Abstract
Background
Medications endorsed by clinical practice guidelines, such as cardiovascular medications, could still have risks that outweigh potential benefits, and could thus warrant deprescribing.
Objectives
The objective of this study was to develop a framework of facilitators and barriers specific to deprescribing cardiovascular medications in the setting of uncertain benefit. Given the frequent use of beta-blockers in heart failure with preserved ejection fraction and its uncertain benefits with potential for harm, we used this scenario as an example case for a cardiovascular medication that may be reasonable to deprescribe.
Methods
We conducted one-on-one, semi-structured interviews of older adults until we reached thematic saturation. Two coders independently reviewed each interview, and developed codes using deductive thematic analysis based on a prior conceptual framework for deprescribing. Subthemes and themes were finalized with a third coder.
Results
We interviewed 10 participants. We identified three key previously-described patient-reported facilitators to deprescribing: 1) Appropriateness of cessation, 2) Process of cessation, and 3) Dislike of medications; and identified three key previously-described patient-reported barriers: 1) Appropriateness of cessation, 2) Process of cessation, and 3) Fear. We found that these facilitators and barriers often co-occurred within the same individual. This observation coupled with subthemes from our patient interviews yielded two barriers to deprescribing specific to cardiovascular medications—uncertainty and conflicting attitudes.
Conclusion
We adapted a new framework of patient-reported barriers and facilitators specific to deprescribing cardiovascular medications. In addition to addressing barriers previously described, future deprescribing interventions targeting cardiovascular medications must also address uncertainty and conflicting attitudes.
Keywords: deprescribing, polypharmacy, heart failure
1. INTRODUCTION
The prevalence of polypharmacy, broadly defined as the use of more medications than are medically necessary or beneficial[1], has risen substantially over the past 2 decades[2]. This largely relates to an aging population that frequently contends with multiple chronic conditions[3, 4], for which multiple pharmacologic agents may be prescribed. While a high number of medications may reflect optimal therapy for several unique chronic medical conditions, high medication burden is associated with the risk of several adverse outcomes including falls[5–8] disability[9–11], and hospitalizations[12–15]. Consequently, developing tools and strategies to address polypharmacy has become a major priority for patient safety[16, 17].
Deprescribing has emerged as a key strategy to address polypharmacy. The systematic review of definitions of deprescribing defines deprescribing as the process of discontinuing drugs when existing or potential harms outweigh existing or potential benefits in the context of an individual’s care goals, level of functioning, life expectancy, values, and preferences, under the supervision of a health care professional[18]. Despite its role as an integral part of patient-centric and goal-concordant prescribing practice[19], deprescribing is seldom incorporated into usual clinical practice.
While there is an expanding evidence base for deprescribing across several different medication classes based on explicit criteria as well as implicit criteria via tools like the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP),[20] the majority of studies on deprescribing to date have focused on potentially inappropriate medications (PIMs) [21]—a group of medications (such as benzodiazepines and antipsychotics) that expert panels recommend should be avoided due to risks that outweigh potential benefits in most older adults[22, 23]. While deprescribing PIMs can reduce medication burden, this strategy has not consistently improved clinical outcomes[24, 25]. The limited impact of targeting only PIMs could relate to the fact that the majority of medications taken by older adults with multiple chronic conditions are not PIMs.[26] Importantly, medications with potential benefits, even those that are endorsed by clinical practice guidelines, could still have risks that outweigh potential benefits[19, 27]. This is especially relevant for cardiovascular medications, which can provide benefits in the setting of primary or secondary prevention, but are also responsible for a significant proportion of adverse drug events.[28] The risk-benefit ratio of cardiovascular medications can also change over time; [29] indeed, increased risks for harm[30, 31] and decreased potential for benefit[32, 33] are often observed with advancing age in the setting of declines in function, cognition, and overall life expectancy. Consequently, there are clinical circumstances where a cardiovascular medication may not be appropriate, and should thus be targeted for deprescribing.[34] A recent national survey of physicians reported that one of the barriers to deprescribing cardiovascular medications is the perception that patients are not amenable to deprescribing their cardiovascular medications.[35] Although patient-reported barriers and facilitators to deprescribing have previously been described in several prior studies, the majority of these studies have focused on PIMs. [36] Because barriers and facilitators to deprescribing cardiovascular medications might differ, we sought to develop a framework of patient-reported barriers and facilitators specific to deprescribing cardiovascular medications by conducting a qualitative study of older adults with multiple chronic conditions and polypharmacy. To conduct this study, we opted to specifically focus on beta-blockers in heart failure with preserved ejection fraction (HFpEF) due to the frequent use of beta-blockers in this condition [37] and their inclusion on the clinical practice guidelines [38] despite substantial uncertainty regarding their benefits[39–41] and their potential to cause harm.[42–46, 13].
2. METHODS
2.1. Study sample and setting
We conducted one-on-one, semi-structured interviews of consecutive patients (age ≥55 years) hospitalized for HF who were taking beta-blockers for HFpEF. Examining patients with HFpEF is well-suited for a study about deprescribing because multimorbidity (the condition of having multiple chronic conditions)[47] and polypharmacy are essentially universal in this condition[44, 48]. Examining beta-blockers is well-suited because, despite their frequent use (up to 80% of patients with HFpEF take beta-blockers[37]) and inclusion in the clinical practice guidelines for HFpEF [38], there is substantial uncertainty regarding their potential benefits in HFpEF. Studies of beta-blockers in HFpEF have been neutral with regard to mortality, hospitalization rates, quality of life, and echocardiographic parameters[39–41]; and there is a rationale that beta-blockers can cause harm in HFpEF by potentially worsening pathophysiological features of HFpEF,[42, 43, 45], worsening function[46], and increasing the risk for adverse drug reactions[13]. Since beta-blocker use in HFpEF may be a reasonable target for deprescribing, we used this common clinical circumstance as an archetype to examine barriers and facilitators to deprescribing cardiovascular medications.
We recruited hospitalized patients from a quaternary academic center in New York City. We identified potentially eligible participants based on chart review of HFpEF patients admitted to the hospital between July 2018 and August 2018, regardless of gender. Eligibility criteria included age ≥55 years, diagnosis of HFpEF, current hospitalization for heart failure, and outpatient prescription of beta-blocker prior to hospitalization. We excluded individuals who had other indications for beta-blockers such as arrhythmias and/or a history of myocardial infarction based on chart review.[44] We also excluded individuals with conditions that would preclude their participation in a qualitative interview, such as severe cognitive impairment, severe psychiatric disorder, and delirium. Among the 16 patients who were eligible to participate, 6 declined to participate and 10 provided in-person written consent and participated in the study. We conducted interviews until we reached thematic saturation (the point at which no additional themes emerged)[49]. After reaching thematic saturation, we interviewed two additional participants to increase the robustness of our findings. This study adhered to the Consolidated Criteria for Reporting Qualitative research (COREQ)[50] (Online Resource 1).
This study was approved by the Weill Cornell Medicine Institutional Review Board, and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all study participants.
2.2. Prior framework of patient-reported barriers and facilitators to deprescribing
Reeve et al[36] previously developed a framework of patient-reported barriers and facilitators to deprescribing based on a systematic review. The authors identified 21 eligible qualitative, quantitative, and/or mixed methods studies describing patient-reported barriers and facilitators to the withdrawal of medications. The authors subsequently conducted content analysis, iteratively developing codes and themes to produce a framework. Notably, the studies included in this systematic review predominantly examined barriers and facilitators to deprescribing PIMs like benzodiazepines, anti-psychotics, and proton pump inhibitors. Key subthemes from this framework included appropriateness of cessation, process of cessation, external influences, dislike of medications, and fear (Online Resource 2). We used this prior framework for our interview guide and our analysis, building on key themes to ultimately develop a modified framework that specifically reflected patient-reported barriers and facilitators to deprescribing cardiovascular medications.
2.3. Data collection and analysis
Based on prior literature about deprescribing and the authors’ own clinical experience with caring for older adults with heart failure, we developed an interview guide of open-ended questions encouraging participants to explain their perspectives on deprescribing (see Online Resource 3 for the full interview guide). Since there is variation in the definition of deprescribing, we chose to define deprescribing according to the systematic review of definitions of deprescribing, which defines it as the process of discontinuing drugs when existing or potential harms outweigh existing or potential benefits in the context of an individual’s care goals, level of functioning, life expectancy, values, and preferences, under the supervision of a health care professional[18]. This definition does not include dose reduction, and was therefore was not a focus of this project. The interview guide included five key questions: first, we inquired about participants’ feelings toward cardiovascular medication discontinuation, scenarios in which participants would be comfortable with cardiovascular medication discontinuation, and scenarios in which participants would be uncomfortable with cardiovascular medication discontinuation; then we inquired about participant-reported priorities and considerations for discussing beta-blocker discontinuation, and participant feelings toward beta-blocker discontinuation. We also included several probes to elicit additional details, when relevant. To provide context for the interview questions, we explained to participants that there is sometimes uncertainty regarding the risks and benefits of cardiovascular medications in selected circumstances; and emphasized the limitations in the evidence supporting the use of beta-blockers in HFpEF. Guided by the interview guide, one author (TR) conducted all in-person semi-structured interviews during each participant’s hospitalization. Each interview lasted approximately 20–40 minutes. Interviews were audio-recorded, professionally transcribed, and de-identified. Transcripts were carefully reviewed by the interviewer (TR) to ensure accuracy.
Two coders (TR and BS) independently reviewed each interview, engaging in an iterative process to develop codes using deductive thematic analysis[51] based on Reeve’s previously-published framework of patient-reported barriers and facilitators to deprescribing[36]. The two coders independently reviewed and coded the first three transcripts, and then met to develop a preliminary codebook with a third study team member (PG). The coders then independently reviewed another five transcripts, and met again with the third study team member to refine the codebook,which included adding and removing some codes. Finally, the coders independently reviewed the last two transcripts, and met with the third study team member one final time; this final meeting did not yield any major changes to the codebook. Subthemes and themes were finalized at this time, and were subsequently reviewed and corroborated by a fourth (MJS) and fifth study member (RMC). The final codebook included 85 codes, coalescing into 25 subthemes and 8 themes (3 facilitators and 5 barriers), which were adapted based on the pre-existing Reeve framework. Throughout the iterative process of coding, disagreements were resolved through discussion and ultimately reconciled.
3. RESULTS
We completed interviews for 10 participants (7 women and 3 men). We reached thematic saturation (consensus among 3 reviewers—TR, BS, PG), defined as the point at which no additional themes emerged[49], after eight interviews; and subsequently interviewed two additional participants to increase the robustness of our findings. Participants had a median age of 80 years (Table 1), and took a median of 12 medications. We classified major themes into patient-reported facilitators and barriers to deprescribing cardiovascular medications. Table 2 provides a summary of these themes and subthemes.
Table 1:
Patient Characteristics
| Participant number | Age (years) | Sex | Comorbid Conditions | Prescription Medication Count |
|---|---|---|---|---|
| 1 | 88 | Female | Hypertension, Hypothyroidism, Atrial fibrillation, GERD, Breast Cancer, Lymphoma, Anxiety/Depression Chronic back pain | 13 |
| 2 | 86 | Female | Hypertension, Hyperlipidemia, Atrial fibrillation, GERD | 10 |
| 3 | 82 | Female | Coronary artery disease, Atrial fibrillation, Hypertension, Hyperlipidemia, Hypothyroidism, Cataracts, Gout, MGUS | 7 |
| 4 | 75 | Male | Diabetes mellitus, Hypertension, Peripheral vascular disease, Glaucoma, Chronic kidney disease, Osteoarthritis, Hyperlipidemia, Coronary artery disease, Atrial fibrillation | 14 |
| 5 | 80 | Male | Atrial fibrillation, COPD, Hypertension, Coronary artery disease, BPH, GERD, Chronic back pain | 12 |
| 6 | 56 | Female | Diabetes mellitus, Chronic kidney disease, Cerebrovascular disease, Hypertension, Hyperlipidemia, Obstructive sleep apnea | 11 |
| 7 | 86 | Female | Diabetes, Hyperlipidemia, Hypertension, Spinal stenosis, Cerebrovascular disease, Breast cancer | 12 |
| 8 | 73 | Female | Multiple myeloma, End-stage renal disease, Coronary artery disease, Atrial fibrillation, Diabetes, Hyperlipidemia, Hypertension | 18 |
| 9 | 67 | Female | Hypertension, End-stage renal disease, HIV, Hepatitis C, Coronary artery disease, Chronic back pain | 15 |
| 10 | 81 | Male | Atrial fibrillation, Obstructive sleep apnea, COPD, Chronic kidney disease, Hypertension, Hyperlipidemia, Diabetes Mellitus | 11 |
Abbreviations: GERD=Gastroesophageal reflux disease; MGUS=Monoclonal gammopathy of undetermined significance; COPD=Chronic obstructive pulmonary disease; BPH=benign prostatic hyperplasia
Table 2.
Themes and Subthemes
| Theme | Subtheme |
|---|---|
| Facilitators | |
| 1. Appropriateness of Cessation | Medication Ineffectiveness |
| Adverse Drug Effects | |
| Therapeutic Competition | |
| Positive Experience | |
| 2. Process of Cessation | Temporary Trial |
| Possibility of Medication Resumption | |
| Rigorous Monitoring | |
| Open Communication | |
| Physician Consensus | |
| 3. Dislike of Medications | Inconvenience |
| High Medication Burden | |
| High Cost | |
| Barriers | |
| 1. Appropriateness of Cessation | Medications Needed to Survive |
| Evidence of Benefit | |
| Maintenance of Status Quo | |
| 2. Process of Cessation | Insufficient Monitoring |
| Prescribing/Deprescribing Discordance | |
| Physician Distrust | |
| 3. Fear | Fear of Health Deterioration |
| Fear of Symptom Recurrence | |
| Fear of Shortened Lifespan | |
| Newly Emergent Themes (New Barriers) | |
| 1. Uncertainty | Uncertain Risks and Benefits |
| Uncertain Personal Derivation of Benefit | |
| 2. Conflicting Attitudes | Simultaneous Concerns |
| Simultaneous Feelings | |
3.1. Facilitators
We identified three key patient-reported facilitators to deprescribing, all of which were present in Reeve’s framework: 1) Appropriateness of cessation, 2) Process of cessation, and 3) Dislike of medications. We describe each facilitator, and provide illustrative quotations below.
Facilitator #1: Appropriateness of cessation
Appropriateness of cessation as a facilitator refers to patients’ acceptance of medication discontinuation based on the perspective that their medications may be unnecessary and/or harmful. Key subthemes included: Medication Ineffectiveness, Adverse Drug Effects, Therapeutic Competition, and Positive Experience with discontinuation.
Medication Ineffectiveness
When patients did not feel that a medication was achieving its intended goal, they were more open to deprescribing: “Carvedilol is supposed to help your irregular heart rhythm, but I always felt that it never did that for me… I feel as though it really doesn’t help my heart rhythms.” (Patient 2)
Adverse Drug Effects
When patients were concerned about adverse effects from their medications, they were more amenable to deprescribing: “Sometimes the medication do you more harm than good. If you just… associate the symptoms you have with what you read in the [drug information sheet], you know it’s time to stop.” (Patient 7)
Therapeutic Competition
When patients felt that a medication was helping one condition but negatively affecting another condition, a scenario called therapeutic competition[52], patients were more interested in deprescribing: “I’m taking [medication] A to help [condition] B but [treating] B is hurting [condition] C. Like A is good for B but you get constipation. If I had a choice, I would… definitely get off them.” (Patient 5)
Positive Experience
If patients felt well after a prior experience with medication discontinuation, they were more open to deprescribing: “I would go with the way I feel. If, without the medication, I feel better, I’m not taking the medication.” (Patient 7)
Facilitator #2: Process of cessation
Process of cessation as a facilitator refers to patients’ acceptance of medication discontinuation based on the presence of a pragmatic discontinuation strategy that ensures safety. Key subthemes included: Temporary Trial of discontinuation, the Possibility of Medication Resumption if harm from discontinuation occurred, Rigorous Monitoring for risks and potential benefits of discontinuation, Open Communication about the risks and potential benefits with a physician, and Physician Consensus.
Temporary Trial
Patients were open to deprescribing in the setting of a temporary trial. “[If my doctor wanted to temporarily stop the medication,] I would be open to that, yes, yeah. I’d definitely want to know if I can do without it. I would just say, ‘Let me try [stopping] it… at least for a while.’” (Patient 7)
Possibility of Medication Resumption
If there remained a possibility to resume the medication at a future time, patients were more willing to consider deprescribing: “Suppose in the long run I don’t feel better and I start feeling worse. I feel that I should be able to say like, ‘Now I think I should be able to get back on the medicine.’” (Patient 6)
Rigorous Monitoring
If there was a rigorous process for monitoring and quantifying how patients were feeling with and without the medication, patients would be more open to try deprescribing: “It would be something that I would have to pay attention to. Is [discontinuation] making a difference now, or is it making me feel better or worse? I would have to like pay attention to it and see without the medicine how I’m feeling.” (Patient 6)
Open Communication
Patients cited open communication between patients and physicians regarding the risks and benefits of various medications as an important facilitator to deprescribing. “I want to know exactly what is metoprolol doing and how it’s doing it. How is it working in my body? Is it harming me? If I feel that it’s helping me but the doctor tells me it’s not, then I want him to explain to me why is it not helping me, make it clear… Put it [on] the table. I would really try to… analyze it myself and maybe compromise.” (Patient 7)
Physician Consensus
Patients were more amenable to deprescribing if their physicians were in agreement about discontinuing a medication: “It doesn’t matter which of them said stop it as long as they both agree… Get together and decide about the right medication. I believe it should be a unity.” (Patient 7)
Facilitator #3: Dislike of medications
Many patients dislike taking medications and this impacts their willingness to take them. Key subthemes included: Inconvenience, High Medication Burden, and High Cost.
Inconvenience
Patients reported interest in deprescribing if their medications created inconvenience: “[Furosemide makes] you have to go to the bathroom a lot… it interrupts your sleep a lot at night… [discontinuing medication] would be helpful, especially when you go out or somewhere… it will cut down on my bathroom usage.” (Patient 4)
High Medication Burden
Patients were amenable to deprescribing if their medication burden was high, which can negatively affect quality of life. [53–55] “The less…[that] I do, the happier I am. I want lesser [medications].” (Patient 5)
High Cost
Patients expressed interest in deprescribing if it would decrease the cost of their medications: “If [stopping a medication] brought the cost down, I’d be very agreeable to that.” (Patient 2)
3.2. Barriers
We identified three key patient-reported barriers to deprescribing, all of which were present in Reeve’s framework: 1) Appropriateness of cessation, 2) Process of cessation, and 3) Fear. We describe each barrier, and provide illustrative quotations below.
Barrier #1: Appropriateness of cessation
Appropriateness of cessation as a barrier refers to patients finding medication discontinuation undesirable based on their perspective that medications are necessary and beneficial. Key subthemes included: Medications Needed to Survive, Evidence of Benefit, and Maintenance of Status Quo.
Medications Needed to Survive
Patients were less amenable to deprescribing if they perceived that a medication was closely linked to their survival: “I have to take the medicine… so, I could live longer.” (Patient 8)
Evidence of Benefit
Patients were less interested in deprescribing if there was evidence of benefit from the medication: “Metoprolol is very helpful… it brings down my blood pressure, and if my heart’s beating fast–which sometimes it does–I take the medication and in about ten minutes, I can feel it slowing until it’s normal… I’m okay with [my medications].” (Patient 7)
Maintenance of Status Quo
Some patients reported a desire to maintain the status quo, and were therefore uninterested in deprescribing. “I been feeling uncomfortable [about stopping a medication] because… I’ve been doing fine with them.” (Patient 10)
Barrier #2: Process of cessation
Process of cessation as a barrier refers to patients’ reluctance for medication discontinuation based on a suboptimal process of discontinuation. Key subthemes included: Insufficient Monitoring following discontinuation, Prescribing/Deprescribing Discordance, and Physician Distrust.
Insufficient Monitoring
Patients were less amenable to deprescribing if there was not close monitoring following medication discontinuation: “I wouldn’t want someone to say ‘stop this’ and you know, ‘see me in six months. That’s your next visit. (Patient 2)
Prescribing/Deprescribing Discordance
Patients were less interested in deprescribing if a physician wanted to discontinue a medication that a different physician had prescribed: “If the heart doctor gave me the medicine, I will question why the primary doctor wants to stop it.” (Patient 6)
Physician Distrust
Patients were not willing to consider deprescribing if they did not trust their physician: “It depends a lot on trusting the doctor. And I think we’re trusting a little too much. Now my question that’s always in my head, does the doctor really know what they’re [doing]?” (Patient 7)
Barrier #3: Fear
Fear refers to patient concerns about the negative effects of discontinuing medications. Key subthemes included: Fear of Health Deterioration, Fear of Symptom Recurrence, and Fear of Shortened Lifespan.
Fear of Health Deterioration
Patients were not interested in deprescribing when they feared health deterioration following medication discontinuation: “I will not feel comfortable [with stopping medication] because I keep on thinking what will happen to me and I’m taking the risk because… you don’t know when [your health] goes,” (Patient 8)
Fear of Symptom Recurrence
Patients were less amenable to deprescribing if they feared symptom recurrence following medication discontinuation: “It’s the fear. It’s the fear. I wouldn’t be totally comfortable [with stopping medication]... that fear would be constantly in my mind, of me accumulating these fluids around my heart, and the other areas of my body… I’m scared that if I stop taking it, I just could go into heart failure.” (Patient 2)
Fear of Shortened Lifespan
Patients were not interested in deprescribing if they feared a shorter lifespan as a result of medication discontinuation: “If [stopping a medication] will shorten my span of life, no, I will not follow... because I want to live longer.” (Patient 8)
3.3. Newly Emergent Themes
Consistent with Reeve’s prior conceptual model, interviews revealed that appropriateness of cessation and process of cessation can serve as either facilitators or barriers to deprescribing. We also found that facilitators and barriers often co-occurred within the same individual. These observations coupled with subthemes from our patient interviews yielded the emergence of two barriers to deprescribing that were not included in Reeve’s prior conceptual framework of patient-reported barriers and facilitators to deprescribing—uncertainty and conflicting attitudes. We provide illustrative quotations below.
New Theme #1: Uncertainty
Uncertainty refers to patients being unsure about the value of deprescribing. Key subthemes included: Uncertain Risks and Benefits and Uncertain Personal Derivation of Benefit.
Uncertain Risks and Benefit
Patients endorsed uncertainty about the risks and potential benefits of their medications, and thus uncertainty about the value of deprescribing: “Sometimes, it’s a decision that is very hard for me to make. Do I take it, and feel worse? Or should I be…not taking it, and making my condition worse?” (Patient 2)
Uncertain Personal Derivation of Benefit
Patients endorsed uncertainty about whether they would personally derive the intended benefits of their medications, and relatedly, were uncertain about the value of deprescribing: “There’s always a question about… the medications I’m taking, and if they’re doing what they are supposed to do.” (Patient 2)
New Theme #2: Conflicting attitudes
Conflicting attitudes refers to patients’ inconsistent feelings toward deprescribing as a result of simultaneously holding seemingly contradictory attitudes about their medications. Key subthemes: included: Simultaneous Concerns about taking and not taking their medications, and Simultaneous Feelings of aversion and obligation to taking medications.
Simultaneous Concerns
Patients harbored simultaneous concerns about taking and not taking their medications: “Sometimes, because I do think [medications] accelerate my heartbeat, I’m afraid to take them… [but] I’m afraid not to take them because of the effect it might have on my heart if I don’t.” (Patient 2)
Simultaneous Feelings
Patients simultaneously felt aversion toward their medications, as well as an obligation to take their medications: I don’t want [medications]... but I have to take it.” (Patient 5)
3.4. New Framework of Patient-based Barriers and Facilitators to Deprescribing
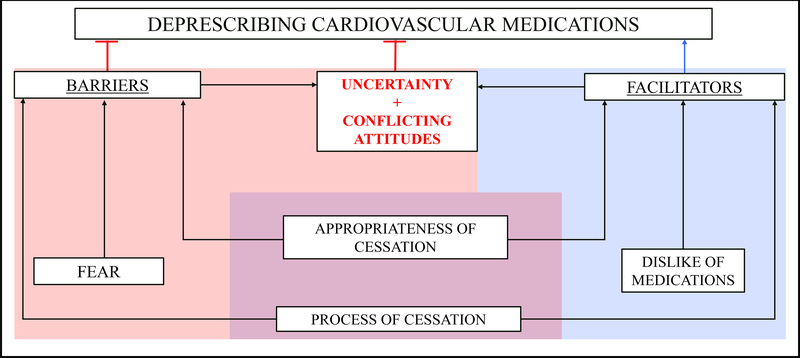
Based on our findings in this qualitative interview study, which build upon Reeve’s prior framework for deprescribing[36], we present a new framework of patient-reported barriers and facilitators to deprescribing cardiovascular medications in Figure 1. Similar to Reeve’s prior framework for deprescribing PIMs[36], appropriateness of cessation and process of cessation were both facilitators and barriers (purple); dislike of medications was an additional facilitator (blue) and fear was an additional barrier (red). Importantly, two new themes (barriers) emerged in this study—uncertainty and conflicting attitudes. As shown in Figure 1, these new themes appear to be strongly influenced by the other facilitators and barriers, and represent proximal influences on deprescribing cardiovascular medications.
Fig 1. Framework for Patient-reported Barriers and Facilitators to Deprescribing Cardiovascular Medications.
Major themes are shown in boxes. Arrows indicate the relationship based on prior work and quotes from this study. The blue shading indicates facilitators, the red indicates barriers, and the purple indicates themes that concurrently serve as facilitators and barriers. Similar to a prior framework for deprescribing, appropriateness of cessation and process of cessation were both facilitators and barriers (purple); dislike of medications was an additional facilitator and fear was an additional barrier. Importantly, two additional themes emerged in this study—uncertainty and conflicting attitudes. As shown, these additional themes appear to be strongly influenced by the other facilitators and barriers, and represent proximal influencers to deprescribing cardiovascular medications.
4. DISCUSSION
Deprescribing has been characterized as an important tool for potentially improving outcomes among adults with multiple chronic conditions and polypharmacy[18]. However, its potential impact may be limited by its primary focus on PIMs, which comprise under 10% of all medication prescriptions.[26] Cardiovascular medications are commonly prescribed to older adults, and contribute to a substantial proportion of adverse drug events in the United States.[28] Accordingly, there may be circumstances where the risks of some cardiovascular medications outweigh their potential benefits, and deprescribing may be warranted.[34] There have been a few randomized controlled trials of deprescribing cardiovascular medications (statins and anti-hypertensive agents), but this has yet to permeate into routine clinical care.[34] Physicians report that a major barrier to deprescribing cardiovascular medications is patient reluctance to deprescribing. However, patient-based barriers (and facilitators) to deprescribing cardiovascular medications have not been well-described. Therefore, to advance efforts toward deprescribing cardiovascular medications, we have generated formative work in this area by conducting qualitative interviews using deductive thematic analysis, and have subsequently developed the first framework, to our knowledge, of patient-reported barriers and facilitators specific to deprescribing cardiovascular medications.
Our study adds to the literature on barriers to deprescribing by uncovering the importance of two key barriers to deprescribing that are specific and particularly relevant to cardiovascular medications—uncertainty and conflicting attitudes. Our findings indicate that some patients are uncertain about the risks and benefits of the cardiovascular medications they take, and some are uncertain about whether they will personally derive benefit from their medications. This uncertainty may be appropriate in many cases—clinicians may not always engage in detailed discussions about the potential benefits and risks when making a recommendation[56]; benefits are frequently discussed, but risks are often underestimated and less commonly discussed[57]. Moreover, when clinicians do share the risks and benefits with patients, they typically use data derived from a selected randomized controlled trial population that reflects population-based average effects. Accordingly, the potential benefits and risks shared with patients may not accurately reflect the benefit and risk for any single individual in the real-world setting[58]. These issues are further complicated by prior observations that patients often struggle with cognitive overload and low self-efficacy when presented with quantitative and probabilistic information in the setting of uncertainty.[59, 60] Our study also found that patients reported conflicting simultaneous feelings of aversion and obligation toward their medications; patients may not want to take the medication, but take them anyway because they feel obligated to do so to ensure disease control and/or survival. The constructs of uncertainty and conflicting attitudes are further strengthened by our observation that both facilitators and barriers often co-occurred within the same individual. While uncertainty and conflicting attitudes have been described previously as important barriers to deprescribing [61–65], our study extends prior findings by highlighting their importance specifically for deprescribing cardiovascular medications, for which the risk-benefit ratios may not be as clear as the risk-benefit ratios for more traditional PIMs (where risks often, though not always, outweigh the benefits)[22].
While patient-physician communication and shared decision-making are especially important in the setting of uncertainty and conflicting attitudes, uncertainty and conflicting attitudes can also serve as barriers to patient-physician communication and shared decision-making. Uncertainty and conflicting attitudes cause heightened risk perceptions and negative affective responses (anxiety and emotional distress), which can lead to decision avoidance and/or impair decision-making[66–68]. Consequently, to expand deprescribing efforts to cardiovascular medications, our findings highlight the need to develop strategies that can combat uncertainty and conflicting attitudes. Decision aids have shown potential for reducing decisional conflict related to proton pump inhibitor use, but their uptake has been limited due to clinician time constraints and workflow.[69, 70] The subthemes identified in this study for “process of cessation” as a facilitator provide some insights on other potential strategies that could mitigate uncertainty and conflicting attitudes. Based on the process of cessation subthemes from this study, a strategy worth developing would be a temporary trial of discontinuation with a quantifiable assessment of the risks and benefits of discontinuation that can lead to a discussion of each patient’s individual risks and potential benefits, and facilitate a discussion and subsequent consensus between the patient and their healthcare team. Serial therapeutic trials (also known as N-of-1 trials) fit this description well, and have accordingly been mentioned as a potential strategy to assess the role of deprescribing among older adults with multiple chronic conditions[71, 72]. Whether such single-person interventions can be implemented to combat uncertainty and conflicting attitudes toward deprescribing cardiovascular medications, and subsequently enhance shared decision making and improve patient-physician communication in the context of deprescribing cardiovascular medications warrants additional investigation.
Our study has several strengths. First, we used a rigorous methodology for analyzing interviews that incorporated two independent coders where disagreements were resolved by consensus and a third coder. We also incorporated a fourth and a fifth coder to confirm the validity of our coding process. The small sample size might give some pause with regard to the generalizability of our findings. However, one of the goals of qualitative research is to develop explanatory models and theory that underlie observed phenomena. Small sample sizes should not necessarily be considered weaknesses of such studies; indeed data saturation, which was the target of this study, frequently occurs between 6 and 12 interviews[49]. In light of the homogeneous population, specific focus, and our primary goal of capturing themes rather than developing theory given the use of a pre-existing conceptual model, we are confident in our findings despite a relatively small sample size[73]. Another limitation was that it was a single site study conducted at a quaternary referral center; we recruited a diverse sample, but patients who seek care at a large academic medical center may not be representative of the average HFpEF patient. We also examined a specific clinical condition—beta-blockers in patients with HFpEF. Although these data provide important formative work on the patient-reported barriers and facilitators to deprescribing cardiovascular medications, future studies are warranted to determine whether our framework applies to other cardiovascular medications, and whether attitudes differ according to primary or secondary prevention. As a complement to this study of patients, future work should also examine physician, pharmacist, and nurse reported barriers and facilitators to deprescribing cardiovascular medications.
5. CONCLUSION
We have developed an adapted framework of patient-reported barriers and facilitators specific to deprescribing cardiovascular medications. Given their primacy as patient-reported barriers to deprescribing cardiovascular medications, uncertainty and conflicting attitudes must be addressed when developing future deprescribing interventions targeting cardiovascular medications.
Supplementary Material
KEY POINTS.
Our study adds to the literature on facilitators and barriers to deprescribing by uncovering two key barriers to deprescribing that are specific and particularly relevant to cardiovascular medications—uncertainty and conflicting attitudes.
In addition to addressing barriers previously described for deprescribing potentially-inappropriate medications, future deprescribing interventions targeting cardiovascular medications must also address uncertainty and conflicting attitudes.
Acknowledgments
Sources of Funding: National Institute on Aging grant R03AG056446 (PI: Goyal); National Cancer Institute grant K07CA207580 (PI: Shen); National Institute of Nursing Research grant R00NR016275 (PI: Masterson Creber). These institutions did not have a role in the design, methods, subject recruitment, data collections, analysis, or preparation of the manuscript.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: Dr. Safford reports research support from Amgen. The other authors report no conflicts.
Footnotes
Informed Consent:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Weill Cornell Medicine Institutional Review Board) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all study participants.
REFERENCES
- 1.Tjia J, Velten SJ, Parsons C, Valluri S, Briesacher BA. Studies to reduce unnecessary medication use in frail older adults: a systematic review. Drugs Aging. 2013;30(5):285–307. doi: 10.1007/s40266-013-0064-1. [DOI] [PubMed] [Google Scholar]
- 2.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA. 2015;314(17):1818–31. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Medicare & Medicaid Services. Chronic Conditions among Medicare Beneficiaries. Available from https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts.html Accessed September 28, 2017.
- 4.Gerteis J, Izrael D, Deitz D, LeRoy L, Ricciardi R, Miller T, Basu J. Multiple Chronic Conditions Chartbook AHRQ Publications No, Q14–0038. Rockville, MD: Agency for Healthcare Research and Quality; April 2014. [Google Scholar]
- 5.Freeland KN, Thompson AN, Zhao Y, Leal JE, Mauldin PD, Moran WP. Medication use and associated risk of falling in a geriatric outpatient population. Ann Pharmacother. 2012;46(9):1188–92. doi: 10.1345/aph.1Q689. [DOI] [PubMed] [Google Scholar]
- 6.Kojima T, Akishita M, Nakamura T, Nomura K, Ogawa S, Iijima K et al. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr Gerontol Int. 2012;12(3):425–30. doi: 10.1111/j.1447-0594.2011.00783.x. [DOI] [PubMed] [Google Scholar]
- 7.Tromp AM, Pluijm SM, Smit JH, Deeg DJ, Bouter LM, Lips P. Fall-risk screening test: a prospective study on predictors for falls in community-dwelling elderly. J Clin Epidemiol. 2001;54(8):837–44. [DOI] [PubMed] [Google Scholar]
- 8.Ziere G, Dieleman JP, Hofman A, Pols HA, van der Cammen TJ, Stricker BH. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2006;61(2):218–23. doi: 10.1111/j.1365-2125.2005.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magaziner J, Cadigan DA. Community resources and mental health of older women living alone. J Aging Health. 1989;1(1):35–49. [DOI] [PubMed] [Google Scholar]
- 10.Crentsil V, Ricks MO, Xue QL, Fried LP. A pharmacoepidemiologic study of community-dwelling, disabled older women: Factors associated with medication use. Am J Geriatr Pharmacother. 2010;8(3):215–24. doi: 10.1016/j.amjopharm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Jyrkka J, Enlund H, Lavikainen P, Sulkava R, Hartikainen S. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf. 2011;20(5):514–22. doi: 10.1002/pds.2116. [DOI] [PubMed] [Google Scholar]
- 12.Akazawa M, Imai H, Igarashi A, Tsutani K. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010;8(2):146–60. doi: 10.1016/j.amjopharm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc. 2012;60(1):34–41. doi: 10.1111/j.1532-5415.2011.03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. doi: 10.1186/s12913-015-0950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinman MA, Landefeld CS, Rosenthal GE, Berthenthal D, Sen S, Kaboli PJ. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54(10):1516–23. doi: 10.1111/j.1532-5415.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz JB, Schmader KE, Hanlon JT, Abernathy DR, Gray S, Dunbar-Jacob J, Holmes HM, Murray MD, Roberts R, Joyner M, Peterson J, Lindeman D, Tai-Seale M, Downey L, Rich MW. Pharmacotherapy in Older Adults with Cardiovascular Disease: Report from an ACC, AGS, NIA Workshop. J Am Geriatr Soc (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny M-P, Sheikh A. Medication Without Harm: WHO’s Third Global Patient Safety Challenge. Lancet (London, England). 2017;389(10080):1680–1. [DOI] [PubMed] [Google Scholar]
- 18.Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–34. doi: 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
- 19.Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–9. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83. [DOI] [PubMed] [Google Scholar]
- 21.Iyer S, Naganathan V, McLachlan AJ, Le Couteur DG. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021–31. doi: 10.2165/0002512-200825120-00004. [DOI] [PubMed] [Google Scholar]
- 22.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–46. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 23.Renom-Guiteras A, Meyer G, Thurmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71(7):861–75. doi: 10.1007/s00228-015-1860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper JA, Cadogan CA, Patterson SM, Kerse N, Bradley MC, Ryan C et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5(12):e009235. doi: 10.1136/bmjopen-2015-009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson T, Abuzahra ME, Keller S, Mann E, Faller B, Sommerauer C et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(2):532–48. doi: 10.1111/bcp.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidoff AJ, Miller GE, Sarpong EM, Yang E, Brandt N, Fick DM. Prevalence of potentially inappropriate medication use in older adults using the 2012 Beers criteria. J Am Geriatr Soc. 2015;63(3):486–500. doi: 10.1111/jgs.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes HM, Min LC, Yee M, Varadhan R, Basran J, Dale W et al. Rationalizing prescribing for older patients with multimorbidity: considering time to benefit. Drugs Aging. 2013;30(9):655–66. doi: 10.1007/s40266-013-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–12. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 29.Tinetti ME, Green AR, Ouellet J, Rich MW, Boyd C. Caring for Patients With Multiple Chronic Conditions. Ann Intern Med. 2019. doi: 10.7326/M18-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson EB, Kukull WA, Buchner D, Reifler BV. Adverse drug reactions associated with global cognitive impairment in elderly persons. Ann Intern Med. 1987;107(2):169–73. [DOI] [PubMed] [Google Scholar]
- 31.Cullinan S, O’Mahony D, O’Sullivan D, Byrne S. Use of a frailty index to identify potentially inappropriate prescribing and adverse drug reaction risks in older patients. Age Ageing. 2016;45(1):115–20. doi: 10.1093/ageing/afv166. [DOI] [PubMed] [Google Scholar]
- 32.Lavan AH, Gallagher P, Parsons C, O’Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46(4):600–7. doi: 10.1093/ageing/afx005. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz JB, Schmader KE, Hanlon JT, Abernethy DR, Gray S, Dunbar-Jacob J et al. Pharmacotherapy in Older Adults with Cardiovascular Disease: Report from an American College of Cardiology, American Geriatrics Society, and National Institute on Aging Workshop. J Am Geriatr Soc. 2019;67(2):371–80. doi: 10.1111/jgs.15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnaswami A, Steinman MA, Goyal P, Zullo AR, Anderson TS, Birtcher KK et al. Deprescribing in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. 2019;73(20):2584–95. doi: 10.1016/j.jacc.2019.03.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goyal P, Anderson TS, Bernacki GM, Marcum ZA, Orkaby AR, Kim D et al. Physician Perspectives on Deprescribing Cardiovascular Medications for Older Adults. J Am Geriatr Soc. 2019. doi: 10.1111/jgs.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793–807. doi: 10.1007/s40266-013-0106-8. [DOI] [PubMed] [Google Scholar]
- 37.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6(2):184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Colvin MM et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, Origasa H, Hori M, Investigators JD. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 2013;15(1):110–8. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 40.Edelmann F, Musial-Bright L, Gelbrich G, Trippel T, Radenovic S, Wachter R et al. Tolerability and Feasibility of Beta-Blocker Titration in HFpEF Versus HFrEF: Insights From the CIBIS-ELD Trial. JACC Heart Fail. 2016;4(2):140–9. doi: 10.1016/j.jchf.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39(1):26–35. doi: 10.1093/eurheartj/ehx564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 43.Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(1):29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 44.Yum B, Archambault A, Levitan EB, Dharamdasani T, Kneifati-Hayek J, Hanlon JT et al. Indications for beta-Blocker Prescriptions in Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc. 2019;67(7):1461–6. doi: 10.1111/jgs.15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer M, LeWinter MM. Heart Rate and Heart Failure With Preserved Ejection Fraction: Time to Slow beta-Blocker Use? Circ Heart Fail. 2019;12(8):e006213. doi: 10.1161/CIRCHEARTFAILURE.119.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinman MA, Zullo AR, Lee Y, Daiello LA, Boscardin WJ, Dore DD et al. Association of beta-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA Intern Med. 2017;177(2):254–62. doi: 10.1001/jamainternmed.2016.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition--multimorbidity. JAMA. 2012;307(23):2493–4. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL et al. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol. 2018;71(17):1921–36. doi: 10.1016/j.jacc.2018.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Method. 2006;18(1):59–82. doi:. [DOI] [Google Scholar]
- 50.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 51.Boyatzis R (1998). Transforming qualitative information: Thematic analysis and code development. Thousand Oaks, CA: Sage. . [Google Scholar]
- 52.Lorgunpai SJ, Grammas M, Lee DS, McAvay G, Charpentier P, Tinetti ME. Potential therapeutic competition in community-living older adults in the U.S.: use of medications that may adversely affect a coexisting condition. PLoS One. 2014;9(2):e89447. doi: 10.1371/journal.pone.0089447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 54.Eton DT, Ramalho de Oliveira D, Egginton JS, Ridgeway JL, Odell L, May CR et al. Building a measurement framework of burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Relat Outcome Meas. 2012;3:39–49. doi: 10.2147/PROM.S34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyd CM, Wolff JL, Giovannetti E, Reider L, Weiss C, Xue Q-l et al. Healthcare task difficulty among older adults with multimorbidity. Medical care. 2014;52 Suppl 3:S118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bogardus ST Jr., Holmboe E, Jekel JF. Perils, pitfalls, and possibilities in talking about medical risk. Jama. 1999;281(11):1037–41. [DOI] [PubMed] [Google Scholar]
- 57.Hoffmann TC, Del Mar C. Clinicians’ Expectations of the Benefits and Harms of Treatments, Screening, and Tests: A Systematic Review. JAMA Intern Med. 2017;177(3):407–19. doi: 10.1001/jamainternmed.2016.8254. [DOI] [PubMed] [Google Scholar]
- 58.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298(10):1209–12. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 59.Jansen J, Naganathan V, Carter SM, McLachlan AJ, Nickel B, Irwig L et al. Too much medicine in older people? Deprescribing through shared decision making. BMJ. 2016;353:i2893. doi: 10.1136/bmj.i2893. [DOI] [PubMed] [Google Scholar]
- 60.Belcher VN, Fried TR, Agostini JV, Tinetti ME. Views of older adults on patient participation in medication-related decision making. J Gen Intern Med. 2006;21(4):298–303. doi: 10.1111/j.1525-1497.2006.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner JP, Edwards S, Stanners M, Shakib S, Bell JS. What factors are important for deprescribing in Australian long-term care facilities? Perspectives of residents and health professionals. BMJ Open. 2016;6(3):e009781. doi: 10.1136/bmjopen-2015-009781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bagge M, Tordoff J, Norris P, Heydon S. Older people’s attitudes towards their regular medicines. J Prim Health Care. 2013;5(3):234–42. [PubMed] [Google Scholar]
- 63.Linsky A, Simon SR, Bokhour B. Patient perceptions of proactive medication discontinuation. Patient Educ Couns. 2015;98(2):220–5. doi: 10.1016/j.pec.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Galazzi A, Lusignani M, Chiarelli MT, Mannucci PM, Franchi C, Tettamanti M et al. Attitudes towards polypharmacy and medication withdrawal among older inpatients in Italy. Int J Clin Pharm. 2016;38(2):454–61. doi: 10.1007/s11096-016-0279-4. [DOI] [PubMed] [Google Scholar]
- 65.Reeve E, Low LF, Hilmer SN. Beliefs and attitudes of older adults and carers about deprescribing of medications: a qualitative focus group study. Br J Gen Pract. 2016;66(649):e552–60. doi: 10.3399/bjgp16X685669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Camerer C, Weber M. Recent Developments in Modeling Preferences - Uncertainty and Ambiguity. J Risk Uncertainty. 1992;5(4):325–70. doi:Doi 10.1007/Bf00122575. [DOI] [Google Scholar]
- 67.Johnson BB, Slovic P. Presenting uncertainty in health risk assessment: initial studies of its effects on risk perception and trust. Risk Anal. 1995;15(4):485–94. [DOI] [PubMed] [Google Scholar]
- 68.Schapira MM, Nattinger AB, McHorney CA. Frequency or probability? A qualitative study of risk communication formats used in health care. Medical decision making : an international journal of the Society for Medical Decision Making. 2001;21(6):459–67. [DOI] [PubMed] [Google Scholar]
- 69.Elwyn G, Scholl I, Tietbohl C, Mann M, Edwards AG, Clay C et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S14. doi: 10.1186/1472-6947-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson W, Farrell B, Welch V, Tugwell P, Way C, Richardson L et al. Continuation or deprescribing of proton pump inhibitors: A consult patient decision aid. Can Pharm J (Ott). 2019;152(1):18–22. doi: 10.1177/1715163518816719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouellet GM, Ouellet JA, Tinetti ME. Principle of rational prescribing and deprescribing in older adults with multiple chronic conditions. Ther Adv Drug Saf. 2018;9(11):639–52. doi: 10.1177/2042098618791371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clough AJ, Hilmer SN, Naismith SL, Kardell LD, Gnjidic D. N-of-1 trials for assessing the effects of deprescribing medications on short-term clinical outcomes in older adults: a systematic review. J Clin Epidemiol. 2018;93:112–9. doi: 10.1016/j.jclinepi.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 73.Hennink MM, Kaiser BN, Marconi VC. Code Saturation Versus Meaning Saturation: How Many Interviews Are Enough? Qual Health Res. 2017;27(4):591–608. doi: 10.1177/1049732316665344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.