Supplemental Digital Content is available in the text.
Abstract
Background:
Hair transplantation is the only method available to regrow new hairs; hence, enhancing the results of this procedure using state-of-the-art methods has become mandatory in clinical practice. Recent studies have suggested that significant improvements in hair density and stimulation of hair growth occur when follicular units are pretreated with platelet plasma growth factors before implantation. This study aimed to investigate and compare the outcomes of this procedure using platelet-rich plasma (PRP)–preserved hair grafts and saline-preserved hair grafts.
Methods:
This is a randomized controlled study. The study included 27 men and 3 women aged 22–51 years. Clinical examination (general and local) and preoperative marking were performed in these patients. The surgical technique involved graft extraction, PRP preparation, and hair implantation. Postsurgical patient satisfaction and clinical improvement were evaluated.
Results:
There were significant differences between the groups in hair uptake and hair thickness after 1 year follow-up, with P value <0.05. Using PRP therapy with follicular unit extraction increases the success of follicular unit extraction hair transplantation. All participants in the PRP group had >75% hair regrowth after 6 months. They had more rapid improvements in hair density and skin recovery than those in the non-PRP group.
Conclusion:
Preserving hair grafts in PRP before implantation increases the hair density, the graft uptake, and the hair thickness compared with pretreatment preservation of hair grafts in saline.
INTRODUCTION
Androgenetic alopecia (AA) is a genetically determined phenomenon defined by a gradual hair loss and reduction in normally thick hair follicles. AA affects up to 30% of men over the age of 30 years and 50% of men over the age of 50 years. It also affects women.1
Platelet-rich plasma (PRP) is created through concentrating platelets found in whole blood. It can aid in tissue regeneration, bone regeneration, and wound repair.2–7 PRP treatment has also been suggested to promote hair growth, encourage cell survival and proliferation, and prolong the anagen phase of the hair cycle.8–12 PRP is thought to exert its effects on AA via delivery of concentrated growth factors to the hair follicle and surrounding area, so it is recommended in the treatment of AA.2
For many years, several procedures have been used for treating male pattern baldness. These include punch grafts, strip grafts, scalp flaps, scalp reductions, and tissue expansion. These techniques tend to take too many sessions, leaving noticeable scarring and yielding unappealing results.4
Recently, an autologous alternative has emerged not only for the intradermal activation of dormant hair follicles but also as an adjuvant therapy for the follicular unit extraction (FUE) technique. The plasma rich in growth factors (PRGF) technology is based on the recovery of a small volume of the patient’s own blood, which is afterward centrifuged and activated to obtain an autologous formulation enriched in proteins and growth factors. The use of this approach enhances the organism’s self-healing ability and promotes tissue renewal, thus providing a therapeutic option for hair follicle regeneration.5
For patients who require significant hair coverage, follicular unit hair transplantation and extraction have become the gold standard surgical methods for providing a natural-looking hairline and the illusion of a headful hair. Various strategies have been developed to increase the efficacy of the transplantation. The crucial discovery of the influence of platelet-derived growth factors in wound healing, tissue regeneration, angiogenesis, chemotaxis, cell proliferation, differentiation, immune modulation, remodeling, and antimicrobial activity has resulted in the development of novel autologous therapeutic methods. A number of platelet concentrates can be prepared for therapeutic applications.6
Promising clinical results were achieved using PRP, though the mechanism of action is not fully understood. It is known that the alpha granules of platelets that have various growth factors are released upon activation of platelets-derived growth factors, beta, fibroblast, which may explain its efficiency.5
Significant improvements in hair density and hair growth stimulation were observed when follicular units were pretreated with platelet plasma growth factors before implantation.7 The aim of our study was to investigate and compare the outcomes of hair transplantation using PRP-preserved hair grafts versus saline-preserved hair grafts.
METHODS
This single, prospective, randomized comparative study was conducted at a private clinic between June 2017 and March 2019. The study was approved by the ethics committee of the Faculty of Medicine, Cairo University, Egypt (IRB 257). The subjects were 30 patients [27 men and 3 women, aged between 22 and 51 years (average, 34 years)] with AA who underwent an FUE type of hair transplantation.
The men were classified according to the Norwood classification (grades 2–6) and the women according to the Ludwig classification (grades 1–3). All patients provided informed consent before participating in the study.
Inclusion Criteria
The inclusion criteria were as follows: (1) age between 20 and 55 years, (2) men with AA and classification of AA into grades 2, 3, 4, 5, or 6 according to the Norwood scale of baldness, (3) women with AA and classification of AA into grades 1, 2, or 3 according to the Ludwig scale of baldness, (4) men with AA, and (5) women with AA.
Exclusion Criteria
The exclusion criteria were as follows: (1) age below 20 years or above 55 years, (2) classification of AA into grade 1 or 7 according to the Norwood scale of baldness, (3) patients with thyroid disorders, (4) patients with bleeding disorders, (5) patients with diabetes, (6) smokers, (7) patients on corticosteroids or immunosuppressive drugs, (8) women with diffuse hair thinning, and (9) men and women with cicatricial alopecia.
Hair Density and Hair Thickness Analysis
Before treatment, patient’s profile photographs (frontal view, lateral view, cephalic view, and occipital view) were taken. Furthermore, the hairs were assessed using a digital trichoscope. Trichoscope is a method of hair and scalp evaluation, which can be used for the diagnosis of certain hair- or scalp-related diseases. Hair and scalp structures can be visualized at higher magnifications. After exclusion of hair or scalp diseases, the trichoscope was used to evaluate and calculate hair density by measuring the number of hairs in 1/2 cm2 area, and then the total number of available hairs for grafting was calculated. Finally, the size of the bald area was assessed and the number of hairs needed in this area was estimated.
The trichoscope (Co Cam, USB-225; SOMETECH Inc, Seoul, Korea) (Fig. 1) was also used to evaluate hair thickness before and after transplantation and to provide an excellent diagnosis of the scalp condition. (See figures, Supplemental Digital Content 1, which display A, The hair system of the trichoscope; B, How to calculate hair density; and C, How to evaluate hair thickness, http://links.lww.com/PRSGO/B407.)
Fig. 1.
The trichoscope device. A, Superior surface. B, Inferior surface.
Fields to be analyzed were as follows: A zone, from hair line to a line 10 cm behind it; C zone, a circle 10 cm diameter centered on vertex; B zone, between A zone and C zone and donor area between both ears (see figures, Supplemental Digital Content 1, http://links.lww.com/PRSGO/B407). A magnification of 100× was used to provide a clear view of hair and scalp (see figures, Supplemental Digital Content 1, http://links.lww.com/PRSGO/B407).
PRP Preparation
During the FUE, 20 ml blood was drawn from the median cubital vein, via venipuncture and then transferred into vacutainer tubes containing acid citrate dextrose or sodium citrate. A ratio of 1 ml of anticoagulant to 7–8 ml of whole blood was maintained. The aspirated blood was gently agitated to thoroughly mix the anticoagulant with the blood.
The blood was centrifuged using a slow speed (soft spin, 101G) for 5 minutes to avoid spinning down the platelets so that they remained suspended in the supernatant plasma. The supernatant plasma containing the platelets was transferred into another sterile tube. The tube was then centrifuged at a higher speed (hard spin, 280G) for another 5 minutes to obtain a platelet at the bottom of the tube and a platelet-poor plasma (PPP) at the top. (See figure, Supplemental Digital Content 2, which displays preparation of PRP, http://links.lww.com/PRSGO/B408.)
The mean platelet count in group 1 was 250 × 109/L, and it was 240 × 109/L in group 2. In the 15 patients undergoing preservation of grafts in PRP, 2 ml of PRP was added to every 500 grafts by pouring from a syringe; thus, grafts were kept in activated PRP from the break to the end of the procedure, that is, for around 3 hours (Fig. 2).
Fig. 2.

Grafts aligned in 10 lines on wet gauze on the sterile dish; the procedure was continued until an adequate number of grafts had been obtained.
The patients were divided randomly using a closed envelope randomization method. The first group (1) included 15 patients for whom grafts were preserved in normal saline. The second group (2) included 15 patients for whom grafts were preserved in PRP. The operative times ranged from 4 to 8 hours, with an average of 6 hours.
Patients were blinded about which treatment they received, and the doctor who evaluated the patients was blinded about which treatment the patients received. Transplantation was performed by the same technician for all patients. FACE-Q scales were used to evaluate the satisfaction of patients undergoing hair transplantation surgery. The FACE-Q scales are a valid and reliable tool for patients undergoing hair transplantation.
Statistical Analysis
Data were described as mean ± SD, median and range, or frequencies (number of cases) and percentages, where appropriate. Microsoft Excel 2013 (Microsoft Corp., Redmond, Wash.) was used for data entry, and the Statistical Package for Social Science (SPSS version 21; IBM Corp., Armonk, N.Y.) was used for data analysis. Simple descriptive statistics (arithmetic mean and SD) were used for summarizing normal quantitative data; median and interquartile ranges were used for summarizing skewed quantitative data, and frequencies were used for qualitative data. A Mann–Whitney U test was used to compare non-normally distributed quantitative data. A P value <0.05 was considered to represent statistical significance.
RESULTS
In the PRP group, the age of the patients ranged from 27 to 51 years (mean 36 years), and in the saline group, from 22 to 48 years (mean 33 years), with an SD of 6 in the PRP group and 7 in the saline group (Fig. 3). There were no significant differences between the 2 groups regarding age (P = 0.136). There was no difference in sex distribution between the 2 study groups (P = 0.640).
Fig. 3.
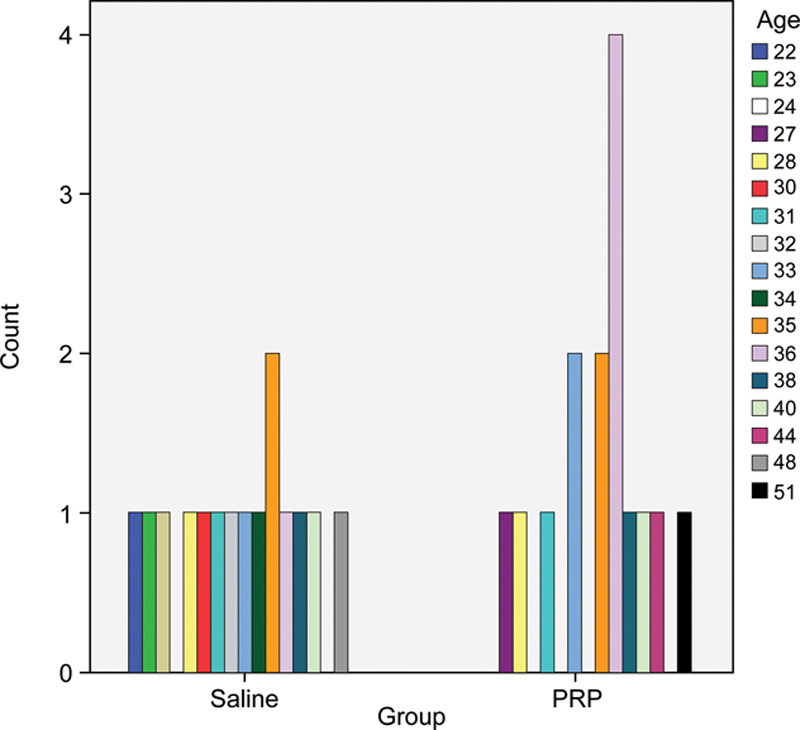
The age distribution in both study groups.
Grades of alopecia ranged from 3–6 (Fig. 4). These did not differ between the 2 study groups (P = 0.580). There was no significant difference between the 2 groups regarding the number of transplanted hairs per session (3500–4500 hairs in the saline group versus 3000–5000 hairs in the PRP group) (P = 0.234). There was no statistical difference between the 2 study groups regarding hair quantity pretreatment (cm2) or hair thickness (Fig. 5).
Fig. 4.
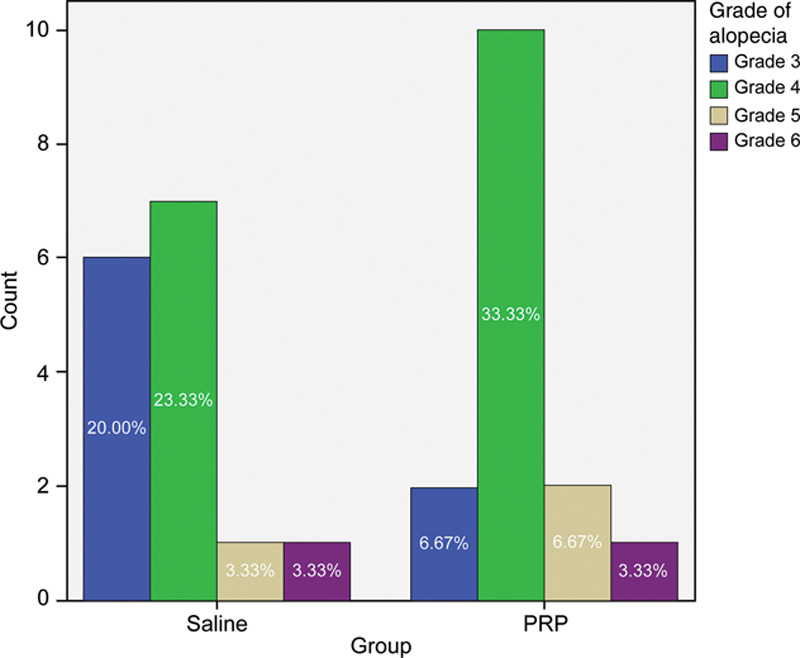
The distribution of the alopecia grade in both study groups.
Fig. 5.
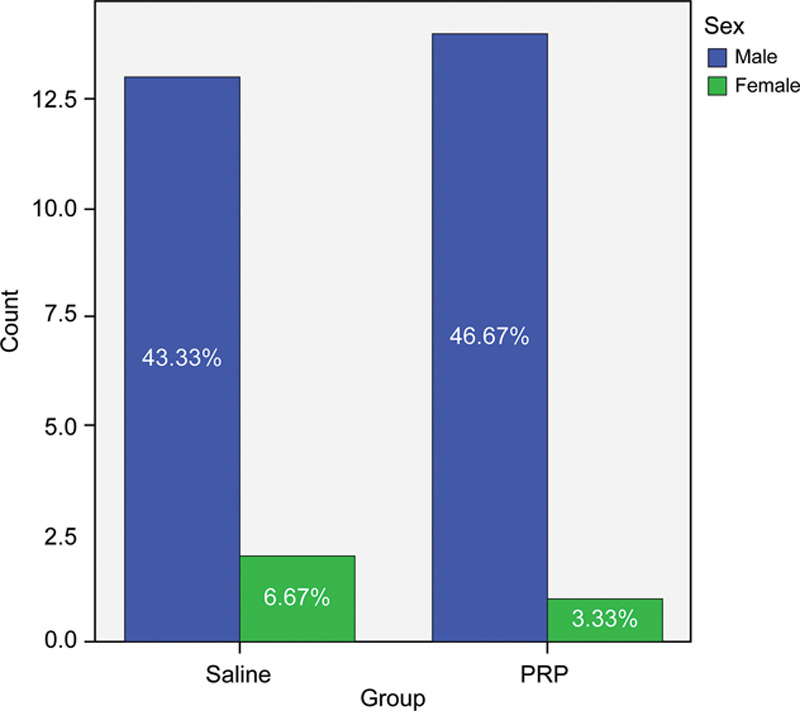
The sex distribution in both study groups.
Our results had significant difference in the yield of follicular units on the scalp in the experimental group compared with the yield in the control group (18.7 follicular units per cm2 versus 16.4 follicular units per cm2), and an increase in follicular density of 15.1% among patients who are treated with saline-preserved hair grafts. A value of P < 0.001 was considered a significant difference (Fig. 6).
Fig. 6.
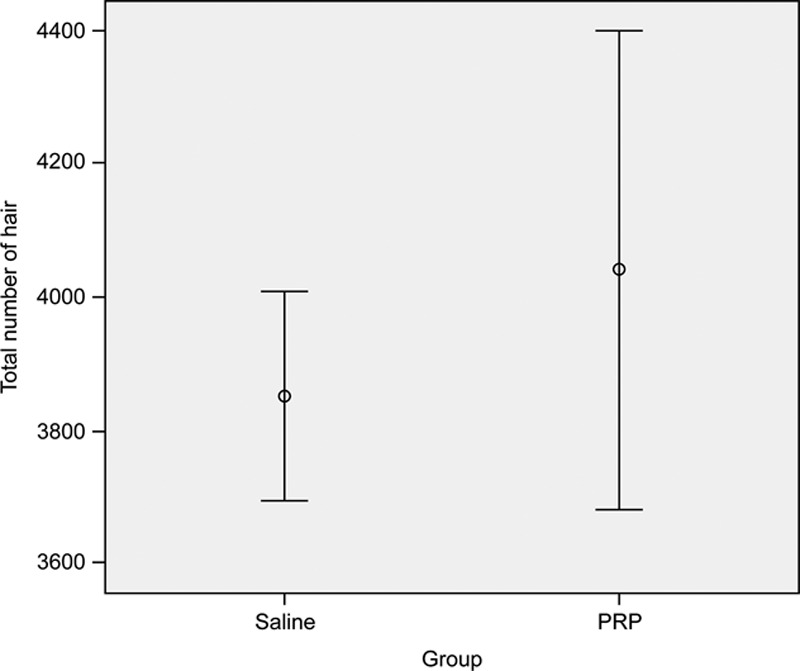
Comparison between the 2 study groups with regard to the total number of transplanted hairs.
In our study, hair follicle density reached its peak at 3 months (170.7 ± 37.8; P < 0.001). At 6 months and 1 year, hair density had increased significantly (196.25 ± 37.7; P < 0.001 and 203.7 ± 39.9; P < 0.001, respectively) compared with that at the baseline. Furthermore, patients were satisfied with a mean satisfaction rate of 7.1 on a scale of 1–10.
In the saline group, hair follicle density reached at 3 months (130.5 ± 27.4; P < 0.001). At 6 months and 1 year, hair density had increased significantly (156.25 ± 37.7; P < 0.001 and 173.7 ± 39.9; P < 0.001, respectively) compared with that at the baseline. Furthermore, patients were satisfied with a mean satisfaction rate of 5 on a scale of 1–10 (Fig. 7). Hair follicle densities in the intervention were 40 units/cm2 for saline, 70 units/cm2 for PRP, and 100 units/cm2 for the control group (Fig. 8).
Fig. 7.
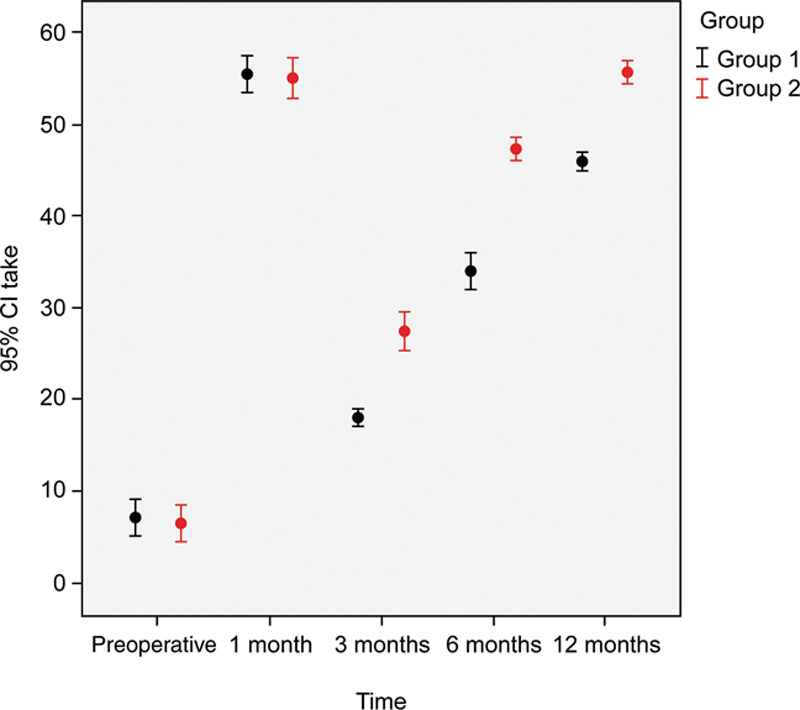
Comparison of graft uptake in both study groups through the study period.
Fig. 8.
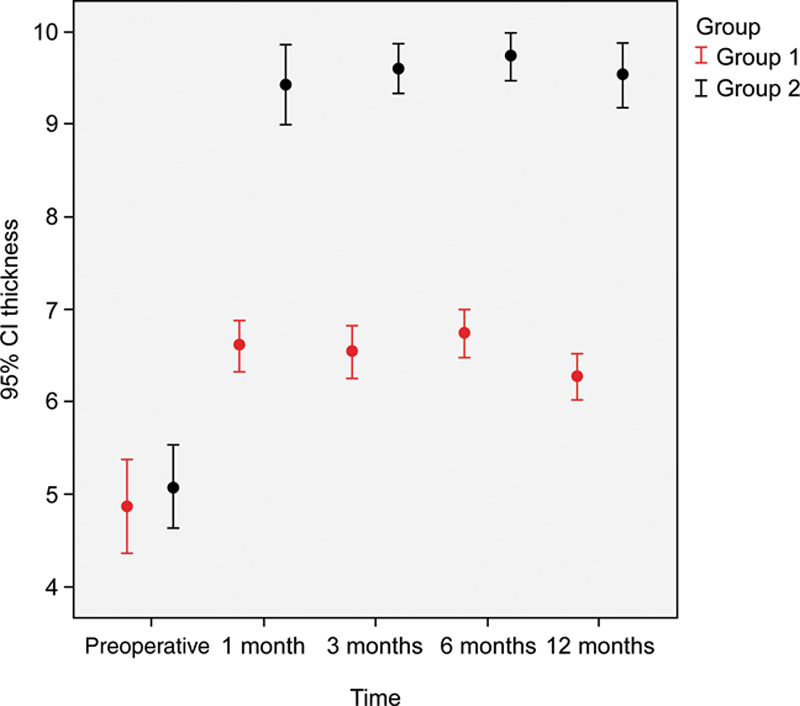
Comparison of hair thickness in both study groups through the study period.
There was no statistical difference between the 2 groups regarding the percentage of hair graft uptake at the 1-month follow-up. On the other hand, there was a significant difference in the percentage of hair graft uptake posttransplantation in both groups at the 3-, 6-, and 12-month follow-ups (P < 0.001). There was also a significant difference in hair thickness at the 1-, 3-, 6-, and 12-month follow-ups.
Using PRP therapy with FUE increases the success of FUE hair transplantation. All participants in the PRP group had >75% hair regrowth after 6 months. They had more rapid improvements in hair density and skin recovery than those in the non-PRP group.3
DISCUSSION
Hair loss is a widespread nonmalignant condition with varying severity, age of onset, and scalp location. Hair is an important part of one’s self-image, and in most societies, hair is associated with youth, health, and virility. A bald scalp is characterized by high levels of potent androgen (dihydro testosterone) and increased expression of the androgen receptor gene.8
This disease affects >2.1 million people worldwide, and by the age of 70 years, 80% of the population will probably develop some type of hair loss. Alopecia is more prevalent in men than in women (70% and 40%, respectively), but severe psychological disturbance has been reported in both genders, including psychiatric symptoms such as depression and anxiety that reduce the quality of life. Hence, an appropriate diagnosis and accurate treatments against follicular degeneration are dermatologic requirements to specifically target the disease.7
Male pattern baldness is classified into 7 types according to the Norwood classification, starting from mild recession of the frontal hair (type I) to loss of the entire scalp hair, except over the temporal and occipital areas (type VII).
Although an extensive list of causes of alopecia exists for both men and women, the most common type remains AA. AA is a genetically determined phenomenon defined by gradual loss and reduction in normally thick hair follicles. AA affects up to 30% of men over the age of 30 years and 50% of men over the age of 50 years. It can also affect women. Various strategies have been developed to increase the efficacy of the method.7
PRP therapy improves the skin milieu of grafted area by cell growth and differentiation, antiapoptotic activity, and neovascularization, making grafted areas more receptive and fertile for newly transplanted hair. It also helps in providing conducive growth environment for dormant hair follicles, leading to their activity and appearance of new regained hair as early as 3 months.9 Most studies suggest that subcutaneous injection of PRP is likely to reduce hair loss and increase hair diameter and density in patients with AA.10
The literature reports a significant improvement in hair density and stimulation of growth when follicular units were pretreated with PRP before implantation1 comparable with our results that there was a significant difference in the yield of follicular units on the scalp in the experimental group compared with the control group (18.7 follicular units per cm2 versus 16.4 follicular units per cm2), which represented an increase in follicular density of 15.1%.
Hair loss was reduced with the use of PRP, and at 3 months, it reached normal levels.7 In our study, hair density reached its peak at 3 months (170.7 ± 37.8; P < 0.001). At 6 months and 1 year, hair density had increased significantly (196.25 ± 37.7; P < 0.001 and 203.7 ± 39.9; P < 0.001, respectively) compared with that at the baseline. Furthermore, patients were satisfied with a mean satisfaction rate of 7.1 on a scale of 1–10.
In this study, we used saline-preserved hair grafts using the FUE technique in group A. While in group B, we used PRP-preserved hair grafts, which resulted in a significantly increased percentage of hair graft uptake in the implanted area after 1 year (93.3% versus 76.6% in the saline group).
In 2012, Araki et al5 reported a significant difference in the number of newly formed follicles in the area of transplantation when PRP was used (344 ± 27 with PRP versus 288 ± 35 without PRP). PRP also shortened the time of hair formation significantly, and the first hairs were observed after 18 days using PRP versus after 20 ± 1 days without PRP. Therefore, PRP has a considerable positive effect on the time of hair formation and the yield of hairs following transplantation.
A significant visual difference in the hair cross-section, but not in hair numbers, was observed with the use of PRP.5 Microscopic findings showed a thickened epithelium, proliferation of collagen fibers and fibroblasts, and increased vessels around the follicles.
The effect of autologous PRP injections on the affected area of alopecia was studied.3 Three months after the treatment, the patients presented with clinical improvements in hair count, hair thickness, hair root strength, and overall alopecia.
In 2016, Mahapatra et al11 conducted a study involving 177 patients. They reported significantly increased local hair numbers per square centimeter after PRP injections, compared with controls (mean difference, 17.90; 95% confidence interval, 5.84–29.95; P = 0.004). Similarly, they reported a significantly increased hair thickness cross section per 10−4 mm2 (mean difference, 0.22; 95% confidence interval, 0.07–0.38; P = 0.005), favoring the PRP group.
This study has several limitations. Our study included a small number of participants and a short follow-up period. In this study, we demonstrated that pretreatment of hair grafts with PRP can significantly increase hair thickness after 12 months. Therefore, the use of PRP-preserved hair grafts is recommended to improve the satisfaction of patients.
Finding the optimal therapeutic protocol appears to be a worthwhile pursuit when invasive surgical techniques like FUE are routinely performed. Although additional studies are needed to elucidate the underlying mechanisms by which PRGF regulates tissue regeneration, the present study demonstrates that PRGF is able to minimize the postsurgical telogenization of the hair and potentiate the performance of grafted hairs. The fibrin clot not only acts as a protective barrier against environmental factors but also provides a biologically active scaffold that induces resident cell proliferation and maintains an optimal integrity of the grafted hair. Randomized clinical trials with a higher number of patients and longer follow-up periods are needed to clarify the suitability of autologous growth factors for hair transplant treatment. However, these preliminary findings suggest that PRGF might offer a safe and effective adjuvant therapy for patients undergoing FUE surgery against alopecia.
Table 1.
The Age Distribution in Both Study Groups
| Group 1 | Group 2 | Total | |
|---|---|---|---|
| Age | |||
| ≤35 years | |||
| Count | 11 | 7 | 18 |
| % within age | 61.1% | 38.9% | 100.0% |
| >35 years | |||
| Count | 4 | 8 | 12 |
| % within age | 33.3% | 66.7% | 100.0% |
| Total | |||
| Count | 15 | 15 | 30 |
Supplementary Material
Footnotes
Published online 25 June 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Amable PR, Carias RB, Teixeira MV, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta KA, Cole J, Deutsch PD, et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatol Surg. 2019;45:1262–1273. [DOI] [PubMed] [Google Scholar]
- 3.Graq S. Outcome of intra-operative injected platelet-rich plasma therapy during follicular unit extraction hair transplant: a prospective randomised study in forty patients. J Cutan Aesthet Surg. 2016;9:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anitua E, Prado R, Sánchez M, et al. Platelet-rich-plasma: preparation and formulation. Oper Tech Orthop. 2012;22:25–32. [Google Scholar]
- 5.Araki J, Jona M, Eto H, et al. Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates: maximization of platelet concentration and removal of fibrinogen. Tissue Eng Part C Methods. 2012;18:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dua A, Dua K. Follicular unit extraction hair transplant. J Cutan Aesthet Surg. 2010;3:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano S, Romeo M, Lankinen P. Platelet-rich plasma for androgenetic alopecia: does it work? Evidence from meta analysis. J Cosmet Dermatol. 2017;16:374–381. [DOI] [PubMed] [Google Scholar]
- 8.Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uebel CO, da Silva JB, Cantarelli D, et al. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458–1466; discussion 1467. [DOI] [PubMed] [Google Scholar]
- 10.Mao G, Zhang M, Fan W. Platelet-rich plasma for treating androgenic alopecia: a systematic review. Aesth Plast Surg. 2019;43:1326–1336. [DOI] [PubMed] [Google Scholar]
- 11.Mahapatra S, Kumar D, Subramanian V, et al. Study on the efficacy of platelet-rich fibrin matrix in hair follicular unit transplantation in androgenetic alopecia patients. J Clin Aesthet Dermatol. 2016;9:29–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro MR, Asín M, Martínez AM, et al. Plasma rich in growth factors (PRGF) for the treatment of androgenetic alopecia. Eur J Plast Surg. 2015;38:437–442. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


