Abstract
UVB radiation mediates inflammatory responses causing skin damage and defects in epidermal differentiation. 1α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) interacts with the vitamin D3 receptor (VDR) to regulate inflammatory responses. Additionally, 1,25(OH)2D3/VDR signaling represents a potential therapeutic target in the treatment of skin disorders associated with inflammation and poor differentiation of keratinocytes. Since the protective effect of 1,25(OH)2D3 against UVB-induced skin damage and inflammation is recognized, CYP11A1-derived vitamin D3-hydroxyderivatives including 20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3 and 1,20,23(OH)3D3 were tested for their anti-inflammatory and skin protection properties in UVB-irradiated human epidermal keratinocytes (HEKn). HEKn were treated with secosteroids for 24 h pre- and post-UVB (50 mJ/cm2) irradiation. Secosteroids modulated the expression of the inflammatory response genes (IL-17, NF-κB p65, and IκB-α), reducing nuclear-NF-κB-p65 activity and increasing cytosolic-IκB-α expression as well as that of pro-inflammatory mediators, IL-17, TNF-α, and IFN-γ. They stimulated the expression of involucrin (IVL) and cytokeratin 10 (CK10), the major markers of epidermal differentiation, in UVB-irradiated cells. We conclude that CYP11A1-derived hydroxyderivatives inhibit UVB-induced epidermal inflammatory responses through activation of IκB-α expression and suppression of NF-kB-p65 activity and its downstream signaling cytokines, TNF-α, and IFN-γ, as well as by inhibiting IL-17 production and activating epidermal differentiation.
Keywords: Photobiology, UV Radiation, Keratinocyte Differentiation, Epidermal Structure
Graphical abstract:
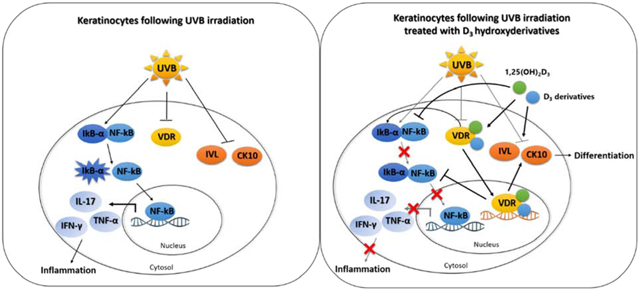
Schematic diagram of the role of 1,25(OH)2D3 (green circle) and CYP11A1-derived D3 derivatives (blue circle) in the modulation of NF-κB/IκB-α and differentiation biomarkers including involucrin (IVL) and cytokeratin 10 (CK10) through VDR pathway [29, 40].
INTRODUCTION
UVB plays an important role in inflammatory responses and the regulation of epidermal keratinocytes differentiation [1, 2]. In resting conditions, nuclear factor kappa B (NF-κB) is bound by the inhibitor kappa B (IκB) to prevent translocation of NF-κB to the nucleus [3, 4]. Degradation of IκB-α releases NF-κB, which is then translocated into the nucleus where it transactivates several genes involved in inflammation [3–5]. NF-κB transcriptional activity plays a crucial role in the production of secreted inflammatory cytokines and chemokines including interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-17 (IL-17), interleukin-33 (IL-33), interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-10 (IL-10), interferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), intercellular adhesion molecule (ICAM) and enzymes, especially cyclooxygenase-2 (COX-2) [6, 7]. UVB irradiation can affect the NF-κB/IκB complex and transcriptional activity of NF-κB through interfering with transmembrane proteins such as toll-like receptor 4 (TLR4) and cluster of differentiation 14 (CD14), and promotes the phosphorylation of IκB [8, 9]. CYP11A1-derived vitamin D3-hydroxyderivatives have previously been shown to protect against skin damage through suppression of UVB-induced DNA damage and reactive oxygen species (ROS) levels [10, 11]. Besides skin inflammation, UVB can interfere in the epidermal differentiation process through the induction of oxidative stress [12]. Keratinocytes respond to UVB exposure to protect the skin against environmental stress with changes in the expression of differentiation-specific markers including keratins and IVL [13].
Vitamin D3 (D3) is a promising precursor for therapeutic agents of skin inflammatory responses and in skin damage prevention [14, 15]. The activation of D3 in the canonical pathway starts with 25-hydroxylation by CYP2R1 or CYP27A1 to produce 25-hydroxyvitamin D3 (25(OH)D3) and is followed 1α-hydroxylation by CYP27B1 to produce 1,25(OH)2D3 (Figure 1a) [16, 17]. In a non-canonical pathway CYP11A1, a steroidogenic enzyme expressed in the skin [18], hydroxylates D3 producing 20(OH)D3 and 20,23(OH)2D3 which can be hydroxylated by CYP27B1 to produce 1,20(OH)2D3 and 1,20,23(OH)3D3 (Figure 1a) [19–21]. The biologically active 1,25(OH)2D3 interacts with the nuclear vitamin D3 receptor (VDR) that also controls inflammatory responses [22]. VDR is a member of the nuclear receptor family regulating the biological actions of vitamin D including the expression of genes involved in inflammation and differentiation of keratinocytes [23, 24]. The active heterodimeric form of the VDR can inhibit NF-κB signaling either by induction of IκB expression or by interference with NF-κB DNA binding [25, 26]. An upregulation of VDR can also increase the levels of proteins accompanying differentiation including involucrin (IVL) and keratins [27]. Thus, signaling pathways downstream of the VDR have been proposed to be potential targets for understanding pathogenesis of skin diseases involving epidermal inflammation and differentiation [28–30].
Figure 1.
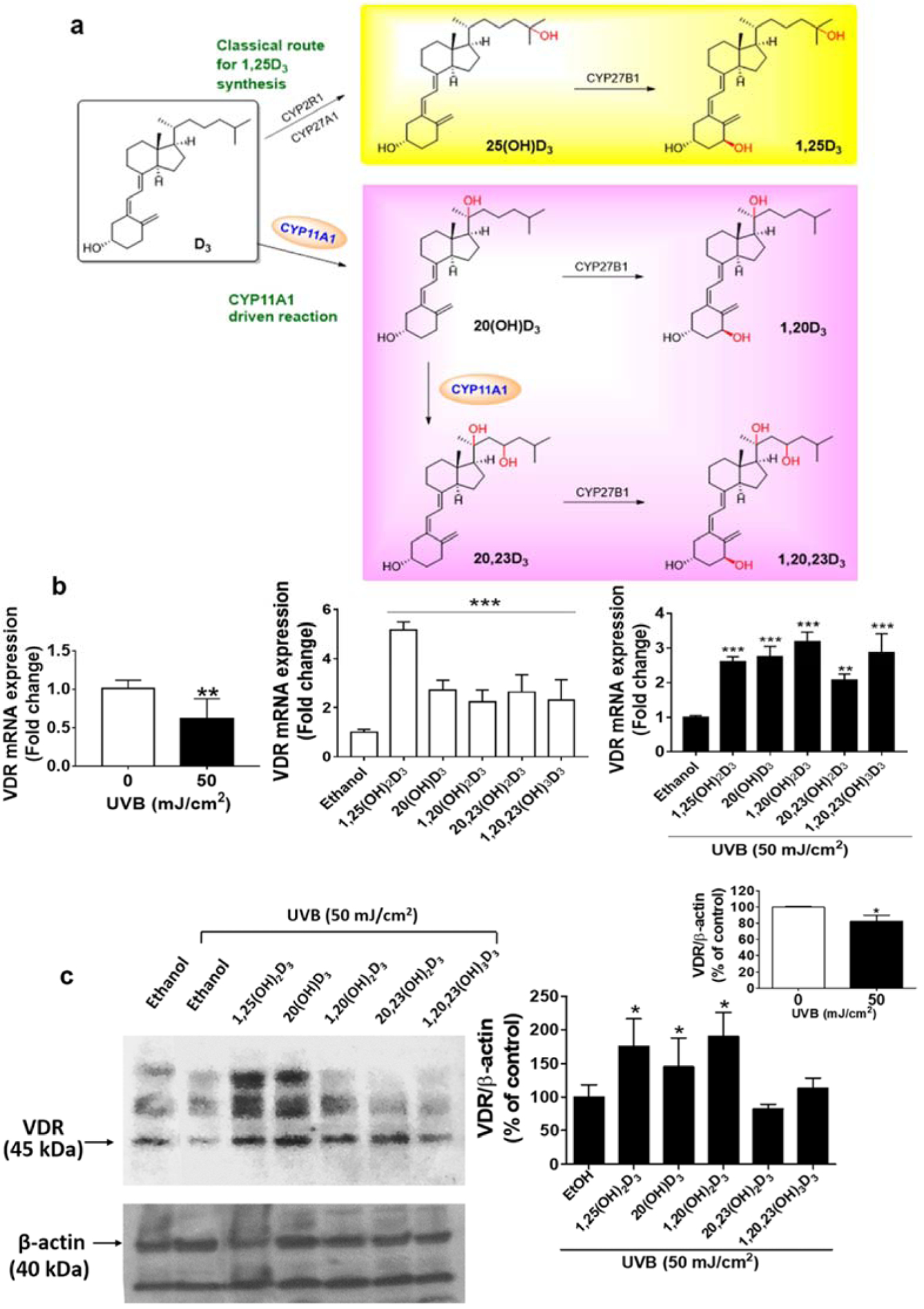
UVB inhibits, while vitamin D3 hydroxyderivatives stimulate VDR expression in human keratinocytes. Keratinocytes were pretreated with 100 nM secosteroids (as indicated) or ethanol (solvent control) for 24 h before UVB irradiation (50 mJ/cm2) and then further incubated with the secosteroids for an additional 24 h. (a) D3 transformation scheme (b) Real-time RT-PCR analyses of UVB-irradiated cells after treatment are shown with black bar charts while non-irradiated cells are shown with white bar charts. VDR mRNA was normalized relative to β-actin, cyclopilin, and GAPDH mRNA levels. Western blot analyses are in (c). VDR protein, detected at 45 kDa, was normalized relative to β-actin and presented as % of control (mean ± S.D.). All are representative of 3–6 independent experiments. Statistical analyses were done by the student t-test, *P<0.05, **P<0.01, ***P<0.001 for all conditions compared to the untreated control.
1,25(OH)2D3 has been shown to have protective effects on skin by inhibiting inflammatory responses [31–33]. Our previous studies on 1,25(OH)2D3 and CYP11A1-derivatived D3 hydroxyderivatives have demonstrated their protective effects against UVB-induced DNA damage [10] and their ability to stimulate VDR and IVL expression and inhibit the expression of inflammatory genes, in non-irradiated epidermal keratinocytes [34–36]. Moreover, we have reported that the anti-inflammatory activity of 20(OH)D3 and 20,23(OH)2D3, which have low calcemic activity compared to1,25(OH)2D3 [37–39], is mediated through the regulation of the NF-kB complex and IkB-α signaling in non-irradiated keratinocytes [29, 40]. This pathway is believed to protect against UV-induced inflammation [41]. In this study, we aimed to investigate the effects of CYP11A1-derivatived D3-hydroxyderivatives on inflammatory responses and differentiation in UVB-irradiated HEKn.
MATERIALS AND METHODS
Source of compounds tested
1,25-dihydroxyvitamin D3 (1,25(OH)2D3) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydroxy-derivatives of vitamin D3, including 20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3, and 1,20,23(OH)3D3, were enzymatically synthesized as previously described [35, 42, 43]. In addition, 20(OH)D3 was chemically or photochemically synthesized as previously described [44, 45].
Cell culture and treatment
HEKn were isolated from neonatal foreskin and cultured as previously described [29, 46]. Patient consent for experiments was not required because the informed consent laws consider human tissue left over from surgery as discarded material. In this study we used secosteroids at a concentration of 100 nM to test the anti-inflammatory and pro-differentiation effects on cells. This concentration exhibited the most potent activity for photoprotection as well as skin cell proliferation, as tested previously [10, 42]. The D3 hydroxyderivative (100 nM) or ethanol were diluted in EpiGRO™ keratinocyte media (Millipore, Burlington, MA, USA) mixed with 0.5% BSA. Our previous study evaluated the effects of UVB on skin cell damage at different doses: 25, 50, 75, or 200 mJ/cm2 [10]. A significant reduction in DNA repair and an induction of oxidant formation was observed in cells with a UVB dose of 50 mJ/cm2. We thus applied this model in the current study. Cells were treated with vitamin D3 or its hydroxyderivatives before UVB (50 mJ/cm2) irradiation (pre-treatment) for 24 h. After irradiation, cells were then immediately incubated in fresh medium supplemented with the vitamin D3 derivatives, for an additional 24 h (post-treatment) as in [10].
Quantitative RT-PCR for mRNA expression
RNA was isolated from treated cells in 60 mm petri dishes using the absolutely RNA RT–PCR Miniprep kit (Stratagene, La Jolla, CA, USA). PCR reactions were performed in triplicate using a Kapa SYBR Fast qPCR Master Mix (Kapa Biosystems, Boston, MA, USA) as indicated in [10]. Sequences of PCR primer are shown in Table S1. The mRNA levels were normalized relative to the amount of housekeeping genes (β-actin, cyclopilin, or GAPDH mRNA) using the ΔΔCt method. The mean of the fold-change and the heat map were generated using Prism software.
The detection of NF-κB p65 phosphorylation
The NF-κB p65 (Total/Phospho) Human InstantOne™ ELISA Kit (Cat No. 85-86083-11, Thermo Fisher Scientific, Waltham, MA, USA) was used to detect the phosphorylation of NF-κB p65 [29, 47]. An antibody cocktail was pre-coated with a capture antibody reagent, the total-NF-κB p65 or phospho-NF-κB p65 antibody, and a detection antibody reagent (1:1 v/v). Cell lysates, a negative control (cell lysis mix), or a positive control (50 μl/well) mixed with the total-NF-κB or phospho-NF-κB antibody cocktail (50 μl/well) were incubated in the pre-coated plate for 1 h at RT. After washing, stop solutions (100 μl/well) were added and the absorbance measured at 450 nm. The phosphorylation level, normalized by the cell number or the total-NF-κB p65, was calculated relative to the control and data are presented as % of control (mean±SD).
Immunofluorescence analysis of NF-κB p65/IκB-α and IVL
Treated cells in a 96-well plate were fixed in 4% paraformaldehyde and tagged with mouse monoclonal antibody against NF-κB p65 (sc-8008; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit polyclonal antibody against IκB-α (sc-371; Santa Cruz Biotechnology, Dallas, TX, USA), or mouse monoclonal antibody against IVL (GTX-14504; Genetex, Irvine, CA, USA), overnight at 4°C (1:200 dilution). Plates were then incubated for 1 h with Alexa-Fluor 488 secondary antibody (Invitrogen Molecular Probes, Eugene, Oregon, USA) diluted 1:300 in blocking solution. Nuclei were stained red with propidium iodide (Vector Laboratories, Burlingame, CA, USA). Stained cells were imaged at 40X magnification and analyzed using Gen5.0 software with a Cytation™ 5 cell imaging reader. The nuclear/cytosolic ratio was calculated from the image intensities of the ImageJ analysis (NIH free download) and presented as % of control (mean±SD) as in [10, 48]. The single color staining represents the expression in cytosol (green), while the dual expression (orange-yellow) represents the expression in both cytosol and nucleus. The cell nuclei were stained red with propidium iodide (red).
The detection of pro-inflammatory cytokines
IL-17, IFN-γ, and TNF-α levels were measured in cell supernatants using RAB0262, RAB022, and RAB1089 ELISA kits, respectively (Sigma-aldrich, St. Louis, MO, USA). Standard or sample cell supernatant was incubated in pre-coated 96-wells plates (100 μl/well) overnight at 4°C. Antibodies (100 μl/well) were then added and plates incubated for 1 h followed by streptavidin (100 μl/well) addition and incubation for 45 min. TMB one-step substrate (100 μl/well) was added and plates incubated for 30 min before adding stop solutions (50 μl/well). Absorbances (450 nm) were measured and cytokine concentrations (pg/ml, mean±SD) calculated from a standard curve.
Western blot analysis of VDR and the proteins involved in inflammation and differentiation
Cytosolic and nuclear fractions were extracted by a nuclear extraction kit (Active motif, Carlsbad, CA, USA). The concentration of protein samples was determined using the Bio-Rad protein Bradford assay kit (BioRad, Hercules, CA, USA). An equal amount of protein (50 μg per well) was loaded into each well of the gel. Proteins were separated using a Mini-PROTEAN® TGX™ gel (BioRad, Hercules, CA, USA) as in [10]. The blot was first probed with anti-VDR Ab, then stripped and reprobed sequentially with antibodies to NFκBp65, IkB-α, IVL, CK10, and beta-actin shown in Figures 1, 3, 4, and 6, respectively. The primary antibodies were as follows: Mouse monoclonal antibody against VDR (sc-13133, Santa Cruz Biotechnology, Dallas, TX, USA; 1:200 dilution), mouse monoclonal antibody against NF-κB p65 (sc-8008, Santa Cruz Biotechnology, Dallas, TX, USA), rabbit polyclonal antibody against IκB-α (sc-371; Santa Cruz Biotechnology, Dallas, TX, USA), mouse monoclonal antibody against IVL (GTX-14504; Genetex, Irvine, CA, USA), and mouse monoclonal antibody against CK10 (sc-23877; Santa Cruz Biotechnology, Dallas, TX, USA), at 1:1000 dilution. The blot was then incubated for 1 h at RT with the HRP-conjugated secondary antibodies: Goat anti-mouse superclonal™ secondary antibody (A28177, Thermo Fisher Scientific, Waltham, MA, USA) or goat anti-rabbit secondary antibody (ab6721, Abcam, Cambridge, MA, USA), at 1:3000 dilution. Immuno-reactivity was detected using supersignal west pico ECL (BioRad, Hercules, CA, USA). The loading controls, β-actin-peroxidase (A3854, Sigma-aldrich, St. Louis, MO, USA; 1:5000 dilution) and lamin A/C (N-18) (sc6215, Santa Cruz Biotechnology, Dallas, TX, USA; 1:200 dilution) were used for the cytosolic and nuclear fractions, respectively. The protein bands of interest were identified according to their molecular weights (kDa) published in the manufacturers’ datasheets, determined relative to the precision plus protein™ kaleidoscope™ standards (BioRad, Hercules, CA, USA). The relevant proteins of interest were detected sequentially with the blots being reprobed with each antibody type, including those for the loading controls. β-actin-peroxidase served as the loading control for whole cell and cytosolic fractions, while lamin A/C served as the loading control of nuclear fraction [49, 50]. ImageJ analysis of blots were performed from 3–6 independent experiments. To analyze the intensity of relevant bands in the blots, an area was selected around the band at the expected molecular weight (kDa) using Image J software, and the pixel intensity measured. Quantitative data were then imported into Microsoft Excel for calculating the percentage intensity of relevant bands compared to the intensities of controls and the ratio of intensities between the nuclear and cytosolic fractions. For the analysis of whole cell and cytosolic fractions, band intensities measured by ImageJ were normalized relative to the loading control and to the band intensities seen in control cells (ethanol without UVB or ethanol with UVB), and are presented as % of control (mean±SD). For the analysis of the nuclear to cytosolic ratio, the nuclear-NFkB p65 pixel intensities were normalized by dividing the pixel intensity value by that for the Lamin A/C loading control. The expression of cytosolic-NFkB p65 was normalized by dividing its pixel intensity by that for the β-actin loading control. The nuclear to cytoplasmic ratio of NFkB-p65 was then calculated by dividing the normalized value for nuclear expression by that for the cytosol, with final data being presented as fold-change (mean±SD).
Figure 3.
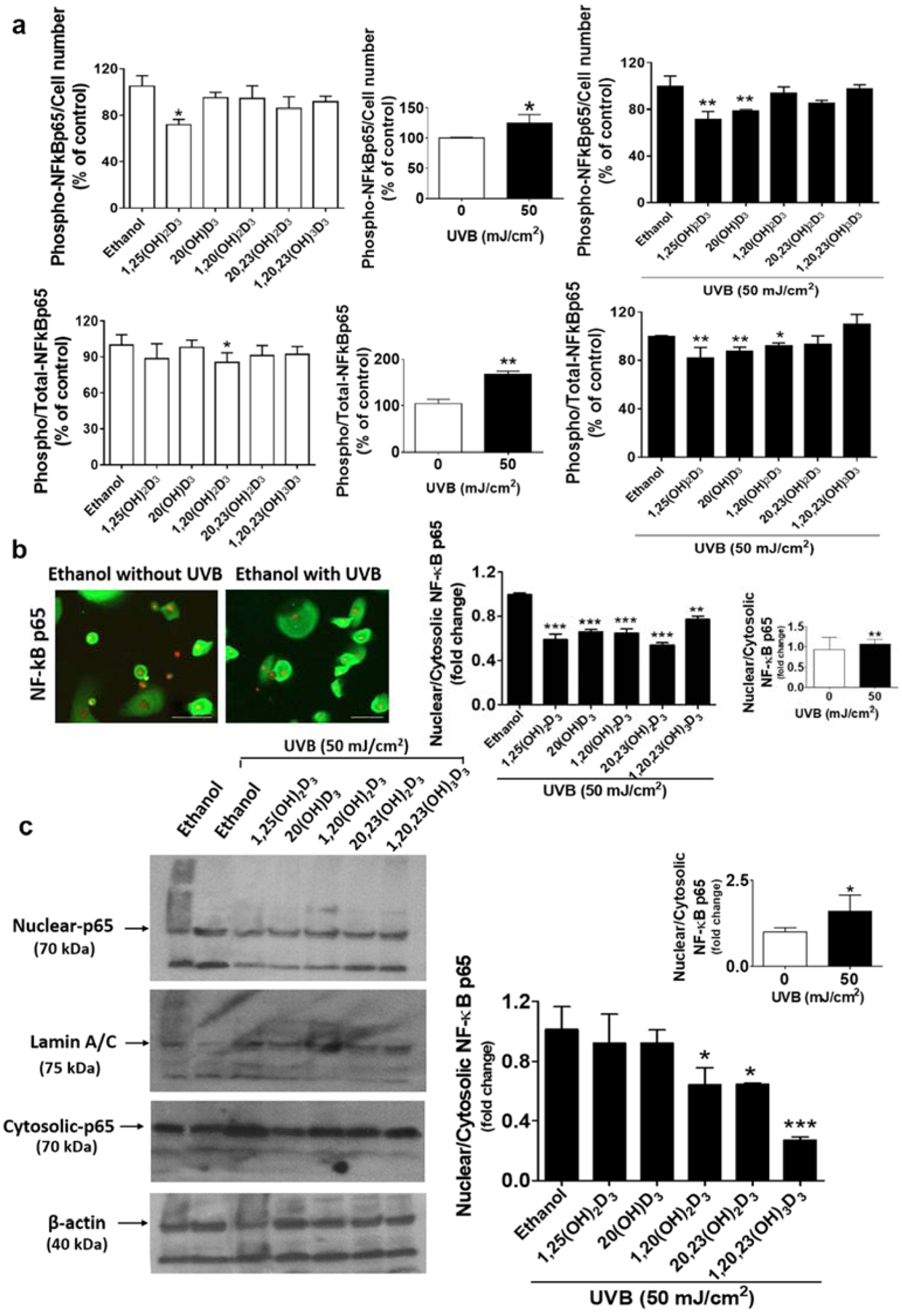
D3 hydroxyderivatives protect against UVB-induced increases in NF-κB p65 phosphorylation and nuclear translocation. (a) ELISA tagged with the phosphorylated-NF-κB p65 or total-NF-κB p65 antibody was used to measure the level of phospho-NF-κB p65/cell number and the phospho/total-NF-κB p65 ratio. (b) NF-κB p65-flourescent positive cells analysis. Lower panel: Quantification of immunofluorescent positive cells presented as the fold-change of the nuclear/cytosol ratio (mean ± S.D.). Scale bar = 100 μm, n ≥ 100 cells for each condition. (c) The blot was stripped and reprobed sequentially with antibodies to nuclear and cytosolic-NF-κB p65 (70 kDa). All are representative of 3–6 independent experiments. Lower panel: Quantification of NF-κB p65 protein is presented as the nuclear/cytosol ratio (mean ± S.D.) and evaluated by the student t-test, *P<0.05, **P<0.01, ***P<0.001 for all conditions compared to the untreated control.
Figure 4.
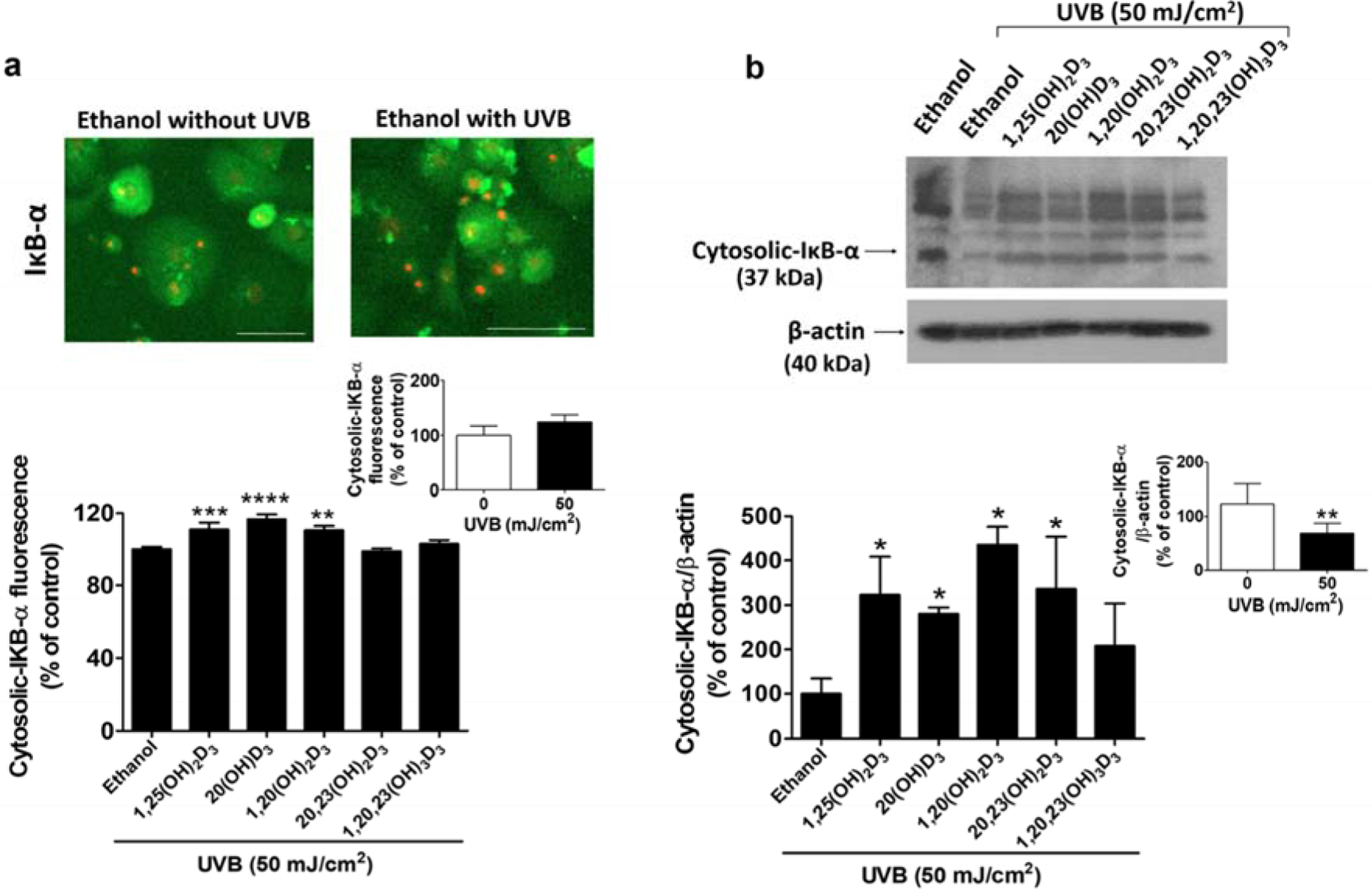
D3 hydroxyderivatives stimulate the inhibitory proteins of the κB-α complex (IκB-α) in UVB-irradiated cells. Treatment with secosteroids (100 nM) was performed as described in materials and methods. Fluorescent microscopy of cells stained with anti-cytosolic-IκB-α is presented in (a). Lower panel: Quantification of immunofluorescent positive cells using the Cytation™ 5 cell imaging, n ≥ 100 cells for each condition. Scale bar = 100 μm. Data are presented as the % of control (mean ± S.D.). (b) The blot was stripped and reprobed sequentially with antibodies to cytosolic- IκB-α (37 kDa). All are representative of 3–6 independent experiments. Lower panel: Quantification of IκB-α protein with data being presented as the % of control (mean ± S.D.) and evaluated by the student t-test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 for all conditions relative to the untreated control.
STATISTICAL ANALYSIS
Data are expressed as mean ± standard deviation of at least three separate experiments (n ≥ 3). The significance of non-irradiated controls or individual treatment groups in comparison to the UVB-irradiated groups was evaluated by the student t-test, *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 for all experiments, using Prism (GraphPad Software Inc.).
RESULTS AND DISCUSSION
CYP11A1-derived D3-hydroxyderivatives stimulate VDR expression in UVB-irradiated keratinocytes
UVB irradiation plays a role in skin inflammation through the reduction of VDR expression in mouse skin and cultured keratinocytes [51, 52]. We and others have previously reported that 1,25(OH)2D3 and its analogs have anti-inflammatory effects mediated via upregulation of VDR signaling [32, 53]. Hence, we investigated the effect of CYP11A1-derived 20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3, and 1,20,23(OH)3D3 which bind with high affinity to VDR [37, 54, 55], on VDR expression. We observed a substantial reduction in VDR mRNA and protein levels in UVB (50 mJ/cm2)-irradiated cells compared to non-irradiated cells (Figure 1b, left panel). All the tested secosteroids significantly reversed the UVB-reduced VDR expression at the mRNA level (Figure 1b, black bar chart). In addition, VDR expression was significantly increased in cells treated with secosteroids that were not exposed to UVB (Figure 1b, white bar chart). We detected the expression of VDR protein as indicated at 45 kDa as shown in the representative blot (Figure 1c, left panel), which was normalized relative to the β-actin control protein. The summary graph of three-independent western blots (Figure 1c, right panel) shows that the treatment with 1,25(OH)2D3, 20(OH)D3 or 1,20(OH)2D3 significantly enhanced VDR protein expression in UVB-irradiated cells, indicating that they can amplify VDR-dependent protective responses.
CYP11A1-derived D3-hydroxyderivatives modulate the expression of inflammatory genes in UVB-irradiated keratinocytes
Since the activation of NF-κB induces the transcription of inflammatory genes including TLR4, COX2, ICAM, IL-6, IL-8, IL-17, IL-33, IL-1α, IL-1β, CD14, NFkB p50, NFkB p65 Rel A, IkB-α, bcl2, and BNIP [5, 6], the expression of the inflammatory genes was determined to investigate the anti-inflammatory role of secosteroids in response to UVB (Figure 2).
Figure 2.
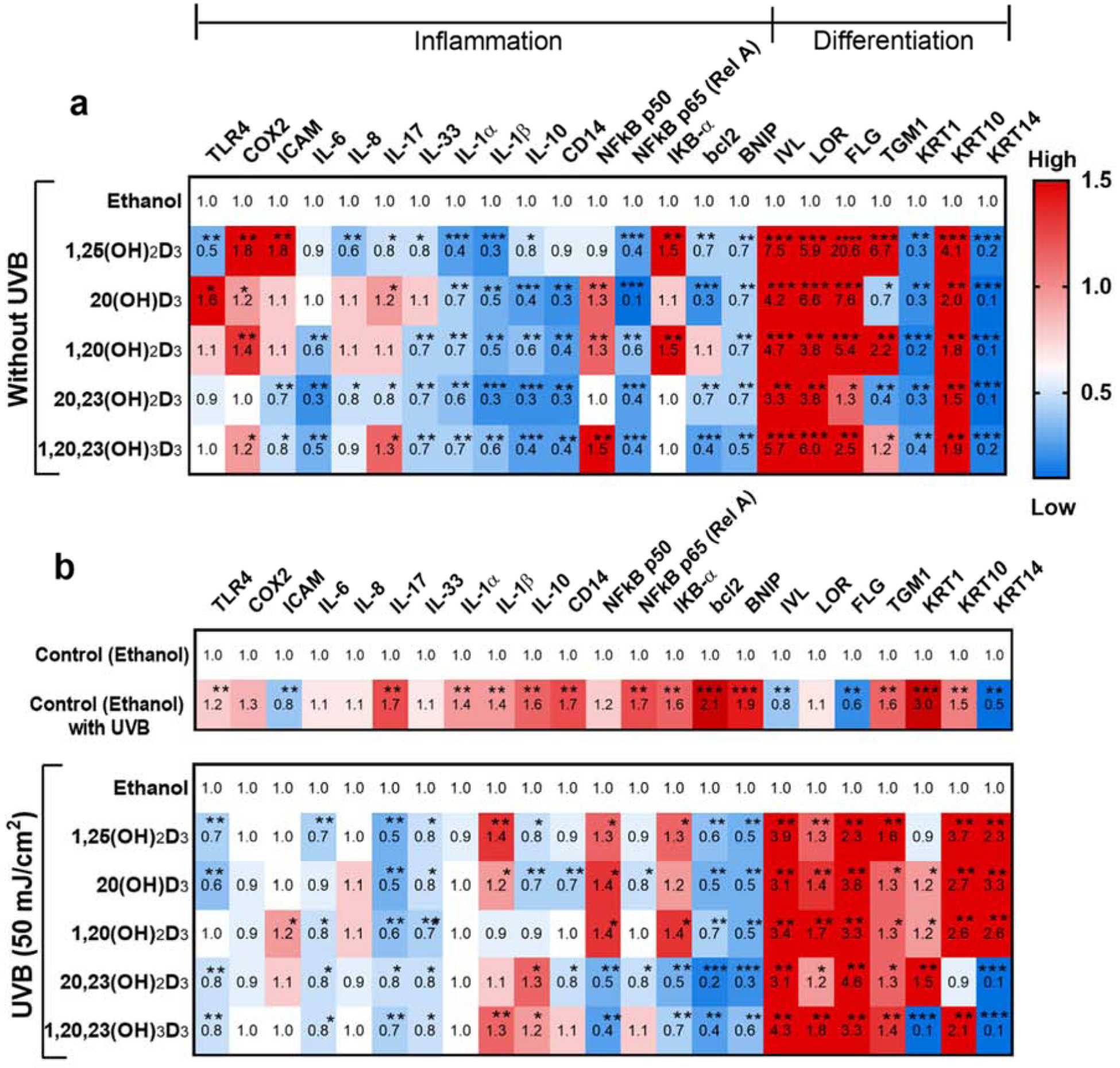
Heat map analysis of the expression of genes involved in inflammation and differentiation following treatment with the hydroxyderivatives of D3 with and without UVB irradiation. Treatment with secosteroids was performed as described in materials and methods. Gene expression was normalized relative to β-actin, cyclopilin, and GAPDH mRNA. Cells treated with secosteroids (or ethanol) without (a) or with UVB irradiation (b). (b) Heat-maps of log2 transformed expression ratios for non-irradiated compared with UVB-irradiated cells. Each vertical row represents the same gene product and each horizontal row each sample. The fluorescence range from high (red) to low (blue) is indicated by the colored bar and reflects the degree of fluorescence intensity/gene expression. Statistical analyses was via the student t-test, *P<0.05, **P<0.01, ***P<0.001 for all conditions, n = 3.
The expression of inflammation-related genes in both non-irradiated and UVB-irradiated cells was modulated by the secosteroids under study (Figure 2). A significant reduction in the expression of eleven genes from a total sixteen, including NFkB p65 (Rel A), was observed in non-irradiated cells. The other five genes: TLR4, COX2, ICAM, NFkB p50, and IkB-α were upregulated in secosteroid- treated cells without prior UVB exposure in comparison to untreated cells (Figure 2a). After UVB exposure (50 mJ/cm2), most of the inflammatory-targeted genes including TLR4, COX2, IL-6, IL-8, IL-17, IL-33, IL-1α, IL-1β, IL-10, CD14, NF-kB p50 and p65, IkB-α, bcl2, as well as BNIP, showed significantly increased expression in UVB-irradiated cells in comparison to non-irradiated cells (Figure 2b, upper map. Nearly all of these genes including TLR4, IL-6, IL-17, IL-33, NFkB p65 (Rel A), bcl2 and BNIP were significantly downregulated by all secosteroids in UVB-irradiated cells (Figure 2b, lower map). However, the expression of some genes such as IL-1β, IL-10, NFkB p50, and IkB-α, was stimulated by some of the secosteroids (Figure 2b, lower map).
We propose that the vitamin D3 derivatives have anti-inflammatory actions following UVB exposure. The mRNA levels of inflammation-related genes, particularly COX2, were increased in treated cells in the absence of UVB exposure. However, the vitamin D3 derivatives slightly inhibited COX2 mRNA levels in cells exposed to UVB, indicating that these compounds do not activate inflammation in cells following UVB irradiation.
D3 hydroxyderivatives down-regulate the phosphorylation of NF-κB p65 in UVB-irradiated keratinocytes
In agreement with previous studies showing that 1,25(OH)2D3, 20(OH)D3 and 20,23(OH)2D3 suppressed the expression of the NF-κB p65 subunit in non-irradiated skin cells [29, 40, 47], this study shows that all the secosteroids tested down-regulated NF-kB p65 (Rel A) in the non-irradiated control while 20(OH)D3 and 20,23(OH)2D3 caused significant down-regulation in the UVB-irradiated group (Figure 2). The level of phospho-NF-κB p65 relative to cell number and total NF-κB p65 levels detected by ELISA were evaluated to assess the activation of NF-κB p65 [56]. In non-irradiated cells, only1,25(OH)2D3 significantly reduced phospho-NF-κB p65 levels/cell number compared to untreated cells (Figure 3a, upper right). A modest decrease (10–20% reduction) in phospho-NF-κB p65 levels/cell number was observed with the other four secosteroids. While treatment with most secosteroids did not cause a significant down-regulation of the phospho-NF-κB p65/total NF-κB p65 levels (5–15% reduction observed), 1,20(OH)2D3 did significantly suppress their levels compared to the untreated cells (Figure 3a, lower right). UVB exposure (50 mJ/cm2) caused up-regulation of phospho-NF-κB p65 levels relative to cell number and the total NF-κB p65 levels (Figure 3a, middle panel). 1,25(OH)2D3 and 20(OH)D3 significantly decreased phospho-NF-κB p65 levels/cell number in UVB-irradiated cells (Figure 3a, upper left). The UVB induced level of NF-κB p65 (NF-κB p65/total NF-κB p65 ratio) was significantly reduced in cells treated with 1,25(OH)2D3, 20(OH)D3, and 1,20(OH)2D3 compared to untreated cells (Figure 3a, lower left). Thus the phosphorylation levels of NF-kB p65 were altered by treatment with some of the secosteroids, presenting their minor effects in phosphorylation levels, as seen in the whole cell fraction.
The effect of D3 hydroxyderivatives on nuclear translocation of NF-κB p65 in UVB-irradiated keratinocytes
Since the phospho-NF-κB p65 levels assessed by a quantitative immunofluorescence assay (Figure 3a) indicated a protective effect of the secosteroids against the UVB-induced inflammatory response, we evaluated nuclear translocation of NF-κB p65 which is essential for its transcriptional activity, by image-based and western blot analyses [57]. There was a significant increase in the nuclear/cytosolic-NF-κB p65 ratio in irradiated cells compared to non-irradiated control cells (Figure 3b, c). The image-based analysis showed that all secosteroids significantly inhibited the translocation of NF-κB p65 caused by UVB exposure (Figure 3b, Figure S1). A significant reduction in the nuclear/cytosol ratio of NF-κB p65 was also observed in secosteroid-treated cells without UVB irradiation (Figure S2). We observed the expression of NF-kB protein (as indicated at 70 kDa) in both nuclear and cytosol fractions as shown in the representative blot (Figure 3c, left panel), which was normalized relative to lamin A/C and β-actin bands, respectively. The expression of NF-kB is reported as the nuclear to cytosolic ratio with Fig 3c (right panel) summarizing the data from three-independent western blots. The data show that treatment with 1,20(OH)2D3, 20,23(OH)2D3 or 1,20,23(OH)3D3 strongly decreases the nuclear-cytosolic NF-kB p65 ratio in UVB-irradiated cells. Similarly, there is a trend for a decrease (approximately 10% inhibition), although not significant, in the nuclear-cytosolic NF-kB p65 ratio in HEKn treated with 1,25(OH)2D3 and 20(OH)D3 in the UVB irradiated cells.
D3-hydroxyderivatives modulate the activity of the IκB-α in UVB-irradiated keratinocytes
Since IκB-α is involved in the inhibition of NF-κB activity by trapping it in the cytosol [9], alterations to the level of cytosolic-IκB-α would indicate whether the secosteroids affect the inflammatory response through modulating IκB-α activity. While the image-based analysis presented a slight induction, western blot analysis showed a strong reduction (almost 50%) in cytosolic-IκB-α in UVB irradiated cells, indicating that UVB irradiation stimulates the inflammatory response in HEKn (Figure 4b). However, treatment with 1,25(OH)2D3, 20(OH)D3, and 1,20(OH)2D3 reversed the decrease in cytosolic IκB-α protein caused by UVB irradiation, as shown in the image-based analysis (Figure 4a, Figure S3). Expression of IkB-α protein at the expected mass of 37 kDa was anlaysed by western blotting of the cytosol fractions, with a representative blot shown in Figure 4b (upper part). Expression was normalized relative to the β-actin control protein. The graph summarizing the data from three-independent western blots shows that the treatment with 1,25(OH)2D3, 20(OH)D3, 1,20(OH)2D3, and 20,23(OH)2D3 significantly enhances cytosolic IκB-α protein levels in comparison to untreated cells (Figure 4b, lower part). Additionally, there was a significant increase in the cytosolic-IκB-α levels in cells treated with 1,25(OH)2D3, 20(OH)D3, and 1,20(OH)2D3 without UVB irradiation, as shown in the image-based analysis (Figure S4).
D3-hydroxyderivatives exert anti-inflammatory effects on UVB-irradiated keratinocytes
Our previous studies on the vitamin D3 hydroxyderivatives showed that they display anti-inflammatory properties including the inhibition of IFN-γ production in splenocytes incubated in vitro [37, 58, 59]. Our current data are consistent with these reports and suggest that the photoprotective effects of compounds under study might include the inhibition of IFN-γ production by keratinocytes. As secosteroids inhibited UVB induced activation and nuclear translocation of NF-kB, we determined if this would lead to inhibition of the expression of NF-kB dependent pro-inflammatory cytokines IL-17, IFN-γ, and TNF-α. UVB exposure (50 mJ/cm2) significantly increased IL-17, IFN-γ, and TNF-α levels compared to non-irradiated cells (Figure 5). Treatment with 1,25(OH)2D3, 20(OH)D3, and 1,20,23(OH)3D3 significantly decreased IL-17 levels in UVB-irradiated cells (Figure 5a). Moreover, there was a significant reduction of IFN-γ levels in cells treated with all secosteroids compared to untreated cells following UVB irradiation (Figure 5b). Interferon (IFN)-γ acts as a crucial biomarker of the immune system in skin cells [60] and its up-regulation has been observed in skin inflammation [61]. IFN-γ expression in treatment groups exhibited the most pronounced change when compared to other cytokines, suggesting that the anti-inflammatory effects of the vitamin D3 derivatives are, at least in part, mediated through the regulation of IFN-γ. Treatment of HEKn with 1,25(OH)2D3, 1,20(OH)2D3, 20,23(OH)2D3, and 1,20,23(OH)3D3 also significantly suppressed UVB-induced TNF-α levels compared to untreated cells (Figure 5c). Moreover, all secosteroids reduced levels of cytokines including IL-17, TNF-α and IFN-γ in non-irradiated cells (Figure S5). Thus, it is apparent that that 1,25(OH)2D3 and the CYP11A-derived secosteroids not only modulate NF-κB p65 and IκB-α pathways, but also inhibit the production of Th1 and Th17 proinflammatory cytokines.
Figure 5.
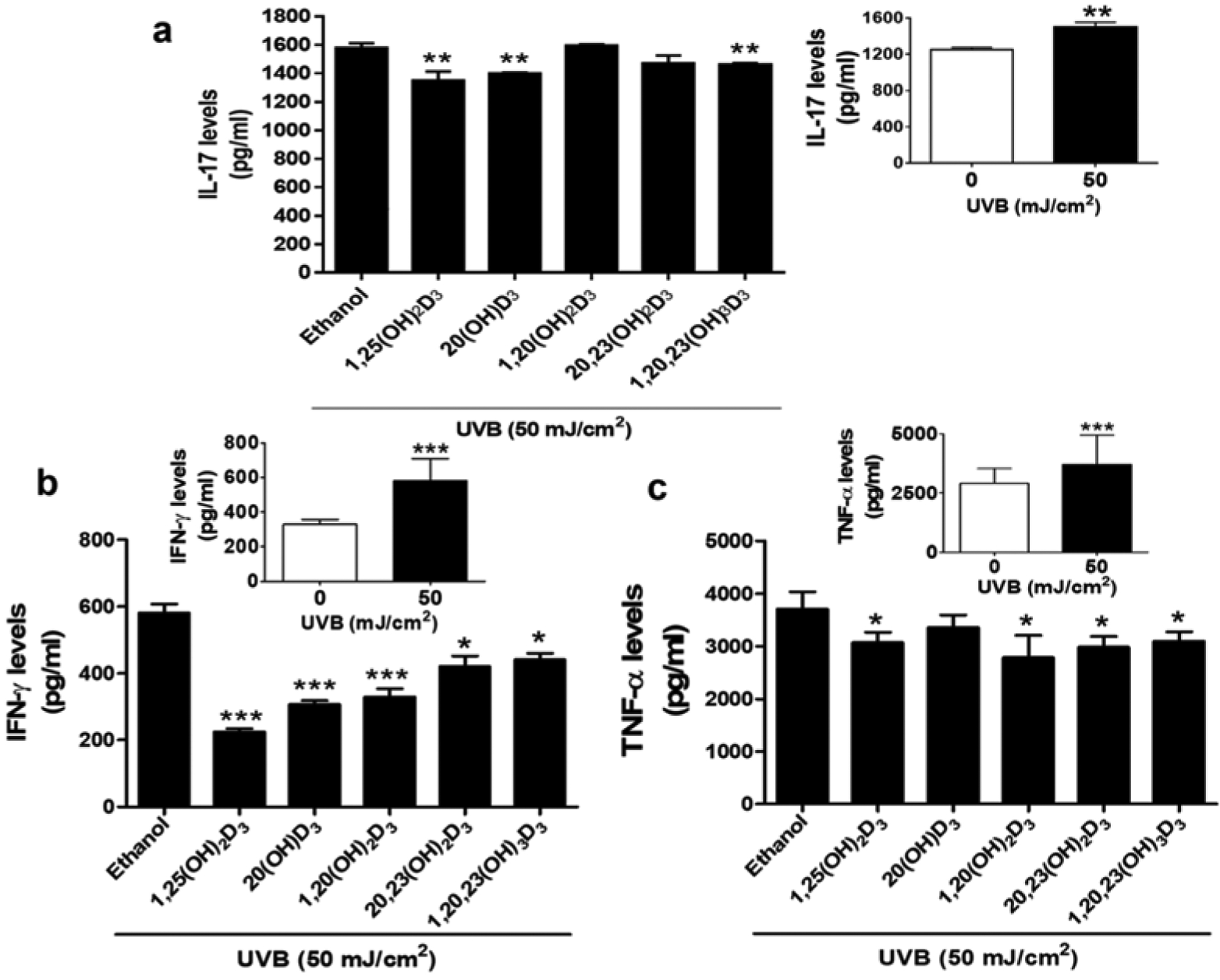
Anti-inflammatory effect of D3 hydroxyderivatives on UVB-irradiated keratinocytes. Treatment with secosteroids (100 nM) was performed as described in materials and methods. Cell supernatants were plated using an ELISA assay kit tagged with (a) IL-17, (b) IFN-γ, or (c) TNF-α antibody. The levels of inflammatory cytokines were calculated from the standard curves. Data are presented as the concentration of cytokines (pg/ml). The statistical significance of differences was evaluated by the student t-test, * P < 0.05, ** P < 0.01, *** P < 0.001, for all conditions relative to the untreated control.
The long-term inhibitory effects of vitamin D3 derivatives on inflammation, without reducing DNA damage, could potentially promote carcinogenesis [62, 63]. However, our previous studies using a range of different methods have shown that the vitamin D3 derivatives under study attenuate direct damage to DNA, stimulate DNA repair, and induce p53 signaling which is a hallmark of the tumor suppressor pathway [10, 11]. Therefore, vitamin D3 derivatives not only protect against UVB-induced inflammation but also against DNA damage, which implies that they have chemopreventive effects on UVB-induced carcinogenesis. It should also be noted that chronic inflammation can promote malignant transformation [64].
D3-hydroxyderivatives modulate the expression of genes involved in cell differentiation in UVB-irradiated keratinocytes
Cell differentiation plays an important role in the repair of damaged skin [65]. Gene expression profiling of IVL, LOR, FLG, TGM1, and KRT (cytokeratin encoded genes) including KRT1, KRT10, and KRT14 was carried out to evaluate the role of the secosteroids under study on epithelial differentiation (Figure 2) [34, 46].
The heat map analysis clearly illustrated a distinct difference in the expression of all genes involved in cell differentiations in HEKn-treated with secosteroids in the presence and absence of UVB. In non-irradiated cells, there was an up-regulation of the expression of five from seven differentiation genes, IVL, LOR, FLG, TGM1, and KRT10, and down-regulation of KRT1 and KRT14 after treatment with secosteroids (Figure 2b, upper map). UVB irradiation significantly suppressed mRNA levels of IVL, FLG, and KRT14 (Figure 2a), but stimulated TGM1, KRT1, and KRT10 mRNA expression (Figure 2a). The expression of almost all genes involved in keratinocyte differentiation, including IVL, LOR, FLG, TGM1, KRT1, KRT10, and KRT14, was significantly upregulated by treatment with secosteroids in UVB-irradiated cells (Figure 2b, lower map). Inversely, there was a down-regulation of KRT1 and KRT14 expression in cells treated with 20,23(OH)2D3 and 1,20,23(OH)3D3 following UVB irradiation (Figure 2b, lower map).
D3-hydroxyderivatives stimulate IVL and CK10 protein expression in UVB-irradiated keratinocytes
The D3 hydroxyderivatives mediated epidermal differentiation through a significant upregulation of IVL, FLG, and KRT10 expression in both the non-irradiated control and UVB-irradiated groups. IVL is a protein commonly used as an early stage differentiation marker of human keratinocytes [13, 66]. While UVB did not significantly affected IVL protein expression as measured by immuno-flourescence (Figure 6a), all secosteroids significantly enhanced expression of IVL protein compared to untreated cells (Figure 6a, Figure S6). Moreover, treatment of non-irradiated cells with secosteroids significantly increased IVL levels (Figure S7). IVL protein expression at the expected mass of 68 kDa in shown in a representative western blot (Figure 6b, upper part). IVL band intensity was normalized relative to the β-actin control protein. The graph of summarized data of six-independent western blots shows that there is a marked increase in IVL expression in UVB-irradiated cells treated with secosteroids (except for 1,20,23(OH)3D3), compared to UVB-irradiated control cells (Figure 6b, lower part).
Figure 6.
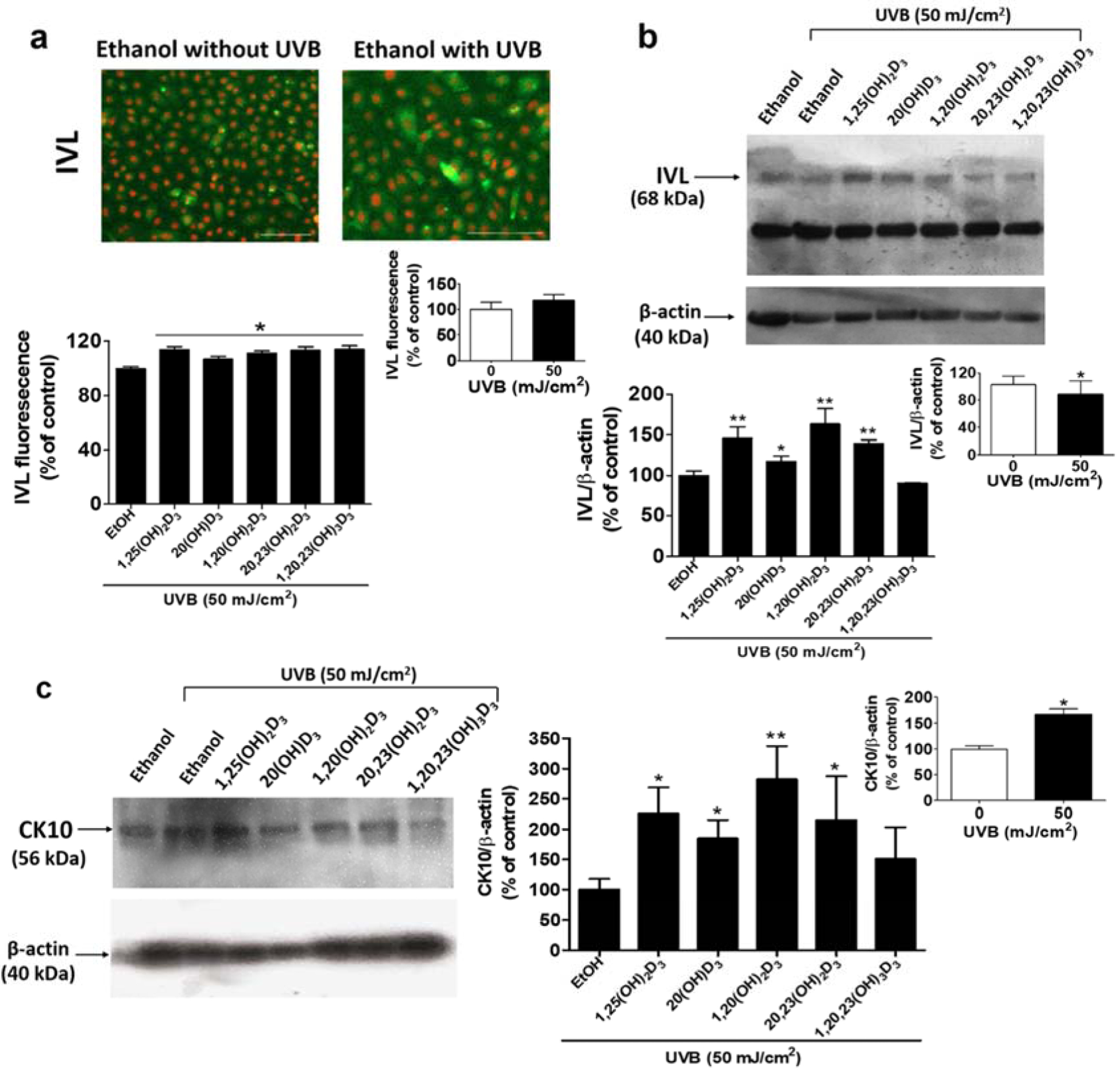
D3 hydroxyderivatives modulate the differentiation of UVB-irradiated keratinocytes as measured by the increased expression of involucrin (IVL) and cytokeratin 10 (CK10). IVL-fluorescent positive cells are presented in (a). Lower panel: Quantification of immunofluorescent positive cells using the Cytation™ 5 cell imaging multi-mode reader, n ≥ 100 cells for each condition. Scale bar = 100 μm. (b) The blot was stripped and reprobed sequentially with antibodies to IVL (68 kDa). Lower panel: Quantification of IVL protein. (c) The blot was stripped and reprobed sequentially with antibodies to CK10 (56 kDa). Lower panel: Quantification of CK10 protein. All are representative of 3–6 independent experiments. Data are presented as the % of control (mean ± S.D.) and evaluated by the student t-test, *P<0.05, **P<0.01, ***P<0.001, for all conditions relative to the untreated control.
Previous studies showed that CK10, highly expressed in suprabasal epidermal keratinocytes [67], was downregulated by the proinflammatory cytokines including IL-17 and TNF-α, causing an impairment of epidermal differentiation [68, 69]. Therefore, the effects of secosteroids on CK10 were evaluated. CK10 protein expression at the expected mas of 56 kDa is shown in a representative western blot (Figure 6c, left panel). CK10 band intensity was normalized relative to the β-actin control protein with a graph summarizing the results from three independent experiments being shown in Figure 6c (right panel). While UVB (50 mJ/cm2) upregulated levels of CK10 compared to non-irradiated cells, the levels of CK10 were further increased in cells treated with 1,25(OH)2D3, 20(OH)D3, 1,20(OH)2D3, and 20,23(OH)2D3 compared with UVB-irradiated untreated cells. Overall the data for IVL and CK10 indicate that the CYP11A1-derivatived secosteroids stimulate epidermal differentiation in UVB treated and untreated cells.
In contrast to the other secosteroids, treatment with 1,20,23(OH)3D3 reduced the expression of genes related to differentiation. However, this effect was not seen at the protein level when tested on the protein markers IVL and CK10. Even though 1,20,23(OH)3D3 could increase VDR mRNA, it could not change VDR protein levels (Figure 1). This suggests that 1,20,23(OH)3D3 has only a weak biological action on the VDR, with minimal effects on downstream targets, including differentiation.
CONCLUDING REMARKS
Here we document that 1,25(OH)2D3 and our synthetic CYP11A1-derived D3-hydroxyderivatives suppress UVB-induced inflammatory responses in keratinocytes by modulating NF-κB/IκB-α and its down-stream signaling pathway, and promote differentiation of keratinocytes. With respect to their mechanism of action, our data indicate that the inhibition of NF-κB signaling by the secosteroids plays a role in inhibition of Th1 and Th17 differentiation directly and indirectly. NF-κBp65 (RelA) binds to enhancer regions within the Ifng locus in T cells to promote expression of IFN-γ and drive Th1 differentiation [70]. Similarly, NF-κB is necessary for the efficient expression of retinoic orphan acid receptor (ROR)γ, the key transcription factor necessary for Th17 differentiation [71]. NF-κB is also important for the expression of IL-12 and IL-1β by innate immune cells, cytokines necessary for Th1 and Th17 differentiation, respectively (Reviewed in [5]. This NF-κB regulation may be mediated by the VDR as reported previously in non-irradiated keratinocytes [29, 40]. The downregulation of Th17 responses may be mediated by the reverse agonistic activity of secosteroids on RORs, which are expressed in keratinocytes, [35, 55], and its γ form in particular, since IL-17 is downstream of RORγ [72]. However, indirect involvement of NF-κB acting on RORγ cannot be excluded [5]. With respect to keratinocyte differentiation, the involvement of VDR is expected, however, the contribution of other nuclear receptors including RORα and γ and of the arylhydrocarbon receptor (AhR) is likely since they are expressed and functional in keratinocytes [34, 35, 46, 55]. They may also contribute to the anti-inflammatory effects of the secosteroids. The precise involvement of the above receptors in photoprotective signaling needs further investigations using selective or combined knock-out mice for these and VDR receptors.
The described photoprotective mechanisms of vitamin D3-hydroxyderivatives involving the targeting of nuclear receptors, including VDR, and NF-κB signaling pathways, represent attractive targets for the development of therapeutic agents for skin disorders associated with UVB-induced inflammation and impaired keratinocyte differentiation.
DATA AVAILABILITY STATEMENT
No datasets were generated or analyzed during the current study.
Supplementary Material
HIGHLIGHTS.
Vitamin D3 hydroxyderivatives protect against UVB-induced skin damage
Vitamin D3 hydroxyderivatives target NF-κB/IκB inflammatory pathways
Vitamin D3 hydroxyderivatives promote keratinocyte differentiation
Vitamin D3 hydroxyderivatives are promising compounds for skin photoprotection
ACKNOWLEDGEMENTS AND FUNDING SOURCES
This work was supported by the National Institutes of Health [grant numbers 1R01AR073004-01A1, R01AR071189-01A1]; and the Veterans Affairs (VA) merit [grant number 1I01BX004293-01A1] to ATS.
ABBREVIATIONS:
- 1,25(OH)2D3
1α,25-dihydroxyvitamin D3
- 20(OH)D3
20S-hydroxyvitamin D3
- 1,20(OH)2D3
1α,20S-dihydroxyvitamin D3
- 20,23(OH)2D3
20S,23S-dihydroxyvitamin D3
- 1,20,23(OH)3D3
1α,20S,23S-trihydroxyvitamin D3
- CYP
cytochrome P450 enzymes
- CYP11A1
cytochrome P450 family 11 subfamily A member 1
- HEKn
human epidermal keratinocytes
- EtOH
ethanol
- UVB
ultraviolet B
- NF-κB
nuclear factor kappa B
- IκB
inhibitor kappa B
- TLR4
toll-like receptor 4
- CD14
cluster of differentiation 14
- COX-2
cyclooxygenase-2
- IL-6
interleukin-6
- IL-8
interleukin-8
- IL-17
interleukin-17
- IL-33
interleukin-33
- IL-1α
interleukin-1α, IL-1β, interleukin-1β
- IL-10
interleukin-10
- IFN-γ
interferon gamma
- TNF-α
tumor necrosis factor-alpha
- Bcl2
B-cell lymphoma 2
- BNIP
B-cell lymphoma 2/adenovirus E1B-19K protein-interacting protein
- VDR
vitamin D3 receptor
- IVL
involucrin
- CK10
cytokeratin 10
- CK1
cytokeratin 1
- CK14
cytokeratin 14
- KRT
cytokeratin encoded gene
- LOR
loricrine
- FLG
filaggrine
- TGM1
transglutaminase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- [1].Wikramanayake TC, Stojadinovic O, Tomic-Canic M, Epidermal Differentiation in Barrier Maintenance and Wound Healing, Adv Wound Care (New Rochelle) 3(3) (2014) 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R, How UV Light Touches the Brain and Endocrine System Through Skin, and Why, Endocrinology 159(5) (2018) 1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hoesel B, Schmid JA, The complexity of NF-kappaB signaling in inflammation and cancer, Mol Cancer 12 (2013) 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu CS, Lan CC, Kuo HY, Chai CY, Chen WT, Chen GS, Differential regulation of nuclear factor-kappa B subunits on epidermal keratinocytes by ultraviolet B and tacrolimus, Kaohsiung J Med Sci 28(11) (2012) 577–85. [DOI] [PubMed] [Google Scholar]
- [5].Liu T, Zhang L, Joo D, Sun SC, NF-kappaB signaling in inflammation, Signal Transduct Target Ther 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Noske K, Secreted immunoregulatory proteins in the skin, J Dermatol Sci 89(1) (2018) 3–10. [DOI] [PubMed] [Google Scholar]
- [7].Bashir MM, Sharma MR, Werth VP, UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription, J Invest Dermatol 129(4) (2009) 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, Armstrong CA, Ansel JC, Human keratinocytes express functional CD14 and toll-like receptor 4, J Invest Dermatol 119(2) (2002) 424–32. [DOI] [PubMed] [Google Scholar]
- [9].Tsuchiya Y, Asano T, Nakayama K, Kato T Jr., Karin M, Kamata H, Nuclear IKKbeta is an adaptor protein for IkappaBalpha ubiquitination and degradation in UV-induced NF-kappaB activation, Mol Cell 39(4) (2010) 570–82. [DOI] [PubMed] [Google Scholar]
- [10].Chaiprasongsuk A, Janjetovic Z, Kim TK, Jarrett SG, D’Orazio JA, Holick MF, Tang EKY, Tuckey RC, Panich U, Li W, Slominski AT, Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms, Redox Biol 24 (2019) 101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Slominski AT, Janjetovic Z, Kim TK, Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W, Tuckey RC, Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation, J Steroid Biochem Mol Biol 148 (2015) 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K, Oxidative stress in aging human skin, Biomolecules 5(2) (2015) 545–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moravcova M, Libra A, Dvorakova J, Viskova A, Muthny T, Velebny V, Kubala L, Modulation of keratin 1, 10 and involucrin expression as part of the complex response of the human keratinocyte cell line HaCaT to ultraviolet radiation, Interdiscip Toxicol 6(4) (2013) 203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Scott JF, Das LM, Ahsanuddin S, Qiu Y, Binko AM, Traylor ZP, Debanne SM, Cooper KD, Boxer R, Lu KQ, Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study, J Invest Dermatol 137(10) (2017) 2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holick MF, Vitamin D and sunlight: strategies for cancer prevention and other health benefits, Clin J Am Soc Nephrol 3(5) (2008) 1548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tuckey RC, Cheng CYS, Slominski AT, The serum vitamin D metabolome: What we know and what is still to discover, J Steroid Biochem Mol Biol 186 (2019) 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jenkinson C, The vitamin D metabolome: An update on analysis and function, Cell Biochem Funct (2019). [DOI] [PubMed] [Google Scholar]
- [18].Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, C.T. R, A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin, Eur J Biochem 271(21) (2004) 4178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EKY, Tuckey RC, Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland, Sci Rep 5 (2015) 14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A, Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc, FEBS J 275(10) (2008) 2585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC, In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1, FASEB J 26(9) (2012) 3901–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu S, Sun J, Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection, Discov Med 11(59) (2011) 325–35. [PMC free article] [PubMed] [Google Scholar]
- [23].McNally P, Coughlan C, Bergsson G, Doyle M, Taggart C, Adorini L, Uskokovic MR, El-Nazir B, Murphy P, Greally P, Greene CM, McElvaney NG, Vitamin D receptor agonists inhibit pro-inflammatory cytokine production from the respiratory epithelium in cystic fibrosis, J Cyst Fibros 10(6) (2011) 428–34. [DOI] [PubMed] [Google Scholar]
- [24].Oda Y, Hu L, Nguyen T, Fong C, Zhang J, Guo P, Bikle DD, Vitamin D Receptor Is Required for Proliferation, Migration, and Differentiation of Epidermal Stem Cells and Progeny during Cutaneous Wound Repair, J Invest Dermatol 138(11) (2018) 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC, Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein, J Biol Chem 288(27) (2013) 19450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Szeto FL, Sun J, Kong J, Duan Y, Liao A, Madara JL, Li YC, Involvement of the vitamin D receptor in the regulation of NF-kappaB activity in fibroblasts, J Steroid Biochem Mol Biol 103(3–5) (2007) 563–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bikle DD, Ng D, Oda Y, Hanley K, Feingold K, Xie Z, The vitamin D response element of the involucrin gene mediates its regulation by 1,25-dihydroxyvitamin D3, J Invest Dermatol 119(5) (2002) 1109–13. [DOI] [PubMed] [Google Scholar]
- [28].Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD, Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth, J Invest Dermatol 118(1) (2002) 11–6. [DOI] [PubMed] [Google Scholar]
- [29].Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM Jr., Slominski AT, 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes, J Cell Physiol 223(1) (2010) 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC, The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions, J Steroid Biochem Mol Biol 144 Pt A (2014) 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mousa A, Naderpoor N, Johnson J, Sourris K, de Courten MPJ, Wilson K, Scragg R, Plebanski M, de Courten B, Effect of vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: a randomized placebo-controlled trial, Sci Rep 7(1) (2017) 15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun D, Luo F, Xing JC, Zhang F, Xu JZ, Zhang ZH, 1,25(OH)2 D3 inhibited Th17 cells differentiation via regulating the NF-kappaB activity and expression of IL-17, Cell Prolif 51(5) (2018) e12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhou Y, Wang GF, Yang L, Liu F, Kang JQ, Wang RL, Gu W, Wang CY, Treatment with 1,25(OH)2D3 induced HDAC2 expression and reduced NF-kappaB p65 expression in a rat model of OVA-induced asthma, Braz J Med Biol Res 48(7) (2015) 654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Slominski AT, Kim TK, Janjetovic Z, Brozyna AA, Zmijewski MA, Xu H, Sutter TR, Tuckey RC, Jetten AM, Crossman DK, Differential and Overlapping Effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)(2)D3, Int J Mol Sci 19(10) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, Jetten AM, RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D, FASEB J 28(7) (2014) 2775–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT, 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation, J Invest Dermatol 128(9) (2008) 2271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lin Z, Marepally SR, Goh ESY, Cheng CYS, Janjetovic Z, Kim TK, Miller DD, Postlethwaite AE, Slominski AT, Tuckey RC, Peluso-Iltis C, Rochel N, Li W, Investigation of 20S-hydroxyvitamin D3 analogs and their 1alpha-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities, Sci Rep 8(1) (2018) 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF, Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity, PLoS One 5(3) (2010) e9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE, 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo, J Clin Endocrinol Metab 98(2) (2013) E298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT, 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes, PLoS One 4(6) (2009) e5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Naik KK, Thangavel S, Alam A, Cytotoxicity and Anti-inflammatory Activity of Flavonoid Derivatives Targeting NF-kappaB, Recent Pat Inflamm Allergy Drug Discov 10(2) (2017) 119–132. [DOI] [PubMed] [Google Scholar]
- [42].Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A, Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells, Drug Metab Dispos 39(9) (2011) 1577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC, The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism, FEBS J 272(16) (2005) 4080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A, Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity, Steroids 75(12) (2010) 926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, Li W, Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity, Anticancer Res 34(5) (2014) 2153–63. [PMC free article] [PubMed] [Google Scholar]
- [46].Slominski AT, Kim TK, Hobrath JV, Janjetovic Z, Oak ASW, Postlethwaite A, Lin Z, Li W, Takeda Y, Jetten AM, Tuckey RC, Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols, Sci Rep 7(1) (2017) 11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oeckinghaus A, Ghosh S, The NF-kappaB family of transcription factors and its regulation, Cold Spring Harb Perspect Biol 1(4) (2009) a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chaiprasongsuk A, Lohakul J, Soontrapa K, Sampattavanich S, Akarasereenont P, Panich U, Activation of Nrf2 Reduces UVA-Mediated MMP-1 Upregulation via MAPK/AP-1 Signaling Cascades: The Photoprotective Effects of Sulforaphane and Hispidulin, J Pharmacol Exp Ther 360(3) (2017) 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yanagisawa S, Baker JR, Vuppusetty C, Koga T, Colley T, Fenwick P, Donnelly LE, Barnes PJ, Ito K, The dynamic shuttling of SIRT1 between cytoplasm and nuclei in bronchial epithelial cells by single and repeated cigarette smoke exposure, PLoS One 13(3) (2018) e0193921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ouyang J, Wu M, Huang C, Cao L, Li G, Overexpression of oxidored-nitro domain containing protein 1 inhibits human nasopharyngeal carcinoma and cervical cancer cell proliferation and induces apoptosis: Involvement of mitochondrial apoptotic pathways, Oncol Rep 29(1) (2013) 79–86. [DOI] [PubMed] [Google Scholar]
- [51].Demetriou SK, Ona-Vu K, Teichert AE, Cleaver JE, Bikle DD, Oh DH, Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes, J Invest Dermatol 132(8) (2012) 2097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Biggs L, Yu C, Fedoric B, Lopez AF, Galli SJ, Grimbaldeston MA, Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice, J Exp Med 207(3) (2010) 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yang M, Xu J, Yu J, Yang B, Gan H, Li S, Li X, Anti-inflammatory effects of 1,25-dihydroxyvitamin D3 in monocytes cultured in serum from patients with type 2 diabetes mellitus and diabetic nephropathy with uremia via Toll-like receptor 4 and nuclear factor-kappaB p65, Mol Med Rep 12(6) (2015) 8215–22. [DOI] [PubMed] [Google Scholar]
- [54].Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W, Slominski AT, Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength, Mol Cell Endocrinol 361(1–2) (2012) 143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, Li W, Tuckey RC, Jetten AM, Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and RORgamma, J Steroid Biochem Mol Biol 173 (2017) 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Karova K, Wainwright JV, Machova-Urdzikova L, Pisal RV, Schmidt M, Jendelova P, Jhanwar-Uniyal M, Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-kappaB pathway inhibition, J Neuroinflammation 16(1) (2019) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Maguire O, Collins C, O’Loughlin K, Miecznikowski J, Minderman H, Quantifying nuclear p65 as a parameter for NF-kappaB activation: Correlation between ImageStream cytometry, microscopy, and Western blot, Cytometry A 79(6) (2011) 461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lin Z, Chen H, Belorusova AY, Bollinger JC, Tang EKY, Janjetovic Z, Kim TK, Wu Z, Miller DD, Slominski AT, Postlethwaite AE, Tuckey RC, Rochel N, Li W, 1alpha,20S-Dihydroxyvitamin D3 Interacts with Vitamin D Receptor: Crystal Structure and Route of Chemical Synthesis, Sci Rep 7(1) (2017) 10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lin Z, Marepally SR, Kim TK, Janjetovic Z, Oak AS, Postlethwaite AE, Myers LK, Tuckey RC, Slominski AT, Miller DD, Li W, Design, Synthesis and Biological Activities of Novel Gemini 20S-Hydroxyvitamin D3 Analogs, Anticancer Res 36(3) (2016) 877–86. [PMC free article] [PubMed] [Google Scholar]
- [60].Rashighi M, Harris JE, Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo, Ann Transl Med 3(21) (2015) 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, Feigenbaum L, Fuchs E, Lyakh L, Young HA, Hornyak TJ, Arnheiter H, Trinchieri G, Meltzer PS, De Fabo EC, Merlino G, Interferon-gamma links ultraviolet radiation to melanomagenesis in mice, Nature 469(7331) (2011) 548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kidane D, Chae WJ, Czochor J, Eckert KA, Glazer PM, Bothwell AL, Sweasy JB, Interplay between DNA repair and inflammation, and the link to cancer, Crit Rev Biochem Mol Biol 49(2) (2014) 116–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Murata M, Crosstalk between DNA Damage and Inflammation in the Multiple Steps of Carcinogenesis, Int J Mol Sci 18(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Amara S, Tiriveedhi V, Inflammatory role of high salt level in tumor microenvironment (Review), Int J Oncol 50(5) (2017) 1477–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Freije A, Molinuevo R, Ceballos L, Cagigas M, Alonso-Lecue P, Rodriguez R, Menendez P, Aberdam D, De Diego E, Gandarillas A, Inactivation of p53 in Human Keratinocytes Leads to Squamous Differentiation and Shedding via Replication Stress and Mitotic Slippage, Cell Rep 9(4) (2014) 1349–60. [DOI] [PubMed] [Google Scholar]
- [66].Nakamura T, Nishida K, Dota A, Matsuki M, Yamanishi K, Kinoshita S, Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease, Invest Ophthalmol Vis Sci 42(3) (2001) 549–56. [PubMed] [Google Scholar]
- [67].Edqvist PH, Fagerberg L, Hallstrom BM, Danielsson A, Edlund K, Uhlen M, Ponten F, Expression of human skin-specific genes defined by transcriptomics and antibody-based profiling, J Histochem Cytochem 63(2) (2015) 129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Skazik C, Amann PM, Heise R, Marquardt Y, Czaja K, Kim A, Ruhl R, Kurschat P, Merk HF, Bickers DR, Baron JM, Downregulation of STRA6 expression in epidermal keratinocytes leads to hyperproliferation-associated differentiation in both in vitro and in vivo skin models, J Invest Dermatol 134(6) (2014) 1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hanel KH, Cornelissen C, Luscher B, Baron JM, Cytokines and the skin barrier, Int J Mol Sci 14(4) (2013) 6720–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Balasubramani A, Shibata Y, Crawford GE, Baldwin AS, Hatton RD, Weaver CT, Modular utilization of distal cis-regulatory elements controls Ifng gene expression in T cells activated by distinct stimuli, Immunity 33(1) (2010) 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH, The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis, J Exp Med 208(11) (2011) 2321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jetten AM, Takeda Y, Slominski A, Kang HS, Retinoic acid-related Orphan Receptor gamma (RORgamma): connecting sterol metabolism to regulation of the immune system and autoimmune disease, Curr Opin Toxicol 8 (2018) 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.


