Abstract
Background
Salmonella is one of the main causative agents of diarrhea which results in substantial disease burden. To determine the prevalence, serotype distribution, and antimicrobial resistance profiles of clinical Salmonella isolates in Shenzhen, a 6-year surveillance study was conducted.
Results
A total of 297 (5.7%) Salmonella strains were isolated from stool samples from 5239 patients. Among the 42 serotypes identified, serotype Typhimurium was the most common one which represented 39.7% of the isolates (118), followed by serotype Enteritidis (71, 23.9%), London (12, 4.0%), 4, 5, 12: i: - (11, 3.7%), and Senftenberg (8, 2.7%). A high frequency of resistance was found in ampicillin (70.6%), piperacillin (64.5%), tetracycline (63.5%), and streptomycin (54.3%). Resistance to ampicillin and tetracycline was observed in 95.3% of S. Typhimurium isolates; and nalidixic acid in 93.1% of S. Enteritidis isolates. Resistance to 5 or more antimicrobial agents was found in 78.8% of S. Typhimurium and 69.0% of S. Enteritidis isolates. A decreased susceptibility to ciprofloxacin and levofloxacin was associated with amino acid alteration in gyrA gene. Point mutations without amino acid changes were seen in gyrB, parC, and parE genes.
Conclusions
A broad range of serotypes are responsible for Salmonellosis in Shenzhen, with Enteritidis and Typhimurium being the most common serotypes. The high level of antibiotic resistance is of public health significance and ongoing monitoring combined with rational use of antibiotics are recommended. Point mutations in gyrA gene might play an important role in the resistance to fluoroquinolones.
Keywords: Salmonella, Prevalence, Serotype, Antimicrobial resistance, Diarrhea
Background
Salmonella is a main foodborne and waterborne pathogen worldwide which causes an annual death of 230,000 [1]. Salmonella-associated foodborne outbreaks were transmitted by contaminated food such as beef, pork, tomato, and cucumbers [2–5]. It was prevalent throughout the year, but was the most commonly detected between April and October in China [6]. Children less than 5 years old accounted for the largest proportion of infections [7, 8].
Over 2500 serotypes have been reported [9], with Enteritidis and Typhimurium being the most common serotypes causing gastroenteritis [10]. Salmonella isolates with different serotypes vary in the pathogenicity, prevalence, and sensitivity to antibiotics. Salmonella Typhi and Salmonella Paratyphi are usually associated with higher mortality [11]. Some serotypes were reported in only single region of the world, such as Salmonella Rissen and Salmonella Weltevreden were only identified in Asia [12, 13]. Multi-drug resistance was the most common in S. Typhimurium [14].
Salmonellosis is not a severe infection, but the emergence of third-generation cephalosporins and multi-drug resistant isolates has raised concerns [15, 16]. The wide use of antibiotics in poultry and for empirical treatment of salmonellosis has led to the rising of drug resistance rate of Salmonella. A high resistance rate to at least one class of clinically important antimicrobials including quinolones was found in the clinical and animal-derived (chicken and pork) isolates [16, 17]. A wide range of mechanisms were associated with cephalosporins and quinolone resistance, including mutation in the quinolone-resistance determining regions (QRDRs), over-expression of an efflux pump and acquisition of drug resistance plasmids [18].
A one-year surveillance was conducted in our previous research and Salmonella was found one of the main causes (12.1%) of acute infectious diarrhea in Shenzhen [19]. Nevertheless, the long-term trend of serotype distribution and antimicrobial resistance pattern of the Salmonella isolates were not defined. To improve our understanding of the prevalence of Salmonella in this region and thus provide basis for designing prevention and control strategies, we investigated the serotypes and antibiotic resistance of the isolates obtained from the surveillance network during 2013 and 2018.
Results
Sample information
A total of 5239 cases (2749 of whom were male) were included during study period. Among these patients, 3870, 425, and 944 cases were enrolled from PKUSZH, SCH, and SYSU8H, respectively. SCH and SYSU8H started participating in the surveillance in 2016 and SCH was only involved in 2016. Patients ranged in age from 0 to 96 years (median 30 years). Local population who have registered permanent residence accounted for 60.4% of the patients. Of all the patients, 310 (5.9%) had fever, 635 (12.1%) had vomiting, and 50 (1.0%) had blood in stools, respectively. Of the patients over 5 years old, 2098 (54.1%) had abdominal pain (Table 1). Overall, Salmonella was isolated from 297 (5.7%) of all cases. The recovery rate in SCH, PKUSZH, and SYSU8H was 13.6%, 4.3%, and 7.7%, respectively.
Table 1.
The epidemiological and clinical characteristics of samples (n = 5239) in this study
| Category | Subcategory | No. (%) |
|---|---|---|
| Year | 2013 | 931 (17.8) |
| 2014 | 793 (15.1) | |
| 2015 | 652 (12.5) | |
| 2016 | 1285 (24.5) | |
| 2017 | 887 (16.9) | |
| 2018 | 691 (13.2) | |
| Age (years) | < 5 | 1361 (26.0) |
| 5 ~ 9 | 73 (1.4) | |
| 10 ~ 19 | 217 (4.1) | |
| 20 ~ 29 | 847 (16.2) | |
| 30 ~ 39 | 984 (18.8) | |
| 40 ~ 49 | 683 (13.0) | |
| 50 ~ 59 | 496 (9.5) | |
| > = 60 | 578 (11.0) | |
| Clinical Symptoms | Abdominal pain | 2098 (54.1) a |
| Fever | 310 (5.9) | |
| Vomiting | 635 (12.1) | |
| Blood in stools | 50 (1.0) |
aOnly 3878 cases aged over 5 years were included for analysis
Serotyping results
A total of 42 serotypes were identified in the 285 Salmonella isolates and additional 12 strains were un-typable. Typhimurium (118, 39.7%) was the most common serotype, followed by serotype Enteritidis (71, 23.9%), London (12, 4.0%), 4, 5, 12: i: - (11, 3.7%), and Senftenberg (8, 2.7%). A total of 46 isolates with uncommon serotypes were found, including serotype Virchow, Corvallis, Vilvoorde, and Sarajane (Table 2). A high recovery rate (4.5%) of S. Typhimurium was observed in 2016 and onwards.
Table 2.
The serotype distribution of clinical Salmonella isolates during 2013 and 2018
| Serotype | No. of isolates by year (recovery rate, %) | ||||||
|---|---|---|---|---|---|---|---|
| 2013 (n = 931) | 2014 (n = 793) | 2015 (n = 652) | 2016 (n = 1285) | 2017 (n = 887) | 2018 (n = 691) | Total (n = 5239) | |
| Typhimurium | 6 (0.6) | 8 (1.0) | 5 (0.8) | 58 (4.5) | 22 (2.5) | 19 (2.7) | 118 (2.3) |
| Enteritidis | 18 (1.9) | 6 (0.8) | 10 (1.5) | 12 (0.9) | 13 (1.5) | 12 (1.7) | 71 (1.4) |
| London | 2 (0.2) | 3 (0.4) | 2 (0.3) | 1 (0.1) | 4 (0.5) | 0 (0.0) | 12 (0.2) |
| 4, 5, 12: i: - | 0 (0.0) | 3 (0.4) | 0 (0.0) | 6 (0.5) | 0 (0.0) | 2 (0.3) | 11 (0.2) |
| Senftenberg | 1 (0.1) | 6 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 8 (0.2) |
| Stanley | 0 (0.0) | 3 (0.4) | 0 (0.0) | 1 (0.1) | 1 (0.1) | 2 (0.3) | 7 (0.1) |
| Agona | 3 (0.3) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 5 (0.1) |
| Litchfield | 0 (0.0) | 0 (0.0) | 5 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.1) |
| Weltevreden | 3 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 4 (0.1) |
| Others | 6 a (0.6) | 2 b (0.3) | 11 c (1.7) | 7 d (0.5) | 10 e (1.1) | 8 f (1.2) | 44 (0.8) |
| Un-typable | 4 (0.4) | 1 (0.1) | 1 (0.2) | 2 (0.2) | 3 (0.3) | 1 (0.1) | 12 (0.2) |
| Total | 43 (4.6) | 33 (4.2) | 34 (5.2) | 87 (6.8) | 53 (6.0) | 47 (6.8) | 297 (5.7) |
a Two strains of serotype Derby, 1 Ruzizi, 1 Meleagridis, and 2 Regent were included.
b One isolate of serotype Gallinarum-pullorum and 1 Drogana were included.
c One strain of Essen, 2 Manchester, 1 Sinstorf, 1 Chester, 1 Chomedey, 1 Tshiongwe, 1 Chennai, 1 Rissen, 1 Papuana, and 1 Fillmore were included.
d Two strains of serotype Virchow, 2 Nigeria, 1 Bovismorbificans, 1 Hidalgo, and 1 Amherstiana were included.
e Two strains of serotype Infantis, 1 Montevideo, 1 Bovismorbificans, 1 Chester, 2 Braenderup, 1 Kottbus, 1 Corvallis, and 1 Kentucky were included.
f One strain of serotype Rissen, 1 Hato, 1 Sarajane, 1 Chester, 1 Assinie, 1 Pomona, 1 Muenster, and 1 Vilvoorde were included.
Age and monthly distribution
The highest detection rate was observed in the age group of 5 ~ 9 years (15.1%), followed by < 5 years (10.1%) and 30 ~ 39 years (4.9%). The age group of over 60 years showed the lowest prevalence (2.8%). A high prevalence of S. Typhimurium infection was seen in the young children aged less than 5 years (6.4%) and 5 ~ 9 years old (5.5%). In the age group of 5 ~ 9 years, serotype Enteritidis and 4, 5, 12: i: - were the other two common serotypes (Table 3).
Table 3.
The serotype distribution of clinical Salmonella isolates in different age groups
| Age group (year) |
No. of tested | No. of isolates (prevalence, %) | Total (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Serotype Typhimurium |
Serotype Enteritidis |
Serotype 4, 5, 12: i: - |
Serotype London |
Serotype Senftenberg |
Serotype Stanley |
Other serotypes |
|||
| < 5 | 1361 | 87 (6.4) | 17 (1.2) | 8 (0.6) | 5 (0.4) | 1 (0.1) | 4 (0.3) | 15 (1.1) | 137 (10.1) |
| 5 ~ 9 | 73 | 4 (5.5) | 6 (8.2) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (15.1) |
| 10 ~ 19 | 217 | 0 (0.0) | 4 (1.8) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 0 (0.0) | 3 (1.4) | 9 (4.1) |
| 20 ~ 29 | 847 | 7 (0.8) | 14 (1.7) | 0 (0.0) | 0 (0.0) | 3 (0.4) | 1 (0.1) | 11 (1.3) | 36 (4.3) |
| 30 ~ 39 | 984 | 9 (0.9) | 14 (1.4) | 0 (0.0) | 1 (0.1) | 2 (0.2) | 0 (0.0) | 22 (2.2) | 48 (4.9) |
| 40 ~ 49 | 683 | 3 (0.4) | 4 (0.6) | 1 (0.1) | 3 (0.4) | 1 (0.1) | 1 (0.1) | 11 (1.6) | 24 (3.5) |
| 50 ~ 59 | 496 | 3 (0.6) | 6 (1.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (1.2) | 16 (3.2) |
| > = 60 | 578 | 5 (0.9) | 6 (1.0) | 0 (0.0) | 2 (0.3) | 0 (0.0) | 1 (0.2) | 2 (0.3) | 16 (2.8) |
| total | 5239 | 118 (2.3) | 71 (1.4) | 11 (0.2) | 12 (0.2) | 8 (0.2) | 7 (0.1) | 70 (1.3) | 297 (5.7) |
Of the 118 S. Typhimurium isolates, 87 (73.7%) were recovered from children aged below 5 years old. Eight of 11 serotype 4, 5, 12: i: - strains were isolated from this age group, while similar detection rate of S. Enteritidis strains was observed in each age group. Of the 72 isolates with other serotypes, 51 (70.8%) were isolated from adults aged between 20 and 59 years old (Table 3).
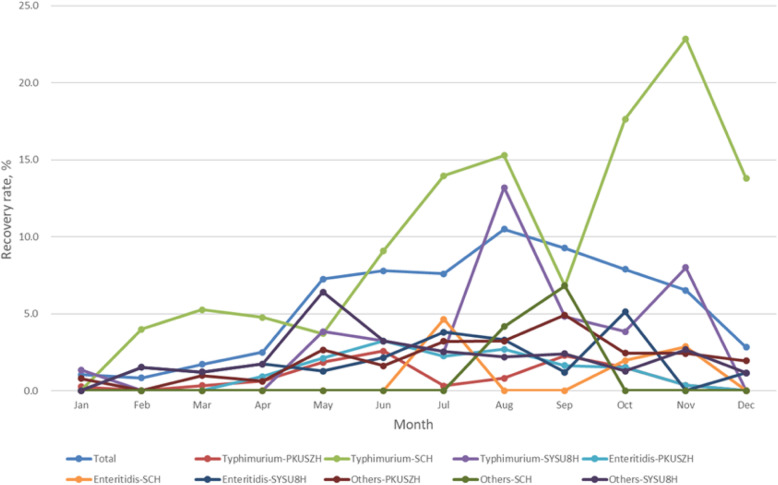
The highest detection rate was seen in August (11.01%), followed by September (8.56%), June (8.49%), and October (7.88%). The detection rate between May and November (8.19%) was significantly higher than that between December and April (1.89%) (χ2 = 93.440, P < 0.001). Similar seasonal distribution was also observed in the serotype Typhimurium isolates (Fig. 1).
Fig. 1.
The monthly distribution of clinical Salmonella isolates. PKUSZH, Peking University Shenzhen Hospital; SCH, Shenzhen Children’s Hospital; SYSU8H, The Eighth Affiliated Hospital, Sun Yat-Sen University
Antimicrobial resistance profile
The highest rate of resistance was found in ampicillin (139, 70.6%), followed by piperacillin (127, 64.5%), tetracycline (125, 63.5%), streptomycin (107, 54.3%), cefazolin (87, 44.2%), and sulphamethoxazole/trimethoprim (75, 38.1%). The resistance to ampicillin and tetracycline was observed in 81 (95.3%) of S. Typhimurium isolates. Twenty-seven (93.1%) of S. Enteritidis isolates were resistant to nalidixic acid (Table 4). Five out of 8 S. Senftenberg isolates were susceptible to all the tested antibiotics. Among the 163 isolates resistant to three or more antimicrobial agents, 82 and 23 were found in S. Typhimurium and S. Enteritidis isolates, respectively. The multiple antibiotic resistance (MAR) index of 123 isolates was over 0.21. The highest MAR index (0.71) was found in a serogroup B un-typable strain.
Table 4.
The antimicrobial susceptibility profiles of Salmonella isolates with different serotypes
| Antimicrobial agent | No. of resistant isolates by serotypes (resistant rate, %) | |||||
|---|---|---|---|---|---|---|
| Typhimurium (n = 85) |
Enteritidis (n = 29) |
London (n = 5) |
Senftenberg (n = 8) |
Others (n = 70) |
Total (n = 197) |
|
| Penicillins | ||||||
| ampicillin | 81 (95.3) | 22 (75.9) | 2 (40.0) | 0 (0.0) | 34 (48.6) | 139 (70.6) |
| piperacillin | 74 (87.1) | 21 (72.4) | 2 (40.0) | 0 (0.0) | 30 (42.9) | 127 (64.5) |
| β-lactam/β-lactamase inhibitors | ||||||
| ampicillin/ sulbactam | 9 (10.6) | 7 (24.1) | 0 (0.0) | 1 (12.5) | 6 (8.6) | 23 (11.7) |
| Cephems | ||||||
| cefazolin | 50 (58.8) | 16 (55.2) | 1 (20.0) | 1 (12.5) | 19 (27.1) | 87 (44.2) |
| cefepime | 23 (27.1) | 5 (17.2) | 0 (0.0) | 0 (0.0) | 9 (12.9) | 37 (18.8) |
| cefotaxime | 40 (47.1) | 8 (27.6) | 0 (0.0) | 1 (12.5) | 15 (21.4) | 64 (32.5) |
| ceftriaxone | 42 (49.4) | 9 (31.0) | 0 (0.0) | 1 (12.5) | 14 (20.0) | 66 (33.5) |
| cefoxitin | 3 (3.5) | 2 (6.9) | 0 (0.0) | 1 (12.5) | 1 (1.4) | 7 (3.6) |
| ceftazidime | 13 (15.3) | 3 (10.3) | 0 (0.0) | 0 (0.0) | 7 (10.0) | 23 (11.7) |
| Monobactams | ||||||
| aztreonam | 23 (27.1) | 3 (10.3) | 0 (0.0) | 0 (0.0) | 9 (12.9) | 35 (17.8) |
| Aminoglycosides | ||||||
| gentamicin | 22 (25.9) | 0 (0.0) | 3 (60.0) | 0 (0.0) | 13 (18.6) | 38 (19.3) |
| amikacin | 2 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) |
| streptomycin | 57 (67.1) | 20 (69.0) | 2 (40.0) | 1 (12.5) | 27 (38.6) | 107 (54.3) |
| Carbapenems | ||||||
| imipenem | 3 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 4 (2.0) |
| meropenem | 2 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) |
| Tetracyclines | ||||||
| tetracycline | 81 (95.3) | 3 (10.3) | 2 (40.0) | 0 (0.0) | 39 (55.7) | 125 (63.5) |
| Quinolones and Fluoroquinolones | ||||||
| ciprofloxacin | 7 (8.2) | 2 (6.9) | 2 (40.0) | 0 (0.0) | 8 (11.4) | 19 (9.6) |
| levofloxacin | 5 (5.9) | 1 (3.4) | 0 (0.0) | 0 (0.0) | 6 (8.6) | 12 (6.1) |
| norfloxacin | 7 (8.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (4.3) | 10 (5.1) |
| nalidixic acid | 26 (30.6) | 27 (93.1) | 3 (60.0) | 0 (0.0) | 18 (25.7) | 74 (37.6) |
| Folate pathway inhibitors | ||||||
| sulphamethoxazole/trimethoprim | 42 (49.4) | 4 (13.8) | 2 (40.0) | 1 (12.5) | 26 (37.1) | 75 (38.1) |
| trimethoprim | 38 (44.7) | 3 (10.3) | 2 (40.0) | 0 (0.0) | 22 (31.4) | 65 (33.0) |
| Phenicols | ||||||
| Chloramphenicol | 41 (48.2) | 2 (6.9) | 1 (20.0) | 0 (0.0) | 21 (30.0) | 65 (33.0) |
| Nitrofurans | ||||||
| nitrofurantoin | 2 (2.4) | 11 (37.9) | 0 (0.0) | 0 (0.0) | 8 (11.4) | 21 (10.7) |
| MAR index | ||||||
| <0.08 | 0 (0.0) | 3 (10.3) | 2 (40.0) | 6 (72.5) | 23 (32.9) | 34 (17.3) |
| 0.08- | 3 (3.5) | 3 (10.3) | 0 (0.0) | 1 (12.5) | 5 (7.1) | 12 (6.1) |
| 0.13- | 5 (5.9) | 1 (3.4) | 1 (20.0) | 0 (0.0) | 3 (4.3) | 10 (5.1) |
| 0.17- | 10 (11.8) | 2 (6.9) | 0 (0.0) | 1 (12.5) | 5 (7.1) | 18 (9.1) |
| ≥0.21 | 67 (78.8) | 20 (69.0) | 2 (40.0) | 0 (0.0) | 34 (48.6) | 123 (62.4) |
Among the common serotypes, the lowest resistant rate was seen in S. Senftenberg. A significant higher frequency of resistance to penicillins, cephems (except for cefoxitin and ceftazidime), monobactams, tetracyclines, folate pathway inhibitors, and phenicols was observed in S. Typhimurium compared with that in other serotypes (P < 0.05), while resistant rate to nalidixic acid (χ2 = 45.227, P < 0.001) and nitrofurantoin (χ2 = 28.897, P < 0.001) was significantly higher in S. Enteritidis. Resistance to third generation cephalosporins and carbapenems was not found, while a higher resistant rate to ciprofloxacin and gentamicin was seen in S. London, compared with that in other serotypes (Table 4).
Mutations with QRDRs
The amino acid alterations in gyrA occurred at codon 87 (Asp-87 → Gly or Asn) in 14 isolates and the MIC to CIP in 9 out of 14 isolates was over 2 μg/ml. The mutation in gyrB occurred at codon 462 and 464, but no amino acid alteration was found (Leu-462 → Leu, Ser-464 → Ser). A single base change in amino acids 67, 75, 77, 117, and 123 in parC were found in 5 of the tested strains. The mutation of codons 500 and 509 in parE was found in 3 and 8 isolates, respectively (Table 5).
Table 5.
The linkage of QRDRs mutations with antimicrobial susceptibility profile
| Isolate No. | Serotype | MAR | MIC (μg/ml) | Mutations-changes in codons | ||||
|---|---|---|---|---|---|---|---|---|
| CIP | LEV | gyrA | gyrB | parC | parE | |||
| 1 | Typhimurium | 0.33 | <=0.25 | <=0.12 | – | – | – | – |
| 2 | Typhimurium | 0.46 | 0.5 | 0.5 | – | – | – | – |
| 3 | Typhimurium | 0.63 | 1 | 2 | – | CTT → CTC, TCC → TCTc | GTT → GTC, CAC → CAT, CAT→CAC, GCG → GCA, TCC → TCTd | ACT→ACGe, CAC → CATf |
| 4 | Bovismorbificans | 0.38 | 1 | 0.5 | GAC → GGCa | CTT → CTC, TCC → TCT | – | ACT, CAC → CAT |
| 5 | Typhimurium | 0.50 | 1 | 0.5 | GAC → GGC | CTT → CTC, TCC → TCT | – | ACT, CAC → CAT |
| 6 | Enteritidis | 0.33 | 0.5 | 2 | – | CTT → CTC, TCC → TCT | – | ACT, CAC → CAT |
| 7 | London | 0.33 | 1 | 1 | – | CTT → CTC, TCC → TCT | GTT → GTC, CAC → CAT, CAT→CAC, GCG → GCA, TCC → TCT | ACT, CAC → CAT |
| 8 | Typhimurium | 0.33 | 2 | 1 | GAC → AACb | – | – | – |
| 9 | Agona | 0.63 | 1 | 1 | – | CTT → CTC, TCC → TCT | GTT → GTC, CAC → CAT, CAT→CAC, GCG → GCA, TCC → TCT | ACT→ACG, CAC → CAT |
| 10 | London | 0.42 | 1 | 1 | GAC → AAC | CTT → CTC, TCC → TCT | GTT → GTC, CAC → CAT, CAT→CAC, GCG → GCA, TCC → TCT | – |
| 11 | Litchfield | 0.25 | 1 | 1 | GAC → AAC | CTT → CTC, TCC → TCT | – | – |
| 12 | 4, 5, 12, i: - | 0.21 | 1 | 1 | – | – | – | – |
| 13 | Enteritidis | 0.08 | 1 | 1 | GAC → AAC | CTT → CTC, TCC → TCT | – | ACT, CAC → CAT |
| 14 | London | 0.13 | 2 | 0.5 | GAC → AAC | CTT → CTC, TCC → TCT | GTT → GTC, CAC → CAT, CAT→CAC, GCG → GCA, TCC → TCT | ACT→ACG, CAC → CAT |
| 15 | 4, 5, 12, i: - | 0.50 | 2 | 2 | GAC → AAC | – | – | – |
| 16 | untypable | 0.42 | 2 | 4 | GAC → AAC | – | – | – |
| 17 | 4, 5, 12, i: - | 0.42 | > = 4 | 4 | GAC → AAC | – | – | – |
| 18 | Typhimurium | 0.42 | 2 | 2 | GAC → AAC | – | – | – |
| 19 | Typhimurium | 0.63 | > = 4 | > = 8 | GAC → AAC | – | – | – |
| 20 | Typhimurium | 0.46 | 2 | 4 | GAC → AAC | – | – | – |
| 21 | Typhimurium | 0.58 | > = 4 | 4 | GAC → AAC | – | – | – |
Base pair changes in bold type.
MIC minimum inhibitory concentration, CIP ciprofloxacin, LEV levofloxacin.
- No mutation
aAmino acid alteration is Asp-87 → Gly.
bAmino acid alteration is Asp-87 → Asn.
cAmino acid alterations are Leu-462 → Leu, Ser-464 → Ser.
dAmino acid alterations are Val-67 → Val, His-75 → His, His-77 → His, Ala-117 → Ala, Ser-123 → Ser.
eAmino acid alteration is Thr-500 → Thr.
fAmino acid alteration is His-509 → His.
Discussion
Salmonella was an important causative microorganism of acute gastroenteritis, which was shown in the detection rate during 2013 and 2018 (4.2% ~ 6.8%). The detection rate was lower compared with that in our previous study (12.1%) which incorporated PCR method [19], but it was comparable to another study (4.8%) conducted in Guangzhou using conventional assay [20]. A detection rate of 4.5% was reported in a large laboratory-based surveillance study in Guangdong province during 2009 and 2012, suggesting the continued prevalence in this region [17].
A broad distribution of serotypes was also reported in another study conducted in Shenzhen [21]. Apart from the detection rate, this study provided the trend of prevalence and antimicrobial resistance data over a long period, and identified some rare serotypes.
The detection rate between May and November was significantly higher compared with that in other months, which might be associated with high temperature. Hot weather contributed to bacterial growth and the chance of consumption of insufficiently heat-treated food or salad was higher. A linear association between temperature and the number of reported cases of salmonellosis was found and it was proven that higher temperature was associated with Salmonella infections [22]. As a result, the sporadic cases and salmonellosis outbreak were commonly found during this period [17].
The high incidence of Salmonella infection in children was consistent with another study where a recovery rate of 17.2% in children was reported [15]. The high frequency of infections in children could be attributed to the behavior such as frequent contact with contaminated limbs, consumption of contaminated food or water, or close contacts with an asymptomatic caretaker [20]. S. Typhimurium and S. Enteritidis were the most common serotypes in children aged below 5 years old, which represented 75.4% (104/138) of the isolates, in accordance with other studies [15, 17]. The high occurrence of S. Typhimurium in the age group of < 5 years and 5 ~ 9 years might be associated with relatively low immune response and behaviors of these young children [23]. Highest recovery rate and highest occurrence of S. Enteritidis in the age group of 5 ~ 9 years was probably due to the small sample size. The high frequency of multi-drug resistance in S. Typhimurium posed the difficulties in treating pediatric patients. Some measures such as good hygiene, proper hand washing, and education of their guardians were recommended to reduce the disease burden of salmonellosis in children.
Wide distribution of serotypes might be another reason of the continued prevalence of Salmonella infections. Apart from S. Typhimurium and S. Enteritidis, a broad range of uncommon serotypes including Vilvoorde and Sarajane were identified, indicating the wide sources of Salmonella. As one of the major pathogenic bacteria in Chinese food commodities [24], Salmonella was commonly isolated from beef, pork, and poultry meat [25, 26]. Agona, Corvallis, and Kentucky were reported the dominant serotypes in chicken samples, while Typhimurium, Rissen, and Derby were the most common in pork samples [16]. The dominant presence of S. Typhimurium in 2016 and onwards was attributed to the participation of SCH and SYSU8H after 2016. Thirty-six of S. Typhimurium strains were isolated from PKUSZH, while 82 isolates were from the other two hospitals.
Serotype Gallinarum-pullorum was isolated from a 24-year-old male patient with mild diarrhea. S. Gallinarum-pullorum was generally regarded as chicken-derived which caused little public health concern. However, occasional infections in human were reported following consumption of heavily contaminated food and low detection rate was found in humans between 1982 and 1992 [27]. Transient illness caused by large number of S. Gallinarum-pullorum was observed in both volunteers [28] and our case. The contaminated raw meat was not considered as a food safety risk due to the thorough cooking tradition, but it was often associated with direct exposure to enteric pathogens and cross-contamination of ready-to-eat foods [29]. As a result, contamination from food handlers, or the consumption of contaminated or cross contaminated food may lead to the Salmonella infections.
Salmonella infections are normally associated with self-limiting diarrhea and antimicrobial therapy is not indicated, but appropriate antimicrobial treatment could be life-saving in severe cases. In addition, antibiotic treatment with ciprofloxacin or fluoroquinolones was recommended for Salmonella infections in infants less than 3 months of age due to the high risk of bacteremia and extraintestinal complications [30, 31]. The occurrence of multiple drug resistant strains raised the importance of antibiotics resistance surveillance. The high level of resistance to the first-line agents: ampicillin, sulphamethoxazole/trimethoprim, and chloramphenicol was also observed in other studies [20]. A similar profile was also found in the isolates from food-producing animals [32], suggesting the careful use of antibiotics in breeding industry was necessary.
The frequency of multiple drug resistance was common. Resistance to 5 or more antibiotics was commonly observed in S. Enteritidis (69.0%) and S. Typhimurium (78.8%). The occurrence of multidrug resistant (MDR) strains was reported to be associated with coexistence of resistance-related genes [14] and their transmission led to great difficulties in treatments. The resistance to first-line drugs of treating severe infections, such as third-generation cephalosporins and fluoroquinolones was of clinical concerns. The emergence of MDR isolates and increasing resistance to important antibiotics suggested the prevention measures and ongoing surveillance of antibiotic resistance are needed to control the infections.
The resistance to ciprofloxacin was mediated by multiple mechanisms [33], and the main mechanism of mutations in QRDRs was investigated in this study. A high frequency of mutations in gyrA, gyrB, parC, and parE genes were found in the quinolone-resistant strains. The amino acid alterations in gyrA was associated with high levels of MIC to CIP and LEV, suggesting that the mutations in gyrA played an important role in the drug resistance. It was also reported in other studies that mutations associated with quinolone resistance were mainly present in the QRDRs of gyrA gene [34, 35], probably due to that the DNA gyrase was the primary target of quinolone action and a single point mutation of gyrA could lead to the reduced susceptibility to fluoroquinolones. In the isolates absence of chromosomal mutations where no amino acid alteration was found, other mechanisms such as over-expression of efflux pump and mutations of other elements might contribute to the resistance [36, 37].
Conclusion
Salmonella was an important causative microorganism of acute diarrhea in this region and a broad range of serotypes were prevalent.
S. Typhimurium and S. Enteritidis were the two most common serotypes. The highest detection rate was found in the age group of less than 9 years old and during June and October.
A high rate of MDR was found in serotype Typhimurium and Enteritidis. An increasing trend of resistant rate to fluoroquinolones was mainly associated with the point mutation in the QRDRs of gyrA gene.
Methods
Stool samples collection
Peking University Shenzhen Hospital (PKUSZH), Shenzhen Children’s Hospital (SCH), and The Eighth Affiliated Hospital, Sun Yat-Sen University (SYSU8H) were selected as sentinel hospitals in this retrospective study. Stool samples were collected from outpatients who visited gastroenteritis clinic due to acute infectious diarrhea and agreed to take part in the surveillance program. Acute diarrhea was defined as over 3 passages of loose, mucus-, watery, or bloody-stools during 24-h period. The stool samples were examined for Salmonella sp. using CHROM agars. The clinical signs and demographic information were retrospectively collected from electronic medical records.
Serotyping and antimicrobial susceptibility testing
The stool specimens were enriched in selenite cysteine (SC) broth and then plated on CHROM agar for isolation of Salmonella spp.. The suspicious colonies were identified using Vitek-2 compact system (bioMerieux, France) and Salmonella spp. isolates were serotyped with a commercial serotyping kit (S&A company, S&A Reagent Lab, Bangkok, Thailand) in the sentinel hospitals. Then part of the isolates were sent to Futian District Center for Disease Control and Prevention (CDC) and Shenzhen Hospital, Southern Medical University and stored in − 80 °C for further analysis.
A total of 197 collected Salmonella isolates representing 26 serotypes were recovered and tested for antimicrobial susceptibility in 2019. Twenty-four antimicrobials (Oxoid, UK) including amikacin (30 micrograms (mcg), AK), ampicillin/sulbactam (20 mcg, SAM), ampicillin (10mcg, AMP), aztreonam (30 mcg, ATM), cefepime (30 mcg, FEP), cefotaxime (30 mcg, CTX), cefoxitin (30 mcg, FOX), ceftazidime (30 mcg, CAZ), ceftriaxone (30 mcg, CRO), cephazolin (30 mcg, KZ), chloramphenicol (30 mcg, C), ciprofloxacin (5 mcg, CIP), gentamicin (10 mcg, CN), imipenem (10 mcg, IPM), levofloxacin (5 mcg, LEV), meropenem (10 mcg, MEM), nalidixic acid (30 mcg, NA), nitrofurantoin (300 mcg, F), norfloxacin (10 mcg, NOR), piperacillin (100 mcg, PRL), streptomycin (10 mcg, S), sulphamethoxazole/trimethoprim (25 mcg, SXT), tetracycline (30 mcg, TE), and trimethoprim (5 mcg, W) were tested using disk diffusion method. The susceptibility to CIP and LEV in quinolone-resistant isolates was confirmed by Vitek-2 compact system (bioMerieux, France).
The MacFarland 0.5 inoculums were prepared and swabbed on the entire surface of Mueller-Hinton agar (Huankai, China) and left to dry for 3–5 min. Antimicrobial susceptibility test discs (Oxoid, UK) were placed on the inoculated agar plate with a disc dispenser (Oxoid, UK) and incubated at 37 °C for 24 h. After incubation, the diameter of inhibition zone was measured and the results were interpreted as susceptible, intermediate, and resistant according to the Clinical and Laboratory Standards Institute guideline (CLSI, 2019) [38]. Escherichia coli strain ATCC 25922 and Pseudomonas aeruginosa strain ATCC 27853 was used as quality control for disk diffusion and Vitek-2 compact system, respectively.
The multiple antibiotic resistance (MAR) index was the ratio between the number of antibiotics to which the organism was resistant and the number of antibiotics tested. Multi-drug resistant (MDR) was defined as resistant to three or more different classes of antimicrobial agents. Statistical analysis was conducted using chi-square test by SPSS version 21 (SPSS Inc., Chicago, IL, USA).
Detection of target gene mutations
A total of 21 isolates that resistant to any of the three antibiotics: ciprofloxacin, levofloxacin, and norfloxacin were chosen to screen for the mutations of the gyrA, gyrB, parC, and parE genes in the QRDRs. The bacterial nucleic acid was extracted using QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) and subjected to PCR amplification using Taq PCR Master Mix Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s recommended protocols. The PCR products were sent to Sangon (Shanghai, China) for sequencing and the results were analyzed using BLAST (PubMed). The primers used for PCR amplification and sequencing were listed in Supplementary Table 1 [39, 40].
Supplementary information
Additional file 1: Table S1. List of primers used in this study.
Acknowledgements
We thank the staff from the clinical laboratory of Peking University Shenzhen Hospital, Shenzhen Children’s Hospital, and The Eighth Affiliated Hospital, Sun Yat-Sen University who took part in the study.
Abbreviations
- AMP
Ampicillin
- AK
Amikacin
- ATM
Aztreonam
- C
Chloramphenicol
- CAZ
Ceftazidime
- CDC
Center for Disease Control and Prevention
- CIP
Ciprofloxacin
- CLSI
Clinical and Laboratory Standards Institute
- CN
Gentamicin
- CRO
Ceftriaxone
- CTX
Cefotaxime
- F
Nitrofurantoin
- FEP
Cefepime
- FOX
Cefoxitin
- IPM
Impenem
- KZ
Cephazolin
- LEV
Levofloxacin
- MAR
Multiple antibiotic resistance
- mcg
Micrograms
- MDR
Multi-drug resistant
- MEM
Meropenem
- MIC
Minimum inhibitory concentration
- NA
Nalidixic acid
- NOR
Norfloxacin
- PCR
Polymerase chain reaction
- PKUSZH
Peking University Shenzhen Hospital
- PRL
Piperacillin
- QRDRs
Quinolone-resistance determining regions
- S
Streptomycin
- SAM
Ampicillin/sulbactam
- SCH
Shenzhen Children’s Hospital
- SXT
Sulphamethoxazole/trimethoprim
- SYSU8H
The Eighth Affiliated Hospital, Sun Yat-Sen University
- TE
Tetracycline
- W
Trimethoprim
Authors’ contributions
QH and WM designed the study. HS, JZ, and YO collected and detected clinical samples. TH detected antibiotic resistance genes. HC, LZ, and SC collected and analyzed data. HS and WM drafted, and revised the manuscript with YZ. All authors read and approved the final manuscript.
Funding
This research was supported by Natural Science Foundation of Guangdong Province (NO.2018A0303130119 and 2020A1515010009), Sanming Project of Medicine in Shenzhen (NO. SZSM201811071 and SZSM201612023), Futian District Public Health Research Project (NO. FTWS2019067 and FTWS2019073), Seeding Program of Shenzhen Hospital of Southern Medical University (NO.2016MM07 and 2018MM01), and Research Foundation of Shenzhen Hospital, Southern Medical University (PY202002YM and PY2020ZY09). We thank the staff from Peking University Shenzhen Hospital, Shenzhen Children’s Hospital, and The Eighth Affiliated Hospital, Sun Yat-Sen University who participated in the study.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics approval and consent to participate
All aspects of the study were performed in accordance with national ethics regulations and approved by the Ethics Committees of the participated organizations, including Shenzhen Center for Disease Control and Prevention, Futian District Center for Disease Control and Prevention, and the sentinel hospitals. Participants received information regarding the purpose of this study and of their right to confidentiality. Written consent was obtained from the participants or the parents or guardians of the pediatric patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12866-020-01886-5.
References
- 1.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12):e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998-2008. Emerg Infect Dis. 2013;19(8):1239–1244. doi: 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller L, Kjelso C, Frank C, Jensen T, Torpdahl M, Soborg B, et al. Outbreak of Salmonella Strathcona caused by datterino tomatoes, Denmark, 2011. Epidemiol Infect. 2016;144(13):2802–2811. doi: 10.1017/S0950268816000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall KEH, Tewell M, Tecle S, Leeper M, Sinatra J, Kissler B, et al. Protracted Outbreak of Salmonella Newport Infections Linked to Ground Beef: Possible Role of Dairy Cows - 21 States, 2016–2017. MMWR Morb Mortal Wkly Rep. 2018;67(15):443–446. doi: 10.15585/mmwr.mm6715a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughlin M, Bottichio L, Weiss J, Higa J, McDonald E, Sowadsky R, et al. Multistate outbreak of Salmonella Poona infections associated with imported cucumbers, 2015-2016. Epidemiol Infect. 2019;147:e270. doi: 10.1017/S0950268819001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ran L, Wu S, Gao Y, Zhang X, Feng Z, Wang Z, et al. Laboratory-based surveillance of nontyphoidal Salmonella infections in China. Foodborne Pathog Dis. 2011;8(8):921–927. doi: 10.1089/fpd.2010.0827. [DOI] [PubMed] [Google Scholar]
- 7.Besser JM. Salmonella epidemiology: a whirlwind of change. Food Microbiol. 2018;71:55–59. doi: 10.1016/j.fm.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Wang LP, Yu JX, Chen X, Wang RN, Yang XZ, et al. Prevalence of Enteropathogens in outpatients with acute diarrhea from urban and rural areas, Southeast China, 2010-2014. Am J Trop Med Hyg. 2019;101(2):310–318. doi: 10.4269/ajtmh.19-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su LH, Chiu CH. Salmonella: clinical importance and evolution of nomenclature. Chang Gung Med J. 2007;30(3):210–219. [PubMed] [Google Scholar]
- 10.Qi X, Li P, Xu X, Yuan Y, Bu S, Lin D. Epidemiological and molecular investigations on Salmonella responsible for gastrointestinal infections in the southwest of Shanghai from 1998 to 2017. Front Microbiol. 2019;10:2025. doi: 10.3389/fmicb.2019.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriksen RS, Vieira AR, Karlsmose S. Lo Fo Wong DM, Jensen AB, Wegener HC, et al. global monitoring of Salmonella serovar distribution from the World Health Organization global foodborne infections network country data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8(8):887–900. doi: 10.1089/fpd.2010.0787. [DOI] [PubMed] [Google Scholar]
- 13.Galanis E. Lo Fo Wong DM, Patrick ME, Binsztein N, Cieslik a, Chalermchikit T, et al. web-based surveillance and global Salmonella distribution, 2000-2002. Emerg Infect Dis. 2006;12(3):381–388. doi: 10.3201/eid1205.050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Li Y, Xu X, Liang B, Wu F, Yang X, et al. Antimicrobial resistance of Salmonella enterica Serovar Typhimurium in Shanghai, China. Front Microbiol. 2017;8:510. doi: 10.3389/fmicb.2017.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Xie X, Xu X, Wang X, Chang H, Wang C, et al. Nontyphoidal salmonella infection in children with acute gastroenteritis: prevalence, serotypes, and antimicrobial resistance in Shanghai, China. Foodborne Pathog Dis. 2014;11(3):200–206. doi: 10.1089/fpd.2013.1629. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Fu Y, Xiong Z, Ma Y, Wei Y, Qu X, et al. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail Markets in Guangdong, China. Front Microbiol. 2018;9:2104. doi: 10.3389/fmicb.2018.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Z, Ke B, Deng X, Liang J, Ran L, Lu L, et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009-2012. BMC Infect Dis. 2015;15:53. doi: 10.1186/s12879-015-0784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguale T, Birungi J, Asrat D, Njahira MN, Njuguna J, Gebreyes WA, et al. Genetic markers associated with resistance to beta-lactam and quinolone antimicrobials in non-typhoidal Salmonella isolates from humans and animals in Central Ethiopia. Antimicrob Resist Infect Control. 2017;6:13. doi: 10.1186/s13756-017-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen H, Zhang J, Li Y, Xie S, Jiang Y, Wu Y, et al. The 12 gastrointestinal pathogens Spectrum of acute infectious diarrhea in a sentinel hospital, Shenzhen, China. Front Microbiol. 2016;7:1926. doi: 10.3389/fmicb.2016.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang B, Xie Y, He S, Mai J, Huang Y, Yang L, et al. Prevalence, serotypes, and drug resistance of nontyphoidal Salmonella among paediatric patients in a tertiary hospital in Guangzhou, China, 2014-2016. J Infect Public Health. 2019;12(2):252–257. doi: 10.1016/j.jiph.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Long LD, Wei HJ, WU SJ, Jiang PS, LI HJ, Zhuo F. Characteristics of drug resistance and molecular typing for Salmonella in diarrhea patients from four hospitals in Shenzhen. Chinese J Zoonoses. 2017;33(10):897–902. [Google Scholar]
- 22.Kovats RS, Edwards SJ, Hajat S, Armstrong BG, Ebi KL, Menne B. The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiol Infect. 2004;132(3):443–453. doi: 10.1017/S0950268804001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broz P, Ohlson MB, Monack DM. Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes. 2012;3(2):62–70. doi: 10.4161/gmic.19141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paudyal N, Pan H, Liao X, Zhang X, Li X, Fang W, et al. A meta-analysis of major foodborne pathogens in Chinese food commodities between 2006 and 2016. Foodborne Pathog Dis. 2018;15(4):187–197. doi: 10.1089/fpd.2017.2417. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Wu Q, Zhang J, Huang J, Chen L, Wu S, et al. Prevalence, bacterial load, and antimicrobial resistance of Salmonella Serovars isolated from retail meat and meat products in China. Front Microbiol. 2019;10:2121. doi: 10.3389/fmicb.2019.02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Pei XY, Zhang XL, Wu LL, Liu Y, Zhou HD, et al. A surveillance of microbiological contamination on raw poultry meat at retail markets in China. Food Control. 2019;104:99–104. doi: 10.1016/j.foodcont.2019.04.037. [DOI] [Google Scholar]
- 27.Shivaprasad HL. Fowl typhoid and pullorum disease. Rev Sci Tech. 2000;19(2):405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- 28.Mc CN, Eisele CW. Experimental human salmonellosis. IV. Pathogenicity of strains of Salmonella pullorum obtained from spray-dried whole egg. J Infect Dis. 1951;89(3):259–265. doi: 10.1093/infdis/89.3.259. [DOI] [PubMed] [Google Scholar]
- 29.Yang B, Xi M, Wang X, Cui S, Yue T, Hao H, et al. Prevalence of Salmonella on raw poultry at retail markets in China. J Food Prot. 2011;74(10):1724–1728. doi: 10.4315/0362-028X.JFP-11-215. [DOI] [PubMed] [Google Scholar]
- 30.Bula-Rudas FJ, Rathore MH, Maraqa NF. Salmonella infections in childhood. Adv Pediatr Infect Dis. 2015;62(1):29–58. doi: 10.1016/j.yapd.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Haeusler GM, Curtis N. Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv Exp Med Biol. 2013;764:13–26. doi: 10.1007/978-1-4614-4726-9_2. [DOI] [PubMed] [Google Scholar]
- 32.Lai J, Wu C, Wu C, Qi J, Wang Y, Wang H, et al. Serotype distribution and antibiotic resistance of Salmonella in food-producing animals in Shandong province of China, 2009 and 2012. Int J Food Microbiol. 2014;180:30–38. doi: 10.1016/j.ijfoodmicro.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Wei Z, Xu X, Yan M, Chang H, Li Y, Kan B, et al. Salmonella Typhimurium and Salmonella Enteritidis infections in sporadic diarrhea in children: source tracing and resistance to third-generation Cephalosporins and ciprofloxacin. Foodborne Pathog Dis. 2019;16(4):244–255. doi: 10.1089/fpd.2018.2557. [DOI] [PubMed] [Google Scholar]
- 34.Gopal M, Elumalai S, Arumugam S, Durairajpandian V, Kannan MA, Selvam E, et al. GyrA ser83 and ParC trp106 mutations in Salmonella enterica Serovar Typhi isolated from typhoid fever patients in tertiary care hospital. J Clin Diagn Res. 2016;10(7):DC14–DC18. doi: 10.7860/JCDR/2016/17677.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eaves DJ, Randall L, Gray DT, Buckley A, Woodward MJ, White AP, et al. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob Agents Chemother. 2004;48(10):4012–4015. doi: 10.1128/AAC.48.10.4012-4015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin D, Chen K, Wai-Chi Chan E, Chen S. Increasing prevalence of ciprofloxacin-resistant food-borne Salmonella strains harboring multiple PMQR elements but not target gene mutations. Sci Rep. 2015;5:14754. doi: 10.1038/srep14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen J, Yang B, Gu Q, Zhang G, Yang J, Xue F, et al. The role of AcrAB-TolC efflux pump in mediating Fluoroquinolone resistance in naturally occurring Salmonella isolates from China. Foodborne Pathog Dis. 2017;14(12):728–734. doi: 10.1089/fpd.2017.2291. [DOI] [PubMed] [Google Scholar]
- 38.Humphries RM, Abbott AN, Hindler JA. Understanding and addressing CLSI breakpoint revisions: a primer for clinical laboratories. J Clin Microbiol. 2019;57(6). 10.1128/JCM.00203-19. [DOI] [PMC free article] [PubMed]
- 39.Fabrega A, du Merle L, Le Bouguenec C, Jimenez de Anta MT, Vila J. Repression of invasion genes and decreased invasion in a high-level fluoroquinolone-resistant Salmonella typhimurium mutant. PLoS One. 2009;4(11):e8029. doi: 10.1371/journal.pone.0008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Randall LP, Coldham NG, Woodward MJ. Detection of mutations in Salmonella enterica gyrA, gyrB, parC and parE genes by denaturing high performance liquid chromatography (DHPLC) using standard HPLC instrumentation. J Antimicrob Chemother. 2005;56(4):619–623. doi: 10.1093/jac/dki293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. List of primers used in this study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.