Abstract
The apoptosis of endothelial cells (ECs) induced by oxidized low-density lipoprotein (ox-LDL) is an important contributing factor in the pathogenesis of atherosclerosis. It has been reported that microRNA (miR)-106a-5p is overexpressed in atherosclerotic plaques and involved in angiogenesis. However, its role and underlying mechanisms in ox-LDL induced EC apoptosis remain to be fully understood. In the present study the expression of miR-106a-5p in human umbilical vein ECs (HUVECs) stimulated with ox-LDL was investigated using reverse transcription-quantitative PCR analysis. Cell viability and apoptosis were assessed by MTT assay and flow cytometry, respectively. Caspase-3 activity and reactive oxygen species (ROS) levels were determined by commercial kits. The interaction between miR-106a-5p and signal transducer and activator of transcription 3 (STAT3) mRNA was examined by luciferase reporter assay. It was found that ox-LDL treatment significantly increased the levels of miR-106a-5p in a dose-dependent manner in HUVECs. Moreover, these results demonstrated that ox-LDL treatment inhibited cell viability, promoted cell apoptosis, increased caspase-3 activity and ROS levels, whereas inhibition of miR-106a-5p reversed the effects of ox-LDL on HUVECs. In addition, it was shown that STAT3 is a direct target of miR-106a-5p in HUVECs, and silencing of STAT3 impaired the protective effects of miR-106a-5p inhibition on cell apoptosis and oxidative injury induced by ox-LDL. Collectively, these results indicated that miR-106a-5p participated in ox-LDL-stimulated apoptosis and oxidative injury in HUVECs by regulating STAT3. Thus, suggesting that miR-106a-5p/STAT3 may serve as a novel therapeutic target for atherosclerosis in the future.
Keywords: endothelial cells, apoptosis, microRNA-106a-5p, oxidized low-density lipoprotein, atherosclerosis, human umbilical vein endothelial cells, cell viability
Introduction
Atherosclerotic cardiovascular disease is the leading cause of death worldwide, causing 247.9 deaths/100,000 persons in 2013, representing 84.5% of cardiovascular deaths (1,2). Endothelial cell (EC) damage is considered to be an early marker in the development of cardiovascular diseases, such as atherosclerosis, myocardial infarction and cardiac failure (3,4). EC injury and dysfunction have been reported in human and animal atherosclerotic lesions, and recovery of the injured endothelium is dependent on the apoptosis and proliferation of ECs, which is vital in the development of atherosclerosis (5–7). It has been demonstrated that oxidized low-density lipoprotein (ox-LDL) is a key factor in the initiation and progression of atherosclerosis due to its roles in inducing endothelial dysfunction and causing oxidative chain reactions (8,9). ox-LDL binds to the lectin-like ox-LDL receptor-1 on the surface of ECs to trigger the accumulation of reactive oxygen species (ROS) and inhibit nitric oxide (9). Following this, ROS production can further upregulate the formation of ox-LDL and activate lipid peroxidation, which may cause apoptosis and increased oxidative stress (10,11). Accumulating evidence has demonstrated that ox-LDL-induced ROS is the main cause of endothelial dysfunction and apoptosis (12,13). Therefore, inhibition of ox-LDL-induced apoptosis and ROS may be a promising strategy to prevent the development of atherosclerosis.
Signal transducer and activator of transcription (STAT)3, a member of the STAT transcription factor family, has been reported to regulate cell apoptosis and growth in various cell types (14). It has been shown that STAT3 modulates inflammation and cell survival processes in ECs (15). In addition, the STAT3 signaling pathway has been shown to act as a therapeutic target in the prevention of atherosclerosis or other cardiovascular diseases via the inhibition of EC apoptosis (16). For example, myricitrin (a glycosyloxyflavone from the plant Myrica cerifera) was demonstrated to have anti-inflammatory, anti-oxidative and anti-nociceptive properties in in vitro and in vivo atherosclerosis models by activating the STAT3 signaling pathway (16). These results indicate that STAT3 acts as a target in the prevention of atherosclerosis or other cardiovascular diseases.
MicroRNAs (miRs/miRNAs) are endogenous small non-coding RNA molecules of 22–25 nucleotides, which act as unique regulators of gene expression at the post-transcriptional level by inhibiting translation or promoting RNA degradation (17). Increasing evidence has revealed that miRNAs are involved in a variety of biological and pathological processes, such as cellular apoptosis, proliferation, differentiation and carcinogenesis (18–20). Several miRNAs have been demonstrated to regulate crucial factors or key pathways in atherosclerosis, which could indicate the importance of miRNAs in the development of cardiovascular disease (21). miR-21 has been shown to suppress apoptosis and induce the proliferation of vascular smooth muscle cells in the progression of atherosclerosis (22). Cheng et al (23), demonstrated that miR-145 inhibited the differentiation of smooth muscle cells and promoted lesion formation in atherosclerosis. Additionally, systemic delivery of miR-181b ameliorated atherosclerosis in apolipoprotein E-deficient mice via the suppression of NF-κB signaling (24). It is well documented that miR-106a-5p has inhibitive effects via the suppression of cell proliferation and migration, as well as having roles in the induction of apoptosis in various types of cancers (25,26). Furthermore, it has been identified that miR-106a-5p is overexpressed in atherosclerotic plaques and involved in biological processes associated with angiogenesis in homozygous LDL receptor-deficient mice (27). However, whether miR-106a-5p also participates in ox-LDL-induced EC apoptosis and oxidative injury remains to be clarified. Therefore, the aim of the present study was to investigate the role and molecular mechanisms of miR-106a-5p in the process of atherosclerosis, using a model of EC injury induced by ox-LDL. This research may provide further evidence concerning the molecular basis of atherosclerosis development.
Materials and methods
Cell culture
The human umbilical vein ECs (HUVECs) were purchased from American Type Culture Collection. They were cultured on DMEM supplemented with 10% FBS (both purchased from Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). Cells were maintained at 37°C in a humidified chamber containing 5% CO2 for 24 h and then treated with different concentrations of ox-LDL (0–80 µg/ml) for 12, 24, 36 and 48 h. ox-LDL was purchased from Hangzhou Union Biotechnology Co., Ltd.
Cell transfection
The miR-106a-5p mimics (5′-AAAAGUGCUUACAGUGCAGGUAG-3′), miR-106a-5p inhibitor (5′-UAUGGCUUUUUAUUCCUAUGUGA-3′) and scrambled mimic or inhibitor were designed and synthesized by Shanghai GenePharma Co., Ltd. si-STAT3 (5′-GCAGCAGCTGAACAACATG-3′) and si-Scramble (5′-UUCUCCGAACGUGUCACGUTT-3′) were also designed and purchased from Shanghai GenePharma Co., Ltd. miR-106a-5p mimics (50 nM), miR-106a-5p inhibitor (50 nM) or si-STAT3 (100 nM) were transfected into cells (2×104 cells) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. After transfection for 48 h, cells were then exposed to ox-LDL for 48 h and collected for further experiments. Inhibitor experiments were performed using 30 µm STA-21 (Enzo Life Sciences, Inc.) as previously described (28). Briefly, HUVEC cells were co-treated with miR-106a-5p inhibitor and either 30 µm STA-21 or DMSO for 24 h and then exposed to ox-LDL for 48 h and collected for further experiments.
RNA extraction and reverse transcription-quantitative (RT-q) PCR
Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) from cells, according to the manufacturer's protocols. For detection of miRNA expression, cDNA was obtained from 10 ng RNA using TaqMan™ MicroRNA assay (Applied Biosystems; Thermo Fisher Scientific, Inc.) at 42°C for 1 h. For detection of mRNA expression, cDNA was prepared from 300 ng RNA using PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.) at 42°C for 1 h. The U6 gene was used as a reference control for miR-106a-5p and GAPDH was used as a reference control for STAT3. RT-qPCR was performed on an Applied Biosystems™ 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with miRNA-specific primers using the TaqMan Gene Expression assay kit. The primer sequences were as follows: miR-106a-5p forward, 5′-GATGCTCAAAAAGTGCTTACAGTGCA-3′ and reverse, 5′-TATGGTTGTTCTGCTCTCTGTCTC-3′; STAT3 forward, 5′-ATCACGCCTTCTACAGACTGC-3′ and reverse, 5′-CATCCTGGAGATTCTCTACCACT-3′; U6 forward, 5′-TCCGATCGTGAAGCGTTC-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; GAPDH forward, 5′-AGCCTCAAGATCATCAGCAATGCC-3′ and reverse, 5′-TGTGGTCATGAGTCCTTCCACGAT-3′. The amplification conditions consisted of 40 cycles of denaturation at 95°C for 5 sec, annealing at 55°C for 20 sec, and extension at 72°C for 20 sec. All reactions were performed in triplicate. The miR-106a-5p relative expression was analyzed using the 2−ΔΔCq method (29).
Cell viability analysis
The CCK-8 assay was used to evaluate cell viability, according to the manufacturer's protocols (30). Briefly, transfected HUVECs were seeded into 96-well plates with complete medium at a density of 2×104 and incubated with 80 µg/ml ox-LDL at 37°C for 48 h. Afterwards, 10 µl of CCK-8 reagent (Dojindo Molecular Technologies, Inc.) was added for another 4-h culture at 37°C. The absorbance was measured at 450 nm to evaluate cell viability.
Apoptosis analysis
After transfection, HUVECs were incubated with 80 µg/ml ox-LDL at 37°C for 48 h. Then, Annexin V-FITC (BD Biosciences) and propidium iodide (PI; 50 µg/ml; BD Biosciences) staining were used to detect cell apoptosis, according to the manufacturer's protocol. Briefly, the cells were collected and washed twice with ice-cold PBS and then resuspended in binding buffer. The cells were then cultured with 5 µl Annexin V-FITC and 10 µl PI at room temperature in the dark for 20 min. Stained cells were analyzed using flow cytometry (BD FACSCalibur™; BD Biosciences) and BD CellQuest™ software version 3.3 (BD Biosciences). The measurements were performed independently for at least three times with similar results.
Analysis of caspase-3 activation
Caspase-3 activity was measured by a fluorometric assay kit (cat. no. K105-25; BioVision, Inc.), according to the manufacturer's protocols. The samples were determined at 405 nm using a microplate reader (model 680; Bio-Rad Laboratories, Inc.). The results are described as fold changes compared with the control group.
Intracellular ROS measurement
H2DCFDA, a cell-permeable fluorogenic probe that can be modified by cellular esterases to form a non-fluorescent H2DCF, was used for ROS detection in this study. The intracellular ROS levels were tested by a total ROS detection assay kit (cat. no. K936; BioVision, Inc.), according to the manufacturer's protocols. Briefly, the HUVECs were harvested by centrifugation at 300 × g for 5 min at room temperature. The cells were re-suspended in culture media with 1X ROS Label and then incubated at 37°C for 30 min in the dark. Subsequently, cells were stained with 500 µl ROS assay buffer at 37°C for 1 h. The staining buffer was replaced by PBS, the DCF fluorescence intensity was measured by the fluorescence microscope. Images were captured with an electronic camera (Olympus Corporation).
Western blot analysis
Total protein from the cell was isolated using RIPA buffer with protease inhibitor cocktail (Pierce; Thermo Fisher Scientific, Inc.). Protein concentration was quantified using a BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein (30 µg) were loaded on 12% SDS-PAGE gel and then transferred to PVDF membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked for 1 h with 5% non-fat milk at room temperature and then incubated overnight at 4°C with primary antibodies against p22phox (1:1,000; cat. no. ab80896; Abcam), STAT3 (1:1,000; cat. no. 9139; Cell Signaling Technology, Inc.) and β-actin (1:10,000; cat. no. sc47778; Santa Cruz Biotechnology, Inc.). Following incubation with the corresponding horseradish peroxidase-conjugated goat anti-mouse secondary antibodies (1:10,000; cat. no. sc2005; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature, the bands were detected using the enhanced chemiluminescence detection system (PerkinElmer, Inc.). The intensity of the bands of interest was analyzed with ImageJ software version 1.46 (National Institutes of Health).
Bioinformatics analysis
miRNA target prediction tool TargetScan Release 7.0 (http://targetscan.org/) was used to search for the putative targets of miR-106a-5p.
Luciferase reporter assay
The miR-106a-5p mimics/inhibitor and the corresponding negative controls (NCs) were designed and synthesized by Shanghai GenePharma Co., Ltd. The fragment of the 3′-UTR of STAT3 [wild-type (wt) or mutant (mut)] was amplified and cloned into the pMIR-REPORT luciferase vector (Ambion; Thermo Fisher Scientific, Inc.). Site-directed mutagenesis of the STAT3 3′-UTR at the putative miR-106a-5p binding site was performed by a QuikChange kit (Qiagen, Inc.). Subsequently, HUVECs at a density of 2×105/well were seeded into 24-well plates and co-transfected with 0.8 μg of pMIR-STAT3-wt-3′-UTR or pMIR-STAT3-mut-3′-UTR, 50 nM miR-106a-5p mimic/inhibitor or the corresponding NC using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The relative firefly luciferase activity was measured 48 h after transfection by using the Dual-Light® luminescent reporter gene assay (Applied Biosystems; Thermo Fisher Scientific, Inc.). Renilla luciferase expression of pRL-TK plasmids (Promega Corporation) was used for normalization.
Statistical analysis
All statistical analyses were performed using SPSS 14.0 software (SPSS, Inc.). Each experiment was repeated at least 3 times. Data are presented as the mean ± SEM. One-way ANOVA followed by Tukey's post hoc test was used to verify statistically differences among the groups. P<0.05 was considered to indicate a statistically significant difference.
Results
ox-LDL induces miR-106a-5p upregulation in HUVECs
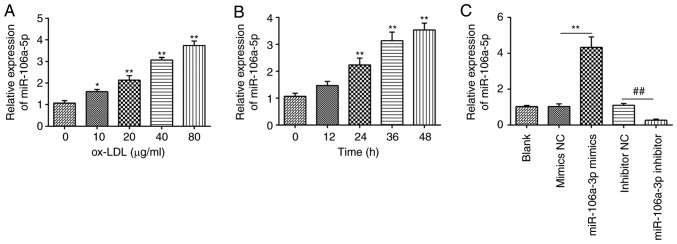
To investigate whether ox-LDL regulates the miR-106a-5p level in HUVECs, cells were treated with ox-LDL at different concentrations (0–80 µg/ml) for 48 h, as previously described (31), and the miR-106a-5p level was determined by RT-qPCR. It was observed that ox-LDL significantly increased miR-106a-5p expression in a dose-dependent manner (Fig. 1A). Based on these results, the HUVECs were subjected to 80 µg/ml ox-LDL for various time durations (0–48 h). As shown in Fig. 1B, treatment of HUVECs with 80 µg/ml of ox-LDL for various durations can lead to a time-dependent upregulation in the levels of miR-106a-5p. Therefore, 80 µg/ml of ox-LDL for 48 h was selected for subsequent experiments. In addition, the efficiency of miR-106a-5p mimics and the miR-106a-5p inhibitor in HUVECs was evaluated. The results showed that miR-106a-5p mimics led to the upregulation of miR-106a-5p compared with the NC mimics, whereas the miR-106a-5p inhibitor resulted in the downregulation of miR-106a-5p (P<0.01; Fig. 1C). These results indicated that miR-106a-5p was upregulated in HUVECs incubated with ox-LDL.
Figure 1.
Effect of ox-LDL on expression levels of miR-106a-5p in HUVECs. (A) HUVECs were treated with ox-LDL at different concentrations (0, 10, 20, 40 or 80 µg/ml) for 24 h. (B) HUVECs were subjected to 80 µg/ml of ox-LDL for various time durations (0, 12, 24 or 48 h). *P<0.05, **P<0.01 vs. 0. (C) HUVECs were transfected with miR-106a-5p mimics or miR-106a-5p inhibitor. Expression of miR-106a-5p was measured using reverse transcription-quantitative PCR. Data are presented as the mean ± SD of three individual experiments. **P<0.01; ##P<0.01. ox-LDL, oxidized low-density lipoproteins; HUVECs, human umbilical vein endothelial cells; miRNA, microRNA; NC, negative control.
Knockdown of miR-106a-5p alleviates ox-LDL-induced HUVECs apoptosis
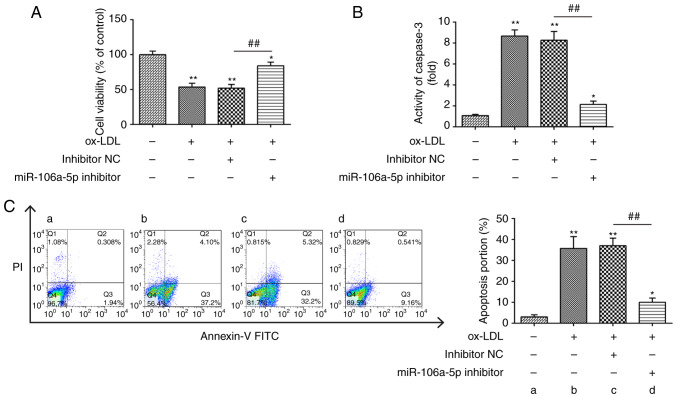
To explore the potential function of miR-106a-5p in HUVECs, the miR-106a-5p inhibitor was transfected into ox-LDL-treated HUVECs, and then the cell viability was measured using a CCK-8 assay. It was found that ox-LDL treatment decreased the viability of HUVECs compared with the blank group, whereas knockdown of miR-106a-5p reversed the ox-LDL-induced effect on cell viability (P<0.01; Fig. 2A). Moreover, ox-LDL treatment led to an increase in caspase-3 activity, which was reversed by the knockdown of miR-106a-5p (P<0.01; Fig. 2B). In addition, ox-LDL treatment significantly increased the percentage of HUVECs in apoptosis, but the knockdown of miR-106a-5p inhibited ox-LDL-induced apoptosis (P<0.01; Fig. 2C). These data suggested that knockdown of miR-106a-5p promoted cell viability and suppressed the apoptosis induced by ox-LDL in HUVECs.
Figure 2.
Knockdown of miR-106a-5p alleviates the effects of ox-LDL on the viability of HUVECs. After transfection with a miR-106a-5p inhibitor or inhibitor NC, the HUVECs were exposed to ox-LDL (80 µg/ml) for 48 h. (A) Cell Counting Kit-8 assay was used to detect cell viability. (B) Caspase-3 activity was tested using a fluorometric assay kit. (C) Cell apoptosis was evaluated using flow cytometry. Data are presented as the mean ± SD of three individual experiments. *P<0.05, **P<0.01 vs. blank group; ##P<0.01 vs. inhibitor NC. ox-LDL, oxidized low-density lipoproteins; HUVECs, human umbilical vein endothelial cells; miR, microRNA; NC, negative control.
Knockdown of miR-106a-5p protects against ox-LDL-induced ROS accumulation
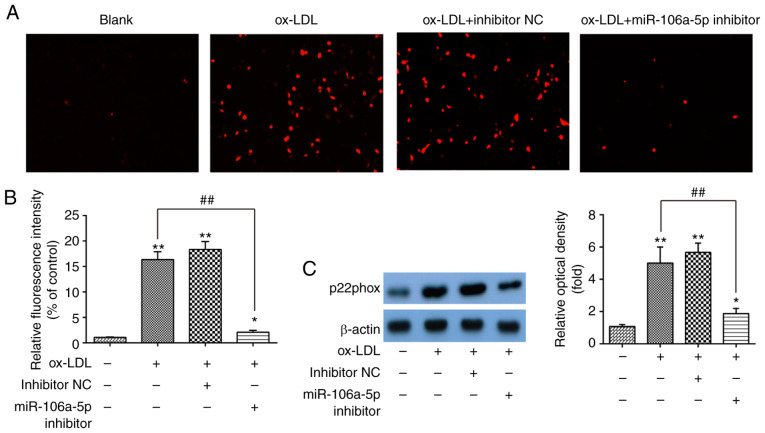
It is reported that the accumulation of ROS is associated with the initiation and progression stages of atherogenesis (32). Therefore, the effects of miR-106a-5p on ox-LDL-induced ROS were investigated in HUVECs. HUVECs were treated with 80 µg/ml of ox-LDL for 48 h after transfection with the miR-106a-5p inhibitor or inhibitor NC, and the ROS levels were detected by a ROS detection kit. As shown in Fig. 3A and B, compared with blank group, ox-LDL-treated HUVECs induced a high ROS level, but the knockdown of miR-106a-5p markedly inhibited the accumulation of ROS induced by ox-LDL (P<0.01). To further investigate the anti-oxidative effects of miR-106a-5p downregulation, western blotting was performed to detect the expression level of the oxidative injury-related protein p22phox (33). As shown in Fig. 3C, knockdown of miR-106a-5p decreased the expression of p22phox in ox-LDL-treated HUVECs (P<0.01). These results indicated that the knockdown of miR-106a-5p led to anti-oxidative effects against ox-LDL-induced ROS levels in HUVECs.
Figure 3.
Knockdown of miR-106a-5p inhibits ox-LDL-induced ROS in HUVECs. After transfection with a miR-106a-5p inhibitor or inhibitor NC, HUVECs were exposed to ox-LDL (80 µg/ml) for 48 h. (A) ROS levels were detected using a ROS detection kit. (B) Relative fluorescence intensity is shown in the bar graphs. (C) p22phox protein level was detected using western blotting; β-actin was used as an internal control. *P<0.05, **P<0.01 vs. blank group; ##P<0.01 vs. inhibitor NC. ROS, reactive oxygen species; ox-LDL, oxidized low-density lipoproteins; miR, microRNA; NC, negative control; HUVECs, human umbilical vein endothelial cells.
STAT3 is a direct target of miR-106a-5p in HUVECs
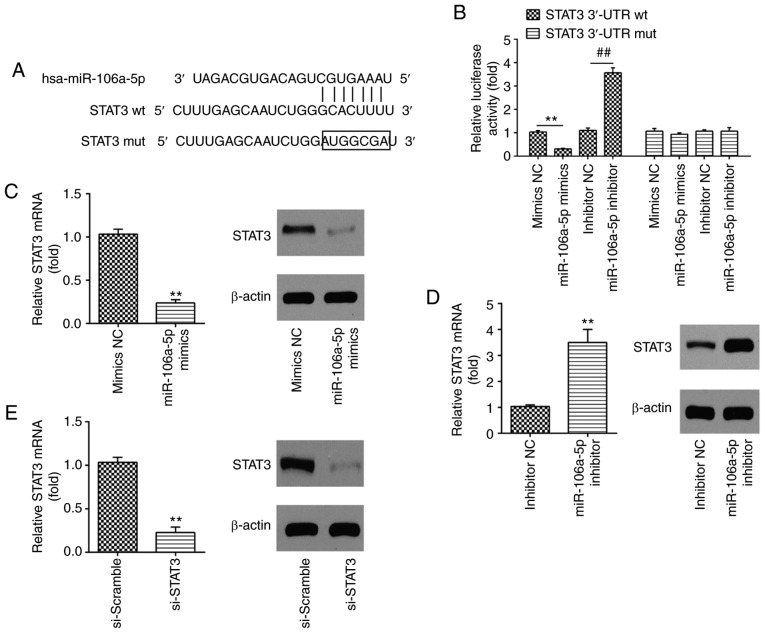
TargetScan was used to predict the putative targets of miR-106a-5p, and it identified that miR-106a-5p has a number of potential targets that are associated with cell apoptosis. This includes programmed cell death 1 ligand 2, apoptosis enhancing nuclease, BCL-2-like protein 2, BCL-2-like protein 11 (apoptosis facilitator) and Fas apoptotic inhibitory molecule 2. In the present study, STAT3 was selected to investigate further due to its association with atherosclerosis development via the regulation of cell apoptosis and inflammation, and because it is a target of miR-106a-5p (34). Thus, STAT3 was identified as a possible target gene of miR-106a-5p (Fig. 4A). To confirm this bioinformatics prediction, a luciferase reporter assay was carried out in HUVECs via co-transfection with a wt or mut reporter plasmid along with miR-106a-5p mimics/inhibitor or NC. The results revealed that the upregulation of miR-106a-5p notably reduced the relative luciferase activity when compared with the mimic NC, while miR-106a-5p inhibitor resulted in the opposite effect in the presence of the wt 3′-UTR (P<0.01; Fig. 4B). As predicted, the miR-106a-5p mimics/inhibitor failed to regulate the relative luciferase activity of the vector containing mut STAT3-3′-UTR in the miR-106a-5p-binding site (Fig. 4B). Western blot analysis and RT-qPCR further confirmed that the overexpression of miR-106a-5p significantly downregulated the mRNA and protein expression level of STAT3 (P<0.01; Fig. 4C), whilst knockdown of miR-106a-5p upregulated the expression level of STAT3 in HUVECs (P<0.01; Fig. 4D). These data indicated that miR-106a-5p suppressed STAT3 expression by targeting its 3′-UTR. Additionally, the knockdown efficiency of si-STAT3 in HUVECs was evaluated, and it was observed that si-STAT3 can significantly downregulate the levels of mRNA and protein expression of STAT3 compared with si-Scramble (P<0.01; Fig. 4E).
Figure 4.
STAT3 is a direct target of miR-106a-5p in HUVECs. (A) STAT3 3′-UTR region containing the wt or mut binding site for miR-106a-5p. (B) HUVECs were co-transfected with either pMIR-STAT3-2-3-UTR or pMIR-STAT3-mut-3′-UTR, and miR-106a-5p mimic/inhibitor or corresponding NC and the relative luciferase activity were measured. **P<0.01; ##P<0.01. HUVECs transfected with miR-106a-5p (C) mimic or (D) inhibitor and corresponding NC, the mRNA and protein level of STAT3 was measured using western blotting; β-actin was used as an internal control. **P<0.01 vs. mimics/inhibitor NC. (E) HUVECs transfected with si-STAT3 or si-Scramble, the STAT3 mRNA and protein level was detected using RT-qPCR and western blot analysis, respectively; β-actin was used as an internal control. **P<0.01 vs. si-Scramble. wt, wild-type; mut, mutant; STAT3, signal transducer and activator of transcription 3; miR, microRNA; HUVECs, human umbilical vein endothelial cells; NC, negative control; RT-qPCR, reverse transcription-quantitative PCR
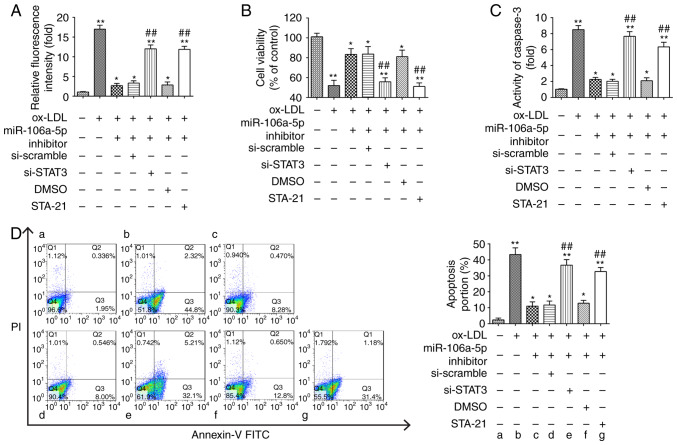
STAT3 silencing interferes with the protective effects of miR-106a-5p downregulation on ox-LDL-induced ROS accumulation and HUVEC apoptosis
To investigate whether the protective effects of miR-106a-5p inhibition on ox-LDL-induced ROS accumulation and cell apoptosis were modulated by STAT3 expression, the HUVECs were co-treated with miR-106a-5p inhibitor and one of the following: si-STAT3; si-scramble; STA-21 (a STAT3 inhibitor); or DMSO. Following this, they were treated with 80 µg/ml of ox-LDL for 48 h. These results showed that the inhibition of miR-106a-5p decreased the ox-LDL-induced ROS level compared with the ox-LDL group, but when cells were transfected with the inhibitor + si-STAT3 or STA-21, the miR-106a-5p inhibitor no longer reduced the ROS level (P<0.01; Fig. 5A). The CCK-8 assay demonstrated that the knockdown of miR-106a-5p increased cell viability, which ox-LDL alone decreased, whereas si-STAT3 and STA-21 reversed the effect of miR-106a-5p inhibition on cell viability in ox-LDL-incubated HUVECs (P<0.01; Fig. 5B). As expected, STA-21 also attenuated the protective effects of miR-106a-5p inhibition on caspase-3 activity and cell apoptosis induced by ox-LDL in HUVECs when compared with the ox-LDL and miR-106a-5p inhibitor group (P<0.01; Fig. 5C and D). These results suggested that the inhibition of STAT3, using STA-21, impaired the protective effects of inhibiting miR-106a-5p in ox-LDL-induced HUVECs, as observed by measuring levels of ROS and apoptosis.
Figure 5.
Inhibition of STAT3 abolishes the protective effects of the miR-106a-5p inhibitor on ox-LDL-induced HUVEC apoptosis. HUVECs were co-transfected with a miR-106a-5p inhibitor and either si-STAT3 or STA-21 (a STAT3 inhibitor), following which they were treated with 80 µg/ml of ox-LDL for 48 h (si-scramble and DMSO groups used as controls). (A) ROS levels were detected using a ROS detection kit. (B) Cell Counting Kit-8 assay was used to detect cell viability. (C) Caspase-3 activity was tested using a fluorometric assay kit. (D) Cell apoptosis was evaluated using flow cytometry. *P<0.05, **P<0.01 vs. blank group; ##P<0.01 vs. ox-LDL group. STAT3, signal transducer and activator of transcription 3; miR, microRNA; HUVECs, human umbilical vein endothelial cells; NC, negative control; ox-LDL, oxidized low-density; ROS, reactive oxygen species
Discussion
The present study demonstrated that ox-LDL can induce the upregulation of miR-106a-5p in a dose-dependent manner in HUVECs, which is associated with decreased cell viability, as well as inhibition of cell apoptosis and ROS accumulation. However, knockdown of miR-106a-5p can prevent these effects and offer protection from ox-LDL. Specifically, STAT3 was identified as a direct target of miR-106a-5p in HUVECs; STAT3 silencing abolished the protective effect of miR-106a-5p inhibitor in ox-LDL-treated HUVECs. These results indicated that the knockdown of miR-106a-5p protects HUVECs from ox-LDL-induced apoptosis and the accumulation of ROS by inhibiting STAT3, suggesting it may act as a target for atherosclerosis treatment.
Increasing evidence has demonstrated that endothelial functions exert a vital effect on the homeostasis of blood vessels (35). ECs play an important role in vascular diseases, and their dysfunction is regarded as a biomarker in the development of atherosclerosis (36). ox-LDL, a key factor in the initiation and progression of atherosclerosis, has been associated with vascular EC dysfunction, including abnormal apoptosis and proliferation (37). Emerging evidence has demonstrated that a number of miRNAs are associated with the modulation of ox-LDL-induced EC apoptosis and inflammation responses in atherosclerosis, including miR-365, miR-26a, miR-221/222 and miR-181a (38–41). It has been reported that miR-106a-5p is upregulated in atherosclerotic plaques and is associated with angiogenesis in homozygous LDL receptor-deficient mice (27). However, whether miR-106a-5p participates in ox-LDL-mediated HUVEC apoptosis and oxidative injury is not known. In this study, it was found that ox-LDL treatment increased the level of miR-106a-5p in a dose and time-dependent manner. Functional experiments showed that ox-LDL treatment significantly inhibited cell viability and induced cell apoptosis, whereas knockdown of miR-106a-5p reversed the ox-LDL-induced effects on viability and apoptosis in HUVECs. These results suggested that the knockdown of miR-106a-5p protected against ox-LDL-induced apoptosis in HUVECs.
Oxidative stress is a potent pathogenic mechanism in the pathogenesis of atherosclerosis due to its role in inducing cellular injury, apoptosis and mitochondrial dysfunction (42, 43). ox-LDL has been shown to cause damage to ECs due to its roles in the production of ROS and apoptosis (44). A previous study showed that miRNAs modulated inflammation and oxidative stress in relation to atherosclerosis (45). A recent study demonstrated that the knockdown of miR-34a suppressed ox-LDL-stimulated apoptosis and oxidative stress in HUVECs (46). Zhang et al (47), reported that miR-34a/sirtuin-1/forkhead box O3 plays an important role in the protective role of genistein against ox-LDL-induced oxidative damage in HUVECs. In the present study, the results demonstrated that knockdown of miR-106a-5p reduced ox-LDL-induced ROS levels via the suppression of oxidation-related proteins. These findings indicated that knockdown of miR-106a-5p exerted protective effects on ox-LDL-induced HUVEC injury by suppressing ROS production.
It has been reported that STAT3 serves as a vital regulator in various types of chronic inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus and psoriasis (48–50). Gharavi et al (51), found that activated STAT3 was upregulated in the inflammatory regions of human atherosclerotic lesions. Additionally, deletion of STAT3 has been found to reduce fatty streak formation in EC-specific STAT3 knock-out mice (51). Vasamsetti et al (52), demonstrated that inhibiting STAT3 suppressed monocyte-to-macrophage differentiation, which is a pivotal facilitation event in atherosclerotic development. Another previous study identified that miR-106a-5p negatively regulated STAT3 activation by targeting its 3′-UTR in human neuroblastoma cells (53). The results of the present study confirmed that miR-106a-5p inhibited STAT3 by targeting its 3′-UTR in HUVECs. Moreover, inhibition of STAT3 reversed the effect of miR-106a-5p downregulation on cell apoptosis and ROS levels induced by ox-LDL in HUVECs. These results indicated that the downregulation of miR-106a-5p had protective effects on ox-LDL-induced HUVEC apoptosis via the modulation of STAT3.
However, there are limitations of this study. Firstly, only STAT3 was selected to investigate further due to its association with atherosclerosis development via the regulation of cell apoptosis and inflammation, and because it is a target of miR-106a-5p (52); it would be useful to study other targets of miR-106a-5p. Subsequent experiments will focus on other possible targets involved in atherosclerosis. Secondly, this study only investigated HUVECs; the use of coronary or the carotid artery ECs would hold greater relevance in atherosclerosis research than HUVECs. Although HUVECs have been extensively used to research atherosclerosis in previous studies, it would be useful to validate the results of the present study in other cells during subsequent experiments.
In conclusion, these results revealed that ox-LDL results in miR-106a-5p upregulation in a dose-dependent manner. Knockdown of miR-106a-5p reversed the ox-LDL-induced effects on apoptosis and ROS production in HUVECs. Furthermore, it was found that inhibition of miR-106a-5p protects against ox-LDL-induced apoptosis and oxidative stress by modulating STAT3 in HUVECs. Taken together, these findings indicated that the miR-106a-5p/STAT3 axis is an important regulator of the development and progression of atherosclerosis, suggesting that miR-106a-5p may serve as a therapeutic target in the treatment of atherosclerosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YHu, RX, YHe, ZZ, XM and LL performed the experiments, contributed to data analysis and wrote the paper. JH conceptualized the study design, contributed to data analysis and experimental materials. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Ustün-Aytekin O, Gürhan ID, Ohura K, Imai T, Ongen G. Monitoring of the effects of transfection with baculovirus on Sf9 cell line and expression of human dipeptidyl peptidase IV. Cytotechnology. 2014;66:159–168. doi: 10.1007/s10616-013-9549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 6.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 7.Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond) 2004;107:343–354. doi: 10.1042/CS20040086. [DOI] [PubMed] [Google Scholar]
- 8.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 9.Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V, Sawamura T. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem. 2001;276:13750–13755. doi: 10.1074/jbc.M010612200. [DOI] [PubMed] [Google Scholar]
- 10.Uchida K. Redox-derived damage-associated molecular patterns: Ligand function of lipid peroxidation adducts. Redox Biol. 2013;1:94–96. doi: 10.1016/j.redox.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou H-C, Chou F-P, Sheu WH-H, Hsu S-L, Lee W-J. Protective effects of magnolol against oxidized LDL-induced apoptosis in endothelial cells. Arch Toxicol. 2007;81:421–432. doi: 10.1007/s00204-006-0172-3. [DOI] [PubMed] [Google Scholar]
- 12.Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci. 2011;342:135–142. doi: 10.1097/MAJ.0b013e318224a147. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Mohamed ASS, Zhou SH. Oxidized low density lipoprotein, stem cells, and atherosclerosis. Lipids Health Dis. 2012;11:85. doi: 10.1186/1476-511X-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin H, Yeh W-I, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL, Fox TH, III, Park K, et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci USA. 2012;109:5004–5009. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan W, Yang Y, Yi W, Yan J, Liang Z, Wang N, Li Y, Chen W, Yu S, Jin Z, et al. New role of JAK2/STAT3 signaling in endothelial cell oxidative stress injury and protective effect of melatonin. PLoS One. 2013;8:e57941. doi: 10.1371/journal.pone.0057941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin M, Luo Y, Meng XB, Wang M, Wang HW, Song SY, Ye JX, Pan RL, Yao F, Wu P, et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vascul Pharmacol. 2015;70:23–34. doi: 10.1016/j.vph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Behrouz Sharif S, Hashemzadeh S, Mousavi Ardehaie R, Eftekharsadat A, Ghojazadeh M, Mehrtash AH, Estiar MA, Teimoori-Toolabi L, Sakhinia E. Detection of aberrant methylated SEPT9 and NTRK3 genes in sporadic colorectal cancer patients as a potential diagnostic biomarker. Oncol Lett. 2016;12:5335–5343. doi: 10.3892/ol.2016.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 19.le Sage C, Agami R. Immense promises for tiny molecules: Uncovering miRNA functions. Cell Cycle. 2006;5:1415–1421. doi: 10.4161/cc.5.13.2890. [DOI] [PubMed] [Google Scholar]
- 20.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menghini R, Stöhr R, Federici M. MicroRNAs in vascular aging and atherosclerosis. Ageing Res Rev. 2014;17:68–78. doi: 10.1016/j.arr.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of microRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, He S, Wara AKM, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, et al. Systemic delivery of microRNA-181b inhibits nuclear factor-κB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2014;114:32–40. doi: 10.1161/CIRCRESAHA.113.302089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhi F, Zhou G, Shao N, Xia X, Shi Y, Wang Q, Zhang Y, Wang R, Xue L, Wang S, et al. miR-106a-5p inhibits the proliferation and migration of astrocytoma cells and promotes apoptosis by targeting FASTK. PLoS One. 2013;8:e72390. doi: 10.1371/journal.pone.0072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Q, Ma Q. MicroRNA-106a inhibits cell proliferation and induces apoptosis in colorectal cancer cells. Oncol Lett. 2018;15:8941–8944. doi: 10.3892/ol.2018.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuevas A, Saavedra N, Cavalcante MF, Salazar LA, Abdalla DSP. Identification of microRNAs involved in the modulation of pro-angiogenic factors in atherosclerosis by a polyphenol-rich extract from propolis. Arch Biochem Biophys. 2014;557:28–35. doi: 10.1016/j.abb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Meng X-M, Huang C, Wu BM, Zhang L, Lv XW, Li J. Long noncoding RNAs: Novel insights into hepatocelluar carcinoma. Cancer Lett. 2014;344:20–27. doi: 10.1016/j.canlet.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Hu MY, Du XB, Hu HB, Shi Y, Chen G, Wang YY. MiR-410 inhibition induces HUVECs proliferation and represses ox-LDL-triggered apoptosis through activating STAT3. Biomed Pharmacother. 2018;101:585–590. doi: 10.1016/j.biopha.2018.02.111. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Liu X, Li Y, Lai J, Zhang N, Ming J, Ma X, Ji Q, Xing Y. Downregulation of microRNA-155 ameliorates high glucose-induced endothelial injury by inhibiting NF-κB activation and promoting HO-1 and NO production. Biomed Pharmacother. 2017;88:1227–1234. doi: 10.1016/j.biopha.2017.01.122. [DOI] [Google Scholar]
- 33.Lu J, Mitra S, Wang X, Khaidakov M, Mehta JL. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal. 2011;15:2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 34.Cui J, Wang JS, Wang SM, Lian C, Qiu JC, Liu Z. microRNA-106a-5p regulated the funcation human umbilical vein endothelial cells by targeting STAT3. Zhonghua Yi Xue Za Zhi. 2019;99:3814–3818. doi: 10.3760/cma.j.issn.0376-2491.2019.48.011. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 35.Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DYR. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet. 2007;39:1397–1402. doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- 36.Mano T, Masuyama T, Yamamoto K, Naito J, Kondo H, Nagano R, Tanouchi J, Hori M, Inoue M, Kamada T. Endothelial dysfunction in the early stage of atherosclerosis precedes appearance of intimal lesions assessable with intravascular ultrasound. Am Heart J. 1996;131:231–238. doi: 10.1016/S0002-8703(96)90346-4. [DOI] [PubMed] [Google Scholar]
- 37.Xu RX, Sun XC, Ma CY, Yao YH, Li XL, Guo YL, Zhang Y, Li S, Li JJ. Impacts of berberine on oxidized LDL-induced proliferation of human umbilical vein endothelial cells. Am J Transl Res. 2017;9:4375–4389. [PMC free article] [PubMed] [Google Scholar]
- 38.Qin B, Xiao B, Liang D, Xia J, Li Y, Yang H. MicroRNAs expression in ox-LDL treated HUVECs: MiR-365 modulates apoptosis and Bcl-2 expression. Biochem Biophys Res Commun. 2011;410:127–133. doi: 10.1016/j.bbrc.2011.05.118. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, Li X, Shen N, Xiao D, Zhang H, et al. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep. 2015;5:9401. doi: 10.1038/srep09401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin B, Cao Y, Yang H, Xiao B, Lu Z. MicroRNA-221/222 regulate ox-LDL-induced endothelial apoptosis via Ets-1/p21 inhibition. Mol Cell Biochem. 2015;405:115–124. doi: 10.1007/s11010-015-2403-5. [DOI] [PubMed] [Google Scholar]
- 41.Wu C, Gong Y, Yuan J, Zhang W, Zhao G, Li H, Sun A, Zou Y, Ge J. microRNA-181a represses ox-LDL-stimulated inflammation response in dendritic cell by targeting c-Fos. J Lipid Res. 2012;53:2355–2363. doi: 10.1194/jlr.M028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 43.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Yao S-T, Zhai L, Feng YL, Song GH, Yu Y, Zhu P, Qin SC. Ox-LDL down-regulates expression of pigment epithelium-derived factor in human umbilical vein endothelial cells. Sheng Li Xue Bao. 2014;66:489–495. (In Chinese) [PubMed] [Google Scholar]
- 45.Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011;25:2515–2527. doi: 10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- 46.Zhong X, Li P, Li J, He R, Cheng G, Li Y. Downregulation of microRNA - 34a inhibits oxidized low - density lipoprotein - induced apoptosis and oxidative stress in human umbilical vein endothelial cells. Int J Mol Med. 2018;42:1134–1144. doi: 10.3892/ijmm.2018.3663. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Zhao Z, Pang X, Yang J, Yu H, Zhang Y, Zhou H, Zhao J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol Lett. 2017;277:115–122. doi: 10.1016/j.toxlet.2017.07.216. [DOI] [PubMed] [Google Scholar]
- 48.Lao M, Shi M, Zou Y, Huang M, Ye Y, Qiu Q, Xiao Y, Zeng S, Liang L, Yang X, Xu H. Protein inhibitor of activated STAT3 regulates migration, invasion, and activation of fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol. 2016;196:596–606. doi: 10.4049/jimmunol.1403254. [DOI] [PubMed] [Google Scholar]
- 49.Kluger MA, Melderis S, Nosko A, Goerke B, Luig M, Meyer MC, Turner JE, Meyer-Schwesinger C, Wegscheid C, Tiegs G, et al. Treg17 cells are programmed by Stat3 to suppress Th17 responses in systemic lupus. Kidney Int. 2016;89:158–166. doi: 10.1038/ki.2015.296. [DOI] [PubMed] [Google Scholar]
- 50.Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, Iiyama T, Asao N, DiGiovanni J, Sano S. Stat3 as a therapeutic target for the treatment of psoriasis: A clinical feasibility study with STA-21, a Stat3 inhibitor. J Invest Dermatol. 2011;131:108–117. doi: 10.1038/jid.2010.255. [DOI] [PubMed] [Google Scholar]
- 51.Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, Johnson J, Szeto WL, Hong L, Fishbein M, et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007;282:31460–31468. doi: 10.1074/jbc.M704267200. [DOI] [PubMed] [Google Scholar]
- 52.Vasamsetti SB, Karnewar S, Kanugula AK, Raj AT, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: Potential role in atherosclerosis. Diabetes. 2014;64:2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, Ye Y, Cong J, Pu D, Liu J, Hu G, Wu J. Regulation of STAT3 by miR-106a is linked to cognitive impairment in ovariectomized mice. Brain Res. 2013;1503:43–52. doi: 10.1016/j.brainres.2013.01.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.