Abstract
Several standard-of-care therapies for the treatment of retinal disease, including aflibercept, inhibit vascular endothelial growth factor (VEGFA). The main shortcoming of these therapies is potential undertreatment due to a lack of compliance resulting from the need for repeated injections. Gene therapy may provide sustained levels of anti-VEGFA proteins in the retina following a single injection. In this nonhuman primate study, we explored whether ADVM-022, a recombinant adeno-associated virus (AAV) vector designed to express aflibercept, could induce anti-VEGFA protein levels comparable with those observed following a single-bolus intravitreal (IVT) injection of the standard-of-care aflibercept recombinant protein. The results demonstrated that intraocular levels of aflibercept measured at 56 days after a single IVT injection of ADVM-022 were equivalent to those in the aflibercept recombinant protein-injected animals measured 21–32 days post-administration. ADVM-022-injected animals exhibited signs of an initial self-limiting inflammatory response, but overall all doses were well tolerated. ADVM-022 administration did not result in systemic exposure to aflibercept at any dose evaluated. These results demonstrated that a single IVT injection of ADVM-022 resulted in safe and efficacious aflibercept levels in the therapeutic range, suggesting the potential of a gene therapy approach for long-term treatment of retinal disease with anti-VEGF therapy.
Graphical Abstract
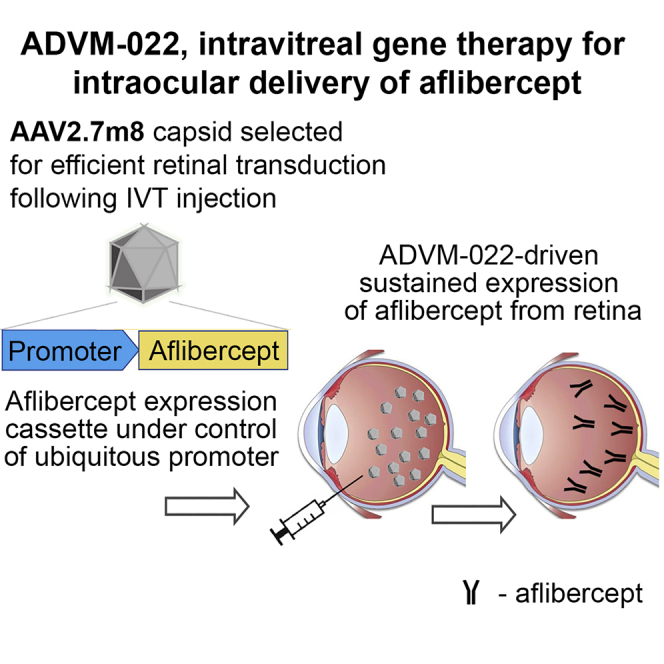
Current treatments of nAMD require frequent intravitreal injections of VEGF inhibitors. ADVM-022 is a one-time intravitreal gene therapy designed to express aflibercept. Kiss et al. demonstrated a range of ADVM-022 doses in non-human primates that resulted in safe ocular expression of aflibercept in the therapeutic range, without systemic exposure.
Introduction
Multiple preclinical and clinical studies have established vascular endothelial growth factor (VEGFA) as a key mediator of the pathological angiogenesis in neovascular age-related macular degeneration (nAMD),1 diabetic retinopathy (DR)2 and diabetic macular edema (DME),3 or macular edema due to retinal vein occlusion (RVO).4 Indeed, anti-VEGFA agents such as ranibizumab, bevacizumab, and aflibercept have demonstrated efficacy in controlling pathological neovascularization and macular edema in these diseases.5, 6, 7, 8, 9 Disease management with these antiangiogenic drugs requires regular intravitreal (IVT) injections, typically every 4–8 weeks, over the patient’s lifetime.10, 11, 12, 13 The necessity of chronic IVT injections for patients suffering from nAMD, DME, or RVO poses a significant burden on patients, caregivers, and the healthcare system, and can result in decreased compliance followed by disease progression.14,15 Minimizing this treatment burden while maintaining treatment efficacy remains an unmet need for therapy of these diseases. Significant effort has therefore focused on extending the dosing intervals between injections.16 Although first-generation anti-VEGFA products, such as Lucentis (ranibizumab) and off label-used Avastin (bevacizumab; Genentech/Roche), generally require up to monthly dosing, Eylea (aflibercept; Regeneron) is a second-generation drug for nAMD that has sufficiently high activity to support a bimonthly treatment regimen in many patients.17 A potential third-generation drug, brolucizumab (Novartis), has a smaller molecular mass of 26 kDa compared with ranibizumab, bevacizumab, or aflibercept. Its small size may provide better tissue penetration and the ability to administer a higher dose of the drug, potentially leading to a longer effect.18 Abicipar pegol (DARPin), an ankyrin repeat protein that targets VEGFA, developed by Allergan, has also shown improved durability of action with the potential to extend the interval between administrations in a phase III clinical trial.19 Additional extended-release systems delivered IVT, including Kodiak Sciences’ KSI-103 anti-VEGF biopolymer conjugate, Graybug Vision’s GB-102 depot microparticle formulation of sunitinib malate (pan VEGF receptor inhibitor), as well as Genentech/Roche’s port delivery system of ranibizumab (phase III); all are proposed to extend the time interval between anti-VEGFA interventions. Although these options are designed to reduce the treatment burden for patients and their caregivers, they still require relatively frequent visits to the physician’s office or invasive ocular surgery and follow-up injections. Gene therapy offers a paradigm-shifting approach to the treatment of nAMD, because it has the potential to provide long-term sustained levels of anti-VEGFA proteins in the retina following a single IVT injection, which could improve clinical outcomes while drastically reducing treatment burden. We previously reported that ADVM-022, an adeno-associated virus (AAV) vector optimized for IVT injection encoding aflibercept, demonstrated sustained expression of the protein out to 16 months in nonhuman primates (NHPs), and in a laser-induced choroidal neovascularization (CNV) animal model of nAMD, ADVM-022 provided the same degree of protection as an IVT bolus of aflibercept recombinant protein delivered at the time of laser treatment.20
Here, we sought to determine whether aflibercept levels measured following a single IVT injection of ADVM-022 were comparable with clinically therapeutic levels achieved following administration of an IVT bolus of commercially available aflibercept recombinant protein. We evaluated aflibercept expression levels in various ocular compartments following IVT ADVM-022 injection in NHPs and determined how these levels relate to the pharmacokinetics (PK) profile following IVT injection of aflibercept recombinant protein. Three doses of ADVM-022 (2 × 1011, 6 × 1011, and 2 × 1012 vector genomes (vg)/eye) were evaluated 56 days post-injection, and the levels were compared with measurements at different time points over 56 days in the same intraocular compartments upon a single IVT injection of 1.2 mg aflibercept recombinant protein. This dose was selected as an approximate equivalent to human dose based on the difference in vitreous volume between NHP and human.21, 22, 23 It is known that IVT administration of AAV vectors with various transgenes can induce intraocular inflammation.20,24,25 Several studies have used concomitant anti-inflammatory interventions as an approach for mitigating or eliminating the inflammatory responses following administration of AAV vectors.24, 25, 26 Consequently, we measured parameters of ocular inflammation as a function of ADVM-022 dose. Our results show that ADVM-022-expressed aflibercept levels in the intraocular tissues were equivalent to those in aflibercept-injected animals measured 21–32 days post-injection. Based on the similarity of aflibercept PK in NHP and humans, the results suggest that ADVM-022 at doses ranging from 2 × 1011 to 2 × 1012 vg/eye provided aflibercept at the levels detected in patients’ eyes treated with the aflibercept IVT dose.27 ADVM-022 treatment did not lead to a systemic exposure to aflibercept, unlike IVT bolus of aflibercept protein, where small amounts of aflibercept were detected in the blood. Overall, ADVM-022 has the potential to provide sustained intraocular expression of aflibercept at pharmacologically active levels in human patients, thus minimizing treatment burden of neovascular ocular diseases with anti-VEGFA drugs and potentially improving visual outcome.
Results
In order to compare ocular levels of aflibercept from ADVM-022 versus aflibercept recombinant protein administration, we examined aflibercept expression levels as a function of ADVM-022 dose and compared them with levels attained following delivery of a single-bolus IVT injection of aflibercept recombinant protein. In this study, three groups of animals (n = 2/group) received a single bilateral IVT administration of ADVM-022 at 2 × 1011, 6 × 1011, or 2 × 1012 vg/eye. ADVM-022-treated animals were monitored for 56 days post-injection, at which time samples were collected for determination of ADVM-022-induced aflibercept expression. Aflibercept levels in animals receiving ADVM-022 were compared with samples collected from a fourth group of animals (n = 2 for each time point) that received a single bilateral IVT injection of aflibercept (1.2 mg/eye) from which tissue was collected at various times out to day 56. This design enabled a direct comparison of expression levels of ADVM-022 aflibercept (as measured in the aqueous and vitreous humor and ocular tissues) with levels resulting from a single bolus injection of aflibercept.
The half-life of IVT-injected aflibercept in African green monkey (Table 1) in vitreous humor was very close to the half-life of aflibercept in human vitreous humor calculated using mathematical modeling,28,29 suggesting similar PK of this drug in human and NHP eyes.
Table 1.
Aflibercept Half-Lives after IVT Administration of Aflibercept Protein Calculated from the Slopes of the Lines in Figure 1
| Matrix | Half-Life (h) |
|---|---|
| Serum | 91 |
| Vitreous humor | 105 |
| Aqueous humor | 106 |
| Retina | 113 |
| Choroid | 98 |
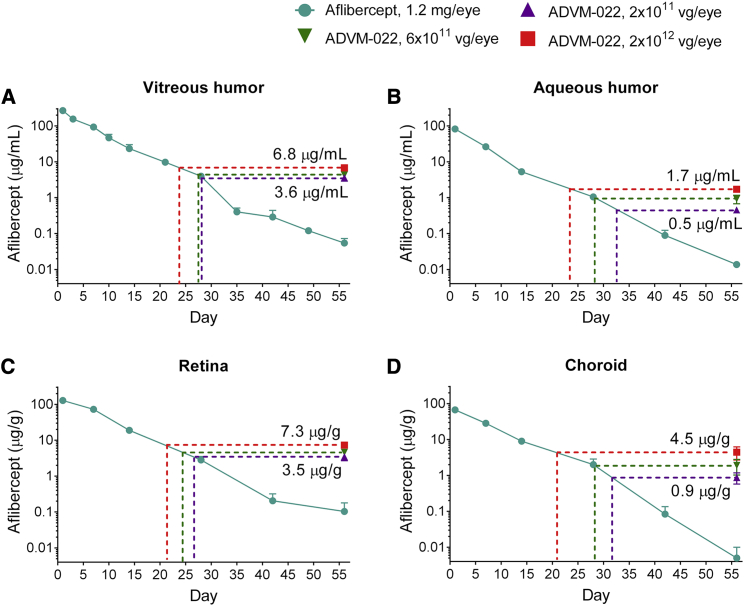
Single IVT injections of either 2 × 1011, 6 × 1011, or 2 × 1012 vg/eye ADVM-022 resulted in post-dose aflibercept concentrations at day 56 that were similar to those observed in the aflibercept protein-injected animals between days 21 and 32 (see purple, green, and red dotted lines in Figure 1; also see Table S1). In comparison, by day 56, animals injected with aflibercept protein displayed concentrations in the different ocular matrices that were ranging from below the level of quantitation to 3% of those expressed from ADVM-022.
Figure 1.
IVT ADVM-022 Provides Levels of Intraocular Aflibercept Expression Comparable with Those Measured 21–32 Days following IVT Injection of Aflibercept Recombinant Protein (n = 4 Eyes, 2 Animals for Each Data Point)
(A) Vitreous humor. (B) Aqueous humor. (C) Retina. (D) Choroid. The dotted lines indicate the times on the decay curves when recombinant aflibercept levels are equivalent to those seen in the ADVM-022-treated groups’ low (2 × 1011 vg/eye, purple), middle (6 × 1011 vg/eye, green), and high (2 × 1012 vg/eye, red) doses tested on day 56. Error bars represent standard deviations.
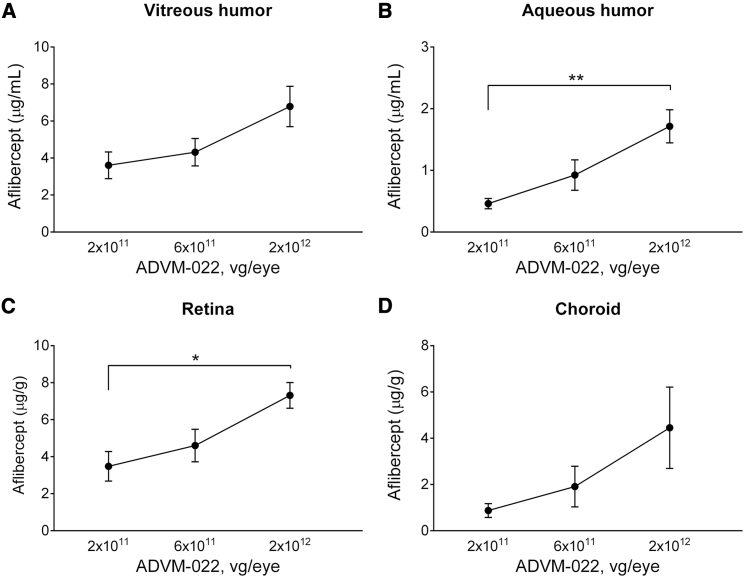
Over the range of ADVM-022 doses injected (2 × 1011–2 × 1012 vg/eye), aflibercept expression levels were less than dose-proportional. For example, a 10-fold increase in vector dose resulted in an approximately 2-fold increase in aflibercept expression in the vitreous humor and retina (Figure 2).
Figure 2.
Aflibercept Expression Levels as a Function of ADVM-022 Dose in vitreous humor (A), aqueous humor (B), retina (C) and choroid (D), collected at Day 56 Post-dose (n = 4 Eyes/2 Animals)
Means ± SEM for treatment groups are shown. ∗p < 0.05, ∗∗p < 0.01 (one-way ANOVA with Tukey’s multiple comparisons test).
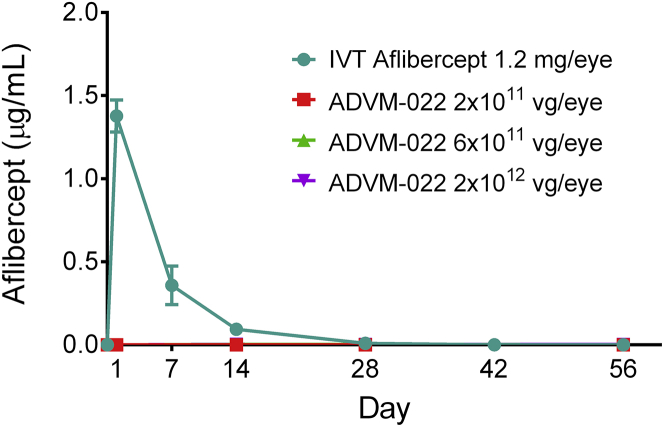
Systemic exposure of aflibercept was evaluated following IVT delivery of either aflibercept recombinant protein or increasing doses of ADVM-022. At all doses of ADVM-022, aflibercept expression in the eye did not result in detectable levels of aflibercept in the systemic circulation (Figure 3). Immediately following IVT injection of aflibercept recombinant protein, serum concentrations were highest at the first post-treatment time point (day 1; 1.4 μg/mL) followed by an exponential decay that reached 0.009 μg/mL at day 28 post-dose.
Figure 3.
Serum Levels of Aflibercept following IVT Delivery of Either Aflibercept Recombinant Protein or Increasing Doses of ADVM-022
Means ± SEM for treatment groups are shown. Note that the data points for each ADVM-022 dose are superimposed on the x axis. For ADVM-022-dosed animals, n = 2 animals per dose at each time point. Due to the serum and other tissue collection schedule, the number of serum specimens from animals dosed with aflibercept was different at each of the time points (n = 12 at baseline [all aflibercept-treated subgroups]; n = 2 at day 1 (subgroup 1a); n = 2 at day 7 (subgroup 1b); n = 8 at day 14 (subgroups 1c, d, e, and f); n = 6 at day 28 (subgroups 1d, e, and f); n = 4 at day 42 (subgroups 1e and f); and n = 2 at day 56 (subgroup 1f).
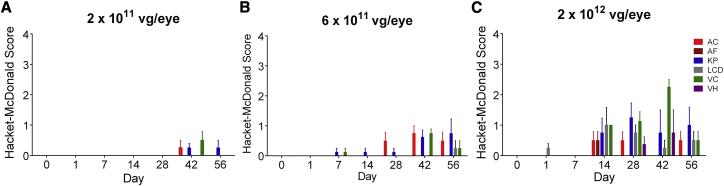
Slit-lamp examinations were performed to evaluate a number of parameters (see Materials and Methods) that could indicate ocular inflammation. The inflammatory responses included aqueous and vitreous cell infiltrates, keratic precipitates, and incidental lens capsule deposit findings. As shown in Figure 4, doses of 2 × 1011 (Figure 4A) and 6 × 1011 vg/eye (Figure 4B) induced minor or mild inflammatory responses through day 56, while the responses to a dose of 2 × 1012 vg/eye (Figure 4C) were more pronounced, but nearly completely resolved by day 56, with the exception of lingering keratic precipitates in the highest dose group. Vitreous haze was transiently observed in the eyes treated with ADVM-022 at 2 × 1012 vg/eye, with no aqueous flare observed at any dose. Representative anterior segment and dilated fundus photographs of eyes receiving a single IVT injection of ADVM-022 at 2 × 1011, 6 × 1011, and 2 × 1012 vg/eye are shown in Figure S1. Evaluation of African green monkey eyes injected with 1.2 mg/eye aflibercept detected sporadic low-grade responses that could be related either to the test article or the procedure (Figure S2).
Figure 4.
Assessment of Ocular Inflammatory Response to Different Doses of ADVM-022
(A–C) Aqueous cell (AC), aqueous flare (AF), keratic precipitate (KP), lens capsule deposit (LCD), vitreous cells (VC), and vitreous haze (VH) were parameters observed in the eyes treated with ADVM-022 injected at 2 × 1011 (A), 6 × 1011 (B), and 2 × 1012 (C) vg/eye, out of 21 parameters scored by slit-lamp examination (see Materials and Methods). Scores are shown as mean ± SEM; n = 4 eyes.
Discussion
ADVM-022 gene therapy has been designed to address the challenges associated with the current standard of care for nAMD, notably the repeated IVT injections for the lifetime of the patient required to maintain visual function,30,31 by providing a continuous supply of an approved anti-VEGFA protein to the eye after a single IVT delivery.
The current study was designed to evaluate whether the levels of the aflibercept expression in ocular tissues driven by IVT-delivered ADVM-022 were comparable with the levels resulting from a single IVT dose of aflibercept recombinant protein (standard of care) within its duration of action period. We also evaluated intraocular aflibercept production, as well as inflammation parameters, as a function of ADVM-022 dose.
The ADVM-022-induced aflibercept expression measured 56 days post-dose reported here was within the range we previously reported in our long-term study, in which aflibercept levels were monitored over a 16-month period post-ADVM-022 administration.20 In this study, aflibercept expression was detected in all ocular tissues sampled (aqueous humor, vitreous humor, choroid, and retina) following ADVM-022 administration.
The levels of aflibercept expression were highest in retina and vitreous humor, followed by choroid and aqueous humor, in agreement with our earlier study.20 This pattern of aflibercept distribution in the intraocular tissues indicates that the neural retina is one of the principal sources of aflibercept, with a potential contribution from anterior ocular tissues, such as iris pigmented epithelium, ciliary body, and ciliary processes that could also be transduced with AAV2.7m8.32
The dose-response of aflibercept expression induced by ADVM-022 indicates that although increased levels of ADVM-022 resulted in a trend toward increased aflibercept expression, a 10-fold increase in vector dose resulted in only a 2-fold increase in aflibercept levels in the retina and vitreous humor. The absence of dose-response linearity in a wide dose range of AAV vector is not unique and was seen in previous in vivo and in vitro studies.33,34 This non-linearity could result from any rate-limiting step in the transduction pathway, from vector distribution in the tissue to its entry to the cell, endosomal processing, nuclear trafficking, and conversion to the transcriptionally active forms, and each of these steps may be affected in a tissue- and cell-type-specific manner.35 Indeed, in the case of IVT/intraocular vector delivery, the dose-proportionality of retina will be affected by the need for vector particles to redistribute through several compartments to reach to the neural retina, such as the redistribution of AAV particles from vitreous to the inner limiting membrane (ILM), with a subsequent dissociation from the ILM with a chance to redistribute to the retinal compartment.36,37
The levels of intraocular aflibercept expressed from ADVM-022 were compared with the levels achieved after IVT bolus injection of 1.2 mg/eye aflibercept recombinant protein in the African green monkey. The PK profile of aflibercept following IVT-injected aflibercept recombinant protein evaluated in this study was similar to the published clearance rates of aflibercept after IVT administration in rabbits and cynomolgus monkeys,38,39 and the half-life in vitreous humor (106 h) was very close to human vitreous humor rates calculated using mathematical modeling (115 h).12,28 The aflibercept levels measured in aqueous humor of ADVM-022-treated NHP eyes were similar to those described by Celik et al.,27 in a study of nAMD patients receiving bimonthly injections aflibercept recombinant protein.
Although ADVM-022 led to expression of aflibercept at levels significantly lower than the peak levels observed after IVT injection of recombinant aflibercept (Figure 2), we have previously reported that its efficacy in suppression of laser-induced CNV lesions was similar to that of the aflibercept standard-of-care bolus IVT injection.20 Pharmacological efficacy of ADVM-022-expressed aflibercept levels was not unexpected because, at all ADVM-022 doses tested, the levels in retina significantly exceeded the half-maximal inhibitory concentration (16 pM, or 1.6 ng/mL) for VEGFA165 interaction with its receptor FLT1.40
Close similarity of the vitreous half-life of IVT-injected aflibercept in the NHP in this study with its modeled half-life in humans implies similar rates of clearance of aflibercept from the human and NHP eyes. Consequently, assuming that ADVM-022 in humans expresses the levels of aflibercept similar to those observed in NHPs, then the time points at which the ADVM-022-expressed aflibercept levels are equivalent to those after IVT injection of aflibercept protein in NHPs (days 21–28 post-injection time points) can be translated to aflibercept PK in humans. Based on this assumption, the ADVM-022-expressed levels of aflibercept in humans would be well within the duration of action of the aflibercept IVT dose: the median duration of aflibercept action reported in the VIEW trials was about 12 weeks,41 although it is typically administered to nAMD patients on average every 4–8 weeks as the current standard of care. This suggests that a single IVT injection of ADVM-022 may lead to a sustained expression of pharmacologically active levels of aflibercept in human patients. Indeed, we cannot exclude the possibility that in some patients, intraocular VEGFA production may exceed the inhibitory capacity of the ADVM-022-expressed aflibercept levels, rendering aflibercept-delivering gene therapy less efficacious; in these patients, there would be a required bolus “rescue” injection of aflibercept in order to completely inhibit nAMD activity. The ongoing clinical trials of ADVM-022 will identify what population of nAMD patients may benefit from the continuous expression of aflibercept delivered by ADVM-022.
Evaluation of inflammatory responses in eyes injected IVT with ADVM-022 at doses ranging from 2 × 1011 to 2 × 1012 vg/eye showed that the eyes receiving ADVM-022 exhibited mostly mild intraocular inflammation, peaking around 1 month post IVT injection. The degree of the inflammatory response correlated with vector dose. The level of inflammation at any dose in this study did not warrant treatment with any anti-inflammatory medication; no topical nor systemic steroids were required to quell the spontaneously resolving inflammatory response to ADVM-022. Importantly, the 2 × 1011 and 6 × 1011 vg/eye doses resulted in aflibercept expression within the range that was previously shown to be efficacious in the laser-induced CNV model of nAMD,20 while inducing only minimal or mild inflammatory responses. The eyes receiving ADVM-022 exhibited mild self-limiting intraocular inflammation, peaking around 1 month post IVT injection. The findings of keratic precipitates and lens capsule deposits in the high-dose group may be, at least in part, related to a pseudo-endophthalmitis-like precipitation of the viral particles themselves, akin to the pseudo-endophthalmitis noted follow-up IVT injections of triamcinolone, rather than a true inflammatory reaction.42
The reduction of systemic VEGFA levels in patients receiving IVT injection of aflibercept or bevacizumab is well documented,43, 44, 45 although the clinical implications of this suppression are not clear. Although this therapy is considered safe in the general population, it has been suggested that a subset of patients, such as those with diabetes, a history of recent myocardial infarction, or cerebrovascular accidents, may be at increased risk for systemic serious adverse events.43, 44, 45, 46 Indeed, a recent meta-analysis identified a possible increased risk for death in diabetic patients receiving monthly IVT anti-VEGFA agents (odds ratio [OR], 2.98; 95% confidence interval [CI], 1.44–6.14; p = 0.003).43 In the current study, ADVM-022 administration did not result in serum exposure to aflibercept, at a time where ocular aflibercept levels from ADVM-022 are present, indicating no leakage into the systemic circulation, and thus ADVM-022 may exhibit an improved safety profile over aflibercept recombinant protein.
In summary, the data from this NHP PK study demonstrate that a single IVT injection of ADVM-022 can induce intraocular levels of aflibercept in the range of therapeutic levels achieved with the standard-of-care recombinant protein from a range of doses spanning one log from 2 × 1011 to 2 × 1012 vg/eye. Together with the published long-term activity of IVT-delivered ADVM-022 in the laser-induced model of neovascular AMD, these results support the concept that sustained aflibercept expression following a single dose of ADVM-022 may provide an effective long-term treatment option for patients suffering from nAMD.
Materials and Methods
Vector
ADVM-022 utilizes the AAV2.7m8 capsid, a variant of AAV2 that includes a 10-amino acid insertion in loop IV of the AAV2 viral capsid proteins (VP1–3). The genome consists of the viral inverted terminal repeats from AAV2 flanking the expression cassette, C11.CO.aflibercept, as previously described.20
In brief, aflibercept expression was controlled by the following regulatory elements: the human cytomegalovirus (CMV) immediate-early enhancer and promoter, an adenovirus tripartite leader sequence (TPL) followed by an enhancer element from the major late promoter (eMLP), a synthetic intron, and a Kozak sequence driving expression of aflibercept. The cDNA of aflibercept was followed by a human scaffold attachment region (SAR) and the human growth hormone (GH) polyadenylation site.
Aflibercept
Aflibercept recombinant protein used as positive control was manufactured by Regeneron Pharmaceuticals and was purchased commercially.
Animals
This study was conducted in naive adult African green monkeys (Chlorocebus sabaeus) of both sexes aged 5–12 years old, as established by dentition criteria.47 All animals were used in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Procedural aspects of the animal studies were approved by the Animal Care and Use Committee overseeing animal welfare at the primate facility (St. Kitts Biomedical Research Foundation, St. Kitts, West Indies) with which RxGen (New Haven, CT, USA) maintains a facility use agreement. All animals were in the normal range at baseline ophthalmic screening, including tonometry, slit-lamp biomicroscopy, fundoscopy, color fundus photography (CFP), fluorescein angiography (FA), and optical coherence tomography (OCT). All animals recruited in the study were pre-screened for the neutralizing antibody (Nab), and only Nab-negative animals were assigned to the ADVM-022 treatment groups.
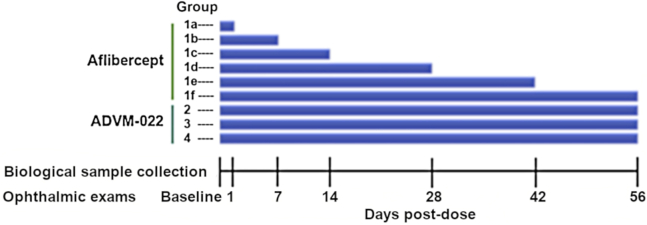
To compare the dose-dependent levels of aflibercept expression following IVT injection of ADVM-022 with the levels achieved following bolus IVT injection of aflibercept recombinant protein, animals of both sexes (n = 20) were randomized into 10 treatment groups by weight, 1 male and 1 female per group (Table 2). On study day 0, groups 1a, 1b, 1c, 1d, 1e, and 1f received bilateral (oculus uterque [OU]) IVT injections of 1.2 mg (30 μL) aflibercept (Eylea, 40 mg/mL, an approved therapy for nAMD). Groups 2, 3, and 4 received IVT OU ADVM-022 at doses of 2 × 1011, 6 × 1011, and 2 × 1012 vg/eye, respectively, at 100 μL/eye.
Table 2.
Animal Group Assignments
| Group | Test Article | Dose/Eye | n and Sex | Sacrifice Day |
|---|---|---|---|---|
| 1a | aflibercept | 1.2 mg | 1 M/1 F | 1 |
| 1b | aflibercept | 1.2 mg | 1 M/1 F | 7 |
| 1c | aflibercept | 1.2 mg | 1 M/1 F | 14 |
| 1d | aflibercept | 1.2 mg | 1 M/1 F | 28 |
| 1e | aflibercept | 1.2 mg | 1 M/1 F | 42 |
| 1f | aflibercept | 1.2 mg | 1 M/1 F | 56 |
| 2 | ADVM-022 | 2 × 1011 vg | 1 M/1 F | 56 |
| 3 | ADVM-022 | 6 × 1011 vg | 1 M/1 F | 56 |
| 4 | ADVM-022 | 2 × 1012 vg | 1 M/1 F | 56 |
F, female; M, male.
The study was performed according to the scheme shown in Figure 5. Animals were examined by slit-lamp biomicroscopy, fundoscopy, FA, tonometry, OCT, and CFP at baseline screening and at specified time points (Figure 5). Slit-lamp examination evaluated eyes for the following parameters: conjunctival congestion, conjunctival swelling, conjunctival discharge, pannus, corneal opacity, corneal clouding, corneal area, inflammatory keratic precipitate, aqueous cells, aqueous flare, fibrin strands, iris hyperemia, iris exfoliation, iris synechia, lens capsule deposit, lens opacity, vitreous cells, vitreous haziness, retinal vasculitis, and papillitis. Evaluation of the posterior wall and vitreous humor was performed by posterior segment slit-lamp examination with a 90-D lens. Eyes also were evaluated for retinal infiltrates and hemorrhage, vascular dilation, tortuosity and sheathing, and optic disc edema during the fundoscopy. Samples of biological material were collected at time points described in Table 3.
Figure 5.
Study Schedule
Animals in groups 1a, b, c, d, e, and f were dosed with aflibercept, 1.2 mg/eye. Animals in groups 2, 3, and 4 were dosed with ADVM-022 OU at 2 × 1011, 6 × 1011, and 2 × 1012 vg/eye, respectively. On day 1, CFP, FA, and OCT data were collected only for group 1a, prior to termination.
Table 3.
Sample Collection Schedule
| Sample | Time Point |
|---|---|
| Serum | baseline (all groups) |
| day 1 (group 1a) | |
| day 7 (group 1b) | |
| day 14 (groups 1c, 1d, 1e, 1f, 2, 3, 4) | |
| day 28 (groups 1d, 1e, 1f, 2, 3, 4) | |
| day 42 (groups 1e, 1f, 2, 3, 4) | |
| day 56 (groups 1f, 2, 3, 4) | |
| Aqueous humor | termination for all groups |
| Vitreous humor | day 1 (group 1a) |
| day 3 (group 1b) | |
| day 7 (group 1b) | |
| day 10 (group 1c) | |
| day 14 (group 1c) | |
| day 21 (group 1d) | |
| day 28 (group 1d) | |
| day 35 (group 1e) | |
| day 42 (group 1e) | |
| day 49 (group 1f) | |
| day 56 (groups 1f, 2, 3, 4) | |
| Ocular tissue (retina, choroid) | at termination for all groups |
Animal Care and Handling
Animals were anesthetized for all procedures and ophthalmic evaluations (8.0 mg/kg ketamine/1.6 mg/kg xylazine, intramuscular [IM] to effect). General well-being was assessed before, during, and after sedation, as well as twice daily on non-procedure days. The daily consumption of food biscuits was monitored. Body weight was measured at the time of ophthalmic examinations.
Test Article Delivery
IVT doses were administered under local anesthesia (0.5% proparacaine) using a 31G 5/16-inch needle (Ulticare Vet RX U-100, #09436) 2 mm posterior to the limbus in the inferior temporal quadrant, targeting the central vitreous. Injections were followed by topical administration of 0.3% ciprofloxacin ophthalmic solution and 1% atropine ophthalmic ointment.
Aqueous and Vitreous Humor Collection
Vitreous humor was sampled in-life using a 27G needle placed 3 mm posterior to the limbus after sterile preparation of the eye as for IVT injections. Vitreous and aqueous humor taps (in-life) were followed by one drop of topical administration of 0.3% ciprofloxacin and 1% atropine ophthalmic ointment. At termination, 1.5 mL vitreous humor and 100 μL aqueous humor per eye were collected. Samples were transferred to pre-labeled and pre-weighed cryotubes, and stored frozen at −80°C until analysis.
Ocular Tissue Collection
Following euthanasia with pentobarbital, and subsequent aqueous and vitreous humor collection, eye globes were enucleated, and excess orbital tissue was trimmed. For all animals sacrificed prior to day 56, both globes were flash frozen in liquid nitrogen and then dissected along frozen tissue planes at room temperature to isolate cornea, iris/ciliary body, lens, vitreous, choroid, retina, sclera, and optic nerve. For animals euthanized on day 56, following euthanasia with pentobarbital, and subsequent aqueous and vitreous humor collection, eye globes were enucleated, and excess orbital tissue was trimmed. The anterior segment of each enucleated eye globe was removed, and the posterior segment was cut in half along the horizontal plane. The retina was removed from the underlying choroid, and the choroid was separated from the outer sclera, weighed, frozen, and stored before the analysis at −80°C.
Prior to the analysis, retina and choroid were homogenized in the tissue lysis buffer (250 mM HEPES, 150 mM NaCl, 1% Triton X-100 and 5 mM EDTA, 1 mM PMSF, protease inhibitor cocktail [P8340; Sigma], supplemented with 0.5% BSA in 1× PBS), with Navy homogenization beads, using Bullet Blender Storm 24 (Next Advance). After homogenization, the lysates were incubated on ice for the remainder of the 20-min (minimum) incubation time, clarified by centrifugation at 14,000 × g for 15 min at 2°C–8°C, and analyzed for aflibercept levels.
Bioanalysis for Aflibercept Expression
Unbound aflibercept levels in aqueous humor, vitreous humor, retina, and choroid were measured using an enzyme-linked immunosorbent assay (ELISA). ELISA plates (NUNC MaxiSorp) were coated with 100 μL/well recombinant human VEGFA (rhVEGFA; R&D Systems) at a concentration of 1 μg/mL in coating buffer (R&D Systems) and incubated overnight at 4°C. After being washed with wash buffer (KPL), the plates were blocked with 300 μL/well protein-free blocking buffer (Pierce). Afterward, the plates were washed, and diluted samples were added (100 μL/well; 1:1,000 for ADVM-022-treated samples and 1:50 for vehicle-treated samples), and incubated for 2 h at ambient temperature. The plates were then washed again, and 100 μL/well anti-human Fcγ-specific antibody conjugated to horseradish peroxidase (HRP) (Jackson ImmunoResearch) at 500 ng/mL in bovine serum albumin (1% in PBS) was added to the wells. After a washing step, 100 μL/well SuperSignal ELISA Pico Chemiluminescent Substrate (Thermo Fisher Scientific) was added to the wells, and luminescence signal was measured using a microplate luminometer.
Data Analysis
Levels of aflibercept expression in the eyes treated with different doses of ADVM-022 (groups 2–4) were compared using one-way AVOVA with Tukey’s post hoc multiple comparisons analysis. All statistics were performed using GraphPad Prism (Version 6) statistical analysis software. The p values <0.05 were considered statistically significant. Half-lives of aflibercept recombinant protein were calculated using linear fits of the log of aflibercept levels (calculated in ng/mL) versus time (days).
Author Contributions
Conceptualization, S.K., C.M.G., M.G.; Investigation, R.G., A.N., R.R., J.S.G., and J.N.; Visualization, R.G., J.N.; Data Curation, R.G., C.M.G.; Data Analysis, R.G., J.S.G.; Writing – Review & Editing, R.G., C.M.G., S.K.
Conflicts of Interest
R.G., A.N., R.R., J.S.., C.M.G., and J.N. are employees of Adverum Biotechnologies and hold stock grants; S.K. and M.G. are paid consultants of Adverum Biotechnologies and hold grants of Adverum Biotechnologies stock.
Acknowledgments
This study was funded by Adverum Biotechnologies. The authors thank S. Seiler for editorial assistance and M. Steel for test article production and quality control.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.06.007.
Supplemental Information
References
- 1.Ferrara N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat. Med. 2010;16:1107–1111. doi: 10.1038/nm1010-1107. [DOI] [PubMed] [Google Scholar]
- 2.Mehanna C.J., Abdul Fattah M., Haddad S., Tamim H., Ghazi N., Salti H. Anti-VEGF Therapy for Persistent Neovascularization after Complete Panretinal Photocoagulation in Proliferative Diabetic Retinopathy. Ophthalmol. Retina. 2019;3:473–477. doi: 10.1016/j.oret.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Cui L., Jiao B., Han Q. Effect of Intravitreal Anti-Vascular Growth Factor Agents With or Without Macular Photocoagulation on Diabetic Macular Edema: A Systematic Review and Meta-Analysis. Diabetes Ther. 2019;10:1283–1296. doi: 10.1007/s13300-019-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noma H., Funatsu H., Yamasaki M., Tsukamoto H., Mimura T., Sone T., Jian K., Sakamoto I., Nakano K., Yamashita H. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am. J. Ophthalmol. 2005;140:256–261. doi: 10.1016/j.ajo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Heier J.S., Brown D.M., Chong V., Korobelnik J.F., Kaiser P.K., Nguyen Q.D., Kirchhof B., Ho A., Ogura Y., Yancopoulos G.D., VIEW 1 and VIEW 2 Study Groups Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Lynch S.S., Cheng C.M. Bevacizumab for neovascular ocular diseases. Ann. Pharmacother. 2007;41:614–625. doi: 10.1345/aph.1H316. [DOI] [PubMed] [Google Scholar]
- 7.Pham B., Thomas S.M., Lillie E., Lee T., Hamid J., Richter T., Janoudi G., Agarwal A., Sharpe J.P., Scott A. Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis. BMJ Open. 2019;9:e022031. doi: 10.1136/bmjopen-2018-022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld P.J., Rich R.M., Lalwani G.A. Ranibizumab: Phase III clinical trial results. Ophthalmol. Clin. North Am. 2006;19:361–372. doi: 10.1016/j.ohc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Virgili G., Parravano M., Evans J.R., Gordon I., Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst. Rev. 2018;10:CD007419. doi: 10.1002/14651858.CD007419.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campa C., Alivernini G., Bolletta E., Parodi M.B., Perri P. Anti-VEGF Therapy for Retinal Vein Occlusions. Curr. Drug Targets. 2016;17:328–336. doi: 10.2174/1573399811666150615151324. [DOI] [PubMed] [Google Scholar]
- 11.Martin D.F., Maguire M.G., Ying G.S., Grunwald J.E., Fine S.L., Jaffe G.J., CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart M.W. Clinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on aflibercept. Clin. Ophthalmol. 2012;6:1175–1186. doi: 10.2147/OPTH.S33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart M.W. Anti-vascular endothelial growth factor drug treatment of diabetic macular edema: the evolution continues. Curr. Diabetes Rev. 2012;8:237–246. doi: 10.2174/157339912800840488. [DOI] [PubMed] [Google Scholar]
- 14.Boyle J., Vukicevic M., Koklanis K., Itsiopoulos C., Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol. Health Med. 2018;23:127–140. doi: 10.1080/13548506.2016.1274040. [DOI] [PubMed] [Google Scholar]
- 15.Sivaprasad S., Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin. Ophthalmol. 2016;10:939–946. doi: 10.2147/OPTH.S100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain R.M., Hariprasad S.M., Ciulla T.A. Treatment Burden in Neovascular AMD:Visual Acuity Outcomes are Associated With Anti-VEGF Injection Frequency. Ophthalmic Surg. Lasers Imaging Retina. 2017;48:780–784. doi: 10.3928/23258160-20170928-01. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Erfurth U., Kaiser P.K., Korobelnik J.F., Brown D.M., Chong V., Nguyen Q.D., Ho A.C., Ogura Y., Simader C., Jaffe G.J. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Hussain R.M., Ciulla T.A. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin. Emerg. Drugs. 2017;22:235–246. doi: 10.1080/14728214.2017.1362390. [DOI] [PubMed] [Google Scholar]
- 19.Callanan D., Kunimoto D., Maturi R.K., Patel S.S., Staurenghi G., Wolf S., Cheetham J.K., Hohman T.C., Kim K., López F.J., Schneider S. Double-Masked, Randomized, Phase 2 Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in Neovascular Age-Related Macular Degeneration. J. Ocul. Pharmacol. Ther. 2018;34:700–709. doi: 10.1089/jop.2018.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grishanin R., Vuillemenot B., Sharma P., Keravala A., Greengard J., Gelfman C., Blumenkrantz M., Lawrence M., Hu W., Kiss S., Gasmi M. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019;27:118–129. doi: 10.1016/j.ymthe.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missel P.J. Simulating intravitreal injections in anatomically accurate models for rabbit, monkey, and human eyes. Pharm. Res. 2012;29:3251–3272. doi: 10.1007/s11095-012-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Short B.G. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol. Pathol. 2008;36:49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 23.Struble C., Howard S., Relph J. Comparison of ocular tissue weights (volumes) and tissue collection techniques in commonly used preclinical animal species. Acta Ophthalmologica. 2014;92 doi: 10.1111/j.1755-3768.2014.S005.x. [DOI] [Google Scholar]
- 24.Heier J.S., Kherani S., Desai S., Dugel P., Kaushal S., Cheng S.H., Delacono C., Purvis A., Richards S., Le-Halpere A. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390:50–61. doi: 10.1016/S0140-6736(17)30979-0. [DOI] [PubMed] [Google Scholar]
- 25.Ye G.J., Budzynski E., Sonnentag P., Miller P.E., Sharma A.K., Ver Hoeve J.N., Howard K., Knop D.R., Neuringer M., McGill T. Safety and Biodistribution Evaluation in Cynomolgus Macaques of rAAV2tYF-CB-hRS1, a Recombinant Adeno-Associated Virus Vector Expressing Retinoschisin. Hum. Gene Ther. Clin. Dev. 2015;26:165–176. doi: 10.1089/humc.2015.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichel F.F., Dauletbekov D.L., Klein R., Peters T., Ochakovski G.A., Seitz I.P., Wilhelm B., Ueffing M., Biel M., Wissinger B., RD-CURE Consortium AAV8 Can Induce Innate and Adaptive Immune Response in the Primate Eye. Mol. Ther. 2017;25:2648–2660. doi: 10.1016/j.ymthe.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celik N., Scheuerle A., Auffarth G.U., Kopitz J., Dithmar S. Intraocular Pharmacokinetics of Aflibercept and Vascular Endothelial Growth Factor-A. Invest. Ophthalmol. Vis. Sci. 2015;56:5574–5578. doi: 10.1167/iovs.15-16418. [DOI] [PubMed] [Google Scholar]
- 28.Stewart M.W., Rosenfeld P.J. Predicted biological activity of intravitreal VEGF Trap. Br. J. Ophthalmol. 2008;92:667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- 29.Stewart M.W., Rosenfeld P.J., Penha F.M., Wang F., Yehoshua Z., Bueno-Lopez E., Lopez P.F. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye) Retina. 2012;32:434–457. doi: 10.1097/IAE.0B013E31822C290F. [DOI] [PubMed] [Google Scholar]
- 30.Askou A.L. Development of gene therapy for treatment of age-related macular degeneration. Acta Ophthalmol. 2014;92(Thesis3):1–38. doi: 10.1111/aos.12452. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez L., Lanitis T., Cele C., Toro-Diaz H., Gibson A., Kuznik A. Intravitreal Aflibercept Versus Ranibizumab for Wet Age-Related Macular Degeneration: A Cost-Effectiveness Analysis. J. Manag. Care Spec. Pharm. 2018;24:608–616. doi: 10.18553/jmcp.2018.24.7.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramachandran P.S., Lee V., Wei Z., Song J.Y., Casal G., Cronin T., Willett K., Huckfeldt R., Morgan J.I., Aleman T.S. Evaluation of Dose and Safety of AAV7m8 and AAV8BP2 in the Non-Human Primate Retina. Hum. Gene Ther. 2017;28:154–167. doi: 10.1089/hum.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakai H., Thomas C.E., Storm T.A., Fuess S., Powell S., Wright J.F., Kay M.A. A limited number of transducible hepatocytes restricts a wide-range linear vector dose response in recombinant adeno-associated virus-mediated liver transduction. J. Virol. 2002;76:11343–11349. doi: 10.1128/JVI.76.22.11343-11349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao X., Li J., Samulski R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry G.E., Asokan A. Cellular transduction mechanisms of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:54–60. doi: 10.1016/j.coviro.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boye S.L., Bennett A., Scalabrino M.L., McCullough K.T., Van Vliet K., Choudhury S., Ruan Q., Peterson J., Agbandje-McKenna M., Boye S.E. Impact of Heparan Sulfate Binding on Transduction of Retina by Recombinant Adeno-Associated Virus Vectors. J. Virol. 2016;90:4215–4231. doi: 10.1128/JVI.00200-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodard K.T., Liang K.J., Bennett W.C., Samulski R.J. Heparan Sulfate Binding Promotes Accumulation of Intravitreally Delivered Adeno-associated Viral Vectors at the Retina for Enhanced Transduction but Weakly Influences Tropism. J. Virol. 2016;90:9878–9888. doi: 10.1128/JVI.01568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niwa Y., Kakinoki M., Sawada T., Wang X., Ohji M. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Invest. Ophthalmol. Vis. Sci. 2015;56:6501–6505. doi: 10.1167/iovs.15-17279. [DOI] [PubMed] [Google Scholar]
- 39.Park S.J., Choi Y., Na Y.M., Hong H.K., Park J.Y., Park K.H., Chung J.Y., Woo S.J. Intraocular Pharmacokinetics of Intravitreal Aflibercept (Eylea) in a Rabbit Model. Invest. Ophthalmol. Vis. Sci. 2016;57:2612–2617. doi: 10.1167/iovs.16-19204. [DOI] [PubMed] [Google Scholar]
- 40.Papadopoulos N., Martin J., Ruan Q., Rafique A., Rosconi M.P., Shi E., Pyles E.A., Yancopoulos G.D., Stahl N., Wiegand S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart M.W. Review of aflibercept for the treatment of neovascular age-related macular degeneration. Clin. Med. Insights Ther. 2013;5:81–93. [Google Scholar]
- 42.Sutter F.K., Gillies M.C. Pseudo-endophthalmitis after intravitreal injection of triamcinolone. Br. J. Ophthalmol. 2003;87:972–974. doi: 10.1136/bjo.87.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avery R.L., Gordon G.M. Systemic Safety of Prolonged Monthly Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2016;134:21–29. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 44.Wang X., Sawada T., Sawada O., Saishin Y., Liu P., Ohji M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am. J. Ophthalmol. 2014;158:738–744.e1. doi: 10.1016/j.ajo.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Zehetner C., Kirchmair R., Huber S., Kralinger M.T., Kieselbach G.F. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br. J. Ophthalmol. 2013;97:454–459. doi: 10.1136/bjophthalmol-2012-302451. [DOI] [PubMed] [Google Scholar]
- 46.Avery R.L., Castellarin A.A., Steinle N.C., Dhoot D.S., Pieramici D.J., See R., Couvillion S., Nasir M.A., Rabena M.D., Le K. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014;98:1636–1641. doi: 10.1136/bjophthalmol-2014-305252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner T.R., Anapol F., Jolly C.J. Growth, development, and sexual dimorphism in vervet monkeys (Cercopithecus aethiops) at four sites in Kenya. Am. J. Phys. Anthropol. 1997;103:19–35. doi: 10.1002/(SICI)1096-8644(199705)103:1<19::AID-AJPA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.