al. (1998). A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat. Genet. 18 25–29. 10.1038/ng0198-25 [DOI] [PubMed] [Google Scholar]
- Steele D. F., Fedida D. (2014). Cytoskeletal roles in cardiac ion channel expression. Biochim. Biophys. Acta Biomembr. 1838 665–673. 10.1016/j.bbamem.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Stott J. B., Barrese V., Greenwood I. A. (2016). Kv7 channel activation underpins EPAC-dependent relaxations of rat arteries. Arterioscler. Thromb. Vasc. Biol. 36 2404–2411. 10.1161/atvbaha.116.308517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott J. B., Barrese V., Suresh M., Masoodi S., Greenwood I. A. (2018). Investigating the role of G protein βγ in Kv7-dependent relaxations of the rat vasculature. Arterioscler. Thromb. Vasc. Biol. 38 2091–2102. 10.1161/atvbaha.118.311360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott J. B., Povstyan O. V., Carr G., Barrese V., Greenwood I. A. (2015). G-protein βγ subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc. Natl. Acad. Sci. U.S.A. 112 6497–6502. 10.1073/pnas.1418605112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng T., Christoph T., Andersson K. E. (2004). Urodynamic effects of the K+ channel (KCNQ) opener retigabine in freely moving, conscious rats. J. Urol. 172 2054–2058. 10.1097/01.ju.0000138155.33749.f4 [DOI] [PubMed] [Google Scholar]
- Suessbrich H., Bleich M., Ecke D., Rizzo M., Waldegger S., Lang F., et al. (1996). Specific blockade of slowly activating I(sK) channels by chromanols - Impact on the role of I(sK) channels in epithelial. FEBS Lett. 396 271–275. 10.1016/0014-5793(96)01113-1 [DOI] [PubMed] [Google Scholar]
- Sunose H., Liu J., Marcus D. C. (1997). cAMP increases K+ secretion via activation of apical IsK/KvLQT1 channels in strial marginal cells. Hear. Res. 114 107–116. 10.1016/s0378-5955(97)00152-4 [DOI] [PubMed] [Google Scholar]
- Surti T. S., Huang L., Jan Y. N., Jan L. Y., Cooper E. C. (2005). Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels. Proc. Natl. Acad. Sci. U.S.A. 102 17828–17833. 10.1073/pnas.0509122102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland E. W., Rall T. W. (1958). Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 232 1077–1091. [PubMed] [Google Scholar]
- Svalø J., Bille M., Parameswaran Theepakaran N., Sheykhzade M., Nordling J., Bouchelouche P. (2013). Bladder contractility is modulated by Kv7 channels in pig detrusor. Eur. J. Pharmacol. 715 312–320. 10.1016/j.ejphar.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Svalø J., Hansen H. H., Rønn L. C. B., Sheykhzade M., Munro G., Rode F. (2012). Kv7 positive modulators reduce detrusor overactivity and increase bladder capacity in rats. Basic Clin. Pharmacol. Toxicol. 110 145–153. 10.1111/j.1742-7843.2011.00765.x [DOI] [PubMed] [Google Scholar]
- Taussig R., Gilman A. G. (1995). Mammalian membrane-bound adenylyl cyclases. J. Biol. Chem. 270 1–4. 10.1074/jbc.270.1.1 [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Ilouz R., Zhang P., Kornev A. P. (2012). Assembly of allosteric macromolecular switches: lessons from PKA. Nat. Rev. Mol. Cell Biol. 13 646–658. 10.1038/nrm3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Zhang P., Steichen J. M., Keshwani M. M., Kornev A. P. (2013). PKA: lessons learned after twenty years. Biochim. Biophys. Acta 1834 1271–1278. 10.1016/j.bbapap.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrenoire C., Clancy C. E., Cormier J. W., Sampson K. J., Kass R. S. (2005). Autonomic control of cardiac action potentials. Circ. Res. 96 e25–e34. [DOI] [PubMed] [Google Scholar]
- Terrenoire C., Houslay M. D., Baillie G. S., Kass R. S. (2009). The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J. Biol. Chem. 284 9140–9146. 10.1074/jbc.m805366200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testai L., Barrese V., Soldovieri M. V., Ambrosino P., Martelli A., Vinciguerra I., et al. (2016). Expression and function of Kv7.4 channels in rat cardiac mitochondria: possible targets for cardioprotection. Cardiovasc. Res. 110 40–50. 10.1093/cvr/cvv281 [DOI] [PubMed] [Google Scholar]
- Torekov S. S., Iepsen E., Christiansen M., Linneberg A., Pedersen O., Holst J. J., et al. (2014). KCNQ1 long QT syndrome patients have hyperinsulinemia and symptomatic hypoglycemia. Diabetes 63 1315–1325. 10.2337/db13-1454 [DOI] [PubMed] [Google Scholar]
- Tresguerres M., Levin L. R., Buck J. (2011). Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 79 1277–1288. 10.1038/ki.2011.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnham R. E., Scott J. D. (2016). Protein kinase A catalytic subunit isoform PRKACA. History, function and physiology. Gene 577 101–108. 10.1016/j.gene.2015.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis A. V., Heidenreich M., Kharkovets T., Spitzmaul G., Jensen H. S., Nicoll R. A., et al. (2010). The KCNQ5 potassium channel mediates a component of the afterhyperpolarization current in mouse hippocampus. Proc. Natl. Acad. Sci. U.S.A. 107 10232–10237. 10.1073/pnas.1004644107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S., Su J., Ranta F., Wittekindt O. H., Ris F., Rösler M., et al. (2005). Effects of IKs channel inhibitors in insulin-secreting INS-1 cells. Pflugers Arch. Eur. J. Physiol. 451 428–436. 10.1007/s00424-005-1479-2 [DOI] [PubMed] [Google Scholar]
- Unoki H., Takahashi A., Kawaguchi T., Hara K., Horikoshi M., Andersen G., et al. (2008). SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat. Genet. 40 1098–1102. 10.1038/ng.208 [DOI] [PubMed] [Google Scholar]
- Vallon V., Grahammer F., Volkl H., Sandu C. D., Richter K., Rexhepaj R., et al. (2005). KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc. Natl. Acad. Sci. U.S.A. 102 17864–17869. 10.1073/pnas.0505860102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. (1991). Distinct voltage-dependent regulation of a heart-delayed IK by protein kinases A and C. Am. J. Physiol. 261 C1081–C1090. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S., Reiken S., Motoike H., D’Armiento J., Marks A. R., et al. (1988). Regulation of a heart potassium channel by protein kinase A and C. Science 242 67–69. 10.1126/science.2845575 [DOI] [PubMed] [Google Scholar]
- Wang H. S., Pan Z., Shi W., Brown B. S., Wymore R. S., Cohen I. S., et al. (1998). KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282 1890–1893. 10.1126/science.282.5395.1890 [DOI] [PubMed] [Google Scholar]
- Wang Q., Curran M. E., Splawski I., Burn T. C., Millholland J. M., VanRaay T. J., et al. (1996). Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat. Genet. 12 17–23. 10.1038/ng0196-17 [DOI] [PubMed] [Google Scholar]
- Warth R., Garcia Alzamora M., Kim J., Zdebik A., Nitschke R., Bleich M., et al. (2002). The role of KCNQ1/KCNE1 K+ channels in intestine and pancreas: lessons from the KCNE1 knockout mouse. Pflügers Arch. Eur. J. Physiol. 443 822–828. 10.1007/s00424-001-0751-3 [DOI] [PubMed] [Google Scholar]
- Weckhuysen S., Ivanovic V., Hendrickx R., Coster R., Van Hjalgrim H., Møller R. S., et al. (2013). Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology 81 1697–1703. 10.1212/01.wnl.0000435296.72400.a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckhuysen S., Mandelstam S., Suls A., Audenaert D., Deconinck T., Claes L. R. F., et al. (2012). KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann. Neurol. 71 15–25. 10.1002/ana.22644 [DOI] [PubMed] [Google Scholar]
- Weigand I., Ronchi C. L., Rizk-Rabin M., Dalmazi G., Di Wild V., Bathon K., et al. (2017). Differential expression of the protein kinase A subunits in normal adrenal glands and adrenocortical adenomas. Sci. Rep. 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch E. J., Jones B. W., Scott J. D. (2010). Network with the AKAPs context-dependent regulation of anchored enzymes. Mol. Interv. 10 86–97. 10.1124/mi.10.2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden A. D., Zou A., Wagoner P. K., Jegla T. (2001). Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br. J. Pharmacol. 132 381–384. 10.1038/sj.bjp.0703861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Scott J. D. (2004). AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 5 959–970. 10.1038/nrm1527 [DOI] [PubMed] [Google Scholar]
- Xu Q., Chang A., Tolia A., Minor D. L. (2013). Structure of a Ca2+/CaM:Kv7.4 (KCNQ4) B-helix complex provides insight into M current modulation. J. Mol. Biol. 425 378–394. 10.1016/j.jmb.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Senokuchi T., Lu M., Takemoto M., Fazlul Karim M., Go C., et al. (2011). Voltage-gated K+ channel KCNQ1 regulates insulin secretion in MIN6 β-cell line. Biochem. Biophys. Res. Commun. 407 620–625. 10.1016/j.bbrc.2011.03.083 [DOI] [PubMed] [Google Scholar]
- Yasuda K., Miyake K., Horikawa Y., Hara K., Osawa H., Furuta H., et al. (2008). Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat. Genet. 40 1092–1097. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. (1990). Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J. Physiol. 421 135–150. 10.1113/jphysiol.1990.sp017937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Juhl C. R., Hylten-Cavallius L., Salling-Olsen M., Linneberg A., Holst J. J., et al. (2020). Gain-of-function mutation in the voltage-gated potassium channel gene KCNQ1 and glucose-stimulated hypoinsulinemia – case report. BMC Endocr. Disord. 20:38. 10.1186/s12902-020-0513-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Shapiro M. S. (2012). Activity-dependent transcriptional regulation of M-type (Kv7) K+ channels by AKAP79/150-mediated NFAT actions. Neuron 76 1133–1146. 10.1016/j.neuron.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Kerlavage A. R., Taylor S. S. (1979). Structural comparisons of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J. Biol. Chem. 254 2408–2412. [PubMed] [Google Scholar]