The crystal structure of the N-terminal membrane anchoring domain of mycobacterial DivIVA/Wag31 reveals a filament-compatible ‘dimer-of-dimers’ assembly state. The results suggest that, in addition to lipid binding, the N-terminal of Wag31 can participate in self-assembly to form filamentous structures.
Keywords: mycobacterial polar growth, dimer assembly, mycobacterium tuberculosis, coiled coil, lipids, filaments
Abstract
Wag31, or DivIVA, is an essential protein and a drug target in the human pathogen Mycobacterium tuberculosis that self-assembles at the negatively curved membrane surface to form a higher-order structural scaffold, maintains rod-shaped cellular morphology and localizes key cell-wall synthesizing enzymes at the pole for exclusive polar growth. The crystal structure of the N-terminal lipid-binding domain of mycobacterial Wag31 was determined at 2.3 Å resolution. The structure revealed a highly polar surface lined with several conserved charged residues that suggest probable sites for interactions with membrane lipids. Crystal-packing analysis revealed a previously unseen ‘dimer-of-dimers’ assembly state of N-terminal Wag31, which is formed by antiparallel stacking of two coiled-coil dimers. Size-exclusion column-chromatography-coupled small-angle solution X-ray scattering data revealed a tetrameric form as a major assembly state of N-terminal Wag31 in solution, further supporting the crystal structure. The results suggest that, in addition to lipid binding, the N-terminal Wag31 can participate in self-assembly to form filamentous structures. Plausible models of linear self-assembly and branching of Wag31 filaments consistent with available data are suggested.
1. Introduction
The sensing of membrane curvature plays a critical role in diverse physiological processes such as the maintenance of cellular morphology, polar or hyphal growth in bacteria and endocytosis in eukaryotes (Cannon et al., 2017 ▸). Prominent examples of positive curvature-sensing proteins include septins and BAR (Bin/amphiphysin/Rvs) domain proteins in eukaryotes, and SpoVM in sporulating bacteria (Cannon et al., 2017 ▸). In Gram-positive bacteria, DivIVA recognizes negative concave membrane curvature, and self-assembles at the cytoplasmic side of the pole and curved region of the cell-division septum to form a structural scaffold (Edwards & Errington, 1997 ▸; Letek et al., 2008 ▸; Lenarcic et al., 2009 ▸; Ramamurthi & Losick, 2009 ▸; Kaval & Halbedel, 2012 ▸; Bach et al., 2014 ▸). DivIVA, which is a filamentous coiled-coil protein, aids in the localization of various target proteins at the pole for cell-wall growth and a variety of biological functions (Rudner & Losick, 2010 ▸; Laloux & Jacobs-Wagner, 2014 ▸; Halbedel & Lewis, 2019 ▸; Hammond et al., 2019 ▸).
DivIVA, also known as Wag31 and antigen 84, is essential in mycobacteria, and is required for its exclusive polar growth, morphology maintenance and other functions (Nguyen et al., 2007 ▸; Kang et al., 2008 ▸; Mukherjee et al., 2009 ▸; Jani et al., 2010 ▸; Plocinski et al., 2012 ▸; Ginda et al., 2013 ▸; Plocinska et al., 2014 ▸; Melzer et al., 2018 ▸). Furthermore, Wag31 is targeted by bactericidal amino-pyrimidine sulfonamides, though the mechanism of antibacterial action is not known (Boshoff, 2017 ▸; Singh et al., 2017 ▸). Wag31 plays a critical role in regulating peptidoglycan biosynthesis and localizing many cell-wall synthesizing enzymes at the pole to support polar growth (Kang et al., 2008 ▸; Jani et al., 2010 ▸; Meniche et al., 2014 ▸; Xu et al., 2014 ▸). Wag31 is phosphorylated at a single site (Thr73) that enables it to be better at localization at the pole and at regulation of peptidoglycan biosynthesis (Kang et al., 2008 ▸; Jani et al., 2010 ▸). A small membrane-bound protein CwsA may assist Wag31 localization at the mycobacterial pole (Plocinski et al., 2012 ▸). While depletion of Wag31 leads to a ‘rod to spherical cell’ transition (Nguyen et al., 2007 ▸; Kang et al., 2008 ▸; Meniche et al., 2014 ▸), Wag31 is shown to contribute to restoration of rod shape in spherical cells (Melzer et al., 2018 ▸).
Oligomerization appears to be a recurring theme in the sensing of mesoscale (∼100 nm to 1 µm) curvature by nanoscale protein subunits in several cases (Antonny, 2011 ▸; Cannon et al., 2017 ▸). A ‘molecular bridging of the curvature’ by oligomers of DivIVA was suggested as a mechanism of DivIVA binding to a concave membrane (Lenarcic et al., 2009 ▸). However, how DivIVA oligomerizes is sparsely understood. Structural data on DivIVA is so far limited to only one study (Oliva et al., 2010 ▸). In this study, a model of a 30 nm long tetrameric form of DivIVA from Bacillus subtilis (bsDivIVA) was proposed based on the crystal structures of individual N-terminal and C-terminal coiled-coil domains of DivIVA (Oliva et al., 2010 ▸). Conserved phenylalanine and arginine residue pairs at both ends of this bent elongated tetrameric structure occupy the proposed sites of polar membrane tethering (Oliva et al., 2010 ▸). In addition, super-resolution microscopy revealed double-ring structures formed by self-assembled bsDivIVA near the division septum (Eswaramoorthy et al., 2011 ▸). Electron micrographs showing oligomerization of bsDivIVA mutants to form ∼22 nm long ‘doggy-bone’ shaped structures, and longer strings and networks in vitro were also reported (Stahlberg et al., 2004 ▸). Recently, it was shown that the mycobacterial Wag31 (tbWag31) forms several micrometre long primarily linear polymers in vitro with occasional bending or branching (Choukate et al., 2019 ▸). The structural basis of these oligomeric formations remains elusive.
Here, we report the crystal structure of the N-terminal lipid-binding domain of tbWag31 (N-Wag31) at 2.3 Å resolution. Crystal-packing analysis reveals a novel tetrameric form of N-Wag31 composed of two dimeric coiled-coil domains stacked in antiparallel fashion, which is compatible with linear filament formation. Accompanying size-exclusion columnchromatography-coupled small-angle X-ray scattering (SEC-SAXS) data further support a tetrameric form of N-Wag31 in solution. Our data suggest that, in addition to membrane lipid binding, the lipid-binding domain of Wag31 can participate in self-assembly. Based on the available data, we proposed possible models of Wag31 self-assembly for linear, and branched, filament formation.
2. Materials and methods
2.1. Recombinant protein expression
The N-terminal domain of Wag31 (residues 2–60 of tbWag31 with an N-terminal tag containing residues ‘MAHHHHHHENLFYQG’, the codon-optimized gene synthesized and sub-cloned in a pET15b vector by Genescript) construct was expressed in Escherichia coli BL21 (DE3) cells. A primary culture of volume 20 ml was started at 37°C in LB broth containing 100 mg ml−1 ampicillin and incubated overnight. This primary culture was transferred and grown in 2 l of LB broth at 37°C until an OD600 of 0.6 was reached. Next, recombinant protein expression was induced by adding 1 mM IPTG, and cells were incubated at 25°C for 16 h. The cells were harvested and stored at −80°C for further use.
2.2. Protein purification
The frozen cell pellets were resuspended in lysis buffer containing 20 mM Tris-HCl pH 7.0 and 150 mM NaCl with 0.2% Triton-X 100 and then lysed by sonication. The cell lysate was loaded onto a nickel IMAC Sepharose fast-flow affinity column (GE healthcare) and bound protein was eluted with a buffer containing 50 mM Tris–HCl at pH 7.0, 150 mM NaCl and 500 mM imidazole. The fractions of interest were pooled, concentrated and further purified by size-exclusion chromatography using a HiLoad 16/60 Superdex 200 pg column (GE healthcare) in the following buffer: 50 mM Tris–HCl at pH 7.0 and 150 mM NaCl. Freshly purified N-Wag31 protein was concentrated to 10 mg ml−1 for crystallization experiments.
2.3. Circular dichroism spectropolarimetry
For secondary structure estimation, CD spectra of purified N-Wag31 at ∼1 mg ml−1 concentration was measured at 25°C using an in-house Jasco J815 spectropolarimeter. Three CD spectra scans were averaged and plotted as molar-residue ellipticity versus wavelength.
2.4. Crystallization and data collection
Crystals of N-Wag31 were grown by both hanging- and sitting-drop vapour-diffusion methods at 19°C in 0.2 M magnesium chloride, 0.1 M sodium cacodylate at pH 6.5 and 50%(v/v) polyethylene glycol 200. These crystals were very small and recalcitrant to growth. Crystals were flash frozen in liquid nitrogen without any additional cryo-protectant and transported to the European Synchrotron Radiation Facility (ESRF) in France. X-ray diffraction data from an N-Wag31 crystal were collected at the microfocus beamline ID30A-3 (MASSIF-3) at ESRF, which is suitable for such small crystals. Only one useful dataset was obtained after testing 37 crystals. Data collection was carried out at 100 K, using an EIGER detector (DECTRIS Ltd). Data indexing, processing, merging and scaling were performed using XDS (Kabsch, 2010 ▸) and the CCP4 suite of software (Winn et al., 2011 ▸).
2.5. Crystal structure determination, model building and refinement
The crystal structure of N-Wag31 was determined using the molecular replacement method (MOLREP, Vagin & Teplyakov, 1997 ▸). The crystal structure of its homolog N-bsDivIVA (PDB code 2wuj; Oliva et al., 2010 ▸) was used as a search model in a dimeric form, after converting it to a poly-alanine model. Following rigid-body minimization of the best solution, positional refinements were performed using REFMAC (Murshudov et al., 1997 ▸) with non-crystallographic symmetry (NCS) restraints. An R free set was used to monitor the progress of refinement and individual isotropic B factors were refined. Model rebuilding was performed using Coot (Emsley et al., 2010 ▸). SIGMAA-weighted difference maps and composite OMIT maps were used for model rebuilding. In the latter stages of refinement, simulated-annealing runs were performed in Phenix (Adams et al., 2010 ▸). NCS restraints were also released during the final rounds of the refinement. In addition to two protein chains and water molecules, the asymmetric unit contains half of a triethylene glycol (PGE) related to itself by a twofold crystallographic symmetry operation. MOLPROBITY was used for structure validation (Chen et al., 2010 ▸).
N-Wag31 contains two chains, A and B, in the asymmetric unit [Figs. 1 ▸(a) and S1 in the Supporting information]. In addition to residues 2–60, chain A contains a large, contiguous segment of the poly-histidine tag region (residues −9 to 1). Chain B contains residues 2–60. An isolated tripeptide interacting with a neighbouring molecule, tentatively containing ‘HHE’ sequence, was assigned to chain B. However, as this tripeptide segment is far from the rest of the B chain, an out of register error may not be ruled out. Electron density at and around the loop region containing residues 17–20 was rather poor, especially in chain A. The main-chain conformation for this loop region was traced and built in the electron-density map for chain B and rebuilt in the same way for chain A. Side-chain densities were poor for several residues around this loop region and at the C-terminal end of N-Wag31, which is reflected in higher than average temperature factors for these side chains. All these side chains were modelled using suitable rotamers from the Coot rotamer library (Lovell et al., 2000 ▸). Loss of side-chain density could be a result of radiation damage caused by X-rays at the microfocus beamline or flexibility. Data-collection and refinement statistics are summarized in Table 1 ▸. Figures were prepared using PyMOL (The PyMOL Molecular Graphics System, Schrodinger, LLC) and CCP4MG from the CCP4 suite (Winn et al., 2011 ▸).
Figure 1.
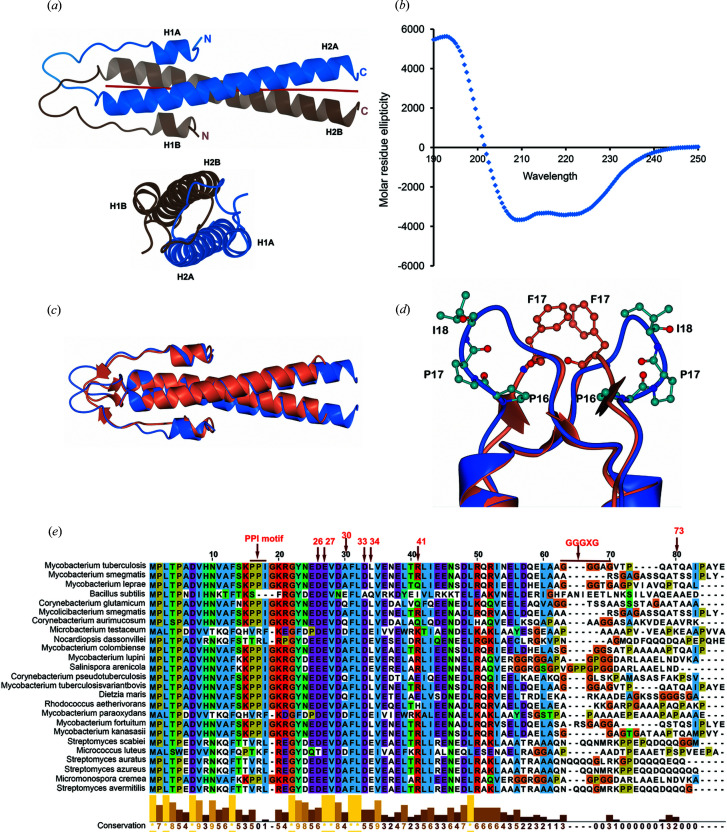
Crystal structure of N-Wag31. (a) Two nearly orthogonal views of the N-Wag31 (residues 2–60) dimer as blue and pale brown ribbons are shown. The calculated local coiled-coil axes are shown as red jointed lines. (b) A CD profile (molar-residue ellipticity versus wavelength) of N-Wag31. (c) Superimposed structures of N-Wag31 (blue) and N-bsDivIVA (coral) are shown. (d) The intertwined loop region in N-Wag31 (blue) containing the P16P17I18 sequence (balls and sticks, with carbon atoms in light blue, oxygen in red and nitrogen in blue) and the equivalent region in N-bsDivIVA (coral) containing the stacked Phe17 residues (balls and sticks, with carbon atoms in coral, oxygen in red and nitrogen in blue), are shown. (e) Multiple sequence alignment of the N-terminal region of tbWag31 (numbering for tbWag31 in red) and homologs, with the degree of conservation, is shown (prepared in Jalview; Waterhouse et al., 2009 ▸).
Table 1. Crystal-diffraction and model-refinement statistics (generated using the Table 1 option of Phenix; Adams et al., 2010 ▸).
Statistics for the highest-resolution shell are shown in parentheses.
| Diffraction-data and refinement statistics | N-Wag31 |
|---|---|
| Experimental station | ID30A-3, ESRF |
| Wavelength (Å) | 0.97 |
| Resolution range (Å) | 30.1–2.3 (2.4–2.3) |
| Space group | C121 |
| Unit-cell parameters (a, b, c in Å and α, β, γ in °) | 44.9, 53.8, 61.2, 90, 100.8, 90 |
| Total reflections | 77318 (5929) |
| Unique reflections | 6374 (592) |
| Multiplicity | 12.1 (9.9) |
| Completeness (%) | 98.6 (92.2) |
| 〈I/σ(I)〉 | 7.45 (2.2) |
| Wilson B factor (Å2) | 21.75 |
| R merge | 0.235 (1.054) |
| R meas | 0.245 (1.112) |
| R pim | 0.070 (0.345) |
| CC1/2 | 0.99 (0.76) |
| CC* | 0.99 (0.93) |
| Reflections used in refinement | 6363 (592) |
| Reflections used for R free | 353 (38) |
| R work | 0.175 (0.180) |
| R free | 0.225 (0.245) |
| Number of non-H atoms | 1137 |
| Macromolecules | 1091 |
| PGE | 10 |
| Water | 36 |
| Protein residues | 132 |
| RMS (bonds, Å) | 0.010 |
| RMS (angles, °) | 1.05 |
| Ramachandran favoured (%) | 98.4 |
| Ramachandran allowed (%) | 1.6 |
| Ramachandran outliers (%) | 0.00 |
| Rotamer outliers (%) | 0.8 |
| Average B factor (Å2) | 32.6 |
| Macromolecules | 32.6 |
| PGE | 35.5 |
| Water | 31.3 |
2.6. Size-exclusion chromatography coupled with SAXS experiment
The SEC-SAXS experiments were performed at the BM29 beamline at ESRF using a Shimadzu HPLC system. The purified N-Wag31 (4.4 mg ml−1) in a buffer containing 20 mM Tris pH 7.5, 150 mM NaCl and 10% glycerol was used for the SAXS experiments. The protein was snap frozen under liquid nitrogen and transported to the synchrotron site, where it was thawed on ice prior to the experiments. Furthermore, 30 µl of protein sample was injected into an Agilent Bio-SEC-3 column. SEC-SAXS data were collected at ∼1 Å X-ray wavelength, with 1 s frame−1 exposure and 2.8 m detector distance, using a Pilatus detector (Dectris Ltd). SEC-SAXS data analysis was performed using the ATSAS suite of software (Franke et al., 2017 ▸).
2.7. Data availability
Protein structure coordinates and structure factors have been deposited in the PDB with accession code 6lfa. SAXS data for peak II are available from SASBDB with accession code SASDHH4.
3. Results
3.1. Crystal structure of the N-terminal domain of Wag31
Mycobacterial Wag31 (P9WMU1, Rv2145c) is a 260-residue long filament-forming protein containing two domains: an N-terminal lipid- or membrane-binding domain and a C-terminal domain that participates in polar protein localization. The crystal structure of the N-terminal domain of tbWag31, or N-Wag31, shows a parallel coiled-coil dimer composed of two chains, A and B, containing residues 2–60 and additional residues from the poly-histidine tag regions [Fig. 1 ▸(a), see Fig. S1 and the experimental methods section]. The two chains of N-Wag31 are similar to each other, with a root-mean-square deviation (RMSD) of 0.65 Å for 59 Cα atoms (calculated using CLICK; Nguyen et al., 2011 ▸). The N-terminal segment of N-Wag31 contains a short helical turn (H1 helix) and loop followed by a sharp turn joining the coiled-coil helix (H2 helix). The loop region is intertwined in the N-Wag31 dimer, as observed for the N-terminal domains of its structural homologs bsDivIVA (N-bsDivIVA) and GpsB (Oliva et al., 2010 ▸; Halbedel & Lewis, 2019 ▸; Cleverley et al., 2019 ▸). CD data obtained from N-Wag31 supported an α-helix rich N-Wag31 structure in solution, with an estimated helical content of ∼60% [Fig. 1 ▸(b), calculated using K2D; Andrade et al., 1993 ▸]. The average coiled-coil pitch is ∼171 Å for residues 26–60 of N-Wag31 (calculated using TWISTER; Strelkov & Burkhard, 2002 ▸).
THe N-Wag31 structure is similar to the structure of its homolog N-bsDivIVA, with which it shares ∼42% sequence identity, with a 1.6 Å RMSD for 56 Cα atoms in a chain [Fig. 1 ▸(c), computed using Dali (Holm, 2019 ▸)]. However, the two structures differ significantly in the intertwined loop region that houses the lipid-binding site [Figs. 1 ▸(c) and 1 ▸(d)]. In the N-bsDivIVA structure, two spatially adjacent phenylalanine residues (Phe17) from the intertwined loops of two subunits in the coiled-coil dimer were shown to participate in lipid binding [Fig. 1 ▸(d); Oliva et al., 2010 ▸; Halbedel & Lewis, 2019 ▸]. The putative lipid-binding region in N-Wag31 houses a conserved ‘P16P17I18’ motif instead of Phe17 [Figs. 1 ▸(d) and 1 ▸(e)]. Exposed non-polar Ile18 at the tip of the intertwined loop is a candidate for direct interaction with membrane lipid. However, distances between the Cα atoms of the two Ile18 residues in N-Wag31 are ∼15 Å. In comparison, the Cα atoms of lipid-binding Phe17 residues in N-bsDivIVA are separated by ∼5 Å, with their side-chain aromatic rings stacked [Fig. 1 ▸(d)]. A conformational change may possibly bring the Ile18 hydrophobic side chains together in N-Wag31 to form a lipid-interacting patch. On the other hand, the presence of two proline residues before Ile18 makes this region structurally rigid. Thus, the putative lipid-binding region is quite different in N-Wag31 than in N-bsDivIVA.
3.2. Crystal-packing analysis suggests a ‘dimer-of-dimers’ organization of N-Wag31
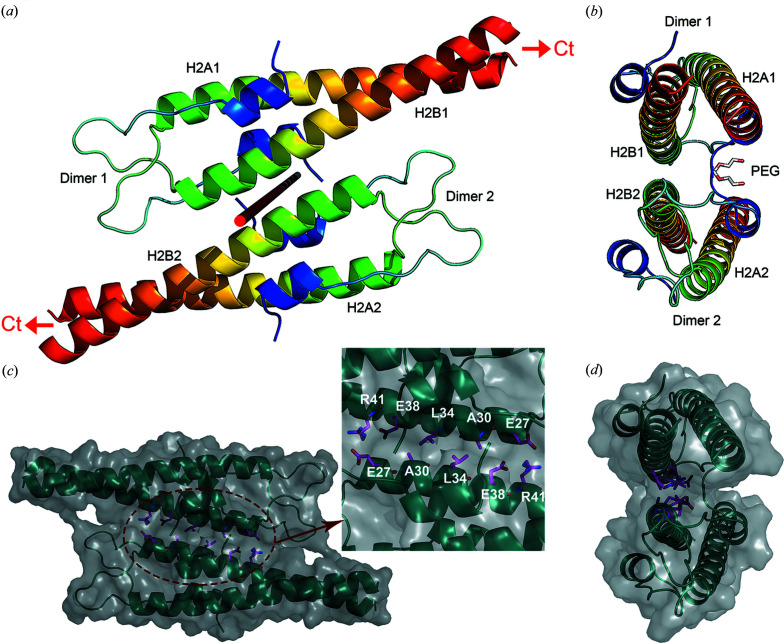
Coiled coils can assemble in a number of ways to form higher-order assemblies (Moutevelis & Woolfson, 2009 ▸). In order to learn about assembly of N-Wag31, we performed crystal-packing analysis. It appears that the dimeric coiled-coil domain of N-Wag31 combines with an adjacent crystallographic twofold-symmetry-related dimer to form a tetramer [Figs. 2 ▸(a) and 2 ▸(b)]. Isologous interface in N-Wag31 was assigned using the PDBePISA server (Krissinel & Henrick, 2007 ▸). The tetramer buries ∼396.0 Å2 of water-accessible surface area upon assembly, with the coiled-coil forming H2 helices of the two B chains with complimentary surface shapes stacking against each other [Figs. 2 ▸(c) and 2 ▸(d)]. The two stacked H2 helices are in near-antiparallel orientation, with an angle of ∼176° between the two local helix axes (computed using residues 26–41).
Figure 2.
Oligomeric organization of N-Wag31. (a), (b) Two views of the ‘dimer-of-dimers’ form of N-Wag31 related by crystallographic twofold symmetry are shown as ribbons (Jones rainbow, blue to red). The coiled-coil forming H2 helices in both A and B chains in both dimers are labelled as follows: H2A1 and H2B1 in A and B chains of dimer 1, and H2A2 and H2B2 in A and B chains of dimer 2. The directions to the C-terminal domains (Ct) are marked with red arrows in (a). The twofold axis of rotation is shown as a red rod in (a). A PGE molecule bound to N-Wag31 is shown as sticks in (b). (c), (d) Two views of the ‘dimer-of-dimers’ of N-Wag31 are shown as ribbons (cyan) within the semi-transparent molecular surface (grey) of the tetramer. Side chains of the interface-forming residues are shown as balls and sticks (carbon in magenta, oxygen in red and nitrogen in blue). A zoomed view of the side-chain residues (Arg41, Glu27, Leu34, Ala30 and Glu38) at the interface is shown in the inset.
The size of the buried surface area is a critical quantity that helps in distinguishing between crystal contacts and biological interfaces (∼370–4750 Å2 for a homodimer; Henrick & Thornton, 1998 ▸; Krissinel & Henrick, 2007 ▸). The buried surface area at the crystallographic twofold-related interface in N-Wag31 is small (∼396.0 Å2). This crystallographic interface is lined by residues that are conserved in many actinobacteria, such as Asp26, Glu27, Ala30, Leu34 and Arg41 [Fig. 2 ▸(c)]. The center of this interface is formed by buried non-polar Leu34 and Ala30. Two salt bridges are formed between the side chains of Glu27 and Arg41 residues at this interface on both sides of the central non-polar region [Fig. 2 ▸(c)]. A second buried surface area (<300 Å2) was detected at the C-terminal end of N-Wag31, which is probably formed because of crystal packing. In addition, a PEG molecule was found buried at one side of this assembly interface, covering a rather small, ∼80 Å2, part of the accessible surface area of the N-terminal H1 helix [Fig. 2 ▸(b)]. Note that formation of a tetrameric form of N-Wag31 in the absence of PEG is supported by solution SAXS data, which are described in the next section.
3.3. Solution scattering supports a ‘dimer-of-dimers’ organization of N-Wag31
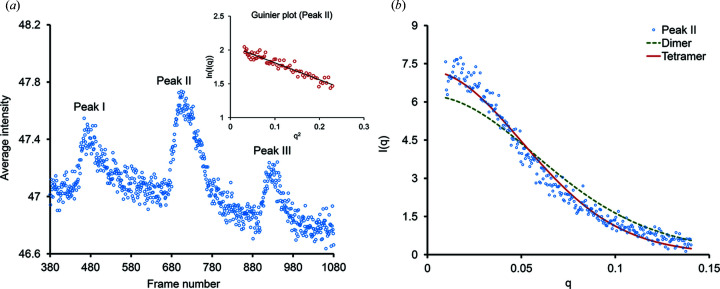
In order to determine the solution assembly states of N-Wag31, we performed a SEC-SAXS experiment. The size-exclusion column elution profile of N-Wag31 revealed the presence of multiple assembly states eluted as separate peaks, numbered from left to right as peaks I, II and III, respectively [Figs. 3 ▸(a) and S2(a)]. The early eluting peak I suggests the presence of large aggregates [Figs. 3 ▸(a) and S2(b)]. The averaged SAXS data obtained from peak III, which we expect to be a dimer, were too weak for analysis. SAXS data analysis suggests that the peak II region contains a tetrameric form of N-Wag31 (∼8.6 kDa monomer−1), with a predicted mass of ∼34.6 kDa based on the Bayesian inference method (Hajizadeh et al., 2018 ▸). The central peak II appears to be the major assembly state in solution [Fig. S2(a)]. Residues −9 to 1 of chain A of N-Wag31 containing a near-complete poly-histidine tag were used to build an equivalent region of chain B of the dimer, and the tetramer, using NCS operations, for all SAXS calculations. However, this tag region might be floppy in solution. The radius of gyration (R g) obtained from the Guinier analysis of averaged SAXS data from peak II was 27.2 Å [q R g ≤ 1.3, Fig. 3 ▸(a), where q refers to momentum transfer in nm−1], while R g calculated from dimeric/tetrameric N-Wag31 coordinates was ∼20.9 and 24.2 Å, respectively. χ2 values for fits between the theoretical scattering intensities derived from the tetrameric and dimeric N-Wag31 models with experimental averaged scattering intensity from peak II were 1.45 and 4.0, respectively, further supporting the tetrameric structure [Fig. 3 ▸(b)]. As the averaged SAXS data were quite noisy in the high-angle region and inadequate for further analysis, shape reconstructions were not performed. To summarize, solution SEC-SAXS data support a tetrameric assembly of N-Wag31, which is consistent with the crystal structure.
Figure 3.
SEC-SAXS data analysis. (a) A SAXS elution profile (average intensity versus frame number obtained from CHROMIXS; Franke et al., 2017 ▸) of N-Wag31. The Guinier plot [lnI(q) versus q 2, I is intensity and q is momentum transfer in nm−1] for peak II is shown in the inset. (b) A scattering profile of peak II data (I versus q in Å−1). Computed scattering profiles of dimeric and tetrameric N-Wag31 (calculated using CRYSOL with suggested parameters; Franke et al., 2017 ▸) are shown fitted to the experimental data.
3.4. Surface-charge distribution suggests putative lipid-binding sites in N-Wag31
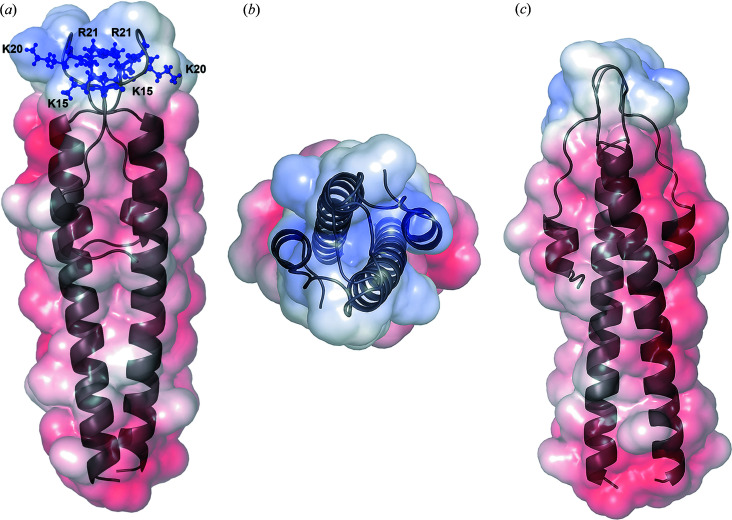
Mapping the Poisson–Boltzmann electrostatic potential into the solvent-accessible surface revealed that the surface of the lipid-binding N-Wag31 dimer is highly polar [Figs. 4 ▸(a)–4 ▸(c)]. The N-Wag31 sequence contains 14 negatively charged and six positively charged residues. The surface of the homologous N-bsDivIVA, with several charged residues, is similarly polar (Oliva et al., 2010 ▸). This is not surprising as charged amino acids are more common in coiled coils than other proteins (Surkont & Pereira-Leal, 2015 ▸). The electrostatic potential calculation shows a positively charged patch at the intertwined loop region of N-Wag31, which is lined with conserved arginine and lysine residues [Figs. 4 ▸(a) and 4 ▸(b)]. This region probably defines the conserved positively charged membrane-associating surface in the Wag31/DivIVA family of proteins and GpsB that interact with membrane phospholipids (Oliva et al., 2010 ▸; Killian & Heijne, 2000 ▸; Halbedel & Lewis, 2019 ▸).
Figure 4.
Surface-charge distribution in N-Wag31. (a)–(c) Different views of the dimeric N-Wag31 (grey) with electrostatic potential mapped into the semi-transparent water-accessible surface (a water radius of 1.4 Å) are shown. Conserved positively charged residues (Lys15, Lys20, Arg21) at the lipid-interacting intertwined loop region of N-Wag31 are shown as blue balls and sticks in (a). Electrostatic potential was computed using APBS with AMBER charges and default parameters (Baker et al., 2001 ▸).
In addition to the positively charged intertwined loop region, the tetrameric form of N-Wag31 contains negatively charged patches on its water-accessible surface [Figs. 4 ▸(a)–4 ▸(c)]. Negatively charged protein residues can interact with ethanolamine lipids in the membrane (Jurásek et al., 2019 ▸). Ethanolamine is present in the mycobacterial membrane (Chiaradia et al., 2017 ▸) and can potentially interact with conserved negatively charged surface patches in the tetrameric N-Wag31.
3.5. A suggested model for Wag31 filament formation
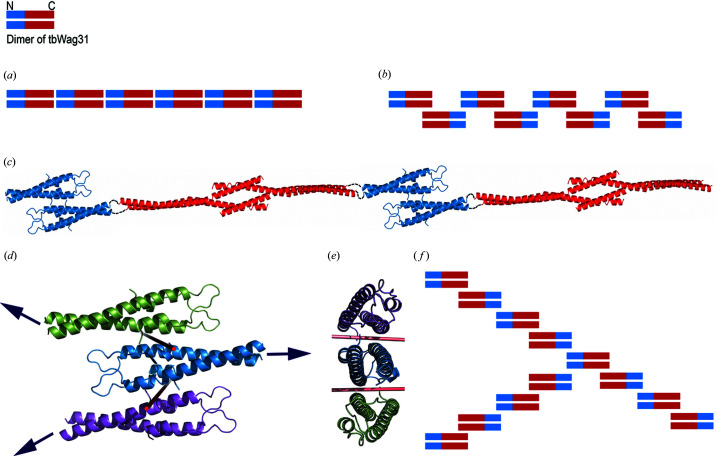
Linear filaments, with sporadic branching, were recently reported for full-length tbWag31 (Choukate et al., 2019 ▸). The average diameters of these full-length tbWag31 filaments (1.4–2.1 nm, Choukate et al., 2019 ▸) are roughly similar to the diameter of a coiled coil, which is ∼2 nm. The N-Wag31 forms a ‘dimer-of-dimers’ with the C-terminal regions extending in opposite directions, which is compatible with such linear filament formation [Fig. 2 ▸(a)]. A protein subunit can be arranged to form a filament in a few ways [Figs. 5 ▸(a) and 5 ▸(b)]. It could polymerize by a pure translational repeat along a certain direction or it could polymerize by a combination of rotation and translation along a helical axis. Alternatively, a protein subunit can form a filament by utilizing twofold symmetry operations. For a protein with an N-terminal domain N and a C-terminal domain C, two such theoretical arrangements would be —NC—NC—NC—NC—NC— or —NC—CN—NC—CN—NC— [Figs. 5 ▸(a) and 5 ▸(b)]. In the latter case, N—N and C—C inter-subunit interfaces related by twofold symmetry would be formed [Fig. 5 ▸(b)]. Wag31 appears to form a linear filament using the second option involving twofold symmetry operations [Fig. 5 ▸(c)].
Figure 5.
A proposed model of Wag31 filament assembly. (a), (b) Two theoretically alternative possible modes of self-assembly of Wag31, using (a) pure translational repeat operations and (b) twofold-symmetry-related repeat operations, are shown. (c) Ribbon diagrams of the tetramers of N-terminal N-Wag31 (blue, this work) and the C-terminal domain of bsDivIVA (red, Oliva et al., 2010 ▸) are shown, joined by linker regions as dashed lines, as a theoretical filament. The linker region is ∼20 residues long in bsDivIVA, and ∼100 residues long in tbWag31. (d), (e) Two views of a theoretical hexamer composed of three dimers of N-Wag31 (ribbons in green, blue and magenta). The twofold axes of rotation are shown as red rods. (f) A theoretically possible mode of 3-way branching in tbWag31 filament.
The structure of the C-terminal domain of tbWag31, which is predicted to be a coiled coil (Choukate et al., 2019 ▸), is currently not available. A low-resolution model of the C-terminal domain of bsDivIVA was shown to form a ‘dimer-of-dimers’, with a central 4-helix bundle region [Fig. 5 ▸(c); Oliva et al., 2010 ▸]. The sequence identity between this truncated C-terminal domain of bsDivIVA and that of the C-terminal region of tbWag31 (residues 165–234) is about 26%, suggesting that the C-terminal Wag31 may assume a bsDivIVA-like ‘dimer-of-dimers’ structure. A combination of the two such N-terminal and C-terminal ‘dimer-of-dimers’ can be used to build a linear filament of Wag31 [Fig. 5 ▸(c)].
Furthermore, the location of the twofold-symmetry-related interface in the N-Wag31 suggests a natural way for lateral or side-way association of dimeric N-Wag31 units to form higher-order oligomers, such as a hexamer [Figs. 5 ▸(d)–5 ▸(f)]. Such a theoretical hexameric association of N-Wag31 can lead to branching or bifurcations in a Wag31 filament (amongst other possibilities), and can be exploited for protein-based scaffold design [Figs. 5 ▸(d) and 5 ▸(e)].
4. Conclusions
The crystal structure of N-Wag31 reported here reveals a tetrameric form of N-Wag31, which is further supported by solution SAXS data. In contrast, homologous N-bsDivIVA is reportedly a dimer in solution (Oliva et al., 2010 ▸). A tetrameric form of bsDivIVA was built by combining the crystal structures of C-terminal ‘dimer-of-dimers’ and two terminally located dimeric N-terminal domains (Oliva et al., 2010 ▸). However, how such a tetramer can be further assembled to build higher-order structures was not obvious. Based on electron micrographs of filaments formed by bsDivIVA mutants, an assembly pathway of ‘doggy-bone’ shaped basic building blocks were suggested (Stahlberg et al., 2004 ▸). However, atomic details of such assembly remained elusive. The tetrameric form of N-Wag31 reported here elucidates the structural basis of such assembly formation involving the N-terminal lipid-binding domain that can lead to higher-order structures. Furthermore, considering that tbWag31 is a high-confidence drug target (Singh et al., 2017 ▸; Boshoff, 2017 ▸), the structural data presented here can be exploited for designing inhibitors.
How N-Wag31 orients itself at the polar membrane surface for curvature sensing is currently not known. Molecular-dynamics simulation studies suggested that lipid composition can influence the orientation of DivIVA at the membrane (Jurásek et al., 2019 ▸). Ethanolamine lipids may interact with negatively charged surface patches causing DivIVA to realign from perpendicular to parallel orientation at the membrane surface (Jurásek et al., 2019 ▸). Likewise, N-Wag31 orientation may be determined by interactions involving both the positively charged intertwined loop region and negatively charged surface patches [Figs. 4 ▸(a)–4 ▸(c)] with the polar membrane lipids.
The crystal structure of N-Wag31 that comprises residues 2–60 of tbWag31 raises questions about the possible structural role of phosphorylated Thr73 residue. The Thr73 residue is in a putative intrinsically unstructured region adjacent to the N-terminal domain of Wag31 (Choukate et al., 2019 ▸) and proximal to several glycine residues, such as G63G64G65X66G67 [Fig. 1 ▸(e)]. Small and flexibility-imparting glycine residues are typically rare in coiled coils (Surkont & Pereira-Leal, 2015 ▸). Within the classical heptad repeat ‘abcdefg’ in a coiled coil, the ‘a’ and ‘d’ regions are buried inside where ‘g’ and ‘e’ regions make ionic interactions between the helices to stabilize the coiled-coil assembly. If the coiled-coil heptad assignment (made by TWISTER; Strelkov & Burkhard, 2002 ▸) is theoretically extended beyond the last residue (Leu60, assigned ‘a’ position) observed in the crystal structure, the 73rd residue will be in the ‘g’ position assuming coiled-coil continuity, and could be critical for providing a stabilizing salt link for coiled-coil continuation in the phosphorylated form of tbWag31 in an otherwise flexible region.
Supplementary Material
Supporting figures. DOI: 10.1107/S2052252520006053/lz5036sup1.pdf
PDB reference: Structure of the N-terminal domain of Wag31, 6lfa
SASBDB reference: N-terminal domain of Wag31 from M. tuberculosis, SASDHH4
Acknowledgments
We thank Dr Nishant Varshney and staff at the BM29 and ID30 beamlines at ESRF for assistance during data collection. We also thank Professor Jan Löwe from MRC, Cambridge, for providing the coordinates of the C-terminal domain of bsDivIVA.
Funding Statement
This work was funded by Council of Scientific and Industrial Research, India, CSIR – Indian Institute of Chemical Biology grant . Department of Biotechnology, Government of West Bengal grant .
References
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. [DOI] [PMC free article] [PubMed]
- Andrade, M. A., Chacón, P., Merelo, J. J. & Morán, F. (1993). Protein Eng. Des. Sel. 6, 383–390. [DOI] [PubMed]
- Antonny, B. (2011). Annu. Rev. Biochem. 80, 101–123. [DOI] [PubMed]
- Bach, J. N., Albrecht, N. & Bramkamp, M. (2014). Front. Microbiol. 5, 59. [DOI] [PMC free article] [PubMed]
- Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. (2001). Proc. Natl Acad. Sci. USA, 98, 10037–10041. [DOI] [PMC free article] [PubMed]
- Boshoff, H. I. (2017). Mol. Microbiol. 103, 2–6. [DOI] [PMC free article] [PubMed]
- Cannon, K. S., Woods, B. L. & Gladfelter, A. S. (2017). Trends Biochem. Sci. 42, 961–976. [DOI] [PMC free article] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Chiaradia, L., Lefebvre, C., Parra, J., Marcoux, J., Burlet-Schiltz, O., Etienne, G., Tropis, M. & Daffé, M. (2017). Sci. Rep. 7, 12807. [DOI] [PMC free article] [PubMed]
- Choukate, K., Gupta, A., Basu, B., Virk, K., Ganguli, M. & Chaudhuri, B. (2019). J. Struct. Biol. 209, 107429. [DOI] [PubMed]
- Cleverley, R. M., Rutter, Z. J., Rismondo, J., Corona, F., Tsui, H. T., Alatawi, F. A., Daniel, R. A., Halbedel, S., Massidda, O., Winkler, M. E. & Lewis, R. J. (2019). Nat. Commun. 10, 261. [DOI] [PMC free article] [PubMed]
- Edwards, D. H. & Errington, J. (1997). Mol. Microbiol. 24, 905–915. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 485–501. [DOI] [PMC free article] [PubMed]
- Eswaramoorthy, P., Erb, M. L., Gregory, J. A., Silverman, J., Pogliano, K., Pogliano, J. & Ramamurthi, K. S. (2011). mBio, 2, e00257-11. [DOI] [PMC free article] [PubMed]
- Franke, D., Petoukhov, M. V., Konarev, P. V., Panjkovich, A., Tuukkanen, A., Mertens, H. D. T., Kikhney, A. G., Hajizadeh, N. R., Franklin, J. M., Jeffries, C. M. & Svergun, D. I. (2017). J. Appl. Cryst. 50, 1212–1225. [DOI] [PMC free article] [PubMed]
- Ginda, K., Bezulska, M., Ziółkiewicz, M., Dziadek, J., Zakrzewska-Czerwińska, J. & Jakimowicz, D. (2013). Mol. Microbiol. 87, 998–1012. [DOI] [PubMed]
- Hajizadeh, N. R., Franke, D., Jeffries, C. M. & Svergun, D. I. (2018). Sci. Rep. 8, 7204. [DOI] [PMC free article] [PubMed]
- Halbedel, S. & Lewis, R. J. (2019). Mol. Microbiol. 111, 1404–1415. [DOI] [PubMed]
- Hammond, L. R., White, M. L. & Eswara, P. J. (2019). J. Bacteriol. p. 201. [DOI] [PMC free article] [PubMed]
- Henrick, K. & Thornton, J. M. (1998). Trends Biochem. Sci. 23, 358–361. [DOI] [PubMed]
- Holm, L. (2019). Bioinformatics, 35, 5326–5327. [DOI] [PubMed]
- Jani, C., Eoh, H., Lee, J. J., Hamasha, K., Sahana, M. B., Han, J. S., Nyayapathy, S., Lee, J. Y., Suh, J. W., Lee, S. H., Rehse, S. J., Crick, D. C. & Kang, C. M. (2010). BMC Microbiol. 10. [DOI] [PMC free article] [PubMed]
- Jurásek, M., Flärdh, K. & Vácha, R. (2019). Biochim. Biophys. Acta Biomembr. p. 183144. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kang, C. M., Nyayapathy, S., Lee, J. Y., Suh, J. W. & Husson, R. N. (2008). Microbiology, 154, 725–735. [DOI] [PubMed]
- Kaval, K. G. & Halbedel, S. (2012). Virulence, 3, 406–407. [DOI] [PMC free article] [PubMed]
- Killian, J. A. & von Heijne, G. (2000). Trends Biochem. Sci. 25, 429–434. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Laloux, G. & Jacobs-Wagner, C. (2014). J. Cell Sci. 127, 11–19. [DOI] [PMC free article] [PubMed]
- Lenarcic, R., Halbedel, S., Visser, L., Shaw, M., Wu, L. J., Errington, J., Marenduzzo, D. & Hamoen, L. W. (2009). EMBO J. 28, 2272–2282. [DOI] [PMC free article] [PubMed]
- Letek, M., Ordóñez, E., Vaquera, J., Margolin, W., Flärdh, K., Mateos, L. M. & Gil, J. A. (2008). J. Bacteriol. 190, 3283–3292. [DOI] [PMC free article] [PubMed]
- Lovell, S. C., Word, J. M., Richardson, J. S. & Richardson, D. C. (2000). Proteins, 40, 389–408. [PubMed]
- Melzer, E. S., Sein, C. E., Chambers, J. J. & Siegrist, M. S. (2018). Cytoskeleton (Hoboken), 75, 498–507. [DOI] [PMC free article] [PubMed]
- Meniche, X., Otten, R., Siegrist, M. S., Baer, C. E., Murphy, K. C., Bertozzi, C. R. & Sassetti, C. M. (2014). Proc. Natl Acad. Sci. 111, E3243–E3251. [DOI] [PMC free article] [PubMed]
- Moutevelis, E. & Woolfson, D. N. (2009). J. Mol. Biol. 385, 726–732. [DOI] [PubMed]
- Mukherjee, P., Sureka, K., Datta, P., Hossain, T., Barik, S., Das, K. P., Kundu, M. & Basu, J. (2009). Mol. Microbiol. 73, 103–119. [DOI] [PubMed]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed]
- Nguyen, L., Scherr, N., Gatfield, J., Walburger, A., Pieters, J. & Thompson, C. J. (2007). J. Bacteriol. 189, 7896–7910. [DOI] [PMC free article] [PubMed]
- Nguyen, M. N., Tan, K. P. & Madhusudhan, M. S. (2011). Nucleic Acids Res. 39, W24–W28. [DOI] [PMC free article] [PubMed]
- Oliva, M. A., Halbedel, S., Freund, S. M., Dutow, P., Leonard, T. A., Veprintsev,D. B., Hamoen, L. W. & Löwe, J. (2010). EMBO J. 29, 1988–2001. [DOI] [PMC free article] [PubMed]
- Plocinska, R., Martinez, L., Gorla, P., Pandeeti, E., Sarva, K., Blaszczyk, E., Dziadek, J., Madiraju, M. V. & Rajagopalan, M. (2014). J. Bacteriol. 196, 4120–4129. [DOI] [PMC free article] [PubMed]
- Plocinski, P., Arora, N., Sarva, K., Blaszczyk, E., Qin, H., Das, N., Plocinska, R., Ziolkiewicz, M., Dziadek, J., Kiran, M., Gorla, P., Cross, T. A., Madiraju, M. & Rajagopalan, M. (2012). J. Bacteriol. 194, 6398–6409. [DOI] [PMC free article] [PubMed]
- Ramamurthi, K. S. & Losick, R. (2009). Proc. Natl Acad. Sci. 106, 13541–13545. [DOI] [PMC free article] [PubMed]
- Rudner, D. Z. & Losick, R. (2010). Cold Spring Harb. Perspect. Biol. 2, a000307. [DOI] [PMC free article] [PubMed]
- Singh, V., Dhar, N., Pató, J., Kolly, G. S., Korduláková, J., Forbak, M., Evans, J. C., Székely, R., Rybniker, J., Palčeková, Z., Zemanová, J., Santi, I., Signorino-Gelo, F., Rodrigues, L., Vocat, A., Covarrubias, A. S., Rengifo, M. G., Johnsson, K., Mowbray, S., Buechler, J., Delorme, V., Brodin, P., Knott, G. W., Aínsa, J. A., Warner, D. F., Kéri, G., Mikušová, K., McKinney, J. D., Cole, S. T., Mizrahi, V. & Hartkoorn, R. C. (2017). Mol. Microbiol. 103, 13–25. [DOI] [PubMed]
- Stahlberg, H., Kutejová, E., Muchová, K., Gregorini, M., Lustig, A., Müller, S. A., Olivieri, V., Engel, A., Wilkinson, A. J. & Barák, I. (2004). Mol. Microbiol. 52, 1281–1290. [DOI] [PubMed]
- Strelkov, S. V. & Burkhard, P. (2002). J. Struct. Biol. 137, 54–64. [DOI] [PubMed]
- Surkont, J. & Pereira-Leal, J. B. (2015). Genome Biol. 7, 545–556. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (1997). J. Appl. Cryst. 30, 1022–1025.
- Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. (2009). Bioinformatics, 25, 1189–1191. [DOI] [PMC free article] [PubMed]
- Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., Keegan, R. M., Krissinel, E. B., Leslie, A. G. W., McCoy, A., McNicholas, S. J., Murshudov, G. N., Pannu, N. S., Potterton, E. A., Powell, H. R., Read, R. J., Vagin, A. & Wilson, K. S. (2011). Acta Cryst. D67, 235–242. [DOI] [PMC free article] [PubMed]
- Xu, W. X., Zhang, L., Mai, J. T., Peng, R. C., Yang, E. Z., Peng, C. & Wang, H. H. (2014). Biochem. Biophys. Res. Commun. 448, 255–260. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting figures. DOI: 10.1107/S2052252520006053/lz5036sup1.pdf
PDB reference: Structure of the N-terminal domain of Wag31, 6lfa
SASBDB reference: N-terminal domain of Wag31 from M. tuberculosis, SASDHH4
Data Availability Statement
Protein structure coordinates and structure factors have been deposited in the PDB with accession code 6lfa. SAXS data for peak II are available from SASBDB with accession code SASDHH4.