Abstract
Colorectal cancer is one of the commoner digestive tract malignant tumor types, and its incidence and mortality rate are high. Accumulating evidence indicates that long-chain non-coding RNAs (lncRNAs) and protein-coding RNAs interact with each other by competing with the same micro(mi)RNA response element (MREs) and serve an important role in the regulation of gene expression in a variety of tumor types. However, the regulatory mechanism and prognostic role of lncRNA-mediated competing endogenous (ce)RNA networks in colon cancer have yet to be elucidated. The expression profiles of mRNAs, lncRNAs and miRNAs from 471 colon cancer and 41 paracancerous tissue samples were downloaded from The Cancer Genome Atlas database. A lncRNA-miRNA-mRNA ceRNA network in colon cancer was constructed and comprised 17 hub lncRNAs, 87 hub miRNA and 144 hub mRNAs. The topological properties of the network were analyzed, and the random walk algorithm was used to identify the nodes significantly associated with colon cancer. Survival analysis using the UALCAN database indicated that 2/17 lncRNAs identified [metastasis-associated lung adenocarcinoma transcript (MALAT1) and maternally expressed gene 3 (MEG3)] and 5/144 mRNAs [FES upstream region (FURIN), nuclear factor of activated T-cells 5 (NFAT5), RNA Binding Motif Protein 12B (RBM12B), Ras related GTP binding A (RRAGA) and WD repeat domain phosphoinositide-interacting protein 2 (WIPI2)] were significantly associated with the overall survival of patients with colon cancer, and may therefore be used as potential prognostic biomarkers of colon cancer. According to extracted lncRNA-miRNA-mRNA interaction pairs, the GSE26334 dataset was used to confirm that the lncRNA MALAT1/miR-129-5p/NFAT5 axis may represent a novel regulatory mechanism concerning the progression of colon cancer. The clusterProfiler package was used to analyze Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in colon cancer. Finally, drugs that significantly interact with the core genes identified in colon cancer were predicted using a hypergeometric test. Of these, fostamatinib was identified to be a targeted drug for colon cancer therapy. The present findings provide a novel perspective for improved understanding of the lncRNA-associated ceRNA network and may facilitate the development of novel targeted therapeutics in colon cancer.
Keywords: colon cancer, competing endogenous RNA network, long non-coding RNAs, prognostic biomarker, targeted drug
Introduction
Colon cancer accounts for the third highest incidence of cancer in the world, and its mortality rate is also increasing (1). Previous studies have revealed that a family or personal history of colorectal cancer, colorectal polyps or chronic inflammatory bowel disease are the most common risk factors for colorectal cancer (2–4). Despite the development of early diagnostic techniques, more than 25% of patients present with metastases when they are diagnosed (5). Notably ~50% of patients with colon cancer will exhibit recurrence and succumb to the disease within 5 years; although chemotherapy and targeted therapy have significantly improved the efficacy of treatment, the internal mechanism underlying colon cancer tumorigenesis has yet to be elucidated (6). Hence, it is imperative to study the molecular mechanism underlying colon cancer and identify novel biomarkers to improve the prognosis of patients with colon cancer.
The competing endogenous (ce)RNA network hypothesis was proposed by Salmena et al (7) as early as 2011 and ceRNA represents a novel mechanism of gene expression regulation. The ceRNA hypothesis is a supplement to the traditional micro(mi)RNA regulation of RNA theory, and there is a reverse RNA to miRNA action mode, that is, ceRNAs, through miRNA response elements (MREs), compete for the same miRNAs, to regulate the expression of target genes. Recently, the molecules that influence ceRNA networks include long non-coding (lnc)RNAs, pseudogenes, circular (circ)RNAs and other molecules (8–10). Numerous experimental studies have supported the theory of ceRNA network regulation (11,12). For example, PTEN, SNHG6, lncRNA-H19 and other molecules regulate the expression of corresponding target genes and influence the occurrence and progression of cancer through their conservative 3′UTR competitive adsorption of miRNA molecules (7,13).
In the present study, the disciplinary advantages of molecular biology and bioinformatics were integrated in order to construct a molecular regulatory network in colon cancer, with lncRNA at the core, which may provide evidence to improve understanding of the mechanism underpinning the occurrence and progression of colon cancer. Similarly, the current methodology provides a novel concept for the study of other cancer-associated mechanisms.
Materials and methods
Data source and processing
The RNA-Seq V2 data and corresponding clinical information of patients with colon cancer were downloaded from The Cancer Genome Atlas (TCGA) data portal (https://tcga-data.nci.nih.gov/tcga/). Registration data from a total of 546 patients were retrieved; 75 patients were excluded because they had only clinical information and not sequencing data. Finally, 471 patients with complete clinical and sequencing information were included in the present study. Of the 471 patients, 41 had paracancerous tissues which were sequenced simultaneously. The mRNA and miRNA sequencing data were processed using R software (R version 3.5.2; http://www.R-project.org/), and any lncRNAs with a description from Ensembl (https://asia.ensembl.org/index.html) were selected for further study. The data of human miRNA-target gene interaction were retrieved from the TargetScan (http://www.targetscan.org/vert_72/), miRDB (http://www.mirdb.org/) (14), PITA (https://genie.weizmann.ac.il/pubs/mir07/mir07 _data.html) and miRanda databases (http://miranda.org.uk/), and the lncRNA-miRNA interaction data came from the miRcode (http://www.mircode.org/) (15), StarBase (http://starbase.sysu.edu.cn/) and lncBase databases (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r-lncbasev2%2Findex) (16). RNA-seq profile data and the clinical characteristics of colon cancer are available on open-access databases; therefore, there was no requirement for approval by the local ethics committee.
miRNA-mRNA interactive data acquisition
The interaction data of human miRNA- target genes were downloaded from TargetScan, miRDB, PITA and miRanda databases. The miRNA-mRNA interacting pairs common to >3 databases were selected as credible miRNA-target gene pairs. Among them, TargetScan, miRanda and PITA are miRNA-gene symbol interacting pairs, while miRDB is miRNA-refseq ID interacting pairs. Therefore, refseq ID was converted to gene symbols via the hg38 annotation file. Then, if the miRNA-mRNA interacting pair was common to >3 databases, it was regarded as a significant miRNA-mRNA interacting pair. Finally, the interacting pairs were de-duplicated.
miRNA-lncRNA interactive data acquisition
The human lncRNA-miRNA interaction data was downloaded from three databases (miRcode, StarBase and lncBase), and the lncRNA-miRNA interacting pairs common to >2 databases were selected as lncRNA-miRNA interacting pairs. Finally, the repeated interacting pairs were removed.
Data acquisition of lncRNA-mRNA expression profiles in colon cancer
The RNASeqV2 data were downloaded from TCGA database. First, the expression values of genes with the same gene name were selected as the mean value, and the lncRNA expression profile and mRNA expression profile were obtained by separating RNA.
Construction of the ceRNA network
Using the target interacting database, it was considered that the number of shared target miRNA between lncRNA and mRNA was >3 and the hypergeometric test false discovery rate (FDR) <0.01 was the cutoff used to identify potential lncRNA-mRNA ceRNA interacting pairs. The Spearman correlation coefficient of potential lncRNA-mRNA ceRNA pairs was calculated using the lncRNA and mRNA expression profile of colon cancer. An R >0, adjusted P-value <0.01, lncRNA-mRNA relationship combined with its shared miRNA constituted a competitive endogenous RNA network of lncRNA-miRNA-mRNA in colon cancer.
Analysis of ceRNA network properties
Cytoscape was used for ceRNA network presentation (17), and Molecular Complex Detection (MCODE) was used to identify four modules in the ceRNA network (17). The topological properties of the network were analyzed (degree distribution, clustering coefficient and hub analysis), in which hub miRNA and hub lncRNA were selected as the core regulatory factors of colon cancer, and hub mRNA was selected as the core gene of colon cancer. Then, the random walk algorithm was used to mine the function factor (lncRNA/miRNA/mRNA) in the ceRNA network. Furthermore, enrichR was used to analyze the function and pathway of the genes (18). Finally, hub mRNA and lncRNA were selected for survival analysis using the UALCAN database (http://ualcan.path.uab.edu/index.html) to verify that whether they could be used as a prognostic biomarker of colon cancer (19).
Identifying the lncRNA-miRNA-mRNA regulatory axis of colon cancer from extracted lncRNA-miRNA-mRNA interaction pairs using GEO datasets
GEPIA (http://gepia.cancer-pku.cn/) was used to analyze the ceRNA interacting pairs extracted from a colon cancer dataset (20). Person correlation analysis was conducted on lncRNA and mRNA, to obtain the most positive correlation between lncRNA and mRNA relationship pairs. Next, LncACTdb 2.0 (http://www.bio-bigdata) was used to verify the regulatory association between lncRNA and miRNA that had been validated by relevant experiments (21), and finally the GSE26334 dataset was used to verify the regulatory association between miRNA and mRNA (22). The expression of mRNAs, lncRNAs and miRNAs in LoVo colon cancer lines was determined using R and visualized using GGPLOT2 3.1.0 (https://cran.r-project.org/web/packages/ggplot2/index.html).
Target set of core regulators and functional enrichment analysis of core genes
The target genes (target set) of the core regulatory factors of top 10 were screened, and the function and pathway enrichment of the target genes were analyzed using enrichR. Then, enrichR was used to analyze the function and pathway of core genes in colon cancer.
Drug design
The core genes of colon cancer were combined with all the drugs on Drugbank5.0 (https://www.drugbank.ca) (23), and significance analysis was performed using a hypergeometric test to identify the drugs that may have therapeutic effect on colon cancer.
Statistical analysis
Student's t test was used to estimate the significance of difference in gene expression levels between groups. The t-test was performed using a PERL script with Comprehensive Perl Archive Network (CPAN) module ‘Statistics: T Test’.
Available TCGA patient survival data were used for Kaplan-Meier survival analyses and to generate overall survival plots. The P-value obtained from log-rank test was used to indicate statistical significance of survival correlation between groups (19). P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the subjects included in the study
The detailed clinical and pathological characteristics of all the 546 colon cancer patients downloaded from TCGA are listed in Table I.
Table I.
Clinicopathological characteristics of all the 546 patients with colon cancer downloaded from TCGA.
| Clinical features | Patients n (%) |
|---|---|
| Age (years) | |
| >67 | 302 (55.31) |
| ≤67 | 244 (44.69) |
| Sex | |
| Male | 284 (52.01) |
| Female | 262 (47.99) |
| Pathology stage | |
| I | 86 (15.75) |
| II | 219 (40.11) |
| III | 152 (27.84) |
| IV | 78 (14.29) |
| Unknown | 11 (2.01) |
| Pathology M stage | |
| M0 | 398 (72.89) |
| M1 | 78 (14.29) |
| MX | 62 (11.36) |
| Unknown | 8 (1.47) |
| Pathology N stage | |
| N0 | 323 (59.16) |
| N1 | 124 (22.71) |
| N2 | 99 (18.13) |
| Pathology T stage | |
| T1 | 11 (2.01) |
| T2 | 90 (16.48) |
| T3 | 377 (69.05) |
| T4 | 67 (12.27) |
| TX | 1 (0.18) |
TCGA, The Cancer Genome Atlas.
Screening miRNA-mRNA interaction pairs
The human miRNA-target gene interaction data were downloaded from four databases (TargetScan, miRDB, PITA and miRanda). If the miRNA-mRNA interacting pair appeared in >3 databases, it was included as a miRNA-mRNA interacting pair. As a result, 160,345 miRNA-mRNA interacting pairs were selected.
Screening lncRNA-miRNA interaction pairs
The human lncRNA-miRNA interaction data was downloaded from three databases including miRcode, StarBase, lncBase, and the lncRNA-miRNA interacting pairs that appeared in more than two databases were selected as lncRNA-miRNA interacting pairs. Finally, 3,158 lncRNA-miRNA interacting pairs were obtained.
Construction of the ceRNA network
Identification of candidate ceRNA interacting pairs
Combined with the miRNA-mRNA interacting pair and lncRNA-miRNA interacting pair data, if each pair of lncRNA and mRNA sharedthe same miRNA, the lncRNA and mRNA were connected as an interacting pair. Using the R package, the hypergeometric test was applied to calculate the significance of association between lncRNA and mRNA pairs and miRNAs for each pair of lncRNA and mRNA. The parameters were as follows: Q, the number of miRNAs shared by the lncRNA and mRNA to be tested; k, number of miRNAs interacting with the gene to be tested; m, number of miRNAs interacting with the lncRNA to be tested; and n, total number of miRNAs minus the number of miRNAs to be tested. lncRNA-mRNA interacting pairs with the number of shared target miRNA >3 and FDR <0.01 between lncRNAs and mRNAs were selected as potential ceRNA interacting pairs. Eventually, 28,668 potential ceRNA interactions were obtained.
Acquisition of the candidate ceRNA interaction pairs
The Spearman correlation coefficient of each potential lncRNA-mRNA ceRNA pair was calculated using the lncRNA and mRNA expression profile in colon cancer. If R>0, the lncRNA-mRNA association of P-adjusted <0.01 was identified as a ceRNA network of lncRNA-mRNA in colon cancer. Finally, a total of 6,933 colon cancer lncRNA-miRNA-mRNA interaction pairs and 1,258 colon cancer lncRNA-mRNA interaction pairs were identified.
Display of the ceRNA network and analysis of topological properties in colon cancer
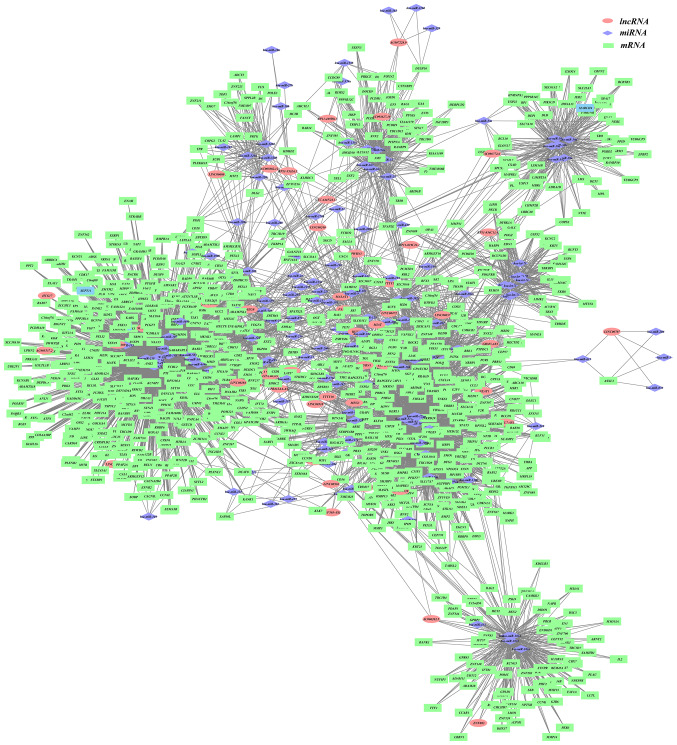
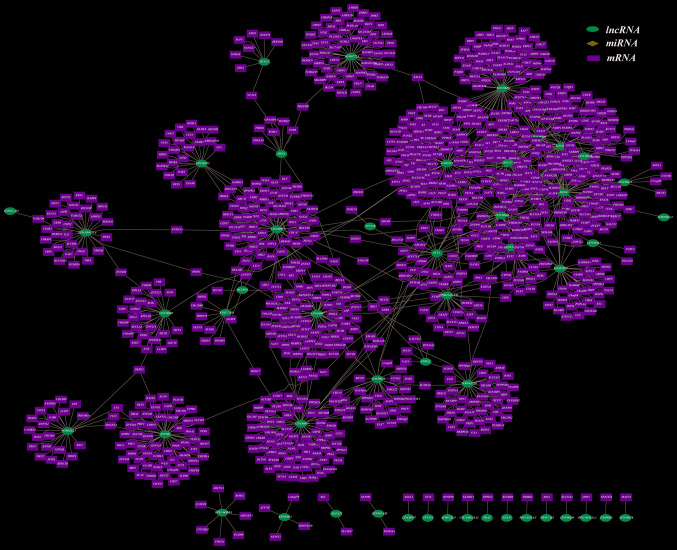
Cytoscape was utilized to demonstrate the colon cancer ceRNA network (lncRNA-miRNA-mRNA), as displayed in Fig. 1. The binary network (lncRNA-mRNA) is shown in Fig. 2.
Figure 1.
lncRNA-miRNA-mRNA competition network in colon cancer. Purple diamonds, miRNAs; pink ellipses, lncRNAs; green rectangles, mRNAs. lncRNA, long non-coding RNA; miRNA, microRNA.
Figure 2.
lncRNA-mRNA competitive network in colon cancer (excluding miRNA). Pink ellipses, lncRNAs; green rectangles, mRNAs. lncRNA, long non-coding RNA.
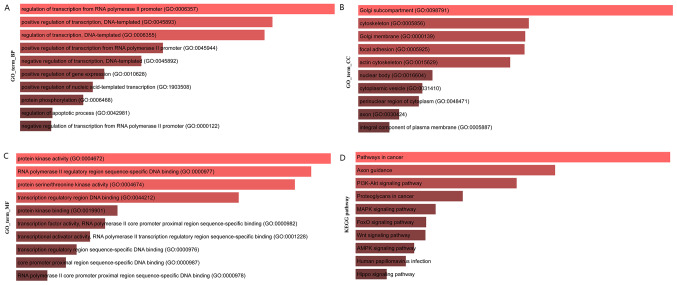
In the present study, the topological properties of the network were analyzed in the network shown in Fig. 1. The miRNA with a degree >10 was selected as the hub miRNA, the lncRNA with a degree >10 was selected as the hub lncRNA and the mRNA with a degree >10 was selected as the hub mRNAs. Finally, a total of 17 hub lncRNAs, 87 hub miRNAs and 144 hub genes of colon cancer were included (Table SI). MCODE was used to identify four modules in the ceRNA network, and the module diagram is presented in Figs. S1-4. The random walk algorithm was used to find the important nodes in the ceRNA network. The functional enrichment analysis of the genes was as listed in Fig. 3.
Figure 3.
Functional enrichment analysis of the genes identified using the random walk algorithm. (A) Functional enrichment map of biological process GO terms for the ceRNA network. (B) Functional enrichment map of cellular components GO terms for ceRNA network. (C) Functional enrichment map of molecular function GO terms for ceRNA network. (D) Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for ceRNA network. The gray area of the bar indicates that the P-value is not significant (P>0.05). GO, gene ontology; ceRNA, competing endogenous RNA.
Screening of biomarkers associated with prognosis via survival analysis
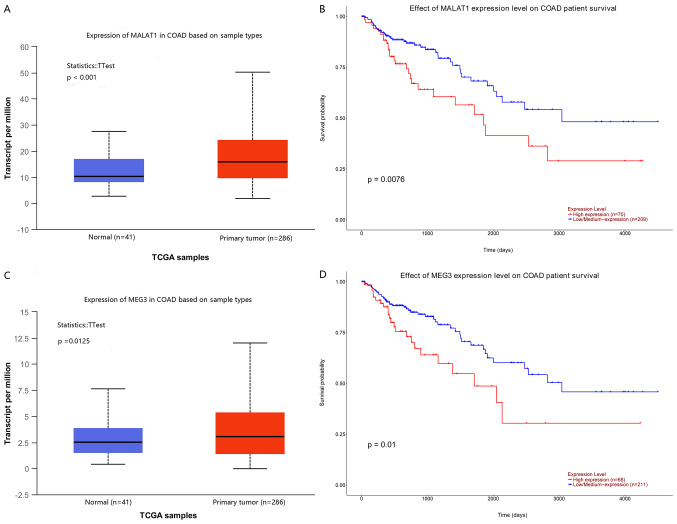
In order to demonstrate that the expression of colon cancer-related lncRNAs was associated with the prognosis of colon cancer, the survival curve of 17 colon cancer-related lncRNAs was identified in the UALCAN database and it was revealed that 2 of these were associated with the overall survival of patients with colon cancer. The results are presented in Fig. 4.
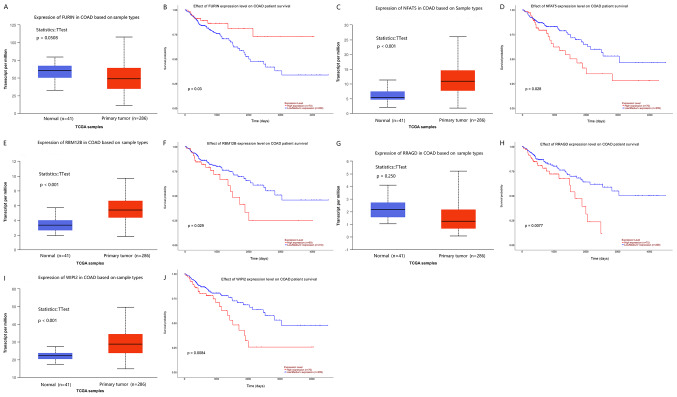
Figure 4.
Association between expression of lncRNAs and prognosis in colon cancer. (A and C) Expression of lncRNAs metastasis-associated lung adenocarcinoma transcript (MALAT1) and maternally expressed gene 3 (MEG3) between normal and colon cancer tissues. (B and D) Kaplan-Meier analysis of the lncRNAs for the overall survival in the colon cancer patients. lncRNAs, long non-coding RNAs.
Among the results, lncRNA metastasis-associated lung adenocarcinoma transcript (MALAT1) and lncRNA maternally expressed gene 3 (MEG3) were upregulated in colon cancer tissue compared with that in normal tissue (Fig. 4A and C), and high expression of these lncRNAs was significantly associated with poor overall survival in patients with colon cancer (Fig. 4B and D).
In addition, 5 out of 144 hub genes (FURIN, NFAT, RBM12B, RRAGD and WIPI2) were closely associated with overall survival of colon cancer. The upregulation of all of these genes, apart from FURIN, was associated with poor overall survival in patients with colon cancer (Fig. 5).
Figure 5.
Association between expression of mRNAs and prognosis in patients with colon cancer. (A) Expression level and (B) overall survival analysis of FURIN expression. (C) Expression level and (D) overall survival analysis of NFAT5 expression. (E) Expression level and (F) overall survival analysis of RBM12B expression. (G) Expression level and (H) overall survival analysis of RRAGD expression. (I) Expression level and (J) overall survival analysis of WIPI2 expression. FURIN, Furin, FES upstream region; NFAT5, nuclear factor of activated T-cells 5; RBM12B, RNA binding motif protein 12B; RRAGA, Ras related GTP binding A; WIPI2, WD repeat domain phosphoinositide-interacting protein 2.
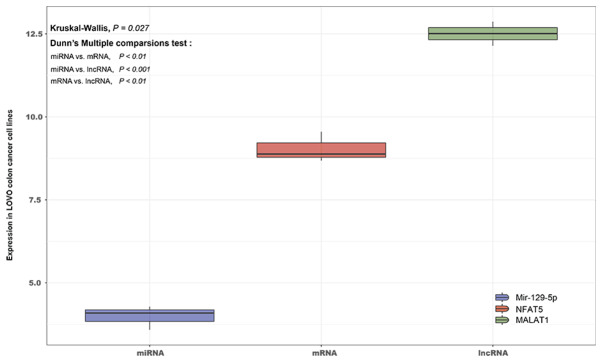
MALAT1 is significantly upregulated, and this is correlated with downregulation of miR-129-5p and upregulation of NFAT5, in LoVo colon cancer cell lines
Using data from the GSE26334 dataset, the full transcriptome sequence of LoVo colon cancer lines was sequenced. The differential expression among mRNAs, lncRNAs and miRNAs in LoVo colon cancer lines was then compared using the Kruskal-Wallis test and visualized by R and GGPLOT2 3.10. The results indicated that the expression levels of lncRNA MALAT1 and mRNA NFAT5 in LoVo colon cancer lines were significantly upregulated, while miR-129-5p was significantly downregulated, with a significant difference between them (P=0.027). This result further confirmed the prediction that the MALAT1/miR-129-5p/NFAT5 axis was significantly associated with colon cancer (Table II; Fig. 6).
Table II.
Extracted lncRNA-miRNA-mRNA interaction pairs that require verification.
| lncRNA | mRNA | miRNA | R | P-value |
|---|---|---|---|---|
| MALAT1 | NFAT5 | hsa-miR-142 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-384 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-519d | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-150 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-494 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-32 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-205 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-149 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-124 | 0.41 | 7.6×10−13 |
| MALAT1 | NFAT5 | hsa-miR-129 | 0.41 | 7.6×10−13 |
| MALAT1 | RBM12B | hsa-miR-519d | 0.32 | 6.6×10−8 |
| MALAT1 | RBM12B | hsa-miR-200b | 0.32 | 6.6×10−8 |
| MALAT1 | RBM12B | hsa-miR-429 | 0.32 | 6.6×10−8 |
| MEG3 | NFAT5 | hsa-miR-215 | 0.26 | 2×10−6 |
| MEG3 | NFAT5 | hsa-miR-361 | 0.26 | 2×10−6 |
| MEG3 | NFAT5 | hsa-miR-122 | 0.26 | 2×10−6 |
| MEG3 | NFAT5 | hsa-miR-129 | 0.26 | 2×10−6 |
| MALAT1 | ABCA1 | hsa-miR-142 | 0.15 | 0.015 |
| MEG3 | RRAGD | hsa-miR-129 | 0.14 | 0.011 |
| MALAT1 | WIPI2 | hsa-miR-383 | 0.13 | 0.026 |
| MALAT1 | WIPI2 | hsa-miR-503 | 0.13 | 0.026 |
| MALAT1 | WIPI2 | hsa-miR-1271 | 0.13 | 0.026 |
| MALAT1 | WIPI2 | hsa-miR-124 | 0.13 | 0.026 |
| MALAT1 | RRAGD | hsa-miR-519d | 0.043 | 0.48 |
| MALAT1 | RRAGD | hsa-miR-144 | 0.043 | 0.48 |
| MALAT1 | RRAGD | hsa-miR-124 | 0.043 | 0.48 |
| MALAT1 | RRAGD | hsa-miR-129 | 0.043 | 0.48 |
| MALAT1 | FURIN | hsa-miR-519d | 0.042 | 0.49 |
| MALAT1 | FURIN | hsa-miR-124 | 0.042 | 0.49 |
The regulatory effect of lncRNA MALAT1/hsa-miR-129/NEAT5 axis (bold) in colon cancer could be verified in GSE26334. MALAT1, metastasis-associated lung adenocarcinoma transcript; MEG3, maternally expressed gene 3; lnRNA, long non-coding RNA; miRNA, microRNA; FURIN, Furin, FES upstream region; NFAT5, nuclear factor of activated T-cells 5; RBM12B, RNA binding motif protein 12B; RRAGA, Ras related GTP binding A; WIPI2, WD repeat domain phosphoinositide-interacting protein 2; ABCA1, ATP-binding cassette subfamily A member 1.
Figure 6.
Expression of lncRNA MALAT1, miR-129-5p and NFAT5 in LoVo colon cancer lines. lncRNA, long non-coding RNA; miRNA, microRNA; MALAT1, metastasis-associated lung adenocarcinoma transcript; NFAT5, nuclear factor of activated T-cells 5.
Target set of core regulators and functional enrichment analysis of core genes
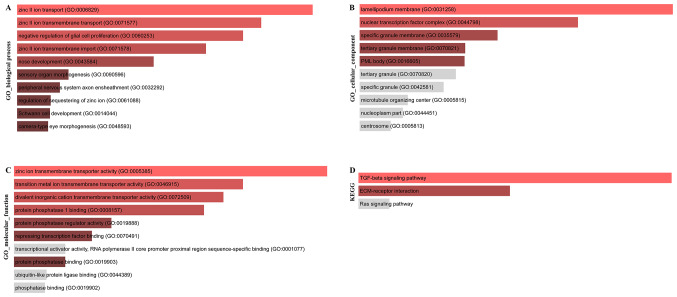
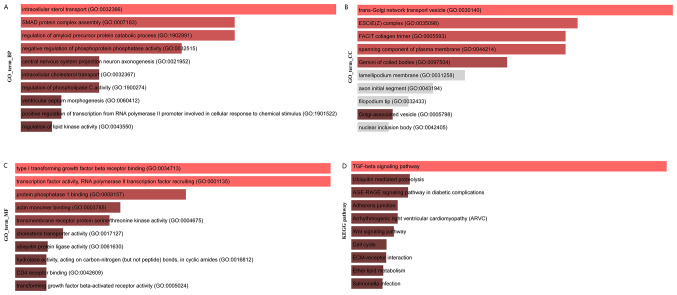
The target genes (target set) of the core regulatory factor (top 10 miRNAs, top10 lncRNAs) were screened, and a total of 11,814 target sets were obtained, and the function and pathway enrichment of the target set were analyzed. The results of Gene Ontology (GO) biological process (BP) enrichment analysis are shown in Fig. 7A, the results of GO cell components (CC) are shown in Fig. 7B, the enrichment analysis results of GO molecular function (MF) enrichment analysis are shown in Fig. 7C and the results of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis are presented in Fig. 7D.
Figure 7.
Target set of core regulators and functional enrichment analysis of core genes. Functional enrichment map of (A) biological process, (B) cellular components and (C) molecular function GO terms for ceRNA network. (D) Enriched KEGG pathways for ceRNA network. GO, gene ontology; ceRNA, competing endogenous RNA; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Then, enrichR was used to analyze the function and pathway of 144 core genes in colon cancer. The results of GO biological process (BP) enrichment analysis are shown in Fig. 8A, the results of GO cell components (CC) enrichment analysis are shown in Fig. 8B, the results of GO molecular function (MF) enrichment analysis are shown in Fig. 8C and the results of KEGG pathway enrichment analysis are shown in Fig. 8D.
Figure 8.
The function enrichment and pathway analysis of 144 core genes in colon cancer. (A) Functional enrichment map of (A) biological process, (B) cellular components and (C) molecular function GO terms for ceRNA network. (D) Enriched KEGG pathways for ceRNA network. The gray area of the bar indicates that the P-value is not significant (P>0.05). GO, gene ontology; ceRNA, competing endogenous RNA; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Drug prediction of colon cancer
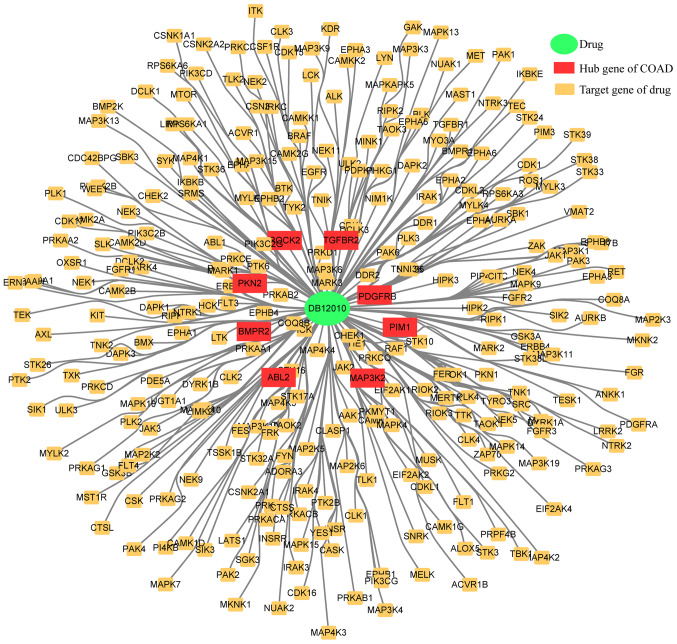
Drug-target relationship data was downloaded from Drugbank, which was used as the interaction background. As a result, 144 colon cancer core genes were selected as the gene set and the drugs that interact significantly with the core genes of colon cancer were calculated using the hypergeometric test. If the hypergeometric test P-value was <0.05 and the number of interactions with the core genes of colon cancer was >2, the drugs were predicted to be associated with colon cancer. After data preprocessing, a total of 9,484 drug-target gene pairs were selected as the background of interaction. Finally, one drug (DB12010) met the threshold values, and was revealed to be fostamatinib; the results are given in Table III and Fig. 9.
Table III.
Association between hub genes and targeted drugs in colon cancer (top 10).
| Drug | P-value | Connection |
|---|---|---|
| DB12010 | 0.00672750878641727 | 8 |
| DB11638 | 0.0393484004831608 | 1 |
| DB01593 | 0.000357387058865982 | 0 |
| DB00142 | 0.010354570549436 | 0 |
| DB00114 | 0.0173304785268529 | 0 |
| DB00898 | 0.033077964206484 | 0 |
| DB00334 | 0.0376561578707151 | 0 |
| DB00543 | 0.042873010623447 | 0 |
DB12010 was revealed to be fostamatinib.
Figure 9.
Targeting drug-gene network map based on 144 hub genes of colon adenocarcinoma.
Discussion
Since the hypothesis of long-chain non-coding RNAs (lncRNAs) as a competing endogenous (ce)RNA network was firstly reported in 2011, it has become a focus of tumor research (7). At present, the network theory of lncRNA-miRNA-mRNA has been gradually confirmed and serves an important role in tumor research. lncRNAs are a class of non-coding products with a length of >200 nucleotides, and account for 68% of all non-coding RNA molecules (24). As an important subgroup of non-coding RNAs, lncRNAs have been confirmed to influence a wide variety of physiological functions and pathological process, such as diabetes, cardiovascular disease, tumors and other diseases (25–31). They serve an important role in regulating gene expression. Since the identification of the ceRNA network, a new method of molecular regulation has been considered. lncRNAs attenuate the biological function of miRNAs by sponging miRNAs with the same microRNA response element (MRE). As a result of the inhibitory effect, lncRNAs are capable of regulating the tumorigenesis and progression of colon cancer.
In the present study, 6,933 lncRNA-miRNA-mRNA interaction pairs were identified in colon cancer, which included 17 hub lncRNAs, 87 hub miRNAs and 144 hub colon cancer core genes. The ceRNA construction of the present study differs from previous methods of ceRNA construction based on differential expression of lncRNAs, miRNAs and mRNAs. However, the present study used multiple databases to screen trusted miRNA-mRNA interaction pairs and lncRNA-miRNA interaction pairs. The significance of lncRNA- and mRNA-shared miRNAs was calculated by using hypergeometric test for each pair of lncRNA and mRNA by using R packages, and the lncRNA-miRNA-mRNA network was successfully constructed. It was revealed that the colon cancer-associated genes identified by random walk analysis were predominantly enriched in GO terms such as ‘zinc ion transport’, ‘nuclear transcription factor complex’, ‘zinc ion transmembrane transporter activity’ and ‘protein phosphate regulator activity’, amongst others. Previous studies have indicated that zinc transporters serve a vital role in carcinogenesis and tumorigenesis (32–34). Similarly, protein phosphate regulator activity is associated with numerous cancer types, such as breast cancer, prostate cancer, non-small cell lung cancer and colon cancer (35–37). Several cancer-associated pathways including transforming growth factor β (TGF-β) signaling, extracellular matrix (ECM) receptor interaction, Ras signaling, PI3K/Akt signaling pathways, and other biological pathways have been identified to be enriched following KEGG analysis. For example, CD51 was revealed to bind TGF-β receptors and further upregulate TGF-β/mothers against decapentaplegic homolog signaling in colorectal stem cells (38). In addition, the TGF-β signaling pathway, a regulator of TWIST1, enhanced stem cell properties in human colorectal cancer (39). The ECM-receptor interaction signaling pathway was found to influence the progression of breast cancer (40). In addition, it was reported that Twist-related protein 2 upregulated the expression of ITGA6 and CD44 which may promote cell proliferation, migration and invasiveness in human kidney cancer via the ECM-receptor interaction pathway (41). Dysregulation of Wnt/β-catenin and Hedgehog/Gli signaling was found to serve a key role in colon cancer progression (42). In addition, the PI3K/Akt pathway was demonstrated to participate in the activation and regulation of cell proliferation, survival, migration and angiogenesis in numerous human cancer types, including colon cancer (43). The aforementioned results confirmed that the core genes identified by random walk algorithm are primarily enriched in cancer-associated pathways.
The colon cancer core genes are a subset of the target genes of colon cancer regulators, which were found to be all enriched in GO terms such as ‘transcriptional regulation of RNA polymerase II promoter’, ‘ubiquitin protein ligase activity’ and ‘nucleosome’, ‘transport vesicles across Golgi network’, and enriched in biological pathways such as ‘TGF-β signaling pathway’, ‘ubiquitin-mediated proteolysis’ and ‘Wnt signaling pathway’. It may be useful to discover the function of these core genes.
lncRNAs directly serve as oncogenes or tumor suppressors via post-transcriptional regulation in the cytoplasm, and serve critical roles in several hallmarks of cancer, such as uncontrollable proliferation, evasion and metastasis (44–46). In the present study, the results indicated that metastasis-associated lung adenocarcinoma transcript (MALAT1) and maternally expressed gene 3 (MEG3) were two important lncRNAs influencing colon cancer, and that a high expression of MALAT1 was associated with poor overall survival of patients with colon adenocarcinoma. MALAT1, an 8.5 kb lncRNA, located at 11q13, was characterized in a study of early-stage non-small cell lung cancer (30). Previous studies have demonstrated that MALAT1 may represent a good diagnostic marker in nasopharyngeal carcinoma, breast and bladder cancer (47). Furthermore, MALAT1 has been demonstrated to sponge miR-106b-5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 (48). However, upregulation of MEG3 may decrease the proliferation and metastasis of gastric cancer cells (49). Additionally, MEG3 was found to be significantly downregulated in acute myeloid leukemia and suppressed leukemogenesis in a p53-independent manner (50). The present study indicated that high expression of MEG3 in colon cancers was associated with poor overall survival via bioinformatic analysis. So far, to the best of the authors' knowledge, there has been no research on the mechanism underpinning the role of MEG3 in the tumorigenesis of colon cancer from previous experimental studies. Therefore, future studies are required to further verify its function in colon cancer.
Previous studies have indicated that tissue- and cell-type specific expression of miRNAs may exhibit either tumor-suppressive or oncogenic effects in a context-dependent manner (51,52). Dysregulation of miRNA function is associated with an increasing number of human disease types, particularly cancer (53). According to the MCODE algorithm, 4 gene modules (MCODE score ≥3) were constructed, including hub miRNAs, including hsa-miR-34a, hsa-miR-449a, hsa-miR-34c, hsa-miR-449b, hsa-miR-33a, hsa-miR-542, hsa-miR-449b, hsa-miR-145 and hsa-miR-212. For example, low miR-449a expression was found to predict a poorer prognosis via regulation of the expression of the target gene HDAC1 in colorectal cancer (54). miR-34a/b/c were found to suppress intestinal tumorigenesis resulting from loss of Apc which downregulates the expression of a large number of protumorigenic factors (55). In addition, miR-145 may serve as a tumor suppressor that downregulates hypoxia-inducible factor 1 and vascular endothelial growth factor expression via targeting of p70S6K1, which results in the inhibition of tumor growth and angiogenesis (56).
In the present study, Kaplan-Meier analysis indicated that hub genes, including FURIN, NFAT5, RBM12B, RRAGD and WIPI2, were closely associated with the overall survival of patients with colon cancer. In a variety of cancer types and human cancer cell lines, high expression of FURIN was found to be positively associated with the high invasiveness of tumors, and it has been proposed as a marker of advanced or malignant tumors (57). In addition, high expression of FURIN has been revealed to predict decreased survival in several human cancer types (58). NFAT5 is activated at multiple levels, and the activation is required for the induction of S100A4 in colon cancer cells (59). In addition, NFAT5 is upregulated in invasive human ductal breast carcinomas and has been demonstrated to participate in promoting carcinoma invasiveness using cell lines derived from human breast and colon carcinomas (60). A previous study reported that RBM12B was associated with both breast cancer incidence and outcome (61). However, there are few reports on the function of RBM12B in colon cancer. The present study indicated that high expression of RBM12B was associated with poor overall survival in patients with colon cancer. Thus, the role of RBM12B in the colon cancer requires further elucidation via more experimentation. RRAGD participates in a variety of molecular processes, improves the formation of breast cancer cell line cell-in-cell structure and may influence the late maturation of external cell vacuoles via regulation of mammalian target of rapamycin complex (62). WIPI2 is a mammalian effector of phosphatidylinositol 3-phosphate and is ubiquitously expressed in a variety of cell lines. WIPI2 is recruited to early autophagosomal structures along with autophagy related 16 like 1 and ULK1 (63). Autophagy influences cellular metabolism, the proteome and organelle numbers and quality. Dysfunctional autophagy contributes to multiple diseases, including cancer (64). Currently, there is no report describing the effect of WIPI2 on colon cancer via autophagy, which requires further investigation. The aforementioned research reveals that the ceRNA-associated hub genes identified in the present study serve an important role in the tumorigenesis and progression of colon cancer.
Among these lncRNAs and mRNAs closely associated with colon cancer survival, it was revealed that the lncRNA MALAT1/miR-129-5p/NFAT5 axis may serve a vital role in the progression of colon cancer. Another study demonstrated that the MALAT/mi-R203/DCP1A axis is involved with the development and contributes to the malignancy of colorectal cancers (65). This study may indicate that MALAT1 may exert a regulating role by targeting small RNAs. However, the present study only used the GSE26334 dataset, and further in vitro and in vivo validation experiments are needed.
In the present study, a targeted drug for colon cancer was predicted using the 144 core genes in the colon cancer ceRNA network for the first time, to the best of the authors' knowledge, which may represent a novel therapeutic target for the treatment of colon cancer. Furthermore, the drug was revealed to be a spleen tyrosine kinase inhibitor, and effective inhibition of B-cell receptor signal transduction in vivo results in a decrease in the proliferation and survival of malignant B cells and a significant prolongation of the survival time of treated animals (66). In addition, this drug appears to represent a promising therapeutic for immunological diseases (67). However, the use of the drug for the clinical treatment of colon cancer would still require further bioinformatic analysis and randomized controlled trials.
Supplementary Material
Acknowledgements
The authors would like to thank GAP BioTech studio for providing bioinformatic analysis in the present study.
Funding
This study was supported by the Chongming Committee of Science and Technology (grant no. CKY2018-11).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in TCGA repository (https://portal.gdc.cancer.gov/) and the GEO repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE26334.
Authors' contributions
DH and BZ contributed to the data analysis and wrote the main manuscript. DH analyzed the relevant data and prepared Figs. 1–5, WS analyzed the relevant data and prepared Fig. 6 and Table II, and BZ analyzed the relevant data and prepared Figs. 7–9. MY created Tables I and III and analyzed the data in the two Tables. WS contributed to the process of revising the manuscript. WS and LZ designed the experiments, reviewed drafts of the paper and approved the final draft. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wong KE, Ngai SC, Chan KG, Lee LH, Goh BH, Chuah LH. Curcumin nanoformulations for colorectal cancer: A review. Front Pharmacol. 2019;10:152. doi: 10.3389/fphar.2019.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao Y, Wang Y, Qi M, He X, Zhu Y, Hong J. Risk factors for recurrent colorectal polyps. Gut Liver. 2019 doi: 10.5009/gnl19097. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohl C, Hombach A, Kruis W. Chronic inflammatory bowel disease and cancer. Hepatogastroenterology. 2000;47:57–70. [PubMed] [Google Scholar]
- 5.Mehta A, Patel BM. Therapeutic opportunities in colon cancer: Focus on phosphodiesterase inhibitors. Life Sci. 2019;230:150–161. doi: 10.1016/j.lfs.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 6.Yu C, Zhang Y. Development and validation of a prognostic nomogram for early onset colon cancer. Biosci Rep. 2019;36:BSR20181781. doi: 10.1042/BSR20181781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, Ren D, Ye X, Li C, Wang Y, et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer. 2018;17:79. doi: 10.1186/s12943-018-0827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Xu P, Chen B, Zhang Z, Zhang C, Zhan Q, Huang S, Xia ZA, Peng W. Identifying circRNA-associated-ceRNA networks in the hippocampus of Aβ1-42-induced Alzheimer's disease-like rats using microarray analysis. Aging (Albany NY) 2018;10:775–788. doi: 10.18632/aging.101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing Q, Huang Y, Wu Y, Ma L, Cai B. Integrated analysis of differentially expressed profiles and construction of a competing endogenous long non-coding RNA network in renal cell carcinoma. PeerJ. 2018;6:e5124. doi: 10.7717/peerj.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Li X, Gao Y, Guo Q, Ning S, Zhang Y, Shang S, Wang J, Wang Y, Zhi H, et al. LnCeVar: A comprehensive database of genomic variations that disturb ceRNA network regulation. Nucleic Acids Res. 2020;48:D111–D117. doi: 10.1093/nar/gkz887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y, Zhang T, Qi L, Zhou C, Wei J, Feng F, Liu R, Sun C. Integrated analysis of co-expression and ceRNA network identifies five lncRNAs as prognostic markers for breast cancer. J Cell Mol Med. 2019;23:8410–8419. doi: 10.1111/jcmm.14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Deng Y, Guo Q, Zhu J, Cao L, Guo X, Xu F, Weng W, Ju X, Wu X. Long non-coding RNA SNHG6 promotes cell proliferation and migration through sponging miR-4465 in ovarian clear cell carcinoma. J Cell Mol Med. 2019;23:5025–5036. doi: 10.1111/jcmm.14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Wang X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019;20:18. doi: 10.1186/s13059-019-1629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeggari A, Marks DS, Larsson E. miRcode: A map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics. 2012;28:2062–2063. doi: 10.1093/bioinformatics/bts344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P, Floros E, Dalamagas T, Hatzigeorgiou AG. DIANA-LncBase v2: Indexing microRNA targets on non-coding transcripts. Nucleic Acids Res. 2016;44:D231–D238. doi: 10.1093/nar/gkv1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma'ayan A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Q, Meng WY, Jie Y, Zhao H. lncRNA MALAT1 induces colon cancer development by regulating miR-129-5p/HMGB1 axis. J Cell Physiol. 2018;233:6750–6757. doi: 10.1002/jcp.26383. [DOI] [PubMed] [Google Scholar]
- 22.King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee JS, Rustgi AK. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71:4260–4268. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng SD, Yang JH, Yao CH, Yang SS, Zhu ZM, Wu D, Ling HY, Zhang L. Potential regulatory mechanisms of lncRNA in diabetes and its complications. Biochem Cell Biol. 2017;95:361–367. doi: 10.1139/bcb-2016-0110. [DOI] [PubMed] [Google Scholar]
- 26.Greco S, Gaetano C, Martelli F. Long noncoding competing endogenous RNA networks in age-associated cardiovascular diseases. Int J Mol Sci. 2019;20:E3079. doi: 10.3390/ijms20123079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ke S, Yang Z, Yang F, Wang X, Tan J, Liao B. Long noncoding RNA NEAT1 aggravates Aβ-induced neuronal damage by targeting miR-107 in Alzheimer's disease. Yonsei Med J. 2019;60:640–650. doi: 10.3349/ymj.2019.60.7.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni S, Lied A, Kulkarni V, Rucevic M, Martin MP, Walker-Sperling V, Anderson SK, Ewy R, Singh S, Nguyen H, et al. CCR5AS lncRNA variation differentially regulates CCR5, influencing HIV disease outcome. Nat Immunol. 2019;20:824–834. doi: 10.1038/s41590-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Liu Y, Cai J. lncRNA MIR155HG regulates M1/M2 macrophage polarization in chronic obstructive pulmonary disease. Biomed Pharmacother. 2019;117:109015. doi: 10.1016/j.biopha.2019.109015. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Li XF, Cheng LN, Li XL. Long non-coding RNA CRNDE promoted cell apoptosis by suppressing miR-495 in inflammatory bowel disease. Exp Cell Res. 2019;382:111484. doi: 10.1016/j.yexcr.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Pan Z, Choi S, Ouadid-Ahidouch H, Yang JM, Beattie JH, Korichneva I. Zinc transporters and dysregulated channels in cancers. Front Biosci (Landmark Ed) 2017;22:623–643. doi: 10.2741/4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gartmann L, Wex T, Grungreiff K, Reinhold D, Kalinski T, Malfertheiner P, Schütte K. Expression of zinc transporters ZIP4, ZIP14 and ZnT9 in hepatic carcinogenesis-An immunohistochemical study. J Trace Elem Med Biol. 2018;49:35–42. doi: 10.1016/j.jtemb.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 34.Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol. 2013;10:219–226. doi: 10.1038/nrurol.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran MH, Seo E, Min S, Nguyen QT, Choi J, Lee UJ, Hong SS, Kang H, Mansukhani A, Jou I, Lee SY. NEDD4-induced degradative ubiquitination of phosphatidylinositol 4-phosphate 5-kinase α and its implication in breast cancer cell proliferation. J Cell Mol Med. 2018;22:4117–4129. doi: 10.1111/jcmm.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodgers SJ, Ferguson DT, Mitchell CA, Ooms LM. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases. Biosci Rep. 2017;37:BSR20160432. doi: 10.1042/BSR20160432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, Shi L, Wang Z, Wang Y, Li Z, Xie C. ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biol Ther. 2015;16:1194–1204. doi: 10.1080/15384047.2015.1056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Zhang B, Wu H, Cai J, Sui X, Wang Y, Li H, Qiu Y, Wang T, Chen Z, et al. CD51 correlates with the TGF-beta pathway and is a functional marker for colorectal cancer stem cells. Oncogene. 2017;36:1351–1363. doi: 10.1038/onc.2016.299. [DOI] [PubMed] [Google Scholar]
- 39.Nakano M, Kikushige Y, Miyawaki K, Kunisaki Y, Mizuno S, Takenaka K, Tamura S, Okumura Y, Ito M, Ariyama H, et al. Dedifferentiation process driven by TGF-beta signaling enhances stem cell properties in human colorectal cancer. Oncogene. 2019;38:780–793. doi: 10.1038/s41388-018-0480-0. [DOI] [PubMed] [Google Scholar]
- 40.Bao Y, Wang L, Shi L, Yun F, Liu X, Chen Y, Chen C, Ren Y, Jia Y. Transcriptome profiling revealed multiple genes and ECM-receptor interaction pathways that may be associated with breast cancer. Cell Mol Biol Lett. 2019;24:38. doi: 10.1186/s11658-019-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HJ, Tao J, Sheng L, Hu X, Rong RM, Xu M, Zhu TY. RETRACTED: Twist2 promotes kidney cancer cell proliferation and invasion via regulating ITGA6 and CD44 expression in the ECM-Receptor-Interaction pathway. Biomed Pharmacother. 2016;81:453–459. doi: 10.1016/j.biopha.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 42.Song L, Li ZY, Liu WP, Zhao MR. Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapy. Cancer Biol Ther. 2015;16:1–7. doi: 10.4161/15384047.2014.972215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seguella L, Capuano R, Pesce M, Annunziata G, Pesce M, de Conno B, Sarnelli G, Aurino L, Esposito G. S100B protein stimulates proliferation and angiogenic mediators release through RAGE/pAkt/mTOR pathway in human colon adenocarcinoma Caco-2 cells. Int J Mol Sci. 2019;20:E3240. doi: 10.3390/ijms20133240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 48.Zhuang M, Zhao S, Jiang Z, Wang S, Sun P, Quan J, Yan D, Wang X. MALAT1 sponges miR-106b-5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 enhanced microtubules mobility. EBioMedicine. 2019;41:286–298. doi: 10.1016/j.ebiom.2018.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:3850–3856. [PubMed] [Google Scholar]
- 50.Lyu Y, Lou J, Yang Y, Feng J, Hao Y, Huang S, Yin L, Xu J, Huang D, Ma B, et al. Dysfunction of the WT1-MEG3 signaling promotes AML leukemogenesis via p53-dependent and -independent pathways. Leukemia. 2017;31:2543–2551. doi: 10.1038/leu.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17:719–732. doi: 10.1038/nrg.2016.134. [DOI] [PubMed] [Google Scholar]
- 52.Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19:E1310. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishikawa D, Takasu C, Kashihara H, Nishi M, Tokunaga T, Higashijima J, Yoshikawa K, Yasutomo K, Shimada M. The significance of microRNA-449a and its potential target HDAC1 in patients with colorectal cancer. Anticancer Res. 2019;39:2855–2860. doi: 10.21873/anticanres.13414. [DOI] [PubMed] [Google Scholar]
- 55.Jiang L, Hermeking H. miR-34a and miR-34b/c suppress intestinal tumorigenesis. Cancer Res. 2017;77:2746–2758. doi: 10.1158/0008-5472.CAN-16-2183. [DOI] [PubMed] [Google Scholar]
- 56.Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li D, Lai L, Jiang BH. miR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coppola JM, Bhojani MS, Ross BD, Rehemtulla A. A small-molecule furin inhibitor inhibits cancer cell motility and invasiveness. Neoplasia. 2008;10:363–370. doi: 10.1593/neo.08166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scamuffa N, Sfaxi F, Ma J, Lalou C, Seidah N, Calvo F, Khatib AM. Prodomain of the proprotein convertase subtilisin/kexin Furin (ppFurin) protects from tumor progression and metastasis. Carcinogenesis. 2014;35:528–536. doi: 10.1093/carcin/bgt345. [DOI] [PubMed] [Google Scholar]
- 59.Chen M, Sastry SK, O'Connor KL. Src kinase pathway is involved in NFAT5-mediated S100A4 induction by hyperosmotic stress in colon cancer cells. Am J Physiol Cell Physiol. 2011;300:C1155–C1163. doi: 10.1152/ajpcell.00407.2010. [DOI] [PubMed] [Google Scholar]
- 60.Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–544. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- 61.Plasterer C, Tsaih SW, Lemke A, Schilling R, Dwinell M, Rau A, Auer P, Rui H, Flister MJ. Identification of a rat mammary tumor risk locus that is syntenic with the commonly amplified 8q12.1 and 8q22.1 regions in human breast cancer patients. G3 (Bethesda) 2019;9:1739–1743. doi: 10.1534/g3.118.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang ZR, Zheng Y, Ruan BZ, et al. Expression and subcellular localization of RRAGD-EGFP fusion protein in MDA-MB-436 cells. Letters in Biotechnology. 2017;28:639–642. (In Chinese) [Google Scholar]
- 63.Polson HE, de Lartigue J, Rigden DJ, Reedijk M, Urbé S, Clague MJ, Tooze SA. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6:506–522. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- 64.Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–1930. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu C, Zhu X, Tao K, Liu W, Ruan T, Wan W, Zhang C, Zhang W. MALAT1 promotes the colorectal cancer malignancy by increasing DCP1A expression and miR203 downregulation. Mol Carcinog. 2018;57:1421–1431. doi: 10.1002/mc.22868. [DOI] [PubMed] [Google Scholar]
- 66.Suljagic M, Longo PG, Bennardo S, Perlas E, Leone G, Laurenti L, Efremov DG. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Eµ-TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116:4894–4905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 67.Bajpai M. Fostamatinib, a Syk inhibitor prodrug for the treatment of inflammatory diseases. IDrugs. 2009;12:174–185. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in TCGA repository (https://portal.gdc.cancer.gov/) and the GEO repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE26334.