Abstract
The study of male and female reproductive tract development requires expertise to two separate disciplines, developmental biology and endocrinology. For ease of experimentation and economy, the mouse has been used extensively as a model for human development and pathogenesis, and for the most part similarities in developmental processes and hormone action provide ample justification for the relevance of mouse models for human reproductive tract development. Indeed, there are many examples describing the phenotype of human genetic disorders that have a reasonably comparable phenotype in mice, attesting to the congruence between mouse and human development. However, anatomic, developmental and endocrinologic differences exist between mice and humans that (1) must be appreciated and (2) considered with caution when extrapolating information between all animal models and humans. It is critical that the investigator be aware of both the similarities and differences in organogenesis and hormone action within male and female reproductive tracts so as to focus on those features of mouse models with clear relevance to human development/pathology. This review, written by a team with extensive expertise in the anatomy, developmental biology and endocrinology of both mouse and human urogenital tracts, focusses upon the significant human/mouse differences, and when appropriate voices a cautionary note regarding extrapolation of mouse models for understanding development of human male and female reproductive tracts.
Keywords: Prostate, Mullerian duct, uterus, vagina, adenosis, penis, clitoris, prepuce, hypospadias, benign prostatic hyperplasia, alpha-fetoprotein
I. Introduction
Developmental biologists have employed a range of species (fish, amphibians, birds and mammals) to decipher that morphogenetic and molecular processes involved in vertebrate development with the underlying view that animal models may reveal human developmental mechanisms and provide an understanding of congenital birth defects. The literature is replete with numerous examples of the value of animal models and their relevance to human development. Embryonic, fetal and neonatal mice have been extensively used to study organogenesis and differentiation with the tacit assumption that morphogenetic and molecular mechanisms in mice are congruent with human development. This belief is fostered by many developmental features shared in common by mice and humans. Both species have, with few exceptions, the same spectrum of organs and numerous developmental processes that appear similar if not identical. Also, many genetic disorders in humans have direct counterparts in spontaneously mutant or genetically manipulated mice. For example, a fundamentally similar feminized phenotype is seen (a) in androgen-insensitive XTfm/Y mice, (b) in genetically engineered androgen- receptor-deficient mice, and (c) in humans with genetically defective androgen receptor (Ohno, 1979; Walters et al, 2010; Wilson, 1987; Yeh et al, 2002).
Androgens play a central role in masculine development in both rodents and humans as is the role/function of Müllerian inhibiting substance in determining the fate of the Müllerian ducts (Cunha et al, 2019b; MacLaughlin and Donahoe, 2002; Ren et al, 2017; Wilson et al, 1981). Tissue recombinant experiments illustrate the concurrence between rodent and human developmental mechanisms in so far as mouse (or rat) urogenital sinus mesenchyme (UGM, prostatic inductor) induces human urinary bladder epithelium to form prostate-like glands that produce human prostate specific antigen (Aboseif et al, 1999; Cunha et al, 1983). The implication of this experiment is that the signaling molecules constituting the dialogue of prostatic mesenchymal-epithelial interactions are sufficiently conserved that mouse and rat UGM can reprogram human epithelial morphogenesis and differentiation.
While the above findings favor the relevance of mouse studies for human biology, critical comparisons of mouse versus human development reveal morphogenetic and anatomical differences. One factor hampering such human/mouse comparisons is the limited literature on human organogenesis, particularly in regard to molecular mechanisms. Also, the technical sophistication of decades-old studies on human organogenesis rests in many cases on histologic examination and to a much limited extent on modern molecular techniques. Extrapolation of mouse models to human development requires expertise of the fine points of both mouse and human organogenesis. The sobering fact is that certain aspects of mouse and human reproductive tract development are fundamentally different and thus must be critically examined for relevance to human developmental biology. This review focuses on mouse/human developmental and anatomical differences and their implications vis a vie human biology/pathology.
II. Preputial Development
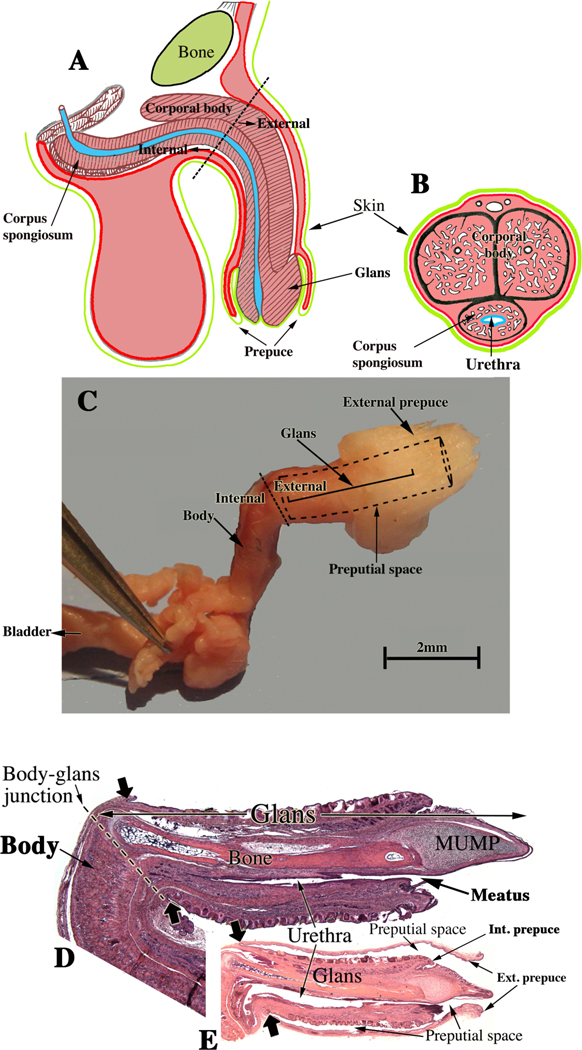
Adult anatomy represents the endpoint of highly orchestrated developmental processes, and accordingly adult anatomic differences between mice and humans are indicative of differences in morphogenetic and molecular mechanisms of development. Mice have two prepuces, while humans have only one prepuce (Fig. 1A–B) (Blaschko et al, 2013). Male mice have an external prepuce that constitutes the prominent perineal appendage (Fig. 1C) (Sinclair et al, 2016c) whose external surface is hair-bearing, while its internal surface defines the voluminous preputial space housing the mouse penis (Fig. 1A) (Blaschko et al, 2013; Rodriguez et al, 2011). Also, the mouse has an internal prepuce that is integral to the distal aspect of the mouse penis and thus is similar/homologous to the human prepuce (Blaschko et al, 2013) (Fig. 1A–B). Development of the internal prepuce of the mouse has not been adequately described.
Figure 1.
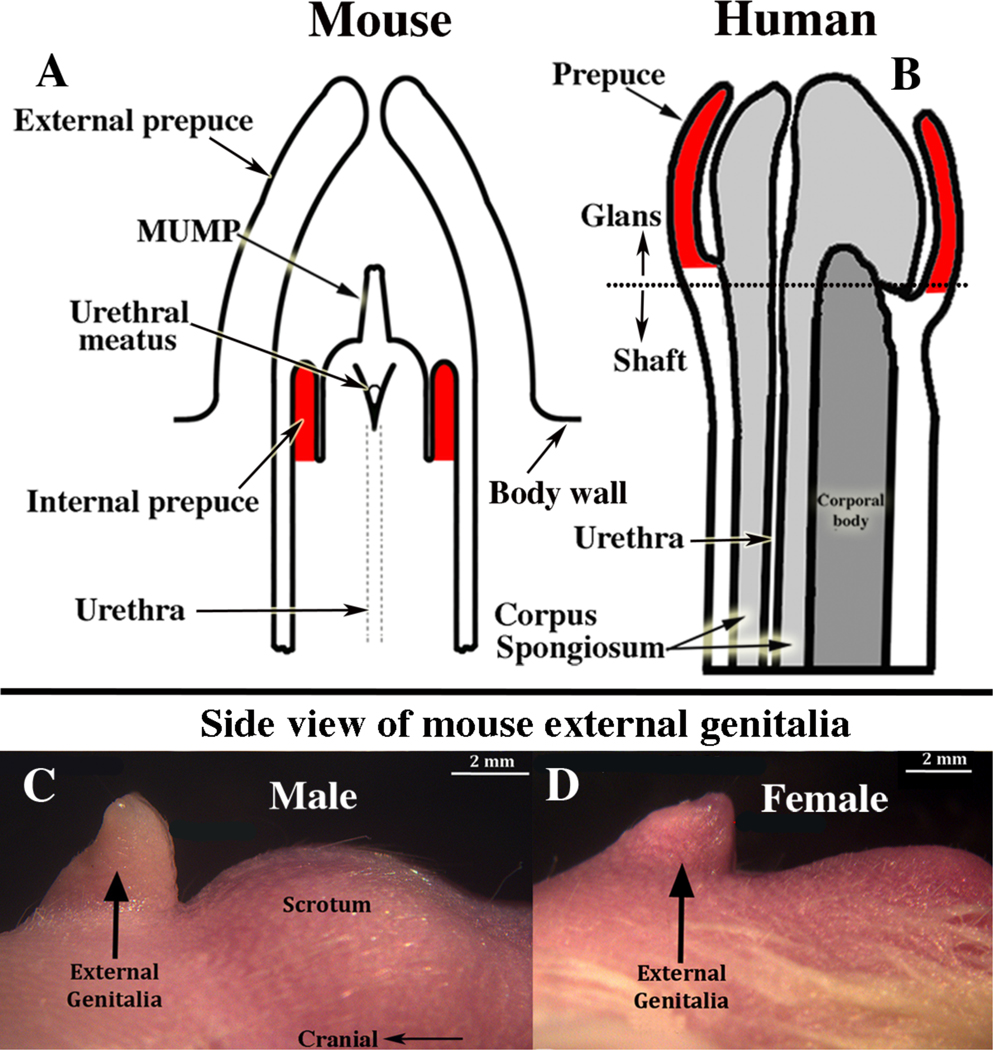
The mouse penile glans (A) lies within an extensive preputial space defined by the hair-bearing external prepuce, which forms the prominent elevation in the perineum (C). Comparison of (A & B) suggests homology between the human prepuce and the mouse “internal prepuce” (both red) in so far as both are integral to the distal aspect of the penis and encircle the glans. (C & D) Side views of male (C) and female (D) mouse perineal appendages (labeled external genitalia). The MUMP (male urogenital mating protuberance) is a fibrocartilaginous process that extends ~1mm beyond the urethral meatus in mice (A).
Morphogenesis of the external prepuce of the mouse occurs via a developmental mechanism entirely different from that of human preputial development (Cunha et al, 2019c; Liu et al, 2018a; Liu et al, 2018b). See companion paper, “Development of the human prepuce“ Cunha et al. 2019c. The mouse prepuce develops as a result of midline fusion of the preputial swellings, for which there is no human counterpart. Moreover, malformations of the mouse external prepuce elicited by developmental exposure to exogenous estrogens differ substantially from spontaneous preputial malformations in humans (Cunha et al, 2019c; Cunha et al, 2015; Mahawong et al, 2014a, b). Development of the mouse internal prepuce has only been described superficially (Schlomer et al, 2013; Sinclair et al, 2016a), but the data clearly establish that the mouse internal prepuce and the human prepuce differ greatly in anatomy and development even though both are integral to the glans penis (Cunha et al, 2019c; Liu et al, 2018a; Liu et al, 2018b).
III. Penile morphology and development
The penis develops from the embryonic genital tubercle in all mammalian species, even though penile morphology is vastly different in humans, mice and many other species (Simmons and Jones, 2007). The mouse penis has a substantial fibrocartilaginous projection extending beyond the urethral meatus (the male urogenital mating protuberance, MUMP) that is absent in humans (Figs. 1, 2D & E) (Rodriguez et al, 2011; Yang et al, 2010). Also, the mouse penis contains bone (os penis) and an associated zone of hyaline cartilage, again structures lacking in humans. Morphogenesis of these anatomic structures relies on developmental processes expressed in mouse and not in humans. Thus, use of the mouse penis to model human penile development requires caution so as to focus on those developmental features shared in common.
Figure 2.
Adult human and mouse penile anatomy. (A) Drawing of human penis in mid-sagittal view and (B) in transverse section. Note junction of the pendulous external portion and the internal portion of the human penis indicated by the dotted line in (A). (C) Photograph of a dissected adult mouse penis. The junction between the internal and external portions of the mouse penis occurs at the right-angle bend denoted by the dotted line and associated labels, “internal” and “external”. The forceps hold one of the bilateral crura. The internal portion contains the body of the mouse penis, while the external portion is called the glans. The glans in (C) cannot be seen because it lies within the preputial space (dotted lines); the position of the glans is indicated by . (D) Mid- sagittal hematoxylin–eosin stained section of the adult mouse penis with the external prepuce removed. (E) The mouse glans penis within the proximal portion of the external prepuce. Note reflection of epithelium of the external prepuce onto the surface of the penis indicated by the large solid arrows in both (D & E). (Adapted from Rodriguez et al. 2011 and Cunha et al. 2015 with permission).
. (D) Mid- sagittal hematoxylin–eosin stained section of the adult mouse penis with the external prepuce removed. (E) The mouse glans penis within the proximal portion of the external prepuce. Note reflection of epithelium of the external prepuce onto the surface of the penis indicated by the large solid arrows in both (D & E). (Adapted from Rodriguez et al. 2011 and Cunha et al. 2015 with permission).
Anatomical nomenclature of the mouse penis also differs substantially from human and must be understood to avoid confusion. For all species the penis consists of two parts: (a) an external portion projecting from the body wall and (b) an internal portion lying beneath the body surface contour. For humans, the internal portion consists of the proximal attachments of the corpora cavernosa (crura) to the inferior ischio-pubic rami (Clemente, 1985). In humans, the external (or pendulous) portion consists of the shaft or body of the penis containing the corporal body (fused corpora cavernosa) and the corpus spongiosum surrounding the urethra (Fig. 2). The distal aspect of the corpus spongiosum forms the glans and contains the urethral meatus (Clemente, 1985). For the mouse, the internal portion of the penis contains the proximal attachments of corpora cavernosa, and the corporal body (fused corpora cavernosa) and also resides internally. These internal elements of the mouse penis lie deep to the preputial space and constitute in part the so- called the body of the mouse penis. Thus, the term body of the penis refers in the human to the external pendulous portion, while in the mouse the body of the penis lies internally (Fig. 2). In mice, a right angle bend (Fig. 2C) corresponds to the junction between the internal element, body of the penis, and the external element, the glans, residing within the preputial space (Fig. 2C–E). The glans contains the os penis proximally, the fibrocartilaginous MUMP distally, as well as the 5 erectile bodies: (a) the corpus cavernosum glandis, (b) the bilateral MUMP corpora cavernosa, and (c) the bilateral corpora cavernosa urethrae (Sinclair et al, 2016b; Weiss et al, 2012). The body of the mouse penis contains the corporal body and thus the mouse has 6 erectile bodies in total. Humans have only two penile erectile bodies, namely the corporal body and the corpus spongiosum (Fig. 2A–B) (Clemente, 1985). The corpus spongiosum in humans entirely surrounds the urethra (Fig. 2B). Its counterpart in mice, the corpora cavernosa urethrae, does not surround the urethra, but instead lies ventral to it (Sinclair et al, 2016b). Little is known about development of the mouse penile erectile bodies, except that they form from mesenchymal cell condensates (Blaschko et al, 2013; Rodriguez et al, 2012). As is all too easily appreciated, differences in the terminology and anatomy of the mouse and human penis can be a source of confusion.
In mice the formation of penile erectile bodies is androgen-dependent. This interpretation rests upon the following observations: (a) Male mice develop 6 penile erectile bodies, while female mice are devoid of erectile bodies in their external genitalia (clitoris) (Weiss et al, 2012); (b) Androgen insensitive male XTfm/Y mice have completely feminized external genitalia, which includes a clitoris devoid of erectile bodies (Weiss et al, 2012); and (c) Mesenchymal condensates, the precursors of penile erectile bodies, are composed of androgen receptor-positive mesenchymal cells (Blaschko et al, 2013; Rodriguez et al, 2012). Clearly, formation of mouse penile erectile bodies depends upon androgen. This mechanistic scenario (androgenic induction of erectile body development) in mice does not apply to humans, in which corporal bodies are present in both the adult and fetal human penis and clitoris (Clemente, 1985; Cunha et al, 2019b). Moreover, corporal bodies form in both sexes as early as 8–9 weeks of development (and probably earlier) when testicular androgen production is either nil or just starting. Thus, embryonic formation of erectile bodies is surely androgen- independent in humans, whereas erectile body formation is androgen-dependent in mice (Cunha et al, 2019b).
Mouse and human penises develop from the genital tubercle, a projection in the embryonic perineum. In both species and both sexes the genital tubercle contains a solid urethral plate that extends to near the distal tip of the genital tubercle (Baskin et al, 2018; Hynes and Fraher, 2004a; Li et al, 2015; Overland et al, 2016; Seifert et al, 2008). Despite these features common to both human and mouse, considerable anatomical differences are seen in penises of these two species, thus profound developmental variances are expected. This is especially true for penile urethral development.
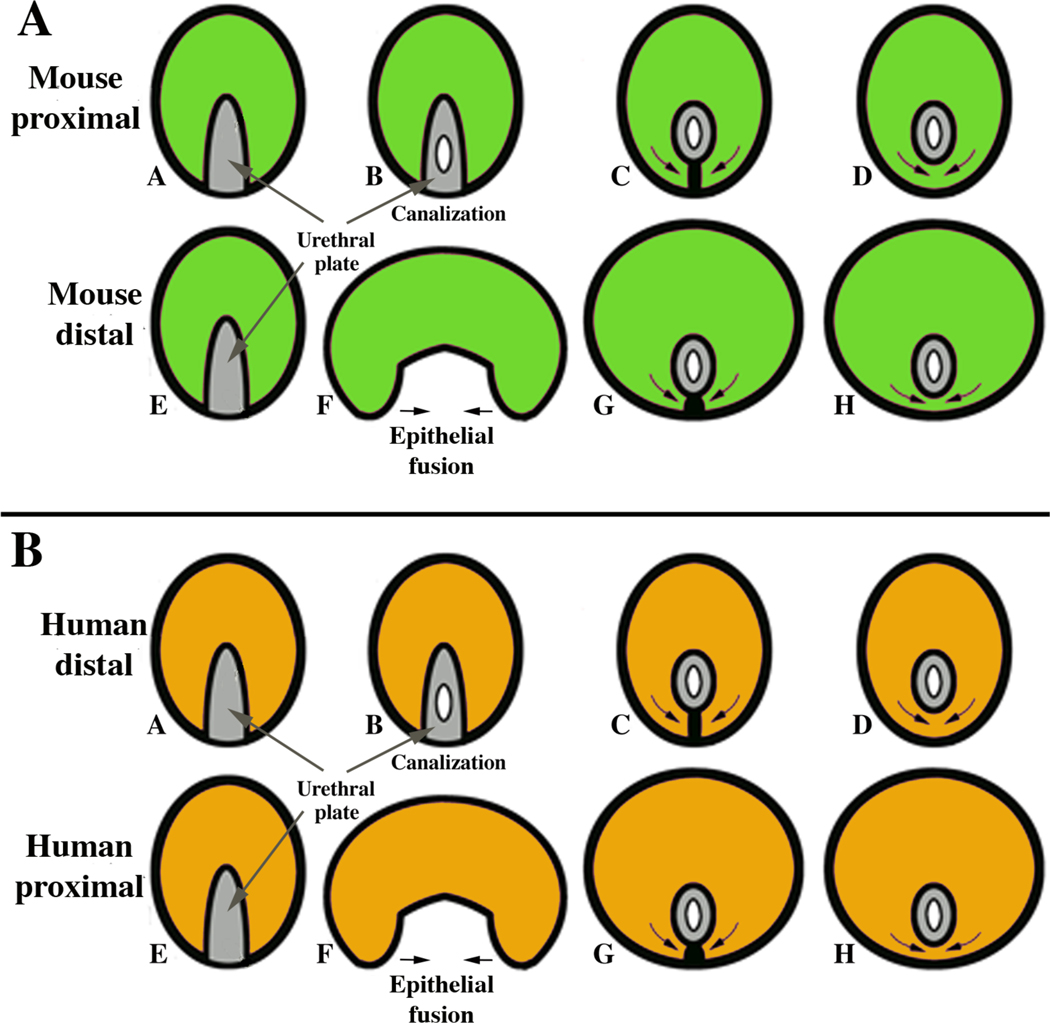
For both mouse and human, two entirely different morphogenetic mechanisms are in play for penile urethral development: (a) epithelial fusion events and (b) direct canalization of the solid urethral plate. In mice, the proximal portion of the penile urethra forms via direct canalization of the urethral plate (Fig. 3A) (Hynes and Fraher, 2004b; Seifert et al, 2008). In contrast, the distal portion of the mouse urethra, and especially the urethral meatus, forms via requisite epithelial fusion events (Liu et al, 2018a). Conversely, in humans (Fig. 3B) formation of the urethra within the glans occurs via direct canalization of the urethral plate, whereas proximally within the human penile shaft, the urethra forms via fusion of the urethral folds (Baskin et al, 2018; Li et al, 2015). Thus, both mechanisms of penile urethral formation occur in mice and humans, but the location of developmental mechanisms is exactly opposite in these species. Of note, hypospadias in humans occurs most frequently at precisely the junction of these two disparate morphogenetic mechanisms, namely at the glans/shaft interface (Baskin and Ebbers, 2006; Baskin et al, 1998; Cunha et al, 2015; Liu et al, 2018a; Liu et al, 2018b)}.
Figure 3.
Diagrams of divergent development of mouse (A) and human (B) urethral development. (A) The proximal portion of the mouse penile urethra (within the glans) develops via direct canalization of the urethral plate, while the distal aspect of the mouse penile urethra and the urethral meatus forms via epithelial fusion. (B) The distal portion of the human penile urethra (within the glans) develops via direct canalization of the urethral plate, while the proximal portion of the human penile urethra within the shaft forms via fusion of the urethral folds.
IV. Hypospadias:
Hypospadias is a common congenital malformation in humans. Indeed, it is the second most common congenital anomaly in boys, occurring in approximately 1:200–1:300 male births (Baskin, 2000). Hypospadias consists of the following spectrum of anomalies: (a) abnormal positioning of the urethral meatus, (b) absence of the ventral prepuce, (c) thinning or absent corpus spongiosum and (d) chordee (abnormal penile curvature) (Baskin et al, 1998). About 50% of patients with hypospadias have defects occurring at the glans–shaft junction or distally in the glans (Baskin and Ebbers, 2006). Spontaneous hypospadias has never been reported in wild-type mice. Moreover, the anatomic patterns of hypospadias in mice (and rats) experimentally induced by exogenous estrogenic differ substantially from that seen in humans both in position and morphology. This is not surprising given the substantial differences in morphology of the human versus the mouse penis. For example, one form of mouse hypospadias induced by exogenous estrogen is defined by whether the os penis is present in the same transverse section with the ventral cleft in the MUMP ridge (Mahawong et al, 2014b). While this may be a valid form of mouse hypospadias, it bears little resemblance to human hypospadias. Likewise, estrogen-induced malformation of the mouse penile urethral meatus (Mahawong et al, 2014a, b; Sinclair et al, 2016a; Sinclair et al, 2016b) is completely different from human hypospadias, which is illustrated in an accompanying paper (Cunha et al, 2019c). These differences led us to question the use of estrogen- induced mouse penile malformations as a model for human hypospadias (Cunha et al, 2019c; Cunha et al, 2015; Sinclair et al, 2016b). Flutamide-induced rat and mouse hypospadias appears to be a better model (Sinclair et al, 2017; Zheng et al, 2015).
V. Clitoral morphology and development:
Mouse and human clitori are entirely different anatomically, and the vast anatomic differences are accounted for by corresponding differences in developmental processes (Baskin et al, 2018; Cunha et al, 2019a; Overland et al, 2016). The human clitoris is a diminutive version of the human penis, except for the absence of the urethra (Clemente, 1985). Like the penis, the human clitoris has a shaft, glans and a corporal body. The human clitoris develops from the embryonic genital tubercle, and like the human penis projects externally from the body surface, albeit minimally (into the vaginal vestibule) (Clemente, 1985). Development of the human clitoris is remarkably similar to that of the human penis (Baskin et al, 2018; Li et al, 2015; Overland et al, 2016) with the exception that the urethra does not form within the human clitoris. An early event in development of both the penis and clitoris is the canalization of the urethral/vestibular plate to form a wide urethral/vestibular groove (Baskin et al, 2018; Li et al, 2015; Overland et al, 2016). In males the edges of the groove (urethral folds) fuse to form the penile urethra (Li et al, 2015). In contrast, in human females the edges of the vestibular groove (vestibular folds) remain separate as the labia minora that define the vaginal vestibule (Baskin et al, 2018; Overland et al, 2016).
Unlike the human clitoris, the mouse clitoris is entirely devoid of erectile bodies and is defined by an internally located U-shaped epithelial lamina (the clitoral lamina) (Martin-Alguacil et al, 2008a; Weiss et al, 2012), whose development is tangentially related to the genital tubercle (Cunha et al, 2019a). First and foremost, it must be recognized that the prominent appendage seen in the perineum of an adult female mouse in not the clitoris, but instead is female prepuce (Sinclair et al, 2016c) that forms from the preputial swellings that are not present in humans (Cunha et al, 2019a). The U-shaped clitoral lamina forms via fusion of the preputial swellings with the epidermis of the genital tubercle to form the circumferential preputial lamina, which remodels into the U- shaped clitoral lamina (Cunha et al, 2019a). See the accompanying paper on mouse clitoral development that emphasizes the many differences in mouse/human clitoral anatomy and development (Cunha et al, 2019a).
VI. Prostate:
Prostatic development in mice and men is similar in many aspects and substantially different in others. The principal common feature is that prostatic development is androgen-dependent in both species. Many common mouse/human features of prostatic development have been reported previously (Cunha et al, 1987; Cunha et al, 2018c; Keil and Vezina, 2015; Timms, 2008; Vezina and Bushman, 2007). Human/mouse prostatic differences include the following:
A. Prostatic anatomy:
The prostate of humans and rodents have similar anatomical and functional roles, however there are also significant differences that distinguish prostates of these species. Knowledge of the anatomy and histology and their ultimate roles in physiology are key to the interpretation of modeling the murine prostate for human benign prostatic hyperplasia.
The mouse prostate consists of four paired lobes: the anterior, ventral, dorsal, and lateral lobes. Due to the close proximity of the dorsal and lateral lobes, they are commonly referred to as the dorso-lateral lobe. Contrasting the lobes of the rodent, the human prostate is categorized by zones (e.g. central, transition and peripheral) and are encompassed together by a thick fibromuscular capsule. The corresponding prostatic lobes of the mouse for human central, transition and peripheral zones remain a matter of continued debate. The lobes of the rodent prostate are individually enclosed in thin delicate capsules and thus loosely surround the prostatic urethra. The rodent prostatic lobes are elongated, opaque, and tube-like in appearance and are described based on their anatomical location around the rhabdosphincter (i.e. dorsally, laterally, ventrally, and anteriorly) near the base of the bladder.
A further anatomical difference between the rodent and human is the location of the anterior prostate, also known as the coagulating gland, which in mice is immediately adjacent to the seminal vesicle and located cranially to the other prostate lobes. The intimate association of the mouse anterior prostate with the seminal vesicle does not have a human counterpart. The mouse prostatic lobes project into the abdominal cavity and the ducts convey prostatic fluids during ejaculation into the urethra (Nicholson et al, 2012).
Mouse and human prostates develop from solid epithelial buds emerging from endodermal urogenital sinus epithelium (UGE) (Cunha et al, 1987; Cunha et al, 2018c). Such buds emerge at specific anatomic points in both species to give rise to specific prostatic lobes in mice (Sugimura et al, 1986a; Sugimura et al, 1986b) and to a specific prostatic zonal anatomy in humans (Lowsley, 1912; McNeal, 1981, 1983). Epithelial buds emerge from the ventral UGS in both species. In mice (and rats) this leads to the formation of the ventral prostatic lobe (Hayashi et al, 1991; Sugimura et al, 1986a). In humans the ventral prostatic buds (Cunha et al, 2018c; Lowsley, 1912) regress during fetal life, and thus a “ventral lobe equivalent” is not to be found in the adult human.
B. Intra-prostatic ejaculatory ducts and prostatic utricle:
In humans, the seminal vesicle duct joins the vas deferens bilaterally to form the paired ejaculatory ducts that traverse the prostate to open into the prostatic urethra on the apex of the verumontanum lateral to the prostatic utricle (Cunha et al, 2018c). In mice, ejaculatory ducts, formed by fusion of seminal vesicle ducts with the vas deferens, have been described in some reports (Lin et al, 2003; Timms and Hofkamp, 2011), while in others, the vas deferens and bilateral seminal vesicle ducts open separately into the prostatic urethra (Cunha et al, 2018c; Green, 1966). Therefore, while some degree of homology exists in the verumontanum and its associated “ducts” between mouse and human, distinct anatomical differences exist and imply differences in developmental processes.
C. Prostatic squamous metaplasia and the complications of alpha-fetoprotein in the neonatal mouse:
The developing human prostate is susceptible to effects of the high levels of maternal estrogens in the third trimester (Boroditsky et al, 1978; Dawson et al, 1983). Prostatic squamous metaplasia is a striking and consistent manifestation of maternal estrogens in the third trimester (Zondek and Zondek, 1979). In contrast, spontaneous prostatic squamous metaplasia has never been reported in mice, even though prostatic squamous metaplasia can be induced in mice by exogenous estrogens such as estradiol or diethylstilbestrol (DES) (Risbridger et al, 2001). The absence of spontaneous prostatic squamous metaplasia in mice can be attributed to high levels of alpha- fetoprotein (AFP) produced prenatally and neonatally by the mouse (and rat) liver. AFP in these species binds estrogen at high affinity (Uriel et al, 1976), and the levels of AFP in rat (and mouse) serum are particularly high. Thus, endogenous serum estrogen of either fetal or maternal origin is sequestered in mice and is not available for entry into target cells. In contrast, human AFP does not bind estrogens (Swartz and Soloff, 1974), and thus in humans, endogenous and exogenous estrogens are free to interact with target cells and to elicit prostatic squamous metaplasia (and in females stimulate breast, uterus and vagina) during the perinatal period (Driscoll and Taylor, 1980; Yonemura et al, 1995; Zondek and Zondek, 1979). After birth prostatic squamous metaplasia disappears.
The presence AFP in neonatal mice has profound effects on estrogen action on all estrogen target organs and this fact must be taken into consideration in experimental design and interpretation of results. An example of problems that can arise in the use of neonatal mice is illustrated in a recent paper by Zheng et al. This elegant paper provides some interesting and important ideas concerning hormone- induced malformations of the external genitalia of mice elicited by disrupting AR signaling via genetic or anti-androgen treatment as well as via treatment of newborn mice with a pharmacological dose of estradiol benzoate (Zheng et al, 2015). Effects of the massive dose of estradiol benzoate included profound down- regulation of androgen receptor (AR) within the developing penis, widespread induction of ERα expression in female external genitalia, and effects on proliferation, apoptosis and gene expression in male and female external genitalia (Zheng et al, 2015). With respect to Esr1 (ERα) signaling, the authors explicitly discuss the relevance of their pharmacologic estrogen studies in relation to normal development of male and female external genitalia. Simply put, it is not possible to interpret outcomes as normal when effects are elicited by a pharmacological dose of estradiol. Such an interpretation is unjustified and certainly inconclusive for many reasons: (a) It is sobering to note that a dose of estradiol at 1μg/kg is sufficient to elicit a strong stimulation of uterine epithelial proliferation in adult mice (AFP is absent) (Nanjappa et al, 2019). The dose used in the Zheng et al paper on neonates was 300μg/kg, that is, 300 times that required to elicit adult uterine epithelial growth. The reason for the pharmacologic dose used by Zheng et al is the well-known fact that in order to elicit estrogenic effects in neonatal mice, pharmacologic doses are required to overcome the “neutralizing” effect of AFP. It is unacceptable and inappropriate to apply the term “normal” to an experiment in which a pharmacologic estrogen dose was administered at least 300 times above physiologic. In addition, the author’s explicit statement that estradiol/ERα signaling is occurring in “normal” (untreated) neonatal female mice can be challenged on several grounds: (1) Several effects of estrogens have been previously reported in neonatal female mice treated with pharmacologic doses of estrogen (Bern et al, 1984; Bern and Talamantes, 1981; Forsberg and Kalland, 1981; McLachlan et al, 1980). None of these classic estrogenic responses have been reported in untreated or oil-treated wild-type female neonatal mice, perhaps for two reasons: (a) estrogen levels are extremely low in neonatal rodents (Pang et al, 1979) and (b) low levels of estrogen in neonatal mice are likely to be sequestered by AFP as discussed above. (2) The body of evidence in the literature support the idea that estrogenic responses may not even occur in untreated wild-type female neonatal mice during the period when AFP is present (birth to ~20 days postnatal). This idea is supported by several observations: (a) Esr1KO, Esr2KO, or Esr1/Esr2KO male mice exhibit normal internal and external genital structures that are indistinguishable from their wild-type littermates (Couse and Korach, 1999a, b; Nef and Parada, 2000). (b) Proliferation of uterine luminal epithelial cells, uterine stromal cells, and glandular epithelium is comparable in untreated wild-type, heterozygotes, and Esr1KO mice examined sequentially from day 2 to 22 postnatal. Adenogenesis, the formation of uterine glands, is also comparable in these 3 groups (Nanjappa et al, 2015). The fact that values for all of these parameters were virtually identical in untreated wild- type and Esr1KO mice suggest that Esr1 signaling is not involved in uterine growth (and perhaps globally in other organs) from birth to at least 22 days postpartum. Moreover, estrogen deprivation via neonatal ovariectomy alone or neonatal ovariectomy plus adrenalectomy causes no significant reduction in neonatal uterine growth relative to control (Ogasawara et al, 1983). Thus, disruption of estrogen receptor genes as well as ablation of estrogens via ovariectomy and adrenalectomy is inconsistent with estrogenic signaling in normal untreated neonatal mice. Moreover, studies using pharmacologic doses of estradiol benzoate cannot be considered to reflect normal physiology/development of estrogen target organs. Finally, knowledge of mouse anatomy is critical. The structure designated as mouse clitoris and illustrated as such (Zheng et al, 2015), is not clitoris, and instead is prepuce (Cunha et al, 2019a; Mahawong et al, 2014b; Martin-Alguacil et al, 2008b; Sinclair et al, 2016c; Weiss et al, 2012; Yang et al, 2010).
Another important mouse/human difference concerns the response to the so-called “anti-estrogen”, tamoxifen. While tamoxifen is used therapeutically as an anti-estrogen in humans (Marth et al, 1984; Moseson et al, 1978), tamoxifen is an estrogen in mice (Diwan et al, 1997; Takamatsu et al, 1992). Thus, the use of tamoxifen-inducible gene constructs at a pharmacologic tamoxifen dose of (80 mg/kg) (Zheng et al, 2015) is potentially a serious complication for prenatal targeting of transgenes to reproductive tract organs. The mechanisms involved in whether tamoxifen acts as an anti-estrogen or an estrogen in either species is complex and depends on dose, target tissue, timing of exposure and molecular regulators present in a particular tissue. Caution is urged.
D. Prostatic basal cells:
The function of prostatic basal cells has been and continues to be a source of interest. Prostatic basal cells lie on the epithelial basement membrane and express a spectrum of differentiation markers distinguishing them from the secretory luminal cells (Table 1). In humans, prostatic basal epithelial cells form a continuous uninterrupted layer beneath the luminal cells (Andrews, 1951; Bonkhoff et al, 1994; Srigley et al, 1990), whereas in mice the basal cell layer is discontinuous (Hayward et al, 1996a). The significance of this difference is unclear. Insight into the function of prostatic basal cells is derived from a study by Takeshi Kurita and colleagues who created mature mouse prostate that lacked basal cells (Kurita et al, 2004a), an achievement produced by “rescuing” prostates from p63-deficient mice by grafting into adult male hosts. This approach was required as p63-deficient mice die at birth, which is well before the prostate has differentiated. p63 is a protein normally expressed in prostatic basal cells, and such p63-knockout prostates are devoid of basal cells. Anomalies found in these basal-cell-deficient prostates include: (a) Abnormal PAS- reactive mucinous goblet cells within the prostatic epithelium; (b) A profoundly exaggerated response to androgen deprivation; (c) Impaired prostatic regeneration from the androgen-deficient state following treatment with exogenous testosterone (Kurita et al, 2004a). The higher proportion of basal epithelial cells in human versus mouse prostates may have important consequences. The role of prostatic basal epithelial cells in carcinogenesis remains to be defined as data on this subject is conflicted. Loss of prostatic basal cells occurs early in the carcinogenic process in humans and rats (Bostwick, 1996; Wong and Tam, 2002), yet prostatic basal epithelial cells are said to regulate and suppress human prostate cancer cells (Miniati et al, 1996).
Table 1.
Protein differentiation markers of prostatic basal and luminal epithelial cells.
| Differentiation marker | Basal cells | Luminal cells | Reference |
|---|---|---|---|
| p63 | Yes | No | (Weinstein et al, 2002) |
| Keratin 14 | Yes | No | (Weinstein et al, 2002) |
| Keratin 5/6 | Yes | No | (Abrahams et al, 2002) |
| Keratin 7 | No | Yes | (Trompetter et al, 2008) |
| Keratin 8 | No | Yes | (Hudson et al, 2001) |
| Keratin 17 | Yes | No | (Trompetter et al, 2008) |
| Keratin 18 | No | Yes | (Hudson et al, 2001) |
| Keratin 19 | No | Yes | (Peehl et al, 1996) |
| Keratin 20 | No | Yes | (Trompetter et al, 2008) |
| Androgen receptor | No* | Yes | (Cunha et al, 1987) |
Most studies indicated that prostatic basal cells are AR-negative
E. Prostatic stroma:
During the fetal period the prostatic connective tissue of both mice and humans is undifferentiated and appropriately designated as urogenital sinus mesenchyme (UGM), which induces and specifies prostatic epithelial differentiation (Cunha et al, 1987). In the course of development, the UGM differentiates into smooth muscle, a process that requires a signal(s) from the developing prostatic epithelium (Cunha et al, 1992). In mice and rats, thin smooth muscle bundles form immediately around the prostatic ducts (Cunha et al, 1992; Cunha et al, 1996). Accordingly, adult mouse (and rat) prostatic stroma contains only a small proportion of smooth muscle cells, as the connective tissue between prostatic ducts is devoid of smooth muscle.
In contrast, adult human prostatic stroma contains vastly more smooth muscle. Indeed, adult human prostatic stroma is predominantly smooth muscle (Cunha et al, 1996; Hayward et al, 1996b; Hayward et al, 1997). The significance of this mouse/human difference is not known, but as prostatic carcinogenesis is initiated in humans, a “reactive stroma” develops in which the smooth muscle cells lose differentiation markers and transform into myofibroblastic cells (Ayala et al, 2003; Tuxhorn et al, 2002). Dedifferentiation of smooth muscle cells also occurs in rat prostatic carcinogenesis (Wong and Tam, 2002). The resultant human carcinoma-associated fibroblasts promote tumorigenesis (Olumi et al, 1998). Moreover, the nature and extent of stromal changes occurring during human prostatic carcinogenesis have predictive/prognostic implications (Ayala et al, 2003; Tuxhorn et al, 2002; Yanagisawa et al, 2007). Finally, evidence suggests that the smooth muscle layer regulates signaling between prostatic mesenchyme and epithelium during prostatic development in rats, and thus comprises part of the mechanism regulating prostatic induction (Thomson et al, 2002). For these reasons, the status of smooth muscle in developing and adult rodent versus human prostates deserves further research.
F. Prostatic ductal branching morphogenesis.
Rat and mouse prostatic buds emerge from the UGE and grow peripherally toward mesenchymal densities called mesenchymal pads in which ductal branching initiates (Thomson and Cunha, 1999). The ventral mesenchymal pad is the most extensively studied (Thomson and Cunha, 1999; Timms et al, 1995), even though there are also dorsal-lateral mesenchymal pads in which the dorsal-lateral prostate develops. The ventral mesenchymal pad is rich in FGF7 and FGF10 (Finch et al, 1995; Thomson and Cunha, 1999). Once the elongating prostatic buds enter the ventral mesenchymal pad, profuse ductal branching occurs. Up to and throughout the period of ductal branching, the mesenchymal pads are devoid of smooth muscle markers (Thomson et al, 2002). This is not the case for human prostatic development as ductal branching occurs within fields of undifferentiated prostatic mesenchyme as well as in fields of smooth muscle (Fig. 4) (Cunha et al, 2018c).
Figure 4.
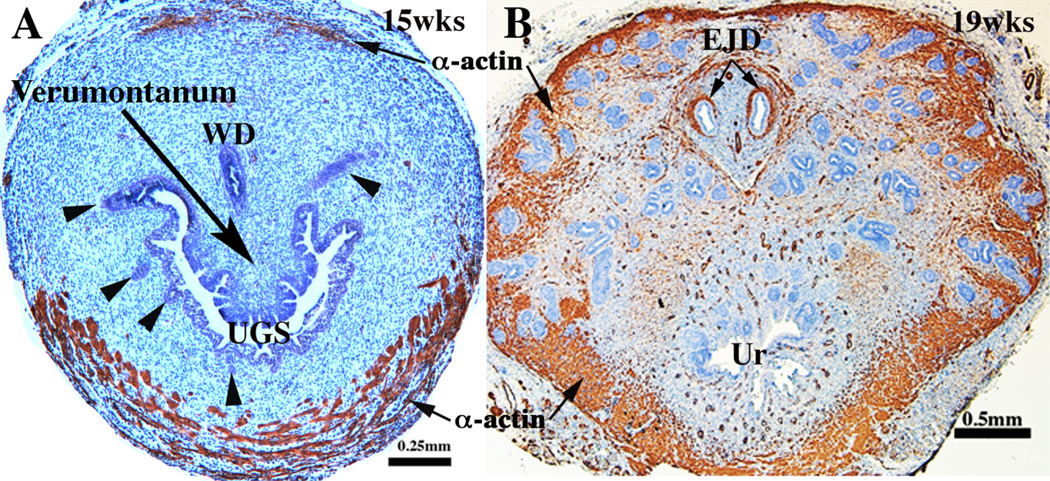
Human fetal prostates at 15 (A) and 19 weeks (B) stained for smooth muscle actin. At 15 weeks (A) smooth muscle has differentiated ventrally and is just beginning to appear dorsally. By 19 weeks (B) smooth muscle has differentiated circumferentially around the developing prostate. Ductal branching has occurred in the smooth-muscle- poor central region as well as in the peripheral smooth-muscle-rich region.
G. Prostate cancer:
Prostate cancer is one of the most common neoplasms affecting men, occurring in up to 80% of men who reach the age of 80 years (Crawford et al, 2001). In contrast, spontaneous prostate cancer is extremely rare in mice and rats. The reasons for this vast difference remain unknown, however exposure to developmental events such as high levels of estrogens may play a role.
H. Benign prostatic hyperplasia (BPH):
BPH afflicts a large proportion of aging men and may lead to urethral obstruction (McNeal, 1985). A key comparative difference between mouse and human prostates is a thick fibromuscular capsule that surrounds the human prostate; mice and rats have no such entity surrounding the prostatic lobes to collectively unify the entire organ. The thick collagenous capsule in humans may be an important contributor in the development of urethral obstruction associated with BPH. The human prostatic capsule has traditionally been described to confine growth, especially nodular growth, thereby creating pressure or impingement on the urethra as it traverses through the prostate, altering urinary flow and ultimately leading to urinary symptoms.
The prevalence of BPH increases with age (Gupta et al, 2006; Prakash et al, 2002). Lower urinary tract symptoms (LUTS) are commonly associated with BPH (Girman et al, 1995; Roehrborn et al, 1999). BPH is postulated to lead to symptoms due to development of a bladder outlet obstruction. This is because the urethra passes through the human prostate gland, and proliferation of tissues within the prostate are confined by the prostatic capsule which serves to restrict outward expansion of prostatic tissues. Instead, enlarging prostatic nodules push inward on the urethra potentially causing urinary obstruction. In this context, expansion of hyperplastic prostatic tissues can impede urinary flow, leading to obstructive symptoms. As such, transurethral resection of the prostate (TURP) is used to remove impinging tissue and allow for an “open” channel within the prostate.
Other than humans, dogs are the only other species to spontaneously develop BPH. Recently, aged mice were found to have cellular, anatomical, and functional aspects of BPH (Liu et al, 2019). For decades, it was believed that mice would not make good models for BPH/LUTD research because the anatomy of distinct prostatic lobes in mice is anatomically dissimilar to that of the human prostate. To this point, mouse prostatic lobes surround the rhabdosphincter/urethra, but are not encompassed by thick connective tissue capsule as found in men. Although these anatomical differences have been cited previously as grounds for not using mice in urinary dysfunction research, a careful examination of the prostatic ducts entering into the mouse urethra suggests that the adult mouse prostatic urethra shares in parallel features with the human transition zone in that it directly surrounds the urethra. Although the mouse urethra is not encompassed by a thick tunica of collagen, it is surrounded by a dense muscular stroma known as the rhabdosphincter, which forms a “border” analogous to the capsule of the human prostate. Indeed, an increase in periurethral prostatic ductal growth is associated with lower urinary tract dysfunction in both mice and men. As such, a similar anatomical/cellular impingement “mechanism” exists in both men and rodents. Moreover, this obstruction is associated with mouse urinary dysfunction (Liu et al, 2019; Nicholson et al, 2015; Nicholson et al, 2012; Ricke et al, 2018). Thus, rodents have been successfully used to model BPH in humans.
Alternatives to prostate hyperplasia as the driving force in BPH/bladder outlet obstruction and subsequent urinary symptoms is development of smooth muscle tone and prostatic urethral fibrosis. Mouse models of prostatic smooth muscle contractility are limited, and it is uncertain if there are physiological consequences in the mouse that lead to LUTD. Nonetheless the mouse prostatic urethra does contain smooth muscle and hence may serve as a model for hypercontractility in LUTD (Liu et al, 2019).
Prostatic fibrosis results in a lack of urethral compliance, and hence may lead to bladder outlet obstruction, an idea currently being assessed in mouse models. Normally, as urine passes through the prostatic urethra, the urethra stretches and expands to achieve maximal urinary flow. If the urethra cannot expand or is smaller in diameter due to fibrosis of the surrounding periurethral tissue, this may explain why some men are not responsive to medical therapies for BPH, which do not target fibrosis. Additionally, it may explain why men with small non-hyperplastic prostates have BPH/LUTS. These processes as well as other experimental concepts are perhaps the most relevant reasons for using rodents as models for BPH/LUTS.
VII. Female reproductive tract development:
A. Müllerian duct development:
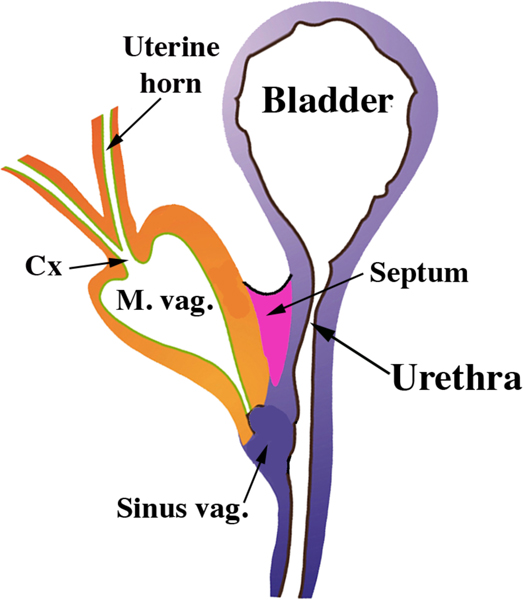
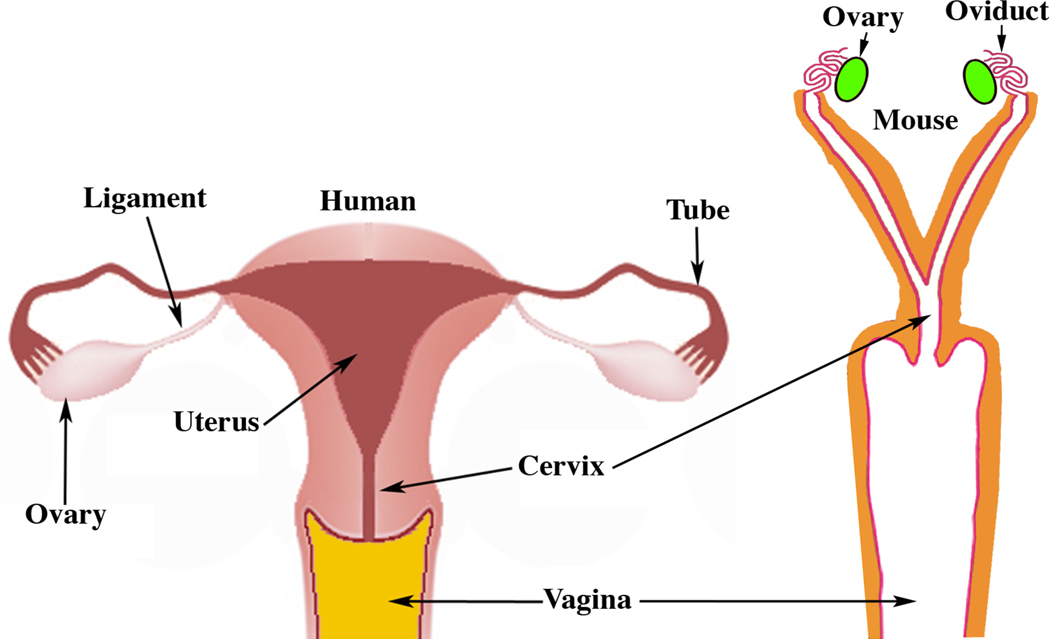
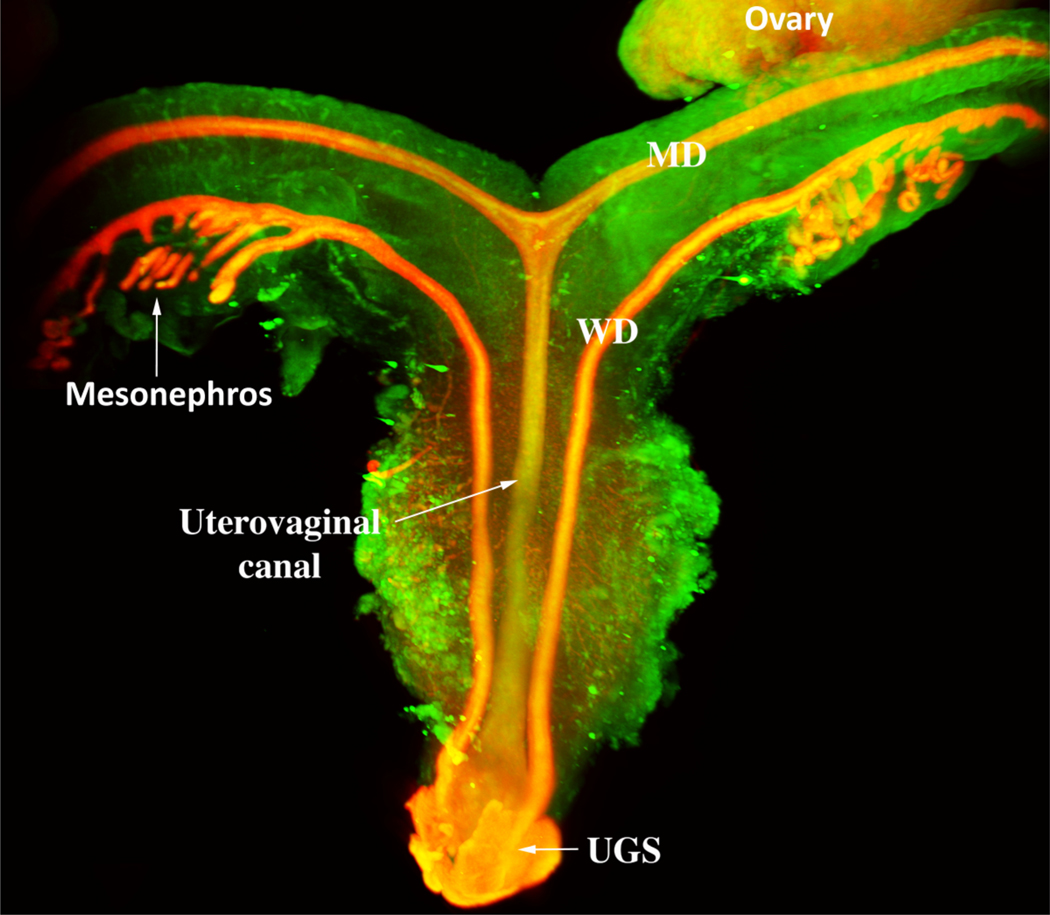
In females the bilateral Müllerian ducts give rise to the oviducts/uterine tubes, uterus, cervix and the vagina (Kurita and Nakamura, 2008; Robboy et al, 2017). In both humans and mice the bilateral Müllerian ducts fuse in the midline, but the degree of fusion differs vastly in these two species (Robboy et al, 2017). In mice, only the caudal-most portions of the Müllerian ducts fuse to form a relatively short cervical canal and the so-called Müllerian vagina, which in the neonate forms the upper portion of the vagina and includes the vaginal fornices (Kurita, 2011) (Fig. 5). Most of the Müllerian ducts in mice remains separate (unfused) and forms the bilateral uterine horns and oviducts (Kurita, 2011; Kurita and Nakamura, 2008; Robboy et al, 2017) (Fig. 6). In contrast, in humans all but the cranial portions of the Müllerian ducts fuse to form the uterovaginal canal, the primordium of the uterine body, cervix and vagina (Cunha et al, 2018b; Robboy et al, 2017). The unfused cranial portions of the human Müllerian ducts form the uterine tubes, also known as the Fallopian tubes (Fig. 6). Thus, from a morphogenetic perspective Müllerian duct development differs considerably in mice and humans (Fig. 6).
Figure 5.
Diagram of the reproductive tract of a neonatal mouse. The fused Müllerian ducts form the cervical canal (Cx) and the Müllerian vagina (M. vag.). The unfused Müllerian ducts form the uterine horns and oviducts. The Müllerian vagina contacts the solid sinus vagina whose epithelium is derived from the urogenital sinus.
Figure 6.
Diagrams of adult human and mouse female reproductive tracts. In humans most of the Müllerian ducts fuse in the midline and form the uterus, cervix and contribute to vaginal development, with the unfused cranial portions forming the uterine tubes. In mice most of the Müllerian ducts remain unfused to form the oviducts and uterine horns, with the fused portion forming the cervix and contributing to the vagina.
During female reproductive tract development the Müllerian ducts grow caudally in both mice and humans to fuse with the epithelium of the urogenital sinus (UGS) (Koff, 1933; Kurita, 2010; O’Rahilly, 1973). In humans, the Müllerian duct/UGS junction fluctuates with time. Initially in humans the Müllerian duct/UGS junction is near the bladder in females (Koff, 1933), but by 12 weeks this junction descends caudally to near the vaginal introitus (Koff, 1933; Robboy et al, 2017). In mice, the Müllerian duct/UGS junction becomes situated cranially in males and more caudally in females, even though the initial point of contact of the Müllerian ducts with the UGS is high (near the bladder) in both sexes. The subsequent male/female positional difference in mice is attributed to the timing and the duration of androgen action during fetal stages (Larkins et al, 2016). The absence of androgen action in females accounts for a more caudal positioning of the Müllerian duct/UGS junction in mice, while androgen action in males accounts for the more cranial positioning of the Müllerian duct/UGS junction. In humans (as in mice) the positioning of the Müllerian duct/UGS junction is cranial in males (Cunha et al, 2018c) and caudal in females (Cunha et al, 2018b; Robboy et al, 2017). The mechanism of this sexually dimorphic positioning of the Müllerian duct/UGS junction in humans has not been explored adequately. However, the proposed role of androgens (based upon mouse studies) in positioning the Müllerian duct/UGS junction in males and females (Larkins et al, 2016) is doubtful in humans for several reasons: (a) The initial cranial position of the human Müllerian duct/UGS junction described previously (Cunha et al, 2018c; Koff, 1933) in males is established at about 8 weeks of gestation when androgen levels are low or non-existent. (b) The fetal testes begin testosterone production at about 8–10 weeks of gestation (Siiteri and Wilson, 1974). (c) The first concrete evidence of androgen action in human males is initiation of prostatic bud formation which occurs at 10 weeks. Thus, it is unlikely that androgens play a role in the cranial positioning of the Mullerian duct/UGS junction in human males. In human female fetuses, the Müllerian duct/UGS junction is established at ~8 weeks shortly below the bladder as in males, but over the next 3–4 weeks the Müllerian duct/UGS junction translocates caudally to the region of the vaginal introitus (Cunha et al, 2018b; Koff, 1933; Robboy et al, 2017). The mechanism of this positional change is not known. Whether in humans these male/female differences in the insertion of the fused Müllerian ducts (uterovaginal canal) with the UGS are attributable to androgen action in males or the lack of androgen action in females is uncertain as the spatial distribution of androgen receptors remains unresolved in developing human male and female reproductive tracts at the required ages.
B. Derivation of vaginal epithelium:
The question of vaginal epithelial derivation was debated for decades both for humans (Cunha et al, 2018b; O’Rahilly, 1977; Robboy et al, 2017) and mice (Kurita, 2010). Initially, speculation on the derivation of human and mouse vaginal epithelium was based mostly upon histologic analysis (Bulmer, 1957; Forsberg, 1973; Koff, 1933). To reveal the derivation of vaginal epithelium in mice, Kurita utilized transgenic mice in which an enhanced green fluorescent protein (EGFP) reporter irreversibly labeled the Müllerian, Wolffian or UGS epithelia (Kurita, 2010). Kurita showed that the vaginal rudiment in mouse embryos and newborns consisted of the fused Müllerian ducts (Müllerian vagina) plus UGS epithelium (sinus vagina) (See Fig. 5), which in the transgenic mice were separately and irreversibly labeled red and green, respectively. However, postnatally in mice the proportion of the UGS vaginal epithelium was progressively reduced as the Müllerian epithelium grew caudally such that at puberty when the vagina opened, urogenital sinus epithelium was detected only in the vulva, and not in the vagina. Thus, in mice adult vaginal epithelium derives solely from Müllerian duct epithelium (MDE) (Kurita, 2010).
In humans, based upon histologic analysis, Koff (1933) asserted that vaginal epithelium is mostly derived from Müllerian epithelium with a minor contribution ascribed to UGE, while Bulmer (1957) concluded the opposite, namely that virtually all of the human vaginal epithelial lining derived from UGS. Reich and Fritsch (2014) asserted that the squamous epithelia of the exocervix and upper vagina are of Müllerian origin, thus supporting Koff’s interpretation. Unfortunately, these previous studies lacked markers indicative of both Müllerian and urogenital sinus epithelia. Our recent studies have used PAX2 (a Müllerian epithelial marker) and FOXA1 (an endodermal UGE marker) to immunohistochemically explore the ontogeny of human vaginal epithelium (Cunha et al, 2018b; Robboy et al, 2017). As in the mouse, the relative distriburion of PAX2 (a Müllerian epithelial marker) and FOXA1 (an endodermal UGE marker) changes temporally with development. In humans by week 21 when vaginal development is essentially complete, FOXA1-positive UGE was detected from the introitus to the level of the cervical os, thus supporting Bulmer’s interpretation (Fig. 7) (Cunha et al, 2018b; Robboy et al, 2017). At the external cervical os the stratified squamous FOXA1-positive epithelium transitions abruptly to a pseudostratified columnar PAX2-positive epithelium (Cunha et al, 2018b; Robboy et al, 2017). The FOXA1 and PAX2 immunostaining was followed sequentially from 12 to 21 weeks with several specimens spread across this age range, and mirror image FOXA1/PAX2 immunostaining moved from near the introitus at 12 weeks to the external cervical os at 21 weeks (Robboy et al, 2017). There are 2 interpretations of this data: (a) FOXA1-positive UGE extends cranially to replace the Müllerian epithelium up to the external cervical os. (b) Alternatively, the PAX2-positive Müllerian epithelium turns off PAX2 expression and turns on FOXA1 expression in a progressive wave beginning caudally near the introitus and extending cranially to the external cervical os. This later possibility seems unlikely. We conclude that the derivation of vaginal epithelium differs vastly in mice versus humans, surely involving different molecular mechanisms. Such discoveries emphasize the need to further decipher how this complicated process proceeds differently in both species.
Figure 7.
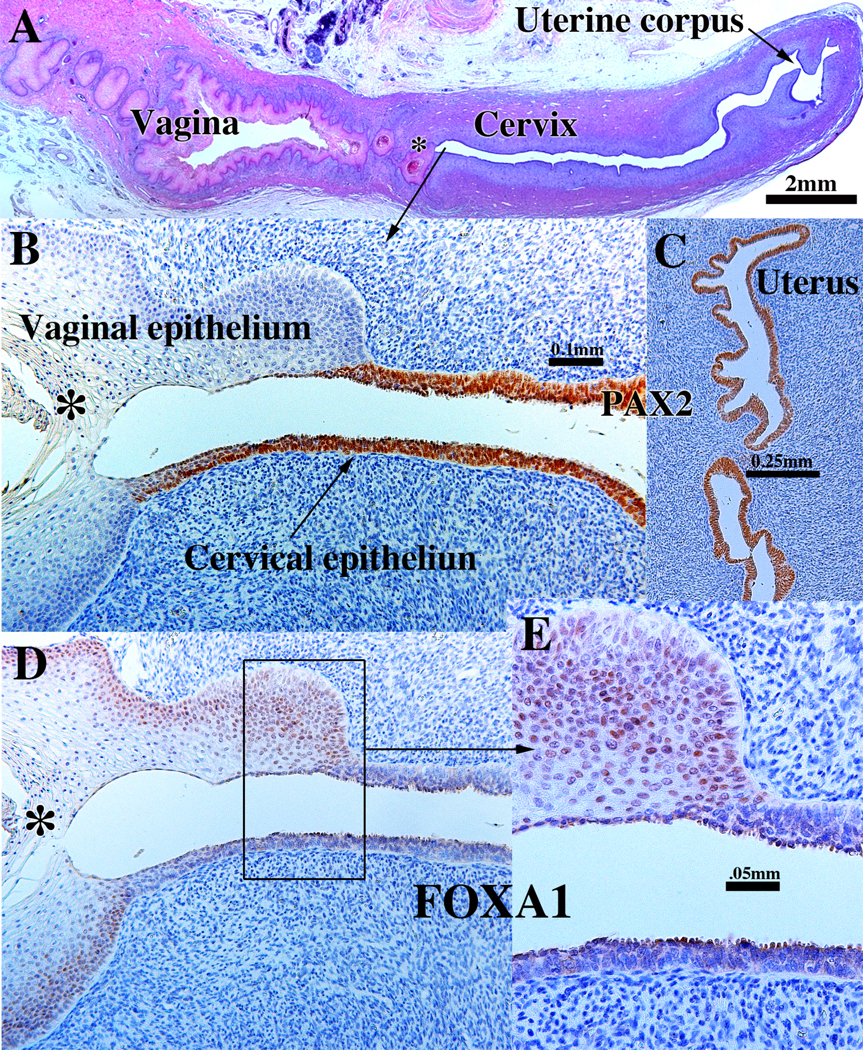
Sagittal sections of a 21-week female reproductive tract. (A) depicts the upper portion of the vagina, cervix and uterus (H&E stain). The vaginal epithelium is many layers thick due to endogenous estrogenic stimulation. Note the abrupt transition in epithelial differentiation at the vaginal/cervical border. (B) PAX2 staining of the vaginal/cervical border shows prominent PAX2 immunostaining (indicative of Müllerian duct origin) of the pseudostratified stratified relatively thin cervical epithelium and PAX2-negative stratified squamous vaginal epithelium. (C) Epithelium of the uterine corpus is strongly PAX2-reactive as expected for a Müllerian epithelium. (D & E) FOXA1 nuclear immunostaining was seen uniformly throughout the entire vagina, and FOXA1 nuclear immunostaining abruptly stopped at the vaginal/cervical border (D-E) in mirror image to PAX2 immunostaining (B). (modified from Robboy et al., 2017 with permission). (B & D) are serial sections of the same specimen.
C. Cervix:
Development of the human cervix is poorly understood. During early fetal life, the future cervix is indistinguishable from the future uterine corpus or future vagina other than by location. The human cervix develops within the uterovaginal canal (Fig. 8), a thin midline epithelial tube formed via midline fusion of the Müllerian ducts, which is the common primordium of the uterine corpus, cervix and vagina (Cunha et al, 2018b; Robboy et al, 2017). An undifferentiated simple columnar epithelium initially lines the embryonic human uterovaginal canal. In mice only the caudal aspect of the Müllerian ducts fuses to form the cervical canal, exocervix, fornices and the so-called Müllerian vagina (Fig. 5). Epithelia of all of these structures will differentiate into a stratified squamous epithelium.
Figure 8.
Light sheet microscopy of a human female reproductive tract at 9.5 weeks stained with an antibody to E-cadherin. The mesonephros and Wolffian ducts (WD) are present. Cranially, the unfused MDs are destined to form the uterine tubes. Midline fusion of the MDs has created the uterovaginal canal that terminates caudally by joining the urogenital sinus (UGS).
Two epithelial types differentiate in the adult human cervix:
(a) A stratified squamous epithelium lining the caudal (lower) segment of the cervical canal and covering the surface of the exocervix (that portion of the cervix projecting into the vaginal vault). These epithelia are the similar to that lining the adult vagina. (b) A simple columnar epithelium lines the endocervix, whose differentiation is similar to that of epithelium of the Fallopian tube and uterine corpus. Beginning at about the 16th gestational week, the Müllerian epithelium lining the endocervix develops simple glandular outpouchings (glands) which later will undergo mucinous differentiation (Robboy et al, 2017).
Thus, even though the cervix of mice and humans arise in total or in part from the Müllerian ducts, adult differentiation of cervical epithelium differs significantly in these two species. Mouse cervical epithelium is stratified squamous and non-glandular (identical to that of vaginal epithelium), whereas human cervical epithelium is stratified squamous on the exocervix and simple columnar in the endocervix (Atkins, 2019; Kurita et al, 2001). These two different types of epithelial differentiation in the human cervix may be elicited via region-specific inductive cues from mesenchyme as has been reported for murine Müllerian epithelial differentiation (Cunha, 1976; Kurita et al, 2001). Such mesenchymal-epithelial interactions are known to be at play in the developing human female reproductive tract (Cunha et al, 2018a). Alternatively, these differences in epithelial differentiation may be attributed to differences in germ layer origin, Müllerian for endocervix and urogenital sinus for exocervix as discussed above.
D. Mouse oviduct and human uterine tube.
The mouse oviduct and human uterine tube are derived from the cranial (unfused) portions of the Müllerian ducts. The development of both of these structures is similar in both species.
E. Progesterone receptor regulation:
Regulation of the progesterone receptor in uterine epithelium differs substantially in the mouse versus human (Horwitz and McGuire, 1979; Janne et al, 1975; Kurita et al, 2000; Kurita et al, 2005b). In the mouse, uterine epithelial progesterone receptor (PGR) is strongly expressed following ovariectomy (that is, in the absence of estrogen) and is profoundly down regulated upon estrogen administration, an effect mediated indirectly via stromal estrogen receptor 1 (ESR1) (paracrine mechanism) (Kurita et al, 2000). In contrast, in humans PGR is up-regulated directly via ESR1 in uterine epithelium (Cunha et al, 2017; Horwitz and McGuire, 1979; Janne et al, 1975; Kurita et al, 2005b). Thus, the progesterone receptor, which has important therapeutic implications in humans, is regulated in uterine epithelium via opposite mechanisms in mice versus humans.
F. Vaginal adenosis and vaginal adenocarcinoma:
Vaginal adenosis is a pathological condition in humans and mice in which glandular tissue is abnormally present in the vagina. The definition also applies in humans and mice to the presence of a similar histological type of glandular tissue found in the exocervix (Kurita et al, 2004b; Robboy et al, 2019). First begun in the early 1940s for therapeutic purposes, diethylstilbestrol (DES), an inexpensive, synthetic estrogen, was given to pregnant mothers who were known to have high risk pregnancies in the belief it would lessen the chance if not prevent miscarriage. It was not until 1971 that it was recognized that about 1:1000 young women exposed in utero to DES would develop a clear-cell adenocarcinoma of the vagina or exocervix (Herbst et al, 1974). The critical period of DES exposure was from about the 8th to 22nd gestational week, the time when the vagina was undergoing substantial fetal development. The link to the cancer topographically (Robboy et al, 1982b) and histologically (Robboy et al, 1984b) was the presence of adenosis.
Human vaginal adenosis develops in three forms, with the clear cell adenocarcinoma being linked to only the tuboendometrial form. This form resembles the Müllerian epithelium typically lining the Fallopian tube or endometrium: namely cuboidal to columnar glandular epithelial cells having an eosinophilic cytoplasm and a luminal surface that often is ciliated. The second form, the mucinous columnar epithelial cell, resembles the typical and normal mucin-rich columnar epithelial cells lining the normal endocervix; this form has no preneoplastic potential related to estrogen exposure. The third form, the embryonic form, is occasionally seen in the adult as an isolated gland (Robboy et al, 1986). This form histologically resembles the embryonic glandular tissue seen in the early fetal female reproductive tract. In the adult, this form is thought to be a vestigial Müllerian epithelial remnant that had never transformed into stratified squamous epithelium. When encountered, it is always an incidental finding. None of these latter two forms are known to be linked to prenatal DES exposure.
DES-induced adenosis in mice is linked to the disruption of p63 expression in Müllerian epithelium of the upper vagina, vaginal fornices and cervical canal. Initially embryonic Mullerian epithelium is simple columnar and devoid of p63 expression. However, shortly before birth and in the early neonatal period p63 is expressed in the epithelial cells of the Müllerian vagina as they transform from simple columnar to stratified squamous. In the female reproductive tract p63 appears to be the earliest vaginal epithelial identity marker, which is followed in turn by other vaginal epithelial markers (Kurita et al, 2005a). Treatment of neonatal mice with DES beginning at birth focally inhibits p63 expression in Müllerian vaginal epithelium resulting in the retention of simple columnar epithelium in the upper vagina, vaginal fornices and cervical canal. The resultant adenotic epithelium expresses a variety of protein markers consistent with uterine epithelial differentiation (Kurita et al, 2004b). While a great deal has been revealed concerning DES-induce adenosis in mice, DES induction of human vaginal adenosis is based upon a limited set of specimens.
We have induced adenosis in grafts of human fetal vagina to athymic mice that were treated with DES (Cunha et al, 2017; Robboy et al, 1982a). The resultant simple columnar adenotic epithelium observed in vaginae so treated with DES expressed PAX2, a marker of Müllerian epithelium, and a spectrum of other markers indicative of simple columnar epithelial cells consistent with a uterine epithelial phenotype (Cunha et al, 2017). Thus, for both species adenosis appears to be retained embryonic-like Müllerian epithelium. However, the mechanistic scenario for DES induction of adenosis has to be considered in the context of the relative contribution of Müllerian versus urogenital sinus epithelium to the vagina, which differs in mice versus humans as discussed above. In mice, the epithelium of the Müllerian vagina grows caudally, and at puberty urogenital sinus epithelium has been completely replaced by Müllerian epithelium (Kurita, 2010). Accordingly, vaginal epithelium of adult mice is entirely derived from Müllerian epithelium. Quite the opposite appears to occur in human in which FOXA1-positive urogenital sinus epithelium initially found in the most caudal segment of the developing vagina near the introitus extends cranially apparently replacing the Müllerian epithelium up to the level of the external cervical os. How this alternate human mechanistic scenario relates to DES-induced adenosis remains to be determined. Nonetheless, the DES- induced adenosis expresses PAX2 indicative of its Müllerian origin.
VIII. Mammary gland
To fully appreciate the validity and limitations of mouse mammary development relative to the molecular mechanisms involved in breast cancer development and progression, it is essential to be aware of the similarities and differences that exist between the mouse mammary gland and the human breast (McNally and Stein, 2017). In all mammalian species examined the mammary epithelial buds that invade from the basal surface of the embryonic epidermis into the underlying mesenchyme (Cunha and Hom, 1996; Veltmaat et al, 2003). In mouse embryos, the epithelial buds invade into an androgen-receptor-rich mesenchyme, and in male embryos endogenous androgens of testicular origin elicit regression of the mammary epithelial rudiments (Kratochwil and Schwartz, 1976). In female mouse embryos due to the absence of androgens, the mammary buds are retained and subsequently form a branched ductal network (Cowin and Wysolmerski, 2010; Richert et al, 2000) which postnatally become lactogenic when hormonally stimulated (Nandi, 1958). In the human male, the mammary rudiments do not disappear, and hence breast development, lactation (male galactorrhea) and even rarely breast cancer can occur in men (Giordano, 2018; Tokunaga et al, 1988; Wieland et al, 1967). Breast cancer is unknown in male mice.
XI. Discussion
The validity, relevance and limitations of mouse (and rat) hormone target organs of male and female reproductive tracts and mammary glands relative to human development and pathogenesis rests on similarities in anatomy and developmental processes in these species. In many respects developmental mechanisms are similar, and in many cases, perhaps identical between humans and mice. Most aspects of androgen, estrogen and progestin action are shared by both rodents and humans. For both species masculine urogenital development depends upon androgens (Cunha et al, 1987). Evidence in mouse, rats and humans indicates that androgen action elicits prostatic bud formation and growth, ductal branching morphogenesis and secretory function. Stabilization and retention of the Wolffian ducts and their subsequent development into the epididymis, vas deferens and seminal vesicle is androgen-dependent in all mammalian species examined and mediated via androgen receptors. The protein hormone, Müllerian inhibiting substance (also called anti-Müllerian hormone) produced by the fetal testes elicits destruction of the embryonic Müllerian ducts in mice and humans. Thus, the basic hormonal mechanisms of sex differentiation are conserved in both mice and humans, even though the mechanism of progesterone receptor regulation is entirely different in uterine epithelium of mice and humans as discussed above.
Growth and secretory cytodifferentiation within the female reproductive tract and mammary glands is stimulated or dependent at least in part upon estrogenic stimulation, even though initial formation of female reproductive tract and mammary glands are independent of estrogen action based upon their development in estrogen receptor deficient mutant mice (Couse and Korach, 1999a, b). In many tissues within the female mouse reproductive tract and mammary gland, estrogens induce expression of the progesterone receptor as is also the case in humans. A striking exception is the regulation of the progesterone receptor in mouse uterine epithelium in which the progesterone receptor is highly expressed in the estrogen-deprived state and down regulated by estrogen (Kurita et al, 2000), a pattern exactly opposite to that in humans.
The challenge for users of mouse models is to recognize those anatomic and developmental features in mice that are substantially different with human development thus requiring caution in extrapolating mouse studies to human biology, while focusing on those features that are shared in common. For certain organs developmental features may be similar/identical to their human counterparts, while other features within the same organ may exhibit substantial mouse/human differences. For example, while penile development in both mice and humans in androgen-dependent, formation of the penile corporal body is androgen-independent in humans but androgen-dependent in mice (Cunha et al, 2019b).
The degree to which organogenesis in mice differs from that in humans varies on an organ basis. Clitoral anatomy and development are completely different in mouse versus human (Cunha et al, 2019a). Clitoral development is a particularly under reported subject. Studies on development of the external genitalia have focused mainly on penile development, perhaps in large part because of experimental models of mouse hypospadias. To fill this void in the literature, we have dedicated our companion paper to development of the mouse clitoris, with comparison to human clitoral development (Cunha et al, 2019a). Human and mouse clitoral development proceed via completely different morphogenetic mechanisms (Baskin et al, 2018; Cunha et al, 2019a; Overland et al, 2016). Whereas the human clitoris develops directly from the embryonic genital tubercle (Baskin et al, 2018; Overland et al, 2016), the U-shaped mouse clitoral lamina, which defines the mouse clitoris (Martin-Alguacil et al, 2008a; Weiss et al, 2012), derives from the preputial lamina (Cunha et al, 2019a).
Penile development and adult anatomy are likewise different in mice and humans. While both the mouse and human penis develop from the genital tubercle, the mouse penis has a fibrocartilaginous MUMP, an os penis and several additional erectile bodies not present in the human penis. Formation of the penile urethra occurs via direct canalization of the urethral plate as well as via epithelial fusion events in both species, but the spatial readout of these morphogenetic mechanisms is exactly opposite in mouse versus human (Liu et al, 2018a). In humans, epithelial fusion events (fusion of the urethral folds) occurs in the shaft, whereas direct canalization of the urethral plate occurs within the glans. In mice, urethral plate canalization occurs proximally within the glans, while epithelial fusion events occur distally and are involved in formation of the urethral meatus (Baskin et al, 2018; Liu et al, 2018a; Liu et al, 2018b). It is not surprising that anomalies interpreted as hypospadias in mice bear little resemblance to human hypospadias (Cunha et al, 2015; Sinclair et al, 2016b).
The therapeutic administration of the synthetic estrogen, diethylstilbestrol (DES), to pregnant women from the mid 1940s to the early 1970s led to the appreciation of estrogens as trans-placental carcinogens and teratogens in humans. It also led to a remarkable confluence between the mouse as an animal model for the deleterious effects in humans of exogenous estrogens on the developing female reproductive tract and mammary gland (Bern et al, 1975). The association of prenatal DES with clear cell adenocarcinoma of the vagina in young women (Herbst et al, 1971) coupled with the pioneering studies from the laboratories of Howard Bern, John McLachlan and John Gunnar Forsberg revealed a remarkable correspondence between perinatally estrogen- induced lesions (carcinomatous and teratogenic) in mouse models and those seen in women exposed prenatally to DES (Bern, 1992; Bern and Talamantes, 1981; Forsberg, 1976; Forsberg and Kalland, 1981; Herbst and Bern, 1981; Jefferies et al, 1984; Kaufman and Adam, 2002; Kaufman et al, 1980; McLachlan, 1981; Robboy et al, 1984a; Titus- Ernstoff et al, 2010). Yet, to this day, no animal model has ever developed clear-cell vaginal adenocarcinoma. One lesion, cervico-vaginal adenosis, consisting of simple columnar epithelium and glands within the vagina, was observed in both perinatally DES-treated mice and women exposed in utero to DES. Adenosis of the tuboendometrial type, a benign abnormality of vaginal epithelial differentiation, is thought to be the substrate from which human clear-cell vaginal adenocarcinoma develops through an intermediate lesion called atypical adenosis (Robboy et al, 1984b). In mice, DES perturbs the normal expression of p63 during squamous differentiation of Müllerian vaginal epithelium leading to the retention of simple columnar p63-negative adenosis in the vagina (Kurita et al, 2004b). Adenosis has also been induced experimentally in human fetal vaginas by administration of DES to host mice bearing grafts of human fetal vagina (Cunha et al, 2017; Robboy et al, 1982a). Despite the remarkable concurrence between the mouse and human literature on this subject, it is surprising that evidence suggests that vaginal epithelial derivation is profoundly different in mice versus humans (Kurita, 2010; Robboy et al, 2017). In mice vaginal development culminates in formation of lining epithelium solely derived from the Müllerian ducts (Kurita, 2010). Studies utilizing Müllerian duct (PAX2) versus urogenital sinus epithelium (FOXA1) markers suggest that human vaginal epithelium derives from urogenital sinus epithelium (Robboy et al, 2017), supporting the earlier findings of Bulmer (Bulmer, 1957). The apparent differences in the derivation of vaginal epithelium in mice versus humans raises questions regarding the etiology of vaginal carcinogenesis in these two species.
The Müllerian ducts are the precursors of female internal genitalia in both mice and humans, and yet many human versus mouse anatomical and developmental differences are noted. Midline fusion of the Müllerian ducts is vastly different in mice versus humans, leading to a unicornuate uterus in humans and bicornuate uterine horns in mice. The cervix of mice is lined by a stratified squamous epithelium, while the human cervix contains a stratified squamous epithelium in the exocervix and simple columnar glandular epithelium in the endocervix. Cervical cancer is a significant health risk in human but is uncommon in mice. Models of cervical cancer in mice frequently utilize transgenic mice expressing proteins associated with human papillomavirus (Shai et al, 2010; Spurgeon et al, 2014). Cervico-vaginal cancer can be induced in mice by perinatal DES (Newbold and McLachlan, 1982). Such treatment also induces uterine carcinoma (Newbold et al, 1990), which is rarely seen in untreated mice.
While prostatic development is universally induced by androgens in all species, significant anatomic and development differences exist in mouse versus human prostate and prostatic pathology that include: (a) absence of a ventral prostatic lobe in humans versus the presence of a ventral prostatic lobe in mice, (b) presence of a thick collagenous capsule in human prostate and its absence in mice, (c) differences in the morphology of the verumontanum and associated ducts in human versus mouse, (d) human/mouse differences in smooth muscle amount and distribution in so far as prostatic ducts are surrounded by thin smooth muscle bundles in mouse, whereas smooth muscle is abundant throughout human prostatic stroma, (e) basal epithelial cells organized into as a continuous layer in human prostate and a discontinuous layer in mouse prostate. (f) ductal branching occurring in a mesenchymal matrix in mice, while ductal branching in human prostate occurs in both smooth muscle rich and smooth muscle deficient connective tissue. (g) Squamous metaplasia of prostatic ducts is a common feature of human prostate in the third trimester and does not occur in mice, presumably due to the sequestration of serum estrogens by alpha-fetoprotein in mice. Spontaneous prostatic hyperplasia and prostatic carcinoma is rare/absent in mice and very common in humans. The high incidence of prostatic carcinoma in humans versus the absence of spontaneous prostatic carcinoma in mice remains to be explained.
The present report highlights the many development and adult anatomic differences in male and female mouse versus human reproductive tract development. In this era of genetically engineered mice used as models of development, differentiation and pathogenesis, it is critical to be aware of useful and relevant homologies in development and adult anatomy. More to the point, the investigator should be aware of significant differences in morphogenesis, development, differentiation and adult anatomy that may render mouse models of questionable relevance to the human condition.
Acknowledgements:
This study was supported by NIH grant K12 DK083021 (to LSB and AWS).
References
- Aboseif S, El-Sakka A, Young P and Cunha G (1999) Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation 65:113–118. [DOI] [PubMed] [Google Scholar]
- Andrews GS (1951) The histology of the human foetal and prepubertal prostates. J Anat 85:44–54. [PMC free article] [PubMed] [Google Scholar]
- Atkins KA (2019) Normal Histology of the Uterus and Fallopian Tubes In: Mills S (ed) Histology for Pathologists. Lippincott, Philadelphia, pp 1059–1106. [Google Scholar]
- Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J and Rowley DR (2003) Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res 9:4792–4801. [PubMed] [Google Scholar]
- Baskin L, Shen J, Sinclair A, Cao M, Liu X, Liu G, Isaacson D, Overland M, Li Y and Cunha GR (2018) Development of the human penis and clitoris. Differentiation 103:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin LS (2000) Hypospadias and urethral development. J Urol 163:951–956. [PubMed] [Google Scholar]
- Baskin LS and Ebbers MB (2006) Hypospadias: anatomy, etiology, and technique. J Pediatr Surg 41:463–472. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Li YW and Cunha GR (1998) Anatomical studies of hypospadias. J Urol 160:1108–1115. [DOI] [PubMed] [Google Scholar]
- Bern HA (1992) The development of the role of hormones in development—a double remembrance. Endocrinology 131:2037–2038. [DOI] [PubMed] [Google Scholar]
- Bern HA, Jones LA, Mori T and Young PN (1975) Exposure of neonatal mice to steroids: longterm effects on the mammary gland and other reproductive structures. J. Steroid Biochem. 6:673–676. [DOI] [PubMed] [Google Scholar]
- Bern HA, Mills KT, Ostrander PL, Schoenrock B, Graveline B and Plapinger L (1984) Cervicovaginal abnormalities in BALB/c mice treated neonatally with sex hormones. Teratology 30:267–274. [DOI] [PubMed] [Google Scholar]
- Bern HA and Talamantes FJ (1981) Neonatal mouse models and their relation to disease in the humal female In: Herbst A and Bern HA (ed) Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc, New York, pp 129–147. [Google Scholar]
- Blaschko SD, Mahawong P, Ferretti M, Cunha TJ, Sinclair A, Wang H, Schlomer BJ, Risbridger G, Baskin LS and Cunha GR (2013) Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestrol-treated mice. Anatomical record 296:1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U and Remberger K (1994) The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 24:114–118. [DOI] [PubMed] [Google Scholar]
- Boroditsky RS, Reyes FI, Winter JS and Faiman C (1978) Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet Gynecol 51:686–691. [PubMed] [Google Scholar]
- Bostwick DG (1996) Prospective origins of prostate carcinoma. Prostatic intraepithelial neoplasia and atypical adenomatous hyperplasia. Cancer 78:330–336. [DOI] [PubMed] [Google Scholar]
- Bulmer D (1957) The development of the human vagina. J. Anat. 91:490–509. [PMC free article] [PubMed] [Google Scholar]
- Clemente CD Gray’s Anatomy. Philadelphia: Lea and Febiger, 1985. [Google Scholar]
- Couse JF and Korach KS (1999a) Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417. [DOI] [PubMed] [Google Scholar]
- Couse JF and Korach KS (1999b) Reproductive phenotypes in the estrogen receptor-alpha knockout mouse. Ann Endocrinol (Paris) 60:143–148. [PubMed] [Google Scholar]
- Cowin P and Wysolmerski J (2010) Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb Perspect Biol 2:a003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford ED, Miller GJ, Labrie F, Hirano D, Batuello J and Glode LM (2001) Prostate cancer pathology, screening, and epidemiology. Reviews in urology 3 Suppl 2:S2–S10. [PMC free article] [PubMed] [Google Scholar]
- Cunha GR (1976) Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J. Exp. Zool. 196:361–370. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N and Kinbara H (1992) Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol. 1:76–83. [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ and Sugimura Y (1987) The endocrinology and developmental biology of the prostate. Endocrine Rev. 8:338–362. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Dahiya R and Foster BA (1996) Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anatomica 155:63–72. [DOI] [PubMed] [Google Scholar]
- Cunha GR and Hom YH (1996) Role of mesenchymal-epithelial interactions in mammary gland development. J. Mammary Gland Biology and Neoplasia 1:21–35. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Kurita T, Cao M, Shen J, Cooke PS, Robboy SJ and Baskin LS (2018a) Tissue interactions and estrogenic response during human female fetal reproductive tract development. Differentiation 101:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Liu G, Sinclair A, Cao M and Baskin L (2019a) Mouse clitoral development and comparison to human clitoral development. Differentiation In Press: [DOI] [PubMed] [Google Scholar]
- Cunha GR, Liu G, Sinclair A, Cao M, Glickman S, Cooke PS and Baskin L (2019b) Androgen-independent events in penile development in humans and animals Differentiation (In Press): [DOI] [PubMed] [Google Scholar]
- Cunha GR, Robboy SJ, Kurita T, Isaacson D, Shen J, Cao M and Baskin LS (2018b) Development of the human female reproductive tract. Differentiation 103:46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Sekkingstad M and Meloy BA (1983) Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit, and human tissues. Differentiation 24:174–180. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Cao M and Baskin L (2019c) Development of the human prepuce. Differentiation (In Press): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Risbridger G, Hutson J and Baskin LS (2015) Current understanding of hypospadias: relevance of animal models. Nat Rev Urol 12:271–280. [DOI] [PubMed] [Google Scholar]
- Cunha GRT,K, Cao M, Shen J, Robboy S and L B (2017) Response of xenografts of developing human female reproductive tracts to the synthetic estrogen, diethylstilbestrol. Differentiation (In Press): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Vezina CM, Isaacson D, Ricke WA, Timms BG, Cao M, Franco O and Baskin LS (2018c) Development of the human prostate. Differentiation 103:24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson EB, Simmons JE and Nagamani M (1983) A comparison of serum free estriol with spontaneous urine total estrogen and estrogen/creatinine ratio. Int J Gynaecol Obstet 21:371–375. [DOI] [PubMed] [Google Scholar]
- Diwan BA, Anderson LM and Ward JM (1997) Proliferative lesions of oviduct and uterus in CD-1 mice exposed prenatally to tamoxifen. Carcinogenesis 18:2009–2014. [DOI] [PubMed] [Google Scholar]
- Driscoll SG and Taylor SH (1980) Effects of prenatal maternal estrogen on the male urogenital system. Obstet Gynecol 56:537–542. [PubMed] [Google Scholar]
- Finch PW, Cunha GR, Rubin JS, Wong J and Ron D (1995) Pattern of KGF and KGFR expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Developmental Dynamics 203:223–240. [DOI] [PubMed] [Google Scholar]
- Forsberg JG (1973) Cervicovaginal epithelium: its origin and development. Am J Obstet Gynecol 115:1025–1043. [DOI] [PubMed] [Google Scholar]
- Forsberg JG (1976) Animal model of human disease: adenosis and clear-cell carcinomas of vagina and cervix. Am J Pathol 84:669–672. [PMC free article] [PubMed] [Google Scholar]
- Forsberg JG and Kalland T (1981) Neonatal estrogen treatment and epithelial abnormalities in the cervicovaginal epithelium of adult mice. Cancer Res 41:721–734. [PubMed] [Google Scholar]
- Giordano SH (2018) Breast Cancer in Men. N Engl J Med 378:2311–2320. [DOI] [PubMed] [Google Scholar]
- Girman CJ, Jacobsen SJ, Guess HA, Oesterling JE, Chute CG, Panser LA and Lieber MM (1995) Natural history of prostatism: relationship among symptoms, prostate volume and peak urinary flow rate. J Urol 153:1510–1515. [DOI] [PubMed] [Google Scholar]
- Green EL (1966) Biology of the laboratory mouse. Jackson Laboratory [Google Scholar]
- Gupta N, Sivaramakrishna, Kumar R, Dogra PN and Seth A (2006) Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int 97:85–89. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Sugimura Y, Kawamura J, Donjacour AA and Cunha GR (1991) Morphological and functional heterogeneity in the rat prostatic gland. Biol. Reprod. 45:308–321. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Brody JR and Cunha GR (1996a) An edgewise look at basal cells: Three-dimensional views of the rat prostate, mammary gland and salivary gland. Differentiation 60:219–227. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Cunha GR and Dahiya R (1996b) Normal development and carcinogenesis of the prostate: A unifying hypothesis. Ann N Y Acad Sci. 784:50–62. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Rosen MA and Cunha GR (1997) Stromal-epithelial interactions in normal and neoplastic prostate. Br. J. Urology 79, Suppl. 2:18–26. [DOI] [PubMed] [Google Scholar]
- Herbst A and Bern H (1981) Developmental effects of DES in pregnancy. Thieme Stratton, New York. [Google Scholar]
- Herbst AL, Robboy SJ, Scully RE and Poskanzer DC (1974) Clear-cell adenocarcinoma of the vagina and cervix in girls: analysis of 170 registry cases. Am J Obstet Gynecol 119:713–724. [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H and Poskanzer DC (1971) Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. New Eng. J. Med. 284:878–881. [DOI] [PubMed] [Google Scholar]
- Horwitz KB and McGuire WL (1979) Estrogen control of progesterone receptor induction in human breast cancer: role of nuclear estrogen receptor. Adv Exp Med Biol 117:95–110. [DOI] [PubMed] [Google Scholar]
- Hynes PJ and Fraher JP (2004a) The development of the male genitourinary system: II. The origin and formation of the urethral plate. Br J Plast Surg 57:112–121. [DOI] [PubMed] [Google Scholar]
- Hynes PJ and Fraher JP (2004b) The development of the male genitourinary system: III. The formation of the spongiose and glandar urethra. Br J Plast Surg 57:203–214. [DOI] [PubMed] [Google Scholar]
- Janne O, Kontula K, Luukkainen T and Vihko R (1975) Oestrogen-induced progesterone receptor in human uterus. J Steroid Biochem 6:501–509. [DOI] [PubMed] [Google Scholar]
- Jefferies JA, Robboy SJ, O’Brien PC, Bergstralh EJ, Labarthe DR, Barnes AB, Noller KL, Hatab PA, Kaufman RH and Townsend DE (1984) Structural anomalies of the cervix and vagina in women enrolled in the Diethylstilbestrol Adenosis (DESAD) Project. Am J Obstet Gynecol 148:59–66. [DOI] [PubMed] [Google Scholar]
- Kaufman RH and Adam E (2002) Findings in female offspring of women exposed in utero to diethylstilbestrol. Obstet Gynecol 99:197–200. [DOI] [PubMed] [Google Scholar]
- Kaufman RH, Adam E, Binder GL and Gerthoffer E (1980) Upper genital tract changes and pregnancy outcome in offspring exposed in utero to diethylstilbestrol. Am J Obstet Gynecol 137:299–308. [DOI] [PubMed] [Google Scholar]
- Keil KP and Vezina CM (2015) DNA methylation as a dynamic regulator of development and disease processes: spotlight on the prostate. Epigenomics 7:413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff AK (1933) Development of the vagina in the human fetus. Contrib. Embryol. Carnegie Inst. Wash. 24:59–90. [PubMed] [Google Scholar]
- Kratochwil K and Schwartz P (1976) Tissue interaction in androgen response of embryonic mammary rudiment of mouse: Identification of target tissue of testosterone. Proc. Natl. Acad. Sci. USA 73:4041–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T (2010) Developmental origin of vaginal epithelium. Differentiation 80:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T (2011) Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation; research in biological diversity 82:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cooke PS and Cunha GR (2001) Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol 240:194–211. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA and Medina RT. (2005a) Differential expression of p63 isoforms in female reproductive organs. Mech Dev 122:1043–1055. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB and Cunha GR (2000) Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod 62:821–830. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL. and Gold LI (2005b) The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation 73:313–322. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA and Cunha GR (2004a) Role of p63 and basal cells in the prostate. Development 131:4955–4964. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA and Cunha GR (2004b) Roles of p63 in the diethylstilbestrol- induced cervicovaginal adenosis. Development 131:1639–1649. [DOI] [PubMed] [Google Scholar]
- Kurita T and Nakamura H (2008) Embryology of the uterus In: Aplin JD, Fazleabas AT, Glasser SR and Giudice LC (ed) Endometrium. Informa UK Ltd, London, pp 1–18. [Google Scholar]
- Larkins CE, Enriquez AB and Cohn MJ (2016) Spatiotemporal dynamics of androgen signaling underlie sexual differentiation and congenital malformations of the urethra and vagina. Proc Natl Acad Sci U S A 113:E7510–E7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sinclair A, Cao M, Shen J, Choudhry S, Botta S, Cunha G and Baskin L (2015) Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. J Urol 193:1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TM, Rasmussen NT, Moore RW, Albrecht RM and Peterson RE (2003) Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8- tetrachlorodibenzo-p-dioxin. Toxicol Sci 76:171–181. [DOI] [PubMed] [Google Scholar]
- Liu G, Liu X, Shen J, Sinclair A, Baskin L and Cunha GR (2018a) Contrasting mechanisms of penile urethral formation in mouse and human. Differentiation 101:46–64. [DOI] [PubMed] [Google Scholar]
- Liu TT, Thomas S, McLean DT, Roldan-Alzate A, Hernando D, Ricke EA and Ricke WA (2019) Prostate enlargement and altered urinary function are part of the aging process. Aging (Albany NY) 11:2653–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu G, Shen J, Yue A, Isaacson D, Sinclair A, Cao M, Liaw A, Cunha GR and Baskin L (2018b) Human glans and preputial development. Differentiation 103:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowsley OS (1912) The development of the human prostate gland with reference to the development of other structures at the neck of the urinary bladder. Am. J. Anat. 13:299–349. [Google Scholar]
- MacLaughlin DT and Donahoe PK (2002) Mullerian inhibiting substance: an update. Adv Exp Med Biol 511:25–38; discussion 38–40. [DOI] [PubMed] [Google Scholar]
- Mahawong P, Sinclair A, Li Y, Schlomer B, Rodriguez E Jr., Ferretti MM, Liu B, Baskin LS and Cunha GR (2014a) Comparative effects of neonatal diethylstilbestrol on external genitalia development in adult males of two mouse strains with differential estrogen sensitivity. Differentiation 88:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahawong P, Sinclair A, Li Y, Schlomer B, Rodriguez E Jr., Ferretti MM, Liu B, Baskin LS and Cunha GR (2014b) Prenatal diethylstilbestrol induces malformation of the external genitalia of male and female mice and persistent second-generation developmental abnormalities of the external genitalia in two mouse strains. Differentiation 88:51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth C, Daxenbichler G, Buehring GC, Hofstadter F and Dapunt O (1984) Inhibition of the estradiol-induced growth of cultured human breast cancer cells by the anti-estrogens tamoxifen, desmethyl-tamoxifen, 4-hydroxy-tamoxifen and enclomiphene. Biochem Pharmacol 33:3951–3956. [DOI] [PubMed] [Google Scholar]
- Martin-Alguacil N, Pfaff DW, Shelley DN and Schober JM (2008a) Clitoral sexual arousal: an immunocytochemical and innervation study of the clitoris. BJU Int 101:1407–1413. [DOI] [PubMed] [Google Scholar]
- Martin-Alguacil N, Schober J, Kow LM and Pfaff D (2008b) Oestrogen receptor expression and neuronal nitric oxide synthase in the clitoris and preputial gland structures of mice. BJU Int 102:1719–1723. [DOI] [PubMed] [Google Scholar]
- McLachlan J (1981) Rodent models for perinatal exposure to diethylstilbestrol and their relation to human disease in the male In: Herbst A and Bern HA (ed) Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc, New York, pp 148–157. [Google Scholar]
- McLachlan JA, Newbold RR and Bullock B (1980) Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 40:3988–3999. [PubMed] [Google Scholar]
- McNally S and Stein T (2017) Overview of Mammary Gland Development: A Comparison of Mouse and Human. Methods Mol Biol 1501:1–17. [DOI] [PubMed] [Google Scholar]
- McNeal JE (1981) The zonal anatomy of the prostate. Prostate 2:35–49. [DOI] [PubMed] [Google Scholar]
- McNeal JE (1983) The prostate gland: morphology and pathobiology. Monogr. Urology 4:3–37. [Google Scholar]
- McNeal JE (1985) Morphology and biology of benign prostatic hyperplasia In: Bruchovsky N, Chapdellaine A and Neumann F (ed) Regulation of androgen action. Congressdruck R. Bruckner, Berlin, pp 191–197. [Google Scholar]
- Miniati DN, Chang Y, Shu WP, Peehl DM and Liu BC (1996) Role of prostatic basal cells in the regulation and suppression of human prostate cancer cells. Cancer Lett 104:137–144. [DOI] [PubMed] [Google Scholar]
- Moseson DL, Sasaki GH, Kraybill WG, Leung BS, Davenport CE and Fletcher WS (1978) The use of antiestrogens tamoxifen and nafoxidine in the treatment of human breast cancer in correlation with estrogen receptor values. A phase II study. Cancer 41:797–802. [DOI] [PubMed] [Google Scholar]
- Nandi S (1958) Endocrine control of mammary gland development and function in the C3H/He Crgl mouse. J. Natl. Inst. 21:1039–1063. [PubMed] [Google Scholar]
- Nanjappa MK, Medrano TI, March AG and Cooke PS (2015) Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biol Reprod 92:78. [DOI] [PubMed] [Google Scholar]
- Nanjappa MK, Mesa AM, Medrano TI, Jefferson WN, DeMayo FJ, Williams CJ, Lydon JP, Levin ER and Cooke PS (2019) The histone methyltransferase EZH2 is required for normal uterine development and function in mice. Biol Reprod [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S and Parada LF (2000) Hormones in male sexual development. Genes Dev 14:3075–3086. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Bullock BC. and McLachlan JA (1990) Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res 50:7677–7681. [PubMed] [Google Scholar]
- Newbold RR and McLachlan JA (1982) Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 42:2003–2011. [PubMed] [Google Scholar]
- Nicholson TM, Moses MA, Uchtmann KS, Keil KP, Bjorling DE, Vezina CM, Wood RW and Ricke WA (2015) Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol 193:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW and Ricke WA (2012) Testosterone and 17beta- estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 153:5556–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly R (1973) The embryology and anatomy of the uterus In: Norris HJ, Hertig AT and Abell MR (ed) The Uterus. Williams and Wilkins Co, Baltimore, pp 17–39. [Google Scholar]
- O’Rahilly R (1977) Prenatal human development In: Wynn RM (ed) Biology of the Uterus. Plenum Press, New York, pp 35–57. [Google Scholar]
- Ogasawara Y, Okamoto S, Kitamura Y and Matsumoto K (1983) Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I]iododeoxyuridine. Endocrinology 113:582–587. [DOI] [PubMed] [Google Scholar]
- Ohno S (1979) Major Sex Determining Genes. Springer-Verlag, New York. [Google Scholar]
- Olumi AF, Dazin P and Tlsty TD (1998) A novel coculture technique demonstrates that normal human prostatic fibroblasts contribute to tumor formation of LNCaP cells by retarding cell death. Cancer Res 58:4525–4530. [PubMed] [Google Scholar]
- Overland M, Li Y, Cao M, Shen J, Yue X, Botta S, Sinclair A, Cunha G and Baskin L (2016) Canalization of the Vestibular Plate in the Absence of Urethral Fusion Characterizes Development of the Human Clitoris: The Single Zipper Hypothesis. J Urol 195:1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang SF, Caggiula AR, Gay VL, Goodman RL and Pang CS (1979) Serum concentrations of testosterone, oestrogens, luteinizing hormone and follicle- stimulating hormone in male and female rats during the critical period of neural sexual differentiation. J Endocrinol 80:103–110. [DOI] [PubMed] [Google Scholar]
- Prakash K, Pirozzi G, Elashoff M, Munger W, Waga I, Dhir R, Kakehi Y and Getzenberg RH (2002) Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci U S A 99:7598–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Wu D and Gong C (2017) Persistent Mullerian duct syndrome: A case report and review. Exp Ther Med 14:5779–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW and Anderson SM (2000) An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 5:227–241. [DOI] [PubMed] [Google Scholar]
- Ricke WA, Timms BG and vom Saal F (2018) Prostate structure In: Skinner M (ed) Encyclopedia of reproduction. Academic Press, London, pp XX. [Google Scholar]
- Risbridger G, Wang H, Frydenberg M and Cunha GR (2001) The metaplastic effects of estrogen on prostate epithelium: Proliferation of cells with basal cell phenotype. Endocrinology 142:2443–2450. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Cunha GR, Kurita T and Strickland KC (2019) Vagina In: Mills S (ed) Histology for Pathologists. Lippincott, Philadelphia, pp 1047–1058. [Google Scholar]
- Robboy SJ, Hill EC, Sandberg EC and Czernobilsky B (1986) Vaginal adenosis in women born prior to the diethylstilbestrol era. Hum Pathol 17:488–492. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Kurita T, Baskin L and Cunha GR (2017) New insights insights into human female reproductive tract development. Differentiation 97:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robboy SJ, Noller KLRH,K, Barnes AB, Townsend D, Gunderson JH, Kurland L and Nash S (1984a) An atlas of findings in the human female after intrauterine exposure to diethylstilbestrol. DHEW publication 84–2344, [Google Scholar]
- Robboy SJ, Taguchi O and Cunha GR (1982a) Normal development of the human female reproductive tract and alterations resulting from experimental exposure to diethylstilbestrol. Human Pathol. 13:190–198. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Welch WR, Young RH, Truslow GY, Herbst AL and Scully RE (1982b) Topographic relation of cervical ectropion and vaginal adenosis to clear cell adenocarcinoma. Obstet Gynecol 60:546–551. [PubMed] [Google Scholar]
- Robboy SJ, Young RH, Welch WR, Truslow GY, Prat J, Herbst AL and Scully RE (1984b) Atypical vaginal adenosis and cervical ectropion. Association with clear cell adenocarcinoma in diethylstilbestrol-exposed offspring. Cancer 54:869–875. [DOI] [PubMed] [Google Scholar]
- Rodriguez E Jr., Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha G and Baskin L (2012) Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation 84:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E Jr., Weiss DA, Yang JH, Menshenina J, Ferretti M, Cunha TJ, Barcellos D, Chan LY, Risbridger G, Cunha GR and Baskin LS (2011) New insights on the morphology of adult mouse penis. Biol Reprod 85:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrborn CG, McConnell JD, Lieber M, Kaplan S, Geller J, Malek GH, Castellanos R, Coffield S, Saltzman B, Resnick M, Cook TJ and Waldstreicher J (1999) Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 53:473–480. [DOI] [PubMed] [Google Scholar]
- Schlomer BJ, Feretti M, Rodriguez E Jr., Blaschko S, Cunha G and Baskin L (2013) Sexual differentiation in the male and female mouse from days 0 to 21: a detailed and novel morphometric description. J Urol 190:1610–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD and Cohn MJ (2008) Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol 318:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai A, Pitot HC and Lambert PF (2010) E6-associated protein is required for human papillomavirus type 16 E6 to cause cervical cancer in mice. Cancer Res 70:5064–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri PK and Wilson JD (1974) Testosterone formation and metabolism during male sexual differentiation in the human embryo. J. Clin. Endocrinol. Metab. 38:113–125. [DOI] [PubMed] [Google Scholar]
- Simmons MN and Jones JS (2007) Male genital morphology and function: an evolutionary perspective. J Urol 177:1625–1631. [DOI] [PubMed] [Google Scholar]
- Sinclair AW, Cao M, Baskin L and Cunha GR (2016a) Diethylstilbestrol-induced mouse hypospadias: “window of susceptibility”. Differentiation 91:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AW, Cao M, Pask A, Baskin L and Cunha GR (2017) Flutamide-induced hypospadias in rats: a critical assessment. Differentiation 94:37–57. [DOI] [PubMed] [Google Scholar]
- Sinclair AW, Cao M, Shen J, Cooke P, Risbridger G, Baskin L and Cunha GR (2016b) Mouse hypospadias: A critical examination and definition. Differentiation 92:306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AW, Glickman SE, Baskin L and Cunha GR (2016c) Anatomy of mole external genitalia: Setting the record straight. Anat Rec (Hoboken) 299:385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon ME, Chung SH and Lambert PF (2014) Recurrence of cervical cancer in mice after selective estrogen receptor modulator therapy. Am J Pathol 184:530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srigley JR, Dardick I, Hartwick RW and Klotz L (1990) Basal epithelial cells of human prostate gland are not myoepithelial cells. A comparative immunohistochemical and ultrastructural study with the human salivary gland. Am J Pathol 136:957–966. [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR and Donjacour AA (1986a) Morphogenesis of ductal networks in the mouse prostate. Biol. Reprod. 34:961–971. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR and Donjacour AA (1986b) Prostatic glandular architecture: Wholemount analysis of morphogenesis and androgen dependency In: Rodgers CH, Coffey DS, Cunha GR, Grayhack JT, Hinman F and Horton R (ed) Benign Prostatic Hyperplasia. U.S. Govt. Printing Office, Washington, D.C., pp 55–72. [Google Scholar]
- Swartz SK and Soloff MS (1974) The lack of estrogen binding by human alpha- fetoprotein. J Clin Endocrinol Metab 39:589–591. [DOI] [PubMed] [Google Scholar]
- Takamatsu Y, Iguchi T and Takasugi N (1992) Effects of postpubertal treatment with diethylstilbestrol and tamoxifen on protein expression in the vagina and uterus of neonatally diethylstilbestrol-exposed mice. In Vivo 6:271–278. [PubMed] [Google Scholar]
- Thomson AA and Cunha GR (1999) Prostatic growth and development are regulated by FGF10. Development 126:3693–3701. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Timms BG, Barton L, Cunha GR and Grace OC (2002) The role of smooth muscle in regulating prostatic induction. Development 129:1905–1912. [DOI] [PubMed] [Google Scholar]
- Timms B, Lee C, Aumuller G and Seitz J (1995) Instructive induction of prostate growth and differentiation by a defined urogenital sinus mesenchyme. Microscopy Res. and Technique 30:319–332. [DOI] [PubMed] [Google Scholar]
- Timms BG (2008) Prostate development: a historical perspective. Differentiation 76:565–577. [DOI] [PubMed] [Google Scholar]
- Timms BG and Hofkamp LE (2011) Prostate development and growth in benign prostatic hyperplasia. Differentiation 82:173–183. [DOI] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K and Hoover RN (2010) Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). Int J Androl 33:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga T, Hayashi T, Honda E, Kikuchi N and Utsunomiya H (1988) [A case of male acromegaly with galactorrhea]. No Shinkei Geka 16:1101–1105. [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD and Rowley DR (2002) Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8:2912–2923. [PubMed] [Google Scholar]
- Uriel J, Bouillon D, Aussel C and Dupiers M (1976) Alpha-fetoprotein: the major high-affinity estrogen binder in rat uterine cytosols. Proc Natl Acad Sci U S A 73:1452–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltmaat JM, Mailleux AA, Thiery JP and Bellusci S (2003) Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 71:1–17. [DOI] [PubMed] [Google Scholar]
- Vezina CM and Bushman AW (2007) Hedgehog signaling in prostate growth and benign prostate hyperplasia. Curr Urol Rep 8:275–280. [DOI] [PubMed] [Google Scholar]
- Walters KA, Simanainen U and Handelsman DJ (2010) Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Human reproduction update 16:543–558. [DOI] [PubMed] [Google Scholar]
- Weiss DA, Rodriguez E Jr., Cunha T, Menshenina J, Barcellos D, Chan LY, Risbridger G, Baskin L and Cunha G (2012) Morphology of the external genitalia of the adult male and female mice as an endpoint of sex differentiation. Molecular and cellular endocrinology 354:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland RG, Folk RL, Taylor JN and Hamwi GJ (1967) Studies of male hypogonadism. I. Androgen metabolism in a male with gynecomastia and galactorrhea. J Clin Endocrinol Metab 27:763–767. [DOI] [PubMed] [Google Scholar]
- Wilson J, George F and Griffin J (1981) The hormonal control of sexual development. Science 211:1278–1284. [DOI] [PubMed] [Google Scholar]
- Wilson JD (1987) Disorders of androgen action. Clinical research 35:1–12. [PubMed] [Google Scholar]
- Wong YC and Tam NN (2002) Dedifferentiation of stromal smooth muscle as a factor in prostate carcinogenesis. Differentiation 70:633–645. [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, Miles BJ, Wheeler TM and Ayala GE (2007) Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol 38:1611–1620. [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N and Baskin LS (2010) Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. The Journal of urology 184:1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L and Chang C (2002) Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A 99:13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura CY, Cunha GR, Sugimura Y and Mee SL (1995) Temporal and spatial factors in diethylstilbestrol-induced squamous metaplasia in the developing human prostate. II. Persistent changes after removal of diethylstilbestrol. Acta Anat (Basel) 153:1–11. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Armfield BA and Cohn MJ (2015) Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc Natl Acad Sci U S A 112:E7194–7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondek LH and Zondek T (1979) Effect of hormones on the human fetal prostate. Contrib Gynecol Obstet 5:145–158. [PubMed] [Google Scholar]