Abstract
Purpose
The human thrifty phenotype hypothesis presupposes that lower 24-hour (24h) energy expenditure (24EE) during famine preserves body mass and promotes survival. The prevailing view defines thrifty individuals as having a lower 24EE during fasting. However, it is also plausible that the greater decline in 24EE during fasting in thrifty individuals is due to higher 24EE during energy balance conditions (ENBAL). Herein, we provide evidence that this is indeed the case.
Methods
In 108 healthy subjects, 24EE was measured in a whole-room indirect calorimeter both during ENBAL and 24h fasting conditions. Subjects were categorized as thrifty or spendthrift based on the median value (−162 kcal/day) of the difference in 24EE (adjusted for body composition) between fasting and ENBAL conditions. Concomitant 24h urinary catecholamines were assessed by liquid chromatography–mass spectrometry.
Results
Compared to ENBAL, 24EE decreased during 24h fasting by 172 kcal/day (standard deviation = 93; range, −470 to 122). A greater-than-median decrease in 24EE (“thriftier” phenotype) was due to higher 24EE during ENBAL (+124 kcal/day; P < 0.0001) but not to lower 24EE during fasting (P = 0.35). Greater fasting-induced increase in epinephrine was associated with concomitant lower decrease in 24EE (r = 0.27; P = 0.006).
Main Conclusion
The greater decrease in 24EE during acute fasting (which characterizes the thrifty phenotype) is not due to reduced metabolic rate during fasting but to a relatively higher 24EE during feeding conditions, and this decrease in 24EE during fasting is accompanied by a smaller increase in epinephrine. These results recharacterize the prevailing view of the short-term 24EE responses that define the human metabolic phenotypes.
Clinical Trials: NCT00523627, NCT00687115, NCT02939404
Keywords: thrifty phenotype, energy balance, fasting, energy expenditure, epinephrine, FGF21
The existence of thrifty versus spendthrift phenotypes, defined by measurement of 24-hour (24h) energy expenditure (24EE) in a whole-room indirect calorimeter during acute fasting and overfeeding, was first proposed in 2001 (1). In this preliminary study, 24EE responses to short-term (24h) fasting and 200% overfeeding were related such that, compared to energy balance (ENBAL), individuals with less increase in 24EE during overfeeding decreased 24EE more during fasting (“thrifty” phenotype), whereas those with a greater increase in 24EE during overfeeding had a smaller decrease in 24EE during fasting (“spendthrift” phenotype) (2, 3). We have demonstrated that thrifty subjects, characterized by greater decline in 24EE from ENBAL during 24h fasting, are more susceptible to weight gain and more resistant to weight loss in 3 separate settings: in free-living conditions (4) and in carefully-controlled inpatient feeding studies during 6-week caloric restriction (5) and during low-protein daily overfeeding (6).
In the aforementioned studies, thriftiness is defined as a greater decrease in 24EE from ENBAL during acute (24h) fasting. The proposed model is that this decline is attributed to lower 24EE during fasting conditions (Fig. 1). However, lower fasting 24EE itself did not predict weight loss or weight gain and, contrary to the original model and counterintuitively, thrifty individuals appeared to have a higher 24EE during ENBAL (7). Thus, we hypothesized that thriftiness is defined not by lower fasting 24EE but by higher ENBAL 24EE which, if so, would fundamentally alter the prevailing model of the thrifty/spendthrift phenotype.
Figure 1.
Original concept of the human metabolic phenotypes, where differences in the decrease in 24EE during 24h fasting between spendthrift and thrifty subjects are due to a lower 24EE during fasting in thrifty subjects compared with spendthrift subjects.
Leptin, catecholamines, and fibroblast growth factor 21 (FGF21) are candidate thermogenic hormones that could mediate the extent of metabolic thriftiness during acute fasting conditions. Leptin is an adipocyte-derived hormone that decreases with fasting and modulates 24EE via its effects on the hypothalamic neural circuitry (8–10). A greater decrease in 24EE during short-term fasting (indicative of a thrifty phenotype) is associated with a lower urinary epinephrine excretion rate in this condition (11). FGF21, a liver-derived hormone associated with thermogenesis in rodent models (12, 13), also decreases following short-term fasting in humans (14, 15).
In this present study, we investigated in a cohort of 108 healthy individuals whether: (1) the greater decrease in 24EE during 24h fasting (which characterizes thrifty subjects) is due to a lower 24EE during fasting or to a higher 24EE during ENBAL; and, (2) if changes in urinary catecholamines, FGF21, and leptin are associated with the concomitant decrease in 24EE during 24h fasting. We also examined the components of 24EE, sleeping metabolic rate (SLEEP) and “awake and fed” thermogenesis (AFT), to determine their contribution to the overall decrease in 24EE during 24h fasting.
Research Design and Methods
Subjects
For this cross-sectional analysis, baseline data from 108 individuals from 3 different studies at our institute were combined (Supplemental Fig. 1; all supplementary material and figures are located in a digital research materials repository (16)). All individuals were 18 years or older and living in and around nearby Phoenix. Subjects were weight stable (< 10% weight variation) for at least 6 months prior to admission and were confirmed to be healthy by history, physical examination, and fasting blood tests. On admission to the clinical research unit, subjects were placed on a standard, normal-protein, weight-maintaining diet (WMD; 50% carbohydrate, 30% fat, and 20% protein), using unit-specific equations based on weight and gender (17). Daily fasting body weight measured by calibrated scale was maintained within 1% of the admission weight by daily adjusting the energy intake of WMD (18). Body composition was assessed by dual-energy x-ray absorptiometry (DXA) (DPX-1, Lunar Corp, Madison, WI) on the second day after admission. Fat mass (FM) and fat-free mass (FFM) were calculated from the percentage body fat obtained from DXA scans. All subjects had normal glucose regulation based on oral glucose tolerance test (OGTT) performed after 3 days on the WMD (19). Plasma glucose concentrations were measured using the Analox GM9 glucose oxidase method (Analox Inst. USA Inc., Lunenburg, MA). Plasma insulin concentrations were measured by an automated immunoenzymometric assay (Tosoh Bioscience Inc., Tessenderlo, Belgium). All participants provided written informed consent prior to beginning the study. The Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) approved all 3 studies.
Measurement of energy metabolism
Twenty-four-hour energy expenditure (EE) was assessed in a large, open-circuit indirect whole-room calorimeter (respiratory chamber), as previously described (5, 20). Briefly, volunteers entered the calorimeter at 08:00am after having fasted overnight and eating breakfast at 07:00am. They remained therein for 23 hours and 15 minutes during which 3 meals were provided at 11:00am , 4:00pm, and 7:00 pm. While in the calorimeter, subjects were asked to remain sedentary. The mean ambient temperature while in the calorimeter was 23.9°C ± 1.3°C (mean ± standard deviation [SD]). Twenty-four-hour EE and respiratory quotient (RQ) were calculated from previously derived equations (21) by extrapolating to 24h the average CO2 production and O2 consumption per minute during the 23 hours and 15 minutes in the calorimeter.
The components of 24EE were as follows: the sleeping metabolic rate (SLEEP), the rate of EE in the inactive-awake state (EE0), and the awake-fed thermogenesis (AFT) during ENBAL or awake-nonfed thermogenesis (ANFT) during fasting. SLEEP was calculated by extrapolating to 24h the average of all minute-by-minute EE time points (kcal/min) calculated from 11:30pm to 5:30am overnight during which spontaneous physical activity (SPA) was < 1.5% (ie, when subjects were motionless). The SPA was measured by radar sensors and expressed as the percentage of time when activity was detected (22). The EE0 was calculated as the intercept of the regression line between EE and SPA minute-by-minute timepoints between 10:00am and 1:00am, as previously described (23). The AFT and ANFT were calculated as the difference between EE0 and SLEEP expressed in kcal/min in their respective time periods and then extrapolated to 15 hours, as previously described (23). The physical activity level (PAL) was calculated as 24EE divided by SLEEP (24).
Energy expenditure during 24h energy balance and fasting conditions
To closely achieve ENBAL during the 24EE assessment in the whole-room calorimeter, 24EE was measured twice during eucaloric conditions in the baseline period in all participants in the clinical trials NCT00523627 and NCT00687115 (n = 95). The first eucaloric EE assessment was obtained while subjects resided for 24h in the calorimeter with total energy intake calculated using a unit-specific formula to achieve 24h ENBAL in the confined environment of the calorimeter (20). Secondly, after 2 days, subjects had another eucaloric 24EE assessment inside the calorimeter when the total energy intake was equal to the 24EE value calculated during the first eucaloric EE assessment, for precise determination of 24EE during ENBAL (24EEENBAL). In the clinical trial NCT02939404, 24EEENBAL was measured only once (n = 13) while obtaining the same degree of deviation from perfect energy balance as in the duplicate assessments. Apart from this difference in the study scheme, the baseline periods of all 3 studies were comparable.
Following ENBAL assessment and after at least 3 days on the WMD, 24EE was assessed during 24h of fasting when no food was provided, and subjects were instructed to keep themselves hydrated by drinking water or no-calorie, no-caffeine beverages.
Hormonal measurements
Fasting blood draws for measurements of FGF21 and leptin concentrations were performed in the morning both prior to and upon exiting the calorimeter during each dietary intervention. Plasma samples were collected in EDTA-containing tubes and stored in a freezer at −70°C for later measurements of hormone concentrations by the NIDDK Core laboratory in Bethesda, MD. Plasma FGF21 concentrations were measured in n = 94 subjects by ELISA (R&D Systems, Minneapolis, MN, USA). The intra-assay coefficient of variation (CV) was 2.5% and the inter-assay CV was 5.2%. Plasma leptin concentrations were measured in n = 87 subjects by ELISA (EMD Millipore, Billerica, MA). The intra-assay CV was 2.6% and the inter-assay CV was 5.0%.
Twenty-four-hour urine was collected in a refrigerator inside the calorimeter during each 24EE measurement and stored in a freezer at −70°C for later measurement of urinary catecholamines in n = 101 subjects at the Mayo Collaborative Services, LLC in Rochester, MN via high-performance liquid chromatography. The diet served in the calorimeter did not contain any caffeinated and alcoholic beverages, as well as catecholamine-rich foods that could have increased urinary catecholamine levels.
Statistical analysis
Statistical analysis was performed using the SAS statistical software package (SAS Enterprise Guide Version 7.15; SAS Institute, Cary, NC). Unless otherwise specified, data were expressed as mean ± SD or mean with 95% confidence interval (CI). Skewed data were log10-transformed before analyses to meet the assumptions of parametric tests (ie, homoscedasticity and normal distribution of data). The Pearson correlation coefficient was used to quantify associations between continuous variables.
Multivariate linear regression analysis was used to adjust EE measures during ENBAL (Supplemental Table 1) and during fasting (Supplemental Table 2) (16) for their known determinants including demographic, environmental, and body composition measures (21, 25) by including age, sex, ethnicity, FM, FFM, SPA (only 24EE analyses), ambient temperature, and calorimeter room (Room 1 or 2) as covariates in the regression models. The change in adjusted 24EE from ENBAL during fasting was calculated as the adjusted 24EEFASTING minus the adjusted 24EEENBAL.
For graphical purposes and ease of interpretation, the aforementioned analyses using continuous EE data were followed up with confirmatory analyses using metabolic groups. In these confirmatory analyses, thrifty/spendthrift subjects were arbitrarily defined as those with lower/higher-than-median decrease in adjusted 24EE during fasting from ENBAL, respectively, as done previously (5, 7). Similarly, additional analyses of the 10 thriftiest and 10 most spendthrift subjects were also carried out. Paired t test was used to evaluate the difference (∆) in EE measures between fasting vs ENBAL conditions while unpaired t test was used to compare EE measures between metabolic groups (thrifty/spendthrift). Sensitivity analyses were conducted including only men, and by additionally adjusting EE measures by clinical protocol and similar results were obtained (data not shown).
Results
Baseline characteristics of the study cohort are presented in Table 1. The relationships between FFM and 24EE during ENBAL and during fasting are presented in Supplemental Fig. 2 (16). Energy intake and 24EEENBAL were closely related (r = 0.96; P < 0.0001) (Fig. 2) and the regression line was almost identical to the identity line, thus indicating successful achievement of 24h energy balance over the entire range of energy intake values. The average deviation from perfect ENBAL was 40 ± 103 kcal/day, equivalent to 2.2 ± 5.0% of measured 24EE.
Table 1.
Baseline Characteristics of the Study Cohort
| Total (n = 108) | Spendthrift (n = 54) | Thrifty (n = 54) | P value | |
|---|---|---|---|---|
| Male, n (%) | 83 (76.9) | 41 (75.9) | 42 (77.8) | 0.82 |
| Ethnicity, n (%) | 0.5 | |||
| African-American | 20 (18.5) | 11 (20.4) | 9 (16.7) | |
| Caucasian | 37 (34.3) | 14 (25.9) | 23 (42.6) | |
| Native American | 36 (33.3) | 18 (33.3) | 18 (33.3) | |
| Hispanic | 15 (13.9) | 11 (20.4) | 4 (7.4) | |
| Age, years | 36.6 ± 10.4 (18, 55.4) | 36.4 ± 10.2 (18, 55.4) | 36.8 ± 10.6 (18.3, 55) | 0.87 |
| Height, cm | 171.8 ± 8.1 (150.0, 196.4) | 172.0 ± 8.0 (156.8, 196.4) | 171.7 ± 8.3 (150.0, 185.0) | 0.89 |
| Weight-maintaining diet intake, kcal/day | 2713 ± 211 (2196, 3295) | 2731 ± 225 (2312, 3295) | 2695 ± 198 (2196, 3106) | 0.39 |
| Body composition measures | ||||
| Body weight, kg | 83.4 ± 18.7 (47.5, 139.2) | 85.5 ± 20.8 (56, 139.2) | 81.4 ± 16.2 (47.5, 119.3) | 0.25 |
| BMI, kg/m2 | 28.3 ± 6.6 (17.8, 49.9) | 29.1 ± 7.6 (20.6, 49.9) | 27.6 ± 5.4 (17.8, 42.9) | 0.24 |
| Body fat, % | 26.8 ± 9.1 (7.6, 46.1) | 27.1 ± 10 (7.9, 45.8) | 26.5 ± 8.1 (7.6, 46.1) | 0.72 |
| FM, kg | 23.3 ± 11.7 (5.4, 56.3) | 24.4 ± 13.4 (5.4, 56.3) | 22.1 ± 9.8 (6.4, 50.7) | 0.31 |
| FFM, kg | 60.1 ± 11 (35.5, 90) | 61.2 ± 11.4 (37.8, 90) | 59.1 ± 10.5 (35.5, 80.6) | 0.32 |
| Measurements of glucose tolerance and insulin sensitivity | ||||
| Fasting glucose, mg/dL | 91.8 ± 6.2 (77.5, 113) | 92.3 ± 5.6 (80, 113) | 91.4 ± 6.7 (77.5, 109.5) | 0.46 |
| Fasting insulin, μIU/mL | 10.1 ± 7.7 (2, 46.5); 7.3, 5.0–13.0* | 10.3 ± 7.6 (2, 32.4); 7.2, 5.3–13.5* | 9.9 ± 8 (2, 46.5); 7.5, 5.0– 13.0* | 0.71 |
| 2-h glucose during OGTT, mg/dL | 110.2 ± 26.2 (50, 185) | 113 ± 26.6 (50, 185) | 107.2 ± 25.7 (65, 183) | 0.26 |
| 2-h insulin during OGTT, μIU/mL | 68.5 ± 82 (3, 523); 45.0, 27.0–73.4* | 75.2 ± 82.8 (3, 509.8); 51.2, 30.0–88.0* | 61.7 ± 81.5 (4, 523); 43.0, 20.0–69.0* | 0.17 |
| Energy expenditure measures during energy balance (ENBAL) | ||||
| RQ ratio | 0.86 ± 0.04 (0.76, 1.00) | 0.87 ± 0.03 (0.80, 0.94) | 0.86 ± 0.04 (0.76, 1.00) | 0.34 |
| 24EE, kcal/day | 2074 ± 357 (1383, 3188) | 2053 ± 393 (1502, 3188) | 2096 ± 319 (1383, 2830) | 0.53 |
| Adjusted 24EE, kcal/daya | 2074 ± 137 (1707, 2461) | 2012 ± 141 (1707, 2461) | 2136 ± 102 (1918, 2438) | <0.0001 |
| ∆ adjusted 24EE during fastinga | –172 ± 93 (–470, 122) | –98 ± 57 (–162, 122) | –245 ± 58 (–470, –162) | <0.0001 |
| 24EE/FFM, kcal/day/kg | 34.7 ± 3.5 (25.5, 42.5) | 33.7 ± 3.7 (25.5, 41.7) | 35.7 ± 3.0 (28.6, 42.5) | 0.003 |
| SLEEP, kcal/day | 1637 ± 279 (1073, 2306) | 1619 ± 292 (1193, 2306) | 1655 ± 268 (1073, 2303) | 0.50 |
| Adjusted SLEEP, kcal/daya | 1637 ± 120 (1384, 2066) | 1594 ± 101 (1384, 1799) | 1681 ± 122 (1386. 2066) | <0.0001 |
| ∆ adjusted SLEEP during fastinga | –15 ± 111 (–403, 262) | +33 ± 89 (-147, 262) | −63 ± 111 (-403, 179) | <0.0001 |
| EE0, kcal/15h | 1375 ± 237 (941, 2098) | 1364 ± 263 (965, 2098) | 1385 ± 210 (941, 1955) | 0.65 |
| Adjusted EE0, kcal/15ha | 1375 ± 91 (1133, 1617) | 1337 ± 95 (1133, 1617) | 1413 ± 68 (1256, 1536) | <0.0001 |
| ∆ adjusted EE0during fastinga | –170 ± 75 (–390, 1) | –117 ± 48 (–204, 1) | –223 ± 58 (–390, –89) | <0.0001 |
| AFT, kcal/15h | 352 ± 109 (145, 695) | 353 ± 114 (172, 657) | 351 ± 105 (145, 695) | 0.95 |
| Adjusted AFT, kcal/15ha | 352 ± 84 (195, 601) | 340 ± 79 (217, 601) | 363 ± 87 (195, 539) | 0.15 |
| 24h Energy intake, kcal/day | 2115 ± 347 (1461, 2956) | 2102 ± 380 (1461, 2956) | 2127 ± 313 (1508, 2829) | 0.72 |
| SPA, % of time | 5.8 ± 3.2 (0.7, 16.4) | 6 ± 3.6 (0.7, 16.4) | 5.6 ± 2.8 (0.7, 15.5) | 0.58 |
| Night time SPA, % of time | 1.8 ± 1.6 (0.0, 9.7) | 1.6 ± 1.6 (0.0, 9.7) | 2.0 ± 1.9 (0.0, 8.1) | 0.17 |
| PAL, 24EE/SLEEP, ratio | 1.3 ± 0.1 (1.1, 1.5) | 1.3 ± 0.1 (1.1, 1.5) | 1.3 ± 0.1 (1.1, 1.4) | 0.85 |
| Energy expenditure measures during 24h fasting | ||||
| RQ ratio | 0.79 ± 0.03 (0.71, 0.90) | 0.79 ± 0.03 (0.73, 0.86) | 0.79 ± 0.03 (0.71, 0.90) | 0.96 |
| 24EE, kcal/day | 1903 ± 311 (1254, 2811) | 1935 ± 344 (1365, 2811) | 1871 ± 273 (1254, 2486) | 0.29 |
| Adjusted 24EE, kcal/daya | 1903 ± 123 (1633, 2315) | 1914 ± 193 (1633, 2315) | 1892 ± 105 (1654, 2184) | 0.35 |
| 24EE/FFM, kcal/day/kg | 31.9 ± 3.0 (24.9, 40.2) | 31.8 ± 3.4 (24.9, 40.2) | 31.9 ± 2.7 (26.5, 40.2) | 0.87 |
| SLEEP (kcal/day) | 1622 ± 258 (1110, 2382) | 1644 ± 266 (1129, 2382) | 1601 ± 250 (1110, 2373) | 0.39 |
| Adjusted SLEEP, kcal/daya | 1622 ± 121 (1364, 2087) | 1627 ± 122 (1370, 1955) | 1618 ± 122 (1364, 2087) | 0.69 |
| EE0, kcal/15h | 1205 ± 200 (809, 1787) | 1234 ± 227 (854, 1787) | 1176 ± 165 (809, 1602) | 0.14 |
| Adjusted EE0, kcal/15ha | 1205 ± 85 (1012, 1426) | 1219 ± 95 (1012, 1426) | 1191 ± 70 (1026, 1359) | 0.08 |
| ANFT, kcal/15h | 194 ± 95 (0, 493) | 210 ± 102 (0, 493) | 177 ± 85 (0, 353) | 0.07 |
| Adjusted ANFT, kcal/15ha | 194 ± 82 (-77, 395) | 206 ± 77 (41, 395) | 182 ± 86 (-77, 346) | 0.12 |
| SPA, % of time | 5.3 ± 3.1 (0.4, 18.3) | 5.2 ± 3.2 (0.8, 18.3) | 5.4 ± 3.1 (0.4, 12.7) | 0.75 |
| Night time SPA, % of time | 1.8 ± 1.6 (0.0, 7.5) | 1.8 ± 1.6 (0.0, 7.0) | 1.8 ± 1.5 (0.0, 7.5) | 0.93 |
| PAL, 24EE/SLEEP, ratio | 1.2 ± 0.1 (1.0, 1.4) | 1.2 ± 0.1 (1.0, 1.3) | 1.2 ± 0.1 (1.0, 1.4) | 0.77 |
Values are presented as mean ± SD for continuous variables or number (frequency) for categorical variables with minimum and maximum in parentheses. Skewed values are also expressed as medians with interquartile ranges (*). Classification of subjects into spendthrift or thrifty metabolic group was based on the median value of change (∆) in adjusted 24EE from energy balance during fasting (−162 kcal/day), such that thrifty subjects were those with a greater-than-median decrease in 24EE.
Significance between thrifty and spendthrift metabolic groups was determined by Student’s unpaired t-test. Significant differences are highlighted in bold.
aAdjusted energy expenditure values were calculated by adding the average value calculated in the whole cohort to the residual values obtained from linear regression models with covariates age, sex, ethnicity, FM, FFM, spontaneous physical activity, ambient temperature, and calorimeter room.
Abbreviations: 24EE, 24h energy expenditure; AFT, awake-fed thermogenesis; ANFT, awake-nonfed thermogenesis; BMI, body mass index; EE0, nonactivity energy expenditure; FM, fat mass; FFM, fat-free mass; OGTT, oral glucose tolerance test; PAL, physical activity level; RQ, respiratory quotient; SLEEP, sleeping energy expenditure; SPA, spontaneous physical activity.
Figure 2.
Close association between 24h food intake and 24EE during energy balance inside the calorimeter. The slope of the regression line is 0.99 (95% CI, 0.93, 1.04) and its X- and Y-intercepts are 8 (CI, −123, 125) and −8 (CI, −130, 114), respectively. Abbreviations: 24EE, 24h energy expenditure; CI, confidence interval.
Relationships between 24EE measures during energy balance and acute fasting conditions
Adjusted 24EE during ENBAL (24EEENBAL) and during 24h fasting (24EEFASTING) were closely related (r = 0.75; P < 0.0001) (Fig. 3A). Except for 4 participants who increased their adjusted 24EE during fasting, all data points were below the identity line, demonstrating that 24EE decreased in nearly all subjects during 24h fasting (Fig. 3A). However, the slope of the regression line was 0.67 (CI, 0.56–0.78) indicating a proportional effect for the decrease in 24EE from ENBAL to fasting. Specifically, subjects with higher adjusted 24EEENBAL decreased 24EE more during 24h fasting (ie, thriftier subjects) compared to subjects with lower 24EEENBAL. This was also confirmed when expressing the absolute fasting-induced change in metabolic rate as percentage of 24EE during ENBAL: subjects with a greater percentage decrease in 24EE during fasting (ie, thriftier subjects) had a higher 24EEENBAL (r = −0.36; P = 0.0002, Supplemental Fig. 3A) (16) but similar 24EEFASTING (P = 0.06, Supplemental Fig. 3C) (16) compared to subjects with smaller percent decrease (ie, more spendthrift subjects).
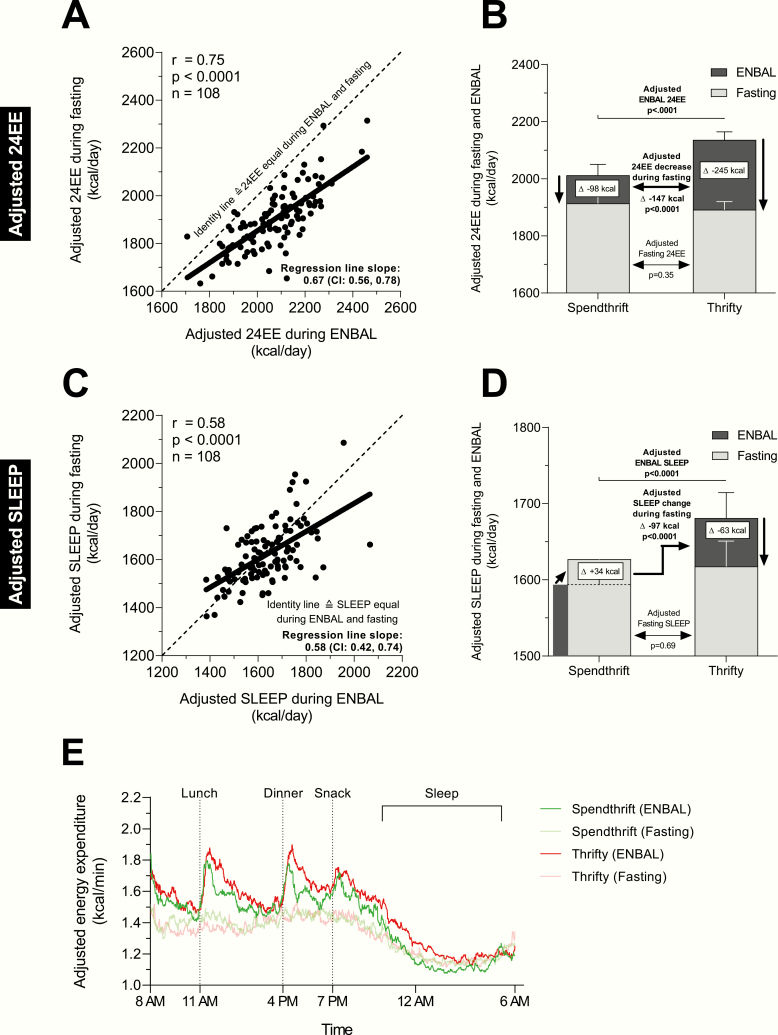
Figure 3.
Relatively higher EE during energy balance (thrifty phenotype) is associated with greater decrease in EE during 24h fasting. Relationships between adjusted 24EE (panel A) and adjusted SLEEP (panel C) during ENBAL (x-axis) and during fasting (y-axis). Adjusted 24EE and SLEEP values were calculated via linear regression models including age, sex, ethnicity, fat mass (FM), fat-free mass (FFM), spontaneous physical activity (SPA) (only 24EE analyses), ambient temperature, and calorimeter room (Room 1 or 2) as covariates in the regression models. Comparison of changes in adjusted 24EE (panel B) and in adjusted SLEEP (panel D) from ENBAL during 24h fasting between thrifty and spendthrift subjects. Classification of subjects as either spendthrift or thrifty is based on the median value of the adjusted decrease in 24EE from ENBAL during 24h fasting, such that thrifty subjects were identified as those with a greater-than-median decrease in adjusted 24EE (< −162 kcal/day). Black bars denote the average 24EE or SLEEP during ENBAL while grey bars denote the average 24EE or SLEEP during 24h fasting. The distance between the black and the grey bars denotes the fasting-induced changes in 24EE or SLEEP. Error bars denote the 95% confidence interval. Statistical significance between metabolic groups was determined by Student unpaired t tests. Average 24h time courses of minute-by-minute energy expenditure in thrifty and spendthrift subjects during ENBAL and during 24h fasting (panel E). Minute-by-minute adjusted energy expenditure values of each time course were calculated from a linear mixed model to account for repeated measures over the 24h with covariates age, sex, ethnicity, FM, FFM, SPA, ambient temperature, and calorimeter room. Four subjects had a 24EE during fasting greater than that during ENBAL. In this case, we substituted the 24EE values during FST of these 4 subjects with their corresponding 24EE value during ENBAL to avoid positive, nonphysiological values for the decrease in 24EE during fasting. In a sensitivity analysis, similar results were obtained after removing these 4 subjects from the analysis. In the scatterplot shown in panel C, 3 subjects can be considered outliers as they have a very high adjusted SLEEP during ENBAL but a very low adjusted SLEEP during fasting. In a sensitivity analysis, similar results were obtained after removing these 3 subjects from the analysis (slope: 0.73; 95% CI, 0.56–0.90). Abbreviations: 24EE, 24h energy expenditure; CI, confidence interval; EE, energy expenditure; ENBAL, energy balance; SLEEP, sleeping metabolic rate.
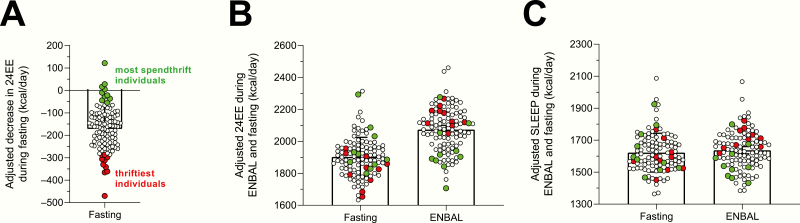
For illustrative purposes and to confirm our results obtained using continuous 24EE data, we also performed a group-wise comparison of thrifty vs spendthrift subjects (Fig. 3B) as arbitrarily defined by the median decrease (−162 kcal/day) in adjusted 24EE from ENBAL during 24h fasting (Table 1). According to this arbitrary definition, on average thrifty subjects decreased 24EE by ~11% while spendthrift subjects decreased it by only ~4% (Fig. 3B). In absolute terms, thrifty subjects decreased 24EE by ~147 kcal/day more than spendthrift subjects (CI, 124–168; P < 0.0001) and had a higher 24EEENBAL (+124 kcal/day, CI, 77–171; P < 0.0001) but similar 24EEFASTING (P = 0.35) compared to spendthrift subjects (Table 1). The analysis of 24h time courses of adjusted EE revealed that thrifty subjects had on average a relatively higher metabolic rate following meals and overnight during ENBAL compared to spendthrift subjects, despite similar kinetics during fasting conditions (Fig. 3E). Furthermore, we also arbitrarily analyzed data from the extremes of the continuous data distribution, namely, the 10 thriftiest vs the 10 most spendthrift subjects based on the largest/smallest decrease in adjusted 24EE during 24h fasting in our cohort. Nearly all the thriftiest subjects, who decreased their adjusted 24EE by an average of 336 kcal/day during 24h fasting (Fig. 4A), had greater-than-mean adjusted 24EE during ENBAL (Fig. 4B), whereas the most spendthrift subjects, who on average decreased their adjusted 24EE by only 2 kcal/day, overall had a lower-than-mean adjusted 24EE during ENBAL.
Figure 4.
Adjusted values of 24h energy expenditure and sleeping metabolic rate of the top 10 thriftiest and top 10 most spendthrift subjects during ENBAL and 24h fasting. Classification of the 10 thriftiest / 10 most spendthrift subjects was based on the decrease in adjusted 24EE from ENBAL during 24h fasting, such that the 10 thriftiest subjects were those who had the greatest decrease in adjusted 24EE during 24h fasting, whereas the 10 most spendthrift subjects were those who had the smallest decrease (or increase) in adjusted 24EE during fasting (panel A). Adjusted 24EE (or SLEEP) values were calculated by adding the average 24EE (or SLEEP) value calculated in the whole cohort to the residual values obtained from a linear regression model with covariates age, sex, ethnicity, fat mass, fat-free mass, spontaneous physical activity (only 24EE), ambient temperature, and calorimeter room. Abbreviations: 24EE, 24-h energy expenditure; ENBAL, energy balance; SLEEP, sleeping metabolic rate.
We also expressed 24EE per kg of FFM (24EE/FFM ratio) to gain further insight into the differences in 24EE between thrifty/spendthrift phenotypes. During energy balance, thrifty subjects had an average higher 24EE/FFM ratio than spendthrift subjects (35.7 vs 33.7 kcal/kg; P = 0.003) (Table 1) while no difference was found during fasting conditions (P = 0.87). To confirm this finding and overcome potential bias due to the use of ratios, we performed linear regression analyses of unadjusted 24EE using FFM and metabolic group (thrifty/spendthrift) as independent variables. Again, we found that thrifty subjects had a relatively higher 24EE at any level of FFM compared to spendthrift subjects (+101 kcal/day, 95% CI, +29, +173; P = 0.006) (Fig. 5A), while there was no such difference during 24h fasting (P = 0.67) (Fig. 5B). Similar results (+135 kcal/day, 95% CI, +84, +186; P < 0.0001) were obtained when including all determinants of 24EE (ie, FM, age, sex, etc.) to the regression model (described in the “Research Design and Methods” section).
Figure 5.
Thrifty subjects have a higher 24EE at any level of fat-free mass than spendthrift subjects Relationships between fat-free mass and 24EE during (A) 24h energy balance and during (B) 24h fasting, separated by metabolic groups. Thrifty subjects are denoted in red, spendthrift subjects are denoted in green. In a linear regression analysis including the phenotypic group (thrifty vs spendthrift) and fat-free mass as determinants, thrifty subjects had higher 24EE than spendthrift subjects at any level of fat-free mass during energy balance (+101 kcal/day, 95% CI, +29, +173; P = 0.006) but not during fasting conditions (P = 0.67). Classification of subjects as either spendthrift or thrifty is based on the median value of the adjusted decrease in 24EE from ENBAL during 24h fasting, such that thrifty subjects were identified as those with a greater-than-median decrease in adjusted 24EE (< −162 kcal/day). Abbreviations: 24EE, 24-h energy expenditure.
There were no associations between the fasting-induced decrease in adjusted 24EE and age, body mass index (BMI), and percentage body fat (all P > 0.67) nor any significant interactions (all P-interaction > 0.82), indicating that thriftiness was independent of these demographic and anthropometric factors.
Relationships between SLEEP measures during energy balance and acute fasting conditions
Adjusted SLEEP during ENBAL (SLEEPENBAL) and during 24h fasting (SLEEPFASTING) were also related with each other (r = 0.58; P < 0.0001, Fig. 3C), but not all data points were below the identity line, indicating a relatively increased SLEEP in some subjects during 24h fasting (Fig. 3C). The slope of the linear regression line was 0.58 (CI, 0.42–0.74) indicating a proportional effect for the change in SLEEP, that is, subjects with relatively higher SLEEPENBAL decreased SLEEP more during 24h fasting compared with subjects with relatively lower SLEEPENBAL. Similar results were obtained when expressing absolute changes in SLEEP as percentage of SLEEPENBAL (Supplemental Fig. 3B–D) (16).
As done for 24EE, we confirmed these results using continuous SLEEP data by comparing the 2 metabolic groups (Fig. 3D). Specifically, spendthrift individuals increased SLEEP by ~2% or 34 kcal/day (CI, 9–58; P = 0.008, Fig. 3D) while thrifty individuals decreased it by ~4% or 63 kcal/day (CI, –94 to –33; P = 0.0001), thus the average difference between groups was –97 kcal/day (CI, –135 to –59; P < 0.0001). Thrifty subjects had a relatively higher SLEEPENBAL (+87 kcal/day, CI, 45–130; P < 0.0001, Table 1) but similar SLEEPFASTING compared with spendthrift subjects (P = 0.87, Table 1). When analyzing the ten thriftiest and 10 most spendthrift subjects, we found that nearly all the thriftiest subjects (Fig. 4A) had a greater-than-mean adjusted SLEEP during ENBAL (Fig. 4C), while the most spendthrift subjects had overall a lower adjusted SLEEP during ENBAL.
The associations between 24EE/SLEEP during ENBAL and fasting were similar after additional adjustment for measures of insulin sensitivity index obtained from OGTT values.
We also analyzed the relationships between adjusted EE0 and AFT during both ENBAL and 24h fasting conditions and found overall similar results to what we reported for 24EE and SLEEP (Supplemental Fig. 4) (16).
Hormonal changes and their relationships with EE changes during 24h fasting
Compared to ENBAL, urinary norepinephrine decreased on average by ~14% or 4.8 μg/24h (P = 0.003) while urinary epinephrine increased by ~36% or 1.5 μg/24h (P < 0.0001) during fasting (Supplemental Fig. 5) (16). After 24h fasting, on average plasma FGF21 concentration decreased by ~34% or 57 pg/mL (P < 0.0001) and leptin decreased by ~70% or 10.9 ng/mL (P < 0.0001)(Table 2).
Table 2.
Hormonal Measurements During Energy Balance and Fasting Conditions
| Energy balance | Fasting | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Absolute Change | Percent Change | P value | Pre | Post | Absolute Change | Percent Change | P value | |
| FGF21 (pg/mL) | 143.9 ± 112.6 | 117.1 ± 80.6 | –26.9 ± 70.7 | –18.7 ± 62.8 | 0.0003 | 167.7 ± 115.2 | 110.5 ± 72.1 | –57.2 ± 96.6 | –34.1 ± 83.9 | <0.0001 |
| Leptin (ng/mL) | 8.2 (6.4, 10.4) | 6.3 (4.9, 8.1) | –2.9 ± 4.0 | –23.1 ± 25.2 | <0.0001 | 8.8 (6.9, 11.2) | 2.6 (2.0, 3.4) | –10.9 ± 16.8 | –69.5 ± 57.7 | <0.0001 |
| Urinary epinephrine (μg/24h) a | - | 3.7 (3.3, 4.1) | - | - | - | - | 5.0 (4.5, 5.6) | 1.5 ± 2.6 | 35.7 ± 101.2 | <0.0001 |
| Urinary norepinephrine (μg/24h) a | - | 25.5 (23.0, 28.2) | - | - | - | - | 22.0 (20.1, 24.2) | –4.8 ± 18.0 | –13.7 ± 63.1 | 0.003 |
Pre/Post values are shown as mean ± SD (FGF21) or as geometric mean with 95%CI in parentheses (leptin, urinary epinephrine, urinary norepinephrine). Absolute and percent changes are shown as mean ± SD with 95% CI in parentheses.
aUrinary epinephrine and norepinephrine concentrations during 24h fasting conditions were compared to their respective 24h concentrations during energy balance. Hormonal data available from n = 94 (FGF21), n = 87 (leptin), and n = 101 subjects (urinary epinephrine and norepinephrine). Statistical significance determined by Student paired t test. Statistically significant results are highlighted in bold.
Abbreviations: CI, confidence interval; FGF21, fibroblast growth factor 21; SD standard deviation
A greater increase in urinary epinephrine during 24h fasting was associated with less decrease in adjusted 24EE (r = 0.27; P = 0.006, Fig. 6A). When analyzing the 2 metabolic groups, on average spendthrift individuals increased epinephrine concentrations during fasting by 2.6 µg/24h (CI, 1.7–3.4; P < 0.0001, Fig. 6B), which was 4.3-fold or 2.0 µg/24h (CI, 1.0–2.9; P < 0.0001) higher than thrifty individuals who increased epinephrine concentrations by only 0.6 µg/24h (CI, 0.04–1.2; P = 0.04). The fasting-induced increase in epinephrine also correlated with concomitant changes in adjusted EE0 and SLEEP (r = 0.24; P = 0.02; and r = 0.24; P = 0.01; Supplemental Fig. 8A and B, respectively (16)) during 24h fasting, but not with concomitant changes in ANFT (P = 0.9) (Supplemental Fig. 6C) (16).
Figure 6.
A greater decrease in 24EE during 24h fasting is associated with less increase in urinary epinephrine excretion rate. Association of fasting-induced changes in (panel A) 24h urinary epinephrine excretion rate, (panel C) plasma leptin, and (panel D) plasma FGF21 concentrations with the concomitant decrease in adjusted 24EE during fasting. Comparison of fasting-induced changes in (panel B) 24h urinary epinephrine excretion rate, (panel D) plasma leptin, and (panel F) plasma FGF21 between thrifty and spendthrift subjects. Classification of subjects as spendthrift or thrifty is based on the median value of adjusted decrease in 24EE from ENBAL during fasting, such that thrifty subjects were those with a greater-than-median decrease in 24EE. Adjusted 24EE values were calculated by adding the average 24EE value calculated in the whole cohort to the residual values obtained from a linear regression model with covariates age, sex, ethnicity, FM, FFM, spontaneous physical activity, ambient temperature, and calorimeter room. In the bar charts, black bars denote mean hormonal concentration during ENBAL, and grey bars denote mean hormonal concentration during 24h fasting. Error bars denote the 95% confidence interval of the respective mean. Abbreviations: 24EE, 24h energy expenditure; FGF21, fibroblast growth factor 21.
A greater decrease in plasma leptin concentration after 24h fasting tended to be associated with concomitant greater decrease in adjusted 24EE (r = 0.19; P = 0.07) (Fig. 6C). On average, thrifty individuals decreased leptin concentrations by 72% (CI, −76 to −67; P < 0.0001) (Fig. 6D) and this decrease was 1.22-fold greater (CI, 1.01–1.48; P = 0.04) than that of spendthrift individuals who decreased leptin by only 66% (CI, −70 to −62; P < 0.0001). The fasting-induced decrease in leptin also correlated with the concomitant decrease in adjusted EE0 (r = 0.21; P = 0.05) (Supplemental Fig. 6D), but not SLEEP (P = 0.27) (Supplemental Fig. 6E) and ANFT (P = 0.36) (Supplemental Fig. 6F) (16).
A greater decrease in plasma FGF21 following 24h fasting was not associated with the concomitant decrease in adjusted 24EE (P = 0.49) (Fig. 6E) with both thrifty and spendthrift groups decreasing to the same extent (P = 0.25) (Fig. 6F). However, a lesser decrease in FGF21 after 24h fasting was associated with a lesser concomitant decrease in adjusted SLEEP (r = 0.38; P = 0.0002) (Fig. 7A) and ANFT (r = −0.37; P = 0.0003) (Supplemental Fig. 6H) (16), but not with EE0 (P = 0.43) (Supplemental Fig. 6G) (16).
Figure 7.
Less decrease in plasma FGF21 concentration after 24h fasting is associated with less decrease in SLEEP and greater urinary epinephrine excretion rate. The y-intercept of the regression line of panel A is 11 (95% confidence interval, −15 to 36). Abbreviations: FGF21, fibroblast growth factor 21; SLEEP, sleeping energy expenditure.
A greater fasting-induced decrease in FGF21 was associated with a greater concomitant decrease in epinephrine (r = 0.28; P = 0.006, Fig. 7B). In a multivariate analysis including both fasting-induced changes in epinephrine and FGF21 as predictors, only FGF21 was independently associated with the change in adjusted SLEEP during 24h fasting (P = 0.0002) while epinephrine was no longer significant (P = 0.23).
No associations were found between the decrease in norepinephrine from ENBAL during 24h fasting and changes in EE measures (all P > 0.11). Hormonal results were similar when considering the absolute difference in unadjusted EE measures between conditions (data not shown).
Discussion
The thrifty phenotype hypothesis claims that some individuals decrease EE to a greater extent during prolonged periods of food restriction to conserve energy stores. Based on our assessment of thriftiness, which is limited to the acute condition of 24h fasting, this concept remained true. However, the decline in 24EE (calculated as the difference between fasting and ENBAL conditions) was due not to relatively lower EE during fasting conditions but relatively higher EE during ENBAL. Specifically, thrifty subjects had a greater decrease in 24EE during fasting because of higher EE requirements during physiologic, eucaloric conditions and they showed greater decreases both in daytime nonactivity thermogenesis and SLEEP during acute fasting. Conversely, spendthrift individuals had relatively lower 24EE during isocaloric conditions and showed smaller decreases both in 24EE and in daytime thermogenesis during 24h fasting, which was accompanied by a slight increase in SLEEP (Fig. 8). Further, we investigated thermogenic hormones as potential physiological mediators of the observed EE changes during short-term fasting. We found that a smaller increase in urinary epinephrine and, to a lesser extent, a greater decrease in plasma leptin following 24h fasting were associated with a greater decrease in adjusted 24EE during 24h fasting (ie, thrifty phenotype), while a smaller decrease in plasma FGF21 concentration was associated with a smaller decrease in adjusted SLEEP during 24h fasting.
Figure 8.
Redefined concept of the human thrifty and spendthrift phenotypes, where the greater decrease in adjusted 24EE in thrifty subjects is a result of higher EE requirements during energy balance and characterized by greater decreases both in daytime thermogenesis (red bold arrows) and in sleeping metabolic rate (blue bold arrows). Conversely, spendthrift subjects decrease 24EE less during fasting as they are able to increase their sleeping metabolic rate while also showing smaller decrease in daytime thermogenesis. The fasting-induced changes in sleeping metabolic rate might be mediated by a hormonal pathway including FGF21 and epinephrine (light blue arrows). Abbreviations: 24EE, 24h energy expenditure; FGF21, fibroblast growth factor 21.
Thrifty subjects have a relatively higher energy expenditure during isocaloric conditions
Our study is unique in its careful assessment of 24EE and its components both during 24h fasting and during ENBAL (average deviation from exact energy balance ~2%), which allowed us to thoroughly phenotype subjects with respect to their changes in energy metabolism during acute fasting. The importance of these phenotypes, revealed by the extent of short-term decrease in daily EE during acute fasting, has been validated in studies that show their predictive role in weight change both in highly controlled inpatient settings (5, 26) and in free-living conditions (4). Our current results recharacterize the proposed phenotypes showing that, counterintuitively, thrifty individuals had relatively higher 24EE, EE0, and SLEEP during isocaloric conditions compared to spendthrift individuals after adjustment for differences in body composition and other known EE determinants. We demonstrated these results using continuous EE data in correlation analyses (eg, Fig. 3A–C) and, subsequently, confirmed by grouping data for visualization purposes (eg, Fig. 3B–2D).
This paradoxical feature of these phenotypes was unexpected, as thriftier individuals are believed to be more “energy efficient” and, as such, should have a lower (instead of higher) rate of EE in any dietary condition. Yet, 24EE during isocaloric conditions was relatively higher (instead of lower) in thriftier individuals and, although 24EE decreased more during acute fasting (as expected by their increased thriftiness), they had similar 24EE, EE0, and SLEEP during acute fasting compared to spendthrift individuals (Fig. 8 and Table 1). These results demonstrate that the greater extent of the decrease in 24EE of thrifty subjects during 24h fasting arises from their higher 24EE during ENBAL rather than a lower 24EE during fasting as it was originally hypothesized (Fig. 1). Importantly, this new characterization of metabolic phenotypes does not invalidate our previous studies based on the short-term change in 24EE during fasting, which still defines the metabolic phenotype (ie, thrifty individuals show greater decrease in 24EE during acute fasting).
Our results indicate that the physiologic state of feeding is responsible for the metabolic differences between thrifty vs spendthrift phenotypes. Thus, as the next steps, it may be possible to identify the biological/hormonal mediators that determine the higher eucaloric requirements in thrifty subjects, such that clinical/pharmacological interventions can be devised to convert a thriftier metabolism into a more spendthrift one. This may be of clinical relevance as the higher eucaloric EE level of thrifty subjects might increase their risk for accelerated aging and early mortality (27, 28).
Herein, we also demonstrate for the first time that the greater decrease in 24EE during 24h fasting in thrifty individuals is due to greater decreases both in AFT (an index of daytime thermogenesis and a proxy for diet-induced thermogenesis) and SLEEP. Conversely, the smaller decrease in 24EE during 24h fasting in spendthrift individuals is accounted for smaller decreases in AFT along with increased SLEEP, which attenuated their overall decrease in 24EE. Thus, the greater fasting-induced decrease in 24EE observed in thrifty compared to spendthrift subjects arises from a greater decrease in both daytime thermogenesis and SLEEP (Fig. 8).
Relatively higher 24EE in thrifty subjects may have an important role in the context of energy homeostasis and daily energy balance. Several studies reported that, in humans, higher resting metabolic rate (RMR) is a predictor of greater habitual food intake (29–31) and that increased eucaloric 24EE in the confined environment of a whole-room calorimeter (32–34) is a driver of greater food intake and overeating (32–34). The causal link between energy expenditure and intake may reflect physiological energy-sensing mechanisms (2, 35, 36) that might constitute one of the determinants of long-term weight gain (33). Notably, especially in studies employing whole-body calorimetry (32, 33), the EE and intake measurements are often obtained in conditions of low energy turnover (~2200–2400 kcal/day), as there are limited physical activity levels and reduced intake compared to outside the calorimeter. According to the framework of energy turnover (37), low energy turnover is associated with poor control of eating and higher body weight. If those we identified as thrifty based on higher 24EE remain in a low-energy-turnover state during free-living conditions, we hypothesize that this leads to increased energy intake driven by their higher 24EE. Consistent with this hypothesis, in a previous study of our group, higher eucaloric 24EE in the confined environment of a whole-room calorimeter was associated with greater ad libitum energy intake and overeating using a vending machine paradigm (32). This increased drive to overeating presumably due to energy sensing might be mediated by altered eating behavior such as lower cognitive restraint, higher dietary disinhibition, and greater susceptibility to hunger cues (38).
We acknowledge that we cannot extend our findings to free-living conditions where energy turnover may be higher due to higher physical activity and higher food intake, which might ultimately be more beneficial with regard to weight gain; for example, higher eucaloric energy turnover (~3200 kcal/day) has been linked to better appetite control (39), improved postprandial glucose and insulin regulation (40), and improved fat balance (41). However, we propose that thrifty individuals who maintain a low energy turnover also in free-living conditions might be at greater risk for weight gain than spendthrift individuals. Therefore, as a clinical implication, it may be beneficial to encourage thrifty individuals to increase their physical activity levels (thus, their energy turnover) to avoid future weight gain and adverse metabolic outcomes.
The higher eucaloric 24EE in thrifty subjects compared to conditions of energy deficit (ie, fasting) might also be relevant in the context of energy surplus (ie, overfeeding). In fact, we have shown in previous studies that thriftiness is not only observed during short-term fasting and acute energy deficit but also during conditions of positive energy balance such as short-term overfeeding (1, 4). That is, thriftier subjects (defined by a greater decrease in 24EE during fasting) show smaller increases in 24EE in response to 200% overfeeding (1, 4). Interestingly, a greater decrease in 24EE during short-term fasting and less increase in 24EE during short-term low-protein overfeeding predict greater future weight gain in free-living conditions and after 6 weeks of controlled low-protein overfeeding, respectively (4, 6).
In light of the new findings presented in this study, we hypothesize that the relatively higher 24EE observed in thrifty subjects during eucaloric conditions cannot only explain their greater decrease in 24EE during fasting but also their blunted increase in 24EE during short-term overfeeding and therefore constitute an important metabolic trait of the thrifty phenotype mediating the adaptive EE responses during fasting and overfeeding. We are currently investigating this question and will present our findings in a separate manuscript.
Additionally, the greater decrease in 24EE (thriftier metabolism) in acute conditions of fasting may explain the resistance to losing weight in conditions of negative energy balance due to increased metabolic efficiency and energy saving (5).
Changes in epinephrine are associated with the decrease in 24EE during acute fasting
Previous studies showed that short- and long-term fasting lead to a reduction in 24EE (20, 42). However, the hormonal mediators underlying the greater decrease in 24EE characterizing the metabolic phenotype during fasting have not been fully identified. Thyroid hormones would seem to be logical hormonal mediators but previous studies have not indicated so (43, 44). In the present study, we found that epinephrine increased more in spendthrift subjects during 24h fasting; thus, it may constitute a hormonal mediator of the fasting-induced changes in 24EE.
The release of catecholamines is a major part of the hormonal response to acute stress. Interestingly, during fasting-stress, seminal studies from Landsberg, Macdonald, Young, and others show that the sympathetic nervous system decreases central sympathetic activity by lowering norepinephrine secretion while, at the same time, independently amplifying sympathoadrenal activity by increasing epinephrine secretion (45–47). However, some studies also reported no change (48, 49) or even a decrease (50) in epinephrine during fasting. In this present study, we found similar results to what Landsberg, Macdonald, and Young reported, that is, decreased urinary norepinephrine excretion but increased urinary epinephrine excretion during 24h fasting. The reason for the “sympathetic dissociation” during fasting could be that energy dissipation needs to be minimized (lower norepinephrine) while glucose and lipids need to be mobilized to ensure survival (higher epinephrine) (45). The fasting-induced decrease in blood glucose levels may directly trigger the release of epinephrine from the adrenal medulla (51) which then stimulates lipolysis, hepatic glucose production, and pancreatic glucagon secretion (51–54). All these processes are known to alter whole-body energy metabolism. Accordingly, intravenous epinephrine infusion increases metabolic rate in humans (55, 56), especially during fasting conditions (57). In line with these findings, we found that a greater increase in epinephrine during fasting was related to a lesser concomitant decrease in 24EE, indicating that epinephrine might be a hormonal mediator differentiating the thrifty vs spendthrift phenotype. In agreement with our previous report (11), spendthrift subjects had higher epinephrine concentration during 24h fasting as a result of an average 4.3-fold greater increase in epinephrine concentration compared with thrifty subjects, whereas during ENBAL, epinephrine concentrations were similar between thrifty and spendthrift individuals. Higher sympathoadrenal activity indicates increased metabolic stress (45), therefore we speculate that a feature of the spendthrift phenotype could be constituted by a fasting-induced “stress response” which may counteract the suppression of central sympathetic activity by an increased adrenal epinephrine secretion that, in turn, may increase thermogenesis and attenuate the overall decrease in 24EE during fasting in spendthrift subjects. This finding also implicates that the modulation of epinephrine secretion in thrifty subjects might be another target for clinical interventions aiming to convert the thrifty into a more spendthrift phenotype.
We also assessed whether leptin may have a role in the extent of 24EE decrease during short-term fasting. Leptin, a hormone that acts as a signal to the brain to inform about body energy stores, substantially decreased following 24h fasting, which qualifies and confirms leptin as a starvation signal (10). Leptin has been shown to increase EE through its effects on the cardiovascular system and brown adipose tissue thermogenesis in mice (9). Interestingly, plasma leptin concentration did decrease more during fasting in those thrifty vs spendthrift subjects. Thus, the greater fasting-induced decrease in leptin in thrifty subjects may determine, in part, the greater decrease in 24EE. However, we did not observe a significant correlation between decreases in 24EE and leptin (Fig. 6C) and previous studies demonstrated that leptin treatment during weight loss in humans did not substantially affect concomitant reductions in EE (8), advocating against clinically significant effects of leptin on the metabolic phenotype.
FGF21 and epinephrine are associated with the fasting-induced changes in sleeping metabolic rate
Interestingly, despite an overall decrease in 24EE, during fasting spendthrift subjects actually increased SLEEP—a surrogate marker for RMR—while SLEEP decreased in thrifty subjects. The existing literature on this matter is inconclusive: in some studies, fasting was associated with a decreased RMR as a mechanism to conserve energy (14, 58), while other studies have reported a fasting-induced increase in RMR by as much as to 14% (48, 57, 59, 60). In one study, this increase was associated with an increased plasma norepinephrine secretion (while plasma epinephrine levels did not change during starvation) (48). However, we found that the fasting-induced increase in epinephrine positively correlated with increased SLEEP during 24h fasting (Supplementary Fig. 6B) (16). Although previously shown in different studies (48, 57, 59, 60), the fact that some individuals relatively increase their SLEEP during 24h fasting may seem counterintuitive as this behavior does not align with the theory of energy conservation during famine. Because the changes in SLEEP and epinephrine during fasting were correlated, we hypothesize that the increase in SLEEP during fasting might be a sign of greater epinephrine-mediated substrate mobilization to ensure energy supply (45). In accordance with our hypothesis, the lipolytic and thermogenic effects of epinephrine are enhanced during fasting (57) which may be due to increased adrenoreceptor sensitivity (61). While we cannot rule out that night-to-night variations in SLEEP might also have influenced our data, we have found that SLEEP is reproducible during energy balance and fasting (20).
Additionally, we found that smaller decrease in plasma FGF21 concentration after 24h fasting was associated with lesser decrease in SLEEP. FGF21 is a liver-derived hormone implicated in the regulation of energy homeostasis (62), mainly via activation of uncoupling protein 1 in brown adipocytes but also via effects on the central nervous system (63). In mice, FGF21 is rapidly induced by fasting (64) while in humans, short-term (24-hour) fasting first leads to a decrease in FGF21 concentration which is then followed by an increase after longer periods of starvation (14, 15). However, in humans, its physiologic role during fasting conditions is still unclear (14). Fazeli et al did not find an association between fasting-induced changes in RMR (measured by a ventilated-hood system) and concomitant changes in FGF21 (14). Conversely, our present data indicate a role for FGF21 in the regulation of EE during sleep. In fact, in those subjects whose SLEEP tended to increase during fasting (more spendthrift), it is notable that FGF21 proportionally increased as well, while thrifty subjects with greater decrease in SLEEP also had a greater decrease in FGF21 (Fig. 7A). Interestingly, the fasting-induced changes in FGF21 and in epinephrine were correlated (Fig. 7B). Thus, FGF21 and epinephrine may share a common regulating pathway regarding their potential effects on SLEEP during fasting. Consistent with the hypothesis of fasting-induced epinephrine secretion as a sign of stress, the concomitant increase in FGF21 in some subjects could also be considered as a metabolic response to “nutrient stress” (65). In this context, it is tempting to speculate that targeted exogenous FGF21 therapy might convert a thriftier into a more spendthrift metabolism and promote weight loss. Interestingly, some clinical trials testing FGF21 treatment already showed beneficial effects on weight loss (66, 67), but new studies are warranted to explore the weight loss mediating effects of FGF21 therapy by assessing concomitant changes in EE and food intake.
Limitations
Our study has limitations. This is a cross-sectional analysis, thus, causality of the associations between epinephrine, FGF21, leptin, and EE measures could not be determined.
Further, urinary catecholamines are spillover measures and may not represent the “real” end-organ effects of the sympathetic nervous system activity during acute fasting.
Further, we did not have measures of physical activity levels in free-living conditions, nor did we assess physical fitness in our inpatient study, the latter being a potential determinant of the metabolic phenotype as higher 24EE during ENBAL is associated with greater physical fitness (68). However, we assessed SPA inside the whole-room calorimeter which is an indicator of free-living PAL (24). As we adjusted our energy expenditure measures for SPA, this adjustment might indirectly control for free-living PAL.
We only admitted weight-stable subjects to this study. However, our arbitrary definition of weight stability was less than 10% weight change in the last 6 months which would be ~14 kg in the case of the heaviest individual of our study. Although we asked subjects whether they recently attempted a weight loss diet, we cannot exclude that we admitted subjects that lost or gained a high absolute amount of weight before being admitted to the study. This could have influenced our results as previous studies reported that weight-loss induced metabolic adaptations can persist for periods greater than 6 months (69). Yet, we conducted our EE assessments after a weight-stabilization run-in period of at least 5 days to achieve a short-term period of weight stability to minimize metabolic adaptations to previous weight change.
In our study, the eucaloric session (ie, energy balance) always preceded the fasting session, which may have influenced our results as subjects (1) were already used to the confined environment of the metabolic chamber during the fasting session, and (2) were on our metabolic ward longer prior to the fasting session.
Conclusion
By measuring short-term changes in energy metabolism under controlled conditions of ENBAL and acute fasting, our lab has established thrifty vs spendthrift phenotypes that quantify the individual susceptibility to weight change. The present results provide crucial new insights into these phenotypes in multiple ways: (1) The greater decrease in 24EE during fasting—which characterizes thrifty subjects—is due to higher EE requirements during eucaloric conditions rather than lower EE requirements during acute fasting as previously believed. (2) Changes in SLEEP during fasting were in opposing directions in thrifty and spendthrift individuals, with the former decreasing SLEEP and the latter actually increasing SLEEP during fasting. (3) Epinephrine and FGF21 might constitute hormonal mediators of these fasting-induced changes in 24EE and SLEEP. Our current results recharacterize the thrifty phenotype and identify potential mediators that govern the acute metabolic response to 24h fasting which defines this phenotype. A better understanding of the metabolic differences among individuals will improve behavioral and pharmacological interventions to control body weight and prevent obesity.
Acknowledgments
The authors thank the dietary, nursing, and technical staff of the National Institutes of Health Clinical Unit in Phoenix, AZ, for their assistance. Most of all, the authors thank the volunteers for their participation in the study.
Glossary
Abbreviations
- 24EE
24h energy expenditure
- 24EEENBAL
24h energy expenditure during energy balance
- 24EEFASTING
24h energy expenditure during fasting
- 24h
24-hour
- AFT
awake and fed thermogenesis
- ANFT
awake-nonfed thermogenesis
- BMI
body mass index
- CI
confidence interval
- CV
coefficient of variation
- DXA
dual-energy x-ray absorptiometry
- EE
energy expenditure
- EE0
nonactivity energy expenditure
- ENBAL
energy balance
- FFM
fat-free mass
- FGF21
fibroblast growth factor 21
- FM
fat mass
- PAL
physical activity level
- RQ
respiratory quotient
- RMR
resting metabolic rate
- SPA
spontaneous physical activity
- SLEEP
sleeping metabolic rate
- WMD
weight-maintaining diet
Financial Support: This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health. P.P. was supported by the program “Rita Levi Montalcini for young researchers” from the Italian Minister of Education and Research.
Author Contributions: Dr. Hollstein carried out the initial analyses, interpreted the results, wrote the manuscript and approved the final manuscript as submitted. Dr. Basolo interpreted the results and approved the final manuscript as submitted. Dr. Ando interpreted the results and approved the final manuscript as submitted. Dr. Krakoff interpreted the results, edited the initial draft of the manuscript, and approved the final manuscript as submitted. Dr. Votruba designed the study, interpreted the results and approved the final manuscript as submitted. Dr. Walter interpreted the results and approved the final manuscript as submitted. Dr. Piaggi designed the study, interpreted the results, edited the manuscript, and approved the final manuscript as submitted. Dr. Piaggi is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information
Disclosure Summary: The authors have nothing to disclose and declare no conflict of interest.
References
- 1. Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes Relat Metab Disord. 2001;25(5):593–600. [DOI] [PubMed] [Google Scholar]
- 2. Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J Endocrinol Invest. 2018;41(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piaggi P. Metabolic determinants of weight gain in humans. Obesity (Silver Spring). 2019;27(5):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schlögl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Diabetes. 2015;64(11):3680–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reinhardt M, Thearle MS, Ibrahim M, et al. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes. 2015;64(8):2859–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hollstein T, Ando T, Basolo A, Krakoff J, Votruba SB, Piaggi P. Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: further evidence for spendthrift and thrifty metabolic phenotypes. Am J Clin Nutr. 2019;110(3):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reinhardt M, Schlögl M, Bonfiglio S, Votruba SB, Krakoff J, Thearle MS. Lower core body temperature and greater body fat are components of a human thrifty phenotype. Int J Obes (Lond). 2016;40(5):754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223(1):T83–T96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandit R, Beerens S, Adan RAH. Role of leptin in energy expenditure: the hypothalamic perspective. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R938–R947. [DOI] [PubMed] [Google Scholar]
- 10. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vinales KL, Schlögl M, Piaggi P, et al. The consistency in macronutrient oxidation and the role for epinephrine in the response to fasting and overfeeding. J Clin Endocrinol Metab. 2017;102(1):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laeger T, Henagan TM, Albarado DC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laeger T, Albarado DC, Burke SJ, et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 2016;16(3):707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fazeli PK, Lun M, Kim SM, et al. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125(12):4601–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vinales KL, Begaye B, Bogardus C, Walter M, Krakoff J, Piaggi P. FGF21 is a hormonal mediator of the human “Thrifty” metabolic phenotype. Diabetes. 2019;68(2):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollstein T. Supplemental material - recharacterizing the metabolic state of energy balance in thrifty and spendthrift phenotypes. Harvard Dataverse. Deposited February 10, 2020. doi: 10.7910/DVN/WQOTYV. [DOI] [PMC free article] [PubMed]
- 17. Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86(3):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller DS, Mumford P. Gluttony. 1. An experimental study of overeating low- or high-protein diets. Am J Clin Nutr. 1967;20(11):1212–1222. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2015;38(Suppl):S8–S16. [DOI] [PubMed] [Google Scholar]
- 20. Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab. 2013;98(7):2791–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schutz Y, Ravussin E, Diethelm R, Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. Int J Obes. 1982;6(1):23–28. [PubMed] [Google Scholar]
- 23. Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62(12):4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snitker S, Tataranni PA, Ravussin E. Spontaneous physical activity in a respiratory chamber is correlated to habitual physical activity. Int J Obes Relat Metab Disord. 2001;25(10):1481–1486. [DOI] [PubMed] [Google Scholar]
- 25. Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98(4):E703–E707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hollstein T, Ando T, Basolo A, Krakoff J, Votruba SB, Piaggi P. Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: further evidence for spendthrift and thrifty metabolic phenotypes. Am J Clin Nutr. 2019;110(3):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jumpertz R, Hanson RL, Sievers ML, Bennett PH, Nelson RG, Krakoff J. Higher energy expenditure in humans predicts natural mortality. J Clin Endocrinol Metab. 2011;96(6):E972–E976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruggiero C, Metter EJ, Melenovsky V, et al. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2008;63(7):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caudwell P, Finlayson G, Gibbons C, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr. 2013;97(1):7–14. [DOI] [PubMed] [Google Scholar]
- 30. McNeil J, Lamothe G, Cameron JD, et al. Investigating predictors of eating: is resting metabolic rate really the strongest proxy of energy intake? Am J Clin Nutr. 2017;106(5):1206–1212. [DOI] [PubMed] [Google Scholar]
- 31. Hopkins M, Finlayson G, Duarte C, et al. Biological and psychological mediators of the relationships between fat mass, fat-free mass and energy intake. Int J Obes (Lond). 2019;43(2):233–242. [DOI] [PubMed] [Google Scholar]
- 32. Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int J Obes (Lond). 2014;38(2):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Basolo A, Votruba SB, Heinitz S, Krakoff J, Piaggi P. Deviations in energy sensing predict long-term weight change in overweight Native Americans. Metabolism. 2018;82:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab. 2015;100(8):3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blundell JE, Caudwell P, Gibbons C, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Model Mech. 2012;5(5):608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blundell JE, Tremblay A. Appetite control and energy (fuel) balance. Nutr Res Rev. 1995;8(1):225–242. [DOI] [PubMed] [Google Scholar]
- 37. Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr. 1956;4(2):169–175. [DOI] [PubMed] [Google Scholar]
- 38. Stinson EJ, Graham AL, Thearle MS, Gluck ME, Krakoff J, Piaggi P. Cognitive dietary restraint, disinhibition, and hunger are associated with 24-h energy expenditure. Int J Obes (Lond). 2019;43(7):1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hägele FA, Büsing F, Nas A, et al. Appetite control is improved by acute increases in energy turnover at different levels of energy balance. J Clin Endocrinol Metab. 2019;104(10):4481–4491. [DOI] [PubMed] [Google Scholar]
- 40. Büsing F, Hägele FA, Nas A, Hasler M, Müller MJ, Bosy-Westphal A. Impact of energy turnover on the regulation of glucose homeostasis in healthy subjects. Nutr Diabetes. 2019;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nas A, Büsing F, Hägele FA, Hasler M, Müller MJ, Bosy-Westphal A. Impact of energy turnover on fat balance in healthy young men during energy balance, caloric restriction and overfeeding. Brit J Nutr. 2020;123(1):30-40. [DOI] [PubMed] [Google Scholar]
- 42. Drenick EJ, Dennin HF. Energy expenditure in fasting obese men. J Lab Clin Med. 1973;81(3):421–430. [PubMed] [Google Scholar]
- 43. McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. 2014;1311:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Basolo A, Begaye B, Hollstein T, et al. Effects of short-term fasting and different overfeeding diets on thyroid hormones in healthy humans. Thyroid. 2019;29(9):1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Landsberg L. Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26(4-6):497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247(1 Pt 1):E35–E40. [DOI] [PubMed] [Google Scholar]
- 47. Palmblad J, Levi L, Burger A, et al. Effects of total energy withdrawal (fasting) on thelevels of growth hormone, thyrotropin, cortisol, adrenaline, noradrenaline, T4, T3, and rT3 in healthy males. Acta Med Scand. 1977;201(1-2):15–22. [DOI] [PubMed] [Google Scholar]
- 48. Zauner C, Schneeweiss B, Kranz A, et al. Resting energy expenditure in short-term starvation is increased as a result of an increase in serum norepinephrine. Am J Clin Nutr. 2000;71(6):1511–1515. [DOI] [PubMed] [Google Scholar]
- 49. Christensen NJ. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974;23(1):1–8. [DOI] [PubMed] [Google Scholar]
- 50. Mansell PI, Fellows IW, Macdonald IA. Enhanced thermogenic response to epinephrine after 48-h starvation in humans. Am J Physiol. 1990;258(1 Pt 2):R87–R93. [DOI] [PubMed] [Google Scholar]
- 51. Wang M, Wang Q, Whim MD. Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia. Proc Natl Acad Sci U S A. 2016;113(21):E3029–E3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79(1):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wolfe RR, Peters EJ, Klein S, Holland OB, Rosenblatt J, Gary H Jr. Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am J Physiol. 1987;252(2 Pt 1):E189–E196. [DOI] [PubMed] [Google Scholar]
- 54. Qvisth V, Hagström-Toft E, Enoksson S, Moberg E, Arner P, Bolinder J. Human skeletal muscle lipolysis is more responsive to epinephrine than to norepinephrine stimulation in vivo. J Clin Endocrinol Metab. 2006;91(2):665–670. [DOI] [PubMed] [Google Scholar]
- 55. Staten MA, Matthews DE, Cryer PE, Bier DM. Physiological increments in epinephrine stimulate metabolic rate in humans. Am J Physiol. 1987;253(3 Pt 1):E322–E330. [DOI] [PubMed] [Google Scholar]
- 56. Macdonald IA, Bennett T, Fellows IW. Catecholamines and the control of metabolism in man. Clin Sci (Lond). 1985;68(6):613–619. [DOI] [PubMed] [Google Scholar]
- 57. Mansell PI, Fellows IW, Macdonald IA. Enhanced thermogenic response to epinephrine after 48-h starvation in humans. Am J Physiol. 1990;258(1 Pt 2):R87–R93. [DOI] [PubMed] [Google Scholar]
- 58. Nair KS, Woolf PD, Welle SL, Matthews DE. Leucine, glucose, and energy metabolism after 3 days of fasting in healthy human subjects. Am J Clin Nutr. 1987;46(4):557–562. [DOI] [PubMed] [Google Scholar]
- 59. Webber J, Macdonald IA. The cardiovascular, metabolic and hormonal changes accompanying acute starvation in men and women. Br J Nutr. 1994;71(3):437–447. [DOI] [PubMed] [Google Scholar]
- 60. Mansell PI, Macdonald IA. The effect of starvation on insulin-induced glucose disposal and thermogenesis in humans. Metabolism. 1990;39(5):502–510. [DOI] [PubMed] [Google Scholar]
- 61. Pollet RJ, Levey GS. Principles of membrane receptor physiology and their application to clinical medicine. Ann Intern Med. 1980;92(5):663–680. [DOI] [PubMed] [Google Scholar]
- 62. Lewis JE, Ebling FJP, Samms RJ, Tsintzas K. Going back to the biology of FGF21: new insights. Trends Endocrinol Metab. 2019;30(8):491–504. [DOI] [PubMed] [Google Scholar]
- 63. BonDurant LD, Ameka M, Naber MC, et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 2017;25(4):935–944.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. [DOI] [PubMed] [Google Scholar]
- 65. Kim KH, Lee MS. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J. 2014;38(4):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gaich G, Chien JY, Fu H, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18(3):333–340. [DOI] [PubMed] [Google Scholar]
- 67. Talukdar S, Zhou Y, Li D, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016;23(3):427–440. [DOI] [PubMed] [Google Scholar]
- 68. Ando T, Piaggi P, Bogardus C, Krakoff J. VO2max is associated with measures of energy expenditure in sedentary condition but does not predict weight change. Metabolism. 2019;90:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97(5):990–994. [DOI] [PubMed] [Google Scholar]