Abstract
Of the various transcription factors that play a role in controlling oxidative stress, the role of FoxO proteins in skin aging has recently become of interest. Unlike other FoxOs, FoxO6 remains in the nucleus due to the lack of nuclear export signal, so that it may respond sensitively to intracellular stimuli for the induction of target genes. However, the role of FoxO6 in melanogenesis and its related signaling pathways are unclear. We used UV exposed and intrinsically aged mice that exhibited skin aging. Our data showed that FoxO6 activation was markedly decreased in the skin of aging mice and UVB-exposed hairless mice that exhibited an increase in melanogenesis. The reduced FoxO6 activity was closely associated with the elevation of oxidative stress in the skin of these animal models. To our interest, siRNA-mediated FoxO6 knockdown markedly increased melanin content and related signaling pathways in B16F10 cells even without any stimulation. On the contrary, adenovirus-mediated FoxO6 activation significantly reduced melanin content in UVB-exposed B16F10 cells, which is closely associated with the induction of antioxidant genes including MnSOD and catalase, leading to a decrease in oxidative stress. Furthermore, vitamin C treatment reversed the elevated melanogenesis by the FoxO6 knockdown, indicating that the decreased antioxidant capacity greatly contributes to increased melanogenesis in the FoxO6 knockdown condition. For the upstream of a FoxO6 signaling pathway in melanocytes, FoxO6 phosphorylation by Akt appears to be essential evidenced by the reduction of FoxO6 activity and the increase in melanogenesis by PI3K/AKT inhibitor treatment. Our study suggests that FoxO6 is an antioxidant gene that prevents oxidative stress-induced melanogenesis.
Keywords: Oxidative stress, FoxO6, Antioxidant, Melanogenesis
Graphical abstract
1. Introduction
Evolutionally conserved forkhead transcription factors consist of FoxO1, FoxO3a, FoxO4, and FoxO6 in mammals. Studies have shown that FoxO proteins play key roles in inducing mRNA expression of target genes involved in energy metabolism, apoptosis, cell cycle, DNA repair, cell death, and oxidative stress response [[1], [2], [3], [4]]. FoxO proteins are activated by various physiological and nutritional stimuli including insulin, IGFs, oxidative stress, cytokines, and macro- and micro-nutrients [5,6].
Of the FoxO family, FoxO6 exhibits fundamental differences from other members. FoxO6 has the lowest protein homology (~30%) in amino acid sequence compared with that of the other members [6]. FoxO6 has only two consensus AKT/PKB phosphorylation sites—at Thr26 and Ser184—within the DNA binding domain, whereas other FoxO proteins have three conserved phosphorylation sites—at Thr24, Ser253, and Ser316 [6]. Unlike other family members, FoxO6 remains in the nucleus, independent of insulin action, because of the lack of the consensus motif that induces the nuclear export signal, while the other family members translocate between the nucleus and cytosol depending on insulin signaling. Instead of subcellular redistribution, insulin suppresses FoxO6 activity in the nucleus by inducing FoxO6 phosphorylation directly [7].
Although FoxO6 is evolutionally conserved across species ranging from frogs to humans [7], the biological functions of FoxO6 have not been well studied, partially due to earlier reports of its limited expression, only in the brain [8,9]. However, later studies reported that FoxO6 was widely expressed in other tissues including the adipose tissue, muscle, intestine, heart, lung, and kidney [10]. Subsequently, various studies have been performed to elucidate the functions of FoxO6 in tissues and its role in metabolic disorders. FoxO6 activation has been associated with metabolic syndromes including insulin resistance, hyperlipidemia, and fatty liver [11,12]. Liver-specific FoxO6-overexpressed mice exhibited hyperlipidemia because of enhancement of hepatic lipogenesis and VLDL secretion, and FoxO6 regulation was dysregulated in insulin-resistant mice [12]. On the contrary, FoxO6 depletion ameliorated diet-induced glucose intolerance and insulin resistance due to the suppression of hepatic gluconeogenesis and macrophage infiltration in the liver and adipose tissues in mice [11]. FoxO6 suppression may be beneficial to reduce metabolic syndromes, partly because of the decrease in lipogenesis and lipid secretion in metabolic tissues.
Independent of its pathogenic roles in metabolic syndromes, FoxO6 plays an essential role in the redox state by regulating antioxidant genes. Our previous study showed that FoxO6 transactivates antioxidant genes including MnSOD and catalase in the HepG2 cells, contributing to the intracellular antioxidant system [13]. Because oxidative stress is one of the major factors to induce photoaging in the skin, the intracellular antioxidant system plays an essential role in ameliorating the problems associated with skin aging including melanogenesis and wrinkle formation [14,15]. In this regard, FoxO6 may be greatly beneficial to control intracellular redox state because FoxO6 remains in the nucleus regardless of insulin action [9], and thereby sensitively responding to external or intracellular stimuli to regulate antioxidant gene expression.
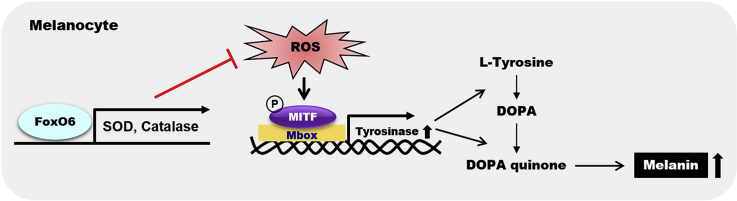
Melanogenesis occurs in the melanocytes located in the epidermis of the skin. Melanocytes transfer the synthesized melanin to keratinocytes via the dendrites, leading to skin darkening. Although melanogenesis is a defense mechanism against ultraviolet (UV) irradiation, melanin overproduction results in undesirable pigmentation [16] and unfavorable cosmetic problems. Therefore, the suppression of melanin overproduction has attracted substantial attention to the maintenance of healthy skin [14,15,17]. Because oxidative stress stimulates skin aging, causing melanogenesis and wrinkle formation, elevating the intracellular antioxidant defense system or finding natural antioxidants is an attractive strategy to suppress intrinsic aging or photoaging [14,[18], [19], [20]]. Here, we investigated whether the FoxO6-mediated antioxidant system contributes to the inhibition of melanogenesis, using intrinsically aged mice, HRM-2 hairless mice with UVB exposure, and B16F10 cells transduced with adenovirus including FoxO6 or FoxO6 siRNA.
2. Methods
2.1. Materials
Kojic acid, DCFDA, DHR123, and other chemical reagents were purchased from Sigma Chemical (St. Louis, MO, USA). Antibodies against MITF, tyrosinase, FoxO6, pFoxO6, pCREB, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Animals
Male C57BL/6 mice (12 and 24 months) were obtained from Samtako (Osan, Korea) (n = 6 per group). After stabilization for one week, the mice were dissected and the dorsal skin was shaved with an electronic razor, immediately excised, frozen in liquid nitrogen, and stored at −80 °C. HRM-2 hairless mice (6-week-old male, n = 5 per group) were obtained from Hoshino Laboratory Animals (Yashino, Saitama, Japan). The mice were exposed to UVB every other day for 26 days in a UVB exposure chamber at 150 mJ/cm2. From days 26 to 28, mice were exposed to UVB every day to maximize the effect of UVB on skin pigmentation. After 28 days of the treatments, mice were sacrificed and the dorsal skin was excised and quickly frozen in a nitrogen tank for further experiments. For staining purposes, the excised skin was fixed in 4% paraformaldehyde. The mice were maintained with 12 h/12 h light/dark cycle and received ad libitum access to standard laboratory diet and water. All animal studies were approved by the Institutional Animal Care Committee of Pusan National University (Approval number 2013-0357) and were performed in accordance with the guidelines for animal experiments issued by Pusan National University.
2.3. Cell culture
B16F10 cells (from the Korean Cell Line Bank) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Nissui Pharmaceutical) with 10% fetal bovine serum (FBS; Sigma Chemical) and penicillin/streptomycin (100 IU/50 μg/mL) in a humidified atmosphere containing 5% CO2 (in air) at 37 °C. B16F10 cells were cultured in 6-well plates for melanin quantification and enzyme activity assays.
2.4. UVB exposure
We used a commercially available UV exposure chamber (Ultraviolet crosslinker, UVP, LLC, Upland, CA, USA) for chronic UVB exposure of mice (Kim et al., 2013; Lee et al., 2015). The UV exposure chamber is designed to measure and control the UV radiation within the exposure chamber. We used UVB-specific tubes and sensors provided by the manufacturer, which continuously measures UVB energy and automatically adjusts to variations in UVB intensity that occurs as the UVB tubes age. Therefore, we could minimize potential contamination of UVA, UVC, and infrared radiation.
2.5. Transduction of FoxO6 CA and FoxO6 knockdown
For FoxO6 activation, adv-FoxO6-CA (from 50 to 200 MOI) was used to transduce the B16F10 cells. FoxO6-CA was obtained from H.H Dong (University of Pittsburgh), which mutates the Akt/PKB phosphorylation site at Ser184 to Ala184. To induce knockdown of FoxO6, the B16F10 cells were grown to 80% confluence in DMEM and treated with adv-FoxO6-siRNA (from 50 to 200 MOI) for 48 h.
2.6. Measurement of melanin contents
Melanin content was measured as described previously (Pacher et al., 2007). In the current study, melanin content was used as an index of melanogenesis. B16F10 melanoma cells (5×104 cells/well) were washed with PBS and dissolved in 100 μL of 1 N NaOH. The samples were incubated at 60 °C for 1 h, and the absorbance of the samples was measured at 409 nm to determine the melanin content.
2.7. Assay of tyrosinase activity
Tyrosinase activity was determined in B16F10 melanoma cells by measuring the rate of l-DOPA oxidation. B16F10 melanoma cells (5×104 cells/well) were washed with PBS and dissolved in 100 μL of 50 mM sodium phosphate buffer (pH 6.5) containing 1% TritonX-100 (Sigma-Aldrich) and 0.1 mM phenylmethyl sulfonyl fluoride (PMSF). The samples were frozen at −80 °C for 30 min and the supernatants were collected by centrifugation at 12,000×g for 30 min at 4 °C. Next, 80 μL of the supernatants were obtained, and 20 μL of l-DOPA (2 mg/mL) was added to the wells of a 96-well plate. Finally, the absorbance was read at 450 nm every 10 min for 1 h at 37 °C using an ELISA plate reader.
2.8. ROS or ONOO− scavenging activity
The scavenging activity of ROS and ONOO- was measured by fluorescent 2,7-dichlorodihydrofluorescein diacetate (DCFDA) and dihydrorhodamine (DHR) 123, respectively. To measure ROS levels, 10 μL of the skin homogenate sample was added with 25 μM DCFDA in black 96-wells to a final volume of 250 μL. Cells were seeded at 1 × 104 cells/well in a black 96-well cell culture dish and 25 μM DCFDA was added. To determine the ONOO- level, the reaction buffer (190 μL) was prepared by adding rhodamine solution (50 mM sodium phosphate buffer, 90 mM sodium chloride solution), 5 mM diethylenetriaminepentaacetic acid (DTPA), and DHR123 to a 10 μL skin homogenate sample of cells seeded at 1 × 104 cells/well in a black 96-well cell culture dish. The reaction buffer was directly added to the plate. ROS and ONOO- scavenging activity values were measured every 5 min for 30 min on a fluorescent plate reader (SpectraMax i3, Molecular Devices) with an excitation of 485 nm and emission of 535 nm.
2.9. Western blotting
Protein samples from skin lysates (15–30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membranes, which were immediately placed in 5% non-fat milk blocking buffer containing 10 mM Tris (pH 7.5), 100 mM NaCl, and 0.1% Tween 20. The membrane was washed in TBS-Tween buffer for 30 min and then incubated with specific primary antibodies indicated in the figure legends (dilution 1:1000) at 4 °C overnight. After washing with TBS-Tween buffer, the membrane was incubated with a horseradish peroxidase-conjugated anti-mouse antibody (Santa Cruz, 1:10,000), an anti-rabbit antibody (Santa Cruz, 1:10,000), or an anti-goat antibody (Santa Cruz, 1:10,000) at 25 °C for 1 h. The immunoblots were visualized using Western Bright Peroxide solution (Advansta, CA, USA) and Davinch-Chemi CAS-400 (Davinch-K, Korea) according to the manufacturer's instructions. Antibodies against FoxO6 and p-FoxO6 (Ser184) were a gift from Dr. H. H. Dong (University of Pittsburgh, PA).
2.10. Quantitative RT-PCR
Total RNA from the tissues was isolated using a rapid extraction method (TRI-Reagent, Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed on cDNA samples using the iQTM SYBR Green Supermix system (Bio-Rad, Hercules, CA, USA). The protocols used are as follows: denaturation at 95 °C for 2 min, followed by 40 cycles of amplification at 95 °C for 15 s, 55 °C for 30 s, 72 °C for 30 s, and an acquisition temperature for 15 s (Bioneer ExicycleTM 96, Daejeon, Korea). The analysis was conducted using the sequence detection software supplied with the instrument. For each sample, the delta-delta cycle threshold cycle (ddCT) (crossing point) values were calculated as the Ct of the target gene minus the Ct of the β-actin gene. Gene expression was derived according to the equation 2-ddCt; changes in gene expression are expressed relative to basal levels. The primer sequences are as follows: m-FoxO6, as-TTC AGC ATC CAC CAT GAA CT, ss-GAA GAG CTC CCG ACG GAA CG; and m-b-actin, as-GGC TGT ATT CCC CTC CAT CG, ss-CCA GTT GGT AAC AAT GCC ATG T.
2.11. Statistical analysis
All results are expressed as mean ± SEM. Treatments were compared using a one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. Student's t-test was used to analyze the differences between the two groups. p values < 0.05 were considered statistically significant. Analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Aging-related oxidative stress is associated with the downregulation of FoxO6 in the skin
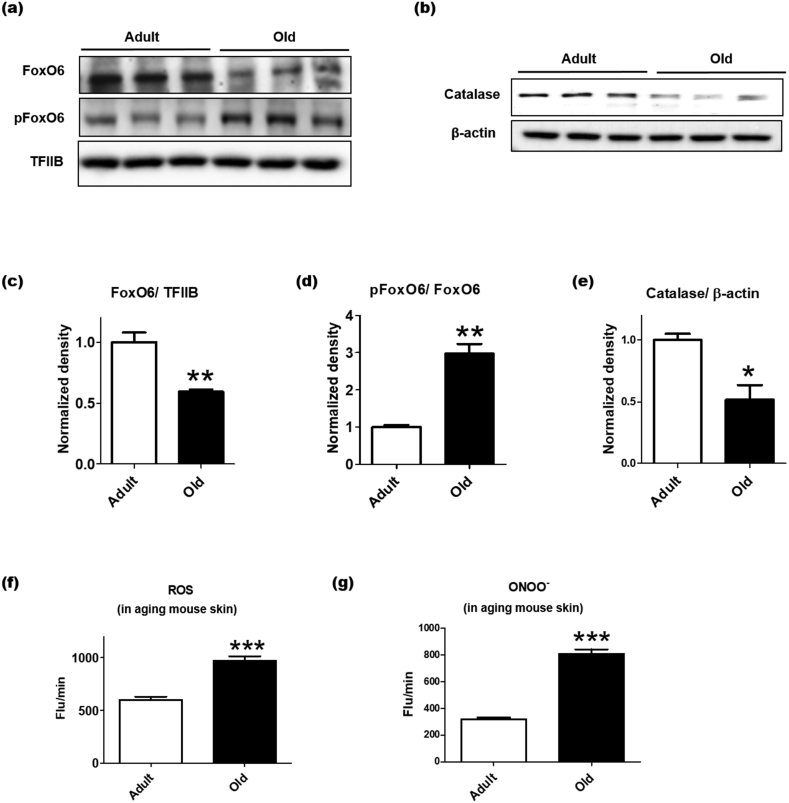
Proper control of oxidative stress is essential to inhibit intrinsic and UV exposure-mediated skin aging, including suppression of uncontrolled melanogenesis and wrinkle formation [21,22]. Our previous study showed that FoxO6 may regulate redox balance in the liver by upregulating antioxidant genes including MnSOD and catalase [13]. We investigated whether the FoxO6 was associated with the intrinsic aging of the skin, using adult and old C57BL/6 mice. Aging not only reduced the total protein level of FoxO6, but also increased phosphorylated FoxO6 (p-FoxO6), an inactive form of FoxO6 in the nucleus of the skin (Fig. 1a, c, and 1d). Consistently, the protein level of catalase as a downstream target of FoxO6 was decreased in the cytosolic fraction of the skin samples (Fig. 1b and e). When the state of oxidative stress was compared, the skin of aging mice exhibited elevated levels of ROS and ONOO-(Fig. 1f and g). These data suggest that the increased oxidative stress in the skin of aging mice may be closely associated with the decrease in FoxO6 activity.
Fig. 1.
Lowered antioxidant capacity in the intrinsic skin aging is related to the decrease in FoxO6 activity. The dorsal skin of adult (age, 12 months) and old (age, 24 months) C57BL/6 mice were homogenized to perform western blotting (n = 6 per group). (A) The protein levels of total FoxO6 and p-FoxO6 in the nucleus. (B) The protein level of catalase in the cytosol. Western blots of (C) FoxO6/TFIIB, (D) p-FoxO6/FoxO6, and (E) catalase/β-actin were semi-quantified by the Image J software (n = 3per group). The western blotting results were confirmed using another cohort (total n = 6 per group). (F) ROS and (G) ONOO- levels were determined in the dorsal skin homogenate of the adult and aged mice. Data are represented as mean ± SEM. Statistical results of student's t-test: *p <0.05, **p <0.01, and ***p <0.001 vs. the adult mice.
3.2. UVB-induced skin darkening is associated with the downregulation of FoxO6 and the elevation of oxidative stress in HRM-2 hairless mice
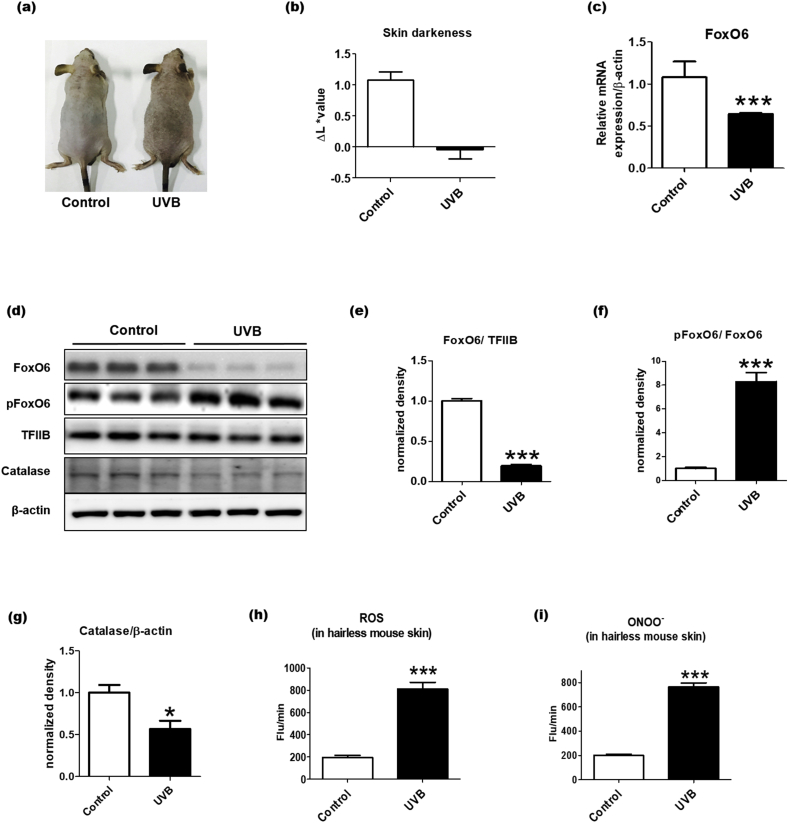
To investigate the relationship between FoxO6 and photoaging, we investigated whether FoxO6 expression was altered in response to UVB exposure, which generates oxidative stress in the skin [14,15]. HRM-2 melanin-possessing hairless mice were exposed to UVB chronically, and we confirmed that the dorsal skin of the mice darkened due to UVB-induced melanogenesis (Fig. 2a–b). To study whether the UVB-induced skin darkening was associated with FoxO6 regulation, the dorsal skin samples were analyzed for FoxO6 mRNA and protein expression. The FoxO6 mRNA expression level was decreased in the UVB-exposed skin (Fig. 2c). Also, the total FoxO6 protein level was notably decreased, and p-FoxO6 protein level was increased in the nucleus of the UVB-exposed skin (Fig. 2d and e). When the western blot results were semi-quantified, the p-FoxO6/FoxO6 ratio was markedly elevated (Fig. 2f), indicating that UVB exposure inactivates FoxO6. When other FoxOs were examined, the mRNA levels of FoxO1 and FoxO3 were decreased in response to UVB but that of FoxO4 was unchanged (Supplementary Fig. 1).
Fig. 2.
UVB-induced skin darkening is associated with the downregulation of FoxO6 and the elevation of oxidative stress in HRM-2 hairless mice. After HRM-2 hairless mice were exposed to UVB for 4weeks (see Methods for more detailed information), skin samples were excised and homogenized (n = 5 per group). (A) The pictures of the dorsal skin were taken after 4 weeks of UVB exposure. (B) The darkness of the dorsal skin was determined on day 28 of the UVB exposure using a CR-10 spectrophotometer. Higher L ∗ values represent brighter colors (n = 5 per group). (C) The mRNA expression levels of FoxO6 in the skin were normalized to β-actin. (D) The protein expression levels of FoxO6 and p-FoxO6 in the nucleus and the protein levels of catalase in the cytosolic fraction in the skin of the UVB exposed hairless mice. Western blots of (E) FoxO6/TFIIB, (F) p-FoxO6/FoxO6, and (G) catalase/β-actin were semi-quantified by the ImageJ software. (H) ROS and (I) ONOO- levels were determined in the dorsal skin homogenate of the HRM-2 hairless mice. Data are represented as mean ± SEM. Statistical results of Student's t-test: *p <0.05 and ***p <0.001 vs. the control mice without UVB exposure. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To further examine FoxO6 downstream signaling, the protein level of catalase was measured in the skin homogenate of the HRM-2 hairless mice. UVB significantly lowered the protein level of catalase (Fig. 2d and g). In conjunction with this, UVB exposure markedly elevated the levels of ROS and ONOO-in the skin (Fig. 2h and i). These data suggest that the UVB-induced inactivation of FoxO6 and the increased oxidative stress were closely related to the skin darkening observed in the HRM-2 hairless mice.
3.3. FoxO6 knockdown induces melanogenesis in B16F10 cells
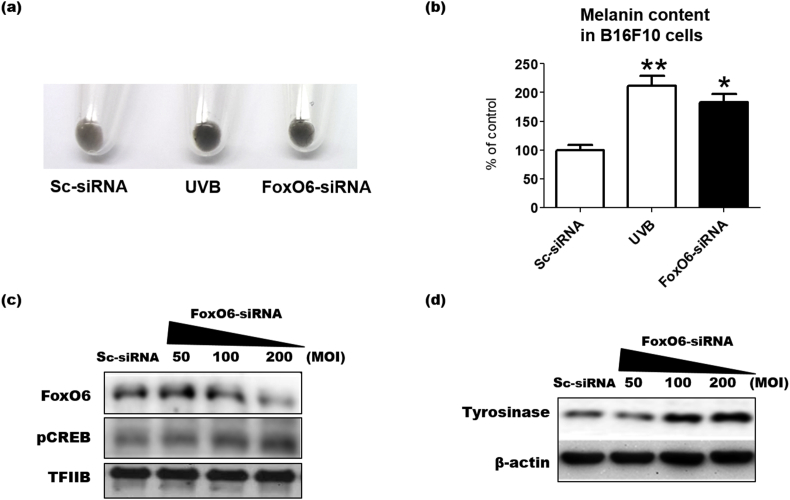
To examine whether FoxO6 regulates melanogenesis, FoxO6 was knocked-down in B16F10 cells by infecting with an adenovirus containing FoxO6 siRNA. No difference in FoxO6 protein level was observed between the non-treated and scrambled siRNA-treated control groups (data not shown). FoxO6 protein levels were decreased by the siRNA-containing adenovirus delivery at 200 MOI compared to the scrambled (SC) siRNA-treated group (Fig. 3c). FoxO6 knockdown darkened the cell pellet and significantly elevated melanin content even without any stimulation (Fig. 3a and b). Compared with the UVB-treated group as a positive control, UVB exposure appears to be a stronger melanogenic inducer than FoxO6 inhibition (Fig. 3a and b), possibly because UVB activates a wide range of melanogenic signaling. When melanogenic signaling was examined by western blotting, FoxO6 knockdown resulted in increase in the protein levels of phosphorylated CREB in the nucleus, which is a melanogenic transcription factor, and increased protein levels of tyrosinase, which is an essential enzyme for melanogenesis (Fig. 3c and d). These data indicated that FoxO6 downregulation induced melanogenesis in the B16F10 cells.
Fig. 3.
FoxO6 knockdown stimulates melanogenesis in B16F10 cells. (A) B16F10 cells were treated with the Adv-FoxO6-siRNA (200 MOI) for 48 h, and melanin levels were observed. (B) Melanin levels were determined by measuring absorbance at 409 nm using a spectrophotometer. Sc-siRNA: scramble siRNA, UVB: UVB exposure at 10 mJ/cm2. After treatment with the Adv-FoxO6-siRNA (50, 100, and 200 MOI), the cell lysates were analyzed by western blotting for (C) pCREB and FoxO6 and (D) tyrosinase. Data are represented as mean ± SEM (n = 3 per group). Statistical results of one-way analysis of variance followed by the Dunnett's multiple comparison test. *p < 0.05 and **p < 0.01 compared with the Sc-siRNA treated group.
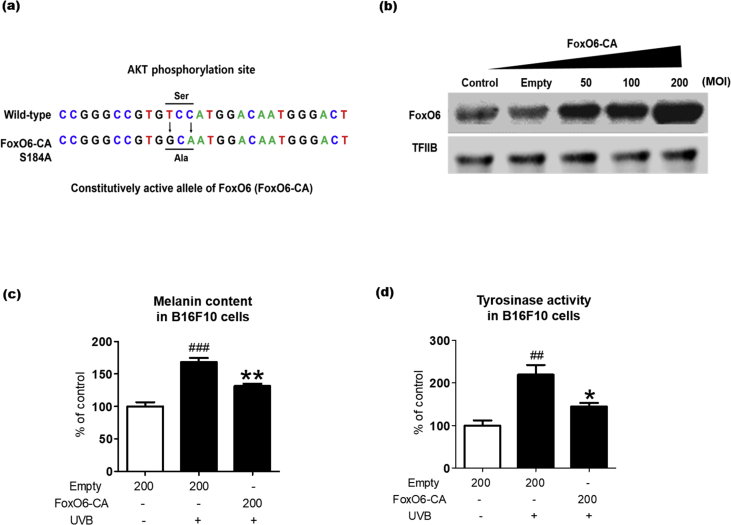
3.4. FoxO6 activation ameliorates UVB-induced melanogenesis
Because FoxO6 deficiency elevated the intracellular melanin levels even without stimulations, including UV exposure and αMSH treatment, we hypothesized that FoxO6 activation ameliorates melanogenesis induced by UVB, the strongest melanogenic inducer. To study this, we used an adenoviral vector expressing a constitutively active allele of FoxO6 (FoxO6-CA). The FoxO6-CA mutant includes 2 nucleotide substitutions resulting in the conversion of Ser184 to Ala184. This mutation impairs the conserved Akt phosphorylation site on FoxO6 [12] (Fig. 4a). We selected the most efficient concentration (200 MOI) for adenoviral transduction based on a preliminary study (Fig. 4b). There was no visible cytotoxicity at this concentration (data not shown). Other studies also indicated no cytotoxicity of the adenovirus up to 200 MOI [10,12]. As expected, UVB exposure with empty virus transduction significantly increased melanin content in the B16F10 cells, whereas the FoxO6-CA adenovirus transduction notably ameliorated it (Fig. 4c). Consistently, the UVB-induced increase in tyrosinase activity was significantly reduced by the FoxO6 viral transduction (Fig. 4d). These data indicated that FoxO6 activation prevented UVB-induced melanogenesis.
Fig. 4.
FoxO6 activation decreases melanogenesis. (A) Schematic description of an adenoviral vector expressing a constitutively active allele of FoxO6 (FoxO6-CA). (B) B16F10 cells were transduced with Adv-FoxO6-CA or Adv-Empty for (B) 24 h or (C and D) 48 h. (B) After treatment with Adv-FoxO6-CA, the cell lysates were analyzed by western blotting. (C) Intracellular melanin levels and (D) tyrosinase activity were assessed by measuring absorbance at 450 nm (n = 4 per group). These experiments were repeated two times. Con: untreated, Empty: Adv-null vectors-transduced. Data are represented as mean ± SEM. Statistical results of one-way analysis of variance followed by the Dunnett's multiple comparison test. ##p < 0.01, ###p < 0.001 compared with the Adv-empty, *p < 0.05 and **p < 0.01 compared with Adv-FoxO6-CA.
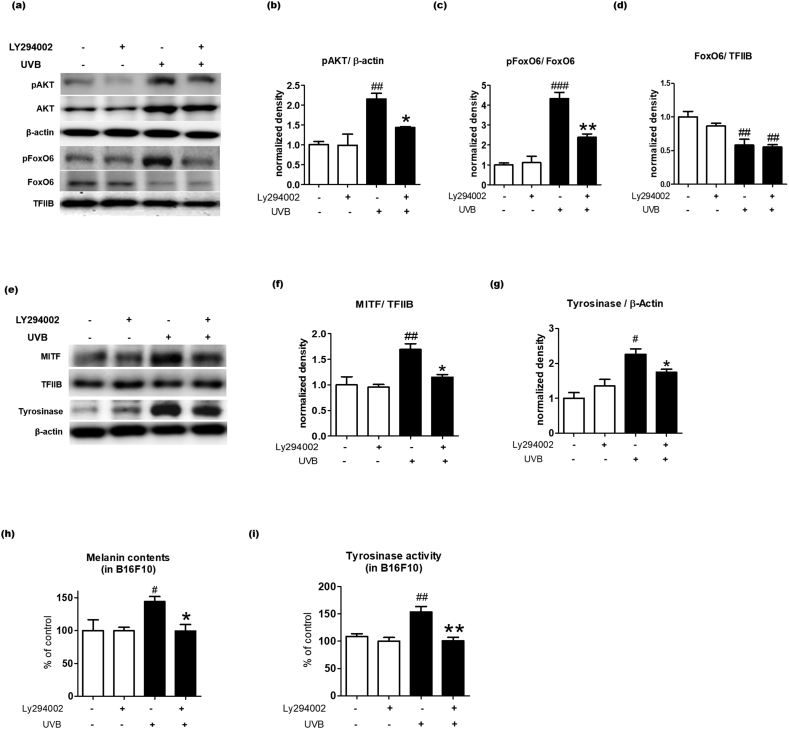
3.5. AKT is an upstream target of FoxO6 in UVB induced melanogenesis
The FoxO family is regulated by multiple factors including AMP-activated protein kinase (AMPK), protein kinase B (also known as AKT), CREB binding protein (CBP), and N-terminal kinase (JNK) [23]. However, FoxO6 regulation has not been investigated, especially in the skin. The present study showed that transduction with the FoxO6-CA adenovirus that impairs the conserved Akt phosphorylation site on FoxO6 results in the inhibition of UVB-induced melanogenesis (Fig. 4). Thus, we tested whether Akt signaling affects FoxO6 phosphorylation and UVB-induced melanogenesis in B16F10 cells. UVB exposure increased protein levels of an active form of AKT (p-AKT) and an inactive form of FoxO6 (p-FoxO6) in B16F10 cells (Fig. 5a–c). On the contrary, the PI3K/AKT inhibitor (LY294002) treatment lowered p-AKT and p-FoxO6 in the UVB-exposed condition (Fig. 5a–c). When the western blot was semi-quantified, UVB exposure decreased the total FoxO6 protein levels in the nucleus regardless of LY294002 treatment (Fig. 5d). Furthermore, the p-FoxO6/FoxO6 ratio was markedly elevated by UVB exposure, but reduced by LY294002 treatment (Fig. 5c). These data indicated that FoxO6 was inactivated by PI3K/AKT signaling in B16F10 cells. We further examined whether the Akt-mediated regulation of FoxO6 was associated with melanogenic signaling. As expected, UVB exposure increased the protein levels of the nuclear MITF and the cytosolic tyrosinase, essential factors for melanogenesis, whereas LY294002 treatment reduced these factors (Fig. 5e–g). Consistently, UVB-exposed B16F10 cells had higher tyrosinase activity and melanin levels, which were reduced by LY294002 treatment, although LY294002 did not affect these factors in the UVB-unexposed B16F10 cells (Fig. 5h and i). These data indicated that the PI3K/AKT inhibitor-mediated decrease in melanogenesis was related to FoxO6 activation.
Fig. 5.
AKT is an upstream target of FoxO6 in UVB-induced melanogenesis. B16F10 cells were pretreated with 10 μM LY290042 followed by 10 mJ/cm2 UVB exposure. (A) Western blotting results of AKT, pAKT in the cytosolic fraction, and FoxO6 and pFoxO6 in the nuclear fraction. Western blots of (B) p-AKT/β-actin, (C) p-FoxO6/FoxO6, and (D) FoxO6/TFIIB were semi-quantified by the Image J software (n = 4 per group). (e) Western blotting results of tyrosinase in the cytosol and MITF in the nuclear fraction. β-Actin and TFIIB were used as loading controls for the cytosolic and nuclear fractions, respectively. Western blots of (F) MITF/TFIIB and (g) tyrosinase/β-actin were semi-quantified by the Image J software (n = 4 per group). The levels of (H) melanin and (I) tyrosinase activity were quantified by measuring absorbance at 450 nm (n = 4 per group). Data are represented as mean ± SEM. Statistical results of one-way analysis of variance followed by the Dunnett's multiple comparison test. #p <0.05, ##p <0.01, and ###p <0.001 compared with the untreated group, *p <0.05 and **p <0.01 compared with the UVB-treated group.
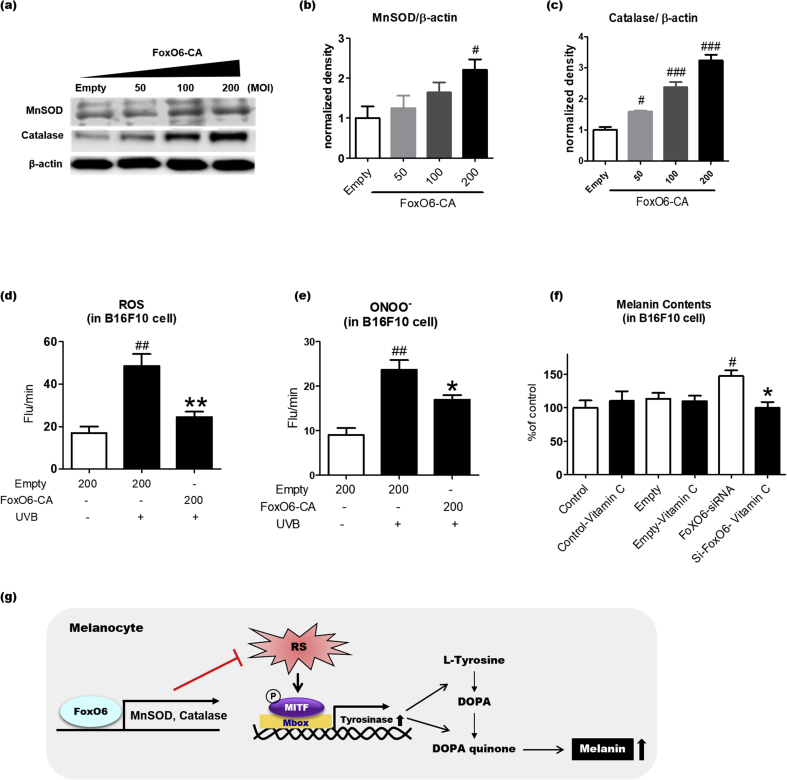
3.6. FoxO6-mediated increase in antioxidant capacity contributes to the anti-melanogenic effect
UVB-induced oxidative stress is a well-known contributor to melanogenesis. FoxO6 has been shown to maintain redox balance in the liver [13]. Thus, we determined whether FoxO6 regulates antioxidant gene expression in the melanocytes. Constitutively active FoxO6-CA-transduced B16F10 cells were homogenized to measure the protein levels of antioxidant genes. FoxO6-CA transduction greatly increased protein levels of catalase and MnSOD compared with that of the control group subjected to empty virus transduction (Fig.6a–c). To determine whether the FoxO6-mediated increase in the antioxidant gene expression was related to antioxidant capacity, we measured ROS and ONOO- levels in B16F10 cells. UVB exposure and FoxO6-CA transduction significantly increased and decreased ROS and ONOO- levels, respectively, further demonstrating that FoxO6 elevates antioxidant capacity in the melanocytes (Fig. 6d and e). Based on these results, we hypothesized that the addition of antioxidants reverses FoxO6 knockdown-induced melanogenesis if the FoxO6-mediated anti-melanogenic effect is at least partially dependent on its antioxidant capacity. The melanin content was unaltered when the B16F10 cells were treated with vitamin C or empty virus (Fig. 6f). However, siRNA-mediated FoxO6 knockdown significantly elevated the melanin content, but vitamin C treatment reversed it (Fig. 6f), suggesting that the FoxO6-mediated elevation of antioxidant capacity plays an important role in its anti-melanogenic effect (Fig. 6g).
Fig. 6.
FoxO6-mediated increase in antioxidant capacity contributes to the anti-melanogenic effect. B16F10 cells were treated with adv-FoxO6-CA or empty virus. (A) Protein levels of MnSOD and catalase. β-actin was used as a loading control. Western blots of (B) MnSOD/β-actin and (C) Catalase/β-actin were semi-quantified by the Image J software (n = 4 per group). Suppression of (D) ROS and (E) ONOO- levels by adv-FoxO6-CA (200 MOI). ROS and ONOO- levels in B16F10 cells were measured by 2′7′-dichlorodihydrofluorescein diacetate (DCFDA) and dihydrohodamine (DHR123), respectively. (F) Melanin content in B16F10 cells treated with the empty virus, FoxO6 siRNA-containing virus, or/and vitamin C. Data are represented as mean ± SEM (n =4/group). (G) Schematic representation of the potential mechanism underlying the FoxO6-mediated anti-melanogenic effect in melanocytes. RS, reactive species; Mbox, TCAYRTG, or CAYRTGA sequences in the promoter regions; MITF, microphthalmia-associated transcription factor. Statistical results of one-way analysis of variance followed by the Dunnett's multiple comparison test.#p <0.05, ##p <0.01, and ###p <0.001 compared with Adv-Empty, *p <0.05 and **p <0.01 compared with Adv-Empty/UVB or Adv-FoxO6-siRNA.
4. Discussion
In the current study, we investigated the roles of FoxO6 in melanogenesis. The data showed that the mRNA or protein levels of FoxO6 were markedly reduced in the skin of the intrinsic aging or photoaging mouse models that exhibit oxidative stress and hyper-pigmentation. In conjunction to this, melanin content and related signaling pathways were significantly increased by FoxO6 knockdown in B16F10 cells, whereas this effect was reversed by FoxO6 activation. For the upstream regulation of FoxO6-mediated antimelanogenic activity, FoxO6 phosphorylation by AKT appears to be essential. The mechanisms underlying the anti-melanogenic effect included but were not limited to the elevation of intracellular antioxidant capacity by FoxO6, because FoxO6 activation upregulated antioxidant genes including catalase and MnSOD, thereby reducing UVB-induced ROS and ONOO- generation. This was further supported by the vitamin C-mediated amelioration of UVB-induced melanogenesis in the FoxO6 knockdown cells. We concluded that FoxO6 is a transcription factor that elevates the intracellular defense system against oxidative stress in the melanocytes, leading to an anti-melanogenic effect.
Despite a distinct regulatory pattern of FoxO6, its biological functions have not been extensively studied. Although studies have shown that FoxO6 was involved in pathologic conditions including gastric cancer [24,25], hyperlipidemia [12], and insulin resistance [10,11], the roles of FoxO6 in the skin is unknown. The current study demonstrated that FoxO6 was essential in ameliorating UVB-induced oxidative stress in the melanocytes, as evidenced by the marked reduction of the UVB-induced ROS and ONOO- production after the adenovirus-mediated FoxO6 activation. The antioxidant functions of FoxO6 have been shown in other tissues. Previous studies have shown that the lowered protein levels of MnSOD and catalase in the kidney of the aging rat were associated with the decrease in FoxO6 expression [26]. FoxO6 overexpression increased the protein levels of antioxidant genes in HepG2 and HEK293T cells [13,26]. Considering FoxO6 is expressed ubiquitously [10], the activation of FoxO6 may act as an intracellular defense system against oxidative stress.
The intracellular antioxidant system is important not only for reducing melanogenesis, but also for ameliorating other problems associated with skin aging including wrinkle formation, decrease in skin elasticity, and skin cancers. It is likely that FoxO6 may respond more sensitively than the other FoxO family members to induce antioxidant gene expression because FoxO6 remained in the nucleus regardless of insulin action [9]. Thus, further studies are necessary to examine whether the FoxO6-mediated cellular defense system against oxidative stress can ameliorate these other problems associated with skin aging.
PI3K/AKT signaling is an important regulator of the FoxO family [7]. Our study examined FoxO6 regulation in melanocytes and the data showed that the nuclear FoxO6 was also regulated by PI3K/AKT signaling in the melanocytes. It appears that UVB-induced activation of AKT is an upstream mechanism underlying FoxO6 inactivation, which consequently lowers the intracellular antioxidant capacity. However, other mechanisms may also exist, because UVB exposure decreased the total protein levels of FoxO6. Further investigations should focus on UV-responsive factors including transcription factors or miRNAs that downregulate FoxO6 expression. Also of interest is how FoxO6 can be regulated in the skin by AKT without its shuttling between the nucleus and the cytosol.
Although our study focused on the AKT/FoxO6 axis in melanogenesis, especially after UV exposure, AKT regulates diverse pathways including cell survival signaling. This raises a question about the consequence of UVB-mediated AKT activation in the skin. FoxO6 is one of the diverse targets regulated by AKT. Focusing on the intracellular defense system against oxidative stress and skin aging angle, AKT activation by UVB may result in FoxO6 inactivation, thereby decreasing antioxidant capacity. On the contrary, it has also been reported that UV irradiation activates the PI3K/AKT survival pathway in the skin [27,28]. The activated AKT signaling may likely protect skin cells from UVB-mediated apoptosis. Therefore, PI3K/AKT activation may have both positive and negative effects on the skin, post-UV exposure.
It has been reported that FoxO3 is an anti-melanogenic protein that mediates antioxidant-induced depigmentation [18]. The expression of melanogenic genes and cellular melanin levels elevated with FoxO3 knockdown and the overexpression of FoxO3 reversed these characteristics [18]. Interestingly, when antioxidants such as vitamin C, N-acetylcysteine, and Trolox were applied to MNT1 cells, melanin content decreased with FoxO3 nuclear translocation, but this phenomenon was disappeared with FoxO3 knockdown, indicating that FoxO3 may orchestrate antioxidants-mediated anti-melanogenic effects. On the other hand, the mechanism underlying the FoxO6-mediated anti-melanogenic effect may be slightly different with FoxO3 because the elevated melanogenesis with FoxO6 knockdown was reversed by vitamin C treatment, suggesting FoxO6 may not be a mandatory factor for vitamin C-mediated anti-melanogenic effects, whereas FoxO3 may be necessary for this effect. Based on our data, FoxO6 exerts anti-melanogenic effects at least partially through up-regulating genes associated with antioxidant defense, thereby reducing UVB induced oxidative stress. It is necessary to further study the differences in anti-melanogenic mechanisms of FoxO3 and FoxO6 at the same experimental conditions.
Taken together, FoxO6 appears to be downregulated in response to biological aging and photoaging in the skin. FoxO6 inactivation downregulates gene expression related to the intracellular antioxidant defense system, thereby leading to an enhancement of oxidative stress in the skin. As a consequence, melanogenesis occurs in the skin. The skin-specific activation of FoxO6 may have inhibitory effects on various skin aging properties, including melanogenesis, at least partially, by enhancing the intracellular antioxidant capacity.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgments
We would like to thank the Aging Tissue Bank (Busan, Republic of Korea) for providing the tissue samples. We also thank Dr. H. Henry Dong at the University of Pittsburgh School of Medicine for kindly providing us with the FoxO6 adenovirus.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101624.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (Grant No. 2009-0083538; Grant No. 2018R1A2A3075425) and by Basic Science Research Program through NRF funded by the Ministry of Education (Grant No. 2016R1A6A3A11933476). This work was also supported by NRF grant (No. 2020R1A2C2006436).
Author contributions
K.M. Moon performed experiments and wrote the manuscript. B. Lee designed the studies and wrote the manuscript. D.H. Kim was involved with data analysis and critical analysis of the manuscript. H.Y. Chung designed the studies and revised the whole manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bicknell K.A. Forkhead (FOX) transcription factors and the cell cycle: measurement of DNA binding by FoxO and FoxM transcription factors. Methods Mol. Biol. 2005;296:247–262. doi: 10.1385/1-59259-857-9:247. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa-Hibi Y., Kobayashi Y., Chen C., Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxidants Redox Signal. 2005;7(5-6):752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 3.Gross D.N., van den Heuvel A.P., Birnbaum M.J. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27(16):2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Tang N., Hadden T.J., Rishi A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Barthel A., Schmoll D., Unterman T.G. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metabol. 2005;16(4):183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Kim D.H., Zhang T., Lee S., Dong H.H. FoxO6 in glucose metabolism (FoxO6) J. Diabetes. 2013;5(3):233–240. doi: 10.1111/1753-0407.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S., Dong H.H. FoxO integration of insulin signaling with glucose and lipid metabolism. J. Endocrinol. 2017;233(2):R67–R79. doi: 10.1530/JOE-17-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoekman M.F., Jacobs F.M., Smidt M.P., Burbach J.P. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr. Patterns. 2006;6(2):134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs F.M., van der Heide L.P., Wijchers P.J., Burbach J.P., Hoekman M.F., Smidt M.P. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J. Biol. Chem. 2003;278(38):35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- 10.Kim D.H., Perdomo G., Zhang T., Slusher S., Lee S., Phillips B.E., Fan Y., Giannoukakis N., Gramignoli R., Strom S., Ringquist S., Dong H.H. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60(11):2763–2774. doi: 10.2337/db11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabuig-Navarro V., Yamauchi J., Lee S., Zhang T., Liu Y.Z., Sadlek K., Coudriet G.M., Piganelli J.D., Jiang C.L., Miller R., Lowe M., Harashima H., Dong H.H. Forkhead box O6 (FoxO6) depletion attenuates hepatic gluconeogenesis and protects against fat-induced glucose disorder in mice. J. Biol. Chem. 2015;290(25):15581–15594. doi: 10.1074/jbc.M115.650994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D.H., Zhang T., Lee S., Calabuig-Navarro V., Yamauchi J., Piccirillo A., Fan Y., Uppala R., Goetzman E., Dong H.H. FoxO6 integrates insulin signaling with MTP for regulating VLDL production in the liver. Endocrinology. 2014;155(4):1255–1267. doi: 10.1210/en.2013-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D.H., Park M.H., Chung K.W., Kim M.J., Park D., Lee B., Lee E.K., Choi Y.J., Kim N.D., Yu B.P., Chung H.Y. Suppression of FoxO6 by lipopolysaccharide in aged rat liver. Oncotarget. 2015;6(33):34143–34157. doi: 10.18632/oncotarget.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee B., Moon K.M., Kim S.J., Kim S.H., Kim D.H., An H.J., Jeong J.W., Kim Y.R., Son S., Kim M.J., Chung K.W., Lee E.K., Chun P., Ha Y.M., Kim M.S., Mo S.H., Moon H.R., Chung H.Y. (Z)-5-(2,4-Dihydroxybenzylidene)thiazolidine-2,4-dione prevents UVB-induced melanogenesis and wrinkle formation through suppressing oxidative stress in HRM-2 hairless mice. Oxid Med Cell Longev. 2016;2016:2761463. doi: 10.1155/2016/2761463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B., Moon K.M., Son S., Yun H.Y., Han Y.K., Ha Y.M., Kim D.H., Chung K.W., Lee E.K., An H.J., Ullah S., Chun P., Moon H.R., Chung H.Y. Dihy(2R/S,4R)-2-(2,4-droxyphenyl)thiazolidine-4-carboxylic acid prevents UV-induced wrinkle formation through inhibiting NF-kappaB-mediated inflammation. J. Dermatol. Sci. 2015;79(3):313–316. doi: 10.1016/j.jdermsci.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Briganti S., Camera E., Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigm. Cell Res. 2003;16(2):101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 17.Kang K.H., Lee B., Son S., Yun H.Y., Moon K.M., Jeong H.O., Kim D.H., Lee E.K., Choi Y.J., Kim D.H., Chun P., Moon H.R., Chung H.Y. (Z)-2-(Benzo[d]thiazol-2-ylamino)-5-(substituted benzylidene)thiazol-4(5H)-one Derivatives as Novel Tyrosinase Inhibitors. Biol. Pharm. Bull. 2015;38(8):1227–1233. doi: 10.1248/bpb.b15-00300. [DOI] [PubMed] [Google Scholar]
- 18.Kim J., Choi H., Cho E.G., Lee T.R. FoxO3a is an antimelanogenic factor that mediates antioxidant-induced depigmentation. J. Invest. Dermatol. 2014;134(5):1378–1388. doi: 10.1038/jid.2013.510. [DOI] [PubMed] [Google Scholar]
- 19.Lee B., Moon K.M., Lee B.S., Yang J.H., Park K.I., Cho W.K., Ma J.Y. Swertiajaponin inhibits skin pigmentation by dual mechanisms to suppress tyrosinase. Oncotarget. 2017;8(56):95530–95541. doi: 10.18632/oncotarget.20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee B., Moon K.M., Lim J.S., Park Y., Kim D.H., Son S., Jeong H.O., Kim D.H., Lee E.K., Chung K.W., An H.J., Chun P., Seo A.Y., Yang J.H., Lee B.S., Ma J.Y., Cho W.K., Moon H.R., Chung H.Y. 2-(3, 4-dihydroxybenzylidene)malononitrile as a novel anti-melanogenic compound. Oncotarget. 2017;8(53):91481–91493. doi: 10.18632/oncotarget.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poljsak B., Dahmane R.G., Godic A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol. Alpina Pannonica Adriatica. 2012;21(2):33–36. [PubMed] [Google Scholar]
- 22.Rinnerthaler M., Bischof J., Streubel M.K., Trost A., Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boccitto M., Kalb R.G. Regulation of Foxo-dependent transcription by post-translational modifications. Curr. Drug Targets. 2011;12(9):1303–1310. doi: 10.2174/138945011796150316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qinyu L., Long C., Zhen-dong D., Min-min S., Wei-ze W., Wei-ping Y., Cheng-hong P. FOXO6 promotes gastric cancer cell tumorigenicity via upregulation of C-myc. FEBS Lett. 2013;587(14):2105–2111. doi: 10.1016/j.febslet.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Wang J.H., Tang H.S., Li X.S., Zhang X.L., Yang X.Z., Zeng L.S., Ruan Q., Huang Y.H., Liu G.J., Wang J., Cui S.Z. Elevated FOXO6 expression correlates with progression and prognosis in gastric cancer. Oncotarget. 2017;8(19):31682–31691. doi: 10.18632/oncotarget.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D.H., Park M.H., Chung K.W., Kim M.J., Jung Y.R., Bae H.R., Jang E.J., Lee J.S., Im D.S., Yu B.P., Chung H.Y. The essential role of FoxO6 phosphorylation in aging and calorie restriction. Age. 2014;36(4):9679. doi: 10.1007/s11357-014-9679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi Y.J., Moon K.M., Chung K.W., Jeong J.W., Park D., Kim D.H., Yu B.P., Chung H.Y. The underlying mechanism of proinflammatory NF-kappaB activation by the mTORC2/Akt/IKKalpha pathway during skin aging. Oncotarget. 2016;7(33):52685–52694. doi: 10.18632/oncotarget.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan Y.S., Wang Z.Q., Shao Y., Voorhees J.J., Fisher G.J. Ultraviolet irradiation activates PI 3-kinase/AKT survival pathway via EGF receptors in human skin in vivo. Int. J. Oncol. 2001;18(3):461–466. doi: 10.3892/ijo.18.3.461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.