Abstract
Background
Recent studies have reported inconsistent associations between maternal residential green space and preterm birth (PTB, born < 37 completed gestational weeks). In addition, windows of susceptibility during pregnancy have not been explored and potential interactions of green space with air pollution exposures during pregnancy are still unclear.
Objectives
To evaluate the relationships between green space and PTB, identify windows of susceptibility, and explore potential interactions between green space and air pollution.
Methods
Birth certificate records for all births in California (2001–2008) were obtained. The Normalized Difference Vegetation Index (NDVI) was used to characterized green space exposure. Gestational age was treated as a time-to-event outcome; Cox proportional hazard models were applied to estimate the association between green space exposure and PTB, moderately PTB (MPTB, gestational age < 35 weeks), and very PTB (VPTB, gestational age < 30 weeks), after controlling for maternal age, race/ethnicity, education, and median household income. Month-specific green space exposure was used to identify potential windows of susceptibility. Potential interactions between green space and air pollution [fine particulate matter <2.5 μm (PM2.5), nitrogen dioxide (NO2), and ozone (O3)] were examined on both additive and multiplicative scales.
Results
In total, 3,753,799 eligible births were identified, including 341,123 (9.09%) PTBs, 124,631 (3.32%) MPTBs, and 22,313 (0.59%) VPTBs. A reduced risk of PTB was associated with increases in residential NDVI exposure in 250 m, 500 m, 1000 m, and 2000 m buffers. In the 2000 m buffer, the association was strongest for VPTB [adjusted hazard ratio (HR) per interquartile range increase in NDVI: 0.959, 95% confidence interval (CI): 0.942–0.976)], followed by MPTB (HR=0.970, 95% CI: 0.962–0.978) and overall PTB (HR=0.972, 95% CI: 0.966–0.978). For PTB, green space during the 3rd – 5th gestational months had stronger associations than those in the other time periods, especially during the 4th gestational month (NDVI 2000 m: HR=0.970, 95% CI: 0.965–0.975). We identified consistent positive additive and multiplicative interactions between decreasing green space and higher air pollution.
Conclusion
This large study found that maternal exposure to residential green space was associated with decreased risk of PTB, MPTB, and VPTB, especially in the second trimester. There is a synergistic effect between low green space and high air pollution levels on PTB, indicating that increasing exposure to green space may be more beneficial for women with higher air pollution exposures during pregnancy.
Keywords: Preterm birth, green space, air pollution, interaction, exposure time window
INTRODUCTION
Preterm birth (PTB), defined as birth before 37 completed gestational weeks (March of Dimes et al., 2012), is a major cause of infant mortality and morbidity (Liu et al., 2016), and the leading cause of childhood mortality (Harrison & Goldenberg, 2016). Globally, preterm birth accounts for 11% of all deliveries, and the rate is increasing in most countries, causing a substantial public health burden (Harrison & Goldenberg, 2016; Petrou, 2003). Preterm infants remain at higher risk for multiple long-term adverse effects later in life, including delay in cognitive development, decreased motor function, behavioral disorders, altered cardiovascular and renal functions, as well as an increased risk for asthma and other respiratory diseases (Chehade et al., 2018; McCormick et al., 2011; Saigal & Doyle, 2008). Additionally, women with a history of PTB may have an increased risk of subsequent cardiovascular diseases (Robbins et al., 2014).
Currently about 55% of the global population lives in urban areas and this percentage is predicted to reach 68% by 2050 (United Nations, 2018). Due to rapid urbanization, an increasing number of people live in complex environments with many high-rise buildings, high population density and low-level green space (H. Li et al., 2015; Skyscrapercity, 2015; Urban Audit, 2007). Concerns are mounting about the association between lack of green space and various adverse health outcomes in urban-dwelling populations (Bettencourt LM, 2007; Fong et al., 2018; James et al., 2015; Nieuwenhuijsen et al., 2017; World Health Organization, 2016). Underlying pathways linking green space to health include stress reduction and psychological restoration; encouragement of health-enhancing behaviours, such as physical activity and social cohesion; and mitigation of harmful environmental exposures and nuisances caused by air pollution and noise (Bowler et al., 2010a; Hartig et al., 2014; Lee & Maheswaran, 2011; Markevych et al., 2017; Twohig-Bennett & Jones, 2018; Vienneau et al., 2017). Green space exposure has been associated with a wide range of health benefits, such as reduced risks of all-cause mortality, cardiovascular diseases, type 2 diabetes, and improved mental health and some pregnancy outcomes, including decreased risks of low birth weight (Fong et al., 2018; Gascon et al., 2016; James et al., 2015; Laurent et al., 2019; Laurent et al., 2016a; Twohig-Bennett & Jones, 2018; World Health Organization, 2016). Only few studies have examined the associations between PTB and green space, showing heterogeneous results. While a possible protective effect of green space against PTB has been reported in previous studies (Casey et al., 2016; Grazuleviciene et al., 2015; Hystad et al., 2014; Laurent et al., 2013a), other findings are inconsistent (Abelt & McLafferty, 2017; Agay-Shay et al., 2014; Cusack et al., 2017; Dadvand et al., 2012a; Dzhambov et al., 2019; Glazer et al., 2018), likely due to the variation in study design, exposure measurement, study location (e.g. urban vs. rural area), study population, and other factors. Therefore, this study endeavors to investigate the relationship between green space and PTB.
Pregnancy is a sensitive time period during which intrauterine exposures can modulate fetal development and confer a long-lasting effect on the offspring (Marques et al., 2013). Considering different time windows of exposure during pregnancy with an appropriate temporal scale (e.g., months or trimesters) could be as important as considering the intensity of exposure (Ballester & Iniguez, 2011). Exploring critical windows of exposure could help design experimental studies to explore the underlying mechanisms and provide guidance to pregnant women by developing timely interventions (e.g. promote behavior changes focusing the most effective time windows) (Bennett et al., 2019; Sheridan et al., 2019; Wang et al., 2018). Therefore, it is prudent to identify the susceptible window of green space exposure associated with PTB.
In parallel, exposure to air pollution during pregnancy is increasingly recognized as a risk factor for PTB (Kloog et al., 2012; Lamichhane et al., 2015; Laurent et al., 2016c; X. Li et al., 2017; Malley et al., 2017; Olsson et al., 2013; Pereira et al., 2014; Stieb et al., 2012; Wang et al., 2018; Wilhelm et al., 2011). Many studies showed that ambient air pollution exposure during pregnancy increases the risk of PTB in California (Laurent et al., 2016c; Ritz et al., 2000; Sheridan et al., 2019; Wu et al., 2009). Investigating the potential interactions between green space and air pollution exposure during pregnancy may contribute to better understanding how to maximize the potential benefits of increasing exposure to residential green space on PTB (Kloog, 2019). A joint intervention on green space and air pollution during pregnancy may lead to more important benefits than isolated efforts. Moreover, the effect modification may be different depending on different air pollutants. To our knowledge, no study investigated interactions between green space and air pollutants during pregnancy and their joint impact on PTB as a time-to-event outcome (Kumar et al., 2019).
In this study, we aimed to 1) investigate the relationships between PTB and green space using 2001–2008 birth certificate data from a large study population of the state of California, U.S., 2) identify windows of susceptibility to green space exposure during pregnancy, and 3) examine the potential joint effects of green space and air pollution exposures during pregnancy.
METHODS
Study population
Birth certificate records for all births occurring from January 1, 2001 to December 31, 2008 in California (n = 4,385,997) were obtained from the California Department of Public Health. Maternal addresses of residence recorded on birth certificates were geocoded with the Texas A&M, NAACCR, Automated Geospatial Geocoding Interface Environment (AGGIE) Geocoder (Goldberg et al., 2008). In total, 4,370,371 births were included in this study after excluding infants whose address of record could not be geocoded (n = 1,361, 0.03%), who were born to women residing outside of California (n = 7,512, 0.17%), or who had State File Number information missing which prevented data linkage (n = 8,119, 0.19%).
After the address linkages were completed, a series of exclusions were made sequentially. Births with multiple gestations (n = 132,369) were excluded as well as infants with recorded birth defects or unknown birth defects status (n = 18,811 and n = 675, respectively). We also excluded births with missing information for gestational age (n = 196,247), estimated gestational age < 121 or > 319 days (n = 2,051 and 43,187, respectively), implausible combinations of birth weight and gestational age (n = 17,026) (Alexander et al., 1996), and infants born to women > 60 years of age (n = 43). Lastly, infants conceived > 19 weeks before 1 January 2001 (n = 389,611), or < 43 weeks before 31 December 2008 (n = 38,598) were excluded to avoid fixed cohort bias (Laurent et al., 2016c; Strand et al., 2011). The time of conception and resulting gestational age were estimated based on the first day of the last menstrual period (LMP) reported by mothers on the birth certificate (Barradas et al., 2014). These exclusions were made a priori to avoid accusations of p-hacking. After all exclusions, several of which were overlapping, 3,753,799 births from the source population were eligible for the analysis.
Green space exposure
The Normalized Difference Vegetation Index (NDVI) (Tucker, 1979) was used to characterize green space exposure as has been done in previous studies (Dadvand et al., 2012a; Laurent et al., 2019; Villeneuve et al., 2012). The NDVI captures the vegetation density on the ground from satellite data, which is calculated based on land surface reflectance of near-infrared (NIR) and visible red (VIS R) wavelengths: NDVI = (NIR – VIS R) / (NIR + VIS R). Values for NDVI range between −1 and 1, with higher values indicating a higher density of greenness. Negative values, representing water bodies, were removed from the analysis (Markevych et al., 2017). In this study, we used smoothed and gap-filled composite Moderate Resolution Imaging Spectroradiometer (MODIS) NDVI data that combined measurements from both the Terra and the Aqua satellite instruments (Spruce et al., 2016). The data were generated using the NASA Stennis Time Series Product Tool that produced NDVI data less affected by clouds and bad pixels. The data had a spatial resolution of 250 m x 250 m and a temporal resolution of every 8-days (46 time-points annually). Average NDVI values were calculated based on circular buffers of 250 m, 500 m, 1000 m, and 2000 m of each residential location. We temporally interpolated the every eight-day NDVI to generate daily NDVI values using the TIMESERIES Procedure of the SAS 9.4 software. We then calculated both month-specific and entire-pregnancy exposures by averaging the NDVI measurements in each specific time period. Gestational month was defined as every 30 consecutive days starting from the date of conception. Entire pregnancy was defined for the period from the date of conception to delivery.
Air pollution exposure
The air pollution metrics used in this study have been extensively described in previous papers (Laurent et al., 2019; Laurent et al., 2014; Laurent et al., 2016b, 2016c; Laurent et al., 2013b) and a report (Wu et al., 2016). Briefly, hourly air pollution measurements for fine particulate matter with diameter <2.5 μm (PM2.5), nitrogen dioxide (NO2) and ozone (O3) were obtained for the entire State of California from 2000 to 2008 from U.S. EPA’s monitoring stations. Daily averages (24 hours for PM2.5 and NO2 and an eight-hour window of 10 AM – 6 PM for O3) (Medina-Ramon et al., 2006), and then monthly averages were calculated. Monthly averaged concentrations of PM2.5, NO2, and O3 were spatially interpolated between stations using an empirical Bayesian kriging (EBK) model, which is a spatial interpolation approach that automates variogram modeling in a kriging model through a process of sub-setting and simulation. Compared to the common kriging approaches, the EBK model accounts for the uncertainties in estimating the underlying semivariogram. Thus, EBK has several advantages, including automatic variogram modeling (minimal manual interaction); standard error estimation; more accurate predictions of moderately nonstationary data; and more accurate prediction for small datasets (Krivoruchko, 2012; Pilz & Spöck, 2007). ArcGIS 10.1 (ESRI, Redlands, CA) was used to generate surface predictions of air pollutants at a 200 m x 200 m grid resolution.
Statistical analyses
In this study, the association between gestational age and green space exposure was examined using time-to-event regression models, and term births were censored at week 37. Specifically, Cox proportional hazard models were applied to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for per interquartile range (IQR) increase in NDVI during the entire pregnancy in California. Preterm birth was further categorized into moderately preterm birth (MPTB, born < 35 weeks), and very preterm birth (VPTB, born < 30 weeks). Time point beyond week 35 and 30 were excluded for MPTB and VPTB, respectively. Pearson’s correlation was used to examine the correlation between NDVI and air pollution exposures.
Confounders in the relationship between green space and PTB were identified by a causal diagram based on the existing literature (Appendix 1) (Greenland et al., 1999). In our primary analysis, we adjusted for a minimal set of potential confounders: maternal age, race/ethnicity (African American, Asian, Hispanic, non-Hispanic white, and others including Hawaiian/Pacific Islanders, American Indian/Alaskan native and mothers with multiple race/ethnicities specified), educational level (⩽ 8th grade, 9th grade to high school, college < 4 years, and college ⩾ 4 years), and median household income by census block group (U.S. et al., 2004). Given the large spatial scale of the data, we tested for spatial clustering using Moran’ I. The Moran’s I was 0.047 (z=14.119, p<0.001). The spatial correlation is very weak despite its significance. ZIP codes were fitted as a random effect in the models to account for potential spatial clustering for PTB. Furthermore, we performed sensitivity analyses to examine the influence of adjusting for season of conception (warm: May-October; cool: November–April), year of birth (2001–2008), maternal smoking status during pregnancy, and maternal comorbidities (chronic hypertension, diabetes, preeclampsia, gestational diabetes). Data for maternal smoking during pregnancy were only available years 2007 and 2008. We also performed analyses stratified by region to explore the differences between rural and urban population. Cochran Q tests were used to measure the heterogeneity among subgroups.
Since entire pregnancy or trimester-specific exposures may not be sufficiently refined to identify windows of susceptibility, gestational month-specific exposure was used to assess impacts of maternal residential green space on PTB. Potential interaction of green space and air pollution were measured on both multiplicative and additive scales. Multiplicative interactions of green space and air pollution were evaluated by introducing a product term (“NDVI* air pollutant”) in our models. Additive interactions were quantified by calculating the relative excess risks due to interactions (RERIs) (Li & Chambless, 2007). Both decreasing green space (1-NDVI) and air pollutant concentrations were treated as continuous exposures. HRs of interaction term >1 indicate positive multiplicative interaction; RERI values above 0 indicate a positive additive effect modification. A positive additive effect measure modification would mean that if we were to intervene on exposure to green space during pregnancy on the study population, those with higher levels of air pollution would benefit more than participants with lower exposure to air pollution (in our case the contrast is expressed for a 10ug/m3 unit change in PM2.5, 10 parts per billion unit change in NO2 and O3, and 0.1 unit change in NDVI). All analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC).
This study was approved by the Institutional Review Board of the University of California, Irvine.
RESULTS
Among 3,753,799 births included in our study population, 341,123 (9.09%) PTBs, 124,631 (3.32%) MPTBs, and 22,313 (0.59%) VPTBs were identified. The distribution of selected population characteristics and green space exposure levels is presented in Table 1. Compared to term births, PTBs were observed more frequently among younger or older mothers, African American or Hispanic mothers, mothers with low education, and mothers who lived in low-income neighborhoods. On average, residential surrounding NDVI levels for the entire pregnancy increased with maternal age, education, and neighborhood median income. Residential NDVI levels were the highest among Non-Hispanic white women and lowest among African American or Hispanic women. In addition, NDVI levels were lower in urban areas than in rural areas. NDVI was negatively correlated with all air pollution indicators (Appendix 2). For the 2000 m buffer, the negative correlations were most pronounced with NO2 (r = −0.44), followed by and PM2.5 (−0.29) and ozone (r = −0.13). The negative correlations were stronger with increasing green space buffer size for NO2 and PM2.5; the correlations with ozone were similar across different buffer sizes.
Table 1.
Description of the study population and green space exposure levels by maternal characteristics.
| Characteristics | Preterm birth (< 37 weeks) n = 341,123 | Term birth (≥ 37 weeks) n = 3,412,676 | Total births n = 3,753,799 | NDVI 250m mean (SD) |
|---|---|---|---|---|
| Maternal age, years, n (%) | ||||
| <15 | 759 (0.002) | 3857 (0.001) | 4616 (0.001) | 0.33 (0.10) |
| 15 to 19 | 38116 (11.2) | 311818 (9.1) | 349934 (9.3) | 0.34 (0.10) |
| 20 to 24 | 77511 (22.7) | 778706 (22.8) | 856217 (22.8) | 0.34 (0.10) |
| 25 to 29 | 82171 (24.1) | 908515 (26.6) | 990686 (26.4) | 0.35 (0.10) |
| 30 to 34 | 78026 (22.9) | 840404 (24.6) | 918430 (24.5) | 0.36 (0.11) |
| 35 to 39 | 49611 (14.5) | 459108 (13.5) | 508769 (13.6) | 0.37 (0.11) |
| 40 to 44 | 13904 (4.1) | 104481 (3.1) | 118385 (3.2) | 0.38 (0.12) |
| ≥ 45 | 961 (0.003) | 5362 (0.002) | 6259 (0.002) | 0.38 (0.12) |
| Maternal race/ethnicity, n (%) | ||||
| African American | 26111 (7.8) | 169693 (5.0) | 195804 (5.3) | 0.33 (0.09) |
| Asian | 37326 (11.1) | 397742 (11.8) | 435068 (11.7) | 0.36 (0.10) |
| Hispanic | 185293 (55.0) | 1746125 (51.8) | 1931418 (52.1) | 0.33 (0.09) |
| Non-Hispanic white | 79999 (23.7) | 982680 (29.1) | 1062679 (29.0) | 0.40 (0.12) |
| Multiple/other | 8365 (2.5) | 76790 (2.3) | 85155 (2.3) | 0.37 (0.12) |
| Maternal education, n (%) | ||||
| ≤ 8th grade | 39709 (12.0) | 350658 (10.5) | 390367 (10.7) | 0.33 (0.10) |
| 9th grade – high school | 164595 (49.6) | 1492342 (44.8) | 1656937 (45.3) | 0.34 (0.10) |
| College (< 4 years) | 66386 (20.0) | 678255 (20.4) | 744641 (20.3) | 0.36 (0.11) |
| College (≥ 4 years) | 61311 (18.5) | 806717 (24.2) | 868028 (23.7) | 0.39 (0.11) |
| Median annual income by census block group, n (%) | ||||
| ≤ $31,033 | 98646 (29.0) | 836881 (24.6) | 935527 (25.0) | 0.31 (0.10) |
| $31,034–$42,534 | 87820 (25.8) | 847690 (24.9) | 935510 (25.0) | 0.34 (0.10) |
| $42,535–$60,221 | 82012 (24.1) | 853496 (25.1) | 935508 (25.0) | 0.36 (0.10) |
| ≥ $60,222 | 71664 (21.1) | 863678 (25.4) | 935342 (25.0) | 0.40 (0.10) |
| Season of conception, n (%) | ||||
| Warm season | 166990 (49.0) | 1652314 (48.4) | 1819304 (48.5) | 0.35 (0.11) |
| Cool season | 174133 (51.0) | 1760362 (51.6) | 1934495 (51.5) | 0.35 (0.11) |
| Rural/urban status, n (%) | ||||
| Rural | 30612 (9.0) | 317371 (9.3) | 347983 (9.3) | 0.45 (0.16) |
| Urban | 310084 (91.0) | 3089453 (90.7) | 3399537 (90.7) | 0.35 (0.09) |
SD, standard deviation.
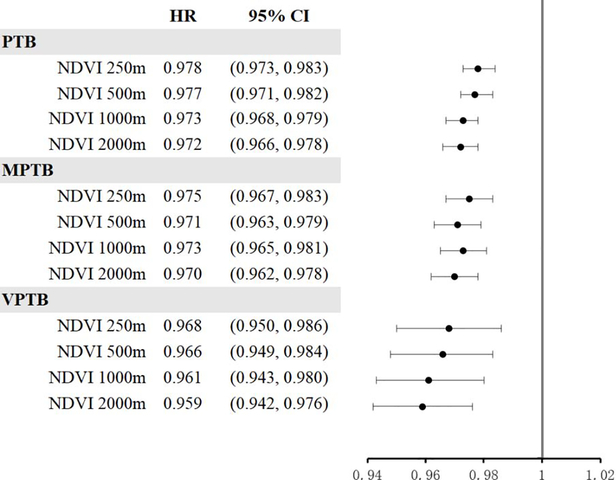
Figure 1 shows adjusted hazard ratios of PTB associated with average NDVI exposure during the entire pregnancy. HRs for overall PTB in association with IQR increases (Appendix 2) in green space exposure were precisely less than 1 for all buffers. The association was strongest for the largest buffer size (NDVI 2000 m: HR=0.972, 95% CI: 0.966–0.978). We found similar patterns for MPTB (NDVI 2000 m: HR=0.970, 95% CI: 0.962–0.978) and VPTB (NDVI 2000 m: HR=0.959, 95% CI: 0.942–0.976). In sensitivity analyses, associations between green space and overall PTB were slightly stronger after further adjusting for season of conception and year of birth, maternal smoking status during pregnancy, and maternal comorbidities, respectively (Appendix 3). In subgroup analyses, HRs for overall PTB in association with IQR increases in green space exposure were found to be protective for all buffer sizes in both rural and urban areas (NDVI 2000 m: HR=0.969, 95% CI: 0.959–0.980 and HR=0.967, 95% CI: 0.960–0.974, respectively) (Appendix 4). Cochrane’s Q tests did not reveal any heterogeneity between the two subgroups.
Figure 1. Hazard ratios (HRs) of PTB associated with average green space exposure during the entire pregnancy.
HRs and 95% confidence intervals (CIs) were calculated for per interquartile range (IQR) increment for green space (NDVI); PTB, preterm birth; MPTB, moderately PTB; VPTB, very PTB.
All models adjusted for maternal age, race/ethnicity, educational level, and median household income at census block-group level; maternal address ZIP code was fitted as a random effect.
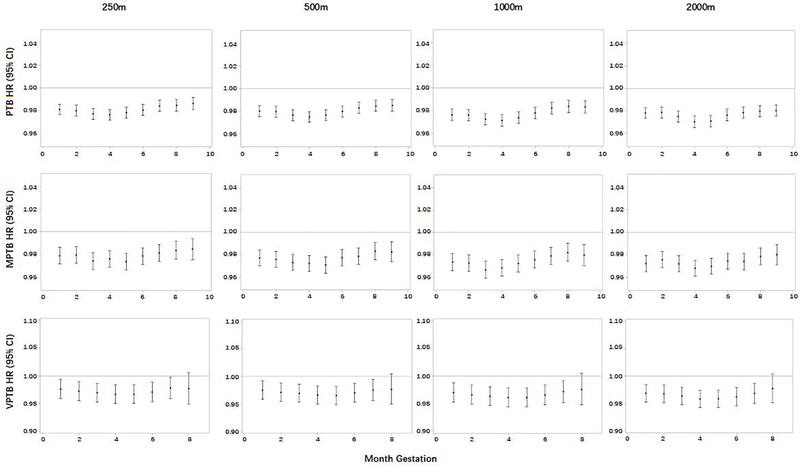
In the monthly time window analyses (Figure 2), the NDVI levels showed protective effects on the three outcome variables (PTB, MPTB, and VPTB) and for all gestational months. Overall, relatively stronger protective associations were observed for the 3rd – 5th gestational months (late first trimester and early second trimester) than the other time periods. For PTB, the strongest association was observed for the 4th gestational month within a 2000 m buffer around the maternal home (HR=0.970, 95% CI: 0.965–0.975). Cochrane’s Q tests showed that there were statistically significant differences across the monthly HRs for PTB and MPTB (Appendix 5), but no significant heterogeneity across time windows for VPTB.
Figure 2. Hazard ratios (HRs) of PTB associated with month-specific green space exposure.
HRs and 95% confidence intervals (CIs) were calculated for per interquartile range (IQR) increment for green space (NDVI); PTB, preterm birth; MPTB, moderately PTB; VPTB, very PTB.
All models adjusted for maternal age, race/ethnicity, educational level, and median household income at census block-group level; maternal address ZIP code was fitted as a random effect.
Results from the interaction analysis are presented in Table 2. For all the air pollutants in our study (PM2.5, NO2, and O3), we observed consistent positive multiplicative and additive interactions between decreasing green space (1- NDVI) and higher air pollution. HRs of the product term >1 indicated positive multiplicative interaction, and RERIs >0 indicated the presence of positive additive interaction. Both of them indicated joint effects of decreasing green space and air pollution on PTB that are greater than expected (i.e., synergistic) based on the estimated effects of each exposure alone, suggesting decreasing green space may have more deleterious effects on PTB for women who are exposed to higher air pollution levels.
Table 2.
Interactions of green space and air pollution exposure on PTB during the entire pregnancy.
| Exposures | Additive interaction RERI | 95% CI | Multiplicative interaction HR* | 95% CI |
|---|---|---|---|---|
| PM2.5 | ||||
| NDVI 250 m | 0.009 | (0.003, 0.016) | 1.009 | (1.002, 1.017) |
| NDVI 500 m | 0.006 | (−0.001, 0.014) | 1.006 | (0.998, 1.014) |
| NDVI 1000 m | 0.008 | (0.001, 0.016) | 1.008 | (1.001, 1.016) |
| NDVI 2000 m | 0.009 | (0.002, 0.017) | 1.009 | (1.001, 1.018) |
| NO2 | ||||
| NDVI 250 m | 0.007 | (0.003, 0.012) | 1.007 | (1.003, 1.012) |
| NDVI 500 m | 0.009 | (0.004, 0.014) | 1.009 | (1.005, 1.014) |
| NDVI 1000 m | 0.011 | (0.006, 0.016) | 1.011 | (1.006, 1.016) |
| NDVI 2000 m | 0.010 | (0.005, 0.015) | 1.010 | (1.005, 1.016) |
| O3 | ||||
| NDVI 250 m | 0.007 | (0.004, 0.010) | 1.007 | (1.004, 1.010) |
| NDVI 500 m | 0.014 | (0.011, 0.017) | 1.014 | (1.011, 1.017) |
| NDVI 1000 m | 0.014 | (0.011, 0.017) | 1.014 | (1.011, 1.018) |
| NDVI 2000 m | 0.013 | (0.010, 0.017) | 1.014 | (1.010, 1.017) |
RERI: Relative excess risks due to interaction; HR: hazard ratio.
The interaction term in Cox proportional hazard models.
DISCUSSION
In this large population covering the State of California from 2001 to 2008, maternal exposure to residential NDVI-based green space was associated with a decreased risk of PTB in both urban and rural areas. These protective effects of green space were stronger during the 3rd – 5th gestational month for PTB and MPTB. We also found consistent positive interaction between decreasing green space and air pollution on both multiplicative and additive scales. This indicates that mothers who are exposed to more air pollution during pregnancy may benefit more from an increase in green space exposure, when compared with mothers exposed to less air pollution.
Green space may have a positive effect on health outcomes through several pathways, including reducing stress, increasing social cohesion, promoting physical activity, improving immune status, and lowering levels of environmental nuisances such as air pollution, ambient noise, and outdoor temperature (Bowler et al., 2010a; Hartig et al., 2014; Lee & Maheswaran, 2011; Markevych et al., 2017; Twohig-Bennett & Jones, 2018; Vienneau et al., 2017). Reduced PTB risk has been associated with maternal green space exposure in a few previous studies (Hystad et al., 2014; Laurent et al., 2013a). To our knowledge, this is the first study applying the time-to-event analysis to examine the association between residential surrounding green space and PTB. We found consistent protective effects between green space exposure and PTB after controlling for maternal age, race/ethnicity, education, and neighborhood income. In contrast, several other studies found inconsistent association (Abelt & McLafferty, 2017; Agay-Shay et al., 2014; Cusack et al., 2017; Dadvand et al., 2012a; Glazer et al., 2018). Reasons that may partially explain the variation in results include differences in study design, methods used to assess green space exposure, study location (urban vs. rural area), study population, and other factors. Grazuleviciene et al. found NDVI within 100 m, 300 m, and 500 m of residences was not associated with PTB, however an association was found between the distance to city park and the risk of PTB (Grazuleviciene et al., 2015), indicating inconsistent results caused by various exposure measures reflecting different potential pathways. The higher maternal physical activity during pregnancy, such as park use, might explain beneficial effect of green space in this study. Contact with ambient green space (such as road side trees, and small lawns) might be important if green space has the greatest influence on health via restorative properties and stress reduction (Mitchell et al., 2011). Another study reported a protective association between green space and PTB in urban areas [odds ratio (OR) = 0.78, upper tertiles vs. lowest tertile, 95% CI: 0.61–0.99], but not in rural areas (Casey et al., 2016), indicating urban and rural differences. However, we observed similar protective associations between green space and PTB in both rural and urban areas. Hystad et al. reported a decreased risk of moderate PTB (30–36 gestational weeks) with higher green space exposure (OR=0.95, 95% CI: 0.91–0.99 for an IQR increase in NDVI within 100 m buffer), but no association for VPTB (Hystad et al., 2014). Our results showed protective effects of green space exposure on PTB, MPTB, and VPTB. The strongest associations were observed for VPTB, suggesting green space may have stronger protective effects for more serious conditions.
In time window analysis, we found protective associations between green spaces and PTB in each of the gestational months, with the stronger associations during the 3rd – 5th gestational months (from the end of the first trimester to the middle of second trimester). In early pregnancy, women experience biological changes associated with hormonal fluctuations and to accommodate a growing baby (Kumar & Magon, 2012). Nausea, vomiting and other pregnancy symptoms might be due to the high levels of human chorionic gonadotropin (hCG) during the first trimester (Lee & Saha, 2011). For most women, the early pregnancy symptoms gradually disappear at the end of the first trimester largely because hCG tends to plateau at a lower level for the remainder of pregnancy (Kumar & Magon, 2012). For the majority of pregnant women, the second trimester might be the most comfortable time during pregnancy when they are not bothered by early pregnancy symptoms and are less physically challenged compared to late pregnancy with rapidly-growing fetus, increased weight, volume, and loss of balance (Committee on Obstetric Practice, 2018; Takeda K et al., 2015). Therefore, pregnant women may be more likely to walk or exercise outdoors and be in contact with green space during this period of time. Thus, green space might benefit pregnant women most during this period through reduced stress and enhanced physical activity. Green space has been shown to reduce depressive symptoms in pregnant women, and physical activity partially mediated the association of green space and PTB (McEachan et al., 2016). In addition, previous studies associated prenatal maternal stress with PTB (Hobel et al., 2008), and stress in the second trimester appears most strongly associated with PTB (Hoffman et al., 2016). There is also evidence suggesting that stress-related alterations in the hypothalamic-pituitary-adrenal-placental system in mid-pregnancy, but not in early or late pregnancy, may be predictive of postpartum depressive symptoms, suggesting a possible window or vulnerability at that time (Yim et al., 2009; Yim et al., 2015). Furthermore, higher leisure-time physical activity could reduce the risk of PTB (Aune et al., 2017). Mild physical activity during the second trimester of pregnancy such as walking has a protective effect on PTB (Takito & Benicio, 2010). Our results support the previous findings that the second trimester is likely the most influential time window for green space-related factors, such as stress and physical activity.
The interaction of green space and air pollution on PTB may imply potential biological mechanisms. In our study, we found consistent positive interactions of green space and air pollution during pregnancy on the risk of PTB. This is likely because air pollution may add oxidative stress and inflammatory responses in the body (Vadillo-Ortega et al., 2014), which may counter the benefits of green space exposure during the pregnancy. Findings suggested that an imbalance between oxidants and antioxidants may be associated with PTB. Analyses of reactive oxygen species or by-products of oxidative stress reported higher levels of these molecules for preterm maternal and fetal blood, amniotic membranes and fluid and placental specimens compared to term birth specimens (Moore et al., 2018). Maternal lifestyle and health status may influence oxidative stress resulting in PTB. For instance, moderate exercise and an active lifestyle have been demonstrated to be useful in reducing oxidative stress (Baltaci et al., 2016; Tarquini et al., 2018). Further, chronic psychological stress promotes oxidative damage through frequent and sustained activation of the hypothalamic-pituitary-adrenal axis (Aschbacher et al., 2013). Therefore, green space may reduce oxidative stress by promoting physical activity and reducing stress. In the interaction analysis, all the air pollution metrics showed positive interactions with green space on PTB, indicating that increasing exposure to green space may be more beneficial for women who are exposed to higher levels of air pollution during pregnancy. Our results suggest that targeting women exposed to high levels of air pollution when implementing interventions to increase green space exposure may lead to a more pronounced reduction of PTBs in California. Given the documented social inequalities in exposure to air pollution (Hajat et al., 2015) and its role on the risk of PTB (Benmarhnia et al., 2017), such targeted interventions on green space have the potential to reduce important social inequalities in PTB in California.
Relationships between green space and air pollution are complex. Studies without considering combined exposures may overestimate the influence of only one exposure, while underestimate their joint effects (Klompmaker et al., 2019b; Klompmaker et al., 2019a). Although we focused on the effect modification of air pollution on green space associated with PTB in this study, air pollution may play different roles on how green space affects health outcomes, as confounder, and mediator. Air pollution has been associated with both green space and PTB. Ignoring the potential confounding by air pollution may lead to distortion of the true effect. In addition, green space affects air pollution levels. In greener areas, air pollution levels may be lower due to the competitive land use between green space and sources of air pollution. Green space is also linked to air quality by various mechanisms (Nowak et al., 2006). Gaseous air pollutants can be directly removed by absorption through leaf stomata (Salmond et al., 2016), and filtering/intercepting and dry deposition of vegetation is one of the major mechanisms in removing particulate matter (Givoni, 1991; Janhäll, 2015). Vegetation also influences air quality by changing dispersion or local air flow and ventilation patterns (Abhijith et al., 2017; Givoni, 1991; Janhäll, 2015), which may positively or negatively affect personal air pollution exposure and thus health outcomes (Abhijith et al., 2017; Dadvand et al., 2012b). Further studies are warranted to investigate the relationships between green space and air pollution and their combined effects on birth outcomes.
The major strengths of our study include the large size and diversity of our study population, the wide range of green space exposure buffer size, and the time to event analytical framework. In logistic regression models, where PTB is treated as a binary outcome, time-varying exposures (e.g. green space) can’t be account for, which may lead to bias in the estimates, since the duration of exposure differs between preterm births and term births. This kind of bias could be avoided with the survival approach, in which gestational age is treated as a time-to-event variable and green space was included as a time-varying exposure variable. Moreover, the critical exposure windows can be examined within the time-to-event framework. We also conducted analyses using logistic regression model. The associations between green space and overall PTB in logistic regression models were slightly smaller than those in cox models (Appendix 6). To the best of our knowledge, this is the first study investigating interactions between green space and air pollutants during pregnancy and their joint impact on PTB as a time-to-event outcome. However, several limitations should be considered when interpreting the study findings. First, although NDVI is the most commonly-used metric in remote sensing for green space exposure in epidemiological studies (Fong et al., 2018), it cannot reflect the use and types of vegetation. The differences in the contact with green space and its composition, such as the proportion of trees and grass, might have different impacts on health through different pathways (Astell-Burt & Feng, 2019). Different green space species could also influence the air pollution levels (Alonso et al., 2011; Tallis et al., 2011; Tiwary et al., 2009). For example, Astell-Burt and Feng reported urban tree canopy may be a good option for promotion of community mental health rather than any other urban greening in Australia (Astell-Burt & Feng, 2019). Coniferous trees are found to be the best for capturing particulate matter over evergreen broadleaf and deciduous species (Tallis et al., 2011; Tiwary et al., 2009), while evergreen broadleaf and deciduous tree species could remove more atmospheric ozone than conifer forests (Alonso et al., 2011). Further research is needed using more informative and multiple green space indicators to represent different aspects of green space exposure and potentially help in identifying the pathways. For instance, street view image in combination with machine learning approach may be valuable tools for green space assessment (Helbich et al., 2019; Lu, 2018). Nieuwenhuijsen et al. evaluated multiple urban exposures simultaneously, including the built environment, air pollution, road traffic noise, meteorology, natural space, and road traffic, to provide a holistic view of environmental exposures and their effects on birth weight (Nieuwenhuijsen et al., 2019). In addition, our air pollution exposure assessment relied on the measurements from monitoring stations that are relatively sparsely distributed, which could not capture local-scale pollutant variation from emission sources. In addition, we relied solely on estimated exposures at maternal residential address reported on the infant birth certificate for both green space and air pollution exposure. The mobility of women during pregnancy were unavailable from birth certificate data. A recent study in Washington State found that moving during the first trimester of pregnancy may be a risk factor for preterm birth and low birth weight (Bond et al., 2019). However, we could not account for its influence without information on residential change. Further, indoor and personal exposure levels could not be estimated without time-location and physical activity data. Future studies may include maternal addresses prior to and throughout pregnancy and use global positioning system tracking and personal monitors to examine real-time personal-level green space and air pollution exposures, although the latter option would be very costly. Another limitation of using the administrative dataset is the lack of information on individual lifestyle and behavioral factors, such as physical activity, social cohesion, noise exposure, and stress reduction, which may be important mediators linking green space to PTB. More research is needed to examine these potential mediators and pathways to develop a more comprehensive understanding of the beneficial effects of green space on birth outcomes. Most recent studies were limited to America and Europe (Abelt & McLafferty, 2017; Agay-Shay et al., 2014; Casey et al., 2016; Cusack et al., 2017; Dadvand et al., 2012a; Glazer et al., 2018; Grazuleviciene et al., 2015; Hystad et al., 2014; Laurent et al., 2013a). Green space and air pollution exposure levels could vary in different regions and might cause different health impacts. Thus, there is also a strong need for studies conducting in other geographical settings (Dzhambov et al., 2019), particularly in low-income and middle-income countries, where air pollution exposure is most severe and require better urban planning in mega cities.
CONCLUSIONS
This large study found that maternal exposure to residential green space was associated with a decreased risk of PTB, including overall, moderately, and very PTB. The stronger associations were observed for larger green space buffer size, especially during the 3rd – 5th gestational months. Our findings also suggest that there are potential positive interactions between green space exposure and air pollution indicators during pregnancy. Further multi-disciplinary studies would be needed to examine this association in other settings and fully understand how the complexity of the pregnant woman’s biopsychosocial and environmental experience influences PTB.
Supplementary Material
Highlights.
Exposure to residential green space is associated with decreased risk of PTB.
Green space has stronger protective associations with PTB in the second trimester.
There are positive interactions between green space and air pollution on PTB.
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Environmental Health Sciences (NIEHS; ES030353). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelt K, & McLafferty S (2017). Green Streets: Urban Green and Birth Outcomes. Int J Environ Res Public Health, 14(7). doi: 10.3390/ijerph14070771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abhijith KV, Kumar P, Gallagher J, McNabola A, Baldauf R, Pilla F, … Pulvirenti B (2017). Air pollution abatement performances of green infrastructure in open road and built-up street canyon environments – A review. Atmospheric Environment, 162, 71–86. doi: 10.1016/j.atmosenv.2017.05.014 [DOI] [Google Scholar]
- Agay-Shay K, Peled A, Crespo AV, Peretz C, Amitai Y, Linn S, … Nieuwenhuijsen MJ (2014). Green spaces and adverse pregnancy outcomes. Occup Environ Med, 71(8), 562–569. doi: 10.1136/oemed-2013-101961 [DOI] [PubMed] [Google Scholar]
- Alexander GR, Himes JH, Kaufman RB, Mor J, & Kogan M (1996). A United States national reference for fetal growth. Obstet Gynecol, 87(2), 163–168. doi: 10.1016/0029-7844(95)00386-x [DOI] [PubMed] [Google Scholar]
- Alonso R, Vivanco MG, Gonzalez-Fernandez I, Bermejo V, Palomino I, Garrido JL, … Artinano B (2011). Modelling the influence of peri-urban trees in the air quality of Madrid region (Spain). Environ Pollut, 159(8–9), 2138–2147. doi: 10.1016/j.envpol.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, & Epel E (2013). Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38(9), 1698–1708. doi: 10.1016/j.psyneuen.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell-Burt T, & Feng X (2019). Association of Urban Green Space With Mental Health and General Health Among Adults in Australia. JAMA Netw Open, 2(7), e198209. doi: 10.1001/jamanetworkopen.2019.8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D, Schlesinger S, Henriksen T, Saugstad OD, & Tonstad S (2017). Physical activity and the risk of preterm birth: a systematic review and meta-analysis of epidemiological studies. BJOG, 124(12), 1816–1826. doi: 10.1111/1471-0528.14672 [DOI] [PubMed] [Google Scholar]
- Ballester F, & Iniguez C (2011). Air Pollution Exposure During Pregnancy and Reproductive Outcomes. [Google Scholar]
- Baltaci SB, Mogulkoc R, & Baltaci AK (2016). Resveratrol and exercise. Biomed Rep, 5(5), 525–530. doi: 10.3892/br.2016.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barradas DT, Dietz PM, Pearl M, England LJ, Callaghan WM, & Kharrazi M (2014). Validation of obstetric estimate using early ultrasound: 2007 California birth certificates. Paediatr Perinat Epidemiol, 28(1), 3–10. doi: 10.1111/ppe.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmarhnia T, Huang J, Basu R, Wu J, & Bruckner TA (2017). Decomposition Analysis of Black-White Disparities in Birth Outcomes: The Relative Contribution of Air Pollution and Social Factors in California. Environ Health Perspect, 125(10), 107003. doi: 10.1289/EHP490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KP, Brown EM, Santos HL, Poegel M, Kiehl TR, Patton EW, … Boles NC (2019). Identifying Windows of Susceptibility by Temporal Gene Analysis. Sci Rep, 9(1), 2740. doi: 10.1038/s41598-019-39318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt LM LJ, Helbing D, Kühnert C, West GB.. (2007). Growth, innovation,scaling, and the pace of life in cities. Proc Natl Acad Sci USA, 104, 7301–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JC, Mancenido AL, Patil DM, Rowley SS, Goldberg J, & Littman AJ (2019). Residence change during the first trimester of pregnancy and adverse birth outcomes. Journal of Epidemiology and Community Health, 73(10), 913. doi: 10.1136/jech-2018-211937 [DOI] [PubMed] [Google Scholar]
- Bowler D, Buyung-Ali L, Knight T, & Pullin A (2010a). A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health, 10, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JA, James P, Rudolph KE, Wu CD, & Schwartz BS (2016). Greenness and Birth Outcomes in a Range of Pennsylvania Communities. Int J Environ Res Public Health, 13(3). doi: 10.3390/ijerph13030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehade H, Simeoni U, Guignard JP, & Boubred F (2018). Preterm Birth: Long Term Cardiovascular and Renal Consequences. Curr Pediatr Rev, 14(4), 219–226. doi: 10.2174/1573396314666180813121652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Obstetric Practice. (2018). ACOG Practice Bulletin No. 189 Summary: Nausea And Vomiting Of Pregnancy. Obstetrics & Gynecology, 131(1), 190–193. doi: 10.1097/aog.0000000000002450 [DOI] [PubMed] [Google Scholar]
- Cusack L, Larkin A, Carozza S, & Hystad P (2017). Associations between residential greenness and birth outcomes across Texas. Environ Res, 152, 88–95. doi: 10.1016/j.envres.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Dadvand P, de Nazelle A, Triguero-Mas M, Schembari A, Cirach M, Amoly E, … Nieuwenhuijsen M (2012b). Surrounding greenness and exposure to air pollution during pregnancy: an analysis of personal monitoring data. Environ Health Perspect, 120(9), 1286–1290. doi: 10.1289/ehp.1104609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Sunyer J, Basagana X, Ballester F, Lertxundi A, Fernandez-Somoano A, … Nieuwenhuijsen MJ (2012a). Surrounding greenness and pregnancy outcomes in four Spanish birth cohorts. Environ Health Perspect, 120(10), 1481–1487. doi: 10.1289/ehp.1205244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhambov AM, Markevych I, & Lercher P (2019). Associations of residential greenness, traffic noise, and air pollution with birth outcomes across Alpine areas. Science of The Total Environment, 678, 399–408. doi: 10.1016/j.scitotenv.2019.05.019 [DOI] [PubMed] [Google Scholar]
- Fong KC, Hart JE, & James P (2018). A Review of Epidemiologic Studies on Greenness and Health: Updated Literature Through 2017. Curr Environ Health Rep, 5(1), 77–87. doi: 10.1007/s40572-018-0179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Triguero-Mas M, Martínez D, & et al. (2016). Residential green spaces and mortality: a systematic review. Environ Int, 86, 60–67. [DOI] [PubMed] [Google Scholar]
- Givoni B (1991). Impact of Planted Areas on Urban Environmental-Quality – a Review.. Atmospheric Environment Part B-Urban Atmosphere, 25, 289–299. [Google Scholar]
- Glazer KB, Eliot MN, Danilack VA, Carlson L, Phipps MG, Dadvand P, … Wellenius GA (2018). Residential green space and birth outcomes in a coastal setting. Environ Res, 163, 97–107. doi: 10.1016/j.envres.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DW, Wilson JP, Knoblock CA, Ritz B, & Cockburn MG (2008). An effective and efficient approach for manually improving geocoded data. Int J Health Geogr, 7, 60. doi: 10.1186/1476-072X-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazuleviciene R, Danileviciute A, Dedele A, Vencloviene J, Andrusaityte S, & et al. (2015). Surrounding greenness, proximity to city parks and pregnancy outcomes in Kaunas cohort study. Int J Hyg Environ Health, 218, 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, & Robins JM (1999). Causal diagrams for epidemiologic research. Epidemiology, 10(1), 37–48. [PubMed] [Google Scholar]
- Hajat A, Hsia C, & O’Neill MS (2015). Socioeconomic Disparities and Air Pollution Exposure: a Global Review. Curr Environ Health Rep, 2(4), 440–450. doi: 10.1007/s40572-015-0069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MS, & Goldenberg RL (2016). Global burden of prematurity. Semin Fetal Neonatal Med, 21, 74–79. [DOI] [PubMed] [Google Scholar]
- Hartig T, Mitchell R, de Vries S, & Frumkin H (2014). Nature and health. Annu Rev Public Health, 35, 207–228. doi: 10.1146/annurev-publhealth-032013-182443 [DOI] [PubMed] [Google Scholar]
- Helbich M, Yao Y, Liu Y, Zhang J, Liu P, & Wang R (2019). Using deep learning to examine street view green and blue spaces and their associations with geriatric depression in Beijing, China. Environ Int, 126, 107–117. doi: 10.1016/j.envint.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobel CJ, Goldstein A, & Barrett ES (2008). Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol, 51(2), 333–348. doi: 10.1097/GRF.0b013e31816f2709 [DOI] [PubMed] [Google Scholar]
- Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, & Ross RG (2016). Measures of Maternal Stress and Mood in Relation to Preterm Birth. Obstet Gynecol, 127(3), 545–552. doi: 10.1097/AOG.0000000000001287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hystad P, Davies HW, Frank L, Van Loon J, Gehring U, Tamburic L, & Brauer M (2014). Residential greenness and birth outcomes: evaluating the influence of spatially correlated built-environment factors. Environ Health Perspect, 122(10), 1095–1102. doi: 10.1289/ehp.1308049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Banay RF, Hart JE, & Laden F (2015). A Review of the Health Benefits of Greenness. Curr Epidemiol Rep, 2(2), 131–142. doi: 10.1007/s40471-015-0043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhäll S (2015). Review on urban vegetation and particle air pollution – Deposition and dispersion. Atmospheric Environment, 105, 130–137. doi: 10.1016/j.atmosenv.2015.01.052 [DOI] [Google Scholar]
- Klompmaker JO, Hoek G, Bloemsma LD, Wijga AH, van den Brink C, Brunekreef B, … Janssen NAH (2019b). Associations of combined exposures to surrounding green, air pollution and traffic noise on mental health. Environ Int, 129, 525–537. doi: 10.1016/j.envint.2019.05.040 [DOI] [PubMed] [Google Scholar]
- Klompmaker JO, Janssen NAH, Bloemsma LD, Gehring U, Wijga AH, van den Brink C, … Hoek G (2019a). Residential surrounding green, air pollution, traffic noise and self-perceived general health. Environ Res, 179(Pt A), 108751. doi: 10.1016/j.envres.2019.108751 [DOI] [PubMed] [Google Scholar]
- Kloog I (2019). Air pollution, ambient temperature, green space and preterm birth. Curr Opin Pediatr, 31(2), 237–243. doi: 10.1097/MOP.0000000000000736 [DOI] [PubMed] [Google Scholar]
- Kloog I, Melly S, Ridgway W, Coull B, & Schwartz J (2012). Using new satellite based exposure methods to study the association between pregnancy PM2.5 exposure, premature birth and birth weight in Massachusetts. Environ Health, 11, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoruchko K (2012). Empirical Bayesian Kriging, Implemented in ArcGIS Geostatistical Analyst: ESRI. [Google Scholar]
- Kumar P, Druckman A, Gallagher J, Gatersleben B, Allison S, Eisenman TS, … Morawska L (2019). The nexus between air pollution, green infrastructure and human health. Environ Int, 133(Pt A), 105181. doi: 10.1016/j.envint.2019.105181 [DOI] [PubMed] [Google Scholar]
- Kumar P, & Magon N (2012). Hormones in pregnancy. Nigerian medical journal : journal of the Nigeria Medical Association, 53(4), 179–183. doi: 10.4103/0300-1652.107549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane DK, Leem JH, Lee JY, & Kim HC (2015). A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol, 30, e2015011. doi: 10.5620/eht.e2015011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Benmarhnia T, Milesi C, Hu J, Kleeman MJ, Cockburn M, & Wu J (2019). Relationships between greenness and low birth weight: Investigating the interaction and mediation effects of air pollution. Environ Res, 175, 124–132. doi: 10.1016/j.envres.2019.05.002 [DOI] [PubMed] [Google Scholar]
- Laurent O, Gomolka M, Haylock R, Blanchardon E, Giussani A, Atkinson W, … Laurier D (2016a). Concerted Uranium Research in Europe (CURE): toward a collaborative project integrating dosimetry, epidemiology and radiobiology to study the effects of occupational uranium exposure. J Radiol Prot, 36(2), 319–345. doi: 10.1088/0952-4746/36/2/319 [DOI] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Cockburn M, Escobedo L, Kleeman MJ, & Wu J (2014). Sources and contents of air pollution affecting term low birth weight in Los Angeles County, California, 2001–2008. Environ Res, 134, 488–495. doi: 10.1016/j.envres.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, … Wu J (2016b). Low birth weight and air pollution in California: Which sources and components drive the risk? Environ Int, 92–93, 471–477. doi: 10.1016/j.envint.2016.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, … Wu J (2016c). A Statewide Nested Case-Control Study of Preterm Birth and Air Pollution by Source and Composition: California, 2001–2008. Environ Health Perspect, 124(9), 1479–1486. doi: 10.1289/ehp.1510133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Wu J, Li L, Chung J, & Bartell S (2013b). Investigating the association between birth weight and complementary air pollution metrics: a cohort study. Environ Health, 12, 18. doi: 10.1186/1476-069x-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Wu J, Li L, & Milesi C (2013a). Green spaces and pregnancy outcomes in Southern California. Health Place, 24, 190–195. doi: 10.1016/j.healthplace.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, & Maheswaran R (2011). The health benefits of urban green spaces: a review of the evidence. J Public Health (Oxf), 33(2), 212–222. doi: 10.1093/pubmed/fdq068 [DOI] [PubMed] [Google Scholar]
- Lee NM, & Saha S (2011). Nausea and vomiting of pregnancy. Gastroenterol Clin North Am, 40(2), 309–334, vii. doi: 10.1016/j.gtc.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen W, & He W (2015). Planning of Green Space Ecological Network in Urban Areas: An Example of Nanchang, China. Int J Environ Res Public Health, 12(10), 12889–12904. doi: 10.3390/ijerph121012889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, & Chambless L (2007). Test for additive interaction in proportional hazards models. Ann Epidemiol, 17(3), 227–236. doi: 10.1016/j.annepidem.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, … Xiang H (2017). Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ Pollut, 227, 596–605. doi: 10.1016/j.envpol.2017.03.055 [DOI] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, … Black RE (2016). Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet, 388(10063), 3027–3035. doi: 10.1016/s0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y (2018). The Association of Urban Greenness and Walking Behavior: Using Google Street View and Deep Learning Techniques to Estimate Residents’ Exposure to Urban Greenness. Int J Environ Res Public Health, 15(8). doi: 10.3390/ijerph15081576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley CS, Kuylenstierna JC, Vallack HW, Henze DK, Blencowe H, & Ashmore MR (2017). Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ Int, 101, 173–182. doi: 10.1016/j.envint.2017.01.023 [DOI] [PubMed] [Google Scholar]
- March of Dimes, PMNCH, Save the Children, & WHO. (2012). Born Too Soon: The Global Action Report on Preterm Birth.
- Markevych I, Schoierer J, Hartig T, Chudnovsky A, Hystad P, Dzhambov AM, & et al. (2017). Exploring pathways linking greenspace to health: theoretical and methodological guidance. Environmental Research, 158, 301–317. [DOI] [PubMed] [Google Scholar]
- Marques AH, O’Connor TG, Roth C, Susser E, & Bjorke-Monsen AL (2013). The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci, 7, 120. doi: 10.3389/fnins.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MC, Litt JS, Smith VC, & Zupancic JA (2011). Prematurity: an overview and public health implications. Annu Rev Public Health, 32, 367–379. doi: 10.1146/annurev-publhealth-090810-182459 [DOI] [PubMed] [Google Scholar]
- McEachan RR, Prady SL, Smith G, Fairley L, Cabieses B, Gidlow C, … Nieuwenhuijsen MJ (2016). The association between green space and depressive symptoms in pregnant women: moderating roles of socioeconomic status and physical activity. J Epidemiol Community Health, 70(3), 253–259. doi: 10.1136/jech-2015-205954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Ramon M, Zanobetti A, & Schwartz J (2006). The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol, 163(6), 579–588. doi: 10.1093/aje/kwj078 [DOI] [PubMed] [Google Scholar]
- Mitchell R, Astell-Burt T, & Richardson EA (2011). A comparison of green space indicators for epidemiological research. J Epidemiol Community Health, 65(10), 853–858. doi: 10.1136/jech.2010.119172 [DOI] [PubMed] [Google Scholar]
- Moore TA, Ahmad IM, & Zimmerman MC (2018). Oxidative Stress and Preterm Birth: An Integrative Review. Biol Res Nurs, 20(5), 497–512. doi: 10.1177/1099800418791028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen M, Agier L, Basagana X, Urquiza J, Tamayo-Uria I, Giorgis-Allemand L, … Slama R (2019). Influence of the Urban Exposome on Birth Weight. Environ Health Perspect, 127(4), 47007. doi: 10.1289/EHP3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen M, Khreis H, Triguero-Mas M, Gascon M, & Dadvand P (2017). Fifty Shades of Green: Pathway to Healthy Urban Living. Epidemiology, 28, 63–71. [DOI] [PubMed] [Google Scholar]
- Nowak DJ, Crane DE, & Stevens JC (2006). Air pollution removal by urban trees and shrubs in the United States. Urban Forestry & Urban Greening, 4(3–4), 115–123. doi: 10.1016/j.ufug.2006.01.007 [DOI] [Google Scholar]
- Olsson D, Mogren I, & Forsberg B (2013). Air pollution exposure in early pregnancy and adverse pregnancy outcomes: a register-based cohort study. BMJ Open, 3(2). doi: 10.1136/bmjopen-2012-001955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G, Belanger K, Ebisu K, & Bell ML (2014). Fine particulate matter and risk of preterm birth in Connecticut in 2000–2006: a longitudinal study. Am J Epidemiol, 179(1), 67–74. doi: 10.1093/aje/kwt216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou S (2003). Economic consequences of preterm birth and low birthweight. BJOG: An International Journal of Obstetrics and Gynaecology, 110, 17–23. doi: 10.1016/s1470-0328(03)00013-2 [DOI] [PubMed] [Google Scholar]
- Pilz J, & Spöck G (2007). Why Do We Need and How Should We Implement Bayesian Kriging Methods. Stochastic Environmental Research and Risk Assessment, 22, 621–632. [Google Scholar]
- Ritz B, Yu F, Chapa G, & Fruin S (2000). Effect of Air Pollution on Preterm Birth among Children Born in Southern California between 1989 and 1993. Epidemiology, 11(5), 502–511. [DOI] [PubMed] [Google Scholar]
- Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, & Callaghan WM (2014). History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol, 210(4), 285–297. doi: 10.1016/j.ajog.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, & Doyle LW (2008). An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet, 371(9608), 261–269. doi: 10.1016/s0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- Salmond JA, Tadaki M, Vardoulakis S, Arbuthnott K, Coutts A, Demuzere M, … Wheeler BW (2016). Health and climate related ecosystem services provided by street trees in the urban environment. Environ Health, 15 Suppl 1, 36. doi: 10.1186/s12940-016-0103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan P, Ilango S, Bruckner TA, Wang Q, Basu R, & Benmarhnia T (2019). Ambient Fine Particulate Matter and Preterm Birth in California: Identification of Critical Exposure Windows. Am J Epidemiol, 188(9), 1608–1615. doi: 10.1093/aje/kwz120 [DOI] [PubMed] [Google Scholar]
- Skyscrapercity. (2015). http://www.skyscrapercity.com/showthread.php?t=1660203.
- Spruce JP, Gasser GE, & Hargrove WW (2016). MODIS NDVI Data, Smoothed and Gap-filled, for the Conterminous US: 2000–2015. ORNL DAAC, Oak Ridge, Tennessee, USA.. [Google Scholar]
- Stieb DM, Chen L, Eshoul M, & Judek S (2012). Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res, 117, 100–111. doi: 10.1016/j.envres.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, & Tong S (2011). The influence of season and ambient temperature on birth outcomes: a review of the epidemiological literature. Environ Res, 111(3), 451–462. doi: 10.1016/j.envres.2011.01.023 [DOI] [PubMed] [Google Scholar]
- Takeda K, Shimizu K, & Sci, I. M. J. P. T. (2015). Changes in balance strategy in the third trimester. Imura M. J Phys Ther Sci, 27(6), 1813–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takito MY, & Benicio MH (2010). Physical activity during pregnancy and fetal outcomes: a case-control study. Rev Saude Publica, 44(1), 90–101. doi: 10.1590/s0034-89102010000100010 [DOI] [PubMed] [Google Scholar]
- Tallis M, Taylor G, Sinnett D, & Freer-Smith P (2011). Estimating the removal of atmospheric particulate pollution by the urban tree canopy of London, under current and future environments. Landscape and Urban Planning, 103(2), 129–138. doi: 10.1016/j.landurbplan.2011.07.003 [DOI] [Google Scholar]
- Tarquini F, Picchiassi E, Coata G, Centra M, Bini V, Meniconi S, … Di Renzo GC (2018). Induction of the apoptotic pathway by oxidative stress in spontaneous preterm birth: Single nucleotide polymorphisms, maternal lifestyle factors and health status. Biomed Rep, 9(1), 81–89. doi: 10.3892/br.2018.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary A, Sinnett D, Peachey C, Chalabi Z, Vardoulakis S, Fletcher T, … Hutchings TR (2009). An integrated tool to assess the role of new planting in PM10 capture and the human health benefits: a case study in London. Environ Pollut, 157(10), 2645–2653. doi: 10.1016/j.envpol.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Tucker CJ (1979). Red and photographic infrared linear combinations for monitoring vegetation. Remote Sensing of Environment, 8(2), 127–150. [Google Scholar]
- Twohig-Bennett C, & Jones A (2018). The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ Res, 166, 628–637. doi: 10.1016/j.envres.2018.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S., Census, & Bureau. (2004). 2000 Census of Population and Housing Summary Tape File 3A. Washington, DC: U.S. Census Bureau. [Google Scholar]
- United Nations. (2018). Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision. [Google Scholar]
- Urban Audit. (2007). Survey on perceptions of quality of life in 75 European cities. European Commission. [Google Scholar]
- Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sanchez BN, Rojas-Bracho L, Viveros-Alcaraz M, … O’Neill MS (2014). Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses, 82(2), 219–224. doi: 10.1016/j.mehy.2013.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienneau D, de Hoogh K, Faeh D, Kaufmann M, Wunderli JM, Roosli M, & Group SNCS (2017). More than clean air and tranquillity: Residential green is independently associated with decreasing mortality. Environ Int, 108, 176–184. doi: 10.1016/j.envint.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Jerrett M, Su JG, Burnett RT, Chen H, Wheeler AJ, & Goldberg MS (2012). A cohort study relating urban green space with mortality in Ontario, Canada. Environ Res, 115, 51–58. doi: 10.1016/j.envres.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Wang Q, Benmarhnia T, Zhang H, Knibbs LD, Sheridan P, Li C, … Huang C (2018). Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ Int, 121(Pt 1), 317–324. doi: 10.1016/j.envint.2018.09.021 [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh J, Su J, Cockburn M, Jerrett M, & Ritz B (2011). Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles County, California. Environ Health, 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). Urban green spaces and health. A review of evidence. http://www.euro.who.int/__data/assets/pdf_file/0005/321971/Urban-green-spaces-and-health-review-evidence.pdf?ua=1 Copenhagen: WHO Regional Office for Europe. [Google Scholar]
- Wu J, Laurent O, Li L, Hu J, & Kleeman M (2016). Adverse Reproductive Health Outcomes and Exposure to Gaseous and Particulate-Matter Air Pollution in Pregnant Women. Res Rep Health Eff Inst(188), 1–58. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, & Ritz B (2009). Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect, 117(11), 1773–1779. doi: 10.1289/ehp.0800334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel Schetter C, Hobel CJ, Chicz-DeMet A, & Sandman CA (2009). Risk of Postpartum Depressive Symptoms With Elevated Corticotropin-Releasing Hormone in Human Pregnancy. Archives of General Psychiatry, 66(2), 162–169. doi: 10.1001/archgenpsychiatry.2008.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, & Dunkel Schetter C (2015). Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol, 11, 99–137. doi: 10.1146/annurev-clinpsy-101414-020426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.