Abstract
Pulmonary hypertension (PH) encompasses a syndrome of diseases that are characterized by elevated pulmonary artery pressure and pulmonary vascular remodeling and that frequently lead to right ventricular (RV) failure and death. Several types of PH exhibit sexually dimorphic features in disease penetrance, presentation, and progression. Most sexually dimorphic features in PH have been described in pulmonary arterial hypertension (PAH), a devastating and progressive pulmonary vasculopathy with a 3-year survival rate <60%. While patient registries show that women are more susceptible to development of PAH, female PAH patients display better RV function and increased survival compared to their male counterparts, a phenomenon referred to as the “estrogen paradox” or “estrogen puzzle” of PAH. Recent advances in the field have demonstrated that multiple sex hormones, receptors, and metabolites play a role in the estrogen puzzle and that the effects of hormone signaling may be time and compartment specific. While the underlying physiological mechanisms are complex, unraveling the estrogen puzzle may reveal novel therapeutic strategies to treat and reverse the effects of PAH/PH. In this article, we (i) review PH classification and pathophysiology; (ii) discuss sex/gender differences observed in patients and animal models; (iii) review sex hormone synthesis and metabolism; (iv) review in detail the scientific literature of sex hormone signaling in PAH/PH, particularly estrogen-, testosterone-, progesterone-, and dehydroepiandrosterone (DHEA)-mediated effects in the pulmonary vasculature and RV; (v) discuss hormone-independent variables contributing to sexually dimorphic disease presentation; and (vi) identify knowledge gaps and pathways forward.
Introduction
Several cardiopulmonary diseases are characterized by sex and gender differences and have been the focus of comprehensive research efforts (145). However, few of these diseases have seen as much progress in understanding the biological basis of these differences as pulmonary hypertension (PH), a pulmonary vasculopathy resulting in elevated pulmonary artery (PA) pressures (376). PH is not a single disease but rather a syndrome that encompasses a heterogeneous group of acute and chronic diseases of different origins and etiologies that share the common feature of mean pulmonary artery pressure (mPAP) higher than 20 to 25 mmHg (377). The current PH classification guidelines differentiate five major groups that differ in their etiologies and phenotypes (Figure 1) (377). If left untreated, PH of any etiology can lead to right ventricular (RV) failure and death. The majority of sex and gender differences in PH have been described in pulmonary arterial hypertension (PAH; Group 1 PH), a disease characterized by progressive pulmonary vascular remodeling resulting in severely increased pulmonary vascular resistance (PVR) and a high likelihood of RV failure and death (326, 429, 430). Sexually dimorphic features have also been described in other types of PH but are typically not as prevalent or pronounced as in PAH.
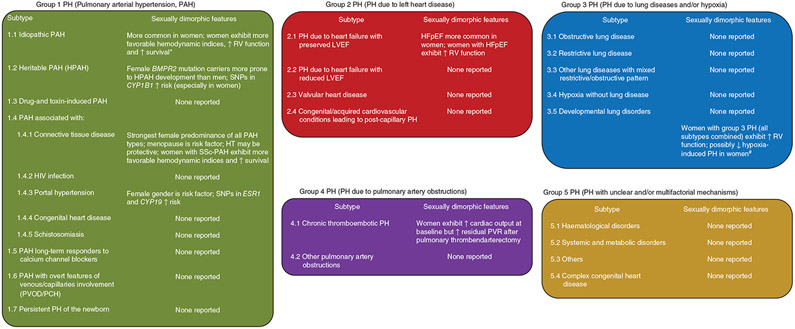
Figure 1. Current classification of pulmonary hypertension (PH) and subtypes with evidence for sexually dimorphic features.
PH classification from 6th World Symposium (Nice, 2018) according to Simonneau et al. (351). In addition to the data presented here, one study in a large cohort of veterans with all types of PH (predominantly Group 2 and 3 PH; n = 15,464 patients) demonstrated that women with PH exhibit higher pulmonary vascular resistance and pulmonary artery pulse pressure, yet lower RAP as well as 18% greater survival compared to men with PH. *These analyses predominantly included patients with idiopathic PAH and also patients with heritable PAH and drug- and toxin-associated PAH (no subgroup analyses performed). #Attenuated hypoxia-induced PH in women not consistently found across studies. BMPR2, gene encoding bone morphogenic protein receptor 2; CYP1B1, gene encoding cytochrome P450 1B1; CYP19A1, gene encoding aromatase; ESR1, gene encoding estrogen receptor α; HFpEF, heart failure with preserved ejection fraction; HIV, human immunodeficiency virus; HT, hormone therapy; LVEF, left ventricular ejection fraction; PCH, pulmonary capillary hemangiomatosis; PVOD, pulmonary veno-occlusive disease; PVR, pulmonary vascular resistance; RV, right ventricle; SNP, single-nucleotide polymorphism; SSc, systemic sclerosis.
Sexual dimorphism in PAH exists in disease prevalence, severity of hemodynamic alterations, RV adaptation, treatment responses, and, importantly, survival. In particular, women are more prone to develop PAH, but exhibit a more favorable hemodynamic profile, better RV function, a better response to treatment with endothelin receptor antagonists (ERAs), and better survival. Men, on the other hand, are less prone to develop PAH and are more likely to respond to treatment with phosphodiesterase type 5 inhibitors but are more likely to die from this disease. More favorable hemodynamic profiles and higher survival rates have also been described in women with non-PAH types of PH; however, data from these cohorts is less abundant than for PAH.
This article comprehensively reviews the rapidly expanding biological and epidemiological knowledge regarding sex and gender differences in PAH and PH. We review the role of sex hormones, their metabolites and their receptors, and the role of nonhormonal factors in the pulmonary vasculature and RV in health and disease. We discuss cell culture systems, animal studies, and studies in humans. Knowledge gaps will be identified, and pathways forward will be proposed.
We use the term “PH” when discussing PH in general and the term “PAH” when specifically referring to this disease. By convention, elevated PA pressure in animal models is referred to as “PH,” while “PAH” is reserved for the human condition. According to the definitions published by the Institute of Medicine and embraced by the APS Journals (279, 468), we use the term “sex” when biologic concepts are described, but use the term “gender” when cultural or behavioral influences may play a role (e.g., in human studies).
A list of commonly used abbreviations is provided in the Abbreviations and Acronyms Section.
Overview of PH Classifications and Pathophysiology
PH Classification and Definitions
PH has traditionally been defined as an mPAP > 25 mmHg with a classification scheme divided into five groups based on the predominant underlying pathology and clinical phenotype (Figure 1). These groups encompass Group 1 (PAH), Group 2 (PH due to left heart disease), Group 3 (PH due to lung disease and/or hypoxia), Group (4 PH due to PA obstructions such as chronic thromboembolic pulmonary hypertension [CTEPH]), and Group 5 (PH due to unclear or multifactorial mechanisms) (377). A detailed discussion of all five PH groups is beyond the scope of this article; the most up-to-date classification from the Proceedings of the 6th World Symposium on Pulmonary Hypertension in Nice is presented by Simonneau et al. (377).
Most recently, the hemodynamic definition of PH was changed to an mPAP cut-off of >20 mmHg (377), two standard deviations above the upper limit of normal for the pulmonary circulation, although this remains controversial. Regardless of the threshold used for mPAP, the various PH phenotypes can also be classified based on the localization of the pathology in the pulmonary vascular compartment. Precapillary PH is characterized by (i) an elevated mPAP, (ii) a pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg, and (iii) a PVR ≥ 3 Wood units. Precapillary PH occurs in Groups 1, 2, 3, and in some cases of Group 5 PH (377). Postcapillary PH, on the other hand, is characterized by an elevation of both mPAP (to >20 or 25 mmHg) and PAWP (≥ 15 mmHg). This may occur in isolation (without an elevation in PVR to >3 Wood units) or combined with precapillary PH such that mPAP, PAWP, and PVR are increased. Both isolated and combined postcapillary PH occur in Group 2 PH and in some forms of Group 5 PH.
Pathophysiology of PAH and PH
The pathophysiology of PAH and PH has been reviewed in detail elsewhere (166, 326). An overview is provided in Figure 2. Briefly, PH occurs as a consequence of lesions in the arterial, capillary, or venous compartment of the pulmonary vasculature. In certain subtypes and associated conditions (e.g., pulmonary veno-occlusive disease (PVOD), drug- and toxin- and connective tissue disease-associated PAH, and CTEPH), a spectrum of lesions may occur that span more than one compartment. PH can also occur in a fairly normal pulmonary vasculature as a consequence of venous congestion due to left heart disease or increased pulmonary blood flow in the setting of hypervolemia or hyperdynamic states.
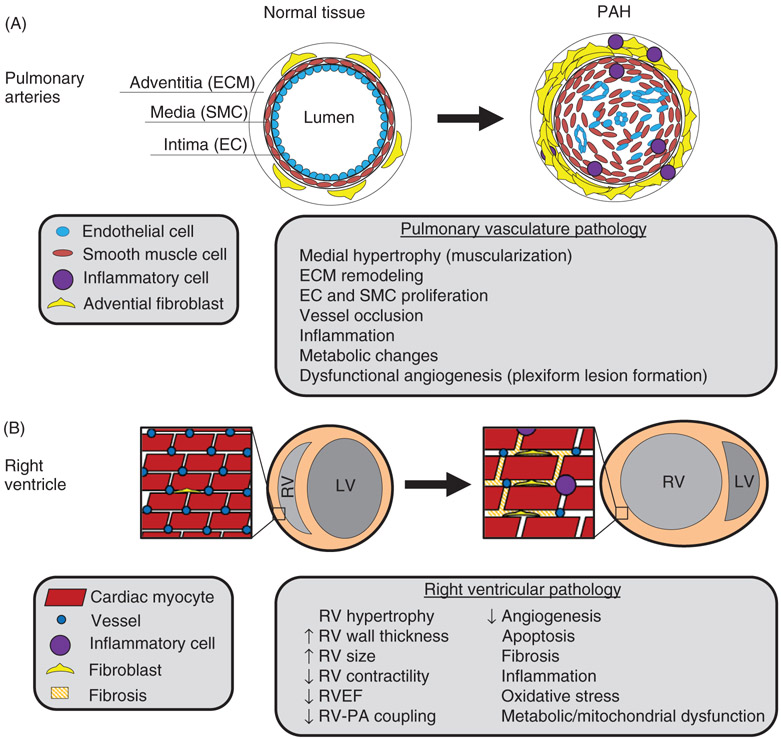
Figure 2. Pathophysiology of PAH.
(A) Arterial cross section illustrating PAH pathology in the pulmonary arteries. Proliferation of endothelial cells (ECs), smooth muscle cells (SMCs), and fibroblasts leads to vascular remodeling with eventual occlusion of diseased vessels. Neoangiogenesis driven by apoptosis-resistant proliferative ECs, SMCs, and other resident PA and recruited cells promotes formation of plexiform vascular lesions, which are the hallmark of PAH. Plexiform lesions may be seen within pulmonary vessels as well as extending into the adventitial tissue (not shown). Infiltration of PH vascular lesions by immune cells and bone marrow-derived cells drives a pro-inflammatory and pro-proliferative state in the tissue. (B) Transverse section of the heart. High pulmonary vascular resistance in PAH produces increased afterload on the RV, resulting in adaptation and RV failure in PAH. RV hypertrophy may be adaptive and compensatory to overcome PVR and maintain cardiac output (not shown). On the other hand, RV hypertrophy may be maladaptive, marked by vessel rarefaction, metabolic dysfunction, inflammation, cell death, fibrosis, and increased RV dilatation. Maladaptive RV remodeling is associated with RV ischemia and decreased RV ejection fraction and cardiac output, resulting in RV failure.
A tremendous amount of progress has been made in our understanding of PAH pathobiology (166, 326). Over the past several decades, discoveries that endothelial dysfunction and vascular remodeling occur in PAH from dysregulation of nitric oxide (NO), endothelin-1 (ET-1), and prostacyclin pathways informed drug development and led to the approval of numerous pulmonary vasodilators (169). We now understand PAH to be an even more complex and systemic disease. Numerous cells in and around the vascular compartment, such as endothelial cells (ECs), smooth muscle cells (SMCs), adventitial fibroblasts, and inflammatory cells contribute to disease pathogenesis and are influenced by the immune and hematopoietic systems as well as abnormalities in cellular energetics and metabolism. The current paradigm is that PAH occurs as a consequence of a single or repetitive pulmonary vascular injury mediated by increased pulmonary blood flow, shear stress, inflammatory processes, excessive vasoconstrictor stimuli, and/or EC damage. While not all individuals with a pulmonary vascular injury develop PAH, disease development is more likely to occur in the setting of genetic predisposition (e.g., mutations in bone morphogenetic protein receptor 2 [BMPR2]), previous vascular injury (e.g., premature birth and environmental exposures), and/or coexposures (e.g., hormonal and metabolic abnormalities, and substance abuse in HIV infection) (166, 291). Epigenetic changes may further modify the disease course; these multiple potential “hits” to the pulmonary vasculature are being targeted for potential intervention. Deep phenotyping efforts are also underway to understand common molecular mechanisms that may underpin and influence the severity of various forms of PH across all five PH groups and provide targets for precision-based medicine (152).
Right Ventricular (RV) Adaptation in PH
RV failure is an important cause of morbidity and mortality in PAH as well as Group 2 and 3 PH from highly prevalent chronic heart and lung diseases. An estimated 70 million individuals in the United States may have right heart dysfunction (171, 239, 274, 282, 292, 334, 434), yet there are no well-established biologic or clinical determinants of RV structure and function and no approved treatments for right heart failure. Unlike the left ventricle (LV), the thin-walled, compliant RV has difficulty accommodating increases in resistance such that even incremental increases or fluctuations in afterload over time may lead to RV sequelae (448, 449). There is, however, great variability in the clinical trajectory of patients, and they often present at later stages of disease, when RV dysfunction has already occurred. While RV failure is the proximate cause of death in PAH, mechanisms of RV adaptation (and maladaptation) have garnered much interest but remain understudied (212).
Current knowledge of the pathophysiology of RV failure has been discussed in detail elsewhere (346, 447, 449) and is beyond the scope of this article. A brief overview is presented here and in Figure 2. Initially, as RV afterload increases during PH development, the RV employs compensatory mechanisms that include structural changes, neurohormonal activation, and increased contractility (346, 449). At the cellular level, these changes are accompanied by increased angiogenesis, changes in mitochondrial function and substrate utilization, increased production of reactive oxygen species, changes in myosin isoform expression, and changes in sarcomere organization and structure (346). It is thought that these changes allow for a state of adaptive (or compensated) RV hypertrophy, characterized by a cardiac output that is still sufficient to meet the metabolic demands of the body (448, 449). However, with ongoing increases in RV afterload, the RV’s compensatory mechanisms will eventually be exhausted and cause a transition to a maladaptive (or decompensated) form of RV hypertrophy (448,449). Consequently, RV failure with decreased cardiac output and decreased oxygen delivery occurs. At a cellular and molecular level, maladaptive RV hypertrophy purportedly is characterized by ischemia, impaired or insufficient angiogenesis, inflammation, oxidative stress, metabolic dysfunction, and impaired calcium handling, all associated with myocardial fibrosis and cell death (34, 447, 449). The individual contribution of each of these processes may vary from patient to patient and exhibit marked temporal and spatial variations (212).
A brief overview of PAH/PH epidemiology and subtypes, with a focus on those subgroups with a known gender bias, as well as a review of gender differences in RV adaptation across all forms of pulmonary vascular disease follows.
Overview of Gender Differences in PAH and PH
Gender Bias in PAH Epidemiology
The earliest modern description of idiopathic PAH by Dresdale et al. (80) in 1951 included three young women. The first prospective multicenter registry from the National Institutes of Health (NIH), which included patients with idiopathic, heritable PAH and PAH associated with anorexigen use, reported a mean age of 36 ± 15 years and a ratio of women:men of 1.7:1. Before the advent of targeted PAH therapy, 1-, 3-, and 5-year survival for this cohort was 68%, 48%, and 34%, respectively, with an estimated median survival of 2.8 years (69). This early description of then “primary pulmonary hypertension,” a rare disease affecting young women of child-bearing age, has evolved in recent years.
The prevalence of Group 1 PAH is estimated between 15 cases/million (5.9 cases/million for idiopathic PAH) with an incidence of 1.1 to 3.7 cases/million/year (96, 168, 231, 318). Multiple registries have captured survival in both the pre- and posttreatment era (28, 69, 96, 98, 167, 177, 185, 231, 307, 412, 487). Short-term survival has improved over time and is approximately 90% at 1 year and 75% at 3 years. Longer term survival remains poor, however, with registries survival rates between 21% and 75% at 5 years.
While a gender bias similar to that reported in the NIH registry has been noted in recent registries throughout the world (60, 96, 98, 158, 168, 177, 224, 231, 268, 318, 412, 450), others have described a more marked predominance among women. In modern registries including various Group 1 etiologies, as many as 70% of participants are women, and the average age of all participants is older (5th decade of life) (168, 185, 411, 412). A large European registry, which enrolled patients from 2007 to 2011 (The Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension [COMPERA]), demonstrated a ratio of 1.8 women:1 men that was most pronounced among younger patients (158). The largest US-based registry, the Registry to Evaluate Early And Long-term pulmonary arterial hypertension disease management (REVEAL), began enrolling patients in 2006 and reported among idiopathic PAH patients an average age of 53 ± 15 years, 80% of whom were women (18, 262). Whether these observations signal a true change in disease biology or a significant survival bias among women because of a predominantly prevalent (vs incident) study population is not known. In China, where targeted PAH therapies have only recently become available, the earliest registry of incident (i.e., untreated) patients included 71% women (a ratio of 2.4 women:1 men), similar to the US NIH registry (177, 338). In a follow-up Chinese study from a treated/prevalent cohort, 76% of participants were women, and there were 3.1 times as many women enrolled as men (487). Table 1 summarizes the gender biases of modern population-based registries for Group 1 PH.
Table 1.
Gender ratios in major PH registries across the world.
| Registry | References | Enrollment period |
P(A)H types included |
Patient age (years; mean unless indicated otherwise) |
Number of patients included |
Ratio, Women:men |
|---|---|---|---|---|---|---|
| Latvian | (380) | 2007–2016 | IPAH, APAH, drug-induced PAH, CTEPH | PAH: 65 (median) CTEPH: 67 (median) | PAH: 130 CTEPH: 44 | PAH: 2.7:1 CTEPH: 1.6:1 |
| Swedish (SPHAR) | (329) | 2008–2014 | IPAH, HPAH, APAH, CTEPH |
PAH: 67 (median) CTEPH: 70 (median) | PAH: 457 CTEPH: 183 | PAH: 1.8:1 CTEPH: 1:1 |
| European (COMPERA) | (307) | 2007–2013 | IPAH, HPAH, APAH | 68 (median) | 1283 | 1.8:1 |
| International CTEPH Registry | (319) | 2007–2009 | CTEPH | 63 (median) | 679 | 1:1 |
| UK/Ireland | (231) | 2001–2009 | IPAH, HPAH, anorexigen-induced PAH |
50 | 493 | 1.4:1 |
| Spanish | (96) | 2007–2008 | IPAH, APAH, TOS-PAH, PVOD, CTEPH |
PAH: 45 CTEPH: 61 | PAH: 866 CTEPH: 162 | PAH: 2.4:1 CTEPH: 1.5:1 |
| REVEAL | (28) | 2006–2007 | IPAH, HPAH, APAH, anorexigen-induced PAH |
53 | 2525 | 4.1:1 IPAH 3.8:1 APAH |
| Chinese | (177) | 1999–2004 | IPAH, HPAH | 36 | 72 | 2.4:1 |
| French | (170) | 2002–2003 | IPAH, HPAH, APAH, anorexigen-induced PAH |
50 | 674 | 1.9:1 |
| Scottish | (318) | 1986–2001 | IPAH, APAH | 51 | 374 | 2.3:1 |
| NIH | (338) | 1981–1985 | “Primary PH” (IPAH and HPAH) | 36 | 187 | 1.7:1 |
APAH, associated pulmonary arterial hypertension; CTEPH, chronic thromboembolic pulmonary hypertension; HPAH, hereditary pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; NIH, National Institutes of Health; PVOD, pulmonary veno-occlusive disease; TOS-PAH, toxic oil syndrome-induced pulmonary arterial hypertension.
While female gender has long been established as the major clinical risk factor for PAH, in 2010, both the French (167) and US REVEAL (28) registries published similar findings and found male gender to double the risk of death in PAH. This risk was shown to be independent of established measures of disease such as the six-minute walk distance (6MWD) and cardiac index (CI) (28, 167, 170). Follow-up data from REVEAL continued to demonstrate significant differences in outcome based on gender irrespective of whether the PAH diagnosis was incident or prevalent, such that 5-year survival estimates for newly diagnosed (incident) men were 53% ± 4% versus 63% ± 2% for women and 57% ± 2% versus 68% ± 1% for previously diagnosed (prevalent) men versus women (98). Interestingly, this survival benefit occurs despite more profound vascular remodeling and more plexiform lesions in PAH women (390), a constellation suggestive of better adaptation to vascular remodeling in women (reviewed in more detail below). Table 2 provides a summary of gender differences in survival, hemodynamic alterations, and treatment responses in PAH.
Table 2.
Major findings from studies investigating gender differences, sex hormones, or sex hormone pathway components in human PAH.
| Study finding | References |
|---|---|
| General findings | |
| ↑ Susceptibility to PAH development in women | See Table 1 |
| ↑ Pulmonary vascular remodeling in female PAH patients | (390) |
| ↑ Survival in female PAH patients | (28, 167, 170, 175, 307, 365) |
| ↓ Hemodynamic alterations in female PAH patients (↓ RAP, ↓ mPAP, ↑ CI) | (365, 445) |
| ↑ RVEF in female PAH patients | (175, 192,405) |
| ↑ Improvement in RVEF in females after initiation of PAH treatment responsible for survival advantage in females | (175) |
| ↑ Response to treatment with endothelin receptor antagonists or intravenous prostacyclins in female PAH patients | (108, 118) |
| ↑ Response to treatment with tadalafil in male PAH patients | (258) |
| Menopause is risk factor for scleroderma-associated PAH (SSc-PAH); HT attenuates SSc-PAH | (30, 363) |
| Findings specific to sex hormone signaling | |
| Altered estrogen metabolism ↑ penetrance of hereditary PAH; ↓ urine 2-hydroxyestradiol/16α-hydroxyestrone ratios in patients with hereditary PAH vs. unaffected BMPR2 mutation carriers |
(12) |
| ↑ ESR1 mRNA expression in PAH patients | (331, 391) |
| SNPs in ESR1 and CYP19A1 associated with ↑ risk for development of portopulmonary hypertension; SNPs in CYP19A1 associated with ↑ E2 plasma levels |
(340) |
| E2 plasma levels correlate negatively with 6MWD and functional class in male and female PAH patients | (19, 441) |
| ↑ E2 and E2/testosterone ratio and ↓ testosterone and γ progesterone associated with ↑ risk of PAH in males; ↑ E2 associated with ↑ mortality in male patients | (471) |
| Genetic variations in E2 metabolism and androgen signaling associated with RV morphology in a gender-specific manner (MESA-RV, healthy cohort) | (443) |
| Aromatase inhibition safe and ↑ 6MWD in postmenopausal and male PAH patients (small proof-of-concept study) | (193) |
| Lower DHEA-S levels in men and postmenopausal women with PAH compared to matched controls; lower DHEA-S associated with more severe PAH, RV dysfunction | (19, 441) |
| Lower DHEA-S and metabolites associated with poor survival | (19) |
| Open-label DHEA treatment in small (n = 8) study of COPD-PH improved 6MWD and hemodynamics | (90) |
The upper section lists general findings; the lower section lists findings related to the study of specific sex hormones. BMPR2, bone morphogenetic protein receptor 2 gene; CI, cardiac index; CYP19A1, aromatase gene; ESR1, estrogen receptor α gene; HT, hormone therapy; mPAP, mean pulmonary artery pressure; RAP, right atrial pressure; RVEF, right ventricular ejection fraction; SNP, single-nucleotide polymorphism; 6MWD, six-minute walking distance.
Age may be an important modifier of the relationship between gender and outcomes in PAH (28, 96, 167, 171, 307, 445), which suggests that temporal changes in the hormonal milieu may impact disease risk and severity throughout the lifespan. Among older patients, gender-based differences in PAH prevalence appear to be diminished (158, 231, 445). In a large study (n = 1211) of patient-level pooled data from 11 clinical trials in PAH, women with idiopathic PAH and connective tissue disease (CTD)-associated PAH had more favorable hemodynamic indices (lower right atrial pressure [RAP], lower PVR, and higher CI) as compared to men with idiopathic and CTD-associated PAH (445). Younger men had higher mPAP than younger women, but this difference was attenuated after age 45 years. In both men and women with idiopathic PAH, hemodynamic burden, including mPAP, tended to decrease to similar levels with age, such that a gender difference in mPAP was only seen in patients <45 years old. Similar observations have been made in the COMPERA registry, which demonstrated a strong gender bias toward women among younger patients with PAH that dissipated after age 65 (158), and in the REVEAL registry, men had higher RAP and mPAP at diagnosis (as well as worse survival especially in those older than 60 years of age) (28, 365). These observations have not been consistent across all registries, and further work is needed to refine the sex-age interaction in pulmonary vascular disease (231,487).
Race/ethnicity may also modify the relationship between sex and PAH. In the NIH registry, the gender ratio was even more skewed toward women (4.3:1) among African Americans (69). This observation was also made in the United Kingdom and Ireland, where 85% of nonwhite patients were women (as compared to 70% women in white patients) and in the United States, where the ratio of women:men was 5.4:1 in African Americans from the REVEAL registry (18, 231).
Gender Bias in PAH Subtypes
Mutations in BMPR2, a gene encoding a member of the transforming growth factor (TGF)-β family, are present in 70% to 80% of families with PAH and roughly 25% to 30% of patients with idiopathic PAH (73, 291, 388). These mutations are transmitted in an autosomal dominant fashion with incomplete penetrance. Female mutation carriers are more than twice as likely to be affected with PAH as carrier men (31); in a large cohort of individuals with BMPR2 mutations, roughly 70% of the population were women (97). Cross talk between BMPR2 pathways and estrogen signaling has been a major area of study as reviewed below. Rarer mutations in activin-like receptor kinase-1 (ALK-1), endoglin (ENG), mothers against decapentaplegic homologue (SMADs), caveolin 1 (CAV1), and the potassium channel subfamily K member 3 (KCNK3) genes have also been identified (15, 245, 302, 372). Recent studies identified that rare variants in ATP13A3, AQP1, and SOX17 and common genetic variations at loci in an enhancer near SOX17 and in HLA-DPA1/DPB1 are associated with PAH (129, 336). Biallelic mutations in EIF2AK4 have been linked to pulmonary capillary hemangiomatosis (PCH) and PVOD, very rare forms of PAH (31). Mutations in TBX4 are associated with childhood-onset PAH (198). The penetrance of PAH in these rare mutations is not known to vary by gender.
A number of systemic diseases are associated with the development of pulmonary vasculopathy, although the mechanisms by which PAH develops in these varied conditions are poorly understood. In some of these subgroups, female gender has been described as a risk factor for the development of PAH, including CTD-associated PAH and portopulmonary hypertension. Approximately 12% of systemic sclerosis (SSc) patients develop PAH, and it is a major cause of death (137, 295, 392). Additional CTDs such as systemic lupus erythematosus, mixed CTD, and rheumatoid arthritis are also associated with PAH. While the true prevalence of PAH in these conditions is unknown, PAH occurs less commonly than in SSc and is associated with better outcomes than when associated with SSc (60, 64). After idiopathic PAH, CTD-PAH patients are the second most represented subgroup in registry studies (18, 96, 168, 318). CTD-PAH patients tend to be older, have less hemodynamic impairment, and are more likely to have mixed phenotype PH from concurrent interstitial lung disease, pulmonary venous involvement, and left heart disease (18, 487). As in idiopathic PAH, female sex is arisk factor in CTD-PAH (18,487). CTD itself occurs more commonly in women than in men, and when associated with PAH patients are 3.8 to 10 times more likely to be women (18, 60,168,185,487). Women with SSc are eight times more likely than men with SSc to be affected by PAH (59). While scleroderma-associated PAH is more common in women, it is interesting to note that PAH in scleroderma patients frequently does not occur until after menopause (30, 363). In patients with systemic lupus erythematosus, women are 17 times more likely to be affected with PAH than men (60). Chung et al. demonstrated an almost fourfold increase in the risk of death (hazard ratio 3.9, 95% CI 1.1–13.9, p = 0.03) among men as compared to women with PAH associated with SSc (58), however.
The presence of portal hypertension without other clinical risk factors or associated conditions in a patient with PAH is designated as portopulmonary hypertension. This condition occurs in roughly 3% to 6% of patients with cirrhosis referred for liver transplantation (110). The French Registry reported that 40% of patients with portopulmonary hypertension were women (168). While the degree of cirrhosis does not influence the risk of portopulmonary hypertension, female gender and autoimmune hepatitis are independent risk factors for the development of PAH in these patients (63, 138, 195). Female gender as a risk factor for portopulmonary hypertension has been confirmed in several registry studies (168, 195, 205). Although the pathobiology of portopulmonary hypertension has not been entirely elucidated, abnormalities in sex hormone signaling have been implicated in disease development, as discussed below (303, 340). Survival is generally poorer in portopulmonary hypertension than in idiopathic PAH (205).
Additional PAH subtypes and their associated conditions do not appear to have a strong gender bias. These include drug- and toxin-induced PAH, human immunodeficiency virus (HIV) infection-associated PAH, congenital heart disease (CHD)-associated PAH, and schistosomiasis-induced PAH. This may be because hormonal factors do not play a pathobiologic role in all forms of PAH or because these conditions are less well studied. Certain drugs and toxins have been implicated in the development of PAH, some as “definite” causes of PAH and others as “possible” (377). The most classic example of drug-induced PAH are the anorexigens such as fenfluramine (1, 40, 79, 255, 339, 357, 450). Although tyrosine kinase inhibitors have been studied to treat PAH, the use of dasatinib for chronic myelogenous leukemia has been associated with the development of PAH (285, 333, 353). Methamphetamine was recently reclassified as a “definite” cause of PAH; in one study, patients with methamphetamine-associated PAH were less likely to be women, had more severe disease, and worse outcomes as compared to patients with idiopathic PAH (486). Treatment with interferon has also been identified as a possible risk factor for PAH (44, 74, 111, 178, 352, 358, 376).
The prevalence of pulmonary vascular disease in HIV-infected patients is approximately 0.5% (308, 379, 389), which has not decreased in frequency despite the advent of antiretroviral therapy (379). Disease characteristics are similar to idiopathic PAH patients, although gender does not appear to be a risk factor for the development of PAH in HIV (168, 321). Pulmonary vascular disease has been reported in 4% to 34% of adults with CHD (87, 93, 242). Modern PAH registries have reported that CHD-associated PAH makes up 11% to 24% of Group 1 PAH patients; this does not appear to vary by gender (18, 26, 96, 168, 231). In recent years, consensus guidelines have included a more detailed subclassification of pulmonary vascular disease associated with CHD, which distinguishes between precapillary/Group 1 PAH and Group 2 PH due to congenital/acquired cardiovascular conditions leading to postcapillary PH (376, 377). Chronic schistosomiasis is likely the most common cause of PAH worldwide given the widespread prevalence of schistosomiasis mansoni infection but is incompletely understood. While direct pulmonary vascular exposure to egg antigens does not appear to cause schistosomiasis-PAH, many of the mechanistic pathways implicated in idiopathic PAH (e.g., TGF-β and inflammatory cytokines) have been implicated in the development of Schistosoma-related pulmonary vascular injury (130-132). A gender bias in Schistosoma-associated PAH has not been described.
Gender Bias in RV Function in PAH
Women have better RV systolic function in both health and PH, including Group 1 (PAH), Group 2 (left heart disease), and Group 3 (chronic lung disease/hypoxia) (192, 196, 269, 323, 444). The Multi-Ethnic Study of Atherosclerosis (MESA)-RV is the largest population-based, cardiovascular disease-free cohort with available RV indices measured via cardiac magnetic resonance imaging (MRI), the gold standard for RV assessment. Female gender was associated with higher right ventricular ejection fraction (RVEF), lower RV mass, and smaller RV volumes after adjustment for multiple comorbid factors and body size in MESA-RV (194, 196). These gender-based differences in RVEF and volumes were replicated in The Framingham Heart Study Offspring cohort (106). Both the MESA and Framingham studies showed age to be an important modifier of the relationships between gender and measures of RV morphology (106, 194, 196).
Until recently, the RV had not been robustly studied in PAH. This is important, since changes in RV structure and function with PAH therapies are more strongly tied to survival in PAH than changes in PVR (434-436). Kawut et al. demonstrated that male gender is associated with lower RVEF measured by radionuclide angiography in a single-center cohort of subjects with PAH (192). This finding has since been corroborated by several other investigators (175, 405). Among a Dutch cohort of 101 patients with idiopathic PAH, heritable PAH, or anorexigen associated PAH, men and women had similar reductions in PVR, but RVEF declined in men and improved in women over time with PAH therapies (175). A significant proportion (39%) of the transplant-free survival difference seen between men and women was explained by treatment-related improvements in RVEF. This suggests that the survival bias conferred by female gender in PAH may be explained at least in part by gender or sex hormone-mediated effects on the RV. These observations have led to increased interest in the study of sexual dimorphism in RV function and failure in PAH. Studies of gender differences in RV function in PAH are listed in Table 2.
Gender Bias in Treatment Responses in PAH
In addition to the observational studies reviewed above that demonstrate gender-based differences in PAH prevalence, RV function, and survival, differential responses to PAH-specific treatments have also been described. In a patient-level pooled analysis from six randomized placebo-controlled trials of ERAs submitted to the US Food and Drug Administration, Gabler et al. noted that women exhibited a better response in 6MWD to treatment with ERAs (118). Similarly, women were more likely to respond to treatment with prostacyclin analogues (108). On the other hand, a post-hoc analysis of subjects enrolled in a trial of the phosphodiesterase type 5 inhibitor tadalafil demonstrated that men were more likely to improve their 6MWD and quality of life after starting tadalafil treatment as compared to women (258).
Gender Bias in Non-PAH PH
Studies in non-PAH PH are sparse and in general less robust than those in PAH. No clear signal exists indicating that female gender is a risk factor for disease development in non-PAH PH. Heart failure with preserved ejection fraction (HFpEF) is frequently associated with PH and is more common in postmenopausal women (257, 359), but female gender is not a risk factor for HFpEF-PH per se. On the contrary, some studies exist suggesting that women exhibit less hypoxia-induced PH and less chronic mountain sickness than men; however, such effects are not consistently found across studies (39, 225, 490). Two retrospective studies identified male gender as a risk factor for the development of high-altitude pulmonary edema (HAPE), a disease characterized by exaggerated and uneven hypoxic pulmonary vasoconstriction (HPV) (165, 386). Similar to PAH, there is evidence that women with non-PAH PH demonstrate superior RV function and higher survival rates. A review of the Veterans Affairs Clinical Assessment Reporting and Tracking (CART) Program database demonstrated that in a large cohort (n = 15,464 patients) of veterans with all types of PH (but predominantly Group 2 and 3 PH), women exhibited higher PVR and PA pulse pressure, yet lower RAP (442). This constellation of findings is indicative of better RV adaptation despite higher RV afterload. Interestingly, women veterans with PH had 18% greater survival compared to men with PH. When the cohort was limited to veterans with precapillary PH, women with PH were 29% more likely to survive. Women are also less likely to develop RV dysfunction in the setting of HFpEF (269) and exhibit better RV function in the setting of chronic lung disease (323). No gender bias in prevalence has been demonstrated in CTEPH (319). In a Japanese cohort of CTEPH patients, women exhibited better cardiac output at baseline but higher residual PVR after pulmonary thrombendarterectomy (371).
Proposed Mechanisms of Gender Differences in Human PAH/PH
Taken together, the data reviewed above suggest profound gender differences in PAH and other types of PH (Tables 1 and 2). Female gender is one of the strongest risk factors for PAH development, but also a robust protective factor once the disease has been acquired. On the other hand, with the possible exception of PH from HFpEF, female gender does not appear to be a risk factor for non-PAH types of PH. In both PAH as well as non-PAH PH, female gender is associated with better RV adaptation, indicative of persistent gender-based phenotypes across various types of pulmonary vascular disease.
These findings could be due to direct effects of sex hormones on cardiopulmonary function, genetically determined factors, environmental or epigenetic influences, and/or cultural phenomena. Several lines of evidence in humans suggest that biologically relevant effects of sex hormones indeed play a role in mediating gender differences in the pulmonary vasculature and RV. First, there is a high prevalence of exposure to hormone therapy (HT) in women with PAH (404). Second, genetic alterations in estrogen-metabolizing enzymes and estrogen receptors (ERs) have been found in various forms of PAH (12, 331, 340). Third, 17β-estradiol (E2) plasma levels correlate positively with RV function in healthy postmenopausal HT users yet negatively with 6MWD and functional class in PAH patients (19, 441, 444), and lower dehydroepiandrosterone-sulfate (DHEA-S) levels correlate with worse hemodynamics, RV function, 6MWD, and functional class in PAH patients (19, 441). Of note, lower DHEA-S levels are also associated with lower survival (19, 337). Fourth, at least one study described an absence of hemodynamic differences between men and women with PAH once they are older than 45 years, suggesting that the menopausal transition (and other hormone-related life cycle changes like waning testosterone and/or DHEA) may modify disease risk (445). In addition, as mentioned above, menopause represents a risk factor for the development of PAH in scleroderma patients, while HT may attenuate the risk of PAH in these patients (30, 363). Lastly, genetic variations in 17β-estradiol metabolism and androgen signaling are associated with RV morphology in a gender-specific manner in cohort of subjects without clinical cardiovascular disease (MESA-RV) (443). The contributions of sex hormones in human PAH/PH will be reviewed in detail within this article. The roles of genetics, epigenetics, environmental exposures, and cultural factors have been much less well studied and represent a significant knowledge gap in the field.
The “Estrogen Puzzle” in PAH
The marked discrepancy between increased susceptibility to PAH among women on the one hand and better disease outcomes in women on the other hand has been described as the “estrogen paradox” and has been the topic of many editorials, reviews, and discussions at scientific meetings. A second “estrogen paradox” has been identified in the area of basic and cellular investigation. In particular, this refers to the finding that estrogens are protective against disease development in several animal models of PAH, but detrimental (disease promoting) in others. Lastly, some investigators refer to a paradox in the observation that estrogens have been uniformly shown to be cardioprotective in the RV, whereas in the pulmonary vasculature they may exert disease-promoting effects. Together, these inconsistencies have led to increased interest in the study of sexual dimorphism in PAH and RV failure. However, since the term “paradox” implies an observation or finding that is logically unacceptable or self-contradictory, we prefer the terms “estrogen puzzle” or “estrogen conundrum.” This is based on the rationale that we believe biological explanations exist for the observed sex/gender differences in PAH but have not yet been fully identified. For example, many “paradoxical” effects can be explained with dose-, timing-, or compartment-specific effects of estrogen. We would also argue that to focus solely on estrogen as the hormone of interest is too narrow a scope. While estrogens clearly have been implicated as clinically important disease modifiers in PAH, one should note that estrogen-independent factors, such as other sex hormones, sex chromosomes, genetics, and epidemiological factors, likely play significant roles as well.
Sex Differences in Animal Models of PAH
Much of the knowledge about mechanisms of gender and sex differences in PAH and PH has been obtained from the study of animal models. While several animal models have been developed that display features of the pulmonary vascular remodeling and/or right heart hypertrophy/failure that are common to PAH, recapitulating the sexually dimorphic disease presentation and progression in these models has been challenging. In contrast to human PAH, female sex is protective in many classical models of PAH, such as hypoxia- or monocrotaline (MCT)-induced PH. However, several models (many of them transgenic) have been developed over the past two decades that demonstrate a female bias with regard to disease susceptibility or severity, and animal studies have allowed for a more nuanced understanding of the effects of sex and sex hormones on disease development. In addition, progress has been made in understanding sex differences in RV function and failure. Commonly used animal models of PH and RV failure have been reviewed extensively elsewhere (126, 212, 347, 394). Here, we briefly describe each model of PAH and the impact of sex on pulmonary and RV remodeling.
A synopsis is provided in Table 3. Contributions of individual sex hormones to PH development in these models and their interactions with specific pathways will be described later in this article.
Table 3.
Overview of sex differences observed in animal models of PH.
| Species | Male bias | References | Female bias | References |
|---|---|---|---|---|
| Mouse | Hypoxia-induced PH | (478, 479) | SERT+ (with or without hypoxia) | (461, 463) |
| eNOS−/− | (278) | S100A4/Mts1+ | (72) | |
| VIP−/− | (349) | Dexfenfluramine-induced PH | (71) | |
| ApoE−/− + high-fat diet | (147) | Smooth muscle-specific STAT5++/− or STAT5−/− + chronic hypoxia |
(479) | |
| miR-214−/− + Su5416/chronic hypoxiaa | (395, 479) | Cyp2c44−/− + chronic hypoxia | (182) | |
| Gonadectomized “four core genotypes” mouseb | (431) | |||
| Rat | Monocrotaline-induced PH | (421,432, 483) | Athymic rnu/rnu rats + Su5416 or chronic hypoxiac | (408) |
| Hypoxia-induced PH/HPV | (92, 208, 327, 335) | 4,4′-Methylenedianiline (DAPM)-induced PH | (46) | |
| Su5416/hypoxiad | (114, 213) | |||
| Athymic rnu/rnu rats + semaxanib | (135) | |||
| Chicken | Hypoxia-induced PH | (42, 350) | n/a | |
| Sheep | HPV | (459, 460) | n/a | |
| Swine | Hypoxia-induced PH | (267) | n/a |
ApoE, apolipoprotein E; athymic rnu/rnu rats, T-cell-deficient athymic rats; Cyp2c44e, cytochrome P450 2c44e; eNOS, endothelial nitric oxide synthase; HPV, hypoxic pulmonary vasoconstriction; miR-214, microRNA 214; n/a, no studies available; SERT+, serotonin transporter overexpression; STAT, signal transducer and activator of transcription; Su5416; sugen (VEGF receptor 2 inhibitor); S100A4/Mts1 +, S100A4/Mts1 overexpression; VIP, vasoactive intestinal peptide.
More RV hypertrophy in males; no difference in RV systolic pressure or pulmonary artery remodeling.
Not a typical study comparing sexes, but Y-chromosome protective.
Sex differences abolished after repletion with CD4+CD25hlgh T regulatory cells.
No published sex differences in RV systolic pressure, but better RV function, less RV remodeling, and higher survival in females, as well as disparate pulmonary artery remodeling in males and females.
Chronic Hypoxia-Induced Pulmonary Hypertension (HPH)
Hypoxia has classically been used in both rodent and non-rodent models to induce pulmonary vascular remodeling. Histologically, HPH (hypoxia-induced pulmonary hyper-tension) induces media hypertrophy in the pulmonary vasculature, but plexiform or vaso-occlusive lesions are not seen (394). In addition, damage to the pulmonary vasculature is reversible after reexposure to room air, and this model induces RV hypertrophy but not failure (212, 393). While hypoxia alone does not recapitulate Group 1 PAH pathology, HPH shares certain signaling pathways and disease mechanisms with human PAH and could be considered a model of mild or early PAH (393). Some investigators, on the other hand, suggest that HPH may be a better model for Group 3 PH (345). Contrary to human data, females of many HPH model species are more resistant to HPH, with smaller increases in right ventricular systolic pressure (RVSP), RV hypertrophy, and pulmonary vascular remodeling. This effect has been shown in vivo in rats, mice, swine, and chickens (42, 92, 208, 267, 327, 335, 350), as well as in isolated ovine lungs, which display reduced HPV (459, 460). Reduced HPV has also been demonstrated in isolated pulmonary arteries (PAs) from female rats (214). While the mechanisms of contraction during the acute phase of HPV are separate from the mechanisms governing contraction and remodeling during chronic HPH, the observation that sex impacts the contractile response of isolated pulmonary vessels illustrates the dramatic sexual dimorphism of tissues implicated in PH pathogenesis. The in vivo data in rats is particularly compelling, as ovariectomized rats become vulnerable to severe HPH, and supplemental E2 treatment in ovariectomized animals rescues this effect (92, 335). HPH is driven in part by hypoxia-induced erythrocytosis (which leads to increased blood viscosity and increases in PA pressure) (299, 437), and it has been demonstrated that the female resilience in HPH is at least in part due to lower hematocrit levels (208, 296). However, direct effects on the pulmonary vasculature (less vasoconstriction and remodeling) play a role as well. Compared with rats, mice are relatively resistant to HPH regardless of sex (126, 161, 406). Nevertheless, female HPH mice also display more favorable hemodynamics, less RV hypertrophy, and less PA remodeling (478, 479). While favorable hemodynamics and decreased RV hypertrophy are akin to humans, decreased PA remodeling is not. Female HPH mice also express higher levels of angiogenic factors such as VEGF-A in the RV as compared with hypoxic males (35).
Monocrotaline-Induced PH (MCT-PH)
Administration of the toxic pyrrolizidine alkaloid MCT in rats is another classical model of PAH. Circulating MCT is converted to its bioactive form dehydromonocrotaline by the cytochrome P450 (CYP) enzyme CYP3A family in the liver (189). The exact mechanism through which dehydromonocrotaline induces PH is unknown, but it likely acts primarily through damage to pulmonary artery endothelial cells (PAECs) (342). For reasons that are not entirely clear, this model does not work in mice; this may be due to unpredictable CYP3A subtype 4 activity and/or species-specific resistance to MCT-induced vascular injury (127). Muscularization of PAs, increased PVR, RV hypertrophy, and eventual RV failure and death are seen in this model. MCT-PH is accompanied by systemic inflammation and possibly myocarditis and hepatic veno-occlusive disease and has therefore been proposed as a model of inflammation-induced PAH, such as PAH associated with CTD (127). Similar to findings in hypoxia, female sex is protective in the rat model of MCT-PH. Specifically, female sex or exogenous estrogens ameliorate the phenotype of MCT-PH compared with males, while ovariectomy (OVX) exacerbates disease progress (3, 99, 298, 421, 432, 483). Sex differences in the MCT model may result at least in part from decreased CYP3A activity in the female liver, leading to reduced levels of dehydromonocrotaline (189). Recently, MCT administration has been combined with chronic hypoxia to develop a more severe phenotype of PH, characterized by thrombotic, neointimal, and plexiform-like lesions in the pulmonary vasculature (66, 289). Only data from male rats has been published using this combined injury model. Similarly, MCT in combination with pneumonectomy causes more severe PH and vascular remodeling but has only been published in males (466).
Sugen/Hypoxia-Induced PH (SuHx-PH)
A more recent model of PAH was published in 2001, which more closely resembles the human phenotype (409). Here, administration of the vascular endothelial growth factor (VEGF) receptor 2 antagonist Su5416 (sugen) to young rats, followed by hypoxia and subsequent reexposure to normoxia produces severe PH with RV failure and mortality. In this model, Su5416 administration leads to initial PAEC apoptosis, followed by exuberant proliferation of the remaining PAECs, resulting in pronounced and progressive PA remodeling and formation of vaso-occlusive lesions (409). Two recent studies demonstrated that female sugen/hypoxia-induced pulmonary hypertension (SuHx-PH) rats exhibit better RV function than their male counterparts (both at rest and after acute exercise), including improved stroke volume index, CI, RV compliance, and reduced RV hypertrophy (114,213). These findings were accompanied by more favorable antioxidant, pro-survival, and pro-angiogenic responses as well as less fibrosis and lower pro-inflammatory cytokine expression in female RVs. Interestingly, higher cardiac indices were also noted in healthy females versus males, mirroring the better RV function noted in healthy humans (167, 175, 192, 405). In both studies, no sex differences were found in RVSP increase or pulmonary vascular remodeling (114, 213). A study by Rafikova et al. (330), on the other hand, demonstrated sexually dimorphic pathology in the pulmonary vasculature, with female SuHx-PH rats displaying increased pulmonary vascular wall thickness compared to male SuHx-PH animals. However, the pulmonary vasculature from male SuHx-PH rats displayed increased fibrosis and inflammatory markers, and female SuHx-PH rats displayed less RV hypertrophy and increased survival (330). Taken together, these studies show that SuHx-PH exhibits sexually dimorphic features in rats, with better RV adaptation and survival in females, despite potentially more pronounced vascular remodeling. Importantly, this mirrors the human PAH phenotype and suggests that SuHx-PH is a suitable model to study sex differences. The lung vascular remodeling, fibrosis, and inflammation data by Rafikova et al. suggest that sex differences in the pulmonary vasculature can be nuanced and that a detailed examination of different compartments and pathways is necessary to capture the full spectrum of sex differences in the lung vasculature in experimental PH and human PAH. SuHx-PH has also been employed in mice, albeit with a less-consistent and less-severe phenotype (126, 212, 347, 394). Induction of SuHx-PH in mice requires maintained hypoxia as well as weekly injections of Su5416. In the only published male-female comparison in SuHx-PH mice, no sex differences were noted in RVSP or RV hypertrophy (61).
Mutant and Transgenic Rodent Strains
Genetically modified rodents have produced mixed and occasionally contradictory findings in regard to sex-based differences in PH. While some models display a female bias, others show the opposite. For example, overexpression of the calcium-binding protein S100A4/Mts1 (72) or the serotonin transporter SERT (462, 464) increases female penetrance of PH and disease severity, while ovariectomy attenuates these effects. Disease outcomes are similarly more severe in female mice globally lacking the epoxygenase CYP2c44 (182) or lacking the transcription factor Stat5 in SMCs (479). Conversely, female mice lacking genes for vasoactive intestinal peptide (VIP) (349), endothelial nitric oxide synthase (eNOS) (278), or apolipoprotein E (ApoE) (the latter in combination with a high-fat diet) (147) develop a much less-severe PH phenotype compared with male littermates. Furthermore, hypoxic female miR-214 knockout mice develop similar hemodynamic and PA remodeling alterations as their male counterparts but exhibit less RV hypertrophy (395). Two studies demonstrated that certain transgenic manipulations can abolish the male bias in experimental PH, suggesting that these pathways may be involved in making males more susceptible or females less susceptible. Specifically, smooth muscle-specific deletion of Stat5 (479) or the transcriptional repressor Bcl6 (478) abrogates female protection in female rats with HPH. In the case of Stat5 deletion, female protection is reversed to female susceptibility.
As mutations in BMPR2 underlie most cases of heritable PAH, and since decreased BMPR2 activation has been noted in idiopathic PAH (246), several strains of Bmpr2 mutant mice have been developed to examine the role of this signaling pathway in disease progression (reviewed in Ref. 345). Global knockout of the Bmpr2 gene is embryonically lethal in mice, while heterozygous mutant mice spontaneously develop a mild form of PH (29). Targeted deletion of Bmpr2 in either ECs (160) or SMCs (458) is sufficient to produce PH features such as increased RVSP and pulmonary vascular remodeling. A recent report from Hautefort et al. describes a similar phenotype in Bmpr2 mutant rats (150). A gender bias has not been reported in Bmpr2 mutants, with similar penetrance of PH seen in both male and female mice. However, Bmpr2 mutant mice are more vulnerable to 16-OHE1 administration than wild-type controls, leading to increased PH penetrance (52). This phenomenon is associated with aberrant estrogen signaling within cells of the pulmonary vasculature (101). Even though there appear to be no sexually dimorphic features in Bmpr2 mutant strains, altered estrogen signaling in Bmpr2 mutants may be relevant to unraveling the estrogen puzzle in human PAH.
While these transgenic mouse models allow focused hypothesis testing regarding the role of a particular gene/protein in PH and are useful in identifying mechanisms that may explain sex differences in PH, it is important to remember that many transgenic models display only a mild hemodynamic or vascular phenotype of PH, thus limiting their clinical relevance (24). Additionally, knockout models are primarily available in mice, which generally display a less-severe PH phenotype than rats, and robust models of PH such as MCT or SuHx either do not work in mice (in the case of MCT) or result in only a mild phenotype (in the case of SuHx) (61, 126). Despite these caveats, transgenic mouse models have been successfully used to investigate the estrogen puzzle and have advanced the field. Recent advances in genetic manipulation such as CRISPR/Cas9 are increasing the number of available transgenic rat strains (105); this generates an exciting opportunity to perform gain- and loss-of-function studies in more robust PH models.
Rodent Models of Immunity and PH
Two studies have been published employing T-cell-deficient athymic rnu/rnu rats. In the first study, administration of the VEGFR 1 and 2 antagonist semaxanib induced more severe PA remodeling, more RV hypertrophy, and more profound RV systolic dysfunction in males relative to females (135). Impairment of RV-PA coupling efficiency was observed only in males, and pulmonary artery smooth muscle cells (PASMCs) switched from a contractile state to a dedifferentiated state in males only. However, a more recent study demonstrated the opposite effect (408). Here, sugen administration or chronic hypoxia led to a more severe phenotype in female rats. In particular, female rats exhibited greater pulmonary inflammation; augmented RV fibrosis; lower plasma levels of prostacyclin; decreased lung expression of cyclooxygenase, prostacyclin synthase, programmed death ligand-1 (PDL-1), and heme oxygenase-1; and reduced PDL-1 levels in the RV. Treg immune reconstitution protected against PH development in both sexes and abrogated sex differences in Treg-deficient animals. While the reason for the contradictory findings between the two studies in athymic rats is unclear and must be resolved, the implication that immunity may underlie sex differences in PH is intriguing and parallels data showing sex- and sex hormone-mediated differences in immune function and dysfunction (227) and studies that identify immune dysregulation as a contributing factor to PAH in humans (328). More studies are required to investigate the interplay among sex, sex hormones, and immunity in PAH/PH. Ultimately, such studies would be expected to shed further light on the underlying mechanisms of sex differences in experimental PH and human PAH.
A critical role of immune cells in PH was recently demonstrated by Hu et al. in a study where humanized mice were engrafted with human hematopoietic CD34+ progenitor cells (resulting in circulating human leukocytes) and subsequently exposed to chronic hypoxia. In contrast to nonhumanized mice, humanized mice displayed significantly increased RVSP and PA muscularization (163), suggesting that species-specific immune responses may underlie the reduced acuity of murine PH models at least in part.
Drug-Induced PH
While gender does not play a clear role in human drug- and toxin-induced PAH (perhaps because of psychosocial or behavior influences), two models of drug-induced PAH predominantly affect female rodents. Specifically, administration of the anorexigen dexfenfluramine or the industrial compound 4,4′-diaminodiphenylmethane (DAPM) results in PH in female animals only (46, 71). The mechanism of both drugs appears to involve increased serotonergic signaling and altered estrogen metabolism to favor pro-proliferative metabolites. Interestingly, dexfenfluramine-induced PH involves upregulation of the estrogen-metabolizing enzyme CYP1B1, and CYP1B1 knockout animals are not susceptible to dexfenfluramine-induced PH (71).
Pulmonary Artery Banding (PAB)
Pulmonary artery banding (PAB) is a model of RV hypertrophy with or without RV failure that is independent of changes in the pulmonary vasculature (212). No investigations focusing on sex differences in this model have been published to date.
Summary of Studies of Sex as a Disease Modifier in Animal Models
Taken together, animal models have identified sex as an important disease modifier in experimental PH. As in humans, animal models of PAH demonstrate an effect of sex on disease penetrance and severity. As in humans, the results from animal models can be complex and occasionally contradictory. In the classical models of PAH (HPH and MCT-PH), female sex is protective. Female sex is also protective in several transgenic mouse models. On the other hand, a female bias with regard to disease susceptibility or severity has been noted in transgenic mice, Treg-deficient rats and models of drug exposure. SuHx-PH rats exhibit complex sex-specific features in the pulmonary vasculature but better RV function and survival in females. It is worth noting, however, that the pulmonary hypertensive or RV failure phenotype in some of these models is modest, thus limiting their relevance to human PAH. In particular, results from studies in mice may be of limited translational value because of the mild PH phenotype produced. New animal models, including transgenic, immunological, pharmacological, and surgical models that employ various “hits” to the pulmonary vasculature rather than one single insult (thus mimicking the pathogenesis of human PAH) are likely to provide novel information on the role of sex in PH (212). For example, SuHx-PH rats recapitulate many of the hallmarks of human PAH (126, 212, 347, 394). However, it is critical to look at animal models as tools to dissect specific components or mechanisms of disease development rather than use one single model to represent and study the entire spectrum of gender differences in human PAH (38, 324). In addition, important modifiers such as animal age, phase of the estrous cycle, and external influences (e.g., dietary phytoestrogens (149) and gender of the animal handler (387)) need to be considered. Only the study of several animal models in conjunction and the consideration of these modifiers will generate sufficient and relevant new data to solve the estrogen puzzle.
What are Possible Explanations for Discrepancies in Sex/Gender Differences and Sex Hormone Effects between Rodent Models and Human PAH?
While the animal models described here recapitulate many features of PAH, no animal model is a perfect analog for human physiology. Animal models of estrogen signaling are particularly difficult as the human menstrual cycle is a radically different physiological phenomenon than the much shorter estrous cycles of laboratory animal rodents (356). Hormone levels, cycle duration, and the aging endocrine profile are all species specific and may play a vital role in PAH/PH penetrance and progression. These differences may underlie seemingly contradictory findings, such as an association of high E2 levels with PH in humans (19, 441, 471), but reduced E2 levels in rodent models of PH (114, 482). It is possible that PAH/PH alters hormone metabolism and secretion differently between humans and model species and that the profile of estrogen metabolite production and distribution is different between species. The nature and physiological impact of increased E2 levels in human PAH patients is also unclear. Without a baseline endocrine profile that predates disease onset, it is impossible to tell if increased E2 levels are a result of PAH onset or if elevated E2 levels instead directly contribute to PAH development. Also, because hormone secretion is controlled via a negative feedback mechanism at the level of the hypothalamus and pituitary, it is possible that impaired ER signaling could lead to a surplus of circulating hormone in the circulation. In this scenario, elevated E2 may be serving as a biomarker of impaired downstream signaling, rather than as a disease mediator per se. More detailed investigations into hormone metabolism and species-specific endocrine profiles are warranted to develop new models of PAH that more closely align with human physiology.
What are the Underlying Mechanisms Mediating Sex and Gender Differences in Rodent Models and Human PAH?
The data reviewed above clearly demonstrate that profound gender and sex differences exist in human PAH and PH as well as in experimental PH. Substantial research over the past two decades has identified sex hormone-dependent and -independent mechanisms as mediators of these differences. The latter include genetic, epigenetic, environmental, and behavioral factors. In the remainder of this article, we review sex hormone-dependent and -independent factors involved in sex and gender differences in etiology, physiology, hemodynamics, treatment responses, and outcomes in PAH and PH. Given the critical role of RV function in PAH, we highlight sex and gender differences in RV function in dedicated s. Since the majority of the published literature involves data on estrogen signaling and metabolism, a large part of this article will focus on this area, but we also discuss the currently available knowledge about testosterone, progesterone, and DHEA(-S) as well as genetic, environmental, and behavioral modifiers. The conglomerate of the data reviewed demonstrates that there has been significant progress in the field and that we are getting closer to solving the “gender puzzle” in PAH and PH.
Overview of Sex Hormone Synthesis and Metabolism
Detailed reviews of steroid hormone production and metabolism are available in the literature (317, 417) and are beyond the scope of this article. However, since basic concepts of steroidogenesis and sex hormone signaling are key to understanding the sexually dimorphic pathogenesis and presentation of PAH/PH, clinically important key concepts will be reviewed here. The steroidogenic pathway is illustrated in Figure 3.
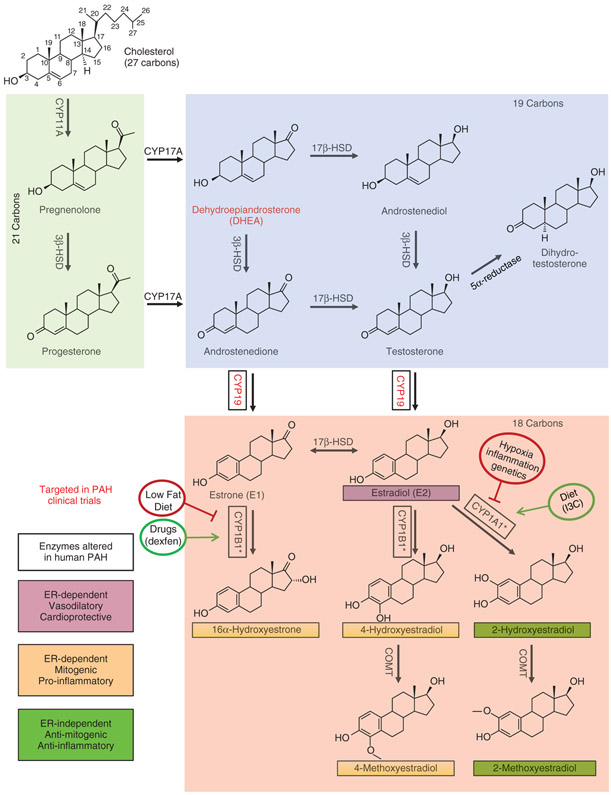
Figure 3. Sex hormone synthesis and estrogen metabolism.
Steroidogenic enzymes represented here are present in the lung, heart, and/or vascular tissue (148, 197, 314, 317, 480). Abundance of boxed enzymes is altered in PH/PAH (12, 326, 340, 457, 462-464). Compounds in red have been targeted in clinical trials of PAH therapies (193). 2-Hydroxyestradiol and 2-methoxyestradiol exert ER-independent antiproliferative, anti-inflammatory effects that appear to mitigate vascular remodeling in animal models of PAH (22, 82, 421). Conversely, the estrogen metabolites 4-hydroxyestradiol, 4-methoxyestradiol, and 16α-hydroxyestrone signal via estrogen receptors and promote a mitogenic, inflammatory, and antiapoptotic phenotype that exacerbates PH/PAH (54, 463). *Multiple CYP enzymes are capable of catalyzing estrogen hydroxylation; CYP1A1 and CYP1B1 appear to be the most relevant isoforms in PAH pathology. Factors including diet (240, 275), hypoxia (107), inflammation (11), genetics (12, 146), and drug exposure (71) may alter estrogen metabolism. Effects of enzymes and metabolites depicted here may be cell-, tissue-, and/or organ specific.
All steroid hormones are derived from cholesterol, a 27-carbon sterol consisting of four hydrocarbon rings attached to a hydrocarbon tail (top left in Figure 3). Sex hormone synthesis is characterized by the progressive cleavage of carbon atoms surrounding the hydrocarbon ring structure. Sex hormones can therefore be broken into three classes based on the number of carbon atoms present in their structure: 21-C, 19-C, and 18-C hormones (green, blue, and pink boxes, respectively, in Figure 3). Hormone synthesis and modification within each of these classes will be briefly discussed before examining the role of specific sex hormones in PAH/PH.
In the first step of steroidogenesis (Figure 3), CYP11A (side-chain cleavage enzyme) cleaves the hydrocarbon tail from cholesterol to yield pregnenolone, a 21-carbon compound that is the common precursor of all steroid hormones. CYP11A is located on the inner mitochondrial membrane, and transport of cholesterol across the outer mitochondrial membrane by steroidogenic acute regulatory (StAR) protein is the rate-limiting step in steroid hormone synthesis (396). The enzyme 3β-hydroxysteroid dehydrogenase (3β-HSD) converts pregnenolone to progesterone, a biologically active steroid critical to establish and maintain pregnancy. Progesterone is primarily secreted by the ovary and placenta, but also serves as a metabolic intermediary in the adrenal gland. The role of progesterone in PAH/PH is a fairly unexplored avenue of research that will be discussed later.
CYP17A catalyzes conversion of either pregnenolone or progesterone to the 19-carbon hormones dehydroepiandrosterone (DHEA) or androstenedione, respectively (blue box in Figure 3). Circulating DHEA and its sulfate ester DHEA-S are the most abundant steroid hormones in the human body (252). DHEA can be converted to the weak androgens androstenedione or androstenediol, both of which are immediate precursors of the potent androgen testosterone. The enzyme 5a-reductase converts testosterone to dihydrotestosterone (DHT), the most potent biological androgen.
18-Carbon steroid hormones (estrogens) are derived from androstenedione or testosterone. CYP19 (aromatase) catalyzes the conversion of androstenedione or testosterone into estrone (E1) or estradiol (E2), respectively (pink box in Figure 3). Estrone may be converted to estradiol through the action of 17β-hydroxysteroid dehydrogenase (17β-HSD). Estrogens may be further processed into bioactive metabolites via hydroxylation by multiple CYP enzymes including CYP1A1 and CYP1B1. The resulting hydroxyestrogens may then undergo methylation by the enzyme catechol-O-methyl transferase (COMT) to form methoxyestrogens (220, 427, 476). One hydroxylated metabolite, 16α-hydroxyestradiol, is commonly referred to as estriol (E3), an estrogenic compound that plays an important role during pregnancy (4, 310). The various estrogen metabolites exert radically different effects on cell signaling and function that may impact many proliferative and inflammatory processes, such as cancer, chronic inflammatory diseases, and PAH (86). The role of these metabolites in PAH is described in the following section. Sex steroids typically signal through specific receptors (e.g., progesterone-, androgen-, and estrogen-receptors), even though receptor-independent actions of certain metabolites have been described as well (described in detail later).
Circulating hormone levels vary widely among individuals and tend to decline with age (Table 4). Women of reproductive age also exhibit cyclical surges and withdrawal of estrogens and progesterone according to the phase of each menstrual cycle. Pregnancy and menopause are marked by their own distinct endocrine profiles. While serum hormone levels are constantly in flux, a high proportion of circulating sex hormones are bound to carrier proteins such as albumin or sex hormone-binding globulin (SHBG), which sequester hormones from receptors in surrounding tissues. This results in a relatively small percentage of circulating hormones being biologically active (143). Further complicating the issue, many nongonadal tissues contain the necessary enzymes (e.g., 3β-HSD, 17β-HSD, and aromatase) to synthesize both androgens and estrogens from DHEA. Peripheral production of androgens and/or estrogens is possible in many organs and occurs in adipocytes, ECs, SMCs, cardiac fibroblasts, and cardiomyocytes (148, 197, 314, 317, 465, 480). Peripheral aromatization to form E1 and E2 is particularly important as this is the main source of estrogens in men and post-menopausal women (183, 378). Local production of steroids by a target cell has been termed “intracrinology” (207, 360). This is biologically and clinically relevant as it may lead to significant local steroid signaling not reflected in measurements of circulating hormone levels.
Table 4.
Normal values for sex hormone levels in humans across the life span.
| Women of reproductive age |
|||||||
|---|---|---|---|---|---|---|---|
| Hormone | Men <60 | Men >60 | Follicular phase | Preovulatory phase | Luteal phase | Pregnancy | Menopause |
| Progesterone | 0.25–0.90 ng/ml | 0.25–0.90 ng/ml | 0.3–1.2 ng/ml | 0.7–2.5 ng/ml | 1–30 ng/ml | 9–300 ng/ml | <0.2–1.1 ng/ml |
| 1–3 nM | 1−3 nM | 1–4 nM | 2–9 nM | 3–100 nM | 25–1000 nM | 0.6–3.5 nM | |
| DHEA | 1.5–10.4 ng/ml | 0.3–3.5 ng/ml | 3–10 ng/ml | 3–10 ng/ml | 3–10 ng/ml | 1.5–17 ng/ml | 0.4–4.6 ng/ml |
| 5–36 nM | 1–12 nM | 10–35 nM | 10–35 nM | 10–35 nM | 5–60 nM | 1.5–16 nM | |
| Testosterone | 2–15 ng/ml | 1–10 ng/ml | 0.2–0.8 ng/ml | 0.2–0.8 ng/ml | 0.2–0.8 ng/ml | 1–1.4 ng/ml | 0.2–0.8 ng/ml |
| 6–50 nM | 3.5–33 nM | 0.7–2.5 nM | 0.7–2.5 nM | 0.7–2.5 nM | 3.5–5n M | 0.7–2.5 nM | |
| Estradiol | 0.015–0.05 ng/ml | 0.002–0.04 ng/ml | 0.02–0.1 ng/ml | 0.15–0.4 ng/ml | 0.06–0.20 ng/ml | 1−40 ng/ml | 0.01–0.03 ng/ml |
| 0.05–0.2 nM | 0.007–0.15 nM | 0.07–0.4 nM | 0.5–1.5 nM | 0.2–0.8 nM | 4–150 nM | 0.04–0.12 nM | |
Gauging the effect of sex and sex hormones on physiology is complex due to conversion between hormone classes, changing levels of bioactivity, steroid metabolism into other bioactive compounds, and hormone synthesis in nonclassical steroidogenic tissues. In target cells, changes in hormone-receptor isotype abundance or localization (91, 294, 325, 355), variations in activity of enzymes responsible for sex hormone synthesis (47, 202, 253), interactions with genomic and nongenomic cofactors (265), additive or opposing effects of multiple hormones or receptors (142, 191), and epigenetic phenomena (219) can drastically alter the downstream effect of hormone-receptor binding. At a broader level, the expression and activity of sex steroid receptors, as well as hormone-metabolizing enzymes, may be affected by sex, age, diet, environmental exposures, fluctuations in endogenous sex hormone levels (e.g., menstrual cycle and menopause), and various disease states (reviewed in Ref. 297). It is therefore not surprising that many sex hormone-mediated effects are compartment-, time-, and concentration dependent. Carefully devised experimental systems are required to determine the impact of steroid hormones, particularly in a complicated syndrome like PH. The development of new animal models, new biological tools, and the dedication of many researchers has allowed recent advances in unraveling the role of steroid hormones in PAH/PH and moved the field closer to unraveling the “estrogen puzzle” in PAH.
Estrogen in PH
Estrogen Signaling and Metabolism
E1, E2, and E3 are the three main estrogens in the human body. Of these, E2 is the most potent estrogen and the primary mediator of estrogen signaling. An overview of E2 levels in humans across the life span is provided in Table 4. E1, E2, and E3, as well as many of their metabolites, signal through interaction with three ERs. Two of these receptors, ERα and ERβ, are members of the nuclear receptor superfamily (reviewed in Refs. 151, 271, 272, 297, 375). ERα and ERβ are encoded by independent genes located on separate chromosomes. In humans, ESR1 (estrogen receptor α gene) encodes ERα, a protein composed of 595 amino acid residues with a molecular weight of 66.2 kDa. ESR2 (estrogen receptor β gene) encodes ERβ, a slightly smaller protein composed of 530 amino acids and a molecular weight of 59.2 kDa (305). The two receptors share high homology for the DNA-binding domain, as well as the ligand-binding domain (95% and 60%, respectively) (206). Both receptors also contain two activation function domains (AF-1/AF-2) which alter transcription through interaction with nuclear coactivators/repressors (201, 454). ERα and ERβ share less than 30% homology of the AF domains, which contributes to the unique transcriptional profile of each receptor despite near-perfect homology of the DNA-binding domain (488).
ERα and ERβ are widely expressed throughout the body (reproductive, cardiovascular, respiratory, central nervous, endocrine, immune, and skeletal systems). In the pulmonary vasculature, ERα and ERβ are expressed in PAECs (141, 439, 440), PASMCs (141, 187), and fibroblasts (78), all of which are involved in vascular remodeling during PH. ERs are also expressed in other lung cells including epithelial cells (141, 176), alveolar cells (315), and alveolar macrophages (401, 439). In the heart, ERα and ERβ are expressed in ECs, cardiomyocytes, and fibroblasts (reviewed in Refs. 271, 272). ERα and ERβ are also expressed in progenitor cells (140) and immune cells (204, 397), where they modify developmental, regenerative, and immune signaling processes in health and disease. While there is overlap in functions of ERα and ERβ, significant differences exist between the two ERs in tissue expression and function.
Classical estrogen signaling occurs via these two receptors in what is termed the genomic pathway. Here, estrogen diffuses through the cell membrane and binds to cytoplasmic ERα or ERβ. This estrogen-ER complex subsequently dimerizes with another estrogen-ER complex (either as a homo- or heterodimer) and translocates to the nucleus. The estrogen-ER dimer then binds to an estrogen responsive element (ERE) in the DNA. In this context, estrogen acts as a classic transcription factor to alter gene expression. Coactivators and corepressors of gene transcription interact with the estrogen-ER complex and contribute to regulating gene expression. In a variation of this pathway, referred to as tethered estrogen signaling, the estrogen-ER complex indirectly regulates gene expression by binding to and modulating the activity of other transcription factors. In a third pathway, nonestrogen ligands such as epidermal growth factor phosphorylate and activate cytoplasmic ER in the absence of ER binding (67). Lastly, estrogens can bind to ERα and ERβ anchored to the cell membrane. Activation of these membrane-bound receptors leads to rapid, nongenomic cellular effects. In this signaling pathway, estrogen binds to a membrane-bound receptor, leading to activation of a second messenger such as MAPK (367). This pathway does not require altered gene transcription and can induce rapid and diverse cellular effects such as ion channel activation, or activation of eNOS or prostacyclin synthase (374). Nongenomic signaling occurs within seconds to minutes and is particularly important in the cardiovascular and respiratory systems (16, 49, 53, 157, 367, 370, 402, 481). While nongenomic estrogen signaling initiates rapid dynamic cellular responses, this pathway may also ultimately produce changes in gene transcription through activation and phosphorylation of downstream transcription factors and activators (33, 151, 297). A third ER, GPR30 (G-protein-coupled receptor 30), is a G-protein-coupled receptor that has been shown to bind estrogen and signal exclusively through the nongenomic pathway.
Estrogen signaling can be altered by changes to either the ligand or receptor. Each endogenous estrogen (E1, E2, and E3) has a unique ER affinity, and the downstream effects of estrogen signaling also depend on the type of ER (ERα, ERβ, and GPR30), their interactions with each other, and their tissue-specific expression patterns. Both circulating estrogen levels and ER expression patterns change based on sex, age, disease state, and fluctuations during the menstrual cycle (reviewed in Ref. 297). Changes in ER ubiquitination can increase proteosomal degradation of ERs, while posttranslational modifications including methylation, acetylation, or S-nitrosylation may drastically impact ER signaling. In the genomic pathway, nuclear coactivators and corepressors may alter transcription by interacting with the ER in the nucleus (264). One final modulator of ER signaling is the existence of several ER splice variants. These variants demonstrate a range of activity during in vitro studies from constitutive activation to dominant-negative regulation of ER signaling (156). A relative increase in the expression of ER splice variants (as a percentage of total ER isoforms) is associated with human disease pathology, particularly tumorigenesis (156,410). The role of ER splice variants has not been investigated in PH.
E1 or E2 may be further metabolized to form new bioactive compounds, usually by hydroxylation at carbon 2, 4, or 16. The 2-hydroxylation pathway transiently produces 2-hydroxyestradiol (2-OHE2) or 2-hydroxyestrone (2-OHE1) before conversion to methoxyestrogens by the enzyme COMT (220, 427, 476). 2-Methoxyestradiol (2-ME2) is a potent metabolite with no ER affinity, which displays antimitogenic and proapoptotic properties in both tumor (215, 322, 362) and vascular SMCs (82, 428, 484). All enzymes necessary for conversion of E2 to 2-ME2 (CYP1A1/2, CYP1B1, and COMT) are present in cardiovascular tissue, and 2-ME2 synthesis occurs in vascular ECs and SMCs (81, 82, 220, 417, 485). In contrast to the antimitogenic, nonestrogenic metabolites resulting from the 2-hydoxylation pathway, the 16-hydroxylation pathway produces 16α-hydroxyestradiol (E3) or 16α-hydroxyestrone (16α-OHE1). While E3 is a weak estrogen, 16α-OHE1 exerts comparable estrogenic effects to E2 (103) and promotes inflammatory, proliferative, and angiogenic cellular processes (417). 16α-OHE1 has lower affinity for SHBG than E2 and may covalently bind to ERs causing hyperestrogenic signaling (103, 403, 417). Several CYP450 isoforms are capable of catalyzing 16α-hydroxylation (17), and the enzyme CYP1B1 has been the primary focus of PAH research. CYP1B1 is expressed in cardiovascular tissue (314) and is upregulated in PASMCs in several animal models of PAH (71, 179). Because of its pro-mitogenic properties, 16α-OHE1 is currently explored as a therapeutic target in clinical trials for various cancers (e.g., NCT02525159). The role of CYP1B1 and 16α-OHE1 metabolism in PAH is currently under investigation and will be discussed in detail later in this article. A minor pathway of estrogen metabolism is the 4-hydroxylation pathway, leading to the formation of 4-hydroxyestradiol (4-OHE2), which exerts estrogenic and carcinogenic effects (54, 228, 417). It should be noted that the biological effects and properties of estrogen metabolites have primarily been explored in reproductive, endocrine, and malignant tissues and that several of the reported effects may be tissue-, time-, and/or context dependent and have not been fully explored in the cardiopulmonary system. The ratio of various hormones and metabolites may also be more important for net biological effects than the absolute levels of one single hormone (145).
Estrogen in the Pulmonary Vasculature
Cell Culture and Animal Studies
In the systemic vasculature, estrogen signaling promotes healthy vessel function and is protective in the face of disease or vascular injury (48, 136, 313,425,470). In particular, ERα facilitates EC recovery after vascular injury, blocks monocyte adhesion to ECs, and inhibits vasoconstrictor responses (32, 36, 244, 313, 381, 467, 475). Furthermore, loss-of-function mutations in ESR1 have been linked to endothelial dysfunction, coronary artery disease, myocardial infarction, and stroke (361, 368, 369, 398, 399). Many studies support a similar role of estrogen and its receptors in the pulmonary vasculature. Both ERα and ERβ activation in cultured PAECs increases eNOS activity (49, 157, 216, 247, 370), while ERβ-mediated signaling has been shown to increase prostacyclin synthesis (247). Increased activation and synthesis of these potent vasodilators suggests that estrogen signaling in PAECs promotes vasodilation. Indeed, endogenous (214) or exogenous (210) estrogen exposure attenuates both phenylephrine-induced vasoconstriction and HPV in isolated rat PA rings. These effects were later shown to be mediated by specific ER isoforms as the ERa-agonist propylpyrazole triol (PPT) attenuates phenylephrine-induced vasoconstriction, while the ERβ agonist DPN (diarylpropionitrile) ameliorates HPV (209). Inhibition of eNOS eliminates the vasodilatory effect of both ERα and ERβ, indicating that NO plays a central role in estrogen’s effect in the pulmonary vasculature (209). Several other groups have demonstrated that estrogens, and in particular E2, attenuate HPV (116, 210, 211, 214,473) (see Table 5 for details).
Table 5.
Major animal studies of E2 or estrogen metabolites in PH.
| Model | Species | Major findings | References |
|---|---|---|---|
| Isolated lungs or isolated PAs | Rat, sheep | Female sex, high estrogen states (pregnancy, proestrus), exogenous E2 or selective ERα or ERβ agonist ↓ HPV and/or drug-induced PA vasocontraction | (116, 208-210, 214, 335, 473) |
| Hypoxia-induced PH (HPH) | Rat, chicken, swine, mouse | Females protected; OVX ↑ PH, E2 replacement in OVX ↓ PH |
(42, 92, 267, 327, 335) |
| E2 administration ↓ HPH in male rats | (208, 474) | ||
| E2 ↓ ET-1, ERK1/2, Akt, Skp2 | (92, 208, 474) | ||
| E2 ↑ p27Ki1, LC3B | (208, 474) | ||
| ERα and ERβ ↓ pro-proliferative signaling | (208) | ||
| ER-mediated anti-proliferative E2 effects on PAECs | (208) | ||
| ↓ proliferation in hypoxic PASMCs from proestrus rats | (463) | ||
| CYP1B1 ↑ in male and female mice; inhibition protective; knockout ↓ PH and PA remodeling in male mice only |
(473) | ||
| 16α-OHE1 ↑ in HPH; treatment of HPH mice with 16α-OHE1 ↑ PH | (463) | ||
| Hypoxia ↑ ERβ in rat lungs and cultured PAEC from rats and humans; ERβ ↓ HIF-activation and proliferative processes in PAECs and HPH lungs | (115) | ||
| E2 regulates proliferative and inflammatory gene expression via ER during hypoxia; E2 ↓ gremlin expression and ↑ BMPR2 signaling in hypoxic lungs | (112) | ||
| Aromatase inhibition ↓ PH in hypoxic female mice | (248) | ||
| Monocrotaline-induced PH (MCT-PH) | Rat | Females protected; OVX ↑ PH, 2ME2 replacement in OVX ↓ PH E2 metabolites (2OHE2, 2ME2, 2EE) protective E2 pro-angiogenic and anti-inflammatory |
(421, 432, 483) (418, 420, 421, 423) (432) |
| ERβ-mediated protection | (432) | ||
| MCT-PH “estrogen-deficient” state (↓ lung aromatase, lung ERa, plasma E2, ↑ CYP1B1) | (483) | ||
| E2 ↓ ET-1, ↑ NO, ↑ PGI2 | (483) | ||
| Activation of nongenomic ER (GPR30) in male or OVX female MCT rats: ↓ RVH, ↓ RVSP, ↑ exercise endurance | (5, 6) | ||
| E2 treatment ↓ RVSP, ↓ RVH, ↓ pulmonary vascular remodeling after MCT injection of aged ApoE~−/− mice | (433) | ||
| Phytoestrogen genistein ↓ MCT-PH by ↓ miR206 and ↑ pulmonary angiogenesis | (260, 366) | ||
| Sugen/hypoxia-induced PH | Rat, mouse | Only mild hemodynamic alterations in female rats CYP1B1 ↑ in male and female mice; CYP1B1 inhibition ↓ PH |
(348) (463) |
| E2 protective in OVX SuHx rats ↓ RVSP, ↓ PA muscularization, ↓ RVH, ↑ CI, ↑ VO2max ERα agonist replicates E2 effects | (114) | ||
| E2 administration in males: ↓ RVH, ↓ apoptotic signaling, ↑ apelin | (114) | ||
| E2 ↑ RV adaptation after acute exercise in SuHx-PH rats | (213) | ||
| E2 treatment in SuHx-PH OVX mice: ↓ RV afterload, ↓ PA muscularization, ↑ PA compliance, ↑ RVEF, ↑ CO, maintains pulmonary hemodynamics | (232, 234, 235) | ||
| E2 treatment preserves RV mitochondria number and function in OVX rats | (233) | ||
| SuHx-PH ↑ CYP1A1, ↑ aromatase. Inhibition of aryl hydrocarbon receptor (AhR) reversed this effect | (70) | ||
| Aromatase inhibition ↓ PH in female rats | (248) | ||
| Serotonin transporter overexpression (SERT+) |
Mouse | Female mice develop ↑ PA pressure at normoxia and ↑ PH during hypoxia exposure; OVX protective; E2 detrimental | (462) |
| E2 ↑ proliferation, Tph-1, 5-HT1B receptor, and SERT expression in human PASMCs | (462) | ||
| CEBPβ, FOS, CYP1B1 ↑ in female hypoxic SERT+ mice; E2 ↑ these factors in human PASMCs | (464) | ||
| SERT+ mice overexpress CYP1B1; CYP1B1 inhibition prevents spontaneous PH phenotype | (179) | ||
| S100A4/Mts1 overexpression |
Mouse | Female MTS1+ mice more susceptible to PH development than males: ↑ RVSP, ↑ pulmonary vascular remodeling in females | (72) |
| E2 treatment ↑ Mts1 and ↑ proliferation in hPASMCs in a RAGE-dependent manner | (72) | ||
| Dexfenfluramine (Dfen)-induced PH |
Mouse | Only females develop PH; OVX protective CYP1B1 necessary for PAH development in Dfen-treated mice |
(71) (71) |
| Dfen and E2 treatments ↑ CYP1B1 and Tph1 expression in cultured PAH-PASMCs |
(71) | ||
| BMPR2 mutation- induced PH | Mouse | 16α-OHE1 ↑ disease penetrance and ↑ RV dysfunction 16α-OHE1 ↓ BMPR2 signaling in control mice but not in BMPR2 mutants |
(101) (101) |
| 16α-OHE1 ↓ cytokine expression but ↑ alterations in genes related to platelet function, angiogenesis, Wnt pathway, and energy metabolism | (101) | ||
| Lack of protective effect of 2-ME2 | (101) | ||
| Altered intracellular localization of ERα in BMPR2 mutant pulmonary microvascular endothelial cells (associated with insensitivity to activation by E2) | (101) | ||
| Estrogen inhibition ↓ PH | (51) | ||
| 16α-OHE1 ↑ PH via upregulation of microRNA-29 (miR-29) | (52) |
Studies organized by model system. 16α-OHE1, 16-alpha hydroxyestrone; 2EE, 2-ethoxyestradiol; 2ME2, 2-methoxyestradiol, Akt, RAC-alpha serine/threonine-protein kinase; ApoE, apolipoprotein E; Bmpr2, bone morphogenetic protein receptor 2; CEBPβ, CCAAT enhancer binding protein beta; CI, cardiac index; CO, cardiac output; CYP1B1, cytochrome P450 1 subfamily B member 1; ERK1, extracellular signal-regulated kinase 1; ET1, endothelin 1; FOS, Fos proto-oncogene, AP-1 transcription factor subunit; eNOS, endothelial nitric oxide synthase; HIF, hypoxia-inducible factor; HPH, hypoxia-induced pulmonary hypertension; HPV, hypoxic pulmonary vasoconstriction; LC3B, autophagy-related ubiquitin-like modifier LC3 B; MCT, monocrotaline; NO, nitric oxide; OVX, ovariectomy; P27Kip1, cyclin-dependent kinase inhibitor 1B; PGI2, prostacyclin; RAGE, receptor for advanced glycation end products; RVEF, right ventricular ejection fraction, RVH, right ventricular hypertrophy, SERT+, serotonin transporter over-expression; SKP2, S-phase kinase-associated protein 2; SuHx, sugen-hypoxia; Tph1, tryptophan hydroxylase 1; VO2max, maximal oxygen uptake.
The Estrogen Puzzle in Animal Models of PAH
Studies in classic (HPH and MCT) animal models of PH have shown that estrogen ameliorates the progression of PH in the pulmonary vasculature, a finding that is not surprising given the protective role of female sex in these animal models. The SuHx-PH rodent models have produced seemingly contradictory findings as both estrogen administration and estrogen antagonism have been shown to ameliorate PH outcomes. Still other research models have identified estrogen as a potent disease mediator in the pulmonary vasculature. In the following sections, we review what is known about estrogen’s effect in the lung during PH progression, followed by a summary of estrogen’s effects on the RV. The results of studies focusing on bioactive estrogen metabolites will be discussed separately. An overview of the major studies of E2 signaling in experimental PH is provided in Table 5.
Animal Studies Demonstrating Protective Estrogen Effects in the Pulmonary Vasculature
Protective effects of endogenous and exogenous estrogen exposure in female and male animals during chronic hypoxia exposure have been demonstrated by multiple research groups (92, 115, 208, 335, 473, 474). Specifically, E2 administration has been shown to oppose hemodynamic alterations and pulmonary vascular remodeling in HPH both by attenuating hypoxia-induced upregulation of pro-angiogenic/pro-proliferative factors such as ET-1 (92), erythropoietin (296), and ERK1/2 (208) and promoting antiproliferative factors including the cell cycle inhibitor p27Kip1 (208, 474) and the autophagy marker LC3-B (208).
Several studies have focused on identifying the role of ER signaling and activation in the pulmonary vasculature during HPH. Lahm et al. found that E2 attenuates HPH in an ER-dependent manner, and that E2 exerts antiproliferative effects on hypoxic, but not normoxic, PAECs (208). In a study by Frump et al., a microarray analysis of HPH rat lungs treated with E2 or E2 plus the ER-antagonist ICI182,780 revealed that E2 regulates several genes that mediate proliferative and inflammatory processes during hypoxia and that these processes are dependent on ER (112). This method also revealed new ER targets in HPH including the bone morphogenic protein antagonist gremlin 1, which was upregulated in hypoxia but reduced by E2 treatment (112). Other studies sought to identify the relative importance of each ER subtype in protecting the pulmonary vasculature during HPH. These studies revealed that both ERα and ERβ play a role in E2-mediated protection during HPH as blockade of either receptor eliminates E2’s inhibitory effects on PA muscularization and ERK1/2 activation in hypoxic PAECs (208). However, multiple lines of evidence suggest that ERβ-mediated signaling may be more vital for E2-mediated protection in HPH. For example, chronic hypoxia upregulates ERβ but not ERα in both rat and human PAECs (115, 208). This occurs in a hypoxia-inducible factor 1α (HIF-1α)-dependent manner. ERβ activation, in turn, induces expression of the HIF inhibitor prolyl hydroxylase 2 (PHD2). Esr2 knockout mice display increased hypoxia-induced PA muscularization compared with wild-type or Esr1 knockout mice when treated with E2 during chronic hypoxia (115). ERβ and PHD2 therefore constitute a negative feedback loop that limits hypoxia-induced HIF-1α signaling and pulmonary vascular remodeling (115).
E2 supplementation is protective against pulmonary remodeling in MCT-PH. Farhat and colleagues demonstrated that E2 supplementation in male rats reduces PA remodeling 4 weeks after MCT administration (99). A study by Yuan et al. in ovariectomized female rats demonstrated that E2 administration reduced pulmonary vascular muscularization and reduced total pulmonary resistance after MCT injection (483). Protective effects of E2 in the pulmonary vasculature were associated with suppressed macrophage infiltration in the pulmonary vasculature, as well as increased lung NO and prostacyclin levels, and reduced ET-1 expression. The authors also demonstrated that MCT administration reduced plasma E2 levels and reduced aromatase expression in the lung tissue. In contrast, the E2-metabolizing enzymes CYP1A1 and CYP1B1 were upregulated in lung tissue. The authors suggested that MCT administration elicits an estrogen-deficient state. However, studies in PAH patients suggest a more complicated picture: while decreased E2 levels indeed have been reported in premenopausal women with PAH (482), another study demonstrated increased E2 levels in postmenopausal PAH patients (19), suggesting that E2 levels in PAH may be age dependent.
Two studies investigated the role of ERs in MCT-PH. A study by Umar et al. demonstrated that E2 administration attenuates MCT-induced PH and prevents disease progression to RV failure and death in male rats (432). In that study, E2 administration reduced PA muscularization, reduced lung inflammation and fibrosis, and induced pulmonary neoan-giogenesis. Most impressively, these results were shown in a rescue model, in which the rats received E2 supplementation after PH was established. E2’s protective effects were mediated by ERβ signaling, as coadministration of an ERβ antagonist removed E2-mediated protection, while treatment with the ERβ agonist DPN recapitulated E2’s effects (432). Another study, however, suggested that the protective effects of E2 in this model are not mediated solely by ERβ. Here, chronic treatment with the GPR30 agonist G1 after MCT exposure was also able to ameliorate the PH phenotype (5). Specifically, G1 treatment was associated with decreased MCT-induced pulmonary vascular remodeling, decreased pulmonary fibrosis, and higher levels of eNOS protein in the pulmonary vasculature compared with vehicle treated rats.
Several recent studies interrogated E2 effects on vascular remodeling and disease progression in the SuHx-PH model. SuHx-PH in rats is associated with reduced circulating E2 levels in female rats (114), similar to what has been described in MCT-PH (482). Ovariectomy-induced E2 depletion increased RVSP, whereas pulmonary vascular remodeling was not affected. E2 supplementation in ovariectomized female SuHx-PH rats, on the other hand, reduced RVSP and PA muscularization compared with ovariectomized SuHx-PH females and even intact SuHx-PH females. This suggests that E2 repletion in ovariectomized SuHx-PH females creates “super responders”, a phenomenon whose underlying mechanisms require further study. In the same study, male SuHx-PH rats were implanted with subcutaneous E2 pellets to elevate circulating E2 levels to those of female rats, but E2, while attenuating right ventricular hypertrophy (RVH) and rescuing CI to control levels, did not attenuate RVSP increases or prevent PA vascular remodeling (114). In a separate study, the same group employed the SuHx-PH rat model to examine the interaction between PH and exercise tolerance (213). In this experiment, ovariectomy worsened increases in RVSP (but not PA remodeling), and E2 supplementation in ovariectomized rats decreased PA pressures and reduced the abundance of fully muscularized vessels in the pulmonary vasculature. More critically, E2 supplementation was associated with a significant reduction in postexercise total pulmonary resistance (a surrogate for PVR (203)), indicating superior performance of the E2-supplemented pulmonary vasculature after strenuous exercise. Liu et al. examined the effect of E2 supplementation on pulmonary hemodynamics in ovariectomized females in the SuHx-PH mouse model and found that E2 decreased PA elastance (a marker of RV afterload) and increased PA global compliance and transpulmonary vascular efficiency (defined as the ratio of cardiac output to total hydraulic power over the cardiac cycle) (232, 234, 235). Structurally, the authors demonstrated that E2 supplementation rescued SuHx-induced increases in PA wall thickness and collagen content. One curiosity is that Liu et al. did not find medial hypertrophy in the distal pulmonary arterioles as a result of SuHx-PH, likely because mice show a less robust response to this treatment than rats (446). Taken together, the results from the SuHx-PH studies suggest that E2, while being able to attenuate PA pressure increases and remodeling, has somewhat less-consistent effects on the PA than in the traditional PAH models such as HPH and MCT-PH (with the exception of E2 repletion in ovariectomized females, which dramatically rescues the PH phenotype). This is in stark contrast to E2’s profound RV effects in this model, which will be discussed further below. One important caveat is that sugen administration has been shown to upregulate both aromatase and CYP1A1 in rat lung tissue, indicating that pulmonary estrogen metabolism may be changed in this model of PAH (70). How these changes correlate with estrogen signaling in human PAH is currently under investigation.
Animal Studies Identifying Estrogen as a Disease Mediator in the Pulmonary Vasculature
Not all studies have demonstrated a protective effect of estrogen in the pulmonary vasculature. In fact, estrogen has been shown to promote PH development in several transgenic mouse models of PH—SERT+ mice (462), BMPR2−/− mice (51), and S100A4/Mts1 overexpressing mice (72). For example, female mice overexpressing the serotonin transporter (SERT+) develop severe PH when exposed to hypoxia, an effect not seen after ovariectomy. Additionally, exogenous E2 administration restores PH susceptibility after ovariectomy in SERT+ females and induces proliferation in cultured human PASMCs in a manner dependent on de novo serotonin synthesis and activation of the serotonin receptor 5HTB (1B) (462). A latter study by the same research group demonstrated that the PH phenotype of SERT+ mice could be ameliorated by treatment with the ERα inhibitor MPP, which eliminated the proliferative pulmonary vascular phenotype while increasing expression of BMPR2 in the lungs of SERT+ mice (469).
Heritable PAH in humans is associated with mutations in the TGFβ superfamily receptor BMPR2 (62), and BMPR2 knockout mice spontaneously develop a mild form of PH (160, 458). Recently, Chen et al. demonstrated that the PH phenotype and the occurrence of muscularized pulmonary arterioles were reduced by estrogen inhibition (with fulvestrant, anastrozole, or tamoxifen) in female BMPR2 mutants (51). Interestingly, when BMPR mutants were crossed with Esr1 or Esr2 knockout mice, loss of Esr1 reduced the rate of total pulmonary vessel occlusion while knockout of Esr2 completely eliminated vessel occlusion. This implies that ERβ signaling mediates the pathologic effects of estrogen in this model. The authors also linked estrogen signaling and Esr2 to the occurrence of metabolic defects such as oxidized lipid formation and insulin resistance as well as decreased abundance of the metabolic modulators peroxisome proliferator-activated receptor-γ and CD36.
In mice overexpressing the calcium-binding protein S100A4/Mts1, E2 administration further upregulated the expression of S100A4/Mts1 in PASMCs (72). This effect was associated with increased activation of S100A4’s receptor RAGE (receptor for advanced glycosylation end products) and a proliferative PASMC phenotype, leading to occlusive lesions in the pulmonary vasculature. There is convincing evidence that RAGE overexpression by PASMC contributes to PAH in humans, and RAGE inhibitors are effective in treating both MCT-PH and SuHx-PH in rats (270). Effects of endogenous and exogenous estrogens on mediating RAGE-induced SMC proliferation remain under investigation.
In another study of detrimental pulmonary estrogen signaling, Mair et al. showed that aromatase inhibition attenuated disease progression in both a mouse HPH model and a rat SuHx-PH model, but only in female animals (248). In both disease models, the aromatase inhibitor anastrozole decreased pulmonary vascular remodeling in a dose-dependent manner in females. Anastrozole also rescued PH-mediated decreases in BMPR2 protein levels in the lungs of female animals. Interestingly, this study showed that SuHx treatment increases the level of endogenous circulating estrogens in female rats, contrary to studies from other research groups (114). Mair et al. also demonstrated that E2 can increase proliferation and inhibit BMPR2 signaling in PASMCs from healthy controls (249).
In summary, animal studies examining estrogen signaling in the pulmonary vasculature during PH have produced conflicting and paradoxical results (Table 5). Exogenous E2 administration improves PH outcomes and limits pulmonary vascular remodeling in HPH (92, 115, 208, 296, 335, 474), MCT-PH (99, 432, 483), and SuHx-PH (114, 213, 232, 234). On the other hand, attenuation of estrogen signaling by aromatase inhibition or ER antagonists appears to be protective in the HPH, SuHx-PH, and BMPR2 mutation models of disease in female rodents (248). Transgenic mouse models of PH have also identified estrogen as a necessary cofactor and mediator of specific disease pathways that contribute to pulmonary vascular remodeling (51, 72, 462). Differences in model species (mouse vs rat), animal age, or estrogen source (endogenous vs. exogenous) between studies may contribute to these conflicting results. Dosing strategies for estrogen or its inhibitors need to be taken into consideration. This is of particular importance, as E2 clearly can exhibit dose-dependent effects and since ER inhibitors can also be partial agonists (109, 263). Tissue- or cell-type-specific effects of estrogen or its inhibitors can be pronounced and induce variability as well. Furthermore, the timing of estrogen administration or inhibition relative to the onset and progression of PH may be an important factor. Lastly, confounders such as diurnal variations, estrogen coexposure in animal feeds, and even the gender of the animal handler need to be considered (383). More research into estrogen signaling in the lung considering these modifiers is required to parse the differential and contradictory effects shown here.
Estrogen Metabolites in Pulmonary Hypertension
Several estrogen metabolites have been identified as disease modifiers in PAH and may explain some of the divergent and paradoxical effects of estrogen signaling in disease models. 2-ME2, a nonestrogenic metabolite of E2, has been shown to be protective in several models of PH. Tofovic et al. were the first to demonstrate that 2-ME2 administration attenuates MCT-PH in male rats (420). 2-ME2’s protective effects were further characterized in several studies in which this metabolite attenuated MCT-PH in both male and female rats (418, 421) as well as in a model of bleomycin-induced PH and fibrosis (using ovariectomized female rats (422)). The synthetic analogue of 2-ME2, 2-ethoxyestradiol, exerts similar antimitogenic effects in the pulmonary vasculature and attenuates MCT-PH (423). 2-ME2 exerts strong antimitotic effects in ECs and inhibits ET-1 and MAPK activity even more acutely than E2 (81, 84). It has also been shown to inhibit systemic vascular remodeling by downregulating Akt and ERK1/2 activation, while upregulating cyclooxygenase-2 and the cell cycle inhibitor p27 (22). As 2-ME2 can be synthesized from circulating E2 by ECs and SMCs (82,485), it is possible that the protective effects of E2 are mediated at least in part by conversion to 2-ME2. Indeed, E2 does not inhibit proliferation of SMCs collected from Comt knockout mice (484), which do not express the necessary enzyme for formation of methoxyestradiol metabolites (2-ME2 and/or 4-ME2), indicating that synthesis of 2-ME2 may be important for estrogen effects in some cell types. Furthermore, pathological conditions such as hypoxia and inflammation (both factors in PAH/PH etiology and progression) have been shown to decrease activity of CYP1A1 (one of the enzymes responsible for E2 conversion to the 2-ME2 precursor 2-OHE2). Since this would result in limiting the 2-hydroxylation pathway (107, 407) and 2-ME2 production, it is conceivable that decreased 2-ME2 production could contribute to hypoxia- or inflammation-mediated PH. However, at least in hypoxia, this conversion is not necessary for E2 to attenuate PH development (208). In addition, selective ER agonists recapitulate some or all of the effects of E2 administration in many PH models, indicating that 2-ME2 is not the sole mediator of E2’s salutary effects. Lastly, at least in one study, 2-ME2 administration exhibited only modest effects (101).
In contrast to the antiproliferative effects of 2-ME2, the estrogen metabolite 16α-OHE1 has been identified as a contributor to PAH development. An association among BMPR2 genetic status, 16α-OHE1 production, and PAH development was first identified by West et al. (457) and Austin et al. (12). The authors found that a single-nucleotide polymorphism (SNP) in CYP1B1 (one of the enzymes responsible for production of 16α-OHE1 as well as 2-OHE2; Figure 3) results in lower CYP1B1 activity and a lower urinary 2-OHE2/16α-OHE1 ratio in BMPR2 carriers that developed PAH as compared to nonaffected carriers. The data from animal models of PH supports the theory that increased 16α-OHE1 activity promotes PH. White et al. demonstrated that pulmonary CYP1B1 levels are increased by both hypoxia and SuHx-PH, that Cyplbl knockout mice are resistant to HPH, and that administration of the CYP1B1 inhibitor 2,3′,4,5′-tetramethoxystilbene (TMS) attenuates PH resulting from both hypoxia or SuHx (463). While it is unclear why White et al. found increased CYP1B1 expression as compared to the decreased activity noted by West and Austin, both groups implicated 16α-OHE1 in PAH development. In vitro experiments by White et al. confirmed that 16α-OHE1 provoked proliferation in human PASMCs, particularly PASMCs collected from PAH patients. Finally, the authors demonstrated that 16α-OHE1 injection could elicit a PH phenotype in mice (463). Other models of PH have also been shown to exhibit upregulated CYP1B1 expression in the lung, including SERT+ mice, anorexigen-induced PH female mice, and female MCT-PH rats (71, 464, 483), identifying altered CYP1B1 activity as a disease mediator in experimental PH. Interestingly, administering 16α-OHE1 to BMPR2 mutant mice doubles the penetrance of PH (101) and disrupts cellular metabolism through upregulation of miRNA-29 (52). Both CYP1B1 and 16α-OHE1 are attractive therapeutic targets due to a conserved expression pattern between humans and animal models, and the dramatic effect of inhibition in animal models.
Taken together, while there is a discrepancy between animal and human studies with regard to CYP1B1 activity/expression, there is robust evidence that 16α-OHE1 promotes a PAH phenotype in vivo and in vitro. 2-ME2, on the other hand, can promote adaptive processes. These studies have led to a paradigm of “bad” and “good” estrogen metabolites and altered estrogen metabolism in PAH.
Human Studies
Despite the epidemiologic observations reviewed above, until recently few clinical studies have explored the role of estrogens in PAH pathogenesis, risk, and outcomes. Higher levels of circulating E2 have been found in both men and postmenopausal women with PAH as compared to age- and body mass index-matched healthy controls (19, 441, 471). In a study comparing 23 men with WHO Group 1 PAH (including idiopathic PAH, heritable PAH, and CTD-associated PAH) and 67 sex-, age-, and weight-matched healthy controls, higher E2 levels were associated with the risk of PAH, such that a 1-unit increase in E2 increased the risk of PAH 50-fold, and also shorter 6MWD in PAH cases (441). These results were replicated in a larger prospective cohort of 95 men with idiopathic PAH from China; in this study, higher E2 levels were also independently associated with death in PAH patients (471). In 112 postmenopausal women ≥ age 55 with idiopathic PAH, heritable PAH, or CTD-associated PAH, PAH cases had higher circulating E2 levels as compared to matched controls, and higher E2 levels were associated with shorter 6MWD (each per unit increase was associated with a 32-m decrement in 6MWD, the minimally important difference for the 6MWD in PAH), higher RAP, and worse functional class (19, 259). In postmenopausal women with limited cutaneous SSc, the use of HT (most commonly combination estrogen/progesterone) after menopause decreased the risk of the development of echocardiographic PH, although there were no differences in single measurements of E2 levels between those who did and did not develop PH (30). A second study demonstrated that menopause was an independent risk factor for the development of PH in SSc (363). There have been no published studies that measure circulating estrogens in premenopausal women with PAH, perhaps because it is difficult to control for the impact of menstrual cycle variation on these levels.
As mentioned above, 2-OHE2 and 16α-OHE1 have different effects on inflammation and mitogenesis and have previously been implicated in oncogenesis and vascular metastatic invasion (83). Austin and colleagues discovered alterations in E2 metabolism in heritable PAH and demon-strated that a CYP1B1 polymorphism increased the risk of PAH penetrance in women with BMPR2 mutations, but not in carrier men (12). In addition, the presence of the CYP1B1 mutation in PAH patients was associated with lower urinary 2-OHE2/16α-OHE1 ratios (12, 13, 463). In a small study, 10 men with heritable PAH had a higher proportion of the mitogenic metabolite (16α-OHE1) compared to the anti-inflammatory/antiproliferative metabolite (2-OHE2) as compared to healthy controls (101). This same group described direct ERα binding to the BMPR2 promoter, leading to reduced BMPR2 gene expression in females, and demonstrated that 16α-OHE1 promotes the development of heritable PAH via upregulation of microRNA-29, which plays a key role in cellular energetics and metabolism (13, 52). Interestingly, increased expression of 16α-OHE1 has been demonstrated in pulmonary arterioles from the explanted or autopsied lungs of PAH patients as compared to non-PAH lungs (463). These important connections among sex, E2 metabolite balance, altered BMPR2 expression, and effects on cellular metabolism may underpin the female predominance of heritable (and perhaps idiopathic) PAH. The role of these pathways in men with PAH and nonheritable disease has not been elucidated.
The Pulmonary Vascular Complications of Liver Disease Cohort enrolled patients with chronic liver disease and then performed a case-control study to determine genetic risk factors for portopulmonary hypertension in 15 candidate genes of interest (340). Polymorphisms in ESR1 and CYP19A1 (which encodes for aromatase) were associated with altered risk of developing portopulmonary hypertension. Moreover, biologic activity of the aromatase variants was suggested by a correlation between increased circulating E2 levels and genotype (340). Both ERα and aromatase are present in human lungs. Specifically, increased ERα expression has been demonstrated predominantly in PASMCs from explanted lungs of women with PAH as compared to control lungs (469). Peripheral aromatase activity (which accounts for most of the estrogen production in postmenopausal women and men) is present in SMCs of the small muscular PAs of explanted lungs from women with PAH. Complementary animal experiments implicate this enzyme in pulmonary vascular disease pathogenesis (248, 469).
A recently completed randomized, double-blind, and placebo-controlled trial demonstrated a significant reduction in circulating E2 levels with anastrozole treatment versus placebo, but no effect on echocardiographic RV measures at 12 weeks in 18 men and postmenopausal women with Group 1 PAH (193). Other hormone levels (specifically testosterone, progesterone, and DHEA-S) were not affected. Active treatment also resulted in an improvement in 6MWD (+26 m) compared to placebo (−12 m) (median % change from baseline was +8% versus −2%, respec-tively [p = 0.042]), and there were no adverse events. This small pilot study demonstrates the feasibility of hormonal manipulation as a treatment strategy in PAH. A longer, larger Phase II multicenter, randomized, double-blind, and placebo-controlled trial of anastrozole is ongoing (NCT03229499) in men and postmenopausal women with WHO Group 1 PAH. Tamoxifen, a selective ER modulator, is also being studied in a single-center, randomized, double-blind, and placebo-controlled Phase II trial in subjects with idiopathic, heritable, drug- or toxin-induced, or CTD-associated PAH (NCT03528902).
Estrogens and RV function
Cell Culture and Animal Studies
Previous studies in the LV demonstrated that estrogen signaling promotes the maintenance of myocardial metabolism and inhibits inflammation, fibrosis, and apoptosis in response to acute or chronic injuries such as pressure overload (104, 173, 216, 230, 452, 453). Of note, cardioprotective effects of the PDE5 inhibitor sildenafil in two animal models of left ventricular dysfunction are estrogen dependent (354). Healthy women exhibit superior RV systolic function compared to men, a relationship that correlates with E2 levels and that persists among patients with PAH (192, 196, 444). These observations indicate that E2 may exert direct RV-protective effects during PAH/PH, altering disease progression independent of its effects in the pulmonary vasculature. Indeed, several animal studies have confirmed this notion.
E2 supplementation of male rats during hypoxia reduced RVSP and RV hypertrophy, while increasing cardiac output (208). Cotreatment with the ERα antagonist MPP increased RV hypertrophy and reduced cardiac output to levels seen in untreated hypoxic animals. Nonselective (dual) ER blockade was required to attenuate other E2-mediated effects inthe RV such as ERK1/2 inhibition. These results indicate that estrogen signaling in the chronically hypoxic RV may involve multiple ERs, which initiate both unique and redundant downstream signaling. However, it should be noted that these effects on RV structure, function, and signaling may be secondary to the lower RVSP and PA remodeling noted with E2 treatment.
Several studies found that E2 mediates RV-protective effects in MCT-PH. Umar et al. treated male rats with E2, ERα agonist (PPT), or ERβ agonist (DPN) after MCT injection (432). E2 supplementation improved RVEF and decreased RV hypertrophy. E2 also promoted neoangiogenesis in the RV, thereby increasing vessel density. This observation is critical as vessel rarefaction and impaired angiogenesis in the RV is suggested to play a major role in RV failure during PH (reviewed in Ref. 113). Protective effects of E2 were recapitulated by DPN, suggesting that ERβ is involved in mediating its cardioprotective effects. Another study by the same group demonstrated similar effects of E2 administration after MCT injection of aged (12–14 months) female ApoE knockout mice, indicating that estrogen may be vital in attenuating PH in aged subjects with a disrupted reproductive cycle (433). Nadadur et al. demonstrated that E2 treatment reduces RV fibrosis after MCT administration (298). Effects of E2 in this study were largely recapitulated by the ERβ agonist DPN, similar to the effects noted by Umar et al. In recent studies from Alencar et al., activation of GPR30 with the GPR30 agonist G1 attenuated the effects of MCT in a rat model (5, 6), indicating that nongenomic estrogen signaling may also be critical to RV protection. As in the studies performed in HPH models, E2 or ER agonist also affected RVSP and PA remodeling in all these studies, making it difficult to dissect whether ER signaling exerts direct effects on the RV or whether RV structure and function improved as a result of lower RV afterload. However, Nadadur et al. demonstrated that E2 administration decreases fibrosis markers in cultured cardiac fibroblasts (298), indicating that this cell population is a direct target of E2.
More recent studies evaluated E2’s effects in angiopro-liferative PH. Using the SuHx-PH animal model, multiple groups have identified robust RV-protective effects of E2 (114, 213, 232-235). In one study, both endogenous (intact females) and exogenous (E2 repletion in ovariectomized rats) estrogens improve cardiac output and exercise capacity, and attenuate SuHx-induced increases in expression of pro-inflammatory and proapoptotic mediators as well as markers of mitochondrial dysfunction and oxidative stress. In addition, E2 increased abundance of the pro-angiogenic and pro-contractile peptide apelin (114). SuHx-PH also decreases expression of ERα in the RV, while E2 repletion increased ERα abundance. ERβ, GPR30, and aromatase, on the other hand, were not altered. E2 supplementation was also RV protective in male SuHx-PH rats, and administration of an ERα agonist replicated these effects. ERβ agonist treatment, on the other hand, was less efficacious, suggesting that ERα is primarily mediating E2’s RV-protective effects. A later study demonstrated that E2 abrogates decreases in RV function in SuHx-PH induced by an acute exercise challenge, an effect that was accompanied by increased RV antiapoptotic signaling, eNOS activation, and signs of improved autophagic flux. Similar to the results from Nadadur et al., Lahm et al. noted that E2 inhibited RV fibrosis (213). Concomitant inhibitory effects on RVSP and PA remodeling in both studies precluded determining whether E2’s effects on the RV were direct or indirect. This question was elegantly addressed by Liu et al. (233, 234). Using a SuHx-PH mouse model with E2 repletion in ovariectomized females, these authors demonstrated that E2-mediated cardioprotective effects in this model were both direct (by increasing RV contractile function) and indirect (by decreasing collagen accumulation and increasing compliance in the proximal PA). These authors also examined mitochondrial function in the RV and found that E2 supplementation improved both mitochondrial density and respiratory function compared with placebo-treated animals (233). Finally, this group demonstrated that E2 treatment preserves PA compliance after SuHx treatment, which reduces the pulsatile load on the RV, leading to improved RV function and improved ventricular-vascular coupling (232, 235).
In summary, data from multiple animal models clearly demonstrate that E2 improves RV function, structure, and biochemical processes in PH (Table 5). Interestingly, even in animal models of E2-mediated pulmonary vascular prolifer-ation, RV-protective effects such as reduced RV hypertrophy are often observed (419, 462). We are now beginning to understand the mechanisms of estrogenic signaling in the RV. All three ERs have been implicated in mediating RV protection (albeit with differences between model system), but their individual contributions and importance must be studied in more detail. In addition, PA-independent effects of estrogenic signaling (e.g., using a PAB model) need to be studied in more detail.
Human Studies
A key and unanswered question in human PAH is whether estrogens have a direct role in RV adaptation, which may serve to explain why more women than men develop PAH but have preserved RV function and better survival (Table 2). While such a role of estrogens is clearly suggested by the animal studies reviewed above, there are no human studies directly linking circulating estrogens to RV morphology in PAH, and the only observational data to date has been performed in an epidemiologic cohort without clinical cardiovascular disease (the MESA-RV Study). In postmenopausal women from MESA-RV using HT, higher E2 levels were associated with higher RVEF and lower RV end-systolic volume, but this relationship was not seen in non-HT users or men (444). The association seen in HT users only may be explained by greater E2 levels, a higher degree of variation in E2, altered or unmeasured estrogen metabolites, or protein/receptor interactions. Exogenous HT may lead to upregulated ER tissue expression and altered E2 sensitivity (76). High E2 states have been associated with heart neovascularization, and human ventricular myocardium contains functional ERs, which may result in adaptive remodeling and better RV systolic function (174, 272).
Cytochrome P450 enzymes are preferentially expressed in the RV (as compared to the LV) in humans (414). A follow-up study in MESA-RV demonstrated that genetic variation in CYP1B1 was independently associated with increased RVEF in postmenopausal women (443). This polymorphism is tightly linked to the variant that increased the risk of PAH in BMPR2 carriers (12) described above as well as variants tied to angioinvasion in cancer (75, 190, 312, 456). There were no associations noted in other candidate genes important in estrogen signaling and metabolism including ESR1 or ESR2 or CYP19A1. Urinary estrogen metabolites were also measured and were associated with RVEF but did not mediate the CYP1B1 SNP-RVEF relationship. Interestingly, the CYP1B1 polymorphism-RVEF association was strongest in black women, who have the highest female predominance in PAH. Activity and by-products of the cytochrome P450 subfamilies and E2 metabolite balance can be altered acutely during hypoxia, inflammation, with the onset of vascular disease, and with daily dietary changes (184, 275). This suggests that the impact of these metabolites on the cardiopulmonary unit is complex and may vary depending on an individual’s race/ethnicity, age, hormonal milieu (endogenous and exogenous), and disease course. These observations lend support to the hypothesis that sex hormones and their genotypes may have pleiotropic effects on the pulmonary circulation and RV and give rise to unique sex-based phenotypes in PAH. Given the profound effects of E2 on RV function, the two ongoing trials of E2 reduction as a treatment strategy in PAH (anastrozole, NCT03229499 and tamoxifen, NCT03528902) are incorporating echocardiographic measures of RV function as safety and efficacy end points in subjects with PAH.
In summary, data from animal and human studies confirm that estrogens are a clinically relevant modifier of RV function in PAH/PH (Tables 2 and 5). Elucidating the exact role of estrogenic signaling during RV adaptation to increased afterload may allow for the development of targeted therapies that improve cardiac adaptation in PAH/PH while avoiding potential off-target effects in the pulmonary vasculature.
Testosterone in PH
Testosterone Signaling and Metabolism
Testosterone and its metabolite DHT are ligands for the androgen receptor (AR). DHT is a far more potent androgen than testosterone (approximately 10 times) due to its relatively higher binding affinity and slower dissociation rate from the AR (134). Like other steroid hormones, androgens signal primarily through a genomic pathway to alter gene expression. Ligand binding initiates AR dimerization, nuclear localization, binding to androgen response elements in the DNA, and transcriptional modification of target genes. Nuclear cofactors (coactivators/corepressors), chromatinmodifying enzymes, and posttranslational modification of the AR all play a role in modifying androgen signaling in target cells (261). Like estrogens, androgens may also exert effects through a nongenomic signaling pathway acting through cytoplasmic or membrane-bound ARs. By doing so, testos-terone or DHT can rapidly initiate cell signaling pathways including rapid calcium influx (128), MAPK signaling (320), PI3K/AKT activation (121), cytoskeletal reorganization (311), or apoptosis (65, 413). Androgen signaling is vital for development and function of the male reproductive tract and development of secondary sex characteristics in males, but also plays a critical role in the cardiovascular system (85). Particularly relevant to PAH/PH, the AR is expressed in vascular SMCs, EC, lung tissue, and both atrial and ventricular cardiomyocytes (85, 237, 238, 276). Circulating testosterone levels fall in aged males (Table 4), and animal models indicate that this effect may be compounded by a reduced androgen sensitivity of the vasculature (94). Isolated coronary arteries from aged male rats display a muted response to testosterone in vitro (94), while AR expression in heart tissue is dependent on circulating testosterone levels (124). Careful study of androgen signaling in the cardiovascular systems of men and women in the context of aging, estrogen/androgen balance, and pulmonary vascular disease may provide data relevant to the sexually dimorphic progression of PAH.
As discussed in the hormone synthesis section, circulating testosterone may be converted to E2 by aromatase in peripheral tissues. It is therefore conceivable that any experimental effects of endogenous or exogenous testosterone administration could be mediated by estrogen signaling in target tissues after aromatization. To this point, the ratio of circulating E2/testosterone has been associated with cardiovascular disease risk in epidemiologic studies (68, 489). Careful monitoring of hormone levels and/or administration of nonaromatizable DHT is required in experimental systems to accurately identify the effect of androgens in PH.
Testosterone in the Pulmonary Vasculature
Cell Culture and Animal Studies
Studies in isolated human (343, 382) and rat (95) pulmonary vessels demonstrated that testosterone is a powerful vasodilator in this vascular bed. While testosterone elicits vasodilation in tissue collected from either sex, the vasodilatory response appears to be greater in male tissue (95, 343). Acute testosterone-induced vasodilation is nongenomically mediated by antagonistic effects on voltage-gated calcium channels and a subsequent reduction in calcium influx into SMCs (139, 181, 364). While the acute vasodilation response to testosterone involves vascular SMCs, androgens also act through genomic signaling pathways to promote NO synthesis in cultured systemic ECs (123, 280). Both classical AR signaling and activation of ER signaling after aromatization have been implicated in this process (reviewed in Ref. 241), and the pulmonary vasculature of male eNOS knockout mice exhibits increased muscularization compared to female knockouts (278). While testosterone-induced vasodilation would be expected to protect the male lung against incipient PAH/PH, effects of testosterone on other clinically relevant processes in PAH (e.g., proliferation, metabolism, and inflammation) are largely unknown. In particular, the role of androgens in pulmonary vascular remodeling during PAH has not been studied. In cultured systemic vascular SMCs and ECs, androgenic signaling promotes proliferation (117, 384). In addition, androgen signaling opposes EC dysfunction, oxidative stress, and inflammation (55). These data suggest that testosterone may exert biologically relevant effects in the pulmonary vasculature (Table 6) as well and provide a rationale to study these pathways in more detail.
Table 6.
Animal studies of progesterone and testosterone in PH.
| Model | Species | Major findings | References |
|---|---|---|---|
| Isolated lungs or isolated PAs | Rat, sheep | Testosterone, progesterone ↑ PA vasorelaxation | (95, 116, 180, 226, 382) |
| Hypoxia-induced PH (HPH) | Rat | Testosterone ↑ RV hypertrophy in castrated male rats | (286) |
| Monocrotaline-induced PH (MCT-PH) | Rat | Progesterone protective in OVX females; ↓ RVSP, ↓ RV hypertrophy, ↓ PA remodeling, ↓ mortality | (416) |
| Pulmonary artery banding | Mouse | Testosterone ↓ RV function and ↑ RV remodeling | (154) |
OVX, ovariectomy; PA, pulmonary artery; RV, right ventricle; RVSP, right ventricular systolic pressure.
Human Studies
Testosterone deficiency has been demonstrated in a number of chronic diseases (341, 424). Two studies have measured circulating testosterone levels in men with PAH with discordant results. In a cohort of 95 Chinese men with idiopathic PAH, testosterone deficiency was found in 54% of PAH patients as compared to matched healthy controls (471). In 23 men with idiopathic, heritable, or CTD-associated PAH, there were no differences between total and bioavailable testosterone levels in PAH cases as compared to matched controls, perhaps due to the smaller sample size or greater variation in PAH diagnoses and race/ethnicity in this study (441). In both studies, the findings with circulating E2 levels (higher levels associated with PAH and more severe disease) were more robust and drove associations between greater E2/testosterone ratios and the risk of PAH. Total testosterone levels were not associated with disease severity in either study nor survival in the Chinese cohort. Lower levels of total testosterone and bioavailable testosterone increased the odds of PAH threefold in a study of postmenopausal women with Group 1 PH, but there were no consistent associations observed between lower testosterone (or bioavailable testosterone) levels and markers of PAH severity (19). Taken together, testosterone levels may be lower in PAH than in health (as has been described for many chronic diseases), but currently available human studies do not suggest that lower circulating testosterone levels are associated with worse pulmonary vascular disease.
Testosterone in the RV
Cell Culture and Animal Studies
RV hypertrophy in response to increased PVR is a hallmark of PH. Androgens promote cardiomyocyte hypertrophy in vitro (256), in animal models (43), and in cases of anabolic steroid abuse (2). However, this effect has primarily been studied in the LV. Compounding this effect, cardiac hypertrophy significantly elevates expression of 5α-reductase, increasing conversion of testosterone to DHT and promoting a positive feedback loop of androgen signaling and hypertrophy (415). Two recent studies have examined the role of androgen signaling in cardiac hypertrophy resulting from pressure overload. Montalvo et al. showed that male mice exhibit more severe LV dilatation in response to transverse aortic constriction (TAC) compared with female or castrated animals, and that this effect was TGFβ dependent (284). Zwadlo et al. demonstrated that DHT appears to drive this phenotype as inhibition of 5α-reductase significantly reduced LV hypertrophy in male mice after TAC (491). While there are physiological differences between the left and right ventricle, animal studies focusing on the RV demonstrate a similar hypertrophic effect of androgens. Specifically, castrated male rats demonstrate RV hypertrophy when administered testosterone (286). Interestingly, the effects of testosterone on RV hypertrophy are additive to those of hypoxia, an effect not seen with other steroid hormones. In a PAB model of RV dysfunction, castrated male mice exhibit less RV hypertrophy, while testosterone replacement after castration leads to increased levels of hypertrophy (154). In parallel, testosterone promotes RV fibrosis, whereas testosterone deprivation appears to improve survival. This indicates that testosterone may promote a maladaptive type of RV hypertrophy.
Human Studies
Testosterone increases the myocardial inflammatory response and promotes cardiac remodeling (200, 300). Epidemiologic studies have shown that left heart failure is characterized by testosterone deficiency and is associated with worse cardiovascular outcomes in men (199, 251). The role of androgens in cardiovascular health remains controversial, however, because of mixed results with testosterone supplementation in human studies (41, 102). In the same study from MESA-RV, which demonstrated that higher E2 levels were linked to higher RVEF in postmenopausal women HT users, bioavailable and total testosterone levels were associated with greater RV mass and larger RV volumes (including RV stroke volume) in men only and were independent of LV measures (444). It is unknown whether these associations may be adaptive or maladaptive in pulmonary vascular disease as this study was cross-sectional in nature and performed in a cohort without clinical cardiovascular disease. There were no relationships noted between circulating testosterone or bioavailable testosterone levels and echocardiographic RV structure or function or natriuretic peptide levels in postmenopausal women with Group 1 PH; studies performed in men with PAH did not assess RV function (19).
In the follow-up genotype-RV phenotype study from MESA-RV, two polymorphisms in the AR gene were associated with RV end-diastolic volume and mass in men only and were dependent on circulating testosterone levels, indicating that these variants may have biologic relevance (443). ARs are present in human cardiomyocytes and stimulate hypertrophy with testosterone binding (256). Testosterone also directly regulates AR transcription during left ventricular hypertrophy in human hearts (415). AR interactions may lead to changes in RV morphology via both genomic and nongenomic effects that depend on the androgen, hormone concentration, cardiac receptor density, and sex of the individual.
Taken together, it appears that androgens may be protective in the pulmonary vasculature by promoting vasodilation, but detrimental to RV remodeling in the face of increased afterload (Tables 2 and 6). This hypothesis fits the clinical data in which men are less likely to develop PAH but display decreased survival rates compared with women. However, there is a paucity of mechanistic studies examining the role of androgen signaling in proliferative processes in the pulmonary vasculature. Studies from the systemic vasculature suggest that testosterone and androgenic signaling may promote PA wall cell homeostasis and also enhance proliferation, suggesting that pulmonary vascular effects of testosterone need to be studied in more detail. More mechanistic studies evaluating androgenic signaling in the RV are required as well.
Progesterone in PH
Progesterone Signaling and Metabolism
Progesterone is primarily synthesized by the ovarian corpus luteum during the menstrual cycle as well as by the placenta during pregnancy. Progesterone is one of the most critical hormones during pregnancy, and many physio-logical changes of pregnancy are progesterone mediated (reviewed in Ref. 153). Progesterone signaling is necessary for differentiation and maintenance of female reproductive tissues including the uterine and mammary epithelium (133). Like other steroid hormones, progesterone signals through binds to a steroid receptor (progesterone receptor, PR) and subsequently modulates gene transcription as well as through nonclassical pathways, which include genomic and nongenomic signaling cascades (120). Cofactors including transcriptional activators/repressors, chromatin-modifying enzymes, and posttranslational modification of the PR may alter progesterone signaling in target cells (133).
PR is expressed in ECs, including proliferative ECs and myofibroblasts found in plexiform lesions isolated from PAH patients (21,455). PR is also highly expressed in the systemic vasculature and in cardiac tissue, such as ECs and SMCs of the aorta, carotid and coronary arteries, and cardiomyocytes (172). Vascular expression of PR in the uterine vasculature varies according to the phase of the menstrual cycle and tends to decrease with age (217). It is possible that PR expression in other tissues demonstrates similar cyclicity. Interestingly, estrogen upregulates expression of the PR in cardiovascular tissue (186, 229), an effect that may be relevant to PAH disease progression.
In vitro culture of ECs revealed that PR activation suppresses cytokine production (122) and inhibits ET-1 synthesis (288). PR knockout mice demonstrate increased vascular medial hypertrophy and SMC proliferation after vascular injury, and isolated vascular SMCs from PR knockout mice are hyperproliferative in culture (188). These studies indicate that PR signaling might play a protective role by limiting the inflammatory, angiogenic, and proliferative phenotypes of PAH/PH; however, exogenous progesterone has also been shown to intensify vascular injury response in wild-type mice (188). Studies to clearly define the role of progesterone signaling in the pulmonary vasculature generally and in the context of PAH specifically are clearly needed.
Progesterone in the Pulmonary Vasculature and RV
Cell Culture and Animal Studies
Investigations of sex and gender differences in PAH/PH have focused on the role of estrogens, while remarkably few studies have examined the role of progesterone. This imbalance is surprising given that both hormones are much more abundant in women compared to men (Table 4).
Progesterone regulates proliferation of both ECs and SMCs (222, 223, 287, 438) and has been shown to be vasodilatory in pulmonary vessels isolated from both rats (95) and rabbits (226). While one study in humans found that oral progesterone supplementation opposed vasodilatory effects of estradiol (281), this study was not conducted in the pulmonary vasculature.
Tofovic et al. demonstrated that progesterone administration mitigates MCT-induced PH in rats (416). Specifically, progesterone supplementation in ovariectomized MCT-PH rats attenuated MCT-induced increases in RVSP, RV hypertrophy, PA remodeling, and mortality compared with untreated ovariectomized MCT-PH rats. Despite the encouraging results of this study, no data on the role of progesterone in animal models of PH has been published in the subsequent decade. Further studies that mechanistically evaluate pulmonary vascular effects of progesterone as well as the interaction between progesterone and estradiol in the context of PAH/PH could generate new knowledge to expand our understanding of sex steroid signaling and sex/gender differences in this field.
Little is also known about the role of progesterone during PH-induced RV remodeling. Progesterone promotes cardiac hypertrophy in other contexts, implying that it may drive RV adaptation as well. Progesterone can induce cardiac protein synthesis (125) as well as initiate hypertrophy in isolated rat cardiomyocytes (57). Additionally, progesterone is the dominant hormone of pregnancy, a condition that results in transient cardiac hypertrophy. Progesterone promotes cardiac hypertrophy in vitro, and PR activation inhibits apoptosis in cultured rat cardiomyocytes (293). One intriguing hypothesis is that progesterone may promote “physiological” (or adaptive) cardiac hypertrophy (56, 57, 283, 472) rather than the maladaptive cardiac hypertrophy associated with severe PH. However, this hypothesis has yet to be tested in the context of PAH/PH. On the other hand, pregnant women with PAH are at a particular high risk for increased morbidity and mortality (153, 273, 400). Whether this is directly or indirectly linked to the increased progesterone levels of pregnancy is unknown. A better understanding of the cardiopulmonary effects of progesterone in health and disease is critical for understanding the mechanisms of adaptive and maladaptive changes in both pregnant and nonpregnant women with PAH.
Human Studies
While progesterone has known effects on the respiratory system, its impact on the cardiovascular system is less well understood (20, 77). Two studies (described above) measured progesterone levels in PAH patients (19, 471). In postmenopausal women, there were no differences in circulating progesterone levels in subjects with PAH as compared to matched controls and no associations between progesterone levels and disease severity in PAH subjects including RV function assessed by echocardiography (19). In the study of men with idiopathic PAH from China, lower progesterone levels increased the risk of PAH and were associated with worse functional class, shorter 6MWD, and more severe hemodynamic impairment (471). There was no association between progesterone levels and mortality in this study, and RV structure and function were not assessed. There have been no studies of circulating progesterone levels in premenopausal women with PAH, which may be more informative than in postmenopausal women, and no human studies to date of the relationship between progesterone and RV performance in PAH or in health.
Taken together, the limited body of literature on progesterone in PAH/PH demonstrates that this hormone may be beneficial in the pulmonary vasculature and possibly even in the RV (Tables 2 and 6). However, more studies of progesterone in PH are needed to more clearly define its role.
DHEA in PH
DHEA Signaling and Metabolism
Pregnenolone is the prohormone to progesterone and DHEA. DHEA and DHEA-S are precursors in the biosynthesis of androgens and metabolized directly to androstenedione, testosterone, and subsequently estrogens. DHEA and DHEA-S are produced predominantly in the adrenal cortex and are the most abundant circulating endogenous steroids but wane with aging. The hormone has been shown to have direct genomic and nongenomic effects on vasculature as well as cardiomyocytes (254). DHEA binds directly to vascular endothelium to activate NO synthase and regulates ET-1 production, two key drivers in PAH pathobiology that are also major treatment targets (50, 236, 254, 301). When human systemic vein ECs are exposed to DHEA, inflammatory signaling is reduced (7, 451). DHEA has been shown to rescue cardiomyocyte hypertrophy induced by ET-1 (301) and to prevent myocardial fibrosis and contractile dysfunction through the restoration oxidative balance and downregulation of advanced glycation end products (AGEs) and its receptors, reducing tissue levels of collagen and fibronectin (9, 10).
DHEA in the Lung Vasculature
Cell Culture and Animal Studies
DHEA exposure at variable concentrations has been shown to induce phenotypic changes in human ECs in vitro (7, 23, 164, 221, 316, 451). Effects include enhanced eNOS expression, NO synthesis, and variable ET-1 secretion (50, 164). Human PAECs actively metabolize DHEA, and treatment of PAECs from PAH patients decreases activation of STAT3 (277, 316), an important mediator of pulmonary vascular remodeling. DHEA appears to be consistently beneficial in experimental PH. Although these studies have been done in predominantly male animals, DHEA has been used as both a prevention and rescue strategy following exposure to hypoxia, altitude, MCT, MCT-pneumonectomy, and SuHx (8, 37, 88, 144, 159, 316). These studies and the mechanisms by which DHEA is proposed to prevent or reverse experimental PH as well as the effects on cardiomyocytes are summarized in Table 7.
Table 7.
Experimental data supporting beneficial effects of DHEA in PH.
| Model | DHEA effects | Proposed mechanism |
|---|---|---|
| Hypoxia | Prevent, rescue PH | BKCa channels (37, 144) |
| Rescue PH | BKCa inhibits 5HT-, KCl-induced SMC growth (89) | |
| Rescue RV | Reduces cardiomyocyte proliferation (88) | |
| Altitude | Prevent, rescue PH | Enhances sGC (306) |
| MCT | Prevent, rescue PH | Inhibits Src, STAT3, Pim1 |
| PASMCs | Increases BMPR2, miR-204 (316) | |
| MCT-PNX | Prevent, rescue | Inhibits RhoA/Rho kinase (159) |
| Sugen/hypoxia | Rescue RV > PH | Reduces RV capillary rarefaction, apoptosis, ROS via STAT3 (8) |
| Cardiomyocytes | Antichronotropic, antihypertrophic | Reduces T-type Ca channels (254) Inhibition of natriuretic peptide expression |
| Antihypertrophic | Reduces ET-1 induced hypertrophy (301) Inhibits BNP |
BKCa, Large conductance Ca2+-activated K channel; 5HT, serotonin; KCl, potassium chloride; SMC, smooth muscle cells; RV, right ventricle; GC, soluble guanylate cyclase; MCT, monocrotaline; STAT3, signal transducer and activator of transcription 3; BMPR2, bone morphogenetic receptor type II; PNX, pneumonectomy; ET-1, endothelin 1; BNP, B-type natriuretic peptide.
Human Studies
In addition to high levels of circulating E2 in PAH, studies of both men and postmenopausal women have demonstrated significantly lower (50%) levels of DHEA-S (which is more stable in blood samples than DHEA) in PAH subjects as compared to age- and body mass index-matched controls (19, 441). In men with PAH, lower levels of DHEA-S were associated with worse hemodynamics (higher RAP and higher PVR). There were more robust associations with disease severity in postmenopausal women (i.e., lower levels of DHEA-S were associated with worse 6MWD, functional class, hemodynamics, and worse RV function by echocar-diography) as well as an association with worse survival such that every unit decrease in DHEA-S was associated with a doubling in the risk of death (19). While DHEA is a prohormone of E2 and testosterone (and testosterone can be aromatized to E2), there were no strong correlations among hormone levels (DHEA-S, E2, and testosterone) and no evidence of effect modification among interrelated hormones, implying that DHEA may have a direct role in the development and progression of PAH and RV adaptation. A second study of unbiased metabolomic profiling demonstrated that DHEA-S and its metabolites were reduced in PAH patients compared to healthy controls and that lower circulating levels of DHEA-S were associated with mortality in PAH patients (337). In eight patients with PH related to chronic obstructive pulmonary disease, open-label treatment with 3 months of DHEA was associated with a significant increase in 6MWD and improvements in hemodynamics without adverse effects (90). These studies have led to the third randomized clinical trial of hormonal modulation as a treatment strategy in PAH; a single-center, double-blind, and placebo-controlled crossover trial of DHEA supplementation in subjects with Group 1 PH is ongoing (NCT03648385).
DHEA and the RV
Animal Studies
Several studies have demonstrated a role for DHEA in cardiomyocyte adaptation to injury (Table 7). In a PH/RV failure model, chronic DHEA treatment over 5 weeks in rats exposed to SuHx reduced RVSP and rescued CI and echocardiographic RV function (8). Treatment with DHEA inhibited capillary rarefaction, apoptosis, oxidative stress and NADPH levels, and fibrosis in the RV of these animals via reduced Rho kinase activity and inhibition of transcription factors implicated in maladaptive cardiac remodeling, STAT3 and NFATc3 (8). In rats exposed to chronic hypoxia followed by reoxygenation, DHEA increased RV myocyte density and proliferation, reduced mitochondrial fragmentation, and prevented RV dysfunction during the recovery phase of these experiments (88). These studies demonstrate that DHEA may have a direct and RV-specific effect independent of downstream hormones like E2.
Human Studies
Low circulating DHEA-S levels have been associated with an increased risk of death in heart disease, cardiac allograft vasculopathy, and heart failure severity (25, 100, 155, 290, 373) (Table 2). In the MESA-RV study, higher levels of DHEA were associated with lower RVEF (calculated from RV stroke volume/RV end-diastolic volume), higher RV stroke volume (calculated from RV end-diastolic—RV end-systolic volume), and larger RV end-diastolic volume in postmenopausal women who did not have any clinical cardiovascular disease. While a lower RVEF and larger RV stroke volume seem difficult to reconcile, higher RV end-diastolic volume with higher DHEA levels would result in larger RV stroke volume and numerically but not pathologically lower RVEF given the derivation of these parameters. In fact, virtually all participants had a normal RVEF (these are “disease-free” adults) in MESA and, as reviewed above, in postmenopausal women with PAH, lower DHEA-S levels were associated with worse RV dilatation and dysfunction by echocardiogram (444). The primary end point of the ongoing trial of DHEA supplementation in PAH is RV contractile function measured by cardiac MRI (NCT03648385).
In summary, the conglomerate of basic and clinical studies of DHEA in PH suggests beneficial effects on both pulmonary vascular and RV function (Tables 2 and 7).
Sex Hormone-Independent Effects
Sex steroid signaling clearly is a major driver of sex differences in susceptibility and disease progression in PH/PAH. However, the hormonal milieu is not the sole factor that impacts gender and sex-based differences in pulmonary vascular disease. Emerging research indicates that nonhormonal factors such as immune cell regulation, iron metabolism, and the Y chromosome itself may lead to sex-based differences in PH penetrance and progression.
A recent study demonstrated sexually dimorphic immune responses in experimental PH. In particular, the authors demonstrated that in regulatory T-cell (Tregs)-deficient rats exposed to sugen or hypoxia, females developed more severe PH than males (408). Interestingly, Treg repletion abolished this sex difference. In additional studies, the authors showed that protective vascular effects of Tregs were ER dependent, suggesting a cross talk between the immune system and sex steroid signaling. This observation suggests that females are reliant on normal Treg function to counteract detrimental effects of pulmonary vascular insults. Patient registry data supports this hypothesis as PAH is often associated with autoimmune disorders, and many of these disorders share similar or more skewed gender ratios as in idiopathic PAH (27). This indicates that altered immune responses may contribute to the female predominance in PAH. How sex-based differences in immunity impact PAH, as well as the role of sex steroid signaling in immune cell regulation, remains to be investigated.
Iron deficiency is a common comorbidity with PAH in humans (344, 385). Since iron is a required for degradation of HIFs (prominent drivers of PAH development), iron deficiency may contribute to the development and progression of PAH in some patients (332). Intravenous iron supplementation is currently the focus of a phase II clinical trial in PAH patients (162). Both globally (266) and in the United States (119, 218), iron deficiency is two to three times more prevalent in women than in age-matched men, suggesting that women may be disproportionately vulnerable to iron deficiency-related HIF activation in the pulmonary vasculature. However, this hypothesis has yet to be tested.
Finally, recent research indicates that the Y chromosome itself may play a protective role in PAH progression. Umar et al. used the Four Core Genotype (FGC) mouse model, in which the chromosome complement is independent of gonadal sex, to demonstrate that the presence of the Y chromosome protects mice from HPH development regardless of sex (431). An explanatory mechanism for this phenomenon was proposed by Yan et al. who demonstrated that the Y-chromosome-encoded transcription factor SRY (sex-determining region of the Y chromosome) promotes Bmpr2 expression in cultured male dermal fibroblasts (477). Altered BMP signaling plays a major role in vascular dysfunction in PH/PAH (reviewed in Ref. 243), and germ line mutations in BMPR2 are found in most cases of heritable PAH (14), making the mechanism discovered by Yan et al. conceptually sound. Additional studies evaluating potential SRY-regulated Bmpr2 expression in the lung vasculature and in vivo would be of tremendous value to the field.
How to Put it all Together: Common Themes, Knowledge Gaps, and Pathways Forward
The aggregate of studies reviewed in this article demonstrates that there has been significant progress in the study of sex/gender differences and sex hormone signaling in PAH/PH. In a relatively short time span, the field has moved from mechanistic cell culture and animal studies to human studies including clinical trials of hormonal modulation, suggesting that harnessing sex hormone signaling may provide a powerful new strategy to treat PAH/PH. The observation that sex hormones interact with several major disease modifiers such as BMPR2 signaling, metabolic function, and RV adaptation indicates that sex hormone signaling is indeed a major disease modifier. The major biological effects of the most abundant sex hormones as well as their major effects in animal studies and their association with PAH outcomes in human studies are depicted in Figure 4. Several “themes” have emerged: E2 has pleiotropic and compartment-specific effects, 16α-OHE1 promotes PAH development, and DHEA seems to be uniformly protective (Figure 4). E2’s RV-protective effects may explain why women with PAH have better RV function and live longer than their male counterparts. At the same time, E2 or its metabolites (in particular, 16α-OHE1) may promote pulmonary vascular remodeling and make certain women more prone to PAH development, especially in the context of additional predispositions, such as a BMPR2 mutation. The roles of testosterone and progesterone, on the other hand, have not been well studied. Since sex hormones exert diverse and pleiotropic effects, and since sex/gender differences are mediated by multiple factors (Figure 5), further research is needed to identify context- and compartment-specific signaling pathways and sex-based phenotypes. Nonhormonal factors, such as Y-chromosome-mediated effects, aging, and immunity, have recently been identified and may affect disease development and/or progression. Consideration of these (as well as potential unidentified) factors and nuances will ultimately solve the “estrogen puzzle” of PAH. An overview of the major current knowledge gaps in the field is provided in Table 8. Given the pleiotropic effects of many sex hormones (and in particular, E2), it remains to be determined whether inhibiting or enhancing one specific hormone will be meritorious. Selectively targeting one receptor or one metabolite may be a more precise approach with less off-target effects. In addition, sex-based treatment strategies may depend on factors such as receptor expression, sex hormone abundance, ratios between various sex hormones, age, comorbidities, or specific genetic or epigenetic landscapes.
Figure 4. Overview of major biological effects of the most abundant sex hormones (A) and their net effects in animal studies as well as reported associations with PAH risk and outcomes in human studies (B).
Note that effects of specific sex hormones on outcomes in human studies may be limited to men or pre- or postmenopausal women only. Human studies measured DHEA-S (dehydroepiandrosterone sulfate). *Data based on study only. #Inconsistent associations across studies. DHEA, dehydroepiandrosterone; PA, pulmonary artery; PAEC, pulmonary artery endothelial cell; PAH, pulmonary arterial hypertension (human studies); PASMCs, pulmonary artery smooth muscle cells; PH, pulmonary hypertension; RV, right ventricle.
Figure 5. Simplified overview of factors contributing to sex/gender differences and sexual dimorphism in PAH and PH.
Note that significant cross talk exists between the factors and mediators listed in this figure. BMPR2, gene encoding bone morphogenic protein receptor 2; CYP1B1, gene encoding cytochrome P450 1B1; CYP19A1, gene encoding aromatase; DHEA, dehydroepiandrosterone; ESR1, gene encoding estrogen receptor α; SNP, single-nucleotide polymorphism.
Table 8.
Knowledge gaps and pathways forward.
| Are there gender differences in RV remodeling/adaptive or maladaptive signaling in human RV failure? |
| What is the role of sex steroid receptors in the human RV? |
| Study of the entire “hormonosome” and interactions with cellular metabolism in experimental and human studies |
| What are the roles of genetics, epigenetics, environmental exposures, and cultural factors in mediating gender differences in human PAH? |
| Need for carefully executed human studies in premenopausal women with PAH and other life cycle transitions (e.g., puberty, menopause, and transgender individuals) |
| Need to study X- and Y-chromosome-mediated effects |
| Improve translational potential in animal studies—e.g., sex balanced experiments, age and estrous as a modifiers of experimental conditions |
Summary and Conclusion
This article has comprehensively reviewed gender differences in human PAH and sex differences in animal studies as well as the physiology of sex steroid signaling in health and PAH. The role of nonhormonal contributors to sex and gender differences in PAH/PH is less well described, but these factors may play a significant role as well. Sex, gender, and sex hormones clearly are major disease modifiers in experimental PH as well as human PAH. Manipulation of sex steroid signaling pathways may open up several new treatment strategies. In addition, several sex-based phenotypes exist, suggesting that therapeutic strategies may need to be tailored toward such specific phenotypes. A better understanding of sex hormone signaling and sex steroid-independent factors will lead to novel and targeted therapeutic approaches for PAH and PH patients of either sex.
Didactic Synopsis.
Major Teaching Points
Pulmonary hypertension (PH) encompasses a heterogeneous group of diseases organized into five groups based on their predominant underlying pathology and clinical phenotype (Figure 1).
The “estrogen puzzle” refers to two observations in PH research: (i) Many PH classes, particularly group 1 (PAH), are marked by sexually dimorphic disease presentation wherein women are at increased risk for disease development but display increased survival compared with men and (ii) animal models demonstrate contradictory effects of estrogen signaling in PH disease progression (protective as well as detrimental).
Human and animal studies have shown varied effects of 17β estradiol (E2) in the pulmonary vasculature in PAH/PH, but consistently show that E2 promotes healthy RV function and adaptation. Disease-promoting effects of E2 in the pulmonary vasculature are mediated at least in part by pro-proliferative metabolites (e.g., 16α-hydroxyestrone).
Few studies have examined the effect of progesterone or androgen signaling in PH though these hormones possibly play a role in disease susceptibility and progression.
DHEA is protective in animal models of PH, and circulating DHEA levels in PAH patients correspond positively with PH endpoints and RV function in humans.
List of Abbreviations
- ApoE
apolipoprotein E
- AR
androgen receptor
- BMPR2
bone morphogenetic protein receptor 2
- COMT
catechol-O-methyl transferase
- CYP
cytochrome P450
- DHEA
dehydroepiandrosterone
- DHEA-S
dehydroepiandrosterone-sulfate
- DHT
dihydrotestosterone
- DPN
diarylpropionitrile (ERβ agonist)
- E1
estrone
- E2
17β-estradiol
- E3
estriol
- ER
estrogen receptor
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- ESR1
estrogen receptor α gene
- ESR2
estrogen receptor β gene
- ET-1
endothelin-1
- GPR30
G-protein-coupled receptor 30
- HIF-1α
hypoxia-inducible factor 1-alpha
- HPAH
hereditary pulmonary arterial hypertension
- HPH
hypoxia-induced pulmonary hypertension
- HPV
hypoxic pulmonary vasoconstriction
- HT
hormone therapy
- IPAH
idiopathic pulmonary arterial hypertension
- LV
left ventricle/left ventricular
- MCT
monocrotaline
- NO
nitric oxide
- OVX
ovariectomy/ovariectomized
- PA
pulmonary artery
- PAB
pulmonary artery banding
- PAEC
pulmonary artery endothelial cell
- PAH
pulmonary arterial hypertension (Group 1 PH)
- PASMC
pulmonary artery smooth muscle cell
- PH
pulmonary hypertension (all groups)
- PPT
propylpyrazole triol (ERα agonist)
- PR
progesterone receptor
- PVR
pulmonary vascular resistance
- RV
right ventricle/right ventricular
- RVEF
right ventricular ejection faction
- RVH
right ventricular hypertrophy
- RVSP
right ventricular systolic pressure
- SERT+
serotonin transporter overexpression
- SMC
smooth muscle cell
- SNP
single-nucleotide polymorphism
- SuHx-PH
PH induced by sugen combined with chronic hypoxia
- 16α-OHE1
16α-hydroxyestrone
- 2-OHE2
2-hydroxyestradiol
- 2-ME2
2-methoxyestradiol
- 4-OHE2
4-hydroxyestradiol
References
- 1.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med 335: 609–616, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ven-tricular dimensions, and rhythm. Am J Cardiol 106 (6): 893–901, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn BH, Park HK, Cho HG, Lee HA, Lee YM, Yang EK, Lee WJ. Estrogen and enalapril attenuate the development of right ventricular hypertrophy induced by monocrotaline in ovariectomized rats. J Korean MedSci 18: 641–648, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev 11 (1): 124–150, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Alencar AK, Montes GC, Montagnoli T, Silva AMS, Martinez ST, Fraga AG, Wang H, Groban L, Sudo RT, Zapata-Sudo G. Activation of GPER ameliorates experimental pulmonary hypertension in male rats. Eur J Pharm Sci 97: 208–217, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alencar AKN, Montes GC, Costa DG, Mendes LVP, Silva AMS, Mar-tinez ST, Trachez MM, Cunha V, Montagnoli TL, Fraga AGM, Wang H, Groban L, Fraga CAM, Sudo RT, Zapata-Sudo G. Cardioprotection induced by activation of GPER in ovariectomized rats with pulmonary hypertension. J Gerontol A Biol Sci Med Sci 73: 1158–1166, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman R, Motton DD, Kota RS, Rutledge JC. Inhibition of vascular inflammation by dehydroepiandrosterone sulfate in human aortic endothelial cells: Roles of PPARalpha and NF-kappaB. Vascul Phar-macol 48: 76–84, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzoubi A, Toba M, Abe K, O’Neill KD, Rocic P, Fagan KA, McMurtry IF, Oka M. Dehydroepiandrosterone restores right ven-tricular structure and function in rats with severe pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 304: H1708–H1718, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, Catalano MG, Danni O, Boccuzzi G. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology 149: 380–388, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Aragno M, Meineri G, Vercellinatto I, Bardini P, Raimondo S, Peiretti PG, Vercelli A, Alloatti G, Tomasinelli CE, Danni O, Boccuzzi G. Cardiac impairment in rabbits fed a high-fat diet is counteracted by dehydroepiandrosterone supplementation. Life Sci 85: 77–84, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Arlt VM, Krais AM, Godschalk RW, Riffo-Vasquez Y, Mrizova I, Roufosse CA, Corbin C, Shi Q, Frei E, Stiborova M, van Schooten FJ, Phillips DH, Spina D. Pulmonary inflammation impacts on CYP1A1-mediated respiratory tract DNA damage induced by the carcinogenic air pollutant benzopyrene. Toxicol Sci 146: 213–225, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JAIII. Alterations in oestrogen metabolism: Implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J 34: 1093–1099, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, Phillips Iii JA, Gaddipati R, Gladson S, Gu E, West J, Lane KB. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ 3: 6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin ED, Loyd JE. Heritable forms of pulmonary arterial hypertension. Semin Respir Crit Care Med 34: 568–580, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA III, Palomero T, Sumazin P, Kim HR, Talati MH, West J, Loyd JE, Chung WK. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 5: 336–343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ba ZF, Lu A, Shimizu T, Szalay L, Schwacha mG, Rue LW 3rd, Bland KI, Chaudry IH. 17beta-Estradiol modulates vasoconstriction induced by endothelin-1 following trauma-hemorrhage. Am J Physiol Heart Circ Physiol 292: H245–H250, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism 50: 1001–1003, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: Baseline characteristics fromthe REVEAL Registry. Chest 137: 376–387, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Baird GL, Archer-Chicko C, Barr RG, Bluemke DA, Foderaro AE, Fritz JS, Hill NS, Kawut SM, Klinger JR, Lima JAC, Mullin CJ, Ouyang P, Palevsky HI, Palmisicano AJ, Pinder D, Preston IR, Roberts KE, Smith KA, Walsh T, Whittenhall M, Ventetuolo CE. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur Respir J 51, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee D, Ventetuolo CE. Pulmonary hypertension in pregnancy. Semin Respir Crit Care Med 38: 148–159, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Barberis MC, Veronese S, Bauer D, De Juli E, Harari S. Immunocyto-chemical detection of progesterone receptors. A study in a patient with primary pulmonary hypertension. Chest 107: 869–872, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res 99: 266–274, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Barkhausen T, Westphal BM, Putz C, Krettek C, van Griensven M. Dehydroepiandrosterone administration modulates endothelial and neutrophil adhesion molecule expression in vitro. Crit Care 10: R109, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron RM, Choi AJ, Owen CA, Choi AM. Genetically manipulated mouse models oflung disease: Potential and pitfalls. Am J Physiol Lung Cell Mol Physiol 302: L485–L497, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med 315: 1519–1524, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Barst RJ, Ivy DD, Foreman AJ, McGoon MD, Rosenzweig EB. Four- and seven-year outcomes of patients with congenital heart disease-associated pulmonary arterial hypertension (from the REVEAL Registry). Am J Cardiol 113: 147–155, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Batton KA, Austin CO, Bruno KA, Burger CD, Shapiro BP, Fairweather D. Sex differences in pulmonary arterial hypertension: Role of infection and autoimmunity in the pathogenesis of disease. Biol Sex Differ 9: 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hyper-tension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pul-monary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L1241–L1247, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Beretta L, Caronni M, Origgi L, Ponti A, Santaniello A, Scorza R. Hormone replacement therapy may prevent the development of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Scand J Rheumatol 35: 468–471, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Best DH, Sumner KL, Smith BP, Damjanovich-Colmenares K, Nakayama I, Brown LM, Ha Y, Paul E, Morris A, Jama MA, Dodson MW, Bayrak-Toydemir P, Elliott CG. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest 151: 821–828, 2017. [DOI] [PubMed] [Google Scholar]
- 32.Billon-Gales A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guery JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation 120: 2567–2576, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Björnström L Department of Cell and Molecular Biology KI, SE-171 77 Stockholm, Sweden, Sjöberg M, and Department of Cell and Molecular Biology KI, SE-171 77 Stockholm, Sweden. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19: 833–842, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure. Chest 135: 794–804, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Bohuslavova R, Kolar F, Kuthanova L, Neckar J, Tichopad A, Pavlinkova G. Gene expression profiling of sex differences in HIF1-dependent adaptive cardiac responses to chronic hypoxia. J Appl Physiol (1985) 109: 1195–1202, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Bolego C, Rossoni G, Fadini GP, Vegeto E, Pinna C, Albiero M, Boscaro E, Agostini C, Avogaro A, Gaion RM, Cignarella A. Selective estrogen receptor-alpha agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J 24: 2262–2272, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, Savineau J, Baulieu E. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100: 9488–9493, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, Hassoun PM, Rabinovitch M, Nicolls MR, Humbert M. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med 195: 583–595, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boos CJ, Mellor A, O’Hara JP, Tsakirides C, Woods DR. The effects of sex on cardiopulmonary responses to acute normobaric hypoxia. High Alt Med Biol 17: 108–115, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Boutet K, Frachon I, Jobic Y, Gut-Gobert C, Leroyer C, Carlhant-Kowalski D, Sitbon O, Simonneau G, Humbert M. Fenfluramine-like cardiovascular side-effects of benfluorex. Eur Respir J 33: 684–688, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Bremner WJ. Testosterone deficiency and replacement in older men. N Engl J Med 363: 189–191, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Burton RR, Besch EL, Smith AH. Effect of chronic hypoxia on the pulmonary arterial blood pressure of the chicken. Am J Physiol 214: 1438–1442, 1968. [DOI] [PubMed] [Google Scholar]
- 43.Cabral AM, Vasquez EC, Moyses MR, Antonio A. Sex hormone modulation of ventricular hypertrophy in sinoaortic denervated rats. Hypertension 11: I93–I97, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Caravita S, Secchi MB, Wu SC, Pierini S, Paggi A. Sildenafil therapy for interferon-beta-1a-induced pulmonary arterial hypertension: A case report. Cardiology 120: 187–189, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Carlström K, Brody S, Lunell NO, Lagrelius A, Möllerström G, Pousette A, Rannevik G, Stege R, von Schoultz B. Dehydroepiandrosterone sulphate and dehydroepiandrosterone in serum: Differences related to age and sex. Maturitas 10: 297–306, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Carroll-Turpin M, Hebert V, Chotibut T, Wensler H, Krentzel D, Varner KJ, Burn BR, Chen YF, Abreo F, Dugas TR. 4,4’-Methylenedianiline alters serotonergic transport in a novel, sex-specific model of pul-monary arterial hypertension in rats. Toxicol Sci 147: 235–245, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo AF, Orlando U, Helfenberger KE, Poderoso C, Podesta EJ. The role of mitochondrial fusion and StAR phosphorylation in the regulation of StAR activity and steroidogenesis. Mol Cell Endocrinol 408: 73–79, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim sH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120: 2319–2330, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol 16: 938–946, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Lin AS, Li Y, Reiter CEN, Ver MR, Quon MJ. Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. J Biol Chem 283: 29228–29238, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Austin ED, Talati M, Fessel JP, Farber-Eger EH, Brittain EL, Hemnes AR, Loyd JE, West J. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur Respir J 50: 1602337, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, Shay S, Trammell A, Phillips JA, Hamid R, Cogan JD, Dawson EP, Womble KE, Hedges LK, Martinez EG, Wheeler LA, Loyd JE, Majka SJ, West J, Austin ED. Estrogen metabolite 16alpha-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation 133: 82–97, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103:401–406, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng Y, Chang LW, Cheng LC, Tsai MH, Lin P. 4-Methoxyestradiol-induced oxidative injuries in human lung epithelial cells. Toxicol Appl Pharmacol 220: 271–277, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Chistiakov DA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Role of androgens in cardiovascular pathology. Vasc Health Risk Manag 14: 283–290, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res 101: 561–570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol (1985) 112: 1564–1575, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung L DR, Lingala B, Alkassab F, Boster M, Csuka ME, Derk C, Fischer A, Frech T, Furst DE, Gomberg-Maitland M, Hinchcliff M, Hsu V, Hummers LK, Khanna D, Medsger TA Jr, Molitor JA, Preston IR, Schiopu E, Shapiro L, Silver R, Simms R, Varga J, Gordon JK, Steen VD. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: Outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res 66: 489–495, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Chung L FH, Benza R, Miller DP, Parsons L, Hassoun PM, McGoon M, Nicolls MR, Zamanian RT. Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest 146: 1494–1504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, Miller DP, Nicolls MR, Zamanian RT. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: Identifying systemic sclerosis as a unique phenotype. Chest 138: 1383–1394, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 1171–1182, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Cogan JD, Vnencak-Jones CL, Phillips JA III, Lane KB, Wheeler LA, Robbins IM, Garrison G, Hedges LK, Loyd JE. Gross BMPR2 gene rearrangements constitute a new cause for primary pulmonary hypertension. Genet Med 7: 169, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Colle IO, Moreau R, Godinho E, Belghiti J, Ettori F, Cohen-Solal A, Mal H, Bernuau J, Marty J, Lebrec D, Valla D, Durand F. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: Aprospective study. Hepatology 37: 401–409, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Condliffe R, Kiely DG, Peacock AJ, Corris PA, Gibbs JS, Vrapi F, Das C, Elliot CA, Johnson M, DeSoyza J, Torpy C, Goldsmith K, Hodgkins D, Hughes RJ, Pepke-Zaba J, Coghlan JG. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 179: 151–157, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Converse A, Zhang C, Thomas P. Membrane androgen receptor ZIP9 induces croaker ovarian cell apoptosis via stimulatory G protein alpha subunit and MAP kinase signaling. Endocrinology 158: 3015–3029, 2017. [DOI] [PubMed] [Google Scholar]
- 66.Coste F, Guibert C, Magat J, Abell E, Vaillant F, Dubois M, Courtois A, Diolez P, Quesson B, Marthan R, Savineau JP, Muller B, Freund-Michel V. Chronic hypoxia aggravates monocrotaline-induced pulmonary arterial hypertension: A rodent relevant model to the human severe form of the disease. Respir Res 18: 47, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS. Physiological coupling of growth factor and steroid receptor signaling pathways: Estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA 93: 12626–12630, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai W, Li Y, Zheng H. Estradiol/Testosterone imbalance: Impact on coronary heart disease risk factors in postmenopausal women. Cardiology 121: 249–254, 2012. [DOI] [PubMed] [Google Scholar]
- 69.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343–349, 1991. [DOI] [PubMed] [Google Scholar]
- 70.Dean A, Gregorc T, Docherty CK, Harvey KY, Nilsen M, Morrell NW, MacLean MR. Role of the aryl hydrocarbon receptor in sugen 5416-induced experimental pulmonary hypertension. Am J Respir Cell Mol Biol 58: 320–330, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dempsie Y, MacRitchie NA, White K, Morecroft I, Wright AF, Nilsen M, Loughlin L, Mair KM, MacLean MR. Dexfenfluramine and the oestrogen-metabolizing enzyme CYP1B1 in the development of pulmonary arterial hypertension. Cardiovasc Res 99: 24–34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, Rabinovitch M, Maclean MR. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. RespirRes 12: 159, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67: 737–744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhillon S, Kaker A, Dosanjh A, Japra D, VanThiel DH. Irreversible pulmonary hypertension associated with the use of interferon alpha for chronic hepatitis C. Dig Dis Sci 55: 1785–1790, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diergaarde B, Potter JD, Jupe ER, Manjeshwar S, Shimasaki CD, Pugh TW, Defreese DC, Gramling BA, Evans I, White E. Polymorphisms in genes involved in sex hormone metabolism, estrogen plus progestin hormone therapy use, and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 17: 1751–1759, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, Patten RD. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res 104: 265–275, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.dos Santos RL, da Silva FB, Ribeiro RF Jr, Stefanon I. Sex hormones in the cardiovascular system. Horm Mol Biol Clin Invest 18: 89–103, 2014. [DOI] [PubMed] [Google Scholar]
- 78.Dougherty SM, Mazhawidza W, Bohn AR, Robinson KA, Mattingly KA, Blankenship KA, Huff MO, McGregor WG, Klinge CM. Gen-der difference in the activity but not expression of estrogen receptors α and β in human lung adenocarcinoma cells. Endocr Relat Cancer 13: 113–134, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Douglas JG, Munro JF, Kitchin AH, Muir AL, Proudfoot AT. Pulmonary hypertension and fenfluramine. Br Med J (Clin Res Ed) 283: 881–883, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dresdale DT, Schultz M, Michtom RJ. Primary pulmonary hypertension: I. Clinical and hemodynamic study. Am J Med 11 (6): 688–705, 1951. [DOI] [PubMed] [Google Scholar]
- 81.Dubey RK, Gillespie DG, Zacharia LC, Rosselli M, Imthurn B, Jackson EK. Methoxyestradiols mediate the antimitogenic effects of locally applied estradiol on cardiac fibroblast growth. Hypertension 39: 412–417, 2002. [DOI] [PubMed] [Google Scholar]
- 82.Dubey RK, Gillespie DG, Zacharia LC, Rosselli M, Korzekwa KR, Fingerle J, Jackson EK. Methoxyestradiols mediate the antimitogenic effects of estradiol on vascular smooth muscle cells via estrogen receptor-independent mechanisms. Biochem Biophys Res Commun 278: 27–33, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Dubey RK, Jackson EK, Gillespie DG, Rosselli M, Barchiesi F, Krust A, Keller H, Zacharia LC, Imthurn B. Cytochromes 1A1/1B1- and catechol-O-methyltransferase-derived metabolites mediate estradiol-induced antimitogenesis in human cardiac fibroblast. J Clin Endocrinol Metab 90: 247–255, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension 37: 640–644, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 53: 688–708, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther 308: 403–409, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Duffels MG, Engelfriet PM, Berger RM, van Loon RL, Hoendermis E, Vriend JW, van der Velde ET, Bresser P, Mulder BJ. Pulmonary arterial hypertension in congenital heart disease: An epidemiologic perspective from a Dutch registry. Int J Cardiol 120: 198–204, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Dumas de La Roque E, Bellance N, Rossignol R, Begueret H, Billaud M, dos Santos P, Ducret T, Marthan R, Dahan D, Ramos-Barbon D, Amor-Carro O, Savineau JP, Fayon M. Dehydroepiandrosterone reverses chronic hypoxia/reoxygenation-induced right ventricular dysfunction in rats. Eur Respir J 40: 1420–1429, 2012. [DOI] [PubMed] [Google Scholar]
- 89.Dumas de la Roque E, Quignard JF, Ducret T, Dahan D, Courtois A, Begueret H, Marthan R, Savineau JP. Beneficial effect of dehydroepiandrosterone on pulmonary hypertension in a rodent model of pulmonary hypertension in infants. Pediatr Res 74: 163–169, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Dumas de la Roque E, Savineau JP, Metivier AC, Billes MA, Kraemer JP, Doutreleau S, Jougon J, Marthan R, Moore N, Fayon M, Baulieu EE, Dromer C. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): A pilot study. Ann Endocrinol 73: 20–25, 2012. [DOI] [PubMed] [Google Scholar]
- 91.Duong V, Licznar A, Margueron R, Boulle N, Busson M, Lacroix M, Katzenellenbogen BS, Cavailles V, Lazennec G. ERalpha and ERbeta expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene 25: 1799–1806, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Earley S, Resta TC. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 283: L86–L93, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Engelfriet PM, Duffels MG, Moller T, Boersma E, Tijssen JG, Thaulow D, Gatzoulis MA, Mulder BJ. Pulmonary arterial hypertension in adults born with a heart septal defect: The Euro Heart Survey on adult con-genital heart disease. Heart 93: 682–687, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Aging reduces the responsiveness of coronary arteries from male Wistar rats to the vasodilatory action of testosterone. Clin Sci (Lond) 99: 77–82, 2000. [PubMed] [Google Scholar]
- 95.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res 33: 645–652, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, Castillo-Palma MJ, Segovia J, Gomez-Sanchez MA, Barbera JA, REHAP investigators. Survival in pulmonary hyper-tension in Spain: Insights from the Spanish registry. Eur Respir J 40: 596–603, 2012. [DOI] [PubMed] [Google Scholar]
- 97.Evans JDW, Girerd B, Montani D, Wang X-J, Galiè N, Austin ED, Elliott G, Asano K, Grnnig E, Yan Y, Jing Z-C, Manes A, Palazzini M, Wheeler LA, Nakayama I, Satoh T, Eichstaedt C, Hinderhofer K, Wolf M, Rosenzweig EB, Chung WK, Soubrier F, Simonneau G, Sitbon O, Graf S, Kaptoge S, Di Angelantonio E, Humbert M, Morrell NW. BMPR2 mutations and survival in pulmonary arterial hyperten-sion: An individual participant data meta-analysis. Lancet Respir Med 4: 129–137, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, Romero AJ, Benton WW, Elliott CG, McGoon MD, Benza RL. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest 148: 1043–1054, 2015. [DOI] [PubMed] [Google Scholar]
- 99.Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. Protection by oestradiol against the development of cardiovascu-lar changes associated with monocrotaline pulmonary hypertension in rats. BrJPharmacol 110:719–723, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feldman HA, Johannes CB, Araujo AB, Mohr BA, Longcope C, McKinlay JB. Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: Prospective results from the Massachusetts Male Aging Study. Am J Epidemiol 153: 79–89, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, Johnson J, Loyd JE, Hemnes A, Austin E, West J. Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ 3: 564–577, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Finkle wD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF Jr, Hoover RN. Increased risk of non-fatal myocar-dial infarction following testosterone therapy prescription in men. PLoS One 9: e85805, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fishman J, Martucci C. Biological properties of 16 alpha-hydroxyestrone: Implications in estrogen physiology and pathophysiology. J Clin Endocrinol Metab 51:611–615, 1980. [DOI] [PubMed] [Google Scholar]
- 104.Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz-Zagrosek V. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298: R1597–R1606, 2010. [DOI] [PubMed] [Google Scholar]
- 105.Flister MJ, Prokop JW, Lazar J, Shimoyama M, Dwinell M, Geurts A. 2015 Guidelines for establishing genetically modified rat models for cardiovascular research. J Cardiovasc Transl Res 8: 269–277, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foppa M, Arora G, Gona P, Ashrafi A, Salton CJ, Yeon SB, Blease SJ, Levy D, O’Donnell CJ, Manning WJ, Chuang Ml. Right ventricular volumes and systolic function by cardiac magnetic resonance and the impact of sex, age, and obesity in a longitudinally followed cohort free of pulmonary and cardiovascular disease: The Framingham Heart Study. Circ Cardiovasc Imaging 9: e003810, 2016. [DOI] [PubMed] [Google Scholar]
- 107.Fradette C, Batonga J, Teng S, Piquette-Miller M, du Souich P. Animal models of acute moderate hypoxia are associated with a down-regulation of CYP1A1, 1A2, 2B4, 2C5, and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in liver. Drug Metab Dispos 35: 765–771, 2007. [DOI] [PubMed] [Google Scholar]
- 108.Frantz RP, Schilz RJ, Chakinala MM, Badesch DB, Frost AE, McLaughlin VV, Barst RJ, Rosenberg DM, Miller DP, Hartline BK, Benton WW, Farber HW. Hospitalization and survival in patients using epoprostenol for injection in the PROSPECT observational study. Chest 147: 484–494, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: Discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res 64: 1522–1533, 2004. [DOI] [PubMed] [Google Scholar]
- 110.Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med 187: 133–143, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fruehauf S, Steiger S, Topaly J, Ho AD. Pulmonary artery hypertension during interferon-alpha therapy for chronic myelogenous leukemia. Ann Hematol 80: 308–310, 2001. [DOI] [PubMed] [Google Scholar]
- 112.Frump AL, Albrecht ME, McClintick JN, Lahm T. Estrogen receptor-dependent attenuation of hypoxia-induced changes in the lung genome of pulmonary hypertension rats. Pulm Circ 7: 232–243, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frump AL, Bonnet S, de Jesus Perez VA, Lahm T. Emerging role of angiogenesis in adaptive and maladaptive right ventricular remodeling in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 314: L443–Ll460, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, Lahm T. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: Effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol 308: L873–L890, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frump AL, Selej M, Wood JA, Albrecht M, Yakubov B, Petrache I, Lahm T. Hypoxia upregulates estrogen receptor beta in pulmonary artery endothelial cells in a HIF-1alpha-dependent manner. Am J Respir Cell Mol Biol 59: 114–126, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fuchs KI, Moore LG, Rounds S. Pulmonary vascular reactivity is blunted in pregnant rats. J Appl Physiol 53: 703–707, 1982. [DOI] [PubMed] [Google Scholar]
- 117.Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol 50: 169–174, 1994. [DOI] [PubMed] [Google Scholar]
- 118.Nb Gabler, French B Strom BL, Liu Z Palevsky HI, Taichman DB Kawut SM, Halpern SD. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest 141: 20–26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gandhi SJ, Hagans I, Nathan K, Hunter K, Roy S. Prevalence, comorbidity and investigation of anemia in the primary care office. J Clin Med Res 9: 970–980, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garg D, Ng SSM, Baig KM, Driggers P, Segars J. Progesterone-mediated non-classical signaling. Trends Endocrinol Metab 28: 656–668, 2017. [DOI] [PubMed] [Google Scholar]
- 121.Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology 147: 2028–2034, 2006. [DOI] [PubMed] [Google Scholar]
- 122.Goddard LM, Ton AN, Org T, Mikkola HK, Iruela-Arispe ML. Selective suppression of endothelial cytokine production by progesterone receptor. Vascul Pharmacol 59: 36–43, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goglia L, Tosi V, Sanchez AM, Flamini MI, Fu XD, Zullino S, Genazzani AR, Simoncini T. Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Mol Hum Reprod 16:761–769, 2010. [DOI] [PubMed] [Google Scholar]
- 124.Golden KL, Marsh JD, Jiang Y. Castration reduces mRNA levels for calcium regulatory proteins in rat heart. Endocrine 19: 339–344, 2002. [DOI] [PubMed] [Google Scholar]
- 125.Goldstein J, Sites CK, Toth MJ. Progesterone stimulates cardiac muscle protein synthesis via receptor-dependent pathway. Fertil Steril 82:430–436, 2004. [DOI] [PubMed] [Google Scholar]
- 126.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR, Voelkel NF. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am J Physiol Lung Cell Mol Physiol 302: L977–L991, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 302: L363–L369, 2012. [DOI] [PubMed] [Google Scholar]
- 128.Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology 136: 2052–2059, 1995. [DOI] [PubMed] [Google Scholar]
- 129.Graf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, Hodgson J, Liu B, Salmon RM, Southwood M, Machado RD, Martin JM, Treacy CM, Yates K, Daugherty LC, Shamardina O, Whitehorn D, Holden S, Aldred M, Bogaard HJ, Church C, Coghlan G, Condliffe R, Corris PA, Danesino C, Eyries M, Gall H, Ghio S, Ghofrani HA, Gibbs JSR, Girerd B, Houweling AC, Howard L, Humbert M, Kiely DG, Kovacs G, MacKenzie Ross RV, Moledina S, Montani D, Newnham M, Olschewski A, Olschewski H, Peacock AJ, Pepke-Zaba J, Prokopenko I, Rhodes CJ, Scelsi L, Seeger W, Soubrier F, Stein DF, Suntharalingam J, Swietlik EM, Toshner MR, van Heel DA, Vonk Noordegraaf A, Waisfisz Q, Wharton J, Wort SJ, Ouwehand WH, Soranzo N, Lawrie A, Upton PD, Wilkins MR, Trembath RC, Morrell NW. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 9: 1416, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Graham BB, Chabon J, Bandeira A, Espinheira L, Butrous G, Tuder RM. Significant intrapulmonary Schistosoma egg antigens are not present in schistosomiasis-associated pulmonary hypertension. Pulm Circ 1: 456–461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Graham BB, Chabon J, Gebreab L, Poole J, Debella E, Davis L, Tanaka T, Sanders L, Dropcho N, Bandeira A, Vandivier RW, Champion HC, Butrous G, Wang XJ, Wynn TA, Tuder RM. Transforming growth factor-beta signaling promotes pulmonary hypertension caused by Schistosoma mansoni. Circulation 128: 1354–1364, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Graham BB, Chabon J, Kumar R, Kolosionek E, Gebreab L, Debella D, Edwards M, Diener K, Shade T, Bifeng G, Bandeira A, Butrous G, Jones K, Geraci M, Tuder RM. Protective role of IL-6 in vascular remodeling in Schistosoma pulmonary hypertension. Am J Respir Cell MolBiol 49:951–959, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grimm SL, Hartig SM, Edwards DP. Progesterone receptor signaling mechanisms. J Mol Biol 428: 3831–3849, 2016. [DOI] [PubMed] [Google Scholar]
- 134.Grino PB. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology 126: 1165–1172, 2018. [DOI] [PubMed] [Google Scholar]
- 135.Guihaire J, Deuse T, Wang D, Fadel E, Reichenspurner H, Schrepfer S. Sex differences in immunology: More severe development of experimental pulmonary hypertension in male rats exposed to vascular endothelial growth factor receptor blockade. Biomed Res Int 2015: 765292, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104: 288–291, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Frances C, Launay D, Mouthon L, Allanore Y, Tiev KP, Clerson P, de Groote P, Humbert M. Early detection of pulmonary arterial hypertension in systemic sclerosis: A French nationwide prospective multicenter study. Arthritis Rheum 52: 3792–3800, 2005. [DOI] [PubMed] [Google Scholar]
- 138.Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: Prevalence and relation to splanchnic hemodynamics. Gastroenterology 100: 520–528, 1991. [DOI] [PubMed] [Google Scholar]
- 139.Hall J, Jones RD, Jones TH, Channer KS, Peers C. Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology 147: 2675–2680, 2006. [DOI] [PubMed] [Google Scholar]
- 140.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 114: 2261–2270, 2006. [DOI] [PubMed] [Google Scholar]
- 141.Hamidi SA, Dickman KG, Berisha H, Said SI. 17beta-estradiol protects the lung against acute injury: Possible mediation by vasoactive intestinalpolypeptide. Endocrinology 152: 4729–4737, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hammes SR, Davis PJ. Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Best Pract Res Clin Endocrinol Metab 29: 581–593, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hammond GL. Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J Endocrinol 230: R13–R25, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hampl V, Bibova J, Povysilova V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J 21: 862–865, 2003. [DOI] [PubMed] [Google Scholar]
- 145.Han MK, Arteaga-Solis E, Blenis J, Bourjeily G, Clegg DJ, DeMeo D, Duffy J, Gaston B, Heller NM, Hemnes A, Henske EP, Jain R, Lahm T, Lancaster LH, Lee J, Legato MJ, McKee S, Mehra R, Morris A, Prakash YS, Stampfli MR, Gopal-Srivastava R, Laposky AD, Punturieri A, Reineck L, Tigno X, Clayton J. Female sex and gender in lung/sleep health and disease. Increased understanding of basic biological, pathophysio-logical, and behavioral mechanisms leading to better health for female patients with lung disease. Am J Respir Crit Care Med 198: 850–858, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: Association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res 60: 3440–3444, 2000. [PubMed] [Google Scholar]
- 147.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 115: 1275–1284, 2007. [DOI] [PubMed] [Google Scholar]
- 148.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res 84: 1285–1291, 1999. [DOI] [PubMed] [Google Scholar]
- 149.Harvey PA, Leinwand LA. Dietary phytoestrogens present in soy dramatically increase cardiotoxicity in male mice receiving a chemotherapeutic tyrosine kinase inhibitor. Mol Cell Endocrinol 399: 330–335, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hautefort A, Mendes-Ferreira P, Sabourin J, Manaud G, Bertero T, Rucker-Martin C, Riou M, Adao R, Manoury B, Lambert M, Boet A, Lecerf F, Domergue V, Bras-Silva C, Gomez AM, Montani D, Girerd B, Humbert M, Antigny F, Perros F. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation 139: 932–948, 2019. [DOI] [PubMed] [Google Scholar]
- 151.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: How do they signal and what are their targets. Physiol Rev 87: 905–931, 2007. [DOI] [PubMed] [Google Scholar]
- 152.Hemnes AR, Beck GJ, Newman JH, Abidov A, Aldred MA, Barnard J, Berman Rosenzweig E, Borlaug BA, Chung WK, Comhair SAA, Erzurum SC, Frantz RP, Gray MP, Grunig G, Hassoun PM, Hill NS, Horn EM, Hu B, Lempel JK, Maron BA, Mathai SC, Olman MA, Rischard FP, Systrom DM, Tang WHW, Waxman AB, Xiao L, Yuan JX, Leopold JA, Group PS. PVDOMICS: A multi-center study to improve understanding of pulmonary vascular disease through phenomics. Circ Res 121: 1136–1139, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hemnes AR, Kiely DG, Cockrill BA, Safdar Z, Wilson VJ, Al Hazmi M, Preston IR, MacLean MR, Lahm T. Statement on pregnancy in pulmonary hypertension from the Pulmonary Vascular Research Institute. Pulm Circ 5: 435–465, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hemnes AR, Maynard KB, Champion HC, Gleaves L, Penner N, West J, Newman JH. Testosterone negatively regulates right ventricular load stress responses in mice. Pulm Circ 2: 352–358, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herrington DM. Dehydroepiandrosterone and coronary atherosclerosis. Ann N Y Acad Sci 774: 271–280, 1995. [DOI] [PubMed] [Google Scholar]
- 156.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev 25: 869–898, 2004. [DOI] [PubMed] [Google Scholar]
- 157.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitricoxide synthase in vascular endothelial cells. J Biol Chem 276: 3459–3467, 2001. [DOI] [PubMed] [Google Scholar]
- 158.Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Grohe C, Lange TJ, Behr J, Klose H, Wilkens H, Filusch A, Germann M, Ewert R, Seyfarth HJ, Olsson KM, Opitz CF, Gaine SP, Vizza CD, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Pittrow D. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: Results from the COMPERA registry. Int J Cardiol 168: 871–880, 2013. [DOI] [PubMed] [Google Scholar]
- 159.Homma N, Nagaoka T, Karoor V, Imamura M, Taraseviciene-Stewart L, Walker LA, Fagan KA, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell Mol Physiol 295: L71–L78, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118: 722–730, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoshikawa Y, Nana-Sinkam P, Moore MD, Sotto-Santiago S, Phang T, Keith RL, Morris KG, Kondo T, Tuder RM, Voelkel NF, Geraci MW. Hypoxia induces different genes in the lungs of rats compared with mice. Physiol Genomics 12: 209–219, 2003. [DOI] [PubMed] [Google Scholar]
- 162.Howard LS, Watson GM, Wharton J, Rhodes CJ, Chan K, Khengar R, Robbins PA, Kiely DG, Condliffe R, Elliott CA, Pepke-Zaba J, Sheares K, Morrell NW, Davies R, Ashby D, Gibbs JSR, Wilkins MR. Supplementation of iron in pulmonary hypertension: Rationale and design of a phase II clinical trial in idiopathic pulmonary arterial hypertension. Pulm Circ 3: 100–107, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hu Y, Zabini D, Gu W, Goldenberg NM, Breitling S, Kabir G, Connelly KA, Kuebler WM. The role of the human immune system in chronic hypoxic pulmonary hypertension. Am J Respir Crit Care Med 198: 528–531, 2018. [DOI] [PubMed] [Google Scholar]
- 164.Huerta-Garcia E, Ventura-Gallegos JL, Victoriano ME, Montiel-Davalos A, Tinoco-Jaramillo G, Lopez-Marure R. Dehydroepiandrosterone inhibits the activation and dysfunction of endothelial cells induced by high glucose concentration. Steroids 77: 233–240, 2012. [DOI] [PubMed] [Google Scholar]
- 165.Hultgren HN, Honigman B, Theis K, Nicholas D. High-altitude pulmonary edema at a ski resort. West J Med 164: 222–227, 1996. [PMC free article] [PubMed] [Google Scholar]
- 166.Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur Respir J 53: 1801887, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. [DOI] [PubMed] [Google Scholar]
- 168.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier J-F, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: Results from a National Registry. Am J Respir Crit Care Med 173: 1023–1030, 2006. [DOI] [PubMed] [Google Scholar]
- 169.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004. [DOI] [PubMed] [Google Scholar]
- 170.Humbert M, Sitbon O, Yaici A, Montani D, O’Callaghan DS, Jais X, Parent F, Savale L, Natali D, Gunther S, Chaouat A, Chabot F, Cordier JF, Habib G, Gressin V, Jing ZC, Souza R, Simonneau G, French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 36: 549–555, 2010. [DOI] [PubMed] [Google Scholar]
- 171.Hyduk A, Croft J, Ayala C, Zheng K, Zheng Z, Mensah G. Pulmonary hypertension surveillance—United States, 1980-2002. MMWR Surveill Summ 54: 1–28, 2005. [PubMed] [Google Scholar]
- 172.Ingegno MD, Money SR, Thelmo W, Greene GL, Davidian M, Jaffe BM, Pertschuk LP. Progesterone receptors in the human heart and great vessels. Lab Invest 59: 353–356, 1988. [PubMed] [Google Scholar]
- 173.Iorga A, Li J, Sharma S, Umar S, Bopassa JC, Nadadur RD, Centala A, Ren S, Saito T, Toro L, Wang Y, Stefani E, Eghbali M. Rescue of pressure overload-induced heart failure by estrogen therapy. J Am Heart Assoc 5: e002482, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation 113: 1605–1614, 2006. [DOI] [PubMed] [Google Scholar]
- 175.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard HJ, Boonstra A, Vonk Noordegraaf The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 145: 1230–1236, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jia S, Zhang X, He DZ, Segal M, Berro A, Gerson T, Wang Z, Casale TB. Expression and function of a novel variant of estrogen receptor-alpha36 in murine airways. Am J Respir Cell Mol Biol 45: 1084–1089, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW, Wang H, Wang ZW, Cheng XS, Xu B, Hu SS, Hui RT, Yang YJ. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arte-rial hypertension. Chest 132: 373–379, 2007. [DOI] [PubMed] [Google Scholar]
- 178.Jochmann N, Kiecker F, Borges AC, Hofmann MA, Eddicks S, Sterry W, Baumann G, Trefzer U. Long-term therapy of interferon-alpha induced pulmonary arterial hypertension with different PDE-5 inhibitors: A case report. Cardiovasc Ultrasound 3: 26, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Johansen AK, Dean A, Morecroft I, Hood K, Nilsen M, Loughlin L, Anagnostopoulou A, Touyz RM, White K, MacLean MR. The serotonin transporter promotes a pathological estrogen metabolic pathway in pulmonary hypertension via cytochrome P450 1B1. Pulm Circ 6: 82–92, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS. Pulmonary vasodilatory action of testosterone: Evidence of a calcium antagonistic action. J Cardiovasc Pharmacol 39: 814–823, 2002. [DOI] [PubMed] [Google Scholar]
- 181.Jones RD, Pugh PJ, Jones TH, Channer KS. The vasodilatory action of testosterone: A potassium-channel opening or a calcium antagonistic action? Br J Pharmacol 138: 733–744, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Joshi SR, Lakhkar A, Dhagia V, Zias AL, Soldatos V, Oshima K, Jiang H, Gotlinger K, Capdevila JH, Schwartzman ML, McMurtry IF, Gupte SA. Cyp2c44 gene disruption exacerbated pulmonary hypertension and heart failure in female but not male mice. Pulm Circ 6: 360–368, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab 13: 122–128, 2002. [DOI] [PubMed] [Google Scholar]
- 184.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453: 1117–1121, 2008. [DOI] [PubMed] [Google Scholar]
- 185.Kane GC, Maradit-Kremers H, Slusser JP, Scott CG, Frantz RP, McGoon MD. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest 139: 1285–1293, 2011. [DOI] [PubMed] [Google Scholar]
- 186.Kararigas G, Becher E, Mahmoodzadeh S, Knosalla C, Hetzer R, Regitz-Zagrosek V. Sex-specific modification of progesterone receptor expression by 17β-oestradiol in human cardiac tissues. Biol Sex Differ 1: 2, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation 89: 1943–1950, 1994. [DOI] [PubMed] [Google Scholar]
- 188.Karas RH, van Eickels M, Lydon JP, Roddy S, Kwoun M, Aronovitz M, Baur WE, Conneely O, O’Malley BW, Mendelsohn ME. A complex role for the progesterone receptor in the response to vascular injury. J Clin Invest 108: 611–618, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kasahara Y, Kiyatake K, Tatsumi K, Sugito K, Kakusaka I, Yamagata S, Ohmori S, Kitada M, Kuriyama T. Bioactivation of monocrotaline byP-450 3A in rat liver. J Cardiovasc Pharmacol 30: 124–129, 1997. [DOI] [PubMed] [Google Scholar]
- 190.Kato I, Cichon M, Yee CL, Land S, Korczak JF. African American-preponderant single nucleotide polymorphisms (SNPs) and risk of breast cancer. Cancer Epidemiol 33: 24–30, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Katzenellenbogen BS. Mechanisms of action and cross-talk between estrogen receptor and progesterone receptor pathways. J Soc Gynecol Investig 7: S33–S37, 2000. [DOI] [PubMed] [Google Scholar]
- 192.Kawut SM, Al-Naamani N, Agerstrand C, Rosenzweig EB, Rowan C, Barst RJ, Bergmann S, Horn EM. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest 135: 752–759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, Palevsky HI, Palmisciano AJ, Patel M, Pinder D, Propert KJ, Smith KA, Stanczyk F, Tracy R, Vaidya A, Whittenhall ME, Ventetuolo CE. Anastrozole in pulmonary arterial hypertension. A randomized, doubleblind, placebo-controlled trial. Am J Respir Crit Care Med 195: 360–368, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, Ogunyankin KO, Bristow MR, Kizer JR, Tandri H, Bluemke DA. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: The Multi-Ethnic Study of Atherosclerosis (MESA)—Right ventricle study. Circulation 126: 1681–1688, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sm Kawut, Krowka MJ Trotter JF, Roberts KE Benza RL, Badesch DB Taichman DB, Horn EM, Zacks S, Kaplowitz N, Brown RS Jr, Fallon MB. Clinical risk factors for portopulmonary hypertension. Hepatology 48: 196–203, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, Kronmal RA, Bluemke DA. Sex and race differences in right ventricular structure and function: The multi-ethnic study of atherosclerosis-right ventricle study. Circulation 123: 2542–2551, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 85: 2519–2525, 2000. [DOI] [PubMed] [Google Scholar]
- 198.Kerstjens-Frederikse WS, Bongers EM, Roofthooft MT, Leter EM, Douwes JM, Van Dijk A, Vonk-Noordegraaf A, Dijk-Bos KK, Hoefsloot LH, Hoendermis ES, Gille JJ, Sikkema-Raddatz B, Hofstra RM, Berger RM. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet 50: 500–506, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khaw K, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective population study. Circulation 116: 2694–2701, 2007. [DOI] [PubMed] [Google Scholar]
- 200.Kłapcińska B, Jagsz S, Sadowska-Krℙpa E, Górski J, Kempa K, Langfort L. Effects of castration and testosterone replacement on the antioxidant defense system in rat left ventricle. J Physiol Sci 58: 173–177, 2008. [DOI] [PubMed] [Google Scholar]
- 201.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids 65: 227–251, 2000. [DOI] [PubMed] [Google Scholar]
- 202.Konings G, Brentjens L, Delvoux B, Linnanen T, Cornel K, Koskimies P, Bongers M, Kruitwagen R, Xanthoulea S, Romano A. Intracrine regulation of estrogen and other sex steroid levels in endometrium and non-gynecological tissues; pathology, physiology, and drug discovery. Front Pharmacol 9: 940, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: A systematic review. Eur Respir J 39:319–328, 2012. [DOI] [PubMed] [Google Scholar]
- 204.Kovats S Estrogen receptors regulate innate immune cells and signal-ing pathways. Cell Immunol 294: 63–69, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, McGoon MD. Portopulmonary hypertension: A report from the US-based REVEAL Registry. Chest 141: 906–915, 2012. [DOI] [PubMed] [Google Scholar]
- 206.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93: 5925–5930, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Labrie F, Belanger A, Pelletier G, Martel C, Archer DF, Utian WH. Science of intracrinology in postmenopausal women. Menopause 24: 702–712, 2017. [DOI] [PubMed] [Google Scholar]
- 208.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache 1.17beta-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185: 965–980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Tan J, Meldrum DR. Selective estrogen receptor-{alpha} and estrogen receptor-{beta} agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 295: R1486–R1493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock 30: 660–667, 2008. [DOI] [PubMed] [Google Scholar]
- 211.Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: Potential new clinical implications for an old hormone. Crit Care Med 36: 2174–2183, 2008. [DOI] [PubMed] [Google Scholar]
- 212.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline Ja, Kolb TM, Mathai SC, Mercier O, Michelakis ED, Naeije R, Tuder RM, Ventetuolo CE, Vieillard-Baron A, Voelkel NF, Vonk-Noordegraaf A, Hassoun PM, American Thoracic Society Assembly on Pulmonary C. Assessment of right ventricular function in the research setting: Knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 198: e15–e43, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lahm T, Frump AL, Albrecht ME, Fisher AJ, Cook TG, Jones TJ, Yakubov B, Whitson J, Fuchs RK, Liu A, Chesler NC, Brown MB. 17beta-Estradiol mediates superior adaptation of right ventricular func-tion to acute strenuous exercise in female rats with severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L375–L388, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, Meldrum DR. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: The effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab 293: E865–E871, 2007. [DOI] [PubMed] [Google Scholar]
- 215.Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy 23: 165–172, 2003. [DOI] [PubMed] [Google Scholar]
- 216.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol 273: L119–L126, 1997. [DOI] [PubMed] [Google Scholar]
- 217.Lantta M, Kärkkäinen J, Lehtovirta P. Progesterone and estradiol receptors in the cytosol of the human uterine artery. Am J Obstet Gynecol 147: 627–633, 1983. [DOI] [PubMed] [Google Scholar]
- 218.Le CHH. The prevalence of anemia and moderate-severe anemia in the US Population (NHANES 2003–2012). PLoS One 11:e0166635, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Le Dily F, Beato M. Signaling by steroid hormones in the 3D nuclear space. Int J Mol Sci 19 (2): 306, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology 144: 3382–3398, 2003. [DOI] [PubMed] [Google Scholar]
- 221.Lee MJ, Kim EH, Lee SA, Kang YM, Jung CH, Yoon HK, Seol SM, Lee YL, Lee WJ, Park JY. Dehydroepiandrosterone prevents linoleic acid-induced endothelial cell senescence by increasing autophagy. Metabolism 64: 1134–1145, 2015. [DOI] [PubMed] [Google Scholar]
- 222.Lee WS, Harder JA, Yoshizumi M, Lee ME, Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med 3: 1005–1008, 1997. [DOI] [PubMed] [Google Scholar]
- 223.Lee WS, Liu CW, Juan SH, Liang YC, Ho PY, Lee YH. Molecular mechanism of progesterone-induced antiproliferation in rat aortic smooth muscle cells. Endocrinology 144: 2785–2790, 2003. [DOI] [PubMed] [Google Scholar]
- 224.Lee WT, Ling Y, Sheares KK, Pepke-Zaba J, Peacock AJ, Johnson MK. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J 40: 604–611, 2012. [DOI] [PubMed] [Google Scholar]
- 225.Leön-Velarde FG, Gamboa A, Chuquiza JA, Esteba WA, Rivera-Chira M, Monge CC. Hematological parameters in high altitude residents living at 4355, 4660, and 5500 meters above sea level. High Alt Med Biol 1: 97–104, 2004. [DOI] [PubMed] [Google Scholar]
- 226.Li HF, Zheng TZ, Li W, Qu SY, Zhang CL. Effect of progesterone on the contractile response of isolated pulmonary artery in rabbits. Can J Physiol Pharmacol 79: 545–550, 2001. [PubMed] [Google Scholar]
- 227.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: When a chromosome makes the difference. Nat Rev Immunol 10: 594–604, 2010. [DOI] [PubMed] [Google Scholar]
- 228.Liehr JG, Ricci MJ. 4-Hydroxylation of estrogens as marker of human mammary tumors. Proc Natl Acad Sci USA 93: 3294–3296, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lin AL, Gonzalez R Jr, Carey KD, Shain SA. Estradiol-17 beta affects estrogen receptor distribution and elevates progesterone receptor content in baboon aorta. Arteriosclerosis 6: 495–504, 1986. [DOI] [PubMed] [Google Scholar]
- 230.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation 120: 245–254, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke-Zaba J, Sheares KK, Corris PA, Fisher AJ, Lordan JL, Gaine S, Coghlan JG, Wort SJ, Gatzoulis MA, Peacock AJ. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: Results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 186: 790–796, 2012. [DOI] [PubMed] [Google Scholar]
- 232.Liu A, Hacker T, Eickhoff JC, Chesler NC. Estrogen preserves pulsatile pulmonary arterial hemodynamics in pulmonary arterial hypertension. Ann Biomed Eng 45: 632–643, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu A, Philip J, Vinnakota KC, Van den Bergh F, Tabima DM, Hacker T, Beard DA, Chesler NC. Estrogen maintains mitochondrial content and function in the right ventricle of rats with pulmonary hypertension. Physiol Rep 5: e013157, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liu A, Schreier D, Tian L, Eickhoff JC, Wang Z, Hacker TA, Chesler NC. Direct and indirect protection of right ventricular function by estrogen in an experimental model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 307: H273–H283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu A, Tian L, Golob M, Eickhoff JC, Boston M, Chesler NC. 17beta-Estradiol attenuates conduit pulmonary artery mechanical property changes with pulmonary arterial hypertension. Hypertension 66: 1082–1088, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3). J Biol Chem 277: 21379–21388, 2002. [DOI] [PubMed] [Google Scholar]
- 237.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 24: 313–340, 2003. [DOI] [PubMed] [Google Scholar]
- 238.Lizotte E, Grandy SA, Tremblay A, Allen BG, Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem 23: 75–86, 2009. [DOI] [PubMed] [Google Scholar]
- 239.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statis-tics Subcommittee. Heart disease and stroke statistics—2010 update. Circulation 121: e46–e215, 2010. [DOI] [PubMed] [Google Scholar]
- 240.Longcope C, Gorbach S, Goldin B, Woods M, Dwyer J, Morrill A, Warram J. The effect of a low fat diet on estrogen metabolism. J Clin Endocrinol Metab 64: 1246–1250, 1987. [DOI] [PubMed] [Google Scholar]
- 241.Lopes RAM, Neves KB, Carneiro FS, Tostes RC. Testosterone and vascular function in aging. Front Physiol 3: 89, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lowe BS, Therrien J, Ionescu-Ittu R, Pilote L, Martucci G, Marelli AJ. Diagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomes. J Am Coll Cardiol 58: 538–546, 2011. [DOI] [PubMed] [Google Scholar]
- 243.Lowery JW, de Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev 21: 287–298, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lu Q, Schnitzler GR, Ueda K, Iyer LK, Diomede OI, Andrade T, Karas RH. ER alpha rapid signaling is required for estrogen induced pro-liferation and migration of vascular endothelial cells. PLoS One 11: e0152807, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS, Chung WK. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 369: 351–361, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, Newman JH, Phillips JA III, Soubrier F, Trembath RC, Chung WK. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 54: S32–S42, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res 81: 355–362, 1997. [DOI] [PubMed] [Google Scholar]
- 248.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, MacLean Mr. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med 190: 456–467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mair KM, Yang XD, Long L, White K, Wallace E, Ewart MA, Docherty CK, Morrell NW, MacLean MR. Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 191: 693–703, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Makieva S, Saunders PT, Norman JE. Androgens in pregnancy: Roles in parturition. Hum Reprod Update 20: 542–559, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Malkin CJ, Pugh PJ, West JN, van Beek EJR, Jones TH, Channer KS. Testosterone therapy in men with moderate severity heart failure: A double-blind randomized placebo controlled trial. Eur Heart J 27: 57–64, 2006. [DOI] [PubMed] [Google Scholar]
- 252.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30: 65–91, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Manna PR, Stetson CL, Daugherty C, Shimizu I, Syapin PJ, Garrel G, Cohen-Tannoudji J, Huhtaniemi I, Slominski AT, Pruitt K, Stocco DM. Up-regulation of steroid biosynthesis by retinoid signaling: Implications for aging. Mech Ageing Dev 150: 74–82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mannic T, Mouffok M, Python M, Yoshida T, Maturana AD, Vuilleumier N, Rossier MF. DHEA prevents mineralo- and glucocorticoid receptor-induced chronotropic and hypertrophic actions in isolated rat cardiomyocytes. Endocrinology 154: 1271–1281, 2013. [DOI] [PubMed] [Google Scholar]
- 255.Mark EJ, Patalas ED, Chang HT, Evans RJ, Kessler SC. Fatal pulmonary hypertension associated with short-term use of fenfluramine and phentermine. N Engl J Med 337: 602–606, 1997. [DOI] [PubMed] [Google Scholar]
- 256.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 98: 256–261, 1998. [DOI] [PubMed] [Google Scholar]
- 257.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 41: 217–223, 2003. [DOI] [PubMed] [Google Scholar]
- 258.Mathai SC, Hassoun PM, Puhan MA, Zhou Y, Wise RA. Sex differences in response to tadalafil in pulmonary arterial hypertension. Chest 147: 188–197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 428–433, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Matori H, Umar S, Nadadur RD, Sharma S, Partow-Navid R, Afkhami M, Amjedi M, Eghbali M. Genistein, a soy phytoestrogen, reverses severe pulmonary hypertension and prevents right heart failure in rats. Hypertension 60: 425–430, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Matsumoto T, Sakari M, Okada M, Yokoyama A, Takahashi S, Kouzmenko A, Kato S. The androgen receptor in health and disease. Annu Rev Physiol 75: 201–224, 2013. [DOI] [PubMed] [Google Scholar]
- 262.McGoon MD, Krichman A, Farber HW, Barst RJ, Raskob GE, Liou TG, Miller DP, Feldkircher K, Giles S. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc 83: 923–931, 2008. [DOI] [PubMed] [Google Scholar]
- 263.McKenna NJ, O’Malley BW. An issue of tissues: Divining the split personalities of selective estrogen receptor modulators. Nat Med 6: 960–962, 2000. [DOI] [PubMed] [Google Scholar]
- 264.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108: 465–474, 2002. [DOI] [PubMed] [Google Scholar]
- 265.McKenna NJ, O’Malley BW. Minireview: Nuclear receptor coactivators—An update. Endocrinology 143: 2461–2465, 2002. [DOI] [PubMed] [Google Scholar]
- 266.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr 12: 444–454, 2009. [DOI] [PubMed] [Google Scholar]
- 267.McMurtry IF, Frith CH, Will DH. Cardiopulmonary responses of male and female swine to simulated high altitude. J Appl Physiol 35: 459–462, 1973. [DOI] [PubMed] [Google Scholar]
- 268.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, Pepke-Zaba J, Pulido T, Rich S, Rosenkranz S, Suissa S. Pulmonary arterial hypertension: Epidemiology and registries. J Am Coll Cardiol 62: D51–D59, 2013. [DOI] [PubMed] [Google Scholar]
- 269.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35: 3452–3462, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, Lauzon-Joset JF, Breuils-Bonnet S, Tremblay E, Biardel S, Racine C, Courture C, Bonnet P, Majka SM, Deshaies Y, Picard F, Provencher S, Bonnet S. Critical role for the advanced glycation end-products recep-tor in pulmonary arterial hypertension etiology. J Am Heart Assoc 2: e005157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. [DOI] [PubMed] [Google Scholar]
- 272.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. [DOI] [PubMed] [Google Scholar]
- 273.Meng ML, Landau R, Viktorsdottir O, Banayan J, Grant T, Bateman B, Smiley R, Reitman E. Pulmonary hypertension in pregnancy: A report of 49 cases at four tertiary North American sites. Obstet Gynecol 129: 511–520, 2017. [DOI] [PubMed] [Google Scholar]
- 274.Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell’Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 121: 252–258, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Michnovicz JJ, Bradlow HL. Altered estrogen metabolism and excretion in humans following consumption of indole-3-carbinol. Nutr Cancer 16: 59–66, 1991. [DOI] [PubMed] [Google Scholar]
- 276.Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Janne OA. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 317: 14–24, 2010. [DOI] [PubMed] [Google Scholar]
- 277.Milewich L, Hendricks TS, Johnson AR. Metabolism of dehydroisoan-drosterone and androstenedione in human pulmonary endothelial cells in culture. J Clin Endocrinol Metab 56: 930–935, 1983. [DOI] [PubMed] [Google Scholar]
- 278.Miller AA, Hislop AA, Vallance PJ, Haworth SG. Deletion of the eNOS gene has a greater impact on the pulmonary circulation of male than female mice. Am J Physiol Lung Cell Mol Physiol 289: L299–L306, 2005. [DOI] [PubMed] [Google Scholar]
- 279.Miller VM. In pursuit of scientific excellence: Sex matters. Am J Physiol Lung Cell Mol Physiol 302: L801–L802, 2012. [DOI] [PubMed] [Google Scholar]
- 280.Miller VM, Mulvagh SL. Sex steroids and endothelial function: Translating basic science to clinical practice. Trends Pharmacol Sci 28: 263–270, 2007. [DOI] [PubMed] [Google Scholar]
- 281.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation 130: 2310–2320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Molkentin JHJD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589, 2006. [DOI] [PubMed] [Google Scholar]
- 284.Montalvo C, Villar AV, Merino D, García R, Ares M, Llano M, Cobo M, Hurlé MA, Nistal JF. Androgens contribute to sex differences in myocardial remodeling under pressure overload by a mechanism involving TGF-β. PLoS One 7: e35635, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Montani D, Bergot E, Gunther S, Savale L, Bergeron A, Bourdin A, Bouvaist H, Canuet M, Pison C, Macro M, Poubeau P, Girerd B, Natali D, Guignabert C, Perros F, O’Callaghan DS, Jais X, Tubert-Bitter P, Zalcman G, Sitbon O, Simonneau G, Humbert M. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation 125: 2128–2137, 2012. [DOI] [PubMed] [Google Scholar]
- 286.Moore LG, McMurtry IF, Reeves JT. Effects of sex hormones on cardiovascular and hematologic responses to chronic hypoxia in rats. Proc Soc Exp Biol Med 158: 658–662, 1978. [DOI] [PubMed] [Google Scholar]
- 287.Morey AK, Pedram A, Razandi M, Prins BA, Hu R-M, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology 138: 3330–3339, 1997. [DOI] [PubMed] [Google Scholar]
- 288.Morey AK, Razandi M, Pedram A, Hu RM, Prins BA, Levin ER. Oestrogen and progesterone inhibit the stimulated production of endothelin-1. Biochem J 330: 1097–1105, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Morimatsu Y, Sakashita N, Komohara Y, Ohnishi K, Masuda H, Dahan D, Takeya M, Guibert C, Marthan R. Development and characterization of an animal model of severe pulmonary arterial hypertension. J Vasc Res 49: 33–42, 2012. [DOI] [PubMed] [Google Scholar]
- 290.Moriyama Y, Yasue H, Yoshimura M, Mizuno Y, Nishiyama K, Tsunoda R, Kawano H, Kugiyama K, Ogawa H, Saito Y, Nakao K. The plasma levels of dehydroepiandrosterone sulfate are decreased in patients with chronic heart failure in proportion to the severity. J Clin Endocrinol Metab 85: 1834–1840, 2000. [DOI] [PubMed] [Google Scholar]
- 291.Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, Trembath RC, Loyd JE. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 53: 1801899, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Morrison DA, Adcock K, Collins CM, Goldman S, Caldwell JH, Schwarz MI. Right ventricular dysfunction and the exercise limitation of chronic obstructive pulmonary disease. J Am Coll Cardiol 9: 1219–1229, 1987. [DOI] [PubMed] [Google Scholar]
- 293.Morrissy S, Xu B, Aguilar D, Zhang J, Chen QM. Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell 9: 799–809, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mott NN, Pak TR. Estrogen signaling and the aging brain: Context-dependent considerations for postmenopausal hormone therapy. ISRN Endocrinol 2013: 814690, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, Black CM, Coghlan JG. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: Application of a registry approach. Ann Rheum Dis 62: 1088–1093, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mukundan H, Resta TC, Kanagy NL. 17Beta-estradiol decreases hypoxic induction of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol 283: R496–R504, 2002. [DOI] [PubMed] [Google Scholar]
- 297.Murphy E Estrogen signaling and cardiovascular disease. Circ Res 109: 687–696, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nadadur RD, Umar S, Wong G, Eghbali M, Iorga A, Matori H, Partow-Navid R, Eghbali M. Reverse right ventricular structural and extracellular matrix remodeling by estrogen in severe pulmonary hypertension. JAppl Physiol (1985) 113: 149–158, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Naeije R, Vanderpool R. Pulmonary hypertension and chronic mountain sickness. High Alt Med Biol 14: 117–125, 2013. [DOI] [PubMed] [Google Scholar]
- 300.Nahrendorf M, Frantz S, Hu K, von zur Muhlen C, Tomaszewski M, Scheuermann H, Kaiser R, Jazbutyte V, Beer S, Bauer W, Neubauer S, Ertl G, Allolio B, Callies F. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovasc Res 57: 370–378, 2003. [DOI] [PubMed] [Google Scholar]
- 301.Nakamura S, Yoshimura M, Nakayama M, Ito T, Mizuno Y, Harada E, Sakamoto T, Saito Y, Nakao K, Yasue H, Ogawa H. Possible association of heart failure status with synthetic balance between aldosterone and dehydroepiandrosterone in human heart. Circulation 110: 1787–1793, 2004. [DOI] [PubMed] [Google Scholar]
- 302.Nasim MT, Ogo T, Ahmed M, Randall R, Chowdhury HM, Snape KM, Bradshaw TY, Southgate L, Lee GJ, Jackson I, Lord GM, Gibbs JS, Wilkins MR, Ohta-Ogo K, Nakamura K, Girerd B, Coulet F, Soubrier F, Humbert M, Morrell NW, Trembath RC, Machado RD. Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum Mutat 32: 1385–1389, 2011. [DOI] [PubMed] [Google Scholar]
- 303.Neuhofer W, Gulberg V, Gerbes AL. Endothelin and endothelin receptor antagonism in portopulmonary hypertension. Eur J Clin Invest 3: 54–61, 2006. [DOI] [PubMed] [Google Scholar]
- 304.Oettel M, Mukhopadhyay AK. Progesterone: The forgotten hormone in men? Aging Male 7: 236–257, 2004. [DOI] [PubMed] [Google Scholar]
- 305.Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Commun 243: 122–126, 1998. [DOI] [PubMed] [Google Scholar]
- 306.Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehy-droepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res 74: 377–387, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Lange TJ, Behr J, Klose H, Claussen M, Ewert R, Opitz CF, Vizza CD, Scelsi L, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Coghlan G, Pepke-Zaba J, Schulz U, Gorenflo M, Pittrow D, Hoeper MM. Anticoagulation and survival in pulmonary arterial hypertension: Results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 129: 57–65, 2014. [DOI] [PubMed] [Google Scholar]
- 308.Opravil M, Pechere M, Speich R, Joller Jemelka HI, Jenni R, Russi EW, Hirschel B, Luthy R, Battegay M, Burgisser P, Doorly R, Egger M, Erb P, Fierz W, Flepp M, Francioli P, Grob P, Gruninger U, Ledergerber B, Malinverni R, Matter L, Paccaud F, Perrin L, Pichler W, Rickenbach M, Rutschmann O, Vernazza P, vonOverbeck J. HIV-associated primary pulmonary hypertension—A case control study. Am J Respir Crit Care Med 155: 990–995, 1997. [DOI] [PubMed] [Google Scholar]
- 309.Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings S. Testosterone and estradiol among older men. J Clin Endocrinol Metab 91: 1336–1344, 2006. [DOI] [PubMed] [Google Scholar]
- 310.Ostergard DR. Estriol in pregnancy. Obstet Gynecol Surv 28:215–231, 1973. [DOI] [PubMed] [Google Scholar]
- 311.Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol 17: 870–881, 2003. [DOI] [PubMed] [Google Scholar]
- 312.Paracchini V, Raimondi S, Gram IT, Kang D, Kocabas NA, Kristensen VN, Li D, Parl FF, Rylander-Rudqvist T, Soucek P, Zheng W, Wedren S, Taioli E. Meta- and pooled analyses of the cytochrome P-450 1B1 Val432Leu polymorphism and breast cancer: A HuGE-GSEC review. Am J Epidemiol 165: 115–125, 2007. [DOI] [PubMed] [Google Scholar]
- 313.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res 90: 1087–1092, 2002. [DOI] [PubMed] [Google Scholar]
- 314.Park BK. Cytochrome P450 enzymes in the heart. Lancet 355:945–946, 2000. [DOI] [PubMed] [Google Scholar]
- 315.Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol 23: 8542–8552, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 301: H1798–H1809, 2011. [DOI] [PubMed] [Google Scholar]
- 317.Payne AH, Hales DB. Overview of steroidogenic enzymes in the path-way from cholesterol to active steroid hormones. Endocr Rev 25: 947–970, 2004. [DOI] [PubMed] [Google Scholar]
- 318.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 30: 104–109, 2007. [DOI] [PubMed] [Google Scholar]
- 319.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D’Armini AM, Morsolini M, Snijder R, Bresser P, Torbicki A, Kristensen B, Lewczuk J, Simkova I, Barbera JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez-Sanchez MA, Kovacs G, Hamid AM, Jais X, Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 124: 1973–1981, 2011. [DOI] [PubMed] [Google Scholar]
- 320.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene 18: 6322–6329, 1999. [DOI] [PubMed] [Google Scholar]
- 321.Petitpretz P, Brenot F, Azarian R, Parent F, Rain B, Herve P, Simonneau G. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation 89: 2722–2727, 1994. [DOI] [PubMed] [Google Scholar]
- 322.Pribluda VS, Gubish ER Jr, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: An endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev 19: 173–179, 2000. [DOI] [PubMed] [Google Scholar]
- 323.Prins KW, Rose L, Archer SL, Pritzker M, Weir EK, Olson MD, Thenappan T. Clinical determinants and prognostic implications of right ventricular dysfunction in pulmonary hypertension caused by chronic lung disease. J Am Heart Assoc 8: e011464, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Provencher S, Archer SL, Ramirez FD, Hibbert B, Paulin R, Boucherat O, Lacasse Y, Bonnet S. Standards and methodological rigor in pulmonary arterial hypertension preclinical and translational research. Circ Res 122: 1021–1032, 2018. [DOI] [PubMed] [Google Scholar]
- 325.Quong J, Eppenberger-Castori S, Moore D III, Scott GK, Birrer MJ, Kueng W, Eppenberger U, Benz CC. Age-dependent changes in breast cancer hormone receptors and oxidant stress markers. Breast Cancer Res Treat 76: 221–236, 2002. [DOI] [PubMed] [Google Scholar]
- 326.Rabinovitch M Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol 240: H62–H72, 1981. [DOI] [PubMed] [Google Scholar]
- 328.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Radegran G, Kjellstrom B, Ekmehag B, Larsen F, Rundqvist B, Blomquist SB, Gustafsson C, Hesselstrand R, Karlsson M, Kornhall A, Nisell M, Persson L, Ryftenius H, Selin M, Ullman B, Wall K, Wikstrom G, Willehadson M, Jansson K, Stefan Soderberg J, on behalf of SveFPH and SPAHR. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand Cardiovasc J 50: 243–250, 2016. [DOI] [PubMed] [Google Scholar]
- 330.Rafikova O, Rafikov R, Meadows ML, Kangath A, Jonigk D, Black SM. The sexual dimorphism associated with pulmonary hypertension corresponds to a fibrotic phenotype. Pulm Circ 5: 184–197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol 298: H1235–H1248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ramakrishnan L, Pedersen SL, Toe QK, Quinlan GJ, Wort SJ. Pul-monary arterial hypertension: Iron matters. Front Physiol 9:641, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rasheed W, Flaim B, Seymour JF. Reversible severe pulmonary hypertension secondary to dasatinib in a patient with chronic myeloid leukemia. Leuk Res 33: 861–864, 2009. [DOI] [PubMed] [Google Scholar]
- 334.Redfield MM, Jacobsen SJ, Burnett J, John C, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA 289: 194–202, 2003. [DOI] [PubMed] [Google Scholar]
- 335.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol 280: L88–L97, 2001. [DOI] [PubMed] [Google Scholar]
- 336.Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, Pauciulo MW, Hadinnapola C, Aman J, Girerd B, Arora A, Knight J, Hanscombe KB, Karnes JH, Kaakinen M, Gall H, Ulrich A, Harbaum L, Cebola I, Ferrer J, Lutz K, Swietlik EM, Ahmad F, Amouyel P, Archer SL, Argula R, Austin ED, Badesch D, Bakshi S, Barnett C, Benza R, Bhatt N, Bogaard HJ, Burger CD, Chakinala M, Church C, Coghlan JG, Condliffe R, Corris PA, Danesino C, Debette S, Elliott CG, Elwing J, Eyries M, Fortin T, Franke A, Frantz RP, Frost A, Garcia JGN, Ghio S, Ghofrani HA, Gibbs JSR, Harley J, He H, Hill NS, Hirsch R, Houweling AC, Howard LS, Ivy D, Kiely DG, Klinger J, Kovacs G, Lahm T, Laudes M, Machado RD, Ross RVM, Marsolo K, Martin LJ, Moledina S, Montani D, Nathan SD, Newnham M, Olschewski A, Olschewski H, Oudiz RJ, Ouwehand WH, Peacock AJ, Pepke-Zaba J, Rehman Z, Robbins I, Roden DM, Rosenzweig EB, Saydain G, Scelsi L, Schilz R, Seeger W, Shaffer CM, Simms RW, Simon M, Sitbon O, Suntharalingam J, Tang H, Tchourbanov AY, Thenappan T, Torres F, Toshner MR, Treacy CM, Vonk Noordegraaf A, Waisfisz Q, Walsworth AK, Walter RE, Wharton J, White RJ, Wilt J, Wort SJ, Yung D, Lawrie A, Humbert M, Soubrier F, Trégouët DA, Prokopenko I, Kittles R, Gräf S, Nichols WC, Trembath RC, Desai AA, Morrell NW, Wilkins MR, UK NIHR BioResource Rare Diseases Consortium, UK PAH Cohort. Genetic determinants of risk in pulmonary arterial hypertension: International genome-wide association studies and meta-analysis. Lancet Respir Med 7: 227–238, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, Bleda M, Haimel M, Coghlan G, Corris PA, Howard LS, Kiely DG, Peacock AJ, Pepke-Zaba J, Toshner MR, Wort SJ, Gibbs JS, Lawrie A, Graf S, Morrell NW, Wilkins MR. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 135: 460–475, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, Williams GW. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 107: 216–223, 1987. [DOI] [PubMed] [Google Scholar]
- 339.Rich S, Rubin L, Walker AM, Schneeweiss S, Abenhaim L. Anorexigens and pulmonary hypertension in the United States: Results from the surveillance of North American pulmonary hypertension. Chest 117: 870–874, 2000. [DOI] [PubMed] [Google Scholar]
- 340.Roberts KE, Fallon MB, Krowka MJ, Brown RS, Trotter JF, Peter I, Tighiouart H, Knowles JA, Rabinowitz D, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, Kawut SM. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med 179: 835–842, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rochira V, Guaraldi G. Hypogonadism in the HIV-infected man. Endocrinol Metab Clin North Am 43: 709–730, 2014. [DOI] [PubMed] [Google Scholar]
- 342.Rosenberg HC, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol 255: H1484–H1491, 1988. [DOI] [PubMed] [Google Scholar]
- 343.Rowell KO, Hall J, Pugh PJ, Jones TH, Channer KS, Jones RD. Testosterone acts as an efficacious vasodilator in isolated human pulmonary arteries and veins: Evidence for a biphasic effect at physiological and supra-physiological concentrations. J Endocrinol Invest 32: 718–723, 2009. [DOI] [PubMed] [Google Scholar]
- 344.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, van der Laarse WJ, Vonk-Noordegraaf A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J 37: 1386–1391, 2011. [DOI] [PubMed] [Google Scholar]
- 345.Ryan J, Bloch K, Archer SL. Rodent models of pulmonary hypertension: Harmonisation with the world health organisation’s categorisation of human PH. Int J Clin Pract Suppl 172: 15–34, 2011. [DOI] [PubMed] [Google Scholar]
- 346.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: Disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 115: 176–188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ryan JJ, Marsboom G, Archer SL. Rodent models of group 1 pulmonary hypertension. Handb Exp Pharmacol 218: 105–149, 2013. [DOI] [PubMed] [Google Scholar]
- 348.Ryan JJ, Marsboom G, Fang yH, Toth PT, Morrow E, Luo N, Piao L, Hong Z, Ericson K, Zhang HJ, Han M, Haney CR, Chen cT, Sharp WW, Archer SL. PGC1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am J Respir Crit Care Med 187: 865–878, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 115: 1260–1268, 2007. [DOI] [PubMed] [Google Scholar]
- 350.Salinas CE, Blanco CE, Villena M, Giussani DA. High-altitude hypoxia and echocardiographic indices of pulmonary hypertension in male and female chickens at adulthood. Circ J 78: 1459–1464, 2014. [DOI] [PubMed] [Google Scholar]
- 351.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 8(1): 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sangal RB, Taylor LE, Gillani F, Poppas A, Klinger JR, Ventetuolo CE. Risk of echocardiographic pulmonary hypertension in individuals with human immunodeficiency virus-hepatitis C virus coinfection. Ann Am Thorac Soc 11: 1553–1559, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sano M, Saotome M, Urushida T, Katoh H, Satoh H, Ohnishi K, Hayashi H. Pulmonary arterial hypertension caused by treatment with dasatinib for chronic myeloid leukemia-critical alert. Intern Med 51: 2337–2340, 2012. [DOI] [PubMed] [Google Scholar]
- 354.Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, Ohta H, Moritoki Y, Iwatani C, Tsuchiya H, Nakamura S, Sekiguchi K, Sakuma T, Yamamoto T, Mori T, Woltjen K, Nakagawa M, Takahashi K, Yamanaka S, Saitou M. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 17: 178–194, 2015. [DOI] [PubMed] [Google Scholar]
- 355.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: Implications for lung diseases. Pharmacol Ther 150: 94–108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sato J, Nasu M, Tsuchitani M. Comparative histopathology of the estrous or menstrual cycle in laboratory animals. J Toxicol Pathol 29: 155–162, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Savale L, Chaumais MC, Cottin V, Bergot E, Frachon I, Prevot G, Pison C, Dromer C, Poubeau P, Lamblin N, Habib G, Reynaud-Gaubert M, Bourdin A, Sanchez O, Tubert-Bitter P, Jais X, Montani D, Sitbon O, Simonneau G, Humbert M. Pulmonary hypertension associated with benfluorex exposure. Eur Respir J 40: 1164–1172, 2012. [DOI] [PubMed] [Google Scholar]
- 358.Savale L, Gunther S, Chaumais MC, Jais X, Sattler C, Macari EA, Montani D, Simonneau G, Humbert M, Sitbon O. Pulmonary Arterial Hypertension in Patients Treated with Interferon. Clamart, France: Le Kremlin Bicetre, 2013. [Google Scholar]
- 359.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol 26:562–568, 2011. [DOI] [PubMed] [Google Scholar]
- 360.Schiffer L, Arlt W, Storbeck KH. Intracrine androgen biosynthesis, metabolism and action revisited. Mol Cell Endocrinol 465: 4–26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Schuit SC, Oei HH, Witteman JC, Geurts van Kessel CH, van Meurs JB, Nijhuis RL, van Leeuwen JP, de Jong FH, Zillikens MC, Hofman A, Pols HA, Uitterlinden AG. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA 291: 2969–2977, 2004. [DOI] [PubMed] [Google Scholar]
- 362.Schumacher G, Neuhaus P. The physiological estrogen metabolite 2-methoxyestradiol reduces tumor growth and induces apoptosis in human solid tumors. J Cancer Res Clin Oncol 127: 405–410, 2001. [DOI] [PubMed] [Google Scholar]
- 363.Scorza R, Caronni M, Bazzi S, Nador F, Beretta L, Antonioli R, Origgi L, Ponti A, Marchini M, Vanoli M. Post-menopause is the main risk factor for developing isolated pulmonary hypertension in systemic sclerosis. Ann N Y Acad Sci 966: 238–246, 2002. [DOI] [PubMed] [Google Scholar]
- 364.Scragg JL, Jones RD, Channer KS, Jones TH, Peers C. Testosterone is a potent inhibitor of L-type Ca(2+) channels. Biochem Biophys Res Commun 318: 503–506, 2004. [DOI] [PubMed] [Google Scholar]
- 365.Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 141: 363–373, 2012. [DOI] [PubMed] [Google Scholar]
- 366.Sharma S, Umar S, Centala A, Eghbali M. Role of miR206 in genistein-induced rescue of pulmonary hypertension in monocrotaline model. J Appl Physiol (1985) 119: 1374–1382, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Shaul PW. Rapid activation of endothelial nitric oxide synthase by estrogen. Steroids 64: 28–34, 1999. [DOI] [PubMed] [Google Scholar]
- 368.Shearman AM, Cooper JA, Kotwinski PJ, Humphries SE, Mendelsohn ME, Housman DE, Miller GJ. Estrogen receptor alpha gene variation and the risk of stroke. Stroke 36: 2281–2282, 2005. [DOI] [PubMed] [Google Scholar]
- 369.Shearman AM, Cooper JA, Kotwinski PJ, Miller GJ, Humphries SE, Ardlie KG, Jordan B, Irenze K, Lunetta KL, Schuit SC, Uitterlinden AG, Pols HA, Demissie S, Cupples LA, Mendelsohn ME, Levy D, Housman DE. Estrogen receptor alpha gene variation is associated with risk of myocardial infarction in more than seven thousand men from five cohorts. Circ Res 98: 590–592, 2006. [DOI] [PubMed] [Google Scholar]
- 370.Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, Shaul PW. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol 26:610–616, 2002. [DOI] [PubMed] [Google Scholar]
- 371.Shigeta A, Tanabe N, Shimizu H, Hoshino S, Maruoka M, Sakao S, Tada Y, Kasahara Y, Takiguchi Y, Tatsumi K, Masuda M, Kuriyama T. Gender differences in chronic thromboembolic pulmonary hypertension in Japan. Circ J 72: 2069–2074, 2008. [DOI] [PubMed] [Google Scholar]
- 372.Shintani M, Yagi H, Nakayama T, Saji T, Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet 46: 331–337, 2009. [DOI] [PubMed] [Google Scholar]
- 373.Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, Braunstein GD, Pepine CJ, Bittner V, Vido DA, Stanczyk FZ, Bairey Merz CN. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: Results from the National Institutes of Health—National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab 95: 4985–4992, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407: 538–541, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. Genomic and non-genomic effects of estrogens on endothelial cells. Steroids 69: 537–542, 2004. [DOI] [PubMed] [Google Scholar]
- 376.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62: D34–D41, 2013. [DOI] [PubMed] [Google Scholar]
- 377.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53: 1801913, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Simpson, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase—A brief overview. Annu Rev Physiol 64: 93–127, 2002. [DOI] [PubMed] [Google Scholar]
- 379.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 177: 108–113, 2008. [DOI] [PubMed] [Google Scholar]
- 380.Skride A, Sablinskis K, Lejnieks A, Rudzitis A, Lang I. Characteristics and survival data from Latvian pulmonary hypertension registry: Comparison of prospective pulmonary hypertension registries in Europe. Pulm Circ 8: 2045894018780521, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Smirnova NF, Fontaine C, Buscato M, Lupieri A, Vinel A, Valera MC, Guillaume M, Malet N, Foidart JM, Raymond-Letron I, Lenfant F, Gourdy P, Katzenellenbogen BS, Katzenellenbogen JA, Laffargue M, Arnal JF. The activation function-1 of estrogen receptor alpha prevents arterial neointima development through a direct effect on smooth muscle cells. Circ Res 117: 770–778, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Smith AM, Bennett RT, Jones TH, Cowen ME, Channer KS, Jones RD. Characterization of the vasodilatory action of testosterone in the human pulmonary circulation. Vasc Health Risk Manag 4: 1459–1466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Smith MM, Clarke EC, Little CB. Considerations for the design and execution of protocols for animal research and treatment to improve reproducibility and standardization: "DEPART well-prepared and ARRIVE safely". Osteoarthr Cartil 25: 354–363, 2017. [DOI] [PubMed] [Google Scholar]
- 384.Somjen D, Kohen F, Jaffe A, Amir-Zaltsman Y, Knoll E, Stern N. Effects of gonadal steroids and their antagonists on DNA synthesis in human vascular cells. Hypertension 32: 39–45, 1998. [DOI] [PubMed] [Google Scholar]
- 385.Soon E, Treacy CM, Toshner MR, MacKenzie-Ross R, Manglam V, Busbridge M, Sinclair-McGarvie M, Arnold J, Sheares KK, Morrell NW, Pepke-Zaba J. Unexplained iron deficiency in idiopathic and her-itable pulmonary arterial hypertension. Thorax 66: 326–332, 2011. [DOI] [PubMed] [Google Scholar]
- 386.Sophocles AM Jr. High-altitude pulmonary edema in Vail, Colorado, 1975–1982. West J Med 144: 569–573, 1986. [PMC free article] [PubMed] [Google Scholar]
- 387.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JCS, McPhail M, Delaney A, Wigerblad G, Schumann Ap, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 11: 629, 2014. [DOI] [PubMed] [Google Scholar]
- 388.Soubrier F, Chung WK, Machado R, Grunig E, Aldred M, Geraci M, Loyd JE, Elliott CG, Trembath RC, Newman JH, Humbert M. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 62: 035, 2013. [DOI] [PubMed] [Google Scholar]
- 389.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary-hypertension in HIV infection. Chest 100: 1268–1271, 1991. [DOI] [PubMed] [Google Scholar]
- 390.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Stearman RS, Bui QM, Speyer G, Handen A, Cornelius AR, Graham BB, Kim S, Mickler EA, Tuder RM, Chan SY, Geraci MW. Systems analysis of the human pulmonary arterial hypertension lung transcriptome. Am J Respir Cell Mol Biol 60: 637–649, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 66: 940–944, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. [DOI] [PubMed] [Google Scholar]
- 394.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. [DOI] [PubMed] [Google Scholar]
- 395.Stevens HC, Deng L, Grant JS, Pinel K, Thomas M, Morrell NW, MacLean MR, Baker AH, Denby L. Regulation and function of miR-214 in pulmonary arterial hypertension. Pulm Circ 6: 109–117, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol 63: 193–213, 2001. [DOI] [PubMed] [Google Scholar]
- 397.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 28:521–574, 2007. [DOI] [PubMed] [Google Scholar]
- 398.Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM. Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation 96: 3774–3777, 1997. [DOI] [PubMed] [Google Scholar]
- 399.Sudhir K, Chou TM, Messina LM, Hutchison SJ, Korach KS, Chatterjee K, Rubanyi GM. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet 349: 1146–1147, 1997. [DOI] [PubMed] [Google Scholar]
- 400.Sun X, Feng J, Shi J. Pregnancy and pulmonary hypertension: An exploratory analysis of risk factors and outcomes. Medicine (Baltimore) 97: e13035, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Schwacha MG, Chaudry IH. Tissue compartment-specific role of estrogen receptor subtypes in immune cell cytokine production following trauma-hemorrhage. J Appl Physiol 102: 163–168, 2007. [DOI] [PubMed] [Google Scholar]
- 402.Suzuki T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Estrogen-mediated activation of non-genomic pathway improves macrophages cytokine production following trauma-hemorrhage. J Cell Physiol 214: 662–672, 2007. [DOI] [PubMed] [Google Scholar]
- 403.Swaneck GE, Fishman J. Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: Characterization and intranuclear localization. Proc Natl Acad Sci USA 85: 7831–7835, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Sweeney L, Voelkel NF. Estrogen exposure, obesity and thyroid disease in women with severe pulmonary hypertension. Eur J Med Res 14:433–442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Swift AJ, Capener D, Hammerton C, Thomas SM, Elliot C, Condliffe R, Wild JM, Kiely DG. Right ventricular sex differences in patients with idiopathic pulmonary arterial hypertension characterised by magnetic resonance imaging: Pair-matched case controlled study. PLoS One 10: e0127415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Tabima DM, Hacker TA, Chesler NC. Measuring right ventricular function in the normal and hypertensive mouse hearts using admittance-derived pressure-volume loops. Am J Physiol Heart Circ Physiol 299: H2069–H2075, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Taherzadeh M, Fradette C, Bleau AM, Jomphe C, Trudeau LE, du Souich P. The 21-aminosteroid U74389G prevents the down-regulation and decrease in activity of CYP1A1, 1A2 and 3A6 induced by an inflammatory reaction. Biochem Pharmacol 71: 366–376, 2006. [DOI] [PubMed] [Google Scholar]
- 408.Tamosiuniene R, Manouvakhova O, Mesange P, Saito T, Qian J, Sanyal M, Lin YC, Nguyen LP, Luria A, Tu AB, Sante JM, Rabinovitch M, Fitzgerald DJ, Graham BB, Habtezion A, Voelkel NF, Aurelian L, Nicolls MR. Dominant role for regulatory T cells in protecting females against pulmonary hypertension. Circ Res 122: 1689–1702, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. [DOI] [PubMed] [Google Scholar]
- 410.Taylor SE, Martin-Hirsch PL, Martin FL. Oestrogen receptor splice variants in thepathogenesis of disease. Cancer Lett 288:133–148, 2010. [DOI] [PubMed] [Google Scholar]
- 411.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J 30: 1103–1110, 2007. [DOI] [PubMed] [Google Scholar]
- 412.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: A reappraisal of the NIH risk stratification equation. Eur Respir J 35: 1079–1087, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Thomas P, Marine Science Institute (p.T. YP, J.D., A.H.B.), The University of Texas at Austin, Port Aransas, Texas 78373, Pang Y, Marine Science Institute (P.T. YP, J.D., A.H.B.), The University of Texas at Austin, Port Aransas, Texas 78373, Dong J, Marine Science Institute (P.T. YP, J.D., A.H.B.), The University of Texas at Austin, Port Aransas, Texas 78373, Berg AH, Marine Science Institute (P.T. YP, J.D., A.H.B.), The University of Texas at Austin, Port Aransas, Texas 78373, Department of Science and Technology (A.H.B.) ÖU, Örebro, Sweden SE-70182. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 155: 4250–4265, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Thum T, Borlak J. Gene expression in distinct regions of the heart. Lancet 355: 979–983, 2000. [DOI] [PubMed] [Google Scholar]
- 415.Thum T, Borlak J. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J 16: 1537–1549, 2002. [DOI] [PubMed] [Google Scholar]
- 416.Tofovic PS, Zhang X, Petrusevska G. Progesterone inhibits vascular remodeling and attenuates monocrotaline-induced pulmonary hypertension in estrogen-deficient rats. Prilozi 30: 25–44, 2009. [PubMed] [Google Scholar]
- 417.Tofovic SP. Estrogens and development of pulmonary hypertension: Interaction of estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol 56: 696–708, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Tofovic SP, Jones TJ, Bilan VP, Jackson EK, Petrusevska G. Synergistic therapeutic effects of 2-methoxyestradiol with either sildenafil or bosentan on amelioration of monocrotaline-induced pulmonary hypertension and vascularremodeling. J Cardiovasc Pharmacol 56:475–483, 2010. [DOI] [PubMed] [Google Scholar]
- 419.Tofovic SP, Rafikova O, Champion H, Schneider F. Estrogens exacerbate development of occlusive pulmonary arterial hypertension and formation of plexiform lesions. In: B63. Experimental Models in Pulmonary hypertension I. American Thoracic Society, 2012, p. A6803–A6803. [Google Scholar]
- 420.Tofovic SP, Salah EM, Mady HH, Jackson EK, Melhem MF. Estradiol metabolites attenuate monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol 46: 430–437, 2005. [DOI] [PubMed] [Google Scholar]
- 421.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascul Pharmacol 45: 358–367, 2006. [DOI] [PubMed] [Google Scholar]
- 422.Tofovic SP, Zhang X, Jackson EK, Zhu H, Petrusevska G. 2-Methoxyestradiol attenuates bleomycin-induced pulmonary hypertension and fibrosis in estrogen-deficient rats. Vascul Pharmacol 51: 190–197, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Tofovic SP, Zhang X, Zhu H, Jackson EK, Rafikova O, Petrusevska G. 2-Ethoxyestradiol is antimitogenic and attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling. Vascul Pharmacol 48: 174–183, 2008. [DOI] [PubMed] [Google Scholar]
- 424.Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, Bakal JA, Armstrong PW, Ezekowitz JA. Testosterone supplementation in heart failure: A meta-analysis. Circ Heart Fail 5: 315–321, 2012. [DOI] [PubMed] [Google Scholar]
- 425.Toutain CE, Filipe C, Billon A, Fontaine C, Brouchet L, Guery JC, Gourdy P, Arnal JF, Lenfant F. Estrogen receptor alpha expression in both endothelium and hematopoietic cells is required for the accelerative effect of estradiol on reendothelialization. Arterioscler Thromb Vasc Biol 29: 1543–1550, 2009. [DOI] [PubMed] [Google Scholar]
- 426.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 33: 1–47, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227: 115–124, 2005. [DOI] [PubMed] [Google Scholar]
- 428.Tsukamoto A, Kaneko Y, Yoshida T, Han K, Ichinose M, Kimura S. 2-Methoxyestradiol, an endogenous metabolite of estrogen, enhances apoptosis and beta-galactosidase expression in vascular endothelial cells. Biochem Biophys Res Commun 248: 9–12, 1998. [DOI] [PubMed] [Google Scholar]
- 429.Tuder RM, Archer SL, Dorfmuller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 62: D4–D12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB. Pathology of pulmonary hypertension. Clin Chest Med 34: 639–650, 2013. [DOI] [PubMed] [Google Scholar]
- 431.Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, Centala A, Arnold AP, Eghbali M. The Y chromosome plays a protective role in experimental hypoxic pulmonary hypertension. Am J Respir Crit Care Med 197: 952–955, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Umar S, Iorga A, Matori H, Nadadur RD, Li J, Maltese F, van der Laarse A, Eghbali M. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med 184: 715–723, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Umar S, Partow-Navid R, Ruffenach G, Iorga A, Moazeni S, Eghbali M. Severe pulmonary hypertension in aging female apolipoprotein E-deficient mice is rescued by estrogen replacement therapy. Biol Sex Differ 8: 9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 58: 2511–2519, 2011. [DOI] [PubMed] [Google Scholar]
- 435.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 28: 1250–1257, 2007. [DOI] [PubMed] [Google Scholar]
- 436.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, Jacobs W, Marcus JT, Marques KM, Bronzwaer JG, Heymans MW, Boonstra A, Postmus PE, Westerhof N, Vonk Noordegraaf A. Clinically significant change in stroke volume in pulmonary hypertension. Chest 139: 1003–1009, 2011. [DOI] [PubMed] [Google Scholar]
- 437.Vanderpool RR, Naeije R. Hematocrit-corrected pulmonary vascular resistance. Am J Respir Crit Care Med 198: 305–309, 2018. [DOI] [PubMed] [Google Scholar]
- 438.Vázquez F, Rodríguez-Manzaneque JC, Lydon JP, Edwards DP, O’Malley BW, Iruela-Arispe ML. Progesterone regulates proliferation of endothelial cells. J Biol Chem 274: 2185–2192, 1999. [DOI] [PubMed] [Google Scholar]
- 439.VegetoE CuzzocreaS, Crisafulli C MazzonE, SalaA KrustA, Maggi A. Estrogen receptor-alpha as a drug target candidate for preventing lung inflammation. Endocrinology 151: 174–184, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Venkov CD, Rankin AB, Vaughan DE. Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation 94: 727–733, 1996. [DOI] [PubMed] [Google Scholar]
- 441.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, Palmisciano AJ, Krishnan I, Pinder D, Preston IR, Roberts KE, Kawut SM. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med 193:1168–1175, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Ventetuolo CE, Hess E, Austin ED, Baron AE, Klinger JR, Lahm T, Maddox TM, Plomondon ME, Thompson L, Zamanian RT, Choudhary G, Maron BA. Sex-based differences in veterans with pulmonary hypertension: Results from the veterans affairs-clinical assessment reporting and tracking database. PLoS One 12: e0187734, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Ventetuolo CE, Mitra N, Wan F, Manichaikul A, Barr RG, Johnson C, Bluemke DA, Lima JA, Tandri H, Ouyang P, Kawut SM. Oestradiol metabolism and androgen receptor genotypes are associated with right ventricular function. Eur Respir J 47: 553–563, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am J Respir Crit Care Med 183:659–667, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Ventetuolo CE, Praestgaard A, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Sex and haemodynamics in pulmonary arterial hypertension. Eur Respir J 43: 523–530, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Vitali SH, Hansmann G, Rose C, Fernandez-Gonzalez A, Scheid A, Mitsialis SA, Kourembanas S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: Long-term follow-up. Pulm Circ 4: 619–629, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin lJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006. [DOI] [PubMed] [Google Scholar]
- 448.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 69: 236–243, 2017. [DOI] [PubMed] [Google Scholar]
- 449.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J Am Coll Cardiol 62: D22–D33, 2013. [DOI] [PubMed] [Google Scholar]
- 450.Walker AM, Langleben D, Korelitz JJ, Rich S, Rubin LJ, Strom BL, Gonin R, Keast S, Badesch D, Barst RJ, Bourge RC, Channick R, Frost A, Gaine S, McGoon M, McLaughlin V, Murali S, Oudiz RJ, Robbins IM, Tapson V, Abenhaim L, Constantine G. Temporal trends and drug exposures in pulmonary hypertension: An American experience. Am Heart J 152: 521–526, 2006. [DOI] [PubMed] [Google Scholar]
- 451.Wang L, Hao Q, Wang YD, Wang WJ, Li DJ. Protective effects of dehydroepiandrosterone on atherosclerosis in ovariectomized rabbits via alleviating inflammatory injury in endothelial cells. Atherosclerosis 214: 47–57, 2011. [DOI] [PubMed] [Google Scholar]
- 452.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol 290: H2204–H2209, 2006. [DOI] [PubMed] [Google Scholar]
- 453.Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, Kelly M, Meldrum DR. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol 296: R972–R978, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Warnmark A, Treuter E, Wright AP, Gustafsson JA. Activation functions 1 and 2 of nuclear receptors: Molecular strategies for transcriptional activation. Mol Endocrinol 17: 1901–1909, 2003. [DOI] [PubMed] [Google Scholar]
- 455.Welter BH, Hansen EL, Saner KJ, Wei Y, Price TM. Membrane-bound progesterone receptor expression in human aortic endothelial cells. J Histochem Cytochem 51: 1049–1055, 2003. [DOI] [PubMed] [Google Scholar]
- 456.Wenzlaff AS, Cote ML, Bock CH, Land SJ, Santer SK, Schwartz DR, Schwartz AG. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: A population-based study. Carcinogenesis 26: 2207–2212, 2005. [DOI] [PubMed] [Google Scholar]
- 457.West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, Lane K, Meyrick B, Loyd J. Gene expression in BMPR2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC Med Genomics 1: 45, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hyperten-sion in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 459.Wetzel RC, Sylvester JT. Gender differences in hypoxic vascular response of isolated sheep lungs. J Appl Physiol 55: 100–104, 1983. [DOI] [PubMed] [Google Scholar]
- 460.Wetzel RC, Zacur HA, Sylvester JT. Effect of puberty and estradiol on hypoxic vasomotor response in isolated sheep lungs. J Appl Physiol 56: 1199–1203, 1984. [DOI] [PubMed] [Google Scholar]
- 461.White K, Dempsie Y, Nilsen M, Wright AF, LoughlinL, MacLean MR. The serotonin transporter, gender, and 17beta oestradiol in the development of pulmonary arterial hypertension. Cardiovasc Res 90: 373–382, 2011. [DOI] [PubMed] [Google Scholar]
- 462.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, Maclean MR. The serotonin transporter, gender, and 17{beta} oestradiol in the development of pulmonary arterial hypertension. Cardiovasc Res 90: 373–382, 2011. [DOI] [PubMed] [Google Scholar]
- 463.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, Thomas M, Mair KM, MacLean MR. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation 126: 1087–1098, 2012. [DOI] [PubMed] [Google Scholar]
- 464.White K, Loughlin L, Maqbool Z, Nilsen M, McClure J, Dempsie Y, Baker AH, MacLean MR. Serotonin transporter, sex, and hypoxia: Microarray analysis in the pulmonary arteries of mice identifies genes with relevance to human PAH. Physiol Genomics 43: 417–437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.White PC. Aldosterone: Direct effects on and production by the heart. J Clin Endocrinol Metab 88: 2376–2383, 2003. [DOI] [PubMed] [Google Scholar]
- 466.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L583–L590, 2007. [DOI] [PubMed] [Google Scholar]
- 467.Widder J, Pelzer T, von Poser-Klein C, Hu K, Jazbutyte V, Fritzemeier KH, Hegele-Hartung C, Neyses L, Bauersachs J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension 42: 991–996, 2003. [DOI] [PubMed] [Google Scholar]
- 468.Wizeman TM, Pardue ML. Exploring the biological contributions to human health: Does sex matter? In: Wizemann TM, Pardue ML editors. Board on Health Sciences Policy. Washington, DC: National Academy Press, 2001, p. 288. [PubMed] [Google Scholar]
- 469.Wright AF, Ewart MA, Mair K, Nilsen M, Dempsie Y, Loughlin L, Maclean MR. Oestrogen receptor alpha in pulmonary hypertension. Cardiovasc Res 106: 206–216, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear estrogen receptor signaling in the endothelium. J Biol Chem 286: 14737–14743, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Wu WH, Yuan P, Zhang SJ, Jiang X, Wu C, Li Y, Liu SF, Liu QQ, Li JH, Pudasaini B, Hu QH, Dupuis J, Jing ZC. Impact of pituitary-gonadal axis hormones on pulmonary arterial hypertension in men. Hypertension (Dallas, Tex: 1979) 72: 151–158, 2018. [DOI] [PubMed] [Google Scholar]
- 472.Xiao J, Li J, Xu T, Lv D, Shen B, Song Y, Xu J. Pregnancy-induced physiological hypertrophy protects against cardiac ischemia-reperfusion injury. Int J Clin Exp Pathol 7: 229–235, 2014. [PMC free article] [PubMed] [Google Scholar]
- 473.Xu D, Niu W, Luo Y, Zhang B, Liu M, Dong H, Liu Y, Li Z. Endogenous estrogen attenuates hypoxia-induced pulmonary hypertension by inhibiting pulmonary arterial vasoconstriction and pulmonary arterial smooth muscle cells proliferation. Int J Med Sci 10: 771–781, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Xu DQ, Luo Y, Liu Y, Wang J, Zhang B, Xu M, Wang YX, Dong HY, Dong MQ, Zhao PT, Niu W, Liu ML, Gao YQ, Li ZC. Beta-estradiol attenuates hypoxic pulmonary hypertension by stabilizing the expression of p27kip1 in rats. Respir Res 11: 182, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007. [DOI] [PubMed] [Google Scholar]
- 476.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med 354: 270–282, 2006. [DOI] [PubMed] [Google Scholar]
- 477.Yan L, Cogan JD, Hedges LK, Nunley B, Hamid R, Austin ED. The Y chromosome regulates BMPR2 expression via SRY: A possible reason “why” fewer males develop pulmonary arterial hypertension. Am J Respir Crit Care Med 198: 1581–1583, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Yang YM, Sehgal PB. Smooth muscle-specific BCL6+/− knockout abrogates sex bias in chronic hypoxia-induced pulmonary arterial hypertension in mice. Int J Endocrinol 2018: 3473105, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Yang YM, Yuan H, Edwards JG, Skayian Y, Ochani K, Miller EJ, Sehgal PB. Deletion of STAT5a/b in vascular smooth muscle abrogates the male bias in hypoxic pulmonary hypertension in mice: Implications in the human disease. Mol Med 20: 625–638, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Young MJ, Clyne CD, Cole TJ, Funder JW. Cardiac steroidogenesis in the normal and failing heart. J Clin Endocrinol Metab 86: 5121–5126, 2001. [DOI] [PubMed] [Google Scholar]
- 481.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: Upregulation of the PI-3K/Akt pathway. J Leukoc Biol 82: 774–780, 2007. [DOI] [PubMed] [Google Scholar]
- 482.Yuan P, Gao L, Jing ZC. Complexities of oestradiol pharmacology in pulmonary arterial hypertension. Eur Respir J 41: 1466–1467, 2013. [DOI] [PubMed] [Google Scholar]
- 483.Yuan P, Wu WH, Gao L, Zheng ZQ, Liu D, Mei HY, Zhang ZL, Jing ZC. Oestradiol ameliorates monocrotaline pulmonary hypertension via NO, prostacyclin and endothelin-1 pathways. Eur Respir J 41: 1116–1125, 2013. [DOI] [PubMed] [Google Scholar]
- 484.Zacharia LC, Gogos JA, Karayiorgou M, Jackson EK, Gillespie DG, Barchiesi F, Dubey RK. Methoxyestradiols mediate the antimitogenic effects of 17beta-estradiol: Direct evidence from catechol-O-methyltransferase-knockout mice. Circulation 108: 2974–2978, 2003. [DOI] [PubMed] [Google Scholar]
- 485.Zacharia LC, Jackson EK, Gillespie DG, Dubey RK. Increased 2-methoxyestradiol production in human coronary versus aortic vascular cells. Hypertension 37: 658–662, 2001. [DOI] [PubMed] [Google Scholar]
- 486.Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, Kudelko K, Liu J, Hsi A, Rupp A, Sweatt AJ, Tuder R, Berry GJ, Rabinovitch M, Doyle RL, de Jesus Perez V, Kawut SM. Features and outcomes of methamphetamine-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 197: 788–800, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Zhang R, Dai LZ, Xie WP, Yu ZX, Wu BX, Pan L, Yuan P, Jiang X, He J, Humbert M, Jing ZC. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest 140: 301–309, 2011. [DOI] [PubMed] [Google Scholar]
- 488.Zhao C, Dahlman-Wright K, Gustafsson J. Estrogen receptor β: An overview and update. Nucl Recept Signal 6: 003, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Zheng HY, Li Y, Dai W, Wei CD, Sun KS, Tong YQ. Imbalance of testosterone/estradiol promotes male CHD development. Biomed Mater Eng 22: 179–185, 2012. [DOI] [PubMed] [Google Scholar]
- 490.Zubieta-Castillo G, Zubieta-Calleja G, Arano E, Zubieta-Calleja L. Respiratory disease, chronic mountain sickness and gender differences at high altitude In: Ohno H, Kobayashi T, Ma-suyama S, Nakashima M, editors. Progress in Mountain Medicine and High Altitude Physiology. Matsumoto, Japan: Press Committee of the 3rd World Congress on Mountain Medicine and High Altitude Physiology, 1998, p. 132–137. [Google Scholar]
- 491.Zwadlo C, Schmidtmann E, Szaroszyk M, Kattih B, Froese N, Hinz H, Schmitto Jd, Widder J, Batkai S, Bahre H, Kaever V, Thum T, Bauersachs J, Heineke J. Antiandrogenic therapy with finasteride attenuates cardiac hypertrophy and left ventricular dysfunction. Circulation 131: 1071–1081, 2015. [DOI] [PubMed] [Google Scholar]